Note: Descriptions are shown in the official language in which they were submitted.
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
METHODS FOR PRODUCING- BASESTOCKS FROM
RENEWABLE SOURCES USING DEWAXING CATALYST
FIELD
10001] The present disclosure relates to catalysts for use in dewaxing and
other
hydrocarbon conversion processes and methods of using such catalysts.
Specifically, this disclosure relates to a dewaxing catalyst comprising a
zeolite
component, a metal component for promoting -hydrogenation and a
hydrothermally stable binder componentõ and methods of using such catalysts.
BACKGROUND
10002] Waxy feedstocks may be used to prepare basestocks having a high
viscosity index (VI). However, in order to obtain a basestock having the low
temperature properties suitable for most uses, it is usually necessary to
dewax the
feedstock, Dewaxing may be accomplished by means of a solvent or
catalytically. Solvent dewaxing is a physical process whereby waxes are
removed
by contacting with a solvent, such as methyl ethyl ketone, followed by
chilling to
crystallize the wax and filtration to remove the wax. Catalytic dewaxing
involves
chemically converting the hydrocarbons leading to unfavorable low temperature
properties to hydrocarbons having more favorable low temperature properties.
Long chain normal paraffins and. slightly branched paraffins readily solidify
and.
thus result in generally unfavorable low temperature properties. Catalytic
dewaxing is a process for converting these long chain normal paraffins and
slightly branched paraffins to molecules having improved low temperature
properties.
100031 Catalytic &waxing- may be accomplished using catalysts that function
primarily by cracking waxes to lower boiling products, or by catalysts that
primarily isomerize waxes to more highly branched products. Catalysts that
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
2
dewax by cracking decrease the yield of lubricating oils while increasing the
yield
of lower boiling distillates. Catalysts that isomerize do not normally result
in
significant -boiling point conversion. Catalysts that dewax primarily by
cracking
are exemplified by the zeolites ZSM-5, ZSM-11, ZSM-12, beta and offretite.
Catalysts that dewax primarily by isomerization are exemplified by the
zeolites
ZSM-22, ZSM-23, SSZ-32, ZSM-35, ZSM-48 and ZSM-50. To ensure adequate
mechanical strength for use in a dewaxing reactor, such zeolite catalysts are
generally combined with an inorganic oxide binder, such as alumina,
10004] Catalysts are needed for the upgrading of renewable basestocks for
fuels and lubricant applications. For example, a catalyst for fatty acid
coupling
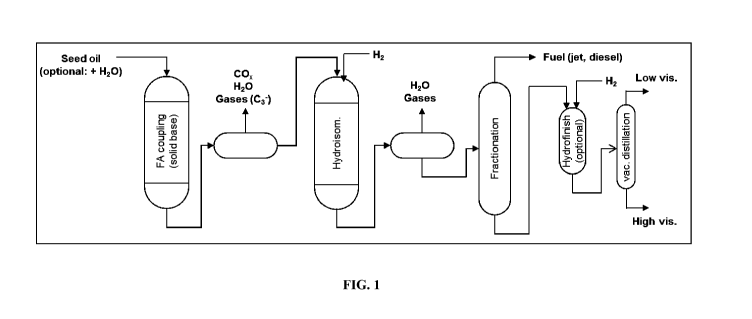
helps production of a highly flexible feedstock. As shown in Figure 1, this
feedstock can then be hydrogenated and/or isomerized using conventional
refinery
processing, thereby producing high value products consisting of a mixture of
fuels, -high viscosity, and low viscosity lubricants. This product stream can
easily
be separated using conventional fractionation and distillation equipment.
100051 The hydrogenationlisomerization catalyst for renewable feedstocks
has several challenges to deal with: 1) a highly oxygenated feed (10% oxygen),
2)
high heats of reaction, and 3) generation of water which is converted into
steam in
the reactor. The last challenge is of major concern to current dewaxing
catalysts
because steam can cause issues with the hydrothermal stability of the catalyst
and
can cause deactivation by dealuminating the zeolite catalyst and/or
degradation of
the oxide support/binder leading to agglomeration of the metal.
100061 Conventional dewaxing catalysts are, however, susceptible to
poisoning by contaminants i.n a feedstock. To mitigate the problem of catalyst
poisoning and to allow effective dewaxing of feedstocks with very high levels
of
waxy materials, it is often desirable to be able to maximize the dewaxing
activity
of the catalyst. However, in seeking maximize activity, it is also important
to
maintain the mechanical strength of the catalyst.
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
3
100071 U.S. Patent No. 8,263,517 to Christine N. Elia, describes a &waxing
catalyst comprising a zeolite with a low silica to alumina ratio in
combination
with a low surface area binder. The low surface area binder is believed to
increase access to the active sites of the zeolite. Especially for bulky
feeds,
increased access to zeolite active sites is expected to lead to an overall
increase in
activity.
10008j U.S. Patent Publication No. 2011/0192766 mentions a supported
catalyst comprising a zeolite having a silica to alumina molar ratio of 500 or
less,
a first metal oxide binder having a crystallite size greater than 200 A and a
second
metal oxide binder having a crystal.lite size less than 100 A, wherein the
second
metal oxide binder is present in an amount less than 15 wt % of the total
weight
of the catalyst,
SUMMARY
[00091 The present disclosure relates to catalysts for use in dewaxing and
other
hydrocarbon conversion processes and methods of using such catalysts. in an
embodiment, there is provided a method for producing a lube base stock and/or
a
fuel from a feedstock of biological origin, the tuethod comprising: contacting
the
feedstock in the presence of a catalyst to produce a lube base stock and/or a
fuel,
wherein the catalyst comprises: a zeolite component selected from a zeolite
having 10-member ring pores, a zeolite having 12-lumber ring pores and a
combination thereof, 0.1 to 5 weight % of a hydrogenation component selected
from Pt, PdõAg, Ni, Mo, Co, W, Rh, Re, Ru, 1r and a mixture thereof, and a
hydrothermally stable binder component selected from silica, alumina, silica-
alumina, titania, zirconia, tantalum oxide, tungsten oxide, molybdenum oxide,
vanadium oxide, magnesium oxide, calcium oxide, yttrium oxide, lanthanum
oxide, cerium oxide, niobiuni oxide, tungstated zirconia, cobalt molybdenum
oxide, cobalt molybdenum sulfide, nickel molybdenum oxide, nickel
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
4
molybdenum sulfide, nickel tungsten oxide, nickel tungsten sulfide, cobalt
tungsten oxide, cobalt tungsten sulfide, nickel molybdenum tungsten oxide and
nickel molybdenum tungsten sulfide, cobalt molybdenum tungsten oxide and
cobal.t molybdenum tungsten sulfide, wherein the weight ratio of the zeolite
to the
hydrothermally stable binder is 85:15 to 25:75.
10010] In another embodiment, there is provided a method for producing a
lube
basestock and/or a fuel from a feedstock of biological origin, the method
comprising: contacting the feedstock in the presence of a catalyst to produce
a
lube base stock and/or a fuel, wherein the catalyst comprises: a zeolite
selected
from ZSM-48, ZSM-23, ZSM-50, ZSM-5, ZSM-22, ZSM-11, ferrierite, faujasite,
beta., ZSM-12, MOR, and a mixture thereof, and a hydrogenation component
comprising at least three metals selected from the group consisting of Pt, Pd,
Ag,
Ni, Mo, Co, W. Rh, Re, and Ru, wherein at least one of the at least three
metals is
in either an oxide or sulfide form. In an aspect of the present embodiment,
the
catalyst further comprises a binder component.
BRIEF DESCRIPTION OF THE DRAWINGS
[00111 Figure 11 is a schem.e illustrating process flow schem.atic for the
conversion of renewable feedstocks to higher value fuels and lubes products
where a catalyst of the present disclosure can be placed into the
hydroisomerization unit.
10012j Figure 2 is a scheme illustrating process chemistry for the
conversion of
renewable feedstocks.
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
DETAILED DESCRIPTION
[00131 All numerical values within the detailed description and the claims
herein are modified by "about" or "approximately" the indicated value, and
take
into account experimental error and variations that would be expected by- a
person
having ordinary skill in the art.
[00141 The present disclosure provides a method for producing a lube base
stock and/or a fuel from a feedstock of biological origin, the method
comprising:
contacting the feedstock in the presence of a catalyst to produce a lube base
stock
and/or a fuel, wherein the catalyst comprises a zeolite, a metal for promoting
hydrogenation and a hydrothermally stable binder. In various embodiments, the
zeolite is selected from selected from a zeolite having 1.0-member ring pores,
a
zeolite having 12-member ring pores and a combination thereof; the metai
component is selected from the group consisting of Pt, Pd, Ag, Ni, Mo, W. Rh,
Re, Ril and a mixture thereof; and the hydrothermally stable binder is
selected
from silica, alumina, silica-alumina, titania, zirconia, tantalurn oxide,
tungsten
oxide, molybdenum oxide, vanadium oxide, magnesium oxide, calcium oxide,
yttrium oxide, lanthanum oxide, cerium oxide, niobium oxide, tungstated
zirconia,
cobalt molybdenurn oxide, cobalt molybdenum sulfide, nickel molybdenurn
oxide, nickel molybdenum sulfide, nickel tungsten oxide, nickel tungsten
sulfide,
cobalt tungsten oxide, cobalt tungsten sulfide, nickel molybdenum tungsten
oxide
and nickel molybdenum tungsten sulfide, cobalt molybdenum_ tungsten oxide and
cobalt molybdenum tungsten sulfide. The catalysts provided herein have
improved hydrothermal stability of the dewaxing catalysts which are, for
example,
used in conversion of renewable basestock. Also, the catalysts can minimize
metal agglomeration, thereby- improving catalytic selectivity and activity.
100151 As shown in Figure 2, a solid base catalyst such as La/ZrO2 converts
natural oils via coupling reactions to ketone or acid functionalized
feedstocks.
The catalyst of the present disclosure is used in the next stage and is
capable of
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
6
doing the hydrogenation and/or isomerization in the presence of water and CO2
without significantly cracking the molecules to gaseous products.
