Note: Descriptions are shown in the official language in which they were submitted.
CA 02900418 2015-08-06
1
WO 2014/124970 Al
Cathode block having an abrasion-resistant surface that can be wetted
The present invention relates to a cathode block for an aluminium electrolysis
cell, to a
method for the production thereof, to the use thereof and to a cathode
comprising it.
Electrolysis cells are used for example for the electrolytic production of
aluminium, which is
usually carried out industrially by the Hall-Fleroult process. In the Hall-
Heroult process, a
melt composed of aluminium oxide and cryolite is electrolysed. The cryolite,
Na3[AlF6] serves
to reduce the melting point from 2,045 C for pure aluminium oxide to
approximately 950 C
for a mixture containing cryolite, aluminium oxide and additives such as
aluminium fluoride
and calcium fluoride.
The electrolysis cell used in this method comprises a cathode base which may
be composed
of a multiplicity of mutually adjacent cathode blocks which form the cathode.
To withstand
the thermal and chemical conditions which prevail during the operation of the
cell, the
cathode is usually composed of a carbon-containing material. Usually, grooves
are provided
on each lower face of the cathode, in each of which at least one busbar is
arranged through
which the current supplied via the anodes is dissipated. An anode, in
particular formed from
individual anode blocks, is usually arranged approximately 3 to 5 cm above the
layer of liquid
aluminium, usually 15 to 50 cm high, which is located on the upper face of the
cathode, the
electrolyte, in other words the melt containing aluminium oxide and cryolite,
being located
between said anode and the surface of the aluminium. During the electrolysis,
which is
carried out at approximately 1,000 C, the aluminium formed is deposited below
the
electrolyte layer, in other words as an intermediate layer between the upper
face of the
cathode and the electrolyte layer, because of the density thereof, which is
greater than that
of the electrolyte. During the electrolysis, the aluminium oxide dissolved in
the melt is split by
an electrical current into aluminium and oxygen. In electrochemical terms, the
layer of liquid
aluminium is the actual cathode, since aluminium ions are reduced to elemental
aluminium
on the surface thereof. Nevertheless, in the following the term cathode refers
not to the
cathode in electrochemical terms, in other words the layer of liquid
aluminium, but rather to
the component, for example composed of one or more cathode blocks, which forms
the
electrolysis cell base.
CA 02900418 2015-08-06
2
A major drawback of the Hall-Heroult process is that it is highly energy-
intensive.
Approximately 12 to 15 kWh of electrical energy are needed to produce 1 kg of
aluminium,
and this constitutes up to 40 % of the production costs. To reduce the
production costs, it is
'therefore desirable to reduce the specific energy consumption in this method
as much as
possible.
Therefore, recently graphite cathodes have increasingly been used, in other
words ones
consisting of cathode blocks containing graphite as a primary constituent. A
distinction is
made between graphitic cathode blocks, in the production of which graphite is
used as the
starting material, and graphitised cathode blocks, for the production of which
a graphite
precursor, which contains a carbon and which is converted to graphite by a
subsequent heat
treatment known as graphitisation at 2,100 to 3,000 C, is used as a starting
material. By
comparison with amorphous carbon, graphite is distinguished by a much lower
electrical
resistivity and by a significantly higher thermal conductivity, meaning that
the use of graphite
cathodes during the electrolysis can reduce the specific energy consumption of
the
electrolysis and also makes it possible to carry out the electrolysis at a
higher current
intensity, making it possible to increase the aluminium production. However,
cathodes or
cathode blocks made of graphite, and in particular graphitised cathode blocks,
are subjected
to considerable wear during the electrolysis as a result of surface abrasion,
this wear being
much greater than the wear of cathode blocks made of amorphous carbon. Aside
from this,
cathodes or cathode blocks made of amorphous carbon or graphite have
comparatively poor
wettability with aluminium.
So as to increase the wettability of cathode blocks, and in particular of
those made of
graphite, with aluminium, and also to increase the wear resistance of cathode
blocks made
of graphite, it has previously been proposed to form at least the face of the
cathode block
forming the upper face thereof during operation of the cathode block from a
graphite material
containing for example titanium diboride. For example, WO 2012/107400 A2
discloses a
cathode block for an aluminium electrolysis cell, which comprises a base layer
and a cover
layer, the base layer containing graphite and the cover layer containing a
graphite composite
material containing 1 to less than 50 % by weight hard material having a
melting point of at
least 1,000 C. Either a material containing carbon, such as coke, anthracite,
soot or
vitreous carbon, or alternatively a non-oxidic ceramic, preferably titanium
diboride, may be
used as the hard material. The addition of the hard material is intended to
increase the wear-
CA 02900418 2015-08-06
3
resistance of the cathode block made of graphite, whilst the use of preferably
titanium boride
is intended to improve the wettability for aluminium.
