Note: Descriptions are shown in the official language in which they were submitted.
CA 02888776 2016-09-26
DRUG DELIVERY MEDICAL DEVICE
10001]
BACKGROUND OF THE INVENTION
[00021 There is a need for medical device technology that can rapidly,
efficiently, reproducibly and
safely transfer a Drug Delivery Formulation from the surface of a percutaneous
medical device (a
coating) onto/into a specific site in the body.
SUMMARY OF THE INVENTION
100031 Provided herein is a medical device comprising a balloon, and a coating
on at least a portion
of the balloon, wherein the coating comprises particles of a pharmaceutical
agent, and wherein each
particle of the pharmaceutical agent are at least partially encapsulated in a
polymer material.
[0004] In one embodiment, at least 50% of the surface area of the
pharmaceutical agent is
encapsulated in the polymer material. In another embodiment, at least 75% of
the surface area of the
pharmaceutical agent is encapsulated in the polymer material. In another
embodiment, at least 90% of
the surface area of the pharmaceutical agent is encapsulated in the polymer
material. In another
embodiment, at least 95% of the surface area of the pharmaceutical agent is
encapsulated in the
polymer material.
[0005] In one embodiment, the polymer layer has an average thickness of from 2
microns to 20
microns. In another embodiment, the polymer layer has an average thickness of
from 5 microns to 15
microns. In another embodiment, the polymer layer has an average thickness of
about 10 microns.
[0006] In one embodiment, the pharmaceutical agent has a crystallinity of at
least 5%. In another
embodiment, the pharmaceutical agent has a crystallinity of at least 20%. In
another embodiment, the
pharmaceutical agent has a crystallinity of from 25% to 95%.
[0007] In one embodiment, an average particle size of the encapsulated
pharmaceutical agent is from
5 nm to 150 nm. In a further refinement, an average particle size of the
pharmaceutical agent is from
10 nm to 100 nm. In another embodiment, an average particle size of the
encapsulated
pharmaceutical agent is from 1 micron to 50 micron. In another embodiment, an
average particle size
of the encapsulated pharmaceutical agent is from 1 micron to 20 micron. In
another embodiment, an
average particle size of the encapsulated pharmaceutical agent is from 1
micron to 10 micron. In a
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
refinement, an average particle size of the encapsulated pharmaceutical agent
is from 1 micron to 5
micron.
[0008] In one embodiment, a weight ratio between the pharmaceutical agent and
the polymer
material is from 1:99 to 70:30. In another embodiment, a weight ratio between
the pharmaceutical
agent and the polymer material is from 25:75 to 40:60. In another embodiment,
a weight ratio of the
pharmaceutical agent and the polymer material is from 40:60 to 60:40.
[0009] In one embodiment, the pharmaceutical agent is at least partially
encapsulated in the polymer
layer by spray-drying a mixture of the pharmaceutical agent, the polymer, and
a solvent. In a
refinement, the mixture is a solution and the solvent is a polar aprotic
solvent. In a further refinement,
.. the polar aprotic solvent is selected from tetrahydrofuran, acetonitrile,
and mixtures thereof In
another refinement, the mixture is a slurry and the solvent is water.
[0010] In one embodiment, the pharmaceutical agent is at least partially
encapsulated in the polymer
material by spraying and drying a solution of the bioabsorbable polymer on the
particles of the
pharmaceutical agent. In a refinement, the solution comprises the polymer and
a polar aprotic
solvent. In a further refinement, the polar aprotic solvent is selected from
tetrahydrofuran,
acetonitrile, and mixtures thereof
[0011] In one embodiment, the pharmaceutical agent is at least partially
encapsulated in the polymer
material by adding particles of the polymer to the pharmaceutical agent in a
tumbling vessel. In a
refinement, the particles of the polymer havean average particle size of from
10 microns to 100
microns. In another refinement, the particles of the polymer are added at a
rate of from 100 [tg to
1000 [tg per minute. In another refinement, the particles of the polymer are
added at a rate of from
300 [tg to 500 [tg per minute.
[0012] In one embodiment, the pharamaceutical agent is at least partially
encapsulated in the
polymer layer by forming an emulsion-based mixture comprising the
pharmaceutical agent and the
polymer material, and separating the polymer-encapsulated pharmaceutical agent
from the emulsion-
based mixture. In a refinement, the polymer material is a mixture of PVA or
PLGA. In further
refinement, the PLGA has a weight ratio from about 40:60 to about 60:40 lactic
acid: glycolic acid.
In another refinement, the pharmaceutical agent is amorphous.
[0013] In a further refinement the pharmaceutical agent is at least partially
encapsulated in the
polymer material by forming an emulsion-based mixture comprising the
pharmaceutical agent and the
polymer material, evaporating a portion of the emulsion, and filtering the
remaining emulsion. In a
further refinement, the encapsulated pharmaceutical agent is resuspended and
lyophilized. In another
refinement, the emulsion-based mixture is formed by combining a first polymer
solution, a second
polymer solution, a third polymer solution, and a pharmaceutical agent
solution. In a further
refinement, the first and third polymer solutions comprise a first polymer and
water. In a further
refinement, the first polymer is PVA. In a further refinement, the first
polymer solution has a polymer
- 2 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
concentration of from about 1% to about 5%. In another refinement, the third
polymer solution has a
polymer concentration of from about 0.5% to about 2%. In another refinement
the second polymer
solution is comprised of a second polymer and an organic solvent. In a further
refinement the second
polymer is PLGA. In another refinement the organic solvent is dochloromethane.
In another
refinement the pharmaceutical agent solution comprises the pharmaceutical
agent and a polar aprotic
solvent. In a further refinement the aprotic solvent is dimethylsulfoxide. In
another refinement, the
emulsion is formed by mixing the pharmaceutical agent solution and the second
polymer solution,
adding the first polymer solution to that mixture, homogenizing the mixture,
and adding the third
polymer solution.
[0014] In another refinement the pharmaceutical agent is crystalline. In a
further embodiement the
pharmaceutical agent is at least partially encapsulated in the polymer
material by forming an
emulsion-based mixture comprising the pharmaceutical agent and at least one
polymer and filtering
the emulsion. In a further refinement the encapsulated pharmaceutical agent is
resuspended and
lyophilized. In another refinement the emulsion-based mixture is formed by
combining a first
.. polymer solution, a second polymer solution, a third polymer solution, and
the crystalline
pharmaceutical agent. In a further refinement the first polymer solution
comprises a first polymer and
an organic solvent. In a further refinement the first polymer solution is
combined with the second
polymer solution and the organic solvent is allowed to evaporate. In a further
refinement, the first
polymer is PLGA. In another refinement the organic solvent is dichloromethane.
In another
refinement, the second and third polymer solutions comprise a second polymer
and water. In a further
refinement the second polymer is PVA. In a further refinement the second
polymer solution has a
polymer concentration of from about 0.5% to about 2%. In another refinement,
the third polymer
solution has a polymer concentration of from about 1% to about 5%. In another
refinement the
emulsion is formed by mixing the first polymer solution and the secdon polymer
solution to form an
emulsion, mixing the pharmaceutical agent and the third polymer solution to
form a suspension, and
combining the emulsion and suspension.
[0015] In one embodiment, the pharmaceutical agent is a macrolide
immunosuppressant. In a
refinement, the macrolide immunosuppressant is rapamycin or a derivative, a
prodrug, a hydrate, an
ester, a salt, a polymorph, a derivative or an analog thereof In another
refinement, the macrolide
immunosuppressant is selected from the group consisting of rapamycin, 40-042-
Hydroxyethyl)rapamycin (everolimus), 40-0-Benzyl-rapamycin, 40-0-(4'-
Hydroxymethyl)benzyl-
rapamycin, 40-0-[4'-(1,2-Dihydroxyethyl)]benzyl-rapamycin, 40-0-Allyl-
rapamycin, 40-0-[3'-(2,2-
Dimethy1-1,3-dioxolan-4(S)-y1)-prop-2'-en-l'-yl] -rap amycin, (2':E,4'S)-40-0-
(4',5'-Dihydroxypent-2'-
en-l'-y1)-rapamycin 40-0-(2-Hydroxy)ethoxycar-bonylmethyl-rapamycin, 40-0-(3-
Hydroxy)propyl-
rapamycin 40-0-(6-Hydroxy)hexyl-rapamycin 40-0-[2-(2-Hydroxy)ethoxy]ethyl-
rapamycin 40-0-
[(3 S)-2,2-Dimethyldioxo lan-3-yl] methyl-rap amycin, 40-0- [(2S)-2,3 -
Dihydroxyprop-1 -yl] -rapamycin,
40-0-(2-Acetoxy)ethyl-rapamycin 40-0-(2-Nicotinoyloxy)ethyl-rapamycin, 40-0-[2-
(N-
- 3 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
Morph lino)acetoxy]ethyl-rapamycin 40-0-(2-N-Imidazolylacetoxy)ethyl-
rapamycin, 40-0-[2-(N-
Methyl-N'-piperazinyl)acetoxy]ethyl-rapamycin, 39-0-Desmethy1-39,40-0,0-
ethylene-rapamycin,
(26R)-26-Dihydro-40-0-(2-hydroxy)ethyl-rapamycin, 28-0-Methyl-rapamycin, 40-
042-
Aminoethyl)-rapamycin, 40-0-(2-Acetaminoethyl)-rapamycin 40-0-(2-
Nicotinamidoethyl)-
rapamycin, 40-0-(2-(N-Methyl-imidazo-2'-ylcarbethoxamido)ethyl)-rapamycin, 40-
042-
Ethoxycarbonylaminoethyl)-rapamycin, 40-0-(2-Tolylsulfonamidoethyl)-rapamycin,
40-0-[2-(4',5'-
Dicarboethoxy-1',2',3'-triazol-1'-y1)-ethyThrapamycin, 42-Epi-
(tetrazolyl)rapamycin (tacrolimus), and
42- [3 In another refinement,
the
pharmaceutical agent is rapamycin.
[0016] In one embodiment, the polymer material comprises a bioabsorbable
polymer. In a
refinement, the bioabsorbable polymer is selected from the group consisting of
polylactides (PLA);
poly(lactide-co-glycolide) (PLGA); polyanhydrides; polyorthoesters; poly(N-(2-
hydroxypropyl)
methacrylamide); poly(dl-lactide) (DLPLA); poly(1-lactide) (LPLA);
polyglycolide (PGA);
poly(dioxanone) (PD0); poly(glycolide-co-trimethylene carbonate) (PGA-TMC);
poly(1-lactide-co-
glycolide) (PGA-LPLA); poly(dl-lactide-co-glycolide) (PGA-DLPLA); poly(1-
lactide-co-dl-lactide)
(LPLA-DLPLA); poly(glycolide-co-trimethylene carbonate-co-dioxanone) (PDO-PGA-
TMC),
polyarginine, polyvinyl alcohol (PVA), and mixtures or co-polymers thereof In
another refinement,
the bioabsorbable polymer is PLGA, PVA, polyarginine, or mixtures thereof In a
further refinement,
the bioabsorbable polymer is a mixture of PLGA and PVA.
[0017] In one embodiment, the polymer layer comprises PLGA. In a refinement,
the polymer layer
is made of PLGA. In another embodiment, the polymer layer comprises
polyarginine. In a
refinement, the polymer layer is made of polyarginine. In a refinement, the
PLGA comprises about
50:50 lactic acid: glycolic acid. In another embodiment, the polymer layer
comprises a durable
polymer.
[0018] In one embodiment, the medical device further comprises a binding agent
deposited on an
exterior surface of the encapsulated particles of the pharmaceutical agent. In
a refinement, a weight
ratio between the binding agent and the polymer is from 1:99 to 25:75. In
another refinement, a
weight ratio between the binding agent and the polymer is from 1:99 to 10:90.
[0019] In one embodiment, the binding agent is deposited by spraying and
drying a solution of the
binging agent on the encapsulated particles of the pharmaceutical agent. In a
refinement, the solution
comprises the binding agent and water.
[0020] In one embodiment, the binding agent comprises at least one of:
Polyarginine, Polyarginine 9-
L-pArg, DEAE-Dextran (Diethylaminoethyl cellulose- Dextran), DMAB
(Didodecyldimethylammonium bromide), PEI (Polyethyleneimine), TAB
(Tetradodecylammonium
bromide), and DMTAB (Dimethylditetradecylammonium bromide). In a refinement,
the binding
- 4 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
agent is Polyarginine. In a further refinement, the Polyarginine has an
average molecular weight of
about 70kDa. In another refinement, the Polyarginine has an average molecular
weight of 5-15kDa.
[0021] In one embodiment, the encapsulated particles of the pharmaceutical
agent are deposited on
the balloon using an eSTAT coating process.
[0022] In one embodiment, the medical device releases at least 3% of the
pharmaceutical agent upon
inflation of the balloon. In another embodiment, the medical device releases
at least 5% or at least
10% of the pharmaceutical agent upon inflation of the balloon. In another
embodiment, the medical
device releases at least about 2 ng/mg, at least about 3 ng/mg, at least about
5 ng/mg, at least about 10
ng/mg, at least about 20 ng/mg, at least about 30 ng/mg, or at least about 40
ng/mg of the
pharmaceutical agent 72 hours after inflation of the balloon.
[0023] In one embodiment, the balloon is an invertable balloon having an
abluminal side; wherein
the coating is provided on the abluminal side of the invertable balloon. In a
refinement, the balloon is
inverted within a catheter. In a further refinement, the balloon is capable of
being pushed out of the
catheter using balloon inflation pressure or by moving the distal end of the
balloon distally through
the balloon, or a combination thereof In another refinement, the invertible
balloon is inverted on an
outside of a catheter. In a further refinement, a treatment length of the
invertible balloon is controlled
by partially un-inverting the invertible balloon on the outside of the
catheter. In another further
refinement, the medical device further comprises a sheath provided over the
invertible balloon. In
another further refinement, the sheath is retractable once the coated balloon
reaches the treatment site,
is positioned near the treatment site, is positioned at the treatment site, is
proximal to the treatment
site, is distal to the treatment site, or is within the treatment site.
[0024] In one embodiment, the medical device further comprises an occluder
configured to block the
flow of bodily fluids toward the balloon before the balloon is inflated. In a
refinement, the occluder
comprises a second balloon. In another refinement, the balloon comprises first
and second sections,
the first section comprising the occluder and the second section comprising
the coating. In another
refinement, the balloon comprises a distal node and a proximal node wherein
the distal node
comprises the coating and wherein the proximal node comprises the occluder, or
wherein the proximal
node comprises the coating and wherein the distal node comprises the occluder.
In still another
refinement, a distal portion of the balloon is coated and wherein a proximal
portion of the balloon is
not coated, and wherein the proximal portion of the balloon is the occlude, or
wherein a proximal
portion of the balloon is coated and wherein a distal portion of the balloon
is not coated, and wherein
the distal portion of the balloon is the occluder.
[0025] Also provided herein is a method of releasing a pharmaceutical agent at
a target site,
comprising providing a device comprising a balloon, and a coating on at least
a portion of the
balloon, wherein the coating comprises particles of a pharmaceutical agent,
and wherein each particle
of the pharmaceutical agent is at least partially encapsulated in a polymer
material; positioning the
- 5 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
device to allow the balloon to reach the target site; and inflating the
balloon of the device, wherein at
least some of the pharmaceutical agent is released to the target site upon
inflating the balloon.
[0026] In one embodiment, the target site is a blood vessel, such as an
artery.
INCORPORATION BY REFERENCE
[0027] All publications and patent applications mentioned in this
specification are herein
incorporated by reference to the same extent as if each individual publication
or patent application
was specifically and individually indicated to be incorporated by reference.
This application relates to
U.S. Provisional Application No. 61/081,691, filed July 17, 2008, U.S.
Provisional Application No.
61/226,239 filed July 16, 2009, U.S. Provisional Application No. 61/212,964,
filed April 17, 2009,
U.S. Application No. 12/504,597, filed July 16, 2009, U.S. Application No.
12/729,580, filed March
23, 2010, PCT Application No. PCT/U52009/050883, filed July 16, 2009, PCT
Application No.
PCT/US2010/028253, filed March 23, 2010, and PCT Application No.
PCT/US2010/042355, filed
July 16, 2010. The contents of these applications are incorporated herein by
reference in their
entirety.
BRIEF DESCRIPTION OF THE DRAWINGS
[0028] The novel features of the invention are set forth with particularity in
the appended claims. A
better understanding of the features and advantages of the present invention
will be obtained by
reference to the following detailed description that sets forth illustrative
embodiments, in which the
principles of the invention are utilized, and the accompanying drawings of
which:
[0029] Figure 1 indicates the Average percent Sirolimus Eluted from the
balloons at various time
points for Formulations F3, F5, and F7;
[0030] Figure 2 depicts an example eSTAT process for coating 12 angioplasty
balloons with
sirolimus;
[0031] Figure 3 depicts coating balloons according to an RESS process;
[0032] Figure 4 depicts a method of preparing a coating formulation according
to an embodiment
herein.
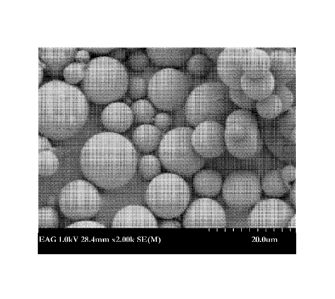
[0033] Figure 5 is an SEM image of microparticles of encapsulated rapamycin
according to one
emdbodiment herein; and
[0034] Figure 6 is an SEM image of microparticles of encapsulated rapamycin
according to another
emdbodiment herein.
DETAILED DESCRIPTION OF THE INVENTION
[0035] The present invention is explained in greater detail below. This
description is not intended to
be a detailed catalog of all the different ways in which the invention may be
implemented, or all the
features that may be added to the instant invention. For example, features
illustrated with respect to
one embodiment may be incorporated into other embodiments, and features
illustrated with respect to
- 6 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
a particular embodiment may be deleted from that embodiment. In addition,
numerous variations and
additions to the various embodiments suggested herein will be apparent to
those skilled in the art in
light of the instant disclosure, which do not depart from the instant
invention. Hence, the following
specification is intended to illustrate some particular embodiments of the
invention, and not to
exhaustively specify all permutations, combinations and variations thereof
Definitions
[0036] As used in the present specification, the following words and phrases
are generally intended
to have the meanings as set forth below, except to the extent that the context
in which they are used
indicates otherwise.
[0037] "Substrate" as used herein, refers to any surface upon which it is
desirable to deposit a
coating. Biomedical implants are of particular interest for the present
invention; however the present
invention is not intended to be restricted to this class of substrates. Those
of skill in the art will
appreciate alternate substrates that could benefit from the coating process
described herein, such as
pharmaceutical tablet cores, as part of an assay apparatus or as components in
a diagnostic kit (e.g. a
test strip). Examples of substrates that can be coated using the methods of
the invention include
surgery devices or medical devices, e.g., a catheter, a balloon, a cutting
balloon, a wire guide, a
cannula, tooling, an orthopedic device, a structural implant, stent, stent-
graft, graft, vena cava filter, a
heart valve, cerebrospinal fluid shunts, pacemaker electrodes, axius coronary
shunts, endocardial
leads, an artificial heart, and the like.
[0038] "Biomedical implant" as used herein refers to any implant for insertion
into the body of a
human or animal subject, including but not limited to stents (e.g., coronary
stents, vascular stents
including peripheral stents and graft stents, urinary tract stents,
urethral/prostatic stents, rectal stent,
oesophageal stent, biliary stent, pancreatic stent), electrodes, catheters,
leads, implantable pacemaker,
cardioverter or defibrillator housings, joints, screws, rods, ophthalmic
implants, femoral pins, bone
plates, grafts, anastomotic devices, perivascular wraps, sutures, staples,
shunts for hydrocephalus,
dialysis grafts, colostomy bag attachment devices, ear drainage tubes, leads
for pace makers and
implantable cardioverters and defibrillators, vertebral disks, bone pins,
suture anchors, hemostatic
barriers, clamps, screws, plates, clips, vascular implants, tissue adhesives
and sealants, tissue
scaffolds, various types of dressings (e.g., wound dressings), bone
substitutes, intraluminal devices,
vascular supports, etc.
[0039] The implants may be formed from any suitable material, including but
not limited to polymers
(including stable or inert polymers, organic polymers, organic-inorganic
copolymers, inorganic
polymers, and biodegradable polymers), metals, metal alloys, inorganic
materials such as silicon, and
composites thereof, including layered structures with a core of one material
and one or more coatings
of a different material. Substrates made of a conducting material facilitate
electrostatic capture.
However, the invention contemplates the use of electrostatic capture, as
described herein, in
- 7 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
conjunction with substrate having low conductivity or which are non-
conductive. To enhance
electrostatic capture when a non-conductive substrate is employed, the
substrate is processed for
example while maintaining a strong electrical field in the vicinity of the
substrate. In some
embodiments, however, no electrostatic capture is employed in applying a
coating to the substrate. In
some embodiments of the methods and/or devices provided herein, the substrate
is not charged in the
coating process. In some embodiments of the methods and/or devices provided
herein, an electrical
potential is not created between the substrate and the coating apparatus.
[0040] Subjects into which biomedical implants of the invention may be applied
or inserted include
both human subjects (including male and female subjects and infant, juvenile,
adolescent, adult and
geriatric subjects) as well as animal subjects (including but not limited to
pig, rabbit, mouse, dog, cat,
horse, monkey, etc.) for veterinary purposes and/or medical research.
[0041] As used herein, a biological implant may include a medical device that
is not permanently
implanted. A biological implant in some embodiments may comprise a device
which is used in a
subject on a transient basis. For non-limiting example, the biomedical implant
may be a balloon,
which is used transiently to dilate a lumen and thereafter may be deflated
and/or removed from the
subject during the medical procedure or thereafter. In some embodiments, the
biological implant may
be temporarily implanted for a limited time, such as during a portion of a
medical procedure, or for
only a limited time (some time less than permanently implanted), or may be
transiently implanted
and/or momentarily placed in the subject. In some embodiments, the biological
implant is not
implanted at all, rather it is merely inserted into a subject during a medical
procedure, and
subsequently removed from the subject prior to or at the time the medical
procedure is completed. In
some embodiments, the biological implant is not permanently implanted since it
completely resorbs
into the subject (i.e. is completely resorbed by the subject). In a preferred
embodiment the biomedical
implant is an expandable balloon that can be expanded within a lumen
(naturally occurring or non-
naturally occurring) having a coating thereon that is freed (at least in part)
from the balloon and left
behind in the lumen when the balloon is removed from the lumen.
[0042] Examples of pharmaceutical agents employed in conjunction with the
invention include,
rapamycin, 40-0-(2-Hydroxyethyl)rapamycin (everolimus), 40-0-Benzyl-rapamycin,
40-0-(4'-
Hydroxymethyl)benzyl-rapamycin, 40-0-[4'-(1,2-Dihydroxyethyl)]benzyl-
rapamycin, 40-0-Allyl-
rapamycin, 40-0- [3'-(2,2-Dimethy1-1,3 -diox olan-4 (S)-y1)-prop-2'- en-1 '-
y1] -rap amycin, (2':E,4'S)-40-
0-(4',5'-Dihydroxypent-2'-en-1'-y1)-rapamycin 40-0-(2-Hydroxy)ethoxycar-
bonylmethyl-rapamycin,
40-0-(3-Hydroxy)propyl-rapamycin 40-0-(6-Hydroxy)hexyl-rapamycin 40-0-[2-(2-
Hydroxy)ethoxy]ethyl-rapamycin 40-0-[(3S)-2,2-Dimethyldioxolan-3-yl]methyl-
rapamycin, 40-0-
[(25)-2,3-Dihydroxyprop-1-y1]-rapamycin, 40-0-(2-Acetoxy)ethyl-rapamycin 40-0-
(2-
Nicotinoyloxy)ethyl-rapamycin, 40-0-[2-(N-Morpholino)acetoxy]ethyl-rapamycin
40-0-(2-N-
Imidazolylacetoxy)ethyl-rapamycin, 40-0-[2-(N-Methyl-N'-
piperazinyl)acetoxy]ethyl-rapamycin, 39-
- 8 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
0-Desmethy1-39,40-0,0-ethylene-rapamycin, (26R)-26-Dihydro-40-0-(2-
hydroxy)ethyl-rapamycin,
28-0-Methyl-rapamycin, 40-0-(2-Aminoethyl)-rapamycin, 40-0-(2-Acetaminoethyl)-
rapamycin
40-0-(2-Nicotinamidoethyl)-rapamycin, 40-0-(2-(N-Methyl-imidazo-2'-
ylcarbethoxamido)ethyl)-
rapamycin, 40-0-(2-Ethoxycarbonylaminoethyl)-rapamycin, 40-0-(2-
Tolylsulfonamidoethyl)-
rapamycin, 40-0-[2-(4',5'-Dicarboethoxy-1',2',3'-triazol-1'-y1)-ethyl]-
rapamycin, 42-Epi-
(tetrazolyl)rapamycin (tacrolimus), and 42- [3
(temsirolimus). The active agent in some embodiments of the devices,
coatings and/or methods provided herein comprises a macrolide
immunosuppressive drug. In some
embodiments the macrolide immunosuppressive drug comprises one or more of
rapamycin, 40-0-(2-
Hydroxyethyl)rapamycin (everolimus), 40-0-Benzyl-rapamycin, 40-0-(4'-
Hydroxymethyl)benzyl-
rapamycin, 40-0-[4'-(1,2-Dihydroxyethyl)]benzyl-rapamycin, 40-0-Allyl-
rapamycin, 40-0-[3'-(2,2-
Dimethyl-1,3 - diox o lan-4(S)-y1)-prop-2'- en-l'-yl] -rap amycin, (2':E,4'S)-
40-0-(4',5'-Dihydroxypent-2'-
en-l'-y1)-rapamycin 40-0-(2-Hydroxy)ethoxycar-bonylmethyl-rapamycin, 40-0-(3-
Hydroxy)propyl-
rapamycin 40-0-(6-Hydroxy)hexyl-rapamycin 40-0-[2-(2-Hydroxy)ethoxy]ethyl-
rapamycin 40-0-
[(3S)-2,2-Dimethyldioxolan-3-yl]methyl-rapamycin, 40-0-[(2S)-2,3-Dihydroxyprop-
1-y1]-rapamycin,
40-0-(2-Acetoxy)ethyl-rapamycin 40-0-(2-Nicotinoyloxy)ethyl-rapamycin, 40-0-[2-
(N-
Morpholino)acetoxy]ethyl-rapamycin 40-0-(2-N-Imidazolylacetoxy)ethyl-
rapamycin, 40-0-[2-(N-
Methyl-N'-piperazinyl)acetoxy]ethyl-rapamycin, 39-0-Desmethy1-39,40-0,0-
ethylene-rapamycin,
(26R)-26-Dihydro-40-0-(2-hydroxy)ethyl-rapamycin, 28-0-Methyl-rapamycin, 40-0-
(2-
Aminoethyl)-rapamycin, 40-0-(2-Acetaminoethyl)-rapamycin 40-0-(2-
Nicotinamidoethyl)-
rapamycin, 40-0-(2-(N-Methyl-imidazo-2'-ylcarbethoxamido)ethyl)-rapamycin, 40-
042-
Ethoxycarbonylaminoethyl)-rapamycin, 40-0-(2-Tolylsulfonamidoethyl)-rapamycin,
40-0-[2-(4',5'-
Dicarboethoxy-1',2',3'-triazol-1'-y1)-ethyl]-rapamycin, 42-Epi-
(tetrazolyl)rapamycin (tacrolimus), and
42- [3
(temsirolimus). The active agent
may be selected from a macrolide immunosuppressive drug, a prodrug, a hydrate,
an ester, a salt, a
polymorph, a derivative, and an analog thereof The active agent may be
selected from sirolimus, a
prodrug, a hydrate, an ester, a salt, a polymorph, a derivative, and an analog
thereof
[0043] The pharmaceutical agents may, if desired, also be used in the form of
their pharmaceutically
acceptable salts or derivatives (meaning salts which retain the biological
effectiveness and properties
of the compounds of this invention and which are not biologically or otherwise
undesirable), and in
the case of chiral active ingredients it is possible to employ both optically
active isomers and
racemates or mixtures of diastereoisomers. As well, the pharmaceutical agent
may include at least
one of: a prodrug, a hydrate, an ester, a salt, a polymorph, a derivative, and
an analog thereof
[0044] The pharmaceutical agent may be an antibiotic agent, as described
herein.
[0045] In some embodiments of the methods, coatings, and/or devices provided
herein, the size of
the active agent in the coating is controlled. In some embodiments, the active
agent is sirolimus and
- 9 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
wherein the sirolimus has an average size (mean diameter) of at least one of:
1.5 [tin, 2.5 [tin, 645nm,
100-200 nm, another controlled size, or a combination thereof In some
embodiments, the active
agent is sirolimus and wherein the sirolimus has a median size of at least one
of: 1.5 [tin, 2.5 [tin,
645nm, 100-200 nm, another controlled size, or a combination thereof In some
embodiments, the
active agent is sirolimus and wherein the sirolimus has an average size (mean
diameter) of at least one
of: about 1.5 [tin, about 2.5 [tin, about 645nm, about 100-200 nm, another
controlled size, or a
combination thereof In some embodiments, the active agent is sirolimus and
wherein the sirolimus
has a median size of at least one of: about 1.5 [tin, about 2.5 [tin, about
645nm, about 100-200 nm,
another controlled size, or a combination thereof In some embodiments, the
active agent is sirolimus
.. and wherein sirolimus at least 75% of the sirolimus as is 1.5 [tin, 2.5
[tin, 645nm, 100-200 nm, or
another controlled size. In some embodiments, the active agent is sirolimus
and wherein sirolimus at
least 50% of the sirolimus as is 1.5 [tin, 2.5 [tin, 645nm, 100-200 nm, or
another controlled size. In
some embodiments, the active agent is sirolimus and wherein sirolimus at least
90% of the sirolimus
as is 1.5 [tin, 2.5 [tin, 645nm, 100-200 nm, or another controlled size.
[0046] In some embodiments of the methods and/or devices provided herein, the
macrolide
immunosuppressive drug is at least 50% crystalline. In some embodiments, the
macrolide
immunosuppressive drug is at least 75% crystalline. In some embodiments, the
macrolide
immunosuppressive drug is at least 90% crystalline. . In some embodiments of
the methods and/or
devices provided herein the macrolide immunosuppressive drug is at least 95%
crystalline. In some
embodiments of the methods and/or devices provided herein the macrolide
immunosuppressive drug
is at least 97% crystalline. In some embodiments of the methods and/or devices
provided herein
macrolide immunosuppressive drug is at least 98% crystalline. In some
embodiments of the methods
and/or devices provided herein the macrolide immunosuppressive drug is at
least 99% crystalline.
[0047] In some embodiments of the methods and/or devices provided herein the
pharmaceutical
.. agent is at least 50% crystalline. In some embodiments of the methods
and/or devices provided herein
the pharmaceutical agent is at least 75% crystalline. In some embodiments of
the methods and/or
devices provided herein the pharmaceutical agent is at least 90% crystalline.
In some embodiments of
the methods and/or devices provided herein the pharmaceutical agent is at
least 95% crystalline. In
some embodiments of the methods and/or devices provided herein the
pharmaceutical agent is at least
97% crystalline. In some embodiments of the methods and/or devices provided
herein pharmaceutical
agent is at least 98% crystalline. In some embodiments of the methods and/or
devices provided herein
the pharmaceutical agent is at least 99% crystalline.
[0048] "Prodrugs" are derivative compounds derivatized by the addition of a
group that endows
greater solubility to the compound desired to be delivered. Once in the body,
the prodrug is typically
acted upon by an enzyme, e.g., an esterase, amidase, or phosphatase, to
generate the active compound.
- 10 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[0049] An "anti-cancer agent", "anti-tumor agent" or "chemotherapeutic agent"
refers to any agent
useful in the treatment of a neoplastic condition. There are many
chemotherapeutic agents available
in commercial use, in clinical evaluation and in pre-clinical development that
are useful in the devices
and methods of the present invention for treatment of cancers.
[0050] "Stability" as used herein in refers to the stability of the drug in a
coating deposited on a
substrate in its final product form (e.g., stability of the drug in a coated
stent). The term "stability"
and/or "stable" in some embodiments is defined by 5% or less degradation of
the drug in the final
product form. The term stability in some embodiments is defined by 3% or less
degradation of the
drug in the final product form. The term stability in some embodiments is
defined by 2% or less
degradation of the drug in the final product form. The term stability in some
embodiments is defined
by 1% or less degradation of the drug in the final product form.
[0051] In some embodiments, the pharmaceutical agent is at least one of: 50%
crystalline, 75%
crystalline, 80% crystalline, 90% crystalline, 95% crystalline, 97%
crystalline, and 99% crystalline
following sterilization of the device. In some embodiments, the pharmaceutical
agent crystallinity is
stable wherein the crystallinity of the pharmaceutical agent following
sterilization is compared to the
crystallinity of the pharmaceutical agent at least one of: 1 week after
sterilization, 2 weeks after
sterilization, 4 weeks after sterilization, 1 month after sterilization, 2
months after sterilization, 45
days after sterilization, 60 days after sterilization, 90 days after
sterilization, 3 months after
sterilization, 4 months after sterilization, 6 months after sterilization, 9
months after sterilization, 12
months after sterilization, 18 months after sterilization, and 2 years after
sterilization. In some
embodiments, the pharmaceutical agent crystallinity is stable wherein the
crystallinity of the
pharmaceutical agent prior to sterilization is compared to the crystallinity
of the pharmaceutical agent
at least one of: 1 week after sterilization, 2 weeks after sterilization, 4
weeks after sterilization, 1
month after sterilization, 2 months after sterilization, 45 days after
sterilization, 60 days after
sterilization, 90 days after sterilization, 3 months after sterilization, 4
months after sterilization, 6
months after sterilization, 9 months after sterilization, 12 months after
sterilization, 18 months after
sterilization, and 2 years after sterilization. In such embodiments, different
devices may be tested
from the same manufacturing lot to determine stability of the pharmaceutical
agent at the desired time
points.
[0052] In some embodiments, the pharmaceutical agent crystallinity is stable
at least one of: 1 week
after sterilization, 2 weeks after sterilization, 4 weeks after sterilization,
1 month after sterilization, 2
months after sterilization, 45 days after sterilization, 60 days after
sterilization, 90 days after
sterilization, 3 months after sterilization, 4 months after sterilization, 6
months after sterilization, 9
months after sterilization, 12 months after sterilization, 18 months after
sterilization, and 2 years after
.. sterilization.
- 11 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[0053] In some embodiments, the pharmaceutical agent crystallinity on the
device tested at a time
point after sterilization does not differ more than 1%, 2%, 3%, 4%, and/or 5%
from the crystallinity
tested on a second device manufactured from the same lot of devices and the
same lot of
pharmaceutical agent at testing time point before sterilization (i.e. the
crystallinity drops no more than
from 99 to 94% crystalline, for example, which is a 5 % difference in
crystallinity; the crystallinity
drops no more than from 99 to 95% crystalline, which is a 4 % difference in
crystallinity; the
crystallinity drops no more than from 99 to 96% crystalline, for example,
which is a 3 % difference in
crystallinity; the crystallinity drops no more than from 99 to 97%
crystalline, for example, which is a
2 % difference in crystallinity; the crystallinity drops no more than from 99
to 98% crystalline, for
example, which is a 1 % difference in crystallinity; in other examples, the
starting crystallinity
percentage is one of 100%, 98%, 96%, 97%, 96%, 95%, 90%, 85%, 80%, 75%, 70%,
60%, 50%,
30%, 25%, and/or anything in between).
[0054] In some embodiments, crystallinity of the pharmaceutical agent on the
device tested at a time
point after sterilization does not differ more than 1%, 2%, 3%, 4%, and/or 5%
from the crystallinity of
pharmaceutical from the same lot of pharmaceutical agent tested at testing
time point before
sterilization of the pharmaceutical agent.
[0055] In some embodiments, crystallinity of the pharmaceutical agent does not
drop more than 1%,
2%, 3%, 4%, and/or 5% between two testing time points after sterilization
neither of which time point
being greater than 2 years after sterilization. In some embodiments,
crystallinity of the
pharmaceutical agent does not drop more than 1%, 2%, 3%, 4%, and/or 5% between
two testing time
points after sterilization neither of which time point being greater than 5
years after sterilization. In
some embodiments, two time points comprise two of: 1 week after sterilization,
2 weeks after
sterilization, 4 weeks after sterilization, 1 month after sterilization, 2
months after sterilization, 45
days after sterilization, 60 days after sterilization, 90 days after
sterilization, 3 months after
sterilization, 4 months after sterilization, 6 months after sterilization, 9
months after sterilization, 12
months after sterilization, 18 months after sterilization, 2 years after
sterilization, 3 years after
sterilization, 4 years after sterilization, and 5 years after sterilization.
[0056] "Polymer" as used herein, refers to a series of repeating monomeric
units that have been
cross-linked or polymerized. Any suitable polymer can be used to carry out the
present invention. It
is possible that the polymers of the invention may also comprise two, three,
four or more different
polymers. In some embodiments of the invention only one polymer is used. In
certain embodiments
a combination of two polymers is used. Combinations of polymers can be in
varying ratios, to
provide coatings with differing properties. Polymers useful in the devices and
methods of the present
invention include, for example, stable or inert polymers, organic polymers,
organic-inorganic
copolymers, inorganic polymers, bioabsorbable, bioresorbable, resorbable,
degradable, and
- 12 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
biodegradable polymers. Those of skill in the art of polymer chemistry will be
familiar with the
different properties of polymeric compounds.
[0057] In some embodiments, the coating further comprises a polymer. In some
embodiments, the
active agent comprises a polymer. In some embodiments, the polymer comprises
at least one of
polyalkyl methacrylates, polyalkylene-co-vinyl acetates, polyalkylenes,
polyurethanes,
polyanhydrides, aliphatic polycarbonates, polyhydroxyalkanoates, silicone
containing polymers,
polyalkyl siloxanes, aliphatic polyesters, polyglycolides, polylactides,
polylactide-co-glycolides,
poly(e-caprolactone)s, polytetrahalooalkylenes, polystyrenes,
poly(phosphasones), copolymers
thereof, and combinations thereof
[0058] In embodiments, the polymer is capable of becoming soft after
implantation, for example, due
to hydration, degradation or by a combination of hydration and degradation. In
embodiments, the
polymer is adapted to transfer, free, and/or dissociate from the substrate
when at the intervention site
due to hydrolysis of the polymer. In various embodiments, the device is coated
with a bioabsorbable
polymer that is capable of resorbtion in at least one of: about 1 day, about 3
days, about 5 days, about
7 days, about 14 days, about 3 weeks, about 4 weeks, about 45 days, about 60
days, about 90 days,
about 180 days, about 6 months, about 9 months, about 1 year, about 1 to about
2 days, about 1 to
about 5 days, about 1 to about 2 weeks, about 2 to about 4 weeks, about 45 to
about 60 days, about 45
to about 90 days, about 30 to about 90 days, about 60 to about 90 days, about
90 to about 180 days,
about 60 to about 180 days, about 180 to about 365 days, about 6 months to
about 9 months, about 9
months to about 12 months, about 9 months to about 15 months, and about 1 year
to about 2 years.
[0059] Examples of polymers that may be used in the present invention include,
but are not limited to
polycarboxylic acids, cellulosic polymers, proteins, polypeptides,
polyvinylpyrrolidone, maleic
anhydride polymers, polyamides, polyvinyl alcohols, polyethylene oxides,
glycosaminoglycans,
polysaccharides, polyesters, aliphatic polyesters, polyurethanes,
polystyrenes, copolymers, silicones,
silicone containing polymers, polyalkyl siloxanes, polyorthoesters,
polyanhydrides, copolymers of
vinyl monomers, polycarbonates, polyethylenes, polypropytenes, polylactic
acids, polylactides,
polyglycolic acids, polyglycolides, polylactide-co-glycolides,
polycaprolactones, poly(e-
caprolactone)s, polyhydroxybutyrate valerates, polyacrylamides, polyethers,
polyurethane dispersions,
polyacrylates, acrylic latex dispersions, polyacrylic acid, polyalkyl
methacrylates, polyalkylene-co-
vinyl acetates, polyalkylenes, aliphatic polycarbonates polyhydroxyalkanoates,
polytetrahalooalkylenes, poly(phosphasones), polytetrahalooalkylenes,
poly(phosphasones), and
mixtures, combinations, and copolymers thereof
[0060] The polymers of the present invention may be natural or synthetic in
origin, including gelatin,
chitosan, dextrin, cyclodextrin, Poly(urethanes), Poly(siloxanes) or
silicones, Poly(acrylates) such as
[rho]oly(methyl methacrylate), poly(butyl methacrylate), and Poly(2-hydroxy
ethyl methacrylate),
Poly( vinyl alcohol) Poly(olefins) such as poly(ethylene), [rho]oly(isoprene),
halogenated polymers
- 13 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
such as Poly(tetrafluoroethylene) - and derivatives and copolymers such as
those commonly sold as
Teflon(R) products, Poly(vinylidine fluoride), Poly(vinyl acetate), Poly(vinyl
pyrrolidone),
Poly(acrylic acid), Polyacrylamide, Poly(ethylene-co-vinyl acetate),
Poly(ethylene glycol),
Poly(propylene glycol), Poly(methacrylic acid); etc.
[0061] Suitable polymers also include absorbable and/or resorbable polymers
including the
following, combinations, copolymers and derivatives of the following:
Polylactides (PLA),
Polyglycolides (PGA), PolyLactide-co-glycolides (PLGA), Polyanhydrides,
Polyorthoesters, Poly(N-
(2- hydroxypropyl) methacrylamide), Poly(1-aspartamide), including the
derivatives DLPLA ¨
poly(dl-lactide); LPLA ¨ poly(1-lactide); PDO ¨ poly(dioxanone); PGA-TMC ¨
poly(glycolide-
co-trimethylene carbonate); PGA-LPLA ¨ poly(1-lactide-co-glycolide); PGA-DLPLA
¨ poly(dl-
lactide-co-glycolide); LPLA-DLPLA ¨ poly(1-lactide-co-dl-lactide); and PDO-PGA-
TMC ¨
poly(glycolide-co-trimethylene carbonate-co-dioxanone), and combinations
thereof
[0062] In some embodiments of the devices, coatings and/or methods provided
herein the polymer
comprises PLGA. In some embodiments of the methods, coatings, or devices
provided herein, the
PLGA comprises about 50:50 Lactic acid: Glycolic acid. The PLGA may have at
least one of: a MW
of about 30KDa and a Mn of about 15KDa, a Mn of about 10KDa to about 25 KDa,
and a MW of
about 15 KDa to about 40KDa. In some embodiments of the methods, coatings, or
devices provided
herein, the PLGA comprises 50:50 Lactic acid: Glycolic acid. In some
embodiments of the methods,
coatings, or devices provided herein, the PLGA comprises from 40:60 to 60:40
Lactic acid: Glycolic
acid. In some embodiments of the methods, coatings, or devices provided
herein, the PLGA
comprises from 45:55 to 55:45 Lactic acid: Glycolic acid. In some embodiments
of the methods,
coatings, or devices provided herein, the PLGA comprises from 48:52 to 52:48
Lactic acid: Glycolic
acid. In some embodiments of the methods, coatings, or devices provided
herein, the PLGA
comprises from 49:51 to 51:49 Lactic acid: Glycolic acid. The use of the term
"about" with regard to
the ratio of Lactic acid to Glycolic acid in the PLGA, as used herein, refers
to ranges of ratios from
40:60 to 60:40, or from 45:55 to 55:45, or from 48:52 to 52:48 or from 49:51
to 51:49, depending on
the embodiment.
[0063] "Copolymer" as used herein refers to a polymer being composed of two or
more different
monomers. A copolymer may also and/or alternatively refer to random, block,
graft, copolymers
known to those of skill in the art.
[0064] "Biocompatible" as used herein, refers to any material that does not
cause injury or death to
the animal or induce an adverse reaction in an animal when placed in intimate
contact with the
animal's tissues. Adverse reactions include for example inflammation,
infection, fibrotic tissue
formation, cell death, or thrombosis. The terms "biocompatible " and
"biocompatibility" when used
herein are art-recognized and mean that the referent is neither itself toxic
to a host (e.g., an animal or
human), nor degrades (if it degrades) at a rate that produces byproducts
(e.g., monomeric or
- 14 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
oligomeric subunits or other byproducts) at toxic concentrations, causes
inflammation or irritation, or
induces an immune reaction in the host. It is not necessary that any subject
composition have a purity
of 100% to be deemed biocompatible. Hence, a subject composition may comprise
99%, 98%, 97%,
96%, 95%, 90% 85%, 80%, 75% or even less of biocompatible agents, e.g.,
including polymers and
.. other materials and excipients described herein, and still be
biocompatible. "Non-biocompatible" as
used herein, refers to any material that may cause injury or death to the
animal or induce an adverse
reaction in the animal when placed in intimate contact with the animal's
tissues. Such adverse
reactions are as noted above, for example.
[0065] The terms "bioabsorbable," "biodegradable," "bioerodible,"
"bioresorbable," and
"resorbable" are art-recognized synonyms. These terms are used herein
interchangeably.
Bioabsorbable polymers typically differ from non-bioabsorbable polymers in
that the former may be
absorbed (e.g.; degraded) during use. In certain embodiments, such use
involves in vivo use, such as
in vivo therapy, and in other certain embodiments, such use involves in vitro
use. In general,
degradation attributable to biodegradability involves the degradation of a
bioabsorbable polymer into
its component subunits, or digestion, e.g., by a biochemical process, of the
polymer into smaller, non-
polymeric subunits. In certain embodiments, biodegradation may occur by
enzymatic mediation,
degradation in the presence of water (hydrolysis) and/or other chemical
species in the body, or both.
The bioabsorbability of a polymer may be indicated in-vitro as described
herein or by methods known
to one of skill in the art. An in-vitro test for bioabsorbability of a polymer
does not require living
.. cells or other biologic materials to indicate bioabsorption properties
(e.g. degradation, digestion).
Thus, resorbtion, resorption, absorption, absorbtion, erosion may also be used
synonymously with the
terms "bioabsorbable," "biodegradable," "bioerodible," and "bioresorbable."
Mechanisms of
degradation of a bioabsorbable polymer may include, but are not limited to,
bulk degradation, surface
erosion, and combinations thereof
[0066] As used herein, the term "biodegradation" encompasses both general
types of biodegradation.
The degradation rate of a biodegradable polymer often depends in part on a
variety of factors,
including the chemical identity of the linkage responsible for any
degradation, the molecular weight,
crystallinity, biostability, and degree of cross-linking of such polymer, the
physical characteristics
(e.g., shape and size) of the implant, and the mode and location of
administration. For example, the
greater the molecular weight, the higher the degree of crystallinity, and/or
the greater the biostability,
the biodegradation of any bioabsorbable polymer is usually slower.
[0067] "Degradation" as used herein refers to the conversion or reduction of a
chemical compound
to one less complex, e.g., by splitting off one or more groups of atoms.
Degradation of the coating
may reduce the coating's cohesive and adhesive binding to the device, thereby
facilitating transfer of
the coating to the intervention site
- 15 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[0068] As used herein, the term "durable polymer" refers to a polymer that is
not bioabsorbable
(and/or is not bioerodable, and/or is not biodegradable, and/or is not
bioresorbable) and is, thus
biostable. In some embodiments, the device comprises a durable polymer. The
polymer may include a
cross-linked durable polymer. Example biocomaptible durable polymers include,
but are not limited
to: polyester, aliphatic polyester, polyanhydride, polyethylene,
polyorthoester, polyphosphazene,
polyurethane, polycarbonate urethane, aliphatic polycarbonate, silicone, a
silicone containing
polymer, polyolefin, polyamide, polycaprolactam, polyamide, polyvinyl alcohol,
acrylic polymer,
acrylate, polystyrene, epoxy, polyethers, celluiosics, expanded
polytetrafluoroethylene,
phosphorylcholine, polyethyleneyerphthalate, polymethylmethavrylate,
poly(ethylmethacrylate/n-
butylmethacrylate), parylene C, polyethylene-co-vinyl acetate, polyalkyl
methacrylates, polyalkylene-
co-vinyl acetate, polyalkylene, polyalkyl siloxanes, polyhydroxyalkanoate,
polyfluoroalkoxyphasphazine, poly(styrene-b-isobutylene-b-styrene), poly-butyl
methacrylate, poly-
byta-diene, and blends, combinations, homopolymers, condensation polymers,
alternating, block,
dendritic, crosslinked, and copolymers thereof The polymer may include a
thermoset material. The
polymer may provide strength for the coated implanable medical device. The
polymer may provide
durability for the coated implanable medical device. The coatings and coating
methods provided
herein provide substantial protection from these by establishing a multi-layer
coating which can be
bioabsorbable or durable or a combination thereof, and which can both deliver
active agents and
provide elasticity and radial strength for the vessel in which it is
delivered.