10016] In various embodiments, a weight ratio of the zeolite to the
hydrothermally stable binder can be controlled. In an enthodirnent, for
exarnple,
the weight ratio of the zeolite to the hydrothermally stable binder is 85:15
to
25:75, particularly, 80:20 to 65:35. In particular embodiments, the ratio is
80:20
or 65:35.
1001171 In another embodiment, there is provided a method for producing a
lube
base stock and/or a fuel from a feed.stock of biological origin, the method
comprising: contacting the feedstock in the presence of a catalyst to produce
a
lube base stock and/or a fuel, wherein the catalyst comprises: a zeolite
selected
from ZSM-48, ZSM-23, ZSM-50, ZSM-5, ZSM-22, ZSNI-11, ferrierite, faujasite,
beta, ZSM-12, MOR and a mixture thereof, and a hydrogenation component
comprising at least three metals selected from the group consisting of Pt, Pd,
Ag,
Ni, Co, Mo, W, Rh, Re, and Ru, wherein at least one of the at least three
metals is
in either an oxide or sulfide form. The catalyst comprising a ternary metal
component can be used in a conversion reaction without additional binder
component. in an. aspect of the present embodiment, the catalyst further
comprises a hydrothermally stable binder.
Zeolite component
100181 A zeolite to be employed in the present catalyst composition can be
selected based on the intended use of the catalyst. When the catalyst is to be
used
in isomerization dewaxing, suitable zeolites include those having 10-menibered
ring pores and particularly those having unidirectional 10-membered ring
pores.
Examples of suitable zeolites include ZSM-48, ZSNI-23, ZSNI-50,
ZSM-22, ZSM-11, ferrierite and combinations thereof. Other suitable zeolites
include those having 12-membered ring pores arid examples of suitable zeolites
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
7
include from faujasite, beta, ZSM-12, MOR and combinations thereof. Also,
suitable zeolites include a combination of a zeolite having 10-membered ring
pores and a zeolite having 12-membered ring pores: tor example, a combination
of beta and ZSM-48.
100191 In particular embodiments, ZSM-48 or ZSM-23 is used as the zeolite
component, and the catalysts are particularly useful in the isomerization
dewaxing
of lube oil -basestocks. Such feedstocks are wax-containing feeds that boil in
the
lubricating oil range, typically having. a 1_0% distillation point greater
than 650 F
(343 C), measured by ASTM 1)86 or ASTM D2887. Such feeds may be derived
from a number of sources such as natural oils like seed oils and animal fats,
oils
derived from solvent refining processes such as raffinates, partially solvent
dewaxed oils, deasphalted oils, distillates, vacuum gas oils, coker gas oils,
slack
waxes, foots oils and the like, and Fischer-Tropsch waxes.
100201 In a particular embodiment, the zeolite component is ZSM-48. ZSM-48
crystals, as used herein, is described variously in terms of "as-synthesized"
crystals that still contain the organic ternplate,; calcined crystals, such as
-Na-form
ZSM-48 crystals; or calcined and ion-exchanged crystals, such as H-form
ZSM-48 crystals. ZSM-48 crystals after removal of the structural directing
agent
have a particular morphology and a molar composition according to the general
formula:
(n)Sia? :A1203
where n is from. 70 to 210. In another embodiment, n is 80 to 100. In yet
another
particular embodiment, n is 85 to 95. In still other embodiments, Si may be
replaced by Cie and Al may be replaced by Ga., B, Fe, Ti, V, and Zr.
100211 The as-synthesized form of ZSM-48 crystals is prepared from a
mixture
having silica, alumina, base and hexamethonium salt directing agent. In an
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
8
embodiment, the molar ratio of structural directing agent:silica in the
mixture is
less than 0.05, less than 0.025, or less than 0.022. In another embodiment,
the
molar ratio of structural directing agent:silica in the mixture is at least
0.01, at
least 0.015, or at least 0.016. In still another embodiment, the molar ratio
of
structural directing agent:silica in the mixture is from 0,015 to 0.025,
preferably
0.016 to 0.022.
10022j Particularly, the catalysts used in processes according to the
disclosure
have a zeolite component with a low ratio of silica. to alumina. For example,
for
ZS14-48, the ratio of silica to alumina in the zeolite can be less than 200:1,
less
than 110:1, less than 100:1, less than 90:1, or less than 80:1. In a
particular
embodiment, the ratio of silica to alumina in the zeolite is less than 80:1,
for
example, particularly '70:1.
Hydrogenation component
100231 A hydrogenation component proniotes the reaction of hydrogen 'with
olefinic unsaturation in fatty acids, fatty acid dimers and oligomers,
ketones,
heavier oxygenates, and other intermediate reaction products. It further acts
to
reduce carbonyl, carboxyl, hydroxyl, and other oxygen containing groups to
provide the saturated. hydrocarbons as reaction products. Working in concert
with
other components in the dewaxing catalysts, it also provides isomerization
functionality, helping to introduce sufficient branching in the final
hydrocarbon
products, where needed, to give basestocks with suitable pour point and low
temperature properties.
10024j Catalysts suitable for hydrogenation include metals such as Pt, Pd,
Ag,
Ni, Co, Mo, W, Rh, Re, Ru, Ir as well as binary or ternary mixtures thereof.
hi
various embodiments, the metal hydrogenation component is a Group VIII noble
metal. In non-limiting fashion, the metal hydrogenation component is Pt. Pd or
a
mixture thereof. In another embodiment, the metal hydrogenation component is a
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
9
binary mixture, such as, for example, a combination of a non-noble Group VIII
metal and a Group VI metal. Suitable combinations include Ni or Co with Mo or
W, particularly Ni with Mo or W. In yet another embodiment, the hydrogenation
component comprises at least three metals selected from the group consisting
of
N, Pd, Ag, Ni, Mo, Co, W, Rh, Re, and RU, wherein at least one of the at least
three metals is in either an oxide or sulfide :form. In a particular
embodiment, the
metal component is (a) Ni, IVIo0x and W0x; or (b) Co, IVIo0x and W0x, wherein
x is in the range of 0.5 to 3.
10025] The
metal hydrogenation component may be added to the catalyst in
any convenient manner. One technique for adding the metal hydrogenation
component is by incipient wetness. For example, after combining a zeolite and
a
hydrothermally stable binder, the combined zeolite and binder are extruded
into
catalyst particles. The catalyst particles are exposed to a solution
containing a
suitable metal precursor containing the Group VI or Group VIII metal.
Alternatively, metal can be added to the catalyst by ion exchange, where a
metal
precursor i.s added to a mixture of zeolite (or zeolite and binder) prior to
extrusion.
The inetal hydrogenation component may be steamed prior to use.
[00261 The
amount of hydrogenation metal component may range from 0.1 to
wt %, based on catalyst. In an embodiment, the amount of metal component is
at least 0.1 wt %, at least 0.25 wt %, at least 0.5 wt %, at least 0.6 wt %,
or at least
0.75 wt %.
Hydrothermally stable binders
10027j The
catalyst needs to be stable in the presence of water especially when
excessive water is generated during a conversion reaction. In
various
embodiments, a catalyst of the present disclosure comprises a binder component
to increase mechanical strength and stability of the catalyst in the presence
of
water under effective hydrogenation conditions. Such a binder is referred to
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
herein as a "hydrothermally stable binder," Non-limiting examples of suitable
binder components include silica, alumina, silica-alumina, titania, zirconia,
tantalum oxide, tungsten oxide, molybdenum oxide, vanadium oxide, magnesium
oxide, calcium oxide, yttrium oxide, lanthanum oxide, cerium oxide, niobium
oxide, tungstated zirconia, cobalt inolybdenum oxide, cobalt molybdenum
sulfide,
nickel molybdenum oxide, nickel molybdenum sulfide, nickel tungsten oxide,
nickel tungsten sulfide, cobalt tungsten oxide, cobal.t tungsten sulfide,
nickel
molybdenum tungsten oxide and nickel molybdenum tungsten sulfide, cobalt
molybdenum tungsten oxide and cobalt molybdenum tungsten sulfide.
100281 In an embodiment, a hydrothermally stable binder component is
selected from binders capable of storing hydrogen, thereby keeping the metal
in a
reduced, highly dispersed state. Non-limiting examples of such binders include
tungsten oxide, molybdenum oxide, vanadium oxide, and a mixture thereof.
100291 In another embodiment, a hydrothermally stable binder component is a
basic oxide, a binder capable of adsorbing carbon dioxide selectively or a
binder
which does not change to a denser phase upon exposure to steam and
temperatures above 350 C. Non-limiting examples of such binders include
magnesium oxide, calcium oxide, yttrium oxide, cerium oxide, niobium oxide,
lanthanum oxide, zirconium oxide, and a mixture thereof.
100301 in another embodiment, a hydrothermally stable binder component is a
complex metal oxide used in. hydroprocessing. Non-limiting examples of such
binders include cobalt molybdenum oxide, cobalt molybdenum sulfide, nickel
molybdenum oxide, nickel molybdenum sulfide, nickel tungsten oxide, nickel
tungsten sulfide, nickel molybdenurn tungsten oxide and nickel molybdenum
tungsten sulfide.
10031j In particular embodiments, the hydrothermally stable binder
component
is selected from lanthanum, cerium, niobium, nickel tungsten oxides, nickel
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
11
tungsten sulfides, nickei molybdenum tungsten oxides, and nickel molybdenum
tungsten sulfide.
10032] A zeolite can be combined with a binder in any convenient manner.
For example, a bound catalyst cari be produced by starting with powders of
both
the zeolite and binder, combining and mulling the powders with added water to
form a mixture, and then extruding the mixture to produce a bound catalyst of
a
desired size. Extrusion aids can also be used to modify the extrusion now
properties of the zeolite and binder mixture.
