Note: Descriptions are shown in the official language in which they were submitted.
CA 02883815 2015-03-03
Title
PLATING SOLUTION AND PLATING PROCESS FOR MULTI-LAYER
CYANIDE-FREE PLATING COPPER-TIN ALLOY COATING, AND
COINS MADE BY THE PROCESS
Background of the Present Invention
Field of Invention
The present invention relates to the technology field of coinage, and
particularly to
electroplating liquid of multi-layer cyanide-free electroplated copper-tin
alloy plating, an
electroplating technology and a coin produced by the technology.
Description of Related Arts
The history of cyanide plating may be traced back to 1831, and the practical
electroplating
technology begins from the patent of cyanide silver plating granted to
Elkington in 1840.
Cyanide zinc plating has been practically applied during the First World War,
and then, cyanide
plating technology is widely used in the electroplating of many single metals
such as zinc,
copper, cadmium, silver and gold or alloy plating. However, cyanides are
highly toxic substances,
and the lethal dose is only 5 mg, as a result, the requirements for management
of toxic cyanide
and wastewater treatment of cyanide electroplating liquid are relative high.
In 1970s, research on
cyanide-free zinc plating technology first got a breakthrough, and until now,
cyanide-free copper
plating, cyanide-free gold and silver plating, and cyanide-free copper alloy
plating and other
CA 02883815 2015-03-03
technologies have been developed one after another, and have been applied in
certain industrial
fields. At present, electroplating materials used in the international coinage
industry mainly
include plated copper, plated nickel, plated copper alloy, and the like,
wherein the electroplating
copper-tin alloy technology still adopts a cyanide electroplating or adopts a
heat treatment
method to form an alloy layer for respective single metal plating.
Copper-tin alloy electroplating is a conventional plating technology that
substitutes for
nickel plating, and can be used for barreling and rack plating. Cyanide system
copper-tin alloy
plating is a relative mature nickel substitute plating technology. Due to the
requirements for
environmental protection and human health, in recent years, development and
researches on
new process of cyanide-free copper-tin alloy electroplating have attracted
extensive attention.
The current reported solution systems for cyanide-free copper-tin alloy
electroplating mainly
include pyrophosphate, pyrophosphate-stannate, citrate-stannate and HEDP and
the like,
wherein the pyrophosphate solution system has the great potential for
substituting the cyanide
solution system. For the cyanide-free brass-tin plating process at the present
stage, the
electroplating time is very long, and thus problems of fogged and loose
plating will occur. As a
result, this process is mostly used in decorative platings, but has fewer
breakthroughs in
functional plating. Therefore, the object of the present invention is to solve
the existing
problems as brass-tin plating is used in functional plating, i.e.,
continuously thickening of the
plating, uniformity and compactness of the plating, stability of the plating
solution, and the like.
Summary of the Present Invention
In view of the disadvantages in the existing technology that the cyanide-free
electroplated brass-tin cannot be plated thicker and the plating is uniform,
the object of the
present invention is to provide a pyrophosphate electroplating liquid of multi-
layer cyanide-free
electroplated copper-tin alloy plating, an electroplating technology for the
multi-layer
2
CA 02883815 2016-09-26
cyanide-free electroplated copper-tin alloy plating, and a coin product
fabricated by such
electroplating technology. By using the pyrophosphate electroplating solution
and the
electroplating technology for multi-layer cyanide-free electroplated copper-
tin alloy plating, a
coin product with a uniform and compact plating having a thickness of up to 20
m can be
obtained.
In an aspect, there is provided a pyrophosphate electroplating solution of
multi-layer
cyanide-free electroplated copper-tin alloy plating, comprising: a cyanide-
free brass-tin
brightening agent consisting of (1) 1 to 10 g/L of MirapolTM WT and (2) 0.05
to 0.5 g/L of
2-mercapto benzimidazole, wherein the concentration of the cyanide-free brass-
tin brightening
agent in the pyrophosphate electroplating solution is 3 to 20 ml/L.
In another aspect, there is provided an electroplating method for multi-layer
cyanide-free electroplated copper-tin alloy plating, comprising: sequentially
electroplating 2 to 4
plating layers of copper-tin alloy on a coin substrate; and subjecting the
plating layers on the coin
substrate to a high-temperature treatment, to form a coin with multi-layer
cyanide-free
electroplated copper-tin alloy platting; wherein the even layer(s) of plating
and the surface layer
are electroplated by adopting the pyrophosphate electroplating solution as
disclosed herein.
According to the electroplating technology for multi-layer cyanide-free
electroplated
copper-tin alloy plating and the coin product fabricated by adopting the
electroplating technology
of the present invention, its billet adopts a low-carbon steel coinage blank
as a substrate, on
which a first layer, a second layer, a third layer and a surface layer are
electroplated in sequence.
The electroplating technology is a multi-layer electroplating technology using
a pyrophosphate
solution system, and the entire electroplating technology features an
identical main salt system,
so that the risk of cross contamination of electroplating liquid among
different platings can be
avoided, and the electroplating liquid can be rinsed with water after each
layer is plated, thereby
omitting the activation process. The problem that the monolayer cyanide-free
electroplated
copper-tin alloy plating is thinner than the cyanide electroplated copper-tin
alloy plating is
solved by adopting a multilayer electroplating. Also, the electroplating
liquid for the plating of
3
CA 02883815 2016-09-26
each layer in the electroplating technology is a pyrophosphate solution
system, which is a
cyanide-free environment friendly system, thereby greatly reducing the
management cost of the
high toxic cyanide, improving the plating environment, decreasing the pressure
of wastewater on
environmental influence, and significantly improving the fabrication level of
the coin plating
cladding material as well.
In accordance with the present invention, the provided electroplating
technology for
multi-layer cyanide-free electroplated copper-tin alloy plating and the coin
structure fabricated
by the electroplating technology differ from the conventional cyanide plated
copper-tin alloy
coins and the current fabrication process and coin structure of the other
electroplated copper-tin
alloy coins at home and abroad. The conventional process for cyanide
electroplated copper-tin
alloy coin is directly electroplating a copper-tin alloy on steel-cored
billet, and the coin has a
monolayer structure. Other copper-tin alloy electroplating technologies
provided at home and
abroad include, for example, firstly plating a base layer on an discus, then
performing single
metal alternating electroplating, and performing heat treatment diffusion
after the plating is
completed, so as to obtain an alloy layer of a certain thickness.
In order to achieve the above object and other objects, the present invention
adopts the
following technical solutions:
A pyrophosphate electroplating solution of multi-layer cyanide-free
electroplated
copper-tin alloy plating comprises a cyanide-free brass-tin major brightening
agent, and the
solute of the cyanide-free brass-tin major brightening agent consists of a
brightening agent A and
a brightening agent B; wherein, the concentration of the brightening agent A
in the cyanide-free
brass-tin major brightening agent is 1 to 10 g/L; and the concentration of the
brightening agent B
in the cyanide-free brass-tin major brightening agent is 0.05 to 0.5 g/L.
Preferably, the concentration of the cyanide-free brass-tin major brightening
agent in the
pyrophosphate electroplating solution is 3 to 20 ml/L.
