Note: Descriptions are shown in the official language in which they were submitted.
. 81790705
APPARATUS AND METHOD FOR RECOGNITION OF SUSPICIOUS ACTIVITIES
[001]
Field
[002] This invention relates generally to patient compliance in medication
administration
protocol scenarios including ingestion of medication, and more particularly to
an apparatus and
method for the collection, analysis and transmission of data related to
patient movements related
to such medication administration and ingestion of the medication into the
body of a user, placing
the medication in their mouth, or other method of transferring medication into
their body,
including features for identifying suspicious or purposefully malicious
activity on the part of the
patient.
1
CA 2902215 2020-03-09
CA 02902215 2015-08-20
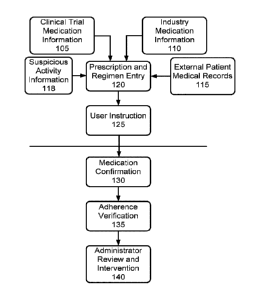
WO 2014/152828 PCT/US2014/027901
Background
1003] Dr Lars Osterberg, M.D. and Dr, Terence Blaschke have reported in the
New
England Journal of Medicine, Adherence to Medication, (N Engl J Med 2005;
353:487-97) 2005
an alarming lack of adherence to required medication protocol, further noting
that while the
average rates of adherence in clinical trials is categorized as "high", this
number still comprises
only rates of 43 to 78 percent. Most importantly, the authors note "The
ability of physicians to
recognize nonadherence is poor, and interventions to improve adherence have
had mixed
results." Adherence, p. 487.The authors conclude "Poor adherence to medication
regimens is
common, contributing to substantial worsening of disease, death and increased
healthcare costs."
Adherence, p. 494. The Trend Repot Series, 2008 Patient Adherence Update: New
Approaches
for Success, October 2008, report similar discouraging statistics. This broad
range may possibly
contribute to the public confidence in the FDA approval process and the
importance of continued
surveillance of a drug throughout the process. Furthermore, it may help to
explain why,
according to the Journal of the American Medical Association (JAMA May 1,
2002), one out of
every five new drugs that comes to market in the US is found to have serious
or life-threatening
adverse effects - unknown or undisclosed at the time of approval. It is
against this backdrop of
poor adherence, and potential danger to patients, that the present invention
operates.
[004] It has been widely recognized that methods and systems for insuring
proper
medication ingestion or administration by individuals are very important in
defending against
unnecessary sickness, deaths and other problems. Giving instructions and then
letting patients
fend for themselves has been shown not to work particularly well. This is
because it is not only
the improper ingestion of medicines that is the primary cause of medical
danger. Rather, an
overall lack of sufficient patient guidance is also part of the problem.
Further, the inability to
confirm a proper prescription regimen being provided to a user in the first
place may cause a
number of other problems with the use of such medication. As has been shown in
regards to
various public health medication administration situation, such as
administration of tuberculosis
medication by the WHO, Directly Observed Treatment (DOT) improves compliance
of patients.
Global Tuberculosis Control: A Short Update to the 2009 Report, World Health
Organization,
2009. As is shown in this report, funding for implementing DOT programs is
high. Thus, the
ability to implement such a program with less of a financial burden would be
desirable.
2
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
[005] Traditionally, participants attend introductions and follow ups for
clinical trials in-
person. Other patients outside of the clinical trial setting attempting to
adhere to a particular
medication protocol similarly are given a prescription and a particular set of
instructions from a
prescribing medical provider or prescribing doctor. Compliance is then
measured at a next visit
with that prescribing professional through traditional methods of counting
unused medication,
and patient interviews. Thus, data collection is similarly limited to patient
visits, rather than on a
daily basis. These old methods such as patient questioning and medication
counting have been
proven to be inadequate measures of adherence and offer no information on dose
timing and drug
holidays (omission of medication for three or more sequential days).
[006] Compliance and medication adherence technologies can increase the
statistical
power of clinical trials. Through the use of such technology, clinical events
can be precisely
linked to medication use history. Captured data can be linked to other sources
such as EDC,
patient diaries and data collected by the physician. Technologies can create
many possibilities for
remote visits and data capture. While smart packaging technologies exist such
as RFID-enabled
computer chip technology, smart blister packs and MEMS caps (microprocessor in
a bottle cap),
they are: a) invasive and need to be physically attached to the medications;
b) are non-conclusive
regarding compliance ¨ a patient may activate the technology without ingestion
of the
medication; c) remain largely unadopted in clinical trials by the
pharmaceutical and biotech
companies due to their high cost; and d) take a longer time to implement.
Further, electronic
patient diaries allow for ease of entry of data by a patient. These diaries,
however, are still
subject to issues related to compliance with medication adherence. Thus, even
if a patient is
meticulous about entering information into the diary, and thus complying with
the requirements
for data entry, there is still no guarantee that they arc properly taking
medication at prescribed
times. This problem is even more acute when a participant is performing a
suspicious action, or
is otherwise maliciously avoiding taking their medication.
[007] Jo Carol et al. stated that "The most reliable method for research
purposes,
although not practical in a clinical setting, may be a combination approach
that includes pill
counts, patient self-report, and electronic monitoring." (Carol J. et al,
Patterns to Antiretroviral
Medication, The Value of Electronic Monitoring, AIDS, 17 (12), ppl, 763-767,
Oct 2003. To
date, technologies alone have only been used to monitor compliance rather than
to encourage it.
3
81790705
These technologies also provide no specific defense against patients who are
purposefully not
taking their medication.
1008] A number of systems exist that provide instructions to a user regarding
when to take
a medication and records when the user indicates that a medication has been
taken. US Patent
No. 7,395,214 describes such a system. A device is provided that provides
instruction to a patient
regarding medications to take. Furthermore, the system may provide a method
for determining
that the prescription is appropriate given the patient's conditions, and other
medications he or she
may already be taking. The system may monitor the dispensing of medicine in
accordance with a
predetermined treatment protocol. While such a system provides many
improvements for easing a
burden on the patient, this system suffers in many ways and in particular in
ways relevant to the
administration of clinical trials and other active patient monitoring of
medication adherence.
[009] Most importantly, this system provides no mechanism for actually
confirming that a
patient is in fact properly administering required medication, including
placing a medication pill
into their mouth, or injecting or inhaling medication following a predetermine
series of steps as
required in a clinical drug trial, as prescribed by a prescribing physician in
the case where
adherence to a particular regimen may prove to be critical to efficacy of the
prescription regimen,
in various public health scenarios, in situations where failure to keep up a
prescription regimen
can potentially harm a population as a whole, such as the generation of
antibiotic-resistant bacteria
strains, in various disease management scenarios, or in home care situations
where maintaining
proper control of administering healthcare professionals is critical. Further,
while the system may
be sufficient for one who is in full possession of their mental faculties, any
individual who may
have difficulty following directions, or one who is actively avoiding
medication may still not be
taking required medication after it is dispensed. Thus, participants may be
forgetful, visually
impaired, purposefully avoiding taking their medication, or otherwise do not
believe in the benefit
of taking such medication, and may thus not properly log medication
administration. Furthermore,
the system requires preloading of various medications into a dispenser, and
thus likely requires
regular visits by an administering manager to be sure appropriate medications
are in fact properly
loaded therein. It is surely possible that an inexperienced user may place
incorrect medications
into the device, or may somehow provide incorrect dosages into the device.
