Note: Descriptions are shown in the official language in which they were submitted.
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
PROCESSES AND SYSTEMS FOR THE PRODUCTION
OF FERMENTATION PRODUCTS
[0001] This
application claims the benefit of U.S. Provisional Application No. 61/699,976,
filed on September 12, 2012; U.S. Provisional Application No. 61/712,385,
filed on October
11, 2012; U.S. Patent Application No. 13/828,353, filed on March 14, 2013; and
U.S. Patent
Application No. 13/836,115, filed on March 15, 2013; the entire contents of
each are herein
incorporated by reference.
[0002] The
Sequence Listing associated with this application is filed in electronic form
via
EFS-Web and hereby incorporated by reference into the specification in its
entirety.
FIELD OF THE INVENTION
[0003] The
present invention relates to the production of fermentation products such as
alcohols including ethanol and butanol, and processes employing in situ
product removal
methods.
BACKGROUND OF THE INVENTION
[0004] A number of chemicals and consumer products may be produced utilizing
fermentation as the manufacturing process. For example, alcohols such as
ethanol and
butanol have a variety of industrial and scientific applications such as
fuels, reagents, and
solvents. Butanol is an important industrial chemical with a variety of
applications including
use as a fuel additive, as a feedstock chemical in the plastics industry, and
as a food-grade
extractant in the food and flavor industry. The production of butanol or
butanol isomers
from materials such as plant-derived materials could minimize the use of
petrochemicals and
would represent an advance in the art. Furthermore, production of chemicals
and fuels using
plant-derived materials or other biomass sources would provide eco-friendly
and sustainable
alternatives to petrochemical processes.
[0005]
Techniques such as genetic engineering and metabolic engineering may be
utilized
to modify a microorganism to produce a certain product from plant-derived
materials or other
sources of biomass.
However, in the production of butanol, for example, some
- 1 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
microorganisms that produce butanol in high yields also have low butanol
toxicity
thresholds. Removal of butanol from the fermentation as it is being produced
is a means to
manage these low butanol toxicity thresholds. Thus, there is a continuing need
to develop
efficient methods and systems for producing butanol in high yields despite the
low butanol
toxicity thresholds of the butanol-producing microorganisms.
[0006] In situ product removal (ISPR) (also referred to as extractive
fermentation) can be
used to remove butanol or other fermentation products from the fermentation as
it is
produced, thereby allowing the microorganism to produce butanol at high
yields. One ISPR
method for removing fermentative alcohol that has been described in the art is
liquid-liquid
extraction (see, e.g., U.S. Patent Application Publication No. 2009/0305370).
In general,
with regard to butanol fermentation, the fermentation broth which includes the
microorganism is contacted with an extractant at a time before the butanol
concentration
reaches, for example, a toxic level. Butanol partitions into the extractant
decreasing the
concentration of butanol in the fermentation broth containing the
microorganism, thereby
limiting the exposure of the microorganism to the inhibitory butanol.
[0007] In order to be technically and economically viable, liquid-liquid
extraction requires
contact between the extractant and the fermentation broth for efficient mass
transfer of the
alcohol into the extractant; phase separation of the extractant from the
fermentation broth
(during and/or after fermentation); efficient recovery and recycle of the
extractant; and
minimal decrease of the partition coefficient of the extractant over long-term
operation.
Extractant can become contaminated over time with each recycle, for example,
by the build-
up of lipids present in the biomass used as feedstock for fermentation, and
this contamination
can lead to a concomitant reduction in the partition coefficient of the
extractant.
[0008] In addition, the presence of undissolved solids during extractive
fermentation can
negatively affect the efficiency of alcohol production. For example, the
presence of the
undissolved solids may lower the mass transfer coefficient, impede phase
separation, result
in the accumulation of oil from the undissolved solids in the extractant
leading to reduced
extraction efficiency over time, slow the disengagement of extractant drops
from the
fermentation broth, result in a lower fermentation vessel volume efficiency,
and increase the
loss of extractant because it becomes trapped in the solids and ultimately
removed as Dried
Distillers' Grains with Solubles (DDGS).
- 2 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[0009] Thus, there is a continuing need for alternative extractive
fermentation processes that
reduce the toxic effect of the fermentative alcohol such as butanol on the
microorganism, and
which can also reduce the degradation of the partition coefficient of an
extractant. The
present invention satisfies the needs described herein and provides methods,
processes, and
systems for the fermentative production of alcohols such as ethanol and
butanol.
SUMMARY OF THE INVENTION
[0010] The present invention is directed to a method for recovering a
fermentation product
from a fermentation broth comprising providing a fermentation broth comprising
a
microorganism, wherein the microorganism produces fermentation product in a
fermentor;
contacting the fermentation broth with at least one extractant; and recovering
the
fermentation product. In some embodiments, the contacting of the fermentation
broth with at
least one extractant occurs in the fermentor, an external unit, or both. In
some embodiments,
the external unit is an extractor. In some embodiments, the extractor is
selected from siphon,
decanter, centrifuge, gravity settler, phase splitter, mixer-settler, column
extractor,
centrifugal extractor, agitated extractor, hydrocyclone, spray tower, and
combinations
thereof In some embodiments, the extractant is selected from C7 to C22 fatty
alcohols, C7 to
C22 fatty acids, esters of C7 to C22 fatty acids, C7 to C22 fatty aldehydes,
C7 to C22 fatty
amides, and mixtures thereof In some embodiments, the extractant is selected
from oleyl
alcohol, behenyl alcohol, cetyl alcohol, lauryl alcohol, myristyl alcohol,
stearyl alcohol, oleic
acid, lauric acid, linoleic acid, linolenic acid, myristic acid, stearic acid,
octanoic acid,
decanoic acid, undecanoic acid, methyl myristate, methyl oleate, 1-nonanol, 1-
decanol, 2-
undecanol, 1-nonanal, 1-undecanol, undecanal, lauric aldehyde, 2-
methylundecanal,
oleamide, linoleamide, palmitamide, stearylamide, 2-ethyl- 1 -hexanol, 2-hexyl-
1 -decanol, 2-
octyl- 1 -dodecanol, and mixtures thereof In some embodiments, a hydrophilic
solute is
added to the fermentation broth. In some embodiments, the hydrophilic solute
is selected
from polyhydroxlated compounds, polycarboxylic acids, polyol compounds, ionic
salts, and
mixtures thereof In some embodiments, the contacting of the fermentation broth
with at
least one extractant occurs in two or more external units. In some
embodiments, the
contacting of the fermentation broth with at least one extractant occurs in
two or more
fermentors. In some embodiments, the fermentors comprise internals or devices
to improve
phase separation. In some embodiments, the internals or devices are selected
from
- 3 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
coalescers, baffles, perforated plates, wells, lamella separators, cones, and
combinations
thereof In some embodiments, real-time measurements are used to monitor
extraction of the
fermentation product. In some embodiments, extraction of the fermentation
product is
monitored by real-time measurements of phase separation. In some embodiments,
phase
separation is monitored by measuring rate of phase separation, extractant
droplet size, and/or
composition of fermentation broth. In some embodiments, phase separation is
monitored by
conductivity measurements, dielectric measurements, viscoelastic measurements,
and/or
ultrasonic measurements. In some embodiments, providing a fermentation broth
comprising
a microorganism occurs in two or more fermentors. In some embodiments, the
fermentation
product may be a product alcohol. In some embodiments, the product alcohol is
selected
from ethanol, propanol, butanol, pentanol, hexanol, and fusel alcohols. In
some
embodiments, the microorganism comprises a butanol biosynthetic pathway. In
some
embodiments, the butanol biosynthetic pathway is a 1-butanol biosynthetic
pathway, a 2-
butanol biosynthetic pathway, an isobutanol biosynthetic pathway, or a 2-
butanone pathway.
In some embodiments, the microorganism is a recombinant microorganism. In some
embodiments, the method further comprises the steps of providing a feedstock
slurry
comprising fermentable carbon source, undissolved solids, oil, and water;
separating the
feedstock slurry forming three streams: (i) an aqueous solution comprising
fermentable
carbon source, (ii) a wet cake comprising solids, and (iii) oil; and adding
the aqueous
solution to the fermentation broth. In some embodiments, the oil is hydrolyzed
to form fatty
acids. In some embodiments, the fermentation broth is contacted with the fatty
acids. In
some embodiments, the oil is hydrolyzed by an enzyme. In some embodiments, the
enzyme
is one or more lipases or phospholipases. In some embodiments, the feedstock
slurry is
generated by hydrolysis of feedstock. In some embodiments, feedstock is
selected from rye,
wheat, corn, cane, barley, cellulosic or lignocellulosic material, and
combinations thereof In
some embodiments, the feedstock slurry is separated by decanter bowl
centrifugation, three-
phase centrifugation, disk stack centrifugation, filtering centrifugation,
decanter
centrifugation, filtration, membrane filtration, microfiltration, vacuum
filtration, beltfilter,
pressure filtration, filtration using a screen, screen separation, grating,
porous grating,
flotation, hydrocyclone, filter press, screwpress, gravity settler, vortex
separator, or
combination thereof In some embodiments, separating the feedstock is a single
step process.
In some embodiments, the wet cake is combined with the aqueous solution. In
some
- 4 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
embodiments, the method further comprises contacting the aqueous solution with
a catalyst
converting oil in the aqueous solution to fatty acids. In some embodiments,
the aqueous
solution and fatty acids are added to the fermentation broth. In some
embodiments, the
catalyst is deactivated.
[0011] The
present invention is also directed to a system comprising one or more
fermentors comprising: an inlet for receiving feedstock slurry; and an outlet
for discharging
fermentation broth comprising fermentation product; and one or more extractors
comprising:
a first inlet for receiving the fermentation broth; a second inlet for
receiving extractant; a first
outlet for discharging a lean fermentation broth; and a second outlet for
discharging a rich
extractant. In some embodiments, the system further comprises one or more
liquefaction
units; one or more separation means; and optionally one or more wash systems.
In some
embodiments, the separation means is selected from decanter bowl
centrifugation, three-
phase centrifugation, disk stack centrifugation, filtering centrifugation,
decanter
centrifugation, filtration, vacuum filtration, belt filter, pressure
filtration, membrane
filtration, microfiltration, filtration using a screen, screen separation,
grating, porous grating,
flotation, hydrocyclone, filter press, screwpress, gravity settler, vortex
separator, and
combinations thereof In some
embodiments, the system also comprises on-line
measurement devices. In some embodiments, the on-line measurement devices are
selected
from particle size analyzers, Fourier transform infrared spectroscopes, near-
infrared
spectroscopes, Raman spectroscopes, high pressure liquid chromatography,
viscometers,
densitometers, tensiometers, droplet size analyzers, pH meters, dissolved
oxygen probes, and
combinations thereof
DESCRIPTION OF THE DRAWINGS
[0012] The accompanying drawings, which are incorporated herein and form a
part of the
specification, illustrate the present invention and, together with the
description, further serve
to explain the principles of the invention and to enable a person skilled in
the pertinent art to
make and use the invention.
[0013] Figure
1 schematically illustrates an exemplary process and system of the present
invention, in which undissolved solids are removed via separation after
liquefaction and
before fermentation.
- 5 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[0014] Figure 2 schematically illustrates an exemplary process and system
of the present
invention, in which ISPR is conducted downstream of fermentation.
[0015] Figure 3 schematically illustrates another exemplary alternative
process and system
of the present invention, in which an oil stream is discharged.
[0016] Figure 4 schematically illustrates another exemplary alternative
process and system
of the present invention, in which the wet cake is subjected to wash cycles.
[0017] Figure 5 schematically illustrates another exemplary alternative
process and system
of the present invention, in which an oil stream is discharged and wet cake is
subjected to
wash cycles.
[0018] Figures 6A and 6B schematically illustrates another exemplary
alternative process
and system of the present invention, in which the aqueous solution and wet
cake are
combined and conducted to fermentation (Figure 6A) and aqueous solution, oil,
and wet cake
are combined and conducted to fermentation (Figure 6B).
[0019] Figures 7A-7D schematically illustrates exemplary alternative
processes and systems
of the present invention, in which the aqueous solution is subjected to
conversion (e.g.,
hydrolysis, transesterification) and/or deactivation.
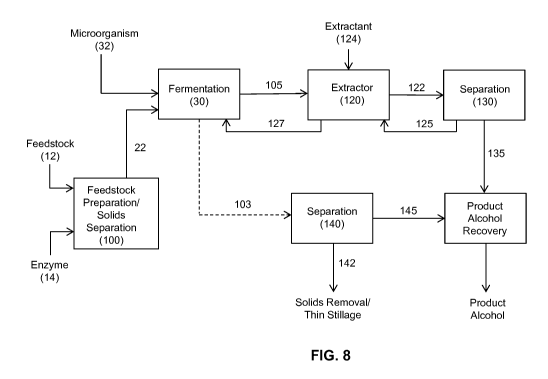
[0020] Figure 8 schematically illustrates an exemplary fermentation process
of the present
invention including downstream processing.
[0021] Figure 9 schematically illustrates an exemplary fermentation process
of the present
invention including downstream processing.
[0022] Figures 10A-10M illustrated various systems that may be used in the
processes
described herein.
[0023] Figures 11A and 11B schematically illustrate multiple pass
extractant flow systems.
[0024] Figure 12 schematically illustrates an exemplary fermentation
process of the present
invention utilizing on-line, in-line, at-line, and/or real-time measurements
for monitoring
fermentation processes.
[0025] Figures 13A and 13B schematically illustrates exemplary processes of
the present
invention for mitigating formation of a rag layer.
[0026] Figure 14 schematically illustrates an exemplary process of the
present invention
including fermentation, extraction, and distillation processes.
[0027] Figure 15 shows the effects of the fermentation broth to extractant
ratios (aq/org) on
extraction column efficiency.
- 6 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[0028] Figures 16A and 16B show the effects of ISPR using an external
extraction column
on isobutanol concentrations and glucose profiles.
[0029] Figure 17 show the effects of ISPR using a mixer-settler on
isobutanol removal
rates.
[0030] Figure 18 shows FTIR spectra of the range of starch concentrations
using in-line
measurements.
[0031] Figure 19 shows FTIR spectra of the starch concentration of wet cake
during
processing of corn mash.
[0032] Figure 20 shows FTIR spectra of corn oil during processing of corn
mash.
[0033] Figure 21 demonstrates a real-time measurement of isobutanol in
COFA.
DESCRIPTION OF THE INVENTION
[0034] Unless defined otherwise, all technical and scientific terms used
herein have the
same meaning as commonly understood by one of ordinary skill in the art to
which this
invention belongs. In case of conflict, the present application including the
definitions will
control. Also, unless otherwise required by context, singular terms shall
include pluralities
and plural terms shall include the singular. All publications, patents, and
other references
mentioned herein are incorporated by reference in their entireties for all
purposes.
[0035] In order to further define this invention, the following terms and
definitions are
herein provided.
[0036] As used herein, the terms "comprises," "comprising," "includes,"
"including," "has,"
"having," "contains," or "containing," or any other variation thereof, will be
understood to
imply the inclusion of a stated integer or group of integers but not the
exclusion of any other
integer or group of integers. For example, a composition, a mixture, a
process, a method, an
article, or an apparatus that comprises a list of elements is not necessarily
limited to only
those elements but can include other elements not expressly listed or inherent
to such
composition, mixture, process, method, article, or apparatus. Further, unless
expressly stated
to the contrary, "or" refers to an inclusive or and not to an exclusive or.
For example, a
condition A or B is satisfied by any one of the following: A is true (or
present) and B is false
(or not present), A is false (or not present) and B is true (or present), and
both A and B are
true (or present).
- 7 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[0037] Also, the indefinite articles "a" and "an" preceding an element or
component of the
invention are intended to be nonrestrictive regarding the number of instances,
that is,
occurrences of the element or component. Therefore, "a" or "an" should be read
to include
one or at least one, and the singular word form of the element or component
also includes the
plural unless the number is obviously meant to be singular.
[0038] The term "invention" or "present invention" as used herein is a non-
limiting term
and is not intended to refer to any single embodiment of the invention but
encompasses all
possible embodiments as described in the application.
[0039] As used herein, the term "about" modifying the quantity of an
ingredient or reactant
of the invention employed refers to variation in the numerical quantity that
can occur, for
example, through typical measuring and liquid handling procedures used for
making
concentrates or solutions in the real world; through inadvertent error in
these procedures;
through differences in the manufacture, source, or purity of the ingredients
employed to
make the compositions or to carry out the methods; and the like. The term
"about" also
encompasses amounts that differ due to different equilibrium conditions for a
composition
resulting from a particular initial mixture. Whether or not modified by the
term "about," the
claims include equivalents to the quantities. In one embodiment, the term
"about" means
within 10% of the reported numerical value, alternatively within 5% of the
reported
numerical value.
[0040] "Biomass" as used herein refers to a natural product containing
hydrolyzable
polysaccharides that provide fermentable sugars and/or starches including any
sugar and
starch derived from natural resources such as corn, sugar cane, wheat,
cellulosic or
lignocellulosic material, and materials comprising cellulose, hemicellulose,
lignin, starch,
oligosaccharides, disaccharides, and/or monosaccharides, and mixtures thereof
Biomass
may also comprise additional components such as protein and/or lipids. Biomass
may be
derived from a single source or biomass may comprise a mixture derived from
more than one
source. For example, biomass may comprise a mixture of corn cobs and corn
stover, or a
mixture of grass and leaves. Biomass includes, but is not limited to,
bioenergy crops,
agricultural residues, municipal solid waste, industrial solid waste, sludge
from paper
manufacture, yard waste, wood and forestry waste (e.g., forest thinnings).
Examples of
biomass include, but are not limited to, corn, corn cobs, crop residues such
as corn husks,
corn stover, grasses, wheat, rye, wheat straw, spelt, triticale, barley,
barley straw, oats, hay,
- 8 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
rice, rice straw, switchgrass, potato, sweet potato, cassava, Jerusalem
artichoke, sugar cane
bagasse, sorghum, sugar cane, sugar beet, fodder beet, soy, palm, coconut,
rapeseed,
safflower, sunflower, millet, eucalyptus, miscanthus, components obtained from
milling of
grains, trees (e.g., branches, roots, leaves), wood chips, sawdust, shrubs and
bushes,
vegetables, fruits, flowers, manure, and mixtures thereof For example, mash,
juice,
molasses, or hydrolysate may be formed from biomass by any processing known in
the art
for processing biomass for purposes of fermentation such as milling and
liquefaction. For
example, cellulosic and/or lignocellulosic biomass may be processed to obtain
a hydrolysate
containing fermentable sugars by any method known to one skilled in the art,
such as low
ammonia pretreatment disclosed in U.S. Patent Application Publication No.
2007/0031918,
which is herein incorporated by reference. Enzymatic saccharification of
cellulosic and/or
lignocellulosic biomass typically makes use of an enzyme consortium (e.g.,
cellulases,
xylanases, glucosidases, glucanases, lyases) for breaking down cellulose and
hemicellulose
to produce a hydrolysate containing sugars including glucose, xylose, and
arabinose.
Saccharification enzymes suitable for cellulosic and/or lignocellulosic
biomass are reviewed
in Lynd, et al. (Microbiol. Mol. Biol. Rev. 66:506-577, 2002).
[0041] "Fermentable carbon source" or "fermentable carbon substrate" as
used herein refers
to a carbon source capable of being metabolized by microorganisms. Suitable
fermentable
carbon sources include, but are not limited to, monosaccharides such as
glucose or fructose;
disaccharides such as lactose or sucrose; oligosaccharides; polysaccharides
such as starch or
cellulose; one carbon substrates; and mixtures thereof
[0042] "Fermentable sugar" as used herein refers to one or more sugars
capable of being
metabolized by the microorganisms disclosed herein for the production of
fermentation
products.
[0043] "Feedstock" as used herein refers to a feed in a fermentation
process, the feed
containing a fermentable carbon source with or without undissolved solids and
oil, and where
applicable, the feed containing the fermentable carbon source before or after
the fermentable
carbon source has been removed from starch or obtained from the breakdown of
complex
sugars by further processing such as by liquefaction, saccharification, or
other process.
Feedstock includes or may be derived from biomass. Suitable feedstocks
include, but are not
limited to, rye, wheat, corn, corn mash, cane, cane mash, barley, cellulosic
material,
- 9 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
lignocellulosic material, or mixtures thereof Where reference is made to
"feedstock oil," it
will be appreciated that the term encompasses the oil produced from a given
feedstock.
[0044] "Fermentation broth" as used herein refers to a mixture of water,
fermentable carbon
sources (e.g., sugars), dissolved solids, optionally microorganisms producing
fermentation
products (e.g., product alcohol), optionally fermentation products (e.g.,
product alcohol), and
other constituents. In some embodiments, fermentation broth refers to the
material held in
the fermentor in which the fermentation product (e.g., product alcohol) is
being made by the
metabolism of fermentable carbon sources by the microorganisms. From time to
time as
used herein, the term "fermentation broth" may be used synonymously with
"fermentation
medium" or "fermented mixture." In some embodiments, fermentation broth
comprising
product alcohol may be referred to as fermentation beer or beer.
[0045] "Fermentor" or "fermentation vessel" as used herein refers to the
unit in which the
fermentation reaction is carried out whereby fermentation product (e.g.,
product alcohol such
as ethanol or butanol) is produced from fermentable carbon sources. The term
"fermentor"
may be used synonymously herein with "fermentation vessel."
[0046] "Liquefaction unit" as used herein refers to the unit in which
liquefaction is carried
out. Liquefaction is the process in which oligosaccharides are released from
feedstock. In
some embodiments where the feedstock is corn, oligosaccharides are released
from the corn
starch content during liquefaction.
[0047] "Saccharification unit" as used herein refers to the unit in which
saccharification
(i.e., the breakdown of oligosaccharides into monosaccharides) is carried out.
Where
fermentation and saccharification occur simultaneously, the saccharification
unit and the
fermentor may be the same unit.
[0048] "Sugar" as used herein refers to oligosaccharides, disaccharides,
monosaccharides,
and/or mixtures thereof The term "saccharide" also includes carbohydrates
including
starches, dextrans, glycogens, cellulose, pentosans, as well as sugars.
[0049] As used herein, "saccharification enzyme" refers to one or more
enzymes that are
capable of hydrolyzing polysaccharides and/or oligosaccharides, for example,
alpha-1,4-
glucosidic bonds of glycogen or starch. Saccharification enzymes may include
enzymes
capable of hydrolyzing cellulosic or lignocellulosic materials as well.
[0050] "Undissolved solids" as used herein refers to non-fermentable
portions of feedstock,
for example, germ, fiber, gluten, and any additional components that do not
dissolve in
- 10 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
aqueous media. For example, the non-fermentable portions of feedstock include
the portion
of feedstock that remains as solids and can absorb liquid from the
fermentation broth.
[0051] "Oil" as used herein refers to lipids obtained from plants (e.g.,
biomass) or animals.
Examples of oils include, but are not limited to, tallow, corn, canola,
capric/caprylic
triglycerides, castor, coconut, cottonseed, fish, jojoba, lard, linseed,
neetsfoot, oiticica, palm,
peanut, rapeseed, rice, safflower, soya, sunflower, tung, jatropha, and
vegetable oil blends.
[0052] "Product alcohol" as used herein refers to any alcohol that can be
produced by a
microorganism in a fermentation process that utilizes biomass as a source of
fermentable
carbon substrate. Product alcohols include, but are not limited to, C1 to C8
alkyl alcohols. In
some embodiments, the product alcohols are C2 to C8 alkyl alcohols. In other
embodiments,
the product alcohols are C2 to C5 alkyl alcohols. It will be appreciated that
Ci to C8 alkyl
alcohols include, but are not limited to, methanol, ethanol, propanol,
butanol, pentanol, and
hexanol. Likewise C2 to C8 alkyl alcohols include, but are not limited to,
ethanol, propanol,
butanol, pentanol, and hexanol. In some embodiments, product alcohol may also
include
fusel alcohols (or fusel oils) and glycerol. "Alcohol" is also used herein
with reference to a
product alcohol.
[0053] "Butanol" as used herein refers to the butanol isomers 1-butanol (1-
BuOH), 2-
butanol (2-BuOH), tert-butanol (t-BuOH), and/or isobutanol (iBuOH, i-BuOH, I-
BUOH, iB
also known as 2-methyl- 1-propanol), either individually or as mixtures
thereof From time to
time, when referring to esters of butanol, the terms "butyl esters" and
"butanol esters" may be
used interchangeably.
[0054] "Propanol" as used herein refers to the propanol isomers isopropanol
or 1-propanol.
[0055] "Pentanol" as used herein refers to the pentanol isomers 1-pentanol,
3-methyl-l-
butanol, 2-methyl-I -butanol, 2,2-dimethyl- 1 -propanol, 3 -pentanol, 2 -p
entanol, 3 -methyl-2-
butanol, or 2-methyl-2-butanol.
[0056] "In Situ Product Removal (ISPR)" as used herein refers to the
selective removal of a
specific product from a biological process such as fermentation to control the
product
concentration in the biological process as the product is produced.
[0057] "Extractant" as used herein refers to a solvent used to extract a
fermentation product
(e.g., product alcohol). From time to time as used herein, the term
"extractant" may be used
synonymously with "solvent."
-11-
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[0058] "Water-immiscible" as used herein refers to a chemical component
such as an
extractant or solvent, which is incapable of mixing with an aqueous solution
such as
fermentation broth, in such a manner as to form one liquid phase.
[0059] "Carboxylic acid" as used herein refers to any organic compound with
the general
chemical formula ¨COOH in which a carbon atom is bonded to an oxygen atom by a
double
bond to make a carbonyl group (¨C=0) and to a hydroxyl group (¨OH) by a single
bond. A
carboxylic acid may be in the form of the protonated carboxylic acid, in the
form of a salt of
a carboxylic acid (e.g., an ammonium, sodium, or potassium salt), or as a
mixture of
protonated carboxylic acid and salt of a carboxylic acid. The term carboxylic
acid may
describe a single chemical species (e.g., oleic acid) or a mixture of
carboxylic acids as can be
produced, for example, by the hydrolysis of biomass-derived fatty acid esters
or triglycerides,
diglycerides, monoglycerides, and phospholipids.
[0060] "Fatty acid" as used herein refers to a carboxylic acid (e.g.,
aliphatic monocarboxylic
acid) having C4 to C28 carbon atoms (most commonly C12 to C24 carbon atoms),
which is
either saturated or unsaturated. Fatty acids may also be branched or
unbranched. Fatty acids
may be derived from, or contained in esterified form, an animal or vegetable
fat, oil, or wax.
Fatty acids may occur naturally in the form of glycerides in fats and fatty
oils or may be
obtained by hydrolysis of fats or by synthesis. The term fatty acid may
describe a single
chemical species or a mixture of fatty acids. In addition, the term fatty acid
also
encompasses free fatty acids.
[0061] "Fatty alcohol" as used herein refers to an alcohol having an
aliphatic chain of C4 to
C22 carbon atoms, which is either saturated or unsaturated.
[0062] "Fatty aldehyde" as used herein refers to an aldehyde having an
aliphatic chain of C4
to C22 carbon atoms, which is either saturated or unsaturated.
[0063] "Fatty amide" as used herein refers to an amide having an aliphatic
chain of C4 to
C22 carbon atoms, which is either saturated or unsaturated.
[0064] "Fatty ester" as used herein refers to an ester having an aliphatic
chain of C4 to C22
carbon atoms, which is either saturated or unsaturated.
[0065] "Aqueous phase" as used herein refers to the aqueous phase of, for
example, a
biphasic mixture containing, for example, a liquid phase and a vapor phase, to
the aqueous
phase of a triphasic mixture containing two liquid phases (e.g., an organic
phase and an
aqueous phase) and a vapor phase, to the aqueous phase of either a biphasic or
triphasic
- 12 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
mixture where the aqueous phase contains some amount of suspended solids, or
to a
quartphasic mixture comprising a vapor phase, an organic phase, an aqueous
phase and a
solid phase. In some embodiments, a triphasic mixture may comprise a vapor
phase, a liquid
phase, and a solid phase. In some embodiments, an aqueous phase may be
obtained by
contacting a fermentation broth with a water-immiscible organic extractant. In
an
embodiment of a process described herein that includes fermentative
extraction, the term
"fermentation broth" then may refer to the aqueous phase in biphasic
fermentative extraction.
[0066] "Organic phase" as used herein refers to the non-aqueous phase of a
mixture (e.g.,
biphasic mixture, triphasic mixture, quartphasic mixture) obtained by
contacting a
fermentation broth with a water-immiscible organic extractant. From time to
time as used
herein, the terms "organic phase" may be used synonymously with "extractant
phase."
[0067] "Effective titer" as used herein refers to the total amount of a
particular fermentation
product (e.g., product alcohol) produced by fermentation per liter of
fermentation broth.
[0068] "Portion" as used herein with reference to a process stream refers
to any fractional
part of the stream which retains the composition of the stream, including the
entire stream, as
well as any component or components of the stream, including all components of
the stream.
[0069] The present invention provides processes and methods for producing
fermentation
products such as product alcohols using fermentation. Other fermentation
products that may
be produced using the processes and methods described herein include
propanediol,
butanediol, acetone, acids such as lactic acid, acetic acid, butyric acid, and
propionic acid;
gases such as hydrogen methane, and carbon dioxide; amino acids; vitamins such
as biotin,
vitamin B2 (riboflavin), vitamin B12 (e.g., cobalamin), ascorbic acid (e.g.,
vitamin C), vitamin E (e.g.,
a-tocopherol), and vitamin K (e.g., menaquinone); antibiotics such as
erythromycin, penicillin,
streptomycin, and tetracycline; and other products such as citric acid,
invertase, sorbitol,
pectinase, and xylitol.
