Note: Descriptions are shown in the official language in which they were submitted.
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
TITLE
[0001] Methods and Systems for Enhanced Microfluidic Processing
CROSS REFERENCE TO RELATED APPLICATIONS
[0002] This application claims the benefit of U.S. Provisional Application
No.
61/771,708, filed on Mar. 1, 2013, which is hereby incorporated in its
entirety by reference.
[0003] This application is related to U.S. Provisional Application No.
61/771,694, filed
on Mar. 1, 2013, which is hereby incorporated in its entirety by reference.
STATEMENT REGARDING FEDERALLY SPONSORED RESEARCH OR
DEVELOPMENT
[0004] This invention was made with government support under NIH contract
HH5N272200900029C and NIH grant 2R44AI073221, awarded by the National
Institutes of
Health. The government has certain rights in the invention.
BACKGROUND OF THE INVENTION
Field of the invention
[0005] The invention relates to methods and systems for moving and
processing fluid
through an assay system.
Description of the Related Art
[0006] Microfluidic processing systems are to carry out chemical or
biochemical
reactions, or sequences of reactions, with small volumes (typically between 1
microliter and
milliliters) of reactants and products. A microfluidic processing system can
comprise a
network of tubes interfaced with discrete components such as valves and
sensors, or an
integrated device made of plastic, glass, metal, or other materials, or a
combination of
materials, with components such as valves and sensors built into the device
and connected by
flow passageways formed in the material.
[0007] Conventional microfluidic processing systems use reciprocating
displacement
pumps, peristaltic effects, syringe pumps, surface tension effects, body
forces on magnetic
beads from external or internal magnetic field sources, vacuum manifolds,
electrokinetic
effects, electrochemical effects, or a combination of these to carry out
chemical or
biochemical reactions or sequences of reactions.
[0008] Flows in microfluidic processing systems are typically associated
with dominance
of viscous effects over inertial effects, referred to as a low Reynolds number
regime. Many
applications of microfluidic processing systems involve one or more high-
molecular-weight
1
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
reactants with correspondingly low binary diffusivities. For example,
molecular dynamic
simulations indicate that the ribonucleic acid chain of approximately 9800
bases which
constitutes the genomic material of the human immunodeficiency virus (HIV),
with a
molecular weight of 3.1 x 106 daltons, has a diffusivity in water of
approximately D=2x10-12
m2 s-1, such that, in 10 minutes, one-dimensional diffusion is associated with
displacement of
only 50 microns. The combination of the dominance of viscous effects over
inertial effects
and the relatively slow diffusivities of reactants of high interest imposes a
need for fluid
mechanical mechanisms for macroscopically mixing two or more solutions in
microfluidic
systems.
[0009] Small volumes of gases are often found in microfluidic systems,
having been
either introduced as part of the process to be carried out or arising
inadvertently, such as
when an expansion or contraction of a fluid passageway in the direction of
flow tends to trap
bubbles during filling. A volume of gas in a microfluidic system can act as a
low-pass filter
with respect to mechanical forcing functions acting on the system. This is
sometimes
referred to as fluidic capacitance. Tubing can also be a source of fluidic
capacitance.
[0010] The tendency of trapped air to act as a low-pass filter creates an
incentive to locate
a fluidic actuator in close physical proximity to the fluid volume on which
said actuator is to
apply force and do mechanical work.
[0011] In some applications of microfluidic systems, there is a need for
the reactions to
take place within fluid passageways which can be discarded after a single use.
For example,
in infectious disease diagnostics, a microfluidic system used to process a
body fluid sample
can be considered a biohazardous waste after completion of the assay. The very
high
negative impact of contamination between production runs creates an incentive
for
microfluidic systems used for antibody purification to be fully disposable
after a single use.
Many types of microfluidic actuators, such as piezoelectric actuators and
electromagnetic
actuators, are too expensive to include in a microfluidic cartridge for a
single use.
Piezoelectric actuators and electromagnetic actuators require mechanical
energy transfer into
the cartridge and can be prone to failure associated with misalignment of the
actuator and the
cartridge. Actuation mechanisms, such as electrochemical gas generation and
surface
tension-based actuation, can be economically built into cartridges, but are
associated with
slow response times, low power output, lack of range, and other limitations.
[0012] There is a need for microfluidic systems which can carry out rapid
macroscopic
mixing of one or more reactants. A fast response time and high power of a
fluidic actuator
2
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
are important for mixing two or more fluids or for reacting two or more
species in a mixture
in the cartridge. Current microfluidic actuators have limitations of low fluid
power
generation capacity, sustaining power and slow response times. While
electroosmotic flow
generation can be associated with high power and fast response times, in some
cases, samples
cannot be transported through an EO microfluidic device because particles in
the sample
could block the EO device, and the fluid would be adversely affected by the
high electric
fields inside the EO device.
[0013] The present invention addresses these and other shortcomings of the
prior art.
SUMMARY OF THE INVENTION
[0014] The invention comprises a microfluidic processing system including a
plurality of
fluid passageways, at least one junction connecting the plurality of fluid
passageways, at least
two mechanisms for fluid transport including at least one high-performance
fluidic actuator.
The high-performance fluidic actuator has a fluid power generation capacity of
at least 10-8
watts, is capable of sustaining the power for at least 30 seconds, and has a
response time for
fluid power generation of less than 10 seconds.
[0015] In some embodiments, the microfluidic processing system is an
integrated system,
referred to as a cartridge. In some embodiments, the cartridge has a displaced
volume less
than or equal to five hundred cubic centimeters, or less than or equal to
fifty cubic
centimeters.
[0016] In some embodiments, the high-performance fluidic actuator is
capable of
transducing electrical power directly into fluidic power. In some embodiments,
operation of
the high-performance fluidic actuator does not comprise a transfer of
mechanical energy from
an external device to the at least one high-performance fluidic actuator.
[0017] In some embodiments, the response time for power generation is less
than 2
seconds, less than 0.2 seconds, or less than 0.04 seconds. In one embodiment,
the actuator is
capable of acting on at least 10 microliters of liquid, such that the liquid
flows through a
fluidic resistance associated with a pressure drop of at least 1 kPa at a flow
rate of at least 0.1
mL per minute.
[0018] In another embodiment, the high-performance actuator is coupled to a
pulse
generator or other controlled time-varying voltage source. In some
embodiments, the high-
performance fluidic actuator is capable of producing fluidic power through
electrokinetic
effects. In some embodiments, the electrokinetic effect is electroosmotic
flow. The
3
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
electroosmotic flow may be generated within a slit capillary or within the
interstices of a slat
structure within each at least one fluidic actuator.
[0019] In another embodiment, the electroosmotic flow is generated within a
bed of
packed beads, a monolithic porous structure, or an array of cylindrical
channels within each
of the fluidic actuators.
[0020] In some embodiments, the microfluidic cartridge includes an opening
for
receiving a starting material into the network of fluid passageways. The
opening can be
closed with a plug or a capping element. The plug or capping element is
capable of receiving
a fluid conduit and sealing shut when the fluid conduit is withdrawn. In other
embodiments,
the fluid conduit is capable of being received by the plug or capping element
and can
comprise a needle, a tube, a rigid fluid conduit, or a semi-rigid fluid
conduit. The plug or
capping element can comprise an elastomeric material. In another embodiment,
the plug or
capping element has a closing mechanism.
[0021] In other embodiments, the cartridge includes a controller capable of
controlling
power delivery from a power source to the high-performance fluidic actuator.
The cartridge
can include a power source operatively coupled to the at least one high-
performance fluidic
actuator. The power source can be located in an external device and coupled to
the cartridge
by an electrical connection. In some embodiments, the power source is
electrical or
pneumatic. The power source can be a battery that can be located inside the
cartridge. In
other embodiments, the battery can be located in an external device and
coupled to the
cartridge by an electrical connection.
[0022] The cartridge can include a second opening for receiving a
processing fluid that is
coupled to the network of fluid passageways. The processing fluid can be
contained within
the network of fluid passageways. The processing fluid can include a first
reagent capable of
lysing a cell or a cellular organelle. The first reagent comprises a detergent
or other
surfactant. In another embodiment, the first reagent comprises an enzyme, such
as a
lysozyme.
[0023] In some embodiments, the processing fluid comprises a homogenization
solution
capable of homogenizing a tissue sample or other heterogeneous biological
material.
[0024] In other embodiments, the processing fluid comprises a solution
capable of
diminishing or eliminating biological activity of a living cell, tissue, or
organism. The
processing fluid can comprise a glass bead or other solid material capable of
causing
mechanical disruption of the starting material. In some embodiments, the
processing fluid
4
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
can comprise a glycogen or other polysaccharide. The processing fluid can
include a carrier
RNA.
[0025] In some embodiments, the cartridge includes a third opening for
receiving an
actuator fluid that is coupled to the high-performance fluidic actuator. The
actuator working
fluid can be situated within the at least one high-performance fluidic
actuator.
[0026] In another embodiment, a portion of the network of fluidic
passageways
comprises a second reagent. The second reagent can include a silica bead, a
particle, or a
paramagnetic bead. The second reagent can also be a fluorescent bead or a
fluorescent
molecule. The second reagent can be a chemiluminescent molecule, such an
alkaline
phosphatase substrate, or a lanthanide or a lanthanide chelate. In other
embodiments, the
second reagent comprises a monoclonal or a polyclonal antibody, and the
monoclonal or
polyclonal antibody can be linked to a signaling molecule.
[0027] The second reagent can be an oligonucleotide probe or primer, or a
combination
of probes or a combination of primers. The oligonucleotide probe specifically
can bind to a
defined region of the genetic material of human immunodeficiency virus, a
hepatitis C virus,
a hepatitis B virus, a M. tuberculosis bacterium, a C. trachomatis bacterium,
an influenza
virus, respiratory syncytial virus, or another virus of the human respiratory
tract. The
oligonucleotide probe can bind to a defined region of the DNA or RNA of a
cancer gene. In
some embodiments, the oligonucleotide probe is labeled, and the label can be a
fluorescent or
a luminescent signaling molecule or a quencher thereof, an aptamer, a
photosensitizer
molecule, a photoactive indicator precursor molecule, or a photosensitizer
molecule and a
photoactive indicator precursor molecule.
[0028] In some embodiments, the photosensitizer molecule and the
photoactive indicator
precursor molecule comprise: at least one sensitizer label particle comprising
one or more
sensitizer agents, one or more sensitizer oligonucleotides, and a matrix for
co-locating such
sensitizer agents and sensitizer oligonucleotide(s); and at least one emitter
label particle
comprising one or more emitter agents, one or more sensitizer
oligonucleotides, and a matrix
for co-locating such emitter agent(s) and emitter oligonucleotide(s). The
photosensitizer
molecule is capable in an excited state of generating a singlet oxygen
molecule. The
photoactive indicator precursor molecule is capable of reacting with a singlet
oxygen
molecule to form a photoactive indicator.
[0029] In other embodiments, the second reagent can be a quantum dot or
other
crystalline semiconductor particle. The second reagent can be a nucleic acid-
specific
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
fluorescent or luminescent dye for sequence-independent measurement of nucleic
acids. The
second reagent can be a molecule capable of participating in Forster Resonance
Energy
Transfer (FRET) or other resonance energy transfer process. In another
embodiment, the
second reagent comprises a labeled protein, a labeled nucleic acid, or a
labeled carbohydrate
species for measurement of a specific cellular compound.
[0030] The second reagent can include a solution having a dye for specific
or non-
specific labeling of a cell. The second reagent can include a primer, a probe,
a combination
of a primer and a probe, or an enzyme capable of catalyzing a polymerase chain
reaction, a
transcription-mediated amplification, a nucleic acid sequence-based
amplification, or another
chemical reaction for amplifying at least one specified nucleic acid sequence.
The enzyme
can be a DNA polymerase, a reverse transcriptase, an RNA polymerase, an RNAse
H, a DNA
helicase, or a recombinase.
[0031] In another embodiment, the starting material comprises a fluid
phase, a fluid-laden
matrix, or a solid phase. The starting material can be blood, sputum, or other
bodily fluid.
The starting material can include a biological tissue, a raw material or
intermediary for a
pharmacological agent or a vaccine, an agricultural product, soil or another
environmental
sample.