Additionally, for
potentially more complex regimens, there is
4
CA 2902215 2020-03-09
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
no method provided for insuring that a user is able to follow such a protocol,
and to thereafter
confirm that the user has in fact taken all required medications in accordance
with any provided
instructions or the like, or has taken the medications according to one or
more specifications or
followed suggested procedures. Furthermore, this system is expensive and
requires constant
maintenance to confirm that the various mechanical parts are in working order.
Finally, there is
no consideration of participants who may be trying to trick the system and
purposefully avoid
taking their medication as may be the case in various patient populations such
as schizophrenia
populations, prison populations, or other groups.
1010] US Patent Application Serial No. 11/839,723, filed August 16, 2007,
titled Mobile
Wireless Medication Management System provides a medication management system
employing
mobile devices and an imaging technology so that a user is able to show a pill
to be taken to the
system, and the system can then identify the medication. Patient histories are
available to an
administrator, including various vital signs as measured by the system. Images
may also be
taken of the patient, provider, medication container or the like. While the
system professes to
ensure adherence to a protocol, the system only provides such help if
requested by a user. There
is in fact no particular manner in which to ensure actual adherence, including
ingestion,
inhalation, injection of the medication, or the relationship of adherence to
the efficacy or safety
of the drug over time. When requiring adherence to a predetermined protocol
for a clinical trial,
this is particularly relevant.
[011] Additionally, existing systems fail to maintain an audit trail for post
administration review by a medical official or other clinical trial
administrator, and further
cannot therefore confirm confirmation of proper medication administration or
population
management. This once again thus fails to defend against suspicious or
malicious medication
users who purposefully do not take their medications.
[012] Therefore, it would be desirable to provide an apparatus that overcomes
the
drawbacks of the prior art.
81790705
Summary
[013] In US Patent Application Serial No. 12/620,686, US Patent Publication
No. US2011-0119073, filed November 18, 2009, titled Method and Apparatus for
Verification of
Medication Administration Adherence; currently pending, US Patent Application
Serial
No. 12/646,383, US Patent Publication No. US2011-0153360, filed December 23,
2009, titled
Method and Apparatus for Verification of Clinical Trial Adherence, currently
pending; US Patent
Application Serial No. 12/646,603, US Patent No. 8,666,781, filed December 23,
2009, titled
Method and Apparatus for Management of Clinical Trials, currently pending; and
US Patent
Application Serial No. 12/728,721, US Patent No. 9,183,601, filed March 22,
2010, titled
Apparatus and Method for Collection of Protocol Adherence Data, currently
pending, the
inventors of the present invention have proposed a system, method and
apparatus that allow for
complete control and verification of adherence to a prescribed medication
protocol or machine or
apparatus use in a clinical trial setting, whether in a health care provider's
care, or when self
administered in a homecare situation by a patient.
[014] These applications present the only medication management system that
may
determine whether a user is actually following a protocol, provide additional
assistance to a user,
starting with instructions preferably including one or more interactive and
real-time audio, visual
textual or the like prompts based upon one or more actions detected of the
user, and moving up to
contact from a medication administrator if it is determined that the user
would need such
assistance in any medical adherence situation, including clinical trial
settings, home care settings,
healthcare administration locations, such as nursing homes, clinics, hospitals
and the like, and in
clinical trial settings.
[015] The inventive solution builds on these initial inventions and provides
one or more
features that may be employed in accordance with these systems to detect
suspicious activity of a
user, and to thwart users who purposefully do not take their medication. The
inventive system
may visually and audibly recognize a fixed series of actions, each comprising
part of the
medication administration process, and may further recognize deviations from
the fixed series of
actions, whether audio, video, and whether used for monitoring orally
ingested, injectable,
inhalable or other administered medications. The system may also interact in
real-time with the
patient when something is identified as incorrect, wrong or suspicious and
either prompt a
different action or raise an alert and recommend an intervention by a
healthcare provider.
6
CA 2902215 2020-03-09
81790705
[016] According to an aspect of the present invention, there is provided a
medication
administration confirmation apparatus, comprising: a display for displaying a
first set of one or
more instructions to a user encouraging proper perfoimance of one or more
steps of a medication
administration sequence; a video capture device operable to capture one or
more video sequences
of the user administering medication; a memory operable to store the one or
more video sequences
as stored video sequences; and a processor operable to during the medication
administration
sequence, cause the display to display a first portion of a field of view of
the video capture device,
wherein a second portion of the field of view is not displayed to the user,
analyze, in at least one
of the one or more video sequences, the second portion of the field of view,
determine that
suspicious activity on behalf of the user is shown in the second portion of
the field of view, mark
the at least one of the stored video sequences as including suspicious
activity, and cause the
display to display one or more further instructions to the user encouraging
proper performance of
the one or more steps of the medication administration sequence in response to
the determination
that suspicious activity is shown in the second portion of the field of view.
[016a] According to another aspect, there is provided a medication
administration
confirmation apparatus, comprising: a display for displaying a first set of
one or more instructions
to a user encouraging proper performance of one or more steps of a medication
administration
sequence; a video capture device operable to capture one or more video
sequences of the user
administering medication in response to the first set of one or more
instructions; a memory
operable to store the one or more video sequences as stored video sequences;
and a processor
operable to analyze at least one of the stored video sequences to identify
suspicious activity on
behalf of the user, the suspicious activity comprising the user concealing at
least a portion of the
one or more steps of the medication administration sequence from capture by
the video capture
device, mark the at least one of the stored video sequences as including
suspicious activity, and
cause the display to display one or more further instructions to the user
encouraging proper
performance of the one or more steps of the medication administration sequence
in response to the
identification of the suspicious activity.
[016b] According to another aspect, there is provided a method for confirming
medication
administration, comprising: displaying, by a medication administration
confirmation apparatus,
a first set of one or more instructions to a user encouraging proper
performance of one or
more steps of a medication administration sequence; capturing one or more
video sequences
of the user administering medication by a video capture device of the
7
Date Recue/Date Received 2022-12-28
81790705
medication administration confirmation apparatus; analyzing, by the medication
administration confirmation apparatus, at least one of the one or more video
sequences to
identify two or more predetermined indications of suspicious activity on
behalf of the user;
marking the at least one of the one or more video sequences as including
suspicious activity,
displaying, by the medication administration confirmation apparatus, one or
more further
instructions to the user identifying a first of the two or more predetermined
indications of
suspicious activity and encouraging proper performance of the one or more
steps of the
medication administration sequence in response to the identification of the
first of the two or
more predetermined indications of suspicious activity; and not displaying to
the user any
information identifying a second of the two or more predetermined indications
of suspicious
activity, and storing a record of the second of the two or more predetermined
indications of
suspicious activity.
[017] In accordance with an embodiment of the present invention, a motion
capture
procedure for capturing motion information related to the administration of
pill or film based oral
medications, or injectable, inhaler-based, other non-pill based medication, or
any other form of
patient administration task that may be performed, may be utilized in
accordance with one or more
of the inventions noted in the above -referenced applications. Therefore, in
accordance with an
embodiment of the present invention, a method and apparatus may be provided
for analyzing
captured patient motion data, preferably in near real time to provide feedback
to the user, to
deteunine a number of times a participant performs some action that is
considered suspicious.
Additionally, the patient performance information may be analyzed
asynchronously to determine
other features of data that may suggest some malicious intent on the part of
the patient.
