Note: Descriptions are shown in the official language in which they were submitted.
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME 1 ________________ DE 2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 1 -
TRIAZOLO[4,5-WYRIMIDINE DERIVATIVES FOR THE TREATMENT OF DISEASES SUCH AS
CANCER
BACKGROUND OF THE INVENTION
The invention had the object of finding novel compounds having valuable
properties, in particular those which can be used for the preparation of
medicaments.
The present invention relates to compounds and to the use of compounds in
which the inhibition, regulation and/or modulation of signal transduction by
protein
kinases, in particular immune-modulatory or stress response kinases,
furthermore
to pharmaceutical compositions which comprise these compounds, and to the use
of the compounds for the treatment of kinase-induced diseases.
Because protein kinases regulate nearly every cellular process, including
metabolism, cell proliferation, cell differentiation, and cell survival, they
are
attractive targets for therapeutic intervention for various disease states.
For
example, cell-cycle control, immune modulation, stress response and
angiogenesis, in which protein kinases play a pivotal role are cellular
processes
associated with numerous disease conditions such as but not limited to cancer,
inflammatory diseases, neurodegenerative diseases, chronic infections,
abnormal
angiogenesis and diseases related thereto, atherosclerosis, macular
degeneration, diabetes, obesity, and pain.
Compounds of formula I inhibit the stress response elF2 kinase ElF2AK4
called general control nonderepressible 2 (GCN2).
Many strategies of cancer treatment of solid tumors focus on the surgically
removal of the tumor mass as far as possible and the subsequent eradication
of any residual tumor cells by radiotherapy and chemotherapy with cytotoxic
agents or inhibitors that target cancer cell pathways more specifically.
However, the success of such approach is limited and often does not persist.
This is mainly due to the narrow therapeutic window for such cytotoxic agents
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 2 -
(specificity and side effects) and to the capability of cancer calls to adapt
to the
selective pressure applied by cytotoxic or other inhibitory agents. The
survival
of a small number of tumor (stem) cells that acquired resistance to the
initial
treatment can be sufficient to seed the regrowth of a tumor. These relapses
are in most cases more difficult to treat compared to that of the initial
tumors.
As a consequence the more successful targeting of tumor cells may require
targeting multiple survival and escape mechanism of tumor cells in parallel
(Muller & Prendegast 2007).
Development of malignancies is accompanied by a major roll up of the
cellular physiology. During this process several qualities are acquired by the
cancer cells that are basis for immortalization or insensitivity to growth
inhibitory signals. In addition the tumor cells also modify the interaction
with
the microenvironment and beyond. The latter area includes the strategies of
tumor cells to escape from the immunological surveillance (Muller &
Prendegast 2007). The immune surveillance limits malignant growth but
also provides a selective pressure triggering the evolution of mechanisms
for evading the immune response as reviewed by [Dunn et al. 2004].
Essentially it has been frequently observed that ablation of T cell immunity
is sufficient to increase tumor incidence [Shankaran et al. 2001] and it is
believed that immune escape is affecting tumor dormancy versus
progression, promoting invasion and metastasis and negatively impacts on
therapeutic response.
Several mechanistic studies discovered that immune escape has an important
interface with metabolic alterations within the tumor microenvironment. Here
important roles in mediating immune tolerance to antigens have been
associated to the catabolism of the essential amino acids tryptophan and
arginine, carried out by the enzymes indoleamine 2,3-dioxygenase (IDO) and
arginase I (ARG), respectively (Bronte and Zanovello, 2005; Muller et al.,
2005b; Muller and Prendergast, 2007; Munn and Mellor, 2007; Popovic et al.,
2007).
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 3
IDO is a single-chain oxidoreductase that catalyzes the degradation of
tryptophan to kynurenine. IDO is not responsible for catabolizing excess
dietary tryptophan but to modulate tryptophan level in a local environment.
Elevations in tryptophan catabolism in cancer patients manifest in
significantly
altered serum concentration of tryptophan or catabolites and this was
correlated to IDO which is commonly elevated in tumors and draining lymph
nodes. According to several publications IDO over-expression is associated
with poor prognosis in cancer [Okamoto et at 2005; Brandacher et al, 2006].
T cells appear to be preferentially sensitive to IDO activation, such that
when
starved for tryptophan they cannot divide and as a result cannot become
activated by an antigen presented to them. Munn and Mellor and their
colleagues, revealed that IDO modulates immunity by suppressing T-cell
activation and by creating peripheral tolerance to tumor antigens (Mellor and
Munn, 2004). These mechanism encompass the subversion of immune cells
recruited by the tumor cell to its immediate microenvironment or to the tumor-
draining lymph nodes Here the tumor antigens that were scavenged by
antigen-presenting cells are cross-presented to the adaptive immune system.
In addition to being directly toleragenic, mature DCs have the capacity to
expand regulatory TceIls (Tregs) [Moser 20031.
Beside tryptophan catabolism the conversion of arginine is increased in a
tumor-conditioned microenvironment, and numerous reports indicate a role for
the activation of arginases during tumor growth and development. In tumor-
infiltrating myeloid cells, arginine is converted by arginase I (ARG1),
arginase II
(ARG2) to urea and ornithine and oxidized by the inducible form of nitric
oxide
synthase (NOS2) to citrulline and nitric oxide (NO).
Increased ARG activity is frequently observed in patients with colon, breast,
lung, and prostate cancer [Cederbaum 20041 correlating with the over-
expression of ARG and NOS found in prostate cancers [Keskinege et at. 2001,
Aaltoma et at. 2001, Wang et at. 2003]. It was shown that ARG activity in
infiltrating macrophages impairs antigen-specific T cell responses and the
expression of the CD3 receptor. Moreover the cumulative activity of ARG and
NOS in tumor associated myeloid cells can generate inhibitory signals to
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 4 -
antigen-specific T lymphocytes that eventually lead to apoptosis [Bronte 2003
a; 2003b].
Both, the IDO and the ARG related mechanism merge at the point of sensing
the depleted concentration of the respective amino acid concentration. During
amino acid deprivation, the elF2 kinase ElF2AK4 called general control
nonderepressible 2 (GCN2) is interacting with the intracellular accumulating
deacylated tRNA. As a consequence the GCN2 is assumed to change from an
auto-inhibited to an active conformation and further activate by auto-
phosphorylation. Then the only known substrate protein elF2a becomes
phosphorylated and as a consequence the complex for translation initiation is
inhibited [Harding et al. 2000,]. This diminishes the general Cap-dependent
translation initiation and by this the corresponding protein production. On
the
other hand this induces the specific expression of stress related target genes
mainly by cap-independent initiation via the activating transcription factor 4
(ATF4). By expressing the respective stress response proteins, e.g. enzymes
in the in amino acid metabolism, the cell tries to compensate the particular
cell
stress [Wek et al. 2006]. If the stress persists, the same pathway will switch
to
promoting cell death via transcription of the pro-apoptotic transcription
factor,
CCAAT/enhancer-binding protein homologous protein (CHOP) [Oyadomari
2004]. It was shown that tryptophan starvation triggers a GCN2- dependent
stress signaling pathway. In T cells altering elF2aphosphorylation and
translational initiation leading to a cell growth arrest (Munn et al. 2005).
Sharma, et al. [2007] published on the direct IDO-induced and GCN2-
dependent activation of mature Tregs. Analogously Fallarino et al [2006] found
a GCN2-dependent conversion of CD4+CD25- cells to CD25+FoxP3+ Tregs
producing IL-10 and TGF13. Rodriguez et al. [2007] identified that activation
of
the GCN2 pathway via tryptophan or arginine depletion in combination with
TCR signaling leads to CD3 chain down regulation, cell cycle arrest and
anergy.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 5 -
Importantly the GCN2 pathway is not only important for the tumoral immune
escape but also plays an active role in modulating tumor survival directly. Ye
et
al [2010] found that the aforementioned transcription factor ATF4 is over-
expressed inhuman solid tumors, suggesting an important function in tumour
progression. Amino acid and glucose deprivation are typical stresses found in
solid tumours and activated the GCN2 pathway to up-regulate ATF4 target
genes involved in amino acid synthesis and transport. GCN2 activation /
overexpression and increased phospho-elF2a were observed in human and
mouse tumors compared with normal tissues and abrogation of ATF4 or GCN2
expression significantly inhibited tumor growth in vivo. It was concluded that
the GCN2-elF2a-ATF4 pathway is critical for maintaining metabolic
homeostasis in tumor cells.
Over all the present biology makes an interference with the ARG/IDO pathway
attractive for braking up the tumoral immune escape by adaptive mechanism.
The interference of GCN2 function is here of particular interest as it is a
merging point of the two pathways, the IDO and ARC, as well as it provides
additional opportunities to impede with the tumor metabolism directly.
Several pathway inhibitors are already considered as immune modulators.
These inhibitors address mainly the enzymatic function of the IDO or ARC
proteins (Muller and Scherle, 2006). The application of the arginase
inhibitor,
N-hydroxy-nor-L-Arg blocks growth of s.c. 3LL lung carcinoma in mice
[Rodriguez 2004]. The NO-donating aspirins like NCX 4016 (2-(acetyloxy)-
benzoic acid 3-(nitrooxymethyl) phenyl ester) have been reported to interfere
with the inhibitory enzymatic activities of myeloid cells. Orally administered
NO
aspirin normalized the immune status of tumor-bearing hosts, increased the
number and function of tumor-antigen-specific T lymphocytes, and enhanced
the preventive and therapeutic effectiveness of the antitumor immunity
elicited
by cancer vaccination (DeSanto 2005).
The substrate analogue 1 methyl-tryptophan (1MT) and related molecules
have been used widely to target IDO in the cancer context and other settings.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 6 -
Studies by Friberg et at. (2002) and Uyttenhove et al. (2003) demonstrated
that 1MT can limit the growth of tumors over-expressing IDO. However 1MT
was unable to elicit tumor regression in several tumor models, suggesting only
modest antitumor efficacy when IDO inhibition was applied as a monotherapy.
In contrast, the combinatory treatment with 1MT and a variety of cytotoxic
chemotherapeutic agents elicited regression of established MMTV-neu/HER2
tumors, which responded poorly to any single-agent therapy [Muller et at
2005a]. lmmunodepletion of CD4+ or CD8+ T cells from the mice before
treatment abolished the combinatorial efficacy observed in this model,
confirming the expectation that 1MT acted indirectly through activation of T
cell-mediated antitumor immunity. Important evidence that IDO targeting is
essential to 1M1 action was provided by the demonstration that 1MT lacks
antitumor activity in mice that are genetically deficient for IDO [Hou et al.,
2007]
The inhibition of GCN2 would enable to combine the two pathway branches of
amino acid starvation induced immunoediting and would reduce the options for
the tumor to circumvent the inhibition of either branch. Moreover, as detailed
above, the GCN2 inhibition provides the opportunity for interfering with the
tumor metabolism at the same time what may enhance the efficacy of a
monotherapy or a combination therapy with other anticancer approaches.
As mentioned above, the elF2 kinase GCN2 is activated by interacting with
deacylated tRNA that is accumulating as direct consequence of nutritional
deprivation stress. Other cellular stress factors like UV irridation, redox
stress
or proteasome inhibition can induce GCN2 activation indirectly [Wek et al
2006]. In all known cases elF2a becomes phosphorylated and this induces
the specific expression of stress related target genes mainly by cap-
independent initiation via the activating transcription factor 4 (ATF4).
Mitsuda et at (2007) showed that presenilin-1 is induced by activating
transcription factor 4 (ATF4), regulated by GCN2. Accumulation of amyloid-p
(AP), which is generated from amyloid precursor protein by y-secretase, in
cerebral cortex is common and critical incident in Alzheimer disease.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 7 -
Specifically, presenilin is an essential for y-secretase activity. Ohata et
al.
(2010) describe a role of GCN2-elF2a-ATF4 signaling in the regulation of y-
secretase activity in autophagy impaired cells: The impairment of the
autophagy-lysosomal system may cause amino acid imbalance in the cell
because autophagy is required for maintenance of amino acid level. The
autophagy-lysosomal system is discussed as a vital modulator of y-secretase
activity through GCN2, leading to Ap accumulation in autophagy deterioration,
which may be a possible therapeutic target for reducing AP production. y-
Secretase plays an important role in the development of Alzheimer disease
(AD). y-Secretase activity is enriched in autophagic vacuoles and it augments
amyloid-p (A13) synthesis.
Senile plaques are primarily composed of p-amyloid peptides (Ap) derived
from amyloid precursor protein (APP) that has undergone proteolytic
processing by p-secretase (BACE-1) and y-secretase. O'Connor et al.(2008)
found that BACE-1 levels are translationally increased by phosphorylation of
elF2a.
Inhibition of GCN2 under such disease conditions that promote activation of y-
se c reta se or induction of BACE-1 with consequence of accumulation of A13
and plaque formation in the brain would provide a valuable avenue to temper
or even stop the progression of neurodegenerative diseases.
It was described that persistent, not acute, parasite or viral infections are
associated to the establishment of immune privileged conditions of even
immune competent host towards the infectious organism or particles. This has
been associated to the local induction of IDO expression. Makala et al (J
Infect
Dis. 2011 Mar 1;203(5):715-25) show that cutaneous Leishmania major
infection stimulated expression of the immune regulatory enzyme indoleamine
2,3 dioxygenase (IDO) in local lymph nodes. Induced IDO attenuated the T cell
stimulatory functions of dendritic cells and suppressed local T cell responses
to exogenous and nominal parasite antigens. 100 ablation reduced local
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 8 -
inflammation and parasite burdens, as did pharmacologic inhibition of MO in
mice with established infections. de Souza Sales (Clin Exp lmmunol. 2011
Aug;165(2):251-63) corroborated the role of indoleamine 2, 3-dioxygenase in
lepromatous leprosy immunosuppression. Boasso et al (Blood. 2007 Apr
15;109(8):3351-9) found that HIV inhibits CD4+ T-cell proliferation by
inducing
indoleamine 2,3-dioxygenase in plasmacytoid dendritic cells and that in vitro
inhibition of IDO results in increased CD4(+) T-cell proliferative response in
PBMCs from HIV-infected patients
Inhibitor drugs of the IDO/GCN2 pathway could be used to enhance host
immunity to chronic and persistent infections.
Literature:
1. Aaltoma, S.H., P.K. Lipponen, and V.M. Kosma. 2001. Inducible nitric oxide
synthase (iNOS) expression and its prognostic value in prostate cancer.
Anticancer Res. 21:3101-3106.
2. Brandacher, G.; Perathoner, A.; Ladurner, R.; Schneeberger, S.; Obrist, P.;
Winkler, C.; Werner, E. R.; Werner-Felmayer, G.; Weiss, H. G.; Gobel, G.;
Margreiter, R.; Konigsrainer, A.; Fuchs, D.; Amberger, A. Prognostic value of
indoleamine 2,3- dioxygenase expression in colorectal cancer: effect on
tumorinfiltrating T cells. Clin. Cancer Res. 2006, 12, 1144-1151.
3. Bronte V, Zanovello P. (2005). Regulation of immune responses by L-
arginine metabolism. Nat Rev Immunol 5: 641-654.
4. Bronte, V., P. Serafini, C. De Santo, I. Mango, V. Tosello, A. Mazzoni,
D.M.
Segal, C. Staib, M. Lowel, G. Sutter, et al. 2003a. IL-4- induced arginase 1
suppresses alloreactive T cells in tumor-bearing mice. J. Immunol. 170:270¨
278.
5. Bronte, V., P. Serafini, A. Mazzoni, D.M. Segal, and P. Zanovello. 2003b. L-
arginine metabolism in myeloid cells controls T-lymphocyte functions. Trends
lmmunol. 24:302-306
6. Carmela De Santo, Paolo Serafini, Ilaria Mango, Luigi Dolcetti, Manlio
Bolla, Piero Del Soldato, Cecilia Melani, Cristiana Guiducci, Mario P.
Colombo, Manuela lezzi, Piero Musiani, Paola Zanovello, and Vincenzo
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 9 -
Bronte. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and
promotes tumor eradication by cancer vaccination. Proc Nati Acad Sci U S A.
2005 March 15; 102(11): 4185-4190
7. Cederbaum, S.D., H. Yu, W.W. Grody, R.M. Kern, P. Yoo, and R.K. lyer.
2004. Arginases I and II: do their functions overlap? Mol. Genet. Metab.
81:S38-44.
8. T. O'Connor, K.R. Sadleir, E. Maus, R.A. Velliquette, J. Zhao, S.L. Cole,
W.A. Eimer, B. Hitt, L.A. Bembinster, S. Lammich, S.F. Lichtenthaler, S.S.
Hebert, S.B. De, C. Haass, D.A. Bennett, R. Vassar, Phosphorylation of the
translation initiation factor elF2alpha increases BACE1 levels and promotes
amyloidogenesis. Neuron, 60 (2008), pp. 988-1009
9. Dey, M., Cao, C., Sicheri, F. and T.E. Dever. Conserved Intermolecular Salt
Bridge Required for Activation of Protein Kinases PKR, GCN2, and PERK.
JBC 282(9): 6653, 2007.
10. Dunn, G. P.; Old, L. J.; Schreiber, R. D. The immunobiology of cancer
immunosurveillance and immunoediting. Immunity 2004, 21, 137-148.
11. Fallarino, F. U. Grohmann, S. You, B.C. et al. The combined effects fo
tryptophan starvation and tryptophan catabolites down-regulate T cell receptor
zeta-chain and induce a regulatory phenotype in naïve T cells. J. Immunol.
176:6752, 2006.
12. Friberg M, Jennings R, Alsarraj M, Dessureault S, Cantor A,
Extermann M et al. (2002). Indoleamine 2,3-dioxygenase contributes to tumor
cell evasion of T cell-mediated rejection. Int. J Cancer 101: 151-155
13. Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D.
Regulated translation initiation controls stress-induced gene expression in
mammalian cells. Mol Cell. 2000 Nov;6(5):1099-108.
14. Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson
M et al. (2007). Inhibition of indoleamine 2,3-dioxygenase in dendritic cells
by
stereoisomers of 1-methyl-tryptophan correlates with antitumor responses.
Cancer Res 67: 792-801.
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 10 -
15. Keskinege, A., S. Elgun, and E. Yilmaz. 2001. Possible implications of
arginase and diamine oxidase in prostatic carcinoma. Cancer Detect. Prey.
25:76-79.
16. Mellor AL, Munn DH. (2004). IDO expression by dendritic cells:
tolerance and tryptophan catabolism. Nat Rev Immunol 4: 762-774.
17. Mitsuda T, Hayakawa Y, Itoh M, Ohta K, Nakagawa T. ATF4 regulates
gamma-secretase activity during amino acid imbalance, Biochem Biophys Res
Commun. 2007 Jan 19;352(3):722-7.
18. Moser, M.
Dendritic cells in immunity and tolerance-do they display
opposite functions? Immunity 2003, 19, 5-8.
19. Muller, A.J. and P.A. Scherle. Targeting the mechanisms of tumoral
immune tolerance with small-molecule inhibitors. Nat. Rev. Cancer. 6:613,
2006.
20. Muller AJ, Prendergast GC. (2007). Indoleamine 2,3-dioxygenase in
immune suppression and cancer. Curr Cancer Drug Targets 7: 31-40.
21. Muller AJ, DuHadaway JB, Sutanto-Ward E, Donover PS, Prendergast
GC. (2005a). Inhibition of indoleamine 2,3-dioxygenase, an immunomodulatory
target of the tumor suppressor gene Binl, potentiates cancer chemotherapy.
Nature Med 11:312-319.
22. Muller AJ, Malachowski WP, Prendergast GC. (2005b). Indoleamine
2,3-dioxygenase in cancer: targeting pathological immune tolerance with small-
molecule inhibitors. Expert Opin Ther Targets 9: 831-849.
23. Munn, D.H., M.D. Sharma, B. Baban, H.P. Harding, Y. Zhang, D. Ron,
A.L. Mellor. GCN2 kinase in T cells mediates proliferative arrest and anergy
induction in response to indoleamine 2,3-dioxygenase_ Immunity. 22:633,
2005
24. Ohta K, Mizuno A, Ueda M, Li S, Suzuki Y, Hida Y, Hayakawa-Yano Y,
ltoh M, Ohta E, Kobori M, Nakagawa T. Autophagy impairment stimulates PS1
expression and gamma-secretase activity. Autophagy. 2010 ;6(3):345-52
25. Okamoto, A.; Nikaido, T.; Ochiai, K.; Takakura, S.; Saito, M.; Aoki,
Y.;
Ishii, N.; Yanaihara, N.; Yamada, K.; Takikawa, 0.; Kawaguchi, R.; lsonishi,
S.;
Tanaka, T.; Urashima, M. lndoleamine 2,3-dioxygenase serves as a marker of
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 1 1 -
poor prognosis in gene expression profiles of serous ovarian cancer cells.
Clin.
Cancer Res. 2005, 11, 6030-6039.
26. Oyadomari S, Mori M. Roles of CHOP/GADD153 in endoplasmic
reticulum stress. Cell Death Differ. 2004 Apr:11(4):381-9.
27. GC Prendergast, Immune escape as a fundamental trait of cancer:
focus on IDO. Oncogene (2008) 27, 3889-3900
28. Popovic PJ, Zeh III HJ, Ochoa JB. (2007). Arginine and immunity. J
Nutr 137: 1681S-1686 S.
29. Rodriguez, P.C., D.G. Quiceno, J. Zabaleta, B. Ortiz, A.H. Zea, M.B.
Piazuelo,A.Delgado, P.Correa, J.Brayer, E.M. Sotomayor, S.Antonia, J.B.
Ochoa, and A.C. Ochoa. Arginase I Production in. the Tumor
Microenvironment by Mature Myeloid Cells Inhibits T-Cell Receptor Expression
and Antigen-Specific T-Cell Responses. Canc. Res. 64:5839, 2004
30. Rodriguez, P.C., D.G. Quiceno, and A.C. Ochoa. L-arginine
availability regulates T-lymphocyte cell-cycle progresion. Blood. 109:1568,
2007.
31. Shankaran, V.; Ikeda, H.; Bruce, A. T.; White, J. M.; Swanson, P. E.;
Old, L. J.; Schreiber, R. D. 1FNgamma and lymphocytes prevent primary
tumour development and shape tumour immunogenicity. Nature 2001, 410,
1107-1111.
32. Sharma, M.D., B. Baban, P. Chandler, D-Y. Hou, N. Singh, H. Yagita,
M. Azuma, B.R. Blazar, A.L. Mellor, and D.H. Munn. Plasmacytoid dendritic
cells from mouse tumor-draining lymph nodes directly activate mature Tregs
via indoleamine 2,3-dioxygenase. J. Clin. Invest. 117:2570, 2007.
33. Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N
et al. (2003). Evidence for a tumoral immune resistance mechanism based on
tryptophan degradation by indoleamine 2,3- dioxygenase. Nat Med 9:1269-
1274
34. Wang, J., M. Torbenson, Q. Wang, J.Y. Ro, and M. Becich. 2003.
Expression of inducible nitric oxide synthase in paired neoplastic and non-
neoplastic primary prostate cell cultures and prostatectomy specimen. Urol.
Oncol. 21:117-122.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 12 -
35. Wek RC, Jiang HY, Anthony TG. Coping with stress: elF2 kinases and
translational control. Biochem Soc Trans. 2006 Feb;34 (Pt 1):7-11.
36. Ye J, Kumanova M, Hart LS, Sloane K, Zhang H, De Panis DN,
Bobrovnikova-Marjon E, Diehl JA, Ron D, Koumenis C. The GCN2-ATF4
pathway is critical for tumour cell survival and proliferation in response to
nutrient deprivation. EMBO J. 2010 Jun 16;29(12):2082-96.
In particular, the present invention relates to compounds and to the use of
compounds in which the inhibition, regulation and/or modulation of signal
transduction by GCN2 plays a role.
The synthesis of small compounds which specifically inhibit, regulate and/or
modulate signal transduction by immune-modulatory or stress response
kinases in particular GCN2, is therefore desirable and an aim of the present
invention.
Moreover, aim of this invention is the synthesis of new compounds for the
prevention and treatment of neoplastic malignancies including, but without
being limited to, solid tumor cancers, cancers of the lymphatic or blood
system,
of neurodegenerative diseases and chronic infections.
It has been found that the compounds according to the invention and salts
thereof have very valuable pharmacological properties while being well tol-
erated.
The compounds of the formula I can furthermore be used for the isolation and
investigation of the activity or expression of GCN2. In addition, they are
particularly suitable for use in diagnostic methods for diseases in connection
with unregulated or disturbed GCN2 activity.
Compounds of formula I can also inhibit tyrosine kinases FMS (CSF1R), GSK3a,
GSK3p, FLT3 or FLT4 or combinations of these kinases, preferentially in
addition
to inhibitory activity towards GCN2.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
=
- 13 -
Fms-like tyrosine kinase 3 (FLT3), which is also known as FLK-2 (fetal liver
kinase
2) and STK-I (stem cell kinase 1), plays an important role in the
proliferation and
differentiation of hematopoietic stem cells. FLT3 receptor kinase is expressed
at
very high levels on the cells of more than 80% of myelogenous patients and of
a
fraction of acute lymphoblastic leukemia cells. Furthermore, the enzyme can
also
be found on cells from patients with chronic myelogenous leukemia in lymphoid
blast crisis. It has been reported that FLT3 kinase is mutated in 30% of acute
myeloid leukemia (AML) and in a subset of acute lymphoblastic leukemia (ALL)
as
well (Gilliland et al, Blood 100, 1532-1542 (2002); Stirewalt et al., Nat.
Rev.
Cancer, 3, 650-665 (2003). Activating mutations in FLT3 mutations have been
associated with a poor prognosis (Malempati et al., Blood, 104, 11 (2004).
FLT3
inhibitors are being developed and some have shown promising clinical effects
against AML (Levis et at Int. J. Hematol, 52, 100- 107 (2005).
It has been reported that some of small-molecule FLT3 inhibitors are effective
in inducing apoptosis in cell lines with FLT3-activating mutations and
prolonging survival of mice that express mutant FLT3 in their bone marrow
cells (Levis et al, Blood, 99, 3885-3891 (2002); Kelly et at, Cancer Cell, 1,
421-
432 (2002); Weisberg et al, Cancer Cell, 1, 433-443 (2002); Yee et at, Blood,
100, 2941-2949 (2002).
US patent application 20090054358 describes Flt3 inhibitors for immune
suppression and in particular for the treatment of immune related disorders
like
organ rejection, bone marrow transplant rejection, non-myeloablative bone
marrow transplant rejection, ankylosing spondylitis, arthritis, aplastic
anemia,
Behcet's disease, type 1 diabetes mellitus, graft-versus-host disease, Graves'
disease, autoimmune hemolytic anemia, Wegener's granulomatosis, hyper IgE
syndrome, idiopathic thrombocytopenia purpura, rheumatoid arthritis, Crohn's
disease, multiple sclerosis, Myasthenia gravis, psoriasis, and lupus, among
other autoimmune diseases. Flt3 Inhibitors might also be used to treat
neurological disorder as neurodegenerative disease, for example a disease
caused by axonal degeneration. Neurodegenerative diseases include, for
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 14 -
example, multiple sclerosis; dennyelinating core disorders, such as multiple
sclerosis, acute transverse myelitis without being limited thereto.
Scott et al (Bioorg. Med Chem Let. (2008) 18(17) p4794) describe CSF-1R
inhibitors for the treatment of cancer. CSF-1R is a member of the class III
receptor tyrosine kinases. Colony stimulatory factor 1 (CSF-1), also known as
macrophage/monocyte colony stimulatory factor (M-CSF), binds to CSF-1R,
resulting in dimerization, autophosphorylation, and activation of signal
transduction.1 CSF-1/CSF-1R signaling is essential for normal monocyte
development. In cancer, pro-tumorigenic macrophages have been identified
and linked to poor prognosis in breast, ovarian, and prostate cancers.
Elevated
levels of CSF-1 and CSF-1R have been reported in several tumor types,
including breast, ovarian, and endometrial cancers, and have also been linked
to invasion and metastasis. Inhibition of CSF-1R activity could therefore have
multiple effects on the tumor through reduction in the levels of tumor-
associated macrophages (TAMs) and have direct effects on the tumor itself
(C.E. Lewis, J.W. Pollard, Cancer Res., 66 (2006), p. 605; I. Bingle, N. et
al., J.
Pathol., 196 (2002), p. 254; B.M. Kacinski, Ann. Med., 27 (1995), p. 79; E.
Garwood et at. J Clin Oncol 26: 2008).
Su JL et at. (Cancer Cell. 2006 Mar;9(3):209-23) report that the VEGF-C/Flt-4
axis promotes invasion and metastasis of cancer cells. Flt-4, a VEGF receptor,
is activated by its specific ligand, VEGF-C. The resultant signaling pathway
promotes angiogenesis and/or lymphangiogenesis. VEGF-C/Flt-4 axis
enhances cancer cell mobility and invasiveness and contributes to the
promotion of cancer cell metastasis. Examination of tumor tissues from various
types of cancers revealed high levels of Flt-4 and VEGF-C expression that
correlated closely with clinical metastasis and patient survival. Inhibition
of Flt-
4 kinase could reduce the invasive capacity in different types of cancer
Combining the inhibitory specificity towards GCN2 with that towards FMS
(CSF1R), FLT3 or FLT4 or combinations of these kinases can be of particular
advantages for the treatment of neoplastic malignancies at different disease
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 15 -
stages. It could combine the effects of stimulating the immune response
towards cancer/tumor cells, to reduce the levels of tumor-associated
macrophages as well as the invasive capacity of cancers for metastasis
formation. In a further aspect the combination of inhibitory activities on
GCN2
particularly with inhibition of FLT3 could be advantageous for the treatment
of
neurodegenerative disorders as it could synergize suppressive effects on
inflammatory processes with the modulation of protein deposites generation in
the brain. In another aspect the combination of inhibitory activities on GCN2
particularly with inhibition of FLT3 could provide advantages for modulating
the
immune response to treat immune related disorders and inflammatory or auto-
immune diseases.
In a further embodiment the present invention specifically relates to
compounds of the formula I which inhibit, regulate and/or modulate signal
transduction by GCN2, FMS (CSF1R), GSK3a, GSK3(3, FLT3 or FLT4 or
combinations of these kinases, to compositions which comprise these
compounds, and to processes for the use thereof for the treatment of diseases
and complaints that are -induced or modulated by GCN2, FMS (CSF1R),
GSK3a, GSK313, FLT3 or FLT4 or combinations of these kinases. .
Further aim of this invention is the synthesis of new compounds for the
prevention and treatment of neoplastic malignancies including, but without
being limited to, solid tumor cancers, cancers of the lymphatic or blood
system,
of neurodegenerative diseases, immune related disorders like arthritis,
psoriasis, lupus, multiple sclerosis or other autoimmune diseases as well as
chronic infections.
The compounds of the formula I can furthermore be used for the isolation and
investigation of the activity or expression of GCN2, FMS (CSF1R), GSK3a,
GSK313, FLT3 or FLT4. In addition, they are particularly suitable for use in
diagnostic methods for diseases in connection with unregulated or disturbed
GCN2, FMS (CSF1R), GSK3a, GSK3f3, FLT3 or FLT4activity. The host or
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 16 -
patient can belong to any mammalian species, for example a primate species,
particularly humans; rodents, including mice, rats and hamsters; rabbits;
horses, cows, dogs, cats, etc. Animal models are of interest for experimental
investigations, providing a model for treatment of human disease.
The susceptibility of a particular cell to treatment with the compounds
according to the invention can be determined by in vitro tests. Typically, a
culture of the cell is combined with a compound according to the invention at
various concentrations for a period of time which is sufficient to allow
active
agents such as anti IgM to induce a cellular response such as expression of a
surface marker, usually between about one hour and one week. In vitro testing
can be carried out using cultivated cells from blood or from a biopsy sample.
The amount of surface marker expressed are assessed by flow cytometry
using specific antibodies recognising the marker.
The dose varies depending on the specific compound used, the specific
disease, the patient status, etc. A therapeutic dose is typically sufficient
considerably to reduce the undesired cell population in the target tissue
while
the viability of the patient is maintained. The treatment is generally
continued
until a considerable reduction has occurred, for example an at least about 50%
reduction in the cell burden, and may be continued until essentially no more
undesired cells are detected in the body.
For identification of a signal transduction pathway and for detection of
interactions between various signal transduction pathways, various scientists
have developed suitable models or model systems, for example cell culture
models (for example Khwaja et al., EMBO, 1997, 16, 2783-93) and models of
transgenic animals (for example White et al., Oncogene, 2001, 20, 7064-
7072). For the determination of certain stages in the signal transduction
cascade, interacting compounds can be utilised in order to modulate the signal
(for example Stephens et al., Biochemical J., 2000, 351, 95-105). The
compounds according to the invention can also be used as reagents for testing
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 17 -
kinase-dependent signal transduction pathways in animals and/or cell culture
models or in the clinical diseases mentioned in this application.
Measurement of the kinase activity is a technique which is well known to the
person skilled in the art. Generic test systems for the determination of the
kinase activity using substrates, for example histone (for example Alessi et
al.,
FEBS Lett. 1996, 399, 3, pages 333-338) or the basic myelin protein, are
described in the literature (for example Campos-Gonzalez, R. and Glenney,
Jr., J.R. 1992, J. Biol. Chem. 267, page 14535).
For the identification of kinase inhibitors, various assay systems are
available.
In scintillation proximity assay (Sorg et al., J. of. Biomolecular Screening,
2002,
7, 11-19) and flashplate assay, the radioactive phosphorylation of a protein
or
peptide as substrate with 'ATP is measured. In the presence of an inhibitory
compound, a decreased radioactive signal, or none at all, is detectable.
Furthermore, homogeneous time-resolved fluorescence resonance energy
transfer (HTR-FRET) and fluorescence polarisation (FP) technologies are
suitable as assay methods (Sills et al., J. of Biomolecular Screening, 2002,
191-214).
Other non-radioactive EL1SA assay methods use specific phospho-antibodies
(phospho-ABs). The phospho-AB binds only the phosphorylated substrate.
This binding can be detected by chemiluminescence using a second
peroxidase-conjugated anti-sheep antibody (Ross et al., 2002, Biochem. J.).
Moreover, compounds of formula I inhibit GSK3a and GSK313
(glycogen synthase kinase-3 alpha and beta).
GSK3 inhibitors inhibit breast cancer cell growth and thus offer an innovative
approach for breast cancers resistant to hormone-bases therapy (H.M. Kim et
al., PLoS One, 2013; 8(4):e60383).
Antimyeloma activity of GSK3 inhibitors is reported by C. Fionda et al., J.
lmmunol. 2013 Jun 15; 190(12):6662-72).
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 18 -
Antic Ion cancer activity of GSK3 inhibitors is reported by E. Grissilli et
al.,
Clin. Cancer Res. 2013 Jul 15; 19(14):3820-31.
Antimyeloma activity of GSK3a and GSK3 8 inhibitors is reported by S.V.
Madhunapantula et al., Pigment Cell Melanoma Res. 2013 Aug 19doi:
10.1111/pcmr.12156.
GSK3 has been implicated in various diseases such as diabetes, inflammation,
cancer, Alzheimer's and bipolar disorder. GSK3 negatively regulates insulin-
mediated glycogen synthesis and glucose homeostasis, and increased
expression and activity of GSK3 has been reported in type II diabetics and
obese animal models (Geetha Vani Rayasam et al., British Journal of
Pharmacology (2009), 156, 885-898).
PRIOR ART
Triazolopyrimidine derivatives are described as GSK3 inhibitors for the
treatment of diseases like Alzheimer or diabetes in WO 2005/012307 Al and
in WO 2006/075023 A2.
SUMMARY OF THE INVENTION
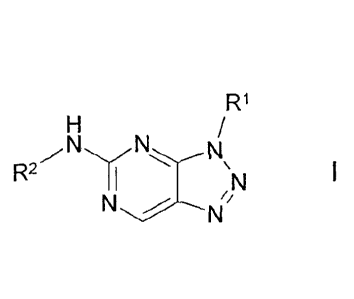
The invention relates to compounds of the formula I
R
1
R2
in which
R1 denotes Ar or Het,
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 19 -
R2 denotes unbranched or branched alkyl with 1-7 C-atoms, wherein
two
adjacent carbon atoms may form a double or triple bond and which
can be mono-, di-, tri-, tetra- or pentasubstituted by Hal and/or which
can be mono- or disubstituted by OR3, N(R3)2, Heti, CN, 000R3,
OCOOR3, CONH2, CONHA, CONA2, NR3COA, NR3S02A, SO2N(R3)2,
S(0)A, COHeti, 0[C(R3)21mN(R3)2, 0[C(R3)2]Het1, NHCOOA,
NHCON(R3)2, NHCOO[C(R3)21mN(R3)2, NHCO0[C(R3)21õHet1
,
NHCONH[C(R3)2]nN(R3)2, NHCONH[C(R3)2]Het1, OCONH[C(R3)21m-
- N(R3)2, OCONH[C(R3)2}pHet1 and/or COA,
or
denotes cycloalkyl with 3-7 C-atoms or cyclopentenyl, each of which
can be mono-, di-, tri-, tetra- or pentasubstituted by A and/or Hal
and/or which can be mono- or disubstituted by [C(R3)2]p0R3,
0[C(R3)210R3, [C(R3)2LN(R3)2, 0[C(R3)2]N(R3)2, [C(R3)2]pAr,
[C(R3)2],Het1, 0[C(R3)2],Het1, CN, [C(R3)2]COOR3, 0[C(R3)2]COOR3,
CONH2, GONNA, CONA2, [C(R3)2]NR3COA, CONR3S02A, NR3S02A,
SO2N(R3)2, S(0)A, COHetl, 0[C(R3)2jmN(R3)2, [C(R3)2]NR3C00A,
[C(R3)2],NR3COOCH2Ph, NR3CON(R3)2, NHCOO[C(R3)21niN(R3)2,
NHCO0[C(R3)2JpHet1, NHCONH[C(R3)2],-nN(R3)2, NHCONH[C(R3)2lp-
Het1, OCONH[C(R3)2],,N(R3)2, OCONH[C(R3)2LHet1
,
CONH(CH2)pHet2, CONR3NR3COA, CONR3N(R3)2, CONHCyc, COA,
=S, =NR3 and/or =0,
or
denotes piperidinyl, tetrahydropyranyl, pyrrolidinyl, azetidinyl, thiolanyl,
oxetanyl, 3-aza-bicyclo[3.1.0Thexyl, tetrahydrothiophenyl,
tetrahydrothiopyranyl, 4,5,6,7-tetrahydro-1H-indazol-5-yl, 4,5,6,7-
tetrahydro-1H-indazol-6-yl, tetrahydropyrazolyl, tetrahydrofuranyl or
hexahydropyridazinyl, each of which can be mono-, di-, tri-, tetra- or
pentasubstituted by A and/or Hal and/or which can be mono- or
disubstituted by [C(R3)2]p0R3, 0[C(R3)2LOR3, [C(R3)2LN(R3)2,
[C(R3)2]pAr, [C(R3)2]pHeti, [C(R3)2],Het3, CN, [C(R3)2LCOOR3,
CO[C(R3)2]pN(R3)2, CO[C(R3)2]0Het1, C0[C(R3)2]pHet3, NR3COA,
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 20 -
NR3S02A, SO2N(R3)2, S(0),-,A, COHetl, 0[C(R3)2bIN(R3)2,
0[C(R3)21,Het1, NHCOOA, NHCON(R3)2, NHCOO[C(R3)2]-nN(R3)2,
NHCOO[C(R3)2],Het1, NHCONH[C(R3)21-,,N(R3)2, NHCONH[C(R3)2]p-
Het1, OCONH[C(R3)2]mN(R3)2, OCONH[C(R3)2]pHet1, C(0)R3, =S,
=NR3 and/or =0,
R3 denotes H or unbranched or branched alkyl with 1-6 C-atoms,
Ar denotes phenyl or naphthyl, each of which is unsubstituted or
mono-,
di- or trisubstituted by Hal, A, [C(R3)2jp0R3, 0[C(R3)2]OR3, [C(R3)2]-
N(R3)2, 0[C(R3)2]N(R3)2, [C(R3)21pHet1, [C(R3)2LHet3, NO2, CN,
[C(R3)2]COOR3, 0[C(R3)2]COOR3, CON(R3)2, NR3COA, NR3S02A,
SO2N(R3)2, S(0)A, COHetl, 0[C(R3)2]pHet1, NHCOOA, NHCON(R3)2,
NHCOO[C(R3)2]mN(R3)2, NHCOO[C(R3)2]Het1, NHCONH[C(R3)2]m-
N(R3)2, NHCONH[C(R3)21pHet1, OCONH[C(R3)2]mN(R3)2,
OCONH[C(R3)2]Het1, S(0),Flet1, CHO and/or COA,
or denotes azulenyl,
Het denotes furyl, thienyl, pyrrolyl, imidazolyl, pyrazolyl,
oxazolyl,
isoxazolyl, oxadiazolyl, thiazolyl, triazolyl, tetrazolyl, pyridyl, pyrimidyl,
pyridazinyl, pyrazinyl, indolyl, isoindolyl, benzimidazolyl, indazolyl,
indolizinyl, cinnolinyl, quinolyl, isoquinolyl, benzoxazolyl, 1,3-benzo-
dioxolyl, benzothiophenyl, benzofuranyl, imidazopyridyl, dihydroindolyl,
quinoxalinyl, imidazo[1,2-a]pyridinyl, benzo[1,2,51thiadiazolyl,
naphthyridinyl, 2,3-dihydro-benzo[1,41clioxinyl, quinazolinyl,
benzothiazolyl or furo[3,2-b]pyridyl, each of which is unsubstituted or
mono- or disubstituted by Hal, A, [C(R3)21OR3, 01C(R3)2]0R3
,
[C(R3)2]pN(R3)2, 0[C(R3)2],N(R3)2, [C(R3)21Het1, NO2, CN,
[C(R3)2]COOR3, 0[C(R3)2]pCOOR3, CON(R3)2, NR3COA, NR3S02A,
SO2N(R3)2, S(0)A, COHetl, 0[C(R3)2],Het1, NHCOOA, NHCON(R3)2,
NH000[C(R3)2],,N(R3)2, NHC0O[C(R3)21pHet1, NHCONH[C(R3)2Im-
N(R3)2, NHCONH[C(R3)21pHet1, 000NH[C(R3)2]mN(R3)2,
OCONH[C(R3)2]pHet1, S(0),,Het1, CHO, COA, =S and/or =0,
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 21 -
Heti denotes pyrazolyl, pyridazinyl, pyridinyl, oxazolyl, isoxazoyl,
oxadiazolyl, pyrazinyl, indolyl, dihydropyrrolyl, pyrrolidinyl, azetidinyl,
oxetanyl, tetrahydroimidazolyl, dihydropyrazolyl, tetrahydropyrazolyi,
tetrahydrofuranyl, dihydropyridyl, tetrahydropyridyl, piperidinyl,
morpholinyl, hexahydropyridazinyl, hexahydropyrimidinyl,
[1,3]dioxolanyl, tetrahydropyranyl or piperazinyl, each of which is
unsubstituted or mono- or disubstituted by Hal, A, [C(R3)21,0R3,
0[C(R3)2]0R3, [C(R3)2],N(R3)2, [C(R3)21pHet2, NO2, [C(R3)2]pCN,
[C(R3)21pCOOR3, 0[C(R3)2]COOR3, [C(R3)2]CON(R3)2, NR3COA,
NR3S02A, SO2N(R3)2, S(0)A, COHet2, 0[C(R3)21mN(R3)2,
0[C(R3)21pHet2, NHCOOA, NHCON(R3)2, NHCOO[C(R3)2]mN(R3)2,
NHCOO[C(R3)2]pHet2, NHCONH[C(R3)2],-,,N(R3)2, NHCONH[C(R3)2]0-
Het2, OCONH[C(R3)2],,N(R3)2, OCONH[C(R3)2LHet2, S(0)Het2, CHO,
COA, =S and/or =0,
Het2 denotes pyrazolyl, oxadiazolyl, pyridinyl, triazolyl, tetrazolyl,
pyridazinyl,
pyrrolidinyl, azetidinyl, azoxetanyl, tetrahydroimidazolyl,
tetrahydropyrazolyl, tetrahydrofuranyl, piperidinyl, morpholinyl,
tetrahydropyranyl or piperazinyl, each of which is unsubstituted or
mono- or disubstituted by Hal, A, OA, CN, COOA, CONH2, S(0)A,
S(0)Ar, COA and/or =0,
Het3 denotes pyrazolyl, pyridinyl, pyrimidinyl or pyrazinyl, each of which
is
unsubstituted or mono- or disubstituted by Hal, A, [C(R3)2],0R3,
0[C(R3)2]p0R3, [C(R3)21pN(R3)2, [C(R3)2]Idet2, NO2, CN,
[C(R3)21pCOOR3, 0[C(R3)2}pCOOR3, CON(R3)2, NR300A, NR3S02A,
SO2N(R3)2, S(0)A, COHet2, 0[C(R3)2]mN(R3)2, 0[C(R3)21,Het2,
NHCOOA, NHCON(R3)2, NHCOO[C(R3)21,,,N(R3)2, NHC0O[C(R3)21p-
Het2, NHCONH[C(R3)2]mN(R3)2, NHCONH[C(R3)2]pHet2,
OCONH[C(R3)21-nN(R3)2, OCONH[C(R3)2]pHet2, S(0),-,Het2, CHO, COA,
=S and/or =0,
Ph denotes phenyl,
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 22 -
Cyc cycloalkyl with 3-7 C-atoms, which can be mono- or disubstituted
by
OH,
A denotes unbranched or branched alkyl with 1-10 C-atoms, wherein
one, two or three non-adjacent CH- and/or CH2-groups may be
replaced by N-, 0- and/or S-atoms and wherein 1-7 H-atoms may be
replaced by OH, F and/or Cl,
Hal denotes F, Cl, Br or I,
n denotes 0, 1 or 2,
denotes 1, 2 or 3,
denotes 0, 1, 2, 3 or 4,
and pharmaceutically usable derivatives, solvates, salts, tautomers and
stereoisomers thereof, including mixtures thereof in all ratios.
The invention also relates to the optically active forms (stereoisomers), the
enantiomers, the racemates, the diastereomers and the hydrates and solvates
of these compounds.
The invention also relates to the solvates of the salts of the compounds of
formula I, e.g. the mono- or dihydrate of the hydrochloride.
Moreover, the invention relates to pharmaceutically acceptable derivatives of
compounds of formula I.
The term solvates of the compounds is taken to mean adductions of inert
solvent molecules onto the compounds which form owing to their mutual
attractive force. Solvates are, for example, mono- or dihydrates or
alcoholates.
The term pharmaceutically acceptable derivatives is taken to mean, for exam-
ple, the salts of the compounds according to the invention and also so-called
prodrug compounds.
As used herein and unless otherwise indicated, the term "prodrug" means a
derivative of a compound of formula I that can hydrolyze, oxidize, or
otherwise
react under biological conditions (in vitro or in vivo) to provide an active
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 23 -
compound, particularly a compound of formula I. Examples of prodrugs include,
but are not limited to, derivatives and metabolites of a compound of formula I
that
include biohydrolyzable moieties such as biohydrolyzable amides,
biohydrolyzable
esters, biohydrolyzable carbamates, biohydrolyzable carbonates,
biohydrolyzable
ureides, and biohydrolyzable phosphate analogues. In certain embodiments,
prodrugs of compounds with carboxyl functional groups are the lower alkyl
esters
of the carboxylic acid. The carboxylate esters are conveniently formed by
esterifying any of the carboxylic acid moieties present on the molecule. Prod
rugs
can typically be prepared using well- known methods, such as those described
by
Burger 's Medicinal Chemistry and Drug Discovery 6th ed. (Donald J. Abraham
ed., 2001, Wiley) and Design and Application of Prodrugs (H.Bundgaard ed.,
1985, Harwood Academic Publishers Gmfh).
The expression "effective amount" denotes the amount of a medicament or of
a pharmaceutical active ingredient which causes in a tissue, system, animal or
human a biological or medical response which is sought or desired, for
example, by a researcher or physician.
In addition, the expression "therapeutically effective amount" denotes an
amount which, compared with a corresponding subject who has not received
this amount, has the following consequence:
improved treatment, healing, prevention or elimination of a disease, syndrome,
condition, complaint, disorder or side-effects or also the reduction in the
advance of a disease, complaint or disorder.
The expression "therapeutically effective amount" also encompasses the
amounts which are effective for increasing normal physiological function.
The invention also relates to the use of mixtures of the compounds of the
formula I, for example mixtures of two diastereomers, for example in the ratio
1:1, 1:2, 1:3, 1:4, 1:5, 1:10, 1:100 or 1:1000.
These are particularly preferably mixtures of stereoisomeric compounds.
814789805
24
"Tautomers" refers to isomeric forms of a compound that are in equilibrium
with each
other. The concentrations of the isomeric forms will depend on the environment
the
compound is found in and may be different depending upon, for example, whether
the compound is a solid or is in an organic or aqueous solution.
The invention relates to the compounds of the formula I and salts thereof and
to a
process for the preparation of compounds of the formula I and pharmaceutically
usable salts, solvates, tautomers and stereoisomers thereof, characterised in
that a
compound of the formula ll
C k N
1 ,N
N
in which R1 denotes Ar or Het,
is reacted with a compound of the formula III
R2-NH2 Ill
in which R2 denotes unbranched or branched alkyl with 1-7 C-atoms,
wherein two adjacent carbon atoms may form a double or triple bond
and which can be mono-, di-, tri-, tetra- or pentasubstituted by Hal
and/or which can be mono- or disubstituted by OR3, N(R3)2, Heti, CN,
COOR3, OCOOR3, CONH2, CONHA, CONA2, NR3COA, NR3S02A,
SO2N(R3)2, S(0)A, COHetl, 0[C(R3)2]rnN(R3)2, 0[C(R3)2]pHet1,
NHCOOA, NHCON(R3)2, NHCOO[C(R3)2]mN(R3)2,
NHCOO[C(R3)21pHet1, NHCONH[C(R3)2]mN(R3)2, NHCONH[C(R3)2]pHet,
OCONH[C(R3)2]-nN(R3)2), OCONH[C(R3)2]pHet1 and/or COA, or
denotes cycloalkyl with 3-7 C-atoms or cyclopentenyl, each of which
can be mono-, di-, tri-, tetra- or pentasubstituted by A and/or Hal and/or
which can be mono- or disubstituted by
CA 2903903 2020-03-20
814789805
24a
[C(R3)2],0R3, 0[C(R3)2]pOR3, [C(R3)2]3N(R3)2, 0[C(R3)2]p-N(R3)2,
[C(R3)2]pAr, [C(R3)2]pHet1, 0[C(R3)2],Het1, CN,[C(R3)2]pCOOR3,
0[C(R3)2]DCOOR3, CONH2, CONHA, CONA2, [C(R3)2])NR3COA,
CONR3S02A, NR3S02A, SO2N(R3)2, S(0)nA, COHetl,
0[C(R3)2]mN(R3)2, [C(R3)2]3NR3C00A,
[C(R3)2]pNR3COOCH2Ph, NR3CON(R3)2,
NHCOO[C(R3)2NN(R3)2, NHCOO[C(R3)2]pHetl,
NHCONH[C(R3)2]mN(R3)2, NHCONH[C(R3)2]pHet,
OCONH[C(R3)21mN(R3)2, OCONH[C(R3)2]pHet1,
CONH(CH2)pHet2, CONR3NR3COA, CONR3N(R3)2, CONHCyc, COA,
=S, =NR3 and/or =0,
or
denotes piperidinyl, tetrahydropyranyl, pyrrolidinyl, azetidinyl, thiolanyl,
oxetanyl, 3-aza-bicyclo[3.1.0]hexyl, tetrahydrothiophenyl,
tetrahydrothiopyranyl, 4,5,6,7-tetrahydro-1H-indazol-5-yl, 4,5,6,7-
tetrahydro-1H-indazol-6-yl, tetrahydropyrazolyl, tetrahydrofuranyl or
hexahydropyridazinyl, each of which can be mono-, di-, tri-, tetra- or
pentasubstituted by A and/or Hal and/or which can be mono- or
disubstituted by [C(R3)2]30R3, 0[C(R3)2]p0R3, [C(R3)2])N(R3)2,
[C(R3)2]pAr, [C(R3)2]pHet1, [C(R3)2]3Het3, CN, [C(R3)2]pCOOR3,
CO[C(R3)2]pN(R3)2, CO[C(R3)2]pHet1, CO[C(R3)2]3Het3, NR3COA,
NR3S02A, SO2N(R3)2, S(0)nA, COHetl, 0[C(R3)2]mN(R3)2,
0[C(R3)2]pHet1, NHCOOA, NHCON(R3)2, NHCOO[C(R3)2]mN(R3)2,
NHCOO[C(R3)2]pHet1, NHCONH[C(R3)2]A(R3)2,
NHCONH[C(R3)2],Het1, OCONH[C(R3)2]mN(R3)2, OCONH[C(R3)2],Het1,
C(0)R3, =S, =NR3 and/or =0,
and/or
a base or acid of the formula I is converted into one of its salts.
CA 2903903 2020-03-20
814789805
24b
Above and below, the radicals R1 and R2 have the meanings indicated for the
formula I, unless expressly stated otherwise.
For all radicals which occur more than once, their meanings are independent of
one
another.
A denotes alkyl, this is unbranched (linear) or branched, and has 1, 2, 3, 4,
5, 6, 7, 8,
9 or 10 C atoms. A preferably denotes methyl, furthermore ethyl,
CA 2903903 2020-03-20
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 25 -
propyl, isopropyl, butyl, isobutyl, sec-butyl or tert-butyl, furthermore also
pentyl,
1-, 2-or 3-rnethylbutyl, 1,1-, 1,2- or 2,2-dimethylpropyl, 1-ethylpropyl,
hexyl, 1-
, 2- , 3- or 4-methylpentyl, 1,1-, 1,2-, 1,3- , 2,2- , 2,3- or 3,3-
dimethylbutyl, 1-
or 2-ethylbutyl, 1-ethyl-1-methylpropyl, 1-ethyl-2-methylpropyl, 1,1,2- or
1,2,2-
trimethylpropyl, furthermore preferably, for example, trifluoromethyl.
A very particularly preferably denotes alkyl having 1, 2, 3, 4, 5 or 6 C
atoms,
preferably methyl, ethyl, propyl, isopropyl, butyl, isobutyl, sec-butyl, tert-
butyl,
pentyl, hexyl, trifluoromethyl, pentafluoroethyl or 1,1,1-trifluoroethyl.
Moreover, A denotes e.g. CH2OCH3, CH2CH2OH, OCH2CH2NH2, CH2NHCH2
or NHCH2CH3.
Cycloalkyl with 3-7 C-atoms preferably denotes cyclopropyl, cyclobutyl, cyclo-
pentyl, cyclohexyl or cycloheptyl.
R1 particularly preferably denotes 4-methoxyphenyl, 4-ethoxyphenyl, 4-[1-(2-
hydroxyethyl)pyrazol-4-yl]phenyl, 411-(2-methoxyethyppyrazol-4-yl]phenyl, 2-
methyl-quinolin-6-yl, 4-(2-methoxy-ethoxy)phenyl, 4-(2-methoxy-1-
methoxymethyl-ethoxy)phenyl, 4-(tetrahydro-pyran-4-yloxy)phenyl or 4-(3-
hydroxy-3-methyl-butoxy)phenyl.
