Note: Descriptions are shown in the official language in which they were submitted.
CA 02901178 2016-12-15
DRUG DELIVERY BALLOON APPARATUS
Background of the Invention
Local drug delivery is the process by which therapeutic agents are delivered
to
specific areas within the vasculature of a human or animal patient. This
localized treatment
permits an increased concentration of the drug or therapeutic agent at the
intended target area
but avoids toxicity that may result through general systemic delivery within
the circulatory
system.' Known localized drug delivery methods include drug-eluting stents or
balloons.
porous drug infusion balloons and direct catheter delivery.
I 0
Summary of the Invention
The present invention is directed to methods and apparatus fbr the delivery of
a drug
solution or a therapeutic agent to a selected sire within the vascular system
using a drug
delivery balloon apparatus. The. drug delivery balloon apparatus of the
present invention may
beneficially permit an increased balloon length that may be up to four times
longer than that
of other brown balloons providing the advantage of treating larger injury
sites in a single
procedure. The drug delivery balloon apparatus of the present invention may
also provide a
plurality of gooves for receiving the drug solution during delivery to the
target passage.
These grooves may beneficially guide the flow of the drug solution through the
target
/ID passage, while at the same time slowing the drug flow to increase the
amount of time that the
drug is in contact with the wall of the target passage. The drug delivery
balloon apparatus
and its associated channels also can help to minimize the volume of drug
solution required by
1
CA 02901178 2015-08-12
WO 2014/165751
PCT/US2014/032964
occupying a portion of the luminal volume: In addition, the drug delivery
balloon apparatus
may further include an occlusion balloon that may inflate upstream from the
drug delivery
balloon to permit adequate pressure to be maintained in the system during
infusion to
effectively advance the drug or therapeutic agent into and along the plurality
of grooves on
the outer surface of the drug delivery balloon. The occlusion balloon also
helps to prevent
peripheral washout by blocking blood flow from the treatment area.
Thus, in a first aspect, the present invention provides a drug delivery
balloon
apparatus comprising: (a) at least two lumens, comprising a first lumen and a
second lumen,
(b) a balloon inflation port in communication with the first lumen, (c) a drug
delivery port in.
communication with the second lumen, (d) a guidewire port in communication
with either the
second lumen or a third lumen, (e) an occlusion balloon, (f) a. drug delivery
balloon, wherein
an outer surface of the drug delivery balloon defines a plurality of grooves
extending from a
first end of the drug delivery balloon to a second end of the ding delivery
balloon, wherein
the occlusion balloon is disposed between the drug delivery balloon and the
balloon inflation
port, wherein the occlusion balloon and the drug delivery balloon are in
communication with
the first lumen, (g) one or more drug delivery channels extending the length
of the second
lumen, (h) one or more drug delivery ducts extending from the one or more drug
delivery
channels to an exterior surface of the second lumen, and wherein the one or
more drug
delivery ducts are defined only in a portion of the second lumen that is
disposed between the
occlusion balloon and the drug delivery balloon.
In one embodiment, the invention provides that the plurality of grooves may be
axially aligned with a. central axis of the drug delivery balloon. In various
other
embodiments, the plurality of grooves may be spiraled, helical, substantially
straight,
sinusoidal, or cross-hatched, for example. Further, in one example the drug
delivery port
CA 02901178 2015-08-12
WO 2014/165751
PCT/US2014/032964
may be branched such that two, three, four or more different drug solutions or
other solutions
may be introduced into the drug delivery port.
In another embodiment, the invention may provide that the one or more drug
delivery
channels comprises four channels and each drug delivery channel may be in
communication
with three drug delivery ducts such that there are a total of twelve drug
delivery ducts.
In a second aspect, the present invention also provides a method for
administering at
least one drug to a subject in need thereof using a drug delivery balloon
apparatus, the
method comprising: (a) introducing the drug delivery balloon apparatus
according to the first
aspect of the invention to a target passage, (b) inflating the occlusion
balloon and the drug
delivery balloon, (c) injecting a drug solution into the drug delivery port,
and (d) advancing
the drug solution through the second lumen to the one or more drug delivery
ducts into the
target passage in the subject and then into and along a plurality of grooves
defined in an outer
surface of the drug delivery balloon.
