Note: Descriptions are shown in the official language in which they were submitted.
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
BIOMASS CONVERSION SYSTEM HAVING A SINGLE-VESSEL
HYDROTHERMAL DIGESTION UNIT AND A CATALYTIC REDUCTION
REACTOR UNIT FOR INTEGRATED STABILIZATION OF A HYDROLYSATE
AND METHOD FOR USE THEREOF
Field of the Invention
The present disclosure generally relates to digestion of cellulosic biomass
solids,
and, more specifically, to biomass conversion systems and methods for use
thereof that
allow a hydrolysate comprising soluble carbohydrates to be catalytically
transformed into a
more stable reaction product using a pressurized vessel that contains a
fluidly coupled
hydrothermal digestion unit and a catalytic reduction reactor unit.
Background of the Invention
A number of substances of commercial significance may be produced from natural
sources, particularly biomass. Cellulosic biomass may be particularly
advantageous in this
regard due to the versatility of the abundant carbohydrates found therein in
various forms.
As used herein, the term "cellulosic biomass" refers to a living or recently
living biological
material that contains cellulose. The lignocellulosic material found in the
cell walls of
higher plants is the world's most abundant source of carbohydrates. Materials
commonly
produced from cellulosic biomass may include, for example, paper and pulpwood
via
partial digestion, and bioethanol by fermentation.
Plant cell walls are divided into two sections: primary cell walls and
secondary cell
walls. The primary cell wall provides structural support for expanding cells
and contains
three major polysaccharides (cellulose, pectin, and hemicellulose) and one
group of
glycoproteins. The secondary cell wall, which is produced after the cell has
finished
growing, also contains polysaccharides and is strengthened through polymeric
lignin that is
covalently crosslinked to hemicellulose. Hemicellulose and pectin are
typically found in
abundance, but cellulose is the predominant polysaccharide and the most
abundant source
of carbohydrates. The complex mixture of constituents that is co-present with
the cellulose
can make its processing difficult, as discussed hereinafter.
Significant attention has been placed on developing fossil fuel alternatives
derived
from renewable resources. Cellulosic biomass has garnered particular attention
in this
regard due to its abundance and the versatility of the various components
found therein,
particularly cellulose and other carbohydrates. Despite promise and intense
interest, the
development and implementation of bio-based fuel technology has been slow.
Existing
1
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
technologies have heretofore produced fuels having a low energy density (e.g.,
bioethanol)
and/or that are not fully compatible with existing engine designs and
transportation
infrastructure (e.g., methanol, biodiesel, Fischer-Tropsch diesel, hydrogen,
and methane).
Energy- and cost-efficient processes for processing cellulosic biomass into
fuel blends
having similar compositions to fossil fuels would be highly desirable to
address the
foregoing issues and others.
When converting cellulosic biomass into fuel blends and other materials,
cellulose
and other complex carbohydrates therein can be extracted and transformed into
simpler
organic molecules, which can be further reformed thereafter. Fermentation is
one process
whereby complex carbohydrates from cellulosic biomass may be converted into a
more
usable form. However, fermentation processes are typically slow, require large
volume
reactors and high dilution conditions, and produce an initial reaction product
having a low
energy density (ethanol). Digestion is another way in which cellulose and
other complex
carbohydrates may be converted into a more usable form. Digestion processes
can break
down cellulose and other complex carbohydrates within cellulosic biomass into
simpler,
soluble carbohydrates that are suitable for further transformation through
downstream
reforming reactions. As used herein, the term "soluble carbohydrates" refers
to
monosaccharides or polysaccharides that become solubilized in a digestion
process.
Although the underlying chemistry is understood behind digesting cellulose and
other
complex carbohydrates and further transforming simple carbohydrates into
organic
compounds reminiscent of those present in fossil fuels, high-yield and energy-
efficient
digestion processes suitable for converting cellulosic biomass into fuel
blends have yet to
be developed. In this regard, the most basic requirement associated with
converting
cellulosic biomass into fuel blends using digestion and other processes is
that the energy
input needed to bring about the conversion should not be greater than the
available energy
output of the product fuel blends. This basic requirement leads to a number of
secondary
issues that collectively present an immense engineering challenge that has not
been solved
heretofore.
The issues associated with converting cellulosic biomass into fuel blends in
an
energy- and cost-efficient manner using digestion are not only complex, but
they are
entirely different than those that are encountered in the digestion processes
commonly used
in the paper and pulpwood industry. Since the intent of cellulosic biomass
digestion in the
paper and pulpwood industry is to retain a solid material (e.g., wood pulp),
incomplete
2
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
digestion is usually performed at low temperatures (e.g., less than about 100
C) for a fairly
short period of time. In contrast, digestion processes suitable for converting
cellulosic
biomass into fuel blends and other materials are ideally configured to
maximize yields by
solubilizing as much of the original cellulosic biomass charge as possible in
a high-
throughput manner.
Production of soluble carbohydrates for use in fuel blends and other materials
via
routine modification of paper and pulpwood digestion processes is not believed
to be
economically feasible for a number of reasons. Simply running the digestion
processes of
the paper and pulpwood industry for a longer period of time to produce more
soluble
carbohydrates is undesirable from a throughput standpoint. Use of digestion
promoters
such as strong alkalis, strong acids, or sulfites to accelerate the digestion
rate can increase
process costs and complexity due to post-processing separation steps and the
possible need
to protect downstream components from these agents. Accelerating the digestion
rate by
increasing the digestion temperature can actually reduce yields due to thermal
degradation
of soluble carbohydrates that can occur at elevated digestion temperatures,
particularly
over extended periods of time. Once produced by digestion, soluble
carbohydrates are
very reactive and can rapidly degrade to produce caramelans and other heavy
ends
degradation products, especially under higher temperature conditions, such as
above
150 C. Use of higher digestion temperatures can also be undesirable from an
energy
efficiency standpoint. Any of these difficulties can defeat the economic
viability of fuel
blends derived from cellulosic biomass.
One way in which soluble carbohydrates can be protected from thermal
degradation
is through subjecting them to one or more catalytic reduction reactions, which
may include
hydrogenation and/or hydrogenolysis reactions. Stabilizing soluble
carbohydrates through
conducting one or more catalytic reduction reactions may allow digestion of
cellulosic
biomass to take place at higher temperatures than would otherwise be possible
without
unduly sacrificing yields. Depending on the reaction conditions and catalyst
used, reaction
products formed as a result of conducting one or more catalytic reduction
reactions on
soluble carbohydrates may include triols, diols, monohydric alcohols, or any
combination
thereof, some of which may also include a residual carbonyl functionality
(e.g., an
aldehyde or ketone). Such reaction products may be more thermally stable than
soluble
carbohydrates and are readily transformable into fuel blends and other
materials through
conducting one or more downstream reforming reactions. In addition, the
foregoing types
3
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
of reaction products are good solvents in which a hydrothermal digestion may
be
performed, thereby promoting solubilzation of soluble carbohydrates as their
reaction
products and cellulosic biomass components such as lignin, for example.
Another issue associated with the processing of cellulosic biomass into fuel
blends
and other materials is created by the need for high conversion percentages of
a cellulosic
biomass charge into soluble carbohydrates. Specifically, as cellulosic biomass
solids are
digested, their size gradually decreases to the point that they can become
fluidly mobile.
As used herein, cellulosic biomass solids that are fluidly mobile,
particularly cellulosic
biomass solids that are about 3 mm in size or less, will be referred to as
"cellulosic biomass
fines." Cellulosic biomass fines can be transported out of a digestion zone of
a system for
converting cellulosic biomass and into one or more zones where solids are
unwanted and
can be detrimental. For example, cellulosic biomass fines have the potential
to plug
catalyst beds, transfer lines, and the like. Furthermore, although small in
size, cellulosic
biomass fines may represent a non-trivial fraction of the cellulosic biomass
charge, and if
they are not further converted into soluble carbohydrates, the ability to
attain a satisfactory
conversion percentage may be impacted. Since the digestion processes of the
paper and
pulpwood industry are run at relatively low cellulosic biomass conversion
percentages,
smaller amounts of cellulosic biomass fines are believed to be generated and
have a lesser
impact on those digestion processes.
In addition to the desired carbohydrates, other materials may be present
within
cellulosic biomass that can be especially problematic to deal with in an
energy- and cost-
efficient manner. Sulfur- and/or nitrogen-containing amino acids or other
catalyst poisons
may be present in cellulosic biomass. If not removed, these catalyst poisons
can impact the
catalytic reduction reaction(s) used to stabilize soluble carbohydrates,
thereby resulting in
process downtime for catalyst regeneration and/or replacement and reducing the
overall
energy efficiency when restarting the process. On the other hand, in-process
removal of
these catalyst poisons can also impact the energy efficiency of the biomass
conversion
process, since the ion-exchange processes typically needed to affect their
removal are
usually conducted at temperatures below those at which soluble carbohydrates
are
produced by digestion, thereby introducing heat exchange operations that add
to design
complexity and may increase operational costs. In addition to catalyst
poisons, lignin,
which is a non-cellulosic biopolymer, may become solubilized in conjunction
with the
production of soluble carbohydrates.
If not addressed in some manner, lignin
4
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
concentrations may become sufficiently high during biomass conversion that
precipitation
eventually occurs, thereby resulting in costly system downtime. In the
alternative, some
lignin may remain unsolubilized, and costly system downtime may eventually be
needed to
affect its removal.
As evidenced by the foregoing, the efficient conversion of cellulosic biomass
into
fuel blends is a complex problem that presents immense engineering challenges.
The
present disclosure addresses these challenges and provides related advantages
as well.
Summary of the Invention
The present disclosure generally relates to digestion of cellulosic biomass
solids,
and, more specifically, to biomass conversion systems and methods for use
thereof that
allow a hydrolysate comprising soluble carbohydrates to be catalytically
transformed into a
more stable reaction product using a pressurized vessel that contains a
fluidly coupled
hydrothermal digestion unit and a catalytic reduction reactor unit.
In some embodiments, the present invention provides biomass conversion systems
comprising: a pressure vessel comprising a first section and a second section,
the first
section comprising a hydrothermal digestion unit and the second section
comprising a first
catalytic reduction reactor unit that contains a first catalyst capable of
activating molecular
hydrogen; wherein the hydrothermal digestion unit and the first catalytic
reduction reactor
unit are in fluid communication with one another; a biomass feed mechanism
that is
operatively connected to the pressure vessel, the biomass feed mechanism being
capable of
introducing cellulosic biomass solids to the pressure vessel and also capable
of
withdrawing a reaction product from the first catalytic reduction reactor
unit; and a
hydrogen feed line that is operatively connected to the first catalytic
reduction reactor unit.
In some embodiments, the present invention provides biomass conversion systems
comprising: a pressure vessel comprising a first section and a second section,
the first
section comprising a hydrothermal digestion unit and the second section
comprising a first
catalytic reduction reactor unit that contains a first catalyst capable of
activating molecular
hydrogen; wherein the hydrothermal digestion unit and the first catalytic
reduction reactor
unit are in fluid communication with one another; a biomass feed mechanism
that is
operatively connected to the pressure vessel, the biomass feed mechanism being
capable of
introducing cellulosic biomass solids to the pressure vessel while the
pressure vessel
maintains a pressurized state; a hydrogen feed line that is operatively
connected to the first
catalytic reduction reactor unit; and a fluid circulation loop comprising the
pressure vessel
5
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
and a second catalytic reduction reactor unit that contains a second catalyst
capable of
activating molecular hydrogen.
In some embodiments, the present invention provides methods comprising:
providing a pressure vessel comprising a first section and a second section,
the first section
comprising a hydrothermal digestion unit and the second section comprising a
first
catalytic reduction reactor unit that contains a first catalyst capable of
activating molecular
hydrogen (first hydrocatalytic catalyst); wherein the hydrothermal digestion
unit and the
first catalytic reduction reactor unit are in fluid communication with one
another; adding
cellulosic biomass solids to the pressure vessel; heating the cellulosic
biomass solids in the
hydrothermal digestion unit of the pressure vessel, thereby forming a
hydrolysate
comprising soluble carbohydrates within a liquor phase; conveying the liquor
phase
through the first catalytic reduction reactor unit in the presence of
molecular hydrogen so
as to at least partially transform the soluble carbohydrates into a reaction
product; and
conveying at least a portion of the liquor phase from the pressure vessel to a
second
catalytic reduction reactor unit that contains a second catalyst capable of
activating
molecular hydrogen (second hydrocatalytic catalyst), so as to further
transform the soluble
carbohydrates into the reaction product.
The features and advantages of the present disclosure will be readily apparent
to
one having ordinary skill in the art upon a reading of the description of the
preferred
embodiments that follows.
Brief Description of the Drawings
The following figures are included to illustrate certain aspects of the
present
disclosure, and should not be viewed as exclusive embodiments. The subject
matter
disclosed is capable of considerable modifications, alterations, combinations,
and
equivalents in form and function, as will occur to one having ordinary in the
art and the
benefit of this disclosure.
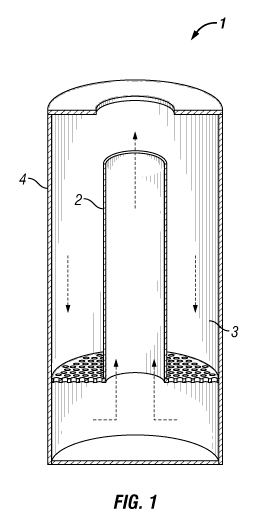
FIGURE 1 shows a schematic of an illustrative annular pressure vessel
containing
an inner catalytic reduction reactor unit and an outer hydrothermal digestion
unit housed
within an outer pressure housing.
FIGURE 2 shows a schematic of an illustrative pressure vessel in which a
hydrothermal digestion unit and a catalytic reduction reactor unit are located
alongside one
another within an outer pressure housing.
FIGURE 3 shows a schematic of an illustrative biomass conversion system having
6
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
a hydrothermal digestion unit and a catalytic reduction reactor unit housed
within a
pressure vessel.
FIGURE 4 shows a schematic of an illustrative biomass conversion system having
a hydrothermal digestion unit and a catalytic reduction reactor unit housed
within a
pressure vessel in which a fluid circulation loop establishes direct fluid
communication
between a fluid inlet and a fluid outlet of the pressure vessel.
Detailed Description
The present disclosure generally relates to digestion of cellulosic biomass
solids,
and, more specifically, to biomass conversion systems and methods for use
thereof that
allow a hydrolysate comprising soluble carbohydrates to be catalytically
transformed into a
more stable reaction product using a pressurized vessel that contains a
fluidly coupled
hydrothermal digestion unit and a catalytic reduction reactor unit.
In the embodiments described herein, the digestion rate of cellulosic biomass
may
be accelerated in the presence of a digestion solvent at elevated temperatures
and pressures
that maintain the digestion solvent in a liquid state above its normal boiling
point. The
more rapid rate of digestion may be desirable from the standpoint of
throughput, but
soluble carbohydrates may be susceptible to degradation under these
conditions, as
discussed in more detail hereinafter. In various embodiments, the digestion
solvent may
contain an organic solvent, particularly an in situ-generated organic solvent,
which may
provide certain advantages, as described hereinafter.
The present disclosure provides systems and methods that allow cellulosic
biomass
solids to be efficiently digested to form soluble carbohydrates, which may
subsequently be
converted through one or more catalytic reduction reactions (e.g.,
hydrogenolysis and/or
hydrogenation) into more stable reaction products comprising oxygenated
intermediates
that may be further processed into higher hydrocarbons. The higher
hydrocarbons may be
useful in forming industrial chemicals and transportation fuels (i.e., a
biofuel), including,
for example, synthetic gasoline, diesel fuels, jet fuels, and the like. As
used herein, the
term "biofuel" will refer to any transportation fuel formed from a biological
source. Such
biofuels may be referred to herein as "fuel blends." In particular, the
systems and methods
described herein are configured such that cellulosic biomass solids can be
digested to
produce a hydrolysate that comprises soluble carbohydrates, where at least a
portion of the
soluble carbohydrates in the hydrolysate may be quickly transformed into a
more stable
reaction product following digestion through a catalytic reduction reaction
before
7
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
degradation has had an opportunity to take place. In the embodiments described
herein,
the foregoing is accomplished by fluidly coupling a hydrothermal digestion
unit and a
catalytic reduction reactor unit together in a pressure vessel. Various
configurations for the
fluidly coupled hydrothermal digestion unit and catalytic reduction reactor
unit are
possible, as described hereinafter. Several types of catalysts that are
capable of activating
molecular hydrogen (also referred to herein as "hydrogen-activating catalysts"
or
"hydrocatalytic catalyst") may be used to mediate the catalytic reduction
reaction.
