Note: Descriptions are shown in the official language in which they were submitted.
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
METHODS AND SYSTEMS FOR WATER RECOVERY
RELATED APPLICATIONS
The present application gains priority from U.S. Provisional Patent
Applications US
61/757,369 filed on 28 January 2013; US 61/819,195 filed on 03 May 2013; US
61/902,174
filed on 09 November 2013; and US 61/906,112 filed on 19 November 2013 all
which are
incorporated by referencer as if fully set-forth herein.
FIELD OF THE INVENTION
The invention is in the field of water treatment.
BACKGROUND OF THE INVENTION
Water, like many other natural resources, is present on earth in a finite
amount. More
than 95% of the water on Earth is present as brackish water or sea water
containing a salt
concentration which renders it unsuitable for many purposes.
It is estimated that more than two thirds of the remaining non-salty water is
present as
ice, primarily in polar caps and glaciers.
This means less than 1% of the water on earth is available as fresh water.
This small fraction of fresh water must sustain not only life, but industry.
Although
the demand for potable water increases with the world's population, direct
consumption of
water by man (i.e. drinking water) and indirect consumption by man (e.g.
bathing, laundry, in
sanitary installations) makes up a relatively small percentage of total water
consumption in
the world.
The bulk of total water consumption in the world is in industrial processes,
including
use as a cooling medium.
For example, The National Energy Board of Canada (2006) estimated that about 2
to
4.5 barrels of fresh water are used to produce a barrel of synthetic crude
oil. Total water
consumption for production of synthetic crude was projected to reach 529
million cubic
meters/year. Wastewater from synthetic crude oil production is alkaline, and
brackish.
Induced hydraulic fracturing (A.K.A. fracking) for production of natural gas
and other
petrochemicals from shale also consumes significant amounts of water. It is
estimated that
20% to more than 40% of tracking water is recovered either as flow-back water
or as
produced water.
1
RECTIFIED SHEET (RULE 91)
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
In the state of Pennsylvania alone the amount of high-TDS (total dissolved
solids)
wastewater produced by fracking and needing disposal was projected to reach to
7300 million
gallons per year in 2011 by the natural gas industry. Levels of salt in
fracking water can be
more than six times higher than in sea water.
SUMMARY OF THE INVENTION
A broad aspect of the invention relates to separation of usable water from a
stream of
water containing hydrophilic, or water soluble, contaminants. In some
exemplary
embodiments of the invention, the stream is an effluent from an industrial
process and the
usable water is sufficiently purified to be re-used in the same industrial
process.
As used in this specification and the accompanying claims the term "bi-
directional
solvent" indicates an organic solvent, which is characterized in that on
equilibrating at 20 C
with 5% (w/w) NaC1 aqueous solution, solvent concentration in the aqueous
phase is at least
1% and less than 50% (W/W) and water concentration in the solvent phase is at
least 5% and
less than 50% (W/W) or a mixture of two or more such solvents.
Examples of bi-directional solvents include, but are not limited to alcohols
of 3 to 6
carbons and/or ketones of 3 to 6 carbons and/or esters of 3 to 6 carbons
and/or organic acids
of 3 to 6 carbons and amines. In some embodiments, the bi-directional solvent
includes
butanol. In some embodiments, butanol is the primary active component in a
mixture of bi-
directional solvents. In some embodiments, butanol serves as the sole active
bi-directional
solvent. Alternatively or additionally, in some embodiments one or more bi-
directional
solvents comprises one or more members of the group consisting of normal
butanol,
secondary butanol, isobutanol, tertiary butanol, normal pentanol, secondary
pentanol,
isopentanol and tertiary pentanol. Alternatively or additionally, in some
embodiments one or
more bi-directional solvents are provided as an extractant. Optionally, the
extractant includes
components which are not bi-directional solvents. According to an embodiment,
the
extractant comprises water.
Another aspect of some embodiments of the invention relates to recovery of
usable
water from the contaminated wastewater stream without solidification (e.g.
precipitation
and/or crystallization) of contaminants.
According to various exemplary embodiments of the invention a water stream to
be
treated includes one or more hydrophilic solutes and optionally one or more
hydrophobic
solutes.
2
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
As used in this specification and the accompanying claims the term
"hydrophilic
solute" indicates a solute with a log P < - 0.5. According to various
exemplary embodiments
of the invention the log P of the hydrophilic solute is -0.55; -0.6 -0.65; -
0.7; -0.75; -0.8 or
intermediate or lesser values. According to various exemplary embodiments of
the invention,
the term "hydrophilic solute" includes ionic compounds.
As used in this specification and the accompanying claims the term
"hydrophobic
solute" indicates a solute with a log P? 0Ø According to various exemplary
embodiments of
the invention the log P of the hydrophobic solute is 0.1; 0.15; 0.2; 0.25;
0.3; 0.35 or
intermediate or greater values. According to various exemplary embodiments of
the invention,
the term "hydrophobic solute" indicates organic compounds with C:0 atom ratio
greater than
3.
One aspect of some embodiments of the invention relates to treatment of
product
process water containing both hydrophilic solutes and hydrophobic solutes. In
some
exemplary embodiments of the invention, the hydrophobic solutes are crude-oil-
associated.
As used in this specification and the accompanying claims the terms
"wastewater
stream" and "product process water" are interchangeable or said "wastewater
stream"
comprises "product process water". Hence, according to an embodiment, said
wastewater
stream comprises product process water mixture with another stream. According
to an
embodiment, said other stream comprises make-up water. According to a related
embodiment,
said make-up water comprises brackish water or sea water.
As used in this specification and the accompanying claims the term "crude-oil-
associated" indicates materials present in crude oil (i.e. unrefined oil),
materials produced
during refining of crude oil or chemical conversion of crude oil, materials
present in produced
gas, materials produced during refining of produced gas or chemical conversion
of produced
gas. According to various exemplary embodiments of the invention, the term
crude oil
includes fossil oil and/or vegetable oil (e.g. Palm Oil Mill Effluent - POME).
In some
embodiments, crude-oil-associated hydrophobic solutes are present in the
wastewater stream
at concentrations of 10 PPM, 25 PPM, 50 PPM 100 PPM, 200 PPM, 300 PPM, 400 PPM
or
500 PPM or intermediate or higher concentrations.
As used in this specification and the accompanying claims, the terms
"distillation" and
"evaporation" are used interchangeably.
3
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
As used in this specification and the accompanying claims, the terms "water-
depleted"
and "water-enriched" mean containing less water and more water, respectively,
compared with
the content prior to contacting, in terms of amount or flux or concentration.
In some exemplary embodiments of the invention, there is provided a method
including:
(a) first contacting at least a portion of a wastewater stream including one
or more
hydrophilic solutes with an extractant including a bi-directional solvent to
form a water-
depleted first aqueous solution and a water-enriched first organic phase;
(b) second contacting the first organic phase with a concentrated aqueous
solution, to
form a second organic phase and a second aqueous solution;
(c) separating water from the second aqueous solution to form a concentrated
aqueous
solution and separated water;
(d) recycling the concentrated aqueous solution to the second contacting; and
(e) recycling bi-directional solvent from the second organic phase to the
first
contacting;
wherein water partial vapor pressure at 50 C of the wastewater stream, the
water-depleted
first aqueous solution, the concentrated aqueous solution and the second
aqueous solution are
P1, P2, P3 and P4, respectively; and wherein the bi-directional solvent is
selected so that
P1>P2; P1>P3 and P4>P3.
According to various exemplary embodiments of the invention the first
contacting is
done at temperature higher than 1 C, 10 C, 20 C, 30 C or 40 C and lower than
99 C, 90 C,
80 C, 70 C or 60 C.
According to various exemplary embodiments of the invention the second
contacting
is done at temperature higher than 1 C, 10 C, 20 C, 30 C or 40 C and lower
than 99 C,
90 C, 80 C, 70 C or 60 C.
Alternatively or additionally, in some embodiments, the wastewater stream
includes
one or more crude-oil-associated hydrophobic solutes. Alternatively or
additionally, in some
embodiments, the method includes separating at least a portion of the one or
more crude-oil-
associated hydrophobic solutes from at least a portion of the second organic
phase.
Alternatively or additionally, in some embodiments separating water includes
heating
the second aqueous solution.
4
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
Alternatively or additionally, in some embodiments the heating separates a
gaseous
and/or solid compound, and the method comprises contacting the separated
gaseous and/or
solid compound with a third aqueous solution to form the concentrated aqueous
solution.
Alternatively or additionally, in some embodiments the gaseous or solid
compound
includes at least one member of the group consisting of NH3, CO, CO2, CaC12,
Ca(NO2)3,
KBr, KC1, KHCO3, K2SO4, MgC12, MgSO4, NaC1, NaHCO3, Na2SO4, NH4C1, (NH4)2CO3,
(NH4)HCO3, H2NCOONH4 and (NH4)2SO4.