[0032] In one embodiment, the cartridge includes a first fluid passageway
comprising a
first substance and a second fluid passageway comprising a second substance,
wherein said
first fluid passageway and said second fluid passageway form a junction in
said microfluidic
cartridge. In another embodiment, the junction is a T-junction or a Y-
junction. In yet
another embodiment, the junction allows formation of one or more microfluidic
droplets
generated from merging of said first and second substances from said first and
second fluid
passageways. In other embodiments, the one or more droplets each comprise an
analyte or a
reagent. In another embodiment, the one or more droplets each comprise at
least one primer
and an enzyme capable of catalyzing a polymerase chain reaction, a
transcription-mediated
amplification, a nucleic acid sequence-based amplification, or another
chemical reaction for
amplifying at least one target nucleic acid sequence. In some embodiments, the
one or more
droplets each comprise a label. In other embodiments, the first or second
substances
comprise a processing fluid. In another embodiment, the one or more droplets
each comprise
a cell.
[0033] In another embodiment, the cartridge includes a plurality of fluid
passageways
comprising different temperature zones for performing stages of an
amplification reaction. In
6
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
one embodiment, a plurality of fluids are combined in said plurality of fluid
passageways to
trigger a labeling or hybridization reaction.
[0034] The invention comprises a system including the microfluidic
cartridge described
above and an apparatus comprising a power source and adapted in some
embodiments for
sourcing electrical power to the microfluidic cartridge. In other embodiments,
the
microfluidic cartridge has an onboard power source. The apparatus is further
adapted for
sensing an indicator of assay outcome. The sensor can sense visible light or
another type of
electromagnetic radiation generated within the cartridge. In some embodiments,
the
apparatus is further adapted for sensing a location or a distribution of
paramagnetic beads
within the cartridge. The apparatus can be adapted for sensing electron spin
nuclear magnetic
resonance or other physical property of a species within the cartridge.
[0035] Another embodiment includes a method comprising providing a first
fluid to a
channel connected to a plurality of fluid passageways, including at least one
junction among
such fluid passageways, in a microfluidic cartridge. The microfluidic
cartridge includes at
least one high-speed microfluidic actuators having a fluid power generation
capacity of at
least 108 wattsand capable of sustaining the power for at least 30 seconds and
a response
time for power generation of less than 10 seconds. The method includes
operating the
microfluidic actuators in a time-varying manner, such that the first fluid and
a second fluid
are introduced into the network of fluid passageways to generate alternating
plugs of fluids,
wherein a length of each plug volume is less than 5 times the smallest average
diameter
among such fluid passageways. The high-speed microfluidic actuator can produce
fluid
power by an electrokinetic effect. The electrokinetic effect can be generated
by an
electroosmotic flow generated within an array of slits, a packed bead bed, or
a monolithic
porous structure.
[0036] The method includes labeling a subset of cells within the first
fluid with a labeling
molecule or a labeling particle within the second fluid specific for at least
one type of
molecule in a cell membrane. The method can include dying a cell in the first
fluid with a
cell permeating dye contained in the second fluid.
[0037] In other embodiments, the method includes labeling a subset of DNA
or RNA
contained within the first fluid with a photosensitizer molecule or a
photoactive indicator
precursor molecule or a combination thereof contained in the second fluid. The
method can
also include labeling a subset of DNA or RNA contained within the first fluid
with a
lanthanide chelate contained in the second fluid. The method includes lysing a
cell or other
7
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
biological material within the first fluid with a detergent or other
surfactant contained in the
second fluid. The detergent can be sodium lauryl sulfate,
hexadecyltrimethylammonium
bromide, or another cationic detergent.
[0038] In another embodiment, the method includes lysing a cell or other
biological
material within the first fluid with an enzyme. The enzyme can be a lysozyme.
The method
further comprises homogenizing a tissue sample or other heterogeneous
biological material
from the first fluid. The method also includes reducing the biological
activity of a living cell,
tissue, or organism in the first fluid. The reducing of biological activity
step can include
using a highly basic solution, such as sodium hydroxide or sodium
hypochlorite.
[0039] The method further includes lysing a cell or other biological
material in the first
fluid with a glass bead or other solid material for mechanical disruption in
the second fluid.
The method includes mixing a swab or a porous matrix with the first fluid and
releasing soil
or other environmental samples bound within the swab or the porous matrix.
[0040] In one embodiment, the first fluid comprises a dendritic cell, and
the method
includes pulsing the dendritic cells to induce an element of an immune
response to insult.
[0041] The method can include producing a pharmacological substance or a
vaccine. The
method includes increasing the bioactivity of a pharmacological substance. The
method can
also include binding a DNA or an RNA molecule contained within the first fluid
to glycogen
or silica. The method also includes purifying the glycogen-complexed or co-
precipitated
DNA and RNA or purifying the DNA or RNA molecule bound to a silica bead or a
silica-
containing structure. The method includes eluting the DNA and RNA from the
glycogen or
silica bead or silica-containing structure.
[0042] The method also includes detecting a presence or an absence of an
analyte in the
first fluid. The detecting comprises sensing visible light or another type of
electromagnetic
radiation from a chemiluminescent or fluorescent molecule coupled to the
analyte. Detecting
can include sensing a location or a distribution of paramagnetic beads coupled
to the analyte
or sensing nuclear magnetic resonance or other physical properties of a
species coupled to the
analyte.
[0043] In one embodiment, the method also includes steps for generating a
plurality of
microdroplets in the plurality of fluid passageways. In another embodiment,
the plurality of
microdroplets are formed by pulsating at least two fluids, wherein pulsating
is generated by a
plurality of high-speed microfluidic actuators in the microfluidic cartridge.
The method can
also include detecting a presence or an absence of an analyte in each of the
plurality of
8
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
microdroplets. In another embodiment, the method includes performing an
amplification
reaction in each of the plurality of microdroplets by moving the plurality of
microdroplets
through a plurality of temperature zones in the microfluidic cartridge. In yet
another
embodiment, the method includes detecting a presence of a target amplicon in
each of the
plurality of microdroplets. The method also includes measuring a melting
temperature (Tõ)
of a target nucleic acid molecule in each of said plurality of microdroplets.
In one
embodiment, the method includes performing a melting temperature (Tõ) analysis
of genetic
divergence of a virus RNA from a reference strain.
BRIEF DESCRIPTION OF THE SEVERAL VIEWS OF THE DRAWINGS
[0044] The figures depict various embodiments of the present invention for
purposes of
illustration only. One skilled in the art will readily recognize from the
following discussion
that alternative embodiments of the structures and methods illustrated herein
may be
employed without departing from the principles of the invention described
herein.
[0045] Figure (FIG.) 1 is an example of a top-down, cut-away view of the
interior of a
microfluidic cartridge, according to one embodiment of the invention.
[0046] FIG. 2 illustrates the processing of fluids each containing a single
dissolved
substance and the concentrations of each of the two dissolved substances as
spatially
averaged across a short channel section of the fluid passageway downstream of
junction and
plotted as a function of time, according to one embodiment of the invention.
[0047] FIG. 3 illustrates a graph showing a functional relationship between
the maximum
voltage and plug width for two fluids downstream of the junction, according to
one
embodiment of the invention.
[0048] FIG. 4 illustrates various flow passageway junction geometries,
according to one
embodiment of the invention.
[0049] FIG. 5 illustrates a graph showing the plug width vs. microactuator
voltage for
microfluidic cartridges, according to one embodiment of the invention.
[0050] FIG. 6 illustrates a graph showing the plug width vs. microactuator
voltage for a
various neck-down diffuser junction designs of the short-plug-width region,
according to one
embodiment of the invention.
[0051] FIG. 7 is an example of a side, cut-away view of the microfluidic
cartridge,
according to one embodiment of the invention.
[0052] FIG. 8 is an example of a side, cut-away view of the microfluidic
cartridge,
including an opening, according to one embodiment of the invention.
9
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
[0053] FIG. 9 is an example of a side, cut-away view of the microfluidic
cartridge,
including viewing window, according to one embodiment of the invention.
[0054] FIG. 10 is an example of a side, cut-away view of the microfluidic
cartridge,
including a microfluidic actuator and electrodes, according to one embodiment
of the
invention.
[0055] FIG. 11 is an example of fluidic plugs in the interior channel of
the microfluidic
cartridge, according to one embodiment of the invention.
[0056] FIG. 12 is an example of an instrument that docks to the
microfluidic cartridge,
according to one embodiment of the invention.
[0057] FIG. 13 illustrates an example of the fluidic plugs generated in the
fluid
passageways of the microfluidic cartridge, according to one embodiment of the
invention.
[0058] FIG. 14 is a photograph of a microfluidic cartridge and an
instrument for
enhanced microfluidic processing, according to an embodiment of the invention.
[0059] FIG. 15 is a photograph of a microfluidic cartridge for enhanced
microfluidic
processing, according to an embodiment of the invention.
[0060] FIG. 16 is an isometric view of an exemplary microfluidic cartridge
for carrying
out processing steps on a sample, according to an embodiment of the invention.
[0061] FIG. 17 is top view of a microfluidic cartridge, according to an
embodiment of the
invention.
[0062] FIG. 18 shows that the high uniformity of the microfluidic cartridge
for use in
biochemical processes under identical prescribed conditions at different
times, according to
an embodiment of the invention. A series of bead-binding experiments were
conducted with
an oligonucleotide target present in the starting solution at concentrations
of 1x10-13 M, 1x10
12
M, and 1x1 0-11 M. Under the control of a high performance actuator, the
target-containing
solution was mixed with a solution containing two types of beads, and
fluorescence was
measured at approximately 610 nanometers, with singlet oxygen as an
intermediary, such that
the emitted light persists after extinguishing of the excitation source. The
plotted values are
indications of the starting concentration of target. At least ten assays were
carried out at each
concentration.
[0063] FIG. 19 shows that the amount of time required for a biochemical
reaction to
reach a desired endpoint, using a microfluidic cartridge of the invention. An
assay similar to
that described for FIG. 18 was performed.
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
[0064] FIG. 20 is an example of electrical potential waveforms applied to
pairs of high-
performance actuators to achieve rapid pulsatile flow at a microfluidic
junction, according to
an embodiment of the invention.
[0065] FIG. 21 illustrates an example junction geometry for synchronizing
mixing of
fluids, according to an embodiment of the invention.
[0066] FIG. 22 is a process flow diagram for a quantitative real-time
polymerase chain
reaction assay using a microfluidic cartridge of the invention for
applications such as
quantitation of HIV genetic material, according to an embodiment of the
invention.
[0067] FIG. 23 depicts an exemplary architecture for using a microfluidic
cartridge of the
invention in the processing of partitions of fluids, where each partition, or
set of partitions,
can undergo a process selected for that partition or set of partitions,
according to an
embodiment of the invention.
[0068] FIG. 24 depicts discrete processing of fluid partitions or sets of
partitions using
the microfluidic cartridge, according to an embodiment of the invention.
[0069] FIG. 25 shows an example of discrete processing of fluid partitions
or sets of
partitions using the microfluidic cartridge, according to an embodiment of the
invention.
DETAILED DESCRIPTION OF THE INVENTION
Overview
[0070] Flows in microfluidic processing systems are typically associated
with dominance
of viscous effects over inertial effects, referred to as a low Reynolds number
regime [1], [2].
Many applications of microfluidic processing systems involve one or more high-
molecular-
weight reactants [3], [4], with correspondingly low binary diffusivities. For
example,
molecular dynamic simulations [5] indicate that the ribonucleic acid chain of
approximately
9800 bases which constitutes the genomic material of the human
immunodeficiency virus
(HIV), with a molecular weight of 3.1 x 106 daltons, has a diffusivity in
water of
approximately D=2x10-12 m2 s-1, such that, in 10 minutes, one-dimensional
diffusion is
associated with displacement of only 50 microns. The combination of the
dominance of
viscous effects over inertial effects and the relatively slow diffusivities of
reactants of high
interest imposes a need for fluid mechanical mechanisms for macroscopically
mixing two or
more solutions in microfluidic systems.
[0071] When an aqueous solution contacts a surface such as glass or silica,
the surface
becomes negatively charged due to the depronation of surface silanol groups.
An electrical
double layer forms as a result of the depronation. The surface charge attracts
dissolved
11
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
counter-ions and repels co-ions, resulting in a charge separation. The Debye
length is the
characteristic thickness of the double layer. The mobile ions in the diffuse
counter-ion layer
are driven by an externally applied electrical field, and the moving ions drag
along bulk
liquid through viscous force interaction.
[0072] The average velocity of electroosmotic flow generated between two
wide parallel
surfaces by the application of an axial electric field Ex is:
a- d p
= - ¨ - 1 - Ka)]
1,1 d v u-
[0073] where a is one-half the separation distance between the two pumping
surfaces, t is
the fluid viscosity, dp/dx is the pressure gradient counter to the flow, e is
the fluid
permittivity, is the zeta potential, a is the ionic energy parameter, and G is
the correction
term for the thickness of the double layer. The wide parallel surfaces become
charged,
attracting counter-ions and repelling co-ions, to form a charge double layer.
