Note: Descriptions are shown in the official language in which they were submitted.
CA 02901532 2015-08-14
WO 2014/152461 PCT/US2014/027364
SYSTEMS AND METHODS FOR MAKING A LAMINAR VENTRICULAR
PARTITIONING DEVICE
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This patent application claims priority as a continuation-in-part
of U.S. patent
application Ser. No. 13/827,927, filed March 14, 2013, which is a continuation
in part of U.S.
patent application Ser. No. 12/893,832, filed on Sep. 29, 2010, which is a
continuation-in-part of
U.S. patent application Ser. No. 11/860,438, filed on Sep. 24, 2007 (which
issued as U.S. Pat.
No. 7,897,086 on Mar. 1, 2011), which is a continuation-in-part of U.S. patent
application Ser.
No. 10/913,608, filed on Aug. 5, 2004 (now abandoned). Each of these patent
applications is
herein incorporated by reference in their entirety.
[0002] U.S. patent application Ser. No. 12/893,832, filed on Sep. 29, 2010
also claims
priority as a continuation-in-part of U.S. patent application Ser. No.
12/509,289, filed on Jul. 24,
2009, which is a continuation of U.S. patent application Ser. No. 11/151,164,
filed on Jun. 10,
2005 (which issued as U.S. Pat. No. 7,582,051 on Sep. 1, 2009). U.S. patent
application Ser. No.
12/893,832 also claims priority to U.S. provisional patent application Ser.
No. 61/246,920, filed
Sep. 29, 2009. Each of these patent applications is herein incorporated by
reference in their
entirety.
INCORPORATION BY REFERENCE
[0003] All publications and patent applications mentioned in this
specification are herein
incorporated by reference in their entirety, as if each individual publication
or patent application
was specifically and individually indicated to be incorporated by reference in
its entirety.
TECHNICAL FIELD
[0004] The present invention relates generally to the field of treating
heart diseases and
more specifically, to a device and method for making a laminar ventricular
partitioning device.
BACKGROUND
[0005] Congestive heart failure (CHF), characterized by a progressive
enlargement of the
heart, particularly the left ventricle, is a major cause of death and
disability in the United States
- 1 -
CA 02901532 2015-08-14
WO 2014/152461 PCT/US2014/027364
and elsewhere. As a patient's heart enlarges, it pumps less efficiently and,
in time, the heart
becomes so enlarged that it cannot adequately supply blood to the body. The
fraction of blood
within the left ventricle that is pumped forward at each stroke, commonly
referred to as the
"ejection fraction", is typically about sixty percent for a healthy heart. A
congestive heart failure
patient typically has an ejection fraction of 40% or less, and as a
consequence, is chronically
fatigued, physically disabled, and burdened with pain and discomfort. Further,
as the heart
enlarges, heart valves lose the ability to close adequately. An incompetent
mitral valve allows
regurgitation of blood from the left ventricle back into the left atrium,
further reducing the heart's
ability to pump blood.
[0006] Congestive heart failure can result from a variety of conditions,
including viral
infections, incompetent heart valves, ischemic conditions in the heart wall,
or a combination of
these conditions. Prolonged ischemia and occlusion of coronary arteries can
result in myocardial
tissue in the ventricular wall dying and becoming scar tissue. Once a portion
of myocardial tissue
dies, that portion no longer contributes to the pumping action of the heart.
As the disease
progresses, a local area of compromised myocardium can bulge during the heart
contractions,
further decreasing the heart's ability to pump blood, and further reducing the
ejection fraction.
[0007] In the early stages of congestive heart failure, drug therapy is
presently the most
commonly prescribed treatment. Drug therapy typically treats the symptoms of
the disease and
may slow the progression of the disease, but it does not cure the disease.
Presently, the only
treatment considered curative for congestive heart disease is heart
transplantation, but these
procedures are high risk, invasive, and costly. Further, there is a shortage
of hearts available for
transplant, many patients fail to meet transplant-recipient qualifying
criteria.
[0008] Much effort has been directed toward the development of surgical
and device-
based treatments for congestive heart disease. Surgical procedures have been
developed to
dissect and remove weakened portions of the ventricular wall in order to
reduce heart volume. As
is the case with heart transplant, these procedures are invasive, risky, and
costly, and many
patients do not qualify medically for the procedure. Other efforts to treat
CHF include the use of
an elastic support placed around the heart to prevent further deleterious
remodeling, and
mechanical assist devices and completely mechanical hearts have been
developed. Recently,
improvements have been made in treating patients with CHF by implanting pacing
leads in both
sides of the heart in order to coordinate the contraction of both ventricles
of the heart. While
these various procedures and devices have been found to be successful in
providing some relief
from CHF symptoms and in slowing disease progression, none has been able to
stop the course
of the disease.
- 2 -
CA 02901532 2015-08-14
WO 2014/152461
PCT/US2014/027364
SUMMARY
[0009] The present invention relates to a ventricular partitioning device
and a method of
employing the device in the treatment of a patient with congestive heart
failure (CHF).
Embodiments of the device are adapted to span a chamber of the heart,
typically the left
ventricle, and partition the chamber into a main productive portion and a
secondary non-
productive portion. This partitioning reduces the total volume of the heart
chamber, reduces the
stress applied to the heart and, as a result, improves the blood ejection
fraction thereof.
[0010] Embodiments of the device have a reinforced partitioning component
with a
concave, pressure-receiving surface which, in part, defines the main
productive portion of the
partitioned heart chamber when secured therein. The reinforced partitioning
component
preferably includes a hub and a membrane forming the pressure receiving
surface. The
partitioning component is reinforced by a radially expandable frame component
formed of a
plurality of ribs.
[0011] The ribs of the expandable frame have distal ends secured to the
central hub and
free proximal ends. The distal ends are preferably secured to the central hub
to facilitate radial
self expansion of the free proximal ends of the ribs away from a centerline
axis. The distal ends
of the ribs may be pivotally mounted to the hub and biased outwardly or fixed
to the hub. The
ribs may be formed of material such as superelastic NiTi alloy that permits
compression if the
free proximal ends of the ribs toward a centerline axis into a contracted
configuration, and when
released, allows for their self expansion to an expanded configuration.
[0012] The free proximal ends of the ribs are configured to engage and
preferably
penetrate the tissue lining a heart chamber, typically the left ventricle, to
be partitioned so as to
secure the peripheral edge of the partitioning component to the heart wall and
to fix the
partitioning component within the chamber so as to partition the chamber in a
desired manner.
The tissue-penetrating proximal tips are configured to penetrate the tissue
lining at an angle
approximately perpendicular to a center line axis of the partitioning device.
The tissue
penetrating proximal tips of the ribs may be provided with attachments such as
barbs or hooks
that prevent withdrawal of the tips from the heart wall.
[0013] The ribs in their expanded configuration angle outwardly from the
hub and the
free proximal ends curve outwardly so that the membrane secured to the ribs of
the expanded
frame forms a trumpet-shaped, pressure receiving surface. The partitioning
membrane in the
expanded configuration has radial dimensions from about 10 to about 160 mm,
preferably about
50 to about 100 mm, as measured from the center line axis.
[0014] The partitioning device may be delivered percutaneously or
intraoperatively. One
particularly suitable delivery catheter has an elongated shaft, a releasable
securing device on the
- 3 -
CA 02901532 2015-08-14
WO 2014/152461
PCT/US2014/027364
distal end of the shaft for holding the partitioning device on the distal end,
and an expandable
member such as an inflatable balloon on a distal portion of the shaft proximal
to the distal end to
press the interior of the recess formed by the pressure-receiving surface to
ensure that the tissue
penetrating tips or elements on the periphery of the partitioning device
penetrate sufficiently into
the heart wall to hold the partitioning device in a desired position to
effectively partition the
heart chamber.
[0015] More particularly, the invention relates to an intracorporeal
partitioning
component that includes a frame with a plurality of ribs that is integrated
with one or more sheets
of fabric to form a unified unilaminar, bilaminar, or multilaminar structure,
as well as methods
for making the partitioning component. Embodiments of the invention thus
include an intra
partitioning component that includes a frame having a plurality of ribs with
radially extending
proximal ends and with distal ends secured to a hub; and a bilaminar sheet
secured to the ribs of
the frame by fused thermoplastic material within the bilaminar sheet of
material. In some of
these embodiments, the bilaminar sheet of material comprises ePTFE. In some
embodiments, the
bilaminar sheet includes a porous material; in other embodiments the bilaminar
sheet includes a
non-porous material.
