Note: Descriptions are shown in the official language in which they were submitted.
CA 02902709 2015-08-26
WO 2014/150256
PCT/US2014/022733
COMPOSITIONS AND METHODS FOR THE MODULATION OF
HEMOGLOBIN (S)
FIELD OF THE INVENTION
100011 This invention provides pharmaceutical compositions for the allosterie
modulation
of hemoglobin (S) and methods for their use in treating disorders mediated by
hemoglobin
(S) and disorders that would benefit from tissue and/or cellular oxygenation.
STATE OF THE ART
100021 Sickle cell disease is a disorder of the red blood cells, found
particularly among
those of African and Mediterranean descent. The basis for sickle cell disease
is found in
sickle hemoglobin (1-lbS or hemoglobin (S)), which contains a point mutation
relative to the
prevalent peptide sequence of hemoglobin (Hb).
100031 Hemoglobin (fib) transports oxygen molecules from the lungs to various
tissues
and organs throughout the body. Hemoglobin binds and releases oxygen through
confbrmational changes. Sickle hemoglobin (HbS) contains a point mutation
where
glutamic acid is replaced with va.line, allowing HbS to become susceptible to
polymerization
to give the HbS containing red blood cells having their characteristic sickle
shape. The
sickled cells are also more rigid than normal red blood cells, and their lack
of flexibility can
lead to blockage of blood vessels. U.S. 7,160,910 discloses compounds that are
allosterie
modulators of hemoglobin. However, a need exists for additional therapeutics
that can treat
disorders that are mediated by I-lb or by abnormal Hb such as HbS.
SUMMARY OF THE INVENTION
100041 This invention relates generally to compositions suitable as allosteric
modulators of
hemoglobin (S). In some aspects, this invention relates to methods for
treating disorders
mediated by hemoglobin (S) and disorders that would benefit from tissue and/or
cellular
oxygenation.
100051 In further aspects, this invention relates to a pharmaceutical
composition comprising
from about 1 mg to about 10 g of a compound selected from the group consisting
()la
compound in Table 1 and at least a pharmaceutically acceptable excipient.
carrier or diluent.
100061 In still further aspects, this invention relates to a blood composition
comprising
blood and one or more compounds selected from the group consisting of a
compound in
CA 02902709 2015-08-26
WO 2(114/15(1256
PCT/US2014/022733
Table I, wherein said blood is comprised of red blood cells and plasma, and
wherein at least
20%, preferably at least 30%, more preferably at least 50%, yet more
preferably at least 80%,
and still more preferably at least 90% of said one or more compounds in the
blood will bind
to red blood cells containing hemoglobin (S) under physiological conditions.
100071 In further aspects of this invention, a blood composition is provided
wherein said
composition comprises a compound in Table 1 and blood, said blood comprising
red blood
cells comprising hemoglobin, at least a part of the hemoglobin being
hemoglobin (S), and at
least a part of said hemoglobin (S) is present as an adduct with said
compound.
100081 In another aspect of the invention, provided herein are adducts of
hemoglobin (S)
and a compound selected from the group consisting of a compound in Table I.
[00091 In a preferred embodiment, a compound selected from the group
consisting of Table
I. present in the adduct of the red blood cells and Hb-S, has a volume of
distribution between
the vascular space and the extra-vascular space under steady state conditions
such that at least
a portion of the compound remains in the vascular space as part of said
adduct. In one aspect,
at least 20%, preferably at least 40%, yet more preferably at least 60%, still
more preferably
at least 80% and even more preferably at least 95% of said compound remains in
the vascular
space as part of said adduct.
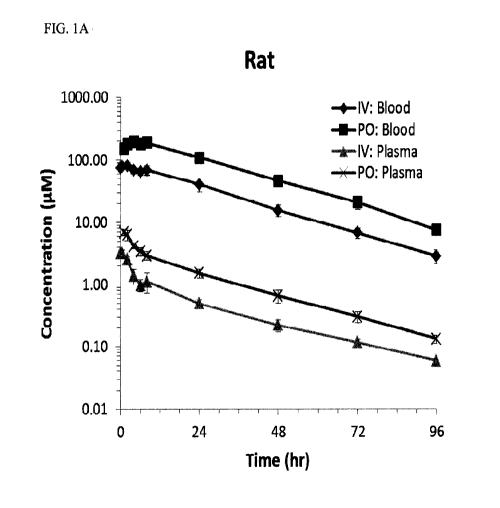
100101 FIG. I graphically illustrates the high oral bioavailability, sustained
exposure and
dramatic RBC partitioning following single dose of GBT440. Certain relevant
pharmacokinetic parameters are tabulated below:
(;I31-440 Rat Dog Monkey
IV Dose (mg/kg) 1.6
PO Dose (mg/kg) 7.2 2.5 4.25
Oral bioavai lability (% 59.8 36.6 36.1
Whole Blood/Plasma Ratio 69.0 74.4 70.9
100111 In a further aspect, the invention relates to a method for treating a
subject in need
thereof, comprising administering to the subject an effective amount of a
pharmaceutical
2
CA 02902709 2015-08-26
WO 2014/150256
PCT/US2014/022733
composition according to the invention. As used herein, a subject refers to a
mammal, such
as a primate, preferably a human.
100121 These and other aspects of the invention are further described below.
BRIEF DESCRIOPTION OF THE FIGURE
100131 FIG. I in parts A, B. and C graphically illustrates the high in vivo
oral
bioavailability, sustained exposure and the high RBC partitioning following
single dose of
GB1440.
DETAILED DESCRIPTION OF THE INVENTION
Definitions
100141 it must be noted that. as used herein and in the appended claims, the
singular forms
"a", "an", and "the" include plural referents unless the context clearly
dictates otherwise.
Thus, for example, reference to "a solvent" includes a plurality of such
solvents.
100151 As used herein, the term "comprising" or "comprises" is intended to
mean that the
compositions and methods include the recited elements, but not excluding
others.
"Consisting essentially or when used to define compositions and methods, shall
mean
excluding other elements of any essential significance to the combination ibr
the stated
purpose. Thus, a composition or process consisting essentially of the elements
as defined
herein would not exclude other materials or steps that do not materially
affect the basic and
novel characteristic(s) of the claimed invention. "Consisting or shall mean
excluding more
than trace elements of other ingredients and substantial method steps.
Embodiments defined
by each of these transition terms are within the scope of this invention.
100161 Unless otherwise indicated, all numbers expressing quantities of
ingredients,
reaction conditions, and so forth used in the specification and claims are to
be understood as
being modified in all instances by the term "about.- Accordingly, unless
indicated to the
contrary, the numerical parameters set forth in the following specification
and attached
claims are approximations. Each numerical parameter should at least be
construed in light of
the number of reported significant digits and by applying ordinary rounding
techniques. The
term "about" when used before a numerical designation, e.g., temperature,
time, amount, and
concentration, including range, indicates approximations which may vary by (
) or - ) 10
%, 5 % or I %.