CA 02901178 2015-08-12
WO 2014/165751
PCT/US2014/032964
Brief Description of the Drawin2s
Figure IA is a side view of drug delivery balloon apparatus, in accordance
with one
embodiment of the invention.
Figure 1B is a front cross-sectional view of a two lumen configuration of the
drug delivery
balloon apparatus, in accordance with one embodiment of the invention.
Figure IC is a front cross-sectional view of a three lumen configuration of
the drug delivery
balloon apparatus, in accordance with one embodiment of the invention.
Figure 1D is a side view of the occlusion balloon and the drug delivery
balloon of the drug
delivery balloon apparatus, in accordance with one embodiment of the
invention.
Figure IF is a detail cross-sectional side view of the drug delivery balloon,
in accordance
with one embodiment of the invention.
Figure 2 is a flow chart depicting functions that can be carried out in
accordance with example
embodiments of the disclosed methods.
4
CA 02901178 2015-08-12
WO 2014/165751
PCT/US2014/032964
Detailed Description of. the Invention
Exemplary methods and systems are described herein. It should be understood
that
the word "exemplary" is used herein to mean "serving as an example, instance,
or
illustration." Any embodiment or feature described herein as "exemplary" is
not necessarily
to be construed as preferred Of advantageous over other embodiments or
features. The
exemplary embodiments described herein are not meant to be limiting. It will
be readily
understood that certain aspects of the disclosed systems and methods can be
arranged and
combined in a wide variety of different configurations, all of which are
contemplated herein.
Furthermore, the particular arrangements shown in the Figures should not be
viewed
as limiting. It should be understood that other embodiments may include more
or less of each
element shown in a given Figure. Further, some of the illustrated elements may
be combined
or omitted. Yet further, an exemplary embodiment may include elements that are
not
illustrated in the Figures.
As used herein, with respect to measurements, -about" means +/- 5
Further, as
used herein, -target passage- refers to the blood vessel or artery in which
the drug delivery
balloon is deployed to effectively administer a drug solution. The target
passage may further
include artificial lumens used, for example, as teaching aids.
In addition, as used herein, -drug solution" refers to any flowable material
that may
be administered into a target passage. When the drug solution comprises a
therapeutic to be
administered to a. subject, any suitable drug that can be administered in
solution can be used,
In various non-limiting embodiments, the therapeutic may comprise sirolimus,
heparin, and
cell-based therapies; and antineoplastic, anti-inflammatory, antiplateletõ
anticoagulant,
antifibrin; antithrombin, .antimitoticõ antibiotic, antiallergic and
antioxidant substances.
Examples of such antineoplastics and/or antimitotics include paclitaxel,
(e.g., TAXOL®
by Bristol-Myers Squibb Co., Stamford, Comm.), docetaxel (e.g..
Taxotere.RTN1.õ from
5
CA 02901178 2015-08-12
WO 2014/165751
PCT/US2014/032964
A.ventis S.A., Frankfurt, Germany.), methotrexate., azathioprine, vincristine,
vinblastine,
fluorouracil, doxorubicin hydrochloride (e.g., A.driamycin® from
Pharmacia. & Upjohn,
Peapack NJ.), and mitomycin. (e.g., .Mutamycin® from Bristol-Myers Squibb
Co.,
Stamfbrdõ Conn.). Examples of such antiplatelets, anticoagulants,
antifibrin, and
antithrombins include aspirin, sodium heparin, low molecular weight heparins,
heparinoids,
hirudin, argatroban, forskolin, vapiprost, prostacyclin and prostacyclin
analogues, dextran, D-
phe-pro-arg-chloromethylketone (synthetic .antithrombin), dipyridamole,
glycoprotein Hbflf
platelet membrane receptor antagonist antibody, recombinant hirudin, and
thrombin
inhibitors such as Angiomax. a (Biogen, Inc., Cambridge, Mass.). Examples of
such.