Alternatively or additionally, in some embodiments the concentrated aqueous
solution
includes an ammonium compound.
Alternatively or additionally, in some embodiments the second aqueous solution
comprises at least a portion of the bi-directional solvent and the heating
separate a third
organic phase.
Alternatively or additionally, in some embodiments separating water includes
contacting the second aqueous solution with a membrane to form separated water
and a
retentate.
Alternatively or additionally, in some embodiments the method is characterized
in that
the membrane is a reverse osmosis membrane.
Alternatively or additionally, in some embodiments the second aqueous solution
comprises at least a portion of the bi-directional solvent and the retentate
comprises a fourth
organic phase.
Alternatively or additionally, in some embodiments the method includes
recycling at
least a portion of the third organic phase or at least a portion of the fourth
organic phase to the
first contacting.
Alternatively or additionally, in some embodiments the separating water
includes
heating the second aqueous solution and contacting the second aqueous solution
with a
membrane.
Alternatively or additionally, in some embodiments the method is characterized
in that
the separated water comprises at least 60% of the water in said at least a
portion of the
wastewater stream.
Alternatively or additionally, in some embodiments P2>P3. Alternatively or
additionally, in some embodiments P 1>P4.
Alternatively or additionally, in some embodiments the bi-directional solvent
has a
greater affinity to monovalent ions compared to divalent ions; the wastewater
stream includes
5
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
at least one multivalent ion and at least one monovalent ion at a multivalent
to monovalent
ion ratio R1, the first aqueous solution includes at least one multivalent ion
and at least one
monovalent ion at a multivalent to monovalent ion ratio R2, and R2 is similar
to Rl.
Alternatively or additionally, in some embodiments the wastewater stream
includes at
least one multivalent ion and at least one monovalent ion at a multivalent to
monovalent ion
ratio R1, the concentrated aqueous solution includes at least one multivalent
ion and at least
one monovalent ion at a multivalent to monovalent ion ratio R3, and R3>R1.
Alternatively or additionally, in some embodiments both the wastewater stream
and
the concentrates aqueous solution include at least one multivalent ion and the
composition of
multivalent ions in said wastewater stream is different from the composition
of multivalent
ions in the concentrated aqueous solution.
Alternatively or additionally, in some embodiments the method includes
contacting at
least a fraction of at least one of the first organic phase and the second
organic phase with a
hydrophobic solvent having a C:0 ratio at least 2 times greater than the C:0
ratio in the bi-
directional solvent.
Alternatively or additionally, in some embodiments the one or more crude-oil-
associated hydrophobic solutes include at least one member of the group
consisting of
naphthenic acid, other organic acids comprising at least 5 carbon atoms, 1,4-
dioxane, acetone,
bromoform, dibenzo(a,h)anthracene, pyridine, phenols and oil.
Alternatively or additionally, in some embodiments the second organic phase
includes
at least 85% of the one or more crude-oil-associated hydrophobic solutes in
said wastewater
stream.
Alternatively or additionally, in some embodiments the water-depleted first
aqueous
solution comprises at least 80% of the one or more hydrophilic solutes in the
wastewater
stream.
Alternatively or additionally, in some embodiments the method includes
recycling at
least 50% of water from the wastewater stream to an industrial process
producing the
wastewater stream.
Alternatively or additionally, in some embodiments the wastewater stream
includes
blowdown of steam generator.
Alternatively or additionally, in some embodiments the method includes
softening
said wastewater stream to form a softened feed stream and feeding the softened
feed stream
to a steam generator to form steam and a blowdown stream.
6
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
Alternatively or additionally, in some embodiments the wastewater stream is
produced by an industrial process selected from the group consisting of
hydraulic fracturing
(fracking), crude oil production from oil sand, steam-assisted gravity
drainage (SAGD),
petroleum industry processing, enhanced oil recovery (EOR) and vegetable oil
production.
Alternatively or additionally, in some embodiments the wastewater stream is
produced
by an industrial process selected from the group consisting of recovering
crude oil and
processing crude oil.
Alternatively or additionally, in some embodiments the method includes
contacting
crude oil with the separated water to produce the wastewater stream.
Alternatively or additionally, in some embodiments the bi-directional solvent
includes
one or more oxygen-comprising organic molecules with 3 to 6 carbon atoms.
Alternatively or additionally, in some embodiments the bi-directional solvent
includes
one or more members of the group consisting of alcohols, ketones, esters,
phenols and
organic acids.
Alternatively or additionally, in some embodiments the bi-directional solvent
includes
one or more members of the group consisting of normal butanol, secondary
butanol,
isobutanol, tertiary butanol, normal pentanol, secondary pentanol, isopentanol
and tertiary
pentanol
Alternatively or additionally, in some embodiments the bidirectional solvent
is
selected so that the ratio of the one or more hydrophilic solutes to the one
or more crude-oil-
associated hydrophobic solutes is at least ten times higher in the water-
depleted first aqueous
solution than in the wastewater stream.
Alternatively or additionally, in some embodiments the concentration of at
least one
of the one or more crude-oil-associated hydrophobic solutes in the extractant
is at least three
times higher than the concentration of the at least one of the one or more
crude-oil-associated
hydrophobic solutes in the wastewater stream just prior to the first
contacting.
Alternatively or additionally, in some embodiments the separating at least a
portion of
the one or more crude-oil-associated hydrophobic solutes from the second
organic phase
includes evaporation.
Alternatively or additionally, in some embodiments the method includes
conducting
the first contacting, the second contacting or both in a counter current mode.
7
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
Alternatively or additionally, in some embodiments between 2 and 20 weight
units of
the bi-directional solvent are provided for each weight unit of water in the
wastewater stream
at the first contacting.
Alternatively or additionally, in some embodiments the bi-directional solvent
includes
one or more phenols.
Alternatively or additionally, in some embodiments the one or more crude-oil-
associated hydrophobic solutes include one or more oils.
In some exemplary embodiments of the invention, there is provided a system
including:
(a) a wastewater source producing a wastewater stream comprising one or more
hydrophilic solutes;
(b) an extractant source comprising an extractant including a bi-directional
solvent;
(c) a first extraction module in fluid communication with the extractant
source and
adapted to contact the extractant with at least a portion of the wastewater
stream to form a
water-depleted first aqueous solution and a water-enriched first organic
phase;
(d) a second extraction module adapted to receive the first organic phase and
contact
the first organic phase with a concentrated aqueous solution, to produce a
second organic
phase and a second aqueous solution;
(e) a separation module adapted to separate water and a solute from the second
aqueous solution; and
(f) a pump adapted to route at least a portion of the solute to the second
water
extraction module as recycled aqueous solution.
Alternatively or additionally, in some embodiments the system includes a
solvent
pump directing at least a portion of the second organic phase to the first
water extraction
module.
It will be appreciated that the various aspects described above relate to
solution of
technical problems associated with production of usable water and/or recycling
of water in an
industrial process.
Alternatively or additionally, it will be appreciated that the various aspects
described
above relate to solution of technical problems related to conservation of
energy in water
purification processes.
8
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
Unless otherwise defined, all technical and scientific terms used herein have
the same
meaning as commonly understood by one of ordinary skill in the art to which
this invention
belongs. Although suitable methods and materials are described below, methods
and materials
similar or equivalent to those described herein can be used in the practice of
the present
invention. In case of conflict, the patent specification, including
definitions, will control. All
materials, methods, and examples are illustrative only and are not intended to
be limiting.
As used herein, the terms "comprising" and "including" or grammatical variants
thereof are to be taken as specifying inclusion of the stated features,
integers, actions or
components without precluding the addition of one or more additional features,
integers,
actions, components or groups thereof This term is broader than, and includes
the terms
"consisting of' and "consisting essentially of' as defined by the Manual of
Patent
Examination Procedure of the United States Patent and Trademark Office.
The phrase "consisting essentially of' or grammatical variants thereof when
used
herein are to be taken as specifying the stated features, integers, steps or
components but do
not preclude the addition of one or more additional features, integers, steps,
components or
groups thereof but only if the additional features, integers, steps,
components or groups
thereof do not materially alter the basic and novel characteristics of the
claimed composition,
device or method.
The phrase "adapted to" as used in this specification and the accompanying
claims
imposes additional structural limitations on a previously recited component.
The term "method" refers to manners, means, techniques and procedures for
accomplishing a given task including, but not limited to, those manners,
means, techniques
and procedures either known to, or readily developed from known manners,
means,
techniques and procedures by practitioners of architecture and/or computer
science.