Preferably, the solute of the cyanide-free brass-tin major brightening agent
consists of
4
CA 02883815 2016-09-26
the brightening agent A and the brightening agent B, and the solvent thereof
is a mixture of water
and organic solvent; wherein the optimum ratio of water and the organic
solvent is such a value
that the brightening agent A and the brightening agent B can be just
dissolved. In the mixture of
water and the organic solvent, the organic solvent is selected from a mixture
of an organic
solvent and water capable of dissolving the brightening agent A and the
brightening agent B.
Preferably, the brightening agent A is the brightening agent MirapolTM WT
manufactured by Rhodia Inc., France; and the brightening agent B is 2-mercapto
benzimidazole.
4a
CA 02883815 2015-03-03
The addition of the Mirapol WT may significantly shorten the electroplating
time,
improve the uniformity and throwing power of plating, and improve the
corrosion resistance of
plating as well; also, the plating may have good salt and fog resistance and
flexibility. In the
present invention, both the brightening agent A and the brightening agent B
are added to the
electroplating liquid, and thus by virtue of the synergism thereof, a uniform
and compact
brass-tin plating can be obtained in a wide range of current density.
Preferably, the pH value of the pyrophosphate electroplating solution of multi-
layer
cyanide-free electroplated copper-tin alloy plating is 8.0 to 10.0, and the
density thereof is 1.30
to 1.45 g/cm3. The pH value of the pyrophosphate electroplating solution of
the present invention
can be adjusted to a desired pH value by using hydrophosphate and phosphoric
acid.
Further, the pyrophosphate electroplating solution of multi-layer cyanide-free
electroplated copper-tin alloy plating also contains the following components
and concentrations:
pyrophosphate 350 to 450 g/L;
soluble copper salt 20 to 35 g/L;
soluble tin salt 1.8 to 3.0 g/L;
conductive salt 0 to 80 g/L; and
cyanide-free brass-tin adjuvant 10 to 50 ml/L.
The solvent of the pyrophosphate electroplating solution is water.
Preferably, the pyrophosphate is one selected from potassium pyrophosphate and
sodium pyrophosphate. Preferably, the pyrophosphate is potassium
pyrophosphate.
Preferably, the soluble copper salt is one, two or more selected from copper
pyrophosphate, copper sulfate, copper chloride, basic copper carbonate, copper
methane
sulfonate and copper sulfamate. Preferably, the soluble copper salt is copper
pyrophosphate.
CA 02883815 2015-03-03
Preferably, the soluble tin salt is one, two or more selected from stannous
pyrophosphate, stannous sulfate, stannous chloride, tin fluoborate and tin
alkylsulfonate.
Preferably, the soluble tin salt is stannous pyrophosphate.
Preferably, the conductive salt is one, two or more selected from potassium
chloride,
sodium chloride, dipotassium hydrogen phosphate, ammonium chloride, potassium
sulphate,
sodium sulphate, potassium carbonate and sodium carbonate. Preferably, the
conductive salt is
dipotassium hydrogen phosphate.
Preferably, the solute of the cyanide-free brass-tin adjuvant consists of an
auxiliary
complexing agent A and an auxiliary complexing agent B; wherein the
concentration of the
auxiliary complexing agent A in the cyanide-free brass-tin adjuvant is 5 to 10
g/L, and the
concentration of the auxiliary complexing agent B in the cyanide-free brass-
tin adjuvant is 5 to
g/L. The solvent of the cyanide-free brass-tin adjuvant is water.
More preferably, both the auxiliary complexing agent A and the auxiliary
complexing
agent B are one, two or more selected from glycolic acid, sodium gluconate,
HEDP (hydroxy
ethidene diphosphonic acid), citric acid, sodium citrate, ammonium citrate,
potassium sodium
tartrate, methanesulfonic acid, triethanolamine, oxalic acid and glycine;
while the auxiliary
complexing agent A and the auxiliary complexing agent B will not select the
same substance
simultaneously. Preferably, the auxiliary complexing agent A is glycolic acid;
and the auxiliary
complexing agent B is sodium gluconate.
In accordance with the present invention, the pyrophosphate electroplating
solution of
multi-layer cyanide-free electroplated copper-tin alloy plating may further
include a stabilizer;
and the concentration of the stabilizer is 0.01 to 0.05 g/L.
Preferably, the stabilizer is one selected from hydroquinone, catechol,
resorcinol,
13-naphthol, ascorbic acid and hydroxy benzenesulfonic acid.
6
CA 02883815 2015-03-03
In accordance with the present invention, the electroplating solution has a
simple
composition and is easy to maintain, and is applicable to a wide range of
current density, and the
plating thickness can be up to 20 j_im without the occurrence of
embrittlement. The content of tin
in the plating is 11% to 14% by weight; and the plating appearance is in
uniform golden yellow
color without chromatic aberration.
The present invention further provides a electroplating method for multi-layer
cyanide-free electroplated copper-tin alloy plating, in which 2 to 4 plating
layers of copper-tin
alloy are sequentially electroplated on a coin substrate, and then, after
performing
high-temperature treatment, a coin with multi-layer cyanide-free electroplated
copper-tin alloy
platting is obtained; wherein, the even layer(s) of plating and the surface
layer are electroplated
by adopting the above pyrophosphate electroplating solution of multi-layer
cyanide-free
electroplated copper-tin alloy plating.
Preferably, the number of the layers of copper-tin alloy plating is 2 or 4.
Preferably, the temperature of the high-temperature treatment is 600 C to 800
C.
In accordance with the present invention, the electroplating method for multi-
layer
cyanide-free electroplated copper-tin alloy plating specifically comprises the
following steps:
1. Electroplating a first layer: taking a coinage blank of low-carbon steel as
a coin
substrate, after removing oil, pickling and activating, the coinage blank is
placed in a first
electroplating liquid, to electroplate a first layer with a thickness of about
1 to 5 micrometers at a
temperature of 20 C to 30 C, so as to obtain the first layer of copper-tin
alloy with a tin content
of less than 2%; and then wash with water.
Preferably, in Step 1, the current density for electroplating the first layer
is 0.5 to 1.5
A/dm2; and the electroplating time is 30 to 60 min.
The adopted first electroplating liquid in Step 1 and Step 5 is electroplating
liquid of
7
CA 02883815 2015-03-03
cyanide-free low tin copper-tin alloy, which may adopt commonly used
electroplating liquid of
cyanide-free low tin copper-tin alloy in the prior art, such as electroplating
liquid containing the
following solute concentrations: potassium pyrophosphate of 250 to 370 g/L;
copper
pyrophosphate of 20 to 30 g/L; stannous pyrophosphate of 0.2 to 0.5 g/L;
dipotassium hydrogen
phosphate of 0 to 80 g/L; cyanide-free alkaline copper additive of 10 to 20
ml/L; wherein the
density is 1.25 to 1.35; and the solvent is water.
Preferably, the water washing after electroplating the first layer is to place
the first layer
electroplated coinage blank in deionized water at room temperature for
rinsing.
In accordance with the present invention, the total thickness of the plating
of the blank
or billet is no less than 20 micrometers; all the binding force, corrosion
resistance, abrasion
resistance, hardness and other indexes of the plating of the blank or billet
meet the requirements
of mint application.
2. Electroplating a second layer: the obtained water washed coinage blank in
Step 1 is
placed in the pyrophosphate electroplating solution of multi-layer cyanide-
free electroplated
copper-tin alloy plating of the present invention, to electroplate a second
layer with a thickness
of about 10 to 20 micrometers at a temperature of 25 C to 35 C, so as to
obtain a second layer of
copper-tin alloy with a tin content of 14% to 18%; and then wash with water.