[0069] "Therapeutically desirable morphology" as used herein refers to the
gross form and structure
of the pharmaceutical agent, once deposited on the substrate, so as to provide
for optimal conditions
of ex vivo storage, in vivo preservation and/or in vivo release. Such optimal
conditions may include,
but are not limited to increased shelf life (i.e., shelf stability), increased
in vivo stability, good
biocompatibility, good bioavailability or modified release rates. Typically,
for the present invention,
the desired morphology of a pharmaceutical agent would be crystalline or semi-
crystalline or
amorphous, although this may vary widely depending on many factors including,
but not limited to,
the nature of the pharmaceutical agent, the disease to be treated/prevented,
the intended storage
conditions for the substrate prior to use or the location within the body of
any biomedical implant.
Preferably at least 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%, 95%, 97%,
98%, 99%, 99.5%,
and/or 100% of the pharmaceutical agent is in crystalline or semi-crystalline
form.
[0070] In some embodiments of the methods and/or devices provided herein, the
macrolide
immunosuppressive drug is at least 50% crystalline. In some embodiments, the
macrolide
immunosuppressive drug is at least 75% crystalline. In some embodiments, the
macrolide
immunosuppressive drug is at least 90% crystalline. In some embodiments of the
methods and/or
.. devices provided herein the macrolide immunosuppressive drug is at least
95% crystalline. In some
embodiments of the methods and/or devices provided herein the macrolide
immunosuppressive drug
- 16-
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
is at least 97% crystalline. In some embodiments of the methods and/or devices
provided herein
macrolide immunosuppressive drug is at least 98% crystalline. In some
embodiments of the methods
and/or devices provided herein the macrolide immunosuppressive drug is at
least 99% crystalline.
[0071] In some embodiments of the methods and/or devices provided herein
wherein the
pharmaceutical agent is at least 50% crystalline. In some embodiments of the
methods and/or devices
provided herein the pharmaceutical agent is at least 75% crystalline. In some
embodiments of the
methods and/or devices provided herein the pharmaceutical agent is at least
90% crystalline. In some
embodiments of the methods and/or devices provided herein the pharmaceutical
agent is at least 95%
crystalline. In some embodiments of the methods and/or devices provided herein
the pharmaceutical
agent is at least 97% crystalline. In some embodiments of the methods and/or
devices provided herein
pharmaceutical agent is at least 98% crystalline. In some embodiments of the
methods and/or devices
provided herein the pharmaceutical agent is at least 99% crystalline.
[0072] "Stabilizing agent" as used herein refers to any substance that
maintains or enhances the
stability of the biological agent. Ideally these stabilizing agents are
classified as Generally Regarded
As Safe (GRAS) materials by the US Food and Drug Administration (FDA).
Examples of stabilizing
agents include, but are not limited to carrier proteins, such as albumin,
gelatin, metals or inorganic
salts. Pharmaceutically acceptable excipient that may be present can further
be found in the relevant
literature, for example in the Handbook of Pharmaceutical Additives: An
International Guide to More
Than 6000 Products by Trade Name, Chemical, Function, and Manufacturer;
Michael and Irene Ash
(Eds.); Gower Publishing Ltd.; Aldershot, Hampshire, England, 1995.
[0073] "Intervention site" as used herein refers to the location in the body
where the coating is
intended to be delivered (by transfer from, freeing from, and/or dissociating
from the substrate). The
intervention site can be any substance in the medium surrounding the device,
e.g., tissue, cartilage, a
body fluid, etc. The intervention site can be the same as the treatment site,
i.e., the substance to which
the coating is delivered is the same tissue that requires treatment.
Alternatively, the intervention site
can be separate from the treatment site, requiring subsequent diffusion or
transport of the
pharmaceutical or other agent away from the intervention site.
[0074] "Compressed fluid" as used herein refers to a fluid of appreciable
density (e.g., >0.2 g/cc) that
is a gas at standard temperature and pressure. "Supercritical fluid," "near-
critical fluid," "near-
supercritical fluid," "critical fluid," "densified fluid," or "densified gas,"
as used herein refers to a
compressed fluid under conditions wherein the temperature is at least 80% of
the critical temperature
of the fluid and the pressure is at least 50% of the critical pressure of the
fluid, and/or a density of
+50% of the critical density of the fluid.
[0075] Examples of substances that demonstrate supercritical or near critical
behavior suitable for the
present invention include, but are not limited to carbon dioxide, isobutylene,
ammonia, water,
methanol, ethanol, ethane, propane, butane, pentane, dimethyl ether, xenon,
sulfur hexafluoride,
- 17 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
halogenated and partially halogenated materials such as chlorofluorocarbons,
hydrochlorofluorocarbons, hydrofluorocarbons, perfluorocarbons (such as
perfluoromethane and
perfluoropropane, chloroform, trichloro-fluoromethane, dichloro-
difluoromethane, dichloro-
tetrafluoroethane) and mixtures thereof Preferably, the supercritical fluid is
hexafluoropropane (FC-
.. 236EA), or 1,1,1,2,3,3-hexafluoropropane. Preferably, the supercritical
fluid is hexafluoropropane
(FC-236EA), or 1,1,1,2,3,3-hexafluoropropane for use in PLGA polymer coatings.
[0076] "Sintering" as used herein refers to the process by which parts of the
polymer or the entire
polymer becomes continuous (e.g., formation of a continuous polymer film). As
discussed herein, the
sintering process is controlled to produce a fully conformal continuous
polymer (complete sintering)
or to produce regions or domains of continuous coating while producing voids
(discontinuities) in the
polymer. As well, the sintering process is controlled such that some phase
separation is obtained or
maintained between polymer different polymers (e.g., polymers A and B) and/or
to produce phase
separation between discrete polymer particles. Through the sintering process,
the adhesions
properties of the coating are improved to reduce flaking of detachment of the
coating from the
substrate during manipulation in use. As described herein, in some
embodiments, the sintering
process is controlled to provide incomplete sintering of the polymer. In
embodiments involving
incomplete sintering, a polymer is formed with continuous domains, and voids,
gaps, cavities, pores,
channels or, interstices that provide space for sequestering a therapeutic
agent which is released under
controlled conditions. Depending on the nature of the polymer, the size of
polymer particles and/or
other polymer properties, a compressed gas, a densified gas, a near critical
fluid or a super-critical
fluid may be employed. In one example, carbon dioxide is used to treat a
substrate that has been
coated with a polymer and a drug, using dry powder and RESS electrostatic
coating processes. In
another example, isobutylene is employed in the sintering process. In other
examples a mixture of
carbon dioxide and isobutylene is employed. In another example, 1,1,2,3,3-
hexafluoropropane is
.. employed in the sintering process.
[0077] When an amorphous material is heated to a temperature above its glass
transition temperature,
or when a crystalline material is heated to a temperature above a phase
transition temperature, the
molecules comprising the material are more mobile, which in turn means that
they are more active
and thus more prone to reactions such as oxidation. However, when an amorphous
material is
maintained at a temperature below its glass transition temperature, its
molecules are substantially
immobilized and thus less prone to reactions. Likewise, when a crystalline
material is maintained at a
temperature below its phase transition temperature, its molecules are
substantially immobilized and
thus less prone to reactions. Accordingly, processing drug components at mild
conditions, such as the
deposition and sintering conditions described herein, minimizes cross-
reactions and degradation of the
drug component. One type of reaction that is minimized by the processes of the
invention relates to
the ability to avoid conventional solvents which in turn minimizes -oxidation
of drug, whether in
amorphous, semi-crystalline, or crystalline form, by reducing exposure thereof
to free radicals,
- 18 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
residual solvents, protic materials, polar-protic materials, oxidation
initiators, and autoxidation
initiators.
[0078] "Rapid Expansion of Supercritical Solutions" or "RESS" as used herein
involves the
dissolution of a polymer into a compressed fluid, typically a supercritical
fluid, followed by rapid
expansion into a chamber at lower pressure, typically near atmospheric
conditions. The rapid
expansion of the supercritical fluid solution through a small opening, with
its accompanying decrease
in density, reduces the dissolution capacity of the fluid and results in the
nucleation and growth of
polymer particles. The atmosphere of the chamber is maintained in an
electrically neutral state by
maintaining an isolating "cloud" of gas in the chamber. Carbon dioxide,
nitrogen, argon, helium, or
other appropriate gas is employed to prevent electrical charge is transferred
from the substrate to the
surrounding environment.
[0079] "Electrostatic Rapid Expansion of Supercritical Solutions" or "e-RESS"
or "eRESS" as used
herein refers to Electrostatic Capture as described herein combined with Rapid
Expansion of
Supercritical Solutions as described herein. In some embodiments,
Electrostatic Rapid Expansion of
Supercritical Solutions refers to Electrostatic capture as described in the
art, e.g., in U.S. Pat. No.
6,756,084, "Electrostatic deposition of particles generated from rapid
expansion of supercritical fluid
solutions," incorporated herein by reference in its entirety.
[0080] Electrostatic Capture may be used for depositing a coating on a device
(e.g. a balloon), and
may be referred to as "eSTAT" herein. Coating is applied to the balloons via
eSTAT attraction, where
the positively charged coating coat a negatively charged device. For example,
in some embodiments,
sirolimus in crystalline form is applied to the balloons via eSTAT attraction
where the positively
charged drug particles coat the negatively charged balloons. The sirolimus
coated on the balloon, in
some embodiments, has an inherently positive charge.
[0081] Figure 2 depicts an example eSTAT process for coating 12 angioplasty
balloons with
sirolimus. In this example process, an eight liter aluminum foil coated bell
jar 2 is kept in place, but is
not electrically grounded. Milled sirolimus (15.5 mg) is placed in a Swagelok
1/2" tee filter 18
(Swagelok, Inc., Supplemental Figure S15) connected to a pulsed pneumatic
valve 20 (Swagelok,
Inc., Supplemental Figure S16) attached to a cylinder of compressed nitrogen
22. The tee filter is
connected on the other end to the eSTAT nozzle 14, a 1/2" x 3/8" Swagelok
reducing union fitted to a
modified 3/8" Swagelok bulkhead union (Swagelok, Inc., Supplemental Figure
S17) via 1/2" (outer
diameter) polypropylene tubing 16. Balloon(s) 4 are mounted in place under the
bell jar 2. In this
example, twelve 3.0 mm width balloons at a time are coated with the positively
charged milled
sirolimus 6. The balloons 4 may be of various lengths, such as lengths ranging
from 17 mm to 23
mm, however, in other embodiments, other sizes may be used. In some
embodiments, fewer or more
balloons may be coated at a time. The balloons 4 used during the coating
process are typically
mounted on catheters having wires 10 disposed therein 8 which are coupled to a
high voltage power
- 19 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
supply 12 (such as a Spellman SL30 high voltage power supply), which may be
set at -15kV, for
example.
[0082] In some embodiments, the lengths of the balloons may be any length from
5 mm to 35 mm, or
any of the following lengths, for example: about 5 mm, about 7 mm, about 8 mm,
about 10 mm, about
12 mm, about 13 mm, about 15 mm, about 18 mm, about 20 mm, about 21 mm, about
23 mm, about
25 mm, about 28 mm, about 30 mm, about 32 mm, about 33 mm, and about 35 mm.
The term
"about" when used in the context of balloon length, can mean variations of for
example, 10%, 25%,
50%, 0.1 mm, 0.25 mm, 0.5 mm, 1 mm, 2 mm, and 5 mm, depending on the
embodiment.
[0083] In some embodiments, the diameters (i e. widths) of the balloons may be
any diameter from
1.5 mm to 6.0 mm, or any of the following diameters, for example: about 1.5
mm, about 1.8 mm,
about 2.0 mm, about 2.25 mm, about 2.5 mm, about 2.75 mm, about 3.0 mm, about
3.25 mm, about
3.5 mm, about 3.75 mm, about 4.0 mm, about 4.25 mm, about 4.5 mm, about 4.75
mm, about 5.0 mm,
about 5.25 mm, and about 5.5 mm. The term "about" when used in the context of
balloon diameter (or
width), can mean variations of for example, 10%, 25%, 50%, 0.1 mm, 0.25 mm,
0.3 mm, 0.4 mm, 0.5
mm, 0.75 mm, 1 mm, and 2 mm, depending on the embodiment.
[0084] In some embodiments a minimum of one balloon is coated at a time. In
some embodiments,
at least one of: at least 3 balloons, at least 5 balloons, at least 6
balloons, at least 8 balloons, at least 10
balloons, at least 12 balloons, at least 15 balloons, at least 16 balloons, at
least 20 balloons, at least 24
balloons, and at least 30 balloons are coated at a time.
[0085] These balloons may or may not be pre-coated with a polymer, such as
PLGA. Coating of the
balloons may be achieved by various means, such as dip coating, spray coating,
or coating using an
RESS method. For example, a polymer (e.g. PLGA) is applied to the balloons via
rapid expansion of
supercritical solutions (RESS), where the solute (e.g. PLGA) is dissolved in a
supercritical fluid then
rapidly expanded with sudden decompression by passing through a short nozzle
into an area of low
temperature and pressure. These conditions cause the dissolved PLGA to rapidly
precipitate as a fine
powder with a narrow distribution of particle size resulting in a uniform
coating on the angioplasty
balloons.
[0086] Figure 3 depicts an example RESS process for coating balloons 4 with
PLGA. The PLGA is
loaded into a vessel 24 in which it is dissolved in HFC236ea from a HFC236ea
cylinder 26 which is
sent to the vessel 24 through a syringe pump 28 (for example, an Isco 260D
syringe pump). The
PLGA thus forms a supercritical solution with the HFC236 ea, which is stirred
at a high pressure
(5500 psi) in mixing view cells (50cc). The PLGA solution is sent through a
syringe pump 30 (for
example, an Isco 260D syringe pump) which sends the solution through a heater
block 32 (with
temperature control feedback) and then through a timed pneumatic valve 34
which is heated at 137C.
The PLGA solution is then sent through a capillary tube 36 (e.g. PEEKsil
capillary tube 1/16" outer
diameter by 100 micron inner diameter by 10 cm long) which is surrounded by a
stainless steel sheath
- 20 -
CA 02888776 2016-09-26
(e.g. 1/2 inches thick stainless steel sheath). The PLGA is then ejected
through a nozzle 40 which is
electrically grounded (for example, via a stainless steel sheath). When the
PLGA solution exits the
nozzle 40, the PLGA is ejected as dry PLGA particles 42, as the solution
comprising PLGA and
HFC236ea rapidly expands. The balloons 4 used during the coating process are
typically mounted on
catheters having wires 10 disposed therein 8 which are electrically grounded
44. The wires 10 may be
coupled to a high voltage power supply 12 (such as a Spellman SL30 high
voltage power supply), in
order to facilitate the eSTAT coating of the balloons with the active agent,
however, during the RESS
process described in this embodiment, the balloons are electrically grounded
and no current flows
from the power supply 12.
[0087] When viewed in combination, Figure 2 and 3 indicate a single apparatus
that can both coat
according to an RESS process and an eSTAT process. Elements called out and
depicted in Figure 2
may similarly be called out in Figure 3, and vice versa. Alternatively,
separate coating apparatuses
may be used to separately coat according to an RESS process and an eSTAT
process.
[0088] "Solution Enhanced Dispersion of Supercritical Solutions" or "SEDS" as
used herein
involves a spray process for the generation of polymer particles, which are
formed when a
compressed fluid (e.g. supercritical fluid, preferably supercritical CO2) is
used as a diluent to a vehicle
in which a polymer is dissolved (one that can dissolve both the polymer and
the compressed fluid).
The mixing of the compressed fluid diluent with the polymer-containing
solution may be achieved by
encounter of a first stream containing the polymer solution and a second
stream containing the diluent
compressed fluid, for example, within one spray nozzle or by the use of
multiple spray nozzles. The
solvent in the polymer solution may be one compound or a mixture of two or
more ingredients and
may be or comprise an alcohol (including diols, triols, etc.), ether, amine,
ketone, carbonate, or
alkanes, or hydrocarbon (aliphatic or aromatic) or may be a mixture of
compounds, such as mixtures
of alkanes, or mixtures of one or more alkalies in combination with additional
compounds such as one
or more alcohols, (e.g., from 0 or 0.1 to 5% of a Ci to Cis alcohol, including
diols, triols, etc.). See for
example U.S. Pat. No. 6,669,785, The solvent may
optionally contain a surfactant, as also described in, e.g., U.S. Pat. No.
6,669,785.
[0089] In one embodiment of the SEDS process, a first stream of fluid
comprising a polymer
dissolved in a common solvent is co-sprayed with a second stream of compressed
fluid. Polymer
particles are produced as the second stream acts as a diluent that weakens the
solvent in the polymer
solution of the first stream. The now combined streams of fluid, along with
the polymer particles,
flow out of the nozzle assembly into a collection vessel. Control of particle
size, particle size
distribution, and morphology is achieved by tailoring the following process
variables: temperature,
pressure, solvent composition of the first stream, flow-rate of the first
stream, flow-rate of the second
stream, composition of the second stream (where soluble additives may be added
to the compressed
- 2 1 -
CA 02888776 2016-09-26
gas), and conditions of the capture vessel. Typically the capture vessel
contains a fluid phase that is at
least five to ten times (5-10x) atmospheric pressure.
[0090] "Electrostatic Dry Powder Coating" or "e-DPC" or "eDPC" as used herein
refers to
Electrostatic Capture as described herein combined with Dry Powder Coating. e-
DPC deposits
material (including, for example, polymer or impermeable dispersed solid) on
the device or other
substrate as dry powder, using electrostatic capture to attract the powder
particles to the substrate. Dry
powder spraying ("Dry Powder Coating" or "DPC") is well known in the art, and
dry powder
spraying coupled with electrostatic capture has been described, for example in
U.S. Pat. Nos:
5,470,603, 6,319,541, and 6,372,246. Methods
for depositing coatings are described, e.g., in WO 2008/148013, "Polymer Films
for Medical Device
Coating,"
[0091] "Dipping Process" and "Spraying Process" as used herein refer to
methods of coating
substrates that have been described at length in the art. These processes can
be used for coating
medical devices with pharmaceutical agents. Spray coating, described in, e.g.,
U.S. Pat. No.
7,419,696, "Medical devices for delivering a therapeutic agent and method of
preparation" and
elsewhere herein, can involve spraying or airbrushing a thin layer of
solubilized coating or dry
powder coating onto a substrate. Dip coating involves, e.g., dipping a
substrate in a liquid, and then
removing and drying it. Dip coating is described in, e.g., U.S. Pat. No.
5,837,313 "Drug release stent
coating process."
[0092] "Bulk properties" properties of a coating including a pharmaceutical or
a biological agent that
can be enhanced through the methods of the invention include for example:
adhesion, smoothness,
conformality, thickness, and compositional mixing.
[0093J "Electrostatically charged" or "electrical potential" or "electrostatic
capture" as used herein
refers to the collection of the spray-produced particles upon a substrate that
has a different
electrostatic potential than the sprayed particles. Thus, the substrate is at
an attractive electronic
potential with respect to the particles exiting, which results in the capture
of the particles upon the
substrate. i.e. the substrate and particles are oppositely charged, and the
particles transport through the
gaseous medium of the capture vessel onto the surface of the substrate is
enhanced via electrostatic
attraction. This may be achieved by charging the particles and grounding the
substrate or conversely
charging the substrate and grounding the particles, by charging the particles
at one potential (e.g.
negative charge) and charging the substrate at an opposite potential (e.g.
positive charge), or by some
other process, which would be easily envisaged by one of skill in the art of
electrostatic capture.
[0094] "Depositing the active agent by an e-RESS, an e-SEDS, or an e-DPC
process without
electrically charging the substrate" as used herein refers to any of these
processes as performed
without intentionally electrically charging the substrate. It is understood
that the substrate might
become electrically charged unintentionally during any of these processes.
- 22 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[0095] "Depositing the active agent by an e-RESS, an e-SEDS, or an e-DPC
process without creating
an electrical potential between the substrate and a coating apparatus" as used
herein refers to any of
these processes as performed without intentionally generating an electrical
potential between the
substrate and the coating apparatus. It is understood that electrical
potential between the substrate and
the coating apparatus might be generated unintentionally during any of these
processes.
[0096] "Intimate mixture" as used herein, refers to two or more materials,
compounds, or substances
that are uniformly distributed or dispersed together.
[0097] "Layer" as used herein refers to a material covering a surface or
forming an overlying part or
segment. Two different layers may have overlapping portions whereby material
from one layer may
be in contact with material from another layer. Contact between materials of
different layers can be
measured by determining a distance between the materials. For example, Raman
spectroscopy may
be employed in identifying materials from two layers present in close
proximity to each other.
[0098] While layers defined by uniform thickness and/or regular shape are
contemplated herein,
several embodiments described herein relate to layers having varying thickness
and/or irregular shape.
Material of one layer may extend into the space largely occupied by material
of another layer. For
example, in a coating having three layers formed in sequence as a first
polymer layer, a
pharmaceutical agent layer and a second polymer layer, material from the
second polymer layer which
is deposited last in this sequence may extend into the space largely occupied
by material of the
pharmaceutical agent layer whereby material from the second polymer layer may
have contact with
material from the pharmaceutical layer. It is also contemplated that material
from the second polymer
layer may extend through the entire layer largely occupied by pharmaceutical
agent and contact
material from the first polymer layer.
[0099] It should be noted however that contact between material from the
second polymer layer (or
the first polymer layer) and material from the pharmaceutical agent layer
(e.g.; a pharmaceutical agent
crystal particle or a portion thereof) does not necessarily imply formation of
a mixture between the
material from the first or second polymer layers and material from the
pharmaceutical agent layer. In
some embodiments, a layer may be defined by the physical three-dimensional
space occupied by
crystalline particles of a pharmaceutical agent (and/or biological agent). It
is contemplated that such
layer may or may not be continuous as physical space occupied by the crystal
particles of
pharmaceutical agents may be interrupted, for example, by polymer material
from an adjacent
polymer layer. An adjacent polymer layer may be a layer that is in physical
proximity to be
pharmaceutical agent particles in the pharmaceutical agent layer. Similarly,
an adjacent layer may be
the layer formed in a process step right before or right after the process
step in which pharmaceutical
agent particles are deposited to form the pharmaceutical agent layer.
[00100] As described herein, material deposition and layer formation
provided herein are
advantageous in that the pharmaceutical agent remains largely in crystalline
form during the entire
- 23 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
process. While the polymer particles and the pharmaceutical agent particles
may be in contact, the
layer formation process is controlled to avoid formation of a mixture between
the pharmaceutical
agent particles the polymer particles during formation of a coated device.
[00101] In some embodiments, the coating comprises a plurality of
layers deposited on the
substrate, wherein at least one of the layers comprises the active agent. In
some embodiments, at least
one of the layers comprises a polymer. In some embodiments, the polymer is
bioabsorbable. In some
embodiments, the active agent and the polymer are in the same layer, in
separate layers, or form
overlapping layers. In some embodiments, the plurality of layers comprise five
layers deposited as
follows: a first polymer layer, a first active agent layer, a second polymer
layer, a second active agent
layer and a third polymer layer.
[00102] In some embodiments of the methods and/or devices provided
herein, the coating
comprises a plurality of layers deposited on the substrate, wherein at least
one of the layers comprises
the active agent. In some embodiments, at least one of the layers comprises a
polymer. In some
embodiments, the polymer is bioabsorbable. In some embodiments, the active
agent and the polymer
are in the same layer, in separate layers, or form overlapping layers. In some
embodiments, the
coating comprises a plurality of layers deposited on the substrate, wherein at
least one of the layers
comprises the pharmaceutical agent. In some embodiments, the pharmaceutical
agent and the
polymer are in the same layer, in separate layers, or form overlapping layers.
In some embodiments,
the plurality of layers comprise five layers deposited as follows: a first
polymer layer, a first active
agent layer, a second polymer layer, a second active agent layer and a third
polymer layer. In some
embodiments, the plurality of layers comprise five layers deposited as
follows: a first polymer layer, a
first pharmaceutical agent layer, a second polymer layer, a second
pharmaceutical agent layer and a
third polymer layer. In some embodiments, the plurality of layers comprise
five layers deposited as
follows: a first polymer layer, a first active biological agent layer, a
second polymer layer, a second
active biological agent layer and a third polymer layer.
[00103] In some embodiments, the device provides the coating to the
intervention site over an
area of delivery greater than the outer surface contact area of the substrate.
In some embodiments, the
area of delivery is at least 110% greater than the outer surface contact area
of the substrate. In some
embodiments, the area of delivery is at least 110% to 200% greater than the
outer surface contact area
of the substrate. In some embodiments, the area of delivery is at least 200%
greater than the outer
surface contact area of the substrate.
[00104] "Laminate coating" as used herein refers to a coating made up
of two or more layers
of material. Means for creating a laminate coating as described herein (e.g.;
a laminate coating
comprising bioabsorbable polymer(s) and pharmaceutical agent) may include
coating the stent with
drug and polymer as described herein (e-RESS, e-DPC, compressed-gas
sintering). The process
comprises performing multiple and sequential coating steps (with sintering
steps for polymer
- 24 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
materials) wherein different materials may be deposited in each step, thus
creating a laminated
structure with a multitude of layers (at least 2 layers) including polymer
layers and pharmaceutical
agent layers to build the final device (e.g.; laminate coated stent).
[00105] "Portion of the coating" and "portion of the active agent" as
used herein refer to an
.. amount or percentage of the coating or active agent that is freed,
dissociated, and/or transferred from
the substrate to the intervention site, either at a designated point in
delivery, during a certain period of
delivery, or in total throughout the entire delivery process. In embodiments,
the device and methods
of the invention are adapted to free, dissociate, and/or transfer a certain
amount of the coating and/or
active agent.
[00106] For example, in embodiments, at least about 10%, at least about
20%, at least about
30%, at least about 50%, at least about 75%, at least about 85%, at least
about 90%, at least about
95%, and/or at least about 99% of the coating is adapted to be freed,
dissociated, and/or to be
transferred from the substrate to the intervention site. In embodiments, at
least about 10%, at least
about 20%, at least about 30%, at least about 50%, at least about 75%, at
least about 85%, at least
.. about 90%, at least about 95%, and/or at least about 99% of the active
agent is adapted to be freed,
dissociated, and/or to be transferred from the substrate to the intervention
site.
[00107] The portion of the coating and/or that is freed, dissociated,
or transferred from the
device substrate is influenced by any or a combination of, e.g., the size,
shape, and flexibility of the
device substrate, the size, shape, surface qualities of and conditions (e.g.,
blood or lymph circulation,
temperature, etc.) at the intervention site, the composition of the coating,
including the particular
active agent(s) and specific polymer component(s) used in the coating, the
relative proportions of
these components, the use of any release agent(s), and substrate
characteristics. Any one or more of
these and other aspects of the device and methods of the invention can be
adapted to influence the
portion of the coating and/or active agent freed, dissociated, and/or
transferred, as desired to produce
the desired clinical outcome.
[00108] "Substantially all of the coating" as used herein refers to at
least about 50%, at least
about 75%, at least about 85%, at least about 90%, at least about 95%, at
least about 97%, and/or at
least about 99% percent of the coating that was present on the device prior to
use.
[00109] "At least a portion of the substrate" as used herein refers to
an amount and/or
percentage of the substrate. In embodiments of the device and methods of the
invention wherein a
coating is on "at least a portion of the substrate," at least about 10%, at
least about 20%, at least about
30%, at least about 50%, at least about 75%, at least about 85%, at least
about 90%, at least about
95%, and/or at least about 99% of the substrate is coated. In embodiments
wherein "at least a portion
of the substrate" is bioabsorbable, at least about 10%, at least about 20%, at
least about 30%, at least
.. about 50%, at least about 75%, at least about 85%, at least about 90%, at
least about 95%, and/or at
least about 99% of the substrate is bioabsorbable.
- 25 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00110] "Transferring at least a portion" as used herein in the
context of transferring a coating
or active agent from the substrate to an intervention site refers to an amount
and/or percentage of the
coating or active agent that is transferred from the substrate to an
intervention site. In embodiments
of the device and methods of the invention wherein at least a portion of a
coating or active agent is
transferred from the substrate to an intervention site, at least about 10%, at
least about 20%, at least
about 30%, at least about 50%, at least about 75%, at least about 85%, at
least about 90%, at least
about 95%, and/or at least about 99% of the coating or active agent is
transferred from the substrate to
the intervention site. In some embodiments, at least about 10%, at least about
20%, at least about
30%, at least about 50%, at least about 75%, at least about 85%, at least
about 90%, at least about
95%, and/or at least about 99% of the coating is adapted to transfer from the
substrate to the
intervention site. In some embodiments, at least about 10% of the coating is
adapted to transfer from
the substrate to the intervention site. In some embodiments, at least about
20% of the coating is
adapted to transfer from the substrate to the intervention site. In some
embodiments, at least about
30% of the coating is adapted to transfer from the substrate to the
intervention site. In some
embodiments, at least about 50% of the coating is adapted to transfer from the
substrate to the
intervention site. In some embodiments, at least about 75% of the coating is
adapted to transfer from
the substrate to the intervention site. In some embodiments, at least about
85% of the coating is
adapted to transfer from the substrate to the intervention site. In some
embodiments, at least about
90% of the coating is adapted to transfer from the substrate to the
intervention site. In some
embodiments, at least about 95% of the coating is adapted to transfer from the
substrate to the
intervention site. In some embodiments, at least about 99% of the coating is
adapted to transfer from
the substrate to the intervention site. As used herein, "about" when used in
reference to a percentage
of the coating can mean ranges of 1%-5%, of 5%-10%, of 10%- 20%, and/or of 10%-
50% (as a
percent of the percentage of the coating transferred, or as a variation of the
percentage of the coating
transferred).
[00111] In some embodiments, the coating portion that is adapted to
transfer upon stimulation
is on at least one of a distal surface of the substrate, a middle surface of
the substrate, a proximal
surface of the substrate, and an abluminal surface of the substrate. In some
embodiments, the
stimulation decreases the contact between the coating and the substrate. In
some embodiments,
device is adapted to transfer less than about 1%, less than about 5%, less
than about 10%. less than
about 15%, less than about 25%, less than about 50%, less than about 70%, less
than about 80%,
and/or less than about 90% of the coating absent stimulation of the coating.
[00112] In some embodiments, at least about 10%, at least about 20%,
at least about 30%, at
least about 50%, at least about 75%, at least about 85%, at least about 90%,
at least about 95%, and/or
at least about 99% of the active agent is adapted to transfer from the
substrate to the intervention site.
In some embodiments, at least about 10% of the active agent is adapted to
transfer from the substrate
- 26 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
to the intervention site. In some embodiments, at least about 20% of the
active agent is adapted to
transfer from the substrate to the intervention site. In some embodiments, at
least about 30% of the
active agent is adapted to transfer from the substrate to the intervention
site. In some embodiments, at
least about 50% of the active agent is adapted to transfer from the substrate
to the intervention site. In
some embodiments, at least about 75% of the active agent is adapted to
transfer from the substrate to
the intervention site. In some embodiments, at least about 85% of the active
agent is adapted to
transfer from the substrate to the intervention site. In some embodiments, at
least about 90% of the
active agent is adapted to transfer from the substrate to the intervention
site. In some embodiments, at
least about 95% of the active agent is adapted to transfer from the substrate
to the intervention site. In
some embodiments, at least about 99% of the active agent is adapted to
transfer from the substrate to
the intervention site. As used herein, "about" when used in reference to a
percentage of the active
agent can mean ranges of 1%-5%, of 5%-10%, of 10%- 20%, and/or of 10%-50% (as
a percent of the
percentage of the active agent transferred, or as a variation of the
percentage of the active agent
transferred).
[00113] In some embodiments, the active agent portion that is adapted to
transfer upon
stimulation is on at least one of a distal surface of the substrate, a middle
surface of the substrate, a
proximal surface of the substrate, and an abluminal surface of the substrate.
In some embodiments,
the stimulation decreases the contact between the coating and the substrate.
In some embodiments,
the device is adapted to transfer less than about 1%, less than about 5%, less
than about 10%. less than
about 15%, less than about 25%, less than about 50%, less than about 70%, less
than about 80%,
and/or less than about 90% of the active agent absent stimulation of the
coating.
[00114] In some embodiments, the device is adapted to transfer at
least about 10%, at least
about 20%, at least about 30%, at least about 50%, at least about 75%, at
least about 85%, at least
about 90%, at least about 95%, and/or at least about 99% of the coating from
the substrate to the
intervention site. In some embodiments, the device is adapted to transfer at
least about 10% of the
coating from the substrate to the intervention site. In some embodiments, the
device is adapted to
transfer at least about 20% of the coating from the substrate to the
intervention site. In some
embodiments, the device is adapted to transfer at least about 30% of the
coating from the substrate to
the intervention site. In some embodiments, the device is adapted to transfer
at least about 50% of the
coating from the substrate to the intervention site. In some embodiments, the
device is adapted to
transfer at least about 75% of the coating from the substrate to the
intervention site. In some
embodiments, the device is adapted to transfer at least about 85% of the
coating from the substrate to
the intervention site. In some embodiments, the device is adapted to transfer
at least about 90% of the
coating from the substrate to the intervention site. In some embodiments, the
device is adapted to
transfer at least about 95% of the coating from the substrate to the
intervention site. In some
embodiments, the device is adapted to transfer at least about 99% of the
coating from the substrate to
the intervention site. As used herein, "about" when used in reference to a
percentage of the coating
- 27 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
can mean ranges of 1%-5%, of 5%-10%, of 10%- 20%, and/or of 10%-50% (as a
percent of the
percentage of the coating transferred, or as a variation of the percentage of
the coating transferred).
[00115] In some embodiments, the coating portion that transfers upon
stimulation is on at
least one of a distal surface of the substrate, a middle surface of the
substrate, a proximal surface of
the substrate, and an abluminal surface of the substrate. In some embodiments,
stimulation decreases
the contact between the coating and the substrate. In some embodiments, the
device is adapted to
transfer less than about 1%, less than about 5%, less than about 10%. less
than about 15%, less than
about 25%, less than about 50%, less than about 70%, less than about 80%,
and/or less than about
90% of the coating absent stimulation of the coating.
[00116] In some embodiments, the device is adapted to transfer at least
about 10%, at least
about 20%, at least about 30%, at least about 50%, at least about 75%, at
least about 85%, at least
about 90%, at least about 95%, and/or at least about 99% of the active agent
from the substrate to the
intervention site. In some embodiments, the device is adapted to transfer at
least about 10% of the
active agent from the substrate to the intervention site. In some embodiments,
the device is adapted to
transfer at least about 20% of the active agent from the substrate to the
intervention site. In some
embodiments, the device is adapted to transfer at least about 30% of the
active agent from the
substrate to the intervention site. In some embodiments, the device is adapted
to transfer at least about
50% of the active agent from the substrate to the intervention site. In some
embodiments, the device is
adapted to transfer at least about 75% of the active agent from the substrate
to the intervention site. In
some embodiments, the device is adapted to transfer at least about 85% of the
active agent from the
substrate to the intervention site. In some embodiments, the device is adapted
to transfer at least about
90% of the active agent from the substrate to the intervention site. In some
embodiments, the device is
adapted to transfer at least about 95% of the active agent from the substrate
to the intervention site. In
some embodiments, the device is adapted to transfer at least about 99% of the
active agent from the
substrate to the intervention site. As used herein, "about" when used in
reference to a percentage of
the active agent can mean ranges of 1%-5%, of 5%-10%, of 10%- 20%, and/or of
10%-50% (as a
percent of the percentage of the active agent transferred, or as a variation
of the percentage of the
active agent transferred).
[00117] In some embodiments, the coating portion that transfers upon
stimulation is on at
least one of a distal surface of the substrate, a middle surface of the
substrate, a proximal surface of
the substrate, and an abluminal surface of the substrate. In some embodiments,
the stimulation
decreases the contact between the coating and the substrate. In some
embodiments, the device is
adapted to transfer less than about 1%, less than about 5%, less than about
10%. less than about 15%,
less than about 25%, less than about 50%, less than about 70%, less than about
80%, less than about
90% of the active agent absent stimulation of the coating.
- 28 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00118] "Freeing at least a portion" as used herein in the context of
freeing a coating and/or
active agent from the substrate at an intervention site refers to an amount
and/or percentage of a
coating or active agent that is freed from the substrate at an intervention
site. In embodiments of the
device and methods of the invention wherein at least a portion of a coating or
active agent is freed
from the substrate at an intervention site, at least about 10%, at least about
20%, at least about 30%, at
least about 50%, at least about 75%, at least about 85%, at least about 90%,
at least about 95%, and/or
at least about 99% of the coating or active agent is freed from the substrate
at the intervention site. In
some embodiments, the device is adapted to free at least about 10%, at least
about 20%, at least about
30%, at least about 50%, at least about 75%, at least about 85%, at least
about 90%, at least about
95%, and/or at least about 99% of the coating from the substrate. In some
embodiments, the device is
adapted to free at least about 10% of the coating from the substrate to the
intervention site. In some
embodiments, the device is adapted to free at least about 20% of the coating
from the substrate to the
intervention site. In some embodiments, the device is adapted to free at least
about 30% of the coating
from the substrate to the intervention site. In some embodiments, the device
is adapted to free at least
about 50% of the coating from the substrate to the intervention site. In some
embodiments, the device
is adapted to free at least about 75% of the coating from the substrate to the
intervention site. In some
embodiments, the device is adapted to free at least about 85% of the coating
from the substrate to the
intervention site. In some embodiments, the device is adapted to free at least
about 90% of the coating
from the substrate to the intervention site. In some embodiments, the device
is adapted to free at least
about 95% of the coating from the substrate to the intervention site. In some
embodiments, the device
is adapted to free at least about 99% of the coating from the substrate to the
intervention site. As used
herein, "about" when used in reference to a percentage of the coating can mean
ranges of 1%-5%, of
5%-10%, of 10%- 20%, and/or of 10%-50% (as a percent of the percentage of the
coating freed, or as
a variation of the percentage of the coating freed).
[00119] In some embodiments, the coating portion that frees upon
stimulation is on at least
one of a distal surface of the substrate, a middle surface of the substrate, a
proximal surface of the
substrate, and an abluminal surface of the substrate.
[00120] In some embodiments, the stimulation decreases the contact
between the coating and
the substrate. In some embodiments, the device is adapted to free less than
about 1%, less than about
5%, less than about 10%. less than about 15%, less than about 25%, less than
about 50%, less than
about 70%, less than about 80%, less than about 90% of the coating absent
stimulation of the coating.
[00121] "Dissociating at least a portion" as used herein in the
context of dissociating a coating
and/or active agent from the substrate at an intervention site refers to an
amount and/or percentage of
a coating and/or active agent that is dissociated from the substrate at an
intervention site. In
embodiments of the device and methods of the invention wherein at least a
portion of a coating and/or
active agent is dissociated from the substrate at an intervention site, at
least about 10%, at least about
- 29 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
20%, at least about 30%, at least about 50%, at least about 75%, at least
about 85%, at least about
90%, at least about 95%, and/or at least about 99% of the coating and/or
active agent is dissociated
from the substrate at the intervention site.
[00122] In some embodiments, the device is adapted to dissociate at
least about 10%, at least
about 20%, at least about 30%, at least about 50%, at least about 75%, at
least about 85%, at least
about 90%, at least about 95%, and/or at least about 99% of the coating from
the substrate. In some
embodiments, the device is adapted to dissociate at least about 10% of the
coating from the substrate
to the intervention site. In some embodiments, the device is adapted to
dissociate at least about 20%
of the coating from the substrate to the intervention site. In some
embodiments, the device is adapted
to dissociate at least about 30% of the coating from the substrate to the
intervention site. In some
embodiments, the device is adapted to dissociate at least about 50% of the
coating from the substrate
to the intervention site. In some embodiments, the device is adapted to
dissociate at least about 75%
of the coating from the substrate to the intervention site. In some
embodiments, the device is adapted
to dissociate at least about 85% of the coating from the substrate to the
intervention site. In some
embodiments, the device is adapted to dissociate at least about 90% of the
coating from the substrate
to the intervention site. In some embodiments, the device is adapted to
dissociate at least about 95%
of the coating from the substrate to the intervention site. In some
embodiments, the device is adapted
to dissociate at least about 99% of the coating from the substrate to the
intervention site. As used
herein, "about" when used in reference to a percentage of the coating can mean
ranges of 1%-5%, of
5%-10%, of 10%- 20%, and/or of 10%-50% (as a percent of the percentage of the
coating dissociated,
or as a variation of the percentage of the coating dissociated).
[00123] In some embodiments, the coating portion that dissociates upon
stimulation is on at
least one of a distal surface of the substrate, a middle surface of the
substrate, a proximal surface of
the substrate, and an abluminal surface of the substrate. In some embodiments,
stimulation decreases
the contact between the coating and the substrate. In some embodiments, the
device is adapted to
dissociate less than about 1%, less than about 5%, less than about 10%. less
than about 15%, less than
about 25%, less than about 50%, less than about 70%, less than about 80%, less
than about 90% of the
coating absent stimulation of the coating.
[00124] "Depositing at least a portion" as used herein in the context
of a coating and/or active
agent at an intervention site refers to an amount and/or percentage of a
coating and/or active agent that
is deposited at an intervention site. In embodiments of the device and methods
of the invention
wherein at least a portion of a coating and/or active agent is deposited at an
intervention site, at least
about 10%, at least about 20%, at least about 30%, at least about 50%, at
least about 75%, at least
about 85%, at least about 90%, at least about 95%, and/or at least about 99%
of the coating and/or
active agent is deposited at the intervention site. In some embodiments,
stimulating decreases the
contact between the coating and the substrate. In some embodiments, depositing
deposits less than
- 30 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
about 1%, less than about 5%, less than about 10%. less than about 15%, less
than about 25%, less
than about 50%, less than about 70%, less than about 80%, and/or less than
about 90% of the coating
absent stimulating at least one of the coating and the substrate.
[00125] "Delivering at least a portion" as used herein in the context
of a coating and/or active
agent at an intervention site refers to an amount and/or percentage of a
coating and/or active agent that
is delivered to an intervention site. In embodiments of the device and methods
of the invention
wherein at least a portion of a coating and/or active agent is delivered to an
intervention site, at least
about 10%, at least about 20%, at least about 30%, at least about 50%, at
least about 75%, at least
about 85%, at least about 90%, at least about 95%, and/or at least about 99%
of the coating and/or
active agent is delivered to the intervention site.
[00126] In some embodiments, the device is adapted to deliver at least
about 10%, at least
about 20%, at least about 30%, at least about 50%, at least about 75%, at
least about 85%, at least
about 90%, at least about 95%, and/or at least about 99% of the coating to the
intervention site. In
some embodiments, the device is adapted to deliver at least about 10% of the
coating to the
intervention site. In some embodiments, the device is adapted to deliver at
least about 20% of the
coating to the intervention site. In some embodiments, the device is adapted
to deliver at least about
30% of the coating to the intervention site. In some embodiments, the device
is adapted to deliver at
least about 50% of the coating to the intervention site. In some embodiments,
the device is adapted to
deliver at least about 75% of the coating to the intervention site. In some
embodiments, the device is
adapted to deliver at least about 85% of the coating to the intervention site.
In some embodiments,
the device is adapted to deliver at least about 90% of the coating to the
intervention site. In some
embodiments, the device is adapted to deliver at least about 95% of the
coating to the intervention
site. In some embodiments, the device is adapted to deliver at least about 99%
of the coating to the
intervention site. As used herein, "about" when used in reference to a
percentage of the coating can
mean ranges of 1%-5%, of 5%-10%, of 10%- 20%, and/or of 10%-50% (as a percent
of the
percentage of the coating delivered, or as a variation of the percentage of
the coating delivered).
[00127] In some embodiments, the coating portion that is delivered
upon stimulation is on at
least one of a distal surface of the substrate, a middle surface of the
substrate, a proximal surface of
the substrate, and an abluminal surface of the substrate. In some embodiments,
the stimulation
decreases the contact between the coating and the substrate. In some
embodiments, the device is
adapted to deliver less than about 1%, less than about 5%, less than about
10%. less than about 15%,
less than about 25%, less than about 50%, less than about 70%, less than about
80%, less than about
90% of the coating absent stimulation of the coating.
[00128] In some embodiments, depositing at least a portion of the
coating comprises
depositing at least about 10%, at least about 20%, at least about 30%, at
least about 50%, at least
about 75%, at least about 85%, at least about 90%, at least about 95%, and/or
at least about 99% of
-31 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
the coating at the intervention site. In some embodiments, stimulating
decreases the contact between
the coating and the substrate. In some embodiments, depositing deposits less
than about 1%, less than
about 5%, less than about 10%. less than about 15%, less than about 25%, less
than about 50%, less
than about 70%, less than about 80%, and/or less than about 90% of the coating
absent stimulating at
least one of the coating and the substrate.
[00129] "Tacking at least a portion" as used herein in the context of
tacking at least a portion
of the coating to an intervention site refers to an amount and/or percentage
of a coating and/or active
agent that is tacked at an intervention site. In embodiments of the device and
methods of the
invention wherein at least a portion of a coating and/or active agent is
tacked at an intervention site, at
least about 10%, at least about 20%, at least about 30%, at least about 50%,
at least about 75%, at
least about 85%, at least about 90%, at least about 95%, and/or at least about
99% of the coating
and/or active agent is tacked at the intervention site. In some embodiments,
stimulating decreases the
contact between the coating and the substrate. In some embodiments, tacking
tacks less than about
1%, less than about 5%, less than about 10%. less than about 15%, less than
about 25%, less than
about 50%, less than about 70%, less than about 80%, and/or less than about
90% of the coating
absent stimulating at least one of the coating and the substrate. In some
embodiments, the device
comprises a tacking element that cooperates with the stimulation to tack the
coating to the
intervention site. In some embodiments, the device comprises a tacking element
that tacks the
coating to the substrate until stimulating with a stimulation.
[00130] "Adhere," "adherence," "adhered," "cohere," "coherence," "cohered,"
and related
terms, as used herein in the context of adherence or coherence of the
substrate to the coating refer to
an interaction between the substrate and the coating that is sufficiently
strong to maintain the
association of the coating with the substrate for an amount of time prior to
the stimulation, e.g.,
mechanical, chemical, thermal, electromagnetic, or sonic stimulation, that is
intended to cause the
coating to be freed, dissociated, and/or transferred. These same terms, as
used in the context of an
interaction between the coating and the target tissue area and/or intervention
site refer to an
interaction between the coating and the target tissue area and/or intervention
site that is sufficient to
keep the coating associated with the target tissue area and/or intervention
site for an amount of time as
desired for treatment, e.g., at least about 12 hours, about 1 day, about 3
days, about 5 days, about 7
days, about 14 days, about 3 weeks, about 4 weeks, about 45 days, about 60
days, about 90 days,
about 180 days, about 6 months, about 9 months, about 1 year, about 1 to about
2 days, about 1 to
about 5 days, about 1 to about 2 weeks, about 2 to about 4 weeks, about 45 to
about 60 days, about 45
to about 90 days, about 30 to about 90 days, about 60 to about 90 days, about
90 to about 180 days,
about 60 to about 180 days, about 180 to about 365 days, about 6 months to
about 9 months, about 9
months to about 12 months, about 9 months to about 15 months, and about 1 year
to about 2 years.
- 32 -
CA 02888776 2016-09-26
1001311 "Balloon" as used herein refers to a flexible sac that can be
inflated within a natural
or non-natural body lumen or cavity, or used to create a cavity, or used to
enlarge an existing cavity.