It has been shown in experiments that the addition of for example titanium
diboride does
increase the wettability of cathode blocks made of graphite, making it
possible to reduce the
thickness of the aluminium layer in the electrolysis cell and thus the anode-
cathode distance
in the electrolysis cell, leading to a reduction in the specific energy
consumption of the
electrolysis cell; however, the wear-resistance of these cathode blocks needs
to be
improved. As a result of the wear resistance which needs to be improved,
cathodes
composed of cathode blocks of this type are unsuitable or only sometimes
suitable for
modern electrolysis cells in particular, which are operated at high current
intensities of up to
600 kA, because of the short service life thereof in these operating
conditions.
The object of the present invention is therefore to provide a cathode block
which is suitable
in particular for use for an aluminium electrolysis cell and which not only
has a low electrical
resistivity and is highly wettable with aluminium melt, but is also
distinguished in particular by
a high wear-resistance towards the abrasive chemical and thermal conditions
prevailing
during the operation during melt-flow electrolysis, in particular including at
high current
intensities of for example 600 kA.
According to the invention, this object is achieved by a cathode block for an
aluminium
electrolysis cell which is composed at least in portions of a material which
is obtainable by
combusting a mixture which contains at least one carbon-containing material
having a
graphitisation level, calculated according to Maire and Mehring from the
average layer
spacing c/2 after a heat treatment at 2,800 C, of at most 0.50 and at least
one non-oxidic
ceramic.
The cathode block according to the invention is preferably formed from a base
layer and a
cover layer, the at least one portion which contains the aforementioned
mixture being part of
the cover layer. Within the meaning of the invention, this portion may extend
over the entire
cover layer or the portion may merely form part of this cover layer.
This solution is based on the finding that the combined addition of material
containing
comparatively poorly or not at all graphitisable carbon, specifically of
carbon-containing
material having a graphitisation level, calculated according to Maire and
Mehring from the
CA 02900418 2015-08-06
4
average layer spacing c/2 after a heat treatment at 2,800 C, of at most 0.50,
and of non-
oxidic ceramic to the material from which at least a portion of the cathode
block is produced
great)y increases the wear-resistance of the cathode block, and this is
accompanied by
excellent wettability of the cathode block with aluminium and outstanding
electrical and
thermal conductivity of the cathode block. Whilst the addition of non-oxidic
ceramic, such as
titanium diboride, because of the catalytic activity thereof for the
graphitisation, increases
both the electrical conductivity and the thermal conductivity of the cathode
block as well as
increasing the wettability of the of the cathode block with aluminium, the
addition of material
containing comparatively poorly or not at all graphitisable carbon greatly
increases the wear-
resistance of the cathode block. Therefore, the cathode block according to the
invention can
be used in particular even at high current intensities of for example 600 kA
and has a long
service life even in operating conditions of this type. Meanwhile, the
comparatively poor
graphitisability of the carbon-containing material is also advantageous
because it adjusts an
excessively high electrical conductivity, which the mere addition of the non-
oxidic ceramic
might confer on the cathode block, into an acceptable range. In principle as
high an electrical
conductivity as possible is desirable for a cathode; however, an excessively
high electrical
conductivity is undesirable because it can lead for example to an
inhomogeneous current
distribution in the cathode block, and this can lead both to electrochemically
induced
corrosion resulting from aluminium carbide formation and to a decrease in
energy efficiency
resulting from increased horizontal currents in liquid aluminium and thus to
reduced stability
of the electrolysis cell. Overall, as a result of the combined addition of
material containing
comparatively poorly or not at all graphitisable carbon, specifically of
carbon-containing
material having a graphitisation level, calculated according to Maire and
Mehring from the
average layer spacing c/2 after a heat treatment at 2,800 C, of at most 0.50,
and of non-
oxidic ceramic to the material from which at least a portion of the cathode
block is produced,
the cathode block according to the invention not only has a low electrical
resistivity and good
wettability with aluminium melt, but is also distinguished in particular by a
high wear-
resistance towards the abrasive chemical and thermal conditions prevailing
during the
operation during melt-flow electrolysis, in particular including at high
current intensities of for
example 600 kA.
Within the meaning of the present invention, carbon material means in
particular a material
containing more than 60 % by weight, preferably more than 70 % by weight,
particularly
preferably more than 80 % by weight and more preferably more than 90 % by
weight carbon,
in particular coke.
CA 02900418 2015-08-06
As a result of the aforementioned properties and advantages, the cathode block
according to
the invention is preferably a graphite-based cathode block, in other words a
cathode block
obtainable by combusting and subsequently graphitising the material from which
it is
produced. As a result of the comparatively poor or completely absent
graphitisability of the
carbon-containing material used according to the invention, having a
graphitisation level,
calculated according to Maire and Mehring from the average layer spacing c/2
after a heat
treatment at 2,800 C, of at most 0.50, only very limited transformation into
a graphite
structure takes place during the graphitisation, and none at all takes place
in the non-oxidic
ceramic, and so the graphite proportion of the cathode block in this
embodiment stems
virtually exclusively from the other constituents of the material, which are
described in detail
below.
