Note: Descriptions are shown in the official language in which they were submitted.
81791389
DNA-PK INHIBITORS
This application claims the benefit to U.S. Provisional Application No.
61/777,816,
filed on March 12, 2013.
TECHNICAL FIELD OF THE INVENTION
[0001] The present invention relates to compounds useful as inhibitors of
DNA-dependent
protein kinase (DNA-PK). The invention also provides pharmaceutically
acceptable
compositions comprising the compounds of the invention and methods of using
the
compositions in the treatment of cancer,
BACKGROUND OF THE INVENTION
[0002] Ionizing radiation (IR) induces a variety of DNA damage of which
double strand
breaks (DSBs) are the most cytotoxic. These DSBs can lead to cell death via
apoptosis and/or
mitotic catastrophe if not rapidly and completely repaired. In addition to IR,
certain
chemotherapeutic agents including topoisomerase II inhibitors, bleomycin, and
doxorubicin
also cause DSBs. These DNA lesions trigger a complex set of signals through
the DNA
damage response network that function to repair the damaged DNA and maintain
cell viability
and genomic stability. In mammalian cells, the predominant repair pathway for
DSBs is the
Non-Homologous End Joining Pathway (NHEJ). This pathway functions regardless
of the
phase of the cell cycle and does not require a template to re-ligate the
broken DNA ends.
NHEJ requires coordination of many proteins and signaling pathways. The core
NHEJ
machinery consists of the Ku70/80 heterodimer and the catalytic subunit of DNA-
dependent
protein kinase (DNA-PKcs), which together comprise the active DNA-PK enzyme
complex.
DNA-PKcs is a member of the phosphatidylinositol 3-kinase-related kinase
(PIKK) family of
serine/threonine protein kinases that also includes ataxia telangiectasia
mutated (ATM), ataxia
telangiectasia and Rad3-related (ATR), mTOR, and four PI3K isoforms. However,
while
DNA-PKcs is in the same protein kinase family as ATM and ATR, these latter
kinases
function to repair DNA damage through the Homologous Recombination (HR)
pathway and
are restricted to the S
1
Date Recue/Date Received 2020-08-10
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
and G2 phases of the cell cycle. While ATM is also recruited to sites of DSBs,
ATR is
recruited to sites of single stranded DNA breaks.
[0003] NHEJ is thought to proceed through three key steps: recognition of
the DSBs,
DNA processing to remove non-ligatable ends or other forms of damage at the
termini,
and finally ligation of the DNA ends. Recognition of the DSB is carried out by
binding of
the Ku heterodimer to the ragged DNA ends followed by recruitment of two
molecules of
DNA-PKcs to adjacent sides of the DSB; this serves to protect the broken
termini until
additional processing enzymes are recruited. Recent data supports the
hypothesis that
DNA-PKcs phosphorylates the processing enzyme, Artemis, as well as itself to
prepare the
DNA ends for additional processing. In some cases DNA polymerase may be
required to
synthesize new ends prior to the ligation step. The auto-phosphorylation of
DNA-PKcs is
believed to induce a conformational change that opens the central DNA binding
cavity,
releases DNA-PKcs from DNA, and facilitates the ultimate religation of the DNA
ends.
[0004] It has been known for some time that DNA-PK-I- mice are
hypersensitive to the
effects of IR and that some non-selective small molecule inhibitors of DNA-
PKcs can
radiosensitize a variety of tumor cell types across a broad set of genetic
backgrounds.
While it is expected that inhibition of DNA-PK will radiosensitize normal
cells to some
extent, this has been observed to a lesser degree than with tumor cells likely
due to the fact
that tumor cells possess higher basal levels of endogenous replication stress
and DNA
damage (oncogene-induced replication stress) and DNA repair mechanisms are
less
efficient in tumor cells. Most importantly, an improved therapeutic window
with greater
sparing of normal tissue will be imparted from the combination of a DNA-PK
inhibitor
with recent advances in precision delivery of focused IR, including image-
guide RT
(IGRT) and intensity-modulated RT (IMRT).
[0005] Inhibition of DNA-PK activity induces effects in both cycling and
non-cycling
cells. This is highly significant since the majority of cells in a solid tumor
are not actively
replicating at any given moment, which limits the efficacy of many agents
targeting the
cell cycle. Equally intriguing are recent reports that suggest a strong
connection between
inhibition of the NHEJ pathway and the ability to kill traditionally
radiorcsistant cancer
stem cells (CSCs). It has been shown in some tumor cells that DSBs in dormant
CSCs
predominantly activate DNA repair through the NHEJ pathway; it is believed
that CSCs
are usually in the quiescent phase of the cell cycle. This may explain why
half of cancer
2
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
patients may experience local or distant tumor relapse despite treatment as
current
strategies are not able to effectively target CSCs. A DNA-PK inhibitor may
have the
ability to sensitize these potential metastatic progenitor cells to the
effects of IR and select
DSB-inducing chemotherapeutic agents.
[0006] Given the involvement of DNA-PK in DNA repair processes, an
application of
specific DNA-PK inhibitory drugs would be to act as agents that will enhance
the efficacy
of both cancer chemotherapy and radiotherapy. Accordingly, it would be
desirable to
develop compounds useful as inhibitors of DNA-PK.
SUMMARY OF THE INVENTION
[0007] It has been found that compounds of this invention, and
pharmaceutically
acceptable compositions thereof, are effective as inhibitors of DNA-PK.
Accordingly, the
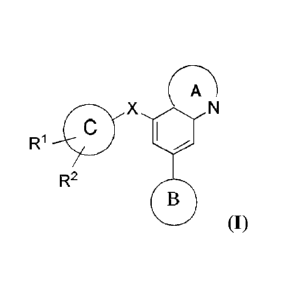
invention features compounds having the general formula:
R1 ID X 1110
R2
(I),
or a pharmaceutically acceptable salt thereof, where each of RI-, R2, X, Ring
A, Ring B and
Ring C is as defined elsewhere herein.
[0008] The invention also provides pharmaceutical compositions that include
a
compound of formula I and a pharmaceutically acceptable carrier, adjuvant, or
vehicle.
These compounds and pharmaceutical compositions are useful for treating or
lessening the
severity of cancer.
[0009] The compounds and compositions provided by this invention are also
useful for
the study of DNA-PK in biological and pathological phenomena; the study of
intracellular
signal transduction pathways mediated by such kinases; and the comparative
evaluation of
new kinase inhibitors.
3
81791389
DETAILED DESCRIPTION OF THE INVENTION
Definitions and General Terminology
[0010] As used herein, the following definitions shall apply unless
otherwise indicated.
For purposes of this invention, the chemical elements are identified in
accordance with the
Periodic Table of the Elements, CAS version, and the Handbook of Chemistry and
Physics,
75th Ed. 1994. Additionally, general principles of organic chemistry are
described in "Organic
Chemistry," Thomas Sorrell, University Science Books, Sausalito: 1999, and
"March's
Advanced Organic Chemistry," 5th Ed., Smith, M.B. and March, J., eds. John
Wiley & Sons,
New York: 2001.
[0011] As described herein, compounds of the invention may optionally be
substituted
with one or more substituents, such as are illustrated generally above, or as
exemplified by
particular classes, subclasses, and species of the invention. It will be
appreciated that the
phrase "optionally substituted" is used interchangeably with the phrase
"substituted or
unsubstituted." In general, the term "substituted," whether preceded by the
term "optionally"
or not, refers to the replacement of one or more hydrogen radicals in a given
structure with the
radical of a specified substituent. Unless otherwise indicated, an optionally
substituted group
may have a substituent at each substitutable position of the group. When more
than one
position in a given structure can be substituted with more than one
substituent selected from a
specified group, the substituent may be either the same or different at each
position.
[0012] As described herein, when the term "optionally substituted" precedes
a list, said
term refers to all of the subsequent substitutable groups in that list. For
example, if X is
halogen; optionally substituted C1_3 alkyl or phenyl; X may be either
optionally substituted
alkyl or optionally substituted phenyl. Likewise, if the term "optionally
substituted" follows a
list, said term also refers to all of the substitutable groups in the prior
list unless otherwise
indicated. For example: if X is halogen, C1-3 alkyl, or phenyl, wherein X is
optionally
substituted by Jx, then both C1_3 alkyl and phenyl may be optionally
substituted by Jx. As is
apparent to one having ordinary skill in the art, groups such as H, halogen,
NO2, CN, NH2,
OH, or OCF3 would not be included because they are not substitutable groups.
As is also
apparent to a skilled person, a heteroaryl or heterocyclic ring containing an
NH group can be
optionally substituted by replacing the
4
Date Recue/Date Received 2020-08-10
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
hydrogen atom with the substituent. If a substituent radical or structure is
not identified or
defined as "optionally substituted," the substituent radical or structure is
unsubstituted.
[0013] Combinations of substituents envisioned by this invention are
preferably those
that result in the formation of stable or chemically feasible compounds. The
term "stable,"
as used herein, refers to compounds that are not substantially altered when
subjected to
conditions to allow for their production, detection, and, preferably, their
recovery,
purification, and use for one or more of the purposes disclosed herein. In
some
embodiments, a stable compound or chemically feasible compound is one that is
not
substantially altered when kept at a temperature of 40 C or less, in the
absence of moisture
or other chemically reactive conditions, for at least a week.
[0014] The term "alkyl" or "alkyl group," as used herein, means a straight-
chain (i.e.,
unbranched) or branched, substituted or unsubstituted hydrocarbon chain that
is
completely saturated. Unless otherwise specified, alkyl groups contain 1-8
carbon atoms.
In some embodiments, alkyl groups contain 1-6 carbon atoms, and in yet other
embodiments, alkyl groups contain 1-4 carbon atoms (represented as "Ci 4
alkyl"). In
other embodiments, alkyl groups are characterized as "Co_4 alkyl" representing
either a
covalent bond or a C1-4 alkyl chain. Examples of alkyl groups include methyl,
ethyl,
propyl, butyl, isopropyl, isobutyl, sec-butyl, and tert-butyl. The term
"alkylene," as used
herein, represents a saturated divalent straight or branched chain hydrocarbon
group and is
exemplified by methylene, ethylene, isopropylene and the like. The term
"alkylidene," as
used herein, represents a divalent straight chain alkyl linking group. The
term "alkenyl,"
as used herein, represents monovalent straight or branched chain hydrocarbon
group
containing one or more carbon-carbon double bonds. The term "alkynyl," as used
herein,
represents a monovalent straight or branched chain hydrocarbon group
containing one or
more carbon-carbon triple bonds.
[0015] The term "cycloalkyl" (or "carbocycle") refers to a monocyclic C3-C8
hydrocarbon or bicyclic C8-C12 hydrocarbon that is completely saturated and
has a single
point of attachment to the rest of the molecule, and wherein any individual
ring in said
bicyclic ring system has 3-7 members. Suitable cycloalkyl groups include, but
are not
limited to, cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, and cycloheptyl.
[0016] The term "heterocycle," "heterocyclyl," "heterocycloalkyl," or
"heterocyclic"
as used herein refers to a monocyclic, bicyclic, or tricyclic ring system in
which at least
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
one ring in the system contains one or more heteroatoms, which is the same or
different,
and that is completely saturated or that contains one or more units of
unsaturation, but
which is not aromatic, and that has a single point of attachment to the rest
of the molecule.
In some embodiments, the "heterocycle," "heterocyclyl," "heterocycloalkyl," or
"heterocyclic" group has three to fourteen ring members in which one or more
ring
members is a heteroatom independently selected from oxygen, sulfur, nitrogen,
or
phosphorus, and each ring in the system contains 3 to 8 ring members.
[0017] Examples of heterocyclic rings include, but are not limited to, the
following
monocycles: 2-tetrabydrofuranyl, 3-tetrahydrofuranyl, 2-tetrabydrothiophenyl,
3-tetrahydrothiophenyl, 2-morpholino, 3-morpholino, 4-momholino, 2-
thiomorpholino, 3-
thiomorpholino, 4-thiomorpholino, 1-pyrrolidinyl, 2-pyrrolidinyl, 3-
pyrrolidinyl,
1-tetrahydropiperazinyl, 2-tetrahydropiperazinyl, 3-tetrahydropiperazinyl, 1-
piperidinyl, 2-
piperidinyl, 3-piperidinyl, 1-pyrazolinyl, 3-pyrazolinyl, 4-pyrazolinyl, 5-
pyrazolinyl, 1-
piperidinyl, 2-piperidinyl, 3-piperidinyl, 4-piperidinyl, 2-thiazolidinyl, 3-
thiazolidinyl,
4-thiazolidinyl, 1-imidazolidinyl, 2-imidazolidinyl, 4-imidazolidinyl, 5-
imidazolidinyl;
and the following bicycles: 3-1H-benzimidazol-2-one, 3-(1-alkyl)-benzimidazol-
2-one,
indolinyl, tetrahydroquinolinyl, tetrahydroisoquinolinyl, benzothiolane,
benzodithiane, and
1,3-dihydro-imidazol-2-one.
[0018] The term "heteroatom" means one or more of oxygen, sulfur, nitrogen,
or
phosphorus, including any oxidized form of nitrogen, sulfur, or phosphorus;
the
quatemized form of any basic nitrogen; or a substitutable nitrogen of a
heterocyclic ring,
for example N (as in 3,4-dihydro-2H-pyrroly1), NH (as in pyiTolidinyl) or NIZ
(as in N-
substituted pyn-olidinyl).
[0019] The term "unsaturated," as used herein, means that a moiety has one
or more
units of unsaturation.
[0020] The term "alkoxy," or "thioalkyl," as used herein, refers to an
alkyl group, as
previously defined, attached to the principal carbon chain through an oxygen
("alkoxy") or
sulfur ("tbioalkyl") atom.
[0021] The terms "haloalkyl," "haloalkenyl," and "haloalkoxy" mean alkyl,
alkenyl, or
alkoxy, as the case may be, substituted with one or more halogen atoms. The
term
"halogen" means F, Cl, Br, or I.
6
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
[0022] The term "aryl" used alone or as part of a larger moiety as in
"aralkyl,"
"aralkoxy," or "aryloxyalkyl," refers to a monocyclic, bicyclic, or tricyclic
carbocyclic
ring system having a total of six to fourteen ring members, wherein said ring
system has a
single point of attachment to the rest of the molecule, at least one ring in
the system is
aromatic and wherein each ring in the system contains 4 to 7 ring members. The
term
"aryl" may be used interchangeably with the term "aryl ring." Examples of aryl
rings
include phenyl, naphthyl, and anthracene.
[0023] The term "heteroaryl," used alone or as part of a larger moiety as
in
"heteroaralkyl," or "heteroarylalkoxy," refers to a monocyclic, bicyclic, and
tricyclic ring
system having a total of five to fourteen ring members, wherein said ring
system has a
single point of attachment to the rest of the molecule, at least one ring in
the system is
aromatic, at least one ring in the system contains one or more heteroatoms
independently
selected from nitrogen, oxygen, sulfur or phosphorus, and wherein each ring in
the system
contains 4 to 7 ring members. The term "heteroaryl" may be used
interchangeably with
the term "heteroaryl ring" or the term "heteroaromatic."
[0024] Further examples of heteroaryl rings include the following
monocycles:
2-furanyl, 3-furanyl, N-imidazolyl, 2-imidazolyl, 4-imidazolyl, 5-imidazolyl,
3-isoxazolyl,
4-isoxazolyl, 5-isoxazolyl, 2-oxazolyl, 4-oxazolyl, 5-oxazolyl, N-pyrrolyl, 2-
pyrrolyl, 3-
2-pyridyl, 3-pyridyl, 4-pyridyl, 2-pyrimidinyl, 4-pyrimidinyl, 5-pyrimidinyl,
pyridazinyl (e.g., 3-pyridazinyl), 2-thiazolyl, 4-thiazolyl, 5-thiazolyl,
tetrazolyl (e.g., 5-
tetrazolyl), triazolyl (e.g., 2-triazoly1 and 5-triazoly1), 2-thienyl, 3-
thienyl, pyrazolyl (e.g.,
2-pyrazoly1), isothiazolyl, 1,2,3-oxadiazolyl, 1,2,5-oxadiazolyl, 1,2,4-
oxadiazolyl,
1,2,3-triazolyl, 1,2,3-thiadiazolyl, 1,3,4-thiadiazolyl, 1,2,5-thiadiazolyl,
pyrazinyl, 1,3,5-
triazinyl, and the following bicycles: benzimidazolyl, benzofuryl,
benzothiophenyl,
indolyl (e.g., 2-indoly1), purinyl, quinolinyl (e.g., 2-quinolinyl, 3-
quinolinyl, 4-quinolinyl),
and isoquinolinyl (e.g., 1-isoquinolinyl, 3-isoquinolinyl, or 4-
isoquinoliny1)..
[0025] As described herein, a bond drawn from a substituent to the center
of one ring
within a multiple-ring system (as shown below) represents substitution of the
substituent
at any substitutable position in any of the rings within the multiple ring
system. For
example, Structure a represents possible substitution in any of the positions
shown in
Structure b.
7
81791389
x
x x
____________________________ ii x
I
f\I X N X
H
X X
Structure a Structure b
[0026] This also applies to multiple ring systems fused to optional ring
systems (which
would be represented by dotted lines). For example, in Structure c, X is an
optional
substituent both for ring A and ring B.
/-- --,
1 A B 2,)(
Structure c
[0027] If, however, two rings in a multiple ring system each have different
substituents
drawn from the center of each ring, then, unless otherwise specified, each
substituent only
represents substitution on the ring to which it is attached. For example, in
Structure d, Y is an
optionally substituent for ring A only, and X is an optional substituent for
ring B only.
Y
1 A B--)(
Structure d
[0028] The term "protecting group," as used herein, represent those groups
intended to
protect a functional group, such as, for example, an alcohol, amine, carboxyl,
carbonyl, etc.,
against undesirable reactions during synthetic procedures. Commonly used
protecting groups
are disclosed in Greene and Wuts, Protective Groups In Organic Synthesis, 3rd
Edition (John
Wiley & Sons, New York, 1999). Examples of nitrogen protecting groups include
acyl, aroyl,
or carbamyl groups such as formyl, acetyl, propionyl, pivaloyl, t-butylacetyl,
2-chloroacetyl,
2-bromoacetyl, trifluoroacetyl, trichloroacetyl, phthalyl, o-
nitrophenoxyacetyl, oc-
chlorobutyryl, benzoyl, 4-chlorobenzoyl, 4-bromobenzoyl, 4-nitrobenzoyl and
chiral
auxiliaries such as protected or unprotected D, L or D, L-amino acids such as
alanine, leucine,
phenylalanine and the like; sulfonyl groups such as benzenesulfonyl, p-
toluenesulfonyl and
the like; carbamate groups such as benzyloxycarbonyl, p-
chlorobenzyloxycarbonyl, p-
methoxybenzyloxycarbonyl, p-nitrobenzyloxycarbonyl, 2-
8
Date Recue/Date Received 2020-08-10
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
nitrobenzyloxycarbonyl, p-bromobenzyloxycarbonyl, 3,4-
dimethoxybenzyloxycarbonyl,
3,5-dimethoxybenzyloxycarbonyl, 2,4-dimethoxybenzyloxycarbonyl, 4-
methoxybenzyloxycarbonyl, 2-nitro-4,5-dimethoxybenzyloxycarbonyl, 3,4,5-
trimethoxybenzyloxycarbonyl, 1-(p-biphenyly1)-1-methylethoxycarbonyl, a,ot-
dimethy1-
3,5-dimethoxybenzyloxycarbonyl, benzhydryloxycarbonyl, t-butyloxycarbonyl,
diisopropylmethoxycarbonyl, isopropyloxycarbonyl, ethoxycarbonyl,
methoxycarbonyl,
allyloxycarbonyl, 2,2,2,-trichloroethoxycarbonyl, phenoxycarbonyl, 4-
nitrophenoxy
carbonyl, fluoreny1-9-methoxycarbonyl, cyclopentyloxycarbonyl,
adamantyloxycarbonyl,
cyclohexyloxycarbonyl, phenylthiocarbonyl and the like, arylalkyl groups such
as benzyl,
triphenylmethyl, benzyloxymethyl and the like and silyl groups such as
trimethylsilyl and
the like. Preferred N-protecting groups are formyl, acetyl, benzoyl, pivaloyl,
t-butylacetyl,
alanyl, phenylsulfonyl, benzyl, t-butyloxycarbonyl (Boc) and benzyloxycarbonyl
(Cbz).
Examples of hydroxyl protecting groups include ethers, such as
tetrahydropyranyl, tert
butyl, benzyl, allyl, and the like; silyl ethers such as trimethyl silyl,
triethyl silyl,
triisopropylsilyl, tert-butyl diphenyl silyl, and the like; esters such as
acetyl,
trifluoroacetyl, and the like; and carbonates. Hydroxyl protecting groups also
include
those appropriate for the protection of phenols.
[0029] Unless otherwise depicted or stated, structures recited herein are
meant to
include all isomeric (e.g., enantiomeric, diastereomeric, and geometric (or
conformational)) forms of the structure; for example, the R and S
configurations for each
asymmetric center, (Z) and (E) double bond isomers, and (Z) and (E)
conformational
isomers. Therefore, single stereochemical isomers as well as enantiomeric,
diastereomeric, and geometric (or conformational) mixtures of the present
compounds are
within the scope of the invention. Compounds that have been drawn with
stereochemical
centers defined, usually through the use of a hatched (¨ill) or bolded (---=)
bond, are
stereochemically pure, but with the absolute stereochemistry still undefined.
Such
compounds can have either the R or S configuration. In those cases where the
absolute
configuration has been determined, the chiral center(s) are labeled (R) or (S)
in the
drawing.
[0030] Unless otherwise stated, all tautomeric forms of the compounds of
the
invention are within the scope of the invention. Additionally, unless
otherwise stated,
structures depicted herein are also meant to include compounds that differ
only in the
9
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
presence of one or more isotopically enriched atoms. For example, compounds
having the
present structures except for the replacement of hydrogen by deuterium or
tritium, or the
replacement of a carbon by a 11C- or 14C-enriched carbon are within the scope
of this
invention. Such compounds are useful, for example, as analytical tools, probes
in
biological assays, or as DNA-PK inhibitors with an improved therapeutic
profile.