3
CA 02902709 2015-08-26
WO 2014/150256
PCT/US2014/022733
100171 The term "pharmaceutically acceptable" refers to safe and non-toxic for
in vivo,
preferably, human administration.
100181 The term "pharmaceutically acceptable salt" refers to a salt that is
pharmaceutically
acceptable.
100191 The term "salt" refers to an ionic compound formed between an acid and
a base.
When the compound provided herein contains an acidic functionality, such salts
include,
without limitation, alkali metal, alkaline earth metal, and ammonium salts. As
used herein.
ammonium salts include, salts containing protonated nitrogen bases and
alkylated nitrogen
bases. Exemplary, and non-limiting cations useful in pharmaceutically
acceptable salts
include Na, K, RA), Cs, NH4, Ca, Ba, itnidazolium, and ammonium cations based
on naturally
occurring amino acids. When the compounds utilized herein contain basic
functionality, such
salts include, without limitation, salts of organic acids, such as caroboxylic
acids and sulfonic
acids, and mineral acids, such as hydrogen halides, sulfuric acid, phosphoric
acid, and the
likes. Exemplary and non-limiting anions useful in pharmaceutically acceptable
salts include
oxalate, maleate, acetate, propionate, succinate, tartrate, chloride, sulfate,
bisalfate, mono-,
di-, and tribasic phosphate, mesylate, tosylate, and the likes.
100201 The term "whole blood" refers to blood containing all its natural
constituents,
components, or elements or a substantial amount of the natural constituents,
components, or
elements. For example, it is envisioned that some components may be removed by
the
purification process before administering the blood to a subject.
100211 The terms "treat", "treating" or "treatment". as used herein, include
alleviating,
abating or ameliorating a disease or condition or one or more symptoms
thereof, preventing
additional symptoms, ameliorating or preventing the underlying metabolic
causes of
symptoms, inhibiting the disease or condition, e.g., arresting or suppressing
the development
of the disease or condition, relieving the disease or condition, causing
regression of the
disease or condition, relieving a condition caused by the disease or
condition, or suppressing
the symptoms of' the disease or condition, and are intended to include
prophylaxis. The terms
also include relieving the disease or conditions, e.g., causing the regression
of clinical
symptoms. The terms further include achieving a therapeutic benefit and/or a
prophylactic
benefit. By therapeutic benefit is meant eradication or amelioration of the
underlying disorder
being treated. Also, a therapeutic benefit is achieved with the eradication or
amelioration of
one or more of the physiological symptoms associated with the underlying
disorder such that
4
CA 02902709 2015-08-26
WO 2014/150256
PCT/US2014/022733
an improvement is observed in the individual, notwithstanding that the
individual is still
afflicted with the underlying disorder. For prophylactic benefit, the
compositions are
administered to an individual at risk of developing a particular disease, or
to an individual
reporting one or more of the physiological symptoms of a disease, even though
a diagnosis of
this disease has not been made.
100221 The terms "preventing" or "prevention" refer to a reduction in risk of
acquiring a
disease or disorder (i.e., causing at least one of the clinical symptoms of
the disease not to
develop in a subject that may he exposed to or predisposed to the disease but
does not yet
experience or display symptoms of the disease). The terms further include
causing the clinical
symptoms not to develop, for example in a subject at risk of suffering from
such a disease or
disorder, thereby substantially averting onset of the disease or disorder.
100231 The term "effective amount" refers to an amount that is effective for
the treatment of
a condition or disorder by an intranasal administration of a compound or
composition
described herein. In some embodiments, an effective amount of any of the
compositions or
dosage forms described herein is the amount used to treat a disorder mediated
by hemoglobin
or a disorder that would benefit from tissue and/or cellular oxygenation of
any of the
compositions or dosage forms described herein to a subject in need thereof.
100241 The term "carrier" as used herein, refers to relatively nontoxic
chemical compounds
or agents that facilitate the incorporation of a compound into cells, e.g.,
red blood cells, or
tissues.
Compounds
100251 A compound utilized herein is selected from Table 1 below or an N-oxide
thereof,
or a pharmaceutically acceptable salt of each thereof. The N-oxides of the
compounds set
forth below are believed to be novel and each of the N-oxide compounds and
their salts
thereof form a further embodiment of the invention.
100261 The compounds in Table I represent compounds capable of meeting one or
more
biological criteria for activity as measured based on one or more biological
parameters such
as, but not limited to, partitioning between red blood cells and blood plasma.
volume of
distribution, oxygen equilibrium curves, oxygen affinity and polymerization
activity.
CA 02902709 2015-08-26
W02(114/15(1256 PCT/US2014/022733
Table I
Compound 1 Chemical Structure Chemical Name
1 =
I Number !
;
I , ;
....................................................................... i
1 i N"---<:-. 2-
methoxy-51124 1 H-pyrazol-5-yOpyridin-3- i
I
1 H
i
N. .,-11,,õ..-,.* yllmethoxyjpyri di ne-4-carbaldehyde
I,
I NJ 1
--,
0 0 I I
I I
I I ,
1
T
i 0 ss.
1 .
i
2 I 2-methoxy-5-[[5-(2-rnethylpyrazol-3- I
I
N ."... N'N yl)pyridin-3 -yl imethoxy]pyridine-4-
I Ik
..--- carbaldehyde
I .
0 0 I
I
! 1
1
i
1 0',
3 1
N---:--;--. 2-inethoxy-5-R24 1-methylpyrazol-3-
I ..--" i yl )pyrid in-3 -ylimethoxylpyri d ine-4-
N¨N
carbaldehyde
0 0
I
/ 1 I
,--r)-)1 i
,
I N,...
[1 0N. .
I 4 I N ---:s"-',`,..1 2-metboxy-54[2-(2H-ietrazol-5-
y1)pyridin-3 -
N.,õ....,k.i,j I vl]methoxylpyridine-4-carbaldehyde
. HN: "I
:
N::N
0 0 '
1 '
T 1
= 0 1
-,.
I I
.............................. =
6
CA 02902709 2015-08-26
WO 2(114/15(1256 PCT/US2014/022733
,
2-methoxy-5-[[2-(4-methyl - 1H- p) ra/o1-5-
:
I .
= ..---- 1 yl)pyrid in-3-A methoxy}pyridinc-4-
carbakiehyde i
i 1 N-NH !
I i
! 0 0 I
I
N---) 1 ,
1 i
. 1 i
: N'..y#
I
0 I
I 6 N "...-- I 2-methoxy-5-[(2-pyrazol- I -ylpyridi
n-3-
N A,....-:::- . yDinethoxy]pyridine-4-carbaldehyde
I
. 0 0
I r.--"k=}1 I
N'...õy1 I
1
5.1[24 I ,5-dimethylpyrazol-4-yljpyrid in-3- .
..----
N ylimethoxy]-2-methoxypyridine-4-
N i
--- I
I earbaidehyde
0 0
I
r-)NN)ji I
Yi
i 1 o
i ___________
8N-----z-N-=... 54[2-(2-ethylpyrazol-3-yl)pyridin-3-
:
I 1 ylimethoxy}-2-inethoxypyridine-4-
\
.. carbaldehyde
1
) 0 0
1
I
NC-.).....)