Preferably, in Step 2, the current density for electroplating the second layer
is 0.5 to 1.5
A/dm2; and the electroplating time is 200 to 550 min.
Preferably, the water washing after electroplating the second layer is to
place the second
layer electroplated coinage blank in deionized water at room temperature for
rinsing.
3. Electroplating a third layer: the obtained water washed coinage blank in
Step 2 is
placed in the first electroplating liquid, to electroplate a third layer with
a thickness of about 3 to
micrometers at a temperature of 20 C to 30 C, so as to obtain a third layer of
copper-tin alloy
8
CA 02883815 2015-03-03
with a tin content of less than 2%; and then wash with water.
Preferably, in Step 3, the current density for electroplating the third layer
is 0.5 to 1.5
A/dm2; and the electroplating time is 60 to 90 min.
Preferably, the water washing after electroplating the third layer is to place
the third
layer electroplated coinage blank in deionized water at room temperature for
rinsing.
4. Electroplating a fourth layer (also called as a surface layer): the
obtained rinsed
coinage blank in Step 3 is placed in a pyrophosphate electroplating solution
of multi-layer
cyanide-free electroplated copper-tin alloy plating, to electroplate a fourth
layer with a thickness
of about 10 to 12 micrometers at a temperature of 20 C to 30 C, so as to
obtain the fourth layer
of copper-tin alloy with a tin content of 14% to 18%; and then wash with
water.
Preferably, in Step 4, the current density for electroplating the fourth layer
is 0.5 to 1.5
A/dm2; and the electroplating time is 200 to 270 min.
Preferably, the water washing after electroplating the fourth layer is to
place the fourth
layer electroplated coinage blank in deionized water at room temperature for
rinsing.
5. The obtained water washed coinage blank with two layers of plating in Step
2 or the
obtained water washed with four layers of plating in Step 4 is dried and
subjected to
high-temperature heat treatment in sequence, to obtain a coin of multi-layer
cyanide-free
electroplated copper-tin alloy plating, i.e., a mono-plating coin of copper-
tin alloy.
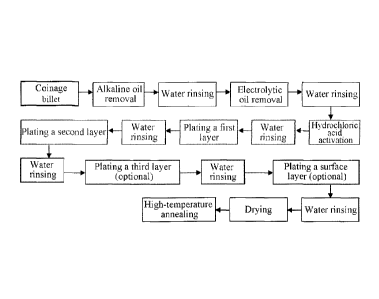
Further, the oil removal step in Step 1 sequentially includes an alkaline oil
removal step
and an electrolytic oil removal step; the pickling and activating step in Step
1 is to perform
pickling and activating on the coinage blank with hydrochloric acid solution.
Preferably, there is a water washing step after each of the alkaline oil
removal step,
electrolytic oil removal step and pickling and activating step. The water
washing is preferably to
perform rinsing with deionized water at room temperature. The alkaline oil
removal step,
9
CA 02883815 2015-03-03
electrolytic oil removal step and pickling and activating step of the present
invention may adopt
a conventional alkaline oil removal step, electrolytic oil removal step and
pickling and activating
step in the prior art.
The present invention further provides a coin product, which is a coin of
multi-layer
cyanide-free electroplated copper-tin alloy plating obtained by using the
above electroplating
method for multi-layer cyanide-free electroplated copper-tin alloy plating of
the present
invention; in the coin of monolayer copper-tin alloy plating formed after high-
temperature heat
treatment, the content of tin in the monolayer plating is 11% to 14% by
weight; and the plating
appearance is in uniform golden yellow without chromatic aberration.
Further, the obtained coin of multi-layer cyanide-free electroplated copper-
tin alloy
plating has a thickness of coin plating of 20 to 24 micrometers when adopting
two layers of
plating; and the obtained coin has a thickness of coin plating of 25 to 31
micrometers when
adopting four layers of plating.
In accordance with the present invention, the adopted electroplating liquid
for each of
plating of the coin product is pyrophosphate solution system. By taking full
advantage of the
advancement and superiority of cyanide-free alloy electroplating, combining
with the manner of
multi-layer electroplating, and rationally considering the combination between
the thickness of
the multi-layer plating and the alloy composition, it is able to solve the
current difficult problem
in the field of electroplating that the monolayer cyanide-free electroplated
alloy plating is thin.
In accordance with the present invention, by adopting the electroplating
method of
multi-layer cyanide-free electroplated copper-tin alloy plating, it enables to
save the
management cost of highly toxic cyanide, and significantly improve the
electroplating
conditions, which is conducive to the health of workers and environmental
protection; the entire
electroplating liquid system is pyrophosphate system, which prevents the risk
of cross
contamination among plating solutions, and makes the whole process more smooth
and easier
CA 02883815 2015-03-03
for controlling.
Brief Description of the Drawings
FIG. 1 is a flow chart of an electroplating process of the present invention;
FIG. 2 shows the influence of a brightening agent A on the appearance of a
Hull cell test
piece;
FIG. 3 shows the influence of a brightening agent B on the appearance of a
Hull cell test
piece;
FIG. 4 shows the appearance of a Hull cell test piece under different current
with the
addition of a brightening agent A and a brightening agent B;
FIG. 5 shows the influence of the addition of an auxiliary complexing agent A
on the
components of plating;
FIG. 6 shows the influence of the addition of an auxiliary complexing agent B
on the
components of plating; and
FIG. 7 shows the influence of the addition of an auxiliary complexing agent A
and an
auxiliary complexing agent B on the components of plating.
Detailed Description of the Preferred Embodiments
Hereinafter, the implementation manners of the present invention are
illustrated with
specific examples, so that persons of ordinary skill in the art can easily
understand other
advantages and efficacies of the present invention from the disclosure of the
specification. The
present invention can also be implemented or applied in other different
specific implementation
11
CA 02883815 2015-03-03
manners, and various modifications and alternations can be made on details in
the specification
based on different views and application without departing from the spirit of
the present
invention.
In accordance with the following Table 1 to Table 7 and Embodiments 1 to 6,
the
cyanide-free brass-tin major brightening agent consists of a brightening agent
Mirapol WT (a
brightening agent A) with concentration of 1 to 10 g/L and 2-mercapto
benzimidazole (a
brightening agent B) with concentration of 0.05 to 0.5 g/L.
In accordance with the following Table 1 to Table 7 and Embodiments 1 to 6,
the
cyanide-free brass-tin adjuvant consists of glycolic acid with concentration
of 5 to 10 g/L and
sodium gluconate with concentration of 5 to 10 g/L.
In accordance with the following Table 1 to Table 7 and Embodiments 1 to 6,the
cyanide-free alkaline copper additive consists of glycolic acid with
concentration of 50 to 100
g/L and 2-mercapto benzimidazole with concentration of 0.05 to 0.5 g/L.
In accordance with the following Table 1 to Table 7 and various embodiments,
the
process parameters of the electroplating method for multi-layer cyanide-free
electroplated
copper-tin alloy plating are preferably shown in Table 1 to Table 7.