R2 preferably denotes unbranched or branched alkyl with 1-7 C-atoms,
wherein two adjacent carbon atoms may form a double or triple bond and
which can be mono-, di-, tri-, tetra- or pentasubstituted by Hal and/or which
can
be mono- or disubstituted by CN, CONH2, CONHA, CONA2 and/or
0[C(R3)2]mN(R3)2,
or
preferably denotes cycloalkyl with 3-7 C-atoms or cyclopentenyl, each of which
can be mono-, di-, tri-, tetra- or pentasubstituted by A and/or Hal and/or
which
can be mono- or disubstituted by [C(R3)2]p0R3, [C(R3)2]N(R3)2, [C(R3)2]pAr,
[C(R3)21pHet1, [C(R3)2]pCOOR3, [C(R3)2}pNR3C00A, S(0)A, ON, CONR3S02A,
SO2N(R3)2, CONH2, CONHA, CONA2, CONH(CH2)pHet2, [C(R3)2}pHet1
,
COHetl, CONR3NR300A, CONR3N(R3)2, CONHCyc and/or
[C(R3)21pNR3C000H2Ph,
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 26 -
or
preferably denotes piperidinyl, tetrahydropyranyl, pyrrolidinyl, azetidinyl,
thiolanyl, oxetanyl, 3-aza-bicyclo[3.1.0]hexyl, tetrahydrothiophenyl,
tetrahydrothiopyranyl, 4,5,6,7-tetrahydro-1H-indazol-5-yl, 4,5,6,7-tetrahydro-
1H-indazol-6-yl, tetrahydropyrazolyl, tetrahydrofuranyl or
hexahydropyridazinyl,
each of which can be mono-, di-, tri-, tetra- or pentasubstituted by A and/or
Hal
and/or which can be mono- or disubstituted by {C(R3)2}2Het1, [C(R3)21pHet3,
SO2N(R3)2, S(0)A, [C(R3)2]pC00R3, C0[C(R3)2]N(R3)2, C0[C(R3)2]Het1
,
C0[C(R3)2]Het3, C(0)R3, and/or =0.
R2 particularly preferably denotes cyclopropyl, cyclobutyl, cyclopentyl,
cyclohexyl, 4-hydroxy-cyclohexyl, 4-amino-cyclohexyl, 4-amino-cyclobutyl, 4-
hydroxy-cyclobutyl, 3-hydroxy-cyclopentyl, 3-amino-cyclopentyl, 3-
aminocarbonyl-cyclobutyl, 3-aminocarbonyl-cyclopentyl, 3-aminocarbonyl-
cyclohexyl, tetrahydrofuranyl or 34(3-hydroxy-3-methyl-
butyl)aminocarbonylIcyclopentyl.
R3 preferably denotes H or alkyl haying 1, 2, 3 or 4 C atoms, particularly
preferably H or methyl.
Ar denotes, for example, o-, m- or p-tolyl, o-, m- or p-ethylphenyl, o-, m- or
p-propylphenyl, o-, m- or p-isopropylphenyl, o-, m- or p-tert-butylphenyl, o-,
m-
or p-hydroxyphenyl, o-, m- or p-nitrophenyl, o-, m- or p-aminophenyl, o-, m-
or
p-(N-methylamino)phenyl, o-, m- or p-(N-methylaminocarbonyl)phenyl, o-, m-
or p-methoxyphenyl, o-, m- or p-ethoxyphenyl, o-, m- or p-ethoxycarbonyl-
phenyl, o-, m- or p-(N,N-dinnethylamino)phenyl, o-, m- or p-(N,N-dimethyl-
aminocarbonyl)phenyl, o-, m- or p-(N-ethylamino)phenyl, o-, m- or p-(N,N-
diethylamino)phenyl, o-, m- or p-fluorophenyl, o-, m- or p-bromophenyl, o-, m-
or p-chlorophenyl, o-, m- or p-(methylsulfonamido)phenyl, o-, m- or p-(methyl-
sulfonyl)phenyl, a-, m- or p-cyanophenyl, o-, m- or p-carboxyphenyl, o-, m- or
p-methoxycarbonylphenyl, o-, m- or p-formylphenyl, o-, m- or p-acetylphenyl,
o-, m- or p-aminosulfonylphenyl, o-, m- or p[2-(morpholin-4-yl)ethoxylphenyl,
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 27 -
o-, m- or p-[3-(N,N-diethylamino)propoxy]phenyl, furthermore preferably 2,3-,
2,4-, 2,5-, 2,6-, 3,4- or 3,5-difluorophenyl, 2,3-, 2,4-, 2,5-, 2,6-, 3,4- or
3,5-
dichlorophenyl, 2,3-, 2,4-, 2,5-, 2,6-, 3,4- or 3,5-dibromophenyl, 2,4- or 2,5-
dinitrophenyl, 2,5- or 3,4-dimethoxyphenyl, 3-nitro-4-chlorophenyl, 3-amino-4-
chloro-, 2-amino-3-chloro-, 2-amino-4-chloro-, 2-amino-5-chloro- or 2-amino-6-
chlorophenyl, 2-nitro-4-N,N-dimethylamino- or 3-nitro-4-N,N-dimethylamino-
phenyl, 2,3-diaminophenyl, 2,3,4-, 2,3,5-, 2,3,6-, 2,4,6- or 3,4,5-
trichlorophenyl,
2,4,6-trimethoxyphenyl, 2-hydroxy-3,5-dichlorophenyl, p-iodophenyl, 3,6-di-
chloro-4-aminophenyl, 4-fluoro-3-chlorophenyl, 2-fluoro-4-bromophenyl, 2,5-
difluoro-4-bromophenyl, 3-bromo-6-methoxyphenyl, 3-chloro-6-methoxyphenyl,
3-chloro-4-acetamidophenyl, 3-fluoro-4-methoxyphenyl, 3-amino-6-methyl-
phenyl, 3-chloro-4-acetamidophenyl or 2,5-dimethy1-4-chlorophenyl.
Ar furthermore preferably denotes phenyl or naphthyl, each of which is
unsubstituted or mono-, di- or trisubstituted by Hal, A, [C(R3)2LOR3,
0[C(R3)2],COOR3, S(0)A, [C(R3)2]pHet1, 0[C(R3)2],Het1 and/or [C(R3)21,Het3.
Moreover, Ar denotes azulenyl.
Het preferably denotes fury!, thienyl, pyrrolyl, imidazolyl, pyrazolyl,
oxazolyl,
isoxazolyl, oxadiazolyl, thiazolyl, triazolyl, tetrazolyl, pyridyl, pyrimidyl,
pyridazinyl, pyrazinyl, indolyl, isoindolyl, benzimidazolyl, indazolyl,
indolizinyl,
cinnolinyl, quinolyl, isoquinolyl, benzoxazolyl, 1,3-benzodioxolyl,
benzothiophenyl, benzofuranyl, imidazopyridyl, dihydroindolyl, quinoxalinyl,
imidazo[1,2-a]pyridinyl, benzo[1,2,5]thiadiazolyl, naphthyridinyl, 2,3-dihydro-
benzo[1,4]dioxinyl, quinazolinyl, benzothiazolyl or furo[3,2-b]pyridyl, each
of
which is unsubstituted or mono- or disubstituted by A, Hal, [C(R3)2]Het1
,
and/or [C(R3)2],0R3.
Heti preferably denotes pyrazolyl, pyridazinyl, oxazolyl, isoxazoyl,
oxadiazolyl, tetrahydro-pyranyl, dihydropyrrolyl, pyrrolidinyl, azetidinyl,
oxetanyl, tetrahydroimidazolyl, piperidinyl, morpholinyl or piperazinyl, each
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 28 -
of which is unsubstituted or mono- or disubstituted by A, [C(R3)2]pHet2,
[C(R3)21,CN, [C(R3)21N(R3)2, [C(R3)2}pC0N(R3)2, [C(R3)210R3 and/or =0.
Het2 preferably denotes pyrazolyl, oxadiazolyl, pyridinyl, tetrazolyl,
pyridazinyl, pyrrolidinyl, azetidinyl, azoxetanyl, tetrahydroimidazolyl,
tetrahydropyrazolyl, tetrahydrofuranyl, piperidinyl, morpholinyl,
tetrahydropyranyl or piperazinyl, each of which is unsubstituted or
monosubstituted by A and/or =0.
Hal preferably denotes F, Cl or Br, but also I, particularly preferably F or
Cl.
Throughout the invention, all radicals which occur more than once may be
identical or different, i.e. are independent of one another.
The compounds of the formula I may have one or more chiral centres and can
therefore occur in various stereoisomeric forms. The formula I encompasses
all these forms.
Accordingly, the invention relates, in particular, to the compounds of the
formula I in which at least one of the said radicals has one of the preferred
meanings indicated above. Some preferred groups of compounds may be
expressed by the following sub-formulae la to Id, which conform to the formula
I and in which the radicals not designated in greater detail have the meaning
indicated for the formula I, but in which
in la R2 denotes unbranched or branched alkyl with 1-7 C-atoms,
wherein two adjacent carbon atoms may form a double or
triple bond and which can be mono-, di-, tri-, tetra- or
pentasubstituted by Hal and/or which can be mono- or
disubstituted by CN, CONH2, CONHA, CONA2 and/or
0[C(R3)2]mN(R3)2,
or
denotes cycloalkyl with 3-7 C-atoms or cyclopentenyl, each
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 29 -
of which can be mono-, di-, tri-, tetra- or pentasubstituted by
A and/or Hal and/or which can be mono- or disubstituted by
[C(R3)2]p0R3, [C(R3)2]N(R3)2, [C(R3)2]pAr, [C(R3)2]pHet1
,
[C(R3)2]pCOOR3, [C(R3)2LNR3C00A, S(0)A, [C(R3)2]pHet1
,
COHetl, CN, CONR3S02A, SO2N(R3)2, CONH2, CONHA,
CONA2, CONH(CH2)pHet2, CONR3NR3COA, CONR3N(R3)2,
CON HCyc and/or [C(R3)21pNR3COOCH2Ph,
or
denotes piperidinyl, tetrahydropyranyl, pyrrolidinyl,
azetidinyl, thiolanyl, oxetanyl, 3-aza-bicyclo[3.1.0Thexyl,
tetrahydrothiophenyl, tetrahydrothiopyranyl, 4,5,6,7-
tetrahydro-1H-indazol-5-yl, 4,5,6,7-tetrahydro-1H-indazol-6-
yl, tetrahydropyrazolyl, tetrahydrofuranyl or
hexahydropyridazinyl, each of which can be mono-, di-, tri-,
tetra- or pentasubstituted by A and/or Hal and/or which can
be mono- or disubstituted by [C(R3)2]pHet1, [C(R3)2]pHet3,
SO2N(R3)2, S(0)A, [C(R3)2]pCOOR3, CO[C(R3)21pN(R3)2,
CO[C(R3)2]pHet1, CO[C(R3)2]pHet3, C(0)R3, and/or =0;
in lb Ar denotes phenyl or naphthyl, each of which is
unsubstituted
or mono-, di- or trisubstituted by Hal, A, [C(R3)2LOR3,
0[C(R3)2],CO0R3, S(0)A, [C(R3)2]Het1, 0[C(R3)2]pHet1
and/or [C(R3)2]pHet3,
or denotes azulenyl;
in lc Heti denotes pyrazolyl, pyridazinyl, oxazolyl, isoxazoyl,
oxadiazolyl, tetrahydro-pyranyl, tetra hydrofuranyl,
dihydropyrrolyl, pyrrolidinyl, azetidinyl, oxetanyl, tetrahydro-
imidazolyl, piperidinyl, morpholinyl or piperazinyl, each of
which is unsubstituted or mono- or disubstituted by A,
[C(R3)2]pHet2, [C(R3)2]pCN, [C(R3)2]3N(R3)2,
{C(R3)2}CON(R3)2, [C(R3)2]OR3 and/or =0;
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 30 -
in Id R1 denotes Ar or Het,
R2 denotes unbranched or branched alkyl with 1-7 C-atoms,
wherein two adjacent carbon atoms may form a double or
triple bond and which can be mono-, di-, tri-, tetra- or
pentasubstituted by Hal and/or which can be mono- or
disubstituted by CN, CONH2, CONHA, CONA2 and/or
0[C(R3)2],-,,N(R3)2,
Or
denotes cycloalkyl with 3-7 C-atoms or cyclopentenyl,
each of which can be mono-, di-, tri-, tetra- or
pentasubstituted by A and/or Hal and/or which can be
mono- or disubstituted by [C(R3)2]p0R3, [C(R3)21N(R3)2,
[C(R3)2]pAr, [C(R3)21pHet1, [C(R3)2}pCOOR3,
[C(R3)2LNR3C00A, S(0)A, CN, CONR3S02A,
SO2N(R3)2, CONH2, CONHA, CONA2, CONH(CH2)pHet2,
[C(R3)2]pHet1, COHetl, CONR3NR3COA, CONR3N(R3)2,
CONHCyc and/or [C(R3)2]pNR3COOCH2Ph,
or
denotes piperidinyl, tetrahydropyranyl, pyrrolidinyl,
azetidinyl, thiolanyl, oxetanyl, 3-aza-bicyclo[3.1.0Thexyl,
tetrahydrothiophenyl, tetra hydrothiopyranyl, 4,5,6,7-
tetrahydro-1H-indazol-5-yl, 4,5,6,7-tetrahydro-1H-indazol-
6-yl, tetrahydropyrazolyl, tetrahydrofuranyl or
hexahydropyridazinyl, each of which can be mono-, di-,
tri-, tetra- or pentasubstituted by A and/or Hal and/or
which can be mono- or disubstituted by [C(R3)2]pHet1
,
SO2N(R3)2, S(0)A, [C(R3)2]pHet3, [C(R3)2]pCOOR3,
CO[C(R3)2]N(R3)2, CO[C(R3)2]pHet1, CO[C(R3)2]pHet3,
C(0)R3, and/or =0,
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 31 -
R3 denotes H or unbranched or branched alkyl with 1-6 C-
atoms,
Ar denotes phenyl or naphthyl, each of which is
unsubstituted or mono-, di- or trisubstituted by Hal, A,
[C(R3)2]p0R3, 0[C(R3)2],CO0R3, S(0)A, [C(R3)2]pHet1
,
0[C(R3)2]pHet1 and/or [C(R3)2]pHet3,
or denotes azulenyl,
Het denotes furyl, thienyl, pyrrolyl, imidazolyl, pyrazolyl,
oxazolyl, isoxazolyl, oxadiazolyl, thiazolyl, triazolyl,
tetrazolyl, pyridyl, pyrimidyl, pyridazinyl, pyrazinyl, indolyl,
isoindolyl, benzimidazolyl, indazolyl, indolizinyl, cinnolinyl,
quinolyl, isoquinolyl, benzoxazolyl, 1,3-benzodioxolyl,
benzothiophenyl, benzofuranyl, imidazopyridyl,
dihydroindolyl, quinoxalinyl, imidazo[1,2-a]pyridinyl,
benzo[1,2,5]thiadiazolyl, naphthyridinyl, 2,3-dihydro-
benzo[1,4]clioxinyl, quinazolinyl, benzothiazolyl or
furo[3,2-b]pyridyl, each of which is unsubstituted or
mono- or disubstituted by A, Hal, [C(R3)2],Flet1, =0 and/or
[C(R3)2]0R3,
Heti denotes pyrazolyl, pyridazinyl, oxazolyl, isoxazoyl,
oxadiazolyl, tetrahydro-pyranyl, tetrahydrofuranyl,
dihydropyrrolyl, pyrrolidinyl, azetidinyl, oxetanyl, tetra-
hydroimidazolyl, piperidinyl, morpholinyl or piperazinyl,
each of which is unsubstituted or mono- or disubstituted
by A, [C(R3)2]DFlet2, [C(R3)2]CN, [C(R
3)2]N(R3)2>
[C(R3)2]pC0N(R3)2, [C(R3)2}0R3 and/or =0,
Het2 denotes denotes pyrazolyl, oxadiazolyl, pyridinyl,
triazolyl,
tetrazolyl, pyridazinyl, pyrrolidinyl, azetidinyl, azoxetanyl,
tetrahydroimidazolyl, tetrahydropyrazolyl,
tetrahydrofuranyl, piperidinyl, morpholinyl,
81789805
32
tetrahydropyranyl or piperazinyl, each of which is
unsubstituted or monosubstitued by A and/or =0,
Het3 denotes pyrazolyl, pyridinyl, pyrimidinyl or
pyrazinyl, each
of which is unsubstituted or mono- or disubstituted by A,
[C(R3)2]pHet2 and/or [C(R3)2],0R3,
Ph denotes phenyl,
Cyc cycloakyl with 3-7 C-atoms, which can be mono- or
disubstituted by OH,
A denotes unbranched or branched alkyl with 1-10 C-
atoms,
wherein one, two or three non-adjacent CH- and/or CH2-
groups may be replaced by N-, 0- and/or S-atoms and
wherein 1-7 H-atoms may be replaced by OH, F and/or Cl,
Hal denotes F, Cl, Br or I
denotes 0, 1 or 2,
m denotes 1, 2 or 3,
denotes 0, 1, 2, 3 or 4
and pharmaceutically acceptable salts, solvates, tautomers and stereoisomers
thereof, including mixtures thereof in all rations.
According to one aspect of the present invention, there is provided compounds
selected from the group
CA 2903903 2020-03-20
81789805
32a
1 ___ No. Name
[3-(4-ethoxy-phenyl)-3H-0,2,31triazolo[4,5-d]pyrimidin-5-A-
prop-2-ynyl-amine
"A2" Isopropy143-(4-methoxy-pheny1)-3H-0,2,31triazolo[4,5-d1-
pyrimidin-5-y1]-amine
,
"A3" [3-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-0]-
(tetrahydro-pyran-4-y1)-amine
cyclopropy143-(4-methoxy-pheny1)-3H-0 2,31triazolo[4,5-d)-
pyrimidin-5-y11-amine
r [3-(4-methoxy-phenyl)-3H-0,2,31triazolo[4,5-d]pyrimidin-5-A-
oxetan-3-yl-amine
CA 2903903 2020-03-20
81789805
32b
tert-buty143-(4-tnethoxy-phenyt)-3H-[1,2,31tnazolo(4.5-4-- 1
pyrimidin-5-yr]-amine
. - -".Ati. ¨ N-(1 .1 -dioxothiolan-3-0)-3-(4-methoxyphenyl)triazolo[4.5-dj-
pyrimidin-5-a mine
- -"A.9" ¨ 3-(4-methoxy-phenyl)-3it-[1,2,3jtriazoiof4,6Td-jpyrimiiin:=54f:
(1 -propy 1-cyclepropyl)-amine
- A10" - I trans-343-(4-metiox-y--phe----nyl)-3H-(1,2,3)thazoia-14 ,5-4]-
--
pyrirnidin-5-ylaminol-cyclopentanol
- 'Al 1" (4-1344-Methoxy-phenyl)-3H-11.2.31triazolor4,5-dbpyrimidin-5-
ylamino)-cyclottexylymethanol
= -;A1-2; - cyc1openty113-(4-m-ithoxy-pheny1)-311-(1.2.3griazolo(4,54i)---
pyrimidin-5-y1)-a mine
k "A i 3 1
" IY(4-methoxy-p-y-10A-41-,-2-.3)triaz- ok){4,11pyrimid in-5-y+ :
i_ 13_44-rnethyl-piperazin-l-ylypropyij-7ine
"A14" 13-(4-methoxy-pheny1Y3H-11,2,31triazolor4,5-djpyrimidin-5-ylj- ,
methyl-amine
- "Al ' ¨1-3-2(4-riCerh6xy---p-henayiT5i441:-Iiiiiiii7614,5-dipyrimidin-5-11-
(1,2,2 .6,6-pentamethyl-p1per1d ia-4-yI)-amine
1 "A16" ethyl-( 3-(4-methoxy-phenyl)-3H41,2,31tr iazolo[4.5-djpyrirnid in-
5-yif-am ine
- "A17" -13-(4-methoxy-phenyly3H-fl ,2,3jtria-ia6[4:5--alpyiinlIctin-5-y11-
(2.2 ,6,6-tetramethyl-piperidin-4-yi)-amine
"A18" (3-(4-methoxy-phenyt)3H41,2,31triazolo[4,5-djpyrimidin-5-yij-
(1-methyl-piperidin-4-yl)-amine
¨ "A19" -(3-(4-methoxy-phenyl).3H-(1,2,3priazolo[4.5-dipyrimid-in-5-y1)-
0-methyt-3-aza-bicyclo(3,1.0jhex-6-y0-arnine
"A20" 4-(344-rnethoxy-phenyly3H-(1.2,3)Inazolo[4,5-dipytimidin-5-
. yiaminaj-l-methyi-pyrnalidin-2-one
r--mi-- - -cyclobutyl-[3:(4-methoxy-phenyt) 31-1-(1,2,3jtriazoto[4.,5417
1 pyrimidin-5-y1)-amine .
CA 2903903 2020-03-20
81789805
32c
' -"-A2-2" (2-(4-chloro-phony1)-cyciopropy11-13-(4-metboxy-phenyi)-3H-
'
(1,2.3)triazok3(4.5-dlpyrimidin-5-ylfamine
---;;-A2---- 113-(4-methoxy-p- henyl)-3H-{1.2.3)triazolo14 .5-djpyrirnidin-5-A-
--
1
(4.5.8,7-tetrahydro-1H-indaz01-5-y1)-amine
"A2-21" trarts-34344-methoxy-phenyl)-3H-11,2,3)triazoici(4.5-dj----
pyrimidin-5-ylamino)-cyclobutanol
i" "A2--5"--e cis-343-(4-methoxy-phenyI)-3H-11 2,31triazoloi4 .5-dipyrimid
in-
5-ylaminoi-cyclobutanot
¨"Ale-- cis-4-I3-(4-methoxy-pherwl)-3H-11.2.31triazotol4,5-djoyrimidin-
5-ylaminol-cyclohexanol
¨"Air cis-313-(4-metboxy-pheny1)-3H-112.31triazolo[4,5-dipyrirriidin-
5.yiamino)-cyclopentanoi
"A28" , (3-(4-rnethoxy-pheny1)-3H-11,2,31triazolo[4,5-djpyrimidio-5-y1)--
(S)-tetrahydro-furari-3-0-amine
i
"A29" tra ri.s-343-(4-brorno-p heny1)-3H-11.2,3)triazoloi4.5-djoyrimid in-
1
5-yiaminoi-cyclopentanol
-"A30-'7 i (cis-3-(3-(4-methoxy-pheny1)-3H41.2-.31triazokii4.5-0
I pyrimidin-511aminol-cyclobutylytarbarnic acid tert-butyl ester
_4
- "A31"-- i ---cis-N4344-methoxy-phenA":3-441.2.31triaible(i,5-d} - ' .1
PYrimidin-5-01-cyclobutarie-1,3-diamine
_
"A32 {trans-3-1344-rnethoxy-pheny1)-3H-(1.2.3)triazo1o(4,5411- I
pyrimidin-5-ytaminol-cyclobuty1}-carbamic acid tort-butyl ester
"A33" (4-13-(4-methoi-ylpheny1)-3H11,2.31triazo10(4.5-d)pyrirnid in-5-
ytaminol-cyciohexylmettlyiycarbarnic acid tert-butyl ester
- "A34" 4-(3---(41-
methoxy-phenyli-3t1-(1,2,3]triazolo[4,5-dip-y-ririiiiiir-il-5- 1
yiarninol-piperidine-1-carboxytic acid tert-butyl ester 1
"A35" {trans-3-1344-
rnethoxy-pbenyl)-3H41,2,31triazolof4,5-di- -11
pyrimidin-5-ylaminej-cyclopentylYcarbamic acid tert-butyl '
ester
¨ 3107(4---ineFie--xY-pli-enyli-311.2.3)triazoloi4lpyriril1diii:57-
-
! ylaminoFazetidine-l-carboxylic acid tert-butyl ester
CA 2903903 2020-03-20
81 789805
32d
1.---"A3f= -(3-aza-
6icyclo(3.1.01hex-6-191)-(3-(4-inethoxy-phenyl)-3H- -1
(1.2.3priazolo(4.5-d]pytimidin-5-01-a mine
I
7'4387-- trini--N-13-(4-methoxy-pheny1)-3H-(1.2,3]triazolo[4,5-d)- - -
t
pyrimidin-5-y1)-cyclobutane-1 ,3-diamine
a.---(4-ar-iiinornethyl-cyclohexyl)-(3-(4-methoxy-phenyl)23W- -
(1 ,2 ,3)triazolof 4,5-dlpyrimid 4i-5-y11-amine
[3-(4-rnethoxy-pheny1)-3H11,2,3]triazolo[4,5-d)pyrimidin-5-4 ,
,
,
piperidin-4-0-amine ,
MA41'H. trans-N-[3-(4-methoxy-
phenyI)-3H-[ 1 .2,3)triazolo(4.541)-- ---1
pyrimedin-5-y1)-cyc1opentane- 1,3-d iamine
r-.1ik4" I 1-443-(4-rnethoxy-pheny1)-3H-(1.2 , 3It riazolo(4, 5-d 1pyrimidin-
5-ylamino)-pipendin- 1 -yI)-ethanone
- --, -.
"A43" .4-
13-(4-methoxy-pheny1)-31-111 2,31triazolo[4.-5---d]pyrimidin-5-y1)-
pipendin-3-y1-amine
- -- ¨ 4
=
"A44" , -
trans-N-I3-(4-methoxy-phenyl)-3H-1 1 .2,3)triazolot4õ5-0
pyrimidin-5-yI)-cyclohexane-1 ,4-diamine
- "A45" ' 7 cis-N-13-(4-methoxy-pheny1)-3H-(1,2,3)171-azolo[4,5-d]-
, pyrimid in-5-yI)-cyclohexane- 1 ,4-diamine
-"448" .-- 2-d imethylamino-1-(4-(3-(4-methoxy-phenyt)-3H-
( 1,2,31triazolo(4,5,d0 yrimidin-5-ylaminol-piporidin- 1 -yI)- .
ethanone
inlet hylamino-1 44-(3-(4-rnethoxy-pheny1)-3/17 1
11,2,3)triazolo(4,5-djpyrimiden-5-ylamino)-pipend in- 1-y1)-
propan- 1 -one
¨ - I3-(4-metho--xy-:pheny1)-3H-E 1,2,31tnazolo[4,5-d]pyrimidin -5-
y1)-
(2-(4-methyl-piperazin- 1-y1)-e th yI)-amine
-"A5 .1-. - t-- - trans-3-{-[4-( 1-methyl-1 H-pyrazol-4-y1)-pheny1)-3H-
[
i
: (1 ,2.31tnazolo[4,5-djpyrimidin-5-ylaminoycyclopentanoi
(1,2, 3 priazolo[4,5-dipyrirrodin-511)-cyclohexane-1,4-diamine
CA 2903903 2020-03-20
81789805
32e
- -A53-"--T-N-[3-(4-iodo-pheny1)-3H-(1,2,31triazolo(4,5-dIpyrimn-5-y11-
cyclohexane-1.4-diamine
- ;7A54"--1--- ----hi-1314-4I-e-thyl-1H-pyrazol-4-y1)-pheny1)-311:---- -----.
I[1 ,2,3)tnazolo(4,5-dlpyrimidin-5-yI)-(trans)-cyclohexane-1.4-
1
diamine
"A57" trans-3-(344-11-(2-hydroxy- -ethyl)-1H-pyrazol-41-y1)-pheny1)-3H-
11,2,31triazolo(4,5-dlpynmidin-5-ylamino)-cyclopentanol
"A58" (1 S.314)-N413-(4:r-nethoxy-pheny1)-3H-(1,2,31triaz03o(4,5-411-
I pyrimidin-5-yI)-cyclopentane-1,3-diamine
¨71A-59- (1R.3S)-N-(3-(4-inethoxy-phenyl)-3H41,2,3)triazolot4,5-dT- -
pyrimidin-5-ylkyclopentane-1.3-diamine
E -7-A60' R1R,3S)-343-(4-methoxy- -pheny1)-31-14-1,2,-Spriazolo[4.5-411-
I
pynmidin-5-ylamincij-cyclopenty1)-(4-methyl-piperazin-1-y1)-
methanone
-A61- [3-(4-met-hoxy-phenyl)-
3H-(1,2,31triazolo(4,54)pyrirnidin-5-y1)- ;
(3-pyrrolidin-1-yl-propyI)-amine
__
"A62- 2-dimethy1amino-1-(343-(4-methoxy-pheny-1)-3Ht-- -- -1
(1,2,3)triazok,(4,5-cilpyrimidin-5-ylaminoj-azetidin-1-y1)- '
ethanone
-=-=====-===-- - = -- ======-- -....
"A63- 3-dimethylamino-1-(3-{3-(4-methoxy-pheny1)~3H-
11.2,3)tnazolo[4,5-d)pyrimidin-5-ylaminol-azetidin-1-y1)-
propan-1-one
;A6-4" N-(3-(4-morpholin-4-yi-pheny1)-3H(1,2,31triazolot4.5-
_
dipyrimidin-5-y1)-cyclohexane-1,4-diamine
---'''.A-e-Y.--- 1--
(R)-313-(4-methoxy-phenyl)-3H-(1,2.3jtriazolO14,541-
pyrimidin-5-ytaminoj-pyrrolidine-1-carboxyhc acid tert-butyl
ester
"A66- I (S)-343-(4-methoxy-pheny1)-3i4-[1,2,3)ITiazo1o(4,5-dj-
1 pyiimidin-5-ylamino)-pyrrolidine-1-carboxylic acid tert-butyl
, I
ester
CA 2903903 2020-03-20
81789805
32f
- "A6-7" ¨trans-3-
(3444nothoxy-phenyl}-3H11,2,31tnazolot4,5-df-
pyrimidin-511amino1-1-triflUorornethyl-cyclopentanol
"A69" -I3-(4-methoxy-phenyty3H11,2,31triazo1o(4,5Apyrirnidin-5-y1)-
(R)-pyrrolidin-3-yl-amine
-7706-- - (R)-1-{4454-(trans)-4-amino-cyclohexylamino}-
(1,2,3)triazotoK6-dipyrin-3-141-phenylypyttolichn-3-o1
( '--"k7T"---i:{4-(5-((trans)-4-iinino:Cyclohexylarnino)-(1.2,3)triazoto[4-.5-
dipyr4midin-3-y1}-phenytypyrrolidin-2-one
--;A-7-2"¨ trans-244-(3-(4-methoxy-pheny1)-3H-(1.2,3)tnazolo(4,5-0 1-
I pyrimidin-5-ylaminci)-cyclohexyr)-ethanoi
-"A-73"- '-' --cis-2-4413:(4-methOW-pheny1)-3H-11,2.31triazolo(4,5-4:11- -
pyrinitdin-5-ylaminoi-cyclohexylyethanol
"A74" [242-amino-ethoxy}eth-yij-f344-methoxy-phenyl)-3H-
11.2.31tnazokit4,5-clipyrimidin-5-4amine
'-"-A757-114-13-(4-fluoro-phenyt)-3H-11,2,3)triazoio{4.5-djpyrimidin-5-y1)-
(trane)-cyclohexane-1,4-diamine
- 0A76 j [3-(3-amino-propoxy)-pt0PYq-1344-methoxy-phenyl)-3H-
(1.2,3)triazolo[4,5-d)pyrimidin-5-yli-amine
*477; --1-1144-rnethoxy-phenyl)-3H-(1.2,31triazolig4.5--4;yrirnidin-5-y1)--
(S)-pyrrolidin-314.amine
*A78" 1 f3-(4-methoxy-phenyi)-31141,2,3)triazolo[4,5-0)pyrimidin-5-0)-
(1-(2-rnorphohn-4-y14-ethyl)-pipendin-4-y1)-amine
"-A 7 6; ...4 -(1 6,3k):5-[3-(4-methoxy-phe-n-yi)-3H-f 1-,1-,31ihazolo[4:6--di-
pyrirnidin-5-ytamino)-cyclopentanecarboxylic acid
- 481" - (1R,36)-343-(4-ntethoxy-phenyl)4H41,2,3)triazolo[4751d1-
pyrimidm-5-yiantinotcyclopentanec.arboxylic acid
- "A82* - 1.344-rn-etiidicy-
phenyiF3H41,273)triazolo{4.54ipyrirnidin.5.y1}(3,4,5,6-tetrahydro-
2H.(1,4160yridinyl-4-y1)-amine
- itk8i."----- 2-dirile-s-i-hifamiiio-1-t(R):3-43-(4-tnethoxy-pheny1)-3H-
(1 2, 31triazolo(4,5-d]pyrimidin-5-ylameno)-pyrrolidin-1-A-
,
ethanone
CA 2903903 2020-03-20
81 789805
32g
- -;A8.4.7.- - 12-----(5-flu-oro-1 H-indo1-3-y1)-ettly1)-{3-(4-(2-met-h-Oxy-
othoxy)-
phenyI)-3H-[ 1.2, 3Priazo3o[4,5-dlpyt imid in-5-0-a mine -
3-dimethylamino-1 -{(R)-3-13-(4-methoxy-pheny1)-5-H-
( 1 ,2 , 3)triazolo(4,5-djpyrimidin-5-ylaminoj-pyrrolidin- 1 -yI}-
propan-1 -one
"4-861; - 13-(4-methoxy-phenyl)-3H-(1,2,31tnazolo(4,5-d)pyrimidin-5Ly111
( 1 -(2-pyrrolidin-1-yl-ethyl)-piperidin-4-yli-amine 1
--7'-k87" 44344-methoxy-pheny1)-3H41.2.31triazolop4.5-dipyrimidin-5-
ylamino)-piperidin-2-one
---;-A6-6" - (3-(4-methoxy-pheny1)-3H-il ,2.3)triazolc44,5-djpyrimidi n-5-:yI)-
1-
((S)- 1 -pyndin-4-yl-pyrrolidin-3-yI)-amine A89" ((1R .3S)-3-(3-(4-methoxy-
phenyl)-3H-[1.2,311riazolo[4, 5-
d)pyrirnidin-5-ylamino)-cyclopenty1)-carbamic acid benzyl
ester
- ' "A9-Ci"---+- - 'I -f(R)--3-13-(4---rnethoxy-pheny1)-3H11
,2.3)triazolo(4.5-4 :
pyrimidin-5-ylamino)-pyrrolidin-1 -yI)-2-pyrazol- 1 -yl-etha none '
- "A91" - I-iiiiiiithylamino-14(S)-3-(3-(4-methoxy=phionyl)-47- '
(1 2 ,3)1r1azo1044. 5-dipyrernidin-5-ylaminoFpyrrolidin-1 -yI)-
ethanone
--"T k92" - fr-- 3-dimethylamino-14(S)-3-(3-(4-methoxy-phenyl)-3H-
11.2 .31tr1azolo(4.54)pyrimidin-5-yla mino)-pyr rolidin- 1 -yI)-
propa n- 1 -one
....
"A93" 1-{(S)-313-(4-rnethoxy-pheny1)-3H2.31triazolo(4,5-d)-
pyrimidin-5-ylamirio)-pyrrolidin-111)-2-pyrazol-1-yl-ethanone
---7A-64.7--- trans-34345-4 1 -methyl- 1 H-pyrazol-4-yI)-py rid in-2-yI)-3H -
( 1 2. 31triazolo[4,5-d jpyrimidin-5-ylaminoycyclopentanol
"A96" '.- trans-N-(3-14-(2-molp-holin-4-yi-ethoxy)-phenylj-3H- ¨
Ii .2.3)triazolo[4,5-djpyrimid in-5-0)-cyclohexane- 1 .4-diamine
"A96" - trans---k-(3-(5-morpholin-4-yl-pyrn-2--Yi)-3H----
1
1 (1 2,3jtriazolo[4, 5-d]pyrimidin-5-4cyclohexane- 1,4-diamine j
i
CA 2903903 2020-03-20
81789805
32h
"A97" {(15,3R)-3-13-(4-methoxy-pheny1)-311-(1,2,31triazcTio(4.5--
d)pyrimidin-5-ylamino)-cyclopenty1)-(4-methyl-piperazin-1-y1)-
methanone
- -4
"A98" (3-(4-methoxy-pheny1)-3H-(1,2,3itriazolo[4,5-dipirimidin-5-y11-
(R)-tetrahydro-furan-3-0-amine
"A99 I trans-3-13-(5-methoxy-pyridin-2-y1)-3H-(1,2,3)triazolo(4,5- --
djpyrimidin-5-ylaminoi-cyclopentanol
"Al 00" N-(3-quinolin-
6-y1-3H41,2,3)triaiolo[4,5-d]pyrirnidin-511)-
cyclohexane-1,4-diamine
(1S.3R)-3-(3-(4-iodo-pheny1)-3H-E1.2.31triaiolo[4,5-
djpyrimidin-5-ylaminol-cyclopentanecarboxylic acid
E Af02T (trans)-3-(3--quinolin-6-y1-3H11,2,31triazolot4-,-5-'djpyriiii-idin-5-
,
ylamino)-cyclopentanol
"A1-03" (S)-3-13-(4-rnethoxy-phenyl)-3H-P ,2,3itriazolo(4.5-
d)pynmidin-5-ylaminol-piperidine-1-carboxylic acid tert-butyl
ester
13-(4-metho-XY2Pheny1)-3H11-:2.31triazoloi4.5-dipyrimidIn75---y1)-
(1-methyl-piperidin-3-y1)-amine
--"Al 65 (R)-- .--(3---(4-methoxy-pheny1)-3H-(1,2,31tria-iolot4 .5-
dipyrimidin-5-ylaminol-piperidine-l-carboxylic acid tert-butyl
ester
"A106" trans-T-{443-(4-methoxy-pheny1)-3H-[1,2,3)triazolo(4.5-di-
pyrimidin-5-ylaminol-cyclohexyl)-piperidin-4-ol
¨cis-11{4-13-(4-methoxy-pheny1)-3H-(1,2,3jt-r-iizolo[4.5-
;
dipyrimidin-5-ylaminoj-cyclohexylypiperidin-4-ol
"A108" (cis)-N-(3-(4-
methoxy-pheny1)-31-1-[1,2,31triazolo[4,5-
d)pyrimidin-5-y1)-14',N-dimethyl-cyclopentane-1.3-diamine
13-(4-methoxy-phen¨y1-)4H-(1,2,3)triaiolOR5=d1Pirimidin=5;y1):-
[ ((R)-1-pyridin-4-yl-pyrrolidin-3-yI)-amine
CA 2903903 2020-03-20
81789805
321
-"A-110% ' (1S,3S)-343-(4-methoxy-phenyt)-3H-[1,2,.31triazolof4.5-
dipyrimidin-5-ylamino)-cyclopentanecarboxylic acid methyl
ester
.- 1-" 7 -taS,3-
t--)--3431(44-Tie--thoxy-phenyt)--3-1-1--11.2,3)triiidldt4'.5-------
/ dlpyrimidin-5-ylaminol-cyclopentylymethanol
i
"/--0-1271 7 - f(715.3R)-3-(3-44-metho;cy-phenyl).-3H-(1,2,3Ttriazolo[4,5------
-
djpyrimidin-5-ylaminol-cyclopentylymethanol
"Al fr 7 7 (i R,3R)-3-(3-(4-methoxy-phenyl)-3H-(1 ,2.31triazolo(4. 5-
i
djpyrimidin-5-ylaminoj-cyclopentanecarboxylic acid methyl
it
ester
¨ -;A--114" 'I-- ((1R,3R)-343-(4-methoxy-phenYl)-3H41 ,2,31triaz0lo(4,5-
i, dipyrimidin-5-ylarninoleyclopentyg-methandl
11--5;713-(4-methoxy-phenyly3H-j1,2,31triazolo[4.5-dipyrimidin-.5-ylr
"11;
I f(R)-1-(1-methyl-1H-pyrazol-4-y1)-pyrrolidin-111)-amine
"A116 (3-(4-(1-methy1-1H-pyrazol-4-y1)-pheny11-3H- ,
, . [1 ,2,31triazolo(4,5-djpyrimidin-5-y1)-(R)-pyrrolidin-3-yl-
amine
,
,
"A117" 1 Rees)-3-13-(4-methoxy-phenyl)-3H-(1,2,3jtriazolo44,5-
i dipyrimidin-5-ylaminol-cyclopentylrnethylycarbamic acid tert.
butyl ester
- "A118 (1S,3R)-N3-{3-(4.(1-rnethyl-1H-pyrazol-4-y1)-phenyq-3H.
1 (1,2,31triazolo[4,5-dipyrimidin-5-yll-cyclopentane-1,3-diamine
- "Al 19" I(1S.3R)--N3-(3-qu'inolin-6-y1-3H-(1,2,3)triazolo14,5--dipyrimidiril
-
_ _I 5-yI)-cyclopentane-1.3-diamine
' "A120" : -- (1R,3R)-N3-(3-(4-methoxy-P-h-eny1)-3H-(1.2,31triazold[4--,5-
Idipyrimdin-5-y1]-cyclopentane-1,3-diamine
"A121" I (1S,3R)-N3--(3-(441-(2--metili>iy-eth-Y4-1H-pyrazol-4-0-1: .-
phenyl}-3H-112,3jtriazolo[4,5-dipyrimidin-511)-cyclopentane-
1 1,3-diamine
.._.__
"A122" 1 ((1R,3S)-3-aminomettlyl-cyclopenty1H3-(4-metholy-phen0- ,
3H-(1.2.3jtr1az010(4,5-djpyrimodin-5-y11-amtne
1
CA 2903903 2020-03-20
81789805
32j
¨ ;;K1-23;r1¨ ( 1R,3Ft )-N-(3-(4-(1-(2-methoxy-ethyi)--1a:pyriiol-4-y11-
4 pheny1)-3H-[1.2.310 iazolo14.5-dlpyrimidin-5-y1)-cyclopentane-
1,3-diamine
(1R,3R)=N-43-(2-methyl-quinolin-6-y1)-3H-(1,2,31triazolor4, 5-
I d)pyrimtdin-5-y11-cyclopentane-1,3-d1am1ne
71--A-116-'i (112.3R)-N-(34411-(2-3thoxy-ethyl)-1H-pyrazol-4-y11-
pfinii)- -
3H-(1,2,31triazolo[4, 5-djpyrimidin- 5-y1)-cyclopentane- 1,3-
diamine
_________________________________________________________________ - ---i
"A126" 13-(2-
methyl-quinolin-6-y1)-3H-[1.2,3)triazolo(4,5-d)pynmidin-5- 1
y11-(R)-pyrrolidin-3-yl-amine
--- "A-CT' - ¨ --N-1-3-14-Tte-trahydr0-p--yr--an-4-yloxy)-phenyI0H- -
[1,2,31triazolo(4,5-dipyrimidin-5-y1)-trans-cyc1ohexane-1,4-
diamine
¨"Al2-87* -(S)-1-0-(5(4-amino-cyclohexylammo}-11,2,31triazolo[4.5- -
, dipyrimodin-3-0)-pheny1)-pyrrolidin-3-ol
1¨"A129" ii-{.3-(4-(1-rnethyl-1H-pyraz01-4-y1)-phenylj-3H- ------'
[1,2 ,3jtriazolo14 ,5-dlpyrimid in-511)-trans-cyclohexane-1,4-
diamine _
- "-A130" -r- 3--dimethylamino-1-((R)-3-{3-(4-(1 -methy1-1H-pyrazol-4-y1) .--
phenyI)-3H-( 1,2,3)1riaz010f4,5-0yrimidin-5-ylaminol-
pyrrolidin-1-yI)-propan-1 -one
1--"ki3-1-:¨ 3-(4-methyl-piperazi n- 1 -yI)- 1-((R)-3.--1-3-(4--(1 -methyI-1H-
1
, pyrazol-4-y1)-pheny1)-3H-(1,2,3)triazolo14,5-dlpyrimid in-5-
ylamino)-pyrrolidin-1-yI)-propan- 1-one
"Al 32" i 1-t(R)-343-(4-
rnethoxy7pheny1)-3H-[1,2,31triaiol-o(4,5- -- :
d]pyrimidin-5-ylaminoi-pyrroliclin-1-0)-3-(4-methyl-piperazin-1-
yI)-propan-1-one
"A133" i 3-(4-methyl-pipe razi n-1-
y1)-11(R)-3-(3-quinolin-6-y1-3H-
I
[1,2,3]thazolo(4.5-dipyrimidin-5-ylamino)-pyrrolidin-1-y1)-
i propan-l-one
_..1___._...__ ____________________ _ . _ .,.,.._ . . ___ _ ... .
,...._._. . õ
CA 2903903 2020-03-20
81789805
32k
- "Al 3,-1- - 3-(4-methyl-piperazin-l-y1)-1-{(R)-3-(3-(2-mitnY1-quinolin-6-
0-311-[1.2.3priaz0lop4.5-dipyrimidin-5-ylaminol-pyrrolidin-1-
L y1}-propan-1-one
.__ õõ
"A135" (1k3R)-3--(3-(4-(2-methoxy-ethoxy)-phenyl)-3H-
(1,2,3)triazolot4,5-dipyrimidin-5-ylamino}-
cyclopentanecarboxylic acid amide
/---;-A136.;-- - --(1-S,3R)-3-(3-(4-inethoxy-phenyl)-3H-(1,2,3ftr1az0144-75-- -
-
djpyrimidin-5-ylaminoi-cyc1opentanecarboxylic acid amide
-76/5-537'; (1S,3S)-343-(4-methoxy-pheny1)-3H-(1,2,31triazolo14,-5-- .--
[._
dipyrimidin-5-ylaminol-cyclopentanecartioxylic acid amide
1(1S.3R)-3-(3-(4-pyrrolidin-1-yl-pheny1)13H-11 2,3]triazo1o14 ,5-
-
dipyrimidin-5-ylarninoj-cyclopentanecarboxylic acid amide
"-A1i-97 (1R3R)-343-(4-methoxy-phenyt)-3H-(1,2,3priazolo(4,5-
dipyrimidin-5-ylamino)-cyclopentanecarboxylic acid
--7'A14e1" ( ii:1- . 3R)- 3 i 3- ( 4 - m e tho x y - p he ny 1)- 3H 4 1,--
i,31tnazolO1475--
djpyrimidin-5-ylaminoj-cyclopentanecarboxylic acid amide
--(1S',3-12--)-3-1-3---(2-Methyl-benzoxazol-6-y1)-3H-(1,tr7i-azolo- i
[4,54lpyrimidin-5-ylaminoycyclopentanecarboxylic acid I
amide
r-z-A-142----I 443-(4-methoxy-pheny1)-3H-11,2,3itnazolo(4,5-dipyrimidin-5-
ylaminol-butyramide
.-----:% ____
443-(4-methoxy-pheny1)-3H-(1.2.31triazolo(4.5-djpyrimidin-5-
ylamino)-cyclohexanocarboxylic acid amide
¨ "A 1 44" 343-(4-methoycy-pheny1)-3H-(1.2.31triazolo[4.5-dipyrimidin-5-
ylaminol-butyramide
,----- -
"A145" I (trans)-3-13-(4-metkixy-phenyl)-3H-(1.2.3priazolo(4,5- -
clipyrimidin-5-ylarninoj-cyclobutanecarboxylic acid amide
'¨'.A1-46;--1(1-34,-3R--)--3-(344-pyrrolidirv-1-yl-pheny1)-3H-(1:2,3itriaz-
olo(4,5--
. djpyrimidin-5--ylamino)-cyclopentanecarboxylic acid amide
CA 2903903 2020-03-20
81789805
321
1--------41-4-7;-- i(1R,3R)-3-(3-(2-methyl-benzothiazol-6-y1)-3H-11,2,3)-
triazolo-
i i [4,5-d)pyrimidin-5-ylaminoj-cyclopentanecarboxylic acid
1 _. ,_:. amide
..... ._ -....
"Ai48"- i (trans)-343-(4-methoxy-
pheny1}-3H-(1,2,3)triazolo[4,5- ,
4J-
pyrirnidin-5-ylamino)-cycionexanecarboxylic acid amide
-;Ailt- "-- Z, (1011)-3-13-(44-thoxy-pheny1)-3H41,2,3itnatO-lo(4.5-d)- -
i pyrimidin-5-ylaminol-cyclopentanecarboxylic acid amide
r---;A-f5-o" - ; - (1R,31:2)-3-(3-(4-ettioxy--pheny1)-3H-(1.2,31tnazolo(4.5-d}-
----
;
1 pyrimidin-5-ylamino}-cyc3opentanecarboxylic acid amide
i _____
- "A1--1"- I: - (1R,3R)-3-{344-((S)-3,3,3-irifluoro-2-hydroxy-propoxy)--
phenyll-3H-L1,2.31triazolo(4,5-d]pyrimidin-5-ylamino}-
cyclopentanecarboxylic acid amide
¨ "A152" (1R,3R)-343-(3-fluoco-4-mettioxy-pheny1)-3H-(1,2,31triazoto-
f4,5-d)pyrimidin-5-ylamino)-cyclopentanecarboxylic acid
amide
- "-A-1-53"' (11:0R)--
3-{3-[4-(2-hydroxy-ethoxy}-pheny1)-3H-(1,2,3)triazO-6--1
(4,5-d)pyrirrodin-5-ylaminoycyclopentanecarboxylic acid !
amide
-----;;A154;; .!--(fR,fR)-3-{3-(3-fluoro-4-(3-oxo-morpholin-4-y1)-phenyij-31i--
--
[1,2,31triazoloi4,5-dipynmidin-5-ylaminoycyclopentane-
carboxylic acid amide
71 Ki 6.5;- I-- - (1k3R)-3-{3444(R)-35.-34ifluOi-o---2---ii.ydroxy---prop-o-
xy)- ---
pheny1}-31-141,2.3}triazolo[4,5-dlpyrimidin-5-ylamino}-
cyclopentanecarboxylic acid amide
i "A156" ' (1R-3R)-3-(3-(2,-3-dihYdro-benzo[1,4}dioxin-6-y1)-3H-(1,2,1-
triazolo14,5-d)pyrimidin-5-ylamino)-cyclopentanecarboxylic
acid amide
"Al 5--" .! (1k3R)-343-(4-ethoxy-3-flociro-pheny1)-3H-i1,2,31triazolo(4.5-
dipynmidin-5-ylamino)-cyclopentanecarboxylic acid amide
CA 2903903 2020-03-20
81789805
32m
"A158"T (1R,3R)-343-(3-fluoro-4-morpholin-4-yl-pheny0-3H-
1 11,2,4triazolo[4,5-dipyrimidin-5-ylaminol-
I cyclopentanecarboxylic acid amide
rA161"A160" (1R,3R)-3-(344-(2-pyrazol-1-yi-ethoxy)-phenylj-3H-
0,2,3)triazolo[4,5--d]pyrimidin-5-ylamino}-
cyclopentanecarboxylic acid amide
"" (1R,3R)-3-{314-(3,3,3-trifluoro-2-hydroxy-2-trif)uoromethyl- _
i propoxyyphenyli-3H-11,2,31triazolo[4,5-d]pyrimidin-5-
ylamino}-cyclopentanecarboxylic acid amide
"A162" 1 (11213R)-343-(4-methoxy-3-methyl-phenyl)-3F1-f1,2,31triazolo- -
1 14,5-dipyrimidin-5-ylaminol-cyclopentanecarboxylic acid
amide
_______________ _ "A163" (1R,3R)-343-
(3,5-difluoro-4-methoxy-pheny1)-3H-0,2,31-
I triazolo[4,5-d]pyrimidin-5-ylaminol-cyclopentanecarboxylic
1 acid amide
"A164" (1R,3R)-343-(4-isopropoxy-phenyi)-3H41,2,31triazolo[4,5-dd r
_ pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide i
"A166" (1S,3R)-3-(344-(2-methoxy-ethoxy)-pheny1)-3H-11,2,3)-
triazotof4,5-djpyrimidin-5-ylaminoycyclopentanecarboxylic
acid amide
_
"A1677 (1R,3R)-3-{314-
(2-methoxy-1-methoxymethyl-ethoxy)-
pheny1]-3H41,2,3)triazoio[4.5-dlpyrimidin-5-ylaminoy
cyclopentanecarboxylic acid amide
_____________________________________________________________ _
CA 2903903 2020-03-20
81789805
32n
1----A-166'.-- ¨(1K3R)-3-{3-(4-(tetrahydna-pyran-4-yloxy)-pheny11-31-1- 1
11.2,3}triazolo[4.5-dipyrimidin-6-ylamino)-
cyclopentanecarboxylc acid amide
"A16-9" - (1R,3R)-3-(144-(2-ethoxy-ethoxy)-pheny9-3k41.31-
triazolo[4,5-dipyrimidin-5-ylamino)-cyclopentanecarboxylic
acid amide
"A169a" ( 1R,3R)-3--((3-azule n-2-yltriazolo(4 ,5-dJpyrimid in-5-yI)-
-a I aminolcyclopentanecarboxamide
( 1R3R)-3-(13-
-"A1 witi" .(6-fluoro-2-naphthyl)triazolof4 ,5-djpyiim1din- 5-1
yljaminojcyclopentanecarboxamide ,
-1-0-66-cri-t( 1F3R)j3-1(3-(4-tert-butoxypheny1)triazolo14,5-dipyrimidin-5- 1
,
yljaminojcyclopentanecarboxarnide _______________________________ ,
"A 169d" (1R,314:3-0--(4-(2-hydroxy-1.1-dimethyl-ethoxy)Phen01- I
triazolo14,5-djpyrirrvidin-5-yljamincijcyclopentariecarboxamide
-7ifk-f69e (1R,314)-34[1-14-1(3R)-tetrahydroturan-3-y1}oxypheny1)-
triazolof4.5-dipyrimidin-5-yliaminoicyclopentanecarboxamide
- "A16-of" (1 R,3R)-3-ii344((3S)-tetrahydrofuran-3-yl)oxyphenyi-
triazolo{4,5-d)pyrimidin-5-yllaminolcyclopentanecarboxamide ,
--7/1/41-70" 1.1 (1R ,3R-)-3-(3-(4- iodo-phenyI)-3H -(1 ,2,31triazolof 4 ,5-
d1- ¨
1 pyrimidin-5-ylaming-cyclopentanecarboxylic acid amide
__-__-_ I
"A171" ' - ( iS,3R)-3-13-(4-iodo-pheny1)-3H11 2,31triazolo(4,5-d)-
1 pyrimiiiin-S-ylaminoycyclopentanecarboxylic acid amide .,
'...¨A1727.- 1¨ (1S,3R)-3-(3-benzoi1 .2 .5/thiadiazol-5-0-3H11.2 , 3j- 1
Itriazolo[4.5-dipyrimidin-5-ylamino)-cyclopentanecarboxylic ;
1 acid amide
"Al -fa." 1 ( 1R.3R)-3-13-(6-methoxy-pyridin-3-y1)-3H-1-1,2,3)triazoio[4,5--H
' d)pyrimidin-5-ylaminol-
cyclopentanecarboxylic acid amide
I
---"-A174" (1R73R)-3-(3-be-rizo[1,2 ,5]thiaciiazoi-5:ii:31-4-171,73)
Itnazol0(4 ,5-dlpyrimid in-5-yiaminoycycloperitaneca rboxylic
' 1 acid amide
CA 2903903 2020-03-20
81789805
320
r---;-A175"' I-1-1 R,3R)-3-13-(5-rnethoxy-pyridin-2-y1)-3H-(1
,2,31triazolo(4,g71
i djpyrimidin-5-ylarninol-cyclopentanecarboxylic acid amide
Ido-pheny1)-3H-(1 .2,31triazolo44,5-di-
pyrimidin-5-ylaminol-cyclopentanecarboxytic acid amide
L t--
--71-141t7;1- (1R,3R)-343-(5-iodo-pyridin-2-y1)-3H-(1,2.3ftriazolo[4,5-4 '
pyrimidin-5-ylaminol-cyclopentanecarboxylic acid amide
(1R.3R)-343.(5-ethoxy--pyridin-2-y1)-3H-[1.2,31triazoto(4,5-di-
pyrimidin-5-ylaminoi-cyclopentanecarboxylic acid amide
'7A-i-79" (1R,3R)-3-(3-(6-ethoxy-pyridin-3-y1)-3H-11,2,3)triazolot4,5-dj-
pyrimidin-5-ylaminol-cyclopentanecarboxylic acid amide
r ;A1-130"--f-- (111.3R)-3-(3-(2-methyl-benzoturan-5-y1)-3H- _
i [1,2.3jtriazolo(4,5-djpyrimidin-5-ylaminoj-
i i
cyclopentanecarboxylic acid amide
---;A-181" -4:. (1R,3R)-313-(2-methoxy-quir-iolin-6-0-3H11.2,3jtriazolo1-475-1
; dipyrimidin-5-ytamino)-cyclopentanecarboxylic acid amide
'A182" (1R.3R)-3-(342-(2-etboxy-ethoxyyquinotin-6-y11-311-
(1,2,3)triazolo14,5-djpyrimidin-5-ylamino)-
.