To achieve the advantages, described above in relation to the addition of the
material
containing comparatively poorly or not at all graphitisable carbon, to a great
extent, a
development of the inventive idea proposes providing carbon-containing
material having a
graphitisation level, calculated according to Maire and Mehring from the
average layer
spacing c/2 after a heat treatment at 2,800 C, of at most 0.40 and
particularly preferably of
at most 0.30 in the material of which the cathode block is composed at least
in portions. This
leads to a particularly good wear-resistance of the cathode block and makes
the electrical
conductivity particularly reliably controllable. In the context of the present
invention, the
graphitisability is determined according to Maire and Mehring as disclosed by
J. Maire and J.
Mehring in "Graphitization of soft carbons" in Chemistry and Physics of
Carbon, Vol. 6,
Marcel Dekker, P.K. Walker Jr. (Ed.), New York 1970, pages 125 to 190. Briefly
summarised, in this context the lattice spacing is determined from the
diffraction peak of the
(002) plane, and the graphitisation level is calculated therefrom by the
formula g = [0.3440 ¨
d(002)]/0.0086, in which g is the graphitisation level and d(002) is the
lattice spacing from
the diffraction peak of the (002) plane in nm.
For the same reason, in a further preferred embodiment it is preferred for the
at least one
carbon-containing material having a graphitisation level, calculated according
to Maire and
Mehring from the average layer spacing c/2 after a heat treatment at 2,800 C,
of at most
0.50 to be coke, particularly preferably coke having an average layer spacing
c/2 of at least
0.339 nm as determined by X-ray diffraction interference. Coke of this type
has a suitably
low graphitisability, very good results being obtained in particular with coke
which has an
CA 02900418 2015-08-06
6
average layer spacing c/2 of 0.340 to 0.344 nm as determined by X-ray
diffraction
interference.
Preferably, particulate carbon material having a graphitisation level,
calculated according to
Maire and Mehring from the average layer spacing c/2 after a heat treatment at
2,800 C, of
at most 0.50 is used, the specific BET area of the particles of the carbon
material preferably
being 10 to 40 m2/g and particularly preferably 20 to 30 m2/g.
A preferred example of coke having a low graphitisability of the
aforementioned type is coke
which accumulates as a side product in the production of unsaturated
hydrocarbons, in
particular of acetylene, and which is referred to in the following as
acetylene coke
irrespective of the nature of the unsaturated hydrocarbon during the
production of which it
accumulates. Acetylene coke obtainable from the crude oil fractions or steam
crack residues
used when quenching reaction gas in the synthesis of unsaturated hydrocarbons,
in
particular of acetylene, has been found to be particularly suitable for this
purpose. To
produce this coke, the quenching oil or soot mixture is passed to a coker
which is heated to
approximately 500 C. In the coker, liquid components of the quenching oil
evaporate, whilst
the coke collects on the base of the coker. A corresponding method is
described for example
in DE 29 47 005 Al. In this way, a fine-grained onion-skin-like coke is
obtained, which
preferably has a carbon content of at least 96 % by weight and an ash content
of at most
0.05 % by weight and preferably of at most 0.01 % by weight.
The acetylene coke preferably has an average layer spacing c/2, as determined
by X-ray
diffraction interference, of at least 0.34 nm, the crystallite size in the c
direction Lc preferably
being less than 20 nm and the crystallite size in the a direction La
preferably being less than
50 nm and particularly preferably less than 40 nm.
In addition, it is preferred for the acetylene coke to be in the form of
spherical particles
having a grain size of more than 0.2 mm and preferably of more than 0.5 mm.
Good results are obtained in particular if the acetylene coke has a BET area
of 20 to 40
m2/g.
CA 02900418 2015-08-06
7
A further preferred example of coke, which may be used in addition or as an
alternative to
acetylene coke, is coke produced in fluidised bed methods. With these methods,
coke of a
spherical to ellipsoid form is obtained, and is of an onion-skin-like
construction.
Another further preferred example of coke, which may be used in addition or as
an
alternative to the above-disclosed acetylene coke and/or coke obtained by
flexicoking
methods, is shot coke, which is produced by delayed coking. The particles of
this coke are of
a spherical morphology. It is preferred for this coke to have an average layer
spacing c/2, as
determined by X-ray diffraction interference, of at least 0.339 nm and for the
crystallite size
in the c direction Lc to be less than 30 nm.
Good results are obtained in particular if the at least one carbon-containing
material having a
graphitisation level, calculated according to Maire and Mehring from the
average layer
spacing c/2 after a heat treatment at 2,800 C, of at most 0.50 consists of
particles having a
grain size of 0.2 mm to 3 mm and preferably of 0.5 mm to 20 mm.
A development of the inventive idea proposes to provide carbon-containing
material having a
graphitisation level, calculated according to Maire and Mehring from the
average layer
spacing c/2 after a heat treatment at 2,800 C, of at most 0.50, which is
composed of
particles having a spherical morphology, in other words a spherical to
ellipsoidal form, in the
mixture. Because of the high flowability thereof, a carbon material consisting
of particles of
this type leads to a material having a higher bulk density, and this
contributes to an increase
in the wear-resistance. Preferably, the particles of the carbon material have
a length-to-
diameter ratio of 1 to 5, particularly preferably of 1 to 3. This is because
the flowability of the
carbon material and thus the bulk density and wear-resistance of the cathode
block
increases more the closer the form of the particles is to an ideal spherical
shape.
