Note: Descriptions are shown in the official language in which they were submitted.
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
TITLE
LYOPHILIZED FIBRIN SEALANT FOR HIGH VOLUME HEMORRHAGE
RELATED APPLICATIONS
This application is a Continuation-ln-Part of U.S. Patent Application No.
12/487,057
(allowed) filed on June 18, 2009 which has received a Notice of Allowance on
November 6,
2012 and with the Issue Fee to be paid before February 6, 2013. All
description, drawings
and teachings set forth therein are expressly incorporated by reference herein
and we
claim priority upon the teachings expressly made therein.
FIELD OF THE INVENTION
The present invention is related to a method to produce a two component or bi-
layer
system consisting of a sterile biocompatible lyophilized desAB fibrin polymer
or fibrin II
polymer composition from concentrated desAB fibrin monomer or fibrin II
monomer in acid
solution, and lyophilized thrombin for application as an adhesive sealant
component and
hemostatic agent. The preparation (invention) trademarked ClotBlock is
presented in
various solid shapes and thickness that may be used to stop bleeding or seal
tissue in vivo
with and without compression. It is particularly related to need of affixing a
fibrin Clot
without a biodegradable support or a support that can be detached after
application over a
bleeding wound in order to seal tissue and control vascular, epidermal, bone
or internal
hemorrhage. The invention may include a biodegradable support made of
hyaluronic acid
or other biodegradable polymer,
BACKGROUND OF THE INVENTION
Severe bleeding for organ resection, trauma, or large dermal wounds is
sometimes
difficult to control. Recently fibrin-based patches have been tested to seal
grade IV/V
wounds using cellulose or gelatin supports. These technologies however cannot
be applied
to certain types of procedures such as interventional radiology, orthopedic or
laparoscopic
1
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
surgery. In addition the "support" used by these products remains in the body
until
biodegraded causing severe inflammatory reaction to foreign body.
Current solutions and limitations. In general, the synthetic adhesives are
used for the
tight sealing of vessels or of lungs and for "gluing" the edges of skin
incisions. These glues
are eliminated, in general after the scaring of the wound, by biodegradation,
absorption or
by simple detachment in the form of scabs. Various technologies have been
developed for
the formulation of tissue adhesives. Some of them are of synthetic origin,
such as the glues
based on cyanoacrylates (2-butyl cyanoacrylate, 2-octyl cyanoacrylate), or
synthetic
polymers, and others contain biological materials such as collagen or fibrin
which in
addition have hemostatic properties.
As a result of their hemostatic and adhesive properties, sealants, and
particularly fibrin
sealants have been extensively used in most surgical specialties for over two
decades to
reduce blood loss and post-operative bleeding because of the ability to adhere
to human
tissue as they polymerize (1, 2, 3). These compounds are used to seal or
reinforce the
sealing of wounds that have been sutured or stapled; they can also be used
with pressure
over an injured area. Fibrin sealants are biological adhesives that mimic the
final step of the
coagulation cascade. (4) The main components of the sealant are fibrinogen,
plasma
proteins and factor XIII on the one hand and thrombin, and calcium chloride on
the other.
The components are often extracted from human plasma or produced by
recombinant
techniques. Mixing fibrinogen and thrombin creates a polymer barrier (fibrin)
that simulates
the last stages of the natural coagulation cascade to form a structured fibrin
clot similar to a
physiological clot.
There are several commercial products available (Floseal, Gelfoam, Evicel,
Bioglue,
surgicel, tachoseal, etc) (3, 5). However, these products have significant
limitations, which
have prevented their widespread use in cases of severe bleeding in surgery and
in
emergency medicine, orthopedic and interventional radiology and laparoscopic
surgery. All
existing haemostatic agents for intracavitary bleeding are designed to be used
as adjuncts
in light to moderate bleeding and require hard compression. One of the major
limitations
encountered in the development and/or use of tissue adhesive and sealant
compositions
for minimally compressible hemorrhage is their inability to form a
sufficiently strong bond to
tissues when there is profuse bleeding and to produce a stable clot within 10
minutes of
2
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
application. Therefore, tissue adhesives and sealants have to be employed in
combination
with compression methods, sutures and/or staples, and adhesive patches so as
to reduce
the tissue-bonding strength required for acceptable performance. However,
there are many
situations where the use of strong compression, sutures and/or staples is
undesirable,
inappropriate or impossible, (e.g. in bone, interventional radiology).
The present alternative approach: In order to form a physical barrier that
resists the flow
of blood, the adhesive matrix must form in a matter of seconds a strong fibrin
interface,
bond with tissues in the midst of flowing blood and remain at the lacerated
site to form a
clot. The ability to adhere to human tissue of each of the product
presentations in a form
of a square, a round patch a sphere , a cylinder, or a cone is related to the
composition
and method of production of fibrin and its interaction (combination) with
thrombin to
stimulate the coagulatory cascade. The essential aspect of the technology is
the ability to
bypass the cleavage process of fibrinogen to produce a fibrin monomer and its
subsequent
polymerized, lyophilized and capable to absorb blood to form a fibrin clot.
The agent starts
the Clot formation process from an already stabilized I fibrin polymer that
absorb the blood
into a lyophilized crosslinked polymer containing the necessary components to
stimulate
the coagulatory cascade (thrombin) and form a physical barrier that turns into
a functional
fibrin clot within two minutes of application. (6)
In our approach these results are obtained through a) the application of a
polymeric
cross-linked lyophilized fibrin network that absorbs the blood forming a very
sticky gel-like
matrix, which attaches to lacerated tissue; and b) the incorporation of a
thrombin layer that
contributes to the rapid formation of a strong fibrin clot stabilized by
calcium independent
transglutaminase enzyme incorporated in the product, and by Factor XIII from
the blood .