The outer layer
of ions of the double layer is mobile. Applying an axial electric field exerts
forces on the
mobile ions and electromigration of the mobile ions drag the bulk fluid
through viscous
interaction. The zeta potential characterizes the effect of the surface
condition on the
electroosmotic flow. The zeta potential is an empirical parameter associated
with the net
excess of surface charge-balancing ions near the surface/fluid interface.
Definitions
[0074] Terms used in the claims and specification are defined as set forth
below unless
otherwise specified.
[0075] "Electroosmotic flow" refers to the movement of liquid induced by an
applied
potential across a fluid conduit. The fluid conduit can be any porous
material, capillary tube,
membrane, substrate, microchannel or passageway for allowing the flow of
liquid. The
electric potential can be applied between any two parallel surfaces.
[0076] A "microfluidic actuator" or "fluidic actuator" refers to a
component that
converts electrical power or another readily stored or generated form of
energy into fluid
power, meaning the application of force on a mass of fluid to transport said
mass of fluid
through a pressure gradient [6].
[0077] "Taylor dispersion" refers to the transport and spreading of a mass
of solute in
laminar flow through a long, straight tube or other similar flow passageway,
such mass of
12
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
solute initially confined within a plug (or a plurality of plugs) within the
flow, such plugs
having axial dimensions on the same order as the tube cross-section [2].
[0078] "Zeta potential" refers to an empirical or semi-empirical parameter
included in
many mathematical models of electroosmotic flow, where, other factors being
equal, a higher
absolute value of a zeta potential is generally associated with higher flow
rates and/or higher
maximum back pressures [7], [8].
[0079] It must be noted that, as used in the specification and the appended
claims, the
singular forms "a," "an" and "the" include plural references unless the
context clearly dictates
otherwise.
[0080] Overview of Microfluidic System
[0081] The invention includes a microfluidic system, such as a cartridge or
similarly
enclosed fluid processing device. In some embodiments, the microfluidic
cartridge has no
moving mechanical parts and eliminates failure modes associated with sliding
contacts,
fluidic fittings, etc. In one embodiment, the microfluidic cartridge runs on
battery power and
incorporates EO fluidic actuation without the need of an external syringe pump
or some other
means of fluidic actuation. In another embodiment, the microfluidic cartridge
includes an
internal mechanism for moving fluid that is pressurized by a microfluidic
actuator.
[0082] In some embodiments, the microfluidic cartridge is small in size and
can be used
with a hand-held, portable device. For example, the cartridge may be less than
40 cm3 in
volume (2 cm x 2 cm x 10 cm = 40 cm3). In addition, the cartridge can have a
displacement
volume of 50-500 cc's. For example, the cartridge can be small enough to fit
in a person's
hand and sized for manufacturing in large quantities at low cost.
[0083] The microfluidic system includes a network of fluidic passageways.
The
passageways can include pipes, tubes, enclosed channels, or other enclosed
structures for
holding and allowing transport of fluids. The fluidic passageways can be
loaded with small
quantities of at least two different fluids. The fluids can have a volume of
less than 10
milliliters each, for example. In one embodiment, at least one of the fluids
is loaded into the
cartridge at or around the time of operation through a port. In other
embodiments, fluids are
pre-loaded into the cartridge.
[0084] The network of fluidic passageways can be connected by one or more
junctions.
Each junction joins two or more fluidic passageways and can be configured in
various
arrangements and designs.
13
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
[0085] The microfluidic cartridge includes at least two microfluidic
actuators, and at least
one microfluidic actuator is a high performance microfluidic actuator. The at
least one high
performance microfluidic actuator has a fluid power generation capacity of at
least 10-8 watts,
is capable of sustaining power for at least 30 seconds, and has a response
time for power
generation of less than 10 seconds. The network of fluidic passageways is in
fluid
communication with the microfluidic actuators.
[0086] The microfluidic system may be a cartridge made of plastic, glass,
or other
materials. Fluid passageways and other features within the cartridge may be
produced by
machining, hot-embossing, injection molding, or other means. The cartridge may
be
assembled from multiple pieces by thermal bonding, laser welding, ultrasonic
welding, or
through the use of epoxies or pressure-sensitive adhesives or other adhesive
means.
[0087] The microfluidic actuators of the invention may operate through the
generation of
electroosmotic flow.
[0088] The microfluidic actuators of the invention may be made from
silicon, glass,
plastic, or other materials. In some embodiments, the microfluidic actuator is
made from a
single-crystal silicon wafer coated with multiple layers of silicon oxide and
silicon nitride,
with the openings in the single-crystal silicon wafer made by a
photolithographic feature
definition process followed by time-multiplexed inductively coupled plasma (TM-
ICP)
etching, also known as deep-reactive ion enhanced (DRIE) etching [9]. The
microfluidic
actuators can be produced from a single-crystal silicon wafer by a simple, one-
step
photolithographic process. These microfluidic actuators are economical for
incorporation
into single-use microfluidic cartridges for a variety of applications.
[0089] In some embodiments, the microfluidic cartridge is designed to dock
or couple
with an instrument for analyzing or processing the fluids or samples inside
the cartridge. The
instrument can include various detection or monitoring components for
analyzing the fluids
or samples, and can include a power supply or electrical circuitry for
providing energy to the
cartridge.
[0090] In some embodiments, electrical power source and associated
circuitry is built into
the cartridge, which operates without connection to external hardware.
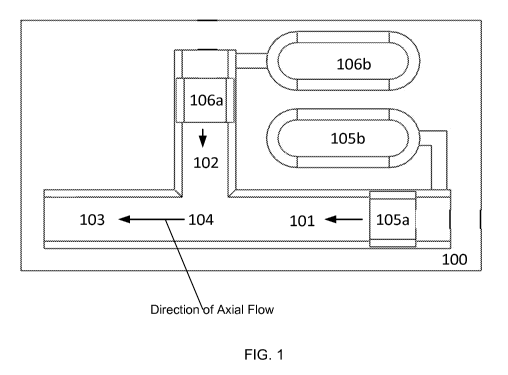
[0091] In FIG. 1, an example of a microfluidic cartridge 100 is shown from
a top-down,
cut-away perspective of the interior of the cartridge 100. The microfluidic
cartridge includes
a first fluidic passageway 101, a second fluidic passageway 102, a third
fluidic passageway
103, and a junction 104 that connects the first, second, and third fluidic
passageways. The
14
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
microfluidic cartridge includes a first pressure source and a second pressure
source. Each of
the pressure sources can be a microfluidic actuator 105b, 106b, at least one
of which is a high
performance microfluidic actuator. In some embodiments, the actuators may also
include one
or more pistons or piston-like elements, 105a and 106a. In some embodiments,
the piston-
like elements 105a and 105b may be plugs of solid material which form a
perimeter seal with
the inside of the fluid passageways within which said plugs travel.
[0092] In one embodiment, the microfluidic actuator 105b, 106b acts on a
processing
fluid contained within a fluidic passageway 101, 102 via the piston or piston-
like element
105a and 106a. For example, operation of the first microfluidic actuator 105b
pushes the
actuator's piston 105a forward. The movement of the piston 105a pressurizes a
fluid within
the fluidic passageway 101, causing such fluid to travel toward the junction
104. Similarly,
operation of the second microfluidic actuator 106b pushes the second piston
106a forward.
The movement of the piston 106a pressurizes a fluid within the fluidic
passageway 102,
causing such fluid to travel toward the junction 104. The two processing
fluids are joined
and mixed at the junction 104.
[0093] In other embodiments, pistons 105a, 106a are not present in the
cartridge as solid
elements. The actuator fluid is contained within or in fluidic contact with
the microfluidic
actuator 105b, 106b and is separated from the processing fluid in the fluidic
passageway by a
plug of a barrier fluid. In some embodiments, the barrier fluid is air or
another gas. Fluidic
movement of the actuator fluid causes the air plug to become pressurized and
to move
forward, which in turn, pressurizes and generates fluidic movement of the
processing fluid in
the fluidic passageway. The function of the air plug as a piston is enhanced
through surface
tension effects. In some embodiments, the interior surface of the fluid
passageway within
which the air plug travels is hydrophobic and free of sharp axial features
conducive to the
flow of the actuator working fluid along the wall of the passageway past the
air plug. In
some embodiments, a plug of an immiscible fluid functions as a piston. In some
embodiments, there is no plug of fluid separating the actuator fluid from the
processing fluid,
and the actuator fluid is in direct contact with the processing fluid in the
fluidic passageway,
but does not mix with the processing fluid (e.g., two immiscible fluids). The
movement of
the actuator fluid causes corresponding pressurization and movement of the
processing fluid.
[0094] Moving fluid through the fluidic passageways toward the junction 104
results in at
least one fluid passing through the junction 104 and into the third fluidic
passageway 103.
For fluidic passageways with cross-sectional dimensions less than 10 mm and
containing
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
liquid phase fluids, the flow of fluid within the passageways 101, 102 and
junction 104 can
be characteristically laminar.
[0095] The first and second fluidic actuators 105b and 106b can be operated
so that the
velocities and flow rates of the fluids within the first and second fluid
passageways 101 and
102 are nearly invariant over time, constant and result in semi-discrete fluid
laminae in the
region of the fluidic passageway 103 immediately beyond the junction 104.
Where there are
a series of cross-sections of fluid passageways over a distance of several
millimeters beyond
the junction 104 in the direction of flow (referred to as the axial
direction), the concentration
of a first fluid can be nearly 100% in one region of the cross-section, and
the concentration of
a second fluid is nearly 100% in another region. The persistence of such
spatial localization
as a function of the axial distance from the junction 104 is approximately
inversely
proportional to the diffusivities of the species in the processing fluids.
[0096] Deviations from the laminar flow operation described above, such as
alternating
plugs of processing fluid, can result from the time-varying action of one or
more of the
microfluidic actuators with corresponding time-varying pressurization and flow
of one or
more of the processing fluids. In one example, the first microfluidic actuator
105b is
operated with a square wave voltage input at a given frequency and a duty
cycle less than
100%, and the second microfluidic actuator 106b is operated with a square wave
voltage
input at the same frequency and at a duty cycle less than 100%, with the first
actuator square
wave out of phase with the second.
[0097] FIG. 2 is an example illustrating that the processing fluids are
aqueous solutions
each containing a single dissolved substance and the concentrations of each of
the two
dissolved substances are spatially averaged across a short channel section of
the fluid
passageway 103 downstream of junction 104 and plotted as a function of time.
The out-of-
phase operation of the actuators results in a sequential injection of
alternating plugs of fluids
contained in the fluid passageways 101 and 102. Because of predominance of
viscous forces
over inertial forces, molecular diffusion can be the primary mechanism by
which chemical
and biochemical constituents of two fluids intermingle when such fluids are
combined within
a microfluidic cartridge. Spatially non-uniform distributions of fluids can
shorten the
distances over which such diffusion takes place, speeding chemical and
biochemical
reactions.
[0098] FIG. 3 is an example of a functional relationship between the
maximum voltage,
duty cycle, and period of microactuator operation and plug width of the two
species
16
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
downstream of the junction 104. The data plotted in FIG. 3 were collected with
a cartridge of
the invention where the fluid passageways are cylindrical with diameter
approximately lmm.
The microfluidic actuators transduce electrical power into fluid power through
the generation
of electroosmotic flow in the interstices within a slat structure comprising
silicon coated with
thin films of silicon nitride and silicon oxide. One of the two solutions
contains a fluorescent
species, such that plug widths could be monitored by epifluorescent microscopy
with a CCD
camera. Voltages ranging from 75 V to 175 V were applied to the two actuators
operating
out of phase with a 50% duty cycle and on-state durations of 100 and 200
milliseconds. As
shown, fluid plugs measuring 2mm axial direction could be produced. Downstream
mixing
of the short plugs can occur through Taylor dispersion.
[0099] The minimum axial dimension of fluid plugs can be constrained by the
cross-
sectional dimensions of the flow passageways at the junction. FIG. 4 is an
example of a flow
passageway junction geometry in which the flow passageways neck down, or
decrease in
cross-sectional dimension, in the region immediately adjoining the junction.
[00100] FIG. 5 shows that a combination of the neck-down geometry and fast
microactuator response can produce very short plugs of fluid.
[00101] FIG. 6 illustrates a graph showing the plug width vs. microactuator
voltage for a
various neck-down diffuser junction designs of the short-plug-width region of
FIG. 4. A 50
millisecond on-state duration with a junction where the channels neck down
from lmm
diameter to 0.25 mm diameter produced plugs less than 50 microns in the axial
direction.