[0070] The present invention provides processes and systems for producing a
product
alcohol by fermentative processes and recovering a product alcohol produced by
a
fermentative process. As an example of an embodiment of the processes
described herein,
fermentation may be initiated by introducing feedstock directly into a
fermentor. In some
embodiments, one or more fermentors may be used in the processes described
herein.
Suitable feedstocks include, but are not limited to, rye, wheat, corn, corn
mash, cane, cane
mash, barley, cellulosic material, lignocellulosic material, or mixtures
thereof These
feedstocks may be processed using methods such as dry milling or wet milling.
In some
- 13 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
embodiments, prior to the introduction to the fermentor, the feedstock may be
liquefied to
create feedstock slurry which may comprise undissolved solids, a fermentable
carbon source
(e.g., sugar), and oil. Liquefaction of the feedstock may be accomplished by
any known
liquefying processes including, but not limited to, acid process, enzyme
process (e.g., alpha-
amylase), acid-enzyme process, or combinations thereof In some embodiments,
liquefaction
may take place in a liquefaction unit.
[0071] If the feedstock slurry is fed directly to the fermentor, the
undissolved solids and/or
oil may interfere with efficient removal and recovery of a product alcohol. In
particular,
when liquid-liquid extraction is utilized to extract a product alcohol from
the fermentation
broth, the presence of the undissolved solids (e.g., particulates) may cause
system
inefficiencies including, but not limited to, decreasing the mass transfer
rate of the product
alcohol to the extractant by interfering with the contact between the
extractant and the
fermentation broth; creating or promoting an emulsion in the fermentor and
thereby
interfering with phase separation of the extractant and the fermentation
broth; reducing the
efficiency of recovering and recycling the extractant because at least a
portion of the
extractant and product alcohol becomes "trapped" in the solids which may be
removed as
Distillers' Dried Grains with Solubles (DDGS); lowering fermentor volume
efficiency
because there are solids taking up volume in the fermentor and because there
is a slower
disengagement of the extractant from the fermentation broth; and shortening
the life cycle of
the extractant by contamination with oil. These effects may result in higher
capital and
operating costs. In addition, extractant "trapped" in the DDGS may detract
from the DDGS
value and qualification for sale as animal feed. Thus, in order to avoid
and/or minimize these
problems, at least a portion of the undissolved solids may be removed from the
feedstock
slurry prior to the addition of the feedstock slurry to the fermentor.
Extraction activity and
efficiency of product alcohol production may be increased when extraction is
performed on a
fermentation broth where the undissolved solids have been removed.
[0072] Processes and systems to process feedstock generating a feedstock
slurry and to
separate feedstock slurry generating an aqueous phase comprising fermentable
carbon source
and a solid phase (e.g., wet cake) are described herein with reference to the
Figures. As
shown in Figure 1, in some embodiments, the system includes liquefaction 10
configured to
liquefy feedstock to create a feedstock slurry. For example, feedstock 12 may
be introduced
to liquefaction 10 (e.g., via an inlet in the liquefaction unit). Feedstock 12
can be any
- 14 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
suitable biomass material known in the industry including, but not limited to,
barley, oat, rye,
sorghum, wheat, triticale, spelt, millet, cane, corn, or combinations thereof
that contains a
fermentable carbon source such as sugar and/or starch. Water may also be
introduced to
liquefaction 10.
[0073] The process of liquefying feedstock 12 involves hydrolysis of starch
in feedstock 12
to water-soluble sugars. Any known liquefying processes, as well as
liquefaction unit,
utilized by the industry can be used including, but not limited to, an acid
process, an enzyme
process, or an acid-enzyme process. Such processes can be used alone or in
combination. In
some embodiments, the enzyme process may be utilized and an appropriate enzyme
14, for
example, alpha-amylase, is introduced to liquefaction 10. Examples of alpha-
amylases that
may be used in the systems and processes of the present invention are
described in U.S.
Patent No. 7,541,026; U.S. Patent Application Publication No. 2009/0209026;
U.S. Patent
Application Publication No. 2009/0238923; U.S. Patent Application Publication
No.
2009/0252828; U.S. Patent Application Publication No. 2009/0314286; U.S.
Patent
Application Publication No. 2010/02278970; U.S. Patent Application Publication
No.
2010/0048446; U.S. Patent Application Publication No. 2010/0021587, the entire
contents of
each are herein incorporated by reference.
[0074] In some embodiments, the enzymes for liquefaction and/or
saccharification may be
produced by the microorganism. Examples of microorganisms producing such
enzymes are
described in U.S. Patent No. 7,498,159; U.S. Patent Application Publication
No.
2012/0003701; U.S. Patent Application Publication No. 2012/0129229; PCT
International
Publication No. WO 2010/096562; and PCT International Publication No. WO
2011/153516,
the entire contents of each are herein incorporated by reference. In some
embodiments,
enzymes for liquefaction and/or saccharification may be expressed by a
microorganism that
also produces a product alcohol. In some embodiments, enzymes for liquefaction
and/or
saccharification may be expressed by a microorganism that also expresses a
butanol
biosynthetic pathway. In some embodiments, the butanol biosynthetic pathway
may be 1-
butanol biosynthetic pathway, 2-butanol biosynthetic pathway, isobutanol
biosynthetic
pathway, or 2-butanone biosynthetic pathway.
[0075] The process of liquefying feedstock 12 creates feedstock slurry 16
(also referred to
as mash or thick mash) that includes fermentable carbon source (e.g., sugar)
and undissolved
solids. In some embodiments, feedstock slurry 16 may include fermentable
carbon source
- 15 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
(e.g., sugar), oil, and undissolved solids. The undissolved solids may be non-
fermentable
portions of feedstock 12. In some embodiments, feedstock 12 may be corn, such
as dry
milled, unfractionated corn kernels, and feedstock slurry 16 is corn mash
slurry. Feedstock
slurry 16 may be discharged from an outlet of liquefaction 10, and may be
conducted to
separation 20.
[0076] Separation 20 has an inlet for receiving feedstock slurry 16, and
may be configured
to remove undissolved solids from feedstock slurry 16. Separation 20 may also
be
configured to remove oil, and/or oil and undissolved solids. Separation 20 may
agitate or
spin feedstock slurry 16 to create a liquid phase or aqueous solution 22 and a
solid phase or
wet cake 24.
[0077] Aqueous solution 22 may include sugar, for example, in the form of
oligosaccharides, and water. Aqueous solution 22 may comprise at least about
10% by
weight oligosaccharides, at least about 20% by weight of oligosaccharides, or
at least about
30% by weight of oligosaccharides. Aqueous solution 22 may be discharged from
separation 20 via an outlet. In some embodiments, the outlet may be located
near the top of
separation 20.
[0078] Wet cake 24 may include undissolved solids. Wet cake 24 may be
discharged from
separation 20 via an outlet. In some embodiments, the outlet may be located
near the bottom
of separation 20. Wet cake 24 may also include a portion of sugar and water.
Wet cake 24
may be washed with additional water in separation 20 after aqueous solution 22
has been
discharged from separation 20. Alternatively, wet cake 24 may be washed with
additional
water by additional separation devices. Washing wet cake 24 will recover the
sugar (e.g.,
oligosaccharides) present in the wet cake, and the recovered sugar and water
may be recycled
to liquefaction 10. After washing, wet cake 24 may be further processed to
form Dried
Distillers' Grains with Solubles (DDGS) through any suitable known process.
The formation
of DDGS from wet cake 24 formed in separation 20 has several benefits. Since
the
undissolved solids do not go to the fermentor, DDGS is not subjected to the
conditions of the
fermentor. For example, DDGS does not contact the microorganisms present in
the
fermentor or any other substances that may be present in the fermentor (e.g.,
extractant
and/or product alcohol) and therefore, the microorganism and/or other
substances are not
trapped in the DDGS. These effects provide benefits to subsequent processing
and selling of
DDGS, for example, as animal feed.
- 16-
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[0079]
Separation 20 may be any conventional separation device utilized in the
industry,
including, for example, centrifuges such as a decanter bowl centrifuge, three-
phase
centrifuge, disk stack centrifuge, filtering centrifuge, or decanter
centrifuge. In some
embodiments, removal of the undissolved solids from feedstock slurry 16 may be
accomplished by filtration, vacuum filtration, belt filter, pressure
filtration, membrane
filtration, microfiltration, filtration using a screen, screen separation,
grates or grating, porous
grating, flotation, hydrocyclone, filter press, screwpress, gravity settler,
vortex separator, or
any method or device that may be used to separate solids from liquids. In some
embodiments, separation 20 is a single step process. In one embodiment,
undissolved solids
may be removed from feedstock slurry 16 to form two product streams, for
example, an
aqueous solution of oligosaccharides which contains a lower concentration of
solids as
compared to feedstock slurry 16 and a wet cake which contains a higher
concentration of
solids as compared to feedstock slurry 16. In addition, a third stream
containing oil may be
generated, for example, if three-phase centrifugation is utilized for solids
removal from
feedstock slurry 16. As such, a number of product streams may be generated by
using
different separation techniques or combinations thereof
[0080] A three-
phase centrifuge may be used for three-phase separation of feedstock slurry
such as separation of the feedstock slurry to generate two liquid phases
(e.g., aqueous stream
and oil stream) and a solid phase (e.g., solids or wet cake) (see, e.g.,
Flottweg Tricanter0,
Flottweg AG, Vilsibiburg, Germany). The two liquid phases may be separated and
decanted,
for example, from the bowl of the centrifuge via two discharge systems to
prevent cross
contamination and the solids phase may be removed via a separate discharge
system.
[0081] In some embodiments using corn as feedstock, a three-phase centrifuge
may be used
to remove solids and corn oil simultaneously from liquefied corn mash. The
solids may be
undissolved solids remaining after starch is hydrolyzed to soluble
oligosaccharides during
liquefaction. The corn oil may be released from the germ of the corn kernel
during grinding
and/or liquefaction. In some embodiments, the three-phase centrifuge may have
one feed
stream and three outlet streams. The feed stream may consist of liquefied corn
mash
produced during liquefaction. The
mash may consist of an aqueous solution of
oligosaccharides (e.g., liquefied starch); undissolved solids which consist of
insoluble, non-
starch components from the corn; and corn oil which consists of glycerides and
free fatty
acids. The three outlet streams from the three-phase centrifuge may be a wet
cake which
- 17-
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
contains most of the undissolved solids from the mash; a heavy centrate stream
which
contains most of the liquefied starch from the mash; and a light centrate
stream which
contains most of the corn oil from the mash. The heavy centrate stream may be
fed to
fermentation. The wet cake may be washed with process recycle water, such as
evaporator
condensate and/or backset as described herein, to recover soluble starch from
the wet cake.
The light centrate stream may be sold as a co-product, converted to another co-
product, or
used in processing such as converting the corn oil to corn oil fatty acids
(COFA). In some
embodiments, COFA may be used as an extractant.
[0082]
Referring to Figure 1, fermentation 30 (or fermentor 30), configured to
ferment
aqueous solution 22 to produce a product alcohol, has an inlet for receiving
aqueous
solution 22.
Fermentation 30 may be any suitable fermentor known in the art.
Fermentation 30 may include fermentation broth. In some embodiments,
simultaneous
saccharification and fermentation (SSF) may occur inside fermentation 30. Any
known
saccharification process utilized by the industry may be used including, but
not limited to, an
acid process, an enzyme process, or an acid-enzyme process. In some
embodiments,
enzyme 38 (e.g., such as glucoamylase) may be introduced to an inlet in
fermentation 30 in
order to hydrolyze oligosaccharides in aqueous solution 22 forming
monosaccharides.
Examples of glucoamylases that may be used in the systems and processes of the
present
invention are described in U.S. Patent No. 7,413,887; U.S. Patent No.
7,723,079; U.S. Patent
Application Publication No. 2009/0275080; U.S. Patent Application Publication
No.
2010/0267114; U.S. Patent Application Publication No. 2011/0014681; and U.S.
Patent
Application Publication No. 2011/0020899, the entire contents of each are
herein
incorporated by reference. In some embodiments, glucoamylase may be expressed
by the
microorganism. In some embodiments, glucoamylase may be expressed by a
microorganism
that also produces a product alcohol. In some embodiments, glucoamylase may be
expressed
by a microorganism that also expresses a butanol biosynthetic pathway. In some
embodiments, the butanol biosynthetic pathway may be 1-butanol biosynthetic
pathway, 2-
butanol biosynthetic pathway, isobutanol biosynthetic pathway, or 2-butanone
biosynthetic
pathway.
[0083] In some embodiments, enzymes such as glucoamylases may be added to
liquefaction. The addition of enzymes such as glucoamylases to liquefaction
may reduce the
viscosity of the feedstock slurry or liquefied mash and may improve separation
efficiency. In
- 18-
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
some embodiments, any enzyme capable of reducing the viscosity of the
feedstock slurry
may be used (e.g., Viscozyme0, Sigma-Aldrich, St. Louis, MO). Viscosity of the
feedstock
may be measured by any method known in the art (e.g., viscometers,
rheometers).
[0084] Microorganism 32 may be introduced to fermentation 30. In some
embodiments,
microorganism 32 may be included in the fermentation broth. In some
embodiments,
microorganism 32 may be propagated in a separate vessel or tank (e.g.,
propagation tank). In
some embodiments, microorganisms from the propagation tank may be used to
inoculate one
or more fermentors. In some embodiments, one or more propagation tanks may be
used in
the processes and systems described herein. In some embodiments, the
propagation tank
may be about 2% to about 5% the size of the fermentor. In some embodiments,
the
propagation tank may comprise one or more of the following mash, water,
enzymes,
nutrients, extractant, and microorganisms. In some embodiments, product
alcohol may be
produced in the propagation tank.
[0085] In some embodiments, microorganism 32 may be bacteria, cyanobacteria,
filamentous fungi, or yeast. In some embodiments, microorganism 32 metabolizes
the sugar
in aqueous solution 22 and produces product alcohol. In some
embodiments,
microorganism 32 may be a recombinant microorganism. In some
embodiments,
microorganism 32 may be immobilized, such as by adsorption, covalent bonding,
crosslinking, entrapment, and encapsulation. Methods for encapsulating cells
are known in
the art, for example, as described in U.S. Patent Application Publication No.
2011/0306116,
which is incorporated herein by reference.
[0086] In some embodiments, in situ product removal (ISPR) may be utilized to
remove
product alcohol from fermentation 30 as the product alcohol is produced by
microorganism 32. In some embodiments, liquid-liquid extraction may be
utilized for ISPR.
In some embodiments, fermentation 30 may have an inlet for receiving
extractant 34. In
some embodiments, extractant 34 may be added to the fermentation broth
downstream of
fermentation 30. Alternative means of additions of extractant 34 to
fermentation 30 or
downstream of fermentation 30 are represented by the dotted lines. In some
embodiments,
ISPR may be conducted in a propagation tank. In some embodiments, ISPR may be
conducted in the fermentor and the propagation tank. In some embodiments, ISPR
may be
performed at the initiation (e.g., time 0) of fermentation and/or propagation.
By initiating
ISPR at the beginning of fermentation and/or propagation, the concentration of
product
- 19-
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
alcohol in the fermentor and propagation tank may be maintained at low levels,
and thereby
minimize the effects of product alcohol on the microorganism and allowing the
microorganism to achieve increased cell mass. In some embodiments, extractant
may be
added to the propagation tank. In some embodiments, extractant may be added
prior to
inoculation of the propagation tank. In some embodiments, extractant may be
added after
inoculation of the propagation tank. In some embodiments, extractant may be
added at
various time points after inoculation of the propagation tank. In some
embodiments,
extractant may be added to the fermentor. In some embodiments, extractant may
be added
prior to inoculation of the fermentor. In some embodiments, extractant may be
added after
inoculation of the fermentor. In some embodiments, extractant may be added at
various time
points after inoculation of the fermentor. In some embodiments, extractant may
be added to
the fermentor and the propagation tank. Examples of liquid-liquid extraction
are described
herein. Processes for producing and recovering alcohols from fermentation
broth using
extractive fermentation are described in U.S. Patent Application Publication
No.
2009/0305370; U.S. Patent Application Publication No. 2010/0221802; U.S.
Patent
Application Publication No. 2011/0097773; U.S. Patent Application Publication
No.
2011/0312044; U.S. Patent Application Publication No. 2011/0312043; and PCT
International Publication No. WO 2011/159998; the entire contents of each are
herein
incorporated by reference.
[0087] Extractant 34 contacts the fermentation broth forming stream 36
comprising, for
example, a biphasic mixture (e.g., extractant-rich phase with product alcohol
and aqueous
phase depleted of product alcohol). In some embodiments, stream 36 may be a
quartphasic
mixture comprising, for example, a vapor phase, an organic phase, an aqueous
phase, and a
solid phase. Product alcohol, or a portion thereof, in the fermentation broth
is transferred to
extractant 34. In some embodiments, stream 36 may be discharged through an
outlet in
fermentation 30. Product alcohol may be separated from the extractant in
stream 36 using
conventional techniques.
[0088] In some embodiments, fermentor internals or devices may be used to
improve phase
separation between fermentation broth and extractant. For example, the
internal or device
may serve as a coalescer to promote phase separation between fermentation
broth and
extractant and/or act as a physical barrier to improve phase separation. These
fermentor
internals or devices may also prevent solids from settling in the extractant
phase (or layer),
- 20 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
promote coalescensce of aqueous droplets that may be entrained in the
extractant layer, and
promote removal of off-gases (e.g., CO2, air), and thereby minimize
disturbance of the
extractant phase and/or liquid-liquid interface. Examples of internals or
devices that may be
used in the processes and systems described herein include, but are not
limited to, baffles,
perforated plates, deep wells, lamella separators, cones, and the like. In
some embodiments,
the perforated plate may be a flat horizontal perforated plate. In some
embodiments, the
cone may be an inverted cone or concentric cone(s). In some embodiments, the
internals
may be rotating. In some embodiments, the internals or devices may be located
at or about
the level of the liquid-liquid interface of fermentation broth and extractant.
In some
embodiments, a coalescing pad may be added and/or exit ports may be relocated
to improve
coalescence and recovery of the aqueous phase.
[0089] In some embodiments prior to ISPR and/or completion of fermentation,
stream 35
may be discharged from an outlet in fermentation 30. Discharged stream 35 may
include
microorganism 32. Microorganism 32 may be separated from stream 35, for
example, by
centrifugation or membrane filtration. In some
embodiments, by removing the
microorganism prior to addition of extractant to the fermentation broth, the
microorganism is
not exposed to the extractant and therefore, not exposed to any negative
impact that the
extractant may have on the microorganism. In addition, by removing the
microorganism
upstream of the extraction process, a more aggressive extraction process
(e.g., heating or
cooling the mixture to enhance separation, using a higher KD and/or higher
selectivity
extractant, or an extractant with improved properties but lower
biocompatibility) may be
employed to recover the product alcohol. In some embodiments, microorganism 32
may be
recycled to fermentation 30 which can increase the production rate of product
alcohol,
thereby resulting in an increase in the efficiency of product alcohol
production.
[0090] Referring to Figure 2, in some embodiments, ISPR may be conducted
downstream
of fermentation 30. In some embodiments, stream 33 including product alcohol
and
microorganism 32 may be discharged from an outlet in fermentation 30 and
conducted
downstream, for example, to an extraction column for recovery of product
alcohol. In some
embodiments, stream 33 may be processed by separating microorganism 32 prior
to ISPR.
For example, removal of microorganism 32 from stream 33 may be accomplished by
centrifugation, filtration, vacuum filtration, belt filter, pressure
filtration, membrane
filtration, microfiltration, filtration using a screen, screen separation,
grates or grating, porous
-21 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
grating, flotation, hydrocyclone, filter press, screwpress, gravity settler,
vortex separator, or
any method or separation device that may be used to separate solids (e.g.,
microorganisms)
from liquids. Following removal of microorganism 32, stream 33 may be
conducted to an
extraction column for recovery of product alcohol.
[0091] Additional embodiments of the processes and systems described herein
are
illustrated in Figures 3 to 6. Figures 3 to 6, including the options for the
addition of
extractant to the fermentor (e.g., generating stream 36) or extraction
conducted downstream
of the fermentor (e.g., generating stream 33), are similar to Figures 1 and 2,
respectively, and
therefore will not be described in detail again.
[0092] Referring to Figure 3, the systems and processes of the present
invention may
include discharging oil 26 from an outlet of separation 20. Feedstock slurry
16 may be
separated into a first liquid phase or aqueous solution 22 comprising a
fermentable sugar, a
solid phase or wet cake 24 comprising undissolved solids, and a second liquid
phase
comprising oil 26 which may exit separation 20. In some embodiments,
separation of
feedstock slurry 16 into a first liquid phase, a second liquid phase, and a
solid phase may
occur in a single step. In some embodiments, feedstock 12 is corn and oil 26
is corn oil. In
some embodiments, oil 26 may be conducted to a storage tank or any unit that
is suitable for
oil storage. Any suitable separation device may be used to discharge aqueous
solution 22,
wet cake 24, and oil 26, for example, a three-phase centrifuge. In some
embodiments, a
portion of the oil from feedstock 12 such as corn oil when the feedstock is
corn, remains in
wet cake 24. In some embodiments, when oil 26 is removed via separation 20
from
feedstock 12 (e.g., corn), the fermentation broth in fermentation 30 includes
a reduced
amount of corn oil.
[0093] As described herein, in some embodiments, oil may be separated from the
feedstock
or feedstock slurry and may be stored in an oil storage unit. For example, oil
may be
separated from the feedstock or feedstock slurry using any suitable means for
separation
including a three-phase centrifuge or mechanical extraction. To improve the
removal of oil
from the feedstock or feedstock slurry, oil extraction aids such surfactants,
anti-emulsifiers,
or flocculents as well as enzymes may be utilized. Examples of oil extraction
aids include,
but are not limited to, non-polymeric, liquid surfactants; talcum powder;
microtalcum
powder; salts (NaOH); calcium carbonate; and enzymes such as Pectinex0 Ultra
SP-L,
- 22 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
CelluclastO, and Viscozyme0 L (Sigma-Aldrich, St. Louis, MO), and NZ 33095
(Noyozymes, Franklinton, NC).
[0094] As illustrated in Figure 4, if oil is not discharged separately it
may be removed with
wet cake 24. When wet cake 24 is removed via separation 20, in some
embodiments, a
portion of the oil from feedstock 12, such as corn oil when the feedstock is
corn, remains in
wet cake 24. Wet cake 24 may be conducted to mix 60 and combined with water or
other
solvents forming wet cake mixture 65. In some embodiments, water may be fresh
water,
backset, cook water, process water, 'utter water, evaporation water, or any
water source
available in the fermentation processing facility, or any combination thereof
Wet cake
mixture 65 may be conducted to separation 70 producing wash centrate 75
comprising
fermentable sugars recovered from wet cake 24, and wet cake 74. Wash centrate
75 may be
recycled to liquefaction 10.
[0095] In some embodiments, separation 70 may be any separation device capable
of
separating solids and liquids including, for example, decanter bowl
centrifugation, three-
phase centrifugation, disk stack centrifugation, filtering centrifugation,
decanter
centrifugation, filtration, vacuum filtration, belt filter, pressure
filtration, membrane
filtration, filtration using a screen, screen separation, grating, porous
grating, flotation,
hydrocyclone, filter press, screwpress, gravity settler, vortex separator, or
combinations
thereof
[0096] In some embodiments, wet cake may be subjected to one or more wash
cycles or
wash systems. For example, wet cake 74 may be further processed by conducting
wet
cake 74 to a second wash system. In some embodiments, wet cake 74 may be
conducted to a
second mix 60' forming wet cake mixture 65'. Wet cake mixture 65' may be
conducted to a
second separation 70' producing wash centrate 75' and wet cake 74'. Wash
centrate 75' may
be recycled to liquefaction 10. In some embodiments, wash centrate 75' may be
combined
with wash centrate 75, and recycled to liquefaction 10. In some embodiments,
wet cake 74'
may be combined with wet cake 74 for further processing as described herein.
In some
embodiments, separation 70' may be any separation device capable of separating
solids and
liquids including, for example, decanter bowl centrifugation, three-phase
centrifugation, disk
stack centrifugation, filtering centrifugation, decanter centrifugation,
filtration, vacuum
filtration, belt filter, pressure filtration, membrane filtration,
microfiltration, filtration using a
screen, screen separation, grating, porous grating, flotation, hydrocyclone,
filter press,
- 23 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
screwpress, gravity settler, vortex separator, or combination thereof In some
embodiments,
the wet cake may be subjected to one, two, three, four, five, or more wash
cycles or wash
systems.
[0097] Wet cake 74 may be combined with syrup and then dried to form DDGS
through any
suitable known process. The formation of the DDGS from wet cake 74 has several
benefits.
Since the undissolved solids do not go to the fermentor, the DDGS does not
have trapped
extractant and/or product alcohol, it is not subjected to the conditions of
the fermentor, and it
does not contact the microorganisms present in the fermentor. These benefits
make it easier
to process DDGS, for example, as animal feed.
[0098] In some embodiments, a portion of undissolved solids may be conducted
to
fermentation 30. In some embodiments, this portion of undissolved solids may
have smaller
particle sizes (e.g., fines). In some embodiments, this portion of undissolved
solids may
form whole stillage. In some embodiments, this whole stillage may be processed
to form
thin stillage and a wet cake. In some embodiments, the wet cake formed from
whole stillage
and wet cake 74 and/or 74' may be combined and further processed to produce
DDGS.
[0099] As shown in Figure 4, oil is not discharged separately from the wet
cake, but rather
oil is included as part of the wet cake and is ultimately present in the DDGS.
If corn is
utilized as feedstock, corn oil contains triglycerides, diglycerides,
monoglycerides, fatty
acids, and phospholipids, which provide a source of metabolizable energy for
animals. The
presence of oil (e.g., corn oil) in the wet cake and ultimately DDGS may
provide a desirable
animal feed, for example, a high fat content animal feed.
[00100] In some embodiments, oil may be separated from wet cake and DDGS and
converted to an ISPR extractant for subsequent use in the same or different
alcohol
fermentation processes. Methods for deriving extractants from biomass are
described in U.S.
Patent Application Publication No. 2011/0312044; U.S. Patent Application
Publication No.
2011/0312043; and U.S. Patent Application Publication No. 2012/0156738; the
entire
contents of each are herein incorporated by reference. Oil may be separated
from wet cake
and DDGS using any suitable process including, for example, a solvent
extraction process.
In one embodiment of the invention, wet cake or DDGS may be added to an
extraction unit
and washed with a solvent such as hexane to remove oil. Other solvents that
may be utilized
include, for example, butanol, isohexane, ethanol, petroleum distillates such
as petroleum
ether, or mixtures thereof Following oil extraction, wet cake or DDGS may be
treated to
- 24 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
remove any residual solvent. For example, wet cake or DDGS may be heated to
vaporize
any residual solvent using any method known in the art. Following solvent
removal, wet
cake or DDGS may be subjected to a drying process to remove any residual
water. The
processed wet cake may be used to generate DDGS. The processed DDGS may be
used as a
feed supplement for animals such as dairy and beef cattle, poultry, swine,
livestock, equine,
aquaculture, and domestic pets.
[00101] In some embodiments, extractant may be used as a means to modify the
color of the
wet cake. For example, feedstocks such as corn contain pigments (e.g.,
xanthophylls) which
may be used as a coloring agent in food products including animal feeds (e.g.,
poultry feed).
Exposure to extractants can modify these pigments resulting in a wet cake that
is, for
example, lighter in color. A lighter color wet cake may produce DDGS with a
lighter color,
which may be a desirable quality for certain animal feeds.
[00102] In some embodiments, where corn is used as the feedstock, xanthophylls
may be
isolated from corn and/or undissolved solids and used as a pigment ingredient
in DDGS or
animal feed, or as a supplement for pharmaceutical and nutraceutical
applications. Methods
for isolating xanthophylls include, but are not limited to, chromatography
such as size
exclusion chromatography, solvent extraction such as ethanol extraction, and
enzyme
treatment such as alcalase hydrolysis (see, e.g., Tsui, et al., J. Food Eng.
83:590-595, 2007;
Li, et al., Food Science 31: 72-77, 2010: U.S. Patent No. 5,648,564; U.S.
Patent No.
6,169,217; U.S. Patent No. 6,329,557; U.S. Patent No. 8,236,929; the entire
contents of each
are herein incorporated by reference). In some embodiments, xanthophylls may
be isolated
from corn and/or undissolved solids and added to COFA. In some embodiments,
COFA
and/or xanthophylls may be used for food, pharmaceutical, and nutraceutical
applications.
[00103] After extraction from wet cake or DDGS, the resulting oil and solvent
mixture may
be collected for separation of oil and solvent. In one embodiment, the
oil/solvent mixture
may be processed by evaporation whereby the solvent is evaporated and may be
collected
and recycled. The recovered oil may be converted to an ISPR extractant for
subsequent use
in the same or different alcohol fermentation processes.
[00104] Removal of the oil component of the feedstock is advantageous to
product alcohol
production because oil present in the fermentor can break down into fatty
acids and glycerin.
