Note: Descriptions are shown in the official language in which they were submitted.
CA 02901331 2015-08-21
TITLE: METHOD, KIT, AND TAPE FOR WOUND CARE
FIELD
[0001] The disclosure relates to wound care. More
specifically, the
disclosure relates to wound care tapes, methods for using wound care tapes,
and
kits containing wound care tapes.
BACKGROUND
[0002] U.S.
Patent Application Publication No. 2013/0334084 (Arbesman)
purports to disclose a contusion patch comprising a high stretch therapeutic
tape
with an adhesive backing. The tape has an anchoring portion that adheres to a
contusion and a plurality of fingers that extend from the anchoring portion.
The
tape is less than about 5 inches in length and about 2 inches in width when
unstretched. A frangible release liner covers the adhesive backing of the tape
prior to application. The release liner has a greater surface area than the
tape, so
that an exposed strip of the release liner extends around the tapes perimeter.
The release liner is scored at junctions between the anchoring portion and the
fingers to allow selective removal during staggered application of the tape.
SUMMARY
[0003] The
following summary is intended to introduce the reader to
various aspects of the disclosure, but not to define any invention.
[0004]
According to some aspects, a kit for wound care is disclosed. The
kit comprises a package having a sealed and sterile interior volume. A wound
care tape is in the interior volume. The wound care tape comprises a high-
stretch strip of woven material having a first face and an opposed second
face,
and an adhesive on the first face in a discontinuous pattern. A set of spaced
apart adhesive-covered portions of the first face are covered by the adhesive,
and a set of spaced apart adhesive-free portions of the first face are free of
the
CA 02901331 2015-08-21
. .
- 2 -
adhesive. The wound care tape does not include an absorbent pad adjacent the
first face. A release liner is in the sterile interior volume and adhered to
the first
face by the adhesive.
[0005] The woven material may be woven cotton. The adhesive may
be a
poly-acrylic adhesive.
[0006] At least some of the adhesive-covered portions and some
of the
adhesive-free portions may be arranged in alternating curved bands. The tape
may extend along a longitudinal axis, and at least some of the adhesive-free
portions may extend transverse to the longitudinal axis.
[0007] The strip of woven material may comprise a pair of
adjacent lobes.
The tape may be generally lemniscate shaped.
[0008] The kit may comprise a plurality of the wound care tapes
in the
package, and the release liner may be adhered to the plurality of the wound
care
tapes. The release liner may be perforated along a boundary between each
adjacent pair of the wound care tapes. The plurality of the wound care tapes
may be arranged in a grid on the release liner.
[0009] The strip of woven material may have an unstretched
length, and
may be elastically stretchable to a stretched length that is at least 1.4
times the
unstretched length, or at least 1.5 times the unstretched length.
[0010] According to some aspects, a wound care tape is
disclosed. The
wound care tape comprises a sterile high-stretch strip of woven material
having a
first face and an opposed second face. A sterile adhesive is on the first face
in a
discontinuous pattern. A set of spaced apart adhesive-covered portions of the
first face are covered by the adhesive, and a set of spaced apart adhesive-
free
portions of the first face are free of the adhesive. The wound care tape does
not
include an absorbent pad adjacent the first face.
CA 02901331 2015-08-21
. .
- 3 -
[0011]
The woven material may be woven cotton. The adhesive may be a
poly-acrylic adhesive.
[0012]
At least some of the adhesive-covered portions and at least some
of the adhesive-free portions may be arranged in alternating curved bands. The
tape may extend along a longitudinal axis, and at least some of the adhesive-
free
portions may extend transverse to the longitudinal axis.
[0013]
The strip of woven material may comprise a pair of adjacent lobes.
The tape may be generally lemniscate shaped.
[0014]
The strip of woven material may have an unstretched length, and
may be elastically stretchable to a stretched length that is at least 1.4
times the
unstretched length, or at least 1.5 times the unstretched length.
[0015]
The tape may be adherable to skin for at least 7 days. The tape
may be adherable to skin for at least 7 to 14 days.
[0016]
Also provided is a use of the tape for treating a wound, and a tape
for the use in treating a wound. The tape may be used for treating a wound.
