Note: Descriptions are shown in the official language in which they were submitted.
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
METHOD OF PRODUCING COUPLED RADICAL PRODUCTS VIA
DE SULF OXYLATION
CROSS-REFERENCE TO PRIOR APPLICATIONS
[0001] This application claims priority to U.S. Provisional Patent
Application No.
61/773,610 filed March 6, 2013. This application is also a continuation-in-
part of U.S. Patent
Application Ser. No. 12/840,508 filed July 21, 2010 (the '508 application).
The '508
application claimed the benefit of U.S. Provisional Patent Application No.
61/228,078, filed
on July 23, 2009, U.S. Provisional Patent Application No. 61/258,557, filed on
Nov. 5, 2009,
and U.S. Provisional Patent Application No. 61/260,961, filed on Nov. 13,
2009. This
application is also a continuation in part of U.S. Patent Application Ser. No.
12/840,401 filed
on July 21, 2010. This application is also a continuation in part of U.S.
Patent Application
Ser. No. 12/840,913. This application is also a continuation in part of U.S.
Patent
Application Ser. No. 13/612,192.
[0002] These provisional and non-provisional patent applications are expressly
incorporated herein by reference.
BACKGROUND OF THE INVENTION
[0003] Sodium salts of alkyl sulfates are useful chemicals that are readily
produced.
These chemicals generally have the following structure:
[R-503]- Na+
One specific example of this type of chemical is Sodium
dodecylbenzenesulfonate that is
commonly used in detergents:
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
00
R1
Na+
f
R2
R + R2 = C11 H24
Of course, any "R" group may be added to the alkyl sulfate.
[0004] Sodium salts of alkyl sulfates are commonly found in detergents,
cosmetics,
surfactants, shampoos, chromatography and other useful products/processes.
Accordingly,
these chemicals are readily available and are considered safe and
biodegradable.
[0005] At the same time, there is a need for a new method by which sodium
salts of alkyl
sulfates may be reacted to form different, organic chemicals. Such a process
is disclosed
herein.
SUMMARY OF THE INVENTION
[0006] A sodium salt of an alkyl sulfonate ([R-503]- Na + ) will be
obtained. Once
obtained, this alkyl sulfonate may be incorporated into an anolyte for use in
an electrolytic
cell. This anolyte may also include a solvent (such as water, methanol, etc.)
and optionally a
supporting electrolyte (in addition to the ([R-503]- Na + ). For convenience,
the alkyl
sulfonate ([R-503]- Na + may also be shown herein as R-503-Na.
[0007] The anolyte is fed into an electrolytic cell that uses a sodium ion
conductive
ceramic membrane that divides the cell into two compartments: an anolyte
compartment and
a catholyte compartment. A typical membrane is a NaSICON membrane. NaSICON
typically has a relatively high ionic conductivity for sodium ions at room
temperature.
Alternatively, if the alkali metal is lithium, then a particularly well suited
material that may
be used to construct an embodiment of the membrane is LiSICON. Alternatively,
if the alkali
metal is potassium, then a particularly well suited material that may be used
to construct an
embodiment of the membrane is KSICON. Other examples of such solid electrolyte
membranes include those based on NaSICON structure, sodium conducting glasses,
beta
alumina and solid polymeric sodium ion conductors. Such materials are
commercially
available. Moreover, such membranes are tolerant of impurities that may be in
the anolyte
and will not allow the impurities to mix with the catholyte. Thus, the
impurities (which were
-2-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
derived from the biomass) do not necessarily have to be removed prior to
placing the anolyte
in the cell.
[0008] The electrolytic cell may use standard parallel plate electrodes,
where flat plate
electrodes and/or flat membranes are used. In other embodiments, the
electrolytic cell may
be a tubular type cell, where tubular electrodes and/or tubular membranes are
used.
[0009] An electrochemically active first anode may be found in the cell and
may be
housed in the first anolyte compartment. The anode may be made of smooth
platinum,
stainless steel, or may be a carbon based electrode. Examples of carbon based
electrodes
include boron doped diamond, glassy carbon, synthetic carbon, Dimensionally
Stable Anodes
(DSA), and lead dioxide. Other materials may also be used for the electrode.
The first anode
allows the desired reaction to take place. In this anolyte compartment of the
cell, the
oxidation (desulfoxylation) reaction and subsequent radical-radical coupling
takes place. In
one embodiment, the anodic desulfoxylation/oxidative coupling of sulfonic
acids occurs via a
reaction that is similar to the known "Kolbe reaction." The standard Kolbe
reaction is a free
radical reaction and is shown below:
2R-COOH ¨> R-R + 2CO2 +26+ 2H+
(Carboxylic Acid) (coupled radical product)
This Kolbe reaction is typically conducted in non-aqueous methanolic
solutions, with
partially neutralized acid (in the form of alkali salt) used with a parallel
plate type
electrochemical cell. The anolyte used in the cell may have a high density.
[0010] As can be seen from the Kolbe reaction, the "R" groups of two
carboxylic acid
molecules are coupled together, thereby resulting in a coupled radical
product. In one
embodiment, the Kolbe reaction is a free radical reaction in which two "R
radicals" (R.) are
formed and are subsequently combined together to form a carbon-carbon bond. It
will be
appreciated by those of skill in the art, that depending upon the starting
material used, the
coupled radical product may be a hydrocarbon or some other chain. The coupled
radical
product may be a dimer, or a mixed product comprising one or more high- or low-
carbon
containing material. The radical in the coupled radical product may include an
alkyl-based
radical, a hydrogen-based radical, an oxygen-based radical, a nitrogen-based
radical, other
hydrocarbon radicals, and combinations thereof. Thus, although hydrocarbons
are shown in
the examples below as the coupled radical product, the hydrocarbon may be
freely substituted
for some other appropriate coupled radical product.