[00331 A catalyst comprising a ternary metal hydrogenation component has
good hydrothermal stability with or without a binder. In an embodiment, to
achieve improved stability, the catalyst may further comprise a binder
selected
from various metal oxides. Non-limiting examples of such binders include
silica,
alumina, silica-alumina, titania, zirconia, tantalum oxide, tungsten oxide,
molybdenum oxide, vanadium oxide, m.agnesium oxide, calcium oxide, yttrium
oxide, lanthanum oxide, cerium oxide, niobium_ oxide, titanium oxide,
lanthanum_
oxide, zirconiurrì oxide, tungstated zirconia, cobalt molybdenum oxide, cobalt
molybdenum sulfide, nickei tungsten oxide, nickel tungsten sulfide, nickel
molybdenum_ tungsten oxide, nickel molybdenum tungsten sulfide, and a mixture
thereof. In particular embodiments, the hydrothermally stable binder is
selected
from lanthanum, cerium, niobium, nickel tungsten oxides, nickei tungsten
sulfides, nickel mo13,rbdenum tungsten oxides, and nickel. molybdenum tungsten
sulfide.
Dewaxinr catalysts
10034] A catalyst of this disclosure can be prepared by combining the three
components, i.e., a zeolite, a hydrogenation component and a binder. Each of
the
three components can be selected from various components described herein,
particularly choosing specific examples listed herein. In various embodiments,
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
12
for example, the hydrogenation component is selected from Ni and Pt; the
zeolite
is ZSM-48 or ZSM-23; and the hydrothermally stable binder is selected from
nickel molybdenum tungsten oxides, nickel molybdenum tungsten sulfide, W03,
La203, Ce02, and Nb205. Non-limiting examples of such catalysts include: (a) a
catalyst comprising Ni, ZSM-48 and W03; (b) a catalyst comprising Ni, ZSM-23
and W03; (c) a catalyst comprising Pt, ZSM-48 and La203; (d) a catalyst
comprising Pt, ZSM-48 and Ce02; (e) a catalyst comprising Pt, ZSM-48 and
Nb205; (f) a catalyst comprising Pt, ZSM-23 and La203; (g) a catalyst
comprising
Pt, ZSM-23 and Ce02; (h) a catalyst comprising Pt, ZSM-23 and Nb205; (i) a
catalyst comprising Pt, ZSM-48 and W03; and (j) a catalyst comprising Pt,
ZSM-23 and W03, where each of (a) to (j) represents a catalyst comprising
three
components.
100351 In a particular embodiment, the catalyst comprises 0.6 wt % Ni,
ZSM-48 and W03, wherein the ratio of Si02:A1703 is 80:1 or less, and wherein
the weight ratio of ZSM-48 to W03 is 8:2.
100361 in another particular embodiment, the catalyst comprises 3 wt % Ni
and
20 wt % W, ZSM-48 and alumina, wherein the ratio of Si02:A1201 is 80: or less,
and wherein the weight ratio of ZSM-48 to alumina is 65:35.
10037j In yet another particular embodiment, the catalyst comprises 0.6 wt
%
Pt, ZSM-48 and Nb205, wherein the ratio of Si02:A1.703 is 80:1 or less, and
wherein the weight ratio of ZSM-48 to Nb205 is 8:2.
100381 In yet another particular embodiment, the catalyst comprises 0.6 wt
%
Pt, ZSM-48 and La203, wherein the ratio of Si02:A1203 is 80:1 or less, and
wherein the weight ratio of ZSM-48 to 11,a703 is 8:2.
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
13
100391 In yet another particular embodiment, the catalyst comprises 0.6 wt
%
Pt, ZSM-48 and Ce02, wherein the ratio of Si07:A1203 is 80:1 or less, and
wherein the weight ratio of ZSM-48 to Ca), is 8:2.
100401 In yet another particular embodiment, the catalyst comprises 0.6 wt
%
Pt, CBV-901 and alumina, wherein the weight ratio of ZSM-48 to alumina is 8:2.
[00411 In yet another -particular embodiment, the catalyst comprises 0.6 wt
%
Pt, ZSM-48 and TiO2, wherein the ratio of Si02:A1203 is 90:1 or less, and
wherein the weight ratio of ZSM-48 to TiO2 is 65:35.
100421 In yet another particular embodiment, the catalyst comprises 0.6 wt
%
Pt, ZSM-23 and alumina, wherein the weight ratio of ZSM-23 to alumina is
65:35.
10043j In yet another particular embodiment, the catalyst comprises 0.6 wt
%
Pt, ZSM-48 and alumina, wherein the ratio of Si.02:A1203 is 90 or less, and
wherein the weight ratio of ZSM-48 to alumina is 65:35.
100441 When a catalyst comprises a ternary metal component, a zeolite is
selected from ZSM-48, ZSM-23, ZSM-50, ZSM-5, ZSM-22, ZSM-11, ferrierite,
faujasite, beta, ZSM-12, MOR and a mixture thereof, and a hydrogenation
component comprises at least three metals sel.ected from Pt, Pd, A.g, Ni, Co,
Mo,
W, Rh, Re, and Ru, wherein at least one of the at least three metals is in
either an
oxi.de or sulfide form. In an embodiment, the zeolite is ZSM-48 or ZSM-23; and
the hydrogenation component comprises (a.) Ni, MoOx and WOx or (b) Co,
MoOx and W0x, wherein x is in the range of 0.5 to 3.
10045j In a particular embodiment, the catalyst comprises ZSM-48 and a
hydrogenation component comprising Ni, MoOx and W0x., where x is in the
range of 0.5 to 3, wherein the ratio of Si07:A1203 is 90 or less, and wherein
the
weight ratio of ZSM-48 to the hydrogenation cornponent is 8:2.
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
14
Feedstock s
[00461 In one embodiment, a process for producing a lube basestock and/or a
fuel hydrocarbon from a feedstock of biological origin, the method comprising:
contacting the feedstock in the presence of a catalyst 'which comprises a
zeolite
component, a hydrogenation component and a hydrothermally stable binder. The
feedstock of biological origin normally comprises one or more components
selected from the group consisting of fatty acids, fatty acid esters, fatty
alcohols,
fatty olefins, mono-glycerides, di-glycerides, tri-glycerides, phospholipids
and
saccharolipids. Optionally water can be co-fed with the biological feedstock,
with
the water content of 0.5-5 wt% of the total feed.
100471 Feedstocks for the process are drawn from renewable sources of
biological origin, e.g., plant, algae or animal (including insect) origin.
.A.nimal,
algae and plant oils containing tri-glycerides, as well as partially processed
oils
containing mono-glycerides and di-glycerides are included in this group.
Another
source of feedstock is phospholipids or saccharolipids containing fatty acid
esters
in their structure, such as phosphatidyl choline and the like present in plant
cell
Carbon numbers for the fatty acid component of such feedstocks are
generally i.n the range of Cr, or greater, up to C30=
10048j Other components of the feed can include a) plant fats, plant oils,
plant
waxes; animal fats, animal oils, animal waxes; fish fats, fish oils, fish
waxes, and
mixtures thereof; b) free fatty acids or fatty acids obtained by hydrolysis,
acid
trans-esterification or pyrolysis reactions from plant fats, plant oils, plant
waxes,
animal fats, animal oils, animal waxes, fish -fats, fish oils, fish waxes, and
mixtures thereof; c) esters obtained by trans-esterification from plant fats,
plant
oils, plant waxes, animal fats, animal oils, animal waxes, fish fats, fish
oils, fish
waxes, and mixtures thereof, d) esters obtained by esterification of free
fatty acids
of plant, animal and fish origin with alcohols, and. mixtures thereof; e)
fatty
alcohols obtained as reduction products of fatty acids from plant fats, plant
oils,
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
plant wax.es, animal fats, animal oils, animal waxes, fish fats, fish oils,
fish. waxes,
and mixtures thereof; and f) waste and recycled food grade fats and oils, and
fats,
oils and waxes obtained by genetic engineering, and mixtures thereof.
[00491 Examples of vegetable oils that can be used in accordance with this
disclosure include, but are not limited to rapeseed (canola) oil, soybean oil,
coconut oil, sunflower oil, pahn oil, palm kernel oil, peanut oil, linseed
oil, tall
oil, corn oil, castor oil, jatropha oil, jojoba oil, olive oil, flaxseed oil.,
catnelina oil,
safflower oil, babassu oil, tallow oil and rice bran oil. Vegetable oils as
referred to
herein can also include processed vegetable oil material as a portion of the
feedstock. Non-limiting examples of processed vegetable oil material include
fatty acids and. fatty acid. alkyl esters. .A.lkyl esters typically include CI-
05 alkyl
esters. One or more of methyl, ethyl, and propyl esters are desirable.
[0050] Examples of animal fats that can be used in accordance with the
disclosure include, but are not limited to, beef fat (tallow), hog fat (lard),
turkey
fat, fish fat/oil, and chicken fat. The animal fats can. be obtained froni any
suitable
source including restaurants and meat production facilities.
[0051] Animal fats as referred to herein also include processed animal fat
material.. Non-limiting examples of processed animal fat material include
fatty
acids and fatty acid alkyl esters. Alkyl esters typically include CI-05 alkyl
esters.
In particular embodiments, alkyl esters are one or more of methyl; ethyl, and
propyl esters.