The lyophilization process in subsequent layers over a biodegradable removable
support facilitates its application, and allows for long-term storage,
transportation and
readiness.
In addition, no fibrin-based products have been developed to address the need
arresting hemorrhage from deep cuts or large bed cutaneous wounds, which cause
such
severe bleeding that often require stitches or sutures.
Composition. All the forms of the present technology - square, sphere,
cylinder or
cone incorporate fibrin monomer in acid solution polymerized by a change of
pH, which is
3
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
neutralized by a buffer solution in the presence of activated transglutaminase
enzyme
(calcium independent) and Factor XIII (calcium dependent) (Calcium dependent
and
Calcium independent), and calcium chloride. Once fibrin polymer is formed and
cross-
linked, a second layer of thrombin is incorporated and subsequently
lyophilized. (Fig 1)
The monomer can be mixed with a volume of about 1`)/0 to about 5% of glycerol
to
achieve a specific viscoelastic profile that is adapted to the type of
application. The
absorption of blood by the cake turns the lyophilized fibrin into a gel, which
forms the fibrin
clot at sites of injury (7).
Under coagulant conditions, calcium independent transglutaminase and activated
Factor
XIII from the blood contribute to this process by stabilizing the fibrin clot
through covalent
bonds.
Key Attributes. Polymerization/Adhesion. The fibrin gel that seals the wound
is formed
as a result of the absorption of blood by the lyophilized bilayer material,
which maintains
covalent bonds while changing from solid to gel state. The clot is
mechanically stable, well
integrated into the wound and more resistant to lysis by plasmin compared with
a non-
cross-linked clot [8] or other fibrin sealants. The inclusion of calcium
independent
transglutaminase facilitates the transglutaminase-mediated cross-linking of
the aC-domains
polymers in fibrin promoting integrin clustering and thereby increasing cell
adhesion and
spreading, which stimulates fibrin to bind avb3-, avb5-and a5b1-integrins on
endothelial
cells [9]. The oligomerization also promotes integrin-dependent cell signaling
via focal
adhesion kinase (FAK) and extracellular signal-regulated kinase (ERK), which
results in an
increased cell adhesion and cell migration [10]. The presence of calcium ions
enhances
the progression from the inflammatory response to the coagulation cascade
(first stage)
and activates Factor XIII.
The adhesion characteristics to vital human tissue and the kinetics of
polymerization
of the proposed agent have been tested in vitro and in vivo. The data obtained
provide
ample evidence of the ability of the various presentation of ClotBlock to stop
bleeding and
achieve hemostasis with minimal compression in induced intraperitoneal wounds
in solid
organs or soft tissue. And to stop intramedulary bone bleeding in knee and hip
replacement
in the swine models.
4
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
Lyophilized Fibrin II is obtained from fibrin II monomer polymerization: US
patent
application 12/487,057 (allowed), which describes a method of preparing a
fibrin monomer.
The ClotBlock sealant composition uses a lyophilized fibrin polymer obtained
from
neutralization of fibrin monomer. The composition of parts and method of
production of the
fibrin II described in this patent application as well as the process of
neutralization and
crossl inking of the polymer are critical to the performance of the proposed
technology
which depends on the characteristics of fibrin itself (thickness of the
fibers, the number of
branch points, the porosity, and the permeability and other polymerization
characteristics
define clotting factors. The Clot produced by ClotBlock creates opaque
matrices of thick
fibers, and therefore tube formation proceeds at a faster rate than in
transparent matrices.
The concentration of thrombin to produce a fibrin monomer and thus the release-
rate of
FPA also has an important impact on the polymerization process. The described
concentrations, dilutions and pH established for ClotBlock produce an optimal
fibrin
structure at an accelerated rate.
ClotBlock presentations:
The fibrin polymer can be produced and lyophilized in various sizes, thickness
and forms in
order to adapt to the type of application (Fig 2A, 2B, 2C). It can be
configured in small
spheres or cylinders 1/4 inch diameter to be introduced through a laparoscopic
port or a
vessel in cases on interventional radiology; it can also be molded in round or
square flat
solid blocks of various sizes in 1/4; 1/2 a 1" thickness for use in spleen
laceration, or organ
resection, or placed over an adhesive bandage to cover deep skin cuts. The
lyophilized
form can also be soaked in water and used as a sealing paste or gel.
"SUMMARY OF THE INVENTION"
The present invention lies within the domain of biological adhesives and
tissue
sealants, which are biodegradable and nontoxic, intended for therapeutic use,
for example,
as an adjunct to hemostasis in laparotomy or laparoscopic surgery, or as
primary treatment
in orthopedic surgery, trauma (spleen laceration), interventional radiology
and large¨bed
wounds.
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
In one aspect, the present invention relates to biocompatible adhesive fibrin
polymer,
which is bio-reabsorbable and nontoxic, for surgical or therapeutic use. It
also relates to a
bilayer application containing bioactive substances, which can be released in
a given site to
stimulate coagulation. In another aspect, the invention relates to a process
for producing
such an adhesive polymer.
Extensive in vivo studies show that ClotBlock is an excellent hemostatic agent
candidate
control moderate to severe bleeding. Its different presentations maximize the
hemostatic
effect in various types of surgical and trauma applications. The agent is
durable, easy to
store, poses minimal risk, requires little training to use, and is highly
effective against
moderate to severe bleeding.