With plugs of this size, a first solution containing a relatively slow-
diffusing species such as
200 nm diameter beads will fully mix with a second solution in less than 10
minutes.
[00102] For greater control over differential fluid transport and/or to mix
multiple fluids
together, multiple microfluidic actuators can be used with multiple channels
and junctions for
moving and combining fluids. Each microfluidic actuator 105b, 106b is fluidly
connected to
an actuator fluid and generates flow of a processing fluid. For example, two
microfluidic
actuators 105b, 106b can generate mixing of two processing fluids. Next, the
mixture can be
joined with a third fluid in another fluidic passageway using the fluidic
pressure of two
additional microfluidic actuators.
[00103] In some embodiments, the microfluidic cartridge 100 is loaded with two
fluids,
one in the first fluidic passageway 101 and the other in the second fluidic
passageway 102.
In some embodiments, the fluids are loaded at or around the time of
manufacture of the
17
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
microfluidic cartridge. The microfluidic cartridge 100 can include actuator
fluid in fluidic
contact with each of the microfluidic actuators 105b, 106b.
[00104] In other embodiments, the microfluidic cartridge 100 is loaded with a
reagent in a
fluidic passageway 101, 102. The reagent can be a fluid phase form, a dried
reagent, or
attached to a surface or wall of the fluidic passageway (e.g., a bead or
particle). In some
embodiments, the reagent is in a processing fluid and includes a detergent or
other surfactant
for lysing a cell or cellular organelle. The reagent can be an enzyme, such as
a lysozyme. In
other embodiments, the reagent is an antibody, protein, peptide,
oligonucleotide, or particle
for binding, hybridizing or interacting with an analyte in the sample or
processing fluid.
Other examples of reagents are described in detail below.
[00105] FIG. 7 is an example of the microfluidic cartridge 100 of FIG. 1,
shown from a
side perspective of the interior of the cartridge 100. As in FIG. 1, the
microfluidic cartridge
includes a first fluidic passageway 101, a second fluidic passageway 102, a
third fluidic
passageway 103, and a junction 104, where the first and second fluidic
passageways meet.
The microfluidic cartridge includes a first fluidic actuator 105b and a second
fluidic actuator
106b. The cartridge also includes one or more pistons or piston-like elements
105a and 106a
that are pushed forward by the first and second fluidic actuators 105b, 106b.
[00106] Referring now to FIG. 8, an opening 801 is shown on the top of the
microfluidic
cartridge 100, which can be used to admit a starting material, sample, or
fluid for subsequent
processing. The opening is connected to the network of fluidic passageways.
The opening
can be connected to a fluidic passageway for processing the starting material.
To prevent
fluid from flowing out of the opening 801 during operation of the microfluidic
cartridge 100,
the opening 801 can have a cap, capping element, plug or other type of closure
802. In some
embodiments, the opening 801 can seal closed by a mechanism, such as a
pneumatic valve.
The opening 801 can be self-sealing through a passive mechanism, such as a
perforated
elastomeric structure that can elastically deform when acted upon by a narrow
conduit, such
as a syringe. In other embodiments, the plug or capping element 802 is capable
of receiving
a fluid conduit and sealing shut when the fluid conduit is withdrawn. The
fluid conduit can
be a needle, a tube, a rigid fluid conduit, or a semi-rigid fluid conduit. The
opening can also
be closed by a thermopheumatic effect, an electromagnetic effect or an
electrostatic effect.
[00107] In one embodiment, the microfluidic cartridge 100 includes at least
one
component or module to facilitate monitoring of a fluid process or for
analyzing the output of
a fluid process. In FIG. 9, the microfluidic cartridge 100 includes an
optically transparent
18
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
region 901 that allows the viewing or monitoring of the fluid in the third
fluidic passageway
103, such as the color, opacity, and other such physical properties of the
fluid. The
transparent region 901 can allow analysis of the fluid within the fluidic
passageway 103,
using techniques such as fluorescence, chemiluminescence, or other analytical
methods, such
as those described herein.
[00108] In FIG. 10, a first microfluidic actuator 1001 is shown and
includes a perforated
structure 1001a with fluidic passageways having at least one cross-sectional
within three
orders of magnitude of the characteristic thickness of the electric double
layer and at least one
electrode on each side of the perforated structure. The electrodes are
electrically connected to
metal contacts and are situated on either side of the microfluidic actuator
1001. An electric
field is applied across the electrodes. In one embodiment, an electric field
is applied across
the electrodes through traces or wires 1002 running through or along a portion
of the
microfluidic cartridge and terminating at contacts 1003.
[00109] In other embodiments, the microfluidic actuator 1001 is coupled to a
pulse
generator or other controlled time-varying voltage source and at least one
pair of electrodes.
The pulse generator or controlled time-varying voltage source can produce a
pattern of
voltage pulses or staggered voltage pulses to the microfluidic actuator 1001.
[00110] In some embodiments, the electroosmotic flow is generated within a
plurality of
slit capillaries within the microfluidic actuator 1001. The electroosmotic
flow can also be
generated within a bed of packed beads, within a monolithic porous structure,
or within an
array of cylindrical channels in the microfluidic actuator 1001.
[00111] In other embodiments, the microfluidic actuator 1001 is filled with an
actuator
fluid with chemical properties conducive to formation at the fluid-solid
interface of an
electric double layer with a high effective zeta potential (e.g. an aqueous
solution for a
perforated structure with internal perforation surfaces containing
predominantly oxygen and
silicon). Application of an electric field generates electroosmotic flow
within the
perforations of the microfluidic actuator 1001. For a perforated structure of
insulated silicon
with slit-like perforations with the smaller cross-sectional dimension between
1 and 10
microns, such electroosmotic flow can drive the fluid into the passageway 101
through fluidic
resistances and/or against pressure heads of 10 kPa or greater. The pressure
associated with
electroosmotic flow can develop within microseconds, with the primary
fundamental
limitation being the rate of momentum diffusion from the wall of each slit-
like perforation to
the center plane of each perforation.
19
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
[00112] In one embodiment, the microfluidic actuator 1001 has a fluid power
generation
capacity of at least 10-8 watts, is capable of sustaining power for at least
30 seconds, and has a
response time for power generation of less than 10 seconds, less than 2
seconds, less than 0.2
seconds, or less than 0.04 seconds, for example. The microfluidic actuator is
also capable of
pressurizing at least 10 microliters of liquid, such that the liquid flows
through a fluidic
resistance associated with a pressure drop of at least 1 kPa at a flow rate of
at least 0.1 mL
per minute.
[00113] The microfluidic actuators in the invention are distinguished by being
small
enough to fit into a cartridge of the prescribed size, by drawing
comparatively little power,
and by a fast response time. Each microfluidic actuator can be cycled on and
off (or
transition between different fluidic power generating states) at 0.1 hertz or
faster, and
preferably at 1 hertz or faster, and more preferably at 10 hertz or faster.
Equivalently, the
microfluidic actuators have a rise time of 10 seconds or less, or a rise time
of 1 second or
less, or a rise time of 0.1 second or less.
[00114] A fast response time and high power are important because the reaction
rate for
two species initially contained within separate fluid phases is markedly
faster when the two
fluid phases are introduced into a reaction channel in short, discrete plugs
compared to when
the two fluids are introduced into a reaction channel continuously or in long
plugs, or when
the two fluids are introduced into a well instead of a channel (i.e., a vessel
with interior
dimension aspect ratios of approximate unity as opposed to a fluid container
with one
dimension much greater than the other two dimensions, as in a pipe or enclosed
channel).
[00115] Referring now to FIG. 11, a diagram is shown of the interior of a
passageway in
the microfluidic cartridge 100. Spatial non-uniformity can facilitate reaction
of two fluid
phases through sequential injection of alternating plugs of the fluids
followed by pressure-
driven flow of the train of plugs through a fluid passageway 1100. Fluid flows
in the low
Reynolds number regime can be well modeled by assuming the flow velocity at
the fluid
passageway 1100 wall to be zero (the no-slip boundary condition). For a
cylindrical
passageway, the radial flow velocity profile is parabolic, described by the
equation:
u(r) = 2U [1 (r\ 2 i
a) i
[00116] where U is the average velocity, r is the radial coordinate, and a is
the radius of
the cylindrical passageway. As the plugs move down the fluid passageway, the
parabolic
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
flow profile causes corresponding plug distortion 1101, 1102. Particles
contained with the
plugs can diffuse radially from the distorted plugs 1103. The particles
diffuse radially
outward 1103a from the plug fronts near the fluid passageway centerline and
radially inward
1103b from the plug tails near the walls. This phenomenon is known as Taylor
dispersion,
which generates efficient mixing of two or more fluids. Similar diffusion
effects can arise in
non-cylindrical fluid passageways.
[00117] In FIG. 12, the microfluidic cartridge 100 is shown docking to an
instrument 1200
useful for facilitating enhanced fluid processing, for monitoring the
processing, for analyzing
the output of the process, or for other processing steps. The microfluidic
cartridge 100 can
include a sensor that senses visible light or another type of electromagnetic
radiation
generated within the cartridge. In one embodiment, the instrument 1200
includes an optical
detector, such as a CCD imager or photomultiplier tube, or other sensor 1201.
In another
embodiment, the microfluidic cartridge 100 includes a detector for detecting
fluorescent
emissions from fluorescently-labeled molecules.
[00118] In one embodiment, the instrument 1200 contains a power supply and
electrical
circuitry 1202 for supplying a time-varying voltage or other input to the
microfluidic actuator
1001. In another embodiment, the controlling voltage is supplied through a pin-
based
interconnect 1203 connected to the power supply/controller by a ribbon cable
1204. In some
embodiments, the power supply is a battery.
[00119] In other embodiments, the microfluidic cartridge 100 is coupled to an
external
power source. The external power source can be coupled to the microfluidic
cartridge 100 by
an electrical connection. The microfluidic cartridge 100 can include a
controller capable of
controlling power delivery from the power source. The power source can be
operatively
coupled to the microfluidic actuator 1001. In some embodiments, the power
source is
electrical, pneumatic, or is a battery. The battery can be located inside the
external device or
coupled to the microfluidic cartridge 100 by an electrical connection.
[00120] In some embodiments, the cartridge components are produced from
specialized
polystyrene and/or ABS plastic resins by injection molding. Cartridge
component joining
can be by die-cut pressure-sensitive adhesives by thermal bonding, by
ultrasonic welding, by
laser welding, by epoxies, by a combination of these means, or by other means.
[00121] FIG. 13 is an example of the alternating fluidic plugs generated in
the fluidic
passageways of the microfluidic cartridge, according to one embodiment of the
invention.
21
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
[00122] FIG. 14 shows an example microfluidic cartridge 1400 and an instrument
1401 for
enhanced microfluidic processing, according to an embodiment of the invention.
The
external housing of the instrument 1401 has been removed to show the internal
configuration. The microfluidic cartridge 1400 includes a microfluidic
actuator 1401 (four
actuators are outlined in black within the cartridge). FIG. 14 shows four
microfluidic
actuators 1401 inside the cartridge 1400. A network of microchannels 1405 is
formed in the
plastic material of the cartridge 1400. The network of microchannels 1405
includes channels
connecting to each of the two fluid ports of the microfluidic actuator 1401
and to each of the
two fluid ports of the other three microfluidic actuators. A circuit board
1404 includes
electrical contacts for each of the microfluidic actuator electrodes. The
electrical contacts are
routed to the instrument 1401 through a cable 1406 with interconnects. The
instrument 1401
includes a microprocessor, power management hardware, and other components for
controlling the voltages applied across the actuator's 1401 electrode pair and
across the
electrode pairs of the other three actuators. The functionality of the
instrument includes
sourcing independently controlled electrical potentials of 100 volts, 200
volts, 400 volts, or
other voltages, such electrical potentials being switchable under
microprocessor control at
frequencies of greater than 10 Hz.
[00123] FIG. 15 shows a microfluidic cartridge 1400 for enhanced microfluidic
processing, according to an embodiment of the invention. A bottom plate 1500
and a top
plate 1501 of the cartridge are shown separately from one another in this
figure to show the
internal configuration. When the two plates are fitted together, they form a
microfluidic
cartridge 1400, similar to one shown in FIG. 14. The cartridge is configured
for four high-
performance microfluidic actuators, which are each in a different stage of
assembly in this
photograph. The cartridge 1400 includes a bottom electrode 1502 and a
semiconductor chip
1503 with a slat structure for generating electroosmotic flow positioned atop
a bottom
electrode, with an intervening chip-sealing gasket. Another semiconductor chip
1504 is
similar to 1503 with an additional gasket placed on top of the slat structure
semiconductor
chip. The cartridge 1400 also includes an upper electrode 1505 with an
additional gasket.