[0016] Embodiments of the invention further include an intracorporeal
partitioning
component that includes a frame having a plurality of ribs with radially
extending proximal ends
and with distal ends secured to a hub; and a single sheet secured to the ribs
of the frame by fused
thermoplastic material on one side of the sheet of material to form a
unilaminar structure.
[0017] Embodiments of the invention also include an intracorporeal product
that includes
a first component configured for intracorporeal deployment, the component
encased in
thermoplastic material; and at least two sheets of ePTFE material secured to
the first component
by fused thermoplastic material therebetween to form at least a bilaminar
sheet of ePTFE
material.
[0018] Embodiments of the invention include a method of securing a
polymeric sheet
material to rib components of a frame structure, including disposing a tube
comprising
thermoplastic material over each of one or more rib components of the frame to
form a
thermoplastic-material-encased rib; forming an assembly by applying the
thermoplastic-encased
rib above a first sheet and a second sheet above the thermoplastic-encased
rib; and heating the
assembly to fuse the first and second sheets to the thermoplastic material to
form a bilaminar
sheet, the fusion occurring by the melting and reforming of the thermoplastic
material between
the sheets, the rib remaining within the melted and reformed thermoplastic
material. These
embodiments include methods wherein the first sheet and second sheet of
material include
ePTFE. In other embodiments, the first sheet and second sheet of material
include a porous
- 4 -
CA 02901532 2015-08-14
WO 2014/152461 PCT/US2014/027364
material. And in still other embodiments, the first sheet and second sheets of
material may
include a porous material, and the other of the first sheet and second sheets
may include a
nonporous material.
[0019] In some of these method embodiments, the heating includes exposure
to a
temperature of about 500° F., and in some of these embodiments the
heating occurs over a
period of about 120 seconds. In some of these embodiments, the method further
includes
applying pressure to the assembly to fuse the thermoplastic material and the
ePTFE sheets to the
rib component, such applied pressure being between about 60 psi and about 90
psi. And in some
of these embodiments wherein the pressure is applied for a period of about 120
seconds.
[0020] Some embodiments of the invention include a method of making an
intracorporeal product, including: (a) providing two ePTFE sheets; (b)
providing a rib
component of a frame structure; (c) deploying a thermoplastic-material
containing element over
at least part of the rib component; (d) applying the ePTFE sheets to at least
a portion of the rib
component covered by the thermoplastic element, the rib component disposed
between the
sheets, to form an assembly; and (e) heating the assembly to fuse the
thermoplastic material and
the ePTFE sheets to the rib component, the ePTFE sheets thereby forming a
bilaminar ePTFE
sheet structure secured to the rib component. In various of these embodiments,
the heating step
includes exposure to a temperature ranging between about 260° F. and
about 530°
F. More particularly, the heating may include exposure to a temperature
ranging between about
375° F. and about 520° F. Still more particularly, the heating
may include exposure
to a temperature ranging between about 490° F. and about 510° F.
And in some
embodiments, the heating may include exposure to a temperature of about
500° F.
[0021] Some embodiments of the method of making an intracorporeal product
further
include applying pressure to the assembly to fuse the thermoplastic material
and the ePTFE
sheets to the rib component. In some of these embodiments, the pressure
applied is between
about 10 psi and about 150 psi. In some particular embodiments, the pressure
applied is between
about 35 psi and about 120 psi. And in some particular embodiments, the
pressure applied is
between about 60 psi and about 90 psi.
[0022] Some embodiments of the method of making an intracorporeal product
include
applying heat and pressure to the assembly for a predetermined period of time
that ranges
between about 30 seconds and about 360 seconds. In some embodiments, the
period of time
ranges between about 75 seconds and about 240 seconds. And in some particular
embodiments,
the period of time is about 120 seconds.
[0023] Some embodiments of the method of making an intracorporeal product
the fusion
of polyethylene material and polytetra-fluoro-ethylene (PTFE) material occurs
by the
- 5 -
CA 02901532 2015-08-14
WO 2014/152461 PCT/US2014/027364
polyethylene melting and intercalating into the ePTFE fabric, cooling, and
reforming to create
interlocking zones of material continuity between polyethylene and
polytetrafluoroethylene
(PTFE).
[0024] Some embodiments of the method of making an intracorporeal product
include (a)
providing one ePTFE sheet; (b) providing a rib component of a frame structure;
(c) deploying a
thermoplastic-material containing element over at least part of the rib
component; (d) applying
the ePTFE sheet to at least a portion of the rib component covered by the
thermoplastic element,
the rib component disposed adjacent to the sheet, to form an assembly; and (e)
heating the
assembly to fuse the thermoplastic material and the ePTFE sheets to the rib
component, the
ePTFE sheet thereby forming a unilaminar ePTFE sheet structure secured to the
rib component.
[0025] Also described herein is a method of securing a polymeric sheet to
rib
components of a frame structure, wherein the rib components are jointed at a
hub to form an
expandable and collapsible implant. In general, the method may include the
steps of disposing a
tube comprising thermoplastic material over each of one or more rib components
of the frame;
forming an assembly by applying the thermoplastic-encased rib adjacent to at
least one
polymeric sheet of material; and heating the assembly to fuse the sheet to the
thermoplastic
material to form a fused sheet, the fusion occurring by the heating and
reforming of the
thermoplastic material to the sheet, the rib remaining within the reformed
thermoplastic material,
wherein the implant is adapted to span a left ventricle. In some embodiments,
the method further
includes the step applying pressure to the assembly to form a fused sheet.
[0026] In some embodiments, the disposing step may further include forming
a
thermoplastic-material-encased rib. In some embodiments, the disposing step
may further
include forming thermoplastic-material-encased ribs having proximal portions
that are not
encased in the thermoplastic material. In some embodiments, the disposing step
may further
include forming thermoplastic-material-encased ribs having tissue-penetrating
proximal ends
that are not encased in the thermoplastic material. In some embodiments, the
disposing step may
further include forming thermoplastic-material-encased ribs, wherein the
thermoplastic material
is disposed over a first portion of a first rib and a second portion of a
second rib, wherein the first
and second ribs are adjacent to one another and the first portion is at a
different position along
the length of the rib than the second portion.
[0027] In some embodiments, at least one polymeric sheet of material
comprises ePTFE.
In some embodiments, the fused sheet is a unilaminar sheet.
[0028] Also described herein are methods of securing a polymeric sheet to
rib
components of a frame structure, wherein the rib components are jointed at a
hub to form an
expandable and collapsible implant, wherein the implant is adapted to span a
left ventricle. In
- 6 -
CA 02901532 2015-08-14
WO 2014/152461
PCT/US2014/027364
general, the method includes the steps of providing an assembly, the assembly
comprising a
frame structure disposed between a first and second polymeric sheet; and
heating the assembly
under pressure to fuse the first polymeric sheet to the second polymeric sheet
around the frame
structure to form a fused sheet. In some embodiments, the first and second
polymeric sheets
comprise ePTFE.
[0029] Also described herein are methods for securing a polymeric sheet to
rib
components of a frame structure, wherein the rib components are jointed at a
hub to form an
expandable and collapsible implant. In general the method may include the
steps of decreasing a
diameter of the frame structure; placing the frame structure into an assembly
fixture, wherein the
assembly fixture is configured to hold the frame structure in a loaded
configuration with a
decreased diameter; placing a polymeric sheet into the assembly fixture; and
heating the
assembly under pressure to fuse the sheet to the frame structure.
[0030] In some embodiments, the method further includes the step of
disposing a tube
comprising thermoplastic material over each of one or more rib components of
the frame. In
some embodiments, the method further includes the step of forming an assembly
by applying the
thermoplastic-encased rib adjacent to at least one polymeric sheet of
material. In some
embodiments, the fusion occurs by the heating and reforming of the
thermoplastic material to the
sheet.
[0031] Also described herein is an assembly fixture for securing a
polymeric sheet to rib
components of a frame structure, wherein the rib components are jointed at a
hub to form an
expandable and collapsible implant. In general, the fixture may include a
first platen having male
shaping portion and a rim portion positioned around the periphery of the first
platen; and a
second platen having female shaping portion and a rim portion positioned
around the periphery
of the second platen; wherein the male and female shaping portions are
configured to hold the rib
components of the frame structure in a loaded configuration with a decreased
diameter.
[0032] In some embodiments, the male and female shaping portions have
complimentary
curved shapes configured to hold the frame in a curved, loaded configuration
with a decreased
diameter.
[0033] In some embodiments, the two rim portions form complementary planar
surfaces
which serve to hold edges of the polymeric sheet. In some embodiments, the
male and female
shaping portions are further configured to press the polymeric sheet. In some
embodiments, the
polymeric sheet comprises ePTFE.