Advantageously, the cellulosic biomass solids may be used to promote retention
of the
catalyst within the pressure vessel, as described hereinafter. Converting the
soluble
carbohydrates into a more stable reaction product nearer their point of origin
may reduce
the amount of thermal decomposition that occurs following hydrothermal
digestion,
thereby increasing yields of the desired reaction product and promoting high
biomass
conversion rates. Other advantages may also be realized by conducting
hydrothermal
digestion and catalytic reduction within a single pressure vessel, as
discussed hereinafter.
As used herein, the term "oxygenated intermediates" refers to alcohols,
polyols,
ketones, aldehydes, and mixtures thereof that are produced from a catalytic
reduction
reaction (e.g., hydrogenolysis and/or hydrogenation) of soluble carbohydrates.
As used
herein, the term "higher hydrocarbons" refers to hydrocarbons having an oxygen
to carbon
ratio less than that of at least one component of the biomass source from
which they are
produced. As used herein, the term "hydrocarbon" refers to an organic compound
comprising primarily hydrogen and carbon, although heteroatoms such as oxygen,
nitrogen, sulfur, and/or phosphorus may be present in some embodiments. Thus,
the term
"hydrocarbon" also encompasses heteroatom-substituted compounds containing
carbon,
hydrogen, and oxygen, for example.
When a digestion solvent is used at high temperatures and pressures in a
hydrothermal digestion, the digestion process may become fairly energy
intensive. If the
energy input requirements for the digestion process become too great, the
economic
feasibility of cellulosic biomass as a feedstock material may be jeopardized.
That is, if the
energy input needed to digest cellulosic biomass becomes too great, processing
costs may
become higher than the actual value of the product being generated. In order
to keep
processing costs low, the amount of externally added heat input to the
digestion process is
desirably kept as low as possible while achieving as high as possible
conversion of the
cellulosic biomass into soluble carbohydrates, which can subsequently be
transformed into
8
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
a more stable reaction product. Conversion of soluble carbohydrates into a
more stable
reaction product is described in more detail hereinafter.
In the embodiments described herein, the soluble carbohydrates may be at least
partially stabilized by a catalytic reduction reaction that takes place within
the same
pressure vessel in which hydrothermal digestion occurs. Once the soluble
carbohydrates
have been at least partially transformed into a more stable reaction product,
completion of
the conversion of the soluble carbohydrates into the reaction product may take
place in a
separate catalytic reduction reactor unit that is not contained within the
pressure vessel.
The described biomass conversion system features can allow a significant
quantity of the
initially solubilized carbohydrates to be converted into a form that is
suitable for
subsequent processing into a biofuel, while forming as small as possible an
amount of
caramelans and other decomposition products in or near the hydrothermal
digestion unit.
A number of advantages may be realized by conducting hydrothermal digestion
and
catalytic reduction within the same pressure vessel, as in the embodiments
described
herein. As discussed above, a leading advantage of the biomass conversion
systems
described herein is that the systems are configured to rapidly stabilize a
significant fraction
of the hydrolysate produced in the hydrothermal digestion unit. Stabilization
of the
hydrolysate may be accomplished by at least partially converting the soluble
carbohydrates
in the hydrolysate into a reaction product through a catalytic reduction
reaction that takes
place in a catalytic reduction reactor unit that is fluidly coupled to the
hydrothermal
digestion unit. By fluidly coupling the hydrothermal digestion unit and the
catalytic
reduction reactor unit together in a single pressure vessel, transit times of
the hydrolysate
to the catalytic reduction reactor unit may be lowered, thereby decreasing the
opportunity
for the soluble carbohydrates to degrade.
Another significant advantage of the presently described biomass conversion
systems is that conducting hydrothermal digestion and catalytic reduction in a
single
pressure vessel may allow excellent heat integration and heat management to be
realized.
As described hereinafter, hydrothermal digestion is an endothermic process,
whereas
catalytic reduction is an exothermic process. Since the two processes occur
within the
same pressure vessel in the biomass conversion systems described herein, the
excess heat
generated by the catalytic reduction reaction may be used to drive the
hydrothermal
digestion process. This can improve the overall energy efficiency of the
biomass
conversion process by limiting the amount of external energy needing to be
input to drive
9
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
the hydrothermal digestion.
As discussed above, the initial reaction product produced in the pressure
vessel may
be conveyed to a second catalytic reduction reactor unit for further
transformation into a
reaction product that is more amenable to being transformed into a biofuel or
other
material. The second catalytic reduction reactor unit in which the further
transformation
takes place may be separate from the pressure vessel in which the initial
catalytic reduction
reaction takes place. The further transformation that takes place may comprise
a further
reduction in the degree of oxidation of the initial reaction product, an
increased conversion
of soluble carbohydrates into oxygenated intermediates, or both. The reaction
product
obtained from the second catalytic reduction reactor unit may be recirculated
to the
pressure vessel, where it may serve as a digestion solvent, and/or it may be
withdrawn
from the second catalytic reduction reactor unit for subsequent conversion
into a biofuel or
other material. By at least partially transforming the soluble carbohydrates
into a reaction
product before the hydrolysate reaches the second catalytic reduction reactor
unit, demands
on the second catalytic reduction reactor unit may be lessened, and it may be
possible to
realize a higher conversion of soluble carbohydrates into the reaction
product. In addition,
it may be possible to use a smaller second catalytic reduction reactor unit
than would
otherwise be feasible, since at least a portion of the soluble carbohydrates
have already
been transformed prior to reaching the second catalytic reduction reactor
unit.
Furthermore, since significant heat integration efficiency may be realized by
conducting
hydrothermal digestion and the initial catalytic reduction reaction in the
same pressure
vessel, there may be a reduced need to recirculate the reaction product from
the second
catalytic reduction reactor unit to the pressure vessel in order to maintain
an energy
efficient process. Thus, lower reaction product recycle ratios may be used,
and a greater
fraction of the reaction product may be withdrawn for subsequent conversion
into a
biofuel. The foregoing factors may also reduce capital and operational costs
associated
with the biomass conversion systems.
In further regard to heat integration efficiency, the present biomass
conversion
systems may also be particularly advantageous, since the pressure vessel of
the systems
may be continuously maintained at elevated temperatures and pressures, in some
embodiments. Thus, hydrothermal digestion may take place continuously as long
as fresh
cellulosic biomass solids can be continuously or semi-continuously supplied to
the pressure
vessel without depressurization taking place. Without the ability to introduce
fresh
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
cellulosic biomass solids to the pressure vessel while maintaining a
pressurized state,
depressurization and cooling may take place during biomass addition,
significantly
reducing the energy- and cost-efficiency of the conversion process. As used
herein, the
term "continuous addition" and grammatical equivalents thereof will refer to a
process in
which biomass is added to a vessel in a substantially uninterrupted manner
without full
depressurization taking place. As used herein, the term "semi-continuous
addition" and
grammatical equivalents thereof will refer to a discontinuous, but as-needed,
addition of
biomass to a vessel without full depressurization taking place. A further
description of
biomass feed mechanisms that may supply biomass to a pressurized vessel are
described in
more detail below.
In the embodiments described herein, various types of fluidly mobile catalysts
that
are capable of activating molecular hydrogen may be used in the catalytic
reduction reactor
unit present within the pressure vessel. As described hereinafter, at least
some cellulosic
biomass solids may also be present in the catalytic reduction reactor unit of
the pressure
vessel. Therefore, catalysts that are susceptible to plugging in the presence
of solids, such
as fixed bed catalysts, for example, are not typically used in this location.
Illustrative types
of catalysts that may be used in the catalytic reduction reactor unit of the
pressure vessel
include, for example, slurry catalysts, ebullating bed catalysts, fluidized
bed catalysts, and
the like. The same catalyst or a different catalyst may be present in the
second catalytic
reduction reactor unit that is not located within the pressure vessel.
A common problem associated with the use of fluidly mobile catalysts is that
suitable containment mechanisms (e.g., catalyst screens, filters, and the
like) are often
needed in order to maintain the catalysts in a desired location. In the
embodiments
described herein, however, it has been discovered that cellulosic biomass
solids may be
advantageously used to help maintain the fluidly mobile catalyst within the
pressure vessel
and/or to provide a mechanism by which the catalyst may be easily returned to
the pressure
vessel during biomass addition thereto. Specifically, it has been discovered
that an
agglomeration of cellulosic biomass solids may effectively sequester catalyst
solids to limit
the free movement of the catalyst. Thus, not only are the cellulosic biomass
solids digested
in the pressure vessel, but they also may effectively serve as a catalyst
screen in the
pressure vessel and/or in a biomass feed mechanism used for introducing the
cellulosic
biomass solids to the pressure vessel. In some embodiments, the cellulosic
biomass solids
may be processed to a size that is more effective for screening the catalyst,
before being
11
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
added to the pressure vessel, if desired. Moreover, free movement of the
fluidly mobile
catalyst through the cellulosic biomass solids is advantageous to promote
distribution of
the catalyst, thereby leading to more ready stabilization of soluble
carbohydrates.
In a similar manner to that which occurs for catalyst sequestration, as
described
above, the biomass conversion systems described herein may aid in reducing the
amount of
cellulosic biomass fines transported from the pressure vessel to the second
catalytic
reduction reactor unit. The cellulosic biomass fines may be natively present
in the biomass
introduced to the pressure vessel or produced in the course of the biomass
undergoing
hydrothermal digestion. Thus, a fluidly mobile catalyst can be used in the
second catalytic
reduction reactor unit, in some embodiments, or a fixed bed catalyst can be
used in the
second catalytic reduction reactor unit, in other embodiments. Optionally, a
solids
separation mechanism may also be used to sequester any catalyst and/or
cellulosic biomass
fines in the liquor phase being conveyed to the second catalytic reduction
reactor unit in
order to confer additional protection thereto.
The biomass conversion systems and associated methods described herein are to
be
further distinguished from those of the paper and pulpwood industry, where the
goal is to
harvest partially digested wood pulp, rather than obtaining as high as
possible a quantity of
soluble carbohydrates, which can be subsequently converted into a reaction
product
comprising oxygenated intermediates. Since the goal of paper and pulpwood
processing is
to obtain raw wood pulp, such digestion processes may be conducted at lower
temperatures
and pressures to remove lower quantities of soluble carbohydrates and non-
cellulosic
components from the biomass, which can be removed at lower temperatures. In
some
embodiments described herein, at least 60% of the cellulosic biomass, on a dry
basis, may
be digested to produce a hydrolysate comprising soluble carbohydrates. In
other
embodiments described herein, at least 90% of the cellulosic biomass, on a dry
basis, may
be digested to produce a hydrolysate comprising soluble carbohydrates. Given
the intent
of paper and pulpwood processing, it is anticipated that much lower quantities
of soluble
carbohydrates are produced in these processes. The design of the present
biomass
conversion systems may enable such high conversion rates by minimizing the
formation of
degradation products during the processing of cellulosic biomass, while
maintaining long
residence times during hydrothermal digestion.
Although conducting a combined hydrothermal digestion of cellulosic biomass
solids and a catalytic reduction reaction of soluble carbohydrates in a single
pressure vessel
12
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
may be advantageous from the standpoint of stabilizing soluble carbohydrates
and
achieving excellent heat integration, biomass conversion systems implementing
such
configurations may present several challenges, as discussed above. Catalyst
poisoning
may also be an issue for some catalysts, since there is little to no
opportunity to remove
catalyst poisons from the hydrolysate when catalytic reduction is conducted in
the same
pressure vessel as the hydrothermal digestion. One way in which this issue can
be
circumvented is to use a poison-tolerant catalyst, some of which are discussed
hereinbelow. Another alternative is to use a catalyst that is regenerable upon
exposure to
conditions that can be established in the pressure vessel. For example, in
some
embodiments, a slurry catalyst may be regenerated through exposure to water at
a
temperature of at least 300 C.
Another alternative to address the issue of catalyst poisoning is to conduct
the
digestion of the cellulosic biomass solids in stages using separate digestion
units. Many of
the poisons that may deactivate a catalyst arise from sulfur-containing
compounds and
nitrogen-containing compounds in the raw cellulosic biomass solids. These
compounds,
along with at least some hemicellulose and lignins, may be at least partially
removed from
cellulosic biomass solids at lower digestion temperatures than those at which
cellulose
produces soluble carbohydrates. By controlling the digestion temperature, a
biomass pulp
may be produced that is enriched in cellulose but depleted in catalyst
poisons,
hemicellulose, and/or lignins, none of which are desirably present in a
process for
producing soluble carbohydrates or a reaction product derived therefrom.
Advantageously,
the catalyst poisons, hemicellulose, and/or lignins can be at least partially
removed from
the biomass pulp before it is added to the pressure vessel and processed to
produce a
reaction product, as described in the embodiments herein. That is, in some
embodiments, a
biomass pulp that has been at least partially depleted in catalyst poisons,
hemicellulose,
and/or lignins may be introduced to the pressure vessel described herein.
Not only may the use of multiple digestion units lessen the likelihood of
catalyst
poisoning, but such use also may advantageously reduce the likelihood of
lignin
precipitation from the liquor phase and formation of undesirable blockages in
the biomass
conversion systems (e.g., in transfer lines and the like). In some
embodiments, the biomass
conversion systems described herein may further comprise a separation
mechanism for
lignin. By removal of at least some of the lignin from the cellulosic biomass
solids before
hydrothermal digestion takes place, separation of the lignin from the liquor
phase may be
13
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
conducted less frequently than would otherwise be possible.
Unless otherwise specified herein, it is to be understood that use of the
terms
"biomass" or "cellulosic biomass" in the description herein refers to
"cellulosic biomass
solids." Solids may be in any size, shape, or form. The cellulosic biomass
solids may be
natively present in any of these solid sizes, shapes, or forms, or they may be
further
processed prior to digestion in the embodiments described herein. The
cellulosic biomass
solids may also be present in a slurry form in the embodiments described
herein.
In practicing the present embodiments, any type of suitable biomass source may
be
used. Suitable cellulosic biomass sources may include, for example, forestry
residues,
agricultural residues, herbaceous material, municipal solid wastes, waste and
recycled
paper, pulp and paper mill residues, and any combination thereof. Thus, in
some
embodiments, a suitable cellulosic biomass may include, for example, corn
stover, straw,
bagasse, miscanthus, sorghum residue, switch grass, bamboo, water hyacinth,
hardwood,
hardwood chips, hardwood pulp, softwood, softwood chips, softwood pulp, and
any
combination thereof. Leaves, roots, seeds, stalks, husks, and the like may be
used as a
source of the cellulosic biomass. Common sources of cellulosic biomass may
include, for
example, agricultural wastes (e.g., corn stalks, straw, seed hulls, sugarcane
leavings, nut
shells, and the like), wood materials (e.g., wood or bark, sawdust, timber
slash, mill scrap,
and the like), municipal waste (e.g., waste paper, yard clippings or debris,
and the like),
and energy crops (e.g., poplars, willows, switch grass, alfalfa, prairie
bluestream, corn,
soybeans, and the like). The cellulosic biomass may be chosen based upon
considerations
such as, for example, cellulose and/or hemicellulose content, lignin content,
growing
time/season, growing location/transportation cost, growing costs, harvesting
costs, and the
like.
Illustrative carbohydrates that may be present in cellulosic biomass may
include,
for example, sugars, sugar alcohols, celluloses, lignocelluloses,
hemicelluloses, and any
combination thereof. Once soluble carbohydrates have been removed from the
biomass
matrix through a digestion process according to the embodiments described
herein, the
soluble carbohydrates may be transformed into a reaction product comprising
oxygenated
intermediates via a catalytic reduction reaction. In some embodiments, the
oxygenated
intermediates comprising the reaction product may be further transformed into
a biofuel
using any combination of further hydrogenolysis reactions, hydrogenation
reactions,
condensation reactions, isomerization reactions, oligomerization reactions,
hydrotreating
14
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
reactions, alkylation reactions, and the like. In some embodiments, at least a
portion of the
oxygenated intermediates may be recirculated to the hydrothermal digestion
unit to
comprise at least a portion of the digestion solvent. Recirculation of at
least a portion of
the oxygenated intermediates to the hydrothermal digestion unit may also be
particularly
advantageous in terms of heat integration and process efficiency.
In some embodiments, biomass conversion systems described herein can comprise:
a pressure vessel comprising a first section and a second section, the first
section
comprising a hydrothermal digestion unit and the second section comprising a
first
catalytic reduction reactor unit that contains a first catalyst capable of
activating molecular
hydrogen; wherein the hydrothermal digestion unit and the first catalytic
reduction reactor
unit are in fluid communication with one another; a biomass feed mechanism
that is
operatively connected to the pressure vessel, the biomass feed mechanism being
capable of
introducing cellulosic biomass solids to the pressure vessel and also capable
of
withdrawing a reaction product from the first catalytic reduction reactor
unit; and a
hydrogen feed line that is operatively connected to the first catalytic
reduction reactor unit.