Table 1 Process parameters of alkaline oil removal
Alkaline oil removal Parameter range
Oil remover 50 to 70 g/L
Temperature 55 C to 65 C
Table 2 Process parameters of electrolytic oil removal
Electrolytic oil removal Parameter range
12
CA 02883815 2015-03-03
Oil remover 60 to 80 g/L
Temperature 55 C to 65 C
Current density 0.5 to 1.3 A/dm2
Table 3 Process parameters of hydrochloric acid activation
Hydrochloric acid activation Parameter range
Concentrated hydrochloric acid (35%) 350 to 500 ml/L
Temperature 20 C to 30 C
Table 4 Process parameters for electroplating a first layer
Process parameters of the electroplating Parameter range
liquid
Potassium pyrophosphate 250 to 370 g/L
Copper pyrophosphate 20 to 30 g/L
Stannous pyrophosphate 0.2 to 0.5 g/L
Dipotassium hydrogen phosphate 0 to 80 g/L
Cyanide-free alkaline copper additive 10 to 20 ml/L
pH value 8.0 to 10.0
Density 1.25 to 1.35
Temperature 20 C to 30 C
Current density 0.5 to 1.5 A/dm2
Anode material oxygen-free
cathode copper
13
CA 02883815 2015-03-03
Table 5 Process parameter for electroplating a second layer
Process parameters of the electroplating Parameter range
liquid
Potassium pyrophosphate 350 to 450 g/L
Copper pyrophosphate 20 to 35 g/L
Starmous pyrophosphate 1.8 to 3.0 g/L
Dipotassium hydrogen phosphate 0 to 80 g/L
Cyanide-free brass-tin major brightening 3 to 20 ml/L
agent
Cyanide-free brass-tin adjuvant 10 to 50 ml/L
pH value 8.0 to 10.0
Density 1.30 to 1.45
Temperature 25 C to 35 C
Current density 0.5 to 1.5 A/dm2
Anode material oxygen-free
cathode copper
Table 6 Process parameters for electroplating a third layer
Process parameters of the electroplating Parameter range
liquid
Potassium pyrophosphate 250 to 370 g/L
Copper pyrophosphate 20 to 30 g/L
Stannous pyrophosphate 0.2 to 0.5 g/L
14
CA 02883815 2015-03-03
Dipotassium hydrogen phosphate 0 to 80 g/L
Cyanide-free alkaline copper additive 10 to 20 ml/L
pH value 8.0 to 10.0
Density 1.25 to 1.35
Temperature 20 C to 30 C
Current density 0.5 to 1.5 A/dm2
Anode material oxygen-
free cathode copper
Table 7 Process parameter for electroplating a surface layer
Process parameters of the electroplating Parameter range
liquid
Potassium pyrophosphate 350 to 450 g/L
Copper pyrophosphate 20 to 35 g/L
Stannous pyrophosphate 1.8 to 3.0 g/L
Dipotassium hydrogen phosphate 0 to 80 g/L
Cyanide-free brass-tin major brightening 3 to 20 ml/L
agent
Cyanide-free brass-tin adjuvant 10 to 50 ml/L
pH value 8.0 to 10.0
Density 1.30 to 1.45
Temperature 25 C to 35 C
Current density 0.5 to 1.5 A/dm2
Anode material oxygen-free cathode copper
CA 02883815 2015-03-03
Embodiment 1:
Take a coinage blank of low-carbon steel as a substrate, thereon electroplate
a first
layer and a second layer in sequence, to obtain a product. The specific steps
are as follows:
(1) Alkaline oil removal
The coinage blank is placed in an alkaline oil remover with concentration of
50 g/L,
and is cleaned for 20 min at a temperature of 55 C, and then rinsed with
deionized water at
60 C.
(2) Electrolytic oil removal
The alkaline washed coinage blank is placed in an electrolytic oil remover
with
concentration of 60 g/L, and is subjected to anode electrolytic cleaning for
20 min at a
temperature of 55 C and with current density of 0.5 A/dm2, and then rinsed
with deionized
water at 60 C.
(3) Hydrochloric acid activation
The coinage blank after electrolytic oil removal is placed in an HC1 solution
with
concentration of 350 ml/L, and is subjected to acid activation for 7 min at a
temperature of
20 C, and then rinsed with deionized water at room temperature.
(4) Electroplating a first layer
The activated coinage blank is placed in a first layer electroplating liquid
with a pH
value of 8.0, and is electroplated a first layer at a temperature of 20 C,
wherein the current
density is 0.5 A/dm2, and the electroplating time is 60 min. The first layer
electroplating liquid
consists of the following components: potassium pyrophosphate, 250 g/L; copper
pyrophosphate, 20 g/L; stannous pyrophosphate, 0.2 g/L; cyanide-free alkaline
copper additive,
16
CA 02883815 2015-03-03
ml/L; and the first layer has a thickness of about 2 to 4 micrometers.
(5) Water washing
The first layer electroplated coinage blank is placed in deionized water, and
rinsed with
deionized water at room temperature.
(6) Electroplating a second layer
After rinsing in deionized water, the coinage blank is placed in a second
layer
electroplating liquid with a pH value of 8.0, and is electroplated a second
layer at a temperature
of 20 C, wherein the current density is 0.5 A/dm2, and the electroplating time
is 540 min. The
second layer electroplating liquid consists of the following components:
potassium
pyrophosphate, 350 g/L; copper pyrophosphate, 20 g/L; stannous pyrophosphate,
1.8 g/L;
cyanide-free brass-tin major brightening agent, 3 ml/L; cyanide-free brass-tin
adjuvant, 10 ml/L;
and the second layer has a thickness of about 18 to 20 micrometers and a tin
content of 14% to
18%.
(7) Water washing and drying
The surface layer electroplated coinage blank is placed in deionized water,
and rinsed
with deionized water at room temperature, and then the coinage blank is dried.
(8) High-temperature heat treatment
The dried coinage blank is placed in a high-temperature heat treatment furnace
being
flowed with reduced protective atmosphere, and is subjected to heat treatment
for 7 min at
650 C and then for 7 min at 680 C. After the heat treatment, the plating of
the product is
diffused into one layer having a tin content of 11% to 14% and a plating
thickness of 20 to 24
micrometers.
Embodiment 2:
17
CA 02883815 2015-03-03
Take a coinage blank of low-carbon steel as a substrate, thereon electroplate
a first
layer and a second layer in sequence, to obtain a product. The specific steps
are as follows:
(1) Alkaline oil removal
The coinage blank is placed in an alkaline oil remover with concentration of
60 g/L,
and is cleaned for 20 min at a temperature of 60 C, and then rinsed with
deionized water at
60 C.
(2) Electrolytic oil removal
The alkaline washed coinage blank is placed in an electrolytic oil remover
with
concentration of 70 g/L, and is subjected to anode electrolytic cleaning for
20 min at a
temperature of 60 C and with current density of 1.0 A/dm2, and then rinsed
with deionized
water at 60 C.
(3) Hydrochloric acid activation
The coinage blank after electrolytic oil removal is placed in an HC1 solution
with
concentration of 480 ml/L, and is subjected to acid activation for 7 min at a
temperature of
25 C, and then rinsed with deionized water at room temperature.
(4) Electrolating a first layer
The activated coinage blank is placed in a first layer electroplating liquid
with a pH
value of 9.0, and is electroplated a first layer at a temperature of 25 C,
wherein the current
density is 1.0 A/dm2, and the electroplating time is 60 min. The first layer
electroplating liquid
consists of the following components: potassium pyrophosphate, 300 g/L; copper
pyrophosphate, 25 g/L; stannous pyrophosphate, 0.35 g/L; cyanide-free alkaline
copper additive,
20 ml/L; and the first layer has a thickness of about 2 to 4 micrometers.