Description of Compounds of the Invention
[0031] In one aspect, the invention features compounds having the formula:
CA)
R1 410 N
R2
(I), wherein
Ring A is a ring system selected from
R3 R3 R3 R3
N-0
I I
,or
Ring B is a ring system selected from
)
0 O FIN---N 0 0 V
, Or
wherein Ring B is optionally substituted with up to 4 fluorine atoms, up to
two OH or
up to two Ci 4alkyl which is optionally substituted with up to 3 fluorine
atoms, up to
two OH, or up to two OCi_zalkyl groups;
Ring C is a cyclohexane or a cyclobutane ring;
X is -NH-, -0-, or -0C14 alkyl-;
each of R1- and R2 is, independently, hydrogen, -C(0)NHR4, -C(0)0R4, -
NHC(0)R4,
-NHC(0)0R4, -NHC(0)NHR4, -NHS(0)2R4, -00-4alkyl-NHR4, or -OW, wherein R1-
and R2 cannot simultaneously be hydrogen, and wherein R1 and R2 and the
intervening
carbon atom can form a dioxane or dioxolane ring;
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
R3 is hydrogen, -C14alkyl, fluoro, chloro, -OCI -C(0)H, -C(0)0H, -C(0)0C1_
2a1ky1, -CN, -C(0)NHC1_2alkyl, or -C(0)NH2, wherein each of said R3 alkyl is
optionally substituted with up to 3 fluorine atoms, up to two OH, or up to two
OCi_
zalkyl groups;
R4 is hydrogen, C1_4alkyl, C2_4alkenyl, C2_4alkynyl, C3_5cycloalkyl, phenyl, a
5-10-
membered monocyclic or bicyclic heteroaryl ring selected from pyrrole,
imidazole,
pyrazole, triazole, thiazole, isothiazole, oxazole, pyridine, pyrimidine,
pyrimidinone,
pyrazine, pyridazine, or quinoline, or a 4-10-membered monocyclic or bicyclic
heterocyclyl ring selected from oxetane, tetrahydrofuran, tetrahydropyran,
dihydroisoxazole, pyrimidine-2,4(1H,3H)-dione, dihydrofuropyrimidine,
dihydropyranopyrimidine, dihydropyrrolopyrimidine, tetrahydropteridine, or
tetrahydropyridopyrimidine, wherein each of said R4 groups is optionally
substituted
with up to four Br, Cl, F, or Ci_4alkyl, up to three CN, NO2, C2_4alkenyl,
C2_4alkynyl,
C3_6cycloalky1, C04 alkyl-C35 cycloalkyl, C0_4 alkyl-O-C1_4 alkyl, Co4 alkyl-O-
Co4
alkyl-C3_5 cycloalkyl, C(0)0C1_4 alkyl, C(0)000_4 alkyl-C3_5 cycloalkyl, C0_4
alkyl-
C(0)NH2, C(0)NHC1_4 alkyl, C(0)N(C1_4alky1)2, C(0)NH(C0_4alkyl-C3_5
cycloalkyl),
CH2OR5, C0_4 alkyl-C(0)R5, C0_4 alkyl-C(0)N(R5)2, C0_4 alkyl-C(0)0R5, C0_4
alkyl-
NHC(0)R5, C0-4 alkyl-N(R5)2, a heterocyclic ring system selected from oxetane,
azetidine, tetrahydrofuran, dihydropyran, tetrahydropyran, morpholine,
piperidine,
pyrrolidine or piperazine, a heteroaryl ring system selected from furan,
oxazole,
oxadiazole, pyrrole, pyrazole, triazole, oxadiazole or tetrazole, or up to two
OR5,
wherein each of said optional R4 substituents is optionally substituted with
up to four
fluorine atoms, up to two Ci 4alkyl groups, up to two OH groups, up to two OCi
4alkyl
groups, up to two SC1_4alkyl groups, a C(0)C1_4 alkyl, a C(0)0C1_4 alkyl, or a
C(0)0C04 alkyl-C35 cycloalkyl; and
each R5 is, independently, hydrogen, Ci_4alkyl, a 5-6-membered heteroaryl
selected from
imidazole, triazole, thiazole, pyridine, or pyrimidine, a 4-6-membered
heterocyclyl
selected from oxetane, tetrahydrofuran, or tetrahydropyran, and each R5 group
is
optionally substituted with chloro, up to three fluorine atoms, up to two
Ci_)alkyl, CH2OH,
CN, up to two OH, up to two OCi_2alkyl, a spirooxetane, pyrrolidine, or
triazole, or two R5
groups together with the intervening nitrogen atom form a morpholine ring,
azetidine ring,
pyrrolidine ring, piperidine ring, or piperazine ring.
11
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
[0032] In one embodiment, Ring C is cyclobutane.
[0033] In another aspect, the invention features compounds having the
formula:
CA)
X N
R1'1115
R2
(II), wherein RI and R2 are as defined for
compounds of formula I.
[0034] In another aspect, the invention features compounds having the
formula:
(II-A), wherein R2 is as defined for compounds
of formula I.
[0035] In another aspect, the invention features compounds having the
formula:
R3
N
N
(II-A-1), wherein R2 is as defined for
compounds of formula I.
[0036] In another aspect, the invention features compounds having the
formula:
12
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
X
R1
(II-B), wherein R1 is as defined for compounds
of formulal.
[0037] In another aspect, the invention features compounds having the
formula:
R3
N
I x CI I
R1
(II-B-1), wherein R1 is as defined for
compounds of formula I.
[0038] In one embodiment, R1 is -00_4 alkyl-NHR4.
100391 In another embodiment, X is ¨0- or -0Ci_4 alkyl-.
[0040] In one embodiment, Ring C is cyclohexane.
[0041] In another aspect, the invention features compounds having the
formula:
A
R1 JX X N
R-2
(III), wherein RI is as defined for compounds of
formula I.
[0042]
[0043] In one embodiment, X is ¨NH-.
[0044] In another aspect, the invention features compounds having the
formula:
13
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
A
R2Nµ'.0'
(III-A), wherein R2 is as defined for compounds of
formula I.
[0045] In another aspect, the invention features compounds having the
formula:
R3
N--x)
N
µØ0N creN =
,v=
R2Nss
(III-A-1) or (III-A-2),
wherein R2 is as defined for compounds of formula I.
[0046] In one embodiment, R2 is -00_4 alkyl-NHR4 or -OW.
[0047] In another embodiment, R2 is -NHR4 or -OW.
[0048] In another aspect, the invention features compounds having the
formula:
A
ecoN N
R1
(III-B), wherein Rl is as defined for compounds of
formula I.
[0049] In another aspect, the invention features compounds having the
formula:
N
1 ' N-R
C
R1 r I.
N
R1 N
Cf.
(III-B-1) or (III-B-
2), wherein R1 is as defined for compounds of formula I.
14
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
[0050] In one embodiment, R1 is -00_4 alkyl-NHR4 or -0R4.
[0051] In another embodiment, R1 is -NHR4 or -0R4.
[0052] In another embodiment, X is ¨0-.
[0053] In another aspect, the invention features compounds having the
formula:
A
0
0#
R2µs
(III-C), wherein R2 is as defined for compounds of formula I.
[0054] In another aspect, the invention features compounds having the
formula:
R3
N N-0
I ' / `N
,== 0
101
N
R2s' R2's
(III-C-1) or (III-C-2), wherein
R2 is as defined for compounds of formula I.
[0055] In another aspect, the invention features compounds having the
formula:
0000 401 N
R1
(III-D), wherein R1 is as defined for compounds of formula I.
[0056] In another aspect, the invention features compounds having the
formula:
R3
I ' N
00,0 or- N icy Or
R1 R1
(III-D-1) or (III-D-2),
wherein
R1 is as defined for compounds of formula I.
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
[0057] In one embodiment, R1 is -00_4 alkyl-NHR4 or -OW.
[0058] In another embodiment, RI is -NHR4 or -ORLI.
[0059] In another aspect, the invention features compounds having the
formula:
R3
ioI-1\1
0
R4"
(III-D-3), wherein Y is -0- or -NH-.
[0060] In one embodiment of compounds having formula I, 11, II-A, II-A-1,
II-B, II-
B-1, III, III-A, III-A-1, III-A-2, III-B, III-B-1, III-B-2, III-C, III-C-1,
III-C-2, III-D,
III-D-1, III-D-2, or III-D-3,
is 0 , , C0), 0 ,or 0
[0061] In another embodiment,
slJart
C
is 0 .
[0062] In another embodiment, fe is hydrogen.
[0063] In another embodiment, R4 is hydrogen, Ci 4alkyl, C2 4alkenyl, C2
4allcynyl, C3
5cycloalkyl, phenyl, wherein each of said R4 groups is optionally substituted
with up to
four Br, Cl, F, or C1_4a1kyl, up to three CN, NO2, C2_4alkeny1, C2_4a1kyny1,
C3_6cycloa1kyl,
Co_4 alkyl-C3_5 cycloalkyl, Co_4 alkyl-O-C1_4 alkyl, Co_4 alkyl-O-00_4 alkyl-
C3_5 cycloalkyl,
C(0)0C1_4 alkyl, C(0)000_4 alkyl-C3_5 cycloalkyl, Co_4 alkyl-C(0)NH2,
C(0)NHC1_4 alkyl,
C(0)N(C1 4 alky1)2, C(0)NH(C0 4 alkyl-C35 cycloalkyl), CH2OR5, Co 4 alkyl-
C(0)R5, Co 4
alkyl-C(0)N(R5)2, Co_4 alkyl-C(0)0R5, Co_4 alkyl-NHC(0)R5, Co_4 alkyl-N(R5)2,
a
heterocyclic ring system selected from oxetane, azetidine, tetrahydrofuran,
dihydropyran,
tetrahydropyran, morpholine, piperidine, pyrrolidine or piperazine, a
heteroaryl ring
system selected from furan, oxazole, oxadiazole, pyrrole, pyrazole, triazole,
oxadiazole or
tetrazole, or up to two OR5, wherein each of said optional R4 substituents is
optionally
substituted with up to four fluorine atoms, up to two Ci_4alkyl groups, up to
two OH
16
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
groups, up to two 0C1_4alkyl groups, up to two SC1_4alkyl groups, a C(0)C14
alkyl, a
C(0)0C1_4 alkyl, or a C(0)000_4 alkyl-C3_5 cycloalkyl; and
each R5 is, independently, hydrogen, Ci_4alkyl, a 5-6-membered heteroaryl
selected from
imidazole, triazole, thiazole, pyridine, or pyrimidine, a 4-6-membered
heterocyclyl
selected from oxetane, tetrahydrofuran, or tetrahydropyran, and each R5 group
is
optionally substituted with chloro, up to three fluorine atoms, up to two
Ci_2alkyl, CH2OH,
CN, up to two OH, up to two OCi_2alkyl, a spirooxetane, pyrrolidine, or
triazole, or two R5
groups together with the intervening nitrogen atom form a morpholine ring,
azetidine ring,
pyrrolidine ring, piperidine ring, or piperazine ring.
[0064] In another embodiment, R4 is a 5-10-membered monocyclic or bicyclic
heteroaryl ring selected from pyrrole, imidazole, pyrazole, triazole,
thiazole, isothiazole,
oxazole, pyridine, pyrimidine, pyrimidinone, pyrazine, pyridazine, or
quinoline, wherein
each of said R4 groups is optionally substituted with up to four Br, Cl, F, or
Ci_4alkyl, up
to three CN, NO2, C2_4alkenyl, C2_4alkynyl, C3_6cycloa1ky1, C0_4 alkyl-C3_5
cycloalkyl, C0-4
alkyl-O-C14 alkyl, Co_4 alkyl-O-00_4 alkyl-C3_5 cycloalkyl, C(0)0C1-1 alkyl,
C(0)000-4
alkyl-C3_5 cycloalkyl, Co_4 alkyl-C(0)NH2, C(0)NHC1_4 alkyl, C(0)N(C1_4
alky1)2,
C(0)NH(C0_4 alkyl-C3_5 cycloalkyl), CH2OR5, Co_4 alkyl-C(0)R5, Co_4 alkyl-
C(0)N(R5)2,
Co_4 alkyl-C(0)0R5, C0-1 alkyl-NHC(0)R5, CO4 alkyl-N(R5)2, a heterocyclic ring
system
selected from oxetane, azetidine, tetrahydrofuran, dihydropyran,
tetrahydropyran,
morpholine, piperidine, pyrrolidine or piperazine, a heteroaryl ring system
selected from
furan, oxazole, oxadiazole, pyrrole, pyrazole, triazole, oxadiazole or
tetrazole, or up to two
OR5, wherein each of said optional R4 substituents is optionally substituted
with up to four
fluorine atoms, up to two CI 4alkyl groups, up to two OH groups, up to two OCi
4alkyl
groups, up to two SCi_4alkyl groups, a C(0)C1_4 alkyl, a C(0)0C1_4 alkyl, or a
C(0)000-4
alkyl-C3_5 cycloalkyl; and
each R5 is, independently, hydrogen, Ci_4alkyl, a 5-6-membered heteroaryl
selected from
imidazole, triazole, thiazole, pyridine, or pyrimidine, a 4-6-membered
heterocyclyl
selected from oxetane, tetrahydrofuran, or tetrahydropyran, and each R5 group
is
optionally substituted with chloro, up to three fluorine atoms, up to two
Ci_)alkyl, CH2OH,
CN, up to two OH, up to two OCi_2alkyl, a spirooxetane, pyrrolidine, or
triazole, or two R5
groups together with the intervening nitrogen atom form a morpholine ring,
azetidine ring,
pyrrolidine ring, piperidine ring, or piperazine ring.
17
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
[0065] In yet another embodiment, R4 is pyridine or pyrimidine, which is
optionally
substituted with up to four Br, Cl, F, or Ci_Alkyl, up to three CN, NO2,
C2_4alkenyl, C2_
4alkynyl, C3_6cycloalkyl, C04 alkyl-C3_5 cycloalkyl, C04 alkyl-O-C14 alkyl,
Co_4 alkyl-O-00_
4 alkyl-C3_5 cycloalkyl, C(0)0C1_4 alkyl, C(0)000_4 alkyl-C3_5 cycloalkyl, C04
alkyl-
C(0)NH2, C(0)NHC1_4 alkyl, C(0)N(C1_4 alky1)2, C(0)NH(C0_4 alkyl-C3_5
cycloalkyl),
CH2OR5, Co_4 alkyl-C(0)R5, Co_4 alkyl-C(0)N(R5)2, Co_4 alkyl-C(0)0R5, Co_4
alkyl-
NHC(0)R5, Co_4 alkyl-N(R5)2, a heterocyclic ring system selected from oxetane,
azetidine,
tetrahydrofuran, dihydropyran, tetrahydropyran, morpholine, piperidine,
pyrrolidine or
piperazine, a heteroaryl ring system selected from furan, oxazole, oxadiazole,
pyrrole,
pyrazole, triazole, oxadiazole or tetrazole, or up to two OR5, wherein each of
said optional
R4 substituents is optionally substituted with up to four fluorine atoms, up
to two Ci_4a1kyl
groups, up to two OH groups, up to two OCi_4alkyl groups, up to two SCi4alkyl
groups, a
C(0)C1_4 alkyl, a C(0)0C1_4 alkyl, or a C(0)000_4 alkyl-C3_5 cycloalkyl; and
each R5 is, independently, hydrogen, Ci_4alkyl, a 5-6-membered heteroaryl
selected from
imidazole, triazole, thiazole, pyridine, or pyrimidine, a 4-6-membered
heterocyclyl
selected from oxetane, tetrahydrofuran, or tetrahydropyran, and each R5 group
is
optionally substituted with chloro, up to three fluorine atoms, up to two
Ci_2alkyl, CH2OH,
CN, up to two OH, up to two OCi_2alkyl, a spirooxetane, pyrrolidine, or
triazole, or two R5
groups together with the intervening nitrogen atom form a morpholine ring,
azetidine ring,
pyrrolidine ring, piperidine ring, or piperazine ring.
100661 In another embodiment, R4 is a 4-10-membered monocyclic or bicyclic
heterocyclyl ring selected from oxetane, tetrahydrofuran, tetrahydropyran,
dihydroisoxazole, pyrimidine-2,4(1H,3H)-dione, dihydrofuropyrimidine,
dihydropyranopyrimidine, dihydropyrrolopyrimidine, tetrahydropteridine, or
tetrahydropyridopyrimidine, wherein each of said R4 groups is optionally
substituted with
up to four Br, Cl, F, or C1_4alkyl, up to three CN, NO2, C2_4alkenyl,
C2_4alkynyl, C3_
6cyc10a1ky1, C04 alkyl-C3_5 cycloalkyl, C0_4 alkyl-O-C14 alkyl, C04 alkyl-O-
00_4 alkyl-C3_5
cycloalkyl, C(0)0C1_4 alkyl, C(0)000_4 alkyl-C3_5 cycloalkyl, C04 alkyl-
C(0)NH2,
C(0)NHC1_4 alkyl, C(0)N(C1_4 alky1)2, C(0)NH(C0_4alkyl-C3_5 cycloalkyl),
CH2OR5, C0-4
alkyl-C(0)R5, C04 alkyl-C(0)N(R5)2, C04 alkyl-C(0)0R5, C04 alkyl-NHC(0)R5, C0-
4
alkyl-N(R5)2, a heterocyclic ring system selected from oxetane, azetidine,
tetrahydrofuran,
dihydropyran, tetrahydropyran, morpholine, piperidine, pyrrolidine or
piperazine, a
18
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
heteroaryl ring system selected from furan, oxazole, oxadiazole, pyrrole,
pyrazole,
triazole, oxadiazole or tetrazole, or up to two OR5, wherein each of said
optional R4
substituents is optionally substituted with up to four fluorine atoms, up to
two Ci_4alkyl
groups, up to two OH groups, up to two OCi_zialkyl groups, up to two
SCi_zialkyl groups, a
C(0)C1_4 alkyl, a C(0)0C14 alkyl, or a C(0)0004 alkyl-C3_5 cycloalkyl; and
each R5 is, independently, hydrogen, Cialkyl, a 5-6-membered heteroaryl
selected from
imidazole, triazole, thiazole, pyridine, or pyrimidine, a 4-6-membered
heterocyclyl
selected from oxetane, tetrahydrofuran, or tetrahydropyran, and each R5 group
is
optionally substituted with chloro, up to three fluorine atoms, up to two
Ci_2alkyl, CH2OH,
CN, up to two OH, up to two OCi_2alkyl, a spirooxetane, pyrrolidinc, or
triazole, or two R5
groups together with the intervening nitrogen atom form a morpholine ring,
azetidine ring,
pyrrolidine ring, piperidine ring, or piperazine ring.
[0067] In another aspect, the invention features compounds having the
formula:
N /1
N
HNC'
144
0 (IV), wherein
R3 is hydrogen, Ci4alkyl, fluoro, chloro, -0C1_2alkyl, or -C(0)N1-12, -
C(0)H, or -CN, wherein each of said R3 alkyl is optionally substituted with OH
or up to 3
fluorine atoms;
R4 is
N X1
R42¨, )(2"
is N, CH, CF, CC1, or CC1_2 alkyl optionally substituted with up to 3
fluorine atoms;
X2 is N or CR4c; wherein Xl and X2 cannot simultaneously be N,
each of R4a , R4b, and R4 is, independently, hydrogen, F, Cl, Br, CN, NO2,
Ci _4 alkyl, C0_4 alkyl-C3_5 cycloalkyl, Co_4 alkyl-O-C1_4 alkyl, Co_4 alkyl-O-
004 alkyl-C3_5
cycloalkyl, C2_4 alkenyl, C2_4 alkynyl, C(0)0C14 alkyl, C(0)000_4 alkyl-C3_5
cycloalkyl,
19
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
C(0)NH2, C(0)NHC1_4 alkyl, C(0)N(C1 _4 alky1)2, C(0)NH(C0_4alkyl-C3_5
cycloalkyl), a
heterocyclic ring system selected from oxetane, azetidine, tetrahydrofuran,
dihydropyran,
tetrahydropyran, morpholine, piperidine, or piperazine, or a heteroaryl ring
system
selected from furan, oxazole, oxadiazole, pyrrole, pyrazole, triazole, or
tetrazole, or R4',
R4a, and the intervening atoms form a dihydrofuran, a dihydropyran, or a
tetrahydropiperidine heterocyclic ring system;
wherein each of said R4a, R4b, or R4e heterocyclic or heteroaryl ring systems
is optionally substituted with up to four fluorine atoms, up to two C1_4
alkyl, up to two OH
groups, a C(0)C1_4 alkyl, a C(0)0C1_4 alkyl, or a C(0)0004 alkyl-C3_5
cycloalkyl; and
wherein each of said R4a , R4b, or R4e alkyl or cycloalkyl is optionally
substituted with up to 2 non-geminal OH groups or up to 3 fluorine atoms.
[0068] In one embodiment, R.' is hydrogen.
[0069] In another embodiment, each of XI and X2 is, independently, CH or N.
[0070] In another embodiment, each of R4a and R4b is, independently, a
heterocyclic
ring system selected from oxetane, azetidine, tetrahydrofuran, dihydropyran,
tetrahydropyran, morpholine, piperidine, or piperazine, wherein each of said
R4a or leb
heterocyclic or heteroaryl ring systems is optionally substituted with up to
four fluorine
atoms, up to two C1_4 alkyl, up to two OH groups, a C(0)C14 alkyl, a C(0)0C14
alkyl, or
a C(0)0004 alkyl-C3_5 cycloalkyl; and wherein each of said R4a or R4b alkyl or
cycloalkyl
is optionally substituted with up to 2 non-geminal OH groups or up to 3
fluorine atoms.