In some embodiments, the biomass conversion systems may further comprise a
fluid circulation loop that establishes fluid communication between a fluid
inlet of the
pressure vessel and a fluid outlet of the biomass feed mechanism. That is, in
such
embodiments, the biomass conversion systems may be configured such that a
liquor phase
may be transported through the biomass feed mechanism and subsequently
returned to the
pressure vessel. In other embodiments, the fluid circulation loop may
establish fluid
communication directly between a fluid inlet and a fluid outlet of the
pressure vessel. That
is, in such embodiments, the liquor phase need not necessarily pass through
the biomass
feed mechanism. Once returned to the pressure vessel, reaction product in the
liquor phase
may serve as a digestion solvent in the hydrothermal digestion unit and/or
unreacted
soluble carbohydrates therein may undergo further catalytic reduction to
produce a reaction
product. In addition, as described above, the liquor phase returned to the
pressure vessel
may aid in maintaining its thermal profile.
In some embodiments, the fluid circulation loop may further comprise a second
catalytic reduction reactor unit that contains a second catalyst capable of
activating
molecular hydrogen. As described above, the second catalytic reduction reactor
unit may
be used to further transform the soluble carbohydrates within the liquor phase
into a
reaction product. In some embodiments, the first catalyst and the second
catalyst may be
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
the same. In other embodiments, the first catalyst and the second catalyst may
be different.
Further description of suitable catalysts for the catalytic reduction reactor
units follows
below.
In some embodiments, biomass conversion systems described herein can comprise:
a pressure vessel comprising a first section and a second section, the first
section
comprising a hydrothermal digestion unit and the second section comprising a
first
catalytic reduction reactor unit that contains a first catalyst capable of
activating molecular
hydrogen; wherein the hydrothermal digestion unit and the first catalytic
reduction reactor
are in fluid communication with one another; a biomass feed mechanism that is
operatively
connected to the pressure vessel, the biomass feed mechanism being capable of
introducing
cellulosic biomass solids to the pressure vessel while the pressure vessel
maintains a
pressurized state; a hydrogen feed line that is operatively connected to the
first catalytic
reduction reactor unit; and a fluid circulation loop comprising the pressure
vessel and a
second catalytic reduction reactor unit that contains a second catalyst
capable of activating
molecular hydrogen.
Various configurations for the pressure vessel having a separated hydrothermal
digestion unit and a catalytic reduction reactor unit are possible. In some
embodiments,
the pressure vessel may comprise an annular structure, with the first section
comprising an
outer portion of the annular structure, and the second section comprising an
inner portion
of the annular structure. That is, in such embodiments, the biomass conversion
systems
may comprise an inner first catalytic reduction reactor unit and an outer
hydrothermal
digestion unit, all maintained within an exterior pressure housing. In other
embodiments,
the first section and the second section may be located alongside one another
in the
pressure vessel. Other configurations for the pressure vessel may be possible,
and, in
general, any pressure vessel having a hydrothermal digestion unit and a
catalytic reduction
reactor unit that are fluidly connected to, but separated from, one another
may be used in
the present embodiments.
FIGURES 1 and 2 show schematics of illustrative pressure vessels containing a
hydrothermal digestion unit and a catalytic reduction reactor unit, which are
fluidly
connected to, but separated from, one another. FIGURE 1 shows a schematic of
an
illustrative annular pressure vessel 1 containing an inner catalytic reduction
reactor unit 2
and an outer hydrothermal digestion unit 3 housed within outer pressure
housing 4.
FIGURE 2 shows a schematic of an illustrative pressure vessel 5 in which a
hydrothermal
16
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
digestion unit 6 and a catalytic reduction reactor unit 7 are located
alongside one another
within outer pressure housing 8. Although FIGURES 1 and 2 have depicted the
pressure
vessel as having a substantially cylindrical configuration, it is to be
recognized that any
shape can be used. The arrows in FIGURES 1 and 2 represent the flow direction
of
hydrolysate and bulk biomass during digestion and that of the liquor phase as
it passes
through the catalytic reduction reactor unit in the process of being
transformed into a
reaction product. As depicted in FIGURES 1 and 2, catalytic reduction reactor
units 2 and
7 are operated as gas lift, slurry, or ebullating bed reactors. However, other
reactor
configurations may also be used. The foregoing pressure vessels may be used in
conjunction with the biomass conversion systems described herein. FIGURES 3
and 4,
which are discussed in more detail hereinbelow, show illustrative biomass
conversion
systems having a hydrothermal digestion unit and a catalytic reduction reactor
unit housed
within a pressure vessel.
In some embodiments, the biomass conversion systems may comprise a biomass
feed mechanism that is operatively connected to the pressure vessel. In
some
embodiments, the biomass feed mechanism may be capable of introducing
cellulosic
biomass solids to the pressure vessel while the pressure vessel maintains a
pressurized
state. In some embodiments, the biomass feed mechanism may also be capable of
withdrawing a reaction product from the pressure vessel. In various
embodiments, the
biomass feed mechanism may comprise a pressure transition zone that cycles
between a
lower pressure state (e.g., atmospheric pressure) and a higher pressure state.
In some
further embodiments, the biomass feed mechanism may further comprise an
atmospheric
pressure zone. Cellulosic biomass solids may be introduced to the pressure
transition zone,
and their pressure may be increased to a level suitable for being introduced
to the pressure
vessel.
When present, suitable atmospheric pressure zones of the biomass feed
mechanism
may include, for example, conveyer belts, vibrational tube conveyers, screw
feeders or
conveyers, holding tanks, surge vessels, bin dispensers, and the like.
Suitable pressure
transition zones that are operable for continuous or semi-continuous addition
of cellulosic
biomass solids to a pressure vessel may include, for example, pressurized
screw feeders,
pressure-cycling chambers, and the like as described in commonly owned United
States
Patent Application Publications 2013/0152457 and 2013/0152458.
In some embodiments, the biomass feed mechanism may allow cellulosic biomass
17
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
solids to be introduced to the pressure vessel without the pressure vessel
being fully
depressurized. Pressurizing the cellulosic biomass solids prior to
introduction to the
pressure vessel may allow the hydrothermal digestion unit to remain
pressurized and
operating continuously. Pressurization can help maintain heat integration and
energy
efficiency of the biomass digestion process, as described above. Additional
benefits of
pressurizing the cellulosic biomass solids are also discussed hereinafter.
In some embodiments, pressurization of the cellulosic biomass solids within
the
biomass feed mechanism may take place, at least in part, by introducing at
least a portion
of the liquor phase in the pressure vessel to the pressure transition zone. In
some or other
embodiments, pressurization of the pressure transition zone may take place, at
least in part,
by introducing a gas to the pressure transition zone. In some embodiments, the
liquor
phase may comprise an organic solvent, which is generated as a reaction
product of a
catalytic reduction reaction. In other embodiments, an external solvent may be
used to
pressurize the pressure transition zone.
At least two benefits may be realized by pressurizing the cellulosic biomass
solids
using a liquor phase from the digestion unit. First, pressurizing the biomass
solids in the
presence of the liquor phase may cause the digestion solvent to infiltrate the
biomass
solids, which may cause the biomass solids to sink in the digestion solvent
once introduced
to the pressure vessel. Further, by adding hot liquor phase to the biomass
solids in the
pressure transition zone, less energy may need to be input to bring the
biomass solids up to
temperature once introduction to the pressure vessel takes place. Both of
these features
may improve the efficiency of the digestion process.
FIGURE 3 shows a schematic of an illustrative biomass conversion system having
a hydrothermal digestion unit and a catalytic reduction reactor unit housed
within a
pressure vessel. For conciseness, FIGURE 3 has been depicted with the annular
pressure
vessel configuration depicted in FIGURE 1. However, it is to be recognized
that other
pressure vessel configurations may be used in the embodiments described
herein.
Moreover, the catalytic reduction reactor unit housed within the pressure
vessel has been
shown in cut-away form so that the cellulosic biomass solids and catalyst
particles therein
may be more clearly depicted.
As shown in FIGURE 3, biomass conversion system 10 includes pressure vessel
12, which contains hydrothermal digestion unit 14 and first catalytic
reduction reactor unit
16 within outer pressure housing 18. Hydrothermal digestion unit 14 and first
catalytic
18
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
reduction reactor unit 16 are fluidly coupled to one another by fluid conduit
region 26.
Fluid conduit region 26 allows a hydrolysate produced within hydrothermal
digestion unit
14 to flow downward by gravity and thereafter be transported upward within
first catalytic
reduction reactor unit 16 in the presence of molecular hydrogen. As depicted
in FIGURE
3, first catalytic reduction reactor unit 16 operates, at least in part, by
gas lift.
Cellulosic biomass solids may be introduced to pressure vessel 12 by biomass
introduction mechanism 20, which comprises atmospheric pressure zone 22 and
pressure
transition zone 24. Cellulosic biomass solids may be housed in atmospheric
pressure zone
22 and added, as needed, to pressure transition zone 24. Cellulosic biomass
solids added to
pressure transition zone 24 may be cycled from atmospheric pressure to an
elevated
pressure state such that they may be introduced to pressure vessel 12. There
may be
various valves or other pressure isolation mechanisms present between
atmospheric
pressure zone 22 and pressure transition zone 24 and between pressure
transition zone 24
and pressure vessel 12, which have not been depicted for purposes of clarity.
Suitable
pressure isolation mechanisms and use thereof will be familiar to one having
ordinary skill
in the art. Suitable biomass feed mechanisms that may supply cellulosic
biomass solids to
a pressurized vessel are described in further detail hereinabove.
During operation of biomass conversion system 10, addition of cellulosic
biomass
solids to pressure vessel 12 may occur on a continuous or semi-continuous
basis. As
described above, cellulosic biomass solids may be added to biomass feed
mechanism 20
and raised to an elevated pressure state. Thereafter, the cellulosic biomass
solids may be
introduced to pressure vessel 12. When introduced to pressure vessel 12, the
cellulosic
biomass solids may enter hydrothermal digestion unit 14 and undergo at least
partial
transformation into soluble carbohydrates. In some embodiments, at least a
portion of the
cellulosic biomass solids may also enter first catalytic reduction reactor
unit 16. As
described hereinafter, the introduction of cellulosic biomass solids to first
catalytic
reduction reactor unit 16 may be particularly advantageous.
Hydrolysate produced in hydrothermal digestion unit 14 may drain into fluid
conduit region 26, where it may subsequently enter first catalytic reduction
reactor unit 16
and flow upward therethrough in the course of being transformed into a
reaction product.
Molecular hydrogen may be introduced to first catalytic reduction reactor unit
16 via
hydrogen feed line 28. First catalytic reduction reactor unit 16 contains
catalyst particles
30 and optionally cellulosic biomass solids 32. As described above, cellulosic
biomass
19
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
solids 32 may aid in retaining catalyst particles 30 within first catalytic
reduction reactor
unit 16. Some of the catalyst particles not retained by first catalytic
reduction reactor unit
16 may be conveyed by the downward biomass and hydrolysate flow within
hydrothermal
digestion unit 14. These catalyst particles may be returned to first catalytic
reduction
reactor unit 16 during the flow of hydrolysate thereto, as described above.
As depicted in FIGURE 3, fluid circulation loop 40 establishes fluid
communication between a fluid inlet 42 of pressure vessel 12 and a fluid
outlet 44 of
biomass feed mechanism 20. Optionally, the fluid circulation loop can
establish a direct
fluid connection between a fluid outlet and a fluid inlet of pressure vessel
12. For
example, in some embodiments, fluid circulation loop 40 may establish a direct
fluid
connection between fluid outlet 46 of pressure vessel 12 and fluid inlet 42 of
pressure
vessel 12 (see FIGURE 4). As depicted in FIGURE 3, fluid circulation loop 40
also
contains second catalytic reduction reactor unit 50 where further
transformation of the
hydrolysate may occur. Fluid circulation loop 40 may be configured to
establish
countercurrent flow in hydrothermal digestion unit 14 once liquor phase
therein is
recirculated to pressure vessel 12. Other flow motifs, including co-current
flow, are also
possible. Reaction product that is not returned to pressure vessel 12 may be
withdrawn
from fluid circulation loop 40 by reaction product takeoff line 64 for further
processing.
During operation of biomass conversion system 10, there may be an open fluid
connection between pressure vessel 10 and biomass feed mechanism 20, such that
liquor
phase may flow into pressure transition zone 24. Reaction product produced in
first
catalytic reduction reactor unit 16 may exit pressure vessel 12 via biomass
feed mechanism
20 (i.e. by fluid circulation loop 40, which is operatively connected to
pressure transition
zone 24). Catalyst particles 30 not retained within first catalytic reduction
reactor unit 16
or returned thereto by bulk biomass and hydrolysate flow may become
sequestered within
cellulosic biomass solids housed within pressure transition zone 24. Once
these cellulosic
biomass solids are introduced to pressure vessel 12, the catalyst particles
may also be
returned thereto. Thus, by flowing the liquor phase from pressure vessel 12
through
cellulosic biomass solids in pressure transition zone 24, an even more
effective retention of
catalyst may be realized.
In some embodiments, it may be desirable that the liquor phase does not flow
through pressure transition zone 24, at least temporarily. For example, when
fresh
cellulosic biomass solids are being added to pressure transition zone 24, the
pressure
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
transition zone may be at atmospheric pressure, in which case it may be
isolated from
pressure vessel 12 by a pressure isolation mechanism, as discussed above. In
such cases,
flow within fluid circulation loop 40 may be maintained by routing the liquor
phase
through bypass line 48, which establishes direct fluid communication to
pressure vessel 12
via fluid outlet 46.
In some embodiments, the fluid circulation loop may directly connect to
pressure
vessel 12, rather than connecting to pressure transition zone 24 of biomass
feed mechanism
20. Although the benefit of flowing the liquor phase through the cellulosic
biomass solids
in pressure transition zone 24 is lost in such embodiments, this configuration
represents a
viable alternative configuration for the fluid connection of fluid circulation
loop 40.
FIGURE 4 shows a schematic of an illustrative biomass conversion system having
a
hydrothermal digestion unit and a catalytic reduction reactor unit housed
within a pressure
vessel in which a fluid circulation loop establishes direct fluid
communication between a
fluid inlet and a fluid outlet of the pressure vessel. Features in FIGURE 4
have identical
reference characters to those depicted in FIGURE 3 and described hereinabove.
For
conciseness, these features will not be described again in detail.
Although the foregoing description has described the benefits afforded by
using
cellulosic biomass solids to sequester catalyst particles, it is to be
recognized that
conventional catalyst screens and filters may be used to retain the catalyst
within first
catalytic reduction reactor unit 14, second catalytic reduction reactor unit
50, or both. Such
catalyst screens and filters will familiar to one having ordinary skill in the
art. Catalyst
filters or screens may include wire mesh or sintered metal or ceramic filters.
Beds of
solids such as, for example, sharp sands or other packed beds of solids,
typically with a
void fraction of 25% or less by volume, may also be deployed as catalytic
filters to
separate slurry catalyst from a liquid filtrate. External filters such cake
filters, for example,
may be deployed, where the filter media can be cloth, sintered metal, metal
screens or
fabrics, porous ceramic, pressed felts or cotton batting, nonwoven fabrics,
filter paper,
polymer membranes, or granular beds of particulate solids. Filters using these
filter media
may include cake filters, horizontal or vertical plate filters, filter
presses, leave filters,
tubular filters, rotary drum filters, centrifugal discharge filters, and the
like. Centrifuges,
hydroclones, and gravity settlers can also be used to separate slurry catalyst
from a liquid
phase and recycle slurry catalyst. A pump, screw, or belt, for example, may be
used to
transport the separated or enriched slurry catalyst back into the catalytic
reduction reactor
21
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
unit.
Various optional elements may also be present within the biomass conversion
systems described herein. In some embodiments, there may be a solids
separation
mechanism 60 within fluid circulation loop 40. Use of a solids separation
mechanism may
protect second catalytic reduction reactor unit 50 from plugging by catalyst
particles and
cellulosic biomass fines, for example. Suitable solids separation mechanisms
are described
in more detail hereinbelow. In some embodiments, there may be a phase
separation
mechanism 62 within fluid circulation loop 40. Phase separation mechanism 62
may be
used to at least partially separate an organic phase from an aqueous of the
reaction product
exiting second catalytic reduction reactor unit 50. Suitable phase separation
mechanisms
are also discussed in more detail hereinbelow.
In various embodiments, suitable materials for the pressure vessel may
include, for
example, carbon steel, stainless steel, or a similar alloy. In some
embodiments, the
pressure vessel may be capable of maintaining a pressure of at least 30 bar.