1005.21 Algae oils or lipids can typically be contained in. algae in the
form of
membrane components, storage products, and/or metabolites. Certain algal
strai.ns, particularly microalgae such. as diatoms and cyanobacteria, can
contain
proportionally high levels of lipids. Algal sources for the algae oils can
contain
varying amounts, e.g., from 2 wt% to 40 wt% of lipids, based on total weight
of
the biomass itself,
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
16
100531 Algal sources for algae oils can include, but are not limited to,
unicellular and multicellular algae. Examples of such algae can include a
rhodophyte, chlorophyte, heterokontophyte, tribophyte, glaucophyte,
ch I orarachniophyte, eugl.enoid, haptophyte, cryptomonad, di nofl agel lum,
phytoplankton, and the like, and combinations thereof. In one embodiment,
algae
can be of the classes Chlorophyceae and/or Haptophyta. Specific species can
include, but are not limited to, Neochloris oleoabundansõicenedesmus
dimorphus, Euglena gracilis, Phaeodactylum tricornutum, Pleurochrysis
carterae, Prymnesium parvum, Tetmselmis chui, and Chlamydomonas
reinharda .A.dditional or alternate algal sources can include one or more
microalgae of the Achnanthes, Amphiprora, Amphora, Ankistrodesmus,
Asteromonas, Boekelovia, Borodinella, Botryococcus, Bracteococcus,
Chaetoceros, Carterics, Chlamydomonas, Chlorococcum, Chlorogonium,
Chlorella, Chroomonas, Chrysosphaera, Cricosphaera, Crypthecodinium,
Cryptomoncts, Cyclotella, Dunaliella, Elhpsoidon, Emiliania, Eremosphaera,
Ernodesmius, Euglena, Franceia, .Fragilaria, Gloeothamnion, Haematococcus,
Halocafeteria, Hymenomonas, Isochrysis, Lepocinclis, Micractinium,
Monoraphidium, Nannochloris, Nannochloropsis, Navicula, Neochloris,
Nephrochloris, Nephroselmis, Nitzschics, Ochromonas, Oedogonium, Oocystis,
Ostreococcus, Pavlova, Parachlorella, Pascheria, Phaeodaciylum, Phagus,
Platymonas, Pleurochrysis, Pleurococcus, Prowtheca, Pseudochlorella,
Pyramimonas, Pyrobotrys, Scenedesmus, Skeletonema, Spyrogyra, Stichococcus,
Tetraselmis, Thalassiosira, Viridiella, and Vo/vox species, and/or one or more
cyanobacteria of the Agmenellum, Anabaena, Anabaenopsis, Anacystis,
Aphanizomenon, Arthrospira, Asterocapsa, Borzia, Calothrix, Chamaesiphon,
Chlorogloeopsis, Chroococcidiopsis, Chroococcus, Crinalium, Cyanobacterium,
Cyanobium, Cyanocystis, Cyanospira, Cyanothece, Cylindrospermopsis,
Cylindrospermum, Dactylococcopsis, Dermocarpella, Fischerella, Fremyella,
Geitleria, Geitlerinema, Gloeobacter, Gloeocapsa, Gloeothece, Halospirulina,
lyengariella, Leptolyngbya, Limnothrfr, Lyngbya, Microcoleus, .Microcystis,
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
17
..41yxosarcina, .Nodularia, Nostoc, Nostochopsis, Oscillatoria, Phormidium,
Planktothrix, Pleurocapsa, Prochlorococcus, Prochlorm Prochlorothrix,
Pseudanabaena, Rivularia, Schizothrix, Scytonema, Spirulina, Stanieria,
Starria,
Stigonema, Symploca, Synechococcus, Synechocystis, Tolypothrix,
Trichodesmium, Tychonema, and Xenococcus species.
10054] Other
feeds usable in the present disclosure can include any of those
that comprise primarily triglycerides and free fatty acids (FFAs). The
triglycerides and FEAs typically contain aliphatic hydrocarbon chains in their
structure having from 8 to 36 carbons, particularly from 10 to 26 carbons, for
example from 14 to 22 carbons. Types of triglycerides can be determined
according to their fatty acid constituents. The fatty acid constituents can be
readily determined using Gas Chromatography (GC) analysis. This analysis
involves extracting the fat or oil, saponifying (hydrolyzing) the fat or oil,
preparing an alkyl (e.g., methyl) ester of the saponified fat or oil, and
determining
the type of (methyl) ester using GC analysis. In one embodiment, a majority
(i.e.,
greater than SO%) of the triglyceride present in the lipid materiai is made of
C10 to
C16 fatty acid constituents, based on total triglyceride present in the lipid
material.
Further, a triglyceride is a molecule having a structure identical to the
reaction
product of glycerol and three fatty acids. Thus, although a triglyceride is
described herein as being comprised of fatty acids, it should be understood
that
the fatty acid component does not necessarily contain a carboxylic acid
hydrogen.
If triglycerides are present, a majority of triglycerides present in the feed
can
particularly be com-prised of Cy, to C22 fatty acid constituents, based on
total
triglyceride content. Other types of feed that are derived from biological raw
materiai components can include fatty acid esters, such as fatty acid alkyl
esters
(e.g., FAME and./or FAEE).
10055j For
reactions with feedstocks having a relatively higher degree of
unsaturationõ an acidic catalyst can be used to promote dimerization and
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
18
oligomerization. The (timers and oligomers are branched or having- cyclic
structures, so that subsequent hydrogenation under the action of the
hydrogenation
catalyst produces saturated -branched or cyclized hydrocarbons than can be
naturally very low in wax and require little if any dewaxing. If the feedstock
is
highly saturated, action of a basic catalyst produces straight chain products
that
are subsequently hydrogenated to relatively straight chain hydrocarbons that
normally require some &waxing to make them suitable lube stocks. Dewaxing
can be provided by the hydrogenation catalyst, as further described 'below.
10056] One method for characterizing the triglycerides in a feedstock is
based
on the number of carbons in the side chain.s. While sonic feedstocks may have
consistent numbers of carbons in. each side chain, such as in a tristearin
feedstock,
many types of triglycerides will have variations in chain length 'between
molecules and even .within molecules. In order to characterize these
variations,
the average number of carbons per side chain in the triglycerides can be
determined. 13y definition, a triglyceride contains three side chains. Each
side
chain contains a number of carbons, as mentioned above. By averaging- the
number of carbons in each side chain for the trig13,rcerides in a feedstock,
an
average side chain length can be determined. 'fhe average number of carbons
(also referred to as average carbon number) per side chain in the feedstock
can be
used as a comparative value for characterizing products. For example, the
average nuMber of carbons per side chain in the feedstock can be compared with
the average number of carbons in hydrocarbons generated by converting and/or
isomerizing the triglyceride-containing feedstock.
10057j In various aspects, the production of fatty acid coupling products
and
corresponding hydrogenated products is based on processing of triglycerides
within the feed. Thus, the presence of at least some triglyceri.des within the
feed
is desirable. The feed can. i.nclude at least 10 wt% of feed. based on a
renewable
source or sources, such as at least 25 wt%. in particular embodiments, the
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
19
renewable portion of the feed is at least 50 wt%, or at least 75 wt%, or at
least 90
wt%, or at least 95 wt%. Such higher amounts of feed -from a rene-wable source
provide an advantage based on the greater amount of renewable material.
Additionally or alternately, the feed can be entirely a feed from a renewable
source, or the feed can include 99 wt% or less of a feed based on a renewable
source, or 90 wt% or less, or 75 wt% or less, or 50 wt% or less.
10058j Higher amounts of feed from a renewable source provide an advantage
based on the greater amount of renewable material, as well as potentially
including a greater amo-unt of triglycerides. Feeds with lower amounts of
renewable materials may have other processing advantages. Such advantages can
include improved flow characteristics within a reaction. systeni, as renewable
feeds often have a relatively high viscosity compared to conventional diesel
or
lubricant feeds in a refinery. Additionally, deoxygenation of a renewable feed
can
generate a substantial am.ount of heat due to formation of highly favorable
products from a free energy standpoint, such as H20 and CO2. For a typical
catalyst bed with a bed length of 25 to 30 feet (9 to 10 meters), it may be
preferable to have a temperature increase across the bed of 100 F (55 C) or
less.
If deoxygenation of a renewable feed with high oxygen content is performed
using a sufficiently reactive catalyst, an exotherm of greater than 100 F
across the
catalyst bed can be generated. Blending a renewable feed with a portion that
does
not contain oxygen can reduce the exotherm generated across a catalyst bed
used
for performing &oxygenation.
100591 Thus the feedstock can. contain a number of components. it can be
supplied as a solution in a suitable solvent (particularly a non-reactive sol-
vent
such as a hydrocarbon), or the feedstock can be supplied neat. The niain
reactions
are thought to be coupling or oligomerizing the fatty acid components (which
produces intermediate products of suitable carbon number to be useful as
diesel
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
fuel and lube base stock.s upon hydrogenation), and hydrogenating the
resulting
products to remove functional groups and produce a saturated hydrocarbon.
10060] The feed may contain various amo-unt of mineral feed as diluent. The
advantages of increased mineral feed content are largely due to dilution of
the
renewable feed, as the processing- conditions effective for deoxygenation of a
rene-wable -feed will have a law or minimal impact on a typical hydroprocessed
mineral feed. Therefore, while the deoxygenation conditions are effective for
&oxygenation of renewable feeds at a variety of blend ratios with mineral
feeds,
it may be preferable to have at least '75 wt% of the feed from a renewable
source,
such as at least 90 wt% or at least 95 wt%.
100611 One option for increasin.g the renewable content of a feed while
retaining some of the benefits of adding a feed with reduced oxygen content is
to
use recycled product from processing of renewable feed as a diluent, A
recycled
product from processing a renewable feed is still derived from a renewable
source, and therefore such a recycled product is counted as a feed portion
from a
renewable source. Thus, a feed containing 60% renewable :feed that has not
been
processed and 40% of a recycled product from processing of the renewable feed
would be considered as a feed that includes 100% of feed frorn a renewable
source, As an example, at least a portion of the product from processing of a
renewable feed can be a diesel boiling range product. Such a recycled diesel
boiling range product will be deoxygenated, and therefore incorporation of the
recycled diesei boiling range product in the feed will reduce the exotherm
generated during deoxygenation. Adding a recycl.ed diesel boiling range
product
is also likely to improve the cold flow properties of a renewable feed. More
generally, any convenient product from processing of a renewable feed can be
recycled for blending with the renewable feed in order to improve the cold
flow
properties and/or reduce the oxygen content of the input flow to a
deoxygenation
process= if a recycled product flow is added to the input to a deoxygenation
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
21
process, the amount of recycled product can correspond to at least 10 wt% of
the
feed to the deoxygenation process, such as at least 25 wt%, or at least 40
wt%.