Percentages (%) of chemicals and/or contaminants are W/W (weight per weight)
unless otherwise indicated. Percentages of solute in solvent (solute
concentration) are W/W. In
those cases where a portion of a solute precipitates or crystallizes, the
weight of solid solute
and dissolved solute are both considered in calculating the solute
concentration.
As used herein, "a proportion of", "a concentration of" or "a ratio between"
"hydrophobic solute", "one or more hydrophobic solute", "at least one of said
one or more
hydrophobic solute", "hydrophilic solute", "one or more hydrophilic solute",
"at least one of
said one or more hydrophilic solute", "monovalent", "at least one monovalent
ion",
"multivalent", "at least one multivalent ion" and similar phrases are to be
taken as specifying
9
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
a proportion of or a concentration of at least one solute/ion, or the ratio
between concentration
of a single solute/ion and the concentration of another single solute/ion.
BRIEF DESCRIPTION OF THE DRAWINGS
In order to understand the invention and to see how it may be carried out in
practice,
embodiments will now be described, by way of non-limiting example only, with
reference to
the accompanying figures. In the figures, identical and similar structures,
elements or parts
thereof that appear in more than one figure are generally labeled with the
same or similar
references in the figures in which they appear. Dimensions of components and
features shown
in the figures are chosen primarily for convenience and clarity of
presentation and are not
necessarily to scale. The attached figures are:
Fig. 1 is a schematic flow plan of a water recovery process according to an
exemplary
embodiment of the invention depicting procedures and streams;
Fig. 2 is a schematic flow plan of a water recovery process according to an
exemplary
embodiment of the invention depicting procedures and streams;
Fig. 3 is a schematic flow plan of a water recovery process according to an
exemplary
embodiment of the invention depicting procedures and streams; and
Fig. 4 is a schematic representation of a water recovery system according to
some
exemplary embodiments of the invention.
DETAILED DESCRIPTION OF EMBODIMENTS
Embodiments of the invention relate to methods and systems for water recovery
as
well as to various streams produced by the recovery process.
Specifically, some embodiments of the invention can be used to recover
wastewater
from product process water in an industrial process.
The principles and operation of a methods and/or systems according to
exemplary
embodiments of the invention may be better understood with reference to the
drawings and
accompanying descriptions.
Before explaining at least one embodiment of the invention in detail, it is to
be
understood that the invention is not limited in its application to the details
set forth in the
following description or exemplified by the Examples. The invention is capable
of other
embodiments or of being practiced or carried out in various ways. Also, it is
to be understood
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
that the phraseology and terminology employed herein is for the purpose of
description and
should not be regarded as limiting.
Exemplary water recovery processes overview
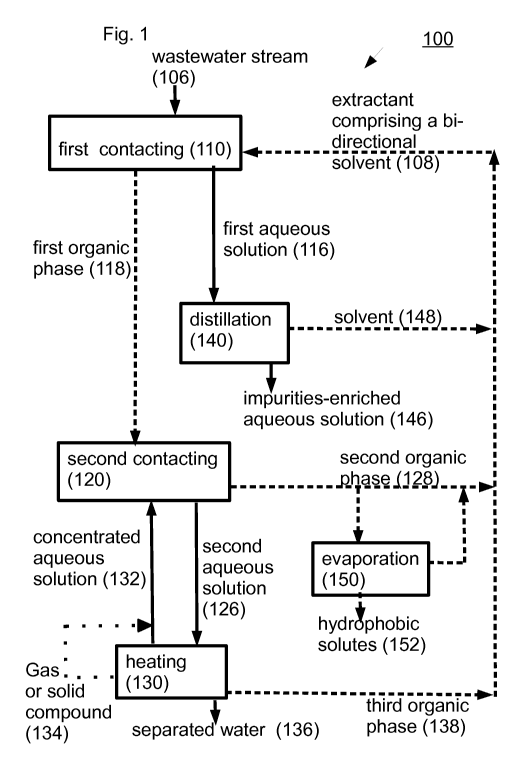
Fig. 1, Fig. 2 and Fig. 3 are a schematic flow plan of a water recovery
process or
methods according to an exemplary embodiments of the invention indicated
generally as 100,
200 or 300 respectively.
In the figures, a flow of organic phases is depicted by dashed arrows, a flow
of
aqueous solutions is depicted by solid arrows and a flow of gas or solid
compound is depicted
by dot arrows.
In the depicted exemplary embodiments, at least a portion of a wastewater
stream
containing one or more hydrophilic solutes 106 is first contacted 110 with an
extractant 108
including a bi-directional solvent to form a water-depleted first aqueous
solution 116 and a
water-enriched first organic phase 118.
In the depicted exemplary embodiments, the first organic phase 118 is second
contacted with a concentrated aqueous solution 132 to form a second organic
phase 128 and a
second aqueous solution 126.
In the depicted exemplary embodiment 100 (Fig. 1), water is separated from the
second aqueous solution 126 (e.g. by heating 130) to form a gas or solid
compounds 134 and
separated water 136.
In the depicted exemplary embodiment 100 (Fig. 1), gas or solid compounds 134
are
contacted with a third aqueous solution to form a concentrated aqueous
solution 1321. n the
depicted exemplary embodiment 200 (Fig. 2), water is separated from the second
aqueous
solution 126 (e.g. by Reverse Osmosis 230) to form a concentrated aqueous
solution 132 and
separated water 136.
In the depicted exemplary embodiment 300 (Fig. 3), gas or solid compounds 134
are
separated from the second aqueous solution 126 (e.g. by heating 330) and water
is separated
from the second aqueous solution 126 (e.g. by Reverse Osmosis 331) to form a
concentrated
aqueous solution 132 and separated water 136.
In the depicted exemplary embodiments, the concentrated aqueous solution 132
is
recycled to the second contacting 120.
In the depicted exemplary embodiments, bi-directional solvent is recycled from
the
second organic phase 128 to the first contacting 110.
11
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
In some exemplary embodiments of the invention, water partial vapor pressure
at
50 C of the wastewater stream 106, the water-depleted first aqueous solution
116, the
recycled aqueous solution 132 and the second aqueous solution 126 are P1, P2,
P3 and P4,
respectively; wherein P1>P2; P1>P3 and P4>P3. According to some
embodiments,P2>P3
and/or P1>P4.
According to some embodiments of the invention, the second contacting 120 is
conducted between concentrated aqueous solution 132 and at least a fraction of
first organic
phase 118.
In some exemplary embodiments of the invention, the wastewater stream 106
comprises one or more crude-oil-associated hydrophobic solutes. According to
various
embodiments of the invention, at least a portion of the one or more crude-oil-
associated
hydrophobic solutes is separated from a portion of the second organic phase
128 (in the
depicted exemplary embodiments, by evaporation 150). According to an
embodiment, the
separating hydrophobic solutes 152 is conducted prior to the recycling of bi-
directional
solvent from the second organic phase 128 to the first contacting 110 or
simultaneously with
it.
In some exemplary embodiments of the invention, first aqueous solution 116 is
substantially free of organic compounds (crude-oil-associated hydrophobic
solutes) other
than the bi-directional solvent. These organic compounds (if present) tend to
migrate into first
organic phase 118. As described below, additional separations by evaporation
lead to
regeneration of the bi-directional solvent and (optionally) to recovery of
desired organic
compounds.
Depicted exemplary embodiments 100, 200 and 300 employs distillation 140 to
recover bi-directional solvent 148 dissolved in first aqueous phase 116. In
other exemplary
embodiments of the invention other separation methods are employed, e.g.
salting out or
using an auxiliary solvent. The amount of solvent 148 to be distilled is
relatively small
because the majority of bi-directional solvent from extractant 108 is present
in first organic
phase 118. In some embodiments, solvent 148 distills as an azeotrope with
water. Optionally,
water in solvent 148 contributes to an increased total water yield as
extractant stream 108 is
recycled.
In the depicted exemplary embodiments, distillation 140 also produces an
impurities-
enriched aqueous solution 146. According to an embodiment, said impurities-
enriched
solution is characterized by water partial vapor pressure at 50 C of P5 and P5
> P1.
12
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
According to various embodiments, said impurities-enriched solution is
disposed as such or
after further treatment. According to various embodiments, such further
treatment comprises
at least one of further concentration, precipitation of at least one component
and addition of a
chemical compound. According to various embodiments the flow rate of said
wastewater is
Fl, the flow rate of said impurities-enriched solution is F2 and Fl/F2 is
greater than 2, 4, 6, 8,
or intermediate of greater ratio.
In the depicted exemplary embodiments, second organic phase 128 (containing
some
water) is recycled to extractant stream 108 without further separation of
water.