cytostatic or antiproliferative agents include angiopeptin, angiotensin
converting enzyme
inhibitors such as captopril (e.g., Capoten® and .Capozide® from
Bristol-Myers
Squibb Co., Stamford, Conn.), cilazapril or lisinopril (e.g.., Piinivii®
and Priazide®
from Merck & Co., Inc., Whitehouse Station, N. J.), calcium Channel blockers
(such as
nifedipine), colchicine, proteins, peptides, fibroblast growth factor (FGF)
antagonists, fish oil
(omega 3-fatty acid), histamine antagonists, lovastatin (an inhibitor of HMG-
CoA reductase,
a Cholesterol lowering drug, brand name Mevacor® from Merck & Co.., Inc.,
Whitehouse Station, N.J.)õ monoclonal antibodies (such as those specific for
Platelet-Derived
Growth Factor (PDGF) receptors), nitroprasside, phosphodiesterase inhibitors,
prostaglandin
inhibitors, suramin, serotonin blockers, steroids, thioprotease inhibitors,
triazolopyrimidine (a
PDGF antagonist), and nitric oxide. An example of an antiallergic agent is
permirolast
potassium. Other therapeutic substances or agents which may be appropriate
agents include
cisplatin, insulin sensitizers, receptor tyrosine kinase inhibitors,
carboplatin, alpha-interferon,
genetically engineered epithelial cells, steroidal anti-inflammatory agents,
non-steroidal anti-
inflammatory agents, antivirals, anticancer drugs, anticoagulant agents, free
radical
scavengers, estradiol, antibiotics, nitric, oxide donors, super oxide
dismutases, super oxide
6
CA 02901178 2015-08-12
WO 2014/165751
PCT/US2014/032964
dismutases mimics, 4-amino-2,2,6,6-tetramethylpiperidine-l-oxyl (4-amino-
TEMPO),
.tacrolimusõ dexamethasone. ABT-578, clobetasol, cytostatic agents, prodrugs
thereof, co-
drugs thereof, and a combination thereof. Other therapeutic substances or
agents may include
rapamycin and structural derivatives or functional analogs thereof, such as 40-
042-
hydroxy)ethyl-rapamycin (known by the trade name of EVEROLIMLIS), 40-043-
hydroxy)propyl-rapamycin, 40-042-(2-hydroxy)ethoxylethyl-rapamycia, methyl
rapamycin,
and 40-0-tetrazole-mpamvcin. In addition, non-therapeutic fluids, such as
water, may be
used, if the drug delivery balloon apparatus is being used in a teaching model
or training
demonstration, for example.
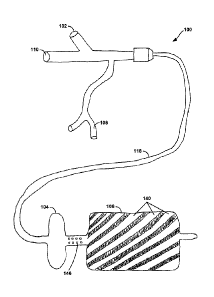
In a first aspect, Figure IA illustrates an example drug delivery balloon
apparatus. 100
in accordance with one embodiment of the invention. The drug delivery balloon
apparatus
100 may include three ports: (1) a balloon inflation port 102 that inflates
both an occlusion
balloon 104 and a drug delivery balloon 106, (2) a drug delivery port 108
through which a
drug solution is administered, and (3) a guidewire port 110 for receiving a
guidewire and the
inflated occlusion balloon 104 and drug delivery balloon 106. In one example
embodiment
as shown in Figure IA, the drug delivery port 108 may be bifurcated, such that
two, three,
four or more different drug solutions or other solutions may be introduced
into the drug
delivery port 108 as deemed appropriate for treatment.
In one example, the three ports lead to two parallel lumens 112. Figure 1B
illustrates
a front cross-sectional view of the two lumens. The balloon apparatus 100 may
include a first
lumen 114 in communication with the balloon inflation port 102 and may be
configured to
receive a saline contrast mixture, or any other suitable fluid medium, to
inflate the occlusion
balloon 104 and the drug delivery balloon 106. Further, the balloon apparatus
100 may
include a second lumen 116 in communication with the drug delivery port 108
and the
guidewire port 110. In one embodiment, the second lumen 116 may be sized and
shaped to
CA 02901178 2015-08-12
WO 2014/165751
PCT/US2014/032964
receive a drug solution. In one embodiment, the second lumen 116 may also be
sized and
shaped to receive a guidewire having a diameter in the range from about 0.25
ram to about 1
mm, and preferably in a range from about 0.254 mm to about 0.9652 mm. In one
embodiment, the first lumen 114 and the second lumen 116 may be enclosed in a
sheath 118.