-3-
CA 02902988 2015-08-27
WO 2014/138252
PCT/US2014/020786
[0011] As noted above, however, the present embodiments may use a sodium salt
(or
alkali metal salt) of the sulfonic acid in the anolyte rather than the
carboxylic acid itself.
Thus, rather than using the standard Kolbe reaction (which uses a carboxylic
acid in the form
of a fatty acid), the present embodiments may involve conducting the following
reaction at
the anode:
2 ([R-S03]- Nat) ¨> R-R + 2S0
+26+ 2Na+
(Sodium salt of alkyl sulfonate) (coupled radical product)
Again, this embodiment results in two "R" groups being coupled together to
form a coupled
radical product such as a hydrocarbon. There are distinct advantages of using
the sodium salt
of the sulfonic acid instead of the sulfonic acid itself:
= ([R-S03]- Nat) is more polar than R-S03-H and so it is more likely to
desulfonate
(react) at lower voltages;
= The electrolyte conductivity may be higher for sodium salts of sulfonic
acids than
sulfonic acids themselves; and
= The anolyte and catholyte may be completely different allowing different
reactions to
take place at either electrode.
[0012] As noted above, the cell contains a membrane that comprises a sodium
ion
conductive membrane. This membrane selectively transfers sodium ions (Nat)
from the
anolyte compartment to the catholyte compartment under the influence of an
electrical
potential, while at the same time preventing the anolyte and catholyte from
mixing.
[0013] The catholyte may be aqueous NaOH or a non aqueous methanol/sodium
methoxide solution. (The anolyte may be aqueous or non-aqueous). An
electrochemically
active cathode is housed in the catholyte compartment, where reduction
reactions take place.
These reduction reactions may be written as:
2Na+ + 2H20 +26 ¨> 2NaOH + H2
2Na+ + 2CH3OH +26 ¨> 2NaOCH3 + H2
Hydrogen gas is the product of the reduction reaction at the cathode. NaOH
(sodium
hydroxide) or NaOCH3 (sodium methoxide) is also produced. This NaOH or NaOCH3
is the
base that was used in a reaction to form R-503-Na. Thus, this reaction may
actually
-4-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
regenerate (in the catholyte compartment) one of the reactants needed in the
overall process.
This NaOH or NaOCH3 may be recovered and re-used in further reactions. The
ability to
regenerate and re-use the NaOH or NaOCH3 is advantageous and may significantly
reduce
the overall costs of the process.
[0014] In an alternative embodiment, a sodium salt of sulfonic acid with a
small number
of carbon atoms (such as CH3S03-Na) may be added to the anolyte in addition to
the R-S03-
Na. The addition of this sulfonate may be advantageous in some embodiments
because:
= It may act as a suitable supporting electrolyte as it is highly soluble
in the solvent,
thereby providing high electrolyte conductivity in the anolyte;
= It will itself desulfonate (in the electrolytic process) and produce CH3.
(methyl
radicals) by the following reaction:
2CH3-S03-Na ¨> CH3. + 2SOõ + 2e- + 2Na+
(Methyl radical)
= In turn, the methyl radical may react with a hydrocarbon group of the
sulfonic acid to
form hydrocarbons with additional CH3- functional group:
CH3. + R. ¨> CH3-R
(Methyl radical) (Radical of the sulfonic acid)
Therefore, in one embodiment, by using CH3-S03-Na as part of the anolyte, this
embodiment
may couple two hydrocarbon radicals from the sulfonic acid together (R-R) or
couple the
radical of the sulfonic acid with a methyl radical from CH3-S03-Na), thereby
producing
mixed hydrocarbon products. This mixture of products may be separated and used
as desired.
Of course, this embodiment is shown using CH3-S03-Na as the additional
reactant. In the
alternative, other sodium salts of a sulfonic acid with a small number of
carbon atoms may
also be used to couple a carbon radical to the radical of the sulfonic acid.
[0015] It will be appreciated that a variety of different hydrocarbons or
coupled radical
products may be formed using the present embodiments. For example, the
particular "R"
group that is selected may be chosen and/or tailored to produce a hydrocarbon
that may be
used for diesel, gasoline, waxes, JP8 ("jet propellant 8"), etc. The
particular application of
the hydrocarbon may depend upon the starting material chosen.
-5-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
[0016] It should be noted that the above-recited embodiments which use a
"desulfoxylation reaction" are similar to the "decarboxylation" reactions
described in the
following published patent applications:
U.S. Patent Application Publication No. 2011/0024288
U.S. Patent Application Publication No. 2011/0027848
U.S. Patent Application Publication No. 2011/0168569
U.S. Patent Application Publication No. 2013/0001095.
All of the above-recited published patent applications are expressly
incorporated herein by
reference. However, those skilled in the art will appreciate that the
reactions, reaction
conditions, reactants, etc. that are disclosed in the "decarboxylation"
processes in the above-
recited documents may be equally applied to the present "desulfoxylation"
reactions.
Further, the examples used herein focus on Na as the alkali metal. Those
skilled in the art
will appreciate that other alkali metals, or alloys of alkali metals, may be
used in conjunction
with or instead of Na.
BRIEF DESCRIPTION OF THE DRAWINGS
[0017] In order that the manner in which the above-recited and other
features and
advantages of the invention are obtained will be readily understood, a more
particular
description of the invention briefly described above will be rendered by
reference to specific
embodiments thereof which are illustrated in the appended drawings.