BRIEF DESCRIPTION OF THE DRAWINGS
Fig. 1. ClotBlock layer distribution
Fig. 2 Possible shapes: A Spheres and cylinders; B Patch or Block; C Bandage
Fig. 3. Comparison of intratissular adherence strength of Clotcake with
Tissel, Floseal, and
Evicel.
Fig. 4. Polymerization, cross-linking and stabilization of fibrin in the
presence of ACTIVA
and Activa + Factor XIII.
Fig. 5. Control of intraoperative bleeding as primary treatment in partial
nephrectomy by
application of ClotBlock showing the formation of a solid clot within 5
minutes* of
application (median of 3.2 1.4 min).
Fig. 6. Control of spleen laceration bleeding as primary treatment without
packing or
sutures. By application of CloBlock7, hemostasis was achieved within 5 minutes
of
application.
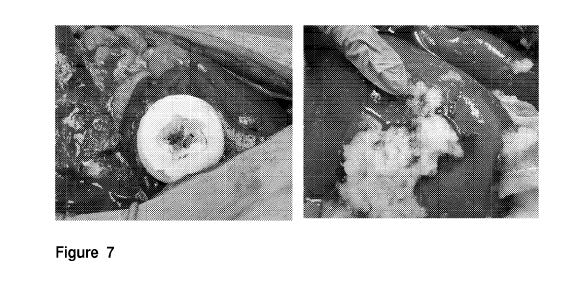
Fig.7. Control of intraoperative bleeding as primary treatment in liver injury
grade IV by
application of ClotBlock. Hemostasis was achieved within 5 minutes .
Hemostatic
effectiveness of ClotBlock in gel form as an adjunct to hemostasis to control
intraoperative
bleeding in partial hepatectomy. Hemostasis was achieved within 5 minutes of
application.
Fig. 8. Microscopic examination under UV light comparing the trace in
fluorescence trapped
in interstitial spaces in kidney and liver at 2 weeks (A) with 5 weeks (B)
after application. .
6
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
Fig 9. Detection of antibodies that might be produced in swine against
Thrombin, using a
sandwich ELISA (enzyme linked immunosorbent assay).
In human fibroblast cultures exposed to ClotBlock preparations
a. HF ¨ Untreated
b. HF + ClotBlock, Day 5
Fig. 10. Detection of antibodies that might be produced in swine against
Thrombin, using a
sandwich ELISA (enzyme linked immunosorbent assay).
Fig 11. Human fibroblast exposed to ClotBlock preparations, there was a total
absence of
damage or toxicity to the cells, and absence of any bacterial or fungal
contamination; the
cells appeared slightly larger than in control untreated cultures.
a. ¨ Untreated, Day 5
b. fibrioblasts + ClotBlock, Day 5
Fig 12. Human epithelial cell cultures (A549) exposed to ClotBlock
preparations, there was
a total absence of damage or toxicity to the cells, and absence of any
bacterial or fungal
contamination; the cells appeared slightly larger than in control untreated
cultures.
c. A549 cells ¨ Untreated, Day 5
d. A549 cells + ClotBlock, Day 5
DETAILED DESCRIPTION
We have developed a hemostatic agent that can be shaped in various forms and
supports, trademarked as Clotblock. The agent is a novel fibrin sealant (pure
fibrin II made
by neutralization of fibrin II monomer) supplemented by thrombin, and designed
to promote
hemostasis in cases of severe bleeding, and to stop hemorrhage with minimal
compression resulting from organ resection, trauma and/or solid organ wounds,
soft tissue,
bone, and large bed wounds. This sealant agent promotes coagulation and
provides
hemostasis as well as adhesiveness between surfaces of damaged tissue. The
present
fibrin sealant can be used 1) as an adjunct or as primary treatment for severe
bleeding; or 2)
shaped for delivery through laparoscopic port or used as a compression in
organ resection,
or 3) placed on support for use in cases of skin laceration; or 4) shaped to
be delivered
7
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
through catheters in cases of interventional radiology; or 5) shape to seal
intramedullary
bleeding arising from orthopedic surgery or trauma.
Each of the presentations consists of a lyophilized bilayer (fig. 1)
comprising: 1) a fibrin II
polymer produced by neutralization of fibrin monomer in acetic acid solution
(pH 3.4) with
HEPES buffer (pH 8.3) and crosslinked, by activated Factor XIII and calcium
independent
tranglutaminase ; 2) a layer of thrombin at a concentration of 20 NIH units/ml
dissolved in
HEPES water solution in a proportion of 1 ml for every 4 ml of fibrin; and 3)
the option of
adding a third layer PLGA fibronectin embedded microspheres between the fibrin
and the
thrombin layers.
The lyophilized bilayer is applied over lacerated bleeding tissue, which
absorbs the
blood to form a sticky, gummy gel barrier and subsequently a fibrin clot as
blood is
absorbed by the fibrin. 1) The agent seals the wound within 2 minutes, and 2)
binds
together the lacerated tissue.
ClotBlock has been developed in several formulations, which vary in shape,
elasticity, and
clotting strength as needed.
Composition and Application
ClotBlock is produced in two layers. It consists of a lyophilized fibrin
polymer topped by a
layer of lyophilized thrombin. The first layer contains crosslinked fibrin
polymer produced by
neutralization of fibrin monomer in acetic acid solution mixed with a buffer
solution
composed of 150 mM NaCI, 50 mM HEPES, 3 mM CaCl2, 0.12 g/mL Activa (calcium
independent transglutaminase enzyme) and 21 Lowey Units of Factor XIII per ml
of
Neutralization buffer, pH 8.5.according to method described in U.S. Patent
Application No.
