Note: Descriptions are shown in the official language in which they were submitted.
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
DOSING INJECTOR
CROSS-REFERENCE TO RELATED APPLICATIONS
[001] This application is a continutation-in-part of U.S. patent
application serial No.
12/121,000, filed May 15, 2008 and claims the benefit of priority pursuant to
35 U.S.C. 119(e)
to United States provisional patent application Serial No. 61/758,698, filed
Jan 30, 2013,
incorporated herein in its entireties by reference.
FIELD
[002] The present invention relates to devices such as a syringe or syringe-
like device
that allow for repeated and accurate dosing of substances such as a
neurotoxin.
BACKGROUND
[003] There are many types of syringes available for medical (injections,
instillations,
etc.) and non-medical (basting/cooking, adhesive, lubrications, other
industrial/manufacturing)
uses. Over the years, there have been many enhancements to the basic syringe.
These include,
but are not limited to syringes that are disposable, include luer locks, have
safety mechanisms to
minimize needle sticks, are dedicated to medication cartridge systems (such as
Carpojects0), as
well as syringes that are factory pre-filled with a unit dose/aliquot of
medication or other fluid.
[004] With most syringes, by applying pressure to the piston, and
controlling where
the piston stops relative to tick marks indicating volumes delivered, the
operator can administer
multiple aliquots (equal or unequal) of fluid from the same syringe in order
to deliver a
predetermined total amount of fluid to a target location.
[005] However, this takes good hand-to-eye coordination. In certain
applications, such
as delivery of multiple doses of medication sequentially during the same
patient visit, it can be
important to deliver very accurate amounts of fluid in a quick, convenient,
precise and accurate
manner.
[006] There is a need for a syringe or a syringe-like device that can
provide for
repeated and accurate dosing of a substance, and allow the operator to
concentrate on other
important aspects of the associated procedures.
SUMMARY
[007] A metered, multiple aliquot/dose syringe in accordance with the
present
invention generally includes a barrel having an open end and a opposing spaced
apart port
1
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
adapted to receive a needle. A piston is provided and slidably disposed within
the barrel through
the barrel open end and a push rod is connected to the piston for sliding the
piston within the
barrel.
[008] In accordance with the present invention, structure is provided which
interconnects the barrel and the push rod for enabling stepwise movement of
the push rod and
the piston within the barrel. This structure further provides concomitant
tactile and/or an audible
(sound) indication of such stepwise movement, thereby enabling the user to
operate the syringe
in delivering multiple doses without the visual observance thereof
[009] Thus, a syringe within the scope of our present invention permits
injection of
accurate doses due to the physical stops or dosage administered indicators
(i.e. elements 58 in
FIG. 1, 22 in FIG. 6, 138 in FIG. 8, and elements 68 in FIG. 10) which can act
to prevent
hydraulic momentum from continuing to deliver fluid after thumb pressure is
lifted off the push
rod (piston).
[010] More particularly, the structure in accordance with one embodiment of
the
present invention may include the plurality of spaced apart ridges on an outer
surface of the push
rod and an engageable ridge disposed on an inner surface of the barrel.
[011] In another embodiment of the present invention, the structure
comprises a
plurality of spaced apart detents in an outer surface of the push rod and a
corresponding
engageable ridge disposed on an inner surface of the barrel.
[012] Still another embodiment of the present invention, the structure
comprises a
plurality of spaced apart ridges disposed on an outer surface of the barrel
and an arm connected
to the push rod. The arm includes a ridge disposed for stepwise engagement of
the spaced apart
ridges. More particularly, the spaced apart ridges may be aligned with one
another only a portion
of the barrel circumference and the arm may have a width smaller than a push
rod
circumference.
[013] Yet another embodiment of the present invention, the structure
includes a
plurality of spaced apart ridges disposed on an outer surface of the barrel
and a sleeve is
provided which surrounds the push rod and includes a ridge disposed on an
inner surface of the
sleeve for stepwise engagement of the spaced apart ridges. More specifically,
in this
embodiment, the spaced apart ridges are circumferential about the barrel and
the sleeve ridge is
circumferential about the sleeve inner surface.
2
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
[014] A further embodiment in accordance with the present invention
includes
structure which comprises a plurality of spaced apart detents disposed on an
outer surface of the
barrel and an arm connected to the push rod with the arm including a ridge
disposed for a
stepwise engagement of the spaced apart detents. More particularly, in this
embodiment, the
spaced apart detents may be aligned with one another over a portion of the
barrel structure and
the arm has a width smaller than a push rod circumference.