[00124] FIG. 16 is an isometric view (mechanical drawing) of an example
microfluidic
cartridge 1600 for carrying out processing steps on a sample, according to an
embodiment of
the invention. The cartridge 1600 includes inlet ports 1601 and 1602, which
can fluidically
interface with a module containing at least one high-performance fluidic
actuator. Fluid
passageways 1604 and 1605 can each hold a volume of a fluid. In one example,
one of the
22
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
fluids can be butanol or another precipitating agent. In another example, one
of the fluids can
contain complexes susceptible to precipitation, such as polysaccharide-bound
nucleic
acids. The internal geometries of the fluid passageways 1604 and 1605 can be
engineered
such that prescribed fluids will exhibit prescribed flow characteristics
within the
passageways. For example, a fluid passageway carrying butanol can be
configured with
smaller cross-sectional dimensions (compared to a fluid passageway for holding
an aqueous
solution) to better maintain the integrity of a butanol flow front during
transport driven by a
microfluidic actuator. The cartridge 1600 can include chambers for receiving a
reactant. The
cartridge 1600 can be made of a cyclic olefin polymer or other polymer. The
cartridge 1600
can comprise elements formed from more than one material such that a cartridge
region
intended to store a solvent resists degradation over time and to achieve other
design
goals. The cartridge 1600 can include a chamber 1606 into which two solutions
are
transported, through the action of one or more microfluidic actuators, at
least one of which is
a high performance microfluidic actuator. The chamber 1606 can be configured
such that
buoyancy effects associated with different densities of two solutions or
phases to facilitate
mixing of the two solutions or phases. The two solutions can be a solvent and
a nucleic acid-
containing solution. The mixing of the solvent and the nucleic acid-containing
solution can
entail transporting the fluids into the chamber 1606 where surface tension
effects, buoyancy
effects, or a combination of these effects causes air bubbles to be retained
within such
chamber upon withdrawal of the liquid phase or phases from the chamber. The
cartridge
1600 includes a component 1607 incorporating a porous structure. A nucleic
acid-containing
solution or other solution, for example, can be passed through the porous
structure. This
passage can be followed by flowing of a solvent such as ethanol through the
porous structure
to wash away unbound material, such as proteins. Nucleic acids can be eluted
into a channel
1608 by passing water through the porous structure.
[00125] FIG. 17 is top view of an example microfluidic cartridge, according to
an
embodiment of the invention.
[00126] FIG. 18 shows data from experiments performed using the microfluidic
cartridge
or the invention. The data demonstrate that biochemical processes and other
processes with
high uniformity in the outcomes of multiple processes run under identical
prescribed
conditions at different times. A series of experiments were conducted with an
oligonucleotide target present in the starting solution at concentrations of
1x1013 M, 1x10-12
M, and 1x10-11 M. Under the control of a high performance actuator, the target-
containing
23
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
solution was mixed with a solution containing two types of beads. The two
types of beads
were functionalized with two types of probes, such that each oligonucleotide
target would
tend to bind one of each bead. The beads were dyed such that excitation by
light at
approximately 680 nanometers would result in emission at approximately 610
nanometers,
with singlet oxygen as an intermediary, such that the emitted light persists
after extinguishing
of the excitation source. The plotted values are indications of the starting
concentration of
target. At least ten assays were carried out at each concentration. The data
has been jittered
for clarity.
[00127] FIG. 19 shows data from experiments performed using the microfluidic
cartridge
or the invention. The data demonstrate that the microfluidic cartridge can be
used to shorten
the time required for a biochemical reaction or other process to reach a
desired endpoint, such
as a signal crossing a minimum threshold value. An assay similar to that
described for FIG.
18 was performed. The target-containing solution and the bead-containing
solution were
mixed at a junction with the flows driven by high-performance microfluidic
actuators. Assays were run under two conditions: 1) with rapid pulsatile flow
of the fluids
while the fluids combined in the junction, and 2) with continuous flow while
the fluids
combined at the junction. The combined solutions were then incubated for 10 or
20 minutes
and then read. As a control, assays were also run with no target in the target
solution. As
shown in FIG. 19, the luminescent signals (being approximately proportional to
the number
of bead pair-target complexes formed) for a 10 minute incubation with rapid
pulsatile flow
are comparable to signal for a 20 minute incubation with laminar flow.
[00128] FIG. 20 is an example of electrical potential waveforms applied to
pairs of high-
performance actuators to achieve rapid pulsatile flow at a microfluidic
junction.
[00129] FIG. 21 illustrates a junction geometry for synchronizing mixing of
fluids using
the invention. Coming into the junction 2100 is a first flow passageway 2101
and a second
flow passageway 2102. The cross-sectional extent of the second flow passageway
2102 in
immediate proximity to the junction is smaller than the cross-sectional of the
first flow
passageway 2101. The first flow passageway 2101 transitions through the
junction and into
the third flow passageway 2103 along an approximately straight line. The
second flow
passageway 2102 forms an angle with both the first and third flow passageways
2101, 2103.
The reduction in cross-section of the second flow passageway 2102 in immediate
proximity
to the junction causes a hydrophobic solution to tend to form a meniscus at
the junction.
Under pulsatile flow driven by a high-performance actuator, the flow front
2104 in the
24
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
secondary flow passageway 2102 can be retained at the junction, with
alternating convex and
concave meniscus formation, notwithstanding the application of net positive
fluid power to
the fluid volume by the actuator. This stalling effect can be maintained until
an advancing
flow front 2005 from the first flow passageway 2101 reaches the junction and
contact occurs
between the two flow fronts. This effect, and similar such effects, can be
used to synchronize
mixing fluids. Synchronized fluid mixing can be associated with better run-to-
run
reproducibility and other favorable assay performance characteristics.
[00130] FIG. 22 is a process flow diagram for a quantitative real-time
polymerase chain
reaction assay using the invention for applications such as quantitation of
HIV genetic
material. A sample 2200 which may contain genetic material of interest, such
as bacterial
DNA or messenger RNA, or viral RNA, is introduced into a processing system,
which
includes microfluidic channels and at least two fluidic actuators 2204, of
which at least one is
a high performance fluidic actuator. The fluidic actuators facilitate a
polymerase chain
reaction involving said genetic material in the sample in a polymerase chain
reaction module
2203. The polymerase chain reaction process can be preceded by reverse
transcription
process in a reverse transcription module 2203. The fluidic actuators
facilitate reverse
transcription of RNA contained within the sample in the reverse transcription
module 2202.
Such reverse transcription may be preceded by a sample preparation process in
a sample
preparation module 2201. Such sample preparation process can be facilitated by
the action of
at least one high performance microfluidic actuator.
[00131] FIG. 23 depicts an architecture for using the microfluidic
cartridge of the
invention in the processing of partitions of fluids, where each partition, or
set of partitions,
can undergo a process selected for that partition or set of partitions. A
cartridge or other
fluidic network 2300 contains a primary channel 2301. Fluidically connecting
to the primary
channel is an array 2302 of at least two side channels. The side channels
comprising the side
channel array are fluidically connected to at least one microfluidic actuator
2304. The
primary channel is fluidically connected to a microfluidic actuator 2303. At
least one of the
microfluidic actuators 2303 and 2304 is a high performance microfluidic
actuator.
[00132] FIG. 24 depicts discrete processing of fluid partitions or sets of
partitions using
the microfluidic cartridge of the invention. A volume of a first fluid 2400
contained within a
channel 2301 can be pressurized and transported through the action of a
microfluidic actuator
2303. A volume of a second fluid 2401 in a side channel within a side channel
array 2302
can be pressurized and transported through the action of the microfluidic
actuator 2304.
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
Additional side channels within the side channel array can be pressurized by
microfluidic
actuators and other means to inject volumes of fluids in side channels into
the volume of fluid
in the first channel at a junction 2402. Volumes of immiscible fluids or air
or other gases
2403 can be injected to cause the fluid in the primary channel to be
partitioned, such
partitions corresponding to particular side channels, such that the fluid in a
specific fluid
partition predominantly comprises the first fluid combined with a prescribed
second fluid
injected from a specific side channel. Such mixtures of the first fluid and
prescribed second
fluid can proceed in a downstream channel, chamber, or other fluidic vessel
2405.
[00133] In some embodiments, the microfluidic cartridge of the invention
comprises an
inlet for a purified sample to be added via pipette (sample prep can be
integrated as needed);
integrated charged slit actuators to drive the assay processes; an
amplification module in
which the sample first reconstitutes PCR primers and then is cycled through
three different
temperature zones for reverse transcriptase step and amplification; droplet
module that
generates a train of droplets, each containing different beacons; a melting
temperature
scanning zone where reassortant resolution takes place with the main actuator
shuttling the
droplets back and forth past the detection zone while a unique optochemical
thermal sensing
method is used to precisely determine the temperature each droplet at each
point during the
ramp.
[00134] FIG. 25 shows an example of a droplet design in a microfluidic
cartridge using
discrete processing of fluid partitions, according to an embodiment of the
invention. Various
side channels under control of individual microfluidic actuators (e.g., an
array of 24 charged-
slit actuators) can sequentially inject a solution comprising lyophilized
beacon probes into an
amplicon solution (output from an amplification module) to generate a droplet
train, which
moves down the primary reaction channel or fluid passageway toward a detection
region
(such as a melt temperature analysis zone or fluorescent detector). In some
embodiments, a
rapid pulsatile flow driven by the individual charged slit microactuators can
be used to
accelerate probe binding to a large, slow-diffusing target amplicon. In one
embodiment, the
charged-slit actuators used for droplet generation operate briefly and
serially. One high-
voltage signal will be applied to the cartridge for the droplet-generation
charged-slit
actuators, and will be routed to the appropriate actuator by an on-cartridge
high voltage de-
multiplexing on the cartridge printed circuit board.
26
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
Methods of the Invention
[00135] A microfluidic cartridge can be produced from individual plastic
components and
discrete microfluidic actuators. The components can be assembled by various
means.
[00136] Description of generating microfluidic actuators is described in
U.S. Provisional
Application No. 61/771,694, filed on Mar. 1,2013, which is hereby incorporated
in its
entirety by reference.
[00137] A. INTRODUCTION AND TRANSPORT OF REACTANTS, INCLUDING
STARTING MATERIAL
[00138] In some embodiments, a reactant or a solution containing a reactant is
added to the
microfluidic cartridge for processing and subsequent analysis. The reactant
may include
blood, sputum, tissue, bodily fluids, cells, cellular components,
extracellular fluids, proteins,
DNA, RNA etc. The starting material may also include dry reagents or
biological materials
for adding to a processing fluid. The starting material can be a fluid phase,
a fluid-laden
matrix, or a solid phase. The starting material can include an intermediary
for a
pharmacological agent or a vaccine. In some instances, the starting material
includes an
agricultural product, soil, or an environmental sample.
[00139] The starting material is mixed with a first fluid in a passageway of
the cartridge.
The first fluid can be mixed with a swab or a porous matrix, which includes
soil or other
environmental samples bound in the swab or porous matrix.
[00140] The starting material can be processed by adding a detergent to lyse
cells or
cellular membranes. Detergents disrupt the cell membrane and include sodium
lauryl
sulfates, hexadecyltrimethylammonium bromide or other cationic or zwitterionic
detergents.
Examples of detergents include Triton X-100, Triton X-114, NP-40, Tween 20,
Tween 80,
SDS (sodium dodecyl sulfate), and CHAPS.
[00141] Enzymes may be used for lysing cells, removing cell walls, or
processing cells or
cellular components in a sample. Examples of enzymes include lysozyme,
lysostaphin,
zymolase, cellulase, mutanolysin, glycanases, proteases, or mannase.
[00142] Processing of the starting material can be performed by mixing the
sample with a
homogenizing solution. For example, a solution can homogenize a tissue sample
or other
biologically heterogeneous sample. The homogenizing solution can include N-
acetyl-L-
cysteine or hypertonic saline. The homogenizing solution can include a
reducing agent, such
as thioredoxin.
27
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
[00143] The homogenizing solution can also include a DNAse or other proteins
for
breaking up of DNA and cell debris. The solution can be capable of diminishing
or
eliminating biological activity of a living cell, tissue, or organism. The
homogenizing
solution can be highly basic and can include sodium hydroxide or sodium
hypochlorite.