[017a] Further in accordance with an embodiment of the present invention, one
or more
predetermined motion sequences may be determined, and one or more related
errors or other
suspicious activities may be defined related to each of these one or more
motion sequences. The
automated system of the invention may then monitor a number of times these one
or more
suspicious activities may take place during a medication administration, and
over multiple
administrations in order to determine a pattern of potentially suspicious
behavior over time.
[018] Still other objects and advantages of some embodiments of the invention
will in part
be obvious and will in part be apparent from the specification and drawings.
[019] The invention accordingly comprises the several steps and the relation
of one or
more of such steps with respect to each of the others, and the apparatus
embodying features of
7a
Date Recue/Date Received 2022-12-28
81790705
construction, combinations of elements and arrangement of parts that are
adapted to affect such
steps, all as exemplified in the following detailed disclosure, and the scope
of the invention will be
indicated in the claims.
7b
Date Regue/Date Received 2022-12-28
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
Brief Description of the Drawings
1020] For a more complete understanding of the invention, reference is made to
the
following description and accompanying drawings, in which:
[021] Figure 1 is a flow chart diagram depicting a method in accordance with
an
embodiment of the invention;
[022] Figure 2 is a flowchart diagram depicting a video sequence capture
method in
accordance with an embodiment of thc invention;
10231 Figure 3 is a depiction of a position determining process in accordance
with an
embodiment of the invention;
1024] Figure 4 is a depiction of another position determining process in
accordance with
an embodiment of the invention;
[025] Figure 5 is a depiction of a motion tracking process in accordance with
an
embodiment of the invention.
8
= 81790705
Detailed Description of Example Embodiments
[026] Examples of embodiments of the invention will now be described making
reference
to the following drawings in which like reference numbers denote like
structure or steps.
Referring to Figure 1, a data flow overview in accordance with the operation
of an embodiment of
the present invention is shown. In accordance with this embodiment of the
invention, information
about a particular drug to be the subject of a clinical trial, to be employed
in a public health or
disease management situation, or the like, other medication administration
program or
prescription, or other patient self-administered medical task such as
performing a home based
urine test or the like may be provided in a database 105, and existing
industry medication
information databases 110 are preferably employed to access prescription,
interaction, application,
and other available information about any number of proposed prescription and
non-prescription
medications and their possible interaction with the clinical trial or other
medications. Further,
patient medical records 115 may be used, and as will be described below, in
conjunction with the
industry medical information and a medical professional's prescribing
expertise to confirm that a
patient is a good candidate for such a clinical trial, or medication
administration program. These
databases may be accessed in a manner known to one of ordinary skill in the
art. This information
may further include information related to associated likely typical elements
of suspicious or
malicious behavior. Such elements may he based upon the type of medication,
method of
medication administration, demographics or other information about the
patient, other features, or
a combination of these elements. Suspicious activity information 118 is
preferably transferred to
the system at this time as well, and as noted above may include one or more
indications of patient
movements or other actions that may indicate suspicious behavior on behalf of
the patient, a
malicious intent to trick the system, or actions of other high risk
population. The various
embodiments of the present invention may also be provided to determine if a
patient is performing
an action or task correctly or if they are simply making a mistake.
[027] Once confirmed, a medication administration and ingestion or other
medication
regimen in accordance with the clinical trial or other prescription
requirements such as in a public
health, medical practice environment or the like may be prescribed and entered
into the system of
the invention at 120. Such medication administration regimen may include one
or
9
CA 2902215 2020-03-09
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
more of ingesting or swallowing a pill, using an inhaler device, using an
injectable medication
device, employing a patch, sublingual administration, a cheek or other skin
located medication
administration device or the like. Once entered into the system, a particular
prescription regimen
may cause a set of user instructions, various training sequences and the like
125 to be generated
and transmitted to an apparatus provided to a patient in accordance with an
embodiment of the
invention for access to the system of the invention. Such an apparatus may
comprise a custom
designed video and audio capture, analysis and transmission apparatus, a smart
phone or other
mobile device including a camera or other video and audio capture apparatuses,
a netbook,
laptop computer, desktop computer, tablet device or the like, free standing,
or built into a mirror
or cabinet or the like, or other computing appliance allowing for the display
of instructions to a
patient, and allowing for the eventual capture, analysis and transmission of
video, audio and
other analysis information. When installing software on a user's own hardware
system, it is
preferred that the software detect and otherwise test or determine that the
hardware attempting to
be utilized by the patient is sufficient to implement the invention and is
sufficient to run a
software package provided in accordance with the invention. Thus, the software
may check that
a camera includes sufficient resolution, that a memory of the device is of
sufficient size to allow
for sufficient captured video storage, that audio may be properly captured,
and that the
transmission system includes sufficient bandwidth to transmit and receive
captured video, audio,
video instructions and the like. Processing may also be performed at a remote
location, thus
allowing the user to include a lighter application or the like on their local
device. Alternatively,
the user may employ the local device as a gateway only, all data being
transmitted to a remote
location for processing, and returning responses as a result of such
processing. Thus, a user may
be able to dial up a video conference number, or otherwise interact with a
remote site, such as by
visiting a particular website or [AIL.
1028] Such user instructions and training sequences may include general
instructions
about the particular medication subject to the current trial or medication
administration protocol,
methods for administration, warnings about side effects, and concerns about
drug interactions
with common substances or medications, or other medications prescribed to the
patient by the
system or by another medical service provider. It is contemplated in
accordance with an
embodiment of the invention that such set of user instructions may be
interactive, allowing a user
to view additional information about such instructions or prescriptions as
desired. These
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
instructions may comprise written, audio or video instructions provided to the
user on a display
of the user apparatus. It is further contemplated that such instructions may
indicate one or more
movement sequences to be associated with a corresponding one or more
medication
administration sequences. These instructions preferably indicate proper and
improper motions
that may be taken by a patient for ingesting a pill, using an inhaler, using
an injectable
medication, and the like, and may indicate various motions that may or may not
be performed by
a user. In such a manner, the patient may be instructed to properly perform
all requested actions,
and avoid actions that may be indicative of a patient trying to trick the
system. In an alternative
embodiment, it may be possible to particularly not describe to the patient the
various suspicious
behaviors that will be tracked in an attempt to "trap" the unsuspecting
malicious patient. These
traps may be maintained and running in a background of the system. Thus, as
will be described
below, detection of one or more errors may generate real time displayed video
and/or audio
feedback to the patient in order to correct actions, while one or more other
errors may be logged
by the system but preferably provide no feedback to the patient, and thus may
be accumulated
and used to analyze patient actions without providing instructions and help to
avoid being caught
by the system. After being "caught" a number of times, a state of the user may
be changed to one
of heightened security awareness. Thus, a user may be first started in a low
level of observation.
After a predetermined number of potentially actions that are caught, the user
may be labeled as a
user that is attempting to trick the system, or that is performing one or more
suspicious acts, thus
warranting a heightened level of security. Additional suspicion will surround
review of all
actions of the patient. After properly using the system correctly for another
predetermined
period of time, the user may be returned to the initial normal state. If on
the other hand,
suspicious activity continues, the user may be moved to yet another state
where the user may be
recommended to be removed from a clinical trial, or taken off a particular
medication, for
example.
[029] In accordance with one or more embodiments of the present invention, one
or
more of these sets of motions or actions may comprise confirmation that a user
has placed a pill
in their mouth and has properly swallowed the pill correctly and has therefore
ingested the
medication properly through visual confirmation of location, confirmation that
the user has
properly used an inhaler device through visual confirmation of position and/or
audio
11
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
confirmation of actuation, confirmation that the user has properly used an
injectable device
through visual confii illation of position and/or audio confirmation of
actuation, or the like.