' cyclopentanecarboxylic acid amide
M83" -t (1R,3-R)-3-(3-(1,2-diTriethyl-114---benzimidazol-5-y1)-3H-- -
i 11,2,31triazolo(4,5-d]pyrimidin-5-ylaminoF
I cyclopentanecarboxylic acid amide
. .
t---"4-1-64"--1-(1R.3R)-3-13-(2-methyl-quinazolin-6-y9-3H-(1,2,3)tria-ibibt4,5-
--
t i dipyrimidin-5-ylaminol-cyclopentanecarboxylic acid amide
t----"A-i8-6"-- ' (1R,3R)-343-(2:methyl-21-1-indazol-6-y1)-311-(1,2-:3-
jiiiiibibi4:5- 1
1 dlpyrimidin-5-ylaminoFcyclobentanecarboxylic acid amide 1
1 "A Tbe¨ (10,3R)-3-13-(2-methy1-2H-indazol-5-y1)-3H-0.2,31triazolo[4,5- i
I dipyrimidin-5-y1am1n0j-cyclopentanecarboxylic acid amide .
_
I "A186a" (1R,3R)-3-((3-cinnotin-6-yttriazolo(4,5-41rinTidin-5-y1)-
1 amino)cyclopentanecarboxamide
r "Al 86b" -(1R,-YR)-3-u3-(7-me-thoxy--3---quinoliiitriazok44,&-ifjp-y-
rimidin:57'
1 yllaminojcyclopentanecarboxamide
CA 2903903 2020-03-20
81789805
32p
. A1 c' '
'(1R,3R)-3-(13--(2--Me¨iiiiiindolizin-7:01.riazoio[4.5-dipyrimidin- -
5-yliaminolcyckventanecarboxamide
"A186d" 1- (1 R,3R)-3-1(3-(2-methylindolizin-611)thiiolo(4,5-d)pyrimidin-
5-yliaminoicyclopentanecarboxamide
'"A186e" (1R.3R)-313-(2-methylimidazo[1 .2-alpyridin-6-yl)triazolo[4,5-
dipyrimidin-5-yljaminoicyclopentanecattoxamide
.1A1-661" ¨ (1R.3R)-3-(3-(2-methy1-1H-indol-S-yi)triazo1o[4.5-dipyritii-idiri--
--1
5-yllamino}cyclopentanecarboxamide
- "A186g" (1R,3R)-31[3-(2-methylisoin-dol-6--y- liriaidiii.j4,5-([3-5-
Maminoicyclopentanecarboxamide
"A186h"- --(1R.3-k)-34[3-(7-methylindoi-iim-2-0iiiaioiet4.5-djpyrimidin-
511jamiriojcyclopentanecarboxamide
I --.7A-1-66-1"- (-I h,3R)-3-
1[3-(1H-indazol-5-yl)triazolo(4,54p-yrimidin-5--
yijaminolcyclopentanecarboxamide
,
' 1-86j" (1R,3R)-3-1[3-(2-me- Ihyl-3H-benzimidazol.511)triazolo[4.5-
dipyrirnidiri-5-y1laminolcyclopentanecarboxamide
I1---;Ai ifrr 7 - , i
pyrimn-5-ylaminoj-cyclopentanecarboxylic acid amide
----;A.-718-8"-- (1S.3R)-3-13-quinolin-6-y1-3H-(1,2.3jtriazolo(4,&41pyrimidin--
5-
_. ., . ____________________________________________ ylamino)-
qclopentanecarboxylic acid
-"A189" (1S,3R)-3-(3-quinan-6-
y1-3H-(12.31triazolo(4,5-dipyrimidiril:-
ylamine)-cyclopentanecarboxylic acid amide
. _
"A 190" (1R,3R).3-(3-quinotin-6-y1-3H-02.31triazolo[4,5-d)pynmidin-5-
ylarnino)-cyclopentanecarboxylic acid amide
- "A191" 1 (1S.3R)-3-(3-(2-methyll--uindlin-62-y1)-311.41,2,31triazolo(4,5-dj-
pyrimidin-5-y1aminoycyc1opentanecarboxylic acid amide
r "Ai92'¨ritrans)-3-(3-(2-methyklulTiolin-611)-3H41,2,31thazoloi4,5-dj-
pyrimidin-5-ylamincii-cyclohexanecarboxylic acid amide I
r "A193;¨(18.3'S)-313-(2-methyl.quinolin-6-y1):311411triazolo[4,54)-1
pyrimidin-5-y1aminoleyclopentanecarboxylic acid amide 1
i.... _ _ . , __ .. .. . ........
..
CA 2903903 2020-03-20
81789805
32q
1----"A1947; (1R,3R)-3-(341 *5inaphthyridin-2-0-3H-0 2.31triazolo(4.5.-df- -
pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide ,
(1 R3R)-313-(1-methyl.1H-indazol-5-y1)-31141,2,31triazolo[4.5-
id]pyrimidin-5-ylaminoi-cyclopentanecarboxylic acid amide
;47.1ia" 1 (1R ,3R)-3=13-(7-methyl-3-quinolyl)triazolo(4,5-dipyrimidin-5-
;Ai 95b" , (1R,3R)-
3.4yE1)3721.nmoejcythcylopeo .on:lec:bci ouix:mlyidoteriazoloo. 5_
dipyrimidin-5-yliaminolcyclopentanecarboxamide
' -41-9877- (1R,3R)-313-(4-iodo-
pheny1)-3H11,2.31triazolo[4,5-41- ,
1--
Ipyrimidin-5-ylaminol-cyclopentanol
--A197-- 1- ¨ ¨(1 ',3S)-343-(4-iodo-phenyl)-3H41.2,3)triazolo[4,5-dj-
____________ , 1 ____ pyrimidin-5-ylaminol-cyclopentanol
"A198" (trans)-343-(4-pyrrolidin-l-yl-phenyl)-3H-f1,2,3ftriazoto[4.541-
pyrimidin-5-ylaminol-cyclopentanol
1-- -:-_,- ¨ _______
"A199" , (trans)-3-(3-(2-methyl-benzoxazol-6-y1)-3H-11,2,3)triazoloiii-,57
djpyrimidin-5-ylaminol-cyclopenta not
----1A/200"- (trans)-3-(3-bianzo(1.2.5)thiadiazol-5-0-
3Fil1,2,31triaibib14,5: -
d)pyrimidin-5-ylamino)-cyclopentano1
r --;;A- 2- 6 *;:- (1 R.3R)-343-(4-
methoxy-pheny1).3H-(1,2,31triazolo[4.5-dj-
pyrimidin-5-yiaminol-cyclonexanol
-.
*A202" (trans)-343-(2-methyl-quinolin-6-y1)-3H-f1 .2.3)tnazolo[4.5-dj-
pyrimidin-5-ylaminoj-cyclopentanoi
;A203-"---1--
(1R,3R)-3-{344:(2-hydroxy-itthoxy)-phenyli-3H-
(1,2,31triazolo(4.5-dkayrimidin-5-yiamino)-cyclopentanol
-A204" (1R,3R)-3-(3-quinotin-6-
0-311-112.31triazolo[4,5-dipyrimidin-5- 1
ylamino)-cyclopen tanot 1
1- --A205" (trans)-3-1314-0S3.3-trifluoro-2-hydroxy-propoxy)-phenylil
i 3H41.2,31triazolo(4,5-dipyrimidin.5-ylaminol-cyclopentanol
I. -;;-A20-6- (trans)-3-[3-(6:etit-oxy-pyndin-3-*-34--i1 ,,2,31triaia-tio
I . pyrimidin-5-yiaminoycyckvontanol
,
,
CA 2903903 2020-03-20
81789805
32 r
r -A207" (trans)-3-(3-(4-ethoxy-pheny1)-3H-11,2,3jtriaz010{4,54}-
pyrimidin-5-ylaminOriciopentanol
r ________ -A208" (1/1,3R)-343-(2-methyl-quiriolin-6-y1)-
3H41,2,31triazolo14,5:dr
pyrimidin-5-ylaminOcyclopentanol
"A209" (trans)-3-(3-(2-methyl-quinazolin-6-y1)-3H-(1,2,31thazolo[4:67--
djpyrimidin-5-ylaminoj-cyclopentanol
¨"-A-2767 -(frans)-3-(3-(4-isopropoxy-pheny1)-3H-f1.2.3(triazolor4;5-df --
pyrimidin-5-ylaminoFcyclopentanol
3H-(1,2,3)triazolo[4.5-djpyrimidin-5-ylamino)-
cyciopentanecarboxylic acid amide
r "A212" (1R,3R)-3-(3-(441-(2-methoxy-ethyl)-1H-pyrazol-4-yli-pheny1)-
3H-(12,3itriazo10[4,5-djoyamidin-5-ylamino).cyclopentanol
"A213" (113,3R)-3-(3-(4-(1-(2-pyrrolidin-l-y1-eth-y1)-1H-pyrazot-4-0)-
phenyl}-3H-(1,2,3(triazolo14,5-djpyrimidin-5-ylamino)-
cyck)pentanol
¨3-(4-(4-15-(trans-3-hydroxy-cyclopentylamino)--
[1 ,2,3]triazolo[4.5-d]pyrimidin-3-y1)-pheny1}-pyrazoi-l-y1)-
propionitrile
-A215" ii-(3-(4-(1-(2-pyraIsig-1-yl-ethyl)-1H-pyrazol-411}-phenyl)-3R--
(1,2,3)triazolo[4,5-dipyrimidin-5-y1)-cyclohexane-1,4-diamine
"A216" (1573-k)-3-(3-(441-(2-pyrtolidin=1-y1-eihyi):5"-pyrazo4-4-y11-
phenyi}-3H-(12,3)triazolo[4,pyrimidin-5-ylemino)-
cyclopentanecarboxytic acid amide
"A21 r (1R,3R)-3-(3-{4-(1-(2-cyano-ethyl)-1H-pyrazol-4-y1J-phenyly -
3H-(1,2,31triazolo[4,5-djpyrimidiri-5-ylamino)-
cyclopentanecarboxylic acid amide
"A218" h1R.3R)-3-(3-(4-(1-(2-methoxy-ettly1)-1H-pyrazol-4-y11-pheny0-
, 3H-(1 ,2,31thaz0ic44,5-dipyrimidin-5-ylamino)-
cyclopentanecarboxylic acid amide
CA 2903903 2020-03-20
81789805
32s
1'A219" (1 R,3R)-3-(3-{5-0-(2-rnethoxy-ethyl)-1H-pyrazol.4-yij-pyrii1in-
2-y1}-3H.I1.2,3)triazolof4,5-dipyrimidin-5-ylamino)-
cyciopentanecarboxylic acid amide
3R)-3-p-(4-(1.12-(5-methyl-(1,2,4joxadiazol-3-y1)-ethyl)-
1H-pyrazol-4-y1)-pheny1)-3H-11,2,31triaz010(4,5-dipyrimidin-5-
ylaminol-cyclopentanecarboxylic acid amide
A22 V (114-.3R)-3-(3-{3-fluoro-441-(2-rnethoxyletf41)-1H-pyrazol-4-.
ylj-phenyt)-3H.[1,2,31triazolo(4.5.dipyrimidin-5-ylamino)-
cyclopentanecarboxylic acid amide
(-1-F4-.3R)-3-(34341-(2-methoxy-ethyl)-1H-pyraz01.4-yll-phenyl)-
3H41.2,3)triazolop,5-dipyrimidin-5-ylamino)-
cyclopentanecarboxylic acid amide
--;A223-';----(1it.3R)-3-13-{411-(2-ettioxy.ethyl)-1H.pyrazol-4-y11-341-67;O:-
.`
pheny1)-3H-11,2,3)triazok44,5-dipyrimidin-5-ylamino)-
cyclopentanecarboxylic acid amide
(1R,3R01(3-{-6--(1-(2.methoxy.ethyl)-1Hpyrazot.4-ylfp¨yrTdin-
3-y1)-3H-f12,31triazolo(4,5-djpyrimidin-5-ylamino)-
cyclopentanecarboxylic acid amide
-;A225" --(1R:3R)-(3-(441-(2-elhoxy-ethyl)-1H-pyrazol-4-y11-phei-iii):--
3H41,2,3)tnazolo[4,5-dipyrimidin-5-ylamino)-
cyclopentanecarboxylic acid (2-methoxy-ethyl)amide
;". (1R T3R).3-(3-{4-(1-(2-ethOxy-ethyl)-1H-pyrazO1-4:Yij:Pninyl)-
3H-(1,2,3)triazolo(4,5-dlpyrimidin-5-ylamino)-
,
cyclopentanecarboxylic acid (2-hydroxy.ethyl).amide
"A227" (14?.3Ri.-3:(3-14-(1µ-(2-ethoxy-ettly1)-1H.pyrazol-4-y1]-iThenil}-
l
L._ 31-1-(1,2,3)triazolo14.5-djpyrimidin-5-ylamino)-cyclopentanol
"A22-8" (1R,3R)-3-(3-P-fluoro-4-(1-(2-methoxy-ethyl)-1H-pyrazol.4-
ylj-phenyl)-3H-(1,2,31tr1azo1o(4,5-dipyrimidin-5-ylamino)- I
cyclopentanol
CA 2903903 2020-03-20
81789805
32t
: -."A-22-9" I '(1R,3R)-3:43-{6-{1-(2-ethoxy-ethy0-1H-pyrazol-4-y1J-pyridin-3-
y1}-3H-(1,2,3)triazolo(4,5-djpyrimidin-5=ylamino)-
Icyclopentanecarboxylic acid amide
"A230" , ( 1 R.3 R)-3-(3-{4-(i-(2'-Meifio- xy-ethyl)-1 H-pyrazo1=4-y1]-3-
1 methyl-pheny1)-3H-(1,2.3)triazolo14,5-dipyrimidin=51tamino)-
i cyclopentanecarboxylic acid amide
-"A-231" 1-(1R.3R)-3={3-14-(1-pyridin-3-ylmethyl-1H=pyrazol-4-y1)-phenyli-
1 311-11.2,11triazolo(4,5-d)pyrimidin-5-ylamino}-cyclopentanol e
--"A2-32;7- 1 ( 1 R.3R)-3-(3-(4-(1..(2-hydroxy-2--met hyl-propyl)- 1 H=pyrai-
Oi---4:1
1 yli-pheny1)-3H-0,2.3itriazo1o[4,5-dlpyrimidin-5-ylamino)-
--1- cyclopentanol
t---';-A-2-33" (1R,314)-3-(344-(1-(2-hydroxy=2-methyl-propy1)-1H-pyrazoi-4-
i yli=phenyll-3H-I1,2,31triazolo(4.5-dipynmidin-5-ylamirio)-
_ I cyclopentanecarboxylic acid amide
' ----4234- ; (trans)=3434641-(2-methoxy-ethyl)-11-1-pyrazol-411)-pyridiri-3-
1
! y1)-3H-[1,2,31triazolo(4,5-dlpyrimidin-5-ylaminoycyclopentanol
- A2354 --1 (1 R,3R)-3-(3-{4i 1-(2-methoxy-ethyl)-1H=pyrazol-411)-phenyli--
3H-(1,2.3)triazolo14,5-dipyrimidin-5-ylamino)-cyclopentane-
carboxylic acid (1-methyl=piperidin-4-y1methyl)=amide
"A236" 2=12-(4-(4-(5-((112.311)-3=hydroxy-cyclopentyLimino)-
(1,2,31tr1azolop4,54)pyrimidin-311j-phenylypyrazol-111)-
ethyll-2H-pyridazin-3-one
(1f1,3R).343-(4-{142-(6-oxo-6H-pyridazin-111)-ethyl)-1W---
pyrazol-4-y1)-pheny1)-3H-11,2,3)Uiazolo14.5-dipyrimidin-5-
ylaminoi-cyc1opentanecarboxylic acid amide
"A23-8'; ----(-1-ii.3ff)-3:(-34:(1:(2--ethoxy-etiiif1)-1H-p--yrazol-41,9=pyrid-
iii--2-
y1)-3H-(1,2,31triazolo14.5-djpyrimidin-5=ylamino)-
cyclopentanecarboxylic acid amide I
r -4-A239". . - i1R,3k1:3-i3---{-5-0-(2--cy¨ano=ethyl)-1H-p¨yr¨aTO1-4-y1J-Py-
ridi-ri:-
! yI}-3H-f 1 ,2,31triazolo(4.5-d)pyrimidin-5-ylamino)-
,' cyciopentanecarboxylic acid amide
______________________________________________________________ ----------;
CA 2903903 2020-03-20
81789805
32u
(1R,3R)-3-1(3-(4-pyrimtdin-5-ylphenyl)triazolo(4,5-djpyrimidiit- I
5-yijamino)cyclopentanecarboxamicie
(1R,3R)-3413-(4-(6-methyl-3-pyridyl)phenylpriazolOr4-75-------1
dipyrimidin-5-Aarninoicyclopentanecarboxamide
(1R,3R)-3-(134441-(2-methoxy-1-(methoxymethyl)ethyt
pyrazoI-4-yljphenylltriazolof4.5-dipyrimidin-5-
= ===.- - - = yllarninoicyclopentanecarboxamide
"A239d* (1R.A)-3.-1[5.---14-(5:in--eifiYiOyn¨c...idi..'"-i-
fyl)Ohenyiltriazolo(4,5-
d]pyrimidin-5-yljamino)cyclopentanecarboxamide
A24 O" -(1R,3R)-3-(3-(4-ithoxy-pheny1)-3H-(1.2,31triaic46[4,54)- ¨
pyrimidin-5-ylaminoycyclopentanecarboxylic acid (3-hydroxy-
3-methyt-buty1)-amide
01R.3R)-3-(3-(4-rnethoxy-p-heny1)-3H-(1,2.3jtriazoto[4,5:--
dipyrimidin-5-ylaminol-cyclopenty1)-(4-methyl-piperazin-1 -y1)-
methanone
---4A242 11--S.3-R-j-3-(3-quinolin-8-0-3H-f1,2,3)triazolo[4.5-djpyrimidin-5-
ylarninoYcyclopentanecarboxylic acid (2-(4-methyl-piperazin-
1hylj-amide
(1R,3R)-3-[3-(4-rnethoxy-phinif)-3H-11.2.3]triazolo(4,5-di-
pyrimidin-5-ylaminol-cyclopentanecarboxylic acid
dimethylamide
A244" f¨(fR,3R)--3---(3-(4-inethoxy-pheny()-3H-(1.2.31triazolo(4,5-d1-
pyrimiclin-5-ylamino)-cyclopentanecarboxylic acid (2-(4-
methyl-piperazin-1-y1)-ethyl)-amide
A2454 (1R,3R)-3-(3-(2-methyt-quinotin-6-y1)-3H-(1,2,31triazo1o(4,5-dj-
pyrimidin-5-ylarninoj-cyclopentanecarboxylic acid (2-hydroxy-
ethyl)-arnide
wA246" (1R,3R)-3-(3-(2-methyl-quinolin-6-0-3H-(1,273friazolo-R5---C-111-
pyrimidin-5-ylarninol-cyclopentanecarboxylic acid (2-methoxy-
ethyl)-amide
CA 2903903 2020-03-20
81789805
32v
--"A-24f (1R,3R)-343-(4-ethoxy-pheny1)-3H-(1,2,3itriazOlo[4.5-d1-
pyrImidin-5-ylaminokyclopentanecarboxylic acid (2-hydroxy-
ethyl)-amide
"A248" (1R,3R)-343-(4-ethoxy-pheny1)-3H-11,2,31tnazo10(4,5-d)- -
pyrimidin-5-ylaminoj-cyclopentanecarboxylic acid (2-methoxy-
ethyl)-amide
(1R,3R)-3-13-(4-ethoxy-pt-Teny1)-3H-(1,2,31triazolof4,5-dj- -
pyrirnidin-5-ylaminoj-cyclopentanecarboxylic acid (1-methyl-
piperidin-4-ylmethyl)-amide
71'4250" (1R,3R)-3-13-(2-methyl-quinolin-6-y1)-3H-(1,2,31triazolo[4.541-
pyrimidin-5-ylaminoj-cyclopentanecarboxylic acid [214-
methyl-piperazin-l-yI)-ethy1}-amide
r -"A251" (1R,3R)-3-(3-(4-ethoxy-phenyl)-3H-(1,2,31triazolo{4,54F¨
pyrimidin-5-ylamino)-cyclopentanecarboxylic acid (2-hydroxy-
2-methyl-propy1)-methyl-amide
"A252" f(1R,3R)-3-(3-(2-methyl-quinolin-6-y1)-3H41,2,31triazolo(4,5-dj-
pyrimidin-5-ylarnino}-cyclopentanecarboxylic acid (1-methyl-
piperidin-4-ylmethyl)-amide
----"A253" (r1R3R)-3-(3-(2-methyl-quinolin-6-y1)-3H-(1.2.3)triazolo(4,5--
CIT
pyrimidin-5-ylamino)-cyclopentanecarboxylic acid (3-hydroxy-
,
3-methyl-butyI)-amide
"A254" (1R,3R)-3-(3-(2-methyl-quinolin-6-y1)-3H-(1,2,31triazo1o[4,5-dj-
pyrimidin-5-ylamingcyclopentanecarboxylic acid ((trans)-3-
hydroxy-cycloperity1)-arnide
----A254a" (1R,3R)-3-(13-(2-methy1-6-quinoly1)triazolo(4,5-d)pyrimidin-5- -
yljaminoj-N-(2-morpholinoethyl)cyclopentanecarboxamide
- "A255" (1R,3R)-3-(3-{441--(2-hydroxiithyl)-1H-pyrazol-4--y-IfpiVeny-1}---
3H-(1,2,31triazo10[4,5-d]pyrimidin-5-ylamino)-
cyclopentanecarboxylic acid amide
- "A255" 2-(4--(445-((rans-4-amino-cyclohexylamino)-(1,2.3jii1-a-z0l0(4,5-:
djpyrimidin-3-ylj-pheny1}-pyrazoi-1-y1)-othanol
CA 2903903 2020-03-20
81789805
32w
--A-2577*---r----- --2--(4-{445-01---R,3S)-3-amino-cyclopentylarnino)- -
I f 1.2,31triazolo[4,5-djpyrimidin-3-y1)-phenyl}-pyrazol-1-y1)-
1
ethanol
i'A258;'' (15.3R)-3-(3-{441-(2-hydroxy-ethyl)-1H-pyrazol-4-y1j-pheny1)---1
3H-11 .2,311riazolo(4,5-dlpynmidin-5-ylamino)-
cyclopentanecarhoxylic acid amide
'-"-A259" (1R3R)-3-(3-(441-(2-hydroxy-ethyl)-1H-pyrazol-4-yll-phenyl}-
I 3H-11,2,3)triazolo[4.5-dlpyrimidin-5-ylamino)-cyclopentanol
- .;-'A-260" (1R,3R)-3-(3-{3-fluoro-411-(2-hydroxy-ethyl)-1H-pyrazol-4-y11-
phenyll-3H-(1,2,3)triazolo(4,5-dipyrimidin-5-ytamino)-
cyclopentanecarboxylic acid amide
-;;A26 iz ---(fR , 3R )--d:(345-(1-(2-hYdroxy-ethyl)-1H-pyrazol-4-y1)-pyridin-
2-0)-3H-(1.2,31tnazolo(4,5-d)pynmidin-5-ylamino)-
cyclopentanecarboxylic acid amide
--7.4-26-2"--tr3"--(4-methoxy-pheny1)-3H41,2,3)tnazolo(4,5-dipyriir-lidin.5-
y1)-
((trans)-3-methylsulfanyl-cyclopenty1)-amine
r---;;A263`;----- ((tiins)-3-methanesulfonyl-cyclopenty1)-(3-(4-rnetho-Xy- ¨
phenyl)-3H-(1.2,3)triazolo(4,5-d[pyrimidin-5-yllamine
... _ ____
"A264" ((cis)-3-methanesulfonyl-cyclopenty1)-(3-(4-methoxy-pheny()-
3H-[1.2.3)triazolo[4.5-djpyrimichn-5111-amine
I- "A265" 1(11,1,3R}:343-(4-{142-(1H-tetrazol-5-y1)-ethyl)-1H-pyrazol-411}-
phenyl)-3H-[1,2.3jtriazolo[4.5-dipyrimidin-5-ylaminol-
cyclopentanecarboxylic acid amide
- A26--6"-- trans-343-(4-(1-(2-( f H-Taiirazol-5-y1)-ethyi)-1H-pyrazol-4-
y1)-
pheny1)-3H41,2,3itnaz01o(4,5-dipyrimidin-5-ylaminoj-
cyclopentanol
- "-A2-6--Fili-(4-rriiiii-o-xy-prieriyi)-3--14-(1,1.31tnazoiof4,5-dipyrimid-in-
i-ylj--
! ((1F2,3S)-3-(5-methyl-oxazol-2-y1)-cyclopentylFamine
¨ 3---(-4---(415-(trans-3-hydroxy-cyclopentylamino)- ----'
I ________ i [1.2,31triazolo[4.5-djpyrimidin-3-y1J-pheny1)-pyra7o1-1-y1)-
1 propionamide
CA 2903903 2020-03-20
81789805
32x
r--"-A26-97.-r-T(R)-1-methanestitionyl-pyrrolidin-3-y1)-(3-quinolin-6-y1-3H-
11,2,3)tnazolo(4,5-djpyrimidin-5-y1)-amine
(-(R)-1-Methanesulfonyl-pyrrolidin-3-y1)-(3-(4-mettiOxy-phen47
3H-(1,2,3itriazolop4
,5-djpyrimidin-5-yll-amine
fl1- (R)-343-(4--methoxy-pheny1)-3H41.2,31triazo1o(4,5-cif-----1
pyrimidin-5-ylaminol-pyrrolidine-l-sulfonic acid amide
1-OR )-3-(3-44-(1 -methy1-1H-pyrazol-4-yl).phenyli-3H-
(1,2.3)triazolo14,5-djpyrimidin-5-ylamino)-pyrrolidin-1-y1)-4-
piperazin-1-y1-butan-1-one
"A272" (1RAS)-4-13-(4-ethoxy-pheny1)-3H-(1.2.3)triazolot4.5-di-
pyrirnylamino}-cyc1opent-2-enecarboxylic acid amide
(IS,4S)-4-[3-(4-Methoxy-phenyl)-3H-Ll2.31triaz010[4.5-dj-
. pyrimidin-5-ylaminoi-cyclopent-2-enecarboxylic acid amide
(1R:3R)-3-{344-(21inethoxy-eibiixyypiier-iyij-311----
(1,2.3/triazolo(4.5-djoyrimidin-5-ylamino)-
cyclopentanecarbonitrile
- 'A2/6" - cis-3-13-(4-Methoxy-P-hen-y-Oa-(1,2.3)triazolo14,5-dipyrimidin-
5-ylaminol-cyclopentanecarbonitri)e
(1k3k):3-(3-(4-ilYdroxy-pheny1)-3H-(1,2,31triiioto(4,5-dj-
pyrimidin-5-y1amino)-cyc)opentanecarboxylic acid amide
-"A277"- (1K3R)-3-{3-[4-(2-morpholin-4-yl-ethoxy)-phenyll--SH- -
(1,2,31triazoto(4,5-d)pynmidin-5-ylamino)-
cyclopentanecarboxylic acid amide
-"A276" (1FaR)-3-(344-(3-hydroxy-1,1-dimein-yl-prOpoxy- -)-pheny1)-3H-
(1,2,3yr1azo10(4.5-djpyrimidin-5-ylamino)-
cyclopentanecarboxylic acid amide
;A2-79';- ¨(1R.51k)-3-(3-(4-(peperidin-4-ylmethoxy)--Phei-iYi)-H-
112.3itriazolo[4,5-dipyrimidin-5-ylamino)-
cyclopentanecarboxylic acid amide
CA 2903903 2020-03-20
81789805
32y
' -"A280'7 --(1R,3R)-343-(4-ethoxy-phenyl)-3H-11,2,31triazolo(4,5-d}-
pyrimidin-5-ylaminoFcyclopentanecarboxylic acid N'.acetyl-
hydrazide
- 'kir (3-(4-ethoxy-ohenyl)-3H-{1,2,3jtriazolo[4,5-dfpyrimidin-5-y11-
[(1R.3R)-3-(5-methyl41,3,4]oxadiazol-2-y1)-cyclopentylyamine
---iik28-F- (1R,3R)-3-(3-(4-ethoxy-phenyl)-3H41,2,3fiTiaz0lo[4,5-4 -
pyrimidin-5-ylaminoj-cyclopentanecarboxylic acid hydrazide ,
----1¨
¨ '7A-283" ((iFOR)-3-(5-amino-11,3,4joxiitazol-2-y1)-cyclopentyl)-13-(4- '
ethoxy-pheny1)-3H-(1,2.3)triazolo[4,5-d}pyrimidin-5-yq-amine
"1._ 142-831"-- --(R)-343-(2-methyl-quinolin-6-0)-3H11,2,31triazolo14,5-d)-
pyrimidin-5-ylaminoj-pyrrolidine-1-carboxylic acid amide
----Ai'd-'= (R)-3-(3-(4-Methoxy-pheny1)-3H-(1,2.31triazolo[4.5-dj-
pyrimidin-5-ylaminol-pyrrolidine-1-carboxylic acid amide
.¨"Tkifier" N-{(1R,3R)-3-13-(4-ethoxy-phenyl)-3H-11,2,31triazolo14,5-d)-
pyrimidin-5-ylaminoj-cyclopentanecarbony1}-
methanesulfonamide
.._..., , ¨
*A287" 2-((trans)-3-(3-(4-ethoxy-pheny1)-3H11,2,31triazolo(4,5-di-
pyrimidin-5-ylaminoj-cydopenty1)-2H-pyridazin-3-one
--A288" f (trans)-3-(3-(4-thethoxy-pheny1)-3H-(1,2,31triaz010(4,5-di- -1
pyrimidin-5-ylaminoycyclooentanesulfonic acid amide 1
"A289" (1R.3R)-3-{3-44-(1-hydroxy-1-methyl-ethyl)-pheny1)-31-1- 1
(1,2.31triazolo14,5-djpyrimidin-5-ylamino)-
cyclopentanec.arboxylic acid amide
L______ ._
"A2-90" . (trans)-3-{344-(1-Hydroxy-1-Methyl-ethyl)-pheny11-3H- ¨
(1,2,31triazolo14,5-dlpyrimidin-5-ylaminoycyclopentanol
---"A--2- i'-' 1-{(1R,3R)-3-(3-(4-methoxy-pheny1)-311-(1.2.3)triezok44.5-dj-
pyrimidin-5-ylaminol-cyclopenty1}-pyrrolidin-2-one
"A292" 3-{415-((1R,3R)-3-carbamoyl-cyclopentylamino)-
[1.2,3}triazolo[4.5-dIpyrimidin-3-A-phenoxyypropionic acid
, methyl ester
=,.._ ______......_
CA 2903903 2020-03-20
81789805
32z
v "A-293-1¨ -(-1 R,3R)-3-(3-14-(3-hydroxy-3-methyl-butoxy)-priinylj-
SH:
[1,2.31triazok44,54i1pyrirnidin-5-ylamino}-
t0 cyclopentariecarboxylic acid amide
.7-A294" - 14-
4:((iik,3k-3-c:a-rbamoyl-cyclopentylamino)-
(1.2.3priazoto[4,5-dipyrimidin-3-yll-phenoxy}-acetic acid
methyl ester
----"A95" --R.3R)-3-(314-(2-hydroxy-2-methyl-propoxy)-phenyq-314- -
(12.31triazolo[4.5-d)byrimidin-5-ylamino}-
cyclopentanecarboxylic acid amide ,
1 -1'4296" - - ¨ (1R,3R)-3-13-(4-met ha nesulionyl-ph-eny1)-3W -
1......... .,.,,.__ (1,2.31triazolo[4.5-djpyrimidn-5-ytaminol-
cyclopentanecarboxylic acid amide
I "A297" 3-f(1-8-.3-6)-3-f13-(4-mettioxyphenyl)triazolo[4,5-dipyrimidin-'5-
I
yfiaminojcyclopenty1)-4H-12.4oxadiazol-5-ofle
[., ' "A298" 3(4-methoxyphe nyI)-N-R 1R, 3-$)-345-meth y1-1 .2,4-
oxadiazol-
3-Acyclopentylftriazolo(4.541pyf imidin-5-amine
5-11/42-99" - (1 A.FR)-3:1(344-(47piperidooxophenyfitriazoto(4-.5-6]-
pyrimidin-5-yliaminoicyclopentanecarboxamide
"A300" (1R-,-3R)-313-14-1(1-methyl-4-
piperidyl)oxylphenyipriazofb[476-
4Apyrimidin-5-yljaminojcyclopentanecarboxarnide
... ...__ ... __________________
and pharmaceutically acceptable solvates, salts, tautomers and stereoisomers
thereof, including mixtures thereof in all ratios.
Particularly preferred are compounds selected from the group "A212", "A140",
"A187", "A218", "A150", "A135", "A167", "A168", "A253", "A293",
and pharmaceutically acceptable salts, solvates, tautomers and stereoisomers
thereof, including mixtures thereof in all rations.
CA 2903903 2020-03-20
81789805
32aa
The compounds of the formula I and also the starting materials for their
preparation
are, in addition, prepared by methods known per se, as described
CA 2903903 2020-03-20
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 33 -
in the literature (for example in the standard works, such as Houben-Weyl,
Methoden der organischen Chemie [Methods of Organic Chemistry], Georg-
Thieme-Verlag, Stuttgart), to be precise Use can also be made here of
variants known per se which are not mentioned here in greater detail.
The starting compounds of the formulae ll and III are generally known. If they
are novel, however, they can be prepared by methods known per se.
Compounds of the formula I can preferably be obtained by reacting a
compound of the formula II with a compound of the formula III.
The reaction is generally carried out under conditions known to the skilled
artisan and which are known and suitable for the said reaction, which belongs
to the nucleophilic substitutions on heteroaromatic ring systems.
Depending on the conditions used, the reaction time is between a few minutes
and 14 days, the reaction temperature is between about 0 and 140 , normally
between 20 and 120 , in particular between about 60 and about 110 .
Examples of suitable inert solvents are hydrocarbons, such as hexane,
petroleum ether, benzene, toluene or xylene; chlorinated hydrocarbons, such
as trichloroethylene, 1,2-dichloroethane, carbon tetrachloride, chloroform or
dichloromethane; alcohols, such as methanol, ethanol, isopropanol,
n-propanol, n-butanol or tert-butanol; ethers, such as diethyl ether,
diisopropyl
ether, tetrahydrofuran (THF) or dioxane; glycol ethers, such as ethylene
glycol
monomethyl or monoethyl ether, ethylene glycol dimethyl ether (diglyme);
ketones, such as acetone or butanone; amides, such as acetamide,
dimethylacetamide or dimethylformamide (DMF); nitriles, such as acetonitrile;
sulfoxides, such as dimethyl sulfoxide (DMS0); carbon disulfide; carboxylic
acids, such as formic acid or acetic acid; nitro compounds, such as
nitromethane or nitrobenzene; esters, such as ethyl acetate, or mixtures of
the
said solvents.
Particular preference is given to 2-methoxyethanol.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 34 -
Free amino groups can furthermore be acylated in a conventional manner
using an acid chloride or anhydride or alkylated using an unsubstituted or
substituted alkyl halide, advantageously in an inert solvent, such as dichloro-
methane or THE, and/or in the presence of a base, such as triethylamine or
pyridine, at temperatures between -60 and +30 .
Pharmaceutical salts and other forms
The said compounds according to the invention can be used in their final non-
salt form. On the other hand, the present invention also encompasses the use
of these compounds in the form of their pharmaceutically acceptable salts,
which can be derived from various organic and inorganic acids and bases by
procedures known in the art. Pharmaceutically acceptable salt forms of the
compounds of the formula I are for the most part prepared by conventional
methods. If the compound of the formula I contains a carboxyl group, one of
its
suitable salts can be formed by reacting the compound with a suitable base to
give the corresponding base-addition salt. Such bases are, for example, alkali
metal hydroxides, including potassium hydroxide, sodium hydroxide and
lithium hydroxide; alkaline earth metal hydroxides, such as barium hydroxide
and calcium hydroxide; alkali metal alkoxides, for example potassium ethoxide
and sodium propoxide; and various organic bases, such as piperidine,
diethanolamine and N-methylglutamine. The aluminium salts of the
compounds of the formula I are likewise included. In the case of certain
compounds of the formula I, acid-addition salts can be formed by treating
these compounds with pharmaceutically acceptable organic and inorganic
acids, for example hydrogen halides, such as hydrogen chloride, hydrogen
bromide or hydrogen iodide, other mineral acids and corresponding salts
thereof, such as sulfate, nitrate or phosphate and the like, and alkyl- and
monoarylsulfonates, such as ethanesulfonate, toluenesulfonate and benzene-
sulfonate, and other organic acids and corresponding salts thereof, such as
acetate, trifluoroacetate, tartrate, maleate, succinate, citrate, benzoate,
salicylate, ascorbate and the like. Accordingly, pharmaceutically acceptable
acid-addition salts of the compounds of the formula I include the following:
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 35 -
acetate, adipate, alginate, arginate, aspartate, benzoate, benzenesulfonate
(besylate), bisulfate, bisulfite, bromide, butyrate, camphorate, camphor-
sulfonate, caprylate, chloride, chlorobenzoate, citrate,
cyclopentanepropionate,
digluconate, dihydrogenphosphate, din itrobenzoate, dodecylsulfate, ethane-
sulfonate, fumarate, galacterate (from mucic acid), galacturonate, gluco-
heptanoate, gluconate, glutamate, glycerophosphate, hemisuccinate, hemi-
sulfate, heptanoate, hexanoate, hippurate, hydrochloride, hydrobromide,
hydroiodide, 2-hydroxyethanesulfonate, iodide, isethionate, isobutyrate,
lactate, lactobionate, nnalate, maleate, malonate, mandelate, metaphosphate,
methanesulfonate, methylbenzoate, monohydrogenphosphate,
2-naphthalenesulfonate, nicotinate, nitrate, oxalate, oleate, palmoate,
pectinate, persulfate, phenylacetate, 3-phenylpropionate, phosphate,
phosphonate, phthalate, but this does not represent a restriction.
Furthermore, the base salts of the compounds according to the invention
include aluminium, ammonium, calcium, copper, iron(111), iron(11), lithium,
magnesium, manganese(111), manganese(11), potassium, sodium and zinc
salts, but this is not intended to represent a restriction. Of the above-men-
tioned salts, preference is given to ammonium; the alkali metal salts sodium
and potassium, and the alkaline earth metal salts calcium and magnesium.
Salts of the compounds of the formula I which are derived from pharma-
ceutically acceptable organic non-toxic bases include salts of primary, sec-
ondary and tertiary amines, substituted amines, also including naturally
occurring substituted amines, cyclic amines, and basic ion exchanger resins,
for example arginine, betaine, caffeine, chloroprocaine, choline, N,N'-
dibenzyl-
ethylenediamine (benzathine), dicyclohexylamine, diethanolamine, diethyl-
amine, 2-diethylaminoethanol, 2-dimethylaminoethanol, ethanolamine,
ethylenediamine, N-ethylmorpholine, N-ethylpiperidine, glucamine,
glucosamine, histidine, hydrabamine, isopropylamine, lidocaine, lysine,
meglumine, N-methyl-D-glucamine, morpholine, piperazine, piperidine,
polyamine resins, procaine, purines, theobromine, triethanolamine, triethyl-
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 36 -
amine, trimethylamine, tripropylamine and tris(hydroxymethyl)methylamine
(tromethamine), but this is not intended to represent a restriction.
Compounds of the present invention which contain basic nitrogen-containing
groups can be quaternised using agents such as (Ci-C4)alkyl halides, for
example methyl, ethyl, isopropyl and tert-butyl chloride, bromide and iodide;
di(C1-C4)alkyl sulfates, for example dimethyl, diethyl and diamyl sulfate;
(C10-
C18)alkyl halides, for example decyl, dodecyl, lauryl, myristyl and stearyl
chloride, bromide and iodide; and aryl(C1-04)alkyl halides, for example benzyl
chloride and phenethyl bromide. Both water- and oil-soluble compounds
according to the invention can be prepared using such salts.
The above-mentioned pharmaceutical salts which are preferred include
acetate, trifluoroacetate, besylate, citrate, fumarate, gluconate,
hemisuccinate,
hippurate, hydrochloride, hydrobromide, isethionate, mandelate, meglumine,
nitrate, oleate, phosphonate, pivalate, sodium phosphate, stearate, sulfate,
sulfosalicylate, tartrate, thiomalate, tosylate and tromethamine, but this is
not
intended to represent a restriction.
Particular preference is given to hydrochloride, dihydrochloride,
hydrobromide,
maleate, mesylate, phosphate, sulfate and succinate.
The acid-addition salts of basic compounds of the formula I are prepared by
bringing the free base form into contact with a sufficient amount of the
desired
acid, causing the formation of the salt in a conventional manner. The free
base
can be regenerated by bringing the salt form into contact with a base and
isolating the free base in a conventional manner. The free base forms differ
in
a certain respect from the corresponding salt forms thereof with respect to
certain physical properties, such as solubility in polar solvents; for the
purposes of the invention, however, the salts otherwise correspond to the
respective free base forms thereof.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 37 -
As mentioned, the pharmaceutically acceptable base-addition salts of the
compounds of the formula I are formed with metals or amines, such as alkali
metals and alkaline earth metals or organic amines. Preferred metals are
sodium, potassium, magnesium and calcium. Preferred organic amines are
N,N'-dibenzylethylenediamine, chloroprocaine, choline, diethanolamine,
ethylenediamine, N-methyl-D-glucamine and procaine.
The base-addition salts of acidic compounds according to the invention are
prepared by bringing the free acid form into contact with a sufficient amount
of
the desired base, causing the formation of the salt in a conventional manner.
The free acid can be regenerated by bringing the salt form into contact with
an
acid and isolating the free acid in a conventional manner. The free acid forms
differ in a certain respect from the corresponding salt forms thereof with
respect to certain physical properties, such as solubility in polar solvents;
for
the purposes of the invention, however, the salts otherwise correspond to the
respective free acid forms thereof.
If a compound according to the invention contains more than one group which
is capable of forming pharmaceutically acceptable salts of this type, the
invention also encompasses multiple salts. Typical multiple salt forms
include,
for example, bitartrate, diacetate, difumarate, dimeglumine, diphosphate,
disodium and trihydrochloride, but this is not intended to represent a
restriction.
With regard to that stated above, it can be seen that the expression "phar-
maceutically acceptable salt" in the present connection is taken to mean an
active ingredient which comprises a compound of the formula I in the form of
one of its salts, in particular if this salt form imparts improved
pharmacokinetic
properties on the active ingredient compared with the free form of the active
ingredient or any other salt form of the active ingredient used earlier. The
pharmaceutically acceptable salt form of the active ingredient can also
provide
this active ingredient for the first time with a desired pharmacokinetic
property
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 38 -
which it did not have earlier and can even have a positive influence on the
pharmacodynannics of this active ingredient with respect to its therapeutic
efficacy in the body.
Isotopes
There is furthermore intended that a compound of the formula I includes
isotope-labelled forms thereof. An isotope-labelled form of a compound of the
formula I is identical to this compound apart from the fact that one or more
atoms of the compound have been replaced by an atom or atoms having an
atomic mass or mass number which differs from the atomic mass or mass
number of the atom which usually occurs naturally. Exam-pies of isotopes
which are readily commercially available and which can be incorporated into a
compound of the formula I by well-known methods include isotopes of
hydrogen, carbon, nitrogen, oxygen, phos-phorus, fluo-rine and chlorine, for
example 2H, 3H, 130, 140, 15N, 180, 170, 31p, 32F), 35s, 18F and -- . 36
CI, respectively.
A compound of the formula I, a prodrug, thereof or a pharmaceutically
acceptable salt of either which contains one or more of the above-mentioned
isotopes and/or other iso-topes of other atoms is intended to be part of the
present invention. An isotope-labelled compound of the formula I can be used
in a number of beneficial ways. For example, an isotope-labelled compound of
the formula I into which, for example, a radioisotope, such as 3H or 14C, has
been incorporated is suitable for medicament and/or substrate tissue
distribution assays. These radioisotopes, i.e. tritium (3H) and carbon-14
(140),
are particularly preferred owing to simple preparation and excellent
detectability. Incor-po-ra-tion of heavier isotopes, for example deuterium
(2H),
into a compound of the formula I has therapeutic advantages owing to the
higher metabolic stability of this isotope-labelled compound. Higher metabolic
stability translates directly into an increased in vivo half-life or lower
dosages,
which under most circumstances would represent a preferred embodi-ment of
the present invention. An isotope-labelled compound of the formula I can
usually be prepared by carrying out the procedures dis-closed in the synthesis
schemes and the related description, in the example part and in the
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 39 -
preparation part in the present text, replacing a non-isotope-labelled
reactant
by a readily available isotope-labelled reactant.
Deuterium (2H) can also be incorporated into a compound of the formula I for
the purpose in order to manipulate the oxidative metabolism of the compound
by way of the primary kinetic isotope effect. The primary kinetic isotope
effect
is a change of the rate for a chemical reaction that results from exchange of
isotopic nuclei, which in turn is caused by the change in ground state
energies
necessary for covalent bond formation after this isotopic exchange. Exchange
of a heavier isotope usually results in a lowering of the ground state energy
for
a chemical bond and thus cause a reduction in the rate in rate-limiting bond
breakage. If the bond breakage occurs in or in the vicinity of a saddle-point
region along the coordinate of a multi-product reaction, the product
distribution
ratios can be altered substantially. For explanation: if deuterium is bonded
to a
carbon atom at a non-exchangeable position, rate differences of km/kD = 2-7
are typical. If this rate difference is successfully applied to a corn-pound
of the
formula I that is susceptible to oxidation, the profile of this compound in
vivo
can be drastically modified and result in improved pharmacokinetic properties.
When discovering and developing therapeutic agents, the person skilled in the
art attempts to optimise pharmacokinetic parameters while retaining desirable
in vitro properties. It is reasonable to assume that many corn-pounds with
poor pharmacokinetic profiles are susceptible to oxidative metabolism. In
vitro
liver microsomal assays currently available provide valuable information on
the
course of oxidative metabolism of this type, which in turn permits the
rational
design of deuterated compounds of the formula I with improved stability
through resistance to such oxidative meta-bolism. Significant improvements in
the pharmacokinetic profiles of compounds of the formula I are thereby
obtained, and can be expressed quantitatively in terms of increases in the in
vivo half-life (t/2), concentration at maximum therapeutic effect (Cmax), area
under the dose response curve (AUC), and F; and in terms of reduced
clearance, dose and materials costs.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 40 -
The following is intended to illustrate the above: a compound of the formula I
which has multiple potential sites of attack for oxidative metabolism, for
example benzylic hydrogen atoms and hydrogen atoms bonded to a nitrogen
atom, is prepared as a series of analogues in which various combinations of
hydrogen atoms are replaced by deuterium atoms, so that some, most or all of
these hydrogen atoms have been replaced by deuterium atoms. Half-life
determinations enable favourable and accurate determination of the extent of
the extent to which the improve-ment in resistance to oxidative metabolism has
improved. In this way, it is deter-mined that the half-life of the parent
compound can be extended by up to 100% as the result of deuterium-
hydrogen exchange of this type.
Deuterium-hydrogen exchange in a compound of the formula I can also be
used to achieve a favourable modification of the metabolite spectrum of the
starting compound in order to diminish or eliminate undesired toxic
metabolites. For example, if a toxic metabolite arises through oxidative
carbon-
hydrogen (C-H) bond cleavage, it can reasonably be assumed that the
deuterated analogue will greatly diminish or eliminate production of the
unwanted metabolite, even if the particular oxidation is not a rate-
determining
step. Further information on the state of the art with respect to deuterium-
hydrogen exchange may be found, for example in Hanzlik et al., J. Org. Chem.
55, 3992-3997, 1990, Reider et al., J. Org. Chem. 52, 3326-3334, 1987,
Foster, Adv. Drug Res. 14, 1-40, 1985, Gillette et al, Biochemistry 33(10)
2927-2937, 1994, and Jarman et al. Carcinogenesis 16(4), 683-688, 1993.
The invention furthermore relates to medicaments comprising at least one
compound of the formula I and/or pharmaceutically acceptable salts, solvates,
tautomers and stereoisomers thereof, including mixtures thereof in all ratios,
and optionally excipients and/or adjuvants.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 41 -
Pharmaceutical formulations can be administered in the form of dosage units
which comprise a predetermined amount of active ingredient per dosage unit.
Such a unit can comprise, for example, 0.5 mg to 1 g, preferably 1 mg to
700 mg, particularly preferably 5 mg to 100 mg, of a compound according to
the invention, depending on the condition treated, the method of
administration
and the age, weight and condition of the patient, or pharmaceutical
formulations can be administered in the form of dosage units which comprise a
predetermined amount of active ingredient per dosage unit. Preferred dosage
unit formulations are those which comprise a daily dose or part-dose, as
indicated above, or a corresponding fraction thereof of an active ingredient.
Furthermore, pharmaceutical formulations of this type can be prepared using a
process which is generally known in the pharmaceutical art.
Pharmaceutical formulations can be adapted for administration via any desired
suitable method, for example by oral (including buccal or sublingual), rectal,
nasal, topical (including buccal, sublingual or transdermal), vaginal or
parenteral (including subcutaneous, intramuscular, intravenous or intradermal)
methods. Such formulations can be prepared using all processes known in the
pharmaceutical art by, for example, combining the active ingredient with the
excipient(s) or adjuvant(s).
Pharmaceutical formulations adapted for oral administration can be adminis-
tered as separate units, such as, for example, capsules or tablets; powders or
granules; solutions or suspensions in aqueous or non-aqueous liquids; edible
foams or foam foods; or oil-in-water liquid emulsions or water-in-oil liquid
emulsions.
Thus, for example, in the case of oral administration in the form of a tablet
or
capsule, the active-ingredient component can be combined with an oral, non-
toxic and pharmaceutically acceptable inert excipient, such as, for example,
ethanol, glycerol, water and the like. Powders are prepared by comminuting
the compound to a suitable fine size and mixing it with a pharmaceutical
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 42 -
excipient comminuted in a similar manner, such as, for example, an edible
carbohydrate, such as, for example, starch or mannitol. A flavour,
preservative,
dispersant and dye may likewise be present.
Capsules are produced by preparing a powder mixture as described above
and filling shaped gelatine shells therewith. Glidants and lubricants, such
as,
for example, highly disperse silicic acid, talc, magnesium stearate, calcium
stearate or polyethylene glycol in solid form, can be added to the powder
mixture before the filling operation. A disintegrant or solubiliser, such as,
for
example, agar-agar, calcium carbonate or sodium carbonate, may likewise be
added in order to improve the availability of the medicament after the capsule
has been taken.
In addition, if desired or necessary, suitable binders, lubricants and disin-
tegrants as well as dyes can likewise be incorporated into the mixture.
Suitable
binders include starch, gelatine, natural sugars, such as, for example,
glucose
or beta-lactose, sweeteners made from maize, natural and synthetic rubber,
such as, for example, acacia, tragacanth or sodium alginate, carboxymethyl-
cellulose, polyethylene glycol, waxes, and the like. The lubricants used in
these dosage forms include sodium oleate, sodium stearate, magnesium
stearate, sodium benzoate, sodium acetate, sodium chloride and the like. The
disintegrants include, without being restricted thereto, starch,
methylcellulose,
agar, bentonite, xanthan gum and the like. The tablets are formulated by, for
example, preparing a powder mixture, granulating or dry-pressing the mixture,
adding a lubricant and a disintegrant and pressing the entire mixture to give
tablets. A powder mixture is prepared by mixing the compound comminuted in
a suitable manner with a diluent or a base, as described above, and optionally
with a binder, such as, for example, carboxymethylcellulose, an alginate,
gelatine or polyvinylpyrrolidone, a dissolution retardant, such as, for
example,
paraffin, an absorption accelerator, such as, for example, a quaternary salt,
and/or an absorbant, such as, for example, bentonite, kaolin or dicalcium
phosphate. The powder mixture can be granulated by wetting it with a binder,
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 43 -
such as, for example, syrup, starch paste, acadia mucilage or solutions of
cellulose or polymer materials and pressing it through a sieve. As an
alternative to granulation, the powder mixture can be run through a tabletting
machine, giving lumps of non-uniform shape, which are broken up to form
granules. The granules can be lubricated by addition of stearic acid, a
stearate
salt, talc or mineral oil in order to prevent sticking to the tablet casting
moulds.
The lubricated mixture is then pressed to give tablets. The compounds
according to the invention can also be combined with a free-flowing inert
excipient and then pressed directly to give tablets without carrying out the
granulation or dry-pressing steps. A transparent or opaque protective layer
consisting of a shellac sealing layer, a layer of sugar or polymer material
and a
gloss layer of wax may be present. Dyes can be added to these coatings in
order to be able to differentiate between different dosage units.
Oral liquids, such as, for example, solution, syrups and elixirs, can be
prepared
in the form of dosage units so that a given quantity comprises a pre-specified
amount of the compound. Syrups can be prepared by dissolving the compound
in an aqueous solution with a suitable flavour, while elixirs are prepared
using
a non-toxic alcoholic vehicle. Suspensions can be formulated by dispersion of
the compound in a non-toxic vehicle. Solubilisers and emulsifiers, such as,
for
example, ethoxylated isostearyl alcohols and polyoxyethylene sorbitol ethers,
preservatives, flavour additives, such as, for example, peppermint oil or
natural
sweeteners or saccharin, or other artificial sweeteners and the like, can
likewise be added.
The dosage unit formulations for oral administration can, if desired, be en-
capsulated in microcapsules. The formulation can also be prepared in such a
way that the release is extended or retarded, such as, for example, by coating
or embedding of particulate material in polymers, wax and the like.
The compounds of the formula I and salts, solvates, tautomers and
stereoisomers thereof can also be administered in the form of liposome
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
-44 -
delivery systems, such as, for example, small unilamellar vesicles, large
unilamellar vesicles and multilamellar vesicles. Liposomes can be formed from
various phospholipids, such as, for example, cholesterol, stearylamine or
phosphatidylcholines.
The compounds of the formula I and the salts, solvates, tautomers and
stereoisomers thereof can also be delivered using monoclonal antibodies as
individual carriers to which the compound molecules are coupled. The
compounds can also be coupled to soluble polymers as targeted medicament
carriers. Such polymers may encompass polyvinylpyrrolidone, pyran
copolymer, polyhydroxypropylmethacrylamidophenol, polyhydroxy-
ethylaspartamidophenol or polyethylene oxide polylysine, substituted by
palmitoyl radicals. The compounds may furthermore be coupled to a class of
biodegradable polymers which are suitable for achieving controlled release of
a medicament, for example polylactic acid, poly-epsilon-caprolactone,
polyhydroxybutyric acid, polyorthoesters, polyacetals, polydihydroxypyrans,
polycyanoacrylates and crosslinked or amphipathic block copolymers of
hydrogels.
Pharmaceutical formulations adapted for transdermal administration can be
administered as independent plasters for extended, close contact with the
epidermis of the recipient. Thus, for example, the active ingredient can be
delivered from the plaster by iontophoresis, as described in general terms in
Pharmaceutical Research, 3(6), 318 (1986).
Pharmaceutical compounds adapted for topical administration can be for-
mulated as ointments, creams, suspensions, lotions, powders, solutions,
pastes, gels, sprays, aerosols or oils.
For the treatment of the eye or other external tissue, for example mouth and
skin, the formulations are preferably applied as topical ointment or cream. In
the case of formulation to give an ointment, the active ingredient can be
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
-45 -
employed either with a paraffinic or a water-miscible cream base.
Alternatively,
the active ingredient can be formulated to give a cream with an oil-in-water
cream base or a water-in-oil base.
Pharmaceutical formulations adapted for topical application to the eye include
eye drops, in which the active ingredient is dissolved or suspended in a
suitable carrier, in particular an aqueous solvent.
Pharmaceutical formulations adapted for topical application in the mouth
encompass lozenges, pastilles and mouthwashes.
Pharmaceutical formulations adapted for rectal administration can be ad-
ministered in the form of suppositories or enemas.
Pharmaceutical formulations adapted for nasal administration in which the
carrier substance is a solid comprise a coarse powder having a particle size,
for example, in the range 20-500 microns, which is administered in the manner
in which snuff is taken, i.e. by rapid inhalation via the nasal passages from
a
container containing the powder held close to the nose. Suitable formulations
for administration as nasal spray or nose drops with a liquid as carrier
substance encompass active-ingredient solutions in water or oil.
