Note: Descriptions are shown in the official language in which they were submitted.
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
BATTERIES HAVING NANOSTRUCTURED COMPOSITE CATHODE
RELATED APPLICATIONS
[0001] This application claims the benefit and priority to U.S. Patent
Application No. 13/795,515, filed March 12, 2013, which claims the benefit and
priority
to U.S. Provisional Patent Application Serial No. 61/692,572, filed August 23,
2012, the
content of each of which is hereby incorporated herein by reference in its
entirety.
[0002] This application is a continuation-in-part of U.S. Patent
Application No.
13/367,572, filed February 7, 2012, which is a continuation-in-part of U.S.
Patent
Application No. 12/437,538, filed May 7, 2009, which claims priority to U.S.
Provisional Patent Application Serial No. 61/051,249, filed May 7, 2008, the
content of
each of which is hereby incorporated herein by reference in its entirety.
TECHNICAL FIELD
[0003] The present invention relates to batteries, and more particularly,
to
secondary batteries employing sheets of non-woven carbon nanotubes.
BACKGROUND
[0004] Carbon nanotubes are known to have extraordinary tensile strength,
including high strain to failure and relatively high tensile modulus. Carbon
nanotubes
may also be highly resistant to fatigue, radiation damage, and heat. To this
end, the
addition of carbon nanotubes to composite materials can increase tensile
strength and
stifthess of the composite materials.
[0005] Within the last fifteen (15) years, as the properties of carbon
nanotubes
have been better understood, interests in carbon nanotubes have greatly
increased within
and outside of the research community. One key to making use of these
properties is
the synthesis of nanotubes in sufficient quantities for them to be broadly
deployed. For
example, large quantities of carbon nanotubes may be needed if they are to be
used as
high strength components of composites in macroscale structures (i.e.,
structures having
dimensions greater than 1 cm.)
[0006] One common route to nanotube synthesis can be through the use of
gas
phase pyrolysis, such as that employed in connection with chemical vapor
deposition.
1
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
In this process, a nanotube may be formed from the surface of a catalytic
nanoparticle.
Specifically, the catalytic nanoparticle may be exposed to a gas mixture
containing
carbon compounds serving as feedstock for the generation of a nanotube from
the
surface of the catalyst particle.
[0007] Recently, one promising route to high-volume nanotube production
has
been to employ a chemical vapor deposition system that grows nanotubes from
catalyst
particles that "float" in the reaction gas. Such a system typically runs a
mixture of
reaction gases through a heated chamber within which the nanotubes may be
generated
from catalyst particles that have precipitated from the reaction gas. Numerous
other
variations may be possible, including ones where the catalyst particles may be
pre-
supplied.
[0008] In cases where large volumes of carbon nanotubes may be generated,
however, the nanotubes may attach to the walls of a reaction chamber,
resulting in the
blockage of nanomaterials from exiting the chamber. Furthermore, these
blockages may
induce a pressure buildup in the reaction chamber, which can result in the
modification
of the overall reaction kinetics. A modification of the kinetics can lead to a
reduction in
the uniformity of the material produced.
[0009] An additional concern with nanomaterials may be that they need to
be
handled and processed without generating large quantities of airborne
particulates, since
the hazards associated with nanoscale materials are not yet well understood.
[00010] The processing of nanotubes or nanoscale materials for macroscale
applications has steadily increased in recent years. The use of nanoscale
materials in
textile fibers and related materials has also been increasing. In the textile
art, fibers that
are of fixed length and that have been processed in a large mass may be
referred to as
staple fibers. Technology for handling staple fibers, such as flax, wool, and
cotton has
long been established. To make use of staple fibers in fabrics or other
structural
elements, the staple fibers may first be formed into bulk structures such as
yarns, tows,
or sheets, which then can be processed into the appropriate materials.
2
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
[0 0 0 1 1] Accordingly, it would be desirable to provide a material that
can take
advantage of the characteristics and properties of carbon nanotubes, so that
sheets made
of carbon nanotubes can be processed for end use applications, such as
batteries.
SUMMARY
[00012] The present invention provides, in accordance with an embodiment,
a
battery comprising a negative electrode including an anode current collector
having at
least one sheet of carbon nanotubes with semiconductor particles, a positive
electrode
including a cathode current collector having at least one sheet of carbon
nanotubes
infiltrated with mixed metal oxides, and a separator, situated between the
negative
electrode and positive electrode. The battery further includes a casing made
from
carbon nanotube composite material. In one embodiment, the composite material
can
comprise carbon nanotubes and polyamide, polyphenylene sulfide, polyether
ether
ketone, polypropylene, bispolyamide, bismaleimide, epoxies or a combination
thereof.
[00013] The carbon nanotube sheets can be made from single wall carbon
nanotubes or multi-wall carbon nanotubes. The sheet of carbon nanotubes can
have
density of about 80 g/m2. The sheet of carbon nanotubes can comprise
substantially
aligned carbon nanotubes and can further include lithium as an intercalation
compound.
[00014] According to one embodiment, the anode includes semiconductor
particles such as silicon or germanium particles. The silicon or germanium
particles can
be ultrasonically welded onto the carbon nanotubes. The cathode, in an
embodiment,
includes a sheet of carbon nanotubes infiltrated with a lithium mixed metal
oxide
comprising lithium, nickel, cobalt or mixtures thereof or alternatively
infiltrated with
zinc-nickel oxide. The separator can be a porous polyethylene membrane, or
polyethylene membrane, or a combination thereof.
[00015] The present invention also provides a method for forming an anode.
The
method includes generating a substantially planar body defined by a matrix of
carbon
nanotubes in the presence of semiconductor particles so as to allow the
formation of a
carbon nanotube sheet with semiconductor particles throughout the matrix of
nanotubes.
In an embodiment, an intercalation compound, such as lithium, can be dispersed
within
the sheet during the formation of the sheet or infiltrated within the sheet
after the
3
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
formation of the sheet. A volume of the mixture can then be deposited onto a
surface so
as to form a substantially planar body defined by a matrix of carbon nanotubes
with
semiconductor particles interdispersed within the matrix of nanotubes. In some
embodiments, a cloud of carbon nanotubes is formed and semiconductor particles
are
introduced within the cloud to form a cloud of a mixture of carbon nanotubes
and
semiconductor particles. In some embodiments, the lithium can be mixed with a
volatile carrier in the volume and the volatile carrier is allowed to
evaporate in a hot
environment so as to form a carbon nanotube composite sheet. In some
embodiments,
the sheet is exposed to an ultrasonic pulse train in the presence of silicon
or germanium
particles.
[00016] The
present invention also provides a method for forming a cathode. The
method includes generating a substantially planar body defined by a matrix of
carbon
nanotubes in the presence lithium mixed metal oxide. The lithium mixed metal
oxide
can comprise lithium and nickel, cobalt or mixtures thereof In one embodiment,
the
mixed metal oxide can be incorporated during formation of the sheet or
alternatively
can be sprayed onto the subsequently formed sheet. In some embodiments, a
cloud of
carbon nanotubes is formed and mixed metal oxide is introduced within the
cloud to
form a cloud of a mixture of carbon nanotubes and mixed metal oxide. A volume
of
the mixture can then be deposited onto a surface so as to form a substantially
planar
body defined by a matrix of carbon nanotubes with mixed metal within the
matrix of
nanotubes. In some embodiments, the lithium mixed metal oxide is mixed with a
volatile carrier in the volume and the volatile carrier is allowed to
evaporate in a hot
environment so as to form a carbon nanotube composite sheet.
[00017] The
present invention also provides a method for forming a battery. The
method includes incorporating a plurality of semiconductor particles into a
first a sheet
of carbon nanotubes to form a negative electrode; infiltrating lithium mixed
metal oxide
within a second sheet of carbon nanotubes to form a positive electrode;
positioning
between the positive and negative electrodes a separator; and sealing the
positive and
negative electrodes and separator into carbon nanotube sheet impregnated with
a
polymer. In some embodiments, the sheet is made from aligned single wall
carbon
nanotubes or multi-wall carbon nanotubes.
4
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
[00018] In an embodiment, a battery including a negative electrode and a
positive electrode is provided. The negative electrode includes an anode
current
collector having at least one sheet of carbon nanotubes and semiconductor
material
deposited on the sheet. The positive electrode includes a cathode current
collector
having at least one sheet of carbon nanotubes having a nickel sulfide or a tin
sulfide
deposited on the sheet. Situated between the negative electrode and positive
electrode
is a separator.
[00019] In accordance with an embodiment, a method for forming a cathode
for
use in a battery is provided. The method includes depositing nickel, such as
by
electrodeposition, on a carbon nanotube sheet, and converting the nickel to
nickel
sulfide such as by a heating process.
[00020] In an embodiment, a method for forming a cathode for use in a
battery is
provided. The method includes depositing tin, such as by electrodeposition, on
a
carbon nanotube sheet, and converting the tin to tin sulfide such as by a
heating
process.
[00021] In an embodiment, a method for forming an anode for use in a
battery is
provided. The method includes providing a substantially planar body defined by
a
matrix of carbon nanotubes, and spraying nickel sulfide or tin sulfide onto
the matrix.
[00022] In an embodiment, a method of manufacturing a battery is provided.
The method includes incorporating a plurality of semiconductor particles into
a first a
sheet of carbon nanotubes to form a negative electrode, and depositing nickel
sulfide or
tin sulfide on a second sheet of carbon nanotubes to from a positive
electrode. A
separator is positioned between the positive and negative electrodes. The
positive and
negative electrodes and separator are sealed with a casing of carbon nanotube
sheet.
[00023] According to one embodiment, a cathode for use in a battery is
provided.
The cathode includes at least one sheet of carbon nanotubes, and nickel
sulfide or tin
sulfide deposited on the sheet.
BRIEF DESCRIPTION OF DRAWINGS
[00024] Fig. 1 illustrates electrical properties of carbon nanotubes made
in
accordance with one embodiment of the present invention.
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
[00025] Fig. 2 illustrates resistivity versus temperature characteristics
of carbon
nanotubes made in accordance with one embodiment of the present invention.
[00026] Fig. 3 illustrates resistivity versus temperature characteristics
of carbon
nanotubes in (and out of) the presence of a magnetic field.
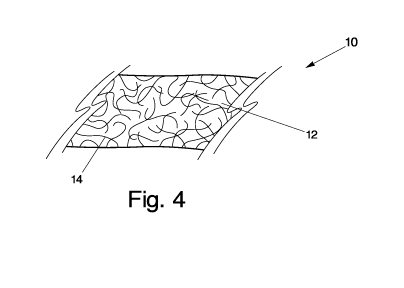
[00027] Fig. 4 illustrates a sheet of nanotubes in accordance with one
embodiment of the present invention.