100711 In another embodiment, each of R4a and R4b is, independently, a
heteroaryl ring
system selected from furan, oxazole, oxadiazole, pyrrole, pyrazole, triazole,
or tetrazole,
wherein each of said R4a or R4b heterocyclic or heteroaryl ring systems is
optionally
substituted with up to four fluorine atoms, up to two Ci_4 alkyl, up to two OH
groups, a
C(0)C1 _4 alkyl, a C(0)0C14 alkyl, or a C(0)0004 alkyl-C3_5 cycloalkyl; and
wherein each
of said R4a or R4b alkyl or cycloalkyl is optionally substituted with up to 2
non-geminal
OH groups or up to 3 fluorine atoms.
[0072] In another embodiment, each of R4a and R4b is, independently,
hydrogen, F, Cl,
Br, CN, NO2, C1_4 alkyl, C0_4 alkyl-C3_5 cycloalkyl, C0_4 alkyl-O-C1_4 alkyl,
Co_4 alkyl-O-00-4
alkyl-C3_5 cycloalkyl, C2_4 alkenyl, C7_4 alkynyl, C(0)0C14 alkyl, C(0)0004
alkyl-C3-5
cycloalkyl, C(0)NH2, C(0)NHC1_4 alkyl, C(0)N(C1_4alky1)2, or C(0)NH(C0_4alkyl-
C3-5
cycloalkyl), wherein each of said R4a or R4b heterocyclic or heteroaryl ring
systems is
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
optionally substituted with up to four fluorine atoms, up to two CI _4 alkyl,
up to two OH
groups, a C(0)C1_4 alkyl, a C(0)0C14 alkyl, or a C(0)0004 alkyl-C3_5
cycloalkyl; and
wherein each of said R4a or R4b alkyl or cycloalkyl is optionally substituted
with up to 2
non-geminal OH groups or up to 3 fluorine atoms.
[0073] In another embodiment, the invention features a compound selected
from the
group of compounds listed in Table I.
[0074] In another embodiment, the invention features a compound selected
from the
group of compounds listed in Table 2.
Compositions, Formulations, and Administration of Compounds of the Invention
[0075] In another embodiment, the invention provides a pharmaceutical
composition
comprising a compound of any of the formulae described herein and a
pharmaceutically
acceptable excipient. In a further embodiment, the invention provides a
pharmaceutical
composition comprising a compound of Table 1. In a further embodiment, the
composition additionally comprises an additional therapeutic agent.
[0076] According to another embodiment, the invention provides a
composition
comprising a compound of this invention or a pharmaceutically acceptable
derivative
thereof and a pharmaceutically acceptable carrier, adjuvant, or vehicle. In
one
embodiment, the amount of compound in a composition of this invention is such
that is
effective to measurably inhibit a DNA-PK in a biological sample or in a
patient. In
another embodiment, the amount of compound in the compositions of this
invention is
such that is effective to measurably inhibit DNA-PK. In one embodiment, the
composition of this invention is formulated for administration to a patient in
need of such
composition. In a further embodiment, the composition of this invention is
formulated for
oral administration to a patient.
[0077] The term "patient," as used herein, means an animal, preferably a
mammal, and
most preferably a human.
[0078] It will also be appreciated that certain of the compounds of present
invention
can exist in free form for treatment, or where appropriate, as a
pharmaceutically
acceptable derivative thereof. According to the present invention, a
pharmaceutically
acceptable derivative includes, but is not limited to, pharmaceutically
acceptable prodntgs,
salts, esters, salts of such esters, or any other adduct or derivative which
upon
21
81791389
administration to a patient in need is capable of providing, directly or
indirectly, a compound
as otherwise described herein, or a metabolite or residue thereof. As used
herein, the term
"inhibitory active metabolite or residue thereof" means that a metabolite or
residue thereof is
also an inhibitor of DNA-PK.
[0079] As used herein, the term "pharmaceutically acceptable salt" refers
to those salts
which are, within the scope of sound medical judgment, suitable for use in
contact with the
tissues of humans and lower animals without undue toxicity, irritation,
allergic response and
the like.
[0080] Pharmaceutically acceptable salts are well known in the art. For
example, S. M.
Berge et al., describe pharmaceutically acceptable salts in detail in I
Pharmaceutical
Sciences, 66:1-19, 1977. Pharmaceutically acceptable salts of the compounds of
this
invention include those derived from suitable inorganic and organic acids and
bases.
Examples of pharmaceutically acceptable, nontoxic acid addition salts are
salts of an amino
group formed with inorganic acids such as hydrochloric acid, hydrobromic acid,
phosphoric
acid, sulfuric acid and perchloric acid or with organic acids such as acetic
acid, oxalic acid,
maleic acid, tartaric acid, citric acid, succinic acid or malonic acid or by
using other methods
used in the art such as ion exchange. Other pharmaceutically acceptable salts
include adipate,
alginate, ascorbate, aspartate, benzenesulfonate, benzoate, bisulfate, borate,
butyrate,
camphorate, camphorsulfonate, citrate, cyclopentanepropionate, digluconate,
dodecylsulfate,
ethanesulfonate, formate, fumarate, glucoheptonate, glycerophosphate,
gluconate,
hemisulfate, heptanoate, hexanoate, hydroiodide, 2-hydroxy-ethanesulfonate,
lactobionate,
lactate, laurate, lauryl sulfate, malate, maleate, malonate, methanesulfonate,
2-
naphthalenesulfonate, nicotinate, nitrate, oleate, oxalate, palmitate,
pamoate, pectinate,
persulfate, 3-phenylpropionate, phosphate, picrate, pivalate, propionate,
stearate, succinate,
sulfate, tartrate, thiocyanate, p-toluenesulfonate, undecanoate, valerate
salts, and the like.
Salts derived from appropriate bases include alkali metal, alkaline earth
metal, ammonium
and N (Ci_4alky1)4 salts. This invention also envisions the quaternization of
any basic
nitrogen-containing groups of the compounds disclosed herein. Water or oil-
soluble or
dispersable products may be obtained by such quaternization. Representative
alkali or
alkaline earth metal salts include sodium, lithium, potassium, calcium,
magnesium, and the
like. Further pharmaceutically acceptable salts include, when appropriate,
nontoxic
22
Date Recue/Date Received 2020-08-10
81791389
ammonium, quaternary ammonium, and amine cations formed using counterions such
as
halide, hydroxide, carboxylate, sulfate, phosphate, nitrate, C1-8 sulfonate
and aryl sulfonate.
[0081] As described above, the pharmaceutically acceptable compositions of
the present
invention additionally comprise a pharmaceutically acceptable carrier,
adjuvant, or vehicle,
which, as used herein, includes any and all solvents, diluents, or other
liquid vehicle,
dispersion or suspension aids, surface active agents, isotonic agents,
thickening or emulsifying
agents, preservatives, solid binders, lubricants and the like, as suited to
the particular dosage
form desired. In Remington: The Science and Practice of Pharmacy, 21st
edition, 2005, ed.
D.B. Troy, Lippincott Williams & Wilkins, Philadelphia, and Encyclopedia of
Pharmaceutical Technology, eds. J. Swarbrick and J. C. Boylan, 1988-1999,
Marcel Dekker,
New York are disclosed various carriers used in formulating pharmaceutically
acceptable
compositions and known techniques for the preparation thereof. Except insofar
as any
conventional carrier medium is incompatible with the compounds of the
invention, such as by
producing any undesirable biological effect or otherwise interacting in a
deleterious manner
with any other component(s) of the pharmaceutically acceptable composition,
its use is
contemplated to be within the scope of this invention.
[0082] Some examples of materials which can serve as pharmaceutically
acceptable
carriers include, but are not limited to, ion exchangers, alumina, aluminum
stearate, lecithin,
serum proteins, such as human serum albumin, buffer substances such as
phosphates, glycine,
sorbic acid, or potassium sorbate, partial glyceride mixtures of saturated
vegetable fatty acids,
water, salts or electrolytes, such as protamine sulfate, disodium hydrogen
phosphate,
potassium hydrogen phosphate, sodium chloride, zinc salts, colloidal silica,
magnesium
trisilicate, polyvinyl pyrrolidone, polyacrylates, waxes, polyethylene-
polyoxypropylene-block
polymers, wool fat, sugars such as lactose, glucose and sucrose; starches such
as corn starch
and potato starch; cellulose and its derivatives such as sodium carboxymethyl
cellulose, ethyl
cellulose and cellulose acetate; powdered tragacanth; malt; gelatin; talc;
excipients such as
cocoa butter and suppository waxes; oils such as peanut oil, cottonseed oil;
safflower oil;
sesame oil; olive oil; corn oil and soybean oil; glycols; such a propylene
glycol or
polyethylene glycol; esters such as ethyl oleate and ethyl laurate; agar;
buffering agents such
as magnesium hydroxide and aluminum
23
Date Recue/Date Received 2020-08-10
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
hydroxide; alginic acid; pyrogen-free water; isotonic saline; Ringer's
solution; ethyl
alcohol, and phosphate buffer solutions, as well as other non-toxic compatible
lubricants
such as sodium lauryl sulfate and magnesium stearate, as well as coloring
agents, releasing
agents, coating agents, sweetening, flavoring and perfuming agents,
preservatives and
antioxidants can also be present in the composition, according to the judgment
of the
formulator.
[0083] The compositions of the present invention may be administered
orally,
parenterally, by inhalation spray, topically, rectally, nasally, buccally,
vaginally or via an
implanted reservoir. The term "parenteral" as used herein includes
subcutaneous,
intravenous, intramuscular, intra-articular, intra-synovial, intrasternal,
intrathecal,
intraocular, intrahepatic, intralesional, epidural, intraspinal, and
intracranial injection or
infusion techniques. Preferably, the compositions are administered orally,
intraperitoneally or intravenously. Sterile injectable forms of the
compositions of this
invention may be aqueous or oleaginous suspension. These suspensions may be
formulated according to techniques known in the art using suitable dispersing
or wetting
agents and suspending agents. The sterile injectable preparation may also be a
sterile
injectable solution or suspension in a non-toxic parenterally acceptable
diluent or solvent,
for example as a solution in 1,3-butanediol. Among the acceptable vehicles and
solvents
that may be employed are water, Ringer's solution and isotonic sodium chloride
solution.
In addition, sterile, fixed oils are conventionally employed as a solvent or
suspending
medium.
[0084] For this purpose, any bland fixed oil may be employed including
synthetic
mono- or diglycerides. Fatty acids, such as oleic acid and its glyceride
derivatives are
useful in the preparation of injectables, as are natural pharmaceutically-
acceptable oils,
such as olive oil or castor oil, especially in their polyoxyethylated
versions. These oil
solutions or suspensions may also contain a long-chain alcohol diluent or
dispersant, such
as carboxymethyl cellulose or similar dispersing agents that are commonly used
in the
formulation of pharmaceutically acceptable dosage forms including emulsions
and
suspensions. Other commonly used surfactants, such as Twecns, Spans and other
emulsifying agents or bioavailability enhancers which are commonly used in the
manufacture of pharmaceutically acceptable solid, liquid, or other dosage
forms may also
be used for the purposes of formulation.
24
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
[0085] The pharmaceutically acceptable compositions of this invention may
be orally
administered in any orally acceptable dosage form including, but not limited
to, capsules,
tablets, aqueous suspensions or solutions. In the case of tablets for oral
use, carriers
commonly used include lactose and corn starch. Lubricating agents, such as
magnesium
stearate, are also typically added. For oral administration in a capsule form,
useful
diluents include lactose and dried cornstarch. When aqueous suspensions are
required for
oral use, the active ingredient is combined with emulsifying and suspending
agents. If
desired, certain sweetening, flavoring or coloring agents may also be added.
[0086] Alternatively, the pharmaceutically acceptable compositions of this
invention
may be administered in the form of suppositories for rectal administration.
These can be
prepared by mixing the agent with a suitable non-irritating excipient that is
solid at room
temperature but liquid at rectal temperature and therefore will melt in the
rectum to release
the drug. Such materials include cocoa butter, beeswax and polyethylene
glycols.
[0087] The pharmaceutically acceptable compositions of this invention may
also be
administered topically, especially when the target of treatment includes areas
or organs
readily accessible by topical application, including diseases of the eye, the
skin, or the
lower intestinal tract. Suitable topical formulations are readily prepared for
each of these
areas or organs.
[0088] Topical application for the lower intestinal tract can be effected
in a rectal
suppository formulation (see above) or in a suitable enema formulation.
Topically-
transdermal patches may also be used.
[0089] For topical applications, the pharmaceutically acceptable
compositions may be
formulated in a suitable ointment containing the active component suspended or
dissolved
in one or more carriers. Carriers for topical administration of the compounds
of this
invention include, but are not limited to, mineral oil, liquid petrolatum,
white petrolatum,
propylene glycol, polyoxyethylene, polyoxypropylene compound, emulsifying wax
and
water. Alternatively, the pharmaceutically acceptable compositions can be
formulated in a
suitable lotion or cream containing the active components suspended or
dissolved in one
or more pharmaceutically acceptable carriers. Suitable carriers include, but
are not limited
to, mineral oil, sorbitan monostearate, polysorbate 60, cetyl esters wax,
cetearyl alcohol,
2-octyldodecanol, benzyl alcohol and water.
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
[0090] For ophthalmic use, the pharmaceutically acceptable compositions may
be
formulated, e.g., as micronized suspensions in isotonic, pH adjusted sterile
saline or other
aqueous solution, or, preferably, as solutions in isotonic, pH adjusted
sterile saline or other
aqueous solution, either with or without a preservative such as benzylalkonium
chloride.
Alternatively, for ophthalmic uses, the pharmaceutically acceptable
compositions may be
formulated in an ointment such as petrolatum. The pharmaceutically acceptable
compositions of this invention may also be administered by nasal aerosol or
inhalation.
Such compositions are prepared according to techniques well-known in the art
of
pharmaceutical formulation and may be prepared as solutions in saline,
employing benzyl
alcohol or other suitable preservatives, absorption promoters to enhance
bioavailability,
fluorocarbons, and/or other conventional solubilizing or dispersing agents.
[0091] Most preferably, the pharmaceutically acceptable compositions of
this
invention are formulated for oral administration.
[0092] Liquid dosage forms for oral administration include, but are not
limited to,
pharmaceutically acceptable emulsions, microemulsions, solutions, suspensions,
syrups
and elixirs. In addition to the active compounds, the liquid dosage forms may
contain
inert diluents commonly used in the art such as, for example, water or other
solvents,
solubilizing agents and emulsifiers such as ethyl alcohol, isopropyl alcohol,
ethyl
carbonate, ethyl acetate, benzyl alcohol, benzyl benzoate, propylene glycol,
1,3-butylene
glycol, dimethylformamide, oils (in particular, cottonseed, groundnut, corn,
germ, olive,
castor, and sesame oils), glycerol, tetrahydrofurfuryl alcohol, polyethylene
glycols and
fatty acid esters of sorbitan, and mixtures thereof. Besides inert diluents,
the oral
compositions can also include adjuvants such as wetting agents, emulsifying
and
suspending agents, sweetening, flavoring, and perfuming agents.
[0093] Injectable preparations, for example, sterile injectable aqueous or
oleaginous
suspensions may be formulated according to the known art using suitable
dispersing or
wetting agents and suspending agents. The sterile injectable preparation may
also be a
sterile injectable solution, suspension or emulsion in a nontoxic parenterally
acceptable
diluent or solvent, for example, as a solution in 1,3-butanediol. Among the
acceptable
vehicles and solvents that may be employed are water, Ringer's solution,
U.S.P. and
isotonic sodium chloride solution. In addition, sterile, fixed oils are
conventionally
employed as a solvent or suspending medium. For this purpose any bland fixed
oil can be
26
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
employed including synthetic mono- or diglycerides. In addition, fatty acids
such as oleic
acid are used in the preparation of injectables.
[0094] The injectable formulations can be sterilized, for example, by
filtration through
a bacterial-retaining filter, or by incorporating sterilizing agents in the
form of sterile solid
compositions which can be dissolved or dispersed in sterile water or other
sterile injectable
medium prior to use.
[0095] In order to prolong the effect of a compound of the present
invention, it is often
desirable to slow the absorption of the compound from subcutaneous or
intramuscular
injection. This may be accomplished by the use of a liquid suspension of
crystalline or
amorphous material with poor water solubility. The rate of absorption of the
compound
then depends upon its rate of dissolution that, in turn, may depend upon
crystal size and
crystalline form. Alternatively, dissolving or suspending the compound in an
oil vehicle
accomplishes delayed absorption of a parenterally administered compound form.
Injectable depot forms are made by forming microencapsule matrices of the
compound in
biodegradable polymers such as polylactide-polyglycolide. Depending upon the
ratio of
compound to polymer and the nature of the particular polymer employed, the
rate of
compound release can be controlled. Examples of other biodegradable polymers
include
poly(orthoesters) and poly(anhydrides). Depot injectable formulations are also
prepared
by entrapping the compound in liposomes or microemulsions that are compatible
with
body tissues.
100961 Compositions for rectal or vaginal administration are preferably
suppositories
which can be prepared by mixing the compounds of this invention with suitable
non-
irritating excipients or carriers such as cocoa butter, polyethylene glycol or
a suppository
wax which are solid at ambient temperature but liquid at body temperature and
therefore
melt in the rectum or vaginal cavity and release the active compound.
[0097] Solid dosage forms for oral administration include capsules,
tablets, pills,
powders, and granules. In such solid dosage forms, the active compound is
mixed with at
least one inert, pharmaceutically acceptable excipient or carrier such as
sodium citrate or
dicalcium phosphate and/or a) fillers or extenders such as starches, lactose,
sucrose,
glucose, mannitol, and silicic acid, b) binders such as, for example,
carboxymethylcellulose, alginates, gelatin, polyvinylpyrrolidinone, sucrose,
and acacia, c)
humectants such as glycerol, d) disintegrating agents such as agar-agar,
calcium carbonate,
27
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
potato or tapioca starch, alginic acid, certain silicates, and sodium
carbonate, e) solution
retarding agents such as paraffin, f) absorption accelerators such as
quaternary ammonium
compounds, g) wetting agents such as, for example, cetyl alcohol and glycerol
monostearate, 11) absorbents such as kaolin and bentonite clay, and i)
lubricants such as
talc, calcium stcarate, magnesium stearate, solid polyethylene glycols, sodium
lauryl
sulfate, and mixtures thereof. In the case of capsules, tablets and pills, the
dosage form
may also comprise buffering agents.
[0098] Solid compositions of a similar type may also be employed as fillers
in soft and
hard-filled gelatin capsules using such excipients as lactose or milk sugar as
well as high
molecular weight polyethylene glycols and the like. The solid dosage forms of
tablets,
dragees, capsules, pills, and granules can be prepared with coatings and
shells such as
enteric coatings and other coatings well known in the pharmaceutical
formulating art.
They may optionally contain opacifying agents and can also be of a composition
that they
release the active ingredient(s) only, or preferentially, in a certain part of
the intestinal
tract, optionally, in a delayed manner. Examples of embedding compositions
that can be
used include polymeric substances and waxes. Solid compositions of a similar
type may
also be employed as fillers in soft and hard-filled gelatin capsules using
such excipients as
lactose or milk sugar as well as high molecular weight polethylene glycols and
the like.
[0099] The active compounds can also be in micro-encapsulated form with one
or
more excipients as noted above. The solid dosage forms of tablets, dragees,
capsules,
pills, and granules can be prepared with coatings and shells such as enteric
coatings,
release controlling coatings and other coatings well known in the
pharmaceutical
formulating art. In such solid dosage forms the active compound may be admixed
with at
least one inert diluent such as sucrose, lactose or starch. Such dosage forms
may also
comprise, as is normal practice, additional substances other than inert
diluents, e.g.,
tableting lubricants and other tableting aids such a magnesium stearate and
microcrystalline cellulose. In the case of capsules, tablets and pills, the
dosage forms may
also comprise buffering agents. They may optionally contain opacifying agents
and can
also be of a composition that they release the active ingredient(s) only, or
preferentially, in
a certain part of the intestinal tract, optionally, in a delayed manner.
Examples of
embedding compositions that can be used include polymeric substances and
waxes.
28
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
[00100] Dosage forms for topical or transdermal administration of a compound
of this
invention include ointments, pastes, creams, lotions, gels, powders,
solutions, sprays,
inhalants or patches. The active component is admixed under sterile conditions
with a
pharmaceutically acceptable carrier and any needed preservatives or buffers as
may be
required. Ophthalmic formulation, eardrops, and eye drops are also
contemplated as being
within the scope of this invention. Additionally, the present invention
contemplates the
use of transdermal patches, which have the added advantage of providing
controlled
delivery of a compound to the body. Such dosage forms can be made by
dissolving or
dispensing the compound in the proper medium. Absorption enhancers can also be
used to
increase the flux of the compound across the skin. The rate can be controlled
by either
providing a rate controlling membrane or by dispersing the compound in a
polymer matrix
or gel.
[00101] The compounds of the invention are preferably formulated in dosage
unit form
for ease of administration and uniformity of dosage. The expression "dosage
unit form" as
used herein refers to a physically discrete unit of agent appropriate for the
patient to be
treated. It will be understood, however, that the total daily usage of the
compounds and
compositions of the present invention will be decided by the attending
physician within
the scope of sound medical judgment. The specific effective dose level for any
particular
patient or organism will depend upon a variety of factors including the
disorder being
treated and the severity of the disorder; the activity of the specific
compound employed;
the specific composition employed; the age, body weight, general health, sex
and diet of
the patient; the time of administration, route of administration, and rate of
excretion of the
specific compound employed; the duration of the treatment; drugs used in
combination or
coincidental with the specific compound employed, and like factors well known
in the
medical arts.
[00102] The amount of the compounds of the present invention that may be
combined
with the carrier materials to produce a composition in a single dosage form
will vary
depending upon the host treated, the particular mode of administration.
Preferably, the
compositions should be formulated so that a dosage of between 0.01 - 100 mg/kg
body
weight/day of the inhibitor can be administered to a patient receiving these
compositions.