[0034] In some embodiments, an implant for partitioning a ventricle is
provided. The
implant can include an expandable frame comprising a central hub and a
plurality of struts
- 7 -
CA 02901532 2015-08-14
WO 2014/152461
PCT/US2014/027364
extending from the hub, the struts having a flared root portion proximate the
central hub; and a
membrane attached to the struts of the expandable frame.
[0035] In some embodiments, each strut terminates in an anchor and
includes a stop
proximate the anchor, the stop locking the membrane in place while also being
adapted to reduce
or prevent over-penetration of the struts into the ventricle wall.
[0036] In some embodiments, the stops and anchors are staggered with
respect to
adjacent stops and anchors.
[0037] In some embodiments, the plurality of struts have staggered
lengths.
[0038] In some embodiments, each strut has a cross-section with a width
and thickness,
wherein the width is greater than the thickness.
[0039] In some embodiments, the plurality of struts are biased to bend
directly outwards
without any twist.
[0040] In some embodiments, the expandable frame has a free diameter
without the
membrane that is oversized relative to an attached diameter where the membrane
is attached.
[0041] In some embodiments, an implant for partitioning a ventricle is
provided. The
implant can include an expandable frame comprising a central hub and a
plurality of struts
extending from the hub, wherein each strut terminates in an anchor and
includes a stop
proximate the anchor; and a membrane attached to the struts of the expandable
frame, wherein
the stop locks the membrane in place while also being adapted to reduce or
prevent over-
penetration of the struts into the ventricle wall.
[0042] In some embodiments, an implant for partitioning a ventricle is
provided. The
implant can include an expandable frame comprising a central hub and a
plurality of struts
extending from the hub, wherein each strut has a cross-section with a width
and thickness,
wherein the width is greater than the thickness; and a membrane attached to
the struts of the
expandable frame.
BRIEF DESCRIPTION OF THE DRAWINGS
[0043] FIG. 1 is an elevational view of a partitioning device embodying
features of the
invention in an expanded configuration.
[0044] FIG. 2 is a plan view of the partitioning device shown in FIG. 1.
[0045] FIG. 3 is a partial longitudinal cross-sectional view of the hub of
the partitioning
device shown in FIG. 1.
[0046] FIG. 4 is a transverse cross sectional view of the hub shown in
FIG. 3 taken along
the lines 4-4.
- 8 -
CA 02901532 2015-08-14
WO 2014/152461 PCT/US2014/027364
[0047] FIG. 5 is a schematic elevational view of a delivery system for the
partitioning
device shown in FIGS. 1 and 2.
[0048] FIG. 6 is a transverse cross-sectional view of the delivery system
shown in FIG. 5
taken along the lines 6-6.
[0049] FIG. 7 is an elevational view, partially in section, of the hub
shown in FIG. 3
secured to the helical coil of the delivery system shown in FIG. 5.
[0050] FIGS. 8A-8E are schematic views of a patient's left ventricular
chamber
illustrating the deployment of the partitioning device shown in FIGS. 1 and 2
with the delivery
system shown in FIG. 5 to partition the heart chamber into a primary
productive portion and a
secondary, non-productive portion.
[0051] FIG. 9 is a partial schematic view of the expandable frame of the
partitioning
device shown in FIGS. 1 and 2 in an unrestricted configuration.
[0052] FIG. 10 is a top view of the expandable frame shown in FIG. 9.
[0053] FIGS. 11 and 12 are schematic illustrations of a method of forming
the
partitioning device shown in FIGS. 1 and 2 from the expandable frame shown in
FIGS. 9 and 10.
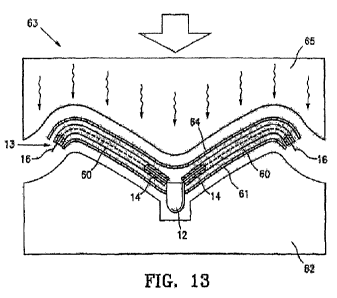
[0054] FIG. 13 is a schematic view of the assembled components shown in
FIG. 12, as
they are situated in a laminating press.
[0055] FIGS. 14A-14D include views of a bilaminar assembly for the making
of an
intracorporeal partitioning device, as well as views of the assembled device.
FIG. 14A shows an
exploded and partially cutaway view of the components of the device assembled
for lamination;
FIG. 14B provides of cutaway view of the device within a press, the press in a
closed position;
FIG. 14C shows a perspective view of an exemplary device; FIG. 14D provides a
frontal view of
the device after assembly.
[0056] FIGS. 15A-15D include views of a unilaminar assembly for the making
of an
intracorporeal partitioning device, as well as views of the assembled device.
FIG. 15A shows an
exploded and partially cutaway view of the components of the device assembled
for lamination;
FIG. 15B provides of cutaway view of the device within the press in a closed
position; FIG. 15C
shows a perspective view of an exemplary device; and FIG. 15D provides a
frontal view of the
device after assembly.
[0057] FIG. 16 provides cross-sectional views of an assembly from which a
bilaminar
partitioning device is formed. FIG. 16A shows a polyethylene-encased rib
sandwiched between
two sheets of ePTFE material as assembled prior to processing in a mold or
press. In this
embodiment, the rib is substantially cylindrical in form, or substantially
circular in cross section.
FIG. 16B shows the same materials after the application of heat and pressure,
to form a
- 9 -
CA 02901532 2015-08-14
WO 2014/152461 PCT/US2014/027364
bilaminar sheet, the sheets held together by melted and reformed polyethylene
material to which
they are both fused, a rib disposed within and adherent to the polyethylene.
[0058] FIG. 17 provides cross-sectional views of an assembly from which a
bilaminar
partitioning device is formed. FIG. 17A shows a polyethylene-encased rib
sandwiched between
two sheets of ePTFE material as assembled prior to processing in a mold or
press. In this
embodiment, the rib is substantially rectangular, but curved in cross section.
FIG. 17B shows the
same materials after the application of heat and pressure, to form a bilaminar
sheet, the sheets
held together by melted and reformed polyethylene material to which they are
both fused, a rib
disposed within and adherent to the polyethylene.
[0059] FIG. 18 provides cross-sectional views of an assembly from which a
unilaminar
partitioning device is formed. FIG. 18A shows a polyethylene-encased rib
overlaying a sheet of
ePTFE material as assembled prior to processing in a mold or press. In this
embodiment, the rib
is substantially circular in cross section. FIG. 18B shows the same materials
after the application
of heat and pressure, to form a unilaminar sheet fused to a rib by the melted
and reformed
polyethylene, the polyethylene interposed between the rib and the ePTFE sheet,
adhering to both.
[0060] FIG. 19 provides cross-sectional views of an assembly from which a
unilaminar
partitioning device is formed. FIG. 19A shows a polyethylene-encased rib
overlaying a sheet of
ePTFE material as assembled prior to processing in a mold or press. In this
embodiment, the rib
is substantially rectangular but curved in cross section. FIG. 19B shows the
same materials after
the application of heat and pressure, to form a unilaminar sheet fused to a
rib by the melted and
reformed polyethylene, the polyethylene interposed between the rib and the
ePTFE sheet,
adhering to both.
[0061] FIGS. 20A and 20B schematically depict the formation of a unilaminar
integrated
structure from the polyethylene-encased rib and ePTFE material by the melting
and solidified
reformed polythethylene to create interlocking continuities between the ePTFE
and the
polyethylene. This structure also depicts a portion of a larger bilaminar
structure, such as a
portion immediately overlaying a rib.
[0062] FIGS. 21A and 21B schematically depict the formation of a bilaminar
integrated
structure from the polyethylene-encased rib and ePTFE material by the melting
and solidified
reformed polythethylene to create interlocking continuities between the ePTFE
and the
polyethylene.
[0063] FIGS. 22-23B include a view of an assembly for the making of an
intracorporeal
partitioning device, as well as views of the assembled device. FIG. 22 shows
an exploded and
partially cutaway view of the components of the assembly for lamination; FIGS.
23A and 23B
illustrate the assembled device.
-10-
CA 02901532 2015-08-14
WO 2014/152461
PCT/US2014/027364
[0064] FIGS. 24A-24C illustrate a cross-section of a loaded frame in its
free state (FIG.
24A), after lamination (FIG. 24B), and implanted (FIG. 24C).
[0065] FIGS. 25A-25C illustrate a first, second, and third embodiment
showing the
frame of the device described herein having sleeves. As shown, the device may
include full
sleeves disposed along the full length of the struts (FIG. 25A), partial
sleeves staggered along the
length of the struts (FIG. 25B), or shortened sleeves (FIG. 25C).
[0066] FIGS. 26A-26E illustrate an embodiment of a frame with various
improvements.