12/487,057 and incorporated by reference herein. These two solutions are mixed
in a ratio
of 1:1 inside a sterile mold. . To this Composition 1`)/0 to 5% of glycerol
can be added,
depending on desired flexibility of the block
This mold is sealed inside a sterile TYVEK (Registered trademark of E.I.
DuPont Co.,
Wilmington (DE) bag and incubated at 37 C for four hours.
8
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
The second layer contains a solution of thrombin in a proportion of 1:4 to
fibrin, which is
dissolved in HEPES buffer at the concentration of 20 units/mL.
Step 1: Each component is sterilized by filtration through a 0.22 micron
Millipore filter. Each
layer is poured into a silicon mold of the desired shape (round, oval or
square) to produce a
"cake" of approximately % to 1" thick, which can be supported by a removable
or
biodegradable polymer of mesh such as polyglactin mesh, or sD,L-lactide
polymer
synthetic mesh, polylactic acid (PLA)/poly(glycolide-co-lactide) copolymer
(PLGA)
membrane or polyglycolic acid (PGA) mesh; or by a self-adhesive bandage for
use in cases
of cutaneous lacerations.
Step 2: Clotblock is then lyophilized at a condenser temperature of -40 C to -
50 C, shelf
temperature of 21 C, during 18-72 hours at a pressure of 200-400 millitor.
Step 3: Each piece of ClotBlock is packaged in plastic bags hermetically
sealed to prevent
moisture loss and maintain sterility.
Step 4: ClotBlock is applied with moderate compression directly over the wound
for 1 to 2
minutes. Within 3 minutes a fibrin clot is formed over the wound.
Alternative Delivery Methods:
ClotBlock can be shaped in small cylinders 1/2" diameter and 1" to 2" long,
which can be
delivered through a laparoscopic port into an intracavitary wound, or through
a catheter to
seal a bleeding vessel.
ClotBlock can be dissolved in water at a proportion of 4:2 to form a liquid
gel that can be
applied with a single syringe on a laceration or wound.
ClotBlock can placed over a self-adhesive or non-self adhesive, which is a bi-
layer system
consisting of flexible fabric adhesive bandage and a sterile biocompatible
lyophilized
desAB fibrin polymer or fibrin 11 polymer composition from concentrated desAB
fibrin
monomer or fibrin 11 monomer in acid solution, that may be used to stop
bleeding or seal
cutaneous tissue. This delivery method is particularly related to need of
arresting bleeding
from large or deep skin cuts by affixing a fibrin Clot compressed by a
bandage.
EXAMPLES
The hemostatic characteristics have been tested in animal studies showing that
the
CLOTBLOCK sealant forms a fibrin clot stronger and faster than other sealants.
The
9
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
adhesive is expected to adhere to lacerated tissue and bind the opposing
tissues together
with a strength that is significantly higher than that observed for fibrin
sealants.
The following laboratory tests were conducted interactively with animal
experiments (rat,
rabbit and swine models for grade III and IV liver wounds).
1. Ex-vivo experiments on baseline formulation: Adhesion and Coagulation
Properties
We conducted adhesion and tensile measurements (Intratissue adherence and clot
strength) with an isometric transducer in Sprague-Dawley rat liver tissue.
Tensile Measurements: The two largest lobes of the liver were separated. One
lobe was
attached to a holder that was fixed later to the isometric transducer. The
other lobe was
placed in a flat bed of gauze in a container that could gradually be elevated
and lowered to
produce contact with the piece of liver in the transducer's holder. A damage
area of 1 cm2
was produced in both liver pieces. The formulation to be tested for tissue
adherence was
deposited between the two pieces. The specimens were placed in contact at a
baseline
pressure of 0 gr. At various time points (1, 5 and 10 minutes of exposure and
contact), the
pressure needed to completely separate them was recorded. We compared
Intratissue
adherence of ClotBlock with Tissel, Floseal, and Evicel. The results of the
intratissue
adherence are depicted in Fig. 5. The force of adherence induced by ClotBlock
after 10 min
is more than 150% stronger than Evicel and 800% stronger than the control in
the
intratissue adhesion model (Fig. 3)
2. Molecular Chemistry of Fibrin Polymerization
We conducted molecular chemistry assays to compare the effectiveness of Fibrin
monomer
polymerization (pH Neutralization) and stabilization (cross-linking) by
activated Factor XIII
versus Ca Independent tranglutaminase enzyme (Fig. 4).
2.1. Studies to determine the effect of ACTIVA on Fibrin stabilization
It is well established that FXIII in the presence of Ca2+ catalyzes fibrin
polymer cross-linking
resulting in insoluble fibrin clot. However, whether the presence of calcium
independent
transglutaminase in the reaction mixture catalyzes crosslinking of fibrin was
not
established. Nor has it been established if there is a synergistic effect of
calcium
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
independent transglutaminase and activated Factor XIII. In order to follow
these reactions,
fibrin was subjected to calcium independent transglutaminase treatment, first
as a
concentration dependent reaction and later as a time dependant reaction.
Concentration-dependent and time-dependent (1, 5, 10 min) reactions were
monitored, A
volume of acidic fibrin solution at 2 mg/mL was quickly mixed with Activa in
60 mM Tris
buffer (pH 8.4), with 2 mM CaC12) in variable concentration (1.0-20.0 U/ml) to
achieve
neutralization. The Fibrin was visualized with anti-fibrinogen antibody
(1:50). Assays
compared a) fibrin and fibrinogen crosslinking by calcium independent
transglutaminase
enzyme at 1, 5 and 10 Minutes (Fig. 4) and fibrin crosslinking by calcium
independent
transglutaminase enzyme at concentrations of 20u/ml, 19U/ml, and 1 U/ml.