[00103] Depending upon the particular proliferative condition or cancer to be
treated,
additional therapeutic agents, which are normally administered to treat or
prevent that
29
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
condition, may also be present in the compositions of this invention. As used
herein,
additional therapeutic agents which are normally administered to treat or
prevent a
particular proliferative condition or cancer are known as "appropriate for the
disease, or
condition, being treated." Examples of additional therapeutic agents are
provided infra.
[00104] The amount of additional therapeutic agent present in the compositions
of this
invention will be no more than the amount that would normally be administered
in a
composition comprising that therapeutic agent as the only active agent.
Preferably the
amount of additional therapeutic agent in the presently disclosed compositions
will range
from about 50% to 100% of the amount normally present in a composition
comprising that
agent as the only therapeutically active agent.
Uses of the Compounds and Compositions of the Invention
[00105] In one embodiment, the invention provides a method of sensitizing a
cell to a
theraputic agent or a disease state that induces a DNA lesion comprising the
step of
contacting the cell with one or more DNA-PK inhibitors of formulae 1, II, or
III, or
subformula thereof (e.g., formulae I-A, I-A-1, I-A-2, I-B, I-B-1, I-B-2, I-C,
I-C-1, I-C-2,
I-C-3, I-C-4, I-D, I-D-1, I-D-2, I-D-3, I-D-4, or I-D-5).
[00106] The invention further provides methods of potentiating a therapeutic
regimen
for treatment of cancer comprising the step of administering to an individual
in need
thereof an effective amount of a DNA-PK inhibitor of formula I, II, or III, or
a
subformula thereof. In one aspect, the therapeutic regimen for treatment of
cancer
includes radiation therapy.
[00107] Compounds of the invention are useful in instances where radiation
therapy is
indicated to enhance the therapeutic benefit of such treatment. In addition,
radiation
therapy frequently is indicated as an adjuvent to surgery in the treatment of
cancer. The
goal of radiation therapy in the adjuvant setting is to reduce the risk of
recurrence and
enhance disease-free survival when the primary tumor has been controlled.
Adjuvant
radiation therapy is indicated in several diseases including colon, rectal,
lung,
gastroesophageal, and breast cancers as described below.
[00108] The invention also can be practiced by including another anti-cancer
chemotherapeutic agent with a compound of the invention in a therapeutic
regimen for the
treatment of cancer, with or without radiation therapy. The combination of a
DNA-PK
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
inhibitor compound of the invention with such other agents can potentiate the
chemotherapeutic protocol. For example, the inhibitor compound of the
invention can be
administered with etoposide or bleomycin, agents known to cause DNA strand
breakage.
[00109] The invention further relates to radiosensitizing tumor cells
utilizing a
compound of formula I, II, or III, or a subformula thereof The preferred
compounds are
those as described for the pharmaceutical compositions of the invention. A
compound that
can "radiosensitize" a cell, as used herein, is defined as a molecule,
preferably a low
molecular weight molecule, administered to animals in therapeutically
effective amount to
increase the sensitivity of cells to electromagnetic radiation and/or to
promote the
treatment of diseases that are treatable with electromagnetic radiation (e.g.,
X-rays).
Diseases that are treatable with electromagnetic radiation include neoplastic
diseases,
benign and malignant tumors, and cancerous cells.
[00110] The present invention also provides methods of treating cancer in an
animal
that includes administering to the animal an effective amount of a DNA-PK
inhibitor such
as, for example, a compound of the invention. The invention further is
directed to
methods of inhibiting cancer cell growth, including processes of cellular
proliferation,
invasiveness, and metastasis in biological systems. Methods include use of a
compound of
the invention as an inhibitor of cancer cell growth. Preferably, the methods
are employed
to inhibit or reduce cancer cell growth, invasiveness, metastasis, or tumor
incidence in
living animals, such as mammals. The compounds of the invention can be used,
either
alone or in combination with the use of IR or one or more chemotherapeutic
agents, in
treating cancer or inhibiting cancer cell growth. Methods of the invention
also are readily
adaptable for use in assay systems, e.g., assaying cancer cell growth and
properties
thereof, as well as identifying compounds that affect cancer cell growth.
1001111 Tumors or neoplasms include growths of tissue cells in which the
multiplication of the cells is uncontrolled and progressive. Some such growths
are benign,
but others are termed "malignant" and can lead to death of the organism.
Malignant
neoplasms or "cancers" are distinguished from benign growths in that, in
addition to
exhibiting aggressive cellular proliferation, they can invade surrounding
tissues and
metastasize. Moreover, malignant neoplasms are characterized in that they show
a greater
loss of differentiation (greater "dedifferentiation") and their organization
relative to one
another and their surrounding tissues. This property is also called
"anaplasia."
31
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
[00112] Neoplasms treatable by the present invention also include solid
tumors, i.e.,
carcinomas and sarcomas. Carcinomas include those malignant neoplasms derived
from
epithelial cells which infiltrate (invade) the surrounding tissues and give
rise to metastases.
Adenocarcinomas are carcinomas derived from glandular tissue, or from tissues
which
form recognizable glandular structures. Another broad category of cancers
includes
sarcomas, which are tumors whose cells are embedded in a fibrillar or
homogeneous
substance like embryonic connective tissue. The invention also enables
treatment of
cancers of the myeloid or lymphoid systems, including leukemias, lymphomas,
and other
cancers that typically do not present as a tumor mass, but are distributed in
the vascular or
lymphoreticular systems.
[00113] DNA-PK activity can be associated with various forms of cancer in, for
example, adult and pediatric oncology, growth of solid tumors/malignancies,
myxoid and
round cell carcinoma, locally advanced tumors, metastatic cancer, human soft
tissue
sarcomas, including Ewing's sarcoma, cancer metastases, including lymphatic
metastases,
squamous cell carcinoma, particularly of the head and neck, esophageal
squamous cell
carcinoma, oral carcinoma, blood cell malignancies, including multiple
myeloma,
leukemias, including acute lymphocytic leukemia, acute nonlymphocytic
leukemia,
chronic lymphocytic leukemia, chronic myelocytic leukemia, and hairy cell
leukemia,
effusion lymphomas (body cavity based lymphomas), thymic lymphoma lung cancer,
including small cell lung carcinoma, cutaneous T cell lymphoma, Hodgkin's
lymphoma,
non-Hodgkin's lymphoma, cancer of the adrenal cortex, ACTH-producing tumors,
nonsmall cell cancers, breast cancer, including small cell carcinoma and
ductal carcinoma,
gastrointestinal cancers, including stomach cancer, colon cancer, colorectal
cancer, polyps
associated with colorectal neoplasia, pancreatic cancer, liver cancer,
urological cancers,
including bladder cancer, including primary superficial bladder tumors,
invasive
transitional cell carcinoma of the bladder, and muscle-invasive bladder
cancer, prostate
cancer, malignancies of the female genital tract, including ovarian carcinoma,
primary
peritoneal epithelial neoplasms, cervical carcinoma, uterine endometrial
cancers, vaginal
cancer, cancer of the vulva, uterine cancer and solid tumors in the ovarian
follicle,
malignancies of the male genital tract, including testicular cancer and penile
cancer,
kidney cancer, including renal cell carcinoma, brain cancer, including
intrinsic brain
tumors, neuroblastoma, astrocytic brain tumors, gliomas, metastatic tumor cell
invasion in
32
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
the central nervous system, bone cancers, including osteomas and
osteosarcomas, skin
cancers, including malignant melanoma, tumor progression of human skin
keratinocytes,
squamous cell cancer, thyroid cancer, retinoblastoma, neuroblastoma,
peritoneal effusion,
malignant pleural effusion, mesothelioma, Wilms's tumors, gall bladder cancer,
trophoblastic neoplasms, hemangiopericytoma, and Kaposi's sarcoma. Methods to
potentiate treatment of these and other forms of cancer are embraced by the
invention.
[00114] The invention provides a method of inhibiting DNA-PK activity in a
biological
sample that includes contacting the biological sample with a compound or
composition of
the invention. The term "biological sample," as used herein, means a sample
outside a
living organism and includes, without limitation, cell cultures or extracts
thereof; biopsied
material obtained from a mammal or extracts thereof; and blood, saliva, urine,
feces,
semen, tears, or other body fluids or extracts thereof. Inhibition of kinase
activity,
particularly DNA-PK activity, in a biological sample is useful for a variety
of purposes
known to one of skill in the art. Examples of such purposes include, but are
not limited to,
biological specimen storage and biological assays. In one embodiment, the
method of
inhibiting DNA-PK activity in a biological sample is limited to non-
therapeutic methods.
Preparation of Compounds of the In
[00115] As used herein, all abbreviations, symbols and conventions are
consistent with
those used in the contemporary scientific literature. See, e.g., Janet S.
Dodd, ed., The ACS
Style Guide: A Manual for Authors and Editors, 2nd Ed., Washington, D.C.:
American
Chemical Society, 1997. The following definitions describe terms and
abbreviations used
herein:
BPin pinacol boronatc ester
Brine a saturated NaCl solution in water
DCM dichloromethane
DIAD diisopropylazodicarboxylate
DIEA diisopropylethylamine
DMA dimethylacetamide
DMF dimethylformamide
DMSO dimethylsulfoxide
DTT dithiothreitol
33
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS electrospray mass spectrometry
Et20 ethyl ether
Et0Ac ethyl acetate
Et0H ethyl alcohol
HEPES 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid
HPLC high performance liquid chromatography
IPA isopropanol
LAH lithium aluminum hydride
LC-MS liquid chromatography-mass spectrometry
LDA lithium diisoproylethylamide
Me methyl
Me0H methanol
MsC1 methanesulfonyl chloride
MTBE methyl t-butyl ether
NMP N-methylpyrrolidine
Pd2(dba)3 tris(dibenzylideneacetone)dipalladium(0)
Pd(dppf)C12 1,1' bis(diphenylphosphino)-ferrocene dichloro-palladium
PG protecting group
Ph phenyl
(rac)-BINAP racemic 2,2'-bis(diphenylphosphino)-1,1'-binaphthyl
RockPhos di-tert-buty1(2',4',6'-triisopropy1-3,6-dimethoxy-[1,1'-biphenyl]-2-
yl)phosphine
RT or rt room temperature
SF C supercritical fluid chromatography
SPhos 2-dicyclohexylphosphino-2',6'-dimethoxybiphenyl
TBAI tetrabutylammonium iodide
tBu tertiary butyl
THF tetrahydrofuran
TEA triethylamine
TMEDA tetramethylethylenediamine
VPhos [3-(2-dicyclohexylphosphanylpheny1)-2,4-dimethoxy-
phenyl]sulfonyloxysodium
34
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
General Synthetic Procedures
001161 In general, the compounds of this invention may be prepared by methods
described herein or by other methods known to those skilled in the art.
Example 1. General preparation of the compounds of formula G
H 1 0Ø,õNH2 A) N A H A
Br 146 N
11, Co)
___________________ D. Br N
IRI [C] N
____________________________________________ ' Rivi:).N
F NMP, 180 C N , Pd2(dba)3 rac-BINAP,
,, 1\ ki
(step 1-i) Cs2CO3, toluene, 100 C r,
[A] -o) (step 1-ii) [D] Lo)
[B]
N
N deprotect N
10' 1101 Riad_
' a N
10. 0 N
PG _____________________ .
'N1216k1 (step 1-iii) H2N (step 1-iv) Pi`N
H H
N N N
[E] Co) [F] C0 ) [G] )
0
Scheme 1
1001171 Compounds of formula 1, wherein X1 is NH (i.e., compounds of formula 1-
A),
can be prepared as outlined below in Scheme 1. Accordingly, as shown in step 1-
i of
Scheme 1, heteroaryl compounds of formula A can be reacted with morpholine or
a
moTholine analog by heating the mixture in a polar, non-protic solvent to
produce
compounds of formula B. Utilizing a palladium-catalyzed, phosphine ligand-
assisted
Buchwald/Hartwig-type coupling, as shown in step 1-ii of Scheme 1, a compound
of
formula B can be reacted with aminocyclohexanes of formula C to produce
compounds of
formula D, wherein R1 and R2 are as described elsewhere herein. In one
example, when
monoprotected meso cyclohexane-1,4-diamines of formula E are prepared, removal
of the
protecting group forms compounds of formula F, as shown in step 1-iii of
Scheme I. The
resulting free amine can then be reacted with various moieties having groups
reactive
towards amines (e.g., Ria-L, where L is a leaving group such as chloro, bromo,
iodo,
toluenesulfonate, methanesulfonate, or trifluoromethanesulfonate; or where L
is a reactive
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
carbonyl-containing moiety such as an active ester or an isocyanato group) to
produce a
compound of formula G, as shown in step 1-iv of Scheme 1.
Example 2. General preparation of the compounds of formula M, N, R, and S
A A C HO A
HO protect PG_0
0 deprotect
I -b.
(step 2-i) NMP, 180 C (step 2-iii)
[H] Br [-I] Br (step 2-ii) [K] 0
R10
0 gain
01 or R2 , WI
R1 'sµ
base [M] [N] (
(step 2-iv)
0 0
Scheme 2a
1001181 Compounds of formula I, wherein X' is 0 can be prepared as outlined
below in
Schemes 2a and 2b. Accordingly, as shown in step 2-i of Scheme 2a, the
hydroxyl group
of heteroaryl compounds of formula H can be protected to produce compounds of
formula
J, which can then be reacted with morpholine or a morpholine analog by heating
the
mixture in a polar, non-protic solvent to produce compounds of formula K after
removal
of the protecting group, as shown in steps 2-ii and 2-iii of Scheme 2a.
Subsequently, as
shown in step 2-iv of Scheme 2a, a compound of formula K can be reacted with a
compound of formula L under conditions sufficient to affect the SN2
displacement of its
leaving group (e.g., where L is a leaving group such as chloro, bromo, iodo,
toluenesulfonate, methanesulfonate, or trifluoromethanesulfonate) to produce a
compound
of formula M or formula M, depending on whether R1 or R2 is hydrogen. In those
instances when Rl or R2 are protected nitrogen or oxygen moieties, compounds
of the
invention can be produced by removal of the protecting group and subsequent
synthetic
manipulation of the resulting free amine/alcohol.
36
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
[00119] Alternatively, as shown in Scheme 2b, the hydroxyl group of a compound
of
formula 0 can be reacted with a compound of formula L to produce a fused
bicycloheteroaryl bromide of formula P, which can subsequently be reacted with
morpholine or a morpholine analog to produce a compound of formula M or
formula N.
,L H
N
CA) R1.-0' A ( )
HO N
IP IR.. [L]
a. R1Ø N 0
NMP, 180 Car [M] or [N]
base
R*1 (step 2-vi)
Br [P] Br
[0] (step 2-v)
Scheme 2b
[00120] Alternatively, as shown in Scheme 2c, compounds of the invention in
which
Ring B is a dihydropyran ring can be prepared by reacting compounds of formula
Q with
dialkyl (3,6-dihydro-2H-pyran-4-yl)boronates to produce compounds of formula
R.
Compounds of formula R can then be subsequently reduced to form compounds of
formula S.
B(OR)2 A
0 N
0 Ria facit- R
A iC INI
)N H2
"=-.. ---- '";:r cataly' st RiOd.
tCr. lip,. 0
(step 2-viii)
R2 /
[Q] Br Pd(dppf), Na2CO3, DMF
[R] [s]
(step 2-vii) 0 0
Scheme 2c
37
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Example 3. Preparation of ethyl (44(7-moipholinoquinoxalin-5-
yl)amino)cyclohexyl)carbamate (Compound 6) and ATI--(7-morpholinoquinoxalin-5-
y1)-
A4-(pyrimidin-2-yl)cyclohexane-1,4-diamine (Compound 18)
H H
N--.1 ocoNH2
N
NH2 H N'l Br AbiI,- N
RP
0 Co) Boc,N H
Br ri&h NH2 Br I ,A\I
0
Me0H 11101 NMP, 180 C l'. I
Pd2(dba)3, rac-BINAP,
N
F (step 3-i) F (step 3-ii) Co) Cs2CO3, toluene,
100 C
[1001] [1002] (step 3-iii)
[1003]
H N1'''.=1
N ,N
CI
0-'LO DIEA, FINcr 40
,
H NI''" H Nr'i CH2r
N N I N
Boc,NõCr 01 N TFA/DCM Zr 0 ..istep3-v) CH3 [6] 0
' H2 *TFA N
N (step3-vi)
H (step 3-iv)
Br
H N1
,N. (,i 0,0,õN
=....N
[1004] ) [1005] L0)
0 N =" N
HN
,J.. r N.,
TEA, NMP, N 'l N
130 C
[18] 0
Scheme 3
[00121] As shown in step 3-i of Scheme 3, to a solution of 3-bromo-5-fluoro-
benzene-
1,2-diamine (compound 1001, 1.11 g, 5.41 mmol) in methanol (11 mL) was added
oxaldehyde (1.57 mL of 40% w/v, 10.8 mmol). The reaction mixture was stirred
at room
temperature under nitrogen. After 2 hours a yellow solid precipitated. The
reaction
mixture was diluted with water (20 mL), stirred an additional 5 minutes,
filtered, and the
collected solid dried under high vacuum to produce 5-bromo-7-fluoroquinoxaline
(compound 1002, 868 mg, 70.6% yield): 1-14-NMR (300 MHz, DMSO-d6) 6 9.06 (s,
2H),
8.36 (dd, J = 8.5, 2.7 Hz, 1H), 8.00 (dd, J = 9.2, 2.7 Hz, 1H); ESMS (M+H+) =
227.14.
[00122] As shown in step 3-ii of Scheme 3, to a solution of 5-bromo-7-
fluoroquinoxaline (4.5 g, 19.8 mmol) in NMP (67.5 mL) was added morpholine
(3.1 mL,
35.6 mmol). The reaction mixture was heated to 140 C and stirred for 15 hours.
After
cooling, the mixture was poured into water (200 mL), extracted with ethyl
acetate (2 x
38
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
100mL), dried over magnesium sulfate, filtered, evaporated under reduced
pressure, and
purified by medium pressure silica gel chromatography (10 to 80% Et0Ac/hexanes
gradient) to provide 4-(8-bromoquinoxalin-6-yl)morpholine (compound 1003,
3.86g, 66%
yield) as a yellow solid: 1H-NMR (400 MHz, DMSO-d6) 6 8.82 (d, J = 1.6 Hz,
1H), 8.73
(d, J = 1.6 Hz, 1H), 8.12 (d, J = 2.5 Hz, 1H), 7.27 (d, J = 2.4 Hz, 1H), 3.87-
3.69 (m, 4H),
3.44-3.34 (m, 4H); ESMS (M+H') = 227.14.
[00123] As shown in step 3-iii of Scheme 3, a mixture of 4-(8-bromoquinoxalin-
6-
yl)morpholine (1.57 g, 5.34 mmol), tert-butyl-N-(4-aminocyclohexyl)carbamate
(1.37 g,
6.40 mmol), (rac)-B1NAP (664 mg, 1.07 mmol), cesium carbonate (5.22 g, 16.0
mmol),
and Pd2(dba); (489 mg, 0.534 mmol) in toluene (50 mL) was heated at 100 C for
12
hours. After cooling, the mixture was diluted with ethyl acetate (150 mL) and
water (25
mL), then filtered through diatomaceous earth which was subsequently washed
with ethyl
acetate. The combined organics were washed with brine, dried over sodium
sulfate,
concentrated under reduced pressure, and purified by medium pressure silica
gel
chromatography (0 to 60% Et0Acihexanes gradient) to provide tert-butyl(-447-
morpholinoquinoxalin-5-yl)amino)cyclohexyl)carbamate (compound 1004, 1.83g,
83.2%
yield): 11-1-NMR (300 MHz, CDC13) 6 8.65 (d, J = 2.0 Hz, 1H), 8.35 (d, J = 2.0
Hz, 1H),
6.60 (d, J = 2.4 Hz, 1H), 6.34 (d, J = 2.4 Hz, 1H), 6.11 (d, J = 7.8 Hz, 1H),
4.60 (s, 1H),
3.97-3.86 (m, 4H), 3.67 (s, 2H), 3.41-3.25 (m, 4H), 1.85 (d, J = 3.0 Hz, 5H),
1.74-1.57 (m,
3H), 1.45 (s, 9H).
1001241 As shown in step 3-iv of Scheme 3, to a solution of tert-butyl
morpholinoquinoxalin-5-yl)amino)cyclohexyl)carbamate (900 mg, 2.00 mmol) in
dichloromethane (16 mL) was added trifluoroacetic acid (3 mL, 38.9 mmol). The
resulting black reaction mixture was stirred under an atmosphere of nitrogen
at room
temperature for 2 hours. Saturated aqueous sodium bicarbonate (150 mL) was
added
slowly until the color turned from black to orange. The mixture was extracted
with
dichloromethane (2 x 100 mL) and the combined organics washed with brine (50
mL),
dried over sodium sulfate, and concentrated under reduced pressure to provide-
N1-(7-
morpholinoquinoxalin-5-yl)cyclohexanc-1,4-diamine, trifluoroacetate (compound
1005):
1H NMR (300 MHz, CDC13) 6 8.64 (d, J = 1.9 Hz, 1H), 8.36 (d, J = 1.9 Hz, 1H),
6.59 (d, J
= 2.3 Hz, 1H), 6.34 (d, J = 2.3 Hz, 1H), 6.20 (d, J = 7.9 Hz, 1H), 3.95-3.84
(m, 4H), 3.69
(s, 1H), 3.41-3.25 (m, 4H), 2.93 (d, J = 8.9 Hz, 1H), 2.09-1.87 (m, 2H), 1.90-
1.68 (m, 6H),
39
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
1.58 (dd, J = 11.2, 8.7 Hz, 2H); ESMS (M+H-) = 328.34. This compound was used
as is
without further purification.