[015] An additional embodiment of the present invention provides for a
syringe in
which the structure comprises a plurality of spaced apart detents disposed on
an outer surface of
the barrel and a sleeve surrounding the push rod includes a ridge disposed on
an inner surface of
the sleeve for stepwise engagement of the spaced apart detents. More
particularly, in this
embodiment, the spaced apart detents may be circumferential about the barrel
and the sleeve
ridge may be circumferential about the sleeve inner surface.
[016] In another aspect, the present invention provides a dosing injector
that includes
a dosing mechanism that allows for repeated and accurate dosing of a substance
such as a
neurotoxin.
[017] In certain embodiments, a dosing injector is provided that includes a
dosing
mechanism that allows for repeated and accurate dosing of a substance, and an
override
mechanism that allows a user to disengage from the dosing mechanism.
DRAWINGS
[018] The advantages and features of the present invention will be better
understood
by the following description when considered in conjunction with the
accompanying drawings,
in which:
[019] FIG. 1 illustrates a metered, multiple aliquot-dose syringe in
accordance with
the present invention which generally includes a barrel, a piston slidably
disposed therein along
with a push rod connected to the piston and a structure interconnected to the
barrel and the
piston for enabling stepwise movement of the push rod and the piston within
the barrel, more
specifically, the structure may include a plurality of spaced apart ridges on
an outer surface of
the piston and an engageable ridge disposed on an inner surface of the barrel;
[020] FIG. 2 is an illustration of the use of the present invention with a
cystoscope for
visualizing and performing procedures in a bladder while at the same time
utilizing tactile and
sound features of the present invention;
3
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
[021] FIGS. 3, 4, and 5 illustrate alternative ridge and detent
configurations
encompassed by the present invention;
[022] FIG. 6 is a plan view of a alternative embodiment of the present
invention
showing a barrel having a plurality of spaced apart ridges aligned with one
another over a
portion of the barrel;
[023] FIG. 7 is a cross sectional view of the barrel illustrated in FIG. 6
further
showing an arm connected to the push rod with the arm including a ridge
disposed for a stepwise
engagement of the spaced apart ridges on the barrel;
[024] FIG. 8 is a plan view of an alternative embodiment of the present
invention in
which the barrel includes circumferential ridges and a sleeve connected to the
push rod includes
a ridge disposed on an inner surface of the sleeve for a stepwise engagement
of the spaced apart
ridges;
[025] FIG. 9 is a cross sectional view of the embodiment shown in FIG. 8;
[026] FIG. 10 is a plan view of yet another embodiment of the present
invention
illustrating a barrel having a plurality of spaced apart detents in an outer
surface thereof along
with a sleeve having a circumferential ridge for engaging the detents;
[027] FIG. 11 is a cross sectional view of the embodiment shown in FIG. 10;
[028] FIG. 12 is a plan view of still another embodiment of the present
invention
utilizing a barrel having a plurality of spaced apart circumferential detent
in a barrel and a
corresponding ridge formed in a sleeve surrounding a push rod;
[029] FIG. 13 is a cross sectional view of the embodiment shown in FIG. 12;
[030] FIGs. 14 to 19 illustrate a dosing device in accordance with another
aspect of
the present invention; wherein:
[031] FIG. 14 is an isometric view of a dosing device in accordance with an
embodiment of the present invention; the device includes a syringe, a plunger
rod slidably
disposed therein and and a control component interconnecting the syringe and
the plunger rod;
[032] FIF. 15 is a cross sectional view of the device shown in FIG. 14;
[033] FIGs. 16A, 16B and 16c show cross-sectional views of the control
component
in three different positions according to aspects of the present invention;
and
4
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
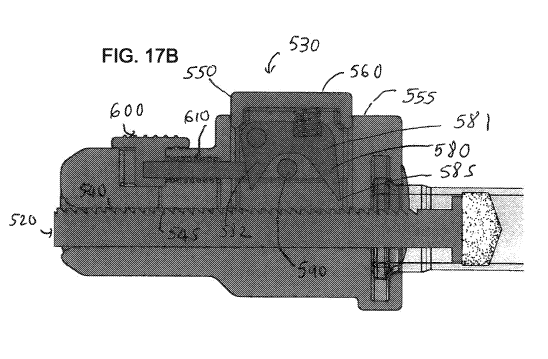
[034] FIGs. 17A and 17B show a control component of an alternative dosing
device in
accordance with aspects of the present invention.
DESCRIPTION
[035] With reference to FIG. 1, there is shown a syringe 10 in accordance
with the
present invention for providing a metered multiple aliquot/dose that includes
a barrel 14 having
an open end 18 and an opposing spaced apart port 22 which is adapted for
receiving a
conventional needle 26, shown in broken line. A piston 30 is slidably disposed
within the barrel
14 through the barrel open end 18 and a push rod 34 is connected to the piston
for sliding the
piston 30 within the barrel 14.