1030] Referring to the lower portion of Figure 1, the horizontal line
indicates a time for
patient administration of medication. At such time, the user may be notified
to take their
medication through any desirable communication and notification system,
including text
messaging, email, telephone call, automated calendar reminder or the like.
While not explicitly
shown, first, preferably the identity of a user may by confirmed through the
use of a facial
recognition sequence, other biometric identification sequence, voice
recognition, other password
identification system, or a combination thereof. Other features of use of the
system, such as time
to turn on, time to perform other tasks, etc. may be recorded to further aid
in determining a
consistent identity of a user of the system. The user may also be asked to say
"hello" or some
other word sequence to allow for audio recognition of the voice of the user.
Upon recognition of
the individual, the system may display one or more data regarding the
individual, such as, by
way of example only, name, patient status, medication to be administered,
calendar indicating to
the patient when medication has been administered and if any administration
times have been
missed, and, selectively, a score indicative of a level of compliance of the
individual with the
medication protocol, if desired. Other metrics may also be tracked, such as if
too many skips
have been registered by a user, or if too many self reports that the user has
taken their medication
without the user of the automated system, each indicating potentially
suspicious activity. This
identification information may be stored to a remote location to aid in
determining whether a
particular user is registering at more than one site, with more than one
device, and thus
attempting to be paid twice for the trial. Such patients arc unlikely to be
interested in taking the
medication at all. Additional attributes of a patient may also be stored in a
centralized database
or the like to potentially alert other clinical trials of potential offenders.
Thus, one or more
patient behavior profiled, facial identification characteristics voice
recognition, combination
thereof, may allow one or more subjects to be placed or a watch list or the
like after having been
identified as non-compliers. Thus, patients trying to get medication from
multiple clinics,
patients trying to sign up for multiple clinical trials, or patients trying to
sign up at multiple sites
in a single clinical trial may be recognized and determined before being able
to be engaged at
these other sites.
12
= 81790705
[031] The system shall preferably be able to learn the pattern of suspicious
patients based
on all data collected (such as number of misses, number of skips, manual
ratios, time on tasks,
number of usability errors, number of suspicious errors, study
coordinator/sponsors notes, flags,
video reviewing results.). Then a patient can be easily classified as
suspicious level 1, level 2...or
not by checking whether the patient fits in certain pattern. The machine will
keep learning each
day when more data are gathered and more patients are enrolled. Thus, a more
accurate placement
of the user in a particular state in a state machine, a will be described
below, can be more
accurately determined.
[0321 Once identified and notified of a type of medication to be administered,
the patient
may display a medication, such as a pill, dissolvable film or the like,
administration apparatus,
such as an inhaler, injectable apparatus, or other medication form (including
a pill bottle or the
like) to confirm that the medication is correct and is the currently
prescribed medication to be
taken through the use of text recognition, medication recognition, barcode or
other code reading of
one or more unique identifiers from the administration apparatus, pill bottle
or the like, or other
appropriate medication recognition scheme. One or more confidence level
measurements may be
employed, such as that described in co-pending application 13/110,500, US
Patent No. 9,665,767,
filed May 18, 2011 to Guan et al., titled Method and Apparatus for Pattern
Tracking.
[033] In addition to recognizing the pill or other medication, the system may
preferably
track how patient holds the pill or other medication, and further may track
continuous motion
from hand to mouth to confirm that no suspicious action has taken place. Thus,
the system may
also check consistent hand usage between identification of the pill and
placing the pill in the
mouth of the user. In addition to tracking the motion of the hand, if the user
is to take the pill out
of a blister pack, the user may track a motion sequence for tracking complete
gestures on screen
from taking out of the pill bottle or blister pack all the way to placing in
mouth / ingesting,
drinking water, showing and tracking water go down, swallowing, and even
showing empty
mouth so that any deviation from the desired sequence may be identified as a
potentially
suspicious activity. Furthermore, the system may view the blister pack to
determine if the correct
number of pills have been removed, that the correct number of pills are
remaining, and whether
other pills or the like have been removed that should not have been removed.
Such suspicious
activity may be used to automatically flag potentially suspicious activity.
13
CA 2902215 2020-03-09
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
[034] Thereafter, the patient may be instructed to administer the medication
in the
prescribed manner. One or more of these administration sequences may comprise
confirmation
that a user has placed a pill in their mouth (ingested the pill) through
visual confirmation of
location, confirmation that the user has properly used an inhaler device
through visual
confirmation of position and/or audio confirmation of actuation, confirmation
that the user has
properly used an injectable device through visual confirmation of position
and/or audio
confirmation of actuation. Thus, for an oral administration, the user may be
asked to place the
pill in their mouth, show the pill in their mouth to the camera to confirm its
presence, drink a
glass of water, and show an empty mouth to the camera to confirm the pill is
gone. The user may
be asked to change the position of their tongue relative to the pill to
provide a more complete
view of the pill in the mouth of the user to the camera. For example, if the
user only presents the
top of their tongue to the camera, and refuses to show under their tongue, a
suspicious activity
may be flagged. Improper tilting of the glass of water, or the head of the
user when drinking the
water may be further automatically recognized and provided as potentially
suspicious activity,
thus changing the state of the user and perhaps changing a level of scrutiny
for reviewing activity
of' the user. Swallowing motions may also be confirmed, the absence thereof
providing further
evidence of potentially suspicious or malicious behavior. For an inhaler, the
user may be asked to
properly position the inhaler and show the positioning to the camera, to
actuate the inhaler in
view of the camera, to breathe in view of the camera, and to hold their breath
for a predetermined
period of time in front of the camera. Different sounds, for example different
frequencies
emitted from the inhaler, may suggest that the user may be blocking the
aerosol or power of the
inhaler with their teeth, either in error or on purpose. Such incorrect
positioning may suggest
incorrect placement, and therefore incorrect medication administration. For an
injectable
medication the user may be asked to show the medication to the camera, to
place the injectable
medication adjacent the proper body part to receive the injection in view of
the camera, to
actuate the injectable medication in view of the camera, and the like.
[035] During this administration, monitoring of the various indicated
potentially
suspicious activities may be provided. Thus, as described above, a
determination of whether the
head or face of the patient leaves the field of view of the camera may be
determined, and as will
be described below, a state of the user may be changed based upon this or
other suspicious
activities noted in accordance with this application. Other suspicious
activities that may be
14
81790705
tracked may include the patient covering their mouth with their hand during
medication
administration of a pill based medication, other body movements indicating an
attempt to remove
the medication or the like. Furthermore, audio recognition may also be
employed to determine
whether the patient is spitting out a pill, for example, or to assist in
determining whether an
inhalable or injectable medication has been properly actuated. Each of these
features may be
preferably monitored over time so that while a single indication of
potentially suspicious behavior
provides important information, continued performance of such suspicious
behavior will provide a
more complete picture of attempts to trick the system, thus allowing for
intervention to identify
the patient, and address the suspicious behavior. Different actions performed
on the part of the
user may allow the user to be classified into one or more states, the state
being changed based
upon one or more actions they may perform over time. Such a state machine may
be performed in
accordance with one or more features as described in copending US Patent
Application Serial
No, 13/189,518, US Patent Publication No. US2012-0316897, filed July 24, 2011
to Hanina et al,
titled Method and Apparatus for Monitoring Medication Adherence. The
medication
administration states noted therein may be employed to classify suspicious
users based upon their
actions.