[00125] As shown in step 3-v of Scheme 3, to solution of N1-(7-
morpholinoquinoxalin-
5-yl)cyclohexane-1,4-diamine (25 mg, 0.07 mmol) and dilsopropylethylamine
(18.0 mg,
24.3 uL, 0.14 mmol) in dichloromethane (750 L) was added ethyl chloroformate
(11.4
mg, 10.0 I, 0.105 mmol). The reaction mixture was stirred for 12 hours,
diluted with
dichloromethane (10mL), washed with saturated aqueous sodium bicarbonate
(5mL), dried
over sodium sulfate, and concentrated under reduced pressure. The resulting
residue was
purified by HPLC preparative chromatography using a 10-90% acetonitrile/water
(0.1%
TFA) gradient as cluant to provide ethyl (44(7-moipholinoquinoxalin-5-
yl)amino)cyclohexyl)carbamate (compound 6, 14 mg, 50% yield): 1H-NMR (300 MHz,
CDC13) 6 8.65 (d, J = 2.0 Hz, 1H), 8.36 (d, J = 2.0 Hz, 1H), 6.61 (d, J = 2.4
Hz, 1H), 6.35
(d, J = 2.4 Hz, 1H), 6.10 (d, J = 7.6 Hz, 1H), 4.72 (s, 1H), 4.12 (q, J = 7.0
Hz, 2H), 3.96-
3.82 (m, 4H), 3.68 (s, 2H), 3.42-3.23 (m, 4H), 1.93-1.78 (m, 6H), 1.69 (dd, J
= 15.0, 6.3
Hz, 2H), 1.25 (t, J = 7.1 Hz, 3H); ESMS (M+H1) = 400.17.
[00126] As shown in step 3-vi of Scheme 3, A mixture of N1-(7-
morpholinoquinoxalin-
5-yl)cyclohexane-1,4-diamine (185 mg, 0.56 mmol), 2-bromopyrimidine (93 mg,
0.58
mmol), and triethylamine (143 mg, 197 uL, 1.41 mmol) in 1-methylpyn-olidin-2-
one (3
mL) was heated to 130 C and stirred for 15 hours. After cooling to room
temperature, the
mixture was diluted with ethyl acetate (70 mL) and methyl tert-butyl ether (20
mL),
washed with water (3 x 20 mL), washed with brine (15 mL), dried over sodium
sulfate,
concentrated under reduced pressure, and purified by medium pressure silica
gel
chromatography (10 to 100% Et0Ac/hexanes gradient) to provide N1-(7-
morpholinoquinoxalin-5-y1)44-(pyrimidin-2-y0cyclohexanc-1,4-diamine (compound
18,
102 mg, 45% yield): 11-1-NMR (300 MHz, CDC13) 6 8.65 (d, J = 2.0 Hz, 1H), 8.37
(d, J =
2.0 Hz, 1H), 8.27 (d, J = 4.8 Hz, 2H), 6.60(d, J = 2.4 Hz, 1H), 6.51 (t, J =
4.8 Hz, 1H),
6.36 (d, J =2.4 Hz, 1H), 6.15 (d, J = 7.8 Hz, 1H), 5.20 (d, J = 7.7 Hz, 1H),
4.04 (d, J = 7.9
Hz, 1H), 3.96-3.82 (m, 4H), 3.70 (s, 1H), 3.39-3.24 (m, 4H), 1.94 (dd, J=
13.7, 4.4 Hz,
6H), 1.78 (dt, J = 28.8, 16.1 Hz, 2H); ESMS (M+H1) = 328.34.
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
Example 4. Preparation of 1-(447-(8-oxa-3-azabicyclo[3.2.1]oetan-3-
yl)quinoxalin-5-
yl)amino)cyclohexyl)-3-ethylurea (Compound 22)\
H N"k) oNH2
H Ni N"
Br -'
N
N'i iii, I
Br riihr N N
I
_______________________ IWP Boc,N
H Boc,N10. 1101
lir CO)
NMP, 180 C w 614.1 I.
Pd2(dba)3, rac-BINAP, H N
F (step 4-i)
( ) Cs2CO3, toluene, 100 C [1007]
[1002] 0 (step 4-ii) CO)
[1006]
H H
- c.0
eTN , N N
0 H3C N -
TFA/DCM r 101
_____________________________________ 1.
H2Nc HNC
(step 4-iii) *TFA N
DIEA, DCM
HN0 N
c ) (step 4-iv)
[1008] u [22] (o)
o
LA13
Scheme 4
[00127] As shown in step 4-i of Scheme 4, to a solution of 5-bromo-7-
fluoroquinoxaline (compound 1002, 150 mg, 0.66 mmol) in NMP (2.3 mL) was added
8-
oxa-3-azabicyclo[3.2.1]octane (178 mg, 1.2 mmol) at RT. The reaction mixture
was
sealed in a microwave vial and heated at 180 C for 20 minutes. Afte cooling to
RT and
pouring into water, the aqueous phase was extracted with Et0Ac (3x). The
combined
extracts were dried over MgSO4, filtered, concentrated under reduced pressure,
and
purified by medium pressure silica gel chromatography (0 to 100% Et0Ac/hexanes
gradient) to provide 3-(8-bromoquinoxalin-6-y1)-8-oxa-3-
azabicyclo[3.2.1]octane
(compound 1006, 87 mg, 41% yield) as a dark orange oil: ESMS (M+1-1f) =
320.07.
[00128] As shown in step 4-ii of Scheme 4, a degassed solution of 3-(8-
bromoquinoxalin-6-y1)-8-oxa-3-azabicyclo[3.2.1]octane (261 mg, 0.815 mmol),
tert-butyl
N-(4-aminocyclohexyl)carbamate (210 mg, 0.98 mmol), rac-BINAP (102 mg, 0.163
mmol), Cs2CO3 (797 mg, 2.45mmo1), and Pd2(dba)3 (75 mg, 0.0815 mmol) in
toluene
(10.5 mL) was heated at 100 C (oil bath temp) in a sealed microwave tube for
15 hours.
After cooling, the mixture was applied directly to a chromatography column and
purified
41
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
by medium pressure silica gel chromatography (0 to 100% Et0Ac/hexanes
gradient) to
afford tert-butyl (4-((7-(8-oxa-3-azabicyclo[3.2.1]octan-3-yl)quinoxalin-5-
yl)amino)cyclohexyl)carbamate (compound 1007, 141 mg, 36% yield) as a white
solid:
11-1-NMR (400 MHz, CDC13) d 8.49 (s, 1H), 8.23 (d, J= 1.5 Hz, 1H), 6.48 (s,
1H), 6.18 (d,
J = 1.9 Hz, 1H), 6.06 (s, 1H), 4.52 (s, 1H), 4.47 (s, 2H), 3.60 (s, 2H), 3.45
(d, J = 11.6 Hz,
2H), 3.14-3.12 (m, 2H), 1.96-1.84 (m, 4H), 1.79 (s, 5H), 1.54 (s, 3H) and 1.38
(s, 9H)
ppm; ESMS (M+H ) = 453.96.
[00129] As shown in step 4-iii of Scheme 4, to a solution of compound 1007
(141 mg,
0.295 mmol) in CH2C12 (2.5 mL) was added TFA (656 mg, 443 uL, 5.75 mmol) at
RT.
The resulting black solution was stirred for 2 hours and then the reaction was
quenched by
the addition of saturated NaHCO3 until the black color gradually turned into
an orange
color. The reaction mixture was extracted with CH2C12 (3x) and the combined
organic
extracts were dried over Na2SO4 and evaporated to dryness to provide N1-(7-(8-
oxa-3-
azabicyclo[3.2.1]octan-3-yl)quinoxalin-5-yl)cyclohexane-1,4-diamine,
trifluoroacetate
(compound 1008): ESMS (M+H) = 354.20. This material was used in subsequent
reactions without any further purification.
[00130] As shown in step 4-iv of Scheme 4, to a solution of compound 1008 (45
mg,
0.071 mmol) and DIEA (36.5 mg, 49.0 lid-, 0.28 mmol) in CH2C12 (1.4 mL) was
added
ethyl isocyanate (20 mg, 0.28 mmol) at RT. The solution was stirred at this
temperature
for 15 hours and then applied directly to a chromatography column and purified
by
medium pressure silica gel chromatograph (0 to 100% Et0Ac/hexanes gradient) to
afford
1-(447-(8-oxa-3-azabicyclo[3.2.1]octan-3-yOquinoxalin-5-yl)amino)cyclohexyl)-3-
ethylurea (compound 22, 8 mg, 27% yield) as a white solid: 1H-NMR (400 MHz,
CDC13)
68.54 (s, 1H), 8.22 (s, 1H), 6.43 (s, 1H), 6.19 (s, 1H), 6.02 (s, 1H), 4.47
(s, 2H), 4.38 (d, J
= 5.2 Hz, 1H), 4.28 (s, 1H), 3.74 (s, 1H), 3.60 (s, 1H), 3.42 (s, 4H), 3.14-
3.09 (m, 4H),
2.05-1.87 (m, 3H), 1.79 (s, 3H), 1.55 (d, J = 7.1 Hz, 2H) and 1.21-1.05 (m,
5H) ppm;
ESMS (M+H) = 425.35.
42
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Example 5. Preparation of M-(6-morpholinobenzo[c][1,2,5]oxadiazol-4-y1)-N4-
(pyrimidin-2-yl)cyclohexane-1,4-diamine (Compound 23)
ii.N,Boc o,,N H2
CI
N N HNc _______________
oCiaNµBoc TEA, DMF 4M HCl/THF
, HNefo
140 C N N
(step 5-ii N) N " Ha
[1010]
H2N
[1009] (step 5-i) I,)[ [1011]
io,N H2
N-0
Br N
HN
* HCI N
[1011] HNf3' 1110
N N
r
C
Pd2(dba)3, rac-BINAP,
0 Cs2003, toluene, 100 C 0
[1012] (step 5-iii) [23]
Scheme 5
1001311 As shown in step 5-i of Scheme 5, a mixture of tert-butyl ((cis)-4-
aminocyclohexyl)carbamate (compound 1009, 490 mg, 2.3 mmol), 2-
chloropyrimidine
(262 mg, 2.3 mmol) and TEA (463 mg, 6371j1, 4.6 mmol) in DMF (10 mL) was
subjected
to microwave irradiation for 20 minutes at 150 C. The reaction mixture was
diluted with
Et0Ac, washed with H20, dried over Na2SO4, concentrated under reduced
pressure, and
purified by medium pressure silica gel chromatography (0 to 50 % Et0Ac/hexanes
gradient) to provide tert-butyl ((cis)-4-(pyrimidin-2-
ylamino)cyclohexyl)carbamate
(compound 1010) as a white solid: 1-H-NMR (300 MHz, CDC13) 8 8.28 (d, .1= 4.8
Hz,
2H), 6.53 (t, J= 4.8 Hz, 1H), 5.12 (s, 1H), 4.56 (s, 1H), 3.99 (dq, J= 7.0,
3.5 Hz, 1H),
3.65 (s, 1H), 1.83 (tq, J= 10.2, 3.6 Hz, 5H), 1.66 (s, 8H), 8.13-7.91 (m, 3H),
1.47 (s, 9H).
1001321 As shown in step 5-ii of Scheme 5, HC1 (3 mL, 4M in THF, 12 mmol) was
added to compound 1010. The mixture was stirred for 30 min and concentrated
under
reduced pressure to produce (cis)-N1-(pyrimidin-2-yl)cyclohexane-1,4-diamine
hydrochloride (compound 1011). This material was used in subsequent reactions
as is
without further purification.
43
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
[00133] As shown in step 5-iii of Scheme 5, a mixture of 4-bromo-6-
morpholinobenzo[c][1,2,5]oxadiazole (compound 1012, 147 mg, 0.5 mmol), (cis)-
N1-
(pyrimidin-2-yl)cyclohexane-1,4-diamine hydrochloride (120 mg, 0.6 mmol),
(rae)-
BINAP (32 mg, 0.05 mmol) , Pd2(dba)3 (24 mg, 0.026 mmol), and cesium carbonate
(506
mg, 1.55 mmol) in toluene (5 mL) was flushed with nitrogen gas and stirred
overnight at
90 C under an atmosphere of nitrogen. The mixture was filtered though a layer
of
diatomaceous earth, concentrated under reduced pressure, and purified by
medium
pressure silica gel chromatography (0 to 80% Et0Ac/hexanes gradient) to
provide (eis)-
-(6-morpholinobenzo[c] [1,2,5]oxadiazol-4-ye-N4-(pyrimidin-2-y0cyclobexane-1,4-
diaminc (compound 23) as an orange solid: 1-1-1-NMR (300 MHz, CDC13) 6 8.20
(d, J = 4.9
Hz, 2H), 6.46 (t, J = 4.8 Hz, 1H), 6.05 (d, J = 1.6 Hz, 1H), 5.82 (s, 1H),
5.24 (s, 1H), 4.82
(d, J = 7.0 Hz, 1H), 3.98 (s, 1H), 3.85-3.72 (m, 4H), 3.60 (s, 1H), 3.23-3.06
(m, 4H), 1.95-
1.62 (m, 8H).
44
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Example 6. Preparation of 5-methoxy-N-((cis)-447-morpholinoquinoxalin-5-
yl)oxy)cyclohexyl)pyrimidin-2-amine (Compound 134)
..,õOH OMs
F
MsCDI, TEA,
-/ CM HN
+ y ___________________________________ _..
H2N N N Et3N, iPrOH HN
(step 6-i) N N (step 6-ii) N-LN
Br y [1013]
y [1014]
Br H Br
NH2 NH2
0
NH2 HO H NH2 0li-F1 HO
NO2 2 HO
NO2 Br2 40 Raney Ni 0
HO 0
dioxane Et0Ac Me0H Br
(step 6-iii) Br (step 6-iv) Br (step 6-v)
[1015] [1016] [1017]
H N
N --\.1 I
1\1 N .''l I 0
TBDMS-CI, H3C, .,c) I .õ N 1. ( ) HO 0,- N 000 0,,, N
imidazole, Si 50 N N
[1014]
DCM H3C-.-2( 61.1 __________ / HN
-," H C CH 3 Pd2(dba)3, rac-BINAP, Cs2CO3 (step 6-vi)
N
3 3
Br Cs2CO3, toluene,
dioxane, y
[1018] 100 C C )
1 0 5 C ?
0 [1020]
2. TBAF (step 6-viii) Br
[1019]
(step 6-vii)
Me0H, I
(Ally1PdC1)2, Cs2CO3, Co.
toluene 100 C
,. HN
(step 6-ix) )-. N
INV N
y coD
0, ,. [134]
Cn3
Scheme 6
[00134] As shown in step 6-i of Scheme 6, to a mixture of 5-bromo-2-fluoro-
pyrimidine
(1 g, 5.651 mmol) in iPrOH (10 mL) was added TEA (1.143 g, 1.574 mL, 11.30
mmol)
and trans-4-aminocyclohexan-1-ol (650.8 mg, 5.651 mmol). The mixture was
microwaved for 20min at 150 C, concentrated under reduced pressure, diluted
with
Et0Ac , washed with water, and dried over Na7SO4. After removal of the
volatiles under
reduced pressure, the residue was purified by medium pressure silica gel
chromatography
(0-80% Et0Ac/hexanes gradient) to provide (trans)-445-bromopyrimidin-2-
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
yl)amino)cyclohexanol (compound 1013, 1.2 g): 1H-NMR (300 MHz, CDC13) 6 8.28
(s,
2H), 5.03 (d, J = 8.1 Hz, 1H), 3.91-3.49 (m, 2H), 2.31-1.90 (m, 4H), 1.56-1.19
(m, 4H)..
[00135] As shown in step 6-ii of Scheme 6, to compound 1013 (1.2 g, 4.41 mmol)
in
DCM (20 mL) was added TEA (1.134 g, 1.84 mL, 13.2 mmol) and MsC1 (505 mg, 341
uL, 4.41 mmol). The reaction mixture was stirred for 1 hour, concentrated
under reduced
pressure, and purified by medium pressure silica gel chromatography (0 to 80%
Et0Ac/hexanes gradient) to provide trans-4((5-bromopyrimidin-2-
yl)amino)cyclohexyl
methanesulfonate (compound 1014): 1H-NMR (300 MHz, CDC13) 6 8.29 (s, 2H), 5.03
(d,
J = 7.8 Hz, 1H), 4.70 (tt, J = 10.6, 3.9 Hz, 1H), 3.80 (dtt, J = 11.2, 7.6,
3.7 Hz, 1H), 3.04
(s, 3H), 2.30-2.12 (m, 4H), 1.93-1.69 (m, 2H), 1.51-1.33 (m, 2H).
[00136] As shown in step 6-iii of Scheme 6, to a solution of 2-amino-3-
nitrophenol
(5.00 g, 32.4 mmol) in dioxane (50 mL) was added bromine (6.22 g, 2.01 mL,
38.9 mmol).
The mixture was stirred for 2 hours and a precipitate formed, which was
collected and
washed with dioxane and ether. The resulting yellow solid treated with a
saturated
NaHCO3 solution, which was extracted with Et0Ac (3x). The combined organics
were
dried over Na2SO4, filtered, and concentrated under reduced pressure to yield
2-amino-5-
bromo-3-nitrophenol (compound 1015) as a brown solid. This material was
carried on as
is in subsequent reactions without futher purification.
1001371 As shown in step 6-iv of Scheme 6, to a solution of 2-amino-5-bromo-3-
nitrophenol (7.5 g, 31.8 mmol) in ethyl acetate (60 mL) was added Raney
nickelTM (1.90
g, 214 uL, 32.4 mmol) and the reaction mixture was shaken for 2 hours under an
atmosphere of H2 at 30 p.s.i. After filtering and drying over Na2SO4, the
mixture was
concentrated under reduced pressure to provide 2,3-diamino-5-bromophenol
(compound
1016), which was used as is in subsequent reactions without futher
purification.
[00138] As shown in step 6-v of Scheme 6, 2,3-diamino-5-bromophenol (6.0 g,
29.5
mmol) was dissolved in methanol and to this solution was added glyoxal (3.77
g, 2.98 mL,
64.9 mmol) and stirred overnight. The reaction mixture was concentrated under
reduced
pressure to a minimum volume and the resulting tan solid collected by
filtration and dried
under high vacuum to produce 7-bromoquinoxalin-5-ol (compound 1017), which was
used
as is in subsequent reactions without futher purification.
[00139] As shown in step 6-vi of Scheme 6, a solution of 7-bromoquinoxalin-5-
ol (2.0
g, 8.89 mmol) in DCM (20 mL) was added imidazole (1.82 g, 26.7 mmol) and tert-
46
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
butyldimethylsilyl chloride (1.34 g, 1.65 mL, 8.89 mmol). The reaction mixture
was
stirred overnight at RT, concentrated under reduced pressure, and purified by
medium
pressure silica gel chromatography (0 to 20% Et0Ac/hexanes gradient) to
provide 7-
bromo-5-((tert-butyldimethylsilyl)oxy)quinoxaline (compound 1018) as a
colorless oil:
1-H-NMR (300 MHz, CDCL3) 68.69 (q, J= 1.8 Hz, 2H), 7.80 (d, J= 2.1 Hz, 1H),
7.22 (d,
J = 2.1 Hz, 1H), 0.96 (s, 9H), 0.81 (s, 7H).
[00140] As shown in step 6-vii of Scheme 6, a mixture of 7-bromo-5-((tert-
butyldimethylsilyl)oxy)quinoxaline (700 mg, 2.06 mmol), morpholine (270 mg,
270 !at,
3.09 mmol), Pd2(dba)3 (94.50 mg, 0.1032 mmol), (rac)-BINAP (129 mg, 0.206
mmol),
cesium carbonate (2.02 g, 6.19 mmol) in toluene (7 mL) was flushed with
nitrogen for 10
minutes. The mixture was then heated overnight at 100 C. After cooling, the
reaction
mixture was diluted with Et0Ac, filtered through a layer of diatomaceous
earth,
concentrated under reduced pressure, and purified by medium pressure silica
gel
chromatography (0 to 30% Et0Ac/hexanes gradient) to provide 7-
morpholinoquinoxalin-
5-ol. This compound (450 mg, 1.3 mmol) was dissolved in THF (20 mL) and tetra-
n-
butylammonium fluoride (539 mg, 2.06 mmol) was added. The reaction mixture was
stirred for 0.5 hour, concentrated under reduced pressure, and purified by
medium pressure
silica gel chromatography (0 to 100% Et0Ac /hexanes gradient) to provide 7-
morpholinoquinoxalin-5-ol (compound 1019) as a yellow solid: 1H-NMR (300 MHz,
CDC13) 6 8.75 (d, J = 2.0 Hz, 1H), 8.46 (d, J = 2.0 Hz, 1H), 7.70 (d, J = 41.8
Hz, 1H), 7.01
(d, J = 2.6 Hz, 1H), 6.89 (d, J = 2.5 Hz, 1H), 4.12-3.78 (m, 4H), 3.51-3.24
(m, 4H).
[00141] As shown in step 6-viii of Scheme 6, a solution of 7-
morpholinoquinoxalin-5-
ol (100 mg, 0.432 mmol), (trans)-4-((5-bromopyrimidin-2-yDamino)cyclohexyl
methanesulfonate (compound 1014, 303 mg, 0.865 mmol), and CsCat (282 mg, 0.865
mmol) in dioxane (1.0 mL was stirred for 16 hours at 105 C. After cooling, the
reaction
mixture was diluted with Et0Ae, filtered through diatomaceous earth,
concentrated under
reduced pressure, and purified by medium pressure silica gel chromatography (0
to 5%
Me0H/DCM gradient) to produce 5-bromo-N-((cis)-447-morpholinoquinoxalin-5-
yl)oxy)cyclohexyl)pyrimidin-2-amine (compound 1020, 110 mg) as a yellow foam:
1H-
NMR (400 MHz, CDC13) 6 8.70 (d, J = 2.0 Hz, 1H), 8.64 (d, J = 1.9 Hz, 1H),
8.29 (s, 2H),
6.98 (d, J = 2.5 Hz, 1H), 6.92 (d, J = 2.5 Hz, 1H), 5.29 (d, J = 8.3 Hz, 1H),
4.81 (s, 1H),
4.04-3.84 (m, 4H), 3.42-3.31 (m, 4H), 2.22 (s, 2H), 1.92 (d, J = 4.9 Hz, 6H).