Pharmaceutical formulations adapted for administration by inhalation encom-
pass finely particulate dusts or mists, which can be generated by various
types
of pressurised dispensers with aerosols, nebulisers or insufflators.
Pharmaceutical formulations adapted for vaginal administration can be
administered as pessaries, tampons, creams, gels, pastes, foams or spray
formulations.
Pharmaceutical formulations adapted for parenteral administration include
aqueous and non-aqueous sterile injection solutions comprising antioxidants,
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
-46 -
buffers, bacteriostatics and solutes, by means of which the formulation is
rendered isotonic with the blood of the recipient to be treated; and aqueous
and non-aqueous sterile suspensions, which may comprise suspension media
and thickeners. The formulations can be administered in single-dose or
multidose containers, for example sealed ampoules and vials, and stored in
freeze-dried (lyophilised) state, so that only the addition of the sterile
carrier
liquid, for example water for injection purposes, immediately before use is
necessary. Injection solutions and suspensions prepared in accordance with
the recipe can be prepared from sterile powders, granules and tablets.
It goes without saying that, in addition to the above particularly mentioned
constituents, the formulations may also comprise other agents usual in the art
with respect to the particular type of formulation; thus, for example, for-
mulations which are suitable for oral administration may comprise flavours.
A therapeutically effective amount of a compound of the formula I depends on
a number of factors, including, for example, the age and weight of the animal,
the precise condition that requires treatment, and its severity, the nature of
the
formulation and the method of administration, and is ultimately determined by
the treating doctor or vet. However, an effective amount of a compound
according to the invention is generally in the range from 0.1 to 100 mg/kg of
body weight of the recipient (mammal) per day and particularly typically in
the
range from 1 to 10 mg/kg of body weight per day. Thus, the actual amount per
day for an adult mammal weighing 70 kg is usually between 70 and 700 mg,
where this amount can be administered as a single dose per day or usually in
a series of part-doses (such as, for example, two, three, four, five or six)
per
day, so that the total daily dose is the same. An effective amount of a salt,
solvate, tautomer and stereoisomer thereof can be determined as the fraction
of the effective amount of the compound according to the invention per se. It
can be assumed that similar doses are suitable for the treatment of other
conditions mentioned above.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 47 -
The disclosed compounds of the formula I can be administered in combination
with other known therapeutic agents including agents for the treatment of RA
(rheumatoid arthritis). As used here, the term "agents for the treatment of
RA"
relates to any agent which is administered to a patient with RA for the
purposes of treating the RA.
The medicaments below are preferably, but not exclusively, combined with the
compounds of the formula I:
1. NSAIDs (non-steroidal anti-inflammatory drugs) and analgesics
2. Glucocorticoids (low oral doses)
3. Conventional disease-modifying antirheumatic drugs (DMARDs)
- Methotrexate
- Leflunomide
- Sulfasalazine
- Hydroxycloroquine
- Azathioprine
- Ciclosporin
- Minocycline
- Gold
4. Biologic response modifiers (BRMs) --> target molecules/ immune cells
involved in the inflammatory process, and include the following agents:
- TNF inhibitors
- etanercept (Enbrel)
- infliximab (Remicade)
adalimumab (Humira)
- B-cell-directed therapy
- rituximab (Rituxan)
- T-cell/B-cell coactivation signal inhibitor
- abatacept (Orencia)
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 48 -
- IL-1 receptor antagonist
- anakinra (Kineret)
MECHANISM OF ACTION
Golimumab Fully humanized monoclonal
antibody to TNF
Certolizumab pegol Anti -TNF agent with just the Fab
portion attached to the
polyethylene glycol
Tocilizumab Humanized monoclonal anti-IL-6
antibody that binds to the soluble
and membrane-expresses IL-6
receptor
Ocrelizumab Humanized-second generation
anti-CD20 antibody that depletes B
cells
Ofatumumab Human monoclonal anti-CD20
IgG1 antibody
Denosumab Fully humanized monoclonal
antibody that binds to and inhibits
the receptor activator for nuclear
factor-kB ligand
TRU-015 New class of CD20-directed
protein therapeutics
Oral small molecules Cytoplasmic targets
(JAK, Syk, MAP kinase
inhibitors)
Tolerogens (dnaJP1) lmmunotherapy based on T-cell
tolerization
A combined treatment of this type can be achieved with the aid of simulta-
neous, consecutive or separate dispensing of the individual components of the
treatment. Combination products of this type employ the compounds according
to the invention.
The invention furthermore relates to medicaments comprising at least one
compound of the formula I and/or pharmaceutically acceptable salts, solvates,
tautomers and stereoisomers thereof, including mixtures thereof in all ratios,
and at least one further medicament active ingredient.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 49 -
The invention also relates to a set (kit) consisting of separate packs of
(a) an effective amount of a compound of the formula I and/or
pharmaceuti-
cally acceptable salts, solvates, tautomers and stereoisomers thereof, in-
cluding mixtures thereof in all ratios,
and
(b) an effective amount of a further medicament active ingredient.
The set comprises suitable containers, such as boxes, individual bottles, bags
or ampoules. The set may, for example, comprise separate ampoules, each
containing an effective amount of a compound of the formula I and/or
pharmaceutically acceptable salts, solvates, tautomers and stereoisomers
thereof, including mixtures thereof in all ratios,
and an effective amount of a further medicament active ingredient in dissolved
or lyophilised form.
"Treating" as used herein, means an alleviation, in whole or in part, of
symptoms associated with a disorder or disease, or slowing, or halting of
further progression or worsening of those symptoms, or prevention or
prophylaxis of the disease or disorder in a subject at risk for developing the
disease or disorder.
The term "effective amount" in connection with a compound of formula (I) can
mean an amount capable of alleviating, in whole or in part, symptoms
associated with a disorder or disease, or slowing or halting further
progression
or worsening of those symptoms, or preventing or providing prophylaxis for the
disease or disorder in a subject having or at risk for developing a disease
disclosed herein, such as inflammatory conditions, immunological conditions,
cancer, metabolic conditions, neurodegenerative conditions, chronic infections
or conditions treatable or preventable by inhibition of a kinase or a kinase
pathway, in one embodiment, the GCN2 pathway. In another embodiment this
relates to conditions treatable or preventable by inhibition of a kinase or a
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 50 -
kinase pathway, from the group of GCN2, FMS (CSF1R), FLT3 or FLT4 or
combinations thereof. In one embodiment an effective amount of a compound
of formula (I) is an amount that inhibits a kinase in a cell, such as, for
example,
in vitro or in vivo. In some embodiments, the effective amount of the
compound of formula (I) inhibits the kinase in a cell by 10%, 20%, 30%, 40%,
50%, 60%, 70%, 80%, 90% or 99%, compared to the activity of the kinase in
an untreated cell. The effective amount of the compound of formula (I), for
example in a pharmaceutical composition, may be at a level that will exercise
the desired effect; for example, about 0.005 mg/kg of a subject's body weight
to about 10 mg/kg of a subject's body weight in unit dosage for both oral and
parenteral administration.
USE
The present compounds are suitable as pharmaceutical active ingredients for
mammals, especially for humans, in the treatment of immune modulatory and
stress response kinase-induced diseases. These diseases include neoplastic
malignancies including, but without being limited to, solid tumor cancers,
cancers of the lymphatic or blood system, the proliferation of tumour cells,
pathological neovascularisation (or angiogenesis) which promotes the growth
of solid tumours, neurodegenerative diseases (Alzheimer, demyelinating core
disorders multiple sclerosis and the like), immune related disorders like
arthritis, psoriasis, lupus, or other autoimmune diseases as well as chronic
infections.
The present invention encompasses the use of the compounds of the formula I
and/or physiologically acceptable salts and solvates thereof for the
preparation
of a medicament for the treatment or prevention of cancer. Preferred
carcinomas for the treatment originate from the group cerebral carcinoma,
urogenital tract carcinoma, carcinoma of the lymphatic system, stomach
carcinoma, laryngeal carcinoma and lung carcinoma. A further group of
preferred forms of cancer are monocytic leukaemia, lung adenocarcinoma,
small-cell lung carcinomas, pancreatic cancer, glioblastomas, melanomas and
81789805
- 51 -
breast carcinoma. A further group of preferred forms of cancer include, but is
not limited to, cervical cancer, neuroblastoma, testicular cancer,
macroglobulinemia and sarcomas.
Also encompassed is the use of the compounds according to the invention
and/or physiologically acceptable salts and solvates thereof for the
preparation
of a medicament for the treatment or prevention of a neurological disorder,
particularly a neurodegenerative disease, for example a disease caused by
axonal degeneration or by protein plaque deposition. Neurodegenerative
diseases include, for example, demyelinating core disorders, such as multiple
sclerosis, acute transverse myelitis, amyotrophic lateral sclerosis,
Creutzfeldt-
Jakob disease or Alzheimer disease.
Further encompassed is the use of the compounds according to the invention
and/or physiologically acceptable salts and solvates thereof for the
preparation
of a medicament for the treatment of chronic infections. Such a chronic
infection could relate to parasites like leishmania to leprosy or to viral
infection
by HIV and the like.
Further encompassed is the use of the compounds according to the invention
and/or physiologically acceptable salts and solvates thereof for the
preparation
of a medicament for the treatment or prevention of a disease in which
angiogenesis is implicated.
Such a disease in which angiogenesis is implicated is an ocular disease, such
as retinal vascularisation, diabetic retinopathy, age-induced macular
degeneration and the like.
.
The present invention encompasses the use of the compounds of the formula I
and/or physiologically acceptable salts and solvates thereof for the
preparation
of a medicament for the treatment or prevention of immune related disorder
like ankylosing spondylitis, arthritis, aplastic anemia, Behcet's disease,
type 1
diabetes mellitus, graft-versus-host disease, Graves' disease, autoimmune
CA 2903903 2020-03-20
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 52 -
hemolytic anemia, Wegener's granulomatosis, hyper IgE syndrome, idiopathic
thrombocytopenia purpura, rheumatoid arthritis, Crohn's disease, multiple
sclerosis, Myasthenia gravis, psoriasis, and lupus, among other autoimmune
diseases. It might also be used treat organ rejection, bone marrow transplant
rejection, non-myeloablative bone marrow transplant rejection,enhance bone
marrow engraftment after non-myeloablative conditioning regimens, and
combinations thereof
Also encompassed is the use of the compounds of the formula I and/or physio-
logically acceptable salts and solvates thereof for the preparation of a
medicament for the treatment or prevention of a immune-modulatory or stress
response kinase-induced disease or a immune-modulatory or stress response
kinase-induced condition in a mammal, in which to this method a
therapeutically effective amount of a compound according to the invention is
administered to a sick mammal in need of such treatment. The therapeutic
amount varies according to the specific disease and can be determined by the
person skilled in the art without undue effort.
The present invention also encompasses the use compounds of the formula I
and/or physiologically acceptable salts and solvates thereof for the
preparation
of a medicament for the treatment or prevention of retinal vascularisation.
The expression "immune-modulatory or stress response kinase-induced
diseases or conditions" refers to pathological conditions that depend on the
activity of one or more immune-modulatory or stress response kinases.
immune-modulatory or stress response kinases either directly or indirectly
participate in the signal transduction pathways of a variety of cellular
activities,
including proliferation, adhesion and migration and differentiation. Diseases
associated with immune-modulatory or stress response kinase activity include
neoplastic malignancies ( solid tumor cancers, cancers of the lymphatic or
blood system and the like), of neurodegenerative diseases, immune related
disorders like arthritis, psoriasis, lupus, multiple sclerosis or other
autoimmune
diseases as well as chronic infections.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 53 -
The present invention specifically relates to compounds of the formula I and
pharmaceutically acceptable salts, solvates, tautomers and stereoisomers
thereof, including mixtures thereof in all ratios,
for the use for the treatment of diseases in which the inhibition, regulation
and/or modulation inhibition of GCN2 plays a role.
The present invention specifically relates to compounds of the formula I and
pharmaceutically acceptable salts, solvates, tautomers and stereoisomers
thereof, including mixtures thereof in all ratios, for the use for the
inhibition of
GCN2.
The present invention specifically relates to compounds of the formula I and
pharmaceutically acceptable salts, solvates, tautomers and stereoisomers
thereof, including mixtures thereof in all ratios, for the use for the
treatment of
neoplastic malignancies (solid tumor cancers, cancers of the lymphatic or
blood system and the like), of neurodegenerative diseases, immune related
disorders like arthritis, psoriasis, lupus, multiple sclerosis or other
autoimmune
diseases as well as chronic infections.
Especial preference is given to the use for the treatment of a disease where
the disease is a neoplastic malignancies.
The neoplastic malignancies is preferably selected from the group of tumours
of the lung, squamous epithelium, the bladder, the stomach, the kidneys, of
head and neck, the oesophagus, the cervix, the thyroid, the intestine, the
liver,
the brain, the prostate, the urogenital tract, the lymphatic system, the
stomach
and/or the larynx.
The neoplastic malignancies is furthermore preferably selected from the group
lung adenocarcinoma, small-cell lung carcinomas, pancreatic cancer, glioblas-
tomas, colon carcinoma and breast carcinoma.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 54 -
Preference is furthermore given to the use for the treatment of a neoplastic
malignancies of the blood and immune system, preferably for the treatment of
a tumour selected from the group of acute myeloid leukaemia, chronic myeloid
leukaemia, acute lymphatic leukaemia and/or chronic lymphatic leukaemia.
The present invention specifically relates to methods for treating or
preventing an
inflammatory condition, immunological condition, autoimrnune condition,
allergic
condition, rheumatic condition, thrombotic condition, cancer, infection,
neurodegenerative disease, neuroinflammatory disease, cardiovascular disease
or metabolic condition, comprising administering to a subject in need thereof
an
effective amount of a compound of formula I or a pharmaceutically acceptable
salt, tautomer, stereoisomer or solvate thereof.
In another aspect provided herein are methods of inhibiting a kinase in a cell
expressing said kinase, comprising contacting said cell with an effective
amount of
a compound of formula I or a pharmaceutically acceptable salt, tautomer,
stereoisomer or solvate thereof. in one embodiment the kinase is GCN2 or
mutants or isoforms thereof, or combinations of two or more thereof.
Representative immunological conditions that compounds of formula I are
useful for treating or preventing include, but are not limited to, Behcet's
.
syndrome, non-allergy mast cell diseases (e.g., mastocytosis and treatment of
anaphylaxis), ankylosing spondylitis, osteoarthritis, rheumatoid arthritis
(RA),
multiple sclerosis, lupus, inflammatory bowel disease, ulcerative colitis,
Crohn's disease, myasthenia gravis, Grave's disease, transplant rejection,
humoral transplant rejection, non-humoral transplant rejection, cellular
transplant rejection, immune thrombocytopenic purpura (ITP), idiopathic
thrombocytopenic purpura, diabetes, immunological response to bacterial,
parasitic, helminth infestation or viral infection, eczema, dermatitis, graft
versus
host disease, Goodpasture's disease, hemolytic disease of the newborn,
autoimmune hemolytic anemia, anti-phospholipid syndrome, ANCA-associated
vasculitis, Churg-Strauss syndrome, Wegeners granulomatosus, pemphigus
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 55 -
vulgaris, serum sickness, mixed cryoglobulinemia, peripheral neuropathy
associated with IgM antibody, microscopic polyangiitis, Hashimoto's
thyroiditis,
Sjogrens syndrome, fibrosing conditions (such as those dependent on the
innate or adaptive immune systems or local mesenchyma cells) or primary
biliary cirrhosis.
Representative autoimmune conditions that compounds of formula I are useful
for
treating or preventing include, but are not limited to, autoimmune hemolytic
anemia (A1HA), Behcet's syndrome, Crohn's disease, type I diabetes,
Goodpasture's disease, Grave's disease, Hashimoto's thyroiditis, idiopathic
thrombocytopenic purpura, lupus, multiple sclerosis, amyotrophic lateral
sclerosis,
myasthenia gravis, pemphigus vulgaris, primary biliary cirrhosis, rheumatoid
arthritis, scleroderma, Sjogren's syndrome, ulcerative colitis, or Wegeners
granulomatosus.
Representative allergic conditions that compounds of formula I are useful for
treating or preventing include, but are not limited to, anaphylaxis, hay
fever,
allergic conjunctivitis, allergic rhinitis, allergic asthma, atopic
dermatitis, eczema,
urticaria, mucosal disorders, tissue disorders and certain gastrointestinal
disorders.
Representative rheumatic conditions that compounds of formula I are useful for
treating or preventing include, but are not limited to, rheumatoid arthritis,
gout,
ankylosing spondylitis, or osteoarthritis.
Representative inflammatory conditions that compounds of formula I are useful
for
treating or preventing include, but are not limited to, non-ANCA (anti-
neutrophil
cytoplasmic autoantibody) vasculitis (e.g., wherein GCN2 function is
associated
with neutrophil adhesion, diapedesis and/or activation), psoriasis, asthma,
allergic
rhinitis, allergic conjunctivitis, chronic urticaria, hives, anaphylaxis,
bronchitis,
chronic obstructive pulmonary disease, cystic fibrosis, inflammatory bowel
disease, irritable bowel syndrome, gout, Crohn's disease, mucous colitis,
ulcerative colitis, allergy to intestinal antigens (such as gluten
enteropathy),
diabetes (e.g., Type I diabetes and Type II diabetes) and obesity. In some
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 56 -
embodiments, the inflammatory condition is a dermatologic condition, such as,
for
example, psoriasis, urticaria, hives, eczema, scleroderma, or dermatitis. In
other
embodiments, the inflammatory condition is an inflammatory pulmonary
condition,
such as, for example, asthma, bronchitis, chronic obstructive pulmonary
disease
(COPD), or adult/acute respiratory distress syndrome (ARDS). In other
embodiments, the inflammatory condition is a gastrointestinal condition, such
as,
for example, inflammatory bowel disease, ulcerative colitis, Crohn's disease,
idiopathic inflammatory bowel disease, irritable bowel syndrome, or spastic
colon.
Representative infections that compounds of formula I are useful for treating
or
preventing include, but are not limited to, bacterial, parasitic, prion, viral
infections
or helminth infestation.
Representative cancers that compounds of formula I are useful for treating or
preventing include, but are not limited to, cancer of the head, neck, eye,
mouth,
throat, esophagus, bronchus, larynx, pharynx, chest, bone, lung, colon,
rectum,
stomach, prostate, urinary bladder, uterine, cervix, breast, ovaries,
testicles or
other reproductive organs, skin, thyroid, blood, lymph nodes, kidney, liver,
pancreas, brain, central nervous system, solid tumors and blood-borne tumors.
Representative cardiovascular diseases that compounds of formula I are useful
for treating or preventing include, but are not limited to, restenosis,
atherosclerosis
and its consequences such as stroke, myocardial infarction, ischemic damage to
the heart, lung, gut, kidney, liver, pancreas, spleen or brain.
Representative metabolic conditions that compounds of formula I are useful for
treating or preventing include, but are not limited to, obesity and diabetes
(e.g. ,
Type I and II diabetes). In a particular embodiment, provided herein are
methods
for the treatment or prevention of insulin resistance. In certain embodiments,
provided herein are methods for the treatment or prevention of insulin
resistance
that leads to diabetes (e.g., Type II diabetes). In another embodiment,
provided
herein are methods for the treatment or prevention of syndrome X or metabolic
syndrome. In another embodiment, provided herein are methods for the treatment
or prevention of Type ll diabetes, Type I diabetes, slow-onset Type I
diabetes,
81789805
- 57 -
diabetes insipidus (e.g., neurogenic diabetes insipidus, nephrogenic diabetes
insipidus, dipsogenic diabetes insipidus, or gestagenic diabetes insipidus),
diabetes mellitus, gestational diabetes mellitus, polycystic ovarian syndrome,
maturity-onset diabetes, juvenile diabetes, insulin-dependant diabetes, non-
insulin
dependant diabetes, malnutrition-related diabetes, ketosis-prone diabetes, pre-
diabetes (e.g. , impaired glucose metabolism), cystic fibrosis related
diabetes,
hemochromatosis and ketosis-resistant diabetes.
Representative neurodegenerative and neuroinflammatory diseases that
compounds of formula I are useful for treating or preventing include, but are
not
limited to, Huntington's disease, Alzheimer's disease, viral (e.g., HIV) or
bacterial-
associated encephalitis and damage.
In another embodiment, provided herein are methods for the treatment or
prevention of fibrotic diseases and disorders. In a particular embodiment,
provided
herein are methods for the treatment or prevention of idiopathic pulmonary
fibrosis, myelofibrosis, hepatic fibrosis, steatofibrosis and steatohepatitis.
In another embodiment, provided herein are methods for the treatment or
prevention of diseases associated with thrombotic events such as but not
limited
to atherosclerosis, myocardial infarction and ischemic stroke.
The present invention specifically relates to compounds of the formula I and
pharmaceutically acceptable salts, solvates, tautomers and stereoisomers
thereof, including mixtures thereof in all ratios, for the use for the
treatment
and/or prevention of inflammatory conditions, immunological conditions,
autoimmune conditions, allergic conditions, rheumatic conditions, thrombotic
conditions, cancer, infections, neurodegenerative diseases, neuroinflammatory
diseases, cardiovascular diseases, and metabolic conditions, the methods
comprising administering to a subject in need thereof an effective amount of a
compound of formula I.
Moreover, the present invention specifically relates to compounds for the use
for the treatment and/or prevention of cancer,
CA 2903903 2020-03-20
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 58 -
where the cancer to be treated is a solid tumour or a tumour of the blood and
immune system.
Moreover, the present invention specifically relates to compounds, for the use
for the treatment and/or prevention of cancer, where the where the tumour
originates from the group of acute myeloid leukaemia, chronic myeloid
leukaemia, acute lymphatic leukaemia and/or chronic lymphatic leukaemia.
Moreover, the present invention specifically relates to compounds, for the use
for the treatment and/or prevention of cancer, where the solid tumour
originates from the group of tumours of the epithelium, the bladder, the
stomach, the kidneys, of head and neck, the esophagus, the cervix, the
thyroid, the intestine, the liver, the brain, the prostate, the uro-genital
tract, the
lymphatic system, the stomach, the larynx, the bones, including
chondosarcoma and Ewing sarcoma, germ cells, including embryonal tissue
tumours, and/or the lung, from the group of monocytic leukaemia, lung
adenocarcinoma, small-cell lung carcinomas, pancreatic cancer,
glioblastomas, neurofibroma, angiosarcoma, breast carcinoma and /or maligna
melanoma.
Moreover, the present invention specifically relates to for the use for the
treatment and/or prevention of diseases selected from the group
rheumatoid arthritis, systemic lupus, asthma, multiple sclerosis,
osteoarthritis,
ischemic injury, giant cell arteritis, inflammatory bowel disease, diabetes,
cystic
fibrosis, psoriasis, Sjogrens syndrom and transplant organ rejection.
Moreover, the present invention specifically relates to compounds for the use
for the treatment and/or prevention of diseases selected from the group
Alzheimer's disease, Down's syndrome, hereditary cerebral hemorrhage with
amyloidosis-Dutch Type, cerebral amyloid angiopathy, Creutzfeldt-Jakob
disease, frontotemporal dementias, Huntington's disease, Parkinson's disease.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 59 -
Moreover, the present invention specifically relates to compounds for the use
for the treatment and/or prevention of diseases selected from the group
leishmania, mycobacteria, including M. leprae, M. tuberculosis and/or M.
avium, leishmania, plasmodium, human immunodeficiency virus, Epstein Barr
virus, Herpes simplex virus, hepatitis C virus.
Moreover, the present invention specifically relates to compounds of the
formula I and pharmaceutically acceptable salts, solvates, tautomers and
stereoisomers thereof, including mixtures thereof in all ratios, for the use
for
the inhibition of GSK3.
The disclosed compounds of the formula I can be administered in combination
with other known therapeutic agents, including anticancer agents. As used
here, the term "anticancer agent" relates to any agent which is administered
to
a patient with cancer for the purposes of treating the cancer.
The anti-cancer treatment defined herein may be applied as a sole therapy or
may involve, in addition to the compound of the invention, conventional
surgery
or radiotherapy or chemotherapy. Such chemotherapy may include one or
more of the following categories of anti- tumour agents:
antiproliferative/antineoplastic/DNA-damaging agents and combina-
tions thereof, as used in medical oncology, such as alkylating agents (for
example cis-platin, carboplatin, cyclophosphamide, nitrogen mustard,
melphalan, chloroambucil, busulphan and nitrosoureas); antimetabolites (for
example antifolates such as fluoropyrimidines like 5-fluorouracil and tegafur,
raltitrexed, methotrexate, cytosine arabinoside, hydroxyurea and gemcitabine);
antitumour antibiotics (for example anthracyclines, like adriamycin,
bleomycin,
doxorubicin, daunomycin, epirubicin, idarubicin, mitomycin-C, dactinomycin
and mithramycin) ; antimitotic agents (for example vinca alkaloids, like
vincristine, vinblastine, vindesine and vinorelbine, and taxoids, like taxa!
and
taxotere) ; topoisomerase inhibitors (for example epipodophyllotoxins, like
etoposide and teniposide, amsacrine, topotecan, irinotecan and camptothecin)
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 60 -
and cell-differentiating agents (for example all-trans-retinoic acid, 13-cis-
retinoic acid and fenretinide);
(ii) cytostatic agents, such as antioestrogens (for example tamoxifen,
toremifene, raloxifene, droloxifene and iodoxyfene), oestrogen receptor
downregulators (for example fulvestrant), antiandrogens (for example bi-
calutamide, flutamide, nilutamide and cyproterone acetate), LHRH antagonists
or LHRH agonists (for example goserelin, leuprorelin and buserelin),
progesterones (for example megestrol acetate), aromatase inhibitors (for
example as anastrozole, letrozole, vorazole and exemestane) and inhibitors of
5a-reductase, such as finasteride;
(iii) agents which inhibit cancer cell invasion (for example metallo-
proteinase inhibitors, like marimastat, and inhibitors of urokinase
plasminogen
activator receptor function);
(iv) inhibitors of growth factor function, for example such inhibitors
include
growth factor antibodies, growth factor receptor antibodies (for example the
anti-erbb2 antibody trastuzumab [HerceptinTMj and the anti-erbbl antibody
cetuximab [C225]), farnesyl transferase inhibitors, tyrosine kinase inhibitors
and serine/threonine kinase inhibitors, for example inhibitors of the
epidermal
growth factor family (for example EGFR family tyrosine kinase inhibitors, such
as N-(3-chloro-4-fluorophenyI)-7-methoxy-6- (3-morpholinopropoxy) quinazolin-
4-amine (gefitinib, AZD1839), N-(3-ethynylphenyI)-6,7-bis (2-
methoxyethoxy)quinazolin-4-amine (erlotinib, 051-774) and 6-acrylamido-N-(3-
chloro-4-fluoropheny1)-7-(3-morpholinopropoxy)quinazolin-4-amine (Cl 1033) ),
for example inhibitors of the platelet-derived growth factor family and for
example inhibitors of the hepatocyte growth factor family;
(v)antiangiogenic agents, such as those which inhibit the effects of vascular
endothelial growth factor, (for example the anti-vascular endothelial cell
growth
factor antibody bevacizumab [AvastinTm], compounds such as those disclosed
in published international patent applications WO 97/22596, WO 97/30035,
WO 97/32856 and WO 98/13354) and compounds that work by other
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
-61 -
mechanisms (for example linomide, inhibitors of integrin av133 function and
angiostatin);
(vi) vessel-damaging agents, such as combretastatin A4 and compounds
disclosed in international patent applications WO 99/02166, WO 00/40529,
WO 00/41669, WO 01/92224, WO 02/04434 and WO 02/08213;
(vii) antisense therapies, for example those which are directed to the tar-
gets listed above, such as ISIS 2503, an anti-Ras antisense;
(viii) gene therapy approaches, including, for example, approaches for re-
placement of aberrant genes, such as aberrant p53 or aberrant BRCA1 or
BRCA2, GDEPT (gene-directed enzyme pro-drug therapy) approaches, such
as those using cytosine deaminase, thymidine kinase or a bacterial
nitroreductase enzyme, and approaches for increasing patient tolerance to
chemotherapy or radiotherapy, such as multi-drug resistance gene therapy;
and
(ix) immunotherapy approaches, including, for example, ex-vivo and in-
vivo approaches for increasing the immunogenicity of patient tumour cells,
such as transfection with cytokines, such as interleukin 2, interleukin 4 or
granulocyte-macrophage colony stimulating factor, approaches for decreasing
T-cell anergy, approaches using transfected immune cells, such as cytokine-
transfected dendritic cells, approaches using cytokine-transfected tumour cell
lines, and approaches using anti-idiotypic antibodies.
The medicaments from Table 1 below are preferably, but not exclusively, com-
bined with the compounds of the formula I.
Table 1.
Alkylating agents Cyclophosphamide Lomustine
Busulfan Procarbazine
Ifosfamide Altretamine
Melphalan Estramustine phosphate
Hexamethylmelamine Mechloroethamine
Thiotepa Streptozocin
chloroambucil Temozolomide
Dacarbazine Semustine
Carmustine
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 62 -
Platinum agents Cisplatin Carboplatin
Oxaliplatin ZD-0473 (AnorMED)
Spiroplatin Lobaplatin (Aetema)
Carboxyphthalatoplatinum Satraplatin (Johnson
Tetraplatin Mafthey)
Ormiplatin BBR-3464
Iproplatin (Hoffrnann-La Roche)
SM-11355 (Sumitomo)
AP-5280 (Access)
Antimetabolites Azacytidine Tomudex
Gemcitabine Trimetrexate
Capecitabine Deoxycoformycin
5-fluorouracil Fludarabine
Floxuridine Pentostatin
2-chlorodesoxyadenosine Raltitrexed
6-Mercaptopurine Hydroxyurea
6-Thioguanine Decitabine (SuperGen)
Cytarabine Clofarabine (Bioenvision)
2-fluorodesoxycytidine Irofulven (MGI Pharrna)
Methotrexate DMDC (Hoffmann-La
Idatrexate Roche)
Ethynylcytidine (Taiho )
Topoisomerase Amsacrine Rubitecan (SuperGen)
inhibitors Epirubicin Exatecan mesylate
Etoposide (Daiichi)
Teniposide or Quinamed (ChemGenex)
mitoxantrone Gimatecan (Sigma- Tau)
Innotecan (CPT-11) Diflomotecan (Beaufour-
7-ethyl-10- 1psen)
hydroxycamptothecin TAS-103 (Taiho)
Topotecan Elsamitrucin (Spectrum)
Dexrazoxa net J-107088 (Merck & Co)
(TopoTarget) BNP-1350 (BioNumerik)
Pixantrone (Novuspharrna) CKD-602 (Chong Kun
Rebeccamycin analogue Dang)
(Exelixis) KW-2170 (Kyowa Hakko)
BBR-3576 (Novuspharrna)
Antitumour Dactinomycin (Actinomycin Amonafide
antibiotics D) Azonafide
Doxorubicin (Adriamycin) Anthrapyrazole
Deoxyrubicin Oxantrazole
Valrubicin Losoxantrone
Daunorubicin Bleomycin sulfate
(Daunomycin) (Blenoxan)
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 63 -
Epirubicin Bleomycinic acid
Therarubicin Bleomycin A
ldarubicin Bleomycin B
Rubidazon Mitomycin C
Plicamycinp MEN-10755 (Menarini)
Porfiromycin GPX-100 (Gem
Cyanomorpholinodoxo- Pharmaceuticals)
rubicin
Mitoxantron (Novantron)
Antimitotic agents Paclitaxel SB 408075
Docetaxel (GlaxoSmithKline)
Colchicine E7010 (Abbott)
Vinblastine PG-TXL (Cell
Vincristine Therapeutics)
Vinorelbine IDN 5109 (Bayer)
Vindesine A 105972 (Abbott)
Dolastatin 10 (NCI) A 204197 (Abbott)
Rhizoxin (Fujisawa) LU 223651 (BASF)
Mivobulin (Warner- D 24851 (ASTA Medica)
Lambert) ER-86526 (Eisai)
Cemadotin (BASF) Comb retastatin A4 (BMS)
RPR 109881A (Aventis) lsohomohalichondrin-B
TXD 258 (Aventis) (PharmaMar)
Epothilone B (Novartis) ZD 6126 (AstraZeneca)
T 900607 (Tularik) PEG-Paclitaxel (Enzon)
T 138067 (Tularik) AZ10992 (Asahi)
Cryptophycin 52 (Eli Lilly) !DN-5109 (Indena)
Vinflunine (Fabre) AVLB (Prescient
Auristatin PE (Teikoku NeuroPharma)
Hormone) Azaepothilon B (BMS)
BMS 247550 (BMS) BNP- 7787 (BioNumerik)
BMS 184476 (BMS) CA-4-prodrug (OXiGENE)
BMS 188797 (BMS) Dolastatin-10 (NrH)
Taxoprexin (Protarga) CA-4 (OXiGENE)
Aromatase Aminoglutethimide Exemestan
inhibitors Letrozole Atamestan (BioMedicines)
Anastrazole YM-511 (Yamanouchi)
Formestan
Thymidylate Pemetrexed (Eli Lilly) Nolatrexed (Eximias)
synthase ZD-9331 (BTG) CoFactor TM (BioKeys)
inhibitors
DNA antagonists Trabectedin (PharmaMar) Mafosfamide (Baxter
Glufosfamide (Baxter International)
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 64 -
International) Apaziquone (Spectrum
Albumin + 32P (Isotope Pharmaceuticals)
Solutions) 06-benzylguanine
Thymectacin (NewBiotics) (Paligent)
Edotreotid (Novartis)
Farnesyl Arglabin (NuOncology Tipifarnib (Johnson &
transferase Labs) Johnson)
inhibitors lonafarnib (Schering- Perillyl alcohol (DOR
Plough) BioPharma)
BAY-43-9006 (Bayer)
Pump inhibitors CBT-1 (CBA Pharma) Zosuquidar
Tariquidar (Xenova) trihydrochloride (Eli Lilly)
MS-209 (Schering AG) Biricodar dicitrate (Vertex)
Histone acetyl Tacedinaline (Pfizer) Pivaloyloxymethyl butyrate
transferase in- SAHA (Aton Pharma) (Titan)
hibitors MS-275 (Schering AG) Depsipeptide (_Fujisawa)
Metalloproteinase Neovastat (Aeterna Labo- CMT -3 (CollaGenex)
inhibitors ratories) BMS-275291 (Celltech)
Ribonucleoside Marimastat (British Bio- Tezacitabine (Aventis)
reductase inhibi- tech) Didox (Molecules for
tors Gallium maltolate (Titan) Health)
Triapin (Vion)
TNF-alpha Virulizin (Lorus Therapeu- Revimid (Celgene)
agonists/ tics)
antagonists CDC-394 (Celgene)
Endothelin-A re- Atrasentan (Abbot) YM-598 (Yamanouchi)
ceptor antagonists ZD-4054 (AstraZeneca)
Retinoic acid re- Fenretinide (Johnson & Alitretinoin (Ligand)
ceptor agonists Johnson)
LGD-1550 (Ligand)
lmmunomodula- Interferon Dexosome therapy (Ano-
tors Oncophage (Antigenics) sys)
GMK (Progenics) Pentrix (Australian Cancer
Adenocarcinoma vaccine Technology)
(Biomira) JSF-154 (Tragen)
CTP-37 (AVI BioPharma) Cancer vaccine (Intercell)
JRX-2 (Immuno-Rx) Norelin (Biostar)
PEP-005 (Peplin Biotech) BLP-25 (Biomira)
Synchrovax vaccines (CTL MGV (Progenics)
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 65 -
Immuno) (3-Alethin (Dovetail)
Melanoma vaccine (CTL CLL-Thera (Vasogen)
Immuno)
p21-RAS vaccine (Gem-
Vax)
Hormonal and Oestrogens Prednisone
antihormonal Conjugated oestrogens Methylprednisolone
agents Ethynyloestradiol Prednisolone
chlorotrianisene Aminoglutethimide
Idenestrol Leuprolide
Hydroxyprogesterone Goserelin
caproate Leuporelin
Medroxyprogesterone Bicalutamide
Testosterone Flutamide
Testosterone propionate Octreotide
Fluoxymesterone Nilutamide
Methyltestosterone Mitotan
Diethylstilbestrol P-04 (Novogen)
Megestrol 2-Methoxyoestradiol (En-
Tamoxifen treMed)
Toremofin Arzoxifen (Eli Lilly)
Dexamethasone
Photodynamic Talaporfin (Light Sciences) Pd-Bacteriopheophorbid
agents Theralux (Theratechnolo- (Yeda)
gies) Lutetium-Texaphyrin
Motexafin-Gadolinium (Pharmacyclics)
_ (Pharmacyclics) Hypericin
Tyrosine kinase Imatinib (Novartis) Kahalide F (PharmaMar)
inhibitors Leflunomide(Sugen/Phar- CEP- 701 (Cephalon)
macia) CEP-751 (Cephalon)
ZDI839 (AstraZeneca) MLN518 (Milleniunn)
Erlotinib (Oncogene Sci- PKC412 (Novartis)
ence) Phenoxodiol 0
Canertj nib (Pfizer) Trastuzumab (Genentech)
Squalamine (Genaera) C225 (ImClone)
SU5416 (Pharmacia) rhu-Mab (Genentech)
SU6668 (Pharmacia) MDX-H210 (Medarex)
ZD4190 (AstraZeneca) 2C4 (Genentech)
1D6474 (AstraZeneca) MDX-447 (Medarex)
Vatalanib (Novartis) ABX-EGF (Abgenix)
PKI166 (Novartis) IMC-1C11 (ImClone)
GW2016 (GlaxoSmith-
Kline)
EKB-509 (Wyeth)
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 66 -
EKB-569 (Wyeth)
Various agents SR-27897 (CCK-A inhibi- BCX-1777 (PNP inhibitor,
tor, Sanofi-Synthelabo) BioCryst)
Tocladesine (cyclic AMP Ranpirnase (ribonuclease
agonist, Ribapharm) stimulant, Alfacell)
Alvocidib (CDK inhibitor, Galarubicin (RNA synthe-
Aventis) sis inhibitor, Dong-A)
CV-247 (COX-2 inhibitor, Tirapazamine (reducing
Ivy Medical) agent, SRI International)
P54 (COX-2 inhibitor, N-Acetylcysteine (reducing
Phytopharm) agent, Zambon)
CapCellTM (CYP450 R-Flurbiprofen (NF-kappaB
stimulant, Bavarian Nordic) inhibitor, Encore)
GCS-I00 (ga13 antagonist, 3CPA (NF-kappaB
GlycoGenesys) inhibitor, Active Biotech)
G17DT immunogen (gas- Seocalcitol (vitamin D
trin inhibitor, Aphton) receptor agonist, Leo)
Efaproxiral (oxygenator, 131-I-TM-601 (DNA
Allos Therapeutics) antagonist,
PI-88 (heparanase inhibi- TransMolecular)
tor, Progen) Eflomithin (ODC inhibitor,
Tesnnilifen (histamine an- ILEX Oncology)
tagonist, YM BioSciences) Minodronic acid
Histamine (histamine H2 (osteoclast inhibitor,
receptor agonist, Maxim) Yamanouchi)
Tiazofurin (IMPDH inhibi- lndisulam (p53 stimulant,
tor, Ribapharm) Eisai)
Cilengitide (integrin an- Aplidin (PPT inhibitor,
tagonist, Merck KGaA) PharmaMar)
SR-31747 (IL-1 antagonist, Rituximab (CD20 antibody,
Sanofi-Synthelabo) Genentech)
CCI-779 (mTOR kinase Gemtuzumab (CD33
inhibitor, Wyeth) antibody, Wyeth Ayerst)
Exisulind (PDE-V inhibitor, PG2 (haematopoiesis
Cell Pathways) promoter, Pharmagenesis)
CP-461 (PDE-V inhibitor, ImmunolTM (triclosan
Cell Pathways) mouthwash, Endo)
AG-2037 (GART inhibitor, Triacetyluridine (uridine
Pfizer) prodrug, Wellstat)
VVX-UK1 (plasminogen SN-4071 (sarcoma agent,
activator inhibitor, Wilex) Signature BioScience)
PBI-1402 (PMN stimulant, TransMID-1071"
ProMetic LifeSciences) (immunotoxin, KS
Bortezomib (proteasome Biomedix)
inhibitor, Millennium) PCK-3145 (apoptosis
SRL-172 (T-cell stimulant, promoter, Procyon)
SR Pharma) Doranidazole Lapoptosis
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 67 -
TLK-286 (glutathione-S promoter, Pola)
transferase inhibitor, Telik) CHS-828 (cytotoxic agent,
PT-100 (growth factor Leo)
agonist, Point Therapeu- Trans-retinic acid
tics) (differentiator, NIH)
Midostaurin (PKC inhibitor, MX6 (apoptosis promoter,
Novartis) MAXIA)
Bryostatin-1 (PKC stimu- Apomine (apoptosis
lant, GPC Biotech) promoter, ILEX Oncology)
CDA-II (apoptosis pro- Urocidin (apoptosis
moter, Everlife) promoter, Bioniche)
SDX-101 (apoptosis pro- Ro-31-7453 (apoptosis
moter, Salmedix) promoter, La Roche)
Ceflatonin (apoptosis pro- Brostallicin (apoptosis
moter, ChemGenex) promoter, Pharmacia)
The disclosed compounds of the formula I can be administered in combination
with other known therapeutic agents, including anticancer agents. As used
here,
the term "anticancer agent" relates to any agent which is administered to a
patient
with cancer for the purposes of treating the cancer.
The anti-cancer treatment defined above may be applied as a monotherapy or
may involve, in addition to the herein disclosed compounds of formula I,
conventional surgery or radiotherapy or medicinal therapy. Such medicinal
therapy, e.g. a chemotherapy or a targeted therapy, may include one or more,
but
preferably one, of the following anti-tumor agents:
Alkylatino agents
such as altretamine, bendamustine, busulfan, carmustine, chlorambucil,
chlormethine, cyclophosphamide, dacarbazine, ifosfamide, improsulfan,
tosilate,
lomustine, melphalan, mitobronitol, mitolactol, nimustine, ranimustine,
temozolomide, thiotepa, treosulfan, mechloretamine, carboquone;
apaziquone, fotemustine, glufosfamide, palifosfamide, pipobroman,
trofosfamide,
uramustine, TH-3024, VAL-0834;
Platinum Compounds
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 68 -
such as carboplatin, cisplatin, eptaplatin, miriplatine hydrate, oxaliplatin,
lobaplatin, nedaplatin, picoplatin, satraplatin;
lobaplatin, nedaplatin, picoplatin, satraplatin;
DNA altering agents
such as amrubicin, bisantrene, decitabine, mitoxantrone, procarbazine,
trabectedin, clofarabine;
amsacrine, brostallicin, pixantrone, laromustine";
Topoisomerase Inhibitors
such as etoposide, irinotecan, razoxane, sobuzoxane, teniposide, topotecan;
amonafide, belotecan, elliptinium acetate, voreloxin;
Microtubule modifiers
such as cabazitaxel, docetaxel, eribulin, ixabepilone, paclitaxel,
vinblastine,
vincristine, vinorelbine, vindesine, vinflunine;
fosbretabulin, tesetaxel;
Antimetabolites
such as asparaginase3, azacitidine, calcium levofolinate, capecitabine,
cladribine, cytarabine, enocitabine, floxuridine, fludarabine, fluorouracil,
gemcitabine, mercaptopurine, methotrexate, nelarabine, pemetrexed,
pralatrexate,
azathioprine, thioguanine, carmofur;
doxifluridine, elacytarabine, raltitrexed, sapacitabine, tegafur2'3,
trimetrexate;
Anticancer antibiotics
such as bleomycin, dactinomycin, doxorubicin, epirubicin, idarubicin,
levamisole,
miltefosine, mitomycin C, romidepsin, streptozocin, valrubicin, zinostatin,
zorubicin, daunurobicin, plicamycin;
aclarubicin, peplomycin, pirarubicin;
Hormones/Antagonists
such as abarelix, abiraterone, bicalutamide, buserelin, calusterone,
chlorotrianisene, degarelix, dexamethasone, estradiol, fluocortolone
fluoxymesterone, flutamide, fulvestrant, goserelin, histrelin, leuprorelin,
megestrol,
mitotane, nafarelin, nandrolone, nilutamide, octreotide, prednisolone,
raloxifene,
tamoxifen, thyrotropin alfa, toremifene, trilostane, triptorelin,
diethylstilbestrol;
acolbifene, danazol, deslorelin, epitiostanol, orteronel, enzalutamide";
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 69 -
Aromatase inhibitors
such as aminoglutethimide, anastrozole, exemestane, fadrozole, letrozole,
testolactone;
formestane;
Small molecule kinase inhibitors
such as crizotinib, dasatinib, erlotinib, imatinib, lapatinib, nilotinib,
pazopanib,
regorafenib, ruxolitinib, sorafenib, sunitinib, vandetanib, vemurafenib,
bosutinib,
gefitinib, axitinib;
afatinib, alisertib, dabrafenib, dacomitinib, dinaciclib, dovitinib,
enzastaurin,
nintedanib, lenvatinib, linifanib, linsitinib, masitinib, midostaurin,
motesanib,
neratinib, orantinib, perifosine, ponatinib, radotinib, rigosertib,
tipifarnib, tivantinib,
tiVozanib, trametinib, pimasertib, brivanib alaninate, cediranib, apatinib4,
cabozantinib S-ma1ate13, ibrutinib1'3, icotinib4, buparlisib2, cipatinib4,
cobimetinib13,
fedratinibl, XL-6474;
Photosensitizers
such as methoxsalen3;
porfimer sodium, talaporfin, temoporfin;
Antibodies
such as alemtuzumab, besilesomab, brentuximab vedotin, cetuximab,
denosumab, ipilimumab, ofatumumab, panitumumab, rituximab, tositumomab,
trastuzumab, bevacizumab, pertuzumab2'3;
catumaxomab, elotuzumab, epratuzumab, farletuzumab, mogamulizumab,
necitumumab, nimotuzumab, obinutuzumab, ocaratuzumab, oregovomab,
ramucirumab, rilotumumab, siltuximab, tocilizumab, zalutumumab, zanolimumab,
matuzumab, dalotuzumab1'2'3, onartuzumab1'3, racotumomabl,
tabalumab1'3, EMD-5257974, nivolumabt 3;
Cytokines
such as aldesleukin, interferon alfa2, interferon a1fa2a3, interferon
a1fa2b2'3;
celmoleukin, tasonermin, teceleukin, oprelvekin13, recombinant interferon
beta-1a4;
Drug Conjugates
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 70 -
such as denileukin diftitox, ibritumomab tiuxetan, iobenguane 1123,
prednimustine,
trastuzumab emtansine, estramustine, gemtuzumab, ozogamicin, aflibercept;
cintredekin besudotox, edotreotide, inotuzumab ozogamicin, naptumomab
estafenatox, oportuzumab monatox, technetium (99mTc) arcitunnomab1'3,
vintafolidet3;
Vaccines
such as sipu1euce13; vitespen3, emepepimut-33, oncoVAX4, rindopepimut3,
troVax4, MGN-16014, MGN-17034;
Miscellaneous
alitretinoin, bexarotene, bortezomib, everolimus, ibandronic acid, imiquimod,
lenalidomide, lentinan, metirosine, mifamurtide, pamidronic acid,
pegaspargase,
pentostatin, sipuleuceI3, sizofiran, tamibarotene, temsirolimus, thalidomide,
tretinoin, vismodegib, zoledronic acid, vorinostat;
celecoxib, cilengitide, entinostat, etanidazole, ganetespib, idronoxil,
iniparib,
ixazomib, lonidamine, nimorazole, panobinostat, peretinoin, plitidepsin,
pomalidomide, procodazol, ridaforolimus, tasquinimod, telotristat,
thymalfasin,
tirapazamine, tosedostat, trabedersen, ubenimex, valspodar, gendicine4,
picibaniI4, reolysin4, retaspimycin hydrochloridet3, trebananib23, v1ru11zin4,
carfilzomibt3, endostatin4, immucotheI4, belinostat3, MGN-17034;
Prop. INN (Proposed International Nonproprietary Name)
2 Rec. INN (Recommended International Nonproprietary Names)
3 USAN (United States Adopted Name)
4 no INN.
The following abbreviations refer respectively to the definitions below:
aq (aqueous), h (hour), g (gram), L (liter), mg (milligram), MHz (Megahertz),
min.
(minute), mm (millimeter), mmol (millimole), mM (millimolar), m.p. (melting
point),
eq (equivalent), ml (milliliter), I (microliter), ACN (acetonitrile), AcOH
(acetic acid),
CDCI3 (deuterated chloroform), CD3OD (deuterated methanol), CH3CN
(acetonitrile), c-hex (cyclohexane), DCC (dicyclohexyl carbodiimide), DCM
(dichloromethane), DIC (diisopropyl carbodiimide), DIEA (diisopropylethyl-
amine),
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 71 -
DMF (dimethylformamide), DMSO (dimethylsulfoxide), DMSO-de, (deuterated
dimethylsulfoxide), EDC (1-(3-dimethyl-amino-propyI)-3-ethylcarbodlimide), ESI
(Electro-spray ionization), Et0Ac (ethyl acetate), Et20 (diethyl ether), Et0H
(ethanol), HATU (dinnethylamino-([1,2,3]triazolo[4,5-blpyridin-3-yloxy)-
methylenel-
dimethyl-ammonium hexafluorophosphate), HPLC (High Performance Liquid
Chromatography), i-PrOH (2-propanol), K2CO3 (potassium carbonate), LC (Liquid
Chromatography), Me0H (methanol), MgSO4 (magnesium sulfate), MS (mass
spectrometry), MTBE (Methyl tert-butyl ether), NaHCO3 (sodium bicarbonate),
NaBH4 (sodium borohydride), NMM (N-methyl morpholine), NMR (Nuclear
Magnetic Resonance), PyBOP (benzotriazole-1-yl-oxy-tris-pyrrolidino-
phosphonium hexafluorophosphate), RT (room temperature), Rt (retention time),
SPE (solid phase extraction), TBTU (2-(1-H-benzotriazole-1-yI)-1,1,3,3-
tetramethyluromium tetrafluoro borate), TEA (triethylamine), TFA
(trifluoroacetic
acid), THE (tetrahydrofuran), TLC (Thin Layer Chromatography), UV
(Ultraviolet).
Description of the in vitro assays
GCN2: Assay principle & conditions
This assay can quantificate the activity of the serin kinase GCN2 (general
control non-derepressible-2).
This kinase is involved in the stress metabolism of cells. It is activated
upon
starvation (amino acid depletion). Its natural substrate is elF2a (eukaryotic
initiation factor 2 alpha subunit), a translation factor, which gets activated
(phosphorylated) by GCN2 in case of an amino acid bottleneck in the cells.
This in turn leads to a halt of the protein synthesis. Inhibition of GCN2
results
in stopping this mechanism: The cell can not stop protein production upon
"starvation" stress.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 72 -
The assay is run in two steps: the enzymatic reaction and the detection step.
In the first step GCN2 is incubated with 10 pM ATP and 80 nM of the GFP-
labelled substrate elF2alpha at room temperature.
The enzymatic reaction is stopped by addition of EDTA. The amount of
phosphorylated elF2alpha is determined by TR-FRET (Lanthascreen): A
complex is formed consisting of antibody and GFP labelled phospho-elF2a,
which allows a FRET upon exitation at 340 nm.
The GCN2-activity is directly proportional to the ratio of fluorescence units
at
the emission wavelenghth 520 nm (phosphopeptide-sensitive wavelength =
emission of GFP) to the units at 495 nm (reference wavelength = emission of
Terbium-chelate).
Final concentrations in the enzymatic reaction
Hepes, pH 7.0 50 mM
MgCl2 10 mM
MnCl2 5 mM
BSA 0.1%
DMSO 1%
ATP 10 uM
DTT 2 mM
GFP-elF2a 80 nM (substrate)
GCN2 30 nM (enzyme)
Assay procedure
4 uL enzyme solution (in assay buffer)
1.5 uL compound (in cmpd dilution buffer/6.3% DMSO)
Incubation 20 min at RT
4 uL substrate/ATP mix (in assay buffer)
Incubation 90 min at RT
10 uL stop/detection mix (in antibody dilution buffer)
Incubation 60 min at RT
Readout Lanthascreen 340/495/520
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 73 -
Cellular assay for the determination of compound activities
Human U2OS cells (2000 cells/well) are seeded into 384-well plates and
incubated for 20 hours.
The next day, the cells are treated with the test compounds and incubated for
2 hours. Then, tryptophanol, at a final concentration of 600 pM, is added to
the
cells and those are incubated for 30 minutes.
The analysis of cellular GCN2 activities is done by immunocytochemistry.
Briefly, cells are fixated on the well surfaces by formaldehyde and
permeabilised with Triton X-100. The primary antibody (anti-phospho-
elF2alpha (Ser51, Cell Signalling Technology, #3398) is incubated on the
treated cells for 20 hours, followed by a 60 minutes incubation of the
secondary antibody (anti-rabbit-IgG-Alexa 488; Molecular Probes # 11008).
The analysis and quantification of phosphorylated GCN2 is done by scanning
the plates in the Acumen Explorer system (TTPLabtech). The obtained data
are normalised against the untreated control wells (DMS0 only) and expressed
as % effect values. The determination of IC50 values is done by using the
Graph Pad Prism software.
GSK3a
GSK3a (h) is incubated with 8 mM MOPS pH 7,0, 0,2 mM EDTA, 20 pM
YRRAAVPPSPSLSRHSSPHQS(p)EDEEE (phosphor GS2 peptide), 10 mM
Mg-acetate and [gamma-33P-ATP] (specific activity approx. 500 cpm/
Pmol, concentration as required). The reaction is initiated by the addition of
the
MgATP. After incubation for 40 minutes at the room temperature, the
Reaction is stopped by the addition of 3% phosphoric acid solution. 10 pL of
reaction is then spotted onto a P30 filtermat and washed three times for
5 minutes in 50 mM phosphoric acid and once in methanol prior to drying and
scintillation counting. The assay is performed at 10 pM ATP or Km ATP. The
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 74 -
ATP concentration used for the Km ATP protocol is 10 pM ATP whereas the
actual KM ATP for GSK3a(h) is 10 pM.
GSK31B
GSK313 (h) is incubated with 8 mM MOPS pH 7,0, 0,2 mM EDTA, 20 pM
YRRAAVPPSPSLSRHSSPHQS(p)EDEEE (phosphor GS2 peptide), 10 mM
Mg-acetate and [gamma-33P-ATP] (specific activity approx. 500 cpm/
Pmol, concentration as required). The reaction is initiated by the addition of
the
MgATP. After incubation for 40 minutes at the room temperature, the
Reaction is stopped by the addition of 3% phosphoric acid solution. 10 pL of
reaction is then spotted onto a P30 filtermat and washed three times for
5 minutes in 50 mM phosphoric acid and once in methanol prior to drying and
scintillation counting. The assay is performed at 10 pM ATP or Km ATP. The
ATP concentration used for the Km ATP protocol is 10 pM ATP whereas the
actual KM ATP for GSK3a(h) is 10 pM.
1H NMR:
Bruker 400- or 500 MHz
HPLC/MS conditions A
column: Chromolith Performance ROD RP-18e, 50 x 4.6 mm2
gradient: A:E3 = 96:4 to 0:100. in 2.8 min
flow rate: 2.40 ml/min
eluent A: water + 0.05 % formic acid
Eluent B: acetonitrile + 0.04 % formic acid
wavelength: 220 nm
mass spectroscopy: positive mode
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 75 -
HPLC/MS conditions B
column: Chromolith Performance ROD RP-18e, 100 x 3 mm2
gradient: A:B = 99:1 to 0:100 in 1.8 min
flow rate: 2.0 ml/min
eluent A: water + 0.05 % formic acid
eluent B: acetonitrile + 0.04 % formic acid
wavelength: 220 nm
mass spectroscopy: positive mode
HPLC/MS conditions C
column: Chromolith Performance ROD RP-18e, 100 x 3 mm2
gradient: A:B = 99:1 to 0:100 in 3.5 min
flow rate: 2.0 ml/min
eluent A: water + 0.05 % formic acid
Eluent B: acetonitrile + 0.04 % formic acid
wavelength: 220 nm
mass spectroscopy: positive mode
Above and below, all temperatures are indicated in C. In the following ex-
amples, "conventional work-up" means: water is added if necessary, the pH is
adjusted, if necessary, to values between 2 and 10, depending on the
constitution of the end product, the mixture is extracted with ethyl acetate
or
dichloromethane, the phases are separated, the organic phase is dried over
sodium sulfate and evaporated, and the residue is purified by chromatography
on silica gel and/or by crystallisation. Rf values on silica gel; eluent:
ethyl
acetate/methanol 9:1.