[036] Structure 40 interconnecting the barrel 14 and the push rod 34
enables stepwise
movement of the push rod 34 and the piston 30 within the barrel 14.
[037] As will be described hereinafter in greater detail, the structure 40
is configured
for producing sound indication as indicated by lines 44, in FIG. 2 of stepwise
movement of the
push rod 34 and piston 30 within the barrel 14 to enable an operator 48 to
deliver multiple doses
of a medicament without visual observation of the syringe 10. This is
particularly useful in the
delivery of a drug using an endoscope 52 as shown in FIG. 2.
[038] As the operator 48 (such as a surgeon or urologist) is busy
manipulating the
scope 52 so that he/she can visualize the proper area of the body tissue 54,
in the absence of an
assistant, he/she also needs to control the syringe 10, place and secure the
scope 52, and then let
go of the scope 52 with one hand to grasp then push the syringe 10.
[039] During this time, the scope 52 and needle 10 have a tendency to move.
In prior
art discoveries, the hydraulic momentum of the initial piston (not shown)
push, may cause the
piston to move past a desired point on a barrel (not shown), leading to too
much fluid being
administered at each injection site. The syringe 10 in accordance with the
present invention
prevents this over-run by having "ratcheted stops" spaced at proper distance
for the desired
volume to be delivered. It will further enable the procedure to be conducted
by one person
without the need for an assistant as the endoscopic operator 48 will not have
to take his eyes off
the image being relayed through the scope 52.
[040] With reference again to FIG. 1, in accordance with the present
invention, the
structure 40 includes a plurality of spaced apart ridges 58 disposed on an
outer surface 62 of the
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
push rod 34 and an engagable ridge 66 disposed on an inner surface 70 of the
barrel 14.
Movement of the push rod 34 in a direction indicated by arrow 72 causes
stepwise engagement
between the ridges 58, 66 resulting not only in a tactile sensation by the
operator 48 but also in
sound generation as indicated at lines 44 in FIG. 2. Selected spacing 76 may
provide for one
milliliter doses, as an example, to be ejected from a multi-milliliter
syringe. Thus, the syringe 10
in accordance with the present invention provides for an accurate and precise
dose/aliquot of
fluid administered in a fast and stepwise convenient manner. An enlargement of
the structure 40
showing a ridges 58, 66 during engagement is illustrated in FIG. 3.
[041] FIG. 4 is an alternative embodiment of the structure 80 in accordance
with the
present invention, wherein structure 80 comprises a plurality of detents 82 in
a push rod outer
surface 86 and a correspondingly engageable ridge 88 disposed on a barrel
inner surface 92.
[042] Control of the tactile sensory indication of stepwise movement
provided by a
structure 96 for a push rod 98 and barrel 100 is shown in FIG. 5 wherein a
detents 104 and ridge
108 have a smoother contour than a corresponding ridges 82, 88 shown in FIG. 4
thereby
changing a tactile and auditory sensing of movement of the push rod 98 within
the barrel 100.
[043] In yet another embodiment syringe 112 is illustrated in FIGS. 6 and 7
which
includes a barrel 114, piston 116, and a push rod 118. The syringe barrel 114,
as illustrated in
FIGS. 6 and 7, includes a plurality of spaced apart ridges 122 disposed on an
outer surface 126
of the barrel 114 and an arm 130 connected to a push rod 118 which includes an
arm ridge 132
for a stepwise engagement with the ridges 122. In this syringe 112, the ridges
122 are aligned
with one another over a portion of the barrel 114 circumference, or outer
surface, 126 and the
arm 130 has a width smaller than a circumference of the push rod 118.
[044] In yet another embodiment syringe 134 is illustrated in FIGS. 8 and 9
with
common character references indicating identical or substantially similar
elements as
hereinbefore discussed in connection with other embodiments of the present
invention.
[045] As shown in FIGS. 8 and 9, the syringe 134 includes a plurality of
spaced apart
circumferential ridges 138 disposed on an outer surface 140 of a barrel 142,
and a sleeve
surrounds and is connected to a push rod 150. A circumferential ridge 154
disposed on an inner
surface of the sleeve 146 enables stepwise engagement of the barrel ridges 138
in a manner as
hereinabove described in connection with earlier described embodiments of the
present
invention.
6
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
[046] FIGS. 10 and 11 illustrate yet another embodiment syringe 162 in
accordance
with the present invention that includes a barrel 164 which includes a
plurality of spaced apart
detents 168 and an outer surface 170 of the barrel 164 and an arm 174 attached
to a push rod 178
and includes a ridge 180 disposed on an arm inner surface 182 for a stepwise
engagement with
the detents 168 in a stepwise manner.