Separated water 136 is the primary product of methods 100, 200 and 300. In
some
10 exemplary embodiments of the invention, the amounts of bi-directional
solvent and/or
hydrophilic solutes and/or hydrophobic solutes in separated water 136 are
sufficiently low at
this stage that it can serve as feed water to an industrial process and/or
agricultural irrigation
water and/or potable water.
In some exemplary embodiments of the invention, wastewater stream 106 contains
one or more crude-oil-associated hydrophobic solutes. These hydrophobic
solutes migrate to
the bi-directional solvent and will tend to accumulate there if not removed.
In the depicted
exemplary embodiments, evaporation 150 is depicted as separating at least a
portion of the
one or more hydrophobic solutes 152 from second organic phase 128 prior to the
contacting
with wastewater stream 106. In some embodiments, hydrophobic solutes 152
include organic
acids (e.g. naphthenic acid).
According to various embodiments, water separating from second aqueous
solution
126 includes at least one of Heating, Evaporation, Reverse Osmosis, Forward
Osmosis,
Electrodialysis and contacting with a solvent.
In some exemplary embodiments 200 (Fig. 2) and 300 (Fig. 3) of the invention,
the
separating of water from second aqueous solution 126 includes contacting the
second
aqueous solution 126 with a membrane 230 (Fig. 2) or 331 (Fig. 3) to form a
separated water
136 and a retentate which includes the concentrated aqueous solution 132. In
the depicted
exemplary embodiments, the membrane is a reverse osmosis membrane (RO). In
other
exemplary embodiments, the membrane is a nano-filtration membrane.
According to some embodiments, second aqueous phase 126 includes the bi-
directional solvent. The concentration of the bi-directional solvent in second
aqueous phase
126 is a function of hydrophilic solutes (e.g. salts) concentration there.
According to some
embodiments 200 (Fig. 2) and 300 (Fig. 3), the bi-directional solvent is at
least partially
13
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
removed from the second aqueous solution 126 prior to the contacting with the
membrane
(depicted as separation membrane 230(Fig. 2), or 331 (Fig. 3)), e.g. by
distillation.
Alternatively or additionally, according to some embodiments the bi-
directional solvent is
separated by the contacting with a membrane (e.g. separation membrane 230(Fig.
2), or 331
(Fig. 3)). According to an embodiment, the bi-directional solvent is rejected
by the membrane
and is retained in the retentate along with the concentrated aqueous solution.
According to an
embodiment, said concentrated aqueous solution 132 is of reduced volume and
higher salt
concentration compared to said second aqueous solution. As a result, the
amount of bi-
directional solvent dissolved in it is small compared with the amount
dissolved in said second
aqueous solution and the vast majority of the bi-directional solvent is
rejected to a third
organic phase 138, which is formed in the retentate.
In some embodiments, at least a portion of the third organic phase 138 is
recycled as
bi-directional solvent to the first contacting 110. According to various
embodiments, said
third organic phase 138 is combined with said second organic phase 128 or
introduced
separately to said first contacting, e.g. at a point closer to the exit of
said first aqueous
solution 116.
In some embodiments, the retentate is included in the concentrated aqueous
solution
which is at least partially recycled to the second contacting 120 as
concentrated aqueous
solution 132. According to a related embodiment, the recycling to the second
contacting 120
is conducted without prior separation of dissolved bi-directional solvent.
In some embodiments, the separated water 136 comprises at least 60%, 70%, 80%,
85%, 90% or at least 95% of the water in the wastewater stream 106.
According to various embodiments, the third organic phase 138 includes the bi-
directional solvent and water. According to an embodiment, the third organic
phase 138 could
be recycled as such to the first contacting in 110.
According to various embodiments, water extraction (first contacting 110) is
selective
to water over ions. Selectivity is particularly high compared to extraction of
divalent ions,
including ones contributing to hardness and scale.
According to some embodiments, wastewater stream 106 includes at least one
multivalent ion and at least one monovalent ion at a multivalent to monovalent
ratio R1, the
first aqueous solution 116 includes at least one multivalent ion and at least
one monovalent
ion at a multivalent to monovalent ratio R2, and R2 is similar to R1 .
According to some
14
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
embodiments, R2/R1 is in the range between 0.75 and 1.25, between 0.8 and 1.2,
between
0.85 and 1.15 or between 0.9 and 1.1.
According to some embodiments, the concentrated aqueous solution 132 includes
at
least one multivalent ion and at least one monovalent ion at a multivalent to
monovalent ratio
R3, and R3>R1. According to some embodiments, R3/R1 is greater than 2, 4, 6, 8
or greater
than 10.
According to some embodiments, both the wastewater stream 106 and the
concentrated aqueous solution 132 include at least one multivalent ion, and
the composition
of multivalent ions in the wastewater stream 106 is different from the
composition of
multivalent ions in the concentrated aqueous solution 132. According to
various
embodiments, one of these solutions (132 and 106) contains at least one
multivalent ions that
do not exist in the other. Alternatively or additionally, in some embodiments
the
concentration of a given multivalent ion in one of these solutions is
different from the
concentration of the same multivalent ion in the other.
Exemplary water separation method
In some exemplary embodiments 100 (Fig. 1) and 300 (fig. 3) of the invention,
the
concentrated aqueous solution 132 contains at least one member of: NH3, CO,
CO2, CaC12,
Ca(NO2)3, KBr, KC1, KHCO3, K2SO4, MgC12, MgSO4, NaC1, NaHCO3, Na2SO4, NH4C1,
(NH4)2CO3, (NH4)HCO3, H2NCOONH4 and (NH4)2SO4. At the second contacting 120
said
concentrated aqueous solution 132 and said first organic phase are contact,
generating second
organic phase 128 and second aqueous solution 126.
According to exemplary embodiment 100 (Fig. 1) The heating 130 of the second
aqueous solution 126 generate the following streams; third organic phase 138,
solid or gas
compounds 134, a third aqueous solution and separated water 136. Contacting
said third
aqueous solution with said solid or gas compound reforms said concentrated
aqueous solution
132.
According to exemplary embodiment 300 (Fig. 3) the secondary aqueous solution
126
is heated 330 to separate a solid and/or gas compound 134. According to
exemplary
embodiments, the separated compound is selected from a group consisting of
NH3, CO, CO2,
CaC12, Ca(NO2)3, KBr, KC1, KHCO3, K2SO4, MgC12, MgSO4, NaC1, NaHCO3, Na2SO4,
NH4C1, (NH4)2CO3, (NH4)HCO3, H2NCOONH4 and (NH4)2SO4. The formed aqueous
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
solution is contacted with a membrane, e.g. a reverse osmosis membrane, to
form a retentate,
separated water 136 and a fourth organic phase.
According to an exemplary embodiment, said separated solid and/or gaseous
compound is contacted with said retentate to reform said concentrated aqueous
solution 132.
According to some embodiments the concentrated aqueous solution 132 comprises
an
ammonium compound. The ammonium compound includes at least one of ammonium
bicarbonate, ammonium carbonate, and ammonium carbamate.
Exemplary water recovery method
Alternatively or additionally, in some embodiments, methods 100, 200 and 300
includes contacting (not depicted) at least a fraction of at least one of
first organic phase 118
and second organic phase 128 with a hydrophobic solvent, characterized in that
C:0 ratio in
the hydrophobic solvent is at least 2 times greater than that ratio in the bi-
directional solvent.
According to these embodiments, the contacting induces water rejection from
the first organic
phase 118 and/or the second organic phase 128. According to various
embodiments, after
separating the rejected water, the hydrophobic solvent is separated (e.g. by
distillation of one
of the two) from the bi-directional solvent in first organic phase 118 and/or
second organic
phase 128 before the solvent is reused in the contacting 120 and/or 110,
respectively.
Various exemplary embodiments
According to various exemplary embodiments of the invention crude-oil-
associated
hydrophobic solutes 152 include naphthenic acid and/or other organic acids
comprising at
least 5 carbons, and/or 1,4-dioxane, and/or acetone, and/or bromoform, and/or
dibenzo(a,h)anthracene, and/or pyridine, and/or phenols and/or oil (e.g.
fossil oil, vegetable
oil). According to some embodiments, in addition to soluble crude-oil-
associated
hydrophobic matter, there could be suspended crude-oil-associated hydrophobic
matter.
Therefore, the amount of the crude-oil-associated hydrophobic matter in 106
may be greater
than saturation concentration.
According to some embodiments, one or more of the crude-oil-associated
hydrophobic solutes is less volatile than water, and is difficult to separate
from the
wastewater stream 106 by known methods, such as evaporation. According to some
embodiments of the invention, such solutes are efficiently removed at low
cost, optionally
without their evaporation.