Understanding that
these drawings depict only typical embodiments of the invention and are not
therefore to be
considered to be limiting of its scope, the invention will be described and
explained with
additional specificity and detail through the use of the accompanying drawings
in which:
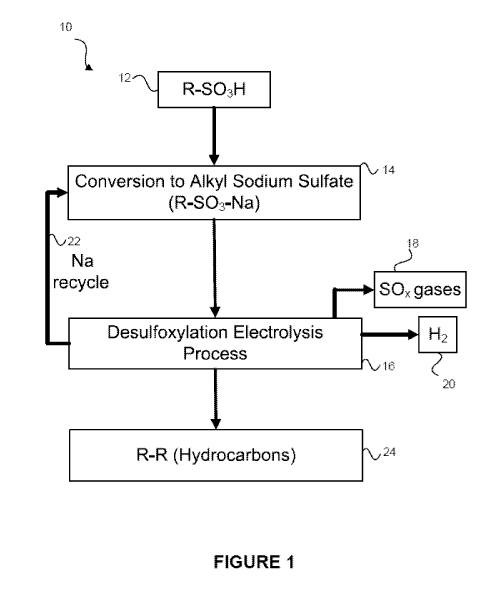
[0018] Figure 1 is a schematic diagram illustrating the overall process
that uses
desulfoxylation to produce coupled hydrocarbon products;
[0019] Figure 2 is a schematic view of an electrolytic cell for conversion
of sodium salts
of sulfonic acids to coupled radical products by anodic desulfooxylation and
subsequent
carbon-carbon bond formation in accordance with the present embodiments;
[0020] Figure 3 is a schematic view of another embodiment of an
electrolytic cell for
conversion of sodium salts of sulfonic acids to coupled radical products; and
[0021] Figure 4 is a schematic view of another embodiment of an
electrolytic cell for
conversion of sodium salts of sulfonic acids to coupled radical products;
DETAILED DESCRIPTION
[0022] Referring now to Figure 1, the overall process 10 for producing a
coupled radical
product is disclosed. As shown in Figure 1, a quantity of a sulfonic acid (R-
503H) may be
-6-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
obtained 12. This sulfonic acid may be an organic acid and may be obtained
from biomass or
from any other source. Those skilled in the art will appreciate the various
different sources of
sulfonic acids. Any and all sources of the sulfonic acid fall within the
present embodiments.
The above-recited published patent applications describe biomass as a
potential starting
material and that the biomass may be converted into sulfonic acids via known
methods.
[0023] Once the sulfonic acid is obtained, the sulfonic acid may be
converted 14 into the
alkyl sodium sulfate (R-S03-Na). This conversion reaction may occur by
reacting the
sulfonic acid with a base, may occur in an electrochemical cell, or may occur
in some other
way. In other embodiments, instead of obtaining the sulfonic acid and reacting
it to form the
alkyl sodium sulfate, the quantity of the alkyl sodium sulfate may be directly
obtained
(through purchase, etc.).
[0024] The alkyl sodium sulfate may then be subjected to a desulfoxylation
electrolysis
process 16, in the manner outlined herein. As described herein, this process
may produce a
quantity of a SO, gas 18 (such as, for example, SO2, SO3, etc.). A quantity of
hydrogen gas
20 may also be produced. Those skilled in the art will appreciate that these
gases may be
collected, re-used, disposed of, etc., as desired. Further, as part of the
desulfoxylation
process 16, the sodium ions may be recycled such as, for example, in the form
of a base such
as NaOH (as shown by arrow 22) and used again to form the R-S03-Na.
[0025] At the same time, the desulfoxylation process 16 operates to couple
organic
radicals together, thereby forming an R-R hydrocarbon product 24. As noted
above, these
hydrocarbons may be valuable products, such as fuels, gasoline additives etc.
[0026] An electrochemical cell may be used to conduct the desulfoxylation
process 16.
An example of a typical embodiment of a cell is shown in Figure 2. This cell
200, which
may also include a quantity of a first solvent 160 (which may be, for example,
water or an
alcohol like methanol, ethanol, and/or glycerol), may be used to conduct an
advanced Kolbe
reaction. The solvent 160 may be obtained from any source. This advanced Kolbe
reaction
produces a hydrocarbon 170 along with a quantity of SO x 172 gases. A quantity
of a base
150 is also produced. (In the embodiment of Figure 2, the base is NaOH.) The
hydrocarbon
170 is but one example of any of a number of coupled radical products that may
be produced
by this process, and may be, for example, a mixture of hydrocarbons.
Similarly, the SO,
gases 172 produced in the process 200 is a naturally-occurring chemical and
may be disposed
of, collected, sold, etc.
[0027] The hydrocarbon 170 produced in the process 200 (and more
specifically in the
advanced Kolbe reaction) may be of significant value. Hydrocarbons have
significant value
-7-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
for use in fuels, diesel fuels, gasoline, medical applications, waxes,
perfumes, oils, and other
applications and products. With the process of the present invention,
different types of
hydrocarbons may be used. Hydrocarbons are often classified by the number of
carbons in
their chain. In addition, hydrocarbons may often be classified into the
following "fractions":
C1 Methane fraction
C2-05 Natural gas fraction
C6-C113 Gasoline fraction
Cio-C13 JP8 fraction
C14-C20 Diesel fraction
C20-C25 Fuel Oil fraction
C20-C30 Waxes
Note that these classifications are not exact and may change according to the
particular
embodiment. For example, the "gasoline fraction" could have a portion of C11,
the JP8
fraction could have some C14, etc.