DETAILED DESCRIPTION
[0067] FIGS. 1-4 illustrate a partitioning component 10 which embodies
features of the
invention and which includes a partitioning membrane 11, a hub 12, preferably
centrally located
on the partitioning device, and a radially expandable reinforcing frame 13
formed of a plurality
of ribs 14. Embodiments of the partitioning component 10 may be alternatively
referred to as an
intracorporeal partitioning component or an intracorporeal product, referring
to its position
within a ventricle of the heart, and to its function in partitioning the
ventricle. Preferably, the
partitioning membrane 11 is secured to the proximal or pressure side of the
frame 13 as shown in
FIG. 1. The ribs of the intracorporeal device 14 have distal ends 15 which are
secured to the hub
12 and free proximal ends 16 which are configured to curve or flare away from
a center line axis
17. Radial expansion of the free proximal ends 16 unfurls the membrane 11
secured to the frame
13 so that the membrane presents a relatively smooth, pressure receiving
surface 18 which
defines in part the productive portion of the patient's partitioned heart
chamber.
[0068] As shown in more detail in FIGS. 3 and 4, the distal ends 15 of the
ribs 14 are
secured within the hub 12 and a transversely disposed connector bar 20 is
secured within the hub
which is configured to secure the hub 12 and thus the partitioning component
10 to a delivery
system such as shown in FIGS. 5 and 6. The curved free proximal ends 16 of
ribs 14 are
provided with sharp tip elements 21 which are configured to hold the frame 13
and the
membrane 11 secured thereto in a deployed position within the patient's heart
chamber.
Preferably, the sharp tip elements 21 of the frame 13 penetrate into tissue of
the patient's heart
wall in order to secure the partitioning component 10 within the heart chamber
so as to partition
the ventricular chamber into a productive portion and a non-productive
portion.
[0069] The connector bar 20 of the hub 12, as will be described later,
allows the
partitioning device 10 to be secured to a delivery system and to be released
from the delivery
system within the patient's heart chamber. The distal ends 15 of the
reinforcing ribs 14 are
secured within the hub 12 in a suitable manner or they may be secured to the
surface defining the
-11-
CA 02901532 2015-08-14
WO 2014/152461 PCT/US2014/027364
inner lumen or they may be disposed within channels or bores in the wall of
the hub 12. The ribs
14 are pre-shaped so that when not constrained other than by the membrane 11
secured thereto
(as shown in FIGS. 1 and 2), the free proximal ends 16 thereof expand to a
desired angular
displacement away from a center line axis 17 which is about 20 degrees to
about 90 degrees,
preferably about 50 degrees to about 80 degrees.
[0070] FIGS. 5-7 illustrate a suitable delivery system 30 delivering the
partitioning
component 10 shown in FIGS. 1 and 2 into a patient's heart chamber and
deploying the
partitioning component 10 to partition the heart chamber as shown in FIGS. 8A-
8E. The delivery
system 30 includes a guide catheter 31 and a delivery catheter 32.
[0071] The guide catheter has an inner lumen 33 extending between the
proximal end 34
and distal end 35. A hemostatic valve (not shown) may be provided at the
proximal end 34 of the
guide catheter 31. A flush port 36 on the proximal end 34 of guide catheter 31
is in fluid
communication with the inner lumen 33.
[0072] The delivery catheter 32 has an outer shaft 40 with an inner lumen
41 and a
proximal injection port 42, an inner shaft 43 disposed within the inner lumen
41 with a first
lumen 44 and a second lumen 45. Balloon inflation port 46 is in fluid
communication with the
first lumen 44 and flush port 47 is in fluid communication with the second
lumen 45. Torque
shaft 48 is rotatably disposed within the second lumen 44 of the inner shaft
43 and has an
injection port 49 provided at its proximal end 50 in fluid communication with
the inner lumen 51
of the torque shaft. The torque shaft 48 is preferably formed at least in part
of a hypotube formed
of suitable material such as superelastic Nitinol or stainless steel. A torque
knob 52 is secured to
the proximal end 50 of torque shaft 48 distal to the injection port 49. A
helical coil screw 53 is
secured to the distal end of the torque shaft 48 and rotation of the torque
knob 52 on the proximal
end 50 of the torque shaft 48 rotates the screw 53 on the distal end of torque
shaft 48 to facilitate
deployment of a partitioning device 10. An inflatable balloon 55 is sealingly
secured to the distal
end of the inner shaft 43 and has an interior 56 in fluid communication with
the first lumen 44.
Inflation fluid may be delivered to the interior 56 through port 44a in the
portion of the inner
shaft 43 extending through the balloon 55. Inflation of the balloon 55 by
inflation fluid through
port 46 facilitates securing the partitioning component 10.
[0073] To deliver the partitioning component 10, it is secured to the
distal end of the
delivery catheter 32 by means of the helical coil screw 53. The partitioning
component 10 is
collapsed to a first, delivery configuration which has small enough transverse
dimensions to be
slidably advanced through the inner lumen 33 of the guide catheter 31.
Preferably, the guide
catheter 31 has been previously percutaneously introduced and advanced through
the patient's
vasculature, such as the femoral artery, in a conventional manner to the
desired heart chamber.
-12-
CA 02901532 2015-08-14
WO 2014/152461 PCT/US2014/027364
The delivery catheter 32 with the partitioning component 10 attached is
advanced through the
inner lumen 33 of the guide catheter 31 until the partitioning component 10 is
ready for
deployment from the distal end of the guide catheter 31 into the patient's
heart chamber 58 to be
partitioned.
[0074] The partitioning component 10 mounted on the screw 53 is urged
partially out of
the inner lumen 33 of the guide catheter 31 until the hub 12 engages the heart
wall as shown in
FIG. 8B with the free proximal ends 16 of the ribs 14 in a contracted
configuration within the
guide catheter. The guiding catheter 31 is withdrawn while the delivery
catheter 32 is held in
place until the proximal ends 16 of the ribs 14 exit the distal end of the
guiding catheter. The free
proximal ends 16 of ribs 14 expand outwardly to press the sharp proximal tips
21 of the ribs 14
against and preferably into the tissue lining the heart chamber, as shown in
FIG. 8C.
[0075] With the partitioning component deployed within the heart chamber
and
preferably partially secured therein, inflation fluid is introduced through
the inflation port 46 into
first lumen 44 of inner shaft 43 of the delivery catheter 32 where it is
directed through port 44a
into the balloon interior 56 to inflate the balloon. The inflated balloon
presses against the
pressure receiving surface 18 of the partitioning component 10 to ensure that
the sharp proximal
tips 21 are pressed well into the tissue lining the heart chamber.
[0076] With the partitioning device 10 properly positioned within the
heart chamber, the
knob 52 on the torque shaft 48 is rotated counter-clockwise to disengage the
helical coil screw
53 of the delivery catheter 32 from the hub 12. The counter-clockwise rotation
of the torque shaft
48 rotates the helical coil screw 53 which rides on the connector bar 20
secured within the hub
12. Once the helical coil screw 53 disengages the connector bar 20, the
delivery system 30,
including the guide catheter 31 and the delivery catheter 32, may then be
removed from the
patient.
[0077] The proximal end of the guide catheter 31 is provided with a flush
port 36 to
inject therapeutic or diagnostic fluids through the inner lumen 33. Similarly,
the proximal end of
the delivery catheter 32 is provided with a flush port 42 in communication
with inner lumen 41
for essentially the same purpose. An inflation port 46 is provided on the
proximal portion of the
delivery catheter for delivery of inflation fluid through the first inner
lumen 44 to the interior 56
of the balloon 55. Flush port 47 is provided in fluid communication with the
second inner lumen
45 of the inner shaft 43. An injection port 49 is provided on the proximal end
of the torque shaft
48 in fluid communication with the inner lumen 51 of the torque shaft for
delivery of a variety of
fluids.
[0078] The partitioning component 10 partitions the patient's heart
chamber 57 into a
main productive or operational portion 58 and a secondary, essentially non-
productive portion
- 13 -
CA 02901532 2015-08-14
WO 2014/152461
PCT/US2014/027364
59. The operational portion 58 is much smaller than the original ventricular
chamber 57 and
provides for an improved ejection fraction. The partitioning increases the
ejection fraction and
provides an improvement in blood flow. Over time, the non-productive portion
59 fills first with
thrombus and subsequently with cellular growth. Bio-resorbable fillers such as
polylactic acid,
polyglycolic acid, polycaprolactone, and copolymers and blends may be employed
to initially fill
the non-productive portion 59. Fillers may be suitably supplied in a suitable
solvent such as
DMSO. Other materials which accelerate tissue growth or thrombus may be
deployed in the non-
productive portion 59. ,
[0079] FIGS. 9 and 10 illustrate the reinforcing frame 13 in an unstressed
configuration
and include the ribs 14 and the hub 12. The ribs 14 have a length L of about 1
to about 8 cm,
preferably, about 1.5 to about 4 cm for most left ventricle deployments. The
proximal ends 16
have a flared construction. To assist in properly locating the device during
advancement and
placement thereof into a patient's heart chamber, parts, e.g. the distal
extremity, of one or more
of the ribs and/or the hub may be provided with markers at desirable locations
that provide
enhanced visualization by eye, by ultrasound, by X-ray, or other imaging or
visualization means.