Examples
Preparation of intermediates
Synthesis of 5-chloro-3-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-
cl]pyrimidine
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 76 -
ClNCl H2N NOPr)2Et N
II (10
431 dioxane NO2
0'
eft
H2/Ni Cl.õ1\1.,.,.N butyl nitrite
___________ ". 11
THF N,..NF121WPo acetonitrile CKCN
NN'N
To a solution of 2,4-dichloro-5-nitro-pyrimidine (5.82 g, 30 mmol) in dioxane
(90
ml) is added N-ethyldiisopropylamine (5.61 ml, 33 mmol) slowly and under
external cooling with ice. Then, still under cooling, a solution of p-
anisidine
(4.06 g, 33 mmol) in dioxane (12 ml) is added slowly. The reaction mixture is
stirred for 3 hours at room temperature. The solvent is evaporated and the
residue is taken up in water (100 m1). The solid is filtered off, washed with
water
and dried under vacuum to afford (2-chloro-5-nitro-pyrimidin-4-yI)-(4-methoxy-
phenyl)-amine as orange crystals; HPLC/MS 1.94 min (B), [M+H] 281; 1FI NMR
(400 MHz, DMSO-d6) 5 [PPm] 10.35 (s, 1H), 9.11 (s, 1H), 7.46 ¨ 7.39 (m, 2H),
7.03 ¨ 6.96 (m, 2H), 3.79 (s, 3H).
A solution of (2-chloro-5-nitro-pyrimidin-4-y1)-(4-methoxy-pheny1)-amine (8.13
g,
29.0 mmol) in THF (90 ml) is hydrogenated with sponge-nickel as catalyst (2.0
g) at room temperature and under atmospheric pressure. The catalyst is
filtered
off and the filtrate is evaporated. The residue is chromatographed on a silica
gel column with cyclohexane/ethyl acetate as eluent to afford 2-chloro-N4-(4-
methoxy-pheny1)-pyrimidine-4,5-diamine as reddish brown solid; HPLC/MS 1.68
min (B), [M+H] 251; 1H NMR (400 MHz, DMSO-d5) 6 [ppm] 8.51 (s, 1H), 7.57 ¨
7.49 (m, 2H), 7.60 (s, 1H), 7.58 ¨ 7.49 (m, 2H), 6.99 ¨6.91 (m, 2H), 5.19 (s,
2H), 3.76 (s, 3H).
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 77 -
To a solution of 2-chloro-N4-(4-methoxy-phenyl)-pyrimidine-4,5-diamine (2.59
g, 10.3 mmol) in acetonitrile (22 ml) is added butyl nitrite (1.81 ml, 15.5
mmol)
and the reaction mixture is stirred for 1 hour at 80 C. The reaction mixture
is
cooled to room temperature and evaporated. The residue is chromatographed
on a silica gel column with cyclohexane/ethyl acetate as eluent to afford 5-
chloro-3-(4-methoxy-pheny1)-3H41,2,3]t1iazo1o[4,5-d]pyrimidine as off-white
crystals; HPLC/MS 2.23 min (A), [M+H] 262; 1H NMR (400 MHz, DMSO-d6) 6
[ppnn] 9.82 (s, 1H), 7.93 (d, J = 9.0 Hz, 2H), 7.26 (d, J = 9.0 Hz, 2H), 3.88
(s,
3H).
The following compounds are prepared analogously:
Cl
(),AN 0
N-N
5-chloro-3-(4-ethoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidine; grey powder;
HPLC/MS 2.87 min (C), [M+H] 276;
Cl
N \O
/
5-chloro-3-(4-morpholin-4-yl-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidine;
yellow
solid;
1 HPLC/MS 1.87 min (B), [M+H] 317; H NMR (400 MHz, DMSO-d6) 6 [ppm] 9.80
(s, 1H), 7.88 ¨ 7.80 (m, 2H), 7.26 ¨ 7.19 (m, 2H), 3.82 ¨ 3.75 (m, 4H), 3.29 ¨
3.22 (m, 4H);
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 78 -
Cl
NJ,
N 411 N
OH
(R)-144-(5-chloro-E1,2,3]triazolo[4,5-d]pyrimidin-3-y1)-phenylkpyrrolidin-3-
ol,
yellow solid; HPLC/MS 1.76 min (B), [M+H] 317; 1H NMR (500 MHz, DMSO-d6)
ö [ppm] 9.78 (s, 1H), 7.73 (d, J = 9.0 Hz, 2H), 6.76 (d, J= 9.0 Hz, 2H), 5.00
(d,
J = 3.8 Hz, 1H), 4.45 (m, 1H), 3.50 (dd, J = 10.3, 4.8 Hz, 1H), 3.47 ¨ 3.34
(m,
2H), 3.21 ¨3.14 (m, 1H), 2.09 (dtd, J = 13.2, 8.5, 4.8 Hz, 1H), 2.01 ¨1.90 (m,
1H).
Synthesis of 6-(5-chloro-[1,2,31triazolo[4,5-d]pyrimidin-3-y1)-quinoline
hydrochloride
CI H2N NOIDO2Et CI N N
N."7-NO2 clioxane NNO2
N
H2/Ni Cl N. N NaNO
\ 2
T1
CI _________________________________________________ N N x HCI
NõNH2 ,
THF HCI
To a solution of 6-aminoquinoline (401 mg, 2.78 mmol) in dioxane (15 ml) is
added 2,4-dichloro-5-nitro-pyrimidine (491 mg, 2.53 mmol) slowly and under
external cooling with ice. Then, still under cooling, N-ethyldiisopropylamine
(0.47 ml, 2.78 mmol) is added slowly. The reaction mixture is stirred for 3
hours
at room temperature. The solvent is evaporated and the residue is taken up in
water. The solid is filtered off, washed with water and dried under vacuum to
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 79 -
afford (2-chloro-5-nitro-pyrimidin-4-y1)-quinolin-6-yl-amine as orange
crystals;
HPLC/MS 1.67 min (A), [M+H]I 302.
A solution of (2-chloro-5-nitro-pyrimidin-4-y1)-quinolin-6-yl-amine (793 mg,
2.63
mmol) in THF (20 ml) is hydrogenated with sponge-nickel as catalyst (1.0 g) at
room temperature and under atmospheric pressure. Ater 20 hours, the catalyst
is filtered off and the filtrate is evaporated to afford 2-chloro-N4-quinolin-
6-yl-
pyrimidine-4,5-diamine as brown-red crystals; HPLC/MS 1.27 min (A), [M+H]
272.
To a slurry of 2-chloro-N4-quinolin-6-yl-pyrimidine-4,5-diamine (562 mg, 2.07
mmol) in 37% aqueous hydrochloric acid (9 ml) is added sodium nitrite (286
mg, 4.14 mmol) and the reaction mixture is stirred for 2 hours at room
temperature. The reaction mixture is evaporated. The residue is triturated
with
heptane to afford crude 6-(5-chloro-[1,2,3]triazolo[4,5-d]pyrimidin-3-y1)-
quinoline
hydrochloride, which is used as such for the following reactions; HPLC/MS 1.91
min (A), [M+El] 283.
The following compounds are prepared analogously:
Cl
N
N
5-chloro-3-(6-methoxy-pyridin-3-y1)-3H[1,2,3]triazolo[4,5-d]pyrimidine; light
brown crystals; HPLC/MS 2.50 min (C), [M+1-1] 263;
CI
N 0
yI(.1
N N
'
N-N
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 80 -
144-(5-chloro-[1,2,3]triazolo[4,5-d]pyrimidin-3-0-phenyil-pyrrolidin-2-one;
HPLC/MS 173 min (B), [M+H] 315;
Cl
N
5-chloro-3-(4-fluoro-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidine; HPLC/MS 2.25
min (A), [M+H] 250; 1H NMR (400 MHz, DMSO-d6) 5 [ppm] 9.85 (s, 1H), 8.11
(dd, J = 9.0, 4.8 Hz, 2H), 7.59 (t, J = 8.7 Hz, 2H).
Synthesis of 5-chloro-3-(4-chloro-3-fluoro-phenyl)-3H41,2,31triazolo[4,5-
d]pyrimidine
H2NF NEt3
THF NNO2 CI
CI
SnCl2 N F NaNO2
fi
N.NH2 Nµ
Et0AciEt0H CI HCI
To a solution of 2,4-dichloro-5-nitro-pyrimidine (1.04 g, 5.36 mmol) in THF
(20
ml) is added 4-chloro-3-fluoro-phenylamine (678 mg, 4.66 mmol) slowly and
under external cooling with ice. Then, still under cooling, a solution of
triethyl-
amine (0.65 ml, 4.66 mmol) in THF (5 ml) is added slowly. The reaction mixture
is stirred for 1 hour at room temperature. The solids are filtered off and
washed
with THE. The filtrate is evaporated and crystallized from methanol to afford
(4-
chloro-3-fluoro-phenyl)-(2-chloro-5-nitro-pyrimidin-4-y1)-amine as yellow
crystals; HPLC/MS 2.98 min (C), [M+H] 303.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 81 -
To a solution of (4-chloro-3-fluoro-pheny1)-(2-chloro-5-nitro-pyrimidin-4-y1)-
amine (1.28 g, 4.23 mmol) in a mixture of ethyl acetate (17 ml) and ethanol
(12
ml) is added tin(I1)chloride (4.01 g, 21.2 mmol) and the reaction mixture is
stirred for 2 hours at 700 C. The reaction mixture is basified with saturated
sodium carbonate solution, filtered over a pad of Celite and the residue is
washed with ethyl acetate. The organic phase of the filtrate is separated,
dried
over sodium sulfate and evaporated. The residue is chromatographed on a
silica gel column with dichloromethane/methanol as eluent to afford 2-chloro-
N4-(4-chloro-3-fluoro-pheny1)-pyrimidine-4,5-diamine as brown crystals;
HPLC/MS 2.60 min (C), [M+H] 273.
To a slurry of 2-chloro-N4-(4-chloro-3-fluoro-phenyl)-pyrimidine-4,5-diamine
(617 mg, 2.26 mmol) in 37% aqueous hydrochloric acid (10 ml) is added
sodium nitrite (312 mg, 4.53 mmol) and the reaction mixture is stirred for 2
hours at room temperature. Ice is added to the reaction mixture. The resulting
precipitate is filtered off, washed with water and dried under vacuum to
afford 5-
chloro-3-(4-chloro-3-fluoro-phenyl)-3H[1,2,31triazolo[4,5-d]pyrimidine as
brown
powder; HPLC/MS 3.03 min (C), [M+H] 284.
The following compounds are prepared analogously:
CI
N 0
- =
6-(5-chloro-[1,2,3]triazolo[4,5-d]pyrimidin-3-y1)-3,3-dimethyl-1,3-dihydro-
indol-2-
one; beige powder; HPLC/MS 2.00 min (A), [M+H] 315;
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 82 -
Cl
N
NN
5-chloro-314-(2-methoxy-ethoxy)-phenyl}-3H41,2,3]triazolo[4,5-d]pyrimidine;
beige powder; HPLC/MS 2.15 min (A), [M+FIJ 306;
CI
IV/ N\I 0,
6-(5-chloro-[1,2,3]triazolo[4,5-d]pyrimidin-3-yI)-quinoxaline; yellow powder;
HPLC/MS 1.93 min (A), [M+H] 284;
Synthesis of 344-(5-chloro-[1,2,3]triazolo[4,5-d]pyrimidin-3-y1)-phenoxyl-
propionic acid
40 HN 40/ DIPEA N
CI N CI 2 0 1 0
0 dioxane NNO2
N NO2 0 0
OH
Jo
0
SnCl2 CI NN NaNO2
,NH12111r1
HCI
Et0Ac/Et0H
To a solution of 2,4-dichloro-5-nitro-pyrimidine (1.02 g, 5.25 mmol) in THF
(20
ml) is added 3-(4-amino-phenoxy)-propionic acid methyl ester (1.02 g, 5.25
mmol) slowly and under external cooling with ice. Then, still under cooling,
diisopropylethylamine (1.07 ml, 6.30 mmol) is added slowly. The reaction
mixture is stirred for 1 hour at room temperature. The solids are filtered off
and
washed with THF. The filtrate is evaporated and the residue is chromate-
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 83 -
graphed on a silica gel column with cyclohexane/ethyl acetate as eluent to
afford 344-(2-chloro-5-nitro-pyrimidin-4-ylamino)-phenoxyl-propionic acid
methyl ester as dark red crystals; HPLC/MS 2.73 min (C), [M+H] 353.
To a solution of 314-(2-chloro-5-nitro-pyrimidin-4-ylannino)-phenoxyl-
propionic
acid methyl ester (1.54 g, 4.37 mmol) in a mixture of ethyl acetate (20 ml)
and
ethanol (5 ml) is added tin(I1)chloride (2.49 g, 13.1 mmol) and the reaction
mixture is stirred for 1 hours at 70 C. The reaction mixture is basified with
saturated sodium carbonate solution, filtered over a pad of Celite and the
residue is washed with ethyl acetate. The organic phase of the filtrate is
separated, dried over sodium sulfate and evaporated. The residue is
chromatographed on a silica gel column with cyclohexane/ethyl acetate as
eluent to afford 344-(5-amino-2-chloro-pyrimidin-4-ylamino)-phenoxyl-propionic
acid methyl ester as brown oil, which crystallizes on standing; HPLC/MS 2.24
min (C), [M+1-1] 323.
To a slurry of 344-(5-amino-2-chloro-pyrimidin-4-ylamino)-phenoxy]-propionic
acid methyl ester (1.39 g, 4.30 mmol) in 37% aqueous hydrochloric acid (13 ml)
is added sodium nitrite (594 mg, 8.61 mmol) and the reaction mixture is
stirred
for 3 hours at room temperature. Ice is added to the reaction mixture. The
resulting precipitate is filtered off, washed with water and dried under
vacuum to
afford 344-(5-chloro-[1,2,3]triazolo[4,5-d]pyrimidin-3-y1)-phenox4propionic
acid
as light yellow powder; HPLC/MS 2.33 min (C), [M+H] 320.
Synthesis of 3-(4-bromo-pheny1)-5-chloro-3H41,2,3]triazo10[4,5-d]pyrimidine
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 84 -
DIPEA
H2N CI,, ,N N
TI
NNO2 Br dioxane NBr
Br
SnCl2 N butyl nitrite
____________________________________________________ Cl N
Et0Ac/Et0H
N
,
NNH2IllIl Br acetonitrile II N
N
To a solution of 4-bromoaniline (3.78 g, 22.0 mmol) in dioxane (100 ml) is
added 2,4-dichloro-5-nitro-pyrimidine (3.88 g, 20 mmol) slowly and under
external cooling with ice. Then, still under cooling, diisopropylethylamine
(3.74
ml, 22.0 mmol) is added slowly. The reaction mixture is stirred for 1 hour at
room temperature. The solids are filtered off and washed with THE. The
filtrate
is taken up in water, the solids are filtered off, washed with water and dried
under vacuum to afford (4-bromo-pheny1)-(2-chloro-5-nitro-pyrimidin-4-y1)-
amine; HPLC/MS 2.08 min (B), [NAM] 331.
To a solution of (4-bromo-phenyl)-(2-chloro-5-nitro-pyrimidin-4-y1)-amine
(5.97
g, 18.13 mmol) in a mixture of ethyl acetate (100 ml) and ethanol (50 ml) is
added tin(I1)chloride (10.3 g, 54.4 mmol) and the reaction mixture is stirred
for 1
hour at 70 C. The reaction mixture is cooled to room temperature, basified
with
saturated sodium carbonate solution, filtered over a pad of Celite and the
residue is washed with ethyl acetate. The organic phase of the filtrate is
separated, dried over sodium sulfate and evaporated. The residue is
chromatographed on a silica gel column with cyclohexane/ethylacetate as
eluent to afford N4-(4-bromo-phenyl)-2-chloro-pyrimidine-4,5-diamine as light
brown powder; HPLC/MS 1.86 min (B), [M+1-1} 301.
To a solution of N4-(4-bromo-phenyl)-2-chloro-pyrimidine-4,5-diamine (3.33 g,
11.1 mmol) in acetonitrile (50 ml) is added butyl nitrite (1.729, 16.7 mmol)
and
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 85 -
the reaction mixture is stirred for 1 hour at 70 C. The reaction mixture is
cooled
to room temperature and evaporated. The residue is chromatographed on a
silica gel column with cyclohexane/ethyl acetate as eluent to afford 3-(4-
bromo-
pheny1)-5-chloro-3H41,2,31triazo1o[4,5-d]pyrimidine as brown powder;
HPLC/MS 2.09 min (B), [M+11] 312.
The following compound is synthesized analogously:
Cl
N
N=N
5-chloro-3-(4-iodo-phenyl)-3H41,2,3jtr1azo1o[4,5-d]pyrimidine;
HPLC/MS 3.10 min (C), [M+H] 358.
Example 1
Synthesis of (1-methyl-1H-pyrazol-4-ylmethyl)-(3-q uinolin-6-y1-3H-
[1,2,3]triazolo[4,5-djpyrimidin-5-y1)-amine ("B1")
N N
N¨ 2-methoxyethanol
¨14
CIN,Z
N x HCI N
100 C
II sN NH2 II
A solution of crude 6-(5-chloro-[1,2,3]triazolo[4,5-clipyrimidin-3-y1)-
quinoline
hydrochloride (85 mg, ca. 0.15 mmol) and (1-methylpyrazol-4-yl)methanamine
(18.4 mg, 0.17 mmol) in 2-methoxyethanol (5 ml) is heated to 100 C and
stirred at this temperature for 2 hours. The reaction mixture is evaporated
and
the residue is chromatographed on a silica gel column with ethyl
acetate/methanol as eluent to afford (1-methy1-1H-pyrazol-4-ylmethyl)-(3-
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 86 -
quinolin-6-y1-3H41,2,31triazolo[4,5-d]pyrimidin-5-y1)-amine as yellow powder;
HPLC/MS 1.99 min (C), [M+H] 358; 1H NMR (400 MHz, DMSO-d6) 6 [ppm]
9.30 (s, 1H), 9.00 (dd, J = 4.2, 1.7 Hz, 1H), 8.83 (s, 1H), 8.65 (d, J = 9.2
Hz,
1H), 8.58 ¨ 8.50 (m, 2H), 8.30 (d, J = 9.3 Hz, 1H), 7.70 ¨7.62 (m, 2H), 7.44
(s,
1H), 4.47 (d, J = 5_3 Hz, 2H), 375 (s, 3H).
The following compounds are prepared analogously:
[3-(4-ethoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-prop-2-ynyl-
amine
("Al")
)7--N
N
HPLC/MS 2.71 min (C), [M+H] 295; 1H NMR (500 MHz, DMSO-d6) 6 [ppm]
9.30 (s, 1H), 8.45 (s, 1H), 8.07 (s, 2H), 7.21 -7.14 (m, 2H), 4.19 -4.09 (m,
41-I), 3.06 (s, 1H), 1.38 (t, J=7.0, 3H);
3,3-dimethy1-6-{5-[(5-methyl-pyrazin-2-ylmethyl)-aminol-[1,2,3]triazolo[4,5-*
pyrimidin-3-y11-1,3-dihydro-indo1-2-one ("B2")
0
HN
HN N
1
N'N
HPLC/MS 1.83 min (A), [M+H] 402; 1H NMR (500 MHz, DMSO-d6) 6 [ppm]
10.61 (s, 1H), 9.29 (s, 1H), 8.84 - 8.71 (m, 1H), 8.59 (s, 1H), 8.52 - 8.44
(m,
1H), 7.73 - 7.60 (m, 2H), 7.55 - 7.45 (m, 1H), 4.77 - 4.67 (m, 2H), 2.45 (s,
3H), 1.32 (s, 6H);
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 87 -
{3-[4-(2-methoxy-ethoxy)-pheny1]-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y11-(5-
methyl-pyrazin-2-ylmethyl)-amine ("B3")
OX
HNN, N
II N
HPLC/MS 1.90 min (A), [M+H] 393; 1H NMR (500 MHz, DMSO-d6) 6 [ppm] 9.28
(s, 1H), 8.76 - 8.69 (m, 1H), 8.56- 8.43 (m, 2H), 7.99 - 7.82 (m, 2H), 7.23 -
7.10 (m, 2H), 4.76 - 4.62 (m, 2H), 4.21 -4.17 (m, 2H), 3.72 - 3.69 (m, 2H),
3.34 (s, 3H), 2.45 (s, 3H);
[3-(4-chloro-3-fluoro-pheny1)-3H-[1,2,3]triazolo[4,5-dipyrimidin-5-y1]-(1-
methyl-
1H-pyrazol-4-ylmethyl)-amine ("B4")
ci
-141\1\31
N
HPLC/MS 2.70 min (C), [M+H] 359; 1H NMR (400 MHz, DMSO-d6) 6 [ppm] 9.27
(s, 1H), 8.60 (t, J= 5.1 Hz, 1H), 8.31 (dd, J- 10.6, 2.4 Hz, 1H), 8.16(d, J-
8.9
Hz, 1H), 7.91 (t, J = 8.5 Hz, 1H), 7.63 (s, 1H), 7.40 (s, 1H), 4.41 (d, J= 5.8
Hz,
2H), 3.76 (s, 3H);
(5-methyl-pyrazin-2-ylmethyl)-(3-quinoxalin-6-y1-3H-[1,2,31triazolo[4,5-d]-
pyrimidin-5-yI)-amine ("B5")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 88 -
Nfr-N
HNN N
ii N
N'
HPLC/MS 1.79 min (A), [M+H] 371; 1H NMR (400 MHz, DMSO-d6) 6 [ppm] 9.38
(s, 1H), 9.08 (d, J = 1.9 Hz, 1H), 9.05 (d, J = 1.9 Hz, 1H), 8.94 (t, J = 5.4
Hz,
1H), 8.86 (s, 1H), 8.64 (s, 2H), 8.53 (s, 1H), 8.34 (d, J- 9.1 Hz, 1H), 4.75
(d, J
= 5.9 Hz, 2H), 2.44 (s, 3H);
{314-(2-methoxy-ethoxy)-pheny1]-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y1}-(1-
methy1-1H-pyrazol-4-ylmethyl)-amine ("B6")
,N
'N
\¨ 0-7-0
HN
,N
N
HPLC/MS 1.91 min (A), [M+H] 381; 1H NMR (500 MHz, DMSO-d6) 6 [ppm] 9.22
(s, 1H), 8.38 (s, 1H), 8.04 (d, J = 8.5 Hz, 2H), 7.59 (s, 1H), 7.38 (s, 1H),
7.22
(m, 2H), 4.38 (s, 2H), 4.19 (t, J = 4.5 Hz, 2H), 3.75 (s, 3H), 3.73 - 3.67 (m,
2H),
3.33 (s, 3H);
Isopropy143-(4-methoxy-phenyl)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-y1]-amine
("A2")
N 0,
,
N
\-
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 89 -
HPLC/MS 1.98 min (B), [M+H] 285; 1H NMR (500 MHz, DMSO-d6) 6 [ppm] 9.26
- 9.19 (m, 1H), 8.08 - 7.93 (m, 3H), 7.19 (d, J=8.9, 2H), 4.28 - 4.06 (m, 1H),
3.85 (s, 3H), 1.24 - 1.18 (m, 6H);
[3-(4-methoxy-pheny1)-3H-[1,2,31triazolo[4,5-d]pyrimidin-5-y1]-(tetrahydro-
pyran-
4-y1)-amine ("A3")
0,
o
/\H
111'
N
N\ t-N\
.N
N
HPLC/MS 1.80 min (B), [M+H] 327; 1H NMR (500 MHz, DMSO-d6) 6 [ppm] 9.33
-9.21 (m, 1H), 8.18 - 7.88 (m, 3H), 7.23 - 7.11 (m, 2H), 4.16 - 3.94 (m, 1H),
3.92 - 3.87 (m, 21-1), 3.85 (s, 3H), 3.47 - 3.37 (m, 2H), 1.95- 1.79 (m, 2H),
1.63 - 1.51 (m, 2H);
cyclopropy143-(4-methoxy-pheny1)-3H-I1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-
amine
("A4")
H 0,
)/--N
N\
HPLC/MS 1.89 min (B), [M+H] 283; 1H NMR (500 MHz, DMSO-d6) 6 [PPrn] 9.23
(s, 1H), 8.31 -8.02 (m, 3H), 7.18 (d, J=8.7, 2H), 3.85 (s, 3H), 2.84 (s, 1H),
0.78 - 0.71 (m, 2H), 0.59 - 0.54 (m, 2H);
[3-(4-methoxy-phenyl)-3H[1,2,31triazolo[4,5-d]pyrimidin-5-y11-oxetan-3-yl-
amine
("A5")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 90 -
0¨
N
-N
N
HPLC/MS 1.29 min (B), [M+1-1] 299;
[3-(4-ethoxy-pheny1)-3H41,2,31triazolo[4,5-d]pyrimidin-5-y1142-(1-methyl-1H-
pyrazol-4-y1)-ethyl]-amine ("B7")
N-N
0
HN N N
N N'j\j
HPLC/MS 1.85 min (6), [M+H} 365; 1H NMR (500 MHz, DMSO-d6) 6 [PPm] 9.23
(s, 1H), 8.20- 8.14 (m, 1H), 8.05 - 7.99 (m, 2H), 7.50 (s, 1H), 7.27 (s, 1H),
7.20 - 7.14 (m, 2H), 4.13 (q, J=7.0, 2H), 3.76 (s, 3H), 3.51 - 3.44 (m, 2H),
2.76 - 2.69 (m, 2H), 1.37 (t, J=7.0, 3H);
2-(3,4-difluoro-pheny1)-243-(6-methoxy-pyridin-3-yr)-3H-[1,2,3]triazolo[4,5-d]-
pyrimidin-5-ylaminoFethanol ("B8")
O
F
OH ¨
HN
it
N',N1
HPLC/MS 2.62 min (C), [M+H} 400; 1H NMR (500 MHz, DMSO-c15) 6 [PPnl]
9.30 (s, 1H), 8.76 (s, 1H), 8.54 (d, J = 7.4 Hz, 1H), 8.32 (s, 1H), 8.26 (dd,
J=
8.8, 2.9 Hz, 1H), 7.46 (ddd, J = 11.8, 7.9, 2.1 Hz, 1H), 7.41 -7.32 (m, 1H),
7.25
(ddd, J = 9.8, 4.7, 2.6 Hz, 1H), 7.09 (d, J = 8.9 Hz, 1H), 5.24 (s, 1H), 4.98
(q, J
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 91 -
=7.1 Hz, 111), 3.97(s, 3H), 3.74 (dd, J = 10.9, 7.5 Hz, 1H), 3.67 (dd, J =
11.0,
5.7 Hz, 1H);
[3-(4-ethoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-[2-(1H-pyrazol-4-
y1)-
ethyll-amine ("B9")
F
N-Ni
HN
\I
'j
N
HPLC/MS 1.77 min (B), [M+H] 351; 1H NMR (400 MHz, DMSO-d5) 5 [ppm]
12.54 (s, 1H), 9.23 (s, 1H), 8.19 (s, 1H), 8.02 (d, J = 8.7 Hz, 2H), 7.56 (s,
1H),
7.37 (s, 1H), 7.17 (d, J = 8.8 Hz, 2H), 4.12 (q, J = 7.0 Hz, 2H), 3.54 - 3.44
(m,
2H), 2.77 (t, J = 7.5 Hz, 2H), 1.37 (t, J = 7.0 Hz, 3H);
trans-413-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclohexanol ("A6")
HO
-N
N
HPLC/MS 1.70 min (B), [M+H] 341; 1H NMR (400 MHz, DMSO-d5) 5 [ppm]
9.21 (s, 1H), 8.05 (d, J = 8.5 Hz, 2H), 7.93 (d, J = 7.8 Hz, 1H), 7.20 (d, J =
8.8
Hz, 3H), 4.63 (d, J = 4.3 Hz, 1H), 3.86 (s, 3H), 3.71 (m, 1H), 3.4 (m, 1H),
1.91
(m, 4H), 1.43 - 1.21 (m, 4H);
tert-butyl43-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-amine
("AT)
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 92
0,
N
N\
N -
HPLC/MS 2.09 min (B), [M+H] 299; 1H NMR (500 MHz, DMSO-c16) 6 [ppm] 9.23
(s, 1H), 8.01 (d, J = 8.5 Hz, 2H), 7.72 (s, 1H), 7.23 ¨ 7.16 (m, 2H), 3.85 (s,
3H),
1.44 (s, 9H);
N-(1,1-dioxothiolan-3-y1)-3-(4-methoxyphenyl)triazolo[4,5-d]pyrimidin-5-amine
("A8")
11?
----p
HNN N
sN
N
HPLC/MS 1.71 min (B), [M+H] 361; 1H NMR (400 MHz, DMSO-c16) 6 [ppm] 9.34
(s, 1H), 8.55 (s, 1H), 8.01 (bs, 2H), 7.19 (d, J = 9.0 Hz, 2H), 4.66 (s, 1H),
3.85
(s, 3H), 3.57 (dd, J = 13.5, 7.6 Hz, 1H), 3.45 ¨ 3.33 (m, 1H), 3.29 ¨ 3.12 (m,
2H), 2.27 (m, 1H);
[3-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-(1-propyl-
cyclopropy1)-amine ("A9")
HN N N
ii N N
NI'
HPLC/MS 2.16 min (B), [M+H] 325; 1H NMR (500 MHz, DMSO-d6) 6 [ppm]
9.21 (s, 1H), 8.39 (s, 1H), 8.17 (d, J=8.6, 2H), 7.20 (d, J=8.7, 2H), 3.85 (s,
3H), 1.70 - 1.59 (m, 2H), 1.45 - 1.34 (m, 2H), 0.87 (t, J=7.4, 3H), 0.80 -
0.63
(m, 4H);
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 93 -
trans-3-[3-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopentanol ("A10") '
OH 0 -----
HN N
N
N';
racemic mixture; HPLC/MS 1.69 min (B), [M+H] 327; 1H NMR (500 MHz,
DMSO-d6) 6 [ppm] 9.21 (s, 1H), 8.14 (d, J = 6.0 Hz, 1H), 8.05 (d, J = 8.3 Hz,
2H), 7.20 (m, J = 8.3 Hz, 2H), 4.51 (m, 1H), 4.37 (m, 1H), 4.23 (m, 1H), 3.85
(s,
3H), 2.15 (m, 1H), 1.92 (m, 2H), 1.76 (m, 1H), 1.52 (m, 1H);
[3-(4-methoxy-phenyl)-3H-E1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-phenethyl-amine
("B10")
0--
HN
s
N N';
HPLC/MS 2.13 min (B), [M+H] 347; 1H NMR (400 MHz, DMSO-d5) 5 [PPrn] 9.23
(s, 1H), 8.21 (s, 1H), 8.04 (d, 2H), 7.33 - 7.24 (m, 4H), 7.23 - 7.15
(m,
3H), 3.86 (s, 3H), 3.62 - 3.50 (m, 2H), 2.95 - 2.87 (m, 2H);
benzy143-(4-methoxy-pheny1)-3H-J1,2,31triazolo[4,5-d]pyrimidin-5-y1]-amine
("B11")
O¨
S
H
N
11
N"N
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 94 -
HPLC/MS 2.05 min (B), [M+H) 333; 1H NMR (500 MHz, DMSO-d6) 5 [ppm] 9.25
(s, 1H), 8.75 - 8.64 (m, 1H), 7.98 - 7.92 (m, 2H), 7.44 - 7.27 (m, 4H),
7.22(t,
J=7.3, 1H), 7.19- 7.12 (m, 2H), 4.54 (d, J=5.7, 2H), 3.85 (s, 3H);
14-[3-(4-Methoxy-pheny1)-3H41,2,31triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclohexylymethanol ("Al 1")
HO 0¨
)--N
N
N
N
HPLC/MS 1.87 min (B), [M+H] 355; 1H NMR (400 MHz, DMSO-d6/TFA-d1) 6
[ppm] 9.50 (s, 1H), 8.09 ¨ 8.02 (m, 2H), 7.23 ¨ T14 (m, 2H), 4.22 ¨4.15 (m,
1H), 3.89 (s, 3H), 3.44 (d, J = 5.9 Hz, 2H), 1.89 (m, 2H), 1.77 ¨ 1.59 (m,
5H),
1.51 (m, 2H);
cyclopenty143-(4-methoxy-phenyl)-3H41,2,3]triazolo[4,5-dlpyrimidin-5-yll-amine
("Al2")
04\
-N
NN
HPLC/MS 2.12 min (B), [M+H] 311; 1H NMR (500 MHz, DMSO-d6) 6 [PPm] 9.25
(s, 1H), 8.75 -8.64 (m, 1H), 7.98 - 7.92 (m, 2H), 7.44 -7.27 (m, 4H), 7.22 (t,
J=7.3, 1H), 7.19- 7.12 (m, 2H), 4.54 (d, J=5.7, 2H), 3.85 (s, 3H);
[3-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-y1H3-(4-methyl-
piperazin-1-y1)-propyl]-amine ("A13")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 95 -
NJ
\ 0_
N\ N
,N
N
HPLC/MS 1.44 min (A), [M+H] 383; 1H NMR (400 MHz, DMSO-d6/TFA-d1)
[ppm] 9.28 (s, 1H), 8.07 (m, 2H), 7.20 (d, J = 8.5 Hz, 2H), 3.89 (s, 3H), 3.82
¨
3.25 (m, 12H), 2.96 (s, 3H), 2.15 ¨ 2.08 (m, 2H);
trans-343-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-1-
trifluoromethyl-cyclopentanol ("A67")
OH
0,
H
N
NtN
N
N
HPLC/MS 1.95 min (B), [M+H] 395;
trans-2-(443-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclohexyl)-ethanol ("A72")
0-
)---N
,N
N
HPLC/MS 2.65 min (C), [M+H] 369; 1H NMR (500 MHz, DMSO-d6, TFA-d1) 5
[ppm] 9.25 (s, 1H), 8.03 (m, 2H), 7.19 (d, J = 7.6 Hz, 2H), 3.87 (s, 3H), 3.76
(m, 1H), 3.47 (t, J = 6.4 Hz, 2H), 2.01 (m, 2H), 1.83 ¨ 1.76 (m, 2H), 1.44 ¨
1.29
(m, 5H), 1.10 ¨ 0.99 (m, 2H);
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 96 -
,
cis-2-{443-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-
cyclohexyI}-ethanol (9A73")
0-
______________________________________ ,N
N
HPLC/MS 2.67min (C), [M+H] 369; 1H NMR (500 MHz, DMSO-d6) 6 [ppm] 9.27
(s, 1H), 8.06 ¨ 8.01 (m, 2H), 7.23 ¨ 7.15 (m, 2H), 4.02 ¨ 3.98 (m, 1H), 3.86
(s,
3H), 3.45 (t, J = 6.8 Hz, 2H), 1.78 (m, 2H), 1.63 ¨ 1.41 (m, 8H), 1.40 ¨ 1.33
(m,
1H);
[2-(2-amino-ethoxy)-ethy1]-[3-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-4-
pyrimidin-5-yI]-amine formate ("A74")
H2N
0,
0--7¨N\_ =
N
N\
HPLC/MS 1.41 min (B), [M+H] 330; 1H NMR (400 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.25 (s, 1H), 8.18- 7.89 (m, 3H), 7.19 (d, J=8.8, 2H), 3.85 (s, 3H),
3.66
- 3.49 (m, 6H), 2.85 (t, J=5.4, 2H);
[3-(3-amino-propoxy)-propy1143-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-
d]pyrimidin-5-yI]-amine formate ("A76")
0¨
H2N\
0\ / ____________________________ N
Ni\ZN\
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 97 -
HPLC/MS 1.43 min (B), [M+H] 358; 1H NMR (400 MHz, DMSO-d6, TFA-d-i) 5
[ppm] 9.34 -9.19 (m, 1H), 8.18 -7.90 (m, 3H), 7.19 (d, J=8.7, 2H), 3.85 (s,
3H), 3.51 - 3.37 (m, 6H), 2.79 -2.71 (m, 2H), 1.91 - 1.77 (m, 2H), 1.71 (p,
J=6.5, 2H);
[3-(4-methoxy-phenyl)-3H-[1,2,31triazolo[4,5-d]pyrimidin-5-y1]-(3,4,5,6-
tetrahydro-2H41,411Dipyridinyl-4-y1)-amine ("A82")
0,
N N _______ >)_N =
N
N'N
HPLC/MS 1.96 min (C), [M+H] 403; 1H NMR (400 MHz, DMSO-d6, TFA-d-i) 5
[ppm] 9.29 (s, 1H), 8.21 (d, J = 7.7 Hz, 2H), 8.08 (m, 1H), 7.22 (d, J = 7.8
Hz,
1H), 7.18 (d, J= 9.1 Hz, 1H), 4.23 (m, 3H), 3.88 (s, 3H), 3.48 (m, 2H), 2.18
(m,
2H) 1.73 (m, 2H);
(cis)-N43-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-01-N',N'-
dimethyl-cyclopentane-1,3-diamine ("A108")
0¨N
>N S=Z¨N
HPLC/MS 1.45 min (B), [M+H] 354.
Example 2
Synthesis of [3-(4-methoxy-pheny1)-3H11,2,31triazolo[4,5-d]pyrimidin-5-y1]-
methyl-amine ("A14")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 98 -
0-
0'
CKNN water HNI,N N
II
N CH3NH2 ______
80 C II
A slurry of 5-chloro-3-(4-methoxy-phenyl)-3H-E1,2,3]triazolo[4,5-d]pyrimidine
(131 mg, 0.50 mmol) in aqueous methylamine solution (40% by weight, 2 ml) is
heated to 80 C in a closed reaction vial and stirred at this temperature for
30
minutes. The mixture is cooled to room temperature and excess water is
added. The solid is filtered off, washed with water and dried under vacuum to
afford [3-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y11-methyl-
amine as off-white crystals; HPLC/MS 2.42 min (C), [M+H] 257; 1H NMR (400
MHz, DMSO-d6) 6 [ppm] 9.22 (s, 1H), 8.05 (, 3H), 7.19 (d, J= 8.6 Hz, 2H),
3.85 (s, 3H), 2.90 (m, 3H).
The following compound is prepared analogously:
ethyl-[3-(4-methoxy-phenyl)-3H-[1,2,3]triaz010[4,5-d]pyrimidin-5-A-amine
("Al 6")
HPLC/MS 2.64 min (C), [M+H] 271; 1H NMR (400 MHz, DMSO-d6) 6 [ppm]
9.36 (s, 1H), 8.08 (d, J = 9.0 Hz, 2H), 7.23 ¨7.14 (m, 2H), 4.32 (p, J = 6.5
Hz, 1H), 3.88 (s, 3H), 2.05 (m, 2H), 1.76 (m, 2H), 1.72 ¨ 1.59 (m, 2H).
Example 3
Synthesis of [3-(4-methoxy-phenyl)-3H[l,2,3]triazolo[4,5-d]pyrimidin-5-y1]-
(1,2,2,6,6-pentamethyl-piperidin-4-yI)-amine ("A15")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 99 -
0'
0"
2-methoxyethanol
NH2 100 C
A solution of 5-chloro-3-(4-methoxy-pheny1)-3H[1,2,31triazolo[4,5-dlpyrimidine
(131 mg, 0.50 mmol) and 4-amino-1,2,2,6,6-pentamethylpiperidine (104 mg,
0.60 mmol) in 2-methoxyethanol (1 ml) is heated to 100 C and stirred at this
temperature for 1 hour. The reaction mixture is cooled to room temperature
and partitioned between dichloromethane and 2 N NaOH. The organic phase
is evaporated and the residue is chromatographed on a silica gel column with
methanol/dichloromethane as eluent to afford [3-(4-methoxy-phenyI)-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-(1,2,2,6,6-pentamethyl-piperidin-4-y1)-
amine
as light yellow crystals; HPLC/MS 1.87 min (C), [M+H] 396; 1H NMR (400 MHz,
DMSO-d6) 6 [ppm] 8.21 -8.08 (m, 2H), 8.05 (d, J = 7.2 Hz, 1H), 7.14 - 7.07
(m, 2H), 4.21 -4.03 (m, 1H), 3.84 (s, 3H), 2.22 (s, 3H), 1.97 - 1.82 (m, 2H),
1.41 (d, J = 12.1 Hz, 2H), 1.11 (s, 12H).
The following compounds are prepared analogously:
[3-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-(2,2,6,6-
tetramethyl-piperidin-4-y1)-amine ("A17")
0-
HN N
)--N 1114
N
,N
N
HPLC/MS 1.89 min (C), [M+H] 382; 1H NMR (400 MHz, DMSO-d6) 6 [ppm]
9.22 (s, 1H), 8.17 - 8.10 (m, 2H), 8.06 (d, J= 7.2 Hz, 1H), 7.14 - 7.07 (m,
2H), 4.23 (dtd, J = 11.6, 7.9, 3.6 Hz, 1H), 3.83 (s, 3H), 1.88 (dd, J= 12.2,
3.5
Hz, 2H), 1.26 - 1.02 (m, 15H);
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 100 -
[3-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-(1-methyl-
piperidin-4-y1)-amine ("A18")
0,
)¨N
N
HPLC/MS 1.72 min (C), [M H] 340;
[3-(4-methoxy-pheny1)-31-111,2,31triazolo[4,5-d]pyrimidin-5-y11-(3-methyl-3-
aza-
bicyclo[3.1.0]hex-6-y1)-amine ("A19")
sot 0,
N
N\
N
HPLC/MS 1.33 min (B), [M+H1338; 1H NMR (400 MHz, DMSO-d6) 6 [PPm] 9.22
(s, 1H), 8.24 (s, 1H), 8.15 (d, J- 8.5 Hz, 2H), 7.20 (d, J= 8.7 Hz, 2H), 3.86
(s,
3H), 3.06 (d, J = 8.8 Hz, 2H), 2.88 (s, 1H), 2.35 (d, J = 7.7 Hz, 2H), 2.25
(s, 3H),
1.62 (s, 2H).
Example 4
Synthesis of 4-[3-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino]-1-methyl-pyrrolidin-2-one ("A20")
0¨
* ¨
1)1 2-methoxyethanol 0 0
0
EtN0P02
CNN
Zµ 1)1
TI x HCI 100 C HN N
NH2
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 101 -
To a solution of 5-chloro-3-(4-methoxy-phenyl)-3H41,2,3]triazolo[4,5-dj-
pyrimidine (97 mg, 0.37 mmol) and 4-amino-1-methyl-pyrrolidin-2-one
hydrochloride (65 mg, 0.37 mmol) in 2-methoxyethanol (3 ml) is added N-
ethyldiisopropylamine (0.13 ml, 0.75 mmol). The mixture is heated to 100 C
and stirred at this temperature for 1 hour. The reaction mixture is evaporated
and the residue is chromatographed on a silica gel column with methanol /
dichloromethane as eluent to afford 443-(4-methoxy-phenyl)-3H-[1,2,31triazolo-
[4,5-d]pyrimidin-5-ylamino]-1-methyl-pyrrolidin-2-one as off-white powder;
HPLC/MS 1.62 min (B), [M+H] 34; 1H NMR (500 MHz, DMSO-d5) 6 [PPril] 9.29
(s, 1H), 8.50 (s, 1H), 8.04 (d, J = 8.8 Hz, 2H), 7.20 (d, J = 8.6 Hz, 3H),
4.53 -
4.47 (m, 1H), 3.85 (s, 3H), 3.74 (dd, J = 10.2, 7.3 Hz, 1H), 3.36 (m, 1H),
2.74
(s, 3H), 2.72 - 2.65 (m, 1H), 2.46 - 2.38 (m, 1H).
The following compounds are prepared analogously:
cyclobuty143-(4-methoxy-phenyl)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-y11-amine
("A21")
N
N\
N
HPLC/MS 2.02 min (B), [M+1-1] 297; 1H NMR (400 MHz, DMSO-d6) 5 [ppm]
9.23 (s, 1H), 8.41 (d, J = 6.8 Hz, 1H), 8.04 (d, J = 8.6 Hz, 2H), 7.21 (d, J =
8.8
Hz, 2H), 4.35 (dd, J = 16.4, 8.4 Hz, 1H), 3.85 (s, 3H), 2.30 (m, 2H), 2.11 -
2.01
(m, 2H), 1.72 (m, 2H);
[2-(4-chloro-phenyl)-cyclopropy1H3-(4-methoxy-phenyl)-3H41,2,31triazolo[4,5-
d]pyrimidin-5-yI]-amine ("A22")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 102 -
0,
=
CI N\
N
HPLC/MS 2.24 min (B), [M+H] 393; 1H NMR (500 MHz, DMSO-d6) 6 [ppm]
9.27 (s, 1H), 8.69 - 8.59 (m, 1H), 7.90 - 7.85 (m, 2H), 7.36 (d, J=8.1, 2H),
7.22 (d, J=8.3, 2H), 6.82 - 6.75 (m, 2H), 3.82 (s, 3H), 2.95 -2.85 (m, 1H),
2.03 - 1.96 (m, 1H), 1.50 - 1.44 (m, 1H), 1.33 - 1.25 (m, 1H);
[3-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-y1]-(4,5,6,7-
tetrahydro-1H-indazol-5-y1)-amine ("A23")
0¨
HII
HPLC/MS 1.70 min (B), [M+H] 363; 1H NMR (500 MHz, DMSO-d6) 6 [ppm]
12.33 (s, 1H), 9.25 (s, 1H), 8.17 (d, J = 7.1 Hz, 1H), 8.03 (d, J= 8.5 Hz,
2H),
7.18 (d, J = 9.0 Hz, 2H), 4.16 - 4.10 (m, 1H), 3.83 (s, 3H), 2.94 - 2.87 (m,
1H),
2.84 - 2.75 (m, 1H), 2.73 2.62 (m, 1H), 2.61 -2.51 (m, 1H), 2.21 - 2.13 (m,
1H), 1.79 (m, 1H);
trans-343-(4-methoxy-pheny1)-3H11,2,3]triazolo[4,5-dipyrimidin-5-ylamino]-
cyclobutanol ("A24")
N 0,
)/¨N
N\
-N
N
HPLC/MS 1.66 min (B), [M+H] 313; 1H NMR (500 MHz, DMSO-d6) 6 [PPril]
9.23 (s, 1H), 8.42 (d, J = 5.3 Hz, 1H), 8.03 (d, J = 8.5 Hz, 2H), 7.20 (d, J=
8.5
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 103 -
Hz, 2H), 5.03 (d, J = 4.4 Hz, 1H), 4.37 ¨ 4.29 (m, 2H), 3.86 (s, 3H), 2.35 ¨
2.26
(m, 2H), 225¨ 2.17 (m, 2H);
cis-343-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclobutanol ("A25")
HON-0,N
)z--N
N N
\ ¨
N
HPLC/MS 1.68 min (13), [M+H] 313; 1H NMR (500 MHz, DMSO-c16) 5 [PPrn]
9.22 (s, 1H), 8.36 (d, J = 5.7 Hz, 1H), 8.04 (d, J = 8.6 Hz, 2H), 7.20 (d, J =
8.8
Hz, 2H), 5.06 (d, J = 5.9 Hz, 1H), 3.91 (m, 1H), 3.85 (s, 3H), 3.84 ¨ 3.76 (m,
1H), 2.70 ¨ 2.57 (m, 2H), 1.94 ¨ 1.85 (m, 2H);
cis-443-(4-methoxy-phenyi)-3H-{1,2,3}triazolo[4,5-cljpyrimidin-5-ylamino]-
cyclohexanol ("A26")
0,
-,N
N
HPLC/MS 1.77 min (B), [M+H] 341; 1H NMR (500 MHz, DMSO-d6) 6 [PPm]
9.22 (s, 1H), 8.18 ¨7.79 (m, 3H), 7.18 (d, J = 8.3 Hz, 2H), 4.40 (m, 1H), 3.85
(s, 3H), 3.75 (m, J = 4.4 Hz, 1H), 3.4 (m, 1H), 1.78 (m, 2H), 1.73 ¨ 1.61 (m,
4H), 1.57¨ 1.47 (m, 2H);
cis-313-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopentanol ("A27")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 104 -
HO
¨N =
NN
__________________________________________ -N
N
HPLC/MS 1.81 min (B), [M+H] 327; 1F1 NMR (400 MHz, DMSO-d6) 6 [ppm]
9.39 (s, 1H), 8.07 (d, J = 9.0 Hz, 2H), 7.23 ¨ 7.14 (m, 2H), 4.40 (tt, J = T7,
5.3
Hz, 1H), 4.27 (if, J = 5.4, 3.7 Hz, 1H), 3.88 (s, 3H), 2.23 (ddd, J = 13.5,
7.8, 5.7
Hz, 1H), 2.14 ¨ 2.01 (m, 1H), 1.78 (m, 4H);
[3-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-y1]-(S)-tetrahydro-
furan-3-yl-amine ("A28")
00FN-1 0,
)/--N
1\1\__t\ N
-N
N
HPLC/MS 1.79 min (B), [M+H] 313; 1H NMR (400 MHz, DMSO-d6) 6 [PP111]
9.27 (s, 1H), 8.37 (m, 1H), 8.03 (m, 2H), 7.20 (d, J = 8.5 Hz, 2H), 4.41 (m,
1H),
3.95 (m, 1H), 3.85 (s, 3H), 3.74 (q, J = 7.9 Hz, 1H), 3.65 (m, 1H), 3.28 (m,
1H),
2.19 (m, 1H), 1.98 (m, 1H);
trans-3-[3-(4-bromo-phenyI)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopentanol ("A29")
Br
041
HO\ss 44,
N\ N\
__________________________________________ õN
HPLC/MS 1.86 min (B), [M+H] 375/378; 1H NMR (400 MHz, DMSO-d6) 6 [ppm]
9.23 (s, 1H), 8.24 (d, J = 6.4 Hz, 1H), 8.17 (d, J= 8.5 Hz, 2H), 7.85 (d, J=
8.5
Hz, 2H), 4.50 (m, 1H), 4.39 (q, J = 6.9 Hz, 1H), 4.24 (m, 1H), 2.16 (m, 1H),
1.99
¨ 1.85 (m, 2H), 1.76 (m, 1H), 1.63 ¨ 1.50 (m, 2H);
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 105 -
{3-[4-(2-methoxy-ethoxy)-pheny1]-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y11-(3-
pyridin-2-yl-propy1)-amine ("A68")
z N
N
HPLC/MS 1.87 min (C), [M+H] 406; 1H NMR (400 MHz, DMSO-d3, TFA-d1) 6
[ppm] 9.27 (s, 1H), 8.75 (d, J = 5.8 Hz, 1H), 8.47 (t, J = 7.8 Hz, 1H), 8.02
(m,
3H), 7.86 (t, J = 6.9 Hz, 1H), 7.23 - 7.14 (m, 2H), 4.26- 4.19 (m, 2H), 3.79 -
3.71 (m, 2H), 3.56 (t, J = 6.4 Hz, 2H), 3.38 (s, 3H), 3.17 (t, J- 7.7 Hz, 2H),
2.17 (p, J = 6.8 Hz, 2H);
[3-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-y1H1-(2-morpholin-
4-yl-ethyl)-piperidin-4-y11-amine ("A78")
/ __ \ 0--
\--N ) ____________________________ NH N
-N
N
HPLC/MS 1.33 min (B), [M+H] 439;
(1S,3R)-343-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopentanecarboxylic acid ("A79")
OH
OH 0
I\!
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 106 -
HPLC/MS 1.80 min (B), [M+H] 355; 1H NMR (400 MHz, DMSO-d6, TFA-d1) 6
[ppm} 9.26 (s, 1H), 8.04 (m, 2H), 7.23¨ 7.16 (m, 2H), 4.27 (m, 1H), 3.87 (s,
3H), 2.81 (p, J = 8.5 Hz, 1H), 2.39¨ 2.26 (m, 1H), 2.07 ¨ 1.81 (m, 4H), 1.70
(m, 1H);
(1R,3S)-34344-methoxy-pheny1)-3H11,2,3]triazolo[4,5-d]pyrimidin-5-ylaminok
cyclopentanecarboxylic acid (A81")
OH
0 ¨
0 *y_)_...H
)--N
1\
__________________________________ -N
N-
HPLC/MS 1.81 min (B), [M+H] 355;
[2-(5-fluoro-1H-indo1-3-yl)-ethylj-{344-(2-methoxy-eth oxy)-p henyI]-3H-
[1,2,31triazolo[4,5-d]pyrimidin-5-yll-amine ("A84")
HN /
HPLC/MS 2.91 min (C), [M-'-H) 448; 1H NMR (500 MHz, DMSO-d6, TFA-d1) 5
[ppm] 9.32 (s, 1H), 8.07 ¨ 8.02 (m, 2H), 7.36 (dd, J = 8.8, 4.5 Hz, 1H), 7.32
(dd, J= 9.9, 2.5 Hz, 1H), 7.28 (s, 1H), 7.22 ¨ 7.15 (m, 2H), 6.90 (td, J= 9.2,
2.5 Hz, 1H), 4.25 ¨ 4.19 (m, 2H), 3.79 ¨ 3.69 (m, 4H), 3.38(s, 3H), 3.08 (t,
J=
7.4 Hz, 2H);
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 107 -
[3-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-dipyrimidin-5-y1H1-(2-pyrrolidin-
1-
ykethy1)-piperidin-4-y1]-amine ("A86")
0-
\ \_11
N/
HPLC/MS 1.26 min (B), [M+H] 423;
4-[3-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
piperidin-
2-one ("A87")
0
HN )¨N
114
N--"N
HPLC/MS 1.59 min (B), [M+H] 340; 1H NMR (500 MHz, DMSO-d6, TFA-d1) 5
[ppm] 9.29(s, 1H), 8.14 ¨ 7.93 (m, 2H), 7.19 (d, J = 9.1 Hz, 2H), 4.35 (m,
1H),
3.88 (s, 3H), 3.41 (m, 1H), 3.33 (ddd, J = 13.1, 8.9, 4.7 Hz, 1H), 2.82 (dd, J
=
17.4, 6.1 Hz, 1H), 2.56 ¨ 2.48 (m, 1H), 2.28 ¨ 2.03 (m, 1H), 1.90 (m, 1H);
[3-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-ylk(S)-1-pyridin-4-
yl-
pyrrolidin-3-y1)-amine ("A88")
0,
NO N\
N\
-
NN
HPLC/MS 1.99 min (C), [M+H] 389; 1H NMR (400 MHz, DMSO-d6) 6 [ppm]13.7
(s, 1H), 9.31 (s, 1H), 8.55 (s, 1H), 8.21 (d, J = 6.8 Hz, 2H), 8.06 (m, 2H),
7.19
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 108 -
(d, J = 8.5 Hz, 2H), 6.85 (d, J = 6.9 Hz, 3H), 4.67 (m, 1H), 3.85 (m, 4H),
3.72
(m, 2H), 3.61 (m, 2H), 2.37 (dq, J = 13.7, 7.3 Hz, 1H), 2.24 (m, 1H);
{(1R,3S)-343-(4-methoxy-phenyl)-3H41,2,31triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopenty1}-carbamic acid benzyl ester ("A89")
N""0 "NH
(31"-
0 r\v/ N
N
Nr--N
HPLC/MS 2.08 min (B), [M+H] 460; 1H NMR (500 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.26 (s, 1H), 8.06 (m, 2H), 7.42 ¨7.34 (m, 4H), 7.34 ¨ 7.28 (m, 1H),
7.19 (d, J= 8.6 Hz, 2H), 5.04 (s, 2H), 4.29 (m, 1H), 3.95 (p, J = 7.2 Hz, 1H),
= 3.87 (s, 3H), 2.45 ¨2.35 (m, 1H), 2.01 (dq, J = 13.8, 7.3 Hz, 1H), 1.91
(dq, J =
= 13.5, 7.4, 7.0 Hz, 1H), 1.81 ¨1.70 (m, 1H), 1.70 ¨ 1.61 (m, 1H), 1.60 ¨
1.51
(m, 1H);
[3-(4-methoxy-pheny1)-3H-E1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-(R)-tetrahydro-
furan-3-yl-amine ("A98")
0,
N
11--N
HPLC/MS 1.81 min (B), [M+H] 313; 1H NMR (400 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.28 (s, 1H), 8.07 (d, J = 8.5 Hz, 2H), 7.24 ¨ 7.16 (m, 2H), 4.51 (m,
1H),
4.02 ¨ 3.85 (m, 5H), 3.77 (td, J = 8.1, 5.7 Hz, 1H), 3.72 (m, 1H), 2.25 (dq, J
=
12.6, 7.6 Hz, 1H), 2.10 ¨ 1.97 (m, 1H);
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 109 -
(trans)-3-[3-(5-methoxy-pyridin-2-y1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminol-cyclopentanol ("A99")
N
N\ N
-N
N
H PLC/MS 1.96 min (C), [M+H] 328; 1H NMR (400 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.26 (s, 1H), 8.39 (d, J = 3.0 Hz, 1H), 8.03 (d, J = 8.2 Hz, 1H), 7.75
(dd,
J- 9.0, 3.1 Hz, 1H), 4.45 (m, 1H), 4.24 (tt, J = 5.9, 3.2 Hz, 1H), 3.96 (s,
3H),
2.14 (m, 1H), 1.94 (m, 2H), 1.75 (dt, J = 13.3, 6.7 Hz, 1H), 1.60 - 1.44 (m,
2H).