Additionally or alternately-, the amount of recycled product in a feed can be
60
wt% or less, such as 50 wt% or less, 40 wt% or less, or 25 wt% or less.
100621 With regard to triglyceride content, the feedstock can include at
least 10
wt%, such as at least 25 wt%, and particularly at least 40 wt%, or at least 60
wt%,
or at least 80 wt%. Additionally or alternately, the feed can be composed
entirely
of triglycerid.es, or the triglyceride content of the feed can be 90 wt% or
less, such
as 75 wt% or less, or 50 wt% or less. The methods described herein are
suitable
for conversion of triglycerides to lubricant and diesel products in a single
reactor,
so higher contents of triglycerides may be advantageous. However, to the
degree
that a recycle loop is used to improve the feed flow properties or reduce the
reaction exotherm across catalyst beds, lower triglyceride contents may be
beneficial.
[00631 While feed dilution can be used to control the exotherm generated
across a catalyst bed used for deoxygenation, it is noted that some processing
options can also impact the exotherm. One alternative is to use a less
reactive
catalyst, so that a larger amount of catalyst is needed at a given liquid
hourly
space velocity (LAW) in order to deoxygenate a feed to a desired level.
Another
option is to reduce the amount of hydrogen provided for the deox.ygenation
process. Still another option could be to introduce additional features into a
reactor to assist in cooling and/or transporting heat away from a
deoxygenation
catalyst bed. In combination with selecting an appropriate amount of product
recycle and/or blending of another non-oxygenated feed, a desired combination
of
a flow characteristics and heat generation during deoxygenation can be
achieved.
100641 Oxygen is the major heteroatom component in renewable base feeds. A
renewable feedstream based on a vegetable oil., prior to hydrotreatment,
includes
up to 10 wt% oxygen, for example up to 12 wt% or up to 14 wt%. Such a
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
22
renewable feedstream, also called a biocomponent feedstream, normally includes
at least 1 wt% oxygen, for example at least 2 wt%, at least 3 wt%, at least 4
wt%,
at least 5 wt%, at least 6 wt%, or at least 8 wt%. Further, the renewable
feedstream, prior to hydrotreatment, can include an olefin content of at least
3
wt%, for example at least 5 wt% or at least 10 wt%.
10065] Biocomponent based feedstreams have a wide range of nitrogen and/or
sulfur contents depending on the feed sources. For exarriple, a feedstream
based
on a vegetable oil source can contain up to 300 wppm nitrogen. In some
embodiments, the sulfur content can be 500 wppm or less, for example 100 wppm
or less, 50 wppm or less, or 10 wpprn or less, where wppm stands for parts per
million by weight.
Reaction conditions and process configurations
100661 Hydrogen is present throughout the reactor, and is consumed by the
reactants during the hydrogenation step. Advantageously, it was found that the
presence of hydrogen did not adversely affect the fatty acid coupling
reactions
believed to be catalyzed primarily by the acidic or basic catalysts. During
the
fatty acid coupling, hydrogen transfer reactions can lead to formation of coke
molecules, which can cause catalyst deactivation. In various embodiments, the
presence of hydrogen can inhibit hydrogen transfer and improve catalyst life.
In
an embodiment, water is added to the renew/able :feed.
10067] Temperature and pressure of -the reactor and reactants is selected
depending on the throughput and turnover required. Non-limiting examples of
temperatures include 100 to 500 C, 200 to 400 C, and 250 to 400 C. Hydrogen
partial pressure is used in the range of from 1.8 to 34.6 MPag (250 to 5000
psig)
or 4.8 to 20.8 MPag, by way of non-limiting example. Also in non-limiting
fashion, a liquid hourly space velocity is from 0.2 to 10 v/v/hr, or 0.5 to
3.0, and a
hydrogen circulation rate is 35.6 to 1781. m3/m3 (200 to 10,000 scf/B),
particularly
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
23
1.78 to 890.6 m3/m3 (1,000 to 5000 scf/B). Further non-limiting examples of
conditions are given in working examples.
[0068] Loading of the catalyst is 1 to 30% by weight of the weight of the
:feedstock in the reactor, for example 2 to 20%, or 5 to 1.0% by weight. The
reaction time or residence time can range from 5 minutes to 50 hours depending
on types of catalysts used, reaction temperature and the amount (wt %) of
catalyst
in the reactor. In a particular embodiment, a residence time is 10 minutes to
10
hours. Shorter residence time gives better efficiency for reactor usage.
Longer
residence time ensures high conversion to pure hydrocarbons. Usually an
optimized reactor time is most desirable.
[0069] In various embodiments, the duration of the reaction (or the average
residence time in the reactor for a continuous process) is 1-48 hours, 1-20
hours,
12-36 hours, or 24-30 hours. In various ernbodiments, the reactions are
carried
out in a fixed bed reactor, a continuous stir tank reactor, or a batch
reactor. In any
of these operations, it is advantageous to maintain partial pressure of
hydrogen
above 300 psi, above 400 psi, above 500 psi, above 600 psi, or above 700 psi.
During conversion, carbon dioxide and water generated from the action of the
acidic or basic catalyst on the feedstock fatty acids are present in gaseous
form,
and thus increase the total reactor pressure. Under this condition, it can be
important to maintain hydrogen partial. pressure. By way of non-limiting
example, this can be achieved by intermittently purging the reactor gas and re-
charging with hydrogen gas in batch or CSTR operation. Alternatively, in a
fixed
bed operation, this can be achieved by withdrawing reactor gas at different
locations along the fixed bed reactor; or alternatively by stage injection of
hydrogen. Other means to maintain hydrogen pressure are also possible.
[0070] Where needed, the hydrogenation catalyst can introduce branches into
the final hydrocarbon products to provide a dewaxi.ng function. For
triglycerides
with only saturated fatty acid side chains, the combination of fatty acid
coupling
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
24
(particularly using a basic material as the first catalyst) and hydrogenation
will be
relatively unbranched hydrocarbons. For triglycerides with both saturated and
unsaturated fatty acid side chains, the combination of fatty acid coupling and
hydrogenation will be mixtures of branched hydrocarbons (containing one or
more branches of various lengths in the range of 1 to 10 carbons) and
naphthenics
substituted 'with various lengths of -hydrocarbon chains. Of course, if the
side
chains of the triglycerides contain other types of heteroatoms, such as
nitrogen or
sulfur, other types of molecules may be generated,
10071] I'or triglycerides with side chains containing between 12 and 22
carbon
atoms, the stacked bed configuration of the fatty acid coupling catalyst and
hydrogenation catalyst will result in production of hydrocarbon molecules that
boil in the lubricant boiling range as a primary product, with some production
of
hydrocarbon molecules that boil in the diesel -boiling range. The lubricant
boiling
range molecules correspond to fatty acid coupling products that were formed
during conversion of the triglycerides in the feedstock. These fatty acid
coupling
products are subsequently hydrogenated and isomerized. However, whil.e the
process of converting triglycerides will typically occur at percentages
approaching
100%, less than all of the side chains in the triglycerides may result in
formation
of coupling products. Instead, at least a portion of the side chains :from the
triglycerides will reach the hydrogenation catalyst without combining with
another side chain to form a lubricant 'boiling range molecule. These
uncombined
side chains are also deoxygenated and isomerized by the hydrogenation
catalyst,
resulting in diesel boiling range molecules. Thus, a stacked bed arrangement
for
the catalysts would be expected to generate a majority portion of lubricant
boiling
range molecules from a triglyceride feed and a minority portion of diesel
boiling
range molecules.
[00721 In order to provide a general way of characterizing the hydrocarbons
resulting from conversion, hydrogenation, and isomerization of a triglyceride
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
feed, the average number of carbons (i.e., average carbon number) in
hydrogenated molecules derived from triglycerides can be compared with the
average number of carbons in the fatty acid side chains of the triglycerides.
The
average number of carbons in hydrocarbon molecules derived from triglyceri.des
in a feed can be at least 1.5 times the average number of carbons in the fatty
acid
side chains of the corresponding triglycerides, such as at least 1.75 time the
average number of carbons in the fatty acid side chains or at least 1.9 times
the
average number of carbons in the fatty acid side chains,
10073] In a particular embodiment, the average carbon number of
hydrocarbons produced by conversion of feedstock based triglycerides or other
fatty esters is two times or more that of the fatty acid components of the
feedstock. The first catalyst is believed to increase carbon number in the
product
by a factor of approximately- two or more comparing to the car-bon numbers of
the
fatty acid side chains in the feed, by the process of coupling
(oligomerization,
ketonization, and aldol condensation).
Further processing,
100741 The product of the reaction described herein is a mixture of
hydrocarbons, largely saturated, having a carbon number in the diesel fuel and
lube base stock range. if desired, the reaction product can be hydrofinished
by
subjecting it to low pressure -hydrogen. This process can clean up residual
unsaturations and oxygenates that may result when the products are being
heated
in the presence of the hydrogenation catalyst, which can have some cracking
power given that it may contain an acidic carrier such as a zeolite. The
hydrofinishing can be carried out either in a fixed-bed or in an autoclave
reactor.
The catalyst can be either noble metal (Pd, Pt, Rh, Ru, Ir, or combination
thereof)
or non-noble metal (Co, Ni., Fe), particularly- supported on a support such as
clay,
alumina, aluminosilicate, silica, titania and. zirconia. The weight hourly
space
velocity can be in the range of 0.5 to 10 11-1, under a hydrogen pressure in
the
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
26
range of ambient to 30 MPag, and a temperature from I 50 C to 400 C. The
resulting product can then be further processed by distillation to separate
out any
diesel fuel from the lube base stock.
