Note: Descriptions are shown in the official language in which they were submitted.
CA 02882728.2015-02-20
1
DESCRIPTION
METHOD FOR NON-FREEZE LOW-TEMPERATURE PRESERVATION OF
MAMMALIAN EMBRYO OR FERTILIZED EGG
TECHNICAL FIELD
[0001]
The present invention relates to a method and a preservative solution for
preserving a mammalian embryo(s) or fertilized egg(s) at non-freezing low
temperature.
BACKGROUND ART
[0002]
Not less than 60,000 bovine embryos are distributed per year in Japan. The
demand for bovine embryos is increasing year by year in North America, South
America, Europe and Asia. Thus, researches on cryopreservation of bovine
embryos are being carried out worldwide, and development of a number of slow
freezing methods and rapid freezing methods has realized relatively stable
conception
rates. However, the conception rate which can be achieved at the current
technological level is only about 50%, and no significant improvement in the
conception rate has been made during the last 20 or more years. This might
indicate
that cryopreservation of mammalian embryos has reached a certain technological
limit.
[0003]
Embryos resistant to damage caused by freezing/thawing are limited to high-
quality embryos. Production of a plurality of embryos by superovulation
treatment
often results in obtaining only low-quality embryos due to various factors
(for
example, bad physical condition of the donor cattle or low vitality of the
donated
sperm). Nevertheless, these low-quality embryos can also be implanted into the
CA 02882728.2015-02-20
2
uteri of recipient cattle to produce calves if the embryos have not undergone
freezing
treatment.
[0004]
Id from donor
there iso an tmethodtliv e non-frozen
a i l abl state et h a s allowstfora h
survivalshort
rot fpbeor period
d while
n eem ryo s
collectenoy
r cae n h suppressing their growth, embryos that have not suffered from injury
by
freezing/thawing can be very efficiently used for production of calves by
utilization
of transportation means such as home delivery services. Moreover, in cases
where
there is a time lag of several days between the estrous cycles of the donor
cattle and
the recipient cattle, the timing of implantation can be adjusted according to
the
estrous cycle of the recipient cattle. Furthermore, development of such a
method for
preserving embryos will eliminate the necessity of liquid nitrogen or an
expensive
refrigeration equipment such as a deep freezer. That is, preservation of
embryos in
a household refrigerator will become possible.
[0005]
It has been reported that the limit of the period of non-freezing preservation
is
3 days in the case of bovine embryos (Non-patent Document 1), and 4 days in
the
case of ovine embryos (Non-patent Document 2). A thermal hysteresis protein
(Nfe8) and thermal hysteresis-like proteins Nfell and Nfe6, which are found in
a fish
species Zoarces elongatus Kner, have been reported to exert a cell life
prolonging
function under low-temperature conditions (Patent Document 1), and their
practical
use as cell life prolonging agents has been expected.
[0006]
The animal species which have been studied for non-freezing low-
temperature preservation include various species such as mouse, pig, sheep,
cattle,
and rare species. The properties of embryos are largely different among the
animal
species. Thus, components effective for embryo preservation, and mixing ratios
and
CA 028827282015-02-20
3
effective concentration ranges of these components, are also largely different
among
the species, and therefore components for the preservative solution need to be
carefully examined for each animal species. The method of preservation of
bovine
embryos at non-freezing low temperature has long been studied from the
viewpoints
of improvement of the conception rate, estrus synchronization between the
donor
cattle and the recipient cattle, and utilization of low-quality embryos.
However, no
effective preservation method has been developed so far.
[0007]
Under such circumstances, development of a preservative solution for
mammalian embryos which not only enables more effective protection or
preservation of mammalian embryos at non-freezing low temperature to prolong
their
life, but also achieves maintenance of hatching ability and a high conception
rate, has
been demanded. Similarly, development of a method for preserving mammalian
embryos which not only enables their preservation for the number of days
required
for transportation, but also realizes their preservation and transportation
using small
general-purpose equipment or a container that consumes less energy, has been
strongly demanded.
PRIOR ART DOCUMENTS
PA ____ 1ENT DOCUMENT
[0008]
Patent Document 1: JP 2010-248160 A
NON-PATENT DOCUMENTS
[0009]
Non-patent Document 1: Lindner GM and Ellis DE. Refrigeration of bovine
embryos. Theriogenology 1985;23:202.
Non-patent Document 2: Baguisi A, Arav A, Crosby TF, Roche JF, Boland
MP. Hypothermic storage of sheep embryos with antifreeze proteins: development
in
81785782
4
vitro and in vivo. Theriogenology 1997;48:1017-1024.
SUMMARY OF THE INVENTION
PROBLEMS TO BE SOLVED BY THE INVENTION
[0010]
Accordingly, an object of the present invention is to provide means which
enables satisfactory preservation of mammalian embryos or fertilized eggs in
the
non-frozen state for a longer period than conventional means, which novel
means
also achieves high hatching ability and conception rate of the embryo after
the
preservation.
MEANS FOR SOLVING THE PROBLEMS
[0011]
As a result of intensive study, the present inventors discovered that use of a
combination of serum and a Good's buffer at specific concentrations enables
long-
term preservation of mammalian embryos in the non-frozen state without using a
cell
life prolonging peptide such as Nfel 1, and that use of HUES produces an
especially
favorable effect which enables achievement of very high survival and
conception
rates even in the case where the embryos are preserved in the non-frozen state
for a
period of as long as 7 days, thereby completing the present invention.
