Note: Descriptions are shown in the official language in which they were submitted.
CA 02897306 2015-07-14
PC72074A
1
Combinations
Field of the Invention
The invention relates to the treatment of pain using combinations of
pharmaceutically
active agents. More particularly, this invention relates to the use of
combinations of an N-
thiazoly1-4-phenoxybenzenesulfonamide derivative and a second pharmaceutically
active
agent, and to pharmaceutical compositions containing them.
Background
The N-thiazolyI-4-phenoxybenzenesulfonamide derivatives that are the first
component of
the combinations of the present invention are modulators of the Nav1.7 sodium
channel.
The compounds are disclosed in International Patent Application
PCT/IB2010/050033,
published as W02010/079443.
Voltage-gated sodium channels are found in all excitable cells including
myocytes of
muscle and neurons of the central and peripheral nervous system. In neuronal
cells,
sodium channels are primarily responsible for generating the rapid upstroke of
the action
potential. In this manner sodium channels are essential to the initiation and
propagation of
electrical signals in the nervous system. Proper and appropriate function of
sodium
channels is therefore necessary for normal function of the neuron.
Consequently, aberrant
sodium channel function is thought to underlie a variety of medical disorders
(see Hubner
C.A., Jentsch T.J., Hum. Mol. Genet., 11(20): 2435-45 (2002) for a general
review of
inherited ion channel disorders) including epilepsy (Yogeeswari et al., Curr.
Drug Targets,
5(7): 589-602 (2004)), arrhythmia (Noble D., Proc. Natl. Acad. Sci. USA,
99(9): 5755-6
(2002)), myotonia (Cannon, S.C., Kidney Int. 57(3): 772-9 (2000)), and pain
(Wood, J.N. et
al., J. Neurobiol., 61(1): 55-71 (2004)).
There are currently at least nine known members of the family of voltage-gated
sodium
channel (VGSC) alpha subunits. Names for this family include SCNx, SCNAx, and
Navx.x.
The VGSC family has been phylogenetically divided into two subfamilies Nav1.x
(all but
SCN6A) and Nav2.x (SCN6A). The Nav1.x subfamily can be functionally subdivided
into
CA 02897306 2015-07-14
PC72074A
2
two groups, those which are sensitive to blocking by tetrodotoxin (TTX-
sensitive or TTX-S)
and those which are resistant to blocking by tetrodotoxin (TTX-resistant or
TTX-R).
An increasing body of evidence suggests that Nav1.7 may play a key role in
various pain
states, including acute, inflammatory and/or neuropathic pain. Deletion of the
SCN9A gene
in nociceptive neurons of mice led to a reduction in mechanical and thermal
pain
thresholds and reduction or abolition of inflammatory pain responses (Nassar
et al., Proc
Nat! Acad Sci USA, 101(34): 12706-11 (2004)). In humans, Nav1.7 protein has
been
shown to accumulate in neuromas, particularly painful neuromas (Kretschmer et
al., Acta.
Neurochir. (Wien), 144(8): 803-10 (2002)). Gain of function mutations of
Nay1.7, both
familial and sporadic, have been linked to primary erythermalgia, a disease
characterized
by burning pain and inflammation of the extremities (Yang et al., J. Med.
Genet., 41(3):
171-4 (2004), and paroxysmal extreme pain disorder (Waxman, SG Neurology.
7;69(6):
505-7 (2007)). Congruent with this observation is the report that the non-
selective sodium
channel blockers lidocaine and mexiletine can provide symptomatic relief in
cases of
familial erythermalgia (Legroux-Crepel etal., Ann. Dermatol Venereol., 130:
429-433) and
carbamazepine is effective in reducing the number and severity of attacks in
PEPD
(Fertleman eta!, Neuron.,52(5):767-74 (2006). Further evidence of the role of
Nav1.7 in
pain is found in the phenotype of loss of function mutations of the SCN9A
gene. Cox and
colleagues (Nature, 444(7121):894-8 (2006)) were the first to report an
association
between loss-of-function mutations of SNC9A and congenital indifference to
pain (CIP), a
rare autosomal recessive disorder characterized by a complete indifference or
insensitivity
to painful stimuli. Subsequent studies have revealed a number of different
mutations that
result in a loss of function of the SCN9A gene and and the CIP phenotype
(Goldberg et al,
Clin Genet. ;71(4): 311-9 (2007), Ahmad eta!, Hum Mol Genet. 1;16(17): 2114-21
(2007)).
Nav1.7 inhibitors are therefore potentially useful in the treatment of a wide
range of
disorders, particularly pain.
The second component of the combinations of the present invention is a
pharmaceutically
active agent selected from gabapentin and pregabalin.
CA 02897306 2015-07-14
PC72074A
3
Gabapentin (2[1-(aminomethyl)cyclohexyliacetic acid) and pregabalin ((S)-3-
(aminomethy)-5-methylhexanoic acid) are ligands for the c2-6 subunit of
calcium channels.
They have been reported to be effective in the treatment of neuropathic pain.
Gabapentin
is marketed under the brand name NeurontinTM and pregabalin is marketed under
the
brand name Lyrica TM.
There is an ongoing need to provide improved methods for managing pain. The
use as
disclosed herein of a combination of a Nav1.7 modulator and a modulator of
activity at the
alpha-2 delta subunit of calcium channels may afford a therapeutic regimen
that provides:
a greater efficacy; and/or the ability to use lower doses of the combination
when compared
to the individual agents; and/or an improved tolerability to the use of either
agent alone.
Summary of the Invention
In a first aspect Al , the present invention provides a use of a combination
for the treatment
of pain.
In a first embodiment Al El , the present invention provides a use of a
combination for the
treatment of pain comprising the separate, sequential or simultaneous use of a
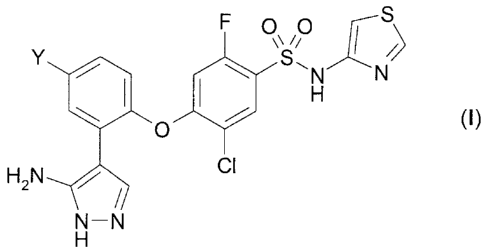
therapeutically effective amount of a compound according to formula (I)
F 0 0
\S,NXN
0 100
(I)
H2N z Cl
N¨N
or a pharmaceutically acceptable salt thereof, wherein ¨Y is ¨Cl or ¨CF3;
and a second pharmaceutically active compound selected from gabapentin and
pregabalin,
or a pharmaceutically acceptable salt thereof.
CA 02897306 2015-07-14
PC72074A
A. 4
In a further embodiment Al E2, the present invention provides the use of
embodiment
Al El which consists essentially of the use of a compound according to formula
(I) and a
second pharmaceutically active compound selected from gabapentin and
pregabalin, or a
pharmaceutically acceptable salt of one or both said compounds.
In a further embodiment Al E3, the present invention provides the use of
embodiments
Al El or Al E2, wherein the compound according to formula (I) is 442-(5-amino-
1H-
pyrazol-4-y1)-4-chlorophenoxy]-5-chloro-2-fluoro-N-(1,3-thiazol-4-
yl)benzenesulfonamide
(IA) or a pharmaceutically acceptable salt thereof.
F 00
X
is 0
Cl S
=(IA)
Cl
H2N z
N¨N
In a further embodiment Al E4, the present invention provides the use of
embodiments
Al El or Al E2, wherein the compound according to formula (I) is 442-(5-amino-
1H-
pyrazol-4-y1)-4-(trifluoromethyl)-phenoxy]-5-chloro-2-fluoro-N-(1,3-thiazol-4-
yl)benzenesulfonamide (IB) or a pharmaceutically acceptable salt thereof.
F =0 0
X
0
F3C opi S
(IB)
H2N(N7 Cl
N¨N
CA 02897306 2015-07-14
PC72074A
In a further embodiment A1E5, the present invention provides the use of any
one of
embodiments A1E1, A1E2, A1E3 and A1E4, wherein the second pharmaceutically
active
compound is gabapentin or a pharmaceutically acceptable salt thereof.
5 In a further embodiment A1E6, the present invention provides the use of
any one of
embodiments A1E1, A1E2, A1E3 and A1E4, wherein the second pharmaceutically
active
compound is pregabalin or a pharmaceutically acceptable salt thereof.
In a further embodiment A1E7, the present invention provides the use of any
one of
embodiments A1E1, A1E2, A1E3, A1E4, A1E5 and AlE6 wherein the compound
according to formula (I) and the second pharmaceutically active compound are
for use
simultaneously.
In a further embodiment A1E8, the present invention provides the use of
embodiment
A1E7, wherein the compound according to formula (I) and the second
pharmaceutically
active compound are formulated together as a single pharmaceutical dosage
form.
In a further embodiment A1E9, the use is oral.