[00028] Fig. 5 illustrates an alternative embodiment of the present
invention.
[00029] Fig. 6 illustrates a Chemical Vapor Deposition system for
fabricating
nanotubes, in accordance with one embodiment of the present invention.
[00030] Fig. 7 illustrates a system of the present invention for formation
and
harvesting of nano fibrous materials.
[00031] Fig. 8 illustrates a system of the present invention for formation
and
harvesting of nano fibrous materials.
[00032] Fig. 9 illustrates a system of the present invention for treating
nanostructured sheets post formation.
[00033] Fig. 10 illustrates insertion loss from nanostructured sheets made
in
accordance with one embodiment of the present invention.
[00034] Fig. 11A illustrates a nanofibrous non-woven sheet generated from
the
system shown in Figs. 1-2, and from which anode and cathode of a battery can
be
fabricated in accordance with one embodiment of the present invention.
[00035] Fig. 11B illustrates a cross section of a matrix of nanotubes in
accordance with one embodiment of the present invention.
[00036] Fig. 12 illustrates prior art battery technology.
[00037] Fig. 13 illustrates a battery using sheets of carbon nanotubes in
accordance with one embodiment.
[00038] Fig. 14 is an image of micro-silicon particle powder dispersed in
the
carbon nanotube (CNT) conductive sheet.
6
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
[00039] Fig. 15 is an image of nano-silicon particle "welded" to carbon
nanotube
matrix.
[00040] Fig. 16 is a graph showing the specific conductivity as a function
of
frequency.
[00041] Fig. 17 is a graph showing stress strain for carbon nanotube
composites.
[00042] Fig. 18a is an image of a CNT sheet, 10 g/m2 electrode coated with
about 30 to 50 nm of silicon deposited on each tube using a silane CVD
process.
[00043] Fig. 18b is an image at higher magnification of the CNT sheet of
Fig.
18a.
[00044] Fig. 19a is a graph showing the charge capacity as a function of
the
discharge capacity for lithiation of silicon coated CNT electrodes.
[00045] Fig. 19b is a graph showing the reversible capacity as a function
of the
charge capacity for lithiation of silicon coated CNT electrodes.
[00046] Fig. 20 is a graph showing dealloying capacity and coulombic
efficiency as a function of the cycle index.
[00047] Fig. 21 represents a schematic of a large CNT battery for an
electric
vehicle.
[00048] Fig. 22 is a graph showing the conductivity as a function of the
temperature for a variety of different CNT sheet treatments.
[00049] Fig. 23 is a graph showing the resistivity of copper as a function
of the
temperature.
DETAILED DESCRIPTION
[00050] The present invention provides, in an embodiment, a composite
material
made from nanostructured sheets designed to promote, for instance, increased
conductivity. In an embodiment, the sheet may include a substantially planar
body.
The planar body, in one embodiment, can be defined by a matrix of nanotubes.
Matrix,
as defined herein, is a lattice-like or net-like structure with opening
between adjacent
nanotubes. As there may exist openings between adjacent nanotubes in the
matrix, a
protonation agent may be applied to enhance contact between nanotubes for
better
7
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
conduction. To the extent desired, a plurality of composite sheets may be then
layered
on one another to enhance thickness of the sheet.
[00051] Presently, there exist multiple processes and variations thereof
for
growing nanotubes, and forming yarns, sheets or cable structures made from
these
nanotubes. These include: (1) Chemical Vapor Deposition (CVD), a common
process
that can occur at near ambient or at high pressures, and at temperatures above
about
400 C, (2) Arc Discharge, a high temperature process that can give rise to
tubes having
a high degree of perfection, and (3) Laser ablation.
[00052] The present invention, in one embodiment, employs a CVD process or
similar gas phase pyrolysis procedures known in the industry to generate the
appropriate
nanostructures, including carbon nanotubes. Growth temperatures for a CVD
process
can be comparatively low ranging, for instance, from about 400 C to about
1350 C.
Carbon nanotubes (CNTs), both single wall (SWNT) or multiwall (MWNT), may be
grown, in an embodiment of the present invention, by exposing nanoscaled
catalyst
particles in the presence of reagent carbon-containing gases (i.e., gaseous
carbon
source). In particular, the nanoscaled catalyst particles may be introduced
into the
reagent carbon-containing gases, either by addition of existing particles or
by in situ
synthesis of the particles from a metal-organic precursor, or even non-
metallic catalysts.
Although both SWNT and MWNT may be grown, in certain instances, SWNT may be
selected due to their relatively higher growth rate and tendency to form rope-
like
structures, which may offer advantages in handling, thermal conductivity,
electronic
properties, and strength.
[00053] The strength of the individual carbon nanotubes generated in
connection
with the present invention may be about 30 GPa or more. Strength, as should be
noted,
is sensitive to defects. However, the elastic modulus of the carbon nanotubes
fabricated
in the present invention may not be sensitive to defects and can vary from
about 1 to
about 1.2 TPa. Moreover, the strain to failure of these nanotubes, which
generally can
be a structure sensitive parameter, may range from a about 10% to a maximum of
about
25% in the present invention.
[00054] Furthermore, the nanotubes of the present invention can be provided
with
relatively small diameter. In an embodiment of the present invention, the
nanotubes
8
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
fabricated in the present invention can be provided with a diameter in a range
of from
less than 1 nm to about 10 nm. It should be appreciated that the carbon
nanotubes
made in accordance with one embodiment of the present invention may be
extended in
length (i.e., long tubes) when compared to commercially available carbon
nanotubes.
In an embodiment of the present invention, the nanotubes fabricated in the
present
invention can be provided with a length in the millimeter (mm) range.
[00055] The nanotubes of the present invention can also be used as a
conducting
member to carry relatively high current similar to a Litz wire or cable.
However, unlike
a Litz wire or cable soldered to a connector portion, the nanotube conducting
member of
the present invention can exhibit relatively lower impedance in comparison. In
particular, it has been observed in the present invention that the shorter the
current
pulses, the better the nanotube-based wire cable or ribbon would perform when
compared with a copper ribbon or Litz wire. One reason for the observed better
performance may be that the effective frequency content of the pulse, which
can be
calculated from the Fourier Transform of the waveform for current pulses that
are
square and short, e.g., about 100 ms to less than about 1 ms, can be very
high.
Specifically, individual carbon nanotubes of the present invention can serve
as
conducting pathways, and due to their small size, when bulk structures are
made from
these nanotubes, the bulk structures can contain extraordinarily large number
of
conducting elements, for instance, on the order of 1014/cm2 or greater.
[00056] Carbon nanotubes of the present invention can also demonstrate
ballistic
conduction as a fundamental means of conductivity. Thus, materials made from
nanotubes of the present invention can represent a significant advance over
copper and
other metallic conducting members under AC current conditions. However,
joining this
type of conducting member to an external circuit requires that essentially
each nanotube
be electrically or thermally contacted to avoid contact resistance at the
junction.
[00057] Carbon nanotubes of the present invention can exhibit certain
characteristics which are shown in Figs. 1-3. Fig. 1 illustrates the
electrical properties
of carbon nanotubes made in accordance with an embodiment of the present
invention.
Fig. 2 illustrates the resistivity of these carbon nanotubes in relation to
temperature.
Typical temperature dependent electrical resistance R (T) of a single MWNT
measured
9
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
in a four-probe configuration, i.e. the current is passed through the outer
contacts and
voltage is measured over the inner ones. Fig. 3 illustrates characteristics of
carbon
nanotube resistivity versus temperature in (and out of) the presence of a
magnetic field.
[00058] It should be noted that although reference is made throughout the
application to nanotubes synthesized from carbon, other compound(s), such as
boron,
MoS2, or a combination thereof may be used in the synthesis of nanotubes in
connection
with the present invention. For instance, it should be understood that boron
nanotubes
may also be grown, but with different chemical precursors. In addition, it
should be
noted that boron may also be used to reduce resistivity in individual carbon
nanotubes.
Furthermore, other methods, such as plasma CVD or the like can also be used to
fabricate the nanotubes of the present invention.
[00059] The present invention provides, in an embodiment, a composite
material
made from nanostructured composite sheets designed to increase conductivity of
the
carbon nanotubes within the sheet. As shown in Fig. 4, the composite material
10 may
include a substantially planar body in the form of a composite sheet 12. A
matrix of
nanotubes 14 may define the planar body. As there may be openings between
adjacent
carbon nanotubes, in order to enable efficient conduction between a nanoscale
environment and a traditional electrical and/or thermal circuit system, the
proximity of
adjacent nanotubes within the planar body may be brought closer to one
another. To
enhance the proximity between adjacent nanotubes, a protonation agent may be
applied.
In an embodiment, the composite material may be a single layer as shown in
Fig. 4, or
may be a plurality of layers on top of one another as shown in Fig. 5.
System for Fabricating Sheets
[00060] With reference now to Fig. 6, there is illustrated a system 30,
similar to
that disclosed in U.S. Patent 7,993,620 (incorporated herein by reference),
for use in the
fabrication of nanotubes. System 30, in an embodiment, may be coupled to a
synthesis
chamber 31. The synthesis chamber 31, in general, includes an entrance end
311, into
which reaction gases (i.e., gaseous carbon source) may be supplied, a hot zone
312,
where synthesis of extended length nanotubes 313 may occur, and an exit end
314 from
which the products of the reaction, namely the nanotubes and exhaust gases,
may exit
and be collected. The synthesis chamber 31, in an embodiment, may include a
quartz
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
tube 315 extending through a furnace 316. The nanotubes generated by system
30, on
the other hand, may be individual single-walled nanotubes, bundles of such
nanotubes,
and/or intertwined single-walled nanotubes. In particular, system 30 may be
used in
the formation of a substantially continuous non-woven sheet generated from
compacted
and intermingled nanotubes and having sufficient structural integrity to be
handled as a
sheet.
[00061] System 30, in one embodiment of the present invention, may also
include
a housing 32 designed to be substantially airtight, so as to minimize the
release of
airborne particulates from within the synthesis chamber 31 into the
environment. The
housing 32 may also act to prevent oxygen from entering into the system 30 and
reaching the synthesis chamber 31. In particular, the presence of oxygen
within the
synthesis chamber 31 can affect the integrity and compromise the production of
the
nanotubes 313. System 30 may also include an injector similar to those
disclosed in
Application Serial Number 12/140,263, incorporated herein by reference in its
entirety.