[0028] By forming the coupled radical products according to the present
embodiments,
various hydrocarbons could be made in some or all of these fractions. For
example,
embodiments may be constructed in which a Cg hydrocarbon (octane) is formed,
which is a
principal ingredient in commercial gasoline. Likewise, a C12 hydrocarbon may
be formed,
which may be used in making JP8. Of course, the exact product that is obtained
depends
upon the particular starting material(s) and/or the reaction conditions used.
Thus, the present
embodiments allow biomass to be converted into synthetic lubricants, gasoline,
JP8, diesel
fuels, or other hydrocarbons.
[0029] Figure 2 shows the cell 200 (which may be an electrochemical cell to
which a
voltage may be applied). The cell 200 includes a catholyte compartment 204 and
an anolyte
compartment 208. The catholyte compartment 204 and the anolyte compartment 208
may be
separated by a membrane 212.
[0030] The particulars of each cell 200 will depend upon the specific
embodiment. For
example, the cell 200 may be a standard parallel plate cell, where flat plate
electrodes and/or
flat plate membranes are used. In other embodiments, the cell 200 may be a
tubular type cell,
where tubular electrodes and/or tubular membranes are used. An
electrochemically active
first anode 218 is housed, at least partially or wholly, within the anolyte
compartment 208.
More than one anode 218 may also be used. The anode 218 may comprise, for
example, a
smooth platinum electrode, a stainless steel electrode, or a carbon based
electrode. Examples
of a typical carbon based electrode include boron doped diamond, glassy
carbon, synthetic
-8-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
carbon, Dimensionally Stable Anodes (DSA) and relatives, and/or lead dioxide.
Other
electrodes may comprise metals and/or alloys of metals, including S = S,
Kovar,
Inconel/monel. Other electrodes may comprise Ru02-Ti02/Ti, Pt0x-Pt02/Ti, IrOx,
C0304,
Mn02, Ta205 and other valve metal oxides. In addition, other materials may be
used to
construct the electrode such as 5n02, Bi2Ru207 (BRO), Bi5n207, noble metals
such as
platinum, titanium, palladium, and platinum clad titanium, carbon materials
such as glassy
carbon, BDD, or Hard carbons. Additional embodiments may have Ru02-Ti02, hard
vitrems
carbon, and/or Pb02. Again, the foregoing serve only as examples of the type
of electrodes
that may be employed. The cathode compartment 204 includes at least one
cathode 214. The
cathode 214 is partially or wholly housed within the cathode compartment 204.
The material
used to construct the cathode 214 may be the same as the material used to
construct the anode
218. Other embodiments may be designed in which a different material is used
to construct
the anode 218 and the cathode 214.
[0031] The anolyte compartment 208 is designed to house a quantity of
anolyte 228. The
catholyte compartment 204 is designed to house a quantity of catholyte 224. In
the
embodiment of Figure 2, the anolyte 228 and the catholyte 224 are both
liquids, although
solid particles and/or gaseous particles may also be included in either the
anolyte 228, the
catholyte 224, and/or both the anolyte 228 and the catholyte 224.
[0032] The anode compartment 208 and the cathode compartment 204 are separated
by an
alkali metal ion conductive membrane 212. The membrane utilizes a selective
alkali metal
transport membrane. For example, in the case of sodium, the membrane is a
sodium ion
conductive membrane 212. The sodium ion conductive solid electrolyte membrane
212
selectively transfers sodium ions (Nat) from the anolyte compartment 208 to
the catholyte
compartment 204 under the influence of an electrical potential, while
preventing the anolyte
228 and the catholyte 224 from mixing. Examples of such solid electrolyte
membranes
include those based on NaSICON structure, sodium conducting glasses, beta
alumina and
solid polymeric sodium ion conductors. NaSICON typically has a relatively high
ionic
conductivity at room temperature. Alternatively, if the alkali metal is
lithium, then a
particularly well suited material that may be used to construct an embodiment
of the
membrane is LiSICON. Alternatively, if the alkali metal is potassium, then a
particularly
well suited material that may be used to construct an embodiment of the
membrane is
KSICON.
[0033] The anolyte compartment 208 may include one or more inlets 240 through
which
the anolyte 228 may be added. Alternatively, the components that make up the
anolyte 228
-9-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
may be separately added to the anolyte compartment 208 via the inlets 240 and
allowed to
mix in the cell. The anolyte includes a quantity of the alkali metal salt of a
sulfonic acid 108
(R-S03-Na). In the specific embodiment shown in Figure 2, sodium is the alkali
metal, so
that alkali metal sulfonic acid salt 108 is a sodium salt. The anolyte 228
also includes a first
solvent 160, which, may be water 160a. Of course, other types of solvents may
also be used.
The anolyte 228 may optionally include other alkali metal salts of sulfonic
acids (such as, for
example, CH3-S03-Na). Other mixtures of different alkali metal salts of
sulfonic acids may
also be used.
[0034] The catholyte compartment 204 may include one or more inlets 242
through which
the catholyte 224 may be added. The catholyte 224 includes a second solvent
160b. The
second solvent 160b may be water (as shown in Figure 2) or may be alcohol or
some other
type of solvent of mixture of solvents. Significantly, the solvent 160b in the
catholyte 224 is
not necessarily the same as the first solvent 160a in the anolyte 228. In some
embodiments,
the solvents 160a, 160b may be the same. The reason for this is that the
membrane 212
isolates the compartments 208, 204 from each other. Thus, the solvents 160a,
160b may be
each separately selected for the reactions in each particular compartment
(and/or to adjust the
solubility of the chemicals in each particular compartment). Thus, the
designer of the cell
200 may tailor the solvents 160a, 160b for the reaction occurring in the
specific compartment,
without having to worry about the solvents mixing and/or the reactions
occurring in the other
compartment. This may be a significant advantage in designing the cell 200. A
typical
Kolbe reaction only allows for one solvent used in both the anolyte and the
catholyte.