I
0"...
j_I_ ............... i
7
CA 02902709 2015-08-26
WO 2014/150256
PCT/US2014/022733
9 -----... 2-methoxy-54[24.2-propaii-2-y I pyrato i-3-
1
; 1
yl)pyridin-3-yllmet.hoxy]pyridine-41-
,-,
carbaldehyde
1,_; =-== t I ====,.
. ' s..."''... 0 0 ,
. J\ H
1 N)...,....-
I I I
N--.."...- ; t ...........
2-tnethoxy-54(2-phenylpyridin-3-
1
yl)methoxylpyridine-4-carbaldchyde
i I I 0,. I 1
I
.,........!:
= 0 0 1
1
N,=,,,r
' 0,..,
I 11
1 N
2-methoxy-5-11[3-(2-propan-2-ylpyrazo1-3-
yl)pyriclin4-yl1methoxylpyridine-4- i
I ,\....7r---`----!--
carbaldehyde
NN- '-,-.
= 1
1
1 ...H...
0,...
,
12f\l`'-z-:C"--. f 2-hydroxy-64[2-(2-propan-2-ylpyrazol-3-
.
=
I 1 yl)pyridin-3-ylimethoxAbenzaldelvde
I -.....,
I
:
)
I
1
14110 .
i .
,
,
,
,
1 OH
....................................................................... 1
8
CA 02902709 2015-08-26
WO 2(114/15(1256 PCT/US2014/022733
13 N¨".''N'',. i 2-
methoxy-5-[(2-pyridin-3-ylpyridin-3- ,
i
N'.......`-'- 1 yOmethoxylpyridine-4-carbaldehyde
i
i
0 0 I
r - 1
N -,
I 1
1
1 0,,,
1
1. I4 INI 7), j . _____________
2-methoxy-54[242-(2-rnethoxyethyl)pyrazol-
(c-' '
3.111pyridin-3-yllinethoxylpyridine-4-
-'
;\ carbaldehyde
!
1 /
I
1
1 0 .
1
s.--.
15 N= 51[212-(2-hydroxyethy1)pyrazo1-3-
yllpyridin-
11 ,
.... õ..., 3-ylimeth.oxy:1-2-
methoxypyridine-4- .
carbaldehyde
1
N---NN) 0 0
HO--1 '' I
I NEY,
1 .
0
I __ 16 1 N"........::"C"1 ____________________________ 2-
methoxy-5-[[2-(2-propy1pyrazo1-3- 1
I
c...TA) yppyridin-3-yi]meth.oxyipyridine-4-
--..õ
\ carbaldehyde
, N---N\.. "=-=
1 / 0 0
I
I Ny
1
1 0 i
`...
.1
9
CA 02902709 2015-08-26
WO 2(114/15(1256 PCT/US2014/022733
.......... , ......................................... - - - - -,
2-methoxy-5-112-12-(2.2.2-
,.
N --::-
1
c
.-.... ..õ..,.....::::,-- tril1uoroethyl)pyrazol-3-yilpyriclii)-3-
- N 1
,...,....i.
1
ylimethoxylpyridine-4-carbaldehyde
. N .,
0 0 ,
i
F
1
I1 N,),,,,,,
I
I 0 1 I
I ..,
I
18 N---z".= 5412-(2-cyclobtaylpyrazol-3-y1)pyridin-
3-
.
õ,,. yljmethoxy.1-2-methoxypyridine-4-
-N
I C-s'r L, 0 0 carbaldehyde
1 N \c3
J J
1
N::=,...rõ.. 1
1 0
19 (Th 5-[[242-cyclohexylpyrazol-3-yl)pyridin-3-
! L---(/) N---:::::. yl]methoxy]-2-methoxypyridine-4-
,N carbaldehyde
N \ I
0 0 1
I
i
...................................................................... I
2 i ) rsi -'<kN 1 5-[[2[2-(cyclohexylmethyl)pyrazol-3-
1
.
yljpyridin-3-yHmethoxy1-2-methoxypyridine- i
1 -I
4-carbaldehyde
IN:N,,_ ,..õ-=
1 I
ON, ,
;
1 1
CA 02902709 2015-08-26
WO 2014/150256 PCT/US2014/022733
r"----' 5-R2-
(2-cyclopentylpyrazoT3;71)p-TriciiI73-77
1 L
N -"...-z.-'-': yljrnethoxy1-2-rnethoxypyridine-4-
= --..? I 1
. carbaldehyde
0 0 I i
(--)\ ij
!
õ,-"If 1
Nz-, '
I 0 1
!
--,
i
1
-1-) 1 51[242-(2.2-difluoroethyl)pyrazol-3-
1
I ¨ N----"-',:s=
-1.1.,.., yllpyridin-3-ylimethoxy1-2-
methoxypyridinc-
!
. 4-carbaldehyde ,
N¨N\
I I
0,,,,
23i I N ""..".'-'->-. 1 2-
methoxy-5-[[2-(2-rnethylphenyl)pyridin-3- 1
yijmethoxy]pyridine-4-carbaldehyde
i 1
i -...,......--'
0 0 I
1
1"---Y1
N,..._,,.-',, 1
1
0 I
..,õ.
,
...................................................................... i
s
I
24 0 N - - - . . > " -- - 2-methoxy-5412-(2-methoxypyridin-
3-
...õ..c- vl)pyridin-3-yrjniethoxylpyridine-4-
carbaldehyde .
1
I I 1
fYJI
, ! 11
0.,
II ............................... j 1.
11
CA 02902709 2015-08-26
WO 2(114/15(1256
PCT/US2014/022733
:
-=:, 2-rnethoxy-5-[[3-(2-propan-2-ylpyrazol-3-
.
-- N"--'"'---)
=
I' il yOpyrazin-2-Amethoxylpyridine-4-
N carbaldehyde
1._ 1
N\.0) 0
H 1
i
W..
=,..
0 =
..,.. =
_______________________________________________________________________ 1
:
I ..
26 1 N'.- ; 2-(difluoromethoxy)-51[2-(2-propan-2-
i
\C=z''-(1......f.;>1 ylpyrazol-3-yHpyridin-3-
yrimethoxy]pyridine-
4-carbaldehyde .
=
i N-N).---- 0 0 1
1
i
Nr.;;.,..:(1
i
I
0,,,..F
i I
F
1
2-(2-methoxyethoxy)-54[2-(2-propan-2-
l
.."-- I ylpyrazol-3-yOpyridin-3-
Amethoxylpyridirie- I
I \N¨N 4-carbaldehyde I
õr__ 0 .
il ,
, . ,
,
0, ......
. ,
,
28I N 21513-[(4-forrnyl-6-methoxypyridin-3-
=
vl)oxyrnethyllpyridin-2-ylipyrazol-I-yi]acctic I
I
0 0 I
i
1 110-3.\\ 0 r.""--)1 I
1 I
Ny-
I0,,...