In a further preferred embodiment of the present invention, the individual
particles of the
carbon-containing material having a graphitisation level, calculated according
to Maire and
Mehring from the average layer spacing c/2 after a heat treatment at 2,800 C,
of at most
0.50 have an onion-skin structure, referring within the meaning of the
invention to a
multilayer construction in which an inner layer of particles of spherical or
ellipsoid form is
covered completely or at least in part by at least an intermediate layer and
an outer layer.
CA 02900418 2015-08-06
8
To achieve the advantages, described above in relation to the addition of the
material
containing comparatively poorly or not at all graphitisable carbon, to a
particularly great
extent, a development of the inventive idea proposes providing carbon-
containing material
having a graphitisation level, calculated according to Maire and Mehring from
the average
layer spacing c/2 after a heat treatment at 2,800 C, of at most 0.50 in an
amount of 1 to 25
% by weight, particularly preferably of 10 to 25 % by weight and most
preferably of 10 to 20
`)/0 by weight in the mixture from which the material of which the cathode
block is composed
at least in portions is obtained by combusting and preferably graphitising. As
a result, a
particularly high wear-resistance of the cathode block is achieved at the same
time as
excellent wettability with aluminium and sufficiently high electrical and
thermal conductivity.
In a preferred embodiment of the present invention, the non-oxidic ceramic is
a non-oxidic
ceramic which is composed of at least one metal from the 4th to 6th transition
groups and at
least one element from the 31-d or 4th main group of the periodic table of
elements.
Particularly preferred examples of ceramics of this type are titanium
diboride, zirconium
diboride, tantalum diboride, titanium carbide, boron carbide, titanium
carbonitride, silicon
carbide, tungsten carbide, vanadium carbide, titanium nitride, boron nitride,
silicon nitride
and any desired chemical combinations and/or mixtures of two or more of said
compounds.
Particularly good results are obtained if the at least one non-oxidic ceramic
is titanium
diboride and/or zirconium diboride, in particular titanium diboride.
A development of the inventive idea proposes for the at least one non-oxidic
ceramic
contained in the cathode block to have a monomodal particle size distribution,
the volume-
average particle size (d3,50) as determined by random light scattering
pursuant to
International Standard ISO 13320-1, being 10 to 20 pm.
In the context of the present invention, it has been found that non-oxidic
ceramic having an
above-defined monomodal particle size distribution not only brings about very
good
wettability of the surface of the cathode block with aluminium, but, by way of
combination
with the at least one carbon-containing material having a graphitisation
level, calculated
according to Maire and Mehring from the average layer spacing c/2 after a heat
treatment at
2,800 C, of at most 0.50, in particular also leads to a cathode block having
excellent wear-
resistance. In addition, in the context of the present invention it has
surprisingly been found
CA 02900418 2015-08-06
9
that this effect is achieved in particular even at comparatively small amounts
of added non-
oxidic ceramic. As a result, a high concentration of non-oxidic ceramic in the
cathode block,
which leads to a brittle cathode block surface, can be dispensed with. Non-
oxidic ceramic
'having an above-defined monomodal particle size distribution is also
distinguished by very
good workability. In particular, the dust formation of a non-oxidic ceramic of
this type is
sufficiently low, for example during filling into a mixing container or during
transport of
powder containing this ceramic, and at most slight agglomerate formation
occurs during the
mixture. Moreover, a powder of this type containing this ceramic has a
sufficiently high
flowability and pourability, in such a way that it can be conveyed to a mixing
device for
example using a conventional conveyor device.
Preferably, the at least one non-oxidic ceramic provided in the cathode block
has a
monomodal particle size distribution, the volume-average particle size
(d3,50), determined as
above, being 12 to 18 pm and particularly preferably 14 to 16 pm.
As an alternative to the aforementioned embodiment, the non-oxidic ceramic
contained in
the cathode block may have a monomodal particle size distribution, the volume-
average
particle size (d3,50) as determined by random light scattering pursuant to
International
Standard ISO 13320-1, being 3 to 10 pm and preferably 4 to 6 pm. In this
embodiment too,
particularly preferably a non-oxidic titanium ceramic and most preferably
titanium diboride
having an above-defined monomodal particle size distribution is used.
A development of the inventive idea proposes that the at least one non-oxidic
ceramic has a
volume-average d3,90 Particle size, determined as above, of 20 to 40 pm and
preferably of 25
to 30 pm. Preferably, the non-oxidic ceramic has a d3,90 value of this type in
combination with
an above-defined d3,50 value. In this embodiment too, the non-oxidic titanium
ceramic and
particularly preferably titanium diboride. As a result, the advantages and
effects mentioned
for the above embodiment are actually achieved to an increased extent.