EXAMPLES
EXAMPLE 1: 0.6 wt% Pt Impretthated 80/20 Steamed H-ZSM-48/W0x
100751 The title catalyst (0.6 wt% Pt impregnated 80/20 ZSM-48/W0x) was
prepared by the following method: material is first extruded as 80 wt% 70:1
Si02:A1703 ZSM-48 and 20 wt% tungsten oxide (designated as NV-0x). Charge
the tungsten oxide to a Lancaster Muller and dry mull for 3 minutes. Dilute
28.6
TEAOH (Tetraethylammonium Hydroxide) in 66.1 g of de-ionized water and
slowly add to the WOx. The WOx was mixed by hand in a beaker due to the low
volume of material. Wet mull the mixture for 3 minutes. Add the ZSM-48 crystal
to the peptized WOx and mull 10 minutes. Dilute 57.2 of TE.A.OH in 627.3g of
deionized water and add to the rnull mix over a five minute period. Wet mull
the
mixture for 20 minutes or until the desired consistency is achieved. Extrude
the
mull mixture on a 2" Bonnot extruder using 1/16" quadrulobe die inserts.
10076j Pre-calcine the bound zeolite in flowing N2 at )50 F (510 C) for
three
hours to start removing the structure directing agent from the zeolite.
Ammonium-exchange the formed material two times (5 ml of i M NII4NO3
solution per gram of catalyst) under ambient conditions to remove the alkali
cations from the structure. After completing the second exchange wash the
material with de-ionized water for one hour. Dry at 250 F (I21 C) overnight in
a
forced draft oven. To create the acid form of the catalyst, calcine the
extrudate in
air for 6 hours at 1,000 F (538 C) in air.
[0077] Place the acid form of the catalyst into a vertical steamer. Bring
catalyst up to 650 F (343 C) in air and hold at temperature for 30 minutes.
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
27
Switch from air to steam over a 30-minute period. Ramp the temperature of the
steamer to '700 F (37 PC), allow the temperature in the bed to stabilize, and
hold
for 3 hours at 700 F in 100% steam. Cool down in air and remove the catalyst
from the steamer.
100781 Impregnate the steamed acid form of the catalyst using a tetraamine
platinum nitrate solution via spray impregnation targeting a metal loading of
0.6
wt% Pt. Spray in the impregnating solution slowly; after the solution has been
applied continue mixing for 20 minutes to insure that the solution is
uniformly
distributed across all of the extrudates. Dry at ambient conditions in an open
dish.
Dry for 2 hours in a forced air oven at 250 F. Complete the impregnation by
calcining the extrudate in air at 680 F (360 C) for three hours,
100791 The finished catalyst had 0.56 wt% Pt on catalyst. Dispersion of Pt
was
measure by EI7 chemisorption, a H/Pt molar ratio of 4.02 was observed,
indicating
high degree of Pt dispersion (equivalent to smaller Pt particles on catalyst).
EXAMPLE 2:80/20 H-ZSM-48/NiMoW0x
100801 The title catalyst (80/20 H-ZSM-48/NiMoW0x) was prepared by the
following method.: charge the NiMoWOx to a Lancaster Muller and dry mull for
3 hours. Dilute 28.6 g of 35 wt% TEAOH in 66.1 g of de-ionized water. Slowly
add the solution to the NiMoW0x. Wet mull the mixture for 3 minutes. Add the
ZSM-48 crystal to the peptized NiMoW0x and mull 10 minutes. Dilute 57.2 g of
35 wt% TEAOH in 680.2g of de-ionized water. Add the solution to the mull mix
over a five minute period. Wet mull for 20 minutes or until reasonable
consistency is achieved. Extrude the mull mix on a 2" Bonnot extruder equipped
with a die plate using 1/16" quadrulobe die inserts. Dry in a forced air oven
at
250 F to dry the extrudate.
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
28
100811 Pre-calcine the extrudate in flowing nitrogen at 950 F for 3 hours.
Ammonium-exchange the extrudate two times under ambient conditions (5 ml of
N NH4NO3 solution per gram of catalyst). After the completion of two
exchanges, wash with DI water for 1 hour at room temperature, drain, and dry
under ambient conditions. Dry at 250 F overnight in a forced draft oven. Heat
the
extrudate under nitrogen to 752 F (300 C) for three hours. Lower the
temperature
of the oven to 700 F and begin introducing air over a three hour period. The
final
air calcination should be completed at ì,000 F under air for 10 hours.
1008.21 Loadings of metal on the finished catalyst were 3.45 wt% W, 2,41
wt%
Ni, and 1.92 wt% Mo.
EXAMPLE 3: 3 wt% Ni and 20 wt% W Impregnated 65/35 ZSM-48/alumina
(V-300)
100831 The title catalyst (3 wt% Ni and 20 wt% W impregnated 65/35
ZSM-48/V-300) was prepared by the following method: ch.arge 1,639 g of
ZSM-48 (Si02:A1203 = 70:1) crystal to the miler and mull for 10 minutes. Add
1.153 g of Versal-300 alumina to the muller and mull for 10 minutes. Slowl.y
add
1547 g of de-ionized water to mull mix -while mulling. Mull the mixture for 40
minutes or until a reasonable mixture consistency is achieved. Extrude the
mixture on the 2" Bonnot extruder using a die plate with 1/20" quadrulobe
inserts.
After extrusion, dry at 250 F in a forced draft oven.
10084] Pre-calcine the extrudate prepared above for 3 hours at 1,000T in
flowing nitrogen. After calcining the extrudate under inert conditions,
exchange
the material two times with ammonium nitrate (5 ml of 1 N NI1IN03 solution per
gram of catalyst). After the second exchange wash the material with de-ionized
water for 1 hour at room temperature, drain, and blow dry with air. Dry the
exchanged material in a forced draft oven at 250 F overnight. Calcine the
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
29
ammonium form of the extrudate for 6 hours at 000 F in air to create the acid
form of the catalyst.
10085] Impregnate the extrudate with 20 wt% W using ammonium
metatungstate hydrate using a rotary spray impregnation technique. For
example,
500 g of extrudate would be impregnated with 134 g of ammonium metatungstate
hydrate dissolved in water. After the material is sprayed onto the catalyst
the
catalyst should be mixed for an additional 30 minutes to improve the
homogeneity
of the metal dispersion. Dry the extrudate for 4 hours at ambient conditions
in a
pan. Dry the catalyst overnight in a forced draft oven at 250 F. Calcine the
extrudates in air at 900 F for 1 hour.
100861 The resulting catalyst had 14 wt% tungsten and 3 wt% Ni as measured
by XR.F analysis.
EXAMPLE 4: 0.6 wt% Ni Impregnated 80/20 Steamed H-ZSM-48/W0x
10087j The title catalyst (0.6 wt% Ni impregnated. 80/20 ZSM-48/W0x) was
prepared by the following method: the 80:20 ratio of ZSM-48 and WOx extrudate
formed in Example 1 is impregnated. with 0.6 wt% Ni instead of 0.6 wt% it.
Impregnate the steamed acid form of the catalyst using a nickel nitrate
hexahydrate solution via spray impregnation targeting a metal loading- of 0.6
wt%
Ni. Spray in the impregnating solution slowly; after the solution has been
applied
continue mixing for 30 minutes to insure that the solution is uniformly
distributed
across all of the extrudates. Dry the extrudate for 4 hours at ambient
conditions in
a pan. Dry the catalyst overnight in a forced draft oven at 250 F. Calcine the
extrudates in air at 900 F for 3 hours. The finished catalyst contained 0.69
wt%
Ni.
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
EXAMPLE 5: 0.6 wt% Pt Impregnated 80/20 Steamed H-ZSM-48/Niobium
Oxide
[0089] The title catalyst (0.6 wt% Pt impregnated 80/20 ZSM-48/niobium
oxide) was prepared by the following method: material is first extruded as 80
wt% 70:1 Si02:A1.203 ZSM-48 and 20 wt% niobium oxide. Charge the niobium
oxide to a Lancaster Muller and dry mull for 3 minutes. Dilute 17.1g of 35 wt%
TEAOH in 39.'7 g of de-ionized water and slowly add to the niobium oxide. Wet
mull the mixture for 3 minutes. Add the ZSM-48 crystal_ to the peptized
niobium_
oxide and mull for 10 minutes, Dilute 34.3 g of 35 wt% TEAOH in 356.2 g of
&ionized water and add to the mull mix over a five minute period. Wet mull the
mixture for 20 minutes or until the desired consistency is achieved. Extrude
the
mull mixture on a 2" Bonnot extruder using 1/16" quadrulobe die inserts.
[0090] Pre-calcine the bound zeolite in flowing N2 at 950T for three hours
to
start removing the structure directing agent from the zeolite. Ammonium-
exchange the forined material two times (5 ml of 1 M NI-14NO3 solution per
gram
of catalyst) under ambient conditions to remove the alkali cations from the
structure. After completing the second exchange wash the material with de-
ionized water for one hour. Dry at 250 F overnight in a forced draft oven. To
create the acid form of the catalyst, calcine the extrudate in air for 6 hours
at
1,000E in air.
[00911 Place the acid form of the catalyst into a vertical steamer. Bring
catalyst up to 650 F in air and hold at temperature for 30 minutes. Switch
from
air to steam over a 30-minute period. Ramp the temperature of the steamer to
700T, allow the temperature in the bed to stabilize, and hold for 3 hours at
700 E
in 100% steam. Cool down in air and remove the catalyst front the steamer.
[00921 Im_pregnate the steamed acid forirr of the catalyst using a
tetraamine
platinum nitrate solution via spray impregnation targeting a metal loading of
0.6
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
31
VA% Pt. Spray in the impregnating solution slowly; after the solution has been
applied continue mixing for 20 minutes to insure that the solution is -
uniformly
distributed across all of the extrudates. Dry at ambient condition in an open
dish.
Dry for 2 hours in a forced air oven at 250 F. Complete the impregnation by
calcining the extrudate in air at 680 F for three hours.