Accordingly, the use of two separate solvents may be advantageous. In other
embodiments,
either the first solvent 160a, the second solvent 160b, and/or the first and
second solvents
160a, 160b may comprise a mixture of solvents.
[0035] The catholyte 224 may also include a base 150. In the embodiment of
Figure 2,
the base 150 may be NaOH or sodium methoxide, or a mixture of these chemicals.
[0036] The reactions that occur at the anode 218 and cathode 214 will now
be described.
As with all electrochemical cells, such reactions may occur when voltage
source 290 applies
a voltage to the cell 200.
[0037] At the cathode 214, a reduction reaction takes place. This reaction
uses the sodium
ions and the solvent to form hydrogen gas 270 as well as an additional
quantity of base 150.
Using the chemicals of Figure 2 as an example, the reduction reaction may be
written as
follows:
-10-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
2Na+ + 2H20 +26 ¨> 2NaOH + H2
2Na+ + 2CH3OH +26 ¨> 2NaOCH3 + H2
The hydrogen gas 270 and/or the base 150 may be extracted through outlets 244.
The
hydrogen gas 270 may be gathered for further processing for use in other
reactions, and/or
disposed of or sold. The production of the base 150 may be a significant
advantage because
the base 150 that was consumed in the conversion reaction 14 of Figure 1 is
regenerated in
this portion of the cell 200. Thus, the base formed in the cell may be
collected and re-used in
future reactions (or other chemical processes). As the base may be re-used,
the hassle and/or
the fees associated with disposing of the base may be avoided.
[0038] The reactions that occur at the anode 218 may involve
desulfoxylation. These
reactions may involve an advanced Kolbe reaction (which is a free radical
reaction) to form a
quantity of a hydrocarbon 170 and carbon dioxide 172. Using the chemicals of
Figure 2 as an
example, the oxidation reactions may be written as follows:
2R-S03-Na R-R + SO, +26+ 2Na+
(Sodium salt of Sulfonic Acid) (coupled radical product)
The SO, gas 172 may be vented off (via outlets 248). The coupled radical
product 170 may
also be collected via an outlet 248. For example, a quantity of the solvent
160/160a may be
extracted via an outlet 248 and recycled, if desired, back to the inlet 240
for future use.
[0039] The advanced Kolbe reaction may comprise a free radical reaction. As
such, the
reaction produces (as an intermediate) a hydrocarbon radical designated as R..
Accordingly,
when two of these R. radicals are formed, these radicals may react together to
form a carbon-
carbon bond:
R. R. R-R
(Hydrocarbon radical) (Hydrocarbon radical) (A new
hydrocarbon)
As shown in Figure 2, this R-R hydrocarbon product is designated as
hydrocarbon 170. In
essence, the R moiety is being desulfoxylated, as the sulfonyl moeity is
removed, leaving
only the R. radical that is capable of reacting to form a hydrocarbon.
[0040] As noted above, additional salts of sulfonic acids may be used in
Figure 2. For
example, if CH3-503-Na (or some other sodium salt of sulfonic acid with a
small number of
-11-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
carbon atoms) may be part of (or added to) the anolyte 228. CH3-S03-Na may act
as a
suitable supporting electrolyte as it is highly soluble in some solvents,
providing high
electrolyte conductivity. At the same time, CH3-S03-Na may itself
desulfoxylate as part of
the advanced Kolbe reaction and produce CH3. (methyl) radicals by the
following reaction:
2CH3-S03-Na ¨> 2CH3. + SO, +26+ 2Na+
(Methyl Radicals)
The methyl radicals may then be reacted with hydrocarbon group of the sulfonic
acid to form
hydrocarbons with additional CH3- functional group:
CH3. + R. ¨> CH3-R
Alternatively or additionally, the methyl radical may react with another
methyl radical to
form ethane:
CH3. + CH3. ¨> CH3-CH3
Ethane (CH3-CH3) is a hydrocarbon that may form a portion of the hydrocarbon
product 170.
The CH3-R formed in the reaction may also be part of the hydrocarbon product
170. (This R-
CH3 product is shown as numeral 170b.) Thus, a mixture of hydrocarbons may be
obtained,
and are represented by the structure R-R. If desired, the various hydrocarbons
may be
separated from each other and/or purified, such as via gas chromatography or
other known
methods. The present embodiments may couple two hydrocarbon radicals or couple
methyl
radicals with hydrocarbon radicals. The amount of the CH3-R or R-R in the
product may
depend upon the particular reaction conditions, quantities of reactants used
in the anolyte, etc.
[0041] The foregoing example involved the use of CH3-S03-Na in addition to
the acid salt
to produce reactive methyl radicals, thereby producing CH3-R in addition to
the R-R product.
However, rather than CH3-S03-Na, other salts that have a small number of
carbons may be
used in place of or in addition to CH3-S03-Na. These salts having a small
number of carbons
may produce, for example, ethyl radicals, propyl radicals, isopropyl radicals,
and butyl
radicals during desulfoxylation. Materials that produce H radicals may also be
used. Thus,
by changing the optional component, additional hydrocarbons may be formed in
the cell 200.
The user may thus tailor the specific product formed by using a different
reactant. Thus, it is
-12-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
possible to create a mixture of products as different alkyl radicals react
together or even react
with a methyl radical, a hydrogen radical, etc. The different alkyl radicals
may be added by
adding CH3-503-Na, H-503-Na, etc. into the anolyte through, for example, an
additional port
in the anolyte compartment. Such a different mixture of products may be, in
some
embodiments, similar to what would occur in a disproportionation reaction.