[00144] In some embodiments, the solution include glass beads, steel beads,
zirconium
silicate beads, zirconium oxide beads, or other solid material used for
mechanical disruption
of the sample material. The beads or solid material are used to disrupt cells
or cellular
material, in a process called beadbeating. The solution can also include
glycogen or
polysaccharides.
[00145] In other embodiments, the solution comprises carrier RNA for DNA
extraction
from the sample. Solvents such as acetone can also be used to extract cellular
proteins.
[00146] In some embodiments, the starting material comprises a dendritic cell
and can be
mixed with a first fluid in the cartridge and pulsed to induce an element of
an immune
response to insult.
[00147] The starting material can be processed and then analyzed using methods
described
herein. In some cases, the processed starting material is combined as a fluid
with other
reagents or fluids in the microfluidic cartridge.
[00148] B. LABELING OF ANALYTES
[00149] Methods are provided for labeling analytes in the processing fluid.
Examples of
analytes include proteins, DNA, RNA, antibodies, peptides, or other compounds
produced by
a host. Analytes can include DNA, RNA, antibodies, peptides or proteins
produced outside
the host, such as proteins released by pathogens during the course of
infection.
[00150] In one embodiment, a process for labeling analytes is provided using
the
microfluidic cartridge. In one embodiment, microfluidic actuators pressurize
pumps, which
propel a processing fluid including an analyte and a fluid comprising a
labeling molecule into
a common fluidic passageway, and the fluids combine such that labeling takes
place. In
some embodiments, the microfluidic actuators generate a Taylor dispersion of
alternating
plugs of fluids to mix the solutions for labeling.
[00151] Exemplary labeling reagents include chemiluminescent species, such as
luminal,
isoluminol, acridinium esters, thioesters, sulfonamides, and phenanthridium
esters, alkaline
phosphatase; fluorescent species like phycoerythrin, colloidal gold or other
colloidal metals;
or quantum dots. Other fluorescent reagents include lanthanides or lanthanide
chelates
28
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
(Europium, Samarium, Terbium, Dysprosium, etc.) and can be used in a SOCLE
assay, as
described below.
[00152] Quantum dots are crystalline semiconductor particles whose electronic
characteristics are closely related to the size and shape of the individual
crystal. Generally,
the smaller the size of the crystal, the larger the band gap, the greater the
difference in energy
between the highest valence band and the lowest conduction band becomes,
therefore more
energy is needed to excite the dot, and concurrently, more energy is released
when the crystal
returns to its resting state. For example, in fluorescent dye applications,
this equates to higher
frequencies of light emitted after excitation of the dot as the crystal size
grows smaller,
resulting in a color shift from red to blue in the light emitted. In addition
to such tuning, a
main advantage with quantum dots is that, because of the high level of control
possible over
the size of the crystals produced, it is possible to have very precise control
over the
conductive properties of the material.
[00153] Fluorescence, chemiluminescence and phosphorescence are three
different types
of luminescence properties (emission of light from a substance). Fluorescence
is a property
where light is absorbed and remitted within a few nanoseconds (approx. 1 Ons)
at a lower
energy (higher wavelength), while bioluminescence is biological
chemiluminescence, a
property where light is generated by a chemical reaction of an enzyme on a
substrate.
Phosphorescence is a property of materials to absorb light and emit the energy
several
milliseconds or more later (due to forbidden transitions to the ground state
of a triplet state,
while fluorescence occurs in exited singlet states).
[00154] Fluorescent labeling is a process of covalently attaching a
fluorophore to another
molecule, such as a protein, nucleic acid molecule, lipid or other small
molecule. A reactive
derivative of a fluorophore can be used to selectively bind to a functional
group in a target
molecule. Common reactive groups include isothiocyanate derivatives, such as
FITC and
TRITC, succinimidyl esters, such as NHS-fluorescein, maleimide activated
fluorophores,
such as fluorescein-5-maleimide, or fluorophore-labeled oligonucleides, such
as 6-FAM
phosphoramidite. Fluorescent proteins or fluorophores can also be non-
specifically or non-
covalently attached to proteins. The fluorescently-labeled molecule is excited
by light (an
excitation source) and emits fluorescence, which can be detected by the
visible eye or
fluorescence detectors. Various light sources may be used as excitation
sources, including
lasers, photodiodes, and lamps, xenon arcs and mercury-vapor lamps in
particular.
29
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
[00155] The analyte can be labeled with a fluorophore for detection by FRET
(Forster
(Fluorescence) resonance energy transfer)), resonance energy transfer (RET) or
electronic
energy transfer (EET). FRET is a mechanism describing energy transfer between
two
chromophores. A donor chromophore, initially in its electronic excited state,
may transfer
energy to an acceptor chromophore through nonradiative dipole¨dipole coupling.
For
example, an analyte that is labeled with a cyan fluorescent protein (CFP) can
transfer energy
after excitation to a yellow fluorescent protein (YFP), which emits
fluorescent signal for
detection.
[00156] Analytes can be labeled and detected using silica beads, particles, or
paramagnetic
beads. The beads or particles hybridize or bind to a target analyte and can be
purified or
separated out from the fluid by magnetic separation, affinity purification,
etc.
[00157] Analytes can also be labeled and detected using oligonucleotide
probes. The
probe can be RNA or DNA, or modified versions thereof The oligonucleotide
probe can be
designed to target a specific nucleic acid sequence, specific to a virus,
bacterium, infectious
organism, or human gene. Examples of target nucleic acid sequences include
sequences
specific to HIV, hepatitis B, hepatitis C, M. tuberculosis, c. trachomatis, an
influenza virus,
respiratory syncytial virus, a virus of the human respiratory tract, or a
cancer-associated gene
(e.g., ERRB2). The oligonucleotide probe can be labeled with a fluorescent
molecule, a
luminescent signaling molecule, or a quencher molecule.
[00158] Other examples of labeling reagents comprise a labeled carbohydrate, a
labeled
nucleic acid, or a labeled protein for measurement of a specific cellular
compound.
[00159] In some embodiments, the fluids in the fluidic passageways comprise a
dye for
specific or non-specific labeling of a cell.
[00160] In other embodiments, the fluids in the fluidic passageways comprise a
primer, a
probe, or a combination of a primer and a probe, and an enzyme capable of
catalyzing a
polymerase chain reaction, a transcription-mediated amplification, a nucleic
acid sequence-
based amplification, or another chemical reaction for amplifying at least one
specified nucleic
acid sequence. The enzyme can comprise a DNA polymerase, a reverse
transcriptase, an
RNA polymerase, an RNAse H, a DNA helicase, or a recombinase.
[00161] In another embodiment, the labeling reagents are attached, bound or
linked to a
wall of a fluidic passageway. When a fluid comprising a target analyte passes
through the
passageway in the cartridge, the target analyte associates or binds to the
bound reagent.
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
[00162] Labeling an analyte can be followed by detection of the analyte or
measurement
of the quantity of analyte present in the sample. For example, a species that
is labeled with
fluorescent particles can be detected by illuminating with light at the
excitation frequency of
the fluorescent label and measuring the emitted light.
[00163] Labeling can also be followed by separation of the analyte. For
example, one
could separate analytes by labeling a species with magnetic particles and by
imposing a
magnetic field in which the labeled species are differentially transported.
[00164] C. LABELING PROTEINS WITH ANTIBODIES
[00165] A sample or starting material can be combined with a first solution
containing a
first set of antibodies, which specifically bind a target protein in the
sample, for example.
The combined fluids can move by electroosmotic flow in a passageway, passing
by a region
of the wall of the fluid passageway. The wall is bound with a second set of
antibodies that
specifically bind a different epitope of the target protein. Target proteins
binding to the wall
region and forming a sandwich with the first set of antibodies can be detected
by an
spectrometer or other instrument that measures fluorescence.
[00166] By using antibodies specific to more than one target and providing
more than one
variety of antibody, e.g., each antibody attached to a separate region of the
wall, multiple
targets can be specifically detected and measured. The use of multiple
fluorescent labels
further extends the utility.
[00167] Many other assays similar to the basic antibody sandwich assay can be
carried out,
such as detecting genomic material using pairs of oligonucleotide probes.
Among many
suitable non-optical assay means are electrochemical assay methods and assay
methods using
paramagnetic beads.
[00168] D. SOCLE DETECTION ASSAY
[00169] An exemplary labeling and detection method for analytes used in a
microfluidic
cartridge is singlet oxygen catalyzed light emission (SOCLE). SOCLE is a
variant on
luminescence oxygen channeling (Ullman et al., Luminescent oxygen channeling
assay
(LOCI): sensitive, broadly applicable homogeneous immunoassay method. (1996).
Clin.
Chem 42, 1518-1526; Ullman et al., Luminescent oxygen channeling immunoassay:
measurement of particle binding kinetics by chemiluminescence. (1994).
Proceedings of the
National Academy of Sciences 91, 5426-5430) and is widely used in well-format
commercial
immunoassay systems.
31
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
[00170] The bipartite SOCLE assay incorporates probe-conjugated
photosensitizer and
chemiluminescent/fluorescent emitter beads. Excitation of a sensitizer bead
with light
generates a fluorescent signal only if hybridization to a target has brought a
sensitizer and an
emitter bead into close proximity (< 200nm). Singlet oxygen acts as an energy-
transporting
intermediary. Because diffusion of the singlet oxygen between the sensitizer
and emitter
beads requires finite time, there is temporal separation of the excitation and
photon counting
steps. Background fluorescence is therefore reduced by several orders of
magnitude, as the
exciting radiation source is shut off prior to reading. The SOCLE assay is non-
enzymatic
(i.e. no thermolabile proteins).
[00171] In one example, a microfluidic cartridge includes a fluid
comprising the first
target nucleic acid and a second fluid comprising a sensitizer oligonucleotide
conjugated to a
sensitizer bead. The sensitizer oligonucleotide includes a complementary
sequence to the
first target nucleic acid. The first target nucleic acid molecule hybridizes
with the sensitizer
oligonucleotide conjugated to a bead by combining the two fluids together,
using the methods
described above. For instance, the two fluids are pressurized and combine by
electroosmotic
flow into a junction that combines the two fluids in the passageway. A third
fluid can include
second target nucleic acid molecule that is complementary to the first target
nucleic acid
molecule and also complementary to an emitter oligonucleotide conjugated to an
emitter
bead. The third fluid can be mixed with a fourth fluid so that the emitter
oligonucleotide-
bead hybridizes with the second target nucleic acid. The sensitizer-bead
complexed
molecules and the emitter-bead complexed molecules can be mixed ((a) first
target nucleic
acid and sensitizer oligonucleotide-bead complex and (b) second target nucleic
acid and
emitter oligonucleotide-bead complex) at another junction that combines two
fluidic
passageways by generating plugs of alternating fluids using the microfluidic
actuators
described above. The hybridization of the first and second target nucleic
acids can produce a
signal when the two complexes hybridize and the emitter bead and the
sensitizer bead are in
near proximity to each other (e.g., <200 nm apart). In some instances, the
sensitizer-bead
complex and the emitter-bead complex both hybridize to a bridge probe
(oligonucleotide) that
is complementary to the first target nucleic acid and the second target
nucleic acid. The
bridge probe helps to form an oligonucleotide complex between the emitter
oligonucleotide-
bead complex and the sensitizer oligonucleotide-bead complex.
[00172] In other example, a fluidic passageway comprises a matrix for co-
localizing the
sensitizer oligonucleotide-bead complexes or a matrix for co-localizing the
emitter
32
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
oligonucleotide-bead complexes. Fluids that comprise the target nucleic acid
molecules can
hybridize to the sensitizer oligonucleotide-bead or hybridize to the emitter
oligonucleotide-
bead complexes.
[00173] From within the cartridge, the sample may require excitation at 680 nm
and
detection at 615 nm. The readout from the sample may be detected from the
detection
window of the cartridge. The excitation steps can occur in sequence with a
transition period
on the order of milliseconds or less. The excitation source can be high
intensity, and the
detector can be highly sensitive. The optics module should be designed to
satisfy these
requirements. The excitation source is a light-emitting diode (LED), an
efficient light source
which emits over a relatively narrow range of wavelengths. Light emitted by
the assay is
detected by a photomultiplier tube (PMT). Lenses, bandpass filters, and a
dichroic
beamsplitter direct light from the LED into the cartridge and from the
cartridge into the PMT.