The second lumen 116 may include one or more drug delivery channels 120
extending the
length of .the second lumen 116. These drug delivery channels 120 may be used
to transport
the drug solution from the drug delivery port 108 to a target passage. The
second lumen 116
may also include a guidewire channel 122 extending the length of the second
lumen 116. In
another example, the second lumen 116 may include a single channel for both
the guidewire
and drug solution.
In such a configuration, the guidewire may be removed after use so that the
drug
solution can pass through the second lumen 116. In operation, the balloon
apparatus 100 may
be configured to infuse the drug solution while the .guidewire is in the
second lumen 116. In
such a configuration, the second lumen 116 would have a larger diameter than
the guidewire
from a location between the guidewire port. 110 and the drug delivery port 108
until just distal
to the drug delivery ducts 146. The second lumen 116 would shrink down to
about the
diameter of the guidewire just distal to the drug delivery ducts 146 to the
distal end of the
balloon. Further, the second lumen 116 would shrink down to about the diameter
of the
guidewire proximal to the drug delivery port 108, so as to prevent the drug
solution from
exiting the guidewire port 110. In another example, a flange or one-way valve
may be used
to prevent the drug solution from exiting the guidewire port 110. Other
configurations are
possible as well.
In another embodiment, the .three ports may be coupled to three concentrically
aligned
lumens 124. For example, Figure 1C illustrates a front cross-sectional view of
the three
lumens 124. As shown in Figure 1C, the three concentrically aligned lumens 124
comprise
CA 02901178 2015-08-12
WO 2014/165751
PCT/US2014/032964
an inner lumen 126, a middle lumen 128, and an outer lumen 130, where the
first lumen is
arranged as the inner lumen, the second lumen is arrani-,ed as the middle
lumen and the third
lumen is arranged as the outer lumen. The inner lumen 126 may be in
communication with.
the guidewire port 110 and may be sized and shaped to receive a guidewire
having a diameter
in the range from about 0.25 mm to about 1 mm, and preferably in a range from
about 0.254
mm to about 0.9652 min. The middle lumen 128 may be ihi communication with the
drug
delivery port 108. The middle lumen 128 may include a plurality of flexible
spacers 132 that
extend between the inner lumen 126 and the outer lumen 130 to maintain the
structural
integrity of the middle lumen 128. These spacers 132, in combination with the
middle lumen.
128 and the inner lumen 126, may further define a one or more drug delivery
channels 134
extending the length of the middle lumen 128. As discussed above, these drug
delivery
channels 134 may be used to transport the drug solution from the drug delivery
port 108 to a
target passage. The outer lumen 130. may be in communication with the balloon
inflation
port 102. The outer lumen 130 may also include a plurality of flexible spacers
136 to help
maintain the structural integrity of the outer lumen 130. These spacers 136,
in combination
with the outer lumen 130 and middle lumen 128, may also define a plurality of
fluid delivery
channels 138 extending the length of the outer lumen 130. These fluid delivery
channels 138
may be in fluid communication with the occlusion balloon 104 and the drug
delivery balloon
106.