Glycerin can accumulate in water and reduce the amount of water that is
available for
recycling throughout the system. Thus, removal of the oil component of
feedstock can
- 25 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
increase the efficiency of product alcohol production by increasing the amount
of water that
can be recycled through the system.
[00105] Referring to Figure 5, oil may be removed at various points during the
processes
described herein. Feedstock slurry 16 may be separated, for example, using a
three-phase
centrifuge, into a first liquid phase or aqueous solution 22, a second liquid
phase comprising
oil 26, and a solid phase or wet cake 24. Wet cake 24 may be further processed
to recover
fermentable sugars and oil. Wet cake 24 may be conducted to mix 60 and
combined with
water or other solvents forming wet cake mixture 65. In some embodiments,
water may be
backset, cook water, process water, 'utter water, water collected from
evaporation, or any
water source available in the fermentation processing facility, or any
combination thereof
Wet cake mixture 65 may be conducted to separation 70 (e.g., three-phase
centrifuge)
producing wash centrate 75 comprising fermentable sugars, oil stream 76, and
wet cake 74.
Wash centrate 75 may be recycled to liquefaction 10.
[00106] As described herein, wet cake may be subjected to one or more wash
cycles or wash
systems. In some embodiments, wet cake 74 may be conducted to a second mix 60'
forming
wet cake mixture 65'. Wet cake mixture 65' may be conducted to a second
separation 70'
producing wash centrate 75', oil stream 76' and wet cake 74'. Wash centrate
75' may be
recycled to liquefaction 10. In some embodiments, wash centrate 75' may be
combined with
wash centrate 75, and recycled to liquefaction 10. In some embodiments, wet
cake 74' may
be combined with wet cake 74 for further processing as described below. In
some
embodiments, oil stream 76' and oil 26 may be combined and further processed
for the
generation of extractant that may be used in the fermentation process or oil
stream 76' and
oil 26 may be combined and further processed for the manufacture of consumer
products.
[00107] Wet cake 74 may be combined with syrup and then dried to form DDGS
utilizing
any suitable process. The formation of DDGS from wet cake 74 has several
benefits. Since
the undissolved solids do not go to the fermentor, the DDGS does not contain
extractant
and/or product alcohol, it is not subjected to the conditions of the
fermentor, and it does not
contact the microorganisms present in the fermentor. These benefits make it
easier to
process DDGS, for example, as animal feed. As described herein, in some
embodiments, wet
cake 74, 74', and wet cake formed from whole stillage may be combined and
further
processed to produce DDGS.
- 26 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[00108] As illustrated in Figure 6A, aqueous solution 22 and wet cake 24 may
be combined,
cooled, and conducted to fermentation 30. Feedstock slurry 16 may be
separated, for
example, using a three-phase centrifuge, into a first liquid phase or aqueous
solution 22, a
second liquid phase comprising oil 26, and a solid phase or wet cake 24. In
some
embodiments, oil 26 may be conducted to a storage tank or any unit that is
suitable for oil
storage. Aqueous solution 22 and wet cake 24 may be conducted to mix 80 and re-
slurried
forming aqueous solution/wet cake mixture 82. Mixture 82 may be conducted to
cooler 90
producing cooled mixture 92 which may be conducted to fermentation 30. In some
embodiments, when oil 26 is removed via separation 20 from feedstock slurry
16, mixtures
82 and 92 include a reduced amount of oil.
[00109] In another embodiment, as illustrated in Figure 6B, feedstock slurry
16 may be
separated using a separation device (e.g., a three-phase centrifuge) to
generate a first liquid
phase or aqueous solution 22, a second liquid phase comprising oil 26, and a
solid phase or
wet cake 24. Aqueous solution 22, wet cake 24, and oil 26, or portions
thereof, may be
conducted to fermentation 30. In some embodiments, aqueous solution 22, wet
cake 24, and
oil 26, or portions thereof, may be combined, for example, by mixing, forming
an aqueous
solution, wet cake, and oil mixture, and the mixture may be conducted to
fermentation 30. In
some embodiments, aqueous solution 22 and wet cake 24 may be combined forming
an
aqueous solution and wet cake mixture, then oil 26 may be added to the mixture
forming an
aqueous solution, wet cake, and oil mixture and this mixture may be conducted
to
fermentation 30. In some embodiments, aqueous solution 22 and wet cake 24 may
be
combined forming an aqueous solution and wet cake mixture, and this mixture
and oil 26, or
a portion thereof, may be conducted to fermentation 30 as separate streams.
[00110] In additional embodiments of the processes and systems described
herein,
saccharification may occur in a separate saccharification system. In some
embodiments, a
saccharification system may be located between liquefaction 10 and separation
20 or
between separation 20 and fermentation 30. In some embodiments, liquefaction
and/or
saccharification may be conducted utilizing raw starch enzymes or low
temperature
hydrolysis enzymes such as StargenTM (Genencor International, Palo Alto, CA)
and BPXTM
(Novozymes, Franklinton, NC). In some embodiments, feedstock slurry may be
subjected to
raw starch hydrolysis (also known as cold cooking or cold hydrolysis).
- 27 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[00111] In some embodiments, the systems and processes of the present
invention may
include a series of two or more separation devices (e.g., centrifuges) for the
removal of
undissolved solids and/or oil. For example, aqueous solution discharged from a
first
separation unit may be conducted to an inlet of a second separation unit. The
first separation
unit and second separation unit may be identical (e.g., two three-phase
centrifuges) or may be
different (e.g., a three-phase centrifuge and a decanter centrifuge).
Separation may be
accomplished by a number of means including, but not limited to, decanter bowl
centrifugation, three-phase centrifugation, disk stack centrifugation,
filtering centrifugation,
decanter centrifugation, filtration, vacuum filtration, belt filter, pressure
filtration, membrane
filtration, filtration using a screen, screen separation, grating, porous
grating, flotation,
hydrocyclone, filter press, screwpress, gravity settler, vortex separator, or
combinations
thereof
[00112] The absence or minimization of undissolved solids in the fermentation
broth has
several benefits. For example, the need for units of operation in the
downstream processing
may be eliminated, thereby resulting in an increased efficiency for product
alcohol
production. Also, some or all of the centrifuges used to process whole
stillage may be
eliminated as a result of less undissolved solids in the fermentation broth
exiting the
fermentor. Removal of undissolved solids from feedstock slurry may improve the
processing
productivity of biomass and cost effectiveness. Improved productivity may
include
increased efficiency of product alcohol production and/or increased extraction
activity
relative to processes and systems that do not remove undissolved solids prior
to fermentation.
For additional description of processes and systems for separating undissolved
solids from
feedstock slurry see, for example, U.S. Patent Application Publication No.
2012/0164302
and PCT International Patent Application No. PCT/US2013/51571, the entire
contents of
each are herein incorporated by reference.
[00113] As described herein, product alcohol may be recovered from
fermentation broth
using a number of methods including liquid-liquid extraction. In some
embodiments of the
processes and systems described herein, an extractant may be used to recover
product alcohol
from fermentation broth. Extractants used herein may have, for example, one or
more of the
following properties and/or characteristics: (i) biocompatible with the
microorganisms,
(ii) immiscible with the fermentation broth, (iii) a high partition
coefficient (Kp) for the
extraction of product alcohol, (iv) a low partition coefficient for the
extraction of nutrients
- 28 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
and/or water, (v) low viscosity (14 (vi) high selectivity for product alcohol
as compared to,
for example, water, (vii) low density (p) relative to the fermentation broth
or a density that is
different as compared to the density of the fermentation broth, (viii) a
boiling point suitable
for downstream processing of the extractant and product alcohol, (ix) a
melting point lower
than ambient temperature, (x) minimal absorbency in solids, (xi) a low
tendency to form
emulsions with the fermentation broth, (xii) stability throughout the
fermentation process,
(xiii) low cost, and (xiv) nonhazardous.
[00114] In some embodiments, the extractant may be selected based upon certain
properties
and/or characteristics as described herein. For example, viscosity of the
extractant can
influence the mass transfer properties of the system, that is, the efficiency
with which the
product alcohol may be extracted from the aqueous phase to the extractant
phase (i.e.,
organic phase). The density of the extractant can affect phase separation. In
some
embodiments, selectivity refers to the relative amounts of product alcohol to
water taken up
by the extractant. The boiling point can affect the cost and method of product
alcohol
recovery. For example, in the case where butanol is recovered from the
extractant phase by
distillation, the boiling point of the extractant should be sufficiently low
as to enable
separation of butanol while minimizing any thermal degradation or side
reactions of the
extractant, or the need for deep vacuum in the distillation process.
[00115] The extractant may be biocompatible with the microorganism, that is,
nontoxic to
the microorganism or toxic only to such an extent that the microorganism is
impaired to an
acceptable level. In some embodiments, biocompatible refers to the measure of
the ability of
a microorganism to utilize fermentable carbon sources in the presence of an
extractant. The
extent of biocompatibility of an extractant may be determined, for example, by
the glucose
utilization rate of the microorganism in the presence of the extractant and
product alcohol. In
some embodiments, a non-biocompatible extractant refers to an extractant that
interferes with
the ability of a microorganism to utilize fermentable carbon sources. For
example, a non-
biocompatible extractant does not permit the microorganism to utilize glucose
at a rate
greater than about 25%, greater than about 30%, greater than about 35%,
greater than about
40%, greater than about 45%, or greater than about 50% of the rate when the
extractant is not
present.
[00116] One skilled in the art may select an extractant to maximize the
desired properties
and/or characteristics as described herein and to optimize recovery of a
product alcohol. One
- 29 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
of skill in the art can also appreciate that it may be advantageous to use a
mixture of
extractants. For example, extractant mixtures may be used to increase the
partition
coefficient for the product alcohol. Additionally, extractant mixtures may be
used to adjust
and optimize physical characteristics of the extractant, such as the density,
boiling point, and
viscosity. For example, the appropriate combination may provide an extractant
which has a
sufficient partition coefficient for the product alcohol and sufficient
biocompatibility to
enable its economical use for removing product alcohol from fermentative
broth.
[00117] In some embodiments, extractants useful in the processes and systems
described
herein may be organic solvents. In some embodiments, extractants useful in the
processes
and systems described herein may be water-immiscible organic solvents. In some
embodiments, the extractant may be an organic extractant selected from the
group consisting
of saturated, mono-unsaturated, poly-unsaturated C12 to C22 fatty alcohols,
C12 to C22 fatty
acids, esters of C12 to C22 fatty acids, C12 to C22 fatty aldehydes, C12 to
C22 fatty amides, and
mixtures thereof In some embodiments, the extractant may also be an organic
extractant
selected from the group consisting of saturated, mono-unsaturated, poly-
unsaturated C4 to
C22 fatty alcohols, C4 to C28 fatty acids, esters of C4 to C28 fatty acids, C4
to C22 fatty
aldehydes, C4 to C22 fatty amides, and mixtures thereof In some embodiments,
the fatty acid
may be a C4 to C24 fatty acid and/or the ester may be an ester of a C4 to C24
fatty acid. In
some embodiments, the extractant may be an organic extractant selected from
the group
consisting of saturated, mono-unsaturated, poly-unsaturated C12 to C18 fatty
alcohols, C12 to
C18 fatty acids, esters of C12 to C18 fatty acids, C12 to C18 fatty aldehydes,
C12 to C18 fatty
amides, and mixtures thereof In some embodiments, the extractant may be an
organic
extractant selected from the group consisting of saturated, mono-unsaturated,
poly-
unsaturated C14 to C18 fatty alcohols, C14 to C18 fatty acids, esters of C14
to C18 fatty acids,
C14 to C18 fatty aldehydes, C14 to C18 fatty amides, and mixtures thereof In
some
embodiments, the extractant may be an organic extractant selected from the
group consisting
of saturated, mono-unsaturated, poly-unsaturated C16 to C18 fatty alcohols,
C16 to C18 fatty
acids, esters of C16 to C18 fatty acids, C16 to C18 fatty aldehydes, C16 to
C18 fatty amides, and
mixtures thereof In some embodiments, the extractant may comprise carboxylic
acids. In
some embodiments, the ester of a fatty acid may be the combination of a fatty
acid with an
alcohol (e.g., fatty ester). In some embodiments, the alcohol may be a product
alcohol. In
- 30 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
some embodiments, the ester may be methyl ester, ethyl ester, propyl ester,
butyl ester,
pentyl ester, hexyl ester, or glycerides.
[00118] In some embodiments, the extractant may include a first extractant
selected from
C12 to C22 fatty alcohols, C12 to C22 fatty acids, esters of C12 to C22 fatty
acids, C12 to C22 fatty
aldehydes, C12 to C22 fatty amides, and mixtures thereof; and a second
extractant selected
from C12 to C22 fatty alcohols, C12 to C22 fatty acids, esters of C12 to C22
fatty acids, C12 to
C22 fatty aldehydes, C12 to C22 fatty amides, and mixtures thereof In some
embodiments, the
extractant may include a first extractant selected from C12 to C22 fatty
alcohols, C12 to C22
fatty acids, esters of C12 to C22 fatty acids, and mixtures thereof; and a
second extractant
selected from C12 to C22 fatty alcohols, C12 to C22 fatty acids, esters of C12
to C22 fatty acids,
and mixtures thereof In some embodiments, the extractant may include a first
extractant
selected from C12 to C18 fatty alcohols, C12 to C18 fatty acids, esters of C12
to C18 fatty acids,
and mixtures thereof; and a second extractant selected from C12 to C18 fatty
alcohols, C12 to
C18 fatty acids, esters of C12 to C18 fatty acids, and mixtures thereof In
some embodiments,
the extractant may include a first extractant selected from C14 to C18 fatty
alcohols, C14 to C18
fatty acids, esters of C14 to C18 fatty acids, and mixtures thereof; and a
second extractant
selected from C14 to C18 fatty alcohols, C14 to C18 fatty acids, esters of C14
to C18 fatty acids,
and mixtures thereof In some embodiments, the extractant may include a first
extractant
selected from C12 to C22 fatty alcohols, C12 to C22 fatty acids, esters of C12
to C22 fatty acids,
C12 to C22 fatty aldehydes, C12 to C22 fatty amides, and mixtures thereof; and
a second
extractant selected from C7 to Cii fatty alcohols, C7 to Cii fatty acids,
esters of C7 to Cii fatty
acids, C7 to C11 fatty aldehydes, and mixtures thereof
[00119] In some embodiments, the extractant may be an organic extractant such
as oleyl
alcohol, behenyl alcohol, cetyl alcohol, lauryl alcohol (also referred to as 1
-dodecanol),
myristyl alcohol, stearyl alcohol, oleic acid, lauric acid, linoleic acid,
linolenic acid, myristic
acid, palmitic acid, stearic acid, octanoic acid, decanoic acid, undecanoic
acid, methyl
myristate, methyl oleate, 1-nonanol, 1-decanol, 2-undecanol, 1 -nonanal, 1 -
undecanol,
undecanal, lauric aldehyde, 2-methylundecanal, oleamide, linoleamide,
palmitamide,
stearylamide, 2-ethyl-1 -hexanol, 2 -hexyl- 1 -decanol, 2 -octyl- 1 -do dec
anol, and mixtures
thereof In some embodiments, the extractant may comprise one or more of the
following
oleic acid, lauric acid, linoleic acid, linolenic acid, myristic acid,
palmitic acid, stearic acid,
octanoic acid, decanoic acid, and undecanoic acid. In some embodiments, the
extractant may
-31-
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
comprise one or more of the following oleic acid, linoleic acid, linolenic
acid, myristic acid,
palmitic acid, and stearic acid. In some embodiments, the extractant may
comprise one or
more of the following oleic acid, linoleic acid, palmitic acid, and stearic
acid. In some
embodiments, the extractant may comprise one or more of the following oleic
acid, lauric
acid, linoleic acid, linolenic acid, myristic acid, palmitic acid, stearic
acid, octanoic acid,
decanoic acid, and undecanoic acid, and one or more esters of oleic acid,
lauric acid, linoleic
acid, linolenic acid, myristic acid, palmitic acid, stearic acid, octanoic
acid, decanoic acid,
and undecanoic acid. In some embodiments, the extractant may comprise one or
more of the
following oleic acid, linoleic acid, linolenic acid, myristic acid, palmitic
acid, and stearic
acid, and one or more esters of oleic acid, linoleic acid, linolenic acid,
myristic acid, palmitic
acid, and stearic acid. In some embodiments, the extractant may comprise one
or more of the
following oleic acid, linoleic acid, palmitic acid, and stearic acid, and one
or more esters of
oleic acid, linoleic acid, palmitic acid, and stearic acid. In some
embodiments, the extractant
may comprise one or more of the following oleyl alcohol, behenyl alcohol,
cetyl alcohol,
lauryl alcohol, myristyl alcohol, stearyl alcohol. In some embodiments, the
extractant may
comprise one or more of the following 1-nonanol, 1-decanol, 2-undecanol, 1-
nonanal, 1-
undecanol, undecanal, 2-ethyl-1 -hexanol, 2-hexyl-1-decanol, 2 -octyl-1 -
dodecanol.
[00120] In some embodiments, the extractant may be a mixture of biocompatible
and non-
biocompatible extractants. Examples of mixtures of biocompatible and non-
biocompatible
extractants include, but are not limited to, oleyl alcohol and nonanol, oleyl
alcohol and 1-
undecanol, oleyl alcohol and 2-undecanol, oleyl alcohol and 1-nonanal, oleyl
alcohol and
decanol, and oleyl alcohol and dodecanol. Additional examples of biocompatible
and non-
biocompatible extractants are described in U.S. Patent Application Publication
No.
2009/0305370 and U.S. Patent Application Publication No. 2011/0097773; the
entire
contents of each herein incorporated by reference. In some embodiments,
biocompatible
extractants may have high atmospheric boiling points. For example,
biocompatible
extractants may have atmospheric boiling points greater than the atmospheric
boiling point of
water.
[00121] In some embodiments, a hydrophilic solute may be added to fermentation
broth that
is contacted with an extractant. The presence of a hydrophilic solute in the
aqueous phase
may improve phase separation and may increase the fraction of product alcohol
that
partitions into the organic phase. Examples of a hydrophilic solute may
include, but are not
- 32 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
limited to, polyhydroxylated compounds, polycarboxylic compounds, polyol
compounds,
and dissociating ionic salts. Sugars such as glucose, fructose, sucrose,
maltose, and
oligosaccharides may serve as a hydrophilic solute. Other polyhydroxylated
compounds may
include glycerol, ethylene glycol, propanediol, polyglycerol, and hydroxylated
fullerene.
Polycarboxylic compounds may include citric acid, tartaric acid, maleic acid,
succinic acid,
polyacrylic acid, and sodium, potassium, or ammonium salts thereof Ionic salts
that may be
used as a hydrophilic solute in fermentation broth comprise cations that
include sodium,
potassium, ammonium, magnesium, calcium, and zinc; and anions that include
sulfate,
phosphate, chloride, and nitrate. The amount of hydrophilic solute in the
fermentation broth
may be selected by one skilled in the art to maximize the transfer of product
alcohol from the
aqueous phase (e.g., fermentation broth) to the organic phase (e.g.,
extractant) while not
having a negative impact on the growth and/or productivity of the product
alcohol-producing
microorganism. High levels of hydrophilic solute may impose osmotic stress
and/or toxicity
on the microorganism. One skilled in the art may use any number of known
methods to
determine an optimal amount of hydrophilic solute to minimize the effects of
osmotic stress
and/or toxicity on the microorganism.
[00122] In some embodiments where the product alcohol is butanol, the
extractant may be
selected for attracting the alkyl portion of butanol and for providing little
or no affinity to
water. An extractant that offers no hydrogen bonding, for example, to water
will absorb the
alcohol selectively. In some embodiments, the extractant may comprise an
aromatic
compound. In some embodiments, the extractant may comprise alkyl substituted
benzenes
including, but not limited to, cumene, para-cymene (also known as 1-methy1-4-
(1-
methylethyl)benzene), meta-cymene (also known as 1-methy1-3-(1-
methylethyl)benzene),
meta-diisopropylbenzene, para-diisopropylbenzene, triethylbenzene, ethyl butyl
benzene, and
tert-butylstyrene. An advantage of using an alkyl-substituted benzene is the
comparatively
higher butanol affinity relative to other hydrocarbons. In addition, isopropyl-
substituted or
isobutyl-substituted benzenes may offer a particular advantage in butanol
affinity over other
substituted benzenes. Another advantage is the lower viscosity, lower surface
tension, lower
density, higher thermal stability, and higher chemical stability that aids in
phase separability
and long-term reuse. In some embodiments, an extractant that attracts the
alkyl portion of
butanol may be combined with another extractant that offers affinity in the
form of hydrogen
bonding, for example, to the hydroxyl portion of butanol such that the mixture
provides an
- 33 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
optimal balance between selectivity and partitioning over water. In some
embodiments, an
extractant containing butanol may be phase separated from fermentation broth
and distilled
in a column operating under vacuum. This distillation may operate with reflux
in order to
maintain a distillate of high purity butanol that contains very little
extractant. The bottoms
may comprise a portion of the butanol contained in the distillation feed such
that the
reboiling temperature under vacuum is suitable for delivering heat indirectly
from available
steam. Distillation may be carried out with a partial condenser where only
reflux liquid is
condensed, and a vapor distillate of substantially butanol composition may be
directed into
the bottom of a rectification column that is simultaneously fed a butanol
stream decanted
from condensed beer column overhead vapor. An advantage of this type of
distillation is that
the need for a reboiler to purify the decanted butanol stream is eliminated by
heat integrating
the vapor generated from stripping butanol out of the extractant.
[00123] In some embodiments, extractant may be generated from feedstock. For
example,
oils such as corn oil present in feedstock may be used for the generation of
extractant for
extractive fermentation. The glycerides in oil may be chemically or
enzymatically converted
into a reaction product, such as fatty acids and/or fatty esters (e.g., ethyl
esters, butyl esters,
fusel esters) which may be used as an extractant for the recovery of the
product alcohol.
Using corn oil as an example, corn oil triglycerides may be reacted with a
base such as
ammonia hydroxide to obtain fatty amides and glycerol. In some embodiments,
oil in the
feedstock may be hydrolyzed by a catalyst to generate fatty acids. In some
embodiments, at
least a portion of the acyl glycerides in oil may be hydrolyzed to carboxylic
acid by
contacting the oil with catalyst. In some embodiments, the resulting acid/oil
composition
includes monoglycerides and/or diglycerides from the partial hydrolysis of the
acyl
glycerides in the oil. In some embodiments, the resulting acid/oil composition
includes
glycerol, a by-product of acyl glyceride hydrolysis. In some embodiments, the
resulting
acid/oil composition includes lysophospholipids from the partial hydrolysis of
phospholipids
in the oil. Methods for deriving extractants from biomass are described in
U.S. Patent
Application Publication No. 2011/0312044; U.S. Patent Application Publication
No.
2011/0312043; and U.S. Patent Application Publication No. 2012/0156738, the
entire
contents of each are all herein incorporated by reference.
[00124] In some embodiments, the conversion of oil (e.g., hydrolysis,
transesterification) in
the feedstock or feedstock slurry may occur in the fermentor by the addition
of a catalyst to
- 34 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
the fermentor. For example, a catalyst such as lipase may be added to the
fermentor,
converting the oil present in the feedstock or feedstock slurry to fatty acids
and/or fatty
esters. In some embodiments, the conversion of oil in the feedstock or
feedstock slurry may
occur in a separate unit. For example, the feedstock or feedstock slurry may
be conducted to
a unit, and a catalyst such as lipase may be added to the unit, converting the
oil present in the
feedstock or feedstock slurry to fatty acids. As another example, the
feedstock or feedstock
slurry may be conducted to a unit, and a catalyst such as lipase and an
alcohol (e.g., ethanol,
butanol, fusel alcohols) may be added to the unit, converting the oil present
in the feedstock
or feedstock slurry to fatty esters. In some embodiments, the fatty acids
and/or fatty esters
may be added to the fermentor and may be used as an extractant for the
recovery of the
product alcohol. In some embodiments, the fatty acids and/or fatty esters may
be added to an
external extractor or extractant column and may be used as an extractant for
the recovery of
the product alcohol.
[00125] In some embodiments, oil may be separated from feedstock slurry and
the oil may
be conducted to a unit, and a catalyst such as lipase may be added to the
unit, generating a
fatty acid stream. The fatty acid stream may be heated to deactivate the
lipase and then the
fatty acid stream may be conducted to an external extractor or a storage tank.
Fatty acids
from the storage tank may be conducted to an external extractor for extraction
of product
alcohol from fermentation broth. In some embodiments, oil separated from
feedstock slurry
may be stored in a storage tank. A catalyst such as lipase may be added to the
storage tank,
generating a fatty acid stream. The fatty acid stream may be heated to
deactivate the lipase,
cooled, and then conducted to an external extractor for extraction of product
alcohol from
fermentation broth. In some embodiments, oil separated from feedstock slurry
may be
conducted to a unit, and a catalyst such as lipase may be added to the unit,
generating a fatty
acid stream. The fatty acid stream may be heated to deactivate the lipase,
cooled, and then
the fatty acid stream may be conducted to a fermentor.
[00126] In some embodiments, the one or more catalysts may be one or more
enzymes, for
example, hydrolase enzymes. In some embodiments, the one or more catalysts may
be one
or more enzymes, for example, lipase enzymes. Lipase enzymes may be derived
from any
source including, for example, Absidia, Achromobacter, Aeromonas, Alcaligenes,
Alternaria,
Aspergillus, Achromobacter, Aureobasidium, Bacillus, Beauveria, Brochothrix,
Candida,
Chromobacter, Coprinus, Fusarium, Geotricum, Hansenula, Humicola, Hyphozyma,
- 35 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
Lactobacillus, Metarhizium, Mucor, Nectria, Neurospora, Paecilomyces,
Penicillium,
Pseudomonas, Rhizoctonia, Rhizomucor, Rhizopus, Rhodosporidium, Rhodotorula,
Saccharomyces, Sus, Sporobolomyces, Thermomyces, Thiarosporella, Trichoderma,
Verticillium, and/or Yarrowia. In some embodiments, the source of the lipase
may be
selected from the group consisting of Absidia blakesleena, Absidia
corymbifera,
Achromobacter iophagus, Alcaligenes sp., Alternaria brassiciola, Aspergillus
flavus,
Aspergillus niger, Aspergillus tubingensis, Aureobasidium pullulans, Bacillus
coagulans,
Bacillus pumilus, Bacillus strearothermophilus, Bacillus subtilis, Brochothrix
thermosohata,
Burkholderia cepacia, Candida cylindracea (Candida rugosa), Candida
paralipolytica,
Candida antarctica lipase A, Candida antarctica lipase B, Candida ernobii,
Candida
deformans, Candida rugosa, Candida parapsilosis, Chromobacter viscosum,
Coprinus
cinerius, Fusarium heterosporum, Fusarium oxysporum, Fusarium solani, Fusarium
solani
pisi, Fusarium roseum culmorum, Geotrichum candidum, Geotricum pen icillatum,
Hansenula anomala, Humicola brevispora, Humicola brevis var. thermoidea,
Humicola
insolens, Lactobacillus curvatus, Rhizopus oryzae, Mucor javanicus, Neurospora
crassa,
Nectria haematococca, Penicillium cyclopium, Penicillium crustosum,
Penicillium
expansum, Penicillium roqueforti, Penicillium camembertii, Penicillium sp. I,
Penicillium sp.
II, Pseudomonas aeruginosa, Pseudomonas alcaligenes, Pseudomonas cepacia (syn.
Burkholderia cepacia), Pseudomonas fluorescens, Pseudomonas fragi, Pseudomonas
maltophilia, Pseudomonas mendocina, Pseudomonas mephitica lipolytica,
Pseudomonas
alcaligenes, Pseudomonas plantari, Pseudomonas pseudoalcaligenes, Pseudomonas
putida,
Pseudomonas stutzeri, and Pseudomonas wisconsinensis, Rhizoctonia solani,
Rhizomucor
miehei, Rhizopus arrhizus, Rhizopus delemar, Rhizopus japonicus, Rhizopus
microsporus,
Rhizopus nodosus, Rhizopus oryzae, Rhodosporidium toruloides, Rhodotorula
glutinis,
Saccharomyces cerevisiae, Sporobolomyces shibatanus, Sus scrofa, Thermomyces
lanuginosus (formerly Humicola lanuginose), Thiarosporella phaseolina,
Trichoderma
harzianum, Trichoderma reesei, and Yarrowia lipolytica.
[00127] In some embodiments, hydrolase and/or lipase may be expressed by the
microorganism. In some embodiments, the microorganism may be engineered to
express
homologous or heterologous hydrolase and/or lipase. In some embodiments,
hydrolase
and/or lipase may be expressed by a microorganism that also produces a product
alcohol. In
some embodiments, hydrolase and/or lipase may be expressed by a microorganism
that also
- 36 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
expresses a butanol biosynthetic pathway. In some embodiments, the butanol
biosynthetic
pathway may be a 1-butanol biosynthetic pathway, 2-butanol biosynthetic
pathway,
isobutanol biosynthetic pathway, or 2-butanone biosynthetic pathway.