[00174] A typical read cycle sequence entails setting the PMT control voltage
low
(typically 0.3V), turning on the LED for 0.5 seconds, turning the LED off, and
finally
increasing the PMT control (typically to 0.8V) to read the results of SOCLE
signaling in the
read well. Custom analog hardware was built to provide current-to-voltage
conversion,
filtering, and amplification or attenuation. Five analog-to-digital converter
channels (three
signal channels with different amplification, temperature, and PMT control
voltage) are read
for the time the experiment is running (typically 0.7 seconds). The data from
the five
channels is streamed in real time to the microprocessor, which integrates the
signal and
compares it to a standard curve or look-up table to determine starting sample
concentration.
The dynamic range over which the starting sample concentration is determined
can be
increased by decreasing the PMT control voltage after a saturating signal is
measured.
[00175] E. DROPLET-BASED ASSAYS
[00176] The microfluidic cartridge of the invention can be used for detecting
and
quantitating a plurality of analytes in a sample or starting material through
an assay that
includes dividing the sample or starting material into a plurality of
partitioned assay mixtures
that are isolated from one another in respective droplets by an intervening,
immiscible carrier
fluid. This division can take place anywhere within the cartridge and can
occur at any stage
of the processing of the sample or starting material. Examples of stages at
which the division
can occur include: immediately upon introduction of a sample or starting
material into a
cartridge; after a filtration process; after a nucleic acid extraction
process; after a process in
which a segment of genomic material in all species of a particular category
(such as the 16S
33
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
region for bacterial targets) is amplified by polymerase chain reaction or
another method; and
after labeling with a fluorescent bead or other signaling particle.
[00177] The microfluidic cartridge of the invention can include a plurality of
fluidic
passageways that meet at junctions in the cartridge. In some embodiments, this
is a T-
junction or a Y-junction. Two immiscible fluids from two separate fluidic
passageways can
meet at the junction in the cartridge and form droplets at the junction as the
two fluids collide
(e.g., oil-in-water droplets, water-in-oil droplets). The junction and fluidic
passageways can
be narrow enough such that one droplet is formed when two immiscible fluids
meet at the
junction. Multiple droplets can be formed as the fluids flow forward at the
junction and are
joined in a single passageway.
[00178] In some embodiments, the microfluidic cartridge of the invention can
be used to
precisely generate a plurality of partitioned assay mixtures, with arbitrarily
chosen volumes,
without fluid transport or mechanical energy transfer into the cartridge from
an external
component. The capability of choosing the volumes of the partitioned mixtures
arbitrarily
differentiates droplet formation using the invention; in contrast, with
droplet formation by a
droplet generator which does not include a high-performance actuator, the
junction geometry
and the fluid properties are primary determinants of partition volumes,
limiting the capacity
to arbitrarily choose these volumes. At least one microfluidic actuator can
act on a
processing fluid contained within a fluidic passageway, causing such fluid to
travel toward a
junction. Similarly, a second microfluidic actuator can act on an immiscible
carrier fluid
within a second fluidic passageway, causing such carrier fluid to travel
toward the junction.
If at least one of the actuators in the microfluidic cartridge is a high-
performance actuator, the
pressurization of either the first fluid or the carrier fluid, or both, can be
rapidly pulsed or
otherwise pressurized in a time-varying manner, such that fluid partitions
with desired
partition volumes are formed at the joining of the two fluids at the junction.
[00179] The microfluidic cartridge of the invention can also be used for
droplet-based
assays where droplet formation facilitates detection and quantitation of
multiple analytes
within a single starting sample, known as multiplexing. Dual plugs of
immiscible carrier
fluid and reagent can be sequentially injected into a plug of sample, forming
a plurality of
fluid partitions, the reagents chosen such that different reactions take place
within certain
partitions, and each such reaction corresponding to detection of a specific
analyte of interest.
Each reaction can take place within a single droplet and the detector in the
microfluidic
cartridge can detect emissions or signals from each reaction in the droplets.
34
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
[00180] In another embodiment, the microfluidic cartridge of the invention can
be used to
generate droplets and to perform PCR amplification in a plurality of droplets
in the cartridge.
In some embodiments, each droplet includes target nucleic acid molecules,
enzymes, and a
PCR primer mix for amplification module of nucleic acid molecules in a sample.
Each
droplet can comprise PCR reagents and can be cycled through three different
temperature
zones in the microfluidic cartridge (e.g., for reverse transcriptase reactions
and
amplification). In some embodiments, the microfluidic cartridge can generate
droplets in one
region of the cartridge (e.g., a junction of at least two fluid passageways is
used to generate a
train of at least 24 droplets). The PCR amplification can occur through
movement of the
droplets (via the microfluidic actuator) through pre-set temperature zones in
the cartridge.
[00181] In further detail, amplification can be accomplished by shuttling a
fluid
comprising the droplets between three temperature zones in the cartridge for
reverse
transcription and amplification. The low thermal mass of the fluid plug allows
the fluid
temperature to equilibrate in each zone in a few seconds, resulting in rapid
amplification
cycles. The fast transient response time of the charged slit actuators further
enhances the
amplification process by shortening the time required to shuttle the solution
between the
zones. In addition to the three zones, the amplification module can include a
reagent
reconstitution zone. A single relatively large (e.g., 4mm x 6mm) charged-slit
microactuator
can be used for executing these steps. For amplification module thermal
engineering, thermal
analysis can be carried out in COMSOL to establish requisite cartridge thermal
mass
allocation to hold zone temperatures to +1- 1 C of nominal as the solution
moves between
zones.
[00182] In other embodiments, the cartridge includes a melting temperature
scanning zone
(where reassortant resolution takes place with the main actuator shuttling the
droplets back
and forth past the detection zone) and an optochemical thermal sensing method
is used to
precisely determine the temperature of each droplet at various time points in
the cartridge.
[00183] In one embodiments, a droplet generator in the cartridge (as shown in
FIGs. 23-
25) is positioned downstream of an amplification module. The droplet generator
can execute
the individual reconstitution of a number of lyophilized reagent plaques
(e.g., 24 plaques
containing 24 beacons) used in a melting temperature scanning assay and then
generates a
droplet train by sequentially pulsing each beacon solution volume into an
amplicon solution.
Flow in the main channel can be driven by a large charge slit actuator used in
the
amplification stage. In one embodiment, a number of small actuators (e.g., 24
small
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
actuators) can each drive one side of the channel, including both dried beacon
reconstitution
(in hybridization buffer) and injection. The side channel actuators and main
actuator can
work together for shuttling the droplet train back and forth past a detector
during a melt
temperature analysis.
[00184] In some embodiments, the microfluidic cartridge is coupled to an
instrument
comprising charged-slit actuator drive electronics, cartridge temperature
control, optical
components for detection, instrument-control electronics, a touchscreen user
interface
controlled by a separate microcontroller, power electronics, and
communications hardware,
including RFID, WiFi, and Ethernet connectivity.
[00185] F. MIXING-ENHANCING JUNCTION GEOMETRIES
[00186] In addition to mixing fluids at a simple t-junction or y-junction, the
invention can
be used to mix fluids in junctions with junction geometries which specifically
facilitate rapid
mixing. A junction configuration where one or more channel cross-sections in
immediate
proximity to the junction are smaller than the cross-sections more distant
from the junction¨
sometimes referred to as a neck-down junction¨can, in combination with the
invention,
facilitate more rapid macroscopic mixing than with either a non-neck-down
junction with the
invention, or a junction (with or without neck-down) without the invention.
The smaller
channel cross-sections in immediate proximity correspond to smaller minimum
fluid plug
volumes for discrete plug injection using the invention. After the pulse train
moves beyond
the necked-down region of the reaction channel in immediate proximity to the
junction,
conservation of mass requires that small-volume fluid plugs expand into plugs
that are shorter
in the axial dimension compared to that within the necked-down channel region.
The
contribution to mixing of Taylor dispersion is correspondingly increased
compared to a
junction process with larger-volume plugs as in a non-neck-down geometry.
[00187] G. CHANNEL AND JUNCTION FEATURES FOR USING SURFACE
TENSION EFFECTS TO IMPROVE CONTROL OVER FLUIDS IN A CARTRIDGE,
REDUCE BUBBLES, SYNCHRONIZE FLUIDS FOR MIXING AT A JUNCTION, OR
OTHERWISE IMPROVE ASSAY PERFORMANCE
[00188] Features of the channels in proximity to the junction can be used to
improve the
performance of mixing using the invention. For fluids which are hydrophobic
relative to the
material comprising the fluid passageway, inclusion of cavity-like features in
proximity to a
junction can be used with the invention to align fluids prior to mixing. For a
cavity feature
which is an approximately uniform radial expansion and contraction of an
approximately
36
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
cylindrical channel, the axial length along which such expansion and
contraction occurs
being small compared to the channel diameter, the flow front of a fluid volume
can be
retained within the cavity when pressurized by a high-performance actuator
acting at a given
duty cycle and average power due to energy storage associated with surface
tension-mediated
flow front deformation at the entrance to the cavity. An increase in the duty
cycle and/or
average power of the high-performance actuator can overcome the meniscus
energy storage
effect and cause the fluid flow front to move past the cavity entrance. If the
flow
characteristics (such as flow rate) of a fluid within a cartridge are subject
to uncertainty, for
example, because of patient-to-patient variation in hematocrit for whole blood
samples, the
low-duty-cycle/low-power stalling effect at a cavity can be used to reduce the
impact of such
uncertainty on assay performance, for example, by maintaining operation of the
high-
performance actuator at the low-duty-cycle/low-power state until such time has
elapsed that a
fluid at the low-flow-rate extreme of the parameter space contemplated for
cartridge design
(e.g. with a hematocrit at the high end of the physiological range). Through a
similar
combination of effects, with the invention, a cavity can function as a trap
for bubbles of air or
another gas entrained in a fluid.
[00189] The invention can also be used to improve assay performance through
the inclusion
of cavity-like feature in the side channel of a t-junction in immediate
proximity to the
junction. For a cavity feature which is an approximately uniform radial
expansion of an
approximately cylindrical channel, the axial length along which such expansion
occurs being
small compared to the channel diameter, the flow front of a first fluid volume
can be retained
within the cavity when pressurized by a high-performance actuator acting at a
given duty
cycle and average power due to energy storage associated with surface tension-
mediated flow
front deformation at the entrance to the cavity. Provided the axial length of
the cavity is
small compared to the channel diameter, a second fluid passing through the
approximately
straight t-junction passageway can overcome the meniscus energy storage effect
and cause
the flow front of the first fluid to pass into the junction.
[00190] H. REACTION WITH SOLID PHASE TO FACILITATE DETECTION OF
CONSTITUENTS
[00191] The invention can be combined with known methods to facilitate
reactions between
constituents of a solution and a solid phase to facilitate detection or
detection and quantitation
of such constituents. A solution can be flowed into a chamber containing one
or more
interior surface regions on which probes are bound. Such surface-bound probes
may be
37
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
oligonucleotide probes, antibody probes, or other probes. The surface-bound
probes may be
positioned relative to sensing elements or sensing systems facilitating
identification of
binding events between surface-bound probes and solution-phase reactants. Such
reactants
may be oligonucleotides, proteins, sugars, cells, or other reactants. The
surface-bound probes
can be configured in a one-dimensional, two-dimensional, or three-dimensional
array. The
sensing elements or sensing systems may be configured for measuring parameters
of interest
of individual elements of said array. The sensing elements may be engineered
to measure
parameters including temperature, pH, and electromagnetic radiation. Two
microfluidic
actuators, at least one of which is a high-performance actuator, can be used
to induce time-
varying flows in the vicinity of some or all of the probe-functionalized
surface regions. The
time-varying flows can result in exchange of volumes of solution in immediate
proximity to
such surface regions, such that fluid volumes containing comparatively large
concentrations
of unbound reactants are brought into proximity to said surfaces such that the
reactants can
bind to such surface-bound probes.
[00192] I. METERING OF REACTANT
[00193] The invention can draw a prescribed volume of a fluid-phase reactant
such as
blood, plasma, urine, or another biological fluid, or a solution containing a
component for a
chemical or biochemical synthesis process, into a cartridge or other
microfluidic network for
subsequent processing or analysis. A volume of reactant can be loaded into a
chamber by
pipetting, by pouring from another container, by flow directly from a source
(such as blood
flowing directly from an opening in the skin produced by the action of a
mechanical lancet),
or by another means. The loading process can be imprecise, such as a nurse or
other
healthcare professional visually ascertaining that the volume of reactant
exceeds a minimal
volume indicated by a fill line on the chamber. A first microfluidic actuator
can then draw a
volume of reactant into a fluid passageway, or a chamber different from the
intake chamber,
by operating for a prescribed period of time and at a prescribed power level,
such operational
parameters having been previously determined by characterization of the
microactuator and
the associated microfluidic network to correspond to a preferred volume for
subsequent
processing. This is referred to herein as open-loop metering. Alternatively,
said first
microfluidic actuator can draw a volume of reactant into a fluid passageway by
operating
until such time as a sensor indicates that the volume of reactant within said
fluid passageway
has reached a prescribed value. Such sensor can be a capacitive sensor that
exhibits a change
38
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
in capacitance when the reactant flow front advances within the channel to a
predetermined
position. This is referred to herein as closed-loop metering.