Radiopaque markers may be made with, for example, stainless steel, platinum,
gold, iridium,
tantalum, tungsten, silver, rhodium, nickel, bismuth, other radiopaque metals,
and alloys and
oxides of these metals.
[0080] Embodiments of the partitioning device 10, both unilaminar and
bilaminar
embodiments, are conveniently formed by placing a thermoplastic tube 60, e.g.
polyethylene or
high density polyethylene (HDPE), over the ribs 14 of the frame 13 as shown in
FIG. 11 until the
proximal ends 16 of the ribs 14 extend out the ends of the thermoplastic tubes
as shown in FIG.
12, to form thermoplastic-encased ribs. Further steps in the process of
forming a unilaminar or
bilaminar partitioning device make use of a press or lamination mold 63 that
includes a female
platen 62 and a male platen 65, one or both of which can be heated and cooled
according to
process specifics. A first expanded polytetrafluoroethylene (ePTFE) sheet 61
of appropriate size
is placed in the female platen 62 of the mold or press 63. The frame 13, with
tubes 60 slidably
disposed or deployed over the ribs 14, is placed in platen 62 on top of the
ePTFE sheet 61. In
some alternative embodiments, the ePTFE sheet may be placed over the ribs. The
center portion
of the sheet 61 may be provided with an opening through which the hub 12
extends. In the case
of forming a bilaminar embodiment, a second ePTFE sheet 64 is placed on top of
the ribs 14 of
frame 13 as shown in FIG. 13. The melting point of the thermoplastic material
is lower than that
of the ePTFE, thus the application of heat and pressure, as detailed below, is
sufficient to melt
the thermoplastic material but does not cause melting of the ePTFE.
-14-
CA 02901532 2015-08-14
WO 2014/152461 PCT/US2014/027364
[0081] Embodiments of methods to form a partitioning device that joins
ePTFE sheet
material, polyethylene material, and ribs into an integral structure include
the application of heat
and pressure. Heat and pressure may be applied through a mold or press 63 for
a period of
predetermined period of time, such as from about 30 seconds to about 360
seconds, or more
particularly from about 75 seconds to about 240 seconds, or still more
particularly, for about 120
seconds. Either the male platen 65 or the female platen 62, or both male and
female platens may
be heated so as to attain an operating temperature of between about
260° F. and
530° F., particularly to a temperature between about 375° F. and
520° F.,
and more particularly to temperature between about 490° F. and about
510° F., and
still more particularly to a temperature of about 500° F. In some
embodiments, the
assembly may be pressed (i.e., pressured or pressurized), the applied pressure
being in the range
of about 10 psi to about 150 psi. In some particular embodiments, the pressure
is between about
35 psi and about 120 psi, and in more particular embodiments, between about 60
psi and about
90 psi. In some embodiments, a single sheet of ePTFE is utilized to make a
unilaminar device,
the single sheet corresponding to the first sheet 61 of FIG. 13.
[0082] PTFE fabric is a woven material that varies with regard to the
thickness of fibers
and in the intemodal distance between fibers. The presence of the space or
volume between
fibers provides the material with a foraminous quality which is advantageous
for fusion or
adhesion processes. Various forms of ePTFE have average internodal distances
that vary from
about one micron up to about 1,000 microns. Typical embodiments of ePTFE
fabric appropriate
for the manufacture of the herein described partitioning device may have
intemodal distances of
between about 5 microns to about 200 microns, more particularly from about 10
microns to
about 100 microns, and still more particularly from about 20 microns to about
50 microns.
Aspects of the lamination process are described further below, and illustrated
in FIGS. 14-21.
Sheets may be formed of either porous or non-porous ePTFE, as well as other
suitable
biocompatible materials, as described further below.
[0083] As described further, below, the ePTFE fabric is typically
stretched during the
lamination process, under the conditions of heat and pressure that are applied
by the press. Such
stretching may not be uniform across the fabric surface, the maximal linear
stretch in portions of
the fabric may be of a magnitude of 2-fold to 4-fold. The stretching of fabric
serves, in general
terms, to reduce the thickness and overall collapsed profile of the device.
[0084] FIGS. 14A-14D include further views of a bilaminar assembly for the
making of
an intracorporeal partitioning device (as also depicted variously in preceding
FIGS. 11-13) and
views of the assembled device. FIG. 14A shows a perspective view of an
exemplary device; FIG.
14B shows an exploded and partially cutaway view of the components of the
device assembled
- 15 -
CA 02901532 2015-08-14
WO 2014/152461 PCT/US2014/027364
for lamination; FIG. 14C provides of cutaway view of the device within the
press in a closed
position; and FIG. 14D provides a frontal view of the device after assembly.
[0085] In FIG. 14A, the upper or male platen 65 of a press 63 and the
lower or female
platen 62 are seen above and below, respectively, an awaiting assembly that
includes, from top
to bottom, a sheet of ePTFE 64, an assembly of polyethylene 60 covered ribs 14
that are formed
into a cone-shaped configuration, and a bottom sheet of ePTFE 61. Around the
periphery of the
upper platen 65 is a rim portion 66A, and around the periphery of the lower
platen 62 is a rim
portion 66B. These two rim portions (66A and 66B) form complementary planar
surfaces which
serve to hold edges of the sheets of ePTFE fabric as the central portion is
being subjected to
being pressed by the complementary surfaces of the central portion or shaping
portion 67A of the
upper platen 65, and the central portion 67B of the lower platen 62. The
closure of the two
halves of the platen is depicted in the cutaway view of FIG. 14B. A
perspective view of the
device as it would emerge post-formation is seen in FIG. 14C; where the
polyethylene encased
ribs 14 may be seen. A frontal plane-flattening view of the device upon
removal from the press is
shown in FIG. 14D, again showing the polyethylene encased ribs 60A, the
polyethylene now
reformed from its native circular configuration. Details of this structure in
a before-pressing form
60 and after-pressing pressing form 60A are shown in FIGS. 16, 17, and 21.
[0086] FIGS. 15A-15D include various views of a unilaminar assembly for
the making of
an intracorporeal partitioning device, as well as views of the assembled
device. FIG. 15A shows
an exploded and partially cutaway view of the components of the device
assembled for
lamination; FIG. 15B provides of cutaway view of the device within a press,
the press in a closed
position; FIG. 15C shows a perspective view of an exemplary device; FIG. 15D
provides a
frontal view of the device after assembly.
[0087] In FIG. 15A, the upper or male platen 65 of a press 63 and the
lower or female
platen 62 are seen above and below, respectively, an awaiting assembly that
includes, from top
to bottom, an assembly of polyethylene 60 covered ribs 14 that are formed into
a cone-shaped
configuration, and a bottom sheet of ePTFE 61 that will ultimately form a
unilaminar device.
Around the periphery of the upper platen 65 is a rim portion 66A, and around
the periphery of
the lower platen 62 is a rim portion 66B. These two rim portions (66A and 66B)
form
complementary planar surfaces which serve to hold edges of the sheets of ePTFE
fabric as the
central portion is being subjected to being pressed by the complementary
surfaces of the central
portion or shaping portion 67A of the upper platen 65, and the central portion
67B of the lower
platen 62. The closure of the two halves of the platen is depicted in the
cutaway view of FIG.
15B. A perspective view of the device as it would emerge post-formation is
seen in FIG. 15C;
where the polyethylene encased ribs 14 may be seen. A frontal plane-flattening
view of the
- 16 -
CA 02901532 2015-08-14
WO 2014/152461 PCT/US2014/027364
device upon removal from the press is shown in FIG. 15D, again showing the
polyethylene
encased ribs 60A, the polyethylene now reformed from its native circular
configuration. Details
of this structure in a before-pressing form 60 and after-pressing pressing
form 60A are shown in
FIGS. 16, 17, and 21.
