Note: Descriptions are shown in the official language in which they were submitted.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
SURGICAL ACCESS ASSEMBLY AND METHOD OF USING SAME
TECHNICAL FIELD
[0001] The present disclosure relates generally to a surgical device for
use with delicate and
critical tissues, as well as methods of accessing and performing surgery using
same.
BACKGROUND
[0002] Diagnosis and treatment of conditions affecting the brain are among
the most difficult
and complex problems that face the medical profession. The brain is a complex
and delicate soft
multi-component tissue structure that controls bodily functions through a
complex neural
network connected to the rest of the body through the spinal cord. The brain
and spinal cord are
contained within and protected by significant bony structures, e.g., the skull
and the spine. Given
the difficulty of accessing the brain through the hard bony protective skull
and the delicate
network and complex interactions that form the neural communication network
contained within
the brain that define the human body's ability to carry on its functions of
speech, sight, hearing,
functional mobility, reasoning, emotions, respiration and other metabolic
functions, the diagnosis
and treatment of brain disorders presents unique challenges not encountered
elsewhere in the
body.
[0003] For example, abnormalities such as intracranial cerebral hematomas
(ICH), abscesses,
Glioblastomas (GB) and metastases (mets) manifest themselves in the
intraparenchymal
subcortical space (i.e., the white matter) of the brain are particularly
challenging to access, let
alone treat. The ventricles of the brain contain eloquent communication
structures (neural
network) which are located in the subcortical space, called fiber tracts and
fascicles. Thus,
1
SUBSTITUTE SHEET (RULE 26)
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
2
traditionally, unless the ICH, GB, and/or mets where considered anything but
"superficial," such
conditions have been considered inoperable, simply because getting to the
abnormality ICH, GB
and/or mets are considered just as damaging as letting the condition take its
course. Similarly,
tissue abnormalities such as tumors, cysts and fibrous membrane growths which
manifest within
the intraventricular space of the brain are considered challenging to safely
access and often
inoperable, due to their locations within the brain.
[0004] In order to assist in diagnosis and subsequent treatment of brain
disorders, clear,
accurate imaging of brain tissue through the skull is required. In recent
years significant
advances have been made in imaging technology, including stereotactic X-ray
imaging,
Computerized Axial Tomography (CAT), Computerized Tomographic Angiography
(CTA),
Position Emission Tomography (PET) and Magnetic Resonance Imaging (MRI),
Diffusion
Tensor Imaging (DTI) and Navigation systems (instrument position tracking
systems). These
imaging devices and techniques permit the surgeon to observe conditions within
the brain in a
non-invasive manner without opening the skull, as well as provide a map of
critical structures
surrounding an area of interest, including structures such as blood vessels,
membranes, tumor
margins, cranial nerves, including fiber tracts and fascicles. If an
abnormality is identified
through the use of one or more imaging modalities and/or techniques, it may be
necessary or
desirable to biopsy or remove the abnormality.
[0005] Once a course of action has been determined based upon one or more
imaging
techniques, a surgical treatment may be necessary or desired. In order to
operate surgically on
the brain, access must be obtained through the skull and delicate brain tissue
containing blood
vessels and nerves that can be adversely affected by even slight disturbances.
Therefore, great
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
3
care must be taken in operating on the brain so as not to disturb delicate
blood vessels and nerves
to prevent adverse consequences resulting from a surgical intervention.
[0006] Traditionally, accessing abnormalities which manifest in deeper
spaces within the
brain has meant a need for a surgery that creates a highly invasive approach.
In some instances,
in order to obtain access to target tissue, a substantial portion of the skull
is removed and entire
sections of the brain are retracted to obtain access. For example, surgical
brain retractors are
used to pull apart or spread delicate brain tissue, which can leave pressure
marks from lateral
edges of the retractor. In some instances, a complication known as "retraction
injury" may occur
due to use of brain retractors. Of course, such techniques are not appropriate
for all situations,
and not all patients are able to tolerate and recover from such invasive
techniques.
[0007] It is also known to access certain portions of the brain by creating
a burr hole
craniotomy, but only limited surgical techniques may be performed through such
smaller
openings. In addition, some techniques have been developed to enter through
the nasal passages,
opening an access hole through the occipital bone to remove tumors located,
for example, in the
area of the pituitary. These approaches are referred to as Expanded Endonasal
Approaches
(EEA) and were pioneered by one of the inventors of this disclosure.
[0008] A significant advance in brain surgery is stereotactic surgery
involving a stereotactic
frame correlated to stereotactic X-ray images to guide a navigational system
probe or other
surgical instrument through an opening formed in the skull through brain
tissue to a target lesion
or other body. A related advance is frameless image guidance, in which an
image of the surgical
instrument is superimposed on a pre-operative image to demonstrate the
location of the
instrument to the surgeon and trajectory of further movement of the probe or
instrument.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
4
[0009] In recent years, surgical access systems have been developed to
provide access to
previously difficult to access areas. One such prior art system is shown in
FIGS. 1A-1C. System
includes a retractor 20 and an introducer 40. Introducer 40 includes a cone-
shaped distal end
42 with an opening 52 therein (best seen in FIG. 1C). The cone-shaped distal
end is configured
to be a generally blunt, flat surface. With introducer 40 positioned within
retractor 10, system 10
is inserted into brain tissue, thereby pushing brain tissue away while
providing access to an area
of interest. Once system 10 is delivered to the area of interest, retractor 10
is rigidly fixed in
position. More specifically, retractor 10 is fixed in space with the use of a
standard or
conventional neurosurgical fixation device. Once, retractor 10 is fixed in
place, introducer 40 is
then removed from retractor 10, while leaving retractor 10 in its fixed place,
thereby creating a
pathway through the brain tissue.
[0010] While access system 10 may provide a manner to access certain brain
tissue, the blunt
shaped distal end of can actually cause transient or even permanent
deformation and trauma of
delicate tissue structures which can manifest itself in temporary or permanent
neurological
deficits after surgical intervention due to damage of blood vessels, cranial
nerves, fiber tracts and
fascicles. Opening 52 may cause scoring of tissue, also leading to damage of
the tissues and
structures as introducer 40 is pushed through tissue. Further, by rigidly
fixing the placement of
refractor 10, manipulation of retractor 10 is impeded and requires constant
attention by loosening
and retightening to re-position for even micro-movement of the retractor 10,
thereby lengthening
procedure time.
[0011] Another issue that needs to be addressed is visibility. Typically
when employing an
access system in a surgical procedure, it is often like operating in a poorly
lit tunnel. To provide
illumination, it is known to place a light source within the introducer
sheath, such as an
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
endoscope. However, when using an endoscope, the light source takes up a
significant amount
of working space within the introducer sheath, thus reducing the functional
working area for
other instruments, as well as minimizing the ability to move other instruments
within the surgical
site.
[0012] Alternatively, light must be delivered from a remote or external
location, such as a
microscope or exoscope. However, in the case of microscopes and exoscopes, the
external light
source is often blocked by the surgeon and/or instruments in the surgical
field. At a minimum,
the effectiveness is greatly diminished at the distal end of the introducer
sheath where the actual
surgical work and/or treatment is occurring, and where effective visualization
is needed the most.
[0013] Notwithstanding the foregoing advances in imaging technology and
both frame and
frameless stereotactic image guidance techniques, there remains a need for
improved surgical
techniques and apparatus for operating on brain tissue, including providing
improved visibility,
while minimizing surgical openings.
BRIEF DESCRIPTION OF THE DRAWINGS
[0014] Exemplary embodiments of the present disclosure will now be
described in greater
detail with reference to the attached figures, in which:
[0015] FIGS. 1A-1C illustrate a prior art surgical access system.
[0016] FIG. 2 is a perspective cross-sectional view of an exemplary
arrangement of a
surgical access assembly.
[0017] FIG. 3 is a perspective view of an outer sheath of the surgical
access assembly of
FIG. 2.
[0018] FIG. 4A is a side elevational view of the outer sheath of FIG. 3.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
6
[0019] FIG. 4B is an enlarged cross-sectional view of a portion of the
distal end of the outer
sheath of FIG. 4A.
[0020] FIG. 4C is an enlarged cross-sectional view of a portion of an
alternative embodiment
of the distal end of the outer sheath of FIG. 4A.
[0021] FIG. 5 is an end view of outer sheath of FIG. 3.
[0022] FIG. 6A is an elevational view of an alternative embodiment of an
outer sheath.
[0023] FIG. 6B is an end view of the outer sheath of FIG. 6A.
[0024] FIG. 7A is a perspective view of an obturator assembly of the
surgical access
assembly of FIG. 2.
[0025] FIG. 7B is an enlarged view of an end face of the obturator assembly
taken from area
7B of FIG. 7A.
[0026] FIG. 8A is a top view of the obturator assembly of FIG. 7A.
[0027] FIG. 8B is an enlarged view of a distal end of the obturator
assembly taken from area
8B of FIG. 8A.
[0028] FIG. 8C is an alternative embodiment of the distal end of the
obturator assembly
taken from area 8B of FIG. 8A.
[0029] FIG. 8D is an alternative embodiment of the distal end of the
obturator assembly
taken from area 8B of FIG. 8A.
[0030] FIG. 9A is a side elevational view of the obturator assembly of FIG.
7A.
[0031] FIG. 9B is an enlarged view of a portion of the obturator assembly
taken from area
9B of FIG. 9A.
[0032] FIG. 10 is an end view of the obturator assembly of FIG. 7A.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
7
[0033] FIG. 11A is a perspective view of an illuminating ring that
operatively connects to an
outer sheath of the surgical access assembly.
[0034] FIG. 11B is a side view of the illuminating ring of FIG. 11A.
[0035] FIG. 11C is a top view of the illuminating ring of FIG. 11A.
[0036] FIG. 11D is a bottom plan view of the illuminating ring of FIG. 11A.
[0037] FIG. 11E is a cross-sectional view of an exemplary arrangement of a
lighting
arrangement for the illuminating of FIG. 11A.
[0038] FIG. 11F is a plan view of a circuit board for use with the
illuminating ring of 11A.
[0039] FIG. 11G is an exemplary electrical schematic for use with the
illuminating ring of
FIG. 11A.
[0040] FIG. 12 illustrates the illuminating ring of FIG. 11A assembled to
an exemplary
embodiment of the outer sheath.
[0041] FIG. 13 is a flow chart illustrating a process flow using the
surgical access assembly.
[0042] FIG. 14A-14B are images of a brain illustrating an area of interest,
taken using an
imaging modality.
[0043] FIG. 15 is an image taken of the brain shown in FIGS. 14A-14B,
illustrating various
critical structures, such as fiber tracts and fascicles of the brain.
[0044] FIG. 16A is an alternative embodiment of an obturator with an
imaging device
operatively connected thereto.
[0045] FIG. 16B is a partially exploded view of an enlarged cross-sectional
view of the
proximal end of the obturator and a post.
[0046] FIG. 16C is an alternative arrangement of a coil sensor for use with
an obturator.
[0047] FIG. 16D is an end view of the coil sensor mounted on the post of
FIG. 16C.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
8
[0048] FIG. 17A is an elevational view of the surgical access system, while
the obturator is
being withdrawn from the outer sheath.
[0049] FIG. 17B is an elevational view of the surgical access system with
the outer sheath in
place within the brain.
[0050] FIG. 18 is a perspective view of an exemplary surgical device used
for cytoreduction.
[0051] FIG. 19A is an elevational view of an exemplary manipulation member.
[0052] FIG. 19B is an elevational view of an alternative manipulation
member.
[0053] FIG. 20 is a partial perspective view of an exemplary delivery
sleeve that may be
used with a surgical device.
[0054] FIG. 21A is an exemplary arrangement for a therapy delivery device.
[0055] FIG. 21B is an alternative arrangement of the therapy delivery
device of FIG. 21A.
[0056] FIG. 22 is a partially exploded view of an alternative arrangement
of an illuminating
ring assembly.
[0057] FIG. 23 is an elevational view of the illuminating ring assembly of
FIG. 22 in an
assembled configuration.
[0058] FIG. 24 is a partially exploded view of the illuminating ring
assembly of FIG. 22 with
an outer sheath operatively engaged therewith.
[0059] FIG. 25 is a perspective view of the illuminating ring assembly of
FIG. 22, in an
assembled configuration.
[0060] FIG. 26 is an enlarged perspective view of the illuminating ring
assembly taken from
areas 26 in FIG. 25.
[0061] FIG. 27 is a top perspective view of the illuminating ring assembly
of FIG. 22.
[0062] FIG. 28 is a top perspective view of an alternative illuminating
ring assembly.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
9
[0063] FIG. 29 is a bottom perspective view of the illuminating ring
assembly of FIG. 28.
[0064] FIG. 30 is a perspective view of an illuminating ring assembly in an
assembled
configuration with the other sheath.
[0065] FIG. 31 is a rear perspective view of the illuminating ring assembly
of FIG. 30.
[0066] FIG. 32 is a perspective view of the illuminating ring assembly of
FIG. 30.