[00194] The volume of reactant drawn into the first fluid passageway can be
mixed with
other fluids at a junction, such mixing being driven by the combined action of
the first and
second microfluidic actuators, one of which is a high performance actuator, as
described
herein. The reactant drawn into the first fluid passageway can reconstitute a
dried-down or
lyophilized material, either immediately upon being drawn into the first
passageway or
chamber, or at a later stage of processing.
[00195] J. LYSING OF CELLS
[00196] The invention can be used to efficiently lyse cells contained within a
biological
matrix. Whole blood or plasma can be drawn into a fluidic passageway by a
first
microactuator. A solution containing one or more compounds tending to degrade
cell walls
and membranes, such as guanidine thiocyanate or another protein denaturant,
polysorbate 20
or another detergent/emulsifier, and proteinase K or another serine
proteinase, may be loaded
into a second fluid passageway. The loading of the second fluid passageway
with the lysis
solution can be preceded by reconstitution of one or more of the lysis
solution constituents
from a dried-down or lyophilized state. The dried-down or lyophilized material
can be in the
form of a pellet, can be a plaque-like formation coating a portion of an
internal surface of a
fluid passageway, or can be distributed in a porous material located within a
passageway or
chamber.
[00197] K. USE WITH TEMPERATURE CONTROLLERS
[00198] The invention can be used with resistive heaters, with thermoelectric
coolers, and
with other elements and systems for increasing, decreasing, or regulating
temperature of a
fluid volume to facilitate reactions.
[00199] L. EXTRACTION OF DNA AND RNA FROM COMPLEX STARTING
SAMPLES
[00200] The invention can be combined with known methods for reversible
binding of
DNA, RNA, and other nucleic acids from a starting sample. A solution
containing nucleic
acids can be caused to flow through a porous structure by the action of a
microfluidic
actuator. The porous structure can be a packed silica bead bed, such beads
being known to
reversibly bind nucleic acids. The porous structure can have a pore size
distribution and be
otherwise configured to capture precipitated material, such as nucleic acids
that have
precipitated out of solution through binding to glycogen. The flowing of the
nucleic acid-
39
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
containing solution through the porous structure can be preceded by a process
in which cells
are exposed to compounds which tend to disrupting cell walls and membranes and
thereby
improve the efficiency of binding to nucleic acids originally contained within
cells. The
flowing of the nucleic acid-containing solution through the porous structure
can be preceded
by a process in which some or all of the nucleic acids contained therein bind
with a
polysaccharide or other substance which causes such sugar-nucleic acid
complexes to tend to
precipitate. The flowing of the nucleic acid-containing solution through the
porous structure
can be preceded by a process in which a first microfluidic actuator and a
second fluidic
actuator, at least one of which is a high performance fluidic actuator, mixes
the nucleic acid-
containing solution with butanol or another solvent, such that sugar-nucleic
acid complexes
or other precipitation-prone complexes precipitate out of solution and can be
retained near or
within the porous structure. The mixing of the solvent and the nucleic acid-
containing
solution can entail transporting the fluids into a chamber where buoyancy
effects associated
with the different densities of the solvent and the nucleic acid-containing
solution facilitate
mixing of the two phases. The mixing of the solvent and the nucleic acid-
containing solution
can entail transporting the fluids into a chamber where surface tension
effects, buoyancy
effects, or a combination of these effects causes air bubbles to be retained
within such
chamber upon withdrawal of the liquid phase or phases from the chamber. The
passing of the
nucleic acid-containing solution through the porous structure can be followed
by flowing of a
solvent such as ethanol through the porous structure to wash away unbound
material, such as
proteins. There can be more than one such wash step. The passing of a nucleic
acid-
containing solution through the porous structure can be followed by passing
water or another
solution tending to reverse binding of nucleic acids, such that nucleic acids
will tend to be
eluted from the porous structure upon transport of such water or other
solution out of said
porous structure.
EXAMPLES
[00201] Below are examples of specific embodiments for carrying out the
present
invention. The examples are offered for illustrative purposes only, and are
not intended to
limit the scope of the present invention in any way. Efforts have been made to
ensure
accuracy with respect to numbers used (e.g., amounts, temperatures, etc.), but
some
experimental error and deviation should, of course, be allowed for.
[00202] The practice of the present invention will employ, unless otherwise
indicated,
conventional methods of protein chemistry, biochemistry, recombinant DNA
techniques and
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
pharmacology, within the skill of the art. Such techniques are explained fully
in the
literature. See, e.g., T.E. Creighton, Proteins: Structures and Molecular
Properties (W.H.
Freeman and Company, 1993); A.L. Lehninger, Biochemistry (Worth Publishers,
Inc., current
addition); Sambrook, et al., Molecular Cloning: A Laboratory Manual (2nd
Edition, 1989);
Methods In Enzymology (S. Colowick and N. Kaplan eds., Academic Press, Inc.);
Remington 's Pharmaceutical Sciences, 18th Edition (Easton, Pennsylvania: Mack
Publishing
Company, 1990); Carey and Sundberg Advanced Organic Chemistry 3rd Ed. (Plenum
Press)
Vols A and B(1992).
Example 1: Application for HIV Testing
[00203] The methods described above can be used to detect or analyze the
genetic material
or viral proteins of HIV-1 RNA. Because the amount of virus in the bloodstream
of even the
sickest HIV patient is comparatively small, directly detecting target species
requires
sophisticated methods.
[00204] A starting material including a patient's blood or bodily sample is
provided to the
microfluidic cartridge. The sample can be processed using a homogenizing
solution, beads,
or enzymes to lyse the cells in the sample in a fluidic passageway. The
homogenizing
solution and the sample can be mixed using the microfluidic actuators in the
cartridge.
[00205] The processed fluid sample can then be mixed with a second fluid by
pulsing the
fluids together using two or more microfluidic actuators in the microfluidic
cartridge. The
second fluid can contain reagents such as an antibody, oligonucleotide probe,
or labeled
molecule that specifically binds to a protein, DNA, RNA or other molecule that
is specific to
the HIV virus.
[00206] Detection of the HIV virus can be performed using any of the detection
methods
provided above.
Example 2: Detection of Dengue Virus
[00207] The methods described above can be used to detect or analyze the
genomic
material of Dengue virus. Dengue virus (DENY) is a potential biodefense
pathogen and is
classified as a major international public health concern by the World Health
Organization
(WHO). Using the microfluidic cartridge and methods described above, the
Dengue Virus
can be detected in a sample.
[00208] A starting material including a patient's blood or bodily sample is
provided to the
microfluidic cartridge. The sample can be processed using a homogenizing
solution, beads,
or enzymes to lyse the cells in the sample in a fluidic passageway. The
homogenizing
41
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
solution and the sample are mixed using the Taylor dispersion of plugs of
fluid generated by
the microfluidic actuators in the cartridge.
[00209] Optimized performance of this assay requires operation in the
microfluidic
cartridge described above. Cartridge-integrated microfluidic actuators drive
sample and
reagent transport with millisecond temporal resolution, substantially
accelerating bead-primer
reactions relative to diffusion-limited well-format reactions, while cartridge-
integrated
heaters control reaction chamber and readout well temperatures with single-
degree precision.
Example 3: Detection of Mycobacterium tuberculosis (MTB) and analysis of MTB
2enomic material to identify drug resistant strains
[00210] MTB can be identified from a sample or starting material using the
above-
described methods.
[00211] Some strains of MTB are resistant to certain antibiotic drugs
widely used to treat
MTB. The capacity to identify drug resistance allows the selection of
different drugs to treat
individuals infected with resistant strains. Examples of MTB resistance of
clinical
importance include resistance to rifampicin, isoniazid, fluoroquinolones, and
pyrazinamide.
[00212] A sample, such as a sputum sample, from a patient known to be infected
with
MTB or suspected to be infected with MTB can be homogenized in a cartridge of
this
invention and the genomic material, including DNA or ribosomal RNA, released
from the
bacteria. The sample can then undergo a sequence of steps including the
addition of primers
to produce a large number of copies of a region of interest, such as the rpoB
gene, in a
process referred to as isothermal amplification. Action of the microfluidic
actuators in the
cartridge can rapidly mix probes and primers to cause the isothermal
amplification steps to
proceed quickly and efficiently. The amplified target can then be labeled,
such as with
molecular beacons.
Example 4: Quantitation of HIV virus by polymerase chain reaction (PCR)
[00213] The microfluidic cartridge of the invention can be used to precisely
determine the
quantity of HIV genetic material in a sample of material known or suspected to
contain HIV
genetic material. Such material can be a whole blood sample, a plasma sample,
or other
sample. A quantity of sample can be combined with reverse transcription
enzymes to
facilitate reverse transcription of viral RNA into cDNA. Such combination can
be preceded
by one or more processes to lyse the viral coating, remove potentially
interfering substances,
or otherwise prepare the sample for reverse transcription. Such sample
preparation can take
place within the same cartridge or other microfluidic channel network module
as the reverse
42
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
transcription process, or can take place wholly or in part externally from
said cartridge or
microfluidic network module. The combination of the material known or
suspected to
contain HIV genetic material with reverse transcription enzymes can occur at a
junction,
where the reverse transcription genetic material is in solution form, or can
occur through
passage of the material into a chamber containing reverse transcription
enzymes in
lyophilized form, in dried-down form, or in another form. Reconstitution of
reverse
transcription enzymes in dried-down form, in lyophilized form, or in another
form requiring
reconstitution can be facilitated by rapid pulsatile flow driven by one or
more high
performance actuators.
[00214] Reverse transcription can occur in a cartridge chamber with associated
elements
for controlling the temperature of the volume of material in which reverse
transcription
occurs. Such elements can include resistive heaters, elements for increasing
or decreasing the
temperature of a mass of material through thermoelectric effects, resistive
temperature
sensors, circuitry for automatically or manually adjusting the heat production
by a heater
element as a function of the output of a temperature sensor, and other
elements. The solution
that has undergone the reverse transcription reaction can be combined with
primers, enzymes,
and other reagents for polymerase chain reaction. The combination with
primers, enzymes,
and other reagents can occur through fluid transport, driven by at least one
high performance
actuator, of the primers, enzymes, and other reagents into the reaction
chamber where reverse
transcribed DNA was produced, or by fluid transport of the solution containing
the reverse
transcribed DNA, driven by at least one high performance actuator, into
another chamber or
more than one other chamber. Amplicon produced by PCR can be detected while
the PCR
reaction is taking place or by end-point methods. Amplicon produced by PCR can
be
analyzed by including probes specific for the amplicon with a fluor or other
luminescent
particle, a quencher particle, and a hairpin structure and which luminesce
upon excitation
when bound to amplicon. The PCR reaction can be analyzed by changing the
temperature of
the solution and monitoring binding with labeled probes. The PCR reaction can
take place in
a plurality of fluid partitions. At least one high performance actuator can
facilitate the
partitioning of the fluid. The fluid partitions can constitute an emulsion.
The volume and
number of the partition elements can be chosen to facilitate quantitation
through analysis of
the fraction of the partition elements found to have contained at least one
copy of reverse
transcribed DNA prior to the start of the PCR.
43
CA 02903382 2015-09-01
WO 2014/137940
PCT/US2014/020029
Example 5: Detection of influenza and other respiratory pathogens and
differentiation
among such pathogens
[00215] The invention can be used for melting temperature (Tn,) analysis of
genetic
divergence of Influenza A Virus (IAV) RNA from a reference strain. A targeted
amplification can be carried out based on possible target identities. The
targeted
amplification can focus on the HA and NA antigens. The targeted amplification
can rapidly
classify IAV relative to a reference strain across some or all 8 genome
segments. The
targeted amplification can incorporate predicted target sequences or can
function as an
unbiased search for genome-level rearrangement.
[00216] While the invention has been particularly shown and described with
reference to a
preferred embodiment and various alternate embodiments, it will be understood
by persons
skilled in the relevant art that various changes in form and details can be
made therein
without departing from the spirit and scope of the invention.
[00217] All references, issued patents and patent applications cited within
the body of the
instant specification are hereby incorporated by reference in their entirety,
for all purposes.
44