The balloon can be used transiently to dilate a lumen or cavity and thereafter
may be deflated and/or
removed from the subject during the medical procedure or thereafter. In
embodiments, the balloon
can be expanded within the body and has a coating thereon that is freed (at
least in part) from the
balloon and left behind in the lumen or cavity when the balloon is removed. A
coating can be applied
to a balloon either after the balloon has been compacted for insertion,
resulting in a coating that
partially covers the surface of the balloon, or it can be applied prior to or
during compaction. In
embodiments, a coating is applied to the balloon both prior to and after
compaction of the balloon. In
embodiments, the balloon is compacted by, e.g., crimping or folding. Methods
of compacting
balloons have been described, e.g., in U.S. Pat. No. 7,308,748, "Method for
compressing an
intraluminal device," and U.S. Pat. No. 7,152,452, "Assembly for crimping an
intraluminal device and
method of use," relating to uniformly crimping a balloon onto a catheter or
other intraluminal device,
and 5,350,361 "Tr-fold balloon for dilatation catheter and related method,"
relating to balloon folding
methods and devices, In some embodiments the
balloon is delivered to the intervention site by a delivery device. In some
embodiments, the delivery
device comprises catheter. In some embodiments, the balloon is an angioplasty
balloon. Balloons
can be delivered, removed, and visualized during delivery and removal by
methods known in the art,
e.g., for inserting angioplasty balloons, stents, and other medical devices.
Methods for visualizing a
treatment area and planning instrument insertion are described, e.g., in U.S.
Pat. No. 7,171,255,
"Virtual reality 3D visualization for surgical procedures" and U.S. Pat. No.
6,610,013, "3D
ultrasound-guided intraoperative prostate brachytherapy."
[00132] "Compliant balloon" as used herein refers to a balloon which
conforms to the
intervention site relatively more than a semi-compliant balloon and still more
so than a non-compliant
balloon. Compliant balloons expand and stretch with increasing pressure within
the balloon, and are
made from such materials as polyethylene or polyolefin copolymers. There is in
the art a general
classification of balloons based on their expandability or "compliance"
relative to each other, as
described e.g., in U.S. Pat. No. 5,556,383, "Block copolymer elastomer
catheter balloons." Generally,
"non-compliant" balloons are the least elastic, increasing in diameter about 2-
7%, typically about 5%,
as the balloon is pressurized from an inflation pressure of about 6 atm to a
pressure of about 12 atm,
that is, they have a "distension" over that pressure range of about 5%. "Semi-
compliant" balloons
have somewhat greater distensions, generally 7-16% and typically 10-12% over
the same
pressurization range. "Compliant" balloons are still more distensible, having
distensions generally in
the range of 16-40% and typically about 21% over the same pressure range.
Maximum distensions,
i.e. distension from nominal diameter to burst, of various balloon materials
may be significantly
higher than the distension percentages discussed above because wall strengths,
and thus burst
- 33 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
pressures, vary widely between balloon materials. These distension ranges are
intended to provide
general guidance, as one of skill in the art will be aware that the compliance
of a balloon is dependent
on the dimensions and/or characteristics of the cavity and/or lumen walls, not
only the expandability
of the balloon.
[00133] A compliant balloon may be used in the vasculature of a subject. A
compliant
balloon might also be used in any tube or hole outside the vasculature
(whether naturally occurring or
man-made, or created during an injury). For a non-limiting example, a
compliant balloon might be
used in a lumpectomy to put a coating at the site where a tumor was removed,
to: treat an abscess,
treat an infection, prevent an infection, aid healing, promote healing, or for
a combination of any of
these purposes. The coating in this embodiment may comprise a growth factor.
[00134] "Non-Compliant balloon" as used herein refers to a balloon
that does not conform to
the intervention site, but rather, tends to cause the intervention site to
conform to the balloon shape.
Non-compliant balloons, commonly made from such materials as polyethylene
terephthalate (PET) or
polyamides, remain at a preselected diameter as the internal balloon pressure
increases beyond that
required to fully inflate the balloon. Non-compliant balloons are often used
to dilate spaces, e.g.,
vascular lumens. As noted with respect to a compliant balloon, one of skill in
the art will be aware
that the compliance of a balloon is dependent on the dimensions and/or
characteristics of the cavity
and/or lumen walls, not only the expandability of the balloon.
[00135] "Cutting balloon" as used herein refers to a balloon commonly
used in angioplasty
having a special balloon tip with cutting elements, e.g., small blades, wires,
etc. The cutting elements
can be activated when the balloon is inflated. In angioplasty procedures,
small blades can be used
score the plaque and the balloon used to compress the fatty matter against the
vessel wall. A cutting
balloon might have tacks or other wire elements which in some embodiments aid
in freeing the
coating from the balloon, and in some embodiments, may promote adherence or
partial adherence of
the coating to the target tissue area, or some combination thereof In some
embodiments, the cutting
balloon cutting elements also score the target tissue to promote the coating's
introduction into the
target tissue. In some embodiments, the cutting elements do not cut tissue at
the intervention site. In
some embodiments, the cutting balloon comprises tacking elements as the
cutting elements.
[00136] "Inflation pressure" as used herein refers to the pressure at
which a balloon is
inflated. As used herein the nominal inflation pressure refers to the pressure
at which a balloon is
inflated in order to achieve a particular balloon dimension, usually a
diameter of the balloon as
designed. The "rated burst pressure" or "RBP" as used herein refers to the
maximum statistically
guaranteed pressure to which a balloon can be inflated without failing. For
PTCA and PTA catheters,
the rated burst pressure is based on the results of in vitro testing to the
PTCA and/or PTA catheters,
and normally means that at least 99.9% of the balloons tested (with 95%
confidence ) will not burst at
or below this pressure.
- 34 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00137] "Tacking element" as used herein refers to an element on the
substrate surface that is
used to influence transfer of the coating to the intervention site. For
example, the tacking element can
comprise a projection, e.g., a bump or a spike, on the surface of the
substrate. In embodiments, the
tacking element is adapted to secure the coating to the cutting balloon until
inflation of the cutting
balloon. In some embodiments, tacking element can comprise a wire, and the
wire can be shaped in
the form of an outward pointing wedge. In certain embodiments, the tacking
element does not cut
tissue at the intervention site.
[00138] As used herein, a "surgical tool" refers to any tool used in a
surgical procedure.
Examples of surgical tools include, but are not limited to: As used herein, a
"surgical tool" refers to
any tool used in a surgical procedure. Examples of surgical tools include, but
are not limited to: a
knife, a scalpel, a guidewire, a guiding catheter, a introduction catheter, a
distracter, a needle, a
syringe, a biopsy device, an articulator, a Galotti articulator, a bone
chisel, a bone crusher, a cottle
cartilage crusher, a bone cutter, a bone distractor, an Ilizarov apparatus, an
intramedullary kinetic
bone distractor, a bone drill, a bone extender, a bone file, a bone lever, a
bone mallet, a bone rasp, a
bone saw, a bone skid, a bone splint, a bone button, a caliper, a cannula, a
catheter, a cautery, a clamp,
a coagulator, a curette, a depressor, a dilator, a dissecting knife, a
distractor, a dermatome, forceps,
dissecting forceps, tissue forceps, sponge forceps, bone forceps, Carmalt
forceps, Cushing forceps,
Dandy forceps, DeBakey forceps, Doyen intestinal forceps, epilation forceps,
Halstead forceps, Kelly
forceps, Kocher forceps, mosquito forceps, a hemostat, a hook, a nerve hook,
an obstetrical hook, a
skin hook, a hypodermic needle, a lancet, a luxator, a lythotome, a
lythotript, a mallet, a partsch
mallet, a mouth prop, a mouth gag, a mammotome, a needle holder, an occluder,
an osteotome, an
Epker osteotome, a periosteal elevator, a Joseph elevator, a Molt periosteal
elevator, an Obweg
periosteal elevator, a septum elevator, a Tessier periosteal elevator, a
probe, a retractor, a Senn
retractor, a Gelpi retractor, a Weitlaner retractor, a USA-Army/Navy
retractor, an O'Connor-
O'Sullivan retractor, a Deaver retractor, a Bookwalter retractor, a Sweetheart
retractor, a Joseph skin
hook, a Lahey retractor, a Blair (Rollet) retractor, a rigid rake retractor, a
flexible rake retractor, a
Ragnell retractor, a Linde-Ragnell retractor, a Davis retractor, a Volkman
retractor, a Mathieu
retractor, a Jackson tracheal hook, a Crile retractor, a Meyerding finger
retractor, a Little retractor, a
Love Nerve retractor, a Green retractor, a Goelet retractor, a Cushing vein
retractor, a Langenbeck
retractor, a Richardson retractor, a Richardson-Eastmann retractor, a Kelly
retractor, a Parker
retractor, a Parker-Mott retractor, a Roux retractor, a Mayo-Collins
retractor, a Ribbon retractor, an
Alm retractor, a self retaining retractor, a Weitlaner retractor, a Beckman-
Weitlaner retractor, a
Beckman-Eaton retractor, a Beckman retractor, an Ads on retractor, a rib
spreader, a rongeur, a
scalpel, an ultrasonic scalpel, a laser scalpel, scissors, iris scissors,
Kiene scissors, Metzenbaum
scissors, Mayo scissors, Tenotomy scissors, a spatula, a speculum, a mouth
speculum, a rectal
speculum, Sim's vaginal speculum, Cusco's vaginal speculum, a sternal saw, a
suction tube, a surgical
elevator, a surgical hook, a surgical knife, surgical mesh, a surgical needle,
a surgical snare, a surgical
- 35 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
sponge, a surgical spoon, a surgical stapler, a suture, a syringe, a tongue
depressor, a tonsillotome, a
tooth extractor, a towel clamp, towel forceps, Backhaus towel forceps, Lorna
towel forceps, a
tracheotome, a tissue expander, a subcutaneus inflatable balloon expander, a
trephine, a trocar,
tweezers, and a venous cliping. In some embodiments, a surgical tool may also
and/or alternatively
be referred to as a tool for performing a medical procedure. In some
embodiments, a surgical tool may
also and/or alternatively be a tool for delivering to the intervention site a
biomedical implant.
[00139] "Stimulation" as used herein refers to any mechanical
stimulation, chemical
stimulation, thermal stimulation, electromagnetic stimulation, and/or sonic
stimulation that influences,
causes, initiates, and/or results in the freeing, dissociation, and/or the
transfer of the coating and/or
active agent from the substrate.
[00140] "Mechanical Stimulation" as used herein refers to use of a
mechanical force that
influences the freeing, dissociation, and/or transfer of the coating and/or
the active agent from the
substrate. For example, mechanical stimulation can comprise a shearing force,
a compressive force, a
force exerted on the coating from a substrate side of the coating, a force
exerted on the coating by the
.. substrate, a force exerted on the coating by an external element, a
translation, a rotation, a vibration,
or a combination thereof In embodiments, the mechanical stimulation comprises
balloon expansion,
stent expansion, etc. In embodiments, the mechanical stimulation is adapted to
augment the freeing,
dissociation and/or transfer of the coating from the substrate. In
embodiments, the mechanical
stimulation is adapted to initiate the freeing, dissociation and/or transfer
of the coating from the
.. substrate. In embodiments, the mechanical stimulation can be adapted to
cause the freeing,
dissociation and/or transference of the coating from the substrate. In
embodiments, an external
element is a part of the subject. In embodiments, the external element is not
part of the device. In
embodiments the external element comprises a liquid, e.g., saline or water. In
certain embodiments
the liquid is forced between the coating and the substrate. In embodiments,
the mechanical
stimulation comprises a geometric configuration of the substrate that
maximizes a shear force on the
coating.
[00141] "Chemical Stimulation" as used herein refers to use of a
chemical force to influence
the freeing, dissociation, and/or transfer of the coating from the substrate.
For example, chemical
stimulation can comprise bulk degradation, interaction with a bodily fluid,
interaction with a bodily
tissue, a chemical interaction with a non-bodily fluid, a chemical interaction
with a chemical, an acid-
base reaction, an enzymatic reaction, hydrolysis, or a combination thereof In
embodiments, the
chemical stimulation is adapted to augment the freeing, dissociation and/or
transfer of the coating
from the substrate. In embodiments, the chemical stimulation is adapted to
initiate the freeing,
dissociation and/or transfer of the coating from the substrate. In
embodiments, the chemical
stimulation is adapted to cause the freeing, dissociation and/or transfer of
the coating from the
substrate. In embodiments, the chemical stimulation is achieved through the
use of a coating that
- 36 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
comprises a material that is adapted to transfer, free, and/or dissociate from
the substrate when at the
intervention site in response to an in-situ enzymatic reaction resulting in a
weak bond between the
coating and the substrate.
[00142] "Thermal Stimulation" as used herein refers to use of a
thermal stimulus to influence
the freeing, dissociation, and/or transfer of the coating from the substrate.
For example, thermal
stimulation can comprise at least one of a hot stimulus and a cold stimulus.
In embodiments, thermal
stimulation comprises at least one of a hot stimulus and a cold stimulus
adapted to augment the
freeing, dissociation and/or transference of the coating from the substrate.
In embodiments, thermal
stimulation comprises at least one of a hot stimulus and a cold stimulus
adapted to initiate the freeing,
dissociation and/or transference of the coating from the substrate. In
embodiments, thermal
stimulation comprises at least one of a hot stimulus and a cold stimulus
adapted to cause the freeing,
dissociation and/or transference of the coating from the substrate.
[00143] "Electromagnetic Stimulation" as used herein refers to use of
an electromagnetic
stimulus to influence the freeing, dissociation, and/or transfer of the
coating from the substrate. For
example, the electromagnetic stimulation is an electromagnetic wave comprising
at least one of, e.g.,
a radio wave, a micro wave, a infrared wave, near infrared wave, a visible
light wave, an ultraviolet
wave, a X-ray wave, and a gamma wave. In embodiments, the electromagnetic
stimulation is adapted
to augment the freeing, dissociation and/or transference of the coating from
the substrate. In
embodiments, the electromagnetic stimulation is adapted to initiate the
freeing, dissociation and/or
transference of the coating from the substrate. In embodiments, the
electromagnetic stimulation is
adapted to cause the freeing, dissociation and/or transference of the coating
from the substrate.
[00144] "Sonic Stimulation" as used herein refers to use of a sonic
stimulus to influence the
freeing, dissociation, and/or transfer of the coating from the substrate. For
example, sonic stimulation
can comprise a sound wave, wherein the sound wave is at least one of an
ultrasound wave, an acoustic
sound wave, and an infrasound wave. In embodiments, the sonic stimulation is
adapted to augment
the freeing, dissociation and/or transfer of the coating from the substrate.
In embodiments, the sonic
stimulation is adapted to initiate the freeing, dissociation and/or transfer
of the coating from the
substrate. In embodiments, the sonic stimulation is adapted to cause the
freeing, dissociation and/or
transfer of the coating from the substrate.
[00145] "Release Agent" as used herein refers to a substance or substrate
structure that
influences the ease, rate, or extent, of release of the coating from the
substrate. In certain
embodiments wherein the device is adapted to transfer a portion of the coating
and/or active agent
from the substrate to the intervention site, the device can be so adapted by,
e.g., substrate attributes
and/or surface modification of the substrate (for non-limiting example:
substrate composition,
substrate materials, substrate shape, substrate deployment attributes,
substrate delivery attributes,
substrate pattern, and/or substrate texture), the delivery system of the
substrate and coating (for non-
- 37-
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
limiting example: control over the substrate, control over the coating using
the delivery system, the
type of delivery system provided, the materials of the delivery system, and/or
combinations thereof),
coating attributes and/or physical characteristics of the coating (for non-
limiting example: selection of
the active agent and/or the polymer and/or the polymer-active agent
composition, or by the coating
having a particular pattern¨e.g. a ribbed pattern, a textured surface, a
smooth surface, and/or another
pattern, coating thickness, coating layers, and/or another physical and/or
compositional attribute),
release agent attributes (for non-limiting example: through the selection a
particular release agent
and/or the manner in which the release agent is employed to transfer the
coating and/or the active
agent, and/or the amount of the release agent used), and/or a combination
thereof Release agents
may include biocompatible release agents, non-biocompatible release agents to
aggravate and/or
otherwise induce a healing response or induce inflammation, powder release
agents, lubricants (e.g.
ePTFE, sugars, other known lubricants), micronized drugs as the release agent
(to create a burst layer
after the coating is freed from the substrate, physical release agents
(patterning of the substrate to free
the coating, others), and/or agents that change properties upon insertion
(e.g. gels, lipid films, vitamin
E, oil, mucosal adhesives, adherent hydrogels, etc.). Methods of patterning a
substrate are described,
e.g., in U.S. Pat. No. 7,537,610, "Method and system for creating a textured
surface on an implantable
medical device." In embodiments, more than one release agent is used, for
example, the substrate can
be patterned and also lubricated. In some embodiments, the release agent
comprises a viscous fluid.
[00146] In some embodiments, the release agent comprises a viscous
fluid. In some
embodiments, the viscous fluid comprises oil. In some embodiments, the viscous
fluid is a fluid that
is viscous relative to water. In some embodiments, the viscous fluid is a
fluid that is viscous relative
to blood. In some embodiments, the viscous fluid is a fluid that is viscous
relative to urine. In some
embodiments, the viscous fluid is a fluid that is viscous relative to bile. In
some embodiments, the
viscous fluid is a fluid that is viscous relative to synovial fluid. In some
embodiments, the viscous
fluid is a fluid that is viscous relative to saline. In some embodiments, the
viscous fluid is a fluid that
is viscous relative to a bodily fluid at the intervention site.
[00147] In some embodiments, the release agent comprises a physical
characteristic of the
substrate. In some embodiments, the physical characteristic of the substrate
comprises at least one of
a patterned coating surface and a ribbed coating surface. In some embodiments,
the patterned coating
surface comprises a stent framework. In some embodiments, the ribbed coating
surface comprises an
undulating substrate surface. In some embodiments, the ribbed coating surface
comprises an substrate
surface having bumps thereon.
[00148] In some embodiments, the release agent comprises a physical
characteristic of the
coating. In some embodiments, the physical characteristic of the coating
comprises a pattern. In some
embodiments, the pattern is a textured surface on the substrate side of the
coating, wherein the
substrate side of the coating is the part of the coating on the substrate. In
some embodiments, the
- 38 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
pattern is a textured surface on the intervention site side of the coating,
wherein the intervention site
side of the coating is the part of the coating that is transferred to, and/or
delivered to, and/or deposited
at the intervention site.
[00149] "Extrusion" and/or "Extruded" and/or to "Extrude" as used
herein refers to the
movement of a substance away from another substance or object, especially upon
stimulation, e.g., by
a mechanical force. For example, in embodiments of the invention, the coating
is extruded from the
substrate.
[00150] Provided herein is a medical device comprising a substrate and
a coating on at least a
portion of the substrate, wherein the coating comprises an active agent,
wherein the coating is
patterned, and wherein at least a portion of the coating is adapted to free
from the substrate upon
stimulation of the coating.
[00151] Provided herein is a medical device comprising a substrate and
a coating on at least a
portion of the substrate, wherein the coating comprises an active agent,
wherein the coating is
patterned, and wherein at least a portion of the coating is adapted to
dissociate from the substrate upon
stimulation of the coating.
[00152] Provided herein is a medical device comprising a substrate and
a coating on at least a
portion of the substrate, wherein the coating comprises an active agent,
wherein the coating is
patterned, and wherein at least a portion of the coating is adapted to
transfer from the substrate to an
intervention site upon stimulation of the coating.
[00153] In some embodiments, the patterned coating comprises at least two
different shapes.
[00154] "Patterned" as used herein in reference to the coating refers
to a coating having at
least two different shapes. The shapes can be formed by various methods,
including for example,
etching, masking, electrostatic capture, and/or by the coating methods
described herein. For example
the coating may have voids that are at least partially through the thickness
of the coating. In some
embodiments, the voids extend fully through the coating. The voids may be in a
regular
configuration, or irregular in shape. The voids may form a repeating
configuration to form the
patterned coating. The voids may have been removed from a smooth or solid
coating to form a
patterned coating. The coating may in some embodiments be patterned by having
a surface that is
ribbed, wavy or bumpy. The coating may in some embodiments be patterned by
having been cut
and/or etched from a coating sheath and/or sheet in a particular design. The
sheath and/or sheet in
such embodiments may have been formed using the coating methods for
manufacture as described
herein. The pattern design may be chosen to improve the freeing, transfer,
and/or dissociation from
the substrate. The pattern design may be chosen to improve the transfer and/or
delivery to the
intervention site.
[00155] Patterned coatings may be created using the methods and processes
described herein,
for non-limiting example, by providing a substrate having a patterned design
thereon comprising, for
- 39 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
example, a material that is chosen to selectively capture the coating
particles (whether active agent,
polymer, or other coating particles) to coat only a desired portion of the
substrate. This portion that is
coated may be the patterned design of the substrate.
[00156] The term "image enhanced polymer" or "imaging agent" as used
herein refer to an
agent that can be used with the devices and methods of the invention to view
at least one component
of the coating, either while the coating is on the substrate or after it is
freed, dissociated and/or
transferred. In embodiments, an image enhanced polymer serves as a tracer,
allowing the movement
or location of the coated device to be identified, e.g., using an imaging
system. In other embodiments,
an image enhanced polymer allows the practitioner to monitor the delivery and
movement of a coating
component. In embodiments, use of an image enhanced polymer enables the
practitioner to determine
the dose of a component of the coating (e.g., the active agent) that is freed,
dissociated and/or
transferred. Information provided by the image enhanced polymer or imaging
agent about the amount
of coating transferred to the intervention site can allow the practitioner to
determine the rate at which
the coating will be released, thereby allowing prediction of dosing over time.
Imaging agents may
comprise barium compounds such as, for non-limiting example, barium sulfate.
Imaging agents may
comprise iodine compounds. Imaging agents may comprise any compound that
improves radiopacity.
[00157] In embodiments, an image enhanced polymer is used with the
device and methods of
the invention for a purpose including, but not limited to, one or more of the
following: monitoring the
location of the substrate, e.g., a balloon or other device; assessing
physiological parameters, e.g., flow
and perfusion; and targeting to a specific molecule. In embodiments, "smart"
agents that activate only
in the presence of their intended target are used with the device and methods
of the invention.
[00158] Provided herein is a method comprising: providing a medical
device, wherein the
medical device comprises a substrate and a coating on at least a portion of
the substrate, wherein the
coating comprises an active agent; and tacking at least a portion of the
coating to an intervention site.
In some embodiments, the tacking the coating portion (i.e. the portion of the
coating) to the
intervention site is upon stimulating the coating with a stimulation.
[00159] In some embodiments, the substrate comprises a balloon. In
some embodiments, the
portion of the balloon having coating thereon comprises an outer surface of
the balloon. In some
embodiments, the outer surface is a surface of the balloon exposed to a
coating prior to balloon
folding. In some embodiments, the outer surface is a surface of the balloon
exposed to a coating
following balloon folding. In some embodiments, the outer surface is a surface
of the balloon
exposed to a coating following balloon crimping. In some embodiments, the
coating comprises a
material that undergoes plastic deformation at pressures provided by inflation
of the balloon. In some
embodiments, the coating comprises a material that undergoes plastic
deformation at a pressure that is
less than the rated burst pressure of the balloon.
- 40 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00160] In some embodiments, the coating comprises a material that
undergoes plastic
deformation at a pressure that is less than the nominal inflation pressure of
the balloon. In some
embodiments, the coating comprises a material that undergoes plastic
deformation with at least 8
ATM of pressure. In some embodiments, the coating comprises a material that
undergoes plastic
deformation with at least 6 ATM of pressure. In some embodiments, the coating
comprises a material
that undergoes plastic deformation with at least 4 ATM of pressure. In some
embodiments, the
coating comprises a material that undergoes plastic deformation with at least
2 ATM of pressure.
[00161] In some embodiments, the balloon is a compliant balloon. In
some embodiments, the
balloon is a semi-compliant balloon. In some embodiments, the balloon is a non-
compliant balloon.
In some embodiments, the balloon conforms to a shape of the intervention site.
[00162] In some embodiments, the balloon comprises a cylindrical
portion. In some
embodiments, the balloon comprises a substantially spherical portion. In some
embodiments, the
balloon comprises a complex shape. In some embodiments, the complex shape
comprises at least one
of a double noded shape, a triple noded shape, a waisted shape, an hourglass
shape, and a ribbed
shape.
[00163] Some embodiments provide devices that can serve interventional
purposes in addition
to delivery of therapeutics, such as a cutting balloon. In some embodiments,
the substrate comprises
a cutting balloon. In some embodiments, the cutting balloon comprises at least
one tacking element
adapted to tack the coating to the intervention site. In some embodiments, the
tacking element is
adapted to secure the coating to the cutting balloon until inflation of the
cutting balloon. In some
embodiments, the tacking element comprises a wire. In some embodiments, the
wire is shaped in the
form of an outward pointing wedge. In some embodiments, the tacking element
does not cut tissue at
the intervention site.
[00164] One illustration devices provided herein include a cutting
balloon for the treatment of
vascular disease (e.g.; occluded lesions in the coronary or peripheral
vasculature). In this
embodiment, the coating may be preferentially located on the 'cutting wire'
portion of the device.
Upon deployment, the wire pushes into the plaque to provide the desired
therapeutic 'cutting' action.
During this cutting, the polymer and drug coating is plastically deformed off
of the wire by the
combination of compressive and shear forces acting on the wire - leaving some
or all of the coating
embedded in the plaque and/or artery wall. A similar approach may be applied
to delivery of
oncology drugs (a) directly to tumors and/or, (b) to the arteries delivering
blood to the tumors for site-
specific chemotherapy, and/or (c) to the voids left after the removal of a
tumor (lumpectomy). These
oncology (as well as other non-vascular) applications may not require the
'cutting' aspects and could
be provided by coatings directly onto the balloon or onto a sheath over the
balloon or according to an
embodiment wherein the coating forms a sheath over the deflated (pleated)
balloon.
- 41 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00165] A cutting balloon embodiment described herein provides several
advantages. Such
embodiment allows for concentrating the mechanical force on the coating/wire
as the balloon is
inflated --- the wire may serve to concentrate the point-of-contact-area of
the balloon expansion
pressure resulting in a much higher force for plastic deformation of the drug
and polymer coating vs.
the non-cutting plain balloon which may distribute the pressure over a much
larger area (therefore
lower force proportional to the ratio of the areas). Embodiments involving a
cutting balloon provide
for the use of polymers that would otherwise be too rigid (higher modulus) to
deform from a non-
cutting balloon.
[00166] Other embodiments provided herein are based on geometric
configurations of the
device that optimize both the deformation and the bulk-migration of the
coating from the device. In
one embodiment wherein the device is a cutting balloon, the (coated) wire of
the cutting balloon is
shaped like a wedge, pointed outward.
[00167] Another embodiment provides catheter-based devices where the
drug-delivery
formulation is delivered to the therapeutic site in the vasculature via
inflation of a balloon.
[00168] One embodiment provides coated percutaneous devices (e.g.;
balloons, whether
cutting balloons or other balloon types) that, upon deployment at a specific
site in the patient, transfer
some or all of the drug-delivery formulation (5-10%, 10-25%, 25-50%, 50-90%,
90-99%, 99-100%)
to the site of therapeutic demand. In certain embodiments, the balloon is at
least in part cylindrical as
expanded or as formed. In certain embodiments, the balloon is at least in part
bulbous as expanded or
as formed. In certain embodiments, the balloon is at least in part spherical
as expanded or as formed.
In certain embodiments, the balloon has a complex shape as expanded or as
formed (such as a double
noded shape, a triple noded shape, has a waist, and/or has an hourglass shape,
for non-limiting
example).
[00169] In some embodiments, transferring at least a portion of the
active agent comprises
transferring at least about 3%, at least about 5%, at least about 10%, at
least about 20%, at least about
30%, greater than 35%, at least about 50%, at least about 75%, at least about
85%, at least about 90%,
at least about 95%, and/or at least about 99% of the active agent from the
substrate. In some
embodiments, stimulating decreases the contact between the coating and the
substrate. In some
embodiments, transferring transfers less than about 1%, less than about 5%,
less than about 10%. less
than about 15%, less than about 25%, at most about 35%, less than about 50%,
less than about 70%,
less than about 80%, and/or less than about 90% of the active agent absent
stimulating at least one of
the coating and the substrate.
[00170] The term "adapted to transfer at least a portion" of the
coating or active agent to an
intervention site refers to a device that is designed to transfer any portion
of the coating or active
agent to an intervention site.
- 42 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00171] The term "adapted to free" a portion of a coating and/or
active agent from the
substrate refers to a device, coating, and/or substrate that is designed to
free a certain percentage of
the coating and/or active agent from the substrate. As used herein, a device,
coating, and/or substrate
that is designed to free a certain percentage of the coating and/or active
agent from the substrate is
designed to unrestrain the coating and/or active agent from the substrate,
and/or to remove any
obstruction and/or connection the coating may have to the substrate (whether
direct or indirect).
[00172] In some embodiments, the device is adapted to free a portion
of the coating and/or
active agent from the substrate. For non-limiting example, the device is so
adapted by substrate
attributes (for non-limiting example: substrate composition, substrate
materials, shape, substrate
deployment attributes, substrate delivery attributes, substrate pattern,
and/or substrate texture), the
delivery system of the substrate and coating (for non-limiting example:
control over the substrate,
control over the coating using the delivery system, the type of delivery
system provided, the materials
of the delivery system, and/or combinations thereof), coating attributes (for
non-limiting example:
selection of the active agent and/or the polymer and/or the polymer-active
agent composition, or by
the coating having a particular pattern¨e.g. a ribbed pattern, a textured
surface, a smooth surface,
and/or another pattern, coating thickness, coating layers, and/or another
physical and/or compositional
attribute), release agent attributes (for non-limiting example: through the
selection a particular release
agent and/or how the release agent is employed to transfer the coating and/or
the active agent, and/or
how much of the release agent is used), and/or a combination thereof
[00173] In some embodiments, the substrate is adapted to free a portion of
the coating and/or
active agent from the substrate. For non-limiting example, the substrate is so
adapted by selection of
the substrate composition, substrate materials, shape, substrate deployment
attributes, substrate
delivery attributes, substrate pattern, and/or substrate texture, and/or
combinations thereof For
example, a balloon can be designed to only partially inflate within the
confines of the intervention
site. Partial inflation can prevent a designated portion of coating from being
freed.
[00174] In some embodiments, the coating is adapted to free a portion
of the coating and/or
active agent from the substrate. For non-limiting example the coating may be
so adapted by selection
of the active agent and/or the polymer and/or the polymer-active agent
composition, or by the coating
having a particular pattern¨e.g. a ribbed pattern, a textured surface, a
smooth surface, and/or another
pattern, coating thickness, coating layers, and/or another physical and/or
compositional attribute.
[00175] In some embodiments, the substrate is adapted to free a
portion of the coating and/or
active agent from the substrate to the intervention site. For non-limiting
example, the substrate is so
adapted by selection of the substrate composition, substrate materials, shape,
substrate deployment
attributes, substrate delivery attributes, substrate pattern, and/or substrate
texture, and/or combinations
thereof For example, a balloon can be designed to only partially inflate
within the confines of the
intervention site. Partial inflation can prevent a designated portion of
coating from being freed.
- 43 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00176] In some embodiments, the coating is adapted to free a portion
of the coating and/or
active agent from the substrate to the intervention site. For non-limiting
example the coating may be
so adapted by selection of the active agent and/or the polymer and/or the
polymer-active agent
composition, or by the coating having a particular pattern¨e.g. a ribbed
pattern, a textured surface, a
.. smooth surface, and/or another pattern, coating thickness, coating layers,
and/or another physical
and/or compositional attribute.
[00177] In some embodiments, freeing at least a portion of the coating
comprises freeing at
least about 10%, at least about 20%, at least about 30%, greater than 35%, at
least about 50%, at least
about 75%, at least about 85%, at least about 90%, at least about 95%, and/or
at least about 99% of
the coating from the substrate. In some embodiments, stimulating decreases the
contact between the
coating and the substrate. In some embodiments, freeing frees less than about
1%, less than about 5%,
less than about 10%. less than about 15%, less than about 25%, at most about
35%, less than about
50%, less than about 70%, less than about 80%, and/or less than about 90% of
the coating absent
stimulating at least one of the coating and the substrate.
[00178] The term "adapted to dissociate" a portion of a coating and/or
active agent from the
substrate refers to a device, coating, and/or substrate that is designed to
dissociate a certain percentage
of the coating and/or active agent from the substrate. As used herein, a
device, coating, and/or
substrate that is designed to dissociate a certain percentage of the coating
and/or active agent from the
substrate is designed to remove from association between the coating (and/or
active agent) and the
substrate. Also and/or alternatively, as used herein, a device, coating,
and/or substrate that is designed
to dissociate a certain percentage of the coating and/or active agent from the
substrate is designed to
separate the coating (and/or active agent) from the substrate. This separation
may be reversible in
some embodiments. This separation may not be reversible in some embodiments.
[00179] In some embodiments, the device is adapted to dissociate a
portion of the coating
and/or active agent from the substrate. For non-limiting example, the device
is so adapted by
substrate attributes (for non-limiting example: substrate composition,
substrate materials, shape,
substrate deployment attributes, substrate delivery attributes, substrate
pattern, and/or substrate
texture), the delivery system of the substrate and coating (for non-limiting
example: control over the
substrate, control over the coating using the delivery system, the type of
delivery system provided, the
materials of the delivery system, and/or combinations thereof), coating
attributes (for non-limiting
example: selection of the active agent and/or the polymer and/or the polymer-
active agent
composition, or by the coating having a particular pattern¨e.g. a ribbed
pattern, a textured surface, a
smooth surface, and/or another pattern, coating thickness, coating layers,
and/or another physical
and/or compositional attribute), release agent attributes (for non-limiting
example: through the
.. selection a particular release agent and/or how the release agent is
employed to transfer the coating
and/or the active agent, and/or how much of the release agent is used), and/or
a combination thereof
- 44 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00180] In some embodiments, the substrate is adapted to dissociate a
portion of the coating
and/or active agent from the substrate. For non-limiting example, the
substrate is so adapted by
selection of the substrate composition, substrate materials, shape, substrate
deployment attributes,
substrate delivery attributes, substrate pattern, and/or substrate texture,
and/or combinations thereof
For example, a balloon can be designed to only partially inflate within the
confines of the intervention
site. Partial inflation can prevent a designated portion of coating from being
freed.
[00181] In some embodiments, the coating is adapted to dissociate a
portion of the coating
and/or active agent from the substrate. For non-limiting example the coating
may be so adapted by
selection of the active agent and/or the polymer and/or the polymer-active
agent composition, or by
the coating having a particular pattern¨e.g. a ribbed pattern, a textured
surface, a smooth surface,
and/or another pattern, coating thickness, coating layers, and/or another
physical and/or compositional
attribute.
[00182] In some embodiments, the substrate is adapted to free a
portion of the coating and/or
active agent from the substrate to the intervention site. For non-limiting
example, the substrate is so
adapted by selection of the substrate composition, substrate materials, shape,
substrate deployment
attributes, substrate delivery attributes, substrate pattern, and/or substrate
texture, and/or combinations
thereof For example, a balloon can be designed to only partially inflate
within the confines of the
intervention site. Partial inflation can prevent a designated portion of
coating from being freed.
[00183] In some embodiments, the coating is adapted to dissociate a
portion of the coating
and/or active agent from the substrate to the intervention site. For non-
limiting example the coating
may be so adapted by selection of the active agent and/or the polymer and/or
the polymer-active agent
composition, or by the coating having a particular pattern¨e.g. a ribbed
pattern, a textured surface, a
smooth surface, and/or another pattern, coating thickness, coating layers,
and/or another physical
and/or compositional attribute.
[00184] In some embodiments, dissociating at least a portion of the coating
comprises
dissociating at least about 10%, at least about 20%, at least about 30%,
greater than 35%, at least
about 50%, at least about 75%, at least about 85%, at least about 90%, at
least about 95%, and/or at
least about 99% of the coating from the substrate. In some embodiments,
stimulating decreases the
contact between the coating and the substrate. In some embodiments,
dissociating dissociates less than
about 1%, less than about 5%, less than about 10%. less than about 15%, less
than about 25%, at most
about 35%, less than about 50%, less than about 70%, less than about 80%,
and/or less than about
90% of the coating absent stimulating at least one of the coating and the
substrate.
[00185] "Plastic deformation" as used herein is the change in the
physical shape of the coating
by forces induced on the device. Plastic deformation results in increasing the
contact area of the
coating on the tissue and decreasing the contact area of the coating on the
device. This change in
contact area results in some or all of the coating being preferentially
exposed to the tissue instead of
- 45 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
the device. The terms "plastic deformation" and "plastically deform," as used
herein in the context of
a coating, are intended to include the expansion of the coating material
beyond the elastic limit of the
material such that the material is permanently deformed. "Elastic deformation"
as used herein refers
to a reversible alteration of the form or dimensions of the object under
stress or strain, e.g., inflation
pressure of a balloon substrate. The terms "plastic deformation" and
"plastically deform," as used
herein in the context of a balloon or other substrate, are intended to include
the expansion of the
substrate beyond the elastic limit of the substrate material such that the
substrate material is
permanently deformed. Once plastically deformed, a material becomes
substantially inelastic and
generally will not, on its own, return to its pre-expansion size and shape.
"Residual plastic
deformation" refers to a deformation capable of remaining at least partially
after removal of the
inflation stress, e.g., when the balloon is deflated. "Elastic deformation" as
used herein refers to a
reversible alteration of the form or dimensions of the object (whether it is
the coating or the substrate)
under stress or strain, e.g., inflation pressure.
[00186] "Shear transfer" as used herein is the force (or component of
forces) orthogonal to the
device that would drive the coating away from the device substrate. This could
be induced on the
device by deployment, pressure-response from the surrounding tissue and/or in-
growth of tissue
around the coating.
[00187] "Bulk migration" as used herein is the incorporation of the
coating onto/into the
tissue provided by the removal of the device and/or provided by degradation of
the coating over time
and/or provided by hydration of the coating over time. Degradation and
hydration of the coating may
reduce the coating's cohesive and adhesive binding to the device, thereby
facilitating transfer of the
coating to the tissue.
[00188] One embodiment may described by analogy to contact printing
whereby a
biochemically active 'ink' (the polymer + drug coating) from a 'die' (the
device) to the 'stock' (the
site in the body).
[00189] The devices and methods described in conjunction with some of
the embodiments
provided herein are advantageously based on specific properties provided for
in the drug-delivery
formulation. One such property, especially well-suited for non-permanent
implants such as balloon
catheters, cutting balloons, etc. is 'soft' coating that undergoes plastic
deformation at pressures
provided by the inflation of the balloon (range 2-25 ATM, typically 10-18
ATM). Another such
property, especially well-suited to permanent implants such as stents is
coatings where the polymer
becomes 'soft' at some point after implant either by hydration or by
degradation or by combinations
of hydration and degradation.
[00190] Some embodiments provide devices that can advantageously be
used in conjunction
with methods that can aid/promote the transfer of the coating. These include
introducing stimuli to
the coated device once on-site in the body (where the device is delivered
either transiently or
- 46 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
permanently). Such stimuli can be provided to induce a chemical response
(light, heat, radiation, etc.)
in the coating or can provide mechanical forces to augment the transfer of the
coating into the tissue
(ultrasound, translation, rotation, vibration and combinations thereof).
[00191]
In some embodiments, the coating is freed, dissociated, and/or transferred
from the
substrate using a mechanical stimulation. In some embodiments, the coating is
freed from the
substrate using a mechanical stimulation. In some embodiments, the coating is
dissociated from the
substrate using a mechanical stimulation. In some embodiments, the coating is
transferred from the
substrate using a mechanical stimulation. In some embodiments, the coating is
transferred to the
intervention site using a mechanical stimulation. In some embodiments, the
coating is delivered to the
intervention site using a mechanical stimulation. In some embodiments, the
mechanical stimulation is
adapted to augment the freeing, dissociation and/or transference of the
coating from the substrate. In
some embodiments, the mechanical stimulation is adapted to initiate the
freeing, dissociation and/or
transference of the coating from the substrate. In some embodiments, the
mechanical stimulation is
adapted to cause the freeing, dissociation and/or transference of the coating
from the substrate. In
some embodiments, the mechanical stimulation comprises at least one of a
compressive force, a shear
force, a tensile force, a force exerted on the coating from a substrate side
of the coating, a force
exerted on the coating by the substrate, a force exerted on the coating from
an external element, a
translation, a rotation, a vibration, and a combination thereof In some
embodiments, the external
element is a part of the subject. In some embodiments, the external element is
not part of the device.
In some embodiments, the external element comprises a liquid. In some
embodiments, the liquid is
forced between the coating and the substrate. In some embodiments, the liquid
comprises saline. In
some embodiments, the liquid comprises water. In some embodiments, the
mechanical stimulation
comprises a geometric configuration of the substrate that maximizes a shear
force on the coating. In
some embodiments, the mechanical stimulation comprises a geometric
configuration of the substrate
that increases a shear force on the coating. In some embodiments, the
mechanical stimulation
comprises a geometric configuration of the substrate that enhances a shear
force on the coating.
[00192]
In some embodiments, the coating is freed, dissociated, and/or transferred
from the
substrate using a chemical stimulation. In some embodiments, the coating is
freed from the substrate
using a chemical stimulation. In some embodiments, the coating is dissociated
from the substrate
using a chemical stimulation. In some embodiments, the coating is transferred
from the substrate
using a chemical stimulation. In some embodiments, the coating is transferred
to the intervention site
using a chemical stimulation. In some embodiments, the coating is delivered to
the intervention site
using a chemical stimulation. In some embodiments, the chemical stimulation
comprises at least one
of bulk degradation, interaction with a bodily fluid, interaction with a
bodily tissue, a chemical
interaction with a non-bodily fluid, a chemical interaction with a chemical,
an acid-base reaction, an
enzymatic reaction, hydrolysis, and combinations thereof In some embodiments,
the chemical
stimulation comprises bulk degradation of the coating. In some embodiments,
the chemical
- 47 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
stimulation comprises interaction of the coating or a portion thereof with a
bodily fluid. In some
embodiments, the chemical stimulation comprises interaction of the coating or
a portion thereof with a
bodily tissue. In some embodiments, the chemical stimulation comprises a
chemical interaction of the
coating or a portion thereof with a non-bodily fluid. In some embodiments, the
chemical stimulation
comprises a chemical interaction of the coating or a portion thereof with a
chemical. In some
embodiments, the chemical stimulation comprises an acid-base reaction. In some
embodiments, the
chemical stimulation comprises an enzymatic reaction. In some embodiments, the
chemical
stimulation comprises hydrolysis.
[00193] In some embodiments, the chemical stimulation is adapted to
augment the freeing,
dissociation and/or transference of the coating from the substrate. In some
embodiments, the
chemical stimulation is adapted to initiate the freeing, dissociation and/or
transference of the coating
from the substrate. In some embodiments, the chemical stimulation is adapted
to cause the freeing,
dissociation and/or transference of the coating from the substrate. In some
embodiments, the coating
comprises a material that is adapted to transfer, free, and/or dissociate from
the substrate when at the
intervention site in response to an in-situ enzymatic reaction resulting in a
weak bond between the
coating and the substrate.
[00194] In some embodiments, the coating is freed, dissociated, and/or
transferred from the
substrate using a thermal stimulation. In some embodiments, the coating is
freed from the substrate
using a thermal stimulation. In some embodiments, the coating is dissociated
from the substrate using
a thermal stimulation. In some embodiments, the coating is transferred from
the substrate using a
thermal stimulation. In some embodiments, the coating is transferred to the
intervention site using a
thermal stimulation. In some embodiments, the coating is delivered to the
intervention site using a
thermal stimulation. In some embodiments, the thermal stimulation comprises at
least one of a hot
stimulus and a cold stimulus adapted to augment the freeing, dissociation
and/or transference of the
.. coating from the substrate. In some embodiments, the thermal stimulation is
adapted to cause the
freeing, dissociation and/or transference of the coating from the substrate.
In some embodiments, the
thermal stimulation comprises at least one of a hot stimulus and a cold
stimulus adapted to initiate the
freeing, dissociation and/or transference of the coating from the substrate.
In some embodiments, the
thermal stimulation comprises at least one of a hot stimulus and a cold
stimulus adapted to initiate the
freeing, dissociation and/or transference of the coating from the substrate.
[00195] In some embodiments, the coating is freed, dissociated, and/or
transferred from the
device by a electromagnetic stimulation. In some embodiments, the coating is
freed from the
substrate using a electromagnetic stimulation. In some embodiments, the
coating is dissociated from
the substrate using a electromagnetic stimulation. In some embodiments, the
coating is transferred
from the substrate using a electromagnetic stimulation. In some embodiments,
the coating is
transferred to the intervention site using a electromagnetic stimulation. In
some embodiments, the
- 48 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
coating is delivered to the intervention site using a electromagnetic
stimulation. In some
embodiments, the electromagnetic stimulation comprises an electromagnetic wave
comprising at least
one of a radio wave, a micro wave, a infrared wave, near infrared wave, a
visible light wave, an
ultraviolet wave, a X-ray wave, and a gamma wave. In some embodiments, the
electromagnetic
stimulation is adapted to augment the freeing, dissociation and/or
transference of the coating from the
substrate. In some embodiments, the electromagnetic stimulation is adapted to
initiate the freeing,
dissociation and/or transference of the coating from the substrate. In some
embodiments, the
electromagnetic stimulation is adapted to cause the freeing, dissociation
and/or transference of the
coating from the substrate.
[00196] In some embodiments, the coating is freed, dissociated, and/or
transferred from the
device by a sonic stimulation. In some embodiments, the coating is freed from
the substrate using a
sonic stimulation. In some embodiments, the coating is dissociated from the
substrate using a sonic
stimulation. In some embodiments, the coating is transferred from the
substrate using a sonic
stimulation. In some embodiments, the coating is transferred to the
intervention site using a sonic
stimulation. In some embodiments, the coating is delivered to the intervention
site using a sonic
stimulation. In some embodiments, the sonic stimulation comprises a sound
wave, wherein the sound
wave is at least one of an ultrasound wave, an acoustic sound wave, and an
infrasound wave. In some
embodiments, the sonic stimulation is adapted to augment the freeing,
dissociation and/or transference
of the coating from the substrate. In some embodiments, the sonic stimulation
is adapted to initiate
the freeing, dissociation and/or transference of the coating from the
substrate. In some embodiments,
the sonic stimulation is adapted to cause the freeing, dissociation and/or
transference of the coating
from the substrate.
[00197] In some embodiments, the coating is freed, dissociated, and/or
transferred from the
device by a combination of at least two of a mechanical stimulation, a
chemical stimulation, an
electromagnetic stimulation, and a sonic stimulation.
[00198] In some embodiments, the coating is freed, dissociated, and/or
transferred from the
substrate by extrusion.
[00199] Provided herein are device geometries that maximize the shear
forces on the coating.
Such geometric design of the device provides two advantages: (1) increases
(concentrates) the force to
plastically deform the drug and polymer coating (2) decreases the force of
adhesion of the coating.
For example, a wedge-shape aligns the forces of deformation along a shear plan
as opposed to direct
compression. This embodiment provides for: (1) increased efficiency in terms
of % of the coating
transferred (2) increased precision in amount transferred on a case-by-case
basis (3) utilization of
'harder/stiffer' materials (biopolymers) that would otherwise not deform
and/or not bulk-migrate
under deployment conditions (4) minimize the chance of particulate shedding
via purposefully
designing the shape and direction of both the deformation and bulk migration.
For example for a
- 49 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
wedge, particles would be less likely because the coating would be pre-
disposed as a shear from the
device in a sheet form ¨ with the use of soft materials, this may be
illustrated as a coating of silicone
caulk being extruded from the pressure of a rod being pushed into a mattress.
[00200] Another embodiment provide a geometric arrangement of the
coating whereby layers,
e.g. a laminate structure, are provided in the coating to modulate and control
the plastic deformation,
shearing and bulk-migration of the coating into the tissue.
[00201] One embodiment provides coated substrates that, upon
deployment at a specific site
in the patient, transfer some or all of the coating (5-10%, 10-25%, 25-50%, 50-
90%, 90-99%, 99-
100%) to the site of therapeutic demand.
[00202] In some embodiments, the device further comprises a release agent.
In some
embodiments, the release agent is biocompatible. In some embodiments, the
release agent is non-
biocompatible. In some embodiments, the release agent comprises a powder. In
some embodiments,
the release agent comprises a lubricant. In some embodiments, the release
agent comprises a surface
modification of the substrate.