[0012]
That is, the present invention provides a method for preserving a mammalian
embryo(s) or fertilized egg(s), the method comprising immersing a mammalian
embryo(s) or fertilized egg(s) in a medium containing 20 to 80% (viv) serum
and 10
to 100 mM Good's buffer, and storing the embryo(s) or fertilized egg(s) at non-
freezing low temperature. The present invention also provides a preservative
solution for a mammalian embryo(s) or fertilized egg(s), essentially
consisting of a
medium containing 20 to 80% (v/v) serum and 10 to 100 mM Good's buffer.
CA 2882728 2018-11-28
81785782
4a
[0012A]
The present invention includes:
- a method for preserving a mammalian embryo(s) or fertilized egg(s), said
method comprising immersing a mammalian embryo(s) or fertilized egg(s) in a
medium
containing 20 to 80% (v/v) serum and 10 to 100 mM Good's buffer, wherein said
Good's buffer is at least one selected from the group consisting of HEPES,
TES, PIPES,
MOPS and EPPS, and storing the embryo(s) or fertilized egg(s) at a temperature
of -1 C
to 15 C; and
- a preservative solution for a mammalian embryo(s) or fertilized egg(s),
essentially consisting of a medium containing 20 to 80% (v/v) serum and 10 to
100 mM
Good's buffer, wherein said Good's buffer is at least one selected from the
group
consisting of HEPES, TES, PIPES, MOPS and EPPS.
EFFECT OF THE INVENTION
CA 2882728 2019-11-21
CA 028827282015-02-20
[0013]
By the present invention, mammalian embryos and fertilized eggs can be
preserved in the non-frozen state for a longer period compared to conventional
methods, without use of a special component as a preservative solution
component.
5 In the case where HEPES is used as the Good's buffer, an especially
favorable effect
can be obtained, and very high survival and conception rates can be achieved
even
after preservation of mammalian embryos in the non-frozen state for a period
of as
long as 7 days. Since non-freezing preservation of mammalian embryos or
fertilized eggs for 7 days has not yet been realized, the present invention
significantly
contributes to breeding of superior livestock as described below.
[0014]
Conventionally, fertilized livestock eggs for commercial purposes (for
example, 7-day-old embryos (morulae) of Japanese Black Cattle) are enclosed in
cylinders called straws having a diameter of about 2 mm and a length of about
15 cm,
= 15 in a state where the eggs are immersed in a preservative
solution, and commercially
distributed in a state where the straws are immersed in liquid nitrogen. In
the state
where the straws are immersed in liquid nitrogen, their air transportation is
prohibited
by the Civil Aeronautics Law, and the straws are therefore transported to
consumers
(animal husbandry associations and livestock farmers) by ground transportation
by
freight train or truck. Thus, there is a problem of an increase in the
transportation
cost, which leads to a rise in the price of fertilized livestock eggs. By the
present
invention, long-term non-freezing low-temperature preservation is possible,
and
fertilized eggs (embryos) can be transported in a state where the eggs are
contained in
a small cold box or the like, without use of liquid nitrogen. This allows
transportation by airplane, and enables low-cost and more rapid transportation
of
fertilized eggs to distant places. In cryopreservation of embryos, the embryos
are
physically damaged during freezing and thawing. On the other hand, in non-
CA 02882728 2015-02-20
6
freezing preservation, there is no such a risk, and an improved conception
rate can be
expected.
[0015]
Conventionally, in production of fertilized bovine eggs for commercial
purposes, about 20% of bovine embryos are discarded because of disagreement
between the estrous cycles of the donor cattle and the recipient cattle, or of
low
quality. By enabling preservation of livestock embryos in the non-frozen
state,
effective utilization of low-quality embryos that are not resistant to
physical damage
caused by freezing/thawing becomes possible. Moreover, even in cases where
there
is a time lag of several days between the estrous cycles of the donor and the
recipient,
it becomes possible to adjust the timing of implantation depending on the
estrous
cycle of the recipient. The present invention enables effective utilization of
livestock embryos that have been discarded because of the disagreement between
the
estrous cycles, or of low quality.
= 15 [0016]
According to the method of the present invention, embryos or fertilized eggs
immersed in a preservative solution can be used without washing, and can be
implanted into the uteri of recipient animals together with the preservative
solution.
The present inventors previously found that, by using a special peptide
(mature
Nfell) having a cell life prolonging effect in combination with about 20 to
50% (v/v)
serum, bovine embryos can be preserved at non-freezing low temperature for up
to 5
days. In cases where a peptide having cell life prolonging activity is
industrially
used, the peptide expressed in E. coli or the like by genetic engineering
techniques
and then purified is usually used. However, since implantation of embryos or
fertilized eggs together with a preservative solution containing a recombinant
peptide
into the uteri of recipient animals cannot be carried out until the safety of
the
recombinant peptide is sufficiently evaluated, the embryos or fertilized eggs
need to
CA 028827282015-02-20
7
be washed before the implantation. Since in the present invention a
recombinant
peptide is not used, embryos after preservation at low temperature can be
implanted
into recipient parents as they are without washing the embryos. This method is
more simple, and can reduce physical damage to the embryos.
BRIEF DESCRIPTION OF THE DRAWINGS
[0017]
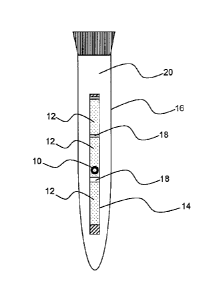
Fig. 1 is a diagram illustrating a specific example in which a bovine embryo
is
enclosed in a straw using the preservative solution of the present invention
to carry
out non-freezing low-temperature preservation.
MODE FOR CARRYING OUT THE INVENTION
[0018]
In the method of the present invention, a mammalian embryo(s) or fertilized
egg(s) is(are) immersed in a medium containing serum and a Good's buffer at
specific concentrations, and the embryo(s) or fertilized egg(s) is(are) stored
at non-
= 1 5 freezing low temperature.