(1S,3R)-343-(4-iodo-pheny1)-3H41,2,31triazolo[4,5-d]pyrimidin-5-ylaminoi-
cyclopentanecarboxylic acid ("A101")
HO,Tr0----N)rN 4114
0 N\ Nil
NN
HPLC/MS 2.02 min (B), [M+H] 451;1H NMR (400 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.27 (s, 1H), 8.09 (d, J = 8.9 Hz, 2H), 8.05 - 7.91 (m, 2H), 4.35 (m,
1H),
2.85 (p, J= 8.5 Hz, 1H), 2.37 (dt, J = 14.6, 7.7 Hz, 1H), 1.99 (m, 4H), 1.76
(m,
1H);
(trans)-3-(3-quinolin-6-y1-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-
cyclopentanol ("A102")
HO \µ'OlF=1\_
HPLC/MS 1.52 min (B), [M+H] 348; 1H NMR (500 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.43 (dd, J = 5.2, 1.4 Hz, 1H), 9.34 - 9.26 (m, 3H), 9.09 (d, J = 10.3
Hz,
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 1 1 -
1 H), 8.56 (d, J= 9.3 Hz, 1H), 8.21 (dd, J = 8.4, 5.2 Hz, 1H), 4.56 (m, 1H),
4.30
(m, 1H), 2.28 ¨ 2.20 (m, 1H), 2.09 (m, 1H), 2.00 (ddt, J = 12.8, 8.4, 6.0 Hz,
1H), 1.80 (m, 1H), 1.63 (m, 2H);
(S)-343-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-yiaminol-
piperidine-1-carboxylic acid tert-butyl ester ("A103")
0õ
1-4
NO -'
0
l\FN
HPLC/MS 2.15 min (B), [M+H] 426;
[3-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-(1-methyl-
piperidin-3-y1)-amine formate ("A104")
0,
410
N
\¨
HPLC/MS 1.39 min (6), [M+H] 340;
(R)-3-[3-(4-methoxy-phenyI)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
piperidine-1-carboxylic acid tert-butyl ester ("A105")
(1-3"J-N-1,
0
0
35 HPLC/MS 2.15 min (B), [M+H] 426;
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 111 -
trans-1-{443-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclohexyl)-piperidin-4-ol formate ("A106")
0,
HO
Nx_t-N
HPLC/MS 1.43 min (B), [M+H] 424; 1H NMR (500 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.28 (m, 1H), 8.13 (s, 1H), 8.11 ¨7.92 (m, 2H), 7.19 (d, J= 8.7 Hz, 2H),
3.97 (m, 1H), 3.87 (s, 3H), 3.7 (m, 1H), 3.44 (d, J = 11.4 Hz, 1H), 3.32¨ 3.18
(m, 3H), 3.05 (t, J = 12.5 Hz, 1H), 2.23 ¨ 2.09 (m, 4H), 2.05 ¨ 1.97 (m, 1H),
1.97 ¨ 1.86 (m, 1H), 1.85 ¨ 1.78 (m, 1H), 1.73 ¨ 1.57 (m, 3H), 1.50 ¨1.37 (m,
2H);
cis-1-{443-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclohexylypiperidin-4-ol formate ("A107")
HO
Nh)¨N
HPLC/MS 1.48 min (B), [M+H] 424; 1H NMR (500 MHz, DMSO-d6) 6 [ppm]
9.30 (s, 1H), 8.13 (s, 1H), 8.04 (m, 2H), 7.19 (d, J = 8.9 Hz, 2H), 4.18 (m,
1H),
3.87 (s, 3H), 3.74 ¨ 3.63 (m, 1H), 3.44 ¨ 3.37 (m, 1 H), 3.32 ¨ 3.21 (m, 3H),
3.11 ¨3.02 (m, 1H), 2.18 ¨ 1.77 (m, 6H), 1.75 ¨ 1.58 (m, 3H);
[3-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-((R)-1-pyridin-
4-
yl-pyrrolidin-3-y1)-amine ("Al 09")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 112 -
0¨
Na
N
"A109" hydrochloride: HPLC/MS 2.00 min (C), [M+H] 389;
(1S,3S)-3-[3-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-
cyclopentanecarboxylic acid methyl ester ("A110")
0 0¨
H
N\
N
HPLC/MS 1.97 min (B), [M+H] 369;
{(1S,3S)-343-(4-methoxy-phenyl)-31-141,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopentylymethanol ("A111")
OH 0-
NL
HPLC/MS 1.80 min (B), [M+H] 341;
{(1S,3R)-343-(4-methoxy-phenyl)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopenty1}-methanol ("A112")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 113 -
0¨
OH
r\il
N
- N
N
HPLC/MS 1/9 min (B), [M+H] 341;
(1R,3R)-343-(4-methoxy-phenyl)-3H41,2,31triaz010[4,5-d]pyrimidin-5-ylaminol-
cyclopentanecarboxylic acid methyl ester ("A113")
0---
0
0 r\jI)--1\1
NI\ZN
NõNI
HPLC/MS 1.89 min (B), [M+H] 369;
{(1 R,3R)-3-[3-(4-methoxy-phenyl)-3H41 ,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminol-
cyclopenty1}-methanol ("A114")
OH
0 -
HPLC/MS 1.77 min (B), [M+H] 341;
[3-(4-methoxy-phenyl)-3H11,2,311r1azolo[4,5-d]pyrimidin-5-y1]-[(R)-1-(1-methyl-
1H-pyrazol-4-y1)-pyrrolidin-3-y11-amine ("A115")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 114 -
NTD¨NH
1\10-
HPLC/MS 1.72 min (B), [M+H] 392.
Example 5
Synthesis of {cis-343-(4-methoxy-phenyl)-3H-[1,2,31triazolo[4,5-dipyrimidin-5-
ylaminc]-cyclobuty1}-carbamic acid tert-butyl ester ("A30") and cis-1\143-(4-
methoxy-phenyl)-3H-0 ,2,31triazolo[4,5-cl]pyrimidin-5-y11-cyclobutane-1,3-
diamine hydrochloride ("A31")
0'
0
HN)-L 0
HNk 0--
2-methoxyethanol
fit
80 C
NH2 HNN N
II :N
NH 2 0--
4 N HCI
in dioxane
HNyNN
= x HCI
A solution of 5-chloro-3-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-
d]pyrimidine
(144 mg, 0.55 mmol) and cis-(3-amino-cyclobutyI)-carbamic acid tert-butyl
ester (113 mg, 0.61 mmol) in 2-methoxyethanol (3 ml) is heated to 80 C and
stirred at this temperature for 18 hours. The reaction mixture is evaporated
and
the residue is chromatographed on a silica gel column with cyclohexane/ethyl
acetate as eluent to afford cis-{343-(4-methoxy-phenyl)-3H11,2,31triazolo[4,5-
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 115 -
d]pyrimidin-5-ylaminoFcyclobuty1}-carbamic acid tert-butyl ester as light pink
powder; HPLC/MS 2.02 min (B), [M+1-1] 412; 1H NMR (500 MHz, DMSO-d6) 6
[ppm] 9.24 (s, 1H), 8.42 (d, J = 5.8 Hz, 1H), 8.05 (d, J = 8.9 Hz, 2H), 7.22
(d, J
= 8.6 Hz, 2H), 7.19¨ 7_15 (m, 1H), 3.92 ¨ 3.87 (m, 1H), 3.85 (s, 3H), 3.74 (m,
1H), 2.64 (m, 2H), 1.98 ¨ 1.88 (m, 2H), 1.38 (s, 9H).
The following compounds are prepared analogously:
{trans-343-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclobutyl}-carbamic acid tert-butyl ester ("A32")
0¨
>0
0 N
,N
N
HPLC/MS 1.99 min (B), [M+H] 412; 1H NMR (400 MHz, DMSO-d6) 6 [ppm]
9.24 (s, 1H), 8.49 (d, J = 4.9 Hz, 1H), 8.03 (d, J = 8.5 Hz, 2H), 7.25 (m,
1H),
7.23 ¨ 7.10 (m, 2H), 4.28 (m, 1H), 4.09 (m, 1H), 3.85 (s, 3H), 2.30 (m, 4H),
1.39 (s, 9H);
{443-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminoi-
cyclohexylmethyI}-carbamic acid tert-butyl ester ("A33")
0¨
N
\ 0 )Nra-N
N
HPLC/MS 2.13 min (B), [M+H] 454;
443-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
piperidine-1-carboxylic acid tert-butyl ester ("A34")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 116 -
O¨
_____________________________________ H
0 \ N\ N
HPLC/MS 2.14 min (B), [M+H] 426; 1H NMR (400 MHz, DMSO-c15) 6 [ppm]
9.24 (s, 1H), 8.02 (m, 3H), 7.19 (d, J = 8.6 Hz, 2H), 3.95 (m, 2H), 3.92 (s,
3H),
3.23 -3.10 (m, 1H), 2.92 (m, 1H), 2.78 (m, 1H), 1.92 -1.83 (m, 2H), 1.40 (m,
11H);
ftrans-343-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-
cyclopentyll-carbamic acid tert-butyl ester ("A35")
0-
0 .0'111 =
H
õN
racemate, HPLC/MS 2.07 min (B), [M+H] 426; 1H NMR (500 MHz, DMSO-d6
TITA-d1) 6 [ppm] 9.30 (s, 1H), 8.08 (d, J= 8.1 Hz, 2H), 7.19 (d, J = 9.1 Hz,
2H), 4.42 (m, 1H), 4.03 (p, J = 7.0 Hz, 1H),.3.88 (s, 3H), 2.23 - 2.14 (m,
1H),
2.05 (m, 1H), 1.98 - 1.91 (m, 2H), 1.64 (m, 1H), 1.57 - 1.49 (m, 1H), 1.42 (s,
9H);
3-[3-(4-methoxy-phenyI)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
azetidine-1-carboxylic acid tert-butyl ester ("A36")
0,
-
0 111
N
.N
N
HPLC/MS 2.05 min (B), [M+H] 398;
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 117 -
(R)-3-[3-(4-methoxy-pheny1)-3H-[1,2,31triazolo[4,5-cl]pyrimidin-5-ylaminol-
pyrrolidine-1-carboxylic acid tert-butyl ester ("A65")
0,
0 ,
N-)q
HPLC/MS 2.04 min (B), [M+H] 412;
(S)-343-(4-methoxy-pheny1)-3H41,2,31triazolo[4,5-d]pyrimidin-5-ylamino]-
pyrrolidine-1-carboxylic acid tert-butyl ester ("A66")
0,
N
0 ID
A o 1110
N
\¨
-N
N
HPLC/MS 2.04 min (B), [M+H] 412.
To a solution of {cis-343-(4-methoxy-pheny1)-3H41,2,31triazolo[4,5-
d]pyrimidin-5-ylaminol-cyclobuty1}-carbamic acid tert-butyl ester (49.4 mg,
0.12 mmol) in a mixture of dioxane (1 ml) and methanol (1 ml) is added 4 N
HCI in dioxane (2 ml). The reaction mixture is stirred for 2 hours at room
temperature. The precipitate that has formed is filtered off, washed with
dioxane and dried under vacuum to afford cis-N-[3-(4-methoxy-pheny1)-3H-
[1,2,3]triazolo[4,5-cl]pyrimidin-5-yli-cyclobutane-1,3-diamine hydrochloride
("A31") as cream-coloured powder; HPLC/MS 1.41 min (B), [M-I-H] 312; 1H
NMR (400 MHz, DMSO-d6) ö [ppm] 9.29 (s, 1H), 8.53 (s, 1H), 8.27 (s, 3H),
8.04 (d, J = 8.4 Hz, 2H), 7.25 - 7.16 (m, 3H), 4.09 (m, 1H), 3.87 (s, 3H),
3.50 (m, 1H), 2.70 (m, 2H), 2.19 (m, 2H).
The following compounds are prepared analogously:
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 118 -
(3-aza-bicyclo[3.1.01hex-6-y1)43-(4-methoxy-phenyl)-3H-[1,2,31triazolo[4,5-
d]pyrimidin-5-y11-amine hydrochloride ("A37")
HNa?-1[4)_
N 410
HPLC/MS 1.32 min (B), [M+H] 324; 1H NMR (500 MHz, DMSO-d6) 6 [PPnl]
9.65 ¨ 9.56 (m, 2H), 9.30 (m, 2H), 8.45¨ 8.41 (m, 1H), 8.20 (d, J = 8.6 Hz,
2H), 7.24 (d, J = 8.5 Hz, 2H), 3.86 (s, 3H), 3.40 (m, 3H), 2.98 ¨ 2.92 (m,
1H), 2.00 (m, 2H);
trans-N43-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-
cyclobutane-1,3-diamine hydrochloride ("A38")
0,
)¨N
N\
N
HPLC/MS 1.33 min (B), [M+H] 312; 1H NMR (400 MHz, DMSO-c16 /TFA-d1)
6 [ppm] 9.28 (s, 1H), 8.05 (d, J = 8.5 Hz, 2H), 7.19 (d, J = 9.1 Hz, 2H), 4.67
(m, 1H), 3.89 (m, 4H), 2.58 (m, 4H);
(4-aminomethyl-cyclohexyl)-[3-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]-
pyrimidin-5-yI]-amine hydrochloride ("A39")
0,
H2N N
)--N =
N\ tN\
HPLC/MS 1.44 min (B), [M+H] 354; 1H NMR (500 MHz, DMSO-d6) 6 [ppm]
9.23 (s, 1H), 8.03 (d, J = 8.2 Hz, 2H), 7.97 (m, 4H), 7.19 (d, J = 8.2 Hz,
2H),
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
-119-
3.85 (m, 4H), 2.72 ¨2.65 (m, 2H), 2.07¨ 2.01 (m, 1H), 1.95 (m, 1H), 1.88 ¨
1.81 (m, 2H), 1.63 ¨ 1.55 (m, 1H), 1.31 (m, 2H), 1.16 ¨1.04 (m, 2H);
[3-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-dipyrimidin-5-y1]-piperidin-4-yl-
amine hydrochloride ("A40")
0,
H NaH
N\
_____________________________________ .N
N
HPLC/MS 1.39 min (B), [M+H] 326; 1H NMR (400 MHz, DMSO-d6r1TA-d1)
6 [ppm] 9.29 (s, 1H), 8.05 (m, 2H), 7.23 ¨ 7.14 (m, 2H), 4.18 (m, 1H), 3.88
(s, 3H), 3.41 (m, 2H), 3.12 (m, 1H), 3.04 (td, J = 12.7,2.9 Hz, 1H), 2.16 (m,
2H), 1.84 (m, 2H);
trans-N43-(4-methoxy-phenyl)-3H-[1,2,31triazolo[4,5-d]pyrimidin-5-y1]-
cyclopentane-1,3-diamine hydrochloride ("A41")
H2N N
.N
N
HPLC/MS 1.39 min (B), [M+H] 326; 1H NMR (400 MHz, DMSO-d6 TWA-d1)
6 [ppm] 9.29 (s, 1H), 8.07 (d, J = 7.6 Hz, 2H), 7.19 (d, J = 9.0 Hz, 2H), 4.53
(m, 1H), 3.88 (s, 3H), 3.77 (p, J = 7.0 Hz, 1H), 2.37 ¨ 2.19 (m, 2H), 2.18 ¨
2.06 (m, 2H), 1.81 ¨1.64 (m, 2H);
[3-(4-methoxy-phenyl)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-y1]-(R)-pyrrolidin-3-
yl-
amine hydrochloride ("A69")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 120 -
H 0,
, 1110
-N
N
HPLC/MS 1.41 min (B), [M+H] 312; 1H NMR (400 MHz, DMSO-c15, TFA-d1)
[ppm] 9.28 (s, 1H), 8.05 (m, 2H), 7.18 (d, J = 9.0 Hz, 1H), 4.66 (m, 1H), 3.88
(s, 3H), 3.57 (m, 1H), 3.52 ¨ 3.42 (m, 11-!), 3.37 (m, 2H), 2.33 (m, 1H), 2.20
(m,
1H);
[3-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-y1]-(S)-pyrrolidin-3-
yl-amine hydrochloride ("A77")
0,
H N
O'
N
HPLC/MS 1.37 min (B), [M+H] 312;
{31441-methyl-I H-pyrazol-4-y1)-pheny1]-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
y1}-
(R)-pyrrolidin-3-yl-amine ("A116")
HND"µN I N
"A116" Hydrochloride HPLC/MS 1.36 min (B), [M+H] 362;
{(cis)-313-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopentylmethyll-carbamic acid tert-butyl ester ("A117")
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 121 -
N
H
0
A 0
N
HPLC/MS 2.12 min (B), [M+H] 440;
(1S,3R)-N3-{344-(1-methy1-1H-pyrazol-4-y1)-phenyl]-3H41,2,31triazolo[4,5-
dlpyrimidin-5-yll-cyclopentane-1,3-diamine ("A118")
N-
H2N N
M11\
-
HPLC/MS 1.43 min (B), [M+H] 376;
(1S,3R)-N3-(3-quinolin-6-y1-3H-[1,2,3]tr1az010[4,5-d]pyrimidin-5-y1)-
cyclopentane-1,3-diamine ("A119")
H2N EN
-N
HPLC/MS 1.33 min (B), [M+H] 347;
(1R,3R)-N343-(4-methoxy-pheny1)-3H11,2,3]triazolo[4,5-d]pyrimidin-5-y1]-
cyclopentane-1,3-diamine ("A120")
0,
H2N, , N
N\
-
N -N
"A120" hydrochloride HPLC/MS 1.42 min (B), [M+H] 326;
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 122 -
(1S,3R)-N3-(3-{441-(2-methoxy-ethyl)-1H-pyrazol-4-y1}-pheny1}-3H-
[1,2,3]triazolo[4,5-cl]pyrimidin-5-y1)-cyclopentane-1,3-diamine ("A121")
H2N0.41\_
\ _______________________________________________ 0
N'N
HPLC/MS 1.42 min (B), [M-FH] 420;
((1R,3S)-3-aminomethyl-cyclopenty1)43-(4-methoxy-phenyl)-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-yli-amine ("A122")
[N-1 0,
)/---N
NH2 N N
\ ¨
N--"N
HPLC/MS 1.46 min (B), [M-FH] 340;
(1R,3R)-N-(3-{441-(2-methoxy-ethyl)-1H-pyrazol-4-yll-pheny1}-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-y1)-cyclopentane-1,3-diamine ("Al 23")
H21\1'00-srvi _N
HPLC/MS 1.44 min (B), [M+H] 420;
(1R,3R)-N-[3-(2-methyl-quinolin-6-y1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y1F
cyclopentane-1,3-diamine ("A124")
041\
H2Nµ.
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 123 -
HPLC/MS 1.57 min (C), [M+1-11 361;
(1R,3R)-N-(3-{441-(2-3thoxy-ethyl)-1H-pyrazol-4-yll-phenyl}-3H-
[1,2,3]triazolo[4,5-cl]pyrimidin-5-y1)-cyclopentane-1,3-diamine ("A125")
H2N
HPLC/MS 1.49 min (C), [WEI] 434;
[3-(2-methyl-quinolin-6-y1)-3H11,2,31triazolo[4,5-dlpyrimidin-5-y1]-(R)-
pyrrolidin-
3-yl-amine ("A126")
7
IN
FIND-^N
)7--N
N\_ N
HPLC/MS 1.25 min (C), [M+NI 390.
Example 6
Synthesis of 1-{343-(4-methoxy-phenyl)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino]-piperidin-1-y1}-ethanone ("A42") and [3-(4-methoxy-phenyl)-3H-
[1,2,3]triazolo[4,5-dlpyrimidin-5-yI]-piperidin-3-yl-amine hydrochloride
("A43")
35
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 124 -
0¨
y2-methoxyethanol y fit
II N 5
NH2
N , DIPEA HNIN
x HCI
80 C
NCI
water
HN N N
90 C
To a solution of 5-chloro-3-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]-
pyrimidine (118 mg, 0.45 mmol) and 1-(3-amino-piperidin-1-yI)-ethanone
hydrochloride (88.4 mg, 0.50 mmol) in 2-methoxyethanol (3 ml) is added N-
ethyldiisopropylamine (0.15 ml, 0.90 mmol). The mixture is heated to 80 C and
stirred at this temperature for 1 hour. The reaction mixture is evaporated and
the residue is chromatographed on a silica gel column with ethyl acetate /
methanol as eluent to afford 1-{343-(4-methoxy-phenyl)-3H11,2,3]triazolo[4,5-
d]pyrimidin-5-ylamino]-piperidin-1-y1}-ethanone as light pink powder; HPLC/MS
1.71 min (B), [M+H] 368.
To a suspension of 1-(343-(4-methoxy-phenyl)-3H41,2,31triazolo[4,5-
d]pyrimidin-5-ylaminokpiperidin-1-yll-ethanone (73 mg, 0.20 mmol) in water
(1 ml) is added 37% aqueous hydrochloric acid (134 pl). The mixture is
heated to 90 C and stirred at this temperature for 44 hours. The reaction
mixture is evaporated and lyophilized to afford [3-(4-methoxy-phenyl)-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-piperidin-3-yl-amine hydrochloride as
orange powder; HPLC/MS 1.38 min (B), [M+FI] 326.
Example 7
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 125 -
Synthesis of trans-N-[3-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-dlpyrimidin-
5-yll-cyclohexane-1,4-diamine ("A44")
0¨ NH
- 2 0
NH2
2-methoxyethanol
ClN4Ik
N
µ1\1 NH 80 C HN N N
2
,
A solution of 5-chloro-3-(4-methoxy-phenyl)-3H[1,2,3]triazolo[4,5-d]pyrimidine
(81 mg, 0.31 mmol) and trans-cyclohexane-1,4-diamine (72 mg, 0.63 mmol) in
2-methoxyethanol (3 ml) is heated to 80 C and stirred at this temperature for
18 hours. The reaction mixture is evaporated and the residue is chromate-
graphed on a silica gel column with dichloromethane/methanol as eluent to
afford trans-N43-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-y11-
cyclohexane-1,4-diamine as light pink powder; HPLC/MS 1.39 min (B), [M+H]
340; 1H NMR (400 MHz, DMSO-d6) 6 [ppm] 9.25 (s, 1H), 8.09 (d, J = 7.5 Hz,
1H), 8.03 (d, J- 8.6 Hz, 2H), 7.19 (d, J = 8.6 Hz, 2H), 6.37 (bs, 2H), 3.86
(s,
3H), 3.70 (m, 1H), 2.87 (m, 1H), 1.99 (m, 4H), 1.37 (m, 4H).
The following compounds are prepared analogously
cis-N-[3-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-
cyclohexane-1,4-diamine ("A45")
HPLC/MS 1.43 min (B), [M+H] 340; 1H NMR (400 MHz, DMSO-d6) 6 [PP711]
9.29 (s, 1H), 8.02 (m, 2H), 7.88 (m, 3H), 7.20 (d, J = 9.1 Hz, 2H), 3.93 (m,
1H), 3.86 (s, 3H), 3.13 (m, 1H), 1.99 (m, 2H), 1.74 (m, 6H),
N-[3-(4-iodo-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-cyclohexane-
1,4-diamine ("A53")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 126 -
NLN
N 111P
-N
N
HPLC/MS 1.98 min (C), [M+H] 436; 1H NMR (400 MHz, DMSO-d6, TFA-c11) 6
[ppm] 9.27 (s, 1H), 8.02 (m, 4H), 3.81 (m, 1H), 3.10 m, 1H), 2.15 ¨2.03 (m,
4H), 1.48 (m, 4H);
N43-(4-morpholin-4-yl-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-y1]-
cyclohexane-1,4-diamine ("A64")
HN
N\
- N
N
HPLC/MS 1.38 min (B), [M+H] 395; 1H NMR (400 MHz, DMSO-d6, TFA-d1)
[ppm] 9.28 (s, 1H), 8.10 (d, J= 9.1 Hz, 2H), 7.40 (d, J = 8.7 Hz, 2H), 3.95 ¨
3.84 (m, 5H), 3.40 (m, 4H), 3.09 (m, 1H), 2.08 (m, 4H), 1.61 ¨ 1.40 (m, 4H);
N13-(4-fluoro-phenyl)-3H41,2,31triazolo[4,5-d]pyrimidin-5-y1]-(trans)-
cyclohexane-1,4-diamine ("A75")
H 2N H
HPLC/MS 1.84 min (C), [M+H] 328; 1H NMR (400 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.28 (s, 1H), 8.21 (dd, J = 8.9, 4.8 Hz, 2H), 7.42 (t, J = 8.8 Hz, 2H),
3.85
(m, 1H), 3.12¨ 3.06 (m, 1H), 2.17 ¨ 2.04 (m, 4H), 1.61 ¨1.44 (m, 4H);
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 127 -
trans-N43-(5-morpholin-4-yl-pyridin-2-y1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-
yll-cyclohexane-1,4-diamine ("A96")
H 2N5
H
Nõ
NN N
HPLC/MS 1.31 min (B), [M+H] 396; 1H NMR (400 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.28 (s, 1H), 8.43 ¨ 8.36 (m, 1H), 7.89 (d, J = 5.2 Hz, 111), 7.65 (dd,
J =
9.0, 3.1 Hz, 1H), 3.93 ¨ 3.63 (m, 5H), 3.34(m, 14H), 3.06 (m, 1H), 2.06 (m,
4H), 1.47 (m, 4H);
N-(3-quinolin-6-y1-3H41,2,3]triazolo[4,5-d]pyrimidin-5-y1)-cyclohexane-1,4-
diamine ("A100")
H2Ni^--0 H
N
HPLC/MS 1.33 min (B), [M+H] 361; 1H NMR (400 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.46 (dd, J = 5.3, 1.5 Hz, 1H), 9.41 ¨9.29 (m, 3H), 9.10 (dd, J= 9.3,
2.3
Hz, 1H), 8.59 (d, J = 9.3 Hz, 1H), 8.24 (dd, J = 8.5, 5.3 Hz, 1H), 4.00 ¨ 3.94
(m, 1H), 3.12 (m, 1H), 2.14 (m, 4H), 1.69 ¨ 1.41 (m, 4H);
N-{3-[4-(tetrahydro-pyran-4-yloxy)-pheny1]-3H-[1,2,3]triazolo[4,5-d]pyrimidin-
5-
yll-trans-cyclohexane-1,4-diamine ("Al 27")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 128 -
N 0
)7--N
N=7-N
HPLC/MS 1.47 min (B), [M+H] 410;
(S)-1-{415-(4-amino-cyclohexylamino)41,2,3]triazolo[4,5-d]pyrimidin-3-y11-
pheny1}-pyrrolidin-3-ol ("A128")
OH
H
N
HPLC/MS 1.37 min (B), [M+H] 395;
N-{344-(1-methy1-1H-pyrazol-411)-pheny1]-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
yll-trans-cyclohexane-1,4-diamine ("A129")
H2Na.Ø H
I 'N
N
N4_ N
HPLC/MS 1.43 min (B), [M+H] 390.
Example 8
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 129 -
Synthesis of 3-(4-{513-(4-nitro-pyrazol-1-y1)-propylaminoj-[1,2,3]triazolo[4,5-
d]-
pyrimidin-3-yll-phenoxy)-propionic acid ("A46") and 3-(4-{513-(4-amino-
pyrazol-1-y1)-propylaminoH1,2,3]triazolo[4,5-d]pyrimidin-3-y1}-phenoxy)-
propionic acid ("A47")
0
0
NO2 HO
HOo
-14\z NO
0
x 2 HCI)
NH2 2-methoxyethanol
triethylamine
CkNN 11
II N
100 C
HO 0
112
r-
0
Pd/C
THE 1\\I
NN
To a solution of 3-(4-nitro-pyrazol-1-y1)-propylamine dihydrochloride (340 mg,
1.40 mmol) in 2-methoxyethanol (20 ml) is added 344-(5-chloro-[1,2,31-
triazolo[4,5-d]pyrimidin-3-y1)-phenoxyl-propionic acid (447 mg, 1.40 mmol) and
triethylamine (581 pl, 4.19 mmol). The mixture is heated to 100 C and stirred
at this temperature for 90 minutes. The reaction mixture is evaporated and the
residue is chromatographed on a silica gel column with dichloromethane /
methanol as eluent to afford 3-(4-{543-(4-nitro-pyrazol-1-y1)-propylamino]-
0,2,3]triazolo[4,5-dipyrimidin-3-y1}-phenoxy)-propionic acid as grey powder;
HPLC/MS 2.33 min (C), [M+11] 454; 1H NMR (500 MHz, DMSO-d6) 6 [PPn11
12.4 (bs, 1H), 9.24 (s, 1H), 8.88 (s, 1H), 8.25 (s, 1H), 8.18 (t, J= 5.8 Hz,
1H),
7.98 (d, J = 8.7 Hz, 2H), 7.16 (d, J = 8.6 Hz, 2H), 4.27 (m, 4H), 3.37 (m,
2H),
2.74 (t, J = 6.0 Hz, 2H), 2.16 (m, 2H).
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 130 -
A solution of 3-(4-{513-(4-nitro-pyrazol-1-y1)-propylaminoH1,2,31triazolo[4,5-
d]pyrimidin-3-ylyphenoxy)-propionic acid (63 mg, 0.14 mmol) in THF (20 ml) is
hydrogenated with palladium on carbon as catalyst (0.1 g) at room temperature
and under atmospheric pressure. After 20 hours, the catalyst is filtered off
and
the filtrate is evaporated. The residue is purified by preparative HPLC to
afford
3-(44543-(4-amino-pyrazol-1-y1)-propylaminoH1,2,31triazolo[4,5-dipyrimidin-3-
yI}-phenoxy)-propionic acid as white amorphous solid; HPLC/MS 1.73 min (C),
[M+H] 424; 1H NMR (500 MHz, DMSO-d5) 5 [IDPril] 9.75 (bs, 3H), 9.27 (s, 1H),
8.22 (s, 1H), 8.04 (d, J = 8.3 Hz, 2H), 7.95 (s, 1H), 7.55 (s, 1H), 7.21 (d, J
= 8.9
Hz, 2H), 4.29 (t, J = 6.0 Hz, 2H), 4.24 (m, 2H), 3,4 (m, 2H), 2.77 (t, J = 6.0
Hz,
3H), 2.12 (m, 2H).
Example 9
Synthesis of 2-dimethylamino-1-{443-(4-methoxy-phenyl)-3H-[1,2,3}triazo10-
[4,5-d]pyrimidin-5-ylamino]-piperidin-1-yll-ethanone ("A48")
O-
N
TBTU
HN N N
AOH HOBt
NMM
HN
N'j\I DMF
x HCI
yNN
N
To a solution of [3-(4-methoxy-phenyl)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-A-
piperidin-4-yl-amine hydrochloride (163 mg, 0.45 mmol) and N,N-dimethyl-
glycine (51.6 mg, 0.50 mmol) in DMF (3 ml) is added 0-(benzotriazol-1-y1)-
N,N,NI,Ni-tetramethyluronium tetrafluoroborate (TBTU) (209 mg, 0.65 mmol),
1-hydroxybenzotriazol hydrate (HOBt) (20. 3 mg, 0.13 mmol) and 4-methyl-
morpholine (NMM) (0.16 ml, 1.50 mmol) and the reaction mixture is stirred for
1 hour at room temperature. The reaction mixture is partitioned between water
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
-131 -
and ethyl acetate. The organic phase is dried over sodium sulfate and
evaporated. The residue is chromatographed on a silica gel column with
methanol/dichloromethane as eluent to afford 2-dimethylamino-144-[3-(4-
methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-piperidin-1-y1}-
ethanone as white powder; HPLC/MS 1.43 min (B), [M+H] 411; 1H NMR (500
MHz, DMSO-d6 /TFA-d1) 6 [ppm] 9.24 (s, 1H), 8.02 (m, 2H), 7.14 (d, J = 9.0
Hz, 2H), 4.35 (d, J = 16.2 Hz, 1H), 4.28 (m, 1H), 4.24 (d, J- 16.1 Hz, 1H),
4.07
(m, 1H), 3.82 (s, 3H), 3.64 (dq, J = 13.8, 3.2 Hz, 1H), 3.20 (t, J = 12.4 Hz,
1H),
3.02 (m, 1H), 2.82 (s, 3H), 2.81 (s, 3H), 2.00 (m, 2H), 1.58 (m, 1H), 1.48 (m,
1H).
The following compounds are prepared analogously:
3-dimethylamino-1-{443-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-
5-ylamino]-piperidin-1-y1}-propan-1-one ("A49")
0¨
/0 /
¨N N
N
=\
õN
HPLC/MS 1.42 min (B), [M+H] 425; 1H NMR (500 MHz, DMSO-d6 /TFA-d1) 6
[ppm] 9.28 (s, 2H), 8.05 (m, 2H), 7.19 (d, J = 8.6 Hz, 2H), 4.32 (m, 1H), 4.06
(m, 1H), 3.92 - 3.87 (m, 1H), 3.86 (s, 3H), 3.32 (t, J = 6.8 Hz, 2H), 3.24 (m,
1H), 2.98 - 2.83 (m, 3H), 2.82 (s, 6H), 2.01 (m, 2H), 1.56 (m, 1H), 1.46 (m,
1H).
2-dimethylamino-14343-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-
5-ylamino]-azetidin-1-ylyethanone ("A62")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 132 -
\ 0,
0
N\4¨N
.N
N
HPLC/MS 1.38 min (B), [M+H] 383;
3-dimethylamino-14343-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-
5-ylaminoFazetidin-1-yll-propan-1-one ("A63")
0,
H
¨Nnr-NIN7N
=
\ 0
N\ tNI\
.N
N
HPLC/MS 1.38 min (B), [M+H] 397;
2-dimethylamino-1-((R)-3[3(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]-
pyrimidin-5-ylaminoFpyrrolidin-1-ylyethanone formate ("A83")
Nr-D'N
Nq--N
NN
HPLC/MS 1.40 min (B), [M+H] 397; 1H NMR (400 MHz, DMSO-d6fTFA-d1) 6
[ppm] 9.19 (s, 3H), 8.00 (m, 3H), 7.09 (m, 2H), 4.52 (m, 1H), 4.08 (m, 2H),
3.79 (s, 3H), 3.68 (dt, J = 11.1, 6.0 Hz, 1H), 3.64 ¨ 3.33 (m, 3H), 2.85 ¨
2.76
(m, 6H), 2.30 ¨ 2.02 (m, 2H);
3-dimethylamino-14(R)-343-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]-
pyrimidin-5-ylamino]-pyrrolidin-1-yll-propan-1-one formate ("A85")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 133 -
yNIrD"-kNE-1
N
N
NN
HPLC/MS 1.39 min (B), [M+H] 411;
1-{(R)-343-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-
pyrrolidin-1-y1}-2-pyrazol-1-yl-ethanone ("A90")
N
N ()
N
HPLC/MS 1.73 min (B), [M+H] 420; 1H NMR (500 MHz, DMSO-d6 /TFA-d1) 6
[ppm] 9.29 (s, 1H), 8.09 (m, 2H), 7.79 (m, 111), 7.74 ¨ 7.56 (m, 1H), 7.20 (m,
2H), 6.36 (m, 1H), 5.27 ¨ 5.03 (m, 2H), 4.55 (m, 1H), 3.88 (s, 3H), 3.84¨ 3.57
(m, 3H), 3.57 ¨ 3.32 (m, 1H), 2.40 ¨ 1.99 (m, 2H);
2-dimethylamino-1-{(S)-3-[3-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]-
pyrimidin-5-ylamino]-pyrrolidin-1-y1}-ethanone formate ("A91")
NO
HPLC/MS 1.43 min (B), [M+H] 397;
3-dimethylamino-1-{(S)-3-[3-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]-
pyrimidin-5-ylaminol-pyrrolidin-1-y1}-propan-1-one formate ("A92")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 134 -
Cr)r
0
>--N
,N
N=N
HPLC/MS 1.47 min (B), [M+H] 411; 1H NMR (500 MHz, DMSO-d6) 6 [PPm]
9.30 (s, 1H), 8.13 (s, 1H), 8.11 ¨7.98 (m, 2H), 7.20 (m, 2H), 4.56 (m, OH),
3.87
(s, 3H), 3.83 (dd, J = 10.7, 6.1 Hz, 1H), 3.77 ¨ 3.64 (m, 1H), 3.64 ¨ 3.41 (m,
2H), 3.37 ¨ 3.26 (m, 2H), 2.81 (m, 7H), 2.38¨ 1.98 (m, 2H);
14(S)-343-(4-methoxy-pheny1)-3H41,2,3]tr1azo1o[4,5-d]pyrimidin-5-ylamino]-
pyrrolidin-1-y1}-2-pyrazol-1-yi-ethanone ("A93")
o,
N
HPLC/MS 1.69 min (B), [M+H] 420;
3-dimethylamino-14(R)-3-{344-(1-methyl-1H-pyrazol-4-y1)-phenyl]-3H-
0,2,31triazolo[4,5-d]pyrimidin-5-ylamino}-pyrrolidin-1-y1)-propan-1-one
("A130")
0 NI/
-`NH
N
N=N
"A130" formate HPLC/MS 1.43 min (B), [M+H] 461;
3-(4-methyl-piperazin-1-y1)-14(R)-3-{344-(1-methy1-1H-pyrazol-4-y1)-pheny1]-
3H-[1 ,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-pyrrolidin-1-y1)-propan-1-one
("A131")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 135 -
NH
N N 1\1%/
N
N
N=N
HPLC/MS 1.39 min (B), [M+H] 516;
1-{(R)-343-(4-methoxy-pheny1)-3H-[1,2,3]tr1azo10[4,5-d]pyrimidin-5-ylamino]-
pyrrolidin-1-y1}-3-(4-methyl-piperazin-1-y1)-propan-1-one ("A132")
NL
NH
ciN N N 0
N=N
"A132" formate HPLC/MS 1.43 min (B), [M+H] 466;
3-(4-methyl-piperazin-1-y1)-1-[(R)-3-(3-quinolin-6-y1-3H-[1,2,3}triazolo[4,5-
d]pyrimidin-5-ylamino)-pyrrolidin-1-yll-propan-1-one ("A133")
0
ry- NO..,NH
N
Cj N N I
"A133" formate HPLC/MS 1.35 min (B), [M+H] 487;
3-(4-methyl-piperazin-1-y1)-1-{(R)-313-(2-methyl-quinolin-6-y1)-311-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-pyrrolidin-1-yll-propan-1-one
("A134")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 136 0
/ N
N?
II N
HPLC/MS 1.31 min (6), [M+H] 501.
io Example 10
Synthesis of [3-(4-methoxy-phenyl)-3H41,2,31triazolo[4,5-d]pyrimidin-5-y1112-
(4-methyl-piperazin-1-y1)-ethyll-amine ("A50")
0¨
triethylamine
THF HNN N
µ,N1
NH2
To a solution of 5-chloro-3-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]-
pyrimidine (200 mg, 0.76 mmol) in THE (4 ml) is added triethylamine (107 pl,
0.76 mmol). The reaction mixture is stirred for 1 hour at room temperature.
The
reaction mixture is partitioned between dichloromethane and water. The
organic phase is dried over sodium sulfate and evaporated. The residue is
chromatographed on a silica gel column with dichloromethane/methanol to
afford [3-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-yI]-[2-(4-
methyl-piperazin-1-y1)-ethy1]-amine as off-white powder; HPLC/MS 1.47 min
(A), [M+H] 369; 1H NMR (500 MHz, DMSO-d6) 6 [ppm] 9.22 (s, 1H), 8.03 (d, J
= 8.2 Hz, 2H), 7.92 (m, 1H), 7.17 (d, J = 9.0 Hz, 2H), 3.85 (s, 3H), 3.49
¨3.41
(m, 2H), 3.30 (m, 2H), 2.53 (m, 2H), 2.45 (m, 2H), 2.30 (m, 4H), 2.14 (s, 3H).
The following compounds are prepared analogously:
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 137 -
[3-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-dipyrimidin-5-y1]-(3-pyrrolidin-1-
yl-
propyI)-amine ("A61")
0-
N\
N
N,
N
HPLC/MS 1.59 min (A), [M+H] 354;1H NMR (500 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.28 (s, 1H), 8.08¨ 8.03 (m, 2H), 7.20 (d, J = 8.9 Hz, 2H), 3.88 (s,
3H),
3.62 ¨ 3.47 (m, 4H), 3.31 ¨ 3.23 (m, 2H), 3.08 ¨ 2.98 (m, 2H), 2.07 ¨ 1.97 (m,
4H), 1.89 (m, 2H);
(R)L1-{445-((trans)-4-amino-cyclohexylamino)41,2,3]triazolo[4,5-d]pyrimidin-3-
yll-phenyll-pyrrolidin-3-ol ("A70")
NI:D..... OH
)7- N
N\
HPLC/MS 1.36min (B), [M+H] 395; 1H NMR (500 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.25 (s, 1H), 7.87 (m, 2H), 6.74 (d, J = 9.0 Hz, 2H), 4.48 (II, J = 4.9,
2.6
Hz, 1H), 3.75 (m, 1H), 3.51 (dd, J = 10.2, 4.8 Hz, 1H), 3.45 (td, J = 9.0, 7.0
Hz,
1H), 3.39 (td, J = 8.7, 3.5 Hz, 1H), 3.24 ¨ 3.17 (m, 1H), 3.10 ¨ 3.01 (m, 1H),
2.17 ¨ 1.94 (m, 6H), 1.54 ¨ 1.37 (m, 4H);
1-{445-((trans)-4-amino-cyclohexylamino)-[1,2,3]triazolo[4,5-d]pyrimidin-3-y11-
phenyll-pyrrolidin-2-one ("A71")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 138
NN
N
N
HPLC/MS 1.36min (B), [M+H] 393; 1H NMR (400 MHz, DMSO-c15, TFA-d1) 6
[ppm] 9.29 (s, 1H), 8.21 (d, J = 8.5 Hz, 2H), 7.98 ¨ 7.91 (m, 2H), 3.95 (t, J
=
7.0 Hz, 2H), 3.88 (m, 1H), 3.10 (m, 1H), 2.57 (m, 2H), 2.23 ¨ 2.07 (m, 6H),
1.51 (m, 4H);
{344-(2-methoxy-ethoxy)-phenyl]-3H11,2,31triazolo[4,5-d]pyrimidin-5-y1}43-(4-
methoxy-pheny1)-propylFamine ("A80")
I\r-N
HPLC/MS 3.05 min (C), [M+H] 435.
Example 11
Synthesis of trans-3-{344-(1-methyl-1H-pyrazol-4-y1)-phenyll-3H-
0,2,3]triazolo[4,5-d]pyrimidin-5-ylamino}-cyclopentanol ("A51")
N-N
Br
HO \ I
N-N
PdCl2dppf HO
HN N N + 0-B. K2 CO3
II N ,
dioxane/water HN N
N
80 C
=
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 139 -
A suspension of (trans)-343-(4-bromo-pheny1)-3H41,2,3]triazolo[4,5-d]-
pyrimidin-5-ylamino]-cyclopentanol (146 mg, 0.39 mmol), 1-methy1-4-(4,4,5,5-
tetramethy141,3,2]dioxaborolan-2-y1)-1H-pyrazole (106 mg, 0.51 mmol) and
potassium carbonate (163 mg, 1.17 mmol) in a mixture of dioxane (1 ml) and
water (0.1 ml) is flushed with nitrogen and heated to 80 C under stirring.
Dichloro[1,1'-bis(diphenylphosphino)ferrocene]palladium(II) dichlorornethane
adduct (32 mg, 0.04 mmol) is added and the reaction is stirred under nitrogen
in a closed reaction vial at 80 C for 18 hours. The reaction mixture is
allowed
to reach room temperature. The precipitate that has formed is filtered off,
washed with water and dried under vacuum to afford (trans)-3-{3-[4-(1-methy1-
1H-pyrazol-4-y1)-phenyl]-3H41,2,31triazolo[4,5-d]pyrimidin-5-ylamino}-
cyclopentanol as grey powder; HPLC/MS 1.66 min (B), [M+H] 377; 1H NMR
(400 MHz, DMSO-d6) 6 [ppm] 9.23 (s, 1H), 8.24 (s, 1H), 8.17 (d, J = 8.1 Hz,
2H), 8.08 (m, 1H), 7.96 (s, 1H), 7.83 (d, J = 8.3 Hz, 2H), 4.55 (s, 1H), 4.42
(m,
1H), 4.25 (m, 1H), 3.89 (s, 3H), 3.57 (s, 3H), 2.18 (m, 1H), 2.00 ¨ 1.86 (m,
2H),
1.82 ¨ 1.74 (m, 1H), 1.54 (m, 2H).
The following compounds are prepared analogously:
N-(3-{441-(2-methoxy-ethyl)-1H-pyrazol-4-y1]-pheny11-3H41,2,31triazolo[4,5-d]-
pyrimidin-5-y1)-cyclohexane-1,4-diamine ("A52")
H2N25
H N
N \ __ 0
)/--N
N\
from N43-(4-iodo-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y1]-cyclohexane-
1,4-diamine ("A53", example 7) and 1-(2-methoxy-ethyl)-4-(4,4,5,5-tetramethyl-
[1,3,2]dioxaborolan-2-y1)-1H-pyrazole;
HPLC/MS 1.89 min (C), [M+H] 434; 1H NMR (400 MHz, DMSO-d6/TFA-d1) 6
[ppm] 9.29 (s, 1H), 8.24 (s, 1H), 8.18 (d, J = 8.4 Hz, 2H), 8.02 (s, 1H),
7.88¨
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 140 -
7.80 (m, 2H), 4.37 (t, J = 5.2 Hz, 2H), 3.87 (m, 1H), 3.79 (t, J = 5.2 Hz,
2H),
3.31 (s, 3H), 3.10 (m, 1H), 2.12 (m, 4H), 1.52 (m, 4H);
N-{344-(1-ethy1-1H-pyrazol-4-y1)-phenyl]-3H41 ,2,3]triazolo[4,5-cilpyrimidin-5-
y1)-(trans)-cyclohexane-1,4-diamine trifluoroacetate ("A54")
H2N.-0 N¨\
from "A53" and 1-ethy1-4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-y1)-1H-
pyrazole;
HPLC/MS 1.95 min (C), [M+H] 404; 1H NMR (400 MHz, DMSO-d6/TFA-d1) 6
[ppm] 9.28 (s, 1H), 8.27 (d, J = 0.8 Hz, 1H), 8.17 (d, J= 8.3 Hz, 2H), 8.01
(s,
1H), 7.92 - 7.70 (m, 2H), 4.24 (q, J = 7.3 Hz, 2H), 3.84 (m, 1H), 3.09 (m,
1H),
2.28 - 1.99 (m, 4H), 1.68 - 1.27 (m, 7H).
Example 12
Chiral separation of "A10" into "A55" and "A56"
¨
HO HO 0¨ HO, 0
chiral column ZD 41,
HN,1\1 N HNN N +
11 µ1\1 µ1\1
N N
"Al 0"
"A55" "A56"
racemic mixture
(trans)-343-(4-Methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-
cyclopentanol ("A10") is chromatographed on a Chiralpak AD column with
heptane/ethanol as eluent to afford two enantiomers:
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 141 -
First eluted enantiomer: "A55", light yellow powder; HPLC/MS 1.69 min (B),
[M+H] 327 (absolute configuration arbitrarily assigned)
Second eluted enantiomer "A56", light yellow powder; HPLC/MS 1.72 min (B),
[M+HJ 327 (absolute configuration arbitrarily assigned).
Example 13
Synthesis of trans-3-(3-{441-(2-hydroxy-ethyl)-1H-pyrazol-4-y1.1-phenyl}-3H-
[1,2,3]triazolo[4,5-cl]pyrimidin-5-ylamino)-cyclopentanol ("A57")
0 0
Br
HO
PdC12dppf HO
-N
Nq K2c 03
HN N N
,
B-0 dioxane/water
,
ONA) 80 C II N
HCI .00" HO
'OH
N
dioxane rj
NN
A suspension of (trans)-3-(3-(4-bromo-phenyl)-3H-[1,2,3]triazolo[4,5-d]-
pyrimidin-5-ylaminokcyclopentanol (195 mg, 0.52 mmol), 142-(tetrahydro-
pyran-2-yloxy)-ethyl]-4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-y1)-1H-
pyrazole (219 mg, 0.68 mmol) and potassium carbonate (216 mg, 1.57 mmol)
in a mixture of dioxane (2 ml) and water (0.2 ml) is flushed with nitrogen and
heated to 80 C under stirring. Dichloro[1,1t-bis(diphenylphosphino)ferrocene]-
palladium(11) dichloromethane adduct (43 mg, 0.05 mmol) is added and the
reaction is stirred under nitrogen in a closed reaction vial at 80 C for 18
hours.
The reaction mixture evaporated and the residue is chromatographed on a
silica gel column with dichloromethane/methanol as eluent to afford (trans)-3-
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 142 -
[3-(44112-(tetrahydro-pyran-2-yloxy)-ethy11-1H-pyrazol-4-yll-pheny1)-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino}-cyclopentanol as yellow powder;
HPLC/MS 1.82 min (B), [M+H] 491.
To a solution of (trans)-343-(4-012-(tetrahydro-pyran-2-yloxy)-ethyl]-1H-
pyrazol-4-yl}-pheny1)-3H11,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-cyclo-
pentanol (72 mg, 0.35 mmol) in dichloromethane (6 ml) is added hydrochloric
acid in dioxane (4 M, 410 pl) and the reaction mixture is stirred for 1 hour
at
room temperature. The solids are filtered off, washed with dichloromethane
and dried under vacuum to afford (trans)-3-(3-(441-(2-hydroxy-ethyl)-1H-
pyrazol-4-y11-phenyl}-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclo-
pentanol hydrochloride as light brown solid; HPLC/MS 1.55 min (B), [M+H] 407
1H NMR (500 MHz, DMSO-d6, TFA-d1) 5 8.29 (s,
1H), 8.18 (m, 2H), 8.03
(s, 1H), 7.86 (d, J = 8.3 Hz, 2H), 4.47 (m, 1H), 4.27 (m, 1H), 4.22 (t, J =
5.6 Hz,
2H), 3.81 (t, J = 5.6 Hz, 2H), 2.19 (m, 1H), 2.04 ¨ 1.89 (m, 2H), 1.80 (m,
1H),
1.56 (m 2H).
Example 14
Synthesis of (1S,3R)-N43-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-
d]pyrimidin-5-y1]-cyclopentane-1,3-diamine ("A58") [first enantiomer] and
(1R,3S)-N-[3-(4-methoxy-phenyl)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-y1]-
cyclopentane-1,3-diamine ("A59") [second enantiomer]
35
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 143 -
0¨
la
+ 04¨
EtN(iPr)2
=
H 0,
HN ----/- 0 j3--N
CIN N
CH3OCH2CH2OH
ll sN
NH2 N--"N
chiral column
H
4=0 )....N
H
rN =+ 4-01 ON )N
H N N
\¨ 1 0 H Nl\ZNI1
HCl/dioxane HCl/dioxane
0---
H
10-4)¨ 0 µ 1).... N
.=
H2Ni N H2N r-N
N0--N
To a solution of 5-chloro-3-(4-methoxy-pheny1)-3H41,2,31triazolo[4,5-
d]pyrimidine (525 mg, 2.01 mmol) and cis-3-amino-cyclopenty1)-carbamic acid
tert-butyl ester hydrochloride (521 mg, 2.20 mmol) in 2-methoxyethanol (15 ml)
is added N-ethyldiisopropylamine (0.75 ml, 4.41 mmol). The mixture is heated
to 80 C and stirred at this temperature for 3 hours. The reaction mixture is
allowed to cool to room temperature. The solids are filtered off, washed with
methanol and dried under vacuum to afford {cis-343-(4-methoxy-pheny1)-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-cyclopenty1}-carbamic acid tert-
butyl
ester as beige powder; HPLC/MS 2.06 min (B), [M+H] 426
{cis-3-[3-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopentyll-carbannic acid tert-butyl ester is chromatographed on a Chiralpak
AD-H column with supercritical carbon dioxide/methanol(containing 0.5%
diethylamine) 80 : 20 as eluent to afford two enantiomers:
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 144 -
First eluted enantiomer: tert-butyl N-[(1S,3R)-31[3-(4-methoxypheny1)-
triazolo[4,5-clipyrimidin-5-yllaminolcyclopentyl]carbamate, white solid.
Second eluted enantiomer: tert-butyl N-[(1R,3S)-3-[[3-(4-methoxypheny1)-
triazolo[4,5-d]pyrimidin-5-yl]amino]cyclopentylicarbamate; light beige solid
tert-Butyl N-[(1S,3R)-34[3-(4-methoxyphenyl)triazolo[4,5-d]pyrimidin-5-
yljaminoJcyclopentylIcarbamate (136 mg, 0.32 mmol) is slurried in hydrogen
chloride in dioxane (4 M solution, 1.1 ml) and methanol (0.5 ml) is added. The
reaction mixture is stirred for 3 hours at room temperature. The solids are
filtered off, washed with dichloromethane and dried under vacuum to afford
(1S,3R)-N343-(4-methoxy-phenyl)-3H41 2, 3]triazolo[4,5-d]pyrimidin-5-y11-
cyclopentane-1,3-diamine hydrochloride as white solid; HPLC/MS 1.47 min
(B), [M+H1326; 1H NMR (500 MHz, DMSO-d6, TFA-d1) 6 [PPn11 9.28 (s, 1H),
8.10 ¨8.04 (m, 2H), 7.23 ¨ 7.16 (m, 2H), 4.30 (m, 1H), 3.87 (s, 3H), 3.60 (q,
J
= 7.5 Hz, 1H), 2.11 ¨1.99 (m, 2H), 1.89 ¨ 1.73 (m, 2H), 1.67 (dt, J = 14.3,
7.5
Hz, 1H).
tert-Butyl N-[(1R,3S)-34[3-(4-methoxyphenyl)triazolo[4,5-d]pyrimidin-5-
yliamino]cyclopentylicarbamate is treated similarly to afford afford (1R,3S)-
N3-
[3-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y11-cyclopentane-
1,3-diamine hydrochloride as white solid; HPLC/MS 1.44 min (B), [M+H] 326.