[036] If a medication is being used to treat a certain symptom, therefore in
accordance
with one or more embodiments of the invention, the system may be employed to
measure visually
if that symptom is improving. For example, concentration, speed to complete
tasks, motor control
etc. If symptoms or behavior (eye movement still erratic for example) fail to
improve, this may
suggest that the user is not taking their medication. Mood may also be
measured and monitored as
a signal of whether the user is taking their medication. Various actions may
once again be used to
classify a user in one or more medication administration states, thus
suggesting a level of review
and follow up that may be required by the system to follow up with the user.
[037] Additionally, determination of movement of the imaging device may be
determined,
through analysis of information provided in the field of view thereof, or
through the use of one or
more gyroscopes or accelerometers thereon. Thus, if the user tampers with the
position of the
imaging device or camera, it will be known and the monitor or healthcare
provider may be
automatically notified. Other clues, such as changes in a background setting
at
CA 2902215 2020-03-09
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
one or more critical times of medication administration, or other indications
of movement of the
device, or the like.
1038] After confirmation or failure of confirmation of such administration,
the patient
may be provided with a progress report regarding how they have performed over
time, and
further providing encouragement for future adherence. Additionally, notice of
a next
administration time may be provided, along with one or more messages from a
healthcare
provider regarding protocol changes, or other desired information.
Furthermore, various
detected instances of potentially suspicious behavior may be reported to a
healthcare provider or
the like via a dashboard reporting general adherence information along with
the noted suspicious
activity information. A warning may also be visually shown to the patient that
unusual activity
has been detected. They may be told that incorrect use of the medication or
protocol may result
in their study coordinator / healthcare provider being contacted, their being
removed from the
trial, or there may be an additional level of monitoring applied to the
patient.
1039] Therefore, in accordance with the invention, confirmation of patient
adherence to
the prescribed administration schedule for the medication as prescribed by the
clinical trial or
other prescription regimen may be determined, while suspicious or malicious
behavior may be
identified. While such confirmation may take a number of forms, in accordance
with the
invention, a preferred method for such confirmation may include capturing a
video and audio
sequence of the patient actually administering the medication. In a further
preferred method,
such a sequence for such confirmation may include employing a facial
recognition sequence or
other biometric confirmation that a particular patient is in fact receiving
treatment, but may also
provide for the ability to obscure the face or other identifying feature of a
user, simplify a
displayed image through rotoscoping or the like, or otherwise encrypt such
information to allow
for the storage and use of such images while protecting the identity of the
patient, a technique
that may be beneficial when a medication administration manager is providing a
general report
about a clinical trial, and not trying to remedy a situation with a particular
patient, or in particular
in a public health or disease management scenario. Activity recognition,
gesture recognition,
computer vision processing or other feature for determining whether a
particular subject
movement meets a predefined movement sequence may be employed to be sure that
the patient
is properly taking prescribed medication. This same gesture recognition may
also be employed
to determine suspicious or malicious behavior on the part of the user, as
described above. Audio
16
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
recognition may also be employed to determine suspicious noises such as
coughing noises or the
like at one or more critical key moments associated with medication
administration. For
example if coughing consistently occurs routinely after the user has pace a
medication pill in
their mouth, then the patient may be escalated to a state providing for a
higher level of scrutiny
of review during the administration process over time. Finally, measurement of
time on task,
indicative of an amount of time required for the user to perform one or more
prescribed steps
may also be employed to aid in determining suspicious behavior. This time on
task may be
measured across many different devices and instances of use of the apparatus
to determine one or
more trends that may be interesting, and may be used to determine suspicious
behavior. For
example, time on task may be employed to determine miming of an activity that
the user I not
really performing the medication administration steps as required.
[040] Referring next to Figure 2, a method in accordance with an embodiment of
the
present invention for performing audio and video capture and recognition of
adherence to a
prescribed protocol, and for determining one or more indications of suspicious
or malicious
activity, is described, as set forth in steps 130 and 135 of Figure 1. In
Figure 2, a patient may
first log into the system of the invention at step 205, employing the facial
recognition, biometric
recognition, password entry, voice recognition, or other patient
identification method, and at step
210 proper medication is preferably confirmed as noted above, through the use
of bar code
reading, text recognition, visual recognition employing video or still image
recognition, or other
medication recognition technique as described above. The patient may be
reminded to log onto
the system to take their medication through any type of reminder, such as a
text message, email,
phone call, automated alarm or the like. Processing then passes to step 225
where the user may
be prompted to perform one or more predetermined actions, the video and/or
audio of each of
these actions preferably being captured. Video capture analysis may then begin
at step 230, such
analysis comprising analysis of the newly captured video and/or audio. At step
235 it may be
determined whether the action has been properly captured, and whether the
captured action has
been properly analyzed by the system in one or more manners as will be
described below.
[041] If it is determined that administration of the medication did not take
place
properly, processing may return to step 225 and the user may be once again
prompted to perform
the action. (Of course, if this process involves actual administration of
pill, film, inhaler,
injectable medication, or any other medication, it may not be proper to
request re-performance of
17
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
the action, unless it can be determined that the user did not actually
administer the medication.)
If the action has been properly performed and is able to be analyzed,
processing ends at step 250
where the various captured video sequences are stored. These stored sequences
may also be
made available for human review and involvement, when it is detei _____ mined
that this would be
beneficial, and may further preferably be employed to determine suspicious or
malicious activity.
Areas of interest for review may be highlighted and marked as potentially
including suspicious
or incorrect activity. Percentage estimates of risk may be automatically
provided by the system
based on risk algorithms that are generated by the state machine, and based
upon a state into
which the user may have been placed based upon their prior activity, and one
or more learned
activities from a plurality of users.
1042] Analysis of the captured video in order to determine suspicious or
malicious
behavior will now be described in greater detail. As is shown in Figure 3, a
box 410 may be
provided on a display viewable by a patient using the system. Indicator box
410 may further be
provided as a circular or other appropriate indicator. Indicator box 410 may
be further hidden
from the view of the user. Alternatively a bezel or other edge of a
smartphone, tablet computer,
other mobile device, computer or the like may be used as a reference, i.e. as
the "box." A
representation of the patient's face may be shown in a position relative to an
optimal filming
position for the use of the system. Thus, while facial representation 400a is
properly positioned,
facial representation 400b is positioned to the left of indicator box 410, and
facial representation
400c is positioned down and to the right of indicator box 410. The inventors
of the present
invention have determined that one indicator of suspicious or malicious
behavior is the face of
the patient leaving the field of view either a great number of times, and/or
at one or more crucial
times. Thus, if the patient's face leaves the display after placing a pill in
their mouth, for
example, but before showing that they have swallowed the pill, it may be that
the patient is
spitting out the pill. If their face leaves the display for one day, this may
simply be user, but if it
is determined that the patient's face is leaving the display on a consistent
basis over time,
suspicious or malicious behavior may be determined. Therefore, positions 400b
and 400c may
be designated as suspicious positions, and that upon this position being
captured for a particular
user at critical times over a number of administrations may result in flagging
the participant for
further review. Thus, an automated system for determining possible suspicious
or malicious
18
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
behavior is provided. The system may report such activity, or alternatively
flag the patient to be
more carefully looked after going forward.