In some
embodiments, the pressure vessel may be capable of maintaining a pressure of
at least 60
bar. In some embodiments, the pressure vessel may be capable of maintaining a
pressure
of at least 90 bar.
Various catalysts may be used in conjunction with the catalytic reduction
reactor
units described herein. In some embodiments, the catalyst in the first
catalytic reduction
reactor unit and the second catalytic reduction reactor unit may be the same.
In other
embodiments, they may be different. In some embodiments, the catalyst in the
first
catalytic reduction reactor unit may comprise a slurry catalyst, an ebullating
bed catalyst,
or a fluidized bed catalyst. In some embodiments, the catalyst in the second
catalytic
reduction reactor unit may comprise a fixed bed catalyst, a slurry catalyst,
an ebullating
bed catalyst, or a fluidized bed catalyst. In some embodiments, the first
catalyst, the
second catalyst, or both may comprise a slurry catalyst.
In some embodiments, the first catalyst, the second catalyst, or both may
comprise
a poison-tolerant catalyst. Use of a poison-tolerant catalyst may be
particularly desirable
when catalyst poisons are not removed from the liquor phase of the hydrolysate
before
catalytic reduction takes place. As used herein, a "poison-tolerant catalyst"
is defined as a
catalyst that is capable of activating molecular hydrogen without needing to
be regenerated
or replaced due to low catalytic activity for at least 12 hours of continuous
operation. Use
of a poison-tolerant catalyst may avoid the disadvantages of process downtime
that are
22
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
associated with catalyst regeneration and replacement. In addition to lost
production time,
considerable energy expenditure may be needed when restarting the hydrothermal
digestion process.
In some embodiments, suitable poison-tolerant catalysts may include, for
example,
a sulfided catalyst. Sulfided catalysts suitable for activating molecular
hydrogen are
described in commonly owned United States Patent Application Publications
2012/0317872, and 2013/0109896. Sulfiding may take place by treating a
catalyst with
hydrogen sulfide or other sulfiding agent, optionally while the catalyst is
deposited on a
solid support. In more particular embodiments, the poison-tolerant catalyst
may comprise
a sulfided cobalt-molybdate catalyst. We have found that sulfided cobalt-
molybdate
catalysts may give a high yield of oxygenated intermediates, while not forming
an excess
amount of C2 ¨ C4 alkanes. The oxygenated intermediates formed may be readily
separated from water via flash vaporization or liquid-liquid phase separation,
and undergo
condensation-oligomerization reactions in separate steps over an acid or base
catalyst, to
produce liquid biofuels in the gasoline, jet, or diesel range. Use of a poison-
tolerant
catalyst may lessen the need to perform stepwise digestion or a purification
of the
hydrolysate (e.g., by ion-exchange) prior to the catalytic reduction reaction
taking place.
Even when catalyst poisons are removed from the hydrolysate, a poison-tolerant
catalyst
may still be used to lessen process downtime.
In some embodiments, the catalyst may be regenerable. In some embodiments, the
catalyst may be a regenerable slurry catalyst. For example, in some
embodiments, a slurry
catalyst may be regenerable through exposure to water at a temperature above
its normal
boiling point. As used herein, a "regenerable catalyst" may have at least some
of its
catalytic activity restored through regeneration, even when poisoned with
nitrogen
compound impurities, sulfur compound impurities, or any combination thereof.
Ideally,
such regenerable catalysts should be regenerable with a minimal amount of
process
downtime. In some embodiments, the slurry catalyst may be regenerated through
exposure
to water having a temperature of at least 200 C. In some embodiments, the
slurry catalyst
may be regenerated through exposure to water having a temperature of at least
250 C. In
some embodiments, the slurry catalyst may be regenerated through exposure to
water
having a temperature of at least 300 C. In some embodiments, the slurry
catalyst may be
regenerated through exposure to water having a temperature of at least 350 C.
In some
embodiments, the slurry catalyst may be regenerated through exposure to water
having a
23
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
temperature of at least 400 C. Exposure to water in a subcritical state or a
supercritical
state may be used for regeneration of the catalyst. Regeneration of the slurry
catalyst may
take place at any location in the biomass conversion system, but more
typically,
regeneration takes place in one of the catalytic reduction reactor units while
hydrolysate is
not being processed therein. Most catalysts effective for mediating a
catalytic reduction
reaction are also regenerable, at least in part, through thermal treatments
with hydrogen. A
particularly suitable slurry catalyst that can be regenerated though exposure
to water above
its normal boiling point is ruthenium disposed on a solid support such as, for
example,
ruthenium on titanium dioxide or ruthenium on carbon. Another suitable slurry
catalyst
may be a platinum or a palladium compound disposed on a solid support.
In some embodiments, the catalytic reduction reactions carried out in the
hydrothermal digestion unit and the catalytic reduction reactor unit may be
hydrogenolysis
reactions. A detailed description of hydrogenolysis reactions is included
hereinbelow.
In some embodiments, the fluid circulation loop may be configured to establish
countercurrent flow in the hydrothermal digestion unit of the pressure vessel.
As used
herein, the term "countercurrent flow" refers to the direction a reaction
product enters the
hydrothermal digestion unit relative to the direction of bulk biomass flow in
the
hydrothermal digestion unit. Other flow configurations such as, for example,
co-current
flow may also be used, if desired.
In some embodiments, there may be a solids separation mechanism located within
the fluid circulation loop between a fluid outlet of the biomass feed
mechanism and a fluid
inlet of the second catalytic reduction reactor unit. In some embodiments,
there may be a
solids separation mechanism located within the fluid circulation loop between
a fluid outlet
of the pressure vessel and a fluid inlet of the second catalytic reduction
reactor unit. A
solids separation mechanism in this location may be used to protect the second
catalytic
reduction reactor unit and reduce the likelihood of plugging.
Solids separation
mechanisms may include any separation technique known in the art including,
for
example, filters, centrifugal force- or centrifugal force-based separation
mechanisms (e.g.,
hydroclones), settling tanks, centrifuges, and the like. Suitable filters may
include, for
example, surface filters and depth filters. Surface filters may include, for
example, filter
papers, membranes, porous solid media, and the like. Depth filters may
include, for
example, a column or plug of porous media designed to trap solids within its
core structure.
In some embodiments, two or more filters may be used within the fluid
circulation loop,
24
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
where at least one of the filters may be backflushed to the hydrothermal
digestion unit
while forward fluid flow continues through at least some of the remaining
filters and
onward to the catalytic reduction reactor unit. That is, two or more filters
may be operated
in a reciprocating manner. In some embodiments, one or more hydroclones may be
used
within the fluid circulation loop. Use of filters and hydroclones within the
fluid circulation
loop are described in commonly owned United States Patent Application
Publications
2013/0152456 and 2013/0158308.
In some embodiments, there may be a phase separation mechanism within the
fluid
circulation loop between a fluid outlet of the second catalytic reduction
reactor unit and a
fluid inlet of the pressure vessel. Suitable phase separation mechanisms and
techniques are
discussed in more detail below.
In some embodiments, there may be a reaction product takeoff line in fluid
communication with the fluid circulation loop, where the reaction product
takeoff line is
located between a fluid inlet of the pressure vessel and a fluid outlet of the
second catalytic
reduction reactor unit. In some embodiments, there may be a solids separation
mechanism
that is operatively connected to the reaction product takeoff line. A solids
separation
mechanism in this location may be used to remove solids from the reaction
product before
it is further transformed downstream into a biofuel or other substance.
Suitable solids
separation mechanisms may include those described previously.
In some embodiments, methods for processing cellulosic biomass solids are
described herein. In some embodiments, methods for processing cellulosic
biomass solids
can comprise: providing a pressure vessel comprising a first section and a
second section,
the first section comprising a hydrothermal digestion unit and the second
section
comprising a first catalytic reduction reactor unit that contains a first
catalyst capable of
activating molecular hydrogen; wherein the hydrothermal digestion unit and the
first
catalytic reduction reactor unit are in fluid communication with one another;
adding
cellulosic biomass solids to the pressure vessel; heating the cellulosic
biomass solids in the
hydrothermal digestion unit of the pressure vessel, thereby forming a
hydrolysate
comprising soluble carbohydrates within a liquor phase; conveying the liquor
phase
through the first catalytic reduction reactor unit in the presence of
molecular hydrogen so
as to at least partially transform the soluble carbohydrates into a reaction
product; and
conveying at least a portion of the liquor phase from the pressure vessel to a
second
catalytic reduction reactor unit that contains a second catalyst capable of
activating
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
molecular hydrogen, so as to further transform the soluble carbohydrates into
the reaction
product.
In some embodiments, the cellulosic biomass solids may be added to the
pressure
vessel while the pressure vessel maintains a pressurized state. In some
embodiments, the
pressure vessel may be maintained at a pressure of at least 30 bar while the
cellulosic
biomass solids are being added. In some embodiments, the cellulosic biomass
solids may
be added to the pressure vessel from a biomass feed mechanism, where the
biomass feed
mechanism is capable of introducing cellulosic biomass solids to the pressure
vessel while
the pressure vessel maintains a pressurized state. Pressure vessels suitable
for the
foregoing purpose have been set forth above. In some embodiments, the biomass
feed
mechanism may add at least some cellulosic biomass solids to the hydrothermal
digestion
unit of the pressure vessel. In some or other embodiments, the biomass feed
mechanism
may add at least some cellulosic biomass solids to the first catalytic
reduction reactor unit
of the pressure vessel.
In some embodiments, the methods may further comprise conveying the liquor
phase to the second catalytic reduction reactor unit through the biomass feed
mechanism.
In some embodiments, the biomass feed mechanism may be empty while conveying
the
liquor phase. In other embodiments, the biomass feed mechanism may contain
cellulosic
biomass solids while the liquor phase is being conveyed therethrough. As
described above,
cellulosic biomass solids in the biomass feed mechanism may be used to
sequester catalyst
solids thereon and limit their transportation to the second catalytic
reduction reactor unit.
Catalyst solids sequestered on the cellulosic biomass solids within the
biomass feed
mechanism may be returned to the pressure vessel during subsequent additions
of
cellulosic biomass solids thereto.
In some embodiments, the methods may further comprise conveying the liquor
phase to the second catalytic reduction reactor unit without the liquor phase
passing
through a biomass feed mechanism. For example, the liquor phase may pass
directly from
a fluid outlet on the pressure vessel to a fluid inlet on the second catalytic
reduction reactor
unit. In some embodiments, direct transfer of the liquor phase may take place
when the
biomass feed mechanism is at atmospheric pressure and being loaded with
additional
cellulosic biomass solids. In other embodiments, the biomass feed mechanism
may be
bypassed entirely. For example, if catalyst solids are effectively retained
within the
pressure vessel it may be possible for the biomass feed mechanism to be
bypassed when
26
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
the liquor phase is being conveyed to the second catalytic reduction reactor
unit.
In some embodiments, prior to digestion, the cellulosic biomass may be washed
and/or reduced in size (e.g., by chopping, crushing, debarking, and the like)
to achieve a
desired size and quality for being digested. The operations may remove
substances that
interfere with further chemical transformation of soluble carbohydrates and/or
improve the
penetration of digestion solvent into the biomass. In some embodiments,
washing may
occur within the hydrothermal digestion unit of the pressure vessel prior to
pressurization.
In other embodiments, washing may occur before the biomass is placed in the
pressure
vessel. For example, in some embodiments, the biomass may be washed in a
biomass feed
mechanism before pressurization takes place.
In general, digestion in the hydrothermal digestion unit may be conducted in a
liquor phase. In some embodiments, the liquor phase may comprise a digestion
solvent
that comprises water. In some embodiments, the liquor phase may further
comprise an
organic solvent. In some embodiments, the organic solvent may comprise
oxygenated
intermediates produced from a catalytic reduction reaction of soluble
carbohydrates. For
example, in some embodiments, a digestion solvent may comprise oxygenated
intermediates produced by a hydrogenolysis reaction or other catalytic
reduction reaction
of soluble carbohydrates. In some embodiments, bio-ethanol may be added to
water as a
startup digestion solvent, with a solvent comprising oxygenated intermediates
being
produced thereafter. Any other organic solvent that is miscible with water may
also be
used as a startup digestion solvent, if desired. In general, a sufficient
amount of liquor
phase may be present in the digestion process such that the biomass surface
remains
wetted. The amount of liquor phase may be further chosen to maintain a
sufficiently high
concentration of soluble carbohydrates to attain a desirably high reaction
rate during
catalytic reduction, but not so high such that degradation becomes
problematic. In some
embodiments, the concentration of soluble carbohydrates may be kept below 5%
by weight
of the liquor phase to minimize degradation. However, it is to be recognized
that higher
concentrations may be used in some embodiments. In some embodiments, organic
acids
such as, for example, acetic acid, oxalic acid, salicylic acid, or
acetylsalicylic acid may be
included in the liquor phase as an acid promoter of the digestion process.
In some embodiments, the digestion solvent may comprise an organic solvent
comprising oxygenated intermediates resulting from a catalytic reduction
reaction of
soluble carbohydrates. The catalytic reduction reaction may take place within
the catalytic
27
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
reduction reactor units. In some embodiments, the organic solvent may comprise
at least
one alcohol, ketone, or polyol. In alternative embodiments, the digestion
solvent may be at
least partially supplied from an external source. For example, in some
embodiments, bio-
ethanol may be used to supplement the organic solvent. Other water-miscible
organic
solvents may be used as well. In some embodiments, the digestion solvent may
be
separated, stored, or selectively injected into the hydrothermal digestion
unit so as to
maintain a desired concentration of soluble carbohydrates or to provide
temperature
regulation in the hydrothermal digestion unit.
In various embodiments, digestion may take place over a period of time at
elevated
temperatures and pressures. In some embodiments, digestion may take place at a
temperature ranging between 100 C to 250 C for a period of time. In some
embodiments,
the period of time may range between 0.25 hours and 24 hours. In some
embodiments, the
digestion to produce soluble carbohydrates may occur at a pressure ranging
between 1 bar
(absolute) and 100 bar. In some embodiments, the digestion process may be
conducted in
stages, with a first stage being conducted at 160 C or below to solubilize and
convert
hemicellulose into a reaction product, and with a second stage being conducted
at 160 C or
above to solubilize and convert cellulose into a reaction product. The lower
temperature
digestion may also remove at least some catalyst poisons and lignin from the
cellulosic
biomass solids.
In various embodiments, suitable biomass digestion techniques may include, for
example, acid digestion, alkaline digestion, enzymatic digestion, and
digestion using hot-
compressed water.
In some embodiments, the methods may further comprise withdrawing at least a
portion of the reaction product from the biomass conversion system. For
example, in some
embodiments, the methods may further comprise withdrawing a portion of the
reaction
product from a fluid outlet of the second catalytic reduction reactor unit,
after further
transforming the soluble carbohydrates into reaction product. In some
embodiments, the
methods may further comprise converting the reaction product into a biofuel,
as described
in further detail hereinafter. In some embodiments, the methods may further
comprise
separating solids from the liquor phase as it is being conveyed to the second
catalytic
reduction reactor unit, as described above.
In some embodiments, the methods may further comprise recirculating at least a
portion of the liquor phase from the second catalytic reduction reactor unit
to the pressure
28
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
vessel. Recirculation of the liquor phase from the second catalytic reduction
reactor unit to
the pressure vessel may, for example, help regulate temperature therein,
provide makeup
digestion solvent for the digestion process, and the like. Recirculation from
the second
catalytic reduction reactor unit to the pressure vessel may take place at
various recycle
ratios. As used herein, the term "recycle ratio" refers to the amount of
liquor phase that is
recirculated to the pressure vessel (e.g., within the fluid circulation loop)
relative to the
amount of liquor phase that is withdrawn from the biomass conversion system
(e.g., by a
reaction product takeoff line).
A particular benefit of performing hydrothermal digestion and catalytic
reduction in
the same pressure vessel is that lower recycle ratios may be used when
recirculating the
liquor phase to the hydrothermal digestion, while still maintaining process
efficiency and
yields. Specifically, there may be less need to supply heat from the
recirculated reaction
product to the digestion process occurring in the pressure vessel, given than
an exothermic
catalytic reduction reaction is already taking place therein. Accordingly, a
relatively high
proportion of the liquor phase exiting the second catalytic reduction reactor
unit may be
withdrawn from the biomass conversion system for subsequent conversion into a
biofuel.
Lower recycle ratios may also allow smaller reactor volumes to be used, as
total liquid
flow velocity in the hydrothermal digestion unit and catalytic reduction
reactor are
reduced. High recycle ratios and high liquid flow velocities may give rise to
excessive
pressure drops, high pump energy and size requirements, and other adverse
features.