In a further aspect A2, the present invention provides a combination of
pharmaceutically
active agents.
In a first embodiment A2E1, the present invention provides a combination
comprising a
compound according to formula (I) and a second pharmaceutically active
compound, as
defined in embodiments A1E1 or A1E2, or a pharmaceutically acceptable salt of
one or
both said compounds.
In a further embodiment A2E2, the present invention provides the combination
according to
embodiment A2E1 selected from:
CA 02897306 2015-07-14
PC72074A
6
4-[2-(5-amino-1H-pyrazol-4-y1)-4-chlorophenoxy1-5-chioro-2-fluoro-N-(1,3-
thiazol-4-
y1)benzenesulfonamide in combination with gabapentin;
4-[2-(5-am ino-1H-pyrazol-4-y1)-4-chlo rophenoxy]-5-chloro-2-fluoro-N-(1,3-th
iazol-4-
yl)benzenesulfonamide in combination with pregabalin;
4-[2-(5-amino-1H-pyrazol-4-y1)-4-(trifluoromethyl)-phenoxy]-5-chloro-2-fluoro-
N-(1,3-
thiazol-4-y1)benzenesulfonamide in combination with gabapentin; and
4-[2-(5-amino-1H-pyrazol-4-y1)-4-(trifluoromethyl)-phenoxy]-5-chloro-2-fluoro-
N-(1,3-
thiazol-4-y1)benzenesulfonamide in combination with pregabalin;
or a pharmaceutically acceptable salt of one or both said compounds.
In a further aspect A3, the present invention provides a pharmaceutical dosage
form.
In a first embodiment A3E1, the present invention provides a pharmaceutical
dosage form
comprising a compound according to formula (I) and a second pharmaceutically
active
compound, as defined in any one of embodiments A1E1, A1E2, A1E3, A1E4, A1E5
and
Al E6, or a pharmaceutically acceptable salt of one or both said compounds;
and one or
more pharmaceutically acceptable excipients.
In a further embodiment A3E2, the present invention provides the
pharmaceutical dosage
form according to embodiment A3E1 for use as a medicament.
In a further embodiment A3E3, the present invention provides the
pharmaceutical dosage
form according to embodiments A3E1 or A3E2 for use in the treatment of pain.
In a further aspect A4, the present invention provides a compound according to
formula (I)
as defined in embodiment A1E1, or a pharmaceutically acceptable salt thereof,
for use in
the treatment of pain, wherein the treatment further comprises the separate,
sequential or
CA 02897306 2015-07-14
PC72074A
7
simultaneous use of a second pharmaceutically active compound as defined in
embodiment Al El, or a pharmaceutically acceptable salt thereof.
In a further embodiment A4E1, the present invention provides the compound
according to
aspect A4 for use in the treatment of pain, wherein the treatment further
consists
essentially of the separate, sequential or simultaneous use of a second
compound as
defined in embodiment Al El, or a pharmaceutically acceptable salt thereof.
In a further aspect A5, the present invention provides a combination as
defined in
embodiment A2E1 or A2E2, or a pharmaceutically acceptable salt of one or both
said
compounds, for use in the treatment of pain.
In a further aspect A6, the present invention provides the use of a compound
according to
formula (I) as defined in embodiment Al El, or a pharmaceutically acceptable
salt thereof,
for the manufacture of a medicament for the treatment of pain, wherein the
treatment
further comprises the separate, sequential or simultaneous use of a second
pharmaceutically active compound as defined in embodiment A1E1, or a
pharmaceutically
acceptable salt thereof.
In a further embodiment A6E1, the present invention provides the use of a
compound
according to aspect A6, wherein the treatment further consists essentially of
the separate,
sequential or simultaneous use of a second compound as defined in claim 1, or
a
pharmaceutically acceptable salt thereof.
In a further aspect A7, the present invention provides the use of a
combination as defined
in embodiment A2E1 or A2E2, or a pharmaceutically acceptable salt of one or
both said
compounds, for the manufacture of a medicament for the treatment of pain.
In a further aspect A8, the present invention provides a kit comprising:
CA 02897306 2015-07-14
PC72074A
8
(i) a pharmaceutical dosage form comprising a compound according to formula
(I) as
defined in embodiment AlE1, or a pharmaceutically acceptable salt thereof, and
a
pharmaceutically acceptable excipient; and
(ii) a pharmaceutical dosage form comprising a second pharmaceutically active
compound as defined in embodiment AIEI, or a pharmaceutically acceptable salt
thereof, and a pharmaceutically acceptable excipient;
with instructions for use of (i) and (ii) in the the treatment of pain.
In a further embodiment A8E1, the present invention provides the kit of aspect
A8 for use
in pain which consists essentially of pharmaceutical dosage forms (i) and
(ii).
In all of the above aspects Al to A8 and embodiments thereof, the pain may be
acute
nociceptive pain.
Brief Description of the Drawings
Fig 1 is a graphical representation of the degree of flinching over
time for the high dose
of pregabalin (Group 5).
Fig 2 is a graphical representation of the degree of flinching over time
for the high dose
of the compound of formula (1B) (Group 3).
Fig 3 is a graphical representation of the degree of flinching over
time for the high dose
combination of the compound of formula (1B) and pregabalin (Group 7).
Fig 4 is a graphical representation of the degree of flinching over
time for the low dose
of pregabalin (Group 4).
Fig 5 is a graphical representation of the degree of flinching over
time for the high dose
of the compound of formula (1B) (Group 2).
Fig 6 is a graphical representation of the degree of flinching over
time for the low dose
combination of the compound of formula (1B) and pregabalin (Group 6).
Fig 7.1 is a visualisation using posterior distribution plots on the log scale
of the high
dose combination during phase 2.
CA 02897306 2015-07-14
PC72074A
9
Fig 7.2 is a visualisation using posterior distribution plots on the log scale
of the high
dose combination during phase 2a.
Detailed description of the Invention
As used herein, the term "combinations of the invention" refers to a
combination of a
compound of formula (I) and a second pharmaceutically active compound,
including:
4-[2-(5-amino-1H-pyrazo1-4-y1 )-4-chlorophenoxy]-5-chloro-2-fluoro-N-(1,3-
thiazol-4-
yl)benzenesulfonamide in combination with gabapentin;
4-[2-(5-amino-1H-pyrazol-4-y1)-4-chlorophenoxy]-5-chloro-2-fluoro-N-(1,3-
thiazol-4-
yObenzenesulfonamide in combination with pregabalin;
4-[2-(5-amino-1H-pyrazol-4-y1)-4-(trifluoromethyl)-phenoxy]-5-chloro-2-fluoro-
N-(1,3-thiazol-
4-y1)benzenesulfonamide in combination with gabapentin; and
4-[2-(5-amino-1H-pyrazol-4-y1)-4-(trifluoromethyl)-phenoxy]-5-chloro-2-fluoro-
N-(1,3-thiazol-
4-yObenzenesulfonamide in combination with pregabalin.
The terms "treatment", "treating" and the like refer to palliative, curative
and prophylactic
treatment. In the context of the treatment of pain resulting from an injury or
pathological
condition, it will be understood that it is the pain that is being treated
rather than the
underlying cause of the pain.
"Separate" use refers to a therapeutic regimen in which the two agents are
used according
to independent schedules. It includes the possibility that, where multiple
doses of each
agent are used, then some of the doses may be taken together.
"Sequential" use refers to a therapeutic regimen in which the two agents are
used
according to the same schedule.
CA 02897306 2015-07-14
PC72074A
"Simultaneous" use refers to a therapeutic regimen in which the two agents are
used
together in a single action.
5 A use of a combination in treatment "which consists essentially of the
use of a compound
according to formula (I) and a second pharmaceutically active compound
selected from
gabapentin and pregabalin, or a pharmaceutically acceptable salt of one or
both said
compounds" refers to a use consisting of the two, and only the two,
pharmaceutically active
agents in combination.
The term "consists essentially of" when used in connection with:
a combination of a compound according to formula (I) and a second
pharmaceutically
active compound;
a compound according to formula (I) for use in the treatment of pain wherein
the
treatment involves a second pharmaceutically active compound;
use of a compound according to formula (I) in the manufacture of a medicament
for the
treatment of pain wherein the treatment involves a second pharmaceutically
active
compound;
a kit of pharmaceutical dosage forms of a compound according to formula (I)
and a
second pharmaceutically active compound;
and the like;
has the same meaning as that described just above in connection with a use of
the
combination in treatment.
The compounds of formula (I) may exist in tautomeric forms. Specifically,
the
aminopyrazole moiety may exist in one or more of the following forms:
H2N H2N HN
H H
CA 02897306 2015-07-14
PC72074A
11
All such tautomers and mixtures of tautomers are included within the scope of
the present
invention. References herein to specific compounds should be understood to
refer to the
compound and/or its tautomer.