16
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
In some embodiments, second organic phase 128 includes at least 85%, at least
90%,
at least 95%, at least 97.5% or at least 99% of the at least one of the one or
more crude-oil-
associated hydrophobic solutes which were present in the wastewater 106.
Alternatively or
additionally, in some embodiments water-depleted first aqueous solution 116
includes at least
80%, at least 85%, at least 90%, at least 95%, at least 97.5% or at least 99%
of the at least
one of the one or more hydrophilic solutes (i.e. in case of multiple solutes,
this could be true
for one of the solutes in some embodiments and more than one of them in other
embodiments) in the wastewater stream 106.
In some exemplary embodiments of the invention, the method includes recycling
at
least 50%, at least 55%, at least 65%, at least 70%, at least 75%, at least
80%, at least 85%, at
least 90% or at least 95% of water from the wastewater stream 106 to an
industrial process
producing the wastewater stream. According to some embodiments, the recycled
water is
derived from the second aqueous solution 126. According to other embodiments,
the recycled
water includes the separated water 136 from the heating 130 (Fig. 1) or
Separation membrane
230 (Fig. 2) or 331 (fig.3). According to some embodiments, the industrial
process generates
different "product process water" stream (i.e. wastewater stream) and/or
consumes
water/aqueous solutions in multiple steps. According to some embodiments, the
recycled
water results from any stream and is used in any step. According to some
embodiments, the
recycled water is at high quality. According to some exemplary embodiments,
the recycled
water is at quality as required for steam production (including steam required
for stripping
solvent from exiting streams). Alternatively or additionally, according to
some embodiments,
the water derived from the second aqueous solution 126 and/or from separated
water 136 has
alternative outlets (e.g. irrigation, emission to rivers and sewage).
According to various exemplary embodiments of the invention the wastewater
stream
106 is produced by an industrial process selected from the group consisting of
induced
hydraulic fracturing (fracking), Steam Assisted Gravity drainage (SAGD), crude
oil
production from oil sand, petroleum industry processing, enhanced oil recovery
(EOR) and
vegetable oil production. In some exemplary embodiments of the invention,
wastewater
stream 106 is produced by an industrial process selected from the group
consisting of
recovering crude oil, recovering gas, and processing crude oil.
In some exemplary embodiments of the invention, methods 100, 200 and 300
includes
contacting crude oil with separated water 136 derived from second aqueous
solution 126 to
produce the wastewater stream 106.
17
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
According to various exemplary embodiments of the invention, the bi-
directional
solvent in extractant 108 includes one or more organic molecules with 3 to 6
carbon atoms. In
some embodiments, the organic molecules include alcohols and/or ketones and/or
esters
and/or organic acids. In some embodiments, the bi-directional solvent in
extractant 108
includes a butanol (e.g. n-butanol or isobutanol). Alternatively or
additionally, in some
embodiments the bi-directional solvent in extractant 108 comprises one or more
amines.
According to some embodiments, the one or more amines include one or more
members of
the group consisting of diethylamine, triethylamine, 1-methyl piperidine, 4-
methyl piperidine
di-isopropylamine, N,N-dietheylmethylamine, dimethylisopropylamine,
ethylisopropylamine,
methylethylisopropylamine, methylethyl-n-propylamine, dimethyl-secondary-
butylamine,
dimethyl-tertiary-butylamine, dimethylisobutylamine,
dimethyl-n-butylamine,
methyldiethylamine, dimethylallylamine, dimethyl-n-propylamine,
diisopropylamine, di-n-
propyl amine, di-allylamine, n-methyl-n-amylamine, n-ethyl-n-butylamine, n-
ethyl-sec-
butylamine, n-ethyl-tertiary-butylamine, n-ethyl-n-pro pylamine, n-ethyl-
isopropylamine, n-
methyl-n-butylamine, n-methyl-sec-butylamine, n-methyl-iso-butylamine, n-
methyl-tertiary
butylamine,dimethyl, 1,1-dimethylpropylamine and dimethyl, 1-methyl
butylamine.
In some exemplary embodiments of the invention, a single amine is employed. In
other exemplary embodiments of the invention, a combination of two or more
amines is
employed. Alternatively or additionally, amines are used in combination with
non-amine
molecules in some embodiments of the invention.
In some exemplary embodiments of the invention, the ratio of at least one of
the
hydrophilic solutes to at least one of the crude-oil-associated hydrophobic
solutes is at least
ten times higher (this ratio does not necessarily apply to the ratio between
total hydrophilic
solutes and total hydrophobic solutes) in the water-depleted first aqueous
solution 116 than in
the wastewater stream 106. Alternatively or additionally, in some embodiments
of the
invention, the concentration of at least one of the one or more crude-oil-
associated
hydrophobic solutes in extractant 108 is at least three times higher than the
concentration of
the at least one of the one or more crude-oil-associated hydrophobic solutes
in the wastewater
stream 106 just prior to first contacting 110 (this ratio does not necessarily
apply to the total
hydrophobic solutes).
Again, in the depicted exemplary embodiment, separating at least a portion of
the one
or more crude-oil-associated hydrophobic solutes 152 from second organic phase
128
includes evaporation 150. In some exemplary embodiments of the invention,
evaporation 150
18
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
includes distillation of the solvent from solutes 152. According to some
embodiments, the
hydrophobic solute is more volatile than the bi-directional solvent. In that
case, the solute is
evaporated out. In other cases, the opposite is true and the bi-directional
solvent is
evaporated. Still there could be both solutes that are more volatile than the
bi-directional
solvent and ones that are less volatile. In such cases, the more volatile are
evaporated first and
then the bi-directional solvent is evaporated. According to an embodiment,
only a small
fraction of the second organic phase 128 is treated for separation of the
hydrophobic solutes
152, e.g. less than 20% of it, less than 15%, less than 10%, or less than 5%.
In some embodiments the one or more crude-oil-associated hydrophobic solutes
include one or more phenols. Alternatively or additionally, in some
embodiments the one or
more crude-oil-associated hydrophobic solutes include one or more oils (e.g.
fossil oil,
vegetable oil).
In some embodiments, the method includes conducting first contacting 110
and/or
second contacting 120 in a counter current mode. According to some
embodiments, the
contacting 110 and/or 120 is conducted in 2-20 stages, 3-15 stages, 4-12
stages or 5-10
stages.
Alternatively or additionally, in some embodiments of methods 100, 200 and 300
the
weight/weight ratio between the amount of bi-directional solvent in stream 108
and the
amount of water in stream 106 is in a range between 2:1 and 20:1, between 3:1
to 17:1,
between 6:1 to 15:1, between 2:1 and 12:1, between 3:1 and 11:1, between 4:1
and 10:1 or in
a range between 8:1 to 12:1. According to some embodiments, first contacting
110 is
conducted in a continuous mode and this ratio is between the weight fluxes of
streams instead
of the amounts.
In some embodiments, stream 106 contains suspended solids. These solids can
include, but are not limited to sand or soil particles. According to various
embodiments, these
solids are removed prior to the first contacting 110. According to various
exemplary
embodiments of the invention solids removal module includes a settling taffl(
and/or filtration
equipment and/or centrifugation equipment (e.g. a flow through centrifuge
and/or a cyclonic
separator). In some embodiments, removal of solids contributes to mechanical
efficiency of
downstream processes.
Alternatively or additionally, in some embodiments stream 106 contains one or
more
dissolved surfactants (e.g. soaps and/or detergents). According to various
embodiments, at
least one of the one or more surfactants is removed from and/or inactivated in
at least a
19
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
portion of stream 106 prior to first contacting 110. In some embodiments, a
surfactant
removal and/or inactivation module is positioned upstream of the first
contacting 110 to
reduce activity of surfactants present in stream 106. According to various
exemplary
embodiments of the invention the surfactant removal and/or inactivation module
employs
surface active material (e.g. activated charcoal) and/or pH adjustment and/or
addition of
multivalent ions.
In some exemplary embodiments of the invention, the surfactant removal and/or
inactivation module contributes to an efficiency of separation of first
aqueous solution 116
from first organic phase 118 and/or to an efficiency of separation of second
aqueous solution
126 from second organic phase 128.
Exemplary wastewater compositions
In some exemplary embodiments of the invention, wastewater stream 106 contains
at
least 10,000 PPM; at least 20,000 PPM; at least 30,000 PPM or at least 40,000
PPM of total
dissolved solids (TDS). In other exemplary embodiments of the invention,
stream 106
contains less than 100,000 PPM, less than 90,000 PPM, less than 80,000 PPM,
less than
70,000 PPM or less than 50,000 PPM of total dissolved solids (TDS).