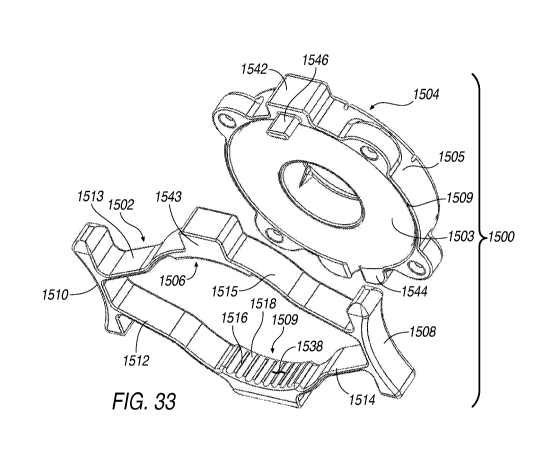
[0067] FIG. 33 is an exploded view of the illuminating ring assembly of
FIG. 32.
[0068] FIG. 34 is a rear elevational view of an alternative arrangement of
an illuminating
ring assembly in an assembled configuration.
[0069] FIG. 35 is a front elevational view of the illuminating ring
assembly of FIG. 34.
[0070] FIG. 36 is a perspective view of the illuminating ring assembly of
FIG. 34.
[0071] FIG. 37 is a top perspective view of an alternative illuminating
ring assembly.
[0072] FIG. 38 is a perspective elevational view of the illuminating ring
assembly of FIG.
37.
[0073] FIG. 39 is a perspective view of the illuminating ring assembly of
FIG. 37, assembled
in an outer sheath.
[0074] FIG. 40 is an enlarged view of the illuminating ring assembly taken
from area 40 in
FIG. 39.
[0075] FIG. 41 is a plan view of the illuminating ring assembly of FIG. 37.
DETAILED DESCRIPTION
[0076] Referring now to the discussion that follows and also to the
drawings, illustrative
approaches to the disclosed assemblies and methods are shown in detail.
Although the drawings
represent some possible approaches, the drawings are not necessarily to scale
and certain features
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
may be exaggerated, removed, or partially sectioned to better illustrate and
explain the present
disclosure. Further, the descriptions set forth herein are not intended to be
exhaustive or
otherwise limit or restrict the claims to the precise forms and configurations
shown in the
drawings and disclosed in the following detailed description.
[0077]
Described herein is surgical access assembly, various components for use in
same, and a method of using the surgical access assembly. The components
disclosed herein
provide surgeons with an enhanced ability to minimize trauma to the patient,
while providing
efficient improved minimally invasive surgical techniques, such as, for
example, during
intracranial surgical techniques.
[0078]
Referring to FIG. 2, a perspective cross-sectional view of a surgical access
assembly
100 is shown. In one exemplary arrangement, surgical access assembly 100
comprises a hollow
outer sheath 102 and a selectively removable obturator 104. As best seen in
FIG. 2, obturator
104 is configured with a length that is longer than a length of outer sheath
102 such that a distal
end 106 of obturator 104 protrudes a predetermined distance from a distal end
108 outer sheath
102, as will be discussed below in greater detail.
[0079]
A locking member 110 may also be provided. Locking member 100 is configured to
operatively retain a separate navigation member 112 (shown in phantom) within
obturator 104,
as will be discussed in greater detail below. A retaining member 114 may be
secured within a
portion of obturator 104 to prevent locking member 110 from being completely
disengaged from
obturator 104.
[0080]
Referring now to FIGS. 3-5, outer sheath 102 will be described in greater
detail.
Outer sheath 102 is defined by distal end 108 and a proximal end 116 and
includes a generally
hollow body portion 118 and a grip ring 120. In one exemplary arrangement,
grip portion 120 is
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
11
configured as a ring, as illustrated in the drawings. However, it is
understood that grip portion
120 need not be configured as a ring. For ease of explanation, grip portion
120 will be referred
to hereinafter as grip ring 120. Grip ring 120 is fixedly secured to body
portion 118 at proximal
end 116. In one exemplary arrangement, body portion 118 is constructed of a
clear
biocompatible material that permits viewing of normal tissue, abnormal tissue,
as well as critical
structures that are disposed outside of body portion 118 when outer sheath 102
is disposed within
such tissue. In one exemplary arrangement, outer sheath 102 is constructed of
polycarbonate,
though other biocompatible materials may be employed, including resins.
[0081] In one exemplary configuration, an imaging mechanism may be
incorporated into
outer sheath 102 that would permit visualization of tumors, vessels, fiber
tracks, fascicles and
even healthy tissue, in real-time. Indeed, as will be explained in further
detail below, the
imaging mechanism will enable physiological functional imaging to provide
information about
the characteristics of the cortical fiber tracks to be visible, thereby
enabling a user to separate and
park such fibers on either side of outer sheath 102 rather than cutting,
stretching and potentially
damaging such fibers while gaining access to a desired location within the
brain. Further, as will
be explained in further detail below, the imaging mechanism may also enable
the surgeon to
have real-time information about the fiber tract and fascicle location, after
placement of outer
sheath 104, and during abnormality resection procedure therethrough. In
addition to white matter
tract imaging, mapping of the characteristics of the cerebral blood flow may
be obtained.
[0082] In one exemplary embodiment, the imaging mechanism may be an
ultrasound probe
incorporated into outer sheath 102. For example, outer sheath 102 may be
provided with one or
more channels within the wall that defines outer sheath 102 that are
configured with one or more
small diameter ultrasound probes. In another arrangement, a single ultrasound
probe that is
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
12
configured to be received within outer sheath 102 may be provided. In yet
another embodiment,
a low field MRI probe may be selectively placed in outer sheath 102 to provide
enhanced
imaging. In yet another embodiment a low field MRI imaging coil may be molded
into or
bonded into outer sheath 102. In still another exemplary arrangement, the
probe may be an
optical coherent tomography (OCT) imaging or spectroscopy.
[0083] Distal end 108 of outer sheath 102 may be configured with a tapered
portion 130 that
extends towards a center axis A-A of outer sheath 102 to a distal edge 132
that surrounds an
opening 134 in distal end 108 of outer sheath 102. Tapered portion 130 serves
to ease the
transition between outer sheath 102 and a distal tip potion 172, without drag,
trauma or coring of
tissue from a diameter that defines a body portion 168 of obturator 104 to a
diameter that defines
body portion 118 of outer sheath 102. In one exemplary configuration, distal
end 108 may be
configured with a radius or other configuration so as to create a
smooth/atraumatic transition of
the brain tissue when surgical access assembly 100 is inserted into the brain.
[0084] For example, as best seen in FIG. 4B, distal edge 132 is configured
so as to be non-
sharpened and radiused. In one exemplary arrangement, distal edge 132 is
configured as a 0.3
mm diameter radiused rim. Tapered portion 130 and radiused distal tip 132
cooperates with
obturator 104 to atraumatically move tissue, as well as various structures
within the brain,
including white matter, away from outer sheath 102 without cutting tissue or
such structures.
Indeed, unlike prior art devices that include either a blunt tip distal end or
a tapered leading edge
such as that shown in FIG. 1C, radiused distal tip 132 cooperates with tapered
portion 130 and
obturator 104 to prevent bruising and damage to various tissue. More
specifically, this
configuration facilitates entry of outer sheath 102 into delicate tissue, but
without cutting such
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
13
delicate tissue. Insertion of surgical access assembly 100 will be explained
in further detail
below.
[0085] Body portion 118 may further be provided with a plurality of spaced
apart indicators
136. Indicators 136 generally extend about the circumference of body portion
118 and each may
further incorporate a secondary indicator 138 that visually illustrates a
predetermined location on
body portion 118, as shown in FIG. 3. While FIG. 3 illustrates four indicators
136, it is
understood that body portion 118 may be provided in a variety of lengths and
that any number of
indicators 136 may be provided. Body portion 118 may also be provided with a
longitudinal
indicator 140. More specifically, as best seen in FIG. 4A, longitudinal
indicator 140 extends
from proximal end 116 to distal end 108. Indicators 136, 138 and 140 may be
printed onto either
an internal or external surface of body portion 118 with an imaging visible
ink such as, for
example ink containing fluro-deoxyglucose (FDG), Technicium 99, Gadolinium,
titanium dust,
barium sulfate, a combination of the above or other suitable imaging material.
Indicators 136
and 138 provide a reference point for the operator of system 100, as
structures may be visible
through body portion 118. Indicator 136, 138 and 140 may also be configured to
be visible
under MRI, CT, PET, or any other suitable imaging modality to enable easy
identification of
areas of interest. In one alternative embodiment, indicators 136, 138 and/or
140 may be etched
or printed onto body portion 118, either on the internal or external surface
of body portion 118.
[0086] Details of grip ring 120 are best seen in FIG. 5. Grip ring 120 is
generally configured
as a flange member 142 defined by an outer periphery 144 and an inner opening
146. Inner
opening 146 may be sized to generally correspond to the diameter of a lumen
148 defined by
body portion 118. Outer periphery 144 is sized to have a diameter that is
larger than lumen 148
of body portion 26. Flange member 142 may further be provided with one or more
small
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
14
openings 150 that are disposed therein. In one exemplary arrangement, a
plurality of small
openings 150 are provided that are spaced generally equi-distantly about inner
opening 146.
Small openings 150 will be described in further detail below. Outer periphery
144 may further
be provided with a textured surface 152 to provide for ease of gripping outer
sheath 102. For
example, in one exemplary arrangement, textured surface 152 comprises a
plurality of alternating
ridges 154 and grooves 156. However, it is understood that other textured
surfaces may be
employed.
[0087] Disposed on a proximal end surface 158 of flange member 142, an
alignment feature
160 may be employed. Alignment feature 160 is used to indicate the location of
longitudinal
indicator 140 when outer sheath 102 is positioned within the brain. Alignment
feature 160 will
be discussed below in greater detail.
[0088] An alternative embodiment of outer sheath 202 is shown in FIGS. 6A-
6B. Outer
sheath 202 is similar to outer sheath 102 in that it is defined by a distal
end 208, a proximal end
216 and a body portion 218. A distal edge 232 is generally configured to be
similar as distal tip
132. A grip ring 220 is fixedly secured to body portion 218.
[0089] Grip ring 220 also includes a textured surface 252. Grip ring 220
further includes a
locating member 262. Locating member 262 is configured to operatively connect
an
illumination ring (best seen in FIG. 11A) 300 to outer sheath 102. As may be
seen, in one
exemplary configuration, locating member 262 extends outwardly from outer
periphery 244 of
grip ring 220. Locating member 262 may also serve as an alignment feature for
indicating the
location of longitudinal indicator 240. Alternatively, a separate alignment
feature 260 may be
provided. For example, in FIG. 6B, alignment feature 260 is positioned
adjacent locating
member 262.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
[0090] Body portion 218 may also be provided with indicators 34, 36, and 38
to assist in
locating outer sheath 202 in operation. However, in another alternative
arrangement, body
portion 218 may be provided with indicators 264 that produce a signal void or
minimal artifact
under certain imaging modalities. In one specific arrangement, indicators 264
may be
configured as small holes that are spaced apart at predetermined distances, as
shown in FIG. 6A.
In yet another alternative arrangement, indicators 264 may be configured as
non-through divots.
In still a further alternative arrangement, indicators 264 may be configured
as a longitudinal
groove (not shown) on either the internal or external surface of body portion
218.
[0091] Referring to FIGS. 7-10, obturator 104 will now be described.
Obturator 104 is
defined by distal end 106, a proximal end 166, a body portion 168 and a handle
portion 170.
Distal end 106 is configured with a generally conical shaped distal tip
portion 172 that tapers to a
tip member 174 to provide atraumatic dilation of tissue. In one exemplary
arrangement, tip
portion 172 tapers toward a closed tip member 174 so as to prevent coring of
tissue as obturator
104 is inserted into the brain.
[0092] There are a number of variables that play the selection of the angle
a that defines the
taper of tip portion 172. These variables include the size of an outer
diameter D1 of obturator
104, the desired length that distal tip portion 172 extends from body portion
168, and the desired
offset for a distal tip of navigation member 112 and tip member 174. More
specifically, it is
contemplated that surgical access assembly 100 will be provided as part of a
kit that may include
multiple sized outer sheaths 102 and obturators 104, to provide the surgeon
with a choice of
different diameter sizes and lengths so as to provide flexibility for
accessing areas of interest
within the brain. However, to insure that the distal tip 174 is determinable
regardless of which
size diameter D1 of obturator 104 is used, taper angle a may be selectively
adjusted. For
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
16
embodiments that utilize navigation member 112 that positions a distal end
thereof at a set
position within obturator 104 (as will be explained in further detail below),
to maintain an
identical offset length between the distal end of navigation member 112 and
distal tip 174 in
different diameter D1 sized obturators 104, taper angle a will need to be
increased, as diameter
D1 increases.