The compounds of formula (I) are capable of forming pharmaceutically
acceptable addition
salts with acids, and these salts may be used in the invention. Suitable acid
addition salts
may be formed from acids which form non-toxic salts. Examples include the
acetate,
adipate, aspartate, benzoate, besylate, bicarbonate/carbonate,
bisulphate/sulphate, borate,
camsylate, citrate, cyclamate, edisylate, esylate, formate, fumarate,
gluceptate, gluconate,
glucuronate, hexafluorophosphate, hibenzate,
hydrochloride/chloride,
hydrobromide/bromide, hydroiodide/iodide, isethionate, lactate, malate,
maleate, malonate,
mesylate, methylsulphate, naphthylate, 2-napsylate, nicotinate, nitrate,
orotate, oxalate,
palmitate, pamoate, phosphate/hydrogen phosphate/dihydrogen
phosphate,
pyroglutamate, saccharate, stearate, succinate, tannate, tartrate, tosylate,
trifluoroacetate
and xinofoate salts.
Hemisalts of acids and bases may also be formed, for example, hemisulphate
salts.
Gabapentin and pregabalin have been reported to form salts with both acids and
bases.
The skilled person will appreciate that the aforementioned salts include ones
wherein the
counterion is optically active, for example d-lactate or 1-lysine, or racemic,
for example dl-
tartrate or dl-arginine.
For a review on suitable salts, see "Handbook of Pharmaceutical Salts:
Properties,
Selection, and Use" by Stahl and Wermuth (Wiley-VCH, Weinheim, Germany, 2002).
Pharmaceutically acceptable salts of compounds of formula (I), gabapentin and
pregabalin
may be prepared by one or more of three methods:
i)
by reacting the compound of formula (I), gabapentin or pregabalin with
the desired acid
or base;
CA 02897306 2015-07-14
PC72074A
12
ii) by removing an acid- or base-labile protecting group from a suitable
precursor of the
compound of formula (I), gabapentin or pregabalin using the desired acid or
base; or
iii) by converting one salt of the compound of formula (I), gabapentin or
pregabalin to
another by reaction with an appropriate acid or base or by means of a suitable
ion
exchange column.
All three reactions are typically carried out in solution. The resulting salt
may precipitate
out and be collected by filtration or may be recovered by evaporation of the
solvent. The
degree of ionisation in the resulting salt may vary from completely ionised to
almost non-
ionised.
The compounds of formula (I), gabapentin and pregabalin, or pharmaceutically
acceptable
salts thereof, may exist in both unsolvated and solvated forms. The term
'solvate' is used
herein to describe a molecular complex comprising a compound of formula (I),
gabapentin
or pregabalin, or a pharmaceutically acceptable salt thereof, and one or more
pharmaceutically acceptable solvent molcules, for example ethanol. The term
'hydrate' is
employed when said solvent is water. Pharmaceutically acceptable solvates in
accordance
with the invention include those wherein the solvent of crystallization may be
isotopically
substituted, e.g. D20, d6-acetone and d6-DMSO.
A currently accepted classification system for organic hydrates is one that
defines isolated
site, channel, or metal-ion coordinated hydrates - see Polymorphism in
Pharmaceutical
Solids by K. R. Morris (Ed. H. G. Brittain, Marcel Dekker, 1995), incorporated
herein by
reference. Isolated site hydrates are ones in which the water molcules are
isolated from
direct contact with each other by intervening organic molcules. In channel
hydrates, the
water molcules lie in lattice channels where they are next to other water
molcules. In
metal-ion coordinated hydrates, the water molcules are bonded to the metal
ion.
When the solvent or water is tightly bound, the complex will have a well-
defined
stoichiometry independent of humidity. When, however, the solvent or water is
weakly
bound, as in channel solvates and hygroscopic compounds, the water/solvent
content will
CA 02897306 2015-07-14
PC72074A
13
be dependent on humidity and drying conditions. In such cases, non-
stoichiometry will be
the norm.
The compounds of formula (I), gabapentin and pregabalin may exist in a
continuum of solid
states ranging from fully amorphous to fully crystalline. The term 'amorphous'
refers to a
state in which the material lacks long range order at the molcular level and,
depending
upon temperature, may exhibit the physical properties of a solid or a liquid.
Typically such
materials do not give distinctive X-ray diffraction patterns and, while
exhibiting the
properties of a solid, are more formally described as a liquid. Upon heating,
a change from
solid to liquid properties occurs which is characterised by a change of state,
typically
second order ('glass transition'). The term 'crystalline' refers to a solid
phase in which the
material has a regular ordered internal structure at the molcular level and
gives a distinctive
X-ray diffraction pattern with defined peaks. Such materials when heated
sufficiently will
also exhibit the properties of a liquid, but the change from solid to liquid
is characterised by
a phase change, typically first order ('melting point').
The compounds of formula (I) may be prepared by the methods disclosed in
International
Patent Application PCT/1132010/050033, published as W02010/079443, the
disclosure of
which is incorporated herein by reference, or by any other method known in the
art for the
preparation of compounds of analogous structure. Compound (IA) is Example 788
of
W02010/079443 and compound (IB) is Example 1029 thereof.
Gabapentin and pregabalin are well known compounds and may be prepared by
methods
disclosed in the literature.
The compounds of formula (I), gabapentin and pregabalin may be obtained, for
example,
as solid plugs, powders, or films by methods such as precipitation,
crystallization, freeze
drying, spray drying, or evaporative drying. Microwave or radio frequency
drying may be
used for this purpose.
CA 02897306 2015-07-14
PC72074A
14
The compounds of formula (I), and gabapentin or pregabalin may be used
separately or in
combination. Generally, they will be used as a formulation in association with
one or more
pharmaceutically acceptable excipients. The term 'excipient' is used herein to
describe
any ingredient other than the compounds of formula (I), gabapentin and
pregabalin. The
choice of excipient will to a large extent depend on factors such as the
particular mode of
administration, the effect of the excipient on solubility and stability, and
the nature of the
dosage form.
Pharmaceutical compositions suitable for the delivery of combinations of the
present
invention and methods for their preparation will be readily apparent to those
skilled in the
art. Such compositions and methods for their preparation may be found, for
example, in
"Remington's Pharmaceutical Sciences", 19th Edition (Mack Publishing Company,
1995).
Suitable modes of administration may include oral, parenteral, topical,
inhaled/intranasal,
rectal/intravaginal, and ocular/aural administration. When the compounds of
formula (I),
gabapentin and pregabalin are to be used separately they may be formulated for
administration by different routes.
Formulations suitable for the aforementioned modes of administration may be
formulated
to be immediate and/or modified release. Modified release formulations may
include
delayed-, sustained-, pulsed-, controlled-, targeted and programmed release.
The compounds of formula (I), gabapentin and pregabalin, or combinations
thereof, may be
formulated for oral administration. Oral administration may involve
swallowing, so that the
compound enters the gastrointestinal tract, or buccal or sublingual
administration may be
employed by which the compound enters the blood stream directly from the
mouth.
Formulations suitable for oral administration may include solid formulations
such as tablets,
capsules containing particulates, liquids, or powders, lozenges (including
liquid-filled),
chews, multi- and nano-particulates, gels, solid solution, liposome, films,
ovules, sprays,
liquid formulations and buccal/mucoadhesive patches.
CA 02897306 2015-07-14
PC72074A
15 =
Liquid formulations may include suspensions, solutions, syrups and elixirs.
Such
formulations may be employed as fillers in soft or hard capsules and typically
comprise a
carrier, for example, water, ethanol, polyethylene glycol, propylene glycol,
methylcellulose,
or a suitable oil, and one or more emulsifying agents and/or suspending
agents. Liquid
formulations may also be prepared by the reconstitution of a solid, for
example, from a
sachet.
The compounds of formula (I), gabapentin and pregabalin, or combinations
thereof, may
also be used in fast-dissolving, fast-disintegrating dosage forms such as
those described in
Expert Opinion in Therapeutic Patents, 11(6), 981-986, by Liang and Chen
(2001).
In a typical tablet dosage form, depending on dose, the drug may make up from
1 weight %
to 80 weight % of the dosage form, more typically from 5 weight % to 60 weight
% of the
dosage form. In addition to the drug, tablets generally contain a
disintegrant. Examples of
disintegrants include sodium starch glycolate, sodium carboxymethyl cellulose,
calcium
carboxymethyl cellulose, croscarmellose sodium, crospovidone,
polyvinylpyrrolidone,
methyl cellulose, microcrystalline cellulose, lower alkyl-substituted
hydroxypropyl cellulose,
starch, pregelatinised starch and sodium alginate. Generally, the disintegrant
comprises
from 1 weight % to 25 weight %, more typically from 5 weight % to 20 weight %
of a
dosage form.