[00062] System 30 may also include a moving belt 320, positioned within
housing 32, designed for collecting synthesized nanotubes 313 made from a CVD
process within synthesis chamber 31 of system 30. In particular, belt 320 may
be used
to permit nanotubes collected thereon to subsequently form a substantially
continuous
extensible structure 321, for instance, a non-woven sheet. Such a sheet may be
generated from a matrix of compacted, substantially non-aligned, and
intermingled
nanotubes 313, bundles of nanotubes, or intertwined nanotubes, with sufficient
structural integrity to be handled as a sheet.
[00063] To collect the fabricated nanotubes 313, belt 320 may be
positioned
adjacent the exit end 314 of the synthesis chamber 31 to permit the nanotubes
to be
deposited on to belt 320. In one embodiment, belt 320 may be positioned
substantially
parallel to the flow of gas from the exit end 314, as illustrated in Fig. 6.
Alternatively,
belt 320 may be positioned substantially perpendicular to the flow of gas from
the exit
end 314 and may be porous in nature to allow the flow of gas carrying the
nanomaterials
to pass therethrough. Belt 320 may be designed as a continuous loop, similar
to a
conventional conveyor belt. To that end, belt 320, in an embodiment, may be
looped
about opposing rotating elements 322 (e.g., rollers) and may be driven by a
mechanical
11
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
device, such as an electric motor. Alternatively, belt 320 may be a rigid
cylinder. In
one embodiment, the motor may be controlled through the use of a control
system, such
as a computer or microprocessor, so that tension and velocity can be
optimized. The
collected nanotubes may then be removed manually or by any other means off the
belt
320 for subsequent use.
[00064] To the extent desired a pressure applicator, such as roller 45,
may be
employed. Referring to Figure 7, the pressure application may be situated
adjacent to
belt 44, that may be positioned substantially perpendicular to the flow of
gas, so as to
apply a compacting force (i.e., pressure) onto the collected nanomaterials. In
particular,
as the nanomaterials get transported toward roller 45, the nanomaterials on
belt 44 may
be forced to move under and against roller 45, such that a pressure may be
applied to the
intermingled nanomaterials while the nanomaterials get compacted between belt
44 and
roller 45 into a coherent substantially-bonded sheet 46. To enhance the
pressure against
the nanomaterials on belt 44, a plate 444 may be positioned behind belt 44 to
provide a
hard surface against which pressure from roller 45 can be applied. It should
be noted
that the use of roller 45 may not be necessary should the collected
nanomaterials be
ample in amount and sufficiently intermingled, such that an adequate number of
contact
sites exists to provide the necessary bonding strength to generate the sheet
46.
[00065] To disengage the sheet 46 of intermingled nanomaterials from belt
44 for
subsequent removal from housing 42, a scalpel or blade 47 may be provided
downstream of the roller 45 with its edge against surface 445 of belt 44. In
this manner,
as sheet 46 moves downstream past roller 45, blade 47 may act to lift the
sheet 46 from
surface 445 of belt 44. In an alternate embodiment, a blade does not have to
be in use to
remove the sheet 46. Rather, removal of the sheet 46 may be manually by hand
or by
other known methods in the art.
[00066] Additionally, a spool or roller 48 may be provided downstream of
blade
47, so that the disengaged sheet 46 may subsequently be directed thereonto and
wound
about roller 48 for harvesting. As the sheet 46 is wound about roller 48, a
plurality of
layers may be formed. Of course, other mechanisms may be used, so long as the
sheet
46 can be collected for removal from the housing 42 thereafter. Roller 48,
like belt 44,
may be driven, in an embodiment, by a mechanical drive, such as an electric
motor 481,
12
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
so that its axis of rotation may be substantially transverse to the direction
of movement
of the sheet 46.
[00067] In order to minimize bonding of the sheet 46 to itself as it is
being wound
about roller 48, a separation material 49 (see Fig. 8) may be applied onto one
side of the
sheet 46 prior to the sheet 46 being wound about roller 48. The separation
material 49
for use in connection with the present invention may be one of various
commercially
available metal sheets or polymers that can be supplied in a continuous roll
491. To that
end, the separation material 49 may be pulled along with the sheet 46 onto
roller 48 as
sheet 46 is being wound about roller 48. It should be noted that the polymer
comprising
the separation material 49 may be provided in a sheet, liquid, or any other
form, so long
as it can be applied to one side of sheet 46. Moreover, since the intermingled
nanotubes
within the sheet 46 may contain catalytic nanoparticles of a ferromagnetic
material, such
as Fe, Co, Ni, etc., the separation material 49, in one embodiment, may be a
non-
magnetic material, e.g., conducting or otherwise, so as to prevent the sheet
46 from
sticking strongly to the separation material 49. In an alternate embodiment, a
separation
material may not be necessary.
[00068] After the sheet 46 is generated, it may be left as a sheet 46 or
it may be
cut into smaller segments, such as strips. In an embodiment, a laser may be
used to cut
the sheet 46 into strips. The laser beam may, in an embodiment, be situated
adjacent the
housing such that the laser may be directed at the sheet 46 as it exits the
housing. A
computer or program may be employed to control the operation of the laser beam
and
also the cutting of the strip. In an alternative embodiment, any mechanical
means or
other means known in the art may be used to cut the sheet 46 into strips.
[00069] To the extent desired, an electrostatic field (not shown) may be
employed to align the nanotubes, generated from synthesis chamber 31,
approximately
in a direction of belt motion. The electrostatic field may be generated, in
one
embodiment, by placing, for instance, two or more electrodes circumferentially
about
the exit end 314 of synthesis chamber 31 and applying a high voltage to the
electrodes.
The voltage, in an embodiment, can vary from about 10 V to about 100 kV, and
preferably from about 4 kV to about 6 kV. If necessary, the electrodes may be
shielded
with an insulator, such as a small quartz or other suitable insulator. The
presence of the
13
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
electric field can cause the nanotubes moving therethrough to substantially
align with
the field, so as to impart an alignment of the nanotubes on moving belt.
[00070] Alternatively, the carbon nanotubes can be aligned by stretching
following the synthesis of the carbon nanotube sheets as provided in co-
pending U.S.
Application 12/170,092, which is incorporated herein by reference in its
entirety.
[00071] System 30, as noted, can provide bulk nanomaterials of high
strength in
a non-woven sheet, such as sheet illustrated in Fig.. 11A. The carbon
nanotubes 14, in
an embodiment, can be deposited in multiple distinct layers 51 to from a
multilayered
structure or morphology in a single CNT sheet 12, as shown in Fig. 11B. As
noted
above, nanofibrous non-woven sheet 110 may be made from the deposition of
multiple
distinct layers of either SWNT or MWNT carbon nanotubes. In an embodiment, the
tensile strength of such a non-woven sheet 110 can be over 40 MPa for SWNT.
Moreover, such a sheet may used with residual catalyst from the formation of
the
nanotubes. However, typical residuals may be less than 2 atomic percent.
[00072] By providing the nanomaterials in a non-woven sheet, the bulk
nanomaterials can be easily handled and subsequently processed for end use
applications, including hydrogen storage, batteries, or capacitor components,
among
others.
[00073] It should be appreciated that the carbon nanotubes made in
accordance
with an embodiment of the present invention may not require treatment with a
surfactant, and may be of at least three orders of magnitude better in
electrical
conductivity and thermal conductivity. Moreover, the carbon nanotube sheets
made in
accordance with an embodiment of the present invention may include a plurality
of
layers. On the other hand, the carbon nanotubes in, for instance, Bucky Paper
are made
of relatively short nanotubes and require treatment with a surfactant. In
addition,
Bucky Paper is made from only one layer of nanotubes as opposed to multilayers
provided with the nonwoven sheet of the present invention.
Treatment Process
[00074] To the extent desired, once a sheet 46 is generated, the sheet 46
may
undergo treatment to enhance conductivity and/or productivity of the nanotubes
in the
14
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
sheet. If strips are generated, the strips may also undergo a treatment
processes to
enhance conductivity and productivity of the nanotubes in the strip.
[00075] Treatment of a sheet 46 after formation may, in an embodiment,
include
subjecting the sheet 46 to a protonation agent. One feature of the protonation
agent may
be to bring the carbon nanotubes in closer proximity with one another. By
bringing the
carbon nanotubes closer together, the protonation agent may act to reduce
surface
tension, reduce resistivity, and increase conductivity of the sheet. Examples
of a
protonation agent may include an acid such as hydronium ion, hydrochloric
acid,
hydrobromic acid, hydrofluoric acid, hydroiodic acid, carbonic acid, sulfuric
acid, nitric
acid, fluorosulfuric acid, chlorosulfonic acid, methane sulfonic acid,
trifluoromethane
sulfonic acid, oleum, an agent thereof, or a combination thereof, or other
materials
capable of being electrically and/or thermally conductive.
[00076] The protonation agent may be applied, in an embodiment, through
the
use of an apparatus 60, such as that shown in Fig. 9. The apparatus may, in an
embodiment, include a plurality of rollers for guiding the sheet through the
application
process. As shown, a first roller 64 and second roller 65 may be situated
adjacent one
another with the second roller 65 being positioned downstream from roller 64.
A tub 61
having a first end 62 and a second end 63 and containing the protonation agent
may be
situated underneath the first roller 64 and the second roller 65. The first
roller may act
to force the sheet through the tub 61 and onto the second roller 65. The
second roller 65
may pull the sheet from the first roller 64 and may wring excess protonation
agent fluid
from the sheet. A third roller 66 may be positioned above the first end 62 of
the tub
near the first roller 64, while the fourth roller 67 may be positioned above
the second
end 63 of the tub near the second roller 65. Rollers 64, 65, 66, and 67 may be
situated
in series to allow the sheet 68 to move smoothly through the rollers. Of
course,
although shown in Fig. 9 as having four rollers, an apparatus for post
treatment of sheets
68 may include a fewer number or a greater number of rollers. To the extent
necessary,
a hood may be situated in such a manner as to prevent fumes from the
protonation agent
to escape. In one embodiment, the apparatus 60 may include a hood such as a
polypropylene hood.
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
[00077] Treating the sheet 68 with a protonation agent may involve
positioning a
bobbin or roll of sheet 68 on the third roller 66. The sheet 68 may then move
downstream, passing from the third roller 66, through the first roller 64,
into the tub 61
containing the protonation agent, and onto the second roller 65 and across the
fourth
roller 67.
[00078] In certain circumstances after treatment, the resulting sheet 68
may be
acidic or basic. To bring the pH of the resulting sheet 68 to approximately
neutral, a
rinsing solution may be applied to the sheet 68. The rinsing solution may, in
an
embodiment, be applied continuously with the protonation agent or it may be
applied
independently of the protonation agent.