(5) Water washing
18
CA 02883815 2015-03-03
The first layer electroplated coinage blank is placed in deionized water, and
rinsed with
deionized water at room temperature.
(6) Electroplating a second layer
After rinsing in deionized water, the coinage blank is placed in a second
layer
electroplating liquid with a pH value of 9.0, and is electroplated a second
layer at a temperature
of 25 C, wherein the current density is 1.2 A/dm2, and the electroplating time
is 540 min. The
second layer electroplating liquid consists of the following components:
potassium
pyrophosphate, 400 g/L; copper pyrophosphate, 25 g/L; stannous pyrophosphate,
2.2 g/L;
dipotassium hydrogen phosphate, 45 g/L; cyanide-free brass-tin major
brightening agent, 20
ml/L; cyanide-free brass-tin adjuvant, 50 ml/L; and the second layer has a
thickness of about 18
to 20 micrometers and a tin content of 14% to 18%.
(7) Water washing and drying
The surface layer electroplated coinage blank is placed in deionized water,
and rinsed
with deionized water at room temperature, and then the coinage blank is dried.
(8) High-temperature heat treatment
The dried coinage blank is placed in a high-temperature heat treatment furnace
being
flowed with reduced protective atmosphere, and is subjected to heat treatment
for 7 min at
650 C and then for 7 mm at 680 C. After the heat treatment, the plating of the
product is
diffused into one layer having a tin content of 11% to 14% and a plating
thickness of 20 to 24
micrometers.
Embodiment 3:
Take a coinage blank of low-carbon steel as a substrate, thereon electroplate
a first
layer and a second layer in sequence, to obtain a product. The specific steps
are as follows:
(1) Alkaline oil removal
19
CA 02883815 2015-03-03
The coinage blank is placed in an alkaline oil remover with concentration of
70 g/L,
and is cleaned for 20 min at a temperature of 65 C, and then rinsed with
deionized water at
60 C.
(2) Electrolytic oil removal
The alkaline washed coinage blank is placed in an electrolytic oil remover
with
concentration of 80 g/L, and is subjected to anode electrolytic cleaning for
20 min at a
temperature of 65 C and with current density of 1.2 A/dm2, and then rinsed
with deionized
water at 60 C.
(3) Hydrochloric acid activation
The coinage blank after electrolytic oil removal is placed in an HC1 solution
with
concentration of 480 ml/L, and is subjected to acid activation for 7 min at a
temperature of
29 C, and then rinsed with deionized water at room temperature.
(4) Electroplating a first layer
The activated coinage blank is placed in a first layer electroplating liquid
with a pH
value of 9.8, and is electroplated a first layer at a temperature of 28 C,
wherein the current
density is 1.4 A/dm2, and the electroplating time is 60 mm. The first layer
electroplating liquid
consists of the following components: potassium pyrophosphate, 360 g/L; copper
pyrophosphate, 28 g/L; stannous pyrophosphate, 0.45 g/L; cyanide-free alkaline
copper additive,
15 ml/L; and the first layer has a thickness of about 2 to 4 micrometers.
(5) Water washing
The first layer electroplated coinage blank is placed in deionized water, and
rinsed with
deionized water at room temperature.
(6) Electroplating a second layer
CA 02883815 2015-03-03
After rinsing in deionized water, the coinage blank is placed in a second
layer
electroplating liquid with a pH value of 9.8, and is electroplated a second
layer at a temperature
of 28 C, wherein the current density is 1.8 A/dm2, and the electroplating time
is 540 min. The
second layer electroplating liquid consists of the following components:
potassium
pyrophosphate, 450 g/L; copper pyrophosphate, 32 g/L; stannous pyrophosphate.
2.8 g/L;
dipotassium hydrogen phosphate, 70 g/L; cyanide-free brass-tin major
brightening agent, 10
ml/L; cyanide-free brass-tin adjuvant, 30 ml/L; and the second layer has a
thickness of about 18
to 20 micrometers and a tin content of 14% to 18%.
(7) Water washing and drying
The surface layer electroplated coinage blank is placed in deionized water,
and rinsed
with deionized water at room temperature, and then the coinage blank is dried.
(8) High-temperature heat treatment
The dried coinage blank is placed in a high-temperature heat treatment furnace
being
flowed with reduced protective atmosphere, and is subjected to heat treatment
for 7 min at
650 C and then for 7 min at 680 C. After the heat treatment, the plating of
the product is
diffused into one layer having a tin content of 11% to 14% and a plating
thickness of 20 to 24
micrometers.
Embodiment 4:
Take a coinage blank of low-carbon steel as a substrate, thereon electroplate
a first
layer, a second layer, a third layer and a surface layer in sequence, to
obtain a product. The
specific steps are as follows:
(1) Alkaline oil removal
The coinage blank is placed in an alkaline oil remover with concentration of
50 g/L,
and is cleaned for 20 min at a temperature of 55 C, and then rinsed with
deionized water at
21
CA 02883815 2015-03-03
60 C.
(2) Electrolytic oil removal
The alkaline washed coinage blank is placed in an electrolytic oil remover
with
concentration of 60 g/L, and is subjected to anode electrolytic cleaning for
20 min at a
temperature of 55 C and with current density of 0.5 A/dm2, and then rinsed
with deionized
water at 60 C.
(3) Hydrochloric acid activation
The coinage blank after electrolytic oil removal is placed in an HC1 solution
with
concentration of 350 ml/L, and is subjected to acid activation for 7 mm at a
temperature of
20 C, and then rinsed with deionized water at room temperature.
(4) Electroplating a first layer
The activated coinage blank is placed in a first layer electroplating liquid
with a pH
value of 8.0, and is electroplated a first layer at a temperature of 20 C,
wherein the current
density is 0.5 A/dm2, and the electroplating time is 30 min. The first layer
electroplating liquid
consists of the following components: potassium pyrophosphate, 250 g/L; copper
pyrophosphate, 20 g/L; stannous pyrophosphate, 0.2 g/L; cyanide-free alkaline
copper additive,
ml/L; and the first layer has a thickness of about 1 to 2 micrometers.
(5) Water washing
The first layer electroplated coinage blank is placed in deionized water, and
rinsed with
deionized water at room temperature.
(6) Plating a second layer
After rinsing in deionized water, the coinage blank is placed in a second
layer
electroplating liquid with a pH value of 8.0, and is electroplated a second
layer at a temperature
22
CA 02883815 2015-03-03
of 20 C, wherein the current density is 0.5 A/din2, and the electroplating
time is 270 min. The
second layer electroplating liquid consists of the following components:
potassium
pyrophosphate, 350 g/L; copper pyrophosphate, 20 g/L; stannous pyrophosphate,
1.8 g/L;
cyanide-free brass-tin major brightening agent, 3 ml/L; cyanide-free brass-tin
adjuvant, 10 ml/L:
and the second layer has a thickness of about 10 to 12 micrometers and a tin
content of 14% to
18%.
(7) Water washing and drying
The second layer electroplated coinage blank is placed in deionized water, and
rinsed
with deionized water at room temperature, and then the coinage blank is dried.