12
CA 02902709 2015-08-26
WO 2(114/15(1256 PCT/US2014/022733
29 . ______________
N'..--""NNN. : 3-1.12-(2-propan-2-ylpyrazoi-3-
y1)pyridin-3- .
, .
I
ylimetboxylpyridine-2-carbaldehyde
i 1 N-tsi - '====0
IIN----- 0 1
)1 1
1 1 :
. i
30 N--"z"-=> ' 6-methy1-3-1[2-(2-propan-2-ylpyrazol-
3-
. yl)pyridin-3-yrlinethoxylpyridine-2-
.
I . \
I carbaldehyde
1 1 'N¨N ---,
1
1 .
H ..................................................................... i
31 .----.. i 51[24.2-hydroxypropan-2-yl)pyridin-3-
1 N ."-- I
1
--.1,.-&---P 1 I yl]metboxy.1-2-
inethoxypyridine-4-
OH
carbaldehyde
..õ
0 0
li
ekT----
1
i !
0
1 -,
1
___________ -r-
32 N'''...." I Hp. 2-(2-tnethoxyethoxy)-5-1[2-(2-
methylpyrazol-
, 3-yl)pyridin-3-yllmethoxyipyricline-4-
. !
1
i
1 carbaldehyde
w_ N ..,
\
1 j'..i'l
i
Ny
, .
.
0..
13
.......
I
.= I - .....
.= 0 . 1
,
i 1 1 1
,
1 ........ 1 .................... i
i .................................................................... 1
13
CA 02902709 2015-08-26
WO 2(114/15(1256 PCT/US2014/022733
. ; __
: 33 1 N'''''-'''''''. ' methyl 34513-1(4-tbrmy1-6-
methoxypyridili- I
,
3-yl)oxymethyllpyridin-2-ylipyrazol- I -
-0--,...1,..,-- ---,---
\ i
1 yllpropanoate
1
:
:
I
1
0 W.,
. .s......,.
;
i 1
i
1 1 0
It 1
34 N ''''l . 3-15-[3-[(4-formyl-6-methoxypyridin-3- 1
õ...yls. ..,.., yl)oxymethyllpyridin-2-y1
I .1pyrazol-1-
(\ 1
yljpropanoic acid
N¨Nõ., L.0 0
I
1 i 0 1 1
I
1
- i
1 HO NZ., I
0 .
'N. .
I
i
35 f\r---' I 3-hydroxy-5-
[t2-(2-propan-2-ylpyrazol-3-
===
yl)pyridin-3-yl]methoxyjpyridine-4-
. -..,
N
\ carbaldehyde
-...
i
I
N',...,
1
---i-
36
1 N''''''.
I 1 3-methoxy-54[2-(2-propan-2-ylpyrazol-3-
L I
yl)pyridin-3-yllmethoxylpyridine-4-
( 1
i -.....7"--C¨N -.."- carbaldehyde
\7---- 0 10
,
N.c...Y :
i 0 i
1 1 1 i
14
CA 02902709 2015-08-26
WO 2(114/15(1256 PCT/US2014/022733
.= 7 T
N ..--:-.... _ ____________
- 2-methoxy-54[244-methyl.-2-propari-2-
''',N
l
1 <
1 i vlpyrazol-3-yl)pyridin-3-
ylimethoxylpyridine-
\ 1 ' 4-carbaldchyde
N-N
. ' r ,0 9
,
i
1-1
1
Nz....:(,..-
I
1 0 1
..."
. I
...Ii
38 I N---.'" 2-hydroxy-6-1[242-(2,2,2- I
!
,
:
...-Ic.------ tritluoroethyl)pyrazol-3-yl]pyridin-3-
L
N-N) yllmethoxylben.zaldehyde
'0 0
F--kF ----"*C-r)
Ii F
11 I
.
.;....s....,,,..,..., .
1 OH
2-hydroxy-6-0-42-(3,3,3-
i õIt. 1 trifluoropropyl)pyrazol-3-ylipyridin-3-
1 1
I
1 N---N 1 ylimethoxylbenzaldehyde I
0 0 I
F 4.) 1
F
SI OH
i
¨r
40 2-(2-methoxyethoxy)-51[242-(2,2,2-
.
trifitioroethyl)pyraz.o1-3-Apyridin-3-
,
yljrnethoxy]pyridine-4-carbaldellyde
0 0
,_/...F e5...c) 1
!
i I 1
N)....,.. 1
1
0,......õ,....õ0., ..............
1
I.. ______________________________________________________________ I
CA 02902709 2015-08-26
WO 2(114/15(1256 PCT/US2014/022733
2-methoxy-54[242-(3,3,3-
:
l
. trifluoropropy)py-razol-3-yl]pyridin-3-
--, .
ylimethoxy]pyridine-4-carbaldehyde 1
0 0 1
1
! F \IF) I 1 ij
7 I
"-----".
i I
F..-
I i
N.;-...,..,õ
I 1 I i
, I ON.
i
.................................. I
1 42 N-"-....."- 2-(2-methoxyethoxy)-54[2-12-(3,3,3-
11
tritluoropropyl)pyrazol-3-yllpyridin-3-
Amethoxylpyridine-4-earbaldehyde i
I
N\) 'N. 0 io I
FF
\ ..b.
I
1
r 1 ,
F N,)
43
=
I I
I
t-
43N ---.-...'. 6-methyl-3-[[2-[2-(2,2,2-
I
...õ...9-' tritluoroethyl)pyrazol-3-yljpyri din-3-
I ' \--..,
yllmethoxylpyridine-2-carbaidehyde
N --N) `NO 0
F---1/4.-F .=-''' J 1
,
F &
1
I .
! i
44NAN%'N`-. 6-methy1-31[212-(3,3,3- '
rifluoropropyl)pyrazol-3-yljpyridin-3-
VI linethoxylpyridine-2-carbaldehyde 1
0 ,
1 Fi =NYjj
= i I
I F 1,../.,N
, I ................................................................. 1
16
CA 02902709 2015-08-26
WO 2014/150256
PCT/US2014/022733
.......... , ....................
2-11tioro-6-R242-(2,2,2-trilluoroethyl)p> razol- !
i
3-ylJpyridin-3-yl]methoxylbenzaidehyde
co o 1 I
ji i
F ---,----- ---,i 1
F
i
I="=-=-=== = F
I
__________ ---r !
46 i N'- 1. 2-fluoro-6-[[242-(3,3,3-
= .//'-'"-.71 1 trilluoropropyl)pyrazol-3-
Apyridin-3-
1
\\ i
I y-limethoxylbenzaldehyde
N-N ---, 1
0 0 :
F.
I F \i----) --.:21
i--.'"ij .
F
47 --1:-. 34[242-(2,2,2-trifluoroethyl)pyrazol-3-
yilpyridin-3-ylimethoxylpyridine-2- :
\\ carbaklehyde
N -N
0 0
LFI a) '
F ----- 1
'-.... N
I .."