[0019]
Good's buffers have common characteristics: for example, they are less likely
to pass through biomembranes; they are highly soluble in water; and they have
low
ability to form complexes with metal ions. Good's buffers are considered to
have
actions to stabilize the pH of the medium during preservation of embryos or
fertilized
eggs, and to protect the cell membrane of the embryos or fertilized eggs
during non-
freezing low-temperature preservation. As described in the Examples below, it
is
assumed that the effect of Good's buffers to prolong the life of embryos and
fertilized
eggs during non-freezing low-temperature preservation is not significantly
dependent
on the stabilization per se of the pH of the medium (preservative solution)
during the
preservation, but is based on a cell-membrane-protecting action other than the
pH
stabilization.
CA 028827282015-02-20
8
[0020]
Specific examples of the buffers known as Good's buffers include the
following:
MES [2-Morpholinoethanesulfonic acid],
Bis-Tris [Bis(2-hydroxyethypiminotris(hydroxymethypmethane],
ADA [N-(2-Acetamido)iminodiacetic acid],
= PIPES [Piperazine-1,4-bis(2-ethanesulfonic acid)],
ACES [N-(2-Acetamido)-2-aminoethanesulfonic acid],
MOPSO [2-Hydroxy-3-morpholinopropanesulfonic Acid],
BES [N,N-Bis(2-hydroxyethyl)-2-aminoethanesulfonic acid],
MOPS [3-Moipholinopropanesulfonic acid],
TES [N-Tris(hydroxymethyl)methy1-2-aminoethanesulfonic acid],
=
HEPES [4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid],
DIPSO [3-[N,N-Bis(2-hydroxyethyl)amino]-2-hydroxy-1-propanesulfonic acid],
= 15 TAPSO [3-(N-tris[Hydroxymethyl]methylamino)-2-
hydroxypropanesulfonic acid],
POPSO [Piperazine-1,4-bis(2-hydroxypropanesulfonic acid)],
HEPPSO [N-(Hydroxyethyl)piperazine-N'-2-hydroxypropanesulfonic acid],
EPPS [344-(2-Hydroxyethyl)-1-piperazinyl]propanesulfonie acid],
Tricine [N-[Tris(hydroxymethyl)methyl]glycine],
Bicine [N,N-Bis(2-hydroxyethyl)glycine],
TAPS [N-Tris(hydroxymethyl)methy1-3-aminopropanesulfonic acid],
CHES [N-Cyclohexy1-2-aminoethanesulfonic acid],
CAPSO [N-cyclohexy1-2-hydroxyl-3-aminopropanesulfonic acid], and
CAPS [N-Cyclohexy1-3-aminopropariesulfonic acid].
[0021]
In the present invention, any of these known Good's buffers can be used. It
is said that the optimum pH for cells is usually 6.8 to 7.2, and that cells
can be ideally
CA 02882728,2015-02-20
9
maintained within this pH range. Thus, a Good's buffer that can exert a
favorable
buffer capacity in the neutral range (pH 6 to 8) at non-freezing low
temperature can
be preferably used. Examples of the Good's buffer that can be favorably used
= include at least one selected from MES, Bis-Tris, ADA, PIPES, ACES,
MOPSO,
BES, MOPS, TES, HEPES, DIPSO, TAPSO, POPSO, HEPPSO, EPPS, Tricine,
Bicine, and TAPS; or at least one selected from HEPES, PIPES, and MOPS. In the
present invention, HEPES is especially preferably used. As a Good's buffer, a
single kind of Good's buffer or a combination of 2 or more kinds of Good's
buffers
= may be used.
10= [0022]
The serum concentration (final concentration) in the medium is 20 to 80%
(v/v), and may be, for example, 25 to 80% (v/v), 30 to 80% (v/v), 35 to 80%
(v/v), 40
to 80% (v/v), 45 to 80% (v/v), 20 to 60% (v/v), 25 to 60% (v/v), 30 to 60%
(v/v), 35
to 60% (v/v), 40 to 60% (v/v), 45 to 60% (v/v), or 45 to 55% (v/v).
[0023]
The Good's buffer concentration (final concentration) in the medium is 10 to
100 mM, and may be, for example, 12.5 to 100 mM, 15 to 100 mM, 20 to 100 mM,
22.5 to 100 mM, 12.5 to 80 mM, 15 to 80 mM, 20 to 80 mM, 22.5 to 80 mM, 20 to
60 mM, 22.5 to 60 mM, 20 to 55 mM, 22.5 to 55 mM, or 22.5 to 52.5 mM. In cases
where HEPES is used alone, the HEPES concentration in the medium can be
preferably selected within these concentration ranges. The concentration may
be
similarly selected also in cases where another Good's buffer is used. In cases
where
two or more kinds of Good's buffers are used in combination, the total
concentration
may be within these ranges.
[0024]
However, in cases where the Good's buffer is used at low concentration even
within the above-described ranges, it is preferred that the serum
concentration be
CA 02882728,2015-02-20
higher. For example, in cases where the Good's buffer is used at a
concentration of
less than 15 mM, the serum concentration is preferably not less than about 30%
(v/v),
e.g., not less than about 40% (v/v).
[0025]
5 By using serum and a Good's buffer at the above-described
concentrations in
combination, a mammalian embryo or fertilized egg can be preserved at non-
freezing
low temperature for a long period. In the present invention, the survival
rate,
hatching rate, and the like of embryos or fertilized eggs after preservation
at non-
freezing low temperature can be improved without using a peptide having cell
life
10 prolonging activity, such as the one described in Patent Document 1.