(8) Electroplating a third layer
The water washed coinage blank is placed in a third layer electroplating
liquid with a
pH value of 8.0, and is electroplated a third layer at a temperature of 20 C,
wherein the current
density is 0.5 A/dm2, and the electroplating time is 90 min. The third layer
electroplating liquid
consists of the following components: potassium pyrophosphate, 250 g/L; copper
pyrophosphate, 20 g/L; stannous pyrophosphate, 0.2 g/L; cyanide-free alkaline
copper additive,
15 ml/L; and the third layer has a thickness of about 3 to 5 micrometers.
(9) Water washing
The third layer electroplated coinage blank is placed in deionized water, and
rinsed
with deionized water at room temperature.
(10) Electroplating a surface layer
After rinsing in deionized water, the coinage blank is placed in a surface
layer
electroplating liquid with a pH value of 8.0, and is electroplated a surface
layer at a temperature
of 20 C, wherein the current density is 0.5 A/dm2, and the electroplating time
is 270 min. The
second layer electroplating liquid consists of the following components:
potassium
23
CA 02883815 2015-03-03
pyrophosphate, 350 g/L; copper pyrophosphate, 20 g/L; stannous pyrophosphate,
1.8 g/L;
cyanide-free brass-tin major brightening agent, 10 ml/L; cyanide-free brass-
tin adjuvant, 30
ml/L; and the surface layer has a thickness of about 10 to 12 micrometers and
a tin content of
14% to 18%.
(11) Water washing and drying
The surface layer electroplated coinage blank is placed in deionized water,
and rinsed
with deionized water at room temperature, and then the coinage blank is dried.
(12) High-temperature heat treatment
The dried coinage blank is placed in a high-temperature heat treatment furnace
being
flowed with reduced protective atmosphere, and is subjected to heat treatment
for 7 min at
650 C and then for 7 min at 680 C. After the heat treatment, the plating of
the product is
diffused into one layer having a tin content of 11% to 14% and a plating
thickness of 25 to 31
micrometers.
Embodiment 5:
Take a coinage blank of low-carbon steel as a substrate, thereon electroplate
a first
layer, a second layer, a third layer and a surface layer in sequence, to
obtain a product. The
specific steps are as follows:
(1) Alkaline oil removal
The coinage blank is placed in an alkaline oil remover with concentration of
60 g/L,
and is cleaned for 20 min at a temperature of 60 C, and then rinsed with
deionized water at
60 C.
(2) Electrolytic oil removal
The alkaline washed coinage blank is placed in an electrolytic oil remover
with
24
CA 02883815 2015-03-03
concentration of 70 g/L, and is subjected to anode electrolytic cleaning for
20 min at a
temperature of 60 C and with current density of 1.0 A/dm2, and then rinsed
with deionized
water at 60 C.
(3) Hydrochloric acid activation
The coinage blank after electrolytic oil removal is placed in an HC1 solution
with
concentration of 400 ml/L, and is subjected to acid activation for 7 min at a
temperature of
25 C, and then rinsed with deionized water at room temperature.
(4) Electroplating a first layer
The activated coinage blank is placed in a first layer electroplating liquid
with a pH
value of 9.0, and is electroplated a first layer at a temperature of 25 C,
wherein the current
density is 1.0 A/dm2, and the electroplating time is 30 min. The first layer
electroplating liquid
consists of the following components: potassium pyrophosphate, 300 g/L; copper
pyrophosphate, 25 g/L; stannous pyrophosphate, 0.3 g/L; cyanide-free alkaline
copper additive,
20 ml/L; and the first layer has a thickness of about 1 to 2 micrometers.
(5) Water washing
The first layer electroplated coinage blank is placed in deionized water, and
rinsed with
deionized water at room temperature.
(6) Electroplating a second layer
After rinsing in deionized water, the coinage blank is placed in a second
layer
electroplating liquid with a pH value of 9.0, and is electroplated a second
layer at a temperature
of 25 C, wherein the current density is 1.2 A/dm2, and the electroplating time
is 270 min. The
second layer electroplating liquid consists of the following components:
potassium
pyrophosphate, 400 g/L; copper pyrophosphate, 25 g/L; stannous pyrophosphate,
2.2 g/L;
cyanide-free brass-tin major brightening agent, 20 ml/L; cyanide-free brass-
tin adjuvant, 50
CA 02883815 2015-03-03
ml/L; and the second layer has a thickness of about 10 to 12 micrometers and a
tin content of
14% to 18%.
(7) Water washing and drying
The second layer electroplated coinage blank is placed in deionized water, and
rinsed
with deionized water at room temperature, and then the coinage blank is dried.
(8) Electroplating a third layer
The water washed coinage blank is placed in a third layer electroplating
liquid with a
pH value of 9.0, and is electroplated a third layer at a temperature of 25 C,
wherein the current
density is 1.0 A/dm2, and the electroplating time is 90 mm. The third layer
electroplating liquid
consists of the following components: potassium pyrophosphate, 300 g/L; copper
pyrophosphate, 25 g/L; stannous pyrophosphate, 0.3 g/L; cyanide-free alkaline
copper additive,
18 ml/L; and the third layer has a thickness of about 3 to 5 micrometers.
(9) Water washing
The third layer electroplated coinage blank is placed in deionized water, and
rinsed
with deionized water at room temperature.
(10) Electroplating a surface layer
After rinsing in deionized water, the coinage blank is placed in a surface
layer
electroplating liquid with a pH value of 9.0, and is electroplated a surface
layer at a temperature
of 25 C, wherein the current density is 1.0 A/dm2, and the electroplating time
is 270 min. The
surface layer electroplating liquid consists of the following components:
potassium
pyrophosphate, 400 g/L; copper pyrophosphate, 25 g/L; stannous pyrophosphate,
2.2 g/L;
cyanide-free brass-tin major brightening agent, 18 ml/L; cyanide-free brass-
tin adjuvant, 40
ml/L; and the surface layer has a thickness of about 10 to 12 micrometers and
a tin content of
14% to 18%.
26
CA 02883815 2015-03-03
(11) Water washing and drying
The surface layer electroplated coinage blank is placed in deionized water,
and rinsed
with deionized water at room temperature, and then the coinage blank is dried.
(12) High-temperature heat treatment
The dried coinage blank is placed in a high-temperature heat treatment furnace
being
flowed with reduced protective atmosphere, and is subjected to heat treatment
for 7 min at
650 C and then for 7 min at 680 C. After the heat treatment, the plating of
the product is
diffused into one layer having a tin content of 11% to 14% and a plating
thickness of 25 to 31
micrometers.
Embodiment 6:
Take a coinage blank of low-carbon steel as a substrate, thereon electroplate
a first
layer, a second layer, a third layer and a surface layer in sequence, to
obtain a product. The
specific steps are as follows:
(1) Alkaline oil removal
The coinage blank is placed in an alkaline oil remover with concentration of
70 g/L,
and is cleaned for 20 min at a temperature of 65 C, and then rinsed with
deionized water at
60 C.
(2) Electrolytic oil removal
The alkaline washed coinage blank is placed in an electrolytic oil remover
with
concentration of 80 g/L, and is subjected to anode electrolytic cleaning for
20 min at a
temperature of 65 C and with current density of 1.3 A/dm2, and then rinsed
with deionized
water at 60 C.