Example 15
Synthesis of {(1R,3S)-343-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-4
pyrimidin-5-ylaminoj-cyclopenty1}-(4-methyl-piperazin-1-y1)-methanone ("A60")
OH
IIIIIfa
(Nõ EDCI, HOBt N)i___N
HN1\1 N + L m 2 >. \,_/
II - DMF 0
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 145 -
To a solution of (1R,3S)-343-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-
d]pyrimidin-5-ylaminokcyclopentanecarboxylic acid (142 mg, 0.40 mmol) in
DMF (2 ml) are added N-(3-dimethylaminopropy1)-N`-ethylcarbodiimide
hydrochloride (153 mg, 0.80 mmol), 1-hydroxybenzotriazole hydrate (122 mg,
0.80 mmol) and 1-methylpiperazine (47.7 mg, 0.48 mmol) and the reaction
mixture is stirred for 3 hours at room temperature. The reaction mixture is
partitioned between water and ethylacetate. The organic phase is dried over
sodium sulfate and evaporated. The residue is chromatographed on a silica
gel column with dichloromethane/methanol as eluent to afford {(1R,3S)-3-[3-(4-
methoxy-phenyl)-3H41,2,31triazolo[4,5-d]pyrimidin-5-ylaminol-cyclopentyll-(4-
methyl-piperazin-1-y1)-methanone as white powder; HPLC/MS 1.49 min (B),
[M+H] 437; 1H NMR (500 MHz, DMSO-d6, TFA-di) 6 [ppm] 9.27 (s, 1H), 8.06
(m, 2H), 7.24¨ 7.15 (m, 2H), 4.56 (m, 1H), 4.37 (m, 1H), 4.21 (m, 1H), 3.88
(s,
3H), 3.45 (m, 3H), 3.24 (m, 1H), 3.01 (m, 3H), 2.86 (s, 3H), 2.25 (dt, J =
14.4,
7.4 Hz, 1H), 2.11 ¨1.60 (m, 5H).
The following compound is prepared analogously:
{(1S,3R)-3-[3-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-
cyclopenty1)-(4-methyl-piperazin-1-y1)-methanone ("A97")
0,
0
HPLC/MS 1.49 min (B), [M+H] 437; 1H NMR (500 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.25 (s, 1H), 8.05 (m, 2H), 7.23 ¨ 7.16 (m, 2H), 4.52 (m, 1H), 4.30 (s,
1H), 4.21 (m, 1H), 3.87 (s, 3H), 3.56 ¨ 3.43 (m, 2H), 3.38 (t, J = 13.5 Hz,
1H),
3.22 (m, 1H), 3.12 ¨ 3.00 (m, 1H), 3.00 ¨ 2.90 (m, 2H), 2.84 (s, 3H), 2.23 (m,
1H), 2.05 ¨ 1.98 (m, 1H), 1.97 ¨ 1.89 (m, 2H), 1.88 ¨ 1.82 (m, 1H), 1.75 (m,
1H).
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 146 -
Example 16
Synthesis of trans-3-{3-[5-(1-methy1-1H-pyrazol-4-y1)-pyridin-2-y11-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino}-cyclopentanol ("A94")
HO
HO
¨N DIEA
II 2-methoxyethanol N
II
i`J. H2 8000
N/ N-N
0, __CµN \ I
P HO
0
\
¨N
PdC12dppf Hf-\.1N N
K2CO3II N
dioxane/water
80 C
HPLC/MS 1.50 min (B), [M+H] 378; 1H NMR (400 MHz, DMSO-d6) PPm [PPm]
9.35 - 9.21 (m, 1H), 8.96 - 8.86 (m, 1H), 8.40 - 8.24 (m, 2H), 8.24 - 7.99 (m,
3H), 4.65 - 4.34 (m, 2H), 4.29 -4.18 (m, 1H), 3.91 (s, 3H), 2.23 - 2.03 (m,
1H), 2.03 - 1.84 (m, 2H), 1.84 - 1.62 (m, 1H), 1.62- 1.41 (m, 2H).
Example 17
Synthesis of trans-N-{344-(2-morpholin-4-yl-ethoxy)-phenyl]-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-y1}-cyclohexane-1,4-diamine ("A95")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 147 -
allylpalladiumchloride
c52i
co_
/\1Z)
rN
NH2 0
II -
OH CS2C 03 HNN N
toluene mli
110 C
HPLC/MS 1.41 min (C), [M+H1439; 1H NMR (500 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.26(s, 1H), 8.13 (s, 1H), 8.12 ¨8.02 (m, 2H), 7.32 ¨ 7.24 (m, 2H), 4.50
(t, J = 4.8 Hz, 2H), 4.07 ¨ 3.99 (m, 2H), 3.85 ¨ 3.71 (m, 3H), 3.67 (t, J =
4.9
Hz, 2H), 3.60 (d, J = 12.5 Hz, 2H), 3.29 m, 2H), 3.07 (m, 1H), 2.13 ¨ 2.01 (m,
4H), 1.54 ¨ 1.38 (m, 4H).
Example 18
Synthesis of (1R,3R)-3-{3-[4-(2-methoxy-ethoxy)-pheny1]-3H41,2,31triazolo[4,5-
d]pyrimidin-5-ylaminoycyclopentanecarboxylic acid amide ("A135")
30
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 148 -
oI
I O
of,o
NEt 0
of
CI NCI 1:3 3
H2/RaNi
4.
II ___________________ >
IP THF
3 - 5 C THF
Cl,õõ..NNH
CI,, ,N NH II
NH2 TI N,,,..-
.--.- NH2
/NO2
0 /
10 Z OH 0
o
0 --,. OH 'o
ifk yx HCI
9
butyl nitrite NH, ilk
x cl..,,NN
II s
_________________________________________________ 3'
acetonitrile N,õ,7----NiN
HN,,N N
DIPEA II µ1\1
15 70 C CH3OCH2CH2OH
80 C
/
0
0 0
NH3 H2N ¨c
?
______________ .-
49
EDCI, HOBt
dioxane HN_,N N
II
N---N"N
In a reaction flask, 2,4-dichloro-5-nitro-pyrimidine (41.7 g, 215 mmol) is
dissolved in THF (250 ml) and the solution is cooled in an ice bath. Under
stirring and external cooling (3-5 C), a solution of 4-(2-methoxy-ethoxy)-
phenylamine (35.9 g, 215 mmol) in THF (250 ml) is added dropwise. An
orange precipitate develops. Then, still under cooling (2-5 C), triethylamine
(29.8 ml, 215 mmol) is added slowly. The reaction mixture is allowed to reach
room temperature and stirred for 30 minutes. The reaction mixture is filtered
with suction and the residue is washed well with THE. The filtrate is
evaporated
and the residue is triturated with tert-butyl methyl ether to afford (2-chloro-
5-
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 149 -
nitro-pyrimidin-4-y1)44-(2-methoxy-ethoxy)-phenylFamine as orange red
crystals; HPLC/MS 1.93 min (B), [M-'-HI 325;
1H NMR (400 MHz, DMSO-d6) 610.36 (s, 1H), 9.12 (s, 1H), 7.41 (d, J= 9.0
Hz, 2H), 7.01 (d, J = 9.0 Hz, 1H), 4.38 - 3.96 (m, 2H), 3.87 - 3.56 (m, 2H),
3.32 (s, 3H).
To a solution of (2-chloro-5-nitro-pyrimidin-4-y1)44-(2-methoxy-ethoxy)-
pheny1]-
amine (68.9 g, 212 mmol) in THE (710 ml) is added sponge-nickel-catalyst
(pH-neutral, THF-wet) and the mixture is hydrogenated at normal pressure and
room temperature for 40 hours. The reaction mixture is filtered with suction.
The filtrate is evaporated and dried under vacuum to afford 2-chloro-N4-[4-(2-
methoxy-ethoxy)-pheny1]-pyrimidine-4,5-diamine as brown solid; HPLC/MS
1.63 min (B), [M+1-1] 295;
1H NMR (400 MHz, DMSO-d6) 6 8.48 (s, 1H), 7.59 (s, 1H), 7.53 (d, J = 9.0 Hz,
1H), 6.94 (d, J = 9.0 Hz, 1H), 5.17 (s, 2H), 4.20 - 3.97 (m, 2H), 3.80 - 3.50
(m,
2H), 3.31 (s, 3H).
To a solution of 2-chloro-N444-(2-methoxy-ethoxy)-phenyl]-pyrimidine-4,5-
diamine (57.0 g, 193 mmol) in acetonitrile (600 ml) is added butyl nitrite
(30.8
g, 34.9 ml, 290 mmol). The solution is heated to 70 C and stirred at this
temperature for 3 hours. The reaction mixture is cooled to room temperature
and concentrated under reduced pressure. The residue is chromatographed
on a silica gel column (1 kg silica gel 60) with tert-butyl methyl ether as
eluent.
The product containing fractions are combined and concentrated under
reduced pressure until a precipitate forms. The solid is filtered off, washed
with
heptane and dried under vacuum to afford 5-chloro-3-14-(2-methoxy-ethoxy)-
phenyl]-3H-[1,2,3]triazolo[4,5-d]pyrimidine as light yellow crystals; HPLC/MS
2.63 min (C), [M+H] 306;
1H NMR (400 MHz, DMSO-d6) 6 9.83 (s, 1H), 7.94 (d, J = 9.1 Hz, 2H), 7.28 (d,
J = 9.0 Hz, 2H), 4.42 4.06 (m, 3H), 3.90 - 3.66 (m, 2H), 3.35 (s, 3H).
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 150 -
To a solution of 5-chloro-314-(2-methoxy-ethoxy)-phenylj-3H-
[1,2,3]triazolo[4,5-d]pyrimidine (3.36 g, 11.0 mmol) in 2-methoxyethanol (50
ml) is added (1R,3R)-3-amino-cyclopentanecarboxylic acid hydrochloride
(1.82 g, 11.0 mmol) and N-ethyldiisopropylamine (3.75 ml, 22.1 mmol). The
mixture is heated to 80 C and stirred at this temperature for 90 minutes.
The reaction mixture is cooled to room temperature and evaporated. The
residue is chromatographed on a silica gel column with
dichloromethane/methanol as eluent to afford (1R,3R)-3-{344-(2-Methoxy-
ethoxy)-phenyl]-3H41,2,31triazolo[4,5-d]pyrimidin-5-ylaminol-
cyclopentanecarboxylic acid as beige amorphous solid; HPLC/MS 1.77 min
(B), [M+H] 399;
1H NMR (500 MHz, DMSO-d6, TFA-d1) 6 9.23 (s, 1H), 8.05 (bs, 2H), 7.17 (d,
J = 8.8 Hz, 2H), 4.31 (bs, 1H), 4.24 ¨ 4.06 (m, 2H), 3.79 ¨ 3.62 (m, 2H),
3.32 (s, 3H), 3.00¨ 2.81 (m, 1H), 2.21 (bs, 1H), 2.10 ¨ 1.94 (m, 2H), 1.84
(ddd, J = 13.2, 8.9, 5.8 Hz, 1H), 1.76 (m, 1H), 1.68 (m, 1H).
In a reaction flask, (1R,3R)-34344-(2-methoxy-ethoxy)-phenyl]-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino}-cyclopentanecarboxylic acid (1.55
g, 3.87 mmol), N-(3-dimethylaminopropyI)-N'-ethylcarbodiimide
hydrochloride (1.50 g, 7.82 mmol) and 1-hydroxybenzotriazole hydrate (525
mg, 3.89 mmol) are dissolved in a 0.5M solution of ammonia in dioxane (40
ml, 20.0 mmol ammonia). The reaction mixture is stirred for 16 hours at 60
C. It is then evaporated and the residue is chromatographed on a silica gel
column with dichloromethane/methanol as eluent to afford (1R,3R)-3-{344-
(2-Methoxy-ethoxy)-phenyl]-3H-[1,2,3]triazolo[4,5-djpyrimidin-5-ylamino}-
cyclopentanecarboxylic acid amide as light yellow solid; HPLC/MS 1.65 min
(B), [M-1-1-1] 398;
1H NMR (500 MHz, DMSO-d6, TFA-d1) 6 9.28 (s, 1H), 8.09 (bs, 2H), 7.32 ¨
6.93 (m, 2H), 4.37 (bs, 1H), 4.32 ¨4.08 (m, 2H), 3.79 ¨ 3.68 (m, 2H), 3.36
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 151 -
(s, 3H), 2.87 (p, J = 8.0 Hz, 1H), 2.23 ¨ 2.05 (m, 2H), 2.06 ¨ 1.96 (m, 1H),
1.87 (m, 1H), 1.74 (m, 2H).
The following compounds are prepared analogously
(1S,3R)-343-(4-methoxy-phenyl)-3H41,2,3]triazolo[4,5-dipyrimidin-5-ylamino]-
cyclopentanecarboxylic acid amide ("A136")
NH2 0¨
0 NH
)--NNN
=
\
HPLC/MS 1.73 min (B), [M+H] 354;
(1S,3S)-343-(4-methoxy-phenyl)-3H-[1,2,31tr1azo1o[4,5-d]pyrimidin-5-ylamino]-
cyclopentanecarboxylic acid amide ("A137")
NH2
0 = 0- 4)¨N
N
HPLC/MS 1.70 min (B), [M+H] 354;
(1S,3R)-343-(4-pyrrolidin-1-yi-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino]-cyclopentanecarboxylic acid amide ("A138")
NH2
0 0. H
N
N =
N\
HPLC/MS 1.91 min (B), [M+H] 393;
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 152 -
(1R,3R)-343-(4-methoxy-pheny1)-3H41,2,31triazolo[4,5-dlpyrimidin-5-ylaminol-
cyclopentanecarboxylic acid ("A139")
OH O-
H
0 N =
N
HPLC/MS 1.79 min (B), [M+11] 355;
(1R,3R)-3-[3-(4-methoxy-pheny1)-3H-[1,2,31triazo10[4,5-d]pyrimidin-5-ylaminol-
cyclopentanecarboxylic acid amide ("A140")
0
NJJN
N
N
HPLC/MS 1.68 min (B), [M+11] 354;
(1S,3R)-313-(2-methyl-benzoxazol-6-y1)-3H-[1,2,3]triazolo[4,5-dlpyrimidin-5-
ylamino]-cyclopentanecarboxylic acid amide ("A141")
NH2
N N 0
-N
N
HPLC/MS 2.20 min (C), [M+Fl] 379;
4-[3-(4-methoxy-phenyl)-3H11,2,3]triazolo[4,5-d]pyrimidin-5-ylaminoF
butyramide ("A142")
0,
NH2 N\
N
N
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 153 -
HPLC/MS 1.61 min (B), [M+H] 328;
1H NMR (400 MHz, DMSO-d6) 8 [ppm] = 9.22 (s, 1H), 8.19 - 7.89 (m, 3H),
7.29- 7.14 (m, 3H), 6.72 (s, 1H), 3.85 (s, 3H), 3.44 - 3.27 (m, 2H), 2.19 -
2.09 (m, 2H), 1.88 -1.73 (m, 2H);
4-[3-(4-methoxy-phenyI)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclohexanecarboxylic acid amide ("A143")
0-
H2 NH
0 ________________________
Nv4-1\,1
N--1\1
HPLC/MS 1.71 min (B), [M+H] 368;
343-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-dlpyrimidin-5-ylamino]-
butyramide ("A144")
0,
N =
H2N NN
HPLC/MS 2.22 min (C), [M+H] 328;
(trans)-343-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-
cyclobutanecarboxylic acid amide ("A145")
0,
H2N
410
)7--N
0
HPLC/MS 1.62 min (B), [M+H] 340;
1H NMR (400 MHz, DMSO-d6) 5 [ppm] = 9.24 (s, 1H), 8.45 (d, J=6.5, 1H),
8.00 (d, J=8.6, 2H), 7.28 - 7.13 (m, 3H), 6.75 (s, 1H), 4.46 -4.35 (m, 1H),
3.86 (s, 3H), 3.02 - 2.89 (m, 1H), 2.53 - 2.37 (m, 2H), 2.34 - 2.14 (m, 2H);
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 154 -
(1R,3R)-3-[3-(4-pyrrolidin-1-yl-phenyl)-3H41,2,31triazolo[4,5-d]pyrimidin-5-
ylamino]-cyclopentanecarboxylic acid amide ("A146")
0
NH2 Ng_
HPLC/MS 1.86 min (B), [M+H] 393;
(1R,3R)-343-(2-methyl-benzothiazol-6-y1)-3H41,2,3]triazolo[4,5-djpyrimidin-5-
ylaminol-cyclopentanecarboxylic acid amide ("A147")
H2N
N\
N--=N
HPLC/MS 1.66 min (B), [M+H] 395;
(trans)-3-[3-(4-methoxy-phenyI)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclohexanecarboxylic acid amide ("A148")
041v_
0 N 4110
NH2
----
N'5-N
HPLC/MS 2.36 min (C), [M+H] 368;
(1S,3R)-343-(4-ethoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminoj-
cyclopentanecarboxylic acid amide ("A149")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 155 ¨
H
S
II
I-12N W
N
HPLC/MS 1.82 min (B), [M+H] 368;
1H NMR (400 MHz, DMSO-d6, TFA-d1) 6 [PPm] 9.24 (s, 1H), 7.98 (d, J= 8.8
Hz, 2H), 7.11 (d, J = 9.1 Hz, 2H), 4.40 -4.25 (m, 1H), 4.08 (q, J = 6.9 Hz,
2H),
2.77 (p, J = 7.8 Hz, 1H), 2.17 (ddd, J = 12.9, 8.5, 6.7 Hz, 1H), 1.98- 1.67
(m,
5H), 1.34 (t, J = 7.0 Hz, 3H);
(1R,3R)-343-(4-ethoxy-pheny1)-3H-[1,2,31triazolo[4,5-d]pyrimidin-5-ylaminol-
cyclopentanecarboxylic acid amide ("A150")
0
NH2 Ns
Nr-N
HPLC/MS 1.75 min (B), [M+H] 368;
1H NMR (500 MHz, DMSO-d6, TFA-d1) 6 [ppm] 9.34 (s, 1H), 8.08 (d, J= 8.5
Hz, 1H), 7.36 - 7.03 (m, 2H), 4.59 -4.37 (m, 1H), 4.15 (q, J = 7.0 Hz, 2H),
2.98 (p, J = 8.0 Hz, 1H), 2.25 (dt, J- 13.7, 7.4 Hz, 1H), 2.22 - 2.14 (m, 1H),
2.09 (dtd, J = 11.7, 7.7, 4.3 Hz, 1H), 1.95 (ddd, J = 13.5, 8.7, 5.2 Hz, 1H),
1.87
- 1.68 (m, 2H), 1.42 (t, J = 7.0 Hz, 3H);
(1R,3R)-3-{3444(S)-3,3,3-trifluoro-2-hydroxy-propoxy)-pheny1]-3H-
[1,2,31triazolo[4,5-d]pyrimidin-5-ylamino}-cyclopentanecarboxylic acid amide
("A151")
OH
0 04 C'CF3
NH2 N
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 156 -
HPLC/MS 1.71 min (B), [M+HJ 352;
(1R,3R)-343-(3-fluoro-4-methoxy-pheny1)-3H-[1,2,31triazolo[4,5-d]pyrimidin-5-
ylaminol-cyclopentanecarboxylic acid amide ("A152")
0 = 0-41k
N
NH2 N\
HPLC/MS 2.33 min (C), [M+H] 372;
(1R,3R)-3-{344-(2-hydroxy-ethoxy)-pheny1]-3H41,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminoycyclopentanecarboxylic acid amide ("A153")
0-11 0,f-OH
=
N
NH2 N,
1\1--N
HPLC/MS 1.53 min (B), [M+Fli 384;
(1R,3R)-3-{343-fluoro-4-(3-oxo-morpholin-4-y1)-pheny1]-3H41,2,3]triazolo[4,5-
d]pyrimidin-5-ylaminoycyclopentanecarboxylic acid amide ("A154")
F r7-0
NH2 N
0
N=N
HPLC/MS 1.54 min (B), [M+H] 441;
(1R,3R)-34314-((R)-3,3,3-trifluoro-2-hydroxy-propoxy)-phenyl]-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino}-cyclopentanecarboxylic acid amide
("A155")
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 157 -
HO
0 ===
Ts's N 410
NH2 N\
N"--N
HPLC/MS 1.70 min (B), [M+H] 452;
(1R,3R)-3-[3-(2,3-dihydro-benzo[1,4]dioxin-6-y1)-3H-[1,2,3]triazolo[4,5-
d]pyrimidin-5-ylamino]-cyclopentanecarboxylic acid amide ("A156")
0 ..0411
0)
0
NH2
N
HPLC/MS 2.26 min (C), [M+H] 382;
(1R,3R)-343-(4-ethoxy-3-fluoro-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminol-cyclopentanecarboxylic acid amide ("A157")
-isssµ
NH2 N
¨UY
N"-N
HPLC/MS 1.79 min (B), [M+H] 386;
(1R,3R)-343-(3-fluoro-4-morpholin-4-yl-pheny1)-3H41 ,2,3]triazolo[4,5-d]-
pyrimidin-5-ylamino}-cyclopentanecarboxylic acid amide ("A158")
H2N
1\F-N
HPLC/MS 1.73 min (B), [M+H] 427;
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 158 -
(1R,3R)-3-{3-[1-(2-methoxy-ethyl)-1H-pyrazol-4-y1]-3H-[1,2,3]triazolo[4,5-d]-
pyrimidin-5-ylamino}-cyclopentanecarboxylic acid amide ("A159")
0-
0 0'4
NH2 N ,CN
I\I=N
HPLC/MS 1.92 min (C), [M+H] 372;
(1R,3R)-3-{344-(2-pyrazol-1-yl-ethoxy)-phenyl]-3H41,2,31triazolo[4,5-d]-
pyrimidin-5-ylamino}-cyclopentanecarboxylic acid amide ("A160")
0 .04 .7-"=-10 N
is 0
NH
2
NN
HPLC/MS 1.65 min (B), [M+H] 434;
(1R,3R)-3-{3-[4-(3,3,3-trifluoro-2-hydroxy-2-trifluoromethyl-propoxy)-pheny1]-
3H-[1,2,31triazolo[4,5-d]pyrimidin-5-ylaminoycyclopentanecarboxylic acid
amide ("A161")
HO
H2N 0
1\1v,1N
Nr---1`1
HPLC/MS 1.88 min (B), [M+H] 520;
(1R,3R)-343-(4-methoxy-3-methyl-pheny1)-3H11,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminol-cyclopentanecarboxylic acid amide ("A162")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 159 -
0 =-&H
-N)/__N
NH2
¨
N'N
HPLC/MS 1.75 min (B), [M+H] 368;
(1R,3R)-343-(3,5-difluoro-4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-
5-ylaminol-cyclopentanecarboxylic acid amide ("A163")
0 0,
f's
NH2 N\
N'N
HPLC/MS 1.79 min (B), [M+H] 390;
(1R,3R)-343-(4-isopropoxy-pheny1)-3H41,2,3]triazolo[4,5-d]oyrimidin-5-
ylaminoj-cyclopentanecarboxylic acid amide ("A164")
0
NH2 NN
N--"N
HPLC/MS 1.80 min (B), [M+H] 382;
(trans)-3-{341-(2-methoxy-ethyl)-1H-pyrazol-4-y11-31-141,2,3]triazolo[4,5-
clipyrimidin-5-ylaminoycyclohexanecarboxylic acid amide ("A165")
04L jo ¨
[FN.
NH2
N
HPLC/MS 1.54 min (B), [M+H] 386;
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 160 -
(1S,3R)-3-{344-(2-methoxy-ethoxy)-phenylj-3H41,2,31triazolo[4,5-dlpyrimidin-
5-ylaminoycyclopentanecarboxylic acid amide ("A166")
of
H2N
HPLC/MS 1.72 min (B), [M+H] 398;
1H NMR (500 MHz, DMSO-d6, TFA-d1) 6 [ppm] 9.28 (s, 1H), 8.05 (d, J = 8.5
Hz, 2H), 7.22 (d, J- 9.0 Hz, 2H), 4.33 (bs, 1H), 4.26 - 4.17 (m, 2H), 3.86 -
3.68 (m, 2H), 3.36 (s, 3H), 2.78 (p, J = 8.1 Hz, 1H), 2.26 - 2.15 (m, 1H),
1.96
(ddt, J = 15.5, 8.1, 4.4 Hz, 1H), 1.91 - 1.83 (m, 2H), 1.78 (td, J = 14.4, 7.3
Hz,
2H);
(1R,3R)-3-{3-[4-(2-methoxy-1-methoxymethyl-ethoxy)-phenyI]-3H-
[1,2,3]triazolo[4,5-dlpyrimidin-5-ylamino}-cyclopentanecarboxylic acid amide
("A167")
0
1-12N 0
411
N=N25
HPLC/MS 1.68 min (B), [M+H] 442;
1H NMR (400 MHz, DMSO-d6, TFA-d1) 6 [ppm] 9.25 (s, 1H), 7.97 (d, J= 8.7
Hz, 2H), 7.14 (d, J = 8.9 Hz, 2H), 4.61 (p, J = 5.1 Hz, 1H), 4.35 (p, J = 6.2
Hz,
1H), 3.52 (d, J = 5.0 Hz, 4H), 3.24 (s, 6H), 2.89 (p, J = 8.0 Hz, 1H), 2.22 -
2.02
(m, 2H), 1.98 (td, J = 8.5, 4.3 Hz, 1H), 1.85 (ddd, J = 13.3, 8.6, 5.1 Hz,
1H),
1.78 - 1.59 (m, 2H),
(1R,3R)-3-{344-(tetrahydro-pyran-4-yloxy)-pheny1]-3H41,2,31triazoIo[4,5-d]-
pyrimidin-5-ylaminol-cyclopentanecarboxylic acid amide ("A168")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 161 -
,ON
'' NH
H2N /--"-N\
N
NN
HPLC/MS 1.72 min (B), [M+H] 424;
1H NMR (500 MHz, DMSO-d6, TFA-d1) 6 [PPm] 9.28 (s, 1H), 8.08 (bs, 2H),
7.25 (d, J = 8.8 Hz, 2H), 4.70 (if, J = 8.5, 4.0 Hz, 1H), 4.37 (bs, 1H), 3.91
(dt, J
= 11.6, 4.4 Hz, 2H), 3.54 (ddd, J = 11.9, 9.4, 2.8 Hz, 2H), 2.87 (p, J = 8.0
Hz,
1H), 2.25 ¨ 1.95 (m, 5H), 1.88 (m, 1H), 1.81 ¨ 1.59 (m, 4H);
(1R,3R)-3-{344-(2-ethoxy-ethoxy)-pheny1]-3H11,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminoycyclopentanecarboxylic acid amide ("A169")
H2N OJ
o
0
N=N
HPLC/MS 1.66 min (B), [M+H] 412;
(1R,3R)-3-[(3-azulen-2-yitriazolo[4,5-d]pyrimidin-5-Aamino]cyclopentane-
carboxamide ("A169a")
H2N 11,
N
0
=
(1R,3R)-3-[[3-(6-fluoro-2-naphthyl)triazolo[4,5-d]pyrimidin-5-yllaminolcyclo-
pentanecarboxamide ("Al 69b")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 162 -
H2N
0 N\
-
N-11
(1R,3R)-34[3-(4-tert-butoxyphenyl)triazolo[4,5-d]pyrimidin-5-ynaminoicyclo-
pentanecarboxamide ("Al 69c")
H2N
0
NN
N
(1R,3R)-34[344-(2-hydroxy-1,1-dimethyl-ethoxy)phenylltriazolo[4,5-d]pyrimidin-
5-ynamino]cyclopentanecarboxamide ("Al 69d")
2H 'N
N 140
0 N\
NN
(1R,3R)-34[314-[(3R)-tetrahydrofuran-3-yl]oxyphenyntriazolo[4,5-d]pyrimidin-5-
yliamino]cyclopentanecarboxamide ("Al 69e")
H2N
0 0
NRS
(1R,3R)-34[344-[(3S)-tetrahydrofuran-3-Aoxyphenylitriazolo[4,5-d]pyrimidin-5-
yllamino]cyclopentanecarboxamide ("A169f)
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 163 -
CoH2N
W" NH
1;1- ,
L-----</-
N,---N .
Example 19
Synthesis of (1R,3R)-343-(4-iodo-phenyl)-3H11,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino]-cyclopentanecarboxylic acid amide ("A170")
1 i
CI
I
0
../. NEt3
N --- N 1101 SnCl2
YLI CI +
3TH_ F CI
5. : NNH
Et0Ac/EtOFT I N NH
" II
NO2 NH2 TI 7000
N."------NH2
OH
1 0 OH
ellk 9 x HCI r_\.
butyl nitrite NH2 Y ili
,. ci.,,N,N,
> HNN N
acetonitrile II N
N .....,--- -NI' DIPEA II 'N
70 C
CH3OCH2CH2OH
80 C
0
NH3 H2N --el.,, I
_______________ ,..-
EDCI, HOBt I1?
THF NH NN==,-- ,
Il
In a reaction flask, 2,4-dichloro-5-nitro-pyrimidine (7.76 g, 40.0 mmol) is
dissolved in THF (40 ml) and the solution is cooled in an ice bath. Under
stirring and external cooling (3-5 C), a solution of 4-iodo-phenylamine
(8.769,
40.0 mmol) in THE (20 ml) is added dropwise. Then, still under cooling (2-5
C),
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 164 -
a solution of triethylamine (5.54 ml, 40.0 mmol) in THF (20 ml) is added
slowly.
The reaction mixture is stirred for 30 minutes under ice cooling and then
allowed to reach room temperature. The reaction mixture is filtered with
suction and the residue is washed well with THE. The filtrate is evaporated
and
the residue is treated with water. The solids are filtered off, washed with
water
and dried under vacuum to afford (2-chloro-5-nitro-pyrimidin-4-y1)-(4-iodo-
pheny1)-amine as yellow powder; HPLC/MS 2.13 min (B), [M+H] 377.
To a solution of (2-chloro-5-nitro-pyrimidin-4-y1)-(4-iodo-phenyl)-amine (11.4
g,
30.3 mmol) in a mixture of ethyl acetate (120 ml) and ethanol (60 ml) is added
tin(I1)chloride (17.2 g, 90.8 mmol) and the reaction mixture is stirred for 60
minutes at 70 C. The reaction mixture is cooled to room temperature, basified
with saturated sodium carbonate solution, filtered over a pad of Celite and
the
residue is washed with ethyl acetate. The organic phase of the filtrate is
separated, dried over sodium sulfate and evaporated. The residue is
chromatographed on a silica gel column with cyclohexane/ethyl acetate as
eluent to afford 2-chloro-N4-(4-iodo-phenyl)-pyrimidine-4,5-diamine as brown
solid; HPLC/MS 2.72 min (C), [M+H] 347.
To a solution of 2-chloro-N4-(4-iodo-phenyl)-pyrimidine-4,5-diamine (8.50 g,
24.5 mmol) in acetonitrile (110 ml) is added butyl nitrite (4.30 ml, 36.8
mmol).
The solution is heated to 70 C and stirred at this temperature for 90
minutes.
The reaction mixture is cooled to room temperature. The solid is filtered off,
washed with acetonitrile and 2-propanol and dried under vacuum to afford 5-
chloro-3-(4-iodo-pheny1)-3H-[1,2,3]triazolo[4,5-d]pyrimidine as brown powder;
HPLC/MS 3.14 min (C), [M+H] 358.
To a solution of 5-chloro-3-(4-iodo-phenyI)-3H-[1,2,3]triaz01o[4,5-
d]pyrimidine (1.799, 5.0 mmol) in 2-methoxyethanol (12.5 ml) is added
(1R,3R)-3-amino-cyclopentanecarboxylic acid hydrochloride (910 mg, 5.50
mmol) and N-ethyldiisopropylamine (1.87 ml, 11.0 mmol). The mixture is
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 165 -
heated to 8000 and stirred at this temperature for 3 hours. The reaction
mixture is cooled to room temperature and water is added. The resulting
precipitate is filtered off, washed with water and dried under vacuum to
afford (1R,3R)-343-(4-iodo-phenyl)-3H41,2,31triazolo[4,5-dipyrimidin-5-
ylamino]-cyclopentanecarboxylic acid as beige solid; HPLC/MS 2.82 min
(C), [M+H] 451.
To a suspension of (1R,3R)-343-(4-iodo-phenyl)-3H-[1,2,3]triazolo[4,5-
d]pyrimidin-5-ylamino]-cyclopentanecarboxylic acid (2.26 g, 5.02 mmol) in
THF (12 ml) are added N-(3-dimethylaminopropyI)-N'-ethylcarbodiimide
hydrochloride (1.93 g, 10.1 mmol), 1-hydroxybenzotriazole hydrate (678 mg,
5.02 mmol) and a 0.5M solution of ammonia in dioxane (50.2 ml, 25.1 mmol
ammonia). The reaction mixture is stirred for 3 days at room temperature. It
is then partitioned between dichloromethane and saturated sodium
hydrogen carbonate solution. The organic phase is dried over sodium
sulfate and evaporated. The residue is chromatographed on a silica gel
column with dichloromethane/methanol as eluent to afford (1R,3R)-3-[3-(4-
iodo-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-cyclopentane-
carboxylic acid amide as off-white crystals; HPLC/MS 2.61 min (C), [M+H]
450;
1H NMR (300 MHz, DMSO-d6, TFA-d1) 6 [PPm] 9.27 (s, 1H), 8.12 (d, J = 8.4
Hz, 2H), 8.01 (d, J = 8.8 Hz, 2H), 4.41 (bs, 1H), 2.91 (p, J = 7.9 Hz, 1H),
2.43 ¨ 1.97 (m, 3H), 1.96 ¨ 1.59 (m, 3H).
The following compounds are prepared analogously:
(1S,3R)-343-(4-iodo-phenyl)-3H41,2,31triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopentanecarboxylic acid amide ("A171")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 166 -
NH2
0 0>_N
=
N'
HPLC/MS 1.94 min (B), [M+H] 450;
(18,3R)-3-(3-benzo[1,2,5]thiadiazol-5-y1-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino)-cyclopentanecarboxylic acid amide ("A172")
NH2 N,
N
N
N"
HPLC/MS 2.43 min (C), [M+H] 382;
(1R,3R)-3-[3-(6-methoxy-pyridin-3-yI)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino]-cyclopentanecarboxylic acid amide ("A173")
0
)z¨N
NH2 1\
HPLC/MS 1.64 min (B), [M+H] 355;
1H NMR (400 MHz, DMSO-d6) 6 [ppm] 9.25 (s, 1H), 8.92 (s, 1H), 8.44- 8.36
(m, 1H), 8.31 -8.23 (m, 1H), 7.23 (s, 1H), 7.11 (d, J=9.0, 1H), 6.70 (s, 1H),
4.34- 4.22 (m, 1H), 3.95 (s, 3H), 2.78 (p, J=7.9, 1H), 2.12 - 2.00 (m, 2H),
1.98- 1.77 (m, 2H), 1.73- 1.56 (m, 2H);
(1R,3R)-3-(3-benzo[1,2,5]thiadiazol-5-y1-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino)-cyclopentanecarboxylic acid amide ("A174")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 167
1 ;NJ
NH2
T
NN
HPLC/MS 2.34 min (C), [M+H] 382;
(1R,3R)-343-(5-methoxy-pyridin-2-y1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino]-cyclopentanecarboxylic acid amide ("A175")
C>41 00,
N
N
NH2
N'N
HPLC/MS 1.50 min (B), [M+H] 355;
(1R,3R)-343-(3-iodo-pheny1)-3H11,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-
cyclopentanecarboxylic acid amide ("A176")
0-211)i¨ N
N N
\___
NH2
N--N1
HPLC/MS 1.84 min (B), [M+H] 450; 1H NMR (400 MHz, DMSO-d6, TFA-d1) 5
[ppm] 9.28 (s, 1H), 8.77 (bs, 1H), 8.30 (d, J = 8.2 Hz, 1H), 7.84 (d, J = 8.0
Hz,
OH), 7.42 (t, J = 8.1 Hz, 1H), 4.42 (bs, 1H), 2.96 (p, J- 8.0 Hz, 1H), 2.22
(m,
2H), 2.15 - 1.91 (m, 2H), 1.90.- 1.65 (m, 2H);
(1R,3R)-343-(5-iodo-pyridin-2-y1)-3H41,2,31triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopentanecarboxylic acid amide ("A177")
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 168
04I\_
0 s-
isss N
NH2 I
¨
HPLC/MS 2.19 min (C), [M+H] 451;
(1R,3R)-343-(5-ethoxy-pyridin-2-y1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino]-cyclopentanecarboxylic acid amide ("A178")
)7¨"N
NH2
N
NN
HPLC/MS 2.06 min (C), [M+H] 369;
(1R,3R)-3-[3-(6-ethoxy-pyridin-3-y1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino]-cyclopentanecarboxylic acid amide ("A179")
NH2
¨ N
HPLC/MS 2.29 min (C), [M+H] 369;
(1R,3R)-343-(2-methyl-benzofuran-5-y1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminol-cyclopentanecarboxylic acid amide ("A180")
0
0
Isµ
NH2 N,
HPLC/MS 2.51 min (C), [M+H] 378;
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 169 -
(1R,3R)-343-(2-methoxy-quino)in-6-y1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminoj-cyclopentanecarboxylic acid amide ("A181")
1
O,H 0
N
H2N
HPLC/MS 2.50 min (C), [M+HJ 405;
(1R,3R)-3-{342-(2-ethoxy-ethoxy)-quinolin-6-y11-3H41,2,3]triazolo[4,5-d]-
pyrimidin-5-ylamino}-cyclopentanecarboxylic acid amide ("A182")
0
H2N NH
rj
N 0
A
T
N-----N
HPLC/MS 2.55 min (C), [WEI] 463;
(1R,3R)-343-(1,2-dimethy1-1H-benzimidazol-5-y1)-3H41,2,31triazolo[4,5-d]-
pyrimidin-5-ylaminol-cyclopentanecarboxylic acid amide ("A183")
0, N¨
Y .
NH2
N
HPLC/MS 1.63 min (C), [M+1-1] 392;
(1R,3R)-313-(2-methyl-quinazolin-6-y1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminol-cyclopentanecarboxylic acid amide ("A184")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 170 -
0
NH2
HPLC/MS 1.37 min (B), [M+H] 390; 1H NMR (500 MHz, DMSO-d6) 6 [ppm] =
9.76 (s, 1H), 9.30 (s, 1H), 9.09- 9.04 (m, 1H), 8.92 -8.83 (m, 1H), 8.41 (d,
J=6.6, 1H), 8.18 (d, J=9.1, 1H), 7.34 - 7.25 (m, 1H), 6.83 -6.73 (m, 1H),
4.46 - 4.37 (m, 1H), 2.88 - 2.77 (m, 4H), 2.35 - 2.27 (m, 1H), 2.16 - 1.91 (m,
2H), 1.84- 1.57 (m, 3H);
(1R,3R)-343-(2-methyl-2H-indazol-6-y1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminol-cyclopentanecarboxylic acid amide ("A185")
Nre-
0 =s()--N)-1=1 ¨N
N/
NH2
HPLC/MS 1.56 min (6), [M+H] 378; 1H NMR (400 MHz, DMSO-d6) 6 [ppm] =
9.31 (s, 1H), 8.55 (s, 1H), 8.48 (s, 1H), 7.97 (2H), 4.45 (s, 1H), 4.28 (s,
3H),
2.93 (p, J = 8.0 Hz, 1H), 2.25 ¨ 2.10 (m, 2H), 2.10 ¨ 1.91 (m, 2H), 1.86 ¨
1.70
(m, 2H);
(1R,3R)-3-[3-(2-methy1-2H-indazol-5-y1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino]-cyclopentanecarboxylic acid amide ("A186")
'N-
)7¨N
N
NH2
WIN/
HPLC/MS 1.56 min (B), [M+H] 378; 1H NMR (500 MHz, DMSO-d5) 6 =
9.29 (s, 1H), 8.60 (m, 2H), 8.07 (bs, 1H), 7.87 (d, J= 9.2 Hz, 1H), 4.59 ¨
4.30
(m, 1H), 4.26(s, 3H), 2.87(p, J = 7.9 Hz, 1H), 2.30 ¨ 2.15 (m, 1H), 2.10 (tt,
J
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
-171 -
11.8, 5.0 Hz, 1H), 2.01 (do, J = 11.0, 8.1 Hz, 1H), 1.84 (ddd, J= 13.6, 8.8,
5.6
Hz, 1H), 1.75 (dtd, J = 18.3, 12.8, 7.0 Hz, 2H);
(1R,3R)-3-[(3-cinnolin-6-yltriazolo[4,5-d]pyrimidin-5-y1)aminolcyclopentane-
carboxamide ("A186a")
N
H2N
0
(1R,3R)-34[3-(7-methoxy-3-quinolyl)triazolo[4,5-d]pyrimidin-5-yliaminoicyclo-
pentanecarboxamide ("A186b")
0
H2N õ0-õH
0
NI
Nj
(1R,3R)-3-[[3-(2-methylindolizin-7-yl)triazolo[4,5-d]pyrimidin-5-
yljamino]cyclo-
pentanecarboxamide ("A186c")
H2N
0 N
I NI\
1\1=N
(1R,3R)-34[3-(2-methylindolizin-6-yl)triazolo[4,5-d]pyrimidin-5-Aamino]cyclo-
pentanecarboxamide ("Al 86d")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 172 -
H
H2N s=O'N
0 N
=
(1R,3R)-31[3-(2-methylimidazo[1,2-a]pyridin-6-yptriazolo[4,5-d]pyrimidin-5-
yliaminolcyclopentanecarboxamide ("Al 86e")
/Cr _________________________________________ r
, _______________________________ N
H2N
0
N'N
(1R,3R)-34[3-(2-methy1-1H-indo1-5-y1)triazolo[4,5-d]pyrimidin-5-yllaminolcyclo-
pentanecarboxamide ("Al 86f")
H2N
NH
0
N
N'N
(1R,3R)-34[3-(2-methylisoindo1-5-yOtriazolo[4,5-d]pyrimidin-5-
yl]aminolcyclopentanecarboxamide ("Al 86g")
H2N , N
o
(1R,3R)-34[3-(7-methylindolizin-2-yl)triazolo[4,5-d]pyrimidin-5-yliaminolcyclo-
pentanecarboxamide ("Al 86h")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 173 -
H N
/
2 It%
N
0 N\
(1R,3R)-34[3-(1H-indazol-5-yl)triazolo[4, 5-d]pyrimidin-5-
yliaminoicyclopentane-
carboxamide ("A186i")
C>41) N.
H2N , __ N
0
N'N
(1R,3R)-34[3-(2-methyl-3H-benzimidazol-5-yl)triazolo[4,5-d]pyrimidin-5-
yljamino]cyclopentanecarboxamide ("Al 86j")
H2N
0
Example 20
Synthesis of (1R,3R)-343-(2-methyl-quinolin-6-y1)-31-141,2,3]triazolo[4,5-c1]-
pyrimidin-5-ylamino]-cyclopentanecarboxylic acid amide ("A187")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 174 -
HN N
+ .--
I
-i I
/ THE N N + Fe/NH4C1
Ly...
cl
CkN NH ___ 3.
CI ,N NH
NO2 NH2 II .- Et0H/H20 11
55 C
2H2
OH
-.,
c-7\ butyl nitrite NH, ? 3 C1,-,...,,.., N x FICI,..... N.
N N
11
acetonitrile i
-.......--.. DIPEA 1- '' '
N,.1\N
80 C CH,OCH,CH,OH
80 C
0 \
HN / N
2
NH3 n
___________________________ -
EDCI, HOBt Nr
dioxane HNN N
TINN---
A solution of 2,4-dichloro-5-nitro-pyrimidine (11.5 g,59.3 mmol) in THF (25
ml)
is cooled to 00 C. To this solution is added a solution of 6-amino-2-methyl-
quinoline (10.4 g, 65.9 mmol) in THF (95 ml) dropwise under stirring. The
reaction mixture is allowed to reach room temperature. The precipitate that
has
formed is filtered off, washed with water and dried under vacuum to afford (2-
chloro-5-nitro-pyrimidin-4-y1)-(2-methyl-quinolin-6-y1)-amine hydrochloride as
yellow crystals; HPLC/MS 1.42 min (A), [M+H] 316; 1F1NMR (400 MHz,
DMSO-d6) 6 [ppm] 10.84 (s, 1H), 9.24 (s, 1H), 8.94 (d, J = 8.6 Hz, 1H), 8.49 ¨
8.34 (m, 2H), 8.22 (dd, J = 9.1, 2.4 Hz, 1H), 7.91 (d, J= 8.6 Hz, 1H), 2.96
(s,
3H).
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 175 -
To a suspension of (2-chloro-5-nitro-pyrimidin-4-y1)-(2-methyl-quinolin-6-y1)-
amine hydrochloride (10.27 g, 29.2 mmol) in water (80 ml) are added
ammonium chloride (935 mg, 17.5 mmol) and ethanol (80 m1). The mixture is
heated to 550 C under stirring and then iron, particle size 10 pm (8.15 g, 146
mmol) is added portionwise. The reaction mixture is stirred for 18 hours at 55
C. The reaction mixture is filtered over Celite and washed with ethanol. The
filtrate is evaporated and dried under vacuum to afford 2-chloro-N4-(2-methyl-
quinolin-6-y1)-pyrimidine-4,5-diamine as yellow solid which was used as such
in
the next step; HPLC/MS 1.26 min (B), [M+H] 286.
To a solution of crude 2-chloro-N4-(2-methyl-quinolin-6-yI)-pyrimidine-4,5-
diamine (12.9 g, approx. 28 mmol) in acetonitrile (100 ml) is added butyl
nitrite
(8.64 ml, 70.2 mmol). The solution is heated to 80 C and stirred at this
temperature for 2 hours. The reaction mixture is cooled to room temperature
and concentrated under reduced pressure. The residue is chromatographed
on a silica gel column with dichloromethane/methanol as eluent to afford 6-(5-
chloro-[1,2,3]triazolo[4,5-d]pyrimidin-3-y1)-2-methyl-quinoline as light
yellow
solid; HPLC/MS 1.61 min (B), [M+H] 297;
1H NMR (400 MHz, DMSO-d6, TFA-d1) 6 [ppm] 9.93 (s, 1H), 9.34 (d, J = 8.6
Hz, 1H), 9.12(d, J = 2.3 Hz, 1H), 8.90 (dd, J- 9.2, 2.3 Hz, 1H), 8.59(d, J=
9.2 Hz, 1H), 8.14 (d, J = 8.6 Hz, 1H), 3.04 (s, 3H).
To a solution of 6-(5-chloro-[1,2,3]triazolo[4,5-d]pyrimidin-3-y1)-2-methyl-
quinoline (1.169, 4.0 mmol) in 2-methoxyethanol (18 ml) is added (1R,3R)-
3-amino-cyclopentanecarboxylic acid hydrochloride (729 mg, 4.4 mmol) and
N-ethyldiisopropylamine (1.36 ml, 8.0 mmol). The mixture is heated to 80 C
and stirred at this temperature for 3 days. The reaction mixture is cooled to
room temperature concentrated. The residue is chromatographed on a silica
gel column with methanol/dichloromethane as eluent to afford (1R,3R)-3-[3-
(2-methyl-quinolin-6-y1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 176 -
cyclopentanecarboxylic acid as light orange solid; HPLC/MS 1.52 min (B),
[M+H} 390.
In a reaction flask, to (1R,3R)-343-(2-methyl-quinolin-6-y1)-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-cyclopentanecarboxylic acid (845
mg, 2.17 mmol) are added N-(3-dimethylaminopropy1)-N'-ethylcarbodiimide
hydrochloride (832 mg, 4.34 mmol), 1-hydroxybenzotriazole hydrate (295
mg, 2.18 mmol) and a 0.5M solution of ammonia in dioxane (22 ml, 11
mmol ammonia). The resulting suspension is stirred for 18 hours at room
temperature. The reaction mixture is concentrated and the residue is
chromatographed on a silica gel column with dichloromethane/methanol as
eluent to afford (1R,3R)-3-[3-(2-methyl-quinolin-6-y1)-3H-[1,2,3]tr1azo1o[4,5-
d]pyrimidin-5-ylamino]-cyclopentanecarboxylic acid amide as light orange
amorphous solid; HPLC/MS 1.41 min (B), [M+H] 389;
1H NMR (400 MHz, DMSO-d6, TFA-d1) 6 [ppm] 9.34 (m, 3H), 9.19 ¨ 9.04
(m, 1H), 8.51 (d, J = 9.3 Hz, 1H), 8.11 (d, J = 8.7 Hz, 1H), 4.56 (m, 1H),
2.95
(p, J= 8.0 Hz, 1H), 2.40 (m, 1H), 2.13 (m, 2H), 1.91 ¨1.67 (m, 3H).
The following compounds are prepared analogously:
(1S,3R)-3-(3-quinolin-6-y1-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-
cyclopentanecarboxylic acid ("A188")
OH
0
N
N
HPLC/MS 1.64 min (B), [M+H] 376;
(1S,3R)-3-(3-quinolin-6-y1-3H41,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-
cyclopentanecarboxylic acid amide ("A189")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 177 _
NH2
HPLC/MS 1.55 min (B), [M+H] 375;
1H NMR (400 MHz, DMSO-d6) 6 [ppm] = 9.29 (s, 1H), 9.00 (dd, J=4.2, 1.7,
1H), 8.86 (d, J=2.4, 1H), 8.67 - 8.60 (m, 1H), 8.58 - 8.52 (m, 1H), 8.38 (d,
J=6.9, 1H), 8.29 (d, J=9.1, 1H), 7.71 -7.63 (m, 1H), 7.39 - 7.30 (m, 1H),
6.87 - 6.76 (m, 1H), 4.39 - 4.28 (m, 1H), 2.79 - 2.71 (m, 1H), 2.29 - 2.19 (m,
1H), 2.06- 1.96 (in, 1H), 1.90- 1.73 (m, 4H);
(1R,3R)-3-(3-quinolin-6-0-3H41,2,31triazolo[4,5-d]pyrimidin-5-y(amino)-
cyclopentanecarboxylic acid amide ("A190")
o
N
1.µ
NH2 N\
NN
HPLC/MS 1.55 min (B), [M+H] 375;
1H NMR (400 MHz, DMSO-c16) 6 [ipPm] = 9.30 (s, 1H), 9.03 - 8.93 (m, 2H),
8.68 (dd, J=9.2, 2.5, 1H), 8.60 (d, J=8.3, 1H), 8.36 (d, J=6.5, 1H), 8.28 (d,
J=9.3, 1H), 7.70 - 7.62 (m, 1H), 7.34 - 7.21 (m, 1H), 6.80 - 6.66 (m, 1H),
4.47 - 4.33 (m, 1H), 2.89 - 2.77 (m, 1H), 2.32 - 1.53 (m, 6H);
(1S,3R)-343-(2-methyl-quinolin-6-y1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminol-cyclopentanecarboxylic acid amide ("A191")
0
NH2
HPLC/MS 1.47 min (B), [M+H] 389;
1H NMR (400 MHz, DMSO-d6, TFA-d1) 6 [ppm] = 9.32 (s, 1H), 9.26 (m, 2H),
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 178 -
9.08 (d, J = 9.4 Hz, 1H), 8.51 (d, J 9.3 Hz, 1H), 8.11 (d, J= 8.7 Hz, 1H),
4.48
(bs, 1H), 3.04 (s, 3H), 2.87 (p, J = 7.5 Hz, 1H), 2.28 (dt, J = 13.3, 6.4 Hz,
1H),
1.91 (m, 5H);
(trans)-343-(2-methyl-quinolin-6-y1)-3H41,2,31triazolo[4,5-dipyrimidin-5-
ylaminol-cyciohexanecarboxylic acid amide ("A192")
0 -N
NH2 NN
N"--N
HPLC/MS 1.95 min (C), [M+1-1] 403;
(1S,3S)-343-(2-methyl-quinolin-6-y1)-3H11,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminoFcyclopentanecarboxylic acid amide ("Al 93")
0
)r-N
NH2 NI,
1\l'N
HPLC/MS 1.44 min (B), [M+1-1] 389;
(1R,3R)-3-(311,5]naphthyridin-2-y1-3H41,2,3]triazolo[4,5-d]pyrimid in-5-
ylamino)-cyclopentanecarboxylic acid amide ("Al 94")
0õ.
\
NH2 N
\¨
HPLC/MS 1.91 min (C), [M+H] 376;
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 179 -
(1R,3R)-343-(1-methy1-1H-indazol-5-y1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino]-cyclopentanecarboxylic acid amide ("A195")
ONN
0 f's
NH2 N
HPLC/MS 1.52 min (B), [M+111378;
(1R,3R)-34[3-(7-methy1-3-quinolyptriazolo[4,5-d]pyrimidin-5-yl]aminolcyclo-
pentanecarboxamide ("Al 95a")
H2N õ0-411
N
(1R ,3R)-31[3-(2-methy1-1 -oxo-6-isoquinolyptriazolo[4,5-d]pyrimidin-5-
yl]amino]-
cyclopentanecarboxamide ("Al 95b")
H2Nfl( 1\(-
0
0
N-=-N
Example 21
Synthesis of (1R,3R)-3-[3-(4-iodo-pheny1)-3H-[1,2,31triazolo[4,5-dipyrimidin-5-
ylamino]-cyclopentanol ("A196") and (1S,3S)-343-(4-iodo-pheny1)-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-cyclopentanol ("Al 97")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 180 -
I
40 HO,
HO,
y x HCI
NH2 DIPEA HN
CH3OCH2CH2OH
(racemic 80 C
mixture)
(racemic
mixture)
chiral separation
HO HO
HNN N HNNN
II N II
N
To a solution of 5-chloro-3-(4-iodo-phenyI)-3H-[1,2,3]triazolo[4,5-
d]pyrimidine
(2.36 g, 6.6 mmol) in 2-methoxyethanol (30 ml) is added trans-3-amino-
cyclopentanol hydrochloride (racemic mixture; 1.0 g, 7.3 mmol) and N-
ethyldiisopropylamine (2.47 ml, 14.5 mmol). The mixture is heated to 80 C
and stirred at this temperature for 90 minutes. The reaction mixture is cooled
to room temperature and water is added. The resulting precipitate is filtered
off, washed with water and dried under vacuum to afford (trans)-3-[3-(4-iodo-
phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-cyclopentanol (racemic
mixture) as beige solid; HPLC/MS 1.94 min (B), [M+H] 423;
1H NMR (400 MHz, DMSO-d6, TFA-d1) 6 [ppm] 9.26 (s, 1H), 8.16 - 8.05 (m,
2H), 8.06 - 7.96 (m, 2H), 4.51 (bs, 1H), 4.32 (tt, J = 6.0, 3.2 Hz, 1H), 2.24
(dt,
J- 12.6, 7.6 Hz, 1H), 2.11 - 1.93 (m, 2H), 1.81 (dt, J = 13.3, 6.6 Hz, 1H),
1.61
(ddt, J = 12.2, 8.1, 5.0 Hz, 2H).
(trans)-3-[3-(4-lodo-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-
cyclopentanol is separated by chiral chromatography on a chiral column with
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 181 -
Lux-Amylose-2 as stationary phase and heptanefethanol 8 : 2 as mobile
phase to afford two enantiomers. Both enantiomers are beige solids. LC/MS
data and NMR data are identical to those of the racemic mixture.