[00128] Commercial lipase preparations suitable as a catalyst include, but
are not limited to,
Lipolase0 100 L, Lipex0 100L, Lipoclean0 2000T, Lipozyme0 CALB L, Novozyme0
CALA L, and Palatase 20000L, available from Novozymes (Franklinton, NC), or
lipases
from Pseudomonas fluorescens, Pseudomonas cepacia, Mucor miehei, hog pancreas,
Candida cylindracea, Candida rugosa, Rhizopus niveus, Candida antarctica,
Rhizopus
arrhizus or Aspergillus available from Sigma Aldrich (St. Louis, MO). In some
embodiments, the lipase may be thermostable and/or thermotolerant, and/or
solvent tolerant.
[00129] In some embodiments, the one or more catalysts may be phospholipases.
A
phospholipase useful in the present invention may be obtained from a variety
of biological
sources, for example, but not limited to, filamentous fungal species within
the genus
Fusarium, such as a strain of Fusarium culmorum, Fusarium heterosporum,
Fusarium
solani, or Fusarium oxysporum; or a filamentous fungal species within the
genus Aspergillus,
such as a strain of Aspergillus awamori, Aspergillus foetidus, Aspergillus
japonicus,
Aspergillus niger or Aspergillus oryzae. Also useful in the present invention
are
Thermomyces lanuginosus phospholipase variants such as the commercial product
Lecitase0
Ultra (Novozymes A'S, Denmark). One or more phospholipases may be applied as
lyophilized powder, immobilized, or in aqueous solution.
[00130] In some embodiments, phospholipase may be expressed by the
microorganism. In
some embodiments, the microorganism may be engineered to express homologous or
heterologous phospholipases. In some embodiments, phospholipase may be
expressed by a
microorganism that also produces a product alcohol. In some embodiments,
phospholipase
may be expressed by a microorganism that also expresses a butanol biosynthetic
pathway. In
some embodiments, the butanol biosynthetic pathway may be a 1-butanol
biosynthetic
pathway, 2-butanol biosynthetic pathway, isobutanol biosynthetic pathway, or 2-
butanone
biosynthetic pathway.
[00131] By-products of fermentation such as isobutyric acid, phenylethanol, 3-
methyl- 1 -
butanol, 2-methyl-1-butanol, isobutyraldehyde, acetic acid, ketoisovaleric
acid, pyruvic acid,
and dihydroxyisovaleric acid may have an inhibitory effect on the
microorganism. In some
embodiments, these by-products may be modified by esterification. For example,
the by-
- 37 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
products may be esterified with carboxylic acids, alcohols, fatty acids, or
other by-products.
In some embodiments, these esterification reactions may be catalyzed by
lipases or
phospholipases. As an example, lipase present in the fermentation broth may
catalyze the
esterification of by-products generated during fermentation. Esterification of
these by-
products may minimize their inhibitory effects on the microorganism.
[00132] Referring to Figure 7A, feedstock 12 may be processed as described in
Figures 1 to
6, and therefore will not be described in detail. Aqueous solution 22 may then
be further
treated to remove any residual oil. In some embodiments, aqueous solution 22
may be
subjected to centrifugation, decantation, or any other method that may be used
for oil
removal. In some embodiments, aqueous solution 22 may be conducted to unit 25
(or vessel)
and catalyst 23 (e.g., lipase) may be added to unit 25, converting the oil
present in aqueous
solution 22 to fatty acids, generating stream 27. Stream 27 may then be
conducted to
fermentation 30 and microorganism 32 may also be added to fermentation 30 for
the
production of product alcohol. Following fermentation 30, stream 31 comprising
product
alcohol and fatty acids may be conducted to an external unit, for example, an
external
extractor or external extraction loop for the recovery of product alcohol.
[00133] Referring to Figure 7B, in some embodiments, catalyst 23 may be
deactivated, for
example, by heating. In some embodiments, stream 27 comprising catalyst 23 may
be heated
(q) to deactivate catalyst 23 prior to addition to fermentation 30. Referring
to Figure 7C, in
some embodiments, deactivation may be conducted in a separate unit, for
example, a
deactivation unit. In some embodiments, stream 27 may be conducted to
deactivation 28.
Following deactivation, stream 27' may be conducted to fermentation 30 and
microorganism 32 may also be added to fermentation 30 for production of
product alcohol.
[00134] Removing oil from aqueous solution 22 by converting the oil to fatty
acids can
result in energy savings for the production plant due to more efficient
fermentation, less
fouling of the equipment due to the removal of the oil, decreased energy
requirements, for
example, the energy needed to dry distillers grains, and improved operation of
evaporators or
evaporation train. In addition, removal of the oil component of the feedstock
is
advantageous to product alcohol production because oil present in the
fermentor can break
down into fatty acids and glycerin. The glycerin can accumulate in the water
and reduce the
amount of water that is available for recycling throughout the system. Thus,
removal of the
oil component of the feedstock increases the efficiency of the product alcohol
production by
- 38 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
increasing the amount of water that can be recycled through the system. Also,
stable
emulsions are less likely to occur by removal of oil. In some embodiments of
the present
invention, in the event that an emulsion forms, emulsions may be readily
broken by
mechanical processing, addition of protic solvents, or by other conventional
means.
[00135] In another embodiment, referring to Figure 7D, aqueous solution 22 may
be
conducted to fermentation 30 and catalyst 23 (e.g., lipase) may be added to
fermentation 30,
converting oil present in aqueous solution 22 to fatty acids and/or fatty
esters. In some
embodiments, fatty esters may be derived from the combination of fatty acids
with an
alcohol. In some embodiments, the alcohol may be any alcohol in fermentation
30 including
a product alcohol. In some embodiments, the amount of oil in aqueous solution
22 converted
to fatty acids and/or fatty esters may be at least about 50%, at least about
60%, at least about
70%, at least about 80%, at least about 90%, or at least about 95%. In some
embodiments,
the ratio of fatty esters and fatty acids generated by the conversion of oil
may be about 75:25.
In some embodiments, the ratio of fatty esters and fatty acids may be about
80:20. In some
embodiments, catalyst 23 may be added to fermentation 30 in an amount to
maintain a
certain oil conversion rate.
[00136] Following fermentation 30, stream 31 comprising product alcohol, fatty
acids, and
fatty esters may be further processed for recovery of product alcohol. For
example,
stream 31 may be conducted to an external unit, for example, an external
extractor or
external extraction loop for the recovery of product alcohol. In some
embodiments, the fatty
acids and fatty esters in stream 31 may be used as an extractant. In some
embodiments, the
external unit may comprise extractant. In some embodiments, the extractant may
comprise
fatty acids and/or esters of fatty acids.
[00137] The present invention also provides processes and systems for
recovering a product
alcohol produced by a fermentative process. One such process for product
alcohol recovery
is liquid-liquid extraction. Using liquid-liquid extraction as an ISPR
technique is best served
by a liquid-liquid extraction process that maximizes the net present value of
the capital
investment required to practice the technology. An aspect of maximizing the
net present
value of a liquid-liquid extraction process is to avoid large capital and
operating cost
expenditures associated with separating extractant from fermentation broth.
[00138] In one embodiment of a liquid-liquid extraction process, extractant
may be added
directly to the fermentor, and fermentation broth and extractant may be mixed
together in a
- 39 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
way that effects mass transfer (e.g., transfer of product alcohol from
fermentation broth to
extractant) and allows the fermentation to proceed to high effective product
alcohol titer. In
such a process, if mixing is too intense or vigorous, the fermentation broth
and extractant
may have to be separated using a separation device such as a centrifuge. If
mixing is not too
intense, phase separation may be achieved through gravity settling brought on
by the density
difference between the extractant and the fermentation broth. In either case,
additional
fermentors may be required to overcome the loss of fermentor volume taken up
by extractant
added to the fermentor. Adding extractant directly to the fermentor may be
carried out in
batch, semi-batch, or continuous modes irrespective of phase separation within
the
fermentor. If continuous mode is employed and gravity separation of
fermentation broth and
extractant is not possible, then a separation device such as a centrifuge may
be required for
the separation of product alcohol from extractant. If the separation process
employed to
remove product alcohol from extractant is such that the microorganism present
in the
fermentation broth is viable the separation process, then separation of
fermentation broth
from product alcohol/extractant may not be required.
[00139] Another embodiment of a liquid-liquid extraction process may include
an external
extractor or extraction column. For example, fermentation broth from the
fermentor may be
conducted to an external extractor where the fermentation broth is mixed with
extractant.
The mixture of fermentation broth and extractant may then be separated,
generating a
fermentation broth stream leaner in product alcohol and an extractant stream
richer in
product alcohol. The leaner fermentation broth stream may be returned to the
fermentor.
The richer extractant stream may be processed further to separate at least a
portion of product
alcohol from the extractant for product alcohol recovery. In some embodiments,
the rate of
product alcohol recovery from the extractant stream may be set at a rate to
maintain plant
production. In some embodiments, the liquid-liquid extraction process may
comprise one or
more external liquid-liquid extractors.
[00140] In some embodiments, fermentation may occur in the fermentor and the
external
extractor. The additional volume of fermentation broth present in the external
extractor may
serve to increase the overall fermentor volume and therefore, may increase the
overall
production of product alcohol.
[00141] The performance of the external extractor with regard to removing
product alcohol
may depend on the surface area available for interfacial contact, the physical
nature of the
- 40 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
fermentation broth and extractant, the relative amounts of the two phases
(e.g., fermentation
broth phase and extractant phase) present in the external extractor, and the
concentration
driving force difference between the fermentation broth and extractant phases.
Maximizing
the efficiency of the external extractor for a given product alcohol
concentration driving
force may be accomplished by reducing the droplet size of the dispersed phase
in the external
extractor, for example, via nozzle design, internals design, and/or agitation.
In some
embodiments, the design and operation of the external extractor may provide
enough mixing
to effect adequate product alcohol transfer between the fermentation broth and
extractant
phases to maintain product alcohol productivity requirements.
[00142] In some instances, CO2 from fermentation may be generated in the
external
extractor, leading to the formation of droplets which may interfere with phase
separation.
For example, droplets of fermentation broth may attach to CO2 rising through
the extractant
phase. In some embodiments, the extractant phase may be maintained as the
continuous
phase to improve the coalescence of droplets. In some embodiments, the
external extractor
may include internals or exit ports for CO2. For example, a coalescing pad may
be added to
the external extractor and/or the exit ports may be located to improve
coalescence and
recovery of the fermentation broth phase.
[00143] Conditions to separate product alcohol from fermentation broth may be
deleterious
to the microorganism present in the fermentation broth. In some embodiments,
the
microorganism may be separated from fermentation broth prior to contacting the
fermentation broth with the extractant. In some embodiments, the microorganism
may be
separated from a mixture of fermentation broth and extractant prior to the
separation (or
processing) of this mixture. Any separation method capable of separating the
microorganism
from fermentation broth or mixture of fermentation broth and extractant may be
used
including, for example, centrifugation. By separating the microorganism prior
to contacting
the fermentation broth with extractant, it may be possible to use more
rigorous extraction
conditions such as higher temperatures and/or non-biocompatible extractants.
If a separation
method was used that was not deleterious to the microorganism, then separating
the
fermentation broth and extractant prior to product alcohol removal may not be
required.
[00144] If extractant and fermentation broth are not separated, then the
extractant may be
included in the evaporator train feed and therefore, become a component of the
syrup formed
during evaporation, and possibly incorporated in animal feed. In some
embodiments,
-41 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
extractant may be separated from the syrup using any separation means
including, for
example, centrifugation. A low boiling point (e.g., comparable to water)
biocompatible
extractant may not require such separation because the extractant and water
may be recycled
for use in the production process.
[00145] In a typical corn-to-product alcohol production plant, the water
balance of the
overall production process may be maintained by recycling water of the
production plant
with recycled water distilled in an evaporator train to remove salts and other
dissolved solids
of the beer. The resulting syrup from the evaporator train may be mixed with
undissolved
solids, and the mixture may be dried and sold as animal feed. Processes and
systems for
processing undissolved solids for animal feed are described, for example, in
U.S. Patent
Application Publication No. 2012/0164302; U.S. Patent Application Publication
No.
2011/0315541; U.S. Patent Application Publication No. 2013/0164795; and PCT
International Patent Application No. PCT/U52013/51571, the entire contents of
each are
herein incorporated by reference.
[00146] As described herein, undissolved solids may be removed from feedstock
(or
feedstock slurry) prior to the addition of the feedstock to fermentation. If
undissolved solids
are not removed upstream of fermentation, then centrifugation of the beer to
remove
undissolved solids may be necessary to avoid fouling of the evaporators. For
example, in a
commercial corn-to-product alcohol dry-grind production plant, undissolved
solids content in
an evaporator train feed may operate at about 3% total suspended solids, and
may be as high
as 3.5-4% total suspended solids. An upstream process that removes enough
solids to
maintain the percentage of total suspended solids at or below these percentage
values may
eliminate the need for centrifugation, for example, prior to conducting the
beer to the
evaporators (or evaporation train). The elimination of this centrifugation
would result in a
savings on the capital required to retrofit a dry-grind corn-to-product
alcohol production
plant.
[00147] By removing at least a portion of the undissolved solids present in
the feedstock
slurry prior to fermentation, the interfacial surface area between the
fermentation broth and
extractant phases in an external extractor may be increased by reducing the
amount of
undissolved solids at the interface, enhancing product alcohol transfer
between the
fermentation broth and the extractant and providing for a clean phase
separation between the
- 42 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
fermentation broth and extractant. A clean phase separation may also eliminate
the need for
additional separation steps (e.g., centrifugation) and therefore, a savings on
capital expenses.
[00148] The separation of fermentation broth and extractant leaving the
external extractor
may be influenced by the solids content and particle size distribution of the
solids content in
the fermentation broth, the gas content and gas bubble size distribution in
the fermentation
broth, the physical properties of the fermentation broth and extractant
including, but not
limited to, viscosity, density, and surface tension as well as the design and
operation of the
external extractor and the design and operation of the fermentor. These
properties may
determine the need for separation devices (e.g., centrifuges) to separate the
fermentation
broth and extractant leaving the external extractor or the fermentor.
Operating under
conditions that eliminate the need for separation devices may minimize the
capital
expenditure to practice liquid-liquid extraction ISPR. In addition, minimizing
the size of the
extractors by maximizing the interfacial area between fermentation broth and
extractant
phases for a given set of fermentation broth and extractant physical
properties can maintain
the ability to inexpensively phase separate fermentation broth and extractant.
By eliminating
the capital and operating cost of separation devices such as centrifuges, the
net present value
of a dry grind corn-to-product alcohol production plant employing a liquid-
liquid extraction
ISPR process may be improved.
[00149] In another embodiment of the processes and systems described herein,
the extractor
design including phase separation capacity may be tailored to accommodate the
physical
properties of the fermentation broth and extractant. If undissolved solids are
not removed
from feedstock slurry or if the concentration of product alcohol in the
fermentation broth is
too low, it may not be possible to remove enough product alcohol to maintain
plant
productivity employing an extractor that does not include phase separation
equipment.
Therefore, the present invention provides for processes and systems that
include solids
removal as well as recovery of product alcohol utilizing an external extractor
wherein the
extractor has been designed to improve phase separation capacity for maximum
product
alcohol recovery.
[00150] An exemplary process of the present invention is described in Figure
8. Some
processes and streams in Figure 8 have been identified using the same name and
numbering
as used in Figures 1-7 and represent the same or similar processes and streams
as described
in Figures 1-7.
- 43 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[00151] Feedstock 12 may be processed and solids separated (100) as described
herein with
reference to Figures 1-7. Briefly, feedstock 12 may be liquefied to generate
feedstock slurry
comprising undissolved solids, fermentable sugars (or fermentable carbon
source), and
depending on the feedstock, oil. The feedstock slurry may then be subjected to
separation
methods to remove suspended solids, generating a wet cake, an aqueous solution
22 (or
centrate) comprising dissolved fermentable sugars, and optionally an oil
stream. Solids
separation may be accomplished by a number of means including, but not limited
to,
decanter bowl centrifugation, three-phase centrifugation, disk stack
centrifugation, filtering
centrifugation, decanter centrifugation, filtration, vacuum filtration, belt
filter, pressure
filtration, membrane filtration, microfiltration, filtration using a screen,
screen separation,
grating, porous grating, flotation, hydrocyclone, filter press, screwpress,
gravity settler,
vortex separator, or combination thereof
[00152] Aqueous solution 22 and microorganism 32 may be added to fermentation
30 where
the fermentable sugars are fermented by microorganism 32 to produce stream 105
comprising product alcohol. In some embodiments, during fermentation, a
portion of
stream 105 may be transferred to extractor 120 (or extraction 120) where
stream 105 is
contacted with extractant 124. In some embodiments, extractant may be stored
in an
extractant storage tank or unit. In some embodiments, stream 105 may be
removed from
fermentation 30 when the concentration of product alcohol and/or other
metabolic products
reach a predetermined concentration. In some embodiments, the predetermined
concentration may be a concentration of product alcohol and/or other metabolic
products
which negatively impact the metabolism of the microorganism. In some
embodiments,
stream 105 may be removed from fermentation 30 when fermentation is initiated.
In some
embodiments, stream 105 may be removed from fermentation 30 to minimize the
effects of
product alcohol on microorganism 32. In some embodiments, fermentation 30 may
comprise
one, two, three, four, five, six, seven, eight, or more fermentors.
[00153] In some embodiments, extractant may be added to fermentation 30. In
some
embodiments, a portion of fermentation broth comprising extractant may be
transferred to
extractor 120, and in some embodiments, extractant may be recovered from the
fermentation
broth comprising extractant. By adding extractant to fermentation 30, ISPR may
be initiated
in fermentation 30.
- 44 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[00154] Product alcohol, or a portion thereof, transfers from stream 105 to
extractant 124,
and stream 122 comprising extractant richer in product alcohol may be
conducted to
separation 130. Stream 127 comprising fermentation broth leaner in product
alcohol may be
returned to fermentation 30. Separation 130 removes a portion of product
alcohol from
stream 122, and stream 125 comprising leaner extractant may be returned to
extractor 120.
In some embodiments, extractor 120 may be external to fermentation 30. In some
embodiments, fermentation 30 may comprise an extractor. In some embodiments,
extractant,
fermentation broth, or both may be at least partially immiscible. Stream 135
may be
conducted downstream for further processing (e.g., distillation) including
recovery of product
alcohol.
[00155] Over the course of extraction, there may be a loss of extractant or a
portion of
extractant. In some embodiments, extractant 124 may be replenished by the
addition of
extractant to extractor 120 or an extractant storage unit. In some
embodiments, for example,
where extractant may be derived from feedstock or feedstock slurry, extractant
124 may be
replenished by converting oil in the feedstock or feedstock slurry to
extractant. For example,
a catalyst may be added to fermentation 30, converting oil in aqueous solution
22 to fatty
acids and/or fatty esters (see, e.g., Figure 7D), and a portion of stream 105
comprising
product alcohol, fatty acids, and/or fatty esters may be transferred to
extractor 120 where
stream 105 may be contacted with extractant 124. Stream 122 comprising product
alcohol-
rich extractant, fatty acids, and/or fatty esters may be conducted to
separation 130 generating
stream 125 comprising leaner extractant, fatty acids, and/or fatty esters.
In some
embodiments, stream 125 may be further processed prior to its return to
extractor 120. For
example, fatty esters present in stream 125 may be subjected to hydrolysis
generating a
stream comprising product alcohol and fatty acids. This stream comprising
product alcohol
and fatty acids may be conducted to extractor 120, or this stream may be
combined with
stream 122 and the combined stream may be conducted to separation 130. In
another
embodiment, this stream comprising product alcohol and fatty acids may be
conducted to
separation 130 or this stream may be conducted to another separation unit
generating a
product alcohol stream and a fatty acid stream. The fatty acid stream may be
conducted to
extractor 120, and the product alcohol stream may be combined with stream 135
and further
processed for product alcohol recovery.
- 45 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[00156] In some embodiments, phase separation of fermentation broth and
extractant after
passing through an extractor may be insufficient such that an unacceptable
level of dispersed
extractant remains in the fermentation broth returning to the fermentor and/or
an
unacceptable level of fermentation broth droplets remain in the extractant
advancing to
distillation. In some embodiments, the phase separation of fermentation broth
and extractant
may be enhanced by processing a heterogeneous mixture exiting the top or
bottom of an
extractor through one or more hydrocyclones or similar vortex device. In some
embodiments, a static mixer may be used in place of an extractor to bring
fermentation broth
and extractant into contact with each other and the heterogeneous mixture that
is formed may
be pumped through one or more hydrocyclones or similar vortex device to effect
a separation
of the aqueous (e.g., fermentation broth) and organic (e.g., extractant)
phases. In some
embodiments, one or more hydrocyclones or similar vortex device may be used to
remove
liquid or liquid droplets from a gas stream. In some embodiments, the gas
stream may be
from the fermentor. In some embodiments, the gas stream may be from a
degassing device.
[00157] In a batch or semi-batch fermentation process, when a portion of the
fermentable
sugars has been metabolized by microorganism 32, stream 103 comprising beer
may be
conducted downstream to separation 140 to separate product alcohol from the
beer.
Stream 145 comprising product alcohol may be conducted downstream for further
processing
(e.g., distillation) including recovery of product alcohol. In a continuous
fermentation
process, stream 103 comprising beer may be conducted downstream to separation
140 to
separate product alcohol from the beer. Stream 142 comprising whole stillage
may be
conducted downstream for further processing including solids removal and
generation of thin
stillage.
[00158] In some embodiments, fermentation 30 may comprise two or more
fermentors, and
stream 105 may comprise combined multiple streams from the two or more
fermentors. In
some embodiments, the combined multiple streams may be conducted to extractor
120. In
some embodiments, stream 127 may be split and portions of stream 127 may be
returned to
the multiple fermentors. In some embodiments, extractor 120 may be a series of
units
connected together in parallel or in series.
[00159] In some embodiments, extraction may be conducted for a certain period
of time.
Extraction may be conducted, for example, until the concentration of product
alcohol in
- 46 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
fermentation 30 is low enough that separation 140 is not required. In some
embodiments,
extraction may be conducted for an extended period of time.
[00160] In some embodiments of the processes and systems described herein, a
decanter
may be used for phase separation. In some embodiments, a decanter may be used
in
combination with an extractor. In some embodiments, the surfaces of the
decanter may be
modified to improve phase separation. For example, surfaces of the decanter
may be
modified by the addition of hydrophilic and/or hydrophobic surfaces.
[00161] In some embodiments, oxygen, air, and/or nutrients may be added to
stream 125
and/or stream 127. In some embodiments, the nutrients may be soluble in
extractant. In
some embodiments, the concentration of oxygen may be measured in the various
streams,
and may be used as part of a control loop to vary the flow of oxygen into the
process. In
some embodiments, mash may be added to extractor 120 to allow for higher
effective titers.
In some embodiments, separation 130 and 140 may be extractors. In some
embodiments,
these extractors may use water to extract product alcohol from extractant, and
product
alcohol may be subsequently separated from an aqueous phase. In some
embodiments,
extractant may be infused with solutes that enhance its capacity to extract
product alcohol
from fermentation broth. In some embodiments, a surge tank may be located
between
extractor 120 and separation 130 as a means to equilibrate the concentration
of product
alcohol in the extractant prior to separation (e.g., distillation).
[00162] In some embodiments, extractor 120 may be designed to utilize CO2
generated
during fermentation for the purpose of mixing fermentation broth and
extractant. In some
embodiments, extractor 120 may be designed to allow for ready disengagement of
CO2 in the
fermentation broth. This design would facilitate the control of the level of
mixing by CO2
bubbles rising through extractor 120. In some embodiments, fermentation broth
may be
removed from fermentation 30 to minimize the concentration of CO2 in stream
105. In some
embodiments, the design of extractor disengagement zones may include surfaces
to promote
phase separation between fermentation broth and extractant. In some
embodiments,
hydrophilic and/or hydrophobic surfaces may be installed in the disengagement
zones to
improve phase separation. In some embodiments, the external extractor may
include
internals or exit ports for CO2. For example, a coalescing pad may be added to
the external
extractor.
- 47 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[00163] In some embodiments to minimize CO2 mixing, the extractor may be
designed with
a small diameter at the bottom of the extractor, graduating to a large
diameter at the top of
the extractor (e.g., conical shape). In some embodiments, the extractor may be
designed with
a stepwise increase in diameter. For example, the extractor may comprise a
first region of
constant diameter flowed by a stepwise increase of diameter to a second region
of constant
diameter. In some embodiments, the extractor may further comprise a second
stepwise
increase of diameter to a third region of constant diameter. In some
embodiments, the
extractor may comprise one or more stepwise increases of diameter. In some
embodiments,
the extractor may comprise one or more regions of constant diameter.
[00164] Over the course of fermentation, the gas content (e.g., CO2) of the
fermentation
broth changes, and these gases may be removed from the fermentation broth by
utilizing a
gas stripper. The amount of gas stripped from the fermentation broth may be
adjusted by
varying the flow through the gas stripper and/or the pressure of the gas
stripper. In some
embodiments, the amount of CO2 in the fermentation broth may be reduced prior
to
transferring the fermentation broth to an extractor. For example, CO2 may be
stripped from
the fermentation broth using a gas stripper or any means known to those
skilled in the art. In
some embodiments, removal of CO2 may be performed at or below ambient
pressure. In
some embodiments, fermentation may continue in the extractor, and CO2 may be
produced
by the microorganism. In some embodiments to minimize CO2 mixing in the
extractor, the
residence time of the fermentation broth in the extractor may be reduced. In
some
embodiments, residence time may be reduced by modifying the height of the
extractor. In
some embodiments, the height of the extractor may be reduced. Reducing the
height of the
extractor may reduce the number of theoretical extraction stages. In some
embodiments, to
maintain the number of theoretical extraction stages, the extractor may be
replaced with two
or more extractors of reduced height. In some embodiments, the two or more
extractors may
be in series. In some embodiments, the two or more extractors may be
connected. In some
embodiments, the two or more extractors may be connected in such a way to
maintain
countercurrent flow. In some embodiments, a degassing stage may be added to
one or more
extraction stages.
[00165] Referring to Figure 8, in some embodiments, the size of dispersed
phase droplets in
extractor 120 may be measured and adjusted through various means to enhance
the rate of
mass transfer. For example, droplet size may be measured using particle size
analysis such
- 48 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
as focused beam reflectance measurement (FBRMO) or particle vision and
measurement
(PVMO) technologies (Mettler-Toledo, LLC, Columbus OH). In some embodiments,
the
fermentation broth may be the dispersed phase and extractant may be the
continuous phase,
and under these conditions, solids present in the fermentation broth may
interact to a lesser
degree with the extractant. In some embodiments, conditions of separation 130
may be
controlled to minimize oxidative and thermal instabilities effects on the
extractant.
[00166] In some embodiments, the quality of the extractant may be monitored
and
extractant replenished at a frequency necessary for successful production of
product alcohol.
In some embodiments, extractant may be taken up by whole stillage solids. The
whole
stillage may be separated into liquid (e.g., thin stillage) and solid streams,
and the solids may
be washed to recover the extractant. In some embodiments, the temperature of
extractor 120
may be adjusted to improve the efficiency of the overall process. In some
embodiments, the
flows of fermentation broth and extractant to extractor 120 may be co-current
or
countercurrent. In some embodiments, membranes may be used to minimize the
mixing of
fermentation broth and extractant. In some embodiments, extractant may be
polymer beads
or inorganic beads that absorb product alcohol. In some embodiments, the
polymer beads or
inorganic beads may be preferentially absorb product alcohol.
[00167] In some embodiments, measurements such as in-line, on-line, at-line,
or real-time
measurements may be used to measure the concentration of product alcohol
and/or metabolic
by-products in the various streams. These measurements may be used as part of
a control
loop to vary the flow between the various units or vessels (e.g., fermentation
30,
extractor 120, separations 130 and 140, etc.) and to improve the overall
process.
[00168] Another exemplary process of the present invention is described in
Figure 9. Some
processes and streams in Figure 9 have been identified using the same name and
numbering
as used in Figures 1-8 and represent the same or similar processes and streams
as described
in Figures 1-8.
[00169] Feedstock 12 may be processed and solids separated (100) as described
herein with
reference to Figures 1-7. In some embodiments, feedstock 12 may be mixed with
recycled
water (e.g., stream 162) generated by evaporation 160. As described herein,
feedstock slurry
may be subjected to separation methods to remove suspended solids, generating
a wet
cake 24, an aqueous solution 22 (or centrate) comprising dissolved fermentable
sugars, and
depending on the feedstock, oil. Wet cake 24 may be dried in dryer 170 and
used to produce
- 49 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
DDGS. In some embodiments, wet cake 24 may be re-slurried with water (e.g.,
recycled
water/stream 162) and subjected to separation to remove additional fermentable
sugars,
generating washed wet cake (e.g., 74, 74' as described in Figures 4 and 5). In
some
embodiments, wet cake streams 24, 74, and 74' may be combined and the combined
wet cake
streams may be dried in a dryer 170 and used to produce DDGS.
[00170] Aqueous solution 22 and microorganism 32 may be added to fermentation
30 where
the fermentable sugars are metabolized by microorganism 32 to produce stream
105
comprising product alcohol. In some embodiments, enzyme may be added to
fermentation 30. Stream 105 may be conducted to extractor 120, and may be
contacted with
extractant 124. Stream 127 comprising fermentation broth leaner in product
alcohol may be
returned to the fermentation 30 and stream 122 comprising extractant richer in
product
alcohol may be conducted to separation 130. In some embodiments, extractor 120
may be
operated in such a way that stream 122 contains minimal cell mass and minimal
substrate.