Failure to minimize residence time prior to stabilization via a catalytic
reduction reaction
may also result in lower yields. Additionally, lower recycle ratios may help
promote
retention of the catalyst within the pressure vessel and lessen the demands on
the cellulosic
biomass solids to retain the catalyst therein. Given the benefit of the
present disclosure,
one having ordinary skill in the art will be able to determine an appropriate
recycle ratio for
liquor phase recirculation that achieves a desired amount of heat integration,
while
balancing a desired rate of downstream biofuel production. In some
embodiments, the
liquor phase may be recirculated from the second catalytic reduction reactor
unit to the
pressure vessel at a recycle ratio ranging between 0.2 and 10. In some
embodiments, the
liquor phase may be recirculated from the second catalytic reduction reactor
unit to the
pressure vessel at a recycle ratio ranging between 1 and 10, or between 1 and
5, or between
0.2 and 2, or between 0.5 and 2, or between 1 and 2, or between 0.2 and 1, or
between 0.5
and 1. In some embodiments, the liquor phase may be recirculated from the
second
29
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
catalytic reduction reactor unit to the pressure vessel at a recycle ratio of
2 or less. In some
embodiments, the liquor phase may be recirculated from the second catalytic
reduction
reactor unit to the pressure vessel at a recycle ratio of 1 or less. In some
embodiments, the
liquor phase may be recirculated from the second catalytic reduction reactor
unit to the
pressure vessel such that countercurrent flow is established in the
hydrothermal digestion
unit. In other embodiments, other flow patterns may be established in the
hydrothermal
digestion unit, including co-current flow, for example.
In some embodiments, heating the cellulosic biomass solids in the pressure
vessel
may take place at a pressure of at least 30 bar. Maintaining digestion at a
pressure of at
least 30 bar may ensure that digestion takes place at a satisfactory rate. In
some
embodiments, heating the cellulosic biomass solids in the pressure vessel may
take place at
a pressure of at least 60 bar. In some embodiments, heating the cellulosic
biomass solids
in the pressure vessel may take place at a pressure of at least 90 bar. In
some
embodiments, heating the cellulosic biomass solids in the pressure vessel may
take place at
a pressure ranging between 30 bar and 430 bar. In some embodiments, heating
the
cellulosic biomass solids in the pressure vessel may take place at a pressure
ranging
between 50 bar and 330 bar. In some embodiments, heating the cellulosic
biomass solids
in the pressure vessel may take place at a pressure ranging between 70 bar and
130 bar. In
some embodiments, heating the cellulosic biomass solids in the pressure vessel
may take
place at a pressure ranging between 30 bar and 130 bar. It is to be noted that
the foregoing
pressures refer to the normal operating pressures at which digestion takes
place.
In general, after digestion in the hydrothermal digestion unit takes place,
only small
percentages of the original cellulosic biomass solids may remain undigested.
In some
embodiments, at least 60% of the cellulosic biomass solids, on a dry basis,
may be digested
to produce hydrolysate. In some embodiments, at least 70% of the cellulosic
biomass
solids, on a dry basis, may be digested to produce hydrolysate. In some
embodiments, at
least 80% of the cellulosic biomass solids, on a dry basis, may be digested to
produce
hydrolysate. In some embodiments, at least 90% of the cellulosic biomass
solids, on a dry
basis, may be digested to produce hydrolysate.
In some embodiments, a poison-tolerant catalyst may be used in the methods as
either the first catalyst, the second catalyst, or both. Suitable poison-
tolerant catalysts have
been set forth above. In some embodiments, a regenerable catalyst may be used
in the
methods as either the first catalyst, the second catalyst, or both. In some
embodiments, the
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
regenerable catalyst may be a slurry catalyst. In some embodiments, the
methods may
further comprise regenerating the slurry catalyst through exposure to water
having a
temperature of at least 200 C, or at least 250 C, or at least 300 C, or at
least 350 C, or at
least 400 C.
In some embodiments, the present methods may further comprise performing a
phase separation of the reaction product. In some embodiments, phase
separation may take
place using a phase separation mechanism that is present following an outlet
of the second
catalytic reduction reactor unit. In various embodiments, performing a phase
separation
may comprise separating a bilayer, conducting a solvent stripping operation,
performing an
extraction, performing a filtration, performing a distillation, or the like.
In some
embodiments, azeotropic distillation may be conducted.
In some embodiments, the methods described herein may further comprise
converting the reaction product into a biofuel. In some embodiments,
conversion of the
reaction product into a biofuel may begin with a catalytic hydrogenolysis
reaction to
transform soluble carbohydrates produced from hydrothermal digestion into a
reaction
product comprising oxygenated intermediates, as described above. As further
described
above and depicted in FIGURES 3 and 4, a liquor phase containing the reaction
product
may be recirculated to the pressure vessel to further aid in the digestion
process. In some
embodiments, the reaction product may be further transformed by any number of
further
catalytic reforming reactions including, for example, further catalytic
reduction reactions
(e.g., hydrogenolysis reactions, hydrogenation reactions, hydrotreating
reactions, and the
like), condensation reactions, isomerization reactions, desulfurization
reactions,
dehydration reactions, oligomerization reactions, alkylation reactions, and
the like. A
description of the initial hydrogenolysis reaction and the further catalytic
reforming
reactions are described hereinafter.
Various processes are known for performing hydrogenolysis of carbohydrates.
One
suitable method includes contacting a carbohydrate or stable hydroxyl
intermediate with
hydrogen, optionally mixed with a diluent gas, and a hydrogenolysis catalyst
under
conditions effective to form a reaction product comprising oxygenated
intermediates such
as, for example, smaller molecules or polyols. As used herein, the term
"smaller molecules
or polyols" includes any molecule that have a lower molecular weight, which
may include
a smaller number of carbon atoms or oxygen atoms, than the starting
carbohydrate. In
some embodiments, the reaction products may include smaller molecules such as,
for
31
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
example, polyols and alcohols. This aspect of hydrogenolysis entails the
breaking of
carbon-carbon bonds
In some embodiments, a soluble carbohydrate may be converted to relatively
stable
oxygenated intermediates such as, for example, propylene glycol, ethylene
glycol, and
glycerol using a hydrogenolysis reaction in the presence of a catalyst that is
capable of
activating molecular hydrogen. Suitable catalysts may include, for example,
Cr, Mo, W,
Re, Mn, Cu, Cd, Fe, Co, Ni, Pt, Pd, Rh, Ru, Ir, Os, and alloys or any
combination thereof,
either alone or with promoters such as Au, Ag, Cr, Zn, Mn, Sn, Bi, B, 0, and
alloys or any
combination thereof. In some embodiments, the catalysts and promoters may
allow for
hydrogenation and hydrogenolysis reactions to occur at the same time or in
succession,
such as the hydrogenation of a carbonyl group to form an alcohol. The catalyst
may also
include a carbonaceous pyropolymer catalyst containing transition metals
(e.g., chromium,
molybdenum, tungsten, rhenium, manganese, copper, and cadmium) or Group VIII
metals
(e.g., iron, cobalt, nickel, platinum, palladium, rhodium, ruthenium, iridium,
and osmium).
In certain embodiments, the catalyst may include any of the above metals
combined with
an alkaline earth metal oxide or adhered to a catalytically active support. In
certain
embodiments, the catalyst described in the hydrogenolysis reaction may include
a catalyst
support.
The conditions under which to carry out the hydrogenolysis reaction will vary
based on the type of biomass starting material and the desired products (e.g.
gasoline or
diesel), for example. One of ordinary skill in the art, with the benefit of
this disclosure,
will recognize the appropriate conditions to use to carry out the reaction. In
general, the
hydrogenolysis reaction may be conducted at temperatures in the range of 110 C
to 300 C,
and preferably from 170 C to 300 C, and most preferably from 180 C to 290 C.
In some embodiments, the hydrogenolysis reaction may be conducted under basic
conditions, preferably at a pH of 8 to 13, and even more preferably at a pH of
10 to 12. In
some embodiments, the hydrogenolysis reaction may be conducted at a pressure
ranging
between 1 bar (absolute) and 150 bar, and preferably at a pressure ranging
between 15 bar
and 140 bar, and even more preferably at a pressure ranging between 50 bar and
110 bar.
The hydrogen used in the hydrogenolysis reaction may include external
hydrogen,
recycled hydrogen, in situ generated hydrogen, or any combination thereof.
In some embodiments, the reaction products of the hydrogenolysis reaction may
comprise greater than 25% by mole, or alternatively, greater than 30% by mole
of polyols,
32
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
which may result in a greater conversion to a biofuel in a subsequent
processing reaction.
In some embodiments, hydrogenolysis may be conducted under neutral or acidic
conditions, as needed to accelerate hydrolysis reactions in addition to the
hydrogenolysis
reaction. For example, hydrolysis of oligomeric carbohydrates may be combined
with
hydrogenation to produce sugar alcohols, which may undergo hydrogenolysis.
A second aspect of hydrogenolysis entails the breaking of -OH bonds such as:
RC(H)2-0H + H2
RCH3 H20. This reaction is also called "hydrodeoxygenation,"
and may occur in parallel with C-C bond breaking hydrogenolysis. Diols may be
converted to mono-oxygenates via this reaction. As reaction severity is
increased with
increased temperature or contact time with catalyst, the concentration of
polyols and diols
relative to mono-oxygenates may diminish as a result of hydrodeoxygenation.
Selectivity
for C-C vs. C-OH bond hydrogenolysis will vary with catalyst type and
formulation. Full
de-oxygenation to alkanes may also occur, but is generally undesirable if the
intent is to
produce mono-oxygenates or diols and polyols which may be condensed or
oligomerized
to higher molecular weight compounds in a subsequent processing step.
Typically, it is
desirable to send only mono-oxygenates or diols to subsequent processing
steps, as higher
polyols may lead to excessive coke formation during condensation or
oligomerization.
Alkanes, in contrast, are essentially unreactive and cannot be readily
combined to produce
higher molecular compounds.
Once oxygenated intermediates have been formed by a hydrogenolysis reaction, a
portion of the reaction product may be recirculated to the hydrothermal
digestion unit to
serve as an internally generated digestion solvent. Another portion of the
reaction product
may be withdrawn and subsequently processed by further reforming reactions to
form a
biofuel. Before being subjected to the further reforming reactions, the
oxygenated
intermediates may optionally be separated into different components. Suitable
separations
may include, for example, phase separation, solvent stripping columns,
extractors, filters,
distillations and the like. In some embodiments, a separation of lignin from
the
oxygenated intermediates may be conducted before the reaction product is
subsequently
processed further or recirculated to the hydrothermal digestion unit.
The oxygenated intermediates may be processed to produce a fuel blend in one
or
more processing reactions. In some embodiments, a condensation reaction may be
used
along with other reactions to generate a fuel blend and may be catalyzed by a
catalyst
comprising an acid, a base, or both. In general, without being limited to any
particular
33
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
theory, it is believed that the basic condensation reactions may involve a
series of steps
involving: (1) an optional dehydrogenation reaction; (2) an optional
dehydration reaction
that may be acid catalyzed; (3) an aldol condensation reaction; (4) an
optional ketonization
reaction; (5) an optional furanic ring opening reaction; (6) hydrogenation of
the resulting
condensation products to form a >C4 hydrocarbon; and (7) any combination
thereof. Acid
catalyzed condensations may similarly entail optional hydrogenation or
dehydrogenation
reactions, dehydration, and oligomerization reactions. Additional polishing
reactions may
also be used to conform the product to a specific fuel standard, including
reactions
conducted in the presence of hydrogen and a hydrogenation catalyst to remove
functional
groups from final fuel product. In some embodiments, a basic catalyst, a
catalyst having
both an acid and a base functional site, and optionally comprising a metal
function, may
also be used to effect the condensation reaction.
In some embodiments, an aldol condensation reaction may be used to produce a
fuel blend meeting the requirements for a diesel fuel or jet fuel. Traditional
diesel fuels are
petroleum distillates rich in paraffinic hydrocarbons. They have boiling
ranges as broad as
187 C to 417 C, which are suitable for combustion in a compression ignition
engine, such
as a diesel engine vehicle. The American Society of Testing and Materials
(ASTM)
establishes the grade of diesel according to the boiling range, along with
allowable ranges
of other fuel properties, such as cetane number, cloud point, flash point,
viscosity, aniline
point, sulfur content, water content, ash content, copper strip corrosion, and
carbon residue.
Thus, any fuel blend meeting ASTM D975 may be defined as diesel fuel.
The present disclosure also provides methods to produce jet fuel. Jet fuel is
clear to
straw colored. The most common fuel is an unleaded/paraffin oil-based fuel
classified as
Aeroplane A-1, which is produced to an internationally standardized set of
specifications.
Jet fuel is a mixture of a large number of different hydrocarbons, possibly as
many as a
thousand or more. The range of their sizes (molecular weights or carbon
numbers) is
restricted by the requirements for the product, for example, freezing point or
smoke point.
Kerosene-type Airplane fuel (including Jet A and Jet A-1) has a carbon number
distribution between C8 and C16. Wide-cut or naphtha-type Airplane fuel
(including Jet
B) typically has a carbon number distribution between C5 and C15. A fuel blend
meeting
ASTM D1655 may be defined as jet fuel.
In certain embodiments, both Airplanes (Jet A and Jet B) contain a number of
additives. Useful additives include, but are not limited to, antioxidants,
antistatic agents,
34
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
corrosion inhibitors, and fuel system icing inhibitor (FSII) agents.
Antioxidants prevent
gumming and usually, are based on alkylated phenols, for example, A0-30, A0-
31, or
A0-37. Antistatic agents dissipate static electricity and prevent sparking.
Stadis 450 with
dinonylnaphthylsulfonic acid (DINNSA) as the active ingredient, is an example.
Corrosion
inhibitors (e.g., DCI-4A) are used for civilian and military fuels, and DCI-6A
is used for
military fuels. FSII agents, include, for example, Di-EGME.
In some embodiments, the oxygenated intermediates may comprise a carbonyl-
containing compound that may take part in a base catalyzed condensation
reaction. In
some embodiments, an optional dehydrogenation reaction may be used to increase
the
amount of carbonyl-containing compounds in the oxygenated intermediate stream
to be
used as a feed to the condensation reaction. In these embodiments, the
oxygenated
intermediates and/or a portion of the bio-based feedstock stream may be
dehydrogenated in
the presence of a catalyst.
In some embodiments, a dehydrogenation catalyst may be preferred for an
oxygenated intermediate stream comprising alcohols, diols, and triols. In
general, alcohols
cannot participate in aldol condensation directly. The hydroxyl group or
groups present
may be converted into carbonyls (e.g., aldehydes, ketones, etc.) in order to
participate in an
aldol condensation reaction. A dehydrogenation catalyst may be included to
effect
dehydrogenation of any alcohols, diols, or polyols present to form ketones and
aldehydes.
The dehydration catalyst is typically formed from the same metals as used for
hydrogenation, hydrogenolysis, or aqueous phase reforming. These catalysts are
described
in more detail above. Dehydrogenation yields may be enhanced by the removal or
consumption of hydrogen as it forms during the reaction. The dehydrogenation
step may
be carried out as a separate reaction step before an aldol condensation
reaction, or the
dehydrogenation reaction may be carried out in concert with the aldol
condensation
reaction.
For concerted dehydrogenation and aldol condensation reactions, the
dehydrogenation and aldol condensation functions may take place on the same
catalyst.
For example, a metal hydrogenation/dehydrogenation functionality may be
present on
catalyst comprising a basic functionality.
The dehydrogenation reaction may result in the production of a carbonyl-
containing
compound. Suitable carbonyl-containing compounds may include, but are not
limited to,
any compound comprising a carbonyl functional group that may form carbanion
species or
may react in a condensation reaction with a carbanion species. In an
embodiment, a
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
carbonyl-containing compound may include, but is not limited to, ketones,
aldehydes,
furfurals, hydroxy carboxylic acids, and, carboxylic acids. Ketones may
include, without
limitation, hydroxyketones, cyclic ketones, diketones, acetone, propanone, 2-
oxopropanal,
butanone, butane-2,3-dione, 3-hydroxybutane-2-one, pentanone, cyclopentanone,
pentane-
2,3-dione, pentane-2,4-dione, hexanone, cyclohexanone, 2-methyl-
cyclopentanone,
heptanone, octanone, nonanone, decanone, undecanone, dodecanone,
methylglyoxal,
butanedione, pentanedione, diketohexane, dihydroxyacetone, and isomers
thereof.