The figure shows the formation of strong gamma dimmers during fibrin cross-
linking with
calcium independent transglutaminase enzyme and factor XIII at 1 minute. At
this time
gamma dimmers are not yet present in the fibrinogen sample.
3. EXPERIMENTS IN ANIMAL MODELS
We conducted studies on intracavitary intraoperative bleeding in the swine
(pig) model in
order to assess the Formation of Fibrin Clot by absorption of blood and to the
determine the
ability to control bleeding.
Study objectives: Compare ClotBlock versus standard surgical practice in
stopping
moderate to severe bleeding during laparoscopic partial nephrectomy; and
determine
whether the hemostatic is safe and can control intraoperative hemorrhage
(minimize
intraoperative blood loss).
3.1. Protocol: Evaluation of ClotBlock for the control of intraoperative
bleeding as
primary treatment in partial nephrectomy.
Six female Yorkshire crossbred swine, age 2.5 months, weighing 37 2 kg, were
used.
The protocol was approved by the Institutional Animal Care and Use Committee
of TMCI.
Animals were subject to a resection of 25% of the kidney via open laparotomy.
After the
damage was induced, a round block of 2.5" in diameter of 60CC ClotBlock bi-
layer of
composition #1F containing 1`)/0 glycerol was compressed against the resection
in the
parenchyma.
11
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
Results: Following a 1-minute profuse bleeding from the interlobular artery,
hemostasis was
achieved in all six animals (Fig. 5) with the formation of a solid clot within
5 minutes* of
application (median of 3.2 1.4 min).
.* The five minute time to hemostasis is defined by the Blood Products
Committee of the
Food and Drug Administration as the maximum time to demonstrate efficacy in
achieving
hemostasis.
3.2 Evaluation of ClotBlock for the control of spleen laceration bleeding as
primary
treatment without packing or sutures.
The purpose of this study is to determine if CloBlock can stop profuse
bleeding within 5
minutes of application in cases traumatic spleen laceration.
Methods: Eight female Yorkshire crossbred swine, age 2.5 months, weighing 37
2 kg,
were used. The protocol was approved by the Institutional Animal Care and Use
Committee.
Animals were subject to a 1inch incision in lateral middle portion of the
spleen (created
sharply by an 11 blade scalpel). After the damage was induced, a round block
of 2.5" in
diameter of 60CC of ClotBlock composition #1F containing 1`)/0 glycerol was
compressed
against the laceration for 2 minutes. Hemostasis was achieved in all animals
within 5
minutes of application (Fig 6).
Results: All animals (n=6) Achieved hemostasis within 5 minutes of
application* with a
median of 3.2 1.4 min.
*The five minute time to hemostasis is defined by the Blood Products Committee
of the
Food and Drug Administration as the maximum time to demonstrate efficacy in
achieving
hemostasis.
3.3 Evaluation of ClotBlock for the control of intraoperative bleeding as
primary
treatment in liver injury grade IV.
Fourteen female Yorkshire crossbred swine, age 2.5 months, weighing 37 2 kg,
were randomized into 3 groups. Group 1 (n= 6) consisted of animals who
underwent grade
4 liver injuries via open laparotomy and were treated with a 40CC ClotBlock.
Group 2 (n=
6) consisted of animals who underwent a similar procedure and were treated
with GelFoam
(Pfizer); and Group 3 (n=4) consisted of animals who underwent a similar
procedure,
bleeding was stopped by suturing the wound, which was further treated with
saline solution.
12
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
In both hemostatic-treated groups either ClotBlock or Gelfoam was applied and
compressed for 2 minutes against the wound. (Fig. 7) For the purposes of this
model, a
grade 4 injury is defined as a 7 cm long full-thickness parenchyma laceration
(created
sharply by an 11 blade scalpel). These injuries were consistent with the
American
Association for the Surgery of Trauma Organ Injury Scaling system..
A spot in the middle of the liver was selected to produce the liver injury
with a scalpel.
The position was calculated by approximation to the suprahepatic vessels and
some
branches of the portal vein. The spot was marked with a marker. After the
damage was
induced, either a 40CC block of ClotBlock of the patch type treate with Alexa
fluorescent
dye with a PLGA membrane as support or a 3x3 inch piece of GelFoam was
compressed
was against the wound for 2 minutes.
Fluid resuscitation with Lactated Ringer's (LR) was begun immediately after
injury.
LR was infused as necessary to re-establish a MAP within at least 80% of the
pre-injury
MAP if possible. Resuscitation was continued for the entire observation
period. At the end
of the 60 minute study, each animal's MAP and the total resuscitation volume
infused were
recorded.
All animals in group 2 (Gelfoam) were euthanized after this procedure was
complete.
Half of Animals in Group 1 (Clotblock n= 3) and Group 3 (Control saline n=2)
were
euthanized at 2 weeks of completion of the study, and the other haLf (Group 1
n=3 and
Group 3 n=2) were euthanized at 4 weeks after completion. Necropsy was
performed and
histological samples were obtained from several organs
Outcome Measures
Primary endpoints: Proportion of successes achieving hemostasis within 5
minutes
following injury. The pre-specified primary endpoint is the time to
hemostasis, defined as
the time interval from application to termination of bleeding or oozing from
the parenchyma.
If recurrent bleeding from the sheath site occurred following initial
hemostasis, the timing
and duration of additional non-compressible application required to
reestablish complete
hemostasis was also recorded.
Secondary endpoints Total blood loss and amount of resuscitation fluid
required to maintain
Median Blood Pressure within 10% of baseline.