The
wound may be an acute wound. The wound may be a surgical incision. The
tape may be used together with stitches, and/or together with skin glue.
Alternatively, the tape may be used alone, without skin glue or stitches.
[0017]
According to some aspects, a method for treating a wound is
disclosed. The method comprises a) adhering a first set of spaced apart
adhesive-covered portions of a high-stretch strip of woven material to a
patient's
skin on a first side of a wound; b) stretching the high-stretch strip of woven
material across the wound to a stretched configuration; and c) adhering
a
second set of spaced apart adhesive-covered portions of the high-stretch strip
of
woven material to the patient's skin on a second side of the wound while the
wound care strip is in the stretched configuration.
CA 02901331 2015-08-21
. .
- 4 -
[0018] During step a), a first set of spaced apart adhesive-
free portions of
the high stretch strip of woven material may be maintained as not adhered to
the
patient's skin. During step c), a second set of spaced apart adhesive-free
portions of the high stretch strip of woven material may be maintained as not
adhered to the patient's skin.
[0019] The method may further comprise leaving the strip of
woven
material adhered to the patient's skin for at least 7 days, for example for 7
to 14
days.
[0020] Steps a) to c) may be carried out without applying an
absorbent
pad between the strip of woven material and the wound.
[0021] The wound may be an acute wound. The wound may be a
surgical
incision.
[0022] The method may further comprise applying a second high-
stretch
strip of woven material to the wound by: a) adhering a first set of spaced
apart
adhesive-covered portions of the second high-stretch strip of woven material
to
the patient's skin on the first side of the wound, while maintaining a first
set of
spaced apart adhesive-free portions of the second high stretch strip of woven
material not adhered to the patient's skin; b) stretching the second high-
stretch
strip of woven material across the wound to a second stretched configuration;
and c) adhering a second set of spaced apart adhesive-covered portions of the
second high-stretch strip of woven material to the patient's skin on the
second
side of the wound while the second wound care strip is in the second stretched
configuration, while maintaining a second set of spaced apart adhesive-free
portions of the second high stretch strip of woven material not adhered to the
patient's skin.
[0023] The first high-stretch strip of woven material may be
applied in a
transverse orientation with respect to the second high-stretch strip of woven
CA 02901331 2015-08-21
. .
- 5 -
material. The second high-stretch strip of woven material may be applied to
overlap with at least a portion of the first high-stretch strip of woven
material.
[0024] The method may further comprise, prior to step a),
applying stitches
to the wound, and/or applying skin glue to the wound. Alternatively, the high
stretch strip of woven material may be applied without prior application of
any
stitches and skin glue.
[0025] The high stretch strip of woven material may be sterile.
For
example, prior to step a), the high stretch strip of woven material may be
sterilized.
[0026] The high stretch strip of woven material may extend
along a
longitudinal axis, and when adhered may be stretchable parallel to the
longitudinal axis and transverse to the longitudinal axis.
BRIEF DESCRIPTION OF THE DRAWINGS
[0027] The drawings included herewith are for illustrating
various
examples of articles, methods, and apparatuses of the present disclosure and
are not intended to limit the scope of what is taught in any way. In the
drawings:
[0028] Figure 1 is a top view of an example wound care tape;
[0029] Figure 2 is a bottom view of the tape of Figure 1;
[0030] Figure 3 is an enlarged side view of the region shown in
box 3 in
Figure 2;
[0031] Figure 4 is a top view of an alternative example wound
care tape;
[0032] Figure 5 is a top view of the wound care tape of Figure
1 on a
release liner;
[0033] Figure 6 is a bottom view of the wound care tape and
release liner
of Figure 5;
CA 02901331 2015-08-21
. .