Figure 1D illustrates the occlusion balloon 104 and the drug delivery balloon
106 of
the drug delivery balloon apparatus 100. 'The occlusion balloon 104 may be
composed of
atraumatic compliant materials such as polyurethane, latex, or silicone, among
other
possibilities, that results in a low burst pressure of about 5 atm, for
example. However, the
occlusion balloon 104 may be configured to withstand greater pressures, for
example up to
about 20 atm. The occlusion balloon 104 may be configured to conform to the
shape and size
9
CA 02901178 2015-08-12
WO 2014/165751
PCT/US2014/032964
of the target passage via low pressure inflation, about 1 to 2 atm. Once
inflated, the occlusion
balloon 104 may provide occlusion in the target passage to allow for drug
delivery into the
target passage downstream from the occlusion balloon 104 to minimize dilution
of the drug
solution from blood flow. The inflated diameter of the occlusion balloon 104
may range
from about 2.5 .mm to about 12 mm and is preferably in a range from about 2.5
rum to about
6 min. The length of the occlusion balloon 104 may range from about 20 mm to
about 40
mm. In one embodiment, the inflated diameter of the occlusion balloon 104
ranges from
about the same as the inflated diameter of the drug delivery balloon 106 to
about 2 min larger
than the inflated diameter of the drug delivery balloon 106. In operation, the
occlusion.
balloon 104 may be inflated prior to the introduction of the drug solution
into the drug
delivery port 108.
The drug delivery balloon 106 may be made of compliant materials such as
polyurethane, latex, or silicone that results in a low burst pressure of about
5 atm, for
example. The length of the drug delivery balloon 106 may range from about 20
mm to about
200 mm. In various embodiments, the length of the drug delivery balloon 106
ranges from
about 80 mm to about 200 mm, from about 100 mm to about 200mm, from about 120
min to
about 200 mm, from about 140 mm to about 200 mm, from about 160 mm to about
200 min,
from about 180 mm to about 200 mm, from about 60 mm to about 120 mm, from
about 60
mm to about 100 mm, and from about 10 min to about 80 min. In one embodiment,
the drug
delivery balloon 106 may have an inflated diameter ranging from about 2.5 mm
to about 12
mm and is preferably in a range from about 2.5 mm to about 6 mm. In various
embodiments,
the inflated diameter of the drug delivery balloon 106 may range from about
2.5 mm to about
3 min from about 4 mm to about 5 mm, and from about 5 mm to about 6 mm.
The outer surface of the drug delivery balloon 106 may define a plurality of
grooves
140 for receiving the drug solution. These grooves 140 may extend from the
first end 142 to
CA 02901178 2015-08-12
WO 2014/165751
PCT/US2014/032964
the second end 144 of the drug delivery balloon 106. The plurality of grooves
140 may serve
to (1) guide the flow of the drug solution and (2) slow the flow of the drug
solution to
increase the time of contact of the drug with the wall of the target passage.
The plurality of
grooves 140 are preferably axially aligned with a central axis of the drug
delivery balloon
106 and may be spiraled, helical, sinusoidal Of substantially straight, among
other
possibilities, in various embodiments. Spiraled, helical or sinusoidal grooves
are preferred
over straight grooves, because the more tortuous grooves provide more surface
area to
contact the vessel wall and further extend the amount of time that the drug
solution contacts
the vessel wall. Further, any pattern of grooves is contemplated including a
cross-hatched or
waffle pattern, for example.
The occlusion balloon 104 may be disposed between the drug delivery balloon
106
and the balloon inflation port 102 such that both the occlusion balloon 104
and the drug
delivery balloon 106 may be in communication with the second lumen 116 or the
outer lumen
130 and receive fluid from the balloon inflation port 102. The occlusion
balloon 104 and the
drug delivery balloon 106 may be separated from each other by a distance
ranging from about
1 mm to about 10 rum, and preferably from about 3 mm to about 5 mm. This
distance allows
adequate pressure to be maintained in the system such that the drug solution
may be
effectively advanced into and along the plurality of grooves 140 on the outer
surface of the
drug delivery balloon 106.
One or more drug delivery ducts 146 may extend from the one or more drug
delivery.
channels 120 defined in the second lumen 116 to an exterior surface of the
second lumen 116..