Aldehydes may include, without limitation, hydroxyaldehydes, acetaldehyde,
glyceraldehyde, propionaldehyde, butyraldehyde, pentanal, hexanal, heptanal,
octanal,
nonal, decanal, undecanal, dodecanal, and isomers thereof. Carboxylic acids
may include,
without limitation, formic acid, acetic acid, propionic acid, butanoic acid,
pentanoic acid,
hexanoic acid, heptanoic acid, isomers and derivatives thereof, including
hydroxylated
derivatives, such as 2-hydroxybutanoic acid and lactic acid. Furfurals may
include,
without limitation, hydroxylmethylfurfural, 5-hydroxymethy1-2(5H)-furanone,
dihydro-5-
(hydroxymethyl)-2(3H)-furanone, tetrahydro-2-furoic acid, dihydro-5-
(hydroxymethyl)-
2(3H)-furanone, tetrahydrofurfuryl alcohol,
1-(2-furyl)ethanol,
hydroxymethyltetrahydrofurfural, and isomers thereof.
In an embodiment, the
dehydrogenation reaction may result in the production of a carbonyl-containing
compound
that is combined with the oxygenated intermediates to become a part of the
oxygenated
intermediates fed to the condensation reaction.
In an embodiment, an acid catalyst may be used to optionally dehydrate at
least a
portion of the oxygenated intermediate stream. Suitable acid catalysts for use
in the
dehydration reaction may include, but are not limited to, mineral acids (e.g.,
HC1, H2504),
solid acids (e.g., zeolites, ion-exchange resins) and acid salts (e.g.,
LaC13). Additional acid
catalysts may include, without limitation, zeolites, carbides, nitrides,
zirconia, alumina,
silica, aluminosilicates, phosphates, titanium oxides, zinc oxides, vanadium
oxides,
lanthanum oxides, yttrium oxides, scandium oxides, magnesium oxides, cerium
oxides,
barium oxides, calcium oxides, hydroxides, heteropolyacids, inorganic acids,
acid modified
resins, base modified resins, and any combination thereof. In some
embodiments, the
dehydration catalyst may also include a modifier. Suitable modifiers may
include, for
example, La, Y, Sc, P, B, Bi, Li, Na, K, Rb, Cs, Mg, Ca, Sr, Ba, and any
combination
thereof. The modifiers may be useful, inter alia, to carry out a concerted
hydrogenation/
dehydrogenation reaction with the dehydration reaction. In some embodiments,
the
36
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
dehydration catalyst may also include a metal. Suitable metals may include,
for example,
Cu, Ag, Au, Pt, Ni, Fe, Co, Ru, Zn, Cd, Ga, In, Rh, Pd, Ir, Re, Mn, Cr, Mo, W,
Sn, Os,
alloys, and any combination thereof. The dehydration catalyst may be self
supporting,
supported on an inert support or resin, or it may be dissolved in solution.
In some embodiments, the dehydration reaction may occur in the vapor phase. In
other embodiments, the dehydration reaction may occur in the liquid phase. For
liquid
phase dehydration reactions, an aqueous solution may be used to carry out the
reaction. In
an embodiment, other solvents in addition to water, may be used to form the
aqueous
solution. For example, water soluble organic solvents may be present. Suitable
solvents
may include, but are not limited to, hydroxymethylfurfural (HMF),
dimethylsulfoxide
(DMSO), 1-methyl-n-pyrollidone (NMP), and any combination thereof. Other
suitable
aprotic solvents may also be used alone or in combination with any of these
solvents.
In an embodiment, the processing reactions may comprise an optional
ketonization
reaction. A ketonization reaction may increase the number of ketone functional
groups
within at least a portion of the oxygenated intermediates. For example, an
alcohol may be
converted into a ketone in a ketonization reaction. Ketonization may be
carried out in the
presence of a basic catalyst. Any of the basic catalysts described above as
the basic
component of the aldol condensation reaction may be used to effect a
ketonization reaction.
Suitable reaction conditions are known to one of ordinary skill in the art and
generally
correspond to the reaction conditions listed above with respect to the aldol
condensation
reaction. The ketonization reaction may be carried out as a separate reaction
step, or it may
be carried out in concert with the aldol condensation reaction. The inclusion
of a basic
functional site on the aldol condensation catalyst may result in concerted
ketonization and
aldol condensation reactions.
In some embodiments, the processing reactions may comprise an optional furanic
ring opening reaction. A furanic ring opening reaction may result in the
conversion of at
least a portion of any oxygenated intermediates comprising a furanic ring into
compounds
that are more reactive in an aldol condensation reaction. A furanic ring
opening reaction
may be carried out in the presence of an acidic catalyst. Any of the acid
catalysts described
above as the acid component of the aldol condensation reaction may be used to
effect a
furanic ring opening reaction. Suitable reaction conditions are known to one
of ordinary
skill in the art and generally correspond to the reaction conditions listed
above with respect
to the aldol condensation reaction. The furanic ring opening reaction may be
carried out as
37
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
a separate reaction step, or it may be carried out in concert with the aldol
condensation
reaction. The inclusion of an acid functional site on the aldol condensation
catalyst may
result in a concerted furanic ring opening reaction and aldol condensation
reactions. Such
an embodiment may be advantageous as any furanic rings may be opened in the
presence
of an acid functionality and reacted in an aldol condensation reaction using a
basic
functionality. Such a concerted reaction scheme may allow for the production
of a greater
amount of higher hydrocarbons to be formed for a given oxygenated intermediate
feed.
In some embodiments, production of a >C4 compound may occur by condensation,
which may include aldol condensation of the oxygenated intermediates in the
presence of a
condensation catalyst. Aldol-condensation generally involves the carbon-carbon
coupling
between two compounds, at least one of which may contain a carbonyl group, to
form a
larger organic molecule. For example, acetone may react with
hydroxymethylfurfural to
form a C9 species, which may subsequently react with another
hydroxymethylfurfural
molecule to form a C15 species. In various embodiments, the reaction is
usually carried
out in the presence of a condensation catalyst. The condensation reaction may
be carried
out in the vapor or liquid phase. In an embodiment, the reaction may take
place at a
temperature ranging from 5 C to 375 C depending on the reactivity of the
carbonyl group.
The condensation catalyst will generally be a catalyst capable of forming
longer
chain compounds by linking two molecules through a new carbon-carbon bond,
such as a
basic catalyst, a multi-functional catalyst having both acid and base
functionalities, or
either type of catalyst also comprising an optional metal functionality. In
some
embodiments, the multi-functional catalyst may be a catalyst having both
strong acid and
strong base functionalities. In some embodiments, aldol catalysts may comprise
Li, Na, K,
Cs, B, Rb, Mg, Ca, Sr, Si, Ba, Al, Zn, Ce, La, Y, Sc, Y, Zr, Ti, hydrotalcite,
zinc-
aluminate, phosphate, base-treated aluminosilicate zeolite, a basic resin,
basic nitride,
alloys or any combination thereof. In some embodiments, the base catalyst may
also
comprise an oxide of Ti, Zr, V, Nb, Ta, Mo, Cr, W, Mn, Re, Al, Ga, In, Co, Ni,
Si, Cu, Zn,
Sn, Cd, Mg, P, Fe, or any combination thereof. In some embodiments, the
condensation
catalyst comprises mixed-oxide base catalysts. Suitable mixed-oxide base
catalysts may
comprise a combination of magnesium, zirconium, and oxygen, which may
comprise,
without limitation: Si--Mg--0, Mg--Ti--0, Y--Mg--0, Y--Zr--0, Ti--Zr--0, Ce--
Zr--0,
Ce--Mg--0, Ca--Zr--0, La--Zr--0, B--Zr--0, La--Ti--0, B--Ti-0, and any
combination
thereof. Different atomic ratios of Mg/Zr or the combinations of various other
elements
38
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
constituting the mixed oxide catalyst may be used ranging from 0.01 to 50. In
some
embodiments, the condensation catalyst may further include a metal or alloys
comprising
metals, such as Cu, Ag, Au, Pt, Ni, Fe, Co, Ru, Zn, Cd, Ga, In, Rh, Pd, Ir,
Re, Mn, Cr, Mo,
W, Sn, Bi, Pb, Os, alloys and combinations thereof. Such metals may be
preferred when a
dehydrogenation reaction is to be carried out in concert with the aldol
condensation
reaction. In some embodiments, preferred Group IA materials may include Li,
Na, K, Cs
and Rb. In some embodiments, preferred Group IIA materials may include Mg, Ca,
Sr and
Ba. In some embodiments, Group JIB materials may include Zn and Cd. In some
embodiments, Group IIIB materials may include Y and La. Basic resins may
include
resins that exhibit basic functionality. The basic catalyst may be self-
supporting or
adhered to any one of the supports further described below, including supports
containing
carbon, silica, alumina, zirconia, titania, vanadia, ceria, nitride, boron
nitride,
heteropolyacids, alloys and mixtures thereof.
In one embodiment, the condensation catalyst may be derived from the
combination of MgO and A1203 to form a hydrotalcite material. Another
preferred
material contains ZnO and A1203 in the form of a zinc aluminate spinel. Yet
another
preferred material is a combination of ZnO, A1203, and CuO. Each of these
materials may
also contain an additional metal function provided by a Group VIIIB metal,
such as Pd or
Pt. Such metals may be preferred when a dehydrogenation reaction is to be
carried out in
concert with the aldol condensation reaction. In some embodiments, the basic
catalyst may
be a metal oxide containing Cu, Ni, Zn, V, Zr, or mixtures thereof. In other
embodiments,
the basic catalyst may be a zinc aluminate metal containing Pt, Pd Cu, Ni, or
mixtures
thereof.
In some embodiments, a base-catalyzed condensation reaction may be performed
using a condensation catalyst with both an acidic and a basic functionality.
The acid-aldol
condensation catalyst may comprise hydrotalcite, zinc-aluminate, phosphate,
Li, Na, K, Cs,
B, Rb, Mg, Si, Ca, Sr, Ba, Al, Ce, La, Sc, Y, Zr, Ti, Zn, Cr, or any
combination thereof. In
further embodiments, the acid-base catalyst may also include one or more
oxides from the
group of Ti, Zr, V, Nb, Ta, Mo, Cr, W, Mn, Re, Al, Ga, In, Fe, Co, Ir, Ni, Si,
Cu, Zn, Sn,
Cd, P, and combinations thereof. In some embodiments, the acid-base catalyst
may
include a metal functionality provided by Cu, Ag, Au, Pt, Ni, Fe, Co, Ru, Zn,
Cd, Ga, In,
Rh, Pd, Ir, Re, Mn, Cr, Mo, W, Sn, Os, alloys or combinations thereof. In some
embodiments, the catalyst may further include Zn, Cd or phosphate. In some
39
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
embodiments, the condensation catalyst may be a metal oxide containing Pd, Pt,
Cu or Ni,
and even more preferably an aluminate or zirconium metal oxide containing Mg
and Cu,
Pt, Pd or Ni. The acid-base catalyst may also include a hydroxyapatite (HAP)
combined
with any one or more of the above metals. The acid-base catalyst may be self-
supporting
or adhered to any one of the supports further described below, including
supports
containing carbon, silica, alumina, zirconia, titania, vanadia, ceria,
nitride, boron nitride,
heteropolyacids, alloys and mixtures thereof.
In some embodiments, the condensation catalyst may also include zeolites and
other microporous supports that contain Group IA compounds, such as Li, Na, K,
Cs and
Rb. Preferably, the Group IA material may be present in an amount less than
that required
to neutralize the acidic nature of the support. A metal function may also be
provided by
the addition of group VIIIB metals, or Cu, Ga, In, Zn or Sn. In one
embodiment, the
condensation catalyst may be derived from the combination of MgO and A1203 to
form a
hydrotalcite material. Another preferred material may contain a combination of
MgO and
Zr02, or a combination of ZnO and A1203. Each of these materials may also
contain an
additional metal function provided by copper or a Group VIIIB metal, such as
Ni, Pd, Pt,
or combinations of the foregoing.
The condensation catalyst may be self-supporting (i.e., the catalyst does not
need
another material to serve as a support), or may require a separate support
suitable for
suspending the catalyst in the reactant stream. One exemplary support is
silica, especially
silica having a high surface area (greater than 100 square meters per gram),
obtained by
sol-gel synthesis, precipitation, or fuming. In other embodiments,
particularly when the
condensation catalyst is a powder, the catalyst system may include a binder to
assist in
forming the catalyst into a desirable catalyst shape. Applicable forming
processes may
include extrusion, pelletization, oil dropping, or other known processes. Zinc
oxide,
alumina, and a peptizing agent may also be mixed together and extruded to
produce a
formed material. After drying, this material may be calcined at a temperature
appropriate
for formation of the catalytically active phase. Other catalyst supports as
known to one
having ordinary skill in the art may also be used.
In some embodiments, a dehydration catalyst, a dehydrogenation catalyst, and
the
condensation catalyst may be present in the same reactor as the reaction
conditions overlap
to some degree. In these embodiments, a dehydration reaction and/or a
dehydrogenation
reaction may occur substantially simultaneously with the condensation
reaction. In some
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
embodiments, a catalyst may comprise active sites for a dehydration reaction
and/or a
dehydrogenation reaction in addition to a condensation reaction. For example,
a catalyst
may comprise active metals for a dehydration reaction and/or a dehydrogenation
reaction
along with a condensation reaction at separate sites on the catalyst or as
alloys. Suitable
active elements may comprise any of those listed above with respect to the
dehydration
catalyst, dehydrogenation catalyst, and the condensation catalyst.
Alternately, a physical
mixture of dehydration, dehydrogenation, and condensation catalysts may be
employed.
While not intending to be limited by theory, it is believed that using a
condensation
catalyst comprising a metal and/or an acid functionality may assist in pushing
the
equilibrium limited aldol condensation reaction toward completion.
Advantageously, this
may be used to effect multiple condensation reactions with dehydration and/or
dehydrogenation of intermediates, in order to form (via condensation,
dehydration, and/or
dehydrogenation) higher molecular weight oligomers as desired to produce jet
or diesel
fuel.
The specific >C4 compounds produced in the condensation reaction may depend on
various factors, including, without limitation, the type of oxygenated
intermediates in the
reactant stream, condensation temperature, condensation pressure, the
reactivity of the
catalyst, and the flow rate of the reactant stream. In general, the
condensation reaction may
be carried out at a temperature at which the thermodynamics of the proposed
reaction are
favorable. For condensed phase liquid reactions, the pressure within the
reactor may be
sufficient to maintain at least a portion of the reactants in the condensed
liquid phase at the
reactor inlet. For vapor phase reactions, the reaction may be carried out at a
temperature
where the vapor pressure of the oxygenates is at least 0.1 bar, and the
thermodynamics of
the reaction are favorable. The condensation temperature will vary depending
upon the
specific oxygenated intermediates used, but may generally range between 75 C
and 500 C
for reactions taking place in the vapor phase, and more preferably range
between 125oC
and 450 C. For liquid phase reactions, the condensation temperature may range
between
5 C and 475 C, and the condensation pressure may range between 0.01 bar and
100 bar.
Preferably, the condensation temperature may range between 15 C and 300 C, or
between
15 C and 250 C.
Varying the factors above, as well as others, will generally result in a
modification
to the specific composition and yields of the >C4 compounds. For example,
varying the
temperature and/or pressure of the reactor system, or the particular catalyst
formulations,
41
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
may result in the production of >C4 alcohols and/or ketones instead of >C4
hydrocarbons.
The >C4 hydrocarbon product may also contain a variety of olefins, and alkanes
of various
sizes (typically branched alkanes). Depending upon the condensation catalyst
used, the
hydrocarbon product may also include aromatic and cyclic hydrocarbon
compounds. The
>C4 hydrocarbon product may also contain undesirably high levels of olefins,
which may
lead to coking or deposits in combustion engines, or other undesirable
hydrocarbon
products. In such cases, the hydrocarbons may optionally be hydrogenated to
reduce the
ketones to alcohols and hydrocarbons, while the alcohols and olefinic
hydrocarbons may
be reduced to alkanes, thereby forming a more desirable hydrocarbon product
having
reduced levels of olefins, aromatics or alcohols.
The condensation reactions may be carried out in any reactor of suitable
design,
including continuous-flow, batch, semi-batch or multi-system reactors, without
limitation
as to design, size, geometry, flow rates, and the like. The reactor system may
also use a
fluidized catalytic bed system, a swing bed system, fixed bed system, a moving
bed
system, or a combination of the above. In some embodiments, bi-phasic (e.g.,
liquid-
liquid) and tri-phasic (e.g., liquid-liquid-solid) reactors may be used to
carry out the
condensation reactions.
In a continuous flow system, the reactor system may include an optional
dehydrogenation bed adapted to produce dehydrogenated oxygenated
intermediates, an
optional dehydration bed adapted to produce dehydrated oxygenated
intermediates, and a
condensation bed adapted to produce >C4 compounds from the oxygenated
intermediates.