In various exemplary embodiments of the invention, total dissolved solids
(TDS) in
said wastewater stream 106 is less than 10,000 ppm; less than 8,000 ppm; less
than 6,000
ppm; less than 4,000 ppm or less than 2,000 ppm. Wastewater stream with these
relatively
low levels of TDS is produced, for example, in cooling towers and/or in the
oil industry.
Alternatively or additionally, in some embodiments the TDS includes barium
and/or
strontium and/or iron and/or other heavy metals and/or radioactive isotopes
and/or cyanides
and/or thiocyanates and/or salts of ammonia and/or sulfides and/or sulfates
and/or calcium
salts and/or silica.
Exemplary extraction conditions
Various exemplary embodiments of the invention described herein relate to
extraction
(110) of water into an extractant comprising bi-directional solvent or back-
extraction (120) of
water from such extractant. According to various embodiments, at least one of
such
extraction and back-extraction is conducted by contacting in a multiple step,
counter-current
operation. According to various embodiments, such contacting is conducted in
industrially
used contactors, e.g. mixer-settlers, extraction columns, centrifugal
contactors and raining-
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
bucket contactor. According to an embodiment, the wastewater comprises
suspended solids
and/or solids are formed during said first contacting and the used contactor
is designed to
handle such solids.
Exemplary optional treatment of said first organic phase
In some exemplary embodiments of the invention, first organic phase 118 is
treated
prior to said second contacting, e.g. by adding an organic solvent or
contacting with an
aqueous solution. According to another embodiment, first organic phase 118
comprises
suspended solids and said treating prior to said second contacting comprises
separating such
suspended solids, e.g. via extended settling or addition of a coagulant.
Exemplary solvent considerations
According to various exemplary embodiments of the invention the bi-directional
solvent employed in extractant stream 108 is selected based upon the total
dissolved solids
(TDS) content of stream 106 and/or the organic compounds (e.g. hydrophobic
solutes)
content of stream 106 and the cost of available energy.
Exemplary advantages
A known method for treating wastewater involves evaporation of the water.
Energy
consumption is high due to the required input of latent heat. Major efforts
are directed to
developing alternatives based on membrane separation (e.g. Reverse Osmosis).
Those require
several pretreatments (e.g. filtration, adsorption, coagulation and softening)
in order to protect
the membrane. These pretreatments substantially increase the cost of the
membranes-based
separation.
One exemplary advantage of some embodiments of the invention is that water is
separated by the extraction with a bi-directional solvent and recovered from
the formed
organic phase without the input of latent heat.
Alternatively or additionally, another exemplary advantage of some embodiments
of
the invention is that the separation membrane 230 (Fig. 2) and 331 (Fig.3) is
not directly
contacted with the wastewater stream; therefore less pretreatment stages are
required.
Alternatively or additionally, those portions of the process that optionally
employ
latent heat (e.g. distillation 140) are applied to smaller portions of the
total mass in the system
21
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
and directed to evaporation of relatively low latent heat solvent, resulting
in significant energy
savings.
Alternatively or additionally, exemplary methods 100, 200 and 300 achieves
efficient
separation of usable water (separated water 136) from the wastewater (106)
forming a
reduced-volume, impurities-concentrated stream (impurities-enriched aqueous
solution 146),
thereby reducing the volume of wastewater to disposal.
Alternatively or additionally, exemplary methods 100, 200 and 300 achieves
good
separation of organic matter (hydrophobic solutes 152), which can be used for
energy or more
specific application.
Alternatively or additionally, exemplary methods 100,200 and 300 results in a
high
quality separated water 136, which may be used e.g. for steam, in a relatively
low costs
compared to alternative treatments.
Alternatively or additionally, exemplary methods described herein are more
suitable
for use in handling hard water (at 106) than previously available
alternatives.
Alternatively or additionally, exemplary methods described herein contribute
to a
reduction in use of chemical reagents.
Alternatively or additionally, exemplary methods described herein are amenable
to
integration with other methods, e.g. gravity separation devices such as the
API (American
Petroleum Institute) oil-water separator.
Exemplary system
Fig. 4 is a schematic representation of a water recovery system indicated
generally as
400. In the figure, a flow of organic phases is depicted by dashed arrows, and
a flow of
aqueous solutions is depicted by solid arrows. Numbers which appear in Fig. 1,
2 and 3 and
are used in Fig. 4 to indicate flows similar to those described above.
Depicted exemplary system 400 includes a first water extraction module 410
adapted
to contact an extractant comprising a bi-directional solvent 108 with at least
a portion of a
wastewater stream including one or more hydrophilic solutes 106 to form a
water-depleted
first aqueous solution 116 and a water-enriched first organic phase 118.
In the depicted exemplary embodiment, system 400 includes a second water
extraction module 420 adapted to contact the first organic phase 118 with a
concentrated
aqueous solution 132, to produce a second organic phase 128 and a second
aqueous solution
126.
22
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
Depicted exemplary system 400 also includes a separation module 430 adapted to
separate a concentrated aqueous solution 132, third organic phase 138 and
separated water
136 from the second aqueous solution 126.
According to various embodiments of the invention, the first contacting 110 at
the
first water extraction module 410 occurs at a first temperature (Ti) and the
second contacting
120 at the second water extraction module 420 occurs at a second temperature
(T2).
According to some embodiments T2 is similar to Ti. According to other
embodiments, T2 is
different than Ti.
According to various embodiments of the invention, system 400 is configured to
allow recycling of at least a portion of the concentrated aqueous solution to
the second water
extraction module 420. In the depicted exemplary system, at least a portion of
the
concentrated aqueous solution 132 is recycled to the second water extraction
module 420.
According to various embodiments of the invention, system 400 is configured to
allow recycling of at least a portion of the second organic phase 128 as bi-
directional solvent
to the first water extraction module 410.
According to some embodiments of the invention, the separation module
comprises a
membrane adapted to form separated water 136 and a retentate in a retentate
compartment
and the retentate is included in the concentrated aqueous solution 132. In the
depicted
exemplary system, the membrane is a Reverse Osmosis membrane.
According to some embodiments of the invention, the system is characterized in
that
the second aqueous solution 126 includes the bi-directional solvent and in
that the retentate
compartment is adapted to include a third organic phase 138.
According to various embodiments of the invention, system 400 is configured to
allow recycling of at least a portion of the third organic phase 138 as bi-
directional solvent to
the first water extraction module 410.
According to various embodiments of the invention, system 400 is characterized
in
being portable. According to some embodiments, system 400 is mobile, moveable,
and can be
transported from one place to another (e.g. from one shale oil play to
another). According to
an embodiment, system 400 is skid mounted.
Exemplary use scenario I: Induced hydraulic fracturing (fracking)
A typical fracking well requires between 4,000 m3 and over 22,000 m3 of water.
Waste
water produced by fracking contains hydrophilic solutes including but not
limited to sodium,
23
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
magnesium and calcium salts, barium, strontium, iron, other heavy metals and
radioactive
isotopes. Total dissolved solids (TDS) are typically in the range of 5,000 PPM
to 100,000
PPM or more.
Waste water produced by fracking also contains hydrophobic materials such as
oil.
Referring again to Figs. 1, 2, 3 and 4: in some exemplary embodiments of the
invention, fracking serves as industrial process and flowback and/or produced
water serve as
wastewater stream 106.
During water recovery processes 100, 200 and 300 the bulk of the hydrophilic
solutes
will separate into first aqueous solution 116 and according to some
embodiments, be removed
from the system at 146 as described in detail hereinabove.
The hydrophobic solutes are selectively and efficiently extracted into the
first organic
phase 118 in the first contacting 110. The hydrophobic solutes remain
practically fully in the
extractant during the second contacting, i.e. in the second organic phase 128.
In the depicted
exemplary embodiments of Fig. 1, 2 and 3 a fraction of the hydrophobic solutes
arrive at
evaporation 150 and is at least partially removed from the system at 152.
Separated water 136
becomes feed process water to the industrial process and can be used as part
of input water for
a subsequent round of fracking.
In some cases, waste water produced by fracking contains a surfactant.
Optionally,
surfactant is removed prior to introduction into methods 100, 200 and 300. In
some exemplary
embodiments of the invention, removal of surfactant contributes to a more
efficient
partitioning between organic phases and aqueous solutions throughout the
process.
Exemplary use scenario II: Synthetic Crude Oil from Oil Sands
Production of a barrel of synthetic crude oil from oil sand requires about 2
to 4.5
barrels of fresh water as an input. In the conventional subterranean process,
this water is
applied as steam to oil sand in a well. In the Surface mining, the oil sand is
removed from the
well and then the water is applied.