Results: End points for animals in Groups 1 and 2 (Grade IV injuries) are
shown in
Table 1. Outcome measures for Grade IV liver injuries treated with ClotBlock
(Group 1) and
with GelFoam (Group 2). All values reported as mean SEM
13
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
Table 1. Survival Time Total Blood Loss (ml) Hemostasis Fluid
Group (min) at Min 5 Requirement (ml)
1 (n= 6) 60 0 100 83 5 200 83
2 (n= 6) 60 0 300 112 0 775 342
3(n=4) 60 0 210 60 5 400 93
Conclusion: All animals treated with ClotBlock achieved hemostasis within 5
minutes of
application. ClotBlock significantly decreases the bleeding time and blood
loss, and
significantly improves the adhesion between lacerated and damaged tissue.
3.4 .Evaluation of ClotBlock placed over a self-adhesive bandage for the
control of
severe cutaneous bleeding as primary treatment.
Ten female Yorkshire crossbred swine, age 2.5 months, weighing 37 2 kg, were
randomized into groups. Group 1 (n= 5) consisted of animals who underwent a
deep shin
laceration 3" long in the groin injuries and treated with ClotBlock placed
over a self-
adhesive bandage for 10 minutes. Group 2 (n= 5) consisted of animals that
underwent a
similar procedure and were not treated.
Outcome Measures
Primary endpoints: Proportion of successes achieving hemostasis within 10
minutes
following injury. Secondary endpoints Total blood loss
Results: End points for animals in Groups 1 and 2 are shown in Table 2.. All
values
reported as mean SEM
Table 2. Total Blood Loss (ml) Hemostasis
Group at Min 10
1 (n= 5) 20 13 5
2 (n= 5) 210 112 0
Conclusion: All animals treated with ClotBlock in a bandage form achieved
hemostasis
within 10 minutes of application. ClotBlock significantly decreases the
bleeding time and
blood loss, and significantly improves the adhesion between lacerated and
damaged tissue.
14
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
4. Safety Studies
Three out of the six swine, Group 1 (n= 6) who underwent grade 4 liver
injuries via open
laparotomy and were treated with a 40CC ClotBlock block were euthanized at
week two
after completion of surgery, and the remaining three at 4 weeks. Similarly,
two controls
(group 3) were followed and euthanized after 2 weeks, and two animals were
euthanized
after 4 weeks. Necropsy was performed and tissue samples from main organs were
obtained.
The safety of the agent was assessed through the evaluation of toxicity,
physiological
adverse effects, biocompatibility, delayed hematoma and/or edema formation and
immunological risks. Physiological and pathological observations included:
Mortality/morbidity; Body weight, Food consumption, Organ weights:
4.1 Acute Toxicity was assessed by macroscopic evaluation at necropsy and by
histological
studies. Irritation of tissues and tissue vessels to which the agents ere in
contact was
assessed looking for evidence of acute and/or chronic inflammation as signs or
irritation in
the histology. Thrombosis, fistula, and abscess formation was assessed for all
organs
4.2 Assessment of delayed hematoma: Risk of subcapsular or parenchymal
hematoma
formation. Delayed hematoma and edema formation was observed macroscopically
and
histologically at 21 days after application. Small hematoma formation is
defined as a visible
or palpable mass of 4 cm in diameter without associated sequelae.
4.3.Gross pathology and Histology
We analyzed the histological damage in lungs, kidneys, liver, spleen from all
treated after 2
and 4 weeks of surgery, and compared sample treated with ClotBlock to control
(treated
with saline solution) Data on inflammation included apoptosis and leukocyte
infiltration.
Inflammation and edema formation was also assessed histologically
Once animals are sacrificed, organs were collected, fixed in 10% formalin and
embedded in
paraffin blocks. Histologic sections were stained with Hematoxylin and Eosin
and examined
at 100X and 400X in optical and microscopes. These slides were evaluated by a
Board-
certified veterinary pathologist as shown in table 3.
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
Results:
Table 3
TDMI PROTOCOL No: 09-019 4-week follow-up
ANIMAL
ID TISSUE MORPHOLOGIC DIAGNOSES
1. Peritonitis, mild, chronic, focal, pleocellular
(granulomatous, neutrophilic, lymphoplasmacytic, eosinophilic)
#04373 Liver with fibrosis, subcapsular hepatitis and foreign
material
2. Hepatitis, periportal, minimal to mild, chronic,
lymphoplasmacytic,
Treated eosinophilic
3. Lipidosis, microvesicular, minimal, diffuse
Lung none
1. Nephritis, interstitial, mild, chronic, multifocal,
Kidney lymphoplasmacytic
1. Peritonitis, mild to moderate, pleocellular (granulomatous,
neutrophilic,
Liver injury lymphoplasmacytic, eosinophilic) with fibrosis,
subcapsular
site hepatitis
2. Hepatitis, periportal, minimal to mild, chronic, diffuse,
ymphoplasmacytic and eosinophilic
3. Lipidosis, microvesicular, minimal to mild, diffuse
Summary of Morphologic Diagnoses:
Heart:
No significant findings
Lung:
1. Atelectasis, mild to moderate, multifocal
16
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
2. Edema, minimal to mild, acute, diffuse
3. Hemaglobin crystals, minimal, focal, with mild neutrophilic inflammation
Kidney:
1. Granular casts, intratubular, minimal, multifocal
2. Hyaline droplets, intracytoplasmic, proximal tubular epithelium, minimal to
mild,
multifocal
Abdominal wall:
Fibrous tract, focal, with a central cavity, mild to moderate granulomatous,
neutrophilic,
lymphoplasmacytic inflammation with hair shafts, and moderate edema
Gross Pathology finding are summarized in table 4.