Binders are generally used to impart cohesive qualities to a tablet
formulation. Examples
of binders include microcrystalline cellulose, gelatin, sugars, polyethylene
glycol, natural
and synthetic gums, polyvinylpyrrolidone, pregelatinised starch, hydroxypropyl
cellulose
and hydroxypropyl methylcellulose. Tablets may also contain diluents, such as
lactose
(monohydrate, spray-dried monohydrate, anhydrous and the like), mannitol,
xylitol,
dextrose, sucrose, sorbitol, microcrystalline cellulose, starch and dibasic
calcium
phosphate dihydrate.
Tablets may also optionally comprise surface active agents, such as sodium
lauryl sulfate
and polysorbate 80, and glidants such as silicon dioxide and talc. When
present, surface
CA 02897306 2015-07-14
PC72074A
16
active agents may comprise from 0.2 weight A) to 5 weight A) of the tablet,
and glidants
may comprise from 0.2 weight % to 1 weight % of the tablet.
Tablets also generally contain lubricants such as magnesium stearate, calcium
stearate,
zinc stearate, sodium stearyl fumarate, and mixtures of magnesium stearate
with sodium
lauryl sulphate. Lubricants generally comprise from 0.25 weight A to 10
weight %, more
typically from 0.5 weight A to 3 weight % of a tablet. Other possible
ingredients include
anti-oxidants, colourants, flavouring agents, preservatives and taste-masking
agents.
Exemplary tablets may contain up to about 80% drug, from about 10 weight % to
about 90
weight % binder, from about 0 weight % to about 85 weight A diluent, from
about 2 weight
% to about 10 weight % disintegrant, and from about 0.25 weight A) to about
10 weight %
lubricant. Tablet blends may be compressed directly or by roller to form
tablets. Tablet
blends or portions of blends may alternatively be wet-, dry-, or melt-
granulated, melt
congealed, or extruded before tabletting. The final formulation may comprise
one or more
layers and may be coated or uncoated; it may even be encapsulated. The
formulation of
tablets is discussed in "Pharmaceutical Dosage Forms: Tablets", Vol. 1, by H.
Lieberman
and L. Lachman (Marcel Dekker, New York, 1980).
Modified release formulations that may be suitable for the purposes of the
invention are
described in US Patent No. 6,106,864. Details of other potentially suitable
release
technologies such as high energy dispersions and osmotic and coated particles
are to be
found in "Pharmaceutical Technology On-line", 25(2), 1-14, by Verma et al
(2001). The
use of chewing gum to achieve controlled release is described in WO 00/35298.
The compounds of formula (I), gabapentin and pregabalin, or combinations
thereof, may
also potentially be formulated for administration directly into the blood
stream, into muscle,
or into an internal organ. Suitable formulations for parenteral administration
may include
intravenous, intraarterial, intraperitoneal, intrathecal, intraventricular,
intraurethral,
intrasternal, intracranial, intramuscular and subcutaneous formulations.
Suitable devices
CA 02897306 2015-07-14
PC72074A
= 17
for parenteral administration may include needle (including microneedle)
injectors, needle-
free injectors and infusion techniques.
Parenteral formulations are typically aqueous solutions which may contain
excipients such
as salts, carbohydrates and buffering agents (preferably to a pH of from 3 to
9), but, for
some applications, they may be more suitably formulated as a sterile non-
aqueous solution
or as a dried form to be used in conjunction with a suitable vehicle such as
sterile, pyrogen-
free water.
The preparation of parenteral formulations under sterile conditions, for
example, by
lyophilisation, may readily be accomplished using standard pharmaceutical
techniques well
known to those skilled in the art.
The solubility of compounds of formula (I), gabapentin and pregabalin used in
the
preparation of parenteral solutions may be increased by the use of appropriate
formulation
techniques, such as the incorporation of solubility-enhancing agents.
Formulations for
parenteral administration may be formulated to be immediate and/or modified
release.
Modified release formulations may include delayed-, sustained-, pulsed-,
controlled-,
targeted and programmed release. Thus combinations of the invention may be
formulated
as a solid, semi-solid, or thixotropic liquid for administration as an
implanted depot
providing modified release of the active compound. Examples of such
formulations may
include drug-coated stents and poly(dl-lactic-coglycolic)acid (PG LA)
microspheres.
The compounds of formula (I), gabapentin and pregabalin, or combinations
thereof, may
also potentially be formulated for topical administration to the skin or
mucosa, that is,
dermally or transdermally. Typical formulations for this purpose may include
gels,
hydrogels, lotions, solutions, creams, ointments, dusting powders, dressings,
foams, films,
skin patches, wafers, implants, sponges, fibres, bandages and microemulsions.
Liposomes may also be used. Typical carriers include alcohol, water, mineral
oil, liquid
petrolatum, white petrolatum, glycerin, polyethylene glycol and propylene
glycol.
CA 02897306 2015-07-14
PC72074A
18
=
Penetration enhancers may be incorporated - see, for example, J Pharm Sci, 88
(10), 955-
958, by Finnin and Morgan (October 1999).
Other topical delivery means may include delivery by electroporation,
iontophoresis,
phonophoresis, sonophoresis and microneedle or needle-free (e.g. PowderjectTM,
BiojectTM, etc.) injection.
The compounds of formula (I), gabapentin and pregabalin, or combinations
thereof, may
also potentially be formulated for administration intranasally or by
inhalation, which may be
in the form of a dry powder (either alone, as a mixture, for example, in a dry
blend with
lactose, or as a mixed component particle, for example, mixed with
phospholipids, such as
phosphatidylcholine) from a dry powder inhaler or as an aerosol spray from a
pressurised
container, pump, spray, atomiser (such as an atomiser using
electrohydrodynamics to
produce a fine mist), or nebuliser, with or without the use of a suitable
propellant, such as
1,1,1,2-tetrafluoroethane or 1,1,1,2,3,3,3-heptafluoropropane.
For intranasal use, the
powder may comprise a bioadhesive agent, for example, chitosan or
cyclodextrin.
The pressurised container, pump, spray, atomizer, or nebuliser may contain a
solution or
suspension of the compound of formula (I), gabapentin or pregabalin, or a
combination
thereof, comprising, for example: ethanol, aqueous ethanol, or a suitable
alternative agent
for dispersing, solubilising, or extending release of the active; a
propellant(s) as solvent;
and an optional surfactant, such as sorbitan trioleate, oleic acid, or an
oligolactic acid.
Prior to use in a dry powder or suspension formulation, the drug product may
be
micronised to a size suitable for delivery by inhalation (typically less than
5 microns). This
may be achieved by any appropriate comminuting method, such as spiral jet
milling, fluid
bed jet milling, supercritical fluid processing to form nanoparticles, high
pressure
homogenisation, or spray drying.
Capsules (made, for example, from gelatin or hydroxypropylmethylcellulose),
blisters and
cartridges for use in an inhaler or insufflator may be formulated to contain a
powder mix of
CA 02897306 2015-07-14
PC72074A
19
the compound of the invention, a suitable powder base such as lactose or
starch and a
performance modifier such as 1-leucine, mannitol, or magnesium stearate. The
lactose
may be anhydrous or in the form of the monohydrate. Other possible excipients
include
dextran, glucose, maltose, sorbitol, xylitol, fructose, sucrose and trehalose.
A suitable solution formulation for use in an atomiser using
electrohydrodynamics to
produce a fine mist may comprise a compound of formula (I), propylene glycol,
sterile
water, ethanol and sodium chloride. Alternative solvents which may be used
instead of
propylene glycol include glycerol and polyethylene glycol.
Suitable flavours, such as menthol and levomenthol, or sweeteners, such as
saccharin or
saccharin sodium, may be added to those formulations of the invention intended
for
inhaled/intranasal administration.
In the case of dry powder inhalers and aerosols, the dosage unit is determined
by means
of a valve which delivers a metered amount. Units in accordance with the
invention are
typically arranged to deliver a metered dose or "puff" containing an
appropriate amount of
the compounds of formula (I), gabapentin or pregabalin.
The compounds of formula (I), gabapentin and pregabalin, or combinations
thereof, may
potentially be used rectally or vaginally, for example, in the form of a
suppository, pessary,
microbicide, vaginal ring or enema. Cocoa butter is a traditional suppository
base, but
various alternatives may be used as appropriate.
The compounds of formula (I), gabapentin and pregabalin, or combinations
thereof, may
also potentially be formulated for administration directly to the eye or ear,
typically in the
form of drops of a micron ised suspension or solution in isotonic, pH-
adjusted, sterile saline.