[0026]
The peptide having cell life prolonging activity (which may be hereinafter
referred to as "cell life prolonging peptide") refers to a peptide which
allows cells
preserved in the presence of the peptide under non-freezing low temperature
= 15 conditions to survive at a significantly higher rate
when compared to cells preserved
in the absence of the peptide. Specific examples of the peptide include the
Nfel I
protein described also in Patent Document 1. The present inventors previously
discovered that use of a combination of mature Nfel 1 and about 20 to 50%
(v/v)
serum enables preservation of bovine embryos at non-freezing low temperature
for
up to 5 days, and applied for a patent. Mature Nfe 1 1 is a peptide composed
of a 66-
residue amino acid sequence formed by removal of the 22-residue secretory
signal
sequence in the N-terminal side of the precursor Nfell protein. Its amino acid
sequence is shown in SEQ ID NO:2. SEQ ID NO:1 is the base sequence of cDNA
encoding mature Nfel 1. The "peptide having cell life prolonging activity"
includes
not only the peptide composed of the same amino acid sequence as the amino
acid
sequence of SEQ ID NO:2, but also a peptide composed of an amino acid sequence
derived from the amino acid sequence of SEQ ID NO:2 by substitution, deletion,
CA 028827282015-02-20
11
insertion and/or addition of a small number (e.g., 1 to several, 2 to 5, or 2
to 3) of the
amino acid residues and having a homology of not less than 80%, for example,
not
less than 85%, not less than 90%, not less than 95%, or not less than 98% to
the
original amino acid sequence shown in SEQ ID NO:2, as long as such a peptide
has a
3 cell life prolonging activity.
[0027]
Whether a peptide has cell life prolonging activity or not can be evaluated
by,
for example, incubating cells in the presence of the peptide and investigating
whether
or not survival of the cells is significantly prolonged when compared to
control cells
incubated in the absence of the peptide. The evaluation can be carried out by,
for
example, a method in which the survival rate is measured using a combination
of a
live cell staining fluorescent dye (e.g., Calcein-AM) and a dead cell staining
fluorescent dye (e.g., propidium iodide) (De Clerck et al., Journal of
Immunological
Methods, 1994, 172(1), pp. 115-124; Nicoletti et al., Journal of Immunological
Methods, 1991, 139(2), pp. 271-279), or a method in which the survival rate of
cells
is measured by quantifying cell damage (i.e., damage of the cell membrane)
using the
level of lactate dehydrogenase (LDH) as an indicator (T. Decker and M. L. L.
Matthes, Journal of Immunological Methods, 1988, 115(1), pp. 61-69).
[0028]
20= In the present invention, since the long-term preservation
at non-freezing low-
temperature is achieved by a combination of serum and a Good's buffer at
specific
concentrations, there is no need to add a peptide having cell life prolonging
activity
to the medium. An example of the mode of the method of the present invention
is a
method in which a mammalian embryo or fertilized egg is immersed in a medium
which contains 20 to 80% (v/v) serum and 10 to 100 mM Good's buffer but
contains
neither (a) nor (b) described below, and the embryo or fertilized egg is then
stored at
non-freezing low temperature.
CA 02882728,2015-02-20
12
(a) The peptide whose amino acid sequence is shown in SEQ ID NO:2
(b) A peptide having an identity of not less than 80% to the amino acid
sequence shown in SEQ ID NO:2 and having cell life prolonging activity.
[0029]
Specific examples of the serum used in the present invention include bovine
serum, pig serum, goat serum, and horse serum. Bovine serum is preferably
used,
but not limited thereto. Bovine serum may be any of fetal bovine serum, calf
serum,
and adult bovine serum.
[0030]
The embryo(s) or fertilized egg(s) preserved by the method of the present
invention is(are) not limited, and preferably an embryo(s) or fertilized
egg(s) of
livestock such as cattle, pig, goat, or horse. The method of the present
invention
can be especially preferably applied to a bovine embryo(s) or fertilized
egg(s). The
beginning
n nh o f nogot the e p e leraevsae gnet oinventionrtoa fe s r t l
applicableized eggaft er either t be to a beginning
innfertilizedn egg be
forecleavage
the
e he
1 5 .
=
embryo).
[0031]
The medium used in the method of the present invention may be a medium
commonly used for culturing mammalian embryos. Specific examples of
commercially available media that can be used for culturing mammalian embryos
include, but are not limited to, the following media. It should be noted that
the
concentrations of the components described below are merely examples in
representative commercially-available products, and not limited to the
specific
examples described below.