[00079] In another embodiment, treatment of the sheet 68 may further
include
spraying the sheet 68 with a second solution as it exits the furnace and is
collected on
the belt 320. The solution may contain, in an embodiment, a mixture of
compounds that
cover the outer surface of the nanotubes in such a manner as to enhance
alignment of the
carbon nanotubes and allow the carbon nanotubes to come into closer proximity
with
one another.
[00080] In an embodiment, the mixture of the second solution may include a
solvent, a polymer, a metal, or a combination thereof The solvent used in
connection
with the solution of the present invention can be used to lubricate the sheet
in order to
gain better alignment and enhancement in the properties of the carbon
nanotubes.
Examples of a solvent that can be used in connection with the solution include
toluene,
kerosene, benzene, hexanes, any alcohol including but not limited to ethanol,
methanol,
butanol, isopropanol, as well as tetrahydrofuran, 1-methyl-2-pyrrolidinone,
dimethyl
formamide, methylene chloride, acetone or any other solvent as the present
invention is
not intended to be limited in this manner. In an embodiment, the solvent may
be used as
a carrier for a polymer, monomer, inorganic salt, or metal oxide to.
[00081] Examples of a polymer that can be used in connection with the
solution
include a small molecule or polymer matrix (thermoset or thermoplastic)
including, but
not limited to, polyurethane, polyethylene, poly(styrene butadiene),
polychloroprene,
poly(vinyl alcohol), poly(vinyl pyrrolidone), poly(acrylonitrile-co-butadiene-
co-
styrene), epoxy, polyureasilazane, bismaleimide, polyamide, polyimide,
polycarbonate,
16
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
or any monomer including styrene, divinyl benzene, methyl acrylate, and tert-
butyl
acrylate. In an embodiment, the polymer may include polymer particles, that
are
difficult to obtain in liquid form.
[00082] Examples of a metal that can be used in connection with the
solution
include a salt (any transition metal, alkali metal, or alkali earth metal salt
or mixture
thereof including, but not limited to, nickel hydroxide, cadmium hydroxide,
nickel
chloride, copper chloride, calcium zincate (CaZn2(OH)6)), or metal oxide (any
transition
metal, alkali metal, or alkali earth metal oxide or mixture thereof, including
but not
limited to: zinc oxide, iron oxide, silver oxide, copper oxide, manganese
oxide, LiCo02,
LiNi02, LiNixCol-x02, LiMn204). In an embodiment, the metal may include
polymers
or volatile solvents to create a carbon nanotube metal matrix composite.
Examples of
such polymers or volatile solvents include powdered forms of aluminum or its
alloys,
nickel, superalloys, copper, silver, tin, cobalt, iron, iron alloys, or any
element that can
be produced in a powdered form including complex binary and ternary alloys or
even
superconductors.
[00083] To disperse the solution, a spraying apparatus may be used. The
spraying apparatus may be any apparatus that is commercially available. In an
embodiment, at one end of the spraying apparatus, there may be a spray head,
through
which the solution may be sprayed onto the sheet 46. In an embodiment, the
spray head
may be flat, round, or any other shape so long as it can permit solution to
exit
therethrough. To the extent desired, the spray head may emit a solution in a
continuous
manner or in a preprogrammed manner.
[00084] Once the sheet 68 has been treated, the treated sheet 68 may be
subject to
a heat source for processing of the sheet. For example, the sheet may be
subject to
sintering, hot isostatic pressing, hot pressing, cold isostatic pressing so as
to yield a
composite sheet or the desired form of the final product.
[00085] Treatment of the composite sheet may, in another embodiment,
further
include infusing the composite sheet with a glassy carbon material so as to
increase the
structural integrity of the sheet and provide substantially low resistance
coupling.
Glassy carbon, in general, may be a form of carbon related to carbon nanotubes
and can
contain a significant amount of graphene like ribbons comprising a matrix of
amorphous
17
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
carbon. These ribbons include sp2 bonded ribbons that can be substantially
similar to
the sp2 bonded nanotubes. As a result, they can have relatively good thermal
and
electrical conductivity. Examples of precursor materials from which glassy
carbon can
be made include furfuryl alcohol, RESOL resin (i.e., catalyzed alkyl-phenyl
formaldehyde), PVA, or liquid resin or any material known to form glassy
carbon when
heat treated. Of course, other commercially available glassy carbon materials
or
precursor materials can be used.
[00086] In accordance with an embodiment of the present invention, the
carbon
nanotube sheet can be chemically treated to allow for substantial alignment of
the
carbon nanotubes within the sheet. The carbon nanotube sheet can be further
treated to
insert an active compound or intercalation element, such as Lithium, as
described
herein.
Battery Applications
[00087] Batteries are generally a type of electrochemical cell that
contain a pair
of electrodes and an electrolyte disposed between the electrodes. One of the
electrodes
may be referred to as a cathode, wherein an active material is reduced during
discharge.
The other electrode may be referred to as an anode, wherein another active
material is
oxidized during discharge. Secondary batteries are generally referred to as
batteries
capable of charging electricity after discharge.
[00088] Research has been conducted on secondary batteries, such as
lithium
secondary batteries, because of their high voltage and high energy density.
Particular
attention has been paid to an electrode material for the cathode of the
secondary
battery. For example, U.S. Pat. No. 4,833,048 discloses a rechargeable battery
obtained
by combining a disulfide compound with metal M which supplies and captures the
cations (M'). The rechargeable battery provides an improved energy density of
at least
150 Wh/kg. However, the difference between the oxidation potential and the
reduction
potential of the disulfide compound is very large. According to the theory of
electrochemical reaction, the electron transfer of the disulfide compound
proceeds
extremely slowly at room temperature. Therefore, it can be difficult to obtain
a
rechargeable battery providing a higher current output of 1 mA/cm2 or more at
room
temperature. The operation of a battery comprising an electrode of disulfide
compound
18
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
is limited to high temperatures in the range of 100 C to 200 C, where the
electron
transfer can proceed faster.
[00089] Lithium based chemistries offer the promise of significant energy
storage; but are limited by the weight of mobile lithium that can be stored
and
burdened by extra weight. One metric of power generating devices is the power
to
weight ratio versus the energy capacity. Since traditional batteries, as well
as presently
available secondary batteries, are made from relatively heavy components, the
amount
of energy generated may not be as efficient or ideal if lighter materials can
be used.
[00090] Lithium based chemistries are almost ideal for batteries as their
cell
potential of 3 to 4.2V provides a very high energy current source. Safety
issues with
lithium batteries have for the most part been addressed by using lithium
intercalation
into graphite based anode as a lithium reservoir instead of pure lithium metal
and by
adding appropriate thermal links and pressure releases. This approach coupled
with the
use of shuttle compounds have minimized the safety issues associated with this
chemistry. Remaining issues concern the weight of the various component parts,
the
potential for thermal run-away and external physical damage resulting from
accidents
that could expose lithium to air or water.
[00091] Existing battery technologies typically use a metal current
collector
coated with a compound that includes anode material, the cathode material, and
current
enhancing additions like graphite and a binder. Anode current collectors are
typically
copper, while cathode current collectors are typically aluminum, and casings
are
typically steel. Sometimes sintered graphite plate is used as a current
collectors.
[00092] The present invention, on the other hand, provides a high power and
high performance battery, for example, rechargeable or secondary battery, that
employs
one or more sheets of carbon nanotubes for both the cathode and the anode. The
present invention also provides a high power and high performance battery for
use in a
variety of energy and power related applications.
[00093] The battery of the present invention, in general, includes a
positive
electrode, a negative electrode, a separator situated between the electrodes,
and a
casing. In some embodiments, the battery utilizes electrodes made from carbon
nanotube sheet for use as current-collectors and can also utilize carbon
nanotube
19
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
composite casing. In some embodiments, the electrodes utilizes aligned carbon
nanotube sheets into which an active compound or intercalation element, such
as
Lithium, is incorporated. In accordance with one embodiment of the present
invention,
the battery can by a Lithium ion type battery. Alternatively, in accordance of
another
embodiment of the present invention, the battery can be a nickel-zinc type
battery.
[00094] The use a free-standing carbon nanotube sheets as an electrode in
electrochemical applications in the present application permits the electrodes
to
maintain bifunctionality (i.e. role as both the active material and current
collector). The
potential for high temperature applications is contemplated since no binder is
required
to fabricate these electrodes, opening up the use in battery applications in
excess of
200 C (a temperature at which most conventional binders are unstable). The
free-
standing electrodes also offer a lightweight, flexible geometry for thin film
batteries
and alternative form factors. The physical properties of free-standing CNT
sheets are
important considerations for any application, but with lithium ion batteries
the
questions surrounding strength and conductivity are paramount. Since battery
manufacturing typically utilizes roll coaters or dye cutting, strength is an
important
consideration for CNT electrodes, since binder and metal substrate are absent.
The
tensile strength of typical SWCNT sheet made in accordance with an embodiment
of
the present invention is about 80-100 MPa, although the synthesis and
processing steps
can dramatically affect this property. The Young's modulus for SWCNT sheets
made in
accordance with an embodiment of the present invention is in the range of
about 5-10
GPa, which indicates that a large force can be applied to these materials
prior to plastic
deformation. Another convenient property is that the CNT sheets of the present
invention can be shaped into any form factor required; they can easily be cut
with
conventional shears.
[00095] The electrical conductivity of purified SWCNT sheets of the
present
invention can be about 106 S/m and can approach pure metal conductivity with
appropriate doping. The temperature-dependent response for free-standing
electrodes of
the present invention also can have attractive electrical properties,
including a
conductivity response that shows less than about 3% variation in conductivity
over the
range of about 100 - 400 K. The electronic transport in free-standing SWCNT
electrodes made in accordance with an embodiment of the present invention can
be
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
attributed to predominant metallic conduction interrupted by tunneling
barriers due to
tube-tube interactions, as well as contributions from phonon scattering and
variable
range hopping.
[00096] The major structural elements of the battery of the present
invention
include the (1) anode current collectors, (2) cathode current collectors and
(3) the
structural casing, all of which can be made from CNT sheets and or CNT
composite
sheets. Fig. 13 illustrates one embodiment of the battery. Battery 50, as
shown,
includes a carbon nanotube cathode 52, a carbon nanotube anode 54, a separator
56
between the cathode and the anode and a casing 58. The battery 50 can also
have an
electrolyte material. During charging of the battery 50, ions (for instance
Lithium ions)
move from the positive electrode (cathode) to the negative electrode (anode),
while
during discharging the ions move from the negative electrode to the positive
electrode.