- 6 -
[0034] Figure 7 is a top plan view of a plurality of the wound
care tapes of
Figure 1 on a common release liner;
[0035] Figure 8 is a top plan view of the wound care tape and
release liner
of Figure 5, inside an example sealed and sterile package;
[0036] Figure 9 is a cross-section taken along line 9-9 in
Figure 8;
[0037] Figure 10 is a schematic top view of a body part with a
wound, and
with stitches applied to the wound;
[0038] Figure 11 shows the body part, wound, and stitches of
Figure 10,
with a first lobe of the wound care tape of Figure 1 being applied to the skin
adjacent the wound;
[0039] Figure 12 shows the body part, wound, stitches, and
wound care
tape of Figure 11, with the wound care tape being stretched across the wound;
[0040] Figure 13 shows the body part, wound, stitches, and
wound care
tape of Figure 12, with the second lobe of the wound care tape being applied
to
the skin adjacent the wound;
[0041] Figure 14 shows the body part, wound, stitches, and
wound care
tape of Figure 13, with additional wound care tapes applied to the wound.
[0042] Figure 15 shows the body part, wound, and stitches of
Figure 10,
with the wound care tape of Figure 4 applied to the wound; and
[0043] Figure 16 shows the body part, wound, and stitches of
Figure 10,
with a plurality of the wound care tapes of Figure 4 applied to the wound.
DETAILED DESCRIPTION
[0044] Various apparatuses or processes will be described
below to
provide an example of an embodiment of the claimed subject matter. No
embodiment described below limits any claim and any claim may cover
processes or apparatuses that differ from those described below. The claims
are
CA 02901331 2015-08-21
. .
- 7 -
not limited to apparatuses or processes having all of the features of any one
apparatus or process described below or to features common to multiple or all
of
the apparatuses described below. It is possible that an apparatus or process
described below is not an embodiment of any exclusive right granted by
issuance
of this patent application. Any subject matter described below and for which
an
exclusive right is not granted by issuance of this patent application may be
the
subject matter of another protective instrument, for example, a continuing
patent
application, and the applicants, inventors or owners do not intend to abandon,
disclaim or dedicate to the public any such subject matter by its disclosure
in this
document.
[0045] Disclosed herein is a tape for wound care (also
referred to herein
as a "wound care tape", or simply as a "tape"), and related kits and methods.
The wound care tapes disclosed herein are related to kinesiology tapes.
However, it is believed that kinesiology tapes have heretofore not been used
directly on broken skin for wound care. That is, kinesiology tapes are
traditionally
used on intact skin for treating conditions associated with muscles, joints,
and/or
contusions. Prior art publications have cautioned against using kinesiology
tape
on broken skin, as it has been believed that applying kinesiology tape to
broken
skin can cause extensive tissue damage (Stockheimer, 2007). Accordingly, in
rare instances where kinesiology tape has been used for wound treatment, care
has been taken to apply a pad between the kinesiology tape and the wound
(Oka, 2010).
[0046] It has presently been determined that kinesiology tapes
can be
used directly on broken skin for wound care. For example, kinesiology tapes,
when cut to a suitable shape and sterilized, can be applied directly to a
wound, to
hold the wound closed. It has been determined that the use of sterilized
kinesiology tapes directly on broken skin for wound care does not necessarily
cause tissue damage, such as the extensive tissue damage purported in the
prior
art. It has further been determined that the use of sterilized kinesiology
tapes
CA 02901331 2015-08-21
- 8 -
directly on broken skin for wound care can be beneficial, in that it may be
worn
for prolonged periods of time, may promote or facilitate healing, may be
considered easy to use by physicians, and may be considered to be comfortable
by patients.
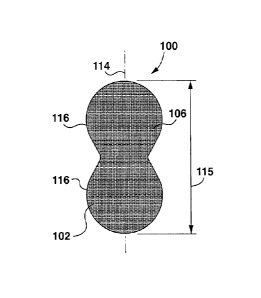
[0047] Referring now to Figures 1 to 3, an example wound care tape 100
is shown. In the example shown, the wound care tape 100 is made from (i.e. cut
from) kinesiology tape, and includes a high stretch strip of woven material
(referred to hereinafter as "strip 102") with an adhesive 108 thereon.
[0048] The woven material may in some examples be a woven cotton.
The use of a woven material allows for breathability of the tape, which is
believed
to facilitate and/or promote wound healing, and is also believed to be
comfortable
for a patient.