[0032]
<Medium 199>
Composition: 200 mg/L calcium chloride, 0.7 mg/L Iron(III) nitrate
81785782
13
nonahydrate, 400 mg/L potassium chloride, 97.67 mg/L magnesium sulfate, 6.1 to
6.8g/L
sodium chloride, 2.2 g/L sodium hydrogen carbonate, 1 mM sodium dihydrogen
phosphate (non-hydrate or monohydrate), 10 mg/L adenine sulfate, 1 mg/L
adenosine-5-
triphosphate, 0.2 mg/L adenosine-5-phosphate, 0.2 mg/L cholesterol, 0.5 mg/L
deoxyribose, 1 g/L D-glucose, 0.05 mg/L reduced glutathione, 0.3 mg/L guanine
hydrochloride, 0 mM or 25 mM HEPES (optional component), 0.4 mg/L sodium
hypoxanthine, 20 mg/L Phenol Red, 0.5 mg/L ribose, 50 mg/L sodium acetate, 0.3
mg/L
thymine, 20 mg/L TweenTM 80, 0.3 mg/L uracil, 0.34 mg/L sodium xanthine, 25
mg/L
L-alanine, 70 mg/L L-arginine hydrochloride, 30 mg/L L-aspartic acid, 0.1 mg/L
L-cysteine hydrochloride monohydrate, 26 mg/L L-cystine dihydrochloride, 75
mg/L
L-glutamic acid, 50 mg/L glycine, 21.88 mg/L L-histidine hydrochloride
monohydrate,
10 mg/L L-hydroxyproline, 40 mg/L L-isoleucine, 60 mg/L L-leucine, 70 mg/L L-
lysine
hydrochloride, 15 mg/L L-methionine, 25 mg/L L-phenylalanine, 40 mg/L L-
proline,
25 mg/L L-serine, 30 mg/L L-threonine, 10 mg/L L-tryptophan, 58 mg/L disodium
L-tyrosine dihydrate, 25 mg/L L-valine, 0.05 mg/L ascorbic acid, 0.01 mg/L a-
tocopherol
phosphate, 0.01 mg/L d-biotin, 0.1 mg/L calciferol, 0.01 mg/L calcium D-
pantothenate,
0.5 mg/L choline chloride, 0.01 mg/L folic acid, 0.05 mg/L i-inositol, 0.01
mg/L
menadione, 0.025 mg/L nicotinic acid, 0.025 mg/L niacinamide, 0.05 mg/L
para-aminobenzoic acid, 0.025 mg/L pyridoxal hydrochloride, 0.025 mg/L
pyridoxine
hydrochloride, 0.01 mg/L riboflavin, 0.01 mg/L thiamine hydrochloride, and 0.1
mg/L
vitamin A.
[0033]
<CR1aa Medium as Modified Medium 199>
Composition: 6.653 g/L sodium chloride, 0.233 g/L potassium chloride,
2.200 g/L sodium bicarbonate, 0.545 g/L calcium lactate, 0.044 g/L sodium
pyruvate,
0.146 g/L L-glutamine, 20 mL/L MEM essential amino acid solution, 10 mL/L MEM
non-essential amino acid solution, and 0.2 to 0.5% bovine serum albumin.
CA 2882728 2018-11-28
CA 02882728,2015-02-20
14
[0034]
<HP-M199 Medium>
Composition: the same composition as medium 199 except that Tween 80 and
para-aminobenzoic acid are not contained; HEPES is not contained.
[0035]
In addition, a medium commonly used for culturing mammalian embryos may
be mixed with one or more of other media such as BME medium, CMRL1066
medium, MEM medium, NCSU medium, and their modified media, to provide the
medium for use. In cases where a commercially available medium already
containing a Good's buffer and/or serum is used, the total concentration(s),
including
the concentration of this Good's buffer and/or the concentration of this
serum, may
be within the above-described concentration range(s) of Good's buffer and/or
serum.
[0036]
To the medium, an additive(s) such as an antioxidant(s) and/or stabilizer(s)
may be added, if necessary. Examples of such components include phosphate
salts,
citrate salts, pyruvate salts, and other organic acids; antioxidants (e.g.,
SOD, vitamin
E, and glutathione); pH adjusters (e.g., hydrochloric acid, acetic acid, and
sodium
hydroxide); low-molecular-weight polypeptides; hydrophilic polymers (e.g.,
polyvinylpyrrolidone); amino acids (e.g., glycine, glutamine, asparagine,
arginine,
and lysine); vitamins (e.g., vitamin A, vitamin Bs, vitamin C, vitamin Ds,
biotin,
folic acid); monosaccharide, disaccharide, and polysaccharide compounds
(including
glucose, mannose, and dextrin); chelating agents (e.g., EDTA); sugar alcohols
(e.g.,
mannitol and sorbitol); salt-forming counterions (e.g., sodium); and nonionic
surfactants (e.g., polyoxyethylene sorbitan ester (Tween (trademark)),
polyoxyethylene-polyoxypropylene block copolymers (Pluronic (trademark)), and
polyethylene glycol).
[0037]
CA 02882728,2015-02-20
To the medium to be used for the embryo preservation, one or more of known
buffer salt solutions such as bicarbonate buffer, phosphate buffer, and
modified
solutions thereof; antibiotics; and the like; may be added, if necessary.
[0038]
5 The mammalian embryos or fertilized eggs can be provided by a known
method known in the art. For example, follicular eggs collected by aspiration
from
the ovary of a mammal may be cultured in vitro, and then be co-cultured with
sperm
of the same mammal (insemination) to obtain a fertilized egg, followed by
developing the fertilized egg to obtain an embryo. In general, from the
10 morphological viewpoint, embryos commercially distributed are at a stage
between
the morula stage and the blastocyst stage. Those skilled in the art can easily
identify
embryos at a stage between the morula stage and the blastocyst stage based on
their
morphologies. For example, such embryos can be easily identified by comparing
their micrographs with the images provided in the International Embryo
Transfer
15 Society (IETS) manual.
[0039]
The container for preservation of the embryo or fertilized egg may be any
container as long as the container shows no toxicity to mammalian embryos or
fertilized eggs, and examples of the container include straws for freezing
semen,
straws for fertilized egg implantation, sampling tubes, cryotubes, cell
culture dishes,
cell culture plates, and vials. For maintenance of a non-freezing low-
temperature
environment, a thermostat, thermostat container, incubation container (whose
type
may be either the gas (normally air) tank type or liquid (normally water) tank
type),
incubation bag, low-temperature chamber, household refrigerator, or the like
may be
used. Although light is preferably eliminated as much as possible during the
preservation of the embryos or fertilized eggs, means commonly used for
maintaining
a non-freezing low-temperature environment usually provides a dark
environment,
CA 02882728,2015-02-20
16
and therefore in many cases no special care is required in this regard.