1043] In an alternative embodiment, a healthcare provider may have a reason to
suspect
a particular patient is acting suspiciously or maliciously, either by
reviewing a recorded video of
the patient taking their medication, or for any other reason. In this
situation, it is contemplated in
accordance with this embodiment of the invention that the healthcare provider
is preferably able
to indicate that the patient should be subject to an increased scrutiny. This
indication may be
made through a dashboard in which the healthcare provider can select a radio
button or the like
thus indicating this increased level of scrutiny, or otherwise make such an
indication. Once
flagged for additional scrutiny, a threshold for automatically determining
suspicious or malicious
behavior, such as the number of times a patient is able to have their face
leave the screed before a
warning is provided to the healthcare provider. Of course, this increased
scrutiny may be applied
to any of the suspicious or malicious activity recognition sequences described
in accordance with
this application, or to any other recognition sequences that may be employed.
[044] Other visual motions or gestures that may be monitored for suspicious
activities
may include the moving of a hand to the patient's mouth after showing a pill
in the mouth of the
patient, indicating that the patient may be removing the pill. Once again, a
one off situation may
not be problematic, while consistent movement of the hand of the user in front
of the mouth at a
particularly sensitive time may warrant additional scrutiny, and a warning
about suspicious or
malicious behavior. Other movements of one or more body parts of the patient
may also be
included. Furthermore, audio indication of performing suspicious or malicious
activity may be
monitored, such as monitoring the user spitting out the pill. Such monitoring
may automatically
be performed based upon audio information, video information, or both. Other
indications of
malicious activity may include tracking a pill spit or thrown by the user
across a screen, etc.
Additionally, the pill or other medication may appear deformed to the camera.
This may be from
misuse or tampering by the user., and may indicate melting, tampering, cutting
or opening the
pill or tablet, r even emptying a capsule of its contents of medication before
ingestion. Alignment
of the portions of a capsule may also be analyzed. Thus, any noted
deformation, color change,
marking change or the like may be automatically determined by the system and
reported to one
or more monitors or key stakeholders.
19
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
[045] If a monitor or other healthcare provider is provided a video review of
one or
more actions performed by a user of the system, it is contemplated in
accordance with one or
more embodiments of the invention that the monitor may be provided with a
continuous one or
more video clips for review. It is contemplated in accordance with one or more
embodiments of
the invention that if the system determines one or more video clips may
include one or more
suspicious activity actions, these video clips may be annotated automatically
by the system, a
risk estimate preferably being provided by the system, and correlated in
accordance with a state
of the user, the risk profile changing as the user moves through one or more
different user states.
Upon review of these video clips by the monitor or other healthcare provider,
the user may
further annotate the video by indicating further information along a timeline
of video
timestamps. This annotation is preferably further stored with the video for
others to see, or for
further analysis by the computer system in accordance with one or more
embodiments of the
invention. Based upon these annotations, and one or more other indicators of
suspicious or
malicious activity, one or more reports may be generated and one or more key
stakeholders may
be notified. Thus, the user may be classified into one or more categories
based upon their
activity, and based upon these categories, reported or further monitored as
noted above.
1046] In addition to monitoring the movement of body parts, it is contemplated
in
accordance with the invention to monitor, either alone or in conjunction with
the movement of
the body parts, movement of one or more medication administration devices,
such as an inhaler
or injector mechanism. This may similarly apply to a cup or the like for
administering a liquid
medication, or a container for holding one or more pill, film or other oral
medications.
Therefore, as is shown in Figure 4, in addition to tracking positioning of the
patient, movement
of one or more objects indicative of suspicious or malicious behavior, either
absolutely or
relative to another body part, may be determined. In the particular embodiment
shown in Figure
4, the positioning of an inhaler, glass of water, etc. relative to the display
area, mouth, or face of
the user may be employed. Similar tracking may be employed for an injectable
medication
delivery device, in accordance with yet another alternative embodiment of the
invention. As is
shown in Figure 4, an inhaler 500 may be determined to be properly positioned
in a box 522, the
box being green, for example, as in the description of Figure 4. Of course,
the box need not be
shown to the patient, in the manner noted above with respect to Figure 3. Such
an object,
however, is more likely to be improperly positioned not only left to right and
up to down, but
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/0279fi1
also in distance to the imaging apparatus. Thus, in addition to a patient
moving the apparatus off
the display side to side or up and down, the patient may also move away from
the imaging
device in an attempt to trick the system. In such a manner the patient may be
attempting to
capitalize on one or more limitations of the imaging device, such as the
resolution thereof, low
light positions, and the like, and any affect such resolution might have on
the ability of the
imaging device to identify shape, color text or other coding, or the like
associated with the object
being imaged. Thus, if positioned too far away from the imaging apparatus, a
sequence of boxes
510, 511, 512 and a small representation of inhaler 500 may be provided to
alert the user to move
the inhaler closer, or may be employed, while invisible to the patient, to
determine whether an
action has been properly determined as suspicious or malicious. Thus, if it is
determined that the
inhaler is not only too far away, but off center, boxes 520, 521, 522 may be
provided (either
visibly or not) to tack the position of the inhaler to determine any potential
suspicious or
malicious movements on the part of the patient.
1047] Similar functionality may be provided for monitoring the position of an
injectable
apparatus relative to a user body part and display of the imaging apparatus to
receive the
injection, including relative angle and distance to the body part. Thus, as
with the facial
movement noted above with respect to Figure 3, movement of the medication
administration
apparatus (inhaler or injectable) off the display (and thus out of view of the
imaging apparatus),
or away from the imaging apparatus to a point where the details of the
medication administration
apparatus are no longer discernible, may be monitored, and if performed
multiple times over a
predetermined timeframc, suspicious or malicious intention may be determined.
1048] Furthermore, as is shown in Figure 5, in addition to using absolute
positioning of
a medication administration apparatus, it is contemplated in accordance with
the various
embodiments of the invention that the movement of a medication administration
apparatus 700
may be tracked, and thus any suspicious or malicious looking movements of the
apparatus 700
may be determined. Thus, if the patient moves the apparatus too quickly,
towards the edge of a
field of view, towards an incorrect body part, faces the apparatus away from
their mouth, or the
like, suspicious or malicious behavior may be determined even thought the
apparatus remains in
the field of view at all times. Thus, as is show in Figure 5, an
administration apparatus 700 is
indicated to be reoriented from a horizontal to a vertical orientation through
movement in the
direction noted by arrows A. A set of guidance tracks 710a, 710b may be
displayed to a patient,
21
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
or may be maintained apart from the patient display, and successive apparatus
positions and
orientations may be determined relative to the guidance tracks. As the
apparatus moves along
the proscribed path, proper location and orientation may be determined, while
looking for any
irregular movement possibly indicating suspicious or malicious behavior on the
part of the
patient. Thus, in accordance with an embodiment of the invention, if a virtual
path is not
properly followed by the patient moving such a medication administration
apparatus, suspicious
or malicious behavior may be determined
[049] As noted above, color and/or audio sequences may also be employed.
Similar
positioning information may be processed relative to an injectable medication.
[050] In addition to determining suspicious or malicious activities taking
place within a
prescribed field of view of an imaging device, and during a time for
monitoring medication
adherence, various additional areas and times may be monitored in accordance
with a preferred
embodiment of the invention. In particular, it may be possible to continue to
record before and
after a medication administration time, thus attempting to determine if a user
removes a pill from
their mouth after they think monitoring has been completed, or before
processing starts. If a user
is to take multiple pills, for example, it is possible to continue to monitor
the user between the
administration, ingestion and the like of pills, even if the display is
providing visual indications
of which pill to take next, and is not displaying the face of the user. In
addition, audio recording
may be employed during these times to determine whether any sounds made by the
user may be
indicative of suspicious or malicious behavior. Such audio sounds may be
indicative of the user
spitting out a pill, actuating an inhaler or injectable device or the like.