These drug delivery ducts 146 may be defined in a portion 148 of the second
lumen 116 that
is disposed between the occlusion balloon 104 and the drug delivery balloon
106. hi other
words, these drug delivery ducts 130 may be downstream from the occlusion
balloon 104 in
operation. In one embodiment, the one or more drug delivery channels 120 may
comprise
11
CA 02901178 2015-08-12
WO 2014/165751
PCT/US2014/032964
four to eight channels. In another embodiment, the one or more drug delivery
channels 120 is
each in fluid communication with one to six drug delivery ducts 146. In a
further
embodiment, the one or more drug delivery channels 120 may comprise four
charnels and
each drug delivery channel may be in fluid communication with three chug
delivery ducts
such that there are a total of twelve drug delivery ducts. The number of drug
delivery ducts
may depend upon the length of portion 148 of the second lumen 116 extending
between the
occlusion balloon 104 and the drug deliver), balloon 106 and/or the diameter
of the drug
delivery ducts 146, among other possibilities..
Figure 1 E illustrates a cross-sectional side view of the drug delivery
balloon 106. As
shown in Figure 1E, the drug delivery balloon 106 includes a plurality of
grooves 140. In
operation, the drug solution advances downstream into and along the plurality
of grooves 140
defined in the outer surface of the drug delivery balloon 106. Once the drug
solution exits the
plurality of grooves 140 at the second end 144 of the drug delivery balloon
106, the drug
solution may be cleared via normal arterial blood flow and ultimate
physiological function.
Figure 2 is a simplified flow chart illustrating a method according to an
exemplary
embodiment. Although the blocks are illustrated in a sequential order, these
blocks may also
be performed in parallel, and/or in a different order than those described
herein. Also, the
various blocks may be combined into fewer blocks, divided into additional
blocks, and/or
removed based upon the desired implementation.
At block 202, the method involves introducing the drug delivery balloon
apparatus
according to any of the foregoing embodiments to a target passage. The drug
delivery
balloon apparatus may be introduced and delivered in a standard coaxial
manner, via over-
the-wire or rapid exchange techniques, as examples.
At block 204, the method involves inflating the occlusion balloon and the drug
delivery balloon. In one embodiment, the occlusion balloon and the drug
delivery balloon
12
CA 02901178 2015-08-12
WO 2014/165751
PCT/US2014/032964
may be inflated by injecting a saline contrast mixture, for example, into the
balloon inflation
port. The saline contrast mixture may then be advanced through a first lumen
to the
occlusion balloon and the drug delivery balloon until both balloons are
inflated. The
occlusion balloon may inflate at a slightly faster rate, since the occlusion
balloon and the drug
delivery balloon are connected in series such that the occlusion balloon
receives the saline
contrast inflation mixture first. In another embodiment, the occlusion balloon
and dmg
delivery balloon may be inflated using any other suitable fluid medium.
After both the occlusion balloon and the drug delivery balloon have been
inflated, the
method continues at block 206 with injecting a drug solution into the drug
delivery port. In.
one embodiment, the drug delivery port is bifurcated, such that two, three,
four or more
different drug solutions or other solutions may be introduced into the drug
delivery port as
deemed appropriate.
At block 208, the method involves advancing the drug solution through a second
lumen to the one or more drug deliver), ducts into a target passage in the
subject. At this
stage, the space between the occlusion balloon and the drug delivery balloon
acts as a
reservoir storing the drug solution as it is delivered via the drug delivery
ducts, Due to the
pressure at which the drug solution is being introduced to the drug delivery
port, the drug
solution advances downstream into and along the plurality of grooves defined
in the outer
surface of the drug delivery balloon. The pressure at which the drug solution
is administered
should not exceed about 2 atm. Once the drug solution exits the plurality of
grooves at the
second end of the drug delivery balloon, the drug solution may be cleared via
normal arterial
blood flow and ultimate physiological function.
While various aspects and embodiments have been disclosed herein, other
aspects and
embodiments will be apparent to those skilled in the art. All embodiments
within and between
different aspects of the invention can be combined unless the context clearly
dictates
13
CA 02901178 2015-08-12
WO 2014/165751
PCT/US2014/032964
otherwise. The various aspects and embodiments disclosed herein are for
purposes of
illustration and are not intended to be limiting, with the true scope and
spirit being indicated
by the tbilowing claims.
14