[0088] An aspect of ePTFE material that relates to the internodal
distances within the
fabric is that such distance is preferably sufficient to accommodate the flow
of melted
polyethylene from the thermoplastic tubes 60 during the heating and pressuring
period of
embodiments of the forming process. As melted polyethylene intercalates into
the ePTFE fabric
and then solidifies in a reformed configuration on cooling, intermingled and
interlocking zones
of material continuity having been created between polyethylene and polytetra-
fluoroethylene
(PTFE). These fusion zones of interlocking zones of material continuity
provide a firm bonding
matrix that (1) secures the still-polyethylene-encased rib 14 to the adjacent
one ePTFE sheet (in a
unilaminar embodiment) or two ePTFE sheets (in a bilaminar embodiment, and
thereby within
the bilaminar structure formed by the two sheets) and (2), in a bilaminar
embodiment, the
adheres the two ePTFE sheets together to form a bilaminar structure.
[0089] FIGS. 16 and 17 provide views of two embodiments of a metallic rib
encased in a
polyethylene tube 60, prior to (A) and subsequent to (B) being fused within
two ePTFE sheets
(61 and 64), to form a bilaminar dPTFE sheet, the two sheets adhering to each
other in the locale
of the zone of fusion between the polyethylene and the ePTFE materials. FIGS.
16A and 16B
depict a rib that is substantially circular in cross section. Similar
embodiments (not shown)
include those with cross sectional profiles that are somewhat flattened or
elliptical. The cross
sectional profile of ribs may vary, and various embodiments may provide
advantages with
regard, for example, to stiffness or to practical aspects of the assembly of
the device. Other
embodiments of ribs are more rectangular in cross section. FIGS. 17A and 17B
depict a rib that
is generally rectangular in cross section, though curved or arched as a whole
in cross section in
this particular embodiment, with a convex upper-facing surface and a concave
lower-facing
surface.
[0090] FIG. 16A provides a cross sectional view of a metallic rib 14,
substantially
circular in cross section, encased in a polyethylene tube 60, the tube
disposed between the two
ePTFE sheets 61 and 64 prior to application of pressure and heat. FIG. 16B
provides a view of
the same materials after heat and pressure to form a bilaminar device. The
thermoplastic material
that originally comprised tube 60 disposed over the rib 14, has reformed as
polyethylene material
60A, which is fused into the porous matrix of the ePTFE sheets 61 and 64. (The
polyethylene
material represented by 60 in its native form and by 60A in its post-melt and
reformed form is
substantially conserved in terms of total volume, but it is redistributed as
schematically depicted
- 17 -
CA 02901532 2015-08-14
WO 2014/152461 PCT/US2014/027364
in FIGS. 16A-16B, as well as in FIGS. 17-21. In addition to the schematically
depicted
polyethylene 60 and 60A, also depicted schematically and not necessarily to
scale are the relative
sizes of the ribs 14 and the PTFE fabric 64.) The first and second ePTFE
sheets thereby form a
bilaminar ePTFE sheet, and at sites where the bilaminar sheet surrounds the
thermoplastic
material; the bilaminar ePTFE and the thermoplastic material solidify, thereby
securing the
sheets 61 and 64 to the ribs 14 and preventing their delamination during use
of the partitioning
device. The encircled detail within FIG. 16A that is labeled 21A is a
reference to FIG. 21A
which provides a more detailed of the ePTFE and polyethelene materials prior
to their fusion
during the lamination process, as described below. The encircled detail within
FIG. 16B that is
labeled 21B is a reference to FIG. 21B which provides a more detailed of the
ePTFE and
polyethelene materials after their fusion during the lamination process, as
described below.
[0091] FIGS. 17A and 17B provide a representation of an embodiment of the
device
wherein the rib 14 is substantially rectangular in cross section, but wherein
the process of
forming a device is otherwise substantially parallel to the sequence shown in
FIGS. 16A and
16B. FIG. 17A provides a cross sectional view of a metallic rib 14,
substantially rectangular in
cross section, encased in a polyethylene tube 60, the tube disposed between
the two ePTFE
sheets 61 and 64 prior to application of pressure and heat to form a bilaminar
device. FIG. 17B
provides a view of the same materials after heat and pressure. The
thermoplastic material that
originally comprised tube 60 disposed over the rib 14 has reformed as
polyethylene material
60A, which is fused into the porous matrix of the ePTFE sheets 61 and 64. The
first and second
ePTFE sheets thereby form a bilaminar ePTFE sheet, and at sites where the
bilaminar sheet
surrounds the thermoplastic material; the bilaminar ePTFE and the
thermoplastic material
solidify, thereby securing the sheets 61 and 64 to the ribs 14 and preventing
their delamination
during use of the partitioning device. Sheets may be formed of either porous
or non-porous
ePTFE, as well as other suitable biocompatible materials, as described further
below.
[0092] In embodiments where only a single sheet of ePTFE is used, a
unilaminar
structure is formed, with the ribs 14 adhering to the ePTFE sheet 61 by way of
the melted and
reformed polyethylene that originally comprised the thermoelastic tube 60
surrounding rib 14.
These unilaminar embodiments are described further below, and depicted in
FIGS. 18 and 19. In
both cases, i.e., the unilaminar and bilaminar embodiments, the reforming of
the polyethylene
which originally encases the rib 14 to a configuration that intercalates
through the ePTFE weave,
it is the reformation of the polyethylene that is substantially responsible
for the integration of the
ePTFE and the polyethylene-encased ribs(s) into an integrated structure.
[0093] In embodiments where only a single sheet of ePTFE is used, a
unilaminar
structure is formed, with the ribs 14 adhering to the single ePTFE sheet 61 by
way of the melted
-18-
CA 02901532 2015-08-14
WO 2014/152461
PCT/US2014/027364
and reformed polyethylene that originally comprised the thermoelastic tube 60
surrounding rib
14, the polyethylene material still encasing the rib. Unilaminar embodiments
of the invention are
depicted in FIGS. 18 and 19. FIG. 18A shows a cross sectional view of a rib,
substantially
circular in cross section, encased in a polyethylene tube 60, the tube
disposed adjacent to ePTFE
sheets 61 prior to application of pressure and heat. FIG. 18B provides a view
of the same
materials after application of heat and pressure. The thermoplastic material
that originally
comprised tube 60 disposed over the rib 14 has fused into the porous matrix of
the ePTFE sheet
61.
[0094] The encircled detail within FIG. 18A that is labeled 20A is a
reference to FIG.
20A which provides a more detailed of the ePTFE and polyethelene materials
prior to their
fusion during the lamination process, as described below. The encircled detail
within FIG. 18B
that is labeled 20B is a reference to FIG. 20B which provides a more detailed
view of the ePTFE
and polyethelene materials after their fusion during the lamination process,
as described below.
[0095] Similarly, FIGS. 19A shows a cross sectional view of a rib,
generally rectangular
in cross section, encased in a polyethylene tube 60, the tube adjacent to
ePTFE sheet 61 prior to
application of pressure and heat. FIG. 19B provides a view of the same
materials after heat and
pressure. The thermoplastic material that originally comprised tube 60
disposed over the rib 14
has fused into the porous matrix of the ePTFE sheet 61.
[0096] In some embodiments of the method, a cooling step is applied
following the
application of pressure and heat. A relatively passive cooling method is
appropriate for some
embodiments, and can be achieved by simply placing the mold on a cold surface
(for example, a
chilled block of copper) or by submerging it in any suitable cold medium such
as chilled water.
In other embodiments, more active, permeative, or quick cooling is preferred,
and may be
accomplished by circulating any suitable coolant (for example, chilled water,
liquid nitrogen)
through cooling channels built into the lamination mold body to bring the
temperature into a
range of about 0° F. to about 32° F.
[0097] While porous ePTFE material is included in typical embodiments, non-
porous
ePTFE may be appropriate for some embodiments. The choice of using non-porous
or porous
ePTFE depends on the intended use or desired features when the partitioning
device is placed in
the heart. A porous membrane can advantageously function as a filter-like
barrier that allows
blood through-flow, but blocks transit of particles or emboli. On the other
hand, in some medical
applications it may be desirable to form a significant seal between two
cardiac compartments
with the intervention of the partitioning device, in which case a non-porous
ePTFE may be
preferred.
- 19 -
CA 02901532 2015-08-14
WO 2014/152461 PCT/US2014/027364
[0098] Further, the membrane 11 may also be formed of other suitable
biocompatible
polymeric materials such as, by way of example, may include Nylon, PET
(polyethylene
terephthalate), and polyesters such as Hytrel. The membrane 11 may
advantageously be
foraminous in nature to facilitate tissue ingrowth after deployment within the
patient's heart, and
further, to provide an advantageous matrix for bonding with melted
polyethylene material, as for
example, from a thermoplastic tube 60. The delivery catheter 32 and the
guiding catheter 31 may
be formed of suitable high strength polymeric material such as, by way of
example,
polyetheretherketone (PEEK), polycarbonate, PET, and/or Nylon. Braided
composite shafts may
also be employed.