[0049] As mentioned above, the strip 102 may be high stretch. As used
herein, the term 'high stretch' indicates that the strip 102 is highly
stretchable in
at least one direction. For example, the strip may be highly stretchable along
its
length. For example, the strip 102 may have an unstretched length 115, and
may be elastically stretchable to a length that is at least 1.4 times the
unstretched
length 115, or at least 1.5 times the unstretched length 115. The strip 102
may
additionally be highly stretchable along its width, or may be minimally-
stretchable
or non-stretchable along its width. The term 'minimally' stretchable indicates
that
the strip 102 is stretchable along its width, but less stretchable than it is
along its
length. The term 'non-stretchable' indicates that the strip 102 is not
stretchable
along its width, or is stretchable to only a negligible extent along its
width.
[0050] The strip 102 has a first face 104 (shown in Figure 2) that in use
faces the skin of a patient, and an opposed second face 106 (shown in Figure
1).
[0051] Referring to Figure 2, in the example shown, the adhesive 108 is
on
the first face 104 in a discontinuous pattern. That is, referring to Figure 3,
the
first face 104 includes a set of adhesive-covered portions 110 (i.e. portions
that
CA 02901331 2015-08-21
. .
- 9 -
are covered by the adhesive), and a set of adhesive-free portions 112 (i.e.
portions that are free of adhesive, so that the first face is exposed). This
may
allow for the tape 100 to stretch, even when adhered to skin. For example,
when
the tape 100 is adhered to skin, the adhesive-free portions 112 may readily
stretch to accommodate patient movement, while the adhesive-covered portions
110 may remain adhered to the skin and remain generally unstretched, minimally
stretched, or less stretched than the adhesive-covered portions. This may
allow
for the tape to be worn for prolonged periods of time, since the tape may not
necessarily peel off the skin when stretched to accommodate patient movement.
[0052] Referring still to Figures 2 and 3, in the example
shown, the
adhesive-covered portions 110 and adhesive-free portions 112 are provided in
an alternating pattern, so that the adhesive-covered portions 110 are spaced
apart from each other, and the adhesive-free portions 112 are spaced apart
from
each other.
[0053] Referring still to Figure 2, the tape 100 extends along
a longitudinal
axis 114. At least some of the adhesive-free portions 112 may extend non-
parallel to the longitudinal axis 114. Furthermore, at least some of the
adhesive-
free portions 112 may extend non-perpendicular to the longitudinal axis. For
example, the adhesive-free portions may be curved, and may extend generally
diagonally with respect to the longitudinal axis 114. This may allow for the
tape
100 to stretch and/or accommodate movement in both a lengthwise direction and
a widthwise direction (as well as additional directions that have both a
lengthwise
and widthwise component), even when adhered to skin. In the example shown,
the adhesive-covered portions 110 and adhesive-free portions 112 are provided
as curved bands that are arranged in an alternating pattern and extend
generally
diagonally with respect to the longitudinal axis.
[0054] The adhesive 108 may be any suitable skin-safe
adhesive, such as
a polyacrylic adhesive. The adhesive 108 may be long lasting, so that the
wound
care tape can be worn on skin for prolonged periods of time. For example, the
CA 02901331 2015-08-21
. .
- 10 -
tape may be adherable to skin for at least 7 days. For further example, the
tape
may be adherable to skin for 7 to 14 days.
[0055] Referring still to Figure 2, in the example shown, the
tape 100 does
not include an absorbent pad adjacent the first face 104. That is, an
absorbent
pad is not provided as part of the tape 100. When the tape 100 is applied to a
wound, the strip 102 and adhesive 108 may be applied directly to the broken
skin
and may contact the wound. It has been determined that despite what has been
cautioned in the prior art, this does not necessarily cause tissue damage.
[0056] Wound care tapes may be provided in a variety of shapes.
In the
example shown in Figures 1 to 3, the strip 102 includes a pair of adjacent
lobes
116, so that the tape 100 is generally lemniscate or figure-8 shaped. It has
been
determined that this shape is particularly useful for wound closure, in that
it can
be easily and readily applied by physicians, is comfortable for patients, and
can
remain on a wound for a prolonged period of time (e.g. up to 7 days or longer)
and thereby facilitate and/or promote wound healing.