47
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
[00142] As shown in step 6-ix of Scheme 6, to a mixture 5-bromo-N-((cis)-447-
morpholinoquinoxalin-5-yl)oxy)cyclohexyl)pyrimidin-2-amine (75 mg, 0.155
mmol),
cesium carbonate (101 mg, 0.309 mmol) , allylpalladium(II) chloride dimer
(0.28 mg,
0.0015 mmol) , RockPlios (2.17 mg, 0.0046 mmol) and Me0H (9.9 mg, 12.5 pt,
0.31
mmol) in toluene (2 mL) was flushed with nitrogen gas and heated to 100 C for
18 hours.
The reaction mixture was iluted with Et0Ac, filtered though a layer of
diatomaceous
earth, and concentrated under reduced pressure. Purification by medium
pressure silica
gel chromatography (0-8% Me0H/DCM gradient) yielded 5-methoxy-N-((eis)-4-((7-
morpholinoquinoxalin-5-yl)oxy)cycloltexyl)pyrimidin-2-amine (compound 134, 43
mg):
1H-NMR (300 MHz, CDC13) 6 8.70 (d, J = 1.9 Hz, 1H), 8.63 (d, J = 1.9 Hz, 1H),
8.07 (s,
2H), 6.96 (d, J = 2.5 Hz, 1H), 6.92 (d, J = 2.5 Hz, 1H), 5.01 (d, J = 8.1 Hz,
1H), 4.80 (q, J
= 5.6, 4.2 Hz, 1H), 4.03-3.87 (m, 5H), 3.80 (s, 3H), 3.42-3.27 (m, 4H), 2.29-
2.10 (m, 2H),
1.99-1.82 (m, 6H).
Example 7. Preparation of 4-(8-(((trans)-4-(pyrimidin-2-
yloxy)cyclohexyl)oxy)quinoxalin-6-yl)morpholine (Compound 34) and 4-(84(cis)-4-
(pyrimidin-2-yloxy)cyclohexyl)oxy)-quinoxalin-6-yl)morpholine (Compound 42)
CI
N N
)0(0,70
1. 6M HCI (aq) OH MsCI, TEA,
DCM
NaH, DMF 2. NaBH4, Me0H (step 7-iii)
HO N N
(step 7-i) N N (step 7-ii)
[1021] 1)J[1022] [1023]
N
dir N
[1019] 0
0
+ 0 N
Cs2CO3,
N N [1024] dioxane, 110 C N N o) N N -- r-
101 (step 7-iv) N=Lj
[34] [42]
Scheme 7
[00143] As shown in step 7-i of Scheme 7, to a solution of 1,4-
dioxaspiro[4.5]decan-8-
ol (compound 1021, 1.0 g, 6.32 mmol) in DMF (10 mL) was added NaH (370 mg,
9.25
mmol). The reaction mixture was stirred for 20 minutes before the addition of
2-
48
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
chloropyrimidine (869 mg, 7.59 mmol). The mixture was stirred for 30 minute at
RT and
then heated to 100 C for 9 hours. After cooling, the mixture was diluted with
Et0Ac,
washed with H20, dried over Na2SO4, concentrated under reduced pressure, and
purified
by medium pressure silica gel chromatography (0-40% Et0Ac/bexanes) to produce
2-(1,4-
dioxaspiro[4.5]decan-8-yloxy)pyrimidine (compound 1022) as a colorless oil: 1H
NMR
(300 MHz, Chloroform-d) 6 8.52 (d, J = 4.8 Hz, 2H), 6.92 (t, J = 4.8 Hz, 1H),
5.15 (ddd, J
= 10.7, 6.5, 4.2 Hz, 1H), 4.05-3.87 (m, 4H), 2.14-1.85 (m, 6H), 1.79-1.65 (m,
2H); ESMS
(M+H') = 237.12.
[00144] As shown in step 7-ii of Scheme 7, to 2-(1,4-dioxaspiro[4.5]decan-8-
yloxy)pyrimidine (620 mg, 2.624 mmol) was added HC1 (4.0 mL of 6 M, 8.86 mmol)
and
the reaction mixture was stirred for 2 hours. The pH of the mixture was
neutralized with
with sat. NaHC01(aq) and the mixture was concentrated under reduced pressure
as a
methanol azeotrope. To the residue was added DCM (30mL) to produce a
precipitate,
followed by stirring for an additional 20 minutes. The solids were filtered
off and the
mother liquor was concentrated under reduced pressure. The resulting residue
was
dissolved in methanol and sodium borohydride (151 mg, 3.99 mmol) was added as
a solid.
The mixture was stirred for 1 hour and the reaction quenched with HC1 (6M,
0.70 mL).
Stirring was continued until gas evolution ceased. The pH of the mixture was
adjusted to
about 8 with 1N sodium hydroxide and extracted with Et0Ac (20rnL). The
organics were
dried over sodium sulfate and concentrated under reduced pressure to produce 4-
(pyrimidin-2-yloxy)cyclohexanol (compound 1023, 248mg, 64% yield) as a mixture
of
(cis)- and (trans)- isomers. A 12 mg aliquot of the sample was purified via
HPLC
preperative reversed-phase chromatography (10-90% CH3CN/water gradient
containing
0.1% TFA) to separate the isomers: (trans)-4-pyrimidin-2-yloxycyclohexanol -
1H NMR
(300 MHz, Chloroform-d) 6 8.54 (d, .1= 4.8 Hz, 2H), 6.95 (t, J = 4.8 Hz, 1H),
5.05 (tt, J =
9.4, 4.0 Hz, 1H), 3.91-3.75 (m, 1H), 2.26-1.99 (m, 4H), 1.76-1.41 (m, 4H);
ESMS (M+FL)
= 195.07, (cis)-4-pyrimidin-2-yloxycyclohexanol -1H NMR (300 MHz, Chloroform-
d) 6
8.62 (d, J = 4.9 Hz, 2H), 7.04 (t, J = 4.9 Hz, 1H), 5.21 (tt, J = 5.3, 2.6 Hz,
1H), 4.56 (s,
1H), 3.85 (p, J = 5.9 Hz, 1H), 2.17-2.02 (m, 2H), 1.88-1.67 (m, 6H); ESMS (M+H-
) =
195.07. The remaining material was used in subsequent reactions as the
cis/trans mixture.
[00145] As shown in step 7-iii of Scheme 7, to a solution of a cis/trans
mixture of 4-
pyrimidin-2-yloxycyclohexanol (244 mg, 1.256 mmol) and triethylamine (350 riL,
2.51
49
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
mmol) in dichloromethane (5 mL) was added methane sulfonyl chloride (145
1.87
mmol). The reaction mixture was stirred for 2 hours, concentrated under
reduced
pressure, and purified by medium pressure silica gel chromatography (0- 20%
Et0Ac/dichloromethane gradient) to provide 4-pyrimidin-2-yloxycyclohexyl)
methanesulfonate (compound 1024, 239 mg, 70% yield) as a mixture of cis/trans
isomers:
1H NMR (300 MHz, Chloroform-d) 6 8.51 (d, J = 4.8 Hz, 2H), 6.93 (t, J = 4.8
Hz, 1H),
5.13 (dq, J = 9.9, 3.0 Hz, 1H), 4.87 (p, J = 3.8 Hz, 1H), 3.04 (d, J = 2.4 Hz,
3H), 2.28-1.99
(m, 4H), 1.99-1.74 (m, 4H); ESMS (M+FL) = 273.52.
[00146] As shown in step 7-iv of Scheme 7, a mixture of (4-pyrimidin-2-
yloxycyclohcxyl) methanesulfonate (105 mg, 0.386 mmol), 7-morpholinoquinoxalin-
5-ol
(178.3 mg, 0.7712 mmol), and Cs2CO3 (125.6 mg, 0.3856 mmol) in dioxane (1.5
mL) was
sealed in a 5 mL microwave tube and heated to 110 C for 14 hours using an oil
bath. The
reaction mixture was cooled to room temperature, diluted with Et0Ac, and
filtered
through diatomaceous earth which was subsequently washed with ethyl acetate.
The
filtrate was concentrated under reduced pressure and the residue purified via
preparative
reversed-phase HPLC (10-90% CH3CN/water gradient containing 0.1% TFA).
Fractions
containing a mixture of cis and trans isomers were further purified via SFC
using a chiral
OJ column and eluting with 40% Me0H in CO2 to provide 21mg of 4-(8-(((trans)-4-
(pyrimidin-2-yloxy)cyclohexyl)oxy)quinoxalin-6-yl)morpholine (compound 34): 1H
NMR
(300 MHz, Chloroform-d) 6 8.69 (dd, J = 3.4, 1.9 Hz, 1H), 8.62 (dd, J = 3.6,
1.9 Hz, 1H),
8.51 (dd, J = 4.8, 2.2 Hz, 2H), 7.01-6.83 (m, 3H), 5.18 (tt, J = 7.0, 3.4 Hz,
1H), 4.79 (tt, J =
6.9, 3.1 Hz, 1H), 4.00-3.85 (m, 4H), 3.34 (dq, J = 4.8, 2.6 Hz, 4H), 2.44-2.16
(m, 4H),
1.92 (tdd, J = 16.4, 7.7, 2.8 Hz, 4H); ESMS (M+H+) = 408.56, and 22 mg of 4-(8-
(((cis)-4-
(pyrimidin-2-yloxy)cyclohexyl)oxy)-quinoxalin-6-yl)morpholine (compound 42):
1H
NMR (300 MHz, Chloroform-d) 6 8.70 (d, J = 1.9 Hz, 1H), 8.63 (d, J = 1.9 Hz,
1H), 8.52
(d, J = 4.8 Hz, 2H), 7.01-6.87 (m, 3H), 5.17 (ddt, J = 8.7, 6.7, 3.4 Hz, 1H),
4.76-4.58 (m,
1H), 4.00-3.87 (m, 4H), 3.40-3.27 (m, 4H), 2.43-2.22 (m, 4H), 2.05-1.87 (m,
2H), 1.86-
1.71 (m, 2H); ESMS (M-i-Hf) = 408.56.
Example 8. N-[(cis)-4-[7-(3,6-dihydro-2H-pyran-4-yl)quinoxalin-5-
yl]oxycyclohexyl]pyrimidin-2-amine (Compound 36)
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
H3C CH3
0Ms
H3C-H-CH3
0 ,0
0 N [1026]
HO 146,1N N
[1014] HN*1:).# 11.-v
HN
CsCO3, CH3CN N N Br
Br Pd(dppf)C12 N
[1018] 90 C [1025] DMF, microwave Lk.,..)
0
(step 8-i) 150 C [36]
(step 8-ii)
Scheme 8
[00147] As shown in step 8-i of Scheme 8, to a mixture of 7-bromoquinoxalin-5-
ol
(compound 1018, 200 mg, 0.89 mmol) and cesium carbonate (579 mg, 1.78 mmol) in
NMP (4.0 mL) was added (trans)-4-(pyrimidin-2-ylamino)cyclohexyl
methanesulfonate
(compound 1014, 241.1 mg, 0.8887 mmol). The mixture was stirred for 18 hours
at 90 C,
at which time an additional 0.5 eq of compound 1014 (241 mg, 0.89 mmol) was
added.
After stirring at 90 C for an additional 6 hours, the reaction mixture was
diluted with
Et0Ac, washed with H20, dried over Na2SO4, concentratedõ and purified by
medium
pressure silica gel chromatography (0-5% Me0H/DCM) to provide N-acis)-4-((7-
bromoquinoxalin-5-yl)oxy)cyclohexyl)pyrimidin-2-amine (compound 1025): 1H-NMR
(300 MHz, CDC13) 6 9.01-8.77 (m, 2H), 8.29 (d, J = 4.8 Hz, 2H), 7.89 (d, J =
1.9 Hz, 1H),
7.25 (d, J = 2.0 Hz, 1H), 6.53 (t, J = 4.8 Hz, 1H), 5.43-5.22 (m, 1H), 4.79
(td, J = 5.2, 2.5
Hz, 1H), 4.18-3.95 (m, 1H), 3.51 (s, 1H), 2.22 (td, J = 10.2, 9.6, 5.4 Hz,
2H), 2.09-1.86
(m, 6H).
[00148] As shown in step 8-ii of Scheme 8, a mixture of N-((cis)-4-((7 -
bromoquinoxalin-5-yl)oxy)cyclohexyppyrimidin-2-amine (compound 1025, 52 mg,
0.1299 mmol) , Pd(dppf)C12 (10.61 mg, 0.01299 mmol), 2-(3,6-dihydro-2H-pyran-4-
y1)-
4,4,5,5-tetramethy1-1,3,2-dioxaborolane (compound 1026, 27.3 mg, 0.13 mmol) ,
Na2CO3
(195 pi of 2M (aq) solution, 0.39 mmol) in DMF (1 mL) was flushed with
nitrogen gas
for 10 minutes. The mixture was subjected to microwave radiation for 20 min at
150 C.
After cooling, the mixture was diluted with Et0Ac , washed with H20, dried
over Na2SO4,
concentrated under reduced pressure and purified by medium pressure silica gel
chromatography (0-5% Me0H/DCM) to provide N-[(cis)-4-[7 -(3 ,6-dihydro-2H-
pyran-4-
51
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
yl)quinoxalin-5-ylloxycyclohexyl]pyrimidin-2-amine (compound 36) as an off-
white
solid: 1-1-1-NMR (300 MHz, CDC13) 6 8.94-8.76 (m, 2H), 8.29 (d, J = 4.8 Hz,
2H), 7.67 (d,
J = 1.7 Hz, 1H), 6.53 (t, J = 4.8 Hz, 1H), 6.37 (tt, J = 3.1, 1.5 Hz, 1H),
5.30 (d, J = 7.9 Hz,
1H), 4.87 (dt, J = 7.5, 3.6 Hz, 1H), 4.43 (q, J = 2.8 Hz, 2H), 4.02 (t, J =
5.5 Hz, 3H), 2.68
(dqd, J = 6.0, 3.4, 3.0, 1.8 Hz, 2H), 2.35-2.11 (m, 2H), 2.07-1.84 (m, 6H);
ESMS (M+H I)
= 404.2.
Example 9. N-((eis)-447-morpholinoquinoxalin-5-yl)oxy)cyclohexyl)pyrimidin-2-
amine
(Compound 28)
N
HO 01 NI
N N''h"N
õOH [1017]
o0 PPh3DIAD 4, N N
0 Br
rac-BINAP JN , ,
THF, 0 C to RT Br Pd2(dba)3,
0 0 0
110 C
[1027] (step 9-i) [1028]
(step 9-ii) [1029] La)
0
0
H2NNI-12 0 I N 0 N' 'CH3
Me0H, RT 2N )0, = N DIEA, 100 C Cr =
HN
L\
H.)
(step 9-iii) (step 9-iv) N N
[28]
[1030] r
Scheme 9
[00149] As shown in step 9-i of Scheme 9, 7-bromoquinoxalin-5-ol (compound
1017,
5.4 g, 24.0 mmol), 2-((trans)-4-hydroxycyclohexypisoindoline-1,3-dione (5.607
g, 22.86
mmol), and triphenylphosphine (8.994 g, 7.945 mL, 34.29 mmol) were dissolved
in
anhydrous THF and the flask was cooled in an ice bath. DIAD (6.93 g, 6.64 mL,
34.3
mmol) was added dropwise and the reaction was stirred at 0 C for 5 minutes,
then warmed
to room temperature and stirred for 18 hours. The reaction mixture was
concentrated
under reduced pressure, the residue was treated with Et20 and stirred for 0.5
hour at RT,
the precipitates filtered off, the filtrate concentrated under reduced
pressure, and the
residue purified by medium pressure silica gel chromatography (0-50%
Et0Acihexanes
52
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
gradient) to produce 2-[(cis)-4-(7-bromoquinoxalin-5-
yl)oxycyclohexyl]isoindoline-1,3-
dione (compound 1028, 6.2 g, 60% yield): 11-1-NMR (300 MHz, CDC13) 6 8.95 (d,
J = 1.8
Hz, 1H), 8.86 (d, J = 1.8 Hz, 1H), 7.91 (d, J = 2.0 Hz, 1H), 7.88-7.80 (m,
2H), 7.77-7.68
(m, 2H), 7.31 (d, J = 2.0 Hz, 1H), 4.96 (t, J = 2.9 Hz, 1H), 4.29 (ft, J =
12.5, 3.8 Hz, 1H),
2.88 (qd, J = 12.9, 3.6 Hz, 2H), 2.54-2.32 (m, 2H), 1.94-1.61 (m, 4H).
[00150] As shown in step 9-ii of Scheme 9, In a round bottom flask fitted with
a
condenser, a mixture of 2-[4-(7-bromoquinoxalin-5-yeoxycyclohexyl]isoindoline-
1,3-
dione (6.2 g, 12.34 mmol) , morpholine (1.61 g, 1.62 mL, 18.5 mmol) , and
Cs2CO3 (12.06
g, 37.0 mmol) in anhydrous toluene (73 mL) was treated with rac-BINAP (768.4
mg,
1.234 mmol) and Pd2(dba); (565 mg, 0.617 mmol). The reaction mixture was
heated at
110 C for 18 hours. After cooling to room temperature, the mixture was
filtered through
diatomaceous earth and concentrated under reduced pressure. The residue was
triturated
with Et20 and the solids collected by filtration and washed with Et20 to
produce 2-((c. is)-
44(7 -morpholinoquinoxalin-5-yl)oxy)cyclohexyDisoindoline-1,3-dione (compound
1029,
4.2 g) as yellow solid. The filterate was concentrated under reduced pressure
and purified
by medium pressure silica gel chromatography (0-100% Et0Ac/hexanes gradient)
to
produce an additional 300 mg of compound 1029: 11-1-NMR (300 MHz, CDC13) 6
8.76-
8.63 (m, 2H), 7.85 (dd, J = 5.4, 3.1 Hz, 2H), 7.79-7.60 (m, 2H), 7.09 (d, J =
2.6 Hz, 1H),
6.99 (d, J = 2.5 Hz, 1H), 5.06 (t, J = 2.8 Hz, 1H), 4.27 (tt, J = 12.3, 3.8
Hz, 1H), 4.02-3.85
(m, 4H), 3.49-3.27 (m, 4H), 3.03-2.75 (m, 2H), 2.37 (d, J = 14.0 Hz, 2H), 1.83-
1.56 (m,
4H).
[00151] As shown in step 9-iii of Scheme 9, to a suspension of 2-Rcis)-4-(7 -
morpholinoquinoxalin-5-yl)oxycyclohexyllisoindoline-1 ,3 -dione (2.3 g, 5.02
mmol) in
Me0H (25 mL) was added hydrazine (321 mg, 315 uL, 10.0 mmol) and the reaction
mixture stirred for 18 hours at RT, over which time the initial suspension
became
homogenenous followed by the appearance of a precipitate. Et20 (30 mL) was
added and
the reaction mixture stirred an additional 30 minutes. The precipitates were
filtered off,
the filtrate concentrated under reduced pressure, the residue treated with DCM
(30 mL),
and any remaining solids removed by filtration. The filtrate was concentrated
under
reduced pressure to provide (cis)-4-((7-moipholinoquinoxalin-5-
yl)oxy)cyclohexanamine
(compound 1030), which was used as is in subsequent reactions: 11-1-NMR (300
MHz,
CDC13) 6 8.69 (d, J = 1.9 Hz, 1H), 8.62 (d, J = 1.9 Hz, 1H), 6.95 (d, J = 2.5
Hz, 1H), 6.90
53
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
(d, J = 2.5 Hz, 1H), 5.00-4.67 (m, 3H), 4.03-3.81 (m, 4H), 3.49 (s, 1H), 3.43-
3.25 (m, 4H),
2.88 (q, J = 6.2 Hz, 2H), 2.36-1.96 (m, 6H).
[00152] As shown in step 9-iv of Scheme 9, to a solution so (cis) 4-(7-
morpholinoquinoxalin-5-yl)oxycyclohexanamine (415 mg, 1.264 mmol) and 2-
methylsulfonylpyrimidine (400 mg, 2.53 mmol) was added DIEA (490 mg, 661 L,
3.79
mmol) and the reaction mixture was sealed in a vessel and heated to 100 C for
16 hours.
After this time, the volatiles were removed under a stream of nitrogen gas and
the crude
residue dissolved in minimal amount of DCM. Purification by medium pressure
silica gel
chromatography (0-10% Me0H/DCM, 1% Et31\1] produced Ar-acis)-4-((7-
morpholinoquinoxalin-5-yl)oxy)cyclohexyl)pyrimidin-2-amine containing
triethylaminc
hydrochloride as an impurity. Dissolved product in DCM and stirred with a
silica-
supported amine (Silabond amine 40-63 gm). The scavenger mixture was
filtered,
concentrated under reduced pressure, and dried under high vacuum to provide N-
((cis)-4-
((7-morpholinoquinoxalin-5-yl)oxy)cyclohexyl)pyrimidin-2-amine (Compound 28,
435
mg): 1-H-NMR (400 MHz, CDC11) 6 8.68 (d, J = 1.9 Hz, 1H), 8.61 (d, J = 1.9 Hz,
1H),
8.27 (s, 1H), 8.26 (s, 1H), 6.94 (d, J = 2.4 Hz, 1H), 6.90 (d, J = 2.4 Hz,
1H), 6.50 (t, J = 4.8
Hz, 1H), 4.78 (s, 1H), 4.08 - 3.97 (m, 1H), 3.94 - 3.86 (m, 4H), 3.37 - 3.28
(m, 4H), 2.20
(d, J = 9.1 Hz, 2H), 1.95 - 1.85 (m, 6H).
54
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Example 10. Preparation of N-[4-[7-(6-oxa-3-azabicyclo[3.1.1]heptan-3-
yequinoxalin-5-
yl]oxycyclohexyl]pyrimidin-2-amine (Compound 291)
0
OH
0
Br') _____________________________________ 111. 0 N410.,.