[00203] In some embodiments, the release agent comprises a physical
characteristic of the
coating. In some embodiments, the physical characteristic of the coating
comprises a pattern. In some
embodiments, the pattern is a textured surface on the substrate side of the
coating, wherein the
substrate side of the coating is the part of the coating on the substrate. In
some embodiments, the
pattern is a textured surface on the intervention site side of the coating,
wherein the intervention site
side of the coating is the part of the coating that is transferred to, and/or
delivered to, and/or deposited
at the intervention site.
[00204] In some embodiments, the release agent comprises a viscous
fluid. In some
embodiments, the viscous fluid comprises oil. In some embodiments, the viscous
fluid is a fluid that
is viscous relative to water. In some embodiments, the viscous fluid is a
fluid that is viscous relative
to blood. In some embodiments, the viscous fluid is a fluid that is viscous
relative to urine. In some
embodiments, the viscous fluid is a fluid that is viscous relative to bile. In
some embodiments, the
viscous fluid is a fluid that is viscous relative to synovial fluid. In some
embodiments, the viscous
fluid is a fluid that is viscous relative to saline. In some embodiments, the
viscous fluid is a fluid that
is viscous relative to a bodily fluid at the intervention site.
[00205] In some embodiments, the release agent comprises a gel.
[00206] In some embodiments, the release agent comprises at least one
of the active agent and
another active agent. The active agent may be placed on the substrate prior to
the coating in order to
act as the release agent. The active agent may be a different active agent
than the active agent in the
coating. The active agent that is the release agent may provide for a second
source of drug to be
delivered to the intervention site or another location once the coating is
released from (or transferred
from, or freed from, or dissociated from) the substrate.
- 50 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00207] In some embodiments, the release agent comprises a physical
characteristic of the
substrate. In some embodiments, the physical characteristic of the substrate
comprises at least one of
a patterned coating surface and a ribbed coating surface. In some embodiments,
the patterned coating
surface comprises a stent framework. In some embodiments, the ribbed coating
surface comprises an
undulating substrate surface. In some embodiments, the ribbed coating surface
comprises an substrate
surface having bumps thereon.
[00208] In some embodiments, the release agent comprises a property
that is capable of
changing at the intervention site. In some embodiments, the property comprises
a physical property.
In some embodiments, the property comprises a chemical property. In some
embodiments, the release
agent is capable of changing a property when in contact with at least one of a
biologic tissue and a
biologic fluid. In some embodiments, the release agent is capable of changing
a property when in
contact with an aqueous liquid.
[00209] In some embodiments, the release agent is between the
substrate and the coating.
[00210] In some embodiments, at least a portion of the pharmaceutical
agent is encapsulated
using a single emulsion/evaporation technique. In some embodiments, the
technique comprises at
least the steps of (1) combining a first polymer (e.g. PVA) and water to form
a first polymer solution;
combining a second polymer (e.g. PLGA) and an organic solvent (e.g.
dichloromethane) to form a
second polymer solution; combining the second polymer (e.g. PLGA) and the
organic solvent (e.g.
dichloromethane) to form a third polymer solution; and combining the
pharmaceutical agent and a
polar aprotic solvent (e.g. DMSO) to form a pharmaceutical agent solution, (2)
mixing the second
polymer solution and pharmaceutical agent solution, (3) adding the first
polymer solution and
homogenizing the resulting mixture; (4) adding the third polymer solution; (5)
optionally, allowing
the organic solvent to evaporate; and (6) filtering the remaining solution. In
some embodiments, the
filtration is accomplished using a centrifuge. In some embodiments, the
filtration is accomplished by
passing the mixture through filter paper. In some embodiments, the
encapsulated pharmaceutical
agent is further resuspended in water and lyophilized.
[00211] In some embodiments, the polymer concentration of the first
polymer solutions is
from about 1% to about 10%, from about 1% to about 9%, from about 1% to about
8%, from about
1% to about 7%, from about 1% to about 6%, from about 1% to about 5%, from
about 1% to about
4%, from about 1% to about 3%, from about 1.5% to about 2.5%, or about 2%. As
used herein, the
term "about" when used in reference to the polymer concentration expressed as
a percentage means a
variation of 0.05%, 0.1%, 0.2%, 0.25%, 0.3%, 0.4%, or 0.5%. For example a
polymer concentration
that is about 2% may be expressed as 2%+/- 0.5% (i.e. 1.5%-2.5%), or may be
from 1.95-2.05%
(2%+/-0.05%), depending on the embodiment. In some embodiments, the polymer
concentration of
the first polymer solutions is from 1% to 10%, from 1% to 9%, from 1% to 8%,
from 1% to 7%, from
1% to 6%, from 1% to 5%, from 1% to 4%, from 1% to 3%, from 1.5% to 2.5%, or
2%.
-51 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00212] In some embodiments, the polymer concentration of the second
polymer solution is
from about 10 mg/mL to about 100mg/mL, from about 15 mg/mL to about 95 mg/mL.
from about 20
mg/mL to about 90 mg/mL, from about 25 mg/mL to about 85 mg/mL, from about 30
mg/mL to
about 80 mg/mL, from about 35 mg/mL to about 75 mg/mL, from about 40 mg/mL to
about 70
mg/mL, from about 40 mg/mL to about 60 mg/mL, from about 45 mg/mL to about 55
mg/mL, or
about 50 mg/mL. As used herein, the term "about" when used in reference to the
polymer
concentration expressed in mg/mL means a variation of 1 mg/mL, 5 mg/mL, 10
mg/mL, or 15
mg/mL, depending on the embodiment. In some embodiments, the polymer
concentration of the
second polymer solution is from 10 mg/mL to 100mg/mL, from 15 mg/mL to 95
mg/mL. from 20
mg/mL to 90 mg/mL, bet from ween 25 mg/mL to 85 mg/mL, from 30 mg/mL to 80
mg/mL, from 35
mg/mL to 75 mg/mL, from 40 mg/mL to 70 mg/mL, from 40 mg/mL to 60 mg/mL, from
45 mg/mL
to 55 mg/mL, or 50 mg/mL
[00213] In some embodiments, the polymer concentration of the third
polymer solutions is
from about 0.5% to about 5%, from about 0.5% to about 4.5%, from about 0.5% to
about 4%, from
about 0.5% to about 3.5%, from about 0.5% to about 3%, from about 0.5% to
about 2.5%, from about
0.5% to about 2%, from about 0.5% to about 1.5%, or about 2%. As used herein,
the term "about"
when used in reference to the polymer concentration expressed as a percentage
means a variation of
0.05%, 0.1%, 0.2%, 0.25%, 0.3%, 0.4%, or 0.5%. For example a polymer
concentration that is about
2% may be expressed as 2%+/- 0.5% (i.e. 1.5%-2.5%), or may be from 1.95-2.05%
(2%+/-0.05%),
depending on the embodiment. In some embodiments, the polymer concentration of
the third polymer
solutions is from 0.5% to 5%, from 0.5% to 4.5%, from 0.5% to 4%, from 0.5% to
3.5%, from 0.5%
to 3%, from 0.5% to 2.5%, from 0.5% to 2%, from 0.5% to 1.5%, or 2%.
[00214] In some embodiments, the concentration of the pharmaceutical
agent in the
pharmaceutical agent solution is from about 1 mg/mL to about 50 mg/mL, from
about 1 mg/mL to
about 40 mg/mL. from about 1 mg/mL to about 30 mg/mL, from about 2 mg/mL to
about 30 mg/mL,
from about 5 mg/mL to about 30 mg/mL, from about 5 mg/mL to about 25 mg/mL,
from about 5
mg/mL to about 20 mg/mL, from about 5 mg/mL to about 15 mg/mL, from about 7
mg/mL to about
12 mg/mL, or about 10 mg/mL. As used herein, the term "about" when used in
reference to the
polymer concentration expressed in mg/mL means a variation of 0.5 mg/mL, 1
mg/mL, 5 mg/mL, 10
mg/mL, or 15 mg/mL, depending on the embodiment. In some embodiments, the
concentration of the
pharmaceutical agent in the pharmaceutical agent solution is from 1 mg/mL to
50 mg/mL, between 1
mg/mL to 40 mg/mL. from 1 mg/mL to 30 mg/mL, from 2 mg/mL to 30 mg/mL, from 5
mg/mL to 30
mg/mL, from 5 mg/mL to 25 mg/mL, from 5 mg/mL to 20 mg/mL, from 5 mg/mL to 15
mg/mL, from
7 mg/mL to 12 mg/mL, or 10 mg/mL.
[00215] In some embodiments, the volume of the organic solvent removed by
evaporation is
at least 5%, at least 10%, at least 15%, at least 20%, at least 30%, at least
40%, at least 45%, at least
- 52 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
50%, at least, 55%, at least 60%, at least 65%, at least 70%, at least 75%, at
least 80%, at least 85%, at
least 90%, or at least 95%. In some embodiments, the volume of the organic
solvent removed by
evaporation is 5%-95%, at 5%-85%, 5%-50%, 5%-25%, 5%-10%, 10%-95%, 10%-50%,
10%-30%,
20%-95%, 30%-75%, 40%-95%, 50%-95%, 70%-90%, 75%-95%, or 80%-95%.
[00216] In some embodiment, a crystalline pharmaceutical agent can be
encapsulated by
emulsion-based encapsulation processes. In some embodiments, at least a
portion of the
pharmaceutical agent is encapsulated using a technique that comprises at least
the steps of (1)
combining a first polymer (e.g. PVA) and water to form a first polymer
solution, combining a second
polymer (e.g. PLGA) and an organic solvent (e.g. dichoromethane) to form a
second polymer
solution, and combining the second polymer and the organic solvent to form a
third polymer solution;
(2) mixing the first and second polymer solutions and allowing the organic
solvent to evaporate to
form an emulsion; (3) mixing the pharmaceutical agent to the third polymer
solution to form a
suspension; (4) combining the emulsion and suspension to form an emulsion-
based mixture; and (5)
filtering the emulsion-based mixture. In some embodiments, the filtration is
accomplished using a
centrifuge. In some embodiments the filtration is accomplished by pouring the
solution through filter
paper. In some embodiments, the encapsulated pharmaceutical agent is further
resuspended in water
and lyophilized.
[00217] In some embodiments, the polymer concentration of the first
polymer solutions is
from about 1% to about 10%, from about 1% to about 9%, from about 1% to about
8%, from about
1% to about 7%, from about 1% to about 6%, from about 1% to about 5%, from
about 1% to about
4%, from about 1% to about 3%, from about 1.5% to about 2.5%, or about 2%. As
used herein, the
term "about" when used in reference to the polymer concentration expressed as
a percentage means a
variation of 0.05%, 0.1%, 0.15%, 0.2%, 0.3%, 0.4%, or 0.5%. For example a
polymer concentration
that is about 2% may be expressed as 2%+/- 0.5% (i.e. 1.5%-2.5%), or may be
from 1.95-2.05%
(2%+/-0.05%), depending on the embodiment. In some embodiments, the polymer
concentration of
the first polymer solutions is from 1% to 10%, from 1% to 9%, from 1% to 8%,
from 1% to 7%, from
1% to 6%, from 1% to 5%, from 1% to 4%, from 1% to 3%, from 1.5% to 2.5%, or
2%.
[00218] In some embodiments, the polymer concentration of the second
polymer solution is
from about 10 mg/mL to about 100mg/mL, from about 15 mg/mL to about 95 mg/mL.
from about 20
mg/mL to about 90 mg/mL, from about 25 mg/mL to about 85 mg/mL, from about 30
mg/mL to
about 80 mg/mL, from about 35 mg/mL to about 75 mg/mL, from about 40 mg/mL to
about 70
mg/mL, from about 40 mg/mL to about 60 mg/mL, from about 45 mg/mL to about 55
mg/mL, or
about 50 mg/mL. As used herein, the term "about" when used in reference to the
polymer
concentration expressed in mg/mL means a variation of 0.5 mg/mL, 1 mg/mL, 5
mg/mL, 10 mg/mL,
or 15 mg/mL, depending on the embodiment. In some embodiments, the polymer
concentration of
the second polymer solution is from 10 mg/mL to 100mg/mL, from 15 mg/mL to 95
mg/mL. from 20
- 53 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
mg/mL to 90 mg/mL, from 25 mg/mL to 85 mg/mL, from 30 mg/mL to 80 mg/mL, from
35 mg/mL
to 75 mg/mL, from 40 mg/mL to 70 mg/mL, from 40 mg/mL to 60 mg/mL, from 45
mg/mL to 55
mg/mL, or 50 mg/mL. .
[00219] In some embodiments, the polymer concentration of the third
polymer solutions is
from about 0.5% to about 5%, from about 0.5% to about 4.5%, from about 0.5% to
about 4%, from
about 0.5% to about 3.5%, from about 0.5% to about 3%, from about 0.5% to
about 2.5%, from about
0.5% to about 2%, from about 0.5% to about 1.5%, or about 2%. As used herein,
the term "about"
when used in reference to the polymer concentration expressed as a percentage
means a variation of
0.05%, 0.1%, 0.15%, 0.2%, 0.3%, 0.4%, or 0.5%. For example a polymer
concentration that is about
2% may be expressed as 2%+/- 0.5% (i.e. 1.5%-2.5%), or may be from 1.95-2.05%
(2%+/-0.05%),
depending on the embodiment. In some embodiments, the polymer concentration of
the third polymer
solutions is from 0.5% to 5%, from 0.5% to 4.5%, from 0.5% to 4%, from 0.5% to
3.5%, from 0.5%
to 3%, from 0.5% to 2.5%, from 0.5% to 2%, from 0.5% to 1.5%, or 2%.
[00220] In some embodiments, the concentration of the pharmaceutical
agent in the
pharmaceutical agent- third polymer solution mixture is from about 0.01 mg/mL
to about 0.1 mg/mL,
from about 0.02 mg/mL to about 0.09 mg/mL. from about 0.03 mg/mL to about 0.08
mg/mL, from
about 0.03 mg/mL to about 0.07 mg/mL, from about 0.04 mg/mL to about 0.07
mg/mL, from about
0.04 mg/mL to about 0.06 mg/mL, or about 0.05 mg/mL. As used herein, the term
"about" when used
in reference to the pharmaceutical agent concentration expressed in mg/mL
means a variation of
0.001 mg/mL, 0.005 mg/mL, 5 mg/mL, 0.002 mg/mL, 0.0025 mg/mL, or 0.1 mg/mL,
depending on
the embodiment. In some embodiments, the concentration of the pharmaceutical
agent in the
pharmaceutical agent- third polymer solution mixture is from 0.01 mg/mL to 0.1
mg/mL, from 0.02
mg/mL to 0.09 mg/m, from about 0.03 mg/mL to 0.08 mg/mL, from about 0.03 mg/mL
to 0.07
mg/mL, from about 0.04 mg/mL to about 0.07 mg/mL, from about 0.04 mg/mL to
about 0.06 mg/mL,
or about 0.05 mg/mL.
[00221] In some embodiments, the volume of the organic solvent removed
by evaporation
during the encapsulation process is at least 5%, at least 10%, at least 15%,
at least 20%, at least 30%,
at least 40%, at least 45%, at least 50%, at least, 55%, at least 60%, at
least 65%, at least 70%, at least
75%, at least 80%, at least 85%, at least 90%, and at least 95%. In some
embodiments, the volume of
the organic solvent removed by evaporation during the encapsulation process is
5%-95%, at 5%-85%,
5%-50%, 5%-25%, 5%-10%, 10%-95%, 10%-50%, 10%-30%, 20%-95%, 30%-75%, 40%-95%,
50%-95%, 70%-90%, 75%-95%, or 80%-95%.
[00222] In some embodiment, the polymer-encapsulated pharmaceutical
agent is in the form
of microspheres or microparticles. Examples of microspheres relevant to the
present disclosure
include: Luzzi, L. A., J. Pharm. Psy. 59:1367 (1970); U.S. Pat. No. 4,530,840;
Lewis, D. H.,
"Controlled Release of Bioactive Agents from Lactides/Glycolide Polymers" in
Biodegradable
- 54 -
CA 02888776 2016-09-26
Polymers as Drug Delivery Systems, Chasin, M. and Langer, R., eds., Marcel
Decker (1990); U.S.
Pat. No. 4,675,1 89; Beck et al., "Poly(lactic acid) and Poly(lactic acid-co-
glycolic acid) Contraceptive
Delivery Systems," in Long Acting Steroid Contraception, Mishell, D. R., ed.,
Raven Press (1983);
U.S. Pat. No. 4,758,435; U.S. Pat. No. 3,773,919; U.S. Pat. No. 4,474,572.
Examples of protein
therapeutics formulated as microspheres include: U.S. Pat. No. 6,458,387; U.S.
Pat. No. 6,268,053;
U.S. Pat. No. 6,090,925; U.S. Pat. No. 5,981,719; and U.S. Pat. No. 5,578,709.
[00223] Microspheres usually have a spherical shape, although
irregularly-shaped
microparticles are possible. Microspheres may vary in size, ranging from
submicron to 1000 micron
diameters. Microspheres suitable for use with the medical device coating
disclosed herein are
submicron to 250 micron diameter microspheres. The microspheres are prepared
by any method
which produces microspheres in a size range acceptable for use in the coating
method disclosed
herein.
[00224] Suitable examples of polymeric materials for use in the
microsphere or microparticles
herein include poly(glycolic acid), poly-d,1-lactic acid, poly-1-lactic acid,
copolymers of the foregoing,
poly(aliphatic carboxylic acids), copolyoxalates, polycaprolactone,
polydioxonene,
poly(orthocarbonates), poly(acetals), poly(lactic acid-caprolactone),
polyorthoesters, poly(glycolic
acid-caprolactone), polydioxoncnc, polyanhyd rides, polyphosphazines, and
natural polymers
including albumin, casein, and some waxes, such as, glycerol mono- and
distearate, and the like.
Various commercially available poly (lactide-co-glycolide) materials (PLGA)
are optionally used in
the method disclosed herein. For example, poly (d,l-lactic-co-glycolic acid)
is commercially available
from Boehringer-Ingelheim as RESOMER RG 503 H. This product has a mole percent
composition
of 50% lactide and 50% glycolide. These copolymers are available in a wide
range of molecular
weights and ratios of lactic acid to glycolic acid. One embodiment includes
the use of the polymer
poly(d,l-lactide-co-glycol ide). The molar ratio of lactide to glycolide in
such a copolymer includes the
range of from about 95:5 to about 50:50.
[00225] The molecular weight of the polymeric material is of some
importance. In some
embodiment, the molecular weight is high enough so that it forms satisfactory
polymer coatings, i.e.,
the polymer should be a good film former. Usually, a satisfactory molecular
weight is in the range of
5,000 to 500,000 daltons. The molecular weight of a polymer is also important
from the point of view
that molecular weight influences the biodegradation rate of the polymer. The
pharmaceutical agent is
released from the microparticles through di ffusional through the polymer
material, through
biodegradation of the polymer material, or through a combination of both. By
an appropriate
selection of polymeric materials a microsphere formulation is made such that
the resulting
microspheres exhibit both diffusional release and biodegradation release
properties. This is useful in
affording multiphasic release patterns.
- 55 -
CA 02888776 2016-09-26
[00226] A variety of methods are known by which compounds are
encapsulated in
microspheres. In these methods, the pharmaceutical agent is generally
dispersed or emulsified, using
stirrers, agitators, or other dynamic mixing techniques, in a solvent
containing a wall-forming
material. Solvent is then removed from the microspheres, and thereafter the
microsphere product is
.. obtained.
[00227] In one embodiment, the polymer-encapsulated pharmaceutical
agent used herein are
made through the incorporation of the pharmaceutical agents and/or other
pharmaceutical agents into
poly (lactic-glycolic acid) - polyvinyl alcohol microspheres. In another
embodiment, the auris sensory
cell modulating agents are encapsulated into alginate microspheres. (See U.S.
Patent No. 6,036,978,
incorporated herein for such disclosure). Biocornpatible methacrylate-based
polymers to encapsulate
the pharmaceutical agent are optionally used in the formulations and methods
disclosed herein. A
wide range of methacrylate-bascd polymer systems are commercially available,
such as the
EUDRAGIT polymers marketed by Evonik. One useful aspect of methacrylate
polymers is that the
properties of the formulation are varied by incorporating various co-polymers.
For example,
poly(acrylic acid-co-methylmethacrylate) microparticles exhibit enhanced
mucoadhesion properties as
the carboxylic acid groups in the poly(acrylic acid) form hydrogen bonds with
mucin (Park etal,
Pharm. Res. (1987) 4(6):457-464). Variation of the ratio between acrylic acid
and methylmethacrylate
monomers serves to modulate the properties of the co-polymer. Methacrylate-
based microparticles
have also been used in protein therapeutic formulations (Naha et al, Journal
of Microencapsulation 04
February, 2008 (online publication)).
[00228] An example of a conventional microencapsulation process for
pharmaceutical
preparations is shown in U.S. Pat. No. 3,737,337.
The pharmaceutical agent to be encapsulated or embedded are dissolved or
dispersed in
the organic solution of the polymer (phase A), using conventional mixers,
including (in the
preparation of dispersion) vibrators and high-speed stirrers, etc. The
dispersion of phase (A),
containing the core material in solution or in suspension, is carried out in
the aqueous phase (B), again
using conventional mixers, such as high-speed mixers, vibration mixers, or
even spray nozzles, in
which case the particle size of the microspheres will be determined not only
by the concentration of
phase (A), but also by the emulsate or microsphere size. With conventional
techniques for the
microencapsulation of auris sensory cell modulating agents, the microspheres
form when the solvent
containing an active agent and a polymer is emulsified or dispersed in an
immiscible solution by
stirring, agitating, vibrating, or some other dynamic mixing technique.
[00229] Methods for the construction of microspheres are also described
in U.S. Pat. No.
4,389,330, and U.S. Pat. No. 4,530,840. The
pharmaceutical agent is dissolved or dispersed in an appropriate solvent. To
the agent-containing
medium is added the polymeric matrix material in an amount relative to the
active ingredient which
- 56 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
gives a product of the desired loading of active agent. Optionally, all of the
ingredients of the auris
sensory cell modulating agent microsphere product can be blended in the
solvent medium together.
Suitable solvents for the agent and the polymeric matrix material include
organic solvents such as
acetone, halogenated hydrocarbons such as chloroform, methylene chloride and
the like, aromatic
hydrocarbon compounds, halogenated aromatic hydrocarbon compounds, cyclic
ethers, alcohols, ethyl
acetate and the like.
[00230] The mixture of ingredients in the solvent is emulsified in a
continuous-phase
processing medium; the continuous-phase medium being such that a dispersion of
microdroplets
containing the indicated ingredients is formed in the continuous-phase medium.
Naturally, the
continuous-phase processing medium and the organic solvent must be immiscible,
and includes water
although nonaqueous media such as xylene and toluene and synthetic oils and
natural oils are
optionally used. Optionally, a surfactant is added to the continuous-phase
processing medium to
prevent the microparticles from agglomerating and to control the size of the
solvent microdroplets in
the emulsion. A preferred surfactant-dispersing medium combination is a 1 to
10 wt. % poly (vinyl
alcohol) in water mixture. The dispersion is formed by mechanical agitation of
the mixed materials.
An emulsion is optionally formed by adding small drops of the active agent-
wall forming material
solution to the continuous phase processing medium. The temperature during the
formation of the
emulsion is not especially critical but influences the size and quality of the
microspheres and the
solubility of the drug in the continuous phase. It is desirable to have as
little of the agent in the
continuous phase as possible. Moreover, depending on the solvent and
continuous-phase processing
medium employed, the temperature must not be too low or the solvent and
processing medium will
solidify or the processing medium will become too viscous for practical
purposes, or too high that the
processing medium will evaporate, or that the liquid processing medium will
not be maintained.
Moreover, the temperature of the medium cannot be so high that the stability
of the particular agent
being incorporated in the microspheres is adversely affected. Accordingly, the
dispersion process is
conducted at any temperature which maintains stable operating conditions,
which preferred
temperature being about 15 C to 60 C, depending upon the drug and excipient
selected.
[00231] The dispersion which is formed is a stable emulsion and from
this dispersion the
organic solvent immiscible fluid is optionally partially removed in the first
step of the solvent removal
process. The solvent is removed by techniques such as through evaporation,
heating, the application
of a reduced pressure or a combination of both. The temperature employed to
evaporate solvent from
the microdroplets generally should not degrade the pharmaceutial agent
employed in the preparation
of a given microparticle, nor should it be so high as to evaporate solvent at
such a rapid rate to cause
defects in the wall forming material. Generally, from 5 to 95%, of the solvent
is removed in the first
solvent removal step.
- 57 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00232] After the first stage, the dispersed microparticles in the
solvent immiscible fluid
medium are isolated from the fluid medium by any convenient means of
separation. Thus, for
example, the fluid is decanted from the microsphere or the microsphere
suspension is filtered. Still
other, various combinations of separation techniques are used if desired.
[00233] Following the isolation of the microspheres from the continuous-
phase processing
medium, the remainder of the solvent in the microspheres is removed by
optional extraction. In this
step, the microspheres are suspended in the same continuous-phase processing
medium used in step
one, with or without surfactant, or in another liquid. The extraction medium
removes the solvent from
the microspheres and yet does not dissolve the microspheres. During the
extraction, the extraction
medium with dissolved solvent is optionally removed and replaced with fresh
extraction medium.
This is best done on a continual basis. The rate of extraction medium
replenishment of a given process
is a variable which is determined at the time the process is performed and,
therefore, no precise limits
for the rate must be predetermined. After the majority of the solvent has been
removed from the
microspheres, the microspheres are dried by exposure to air or by other
conventional drying
techniques such as vacuum drying, drying over a desiccant, or the like. This
process is very efficient
in encapsulating the auris sensory cell modulating agent since core loadings
of up to 80 wt. %,
preferably up to 60 wt. % are obtained.
Methods of Manufacturing Generally
[00234] In some embodiments, a coating is formed on the substrate by a
process comprising
depositing a polymer and/or the active agent by an e-RESS, an e-SEDS, or an e-
DPC process. In some
embodiments, the process of forming the coating provides improved adherence of
the coating to the
substrate prior to deployment of the device at the intervention site and
facilitates dissociation of the
coating from the substrate at the intervention site. In some embodiments, the
coating is formed on the
substrate by a process comprising depositing the active agent by an e-RESS, an
e-SEDS, or an e-DPC
process without electrically charging the substrate. In some embodiments, the
coating is formed on
the substrate by a process comprising depositing the active agent on the
substrate by an e-RESS, an e-
SEDS, or an e-DPC process without creating an electrical potential between the
substrate and a
coating apparatus used to deposit the active agent.
[00235] Means for creating the bioabsorbable polymer(s) + drug (s)
coating of the device with
or without a substrate:
= Spray coat the coating-form with drug and polymer as is done in Micell
process (e-RESS,
e-DPC, compressed-gas sintering).
= Perform multiple and sequential coating¨sintering steps where different
materials may be
deposited in each step, thus creating a laminated structure with a multitude
of thin layers
of drug(s), polymer(s) or drug+polymer that build the final device.
= Perform the deposition of polymer(s) + drug(s) laminates with the
inclusion of a mask on
the inner (luminal) surface of the device. Such a mask could be as simple as a
non-
conductive mandrel inserted through the internal diameter of the coating form.
This
- 58 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
masking could take place prior to any layers being added, or be purposefully
inserted
after several layers are deposited continuously around the entire coating-
form.
[00236] In some embodiments, the coating comprises a microstructure.
In some
embodiments, particles of the active agent are sequestered or encapsulated
within the microstructure.
In some embodiments, the microstructure comprises microchannels, micropores
and/or microcavities.
In some embodiments, the microstructure is selected to allow sustained release
of the active agent. In
some embodiments, the microstructure is selected to allow controlled release
of the active agent.
[00237] Other methods for preparing the coating include solvent based
coating methods and
plasma based coating methods. In some embodiments, the coating is prepared by
a solvent based
coating method. In some embodiments, the coating is prepared by a solvent
plasma based coating
method.
[00238] Another advantage of the present invention is the ability to
create a delivery device
with a controlled (dialed-in) drug-elution profile. Via the ability to have
different materials in each
layer of the laminate structure and the ability to control the location of
drug(s) independently in these
layers, the method enables a device that could release drugs at very specific
elution profiles,
programmed sequential and/or parallel elution profiles. Also, the present
invention allows controlled
elution of one drug without affecting the elution of a second drug (or
different doses of the same
drug).
[00239] Provided herein is a method of forming a medical device
comprising a substrate and a
coating on at least a portion of the substrate, wherein the coating comprises
an active agent, the
method comprising: providing the substrate; and forming the coating on at
least a portion of the
substrate by depositing the active agent by on the substrate by at least one
of an e-RESS, an e-SEDS,
and an e-DPC process, wherein forming the coating results in at least a
portion of the coating being
adapted to transfer from the substrate to an intervention site upon
stimulating the coating with a
stimulation.
[00240] Provided herein is a method of forming a medical device
comprising a substrate and a
coating on at least a portion of the substrate, wherein the coating comprises
an active agent, the
method comprising: providing the substrate; and forming the coating on at
least a portion of the
substrate by depositing the active agent by on the substrate by at least one
of an e-RESS, an e-SEDS,
and an e-DPC process without electrically charging the substrate, wherein
forming the coating results
in at least a portion of the coating being adapted to transfer from the
substrate to an intervention site
upon stimulating the coating with a stimulation.
[00241] Provided herein is a method of forming a medical device
comprising a substrate and a
coating on at least a portion of the substrate, wherein the coating comprises
an active agent, the
method comprising: providing the substrate; and forming the coating on at
least a portion of the
substrate by depositing the active agent by on the substrate by at least one
of an e-RESS, an e-SEDS,
- 59 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
and an e-DPC process without creating an electrical potential between the
substrate and a coating
apparatus used in the at least one e-RESS, an e-SEDS, and an e-DPC process,
wherein forming the
coating results in at least a portion of the coating being adapted to transfer
from the substrate to an
intervention site upon stimulating the coating with a stimulation.
[00242] Provided herein is a method of forming a medical device comprising
a substrate and a
coating on at least a portion of the substrate, wherein the coating comprises
an active agent, the
method comprising: providing the substrate; and forming the coating on at
least a portion of the
substrate by depositing the active agent by on the substrate by at least one
of a dipping and/or a
spraying process, wherein forming the coating results in at least a portion of
the coating being adapted
to transfer from the substrate to an intervention site upon stimulating the
coating with a stimulation.
[00243] Provided herein is a method of forming a medical device
comprising a substrate and a
coating on at least a portion of the substrate, wherein the coating comprises
an active agent, the
method comprising: providing the substrate; and forming the coating on at
least a portion of the
substrate by depositing the active agent by on the substrate by at least one
of an e-RESS, an e-SEDS,
and an e-DPC process, wherein forming the coating results in at least a
portion of the coating being
adapted to free from the substrate upon stimulating the coating with a
stimulation.
[00244] Provided herein is a method of forming a medical device
comprising a substrate and a
coating on at least a portion of the substrate, wherein the coating comprises
an active agent, the
method comprising: providing the substrate; and forming the coating on at
least a portion of the
substrate by depositing the active agent by on the substrate by at least one
of a dipping and/or a
spraying process, wherein forming the coating results in at least a portion of
the coating being adapted
to free from the substrate upon stimulating the coating with a stimulation.
[00245] Provided herein is a method of forming a medical device
comprising a substrate and a
coating on at least a portion of the substrate, wherein the coating comprises
an active agent, the
method comprising: providing the substrate; and forming the coating on at
least a portion of the
substrate by depositing the active agent by on the substrate by at least one
of an e-RESS, an e-SEDS,
and an e-DPC process, wherein forming the coating results in at least a
portion of the coating being
adapted to dissociate from the substrate upon stimulating the coating with a
stimulation.
[00246] Provided herein is a method of forming a medical device
comprising a substrate and a
coating on at least a portion of the substrate, wherein the coating comprises
an active agent, the
method comprising: providing the substrate; and forming the coating on at
least a portion of the
substrate by depositing the active agent by on the substrate by at least one
of a dipping and/or a
spraying process, wherein forming the coating results in at least a portion of
the coating being adapted
to dissociate from the substrate upon stimulating the coating with a
stimulation.
[00247] Provided herein is a method of forming a medical device comprising
a substrate and a
coating on at least a portion of the substrate, wherein the coating comprises
an active agent, the
- 60 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
method comprising: providing the substrate; and forming the coating on at
least a portion of the
substrate by depositing the active agent by on the substrate by at least one
of an e-RESS, an e-SEDS,
and an e-DPC process, wherein forming the coating results in at least a
portion of the coating being
adapted to deliver to the intervention site upon stimulating the coating with
a stimulation.
[00248] Provided herein is a method of forming a medical device comprising
a substrate and a
coating on at least a portion of the substrate, wherein the coating comprises
an active agent, the
method comprising: providing the substrate; and forming the coating on at
least a portion of the
substrate by depositing the active agent by on the substrate by at least one
of a dipping and/or a
spraying process, wherein forming the coating results in at least a portion of
the coating being adapted
.. to deliver to the intervention site upon stimulating the coating with a
stimulation.
[00249] In some embodiments, the e-RESS, the e-SEDS, and/or the e-DPC
process used in
forming the coating is performed without electrically charging the substrate.
In some embodiments,
the e-RESS, the e-SEDS, and/or the e-DPC process used in forming the coating
is performed without
creating an electrical potential between the substrate and the coating
apparatus used in the e-RESS,
the e-SEDS, and/or the e-DPC process.
[00250] In some embodiments, forming the coating results in the
coating adhering to the
substrate prior to the substrate reaching the intervention site.
[00251] Some embodiments further comprise providing a release agent on
the substrate. In
some embodiments, providing the release agent step is performed prior to the
forming the coating
step. In some embodiments, the release agent comprises at least one of: a
biocompatible release
agent, a non-biocompatible release agent, a powder, a lubricant, a surface
modification of the
substrate, a viscous fluid, a gel, the active agent, a second active agent, a
physical characteristic of the
substrate. In some embodiments, the physical characteristic of the substrate
comprises at least one
of: a patterned coating surface of the substrate, and a ribbed surface of the
substrate. In some
embodiments, the release agent comprises a property that is capable of
changing at the intervention
site. In some embodiments, the property comprises a physical property. In some
embodiments, the
property comprises a chemical property. In some embodiments, the release agent
is capable of
changing a property when in contact with at least one of a biologic tissue and
a biologic fluid. In
some embodiments, the release agent is capable of changing a property when in
contact with an
aqueous liquid. In some embodiments, the coating results in a coating property
that facilitates transfer
of the coating to the intervention site. In some embodiments, the coating
property comprises a
physical characteristic of the coating. In some embodiments, the physical
characteristic comprises a
pattern.
[00252] In some embodiments, forming the coating facilitates transfer
of the coating to the
intervention site.
- 61 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00253] In some embodiments, transferring, freeing, dissociating,
depositing, and/or tacking
step comprises softening the polymer by hydration, degradation or by a
combination of hydration and
degradation. In some embodiments, the transferring, freeing, dissociating,
depositing, and/or tacking
step comprises softening the polymer by hydrolysis of the polymer.
[00254] In some embodiments, the providing step comprises forming the
coating by a solvent
based coating method. In some embodiments, the providing step comprises
forming the coating by a
solvent plasma based method.
[00255] In some embodiments, providing the device comprises depositing
a plurality of layers
on the substrate to form the coating, wherein at least one of the layers
comprises the active agent. In
some embodiments, at least one of the layers comprises a polymer. In some
embodiments, the
polymer is bioabsorbable. In some embodiments, the active agent and the
polymer are in the same
layer, in separate layers, or form overlapping layers. In some embodiments,
the plurality of layers
comprise five layers deposited as follows: a first polymer layer, a first
active agent layer, a second
polymer layer, a second active agent layer and a third polymer layer.
EXAMPLES
[00256] The following examples are provided to illustrate selected
embodiments. They
should not be considered as limiting the scope of the invention, but merely as
being illustrative and
representative thereof For each example listed herein, multiple analytical
techniques may be
provided. Any single technique of the multiple techniques listed may be
sufficient to indicate the
parameter and/or characteristic being tested, or any combination of techniques
may be used to indicate
such parameter and/or characteristic. Those skilled in the art will be
familiar with a wide range of
analytical techniques for the characterization of drug/polymer coatings.
Techniques presented here,
but not limited to, may be used to additionally and/or alternatively
characterize specific properties of
the coatings with variations and adjustments employed which would be obvious
to those skilled in the
art.
Sample Preparation
[00257] Generally speaking, coatings on stents, on balloons, on
coupons, on other substrates,
or on samples prepared for in-vivo models are prepared as herein.
Nevertheless, modifications for a
given analytical method are presented within the examples described, and/or
would be obvious to one
having skill in the art. Thus, numerous variations, changes, and substitutions
will now occur to those
skilled in the art without departing from the invention. It should be
understood that various
alternatives to the embodiments of the invention described herein and examples
provided may be
employed in practicing the invention and indicating the parameters and/or
characteristics described.
Coatings on Balloons
- 62 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00258] Coated balloons as described herein and/or made by a method
disclosed herein are
prepared. In some examples, the coated balloons have a targeted coating
thickness of ¨ 15 microns (-
microns of active agent). In some examples, the coating process is PDPDP
(Polymer, sinter, Drug,
Polymer, sinter, Drug, Polymer, sinter) using deposition of drug in dry powder
form and deposition of
5 polymer particles by RESS methods and equipment described herein. In the
illustrations herein,
resulting coated balloons may have a 3-layer coating comprising polymer (for
example, PLGA) in the
first layer, drug (for example, rapamycin) in a second layer and polymer in
the third layer, where a
portion of the third layer is substantially drug free (e.g. a sub-layer within
the third layer having a
thickness equal to a fraction of the thickness of the third layer). As
described layer, the middle layer
(or drug layer) may be overlapping with one or both first (polymer) and third
(polymer) layer. The
overlap between the drug layer and the polymer layers is defined by extension
of polymer material
into physical space largely occupied by the drug. The overlap between the drug
and polymer layers
may relate to partial packing of the drug particles during the formation of
the drug layer. When
crystal drug particles are deposited on top of the first polymer layer, voids
and or gaps may remain
between dry crystal particles. The voids and gaps are available to be occupied
by particles deposited
during the formation of the third (polymer) layer. Some of the particles from
the third (polymer) layer
may rest in the vicinity of drug particles in the second (drug) layer. When
the sintering step is
completed for the third (polymer) layer, the third polymer layer particles
fuse to form a continuous
film that forms the third (polymer) layer. In some embodiments, the third
(polymer) layer however
will have a portion along the longitudinal axis of the stent whereby the
portion is free of contacts
between polymer material and drug particles. The portion of the third layer
that is substantially of
contact with drug particles can be as thin as 1 nanometer.
[00259] Polymer-coated balloons having coatings comprising polymer but
no drug are made
by a method disclosed herein and are prepared having a targeted coating
thickness of, for example,
about 0.5, 1, 2, 3, 4, 5, 10, 15, 20, 25, 30, 35, 40, 45, or 50 microns,
depending in part on whether the
coating expands upon hydration and if so whether it is hydrated. In
embodiments, the coating
thickness is 1-5 microns. In other embodiments, it is 1-10 microns. .
[00260] An example coating process is PPP (PLGA, sinter, PLGA, sinter,
PLGA, sinter) using
RESS methods and equipment described herein. These polymer-coated balloons may
be used as
.. control samples in some of the examples, infra.
[00261] In some examples, the balloons are made of a compliant
polymer. In some examples,
the balloons are made of a non-compliant polymer. The balloons may be, in some
examples, 5 to 50
mm in length, preferably 10-20 mm in length.
[00262] Balloons can be coated while inflated, and later compacted, or
they can be coated
while uninflated. If a balloon is coated while inflated and later folded or
otherwise compacted, then a
portion of the coating can be protected during insertion by virtue of being
disposed within the portion
- 63 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
of the balloon that is not exposed until inflation. The coating can also be
protected by using a sheath
or other covering, as described in the art for facilitating insertion of an
angioplasty balloon.
[00263] The coating released from a balloon may be analyzed (for
example, for analysis of a
coating band and/or coating a portion of the balloon). Alternatively, in some
examples, the coating is
.. analyzed directly on the balloon. This coating, and/or coating and balloon,
may be sliced into sections
which may be turned 90 degrees and visualized using the surface composition
techniques presented
herein or other techniques known in the art for surface composition analysis
(or other characteristics,
such as crystallinity, for example). In this way, what could be an analysis of
coating composition
through a depth when the coating is on the balloon or as removed from the
balloon (i.e. a depth from
the abluminal surface of the coating to the surface of the removed coating
that once contacted the
balloon or a portion thereof), becomes a surface analysis of the coating which
can, for example,
indicate the layers in the slice of coating, at much higher resolution.
Residual coating on an extracted
balloon also can be analyzed and compared to the amount of coating on an
unused balloon, using,
e.g., HPLC, as noted herein. Coating removed from the balloon, or analyzed
without removal and/or
.. release from the balloon, may be treated the same way, and assayed,
visualized, and/or characterized
as presented herein using the techniques described and/or other techniques
known to a person of skill
in the art.
Sample Preparation for In-Vivo Models
[00264] Devices comprising balloons having coatings disclosed herein
are deployed in the
porcine coronary arteries of pigs (domestic swine, juvenile farm pigs, or
Yucatan miniature swine).
Porcine coronary angioplasty is exploited herein since such model yields
results that are comparable
to other investigations assaying neointimal hyperplasia in human subjects. The
balloons are expanded
to a 1:1.1 balloon: artery ratio. At multiple time points, animals are
euthanized (e.g. t = 1 day, 7 days,
14 days, 21 days, and 28 days), the tissue surrounding the intervention site
is extracted, and assayed.
[00265] Devices comprising balloons having coatings disclosed herein
alternatively are
implanted in the common iliac arteries of New Zealand white rabbits. The
balloons are expanded to a
1:1.1 balloon: artery ratio. At multiple time points, animals are euthanized
(e.g., t = 1 day, 7 days, 14
days, 21 days, and 28 days), the tissue surrounding the intervention site is
extracted, and assayed.
.. EXAMPLE 1: CUTTING BALLOONS
CUTTING BALLOON (1)- Mechanical stimulation to free the coating
[00266] A cutting balloon is coated comprising a polymer and an active
agent. The coated
cutting balloon is positioned at the intervention site. The balloon is
inflated to at least 25% below its
nominal inflation pressure. Upon deflation and removal of the cutting balloon
from the intervention
site, at least about 5% to at least about 30% of the coating is freed from the
surface of the cutting
balloon and is deposited at the intervention site.
- 64 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00267] In some examples, the balloon unfolds during inflation,
causing mechanical shearing
forces to at least augment transfer and/or freeing and/or deposition of the
coating from the balloon to
the intervention site.
[00268] In some examples, the balloon twists during inflation, causing
mechanical shearing
forces to at least augment transfer and/or freeing and/or deposition of the
coating from the balloon.
[00269] In one example, the polymer of the coating is about 50:50 PLGA-
Ester End Group,
MW-191(D, degradation rate ¨1-2 months or about 50:50 PLGA-Carboxylate End
Group,
MW-101(D, degradation rate ¨28 days. The active agent is a pharmaceutical
agent such as a
macrolide immunosuppressive drug. Equipment and coating process similar to
Example 1 is
employed. The intervention site is a vascular lumen wall. Upon inflation of
the cutting balloon, at
least about 50% of the coating is freed from the device at the intervention
site.
[00270] In another example, a cutting balloon is coated with a
formulation of PLGA +
sirolimus with total loading of sirolimus ¨20 [tg with the coating
preferentially on the wire of the
cutting balloon. Equipment and process similar to Example 1 is employed. The
intervention site is a
coronary artery. Upon inflation of the cutting balloon, about 5 % to about 15
% of the coating is freed
from the device resulting in delivery of ¨2.0 [tg of drug delivered to the
artery.
[00271] In another example, the polymer of the coating is about 50:50
PLGA-Ester End
Group, MW-191(D, degradation rate ¨1-2 months or about 50:50 PLGA-Carboxylate
End Group,
MW-101(D, degradation rate ¨28 days. The active agent is a chemotherapeutic
agent. Equipment and
coating process similar to Example 1 is employed. The intervention site is a
cavity resulting from
removal of a tumor. Upon inflation of the cutting balloon, at least about 75%
of the coating is
transferred from the device to the intervention site.
[00272] In-vivo testing: A group of 27 New Zealand white rabbits is
prepared for a Seldinger
procedure using a cutting balloon coated with a formulation of about 50:50
PLGA-Ester End Group
(MW-191(D, degradation rate ¨1-2 months) and sirolimus with total loading of
sirolimus ¨20 [tg with
the coating preferentially on the wire of the cutting balloon. The device is
placed at a coronary artery
intervention site with the assistance of fluoroscopy to aid in positioning the
device at the same
location in each subject. Six animals are subjected to the procedure using a
coated balloon that does
not have sirolimus in the coating. After deployment and removal of the device,
3 control animals are
sacrificed at 1 hour post deployment and serum and tissue samples are
collected. The 3 remaining
control animals are sacrificed at 56 days post deployment. During the course
of the study, serum
samples are collected from control and drug-treated animals every five days.
The drug treated
animals, 3 each, are sacrificed at 1 hour, 24 hours, 7 days, 14 days, 28 days,
42 days and 56 days post
deployment. A serum sample as well as a tissue sample from the deployment site
is collected.
[00273] The tissue and serum samples may be subjected to analysis for
sirolimus
concentration. In order to determine the amount of coating freed from the
device and/or delivered to
- 65 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
the intervention site as a percent of the total amount of coating on the
substrate, the tissue
concentration of sirolimus at the one hour time point (or any time point
within the first day following
of the procedure) may be used along with the total content expected for the
coating (based on the total
content for the manufacturing lot) or along with the content of coating
remaining on the device once
removed and the percentage calculated. This percentage is correlative of the
percent of coating freed,
dissociated, and/or transferred from the device and delivered to the
intervention site. Alternatively,
the tissue may be analyzed by various means (noted herein, including but not
limited to SEM, TEM,
and, where image enhanced polymers are used, various imaging means capable of
detecting these
enhanced polymers) to detect the percent of the coating freed, dissociated
and/or transferred from the
substrate and delivered to the intervention site. Again, the amount of coating
known to be on the
substrate based on manufacturing lot characteristics, and/or an assessment of
the coating remaining on
the device following removal of the device from the subject (for example,
wherein the device is an
angioplasty catheter and the substrate is the balloon of the catheter) may be
used to determine the
percent of coating freed, dissociated, and/or transferred from the device. In
some instances, an
assessment of the device following the procedure alone is sufficient to assess
the amount freed or
dissociated from the substrate, without determination of the amount delivered
to the intervention site.
Additionally, where a determination of improvement and/or disease treatment is
desired, levels of
proinflammatory markers could be tested to indicate improvement and/or
treatment of a disease
and/or ailment, for example, by testing high sensitive C-reactive protein
(hsCRP), interleukin-6 (IL-
6), interleukin-lp (IL-1I3), and/or monocyte chemoattractant protein-1 (MCP-
1). The release kinetics
of the drug may be indicated by plotting the sirolimus concentrations at the
timepoints noted above.
[00274] For embodiments using different drugs other than sirolimus,
the biomarkers are
selected based on the disease to be treated and the drugs administered during
the course of therapy as
determined by one of skill in the art. These biomarkers may be used to
indicate the treatment results
for each subject.
[00275] Other in-vivo tests described herein may be used instead of
this test and/or in addition
to this test, adjusted for the particularities of this device, as would be
known to one of ordinary skill in
the art.
[00276] In-vitro testing: One sample of the coated cutting balloon
prepared in Example 1 is
secured to a balloon catheter. A segment of optically clear TYGONO B-44-3
tubing with O.D. =
0.125", I.D. = 0.0625" (Available from McMaster-Carr Part Number: 5114K11
(www.mcmaster.com)) is filled with phosphate-buffered saline solution and
immersed in a water bath
at 37 C to mimic physiological conditions of deployment into a subject. The
coated balloon is
inserted into the tubing and the balloon is inflated to at least 25% below the
balloon's nominal
pressure to mechanically transfer the coating from the balloon to the tubing
wall. The balloon is
deflated and removed from the tubing. Optical microscopy is performed on the
tubing and/or the
- 66 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
balloon (which is inflated to at least 25% below the balloon's nominal
pressure, at least) to determine
the presence and amount of coating transferred to the tubing and/or the amount
of coating freed,
dissociated, and/or transferred from the balloon. Other in-vitro tests
described herein may be used
instead of this test and/or in addition to this test, adjusted for the
particularities of this device, as
would be known to one of ordinary skill in the art.