IO93 i12 chemisorption revealed a Pt dispersion of H/Pt = 0.73,
EXAMPLE 6: 0.6 wt% Pt Impregnated 80/20 Steamed H-ZSM-48/La20:;
100941 The title catalyst (0.6 wt% Pt impregnated 80/20 ZSM-48/La203) was
prepared by the following method: the material is first extruded as 80 wt%
70:1
Si02:A1203 ZSM-48 and 20 wt% lanthanum oxide. Charge 125 g of lanthanum
oxide to a Lancaster Muller and dry mull for 3 minutes. Dilute 17.1 g of 35
wt%
TEAOH in 29.7 g of de-ionized water and slowly add the solution to the
lanthanum. oxide. Wet mull the mixture for 3 minutes, Add the ZSM-48 crystai
to the peptized lanthanum oxide and mull 10 minutes. Dilute 34.3 g of 35 wt%
TEAOH in 356.2 g of deionized water and add to the mull mix over a five-minute
period. Wet mull the mixture for 20 minutes or until the desired consistency
is
achieved. Extrude the mull mixture on a 2" Bormot extruder using 1/16"
quadrulobe die inserts.
100951 Pre-calcine the bound zeol.ite in flowing N2 at 950 F for three
hours to
start removing the structure directing agent from the zeolite. Ammonium-
exchange the formed material two times (5 ml of 1 M NH4NO3 solution per gram
of catalyst) under ambient conditions to remove the alkali cations from the
structure. After completing the second exchange wash the material with de-
ionized water for one hour. Dry at 250 F overnight in a forced draft oven. To
create the acid form of the catalyst, calcine the extrudate in air for 6 hours
at
1,000T in air,
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
32
[0096] Place the acid form of the catalyst into a vertical steamer. Bring
catalyst up to 650 F in air and hold at temperature for 30 minutes. Switch
from
air to steam over a 30-minute period. Ramp the temperature of the steamer to
700 F. allow the temperature in the bed to stabilize, arid hold for 3 hours at
700 F
in 100% steam. Cool down in air and remove the catalyst from the steamer.
[0097] Impregnate the steamed acid form of the catalyst using a tetraamine
platinum nitrate solution via spray impregnation targeting a metal loading of
0.6
wt% Pt. Spray in the impregnating solution slowly; after the solution has been
applied continue mixing for 20 minutes to insure that the solution is
uniformly
distributed across all of the extrudates. Dry at ambient conditions in an open
dish.
Dry for 2 hours in a forced air oven at 250 F. Complete the impregnation by
calcining the extrudate in air at 680 F for three hours.
[0098] The finished catalyst ha.d 0.56 wt% Pt on catalyst. H7 chemisorption
revealed a Pt dispersion of H/Pt = 1.18.
EXAMPLE 7: 0.6 wt% Pt Impregnated 80/20 Steamed H-ZSM-48/Ce0)
[0099] The title catalyst (0.6 wt% Pt impregnated 80/20 ZSM-48/Ce03) was
prepared by the following method: the material is first extruded as 80 wt%
70:1
Si.02:A1201 ZSIV1-48 and 20 wt% cerium oxide. Charge 122 g- of cerium oxide to
a Lancaster Muller and dry mull for 3 minutes, Dilute 17,1 g of 35 wt%
in 39.7 g of de-ionized water and slowly add the solution to the cerium oxide.
Wet mull the mixture for 3 minutes. Add the ZSM-48 crystal to the peptized
lanthanum oxide and mull 10 minutes. Dilute 34.3 g of 35 wt% TEA0I-I in 419.7
g of deionized water and add to the mull mix over a five-minute period. Wet
mull
the mixture for 20 minutes or until the desired consistency is achieved.
Extrude
the mull mixture on a 2" Bonnot extrud.er using 1/16" quadrulobe die inserts.
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
33
[HUM Pre-calcine the bound zeolite in flowing N2 at 950 F for three hours to
start removing the structure directing agent from the zeolite. Ammonium-
exchange the formed materiai two times (5 ml. of 1. M N-144NO3 solution per
gram
of catalyst) under ambient conditions to remove the alkali cations froin the
structure. After completing the second exchange wash the material with
de-ionized water for one hour. Dry at 250 F overnight in a forced draft oven.
To
create the acid form of the catalyst, calcine the extrudate in air for 6 hours
at
1,000T in air.
10011011 Place the acid form of the catalyst into a vertical steamer. Bring
catalyst up to 650 F in air and hold at temperature for 30 minutes, Switch
from
air to stearrì over a 30-minute period. Ramp the temperature of the steamer to
700 F, allow the temperature in the bed to stabilize, and hold for 3 hours at
700 F
in 100% steam. Cool down in air and remove the catalyst from the steamer.
[00102] Impregnate the steamed acid form of the catalyst using a tetraamine
platinuni nitrate solution via spray impregnation targeting a metal loading of
0.6
wt% Pt. Spray in the impregnating solution slowly; after the solution has been
applied continue mixing for 20 minutes to insure that the solution is
uniformly
distributed across all of the extrudates. Dry at ambient conditions in an open
dish.
Dry for 2 hours in a forced air oven at 250 F. Complete the impregnation by
calcining the extrudate in air at 680 F for three hours.
[00103] The finished catalyst had 0.42 wt% Pt on catalyst. H2 chemisorption
revealed a Pt dispersion of .14/Pt = 0.78.
EXAMPLE 8: 0.6 wt% Pt Impregnated 80/20 CBV-901/alumina
[00104] The title catalyst (0.6 wt% Pt impregnated 80/20 CBV-901/alumina)
was prepared by the following method: The material is first extruded as a 80
wt%
CBV-901 and 20 wt% 'Versa' 300 alumina composite using the following
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
34
procedure. Ch.arge 808 g of CBV-9011 USY crystal to a Lancaster Muller and dry
mull for 5 minutes. Dilute 10 g of acetic acid with 690 g of de-ionized water.
Dissolve 5 g of polyvinylacetate (PVA) in the acetic acid solution. Slowly add
the acid/ PVA solution to the zeolite over 5 minutes and mull the mixture for
10
minutes. .A.dd 275 g of Versal-300 alumina. to the muller and mull for an
additional 10 minutes. Add the remaining 173 g of de-ionized water to the mull
mix. over 3 minutes and mull 3 minutes or until reasonable consistency is
achieved. Extrude the mull mixture on a 2" Bonnot extruder using 1/16"
quadrulobe die inserts, Dry the extrudates at 250 F. Calcine the dried
extrudates
in air at 1,000 F for 6 hours,
[001051 Impregnate the acid form of the catalyst using a tetra.a.mine platinum
nitrate solution via spray impregnation targeting a metal loading of 0,6 wt%
Pt.
Spray in the impregnating solution slowly; after -the solution has been
applied
continue mixing for 20 minutes to insure that the solution is uniformly
distributed
across all of the extrudates. Dry at ambient conditions in an open dish, Dry
for 2
hours in a forced air oven at 250 F. Complete the impregnation by calcining
the
extrudate i.n air at 680 F for three hours,
EXAMPLE 9: 0.6 wt% N Impre?-nated 65/35 Steamed 11-ZSM-48/Ti02,
1001061 The title catalyst (0.6 wt% it impregnated 65/35 ZSM-48/Ti02) was
prepared by the following method: the material is first extruded as 65 wt%
90:1
Si02:.A.1203 ZSM-48 and 35 wt% titanium oxid.e, Charge the ZSM-48 to the
muller and mull for 10 minutes. Add 214 g of DT-51 titania to muller and mull
for 10 minutes. Slowly add 488 g of de-ionized water to mull mix while
mulling.
Mull the mixture for 30 minutes or until the mixture reaches the desired
consistency to extrude properly. Extrude mixture on a 2" Bonnot extruder
equipped with a die plate using 1/16" quadrulobe inserts. Dry the extrudate at
250 F i.n a forced draft oven.
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
100107] Pre-calcine the bound zeolite in flowing- N2 at 1,000 F for 3 hours to
start removing the structure directing agent from the zeolite. Ammonium-
exchange the formed material two times (5 ml. of 1M NH4NO3 solution per gram
of catalyst) under ambient conditions to remove the alkali cations from the
structure. After completing the second exchange wash the material with de-
ionized water for one hour. Dry at 250 F overnight in a forced draft oven. To
create the acid form of the catalyst, calcine the extrudate in air for 6 hours
at
1,000 F in air.
[001081 Place the acid form of the catalyst into a vertical steamer. Bring
catalyst up to 700 F in air and hold at temperature for 30 minutes, Switch
from
air to steam over a 30-minute period. Ramp the -temperature of the steamer to
890 F, allow the temperature in the bed to stabilize, and hold for 3 hours at
890 F
in 100% steam. Cool down in air and remove the catalyst from the steamer.
1001091 Impregnate the steamed acid form of the catalyst using a tetraamine
platinum nitrate solution via spray impregnation targeting a metal loading of
0.6
wt% Pt. Spray in the impregnating solution slowly; after the solution has been
applied continue mixing for 20 minutes to insure that the solution is
uniformly
distributed across all of the extrudates. Dry at ambient conditions in an open
dish.
Dr for 2 hours in a forced air oven at 250 F. Complete the impregnation by
calcining the extrudate in air at 680 F for 3 hours.
1001101 112 chemisorption revealed a Pt dispersion of H/Pt = 0.76.
EXAMPLE 10: 0.6 wt% Pt impregnated 65/35 H-ZSM-23/alumina
LOOM] The title catalyst (0.6 wt% Pt impregnated 65/35 ZSM-23/altunina) was
prepared by the following method; the material is first extruded as 65 wt%
ZSM-23 and 35 wt% Versal 300 alumina. Charge the 433 g of ZSM-23 crystal to
muller and dry mull for 15 minutes. Add the 248 g of Versal 300 alumina to the
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
36
muller and dry nuili for an additional 10 minutes. Slowly add 451,3 g of
de-ionized water to the mull mix over 5 minutes and mull the mixture for 10
minutes or until reasonable consistency. Extrude the mixture on a 2" Bonnot
extruder equipped with a die plate using 1/16" quadrulobe inserts. Dry the
extrudate at 250"F in a forced draft oven.