Table 4 Treatment General Adhesions Other Hematoma
Pig Number Condition organs
And at
procedure Necropsy
#04362 40cc normal Moderate 1 Implant No
Grade IV ClotBlock adhesions from growth on hematoma
Liver Injury, #1F Primary abdominal wall and small
no suture treatment on liver bowel 2.All
(2 weeks) 2min else
compression normal
#04366 40cc normal None All normal No
Grade IV ClotBlock hematoma
Liver Injury, #1F Primary
no suture treatment
(2 weeks) 2min
compression
#04372 40cc Normal 1. some adhesions All else No
Grade IV ClotBlock from abdominal normal hematoma
Liver Injury, #1F Primary wall to organs
no suture treatment 2. Dense liver
17
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
(2 weeks) 2min adhesions
compression
#04373 40cc 1. suture 1. dense All else No
Grade IV ClotBlock granuloma adhesions from normal hematoma
Liver Injury, #1F Primary abdominal wall to
no suture treatment liver and spleen
(4 weeks) 2min 2. adhesions on
compression spleen
#04376 40cc normal 1.moderate All else No
Grade IV ClotBlock adhesions to normal hematoma
Liver Injury, #1 F Primary abdominal wall
no suture treatment 2. some adhesions
(4 weeks) 2min on spleen
compression 3. some adhesions
on liver
#04377 40cc normal 1.moderate All else No
Grade IV ClotBlock adhesions to normal hematoma
Liver Injury, #1 F Primary abdominal wall
no suture treatment 2. some adhesions
(4weeks) 2min on spleen
compression 3. some adhesions
on liver
Conclusion: Both the control (sutured) and non-sutured treated animals
contained mild
chronic inflammation and fibrosis at the site of the abdominal wall injury.
Only the treated
animals had evidence of granulomatous inflammation associated with foreign
material
(ClotBlock), at the abdominal wall injury site, hepatic injury site and
seeding adjacent
peritoneal surfaces. While treated animals did not show histologic evidence of
notable
hemorrhage at either clinical location, sutured controls presented evidence of
severe
Peritonitis and subcapsular hepatitis, and hematoma with necrosis and
fibrosis. There was
no evidence of thromboembolism in any other organ. The control animals had
evidence of
extramedullary hematopoiesis in the spleen, interpreted as a response to
hemorrhage from
18
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
previous surgical injury. No changes to suggest significant blood-loss anemia
was seen in
either animal.
All pigs, treated and control developed adhesions when wounded and treated or
sutured.
Therefore adhesions are not caused by ClotBlock, although ClotBlock as other
fibrin
sealants may be a contributing factor.
4.4. Pharmacokinetic profile of the agent through Biodegradation studies.
Elimination
through biodegration by proteolytic enzymes was determined in vivo.
Method: To examine the fate of ClotFoam in vivo, a batch of ClotBlock was
prepared using
fluorescein-tagged human fibrinogen as tracer. This preparation of ClotBlock
was applied to
the six animals of Group 1 in the liver grade IV wound procedure (4.3), which
were
euthnanized at 2 weeks (n=3) and 4 weeks (n=3) following application. Once
animals are
sacrificed, organs were collected, fixed in 10% formalin and embedded in
paraffin blocks.
Histologic sections were examined at 100X and 400X in fluorescence microscope.
The
elimination of ClotBlock was determined by either the total absence of
fluorescent traces in
the samples, or by the level of fluorescense observed at 2 weeks and 4 weeks.
Results: Clotblock was eliminated in all organs within 4 weeks of application
(Fig. 8)
4.5. Evaluation of Immunologic Response
Potential antibody responses to ClotBlock were evaluated.
Methods: Serum samples were collected from experimental animals subjected to
liver
grade IV injury (4.3), pre- and post-treatment on Day 0, Day 7, and Day 21
days post-
surgery and stored frozen at -20 C until analysis. Antibodies generated to the
components
that are used in the formulation of ClotBlock were tested by enzyme-linked
immunosorbent
assay (ELISA).
To detect antibodies that might be produced in swine against components of
ClotBlock, a
sandwich ELISA (enzyme linked immunosorbent assay) was constructed. The bottom
surfaces
of 96-well microtiter plates were coated overnight at 4 C with Fibrin (10
mg/ml in PBS, pH 7.0,
Sigma-Aldrich) or thrombin (n 10 mg/ml in PBS, pH 7.0, Sigma-Aldrich). All
wells were washed
times with PBS. Samples of swine serum were applied at 1:20 final dilution in
PBS,
incubated for 1 hr at room temperature and washed 5 times with PBS. Enzyme
(horseradish
19
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
peroxidase) ¨ conjugated rabbit antibodies to pig IgG (Sigma-Aldrich) were
applied to all wells
at 1:20 dilution in PBS, incubated for 1 hr at room temperature, and washed 5
times with PBS.
Substrate was prepared by dissolving one capsule of substrate (2,2'-Azino-
bis(3-
ethylbenzothiazoline-6-sulfonic acid) di-ammonium salt, 10 mg/capsule, Sigma-
Aldrich) in 100
ml of phosphate-citrate buffer, pH 5.0, and adding H202 (0.25 ml of 3%
solution). Following
incubation for 10 min at room temperature, optical density at 405 nm was
determined using a
BioTek EX800 microplate reader.
Each targeted component was diluted 1:100 in phosphate-buffered saline (PBS),
pH 7.4,
coated onto microtiter plate wells, and incubated overnight at 4 C. The wells
were blocked
with 0.25% (wt/vol) nonfat dry milk/0.2 /0 Tween 20 in PBS (blocking buffer)
and then
incubated with 50 pL of a 1:10 dilution of animal serum in blocking buffer for
1 hour at
37 C. Bound IgG and IgM were detected as standard ELISA system for secondary
antibody.