[047] Still another embodiment syringe 190 is shown in FIGS. 12 and 13
which
includes a barrel 194 having spaced apart circumferential detents 198 in a
barrel outside surface
200 and a sleeve 204 surrounding a push rod 206 includes a ridge 210 disposed
on an inside
surface 214 of the sleeve 204 for stepwise engagement of the spaced apart
detents 198.
[048] A method in accordance with the present invention utilizes any one of
the
syringes 10, 112, 134, 162, 190 hereinabove described and includes with
respect to syringe 10
disposing a medicament in the barrel 14 and operating the structure 40
interconnecting the barrel
114 and the push rod 34 to provide stepwise movement of the push rod 34 and
piston 30 within
the barrel 114 in order to administer metered multiple aliquot/doses of
medicament.
[049] In another aspect, the present invention provides a dosing device
which
provides, among other features, (1) a dosing mechanism allowing for
repetitive, precise and
accurate dosing of a substance; and (2) an override mechanism allowing for a
user to disengage
from the dosing mechanism.
[050] FIG. 14 is a perspective view of an exmplary embodiment of a dosing
device
300 provided in accordance with aspects of the present invention. Briefly, the
dosing device 300
comprises a syringe 310 connected to a plunger rod 320 by a control component
330. The
syringe 310 can be a standard or custom syringe. It may or may not be
prepackaged with the rest
of the dosing device 300. The control component 330 comprises an actuator 360
for actuating
the dosing device 300. In one embodiment, the actuator 360 is a button. In an
alternative
embodiment, the actuator 360 is a lever.
[051] As shown in Fig. 15, the plunger rod 320 comprises an engaging
surface 340. In
one embodiment, the engaging surface 340 comprises a rack having a plurality
of spaced apart
teeth 345. The spaced apart teeth 345 are uniformely spaced apart
longitudinally over at least a
portion of a surface of the push rod 320. The distance between one tooth to
the next tooth
defines an interdental space 365 or pitch, as shown in Fig. 16A. The
interdental space 365
corresponds to a predetermined volume of liquid. In alternative embodiments,
the engaging
surface 340 comprises ridges, detents, or the like.
7
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
[052] In one embodiment, the control component 330 is a plunger assembly.
Plunger
assemblies for use in dispensing devices have been described in for example
U.S. Patent No.
3,161,323, the content of which is incorporated herein in its entireties by
reference.
[053] Referring to Figs. 15 and 16A, the control component 330 comprises a
housing
350 having a top side 355, an actuator 360 for actuating the dosing device 300
to enable stepwise
movement of the push rod 320 within the syringe 310 for repetitive and
accurate delivery of a
predetermined volume of a liquid composition disposed in the syringe. In one
embodiment, the
actuator 360 is a button. In an alternative embodiment, the actuator 360 is a
lever. In one
embodiment, the actuator 360 is movable vertically to various positions
relative to the top side
355. In one embodiment, the actuator 360 has three basic positions,
corresponding to different
levels relative to the top side 355. In a fully extended position, the
actuator 360 is at its
maximum height. In a fully depressed position, the actuator 360 is at a
minimum height relative
to the top side 355. In a partly extended position, the actuator 360 is partly
depressed and having
a height intermediate between the maximum height and the minimum height. The
motive force
that lifts the actuator 360 back up can be provided by a spring or some other
mechanical design
element, as well known to one skilled in the art.
[054] In one embodiment, the housing 350 houses a pawl 380 rotationally
coupled to a
pivot pin 410. In one embodiment, the pawl 380 has a wedge shaped end portion
390 which
reversibly engages with the spaced apart teeth 345 on the engaging surface 340
in a dosing
mechanism as described herein. A stopping pin 400 is positioned adjacent an
inner side of the
pawl 380 and limits clockwise movement of the pawl 380. A biasing element 370
connects the
pawl 380 to the actuator 360. In one embodiment, the biasing element 370 is a
spring.
[055] Referring to FIG. 16A, in a reset or starting position, the actuator
360 is at its
maximum height relative to the top side 355. The biasing element 370 is in an
extended state.
The end portion 390 of the pawl 380 is disengaged from the plurality of spaced
apart teeth 345
on the engaging surface 340.
[056] In a partly actuated position as shown FIG. 16B, the actuator 360 is
partly
depressed such that its height relative to the top side 355 is an intermediate
height between the
maximum height and the minimum height. Pressing the actuator 360 partly
compresses the
biasing element 370 which causes the end portion 390 to engage with an initial
tooth 345 on the
engaging surface 340. The biasing element 370 ensures that the end portion 390
of the pawl 380
remains engaged with the initial tooth 345.