48 I N'-`,.`= I 3-[[21:2-(3,3,3-trifluoropropyl)pyrazol-3-
1
'-'7=Y'''\\ I ''''' Apyridin-3-yllmethoxyjpyridine-2-
carbaldehyde
1
F F) I
I
i I
49 N '''`','" 1 3-chloro-5-[[2-(2-propan-2-ylpyrazol-3-
1
Apyridin-3-ylimethoxy-lpyridine-4-
.
:
carbaldehyde
" ).-----µ"0 0 i
I
1 ..)--...õ-ji
r 1
17
CA 02902709 2015-08-26
WO 2(114/15(1256
PCT/US2014/022733
N 2-fluoro-6-11:2-(2-propan-2-ylpyrazol-3-
yi)pyridin-3-yllmethoxyibenzaldehyde
iI
\\
N¨Ny 0
410
F
51 N ____________ 3-methy1-54[2-(2-propan-2-ylpyrazol-3-
yl)pyridin-3-y1]methoxy]pyridine-4-
.,
carbaldehyde
0
N
3-methy1-5-11.242-(2,2,2-
I
e
vit.! uoroethyppyrazol-3-ylipyridin-3-
yl]methoxy Jpyridine-4-carbaldehyde
N-N
0 0
100271 In a preferred embodiment, the compound is compound 12.
Pharmaceutical Compositions
[00281 In further aspects of the invention, a composition is provided
comprising any of the
compounds described herein, and at least a pharmaceutically acceptable
excipient wherein the
compound of Table 1 is present in the composition in an amount from 1 mg to 10
g.
100291 In another aspect, this invention provides a composition comprising any
of the
compounds described herein, and a pharmaceutically acceptable excipient.
100301 Such compositions can be formulated for different routes of
administration.
Although compositions suitable for oral delivery will probably be used most
frequently, other
routes that may be used include transdermal, intravenous, intraarterial,
pulmonary, rectal,
nasal, vaginal, lingual, intramuscular, intraperitonea], intracutaneous,
intracranial, and
subcutaneous routes. Suitable dosage forms for administering any of the
compounds
18
CA 02902709 2015-08-26
WO 2(114/15(1256
PCT/US2014/022733
described herein include tablets, capsules, pills, powders. aerosols,
suppositories. parenterals.
and oral liquids, including suspensions, solutions and emulsions. Sustained
release dosage
forms may also be used, tbr example, in a transdermal patch form. All dosage
forms may be
prepared using methods that are standard in the art (see e.g., Remington's
Pharmaceutical
Sciences, 16th ed.õA.. Oslo editor, Easton Pa. 1980).
100311 Pharmaceutically acceptable excipients are non-toxic, aid
administration, and do not
adversely affect the therapeutic benefit of the compound of this invention.
Such excipients
may be any solid, liquid, semi-solid or, in the case of an aerosol
composition, g,aseous
excipient that is generally available to one of skill in the art.
Pharmaceutical compositions in
accordance with the invention are prepared by conventional means using methods
known in
the art.
100321 The compositions disclosed herein may be used in conjunction with any
of the
vehicles and excipients commonly employed in pharmaceutical preparations,
e.g., talc, gum
arabic, lactose, starch, magnesium stearate, cocoa butter, aqueous or non-
aqueous solvents,
oils, paraffin derivatives, glycols, etc. Coloring and flavoring agents may
also be added to
preparations, particularly to those for oral administration. Solutions can be
prepared using
water or physiologically compatible organic solvents such as ethanol, 1,2-
propylene Qlycol,
polyglycols, dirnethylsullbxide, fatty alcohols, triglycerides, partial esters
of glycerin and the
like.
100331 Solid pharmaceutical excipients include starch, cellulose,
hydroxypropyl cellulose,
talc, glucose, lactose, sucrose, gelatin, malt, rice, flour, chalk, silica
gel, magnesium stearate,
sodium stearate, glycerol monostearate, sodium chloride, dried skim milk and
the like.
Liquid and semisolid excipients may be selected from glycerol, propylene
glycol, water.
ethanol and various oils, including those of petroleum, animal. vegetable or
synthetic origin.
e g., peanut oil, soybean oil, mineral oil, sesame oil, etc. In certain
embodiments, the
compositions provided herein comprises one or more of a-tocopherol, gum
(rabic, and/or
hydroxypropyl cellulose.
100341 In one embodiment, this invention provides sustained release
formulations such as
drug depots or patches comprising an effective amount of a compound provided
herein. In
another embodiment, the patch fUrther comprises gum Arabic or hydroxypropyl
cellulose
separately or in combination, in the presence of alpha-tocopherol. Preferably,
the
19
CA 02902709 2015-08-26
WO 2(114/15(1256
PCT/US2014/022733
hydroxypropyl cellulose has an average MW of from 10,000 to 100,000. In a more
preferred
embodiment, the hydroxypropyl cellulose has an average MW of from 5,000 to
50.000.
[00351 Compounds and pharmaceutical compositions of this invention may be used
alone
or in combination with other compounds. When administered with another agent,
the co-
administration can be in any manner in which the pharmacological effects or
both are
manifest in the patient at the same time. Thus. co-administration does not
require that a
single pharmaceutical composition, the same dosage form, or even the same
route or
administration be used for administration of both the compound of this
invention and the
other agent or that the two agents be administered at precisely the same time.
However, Co.
administration will be accomplished most conveniently by the same dosage form
and the
same route of administration, at substantially the same time. Obviously, such
administration
most advantageously proceeds by delivering both active ingredients
simultaneously in a
novel pharmaceutical composition in accordance with the present invention.
100361 In further aspects of the invention, one or more adducts of a compound
selected
from Table 1 that is bound to hemoglobin S is contemplated. In one embodiment,
the adduct
is formed from compound 12 and hemoglobin S.
Methods of Treatment
100371 This invention provides a method for increasing the oxygen-carrying
capacity of erythryocytes. In certain embodiments, the invention is related to
a
method of treating red blood cells or whole blood in vivo, in vitro, in situ
or ex vivo
with one or more compounds or pharmaceutical compositions of the invention by
administering or contacting said one or more compound or pharmaceutical
compositions with blood and especially blood containing hemoglobin (S).
100381 In some embodiments, a method for ex vivo storage and/or use of the
compounds and pharmaceutical compositions of the invention is contemplated in
which the compounds and/or pharmaceutical compositions are combined with
whole blood for use in procedures such as, but not limited to, autologous or
non-
autologous blood transfusions, coronary bypass surgery, and any extracorporeal
procedure involving perfusion and/or reperfusion of blood to a subject. In
certain
embodiments, the compounds and/or pharmaceutical compositions may be
combined with whole blood for storage purposes.
CA 02902709 2015-08-26
WO 2(114/15(1256
PCT/US2014/022733
100391 In another of this method aspects, this invention is directed to a
method
tor treating a subject in need thereof (e.g., sickle cell anemia) by
administering to
the subject an effective amount of a pharmaceutical composition of this
invention.
In one preferred aspect, the pharmaceutical composition comprises from about
0.1
mg/kg to about Iglu per day, more preferably, about I mg/kg/day to about I 00
mg/kg/day of a compound or compounds of Table 1.