Other formulations which may be suitable for ocular and aural administration
include
ointments, biodegradable (e.g. absorbable gel sponges, collagen) and non-
biodegradable
(e.g. silicone) implants, wafers, lenses and particulate or vesicular systems,
such as
niosomes or liposomes. A polymer such as crossed-linked polyacrylic acid,
CA 02897306 2015-07-14
PC72074A
polyvinylalcohol, hyaluronic acid, a cellulosic polymer,
for example,
hydroxypropylmethylcellulose, hydroxyethylcellulose, or methyl cellulose, or a
heteropolysaccharide polymer, for example, gelan gum, may be incorporated
together with
a preservative, such as benzalkonium chloride. Such formulations may also be
delivered
5 by iontophoresis.
The compounds of formula (I), gabapentin and pregabalin may be combined with
soluble
macromolcular entities, such as cyclodextrin and suitable derivatives thereof
or
polyethylene glycol-containing polymers, in order to potentially improve their
solubility,
10 dissolution rate, taste-masking, bioavailability and/or stability for
use in any of the
aforementioned modes of administration.
Drug-cyclodextrin complexes, for example, are found to be generally useful for
most
dosage forms and administration routes. Both inclusion and non-inclusion
complexes may
15 be used. As an alternative to direct complexation with the drug, the
cyclodextrin may be
used as an auxiliary additive, i.e. as a carrier, diluent, or solubiliser.
Most commonly used
for these purposes are alpha-, beta- and gamma-cyclodextrins, examples of
which may be
found in International Patent Applications Nos. W091/11172, W094/02518 and
W098/55148.
Disorders for which the combinations of the invention are indicated include
pain. Pain may
be either acute or chronic and additionally may be of central and/or
peripheral origin. Pain
may be of a neuropathic and/or nociceptive and/or inflammatory nature, such as
pain
affecting either the somatic or visceral systems, as well as dysfunctional
pain affecting
multiple systems.
Physiological pain is an important protective mechanism designed to warn of
danger from
potentially injurious stimuli from the external environment. The system
operates through a
specific set of primary sensory neurones and is activated by noxious stimuli
via peripheral
transducing mechanisms (see Meyer et al., 2006, Wall and Melzack's Textbook of
Pain (5th
Ed), Chapter1). These sensory fibres are known as nociceptors, and are
characteristically
CA 02897306 2015-07-14
PC72074A
21
small diameter axons with slow conduction velocities, of which there are two
main types, A-
delta fibres (myelinated) and C fibres (non-myelinated). Nociceptors encode
the intensity,
duration and quality of noxious stimulus and by virtue of their
topographically organised
projection to the spinal cord, the location of the stimulus. The activity
generated by
nociceptor input is transferred, after complex processing in the dorsal horn,
either directly,
or via brain stem relay nuclei, to the ventrobasal thalamus and then on to the
cortex, where
the sensation of pain is generated.
Pain may generally be classified as acute or chronic. Acute pain begins
suddenly and is
short-lived (usually twelve weeks or less). It is usually, although not
always, associated
with a specific cause such as a defined injury, is often sharp and severe and
can result
from numerous origins such as surgery, dental work, a strain or a sprain.
Acute pain does
not generally result in any persistent psychological response. When a
substantial injury
occurs to body tissue, via disease or trauma, the characteristics of
nociceptor activation
may be altered such that there is sensitisation in the periphery, locally
around the injury
and centrally where the nociceptors terminate. These effects lead to a
hightened sensation
of pain. In acute pain these mechanisms can be useful, in promoting protective
behaviours
which may better enable repair processes to take place. The normal expectation
would be
that sensitivity returns to normal once the injury has healed. However, in
many chronic
pain states, the hypersensitivity far outlasts the healing process and is
often due to nervous
system injury or alteration which can be associated with maladaptation and
aberrant
activity (Woolf & Salter, 2000, Science, 288, 1765-1768). As such, chronic
pain is long-
term pain, typically persisting for more than three months and leading to
significant
psychological and emotional problems.
Common examples of chronic pain are
neuropathic pain (e.g. painful diabetic neuropathy or postherpetic neuralgia),
carpal tunnel
syndrome, back pain, headache, cancer pain, arthritic pain and chronic post-
surgical pain,
but may include any chronic painful condition affecting any system, such as
those
described by the International Association for the Study of Pain
(Classification of Chronic
Pain, a publication freely available for download at http://www.iasp-
pain.org).
CA 02897306 2015-07-14
PC72074A
22
The clinical manifestation of pain is present when discomfort and abnormal
sensitivity
feature among the patient's symptoms. Patients tend to be quite heterogeneous
and may
present with various pain symptoms. Such symptoms can include: 1) spontaneous
pain
which may be dull, burning, or stabbing; 2) exaggerated pain responses to
noxious stimuli
(hyperalgesia); and 3) pain produced by normally innocuous stimuli (allodynia)
(Meyer et
al., 2006, Wall and Melzack's Textbook of Pain (5th Ed), Chapter1). Although
patients
suffering from various forms of acute and chronic pain may have similar
symptoms, the
underlying mechanisms may be different and may, therefore, require different
treatment
strategies. Apart from acute or chronic, pain can also be broadly categorized
into:
nociceptive pain, affecting either the somatic or visceral systems, which can
be
inflammatory in nature (associated with tissue damage and the infiltration of
immune cells);
or neuropathic pain.
Nociceptive pain can be defined as the process by which intense thermal,
mechanical, or
chemical stimuli are detected by a subpopulation of peripheral nerve fibers,
called
nociceptors, and can be induced by tissue injury or by intense stimuli with
the potential to
cause injury. Pain afferents are activated by transduction of stimuli by
nociceptors at the
site of injury and activate neurons in the spinal cord at the level of their
termination. This is
then relayed up the spinal tracts to the brain where pain is perceived (Meyer
et al., 2006,
Wall and Melzack's Textbook of Pain (5th Ed), Chapter1). Myelinated A-delta
fibres
transmit rapidly and are responsible for sharp and stabbing pain sensations,
whilst
unmyelinated C fibres transmit at a slower rate and convey a dull or aching
pain. Moderate
to severe acute nociceptive pain is a prominent feature of pain from
strains/sprains, burns,
myocardial infarction and acute pancreatitis, post-operative pain (pain
following any type of
surgical procedure), posttraumatic pain, pain associated with gout, cancer
pain and back
pain. Cancer pain may be chronic pain such as tumour related pain (e.g. bone
pain,
headache, facial pain or visceral pain) or pain associated with cancer therapy
(e.g. in
response to chemotherapy, immunotherapy, hormonal therapy or radiotherapy).
Back pain
may be due to herniated or ruptured intervertabral discs or abnormalities of
the lumber
facet joints, sacroiliac joints, paraspinal muscles or the posterior
longitudinal ligament.
CA 02897306 2015-07-14
PC72074A
23
Back pain may resolve naturally but in some patients, where it lasts over 12
weeks, it
becomes a chronic condition which can be particularly debilitating.
Nociceptive pain can also be related to inflammatory states. The inflammatory
process is a
complex series of biochemical and cellular events, activated in response to
tissue injury or
the presence of foreign substances, which results in swelling and pain
(McMahon et al.,
2006, Wall and Melzack's Textbook of Pain (5th Ed),Chapter3). A common
inflammatory
condition assoiciated with pain is arthritis. It has been estimated that
almost 27 million
Americans have symptomatic osteoarthritis (OA) or degenerative joint disease
(Lawrence
et al., 2008, Arthritis Rheum, 58, 15-35); most patients with osteoarthritis
seek medical
attention because of the associated pain. Arthritis has a significant impact
on psychosocial
and physical function and is known to be the leading cause of disability in
later life.
Rheumatoid arthritis is an immune-mediated, chronic, inflammatory
polyarthritis disease,
mainly affecting peripheral synovial joints. It is one of the commonest
chronic inflammatory
conditions in developed countries and is a major cause of pain.