[00097] The carbon nanotubes composition of the present invention can also
be
useful in minimizing the weight of the battery as compared to existing
batteries and in
increasing the energy to weight and/or energy to volume ratio of the battery
of the
present invention.
[00098] The fabrication of stand-alone CNT electrodes can eliminate the
need
for metal foil substrates and increase capacity and rate capability. The free-
standing
CNT electrodes can store lithium and also support ultra high capacity
semiconductor
particles, like silicon and germanium, which may significantly increase the
usable
anode specific capacity (Ah/kg) in a battery.
[00099] The battery of the present invention has the advantage of not
having
heavy materials such as the aluminum anode and copper cathode or binders and
conductive fillers. The carbon nanotube composition of the battery of the
present
invention can also provide for a high fracture toughness. In addition, the
carbon
nanotube battery of the invention can insure against thermal hot spots that
have the
potential for initiating run-away.
[000100] The anode and the cathode may be formed, in one embodiment, from at
least one sheet of carbon nanotubes. The sheet of carbon nanotubes may be
fabricated
by a CVD process using system 30 shown in Fig. 6. The nanotubes generated in
the
gas phase using a floating catalyst CVD process can form a cloud of nanotubes
that can
21
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
be deposited on a rotating drum. Compounds such as nickel, zinc, nickel-zinc
alloys,
lithium, lithium mixed metal oxides, nickel-zinc oxides, and the like or
combinations
thereof, can be added to form a cloud of nanotubes with the compounds that can
be
deposited on a rotating drum. The drum may translate in front of the furnace
exit to
create a wide mat defined by a plurality of layers of carbon nanotubes within
a single
sheet (see Fig. 11b). Referring to Fig. 5, a plurality of layers 12 of
nanotubes 14 may be
deposited to build a non-woven sheet 20 to a desired density. In an
embodiment, the
density of the non-woven sheet is about 1 mg/cm2. In some embodiments, the
density
of each non-woven sheet can be controlled within a wide range, for instance
from about
0.1 mg/cm2 to about 5 mg/cm2. An example of such a non-woven sheet is shown in
Fig. lla as item 110. Each sheet, in one embodiment, can be made with varying
thickness and/or numbers of layers of carbon nanotubes 14. In some
embodiments, a
plurality of sheets may be necessary to build a carbon nanotube mat having a
density of
about 80 g/m2. For example, a plurality of sheets can be layered on top of one
another
to provide for the desired carbon nanotube density. To the extent desired, the
sheet
may have or may not have a protonation agent.
Cathode
[000101] The cathode, in an embodiment, may be made from, for example, from
one or more sheets of aligned carbon nanotubes infiltrated with mixed metal
oxides. In
accordance to an embodiment, the mixed metal oxide can be a nickel-zinc oxide,
a
nickel-cobalt oxide, a lithium metal oxide or any other similar compound being
utilized
for generation of an electrical current. For example, the carbon nanotube
sheet can be
infiltrated with nano-scaled iron-lithium-phosphate, lithium-nickel-oxides,
lithium-
cobalt-nickel-oxides, lithium-cobalt-oxides, zinc-nickel oxides or combination
thereof.
Other substitutions may include lithium compounded with sulfur (for oxygen),
sulfates,
borates and silicates. Alternatively, the batteries of the present invention
can be zinc air
based, sodium based or lead acid based. It should be noted that although
reference is
made to sheets of aligned carbon nanotubes, sheets of non-aligned carbon
nanotubes
may also be used.
[000102] The mixed metal oxides can be incorporated within the carbon
nanotube
sheets during the construction of the carbon nanotube sheets for use as a
cathode. In
22
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
some embodiments, mixed metal oxide can be added during the nanotube growth
process. For example, mixed metal oxide can be dispersed with the cloud of
nanotubes
during the nanotube growth process using any known methods. In an alternated
embodiments, mixed metal oxide can be added or deposited after formation of
the
carbon nanotube sheet using known methods. For instance, mixed metal oxide can
be
dispersed in a volatile carrier, such as acetone, isopropanol, methanol,
ethanol and the
like, and spayed onto the carbon nanotube sheet. The carrier can be evaporated
from
the sheet by heating the sheet to form a carbon nanotube particulate composite
material.
Anode
[000103] The anode of the battery of the present invention, can include one
or
more sheets of carbon nanotubes into which graphite particles, silicon
particles,
germanium particles or combination thereof are incorporated. The anode can be
formed from aligned CNT sheets having a lithium intercalation compound or a
zinc-
nickel alloy placed into the structure either by adding lithium or zinc-nickel
powder
during the growth process as with the cathode or by spraying pre-formed carbon
nanotube sheets. It should be noted that although reference is made to sheets
of aligned
carbon nanotubes, sheets of non-aligned carbon nanotubes may also be used.
[000104] Silicon or germanium particles, in an embodiment, can be coated onto
the
sheets of carbon nanotube using a Chemical Vapor Deposition process. For
instance,
referring to Figs. 14-15, semiconductor powder, such as silicon powder or
germanium
powder can be incorporated into the carbon nanotube sheets. Alternatively,
semiconductor powder, such as silicon powder or germanium powder, can be
welded to
the carbon nanotube sheet to form the negative current collector or anode. In
some
embodiments, particles of silicon can be dispersed in a solvent or carrier,
such as
acetone, ethanol, methanol and the like, and welded to the CNT sheets by
powerful
ultrasonic pulse. In some embodiments, the particles of silicon or germanium
are added
to the carbon nanotubes using high energy welding, or similar processes known
in the
art, so that the silicon particles become integral with the carbon nanotubes.
For
instance, the particles of silicon or germanium may be positioned on top of
one or more
carbon nanotubes or at the surface of one or more carbon nanotubes.
Alternatively, the
23
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
silicon particles can be attached to the surface of the carbon nanotubes by
any other
suitable methods known in the art.
[000105] In some embodiments, the particles of silicon or germanium are in the
micrometer or nanometer ranges. In some embodiments, the silicon or germanium
particles are 100 nm or less.
[000106] By providing carbon nanotube sheets having silicon or germanium nano-
particles inserted therein, it may not be necessary to add binders or
conductive additives
to the anode. In addition, thermal stability can be improved to the point
where thermal
sensors and venting valves can be potentially be eliminated.
[000107] An important impact of using an electrode made from a free standing
sheet
of carbon nanotubes is that the usable capacity can be increased by removing
the
inactive copper foil. Removal of the copper substrate has additional benefits
including
an increased depth of discharge and the ability to maintain a near-zero volt
state of
charge. It is well documented that prolonged cycling below 2.5 V leads to
oxidation of
the copper substrate.
[000108] In addition, it has been observed that the sheet of carbon nanotubes
having
silicon or germanium particles, produced by the method employed by the present
invention, has an energy density up to three times higher than the energy
density of
commercial graphite anodes (see Table 1).
[000109] In one embodiment, 0.5 mg of nanosilicon particles were dispersed in
250
ml of acetone. Individual carbon nanotube sheets were placed into a metal
holder as
they are suspended in solution at a distance of 0.25 to 0.50 inches from the
ultrasonic
horn (Ultrasonicator VC600, Sonics & Materials, Newton). The ultrasonic horn
was
set to the following settings Duty Cycle: 2 and Micro-tip limit: 2.3. The duty
cycle
dictated the percentage of time that pulses were delivered to the samples. The
amplitude, which is measured in microns, is defined as the up and down
distance
(excursion) that the probe tip travels. The amplitude set point was based upon
the
diameter of the probe and dictated the amount of energy that will be
transmitted into
the liquid. A timer was set to a desired value and ultrasonication was
started. The
ultrasonic horn was maintained in the center of the beaker to ensure that
power was
delivered to the samples evenly.
24
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
Table 1: Comparison of carbon nanotube with commercial anode in terms of
weight volumetric and fade
Commercial PSI/NCTI
Graphite Anode
Si/SWNT Anode
Energy density 350 1500
(mAh/g)
Energy density 525 2250
(mAh/cc)
Capacity Fade ¨ 0.2% 2%
percent cycle
Current collector 9 micron copper SWNT
[000110] Commercially available graphite anode with copper current collector
has an
energy density of 350 mAh/g while anode having silicon vapor deposited on a
SWNT
current collector sheet can provide an energy density of about 1500 mAh/g or
more. In
addition to the above, it has been determined that the sheet of carbon
nanotubes of the
present invention has a capacitance ten times higher or more as compared to
commercially available anodes.
Separator
[000111] The positive and negative electrode can be designed, in one
embodiment, to be separated by a separator. For instance, the separator can be
a
membrane, such as a porous and hydrophilic membrane. In some embodiments, the
separator can be a polypropylene and/or polyethylene electrolytic membrane. It
should
be appreciated that other thin separators/membranes may alternatively be
placed
between the electrodes, so long as the membrane permits diffusion of the
electrolyte
therethrough. In an embodiment, the membrane may be a CELGARDTM porous
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
polyethylene membrane, or polyethylene membrane, or a combination thereof, or
any
other membrane capable of being used with alkaline batteries.
[000112] Any suitable electrolyte solution can be used. Exemplary
electrolytes
include, without limitation, aqueous solutions of KOH, HNO3, HC1,
tetraethylammonium bis(oxaloato)borate (TEABOB), tetraethylammonium
tetrafluoroborate (TEABF4), or triethylmethylammonium tetraflouraborate
dissolved in
acetonitrile.
Casing
[000113] In some embodiments, the battery casing is made from carbon
nanotube
polymeric composite. The battery casing can be formed from a pre-impregnated
CNT
sheet. These sheets can be impregnated with a wide variety of polymeric
matrices such
as toughened epoxy, bismaliamide, polypropylene, polyethylene, PPS, PEEK
(polyether ether ketone), PTFE and the like. In some embodiments, the sheet
can
include a carbon nanotube content from about 20 to about 50%. The density of
the
resultant composite can be less than 2 grams per cc compared with more than
7.8 g/cc
for the standard steel casings.
[000114] The busses to the sheet of carbon nanotubes can either be riveted
or spot
welded. The latter being possible because the edges of the sheet of carbon
nanotubes
can, in an embodiment, be preliminarily electroplated with nickel.
[000115] These battery casings can be massed produced separately or the
battery
material (anode, cathode, separators, inlet values, outlet valves) can be
encapsulated
with the pre-impregnated during the manufacturing process. The casing can be
used to
thermally seal the battery anode, separator and cathode in one step.