The dehydrogenation bed may be configured to receive the reactant stream and
produce the
desired oxygenated intermediates, which may have an increase in the amount of
carbonyl-
containing compounds. The dehydration bed may be configured to receive the
reactant
stream and produce the desired oxygenated intermediates. The condensation bed
may be
configured to receive the oxygenated intermediates for contact with the
condensation
catalyst and production of the desired >C4 compounds. For systems with one or
more
finishing steps, an additional reaction bed for conducting the finishing
process or processes
may be included after the condensation bed.
In some embodiments, the optional dehydration reaction, the optional
dehydrogenation reaction, the optional ketonization reaction, the optional
ring opening
reaction, and the condensation reaction catalyst beds may be positioned within
the same
reactor vessel or in separate reactor vessels in fluid communication with each
other. Each
42
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
reactor vessel preferably may include an outlet adapted to remove the product
stream from
the reactor vessel. For systems with one or more finishing steps, the
finishing reaction bed
or beds may be within the same reactor vessel along with the condensation bed
or in a
separate reactor vessel in fluid communication with the reactor vessel having
the
condensation bed.
In some embodiments, the reactor system also may include additional outlets to
allow for the removal of portions of the reactant stream to further advance or
direct the
reaction to the desired reaction products, and to allow for the collection and
recycling of
reaction byproducts for use in other portions of the system. In some
embodiments, the
reactor system also may include additional inlets to allow for the
introduction of
supplemental materials to further advance or direct the reaction to the
desired reaction
products, and to allow for the recycling of reaction byproducts for use in
other reactions.
In some embodiments, the reactor system also may include elements which allow
for the separation of the reactant stream into different components which may
find use in
different reaction schemes or to simply promote the desired reactions. For
instance, a
separator unit, such as a phase separator, extractor, purifier or distillation
column, may be
installed prior to the condensation step to remove water from the reactant
stream for
purposes of advancing the condensation reaction to favor the production of
higher
hydrocarbons. In some embodiments, a separation unit may be installed to
remove specific
intermediates to allow for the production of a desired product stream
containing
hydrocarbons within a particular carbon number range, or for use as end
products or in
other systems or processes. The condensation reaction may produce a broad
range of
compounds with carbon numbers ranging from C4 to C30 or greater. Exemplary
compounds may include, for example, >C4 alkanes, >C4 alkenes, >C5
cycloalkanes, >C5
cycloalkenes, aryls, fused aryls, >C4 alcohols, >C4 ketones, and mixtures
thereof. The
>C4 alkanes and >C4 alkenes may range from 4 to 30 carbon atoms (i.e. C4 ¨ C30
alkanes
and C4 ¨ C30 alkenes) and may be branched or straight chain alkanes or
alkenes. The >C4
alkanes and >C4 alkenes may also include fractions of C7 ¨ C14, C12 ¨ C24
alkanes and
alkenes, respectively, with the C7 ¨ C14 fraction directed to jet fuel blends,
and the C12 ¨
C24 fraction directed to diesel fuel blends and other industrial applications.
Examples of
various >C4 alkanes and >C4 alkenes may include, without limitation, butane,
butene,
pentane, pentene, 2-methylbutane, hexane, hexene, 2-methylpentane, 3-
methylpentane,
2,2-dimethylbutane, 2,3-dimethylbutane, heptane, heptene, octane, octene,
2,2,4,-
43
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
trimethylpentane, 2,3-dimethyl hexane, 2,3,4-trimethylpentane, 2,3-
dimethylpentane,
nonane, nonene, decane, decene, undecane, undecene, dodecane, dodecene,
tridecane,
tridecene, tetradecane, tetradecene, pentadecane, pentadecene, hexadecane,
hexadecene,
heptyldecane, heptyldecene, octyldecane, octyldecene, nonyldecane,
nonyldecene,
eico sane, eicosene, uneico sane, uneicosene, doeico sane, doeicosene,
trieicosane,
trieicosene, tetraeicosane, tetraeicosene, and isomers thereof.
The >C5 cycloalkanes and >C5 cycloalkenes may have from 5 to 30 carbon atoms
and may be unsubstituted, mono-substituted or multi-substituted. In the case
of mono-
substituted and multi-substituted compounds, the substituted group may include
a branched
>C3 alkyl, a straight chain >C1 alkyl, a branched >C3 alkylene, a straight
chain >C1
alkylene, a straight chain >C2 alkylene, an aryl group, or a combination
thereof. In one
embodiment, at least one of the substituted groups may include a branched C3 ¨
C12 alkyl,
a straight chain Cl ¨ C12 alkyl, a branched C3 ¨ C12 alkylene, a straight
chain Cl ¨ C12
alkylene, a straight chain C2 ¨ C12 alkylene, an aryl group, or a combination
thereof. In
yet other embodiments, at least one of the substituted groups may include a
branched C3 ¨
C4 alkyl, a straight chain Cl ¨ C4 alkyl, a branched C3 ¨ C4 alkylene, a
straight chain Cl
¨ C4 alkylene, a straight chain C2 ¨ C4 alkylene, an aryl group, or any
combination
thereof. Examples of desirable >C5 cycloalkanes and >C5 cycloalkenes may
include,
without limitation, cyclopentane, cyclopentene, cyclohexane, cyclohexene,
methylcyclopentane, methylcyclopentene, ethylcyclopentane, ethylcyclopentene,
ethylcyclohexane, ethylcyclohexene, and isomers thereof.
Aryl groups contain an aromatic hydrocarbon in either an unsubstituted
(phenyl),
mono-substituted or multi-substituted form. In the case of mono-substituted
and multi-
substituted compounds, the substituted group may include a branched >C3 alkyl,
a straight
chain >C1 alkyl, a branched >C3 alkylene, a straight chain >C2 alkylene, a
phenyl group,
or a combination thereof. In some embodiments, at least one of the substituted
groups may
include a branched C3 ¨ C12 alkyl, a straight chain Cl ¨ C12 alkyl, a branched
C3 ¨ C12
alkylene, a straight chain C2 ¨ C12 alkylene, a phenyl group, or any
combination thereof.
In yet other embodiments, at least one of the substituted groups may include a
branched C3
¨ C4 alkyl, a straight chain Cl ¨ C4 alkyl, a branched C3 ¨ C4 alkylene, a
straight chain
C2 ¨ C4 alkylene, a phenyl group, or any combination thereof. Examples of
various aryl
compounds may include, without limitation, benzene, toluene, xylene
(dimethylbenzene),
ethyl benzene, para-xylene, meta-xylene, ortho-xylene, and C9 aromatics.
44
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
Fused aryls contain bicyclic and polycyclic aromatic hydrocarbons, in either
an
unsubstituted, mono-substituted or multi-substituted form. In the case of mono-
substituted
and multi-substituted compounds, the substituted group may include a branched
>C3 alkyl,
a straight chain >C1 alkyl, a branched >C3 alkylene, a straight chain >C2
alkylene, a
phenyl group, or a combination thereof. In other embodiments, at least one of
the
substituted groups may include a branched C3 ¨ C4 alkyl, a straight chain Cl ¨
C4 alkyl, a
branched C3 ¨ C4 alkylene, a straight chain C2 ¨ C4 alkylene, a phenyl group,
or any
combination thereof. Examples of various fused aryls may include, without
limitation,
naphthalene, anthracene, tetrahydronaphthalene, and decahydronaphthalene,
indane,
indene, and isomers thereof.
The moderate fractions, such as C7 ¨ C14, may be separated for jet fuel, while
heavier fractions, such as C12 ¨ C24, may be separated for diesel use. The
heaviest
fractions may be used as lubricants or cracked to produce additional gasoline
and/or diesel
fractions. The >C4 compounds may also find use as industrial chemicals,
whether as an
intermediate or an end product. For example, the aryls toluene, xylene,
ethylbenzene, para-
xylene, meta-xylene, and ortho-xylene may find use as chemical intermediates
for the
production of plastics and other products. Meanwhile, C9 aromatics and fused
aryls, such
as naphthalene, anthracene, tetrahydronaphthalene, and decahydronaphthalene,
may find
use as solvents in industrial processes.
In some embodiments, additional processes may be used to treat the fuel blend
to
remove certain components or further conform the fuel blend to a diesel or jet
fuel
standard. Suitable techniques may include hydrotreating to reduce the amount
of or
remove any remaining oxygen, sulfur, or nitrogen in the fuel blend. The
conditions for
hydrotreating a hydrocarbon stream will be known to one of ordinary skill in
the art.
In some embodiments, hydrogenation may be carried out in place of or after the
hydrotreating process to saturate at least some olefinic bonds. In some
embodiments, a
hydrogenation reaction may be carried out in concert with the aldol
condensation reaction
by including a metal functional group with the aldol condensation catalyst.
Such
hydrogenation may be performed to conform the fuel blend to a specific fuel
standard (e.g.,
a diesel fuel standard or a jet fuel standard). The hydrogenation of the fuel
blend stream
may be carried out according to known procedures, either with the continuous
or batch
method. The hydrogenation reaction may be used to remove remaining carbonyl
groups
and/or hydroxyl groups. In such cases, any of the hydrogenation catalysts
described above
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
may be used. In general, the finishing step may be carried out at finishing
temperatures
ranging between 80 C and 250 C, and finishing pressures may range between 5
bar and
150 bar. In some embodiments, the finishing step may be conducted in the vapor
phase or
liquid phase, and use, external hydrogen, recycled hydrogen, or combinations
thereof, as
necessary.
In some embodiments, isomerization may be used to treat the fuel blend to
introduce a desired degree of branching or other shape selectivity to at least
some
components in the fuel blend. It may also be useful to remove any impurities
before the
hydrocarbons are contacted with the isomerization catalyst. The isomerization
step may
comprise an optional stripping step, wherein the fuel blend from the
oligomerization
reaction may be purified by stripping with water vapor or a suitable gas such
as light
hydrocarbon, nitrogen or hydrogen. The optional stripping step may be carried
out in a
countercurrent manner in a unit upstream of the isomerization catalyst,
wherein the gas and
liquid are contacted with each other, or before the actual isomerization
reactor in a separate
stripping unit utilizing countercurrent principle.
After the optional stripping step the fuel blend may be passed to a reactive
isomerization unit comprising one or more catalyst beds. The catalyst beds of
the
isomerization unit may operate either in co-current or countercurrent manner.
In the
isomerization unit, the pressure may vary between 20 bar to 150 bar,
preferably between 20
bar to 100 bar, the temperature ranging between 195 C and 500 C, preferably
between
300 C and 400 C. In the isomerization unit, any isomerization catalyst known
in the art
may be used. In some embodiments, suitable isomerization catalysts may contain
molecular sieve and/or a metal from Group VII and/or a carrier. In some
embodiments, the
isomerization catalyst may contain SAPO-11 or SAP041 or ZSM-22 or ZSM-23 or
ferrierite and Pt, Pd or Ni and A1203 or Si02. Typical isomerization catalysts
may include,
for example, Pt/SAP0-11/A1203, Pt/ZSM-22/A1203, Pt/ZSM-23/A1203 and Pt/SAP0-
11/Si02.
Other factors, such as the concentration of water or undesired oxygenated
intermediates, may also effect the composition and yields of the >C4
compounds, as well
as the activity and stability of the condensation catalyst. In such cases, the
process may
include a dewatering step that removes a portion of the water prior to the
condensation
reaction and/or the optional dehydration reaction, or a separation unit for
removal of the
undesired oxygenated intermediates. For instance, a separator unit, such as a
phase
46
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
separator, extractor, purifier or distillation column, may be installed prior
to the
condensation reactor so as to remove a portion of the water from the reactant
stream
containing the oxygenated intermediates. A separation unit may also be
installed to
remove specific oxygenated intermediates to allow for the production of a
desired product
stream containing hydrocarbons within a particular carbon range, or for use as
end
products or in other systems or processes.
Thus, in some embodiments, the fuel blend produced by the processes described
herein may be a hydrocarbon mixture that meets the requirements for jet fuel
(e.g.,
conforms with ASTM D1655). In other embodiments, the product of the processes
described herein may be a hydrocarbon mixture that comprises a fuel blend
meeting the
requirements for a diesel fuel (e.g., conforms with ASTM D975).
In other embodiments, a fuel blend comprising gasoline hydrocarbons (i.e., a
gasoline fuel) may be produced. "Gasoline hydrocarbons" refer to hydrocarbons
predominantly comprising C5-9 hydrocarbons, for example, C6-8 hydrocarbons,
and
having a boiling point range from 32 C (90 F) to 204 C (400 F). Gasoline
hydrocarbons
may include, but are not limited to, straight run gasoline, naphtha, fluidized
or thermally
catalytically cracked gasoline, VB gasoline, and coker gasoline. Gasoline
hydrocarbons
content is determined by ASTM Method D2887.
In yet other embodiments, the >C2 olefins may be produced by catalytically
reacting the oxygenated intermediates in the presence of a dehydration
catalyst at a
dehydration temperature and dehydration pressure to produce a reaction stream
comprising
the >C2 olefins. The >C2 olefins may comprise straight or branched
hydrocarbons
containing one or more carbon-carbon double bonds. In general, the >C2 olefins
may
contain from 2 to 8 carbon atoms, and more preferably from 3 to 5 carbon
atoms. In some
embodiments, the olefins may comprise propylene, butylene, pentylene, isomers
of the
foregoing, and mixtures of any two or more of the foregoing. In other
embodiments, the
>C2 olefins may include >C4 olefins produced by catalytically reacting a
portion of the
>C2 olefins over an olefin isomerization catalyst.
The dehydration catalyst may comprise a member selected from the group
consisting of an acidic alumina, aluminum phosphate, silica-alumina phosphate,
amorphous silica-alumina, aluminosilicate, zirconia, sulfated zirconia,
tungstated zirconia,
tungsten carbide, molybdenum carbide, titania, sulfated carbon, phosphated
carbon,
phosphated silica, phosphated alumina, acidic resin, heteropolyacid, inorganic
acid, and a
47
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
combination of any two or more of the foregoing. In some embodiments, the
dehydration
catalyst may further comprise a modifier selected from the group consisting of
Ce, Y, Sc,
La, Li, Na, K, Rb, Cs, Mg, Ca, Sr, Ba, P, B, Bi, and a combination of any two
or more of
the foregoing. In other embodiments, the dehydration catalyst may further
comprise an
oxide of an element, the element selected from the group consisting of Ti, Zr,
V, Nb, Ta,
Mo, Cr, W, Mn, Re, Al, Ga, In, Fe, Co, Ir, Ni, Si, Cu, Zn, Sn, Cd, P, and a
combination of
any two or more of the foregoing. In yet other embodiments, the dehydration
catalyst may
further comprise a metal selected from the group consisting of Cu, Ag, Au, Pt,
Ni, Fe, Co,
Ru, Zn, Cd, Ga, In, Rh, Pd, Ir, Re, Mn, Cr, Mo, W, Sn, Os, an alloy of any two
or more of
the foregoing, and a combination of any two or more of the foregoing.
In yet other embodiments, the dehydration catalyst may comprise an
aluminosilicate zeolite. In some embodiments, the dehydration catalyst may
further
comprise a modifier selected from the group consisting of Ga, In, Zn, Fe, Mo,
Ag, Au, Ni,
P, Sc, Y, Ta, a lanthanide, and a combination of any two or more of the
foregoing. In some
embodiments, the dehydration catalyst may further comprise a metal selected
from the
group consisting of Cu, Ag, Au, Pt, Ni, Fe, Co, Ru, Zn, Cd, Ga, In, Rh, Pd,
Ir, Re, Mn, Cr,
Mo, W, Sn, Os, an alloy of any two or more of the foregoing, and a combination
of any
two or more of the foregoing.
In other embodiments, the dehydration catalyst may comprise a bifunctional
pentasil ring-containing aluminosilicate zeolite. In some embodiments, the
dehydration
catalyst may further comprise a modifier selected from the group consisting of
Ga, In, Zn,
Fe, Mo, Ag, Au, Ni, P, Sc, Y, Ta, a lanthanide, and a combination of any two
or more of
the foregoing. In some embodiments, the dehydration catalyst may further
comprise a
metal selected from the group consisting of Cu, Ag, Au, Pt, Ni, Fe, Co, Ru,
Zn, Cd, Ga, In,
Rh, Pd, Ir, Re, Mn, Cr, Mo, W, Sn, Os, an alloy of any two or more of the
foregoing, and a
combination of any two or more of the foregoing.