In regard to nociceptive pain of visceral origin, visceral pain results from
the activation of
nociceptors of the thoracic, pelvic, or abdominal organs (Bielefeldt and
Gebhart, 2006, Wall
and Melzack's Textbook of Pain (5th Ed),Chapter48). This includes the
reproductive
organs, spleen, liver, gastrointestinal and urinary tracts, airway structures,
cardiovascular
system and other organs contained within the abdominal cavity. As such
visceral pain
refers to pain associated with conditions of such organs, such as painful
bladder syndrome,
interstitial cystitis, prostatitis, ulcerative colitis, Crohn's disease, renal
colic, irritable bowl
syndrome, endometriosis and dysmenorrheal (Classification of Chronic Pain,
available at
http://www.iasp-pain.org). Currently the potential for a neuropathic
contribution (either
through central changes or nerve injury/damage) to visceral pain states is
poorly
understood but may play a role in certain conditions (Aziz et al., 2009, Dig
Dis 27, Suppl 1,
31-41)
Neuropathic pain is currently defined as pain arising as a direct consequence
of a lesion or
disease affecting the somatosensory system. Nerve damage can be caused by
trauma
CA 02897306 2015-07-14
a
PC72074A
. 24,
and disease and thus the term `neuropathic pain' encompasses many disorders
with
diverse aetiologies. These include, but are not limited to, peripheral
neuropathy, diabetic
neuropathy, post herpetic neuralgia, trigeminal neuralgia, back pain, cancer
neuropathy,
HIV neuropathy, phantom limb pain, carpal tunnel syndrome, central post-stroke
pain and
pain associated with chronic alcoholism, hypothyroidism, uremia, multiple
sclerosis, spinal
cord injury, Parkinson's disease, epilepsy and vitamin deficiency. Neuropathic
pain is
pathological as it has no protective role. It is often present well after the
original cause has
dissipated, commonly lasting for years, significantly decreasing a patient's
quality of life
(Dworkin, 2009, Am J Med, 122, S1-S2; Geber et al., 2009, Am J Med, 122, S3-
S12;
Haanpaa et al., 2009, Am J Med, 122, S13-S21). The symptoms of neuropathic
pain are
difficult to treat, as they are often heterogeneous even between patients with
the same
disease (Dworkin, 2009, Am J Med, 122, S1-S2; Geber et al., 2009, Am J Med,
122, S3-
S12; Haanpaa et al., 2009, Am J Med, 122, S13-S21). They include spontaneous
pain,
which can be continuous, and paroxysmal or abnormal evoked pain, such as
hyperalgesia
(increased sensitivity to a noxious stimulus) and allodynia (sensitivity to a
normally
innocuous stimulus).
It should be noted that some types of pain have multiple aetiologies and thus
can be
classified in more than one area, e.g. back pain, cancer pain and even migaine
headaches
may include both nociceptive and neuropathic components.
Similarly other types of chronic pain, perhaps less well understood, are not
easily defined
by the simplistic definitions of nociceptive or neuropathic. Such conditions
include in
particular fibromyalgia and chronic regional pain syndrome, which are often
described as
dysfunctional pain states e.g. fibromyalgia or complex regional pain syndrome
(Woolf,
2010, J Olin Invest, 120, 3742-3744), but which are included in
classifications of chronic
pain states (Classification of Chronic Pain, available at http://www.iasp-
pain.org).
The combinations of the invention may optionally be used in combination with
one or more
further pharmacologically active compounds. Such combinations offer the
possibility of
CA 02897306 2015-07-14
PC72074A
further significant advantages, potentially including patient compliance, ease
of dosing and
synergistic activity.
In the combinations that follow the combination of the invention may be used
5
simultaneously, sequentially or separately in combination with the other
therapeutic agent
or agents.
Useful further pharmacologically active agents that may potentially be used
with the
combination of the invention may include one or more agents selected from:
10 = a selective Nav1.3 channel modulator, such as a compound disclosed in
W02008/118758;
= a selective Nav1.8 channel modulator, such as a compound disclosed in
W02013/114250;
= a selective Nav1.9 channel modulator;
15
= a compound which modulates activity at more than one Nay channel,
including a non-
selective modulator such as bupivacaine, carbamazepine, lamotrigine,
lidocaine,
mexiletine or phenytoin;
= any inhibitor of nerve growth factor (NGF) signaling, such as: an agent
that binds to
NGF and inhibits NGF biological activity and/or downstream pathway(s) mediated
by
20
NGF signaling (e.g. tanezumab), a TrkA antagonist or a p75 antagoinsist, or
an agent
that inhibits downstream signaling in regard to NGF stimulated TrkA or P75
signalling;
= an inhibitor of neurotrophic pathways, where such inhibition is achieved
by: (a) an agent
that binds to nerve growth factor (NGF) (e.g. tanezumab, fasinumab or
fulranumab),
brain-derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) or
neurotrophin-4 (NT-
25
4), or to more than one of the aforementioned neurotrophins (e.g. soluble
P75); or (b)
an agent that inhibits receptor function at one or more of TrKA, TrKB, TrKC or
P75,
either at the orthosteric site, an allosteric site or by inhibition of the
catalytic activity of
the receptor(s);
= a compound which increases the levels of endocannabinoid, such as a
compound with
fatty acid amid hydrolase inhibitory (FAAH) or monoacylglycerol lipase (MAGL)
activity;
= an analgesic, in particular paracetamol;
CA 02897306 2015-07-14
PC72074A
26
= an opioid analgesic, such as: buprenorphine, butorphanol, cocaine,
codeine,
dihydrocodeine, fentanyl, heroin, hydrocodone, hydromorphone, levallorphan
levorphanol, meperidine, methadone, morphine, nalmefene, nalorphine, naloxone,
naltrexone, nalbuphine, oxycodone, oxymorphone, propoxyphene or pentazocine;
= an opioid analgesic which preferentially stimulates a specific intracellular
pathway, for
example G-protein as opposed to beta arrestin recruitment, such as TRV130;an
opioid
analgesic with additional pharmacology, such as: noradrenaline
(norepinephrine)
reuptake inhibitory (NRI) activity, e.g. tapentadol; serotonin and
norepinephrine
reuptake inhibitory (SNRI) activity, e.g. tramadol; or nociceptin receptor
(NOP) agonist
activity, such as GRT6005;
= a nonsteroidal antiinflammatory drug (NSAID), such as a non-selective
cyclooxygenase
(COX) inhibitor, e.g. aspirin, diclofenac, diflusinal, etodolac, fenbufen,
fenoprofen,
flufenisal, flurbiprofen, ibuprofen, indomethacin, ketoprofen, ketorolac,
meclofenamic
acid, mefenamic acid, meloxicam, nabumetone, naproxen, nimesulide,
nitroflurbiprofen,
olsalazine, oxaprozin, phenylbutazone, piroxicam, sulfasalazine, sulindac,
tolmetin or
zomepirac; or a COX-2 selective inhibitor, e.g. celecoxib, deracoxib,
etoricoxib,
mavacoxib or parecoxib;
= a prostaglandin E2 subtype 4 (EP4) antagonist;
= a microsomal prostaglandin E synthase type 1 (mPGES-1) inhibitor;
= a sedative, such as glutethimide, meprobamate, methaqualone or
dichloralphenazone;
= a GABAA modulator with broad subtype modulatory effects mediated via the
benzodiazepine binding site, such as chlordiazepoxide, alprazolam, diazepam,
lorazepam, oxazepam, temazepam, triazolam, clonazepam or clobazam;
= a GABAA modulator with subtype-selective modulatory effects mediated via
the
benzodiazepine binding site with reduced adverse effects, for example
sedation, such
as TPA023, TPA023B, L-838,417, CTP354 or NSD72;
= a GABAA modulator acting via alternative binding sites on the receptor,
such as
barbiturates, e.g. amobarbital, aprobarbital, butabital, mephobarbital,
methohexital,
pentobarbital, phenobartital, secobarbital, or thiopental; neurosteroids such
as
alphaxalone, alphadolone or ganaxolone; p-subunit ligands, such as etifoxine;
or 6-
preferring ligands, such as gaboxadol;
CA 02897306 2015-07-14
PC72074A
27
= a GlyR3 agonist or positive allosteric modulator;
= a skeletal muscle relaxant, e.g. bactofen, carisoprodol, chlorzoxazone,
cyclobenzaprine,
metaxolone, methocarbamol or orphrenadine;
= a glutamate receptor antagonist or negative allosteric modulator, such as
an NMDA
receptor antagonist, e.g. dextromethorphan, dextrorphan, ketamine or,
memantine; or
an mGluR antagonist or modulator;
= an alpha-adrenergic, such as clonidine, guanfacine or dexmetatomidine;
= a beta-adrenergic such as propranolol;
= a tricyclic antidepressant, e.g. desipramine, imipramine, amitriptyline
or nortriptyline;
= a tachykinin (NK) antagonist, such as aprepitant or maropitant;
= a muscarinic antagonist, e.g oxybutynin, tolterodine, propiverine,
tropsium chloride,
darifenacin, solifenacin, temiverine and ipratropium;
= a cholinergic (nicotinic) analgesic, such as ispronicline (TC-1734),
varenicline or
nicotine;
= a Transient Receptor Potential V1 (TRPV1) receptor agonist (e.g.
resinferatoxin or
capsaicin) or antagonist (e.g. capsazepine or mavatrap);
= a Transient Receptor Potential Al (TRPA1) receptor agonist (e.g.