Alternatively,
thermally setting matrix materials can be used to completely seal the battery
following
assembly. If desired safety pressure release values can be co-sealed into the
structure
during assembly.
[000116] Such composite material have, in some embodiments, a breaking
strength higher than steel and are lighter by a factor of about 4. In
addition, such
carbon nanotube composite materials provide for a casing with high fracture
toughness.
It should be noted that casings made from carbon nanotube composites have the
26
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
advantage to be much stronger and have a higher fracture toughness than
conventional
casings made from steel, and can be used as battery structural elements to
mitigate
crash damage.
[000117] A comparison between existing technology and the battery according
to
one embodiment of the present invention is shown in Figs.12-13. In some
embodiments, the anode is a mat having a plurality of carbon nanotube sheets
on top of
one another and comprising particles of silicon infiltrated and welded to the
carbon
nanotubes (Figs. 14 and 15).
[000118] Fig. 16 illustrates the specific conductivity as function of
frequency
were measured for a copper collector and for a carbon nanotube collector.
[000119] Fig. 17 illustrates the stress strain curve of a 60% carbon
nanotube
epoxy composite. As shown, the carbon composite material has a breaking stress
of 1
Gpa.
Battery pack
[000120] In accordance with some embodiments of the present invention, the
battery may be designed to optimize the energy density and/or power. It is
possible to
design the battery to store a desired amount of energy by increasing the
number of cells
in a battery pack. Accordingly, the battery can, in an embodiment, be
optimized to
include two or more cells, each cell having a desired geometry and dimension.
For
instance, the cells can be cylindrical (spiral-wound) or prismatic. Cells can
be
assembled into a module or into a battery pack. Cells or modules can be
packaged in
casings made from carbon nanotube composites, as described herein, which have
the
advantage to be much stronger and have a higher fracture toughness than steel,
and can
mitigate crash damage.
[000121] It should be appreciated that the carbon nanotube composite
material of
the casing can act as a heat sink. Therefore, the design of the battery of the
present
invention having the components as set forth can allow the battery to operate
without
additional protection, such as pressure sensor, temperature sensor, pressure
release
valve or safety vents.
Applications of Mixed Metal Embodiments
27
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
[000122] One of the shortcomings of the metal hydride type battery is the
degradation of electrode integrity in the course of multiple charging-
discharging of the
battery. This is because accompanying the hydriding phase change there can
also be a
change in the lattice contacts that can result in an expansion leading to
cracking in the
alloy. Such alloy typically can withstand about 300 to 500 cycles until the
battery starts
to loose performance.
[000123] On the other hand, when using nanostructure materials fabricated
by the
method of the present invention, the number of cycles that a battery can
withstand can
be increased. In particular, a battery utilizing a sheet of carbon nanotubes
made in
accordance with an embodiment of the present invention can minimize this type
of
degradation, and therefore can withstand an unlimited number of charge-
discharge
cycles.
[000124] It should be appreciated that the sheet of carbon nanotubes have
the
advantage not only to be able to store as much energy as lithium ion batteries
but also
not degrade with charge/discharge cycle. In addition, the sheet of carbon
nanotubes
can offer a substantially high surface area, so that double layer charging can
be
accommodated, so as to hold tremendous energy that can be returned on demand
if very
high but short peak power is required of the battery. This behavior shows the
strong
distinction between the battery of the present invention and other
commercially
available secondary batteries.
[000125] The battery of the present invention, having the components set
forth
above, including sheets of conductive carbon nanotubes, has applications as a
secondary battery. Secondary batteries are generally batteries that are
capable of
charging electricity after discharge. The design of the battery of the present
invention
can also permit the battery to be discharged in two steps. In particular, the
first
discharge of the battery can produce relatively high power, while the second
discharge
can produce relatively high energy.
[000126] Carbon nanotubes of the present invention possess a variety of
useful
properties including a high aspect ratio, channels for lithium ion
intercalation,
improved conductivity, both electrical and thermal, and increased capacity. It
should
be noted that cathodes having SWCNT or MWCNT sheets of the present invention,
as
28
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
well as anodes having SWCNT or MWCNT sheets of the present invention, can show
an improvement in electrical and thermal conductivity and capacity. In
addition, the
cathodes and anodes made using the sheets of the present invention can show a
reduction in weight. Moreover, the use of carbon nanotube sheets of the
present
invention can show an improvement in the power to weight ratios versus the
energy
capacity.
[000127] It should be noted that the design of the battery can be optimized
to
reduce battery mass and volume and to allow for use in for example, electrical
vehicles.
There is currently a worldwide pursuit for sustainable energy systems that
will
simultaneously reduce dependence on fossil fuels and greenhouse gas emissions.
Underlying these initiatives are strategies towards developing more
sustainable
transportation (e.g. electric vehicles) and the ability to increase renewable
energy
consumption from solar and wind technologies. Due to the intermittency of such
production and consumption requirements, these directions require significant
electrical
storage capabilities that are expected to be realized effectively only with
advanced
batteries. Lithium ion technology has emerged as the premier battery chemistry
due to
the increased energy density over other rechargeable technologies. However,
ongoing
technology demands necessitate higher energy densities which can reduce
battery mass
and volume characteristics in portable applications like electrical vehicles,
mobile
communications, and mobile grid storage. Advancements in lithium ion batteries
will
directly support both of ARPA-E mission areas since battery-enabled electric
vehicles
will significantly reduce the use of foreign energy sources and greenhouse gas
emissions.
[000128] The systems and methods of the present invention can provide bulk
nanomaterials of high strength, lower or similar weight, in a composite sheet.
By
providing the nanomaterials in a composite sheet, the bulk nanomaterials can
be easily
handled and subsequently processed for end use applications, including (i)
structural
systems, such as fabrics, armor, composite reinforcements, antennas,
electrical or
thermal conductors, heaters, and electrodes, (ii) mechanical structural
elements, such as
plates and I-beams, and (iii) cabling or ropes. Other applications can include
hydrogen
storage, or capacitor components.
29
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
[000129] The scale-up and commercialization of the aforementioned
technology
depends upon the ability to produce large quantities of materials of
sufficient quality at
a price which is comparable to current lithium ion battery carbons and
substrates. Large
sheets of high quality SWCNTS, produced in accordance with the methods of the
present invention, provide a strong, lightweight and electro-thermally
conductive
material from which the battery can be made.
[000130] Moreover, the composite sheet may be incorporated into composite
structures for additional end use applications, such as sporting goods
products, helmets,
antenna, morphing applications, aerospace, lightning protection flame
proofing, etc.
Composite sheets may further be nickel free, meaning they may be less toxic
than
standard products. Additionally, composite sheets may be repairable to
eliminate the
need to replace the composite sheets entirely or in part. In one embodiment, a
composite material may be formed by impregnating the composite sheet with a
matrix
precursor, such as Krayton, vinyl ester, Polyphenylene Sulfide (PPS),
Polyether ether
ketone (PEEK), bispolyamide, BMI (bismaleimide), epoxies, or polyamides, and
subsequently allowing the matrix to polymerize or thermally cure.
[000131] Composite sheets of carbon nanotubes made from the present
invention
can have a wide variety of applications. Examples of specific applications
include
electromagnetic interference shielding (EMI shielding) which may either
absorb, reflect,
or transmit electromagnetic waves. Shielding may be beneficial to prevent
interference
from surrounding equipment and may be found in stereo systems, telephones,
mobile
phones, televisions, medical devices, computers, and many other appliances.
For these
and similar applications, it may be important that the glassy carbon precursor
be
provided in a substantially thin layer, so that infiltration into the carbon
nanotube sheet
can be minimized to prevent degradation to the properties of the sheet.
[000132] EMI shielding may further be useful in minimizing insertion loss
from
sheets of carbon nanotubes. Insertion loss represents the difference in power
reception
prior to and after the use of a composite sheet. As illustrated in Fig. 10,
there is an
almost immediate drop in power reception followed by a stabilization.
[000133] Composite sheets of carbon nanotubes can have additional
applications,
such as utilizing the resulting assembly in the absorption of radar signal
(EMI shielding)
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
or to provide other desirable properties, such as lighting protection, heat
sinks, or
actuators. For such applications, it may not be critical if the bonding agent
penetrates
the carbon nanotube sheet. Accordingly, the glassy carbon material can be
coated with
less care than for that carried out in capacitor, battery or fuel cell
applications. In one
embodiment, the substrate for applications in this example can be a graphite
epoxy, e-
glass epoxy, or combinations with other types of matrices.
Additional Embodiments
[000134] In another embodiment of the present invention, there is provided
a
battery including (1) anode current collectors, (2) cathode current collectors
and (3) the
structural casing, all of which can be made from CNT sheets and or CNT
composite
sheets. Such battery can also include a separator between the cathode and the
anode
and a casing. The battery of the present invention can also have an
electrolyte material.
In one embodiment, the CNT cathode can have nickel sulfide or tin sulfide
deposited
thereon. In one embodiment, the anode can include a CNT sheet with silicon
deposited thereon and the cathode can include a CNT sheet with nickel sulfide
or tin
sulfide deposited on the sheet.
[000135] To make such CNT cathode having a nickel sulfide deposited
thereon,
nickel such as Damascene nickel may be deposited on a CNT sheet and then
converted
to nickel sulfide such as by a heating process. In another embodiment, to make
the
CNT cathode having a tin sulfide deposited thereon, tin may be deposited on a
CNT
sheet and then converted to tin sulfide such as by a heating process.
[000136] The CNT anode may be produced by silicon coating from silane by
CVD. Nickel produced by electrodeposition on the cathode is then subsequently
converted to nickel sulfide (NiS). The anode including a CNT sheet having
silicon
deposited thereon may be formed using the methods described above.
[000137] It should be appreciated that in some embodiments, a battery may
be
made with conventional materials with the exception that the anode and cathode
are
made from CNT. For example, in some embodiments, only the cathode and anode
are
each made from CNT sheets, with the anode having silicon deposited on the
sheet and
the cathode having nickel sulfide or tin sulfide deposited on the sheet.
31
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
[000138] In some embodiments, the battery of the present invention includes
CNT
anode and CNT cathode current collectors without metal in the system. In some
embodiments, a process to produce a battery and a battery with self-
temperature
regulating materials for the anode and the cathode are disclosed. In some
embodiments,
the battery can use CNT sheets without safety devices, such as temperature
measuring
systems, pressure release valves, etc. CNT sheets may be used as current
collectors
enabling the application of aggressive electrolytes. For example, the battery
may be
designed to include CNT sheet current collectors for both anode and cathode,
taking
advantage of the CNT conductivity and its self-temperature regulation, and
allowing for
the application of very aggressive high-conductivity electrolytes with no
danger of
passive layer breakdown as would occur on aluminum foil. In one embodiment,
the
anode includes a silicon coating (such as by a CVD process) on CNT sheet
material and
the cathode includes a NiS coating on CNT sheets. For example, the cathode may
be
made by first electrochemical coating Damascene nickel on CNT sheets material
followed by a sulfide conversion. It should however be appreciated that other
traditional
cathode materials may be used on CNT current collectors.