[0093] For example, if diameter D1 of obturator 104 is 13.5mm, an exemplary
angle a may
be 45.5 to provide effective atraumatic dilation, as well as a determinable
distal tip 174 location.
However, if diameter D1 of obturator 104 is 15.5mm, an exemplary angle a' may
be 52.8 .
[0094] As best seen in FIG. 8B, distal tip 174 is configured to be radiused
such that tip
member 174 is rounded, and neither blunt, nor sharp. More specifically, tip
member 174 is
configured so as not to have any flat portions which during insertion can
stretch or even tear the
delicate tissues such as the vessels, fiber tracts and fascicles found in the
brain. Further, because
tip member 174 is closed, damage of such delicate tissues and fascicles are
also avoided. In one
exemplary embodiment, tip member 174 is configured with a 0.5 mm radius. As
will be
explained in further detail below, the configuration of tip member 174 is
designed to gently
displace and move the tissue into which it is inserted; i.e., atraumatically
dilate the tissue to
allow for introduction in to an intra-fascilar and para-fascilar manner, as
opposed to cutting
tissue as surgical access assembly 100 is inserted into the tissue.
[0095] Handle portion 170 is positioned at proximal end 166 of obturator
104. As best seen
in FIGS. 7B, 8A and 9A, handle portion 170 comprises a stop member 176 and a
grip member
178. Stop member 176 is positioned distally of grip member 178 and, as best
seen in FIG. 8A, is
configured to have a width W1 that is greater than a diameter D1 of body
portion 168, as well as
a diameter D2 of outer sheath 102 (shown in FIG. 4A). Grip member 178 is
configured with a
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
17
width W2 that is greater than the width W1 of stop member 176, thereby
providing a step-like
configuration. Stop member 176 further defines an engagement surface 177 that
is axially
spaced from a distal surface 179 of grip member 178.
[0096] In one exemplary arrangement, handle portion 170 is configured with
a generally
planar surface 180, as best seen in FIGs. 7A-7B and FIG. 10. Planar surface
180 is configured
with a receiving aperture 182 that is configured to receive locking member
110. In one
exemplary arrangement, receiving aperture 182 is threaded. As best seen in
FIGS. 2, 7B, and
8A, disposed within receiving aperture 182 is an engagement opening 184.
Engagement opening
184 is in communication with a channel 186 (seen in phantom in FIGS. 8A and
9A) that extends
at least partially thorough handle portion 170. After locking member 110 is at
least partially
engaged within receiving aperture 182, retaining member 114 (FIG. 2) is
positioned within
channel 186. Because engagement opening 184 opens into receiving aperture 182,
a portion of
retaining member 114 extends across a portion of receiving aperture 182 such
that locking
member 110 is prevented from being entirely withdrawn from receiving aperture
182. For
example, locking member 110 is illustrated as having threads that cooperate
with corresponding
internal threads in receiving aperture 182. Retaining member 114 is positioned
within channel
186 so as to extend above the threads of locking member 110 such as locking
member 110 is
being removed from receiving aperture 182, threads come into contact retaining
member 114,
thereby preventing complete removal of locking member 110 from handle portion
170.
[0097] An access opening 188 is formed through proximal end 166. Access
opening 188
extends through handle portion 170. In one exemplary arrangement, access
opening 188 may be
provided with an inwardly extending chamfer 189 that tapers toward access
opening 188.
Chamfer 189 provides a self-directing feature for inserting navigation member
112 into access
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
18
opening 188. Access opening 188 is in communication with a first channel
segment 191 that
extends through handle portion 170 and into body portion 168.
[0098] As seen in FIG. 8D, obturator 104 may further be configured to
receive a viewing
member 167 operatively connected thereto. More specifically, conical tip
portion 172 may be
configured with one or more viewing windows 169 that are oriented to be flush
with the surface
of conical tip portion 172. Viewing windows 169 are in communication with a
viewing member
channel 171 that may selectively receive a viewing member such as, for
example, a fiber optic
cable or an ultrasound probe. The viewing member may be in addition to the use
of navigation
member, or in place thereof. The viewing member permits the surgeon to
observe, in real-time
(i.e., during insertion), surrounding tissue and eloquent tissue structures so
as to minimize trauma
during insertion.
[0099] Body portion 168 extends between distal end 106 and proximal end
166. Body
portion 168 includes one or more elongated void areas 190. Void areas 190
serve to reduce
weight of obturator 104, thereby making obturator 104 easier to manipulate
during surgical
procedures. Void areas 190 also facilitate sterilization of obturator 104 by
moisture retention
within body portion 168 of obturator 104. Further, void areas 190 also provide
venting, thereby
preventing a vacuum from being generated as obturator 104 is being withdrawn
from outer
sheath 102 during operation.
[00100] Void areas 190 are separated by web portions 192 that extend axially
through a
portion of the length of body portion 168. Disposed on web portions 192 of
body portion 168
are one or more indicators 194. Indicators 194 may include spaced apart hash
marks (designated
as 194A) that cooperate with an imaging modality to provide information, in
real-time,
concerning the location of obturator 104 relative to various tissue, critical
structures, and
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
19
fascicles within the brain, while obturator 104 is positioned within tissue.
Indicators 194 also
assist with providing information to regarding the relative positions between
obturator 104 and
outer sheath 102. Indicators 194 produce a signal void or minimal artifact
under certain imaging
modalities.
[00101] Body portion 168 may further include one or more cross webs 196. Cross
webs 196
are oriented transverse to web portions 192 and connect web portions 192
together. In one
exemplary arrangement, body portion 168 includes at least one cross web 196
that operatively
defines the outer diameter D2 of body portion 168. Diameter D2 is sized to fit
within lumen 148
of outer sheath 102 such that obturator 104 and outer sheath 102 may be
selectively slid relative
to one another. However, diameter D2 is also sized to minimize or even
eliminate any gaps
between an inner surface of outer sheath 102 and an outer surface of obturator
104. In the
exemplary arrangement shown in FIG. 7-9, three cross webs 196A, 196B and 196C
are provided.
A first cross web 196A is connected to distal tip portion 172, while second
cross web 196B is
spaced proximally from first cross web 196A and separated by a void area 193.
Third cross web
196C is separated from second cross web 196B by void areas 192 and is
positioned distal from
first stop member 176 of handle portion 170. Cross webs 196 serve to provide
for structural
integrity of obturator 104, as well as improved rigidity.
[00102] In one exemplary arrangement, one or more of cross webs 196 may
further be
provided with an annular compensating protuberance 197 to accommodate for
slight
manufacturing variations of the diameter of lumen 148 of outer sheath 102. For
example, as it is
contemplated that outer sheath 102 may be a component that is molded from a
resin, a process
which may produce such slight manufacturing variations. Compensating
protuburance 197
extends slightly radially outwardly from an outer surface of obturator 104 and
cooperates with
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
lumen 148 of outer sheath 102 to create a friction fit between the outer
surface of obturator 104
and lumen 148, due to the slight flexibility of the resin of outer sheath 102.
Use of compensating
protuberance 197 thereby reducing the need for maintaining a high dimensional
tolerance of
outer sheath 102 in production.
[00103] In one embodiment, cross web 196B is provided with a second channel
segment 198
(shown in phantom) that extends there through. Second channel segment 198 is
axially aligned
with first channel segment 191 and is configured to selectively receive
navigation member 112.
In one exemplary arrangement, disposed in first cross web 196A is an inwardly
extending
depression 199, as best seen in FIG. 9B. Depression 199 is configured in such
a manner so as to
align a distal tip of navigation member 112 with distal end 108 of outer
sheath 102, when outer
sheath 102 is assembled to obturator 104.
[00104] Referring to FIGS. 11A-11F, details of an optional illuminating ring
300 will now be
described. Illuminating ring 300 is generally defined by a top surface portion
302, a wall
member 304. A circuit board 306 may also be provided. Top surface 302 includes
at least one
access opening 308 therethrough that is configured to receive one or more
surgical instruments,
as will be described below in further detail. Additional small openings 309
may be provided in
top surface 302. One or more of small openings 309 are configured to be
aligned with small
openings 150 disposed on flange member 142. Wall member 304 extends from top
surface 302
so as to create an open cavity 310 within illuminating ring 300. An outer
surface of wall
member 304 may be textured (not shown), similar to grip ring 120.
[00105] One or more light elements 312 that are supported by a portion of
illuminating ring
300. In one embodiment, shown in FIG. 11E, lights 312 are fixedly mounted to
top surface 304
so as to face inwardly toward open cavity 310, adjacent access opening 308.
Each light 312 is
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
21
electrically connected to a remote power source (not shown) by wires 314. In
one exemplary
arrangement, wires 314 may be retained within channels formed in top surface
302 around
access opening 308.
[00106] In an alternative arrangement (FIG. 11F), lights 312 may be
incorporated in a circuit
board 306. Circuit board 306 is configured with an access opening 316 that may
be aligned with
access opening 308 formed in top surface 302. Further, circuit board 306 is
also sized to be
positioned within open cavity 310, and fixed thereto. In other words, in one
arrangement, circuit
board 306 is sized to have an outer diameter that is smaller than an inner
diameter defined by
wall member 304. A wall opening 318 may be formed through a portion of either
top surface
302 or wall member 304 to provide access for wires 320 to electrically connect
circuit board 306
to a power source. An example of wall opening 318 may be seen in FIGS. 11B,
11D, and 11F.
Circuit board 306 may be configured such that there is a constant output of
light when
illuminating ring 300 is turned on so that there is a steady state.
[00107] An exemplary circuit design 321 is depicted in FIG. 11G for circuit
board 306. In the
exemplary configuration, circuit design 321 is configured to prevent
flickering of lights 312
and/or prevent operation of less than all of the lights 312 during use of
illuminating ring 300.
More specifically, circuit design 321 is configured such that if one light 312
burns out, or if
batteries that supply power to circuit get low, illuminating ring 300 will
simply shut off and a
replacement illuminating ring 300 may be used.
[00108] In one exemplary arrangement, lights 312 are LED lights, although
other light devices
may be utilized. LED lights do not contribute significantly to the weight of
surgical access
assembly 100, and also dissipates a non-clinical significant amount of heat.
Moreover, LED
lights can emit different combinations of colors/frequencies of light that may
be incorporated to
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
22
illuminating ring 300, to provide improved visualization of fluorescing dyes
which allow for the
differentiation of tissues.
[00109] Use of LED lights also allow for an endoscope to be used with surgical
access
assembly 100, but without an accompanying fiber-optic light source. This
arrangement
significantly reduces a required overall outside diameter of the endoscope,
which improves the
working space within lumen 148 of outer sheath 102. More specifically, lumen
148 of outer
sheath 102 has more available working space, thereby providing increased
simultaneous use of
multiple instrumentation, as well as improved visualization. Further, because
traditional
endoscope devices must be attached to a supporting structure that is fixed to
an introducer
cannula, the weight of such an assembly tends to pull on the introducer
cannula, in one direction.
This action can compromise the placement of the introducer cannula during the
procedure and/or
cause trauma to brain tissue. Thus, by incorporating illuminating ring 300 to
outer sheath, such
potential disadvantages may be avoided.
[00110] While illuminating ring 300 may be secured to grip ring 120 of outer
sheath 102 in
any suitable manner, in one exemplary arrangement, illuminating ring 300 is
provided with a
selective locking arrangement to selectively fix illuminating ring 300 to grip
ring 120. In one
exemplary arrangement, wall member 304 is provided with a locking channel 322,
best seen in
FIG. 11B. Locking channel 322 comprises wall opening 318 and that opens into a
first channel
segment 324, and a second channel segment 326 that is in communication with
fist channel
segment 324. Wall opening 318 extends from a bottom surface 328 of wall member
304.
Second channel segment 326 is spaced upwardly from bottom surface 328 of wall
member 304
and is oriented at an angle from first channel segment 324. In one exemplary
arrangement,
second channel segment 326 is oriented 90 from first channel segment 324.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
23
[00111] Locking channel 322 cooperates with locating member 262 to selectively
secure
illuminating ring 300 to grip ring 120. More specifically, illuminating ring
300 is pushed down
over grip ring 120 with locating member 262 entering wall opening 318. As
illuminating ring
300 is pushed downwardly, locating member 262 travels through first channel
segment 324.
Once locating member 262 contacts an terminal end 330 of first channel segment
324,
illuminating ring 300 is rotated relative to outer sheath 102 such that
locating member 262
moves into second channel segment 326, thereby selectively locking
illuminating ring 300 to
outer sheath 102, as shown in FIG. 12. Once connected, illuminating ring 300
thereby provides a
hands-free light source to illuminate lumen 148 of outer sheath 102.
[00112] In one exemplary arrangement, certain segments of outer sheath 102 may
be frosted
so as to reflect light to increase visualization within outer sheath 102. For
example, tapered
portion 130 may be frosted. Similarly, the top of grip ring 120 may also be
frosted.