8
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
[057] In a fully actuated position as shown in FIG. 16C, the actuator 360
is fully
pressed down. The compression of the biasing element 370 causes the pawl 380
to tilt backward,
pushing the plunger rod 320 and the engaging surface 340 forward. In this
position, the pawl 380
is no longer in contact with the stopping pin 400. Once the actuator 360 is
released from this
position, the end portion 390 of the pawl 380 will disengage from the initial
tooth 345 and align
to engage the next tooth the next time the actuator is pressed. The
interdental space between the
spaced apart teeth 345 correlates with a desired dose. Each actuation of the
actuator 360 causes
the plunger rod 320 to advance exactly one increment corresponding to an
interdental space 365
or pitch 365. In one embodiment, each actuation dispenses 0.1m1. In
alternative embodiments,
each actuation dispenses a volume smaller than 0.1m1. In yet other
embodiments, each actuation
dispenses a volume larger than 0.1m1.
[058] In one embodiment, the present dosing device provides an override
mechanism,
wherein a user has an option to freely pull the plunger rod 320 in either
direction for aspiration,
filling, evacuating, or dosing an amount other than an amount specified by the
interdental space
365. To exersize the override mechanism, the user releases the actuator 360
such that it can
return to its maximum height relative to the top side 355 as shown in Fig.
16A. In one
embodiment, a pin (not shown) can be incorporated into the actuator 360 to
maintain it in this
position such that the pawl 380 is disengaged from the engaging surface 340 of
the plunger rod
320. When ready, the user can remove the pin holding the actuator 360 in the
fully extended
position (for example, by using a pull tab accessible from the exterior of the
device). Once the
pin is removed, the biasing element 370 forces the pawl 380 to engage the
engaging surface 340.
This particular embodiment can prove useful when disengagement is only desired
at the
beginning of a procedure. For example, if an injection device is to be
prefilled with a diluent
used for reconstitution, the user will want to inject that diluent freely, and
then freely draw the
reconstituted product back into the device. After the device is loaded, the
user can then pull the
tab and the device is setup to dose incrementally.
[059] Thus, aspects of the present dosing device provides a dosing device,
comprising: a barrel having an open end and an opposing spaced apart port
adapted to receive a
needle; a plunger rod slidably disposed within said barrel through the barrel
open end; the
plunger rod having a plurality of spaced apart teeth over at least a portion
of a top side thereof
and having interdental spaces therebetween; a control component
interconnecting said barrel and
said plunger rod. In one embodiment, the control component comprises a pawl
having an end
point configured for engaging with the plurality of spaced apart teeth; means
for causing the end
9
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
point to enter an interdental space, engage a tooth and move the plunger rod
forwardly an
increment corresponding to the interdental space to allow for stepwise
movement of the plunger
rod within the barrel; and an actuator for actuating the stepwise movement. In
one embodiment,
the actuator is movable from a reset position, wherein the end point is
disengaged from the
plurality of spaced apart teeth, to a partially actuated position, wherein the
end point engages a
first tooth, to a fully actuated position, wherein the end point moves from
the first tooth to a
second tooth; and wherein in the reset position, the plunger rod is freely
slidable longitudinally
the barrel.
[060] In an alternative embodiment shown in FIGs. 17A and 17B, a dosing
device 500
comprises a control component 530. In one embodiment, the control component
530 comprises a
an actuator 560, a housing 550 having a top side 555 which houses a biasing
element 570, a
"double pawl" 580 rotationally coupled to a pivot pin 510 and a stopping pin
590 which limits
clockwise movement of the pawl 580. The double pawl 580 has a first side 581
having an end
point 585 engageable with the engaging surface 540 of the plunger rod 520 and
a second side
582 which does not come into contact with the engaging surface 540. In one
embodiment, the
"double pawl" 580 is normally engaged with the plurality of spaced apart teeth
545 on the
engaging surface 540 of the plunger rod 520. In one embodiment, the actuator
560 is movable
from a resting position to an actuated position. In a resting state, the
actuator 560 is positioned at
a height relative to the top side 555 such that the biasing element 570 is
partly compressed,
keeping the end point 585 in engagement with one of the plurality of spaced
apart teeth 545 on
the engaging surface 540, as shown in Fig. 17A. In an actuated state, the
actuator is depressed,
causing the biasing element 570 to further compress. The compression of the
biasing element
570 causes the pawl 580 to tilt backward, pushing the plunger rod 520 and the
engaging surface
540 forward (not shown), in a similar mechanism as described for Figs. 16A, B
and C.