(3) Hydrochloric acid activation
27
CA 02883815 2015-03-03
The coinage blank after electrolytic oil removal is placed in an HC1 solution
with
concentration of 500 ml/L, and is subjected to acid activation for 7 min at a
temperature of
30 C, and then rinsed with deionized water at room temperature.
(4) Electroplating a first layer
The activated coinage blank is placed in a first layer electroplating liquid
with a pH
value of 9.8, and is electroplated a first layer at a temperature of 30 C,
wherein the current
density is 1.5A/dm2, and the electroplating time is 30 min. The first layer
electroplating liquid
consists of the following components: potassium pyrophosphate, 370 g/L; copper
pyrophosphate, 30 g/L; stannous pyrophosphate, 0.4 g/L; cyanide-free alkaline
copper additive,
15 ml/L; and the first layer has a thickness of about 1 to 2 micrometers.
(5) Water washing
The first layer electroplated coinage blank is placed in deionized water, and
rinsed with
deionized water at room temperature.
(6) Electroplating a second layer
After rinsing in deionized water, the coinage blank is placed in a second
layer
electroplating liquid with a pH value of 10.0, and is electroplated a second
layer at a
temperature of 28 C, wherein the current density is 1.8 A/dm2, and the
electroplating time is
270 min. The second layer electroplating liquid consists of the following
components:
potassium pyrophosphate, 450 g/L; copper pyrophosphate, 35 g/L; stannous
pyrophosphate, 3.0
g/L; cyanide-free brass-tin major brightening agent, 10 ml/L; cyanide-free
brass-tin adjuvant,
30 ml/L; and the second layer has a thickness of about 10 to 12 micrometers
and a tin content of
14% to 18%.
(7) Water washing and drying
The second layer electroplated coinage blank is placed in deionized water, and
rinsed
28
CA 02883815 2015-03-03
with deionized water at room temperature, and then the coinage blank is dried.
(8) Electroplating a third layer
The water washed coinage blank is placed in a third layer electroplating
liquid with a
pH value of 10.0, and is electroplated a third layer at a temperature of 28 C,
wherein the current
density is 1.5 A/dm2, and the electroplating time is 90 min. The third layer
electroplating liquid
consists of the following components: potassium pyrophosphate, 370 g/L; copper
pyrophosphate, 30 g/L; stannous pyrophosphate, 0.5 g/L; cyanide-free alkaline
copper additive,
12 ml/L; and the third layer has a thickness of about 3 to 5 micrometers.
(9) Water washing
The third layer electroplated coinage blank is placed in deionized water, and
rinsed
with deionized water at room temperature.
(10) Electroplating a surface layer
After rinsing in deionized water, the coinage blank is placed in a surface
layer
electroplating liquid with a pH value of 10.0, and is electroplated a surface
layer at a
temperature of 30 C, wherein the current density is 1.8 A/dm2, and the
electroplating time is
270 min. The second layer electroplating liquid consists of the following
components:
potassium pyrophosphate, 450 g/L; copper pyrophosphate, 32 g/L; stannous
pyrophosphate, 2.8
g/L; cyanide-free brass-tin major brightening agent, 18 ml/L; cyanide-free
brass-tin adjuvant,
40 ml/L; and the surface layer has a thickness of about 10 to 12 micrometers
and a tin content
of 14% to 18%.
(11) Water washing and drying
The surface layer electroplated coinage blank is placed in deionized water,
and rinsed
with deionized water at room temperature, and then the coinage blank is dried.
(12) High-temperature heat treatment
29
CA 02883815 2015-03-03
The dried coinage blank is placed in a high-temperature heat treatment furnace
being
flowed with reduced protective atmosphere, and is subjected to heat treatment
for 7 min at
650 C and then for 7 min at 680 C. After the heat treatment, the plating of
the product is
diffused into one layer having a tin content of 11% to 14% and a plating
thickness of 25 to 31
micrometers.
Influence of the Mirapol WT brightening agent A (additive A) and the
brightening
agent B (2-mercapto benzimidazole, i.e., additive B) in the cyanide-free brass-
tin major
brightening agent as well as the cyanide-free brass-tin adjuvant on the
electrodeposition of
copper-tin alloy:
(I) Tests of individual brightening agent A, individual brightening agent B,
and
synergistic effect of both the brightening agent A and the brightening agent B
with test results
as shown below:
1. Brightening agent A (WT) The additive A may effectively improve the burning
in
the high-current density zone and increase the brightness of the plating, and
due to the addition
of A, the electrodeposition characteristic of the alloy electroplating liquid
is changed from
electrochemical step control into diffusion step control. Therefore, in the
high-current density
zone, better electrodeposition can be achieved merely by reducing the
concentration
polarization of metal ions. In the meanwhile, due to the diffusion and
adsorption and inhibition
of the additive A on the electrode surface, the cathode polarization is
increased, so that the alloy
plating crystal is bright and unifoim in appearance. As the plating liquid has
a composition of
Cu2P207.3H20, 25 g/L; Sn2P207, 3.0 g/L; 1(413207.3H20, 450 g/L; K2HPO4-3H20,
60 g/L, with
a pH value of 8.5 and a temperature of 25 C, and as the brightening agent A is
of 0.1 g/L, the
appearance of a Hull cell under different current is as shown in FIG. 2, which
is also shown to
the influence of the additive A on the appearance of a Hull cell test piece.
It can be known from FIG. 2 that the addition of the brightening agent A can
effectively
CA 02883815 2015-03-03
improve the burning in the high-current density zone and has a certain effect
on alloy
co-deposition in the low-current density zone, so that the appearance of Hull
cell test piece is
brighter, and the golden yellow alloy co-deposition area is increased.
However, with the
increase the current, the burning range in the high-current density zone gets
bigger and bigger,
indicating that the operational range of the plating current is narrow.
2. Brightening agent B (2-mercapto benzimidazole) The brightening agent B is
taken
as a grain refiner for the electrodeposition of copper ion, which enhances the
cathode
polarization of copper ions in the low-current density zone, so that the
crystal of metal copper
gets finer, and at the same time, the precipitation of copper is inhibited,
thereby increasing the
tin content in the plating of the low-current density zone. As the plating
liquid has a
composition of: Cu2P207=3H20, 25 g/L; Sn2P207, 3.0 g/L; K4P207=3H20, 450 g/L;
K2HPO4.3H20, 60 g/L; with a pH value of 8.5, a temperature of 25 C, and the
brightening
agent B of 0.0015 g/L, the appearance of a Hull cell under different currents
is as shown in FIG
3, which is also shown to the influence of the brightening agent B on the
appearance of a Hull
cell test piece.
It can be known from FIG. 3 that the addition of the brightening agent B can
effectively
improve the co-deposition in low-current density zone, so that the appearance
of the Hull cell in
the low-current density zone is changed from pink into golden yellow. With the
increase of
the current, the range of the pink plating in the low-current density zone
gets smaller and
smaller. However, with the increase of the current, the burning range in the
high-current density
zone gets bigger and bigger, indicating that the operational range of the
plating current is
narrow.