100401 In aspects of the invention, a method is provided for increasing oxygen
affinity of
hemoglobin S in a subject, the method comprising administering to a subject in
need thereof a
therapeutically effective amount of a pharmaceutical composition of this
invention or a
blood composition comprising one or more compounds of Table L In a preferred
embodiment, the blood composition is free of hemoglobin (S).
10(3411 In aspects of the invention, a method is provided for treating a
condition
associated with oxygen deficiency, the method comprising administering to a
subject in
need thereof a therapeutically effective amount of either the pharmaceutical
or the blood
composition described above.
100421 In further aspects of the invention, a method is provided for treating
oxygen
deficiency associated with sickle cell anemia, the method comprising
administering to a
subject in need thereof a therapeutically effective amount of either the
pharmaceutical or the
blood composition described above.
100431 Additionally, the compounds and pharmaceutical compositions of the
invention can
be added to whole blood or packed cells preferably at thc time of storage or
at the time of
transfUsion. In some embodiments, the compounds and pharmaceutical
compositions may he
added to whole blood or red blood cell fractions in a closed system using an
appropriate
reservoir in which the compound or pharmaceutical composition is placed prior
to storage or
which is present in the anticoatzulating solution in the blood collecting bag.
!...;vnthetie Methods
100441 The synthesis of the compounds of Table I are described in U.S. Patent
Serial Nos.
61/661,320 and 61/581,053, each of which is incorporated herein by reference
in their
entireties, for the sole purpose of describing the synthesis of these
compounds.
21
CA 02902709 2015-08-26
WO 2(114/15(1256
PCT/US2014/022733
EXAMPLES
100451 The following examples are given for the purpose of illustrating
various
embodiments of the invention. They are not meant to limit the invention in any
fashion. One
skilled in the art will appreciate that the invention is well adapted to carry
out the objects and
obtain the ends and advantages mentioned, as well any objects, ends and
advantages inherent
herein. The present examples (along with the methods described herein) are
presently
representative of preferred embodiments. They are exemplary. and are not
intended as
limitations on the scope of the invention. 'Variations and other uses which
are encompassed
within the spirit of the invention as defined by the scope of the claims will
occur to those
skilled in the art.
,Example 1
100461 The compounds provided in the present invention are allosteric
modulators of
hemoglobin. As such, these compounds do not modulate red blood cells by
themselves.
Instead, the response of red blood cells to a concentration of hemoglobin is
increased when
compounds of Table I are present. Compounds of Table I are expected to have
their effect
on red blood cells by virtue of their ability to enhance the function of
hemoglobin.
100471 This experiment was established and used in order to assess the
pharmacokinetic
(PK) properties of the compounds.
100481 Sample collection and data analysis: Rats (Sprague-Dawley, male, 8-12
weeks old)
were dosed with one of three compounds corresponding to compound 12, compound
22 or
compound 23. The rats received oral (I Omg/kg) or intravenous (I mg/kg) doses
of the
compound. Rats were fasted overnight before the experiments and provided with
food after
the 2 hour sampling time point.
100491 Blood samples were collected at different time points. Blood was anti-
coagulated
by 3.2% ISC (trisodiurn citrate) and a portion was separated into plasma
fraction by
centrifugation and removal of blood cells. Plasma and lysed blood samples were
analyzed
for drug concentration using LC-MS/MS. PK parameters were calculated by non
compartmental analysis of the concentration-time profiles using WinNonLin
software
(Pharsight, Mountain View, CA). Apparent elimination half-life (tu2) values
were calculated
as in(2)1. Area under the concentration-time curve (ACC) values were estimated
using the
22
CA 02902709 2015-08-26
WO 2(114/15(1256 PCT/US2014/022733
linear trapezoidal method. AIX!1 values were calculated from the dosing time
to the last
measurable concentration. AUCinf values were calculated as the sum of the
corresponding
AUCIas, and the ratio of the last detectable concentration divided by k.
Plasma clearance (Cl)
is calculated from Dose/AIJCinf. Volume of distribution at steady state (Vss)
is calculated
from Mean Residence Timeinf x Cis,. Maximum concentration (Cmax) and time to
C.õ,õ, (T,õ,,,)
was recorded as observed. The blood/plasma partitioning ratio was calculated
at each
experimental time point.
100501 Results: Table 2 summarizes select PK parameters tbr the compounds
listed below:
Table 2
1 õ . PK Parameter 12 22 23
V(I/kg)
0.14 3.1 3.15
CI (mliminIkg) 0.11 9 13.7
Bioavailability (%) 68.8 6.6 1.8
Blood/plasma (Ratio of peak concentration) 21 5 1 1
Blood/plasma (Ratio of exposure AUCINO 55 33j 4
_________________________________________________________ =
100511 The volume of distribution for compound 12 is 0.14L/kg which indicates
that it is
not significantly distributed into extravascular space in rats (control normal
Vz ::: 0.11.11(g).
Higher V,s are observed for two related compounds (compound 22 and compound
23, 3.1 and
3.15, respectively), indicating that these compounds are more likely to
distribute into the
extravascular space and additional compartments than compound 12.
100521 However, when the red blood cell compartment is considered, compound 12
unexpectedly partitions into blood to a far greater extent than compound 22 or
compound 23.
\Vhen the compounds are dosed orally, the relative proportion in blood (as
compared to
plasma) at peak concentration (C,õaõ) were much higher for compound 12 (21-
fold) than for
compound 22 (5-fold) or compound 23 (3-fold). When the red blood cell/plasma
ratio was
measured at the peak concentration, compound 12 partitioned at a ratio of 70
to 1 into the
erythrocytes attesting to its preferential partition into the compartment
which contains the
drug target hemoglobin. Supportive data was reported in an in vitro system
measuring
binding of compound 12 to hemoglobin and human serum albumin. In this
functional assay,
23
CA 02902709 2015-08-26
WO 2(114/15(1256
PCT/US2014/022733
when both proteins are present in their respective physiologic ratio. compound
12
demonstrated preferentially binding to hemoglobin.
100531 Another surprising and unexpected observation was detected when overall
exposure
was tracked in animals dosed orally with compound 12. There was a 55-fold
higher level of
compound in blood than in plasma compared with blood/plasma ratios for
compound 22 (5-
fold) or compound 23 (1-fold).
1005.11 The ability of compound 12 to partition preferentially in red blood
cells has also
been confirmed in mice treated intravenously. A ratio of blood/plasma of 15.4
(at peak in
vivo concentration) and 30 (at overall exposure) was observed in mice.
Analogous to the
measurements in rats, the volume of distribution (Vss) was low in mice (0.10).
Thus,
compound 12 is not expected to broadly distribute into extravascular space in
mice.
100551 In conclusion, the results shown in Table 2 demonstrate that the lack
of compound
12 distribution into extrava.scular tissues (low Vss) combined with selective
partitioning into
the target compartment (red blood cells) provide a potential basis for reduced
toxicity.