In the Steam Assisted Gravity Drainage (SAGD) process two horizontal wells are
drilled in the oil sands, one at the bottom of the formation and another about
5 metres above
it. These wells are typically drilled in groups off central pads and can
extend for miles in all
directions. In each well pair, steam is injected into the upper well, the heat
melts the bitumen,
which allows it to flow into the lower well, where it is pumped to the
surface.
24
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
The steam for the SAGD process can be generated by a once-through steam
generator
(OTSG). The feed for the OTSG can comprise produced water and optionally also
make-up
water. The OTSG generate a high quality steam for the well injection and a
blowdown stream
that can contain dissolve solids, the blowdown stream needs treatment. The
produced
Synthetic Crude Oil contain water (produces water) that are separated during
the processing
of the Synthetic Crude Oil, Separated water is recycled to the steam
generator.
During the Synthetic Crude Oil production portion of the injected steam
remains in
the ground formation and does not return as produce water. To stand the amount
of steam
required to the Synthetic Crude Oil production make-up water is used in
addition to the
recycled separated produce water.
Make-up water is provided from natural sources as rivers or underground wells
in
some cases the make-up water contains inorganic salts (hydrophilic solutes).
Removal of the inorganic salts and organic acids prior to the OTSG can
decrease the
inorganic salts and organic acids percentage in the blewdown water.
In some exemplary embodiments of the invention produce water (with or without
mixing with make-up water) and blowdown water are wastewater produced during
production of synthetic crude oil.
Waste water produced during production of synthetic crude oil contains
inorganic
salts (hydrophilic solutes), and organic acids (hydrophobic solutes).
Referring again to Figs. 1, 2, 3 and 4: in some exemplary embodiments of the
invention, production of synthetic crude oil serves as industrial process and
wastewater
produced during production of synthetic crude oil serves as wastewater stream
106.
During water recovery process 100, 200 or 300 the bulk of the hydrophilic
inorganic
salts will separate into first aqueous solution 116 and according to some
embodiments, be
removed from the system at 146 as described in detail hereinabove.
The hydrophobic solutes (organic acids) are selectively and efficiently
extracted into
the first organic phase 118 in the first contacting 110. The hydrophobic
solutes remain
practically fully in the extractant during the second contacting, i.e. in the
second organic
phase 128. In the depicted exemplary embodiment of Figs. 1,2 or 3 a fraction
of the
hydrophobic solutes arrive at evaporation 150 and is at least partially
removed from the
system at 152. Separated water (depicted as permeate 136) becomes feed process
water to the
industrial process and can be used as part of input water for a subsequent
round of production
of synthetic crude oil.
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
Exemplary use scenario III: Cooling Water
In Israel water-cooled condensers are estimated to consume some 130 million M3
of
water each year and discharge 35 million M3 ofbrines each year. The brines
contain about 5.6
tons of chlorides/ M3 and about tons of 2.6 tons of sodium/ M3.
Since water-cooled condensers are widely used in large public institutions
throughout
the country, it is estimated that about 50 million M3 of water are consumed
each year for air
conditioning alone.
Even larger amounts of cooling water are used in an industrial context. As an
example, a single refinery can require about 350 M3 /hour of cooling water. Of
this amount,
about 60 to 80% is lost to evaporation in cooling towers and the remaining 20
to 40% is
recovered as cooled water which is, at least theoretically, available for
recycling. Because
minerals do not evaporate, salts are concentrated in the cooling tower by a
factor of about 2.5
to 5.
This means that recycling of cooled water without treatment to remove
dissolved
minerals will cause an increase in the mineral concentration in water
circulating in the
cooling system over time.
Referring again to Figs. 1,2,3 and 4: in some exemplary embodiments of the
invention,
cooling in a cooling tower serves as industrial process and the cooled water
serves as
wastewater stream 106.
During water recovery process 100, the bulk of the hydrophilic inorganic salts
will
separate into first aqueous solution 116 and according to some embodiments, be
removed
from the system at 146 as described in detail hereinabove. Separated water 136
becomes feed
process water and can be used as part of input water for a subsequent round of
cooling.
Water recovery processes 100, 200 and 300are suitable to treat wastewater
stream 106
from the oil industry (e.g. refineries) and cooling towers from various
industries. In some
cases, an oil refinery includes one or more cooling towers so that there are
multiple sources of
wastewater. According to various exemplary embodiments of the invention these
multiple
sources of wastewater are treated according to method 100 either separately or
in combination
with one another.
Exemplary use scenario IV: Effluents from Petroleum Industry processing
In a petroleum refinery, processing includes various treatments (e.g.
cracking, which is
the process in which heavy hydrocarbons are broken down to lighter
hydrocarbons). These
26
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
processing treatments produce wastewater streams including hydrophilic
solutes. These
hydrophilic solutes can include, but are not limited to cyanide salts,
thiocyanate salts, salts of
ammonia and sulfides (e.g. H2S). In addition the waste can include hydrophobic
solutes such
as oils and/or phenols. The phenols can include the monohydrics (having one
hydroxyl group)
such as phenol; o-, m-, and p-cresols, the various xylenols, and the various
ethylphenols. The
phenols may also include polyhydrics (having two or more hydroxyl groups) such
as catechol
and resorcinol which are C6H4(OH)2 isomers. Alternatively or additionally, the
phenols may
include thiophenols such as benzenethiol (or phenyl mercaptan) which is C6H5SH
and
toluenethiols (or tolyl mercaptans) which are CH3C6H4SH isomers.
For example, petroleum industry processing wastewater stream can include < 50
mg
cyanides or thiocyanates and/or? 500 mg/L ammonia or ammonium salts and/or?
500 mg/L
sulfides as hydrophilic solutes. The same stream may also include 50 to 500
mg/L of phenols
and/or 50 to 500 mg/L of oils as hydrophobic solutes.
Referring again to Figs. 1,2,3 and 4: in some exemplary embodiments of the
invention,
petroleum industry processing serves as industrial process and wastewater
produced during
the processing serves as wastewater stream 106.
During water recovery processes 100, 200 and 300 the bulk of the hydrophilic
inorganic salts will separate into first aqueous solution 116 and according to
some
embodiments, be removed from the system at 146 as described in detail
hereinabove.
The hydrophobic solutes (phenols and/or oils) are selectively and efficiently
extracted
into the first organic phase 118 in the first contacting 110. The hydrophobic
solutes remain
practically fully in the extractant during the second contacting, i.e. in the
second organic phase
128. In the depicted exemplary embodiment of Fig. 1, a fraction of the
hydrophobic solutes
arrive at evaporation 150 and is at least partially removed from the system at
152. Separated
water 136 becomes feed process water and can be used as part of input water
for a subsequent
round of any of the processing treatments.
Exemplary use scenario V: Enhanced oil recovery (EOR)
The EOR process is similar production of oil from oil sand (scenario II above)
in that
it involves pumping water down into a well. In EOR liquid water penetrates oil
in the bottom
of the well and accumulates underneath the oil. As the water accumulates it
raises the oil until
the oil reaches a level at which it can be pumped from the well. The oil
pumped from the well
using EOR contains about 20 to 30% water carrying a high concentration of
salts which can
27
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
contain metals and/or radioisotopes. In order to re-use this water it must be
separated from
the oil and the salt concentration must be reduced.
Referring again to Figs. 1,2,3 and 4: in some exemplary embodiments of the
invention,
EOR serves as industrial process and water separated from recovered crude oil
serves as
wastewater stream 106.
During water recovery processes 100, 200 and 300, the bulk of the hydrophilic
inorganic salts, metals and radioisotopes will separate into first aqueous
solution 116 and
according to some embodiments, be removed from the system at 146 as described
in detail
hereinabove.
The hydrophobic solutes (suspended oil droplets) are selectively and
efficiently
extracted into the first organic phase 118 in the first contacting 110. The
hydrophobic solutes
remain practically fully in the extractant during the second contacting, i.e.
in the second
organic phase 128. In the depicted exemplary embodiment of Fig. 1, 2 and 3, a
fraction of the
hydrophobic solutes arrive at evaporation 150 and is at least partially
removed from the
system at 152. Separated water 136 becomes feed process water and can be used
as part of
input water for a subsequent round of EOR.
EXAMPLES
Example 1: Water extraction from a waste stream using recycled n-butanol
extractant
A flowback waste stream was contacted (extracted) with recycled n-butanol (the
bidirectional solvent). The waste stream (Aqueous Feed to extraction)
contained 3% total
dissolved solutes (TDS), mainly salts (hydrophilic solutes), and about 200ppm
oil-related
organic matter (crude-oil-associated hydrophobic solutes). The recycled
(regenerated) n-
butanol (Extractant) contained initially about 11.5% water. The bench scale
extraction was
conducted at 35 C and simulated counter-currently extraction of 8 stages.