[00113] Operation of surgical access assembly will be described in connection
with a process
flow 400 illustrated in FIG. 13. Generally speaking, before any surgical
procedure is decided
upon, a patient will first present with symptoms or deficits requiring
evaluation. Thus, the start
of process flow 400 begins with a surgeon making a determination 402 of the
cause of such
neurological symptoms/deficits. Such a determination may be made through use
of a variety of
imaging modalities, including, but not limited to, MRI or CT imaging. The
process then
proceeds to step 404.
[00114] If the determination from step 402 finds that a brain condition is
found, such as a
tumor or hematoma, an additional determination is required. More specifically,
a location of the
brain condition is determined in step 404. If the imaging determines that an
area of interest is
located in the intra-axial/subcortical space, the process flow continues to
step 406. However, if a
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
24
brain condition is located in other, more easily accessible areas of the
brain, the process flow
stops.
[00115] As discussed above, any suitable imaging modality may be utilized to
determine if a
brain condition exists, and if so, where that brain condition is located.
FIGS. 14A and 14B
illustrate examples of imaging results from an MRI. More specifically, an area
of interest 500, in
this case a tumor, may be seen deep in the subcoritcal space.
[00116] Once area of interest 500 is located, at step 406 an additional
imaging sequence is
employed to determine the location of eloquent structures such as vessels and
fiber tracts and the
associated fascicles so as to plan the safest access route to the area of
interest. Exemplary
arrangements for accomplishing this step include CT-Angiography and MRI with
Diffusion
Tensor Imaging (DTI) sequences. DTI allows for the determination of
directionality as well
as the magnitude of water diffusion along the communication "wiring" pathways
called fiber
tracts and fascicles. This kind of MRI imaging can provide imaging to allow
for the
estimation of potential damage to nerve fibers that connect the areas of the
brain which can
be affected by a stroke, for example, to brain regions that are distant from
it, and can also
be used to visualize white matter fibers in the brain and can map (trace
image) subtle
changes in the white matter associated with diseases such as multiple
sclerosis and
epilepsy, as well as assessing diseases where the brain's wiring is abnormal,
such as
schizophrenia, as well as tumor involvement.
[00117] Diffuse Tensor Tractography (DTT) may also be used. DTT allows for
noninvasive racking of neuronal fiber projections in a living human brain.
White matter
fiber trajectories are reconstructed throughout the brain by tracking the
direction of fastest
diffusion, which is assumed to correspond to the longitudinal axis of the
tract. Diffusion
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
tensor tractography provides insight into white matter integrity, fiber
connectivity, surgical
planning, and patients prognosis. Once the imaging information has been
analyzed, the
process then proceeds to step 408.
[00118] Referring to FIG. 15, an example of DTI imaging of the brain shown in
FIGS. 14A
and 14B is depicted. A map of fascicles and other vessels are illustrated in
FIG. 15, including
major vessels 502 that are shown spread around area of interest 500. Such
images provide the
surgeon with valuable information about potential avenues for access tracts to
area of interest
500.
[00119] In step 408, a plan for the operative trajectory is developed. More
specifically,
imaging information is used to plan (either manually or with software) the
access tract/pathway
to achieve fiber tract involvement during access to the area of interest. In
evaluating fiber tract
involvement from a potential access tract/pathway, consideration of fiber
tract importance may
be based on an individual patient's occupational and personal needs and/or
preference. Once a
pathway has been planned, the process proceeds to step 410.
[00120] In step 410, image data from the MRI/DTI and CT/CTA image sequence
obtained
during step 406 is input into an intraoperative navigation system.
Intraoperative navigation
systems may be used to provide direct visualization of area of interest 500 in
real time, as
surgical access system 100 is being positioned within the brain. The method
then proceeds to
step 412
[00121] Once the procedure has been planned and the image data has been
uploaded to a
navigational system, step 412 requires that the appropriate sized surgical
access assembly 100 is
selected. First the appropriate size of a craniotomy must be determined.
Further, the present
disclosure contemplates that different diameter and length sizes of surgical
access assembly 100
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
26
may be employed, the size depending on the particular location of area of
interest 500.
Accordingly, step 412 requires that the surgeon select the appropriate length
and diameter of
surgical access system 100 to be used, based on the physical and location
characteristics of the
area of interest 500. Once surgical access assembly 100 is selected, the
process proceeds to step
414.
[00122] In step 414, the surgeon creates the craniotomy and Dural access
incision. The
process then proceeds to step 416.
[00123] In step 416, the obturator 104 is inserted into outer sheath 102
until grip ring 120
abuts first stop member 176, as shown in, for example FIG. 2. Navigation
member 112 is then
operatively connected to obturator 104.
[00124] As discussed above, various types of navigation members 112 may be
employed with
surgical access assembly 100. In one exemplary configuration, navigation
member 112 is
configured as a probe (as shown in FIG. 2). In this configuration, navigation
member 112 is
inserted through access opening 188 of grip member 178 until a distal tip 417
of navigation
member 112 is deposited into depression 199 (see FIG. 9B). Depression 199 is
formed so that
distal tip 471 of navigation member 112 is positioned within the same plane as
distal tip 132 of
outer sheath 102, when obturator 102 and outer sheath 104 are assembled
together as shown in
FIG. 2. Locking member 110 may be tightened to fixedly retain navigation
member 112 within
obturator 102. A portion of navigation member 112 will extend proximally from
grip member
178 and will be operatively connected to a navigation system that includes a
screen that visually
illustrates the information obtained from the imaging sequences, along with
the trajectory of
surgical access system 100. Thus, with the navigation member 112 operatively
connected to a
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
27
navigation system, the position of distal tip 132 of outer sheath may be
indicated, in real time,
while surgical access system 100 is being navigated within a body.
[00125] In another configuration, the software operating the navigation system
may further be
provided with an offset dimension that corresponds to a distance D3 between
distal tip 174 of
obturator 104 and distal tip 132 of outer sheath. In this arrangement, a
dotted line may appear on
the navigation screen that indicates where distal tip 174 of obturator 104 is
located, in real-time.
[00126] Navigation member 112 may further be provided with image guidance
position
indicators, such as an array of reflectors of the type use in connection with
optical image
guidance systems. The infrared reflectors used with such a system are mounted
to a handle of a
probe-like navigation member 112 in a customary triangular configuration
calibrated to identify
the tool to the image guidance system. Such imaging systems are available, for
example
Medtronic Surgical Navigation Technologies (Denver, Colo.), Stryker
(Kalamazoo, Mich.), and
Radionics (Burlington Mass.).
[00127] Typically, the positioning of the indicators is calibrated such that
the image guidance
system can project an image of the tool onto a display of images of the
patient's brain, such as
MRI images used to plan surgery. Thus, as discussed above, as surgical access
system 100 is
inserted, the surgeon can see the relative position of system 100 relative to
the structures of the
brain as reflected on images, and particularly with respect to the target
tissue.
[00128] Other guidance systems, such as magnetic or electromagnetic or radio
transmitting
systems may also be used, and the illustration of infrared reflectors and
discussion of optical
image guidance systems are exemplary only and are not intended to be limiting.
In addition,
while the exemplary method has been described in connection with superimposing
an image of
surgical access system 100 onto a pre-operative image, it is contemplated that
real-time imaging
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
28
capability may be utilized and that the image of surgical access system 100
may then be shown
in relation to the surrounding tissue structures on a real time image.
[00129] In another exemplary configuration, an RFID chip may be embedded in
obturator 104
that operatively communicates information to a navigation system or other
surgical system about
the specific attributes, such as, but not limited to, length and diameter.
This information may be
used to facilitate placement with the navigation system or other systems for
information display
or trajectory and location calculations during placement of obturator 104.
[00130] In yet another exemplary arrangement, as shown in FIGS. 16A-16B, an
alternative
embodiment of an obturator 504 may be used, wherein the obturator 504 is
configured with a
post 512 that is configured to operatively attach a navigation array. Post 512
may be detachably
or permanently connected to grip member 578 of obturator 104. For example, as
shown in FIG.
16A, post 512 is configured to be selectively detachable and may be used to
capture a small coil
513 for MRI tracking of surgical access assembly 100. A portion of post 512
may be threaded
and an access opening 588 formed in a proximal face of grip member 578 have be
provided with
corresponding threads (not shown) so as to affix post 512 to obturator 504.
Other manners of
selectively affixing post 512 to obturator 504 are also contemplated,
including, but not limited to,
a locking member 110 arrangement similar that shown in FIG. 2. As also
discussed, post 512
need not be selectively detachable. Indeed, it is contemplated that post 512
may be permanently
affixed to obturator 504, in any suitable manner, whereby the navigation array
may be secured to
post 512. In yet another alternative arrangement, obturator 504 may be
configured such that a
post, which is an element of the array itself, may be attached.
[00131] In still a further alternative arrangement, referring to FIGS. 16C-
16D, a coil sensor
513' may be configured to be disposed about an outer periphery of post 512. In
this
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
29
arrangement, coil sensor 513' is slide or otherwise mounted to post 512 such
that when post 512
is operatively attached to obturator 504 coil sensor 513' is captured between
a portion of grip
member 578 and a proximal end portion 514. A connecting wire 516 operatively
attaches coil
sensor 513' to an image position console 518.
[00132] Once surgical access assembly 100 is assembled and operatively
connected to a
navigational system, the process then proceeds to step 418, in which surgical
access assembly
100 is navigated to area of interest 500. In one exemplary arrangement, distal
tip 178 of
obturator 104 is directed to a furthermost outer margin of area of interest
500. More specifically,
referring to FIG. 14B, for example, surgical access assembly 100 is directed
along a trajectory T
that extends through area of interest 500 to a location 501 that may
positioned within the margins
of area of interest 500 or even slightly beyond the margin.
[00133] Due to the tapered configuration and closed, radiused distal tip 174
of obturator 104,
as well as the radiused distal tip 132 of outer sheath 102, as surgical access
assembly 100 is
inserted into the brain and navigated to area of interest 500, tissue is
gently pushed to either side
of surgical access assembly 100, so as to atraumatically dilate tissue, while
minimizing trauma to
the tissue. Further, because surgical access assembly 100 is operatively
connected to navigation
member 112, as surgical access assembly 100 is being inserted into the brain
tissue, navigation
member 112 may cooperate with an imaging modality to providing real-time
information
concerning fiber tact in trajectory T, thereby allowing the surgeon to
minimize fiber tract
compromise or damage during insertion of surgical access assembly 100. Once
surgical access
assembly 100 is positioned at area of interest 500, the process proceeds to
step 420.
[00134] As step 420, navigation member 112 removed from or detached from
surgical access
assembly 100. The process then proceeds to step 422.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
[00135] Once navigation member 112 is removed, outer sheath 102 is then
operatively
positioned with respect to area of interest 500. More specifically, as shown
in FIG. 17A, outer
sheath 102 is decanted with respect to obturator 104 such that distal end 108
of outer sheath 102
is moved toward distal end 106 of obturator 104, as indicated by arrow M. This
action is
accomplished by grasping grip ring 120 with one hand while maintaining
obturator 104
stationary, such, for example, grasping grip member 178 with another hand.
Grip ring 120 may
be gently rotated and/or swiveled with respect to a central axis of obturator
104 to enable outer
sheath 102 to be moved distally with respect to obturator 104. First stop
member 176 aids in
gripping and manipulating outer sheath 102, in that a gap 423 (see FIG. 2) is
created between
end surface 158 and a distal end surface of grip member 178. Outer sheath 102
is decanted until
grip ring 120 aligns with indicator 194A (see FIG. 7A). Indicator 194A is
spaced from first stop
member 176 a distance that generally corresponds to the length of distal tip
portion 172 of
obturator 104. Accordingly, when grip ring 120 is aligned with indicator 194A,
distal end 108 of
outer sheath 102 is aligned tip member 174 of obturator 104. Moreover, outer
sheath 102 is
positioned within area of interest 500. The process then proceeds to step 424.
[00136] In step 424, once outer sheath 102 is appropriately positioned,
obturator 104 is then
removed from outer sheath 102, as shown in FIG. 17B. More specifically, outer
sheath 102 is
maintained to be relatively stationary at area of interest 500, and obturator
104 is moved in a
proximal direction until fully removed from outer sheath 102. This action
results in outer sheath
102 forming a pathway to area of interest 500. The process then proceeds to
step 426.
[00137] In step 426, outer sheath 102 is then secured in place so as to
prevent cranial pressure
from pushing outer sheath 102 out of the brain tissue. In one exemplary
arrangement, a securing
member may be utilized with small openings 150 on grip ring 120 to temporarily
secure outer
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
31
sheath 102. For instances where illuminating ring 300 is used with surgical
access assembly
100, small openings 309 in illuminating ring 300 align with small opening 150
of grip ring.
Accordingly, securing members may also be utilized with small openings 309. In
the alternative
embodiment shown in FIGS 22-26, the holes 392 defined in the projections 390
which extend
outward from the wall members 304 of the illuminating ring can be utilized to
secure the outer
sheath. However, the securing member may be secured so as to permit a limited
degree of
movement, as will be discussed below, so as to result in a floating system
that permits selective
repositioning. Suitable retaining members include, but are not limited to,
bridle sutures, flexible
bands with retaining hooks, or even repositionable refractor arms. Once outer
sheath 102 is
secured, the process then proceeds to step 428.