CUTTING BALLOON (2)- Mechanical stimulation to free the coating
[00277] A cutting balloon is coated using a solution-based system
(spray or dip coating)
comprising a polymer and an active agent. The coated cutting balloon is
positioned at the intervention
site. The balloon is inflated to at least 25% below its nominal inflation
pressure. At least about 5% to
at least about 30% of the coating is freed from the surface of the cutting
balloon and is deposited at
the intervention site.
[00278] In some examples, the balloon unfolds during inflation,
causing mechanical shearing
forces to at least augment transfer and/or freeing and/or deposition of the
coating from the balloon to
the intervention site.
[00279] In some examples, the balloon twists during inflation, causing
mechanical shearing
forces to at least augment transfer and/or freeing and/or deposition of the
coating from the balloon.
[00280] In one example, the polymer of the coating is about 50:50 PLGA-
Ester End Group,
MW-191(1), degradation rate ¨1-2 months or about 50:50 PLGA-Carboxylate End
Group,
MW-101(1), degradation rate ¨28 days. The active agent is a pharmaceutical
agent such as a
macrolide immunosuppressive drug. Equipment and coating process using a spray
and/or dip coating
process is employed. The intervention site is a vascular lumen wall. Upon
inflation of the cutting
balloon, at least about 50% of the coating is freed from the device at the
intervention site.
[00281] In another example, a cutting balloon is coated with a
formulation of PLGA +
sirolimus with total loading of sirolimus ¨20 [tg with the coating
preferentially on the wire of the
cutting balloon. Equipment and coating process using a spray and/or dip
coating process is employed.
The intervention site is a coronary artery. Upon inflation of the cutting
balloon, about 5 % to about 15
% of the coating is freed from the device resulting in delivery of ¨2.0 [tg of
drug delivered to the
artery.
[00282] In another example, the polymer of the coating is about 50:50 PLGA-
Ester End
Group, MW-191(1), degradation rate ¨1-2 months or about 50:50 PLGA-Carboxylate
End Group,
MW-101(1), degradation rate ¨28 days. The active agent is a chemotherapeutic
agent. Equipment and
coating process using a spray and/or dip coating process is employed. The
intervention site is a cavity
resulting from removal of a tumor. Upon inflation of the cutting balloon, at
least about 75% of the
coating is transferred from the device to the intervention site.
- 67 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00283] In-vivo testing: A group of 27 New Zealand white rabbits is
prepared for a Seldinger
procedure using a cutting balloon coated with a formulation of about 50:50
PLGA-Ester End Group
(MW-191(D, degradation rate ¨1-2 months) and sirolimus with total loading of
sirolimus ¨20 [tg with
the coating preferentially on the wire of the cutting balloon. The device is
placed at a coronary artery
intervention site with the assistance of fluoroscopy to aid in positioning the
device at the same
location in each subject. Six animals are subjected to the procedure using a
coated balloon that does
not have sirolimus in the coating. After deployment and removal of the device,
3 control animals are
sacrificed at 1 hour post deployment and serum and tissue samples are
collected. The 3 remaining
control animals are sacrificed at 56 days post deployment. During the course
of the study, serum
samples are collected from control and drug-treated animals every five days.
The drug treated
animals, 3 each, are sacrificed at 1 hour, 24 hours, 7 days, 14 days, 28 days,
42 days and 56 days post
deployment.
[00284] The tissue and serum samples may be subjected to analysis for
sirolimus
concentration. In order to determine the amount of coating freed from the
device and/or delivered to
the intervention site as a percent of the total amount of coating on the
substrate, the tissue
concentration of sirolimus at the one hour time point (or any time point
within the first day following
of the procedure) may be used along with the total content expected for the
coating (based on the total
content for the manufacturing lot) or along with the content of coating
remaining on the device once
removed and the percentage calculated. This percentage is correlative of the
percent of coating freed,
dissociated, and/or transferred from the device and delivered to the
intervention site. Alternatively,
the tissue may be analyzed by various means (noted herein, including but not
limited to SEM, TEM,
and, where image enhanced polymers are used, various imaging means capable of
detecting these
enhanced polymers) to detect the percent of the coating freed, dissociated
and/or transferred from the
substrate and delivered to the intervention site. Again, the amount of coating
known to be on the
substrate based on manufacturing lot characteristics, and/or an assessment of
the coating remaining on
the device following removal of the device from the subject (for example,
wherein the device is an
angioplasty catheter and the substrate is the balloon of the catheter) may be
used to determine the
percent of coating freed, dissociated, and/or transferred from the device. In
some instances, an
assessment of the device following the procedure alone is sufficient to assess
the amount freed or
dissociated from the substrate, without determination of the amount delivered
to the intervention site.
Additionally, where a determination of improvement and/or disease treatment is
desired, levels of
proinflammatory markers could be tested to indicate improvement and/or
treatment of a disease
and/or ailment, for example, by testing high sensitive C-reactive protein
(hsCRP), interleukin-6 (IL-
6), interleukin-lp (IL-1I3), and/or monocyte chemoattractant protein-1 (MCP-
1). The release kinetics
of the drug may be indicated by plotting the sirolimus concentrations at the
timepoints noted above.
- 68 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00285] For embodiments using different drugs other than sirolimus,
the biomarkers are
selected based on the disease to be treated and the drugs administered during
the course of therapy as
determined by one of skill in the art. These biomarkers may be used to
indicate the treatment results
for each subject.
[00286] Other in-vivo tests described herein may be used instead of this
test and/or in addition
to this test, adjusted for the particularities of this device, as would be
known to one of ordinary skill in
the art.
[00287] In-vitro testing: One sample of the coated cutting balloon
prepared in using spray
and/or dip coating process is secured to a balloon catheter. A segment of
optically clear TYGONO B-
44-3 tubing with O.D. = 0.125", I.D. = 0.0625" (Available from McMaster-Carr
Part Number:
5114K11 (www.mcmaster.com)) is filled with phosphate-buffered saline solution
and immersed in a
water bath at 37 C to mimic physiological conditions of deployment into a
subject. The coated
balloon is inserted into the tubing and the balloon is inflated to at least
25% below the balloon's
nominal pressure to mechanically transfer the coating from the balloon to the
tubing wall. The
balloon is deflated and removed from the tubing. Optical microscopy is
performed on the tubing
and/or the balloon (which is inflated to at least 25% below the balloon's
nominal pressure, at least) to
determine the presence and amount of coating transferred to the tubing and/or
the amount of coating
freed, dissociated, and/or transferred from the balloon. Other in-vitro tests
described herein may be
used instead of this test and/or in addition to this test, adjusted for the
particularities of this device, as
would be known to one of ordinary skill in the art.
CUTTING BALLOON (3)- Mechanical stimulation to free the coating
[00288] A cutting balloon is coated comprising a release agent, a
polymer and an active agent.
The coated cutting balloon is positioned at the intervention site. The balloon
is inflated to at least
25% below its nominal inflation pressure. At least about 5% to at least about
50% of the coating is
freed from the surface of the cutting balloon and is deposited at the
intervention site.
[00289] In some examples, the balloon unfolds during inflation,
causing mechanical shearing
forces to at least augment transfer and/or freeing and/or deposition of the
coating from the balloon to
the intervention site.
[00290] In some examples, the balloon twists during inflation, causing
mechanical shearing
forces to at least augment transfer and/or freeing and/or deposition of the
coating from the balloon.
[00291] In one example, the polymer of the coating is about 50:50 PLGA-
Ester End Group,
MW-191(D, degradation rate ¨1-2 months or about 50:50 PLGA-Carboxylate End
Group,
MW-101(D, degradation rate ¨28 days. The active agent is a pharmaceutical
agent such as a
macrolide immunosuppressive drug. Equipment and coating process similar to
Example 2 is
.. employed. The intervention site is a vascular lumen wall. Upon inflation of
the cutting balloon, at
least about 50% of the coating is freed from the device at the intervention
site.
- 69 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00292] In another example, a cutting balloon is coated with a
formulation of PLGA +
sirolimus with total loading of sirolimus ¨20 [tg with the coating
preferentially on the wire of the
cutting balloon. Equipment and process similar to Example 2 is employed. The
intervention site is a
coronary artery. The release agent is ePTFE powder. Upon inflation of the
cutting balloon, about 5
% to about 15 % of the coating is freed from the device resulting in delivery
of ¨2.0 [tg of drug
delivered to the artery.
[00293] In another example, the polymer of the coating is about 50:50
PLGA-Ester End
Group, MW-191(D, degradation rate ¨1-2 months or about 50:50 PLGA-Carboxylate
End Group,
MW-101(D, degradation rate ¨28 days. The active agent is a chemotherapeutic
agent. Equipment and
coating process similar to Example 2 is employed. The release agent a
micronized active agent or
another active agent in a micronized form. The intervention site is a cavity
resulting from removal of
a tumor. Upon inflation of the cutting balloon, at least about 75% of the
coating is transferred from
the device to the intervention site.
[00294] In-vivo testing: A group of 27 New Zealand white rabbits is
prepared for a Seldinger
procedure using a cutting balloon coated with a formulation of about 50:50
PLGA-Ester End Group
(MW-191(D, degradation rate ¨1-2 months) and sirolimus with total loading of
sirolimus ¨20 [tg with
the coating preferentially on the wire of the cutting balloon. The device is
placed at a coronary artery
intervention site with the assistance of fluoroscopy to aid in positioning the
device at the same
location in each subject. Six animals are subjected to the procedure using a
coated balloon that does
not have sirolimus in the coating. After deployment and removal of the device,
3 control animals are
sacrificed at 1 hour post deployment and serum and tissue samples are
collected. The 3 remaining
control animals are sacrificed at 56 days post deployment. During the course
of the study, serum
samples are collected from control and drug-treated animals every five days.
The drug treated
animals, 3 each, are sacrificed at 1 hour, 24 hours, 7 days, 14 days, 28 days,
42 days and 56 days post
deployment. The tissue and serum samples may be subjected to analysis for
sirolimus concentration.
[00295] In order to determine the amount of coating freed from the
device and/or delivered to
the intervention site as a percent of the total amount of coating on the
substrate, the tissue
concentration of sirolimus at the one hour time point (or any time point
within the first day following
of the procedure) may be used along with the total content expected for the
coating (based on the total
content for the manufacturing lot) or along with the content of coating
remaining on the device once
removed and the percentage calculated. This percentage is correlative of the
percent of coating freed,
dissociated, and/or transferred from the device and delivered to the
intervention site. Alternatively,
the tissue may be analyzed by various means (noted herein, including but not
limited to SEM, TEM,
and, where image enhanced polymers are used, various imaging means capable of
detecting these
enhanced polymers) to detect the percent of the coating freed, dissociated
and/or transferred from the
substrate and delivered to the intervention site. Again, the amount of coating
known to be on the
- 70 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
substrate based on manufacturing lot characteristics, and/or an assessment of
the coating remaining on
the device following removal of the device from the subject (for example,
wherein the device is an
angioplasty catheter and the substrate is the balloon of the catheter) may be
used to determine the
percent of coating freed, dissociated, and/or transferred from the device. In
some instances, an
.. assessment of the device following the procedure alone is sufficient to
assess the amount freed or
dissociated from the substrate, without determination of the amount delivered
to the intervention site.
Additionally, where a determination of improvement and/or disease treatment is
desired, levels of
proinflammatory markers could be tested to indicate improvement and/or
treatment of a disease
and/or ailment, for example, by testing high sensitive C-reactive protein
(hsCRP), interleukin-6 (IL-
6), interleukin-lp (IL-1I3), and/or monocyte chemoattractant protein-1 (MCP-
1). The release kinetics
of the drug may be indicated by plotting the sirolimus concentrations at the
timepoints noted above.
[00296] For embodiments using different drugs other than sirolimus,
the biomarkers are
selected based on the disease to be treated and the drugs administered during
the course of therapy as
determined by one of skill in the art. These biomarkers may be used to
indicate the treatment results
for each subject.
[00297] Other in-vivo tests described herein may be used instead of
this test and/or in addition
to this test, adjusted for the particularities of this device, as would be
known to one of ordinary skill in
the art.
[00298] In-vitro testing: One sample of the coated cutting balloon
prepared in Example 2 is
secured to a balloon catheter. A segment of optically clear TYGONO B-44-3
tubing with O.D. =
0.125", I.D. = 0.0625" (Available from McMaster-Carr Part Number: 5114K11
(www.mcmaster.com)) is filled with phosphate-buffered saline solution and
immersed in a water bath
at 37 C to mimic physiological conditions of deployment into a subject. The
coated balloon is
inserted into the tubing and the balloon is inflated to at least 25% below the
balloon's nominal
pressure to mechanically transfer the coating from the balloon to the tubing
wall. The balloon is
deflated and removed from the tubing. Optical microscopy is performed on the
tubing and/or the
balloon (which is inflated to at least 25% below the balloon's nominal
pressure, at least) to determine
the presence and amount of coating transferred to the tubing and/or the amount
of coating transferred
from the balloon. Other in-vitro tests described herein may be used instead of
this test and/or in
addition to this test, adjusted for the particularities of this device, as
would be known to one of
ordinary skill in the art.
CUTTING BALLOON (4)- Mechanical stimulation to free the coating
[00299] A cutting balloon is coated comprising a polymer and an active
agent. The coated
cutting balloon is positioned at the intervention site. The balloon is
inflated to at least 25% below its
- 71 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
nominal inflation pressure. At least about 10% to at least about 50% of the
coating is freed from the
surface of the cutting balloon and is deposited at the intervention site.
[00300] In some examples, the balloon unfolds during inflation,
causing mechanical shearing
forces to at least augment transfer and/or freeing and/or deposition of the
coating from the balloon to
the intervention site.
[00301] In some examples, the balloon twists during inflation, causing
mechanical shearing
forces to at least augment transfer and/or freeing and/or deposition of the
coating from the balloon.
[00302] In one example, the polymer of the coating is about 50:50 PLGA-
Ester End Group,
MW-191(D, degradation rate ¨1-2 months or about 50:50 PLGA-Carboxylate End
Group,
MW-101(D, degradation rate ¨28 days. The active agent is a pharmaceutical
agent such as a
macrolide immunosuppressive drug. Equipment and coating process similar to
Example 3 is
employed. The intervention site is a vascular lumen wall. Upon inflation of
the cutting balloon, at
least about 50% of the coating is freed from the device at the intervention
site.
[00303] In another example, a cutting balloon is coated with a
formulation of PLGA +
sirolimus with total loading of sirolimus ¨20 [tg with the coating
preferentially on the wire of the
cutting balloon. Equipment and process similar to Example 3 is employed. The
intervention site is a
coronary artery. Upon inflation of the cutting balloon, about 5 % to about 15
% of the coating is freed
from the device resulting in delivery of ¨2.0 [tg of drug delivered to the
artery.
[00304] In another example, the polymer of the coating is about 50:50
PLGA-Ester End
Group, MW-191(D, degradation rate ¨1-2 months or about 50:50 PLGA-Carboxylate
End Group,
MW-101(D, degradation rate ¨28 days. The active agent is a chemotherapeutic
agent. Equipment and
coating process similar to Example 3 is employed. The intervention site is a
cavity resulting from
removal of a tumor. Upon inflation of the cutting balloon, at least about 75%
of the coating is
transferred from the device to the intervention site.
[00305] In-vivo testing: A group of 27 New Zealand white rabbits is
prepared for a Seldinger
procedure using a cutting balloon coated with a formulation of about 50:50
PLGA-Ester End Group
(MW-191(D, degradation rate ¨1-2 months) and sirolimus with total loading of
sirolimus ¨20 [tg with
the coating preferentially on the wire of the cutting balloon. The device is
placed at a coronary artery
intervention site with the assistance of fluoroscopy to aid in positioning the
device at the same
location in each subject. Six animals are subjected to the procedure using a
coated balloon that does
not have sirolimus in the coating. After deployment and removal of the device,
3 control animals are
sacrificed at 1 hour post deployment and serum and tissue samples are
collected. The 3 remaining
control animals are sacrificed at 56 days post deployment. During the course
of the study, serum
samples are collected from control and drug-treated animals every five days.
The drug treated
animals, 3 each, are sacrificed at 1 hour, 24 hours, 7 days, 14 days, 28 days,
42 days and 56 days post
deployment.
- 72 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00306] The tissue and serum samples may be subjected to analysis for
sirolimus
concentration. In order to determine the amount of coating freed from the
device and/or delivered to
the intervention site as a percent of the total amount of coating on the
substrate, the tissue
concentration of sirolimus at the one hour time point (or any time point
within the first day following
of the procedure) may be used along with the total content expected for the
coating (based on the total
content for the manufacturing lot) or along with the content of coating
remaining on the device once
removed and the percentage calculated. This percentage is correlative of the
percent of coating freed,
dissociated, and/or transferred from the device and delivered to the
intervention site. Alternatively,
the tissue may be analyzed by various means (noted herein, including but not
limited to SEM, TEM,
and, where image enhanced polymers are used, various imaging means capable of
detecting these
enhanced polymers) to detect the percent of the coating freed, dissociated
and/or transferred from the
substrate and delivered to the intervention site. Again, the amount of coating
known to be on the
substrate based on manufacturing lot characteristics, and/or an assessment of
the coating remaining on
the device following removal of the device from the subject (for example,
wherein the device is a
cutting angioplasty catheter and the substrate is the cutting balloon of the
catheter) may be used to
determine the percent of coating freed, dissociated, and/or transferred from
the device. In some
instances, an assessment of the device following the procedure alone is
sufficient to assess the amount
freed or dissociated from the substrate, without determination of the amount
delivered to the
intervention site. Additionally, where a determination of improvement and/or
disease treatment is
desired, levels of proinflammatory markers could be tested to indicate
improvement and/or treatment
of a disease and/or ailment, for example, by testing high sensitive C-reactive
protein (hsCRP),
interleukin-6 (IL-6), interleukin-lp (IL-1I3), and/or monocyte chemoattractant
protein-1 (MCP-1).
The release kinetics of the drug may be indicated by plotting the sirolimus
concentrations at the
timepoints noted above.
[00307] For embodiments using different drugs other than sirolimus, the
biomarkers are
selected based on the disease to be treated and the drugs administered during
the course of therapy as
determined by one of skill in the art. These biomarkers may be used to
indicate the treatment results
for each subject.
[00308] Other in-vivo tests described herein may be used instead of
this test and/or in addition
.. to this test, adjusted for the particularities of this device, as would be
known to one of ordinary skill in
the art.
[00309] In-vitro testing: One sample of the coated cutting balloon
prepared in Example 3 is
secured to a balloon catheter. A segment of optically clear TYGONO B-44-3
tubing with O.D. =
0.125", I.D. = 0.0625" (Available from McMaster-Carr Part Number: 5114K11
(www.mcmaster.com)) is filled with phosphate-buffered saline solution and
immersed in a water bath
at 37 C to mimic physiological conditions of deployment into a subject. The
coated balloon is
- 73 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
inserted into the tubing and the balloon is inflated to at least 25% below the
balloon's nominal
pressure to mechanically transfer the coating from the balloon to the tubing
wall. The balloon is
deflated and removed from the tubing. Optical microscopy is performed on the
tubing and/or the
balloon (which is inflated to at least 25% below the balloon's nominal
pressure, at least) to determine
the presence and amount of coating transferred to the tubing and/or the amount
of coating freed,
dissociated, and/or transferred from the balloon. Other in-vitro tests
described herein may be used
instead of this test and/or in addition to this test, adjusted for the
particularities of this device, as
would be known to one of ordinary skill in the art.
CUTTING BALLOON (5)- Mechanical and Chemical stimulation to free the coating
[00310] A cutting balloon is coated with a formulation comprising a
base layer of methyl
acrylate-methacrylic acid copolymer and additional layers of PLGA + paclitaxel
with total dose of
paclitaxel approx. 0.5 [tg/mm2 of the wire. The coating and sintering process
is similar to that as
described in Example 1. The balloon is constructed of a semipermable polymer.
The pressurization
medium is pH 8 phosphate buffer. The coated cutting balloon is positioned at
the intervention site.
The balloon is pressurized to at least to at least 25% below its nominal
inflation pressure. Upon
pressurization of the cutting balloon in the diseased artery, at least about
10% to at least about 30% of
the coating is released into the intervention site and upon depressurization
and removal of the device,
this material is deposited at the intervention site.
[00311] In some examples, the balloon unfolds during inflation, causing
mechanical shearing
forces to at least augment the pH mediated release of the coating from the
balloon to the intervention
site.
[00312] In some examples, the balloon twists during inflation, causing
mechanical shearing
forces to at least augment the pH mediated release of the coating from the
balloon.
[00313] In one example, a base layer of methyl acrylate-methacrylic acid
copolymer is formed
and additional layers of the coating is about 50:50 PLGA-Ester End Group, MW-
191(1), degradation
rate ¨1-2 months or about 50:50 PLGA-Carboxylate End Group, MW-101(1),
degradation rate ¨28
days. The active agent is a pharmaceutical agent such as a macrolide
immunosuppressive drug.
Equipment and coating process similar to Example 1 is employed. The balloon is
constructed of a
semipermable polymer. The pressurization medium is pH 8 phosphate buffer. The
intervention site is
a vascular lumen wall. Upon inflation of the cutting balloon, at least about
50% of the coating is freed
from the device at the intervention site.
[00314] In another example, a cutting balloon is coated with a base
layer of methyl acrylate-
methacrylic acid copolymer and additional layers of PLGA + sirolimus with
total loading of sirolimus
¨20 . Equipment and process similar to Example 1 is employed. The
intervention site is a coronary
artery. The balloon is constructed of a semipermable polymer. The
pressurization medium is pH 8
- 74 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
phosphate buffer. Upon inflation of the cutting balloon, about 5 % to about 15
% of the coating is
freed from the device resulting in delivery of ¨2.0 [tg of drug delivered to
the artery.
[00315] In another example, the polymer of the coating is about 50:50
PLGA-Ester End
Group, MW-191(D, degradation rate ¨1-2 months or about 50:50 PLGA-Carboxylate
End Group,
MW-101(D, degradation rate ¨28 days. The active agent is a chemotherapeutic
agent. Equipment and
coating process similar to Example 1 is employed. The intervention site is a
cavity resulting from
removal of a tumor. Upon inflation of the cutting balloon, at least about 75%
of the coating is
transferred from the device to the intervention site.
[00316] In-vivo testing: A group of 27 New Zealand white rabbits is
prepared for a Seldinger
procedure using a cutting balloon coated with a formulation of about 50:50
PLGA-Ester End Group
(MW-191(D, degradation rate ¨1-2 months) and sirolimus with total loading of
sirolimus ¨20 [tg with
the coating preferentially on the wire of the cutting balloon. The device is
placed at a coronary artery
intervention site with the assistance of fluoroscopy to aid in positioning the
device at the same
location in each subject. Six animals are subjected to the procedure using a
coated balloon that does
not have sirolimus in the coating. After deployment and removal of the device,
3 control animals are
sacrificed at 1 hour post deployment and serum and tissue samples are
collected. The 3 remaining
control animals are sacrificed at 56 days post deployment. During the course
of the study, serum
samples are collected from control and drug-treated animals every five days.
The drug treated
animals, 3 each, are sacrificed at 1 hour, 24 hours, 7 days, 14 days, 28 days,
42 days and 56 days post
.. deployment.
[00317] The tissue and serum samples may be subjected to analysis for
sirolimus
concentration. In order to determine the amount of coating freed from the
device and/or delivered to
the intervention site as a percent of the total amount of coating on the
substrate, the tissue
concentration of sirolimus at the one hour time point (or any time point
within the first day following
of the procedure) may be used along with the total content expected for the
coating (based on the total
content for the manufacturing lot) or along with the content of coating
remaining on the device once
removed and the percentage calculated. This percentage is correlative of the
percent of coating freed,
dissociated, and/or transferred from the device and delivered to the
intervention site. Alternatively,
the tissue may be analyzed by various means (noted herein, including but not
limited to SEM, TEM,
and, where image enhanced polymers are used, various imaging means capable of
detecting these
enhanced polymers) to detect the percent of the coating freed, dissociated
and/or transferred from the
substrate and delivered to the intervention site. Again, the amount of coating
known to be on the
substrate based on manufacturing lot characteristics, and/or an assessment of
the coating remaining on
the device following removal of the device from the subject (for example,
wherein the device is an
cutting angioplasty catheter and the substrate is the cutting balloon of the
catheter) may be used to
determine the percent of coating freed, dissociated, and/or transferred from
the device. In some
- 75 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
instances, an assessment of the device following the procedure alone is
sufficient to assess the amount
freed or dissociated from the substrate, without determination of the amount
delivered to the
intervention site. Additionally, where a determination of improvement and/or
disease treatment is
desired, levels of proinflammatory markers could be tested to indicate
improvement and/or treatment
of a disease and/or ailment, for example, by testing high sensitive C-reactive
protein (hsCRP),
interleukin-6 (IL-6), interleukin-lp (IL-1I3), and/or monocyte chemoattractant
protein-1 (MCP-1).
The release kinetics of the drug may be indicated by plotting the sirolimus
concentrations at the
timepoints noted above.
[00318] For embodiments using different drugs other than sirolimus,
the biomarkers are
selected based on the disease to be treated and the drugs administered during
the course of therapy as
determined by one of skill in the art. These biomarkers may be used to
indicate the treatment results
for each subject.
[00319] Other in-vivo tests described herein may be used instead of
this test and/or in addition
to this test, adjusted for the particularities of this device, as would be
known to one of ordinary skill in
the art.
[00320] In-vitro testing: One sample of the coated cutting balloon
prepared in Example 1 is
secured to a balloon catheter. A segment of optically clear TYGONO B-44-3
tubing with O.D. =
0.125", I.D. = 0.0625" (Available from McMaster-Carr Part Number: 5114K11
(www.mcmaster.com)) is filled with phosphate-buffered saline solution and
immersed in a water bath
at 37 C to mimic physiological conditions of deployment into a subject. The
coated balloon is
inserted into the tubing and the balloon is inflated to at least 25% below the
balloon's nominal
pressure to mechanically transfer the coating from the balloon to the tubing
wall. The balloon is
deflated and removed from the tubing. Optical microscopy is performed on the
tubing and/or the
balloon (which is inflated to at least 25% below the balloon's nominal
pressure, at least) to determine
the presence and amount of coating transferred to the tubing and/or the amount
of coating freed,
dissociated, and/or transferred from the balloon. Other in-vitro tests
described herein may be used
instead of this test and/or in addition to this test, adjusted for the
particularities of this device, as
would be known to one of ordinary skill in the art.
EXAMPLE 2: DRUG-DELIVERY BALLOON CATHETERS
DRUG-DELIVERY BALLOON (1) - Compliant balloon
[00321] A compliant balloon is coated with a material comprising a
polymer and an active
agent. The coated compliant balloon is positioned at the intervention site.
The balloon is inflated to
at least 25% below its nominal inflation pressure. Upon deflation and removal
of the compliant
balloon from the intervention site, at least about 5% to at least about 30% of
the coating is freed from
the surface of the compliant balloon and is deposited at the intervention
site.
- 76 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00322] In some examples, the balloon unfolds during inflation,
causing mechanical shearing
forces to at least augment transfer and/or freeing and/or deposition of the
coating from the balloon to
the intervention site.
[00323] In some examples, the balloon twists during inflation, causing
mechanical shearing
forces to at least augment transfer and/or freeing and/or deposition of the
coating from the balloon.
[00324] In one example, the polymer of the coating is about 50:50 PLGA-
Ester End Group,
MW-191(D, degradation rate ¨1-2 months or about 50:50 PLGA-Carboxylate End
Group,
MW-101(D, degradation rate ¨28 days. The active agent is a pharmaceutical
agent such as a
macrolide immunosuppressive drug. Equipment and coating process similar to
Example 1 is
employed. The intervention site is a vascular lumen wall. Upon inflation of
the compliant balloon, at
least about 50% of the coating is freed from the device at the intervention
site.
[00325] In another example, a compliant balloon is coated with a
formulation of PLGA +
sirolimus with total loading of sirolimus ¨20 [Lg. Equipment and process
similar to Example 1 is
employed. The intervention site is a coronary artery. Upon inflation of the
compliant balloon, about
5 % to about 15 % of the coating is freed from the device resulting in
delivery of ¨2.0 [tg of drug
delivered to the artery.
[00326] In another example, the polymer of the coating is 50:50 PLGA-
Ester End Group,
MW-191(D, degradation rate ¨1-2 months or 50:50 PLGA-Carboxylate End Group, MW-
101(D,
degradation rate ¨28 days. The active agent is a chemotherapeutic agent.
Equipment and coating
process similar to Example 1 is employed. The intervention site is a cavity
resulting from removal of
a tumor. Upon inflation of the compliant balloon, at least about 75% of the
coating is transferred
from the device to the intervention site.
[00327] In-vivo testing: A group of 27 New Zealand white rabbits is
prepared for a Seldinger
procedure using a compliant balloon coated with a formulation of about 50:50
PLGA-Ester End
Group (MW-191(D, degradation rate ¨1-2 months) and sirolimus with total
loading of sirolimus ¨20
[Lg. The device is placed at a coronary artery intervention site with the
assistance of fluoroscopy to
aid in positioning the device at the same location in each subject. Six
animals are subjected to the
procedure using a coated balloon that does not have sirolimus in the coating.
After deployment and
removal of the device, 3 control animals are sacrificed at 1 hour post
deployment and serum and
tissue samples are collected. The 3 remaining control animals are sacrificed
at 56 days post
deployment. During the course of the study, serum samples are collected from
control and drug-
treated animals every five days. The drug treated animals, 3 each, are
sacrificed at 1 hour, 24 hours, 7
days, 14 days, 28 days, 42 days and 56 days post deployment. The tissue and
serum samples may be
subjected to analysis for sirolimus concentration.
[00328] In order to determine the amount of coating freed from the device
and/or delivered to
the intervention site as a percent of the total amount of coating on the
substrate, the tissue
- 77 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
concentration of sirolimus at the one hour time point (or any time point
within the first day following
of the procedure) may be used along with the total content expected for the
coating (based on the total
content for the manufacturing lot) or along with the content of coating
remaining on the device once
removed and the percentage calculated. This percentage is correlative of the
percent of coating freed,
dissociated, and/or transferred from the device and delivered to the
intervention site. Alternatively,
the tissue may be analyzed by various means (noted herein, including but not
limited to SEM, TEM,
and, where image enhanced polymers are used, various imaging means capable of
detecting these
enhanced polymers) to detect the percent of the coating freed, dissociated
and/or transferred from the
substrate and delivered to the intervention site. Again, the amount of coating
known to be on the
substrate based on manufacturing lot characteristics, and/or an assessment of
the coating remaining on
the device following removal of the device from the subject (for example,
wherein the device is a
cutting angioplasty catheter and the substrate is the balloon of the catheter)
may be used to determine
the percent of coating freed, dissociated, and/or transferred from the device.
In some instances, an
assessment of the device following the procedure alone is sufficient to assess
the amount freed or
dissociated from the substrate, without determination of the amount delivered
to the intervention site.
Additionally, where a determination of improvement and/or disease treatment is
desired, levels of
proinflammatory markers could be tested to indicate improvement and/or
treatment of a disease
and/or ailment, for example, by testing high sensitive C-reactive protein
(hsCRP), interleukin-6 (IL-
6), interleukin-lp (IL-1I3), and/or monocyte chemoattractant protein-1 (MCP-
1). The release kinetics
of the drug may be indicated by plotting the sirolimus concentrations at the
timepoints noted above.
[00329] For embodiments using different drugs other than sirolimus,
the biomarkers are
selected based on the disease to be treated and the drugs administered during
the course of therapy as
determined by one of skill in the art. These biomarkers may be used to
indicate the treatment results
for each subject.
[00330] In-vitro testing: One sample of the coated compliant balloon
prepared in Example 1 is
secured to a balloon catheter. A segment of optically clear TYGONO B-44-3
tubing with O.D. =
0.125", I.D. = 0.0625" (Available from McMaster-Carr Part Number: 5114K11
(www.mcmaster.com)) is filled with phosphate-buffered saline solution and
immersed in a water bath
at 37 C to mimic physiological conditions of deployment into a subject. The
coated balloon is
inserted into the tubing and the balloon is inflated to at least 25% below the
balloon's nominal
pressure to mechanically transfer the coating from the balloon to the tubing
wall. The balloon is
deflated and removed from the tubing. Optical microscopy is performed on the
tubing and/or the
balloon (which is inflated to at least 25% below the balloon's nominal
pressure, at least) to determine
the presence and amount of coating transferred to the tubing and/or the amount
of coating freed,
dissociated, and/or transferred from the balloon.
- 78 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00331] Method for the determination of sirolimus levels: Media may be
assayed for
sirolimus content using HPLC. Calibration standards containing known amounts
of drug are to
determine the amount of drug eluted. The multiple peaks present for the
sirolimus (also present in the
calibration standards) are added to give the amount of drug eluted at that
time period (in absolute
amount and as a cumulative amount eluted). HPLC analysis is performed using
Waters HPLC
system, set up and run on each sample as provided in the Table 1 below using
an injection volume of
100 EL.
Table 1
Time point % Acetonitrile % Ammonium Acetate (0.5%), Flow Rate
(minutes) pH 7.4 (mL/min)
0.00 10 90 1.2
1.00 10 90 1.2
12.5 95 5 1.2
13.5 100 0 1.2
14.0 100 0 3
16.0 100 0 3
17.0 10 90 2
20.0 10 90 0
[00332] In-vitro Mass Loss test: One sample of the coated compliant balloon
prepared in
Example 1 is weighed on a microbalance and then secured to a balloon catheter.
A segment of
optically clear TYGONO B-44-3 tubing with O.D. = 0.125", I.D. = 0.0625"
(Available from
McMaster-Carr Part Number: 5114K11 (www.mcmaster.com)) is filled with
phosphate-buffered
saline solution and immersed in a water bath at 37 C to mimic physiological
conditions of
deployment into a subject. The coated balloon is inserted into the tubing and
the balloon is inflated to
at least 25% below the balloon's nominal pressure to mechanically transfer the
coating from the
balloon to the tubing wall. The balloon is deflated and removed from the
tubing. After drying, the
balloon is removed from the guidewire, further dried and weighed on a
microbalance. Comparison of
the pre- and post-deployment weights indicates how much coating is freed,
dissociated, and/or
transferred from the balloon. This analysis may instead and/or alternatively
include testing of the
tubing to determine the amount of coating freed, dissociated, and/or
transferred from the device
during this in-vitro test.
[00333] In-vitro Coating test: One sample of the coated compliant
balloon prepared in
Example 1 is secured to a balloon catheter. A segment of optically clear
TYGONO B-44-3 tubing
with O.D. = 0.125", I.D. = 0.0625" (Available from McMaster-Carr Part Number:
5114K11
- 79 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
(www.mcmaster.com)) is filled with phosphate-buffered saline solution and
immersed in a water bath
at 37 C to mimic physiological conditions of deployment into a subject. The
coated balloon is
inserted into the tubing and the balloon is inflated to at least 25% below the
balloon's nominal
pressure to mechanically transfer the coating from the balloon to the tubing
wall. The balloon is
deflated and removed from the tubing. The section of tubing exposed to the
deployed balloon is cut
away from the remainder of the tubing and the interior of the excised tubing
rinsed with a small
amount of ethanol and an amount of methylene chloride to make up 25 mL total
volume of rinsings
which are collected in a flask for analysis. Analysis by HPLC as described
above is performed to
determine the amount of material freed, dissociated, and/or transferred from
the balloon. This analysis
may instead and/or alternatively include testing of the substrate itself to
determine the amount of
coating freed, dissociated, and/or transferred from the device during this in-
vitro test.
[00334]
In-vitro testing:: One sample of the coated compliant balloon prepared in
Example 1
is secured to a balloon catheter. A segment of resected coronary artery from
Yucatan miniature swine
is positionally fixed and filled with phosphate-buffered saline solution and
immersed in a water bath
.. at 37 C to mimic physiological conditions of deployment into a subject.
The coated balloon is
inserted into the artery and the balloon is inflated to at least 25% below the
balloon's nominal
pressure to mechanically transfer the coating from the balloon to the arterial
wall. The balloon is
deflated and removed from the artery. The section of artery exposed to the
deployed balloon is cut
away from the remainder of the artery section, placed into a tissue
homogonizer and the homogonized
material is extracted with methylene chloride to make up 25 mL total volume of
rinsings which are
collected in a flask for analysis. Analysis by HPLC as described above is
performed to determine the
amount of material freed, dissociated, and/or transferred from the balloon.
This analysis may instead
and/or alternatively include testing of the substrate itself to determine the
amount of coating freed,
dissociated, and/or transferred from the device during this in-vitro test.
[00335] For embodiments related to non-vascular or non-lumenal
applications, e.g. a tumor
site or other cavity or a cannulized site, the same technique is employed with
the modification that the
tissue to be assayed is resected from the tissue adjoining cavity receiving
drug treatment.
[00336]
In-vitro testing:: One sample of the coated compliant balloon prepared in
Example 1
is secured to a balloon catheter. A segment of resected coronary artery from
Yucatan miniature swine
is positionally fixed and filled with phosphate-buffered saline solution and
immersed in a water bath
at 37 C to mimic physiological conditions of deployment into a subject. The
coated balloon is
inserted into the artery and the balloon is inflated to at least 25% below the
balloon's nominal
pressure to mechanically transfer the coating from the balloon to the arterial
wall. The balloon is
deflated and removed from the artery. The section of artery exposed to the
deployed balloon is cut
away from the remainder of the artery section and incised lengthwise to lay
open the artery. Optical
microscopy is performed on the interior of artery to determine the presence
and amount of coating
- 80 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
transferred to the artery and/or the amount of coating transferred from the
balloon. The tissue sample
is also subjected to TEM-SEM analysis.
[00337] In-vitro testing of release kinetics: One sample of the coated
compliant balloon with
total loading of sirolimus ¨20 [tg prepared in Example 1 is secured to a
balloon catheter. A flask
containing exactly 25 mL of pH 7.4 aqueous phosphate buffer equilibrated to 37
C equipped for
magnetic stirring is prepared. Into this flask is placed the coated balloon
and the catheter portion of
the apparatus is secured such that the balloon does not touch the sides of the
flask. The balloon is
inflated to 120 psi with sterile water. Aliquots of 100 EL are removed prior
to addition of the
balloon, after placement of the balloon but prior to inflation of the balloon,
and at regular time
intervals of 2, 4, 6, 8, 10, 12, and 14 minutes. Upon removal of each aliquot
an equivalent volume of
aqueous buffer is added to maintain the volume at 25 mL. The aliquots are
analyzed by HPLC as
described above for the concentration of sirolimus.
[00338] In-vitro testing for distal flow particulates: One sample of
the coated compliant
balloon prepared in Example 1 is secured to a guidewire incorporating a porous
filter of 100 micron
pore size, such as the Cordis AngioGuard emboli capture guidewire. A segment
of optically clear
TYGONO B-44-3 tubing with O.D. = 0.125", I.D. = 0.0625" (Available from
McMaster-Can- Part
Number: 5114K11 (www.mcmaster.com)) is filled with phosphate-buffered saline
solution and
immersed in a water bath at 37 C to mimic physiological conditions of
deployment into a subject.
The coated balloon is inserted into the tubing, the proximal end of the tubing
surrounding the
guidewire sealed with epoxy, and a hypodermic needle which is attached to an
infusion pump and
reservoir of 37 C phosphate-buffered saline solution is inserted into the
tubing proximal to the
balloon assembly. The flow of saline is commenced, the distal filter is
deployed and the balloon is
inflated to at least 25% below the balloon's nominal pressure to mechanically
transfer the coating
from the balloon to the tubing wall. The balloon is deflated and removed from
the tubing. The filter
is deployed for 5 minutes after removal of the balloon, the flow of saline is
halted, the tubing cut
adjacent to the epoxy seal, the filter retracted and removed from the tubing.
The content of the filter
is analyzed.
[00339] In-vitro testing for distal flow particulates: One sample of
the coated compliant
balloon prepared in Example 1 is secured to a guidewire. A segment of
optically clear TYGONO B-
44-3 tubing with O.D. = 0.125", I.D. = 0.0625" (Available from McMaster-Carr
Part Number:
5114K11 (www.mcmaster.com)) is filled with phosphate-buffered saline solution
and immersed in a
water bath at 37 C to mimic physiological conditions of deployment into a
subject and the distal end
of the tubing is connected to a turbidity light scattering detector as
described in Analytical
Ultracentrifugation of Polymers and Nanoparticles, W. Machtle and L. Borger,
(Springer) 2006, p.41.
The coated balloon is inserted into the proximal end of the tubing, the
proximal end of the tubing
surrounding the guidewire sealed with epoxy, and a hypodermic needle which is
attached to an
- 81 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
infusion pump and reservoir of 37 C phosphate-buffered saline solution is
inserted into the tubing
proximal to the balloon assembly. The flow of saline is commenced, a baseline
for light transmission
through the detector is established and the balloon is inflated to at least
25% below the balloon's
nominal pressure to mechanically transfer the coating from the balloon to the
tubing wall. The
balloon is deflated and removed from the tubing. The flow is maintained for 10
minutes after removal
of the balloon, and the flow is analyzed for the presence of particles based
on detector response.
DRUG-DELIVERY BALLOON (2) ¨ Non-Compliant balloon
[00340] A non-compliant balloon is coated with a material comprising a
polymer and an
active agent. The coated non-compliant balloon is positioned at the
intervention site. The balloon is
inflated to at least 25% below its nominal inflation pressure. Upon deflation
and removal of the non-
compliant balloon from the intervention site, at least about 5% to at least
about 30% of the coating is
freed from the surface of the non-compliant balloon and is deposited at the
intervention site.
[00341] In some examples, the balloon unfolds during inflation,
causing mechanical shearing
forces to at least augment transfer and/or freeing and/or deposition of the
coating from the balloon to
the intervention site.
[00342] In some examples, the balloon twists during inflation, causing
mechanical shearing
forces to at least augment transfer and/or freeing and/or deposition of the
coating from the balloon.
[00343] In one example, the polymer of the coating is about 50:50 PLGA-
Ester End Group,
MW-191(1), degradation rate ¨1-2 months or about 50:50 PLGA-Carboxylate End
Group,
MW-101(D, degradation rate ¨28 days. The active agent is a pharmaceutical
agent such as a
macrolide immunosuppressive drug. Equipment and coating process similar to
Example 1 is
employed. The intervention site is a vascular lumen wall. Upon inflation of
the non-compliant
balloon, at least about 50% of the coating is freed from the device at the
intervention site.
[00344] In another example, a non-compliant balloon is coated with a
formulation of PLGA +
sirolimus with total loading of sirolimus ¨20 [Lg. Equipment and process
similar to Example 1 is
employed. The intervention site is a coronary artery. Upon inflation of the
non-compliant balloon,
about 5 % to about 15 % of the coating is freed from the device resulting in
delivery of ¨2.0 [tg of
drug delivered to the artery.
[00345] In another example, the polymer of the coating is about 50:50 PLGA-
Ester End
Group, MW-191(1), degradation rate ¨1-2 months or about 50:50 PLGA-Carboxylate
End Group,
MW-101(D, degradation rate ¨28 days. The active agent is a chemotherapeutic
agent. Equipment and
coating process similar to Example 1 is employed. The intervention site is a
cavity resulting from
removal of a tumor. Upon inflation of the non-compliant balloon, at least
about 75% of the coating is
transferred from the device to the intervention site.
- 82 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00346] In-vivo and/or in-vitro testing may be performed according to
the methods described
herein.
EXAMPLE 3: IN VIVO DELIVERY OF RAPAMYCIN FROM COATED BALLOONS
Sirolimus Coated Balloon Formulation Tested in Rabbits
[00347] GHOST Rapid Exchange (Rx) Catheter was used in this example. Ghost
3.0x18 mm
Rx catheter balloons were coated and used in animal study. The study was
conducted according to
the following design. Several tests were run to determine in-vivo drug
delivery characteristics of the
rapamycin from the coated balloons.
[00348] The first test included expansion of the coated balloons in
rabbit iliac arteries. 8
coated balloons were manufactured and tested in 4 rabbits. Four of the coated
balloons were inflated
in pre-dilated arteries (right iliac) for 60 seconds, and four of the coated
balloons were inflated in non-
dilated arteries (left iliac) for 60 seconds. The amount of drug (sirolimus)
found in the arterial tissue
at the site of expansion was determined. The following table indicates the
results of the testing of
these arteries for sirolimus concentration in arterial tissue and total amount
of sirolimus in each artery.
Drug coated balloons in right iliac arteries were inflated/deflated about 10-
20 min before sacrifice.
Drug coated balloons in left iliac arteries were inflated/deflated about 5-15
min before sacrifice.
Average Sirolimus Total Sirolimus per
Iliac Artery SD SD
(ng/mg) Artery
Average (11g)
Right Iliac (denuded) (n=4) 178.3 32.1 5.4 1.1
Left Iliac (uninjured) (n=4) 216.1 122.4 3.9 1.7
Combined Right + Left Iliac
197.2 85.3 4.7 1.6
Arteries (n=8)
[00349] The following table indicates the raw data of the testing of
these same arteries for the
total amount of sirolimus in each artery. It also indicates a calculated
transfer efficiency of sirolimus
to the rabbit iliac arteries and the estimated time that the artery was
exposed to blood flow. The
percent (%) sirolimus transferred to the artery was calculated using an
estimated total amount of
sirolimus on the balloon. The estimated total amount of sirolimus on the
balloon which was based on
the batch average total amount of sirolimus coated on the same batch of
balloons as the test sample
balloon as determined by UV-Viscometric testing of the balloon. The estimated
time that the artery
was exposed to blood flow was the amount of time between balloon inflation and
the balloon testing,
and/or the time between balloon inflation and until the animal was sacrificed
and the artery extracted
for testing by HPLC for content of drug.
Total % Sirolimus Estimated Time
Rabbit # Balloon # Sirolimus per Transferred Artery
Exposed to
Artery (11g) to Artery Blood Flow (min)
#1 Right Iliac Artery N185 5.0 7.76% 20
#1 Left Iliac Artery N157 3.2 5.58% 15
#2 Right Iliac Artery N164 7.0 12.62% 10
- 83 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
#2 Left Iliac Artery N167 5.0 8.98% 5
#3 Right Iliac Artery N175 5.1 9.17% 10
#3 Left Iliac Artery N178 1.8 2.88% 5
#4 Right Iliac Artery N191 4.5 7.59% 15
#4 Left Iliac Artery N117 5.7 7.95% 10
Tracking Average - 4.7 7.8%
SD - 1.6 2.8%
[00350] The following table indicates the blood concentrations of
sirolimus (whole blood)
taken from the animals used in this test. A baseline concentration of
sirolimus was taken prior to
exposure to the coated balloon for each animal, that is, taken before balloon
inflation. The whole
blood samples were taken 5 to 15 minutes after the second balloon was inflated
in each animal, since
two coated balloons were delivered to each animal in this test. The results
indicated in the table
below, therefore, indicate the cumulative whole blood Sirolimus concentration
from the inflation of 2
drug coated balloons per animal. The total sirolimus in blood is based on 56mL
of blood per kg (i.e.
per kg weight of the rabbit tested).
Rabbit # Extraction Conc. Est. Total Sirolimus
(ng/mL) in Blood (jig)
#1 Baseline Below Quality Level -
#2 Baseline Below Quality Level -
#3 Baseline Below Quality Level -
#4 Baseline Below Quality Level -
#1 (15 min) 11.4 2.7
#2 (5 min) 30.8 8.2
#3 (5 min) 22.2 5.9
#4 (10 min) 19.3 4.8
Average (5-15 min) 20.9 5.4
SD 8.0 2.3
[00351] The following table indicates the concentrations of sirolimus on
each balloon used in
this test following the test itself, to indicate the percent (%) of sirolimus
lost following the test
procedure. As noted above, each of the balloons was tracked to the respective
artery, and inflated for
60 seconds (1 minute), then deflated and removed from the animal and tested
for the percent of
sirolimus remaining on the balloon. The percent (%) sirolimus lost is based on
the amount of
sirolimus remaining on the balloon following the test and the total amount of
sirolimus coated on the
balloon which is estimated from the balloon batch average as tested using UV-
Viscometric methods.