Separation 130 may damage microorganism 32 or substrate resulting in a
decrease in the
fermentation rate. Operating extractor 120 with minimal cell mass and
substrate may
minimize any potential damage by separation 130. Stream 125 comprising leaner
extractant
may be returned to extractor 120. Stream 135 from separation 130 may be
conducted to
purification 150 for further processing including recovery of product alcohol.
In some
embodiments, extractant may be added to fermentation 30. In some embodiments,
a portion
of fermentation broth comprising extractant may be transferred to extractor
120, and in some
embodiments, extractant may be recovered from the fermentation broth
comprising
extractant. In some embodiments, the flow rates of fermentation broth and
extractant to
extractor may be modified to improve phase separation. For example, lower
overall flow
rates entering the extractor in the early or later stages of fermentation can
improve the phase
separation of fermentation broth and extractant.
[00171] As described herein, after a batch fermentation process or as a steady
effluent
stream in a continuous fermentation process, stream 103 comprising beer may be
conducted
downstream to separation 140 to separate product alcohol from the whole
stillage 142.
Utilizing an upstream solids removal process may lower the undissolved solids
content in the
thin mash and therefore, it may not be necessary to centrifuge whole stillage
142 to remove
solids. Thus, whole stillage 142 may be conducted directly to evaporation 160.
Syrup 165
- 50 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
generated by evaporation 160 may be mixed with wet cake 24, 74, 74' in dryer
170 to form
DDGS.
[00172] In some embodiments, backset comprising total suspended solids (TSS)
from whole
stillage may be used (or recycled) for feedstock slurry preparation. In some
embodiments,
whole stillage or a portion of whole stillage may be processed through a
solids separation
system including, but not limited to, turbo filtration or ultracentrifugation
prior to
evaporation, or whole stillage or a portion of whole stillage may be processed
for self-
cleaning water purification.
[00173] In some embodiments where coarse grain solids are removed from
liquefied mash,
the whole stillage that is produced may contain fine solids and insoluble
microorganism
fragments, and these dispersed solids may be removed using turbo filtration.
Turbo filtration
may include subjecting a feed suspension to centrifugal motion through a
strainer that can
retain fine solids. These fine solids when formed into a wet cake may contain
some
extractant that is absorbed both on the surface of and inside the pores of
fine grain particles.
In some instances, washing the wet cake with water is insufficient for
recovering extractant
from the wet cake. In some embodiments, a concentrated product alcohol stream
such as the
organic phase may be used to recover extractant from whole stillage wet cake.
In some
embodiments, this organic phase may be formed in a decanter. In some
embodiments, the
wet cake that has been washed with product alcohol may be subsequently washed
with water
to recover the product alcohol from the wet cake.
[00174] In some embodiments, the processes and systems described herein may
include an
extractant reservoir (or tank or vessel). Extractant may be added to the
extractant reservoir
and this extractant may be circulated to an extractor. In some embodiments,
extractant may
be conducted to an extractor and a stream from the extractor may be returned
to the
extractant reservoir. In some embodiments, extractant from an extractant
reservoir may be
circulated to an extractor and/or fermentor. In some embodiments, an
extractant stream may
be circulated between an extractant reservoir, an extractor, and a fermentor.
In some
embodiments, at the completion of fermentation, the contents of the extractant
reservoir,
extractor, and/or the fermentor may be further processed to recover product
alcohol.
[00175] Separation or extraction of product alcohol from extractant may be
accomplished
using methods known in the art, including but not limited to, siphoning,
decantation,
centrifugation, gravity settler, membrane-assisted phase splitting, and the
like. In some
-51 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
embodiments, extraction may be performed using, for example, mixer-settlers.
Mixer-
settlers are stage-wise extractors and are available with various elements for
mixing such as,
pumps, agitators, static mixers, mixing tees, impingement devices, circulating
screens, or
raining buckets. Examples of mixer-settlers are shown in Figures 10A-10H. For
example,
Figure 10A illustrates a mixer-settler using a pump as the source of mixing.
Figure 10B
illustrates a mixer-settler using a mixer as the source of mixing. Figure 10C
illustrates a
mixer-settler using a static mixer as the source of mixing. Figure 10D
illustrates a mixer-
settler using a mixing tee as the source of mixing. Figure 10E illustrates a
mixer-settler using
an impingement mixer as the source of mixing. Figure 1OF illustrates a mixer-
settler using a
raining bucket or meshed screen as the source of mixing. Figure 10G
illustrates a mixer-
settler using a centrifuge as a settler. Figure 10H illustrates a mixer-
settler using a
hydrocyclone or vortex separator as a settler. In some embodiments, one or
more mixing
devices may be used in the processes and systems as described herein.
[00176] In some embodiments, mixers may comprise agitators such as, for
example, flat
blades, pitched blade turbines, or curved propellers. Droplet size produced by
agitated
mixers may be controlled by agitator design, tank design, agitator speed, and
mode of
operation. For static mixers, droplet size may be controlled by the diameter
of the mixer and
flow rate. For example, droplet size may be controlled by varying the flow
through the
mixer over the course of the fermentation. In some embodiments, gases and
mixers may be
used for mixing purposes.
[00177] In some embodiments, one or more mixer-settlers may be used in the
processes and
systems as described herein. In some embodiments, the one or more mixer-
settlers may be
arranged in series or in countercurrent mode as illustrated in Figures 101 and
10J. In some
embodiments, mixer-settlers may be stacked in a column arrangement, providing
multiple
mixing and settling zones. In some embodiments, the settler may comprise
hydrophilic or
hydrophobic surfaces to promote phase separation.
[00178] In another embodiment, column extractors or centrifugal extractors may
be used in
the processes and systems as described herein. Column extractors are
differential extractors
providing conditions for mass transfer over their length with a steadily
changing
concentration profile. The different types of differential extractors may be
divided into non-
mechanical, pulse-agitated, and rotary-agitated. Centrifugal extractors are a
separate class of
differential extractors with the Podbielniak0 centrifugal contactor being one
such type.
- 52 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[00179] In some embodiments, non-mechanical spray towers may be used in the
processes
and systems as described herein. One example of a non-mechanical spray tower
includes a
non-mechanical spray tower without column internals. The number of nozzles and
nozzle
diameter may be used to determine droplet size. In some embodiments, the spray
tower may
have internals. In some embodiments, a spray tower may comprise helical
piping. Helical
piping may allow for droplet rise and additional mixing of fermentation broth
and extractant.
In some embodiments, non-mechanical extractors such as packed towers, sieve
trays, and
baffle trays may be used in the processes and systems as described herein.
Examples of these
extractors are shown in Figure 10K. In some embodiments, the packing of such
extractors
may be random or structured.
[00180] In some embodiments, pulsed-agitated extractors may be used in the
processes and
systems as described herein. Pulsed-agitated extractors have different designs
as well
including reciprocating trays or vibrating plates where the trays move in
vertical fashion.
The entire packed and/or sieve tray column can also vibrate in a vertical
fashion to promote
smaller dispersed phase droplets and more mass transfer. Examples of these
extractors are
shown in Figure 10L. In some embodiments, rotary-agitated or rotating disc
contactors may
be used in the processes and systems as described herein. Examples of these
extractors are
shown in Figure 10M.
[00181] In some embodiments, agitated extractors may be used in the processes
and systems
as described herein. For example, agitated extractors with centrifuges may
provide high
mass transfer rates and clean phase separation. In some embodiments, agitated
columns may
be used in the processes and systems as described herein. For example,
agitated columns
with internals may provide high mass transfer rates.
[00182] One aspect of a liquid-liquid extraction process is determining
successful operating
conditions for the extractor over the course of the constantly changing
fermentation. For
example, a typical corn-to-product alcohol batch fermentation employs an
initial inoculum of
microorganism (or cell mass) added to a certain volume of fermentation broth
in the
fermentor, followed by further filling of the fermentor to a specified volume.
The
fermentation is permitted to proceed until a pre-determined amount of the
fermentable
carbon source (e.g., sugar) is consumed. Over the course of batch
fermentation, the
concentrations of cell mass, reaction intermediates, reaction by-products, and
substrate
components change with time as do the physical properties of the fermentation
broth
- 53 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
including viscosity, density, and surface tension. To improve performance
parameters of the
fermentation, for example, rate, titer, and yield parameters of production and
plant
economics such as sales volume, return on investment, and profit, the
extractor may be
operated in a variable way to compensate for the changing fermentation broth.
In addition,
properties of a dynamic fermentation may impact the size limits of the
extractor. Proper
integration of the operation of the extractor and the fermentor may be benefit
by use of
mathematical models of the process (see, e.g., Daugulis and Kollerup,
Biotechnology and
Bioengineering 27:1345-1356, 1985). Augmenting the mathematical model, for
example,
setting the key model parameters with experimental data is also valuable.
Design parameters
for differential extractors to consider for improved rate, titer, and yield of
the fermentation
process include the maximum total flow to the extractor per cross-sectional
area of the
extractor column as well as the height of the extractor required to remove
enough product
alcohol at a given fermentation broth to extractant ratio. It may be necessary
to change the
maximum flow per unit area and extractor height during a batch fermentation.
Another
consideration for differential extractors is droplet size of the dispersed
phase. Appropriate
droplet size may be a balance between small enough to provide adequate mass
transfer but
large enough to allow for clean phase separation exiting the extractor. In
stage-wise
extractors, the mixing intensity required for efficient mass transfer, the
corresponding time
needed to settle, and/or energy needed to separate the phases are additional
elements to
consider. In either type of extractor, stage-wise or differential, the ratio
of fermentation broth
to extractant fed to the extractor plays a role in determining the size of the
extractor.
[00183] In some embodiments, if an extractor of a fixed size were utilized and
the
maximum allowable flow that avoids flooding to the extractor varied from a low
value to a
high value (e.g., from 1/3 to 2/3 the maximum for a given extractor design)
over the course of
the fermentation owing to changes in the physical properties and
concentrations of the
fermentation broth, then the flows to the extractor may be varied, not
exceeding the
maximum flow, while still completing the fermentation in a reasonable time. In
some
embodiments, if an extractor is agitated, the speed of the agitation may be
varied over the
course of the fermentation to offset changes in the fermentation broth.
Droplet size may be
measured within the extractor, and the speed to maintain a fixed droplet size
may be
controlled throughout the fermentation to offset changes in the fermentation
broth. The
amount of mass transfer occurring at any time point may be assessed by
measuring the
- 54 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
concentrations of product alcohol in the inlet and outlet streams and
adjusting conditions
(e.g., flow, flow ratio, agitation) to control the mass transfer over the
course of the
fermentation.
[00184] In some embodiments, multiple extractors of different sizes may be
utilized and
conditions (e.g., flow, flow ratio, agitation) in each extractor may be
adjusted to provide
improved control of the fermentation process. In some embodiments, the ratio
of
fermentation broth to extractant may be modified to improve extraction
efficiency, increase
the concentration of product alcohol in the extractant (equivalent to
increased efficiency),
and reduce the required flows through the extractor.
[00185] In additional embodiments of the processes and systems described
herein, there
may be two or more fermentation broth or aqueous streams. An extractant phase
that has
absorbed product alcohol from a first aqueous stream may be brought into
contact with a
second aqueous stream that contains less product alcohol than the first
aqueous stream or
fermentation broth, enabling the transfer of product alcohol from the rich
extractant phase to
the second aqueous phase. In some embodiments, contacting the rich extractant
with a dilute
aqueous stream may take place in a multi-stage contacting device or in a
static mixer
followed by a settler. In some embodiments, contacting the rich extractant
with a dilute
aqueous stream may take place in the same device where lean extractant is
contacted with
fermentation broth. An extractor with perforated baffles would allow downflow
of both
fermentation broth and a dilute aqueous stream in separate compartments while
an extractant
that is lean in product alcohol may form a continuous phase throughout all
compartments.
An advantage of this configuration is a reduced amount of extractant would be
needed in the
production plant if the extractant remains confined to the closed volume of an
extractor.
Another advantage of this configuration is that the extractant is not
subjected to potential
degradation during distillation and therefore, may exhibit a longer service
life. By
transferring product alcohol to a homogeneous aqueous stream, the product
alcohol may be
conducted to more than one stripping column via partitioning of the dilute
aqueous stream,
taking into consideration column capacities and heat integration. The need to
clean
equipment that is exposed to an extractant may be reduced when product alcohol
is extracted
into an aqueous medium during or immediately after the product alcohol is
extracted from
fermentation broth.
- 55 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[00186] In some embodiments, product alcohol may be transferred from
fermentation broth
to a second aqueous stream or an extractant across a barrier that is selective
for product
alcohol transport. In some embodiments, this barrier may be provided by a
membrane
material. The membrane material may be either organic or inorganic. Examples
of
membrane material include polymers and ceramics. In some embodiments, product
alcohol
may be separated from fermentation broth utilizing a hydrogel. In some
embodiments, the
hydrogel may comprise functional elements that promote interaction with a
product alcohol
such as, but not limited to, hydroxyl functionality, hydrocarbon character,
network size, and
the like. In some embodiments, a hydrogel may comprise a polymeric network
structure or
polymer formulations. Examples of polymer formulations include, but are not
limited to, one
or more of the following: acrylic acid, sodium acrylate, hydroxyethyl
acrylate, methacrylate,
hydroxybutyl acrylate, butylacrylate, vinylated polyethylene oxide, vinylated
polypropylene
oxide, vinylated polytetratmethylene oxide, acrylates and diacrylates of
polyglycols,
polyvinyl alcohol and hydrocarbon derivatized polyvinyl alcohol, and styrene
and styrene
derivatives. In some embodiments, the hydrogel may comprise hydroxyethyl
acrylate and
methacrylate, hydroxybutyl acrylate and methacrylate, or butylacrylate and
methacrylate.
[00187] In other embodiments of the processes and systems described herein,
fermentation
broth may be removed from the bottom of the fermentor at above atmospheric
pressure and
passed through a first flash tank operating at atmospheric pressure to release
dissolved gases
such as CO2. This first flash tank may be a degassing cyclone and the vapors
from this first
flash tank may be combined with vapors from the fermentor and directed to a
scrubber. In
some embodiments, the fermentation broth from the first flash tank may be
passed through a
second flash tank operating below atmospheric pressure to release more
dissolved gases such
as CO2. This second flash tank may be a degassing cyclone and the vapors from
this second
flash tank may be re-compressed to atmospheric pressure, cooled, and partially
condensed
prior to being combined with vapors from the fermentor and being directed to a
scrubber.
The fermentation broth exiting this second flash tank may be pumped to an
extraction
column operating at above atmospheric pressure so that any remaining or newly
formed
dissolved gases will not lead to formation of a vapor phase in the extraction
column.
[00188] In another embodiment of the processes and systems described herein,
fermentation
broth may be conducted to an extractor and contacted with extractant
generating an aqueous
stream and organic stream comprising extractant and product alcohol. This
organic stream
- 56 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
may be conducted to a flash tank (e.g., vacuum flash) for separation of
product alcohol from
extractant. In some embodiments, the extractant stream from the flash tank may
be recycled
to the extractor and/or the fermentor. In some embodiments, the organic stream
may be
conducted to a second extractor prior to the flash tank. This second extractor
may be used to
remove, for example, any residual water in the organic stream. The extractors
may be
siphons, decanters, centrifuges, gravity settlers, mixer-settlers, or
combinations thereof In
some embodiments, the extractant may be an oil such as, but are not limited
to, tallow, corn,
canola, capric/caprylic triglycerides, castor, coconut, cottonseed, fish,
jojoba, lard, linseed,
neetsfoot, oiticica, palm, peanut, rapeseed, rice, safflower, soya, sunflower,
tung, jatropha,
and vegetable oil blends, or fatty acids derived therefrom.
[00189] In some embodiments of the processes and systems described herein,
automatic
self-cleaning filtration may be used in these processes and systems.
Fermentation broth may
be removed from a fermentor and may be cooled using a cooler (e.g., an
existing cooler in a
fermentation production facility) before entering an automatic self-cleaning
filter. Some
particulates may be retained on the screen medium of the filter as clarified
mash passes
through the filter. Additional filters may be simultaneously undergoing
backflush where a
portion of the clarified mash flows back through the screen carrying the
particulates with it,
discharging a concentrated solids stream. In some embodiments, a portion of
the clarified
mash may enter the top of an extractor while an extractant is fed in the
bottom of the
extractor. The clarified mash and extractant may be brought into contact
either passively by
density differences or with the aid of mechanical motion (e.g., a Karr
column) by means
commonly used in the art. In some embodiments, an organic liquid stream of
extractant
containing product alcohol emerges from the top of the extractor and an
aqueous liquid
stream of fermentation broth that has been at least partially depleted of
product alcohol
relative to clarified mash emerges from the bottom of the extractor. The
aqueous liquid
stream and concentrated solids stream may be combined and returned to the
fermentor. The
extractant stream rich in product alcohol may be heated in a heat exchanger
that transfers
heat from an extractant stream that is lean in product alcohol and that
originates from the
bottom of the extractor. After releasing some heat, the lean extractant may be
further cooled
with water in a heat exchanger to reach a temperature that is suitable for
fermentation.
Circulation of fermentation broth may include a pathway through a heat
transfer device and
mass transfer device enabling the removal of heat and product alcohol per pass
through an
- 57 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
external cooling loop. Moreover, in some embodiments, the rate of heat and
product alcohol
removal may be balanced with the rate of heat and product alcohol production
during
fermentation by adjusting the circulation flow through the external cooling
loop, adjusting
the flow of cooling fluid in a heat exchanger, and/or adjusting the flow of
extractant.
[00190] In some embodiments of the processes and systems described herein,
phase
separation of extractant from fermentation broth may be enhanced by modifying
the
temperature and/or pH of the process. For example, the process may be operated
at
temperatures and/or pH that are different than the temperature and/or pH of
the fermentor. In
some embodiments, the process may be operated at a reduced pH as compared to
the
fermentor. In some embodiments, the process may be operated at a higher
temperature as
compared to the fermentor. In some embodiments, the process may be operated at
a reduced
pH and a higher temperature as compared to the fermentor. A higher temperature
can
increase the kinetics of mass transfer of product alcohol between the aqueous
and organic
phases and may increase the kinetics of coalescence for extractant droplets
dispersed in the
aqueous phase and for aqueous droplets dispersed in the organic phase. In some
embodiments, the temperature inside an extractor containing fermentation broth
and
extractant may be increased by heating the fermentation broth and/or
extractant entering the
extractor. The fermentation broth may be heated either directly with injection
of water vapor
or steam or indirectly via a heat exchanger. In some embodiments, the
extractant feeding the
extractor may originate from distillation where its temperature may already be
elevated. In
some embodiments, the extractant may be cooled to a temperature higher than
the
fermentation temperature.
[00191] In some embodiments, a reduced pH can minimize the solubility and
dispersibility
of extractant in the aqueous broth phase. In some embodiments, the extractant
may be a fatty
acid with a known associated pKa value. In some embodiments, the pH of the
fermentation
broth may be reduced to below the pKa of the extractant such that the
carboxylic acid groups
of the fatty acid are substantially protonated. In some embodiments, the pH
may be reduced
by introducing CO2 gas into the fermentation broth or by injecting a small
amount of liquid
acids such as sulfuric acid or any other organic or inorganic acid into the
fermentation broth.
In some embodiments, the pH of the fermentation broth after separating from
the extractant
may be adjusted to the pH of fermentation.
- 58 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[00192] In some embodiments where the extractant phase is the continuous
phase, the
aqueous phase may be distributed or dispersed in the extractant phase. For
example,
fermentation broth comprising product alcohol may be conducted to an extractor
(e.g.,
external extractor) via a distributor or dispersal device. In some
embodiments, the distributor
or dispersal device may be a nozzle such as a spray nozzle. In some
embodiments, the
distributor or dispersal device may be a spray tower. As an example, droplets
of
fermentation broth may be passed through extractant, and product alcohol is
transferred to
the extractant. Droplets of fermentation broth coalesce at the bottom of the
extractor and
may be returned to the fermentor. Extractant comprising product alcohol may be
further
processed for recovery of product alcohol as described herein. In addition, at
the completion
of fermentation, residual product alcohol in the fermentor may also be further
processed for
recovery of product alcohol. In some embodiments, the extractant phase may be
countercurrent.
[00193] In some embodiments where the extractant phase is the continuous phase
and the
aqueous phase is the dispersed phase, mass transfer rates may be improved by
using
electrostatic spraying to disperse the aqueous phase in the extractant phase.
In some
embodiments, one or more spray nozzles may be utilized for electrostatic
spraying. In some
embodiments, the one or more spray nozzles may be an anode. In some
embodiments, the
one or more spray nozzles may be a cathode.
[00194] In some embodiments, extractor effluent may be used to enhance phase
separation.
For example, a portion of rich extractant (i.e., extractant rich in product
alcohol) from the top
of the extractor may be returned to the top of the extractor as reflux, and
the remaining rich
extractant may be further processed for recovery of product alcohol. Also, a
portion of lean
fermentation broth from the bottom of the extractor may be returned to the
bottom of the
extractor as reflux and the remaining lean fermentation broth may be returned
to the
fermentor. In another embodiment, rich extractant may exit the top of the
extractor into a
decanter and separated into a heavy phase and light phase. The heavy phase
from the
decanter may be conducted to the top of the extractor to enhance phase
separation. The light
phase from the decanter may be may be further processed for recovery of
product alcohol.
[00195] In some embodiments of the processes and systems described herein,
multiple pass
extractant flow may be utilized for product alcohol recovery. For example,
multiple
fermentors and extractors may be used, where the fermentation cycle of each
fermentor is at
- 59 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
a different timepoint. Referring to Figure 11A as an example, fermentor 300 is
at an earlier
timepoint as compared to fermentor 400 which is at an earlier timepoint as
compared to
fermentor 500. Fermentation broth comprising product alcohol 302 from
fermentor 300 may
be contacted with extractant 307 in extractor 305, and product alcohol may be
transferred to
extractant generating product alcohol-rich extractant 309. Product
alcohol-rich
extractant 309 from extractor 305 may be conducted to extractor 405.
Fermentation broth
comprising product alcohol 402 from fermentor 400 may be conducted to
extractor 405,
producing product alcohol-rich extractant 409. Product alcohol-rich extractant
409 may be
conducted to extractor 505. Fermentation broth comprising product alcohol 502
from
fermentor 500 may be conducted to extractor 505. Product alcohol-rich
extractant 509 from
extractor 505 may be processed for recovery of product alcohol. Product
alcohol-lean
fermentation broth (304, 404, 504) may be returned to fermentors 300, 400, and
500,
respectively. The number of fermentors and extractors may vary depending on
the
operational facility. A benefit of this process is, for example, the reduction
in total extractant
processing and the size of the extractor.
[00196] In another embodiment of this example, there may be an additional
fermentor F'
and an additional extractor E' (Figure 11B). In this embodiment, when
fermentor 500 (which
is at a later timepoint compared to fermentors 300 and 400) has completed
fermentation,
fermentor 500 may be taken off-line, and in some embodiments, fermentor 500
may undergo
sanitation and/or sterilization procedures such as clean-in-place (CIP) and
sterilization-in-
place (SIP) procedures. When fermentor 500 is taken off-line, fermentor F' may
be brought
on-line. In this embodiment, fermentor F' is at an earlier timepoint as
compared to
fermentor 300 which is at an earlier timepoint as compared to fermentor 400.
Similar to the
description for Figure 11A, fermentation broth comprising product alcohol F'-
02 from
fermentor F' may be contacted with extractant in extractor E', and product
alcohol may be
transferred to extractant generating product alcohol-rich extractant E'-09.
Product alcohol-
rich extractant E'-09 from extractor E' may be conducted to extractor 305.
Fermentation
broth comprising product alcohol 302 from fermentor 300 may be conducted to
extractor 305, producing product alcohol-rich extractant 309. Product
alcohol-rich
extractant 309 may be conducted to extractor 405. Fermentation broth
comprising product
alcohol 402 from fermentor 400 may be conducted to extractor 405. Product
alcohol-rich
extractant 409 from extractor 405 may be processed for recovery of product
alcohol. Product
- 60 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
alcohol-lean fermentation broth (F'-04, 304, 404) may be returned to
fermentors F', 300, and
400, respectively. In some embodiments, this process may be repeated for
multiple cycles,
for example, at least one, at least two, at least three, at least four, at
least five, at least ten, at
least fifteen, at least twenty, or more cycles. In some embodiments, the
process of taking
fermentors off-line and putting additional fermentors on-line may be manual or
automated.
A benefit of this process is reduced extractor flow to product recovery (e.g.,
distillation).
[00197] In some embodiments, an extractant may reduce the flashpoint (i.e.,
flammability)
of the product alcohol. Flashpoint refers to the lowest temperature at which
flame
propagation occurs across the surface of a liquid. Flashpoint may be measured,
for example,
using the ASTM D93-02 method ("Standard Test Methods for Flash Point by Pensky-
Martens Closed Tester"). Reduction of the flashpoint of the product alcohol
can improve the
safety conditions of an alcohol production plant, for example, by minimizing
the fire hazard
of the potentially flammable product alcohol. By improving safety conditions,
the risk of
injury is minimized as well as the risk of property damage and revenue loss.
In some
embodiments where inactivation of the microorganism is required, an extractant
may
improve the inactivation of the microorganism.
[00198] In some embodiments, the processes described herein may be integrated
extraction
fermentation processes using on-line, in-line, at-line, and/or real-time
measurements, for
example, of concentrations and other physical properties of the fermentation
broth and
extractant. These measurements may be used, for example, in feed-back loops to
adjust and
control the conditions of the fermentation and/or the conditions of the
extractor. In some
embodiments, the concentration of product alcohol and/or other metabolites and
substrates in
the fermentation broth may be measured using any suitable measurement device
for on-line,
in-line, at-line, and/or real-time measurements. In some embodiments, the
measurement
device may be one or more of the following: Fourier transform infrared
spectroscope (FTIR),
near-infrared spectroscope (NIR), Raman spectroscope, high pressure liquid
chromatography
(HPLC), viscometer, densitometer, tensiometer, droplet size analyzer, pH
meter, dissolved
oxygen (DO) probe, and the like. In some embodiments, off-gas venting from the
fermentor
may be analyzed, for example, by an in-line mass spectrometer. Measuring off-
gas venting
from the fermentor may be used as a means to identify species present in the
fermentation
reaction. The concentration of product alcohol and other metabolites and
substrates
-61 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
dissolved in the extractant may also be measured using the techniques and
devices described
herein.
[00199] In some embodiments, measured inputs may be sent to a controller
and/or control
system, and conditions within the fermentor (temperature, pH, nutrients,
enzyme and/or
substrate concentration) may be varied to maintain a concentration,
concentration profile,
and/or conditions within the extractor (fermentation broth flow, fermentation
broth to
extractant flow, agitation rate, droplet size, temperature, pH, DO content).
Similarly,
conditions within the extractor may be varied to maintain a concentration
and/or
concentration profile within the fermentor. By utilizing such a control
system, process
parameters may be maintained in such a way to improve overall plant
productivity and
economic goals. In some embodiments, real-time control of fermentation may be
achieved
by variation of concentrations of components (e.g., sugars, enzymes,
nutrients, and the like)
in the fermentor, variation of conditions within the extractor, or both.
[00200] As an example of an isobutanol fermentation process, the efficiency of
isobutanol
extraction in a Karr column is continuously changing as the concentrations of
starch,
sugars and isobutanol change in the fermentation broth. In order to maximize
the efficiency
of the extractor, it may be advantageous to alter the rate at which isobutanol
is removed from
the fermentation broth to match the production profile of the isobutanol
fermentation.
Isobutanol concentrations in the extractant may be maximized resulting in more
energy
efficient distillation operations.
[00201] As part of a process control strategy, real-time measurements of
isobutanol in the
fermentation broth (e.g., column feed) may be coupled with real-time
measurements of
isobutanol in the extractant and in the lean fermentation broth. These
measurements may be
used to adjust the fermentation broth to extractant ratio (flows) to the
extractor. The
flexibility to match the rate of isobutanol extraction with the rate of
isobutanol generation
may allow the extractor to be operated efficiently throughout the extraction.
In addition, by
maintaining a high concentration of isobutanol in the extractant, the
volumetric flow rate to
the distillation columns can be minimized, resulting in an energy savings for
distillation
operations. Phase separation may also be monitored using real-time
measurements, for
example, by monitoring the rate of phase separation, extractant droplet size,
and/or
composition of fermentation broth. In some embodiments, phase separation may
be
monitored by conductivity measurements, dielectric measurements, viscoelastic
- 62 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
measurements, or ultrasonic measurements. In some embodiments, an automated
phase
separation detection system may be used to monitor phase separation. This
automated
system may be used to adjust the flow rates of fermentation broth and
extractant to and from
the extractor and/or adjust the droplet size of extractant, for example, after
mixing of
fermentation broth and extractant. By using these real-time monitoring
systems, clean phase
separation of aqueous and organic phases may be accomplished.
[00202] As another example of process control strategy, droplet size may be
measured using
particle size analysis such as a process particle analyzer (JM Canty, Inc.,
Buffalo, NY),
focused beam reflectance measurement (FBRMO), or particle vision and
measurement
(PVMO) technologies (Mettler-Toledo, LLC, Columbus OH). In some embodiments,
these
measurements may be real-time in situ particle system characterizations. By
monitoring
droplet size in real time, changes in droplet shape and dimensions may be
detected and
process steps may be adjusted to modify droplet size and enhance the rate of
mass transfer.