[00138] In step 428, debulking area of interest 500 may be conducted.
Traditionally, a patient
is given medication, such as, for example, Mannitol, before an intracranial
operation to reduce
intracranial pressure (ICP) of the brain prior to the surgery. Indeed, ICP is
often experienced by
patients due to the natural response of the craniotomy and/or the present of
an abnormality
within the brain. The present inventors have found that it may be advantageous
to omit or
minimize the use of medication for reducing ICP. More specifically, but not
reducing IPC,
because the brain tends to occupy the available space within the skull, after
obturator 104 is
removed from outer sheath 102, the target tissue may have a tendency to flow
into, and present
itself into the open distal end 108 of outer sheath 102, due to the cranial
pressure. Area of
interest 500 may actually move into outer sheath 102 on its own, thereby
assisting in delivery
and minimizing manipulation required of outer sheath 102 during the process.
[00139] It is contemplated that a wide range of surgical devices may be
inserted into outer
sheath 102 to remove tissue abnormalities. In one exemplary arrangement, it is
contemplated
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
32
that outer sheath 102 may have an inner diameter up to approximately 20 mm, to
allow multiple
instruments, such as graspers, dissectors, scissors, cautery and suction
instruments to be inserted
through outer sheath 102 to perform surgery.
[00140] One exemplary surgical device that may be used is the NICO MYRIAD
manufactured and distributed by Nico Corporation of Indianapolis, Indiana.
Referring to FIG.
18, an exemplary surgical cutting device 640 is shown, such as that disclosed
in co-pending, and
co-owned with the assignee of the present application, U.S. Patent Appl.
Serial No. 12/389,447,
the contents of which are incorporated by reference in its entirety. Surgical
cutting device 640
includes a handpiece 642 and a cutting element that includes an outer cannula
644 and an inner
cannula (not shown). In one exemplary configuration, handpiece 642 is
configured with a
generally cylindrical shape. Handpiece 642 may be sized and shaped to be
grasped with a single
hand. Handpiece 642 also includes a lower housing 650 comprising a proximal
section 646 and
a distal section 648. A front housing section 655 may be connected to a cam
housing positioned
in distal section 648. An upper housing 652 is also provided. The cutting
element is mounted to
upper housing 652 and may be fluidly connected to a tissue collector 658. In
one exemplary
arrangement, tissue collector 658 may be operatively connected directly to
upper housing 652.
Alternatively, tissue collector 658 may be remotely connected to the cutting
element by
appropriate tubing. A vacuum line (not shown) may be connected to a proximal
end of tissue
collector 658 to direct tissue into the cutting element, as well as to deliver
severed tissue to tissue
collector 658. A rotation dial 660 for selectively rotating the outer cannula
644 with respect to
handpiece 642 is also mounted to upper housing 652, to provide controlled
cutting action.
[00141] Use of surgical device 640 is advantageous in that space is limited to
effectuate tissue
debulking, such that use of traditional surgical scissors may be challenging,
especially when
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
33
other instruments are inserted into outer sheath 102 simultaneously. Moreover,
fibrosity of a
tumor may present challenges for the use traditional suction debulking
devices. Traditional
graspers operate by tearing tissue of interest. However, the tearing action
may become
problematic if vessels or fascicles are too close to the tissue being torn in
that such vessels or
fascicles may also be torn.
[00142] In step 428, as area of interest 500 is cytoreductively debulked, it
may become
necessary to reposition or move outer sheath 102. If repositioning is
necessary, the process
moves to step 432. To that end, in one exemplary arrangement, manipulation
members may be
provided. Examples of manipulation members 700 and 700' are illustrated in
FIGS. 19A-19B.
Manipulation member 700 comprises a handle member 702 that supports an
armature 704, and a
hook element 706 that extends from armature 704. Hook element 706 is sized to
fit within small
openings 150 and 309 disposed within grip ring 120 and illuminating ring 300,
respectively. In
the alternative embodiment shown in FIGS. 22-27, the hook element 706 fits
within small
openings 388 in the exterior face 394 of the housing 380. In operation, hook
element 706 is
engaged with a small opening 150/309/388 and handle member 702 is used to
gently push or pull
outer sheath 102. Because outer sheath 102 is only loosely secured, outer
sheath 102 may be
selectively moved slightly for improved visualization or to access tissue.
After outer sheath 102
has been repositioned, or if repositioning of outer sheath 102 is not
necessary, the process moves
to step 434, and cytoreduction of area of interest 500 continues.
[00143] In an alternative arrangement, manipulation member 700' may be secured
to a
flexible holder member 710. Manipulation member 700' comprises an armature 712
that carries
a hook element 714 and an engagement portion 716. Engagement portion 716
operatively
engages holder member 710 so as to fixedly secure manipulation member 700' to
holder member
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
34
710, thereby freeing a surgeon's hand, once outer sheath 102 is positioned. It
is understood that
multiple manipulation members 700/700' may be utilized to permit a surgeon to
selectively push
or pull outer sheath 102.
[00144] Outer sheath 102 is configured such that multiple instruments may be
inserted
simultaneously therewithin, thereby increasing the speed and safety of
surgical procedures. In
one exemplary arrangement, an endoscope may be partially inserted and held to
one side of outer
sheath 102, to provide an image of area of interest 500 to a monitor, while a
surgical instrument,
such as surgical instrument 640 is also inserted within outer sheath 102.
Illuminating ring 300
may also be used, with the endoscope and the surgical instrument being
inserted through access
opening 308 that aligns with opening 146 of grip ring 120. Because
illuminating ring 300
provides the necessary light for outer sheath 102, a relatively small diameter
endoscope may be
used, thereby increasing the available space within outer sheath 102 for other
surgical
instruments. In another exemplary configuration, the surgeon may have both a
surgical
instrument and a cautery instrument simultaneously inserted into outer sheath
102, thereby
permitting the surgeon to cauterized vessels that are encountered during the
procedure.
[00145] In another exemplary arrangement, during the procedure, fluorescing
dye may be
introduced into the patient, either before surgery or during the surgery. One
such dye is Glioan
(5-Aminolevulinic Acid), however other suitable dyes may also be used. The
fluorescing dye
may be introduced by any suitable methods, including, but not limited to,
injecting the patient
with the dye, providing the dye orally to the patient prior to surgery, or
even injecting the dye in
situ through outer sheath 102. In one exemplary arrangement, the dye is
configured to bond to
proteins of abnormal cells such that the cells are visually distinguishable
from healthy cells.
With this visual indication of healthy vs. abnormal tissue, the surgical
instrument may be more
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
efficiently used to resect abnormal tissue. In other embodiments, light
delivered through outer
sheath 102 has a predetermined wavelength that is configured to interact with
the dye to
illuminate or fluoresce abnormal tissue. For example, illumination cap 300 may
be provided
with LED lights of a preselected wavelength that operatively interacts with a
preselected dye to
illuminate abnormal tissue and assist with differentiating healthy tissue from
diseased tissue.
[00146] In another exemplary configuration, a light probe or fiber optic
bundle (not shown)
may be inserted into outer sheath 102 to assist with differentiation between
healthy tissue and
abnormal tissue. In one arrangement, the probe/bundle is simply inserted into
outer sheath 102
as a separate element, along with a surgical device. The probe/bundle is
operatively connected
to a console such that the reflected light is delivered to the console. A
sensor in the console (i.e.,
the sensor is remotely located from the point of detection, receives the
reflected light to trigger a
signal to the user based on predetermined parameters. In other words, the
natural florescence of
the tissue is then reflected back to the console to inform the user whether or
not the tissue is
diseased or abnormal.
[00147] In another exemplary configuration, the surgical device may be further
provided with
a delivery sleeve 800 that mounts to surgical device 640, and example of which
may be found in
FIG. 20. Various embodiments of delivery sleeve 800 may be found in co-
pending, and co-
owned with the assignee of the present application, U.S. Patent Appl. Serial
No. 13/269,339, the
contents of which are incorporated by reference in its entirety. As may be
seen in FIG. 20,
delivery sleeve 800 generally includes at least two lumens, a first lumen 802
which is configured
to receive outer cannula 644 of surgical device 640, and a second lumen 804
which is configured
to receive an optical device, such as a light probe or a fiber optic bundle
(not shown). Use of this
arrangement permits use of additional surgical tools/instruments within outer
sheath 102. More
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
36
specifically, as the optical device is supported within the delivery sleeve
800, which, in turn, is
connected to the surgical device, the surgeon can simultaneously differentiate
between abnormal
and healthy tissue, and resect tissue, all with by just holding the surgical
device 640. As a result,
the surgeon may also choose to utilize a separate cautery device within outer
sheath 102 to
permit cauterization of any vessels during the resection, in real time, and
without requiring
removal of the surgical device 640.
[00148] Because outer sheath 102 may be directly positioned at area of
interest 500 in such a
manner as to avoid unnecessary damage to critical structures, and because
surgical device 640
may be placed directly at the sight of area of interest, utilizing surgical
access system 100
provides the ability to resect most of an area of interest 500, such as a
tumor. As one of ordinary
skill in the art can appreciate, the more that a tumor is resected and
removed, the less adjuvant
therapy is required for treatment. In other words, the more diseased tissue
there is resected, the
less diseased tissue there is to destroy.
[00149] Once a cytoreductive resection of area of interest 500 has been
completed, the process
then proceeds to step 436. In step 436 a decision is made to either remove
outer sheath 102 or to
leave outer sheath 102 in position. More specifically, for some therapy
applications removal of
outer sheath 102 may be more effective than leaving outer sheath in place to
deliver the therapy.
If the decision is made to remove outer sheath 102, after removal of outer
sheath 102, the process
400 proceeds to step 438.
[00150] As one of ordinary skill in the art may appreciate, the natural
elasticity of brain tissue
will maintain access or a corridor to area of interest 500 for a period of
time. In step 438, while
the corridor is still intact after removal of outer sheath 102, in one
exemplary arrangement, a
delivery device may be inserted into the corridor to deliver irrigation to the
surgical site. In some
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
37
instances, a syringe may be inserted into the corridor to deliver an
irrigating fluid, such as saline
directly to the surgical site. In another exemplary configuration, a drainage
catheter (which is
configured with a plurality of small openings at its distal end) is delivered
into the corridor such
that the distal end of the catheter is placed at or adjacent the surgical
site. Irrigating fluid is then
introduced into the proximal end (such, as for example, by operatively
attaching a syringe barrel
to the proximal end), to deliver the irrigating fluid to the surgical site.
The irrigating fluid
flushes out debris and assists in the brain tissue's natural tendency to close
back in on itself
Once the surgical site has been irrigated, it may also be desirable to deliver
certain therapies to
the surgical site. For example, certain therapies that may be provided in
liquid form may be
directly injected through the corridor, just prior to the tissue closing back
in on itself Because
the corridor is closing, the therapy will be held in place at the surgical
site, thereby increasing its
effectiveness at the site and surrounding tissue.
[00151] In some therapy methodologies, outer sheath 102 may be necessary to
aid in the
delivery and/or placement of such therapy, as will be explained in further
detail below.
Accordingly, if the decision in step 436 is made to keep outer sheath 102 in
place after
completion of cytoreduction, the process 400 proceeds to step 442.
[00152] In step 442, area of interest/surgical site 500 is irrigated to again
remove any debris
from the area. Irrigation may be performed in the same manner as discussed in
step 438, except
through outer sheath 102. Once irrigation is complete, the process proceeds to
step 444.
[00153] In step 444 a therapy is delivered to area of interest 500. In one
exemplary
configuration, intraoperative radiotherapy (IORT) may be employed, so as to
deliver therapy
directly to area of interest 500 through outer sheath 102. In one exemplary
configuration, an
implantable therapy may be applied to area of interest 500. Example of an
implantable therapy
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
38
include: bioabsorbable radiation pellets, wafers or mesh, such as, for
example, those
manufactured by Nano-Rad LLC. Other examples include, but are not limited to,
titanium
capsules or seeds with radiation contents, bioabsorbable gels or foams that
contain radioactive,
chemotherapy or immunotherapy agents.
[00154] In another exemplary configuration, a balloon catheter may be used to
perform
brachytherapy following the removal of diseased tissue at area of interest
500. For example, a
balloon catheter may be inserted through outer sheath 102 and delivered to
area of interest, and
then the balloon catheter may be inserted with a predetermined amount of
radioactive solution
followed by the delivery of radiation to the surrounding tissues. A
commercially available
catheter that may be used includes the GliaSite balloon catheter, with an
Iotrex radioactive
solution. Use of a balloon catheter may provide a more targeted delivery of
liquid radiation,
thereby reducing impact on brain tissues surrounding the diseased tissue.