[0057] A wound care tape 400 of an alternative shape is shown
in Figure
4. The tape 400 includes a central anchor portion 402, with fingers 404
extending from opposed sides of the anchor portion 402. The fingers 404 are
arranged in pairs on opposed sides of the anchor portion. This shape may also
be referred to as an X-shape, since when the tape is applied (as described
below
with respect to Figures 15 and 16), adjacent fingers may be separated from
each
other, so that the tape 400 forms an X-shape.
[0058] Wound care tapes may also be provided in a variety of
sizes. In
some examples, the strip 102 may have an unstretched length 115 of between
about 2.5 cm and 7.5 cm, or between about 4 cm and 6 cm, or about 5cm. In
some examples, the strip 402 may have an unstretched length of between about
4.5 cm and 25 cm, or between about 6 cm and 21 cm. In some particular
examples, the strip 402 may have a length of about 6.5 cm. In some particular
CA 02901331 2015-08-21
. ,
-11 -
examples, the strip 402 may have a length of about 11 cm. In some particular
examples, the strip 402 may have a length of about 20.5 cm.
[0059] Referring now to Figures 5 and 6, the tape 100 may be
provided on
a release liner 118. The release liner 118 may be adhered to the first face
104
by the adhesive 108. The release liner 118 may be perforated along a boundary
120 between the lobes 116, to facilitate staggered application of the lobes to
the
skin. For example, the release liner 118 may be severed along the boundary
120, and the resulting release liner portion removed from one lobe 116 of the
strip 102. The one lobe 116 may then be applied to the skin adjacent a wound.
The remaining release liner portion may then be removed from the other lobe
116, and the other lobe 116 may then be applied to the skin adjacent a wound.
[0060] Referring to Figure 7, in some examples, a plurality of
the tapes
100 may be provided, and a common release liner 122 may be adhered to the
plurality of tapes 100. The tapes 100 may be arranged in a grid on the release
liner 122, and the release liner 122 may be perforated along a boundary 124
between each adjacent pair of the tapes 100.
[0061] As mentioned above, the tapes disclosed herein (e.g.
tapes 100
and/or 400) may be sterile. For example, both the strip 102 and the adhesive
108 may be sterile, in order to be suitable for use on broken skin. Referring
now
to Figures 8 and 9, in some examples, the tape 100 may be provided in a kit
that
includes a package 126 having a sealed and sterile interior volume 128, with
the
tape 100 and the release liner 118 in the interior volume 128. The package 126
may be a medical grade peel pouch. The package 126, with the tape 100 and
the release liner 118 sealed in the interior volume 128, may in some examples
be
sterilized by steam sterilization. The package 126 may be opened immediately
prior to use of the tape 100.
CA 02901331 2015-08-21
. .
- 12 -
[0062]
In alternative examples, a plurality of wound care tapes 100 may be
provided in a single package. For example, the release liner 122 and plurality
of
tapes 100 shown in Figure 6 may be provided in a sealed and sterile package.
[0063]
The wound care tapes described above may be used on various
types of wounds, including but not limited to acute wounds (e.g. due to
accidents), surgical incisions (e.g. for surgical reconstruction of burns or
scars),
minor wounds (e.g. minor cuts), major wounds, flaps (e.g. free flaps or
transpositional flaps), and fresh wounds. The wound care tapes may be used
alone, together with stitches, or together with skin glue, depending on the
severity of the wound. For example, for some wounds, wound care tapes alone
may optionally be used, without skin glue or stitches. For further example,
for
other wounds, wound care tapes may be used together with stitches and/or skin
glue. For example, stitches and/or skin glue may be applied prior to
application
of the tape.
[0064]
The wound care tapes may generally be applied in a stretched
configuration, so that the elasticity of the tapes aids in holding the wound
closed,
and supports the skin surrounding the wound.
[0065]
An example method of using a wound care tape will now be
described. For simplicity, the method will be described mainly with respect to
wound care tape 100; however, the method may be carried out with other tapes
(e.g. tape 400), and the tape 100 may be applied according to other methods.
[0066]
Referring to Figure 10, a body part 1000 with a wound 1002 is
shown. The wound 1002 may be, for example, an acute wound. In the example
shown, prior to use of any tapes 100, the wound is closed with stitches 1004.