The dehydration reaction may be conducted at a temperature and pressure where
the thermodynamics are favorable. In general, the reaction may be performed in
the vapor
phase, liquid phase, or a combination of both. In some embodiments, the
dehydration
temperature may range between 100 C and 500 C, and the dehydration pressure
may range
between 1 bar (absolute) and 60 bar. In some embodiments, the dehydration
temperature
may range between 125 C and 450 C. In some embodiments, the dehydration
temperature
may range between 150 C and 350 C, and the dehydration pressure may range
between 5
48
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
bar and 50 bar. In some embodiments, the dehydration temperature may range
between
175 C and 325 C.
The >C6 paraffins may be produced by catalytically reacting >C2 olefins with a
stream of >C4 isoparaffins in the presence of an alkylation catalyst at an
alkylation
temperature and alkylation pressure to produce a product stream comprising >C6
paraffins.
The >C4 isoparaffins may include alkanes and cycloalkanes having 4 to 7 carbon
atoms,
such as isobutane, isopentane, naphthenes, and higher homologues having a
tertiary carbon
atom (e.g., 2-methylbutane and 2,4-dimethylpentane), isomers of the foregoing,
and
mixtures of any two or more of the foregoing. In some embodiments, the stream
of >C4
isoparaffins may comprise internally generated >C4 isoparaffins, external >C4
isoparaffins, recycled >C4 isoparaffins, or combinations of any two or more of
the
foregoing.
The >C6 paraffins may be branched paraffins, but may also include normal
paraffins. In one version, the >C6 paraffins may comprise a member selected
from the
group consisting of a branched C6-10 alkane, a branched C6 alkane, a branched
C7 alkane,
a branched C8 alkane, a branched C9 alkane, a branched C10 alkane, or a
mixture of any
two or more of the foregoing. In one version, the >C6 paraffins may include,
for example,
dimethylbutane, 2,2-dimethylbutane, 2,3-dimethylbutane, methylpentane, 2-
methylpentane, 3-methylpentane, dimethylpentane, 2,3-dimethylpentane, 2,4-
dimethylpentane, methylhexane, 2,3-dimethylhexane, 2,3,4-trimethylpentane,
2,2,4-
trimethylpentane, 2,2,3-trimethylpentane, 2,3,3-trimethylpentane,
dimethylhexane, or
mixtures of any two or more of the foregoing.
The alkylation catalyst may comprise a member selected from the group of
sulfuric
acid, hydrofluoric acid, aluminum chloride, boron trifluoride, solid
phosphoric acid,
chlorided alumina, acidic alumina, aluminum phosphate, silica-alumina
phosphate,
amorphous silica-alumina, aluminosilicate, aluminosilicate zeolite, zirconia,
sulfated
zirconia, tungstated zirconia, tungsten carbide, molybdenum carbide, titania,
sulfated
carbon, phosphated carbon, phosphated silica, phosphated alumina, acidic
resin,
heteropolyacid, inorganic acid, and a combination of any two or more of the
foregoing.
The alkylation catalyst may also include a mixture of a mineral acid with a
Friedel-Crafts
metal halide, such as aluminum bromide, and other proton donors.
In some embodiments, the alkylation catalyst may comprise an aluminosilicate
zeolite. In some embodiments, the alkylation catalyst may further comprise a
modifier
49
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
selected from the group consisting of Ga, In, Zn, Fe, Mo, Ag, Au, Ni, P, Sc,
Y, Ta, a
lanthanide, and a combination of any two or more of the foregoing. In some
embodiments,
the alkylation catalyst may further comprise a metal selected from the group
consisting of
Cu, Ag, Au, Pt, Ni, Fe, Co, Ru, Zn, Cd, Ga, In, Rh, Pd, Ir, Re, Mn, Cr, Mo, W,
Sn, Os, an
alloy of any two or more of the foregoing, and a combination of any two or
more of the
foregoing.
In some embodiments, the alkylation catalyst may comprise a bifunctional
pentasil
ring-containing aluminosilicate zeolite. In some embodiments, the alkylation
catalyst may
further comprise a modifier selected from the group consisting of Ga, In, Zn,
Fe, Mo, Ag,
Au, Ni, P, Sc, Y, Ta, a lanthanide, and a combination of any two or more of
the foregoing.
In some embodiments, the alkylation catalyst may further comprise a metal
selected from
the group consisting of Cu, Ag, Au, Pt, Ni, Fe, Co, Ru, Zn, Cd, Ga, In, Rh,
Pd, Ir, Re, Mn,
Cr, Mo, W, Sn, Os, an alloy of any two or more of the foregoing, and a
combination of any
two or more of the foregoing. In one version, the dehydration catalyst and the
alkylation
catalyst may be atomically identical.
The alkylation reaction may be conducted at a temperature where the
thermodynamics are favorable. In general, the alkylation temperature may range
between t
-20 C and 300 C, and the alkylation pressure may range between 1 bar
(absolute) and 80
bar. In some embodiments, the alkylation temperature may range between 100 C
and
300 C. In another version, the alkylation temperature may range between 0 C
and 100 C.
In yet other embodiments, the alkylation temperature may range between 0 C and
50 C.
In still other embodiments, the alkylation temperature may range between 70 C
and 250 C,
and the alkylation pressure may range between 5 bar and 80 bar. In some
embodiments,
the alkylation catalyst may comprise a mineral acid or a strong acid. In other
embodiments, the alkylation catalyst may comprise a zeolite and the alkylation
temperature
may be greater than 100 C.
In some embodiments, an olefinic oligomerization reaction may be conducted.
The
oligomerization reaction may be carried out in any suitable reactor
configuration. Suitable
configurations may include, but are not limited to, batch reactors, semi-batch
reactors, or
continuous reactor designs such as, for example, fluidized bed reactors with
external
regeneration vessels. Reactor designs may include, but are not limited to
tubular reactors,
fixed bed reactors, or any other reactor type suitable for carrying out the
oligomerization
reaction. In some embodiments, a continuous oligomerization process for the
production
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
of diesel and jet fuel boiling range hydrocarbons may be carried out using an
oligomerization reactor for contacting an olefinic feed stream comprising
short chain
olefins having a chain length of from 2 to 8 carbon atoms with a zeolite
catalyst under
elevated temperature and pressure so as to convert the short chain olefins to
a fuel blend in
the diesel boiling range. The oligomerization reactor may be operated at
relatively high
pressures of 20 bar to 100 bar, and temperatures ranging between 150 C and 300
C,
preferably between 200 C to 250 C.
The resulting oligomerization stream results in a fuel blend that may have a
wide
variety of products including products comprising C5 to C24 hydrocarbons.
Additional
processing may be used to obtain a fuel blend meeting a desired standard. An
initial
separation step may be used to generate a fuel blend with a narrower range of
carbon
numbers. In some embodiments, a separation process such as a distillation
process may be
used to generate a fuel blend comprising C12 to C24 hydrocarbons for further
processing.
The remaining hydrocarbons may be used to produce a fuel blend for gasoline,
recycled to
the oligomerization reactor, or used in additional processes. For example, a
kerosene
fraction may be derived along with the diesel fraction and may either be used
as an
illuminating paraffin, as a jet fuel blending component in conventional crude
or synthetic
derived jet fuels, or as reactant (especially C10 to C13 fraction) in the
process to produce
LAB (Linear Alkyl Benzene). The naphtha fraction, after hydroproces sing, may
be routed
to a thermal cracker for the production of ethylene and propylene or routed to
a catalytic
cracker to produce ethylene, propylene, and gasoline.
Additional processes may be used to treat the fuel blend to remove certain
components or further conform the fuel blend to a diesel or jet fuel standard.
Suitable
techniques may include hydrotreating to remove any remaining oxygen, sulfur,
or nitrogen
in the fuel blend. Hydrogenation may be carried after the hydrotreating
process to saturate
at least some olefinic bonds. Such hydrogenation may be performed to conform
the fuel
blend to a specific fuel standard (e.g., a diesel fuel standard or a jet fuel
standard). The
hydrogenation step of the fuel blend stream may be carried out according to
the known
procedures, in a continuous or batchwise manner.
To facilitate a better understanding of the present invention, the following
examples
of preferred embodiments are given. In no way should the following examples be
read to
limit, or to define, the scope of the invention.
EXAMPLES
51
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
Batch reaction studies were conducted in a Parr5000 Hastelloy multi-reactor
having
6 x 75-milliliter reactors operated in parallel at pressures up to 135 bar and
temperatures up
to 275 C, stirred by magnetic stir bar.
Example 1: Determination of minimum gas velocity needed for fluidization of a
slurry catalyst. A 100 mL graduated cylinder was filled with 1 gram of nominal
1 ¨ 25 [tm
nickel-molybdenum/alumina slurry catalyst and 50 grams of deionized water,
maintained
at 23 C and atmospheric pressure. A fritted sparging stone (ACE Glass) was
placed at the
bottom of the graduated cylinder and connected to an N2 supply using 1/8-inch
Teflon
tubing. The N2 flow rate was varied to determine minimum linear velocity
needed to
completely fluidize the slurry catalyst to the top of the liquid column. The
linear velocity
of gas corresponding to complete fluidization and mixing of slurry to the top
of the liquid
level was 0.037 cm/sec.
Example 2: Digestion of cellulosic biomass solids. A 1 inch diameter x 12 inch
tall digester-reactor tube was fitted with a 0.5 inch diameter annular draft
tube insert. The
insert was centered via welding two 1.16 inch x 1.5 cm tabs at 2 cm from each
end. Two 1
cm high x 6 mm V-shaped slots were cut diametrically opposed in the bottom of
the tube to
allow unhindered flow of solid and liquid from the outer annulus to the inside
draft tube.
A nominal 0.89 inch diameter metal plate was installed in the bottom of the
one inch O.D.
tube, to retain the inner annulus and prevent solids from dropping into pipe
and tube
fittings used at the bottom of the digester-reactor. A 1/8 inch stainless
steel gas dispersion
tube was inserted from the bottom, up through the bottom plate, and extending
0.5 inches
into the annular draft tube to a position of 1 cm above the top of the v-
shaped bottom slots.
The draft tube extended to within 3.5 inches of the top overflow port on the
digester-
reactor tube, such that liquid and gas flow through the inner annulus could
spill over and
return to the bottom of the assembly via the outer annulus, once a driving
force of gas flow
through the inner annulus was established.
The digester-reactor was charged with 0.762 grams of nominal 1 ¨ 25 micron
nickel-molybdenum/alumina slurry catalyst, added to the bottom and retained by
the metal
plate. The inner annulus was packed with 4.794 grams of southern pine mini
chips (39%
moisture) having a nominal size of 6 mm x 5 mm x 3 mm. Two plugs of glass wool
totaling 0.035 grams were placed diametrically opposed in the outer annulus, 3
cm from
top of inner annulus, to capture a portion of circulating solids in the
digester-reactor
system.
52
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
The digester-reactor was filled with solvent (45% by weight 1,2-propylene
glycol,
5% ethylene glycol in deionized water with 0.05 wt. % potassium carbonate
buffer), to a
level of 1.5 inches above the top of the inner annulus. Hydrogen flow was
introduced at
the bottom of the digester-reactor, and routed to a high pressure product
vessel maintained
at 70 bar via pressure regulator, before venting at 94 mL/min at atmospheric
pressure and
room temperature (23 C). The calculated superficial linear velocity through
the draft tube
was 0.049 cm/sec, which exceeded the minimum linear velocity for complete
mixing of
slurry catalyst in Example 1. In the absence of the draft tube, the linear gas
velocity was
only 0.013 cm/sec, or less than one-third of that needed for complete mixing
and
suspension of slurry catalyst to take place. The digester-reactor was then
heated via band
heaters (Gaumer) to 190 C. After 1.5 hours, temperature was ramped to 230 C
for 2
hours, followed by an increase to 250 C for 3 hours for a total run time of
6.5 hours.
Measurement of the remaining wood chip height at the end of the run indicated
59% digestion of the wood chips. ICP analysis of the solids, which were
retained on the
glass wool plugs extracted from the outer annulus after the run, indicated the
presence of
nickel and molybdenum, corresponding to 2.2 and 2.9 wt. % catalyst present on
the two
filter plugs.
This result indicated that nickel-molybdate catalyst was lifted from the
bottom of the draft tube to the upper portion of the outer annulus via the
motive force of
gas sparging.
Product formation (mono-oxygenates, glycols, diols, alkanes, and acids) was
monitored by gas chromatograph. Gas chromatographic analyses were conducted
using a
60 m x 0.32 mm ID DB-5 column of 1 p.m thickness, with 50:1 split ratio, 2
mL/min
helium flow, and column oven temperature of 40 C for 8 minutes, followed by a
ramp to
285 C at 10 C/min and a hold time of 53.5 minutes. The injector temperature
was set at
250 C, and the detector temperature was set at 300 C.
Analysis of collected liquid drained from the digester-reactor indicated 1.68
wt. %
product formation for components elutable from the GC at an injector
temperature of
375 C, whereby all eluted components exhibited a retention time less than
sorbitol, a C6
sugar alcohol. This corresponds to an expected value of 1.66 wt. % product
formation, if
all hemicellulose and cellulose carbohydrates were selectively converted to
liquid products
of sufficiently small molecular weight to elute from the GC. Yields to desired
product
were thus 102% of those expected for selective conversion of carbohydrates to
lower
molecular weight intermediates.
53
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
Examples 3 - 5: Impact of catalyst on yields. In Example 3, the digester-
reactor
was charged with 20.0 grams of 50 wt. % 2-propanol in deionized water, 0.30
grams of
sodium carbonate buffer, and 0.504 grams of a sulfided cobalt-molybdate
catalyst
(DC2534, Criterion Catalyst & Technologies L.P containing 1-10% cobalt oxide
and
molybdenum trioxide (up to 30 wt. %) on alumina, and less than 2% nickel) that
was
crushed to less than 100 microns in size. The catalyst was previously sulfided
as described
in United States Patent Application publication 20100236988. The reactor was
then
charged with 2.7 grams of southern pine wood chips containing 39% moisture,
ground via
knife mill, before pressuring with 50 ¨ 51 bar of H2, and heating to 240 C for
5 hours with
stirring. The cycle was repeated for three additions of wood chips.
After cooling and venting of pressure, a liquid sample was withdrawn for
analysis
by GC. The reactor contents were filtered through Whatman GF/F filter paper,
and the
paper with solids was dried in a vacuum oven overnight at 90 C to assess
undigested
solids. GC analysis indicated the presence of 6.3 wt. % of desired
intermediates,
oxygenated components having a retention time less than that of sorbitol. This
value
corresponded to an estimated 79% yield of target intermediates relative to the
mass of
carbohydrates charged as wood to the reactor, or a selectivity of 90% relative
to the 88%
digestion of wood charged.
For Example 4, the experiment was repeated with 3 cycles of wood addition,
using
25 wt. % 2-propanol in deionized water as the solvent and the same cobalt-
molybdate
catalyst. GC analysis indicated a yield of 78% of desired intermediates
relative to the total
amount of carbohydrates charged.
For Example 5, the experiment was repeated with 25% ethanol as the solvent,
but
with no catalyst. GC analysis indicated a yield of only 23% of the targeted
intermediates,
while 36% by weight of the wood feed was converted to a bottoms tar layer that
could not
be made to flow upon reheating to 100 C.
Therefore, the present invention is well adapted to attain the ends and
advantages
mentioned as well as those that are inherent therein. The particular
embodiments disclosed
above are illustrative only, as the present invention may be modified and
practiced in
different but equivalent manners apparent to those skilled in the art having
the benefit of
the teachings herein. Furthermore, no limitations are intended to the details
of construction
or design herein shown, other than as described in the claims below. It is
therefore evident
that the particular illustrative embodiments disclosed above may be altered,
combined, or
54
CA 02885919 2015-03-20
WO 2014/052374 PCT/US2013/061561
modified and all such variations are considered within the scope and spirit of
the present
invention. The invention illustratively disclosed herein suitably may be
practiced in the
absence of any element that is not specifically disclosed herein and/or any
optional element
disclosed herein. While compositions and methods are described in terms of
"comprising,"
"containing," or "including" various components or steps, the compositions and
methods
may also "consist essentially of' or "consist of' the various components and
steps. All
numbers and ranges disclosed above may vary by some amount. Whenever a
numerical
range with a lower limit and an upper limit is disclosed, any number and any
included
range falling within the range is specifically disclosed. In particular, every
range of values
(of the form, "from a to b," or, equivalently, "from approximately a to b,"
or, equivalently,
"from approximately a-b") disclosed herein is to be understood to set forth
every number
and range encompassed within the broader range of values. Also, the terms in
the claims
have their plain, ordinary meaning unless otherwise explicitly and clearly
defined by the
patentee. Moreover, the indefinite articles "a" or "an," as used in the
claims, are defined
herein to mean one or more than one of the element that it introduces. If
there is any
conflict in the usages of a word or term in this specification and one or more
patent or
other documents that referenced herein, the definitions that are consistent
with this
specification should be adopted.