[0042] In a similar manner, instead of and/or in addition to using CH3-503-
Na, H-503-Na
may be used as part of the anolyte. During the electrochemical reaction, the H-
503-Na, like
the CH3-503-Na, will undergo desulfoxylation to form a hydrogen radical:
2H-503-Na ¨> 2H. + 250x +26+ 2Na+
(Hydrogen Radicals)
In turn, this hydrogen radical will react to form:
H. + R. ¨> H-R
AND/OR
H. + H. ¨> H2
The use of H-503-Na as an optional reactant may result in the R-R product
being formed as
well as a quantity of an R-H product (and even a quantity of hydrogen gas
(H2)). (The
hydrogen gas may be re-used if desired). The use of H-503-Na may prevent the
unnecessary
formation of ethane and/or may be used to tailor the reaction to from a
specific hydrocarbon
(R-H) product.
[0043] The particular R group that is shown in these reactions may be any
"R" obtained
from biomass, whether the R includes saturated, unsaturated, branched, or
unbranched chains.
When the R-R product is formed, this is essentially a "dimer" of the R group.
For example, if
the R group is CH3, two methyl radicals react (2CH3.) and "dimerize" into
ethane (CH3-CH3).
If the R group is a C18H34 hydrocarbon, then a C36H78 product may be formed.
By using
these simple principles, as well as using the H-503-Na or the small chain
salt, any desired
hydrocarbon may be obtained. For example, by using a C4 sodium salt, a Cg R-R
hydrocarbon may be formed, which may be useable as part of a gasoline.
Likewise, if a C6
sodium salt is used, a C12 R-R hydrocarbon may be formed, which may be useable
as JP8.
Synthetic lubricants, waxes, and/or other hydrocarbons may be formed in the
same or a
-13-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
similar manner. Those skilled in the art will appreciate how to use these
principals to create
desired hydrocarbons.
[0044] An
alternate embodiment to that of Figure 2 will now be described with reference
to the embodiment shown in Figure 3. Because much of the embodiment of Figure
3 is
similar to that which is shown in Figure 2, a discussion of portions of the
similar features will
be omitted for purposes of brevity, but is incorporated herein by this
reference. Because the
anolyte compartment 208 is separate from the catholyte compartment 204, it is
possible to
create a reaction environment in the anolyte compartment 208 that is different
from the
catholyte compartment 204. Figure 3 illustrates this concept. For example,
hydrogen gas
(H2) 320 may be introduced into the anolyte compartment 208. In some
embodiments, the
anolyte compartment 208 may be pressurized by hydrogen gas 320. In some
embodiments,
the anode 208 or anolyte could include a component 310 made of Pd or other
noble metal
(such as Rh, Ni, Pt, Ir, or Ru) or another substrate such as Si, a zeolite,
etc. (This component
may be all or part of the electrode and may be used to immobilize the hydrogen
gas on the
electrode.) Alternatively, Pd or Carbon with Pd could be suspended within the
cell. The
effect of having hydrogen gas in the anolyte compartment 208 is that the
hydrogen gas may
form hydrogen radicals (H.) during the reaction process that react in the
manner noted above.
These radicals would react with the R. radicals so that the resulting products
would be R-H
and R-R. If sufficient hydrogen radicals (H.) are present, the R-H product may
be
predominant, or may be the (nearly) exclusive product. This reaction could be
summarized
as follows (using Pd as an example of a noble metal, noting that any other
noble metal could
be used):
R-503-Na + H2 and Pd ¨> Pd-Hx ¨> Pd
+ H-R + SO x + e- +
Na+
By using one or more of the noble metals with hydrogen gas in the anolyte
compartment, the
particular product (R-H) may be selected. In the embodiment of Figure 3,
hydrogen gas 270
is produced in the catholyte compartment 204 as part of the reduction
reaction. This
hydrogen gas 270 may be collected and used as the hydrogen gas 320 that is
reacted with the
noble metal in the anolyte compartment 208. Thus, the cell 300 actually may
produce its own
hydrogen gas 270 supply that will be used in the reaction. Alternatively, the
hydrogen gas
270 that is collected may be used for further processing of the hydrocarbon,
such as cracking
and/or isomerizing waxes and/or diesel fuel. Other processing using hydrogen
gas may also
-14-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
be used. The R-H product (which is designated as 170e) helps to minimize the
formation of
the R-R group (which, if the R group is sufficiently, large, may be a
hydrocarbon such as a
wax).
[0045] Referring now to Figure 4, an additional embodiment of a cell 400 is
illustrated.
The cell 400 is similar to the cells that have been previously described.
Accordingly, for
purposes of brevity, much of this discussion will not be repeated. In the
embodiment of
Figure 4, the cell 400 is designed such that one or more photolysis reactions
may occur in the
anolyte compartment 208. Specifically, a photolysis device 410 is designed
such that it may
emit (irradiate) radiation 412 into the anolyte compartment 208. This
irradiation may
produce hydrogen radicals (H.). The hydrogen gas 320 may be supplied to the
anolyte
compartment 208 using any of the mechanisms described above, as illustrated by
the
following equation:
H2 -> H. + H.