[0040]
In the present invention, the mammalian embryo(s) or fertilized egg(s) is(are)
enclosed in an appropriate preservation container in a state where the
embryo(s) or
fertilized egg(s) is(are) immersed in a medium containing serum and a Good's
buffer
at the specific concentrations described above, and the enclosed embryo(s) or
fertilized egg(s) is(are) stored under a non-freezing low-temperature
environment.
The non-freezing low temperature means a low temperature at which the medium
and
the mammalian embryo(s) or fertilized egg(s) are not frozen, and examples of
the
non-freezing low temperature include temperatures within the range of -1 to 15
C, 2
to 10 C, or 4 to 5 C.
[0041]
By the method of the present invention, a mammalian embryo(s) or fertilized
egg(s) can be preserved at non-freezing low temperature for as long as 7 days
without
use of a cell life prolonging peptide such as mature Nfel 1. For example, in
the
Examples below, it was found that the embryo preservation performance (the
survival rate, hatching rate, and conception rate after the preservation) is
especially
excellent when medium 199 containing 50% (v/v) fetal bovine serum and, as the
Good's buffer, 25 mM or 50 mM HEPES, is used. Thus, examples of especially
preferred modes of the method of the present invention include, but are not
limited to,
a mode in which 45 to 55% (v/v) serum and, as the Good's buffer, 22.5 to 52.5
mM
HEPES are used.
[0042]
The medium containing serum and a Good's buffer at the specific
concentrations described above to be used for the preservation method of the
present
invention may be provided as a preservative solution for preserving a
mammalian
embryo(s) or fertilized egg(s) at non-freezing low temperature. That is, the
present
CA 02882728,2015-02-20
17
invention also provides a preservative solution for a mammalian embryo(s) or
fertilized egg(s), which preservative solution essentially consists of a
medium
containing 20 to 80% (v/v) serum and 10 to 100 mM Good's buffer.
[0043]
In the present invention, since the long-term preservation at non-freezing low-
temperature is achieved by a combination of serum and a Good's buffer at
specific
concentrations, there is no need to add a peptide having cell life prolonging
activity
to the medium. An example of the mode of the preservative solution of the
present
invention is a preservative solution essentially consisting of a medium which
1 0 contains 20 to 80% (v/v) serum and 10 to 100 mM Good's buffer but
contains neither
(a) nor (b) described below.
(a) The peptide whose amino acid sequence is shown in SEQ ID NO:2
(b) A peptide having an identity of not less than 80% to the amino acid
sequence shown in SEQ ID NO:2 and having cell life prolonging activity.
[0044]
The term "essentially consisting of' means that any other component(s) may
be contained as long as the component(s) do(does) not affect the effect of the
invention. The preservative solution of the present invention may contain an
arbitrary component(s) other than the peptide having cell life prolonging
activity, as
long as the medium contains serum at a specific concentration and a Good's
buffer at
a specific concentration. The component(s) that may be arbitrarily contained
is(are)
as described above. Preferred conditions in terms of the serum concentration,
type
of the serum, concentration of the Good's buffer, and the like are also as
described
above.
[0045]
The preservative solution may be sterilized, as necessary, by filtration
(normally with a filter having a pore size of 0.2 um) or the like after its
preparation,
CA 02882728.2015-02-20
18
and stored in a cool and dark place until use.
EXAMPLES
[0046]
The present invention is described below more concretely by way of
Examples. However, the present invention is not limited to the Examples below.
[0047]
<Materials and Methods>
1. Preparation of Bovine Embryos
Embryos collected from donor bovine animals of Japanese Black Cattle and
Holstein Cattle after superovulation were evaluated for their qualities
according to
the regulation by International Embryo Transfer Society (JETS manual. Manual
of
the international embryo transfer society. In: Stringfellow DA, Seidel SM,
editors.
Savoy, Illinois, USA: IETS 1998.). Low-quality embryos were subjected to an in
vitro culture test after 7 days of non-freezing preservation, and high-quality
embryos
were subjected to investigation of the conception rate after implantation to
recipient
cattle.
[0048]
Preservation of bovine embryos was carried out as follows (Fig. 1). First, a
preservative solution alone was sucked into a straw having a capacity of 0.25
ml, and,
after introduction of an air layer, a bovine embryo contained in the
preservative
solution was sucked. Thereafter, an air layer was introduced, and the
preservative
solution alone was sucked again. The straw was then sealed. Subsequently, the
straw containing the sucked bovine embryo was immersed in water contained in a
plastic container (plastic tube) which had been preliminarily cooled to 4 to 5
C.
The plastic container was then stored in a refrigerator for 7 days at 4 to 5
C.
[0049]
After the non-freezing preservation, the bovine embryo was washed 3 times
CA 02882728 2015-02-20
19
by immersion in PBS(+) containing 5% (v/v) fetal bovine serum (FBS). The
washed bovine embryo was cultured under 5% CO2 at 38 C in CRlaa medium
containing 5% (v/v) FBS for 48 hours. Morphological observation was carried
out
under a stereoscopic microscope, and the embryo was judged to be alive if the
progress of cleavage by the culture could be found and the cytoplasmic
membrane
was not disrupted. Live cells were checked for whether hatching from the zona
pellucida could be observed or not. A bovine embryo washed with PBS(+)
containing 5% (v/v) FBS was nonsurgically implanted into the deep part of the
corpus-luteum-side uterine horn of each recipient cattle on Day 6 to 8 of an
estrous
cycle (the date of estrus was defined as Day 0). On Days 30 and 60 of the
estrous
cycle, pregnancy diagnosis was carried out. For a significance test to verify
the
effect of the embryo preservation solution according to the present invention,
a X2 test
was used.