100561 Accordingly, provided herein are blood compositions comprising one or
more
compounds selected from Table 1, and blood, wherein in the blood, at least 30%
of the
compound or compounds are bound to the red blood cells present in the blood.
Example 2
100571 Another series of assays were conducted in order to assess additional
pharmacokinetic (PK) properties of the compounds from Example 1.
Reverse Hemox Assay
100581 Oxygen Equilibrium Curves (OEC) of whole blood before and after
treatment with
different concentrations of compounds 12, 22 and 23 were performed as follows
using a
HEMOX analyzer (TCS Scientific, New Hope, PA). Blood samples from homozygous
sickle
eel] patients were obtained though the Hemoglobinopathy Center at Children's
Hospital
Oakland Research Institute (CHORD with Institutional Review Board approval.
The
hematocrit was adjusted to 20% using autologous plasma and the blood samples
were
incubated for 1 hour at 37 'V in absence or presence of compounds. 100 1.d of
these samples
were added to 5 ml.. of Hemox buffer (30 rnM TES. 130 mM NaCI, 5 mM KCI, pH:::
7.4) at
24
CA 02902709 2015-08-26
WO 2(114/15(1256
PCT/US2014/022733
37 C and then transferred to the Hemox sample chamber. The samples were
saturated with
oxygen by flushing with compressed air for 10 minutes. The samples were then
flushed with
pure nitrogen and the respective absorbances of oxy- and deoxy-Hb are recorded
as a
function of the solution p02. The oxygen equilibrium data were then fined to
the Hill Model
to obtain values for p50. The deoxygcnation curves for both whole blood alone
(control) and
whole blood in the presence of the compound were collected with the TCS
software,
100591 Results: Table 3 below lists the delta p50% values where "+" indicates
a delta
p50% of between 0 and 29, "++" indicates a delta p50% of between 30 and 50,
and "-H-+"
indicates a delta p50% of 50 or greater. A positive delta p50 value
corresponds to a left
shifted curve and a lower p50 value relative to control, indicating that the
compound acts to
modulate Hb(S) to increase its affinity for oxygen,
R/T Assay
100601 A relaxed-to-tense transition assay ("RIF assay") was used to determine
the ability
of compounds 12. 22 and 23 to mantain the high-oxygen affinity relaxed (R)
state of
hemoglobin under deoxygenated conditions. This ability can be expressed as a
"delta R."
value (i.e., the change in the time-period of the R. state after hemoglobin is
treated with a
compound, as compared to the period without treatment with the comound). Delta
R is the
%R to remaining after the compounds treatment compared with no treatment (e.g.
if R%
without treatment is 8% while with treatment with a target compound is 48% R
at 30 M,
then %R is 40% for that compound.
[00611 A mixture of ElbS/A was purified from blood obtained from homozygous
sickle cell
patients though the Hemoglobinopathy Center at Children's Hospital Oakland
Research
Institute (CHORD with Institutional Review Board approval. libS/A (at a final
concentration
of 3 uM) was incubated for 1 hr at 37 C in presence or absence of compounds in
501.04
potassium phosphate buffer, pH=7.4 and 30 pM 2, 3 diphosphoglycerate (DPG) in
96 well
plates in a final volume of 160 p.1. Compounds were added at different
concentrations (3 1.1M
to 100 p.M final concentrations). Plates were covered with a Mylar film. After
incubation
was completed the Mylar cover was removed and the plates were placed in a
Spectrostar
Nano plate reader previously heated at 37 C. Five minutes later, N.) (flow
rate ¨ 20 Umin)
was flowed through the spectrophotometer. Spectroscopic measurements (300 nm
to 700
CA 02902709 2015-08-26
WO 2(114/15(1256
PCT/US2014/022733
am) were taken every 5 min for 2 hours. Data analysis was performed by using
linear
regression from the data retrieved for all wavelengths.
100621 Results: Table 3 below lists the delta R values where ÷+" indicates a
delta R of
between 0 and 30. "++" indicates a delta It of between 30 and 50, and "'4-
+"+" indicates a delta
R of 50 or greater.
Polvmeyization As.sav
100631 Polymerization assays are carried out in vitro using purified FibS
exchanged into 1.8
M potassium phosphate buffer at pH 7.4. Using a slightly modified protocol
(Antonini and
Brunori, 1971), I113S is purified by the C:RO VIRUSYS, from blood obtained
from
homozygous sickle cell patients through the Hemoglobinopathy Center at
Children's Hospital
Oakland Research Institute (CHORD with Institutional Review Board approval.
Compounds
are prepared in 100% .DMS0 and a desired amount is added to 50 tiM of purified
FIB at a
final DMSO concentration of 0.3%. Final potassium phosphate concentration is
adjusted to
1.8 M using a combination of 2.5 M potassium phosphate stock solution and
water at 7.4.
The reaction mixture is incubated thr an hour at 37 C and then transferred
into a 24-well
plate for deoxygenation in a glove box containing 99.5 % nitrogen and 0.5%
oxygen. The
24-well plate is not covered and incubated at 4 "C on a plate cooler inside
the glove box tbr
one and a half hours. Fifty ut of the reaction mixture is transferred into a
96-we1l plate arid
the absorbance at 700 urn is measured every minute for one hour at 37 "C in a
plate reader
located inside the glove box. A plot of the absorbance against time is fitted
using a Boltzman
sigmoidal fit and the delay time (from zero to time at half Vmax) is measured.
To compare
and rank compounds, delay times arc expressed as percent delay (%DT), which is
defined as
the difference in delay times tbr HbSicompound and FIB alone multiplied by 100
and
divided by the delay time for HbS alone.
100641 Results: Compounds listed below have been tested in the polymerization
assay.
Activity ranges are defined by the number of dagger (t) symbols indicated. t
denotes activity
> 40% but -z; 80%; ft denotes activity > 80% but 120%; i"I't denotes activity
> 120% but
< 140%; till` denotes activity > 160%.
26
CA 02902709 2015-08-26
WO 2014/150256
PCT/US2014/022733
Table 3
In limo Assay Parameter I Unit 12 22 23
Reverse fiemox (1 mN,1) r (delta p50%) 79.83 (+++) 68.69 (+++)
72.45
R-T (91iM) (delta R) 65.45 (-H-+) 31.02 (+1-) 37.15 (++)
R-T (10uAl) (delta R) 62.75 (+++) 36.25 (++) 51.55 (+++)
Polymerization ( 75 uNl) (% DT) 108.56 (tt) 90.22 (-ill ; 98.19 0-11
Example 3
100651 Another set of assays was conducted to determine the effect of
compounds of the
invention on the oxygen affinity of hemoglobin and theological properties of
blood.
Oxygen Dissociation Assay
100661 In a 96-well format oxygen dissociation assay (ODA), compounds 5, 9,
and 12 were
all more potent at increasing the oxygen affinity of HbS than 5-hydroxy
furfural (5-HME), an
agent currently being tested in clinical trials in patients with sickle cell
disease.