The following compounds are prepared analogously:
(trans)-343-(4-pyrrolidin-1-yl-phenyl)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminol-cyclopentanol ("A198")
H 0
11>"
N 14111
N\
HPLC/MS 1.91 min (B), [M+H] 366;
(trans)-343-(2-methyl-benzoxazol-6-y1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminol-cyclopentanol ("A199")
HO
O\
N
HPLC/MS 2.17 min (C), [M+111352;
(trans)-3-(3-benzo[1,2,5]thiadiazol-5-y1-3H-0,2,31triazolo[4,5-d]pyrimidin-5-
ylamino)-cyclopentanol ("A200")
N-S=
H 0 I N
N
,
¨
HPLC/MS 2A0 min (C), [M-1-H] 355;
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 182 -
(1R,3R)-343-(4-methoxy-phenyly3H41,2,3]triazolo[4,5-d]pyrimidin-5-ylaminoy
cyclohexanol ("A201")
HO 0¨
NN
N 411
HPLC/MS 1.79 min (C), [M+H] 341; 1H NMR (400 MHz, DMSO-d5, TFA-d1) 5
[ppm] 9.33 (s, 1H), 8.09 (d, J = 8.5 Hz, 2H), 7.24 ¨ 7.16 (m, 2H), 4.31 (bs,
1H),
4.05 (bs, 1H), 3.88 (s, 3H), 1.92 (m, 1H), 1.85¨ 1.19 (m, 7H);
(trans)-3-[3-(2-methyl-quinolin-6-yI)-3H-{1,2,3]triazolo[4 ,5-d]pyrimidin-5-
ylaminoycyclopentanol ( A202")
HO
kc_N
N\__R
N'N
HPLC/MS 1.48 min (B), [M+H] 362;
(1R,3R)-3-{344-(2-hydroxy-ethoxy)-pheny11-3H41,2,3]triazolo[4,5-dipyrimidin-5-
ylaminoycyclopentanol ("A203")
HO
¨ LI \\
N
N
HPLC/MS 1.53 min (B), [M+H] 357;
(1R,3R)-3-(3-quinolin-6-y1-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminoy
cyclopentanol ("A204")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 183 -
HO /
N
N\
NN
HPLC/MS 1.54 min (B), [M+11] 348;
(trans)-3-{3444(S)-3,3,3-trifluoro-2-hydroxy-propoxy)-phenyl]-3H-
[1,2,31triazolo[4,5-d]pyrimidin-5-ylamino}-cyclopentanol ("A205")
HO">N
OH
=
Nr-N
HPLC/MS 1.73 min (B), [M+1-1] 425;
in-5-ylamino]-
("A206")
HO'
= "
NI\
-
NN
HPLC/MS 1.76 min (B), [M+H] 342; 1H NMR (400 MHz, DMSO-d6) 8 PPm
9.36 - 9.20 (m, 1H), 8.93 - 8.76 (m, 1H), 8.41 - 8.25 (m, 1H), 8.25 - 7.93 (m,
1H), 7.08 (d, J=8.9, 1H), 4.55 - 4.46 (m, 1H), 4.46 - 4.32 (m, 3H), 4.28 -
4.17
(m, MI), 2.22 - 2.02 (m, 1H), 2.02- 1.83 (m, 2H), 1.83- 1.62 (m, 1H), 1.60 -
1.43 (m, 2H), 1.37 (t, J=7.0, 3H);
(trans)-343-(4-ethoxy-pheny1)-3H-0,2,3]thazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopentanol ("A207")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 184 -04\11
HO''s )i-N 1411
N\
HPLC/MS 2.52 min (C), [M+H] 341; 1H NMR (400 MHz, DMSO-d6, TFA-di) 6
[ppm] 9.28 (s, 1H), 8.05 (d, J = 8.5 Hz, 2H), 7.18 (d, J- 9.1 Hz, 2H), 4.50
(bs,
1H), 4.30(11, J= 6.0, 3.2 Hz, 1H), 4.15 (q, J= 6.9 Hz, 2H), 2.30 -2.13 (m,
1H), 1.99 (m, 2H), 1.80 (dt, J = 13.4, 6.7 Hz, 1H), 1.69 - 1.50 (m, 2H), 1.40
(t,
J = 7.0 Hz, 3H);
(1R,3R)-313-(2-methyl-quinolin-6-y1)-3H-[1,2,3]triazoIo[4,5-d]pyrimidin-5-
ylaminol-cyclopentanol ("A208")
HO"'
N'N
HPLC/MS 1.48 min (B), [M+H] 362;
(trans)-3-[3-(2-methyl-quinazolin-6-yI)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminol-cyclopentanol ("A209")
1,)-4
HO
Nq-N
HPLC/MS 1.33 min (B), [M+H] 363; 1H NMR (400 MHz, DMSO-d6) 6 ppm
9.67 - 9.64 (m, 1H), 9.28 (s, 1H), 9.04 - 8.97 (m, 1H), 8.89- 8.81 (m, 1H),
8.37 - 8.29 (m, 1H), 8.18 (d, J9.1, 1H), 4.68 -4.54 (m, 1H), 4.53 -4.44 (m,
1H), 4.31 -4.21 (m, 1H), 2.83 (s, 3H), 2.25 -2.02 (m, 2H), 2.01 -1.88 (m,
1H), 1.82 - 1.69 (m, 1H), 1.67 - 1.45 (m, 2H);
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 185 -
(trans)-3-[3-(4-isopropoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylaminoj-
cyclopentanol ("A210")
'CD-`1E\IL
HO 0
//--N
141111)
N
HPLC/MS 1.87 min (B), [M+111 355.
Example 22
Synthesis of (1R,3R)-3-(3-{441-(2-ethoxy-ethyl)-1H-pyrazol-4-y11-phenyl}-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A211")
0
0
do\----NH2
N
N-N
PdC12(dppf)
K2CO3 NH2
HN 0- dioxane/water
13-
L 80 C
i x 0 HNNN
II N
To a solution of (1R,3R)-3-[3-(4-iodo-phenyl)-3H11,2,3]triazo10[4,5-
d]pyrimidin-
5-ylamino]-cyclopentanecarboxylic acid amide (314 mg, 0.70 mmol) and 1-(2-
ethoxy-ethyl)-4-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yI)-1H-pyrazole
(242
mg, 0.91 mmol) in dioxane (2 ml) are added water (0.2 ml) and potassium
carbonate (290 mg, 2.10 mmol). The mixture is flushed with nitrogen and
heated to 80 C. Dichloro[1,11-bis(diphenylphosphino)ferrocenelpalladium(11)
dichloromethane adduct (57 mg, 0.07 mmol) is added and the mixture is stirred
in a closed reaction vial for 18 hours at 80 C. The reaction mixture is
allowed
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 186 -
to reach room temperature and treated with water. The resulting precipitate is
filtered off, washed with water and dried under vacuum. It is chromatographed
on a silica gel column with dichloromethane/methanol as eluent to afford
(1R,3R)-3-(344-0-(2-ethoxy-ethy1)-1H-pyrazol-4-y11-phenyl}-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
as
beige solid; HPLC/MS 2.33 min (C), [M+H] 462; 1H NMR (500 MHz, DMSO-d6,
TFA-d1) 6 [ppm] 9.30 (s, 1H), 8.26 (m, 3H), 8.06 (s, 1H), 7.90¨ 7.84 (m, 2H),
4.50 ¨4.42 (m, 1H), 4.36 (t, J = 5.4 Hz, 2H), 3.82 (t, J = 5.4 Hz, 2H), 3.49
(q, J
= 7.0 Hz, 2H), 2.94 (p, J = 8.0 Hz, 1H), 2.28 ¨ 2.12 (m, 1H), 2.06 (dtd, J =
11.2,
7.4, 6.8, 4.1 Hz, 1H), 1.95 (ddd, J = 13.3, 8.7, 4.2 Hz, 1H), 1.79 (m, 2H),
1.12
(t, J = 7.0 Hz, 3H).
The following compounds are prepared analogously:
(1R,3R)-3-(3-{441-(2-methoxy-ethyl)-1H-pyrazol-4-yll-phenyll-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanol ("A212")
HO \o
NH N,
N
N
HPLC/MS 1.67 min (B), [M+H] 421;
(1R,3R)-3-(3-{441-(2-pyrrolidin-1-yl-ethyl)-1H-pyrazol-4-yll-phenyl}-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanol ('A213")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 187 -
HO
i\C71-)
N /
5N 7N
HPLC/MS 1.40 min (B), [M+H1460;
3-(44445-(trans-3-hydroxy-cyclopentylamino)[1,2,3]triazolo[4,5-d]pyrimidin-3-
yll-phenyl}-pyrazol-1-y1)-propionitrile ("A214")
HO
CN
N ____________________________________________ '
N
N'N
HPLC/MS 1.65 min (B), [M+H} 416;
N-(3-{441-(2-pyrazol-1-yl-ethyl)-1H-pyrazol-4-ylypheny1}-3H11,2,3]triazolo[4,5-
d]pyrimidin-5-y1)-cyclohexane-1,4-diamine ("A215")
H2N.,0
N-N
/
N r N N
yNN
N=N1
HPLC/MS 1A4 min (B), [M+H] 470;
(1S,3R)-3-(3-{441-(2-pyrrolidin-1-yl-ethyl)-1H-pyrazol-4-A-pheny1}-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A216")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 188-
o
NH2
/-N
C.7"1-1\11\
1\1,
HPLC/MS 1.81 min (C), [M+111487;
(1R,3R)-3-(3-{411-(2-cyano-ethyl)-1H-pyrazol-4-q-phenyll-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A217")
______________________________________________________ N
N
NH2 N.
N'N
HPLC/MS 2.20 min (C), [M+H1443;
(1R,3R)-3-(3-{411-(2-methoxy-ethyl)-1H-pyrazol-4-01-pheny1}-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A218")
NH2
¨ N
N=N
HPLC/MS 2.23 min (C), [M+H] 448;
(1R,3R)-3-(3-{541-(2-methoxy-ethyl)-1H-pyrazol-4-01-pyridin-2-01-3H-
[1,2,3]tr1azo1o[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A219")
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
¨ 189¨
/
=O'N ______________________________________________ / 0
N
NH2
N
N=N
HPLC/MS 1.96 min (C), [M+H] 449;
(1R,3R)-343-(4-{142-(5-methy141,2,4]oxadiazol-3-y1)-ethyl]-1H-pyrazol-4-y1}-
phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-cyclopentanecarboxylic
acid amide ("A220")
N,0
_Ns /
N
0 .0-41\
NH2 Nv4,_
N,
HPLC/MS 1.65 min (B), [M+H] 500;
(1R,3R)-3-(3-{3-fluoro-441-(2-methoxy-ethyl)-1H-pyrazol-4-yll-phenyl}-3H-
[1,2,3]triazolo[4,5-dipyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A221")
0N
Ys' _Ns
H2N
y
HPLC/MS 2.35 min (C), [M+H] 466;
(1R,3R)-3-(3-{341-(2-methoxy-ethyl)-1H-pyrazol-4-y1]-phenyll-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)--cyclopentanecarboxylic acid amide
("A222")
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- -190
0
N
Nj
NH2 N\
¨N
HPLC/MS 1.66 min (B), [M+H] 448;
(1R,3R)-3-(3-{4-[1-(2-ethoxy-ethyl)-1H-pyrazol-4-y1]-3-fluoro-pheny11-3H-
[1,2,31tr1az0io[4,5-d]pyrimidin-5-y(amino)-cyclopentanecarboxylic acid amide
("A223")
NH2 N
-
HPLC/MS 2.46 min (C), [M+H] 480;
(1R,3R)-3-(3-{641-(2-methoxy-ethyl)-1H-pyrazol-4-y1]-pyridin-3-y11-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A224")
0 .04
NH 2 NI, N
NN
HPLC/MS 2.07 min (C), [M+HI 449;
(1R,3R)-3-(3-{441-(2-ethoxy-ethyl)-1H-pyrazol-4-yll-pheny1}-3H-
= [1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid (2-
methoxy-ethyl)-amide ("A225")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 191
NH
N
/
y,
N=-14
HPLC/MS 2.45 min (C), [M+H] 520; 1H NMR (500 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.31 (s, 1H), 8.27 (m, 3H), 8.06 (s, 1H), 7.87 (d, J = 8.3 Hz, 2H), 4.46
(bs,
1H), 4.37 (t, J = 5.3 Hz, 2H), 3.82 (t, J = 5.3 Hz, 2H), 3.49 (q, J = 7.0 Hz,
2H),
3.39 (t, J = 5.6 Hz, 2H), 3.30 (t, J = 5.6 Hz, 2H), 3.27 (s, 3H), 2.93 (p, J =
8.0
Hz, 1H), 2.19 (m, 2H), 2.04 (dt, J = 11.8, 7.9 Hz, 1H), 1.93 (ddd, J = 13.5,
8.3,
4.9 Hz, 1H), 1.84¨ 1.67 (m, 2H), 1.13 (t, J= 7.0 Hz, 3H);
(1R,3R)-3-(3-{411-(2-ethoxy-ethyl)-1H-pyrazol-4-A-pheny11-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid (2-
hydroxy-ethyl)-amide ("A226")
o
H
N N
/
HO NH N
y\N
Ni=N1
HPLC/MS 1.69 min (B), [M+H] 506;
(1R,3R)-3-(3-{441-(2-ethoxy-ethyl)-1H-pyrazol-4-A-pheny1}-3H-
. [1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanol ("A227")
HO "µ0"NH
N N
N=N
HPLC/MS 1.73 min (B), [M+H] 435; 1H NMR (400 MHz, DMSO-d6) 6 [PPm] =
9.36 - 9.19 (m, 1H), 8.27 (s, 1H), 8.24 - 8.14 (m, 2H), 8.14 - 8.04 (m, 1H),
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 192 -
8.00 (s, 1H), 7.89 - 7.77 (m, 2H), 4.64 - 4.49 (m, 1H), 4.48 - 4.36 (m, 1H),
4.34 - 4.18 (m, 3H), 3.78 (t, J=5.4, 2H), 3.45 (q, J=7.0, 2H), 2.25 -2.06 (m,
1H), 2.02- 1.86 (m, 2H), 1.85 - 1.66 (m, 1H), 1.63- 1.44 (m, 2H), 1.08 (t,
J=7.0, 3H);
(1R,3R)-3-(3-{3-fluoro-441-(2-methoxy-ethyl)-1H-pyrazol-4-y1]-pheny1}-3H-
[1,2,31triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanol ("A228")
0¨
/
HO' 11
".'NH
,N(N
NJ'
N=N
HPLC/MS 1.75 min (B), [M-F.H] 439;
(1R,3R)-3-(3-{641-(2-ethoxy-ethyl)-1H-pyrazol-4-y11-pyridin-3-y1}-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A229")
õ.
NH
H2N
y
N
v N N
N N
N=N
HPLC/MS 2.18 min (C), [M-FH] 463;
(1R,3R)-3-(3-{441-(2-methoxy-ethyl)-1H-pyrazol-4-y1]-3-methyl-pheny1}-3H-
[1,2,3]triazolo[4,5-djpyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A230")
0 041 _Ns
NH2
N
Nr=--11
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 193 -
HPLC/MS 1.70 min (B), [M+H] 462;
(1R,3R)-3-{344-(1-pyridin-3-ylmethy1-1H-pyrazol-4-y1)-pheny1]-3H-
[1,2,3]triazolo[4,5-cl]pyrimidin-5-ylaminoycyclopentanol ("A231")
HO".0411\
HPLC/MS 1.50 min (B), [M+H] 454;
(1R,3R)-3-(3-{441-(2-hydroxy-2-methyl-propy1)-1H-pyrazol-4-y1J-pheny11-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanol ("A232")
,:NsNyoH
HO
/7--N
NN
HPLC/MS 1.86 min (A), [M+H} 435; 1H NMR (400 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.29 (s, 1H), 8.28 (d, J = 0.8 Hz, 1H), 8.24 (d, J = 8.5 Hz, 2H), 8.10
(d,
J = 0.8 Hz, 1H), 7.89 (d, J = 8.7 Hz, 2H), 4.54 (bs, 11-1), 4.33 (if, J = 5.8,
3.1
Hz, 1H), 4.15 (s, 2H), 2.25 (q, J = 7.0, 6.6 Hz, 1H), 2.13¨ 1.92 (m, 2H), 1.83
(dt, J= 13.4, 6.6 Hz, 1H), 1.70¨ 1.53 (m, 2H), 1.17 (s, 6H);
(1R,3R)-3-(3-{4-[1-(2-hydroxy-2-methyl-propy1)-1H-pyrazol-4-0]-phenyl}-3H-
[1,2,3]triazolo[4,5-djpyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A233")
0 ..1:1)¨=N yOH
N
NH2
N
N=-N
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 194 -
HPLC/MS 2.17 min (C), [M+H] 462;
(trans)-3-(3-(641-(2-methoxy-ethyl)-1H-pyrazol-4-y1]-pyridin-3-y11-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanol ("A234")
04Iv
N N
Ni="1`1
HPLC/MS 1.62 min (B), [M+H] 422;
(1R,3R)-3-(34441-(2-methoxy-ethyl)-1H-pyrazol-4-A-phenyl}-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid (1-
methyl-piperidin-4-ylmethyl)-amide ("A235")
N
\
N----/
N
0 N
"A235" formate salt: HPLC/MS 1.49 min (B), [M+H] 559; 1H NMR (500 MHz,
DMSO-d6) 8 [ppm] = 9.25 (s, 1H), 8.30 - 8.18 (m, 3H), 8.16 (s, 1H), 7.99 (s,
1H), 7.88 - 7.75 (m, 3H), 4.52 - 4.32 (m, 1H), 4.30 (t, J=5.3, 2H), 3.74 (t,
J=5.3, 2H), 3.26 (s, 3H), 2.94 (t, J=6.3, 2H), 2.90 - 2.77 (m, 3H), 2.29 -
2.18
(m, 3H), 2.14- 1.90 (m, 5H), 1.90 - 1.82 (m, 1H), 1.73 - 1.64 (m, 2H), 1.64 -
1.56 (m, 2H), 1.44 - 1.33 (m, 1H), 1.21 -1.09 (m, 2H);
212-(4-{4454(1R,3R)-3-hydroxy-cyclopentylamino)41,2,3]triazolo[4,5-d]-
pyrimidin-3-yil-phenyl}-pyrazol-1-y1)-ethyl]-2H-pyridazin-3-one ("A236")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 195 -
0
HO
N N
11
HPLC/MS 1.61 min (B), [M+H] 485;
(1R,3R)-3-[3-(4-{1-[2-(6-oxo-6H-pyridazin-1-y1)-ethy1]-1H-pyrazol-4-y1}-
pheny1)-
3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-cyclopentanecarboxylic acid
amide ("A237")
0
_Ns
N
'N-
NH2 NJj
N=N1
HPLC/MS 1.60 min (B), [M+H] 512; 1H NMR (500 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.29 (s, 1H), 8.23 (m, 3H), 7.99 (s, 1H), 7.87 ¨7.77 (m, 3H), 7.38 (dd,
J =
9.4, 3.8 Hz, 1H), 6.95 (dd, J = 9.5, 1.6 Hz, 1H), 4.65 ¨4.58 (m, 2H), 4.58
¨4.51
(m, 2H), 4.41 (bs, 1H), 2.90 (p, J = 8.0 Hz, 1H), 2.16 (m, 2H), 2.03 (dtd, J =
10.7, 7.2, 6.3, 4.0 Hz, 1H), 1.92 (m, 1H), 1.78 (m, 2H);
(1R,3R)-3-(3-{541-(2-ethoxy-ethyl)-1H-pyrazol-4-y11-pyridin-2-y1}-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A238")
H2N 0õ,,H N
0 N
11 N
N=-14
HPLC/MS 1.50 min (B), [M+H] 463;
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 196 -
(1R,3R)-3-(3-{511-(2-cyano-ethyl)-1H-pyrazol-4-y11-pyridin-2-y1}-3H-
[1,2,3}triazolo[4,5-d}pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A239")
H2N
7--CN
0
N
N==-N
HPLC/MS 1.42 min (B), [M+H] 444;
(1R,3R)-34[3-(4-pyrimidin-5-ylphenyl)triazolo[4,5-d]pyrimidin-5-yl]aminoicyclo-
pentanecarboxamide ("A239a")
H2N
N
0
1\1=-1q
(1R,3R)-34[314-(6-methy1-3-pyridyl)phenyl]triazolo[4,5-d]pyrimidin-5-
yl]aminoicyclopentanecarboxamide ("A239b")
NI
H2N
0
Nq¨N
=
(1R,3R)-34[3444142-methoxy-1-(methoxymethypethylipyrazol-4-yl]phenyl]-
triazolo[4,5-d]pyrimidin-5-yliaminoicyclopentanecarboxamide ("A239c")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 197 -
o/
0 Nq_N 0
N=N =
(1R,3R)-3-[[344-(5-methylpyrimidin-2-yl)phenyl]triazolo[4,5-d]pyrimidin-5-
yliamino]cyclopentanecarboxamide ("A239d")
H2N
0
N\NN
4"N
(1R,3R)-34[34541-(2-pyrazol-1-ylethyl)pyrazol-4-y1]-2-pyridyntriazolo[4,5-
d]pyrimidin-5-yl]aminolcyclopentanecarboxamide ("A239e")
H2N
0
I
N
Example 23
Synthesis of (1R,3R)-3-[3-(4-ethoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-
5-ylamino]-cyclopentanecarboxylic acid (3-hydroxy-3-methyl-butyl)-amide
("A240")
35
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 198 -
HO
0
0 o_J
0-1
j--OH
410 HO
EDC1
HNNN sN H2N HOBYTHF
HN N N
N¨NI sN
To a solution of (1R,3R)-343-(4-ethoxy-phenyl)-3H-[1,2,3}triazolo[4,5-
d]pyrimidin-5-ylamino]-cyclopentanecarboxylic acid (184 mg, 0.50 mmol) in THE
(2 ml) are added 4-amino-2-methyl-butan-2-ol (103 mg, 1.00 mmol), N-(3-
dimethylaminopropy1)-N'-ethylcarbodiimide hydrochloride (144 mg, 0.75 mmol)
and 1-hydroxybenzotriazole hydrate (76.5 mg, 0.50 mmol) The reaction mixture
is stirred for 4 hours at room temperature. The reaction mixture is filtered.
The
filtrate is concentrated and the residue is chromatographed on a silica gel
column with dichloromethane/methanol as eluent to afford (1R,3R)-3-[3-(4-
ethoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-cyclopentane-
carboxylic acid (3-hydroxy-3-methyl-butyl)-amide as light yellow solid;
HPLC/MS
1.80 min (B), [M+H] 454;
1H NMR (500 MHz, DMSO-d6, TFA-d1) 6 [ppm] 9.27 (s, 1H), 8.08 (bs, 2H), 7.28
¨7.03 (m, 2H), 4.37 (bs, 1H), 4.14 (q, J = 7.0 Hz, 2H), 3.24¨ 3.08 (m, 2H),
282 (p, J = 8.0 Hz, 1H), 2.10 (m, 2H), 2.03 ¨ 1.91 (m, 1H), 1.91 ¨ 1.80 (m,
1H),
1.78 ¨ 1.62 (m, 2H), 1.60 ¨ 1.52 (m, 2H), 1.40 (t, J = 7.0 Hz, 3H), 1.12 (s,
6H).
The following compounds are prepared analogously:
{(1R,3R)-343-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-
cyclopenty1)-(4-methyl-piperazin-1-y1)-methanone ("A241")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 199 -
0,
`NyTh
0
"A241" formate salt HPLC/MS 1.47 min (B), [M+H} 437;
(1S,3R)-3-(3-quinolin-6-y1-3H-[1,2,31triazolo[4,5-d]pyrimidin-5-ylamino)-
cyclopentanecarboxylic acid [2-(4-methyl-piperazin-1-y)ethyll-amide ("A242")
NH
r- NH
r\N¨I N N
N I
N=N
HPLC/MS 1.37 min (B), [M+H] 501;
(1R,3R)-343-(4-methoxy-pheny1)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopentanecarboxylic acid dimethylamide ("A243")
411 0¨,
0 == N
N N\
N'N
HPLC/MS 1.81 min (B), [M+H] 382;
(1R,3R)-343-(4-methoxy-pheny1)-3H-[1,2,3]triazolo[4,5-d]oyrimidin-5-ylaminol-
cyclopentanecarboxylic acid [2-(4-methyl-piperazin-1-y1)-ethyl]-amide ("A244")
0
N=N
HPLC/MS 1.46 min (B), [M+H] 480;
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 200 -
(1R,3R)-343-(2-methyl-quinolin-6-y1)-3H41,2,31triazolo[4,5-d]pyrimidin-5-
ylamino]-cyclopentanecarboxylic acid (2-hydroxy-ethyl)-amide ("A245")
0 0,,111
....
HON.- NH N 1N
N-r-N
HPLC/MS 1.40 min (B), [M+H] 433; 1H NMR (500 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.43 (d, J = 8.7 Hz, 1H), 9.36 (s, 1H), 9.29 (s, 1H), 9.05 (d, J = 9.0
Hz,
1H), 8.46 (d, J = 9.3 Hz, 1H), 8.09 (d, J = 8.7 Hz, 1H), 4.53 (p, J = 7.0 Hz,
1H),
3.42 (t, J= 6.0 Hz, 2H), 3.19 (t, J = 6.1 Hz, 2H), 3.00 (s, 3H), 2.89 (m 1H),
2.35
(m, 1H), 2.18¨ 1.96 (m, 2H), 1.86¨ 1.59 (m, 311);
(1R,3R)-343-(2-methyl-quinolin-6-y1)-3H-[1,2,31triazolo[4,5-d]pyrimidin-5-
ylamino]-cyclopentanecarboxylic acid (2-methoxy-ethyl)-amide ("A246")
0 .0-411
N
No----Nõ NH NN
HPLC/MS 1.50 min (B), [M+H] 447;
(1R,3R)-343-(4-ethoxy-pheny1)-3H-0,2,31triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopentanecarboxylic acid (2-hydroxy-ethyl)-amide ("A247")
0 µH
NH
HO-)
N=N
HPLC/MS 1.73 min (B), [M+H] 412;
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 201 -
(1R,3R)-343-(4-ethoxy-pheny1)-3H11,2,31triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopentanecarboxylic acid (2-methoxy-ethyl)-amide ("A248")
0 õO.,
NH
j- NH /L-N
0
!\I
N-=-N
HPLC/MS 1.85 min (B), [M+H] 426;
(1R,3R)-343-(4-ethoxy-pheny1)-3H11,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopentanecarboxylic acid (1-methyl-piperidin-4-ylmethyl)-amide ("A249")
0NH
N' N
,LyL 0
N=14
"A249" formate salt:
HPLC/MS 2.02 min (C), [M+H] 479; 1H NMR (500 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.29 (s, 1H), 8.12 (s, 1H), 8.08 (bs, 2H), 7.18 (d, J= 9.0 Hz, 2H), 4.42
(bs, 1H), 4.15 (q, J = 7.0 Hz, 2H), 3.52- 3.41 (m, 2H), 3.21 (m, 1H), 3.10-
2.99 (m, 2H), 2.91 (m, 3H), 2.77 (s, 3H), 2.15 (m, 2H), 2.03 (m, 1H), 1.96-.
1.81 (m, 3H), 1.81 - 1.62 (m, 4H), 1.41 (t, J = 7.0 Hz, 3H);
(1R,3R)-3-[3-(2-methyl-quinolin-6-yI)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino]-cyclopentanecarboxylic acid [2-(4-methyl-piperazin-1-y1)-ethy1]-amide
("A250")
0
NH
N "
-f"
--Nr"\N
N=N
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 202 -
"A250" formate HPLC/MS 1.40 min (B), [M+HJ 516;
(1R,3R)-343-(4-ethoxy-pheny1)-3H11,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-
cyclopentanecarboxylic acid (2-hydroxy-2-methyl-propyI)-methyl-amide
("A251")
)issµ 0
NN
HPLC/MS 2.63 min (C), [M+ Fl] 454;
(1R,3R)-3-[3-(2-methyl-quinolin-6-y1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylamincd-cyclopentanecarboxylic acid (1-methyl-piperidin-4-ylmethyp-amide
("A252")
H JO_
N
0
"A252" formate salt HPLC/MS 1.34 min (B), [M+H] 500;
(1R,3R)-3-[3-(2-methyl-quinolin-6-y1)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino]-cyclopentanecarboxylic acid (3-hydroxy-3-methyl-buty1)-amide
("A253")
HO
N
NI
0
Nr-N
HPLC/MS 1.55 min (B), [M+HJ 475;
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 203 -
(1R,3R)-343-(2-methyl-quinolin-6-y1)-3H-[1,2,31triazolo[4,5-d]pyrimidin-5-
ylamino]-cyclopentanecarboxylic acid ((trans)-3-hydroxy-cyclopentyI)-amide
("A254")
psµNr.o.,
0
HO
WAN!
HPLC/MS 1.41 min (B), [M+11] 473;
(1R,3R)-34[3-(2-methyl-6-quinolyl)triazolo[4,5-d]pyrimidin-5-yl]amino]-N-(2-
morpholinoethyl)cyclopentanecarboxamide ("A254a")
N
\
0¨N N
N
H N
0N,N
Example 24
-Synthesis of (1R,3R)-3-(3-1441-(2-hydroxy-ethyl)-1H-pyrazol-4-ylkpheny1}-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A255")
35
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 204 -
c
0
0
PdC12(dppf)
N'NHN NN
N'N
+ ).õ)1 K2CO3 \ I
11 :11 dioxane/water NH
N 0-Bo 80 C 2
HO
HNyNN
N N''
N-N
0 \
4 N HCI in dioxane d_NH2
CH2C12/Me0H
N
11 st\t
To a solution of (1R,3R)-343-(4-iodo-phenyl)-3H41,2,3]triazolo[4,5-d]pyrimidin-
5-ylaminol-cyclopentanecarboxylic acid amide (179 mg, 0.40 mmol) and 142-
(tetrahydro-pyran-2-yloxy)-ethyl]-4-(4,4,5,5-tetramethyl-[1,3,2]clioxaborolan-
2-
yI)-1H-pyrazole (168 mg, 0.52 mmol) in dioxane (2 ml) are added water (0.2
ml) and potassium carbonate (1660 mg, 1.2 mmol). The mixture is flushed with
nitrogen and heated to 80 C. Dichloro[1,1-bis(diphenylphosphino)ferrocene]-
palladium(11) dichloromethane adduct (33 mg, 0.04 mmol) is added and the
mixture is stirred in a closed reaction vial for 16 hours at 80 C. The
reaction
mixture is allowed to reach room temperature and partitioned between water
and dichloromethane. The organic phase is dried over sodium sulfate and
evaporated. The residue is chromatographed on a silica gel column with
dichloromethane/methanol as eluent to afford (1R,3R)-343-(4-1142-
(tetrahydro-pyran-2-yloxy)-ethyll-1H-pyrazol-4-yll-pheny1)-3H-
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 205 -
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-cyclopentanecarboxylic acid amide
as
beige solid; HPLC/MS 2.44 min (C), 1M+H] 518.
To a solution of (1R,3R)-343-(4-{142-(tetrahydro-pyran-2-yloxy)-ethyl]-1H-
pyrazol-4-y1}-phenyl)-3H11,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-
cyclopentanecarboxylic acid amide (77.6 mg, 0.15 mmol) in dichloromethane
(1.5 ml) are added methanol (0.8 ml) and a 4 N solution of hydrochloric acid
in
dioxane (275 pl) and the reaction mixture is stirred for 60 minutes at 50 C.
The solvent is evaporated and the residue is treated with saturated sodium
hydrogen carbonate solution. The solids are filtered off, washed with water
and
dried under vacuum. The residue is chromatographed on a silica gel column
with dichloromethane/methanol as eluent to afford (1R,3R)-3-(3-{411-(2-
hydroxy-ethyl)-1H-pyrazol-4-yll-phenyl}-3H-{1,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino)-cyclopentanecarboxylic acid amide as white crystals; HPLC/MS 2.03
min (C), [M+H] 434;
1H NMR (400 MHz, DMSO-d6, TFA-d1) 6 [ppm] 9.29 (s, 1H), 8.32 (s, 1H), 8.25
(d, J= 8.3 Hz, 2H), 8.08 (s, 1H), 7.88 (d, J= 8.6 Hz, 2H), 4.42 (bs, 1H), 4.26
(t,
J = 5.5 Hz, 2H), 3.85 (t, J = 5.5 Hz, 2H), 2.90 (p, J = 7.9 Hz, 1H), 2.18 (m,
2H),
2.09 ¨ 1.98 (m, 1H), 1.92(m, 1H), 1.86¨ 1.64(m, 2H).
The following compounds are prepared analogously:
2-(4-{445-(trans-4-amino-cyclohexylamino)41,2,31triazolo[4,5-d]pyrimidin-3-yll-
phenyll-pyrazol-1-y1)-ethanol ("A256")
OH
,
N
HPLC/MS 1.35 min (B), [M+H] 420;
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 206 -
2-(4-{4154(1R,3S)-3-amino-cyclopentylamino)41,2,3]triazolo[4,5-d]pyrimidin-3-
y11-phenyil-pyrazol-1-y1)-ethanol ("A257")
H2N
OH
"NH
N KI)'
z N
1;\1
JNj
Nr--N
HPLC/MS 1.41 min (B), [M+H] 406;
(1S,3R)-3-(3-{441-(2-hydroxy-ethyl)-1H-pyrazol-4-yil-phenyll-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A258")
NH2 OH
0
N
N-=N
HPLC/MS 1.55 min (B), [M+H] 434;
(1R,3R)-3-(3-{441 -(2-hydroxy-ethyl)-1H-pyrazol-4-y1]-phenyll-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanol ("A259")
HO _Ns OH
N
N
N'N
HPLC/MS 1.55 min (B), [M+H] 407;
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 207 -
(1R,3R)-3-(3-{3-fluoro-441-(2-hydroxy-ethyl)-1H-pyrazol-4-y1]-pheny1}-3H-
[1,2,31triazolo[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A260")
OH
'f'sµ
NH2
¨ N
N=N
HPLC/MS 1.60 min (B), [M+111452;
(1R,3R)-3-(3-{541-(2-hydroxy-ethyl)-1H-pyrazol-4-y1}-pyridin-2-0}-3H-
[1,2,3]triaz010[4,5-d]pyrimidin-5-ylamino)-cyclopentanecarboxylic acid amide
("A261")
0
N 10H 41 (
NH2
N-
N=1\1
HPLC/MS 1.82 min (C), [M+11] 435;
Example 25
Synthesis of [3-(4-methoxy-phenyl)-3H11,2,3]triazolo[4,5-d]pyrimidin-5-y1]-
((trans)-3-methylsulfanyl-cyclopentyl)-amine ("A262"), ((trans)-3-
methanesulfonyl-cyclopenty1)13-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-
d]pyrimidin-5-y1Famine ("A263") and ((cis)-3-methanesulfonyl-cyclopentyI)-[3-
(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-yll-amine ("A264")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 208 -
\o
0
DIPEA
CH3OCH2CH2OH
I N
HNNN
80 C
NH2
contains 30% cis-isomer
sodium perborate
formic acid
\o s s 0
410
HN.õ.N N HI\LN N
II N Ii sN
N N--1\j=
"A262": HPLC/MS 2.11 min (B), [M+H] 356; trans-isomer: 1H NMR (500 MHz,
DMSO-d6, TFA-d1) 6 [ppm] 9.27(s, 1H), 8.06 (d, J = 9.6 Hz, 2H), 7.19 (d, J=
9.1 Hz, 1H), 4.31 (bs, 1H), 3.87 (s, 3H), 3.18 ¨ 2.96 (m, 1H), 2.51 ¨ 1.51
(multiplets, 6), signals of the cis-isomer: 6 7.19 (d, J = 9.3 Hz, 2H), 4.43
(bs,
1H), 3.29 (p, J = 6.9 Hz, 1H);
"A263" (trans-isomer): HPLC/MS 1.77 min (B), [M+H] 389; 1H NMR (500 MHz,
DMSO-c16, TFA-d1) 6 [ppm] 9.26 (s, 1H), 8.05 (bs, 2H), 7.20 (d, J = 8.7 Hz,
2H),
4.33 (bs, 1H), 3.87 (s, 3H), 3.81 ¨3.59 (m, 1H), 2.95 (s, 3H), 2.47 (m, 1H),
2.06 (m, 4H), 1.79 (m, 1H);
"A264" (cis-isomer): HPLC/MS 1.79 min (B), [M+H] 389; 1H NMR (500 MHz,
DMSO-c16, TFA-d1) 6 [PPm] 9.22 (s, 1H), 8.30 ¨ 7.78 (m, 2H), 7.14 (d, J = 8.9
Hz, 2H), 4.38 (bs, 1H), 3.82 (s, 3H), 3.77 (td, J= 9.1, 4.6 Hz, 1H), 2.91 (s,
3H),
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 209 -
2.47 ¨2.32 (m, 1H), 2.24 ¨2.12 (m, 1H), 2.11 ¨ 2.04 (m, 11-1), 2.04¨ 1.91 (m,
2H), 1.79 (m, 1H).
Example 26
Synthesis of (1R,3R)-343-(4-{142-(1H-tetrazol-5-y1)-ethyl]-1H-pyrazol-4-y1}-
pheny1)-3H41,2,31triazolo[4,5-d]pyrimidin-5-ylamino]-cyclopentanecarboxylic
acid amide ("A265")
,N=Nti
r,CN Nr NH
N-N)
0
N-NJ
H2N-4-,õ /
TMSN3 Bu2SnO
DMF/DME)-
N 150 C
II 0 N-N
H2N Q_ N
H iv
TMSN3 = trimethylsilylazide
"A265": dark purple solid; HPLC/MS 1.77 min (C), [M+H] 486; 1H NMR (400
MHz, DMSO-d6, TFA-d1) 6 [ppm] 9.30 (s, 1H), 8.31 ¨8.17 (m, 3H), 8.00 (s,
1H), 7.82 (d, J = 8.4 Hz, 2H), 4.67 (t, J = 6.8 Hz, 2H), 4.46 (bs, 1H), 3.56
(t,
J = 6.8 Hz, 2H), 2.95 (p, J = 7.9 Hz, 1H), 2.21 (m, 2H), 2.06 (m, 1H), 1.96
(m, 1H), 1.89 ¨ 1.68 (m, 2H).
trans-3-[3-(4-{1-[2-(1H-Tetrazol-5-y1)-ethyl]-1H-pyrazol-4-y1}-pheny1)-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-cyclopentanol ("A266")
HO
0"" IF\IL
N __ t N-N
N
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 210 -
is prepared similarly: HPLC/MS 1.77 min (B), [M+11] 459.
Example 27
Synthesis of [3-(4-methoxy-phenyl)-3H41,2,3]triazolo[4,5-d]pyrimidin-5-y1]-
[(1R,3S)-3-(5-methyl-oxazol-2-y1)-cyclopentylFamine ("A267")
o
OH 0- 0
0 fa EDCI Ot HOBt/DMF AuC13
2N HNNN CH3 CN
H
N sp 5crc
_14
HPLC/MS 2.89 min (C), [M-FH] 392; 1H NMR (400 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.29 (s, 1H), 8.08 (bs, 2H), 7.28¨ 7.03 (m, 3H), 4.47 (bs, 1H), 3.88 (s,
3H), 3.55 (p, J = 8.4 Hz, 1H), 2.68 ¨ 2.60 (m, 1H), 2.36 (d, J = 1.3 Hz, 3H),
2.31 ¨ 2.00 (m, 4H), 1.92 (d, J = 8.6 Hz, 1H).
Example 28
Synthesis of 3-(4-{445-(trans-3-hydroxy-cyclopentylamino)41,2,3]triazolo[4,5-
d]pyrimidin-3-yll-phenyl}-pyrazol-1-0-propionamide ("A268")
35
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
-211 -
CN 0
NH2
N-NJ
N-N
OH
K2CO3/H202 OH
HN Me0H/DMS0
80 C
N
II
HPLC/MS 1.55 min (B), [M+H] 434; 1H NMR (500 MHz, DMSO-d6, TFA-d1)
[ppm] 8.09 (m, 3H), 7.94 (s, 1H), 7.72 (d, J = 8.6 Hz, 1H), 4.45 (p, J = 6.9
Hz,
1H), 4.39 (t, J = 6.5 Hz, 2H), 4.26 (tt, J = 5.8, 3.1 Hz, 1H), 2.73 (t, J =
6.5 Hz,
2H), 2.18 (m, 1H), 2.01 (ddd, J = 12.9, 7.4, 3.0 Hz, 1H), 1.97 ¨ 1.87 (m, 1H),
1.74 (ddd, J = 13.4, 7.4, 5.9 Hz, 1H), 1.61 ¨ 1.47 (m, 2H).
Example 29
Synthesis of ((R)-1-methanesulfonyl-pyrrolidin-3-y1)-(3-quinolin-6-y1-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-y1)-amine ("A269")
CI- / \ N =
F421\ DIPEA
+ CH3S02C1 ________________________________
HN cH2c12
HN N Al
1
N
HPLC/MS 1.63 min (B), [M+H] 411; 1H NMR (400 MHz, DMSO-d6, TFA-d1) 6
[ppm] 9.47 (dd, J = 5.4, 1.5 Hz, 1H), 9.44 (d, J = 8.5 Hz, 1H), 9.36 (s, 1H),
9.32 (s, 1H), 9.16 (d, J = 9.6 Hz, 1H), 8.61 (d, J = 9.3 Hz, 11-0, 8.25 (dd,
J=
8.5, 5.3 Hz, 1H), 4.69 (bs, 1H), 3.83 (m, 1H), 3.58 (dt, J = 9.9, 7.3 Hz, 1H),
3.53 ¨ 3.36 (m, 2H), 2.94 (s, 3H), 2.37 (m, 1H), 2.20 (m, 1H).
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
-212 -
((R)-1-Methanesulfonyl-pyrrolidin-3-y1)43-(4-methoxy-phenyl)-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-yll-amine ("A269a")
0
0 õN&-"N
=S )z¨N
is prepared analogously.
'10
Example 30
Synthesis of (R)-3-13-(4-methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino]-pyrrolidine-l-sulfonic acid amide ("A270")
\o 9. o
H2N - s: 0
H29 dioxane --\11
NH2S02NH2 _______________________ N
*NI 100 C
h
N
HPLC/MS 1.71 min (B), [M+H] 391; 1H NMR (400 MHz, DMSO-d6) 5 [ppm]
9.22 (s, 1H), 8.04 (bs, 2H), 7.14 (d, J = 8.7 Hz, 2H), 4.45 (bs, 1H), 3.81 (s,
3H),
3.52 (dd, J= 10.4, 6.5 Hz, 1H), 3.34 (dt, J= 9.8, 7.2 Hz, 1H), 3.21 (dt, J=
9.8,
7.6 Hz, 1H), 3.16 (m, 1H), 2.22 (dq, J = 13.9, 7.1 Hz, 1H), 2.02 (m, 1H).
Example 31
Synthesis of 14(R)-3-13-[4-(1-methyl-1H-pyrazol-4-y1)-phenyl]-3H-
[1,2,3]triazolo[4,5-d]pyrimidin-5-ylaminol-pyrrolidin-1-y1)-4-piperazin-1-yl-
butan-
1-one ("A271")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 213 -
¨N
0
CI 0 TBTU N
HOBt/NMM
DMF
HNNN
NFID¨N
H
OH
,
H2N 0 N)
Th CI 0
N = 0
\
4 N HCl/dioxane
¨\N
II 1\1
"A271" dihydrochloride: HPLC/MS 1.34 min (B), [M+H] 516; 1H NMR (400
MHz, DMSO-d6, d-TFA) 6 9.27 (s, 1H), 8.20 (s, 1H), 8.20 ¨ 8.05 (m, 2H), 7.96
(s, 1H), 7.80 (d, J = 8.0 Hz, 2H), 4.51 (d, J = 29.6 Hz, 2H), 3.55 (m, 13H),
2.40
(dt, J= 13.9, 6.9 Hz, 2H), 2.27 (m, 1H), 2.13 m, 2H), 2.01 ¨ 1.86 (m, 2H).
Example 32
Synthesis of (1R,4S)-443-(4-ethoxy-phenyl)-3H41,2,3]triazolo[4,5-d]pyrimidin-
5-ylamino]-cyclopent-2-enecarboxylic acid amide ("A272") and (1S,4S)-4-[3-
(4-Methoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-ylamino]-cyclopent-2-
enecarboxylic acid amide ("A273")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 214 -
0
\ 0¨ \ o-
0 0
0
410 + = _________ 3. . O
DIPEA I-IN NN
CIN.-
.,_.11, NH2
CH3OCH2CH2OH II
I
N'-.-N'N L-tartrate salt 800C N----N,I,N1
o
o _I/ o¨
o¨ HO -;
HO õ
NaOH
04 O + Y 441.0
Me0H
HNN N HN)T'NTN'
II N N.,N''N
N----.Ni
NH3 EDCI, HOBt
dioxane/THF
0 0
0¨ H N -4 0¨
H2N
i 1 40 0O
FIN NN +, N HNN,
II ' .N I
N------Ni Nõ--i\IN
"A272": HPLC/MS 2.35 min (C), [M+H] 352; 1H NMR (500 MHz, DMSO-d6,
TFA-d1) 6 [ppm] 9.31 (s, 1H), 8.07 (d, J = 8.5 Hz, 2H), 7.20 (d, J = 9.0 Hz,
2H),
5.97 (m, 2H), 5.11 (bs, 1H), 3.88 (s, 3H), 3.49 (m, 1H), 2.56 ¨ 2.48 (m, 1H),
1.97 (dt, J = 13.3, 5.2 Hz, 1H).
"A273": HPLC/MS 2.24 min (C), [M+H] 352;1H NMR (500 MHz, DMS0-, TEA-
d1) ö [ppm] 9.28 (s, 1H), 8.20 ¨ 7.85 (m, 2H), 7.21 (d, J = 9.0 Hz, 2H), 5.94
(m,
2H), 5.16 (bs, 1H), 3.87 (s, 3H), 3.66 (ddd, J = 8.6, 4.3, 2.2 Hz, 1H), 2.58-
2.51
(m, 1H), 1.99 (ddd, J = 13.2, 8.7, 4.6 Hz, 1H).
Example 33
CA 02903903 2015-09-03
WO 2014/135244
PCT/EP2014/000361
- 215 -
Synthesis of (1R,3R)-3-{3-[4-(2-methoxy-ethoxy)-phenyl}-3H-[1,2,3]triazolo[4,5-
d]pyrimidin-5-ylamino}-cyclopentanecarbonitrile ("A274")
ro
0
d--NH2
Burgess reagent
HNNN dioxane FINN N
II N 100 C
HPLC/MS 1.88 min (B), [M+H] 380; 1H NMR (400 MHz, DMSO-d6, TFA-d1)
[ppm] 9.28 (s, 1H), 8.08 (bs, 2H), 7.48 ¨ 7.00 (m, 2H), 4.46 (bs, 1H), 4.33¨
4.09 (m, 2H), 3.87 ¨3.63 (m, 2H), 3.37 (s, 3H), 3.20 (p, J = 7.7 Hz, 1H), 2.36
¨1.99 (m, 4H), 1.99 ¨ 1.69 (m, 2H).
cis-3-[3-(4-Methoxy-phenyl)-3 H-[1,2, 3]triazolo[4,5-d]pyrimid in-5-ylamino]-
cyclopentanecarbonitrile ("A275")
..13-1\1}¨ 0
N" ,
NCN
\
N-J\I
is prepared analogously; HPLC/MS 1.90 min (B), [M+11] 336; 1H NMR (500
MHz, DMSO-d6, TFA-d1) 6 [PPrh] 9.31 (s, 1H), 8.07 (d, J = 8.0 Hz, 2H), 7.18
(d, J = 9.0 Hz, 2H), 4.38 (bs, 1H), 3.88 (s, 3H), 3.02 (p, J= 8.1 Hz, 1H),
2.56
(dt, J= 13.1, 7.8 Hz, 1H), 2.19 ¨ 2.05 (m, 3H), 2.05 ¨ 1.96 (m, 1H), 1.91 (m,
1H).
Example 34
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 216 -
Synthesis of (1R,3R)-343-(4-hydroxy-pheny1)-3H41,2,31triazolo[4,5-
d]pyrimidin-5-ylamino]-cyclopentanecarboxylic acid amide ("A276"), (1R,3R)-
3-{344-(2-morpholin-4-yl-ethoxy)-phenyl]-3H41,2,3Jtriazolo[4,5-d]pyrimidin-5-
ylamino}-cyclopentanecarboxylic acid amide ("A277"), (1R,3R)-3-{3-[4-(3-
hydroxy-1,1-dimethyl-propoxy)-phenyl]-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-
ylamino}-cyclopentanecarboxylic acid amide ("A278") and (1R,3R)-3-{344-
(piperidin-4-ylmethoxy)-phenyi]-3H41,2,31triazolo[4,5-d]pyrimidin-5-ylamino}-
cyclopentanecarboxylic acid amide ("A279")
o
oY----
___
H N -- I
0
KOAc H2N --",
it
PdC12(PPh3)2
__________________________ HN-)r'NN',I\J + 7 -0-B-Bb
HN N N
N -- -Ni DMF/80 C "ii ,N 0 .4"----
N----.14
.-.-- 0
0 p
_4
H N -, OH Ho ( \N 0
4
\
sodium . / Cc"-' 0
o
perborate
________ ) HNõvN,..,-N. PPVDIAD/THF
II N
c). = THF/water
HNNN.
OH ll N
N00 PPIVDIAD N,õ;.:-------f\i'
rN1\__ j
THF
HO--/ PPh3/DIAD
OH
THF LIN HCI in dioxane
OH i
r'o
..o sHi2
CI
O
o o
o H N -, -4/ o
H2N -Iµ H2N -õ,
9 . ? fk ..? fk
HN,.,,,, \1 NN HN N N HN N N
)-I- s
II 1 N-----NIN .11
"A276": HPLC/MS 1.98 min (C), [M+1-1] 340; 1H NMR (400 MHz, DMSO-d6,
TFA-di) 5 [ppm] 9.41 (s, 1H), 7.93 (d, J= 8.5 Hz, 2H), 7.07 (d, J = 8.9 Hz,
2H),
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
-217 -
4.46 (p, J = 6.2 Hz, 1H), 3.00 (p, J = 8.0 Hz, 1H), 2.33 - 2.15 (m, 2H), 2.10
(dtd,
J= 11.2, 7.4, 6.8,4.1 Hz, 1H), 1.97 (ddd, J = 13.7, 8_8, 5.1 Hz, 1H), 1.90 -
1.69
(m, 2H);
"A277": HPLC/MS 1.66 min (C), [M+H] 453; H NMR (500 MHz, DMSO-d6,
TFA-d1) 6 [ppm] 9.29 (s, 1H), 8.16 (d, J = 8.4 Hz, 2H), 7.31 (d, J = 9.0 Hz,
2H),
4.55 - 4.51 (m, 2H), 4.39 (bs, 1H), 4.12 - 3.98 (m, 2H), 3.87 - 3.75 (m, 2H),
3.70 (t, J = 4.9 Hz, 2H), 3.63 (d, J = 12.6 Hz, 2H), 3.31 (td, J = 12.3, 3.7
Hz,
2H), 2.92(p, J- 8.0 Hz, 1H), 2.26 - 2.09 (m, 2H), 2.09 - 1.99 (m, 1H), 1.92(m,
1H), 1.84 - 1.66 (m, 2H);
"A278": HPLC/MS 2.20 min (C), [M+H] 426; 1H NMR (500 MHz, DMSO-d6,
TFA-d1) 6 [ppm] 9.30 (s, 1H), 8.21 - 8.05 (m, 2H), 7.25 (d, J = 8.9 Hz, 2H),
4.44
(bs, 1H), 3.73 (t, J= 7.3 Hz, 2H), 2.93 (p, J = 8.0 Hz, 1H), 2.33 - 2.11 (m,
2H),
2.06 (qd, J- 8.1, 4.5 Hz, 1H), 1.95(t, J = 7.3 Hz, 2H), 1.94- 1.89 (m, 1H),
1.77
(m, 2H), 1.36 (s, 6H);
"A279": HPLC/MS 1.78 min (C), [M+H] 437; 1H NMR (500 MHz, DMSO-d6) 6
[ppm] 9.30 (s, 1H), 8.11 (d, J = 9.5 Hz, 2H), 7.21 (d, J- 8.8 Hz, 2H), 4.41
(bs,
1H), 4.01 (d, J = 6.2 Hz, 2H), 3.40 (d, J = 12.7 Hz, 2H), 3.04 - 2.89 (m, 3H),
2.18 (m, 3H), 2.11 -1.97 (m, 3H), 1.92 (ddd, J = 13.4, 9.3, 5.6 Hz, 1H), 1.76
(m, 2H), 1.68- 1.50 (m, 2H).
Example 35
Synthesis of (1R,3R)-343-(4-ethoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-
5-ylaminoj-cyclopentanecarboxylic acid N'-acetyl-hydrazide ("A280") and [3-(4-
ethoxy-phenyl)-3H-[1,2,3]triazolo[4,5-d]pyrimidin-5-y11-[(1R,3R)-3-(5-methyl-
[1,3,4]oxadiazol-2-y1)-cyclopentylFamine ("A281")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 218 -
0
0
(DJ
0 sN4
HO H
(7)H EDCl/HOBt
0 NH2 DMFHNNN. ii
HN N N
ii sN
NO J
Burgess reagent
THF
100 C (microwave)
II
"A280": HPLC/MS 2.29 min (C), [M+H] 425; 1H NMR (500 MHz, DMSO-d5,
TFA-d1) 6 [ppm] 9.29 (s, 1H), 8.27 - 7.95 (m, 2H), 7.19 (d, J = 8.3 Hz, 2H),
4.43 (bs, 1H), 4.15 (q, J= 7.0 Hz, 2H), 2.97 (p, J- 7.9 Hz, 1H), 2.17 (m, 2H),
2.10 - 2.01 (m, 1H), 1.99 - 1.92 (m, 1H), 1.91 (s, 3H), 1.85- 1.68 (m, 2H),
1.41 (t, J = 7.0 Hz, 3H);
"A281": HPLC/MS 2.73 min (C), [M+H] 407; 1H NMR (400 MHz, DMSO-d6,
TFA-d1) 6 [ppm] 9.21 (s, 1H), 8.01 (d, J = 8.4 Hz, 2H), 7.10 (d, J = 9.0 Hz,
2H),
4.45 (bs, 1H), 4.07 (q, J= 6.9 Hz, 2H), 3.53 (p, J= 7.8 Hz, 1H), 2.39 (s, 3H),
2.38 - 2.26 (m, 1H), 2.27 - 2.01 (m, 3H), 1.98 - 1.71 (m, 2H), 1.34 (t, J =
7.0
Hz, 3H).
Example 36
Synthesis of (1R,3R)-343-(4-ethoxy-phenyl)-3H41,2,3]triazolo[4,5-d]pyrimidin-
5-ylaminol-cyclopentanecarboxylic acid hydrazide ("A282") and [(1R,3R)-3-(5-
amino-[1,3,4]oxadiazol-2-y1)-cyclopenty1H3-(4-ethoxy-phenyl)-3H-
[1,2,3]triazolo[4,5-dlpyrimidin-5-y1Famine ("A283")
CA 02903903 2015-09-03
WO 2014/135244 PCT/EP2014/000361
- 219 -
0
HO 1.CDI/DMS0 12N, ,p ¨1
N 0
Ot NMHe 2NHH2 x OH /80 C H
HNN.õN
HNNii N N
NH2 h
NO
BrCN
Krico3
Me0H HNNN
"A282": HPLC/MS 2.27 min (C), [M+H] 383; 1H NMR (400 MHz, DMSO-d6,
TFA-d1) 6 [ppm] 9.20 (s, 1H), 7.98 (d, J = 8.3 Hz, 2H), 7.08(d, J= 9.0 Hz,
2H),
4.40 (bs, 1H), 4.05 (q, J= 6.9 Hz, 2H), 2.95 (p, J¨ 7.8 Hz, 1H), 2.09 (m, 3H),
1.90 (ddt, J = 13.5, 9.0, 5.2 Hz, 1H), 1.73 (m, 2H), 1.32 (t, J = 7.0 Hz, 3H);
"A283": HPLC/MS 1.75 min (B), [M+H] 408; 1H NMR (500 MHz, DMSO-d6,
TFA-d1) 6 [ppm] 9.29 (s, 1H), 8.07 (bs, 2H), 7.17 (d, J= 9.1 Hz, 2H), 4.50
(bs,
1H), 4.14 (q, J = 7.0 Hz, 2H), 3.57 (p, J = 8.0 Hz, 1H), 2.36 (dt, J = 14.3,
7.4
Hz, 1H), 2.32 ¨ 2.25 (m, 1H), 2.23 (m, 2H), 1.97 (dq, J = 12.0, 7.5 Hz, 1H),
1.89 (m, 1H), 1.41 (t, J = 7.0 Hz, 3H).
Example 37
Synthesis of (R)-343-(2-methyl-quinolin-6-y1)-3H-[1,2,3]triazolo[4,5-
d]pyrimidin-
5-ylamino]-pyrrolidine-1-carboxylic acid amide ("A284")
DEMANDES OU BREVETS VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVETS
COMPREND PLUS D'UN TOME.
CECI EST LE TOME 1 ________________ DE 2
NOTE: Pour les tomes additionels, veillez contacter le Bureau Canadien des
Brevets.
JUMBO APPLICATIONS / PATENTS
THIS SECTION OF THE APPLICATION / PATENT CONTAINS MORE
THAN ONE VOLUME.
THIS IS VOLUME 1 OF 2
NOTE: For additional volumes please contact the Canadian Patent Office.