A
set of wound care tapes 100 may then be applied to the wound 1002, over the
stitches 1004, to assist in holding the wound 1002 closed, and to support the
skin
surrounding the wound 1002.
CA 02901331 2015-08-21
- 13 -
[0067] For
example, referring to Figure 11, each wound care tape 100 may
be applied by first adhering a first lobe 116a of the strip 102 to the
patient's skin
on a first side 1006 of the wound 1002. This may be done by pressing the first
lobe 116a to the skin on the first side 1006 of the wound 1002, so that the
adhesive-covered portions 110 (not shown in Figures 11 to 13) on the first
lobe
116a (also referred to herein as a "first set" of adhesive covered portions)
adhere
to the patients skin, while the adhesive-free portions 112 on the first lobe
116a
are maintained non-adhered to the patients skin.
[0068]
Referring to Figure 12, the strip 102 may then be stretched across
the wound to a stretched configuration. For example, the strip 102 may be
stretched lengthwise to up to about 1.4 times its unstretched length, or up to
about 1.5 times its unstretched length.
[0069]
Referring to Figure 13, the second lobe 116b of the strip 102 may
then be adhered to the patient's skin on a second side 1008 of the wound 1002.
This may be done by pressing the second lobe 116b to the skin on the second
side 1008 of the wound 1002, so that the adhesive-covered portions 110 (not
shown in Figures 11 to 13) on the second lobe 116b (also referred to herein as
a
"second set" of adhesive-covered portions) adhere to the patients skin, while
the
adhesive-free portions 112 (not shown in Figures 11 to 13) on the second lobe
116b (also referred to herein as a "second set" of adhesive-free portions) are
maintained non-adhered to the patients skin.
[0070] The
entire strip 102 may then optionally be rubbed against the skin,
in order to facilitate adhesion of the entire strip 102 to the skin.
[0071] As
shown in Figures 11 to 13, the tape 100 may be applied without
applying an absorbent pad between the strip 102 and the wound 1002. That is,
the first lobe 116a is adhered to the skin, the strip 102 is stretched, and
the
second lobe 116b is adhered to the skin, without applying an absorbent pad
between the strip 102 and the wound 1002.
CA 02901331 2015-08-21
. ,
- 14 -
[0072] Referring to Figure 14, the steps shown in Figures 11 to
13 may be
repeated with additional tapes 100 (e.g. repeated with a second tape through a
fourth tape), so that a set of tapes 100 is applied to the wound. For
simplicity,
the steps described with respect to Figures 11 to 13 are not repeated herein
for
the additional tapes.
[0073] The tape 100 may optionally be left adhered to the
patient's skin
until removal of the stitches 1004. For example, the tapes 100 may be left
adhered to the patient's skin for at least 7 days, for example for 7 to 14
days.
[0074] In the example described with respect to Figures 11 to
14, each
tape 100 is applied so that its longitudinal axis 114 (one of which is shown
in
Figure 14) extends generally perpendicularly or transverse to the wound 1002.
In alternative examples, one or more tapes may be applied so that its
longitudinal
axis is generally parallel to the wound. For example, referring to Figure 15,
a
tape 400 as described above with respect to Figure 4 has been applied to a
wound so that its longitudinal axis 414 is parallel to the wound 1002. For
further
example, referring to Figure 16, a set of tapes 400 has been applied to the
wound 1002 so that the longitudinal axis 414a of a first set of the tapes 400a
is
parallel to the wound 1002, and so that the longitudinal axis 414b of a second
set
of the tapes 400b is perpendicular to the wound 1002. In other words, some of
the tapes 400 are applied in a transverse orientation with respect to other of
the
tapes 400. In this example, the tapes 400 are applied in an overlapping
fashion,
so that at least a portion of each tape 400 overlaps with at least a portion
of
another tape 400. More specifically, the fingers 404 of some of the tapes 400a
overlap with fingers 404b of other of the tapes (only some of the overlapping
fingers 404a, 404b are labelled in Figure 16).