0
0
N Br
Scheme 10a
1001531 To a mixture of 7-bromoquinoxalin-5-ol (47.53 g, 211.2 mmol), 2-(4-
hydroxycyclohexyl)isoindoline-1,3-dione (52.41 g, 213.7 mmol), and PPh3 (87.31
g,
332.9 mmol) in THF (740 mL) at 21 C was added tert-butyl (NZ)-N-tert-
butoxycarbonyliminocarbamate (DTBAD) (79.51 g, 328.0 mmol) in portions over 40
min
so as to maintain the temperature below 30 C and the resultant reaction
mixture was
stirred at room temperature for a further 20 h.
1001541 The reaction was evaporated in vacuo. The residual reddish-brown
viscous oil
was dissolved in CH2C12 and filtered through a plug of silica in a glass
column using
applied air pressure (plug was made with 1L of dry silica suspended in
CH2Cl2). The
plug was eluted with CH2C12, the fractions were combined and evaporated in
vacuo to
afford a red-brown viscous oil/foam, that was then dissolved in 700 mL of Me0H
before
precipitating. The mixture was stirred at room temperature for 1 h, filtered,
washed with
cold Me0H (500 mL) and Et20 (100 mL), then dried in vacuo to yield a tan solid
that was
suspended in 300 mL Me0H and brought to reflux for 10 min. The suspension was
cooled
to room temperature and filtered, washed with a further Me0H and Et20 (4:1),
and dried
in vacuo to provide 244-(7-bromoquinoxalin-5-yl)oxycyclohexyllisoindoline-1,3-
dione
(58.43 g, 126.6 mmol, 59.94%). 1H NMR (400 MHz, CDC13) 8 8.96 (d, J = 1.8 Hz,
1H),
8.86 (d, J = 1.8 Hz, 1H), 7.91 (d, J = 1.9 Hz, 1H), 7.89 - 7.82 (m, 2H), 7.78 -
7.67 (m, 2H),
7.30 (d, J = 1.9 Hz, 1H), 4.95 (s, 1H), 4.29 (tt, J = 12.5, 3.7 Hz, 1H), 2.87
(qd, J = 13.1, 3.5
Hz, 2H), 2.44 (d, J = 15.2 Hz, 2H), 1.80 (t, J = 14.1 Hz, 2H), 1.67 (d, 2H).
ESI-MS m/z
calc. 451.05316, found 452.19 (M+1)+; Retention time: 0.92 minutes.
81791389
0 0
0 + 1<NH 0
0 Oj
N N
Br N <1\1 N
oj
Scheme 10b
[00155] A mixture of 244-(7-bromoquinoxalin-5-yl)oxycyclohexyl]isoindoline-1,3-
dione
(1 g, 2.211 mmol), 6-oxa-3-azabicyclo[3.1.1]heptane HCl (180 mg, 1.328 mmol),
cesium
carbonate (2.161 g, 6.633 mmol), Pd2(dba)3 (202.5 mg, 0.2211 mmol) and rac-
BINAP
(275.3 mg, 0.4422 mmol) in dioxane (5 mL) was stirred overnight at 70 C, then
heated in a
microwave reactor for 15 min at 150 C. The reaction was then diluted with
methylene
chloride, filtered through CeliteTm, and concentrated. Silica gel flash column
chromatography
(0-5% Me0H/DCM) yielded 24447-(6-oxa-3-azabicyclo[3.1.1]heptan-3-yOquinoxalin-
5-
yl]oxycyclohexyl]isoindoline-1,3-dione (750 mg, 72.1%) as a yellow solid that
was carried on
to the next reaction.
0
H2N,1/40,..
N.,..o.
0 0
0
________________________________________ ).- N
N
N N N
Oj N
0
Scheme 10c
[00156] To a solution of 2-[4-[7-(6-oxa-3-azabicyclo[3.1.1]heptan-3-
yOquinoxalin-5-
yl]oxycyclohexyl]isoindoline-1,3-dione (800 mg, 1.700 mmol) in Et0H (10 mL)
was added
hydrazine monohydrate (85.10 mg, 83.35 i.iL, 1.700 mmol) and the reaction was
stirred at
reflux overnight, then concentrated, diluted with DCM, and filtered. The
filtrate
56
Date Recue/Date Received 2020-08-10
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
was concentrated, and purified on a 40 g silica gel cartridge with 0-50% (20%
NH3/Me0H) to yield 4-[7-(6-oxa-3-azabicyclo[3.1.1]heptan-3-yl)quinoxalin-5-
yl]oxycyclohexanamine (450 mg, 77.8%) as a yellow solid. 1H NMR (300 MHz,
Chloroform-d) a 8.65 (d, J = 1.9 Hz, 1H), 8.53 (d, J = 1.9 Hz, 1H), 6.83 (q, J
= 2.6 Hz,
2H), 4.83 (t, J = 6.0 Hz, 4H), 3.87 - 3.60 (m, 5H), 3.34 (dl, J = 8.7, 6.6 Hz,
1H), 3.01 -
2.83 (m, 1H), 2.23 (dq, J = 11.3, 5.8, 4.8 Hz, 2H), 2.07 (d, J = 8.7 Hz, 1H),
1.92 - 1.62 (m,
6H).
H2N.õ0,,
I I
0
0
F
I Y'
N
Scheme 10d
1001571 A mixture of 4-[7-(6-oxa-3-azabicyclo[3.1.11heptan-3-yflquinoxalin-
5-
yl]oxycyclohexanamine (190 mg, 0.5581 mmol), 2-fluoropyrimidine (60 mg, 0.6118
mmol) and DIEA (200 L, 1.148 mmol) in 2-propanol (2 mL) was heated in a
microwave reactor for 20 min at 150 C. The reaction mixture was concentrated,
and then
purified from 12 g silica gel cartridge with 0-6% Me0H/DCM to yield N-[4-[7-(6-
oxa-3-
azabicyclo[3.1.1]heptan-3-yl)quinoxalin-5-yl]oxycyclohexyl]pyrimidin-2-amine
(120.2
mg, 48.9%) as a yellow solid. Mass 1: 419.23; Retention Time: 0.72; NMR
Annotation:
1H NMR (400 MHz, Chloroform-d) 6 8.42 (d, J = 1.9 Hz, 1H), 8.30 (d, J = 1.9
Hz, 1H),
8.04 (d, J = 4.8 Hz, 2H), 6.65 - 6.56 (m, 2H), 6.28 (t, J = 4.8 Hz, 1H), 4.99
(d, J = 8.1 Hz,
1H), 4.60 (d, J = 6.5 Hz, 3H), 3.79 (dd, J = 8.2, 4.0 Hz, OH), 3.62 - 3.38 (m,
4H), 3.17 -
3.03 (m, 1H), 2.07 - 1.90 (m, 2H), 1.89 - 1.59 (m, 7H).
57
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Example 11. Preparation of 6-(4-methylpiperazin-1-y1)-N-((ls,4s)-447-
morpholinoquinoxalin-5-yl)oxy)cyclohexyl)pyrimidin-4-amine (Compound 537)
H2N N N40,
eN, CI rij
0 Na0t-Bu N 0
t-BuXPhos Palladacycle
110 CNJ
t-BuON, 100 C N 110
I
Scheme 11
[00158] A suspension of 4-(7-molpholinoquinoxalin-5-yeoxycyclohexanamine (500
mg, 1.52 mmol), 4-chloro-6-(4-methylpiperazin-1-yl)pyrimidine (324 mg, 1.52
mmol),
and Na0t-Bu (440 mg, 4.58 mmol) in t-BuOH (10.1 mL) was degassed by bubbling
N2
through the mixture for 10 min. t-BuXPhos Palladacycle (53 mg, 0.077 mmol) was
added,
and the reaction mixture was sealed hermetically and heated to 100 C in an
oil bath for 2
h. The solvent was removed in vacuo, and the crude residue was purified by
silica gel
chromatography (40 g Isco gold column, linear gradient 0% -> 10% Me0H/CH2C12
[+0.1% Et3N]) to yield 6-(4-methylpiperazin-1-y1)-N-((1s,4s)-447-
motpholinoquinoxalin-5-yl)oxy)cyclohexyl)pyrimidin-4-amine as a yellow solid
(654.7
mg, 83.5% yield). 1H NMR (400 MHz, Chloroform-d) 6 8.69 (d, J = 1.8 Hz, 1H),
8.61 (d,
J = 1.8 Hz, 1H), 8.19 (s, 1H), 6.95 (d, J = 2.4 Hz, 1H), 6.90 (d, J = 2.3 Hz,
1H), 5.42 (s,
1H), 4.78 (s, 1H), 4.70 (d, J = 8.0 Hz, 1H), 3.97 - 3.87 (m, 4H), 3.83 (s,
1H), 3.64 - 3.50
(m, 4H), 3.40 - 3.27 (m, 4H), 2.53 -2.42 (m, 4H), 2.34 (s, 3H), 2.19 (d, J =
9.2 Hz, 2H),
1.97 - 1.77 (m, 6H). ESI-MS m/z calc. 504.2961, found 505.44 (M+1)+; Retention
time:
0.5 minutes.
58
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Example 12. Preparation of 2-(4-methylpiperazin-1-y1)-N-((ls,4s)-447-
morpholinoquinoxalin-5-yl)oxy)cyclohexyl)pyrimidin-4-amine (Compound 535)
H2N
CI NõN N
0 N 0
Et3N
1101
rN N Et0H, reflux
0
LN
N
N N 1.
N-methylpiperazine 0
i-Pr2NEt
i-PrOH, 170 C wave
,õ)
Scheme 12
[00159] To a solution of 4-(7-morpholinoquinoxalin-5-yl)oxycyclobexanamine
(500
mg, 1.522 mmol) and Et3N (212 4, 1.522 mmol) in Et0H (7.25 mL) was added 2,4-
dichloropyrimidine (216.0 mg, 1.450 mmol). The resultant reaction solution was
heated to
reflux for 5 h, then cooled to room temperature. The solvent was removed in
vacuo, and
the crude residue was purified by silica gel chromatography (40 g Isco gold
column, linear
gradient 0% -> 10% Me0H/CH2C12 [+0.1% Et3N]) to provide 2-chloro-N44-(7-
morpholinoquinoxalin-5-yeoxycyclohexyl]pyrimidin-4-amine as a yellow solid
(485 mg,
75% yield). 1H NMR (300 MHz, Chloroform-d) 6 8.70 (d, J = 2.0 Hz, 1H), 8.60
(d, J =
2.0 Hz, 1H), 8.00 (d, J = 4.8 Hz, 1H), 6.96 (d, J = 2.5 Hz, 1H), 6.89 (d, J =
2.5 Hz, 1H),
6.23 (d, J = 5.9 Hz, 1H), 5.18 (d, J = 5.7 Hz, 1H), 4.80 (s, 1H), 3.99 - 3.84
(m, 4H), 3.42 -
3.21 (m, 4H), 2.32 - 2.08 (m, 2H), 2.05- 1.73 (m, 6H). EST-MS m/z calc.
440.17276,
found 441.25 (M+1) ; Retention time: 0.69 minutes.
[00160] A solution of 2-chloro-N14-(7-moipholinoquinoxalin-5-
yl)oxycyclohexyl]pyrimidin-4-amine (236 mg, 0.5352 mmol), 1-methylpiperazine
(190
59
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1.711 mmol), and Hunig's base (315 !IL, 1.808 mmol) in i-PrOH (3.6 mL) was
heated
to 170 C in a microwave for 1 h. The solvent was removed in vacuo. The crude
residue
was purified by silica gel chromatography (40 g Isco gold column, linear
gradient 0% ->
10% Me0H/CH2C12 [+0.1% Et3N]) to provide 2-(4-methylpiperazin-1 -y1)-N-
((1s,4s)-4-
((7-morpholinoquinoxalin-5-yl)oxy)cyclohexyl)pyrimidin-4-amine as a yellow
solid (219
mg, 79% yield). 1H NMR (400 MHz, Chloroform-d) 6 8.69 (d, J = 1.9 Hz, 1H),
8.61 (d, J
= 1.9 Hz, 1H), 7.88 (d, J = 5.7 Hz, 1H), 6.95 (d, J = 2.4 Hz, 1H), 6.89 (d, J
= 2.4 Hz, 1H),
5.67 (d, J = 5.8 Hz, 1H), 4.84 - 4.63 (m, 2H), 3.99 - 3.83 (m, 5H), 3.82 -
3.73 (m, 4H),
3.37 - 3.27 (m, 4H), 2.49 - 2.40 (m, 4H), 2.33 (s, 3H), 2.23 -2.11 (m, 2H),
1.96- 1.82 (m,
6H). ES1-MS calc. 504.2961, found 505.35 (M+1) '; Retention time: 0.51
minutes.
Example 13. Preparation of N-methy1-64(1s,4s)-4-((7-motpholinoquinoxalin-5-
ypoxy)cyclohexyl)amino)pyrimidine-4-carboxamide (Compound 359)
0 0
.µ*\..k.'"..'-**1 OH 1) LtalFly1 chloride CI
NH
N N 2) methyl amine
NaHCO3 N N
Scheme 13a
1001611 A solution of oxalyl chloride (186.2g, 256.0 mmol) and DMF (1.7 mL,
21.96
mmol) was added to a suspension of 6-chloropyrimidine-4-carboxylic acid (9.1
g, 57.40
mmol) in dichloromethane (300 mL) dropwise via dropping funnel over 20min.
Allowed
to stir for 21u- and concentrated the acid chloride under reduced pressure.
The acid
chloride was dissolved in dichloromethane (250mL) and to it was added a
solution of
methylamine (30.71 mL of 40 %w/v, 395.5 mmol) in water and NaHCO3 (188.3 mL of
1.2 M, 226.0 mmol) dropwise. The reaction mixture was allowed to stir
overnight. The
layers were separated and extracted the aqueous layer with dichloromethane
(200mL).
The combined organics were washed with brine, dried over sodium sulfate, and
concentrated under reduced pressure. Chromatographed over 120g silica gel
using 0-50%
Et0Ac/Heptane as eluent. 4.356g (44.94% yield) of 6-chloro-N-methylpyrimidine-
4-
carboxamide was obtained as a white solid. 1H NMR (400 MHz, Chloroform-d) 6
8.99
(d, J = 1.1 Hz, 1H), 8.16 (d, J = 1.1 Hz, 1H), 7.90 (s, 1H), 3.06 (d, J = 5.2
Hz, 3H).
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
0
voANH2
0 0 sodium t-butoxide =
_________________________________________ D.
( = N CI YNyji"N t-BuXPhos palladacycle
N
N' vCiiµ
N N t-BuOH
)
0
Scheme 13b
1001621 A solution of 4-(7-morpholinoquinoxalin-5-yl)oxycyclohexanamine (7.593
g,
23.12 mmol), 6-chloro-N-methyl-pyrimidine-4-carboxamide (4.35 g, 25.35 mmol),
and t-
BuXIThos palladacycle (1.519 g, 2.333 mmol) in tBuOH (100 mL) was added sodium
t-
butoxide (25.5 mL of 2 M, 51.00 mmol). Allowed to stir at room temperature
under N2
overnight. The reaction was diluted with dichloromethane (100mL) and filtered
through
Celite with dichloromethane wash. The filtrate was concentrated under reduced
pressure
and chromatographed over 330g silica gel using 0->14% MethanoLDCM as eluent.
The
resulting product was dissolve in small amount of ethanol (15mL) and the
product
precipitated over lh. Filtered with cold ethanol wash to give a yellow solid,
the solid was
dried under vacuum over for 48h at 50 C to yield N-methy1-6-4(1s,4s)-447-
morpholinoquinoxalin-5-yeoxy)cyclohexyl)amino)pyrimidine-4-carboxamide (2.58g)
as a
yellow solid. 1H NMR (300 MHz, Chloroform-d) 6 8.70 (d, J = 1.9 Hz, 1H), 8.61
(d, J =
1.9 Hz, 1H), 8.53 - 8.44 (m, 1H), 8.00 (d, J = 5.6 Hz, 1H), 7.20 (d, J = 1.2
Hz, 1H), 6.96
(d, J = 2.5 Hz, 1H), 6.90 (d, J = 2.5 Hz, 1H), 5.50 (s, 1H), 4.83 (dt, J =
4.8, 2.5 Hz, 1H),
3.97 -3.87 (m, 4H), 3.39 - 3.29 (m, 4H), 3.01 (d, J = 5.1 Hz, 3H), 2.31 -2.19
(m, 2H),
2.06 - 1.70 (m, 6H). ESI-MS calc.
463.2332, found 464.25 (M+1)+; Retention time:
0.59 minutes.
61
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Example 14. Preparation of N-methy1-24(1s,4s)-447-molpholinoquinoxalin-5-
yl)oxy)cyclohexyl)amino)pyrimidine-4-carboxamide (Compound 350)
0 0
rl. AOH EDCI, HOBT
-31111' rYL, N H
mcthylaminc N N
N N DIVIF
CI CI
Scheme 14a
[00163] A solution of 2-chloropyrimidine-4-carboxylic acid (4.425 g, 27.91
mmol) in
DMF (35.40 mL) was added 1-hydroxybenzotriazole (Water (1)) (2.982 g, 19.47
mmol)
and EDCI (Hydrochloric Acid (1)) (6.372 g, 33.24 mmol) at room temperature.
After
stirred for 10 min, to the mixture was added METHYL AMINE in
tetrahydrofuran(20.93
mL of 2 M, 41.86 mmol) and stirred for 2h. The reaction was diluted with ethyl
acetate,
washed with water, and dried over sodium sulfate then concentrated. The
resuting residue
was purified from silica gel column 0-30% ethyl acetate/ hexanes to obtain 2-
chloro-N-
methylpyrimidine-4-carboxamide as a white solid. 1H NMR (300 MHz, Chloroform-
d) 6
8.85 (d, J = 4.9 Hz, 1H), 8.07 (d, J = 4.9 Hz, 1H), 7.82 (s, 1H), 7.27 (s,
OH), 3.06 (d, J =
5.2 Hz, 3H).
vorNH2
N N
ijKvi
0 sodium t-butoxide Ov NLN>
:0
N/1 101 N N
t-BuXPhos palladacycle 1
NH
t-BuOH
0 N
c/0
Scheme 14b
[00164] A solution of 4-(7-morpholinoquinoxalin-5-yl)oxycyclohexanamine
(6.013 g,
18.31 mmol), 2-chloro-N-methyl-pyrimidine-4-carboxamide (3.41 g, 19.87 mmol)
and t-
BuXPhos palladacycle (1.220 g, 1.873 mmol) in tBuOH (100 mL) was added sodium
t-
butoxide (20.52 mL of 2 M, 41.04 mmol). Allowed to stir at rt under N2
overnight.
Diluted rxn with dichloromethane (100mL), filtered through celite and
concentrated
62
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
filtrate under reduced pressure. The resulting residue was chromatographed
over 220g
silica gel using 0-14% Methanol/dichlomethane. The product was triturated with
ethanol
(40mL) at 70 C for 2h, filtered and dried under vacuum at 50 C overnight to
obtain N-
methy1-2-(((1s,4s)-4-((7-morpholinoquinoxalin-5-
yDoxy)cyclohexyl)amino)pyrimidine-4-
carboxamide (5.03g, 62%yield) as a fine yellow powder. 1H NMR (300 MHz,
Chloroform-d) 6 8.69 (d, J = 1.9 Hz, 1H), 8.62 (d, J = 1.9 Hz, 1H), 8.48 (d, J
= 4.9 Hz,
1H), 7.75 (s, 1H), 7.32 (d, J = 4.9 Hz, 1H), 6.96 (d, J = 2.5 Hz, 1H), 6.91
(d, J = 2.5 Hz,
1H), 5.30 (d, J = 7.9 Hz, 1H), 4.85 - 4.73 (m, 1H), 4.07 (dd, J = 8.5, 4.7 Hz,
1H), 3.99 -
3.82 (m, 4H), 3.41 - 3.20 (m, 4H), 3.01 (d, J = 5.1 Hz, 3H), 2.20 (q, J = 6.0
Hz, 2H), 2.06 -
1.82 (m, 6H). ES1-MS m/z calc. 463.2332, found 464.31 (M+1)+; Retention time:
0.71
minutes
[00165] Tables 1 and 2 provides analytical characterization data for certain
compounds
of formula I (blank cells indicate that the test was not performed).
Table 1.
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure
No (M+H) indicated otherwise)
.
NMR peaks given as 6 values
(CDC13) 6 8.64 (d, J = 2.0 Hz,
H 1\1) 1H), 8.36 (d, J = 2.0 Hz, 1H),
6.61 (d, J = 2.4 Hz, 1H), 6.34
0 =10 O .'