For example, droplet size may be used to monitor the amount of extractant in
fermentation
broth. Following phase separation, some extractant may be present in the
fermentation broth,
and in some embodiments where the fermentation broth is recycled to the
fermentor,
monitoring droplet size would provide a means to minimize the amount of
extractant in the
fermentation broth returning to the fermentor. If the amount of extractant in
the fermentation
broth is too high, then phase separation may be improved, for example, by
adjusting the
droplet size of extractant in the extractor and/or adjusting the flow rates of
fermentation broth
and extractant to the extractor. These adjustments in the process steps can
minimize the
amount of extractant in the fermentation broth, as well as minimize the amount
of extractant
in thin stillage and DDGS.
[00203] In one embodiment of this control strategy, isobutanol in the
fermentation broth
would not exceed a concentration or setpoint at which the concentration of
isobutanol
becomes deleterious to the microorganism. The isobutanol fermentation broth
setpoint may
be adjusted higher or lower as the fermentation progresses based upon the
trajectory of the
fermentation. For example, continuous comparison of the concentration of
isobutanol in the
fermentation broth to a setpoint concentration of isobutanol can be utilized
to modify
fermentation broth to extractant ratios or flow rates of fermentation broth
and extractant to an
extractor. To monitor isobutanol concentrations in the fermentation broth,
in situ
measurements of the fermentation broth may be performed using Fourier
transform infrared
- 63 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
spectroscopy (FTIR), near infrared spectroscopy (NIR), and/or Raman
spectroscopy. In
addition, measurements of the fermentor headspace may be performed using FTIR,
Raman
spectroscopy, and/or mass spectrometry.
[00204] In some embodiments, efficient extractor operation may occur close to
the point of
extractor flooding. The use of real-time process control that utilizes
concentration data from
inlet and outlet streams may allow the extractor to be operated reliably near
the point of
flooding. In some embodiments, real-time extractant monitoring may be used to
detect the
partitioning of by-products from the fermentation broth or contaminants into
the extractant.
By-products such as alcohols, lipids, oils, and other fermentation components
may reduce the
extraction efficiency of the extractant. Numerous process monitoring
techniques may be
applied to this measurement including, but are not limited to, Fourier
transform infrared
spectroscopy (FTIR), near infrared spectroscopy (NIR), high performance liquid
chromatography (HPLC), and nuclear magnetic resonance (NMR). The analytical
technique
selected to monitor the extractant for the presence of by-products or
contamination may be a
different technique than employed for real-time alcohol determination. Real-
time data may
be used to trigger the remediation of contaminated extractant or the purge of
contaminated
extractant from the process. These techniques as well as gas chromatography
(GC) and
supercritical fluid chromatography (SFC) may also be utilized to monitor
thermal breakdown
of extractant.
[00205] Referring to Figure 12, the systems and processes of the present
invention may
include means for on-line, in-line, at-line, and/or real-time measurements
(circles represent
measurement devices and dotted lines represent feedback loops). Figure 12 is
similar to
Figure 9, except for the addition of measurement devices for on-line, in-line,
at-line, and/or
real-time measurements, and therefore will not be described in detail again.
[00206] As an example, on-line measurements of aqueous stream 22 may be
utilized to
monitor the concentration of fermentable carbon sources (e.g.,
polysaccharides), oil, and/or
dissolved oxygen. For example, FTIR may be used to monitor the dispersion of
oil in
aqueous stream 22, and process imaging may be used to monitor the
concentration and size
of oil droplets in the aqueous stream 22. In some embodiments, on-line
measurements of
fermentation 30 may be utilized to monitor removal rates of product alcohol.
Measurements
of fermentable carbon sources, dissolved oxygen, product alcohol, and by-
products may be
used to adjust the removal rate of product alcohol in order to maintain a
concentration of
- 64 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
product alcohol in fermentation 30 that is tolerable to microorganisms. By
maintaining a
setpoint product alcohol concentration, product inhibition and toxicity may be
minimized.
[00207] On-line measurements of stream 105 and stream 122 may be used to
operate
process control feedback loops. For example, the concentration of product
alcohol in
stream 105 may be used to control the flow rate of this stream to extractor
120; and the
concentration of product alcohol in stream 122 may be used to control the flow
rate of this
stream to separation 130 and to set the ratio of fermentation broth to
extractant. In addition,
on-line measurements of stream 105 and stream 122 may also be utilized to
establish real-
time product alcohol mass balance. Process control feedback loops for
extractor 120 and
separation 130 may be used to monitor the quality of phase separation of
extractant and
fermentation broth. For example, on-line measurement devices may be used to
detect the
balance of the separation of extractant and fermentation broth, and feed rates
of extractant
and fermentation broth may be adjusted accordingly to improve phase
separation. On-line
devices such as optical devices may be used to detect the presence of a rag
layer (e.g.,
mixture of oil, aqueous solution, and solids) in, for example, extractor 120,
and the ratio of
fermentation broth to extractant may be adjusted to minimize the formation of
a rag layer.
On-line measurements of stream 135 from separation 130 may be used to monitor
the
presence of fermentation broth in this stream, and the presence of
fermentation broth in
stream 135 may indicate poor phase separation. If the concentration of
fermentation broth in
stream 135 exceeds a certain setpoint, process changes such as flow rate
adjustments or
adjustments to the ratio of fermentation broth to extractant may be
implemented to improve
phase separation. In addition, the concentration of product alcohol in stream
135 may be
used as a process control feedback loop to ensure efficient operation of
separation 130.
[00208] As another example, on-line measurements of the concentration of
product alcohol
in stream 127 may be used to monitor extraction efficiency and to maintain a
concentration
of product alcohol in fermentation 30 that is tolerable to microorganisms. In
addition,
stream 127 may be monitored for the presence of extractant as a means to
minimize the
amount of extractant returning to fermentation 30. For example, spectroscopic
and process
imaging techniques may be used to monitor the presence of extractant in stream
127.
Furthermore, a certain concentration of extractant in stream 127 may be
maintained to
improve extraction efficiency and phase separation.
- 65 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[00209] In another embodiment, stream 135 from separation 130 may be conducted
to
purification 150 for further processing including recovery of product alcohol
and
extractant 152. Extractant 152 may be conducted to extractor 120. On-line
measurements
may be used to monitor stream 152 for contaminants and degradation products.
By
monitoring stream 152, the potential for contamination of extractor 120 and
fermentation 30
is minimized. If there is an increase in contaminants in stream 152, this
stream may be
further processed to remove these contaminants, for example, by absorption or
chemical
reaction.
[00210] During the extraction process, a rag layer may form at the interface
of the aqueous
and organic phases, and the rag layer, composed of solids and extractant
(e.g., droplets of
extractant), can accumulate and possibly interfere with phase separation. To
mitigate the
formation of rag layer, agitation of the aqueous and organic phases may be
employed. For
example, an impeller may be used to disperse the rag layer at the aqueous-
organic interface.
Also, fluid flow such as a recirculating loop or vibrations/oscillations may
be used to disrupt
rag formation. Figures 13A and 13B illustrate exemplary processes for
mitigating formation
of a rag layer. Figure 13A exemplifies the use of a static mixer in
combination with an
agitation unit downstream of the settler or decanter for the treatment of a
rag layer, and
Figure 13B exemplifies the use of a static mixer in combination with an
agitation unit
upstream of the settler or decanter for the treatment of a rag layer. In some
embodiments,
other devices such as coalescers or sonic agitation may be used to disperse
the rag layer. In
some embodiments, these devices may be integrated into the settler or
decanter.
[00211] The processes and systems described herein may be conducted using
batch, fed-
batch, or continuous fermentation. Batch fermentation is a closed system in
which the
composition of the fermentation broth is established at the beginning of the
fermentation and
is not subjected to artificial alterations during the fermentation process.
In some
embodiments of batch fermentation, extractant may be added to the fermentor.
In some
embodiments, the volume of extractant may be about 20% to about 60% of the
fermentor
working volume.
[00212] Fed-batch fermentation is a variation of batch fermentation, in which
substrates
(e.g., fermentable sugars) are added in increments during the fermentation
process.
Fed-batch systems are useful when catabolite repression may inhibit the
metabolism of the
microorganism and where it is desirable to have limited amounts of substrate
in the media.
- 66 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
In some embodiments, concentrations of substrate and/or nutrients may be
monitored during
fermentation. In some embodiments, parameters such as pH, dissolved oxygen,
and gases
(e.g., CO2) may be monitored during fermentation. From these measurements, the
rate or
amount of substrate and/or nutrients addition may be determined. In some
embodiments, as
the level or amount of fermentation broth decreases during fermentation,
additional mash
may be added to the fermentor to maintain the level or amount of fermentation
broth, for
example, maintain the level or amount of fermentation broth at the initiation
of the
fermentation process. In some embodiments of fed-batch fermentation,
extractant may be
added to the fermentor.
[00213] Continuous fermentation is an open system where fermentation broth is
added
continuously to a fermentor and an amount of fermentation broth is removed for
further
processing (e.g., recovery of product alcohol). In some embodiments, addition
and removal
of fermentation broth may be simultaneous. In some embodiments, equal amounts
of
fermentation broth may be added and removed from the fermentor. In some
embodiments of
continuous fermentation, extractant may be added to the fermentor. In some
embodiments,
the volume of extractant may be about 3% to about 50% of the fermentor working
volume.
In some embodiments, the volume of extractant may be about 3% to about 20% of
the
fermentor working volume. In some embodiments, the volume of extractant may be
about
3% to about 10% of the fermentor working volume.
[00214] In some embodiments of the processes and systems described herein, gas
stripping
may be used to remove product alcohol from the fermentation broth. Gas
stripping may be
performed by providing one or more gases such as air, nitrogen, or carbon
dioxide to the
fermentation broth, thereby forming a product alcohol-containing gas phase.
For example,
gas stripping may be performed by sparging one or more gases through the
fermentation
broth. In some embodiments, the gas may be provided by the fermentation
reaction. As an
example, carbon dioxide may be provided as a by-product of the metabolism of a
fermentable carbon source by the microorganism. In some embodiments, gas
stripping may
be used concurrently with extractant to remove product alcohol from the
fermentation broth.
Product alcohol may be recovered from the product alcohol-containing gas phase
using
methods known in the art, such as using a chilled water trap to condense the
product alcohol,
or scrubbing the gas phase with a solvent.
- 67 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
Recombinant microorganisms and biosynthetic pathways
[00215] While not wishing to be bound by theory, it is believed that the
processes described
herein are useful in conjunction with any microorganism capable of producing
fermentation
products including alcohol-producing microorganism, particularly recombinant
microorganisms which produce alcohol at titers above their tolerance levels.
[00216] Alcohol-producing microorganisms are known in the art. For
example,
fermentative oxidation of methane by methanotrophic bacteria (e.g.,
ilirethylosinus
trichosporium) produces methafwl, and the yeast strain CEN.PK113-7D (CBS 8340,
the
Centraal Buro voor Schimmelculture; van Dijken, et al., Enzyme Microb. Techno.
26:706-
714, 2000) produces ethanol. Recombinant microorganisms which produce alcohol
are also
known in the art (e.g., Ohta, et al., Appl. Environ. Microbiol. 57:893-900,
1991; Underwood,
et al., Appl. Environ. Microbiol. 68:1071-1081, 2002; Shen and Liao, Metab.
Eng. 10:312-
320, 2008; Hahnai, et al., Appl. Environ. Microbiol. 73:7814-7818, 2007; U.S.
Patent No.
5,514,583; U.S. Patent No. 5,712,133; PCT Application Publication No. WO
1995/028476;
Feldmann, et al., Appl. Microbiol. Biotechnol. 38: 354-361, 1992; Zhang, et
al., Science
267:240-243, 1995; U.S. Patent Application Publication No. 2007/0031918 Al;
U.S. Patent
No. 7,223,575; U.S. Patent No. 7,741,119; U.S. Patent No. 7,851,188; U.S.
Patent
Application Publication No. 2009/0203099 Al; U.S. Patent Application
Publication No.
2009/0246846 Al; and PCT Application Publication No. WO 2010/075241, which are
all
herein incorporated by reference).
[00217] In addition, microorganisms may be modified using recombinant
technologies to
generate recombinant microorganisms capable of producing product alcohols such
as ethanol
and butanol. Microorganisms that may be recombinantly modified to produce a
product
alcohol via a biosynthetic pathway include members of the genera Clostridium,
Zymomonas,
Escherichia, Salmonella, Serratia, Erwinia, Klebsiella, Shigella, Rhodococcus,
Pseudomonas, Bacillus, Lactobacillus, Enterococcus, Alcaligenes, Klebsiella,
Paenibacillus,
Arthrobacter, Corynebacterium, Brevibacterium, Schizosaccharomyces,
Kluyveromyces,
Yarrowia, Pichia, Candida, Hansenula, Issatchenkia, or Saccharomyces. In
some
embodiments, recombinant microorganisms may be selected from the group
consisting of
Escherichia coli, Lactobacillus plan tarum, Kluyveromyces lactis,
Kluyveromyces marxianus
and Saccharomyces cerevisiae. In some embodiments, the recombinant
microorganism is
yeast. In some embodiments, the recombinant microorganism is crabtree-positive
yeast
- 68 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
selected from Saccharomyces, Zygosaccharomyces, Schizosaccharomyces, Dekkera,
Torulopsis, Brettanomyces, and some species of Candida. Species of crabtree-
positive yeast
include, but are not limited to, Saccharomyces cerevisiae, Saccharomyces
kluyveri,
Schizosaccharomyces pombe, Saccharomyces bayanus, Saccharomyces mikitae,
Saccharomyces paradoxus, Zygosaccharomyces rouxii, and Candida glabrata.
[00218] Saccharomyces cerevisiae are known in the art and are available from a
variety of
sources including, but not limited to, American Type Culture Collection
(Rockville, MD),
Centraalbureau voor Schimmelcultures (CBS) Fungal Biodiversity Centre,
LeSaffre, Gert
Strand AB, Ferm Solutions, North American Bioproducts, Martrex, and Lallemand.
Saccharomyces cerevisiae include, but are not limited to, BY4741, CEN.PK 113-
7D, Ethanol
Red yeast, Ferm Pr0TM yeast, Bio-Ferm XR yeast, Gert Strand Prestige Batch
Turbo
alcohol yeast, Gert Strand Pot Distillers yeast, Gert Strand Distillers Turbo
yeast, FerMaxTm
Green yeast, FerMaxTm Gold yeast, Thermosacc0 yeast, BG-1, PE-2, CAT-1,
CB57959,
CB57960, and CBS7961.
[00219] In some embodiments, the microorganism may be immobilized or
encapsulated.
For example, the microorganism may be immobilized or encapsulated using
alginate,
calcium alginate, or polyacrylamide gels, or through the induction of biofilm
formation onto
a variety of high surface area support matrices such as diatomite, celite,
diatomaceous earth,
silica gels, plastics, or resins. In some embodiments, ISPR may be used in
combination with
immobilized or encapsulated microorganisms. This combination may improve
productivity
such as specific volumetric productivity, metabolic rate, product alcohol
yields, and tolerance
to product alcohol. In addition, immobilization and encapsulation may minimize
the effects
of the process conditions such as shearing on the microorganisms.
[00220] The production of butanol utilizing fermentation, as well as
microorganisms which
produce butanol, is disclosed, for example, in U.S. Patent No. 7,851,188, and
U.S. Patent
Application Publication Nos. 2007/0092957; 2007/0259410; 2007/0292927;
2008/0182308;
2008/0274525; 2009/0155870; 2009/0305363; and 2009/0305370, the entire
contents of each
are herein incorporated by reference. In some embodiments, the microorganism
is
engineered to contain a biosynthetic pathway. In some embodiments, the
biosynthetic
pathway is an engineered butanol biosynthetic pathway. In some embodiments,
the
biosynthetic pathway converts pyruvate to a fermentation product. In some
embodiments,
the biosynthetic pathway converts pyruvate as well as amino acids to a
fermentation product.
- 69 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
In some embodiments, at least one, at least two, at least three, or at least
four polypeptides
catalyzing substrate to product conversions of a pathway are encoded by
heterologous
polynucleotides in the microorganism. In some embodiments, all polypeptides
catalyzing
substrate to product conversions of a pathway are encoded by heterologous
polynucleotides
in the microorganism. In some embodiments, the polypeptide catalyzing the
substrate to
product conversions of acetolactate to 2,3-dihydroxyisovalerate and/or the
polypeptide
catalyzing the substrate to product conversion of isobutyraldehyde to
isobutanol are capable
of utilizing reduced nicotinamide adenine dinucleotide (NADH) as a cofactor.
Biosynthetic Pathways
[00221] Biosynthetic pathways for the production of isobutanol that may be
used include
those described in U.S. Patent No. 7,851,188, which is incorporated herein by
reference. In
one embodiment, the isobutanol biosynthetic pathway comprises the following
substrate to
product conversions:
a) pyruvate to acetolactate, which may be catalyzed, for example, by
acetolactate
synthase;
b) acetolactate to 2,3-dihydroxyisovalerate, which may be catalyzed, for
example, by
acetohydroxy acid reductoisomerase;
c) 2,3-dihydroxyisovalerate to a-ketoisovalerate, which may be catalyzed,
for
example, by acetohydroxy acid dehydratase;
d) a-ketoisovalerate to isobutyraldehyde, which may be catalyzed, for
example, by a
branched-chain a-keto acid decarboxylase; and
e) isobutyraldehyde to isobutanol, which may be catalyzed, for example, by
a
branched-chain alcohol dehydrogenase.
[00222] In another embodiment, the isobutanol biosynthetic pathway comprises
the
following substrate to product conversions:
a) pyruvate to acetolactate, which may be catalyzed, for example, by
acetolactate
synthase;
b) acetolactate to 2,3-dihydroxyisovalerate, which may be catalyzed, for
example, by
ketol-acid reductoisomerase;
- 70 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
c) 2,3-dihydroxyisoyalerate to a-ketoisoyalerate, which may be catalyzed,
for
example, by dihydroxyacid dehydratase;
d) a-ketoisoyalerate to yaline, which may be catalyzed, for example, by
transaminase
or yaline dehydrogenase;
e) yaline to isobutylamine, which may be catalyzed, for example, by yaline
decarboxylase;
f) isobutylamine to isobutyraldehyde, which may be catalyzed by, for
example, omega
trans aminas e; and
g) isobutyraldehyde to isobutanol, which may be catalyzed, for example, by
a
branched-chain alcohol dehydrogenase.
[00223] In another embodiment, the isobutanol biosynthetic pathway comprises
the
following substrate to product conversions:
a) pyruyate to acetolactate, which may be catalyzed, for example, by
acetolactate
synthase;
b) acetolactate to 2,3-dihydroxyisoyalerate, which may be catalyzed, for
example, by
acetohydroxy acid reductoisomerase;
c) 2,3-dihydroxyisoyalerate to a-ketoisoyalerate, which may be catalyzed, for
example, by acetohydroxy acid dehydratase;
d) a-ketoisoyalerate to isobutyryl-CoA, which may be catalyzed, for
example, by
branched-chain keto acid dehydrogenase;
e) isobutyryl-CoA to isobutyraldehyde, which may be catalyzed, for example,
by
acylating aldehyde dehydrogenase; and
f) isobutyraldehyde to isobutanol, which may be catalyzed, for example, by
a
branched-chain alcohol dehydrogenase.
[00224] Biosynthetic pathways for the production of 1-butanol that may be used
include
those described in U.S. Patent Application Publication No. 2008/0182308, which
is
incorporated herein by reference. In one embodiment, the 1-butanol
biosynthetic pathway
comprises the following substrate to product conversions:
a) acetyl-CoA to acetoacetyl-CoA, which may be catalyzed, for example, by
acetyl-
CoA acetyltransferase;
-71 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
b) acetoacetyl-CoA to 3-hydroxybutyryl-CoA, which may be catalyzed, for
example,
by 3-hydroxybutyryl-CoA dehydrogenase;
c) 3-hydroxybutyryl-CoA to crotonyl-CoA, which may be catalyzed, for
example, by
crotonase;
d) crotonyl-CoA to butyryl-CoA, which may be catalyzed, for example, by
butyryl-
CoA dehydrogenase;
e) butyryl-CoA to butyraldehyde, which may be catalyzed, for example, by
butyraldehyde dehydrogenase; and
f) butyraldehyde to 1-butanol, which may be catalyzed, for example, by
butanol
dehydrogenase.
[00225] Biosynthetic pathways for the production of 2-butanol that may be used
include
those described in U.S. Patent Application Publication No. 2007/0259410 and
U.S. Patent
Application Publication No. 2009/0155870, which are incorporated herein by
reference. In
one embodiment, the 2-butanol biosynthetic pathway comprises the following
substrate to
product conversions:
a) pyruvate to alpha-acetolactate, which may be catalyzed, for example, by
acetolactate synthase;
b) alpha-acetolactate to acetoin, which may be catalyzed, for example, by
acetolactate
decarboxylase;
c) acetoin to 3-amino-2-butanol, which may be catalyzed, for example,
acetonin
aminase;
d) 3-amino-2-butanol to 3-amino-2-butanol phosphate, which may be
catalyzed, for
example, by aminobutanol kinase;
e) 3-amino-2-butanol phosphate to 2-butanone, which may be catalyzed, for
example,
by aminobutanol phosphate phosphorylase; and
f) 2-butanone to 2-butanol, which may be catalyzed, for example, by butanol
dehydrogenase.
[00226] In another embodiment, the 2-butanol biosynthetic pathway comprises
the
following substrate to product conversions:
- 72 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
a) pyruvate to alpha-acetolactate, which may be catalyzed, for example, by
acetolactate synthase;
b) alpha-acetolactate to acetoin, which may be catalyzed, for example, by
acetolactate
decarboxylase;
c) acetoin to 2,3-butanediol, which may be catalyzed, for example, by
butanediol
dehydrogenase;
d) 2,3-butanediol to 2-butanone, which may be catalyzed, for example, by
dial
dehydratase; and
e) 2-butanone to 2-butanol, which may be catalyzed, for example, by butanol
dehydrogenase.
[00227] Biosynthetic pathways for the production of 2-butanone that may be
used include
those described in U.S. Patent Application Publication No. 2007/0259410 and
U.S. Patent
Application Publication No. 2009/0155870, which are incorporated herein by
reference. In
one embodiment, the 2-butanone biosynthetic pathway comprises the following
substrate to
product conversions:
a) pyruvate to alpha-acetolactate, which may be catalyzed, for example, by
acetolactate synthase;
b) alpha-acetolactate to acetoin, which may be catalyzed, for example, by
acetolactate
decarboxylase;
c) acetoin to 3-amino-2-butanol, which may be catalyzed, for example,
acetonin
aminase;
d) 3-amino-2-butanol to 3-amino-2-butanol phosphate, which may be
catalyzed, for
example, by aminobutanol kinase; and
e) 3-amino-2-butanol phosphate to 2-butanone, which may be catalyzed, for
example,
by aminobutanol phosphate phosphorylase.
[00228] In another embodiment, the 2-butanone biosynthetic pathway comprises
the
following substrate to product conversions:
a) pyruvate to alpha-acetolactate, which may be catalyzed, for example, by
acetolactate synthase;
- 73 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
b) alpha-acetolactate to acetoin which may be catalyzed, for example, by
acetolactate
decarboxylase;
c) acetoin to 2,3-butanediol, which may be catalyzed, for example, by
butanediol
dehydrogenase; and
d) 2,3-butanediol to 2-butanone, which may be catalyzed, for example, by
diol
dehydratase.
[00229] The terms "acetohydroxyacid synthase," "acetolactate synthase," and
"acetolactate
synthetase" (abbreviated "ALS") may be used interchangeably herein to refer to
a
polypeptide (or polypeptides) having enzyme activity that catalyzes the
conversion of
pyruvate to acetolactate and CO2. Example acetolactate synthases are known by
the EC
number 2.2.1.6 (Enzyme Nomenclature 1992, Academic Press, San Diego). These
unmodified enzymes are available from a number of sources, including, but not
limited to,
Bacillus subtilis (GenBank Nos: CAB15618 (SEQ ID NO: 1), Z99122 (SEQ ID NO:
2),
NCBI (National Center for Biotechnology Information) amino acid sequence, NCBI
nucleotide sequence, respectively), Klebsiella pneumoniae (GenBank Nos:
AAA25079 (SEQ
ID NO: 3), M73842 (SEQ ID NO: 4)), and Lactococcus lactis (GenBank Nos:
AAA25161
(SEQ ID NO: 5), L16975 (SEQ ID NO: 6)).
[00230] The term "ketol-acid reductoisomerase" ("KARI"), "acetohydroxy acid
isomeroreductase," and "acetohydroxy acid reductoisomerase" may be used
interchangeably
and refer to a polypeptide (or polypeptides) having enzyme activity that
catalyzes the
reaction of (S)-acetolactate to 2,3-dihydroxyisovalerate. Example KARI enzymes
may be
classified as EC number EC 1.1.1.86 (Enzyme Nomenclature 1992, Academic Press,
San
Diego), and are available from a vast array of microorganisms, including, but
not limited to,
Escherichia coli (GenBank Nos: NP 418222 (SEQ ID NO: 7), NC 000913 (SEQ ID NO:
8)), Saccharomyces cerevisiae (GenBank Nos: NP 013459 (SEQ ID NO: 9), NC
001144
(SEQ ID NO: 10)), Methanococcus maripaludis (GenBank Nos: CAF30210 (SEQ ID NO:
11), BX957220 (SEQ ID NO: 12)), and Bacillus subtilis (GenBank Nos: CAB14789
(SEQ
ID NO: 13), Z99118 (SEQ ID NO: 14)). KARIs include Anaerostipes caccae KARI
variants
"K9G9" and "K9D3" (SEQ ID NOs: 15 and 16, respectively). Ketol-acid
reductoisomerase
(KARI) enzymes are described in U.S. Patent Application Publication Nos.
2008/0261230,
2009/0163376, and 2010/0197519, and PCT Application Publication No.
WO/2011/041415,
which are incorporated herein by reference. Examples of KARIs disclosed
therein are those
- 74 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
from Lactococcus lactis, Vibrio cholera, Pseudomonas
aeruginosa PA01, and
Pseudomonas fluorescens PF5 mutants In some embodiments, the KARI utilizes
NADH.
In some embodiments, the KARI utilizes reduced nicotinamide adenine
dinucleotide
phosphate (NADPH).
[00231] The term "acetohydroxy acid dehydratase" and "dihydroxyacid
dehydratase"
("DHAD") refers to a polypeptide (or polypeptides) having enzyme activity that
catalyzes the
conversion the conversion of 2,3-dihydroxyisovalerate to a-ketoisovalerate.
Example
acetohydroxy acid dehydratases are known by the EC number 4.2.1.9. Such
enzymes are
available from a vast array of microorganisms, including, but not limited to,
Escherichia coli
(GenBank Nos: YP_026248 (SEQ ID NO: 17), NC000913 (SEQ ID NO: 18)),
Saccharomyces cerevisiae (GenBank Nos: NP 012550 (SEQ ID NO: 19), NC 001142
(SEQ
ID NO: 20), M. maripaludis (GenBank Nos: CAF29874 (SEQ ID NO: 21), BX957219
(SEQ
ID NO: 22)), B. subtilis (GenBank Nos: CAB14105 (SEQ ID NO: 23), Z99115 (SEQ
ID NO:
24)), L. lactis, and N crassa. U.S. Patent Application Publication No.
2010/0081154, and
U.S. Patent No. 7,851,188, which are incorporated herein by reference,
describe
dihydroxyacid dehydratases (DHADs), including a DHAD from Streptococcus
mutans.
[00232] The term "branched-chain a-keto acid decarboxylase," "a-ketoacid
decarboxylase,"
"a-ketoisovalerate decarboxylase," or "2-ketoisovalerate decarboxylase"
("KIVD") refers to a
polypeptide (or polypeptides) having enzyme activity that catalyzes the
conversion of a-
ketoisovalerate to isobutyraldehyde and CO2. Example branched-chain a-keto
acid
decarboxylases are known by the EC number 4.1.1.72 and are available from a
number of
sources, including, but not limited to, Lactococcus lactis (GenBank Nos:
AAS49166 (SEQ
ID NO: 25), AY548760 (SEQ ID NO: 26); CAG34226 (SEQ ID NO: 27), AJ746364 (SEQ
ID NO: 28), Salmonella typhimurium (GenBank Nos: NP 461346 (SEQ ID NO: 29),
NC 003197 (SEQ ID NO: 30)), Clostridium acetobutylicum (GenBank Nos: NP 149189
(SEQ ID NO: 31), NC 001988 (SEQ ID NO: 32)), M. caseolyticus (SEQ ID NO: 33),
and L.
grayi (SEQ ID NO: 34).