[0099] FIGS. 20 and 21 provide schematic views of the lamination zones of
the device,
at microscopic scale. Embodiments of the porous or foraminous ePTFE sheets may
have
internodal distances between woven fabric strands that range between about 5
and about 200
microns, as described above. The internodal areas delineated by the fibers
also provide space into
which polyethylene material from the thermoplastic tubes 60 intercalates as it
melts and reforms
during embodiments of the lamination process. As melted polyethylene material
intercalates into
the unmelted ePTFE material and then solidifies into a reformed configuration
on cooling,
intermingled and interlocking zones of respective material-material continuity
are created
between polyethylene and polytetra-fluoro-ethylene (PTFE). The continuity of
the PTFE fibers
remains substantially unchanged, even though the fibers may be stretched, and
the polyethylene
forms a continuous solid that includes the PTFE fibers within it. These
interlocking zones of
material continuity provide a firm bonding matrix that both (1) adheres the
two sheets of the
bilaminar structure together, and (2) secures the rib 14 to and within the
bilaminar structure. The
formation of integrated laminar structures that include one or two ePTFE
sheets and
thermoplastic material entrapping a rib is depicted in FIGS. 20 and 21; these
are schematic
views, drawn such that the internodal distances appear at a scale that is
larger than that of the
device as a whole.
[00100] FIGS. 20A and 20B schematically depict the formation of a
unilaminar integrated
structure from the polyethylene-encased rib and ePTFE material by the melting
and solidified
reformed polythethylene to create interlocking continuities between the ePTFE
and the
polyethylene. This structure also depicts a unilaminar or split-laminar
portion of a larger
bilaminar structure, such as a portion immediately overlaying a rib 14. FIG.
20A depicts a woven
sheet of ePTFE disposed over or adjacent to a portion of the wall of a
polyethylene tube encasing
a rib before being subjected to pressure and heat within a press. FIG. 20B
depicts the unified
structure after the application of heat and pressure, and after the
polyethylene has melted and
reformed within and around the weave of the ePTFE fabric.
- 20 -
CA 02901532 2015-08-14
WO 2014/152461
PCT/US2014/027364
[00101] FIGS.
21A and 21B schematically depict the formation of a bilaminar integrated
structure from the polyethylene-encased rib and ePTFE material by the melting
and solidified
reformed polythethylene to create interlocking continuities between the ePTFE
and the
polyethylene. FIG. 21A depicts two woven sheets of ePTFE disposed,
respectively, over and
under a portion of the wall of a polyethylene tube encasing a rib before being
subjected to
pressure and heat within a press. FIG. 21B depicts the unified structure after
the application of
heat and pressure, and after the polyethylene has melted and reformed within
and around the
weave of the ePTFE fabric. This bilaminar structure occurs in areas not
immediately overlaying
a rib 14, but rather in the area that lies immediately adjacent to a rib 14,
and spreading out
peripherally, thereby creating a substantial area of mutual connection between
the two ePTFE
sheets.
[00102] FIG.
22 shows an exploded and partially cutaway view of the components of the
assembly for lamination. FIG. 22 illustrates an alternative embodiment of an
assembly for the
making of an intracorporeal partitioning device, wherein the device is
laminated in a partially
compressed, i.e. not-free state. This assembly may be configured to assemble
either a unilaminar
or bilaminar device. The assembly depicted in FIG. 22 is similar to the
assemblies described
above with references to FIGS. 14 and 15, however the assembly of FIG. 22 is
configured to
laminate the device in its non-free state.
[00103] As
described above in reference to FIGS. 14 and 15, the implants are assembled,
or laminated, in their free, heat shaped configuration. A resulting device
2300 is shown in FIG.
23A, having a free diameter of X, for example. The devices described herein
are generally
configured for implantation into a ventricle of a patient's heart. In some
embodiments, the
patient's ventricle may be smaller in diameter than the free size of the
device, or more
specifically, smaller than the diameter X, as shown in FIG. 23A. In some
specific cases, the
diameter of the ventricle may be 20 to 30% smaller than the free diameter X of
the device 2300.
For example, in a healthy heart, the end-diastolic dimension of the left
ventricle may range from
36-56mm and the end-systolic dimension of the left ventricle may range from 20-
40mm (A left
ventricle in heart failure would typically have larger dimensions). Therefore,
once implanted, a
device laminated in its free state would likely be held in a contracted
position (i.e. a loaded
configuration with a decreased diameter) and not return to a free state and
its free, or unloaded,
dimension (e.g. diameter). Therefore, the membrane material will likely bunch
between the struts
to accommodate the device moving into the contracted state upon implantation.
Excess
membrane material may lead to, at least, a more expensive device, a larger
collapsed
configuration (necessitating larger guide and delivery catheters), improper
sealing or engagement
with the ventricle wall, and/or a combination thereof. Therefore, it may be
desirable, in some
- 21 -
CA 02901532 2015-08-14
WO 2014/152461
PCT/US2014/027364
configurations to laminate the frame of the device in a pre-loaded, or non-
free, state, thereby
reducing the amount of membrane material utilized to laminate the device.
[00104] In FIG. 22, the upper or male platen 2205 of a press 2203 and the
lower or female
platen 2202 are seen above and below, respectively. As described above, around
the periphery of
the upper platen 2205 is a rim portion, and around the periphery of the lower
platen 2202 is a rim
portion. These two rim portions form complementary planar surfaces which serve
to hold edges
of the sheets of ePTFE fabric as the central portion is being subjected to
being pressed by the
complementary surfaces of the central portion or shaping portion 2207A of the
upper platen
2205, and the central portion 2207B of the lower platen 2202. A perspective
view of the device
as it would emerge post-formation is seen in FIG. 23A. A comparison of the
assembly in FIG. 22
and FIGS. 14 or 15 will show that the shaping portions 2207A and B have a
steeper angle than
the shaping portions 67A and 67B in FIGS. 14 and 15. Furthermore, the height
of the assembly
(and the resulting device) is taller in the assembly of FIG. 22. The assembly
of FIG. 22 thereby
holds the device components (particularly the frame) in a pre-loaded
configuration with a
decreased diameter. Furthermore, as shown by line 2208, the curve of the
shaping elements
2207A and 2207B may follow the curve the struts will undergo in their pre-
loaded configuration.
Alternatively, an assembly with a straight (not-curved 2208) shaping element
may be utilized,
however, in some instances; a straight shaping element may over constrain the
struts in their pre-
loaded configuration.
[00105] As shown in FIG. 23B, a device resulting from the assembly fixture
shown in
FIG. 22 has a diameter X' which is smaller than diameter X as shown in FIG.
23A, and a height
Y' which is taller than Y as shown in FIG. 23A. In one specific example, an
implant with
diameter X equal to 85 mm might be compared to an implant with diameter X'
equal to 75 mm.
In some embodiments, it may be noted that devices assembled in a pre-loaded
state, may have
increased stability and/or a decreased propensity to inverting (flipping
inside out) during
delivery, implantation, and/or the life of the device.
[00106] FIGS. 24A-24C illustrate a cross-section of a loaded frame in its
free state or
unstressed configuration (FIG. 24A), after lamination with an assembly fixture
as shown in FIG.
22 (FIG. 24B), and implanted (FIG. 24C). The frame as shown in FIG. 24A may be
compared to
the device shown in FIGS. 9 and 10, which illustrate the reinforcing frame 13
in an unstressed
configuration and include the ribs 14 and the hub 12. The ribs 14 have a
length L of about 1 to
about 8 cm, preferably, about 1.5 to about 4 cm for most left ventricle
deployments. The
proximal ends 16 have a flared construction. As shown in FIG. 24A, the frame
in its free, pre-
assembled state, may have a diameter of X (e.g. 80mm). As shown in FIG. 24B,
the frame in its
pre-loaded, assembled state, may have a diameter of X-10% (e.g. 72mm). For
example, the
- 22 -
CA 02901532 2015-08-14
WO 2014/152461
PCT/US2014/027364
frame may be pre-loaded by 10% in the assembly fixture. As shown in FIG. 24C,
the frame in its
loaded, implanted state, may have a diameter of X-30-40% (e.g. 56-64mm). For
example, the
frame may be pre-loaded an additional 20-30% in the patient's ventricle,
specifically during
diastole. Although percentages of loading and/or pre-loading and diameter
reduction are listed by
way of providing exemplary loading configurations, such examples are for
purposes of clarity of
understanding only, and are not intended to be limiting. It should be
understood that the frame
may be loaded and/or pre-loaded and reduced in diameter to any suitable size
and configuration.