CNT Battery Electrode Synthesis and Characterization
[000139] CNT current collectors of the present invention may be synthesized
as
described herein. In one embodiment, the electrical conductivity exceeds 0.5 x
106 S/m.
Such electrodes may be fabricated 10 to 15 grams per square meter from CNT
"doped"
sheets. Characterization may be by four point resistivity and Van der Pauw
measurements or alternatively by measuring the sheet resistance using an eddy
current
technique such as the Delcom (50 MHz) system. Examples of typical properties
for
CNT sheets are shown in Table 2.
Table 2: Some Properties of CNT sheets
2
Property CNT Sheet (15 g/m )
3
Density (g/cm ) 0.4 ¨ 0.8
-3
Resistivity (*cm ) ¨ DC 1 x 10
1 MHz -4
0.5 x 10
4
Specific Conductivity 0.17 x 10
2
(S *cm /g) ¨ DC 1.7 x 104
1 MHz
32
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
important for pulse charge and discharge
Strength ¨ Tensile (MPa) 200 to 500 With Epoxy
Modulus (GPa) 20 to 60 2000
Strain to Failure (%) 20 170
3
Sheet Resistance (f2/o) 1.0 - Raw
0.2 - "Doped"
Thermal Conductivity Tunable: 10 ¨ 100
(W/m*K)
Coefficient of Thermal -3 x 1-60
-1
Expansion (K)
-3
Temperature Coefficient of 1.2 x 10 ¨ Raw
Resistivity -3
1.6 x 10 ¨Treated
[000140] In an embodiment, CNT sheet material may be produced by a CVD
process where a cloud of CNT material is deposited on a rotating belt or drum
using the
system described herein. In this process, material may be taken from the belt
or drum
and seamed into a long roll, for example, a 400 feet long roll, and treated to
produce a
sheet having high electrical conductivity. According to one embodiment, an 8
foot by
15 inch sheet, 10 g/m2, CNT sheet may be synthesized. A CNT sheet of any other
suitable dimensions may be made by this process.
The Anode: Coating CNT Sheet Material With Silicon
[000141] In an embodiment, the anode may be formed by coating a CNT sheet
material with silicon. For example, CNT can be coated with silicon using a CVD
process using Silane (SiH4). An example of a silicon coated CNT electrode is
shown in
Figures 18a and 18b. Fig. 18a is an image of a CNT sheet, 10 g/m2 electrode
coated
with about 30 to 50 nm of silicon deposited on each tube using a silane CVD
process.
Fig. 18b is an image at higher magnification of the CNT sheet of Fig. 18a
showing that
very high surface area of the CNT sheets is magnified by the nodular growth
(5Key,
SEM).
[000142] In some embodiments, a process used to coat the CNT material is a
thermally activated silane process:
= SiH4 (g) ¨> SiH4 (ab)
= SiH4 (ab) ¨> SiH2 (ab) + H2 (ab)
= SiH2 (ab) + H2 (ab) ¨> Si + H2 (ab)
= H2 (ab) ¨> H2 (g)
33
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
Testing
[000143] In an embodiment, capacity can be been measured for several
cycles. In
an exemplary embodiment, capacity of the electrode is measured, for example,
at
loadings of 1000 mA-hrs/gram (ref) and one cycle at 1750 mA-hr/g. When the
loading
was reduced to 500 mA-hrs/gram, the electrode was cycled over 30 times without
fade.
In an embodiment, the higher loaded specimen can also be cycled without fade
as long
as the coating remains thin.
[000144] Some of the charge storage data is shown in Figure 19a where the
charge data is plotted against discharge data, and in Figure 19b where the
specific
charge is illustrated. In particular, Fig. 19a is a graph showing the charge
capacity as a
function of the discharge capacity for lithiation of silicon coated CNT
electrodes. Fig.
19b, on the other hand, is a graph showing the reversible capacity as a
function of the
charge capacity for lithiation of silicon coated CNT electrodes.
[000145] Note that up to 1700 mA-hr/g has been measured on CNT material by
PSI. Cycling (full charge to discharge) data on Si coated CNT sheets have been
obtained
up to 500 Cycles but in this case the full charge has been limited to 500 mA-
hr/g. This
data is shown in Fig. 20. In particular, Fig. 20 is a graph showing dealloying
capacity
and coulombic efficiency as a function of the cycle index. In this latter
case, no
degradation of the material was observed for the few cycles that were
examined.
The Cathode: Damascene Nickel Transformed To NiS
[000146] The cathode, in an embodiment, may be made from, for example, one
or
more sheets of aligned carbon nanotubes infiltrated with nickel sulfide or tin
sulfide or
any other similar compound being utilized for generation of an electrical
current. For
example, the carbon nanotube sheet can be infiltrated with nano-scaled nickel
sulfide or
tin sulfide or a combination thereof Alternatively, the batteries of the
present
invention can be zinc air based, sodium based or lead acid based. It should be
noted
that although reference is made to sheets of aligned carbon nanotubes, sheets
of non-
aligned carbon nanotubes may also be used.
[000147] The nickel sulfide or tin sulfide can be incorporated within the
carbon
nanotube sheets during the construction of the carbon nanotube sheets for use
as a
34
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
cathode. In some embodiments, nickel sulfide or tin sulfide can be added
during the
nanotube growth process. For example, nickel sulfide can be dispersed with the
cloud
of nanotubes during the nanotube growth process using any known methods. In
alternate embodiments, nickel sulfide or tin sulfide can be added or deposited
after
formation of the carbon nanotube sheet using known methods. For instance,
nickel
sulfide can be dispersed in a volatile carrier, such as acetone, isopropanol,
methanol,
ethanol and the like, and sprayed onto the carbon nanotube sheet. The carrier
can be
evaporated from the sheet by heating the sheet to form a carbon nanotube
particulate
composite material.
[000148] In one embodiment of the present invention, it may be desirable to
match
the surface area and the loading of the anode and cathode. In most lithium ion
batteries,
the cathode material ability to store charge limits performance. This problem
may be
overcome by the present invention by: (1) the use of sulfur and compounds
containing
sulfur and (2) coating sulfur electrodes with a protective coating to prevent
desorption.
[000149] There is a strong tendency for sulfur to degrade though a set of
reactions
called the polysulfide decomposition chain. To minimize degradation, the
process of the
present invention stabilizes the sulfur by encapsulating it in a sheath or
organic
molecules, or bonding it to another material. As such, the present invention
utilizes the
compound nickel sulfur which is able to hold about 800 mA-hr/g of charge
making its
capacity comparable to that of the silicon anode material. Loading of the
electrode with
nickel is followed by the conversion to the nickel-sulfur compound. Nickel can
be
deposited at extraordinary rates using the damascene nickel process. Once
deposited,
nickel can be transformed chemically to the desired NiS crystal, for example,
by
exposing the material to H2S in a heated environment. Alternatively, the
conversion of
damascene nickel to NiS crystal can be accomplished by such methods as those
disclosed in Zhang et al. Mater. Sci. Eng. (1999), Zhou et al. Adv. Funct.
Mater. (2010),
Huang et al. Chem. Geol. (2010), Wang et al. J. Solid State Chem. Vol. 183
(2010), p.
223, Salavati-Niasari, et al. Mater. Res. Bull. Vol. 44 (2009), p. 2246,
Khiew, et al.
Mater. Lett. Vol. 58 (2004), p. 762, Denholme, et al. Isr. J. Chem. (2010),
which are
incorporated herein by reference in their entireties.
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
[000150] In one embodiment, the present process includes coating the CNT
tubes
using electrochemically deposited Damascene nickel and loading the CNT area to
match
total charge potential of the anode material. Since nickel is much higher in
density
(8.908 g/cc) than silicon (2.3209 g/cc), problems balancing charges can be
minimized.
Sulfides may be prepared through stoichiometric amounts of the metal and
sulfur heated
in evacuated and sealed quartz ampoules at 500-1000 C or by various solution
methods.
[000151] With the process described herein, (1) the CNT sheet electrodes
theoretically and experimentally exhibit lower electrode resistance than
comparable
copper or aluminum foils and with less Joule (I2R) heating, (2) the CNT sheets
tend to
exhibit temperature self-regulation, so that if there is incipient thermal
excursion, then
the material will increase its thermal conductivity and so suppress runaway,
(3) the
material does not degrade, even under very aggressive electrolytes, enabling
more
conductive electrolytes (such as those containing lithium
trifluoromethanesulfonylimide- LiTflm) to be used, and (4) the storage
capacity of this
system can exceed 1100 mA-hr/g compared with about 180 to 250 mA-hr/g, for the
best
commercial batteries. The nickel sulfide based anode and cathode may each have
a
value of 1100 mA-hr/g. A matched system (anode and cathode) provides more
thermal
stability than the current battery and makes design simpler.
[000152] Although nickel sulfide is disclosed, it should be appreciated
that tin
sulfide (SnS) may also be used in connection with the cathode of the present
invention.
Other methods may also be used for the conversion of Damascene tin to tin
sulfide
including those described in Journal of Power Sources, Volumes 97-98, July
2001,
Pages 198-200, Materials Science and Engineering: B, Volume 128, Issues 1-3,
15
March 2006, Pages 75-79, Journal of Power Sources, Volume 146, Issues 1-2, 26
August 2005, Pages 71-78, and Current Opinion in Solid State and Materials
Science,
Volume 4, Issue 2, April 1999, Pages 113-121, incorporated herein by reference
in their
entireties.
The Electrolyte
[000153] Any suitable electrolyte solution can be used. Exemplary
electrolytes
include, without limitation, aqueous solutions of KOH, HNO3, HC1,
tetraethylammonium bis(oxaloato)borate (TEABOB), tetraethylammonium
36
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
tetrafluoroborate (TEABF4), or triethylmethylammonium tetraflouraborate
dissolved in
acetonitrile.
[000154] In an embodiment, very aggressive high conductivity electrolytes
such
as lithium trifluoromethanesulfonylimide in an ethylene carbonate and
dimethoxy
ethane solvent (Lithium Triflate Imide= LiTflm) may be used. The use of such
electrolytes may result in a battery capable of very high specific power
levels.