[00233] The term "branched-chain alcohol dehydrogenase" ("ADH") refers to a
polypeptide
(or polypeptides) having enzyme activity that catalyzes the conversion of
isobutyraldehyde to
isobutanol. Example branched-chain alcohol dehydrogenases are known by the EC
number
1.1.1.265, but may also be classified under other alcohol dehydrogenases
(specifically, EC
1.1.1.1 or 1.1.1.2). Alcohol dehydrogenases may be NADPH-dependent or NADH-
- 75 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
dependent. Such enzymes are available from a number of sources, including, but
not limited
to, Saccharomyces cerevisiae (GenBank Nos: NP 010656 (SEQ ID NO: 35),
NC_001136
(SEQ ID NO: 36), NP 014051 (SEQ ID NO: 37), NC 001145 (SEQ ID NO: 38)),
Escherichia coli (GenBank Nos: NP_417484 (SEQ ID NO: 39), NC 000913 (SEQ ID
NO:
40)), C. acetobutylicum (GenBank Nos: NP_349892 (SEQ ID NO: 41), NC_003030
(SEQ ID
NO: 42); NP 349891 (SEQ ID NO: 43), NC_003030 (SEQ ID NO: 44)). U.S. Patent
Application Publication No. 2009/0269823 describes SadB, an alcohol
dehydrogenase
(ADH) from Achromobacter xylosoxidans. Alcohol dehydrogenases also include
horse liver
ADH and Beijerinkia indica ADH (as described by U.S. Patent Application
Publication No.
2011/0269199, which is incorporated herein by reference).
[00234] The term "butanol dehydrogenase" refers to a polypeptide (or
polypeptides) having
enzyme activity that catalyzes the conversion of isobutyraldehyde to
isobutanol or the
conversion of 2-butanone and 2-butanol. Butanol dehydrogenases are a subset of
a broad
family of alcohol dehydrogenases. Butanol dehydrogenase may be NAD- or NADP-
dependent. The NAD-dependent enzymes are known as EC 1.1.1.1 and are
available, for
example, from Rhodococcus ruber (GenBank Nos: CAD36475, AJ491307). The NADP
dependent enzymes are known as EC 1.1.1.2 and are available, for example, from
Pyrococcus furiosus (GenBank Nos: AAC25556, AF013169). Additionally, a butanol
dehydrogenase is available from Escherichia coli (GenBank Nos: NP 417484, NC
000913)
and a cyclohexanol dehydrogenase is available from Acinetobacter sp. (GenBank
Nos:
AAG10026, AF282240). The term "butanol dehydrogenase" also refers to an enzyme
that
catalyzes the conversion of butyraldehyde to 1-butanol, using either NADH or
NADPH as
cofactor. Butanol dehydrogenases are available from, for example, C.
acetobutylicum
(GenBank Nos: NP 149325, NC 001988; this enzyme possesses both aldehyde and
alcohol
dehydrogenase activity); NP_349891, NC_003030; and NP_349892, NC_003030) and
Escherichia coli (GenBank Nos: NP 417-484, NC 000913).
[00235] The term "branched-chain keto acid dehydrogenase" refers to a
polypeptide (or
polypeptides) having enzyme activity that catalyzes the conversion of a-
ketoisovalerate to
isobutyryl-CoA (isobutyryl-coenzyme A), typically using NAD+ (nicotinamide
adenine
dinucleotide) as an electron acceptor. Example branched-chain keto acid
dehydrogenases are
known by the EC number 1.2.4.4. Such branched-chain keto acid dehydrogenases
are
comprised of four subunits and sequences from all subunits are available from
a vast array of
- 76 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
microorganisms, including, but not limited to, Bacillus subtilis (GenBank Nos:
CAB14336
(SEQ ID NO: 45), Z99116 (SEQ ID NO: 46); CAB14335 (SEQ ID NO: 47), Z99116 (SEQ
ID NO: 48); CAB14334 (SEQ ID NO: 49), Z99116 (SEQ ID NO: 50); and CAB14337
(SEQ
ID NO: 51), Z99116 (SEQ ID NO: 52)) and Pseudomonas putida (GenBank Nos:
AAA65614 (SEQ ID NO: 53), M57613 (SEQ ID NO: 54); AAA65615 (SEQ ID NO: 55),
M57613 (SEQ ID NO: 56); AAA65617 (SEQ ID NO: 57), M57613 (SEQ ID NO: 58); and
AAA65618 (SEQ ID NO: 59), M57613 (SEQ ID NO: 60)).
[00236] The term "acylating aldehyde dehydrogenase" refers to a polypeptide
(or
polypeptides) having enzyme activity that catalyzes the conversion of
isobutyryl-CoA to
isobutyraldehyde, typically using either NADH or NADPH as an electron donor.
Example
acylating aldehyde dehydrogenases are known by the EC numbers 1.2.1.10 and
1.2.1.57.
Such enzymes are available from multiple sources, including, but not limited
to, Clostridium
beijerinckii (GenBank Nos: AAD31841 (SEQ ID NO: 61), AF157306 (SEQ ID NO:
62)),
Clostridium acetobutylicum (GenBank Nos: NP 149325 (SEQ ID NO: 63), NC 001988
(SEQ ID NO: 64); NP 149199 (SEQ ID NO: 65), NC 001988 (SEQ ID NO: 66)),
Pseudomonas putida (GenBank Nos: AAA89106 (SEQ ID NO: 67), U13232 (SEQ ID NO:
68)), and Thermus thermophilus (GenBank Nos: YP_145486 (SEQ ID NO: 69), NC
006461
(SEQ ID NO: 70)).
[00237] The term "transaminase" refers to a polypeptide (or polypeptides)
having enzyme
activity that catalyzes the conversion of a-ketoisovalerate to L-valine, using
either alanine or
glutamate as an amine donor. Example transaminases are known by the EC numbers
2.6.1.42 and 2.6.1.66. Such enzymes are available from a number of sources.
Examples of
sources for alanine-dependent enzymes include, but are not limited to,
Escherichia coli
(GenBank Nos: YP_026231 (SEQ ID NO: 71), NC 000913 (SEQ ID NO: 72)) and
Bacillus
licheniformis (GenBank Nos: YP_093743 (SEQ ID NO: 73), NC 006322 (SEQ ID NO:
74)).
Examples of sources for glutamate-dependent enzymes include, but are not
limited to,
Escherichia coli (GenBank Nos: YP_026247 (SEQ ID NO: 75), NC 000913 (SEQ ID
NO:
76)), Saccharomyces cerevisiae (GenBank Nos: NP_012682 (SEQ ID NO: 77),
NC_001142
(SEQ ID NO: 78)) and Methanobacterium thermoautotrophicum (GenBank Nos:
NP_276546
(SEQ ID NO: 79), NC 000916 (SEQ ID NO: 80)).
[00238] The term "valine dehydrogenase" refers to a polypeptide (or
polypeptides) having
enzyme activity that catalyzes the conversion of a-ketoisovalerate to L-
valine, typically using
- 77 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
NAD(P)H as an electron donor and ammonia as an amine donor. Example valine
dehydrogenases are known by the EC numbers 1.4.1.8 and 1.4.1.9 and such
enzymes are
available from a number of sources, including, but not limited to,
Streptomyces coelicolor
(GenBank Nos: NP 628270 (SEQ ID NO: 81), NC_003888 (SEQ ID NO: 82)) and
Bacillus
subtilis (GenBank Nos: CAB14339 (SEQ ID NO: 83), Z99116 (SEQ ID NO: 84)).
[00239] The term "valine decarboxylase" refers to a polypeptide (or
polypeptides) having
enzyme activity that catalyzes the conversion of L-valine to isobutylamine and
CO2.
Example valine decarboxylases are known by the EC number 4.1.1.14. Such
enzymes are
found in Streptomyces, such as for example, Streptomyces viridifaciens
(GenBank Nos:
AAN10242 (SEQ ID NO: 85), AY116644 (SEQ ID NO: 86)).
[00240] The term "omega transaminase" refers to a polypeptide (or
polypeptides) having
enzyme activity that catalyzes the conversion of isobutylamine to
isobutyraldehyde using a
suitable amino acid as an amine donor. Example omega transaminases are known
by the EC
number 2.6.1.18 and are available from a number of sources, including, but not
limited to,
Alcaligenes denitrificans (AAP92672 (SEQ ID NO: 87), AY330220 (SEQ ID NO:
88)),
Ralstonia eutropha (GenBank Nos: YP_294474 (SEQ ID NO: 89), NC 007347 (SEQ ID
NO: 90)), Shewanella oneidensis (GenBank Nos: NP_719046 (SEQ ID NO: 91),
NC_004347
(SEQ ID NO: 92)), and Pseudomonas putida (GenBank Nos: AAN66223 (SEQ ID NO:
93),
AE016776 (SEQ ID NO: 94)).
[00241] The term "acetyl-CoA acetyltransferase" refers to a polypeptide (or
polypeptides)
having enzyme activity that catalyzes the conversion of two molecules of
acetyl-CoA to
acetoacetyl-CoA and coenzyme A (CoA). Example acetyl-CoA acetyltransferases
are acetyl-
CoA acetyltransferases with substrate preferences (reaction in the forward
direction) for a
short chain acyl-CoA and acetyl-CoA and are classified as E.C. 2.3.1.9 [Enzyme
Nomenclature 1992, Academic Press, San Diego]; although, enzymes with a
broader
substrate range (E. C. 2.3.1.16) will be functional as well. Acetyl-CoA
acetyltransferases are
available from a number of sources, for example, Escherichia coli (GenBank
Nos:
NP 416728, NC 000913; NCBI amino acid sequence, NCBI nucleotide sequence),
Clostridium acetobutylicum (GenBank Nos: NP_349476.1, NC_003030; NP_149242,
NC 001988, Bacillus subtilis (GenBank Nos: NP 390297, NC 000964), and
Saccharomyces
cerevisiae (GenBank Nos: NP_015297, NC_001148).
- 78 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[00242] The term "3-hydroxybutyryl-CoA dehydrogenase" refers to a polypeptide
(or
polypeptides) having enzyme activity that catalyzes the conversion of
acetoacetyl-CoA to 3-
hydroxybutyryl-CoA. Example 3-hydroxybutyryl-CoA dehydrogenases may be NADH-
dependent, with a substrate preference for (S)-3-hydroxybutyryl-CoA or (R)-3-
hydroxybutyryl-CoA. Examples may be classified as E.C. 1.1.1.35 and E.C.
1.1.1.30,
respectively. Additionally, 3-hydroxybutyryl-CoA dehydrogenases may be NADPH-
dependent, with a substrate preference for (S)-3-hydroxybutyryl-CoA or (R)-3-
hydroxybutyryl-CoA and are classified as E.C. 1.1.1.157 and E.C. 1.1.1.36,
respectively. 3-
Hydroxybutyryl-CoA dehydrogenases are available from a number of sources, for
example,
Clostridium acetobutylicum (GenBank Nos: NP_349314, NC 003030), Bacillus
subtilis
(GenBank Nos: AAB09614, U29084), Ralstonia eutropha (GenBank Nos: YP_294481,
NC 007347), and Alcaligenes eutrophus (GenBank Nos: AAA21973, J04987).
[00243] The term "crotonase" refers to a polypeptide (or polypeptides) having
enzyme
activity that catalyzes the conversion of 3-hydroxybutyryl-CoA to crotonyl-CoA
and H20.
Example crotonases may have a substrate preference for (S)-3-hydroxybutyryl-
00A or (R)-3-
hydroxybutyryl-CoA and may be classified as E.C. 4.2.1.17 and E.C. 4.2.1.55,
respectively.
Crotonases are available from a number of sources, for example, Escherichia
coli (GenBank
Nos: NP 415911, NC 000913), Clostridium acetobutylicum (GenBank Nos: NP
349318,
NC 003030), Bacillus subtilis (GenBank Nos: CAB13705, Z99113), and Aeromonas
caviae
(GenBank Nos: BAA21816, D88825).
[00244] The term "butyryl-CoA dehydrogenase" refers to a polypeptide (or
polypeptides)
having enzyme activity that catalyzes the conversion of crotonyl-CoA to
butyryl-CoA.
Example butyryl-CoA dehydrogenases may be NADH-dependent, NADPH-dependent, or
flavin-dependent and may be classified as E.C. 1.3.1.44, E.C. 1.3.1.38, and
E.C. 1.3.99.2,
respectively. Butyryl-CoA dehydrogenases are available from a number of
sources, for
example, Clostridium acetobutylicum (GenBank Nos: NP 347102, NC_ 003030),
Euglena
gracilis (GenBank Nos: Q5EU90, AY741582), Streptomyces collinus (GenBank Nos:
AAA92890, U37135), and Streptomyces coelicolor (GenBank Nos: CAA22721,
AL939127).
[00245] The term "butyraldehyde dehydrogenase" refers to a polypeptide (or
polypeptides)
having enzyme activity that catalyzes the conversion of butyryl-CoA to
butyraldehyde, using
NADH or NADPH as cofactor. Butyraldehyde dehydrogenases with a preference for
NADH
are known as E.C. 1.2.1.57 and are available from, for example, Clostridium
beijerinckii
- 79 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
(GenBank Nos: AAD31841, AF157306) and Clostridium acetobutylicum (GenBank Nos:
NP--149325, NC--001988).
[00246] The term "isobutyryl-CoA mutase" refers to a polypeptide (or
polypeptides) having
enzyme activity that catalyzes the conversion of butyryl-CoA to isobutyryl-
CoA. This
enzyme uses coenzyme B12 as cofactor. Example isobutyryl-CoA mutases are known
by the
EC number 5.4.99.13. These enzymes are found in a number of Streptomyces,
including, but
not limited to, Streptomyces cinnamonensis (GenBank Nos: AAC08713 (SEQ ID NO:
95),
U67612 (SEQ ID NO: 96); CAB59633 (SEQ ID NO: 97), AJ246005 (SEQ ID NO: 98)),
Streptomyces coelicolor (GenBank Nos: CAB70645 (SEQ ID NO: 99), AL939123 (SEQ
ID
NO: 100); CAB92663 (SEQ ID NO: 101), AL939121 (SEQ ID NO: 102)), and
Streptomyces
avermitilis (GenBank Nos: NP 824008 (SEQ ID NO: 103), NC_003155 (SEQ ID NO:
104);
NP 824637 (SEQ ID NO: 105), NC 003155 (SEQ ID NO: 106)).
[00247] The term "acetolactate decarboxylase" refers to a polypeptide (or
polypeptides)
having enzyme activity that catalyzes the conversion of alpha-acetolactate to
acetoin.
Example acetolactate decarboxylases are known as EC 4.1.1.5 and are available,
for
example, from Bacillus subtilis (GenBank Nos: AAA22223, L04470), Klebsiella
terrigena
(GenBank Nos: AAA25054, L04507) and Klebsiella pneumoniae (GenBank Nos:
AAU43774, AY722056).
[00248] The term "acetoin aminase" or "acetoin transaminase" refers to a
polypeptide (or
polypeptides) having enzyme activity that catalyzes the conversion of acetoin
to 3-amino-2-
butanol. Acetoin aminase may utilize the cofactor pyridoxal 5'-phosphate or
NADH or
NADPH. The resulting product may have (R) or (S) stereochemistry at the 3-
position. The
pyridoxal phosphate-dependent enzyme may use an amino acid such as alanine or
glutamate
as the amino donor. The NADH- and NADPH-dependent enzymes may use ammonia as a
second substrate. A suitable example of an NADH-dependent acetoin aminase,
also known
as amino alcohol dehydrogenase, is described by Ito, et al. (U.S. Patent No.
6,432,688). An
example of a pyridoxal-dependent acetoin aminase is the amine:pyruvate
aminotransferase
(also called amine:pyruvate transaminase) described by Shin and Kim (J. Org.
Chem.
67:2848-2853, 2002).
[00249] The term "acetoin kinase" refers to a polypeptide (or polypeptides)
having enzyme
activity that catalyzes the conversion of acetoin to phosphoacetoin. Acetoin
kinase may
utilize ATP (adenosine triphosphate) or phosphoenolpyruvate as the phosphate
donor in the
- 80 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
reaction. Enzymes that catalyze the analogous reaction on the similar
substrate
dihydroxyacetone, for example, include enzymes known as EC 2.7.1.29 (Garcia-
Alles, et al.,
Biochemistry 43:13037-13046, 2004).
[00250] The term "acetoin phosphate aminase" refers to a polypeptide (or
polypeptides)
having enzyme activity that catalyzes the conversion of phosphoacetoin to 3-
amino-2-
butanol 0-phosphate. Acetoin phosphate aminase may use the cofactor pyridoxal
5'-
phosphate, NADH, or NADPH. The resulting product may have (R) or (S)
stereochemistry
at the 3-position. The pyridoxal phosphate-dependent enzyme may use an amino
acid such
as alanine or glutamate. The NADH-dependent and NADPH-dependent enzymes may
use
ammonia as a second substrate. Although there are no reports of enzymes
catalyzing this
reaction on phosphoacetoin, there is a pyridoxal phosphate-dependent enzyme
that is
proposed to carry out the analogous reaction on the similar substrate serinol
phosphate
(Yasuta, et al., Appl. Environ. Microbial. 67:4999-5009, 2001).
[00251] The term "aminobutanol phosphate phospholyase," also called "amino
alcohol 0-
phosphate lyase," refers to a polypeptide (or polypeptides) having enzyme
activity that
catalyzes the conversion of 3-amino-2-butanol 0-phosphate to 2-butanone. Amino
butanol
phosphate phospho-lyase may utilize the cofactor pyridoxal 5'-phosphate. There
are reports
of enzymes that catalyze the analogous reaction on the similar substrate 1-
amino-2-propanol
phosphate (Jones, et al., Biochem J. 134:167-182, 1973). U.S.
Patent Application
Publication No. 2007/0259410 describes an aminobutanol phosphate phospho-lyase
from the
organism Erwinia carotovora.
[00252] The term "aminobutanol kinase" refers to a polypeptide (or
polypeptides) having
enzyme activity that catalyzes the conversion of 3-amino-2-butanol to 3-amino-
2-butanol 0-
phosphate. Amino butanol kinase may utilize ATP as the phosphate donor.
Although there
are no reports of enzymes catalyzing this reaction on 3-amino-2-butanol, there
are reports of
enzymes that catalyze the analogous reaction on the similar substrates
ethanolamine and 1-
amino-2-propanol (Jones, et al., supra). U.S.
Patent Application Publication No.
2009/0155870 describes, in Example 14, an amino alcohol kinase of Erwinia
carotovora
subsp. Atroseptica.
[00253] The term "butanediol dehydrogenase" also known as "acetoin reductase"
refers to a
polypeptide (or polypeptides) having enzyme activity that catalyzes the
conversion of acetoin
to 2,3-butanediol. Butanedial dehydrogenases are a subset of the broad family
of alcohol
-81-
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
dehydrogenases. Butanediol dehydrogenase enzymes may have specificity for
production of
(R)- or (S)-stereochemistry in the alcohol product. (S)-specific butanediol
dehydrogenases
are known as EC 1.1.1.76 and are available, for example, from Klebsiella
pneumoniae
(GenBank Nos: BBA13085, D86412). (R)-specific butanediol dehydrogenases are
known as
EC 1.1.1.4 and are available, for example, from Bacillus cereus (GenBank Nos.
NP 830481,
NC 004722; AAP07682, AE017000), and Lactococcus lactis (GenBank Nos. AAK04995,
AE006323).
[00254] The term "butanediol dehydratase," also known as "dial dehydratase" or
"propanediol dehydratase" refers to a polypeptide (or polypeptides) having
enzyme activity
that catalyzes the conversion of 2,3-butanediol to 2-butanone. Butanediol
dehydratase may
utilize the cofactor adenosyl cobalamin (also known as coenzyme Bw or vitamin
B12;
although vitamin B12 may refer also to other forms of cobalamin that are not
coenzyme
B12). Adenosyl cobalamin-dependent enzymes are known as EC 4.2.1.28 and are
available,
for example, from Klebsiella oxytoca (GenBank Nos: AA08099 (alpha subunit),
D45071;
BAA08100 (beta subunit), D45071; and BBA08101 (gamma subunit), D45071 (all
three
subunits are required for activity), and Klebsiella pneumonia (GenBank Nos:
AAC98384
(alpha subunit), AF102064; GenBank Nos: AAC98385 (beta subunit), AF102064,
GenBank
Nos: AAC98386 (gamma subunit), AF102064). Other suitable dial dehydratases
include, but
are not limited to, B12-dependent dial dehydratases available from Salmonella
typhimurium
(GenBank Nos: AAB84102 (large subunit), AF026270; GenBank Nos: AAB84103
(medium
subunit), AF026270; GenBank Nos: AAB84104 (small subunit), AF026270); and
Lactobacillus collinoides (GenBank Nos: CAC82541 (large subunit), AJ297723;
GenBank
Nos: CAC82542 (medium subunit); AJ297723; GenBank Nos: CAD01091 (small
subunit),
AJ297723); and enzymes from Lactobacillus brevis (particularly strains CNRZ
734 and
CNRZ 735, Speranza, et al., J. Agric. Food Chem. 45:3476-3480, 1997), and
nucleotide
sequences that encode the corresponding enzymes. Methods of dial dehydratase
gene
isolation are well known in the art (e.g., U.S. Patent No. 5,686,276).
[00255] The term "pyruvate decarboxylase" refers to a polypeptide (or
polypeptides) having
enzyme activity that catalyzes the decarboxylation of pyruvic acid to
acetaldehyde and
carbon dioxide. Pyruvate dehydrogenases are known by the EC number 4.1.1.1.
These
enzymes are found in a number of yeast, including Saccharomyces cerevisiae
(GenBank
- 82 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
Nos: CAA97575 (SEQ ID NO: 107), CAA97705 (SEQ ID NO: 109), CAA97091 (SEQ ID
NO: 111)).
[00256] It will be appreciated that microorganisms comprising an isobutanol
biosynthetic
pathway as provided herein may further comprise one or more additional
modifications.
U.S. Patent Application Publication No. 2009/0305363 (incorporated by
reference) discloses
increased conversion of pyruvate to acetolactate by engineering yeast for
expression of a
cytosol-localized acetolactate synthase and substantial elimination of
pyruvate decarboxylase
activity. In some embodiments, the microorganisms may comprise modifications
to reduce
glycerol-3-phosphate dehydrogenase activity and/or disruption in at least one
gene encoding
a polypeptide having pyruvate decarboxylase activity or a disruption in at
least one gene
encoding a regulatory element controlling pyruvate decarboxylase gene
expression as
described in U.S. Patent Application Publication No. 2009/0305363
(incorporated herein by
reference), and/or modifications that provide for increased carbon flux
through an Entner-
Doudoroff Pathway or reducing equivalents balance as described in U.S. Patent
Application
Publication No. 2010/0120105 (incorporated herein by reference). Other
modifications
include integration of at least one polynucleotide encoding a polypeptide that
catalyzes a step
in a pyruvate-utilizing biosynthetic pathway. Other modifications include at
least one
deletion, mutation, and/or substitution in an endogenous polynucleotide
encoding a
polypeptide having acetolactate reductase activity. In some embodiments, the
polypeptide
having acetolactate reductase activity is YMR226C (SEQ ID NOs: 127, 128) of
Saccharomyces cerevisiae or a homolog thereof Additional modifications include
a
deletion, mutation, and/or substitution in an endogenous polynucleotide
encoding a
polypeptide having aldehyde dehydrogenase and/or aldehyde oxidase activity. In
some
embodiments, the polypeptide having aldehyde dehydrogenase activity is ALD6
from
Saccharomyces cerevisiae or a homolog thereof A genetic modification which has
the effect
of reducing glucose repression wherein the yeast production host cell is pdc-
is described in
U.S. Patent Application Publication No. 2011/0124060, incorporated herein by
reference. In
some embodiments, the pyruvate decarboxylase that is deleted or down-regulated
is selected
from the group consisting of: PDC1, PDC5, PDC6, and combinations thereof In
some
embodiments, the pyruvate decarboxylase is selected from those enzymes in
Table 1. In
some embodiments, microorganisms may contain a deletion or down-regulation of
a
polynucleotide encoding a polypeptide that catalyzes the conversion of
glyceraldehyde-3-
- 83 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
phosphate to glycerate 1,3, bisphosphate. In some embodiments, the enzyme that
catalyzes
this reaction is glyceraldehyde-3-phosphate dehydrogenase.
Table 1. SEQ ID Numbers of PDC Target Gene coding regions and Proteins
Description SEQ ID NO: SEQ ID NO:
(Amino Acid) (Nucleic Acid)
PDC1 pyruvate decarboxylase from 107 108
Saccharomyces cerevisiae
PDC5 pyruvate decarboxylase from 109 110
Saccharomyces cerevisiae
PDC6 pyruvate decarboxylase 111 112
Saccharomyces cerevisiae
pyruvate decarboxylase from 113 114
Candida glabrata
PDC1 pyruvate decarboxylase from 115 116
Pichia stipitis
PDC2 pyruvate decarboxylase from 117 118
Pichia stipitis
pyruvate decarboxylase from 119 120
Kluyveromyces lactis
pyruvate decarboxylase from 121 122
Yarrowia lipolytica
pyruvate decarboxylase from 123 124
Schizosaccharomyces pombe
pyruvate decarboxylase from 125 126
Zygosaccharomyces rouxii
[00257] In some embodiments, any particular nucleic acid molecule or
polypeptide may be
at least 80%, 85%, 90%, 95%, 96%, 97%, 98%,¨
or vv% identical to a nucleotide sequence or
polypeptide sequence described herein. The term "percent identity" as known in
the art, is a
relationship between two or more polypeptide sequences or two or more
polynucleotide
sequences, as determined by comparing the sequences. In the art, "identity"
also means the
degree of sequence relatedness between polypeptide or polynucleotide
sequences, as the case
may be, as determined by the match between strings of such sequences. Identity
and
similarity can be readily calculated by known methods, including but not
limited to those
disclosed in: Computational Molecular Biology (Lesk, A. M., Ed.) Oxford
University: NY
(1988); Biocomputing: Informatics and Genome Projects (Smith, D. W., Ed.)
Academic: NY
(1993); Computer Analysis of Sequence Data, Part I (Griffin, A. M., and
Griffin, H. G., Eds.)
Humania: NJ (1994); Sequence Analysis in Molecular Biology (von Heinje, G.,
Ed.)
- 84 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
Academic (1987); and Sequence Analysis Primer (Gribskov, M. and Devereux, J.,
Eds.)
Stockton: NY (1991).
[00258] Methods to determine identity are designed to give the best match
between the
sequences tested. Methods to determine identity and similarity are codified in
publicly
available computer programs. Sequence alignments and percent identity
calculations may be
performed using the MegAlignTM program of the LASERGENE bioinformatics
computing
suite (DNASTAR Inc., Madison, WI). Multiple alignment of the sequences may be
performed using the Clustal method of alignment which encompasses several
varieties of the
algorithm including the Clustal V method of alignment corresponding to the
alignment
method labeled Clustal V (disclosed by Higgins and Sharp, CABIOS. 5:151-153,
1989;
Higgins, et al., Comput. Appl. Biosci. 8:189-191, 1992) and found in the
MegAlignTM
program of the LASERGENE bioinformatics computing suite (DNASTAR Inc.). For
multiple alignments, the default values correspond to GAP PENALTY=10 and GAP
LENGTH PENALTY=10. Default parameters for pairwise alignments and calculation
of
percent identity of protein sequences using the Clustal method are KTUPLE=1,
GAP
PENALTY=3, WINDOW=5 and DIAGONALS SAVED=5. For nucleic acids these
parameters are KTUPLE=2, GAP PENALTY=5, WINDOW=4 and DIAGONALS
SAVED=4. After alignment of the sequences using the Clustal V program, it is
possible to
obtain a percent identity by viewing the sequence distances table in the same
program.
Additionally the Clustal W method of alignment is available and corresponds to
the
alignment method labeled Clustal W (Higgins and Sharp, CABIOS. 5:151-153,
1989;
Higgins, et al., Comput. Appl. Biosci. 8:189-191, 1992) and found in the
MegAlignTM v6.1
program of the LASERGENE bioinformatics computing suite (DNASTAR Inc.).
Default
parameters for multiple alignment (GAP PENALTY=10, GAP LENGTH PENALTY=0.2,
Delay Divergen Seqs(%)=30, DNA Transition Weight=0.5, Protein Weight
Matrix=Gonnet
Series, DNA Weight Matrix=IUB ). After alignment of the sequences using the
Clustal W
program, it is possible to obtain a percent identity by viewing the sequence
distances table in
the same program.
[00259] Standard recombinant DNA and molecular cloning techniques are well
known in
the art and are described by Sambrook, et al. (Sambrook, J., Fritsch, E. F.
and Maniatis, T.
(Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press,
Cold
Spring Harbor, 1989, here in referred to as Maniatis) and by Ausubel, et al.
(Ausubel, et al.,
- 85 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
Current Protocols in Molecular Biology, pub. by Greene Publishing Assoc. and
Wiley-
Interscience, 1987). Examples of methods to construct microorganisms that
comprise a
butanol biosynthetic pathway are disclosed, for example, in U.S. Patent No.
7,851,188, and
U.S. Patent Application Publication Nos. 2007/0092957; 2007/0259410;
2007/0292927;
2008/0182308; 2008/0274525; 2009/0155870; 2009/0305363; and 2009/0305370, the
entire
contents of each are herein incorporated by reference.
[00260] Further, while various embodiments of the present invention have been
described
herein, it should be understood that they have been presented by way of
example only, and
not limitation. It will be apparent to persons skilled in the relevant art
that various changes in
form and detail can be made therein without departing from the spirit and
scope of the
invention. Thus, the breadth and scope of the present invention should not be
limited by any
of the described exemplary embodiments, but should be defined only in
accordance with the
claims and their equivalents.