[0050]
2. Preparation of Non-freezing Preservative Solutions
To medium 199 (Invitrogen) supplemented with antibiotics (penicillin G
potassium and streptomycin sulfate), FBS was added to 20 to 50% (v/v), and
HEPES
was also added to a concentration of 0 to 100 mM. In the conception test, a
group
in which a special peptide having a protection effect against low temperature
(mature
Nfe 11, SEQ ID NO:2) was added was also provided. In addition, in order to
investigate the effects of other Good's buffers other than HEPES, preservation
solutions were prepared by adding FBS and a Good's buffer (PIPES or MOPS) to
medium 199 such that the concentration of FBS was 50% (v/v) and the
concentration
of the Good's buffer was 25 mM. These preservation solutions were sterilized
by
filtration through a filter having a pore size of 0.2 inn, and stored in a
refrigerator
until use.
[0051]
CA 02882728,2015-02-20
The special peptide (mature Nfell) was prepared according to the following
procedure.
[0052]
According to the procedure described in Patent Document 1, a DNA sequence
5 (SEQ ID NO:1) encoding mature Nfell (SEQ ID NO:2) was incorporated into
pET20b (Novagen) to construct a plasmid pET2ONFE11, and E. coli BL21 (DE3)
(Novagen) was transformed with the resulting expression vector. The obtained
transformant was cultured in 2xYT medium supplemented with 100 ptg/m1
ampicillin
at 28 C for 24 hours with shaking.
10 [0053]
After the shake culture for 24 hours, 1/100 volume of the culture was
subcultured into freshly prepared 2xYT medium supplemented with 100 tg/m1
ampicillin, and shake culture was carried out at 28 C. When the turbidity of
the
culture reached 0.D.600=0.5, IPTG (isopropyl-13-DO-thiogalactopyranoside) was
= 15 added to the culture to a final concentration of 0.5 mM
to induce expression of Nfel 1,
and culture was carried out for additional 18 hours.
[0054]
The culture was then subjected to centrifugation at 5000 rpm for 15 minutes
at 4 C to collect the bacterial cells. To the collected cells, 1/20 volume,
with
20 respect to the volume of the culture, of TE buffer (10 mM Tris-HC1/1 mM
EDTA,
pH 8.0) was added, and PMSF (phenylmethylsulfonyl fluoride) was added to the
resulting mixture to 0.1 mM, followed by suspending the bacterial cells.
[0055]
The resulting suspension was once frozen and then thawed, followed by
carrying out homogenization of the bacterial cells by an ultrasonic device.
The
obtained homogenate was subjected to centrifugation at 11,000 rpm for 20
minutes at
4 C, and the supernatant was collected. In this supernatant, citric acid
monohydrate
81785 782
21
(13.2 g/L) and sodium chloride (29.2 g/L) were dissolved, and the resulting
solution
was left to stand at 4 C for 1 hour. Thereafter, the precipitate generated was
subjected to centrifugation at 6,000 rpm at 4 C for 30 minutes, and a
supernatant
containing the protein of interest was collected.
[0056]
The supernatant containing the protein of interest described above was passed
TM
through a Sephadex G-25 gel filtration column (GE Healthcare) with an inner
diameter of 5.0 cm and a height of 26 cm (capacity, 500 ml) using 5 mM sodium
citrate solution as the mobile phase, to obtain a protein solution in which
the solvent
was replaced with 5 mM sodium citrate solution. Subsequently, the pH of the
protein solution was adjusted to 2.9 with a citric acid solution, and the
solution was
then left to stand at 4 C overnight to allow acid denaturation and
precipitation of
contnminoting proteins. The resulting precipitate was removed by
centrifugation at
6,000 rpm at 4 C for 30 minutes, to obtain a supernatant containing the
protein of
interest.
[0057]
Thereafter, a cation exchange High-S column (Bio-Rad) with an inner
diameter of 5.0 cm and a height of 4.6 cm (capacity, 90 ml) was preliminarily
equilibrated with 50 mM sodium citrate buffer (pH 2.9), and the collected
supernatant containing the protein of interest was passed through the column.
The
protein of interest adsorbed to the resin was eluted with 50 mM sodium
citrate/330
mM NaC1 (pH 2.9), and collected. The aqueous solution of the purified product
was further subjected to 5 times of dialysis with 10 volumes of uhrapure
water, and
then subjected to freeze drying, thereby obtaining freeze-dried powder of the
purified
product of mature Nfell.
[0058]
<Results and Discussion>
CA 2882728 2018-11-28
CA 02832728 2015-02-20
22
1. Preservation Effect of HEPES-containing Preservation Solutions on Embryos
As shown in Table 1, it was found that low-quality bovine embryos preserved
in a non-freezing preservative solution whose HEPES concentration was adjusted
to
12.5 to 100 mM showed a higher survival rate after preservation for 7 days
compared
to the HEPES-free group. The rate of hatching from the zona pellucida was
especially high in the cases where the preservative solution contained HEPES
at a
concentration of 25 to 50 mM and FBS at a concentration of 50% (v/v).