190671 Results: Table 4 below lists the change in oxygen affinity (Aoxy
state). After two
hours of passive deoxygenation, compound 12, at an equimolar concentration to
lib,
increased the lib oxygen affinity by 6-fold. Even when compound 12 was present
at
substoichiometric concentrations (ratio of compound 12 to Fib of 1:3), there
was a two-fold
improvement in oxygen affinity for Hb that translates to 16% more oxygenated
llb present.
100681 The agents were then assayed in a ICS Hemox analyzer using pun i tied
lib at 25
t.tM. At a compound 12:Hb ratio of 1:3, the oxygen affinity was improved by
15%, while at
stoichiometric concentrations, the improvement in oxygen affinity was greater
than 70%
when compared to the Hb control.
Reverse Hemox Assay
100691 Reverse hemox assay was performed essentially as described in Example
2. above.
using either washed red blood cells or whole blood.
(00701 Results: Table 4. below, indicates the percent change in p50 (delta
p50%) for each
compound tested. In the washed red blood cells (RBCs), 5-11MF, compound 5, and
compound 12 (I rnIVI compound) gave p50 of 20, 9 and 7 mm Hg, respectively,
compared to
27
CA 02902709 2015-08-26
WO 2014/150256
PCT/US2014/022733
the control red blood cells (delta p50 = 30 mm Hg). To determine the effects
of plasma
proteins on compound activity. OECs were measured in whole blood from sickle
cell disease
patients. 5-HMF, compound 5, compound 9, and compound 12 gave p50 of 27, 18,
11 and 6
mm Hg, respectively, compared to the control blood p50 of 30 mm
Viscosity Assay
10071] Sickle cell disease patients develop anemia as a means for the
circulatory system to
compensate for increase in blood viscosity caused by the non-deformable sickle
cell red
blood cells (ssRBCs). The effects of 5-HMF, compound 5, compound 9, or
compound 12
were tested on sickle cell disease patient blood rheology to determine if
these compounds
decrease the viscosity of ssRBCs that have undergone hypoxia. Using blood from
patients
with sickle cell disease, whole blood (30% hematocrit, ¨1,5mM Hb) was
incubated with 5-
HMI:. compound 5, compound 9, or compound 12 during exposure to two hours of
hypoxia
(2.4% 02). A cone-plate viscometer was used to measure the viscosity at shear
rates ram4in12.
from 60 s'i to 415 s."1.
[00721 Results: Table 4 below lists the change in centipoise (Ad') values for
each
compound. The compounds of the invention dramatically improved blood
viscosity. For
example, compound 12 improved (decreased) viscosity from 6.33 cP (no compound
12) to
4.32 cP (equimolar compound 12). A cP of 3.69 is the average viscosity Ibr
normoxic sickle
cell disease blood. Such an improvement in blood viscosity has the potential
to decrease the
residence time for ssRBCs in hypoxic tissue, and allow for a lower level of
polymerization in
individual red blood cells during their transit through hypoxic tissue. In
addition, compound
12 has been shown to delay polymerization and sickling of red blood cells from
sickle cell
disease patients. All these properties indicate that compound 12 could elicit
a drastic decrease
in flbS polymer concentration, decreasing the likelihood of forming the rigid
cells which
cause vaso occlusion in patients with sickle cell disease.
28
CA 02902709 2015-08-26
WO 2014/150256
PCT/US2014/022733
Table
Assay Hb in ODA Washed RBC Whole Blood Viscosity
OEC OEC
unit (Aoxy state) (delta p50%) (delta p50%)
(AcP)
1111,1 3ttM 1mM 1mM 1.5m114
lcmp 1tv1 31iM 1mM 3mM - 1mM 3mM I .6mM 8mM
r----
5-HMF <1 <1 30 NA 10 47 0.04 1.4
2 10 - NA1 NA 49 71 NA >2.4
9 6 23
69 NA 63 >80 1,3 >2.4
=
____________________________ 4 ..
12 16 56 76 1 NA 80 >80 2.0 >2.4 I
Example 4
100731 Another set of assays was performed to determine the effectiveness of
compound 12
and 5-HMF at delaying in vitro polymerization and preventing sickling of red
blood cells.
Polymerization Assay
100741 The ability of 5-HMF and compound 12 to delay HbS polymerization was
evaluated
as described in Example 2, above. Purified HbS (50 uM) was pre-incubated with
25 uM, 50
1.1.M, or 100 AN4 of 5-HMF or compound 12, then passively de-oxygenated at 4
C in 1.8 M
potassium phosphate. Polymerization was induced via temperature jump from 4 "C
to 37 'C.
Polymerization was quantified based on turbidity of the HbS solution under
continued
hypoxia.
100751 Results: Table 5 below lists the delay (minutes) in polymerization for
each
compound tested. Table 6 lists the delay in polymerization by carbon monoxide
(CO)
liganded HbA, a well characterized inhibitor of HbS polymerization in both
intracellular and
in vitro assays. DT: delay time. Compound 12 delayed IlbS polymerization in a
dose-
dependent manner. Polymerization delay for untreated HbS control was
relatively longer
than that observed by active de-oxygenation using dithionite or laser
treatment. However,
compound 12 delayed HbS polymerization to a similar extent as CO-liganded HbA.
29
CA 02902709 2015-08-26
WO 2(114/15(1256
PCT/US2014/022733
SigKir.2.-AtWY5
[00761 For sickling experiments, red blood cells were pre-incubated with
compound 12 or
5-1-IM1', then subjected to hypoxia (1)02 of ¨30 mmHg.) in a 37 'C. humidified
chamber for
0.5 hr and subsequently imaged using a light microscope. The percentage of
sickled cells in
each image was calculated using CeilVigene software.
100771 Results: Table 5 below lists the effect of each compound on the percent
of sickled
red blood cells. HCT: hematocrit. Compound 12 prevented sickling of RBCs under
hypoxia
suggesting that compound 12 has the ability to prevent intracellular libS
polymerization. In
this sickling assay, red blood cells were exposed to hypoxia tbr a much longer
time than
typical red blood cell transit time through microcirculation (less than a
minute), thus much
less compound may be required to prevent sickling under physiological
conditions.
Table 5
Assay Polymerization Siekling
Unit IiTcnipd-DTtnis (min) (Y0 sickled)
111bS1 ;,IN1 ¨1 mM (20% !ACT)
1Cmpd1 25 ItNi 10 pM = I 00 MM 1 mM 2 mM 5 mM
Cmpd 12 3.9 12.8 1 22.9
41 33 28
1 1.8 6.3 76 65 50
No Cmpd 0 0 0 85 1 85 85
1
Table 6
IAssay Polymerization
Unit DTubsilibA-DTubs (min)
111b1 Total 50 urs.4
% HbA 20% 30% 1 40%
HbA-CO
100781 From the foregoing it will be appreciated that, although specific
embodiments of the
invention have been described herein for purposes of illustration, various
modifications may
be made without deviating from the spirit and scope of the invention.
CA 02902709 2015-08-26
WO 2(114/15(1256
PCT/US2014/022733
100791 Throughout the description of this invention, reference is made to
various patent
applications and publications, each of which are herein incorporated by
reference in their
entirety.
31