[00155] In another exemplary arrangement, an electron beam driven X-ray source
may be
provided. One such exemplary configuration is the Zeiss 1NTRABEAMO. The
electrons are
generated and accelerated in a main unit and travel via an electron beam drift
tube which is
surrounded by a conical applicator sheath such that its tip lies at an
epicenter of an applicator sphere
to provide a point source of low energy X-rays at the tip. With this
configuration, a nearly isotropic
field of low energy is emitted.
[00156] In operation, the applicator sheath is inserted through outer
sheath 102 and into the
surgical cavity at area of interest 500. An intraoperative ultrasound may be
performed to determine
the distance of the applicator surface to the skin, to avoid significant skin
doses. The applicator
sheath may be secured into place by the surgeon using subcutaneous sutures
around the neck of the
sphere, similar to that described above in connection with outer sheath 102.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
39
[00157] In another exemplary arrangement, a photodynamic therapy may be used,
whereby a
predetermined chemical composition may be provided to the patient and the
chemical
composition may be selectively activated by a predetermine wavelength, thereby
achieving a
therapeutic reaction. For example, in one exemplary configuration,
illuminating ring 300 may be
turned on to achieve the therapeutic reaction. In another exemplary
configuration, a light source,
such as, for example, a fiber optic bundle, may be directed through outer
sheath 102, either
directly through outer sheath 102 or through delivery sleeve 800.
[00158] In yet another exemplary configuration, external beam high frequency
ultrasound or
interstitial high frequency ultrasound may also be delivered through outer
sheath 102 and
directly to area of interest 500.
[00159] In yet a further exemplary configuration, as show in FIGS. 21A-21B, an
implantable
delivery device 900/900' may be provided. Implantable delivery device 900/900'
includes a
neck portion 902 that is connected to a body portion 904/904'. Both neck
portion 902 and body
portion 904/904' may be constructed of a relatively soft and flexible
material. Body portion
904/904' defines a reservoir for holding a therapeutic agent therein. A
proximal end 905 of neck
portion 902 is largely closed, with access to an interior of implantable
delivery device 900/900'
being providing by a luer port 906. More specifically, therapy agents are
introduced into
delivery device 900/900' through luer port 906. A sealing flange 908 may
further be provided,
that operatively connects the neck portion 902 to assist in holding
implantable delivery device
900/900' in place within the brain.
[00160] In the arrangement show in FIG. 21A, body portion 904 may be provided
with at least
one small opening 910. In one exemplary arrangement, a plurality of small
openings 910 are
provided, and such openings may be spaced equi-distance from one another about
the periphery
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
of body portion 904. Small openings 910 are configured to permit the therapy
agent that is
introduced through luer port 906 to weep out of the reservoir formed by body
portion 904 at a
controlled rate to increase effectiveness. Alternatively, body portion 900 may
be configured as a
permeable membrane that permits slow and controlled passage of therapy from
the reservoir to
the brain tissue 1000.
[00161] In an alternative arrangement shown in FIG. 21B, body portion 904' may
be provided
with flexible finger-like projections 912. In one exemplary configuration,
projections 912 are
spaced equi-distance from one another about the periphery of body portion
904'. Projections
912 extend outwardly from an outer periphery of body portion 904' and may be
formed with
channels that provide communication between the reservoir and small openings
914 configured
at distal tips 916 of projections 912. Openings 914 are configured to permit
the therapy agent
that is introduced through luer port 906 to weep out of the reservoir.
Projections 914 assist in
frictionally retaining delivery device 900' at a target site.
[00162] Referring back to process 400, if delivery device 900/900' is
employed, delivery
device 900/900' is inserted at area of interest 500 through outer sheath 102.
Once positioned,
outer sheath 102 is removed, and sealing flange 908 is operatively connected
to neck portion 902
such that luer port 906 is accessible. Sealing flange 908 is configured to
extend over the
periphery of the surgical access opening that was formed through the skull
102, thereby
providing protection for the exposed brain tissue 1000. The therapeutic agent
may be supplied to
the reservoir formed by body portion 904/904' either before delivery device
900/900' is
positioned at area of interest 500, or after sealing flange 908 is in place.
Sealing flange 908, as
well as body portion 904/904' and neck portion 902 may be configured with
flexible material to
allow for sealing against the dura and bone of the brain.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
41
[00163] In yet another alternative arrangement involving delivery device
900/900', a transfer
material may be delivered through outer sheath 102, similar to a foam that is
configured to
conform to the cytoreducted area of interest 500. The foam will allow
continuous contact with
the therapy agent that weeps through body portion 904/904' to provide a
controlled dosage of
therapy to area of interest 500.
[00164] After surgery and therapy on the target tissue is complete, the
process proceeds to
step 446. In this step, the instruments used for surgery and/or therapy are
removed from outer
sheath 102. As the target tissue is removed, brain tissue will fill the void
formed by removing
area of interest 500 so that healthy brain tissue underlying the now removed
target tissue is
adjacent the end of outer sheath 102. Outer sheath 102 is then gently removed
and the brain
tissue will naturally fill and reclaim the space formerly occupied by the
abnormality and outer
cannula 102, aided by the irrigation of area of interest 500. Moreover, as the
brain tissue
reclaims the space formerly occupied by the abnormality and outer cannula 102,
implanted
therapies, such as, for example, bioabsorbable radiation pellets, wafers or
mesh, will be held in
place by area of interest 500 to provide effected treatment. While this
process may take several
minutes, it is relatively atraumatic. Once outer sheath 102 has been removed,
the process
continues to step 448, whereby the dura, skull and scalp are then closed in a
known manner and
the process ends. In the exemplary cases whereby a treatment device may be
implanted, full
reclaiming of the space is delayed due to the implant until implant is
explanted or absorbed.
[00165] Because the location of the area of interest will vary from patient to
patient, in one
exemplary arrangement, it is contemplated that surgical access system 100 will
be provided as
part of a kit. More specifically, it is contemplated that a set of multiple
obturators 104 may be
provided that have different lengths and/or diameters. The set may be provided
in a container
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
42
that is configured be sterilized, with obturators 104 secured therein. It is
also contemplated that a
set of manipulation tools 700/700' may also be provided with the kit, and that
manipulation tools
700/700' may be positioned within the container for selective sterilization.
Outer sheath 102
may be provided with the kit, in various lengths and diameters that correspond
to the lengths and
diameters of obturators 104 provided in the kit. However, in one exemplary
arrangement, outer
sheaths 104 are provided separately as single use devices, in sterilized
pouches.
[00166] Alternative embodiments of illuminating ring assemblies will now be
discussed. A
first alternative embodiment of an illuminating ring assembly 1300, as well as
securing
arrangements operable to secure illuminating ring assembly 1300 to a grip
ring, such as grip ring
120 of outer sheath 102, is shown in FIGS. 22 through 27. In one exemplary
arrangement, as
shown in FIG. 22, illuminating ring assembly 1300 is defined by a housing 1304
having a wall
member 1305 and a cover (best seen in FIG. 27). A light member 1303 is
positioned within
housing 1304. Housing 1304 (and light member 1303) further includes an opening
1307
therethrough that is configured to receive medical instruments therein, as
described above.
Extending outwardly from a bottom surface of wall member 1305 is a securing
member 1346. A
positioning member 1348 that also extends outwardly from a bottom surface of
wall member
1305 may be provided. In one exemplary configuration, wall member 1305 is
provided with one
or more protrusions 1342, 1344 that extend radially outwardly from a periphery
of wall member
1304 upon which securing member 1346 and positioning member 1348 may be
mounted.
Protrusions 1342, 1344 may be located opposite one, although other convenient
arrangements are
also contemplated.
[00167] Illuminating ring assembly 1300 further includes a selectively
closable attachment
mechanism 1350. In one exemplary configuration, attachment mechanism 1350 is a
hinged
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
43
attachment mechanism, as may be seen in FIG. 22, for example. However, it is
understood that
other attachment mechanisms may be employed and are contemplated by the scope
of this
disclosure. With respect to attachment mechanism 1350 in FIG. 22-27, hinged
attachment
mechanism 1350 may include a centrally located hinge member 1356 and two arms
1358, 1360
positioned on either side of hinge 1356. Each arm 1358, 1360 is defined by
first and second
ends, 1322a, 1322b and 1324a, 1324b, respectively.
[00168] In embodiment shown, second end 1324a of arm 1358 has a latching
mechanism
1352 to attach second end 1324a of arm 1358 to second end 1324b of arm 1360.
In one
exemplary arrangement, arm 1360 (i.e., the arm without latching mechanism
1352) may,
depending on configuration of latching mechanism 1352, be configured with a
latching recess
1354 configured to receive a portion of latching mechanism 1352. More
specifically, in one
exemplary arrangement, latching recess 1354 is configured to accept a
protuberance 1372 that is
disposed on latching mechanism 1352. Recess 1354 may be defined by a forward
edge 1355
with a rounded lip.
[00169] Latching mechanism 1352 may further include an actuating end 1327and a
pivot
member 1370. Pivot member 1370 serves to mount latching mechanism 1352 to
second end
1324a of arm 1358. Operation of latching mechanism 1352 will be described
below in further
detail.
[00170] Hinged attachment mechanism 1350 has two sides, an obverse side 1364
and a
reverse side, opposite thereto. As illustrated in FIG. 23, the reverse side is
configured to abut at
least a bottom surface 1309 of illuminating ring assembly 1300. In one
embodiment, hinged
attachment mechanism 1350 further includes a mounting cavity (not shown) that
is configured so
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
44
as to accept positioning member 1348 therein. Alternatively, a cavity may be
located elsewhere
on hinged attachment mechanism 1350.
[00171] Hinged attachment mechanism 1350 is configured such that second ends
1324a,
1324b of arms 1358, 1360 are selectively rotatable toward one another.
Further, in one
exemplary arrangement, when positioning member 1348 is positioned within the
cavity of
hinged attachment mechanism 1350, arms 1350, 1360 may be selectively rotated
around
positioning member 1348. More specifically, arms 1358, 1360 may be rotated in
such a way that
second ends 1324a, 1324b of arms 1358, 1360 are rotated toward securing member
1346.
[00172] In one exemplary arrangement, second ends 1324a, 1324b of arms 1358,
1360 may
each be configured with complementary depressions 1366, 1368 (best seen in
FIG. 22).
Depression 1366, 1368 are oriented toward one another when arms 1358, 1360 are
rotated
together so as to define a securing hole 1325 (see FIG. 23) that is configured
to accept a portion
of securing member 1346. More specifically, securing hole 1325 is configured
to be
substantially the size and shape of securing member 1346, best seen in FIGS
23, 24, and 26.
[00173] As shown in FIG. 23, when second ends 1324a, 1324b of arms 1358, 1360
of hinged
attachment mechanism 1350 are brought together, the second ends 1324a, 1324b
may be locked
together by latching mechanism 1352. When second ends 1324a, 1324b of arms
1358, 1360 of
hinged attachment mechanism 1350 are brought toward one another, a forward
edge of
protuberance 1372 on latching mechanism 1352 is forced upward from a neutral
position by
forward edge 1355 of recess 1354 until protuberance 1372 passes over forward
edge 1355 of
recess 1354 and comes to rest in recess 1354, thus locking arms 1358, 1360 in
position.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
[00174] In one exemplary arrangement, second ends 1324a, 1324b may further
include
cooperating indentations 1374a, 1374b. Indentations 1374a, 1374b are
configured to cooperate
to define a locking member recess 1375 (see, e.g., FIG. 23) when second ends
1324a, 1324b are
locked together, as will be explained below.
[00175] In operation, illumination ring assembly 1300 is mounted onto grip
ring 120 of outer
sheath 102. More specifically, wall member 1305 of housing 1304 of
illumination ring assembly
1300 is sized to receive grip ring 120 therein, as shown in FIG. 24, for
example. Hinged
attachment mechanism 1350 is mounted onto housing 1304 such that positioning
member 1348
is received within the mounting cavity of hinge member 1356. First and second
arms 1358, 1360
are pivoted about hinge member 1356 and second ends 1324a, 1324b are brought
together, as
shown in FIG. 25. As may be seen, depressions 1366, 1368 are also brought
together to form
securing hole 1325 around securing member 1346 such that securing member 1346
is captured
within securing hole 1325. Locking member recess 1375 is also formed by second
ends 1324a,
1324b as those ends come together. Locking member recess 1375 is configured to
receive a
locating member 1362 of grip ring 120 (see FIG. 26) to ensure proper assembly
of hinged
attachment mechanism 1350 to housing 1304.
[00176] Once second ends 1324a, 1324b are brought together, locking mechanism
1352 is
actuated with protuberance 1372 engaging recess 1354, thereby securing housing
1304 to grip
ring 120, as shown in FIG. 25. Thus, grip ring 120 of outer sheath 102 may be
secured to
illuminating ring assembly 1300 by hinged attachment mechanism 1350, which is
configured so
that, when hinged attachment mechanism 1350 is in place and arms 1358, 1360
are brought
together and locked in position, an inner circumference of hinged latching
mechanism 1350
frictionally engages grip ring 120 and holds grip ring 120 in place to housing
1304.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
46
[00177] As shown in FIG. 27, housing 1380 of illuminating ring assembly 1300
has a cover
1380 that defines an exterior face 1394. A central opening 1395 is formed
through cover 1380.