Water transferred
from the waste stream to the Extractant. The Extractant to Aqueous Feed (0/A)
weight/weight ratio was 11. The formed organic phases and aqueous phases were
analyzed to
determine the time when their composition has reached a steady state. The
steady state
organic phase (Extract) and the steady state aqueous phase (Raffinate) were
analyzed.
The TDS of the Raffinate was 12%. Its n-butanol concentration was 2.7% and the
concentration of oil-related organic matter was less than 20ppm. The water
content if the
Extract was 17. These analyses indicate that about 75% of the water and
essentially all the
oil-related organic matter initially present in the waste stream got extracted
into the n-
28
CA 02899377 2015-07-27
WO 2014/114996 PCT/1B2013/060283
butanol. The formed Raffinate is the water-depleted first aqueous solution and
the formed
extract is the water-enriched first organic phase.
Examples 2-6: Water extraction from various waste stream using recycled n-
butanol
extractants
Additional waste stream of varying compositions were extracted with recycled n-
butanol (Extractants) of varying initial water content. The procedure was
similar to that in
Example 1 and the results are summarized in Table 1.
TABLE 1
Extractant Extract Water
Waste 0/A
waterRaffinate water extraction
content
stream (weight/
TDS (%) content yield (%)
TDS (%) weight)
(%) (%) [1]
Example 2 1 8.2 7 20 18.5 95
Example 3 1 11.4 11 12 18.5 90
Example 4 3 12.2 12 10 17 70
Example 5 3 7 7 23.5 17 85
Example 6 10 7 9 23.5 12 55
[1] Calculated as the fraction of water in the waste stream that got extracted
into the Extract.
Examples 7-11: Water extraction from a waste stream using various recycled
extractants
Waste stream of various initial TDS were extracted with various recycled
Extractants.
The procedure was similar to that in Example 1 and the results are summarized
in Table 2.
TABLE 2
waste extractant Extract
0/A raffinate Water
stream waterwater
extractant (weight/ TDS extraction
TDS contentcontent
(%) (%) .
weight) (% (%)
) yield [1]
Example
Sec-BuOH 3 11 5 12 22.5 75
7
Example Tert-butyl
8.5 10.2 2 23 31 68
8 alcohol
Example Tert-amyl 5
9.8 9 20 17.3 75
9 alcohol
Example
Phenol 5 17 7 20 25 75
Methyl-
Example
ethyl 1 6.6 16 11 11.4 90
11
ketone
[1] Calculated as the fraction of water in the waste stream that got extracted
into the Extract.
29
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
Example 12: Back-extraction of extract formed in Example 1 by means of MgC12
solution
The Extract formed in Experiment 1 was contacted (back-extracted) with
recycled
(regenerated) aqueous 11% MgC12 solution. The bench scale back-extraction was
conducted
at 35oC and simulated counter-currently extraction of 8 stages. The Extract to
Aqueous
MgC12 solution (0/A) weight/weight ratio was 5. Water transferred from the
Extract to the
MgC12 solution. The formed organic phases and aqueous phases were analyzed to
determine
the time when their composition has reached a steady state. The steady state
organic phase
(regenerated Extractant) and the steady state aqueous phase (diluted MgC12
solution) were
analyzed.
MgC12 concentration in the diluted MgC12 solution was 8. The water content of
the
regenerated Extract was 11.5%. The water extracted from the waste stream into
the extract
according to Example 1, transferred from the extract to the recycled MgC12
solution in this
back-extraction, regenerating the extractant of Example 1.
Examples 13-17: Back-extraction of the extracts formed in Examples 7-11 by
means of
MgC12 solution
The extracts formed in Examples 7-11 were back-extracted with MgC12 solution.
The
procedure was similar to that in Example 12. The results are summarized in
Table 3. Back-
extraction has transferred into the MgC12 solutions the water that got
extracted in Exp. 7-11,
respectively, and has regenerated the respective extractants.
TABLE 3
Extract Extract MgC12
concentration Water concentration
formed water
Extractant in the recycled in the
regenerated
in Exp. content
MgC12 Extractant (%)
# (0/0) solution (%)
Example
7 Sec-BuOH 22.5 11 11
13
Example
8 Tert-butyl alcohol 31 21 10.2
14
Example
9 Tert-amyl alcohol 17.3 18 9.8
Example
16 10 Phenol 25 18 17
Example ii Methyl-ethyl
11.4 10 6.6
17 ketone
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
Examples 18: Water removal from the diluted MgC12 solution of Example 12 by
means of
evaporation
100g of the diluted MgC12 solution formed in Exp. 12 was heated under vacuum
and
the formed vapors was condensed into a liquid and collected. The collected
liquid contained
water and n-butanol. Heating was stopped when the amount of condensed water
was 27g.
After cooling, the MgC12 solution was analyzed. The MgC12 concentration there
was 11%,
which regenerated the recycled MgC12 solution. Water extracted from the waste
stream into
the extract in Example 1 and back-extracted into the MgC12 solution in Example
12 was
recovered from the diluted MgC12 by evaporation from that diluted MgC12
solution.
Examples 19: Water removal from the diluted MgC12 solution of Example 12 by
means of
reverse osmosis
Diluted MgC12 solution formed in Exp. 12 was concentrated in a reverse osmosis
cell
to reach a concentration of 11%. Water transferred through the membrane, while
MgC12 was
rejected. The concentration of MgC12 was increased to regenerate the recycled
MgC12
solution of Exp. 12. A small organic phase separated from the concentrated
MgC12 solution.
Example 20: Back-extraction of extract formed in Example 1 by means of
ammonium
carbonate solution
A ammonia and CO2 were bubbled through a recycled, dilute aqueous solution of
ammonium carbonate to form a concentrated solution of about 20%.
The Extract formed in Experiment 1 was contacted (back-extracted) with the
concentrated solution. The bench scale back-extraction was conducted at 15oC
and simulated
counter-currently extraction of 8 stages. The Extract to Aqueous (NH4)2CO3
solution (0/A)
weight/weight ratio was 5. Water transferred from the Extract to the (NH4)2CO3
solution.
The formed organic phases and aqueous phases were analyzed to determine the
time when
their composition has reached a steady state. The steady state organic phase
(regenerated
Extractant) and the steady state aqueous phase (diluted (NH4)2CO3 solution)
were analyzed.
The water content of the regenerated Extract was 11.5%. The water extracted
from the
waste stream into the extract according to Example 1, transferred from the
extract to the
recycled (NH4)2CO3 solution in this back-extraction, regenerating the
extractant of Example
1.
31
CA 02899377 2015-07-27
WO 2014/114996
PCT/1B2013/060283
It is expected that during the life of this patent many additional industrial
processes
and/or desalination techniques will be developed and the scope of the
invention is intended to
include all such new technologies a priori.
As used herein the term "about" refers to 10 %.
Although the invention has been described in conjunction with specific
embodiments
thereof, it is evident that many alternatives, modifications and variations
will be apparent to
those skilled in the art. Accordingly, it is intended to embrace all such
alternatives,
modifications and variations that fall within the spirit and broad scope of
the appended
claims.
Specifically, a variety of numerical indicators have been utilized. It should
be
understood that these numerical indicators could vary even further based upon
a variety of
engineering principles, materials, intended use and designs incorporated into
the various
embodiments of the invention. Additionally, components and/or actions ascribed
to
exemplary embodiments of the invention and depicted as a single unit may be
divided into
subunits. Conversely, components and/or actions ascribed to exemplary
embodiments of the
invention and depicted as sub-units/individual actions may be combined into a
single
unit/action with the described/depicted function.
Alternatively, or additionally, features used to describe a method can be used
to
characterize an apparatus and features used to describe an apparatus can be
used to
characterize a method.
It should be further understood that the individual features described
hereinabove can
be combined in all possible combinations and sub-combinations to produce
additional
embodiments of the invention. The examples given above are exemplary in nature
and are not
intended to limit the scope of the invention which is defined solely by the
following claims.
Each recitation of an embodiment of the invention that includes a specific
feature,
part, component, module or process is an explicit statement that additional
embodiments not
including the recited feature, part, component, module or process exist.
Specifically, the invention has been described in the context of industrial
processes
and desalination but might also be used to reduce levels of radioisotopes in
water.
All publications, references, patents and patent applications mentioned in
this
specification are herein incorporated in their entirety by reference into the
specification, to
the same extent as if each individual publication, patent or patent
application was specifically
and individually indicated to be incorporated herein by reference. In
addition, citation or
32
CA 02899377 2015-07-27
WO 2014/114996 PCT/1B2013/060283
identification of any reference in this application shall not be construed as
an admission that
such reference is available as prior art to the present invention.
The terms "include", and "have" and their conjugates as used herein mean
"including
but not necessarily limited to".
33