As an alternative to the aforementioned embodiment, the non-oxidic ceramic
contained in
the cathode block may have a volume-average d3,90 particle size, determined as
above, of
to 20 pm and preferably of 12 to 18 pm. Preferably, the non-oxidic ceramic has
a d3,90
value of this type in combination with an above-defined d3,50 value. In this
embodiment too,
particularly preferably a non-oxidic titanium ceramic and most preferably
titanium diboride
having an above-defined monomodal particle size distribution is used.
CA 02900418 2015-08-06
In a further preferred embodiment of the present invention, the non-oxidic
ceramic has a
volume-average d3,10 particle size, determined as above, of 2 to 7 pm and
preferably 3 to 5
µpm. Preferably, the non-oxidic ceramic has a d3,10 value of this type in
combination with an
above-defined d3,90 value and/or d3,50 value. In this embodiment too, the non-
oxidic ceramic
is preferably a non-oxidic titanium ceramic and particularly preferably
titanium diboride. As a
result, the advantages and effects mentioned for the above embodiments are
actually
achieved to an increased extent.
As an alternative to the aforementioned embodiment, the non-oxidic ceramic
contained in
the cathode block may have a volume-average d3,10 particle size, determined as
above, of 1
to 3 pm and preferably of 1 to 2 pm. Preferably, the non-oxidic ceramic has a
d3,10 value of
this type in combination with an above-defined d3,90 value and/or d3,50 value.
In this
embodiment too, particularly preferably a non-oxidic titanium ceramic and most
preferably
titanium diboride having an above-defined monomodal particle size distribution
is used.
In addition, it is preferred for the non-oxidic ceramic, in particular a non-
oxidic titanium
ceramic and particularly preferably titanium diboride, to have a particle size
distribution
characterised by a span value calculated by the following equation:
span = (d3,90 ¨ d3,10)/c13,50
of 0.65 to 3.80 and particularly preferably of 1.00 to 2.25. Preferably, the
non-oxidic ceramic
has a span value of this type in combination with an above-defined d3,90 value
and/or d3,50
value and/or d3,10 value. As a result, the advantages and effects mentioned
for the above
embodiments are actually achieved to an increased extent.
To achieve the advantages described above, in particular sufficiently high
electrical
conductivity and wettability of the cathode block with aluminium, to a
particularly great
extent, a development of the inventive idea proposes providing non-oxidic
ceramic in an
amount of 1 to 45 % by weight in the mixture from which the material of which
the cathode
block is composed at least in portions is obtained by combusting and
preferably graphitising.
Particularly good results are obtained in this regard if the amount of non-
oxidic ceramic is 10
to 40 % by weight and particularly preferably 15 to 35 % by weight.
CA 02900418 2015-08-06
11
Preferably, the total of the amount of carbon-containing material having a
graphitisation
level, calculated according to Maire and Mehring from the average layer
spacing c/2 after a
heat treatment at 2,800 C, of at most 0.50 and the amount of non-oxidic
ceramic in the
mixture from which the material of which the cathode block is composed at
least in portions
is obtained by combusting and preferably graphitising is 2 to 70 % by weight,
preferably 20
to 65 % by weight and particularly preferably 25 to 55 % by weight. As a
result, the cathode
block according to the invention has a particularly good wear-resistance
towards the
abrasive chemical and thermal conditions prevailing during the operation
during melt-flow
electrolysis, in particular including at high current intensities of for
example 600 kA, at the
same time as a low electrical resistivity and a good wettability with
aluminium melt.
In a further preferred embodiment of the present invention, the proportion of
non-oxidic
ceramic is 20 to 95 % by weight, particularly preferably 50 to 75 % by weight,
based on the
total of non-oxidic ceramic and carbon-containing material having a
graphitisation level,
calculated according to Maire and Mehring from the average layer spacing c/2
after a heat
treatment at 2,800 C, of at most 0.50.
In addition to the at least one carbon-containing material having a
graphitisation level,
calculated according to Maire and Mehring from the average layer spacing c/2
after a heat
treatment at 2,800 C, of at most 0.50 and in addition to the at least one non-
oxidic ceramic,
the mixture from which the material of which the cathode block is composed at
least in
portions is obtained by combusting and preferably graphitising preferably
contains at least
one carbon-containing material having a graphitisation level, calculated
according to Maire
and Mehring from the average layer spacing c/2 after a heat treatment at 2,800
C, of more
than 0.50, preferably of at least 0.60, particularly preferably of at least
0.65 and most
preferably of at least 0.70. During the graphitisation which is preferably
carried out after the
combusting, this carbon forms a graphite structure which subsequently
significantly
contributes to the excellent electrical and thermal conductivity of the
cathode block according
to the invention.
In addition to or instead of the at least one carbon-containing material
having a
comparatively high graphitisability, the mixture from which the material of
which the cathode
block is composed at least in portions is obtained by combusting and
preferably graphitising
preferably contains at least one binder. The binder may for example be pitch,
in particular
CA 02900418 2015-08-06
12
coal tar pitch and/or petroleum pitch, tar, bitumen, phenol resin or furan
resin. A particularly
preferred binder is pitch.