Opening 1395 is configured to provide communication with an inner lumen of
outer sheath 102
so as to permit one or more medical devices to be inserted therein.
[00178] In one exemplary arrangement, cover 1380 may be configured with one or
more
notches 1382. Each notch 1382 is configured to extend from inner perimeter
1386 of opening
1395 to an outer perimeter 1384 of wall member 1305. In one exemplary
arrangement, inner
perimeter 1386 of cover 1380 includes a beveled entry surface 1386a (best seen
in FIG. 27) that
joins an inner periphery surface 1386b that defines opening 1395. In this
arrangement, ends of
each notch 1382 at an edge of inner perimeter entry surface 1386a, notches
1382 may be tapered
slightly outwardly. Inner periphery surface 1386b may further include notches
1389 that are
aligned with notches 1382. Notches 1389 may be configured to taper inwardly in
a V-shape
along inner periphery 1386b. When aligned, each notch 1382 serves to receive
and selectively
retain a string or cord from a Neuro Pattie, other absorbent surgical sponge
or other object to be
temporarily positioned within outer sheath 102. The outwardly tapered notch
ends of notches
1382 allow for some flexibility in movement of the string or cord while in the
outer sheath 102.
[00179] In one exemplary arrangement, notches for retaining a Neuro Pattie, or
the like, may
be formed in an upper surface of grip ring 120 for those embodiments where no
light
illuminating ring assembly 1300 are utilized. Details of this configuration
are further discussed
in co-pending U.S. patent application no. 13/786, 062.
[00180] In addition to notches 1382, a multiplicity of small openings 1388 may
also be
formed in housing 1380. Openings 1388 may be spaced equidistantly around inner
perimeter
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
47
1386 of opening 1395 of housing 1380. In one exemplary configuration, openings
1388 may
extend completely through external face 1394. In another exemplary
arrangement, openings
1388 may extend only partially into external face 1394, but not entirely
through external face
1394. A manipulation member, such as that illustrated in FIGS. 19A-19B, may be
engaged with
small openings 1388 and used to gently adjust the location of outer sheath
102. In yet another
alternative arrangement, one or more radial projections 1390 may extend
radially outwardly
away from wall member 1305 of housing 1380. In one arrangement, projections
1390 may be
equally spaced around wall member 1305 of housing 1380. Each projection 1390
may define a
small hole 1392. Retaining members (not shown) may extend through small holes
1392 to
loosely secure outer sheath 102 in place once outer sheath 102 is positioned
within a patient.
Suitable retaining members include, but are not limited to, bridle sutures,
flexible bands with
retaining hooks, or repositionable retractor arms.
[00181] An alternative exemplary arrangement of illuminating ring 1400 is
shown in FIGS.
28-29. Illuminating ring 1400 has a selectively flexible wall member 1404 that
further includes a
first circumferential edge 1406 (best seen in FIG. 27). A second
circumferential edge 1408 may
be disposed opposite to first circumferential edge 1406. A cover 1420 is
connected to the wall
member 1404. As may be seen in FIG. 29, wall member 1404 serves to define a
cavity 1410 into
which grip ring 120 may be received, as will be explained below in further
detail.
[00182] Cover 1420 includes a generally central opening 1468 therethrough. An
inner wall
member 1460 extends between an inner perimeter of cover 1420 thereby defining
an access
opening configured to receive one or more surgical instruments. In one
exemplary arrangement,
a top portion of inner wall member 1460 may be beveled inwardly so as to
direct surgical
instruments and other items introduced through opening 1468.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
48
[00183] Disposed within cavity 1410 is at least one locating member 1412.
Locating member
1412 extends from an inner surface of cover 1420, so as to extend
substantially perpendicularly
therefrom. Each locating member 1412 has an inside surface 1442 and an outside
surface 1444.
Locating members 1412 are configured to accept a radial portion of an outside
perimeter of a
grip ring 120 attached to an outer sheath 102. In one exemplary arrangement,
locating members
1412 are arranged in an opposing manner.
[00184] One or more retaining tabs 1416 extend radially inward into cavity
1410 from first
edge 1406 of wall member 1404. In one exemplary arrangement, retaining tabs
1416 are
configured to be arranged in a generally perpendicular direction from wall
member 1404. Each
of the retaining tabs 1416 may further include a lip 1424 extending from tab
1416 toward the
center of cavity 1410, as will be explained in further detail below. In one
exemplary
arrangement, two retaining tabs 1416 are provided, which may be arranged in an
opposing
manner, as illustrated in FIG. 29.
[00185] As best seen in FIGS. 28-29, one or more gripping areas 1418 may be
disposed on the
outer perimeter of wall member 1404. Gripping areas 1418 may have a textured
surface, such
as, for example, alternating grooves 1434 and ridges 1436 disposed thereon. In
one exemplary
arrangement, two gripping areas 1418 are disposed opposite one another on wall
member 1404.
In one exemplary arrangement, tabs 1416 and gripping areas 1418 are arranged
in an alternating
manner. Wall member 1404 is sufficiently flexible such that when gripping
areas 1418 are
biased toward one another, wall member 1404 flexes, causing tabs 1416 to flex
upwardly. Tabs
1416 may be flexed a sufficient amount to allow the outer perimeter of grip
ring 120 to be
positioned between lips 1424 of tabs 1416. Grip ring 120 may then be
introduced to cavity 1410
defined by wall member 1404, and positioned adjacent inner surface of cover
1420. Inside
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
49
surface 1442 of the at least one locating member 1412 may be positioned nearly
adjacent the
outer perimeter of grip ring 1426, thereby serving to properly position grip
ring 1426. Once grip
ring 1426 is properly positioned, gripping areas 1418 may be released to their
unbiased positions,
thereby releasing tabs 1416 to their original positions. When tabs 1416 return
to the original
positions, lips 1424 extend a sufficient amount over a bottom edge of grip
ring 1426 to prevent
grip ring 1426 from being easily removed from illuminating ring 1400, thereby
securing grip
ring 1426 in place.
[00186] Illuminating ring 1400 may further include a plurality of openings
1448 in inside
surface of cover 1420. A plurality of light elements (not shown) may be
disposed in alignment
with openings 1448. Light elements may be incorporated into a circuit board
(not shown), which
circuit board may be generally ring-shaped, and configured to fit within outer
perimeter of wall
member 1404. Light elements and circuit board may be disposed between inside
surface of
cover 1420 and a top portion 1430 of illuminating ring 1400. Wires (not shown)
may extend
from circuit board through openings (not shown) in wall member 1404 or top
portion 1430, and
be electrically connected to a remote power source.
[00187] Illuminating ring 1400 may also have one or more retaining elements
1452 extending
radially outward from wall member 1404. In one exemplary arrangement,
retaining elements
1452 extend radially outward from adjacent to first circumferential edge 1406
of wall member
1404. Retaining elements 1452 may each define a small hole 1454. Suitable
retaining members
may extend through small holes 1454 in the plurality of retaining elements
1452, and loosely
attach illuminating ring 1400 to a surface. Suitable retaining members
include, but are not
limited to, bridle sutures, flexible bands with retaining hooks, or
repositionable retractor arms.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
[00188] At least one notch 1456 may extend from an outer perimeter to the
inner perimeter of
cover 1430. The at least one notch 1456 may be configured so to accept and
selectively retain a
string or cord from a Neuro Pattie or other absorbent surgical sponge with an
attached string or
cord, as described above in connection with FIG. 27.
[00189] At least one small opening 1470 may be disposed on cover 1430. The at
least one
small opening 1470 may be configured to accept a portion of a manipulation
member. Examples
of manipulation members 700, 700' are illustrated in FIGS. 19A-19B. A hook
element 706 of
manipulation member 700, 700' may engage with small opening 1470, and handle
702 of
manipulation member 700, 700' may be manipulated to gently reposition
illuminating ring 1400
and outer sheath (not shown).
[00190] Another alternative arrangement of an illuminating ring assembly 1500
is shown in
FIGS. 30-33. Illuminating ring assembly 1500 is similar to illuminating ring
assembly 1300
shown in FIG. 22. Illuminating ring assembly 1500 includes a housing 1504
comprising a wall
member 1505 and a cover 1530. Wall member 1504 extends around the outer
perimeter of
illuminating ring 1500 and extends downwardly from cover 1530 to define a
generally circular
cavity. A light member 1503 is positioned within the cavity formed by housing
1504. Light
member 1503 may be configured as a substantially planar ring, wherein an outer
perimeter of
light member 1503 fits within the cavity defined by wall member 1504.
[00191] Housing 1504 (and light member 1503) further includes an opening 1507
therethrough that is configured to receive medical instruments therein, as
described above.
Extending outwardly from a bottom surface of wall member 1505 is a securing
member 1546. In
one exemplary configuration, wall member 1505 may be provided with one or more
protrusions
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
51
1542, 1544 (best seen in FIG. 33) that extend radially outwardly from a
periphery of wall
member 1504 upon which securing member 1546 may be mounted. While not shown,
it is
understood that a positioning member similar to that shown in FIG. 22 and
described above, may
also be provided. Protrusions 1542, 1544 may be located opposite one another,
although other
arrangements are also contemplated.
[00192] Illuminating ring assembly 1500 further comprises a spring clamp 1502
that may be
selectively secured to wall member 1504. Spring claim 1502 may be configured
as a unitary
member, as illustrated in FIG. 33. Spring clamp 1502 may further include a
first and a second
gripping portion 1506, 1509, an attachment portion 1543, and finger rests
1508, 1510. At least
one opening (not shown) may be formed on an end face of attachment portion
1543 of spring
clamp 1502. The opening is configured to selectively receive securing member
1546 to secure
spring clamp 1502 to housing member 1504.
[00193] First and second gripping portions 1506, 1509 are disposed
substantially opposite one
another. First gripping portion 1506 has a face surface 1536, and second
gripping portion 1509
has a face surface 1538. Face surfaces 1536, 1538 of gripping portions 1506,
1509 may face one
another. Attachment portion 1543 may be disposed on the anterior surface 1552
of first gripping
portion 1506.
[00194] Finger rests 1508, 1510 are disposed substantially opposite one
another, and each
finger rest 1508, 1510 is disposed between first and second gripping portions
1506, 1509. As
shown in FIG. 32, first gripping portion 1506 has two arms 1512, 1513, each
extending from first
gripping portion 1506 to one of finger rests 1508, 1510. Second gripping
portion 1509 also has
two arms, 1514, 1515, each extending from second gripping portion 1509 to one
of finger rests
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
52
1508, 1510. The portion of each arm 1512, 1513, 1514, 1515 adjacent the
gripping portion 1506,
1509 of spring clamp 1502 may be configured so as to accommodate grip ring 120
therein. Each
arm 1512, 1513, 1514, 1515 may have sufficient flexibility to allow it to
deviate from its usual
configuration so as to allow gripping portions 1506, 1509 to move apart from
one another in
response to finger rests 1508, 1510 being biased toward one another to
facilitate engagement and
disengagement of spring clamp 1502, housing 1504 and grip ring 102.
[00195] As illustrated in FIG. 33, face surfaces 1536, 1538 of gripping
portions 1506, 1509
may be configured with sufficient curvature to allow them to accommodate a
portion of a grip
ring 120 of an outer sheath 102 (as best shown in FIG. 31). Face surfaces
1536, 1538 may have
ridges 1516 and grooves 1518 disposed on them, which ridges 1516 and grooves
1518 may be
configured in such a way frictionally engage ridges and grooves that may be
disposed on grip
ring 120. In one exemplary arrangement, the ridges 1516 and grooves 1518 are
sized such that
corresponding ridges and grooves on grip ring 120 may be meshed with ridges
1516 and grooves
1518.
[00196] Finger rests 1508, 1510 may have a curvature that will allow fingers
to comfortably
rest in finger rest 1508, 1510. Additionally, finger rests 1508, 1510 may have
a textured surface
to provide purchase for fingers.
[00197] Finger rests 1508, 1510 may be biased toward one another. Biasing
finger rests 1508,
1510 toward one another will move second gripping portion 1509 away from first
gripping
portion 1506 while first gripping portion 1508, which is secured to housing
1504, remains
stationary in relation to illuminating ring 1500. Once second gripping portion
1509 has been
moved a sufficient distance away from first gripping portion 1506, grip ring
120 may be inserted
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
53
between first and second gripping portions 1506, 1509, and finger rests 1508,
1510 may be
released to their unbiased positions. Releasing finger rests 1508, 1510 may
cause second
gripping portion 1509 to move toward first gripping portion 1506, and cause
both gripping
portions 1506, 1509 to contact outer perimeter of grip ring 1526, thus
securing grip ring 120 to
housing 1504. Spacing of ridges 1516 and grooves 1518 on faces 1536, 1538 of
gripping
portions 1506, 1509 may coincide with the spacing of ridges and grooves of
grip ring 120,
providing a larger contact area between grip ring 120 and spring clamp 1502
for a more secure
connection.