[0059]
[Table 1]
Survival rate of bovine embryos preserved for 7 days in the non-frozen state
in medium 199 containing
HEPES and FBS
Number of
HEPES FBS
Number of Number of hatched pH upon
tested live embryos completion
of
concentration concentration embryos
embryos (%) (%)
preservation
O mM 20% 30 4(13.3)8 1(3.3)8 7.9
50% 30 3(10.0)8 1(3.3)8 71
= 20% 35 9(25.7)8 3(8.6)ab 74
12.5mM
50% 35 19(54.3)1' 1'74
25 mM 20% 36 23(63.9)1' 9(25.0)bc 7.3
= 50% 36 25(69.4)1 13(36.1)8 7.0
50mM
20% 30 19(63.3)b 8(26.7) 6.9
1'
50% 30 22(73.3)b 10(33.3)8 6.8
100mM 50% 20 13(65.0)b 4(20.0)48 6.9
a, b, c; difference is significant between different symbols (P <0.05)
[0060]
While the pH of the non-freezing preservative solution containing HEPES
was within the range of 6.9 to 7.4 upon completion of the preservation, the pH
in the
HEPES-free group was 7.7 to 7.9. It is said that the optimum pH for cells is
usually
6.8 to 7.2, and that cells can be ideally maintained within this pH range.
HEPES,
which is a Good's buffer, is known to have high buffer capacity and high
solubility in
aqueous solutions to stabilize the pH. It has been thought that Good's buffers
do
not easily pass through the cell membrane. In view of this, the protection
effect of
tris(hydroxymethyl)aminomethane (hereinafter referred to as tris), which is
often
CA 02832728 2015-02-20
23
used as a buffer together with HEPES and is not a Good's buffer, against low
temperature was studied by low-temperature preservation of bovine embryos in
medium 199 containing 50% (v/v) FBS supplemented with 25 mM tris (whose pH
was adjusted to 7.2 with hydrochloric acid) for 7 days. As a result, the
survival rate
and the hatching rate after the preservation were very low: 10.0% and 5.0%,
respectively. The pH after the low-temperature preservation was 7.2. These
results suggested that, although HEPES has an effect to stabilize the pH of
the non-
freezing preservative solution, the improvement of the performance in the non-
freezing preservation of embryos may not be significantly dependent on the
10= stabilization per se of the pH, but may be based on a cell-
membrane-protecting effect,
other than the pH stabilization effect, of HEPES during the low-temperature
preservation.
[0061]
2. Investigation of Conception Ability
Subsequently, the conception ability of high-quality bovine embryos that had
been preserved in the non-frozen state for 7 days was investigated. Since the
concentration of HEPES generally recommended for use in cell culture is 10 to
25
mM (Luo S et al., Effect of HEPES buffer on the uptake and transport of P-
glycoprotein substrates and large neutral amino acids. Mol Pharm 2010;7:412-
420.),
the concentration of HEPES in the non-freezing preservative solution used in
the
present test for investigation of the conception ability was set to 25 mM. The
special recombinant peptide having a protection effect against low temperature
(mature Nfell peptide) was added to medium 199 containing 50% (v/v) FBS
supplemented with 25 mM HEPES, to see whether the peptide improves the
conception ability or not. The results are shown in Table 2.
[0062]
[Table 2]
CA 02882728 2015-02-20
=
24 =
Conception rate of bovine embryos preserved for 7 days in the non-frozen state
in
medium 199 containing 50%(VN) FBS supplemented with 25 mM HEPES
Special Number of
Conceptus number Conceptus number
recombinant implanted
on Day 30 (%) on Day 60 (%)
peptide embryos
Not contained 16 12 12
(75.0) (75.0)
Contained 16 12 12
(75.0) (75.0)
[0063]
The conception rate of the high-quality bovine embryos preserved in the non-
frozen state for 7 days in the medium 199 containing 50% (v/v) FBS
supplemented
with 25 mM HEPES was as high as 75.0%. Death of early embryos was not found
between Day 30 and Day 60 of pregnancy, and birth of normal calves could be
observed. No conception-promoting effect was found in the special peptide in
the
present study. The results of the present study indicate that the effect of
the special
recombinant peptide can be substituted by use of HEPES and serum.
[0064]
3. Investigation of Effects of Good's Buffers Other Than HEPES
In order to investigate the effects of Good's buffers other than HEPES, the
following experiment was carried out using, in addition to HEPES, the Good's
buffers shown in Table 3 below.
Using medium 199 to which a Good's buffer was added at 25 mM (FBS
concentration, 50% (v/v) FBS), bovine embryos were preserved at low
temperature
for 7 days, and the survival rate and the hatching rate were investigated by
the
method described above. As a control, medium 199 which does not contain a
Good's buffer and contains FBS at a concentration of 50% (v/v) was used. The
results are shown in Table 3. In Table 3, "pH" indicates the pH of the medium
upon
completion of the refrigerated storage for 7 days.
[0065]
81785782
[Table 3]
Buffer N Survival rate Hatched embryos pH
(%)
TES 20 6(30.0) 3(15.0) 7.2
PIPES 20 8(40.0) 5(25.0) 7.0
MOPS 20 9(45.0) 5(25.0) 7.0
EPPS 20 7(35.0) 2(10.0) 7.3
HEPES 36 25(69.4) 13(36.1) 7.0
Not added 30 3(10.0) 1(3.3) 7.7
[0066]
Also in the cases where a Good's buffer other than HEPES was used, the
5 survival rate and the hatching rate of the embryos after the preservation
for 7 days
tended to be high. Although HEPES had the highest effect, the Good's buffers
other than HEPES could also be confirmed to have the effect to protect embryos
during non-freezing low-temperature preservation.
DESCRIPTION OF SYMBOLS
10 [0067]
10 Bovine embryo
12 Preservative solution
14 Straw
16 Plastic Tube
15 18 Air layer
20 Water
SEQUENCE LISTING IN ELECTRONIC FORM
In accordance with Section 111(1) of the Patent Rules, this
description contains a sequence listing in electronic form in ASCII
text format (file: 72643-137 Seq 19-02-2015 vl.txt).
A copy of the sequence listing in electronic form is available from
the Canadian Intellectual Property Office.
CA 2882728 2019-11-21