'A development of the inventive idea proposes for the material of which the
cathode block is
composed at least in portions to be obtainable by combusting and preferably
subsequently
graphitising a mixture which contains:
- 1 to 25 % by weight of at least one carbon-containing material having a
graphitisation
level, calculated according to Maire and Mehring from the average layer
spacing c/2
after a heat treatment at 2,800 C, of at most 0.50,
- 1 to 45 % by weight of at least one non-oxidic ceramic,
- 10 to 70 % by weight of at least one carbon-containing material having a
graphitisation level, calculated according to Maire and Mehring from the
average
layer spacing c/2 after a heat treatment at 2,800 C, of more than 0.50,
preferably of
at least 0.60, particularly preferably of at least 0.65 and most preferably of
at least
0.70, and
- 10 to 25 % by weight binder,
the total of the amount of carbon-containing material having a graphitisation
level, calculated
according to Maire and Mehring from the average layer spacing c/2 after a heat
treatment at
2,800 C, of at most 0.50 and the amount of non-oxidic ceramic preferably
being 5 to 70 %
by weight, and the total of the individual constituents being 100 % by weight.
Particularly preferably, the material of which the cathode block is composed
at least in
portions is obtainable by combusting and preferably subsequently graphitising
a mixture
which contains:
- 10 to 25 % by weight, and preferably 10 to 20 % by weight, of at least
one carbon-
containing material having a graphitisation level, calculated according to
Maire and
Mehring from the average layer spacing c/2 after a heat treatment at 2,800 C,
of at
most 0.40 and preferably of at most 0.30,
- 10 to 40 % by weight, and preferably 15 to 35 % by weight, of at least
one non-oxidic
ceramic,
- 20 to 40 % by weight, and preferably 25 to 35 % by weight, of at least
one carbon-
containing material having a graphitisation level, calculated according to
Maire and
Mehring from the average layer spacing c/2 after a heat treatment at 2,800 C,
of at
least 0.60, and preferably of at least 0.70, and
- 10 to 25 % by weight binder,
CA 02900418 2015-08-06
13
the total of the amount of carbon-containing material having a graphitisation
level, calculated
according to Maire and Mehring from the average layer spacing c/2 after a heat
treatment at
2,800. C, of at most 0.40 and the amount of non-oxidic ceramic preferably
being 20 to 65 %
'by weight and particularly preferably 25 to 55 % by weight, and the total of
the individual
constituents being 100 % by weight.
As set out above, it is particularly preferred for the material of which the
cathode block is
composed at least in portions to be obtainable by combusting and subsequently
graphitising
the above-described mixture. It is preferred for the graphitisation of the
mixture to take place
at a temperature of more than 1,800 to 3,000 C, preferably of 2,000 to 3,000
C and
particularly preferably of 2,200 to 2,700 C.
As mentioned previously, the cathode block preferably comprises a base layer
and a cover
layer, the cover layer being composed at least in portions of the material
which is obtainable
by combusting and preferably subsequently graphitising the above-described
mixture. In this
context, the addition of the non-oxidic ceramic and of the carbon-containing
material having
a graphitisation level, calculated according to Maire and Mehring from the
average layer
spacing c/2 after a heat treatment at 2,800 C, of at most 0.50 is limited to
the at least one
portion of the cover layer of the cathode block. The cover layer is the layer
which is exposed
to the aluminium melt during operation of the electrolysis cell.
In this context, it is preferred for the thickness of the cover layer to be 1
to 50 %, preferably 5
to 40 %, particularly preferably 10 to 30 % and most preferably 15 to 25 % of
the total height
of the cathode block.
In this context, an addition of non-oxidic ceramic and of the carbon-
containing material
having a graphitisation level, calculated according to Maire and Mehring from
the average
layer spacing c/2 after a heat treatment at 2,800 C, of at most 0.50 into the
base layer is
superfluous. Therefore, a preferred embodiment of the present invention
proposes for the
base layer merely to consist of graphitised, graphitic or graphitisable
materials for the
purpose of achieving a high electrical and thermal conductivity. Preferably
the base layer is
at least 80 % by weight, preferably at least 90 % by weight, more preferably
at least 95 % by
weight, more preferably at least 99 % by weight and most preferably completely
composed
of graphite and binder or the carbonisation and/or graphitisation product
thereof.
CA 02900418 2015-08-06
14
The cover layer may comprise a plurality of portions, two or more of the
portions being
composed of respectively different materials. In this way, each surface region
of the cathode
block can be tailored with respect to the desired wear-resistance, electrical
conductivity,
'thermal conductivity and wettability with aluminium. In this embodiment, it
is possible in
particular to take into account the fact that individual surface portions of
the cathode block
are exposed to higher wear than others during the melt-flow electrolysis, in
such a way that
the surface portions which are subject to particularly high wear are
selectively composed of
a material comprising a correspondingly large amount of carbon, having a
graphitisation
level, calculated according to Maire and Mehring from the average layer
spacing c/2 after a
heat treatment at 2,800 C, of at most 0.50, whilst the other surface portions
which are
subject to low wear are composed of a material containing less carbon, having
a
graphitisation level, calculated according to Maire and Mehring from the
average layer
spacing c/2 after a heat treatment at 2,800 C, of at most 0.50.