[00107] As described above, embodiments of the partitioning device 10, both
unilaminar
and bilaminar embodiments, are conveniently formed by placing a thermoplastic
tube 60, e.g.
polyethylene or high density polyethylene (HDPE), over the ribs 14 of the
frame 13 as shown in
FIG. 25A until the proximal ends 16 of the ribs 14 extend out the ends of the
thermoplastic tubes
to form thermoplastic-encased ribs. FIGS. 25A-25C illustrate a first, second,
and third
embodiment showing the frame of the device described herein having sleeves, or
more
specifically thermoplastic tubes 60. As shown, the device may include full
sleeves 60 disposed
along the full length of the struts (FIG. 25A), partial sleeves 60' staggered
along the length of the
struts (FIG. 25B), or shortened sleeves 60" (FIG. 25C). As shown in FIG. 25B,
by reducing the
amount of tubing used, and by staggering the positioning of the tubing along
the length of the
struts 14, the implants collapsed profile may be reduced. As shown in FIG.
25C, a reduction in
profile could also be accomplished by shortening the length of the tubes,
keeping them away
from the perimeter of the device, or proximal ends 16 of the ribs 14, where
most of the profile
size is accumulated. In an alternative embodiment, a frame may be disposed
between two sheets,
and the sheets may be fused together to form the assembled implant without the
need for sleeves,
or more specifically thermoplastic tubes. For example, a method of securing a
polymeric sheet to
rib components of a frame structure may include the steps of providing an
assembly, the
assembly comprising a frame structure disposed between a first and second
polymeric sheet; and
heating the assembly under pressure to fuse the first polymeric sheet to the
second polymeric
sheet around the frame structure to form a fused sheet. In some embodiments,
the polymeric
sheets of material may be ePTFE.
[00108] FIGS. 26A-26E illustrate an embodiment of a frame 2600 having a
plurality of
ribs or struts 2602 extending from a central hub 2604. The frame may be laser
cut from a metal
tube. The metal may be a shape memory alloy such as nitinol. A plurality of
longitudinal cuts
can extend from one end of the metal tube to a position offset from the other
end of the tube,
leaving a central hub 2604 from which the struts 2602 extend from. The cuts
can result in a
plurality of slots 2606 between the struts 2602.
- 23 -
CA 02901532 2015-08-14
WO 2014/152461 PCT/US2014/027364
[00109] As shown in FIG. 26A, the spacing between the slots 2606 can define
the strut
width while the thickness of the tube can define the strut thickness. In some
embodiments, the
spacing of the slots 2606 around the tube can result in struts 2602 having a
cross-sectional width
that is slightly greater than its cross-sectional thickness. This can be
accomplished by having the
spacing between the struts 2602 be slightly greater than the thickness of the
tube. In some
embodiments, the inside diameter (ID) of the tube can be about 5, 6, 7, 8, 9,
10, 11, 12, 13, 14,
15, 16, 17, 18, 19, 20, 25, 30, 35, or 40 percent greater than the thickness,
or between about 5-40
percent greater than the thickness, with the outside diameter being
correspondingly greater than
the ID based on the thickness and the ID of the tube. For example, in some
embodiments when
the ID is about 10% greater than the thickness, the OD is about 35% greater
than the thickness.
[00110] As shown in FIG. 26B, the root 2608 of the strut 2602 is the
portion of the strut
that extends from the hub 2604. The root 2608 of the strut 2602 can be flared
such that the width
of the strut increases as it approaches the hub 2604. In some embodiments, the
width of the strut
2602 at the hub 2604 can be about 10 to 100 percent larger, or between about
20 to 80 percent
larger, or between about 30 to 50 percent larger, than the width of the strut
at a middle portion of
the strut. In some embodiments, the length of the flared root 2608 can be
about equal to the
width of the flared root 2608 at the hub 2604. In other embodiments, the
length of the flared root
2608 can be about 50 to about 300 percent, or about 100 to about 200 percent,
of the width of the
flared root 2608 at the hub 2604. The flared root 2608 can be formed by
tapering the slot 2606
as it reaches the hub 2604. The flared root 2608 spreads bending strains over
a larger amount of
material, thereby decreasing peak strains during manufacturing, loading of the
implant within a
,
catheter, and cyclical loading in the ventricle after implantation.
[00111] The strut cross-section dimensions described above of having a
width slightly
greater than the thickness, in conjuction with the flared root, can bias the
strut so that it bends
directly outwards without any twist or with little twist. This improves the
strength of the struts
and reduces strain.
[00112] As illustrated in FIG. 26C, in some embodiments, the flared root of
the strut can
have a root bend radius 2610 that is sized to (1) reduce or limit peak strains
during shape setting
to reduce or prevent damage and cracking of the metal frame; (2) reduce or
limit peak strains
when the implant is loaded into the catheter and reduce or prevent plastic
deformation of the
metal; (3) reduce or minimize the height of the implant; and reduce peak
strains after
implantation. In some embodiments, the diameter of the frame in its free shape
2612 can be
slightly oversized relative to its laminated shape 2614 so that the membrane
will stay tight after
lamination. For example, the frame can be oversized by about 3, 4, 5, 6, or 7
mm, or be
oversized between about 2 to 10 mm. The lamination mold is designed to conform
to the natural
- 24 -
CA 02901532 2015-08-14
WO 2014/152461
PCT/US2014/027364
shape of the frame when it is reduced to the lamination diameter. The natural
shape of the frame
is the shape the frame takes when its diameter is reduced, meaning when a
frame with a 100 mm
diameter has its diameter reduced to 95 mm by pushing on the anchors, the
struts of the frame
bows in a particular manner that is its "natural" shape. Conforming the
lamination mold to the
natural shape of the frame ensures that the frame is free to move as designed
with little or no
alternating strain concentrations.
[00113] As shown in FIG. 26D, after lamination, there is a strut curvature
2616 near the
anchor on the free ends of the struts that is designed to optimize the angle
of engagement 2618
with the left ventricle wall which improves retention of the implant in the
left ventricle. In some
embodiments, the strut curvature has a radius of about 0.5 to 1.5 inches. In
some embodiments,
the angle of engagement 2618 is about 30 to 60 degrees.
[00114] As shown in FIG. 26E, the free ends of the struts 2602 can
terminate in anchors
2620, which may be barbed. The barb may be a single barb or a double barb. In
addition, in
some embodiments, a stop 2622 can be located at or near the base of the
anchors 2620. The stop
can be a discrete projection or widening of a portion of the strut which
serves to lock the
membrane in place and reduce or prevent over-penetration of the struts into
the ventricle wall.
The length of the struts may alternate between a short length strut and a long
length strut so that
the anchors and/or stops are staggered, which allows the struts to be
collapsed into a more
compact diameter for delivery.
[00115] Unless defined otherwise, all technical terms used herein have the
same meanings
as commonly understood by one of ordinary skill in the art of interventional
cardiology. Specific
methods, devices, and materials are described in this application, but any
methods and materials
similar or equivalent to those described herein can be used in the practice of
the present
invention. While embodiments of the invention have been described in some
detail and by way
of exemplary illustrations, such illustration is for purposes of clarity of
understanding only, and
is not intended to be limiting. Various terms have been used in the
description to convey an
understanding of the invention; it will be understood that the meaning of
these various terms
extends to common linguistic or grammatical variations or forms thereof. It
will also be
understood that when terminology referring to devices or equipment has used
trade names, brand
names, or common names, that these names are provided as contemporary
examples, and the
invention is not limited by such literal scope. Terminology that is introduced
at a later date that
may be reasonably understood as a derivative of a contemporary term or
designating of a subset
of objects embraced by a contemporary term will be understood as having been
described by the
now contemporary terminology. Further, any one or more features of any
embodiment of the
invention can be combined with any one or more other features of any other
embodiment of the
- 25 -
CA 02901532 2015-08-14
WO 2014/152461
PCT/US2014/027364
invention, without departing from the scope of the invention. Still further,
it should be
understood that the invention is not limited to the embodiments that have been
set forth for
purposes of exemplification, but is to be defined only by a fair reading of
claims that are
appended to the patent application, including the full range of equivalency to
which each element
thereof is entitled.
[00116] Terms such a "element", "member", "device", "section", "portion",
"step",
"means" and words of similar import, when used herein shall not be construed
as invoking the
provisions of 35 U.S.C. §112(6) unless the following claims expressly use
the terms "means"
followed by a particular function without specific structure or "step"
followed by a particular
function without specific action. All patents and patent applications referred
to above are hereby
incorporated by reference in their entirety.
- 26 -