This monitored audio and
video information may be automatically processed, in accordance with one or
more other
features of the invention, to determine whether this material provides
additional evidence of
suspicious or malicious behavior.
1051] Finally, it may also be desirable to record a video field of view wider
than that
presented to the user n a display screen. Thus, if a wide angled view of a
scene is imaged, a
standard vide may be displayed to the user, and thus a user attempting to
trick the system may
think they are out of the field of view of the camera because they are not
shown in the display,
and may thus perfolin a suspicious or malicious act. If this act takes place
within the field of
view of the camera, even if not shown on the display, it may be analyzed or
suspicious or
malicious behavior.
22
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
[052] Therefore, in accordance with various embodiments of the invention,
because a
video image of the patient actually administering an inhalable or other
medication (or other
method of medication administration, including but not limited to injections,
dialysis, and any
other medication administration procedure) may be captured and analyzed,
analysis for
suspicious or malicious behavior may be performed. As compared to a self
reporting system or
other available medication monitoring system, such as electronic medicine
bottle caps, the
various embodiments of the present invention are the only system that allow
for the
determination of one or more users trying to trick the system. This is because
rather than simply
relying on the patient to state that a particular medication was administered,
the system makes an
automated determination of proper administration. Through the accumulation of
data, various
motions, movements or sounds may be associated with suspicious or malicious
behavior. It is
only the various embodiments of the present invention that allow for this
independent analysis of
user activity.
1053] Such a video image may be captured or stored in any appropriate format
given a
selected type of activity or gesture recognition that is employed in
accordance with a particular
embodiment of the invention. Such may include full video, biometric data
points, recording of
movement of an article, such as a bracelet or the like, affixed to the patient
or administrator, use
of mapping to provide a stick figure or other body movement tracking
technique, or gesture or
activity recognition to determine movement or the like. The user may be
encouraged to use a
particular sequence of movement to be confirmed that they are properly
administering the
medication according to the protocol, thus reducing the possibility of the
potential appropriate
movements considered to be "correct." Or, as noted above, capture of
customized video
sequences may be performed so that the user is more likely to repeat these
same actions. Indeed,
various instructional videos or other appropriate training may be provided to
a user to insure they
properly administer the medication. Thus, the level of water before and after
drinking may be
automatically analyzed, determination of one or more additional pills having
been removed from
a blister pack may be automatically performed, or injectables or inhalables
may be automatically
monitored to confirm consistent use, all through visual analysis of collected
video sequences.
[054] Once the video sequences are recorded and stored, preferably in a HIPAA
compliant manner, further analysis may be performed thereon. For example, time
on task may
be measured for each portion of the medication administration sequence to be
performed. In
23
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
such a manner, it may be possible to determine how long a particular user
takes to perform a
portion of the medication administration sequence. Patterns may be determined
to identify users
who perform suspicious or malicious activity. For example, certain actions may
be performed
too quickly and uniformly over time, indicating a problem. As an example, in
one particular
embodiment of the invention, the user may be asked to drink a glass of water
after ingesting a
pill. If the time to drink the water is too consistently too fast, this may
give evidence that the
user is in fact not drinking the water, but rather is faking it to trick the
system. Other time on
task measurements may also be employed. Time stamp markers may thus be
captured to confirm
that the user is taking their medication at appropriate times and a number of
times a user has
taken a particular medication, to confirm whether there are substantial delays
between instruction
and administration, or for any other time sequence determination that may aid
in determination
of suspicious activity. Furthei more, other behavioral markers, such as, by
way of example only,
shaking hands indicating a nervousness, or other movements by a patient that
may give a hint as
to the physical or mental status thereof, and potential for trying to trick
the system may be further
employed.
[055] The system may also preferably attempt to determine whether the use is
employing a substitute or altered medication, such as if a pill appears dented
or otherwise
tampered with, has color changes, marking changes, texture changes,
translucency changes, etc.
A single or multiple consecutive or non-consecutive images may be employed in
order to
determine any changes in visual characteristics of the pill. Thus, confirming
medication identity
as well as determining deformation of, for example, a capsule (potentially
indicative of taking
the capsule apart to remove the medication) may be employed. In this
particular embodiment of
the invention, the ability to determined such malicious activity may be
dependent on the
resolution of the imaging device, the higher the resolution, the more complete
a check for these
issues may be performed. Therefore, it is contemplated in accordance with an
embodiment of
the invention that as the resolution of cameras improves, and as the
processing power of mobile
and other imaging devices increases, more complete checks may be employed.
[056] This captured adherence information, including suspicious or malicious
action
information, may be provided to a healthcare provider, clinical trial manager
or the like through a
dashboard allowing for the review of information about an individual patient,
entire population
of patients, or demographically relevant information. Such information may be
provided to
24
' 81790705
easily notify the healthcare provider, clinical trial manager or the like of
problem patients,
demographic groups, medications or the like. One or more dashboards or other
reporting
mechanisms may be employed as described in copending US Patent Application
Serial
No. 13/189,518, US Patent Publication No. US2012-0316897, filed July 24, 2011
to Hanina et al,
titled "Method and Apparatus for Monitoring Medication Adherence". Thus, any
suspicious or
malicious activity information obtained in accordance with the present
invention may be provided
to one or more individuals in accordance with one or more methods or systems
as described in the
'518 application.
[057] Further, once the information is captured to the dashboard, further
analysis may be
performed that may aid in determining potentially intentionally non-compliant
users. For example,
if the user has a high percentage of medication administration confirmations
not employing the
automated visual administration system (i.e. the user tells their healthcare
provider over the phone
that they took their medication, or otherwise indicate to the automated system
that they took their
medication without employing the automated system for confirmation), this may
be indicative of
an attempt to trick the system. Other such metrics may be employed and may
preferably be
gleaned from collected data for one or more particular patient populations. As
noted above, any
one or more of these situations may place the user in a different state or
category, and may
therefore justify additional scrutiny from the system and any monitors o
healthcare providers. The
user may be moved into or out of such state as noted above in accordance with
actions they
perform.
[058] Through the use of suspicious and malicious activity tracking as
described above, a
type of administration language may be generated, allowing for extension to
other patients, and
also allowing for interpretation of reason for differences from a predefined
sequence by a patient.
Thus, if a patient performs an action differently over time, this difference
may provide insight to a
compliance failure, or active attempt to trick the system. It is further
anticipated that analysis of
large numbers of patients will allow for a more flexible system that may
recognize more of a
patient's suspicious or malicious movements, and thus may improve the ability
of the system to
function properly and identify these malicious patients trying to trick the
system. Filters based
upon these accumulated results may be employed to further identify potentially
malicious users.
[059] Therefore, in accordance with an embodiment of the invention, a user may
be
requested to perform a predetermined sequence of actions designed to ensure
performance of
CA 2902215 2020-03-09
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
medication administration, including medication ingestion of a pill or film
based medication,
inhalable or injectable medication, or the like. During administration, one or
more actions
associated with the administration may be monitored to determine one or more
suspicious or
malicious actions. These actions may comprise movement of the user out of the
display of the
imaging device, other movements associated with suspicious or malicious
activity, such as the
hand of a user covering their mouth at a critical time, indicating possible
removal of the pill,
coughing or the like at a critical time, or the like. These actions may be
tracked over time to
determine a habit of such action but the user to identify potential deliberate
non-compliance.