[00261] All publications, patents, and patent applications mentioned in
this specification are
indicative of the level of skill of those skilled in the art to which this
invention pertains, and
are herein incorporated by reference to the same extent as if each individual
publication,
patent, or patent application was specifically and individually indicated to
be incorporated by
reference.
- 86 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
EXAMPLES
[00262] The following nonlimiting examples will further illustrate the
invention. It should
be understood that, while the following examples involve corn as feedstock,
other biomass
sources such as cane may be used for feedstock without departing from the
present invention.
Moreover, while the following examples involve ethanol and butanol, other
alcohols or
fermentation products may be produced without departing from the present
invention.
[00263] The meaning of abbreviations is as follows: "atm" means atmosphere,
"ccm"
means cubic centimeter(s) per minute, "g/L" means gram(s) per liter, "g" means
gram(s),
"gpl" means gram(s) per liter, "gpm" means gallon(s) per minute, "h" or "hr"
means hour(s),
"HPLC" means high performance liquid chromatography, "kg" means kilogram(s),
"L"
means liter(s), "min" means minute(s), "mL" means milliliter(s), "ppm" means
parts per
million, "psig" means pound(s) per square inch, gauge, and "wt%" means weight
percent.
Example 1
Process for Production and Recovery of Butanol Produced by Fermentation
[00264] The processes described herein may be demonstrated using computational
modeling
such as Aspen modeling (see, e.g., U.S. Patent No. 7,666,282). For example,
the commercial
modeling software Aspen Plus (Aspen Technology, Inc., Burlington, MA) may be
used in
conjunction with physical property databases such as DIPPR, available from
American
Institute of Chemical Engineers, Inc. (New York, NY) to develop an Aspen model
for an
integrated butanol fermentation, purification, and water management process.
This process
modeling can perform many fundamental engineering calculations, for example,
mass and
energy balances, vapor/liquid equilibrium, and reaction rate computations. In
order to
generate an Aspen model, information input may include, for example,
experimental data,
water content and composition of feedstock, temperature for mash cooking and
flashing,
saccharification conditions (e.g., enzyme feed, starch conversion,
temperature, pressure),
fermentation conditions (e.g., microorganism feed, glucose conversion,
temperature,
pressure), degassing conditions, solvent columns, pre-flash columns,
condensers,
evaporators, centrifuges, etc.
[00265] An Aspen model was developed with rigorous material and energy balance
in
which 53400 kg/h of corn was mashed and fermented to produce isobutanol and in
which
most of the isobutanol was extracted during fermentation and distilled. This
model included
- 87 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
an approximation of sequenced batch fermentations as continuous processes. An
example of
this fermentation, extraction, and distillation process is illustrated in
Figure 14.
[00266] Liquefied corn mash 601 that was clarified to comprise 1.5 wt%
suspended solids
was pumped at 170.7 tonnes/h and 85 C through a heat exchanger and a water
cooler and fed
to fermentation 600 at 32 C. Vapor stream 602 was vented at 17.2 tonnes/h at
atmospheric
pressure from fermentation 600 to a scrubber with an average continuous molar
composition
of 95.8% carbon dioxide, 3.4% water, and 0.8% isobutanol. An average beer
stream 603
comprising 12.6 gpl isobutanol was discharged continuously from fermentation
600 and pre-
heated through a heat exchanger by the mash 601 prior to being distilled for
isobutanol
recovery.
[00267] Stream 604 with 3875 tonnes/h combined average flow is removed from
fermentation 600 at an average isobutanol concentration of 11.1 gpl and an
average
temperature of 32 C and circulated through an extractor 610 for partial
removal of
isobutanol. The exiting aqueous broth 605 containing 7.9 gpl isobutanol is
cooled by heat
exchange with cooler tower water (CTW) to 30 C prior to re-entering
fermentation 600. A
solvent comprising diisopropylbenzene enters the extractor 610 and exits as
stream 606
comprising 30.1 gpl isobutanol. The extractor 610 provides effectively five
theoretical
liquid-liquid equilibrium stages for contacting fermentation broth with
solvent. Stream 606
passes at 340 tonnes/h through a heat exchanger and enters the middle of
twelve theoretical
stages of distillation column 620. A reboiler is operating at 0.6 atm and 183
C using
150 psig steam to produce solvent stream 607 comprising diisopropylbenzene and
essentially
no isobutanol that exchanges heat with solvent stream 606 through a heat
exchanger and is
further cooled by cooling water CTW prior to re-entering extractor 610. The
overhead vapor
of distillation column 620 is cooled CTW and condensed 630 to form 23.1
tonnes/h of reflux,
0.2 tonnes/h of a residual vapor off-gas 608, and 13.2 tonnes/h of product
distillate 609 that
comprises 99.2% isobutanol, 0.6% water, and 0.2% diisopropylbenzene.
Example 2
Process for Recovery of Ethanol Using an Extractant Column
[00268] A 1" diameter Karr extraction column (Koch Modular Process Systems,
Paramus,
NJ) was used to process fermentation broth that was produced during ethanol
fermentation.
The column contains a series of plates that run down the length of the column
and which are
- 88 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
attached to a central shaft. The shaft is attached to a drive which can move
the perforated
plates (1/4" diameter perforations) up and down in a reciprocating motion. The
frequency of
the motion was a variable during testing, but both the stroke length of the
oscillation (0.75")
and the spacing of the trays (2") were fixed. The column used had a plate
stack height of
3000 mm.
[00269] The top of the column was provided with an aqueous feed consisting of
fermentation broth, while the bottom of the column was provided with a feed of
corn oil fatty
acids (COFA) as the extractant. The two feeds flowed countercurrent to one
another through
the column, and were collected as product at opposite ends of the column.
[00270] The fermentation broth was obtained using a fermentation protocol for
production
of ethanol from liquefied and saccharified corn mash from which, in some
cases, some of the
solids had been removed via centrifugation. In some cases, the extraction
testing was done
over the course of several days, such that a portion of the testing was done
while CO2 off-
gassing was at or near its maximum, while another portion was done when off-
gassing had
effectively stopped. The COFA used in this work was distilled grade from Emery
Oleochemicals (Cincinnati, OH).
[00271] Some experiments were run with COFA as the continuous phase in the
column,
while others were run with continuous aqueous phase. Experiments were also
conducted
with or without internals in place. Two types of internals were tested:
stainless steel and
polytetrafluoroethylene (PTFE). A range of flow rates were examined, in order
to determine
the flow regimes under which the column could be operated without flooding.
Impact of dynamic feed from fermentation
[00272] During the course of the testing, it was determined that in some cases
the column
performance varied as the fermentation progressed. Early in the fermentation,
the
fermentation broth comprising the feed is high in sugar, at intermediate times
a considerable
amount of CO2 (which can impact fluid flows) evolves from the fermentation
broth, while at
later times the concentration of ethanol in the fermentation broth is high.
This temporal
variation in the feed was reflected in variations in the capacity of the
extraction column.
[00273] With conditions using PTFE plates and continuous COFA phase (no
agitation), a
difference was noted in performance when using fermentation broth collected
when the
fermentation was near the period of peak gas evolution ("intermediate broth")
and toward the
end of fermentation ("end broth"). Using end broth, a liquid throughput rate
of 14 gpm/ft2
- 89 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
(Sample 3E) was achieved without flooding the column. The maximum throughput
for
intermediate broth that could be achieved prior to flooding was less than to 9
gpm/ft2
(Sample 4D), with noticeable differences in the size and appearance of the
aqueous droplets.
The droplet size of the aqueous phase was larger (with the formation of
globules) in end
broth as compared to intermediate broth.
Continuous Phase
[00274] The maximum column throughput was also impacted by the nature of the
continuous phase. For end of fermentation conditions, running with continuous
aqueous
phase and stainless steel (S. Steel) internals, a total liquid capacity of
almost 14 gpm/ft2 was
achieved (Sample 2B). For continuous organic phase and PTFE internals, the
rate was less
than 9 gpm/ft2 (Sample 4D). Results are shown in Table 2. The abbreviation AQ
refers to
the aqueous phase and the abbreviation ORG refers to the organic phase.
Referring to Table
2, the Phase was continuous, Sample refers to run conditions, Internals refer
to the material
of the internals, Nom. AQ refers to the nominal aqueous flow rate, Nom. ORG
refers to
nominal organic flow rate, Total Flow (ccm) refers to the total flow of the
aqueous and
organic feeds, and Total Flow (gpm/ft2) refers to total flow per unit cross-
sectional area.
Table 2
Phase Sample Internals Nom. AQ Nom. ORG Total Flow Total Flow
(ccm) (ccm) (ccm) (gpm/ft2)
AQ lA S. Steel 160 60 220 10.7
AQ 1B S. Steel 100 30 130 6.3
AQ 1C S. Steel 89 20 109 5.3
AQ 1D S. Steel 120 65-101 -
AQ lE S. Steel 135 60 195 9.4
AQ 1F S. Steel 132 50 182 8.8
AQ 1G S. Steel 150 50 200 9.7
AQ 1G' S. Steel 120 50 170 8.2
AQ 1H S. Steel 210 75 285 13.8
AQ 11 S. Steel 210 75 285 13.8
AQ 2A S. Steel 210 75 285 13.8
AQ 2B S. Steel 210 75 285 13.8
AQ 2C S. Steel 87 85 172 4.1
ORG 3A PTFE 100 50 150 7.3
ORG 3B PTFE 100 100 200 9.7
ORG 3C PTFE 200 100 300 14.5
ORG 3D PTFE 180 75 255 12.4
ORG 3E PTFE 180 120 300 14.5
ORG 3F PTFE 180 170 350 17.0
- 90 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
ORG 3G PTFE 180 60 240 11.6
ORG 3G PTFE 180 60 240 11.6
ORG 4A PTFE 110 70 180 8.7
ORG 4B PTFE 100 30 130 6.3
ORG 4C PTFE 100 60 160 7.7
ORG 4D PTFE 100 80 180 8.7
ORG 4E PTFE 85 60 145 7.0
ORG 4F PTFE 90 40 130 6.3
ORG 4G PTFE 70 40 110 5.3
ORG 4M PTFE 60 60 120 5.8
ORG 4N PTFE 80 80 160 7.7
[00275] When the column was operated without internals using feed comprised of
fermentation broth near the end of fermentation, the choice of the continuous
phase affected
the column capacity. For continuous aqueous phase, it was possible to operate
at
approximately 25 gpm/ft2 (Samples 2G and 2H). With continuous COFA phase,
however,
problems with flooding occurred at 18 gpm/ft2 (Sample 21). Results are shown
in Table 3.
Table 3
Phase Nom. Aq Nom. Org. Total Flow Total Flow
(ccm) (ccm) (ccm) (gpm/ft2)
AQ 200 75 275 13.3
AQ 200 75 275 13.3
AQ 200 75 275 13.3
AQ 200 120 320 15.5
AQ 240 160 400 19.4
AQ 240 160 400 19.4
AQ 320 170 490 23.7
AQ 390 170 560 27.1
ORG 210 170 380 18.4
ORG 210 170 380 18.4
ORG 60 60 120 5.8
ORG 80 60 140 6.8
ORG 80 80 160 7.7
ORG 90 90 180 8.7
ORG 100 100 200 9.7
ORG 150 50 200 9.7
ORG 170 60 230 11.1
Example 3
Effect of Fermentation Conditions on Extraction Column Capacity
[00276] The nature of fermentation broth is not static, but changes as the
fermentation
process progresses. In fermentation, the concentration of carbohydrates
decreases as the
-91 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
carbohydrates are metabolized by microorganisms. This compositional change in
the
fermentation broth will alter physical parameters such as viscosity and
surface tension of the
fermentation broth, which have an effect on the extraction process. In
addition to the
changes in concentration, at intermediate times a considerable amount of CO2
is evolved; and
this CO2 will impact the flow of the aqueous and organic liquids through the
column.
[00277] A 1" diameter glass Karr extraction column (Koch Modular Process
Systems,
Paramus, NJ), outfitted with PTFE internals, was used to process fermentation
broth from an
ethanol fermentation. The processing was done at several timepoints during the
course of the
fermentation. Organic extractant (COFA) was the continuous phase in the
column, with the
fermentation broth passing through the column as droplets. Prior to the
introduction of the
fermentation broth to the column, the fermentation broth was passed through a
tee in the line
where CO2 bubbles present in the feed were removed through a vent.
[00278] With static internals (no agitation), a difference was noted in
performance when
using fermentation broth taken during the period of peak gas evolution
("intermediate broth")
compared to broth taken toward the end of fermentation ("end broth"). Using
intermediate
broth, a liquid throughput rate of 14 gpm/ft2 was achieved. The maximum
throughput
(before column flooding) for the end broth was less than to 9 gpm/ft2. There
were noticeable
differences in the size and appearance of the aqueous droplets. The droplet
size of the
aqueous phase was visibly larger for end broth as compared to intermediate
broth.
Example 4
Effect of Isobutanol Concentration on Extraction Column Efficiency
[00279] During a typical fermentation process, the levels of product change
with time. This
dynamic concentration change can affect the mass transfer in an extraction
process.
[00280] To demonstrate the effect of isobutanol concentration, a 1" diameter
glass Karr
extraction column (Koch Modular Process Systems, Paramus, NJ), outfitted with
stainless
steel internals, was used to process fermentation broth from a fermentation
that contained
approximately 3 g/L of isobutanol. The fermentation broth formed the
continuous phase in
the extractor, while the organic extractant (COFA) passed through the column
as droplets.
Although CO2 production had essentially ceased, the fermentation broth was
passed through
a tee in the line where any CO2 bubbles present in the feed were removed prior
to the feed
entering the extraction column.
- 92 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[00281] Samples of the feed and exiting streams were analyzed for isobutanol
by liquid
chromatography (LC) or gas chromatography (GC). Results are shown in Table 4.
Mass
balances were done, and the height of an equilibrium transfer stage (HETS)
calculated using
Kremser equations. For the two data points on as-is fermentation broth, the
HETS values
were 10 and 13 feet.
[00282] Isobutanol was then added to the fermentation broth to bring the
concentration to
20 g/L. An extraction test was conducted and from the data, the HETS was found
to be
18 feet. This value was some 50% higher than the values obtained on plain
broth, and is in
line with data obtained using thin stillage spiked with approximately 20 g/L
isobutanol (see
Figure 15).
Table 4
Flow of Flow of Isobutanol Isobutanol Isobutanol Isobutanol HETS
aqueous organic in the rich in the lean in the rich
in the lean
phase phase aqueous aqueous organic
organic
phase phase phase phase
(LC) (LC) (GC)
Aqueous phase mL/min mL/min g/L g/L g/L g/L
ft
Broth 192.5 85 2.80 1.21 3.3 0 10
Broth 247.5 105 3.14 1.65 3.6 0 13
Broth with added
iBuOH 187.5 89.7 20.6 11.5 20.6 0 18
Example 5
ISPR Using an External Extraction Column
[00283] Fermentation broth from an isobutanol fermentation (10-liter scale)
was circulated
to a 5/8" diameter bench top Karr column. The extraction solvent (COFA) was
recycled
from an extractant reservoir to the Karr column. A control fermentation was
run in which a
volume of COFA was added to the fermentor to continuously extract isobutanol
from the
fermentation broth.
[00284] The Karr column was run twice during the fermentation. The first run
was at
timepoint 4 to 7 hours of the fermentation and the second run was at timepoint
22 to 33 hours
of the fermentation. Parameters such as p02 and pH were monitored for both
fermentations.
The measured p02 was lower for the run in which the Karr column was used, as
compared
to the control run that did not use the Karr column. Absolute pH values were
similar for
the Karr column and the control, but the pH profiles were different for the
two runs. The
- 93 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
pH in the Karr column run peaked early, flattened, then peaked again, versus
a single
gradual peak for the control.
[00285] Two aliquots of extraction solvent (1.8 liters each) were analyzed
from the Karr
column. Samples were taken from each aliquot and analyzed for isobutanol
content. The
amount of isobutanol produced in the fermentation with the Karr column was
comparable
to that produced in the control fermentation. The fermentation using the Karr
column
produced a total of 82.4 grams isobutanol: approximately 34 grams were in 3.6
liters of
organic phase and 48 grams in the aqueous phase. The control (30% by volume
organic
phase added to the fermentor) produced 90 g/L, 60 grams in 3 liters of organic
phase and
30 grams in the aqueous phase. Isobutanol concentration in the aqueous phase
was lower in
the control due to the presence of COFA in the control fermentor from time
zero, versus a
non-zero start of extraction in the Karr column run. For the Karr column at
22 hours,
isobutanol was extracted from the fermentor more quickly than it was being
produced.
Glucose profiles were generated for the control and Karr column. The profiles
were
similar, indicating cell growth and metabolism were comparable. Results are
shown in
Figures 16A and 16B. Brackets indicate the time points (4 to 7 hours and 22 to
33 hours)
when the Karr column was in operation.
Example 6
ISPR Using Mixer-Settler
[00286] An external mixer settler system was used to continuously remove
isobutanol from
an active fermentation broth containing a microorganism that produced
isobutanol (i.e.,
isobutanologen). The study used approximately 100 liters of fermentation broth
inoculated
with an isobutanol-producing microorganism (i.e., isobutanologen). The
contents of the
fermentor were re-circulated from the fermentor through the mixer-settler
extraction system.
The extractant, comprising distilled COFA which contained no isobutanol, was
used on a
once-through basis.
[00287] Two static mixers were tested. The majority of the test used a Kenics0
stainless
steel static mixer (A" in diameter with 36 mixing elements). Between hours 12
and 24 of the
run, a plastic mixer was used (StaMixCo HT-11-12.6-24, StaMixCo LLC, Brooklyn,
NY).
Fermentation broth and COFA were fed to opposite sides of a tee, from which
the mixture
flowed through the static mixer. The material exiting the static mixer was fed
to the settler.
- 94 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
The settler was made from a five-liter glass tank. A dip tube passed through
the top of the
settler, near the perimeter, and extended approximately halfway down the
settler. The
organic phase was withdrawn through a port at the top of the settler, while
fermentation broth
was removed from the bottom of the settler. The settler was fitted with an
agitator that
provided gentle mixing to the aqueous-organic interface in order to aid
disengagement of the
two liquid phases and thereby minimize accumulation of solids at the
interface. Data
collected during the run is presented in Table 5, and Figure 17 shows the
isobutanol removal
rates that were achieved during the course of the fermentation. As can be seen
from the data,
isobutanol levels in the aqueous broth remained relatively constant,
indicating that isobutanol
was removed from the fermentation broth at about the same rate as it is being
produced.
Referring to Table 5, Elapsed Time is time from start of fermentation, AQ Flow
is aqueous
feed flow, ORG flow is organic feed flow, iB in AQ feed is isobutanol in the
aqueous feed,
and iB in ORG product is isobutanol in the rich organic product.
Table 5
Elapsed Type of AQ Flow ORG flow iB in AQ iB in
ORG
Time Mixer* (ccm) (ccm) feed product
(hr) (g/L) (g/L)
0.0 A 648 100 5.97 10.14
4.0 A 648 100 5.30 9.22
8.0 A 648 100 4.56 8.20
12.0 A 648 100 4.03 7.63
16.0 B 648 100 4.23 5.36
20.0 B 842.2 130 4.00 7.15
24.0 B 572.4 170 4.53 6.15
28.0 A 648 100 4.65 9.82
32.0 A 648 100 4.92 9.00
36.0 A 648 100 5.27 11.45
41.0 A 648 100 5.65 10.16
45.0 A 648 100 5.40 6.92
*A: 1/2" stainless Kenics0 mixer, 32 elements
B: StaMixCo HT-11-12.6-24, plastic mixer
Example 7
On-Line, At-Line, and Real-Time Measurements
[00288] A mash stream prepared from corn feedstock was conducted to a three-
phase
centrifuge generating three streams: mash, corn oil, and wet cake. On-line or
at-line process
measurements are employed, for example, to improve the recovery of
starch/sugars and the
quality of corn oil, and to maximize the amount of starch/sugars extracted
from wet cake.
- 95 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
Real-time measurements are used, for example, to control the addition of
backset, cookwater,
or water to slurry tanks to maintain a starch/sugar concentration set-point.
The amount of
starch/sugar extracted from the wet cake is maximized using the minimum amount
of added
water, and reducing the hydraulic load on the three-phase centrifuge.
[00289] Corn mash samples were analyzed using Fourier transform infrared
spectroscopy
(FTIR) with a diamond attenuated total reflectance (ATR) probe that allows for
measurements in the presence of solids. The FTIR was calibrated by collecting
spectra of
standard samples in which total starch/sugar determinations using HPLC had
been
completed. The HPLC data was used to create a multivariate partial least
squares (PLS)
model for the FTIR. FTIR spectra were collected and a total starch
concentration generated.
Figure 18 illustrates the range of starch concentrations used to calibrate the
FTIR.
[00290] Corn mash with an average starting concentration of 250 g/L was fed to
a three-
phase centrifuge. The subsequent wet cake was re-slurried and the
concentration of starch
was measured on two samples: 80 g/L and 70 g/L. This slurry was then separated
using a
three-phase centrifuge and the wet cake re-slurried. The starch concentration
of this slurry
was determined to be 28.9 g/L. Results are shown in Figure 19. These
measurements were
used to determine the correct amount of water to re-slurry the wet cake at
each stage.
Optimizing the water addition maximized the starch concentration and minimized
the
hydraulic load on the separation step. Moisture content of the wet cake was
measured using
near-infrared spectroscopy (NIR).
[00291] Corn oil quality is monitored in real-time and the data is used to
control the three-
phase centrifuge variables (e.g., feed rate, g forces, inlet flow rate, scroll
speed). The quality
of corn oil generated by the three-phase centrifuge was measured by monitoring
the
concentration of water carried into the corn oil during the separation. FTIR
with a diamond
ATR probe was used to collect corn oil spectra as it exited the three-phase
centrifuge. The
detection limit for water using the diamond ATR probe approach was
approximately
500 ppm. Lower detection limits are achieved with the use of a flow cell with
a longer
effective path length.
[00292] Figure 20 contains a series of infrared spectra of corn oils that
contain a range of
water concentrations in excess of percent level concentrations down to 100's
of ppm. Water
concentration was determined using the ¨OH stretching region between 3700 cm-1
and
3050 cm-1. The data indicated that a process FTIR may be used to generate real-
time water
- 96 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
concentration in oil data. Real-time water concentration data may be used to
control the
process variables of the three-phase centrifuge (e.g., feed rate, g forces,
inlet flow rate, scroll
speed). The operation of the three-phase centrifuge may be controlled to yield
the highest
quality corn oil or to maximize throughput while not exceeding a water set
point.
[00293] Real-time extractant monitoring was used to detect and monitor thermal
breakdown
of the extractant. Detection of these thermal breakdown products in real-time
is used to
trigger remediation of the extractant or purging of the contaminated
extractant from the
process.
[00294] Figure 21 is an example of the real-time measurement of isobutanol-
rich COFA.
The data was collected using a Metter-Toledo ReactIRTM 247 using a diamond ATR
sampling probe in a flow cell. The COFA stream was collected from the outlet
of a 1-inch
diameter Karr column and pumped to the FTIR using a peristaltic pump. The
FTIR was
calibrated by creating COFA standards spiked with isobutanol and generating a
multivariate
PLS model.
Example 8
Droplet size Analysis
[00295] This example describes the analysis of liquid extractant droplets
after conducting a
process stream containing fermentation broth and extractant (COFA) to a static
mixer. A
PVMO probe (Mettler-Toledo, LLC, Columbus OH) was inserted into the process
stream
approximately 24 hr after the process stream exited the static mixer. The PVMO
probe was
used to collect images every two minutes during a fermentation run. The images
showed the
presence of both COFA droplets ranging in size from 50 to 80 um in diameter
and CO2
bubbles ranging in size from 200 and 400 um in diameter. Monitoring droplet
size in the
process stream containing fermentation broth and COFA after the static mixer
is used to
ensure that the droplets remain below a particular average diameter to ensure
good mass
transfer of isobutanol into the COFA droplets
[00296] The PVMO probe was also used to image the COFA droplets in the lean
broth
stream prior to return of the stream to the fermentor. The detection of COFA
droplets in this
stream is an indication of the amount of COFA returning to the fermentor. The
PVMO probe
was used to collect an image of the stream every two minutes during a
fermentation. Unlike
the stream exiting the static mixer, the lean broth stream had fewer and
smaller droplets (10-
- 97 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
40 p.m). These measurements demonstrate the feasibility of using process
imaging to
monitor the amount of COFA returning to the fermentor.
[00297] Real-time average droplet size data from both sample points are used
to monitor the
phase separation of fermentation broth and COFA. An increase in the
concentration or
number of small COFA droplets detected in the lean fermentation broth recycle
stream (after
isobutanol extraction) can be an indicator that the phase separation of
fermentation broth and
COFA has degraded and too much COFA is exiting the extractor. To improve the
quality of
the phase separation and reduce the number or concentration of the COFA
droplets returning
to the fermentor in the lean broth stream, the average COFA droplet size is
increased post
static mixer.
[00298] Additional process variables that can impact average COFA droplet size
include the
concentration of polysaccharides in the fermentation broth, the ratio of
fermentation broth to
COFA, and total flow rate through the static mixer. As the fermentation
progresses, flow
rate and/or fermentation broth to COFA ratios may be changed to maintain a
constant
average COFA droplet size.
Example 9
Extractor Design
[00299] This example describes a method to design a large-scale extractor
unit. Data from a
pilot-scale extraction is used to estimate the size of the large-scale
extractor unit. The effects
of flow rate, agitation rate, and the presence or absence of internals on
phase separation of
the streams of the extractor unit from a pilot-scale extraction are
determined. The total flow
and ratio of fermentation broth flow to extractant flow is varied at fixed
temperature over the
course of the fermentation, and the conditions under which phase separation
discontinues are
observed. The maximum achievable flow to the extractor unit per square foot of
extractor
unit flow surface area is recorded. The following equation is used to
determine flow per unit
area:
, F
u ¨ ¨
A (Equation 1)
U = flow per unit area (gallons/minute/square foot)
F = total flow of fermentation broth and extractant to the extractor unit
(gallons/minute)
A = cross-sectional area in direction of flow (square feet)
- 98 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
7-t-D 2
for an extraction column this is given by ¨
4
D = column diameter (feet).
[00300] The diameter of a large-scale extractor unit is estimated by the
expected flow of
fermentation broth and extractant to the extractor unit using the following
equation:
4F/ arg e¨scale
D = (Equation 2)
71-U
Flarge-scale ¨ Total flow of fermentation broth and extractant to the large-
scale extractor
(gallons/minute).
[00301] The height of the pilot-scale extractor unit is measured under
different flow regimes
including different flow rates, with and without internals present, different
agitation rates,
and at different concentrations of the product alcohol. Using this data, the
number of
theoretical stages achieved by the height of the extractor unit is estimated
using the Kremser
Equation (Seader and Henley, Separation Process Principles, 2nd edition, John
Wiley &
Sons, 2006, pp. 358-359):
r
Ys
xf r 1 1
ln _________________________ m 1 __ +
E1 E
x _______________________
n my
n= ________________ -
ln(E) (Equation 3)
m F broth
E = extraction factor = Fextrac tan t
Fbroth = flow of broth to the extractor unit (gallons/minute)
Fextractant ¨ flOW of extractant to the extractor unit (gallons/minute)
m = partition coefficient for product alcohol in fermentation broth and
extractant phases
(g/L per g/L)
Xf = concentration of product alcohol in fermentation broth feed (g/L)
- 99 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
Xn = concentration of product alcohol in fermentation broth leaving the
extractor unit
(g/L)
Ys = concentration of product alcohol in extractant entering the extractor
unit (g/L)
n = number of theoretical stages achieved by the height of the extractor unit
Equation 3 is only valid when E 1.
[00302] The height of a theoretical stage for the extractor unit is given by
the height of the
extraction column used in the pilot-scale extraction divided by the number of
theoretical
stages realized in a given experiment. The number of theoretical stages
required to achieve
the separation at large-scale is estimated using the operating conditions
expected at large-
scale in Equation 4:
x= Ys
r 1
ln n! 1 __ 1, + ,
, y Ei E
xn ______________________
my
n = ______________ -
ln(E') (Equation 4)
where 'indicates the condition of the large-scale extractor unit.
[00303] The product of the number of theoretical stages and height of a
theoretical stage
measured for similar flow conditions provides an estimate of the total height
of the large-
scale extractor unit. The flows and concentrations expected at a large-scale
extractor unit are
estimated using a dynamic fermentation model (e.g., Daugulis, et al., Biotech.
Bioeng.
27:1345-1356, 1985).
[00304] While various embodiments of the present invention have been described
herein, it
should be understood that they have been presented by way of example only, and
not
limitation. It will be apparent to persons skilled in the relevant art that
various changes in
form and detail can be made therein without departing from the spirit and
scope of the
invention. Thus, the breadth and scope of the present invention should not be
limited by any
of the described exemplary embodiments, but should be defined only in
accordance with the
following claims and their equivalents.
- 100 -
CA 02883627 2015-03-02
WO 2014/043288
PCT/US2013/059340
[00305] All publications, patents and patent applications mentioned in this
specification are
indicative of the level of skill of those skilled in the art to which this
invention pertains, and
are herein incorporated by reference to the same extent as if each individual
publication,
patent or patent application was specifically and individually indicated to be
incorporated by
reference.
- 101 -