The normal range was determined with 5 normal animal sera. An elevated
antibody
level is defined as greater than two standard deviations above the normal
mean. Each plate
included wells incubated with all reagents except for the diluted serum, which
provided the
background absorbance that was subtracted from all results. Antibodies to
purified
thrombin were determined as described for antibodies to prothrombin, except
that purified
thrombin (5 pg/mL) was used to coat the microtiter plate wells. Inhibitory
anti-factor V
antibodies binding to the factor V C2 domain are associated with hemorrhagic
manifestations. Antibodies to human factor V were identified by coating
microtiter plate
wells with the murine monoclonal antihuman factor V antibody 6A5 (50 pL of 2.5
pg/mL
overnight at 4 C). The wells were washed and blocked, after which they will
were incubated
with 50 pL of 5 pg/mL human factor V. The wells were incubated with a 1:10
dilution of
animal plasma for 1 hour at 37 C. Bound IgG was detected as described above
(standard
sandwich ELISA).
It is very important to consider that then present is a cross species model
zymogenic system with human components being tested in rats and swine. Even if
the
animal produces antibodies against any of ClotBlock components, it is not
certain that
results could be extrapolated to human. There is not a homologous experimental
system at
the preclinical stage.
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
Statistical Analysis
A comparison of the quantitative variables among the 6 randomized subgroups
within the
two treatment arms will performed using the Kruskall-Wallis test. The Mann-
Whitney U test
will used to assess the differences between each randomized group. Statistical
analyses
were conducted using Stata, version 9.0 (Stata, College Station, TX), with P <
.05
considered statistically significant.
RESULTS AND CONCLUSION: There were no significant differences in OD readings
observed with sera collected on day 0, day 7 or day 21 from control and
ClotBlock treated
pigs when tested against Fibrin or Thrombin (Figures 9 and 10). We conclude
that
experimental pigs produced no detectable antibodies against ClotBlock.
5. Sterilization
Sterile preparations of clotcake were studied.
The acidic Fibrin Monomer was sterile filtered in a biological safety cabinet
using a Nalg-
Nunc 500 mL device (Cat # 450-0045, nitrocellulose membrane, 0.45 m filter).
Growth Study: The general experimental protocol included preparation of sample
solutions
which were then plated on Potato dextrose agar (PDA, Sigma-Aldrich, Cat#P2182)
and
Tryptic soy agar (TSA, Sigma-Aldrich, Cat# T4536) gels in Petri dishes for
growth. The
PDA and TSA gels were incubated and observed at the indicated periods of time
for colony
growth (mold and/or bacteria).
The sample was incubated for 30 min at 37 C and evaluated for colony growth
using the
naked eye at the time periods indicated in the Results and Discussion section.
The
samples were run in duplicate or triplicate with multiple samples indicated
with a 1, 2 and 3
designation in data tables. The scale used for evaluation is as follows:
Table 2. Colony Count Key
Symbol Count
- No visible growth
+ 1-199 visible colonies
21
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
++ 200-399 visible colonies
+++ > 400 visible colonies
Table 3 shows the results of studies of microorganism growth analysis on PDA
and TSA of
the sterile components of FIBRIN_ClotFoam.
Table 3. Sterilization Studies by Bacterial Growth on PDA/TSA at 37 C
Tryptic Soy Agar
Potato Dextrose Agar (PDA)
(TSA)
Time Elapsed (days)
1 1
1 2 3 4 5 6 7 1 2 3 4 5 6 7
1 1
Sample #s$
1 - - - - -
-
C**
2 - - - - -
-
(Fibrin/AcOH, pH
3.5)
3 \! - - - -
-
** "sterile" C used for animal studies in SUNY, stored at 4 C for a week
The growth data indicate that sterile components yielded no significant growth
even after
11 days. Furthermore, the following techniques could be used for
sterilization.
6. Biocompatibility
Two ClottBlock preparations were prepared an d tested under sterile
conditions. These
preparations were tested for biocompatibility with human fibroblasts (HF) and
human
epithelial cells (A549 cell line, ATCC).
Normal human fibroblasts (HFs) were obtained from a commercial source and
cultures
established in 60 mm tissue culture plates in Dulbecco's modified Eagle's
medium
supplemented with 10% fetal bovine serum and maintained at 37 C in a
humidified 5% CO2
atmosphere (CO2 incubator). Human epithelial cell line A549 was maintained in
Minimal
Essential Medium supplemented with 10% fetal bovine serum and 2 mM glutamine.
When fibroblast and epithelial cell cultures reached subconfluence, control
and sodium
benzoate ClotFoam preparations were mixed and immediately delivered into
individual
dishes. The cultures were returned to the CO2 incubator and examined daily for
a total of
22
SUBSTITUTE SHEET (RULE 26)
CA 02896866 2015-06-29
WO 2014/106136 PCT/US2013/078152
five days. ClotFoam material and medium was removed from all cultures, and
adherent
cells were stained with crystal violet (0.1% in 2 % ethanol).
RESULTS: The main observation was a total absence of damage or toxicity to the
cells,
and absence of any bacterial or fungal contamination.
In human fibroblast cultures exposed to ClotBlock preparations, the cells
appeared slightly
larger or more spread out than in control untreated cultures. Fig. 11(, b,)
and well as in
human epithelial cells Fig 12,(a, b, )
Conclusion: ClotBlock is biocompatible, and does not inhibit, but rather
stimulate, the
growth and differentiation of cells; which is an important attribute in wound
healing agents.
23
SUBSTITUTE SHEET (RULE 26)