1001121 Pre-calcine the bound zeolite in flowing N2 at 1000 F for 3 hours to
start removing the structure directing agent from the zeolite. Ammonium-
exchange the formed material two times (5 ml of 1 M NI-14NO3 solution per gram
of catalyst) under ambient conditions to remove the alkali cations from the
structure. After completing the second exchange wash the materi.al with
de-ionized water for one hour. Diy at 250 F overnight in a forced draft oven,
To
create the acid form of the catalyst, calcine the extrudate in air for 8 hours
at
1,000"F in air,
1001131 Impregnate the acid form of the catalyst -using a tetraamine platinum
nitrate solution via spray impregnation targeting a metal loading of 0.6 wt%
Pt.
Spray in the impregnating solution slowly; after the solution has been applied
continue mixing for 20 minutes to insure that the solution is uniformly
distributed
across all of the extrudates. Dry at ambient conditions in an open dish. Dry
for 2
hours in a forced air oven at 250 F. Com.pl.ete the impregn.ation by calcining
the
extrudate in air at 680 F for 3 hours.
1001141 The finished catalyst had 0.52 wt% Pt on catalyst. H2 chemisorption
revealed a Pt dispersion of H/Pt = 1.25.
EXAMPLE 11: 0.6 wt% Pt impregnated 65/35 H-ZSM-48/ alumina
[001151 The title catalyst (0.6 wt% Pt impregnated 65/35 ZSM-48/alumina) was
prepared by the following method: add 245 lbs. of ZSM-48 SiO21A1203 90 to the
muller. Mull the mixture for ten minutes. Add 162 lbs. of -Versal 300 alumina.
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
37
Mull the mixture for ten minutes after adding all of the alumina. Add 292 lbs.
of
de-ionized water while mulling. Mull the mixture for forty minutes or until
reasonable consistency is achieved. Extrude the mixture on an extruder
equipped
with a die plate using 1/16" quadrulobe inserts. Dry the extrudate at 250 F in
a
forced draft oven.
1001161 Pre-calcine the bound zeolite in flowing N2 at 980 F for 3 hours to
start
removing the structure directing agent from the zeolite. Ammonium-exchange the
formed. material two times (5 ml of 1 M NI-14NO3 solution per gram of
catalyst)
under ambient conditions to remove the alkali cations from the structure.
After
completing the second exchange wash the material with de-ionized water for one
hour. Dry at 250 F overnight in a forced draft oven. To create the acid form
of
the catalyst, calcine the extrud.ate in air for 6 hours at 980 F in air.
1001171 Place the acid form of the c,atalyst into a vertical. steamer. Bring
catalyst up to 650 F in air and hold at temperature for 30 minutes, Switch
from
air to steam over a 30-minute period. Ramp the temperature of the steamer to
890 F, allow the temperature in the bed to stabilize, and hold for 3 hours at
890 F
in 100% steam. Cool down in air and remove the catalyst from the steamer.
1001.181 Impregnate the steamed acid form of the catalyst using a tetra-in-
line
platinum nitrate solution via spray impregnation targeting a metal loading of
0.6
wt% Pt. Spray in the impregnating solution slowly; after the solution has been
applied continue mixing for 20 minutes to insure that the solution is
uniformly
distributed across all of the extrudates. Dry at ambient conditions in an open
dish.
Dry for 2 hours in a forced air oven at 250 F. Complete the impregnation by
calcining the extrudate in air at 680 F for three hours.
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
38
EXAMPLE 12: Steaming of the 0.6 wt% Pt impregnated 65/35 ZSM-48/ TiO)
[001191 Place the Pt form of the catalyst from. Example 8 into a vertical
steamer.
Bring catalyst up to 950T in air and hold at temperature for 30 minutes.
Switch
:from air to stearn over a 30 minute period. Ramp the -temperature of the
steamer
to I,000 F, allow the temperature in the bed to stabil.ize, and hold for 24
hours at
1000 F in 100% steam. Cool down in air and remove the catalyst from the
steamer.
EXAMPLE 13: Steamin, of the 0.6 wt% Pt impregnated 65/35 ZSM-23/alumina
[001201 Place the Pt form of the catalyst from Example 10 into a vertical
steamer. Bring catalyst up to 950T in air and hold at temperature for 30
minutes.
Switch from air to stearn over a 30 minute period. Ramp the temperature of the
steamer to ,000 F, allow the temperature in the bed to stabilize, and hold for
24
hours at 1,0007 i.n 100% steam. Cool down in air and remove the catalyst from
the steamer.
100121i Catalyst candidates were first screened through a "severe steaming
process" which consisted of steaming each potential lead. at 1,000T for 24
hours
in order to examine the effects that exposure to water at high temperatures
would
have on the crush strength and metal dispersion of each material. Pt
dispersions
were measured by H2 chemisorption. A promising lead candidate for this
application would maintain its crush strength with minimal metal
agglomeration.
Catalyst from Example 111 was included in the study as a point of reference.
The
results of the severe steaming study are shown in Table 1.
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
39
Table I.: Summary of Steaming Study Results
Crush
Crush Strength Strength after
before steaming steaming H/Pt
before H/Pt after
Example (1b/in) (lb/in) steaming
steaming
18.06 25.33 0.49 0.43
8 152.31 129.38 1.37 0.172
9 30.69 25.75 0.76 0.7
79.01 78.52 1.26 0A07
11 156.05 132.35 1.31 0.195
1001221 It can be seen that the catalyst of Example 1 maintained metal
dispersion (indicted by H/Pt) and showed slightly higher crush strength after
severe steaming.
EXAMPLE 14: Catalytic testing for dewaxi.ng of oxygenated feeds
1001231 Catalytic testing was conducted on a High Pressure Heated Orbital
Shaker high-throughput experimentation device, which is a collection of small
batch reactors contained in a heated, high pressure enclosure. Individual
batch
reactors consist of a 40 mm deep well with an internal volume of 5.15 cm3
each.
Each individual well was charged with a catalyst along with
18-pentatriacontanone feed and run at 800 psig H2, 350 C, and WHSV of 1 to 2
VI over a course of 24 hours. Without being bound to any theory or structurai
details, the reaction is schematically represented below. The results are
shown in
Table 2.
+ H2o
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
Table 2: Catalytic testing results
Example Total Pendant
!Epsilon Pendant Methyl # Side
!Free
Conversion Carbon, Groups, Groups, Chains / Carbon Carbon
(%) mole % mole % mole % Molecule #
Index
1 91 22.03 9.44 7.17 1.87 26M8 5.75
98 10.23 10.94 8.24 2.44 29.57 3.03
3 91 22.ti5 7,95 ti.72 1.52 26.47 5.97
5 99
10.19 13.35 9.70 ' 2.28 23.54 2.40
6 100 22.07 10.04 7.86 2.33 29.64 6.54
7 100 12,13 12,76 9.43 2,28 24.16 2.93
9 100 ' 8.41 13.15 10.08 2.96
29.40 2.47
11 100 4,75 14.22 10.41 2.68 25.78 1,22
100124i -Under the conditions tested, ali catalysts disclosed herein
effectively
dewaxed the ketone feed (conversions of ketone > 90%) giving liquid products.
[001251 The products were characterized using quantitative 13C NMR..
Quantitative 13C NMR spectra were obtained using Cr(acac)3 as a relaxation aid
during acquisition. For example, all normal paraffins with carbon numbers
greater than C9 have only five inequivalent carbon NMR absorptions,
corresponding to the terminal methyl carbons (a), methylene carbons at the
second, third, and fourth positions from the molecular ends (0, y and 6,
respectively), and the other carbon atoms along the backbone that have a
common
shift (.6). The intensities of a, p, y and 6 are equal and the intensity of c
carbons
depends on the length of the molecule. Similarly, side branches on the
backbone
of an iso-paraffin have unique chemical shifts and the presence of side-chain
causes a unique shift at the tertiary site on the backbone to -which it is
anchored. It
also perturbs the chemical shifts within three sites of the tertiary site,
imparting
unique chemical shifts (a', p' and y') to the adjacent sites when they occur
in the
center of a long backbone. The number of free ends of molecules can be
estimated by measuring the number of a, p, y and 6 carbons. Unique shifts also
enable measuring the number of pendant side-chains of different length (which
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
41
are called P-Me, P-Et, P-Pr, and P-Bu). The molecular ends that have a side
branch at the 2, 3, 4, or 5 sites (which are called T-Me, T-Et, T-Pr and T-
13u) can
also be measured. The branching features are particularly valuable in
characterizing lube basestocks.
1001261 The products can be characterized by the "Free Carbon Index", which
represents the measure of carbon atoms in an average molecule that are epsilon
carbons:
FCI = (% epsilon carbons) x (Carbon Number) / 1 00,
where the Carbon Number is determined by 13C NMR as following:
Carbon Number = 2 / ((mole% a carbon mole% T-Me carbon + mole%
T-Et carbon mole% T-Pr carbon)/ 1 00)
1001271 13C NMI{ also revealed that the products are significantly free of
carbonyl carbon, consistent with high conversions seen by GC. The dewaxed
products had, on average, 1-3 side chain per molecule, indicating effective
dewaxing of the ketone feed.
1001.281 All documents described herein are incorporated by reference herein,
including any priority documents and/or testing procedures to the extent they
are
not inconsistent -with this text. As is apparent from the foregoing general
description and the specific embodiments, while forms of the disclosure have
been
ill.ustrated and described, various modifications can be made without
departing
from the spirit and scope of the disclosure. A.ccordingly, it is not intended
that the
disclosure be limited thereby. Likewise, the term "comprising" is considered
synonymous with the term "including" for purposes of Australian law.
CA 02896374 2015-06-23
WO 2014/158843 PCT/US2014/020509
42
100129] When numerical lower limits and numerical upper limits are listed
herein, ranges from any lower limit to any upper limit are contemplated,
1001301 While the illustrative embodiments of the disclosure have been
described with particularity,. it 'will be understood that various other
modifications
will be apparent to and can be readily made by those skilled in the art
without
departing from the spirit and scope of the disclosure. Accordingly, it is not
intended that the scope of the claims appended hereto be limited to the
examples
and descriptions set forth herein but rather that the claims be construed as
encompassing all the features of patentable novelty which reside in the
present
disclosure, including all features which would be treated as equivalents
thereof by
those skilled in the art to which the disclosure pertains.