The variables which contribute to the amount (or percent) of sirolimus lost
include the following:
Balloon insertion into iliac (via jugular + aorta); Blood flow;
Pleat/Fold/Sheath methods and
procedures; ¨10% lost during shipping; and/or Balloon inflation/contact with
artery wall.
- 84 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
Balloon ID Total Sirolimus per % Sirolimus
Balloon (m) Lost
Rabbit #1 RIA Balloon N185 13.2 79.3%
Robbie #1 LIA Balloon N157 17.3 69.9%
Rabbit #2 RIA Balloon N164 4.8 91.5%
Rabbit #2 LIA Balloon N167 11.7 79.1%
Rabbit #3 RIA Balloon N175 16.0 71.4%
Rabbit #3 LIA Balloon N178 14.7 77.0%
Rabbit #4 RIA Balloon N191 9.6 83.6%
Rabbit #4 LIA Balloon N117 14.9 79.1%
Tracking Average 12.8 78.9%
SD 4.0 6.8%
[00352] In another test, tracking studies were conducted. The test
comprises tracking 4 coated
balloons to the aorta of a rabbit. Each of 4 coated balloons was inserted,
tracked to the aorta of the
rabbit, and left in the aorta for 2 minutes without inflating the balloon.
Following insertion, tracking,
and resting the balloon in the aorta for 2 minutes, the catheter including the
coated balloon was
removed from the animal.
[00353] The following table indicates the concentrations of sirolimus
on each balloon used in
this test following the test itself, to indicate the percent (%) of sirolimus
lost following the test
procedure. The percent (%) sirolimus lost is based on the amount of sirolimus
remaining on the
balloon following the test and the total amount of sirolimus coated on the
balloon which is estimated
from the balloon batch average as tested using UV-Viscometric methods. The
variables which
contribute to the amount (or percent) of sirolimus lost include the following:
Balloon insertion into
iliac (via jugular + aorta); Blood flow; Pleat/Fold/Sheath methods and
procedures; and/or -10% lost
during shipping.
Balloon ID Total Sirolimus per %
Sirolimus
Balloon (m) Lost
Tracking #1 Balloon (N120) 23.6 66.9%
Tracking #2 Balloon (N160) 20.8 63.9%
Tracking #3 Balloon (N166) 19.0 66.0%
Tracking #4 Balloon (N176) 22.0 65.5%
Tracking Average 21.3 65.6%
SD 2.0 1.3%
[00354] Sirolimus quantification was performed on the balloons and blood
samples from the
previous two tests (as indicated in the "Total Sirolimus per Balloon (ug)"
columns of the previous two
tables, and as indicated in the blood concentration table generally). That is,
sirolimus content was
determined from the 8 balloons inflated in rabbit iliac arteries, the 4
balloons tracked to but not
inflated in rabbit aorta, and the 8 whole blood samples (2 samples/rabbit).
The liver, kidney, spleens,
hearts and lungs were stored (80 C) for later drug analysis.
[00355] In summary, the tests performed in this Example indicate the
following: 197.2 85.3
ng/mg of Sirolimus embedded in artery walls. The Efficiency of Sirolimus
transferred from balloons
- 85 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
to artery walls was 7.8 2.8 %. The amount of sirolimus washed away into
circulation was 5.4 2.3
[Lg. Following inflation in arteries, 78.9 6.8% of the sirolimus coated on
the balloon was removed
from the balloon. Prior to inflation in the arteries, 65.6 1.3% of the
sirolimus coated on the balloon
was removed from the balloon. For reference, 50-100 [tg of sirolimus was
coated on each balloon.
From 1% to 5% of the drug (sirolimus) was transferred to the artery. 1 ng of
sirolimus per mg tissue
was found during the testing as described in this example.
EXAMPLE 4: IN VIVO DELIVERY OF RAPAMYCIN FROM COATED BALLOONS
[00356] Binding agents may be incorporated into the coating to improve
active agent retention
in the artery. Example binding agents include cationic agents and/or
positively charged molecules.
.. An example binding agent may be a surfactant. Other agents may also and/or
alternatively be used.
Binding agents may include, for non-limiting example, at least one of:
Polyarginine, Polyarginine 9-
L-pArg, DEAE-Dextran (Diethylaminoethyl cellulose- Dextran), DMAB
(Didodecyldimethylammonium bromide), PEI (Polyethyleneimine), TAB
(Tetradodecylammonium
bromide), and DMTAB (Dimethylditetradecylammonium bromide). In some
embodiments of the
devices, coatings and/or methods provided herein the coating comprises a
positive surface charge on a
surface of the coating configured to contact the treatment site.
[00357] In some embodiments the surfactant comprises at least one of a
primary amine having
pH < 10, and a secondary amine having pH < 4. In some embodiments surfactant
comprises
octenidine dihydrochloride. In some embodiments the surfactant comprises a
permanently charged
quaternary ammonium cation. In some embodiments the permanently charged
quaternary ammonium
cation comprises at least one of: an Alkyltrimethylammonium salt such as cetyl
trimethylammonium
bromide (CTAB), hexadecyl trimethyl ammonium bromide, cetyl trimethylammonium
chloride
(CTAC); Cetylpyridinium chloride (CPC); Polyethoxylated tallow amine (POEA);
Benzalkonium
chloride (BAC); Benzethonium chloride (BZT); 5-Bromo-5-nitro-1,3-dioxane;
Dimethyldioctadecylammonium chloride; and Dioctadecyldimethylammonium bromide
(DODAB).
In some embodiments the surfactant comprises at least one of:
didodecyldimethylammonium bromide
(DMAB), linear isoform Polyethylenimine (linear PEI), Branched Low MW
Polyethylenimine (PEI)
(of about <25KDa), Branched Low MW Polyethylenimine (PEI) (of about <15KDa),
Branched Low
MW Polyethylenimine (PEI) (of about <10KDa), Branched High MW Polyethylenimine
(of about
>1=25 KDa), Poly-L-Arginine (average or nominal MW of about 70,000 Da), Poly-L-
Arginine
(average or nominal MW > about 50,000 Da), Poly-L-Arginine (average or nominal
MW of about
5,000 to about 15,000 Da), Poly-L-Lysine (average or nominal MW of about
28,200 Da), Poly-L-
Lysine (average or nominal MW of about 67,000 Da), Poly Histidine,
Ethylhexadecyldimethylammonium Bromide, Dodecyltrimethyl Ammonium Bromide,
.. Tetradodecylammonium bromide, Dimethylditetradecyl Ammonium bromide,
Tetrabutylammonium
iodide, DEAE-Dextran hydrochloride, and Hexadimethrine Bromide. In some
embodiments, the
- 86 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
molecular weight of the binding agent is controlled. In some embodiments, the
average size of the
binding agent is controlled.
[00358] In some embodiments of the devices, coatings and/or methods
provided herein the
binding agent and the active agent are mixed and deposited together on the
device. In some
embodiments, the active agent and binding agent are lyophilized prior to
deposition on the device. In
some embodiments dry particles of the active agent and binding agent are
generated in another
manner familiar to one of skill in the art and then coated on the balloon or
other medical device as
described herein, such as by an eSTAT coating process. In some embodiments of
the devices,
coatings and/or methods provided herein the surfactant is deposited on a
balloon after the active agent
is deposited thereon.
[00359] The positive surface charge of the coating may be about 20 mV
to about 40mV. The
positive surface charge may be at least one of: at least about 1 mV, over
about 1 mV, at least about 5
mV, at least about 10 mV, about 10 mV to about 50 mV, about 20 mV to about 50
mV, about 10 mV
to about 40 mV, about 30 mV to about 40 mV, about 20 mV to about 30 mV, and
about 25 mV to
about 35 mV.
In some embodiments the average molecular weight of the binding agent is
controlled. For example,
Polyarginine may have an average molecular weight of 70kDa, 5-15 kDa, another
controlled
molecular weight, or a combination thereof In some embodiments the molecular
weight of the
binding agent is controlled. For example, in some embodiments, Polyarginine is
the binding agent and
at least 75% of the Polyarginine as is 70kDa, 5-15 kDa, or another controlled
molecular weight. In
some embodiments, Polyarginine is the binding agent and at least 50% of the
Polyarginine as is
70kDa, 5-15 kDa, or another controlled molecular weight. In some embodiments,
Polyarginine is the
binding agent and at least 90% of the Polyarginine as is 70kDa, 5-15 kDa, or
another controlled
molecular weight. In some embodiments, Polyarginine is the binding agent and
at least 95% of the
Polyarginine as is 70kDa, 5-15 kDa, or another controlled molecular weight. In
some embodiments,
Polyarginine is the binding agent and at least 98% of the Polyarginine as is
70kDa, 5-15 kDa, or
another controlled molecular weight. In some embodiments, Polyarginine is the
binding agent and at
least 99% of the Polyarginine as is 70kDa, 5-15 kDa, or another controlled
molecular weight.
[00360] In some embodiments, the size of the active agent in the
coating is controlled in order
to improve drug retention in the artery. For non-limiting example, in the case
of sirolimus as an active
agent, the sirolimus may have an average size (mean diameter) of at least one
of: 1.5 [tin, 2.5 [tin,
645nm, 100-200 nm, another controlled size, or a combination thereof In some
embodiments, the
active agent is sirolimus and wherein the sirolimus has a median size of at
least one of: 1.5 [tin, 2.5
[tin, 645nm, 100-200 nm, another controlled size, or a combination thereof In
some embodiments,
the active agent is sirolimus and wherein the sirolimus has an average size
(mean diameter) of at least
one of: about 1.5 [tin, about 2.5 [tin, about 645nm, about 100-200 nm, another
controlled size, or a
- 87 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
combination thereof In some embodiments, the active agent is sirolimus and
wherein the sirolimus
has a median size of at least one of: about 1.5 [tin, about 2.5 [tin, about
645nm, about 100-200 nm,
another controlled size, or a combination thereof In some embodiments the size
of the active agent is
controlled. For example, in some embodiments, sirolimus is the active agent
and at least 75% of the
sirolimus as is 1.5 [tin, 2.5 [tin, 645nm, 100-200 nm, or another controlled
size. In some
embodiments, sirolimus is the active agent and at least 50% of the sirolimus
as is 1.5 [tin, 2.5 [tin,
645nm, 100-200 nm, or another controlled size. In some embodiments, sirolimus
is the active agent
and at least 90% of the sirolimus as is 1.5 [tin, 2.5 [tin, 645nm, 100-200 nm,
or another controlled
size. In some embodiments, sirolimus is the active agent and at least 95% of
the sirolimus as is 1.5
[tin, 2.5 [tin, 645nm, 100-200 nm, or another controlled size. In some
embodiments, sirolimus is the
active agent and at least 98% of the sirolimus as is 1.5 [tin, 2.5 [tin,
645nm, 100-200 nm, or another
controlled size. In some embodiments, sirolimus is the active agent and at
least 99% of the sirolimus
as is 1.5 [tin, 2.5 [tin, 645nm, 100-200 nm, or another controlled size. The
active agent may be, on
average, at least one of: at most 5 microns, over 1 micrometer, between 1
micrometer and 5
micrometers, about 1.5 micrometers on average, and about 2.5 micrometers on
average.
[00361] In some embodiments, the ratio of the active agent to the
binding agent is controlled.
In some embodiments, the ratio of active agent to binding agent is 1:1, 1:2,
1:3, 1:4, 1:5, 1:10, 1:20,
2:1, 3:1, 4:1, 5:1, 10:1, 15:1, 20:1, 3:2, 2:3, 5:2, 5:3, 2:5, 3:5, or another
controlled ratio.
[00362] In some embodiments, the coating may comprise nanoparticles,
and the nanoparticles
may comprise an active agent and a polymer.
[00363] Multiple coating formulations were coated on balloons 3.0 x 18
or 3.0 x 17 balloons
of GHOST Rapid Exchange (Rx) Catheters and delivered in rabbits to their iliac
arteries. The arterial
tissue of the rabbits was extracted at certain time points up to 72 hours and
the amount of drug found
in the arterial tissue was determined by methods known to one of skill in the
art, such as by HPLC
methods testing the arterial tissue or by UV-Viscometric methods looking at
loss of coating or agent
during the procedure, expressed in ng drug (Sirolimus) per mg of tissue,
indicated in the following
table (sample size indicated therein). In most cases, the necropsy time points
were 5 minutes +/- 5%,
24h +/- 5%, and 72 hrs +/- 5%. For example, the necropsy was conducted about 5
minutes, 24 h, or
72hr after deflation of the second drug coated balloon per animal (+/- 5% of
the time point) where two
vessels were used in the study.
5 minutes 24 hours 72 hours
Formulation Sirolimus Sirolimus Sirolimus
Composition Concentration (ng/mg) Concentration (ng/mg) Concentration
(ng/mg)
Fl 127.9 80.2 (n=20) 0.8 0.9 (n=12) N/A
F2 N/A 10.4 14.7 (n=2) N/A
F3 39.0 11.6 (n=8) 25.2 20.2 (n=10) 4.7 3.7 (n=7)
F4 90.6 59.1 (n=4) 1.3 1.9 (n=2) N/A
F5 6.6 2.1 (n=4) 15.2 27.6 (n=6) BQL
(n=4)
F6 226.0 22.6 (n=2) 5.4 7.6 (n=2) N/A
- 88 -
CA 02888776 2015-04-17
WO 2014/063111 PCT/US2013/065777
F7 97.2 54.7 (n=4) 40.5 25.0 (n=6) 2.7 1.7(n=4)
F8 N/A BQL (n=2) N/A
F9 N/A BQL (n=2) N/A
F10 100.5 17.6 (n=4) 28.4 10.9 (n=6) 10.2 5.6 (n=4)
Fl 1 92.4 18.9 (n=4) 6.4 11.7 (n=6) 3.9 5.7 (n=4)
F12 N/A BQL (n=2) N/A
F13A N/A BQL (n=2) N/A
F13B 74.2 13.1 (n=4) 14.0 11.7 (n=6)* 0.9 1.7
(n=4)
F13C N/A BQL (n=2) N/A
F13D N/A 53.0 5.5 (n=2) N/A
F14A N/A BQL (n=2) N/A
F14B N/A BQL (n=2) N/A
Fl4C Unable to make formulation
F14D N/A BQL (n=2) N/A
F15 114.7 66.2 (n=4) 108.2 119.8 (n=4) 46.5 46.1 (n=4)
F16 73.7 38.5 (n=4) N/A BQL (n=4)
F17 404.5 96.0 (n=4) N/A 0.9 1.1 (n=4)
F18 191.3 40.0 (n=4) N/A 1.4 2.8 (n=4)
*Two studies, were conducted and data was combined here, Study 1: n=2 8.1+/-
5.2, Study 2: n=4
17.0 +/- 13.5.
[00364] The results were calculated as total amount of sirolimus extracted
in the artery, as
indicated in the following table:
Formulation 5 minutes 24 hours 72 hours
Composition Sirolimus amount (11g) Sirolimus amount (11g)
Sirolimus amount (11g)
Fl 4.7 1.6 (n=8)
Fl (2nd lot) 5.9 1.6 (n=12) 0.1 0.1 (n=12)
F2 0.4 0.6 (11=2)
F3 study 1** 2.6 +/- 0.9 (n=4 0.8+/- 0.4 (n=4) 0.3+/- 0.2
(n=4)
F3 study 2** 2.4 +/- 2.3 (n=4) 0.8 +/- 1.0 (n=4) 0.4+/- 0.5
(n=3*)
F3 (1st lot**) 3.0 1.5 (n=6) 0.8 0.5 (n=8) 0.2 0.1
(n=6)
F3 (211d lot**) 1.0 0.4 (n=2) 1.2 1.6 (n=2) 1.0 (n=1)
F4 4.5 3.2 (n=4) 0.07 0.1 (n=2)
F5 0.4 0.2 (11=4) 0.6 1.0 (n=6) BQL (n=4)
F6 8.8 1.2 (11=2) 0.2 0.2 (11=2)
F7 6.2 3.7 (11=4) 2.1 1.3 (n=6) 0.2 0.1 (n=4)
F8 BQL (n=2)
F9 BQL (n=2)
F10 2.0 0.4 (n=4) 1.0 0.8 (n=6) 0.2 0.1 (n=4)
Fl 1 2.3 0.5 (n=4) 0.3 0.5 (n=6) 0.1 0.1 (n=4)
F12 BQL (n=2)
F13A BQL (n=2)
F13B 2.2 0.4 (n=4) 0.4 0.3 (n=6) 0.02 0.03
(n=4)
F13C BQL (n=2)
F13D 0.8 0.2 (n=2)
F14A BQL (n=2)
- 89 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
F14B BQL (n=2)
F14D BQL (n=2)
F15 4.8 0.6 (n=4) 2.0 1.9 (n=4) 1.2
1.0 (n=4)
F16 2.2 0.8 (n=4) BQL (n=4)
F17 9.4 2.7 (n=4) 0.02 0.03 (n=4)
F18 4.4 0.9 (n=4) 0.03 0.07 (n=4)
*one outlier removed
** note that data from F3 was divided two ways for analysis, by study and also
by manufacturing lot, thus the
same results are represented in both groups (study 1,2 and lot 1,2).
[00365] Certain formulations were selected for additional analysis, and
their test results were
normalized for the balloon length and the artery segment size extracted. The
following results were
found for these selected formulations:
Formulation 5 minutes- Sirolimus 24 hours- Sirolimus 72
hours- Sirolimus
Composition Concentration (ng/mg) Concentration (ng/mg) Concentration (ng/mg)
Fl 278.5 112.2 (n=20) 2.3 2.6 (n=12) N/A
F3 97.1 49.3 (n=8) 60.1 39.4 (n=10) 11.9 10.7
(n=7)
F5 19.7 6.4 (n=4) 45.5 82.9 (n=6) BQL (n=4)
F7 291.5 164.0 (n=4) 121.5 75.0 (n=6) 8.0 5.1 (n=4)
F10 167.5 29.4 (n=4) 58.8 33.3 (n=6) 17.1
9.3 (n=4)
Fl 1 153.9 31.4 (n=4) 17.1 34.8 (n=6) 6.5
9.5 (n=4)
F13B 130.9 23.2 (n=4) 24.7 20.6 (n=6) 1.5
3.0 (n=4)
F15 202.5 116.8 (n=4) 190.9 211.5 (n=4) 82.0
81.3 (n=4)
F16 130.0 67.9 (n=4) N/A BQL (n=4)
F17 713.8 169.5 (n=4) N/A 1.6 1.9 (n=4)
F18 337.5 70.6 (n=4) N/A 2.5 5.0 (n=4)
[00366] Certain formulations were selected for another analysis, and
concentration results
were normalized for the artery weight (normalized to 0.025g). The following
results were found for
these selected formulations.
5 minutes- Sirolimus 24 hours- Sirolimus 72 hours- Sirolimus
Formulation Composition
Conc. (ng/mg) Conc. (ng/mg) Conc. (ng/mg)
Fl (LOT 1) (PLGA, Sirolimus 2.5 [tin) 186.5 62.7 (n=8) N/A
N/A
Fl (LOT 2) (PLGA, Sirolimus 2.5 [tin) 234.8 66.0 (n=12) 2.4
2.3 (n=12) N/A
F3 (PLGA, Sirolimus 2.5 lam,
99.0 61.6 (n=8) 36.0
27.3 (n=10) 12.8 13.5 (n=7)
Polyarginine 70 1(Da)
F5 (PLGA, Sirolimus 2.5 [tin, DMAB) 16.3 6.3 (n=4) 23.5
39.9 (n=6) BQL (n=4)
F7 (PLGA, Sirolimus 2.5 [tin, PEI) 248.6 147.7 (n=4)
83.2 53.6 (n=6) 7.6 4.4 (n=4)
F10 (PLGA, Sirolimus 2.5 [tin,
80.3 14.9 (n=4) 38.9 31.8 (n=6)
9.2 3.8 (n=4)
Polyarginine 70 1(Da, PEI)
Fl 1 (PLGA, Sirolimus 2.5 lam, DEAE-
91.5 19.5 (n=4) 10.2 21.3 (n=6)
3.3 4.9 (n=4)
Dextran, TAB)
F13B (PLGA, Sirolimus 645 rim,
87.6 15.4 (n=4) 17.4 13.6 (n=6)
0.6 1.2 (n=4)
Polyarginine 70 1(Da)
F15 (LOT 1) (PLGA, Sirolimus 1.5
192.6 23.6 (n=4) 78.2 76.8 (n=4) 49.8 41.8 (n=4)
[tin, Polyarginine 5-15 1(Da)
- 90 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00367] In the rabbit arterial and blood tests noted in this example,
the following coating
details and formulations were used.
- Fl (Formulation 1) comprised PLGA i.e. about 50:50 Lactic acid: Glycolic
acid, and Sirolimus
having an average size of 2.5 m.
- F2 (Formulation 2) comprised PLGA i.e. about 50:50 Lactic acid: Glycolic
acid, and a 1:1 ratio of
Sirolimus having an average size of 2.5 [tin to Polyarginine 70kDa.
- F3 (Formulation 3) comprised PLGA i.e. about 50:50 Lactic acid: Glycolic
acid, and a 10:1 ratio
of Sirolimus having an average size of 1.5 [tin or 2.5 [tin to Polyarginine
70kDa.
- F4 (Formulation 4) comprised PLGA i.e. about 50:50 Lactic acid: Glycolic
acid, and a 10:1 ratio
of Sirolimus having an average size of 2.5 [tin to DEAE-Dextran
(Diethylaminoethyl cellulose-
Dextran).
- F5 (Formulation 5) comprised PLGA i.e. about 50:50 Lactic acid: Glycolic
acid, and a 1:1 ratio of
Sirolimus having an average size of 2.5 [tin and DMAB
(Didodecyldimethylammonium bromide).
- F6 (Formulation 6) comprised PLGA i.e. about 50:50 Lactic acid: Glycolic
acid, and a 10:1 ratio
of Sirolimus having an average size of 2.5 [tin and DMAB
(Didodecyldimethylammonium
bromide).
- F7 (Formulation 7) comprised PLGA i.e. about 50:50 Lactic acid: Glycolic
acid, and a 10:1 ratio
of Sirolimus having an average size of 2.5 [tin and PEI (Polyethyleneimine).
- F8 (Formulation 8) comprised PLGA i.e. about 50:50 Lactic acid: Glycolic
acid, and a 10:1 ratio
of Sirolimus having an average size of 2.5 [tin and TAB (Tetradodecylammonium
bromide).
- F9 (Formulation 9) comprised PLGA i.e. about 50:50 Lactic acid: Glycolic
acid, and a 10:1 ratio
of Sirolimus having an average size of 2.5 [tin and DMTAB
(Dimethylditetradecylammonium
bromide).
- F10 (Formulation 10) comprised PLGA i.e. about 50:50 Lactic acid:
Glycolic acid, and a 1:1:1
ratio of Sirolimus having an average size of 2.5 [tin to Polyarginine 70kDa to
PEI
(Polyethyleneimine).
- Fll (Formulation 11) comprised PLGA i.e. about 50:50 Lactic acid:
Glycolic acid, and a 10:1:1
ratio of Sirolimus having an average size of 2.5 [tin to TAB
(Tetradodecykammonium bromide to
DEAE-Dextran (Diethylaminoethyl cellulose- Dextran).
- F12 (Formulation 12) comprised PLGA Nanospheres (130nm) where the PLGA is
50:50 Lactic
acid: Glycolic acid, and 6.3% Sirolimus.
- F13A (Formulation 13A) comprised PLGA i.e. about 50:50 Lactic acid:
Glycolic acid, and
Sirolimus having an average size of 645nm.
- F13B (Formulation 13B) comprised PLGA i.e. about 50:50 Lactic acid:
Glycolic acid, and a 10:1
ratio of Sirolimus having an average size of 645nm to Polyarginine 70 kDa.
- 91 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
- F13C (Formulation 13C) comprised PLGA i.e. about 50:50 Lactic acid:
Glycolic acid, and a 1:1
ratio of Sirolimus having an average size of 645nm to DMAB
(Didodecyldimethylammonium
bromide).
- F13D (Formulation 13D) comprised PLGA i.e. about 50:50 Lactic acid:
Glycolic acid, and a 10:1
ratio of Sirolimus having an average size of 645nm to PEI (Polyethyleneimine).
- F14A (Formulation 14A) comprised PLGA i.e. about 50:50 Lactic acid:
Glycolic acid, and
Sirolimus having an average size of 100-200 nm.
- F14B (Formulation 14B) comprised PLGA i.e. about 50:50 Lactic acid:
Glycolic acid, and a 10:1
ratio of Sirolimus having an average size of 100-200 nm to Polyarginine 70
1(Da.
- F14C (Formulation 14C) comprised PLGA i.e. about 50:50 Lactic acid: Glycolic
acid, and a 1:1
ratio of Sirolimus having an average size of 100-200 nm to DMAB
(Didodecyldimethylammonium bromide). Note that this formulation was not able
to be made,
therefore, no animal study results were obtained.
- F14D (Formulation 14D) comprised PLGA i.e. about 50:50 Lactic acid:
Glycolic acid, and a 10:1
ratio of Sirolimus having an average size of 100-200 nm to PEI
(Polyethyleneimine).
- F15 (Formulation 15) comprised PLGA i.e. about 50:50 Lactic acid:
Glycolic acid, and a 10:1
ratio of Sirolimus having an average size of 1.5 [tm to Polyarginine 5-15
1(Da.
- F16 (Formulation 16) comprised PLGA i.e. about 50:50 Lactic acid:
Glycolic acid, and a 10:1
ratio of Sirolimus having an average size of 1.5 [tm to Polyarginine 9-L-pArg.
- F17 (Formulation 17) comprised PLGA i.e. about 50:50 Lactic acid: Glycolic
acid, and a 10:1
ratio of Sirolimus having an average size of 645nm to Polyarginine 5-15 1(Da.
- F18 (Formulation 18) comprised PLGA i.e. about 50:50 Lactic acid:
Glycolic acid, and a 10:1
ratio of Sirolimus having an average size of 645nm to Polyarginine 9-L-pArg.
[00368] With the exception of F12, all methods comprised using an RESS
process for coating
the PLGA on the balloon, and using an eSTAT process for coating the Sirolimus
and the positive
charged molecule to the balloon. The general process for coating was 1)
Polymer coat by RESS
processes, 2) Sirolimus and binding agent coat (or sirolimus alone, if there
was no binding agent used
in the formulation e.g. 14A) by eSTAT processes, 3) sinter the coated balloon.
The binding agent (i.e.
charged particle, surfactant, and/or cationic particle) was part of the
Sirolimus coating step wherein
the balloon was coated with both the Sirolimus and the binding agent using an
eSTAT process.
Formulation 12 was coated on the balloon using only an eSTAT or an RESS
process and a sinter step.
[00369] The sirolimus was mixed with the binding agent (e.g. the
surfactant, the cationic
particle, the charged molecule, for non-limiting example) if present in the
particular formulation in the
following manner. The process may be adapted to different binding agents and
different active
agents, however, it is described herein as used with respect to sirolimus and
the binding agents which
were surfactants in the formulations noted in this example. Lyophilisation or
"freeze drying"
processed produced a dry powder of associated drug and binding agent (e.g.
surfactant) suitable for
- 92 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
depositing onto balloons via the eSTAT method. Other processes familiar to one
of skill in the art
may be used as an alternative to lyophilisation in order to associate the drug
and binding agent in a
form suitable for deposition on the balloons via a method described herein. In
this example,
rapamycin (sirolimus) was suspended in water with a binding agent to coat the
sirolimus with binding
agent. The well-suspended sirolimus and binding agent solution was frozen,
retaining the sirolimus
and binding agent assembly, and the water was removed by sublimation to
produce the dry sirolimus
and binding agent material.
[00370] A pre-lyophilisation set of steps may be used in the process
of creating the dried
sirolimus and binding agent solution for use in the eSTAT coating process. The
solution created
thereby may be used in a freeze dryer. The desired quantity of drug (e.g.
sirolimus) and binding agent
were weighed out into a 100mL Schott bottle. Then 50mL of water is added, in
increments of 10mL,
to the Schott bottle. During each increment the solution is mixed with a stir
rod to insure the sirolimus
is being wetted. After the 50mL is added the solution is sonicated in a bath
sonicator (Branson 1510)
for 1 hr. In the final pre-lyophilisation step, the well-suspended solution is
carefully transferred to a
50mL conical centrifuge tube using a plastic pipette; unsuspended sirolimus
and/or binding agent
particles (typically found floating on the surface of the suspension, are not
transferred. Note: the
efficiency of the sirolimus suspension by the binding agent affects the actual
sirolimus to surfactant
ratio of the transferred solution and the final recovered powder, often
changing it from the initial
sirolimus and binding agent ratio weighed out.
[00371] The Lyophilisation steps may be as follows: The recovered
suspension in the 50mL
centrifuge tube is immersed in liquid nitrogen until the solution is
completely frozen. Parafilm is used
to cover the opening of the tube containing the frozen suspension, while
perforations are made in the
film to allow escape of the vapor phase water. The tube containing the frozen
sample is loaded into a
freeze dryer containment vessel and the vessel is attached to one of the
freeze dryer stations. The
switch above the nozzle for the loaded station is activated to begin the
process. The lyophilisation
step is complete when all of the frozen moisture is visibly absent from the
tube. The sample, which
may exist as a xerogel following lyophilisation, is easily converted to a free-
flowing dry powder by
shaking or stirring when the process is complete. It usually takes 1-2 days
for a sample prepared as
described above to complete the lyophilisation step. Note: the freeze-drier
may need to be
periodically be defrosted to remove the accumulated moisture from the samples
in order to work
effectively.
[00372] The following steps were taken to make the sirolimus and
binding agent dry solution
in the eSTAT coating process of the balloons (which had been pre-coated using
an RESS process with
PLGA as noted elsewhere herein). Measure out required quantities of sirolimus
and binding agent into
a 100mL Schott bottle. Add 50mL of water, in increments of 10mL, to the Schott
bottle. During each
increment use a stir rod to mix the sirolimus and binding agent solution.
After 50mL of water is
- 93 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
added, sonicate the solution for 1 hr. After sonication use a plastic pipette
to transfer the suspended
solution to a 50mL centrifuge tube. Avoid transfer of any unsuspended
sirolimus and binding agent
particles. Place 50mL conical tube (without lid) in liquid nitrogen until
solution is completely frozen.
Cover the top of the conical tube with parafilm and make holes in film for
water to travel through.
Seal the 50mL conical bottle in the lyophilisation vessel and connect the
vessel to a freeze dryer
nozzle station. Turn switch above the nozzle to evacuate the air from the
vessel. Keep sample on the
freeze-drier until all water has been removed (typically 1-2 days)
[00373] Rabbit Blood Concentration results follow. The amount of drug
as a concentration per
mL of blood was determined for several formulations, as indicated in the
following table. BQL herein
means below quantitation limit (1-2ng/m1 with respect to whole blood
quantitation of sirolimus)
Formulation Sirolimus in whole blood (ng/mL)
5 min 24 hours 72 hours
Fl 20.9 8.0 (n=4) N/A N/A
Fl (2nd lot) 5.7 1.6 (n=6) BQL (n=6) N/A
F2 N/A BQL (n=1) N/A
F3 (Study 1)* 8.9 4.4 (n=2) BQL (n=2) BQL (n=2)
F3 (Study 2)* 7.1 2.2 (n=2) BQL (n=2) BQL (n=2)
F3 (1st lot)* 7.7 3.7 (n=3) 0.8 0.7 (n=3) BQL (n=3)
F3 (2nd lot)* 5.5 (n=1) BQL (n=1) BQL (n=1)
F4 N/A BQL (n=1) N/A
F5 0.99 0.1 (n=2) BQL (n=3) BQL (n=2)
F6 N/A 2.72 (n=1) N/A
F7 11.2 3.4 (n=2) 1.7 0.3 (n=3) 1.3 0.9
(n=2)
F8 N/A BQL (n=1) N/A
F9 N/A 2.06 (n=1) N/A
F10 BQL (n=2) 0.36 0.6 (n=3) BQL (n=2)
Fl 1 10.1 0.2 (n=2) BQL (n=3) BQL (n=2)
F12 N/A BQL (n=1) N/A
F13A N/A 1.13 (n=1) N/A
F13B 6.4 3.1 (n=2) 1.0 0.9 (n=3)** BQL (n=2)
F13C N/A BQL (n=1) N/A
F13D N/A 2.99 (n=1) N/A
F14A N/A BQL (n=1) N/A
F14B N/A BQL (n=1) N/A
F14D N/A BQL (n=1) N/A
F15 5.7 3.2 (n=2) 1.8 0.01 (n=2) BQL (n=2)
F16 76.8 89.4 (n=2) N/A BQL (n=2)
F17 25.6 1.3 (n=2) N/A BQL (n=2)
F18 23.5 3.3 (n=2) N/A 0.5 0.8 (n=2)
* note that data from F3 was divided two ways for analysis, by study and also
by manufacturing lot, thus the
same results are represented in both groups (study 1,2 versus lot 1,2).
** This data represents two lots of coated balloons, for one of the lots the
sirolimus in whole blood
(ng/mL) results were: (n=2), 0.7 +/- 1.0
- 94 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
The amount of drug as a total amount found in the arterial tissue was
determined for several
formulations, as indicated in the following table.
Formulation Sirolimus in whole blood (Kg) based on 56mL blood
per kg
min 24 hours 72 hours
Fl 5.4 2.3 (n=4) N/A N/A
Fl (2nd lot) 1.0 0.3 (n=6) BQL (n=6) N/A
F2 N/A BQL (n=1) N/A
F3 (Study 1)* 1.5 0.7 (n=3) BQL (n=2) BQL (n=2)
F3 (Study 2)* 1.6 0.5 (n=1) BQL (n=2) BQL (n=2)
F3(1st lot)* 1.7 0.6 (n=3) 0.1 0.1 (n=3) BQL (n=3)
F3 (2nd lot)* 1.2 (n=1) BQL (n=1) BQL (n=1)
F4 N/A BQL (n=1) N/A
F5 0.18 0.2 (n=2) BQL (n=3) BQL (n=2)
F6 0.4 (n=1) N/A
F7 1.9 0.6 (n=2) 0.3 0.1 (n=3) 0.25 0.01
(n=2)
F8 N/A BQL (n=1) N/A
F9 N/A 0.3 (n=1) N/A
F10 BQL (n=2) 0.1 0.1 (n=3) BQL (n=2)
Fl 1 2.0 0.05 (n=2) BQL (n=3) BQL (n=2)
F12 N/A BQL (n=1) N/A
F13A N/A 0.2 (n=1) N/A
F13B 1.3 0.7 (n=2) 0.2 0.2 (n=3) BQL (n=2)
F13C N/A BQL (n=1) N/A
F13D N/A 0.6 (n=1) N/A
F14A N/A BQL (n=1) N/A
F14B N/A BQL (n=1) N/A
F14D N/A BQL (n=1) N/A
F15 1.2 0.7 (n=2) 0.4 0.0 (n=2) BQL (n=2)
F16 18.7 21.9 (n=2) N/A BQL (n=2)
F17 6.2 0.3 (n=2) N/A BQL (n=2)
F18 5.5 0.6 (n=2) N/A 0.12 0.17 (n=2)
* note that data from F3 was divided two ways for analysis, by study and also
by manufacturing lot, thus the
same results are represented in both groups (study 1,2 versus lot 1,2).
5
[00374] The various formulations had the following average amount of
sirolimus coated on
each of the balloons tested in the rabbit arteries. These were average amounts
of drug found on
sample balloons coated according to the same procedures noted herein and from
the same lots and
batches as those tested in the rabbits as noted above. The amount of sirolimus
coated on the balloon
is the average sirolimus concentration based on UV-Vis analysis before
pleating, folding, and
sterilization of the balloons.
- 95 -
CA 02888776 2015-04-17
WO 2014/063111 PCT/US2013/065777
Formulation Sirolimus Coated on Balloons (11g)
Fl 64.52 8.73
Fl (2nd lot) 63.40 2.89
F2 41.05 7.17
F3 89.54 19.61
F3 (2nd lot) 128.71 26.86
F4 115.12 16.92
F5 68.49 4.73
F6 316.95 82.66
F7 165.25 17.47
F8 97.19 16.46
F9 218.86 26.73
F10 65.38 24.45
Fl 1 170.66 14.30
F12 74.20 15.77
F13A 134.23 17.03
F13B 144.63 51.84
F13C 55.46 13.14
F13D 105.31 16.02
F14A 83.10 15.19
F14B 175.96 78.30
F14D 77.50 31.02
F15 106.53 22.55
F16 75.84 5.98
F17 197.64 15.89
F18 196.43 45.89
[00375]
Following expansion of the balloons in the rabbit arteries, each of the
balloons was
removed from the animal and the residual sirolimus on each balloon was
determined. Using the 5
minute data as an indication of the amount (and therefore percent) of
sirolimus transferred to the
artery, and using the amount of drug remaining on the balloon following the
procedure, and using the
average amount of drug on balloons of the same batch as an estimate of the
total amount of drug on
the original device (see the previous table), the percent of sirolimus
transferred to the rabbit artery and
the total percent of sirolimus released during the entire procedure was
determined. The following
table summarizes the results from the formulations tested in this manner.
- 96 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
Formulation Sirolimus on % Sirolimus Transferred to
% Sirolimus
Balloon Post- Artery (5 min) From Sirolimus
Released
Deployment (11g) Released off Respective Balloon
Fl 12.8 4.0 (n=8) 9.8 3.1% (n=8) 78.9 6.8% (n=8)
Fl (2nd lot) 45.7 7.3 (n=36) 23.0 8.5% (n=12)
35.9 10.2% (n=36)
F2 6.1 2.7 (n=2) N/A 85.1 3.6% (n=2)
F3 Study 1* 25.8 9.6 (n=12) N/A
68.8 16.2%(n=12)
F3 Study 2* N/A
28.1 4.9 (n=6) 73.1 7.6% (n=6)
(2.5 [tin)
F3 (1st lot)* 27.6 9.1 (n=20) 4.8 1.4% (n=6)
68.4 15.4% (n=20)
F3 (2nd lot-
56.8 15.6 (n=6) 1.2 0.6% (n=2) 59.0 8.6%(n=6)
1.511m)*
F4 16.0 2.9 (n=6) 5.1 3.5% (n=4) 84.9 2.5% (n=6)
F5 5.0 2.7 (11=14) 0.6 0.2% (n=4) 92.8 3.7% (n=14)
F6 49.4 6.9 (n=4) 3.5 0.2% (n=2) 84.4 3.7% (n=4)
F7 7.1 3.0 (n=14) 3.9 2.4% (n=4) 95.7 1.7%(n=14)
F8 20.5 0.1 (n=2) N/A 78.1 3.8%(n=2)
F9 37.0 4.1 (n=2) N/A 82.6 0.3%(n=2)
F10 1.4 0.8 (n=14) 4.0 0.3% (n=4)
98.1 0.8% (n=14)
Fl 1 43.6 11.2 (n=14) 1.7 0.4% (n=4)
74.4 6.9%(n=14)
F12 2.3 0.8 (n=2) N/A 97.1 1.0% (n=2)
F13A 21.6 1.0 (n=2) N/A 85.0 0.8%(n2)
F13B 30.4 7.4 (n=14) 3.8 0.6% (n=4) 76.5 7.2% (n=14)
F13C 2.1 0.1 (n=2) N/A 96.6 0.0%(n2)
F13D 9.2 2.6 (n=2) N/A 92.1 2.3% (n=2)
F14A 11.6 0.8 (n=2) N/A 87.5 0.3%(n=2)
F14B 16.4 0.6 (11=2) N/A 91.9 0.8%(n2)
F14D 1.7 0.1 (n=2) N/A 98.5 0.1% (n=2)
F15 24.9 5.6 (n=12) 5.3 0.7% (n=4)
76.4 6.3% (n=12)
F16 21.4 3.9 (n=12) 4.2 1.6% (n=4)
72.0 4.6%(n=12)
F17 49.3 6.9 (n=12) 6.6 1.9% (n=4)
74.6 3.6%(n=12)
F18 44.5 8.7 (n=12) 2.5 0.8% (n=4)
76.4 3.3% (n=12)
* note that several lots of coated balloons were manufactured and tested in
several studies, and the
data presented represents data from at two studies and from at two lots. Some
data is represented,
thus, both in a study and also in a lot listing in the above chart (i.e.
coated balloons from
manufacturing lot 1 were tested in both Study 1 and Study 2, and thus the
results are presented in
groups F3 Study 1, F3 Study 2, and F3 1st lot above).
[00376] The following summary observations may be made with regard to
the Rabbit arterial
and blood testing noted in this Example. Formulation 15 has the most drug
retention at 72 hours of
any other formulation. Formulation 3 had a sirolimus retention of 3.9 +/- 3.4
ng/mg at 72 hours (both
lots combined), and 3.2 % of the drug released from the balloons (both lots
combined) was retained in
- 97 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
the artery five minutes after expansion of the balloon in the artery.
Formulation 13B had a sirolimus
retention of 0.9 +/- 1.7 ng/mg at 72 hours, and 3.8 % of the drug released
from the balloons was
retained in the artery five minutes after expansion of the balloon in the
artery. Formulation 15 had a
sirolimus retention of 46.5 +/- 46.1 ng/mg at 72 hours, and 5.3 % of the drug
released from the
balloons was retained in the artery five minutes after expansion of the
balloon in the artery.
[00377] Additonal findings were as follows, which demonstrate for
certain formulations the
Tissue concentrations versus the total amount of sirolimus per artery.
Sirolimus tissue levels as an
absolute amount instead of a concentration removes experimental variability in
the specific amount of
tissue harvested in the necropsy procedures.
Sirolimus Concentration in Artery (ng/mg) Total Sirolimus per Artery (Kg)
F3 Lot 1(5 minutes) 45.8 11.2 (n=4) 2.6 0.9 (n=4)
F3 Lot 2 (5 minutes) 32.3 8.0 (n=4) 2.4 2.3 (n=4)
F 13B (5 minutes) 74.2 13.1 (n=4) 2.2 0.4 (n=4)
F 15(5 minutes) 114.7 66.2 (n=4) 4.8 0.6 (n=4)
F3 Lot 1(24 h) 16.6 8.5 (n=4) 0.8 0.4 (n=4)
F3 Lot 2 (24h) 31.5 30.9 (n=4) 0.8 1.0 (n=4)
F 13B (24h) 17.0 13.5 (n=4) 0.6 0.3 (n=4)
F 15 (24h) 108.2 119.8 (n=4) 2.0 1.9 (n=4)
F3 Lot 1 (72h) 5.2 4.2 (n=4) 0.3 0.2 (n=4)
F3 Lot 2 (72h) 3.9 3.4 (n=3)* 0.4 0.5 (n=3)*
F 13B (72h) 0.9 1.7 (n=4) 0.02 0.03 (n=4)
F 15 (5 minutes) 46.5 46.1 (n=4) 1.2 1.1 (n=4)
.. * Excludes outlier of 137 ng/mg or 4.99 total [tg at 72 hours
[00378] In some embodiments of the methods, coatings, or devices
provided herein, the
coating comprises and a 10:1 ratio of the active agent to the binding agent,
wherein the active agent
comprises sirolimus wherein the binding agent comprises Polyarginine. In some
embodiments, the
sirolimus has an average size of 1.5 [tin or 2.5 m. In some embodiments, the
Polyarginine average
molecular weight is 701(Da. In some embodiments, the Polyarginine average
molecular weight is 5-
151(Da. In some embodiments, the active agent and the binding agent are
deposited on the balloon
together using an eSTAT coating process. In some embodiments, the active agent
and the binding
agent are lyophilized prior to deposition on the balloon. In some embodiments,
at least about 2 ng/mg
of active agent are found in arterial tissue 72 hours after inflation of the
balloon in the artery. In some
embodiments, at least about 3 ng/mg of active agent are found in arterial
tissue 72 hours after inflation
of the balloon in the artery. In some embodiments, at least about 5 ng/mg of
active agent are found in
arterial tissue 72 hours after inflation of the balloon in the artery. In some
embodiments, at least
about 10 ng/mg of active agent are found in arterial tissue 72 hours after
inflation of the balloon in the
artery. In some embodiments, at least about 20 ng/mg of active agent are found
in arterial tissue 72
hours after inflation of the balloon in the artery. In some embodiments, at
least about 30 ng/mg of
active agent are found in arterial tissue 72 hours after inflation of the
balloon in the artery. In some
- 98 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
embodiments, at least about 40 ng/mg of active agent are found in arterial
tissue 72 hours after
inflation of the balloon in the artery.
[00379] In some embodiments of the methods, coatings, or devices
provided herein, in vivo
measurement comprises inflating the balloon inside the artery of a porcine for
about 1 minute and
wherein the amount of active agent transferred to the artery is measured by UV-
Vis evaluation of the
coating remaining on the balloon as determined about five minutes after
inflation of the balloon in the
artery. In some embodiments of the methods, coatings, or devices provided
herein, in vivo
measurement comprises inflating the balloon inside the artery of a porcine for
about 1 minute and
wherein the amount of active agent transferred to the artery is measured by
extracting the artery about
five minutes after inflation of the balloon in the artery and determining the
amount of drug in the
extracted artery using standard methods described herein and/or known to one
of skill in the art. In
some embodiments of the methods, coatings, or devices provided herein, in vivo
measurement
comprises inflating the balloon inside the artery of a rabbit for about 1
minute and wherein the amount
of active agent transferred to the artery is measured by UV-Vis evaluation of
the coating remaining on
the balloon as determined about five minutes after inflation of the balloon in
the artery. In some
embodiments of the methods, coatings, or devices provided herein, in vivo
measurement comprises
inflating the balloon inside the artery of a rabbit for about 1 minute and
wherein the amount of active
agent transferred to the artery is measured by extracting the artery about
five minutes after inflation of
the balloon in the artery and determining the amount of drug in the extracted
artery using standard
methods described herein and/or known to one of skill in the art.
[00380] Provided herein is a method of forming a coating on a medical
device comprising
depositing a polymer on the medical device using an RESS process, mixing a
binding agent and active
agent to create an active agent-binding agent mixture, lyophilizing the active
agent-binding agent
mixture and depositing the active agent-binding agent mixture on the medical
device using an eSTAT
process. In some embodiments, the binding agent comprises a surfactant.
[00381] Pharmacokinetic Studies in Porcine models:
[00382] Formulation 3 (F3) was coated on balloons of 3.0x17 Ghost
Rapid Exchange (Rx)
Catheters according to the procedures noted above and delivered in porcine to
their coronary and
mammary arteries. The animals were sacrificed and arterial tissue was
extracted at several time
points. The amount of drug found in the coronary arterial tissue was
determined and is expressed in
ng drug (Sirolimus) per mg of tissue, and is expressed in normalized form,
i.e. normalized per mg of
tissue and expressed in micrograms (m) in the following table.
- 99 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
Arterial
Sirolimus Total Sirolimus
Time Point SD SD
Concentration per Artery (11g)
(ng/mg)
Day 1: Coronary (n=5) 5.528 4.806 0.3647 0.3056
Day 3: Coronary (n=6) 2.559 2.927 0.1436 0.1402
Day 7: Coronary (n=5) 1.141 1.324 0.0948 0.1375
Day 14: Coronary (n=5) 0.764 0.858 0.0645 0.0940
Day 30: Coronary (n=5) 0.038 0.085 0.0013 0.0029
[00383] The amount of drug found in the mammary arterial tissue was
determined and is
expressed in ng drug (Sirolimus) per mg of tissue, and is expressed in
normalized form, i.e.
normalized per mg of tissue and expressed in micrograms (m) in the following
table.