(photolysis)
This photolysis process may be combined with the electrolysis process of the
cell described
above:
(electrolysis)
2R-503-Na ¨> 2R. + 2S0 +26+ 2Na+
(Sodium salt of sulfonic acid) (Hydrocarbon radical)
The hydrogen radicals and the hydrocarbon radicals may then combine to form a
mixture of
products:
2H. + 2R. ¨> H-R + R-R + H2
Alternatively, the photolysis device may be used to conduct desulfoxylation
and to generate
hydrocarbon radicals:
(photolysis)
2R-503-Na ¨> 2R. +2 SO x +26+ 2Na+
(Sodium salt of sulfonic acid) (Hydrocarbon radical)
-15-
CA 02902988 2015-08-27
WO 2014/138252
PCT/US2014/020786
Thus, a combination of photolysis and electrolysis may be used to form the
hydrocarbon
radicals and/or hydrogen radicals in the anolyte compartment 208:
(photolysis and electrolysis)
2R-S03-Na + H2 ¨> R-R + R-H + H2
This combination of electrolysis and photolysis may speed up the rate of the
desulfoxylation
reaction.
[0046] Yet additional embodiments may be designed using such photolysis
techniques.
For example, the following reactions may occur:
(electrolysis)
2R-S03-Na ¨> 2R. +2
SO, +26+ 2Na+
(Sodium salt of sulfonic acid) (Hydrocarbon radical)
(photolysis and/or electrolysis)
2R. ¨> 2R+ +26
(photolysis)
H2 +26 ¨> 2H-
2H- + 2R+ ¨> 2R-H
This combination of reactions (using photolysis and electrolysis) forms
carbocations and H-
anions that may combine to form the hydrocarbon. Thus, photolysis may be used
as a further
mechanism for forming hydrocarbons. As has been discussed above, although
hydrocarbons
are being used in these examples, the coupled radical product need not be a
hydrocarbon. In
certain embodiments, the method and apparatus of the present invention may be
used to
create nonhydrocarbon radicals which may couple together to form useful
coupled radical
products.
[0047] Referring now to Figures 2-4 collectively, it is noted that each of
these illustrative
embodiments involve separation of the anolyte compartment 208 and the
catholyte
compartment 204 using the membrane 212. As described herein, specific
advantages may be
-16-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
obtained by having such a membrane 212 to separate the anolyte compartment 208
from the
catholyte compartment 204. These advantages include:
= two separate environments for different reaction conditions--for example,
the anolyte
may be non-aqueous, while the catholyte is aqueous (and vice versa);
= anolyte may be at a higher temperature than the catholyte (and vice
versa);
= anolyte may be pressurized and catholyte not (and vice versa);
= anolyte may be irradiated and catholyte not (and vice versa);
= anolyte and/or anode may be designed to conduct specific reactions that
are not
dependent upon the catholyte and/or cathode reactions (and vice versa);
= the different chambers may have different flow conditions, solvents,
solubilities,
product retrieval/separation mechanisms, polarities, etc.
The ability to have separate reaction conditions in the anolyte compartment
and catholyte
compartment may allow the reactions in each compartment to be tailored to
achieve optimal
results.
[0048] Likewise, a membrane, comprising, for example, NaSICON, has a high
temperature tolerance and thus the anolyte may be heated to a higher
temperature without
substantially affecting the temperature of the catholyte (or vice versa).
(NaSICON can be
heated and still function effectively at higher temperatures). This means that
polar solvents
(or non-polar solvents) that dissolve sulfonic acids and sodium salts at high
temperatures may
be used in the anolyte. At the same time, the catholyte is unaffected by
temperature. In fact,
a different solvent system could simultaneously be used in the catholyte.
Alternatively, other
molten salts or acids may be used to dissolve ionic sodium acids and salts in
the anolyte.
Long chain hydrocarbons, ethers, triglycerides, esters, alcohols, or other
solvents may
dissolve acids and sodium salts. Such compounds could be used as the anolyte
solvent
without affecting the catholyte. Ionic liquids could be used as the anolyte
solvent. These
materials not only would dissolve large quantities of sulfonic acid sodium
salts, but also, may
operate to facilitate the desulfoxylation reaction at higher temperatures.
Ionic liquids are a
class of chemicals with very low vapor pressure and excellent dissolving
abilities/dissolving
properties. A variety of different ionic liquids may be used.
[0049] As explained above, one of the advantages of the present cell is
that it produces a
base 150 in the catholyte compartment 204. As noted above, this base 150 may
then be used
as part of the reaction 14 that produces the sodium salt of the sulfonic acid.
The Na + ions for
this reaction come from the anolyte 228 through the membrane. Alternatively,
embodiments
-17-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
may be made in which the sulfonic acids may be reacted to form alkali metal
salts directly in
the catholyte compartment 208. In other words, the conversion reaction 14
occurs within the
cell itself to produce the sulfonic acid sodium salt, and this sodium salt is
then taken from the
catholyte compartment 204 to the anolyte compartment 208 (such as via a
conduit). This
process thus allows the sulfonic acid to be converted to the alkali metal salt
in situ (e.g.,
within the cell). This process would be a one step process (e.g., simply
running the cell)
rather than a two step process (conversion reaction and desulfoxylation within
the cell).
[0050] It should be noted that the above-recited embodiments operate to
produce SO,
gases as a result of the desulfoxylation reaction. By methods known in the
art, these SO,
gases may be converted into SO3 gas. In turn, this SO3 gas may be reacted (in
a known
manner) with water to form sulfuric acid (H2SO4), which is a valuable and
useful chemical
that may be used, sold, etc.
SO3 +H20 ¨> H2SO4
[0051] Additional embodiments may be designed in which the R-503-Na reacts in
water
in teh following manner:
H20 ¨> 2H+ + 1/202 + 26
R-503-Na + El+ ¨> R-503-H + Na+
2R-503-H ¨> 2R. + 2S0 + 2e- +
[0052] In other words, the above-recited embodiments have the water react
first, thereby
producing the acid (R-503-H) and then this acid product undergoes
desulfoxylation. Those
skilled in the art will appreciate how to control the various conditions in
order to
desulfoxylate the acid (R-503-H) or the sodium salt acid (R-503-Na), as
desired.