[000155] In an embodiment, propylene carbonate and dimethoxy ethane with
LiPF6 conductive salts may be utilized. In an embodiment,
trifluoromethanesulfonylimide with propylene carbonate and dimethoxy ethane
may be
used.
Fabrication of Pouch Cells
[000156] In an embodiment of the present invention, pouch cells may be used
for
the characterization of this material. Problems with alternative coin cells
include current
distribution, high edge to volume ratio, metals potentially in contact with
the electrolyte,
etc. These issues are absent in pouch cells.
[000157] Pouch cells may be fabricated in an oxygen free glove box. The
cells
may be made using a commercial vacuum laminator placed in a dry box, such as a
Braun dry box. In an embodiment, a Si coated CNT anode and/or a NiS coated CNT
cathode may be used.
Testing
[000158] In an embodiment, the battery of the present invention may be
charged to
a pre-determined load and cycle 500 times. Battery testing may be conducted
with a lab
view driven potentiostat with coupled coulometer.
[000159] In an embodiment, the battery may be charged to 500 mA-hr/g, to
1000
mA-hr/g loading.
[000160] Fade, specific energy based on mass and volume, and specific power
based on mass and volume may be measured. Also, energy and volume efficiencies
and
costs per watt and costs per joule may also be measured.
Design and simulation of a battery (3 amp-hours at 14 volts)
37
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
[000161] In an embodiment, a battery may have 4 cells which may be
interconnected flexible pouch cells. Figure 21 showns an example of a large
battery for
an EV.
[000162] The characteristics of an example of a 3 A-hr battery, 3.5 V
characteristics are given below in Table 3.
Table 3 Battery Characteristics
Property Value all CNT Value Quallion' Comments
QL0032A
Size About 20 cm by 20 cm 50 by 120 by 7mm As configured, the CNT
by 3 mm 3.5 amp-hours battery is flexible and can
3 amp-hours 3.7V one cell conform to packaging. The
3.5V/cell x 4 cells 9 amps max draw Quallion is shown by
350 Wh/kg 120 Wh/kg comparison as it has about
Power: ND Power: 2000W/kg the same charge storage.
The Quallion uses a spinel
Packing geometries cathode and a graphite
can be changed as anode both filmed on
long as surface area metallic current collectors.
retained. Both batteries are pouch
cells.
Weight 2.8 g/Si, 3.5 g/Ni, 2 Not given PC=pouch cell,
not
media g/S, 50 PC including feed-through.
Weight 150 grams (see note) 97 grams Packaging involves 4
Total pouch cells; by combining
in one cell, weight for the
entire battery could be
dramatically reduced.
Voltage 14V 3.7
Capacity 3 amp-hours (10,800 12,600 coulombs 1800 to 3600 C/g
coulombs)
[000163] 1 http://www.quallion.com/sub-sp-q10032a.asp
Testing of Large Battery
[000164] In an embodiment of the present invention, a battery may be made
of a
series of pouch cells joined in series. This battery is a test system, so all
cells will be
accessible for monitoring. Current density on this kind of battery is related
to the: (1)
conductivity of the electrolyte, (2) electrode spacing and (3) diffusion rates
of the
battery separator. For this kind of battery a highly porous separator may be
used.
38
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
[000165] Alternative configurations may include electrodes at each end of
the
pouch.
Applications of Nickel Sulfide and Tin Sulfide Embodiments
[000166] The batteries described above with reference to Figs. 18a-21, have
many
advantages and applications. Carbon nanotube (CNT) sheet current collectors of
the
present invention may be used in place of conventional copper foils for the
anode and
aluminum foil for the cathode. This may increase energy density while reducing
the
chance of thermal runaway. Non-metallic CNT sheets comprised of millimeter
length
CNTs, for example, provide enhanced surface area and properties that manage
thermal
energy better than metals. For the first time, dissimilar metal current
collectors such as
copper and aluminum need not be used, saving weight, eliminating corrosion
couples,
and enabling the use of more aggressive and superior conductive electrolytes.
Coating
the CNT current anode collectors of the present invention with silicon results
in a high
capacity electrode with a comparative reduction of electrode resistance
(compared with
the coated copper foil); therefore, the Joule heating associated with high
current
performance is diminished.
[000167] The cathode electrodes of the present invention may be the same
CNT
sheets but coated with a nickel-sulfur. The inverse thermal conductivity
relationship
present in CNT materials provides a thermal stabilizing effect on the battery
system,
e.g., the hotter the system, the more heat is conducted away. This
characteristic not only
improves safety but also reduces costs due to simpler design, i.e., reduction
or
elimination of thermal management systems in the battery. The flexibility of
CNT based
current collector of the present invention enables more efficient packaging
and even
flexible conformable batteries to be produced. Carbon nanotube anode and
cathode
based current collectors for lithium based secondary batteries may also be
made.
[000168] Unlike metal-collector based batteries, such thin CNT sheet-based
systems enable very thin flexible and conformal batteries that reduce both
fatigue and
bending stresses, and allow more efficient and innovative packaging. A lower
cost
battery may be produced given that thermal monitoring elements such as the
integrated
circuit, safety release value, and temperature sensor potentially can be
eliminated. Both
space based batteries and more advanced batteries using sulfur based
chemistries may
39
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
also be produced using the processes and materials described herein. Conformal
batteries are another spin out which has value for defense applications.
[000169] Applications of batteries of the present invention include, but
are not
limited to, satellites, ground stations, timers, and remote sensing devices.
All of these
systems are defined by their (1) weight, (2) reliability, (3) safety and (4)
total energy
content (how long they will operate). Existing lithium ion batteries are
versions of
commercial batteries and have proven to last a minimum of 10 years. Power (the
rate of
delivering energy) seems to be less important and lifetime and reliability
more
important. Existing technologies have adequate power. The batteries described
herein
improve the energy content, safety and reliability, as well as reduce weight.
In an
embodiment, the battery makes use of a current collector which may reduce the
battery
weight and enable more flexible packing, while at the same time permit a
factor of 5
times more energy content. The electrode resistivity is less than that of
existing
commercial batteries enabling higher power and less Joule heating.
[000170] The current collectors of the present invention are made from
sheets
manufactured by the method of the present invention that can be substantially
fatigue
resistant. When using this approach, Joule heating is a factor of 4 times less
that the
current technology. Under high currents, this large factor is expected to be
significant
and translates into a light system without the need for external cooling.
Energy storage
is expected to increase by a factor of 5 so that smaller batteries are
possible.
[000171] Table 4 shows a comparison of the proposed effort to the state-of-
the-art.
CA 02883158 2015-02-20
WO 2014/031440 PCT/US2013/055154
Table 4. Comparison of CNT Battery Parameters with Current Technology.
Theoretical Proven Cycle Comments
Capacity Capacity Life
mA h/g mA h/g
Li/Graphite Anode 372 ¨250 Good Existing technology
Anode expansion during
Li/Si Anode (usually
lithiation causes cracking
particles of Si) 4212 2500 Poor of the SI
Expansion cracking
1025 Very
avoided by thin Si coating
Li/Si/CNT Anode ¨3800 proven Good proven with CNTs
Li Ion Cathode ¨300 100 - 150 Good Existing technology
Li/S Standard
Polysulfide cycle causes
Cathode 1673 ¨150 Poor degradation
NiS Experimental protective coatings
Cathode 1673 ¨1000 Moderate
Present Invention Si
30-50 nm coated CNT
Anode 3800 1500 Good
Present Invention Sulfur (alloy)
Cathode 1506 1000 Good
[000172] One advantage of CNT sheet electrodes made according to the
process of
the present invention is their positive coefficient of resistivity with
temperature.
[000173] Thermal conductivity versus temperature data for a variety of
different
CNT sheet treatments is shown in Fig. 22. Thermal conductivity versus
temperature for
the pure uncoated copper foil is shown in Figure 23. This property provides
the
potential for the battery electrode to be self-temperature regulating. As the
electrode
gets hot, its thermal conductivity increases, so if there is a hot spot the
battery material
will conduct heat away faster to remove it. This is quite different than the
copper/aluminum metal foils currently in use. It may be possible to remove
internal
sensors, valves and temperature measurement probes thereby reducing cost.
[000174] Another advantage of using CNT electrode material of the present
invention is the low electrical resistivity of the entire electrode. Present
technology uses
a paste of active media, binders, and graphite powder for the anode. The
resistivity of
this paste dominates the resistivity of the system so that CNT electrode are
more
41
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
conductive as shown in Table 5. The Joule heating of these electrodes under
high
current load, may be significantly less than current technology and the higher
thermal
conductivity may help carry away any localized heat that is generated.
[000175] Table 5 shows the resistivity of a CNT sheet electrode of the
present
invention compared with a copper paste coated electrode, showing improved
resistivity
and thermal conductivity.
Table 5
Resistivity Thermal
-3 conductivity
1 x 10 n=cm 4 4
W=in K
Si-coated CNT fabric 2.93 0.34 17.59 2.32
Compressed Si nanopowder electrode 327,500 22,000 0.40
Graphite electrode 5.0 1.04
[000176] The replacement of heavy and fatigue prone systems with CNT
electrodes not only permits a much lighter battery. Furthermore the safety
advantages
of the CNT material permit the elimination of temperature monitoring and
regulating
systems so that the weight advantage is increased. The flexible nature of the
CNT
electrodes, enables a new kind of packing that saves space. For example,
conformal
batteries can be fabricated to better use the available space.
[000177] In addition to the applications previously described herein, the
batteries
can also have applications in aircraft, rockets and drones. These are
applications where
weight is a premium. Also, applications include automotive use in which
existing
batteries are very inadequate. For example, the range of the Nissan Leaf is
about 73
miles before it has to undergo a 12 hour recharge at 110V. A practical range
equivalent
to IC powered cars of approximately 400 miles is considered necessary before
electric
vehicles can enter the main-stream market. This technology should meet this
demand
with a system that exceeds the present safety needs.
[000178] All references cited herein are incorporated by reference herein
in their
entirety.
42
CA 02883158 2015-02-20
WO 2014/031440
PCT/US2013/055154
[000179] While the present invention has been described with reference to
certain
embodiments thereof, it should be understood by those skilled in the art that
various
changes may be made and equivalents may be substituted without departing from
the
true spirit and scope of the invention. In addition, many modifications may be
made to
adapt to a particular situation, indication, material and composition of
matter, process
step or steps, without departing from the spirit and scope of the present
invention. All
such modifications are intended to be within the scope of the claims appended
hereto.
43