Arterial
Sirolimus Total Sirolimus
Time Point SD SD
Concentration per Artery (11g)
(ng/mg)
Day 1: Mammary (n=5) 2.722 2.931 0.1303 0.1285
Day 3: Mammary (n=4) 0.243 0.386 0.0129 0.0200
Day 7: Mammary (n=9) 0.277 0.648 0.0100 0.0225
Day 14: Mammary (n=4) 0.105 0.066 0.0058 0.0037
Day 30: Mammary (n=9) 0.030 0.090 0.0014 0.0043
[00384] A comparison was performed between arterial drug retention in
a rabbit versus the
porcine model, using the F3 formulation as described above. The comparison
indicated that at Day 1,
the Rabbit Iliac artery concentration of sirolimus was 25.20 +/- 20.20 in ng
sirolimus per mg tissue, or
0.901 mg +/- 0.684 mg when normalized by the amount of tissue in the sample
(n=7-10). At the same
time point at Day 1, the Porcine Coronary artery concentration of sirolimus
was 5.528 +/- 4.806 in ng
sirolimus per mg tissue, or 0.365 mg +/- 0.306 mg when normalized by the
amount of tissue in the
sample (n=5-6). At Day 3, the rabbit iliac artery concentration of sirolimus
was 4.66 +/- 3.65 in ng
sirolimus per mg tissue, or 0.319 mg +/- 0.338 mg when normalized by the
amount of tissue in the
sample. At the same time point at Day 3, the porcine coronary artery
concentration of sirolimus was
2.559 +/- 2.927 in ng sirolimus per mg tissue, or 0.144 mg +/- 0.144 mg when
normalized by the
amount of tissue in the sample.
[00385] Several formulations that were selected for 72-hour testing in
the rabbit iliac model
were submitted for elution testing using a standard elution method. Figure 1
indicates the Average
percent Sirolimus Eluted from the balloons at various time points for
Formulations F3, F5, and F7. At
time 0 days, the F5 is the highest percent elution at about 60%, and the F3
elution is the next highest
data point at about 45% elution at 0 days, whereas F7 is the lowest line
throughout all time points, at
about 30% eluted at 0 days. The line for F5 is the top line of the graph,
eluting the fastest of the three
- 100 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
formulations indicated in the graph, whereas F3 is the middle line of the
graph, and F7 eluting the
slowest only reaching 100% elution at about day 13.
[00386] The coating may release the active agent into a treatment site
over at least one of:
about 3 days, about 5 days, about 1 week, about 1.5 weeks, about 2 weeks,
about 14 days, about 3
weeks, about 21 days, about 4 weeks, about 28 days, about 1 month, about 1.5
months, about 2
months, at least about 3 days, at least about 5 days, at least about 1 week,
at least about 1.5 weeks, at
least about 2 weeks, at least about 14 days, at least about 3 weeks, at least
about 21 days, at least
about 4 weeks, at least about 28 days, at least about 1 month, at least about
1.5 months, at least about
2 months, about 7 to about 14 days, about 14 to about 21 days, about 14 to
about 28 days, about 21 to
about 28 days, and about 7 to about 28 days.
[00387] Provided herein is a coated medical device comprising: a
medical device for
delivering encapsulated active agent to a treatment site; and a coating on the
medical device
comprising the encapsulated active agent wherein the encapsulated active agent
comprise active agent
encapsulated in a polymer, and wherein the encapsulated active agent has a
positive surface charge.
[00388] Provided herein is a coated medical device comprising: a medical
device for
delivering encapsulated active agent to a treatment site; and a coating on the
medical device
comprising the encapsulated active agent wherein the encapsulated active agent
comprise a polymer
that encapsules at least a portion of an active agent, and wherein the
encapsulated active agent has a
positive surface charge.
[00389] In some embodiments, the active agent is not completely
encapsulated. An active
agent (or a portion thereof) need not be completely surrounded in order to be
encapsulated by the
polymer. In some embodiments, at least 10% of the surface area of the active
agent is encapsulated in
the polymer. In some embodiments, at least 20% of the surface area of the
active agent is
encapsulated in the polymer. In some embodiments, at least 25% of the surface
area of the active
agent is encapsulated in the polymer. In some embodiments, at least 30% of the
surface area of the
active agent is encapsulated in the polymer. In some embodiments, at least 40%
of the surface area of
the active agent is encapsulated in the polymer. In some embodiments, at least
50% of the surface area
of the active agent is encapsulated in the polymer. In some embodiments, at
least 60% of the surface
area of the active agent is encapsulated in the polymer. In some embodiments,
at least 70% of the
.. surface area of the active agent is encapsulated in the polymer. In some
embodiments, at least 75% of
the surface area of the active agent is encapsulated in the polymer. In some
embodiments, at least 80%
of the surface area of the active agent is encapsulated in the polymer. In
some embodiments, at least
90% of the surface area of the active agent is encapsulated in the polymer. In
some embodiments, at
least 95% of the surface area of the active agent is encapsulated in the
polymer. In some
embodiments, at least one of: at least 5% of the surface area of the active
agent is at least partially
surrounded by the polymer, at least 10% of the surface area of the active
agent is at least partially
- 101 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
surrounded by the polymer, at least 15% of the surface area of the active
agent is at least partially
surrounded by the polymer, at least 20% of the surface area of the active
agent is at least partially
surrounded by the polymer, at least 25% of the surface area of the active
agent is at least partially
surrounded by the polymer, at least 30% of the surface area of the active
agent is at least partially
surrounded by the polymer, at least 40% of the surface area of the active
agent is at least partially
surrounded by the polymer, at least 50% of the surface area of the active
agent is at least partially
surrounded by the polymer, at least 60% of the surface area of the active
agent is at least partially
surrounded by the polymer, at least 70% of the surface area of the active
agent is at least partially
surrounded by the polymer, at least 75% of the surface area of the active
agent is at least partially
surrounded by the polymer, at least 80% of the surface area of the active
agent is at least partially
surrounded by the polymer, at least 90% of the surface area of the active
agent is at least partially
surrounded by the polymer, and at least 95% of the surface area of the active
agent is at least partially
surrounded by the polymer.
[00390] Provided herein is a coating for a medical device comprising
encapsulated active
agent comprising active agent encapsulated in a polymer, wherein the
encapsulated active agent has a
positive surface charge, and wherein the coating delivers active agent to a
treatment site over at least
about 1 day.
[00391] Provided herein is a method of forming a coating on a medical
device comprising
providing encapsulated active agent comprising a polymer and active agent,
wherein the encapsulated
active agent have a positive surface charge, depositing the encapsulated
active agent on the medical
device. In some embodiments, the coating delivers the active agent to the
treatment site over at least
about 1 day.
[00392] Provided herein is a method of forming a coating on a medical
device comprising
providing encapsulated active agent comprising a polymer at least partially
encapsulating at least a
portion of an active agent wherein the encapsulated active agent has a
positive surface charge, and
depositing the encapsulated active agent on the medical device. In some
embodiments, the coating
delivers the active agent to the treatment site over at least about 1 day.
[00393] Provided herein is a coated medical device comprising: a
medical device for
delivering an active agent to a treatment site; and a coating on the device
comprising the active agent,
wherein the coated medical device delivers at least a portion of the coating
to the treatment site which
portion releases active agent into the treatment site over at least about 1
day.
[00394] Provided herein is a coating for a medical device comprising
an active agent, wherein
the coating delivers the into a treatment site over at least about 1 day.
[00395] Provided herein is a method of forming coating on a medical
device with of an active
agent comprising depositing the active agent on the medical device using an
eSTAT process.
- 102 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00396] In some embodiments of the devices, coatings and/or methods
provided herein the
polymer comprises PLGA. The PLGA may have at least one of: a MW of about 30KDa
and a Mn of
about 15KDa, a Mn of about 10KDa to about 25 KDa, and a MW of about 15 KDa to
about 40KDa.
[00397] In some embodiments of the methods and/or devices provided
herein, the coating
comprises a bioabsorbable polymer. In some embodiments, the active agent
comprises a
bioabsorbable polymer. In some embodiments, the bioabsorbable polymer
comprises at least one of:
Polylactides (PLA); PLGA (poly(lactide-co-glycolide)); Polyanhydrides;
Polyorthoesters; Poly(N-(2-
hydroxypropyl) methacrylamide); DLPLA ¨ poly(dl-lactide); LPLA ¨ poly(1-
lactide); PGA ¨
polyglycolide; PDO ¨ poly(dioxanone); PGA-TMC ¨ poly(glycolide-co-trimethylene
carbonate);
PGA-LPLA ¨ poly(1-lactide-co-glycolide); PGA-DLPLA ¨ poly(dl-lactide-co-
glycolide); LPLA-
DLPLA ¨ poly(1-lactide-co-dl-lactide); and PDO-PGA-TMC ¨ poly(glycolide-co-
trimethylene
carbonate-co-dioxanone), and combinations, copolymers, and derivatives thereof
In some
embodiments, the bioabsorbable polymer comprises between 1% and 95% glycolic
acid content
PLGA-based polymer.
[00398] In some embodiments of the methods and/or devices provided herein,
the polymer
comprises at least one of polycarboxylic acids, cellulosic polymers, proteins,
polypeptides,
polyvinylpyrrolidone, maleic anhydride polymers, polyamides, polyvinyl
alcohols, polyethylene
oxides, glycosaminoglycans, polysaccharides, polyesters, aliphatic polyesters,
polyurethanes,
polystyrenes, copolymers, silicones, silicone containing polymers, polyalkyl
siloxanes,
polyorthoesters, polyanhydrides, copolymers of vinyl monomers, polycarbonates,
polyethylenes,
polypropytenes, polylactic acids, polylactides, polyglycolic acids,
polyglycolides, polylactide-co-
glycolides, polycaprolactones, poly(e-caprolactone)s, polyhydroxybutyrate
valerates,
polyacrylamides, polyethers, polyurethane dispersions, polyacrylates, acrylic
latex dispersions,
polyacrylic acid, polyalkyl methacrylates, polyalkylene-co-vinyl acetates,
polyalkylenes, aliphatic
polycarbonates polyhydroxyalkanoates, polytetrahalooalkylenes,
poly(phosphasones),
polytetrahalooalkylenes, poly(phosphasones), and mixtures, combinations, and
copolymers thereof
The polymers of the present invention may be natural or synthetic in origin,
including gelatin,
chitosan, dextrin, cyclodextrin, Poly(urethanes), Poly(siloxanes) or
silicones, Poly(acrylates) such as
[rho]oly(methyl methacrylate), poly(butyl methacrylate), and Poly(2-hydroxy
ethyl methacrylate),
Poly( vinyl alcohol) Poly(olefins) such as poly(ethylene), [rho]oly(isoprene),
halogenated polymers
such as Poly(tetrafluoroethylene) - and derivatives and copolymers such as
those commonly sold as
Teflon(R) products, Poly(vinylidine fluoride), Poly(vinyl acetate), Poly(vinyl
pyrrolidone),
Poly(acrylic acid), Polyacrylamide, Poly(ethylene-co-vinyl acetate),
Poly(ethylene glycol),
Poly(propylene glycol), Poly(methacrylic acid); etc. Suitable polymers also
include absorbable
and/or resorbable polymers including the following, combinations, copolymers
and derivatives of the
following: Polylactides (PLA), Polyglycolides (PGA), PolyLactide-co-glycolides
(PLGA),
- 103 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
Polyanhydrides, Polyorthoesters, Poly(N-(2- hydroxypropyl) methacrylamide),
Poly(1-aspartamide),
including the derivatives DLPLA ¨ poly(dl-lactide); LPLA ¨ poly(1-lactide);
PDO ¨
poly(dioxanone); PGA-TMC ¨ poly(glycolide-co-trimethylene carbonate); PGA-LPLA
¨ poly(1-
lactide-co-glycolide); PGA-DLPLA ¨ poly(dl-lactide-co-glycolide); LPLA-DLPLA ¨
poly(1-
lactide-co-dl-lactide); and PDO-PGA-TMC ¨ poly(glycolide-co-trimethylene
carbonate-co-
dioxanone), and combinations thereof
[00399] In some embodiments of the methods and/or devices provided
herein, the polymer
has a dry modulus between 3,000 and 12,000 KPa. In some embodiments, the
polymer is capable of
becoming soft after implantation. In some embodiments, the polymer is capable
of becoming soft
after implantation by hydration, degradation or by a combination of hydration
and degradation. In
some embodiments, the polymer is adapted to transfer, free, and/or dissociate
from the substrate when
at the intervention site due to hydrolysis of the polymer.
[00400] In some embodiments of the methods and/or devices provided
herein, the
bioabsorbable polymer is capable of resorbtion in at least one of: about 1
day, about 3 days, about 5
days, about 7 days, about 14 days, about 3 weeks, about 4 weeks, about 45
days, about 60 days, about
90 days, about 180 days, about 6 months, about 9 months, about 1 year, about 1
to about 2 days,
about 1 to about 5 days, about 1 to about 2 weeks, about 2 to about 4 weeks,
about 45 to about 60
days, about 45 to about 90 days, about 30 to about 90 days, about 60 to about
90 days, about 90 to
about 180 days, about 60 to about 180 days, about 180 to about 365 days, about
6 months to about 9
months, about 9 months to about 12 months, about 9 months to about 15 months,
and about 1 year to
about 2 years.
[00401] In some embodiments of the methods and/or devices provided
herein, the coating
comprises a microstructure. In some embodiments, particles of the active agent
are sequestered or
encapsulated within the microstructure. In some embodiments, the
microstructure comprises
microchannels, micropores and/or microcavities. In some embodiments, the
microstructure is
selected to allow sustained release of the active agent. In some embodiments,
the microstructure is
selected to allow controlled release of the active agent.
[00402] In some embodiments of the devices, coatings and/or methods
provided herein the
coating comprises a positive surface charge. The positive surface charge may
be about 20 mV to
about 40mV. The positive surface charge may be at least one of: at least about
1 mV, over about 1
mV, at least about 5 mV, at least about 10 mV, about 10 mV to about 50 mV,
about 20 mV to about
50 mV, about 10 mV to about 40 mV, about 30 mV to about 40 mV, about 20 mV to
about 30 mV,
and about 25 mV to about 35 mV.
[00403] In some embodiments of the devices, coatings and/or methods
provided herein, the
w/w percent of active agent in the encapsulated active agent is about 5%. In
some embodiments of the
- 104 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
devices, coatings and/or methods provided herein, the w/w percent of active
agent in the encapsulated
active agent is about 10-25%.
[00404] In some embodiments, the encapsulated active agent comprises a
polymer at least
partially encapsulating at least a portion of an active agent wherein the
encapsulated active agent has a
positive surface charge. In some embodiments, the active agent is not
completely encapsulated. An
active agent (or a portion thereof) need not be completely surrounded in order
to be encapsulated by
the polymer. In some embodiments, at least 10% of the surface area of the
active agent is
encapsulated in the polymer. In some embodiments, at least 20% of the surface
area of the active
agent is encapsulated in the polymer. In some embodiments, at least 25% of the
surface area of the
active agent is encapsulated in the polymer. In some embodiments, at least 30%
of the surface area of
the active agent is encapsulated in the polymer. In some embodiments, at least
40% of the surface area
of the active agent is encapsulated in the polymer. In some embodiments, at
least 50% of the surface
area of the active agent is encapsulated in the polymer. In some embodiments,
at least 60% of the
surface area of the active agent is encapsulated in the polymer. In some
embodiments, at least 70% of
.. the surface area of the active agent is encapsulated in the polymer. In
some embodiments, at least 75%
of the surface area of the active agent is encapsulated in the polymer. In
some embodiments, at least
80% of the surface area of the active agent is encapsulated in the polymer. In
some embodiments, at
least 90% of the surface area of the active agent is encapsulated in the
polymer. In some
embodiments, at least 95% of the surface area of the active agent is
encapsulated in the polymer. In
some embodiments, at least one of: at least 5% of the surface area of the
active agent is at least
partially surrounded by the polymer, at least 10% of the surface area of the
active agent is at least
partially surrounded by the polymer, at least 15% of the surface area of the
active agent is at least
partially surrounded by the polymer, at least 20% of the surface area of the
active agent is at least
partially surrounded by the polymer, at least 25% of the surface area of the
active agent is at least
partially surrounded by the polymer, at least 30% of the surface area of the
active agent is at least
partially surrounded by the polymer, at least 40% of the surface area of the
active agent is at least
partially surrounded by the polymer, at least 50% of the surface area of the
active agent is at least
partially surrounded by the polymer, at least 60% of the surface area of the
active agent is at least
partially surrounded by the polymer, at least 70% of the surface area of the
active agent is at least
partially surrounded by the polymer, at least 75% of the surface area of the
active agent is at least
partially surrounded by the polymer, at least 80% of the surface area of the
active agent is at least
partially surrounded by the polymer, at least 90% of the surface area of the
active agent is at least
partially surrounded by the polymer, and at least 95% of the surface area of
the active agent is at least
partially surrounded by the polymer.
[00405] In some embodiments of the devices, coatings and/or methods
provided herein, at
least a portion of the encapsulated active agent are nanoparticles. At least a
portion of the
encapsulated active agent may be at least one of: a spherical shape, a
discoidal shape, a hemispherical
- 105 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
shape, a cylindrical shape, a conical shape, a nanoreef shape, a nanobox
shape, a cluster shape, a
nanotube shape, a whisker shape, a rod shape, a fiber shape, a cup shape, a
jack shape, a hexagonal
shape, an ellipsoid shape, an oblate ellipsoid shape, a prolate ellipsoid
shape, a torus shape, a spheroid
shape, a taco-like shape, a bullet shape, a barrel shape, a lens shape, a
capsule shape, a pulley wheel
shape, a circular disc shape, a rectangular disc shape, a hexagonal disc
shape, a flying saucer-like
shape, a worm shape, a ribbon-like shape, and a ravioli-like shape.
[00406] The active agent in some embodiments of the devices, coatings
and/or methods
provided herein comprises a macrolide immunosuppressive drug. The active agent
may be selected
from sirolimus, a prodrug, a hydrate, an ester, a salt, a polymorph, a
derivative, and an analog thereof
A portion of the active agent may be in crystalline form.
[00407] The active agent may be, on average, at least one of: at most
5 microns, over 1
micrometer, between 1 micrometer and 5 micrometers, about 1.5 micrometers on
average, and about
2.5 micrometers on average. In some embodiments, the size of the active agent
in the coating is
controlled in order to improve drug retention in the artery. For non-limiting
example, in the case of
sirolimus as an active agent, the sirolimus may have an average size (mean
diameter) of at least one
of: 1.5 [tin, 2.5 [tin, 645nm, 100-200 nm, another controlled size, or a
combination thereof In some
embodiments, the active agent is sirolimus and wherein the sirolimus has a
median size of at least one
of: 1.5 [tin, 2.5 [tin, 645nm, 100-200 nm, another controlled size, or a
combination thereof In some
embodiments, the active agent is sirolimus and wherein the sirolimus has an
average size (mean
diameter) of at least one of: about 1.5 [tin, about 2.5 [tin, about 645nm,
about 100-200 nm, another
controlled size, or a combination thereof In some embodiments, the active
agent is sirolimus and
wherein the sirolimus has a median size of at least one of: about 1.5 [tin,
about 2.5 [tin, about 645nm,
about 100-200 nm, another controlled size, or a combination thereof In some
embodiments the size
of the active agent is controlled. For example, in some embodiments, sirolimus
is the active agent and
at least 75% of the sirolimus as is 1.5 [tin, 2.5 [tin, 645nm, 100-200 nm, or
another controlled size. In
some embodiments, sirolimus is the active agent and at least 50% of the
sirolimus as is 1.5 [tin, 2.5
[tin, 645nm, 100-200 nm, or another controlled size. In some embodiments,
sirolimus is the active
agent and at least 90% of the sirolimus as is 1.5 [tin, 2.5 [tin, 645nm, 100-
200 nm, or another
controlled size. In some embodiments, sirolimus is the active agent and at
least 95% of the sirolimus
as is 1.5 [tin, 2.5 [tin, 645nm, 100-200 nm, or another controlled size. In
some embodiments,
sirolimus is the active agent and at least 98% of the sirolimus as is 1.5
[tin, 2.5 [tin, 645nm, 100-200
nm, or another controlled size. In some embodiments, sirolimus is the active
agent and at least 99% of
the sirolimus as is 1.5 [tin, 2.5 [tin, 645nm, 100-200 nm, or another
controlled size.
[00408] In some embodiments of the devices, coatings and/or methods
provided herein the
coating delivers the active agent to the treatment site over at least about 1
day. In some embodiments
of the devices, coatings and/or methods provided herein the coating delivers
the active agent to the
- 106 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
treatment site over at least one of: about 3 days, about 5 days, about 1 week,
about 1.5 weeks, about 2
weeks, about 14 days, about 3 weeks, about 21 days, about 4 weeks, about 28
days, about 1 month,
about 1.5 months, about 2 months, at least about 3 days, at least about 5
days, at least about 1 week, at
least about 1.5 weeks, at least about 2 weeks, at least about 14 days, at
least about 3 weeks, at least
about 21 days, at least about 4 weeks, at least about 28 days, at least about
1 month, at least about 1.5
months, at least about 2 months, about 7 to about 14 days, about 14 to about
21 days, about 14 to
about 28 days, about 21 to about 28 days, and about 7 to about 28 days.
[00409] In some embodiments of the devices, coatings and/or methods
provided herein the
treatment site is a vessel wall. In some embodiments of the devices, coatings
and/or methods provided
herein the treatment site is a coronary artery. In some embodiments of the
devices, coatings and/or
methods provided herein the treatment site is bypass graft. In some
embodiments of the devices,
coatings and/or methods provided herein the treatment site is a bifurcated
lesion. In some
embodiments of the devices, coatings and/or methods provided herein the
treatment site is a small
coronary lesions (for example, with reference diameter < 2.5mm). In some
embodiments of the
devices, coatings and/or methods provided herein the treatment site is a
peripheral artery. In some
embodiments of the devices, coatings and/or methods provided herein the
treatment site is vein. In
some embodiments of the devices, coatings and/or methods provided herein the
treatment site is an
AV graft. In some embodiments of the devices, coatings and/or methods provided
herein the
treatment site is an AV fistula. In some embodiments of the devices, coatings
and/or methods
provided herein the treatment site is a biliary tract. In some embodiments of
the devices, coatings
and/or methods provided herein the treatment site is a biliary duct. In some
embodiments of the
devices, coatings and/or methods provided herein the treatment site is a
sinus. In some embodiments
of the devices, coatings and/or methods provided herein the treatment site is
a vein graft.
[00410] In some embodiments of the devices, coatings and/or methods
provided herein the
coating comprises a positive surface charge on a surface of the coating
configured to contact the
treatment site.
[00411] In some embodiments of the devices, coatings and/or methods
provided herein the
encapsulated active agent are micelles.
[00412] In some embodiments of the devices, coatings and/or methods
provided herein the
medical device comprises a balloon. In some embodiments the medical device is
a balloon of a
balloon catheter.
[00413] In some embodiments of the devices, coatings and/or methods
provided herein
depositing the encapsulated active agent comprises using an eSTAT process. In
some embodiments of
the devices, coatings and/or methods provided herein depositing a second
polymer on the medical
device following depositing the encapsulated active agent on the medical
device.
- 107 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00414] In some embodiments of the methods, coatings or and/or devices
provided herein, the
coating is formed on the substrate by a process comprising depositing a
polymer and/or the active
agent by an RESS, e-RESS, an e-SEDS, or an e-DPC process. In some embodiments
of the methods
and/or devices provided herein, wherein the coating is formed on the substrate
by a process
comprising at least one of: depositing a polymer by an RESS, e-RESS, an e-
SEDS, or an e-DPC
process, and depositing the pharmaceutical agent by an e-RESS, an e-SEDS,
eSTAT, or an e-DPC
process. In some embodiments of the methods and/or devices provided herein,
the coating is formed
on the substrate by a process comprising at least one of: depositing a polymer
by an RESS, e-RESS,
an e-SEDS, or an e-DPC process, and depositing the active agent by an eSTAT, e-
RESS, an e-SEDS,
or an e-DPC process. In some embodiments, the process of forming the coating
provides improved
adherence of the coating to the substrate prior to deployment of the device at
the intervention site and
facilitates dissociation of the coating from the substrate at the intervention
site. In some
embodiments, the coating is formed on the substrate by a process comprising
depositing the active
agent by an eSTAT, e-RESS, an e-SEDS, or an e-DPC process without electrically
charging the
substrate. In some embodiments, the coating is formed on the substrate by a
process comprising
depositing the active agent on the substrate by an e-RESS, an e-SEDS, or an e-
DPC process without
creating an electrical potential between the substrate and a coating apparatus
used to deposit the
coating.
[00415] In some embodiments of the devices, coatings and/or methods
provided herein the
second polymer comprises PLGA. The PLGA may have at least one of: a MW of
about 30KDa and a
Mn of about 15KDa, a Mn of about 10KDa to about 25 KDa, and a MW of about 15
KDa to about
40KDa. Depositing the second polymer on the medical device may use at least
one of a RESS coating
process, an eSTAT coating process, a dip coating process, and a spray coating
process.
[00416] In some embodiments of the methods, coatings, and/or devices
provided herein, the
intervention site is in or on the body of a subject. In some embodiments, the
intervention site is a
vascular wall. In some embodiments, the intervention site is a non-vascular
lumen wall. In some
embodiments, the intervention site is a vascular cavity wall. In some
embodiments of the methods
and/or devices provided herein, the intervention site is a wall of a body
cavity. In some embodiments,
the body cavity is the result of a lumpectomy. In some embodiments, the
intervention site is a
cannulized site within a subject. In some embodiments of the methods and/or
devices provided
herein, the intervention site is a sinus wall. In some embodiments, the
intervention site is a sinus
cavity wall. In some embodiments, the active agent comprises a corticosteroid.
[00417] In some embodiments of the methods, coatings, and/or devices
provided herein, the
coating is capable of at least one of: retarding healing, delaying healing,
and preventing healing. In
some embodiments, the coating is capable of at least one of: retarding,
delaying, and preventing the
inflammatory phase of healing. In some embodiments, the coating is capable of
at least one of:
- 108 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
retarding, delaying, and preventing the proliferative phase of healing. In
some embodiments, the
coating is capable of at least one of: retarding, delaying, and preventing the
maturation phase of
healing. In some embodiments, the coating is capable of at least one of:
retarding, delaying, and
preventing the remodeling phase of healing. In some embodiments, the active
agent comprises an
anti-angiogenic agent.
[00418] Provided herein is a method comprising providing a medical
device, wherein the
medical device comprises a substrate and a coating on at least a portion of
the substrate, and wherein
the coating comprises a plurality of layers, wherein at least one layer
comprises a pharmaceutical
agent in a therapeutically desirable morphology, and transferring at least a
portion of the coating from
the substrate to the intervention site upon stimulating the coating with a
stimulation.
[00419] Other compounds that may be used in lieu of Sirolimus (or in
addition thereto)
include, for non-limiting example: Sirolimus which has a FKBP12 binding (nM)
of 0.4 ¨ 2.3 nM and
an Antiproliferative potency (nM) of 0.1 ¨ 3.5 nM; Everolimus which has a
FKBP12 binding (nM) of
1.8 ¨ 2.6 nM and an Antiproliferative potency (nM) of 0.9 ¨ 3.6 nM;
Zotarolimus which has a
FKBP12 binding (nM) of 2.0¨ 3.2 nM and an Antiproliferative potency (nM) of
0.2 ¨2.7 nM;
Biolimus which has an Antiproliferative potency (nM) of about 10 nM;
Temsirolimus which has a
FKBP12 binding (nM) and an Antiproliferative potency (nM) that is about the
same as Sirolimus;
Tacrolimus which has a FKBP12 binding (nM) of 0.2 ¨ 0.4 nM and an
Antiproliferative potency (nM)
of about 350 nM; Pimecrolimus which has a FKBP12 binding (nM) of about 1.2 nM
and an
Antiproliferative potency (nM) of about 1 [LM.
[00420] Alternative compounds that may be used in lieu of sirolimus
(or in addition thereto)
include drugs that were not sufficiently potent to effectively deliver from a
drug stent platform may be
more effective when delivered from a coated balloon (if the drug is highly
lipophilic), for non-limiting
example: Dipyradamole, Cerivastatin, Troglitazone, and/or Cilostazol.
Dipyradamole may be an
appropriate drug for use on a coating of a balloon, for example, since it
inhibits VSMC (vascular
smooth muscle cell) proliferation, is anti-inflammatory, improves endothelial
function, and provides
local release of t-PA (tissue plasminogen activator). Cerivastatin may be an
appropriate drug for use
on a coating of a balloon, for example, since it inhibits VSMC proliferation,
is anti-inflammatory,
improves endothelial function, and can stabilize vulnerable plaque.
Troglitazone may be an
appropriate drug for use on a coating of a balloon, for example, since it
inhibits VSMC proliferation,
is anti-inflammatory, improves endothelial function, and may provide vascular
lipid reduction.
Cilostazol may be an appropriate drug for use on a coating of a balloon, for
example, since it inhibits
VSMC proliferation, may be anti-inflammatory, improves endothelial function,
and is a vasodilator
and/or increases NO (nitric oxide) release and/or production of NO.
[00421] Other drugs that may be appropriate for use on a drug balloon as a
coating that is
released thereby include the following: Drugs to prevent elastic recoil such
as smooth muscle cell
- 109 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
relaxants and/or agents that bind elastin; Drugs to prevent reperfusion injury
such as ANP,
atorvastatin, erythropoietin, and/or glucagon-like peptide 1; Drugs to
stimulate collateral blood flow
such as Vasodilators and/or Growth factors (GF) and GF activators. Drug coated
balloons may be
useful in lower extremities and in peripheral indications, such as in PTA
(Percutaneous transluminal
angioplasty) and in combination with a bare stent, in situations of in stent
restenosis, following
atherectomy. Drug coated balloons may be particularly useful in certain
coronary indications, such as
following in stent restenosis, in small vessel angioplasty situations, in
bifurcations, and in
combination with a bare metal stent. Other uses include in AV Fistulae and
Grafts (dialysis), in the
nasal sinus, in neurovascular vessels, in renal vessels or applications, in
anti-cancer applications, and
in urological applications. a) Fistulae and Grafts (dialysis) Fistulae and
Grafts (dialysis)Fistulae and
Grafts (dialysis).
[00422]
In some embodiments, the device releases at least 3% of the active agent to
artery in
vivo. In some embodiments, the device releases at least 5% of the active agent
to artery in vivo. In
some embodiments, the device releases at least 10% of the active agent in
vivo. In some embodiments,
the device releases at least 5% of the active agent to artery upon inflation
of the balloon in vivo. In
some embodiments, the device releases at least 7% of the active agent to
artery upon inflation of the
balloon in vivo. In some embodiments, the device releases at least 10% of the
active agent to artery
upon inflation of the balloon in vivo. In some embodiments, the device
releases at least 15% of the
active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device releases
at least 20% of the active agent to artery upon inflation of the balloon in
vivo. In some embodiments,
the device releases at least 25% of the active agent to artery upon inflation
of the balloon in vivo. In
some embodiments, the device releases at least 30% of the active agent to
artery upon inflation of the
balloon in vivo. In some embodiments, the device releases at least 40% of the
active agent to artery
upon inflation of the balloon in vivo. In some embodiments, the device
releases at least 50% of the
active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device releases
between 2% and 50% of the active agent to artery upon inflation of the balloon
in vivo. In some
embodiments, the device releases between 3% and 50% of the active agent to
artery upon inflation of
the balloon in vivo. In some embodiments, the device releases between 5% and
50% of the active
agent to artery upon inflation of the balloon in vivo. In some embodiments,
the device releases
between 3% and 30% of the active agent to artery upon inflation of the balloon
in vivo. In some
embodiments, the device releases between 3% and 25% of the active agent to
artery upon inflation of
the balloon in vivo. In some embodiments, the device releases between 3% and
20% of the active
agent to artery upon inflation of the balloon in vivo. In some embodiments,
the device releases
between 3% and 15% of the active agent to artery upon inflation of the balloon
in vivo. In some
embodiments, the device releases between 1% and 15% of the active agent to
artery upon inflation of
the balloon in vivo. In some embodiments, the device releases between 1% and
10% of the active
agent to artery upon inflation of the balloon in vivo. In some embodiments,
the device releases
- 110 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
between 3% and 10% of the active agent to artery upon inflation of the balloon
in vivo. In some
embodiments, the device releases between 1% and 5% of the active agent to
artery upon inflation of
the balloon in vivo.
[00423] As used herein, depending on the embodiment, "upon inflation"
means as soon as
reasonably possible following removal of the device from the treatment site.
This may include
timings such as about 1 minute, about 5 minutes from removal of the device
from the treatment site,
within 1 to 15 minutes from the removal of the device from the treatment site,
within 1 to 15 minutes
from the removal of the device from the treatment site, within 1 to 20 minutes
from the removal of the
device from the treatment site, within 1 minute to 1 hour from the removal of
the device from the
treatment site, within 1 minute to 2 hour from the removal of the device from
the treatment site,
and/or within 1 minute to 3 hours from the removal of the device from the
treatment site.
EXAMPLE 5: DELIVERY OF RAPAMYCIN FROM COATED INVERTABLE BALLOONS
[00424] Provided herein is a device comprising an invertable balloon,
a coating on the
abluminal side of the invertable balloon, wherein the coating comprises an
active agent and a binding
agent. In some embodiments, the device releases at least 3% of the active
agent to artery upon
inflation of the balloon in vivo.
[00425] In some embodiments, the device releases at least 5% of the
active agent to artery
upon inflation of the balloon in vivo. In some embodiments, the device
releases at least 7% of the
active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device releases
at least 10% of the active agent to artery upon inflation of the balloon in
vivo. In some embodiments,
the device releases at least 15% of the active agent to artery upon inflation
of the balloon in vivo. In
some embodiments, the device releases at least 20% of the active agent to
artery upon inflation of the
balloon in vivo. In some embodiments, the device releases at least 25% of the
active agent to artery
upon inflation of the balloon in vivo. In some embodiments, the device
releases at least 30% of the
active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device releases
at least 40% of the active agent to artery upon inflation of the balloon in
vivo. In some embodiments,
the device releases at least 50% of the active agent to artery upon inflation
of the balloon in vivo. In
some embodiments, the device releases between 2% and 50% of the active agent
to artery upon
inflation of the balloon in vivo. In some embodiments, the device releases
between 3% and 50% of
the active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device
releases between 5% and 50% of the active agent to artery upon inflation of
the balloon in vivo. In
some embodiments, the device releases between 3% and 30% of the active agent
to artery upon
inflation of the balloon in vivo. In some embodiments, the device releases
between 3% and 25% of
the active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device
releases between 3% and 20% of the active agent to artery upon inflation of
the balloon in vivo. In
some embodiments, the device releases between 3% and 15% of the active agent
to artery upon
- 111 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
inflation of the balloon in vivo. In some embodiments, the device releases
between 1% and 15% of
the active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device
releases between 1% and 10% of the active agent to artery upon inflation of
the balloon in vivo. In
some embodiments, the device releases between 3% and 10% of the active agent
to artery upon
inflation of the balloon in vivo. In some embodiments, the device releases
between 1% and 5% of the
active agent to artery upon inflation of the balloon in vivo.
[00426] Example invertable balloons (which may also and/or
alternatively be called evertable
balloons) include, but are not limited to, those described in U.S. 6039721
filed Dec 3, 1997; and U.S.
4606347 filed Aug 8, 1985 which patents are incorporated herein by reference
in their entirety. In
some embodiments the abluminal surface of the balloon is coated prior to
inversion, and once coated,
the balloon is inverted such that the abluminal surface of the balloon is
protected from either blood
flow during tracking or tracking contact with the vessel wall, or both, until
the balloon catheter is
positioned near the treatment site, usually just proximally to the site. As
used herein, "abluminal
side" or "abluminal surface" refers to a portion of the balloon having coating
thereon, and intended to
deliver the coating (agent) to the treatment site or location- i.e. the lumen
of the vessel in the case of a
treatment site that is a vessel. The balloon is then un-inverted such that the
abluminal surface is
positioned within the treatment site.
[00427] In an embodiment wherein the balloon is inverted within a
catheter, it may be pushed
out of the catheter using either pressure from the indeflator or another form
of un-inversion of the
balloon, such as for nonlimiting example, by moving the distal end of the
balloon distally through the
balloon itself, essentially unrolling the balloon into the treatment site such
that the coated portion of
the balloon is adjacent the treatment site. In certain embodiments where the
balloon in inverted on the
outside of the catheter, a similar movement and/or pressure from the
indeflator can move the distal
end of the balloon distally thereby unrolling the coated side of the balloon
into proximity of the
treatment site. In some embodiments, the balloon may be partially un-inverted,
such that the treatment
length may be controlled. The balloon thereafter is inflated such that the
abluminal surface that is
coated contacts and/or dilates the treatment site, thereby delivering the
coating or a portion thereof to
the treatment site.
[00428] Any of the devices, coatings, and/or methods described herein
may be combined with
an inverteable type of balloon to deliver the coating in a manner that reduces
and/or substantially
eliminates loss of coating due to tracking and/or blood flow and/or other in-
transit coating loss prior
to locating the device at the treatment site (i.e. delivering the device to
the treatment site). In some
embodiments, at most 1% of coating is removed from the balloon due to tracking
of the coated
balloon to the treatment site. In some embodiments, at most 3% of coating is
removed from the
balloon due to tracking of the coated balloon to the treatment site. In some
embodiments, at most 5%
of coating is removed from the balloon due to tracking of the coated balloon
to the treatment site. In
- 112 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
some embodiments, at most 10% of coating is removed from the balloon due to
tracking of the coated
balloon to the treatment site. In some embodiments, at most 15% of coating is
removed from the
balloon due to tracking of the coated balloon to the treatment site. In some
embodiments, at most 20%
of coating is removed from the balloon due to tracking of the coated balloon
to the treatment site. In
.. some embodiments, at most 25% of coating is removed from the balloon due to
tracking of the coated
balloon to the treatment site. In some embodiments, at most 30% of coating is
removed from the
balloon due to tracking of the coated balloon to the treatment site.
[00429] As used herein, depending on the embodiment, "upon inflation"
means as soon as
reasonably possible following removal of the device from the treatment site.
This may include
timings such as about 1 minute, about 5 minutes from removal of the device
from the treatment site,
within 1 to 15 minutes from the removal of the device from the treatment site,
within 1 to 15 minutes
from the removal of the device from the treatment site, within 1 to 20 minutes
from the removal of the
device from the treatment site, within 1 minute to 1 hour from the removal of
the device from the
treatment site, within 1 minute to 2 hour from the removal of the device from
the treatment site,
and/or within 1 minute to 3 hours from the removal of the device from the
treatment site.
EXAMPLE 6: DELIVERY OF RAPAMYCIN FROM SHEATHED COATED BALLOONS
[00430] Provided herein is a device comprising an balloon, a coating
on the abluminal side of
the balloon, and a sheath over the balloon, wherein the coating comprises an
active agent and a
binding agent. In some embodiments, the device releases at least 3% of the
active agent to artery
upon inflation of the balloon in vivo. In some embodiments, the sheath may be
retracted. In some
embodiments, the sheath may be retracted to expose the coating to the
treatment site. In some
embodiments, the sheath covers the coated balloon until the balloon reaches
the treatment site. In
some embodiments the sheath may be retracted once the coated balloon is
positioned near and/or at
the treatment site. In some embodiments, the sheath covers the coated balloon
until the balloon is
proximal to the treatment site. In some embodiments, the sheath covers the
coated balloon until the
balloon is distal to the treatment site. In some embodiments, the sheath
covers the coated balloon
until the balloon is within to the treatment site. In some embodiments, the
sheath may be moved over
the balloon following deflation of the balloon after the coating (or a portion
thereof) has been released
to the artery, and the catheter may be removed such that the coated balloon is
covered during removal
from the subject. In some embodiments, the sheath may remain in a retracted
state following
deflation of the balloon after the coating (or a portion thereof) has been
released to the artery, and the
catheter may be removed such that the coated balloon is exposed to the
delivery track during removal
from the subject.
[00431] Any of the devices, coatings, and/or methods described herein may
be combined with
a sheath to deliver the coating in a manner that reduces and/or substantially
eliminates loss of coating
- 113 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
due to tracking and/or blood flow and/or other in-transit coating loss prior
to locating the device at the
treatment site (i.e. delivering the device to the treatment site). In some
embodiments, at most 1% of
coating is removed from the balloon due to tracking of the coated balloon to
the treatment site. In
some embodiments, at most 3% of coating is removed from the balloon due to
tracking of the coated
balloon to the treatment site. In some embodiments, at most 5% of coating is
removed from the
balloon due to tracking of the coated balloon to the treatment site. In some
embodiments, at most 10%
of coating is removed from the balloon due to tracking of the coated balloon
to the treatment site. In
some embodiments, at most 15% of coating is removed from the balloon due to
tracking of the coated
balloon to the treatment site. In some embodiments, at most 20% of coating is
removed from the
balloon due to tracking of the coated balloon to the treatment site. In some
embodiments, at most 25%
of coating is removed from the balloon due to tracking of the coated balloon
to the treatment site. In
some embodiments, at most 30% of coating is removed from the balloon due to
tracking of the coated
balloon to the treatment site.
[00432] In some embodiments, the device releases at least 5% of the
active agent to artery
upon inflation of the balloon in vivo. In some embodiments, the device
releases at least 7% of the
active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device releases
at least 10% of the active agent to artery upon inflation of the balloon in
vivo. In some embodiments,
the device releases at least 15% of the active agent to artery upon inflation
of the balloon in vivo. In
some embodiments, the device releases at least 20% of the active agent to
artery upon inflation of the
balloon in vivo. In some embodiments, the device releases at least 25% of the
active agent to artery
upon inflation of the balloon in vivo. In some embodiments, the device
releases at least 30% of the
active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device releases
at least 40% of the active agent to artery upon inflation of the balloon in
vivo. In some embodiments,
the device releases at least 50% of the active agent to artery upon inflation
of the balloon in vivo. In
some embodiments, the device releases between 2% and 50% of the active agent
to artery upon
inflation of the balloon in vivo. In some embodiments, the device releases
between 3% and 50% of
the active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device
releases between 5% and 50% of the active agent to artery upon inflation of
the balloon in vivo. In
some embodiments, the device releases between 3% and 30% of the active agent
to artery upon
inflation of the balloon in vivo. In some embodiments, the device releases
between 3% and 25% of
the active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device
releases between 3% and 20% of the active agent to artery upon inflation of
the balloon in vivo. In
some embodiments, the device releases between 3% and 15% of the active agent
to artery upon
inflation of the balloon in vivo. In some embodiments, the device releases
between 1% and 15% of
the active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device
releases between 1% and 10% of the active agent to artery upon inflation of
the balloon in vivo. In
some embodiments, the device releases between 3% and 10% of the active agent
to artery upon
- 114 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
inflation of the balloon in vivo. In some embodiments, the device releases
between 1% and 5% of the
active agent to artery upon inflation of the balloon in vivo.
[00433] As used herein, depending on the embodiment, "upon inflation"
means as soon as
reasonably possible following removal of the device from the treatment site.
This may include
timings such as about 1 minute, about 5 minutes from removal of the device
from the treatment site,
within 1 to 15 minutes from the removal of the device from the treatment site,
within 1 to 15 minutes
from the removal of the device from the treatment site, within 1 to 20 minutes
from the removal of the
device from the treatment site, within 1 minute to 1 hour from the removal of
the device from the
treatment site, within 1 minute to 2 hour from the removal of the device from
the treatment site,
and/or within 1 minute to 3 hours from the removal of the device from the
treatment site.
EXAMPLE 7: DELIVERY OF RAPAMYCIN FROM COATED BALLOONS WITH
OCCLUDER
[00434] Provided herein is a device comprising a balloon, a coating on
the balloon, and an
occluder, wherein the coating comprises an active agent and a binding agent.
In some embodiments,
the occluder is a flow occluder configured to block the flow of bodily fluids
(e.g. blood) at the
treatment site during exposure of the coating to the treatment site. In some
embodiments, the device
releases at least 3% of the active agent to artery upon inflation of the
balloon in vivo. In some
embodiments, the occluder comprises a second balloon that occludes the flow of
the blood at the
treatment site. In some embodiments, the balloon comprises the occluder, such
that the balloon has
two sections the flow occluder and the coated portion, wherein the flow
occluder occludes the flow of
the blood at the treatment site. In some embodiments, the balloon is dual-
noded, wherein the distal
node is coated and wherein the proximal node is the occluder. In some
embodiments, the occluder is
located proximally from the balloon, and/or portion thereof, having coating
thereon. In some
embodiments, the balloon is dual-noded, wherein the proximal node is coated
and wherein the distal
node is the occluder. In some embodiments the occluder is located distally
from the balloon, and/or
portion thereof, having coating thereon. In some embodiments, the balloon is a
shape appropriate for
the treatment site, such that the occluder portion of the balloon is the
appropriate shape to occlude
flow of blood at the treatment site. In some embodiments, the occluder
substantially conforms to the
shape of a treatment area near the treatment site such blood flow at the
treatment site is occluded
thereby. In some embodiments, the balloon is only partially coated, such that
either or both the distal
and proximal end of the balloon is not coated, and the distal and/or proximal
end of the balloon is the
occluder.
[00435] Provided herein is a device comprising a first balloon, a
coating on the first balloon,
and a second balloon capable of occluding flow of blood at the treatment site
during expansion of the
first balloon at the treatment site. In some embodiments, the occluder is a
second balloon which is not
the balloon having coating thereon. In some embodiments, the occluder is
located proximally from
- 115 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
the balloon, and/or portion thereof, having coating thereon. In some
embodiments the occluder is
located distally from the balloon, and/or portion thereof, having coating
thereon.
[00436] Provided herein is a device comprising a first balloon, a
coating on the first balloon,
and a second balloon configured such that the second balloon expands prior to
expansion of the first
balloon. In some embodiments the occluder occludes the flow of the blood at
the treatment prior to
expansion of the portion of the balloon. In some embodiments, the occluder is
located proximally
from the balloon, and/or portion thereof, having coating thereon. In some
embodiments the occluder
is located distally from the balloon, and/or portion thereof, having coating
thereon.
[00437] In some embodiments, the occluder is not a balloon, but is
another form of occluder
that is configured to occlude flow of blood at the treatment site. In some
embodiments, the occluder
is deployable and retractable, such that it can be deployed prior to inflation
of the balloon having
coating thereon, and following balloon inflation and delivery of the agent to
the treatment site, the
occluder can be retracted and removed either with the removal of the balloon,
or following removal of
the balloon from the treatment site.
[00438] In some embodiments, the occluder has a second coating thereon,
having a second
agent and/or polymer coated thereon as described elsewhere herein, according
to any of the methods
and processes as noted herein. The second coating in some embodiments
comprises a binding agent.
The second coating in some embodiments does not comprise a binding agent.
[00439] In some embodiments, the device releases at least 5% of the
active agent to artery
upon inflation of the balloon in vivo. In some embodiments, the device
releases at least 7% of the
active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device releases
at least 10% of the active agent to artery upon inflation of the balloon in
vivo. In some embodiments,
the device releases at least 15% of the active agent to artery upon inflation
of the balloon in vivo. In
some embodiments, the device releases at least 20% of the active agent to
artery upon inflation of the
balloon in vivo. In some embodiments, the device releases at least 25% of the
active agent to artery
upon inflation of the balloon in vivo. In some embodiments, the device
releases at least 30% of the
active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device releases
at least 40% of the active agent to artery upon inflation of the balloon in
vivo. In some embodiments,
the device releases at least 50% of the active agent to artery upon inflation
of the balloon in vivo. In
some embodiments, the device releases between 2% and 50% of the active agent
to artery upon
inflation of the balloon in vivo. In some embodiments, the device releases
between 3% and 50% of
the active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device
releases between 5% and 50% of the active agent to artery upon inflation of
the balloon in vivo. In
some embodiments, the device releases between 3% and 30% of the active agent
to artery upon
inflation of the balloon in vivo. In some embodiments, the device releases
between 3% and 25% of
the active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device
- 116 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
releases between 3% and 20% of the active agent to artery upon inflation of
the balloon in vivo. In
some embodiments, the device releases between 3% and 15% of the active agent
to artery upon
inflation of the balloon in vivo. In some embodiments, the device releases
between 1% and 15% of
the active agent to artery upon inflation of the balloon in vivo. In some
embodiments, the device
releases between 1% and 10% of the active agent to artery upon inflation of
the balloon in vivo. In
some embodiments, the device releases between 3% and 10% of the active agent
to artery upon
inflation of the balloon in vivo. In some embodiments, the device releases
between 1% and 5% of the
active agent to artery upon inflation of the balloon in vivo.