[061] In one embodiment, the plunger assembly 530 is connected to a spring
loaded
slider actuator 600. As shown in Fig. 17A, in a locked position, a spring 610
connecting the
slider actuator 600 to the housing 550 is in an extended state, and the slider
actuator 600 is
spaced apart from the second side 582 of the "double pawl" 580. In this locked
position, a user
cannot pull the plunger rod 520 back but he/she can control aspiration without
losing the spot on
the engaging surface 540.
[062] In a released state shown in Fig. 17B, to disengage the "double pawl"
580 from
the engaging surface 540, the user pushes the slider actuator 600 forward such
that now it comes
into contact with the second side 582 of the "double pawl' 580. The
compression of the spring
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
610 causes the "double pawl" 580 to tilt backward and disengage from the
engaging surface 540.
In this position, a user can pull the plunger rod 520 in either direction for
aspiration, filling,
evacuating, or dosing an amount other than an amount specified by the
interdental space or pitch
of the plurality of spaced apart teeth 545.
[063] Thus, aspects of the present dosing device provide a barrel having an
open end
and an opposing spaced apart port adapted to receive a needle; a plunger rod
slidably disposed
within said barrel through the barrel open end; the plunger rod having a
plurality of spaced apart
teeth over at least a portion of a top side thereof and having interdental
spaces therebetween; a
control component interconnecting said barrel and said plunger rod. In one
embodiment, the
control component comprises: a pawl comprising a first side having an end
point engageable
with the plurality of spaced apart teeth and a second end opposing the first
side; means for
causing the end point to enter an interdental space, engage a tooth and move
the plunger rod
forwardly an increment corresponding to the interdental space to allow for
stepwise movement
of the plunger rod within the barrel; an actuator for actuating the stepwise
movement. In one
embodiment, the actuator is movable from a resting position, wherein the end
point engages a
first tooth, to an actuated position, wherein the end point moves from the
first tooth to a second
tooth. In one embodiment, the control component further comprises a
compressible slider
actuator facing the second side of the pawl, the slider actuator is movable
from a locked state to
a released state, wherein in the locked state, the slider actuator is spaced
apart from the second
side of the pawl, and in the reelased state, the slider actuator impacts the
second side of the pawl
at an angle, and causes the end point of the first side to disengage from the
plurality of spaced
apart teeth. In one embodiment, in the released state, the plunger rod is
freely slidable
longitudinally the barrel.
[064] In alternative embodiments, multiple engaging surfaces having
variable teeth
sizes can be used on different side of the plunger rod, such that different
dose sizes can be
dispensed with one single device by rotating, removing or replacing the
plunger rod. The
amount of dose variation is dependent on the pawl geometry, but is still
driven by the interdental
space or pitch of the rack(s).
[065] Further embodiments consider the need, in some instances, to
aspirate. This
may be necessary in situations where the user wants to confirm that he/she is
not injecting into a
blood vessel (for instance, any sign of red within the syringe while
aspirating is an indication
that the needle is within a blood vessel). When the pawl is engaged, it
typically sits somewhere
between the rack teeth of the engaging surface. Therefore, it may allow for
the rack to be pulled
11
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
back slightly, before hitting a hard stop against the pawl (similar to
backlash in gears). This
feature can be utilized as an advantage since it allows for a consistent
aspiration with each
injection without impacting the ultimate dose that will be delivered (the user
won't be able to
skip teeth on the rack).
EXAMPLES
[066] The present invention is especially useful with a cystoscope for
injecting a
pharmaceutical, such as botulinum toxin, into a target tissue, such as a
bladder wall to treat a
disease or condition such as a urological disease or condition (i.e. a bladder
dysfunction such as
overactive bladder), a prostate disorder, an ocular disease or condition or
any other human
ailment, condition or disease.
[067] The normal micturition process is a result of a complex network of
innervation
of the bladder and urethral sphincter that ensures satisfactory bladder
filling followed by timely
voiding in healthy individuals. In order to achieve storage of urine in the
bladder during the
filling phase, the bladder neck and urethra remain closed and the detrusor
muscle is relaxed (via
stimulation of the noradrenalin beta receptors in the dome of the bladder). In
the healthy bladder,
when the pressure within the bladder is greater than that within the urethra,
urination begins. The
sensations of pain and bladder fullness are carried by the afferent fibers,
which relay their
message from the bladder to the micturition center in the pons of the brain
triggering micturition.
The voiding phase involves coordinated relaxation of the base of the bladder
and urinary
sphincter (via stimulation of the noradrenalin alpha receptors) and
contraction of the detrusor
muscle in the bladder wall secondary to inhibition of noradrenergic
stimulation followed by
parasympathetic stimulation via the neurotransmitter acetylcholine.