[0075] It is believed that because the adhesive 108 is in a
discontinuous
pattern on the strip 102, the tape 100 is able to move and stretch as the
patient
moves, and thereby can remain on the skin for prolonged periods of time. For
example, when the tape 100 is adhered and in use, it is stretchable and can
CA 02901331 2015-08-21
- 15 -
accommodate movement parallel to its longitudinal axis 114, and can also
accommodate movement transverse to its longitudinal axis 114, without peeling
off of the skin. It is further believed that because the strip is made from a
woven
material, it is breathable and feels generally soft and comfortable to the
patient.
[0076] In any of the above examples, topical medications may be applied
to the skin prior to the applications of the tape. Such topical medications
may be
used, for example, to protect the skin, enhance adherability of the tapes,
and/or
provide antiseptic properties. For example, compound tincture of benzoin may
be applied to the skin prior to the application of the tapes, in order to
protect the
skin, enhance adherability of the tapes, and provide antiseptic properties. In
other examples, antibiotic creams or ointments may be applied.
[0077] While the above description provides examples of one or more
processes or apparatuses, it will be appreciated that other processes or
apparatuses may be within the scope of the accompanying claims.
EXAMPLES
[0078] Wound care tapes as described above were tested on various
wounds, as described in further detail below. The tests were carried out in
the
Department of Surgery and Plastic Surgery of Sunnybrook Hospital, in Toronto,
Ontario, Canada.
[0079] Wound care tapes were provided by Spidertech Inc., of Toronto,
Ontario, Canada. The wound care tapes included a strip of high stretch woven
cotton, with a polyacrylic adhesive on a first face thereof in a discontinuous
pattern (as shown in Figure 2). The tapes did not include an absorbent pad.
The
tapes were provided in figure-8 shapes (as shown in Figure 1), as well as X-
shapes (as shown in Figure 4). The tapes were provided on a release liner, in
a
sealed and sterile package.
EXAMPLE 1
CA 02901331 2015-08-21
- 16 -
[0080] The wound care tapes were tested on several patients who had
undergone an excision and primary closure of the resulting wound using
standard sutures. The wound care tapes were applied across the wound and
over the sutures in a stretched configuration, in order to facilitate wound
closure.
In some cases the wound care tapes were left exposed; in other cases the
wound care tapes were covered with gauze. The wound care tapes were left on
the wound for 14 days, after which time they were removed in the hospital
setting.
[0081] It was observed by the attending surgeon that the wound care
tapes stayed in place for the duration of the test. It was further observed
that the
wound care tapes did not cause any maturation, and increased healing.
EXAMPLE 2
[0082] The wound care tapes were tested on several patients having skin
flaps, including transpositional and rotational flaps. The wound care tapes
were
used in addition to sutures, both to secure the flaps and at the donor site.
The
wound care tapes were applied across the wound in a stretched configuration,
in
order to facilitate wound closure. The wound care tapes were left on the wound
for 14 days, after which time they were removed in the hospital setting
[0083] A strong success rate and excellent outcome were reported by the
attending surgeon. No adverse outcomes or complications were reported.
EXAMPLE 3
[0084] The wound care tapes were tested on patients requiring webspace
reconstruction. Webspace was created using advancement and transpositional
flaps. The wound care tapes were used in addition to sutures, both to secure
the
flaps and at the donor site. The wound care tapes were applied to the wound in
a stretched configuration, in order to facilitate wound closure. The wound
care
tapes were left on the wound for 14 days, after which time they were removed
in
the hospital setting
CA 02901331 2015-08-21
- 17 -
[0085] A strong success rate and excellent outcome were reported by the
attending surgeon. No adverse outcomes or complications were reported.
EXAMPLE 4
[0086] The wound care tapes were tested on small primary cuts (up to 1
cm in length), without any sutures. The wound care tapes were applied across
the wound in a stretched configuration, in order to facilitate wound closure.
[0087] Excellent cosmetic outcome and excellent healing were reported by
the attending surgeon. No adverse outcomes or complications were reported
REFERENCES
"Management of Scar Tissue", Kim Rock Stockheinner, University of Wisconsin,
LaCrosse Wisconsin, Advance Healing, Summer 2007, page 21
"Kinesio Taping for Skin Wounds", Kiyotaka Oka, Office Ikuno, September 2010