EXAMPLE 8: COATINGS PREPARED WITH AND WITHOUT SHEAR MIXING
[00440] F15 (Formulation 15) as described in Example 4 was produced in
multiple lots and
having various rapamycin: polyarginine ratios. Regardless of the rapamycin:
polyarginine ratio,
however, indicative of F15 is that it comprises PLGA i.e. about 50:50 Lactic
acid: Glycolic acid,
Sirolimus having an average size of 1.5 [tin, and Polyarginine 5-15 kDa. The
sirolimus was in
crystalline form. The following Rapamycin:Polyarginine Ratios were produced
for this Example:1:1,
5:1, 10:1, and 50:1.
[00441] In some lots (generally called F15 Lot 3 1:1, 5:1, 10:1, or
50:1), the production
method for the formulation was as depicted in Figure 4. The method for these
lots was as follows:
Dissolve 25mg Poly-L-arginine hydrochloride (Aldrich P4663) (also called
polyarginine herein) (cas
26982-20-7, 5-15kDa ) in 50m1 deionized water in 100m1 bottle and add 250mg
encapsulated
rapamycin (1.5 micron particle size, crystalline form) (step 46). Sonicate
(Branson 1510 bench top
ultrasonic cleaner) for 2h (step 48). Manually separate well-suspended liquid
portion from
unsuspended solids using pipette (step 50). Centrifuge ¨50m1 suspension for
30min at 10,000 rpm
(ThermoElectronCorp. IEC Multi RF Centrifuge) (step 52). Decant supernatant
without allowing
sediment to come to dryness (step 54). There will be an amount of unsupended
fraction 64 following
centrifuge step 52. Add aqueous solution of poly-L-arginine hydrochloride
concentration to produce
desired encapsulated Sirolimus/poly-L-arginine hydrochloride ratio (step 56).
Re-suspend sediment
by shaking and 10 minute sonication. Lyophilize suspension to produce un-
agglomerated
encapsulated rapamycin/polyarginine powder solid lyophilisate 66 (Flexi-Dry
MP) (step 58). This
step took two to three days to achieve completion. This lyophilized solid 66
was eSTAT coated onto a
balloon or a PLGA-coated balloons as a powder (dry coating as described
herein) (step 60) to produce
a coated balloon 62 comprising PLGA, rapamycin, and polyarginine, wherein the
rapamycin is
crystalline in form.
[00442] For the ratio-forming step, the ratios were produced as
follows: 95m1Masterbatch
(combination of "well-suspended" portions of 4 sonicated solutions), estimated
to be ¨5mg/m1 solids,
was divided into 5 portions. For the 50:1 ratio of sirolimus to polyarginine,
20m1 water was added to
the first 18m1 portion, it was sonicated to re-suspend and lyophilized to
produce 50.3 mg solid
- 117 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
lyophilisate. For the 10:1 ratio of sirolimus to polyarginine, 9 mg
polyarginine was dissolved in 20m1
water and was added to the second 18m1 portion whis was sonicated to re-
suspend and lyophilized to
produce 116.6 mg solid lyophilisate. For the 5:1 ratio of sirolimus to
polyarginine, 18mg polyarginine
was dissolved in 20m1 water and was added to the third 18m1 portion, it was
sonicated to re-suspend
.. and lyophilized to produce 127.4 mg solid lyophilisate. For the 1:1 ratio
of sirolimus to polyarginine,
90mg polyarginine was dissolved in 20m1 water and was added to the fourth 18m1
portion, it was
sonicated to re-suspend and lyophilized to produce 142.4 mg solid
lyophilisate.
[00443] In some lots (generally called F15 Lot 4 5:1 or 10:1), the
production methods for a
F15 formulation having a 5:1 ratio of rapamycin to polyarginine lot and a F15
formulation having a
10:1 ratio of rapamycin to polyarginine lot were as follows. Dissolve 25mg
(10:1) or 50 mg (5:1)
Poly-L-arginine hydrochloride (Aldrich P4663) (cas 26982-20-7) (5-15kDa) (also
called
polyarginine) in 25m1 deionized water in 20m1 vial. Add 250mg Sirolimus
(1.5micron particle size,
crystalline in form). Mix for 10min at 10,000 rpm in Laboratory Mixer
(Silverson L4RT) using micro
mixer head attachment to form a suspension (this mixing leaves little or no
sediment). The Lab Mixer
is a High Shear Mixer having an impeller for mechanical mixing. Run mixer with
25m1 pure water to
recover residual material (rinse water). Combine suspension with rinse water.
Lyophilize suspension
to produce un-agglomerated Sirolimus/polyarginine powder (Flexi-Dry MP), which
took two to three
days to achieve completion. This lyophilized solid 66 was eSTAT coated onto
PLGA coated
balloons as a powder (dry coating as described herein) to produce a coated
balloon comprising PLGA,
rapamycin, and polyarginine, wherein the rapamycin is crystalline in form.
[00444] The amount of rapamycin that was found in the actual coated
balloon was also
determined and could be used to determine an actual rapamycin:polyarginine
ratio (as opposed to the
target ratio provided as noted elsewhere herein). To measure the amount of
sirolimus on individual
balloons ultraviolet-visible spectroscopy (UV-Vis) was employed. After
sintering, coated balloons are
cut (the stylus is removed before cutting) from the catheter wires leaving
only ¨1/4" of the wires
remaining connected to the balloons. Balloons were placed in individual 5 ml
scintillation vials
containing 4 ml of ethanol or methanol (sirolimus is soluble in ethanol up to
50 mg/ml). Sonication
for 3 h removes sirolimus from the balloons. Following sonication UV-Vis is
performed. Due to
sirolimus being a triene (containing three double bonds) it produces UV
absorbance at 3 wavelengths:
1 major peak at 277 nm and two smaller peaks at 267 nm and 288 nm. Uncoated
GHOST rapid
exchange nylon balloons and PLGA have also been individually sonicated for 3 h
in ethanol and
showed no interfering extractives for sirolimus measurements. The absorbance
of sirolimus subtracted
from the absorbance of an uncoated balloon at 277 nm is used in conjunction
with a standard curve to
calculate the amount of sirolimus per coated balloon. A standard of 3 from a
batch of 12 coated
balloons underwent UV-Vis analysis to obtain a batch average of sirolimus per
balloon (measured in
[tg). The UV-Vis analysis could also be used to determine the presence and/or
quantitate the amount
of polymer (in this Example, PLGA) in the coating. UV-Vis testing of both lots
3 and 4 revealed
- 118 -
CA 02888776 2015-04-17
WO 2014/063111 PCT/US2013/065777
presence of both polyarginine and rapamycin on the coated balloons. For Lot 3,
the following actual
ratios were determined: for Lot 3 1:1(ideal) actual was about 1.3:1, for Lot 3
5:1(ideal) actual was
about 7.1:1, Lot 3 10:1(ideal) actual was about 14.3:1, for Lot 3 50:1(ideal)
actual was about 43.1:1.
Likewise, for Lot 4 5:1(ideal) actual was about 6.1:1.
[00445] F15 Lot 3 coated balloons were delivered to arteries of animals of
a rabbit study to
assess if and how much of the rapamycin was retained in rabbit iliac arteries
for 72 hours. The
following coated balloon formulations were as follows in Table 18.
Table 18:
Sirolimus:Polyarginine
Ratio Sirolimus/Balloon (11g) (n=6)* Coating Appearance
1:1 65.37 3.84 Transparent,
Speckled
5:1 79.02 9.83 Translucent,
Very Thick
10:1 89.71 5.27 Translucent, Very Thick
50:1 81.28 4.61 Translucent, Very Thick
* Average Sirolimus concentrations based on UV-Vis analysis before
pleating/folding and
sterilization of balloons.
[00446] Study design for this study was as depcited in Table 19.
Table 19:
Number of Vessels Blood PK Necropsy Time
Test Article Animals
Per Animal Per Animal Points
F15 Sirolimus: n=2 time points
n=3 n=2 denuded rabbit
Polyarginine Ratios: (baseline/before 3 days (
5%)
per ratio iliac arteries
1:1, 5:1, 10:1, 50:1 necropsy)
Totals 12 24 24 3 days ( 5%)
Deployed balloons, blood samples and denuded iliac arteries analyzed for
Sirolimus levels.
[00447] The following results were determined as shown in Tables 20,
21, 22. Most retention
from 10:1 ratio of F15 Lot 3 (1.8 1.2 ng/mg). Sirolimus blood levels below 1
ng/ml by 3 days.
Table 20:
Arterial Sirolimus Total Sirolimus per
F15 Ratio SD SD
, Concentration (ng/mg) , , Artery
(11g) ,
F15 1:1 Lot 3, n=6 1.52 2.63 0.022 0.035
F15 5:1 Lot 3, n=6 0.67 0.53 0.013 0.011
F15 10:1 Lot 3, n=6 1.77 1.18 0.041 0.030
F15 50:1 Lot 3, n=6 0.63 0.18 0.010 0.003
Table 21:
Sirolimus Concentration on Balloon
F15 Ratio % Sirolimus Released/Lost*
After Deployment (ug)
1:1 Lot 3 20.6 9.8 (n=6) 68.9 14.0%
(n=6)
5:1 Lot 3 37.9 6.3 (n=6) 52.0 7.8%
(n=6)
10:1 Lot 3 41.3 7.2 (n=6) 53.7 9.1%
(n=6)
50:1 Lot 3 45.4 6.9 (n=6) 44.2 8.6% (n=6)
* Based on Balloon Batch Averages of Sirolimus
- 119 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00448] The amount of Sirolimus coated on balloons was as follows: F15
(1:1, Lot 3)
Sirolimus coated on balloons = 65.37 3.84; F15 (5:1, Lot 3) Sirolimus coated
on balloons = 79.02
9.83; F15 (10:1, Lot 3) Sirolimus coated on balloons = 89.71 5.27; F15
(50:1, Lot 3) Sirolimus
coated on balloons = 81.28 4.61.
Table 22:
Sirolimus Concentration in Whole Blood
F15 Ratio (ng/mL)
Est. Total Sirolimus in Blood (11g) *
1:1 Lot 3 0.29 0.03 (n=3) 0.054 0.01 (n=3)
5:1 Lot 3 0.50 0.15 (n=3) 0.096 0.03 (n=3)
10:1 Lot 3 0.43 0.12 (n=3) 0.081 0.02 (n=3)
50:1 Lot 3 0.38 0.08 (n=3) 0.064 0.01 (n=3)
* Based on 56 ml- Blood per kg; BQL = below quantitation limit (0.1 ng/ml)
[00449] F15 Lot 4 coated balloons were delivered to arteries of
animals of a rabbit study to
assess if and how much of the rapamycin was retained in rabbit iliac arteries
for 72 hours.
[00450] Provided herein is a method of coating at least a portion of a
medical device
comprising a balloon, thereby forming on the medical device a coating on the
the balloon comprising
an active agent and a binding agent, wherein the method comprises: dissolving
the binding agent to
form a binding agent solution, combining the binding agent solution and the
active agent, mixing the
combined binding agent and active agent using a high shear mixer, forming a
suspension comprising
the combined mixed active agent and binding agent, lyophilising the suspension
to form a lyophilisate
of the active agent and the binding agent, and coating the balloon with the
lyophilisate in powder form
using an eSTAT process, wherein the active agent coated on the balloon
comprises active agent in
crystalline form.
[00451] In some embodiments, the high shear mixer is a mechanical
mixer. In some
embodiments, the mechanical mixer comprises an impeller, propeller, and/or a
high speed saw tooth
disperser. In some embodiments, the mechanical mixer comprise a high pressure
pump. In some
embodiments, the high shear mixer comprises a sonic mixer. In some
embodiments, the sonic mixer
comprises a sonicator. In some embodiments, the sonic mixer comprises a
benchtop bath based
sonicator. In some embodiments, the sonic mixer comprises an ultrasonic mixer.
In some
embodiments, the sonic mixer comprises an megasonic mixer.
[00452] In some embodiments, the mechanical mixer comprise a high pressure
pump (up to
40,000 psi (2578 bar)) that forces particles into an interaction chamber at
speeds up to 400 m/s. The
interaction chamber may comprise engineered microchannels. Inside the chamber,
the product may be
exposed to consistent impact and shear forces and then cooled.
[00453] A high shear mixer disperses, or transports, one phase or
ingredient (liquid, solid,
gas) into a main continuous phase (liquid), with which it would normally be
immiscible. In some
embodiments of a mechanical mixer that is a high shear mixer, a rotor or
impellor, together with a
stationary component known as a stator, or an array of rotors and stators, is
used either in a tank
- 120 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
containing the solution to be mixed, or in a pipe through which the solution
passes, to create shear. A
high shear mixer can be used to create emulsions, suspensions, lyosols (gas
dispersed in liquid), and
granular products.
[00454] Fluid undergoes shear when one area of fluid travels with a
different velocity relative
to an adjacent area. In some embodiments of a mechanical mixer that is a high
shear mixer, the high
shear mixer uses a rotating impeller or high-speed rotor, or a series of such
impellers or inline rotors,
usually powered by an electric motor, to "work" the fluid, creating flow and
shear. The tip velocity, or
speed of the fluid at the outside diameter of the rotor, will be higher than
the velocity at the centre of
the rotor, and it is this velocity difference that creates shear.
[00455] A stationary component may be used in combination with the rotor,
and is referred to
as the stator. The stator creates a close-clearance gap between the rotor and
itself and forms an
extremely high shear zone for the material as it exits the rotor. The rotor
and stator combined together
are often referred to as the mixing head, or generator. A large high shear
rotor¨stator mixer may
contain a number of generators.
[00456] In some embodiments the mechanical mixer comprises a batch high
shear mixer. In a
batch high shear mixer, the components to be mixed (whether immiscible liquids
or powder in liquid)
are fed from the top into a mixing tank containing the mixer on a rotating
shaft at the bottom of the
tank. A batch high shear mixer can process a given volume of material
approximately twice as fast as
an inline rotor¨stator mixer of the same power rating; such mixers continue to
be used where faster
processing by volume is the major requirement, and space is not limited. When
mixing sticky
solutions, some of the product may be left in the tank, necessitating
cleaning. However, there are
designs of batch high shear mixers that clean the tank as part of the
operating run. Some high shear
mixers are designed to run dry, limiting the amount of cleaning needed in the
tank.
[00457] In some embodiments the mechanical mixer comprises an inline
high shear rotor-
stator mixer. Generally speaking this version takse the same rotor and stator
from tbe batch high
shear mixer and installs it in a housing with inlet and outlet connections.
Then the rotor is driven
through a shaft seal thus resuling in a rotor-stator mixer that behaves like a
centrifugal pumping
device. That is, in an inline high shear rotor¨stator mixer, the rotor¨stator
array is contained in a
housing with an inlet at one end and an outlet at the other, and the rotor
driven through a seal. The
components to be mixed are drawn through the generator array in a continuous
stream, with the whole
acting as a centrifugal pumping device. Inline high shear mixers offer a more
controlled mixing
environment, take up less space, and can be used as part of a continuous
process. Equilibrium mixing
can be achieved by passing the product through the inline high shear mixer
more than once. Since the
inline mixer may be positioned in a flowing stream, the mixing may be more
controlled than in a
batch configuration, so the number of passes through the high shear zone can
be monitored.
- 121 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00458] An inline rotor¨stator mixer equipped for powder induction
offers flexibility,
capability, and portability to serve multiple mix vessels of virtually any
size. Its straightforward
operation and convenience further maximize equipment utility while simplifying
material handling.
[00459] When used with a vacuum pump and hopper, an inline shear mixer
can be a very
effective way to incorporate powders into liquid streams. Otherwise known as
high shear powder
inductors, these systems have the advantage of keeping the process on the
floor level instead of
working with heavy bags on mezzanines. High shear powder induction systems
also offer easy
interchangeability with multiple tanks.
[00460] A high shear granulator is a process array consisting of an
inline or batch high shear
mixer and a fluid-bed dryer. In a granulation process, only the solid
component of the mixture is
required. Fluid is used only as an aid to processing. The high shear mixer
processes the solid material
down to the desired particle size, and the mixture is then pumped to the
drying bed where the fluid is
removed, leaving behind the granular product.
[00461] In an ultra-high shear inline mixer, the high shear mixing
takes place in a single or
multiple passes through a rotor¨stator array. The mixer is designed to subject
the product to higher
shear and a larger number of shearing events than a standard inline
rotor¨stator mixer, producing an
exceptionally narrow particle-size distribution. Sub-micrometre particle sizes
are possible using the
ultra-high shear technology. To achieve this, the machine is equipped with
stators with precision-
machined holes or slots through which the product is forced by the rotors. The
rotor¨stator array can
also include a mechanism whereby the momentum of the flow is changed (for
example by forcing it
sideways through the stator), allowing for more processing in a single pass.
[00462] High shear mixers may be used to produce standard mixtures of
ingredients that do
not naturally mix. When the total fluid is composed of two or more liquids,
the final result is an
emulsion; when composed of a solid and a liquid, it is termed a suspension and
when a gas is
dispersed throughout a liquid, the result is a lyosol. Each class may or may
not be homogenized,
depending on the amount of input energy.
[00463] To achieve a standard mix, the technique of equilibrium mixing
may be used. A
target characteristic is identified, such that once the mixed product has
acquired that characteristic, it
will not change significantly thereafter, no matter how long the product is
processed. For dispersions,
this is the equilibrium particle size. For emulsions, it is the equilibrium
droplet size. The amount of
mixing required to achieve equilibrium mixing is measured in tank turnover ¨
the number of times the
volume of material must pass through the high shear zone.
[00464] In some embodiments, the sonic mixer comprises a sonicator. In
some embodiments,
the sonic mixer comprises a benchtop bath based sonicator. In some
embodiments, the sonic mixer
comprises an ultrasonic mixer. The ultrasonic mixer may employ ultrasonic
frequencies of any one or
more of: about 18kHz at least, about 20kHz at least, less than 400 kHz, less
than 500 kHz, about
- 122 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
18kHz to about 400kHz, about 20kHz to about 500 kHz, at most about 400kHz, and
at most about 500
kHz. In some embodiments, the sonic mixer comprises an megasonic mixer. The
megasonic mixer
may employ megasonic frequencies of any one or more of: about 500kHz at least,
about 700kHz at
least, about 800kHz at least, less than about 5MHz, less than about 4MHz,
about 500kHz to about
.. 5MHz, about 700kHz to about 4MHz, at most about 5MHz, at most about 4MHz,
at least about
1MHz, and any frequency in the MHz range.
[00465] In some embodiments, a ratio of the active agent to the
binding agent is 1:1, 1:2, 1:3,
1:4, 1:5, 1:10, 1:20, 2:1, 3:1, 4:1, 5:1, 10:1, 15:1, 20:1, 3:2, 2:3, 5:2,
5:3, 2:5, or 3:5 as a target ratio.
In some embodiments,the actual ratio of the active agent to the binding agent
is +/-10% of the ideal
.. ratio, +/-20% of the ideal ratio, +1-25% of the ideal ratio, or +/-30% of
the target ratio. In some
embodiments,the actual ratio is calculated based on UV-Vis testing of the
medical device.
[00466] In some embodiments when the balloon of the device is
delivered to an an artery in
vivo, at least 3% of the active agent is transferred to tissue of the artery.
In some embodiments, at
least 5% of the active agent is transferred to tissue of the artery. In some
embodiments, at least 10% of
the active agent is transferred to tissue of the artery.
[00467] In some embodiments, the binding agents comprises at least one
of: Polyarginine,
Polyarginine 9-L-pArg, DEAE-Dextran (Diethylaminoethyl cellulose- Dextran),
DMAB
(Didodecyldimethylammonium bromide), PEI (Polyethyleneimine), TAB
(Tetradodecylammonium
bromide), and DMTAB (Dimethylditetradecylammonium bromide).
[00468] In some embodiments, an average molecular weight of the binding
agent is
controlled. In some embodiments, a size of the active agent in the coating is
controlled.
[00469] In some embodiments, the active agent is sirolimus and wherein
the sirolimus has
have an average size of at least one of: about 1.5 [tin, about 2.5 [tin, about
645nm, about 100-200 nm,
another controlled size, or a combination thereof In some embodiments, the
active agent is sirolimus
and wherein sirolimus at least 75% of the sirolimus as is 1.5 [tin, 2.5 [tin,
645nm, 100-200 nm, or
another controlled size. In some embodiments, the active agent is sirolimus
and wherein sirolimus at
least 50% of the sirolimus as is 1.5 [tin, 2.5 [tin, 645nm, 100-200 nm, or
another controlled size. In
some embodiments, the active agent is sirolimus and wherein sirolimus at least
90% of the sirolimus
as is 1.5 [tin, 2.5 [tin, 645nm, 100-200 nm, or another controlled size. In
some embodiments, the
coating may comprise nanoparticles, and the nanoparticles may comprise an
active agent and a
polymer.
[00470] In some embodiments, the coating comprises PLGA comprising
about 50:50 Lactic
acid: Glycolic acid. In some embodiments, the coating comprised and about a
10:1 ratio of the active
agent to the binding agent, wherein the active agent comprises sirolimus
wherein the binding agent
comprises Polyarginine. In some embodiments, the sirolimus has an average size
of 1.5 [tin or 2.5
m. In some embodiments, the Polyarginine average molecular weight is 70kDa. In
some
- 123 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
embodiments, the Polyarginine average molecular weight is 5-15kDa. In some
embodiments, the
active agent and the binding agent are lyophilized prior to deposition on the
balloon. In some
embodiments, at least about 2 ng/mg of active agent, at least about 3 ng/mg of
active agent, at least
about 5 ng/mg of active agent, at least about 10 ng/mg of active agent, at
least about 20 ng/mg of
active agent, at least about 30 ng/mg of active agent, and/or at least about
40 ng/mg of active agent are
found in arterial tissue 72 hours after inflation of the balloon in the
artery.
[00471] In some embodiments, in vivo measurement comprises inflating
the balloon inside
the artery of a porcine for about 1 minute and the amount of active agent
transferred to the artery is
measured by UV-Vis evaluation of the coating remaining on the ballon as
determined five minutes
after inflation of the balloon in the artery. In some embodiments, in vivo
measurement comprises
inflating the balloon inside the artery of a rabbit for about 1 minute and the
amount of active agent
transferred to the artery is measured by UV-Vis evaluation of the coating
remaining on the ballon as
determined five
[00472] In some embodiments, the device releases at least one of: at
least 5% of the active
agent to artery upon inflation of the balloon in vivo, at least 7% of the
active agent to artery upon
inflation of the balloon in vivo, at least 10 % of the active agent to artery
upon inflation of the balloon
in vivo, at least 15 % of the active agent to artery upon inflation of the
balloon in vivo, at least 20 %
of the active agent to artery upon inflation of the balloon in vivo, at least
25 % of the active agent to
artery upon inflation of the balloon in vivo, at least 25 % of the active
agent to artery upon inflation of
the balloon in vivo, at least 30 % of the active agent to artery upon
inflation of the balloon in vivo, at
least 40 % of the active agent to artery upon inflation of the balloon in
vivo, at least 50 % of the active
agent to artery upon inflation of the balloon in vivo, between 2% and 50% of
the active agent to artery
upon inflation of the balloon in vivo, between 3% and 50% of the active agent
to artery upon inflation
of the balloon in vivo, between 5% and 50% of the active agent to artery upon
inflation of the balloon
in vivo, between 3% and 30% of the active agent to artery upon inflation of
the balloon in vivo,
between 3% and 25% of the active agent to artery upon inflation of the balloon
in vivo, between 3%
and 20% of the active agent to artery upon inflation of the balloon in vivo,
between 3% and 15% of
the active agent to artery upon inflation of the balloon in vivo, between 1%
and 15% of the active
agent to artery upon inflation of the balloon in vivo, between 1% and 10% of
the active agent to artery
upon inflation of the balloon in vivo, between 3% and 10% of the active agent
to artery upon inflation
of the balloon in vivo, and between 1% and 5% of the active agent to artery
upon inflation of the
balloon in vivo.
[00473] In some embodiments, at least one of: at most 1% of coating is
removed from the
balloon due to tracking of the coated balloon to a treatment site, at most 5%
of coating is removed
from the balloon due to tracking of the coated balloon to the treatment site,
at most 10% of coating is
removed from the balloon due to tracking of the coated balloon to the
treatment site, at most 15% of
- 124 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
coating is removed from the balloon due to tracking of the coated balloon to
the treatment site, at most
20% of coating is removed from the balloon due to tracking of the coated
balloon to the treatment site,
at most 25% of coating is removed from the balloon due to tracking of the
coated balloon to the
treatment site, and at most 30% of coating is removed from the balloon due to
tracking of the coated
balloon to the treatment site.
[00474] Provided
herein is a device made according to any of the methods provided herein,
and having features as described therein.
EXAMPLE 9: Sirolimus Coated Balloon Animal (Rabbit) Study
[00475] Formulations
3, 19, 20, 21, 22, 23 were coated on balloons and the balloons were
inflated in rabbit iliac arteries, and the arteries were studied at 5 minutes,
72 hours and 14 days after
inflation. The objective was to assess if Sirolimus from a drug coated balloon
having Formulations 3,
19, 20, 21, 22, or 23coated thereon is retained in rabbit iliac arteries up to
72 hours and 14 days. The
Formulation, coating composition, amount of sirolimus coated per balloon (in
micrograms), the
sample size (n), and the coating appearance on the balloon is noted in Table
23. The study outline is
noted in Table 24.
Table 23
Coating
Formulation Coating Composition* Sirolimus/Balloon ( g)**
Appearance
F3 Sirolimus (1.5 m), PLGA,
Translucent, Very
114.49 25.18 (n=18)
(Lot 3) Polyarginine 70 kDa
Thick
F19 Sirolimus (1.5 m), Polyarginine 5-15 Transparent,
138.99 19.33 (n=8)
(Lot 1) kDa
Speckled
F20 Sirolimus (1.511m), 25% PLGA, Opaque,
Extremely
223.36 80.21 (n=15)
(Lot 1) Polyarginine 5-15 kDa
Thick
F21 Sirolimus (1.5 m), Pullulan 200 kDa,
Transparent, Thick,
118.29 27.23 (n=15)
(Lot 1) Polyarginine 5-15 kDa
Speckled
F22 Sirolimus (1.5 m), Ultravist,
Tranparent, Light,
85.19 13.20 (n=6)
(Lot 1) Polyarginine 5-15 kDa
Speckled
F23 Sirolimus (1.511m), 25% PLGA,
(L Pullulan 200 kDa, Polyarginine 5-15
143.97 33.58 (n=6) Translucent, Thick
ot 1)
kDa
* Sirolimus:Polyarginine ratio is 10:1 for all formulations.
** Sirolimus/Balloon based on UV-Vis before pleating/folding and sterilization
of balloons
- 125 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
Table 24
Micell DCB Treated Vessels Per
Animals Blood PK
Necropsy Time Points
Formulation Animal
Formulation 3 2 balloon denuded
5 Minutes, 3 Days, 14
Formulation 19 Days ( 5%)
iliac arteries treated 2
Formulation 20 3 per time with Mice11 DCB via
(baseline/before
Formulation 21 point one 60 second necropsy) 3 days (
5%)
Formulation 22 inflation
Formulation 23
and 14
Totals 30 Animals 60 Vessels 60 Bloods 5 min., 3
Days (
5%)
Deployed balloons, blood samples and denuded iliac arteries analyzed for
Sirolimus levels.
* Day 14 blood samples will be sent for analysis only if drug detected in Day
3 blood samples.
** Day 14 animals may be sacrificed at a later time point based on arterial
drug levels at Day 3.
[00476] As a result of the studies, it was found that Formulations 3 and 20
most retained in
arteries at 3 days. Remaining formulations did not provide high levels of
arterial retention. The 5
Minute Retention results showed that F19 (no PLGA) retained ¨2 times more
compared to F3 (w/
PLGA). The 3 Day Retention results showed that F3 and F20 were within the same
level of retention
(¨ 4 ng/mg). There was retention variability in all formulations. In Whole
Blood results showed that
Sirolimus blood levels were below 1 ng/ml by 3 days. Drug released from
balloons results showed
that 70 %to 97% Sirolimus was released depending on formulation.
[00477]
Arterial sirolimus retention as a concentration in ng/mg, or as a total
sirolimus per
artery in micrograms is shown in Table 25. Whole blood sirolimus concentration
in ng/mL,and
estimated total sirolimus in the blood in micrograms is shown in Table 26. The
quantitation limit for
whole blood sirolimus detection was 0.1 ng/mL. The balloon sirolimus levels
(tested on the balloon
after deployment) and a calculated percent of sirolimus released or lost is
provided in Table 27. The
calculation of percent of sirolimus released or lost was based on batch
average of sirolimus on the
balloon for each formulation, respectively.
Table 25.
Arterial Sirolimus Conc. Total Sirolimus per
Formulation
(ng/mg)
Artery (11g)
F3: 5 Minutes
47.6 30.1 (n=6)
1.2 0.8 (n=6)
Sirolimus (1.511m), PLGA, Polyarginine 70 1(Da
F19: 5 Minutes
88.1 97.6 (n=6)
2.5 3.1 (n=6)
Sirolimus (1.5 m), Polyarginine 5-15 1(Da
F3: 3 Days
Sirolimus (1.511m), PLGA, Polyarginine 70 1(Da 4.6 5.1 (n=6) 0. 103
0.117 (n=6)
F19:3 Days
0.30 0.26 (n=6) 0.008
0.007 (n=6)
Sirolimus (1.5 m), Polyarginine 5-15 1(Da
F20: 3 Days
Sirolimus (1.5 m), 25% PLGA, Polyarginine 5-15 3.8 5.1 (n=6) 0.089
0.119 (n=6)
1(Da
- 126 -
CA 02888776 2015-04-17
WO 2014/063111 PCT/US2013/065777
F21: 3 Days
Sirolimus (1.51am), Pullulan 200 l(Da, Polyarginine 5- 0.11 0.05
(n=6) 0.003 0.002 (n=6)
15 l(Da
F22: 3 Days
Sirolimus (1.51am), Ultravist, Polyarginine 5-15 l(Da 0.34 0.17
(n=6) 0.008 0.004 (n=6)
F23: 3 Days
Sirolimus (1.511m), 25% PLGA, 1.2 1.9 (n=6) 0.028
0.040 (n=6)
Pullulan 200 l(Da, Polyarginine 5-15 l(Da
Table 26.
Sirolimus Conc. in Est. Total Sirolimus in
Formulation: Time Point
Whole Blood (ng/mL) Blood (tig) *
F3: 5 Minutes- Sirolimus (1.511m), PLGA,
6.02 1.43 (n=4) 1.49 0.39 (n=4)
Polyarginine 70 l(Da
F19: 5 Minutes- Sirolimus (1.51am),
25.47 10.08 (n=3) 6.12 2.43 (n=3)
Polyarginine 5-15 l(Da
F3: 3 Days- Sirolimus (1.511m), PLGA,
0.38 0.26 (n=3) 0.09 0.06 (n=3)
Polyarginine 70 l(Da
F19: 3 Days- Sirolimus (1.511m), Polyarginine
0.34 0.08 (n=3) 0.08 0.02 (n=3)
5-15 l(Da
F20: 3 Days- Sirolimus (1.511m), 25% PLGA,
0.66 0.26 (n=3) 0.15 0.06 (n=3)
Polyarginine 5-15 l(Da
F21: 3 Days- Sirolimus (1.511m), Pullulan 200
0.39 0.02 (n=3) 0.09 0.01 (n=3)
l(Da, Polyarginine 5-15 l(Da
F22: 3 Days- Sirolimus (1.511m), Ultravist,
0.23 0.05 (n=3) 0.05 0.02 (n=3)
Polyarginine 5-15 l(Da
F23: 3 Days- Sirolimus (1.511m), 25% PLGA,
0.68 0.13 (n=3) 0.15 0.02 (n=3)
Pullulan 200 l(Da, Polyarginine 5-15 l(Da
* Based on 56 mL Blood per kg
Table 27
Sirolimus Conc. on
% Sirolimus
Formulation Balloon
Released/Lost*
post Deployment (ug)
F3 - Sirolimus (1.511m), PLGA, Polyarginine 70
36.5 7.9 (n=20) 69.7 7.7% (n=20)
l(Da
F19- Sirolimus (1.51am), Polyarginine 5-15 l(Da 6.9 2.1 (n=18)
95.0 1.5% (n=18)
F20 - Sirolimus (1.511m), 25% PLGA,
27.9 8.6 (n=6) 87.4 3.4% (n=6)
Polyarginine 5-15 l(Da
F21 - Sirolimus (1.511m), Pullulan 200 l(Da,
3.9 1.5 (n=6) 96.8 1.2% (n=6)
Polyarginine 5-15 l(Da
F22 - Sirolimus (1.511m), Ultravist, Polyarginine
5.1 0.8 (n=6) 93.9 0.9% (n=6)
5-15 l(Da
F23 - Sirolimus (1.511m), 25% PLGA,
21.0 6.0 (n=6) 85.3 3.6% (n=6)
Pullulan 200 l(Da, Polyarginine 5-15 l(Da
* Based on Balloon Batch Averages of Sirolimus
- 127 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
[00478] Mutilple lots of Formulation 3 were produced to evaluation
variability and effects
generally of the sirolimus size (e.g. sirolimus having an average size of 2.5
microns versus sirolimus
having an average size of 1.5 microns). The results are presented in Table 28,
Table 29, and Table
30. Concentrations in Table 28 were normalized using a normalized artery
weight of 0.025 grams.
The calculation of percent of sirolimus released or lost for Table 29 was
based on batch average of
sirolimus on the balloon for each formulation, respectively. The quantitation
limit for whole blood
sirolimus detection was 0.1 ng/mL (for Table 30).
Table 28
5 minutes 24 hours 72 hours
Formulation Composition Sirolimus Sirolimus
Sirolimus
Concentration (ng/mg) Concentration (ng/mg) Concentration (ng/mg)
F3 (LOT 1) - (PLGA, Sirolimus
119.2 57.6 (n=6) 32.9 18.0
(n=8) 8.1 5.7 (n=6)
2.5 [tm, Polyarginine 701(Da)
F3 (LOT 2) - (PLGA, Sirolimus
38.6 16.3 (n=2) 48.5 63.7
(n=2) 41.1 (n=1)
1.5 [tm, Polyarginine 701(Da)
F3 (LOT 3) - (PLGA, Sirolimus
47.6 31.4 (n=6) N/A
4.1 4.7 (n=6)
1.5 [tm, Polyarginine 701(Da)
Table 29
Sirolimus on Balloon Sirolimus on Balloon % Sirolimus
Formulation Composition Before Deployment
After Deployment (ug) Released/Lost*
(ug)
F3 (LOT 1) - (PLGA, Sirolimus
89.5 19.6 (n=9) 27.6 9.1
(n=20) 68.4 15.4% (n=20)
2.5 [tm, Polyarginine 701(Da)
F3 (LOT 2) - (PLGA, Sirolimus
128.7 26.9 (n=42) 56.8 15.6
(n=6) 59.0 8.6% (n=6)
1.5 [tm, Polyarginine 701(Da)
F3 (LOT 3) - (PLGA, Sirolimus
114.5 25.2 (n=18) 36.5 7.9
(n=20) 69.7 7.7% (n=20)
1.5 [tm, Polyarginine 701(Da)
* Based on Balloon Batch Averages of Sirolimus
Table 30
Sirolimus Concentration in Whole Blood (ng/mL)
Formulation Composition
5 minutes 24 hours
72 hours
F3 (LOT 1) - (PLGA, Sirolimus
7.7 3.7 (n=3) 0.8 0.7 (n=4) BQL (n=3)
2.5 [tin, Polyarginine 70 1(1)a)
F3 (LOT 2) - (PLGA, Sirolimus
5.5(n=1) BQL (n=1)
BQL (n=1)
1.5 [tin, Polyarginine 70 1(1)a)
F3 (LOT 3) - (PLGA, Sirolimus
6.0 1.4 (n=4) N/A 0.4 0.3 (n=3)
1.5 [tin, Polyarginine 701(Da)
EXAMPLE 10: COATING OF RAPAMYCIN WITH PLGA THROUGH SPRAY-DRYING
[00479]
In this example, rapamycin is coated with PLGA through a spray-drying process.
A
mixture of 75 (w/w)% PLGA and 25 (w/w)% rapamycin is dissolved in THF to form
a solution.
Other polar aprotic solvent, such as acetonitrile, or mixtures of polar
aprotic solvents, can also be used
- 128 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
to form the solution. The solution is discharged from a spray nozzle and the
solvent is evaporated in a
drying chamber to form the PLGA-encapsulate rapamycin particles, which are
collected via
continuous discharge from the drying chamber to minimize thermal degradation.
In another example,
a slurry of rapamycin, PLGA, and water are spray dried to form the PLGA-
encapsulated rapamycin
particles.
[00480] The particle size of rapamycin can be controlled by adjusting
spraying parameters
(e.g. pressure, temperature) and nozzle configurations (e.g. size, swirl
chamber, rotory disk). In this
example, the spraying parameters and nozzle configuration are adjusted to
produce rapamycin
particles having an average particle size of from 5 to 150 nm, and more
preferably from 10 to 100 nm.
In other examples, the spraying parameters and nozzle configuration are
adjusted to produce
rapamycin particles having an average particle size of from 1 to 10 microns,
and more preferably from
1 to 5 microns.
EXAMPLE 11: COATING OF RAPAMYCIN WITH PLGA THROUGH FLUID BED
COATING
[00481] In this example, rapamycin particles (preferably crystallined, e.g.
crystallinity of 25%
to 95%) are micronized and suspended on a bed of air, wherein a PLGA solution
(e.g. 10-50 wt% in
THF) is sprayed on the suspended rapamycin particles. Other polar aprotic
solvent may also be used.
The particles are then transported to a drying chamber, where the solvent are
evaporated to form the
PLGA-encapsulated rapamycin particles. If needed, the encapsulated particles
are re-suspended,
sprayed with the PLGA solution, and dried until the coating thickness of the
PLGA-encapsulated
rapamycin particles reaches the desired level, e.g. 2 to 20 micron or about 10
microns.
EXAMPLE 12: COATING OF RAPAMYCIN WITH PLGA THROUGH PAN COATING
[00482] In this example, particles of PLGA, such as PLGA pellets, are
added to rapamycin
particles in a tumbling vessel to form the PLGA-encapsulated rapamycin
particles. For example,
PLGA may be ground, such as through ball mill, jet mill, or cryogenic
grinding, to appropriate
particle size (e.g. about 30 microns). The particle size and PLGA layer
thickness of the encapsulated
rapamycin can be controlled by the feeding rate of the PLGA pellets. In this
example, the PLGA
pellets are fed into the tumbling vessel at a rate of from 100 to 1000 [tg per
minute, preferably from
300 to 500 [tg per minute.
[00483] While Examples 10, 11, and 12 provide mechanical or physical method
for
encapsulation of the rapamycin in the PLGA, they should not be interpreted as
limiting the scope of
the present disclosure. Chemical methods of encapsulation, such as core-shell
emulsion preparation
complex coacervation or in situ polymerization, can also be used to form the
encapsulated rapamycin.
[00484] Much of the description herein is provided with reference to a
balloon and a treatment
site that is an artery or blood vessel for ease of description and brevity.
Nevertheless, the methods,
- 129 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
descriptions, devices, and coatings described herein apply to alternative
devices and treatment
locations.
[00485] Unless otherwise stated, use of the term "about" in this
description can mean
variations of 0.1%, 0.5%, 1%, 2%, 3%, 5%, 10%, 15%, 20%, 25%, 30%, 40%, and/or
50%,
depending on the particular embodiment. Where the element being described is
itself expressed as a
percent, the variations are not meant to be percents of percents, rather they
are variations as an
absolute percent¨i.e. an element that is expressed as "about 5%" may be
actually 5%+/- 1%, or from
4% to 6%, depending on the embodiment. Only the variations that would be
rational to one of
ordinary skill in the art are contemplated herein. For example, where the
element itself is expressed as
a small percent, and a person of ordinary skill would know that the element is
not rational to go below
0, the variations contemplated would not go below zero (i.e. about 5% could
mean 5% +/-5% or 0-
10%, but not 5% +/- 10% or -5% to 15%, where this is not reasonable to one of
skill in the art for the
element being described).
[00486] The foregoing is illustrative of the present invention, and is
not to be construed as
limiting thereof While embodiments of the present invention have been
indicated and described
herein, it will be obvious to those skilled in the art that such embodiments
are provided by way of
example only. Numerous variations, changes, and substitutions will now occur
to those skilled in the
art without departing from the invention. It should be understood that various
alternatives to the
embodiments of the invention described herein may be employed in practicing
the invention. It is
intended that the following claims define the scope of the invention and that
methods and structures
within the scope of these claims and their equivalents be covered thereby.
EXAMPLE 13: COATING OF RAPAMYCIN WITH PLGA THROUGH SINGLE
EMULSION/EVAPORATION WITH CENTRIFUGE
[00487] In this example, rapamycin is coated with PLGA through a
single
emulsion/evaporation technique using a centrifuge to filter the emulsion. 200
mg poly(lactic-co-
glycolic)acid (RG504H 0 viscosity 0.45-0.6) was dissolved in 4 ml
dichloromethane (oil phase). One
hundred 1 of a stock solution of rapamycin in Dimethyl Sulfoxide (10 mg/ml)
was dissolved in the
oil phase, which was then homogenized at 10100 rpm for 1 min in a 2% PVA (MW ¨
25,000, 98%
hydrolyzed) solution, using a homogenizer. This emulsion was immediately
poured into 90 ml of a
1% PVA solution, and dichloromethane was allowed to evaporate. After 3 hours,
the particles were
centrifuged (1500 g, 10 min, 4 C) and washed four times in deionized water.
Microparticles were
then re-suspended in 5 ml of deionized water, frozen on dry ice and
lyophilized. Fluorescently labeled
rapamycin-microparticles were prepared by adding 100 1 of 2 mg/ml Alexa Fluor
647 carboxylic
acid, succinimidyl ester to the oil phase along with the rapamycin and
following the same protocol as
above.
- 130 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
EXAMPLE 14: COATING OF RAPAMYCIN WITH PLGA THROUGH SINGLE
EMULSION/EVAPORATION WITH FILTER PAPER
[00488] In this example, rapamycin is coated with PLGA through a
single
emulsion/evaporation technique using filter paper to filter the emulsion. Five
to 10 grams of PVA
(Polyscience Inc, MW ¨25,000, 98% hyrdolized) was dissolved in warmed HPLC
grade water to
create 1% and 2% PVA solutions. Approximately 400 mg of 50:50 Poly DL-lactide-
co-glycolide
(Ester terminated, IV 0.55-0.75, Durect Corporation, Pelham, AL) was added to
a 50 ml centrifuge
tube and dissolved in 8 ml of Dichloromethane (oil phase). Approximately 10 mg
of rapamycin was
added to 1 ml. Dimethylsulfoxide and swirled until white particles were no
longer visible. 200 iLil of
the stock rapamycin solution was added to the oil phase. This solution was
then poured into ¨100 ml
of 2% PVA and homogenized for 1 min at ¨17,500 rpm. The emulsion was then
poured into 180 ml
of 1% PVA and left uncovered overnight to allow the dichloromethane to
evaporate. A frit funnel
was lined with two size 3 Whatman filter paper pieces (pre-wetted with HPLC
grade water) prior to
filtering the solution. The solution was poured through the filter paper,
leaving the particles on the
filter paper. The particles were re-suspended by submerging the filter paper
in 10 ml of water. The
liquid was transferred to a 100 ml round bottom flask which was frozen on dry
ice and then
lyophilized.
[00489] Referring now to Figure 5, the encapsulated microparticles
prepared in this Example
were examined for size and shape using a Hitachi Model S-4700 scanning
electron microscope
(SEM). The SEM image shows that the encapsulated rapamycin particles prepared
in this Example
are generally smooth and spherical. For example, at least 50%, 60%, 70%, 80%,
or 90% of the
particles have a generally spherical shape. Figure 5 also shows a general
particle size range of from
about 0.5 [tin to about 10 [tin. For example, at least 50%, 60%, 70%, 80%, or
90% of the particles
have a size range of about 0.5 gm to about 10 [tin. The particles prepare in
this Example have an
average paticle size of from about 0.5 [uri to about 10 [(m, from about 1 [uri
to about 10 [tin, from
about 1 [Lin to about 8 [tin, from about 1 [Lin to about 7 [tin, from about 1
[Lin to about 6 [tin, from
about 1 [tin to about 5 pm, or from about 2 [Lin to about 4 [tin. The presence
of rapamycin inside the
mircoparticles was confirmed by UV-VIS spectroscopy (Perkin-Elmer Lambda 25 UV-
Vis
Spectrophotometer).
EXAMPLE 15: CRYSTALLINE RAPAMYCIN ENCAPSULATION
[00490] Five to 10 g of PVA (MW ¨25,000, 98% hydrolyzed) was dissolved
in warmed
HPLC grade water to create 1% and 2% PVA solutions. Approximately 200 mg of
50:50 Poly DL-
lactide-co-glycolide (Ester terminated, IV 0.55-0.75) was added to a 50 ml
centrifuge tube and
dissolved in 4 ml of dichloromethane (oil phase). Approximately 50 ml of 1%
PVA was added to the
oil phase and homogenized for 2 mins at ¨17,500 rpm. The emulsion was left
uncovered over night to
allow the dichloromethane to evaporate. Approximately 1 mg of rapamycin was
homogenized in 20
- 131 -
CA 02888776 2015-04-17
WO 2014/063111
PCT/US2013/065777
ml of 2% PVA for two minutes at ¨17,500 rpm. The rapamycin mixture was then
added to the
polymer emulsion and homogenized for two minutes at ¨17,500 rpm. A frit funnel
was lined with
two size 3 Whatman filter paper pieces (pre-wetted with HPLC grade water)
prior to filtering the
solution. The solution was poured through the filter paper, leaving the
particles on the filter paper.
The particles were re-suspended by submerging the filter paper in 10 ml of
water. The liquid was
transferred to a 100 ml round bottom flask which was frozen on dry ice then
lyophilized.
[00491] Referring now to Figure 6, the particles prepared in this
Example were examined for
size and shape using a Hitachi Model S-4700 scanning electron mircroscope
(SEM). The SEM image
shows that the encapsulated rapamycin particles prepared in this Example are
generally smooth and
spherical. For example, at least 50%, 60%, 70%, 80%, or 90% of the particles
have a generally
spherical shape. Figure 6 also shows a general particle size range of from
about 1 [tin to about 50 [tin.
For example, at least 50%, 60%, 70%, 80%, or 90% of the particles have a size
range of about 1 [tin to
about 50 [tin. The particles prepare in this Example have an average paticle
size of from about 1 [tin
to about 50 lam, from about 1 gm to about 40 gm, from about 5 gm to about 40
gm, from about 5 gm
to about 35 gm, from about 5 pin to about 30 p.m, from about 10 p.m to about
30 p.m, or from about 10
gm to about 20 gm. The presence of Rapamycin inside the microparticles was
confirmed by UV-VIS
spectroscopy (Perkin-Elmer Lambda 25 UV-Vis Spectrophotometer).
EXAMPLE 16: ENCAPSULATED MICROPARTICLE CHARACTERIZATION
[00492] Encapsulated microparticles are sized and counted using volume
impedance
measurements on a Beckman Coulter Counter. Average size was determined by
counting at least
10,000 particles. Microparticle surface morphology and shape were examined
using a scanning
electronic microscope. The surface charge of the microparticles was determined
by zeta potential
measurements.
[00493] SEM images of the particles show smooth surface morphology and
confirm the size
obtained from the volume impedance measurements. The size distribution
observed in SEM images
is characteristic of the microparticle encapsulation process described herein.
EXAMPLE 17: ENCAPSULATION EFFICIENCY CHARACTERIZATION
[00494] Five mg of encapsulated rapamycin microparticles was dissolved
in 1 ml of
acetronitrile (HPLC grade), sonicated for 5 minutes and left under a constant
vortex for 30 min. The
amount of rapamycin was then determined by measuring absorbance of the
solution at 278 nm using a
UV-equipped plate reader. Encapsulation efficiency was calculated as the ratio
of rapamycin present
inside particles to the amount of rapamycin that was initially added.
- 132 -