3. Synergistic effect of the brightening agent A and the brightening agent B:
As the plating liquid has a composition of Cu2P207=3H20, 25 g/L; Sn2P207, 3.0
g/L;
K4P207.3H20, 450 g/L; K2HPO4.3H20, 60 g/L, with a pH value of 8.5, and with
the
31
CA 02883815 2015-03-03
brightening agent A being 0.1 g/L and the brightening agent B being 0.0015
g/L, at a
temperature of 25 C, the appearance of a Hull cell under different currents is
as shown in FIG. 4,
which is also shown to the appearance of a Hull cell test piece under
different currents after the
addition of the additives.
It can be known from FIG. 4 that in the plating liquid having the brightening
agent A
and the brightening agent B simultaneously, as the current is 0.3 A, no
burning phenomenon
occurs in the high-current density zone, and the range of pink plating in the
low-current density
zone is very narrow. As the current is 0.5 A, the entire Hull cell test piece
is golden yellow. With
the increase of the current, the burning in the high-current density zone
occurs, while the range
is narrow. Therefore, As the brightening agent A and the brightening agent B
simultaneously
exist in the plating liquid, synergistic effect is generated, so that not only
the burning
phenomenon in the high-current density zone is effective solved, but also the
occurrence of pink
plating in the low-current density zone is eliminated.
(II) Tests of individual auxiliary complexing agent A, individual auxiliary
complexing
agent B, and synergistic effect of the auxiliary complexing agent A and the
auxiliary
complexing agent B with test results being shown below:
1. Auxiliary complexing agent A (glycolic acid) An auxiliary complexing agent
for tin
ion can enhance the complexing of tin ion, eliminate the generation of
monovalent copper
through reaction of free bivalent tin and copper ions, and at the same time,
can effectively
inhibit the oxidation of tin ion. As the plating liquid has a composition of
Cu2P207.3H20, 25
g/L; Sn2P207, 3.0 g/L; K4P207.3H20, 450 g/L; K2HPO4.3H20, 60 g/L, with a pH
value of 8.5,
and a temperature of 25 C; and the auxiliary complexing agent A being 0.3 g/L,
the plating
components under different current densities are as shown in FIG. 5, which is
also shown to the
influence of the auxiliary complexing agent A on the plating components.
In FIG. 5, the horizontal axis represents the current density, and the
vertical axis
32
CA 02883815 2015-03-03
represents the mass percentage of tin in the plating. The curve B shows the
plating components
of plating liquids under different current densities without the addition of
auxiliary complexing
agent A, and the curve C shows the plating components of plating liquids under
different
current densities with the addition the auxiliary complexing agent A. It can
be known from FIG
that, with the increase of the current density, the tin content in the plating
is gradually
increased; as the current density is 0.1 A/dm2, the tin content in the plating
is 12.45%; and as
the current density is 2 A/dm2, the tin content in the plating can be up to
15.67%. With the
addition of the auxiliary complexing agent A, it has a small influence on the
tin content in the
plating in the low-current density zone; while in the high-current density
zone, the precipitation
of tin is effectively inhibited, and as the current density is 2 A/dm2, the
tin content in the plating
is 14.73%, which indicates a drop of 0.94%. It can be seen that, the addition
of the auxiliary
complexing agent A can effectively inhibit the difference in tin content in
the plating under
different current densities, thereby increasing the uniformity of the plating.
2. Auxiliary complexing, agent B (sodium gluconate) An auxiliary complexing
agent
for copper ion in an alkaline solution system can enhance the complexing of
copper ion, and
have a synergistic effect with glycolic acid, thereby greatly improving the
stability of the
plating liquid. As the plating liquid has a composition of Cu2P207.3H20, 25
g/L; Sn2P207, 3.0
g/L; K413207.3H20, 450 g/L; K2HPO4-3H20, 60 g/L, with a pH value of 8.5, a
temperature of
25 C; and as the auxiliary complexing agent B is 0.3 g/L, the plating
components under
different current densities are as shown in FIG. 6, which is also shown to the
influence of the
auxiliary complexing agent B on the plating components.
In FIG. 6, the horizontal axis represents the current density, and the
vertical axis
represents the mass percentage of copper in the plating. The curve B shows the
plating
components of plating liquid under different current densities without the
addition of the
auxiliary complexing agent B, and the curve C shows the plating components of
plating liquid
33
CA 02883815 2015-03-03
under different current densities with the addition of the auxiliary
complexing agent B. It can be
known from FIG. 6 that, with the increase of the current density, the copper
content in the
plating is gradually increased; as the current density is 0.1 A/dm2, the
copper content in the
plating is 87.62%; and as the current density is 2 A/dm2, the copper content
in the plating is
decreased to 84.33%. With the addition of the auxiliary complexing agent B, it
has a small
influence on the copper content in the plating in the high-current density
zone; while in the
low-current density zone, the precipitation of copper is effectively
inhibited, and as the current
density is 0.1 A/dm2, the copper content in the plating is 86.21%, which
indicates a drop of
1.41%. It can be seen that, the addition of the auxiliary complexing agent B
can effectively
inhibit the difference in copper content in the plating under different
current densities, thereby
increasing the uniformity of the plating.
3. Synergistic effect of the auxiliary complexing agent A and the auxiliary
complexing
agent B:
As the plating liquid has a composition of Cu2P207-3H20, 25 g/L; Sn2P207, 3.0
g/L;
K4P207=3H20, 450 g/L; K2HPO4.3H20, 60 g/L, with a pH value of 8.5 and a
temperature of
25 C; and as the auxiliary complexing agent A is 0.3 g/L and the auxiliary
complexing agent B
is 0.3 g/L, the plating components under different current densities are as
shown in FIG 7,
which is also shown to the influence of addition of auxiliary complexing
agents on the plating
components.
In FIG 7, the horizontal axis represents the current density, and the vertical
axis
represents the mass percentage of copper in the plating. The curve B shows the
plating
components of plating liquid under different current densities without the
addition of the
auxiliary complexing agents, and the curve C shows the plating components of
plating liquid
under different current densities with the addition of the auxiliary
complexing agent of 0.3 g/L
and the auxiliary complexing agent B of 0.3 g/L. It can be known from FIG 7
that, in the case
34
CA 02883815 2015-03-03
that the auxiliary complexing agent A and the auxiliary complexing agent B
exist
simultaneously, and in the low-current density zone, the auxiliary complexing
agent A
effectively inhibits the precipitation of copper, thereby decreasing the mass
percentage of
copper in the plating; while in the high-current density zone, the auxiliary
complexing agent B
effectively inhibits the precipitation of tin, thereby decreasing the mass
percentage of tin in the
plating, and increasing the mass percentage of copper. The auxiliary
complexing agent A and
the auxiliary complexing agent B in the plating liquid inhibit the
electrodeposition of copper
and tin in zones of different current densities and generate the synergistic
effect, so that the
copper-tin alloy plating can maintain relatively stable plating components
under different
current densities.
The above descriptions are merely preferred embodiments of the present
invention, but
not any limitations in the form and substance on the present invention. It
should be noted that,
those of ordinary skill in the art can further make a number of improvements
and supplements
without departing from the method of the present invention, and these
improvements and
supplements should also be considered as falling within the protection scope
of the present
invention. Various alternations, modifications, evolutions and equivalent
changes made by those
skilled in the art based on the technical contents disclosed above without
departing from the
spirit and scope of the present invention are equivalent embodiments of the
present invention;
simultaneously, any equivalent alternations, modifications and evolutions made
on the
embodiments according to the technical essence of the present invention fall
within the scope of
the technical solutions of the present invention.