[068] Overactive bladder is a condition resulting in a disruption to the
normal
micturition process. It is a syndrome complex characterized by urinary
urgency, frequency and
may or may not be accompanied by incontinence. Incontinence is due to
involuntary contraction
of the detrusor muscle during bladder filling (detrusor overactivity). Most
cases of incontinence
arise without obvious pathology. In such cases, abnormal detrusor contractions
are termed
idiopathic bladder overactivity. A smaller number of cases are secondary to
neurogenic
pathology and are termed neuro genic detrusor
overactivity.
Neurogenic Detrusor Overactivity.
[069] The pathophysiology of OAB is complex, involving peripheral and
central
nervous system (CNS) dynamics. Several CNS disorders are associated with the
development of
12
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
OAB, including spinal cord injury and multiple sclerosis. Neurological disease
involving the
spinal cord can result in incontinence secondary to a loss of inhibitory input
from the micturition
center and from interruption of the spinobulbospinal pathways which normally
control bladder
behavior. In the event of a spinal cord lesion, a change of balance of the
effects of the afferent
fibers, located between the muscle and submucosa of the bladder, is seen. The
unmyelinated C
fibers become functionally dominant and the detrusor hyperreflexia described
in such patients is
considered due to the reflex mediated by these unmyelinated C fibers.
[070] The result, demonstrable on urodynamic evaluation, is abnormal
involuntary
detrusor contractions, often leading to incontinence. In addition, such
patients frequently suffer
from loss of coordinated relaxation of the urethral sphincters that normally
precedes micturition.
This lack of coordinated activity can result not only in incontinence but also
in vesico-ureteric
reflux which, if left untreated, can lead to potential renal damage.
Available Treatments.
[071] Clean intermittent self-catheterization (CIC) is commonly used to
drain the
bladder, manage neurogenic incontinence and prevent vesico-ureteric reflex.
When employing
CIC, the patient inserts a catheter via the urethra into the bladder in order
to void urine. CIC,
however, can be associated with infection, which can exacerbate the problem of
urinary
incontinence and, in some circumstances, lead to renal damage. Common
pharmacologic
treatments to reduce bladder contractility include anticholinergics,
antispasmodics and tricyclic
antidepressants. However, these therapies are associated with a high incidence
of side effects.
Side effects of anticholinergics include dry mouth, constipation and blurred
vision. Currently,
the only options available to patients who do not respond to or discontinue
anticholinergic
therapy are invasive procedures such as implantable devices to chronically
stimulate the sacral
nerve or surgical bladder augmentation. While these procedures may be
effective for some
patients, they are highly invasive, do not necessarily guarantee continence,
and may have long
term complications.
BOTOX (Botulinum Toxin Type A Purified Neurotoxin Complex) Treatment
[072] Recently, studies have been carried out using BOTOX (botulinum
toxin) in the
treatment of patients who suffer from bladder overactivity. Suppression of
involuntary detrusor
contractions has been attempted via the local administration of BOTOX
(botulinum toxin) to
the detrusor muscle, which inhibits acetylcholine release by cleaving SNAP 25,
a protein
integral to successful docking and release of vesicles within the nerve
endings, including
13
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
acetylcholine, calcitonin gene-related peptides (CGRP), glutamate and
substance-P. BOTOX
(botulinum toxin) is believed to inhibit the acetylcholine mediated detrusor
contractions and may
also inhibit other vesicle-bound neurotransmitters in both the afferent and
efferent pathways of
the bladder wall, urothelium or lamina propria.
[073] There is evidence for the successful use of BOTOX (botulinum toxin)
in the
management of neurogenic incontinence. It has been shown that botulinum toxin
injections of
200 U to 300 U into the detrusor across 20 to 30 injection sites (10 units per
mL per injection
site) have been effective in restoring continence and enabling reduction or
cessation of
anticholinergic medication in such patients. In one study enrolling 21
patients, 17 of the 19
patients with follow-up data had restored continence within 6 weeks. To date,
treatment of over
900 neurogenic overactive bladder patients with BOTOX (botulinum toxin) at
doses ranging
from 200 U to 300 U in 20 to 30 injection sites has been reported. Treatment
benefit has been
described to last between 6 and 12 months with an acceptable side effect
profile.
[074] Endoscopy of the urinary bladder via the urethra is cystoscopy.
Diagnostic
cystoscopy is usually carried out with local anesthesia. General anesthesia is
sometimes used for
operative cystoscopic procedures.
[075] When a patient has a urinary disease or condition, a physician can
use a
cystoscope 52, see FIG. 2 to see inside of the bladder and urethra. The
urethra is the tube that
carries urine from the bladder to the outside of the body. The cystoscope has
lenses which permit
the physician to focus on the inner surfaces of the urinary tract. Some
cystoscopes use optical
fibres (flexible glass fibres) that carry an image from the tip of the
instrument to a viewing piece
at the other end. The cystoscope is as thick as a pencil and has a light at
the tip. Many
cystoscopes have extra tubes to guide other instruments for surgical
procedures to treat urinary
problems.