In an example variant of the above embodiment of the present invention, the at
least two
portions are composed of different materials, which are each obtainable by
combusting a
mixture which contains at least one carbon-containing material having a
graphitisation level,
calculated according to Maire and Mehring from the average layer spacing c/2
after a heat
treatment at 2,800 C, of at most 0.50 and at least one non-oxidic ceramic.
Alternatively,
however, it is also possible for only one or more of the at least two portions
to be composed
of different materials, which are each obtainable by combusting a mixture
which contains at
least one carbon-containing material having a graphitisation level, calculated
according to
Maire and Mehring from the average layer spacing c/2 after a heat treatment at
2,800 C, of
at most 0.50 and at least one non-oxidic ceramic, whilst at least one of the
at least two
portions is composed of a material which contains no carbon-containing
material having a
graphitisation level, calculated according to Maire and Mehring from the
average layer
spacing c/2 after a heat treatment at 2,800 C, of at most 0.50 and/or no non-
oxidic ceramic.
In principle, the cathode block according to the invention is not limited as
regards the
number of different portions in the cover layer. However, good results are
obtained in
particular when the cover layer of the cathode block according to the
invention comprises 3
to 7, preferably 3 to 5, particularly preferably 3 to 4 and most preferably 3
different portions,
preferably one or two of the portions respectively being composed of a
material which is
obtainable by combusting a mixture which contains at least one carbon-
containing material
having a graphitisation level, calculated according to Maire and Mehring from
the average
CA 02900418 2015-08-06
layer spacing c/2 after a heat treatment at 2,800 C, of at most 0.50 and at
least one non-
oxidic ceramic.
'A further subject matter of the present invention is a method for producing a
cathode block
according to at least one of the preceding claims, comprising the following
steps:
a) producing a mixture which contains at least one carbon-containing material
having a
graphitisation level, calculated according to Maire and Mehring from the
average
layer spacing c/2 after a heat treatment at 2,800 C, of at most 0.50 and at
least one
non-oxidic ceramic,
b) shaping the mixture to form at least one portion of a cathode block, and
c) combusting the mixture at a temperature of 600 to less than 1,500 C.
Preferably, in method step a) a mixture is produced which contains:
- 10 to 25 % by weight of at least one carbon-containing material having a
graphitisation level, calculated according to Maire and Mehring from the
average
layer spacing c/2 after a heat treatment at 2,800 C, of at most 0.50,
- 10 to 40 % by weight of at least one non-oxidic ceramic,
- 20 to 40 % by weight of at least one carbon-containing material having a
graphitisation level, calculated according to Maire and Mehring from the
average
layer spacing c/2 after a heat treatment at 2,800 C, of at least 0.60, and
- 10 to 25 % by weight binder,
the total of the amount of carbon-containing material having a graphitisation
level, calculated
according to Maire and Mehring from the average layer spacing c/2 after a heat
treatment at
2,800 C, of at most 0.50 and the amount of non-oxidic ceramic preferably
being 20 to 65 %
by weight, and the total of the individual constituents being 100 % by weight.
Particularly preferably, in method step a) a mixture is produced which
contains:
- 10 to 20 % by weight of at least one carbon-containing material having a
graphitisation level, calculated according to Maire and Mehring from the
average
layer spacing c/2 after a heat treatment at 2,800 C, of at most 0.40,
- 15 to 35 % by weight of at least one non-oxidic ceramic,
- 25 to 35 % by weight of at least one carbon-containing material having a
graphitisation level, calculated according to Maire and Mehring from the
average
layer spacing c/2 after a heat treatment at 2,800 C, of at least 0.70, and
- 10 to 25 % by weight binder,
CA 02900418 2015-08-06
16
the total of the amount of carbon-containing material having a graphitisation
level, calculated
according to Maire and Mehring from the average layer spacing c/2 after a heat
treatment at
2,806 C, of at most 0.40 and the amount of non-oxidic ceramic preferably
being 30 to 50 %
by weight, and the total of the individual constituents being 100% by weight.
In a development of the inventive idea, it is proposed for the mixture
produced in method
step a) to be applied by a vibration method to a second mixture which
preferably contains
- 40 to 90 % by weight of at least one carbon-containing material having a
graphitisation level, calculated according to Maire and Mehring from the
average
layer spacing c/2 after a heat treatment at 2,800 C, of more than 0.50, and
- 10 to 25 % by weight binder,
the total of the individual constituents being 100 % by weight, and the
overall mixture thus
produced being shaped into a cathode block in method step b), the second
mixture forming
the base layer and the other mixture forming the cover layer of the cathode
block, before the
cathode block is combusted in method step c) and subsequently preferably
graphitised.