Such data from multiple users may be accumulated to determine a number of
profiles that may
be indicative of intentional non-compliance, and may be applied as filters to
future action to
categorize one or more users as high risk for intentional non-compliance.
Video imaging of the
users employing the system may further be stored for future analysis in order
to determine time
on task data, for example, to screen whether any particularly noticeable
actions can be
determined, such as the drinking of a glass of water in too fast a time,
extremely consistent time
for performing each action such that it is unlikely the user is actually
taking the medication, or
the like. Other metrics, such as a large number of manual skips, or other
confirmations of
medication administration not employing the inventive video medication
administration system
may also be indicative of a profile of a user that should be considered to
further scrutiny.
[060] Further uses of the video capture sequences may also be employed,
including
video capture of responses to questionnaires about current patient states of
discomfort, informed
consent, and the like which may be further used to determine whether a user is
attempting to
further trick the system. The patient may be able to send a video message
answering one or more
questions, the answers or any other motion or characteristic of the user when
answering the
questions may be further used to determine whether the user is attempting to
trick the system.
1061] In accordance with various embodiments of the invention, when
considering
administration of an inhalable or injectable medication, analysis of adherence
video sequences
may be employed to determine a likelihood that a patient has actually
administered their
medication, including one or more gestures of administration of the
medication. Thus, based
upon video and audio cues determined related to positioning and use of the
medication
administration apparatus, it may be determined that the patient is likely to
be purposefully
improperly positioning the apparatus, and therefore the system may indicate
that it is possible
26
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
that the patient has administered the medication intentionally improperly. Low
confidence in
proper administration based upon failure to properly position the apparatus,
failure to record
audio signals indicative of proper administration or the like may be employed
to determine
whether a patient should be further watched for additional future suspicious
or malicious activity,
via the automated training system described herein, by automated contact, or
by individual
personal contact. This determination of low confidence of administration, even
if it is ultimately
determined that administration likely took place, may still be utilized to
classify the user as a
potentially malicious user requiring greater scrutiny. Thus, the user may be
classified as a good
patient, a suspicious patient, malicious patient, etc., and may further
provide one or more
notifications to a monitor, healthcare provider, or sponsor (in the case of a
clinical trial). Such
notifications may comprise a dashboard notification (noted below), a text
message, and
automatically generated report or the like. Such confidence levels may be
used, in accordance
with a desired algorithm or the like, to provide an overall picture of
medication administration by
a patients or group of patients, thus allowing for further scrutiny based upon
various
characteristics of the patient population if it appears actions are changing,
but not necessarily
waiting until a critical issue is discovered.
10621 It is further contemplated that the method and apparatus of the
invention allow for
integration with one or more audio or video conferencing systems, thus
receiving and/or
providing information there through. Thus, a user may employ a standard video
conferencing
tool or system, and have this information be coupled to a mobile or other
device being used in
accordance with an embodiment of the present invention.
1063] In addition to processing user data locally in accordance with the
imaging device,
this information may be transmitted to a remote location for processing. Thus,
in accordance
with one or more embodiments of the invention, any and all processing noted
herein may be
performed locally, remotely, on in any combination as appropriate. The local
device is therefore
provided with a processor, imaging device and the like necessary to perform
all actions
necessary, including transmission and receipt of data from a remote location.
Such transmission
preferably takes place over a secure, encrypted transmission system with
encrypted data.
Similarly, any remote computing location preferably includes storage,
processing and
transmission and reception systems sufficient to receive, process and transmit
secure data in
accordance with the present invention. Thus communication between the local
device and
27
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
remote location is provided via a publically available network, private
network or the like.
Alternatively, data may be stored on the local device and transferred to the
remote device as
desired, or all processing may take place locally without any transmission of
data to a remote
location. Additionally, any type of camera may be employed, such as a camera
included in a
mobile device, tablet or laptop computer, cellular or smart phone and the
like.
[064] It is further contemplated in accordance with one or more embodiments of
the
invention that data provided from one or more of the above noted systems,
sensors, cameras or
the like may be combined with data from any one or more others to provided
further information
about suspicious activity. Thus, for example, synchronization of audio and
video may provide an
even better picture of malicious or suspicious behavior.
10651 Furthermore, user of high resolution video images may also be employed.
For
example, if a user is determined to be at high risk for suspicious behavior,
at one or more critical
times during medication administration, higher resolution images may be
obtained to allow for a
greater level of scrutiny. By only retrieving these higher resolution images
at the one or more
critical times, overall processing requirements are reduced while allowing for
a more complete
review of user actions. Alternative, a plurality of captured lower resolution
images may be
combined to generate a high resolution image, using techniques such as Super
Resolution or the
like. In such a manner, higher resolution images are made available to review
more critical
portions of a medication administration sequence, while lower resolution
images can be
employed when suspicious activity is less likely.
[066] In accordance with one or more embodiments of the present invention, the
system
in accordance with one or more embodiments of the invention will learn to
recognize suspicious
activity and may preferably attribute a scoring system based on different
metrics. As noted
above, as more patients are identified, profiles will be defined by the state
machine and
confidence levels attributed to patients who demonstrate these activities.
[067] Various embodiments of the invention further include one or more
automated
processes for employing data mining/machine learning/Al techniques to provide
a smarter
system that can better classify patients, and then more easily monitor their
medication
administration over time. It is contemplated in accordance with the system
that the system will
be able to:
a) Understand the pattern of a patient with suspicious behavior
28
CA 02902215 2015-08-20
WO 2014/152828 PCT/US2014/027901
b) Classify the current patient into different categories. Such as "Good
user", "Suspicious
User -- need to be monitored", "Suspicious User -- need to notify sponsor" and
etc., thus placing
the user into a particular state and driving the engagement of the system by
the user in
accordance with one or more attributes of that class.
c) Suggest a way to the human or even automatically implement a way (such as
sending a
text message) to alert the patient about the behavior based on his usage
pattern.
[068] The system preferably also provides one or more abilities to toggle
between high
res and low res images for capturing suspicious behaviors, as noted above. In
particular this
toggling may be performed by: 1) Capturing a high resolution image or a
sequence of high
resolution images at region of interest or time of interest to help check for
suspicious behavior
either by human or machine; 2) During other time or regions, low resolution
images may be
processed to make sure that the system can provide real-time feedback; and 3)
capture low res
images, and create a high res image from a serious of low res images for
analysis on the server
using techniques such Super Resolution.
[069] Data captured by different sensors may also be fused to make a judgement
whether the patient is suspicious. The fusing of different sources of data may
include data
captured by the system and other systems or patient information or history.
1070] Therefore, in accordance with the invention, a method and apparatus are
provided
that allow for the automated confirmation of adherence to administration
protocol for
medication, and provide for a most sophisticated method for confirming and
studying methods of
administration of such prescription medication.
1071] It will thus be seen that the objects set forth above, among those made
apparent
from the preceding description, arc efficiently attained and, because certain
changes may be
made in carrying out the above method and in the construction(s) set forth
without departing
from the spirit and scope of the invention, it is intended that all matter
contained in the above
description and shown in the accompanying drawings shall be interpreted as
illustrative and not
in a limiting sense.
[072] It is also to be understood that this description is intended to cover
all of the
generic and specific features of the invention herein described and all
statements of the scope of
the invention which, as a matter of language, might be said to fall there
between.
29