[076] There are two main types of cystoscopy--flexible and rigid--differing
in the
flexibility of the cystoscope. Flexible cystoscopy is carried out using local
anesthesia on both
sexes. Typically, lidocaine gel (such as the brand name Instillagel) is used
as an anesthetic,
instilled in the urethra. Rigid cystoscopy can be performed under the same
conditions, but is
generally carried out under general anesthesia, particularly in male subjects,
due to the pain
caused by the probe. The embodiments of our invention set forth herein (see eg
the Figures) can
be used to accurately and precisely inject a metered dose (aliquots) of a
botulinum toxin (such as
BOTOX. DYSPORT, MYOBLOC, or XEOMIN) into the bladder wall (detrusor) of a
patient to
14
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
treat a bladder dysfunction. Our invention is not limited to use to treat a
bladder dysfunction or
to administration of a botulinum toxin, as it can be used for any therapeutic,
cosmetic or research
use in which accurate and precisely metered doses of an aqueous pharmaceutical
is desired.
[077] The present invention is useful for injecting a pharmaceutical, such
as
botulinum toxin, into a target tissue, such as a head and/or neck muscle to
treat a disease or
condition such as chronic migraine, or any other human ailment, condition or
disease.
[078] Studies have been carried out using BOTOXO (botulinum toxin) in the
treatment of patients who suffer from chronic migraine. For example, it has
been shown that
botulinum toxin injections into certain head and neck muscles have been
effective in treating
chronic migraine. The recommended dilution is 200 Units/4 mL or 100 Units/2
mL, with a final
concentration of 5 Units per 0.1 mL. The recommended dose for treating chronic
migraine is 155
Units administered intramuscularly using a sterile 30-gauge, 0.5 inch needle
as 0.1 mL (5 Units)
injections per each site. Injections should be divided across 7 specific
head/neck muscle areas as
specified in the table below. A one inch needle may be needed in the neck
region for patients
with thick neck muscles. With the exception of the procerus muscle, which
should be injected at
one site (midline), all muscles should be injected bilaterally with half the
number of injection
sites administered to the left, and half to the right side of the head and
neck. The recommended
re-treatment schedule is every 12 weeks.
Head/Neck Area Recommended Dose (Number of Sites)
Frontalis 20 Units divided in 4 sites
(10 U each side)
Corrugator 10 Units divided in 2 sites
(5 U each side)
Procerus 5 Units in 1 site
Occipitalis 30 Units divided in 6 sites
(15 U each side)
Temporalis 40 Units divided in 8 sites
(20 U each side)
Trapezius 30 Units divided in 6 sites
(15 U each side)
Cervical Paraspinal Muscle Group 20 Units divided in 4 sites
CA 02899327 2015-07-24
WO 2014/120795 PCT/US2014/013634
(10 U each side)
TOTAL DOSE:
155 Units divided in 31 sites
Each intramuscular injection site = 0.1 mL = 5 Units BOTOXO
[079] The embodiments of our invention set forth herein can be used to
accurately and
precisely inject a metered dose (aliquots) of a botulinum toxin (such as
BOTOXO) into certain
head and/or neck muscles of a patient to treat chronic migraine. For example,
each actuation of
the dose injector can be measured to provide 0.1 ml (5 U) of BOTOXO for each
intramuscular
injection.
[080] The present invention is not limited for use to treat chronic
migraine or for
administration of a botulinum toxin; as it can be used for any therapeutic,
cosmetic or research
use in which accurate and precisely metered doses of an aqueous pharmaceutical
is desired.
Accordingly, any and all modifications, variations or equivalent arrangements
which may occur
to those skilled in the art, should be considered to be within the scope of
the present invention as
defined in the appended claims.
[081] Many alterations and modifications may be made by those having
ordinary' skill
in the art without departing from the spirit and scope of the present
disclosure. For example,
features and aspects specifically discussed for one embodiment but not another
may be
interchangeable provided the modification does not conflict or made inoperable
Therefore, it
must be understood that the illustrated embodiments have been set forth only
for the purposes of
examples, and that the embodiments should not be taken as limiting the
disclosure as defined by
the following claims. The following claims are, therefore, to be read to
include not only the
combination of elements which are literally set forth, but all equivalent
elements Ibr performing
substantially the same function in substantially the same 1.7,Tay to obtain
substantially the same
result. The claims are thus to be understood to include those that have been
illustrated and
described above, those that are conceptually equivalent, and those that
incorporate the ideas of
the present disclosure.
16