cinnamaldehyde or
mustard oil) or antagonist (e.g. GRC17536 or CB-625);
= a Transient Receptor Potential M8 (TRPM8) receptor agonist (e.g. menthol
or icilin) or
antagonist;
= a Transient Receptor Potential V3 (TRPV3) receptor agonist or antagonist
(e.g. GRC-
15300);
= a corticosteroid such as dexamethasone;
= a 5-HT receptor agonist or antagonist, particularly a 5-HT1st10 agonist,
such as
eletriptan, sumatriptan, naratriptan, zolmitriptan or rizatriptan;
= a 5-HT 2A receptor antagonist;
= a PDEV inhibitor, such sildenafil, tadalafil or vardenafil;
= an alpha-2-delta ligand such as gabapentin, gabapentin enacarbil or
pregabalin, ;
= a serotonin reuptake inhibitor (SRI) such as sertraline,
demethylsertraline, fluoxetine,
norfluoxetine, fluvoxamine, paroxetine, citalopram, desmethylcitalopram,
escitalopram,
CA 02897306 2015-07-14
PC72074A
28
d,l-fenfluramine, femoxetine, ifoxetine, cyanodothiepin,
I itoxetine, dapoxetine,
nefazodone, cericlamine and trazodone;
= anNRI, such as maprotiline, lofepramine, mirtazepine, oxaprotiline,
fezolamine,
tomoxetine, mianserin, buproprion, buproprion metabolite hydroxybuproprion,
nomifensine and viloxazine, especially a selective noradrenaline reuptake
inhibitor such
as reboxetine;
= an SNRI, such as venlafaxine, 0-desmethylvenlafaxine, clomipramine,
desmethylclomipramine, duloxetine, milnacipran and imipramine;
= an inducible nitric oxide synthase (iNOS) inhibitor;
= a leukotriene B4 antagonist;
= a 5-lipoxygenase inhibitor, such as zileuton;
= a potassium channel opener or positive modulator, such as an opener or
positive
modulator of KCNQ/Kv7 (e.g. retigabine or flupirtine), a G protein-coupled
inwardly-
rectifying potassium channel (GIRK), a calcium-activated potassium channel
(Kca) or a
potassium voltage-gated channel such as a member of subfamily A (e.g. Kv1.1),
subfamily B (e.g. Kv2.2) or subfamily K (e.g. TASK, TREK or TRESK);
= a P2X3 receptor antagonist (e.g. AF219) or an antagonist of a receptor
which contains
as one of its subunits the P2X3 subunit, such as a P2X213 heteromeric
receptor;
= a Cav2.2 calcium channel blocker (N-type), such as ziconotide; and
= a Cav3.2 calcium channel blocker (T-type), such as ethosuximide.
It is within the scope of the invention that two or more pharmaceutical
compositions, at
least one of which contains a compound of formula (I) and one of which
contains
gabapentin or pregabalin, may conveniently be combined in the form of a kit
with
instructions for use of the compositions. Thus the kit of the invention
comprises two or
more separate pharmaceutical compositions, at least one of which contains a
compound of
formula (I) and one of which contains gabapentin or pregabaiin, and means for
separately
retaining said compositions, such as a container, divided bottle, or divided
foil packet. An
example of such a kit is the familiar blister pack used for the packaging of
tablets, capsules
and the like. The kit of the invention may be particularly suitable for
different dosage forms,
for example, oral and parenteral, for use of the separate compositions at
different dosage
CA 02897306 2015-07-14
PC72074A
= 29
intervals, or for titrating the separate compositions against one another. To
assist
compliance, the kit typically comprises directions for use and may be provided
with a so-
called memory aid.
Experimental
In vivo evaluation in mice
Animals: Male CD-1 mice (Charles River, Raleigh, NC) weighing 27-36 grams on
the day
of dosing were housed five per cage. Animals had free access to food and water
and were
maintained on a 12:12 hour light/dark schedule.
Compounds and dosing solutions: The Compound of formula (IB) and pregabalin
were
formulated as solutions in 0.5% methyl cellulose. The high and low dose
combinations
were formulated together in 0.5% methyl cellulose into a single suspension for
oral dosing.
Compounds were dosed via oral gavage at 10mL/kg.
Experimental procedure: Animals were tested for paw movement responses to an
injection of a 2.5% formalin solution (20 pl in saline) using the Automated
Nociception
Analyzer (Yaksh et al., 2001). This device uses a magnetic detection system to
measure
paw movements defined as flinches. Small metal bands were attached to the left
hind paw
of mice just before placement into individual circular test chambers (4 mice
per session) 60
minutes prior to formalin injection. Mice were orally administered test
compounds 2 hours
prior to formalin injection. To initiate the experiment mice were injected
with 20 pl of 2.5%
formalin subcutaneously on the dorsal surface of the left hind paw and placed
in the test
chambers. The instrument then recorded rapid foot movements counted in one
minute
bins continuously for 60 minutes.
Treatment groups were assigned to balance treatments across days and across
test
chambers.
CA 02897306 2015-07-14
PC72074A
Immediately following the 60 minute flinching observation, the mice were
euthanized and
blood and brain tissue were removed for bioanalysis of the compound of formula
(IB) and
pregabalin levels.
5 Experimental groups: Table 1 summarizes the groups tested in the study.
Group Pregabalin dose Compound (16) dose Vehicle Dose volume No.
of
(mg/kg ¨ `mpk') (mg/kg ¨ `mpk') mL/kg
mice
1 0 0 0.5% methyl 10 14
cellulose
2 0 30 0.5% methyl 10 15
cellulose
3 0 100 0.5% methyl 10 15
cellulose
4 10 0 0.5% methyl 10 16
cellulose
5 30 0 0.5% methyl 10 15
cellulose
6 10 30 0.5% methyl 10 16
cellulose
7 30 100 0.5% methyl 10 16
cellulose
Group 6 low dose combination
Group 7 high dose combination
10 Excluded animals: One mouse was excluded from both the vehicle group and
from the
compound of formula (IB) 30 mg/kg group because of an incomplete formalin
injection.
One mouse was excluded from the compound of formula (IB) 100 mg/kg group
because the
metal paw band fell off during the course of the flinching study. One mouse
was excluded
from the pregabalin 30mg/kg group because it only received a partial dose of
test
15 compound.
CA 02897306 2015-07-14
PC72074A
31
Data analysis: The total flinches were counted over particular time intervals
or phases.
The phases summarized in this study were:
Phase 1: 0-9 minutes
Phase 2: 10-60 minutes
Phase 2a: 10-40 minutes
Flinching events were automatically recorded by the Automated Nociception
Analyzer
(Yaksh et al., 2001) and the total number of flinches for each phase was
calculated
automatically. The results for each experimental group are set out in tables
2.1 to 2.7
below.