[00198] To remove grip ring 1526 from housing 1504 and/or grip ring 120,
finger rests 1508,
1510 may be biased toward one another, causing second gripping portion 1509 to
move away
from first gripping portion 1506, thus releasing grip ring 120. Once grip ring
120 has been
released, it may be separated from housing 1504. Further, housing 1504 may be
separated from
spring clamp 1502. More specifically, protuberance 1546 may be disengaged from
the opening
disposed on attachment mechanism 1543.
[00199] Referring now to FIG. 30, At least one notch 1556 may be formed on an
outer surface
1520 of cover 1530 so as to extend from an outer perimeter of cover 1530 to
the perimeter of
opening 1507. The at least one notch 1556 may be configured so to accept and
selectively retain
a string or cord from a Neuro Pattie or other absorbent surgical sponge with
an attached string or
cord, as described above in connection with FIG. 27. In one exemplary
configuration, a plurality
of notches 1556 may be provided, and the notches 1556 may be disposed
equidistantly on the
cover 1530.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
54
[00200] At least one small opening 1570 may be disposed on cover 1530. The at
least one
small opening 1570 may be configured to accept a portion of a manipulation
member. Examples
of manipulation members 700, 700' are illustrated in FIGS. 19A-19B. A hook
element 706 of
manipulation member 700, 700' may engage with small opening 1470, and handle
702 of
manipulation member 700, 700' may be manipulated to gently reposition
illuminating ring 1400
and outer sheath (not shown).
[00201] At least one retaining element 1544 may extend radially outward from
wall member
1504 of cover 1504. Each of the at least one retaining elements 1544 may
define a hole 1548. A
suitable retaining member may extend through each hole 1548 and may be used to
secure
illuminating ring 1500 to a surface. Suitable retaining members include, but
are not limited to,
bridle sutures, flexible bands with retaining hooks, or repositionable
retractor arms.
[00202] Another exemplary configuration of an illuminating light ring assembly
1600 is
shown in FIGs. 34-36. Light ring assembly 1600 is similar to the configuration
shown in FIGS.
30-33. Light ring assembly 1600 includes a cover 1604 that is configured
substantially the same
as cover 1504, a light ring 1603 disposed within cover 1604, as well as a
spring clamp 1602.
[00203] The exemplary alternative arrangement of spring clamp 1602 may have a
first and a
second gripping portion 1606, 1609, an attachment portion 1643, a slidable
portion 1666 and
finger rests 1608, 1610. First and second gripping portions 1606, 1609 are
disposed substantially
opposite one another, as seen in FIGS. 34-36. Attachment portion 1643 is
disposed on the
anterior surface of first gripping portion 1606, and is configured to be
selectively secured to
cover 1604. More specifically, attachment portion 1643 of spring clamp 1602
includes an
opening that is configured to receive a protuberance 1646 of cover 1604, as
shown in FIG. 34.
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
[00204] Slidable portion 1666 is disposed on the anterior surface of second
gripping portion
1609. Slidable portion 1666 includes an exterior edge 1670 opposite second
gripping portion
1606. At least one slot 1668 is formed in slidable portion 1666. Slot 1668
extends from exterior
edge 1670 toward second gripping portion 1609. In one exemplary arrangement, a
pair of slots
1668 is provided, with a land member 1669 therebetween. Further detail
concerning the slots
1668 will be provided below.
[00205] Housing 1604 may have a projection member 1672 extending radially
outward from
wall member 1605, as illustrated in FIG. 35. At least one gripping member 1678
may be
disposed on projection 1672. Each gripping member 1678 may be configured so as
to be
engageable with one of the at least one slots 1668. In one exemplary
configuration, each
gripping member 1678 is configured as having a foot member 1680 that is
oriented transversely
from a leg member 1681. The leg member 1681 is configured to be disposed
within slot 1668,
with foot member overlying an outside surface 1682 of slidable portion 1666,
as best seen in
FIG. 36.
[00206] Similar to finger rests 1508, 1510 in spring clip 1502, finger
rests 1608, 1610 are
disposed substantially opposite one another, and each finger rest 1608, 1610
is disposed between
first and second gripping portions 1606, 1609. First gripping portion 1606 has
two arms 1612,
1613, each extending from first gripping portion 1606 to one of finger rests
1608, 1610. Second
gripping portion has two arms, 1614, 1615, each extending from second gripping
portion 1609 to
one of finger rests 1608, 1610. The portion of each arm 1612, 1613, 1614, 1615
adjacent the
gripping portion 1606, 1609 of spring clamp 1602 may be configured so as to
accommodate a
radial portion of the outer circumference of grip ring 1626. Each arm 1612,
1613, 1614, 1615
may have sufficient flexibility to allow it to deviate from its usual
configuration so as to allow
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
56
gripping portions 1606, 1607 to move apart from one another if finger rests
1608, 1610 are
biased toward one another.
[00207] Gripping portions 1606, 1609 may be configured with sufficient
curvature to allow
them to accommodate a radial portion of grip ring of an outer sheath (not
shown). Gripping
portions 1606, 1609 may each be provided with a retaining lip 1654, 1656
disposed along an
edge of gripping portion 1606, 1609. Retaining lips 1654, 1656 may be oriented
so as to be
substantially parallel to a plane of the top surface 1620 of cover 1604. Each
lip 1654, 1656 is
configured to allow a grip ring to be secured between cover 1604 and lips
1654, 1656 of spring
clamp 1602, and held in place between gripping portions 1606, 1609.
[00208] Finger rests 1608, 1610 each have an outwardly facing surface that may
have a
curvature that will allow fingers to comfortably rest in finger rest 1608,
1610. Finger rests 1608,
1610 may be selectively biased toward one another. When finger rests 1608,
1610 are so biased,
second gripping portion 1609 may be moved away from first gripping portion
1606 to permit
selective engagement with a grip ring.
[00209] Spring clamp 1602 is also configured to be positively engaged with
cover 1604,
which houses light ring 1603 therein. More specifically, once the grip ring of
an outer sheath is
retained to spring clamp 1602 (as described above), slidable portion 1666 is
engaged with
projection member 1672 of cover 1604. More specifically, gripping portions
1678 are received
within slots 1668 and spring clamp 1602 is slid onto cover 1604 until
attachment portion 1643 of
spring clamp 1602 aligns with a protrusion 1642 disposed on cover 1604.
Protuberance 1646
may then be received within an opening (best seen in FIGS. 34 and 36). In this
manner, spring
clamp 1502 is positively secured to cover 1604 (with light ring 1603 disposed
therein) at two
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
57
locations; via protuberance 1646 with an opening on attachment portion 1643
and via
engagement of gripping portions 1678 with slots 1668. The grip ring (not
shown), will thereby
be secured between first and second gripping portions 1606, 1609 of spring
clamp 1602, within
the cavity of cover 1604 between a top surface 1620 and retaining lips 1654,
1656.
[00210] To remove cover 1604 (and light ring) from a grip ring, the finger
rests 1608, 1610
may be biased toward one another, causing second gripping portion 1609 to move
away from
first gripping portion 1606 a sufficient amount to dislodge protrubarance 1646
from the opening
of attachment portion 1643. Spring clamp 1602 may then be slid with respect to
gripping
member 1678 so as to free gripping member 1678 from slots 1680, thereby
releasing spring
clamp 1602 from cover 1604. Once dislodged from cover 1604, finger rests 1608,
1610 may be
biased toward one another to flex gripping portions 1606, 1609 sufficiently
that the grip ring may
be released from retaining lips 1654, 1656.
[00211] Another exemplary alternative configuration of an illuminating ring
assembly 1700 is
shown in FIGS. 37-41. Illuminating ring assembly 1700 includes a cover portion
1704 and an
attachment portion 1702. In this configuration, attachment portion 1702 is
integral with cover
portion 1704 such that illuminating ring assembly 1700 is a unitary structure.
An annular gap
1707 is formed about a substantial periphery of attachment portion 1702 to
provide flexibility to
attachment portion 1702.
[00212] Cover portion 1704 is generally defined by a cover surface 1720 and a
wall member
1705 extending from cover surface 1720. The cover surface 1720 cooperates with
the wall
member 1705 to define a cavity therein (best seen in FIGS. 38 and 41). An
illuminating ring
1703 is disposed within the cavity, but does not extend the entire depth of
the cavity. Extending
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
58
through cover 1704 (as well as illuminating ring 1703) is an opening 1707,
through which
surgical instruments may be received.
[00213] Attachment portion 1702 also has a generally ring-shape and is defined
by first and
second wall sections 1706, 1708. Wall sections 1706, 1708 are separated from
one another so as
to form a gap 1709 therebetween. Each wall section 1706, 1708 is defined by
first and second
ends 1706a, 1706b and 1708a, 1708b, respectively. First ends 1706a, 1708a each
include a
flexible finger tab 1710. Finger tabs 1710 have a thickness that is
substantially less than the
reminder of wall sections 1706, 1708, to which each finger tab 1710 is
connected. Second ends
1706b, 1708b may slope downwardly toward an end of a retaining lip 1712a,
1712b. Retaining
lips 1712a, 1711b are generally curved along the inside periphery of the
cavity formed by wall
member 1705.
[00214] Connection of grip ring 120 from an outer sheath 102 to illuminating
ring assembly
1700 will now be described. First, finger tabs 1710 are pressed toward one
another. Such an
action causes retaining lips 1712a, 1712b to flex, lifting up a top edge
thereof This action
permits grip ring 120 to be received within the cavity. Finger tabs 1710 may
then be released,
which returns retaining lips 1712a, 1712b to its retaining position. In the
retaining position, the
top edge of the retaining lips 1712a, 1712b overlay an outer periphery of grip
ring 120, thereby
capturing grip ring 120 within the cavity of illuminating ring assembly 1700
so as to secure
illuminating ring assembly 1700 to outer sheath 102.
[00215] Referring to FIG. 37, additional alternative features of illuminating
ring assembly
1700 will be described. Similar to previously described configurations, cover
1720 may be
provided with at least one notch 1756. The at least one notch 1756 may extend
from the outer
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
59
perimeter of cover 1720 to the perimeter of opening 1707. The at least one
notch 1756 may be
configured so as to accommodate a string or cord from a Neuro Pattie or other
absorbent surgical
sponge with a string or cord attached.
[00216] At least one small opening 1742 may be disposed on a surface of cover
1720 of
illuminating ring assembly 1700. A manipulation member may be used in
conjunction with
small openings 1742 to gently adjust position of outer sheath 102. Examples of
manipulation
members 700 and 700' are illustrated in FIGS. 19A-19B.
[00217] At least one retaining element 1744 may be provided. Retaining
elements extend
radially outward from wall member 1705 of cover 1704. Each of the retaining
elements 1744
may include a hole 1748. A suitable retaining member may extend through each
hole 1748 and
may be used to secure illuminating ring assembly 1700 to a surface. Suitable
retaining members
include, but are not limited to, bridle sutures, flexible bands with retaining
hooks, or
repositionable retractor arms.
[00218] It will be appreciated that the surgical access system and methods
described herein
have broad applications. The foregoing embodiments were chosen and described
in order to
illustrate principles of the methods and apparatuses as well as some practical
applications. The
preceding description enables others skilled in the art to utilize methods and
apparatuses in
various embodiments and with various modifications as are suited to the
particular use
contemplated. In accordance with the provisions of the patent statutes, the
principles and modes
of operation of this disclosure have been explained and illustrated in
exemplary embodiments.
[00219] It is intended that the scope of the present methods and apparatuses
be defined by the
following claims. However, it must be understood that this disclosure may be
practiced
CA 02901425 2015-08-14
WO 2014/137530 PCT/US2014/015071
otherwise than is specifically explained and illustrated without departing
from its spirit or scope.
It should be understood by those skilled in the art that various alternatives
to the embodiments
described herein may be employed in practicing the claims without departing
from the spirit and
scope as defined in the following claims. The scope of the disclosure should
be determined, not
with reference to the above description, but should instead be determined with
reference to the
appended claims, along with the full scope of equivalents to which such claims
are entitled. It is
anticipated and intended that future developments will occur in the arts
discussed herein, and that
the disclosed systems and methods will be incorporated into such future
examples. Furthermore,
all terms used in the claims are intended to be given their broadest
reasonable constructions and
their ordinary meanings as understood by those skilled in the art unless an
explicit indication to
the contrary is made herein. In particular, use of the singular articles such
as "a," "the," "said,"
etc. should be read to recite one or more of the indicated elements unless a
claim recites an
explicit limitation to the contrary. It is intended that the following claims
define the scope of the
invention and that the method and apparatus within the scope of these claims
and their
equivalents be covered thereby. In sum, it should be understood that the
invention is capable of
modification and variation and is limited only by the following claims.