Preferably, the combustion in method step c) takes place at a temperature of
600 to less
than 1,500 C, preferably of 800 to 1,200 C and particularly preferably of
900 to 1,100 C.
In a development of the inventive idea, it is proposed to graphitise the
combusted cathode
block after method step c) at a temperature of more than 1,800 to 3,000 C,
preferably of
2,000 to 3,000 C and particularly preferably of 2,200 to 2,700 C.
A further subject matter of the present invention is a cathode which contains
at least one
above-disclosed cathode block.
The present invention further relates to the use of an above-disclosed cathode
block or an
above-disclosed cathode to carry out a melt-flow electrolysis to produce
metal, preferably to
produce aluminium.
In the following, purely by way of example, the present invention is described
by way of
advantageous embodiments and with reference to the accompanying drawings, in
which:
Fig. 1 is a schematic perspective view of a cathode block in accordance with a
first
embodiment of the present invention, and
CA 02900418 2015-08-06
17
Fig. 2 is a schematic perspective view of a cathode block in accordance with a
second embodiment of the present invention.
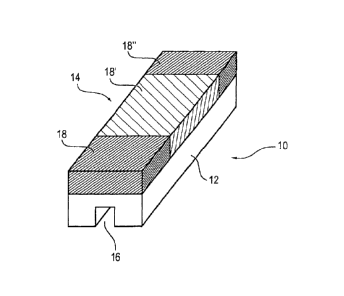
Fig. 1 is a schematic perspective view of a cathode block 10 in accordance
with a first
embodiment of the present invention. The cathode block 10 consists of a lower
base layer
12 and a cover layer 14 arranged above and rigidly connected thereto. The
boundary
surface between the base layer 12 and the cover layer 14 is planar. While the
base layer 12
of the cathode block 10 has a graphite material structure, the cover layer 14
is composed of
a graphite composite material containing acetylene coke and titanium diboride.
The cathode
block 10 has a length of 3,100 mm, a width of 420 mm and a height of 400 mm,
the base
layer 12 having a height of 260 mm and the cover layer 14 having a height of
140 mm.
Finally, the cathode block 10 comprises, on the lower face thereof, a groove
16 having a
right-angled, specifically substantially rectangular cross section.
In practice, a cathode for an aluminium electrolysis cell is composed of 12 to
28 cathode
blocks of this type, a steel busbar (not shown) having a likewise right-angled
or substantially
rectangular cross section being inserted into each of the grooves 16. The gap
between the
busbars and the walls defining the groove 16 is filled with cast iron (not
shown), thereby
connecting the busbars to the walls defining the groove 16.
The cathode block 10 in accordance with a second embodiment of the present
invention
shown in Fig. 2 differs from that shown in Fig. 1 in that the cover layer 14
consists of three
different portions 18, 18', 18". The portions 18, 18" are each composed of the
same
material, which differs from the material of which the portion 18' is composed
and from the
material of which the base layer 12 is composed. Whilst the portions 18, 18"
are composed
of a graphite composite material containing 20 % by weight acetylene coke and
20 % by
weight titanium diboride, the portion 18' is composed of a graphite composite
material
containing 10 % by weight acetylene coke and 30 % by weight titanium diboride,
and the
base layer 12 has a graphite material structure. In this way, the individual
surface portions
are adapted to the cover layer 14 in such a way that the portions 18, 18', 18"
of the cathode
block 10 which are exposed to a higher wear than other parts during the melt-
flow
electrolysis have a correspondingly higher wear resistance.
CA 02900418 2015-08-06
18
In the following, purely by way of example, the present invention is disclosed
by way of an
example, which does not limit the invention.
'Example
A cathode block 10 as shown in Fig. 1 was produced by filling a mixture A,
forming the base
layer 12, and a mixture B, forming the cover layer 14, into a correspondingly
dimensioned
vibration mould.
The mixture A was composed as follows:
- 80 % by weight petroleum coke having a graphitisation level, calculated
according to
Maire and Mehring from the average layer spacing c/2 after a heat treatment at
2,800
C, of 0.7, and
- 20 % by weight coal tar pitch having a Kramer-Sarnow softening point of
90 C as a
binder.
Further, the mixture B was composed as follows:
- 24 % by weight titanium diboride,
- 16 % by weight acetylene coke having a graphitisation level, calculated
according to
Maire and Mehring from the average layer spacing c/2 after a heat treatment at
2,800
C, of 0.3,
- 40 % by weight petroleum coke having a graphitisation level, calculated
according to
Maire and Mehring from the average layer spacing c/2 after a heat treatment at
2,800
C, of 0.7, and
- 20 % by weight coal tar pitch having a Kramer-Sarnow softening point of
90 C as a
binder.
CA 02900418 2015-08-06
19
List of reference numerals
cathode block
12 base layer
14 cover layer
16 groove
18, 18', 18" portions of the cover layer