Table 2.1: Vehicle (Group 1)
Total number of flinches counted at 5 minute intervals
Animal # 5 10 15 20 25 30 35 40 45 50 55
60
1
178 121 97 97 218 195 210 203 102 61 163 93
2 266 149 77 150 213 71 15 61 11 0 0
0
3 302 187 240 267 229 256 32 16 14 97 52
5
4
317 204 210 220 273 275 247 226 227 132 80 96
5 283 105 71 234 240 175 27 30 48 7 18
0
6
290 196 208 293 293 215 68 15 3 0 0 30
7
310 241 198 288 230 188 86 31 26 78 124 206
8
270 217 180 233 228 173 137 79 125 106 103 93
9 225 157 172 190 189 154 149 49 76 2 1
3
10
263 138 116 96 313 210 283 29 15 14 9 136
11
195 51 92 197 149 163 145 137 16 2 4 123
12
201 140 139 178 153 123 6 34 10 33 202 103
13 268 168 238 208 210 235 69 44 96 4 0
0
14 279 237 160 183 273 66 5 4 129 31
7 18
mean
261 165 157 202 229 179 106 68 64 41 55 65
SEM
12 14 16 16 13 16 25 19 17 12 18 18
CA 02897306 2015-07-14
PC72074A
= 32
Table 2.2: Compound (I) 30 mg/kg (Group 2)
Total number of flinches counted at 5 minute intervals
Animal # 5 10 15 20 25 30 35 40 45 50 55
60
1 293 172 194 197 242 282 210 208 134 167 248 200
2 340 260 239 291 242 298 182 125 72 61 60 25
3 302 61 192 209 252 239 119 49 29 30 15 14
4 278 68 51 219 210 164 258 174 56 39 20 16
266 126 128 173 188 26 32 23 14 10 3 4
6 316 239 202 293 299 309 213 141 232 58 57 204
7 294 289 247 221 293 270 134 123 9 10 0 23
8 216 190 161 269 184 102 62 48 17 10 4 7
9 229 150 98 63 186 177 155 110 82 22 58 24
203 96 183 298 227 174 154 103 18 96 25 103
11 285 69 208 155 297 299 230 224 147 126 10 5
12 253 184 85 150 248 114 81 9 11 9 146 48
13 332 202 200 243 221 178 125 190 185 210 7 54
14 332 179 135 290 310 100 160 35 6 4 4 63
291 133 236 238 293 174 260 119 46 147 103 157
mean 282 161 171 221 246 194 158 112 71 67 51 63
SEM
11 18 15 17 11 22 18 18 19 17 18 18
Table 2.3: Compound (18) 100 mg/kg (Group 3)
Total number of flinches counted at 5 minute intervals
Animal # 5 10 15 20 25 30 35 40 I 45 50
55 60
1 318 191 56 32 16 14 117 145 133 225 161 116
2 280 106 219 41 74 76 28 20 7 30 7 9
3 210 63 1 0 36 75 62 56 66 37 42 11
4 296 147 137 215 265 154 199 189 68 67 155 239
5 285 203 138 181 169 130 127 82 60 26 23 6
CA 02897306 2015-07-14
PC72074A
33
Total number of flinches counted at 5 minute intervals
Animal # 5 10 15 20 25 30 35 40 45 50 55 60
6 178 91 22 96 144 68 46 17 15 33 12 24
7 289 111 63 123 189 173 72 45 42 39 11 5
8 214 314 335 84 35 1 12 13 14 0 0 0
9 208 128 175 61 44 114 138 9 0 0 0 74
207 154 94 65 123 85 46 56 75 26 30 9
11 317 205 134 213 210 136 24 23 21 11 9 6
12 300 255 166 60 88 288 143 49 29 35 21 89
13 276 222 185 124 57 171 144 44 51 28 17 132
14 258 183 134 169 142 175 172 124 67 35 3 35
286 94 89 108 133 263 112 116 258 105 24 17
mean
261 164 130 105 115 128 96 66 60 46 34 51
SEM
12 18 22 17 19 21 15 14 17 14 13 17
Table 2.4: Pregabalin 10 mg/kg (Group 4)
Total number of flinches counted at 5 minute intervals
Animal # 5 10 15 20 25 30 35 40 45 50 55 60
1 322 65 5 186 182 202 34 28 56 152 12 8
2 258 109 170 313 318 173 75 328 226 186 107 96
3 291 106 144 197 217 157 163 33 5 6 6 3
4 198 95 7 61 172 110 144 88 29 60 2 0
5 321 217 209 224 210 112 73 170 10 1 4 0
6 239 43 28 283 235 235 158 87 32 5 2 4
7 294 149 163 281 244 198 191 51 8 76 127 34
8 154 49 6 0 32 123 67 96 61 22 13 5
9 259 186 2 74 11 165 144 22 109 114 140 159
10 280 105 71 286 258 24 12 5 8 6 0 0
11 223 63 8 134 177 134 42 12 9 2 12 0
12 192 62 36 154 166 139 54 5 0 2 0 1
CA 02897306 2015-07-14
PC72074A
34
=
Total number of flinches counted at 5 minute intervals
13 249 112 84 148 248 154 122 98 12 13 10 10
14 287 209 159 219 285 260 198 293 219 229 35 11
15 202 52 83 211 181 129 168 35 78 7 2 40
16 176 91 83 21 78 103 114 58 7 2 0 0
mean 247 107 79 175 188 151 110 88 54 55 30 23
SEM 13 14 18 24 21 14 15 24 18 19 12 11
Table 2.5: Pregabalin 30 mg/kg (Group 5)
Total number of flinches counted at 5 minute intervals
Animal # 5 10 15 20 25 30 35 40 45 50 55 60
1 199 63 66 103 126 30 6 5 4 2 113 0
2 243 173 23 118 192 202 251 124 32 79 11 4
3 174 11 27 139 221 95 174 42 28 6 9
7
4 137 7 0 76 112 125 27 7 6 6 16 71
103 28 25 162 79 94 57 82 16 8 14 32
6 197 194 111 145 231 150 35 12 200 155 0 5
7 243 74 76 125 297 248 173 58 22 17 43 1
8 245 86 41 33 195 121 64 0 0 0 0 24
9 78 0 91 157 131 67 1 1 0 0 26 41
241 125 4 135 162 199 88 128 41 26 22 148
11 282 153 118 294 316 213 102 145 1 2 1
0
12 163 5 0 0 67 101 29 0 0 0 0 0
13 229 187 37 102 175 170 72 154 8 10 17 73
14 172 56 0 186 154 173 80 3 4 6 94 22
213 164 103 213 171 43 32 13 1 0 99 91
mean 195 88 48 133 175 135 79 52 24 21 31 35
SEM 15 19 11 18 18 17 18 15 13 11 10 11
Table 2.6: Low Dose Combination (Group 6)
CA 02897306 2015-07-14
PC72074A
Total number of flinches counted at 5 minute intervals
Animal # 5 10 15 20 25 30 35 40 45 50 55 60
1 295 100 48 143 234 156 59 130 18 17 20 11
2 288 75 122 250 198 122 14 44 18 11 5 40
3 199 56 16 88 86 86 84 30 28 64 11 7
4 239 106 69 131 192 56 6 28 6 5 0 0
5 270 119 126 231 181 73 287 134 17 10 6 8
6 258 93 205 322 337 284 168 66 31 0 0 208
7 119 71 165 140 52 224 95 68 66 42 41 114
8 193 23 71 146 264 167 65 53 10 6 1 0
9 281 51 28 32 233 263 111 23 152 91 3 12
10 245 158 161 89 233 223 107 127 51 75 22 14
11 242 108 49 203 180 199 115 12 6 10 5 158
12 135 11 1 1 23 42 49 22 18 10 0 1
13 273 121 27 175 188 191 43 16 11 11 3 43
14 230 142 109 256 178 99 86 5 3 1 3 100
15 184 56 8 132 171 113 80 27 31 1 0 0
16 133 45 35 86 29 58 25 0 0 0 0 22
mean 224 83 78 152 174 147 87 49 29 22 8 46
SEM 14 10 16 21 22 19 17 11 9 7 3 16
Table 2.7: High Dose Combination (Group 7)
Total number of flinches counted at 5 minute intervals
Animal # 5 10 15 20 25 30 35 40 45 50 55 60
1 130 10 11 25 0 61 20 12 1 0 0 0
2 179 2 2 1 2 40 101 101 63 53 20 11
3 247 44 1 2 4 0 1 25 58 15 10 4
4 274 22 4 99 168 105 44 17 7 0 0 2
5 162 14 8 9 0 = 5 109 23 10 1 0 0
6 257 106 11 14 6 = 6 98 58 38 52 25 38
CA 02897306 2015-07-14
PC72074A
36
Total number of flinches counted at 5 minute intervals
Animal # 5 10 15 20 25 30 35 40 45 50 55
60
7 265 69 24 17 153 73 274 275 236 140 24 13
8 120 5 14 0 0 20 4 42 34 50 31 21
9 75 76 12 69 18 19 0 2 51 46 33 20
292 56 2 8 94 147 174 245
76 25 23 28
11 264 58 96 32 35 53 148 100 38 2 7 5
12 239 15 0 38 119 154 35 5 4 2 1 8
13 259 84 14 63 145 180 99 11 20 8 0 0
14 225 19 42 0 53 90 106 165 19 233 17 2
243 135 64 136 126 142 11 2 1 2 3 10
16 201 11 2 1 13 6 37 91 29 0 1 10
mean 215 45 19 32 59 69 79 73 43 39 12 11
SEM 16 10 7 10 16 15 19 22 14 16 3 3
Graphical representation of the time course of flinching for both the high
dose and low dose
combinations are shown in, respectively Figs 1 to 3 and 4 to 6.
5 The total numbers of flinches within each phase were analyzed separately
on the log scale
using a linear model accounting for experimental day. Individual treatment
comparisons
were performed and summarized using means, differences, 95% confidence
intervals and
p-values. Results for the high dose Phase 2 and Phase 2a total flinches, where
a
statistically significant additive effect was seen, are visualized in Figs 7.1
and 7.2 using
10 posterior distribution plots of the treatment means on the log scale.
Results:
The high dose combination resulted in a significant additive effect in Phase 2
and Phase 2a
(p<0.01). In Phase 2 flinching was decreased by 30%, 40% and 69% by the
compound of
15 formula (IB) 100 mg/kg, pregabalin 30 mg/kg and the combination dose,
respectively. In
Phase 2a flinching was decreased by 36%, 38% and 74% by the compound of
formula (IB)
100 mg/kg, pregabalin 30 mg/kg and the combination dose, respectively.
CA 02897306 2015-07-14
PC72074A
37
In vivo evaluation in a man
The combined effects of pregabalin and Nay 1.7 blockade may also be examined
in clinical
pain studies. Clinical studies can examine single or multiple doses of either
or both agents
in exploratory pain endpoints in healthy volunteers or patients. Clinical
studies can also be
carried out in pain patient populations employing, for example, parallel
group, cross-over or
randomised withdrawal study designs.