[0053] In one embodiment, the anolyte comprises G-type solvents, H-Type
solvents,
and/or mixtures thereof G-type solvents are di-hydroxyl compounds. In one
embodiment
the G-type compound comprises two hydroxyl groups in contiguous position. H-
type
solvents are hydrocarbon compounds or solvent which can dissolve hydrocarbons.
For
example, H-type solvents include, hydrocarbons, chlorinated hydrocarbons,
alcohols,
ketones, mono alcohols, and petroleum fractions such as hexane, gasoline,
kerosene,
dodecane, tetrolene, and the like. The H-type solvent can also be a product of
the
desulfoxylation process recycled as a fraction of the hydrocarbon product.
This will obviate
the need of procuring additional solvents and hence improve overall economics
of the
process.
-18-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
[0054] By way of further description, G-type of solvents solvate an ¨S03-Na
group of a
alkali metal salt of acid by hydrogen bonding with two different oxygen atoms,
whereas the
hydrocarbon end of the alkali metal salt of sulfonic acid is solvated by an H-
type of solvent.
For a given G-type solvent, the solvency increases with increase of
hydrocarbons in the H-
type solvent.
[0055]
The table below shows some non-limiting examples of G-type and H-type
solvents:
G-type H-type
ehthylene glycol isopropanol
glycerine methanol
1,2-dihidroxy-4-oxadodecane ethanol
2-methyl-2-propyl- 1 ,3 -propanediol butanol
2-ethyl-1 ,3 -hexanediol amyl alcohol
2-amino-2-methyl- 1 ,3 -propanediol octanol
2,3-butanediol hexane
3 -amino- 1 ,2-propanediol trichloroethane, dichloroethane
1,2-octanediol methylene dichloride
cis- 1 ,2-cyclohexanediol chloroform
rans- 1 ,2- cyclohexanediol carbon tetrachloride
cis- 1 ,2-cyclopentanediol tetralin
1,2-pentanediol decalin
1,2-hexanediol monoglyme
diglyme
tetraglyme
acetone
acetaldehyde
[0056] It should be noted that although there are specific advantages of
using a divided
cell, embodiments may be constructed in which the cell is undivided. This cell
may be
summarized as follows:
Pt 11 R-S03-Na + NaOH + H20 11 Pt
The Pt electrodes may be replaced by other electrodes, as outline herein.
Also, the base
NaOH may be replaced by other bases (such as sodium methylate, or other
bases), as desired.
Likewise, the solvent, water, may be replaced by other solvents, as desired.
In this
embodiment, the anode reaction is a desulfoxylation reaction to form SO, and R-
R. The
cathode reaction is a reduction to form hydrogen gas (the H being provided by
the water). In
-19-
CA 02902988 2015-08-27
WO 2014/138252
PCT/US2014/020786
other embodiments, CH3-S03-Na (or other R groups) may optionally be used.
Similarly, the
acidic form of the sodium salt may be used, provided that there is also base
to convert it to a
sodium salt.
[0057] Although many of the examples provided herein involve the use of
monosulfonic
acids, disulfonic acids or polysulfonic acids may also be used. However, when
using
disulfonic acids or polysulfonic acids, steps (in some embodiments) may be
taken to avoid or
reduce polymerization. This polymerization reaction is summarized below by a
disulfonic
acid, but a similar reaction is possible for a polysulfonic acid:
(desulfoxylation)
Na-503-R-R-503-Na ¨> =R-R.
(sodium salt of a disulfonic acid)
Since these hydrocarbon radicals have reactive sites at each end, these .R-R.
radicals could
then line up to polymerize:
.... =R-R. + =R-R. =R-R. + =R-R. ....
In some embodiments, such polymerization may be desired. In other embodiments,
polymerization is not desired. Accordingly, techniques may be employed to
reduce the
likelihood of polymerization (e.g., "cut off' the polymerization). This may
involve, for
example, forming methyl radicals (CH3.) via acetate, forming H. radicals to
truncate the R
group. Likewise, the techniques associated with using a mixed solvent system
may also
reduce such polymerization. For example, by using a nonpolar solvent in
combination with a
polar solvent in the anolyte, the formed hydrocarbon will be pulled into the
non-polar solvent
quickly, thereby preventing it from polymerizing.
[0058] Various examples of the techniques described herein may be used and
performed
readily. Some of these examples include:
(hydrocarbon (hexane))
2C3H7503-Na ¨> C61114 + 2S0
+ 2e- + 2Na+
(waxy hydrocarbon)
2C18H34503-Na ¨> R-R +2 SO
x +26+ 2Na+
-20-
CA 02902988 2015-08-27
WO 2014/138252 PCT/US2014/020786
(hydrocarbon in an organic layer)
Na-S03-(CH3)3-S03-Na ¨> R-R + 2SOõ +26+ 2Na+
C 1 7H33S03-Na + C 1 7H31 S03-Na + CH3S03-Na
¨> mixture of products including C17 and C18 hydrocarbons
[0059] The present invention may be embodied in other specific forms
without departing
from its structures, methods, or other essential characteristics as broadly
described herein and
claimed hereinafter. The described embodiments are to be considered in all
respects only as
illustrative, and not restrictive. The scope of the invention is, therefore,
indicated by the
appended claims, rather than by the foregoing description. All changes that
come within the
meaning and range of equivalency of the claims are to be embraced within their
scope.
-21-