(d, J = 2.4 Hz, 1H), 5.90 (s,
H3CA N. 370.52 1H), 5.40 (d, J = 7.7 Hz, 1H),
3.98 -3.76 (m, 5H), 3.51 -3.24
Co) (m, 5H), 2.33 - 2.08 (m, 4H),
1.99 (s, 3H), 1.58- 1.31 (m,
4H)
(CDC13) 6 8.63 (d, J = 1.9 Hz,
1H), 8.34 (d, J = 1.9 Hz, 1H),
7.17 (d, J = 8.6 Hz, 2H), 6.90
,,CH3
0 H N (d, J = 8.5 Hz, 2H), 6.59 (d, J =
0N
N"µs. 2.2 Hz, 1H), 6.29 (d, J = 2.2
r- iiHz, 1H), 5.86 (d, J = 7.6 Hz,
2 476.61 1H), 5.29 (d, J = 8.0 Hz, 1H),
3.96 - 3.75 (m, 8H), 3.52 (s,
(o) 2H), 3.39 - 3.22 (m, 5H), 2.19
(d, J = 11.7 Hz, 2H), 2.12 -
1.89 (m, 2H), 1.43 (td, J = 13.0,
2.4 Hz, 2H), 1.32- 1.15 (m,
2H)
63
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.65 (d, J = 2.0 Hz,
H 1H), 8.35 (d, J = 2.0 Hz, 1H),
=N (6d. 6, =( d2, .J4=} {2z. ,41H} {z) 61H.1 )1, 60.3, 4
3 j =
CH3 0
H3C-4,
H3C0 N 428.49 7.8 Hz, 1H), 4.60 (s, 1H), 3.97
- 3.86 (m, 4H), 3.67 (s, 2H),
Co) 3.41 - 3.25 (m, 4H), 1.85 (d, J =
3.0 Hz, 5H), 1.74 - 1.57 (m,
3H), 1.45 (s, 9H)
(CDC13) 6 8.64 (d, J = 1.9 Hz,
H
1H), 8.34 (d, J = 1.9 Hz, 1H),
=1
0 ea#N N 6.61 (d, J = 2.2 Hz, 1H), 6.35
(d, J = 2.3 Hz, 1H), 6.12 ( s,
4 H3CN 370.46 1H), 5.56 (d, J = 7.5 Hz, 1H),
4.11 -3.81 (m, 5H), 3.70 (m,
1H), 3.42 - 3.24 (m, 4H), 1.99
(s, 3H), 1.86 (m, 6H), 1.75 -
1.51 (m, 2H)
(CDC13) 6 8.65 (d, J = 2.0 Hz,
H 1H), 8.36 (d, J = 2.0 Hz, 1H),
6.62 (d, J = 2.4 Hz, 1H), 6.34
Q 0
oel:D#N 4101 (d, J = 2.3 Hz, 1H), 6.09 (d, J =
H3CS,N
406.12 7.6 Hz, 1H), 4.68 (d, J = 7.2
Hz, 1H), 3.97 - 3.82 (m, 4H),
3.76 - 3.47 (m, 2H), 3.40 - 3.23
*ICK (m, 4H), 3.01 (s, 3H), 2.04 -
1.65 (m, 8H)
(CDC13) 6 8.65 (d, J = 2.0 Hz,
1H), 8.36 (d, J = 2.0 Hz, 1H),
H 6.61 (d, J = 2.4 Hz, 1H), 6.35
0
6 H3COAN N
= 400.17 (d, J = 2.4 Hz, 1H), 6.10 (d, J =
7.6 Hz, 1H), 4.72 (s, 1H), 4.12
(q, J = 7.0 Hz, 2H), 3.96 - 3.82
C) (m, 4H), 3.68 (s, 2H), 3.42 -
o 3.23 (m, 4H), 1.93 - 1.78 (m,
6H), 1.69 (dd, J = 15.0, 6.3 Hz,
2H), 1.25 (t, J = 7.1 Hz, 3H)
64
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.65 (d, J = 2.0 Hz,
1H), 8.35 (d, J = 2.0 Hz, 1H),
H
) 6.61 (d, J = 2.4 Hz, 1H), 6.35
I
0 iecji,N1 0 N (d, J = 2.3 Hz, 1H), 6.10 (d, J=
7 0 A
0 N 7.7 Hz, 1H), 5.26 (s, 1H), 4.85
442.14 (d, J = 7.4 Hz, 1H), 4.03 - 3.79
H
.,.N) (m, 8H), 3.68 (s, 2H), 3.41 -
3.24 (m, 4H), 2.16 (dd, J =
0 13.9, 6.0 Hz, 1H), 2.08 - 1.93
(m, 1H), 1.86 (d, J = 3.5 Hz,
6H), 1.76 - 1.54 (m, 2H)
(CDC13) 6 8.65 (d, J = 2.0 Hz,
H
N-'1 1H), 8.36 (d, J = 2.0 Hz, 1H),
I
H01 õ101, N 0 N 6.61 (d, J = 2.4 Hz, 1H), 6.35
(d, J = 2.4 Hz, 1H), 6.10 (d, J =
8 0 N 416.42 7.6 Hz, 1H), 4.88 (d, J = 6.3
H
N Hz, 1H), 4.21 (d, J = 4.1 Hz,
Co) 2H), 3.95 - 3.88 (m, 4H), 3.87 -
3.59 (m, 4H), 3.40 - 3.26 (m,
4H), 1.95 - 1.52 (m, 9H)
(CDC13) 6 8.65 (d, J = 1.9 Hz,
CH3 H 1\11 1H), 8.37 (d, J = 2.0 Hz, 1H),
I
11 0 #0,N is N 6.61 (d, J = 2.3 Hz, 1H), 6.34
(d, J = 2.3 Hz, 1H), 6.09 (d, J =
9 ==0.1.1. N 424.42 7.4 Hz, 1H), 4.81 (s, 1H), 4.66
C N
H (s, 2H), 4.01 - 3.86 (m, 4H), oD
3.65 (s, 2H), 3.42 - 3.24 (m,
4H), 1.87 (t, J = 2.3 Hz, 5H),
1.64 (d, J = 26.9 Hz, 6H)
(CDC13) 6 8.65 (d, J = 1.9 Hz,
1H), 8.36 (d, J = 1.9 Hz, 1H),
H N 1 6.61 (d, J = 2.3 Hz, IH), 6.35
0
-.0 N4) 10 N (d, J = 2.3 Hz, 1H), 6.10 (d, J =
A N
7.7 Hz, 1H), 4.72 (s, 1H), 4.02
ro 414.44
H (t, J = 6.6 Hz, 2H), 3.95 - 3.82
CH3 N (m, 4H), 3.68 (s, 2H), 3.43 -
Cop 3.26 (m, 4H), 1.87 (d, J = 3.7
Hz, 6H), 1.78 - 1.50 (m, 4H),
0.94 (t, J = 7.4 Hz, 3H)
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC1.3) 6 8.65 (d, J = 2.0 Hz,
1H), 8.35 (d, J = 2.0 Hz, 1H),
N
H I 6.60 (d, J = 2.4 Hz, 1H), 6.35
11 H3c 0
eaõ,N 0 N
CH3 0 414.44 (d, J = 2.4 Hz, 1H), 6.10 (d, J =
.). AN 7.7 Hz, 1H), 4.91 (dt, J = 12.5,
H 6.2 Hz, 1H), 4.69 (s, 1H), 4.01
CN) - 3.81 (m, 4H), 3.68 (s, 2H),
o 3.46 - 3.24 (m, 4H), 1.93 - 1.76
(m, 6H), 1.78 - 1.56 (m, 2H),
1.25 (t, J = 9.6 Hz, 6H)
(CDC13) 6 8.65 (d, J = 1.9 Hz,
H I
N--.) 1H), 8.35 (d, J = 1.9 Hz, 1H),
0 0,0,,,N 0 N 6.61 (d, J = 2.3 Hz, 1H), 6.35
(d, J = 2.3 Hz, 1H), 6.11 (d, J =
12 H3Cr.,N.,0,1,N 428.2 7.6 Hz, 1H), 4.74 (s, 1H), 3.99
H
CH3 N - 3.79 (m, 6H), 3.68 (s, 2H),
(c) 3.40 - 3.24 (m, 4H), 1.87 (d, J =
3.5 Hz, 7H), 1.78 - 1.52 (m,
2H), 0.93 (d, J = 6.7 Hz, 6H)
(CDC13) 6 8.65 (t, J = 1.6 Hz,
1H), 8.36 (t, J = 1.6 Hz, 1H),
1
H N*- 1 6.61 (d, J = 2.3 Hz, 1H), 6.35
13 r0 Nif:Do 0 N N
0 418.44 (d, J = 2.0 Hz, 1H), 6.11 (d, J =
A 7.6 Hz, 1H), 4.91 (d, J = 7.2
-.
H Hz, 1H), 4.80 - 4.18 (m, 4H),
F N 4.00 - 3.84 (m, 4H), 3.81 - 3.56
Co) (m, 2H), 3.46 - 3.21 (m, 4H),
1.87 (d, J = 3.5 Hz, 6H), 1.71
(dd, J = 16.0, 8.2 Hz, 2H)
(CDC13) 6 8.65 (d, J = 2.0 Hz,
H N 1H), 8.36 (d, J = 2.0 Hz, 1H),
'''''
H I 6.61 (d, J = 2.4 Hz, 1H), 6.34
0 N (d, J = 2.3 Hz, 1H), 6.10 (d, J =
14 ..0AN0,, 410.44 7.1 Hz, 1H), 4.83 (s, 1H), 4.69
H (s, 2H), 3.96 - 3.83 (m, 4H),
(N 3.68 (s, 2H), 3.40 - 3.23 (m,
o) 4H), 2.48 (t, J = 2.4 Hz, 1H),
1.87 (d, J = 4.2 Hz, 6H), 1.71
(dd, J = 15.7, 7.4 Hz, 2H)
66
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC11) 6 8.66 (d, J = 2.0 Hz,
1H), 8.37 (d, J = 2.0 Hz, 1H),
?I-13 N-*.i
H I 7.10 -6.98 (m, 2H), 6.87 (d, J =
0 N 9.0 Hz, 2H), 6.62 (d, J = 2.4
15 it j) µe(CrN 0
g'ri. 0 N Hz, 1H), 6.36 (d, J = 2.4 Hz,
478.44 1H), 6.13 (d, J = 7.3 Hz, 1H),
H
N 5.06 (d, J = 7.9 Hz, 1H), 3.97 -
Co) 3.84 (m, 4H), 3.84 - 3.61 (m,
5H), 3.42 - 3.25 (m, 4H), 1.91
(d, J = 4.2 Hz, 6H), 1.85 - 1.68
(m, 2H)
(CDC13) 6 8.49 (s, 1H), 8.23 (d,
H
1 J = 1.5 Hz, 1H), 6.48 (s, 1H),
I
ecrN 0 N
4J.=521(.9s,H1Hz,),1H4.)4,76.(0s6,
CH3 0
H3C-71,, A (6s. ,181H(d),
16 0 N 453.96 2H), 3.60 (s, 2H), 3.45 (d, J =
H3C H
N 11.6 Hz, 2H), 3.14 - 3.12 (m,
..-- --..
0 2H), 1.96 - 1.84 (m, 4H), 1.79
0 (s, 5H), 1.54 (s, 3H) and 1.38
(s, 9H) ppm
, N-0, (cDa3) 6 6.13 (d, J= 1.6 Hz,
/ N 1H), 5.89 (d, J = 1.4 Hz, 1H),
CH3 0
H3C¨j, [1
Cr WI 4.87 (d, J = 7.0 Hz, 1H), 4.59
17 ,_, ri 418.4 (s, 1H), 3.26 (dd, J = 9.2, 4.3
1 13,, H
N Hz, 4H), 1.98 - 1.74 (m, 6H),
Co) 1.65 (dd, J = 15.9, 7.3 Hz, 3H),
1.47 (s, 9H)
(CDC13) 6 8.65 (d, J = 2.0 Hz,
1H), 8.37 (d, J = 2.0 Hz, 1H),
8.27 (d, J = 4.8 Hz, 2H), 6.60
H NI (d, J = 2.4 Hz, 1H), 6.51 (t, J =
N 4.8 Hz, 1H), 6.36 (d, J = 2.4
18 HNiCi 116 N 406.48
Hz, 1H), 6.15 (d, J = 7.8 Hz,
1H), 5.20 (d, J = 7.7 Hz, 1H),
N N N 4.04 (d, J = 7.9 Hz, 1H), 3.96 -
Co)
3.82 (m, 4H), 3.70 (s, 1H), 3.39
-3.24 (m, 4H), 1.94 (dd, J =
13.7, 4.4 Hz, 6H), 1.78 (dt, J =
28.8, 16.1 Hz, 2H)
67
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.64 (d, J = 1.9 Hz,
1H), 8.35 (d, J = 1.9 Hz, 1H),
N*-1
H 6.58 (t, J = 5.1 Hz, 2H), 6.34
0 19 H3C-CLN.0,N N
400.46 (d, J = 2.3 Hz, 1H), 6.10 (d, J =
7.3 Hz, 1H), 4.03 (d, J = 8.3
`)L
Hz, 1H), 3.88 (t, J = 4.7 Hz,
C6H), 3.68 (s, 1H), 3.42 (s, 3H),
o) 3.37 - 3.23 (m, 4H), 1.98 - 1.78
(m, 6H), 1.69 (dd, J = 15.8, 7.5
Hz, 2H)
(400.0 MHz, CDC13) 6 8.52 (d,
J= 1.7 Hz, 1H), 8.23 (d, J = 1.6
Hz, 1H), 6.47 (d, J = 1.9 Hz,
H NI 468.23 1H), 6.19 (s, 1H), 6.03 (s, 1H),
20 0
5.19(s, 1H), 4.76 (d, J = 7.6
.õ0 N Hz, 1H), 4.47 (s, 2H), 3.87 -
3.76 (m, 4H), 3.60 (s, 2H), 3.45
(d, J = 11.6 Hz, 2H), 3.13 -
3.10 (m, 2H), 2.61 (s, 1H), 2.13
0 - 2.04 (m, 1H), 1.95 - 1.85 (m,
5H), 1.79 (s, 5H) and 1.62 -
1.58 (m, 2H)
N
H
0 ,CrN N
21 H3c0AN 426.31
H
0
(400.0 MHz, CDC13) 6 8.54 (s,
1H), 8.22 (s, 1H), 6.43 (s, 1H),
H N1 6.19 (s, 1H), 6.02 (s, 1H), 4.47
0 N (s, 2H), 4.38 (d, J = 5.2 Hz,
1H), 4.28 (s, 1H), 3.74 (s, 1H),
22 H3C-N-j."N 425.35
H H 3.60 (s, 1H), 3.42 (s, 4H), 3.14
- 3.09 (m, 4H), 2.05 - 1.87 (m,
3H), 1.79 (s, 3H), 1.55 (d, J =
0 7.1 Hz, 2H) and 1.21 - 1.05 (m,
5H)
68
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC11) 6 8.20 (d, J = 4.9 Hz,
N-0,
H 1 N 2H), 6.46 (t, J = 4.8 Hz, 1H),
N
23 HWCj 396.2 6.05 (d, J = 1.6 Hz, 1H), 5.82
(s, 1H), 5.24 (s, 1H), 4.82 (d, J
.i. II
--L, N = 7.0 Hz, 1H), 3.98 (s, 1H),
N '. N 3.85 - 3.72 (m, 4H), 3.60 (s,
1H), 3.23 - 3.06 (m, 4H), 1.95 -
1.62 (m, 8H). [2]
(400 MHz, CDC13) 6 8.65 (s,
1H), 8.37 (d, J = 1.8 Hz, 1H),
H
I\ I 8.08 (d, J = 4.9 Hz, 1H), 7.41
N 24 HN/Cj. II N (t, J = 7.7 Hz, 1H), 6.61 (s, 1H),
6.58 - 6.53 (m, 1H), 6.46 - 6.30
405.59 (m, 2H), 6.15 (d, J = 7.3 Hz,
N 1H), 4.56 (s, 1H), 3.98 - 3.78
N'i
Co) (m, 5H), 3.76 - 3.61 (m, 1H),
3.42 - 3.24 (m, 4H), 1.97 (d, J =
29.6 Hz, 6H), 1.86 - 1.66 (m,
2H)
(CDC13) 6 8.69 (d, J = 2.0 Hz,
) 1H), 8.42 (d, J = 2.0 Hz, 1H),
H I 8.26 (s, 1H), 7.14 (d, J = 2.1
HN
.fooN 0 N
Hz, 1H), 6.64 (d, J = 2.4 Hz,
1H), 6.38 (d, J = 2.3 Hz, 1H),
'IN N 467.57 6.24 (d, J = 6.8 Hz, 1H), 4.31
N r 0 (q, J = 7.1 Hz, 2H), 3.97 - 3.81
H3C---N / r ,.
(m, 5H), 3.41 - 3.24 (m, 4H),
.-- 0\< L'e
2.22 (d, J = 7.5 Hz, 2H), 1.90
0 (dd, J = 22.4, 10.3 Hz, 6H),
1.35 (t, J = 7.1 Hz, 3H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
1\1-NI i 1H), 8.62 (d, J = 1.9 Hz, 1H),
N 6.95 (d, J = 2.5 Hz, 1H), 6.88
26 10
CH 0 10=4*()
H3C--k 3A (d, J = 2.5 Hz, 1H), 4.82 ? 4.60
H3C/ -0 N 429.62 (m, 2H), 4.00? 3.88 (m, 4H),
H
N 3.69 (d, J = 6.8 Hz, 1H), 3.42?
Co) 3.28 (m, 4H), 2.15 (p, J = 6.7,
5.6 Hz, 2H), 1.94? 1.75 (m,
6H), 1.46 (s, 9H)
69
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.65 (d, J = 2.0 Hz,
1H), 8.36 (d, J = 2.0 Hz, 1H),
7.98 (dd, J = 2.8, 1.5 Hz, 1H),
H I
V-7'.1 7.88 (d, J = 1.5 Hz, 1H), 7.79
N N (d, J = 2.8 Hz, 1H), 6.62 (d, J =
2.4 Hz, 1H), 6.37 (d, J = 2.4
27 HNIP S 406.58 Hz, 1H), 6.17 (s, 1H), 8.69 -
N N 8.61 (m, 1H), 4.60 (d, J = 7.7
Nj Co) Hz, 1H), 4.08 - 3.83 (m, 5H),
8.71 - 8.57 (m, 1H), 3.73 (t, J =
6.9 Hz, 1H), 3.40 - 3.25 (m,
4H), 1.96 (h, J = 4.9 Hz, 6H),
1.77 (q, J= 7.4, 6.1 Hz, 2H)
(400 MHz, CDC13) 6 8.77 -
8.59 (m, 2H), 8.29 (d, J = 4.9
0 aill N
Hz, 2H), 7.01 - 6.87 (m, 2H),
6.61 - 6.48 (m, 1H), 4.82 (s,
28 HNIX:::r. II" 407.3
-i. N 1H), 4.05 (s, 1H), 3.93 (t, J=
N N
4.8 Hz, 4H), 3.35 (t, J = 4.8 Hz,
V (a)
4H), 2.23 (d, J = 13.1 Hz, 2H),
2.05 - 1.82 (m, 6H)
H 1
1\l' 1 7 (DMSO-d6) 6 12.65 (s, 1H),
ocf,N 0 N 8.69 (d, J = 2.0 Hz, 2H), 8.43
(d, J = 2.0 Hz, 1H), 8.00 (d, J =
HN 7.9 Hz, 1H), 6.54 (d, J = 2.5
.1, N 450.61 Hz, 1H), 6.48 (d, J = 2.3 Hz,
29
N N 1H), 6.17 (d, J = 8.2 Hz, 1H),
[,) Co) 4.06 (s, 1H), 3.77 (dd, J = 5.9,
3.8 Hz, 4H), 3.29 (s, 5H), 2.04
0 OH - 1.46 (m, 8H)
(CDC13) 6 8.66 (d, J = 2.0 Hz,
1H), 8.58 (s, 1H), 8.37 (d, J =
H N' 1 2.0 Hz, 1H), 8.13 (s, 2H), 6.63
N N
(d, J = 2.4 Hz, 1H), 6.37 (d, J =
2.4 Hz, 1H), 6.14 (d, J = 7.6
30 HNICT 406.52
Hz, 1H), 3.96 - 3.86 (m, 4H),
(N) 3.76 (d, J = 7.7 Hz, 2H), 3.54
o
N ... N (d, J = 8.3 Hz, 1H), 3.39 - 3.29
-....--
(m, 4H), 2.02 - 1.86 (m, 6H),
1.76 (q, J = 8.9, 8.3 Hz, 2H)
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 12.88 - 12.45 (m,
1H), 8.66 (d, J = 2.0 Hz, 1H),
H N=II 8.37 (d, J = 2.0 Hz, 1H), 7.81
N N (s, 1H), 6.62 (d, J = 2.3 Hz,
1H), 6.36 (d, J = 2.4 Hz, 1H),
31 HN3. 422.49 6.12 (d, J = 7.7 Hz, 1H), 5.27
.)`.-
' N N (s, 1H), 5.05 (d, J = 7.6 Hz,
N Co) 1H), 4.02 - 3.83 (m, 4H), 3.63
0
(d, J = 47.6 Hz, 2H), 3.44 -
3.22 (m, 4H), 1.91 (q, J = 4.8,
4.3 Hz, 6H), 1.82-1.69 (m, 2H)
(CDC13) 6 8.78 (d, J = 1.3 Hz,
H I
Ni'l 1H), 8.66 (d, J = 2.0 Hz, 1H),
#0õ, N IN N 8.37 (d, J = 2.0 Hz, 1H), 7.91
(d, J = 1.3 Hz, 1H), 6.62 (d, J =
HN 2.4 Hz, 1H), 6.37 (d, J = 2.4
32 N 464.6 Hz, 1H), 6.16 (d, J = 7.6 Hz,
1H), 5.13 (d, J = 7.6 Hz, 1H),
1 I (o)
4.10 (s, 1H), 3.99 -3.86 (m,
7H), 3.76 (s, 1H), 3.39 - 3.28
0.1'''CrCH3
(111, 4H), 1.98 (h, J = 4.8 Hz,
6H), 1.80 (t, J = 8.7 Hz, 2H)
(CDC13) 6 8.63 (d, J = 1.9 Hz,
1H), 7.99 (dd, J = 2.8, 1.5 Hz,
N-.7)I 1H), 7.94 - 7.85 (m, 1H), 7.79
0
Cr 0 N
407.3 (d, J = 2.8 Hz, 1H), 7.14 (d, J =
33 HN
1.0 Hz, 1H), 7.03 - 6.87 (m,
2H), 4.82 (d, J = 5.7 Hz, 1H),
N N 4.70 (d, J = 8.0 Hz, 1H), 4.03 -
1 I Co) 3.86 (m, 4H), 3.51 (s, 1H), 3.43
..,..N
-3.30 (m, 4H), 2.35- 1.81 (m,
8H)
(CDC13) 6 8.69 (dd, J = 3.4, 1.9
Hz, 1H), 8.62 (dd, J = 3.6, 1.9
Nr.7) Hz, 1H), 8.51 (dd, J = 4.8, 2.2
34 O''' 0 Cr 110 N
408.5 Hz, 2H), 7.01 - 6.83 (m, 3H),
5.18 (tt, J = 7.0, 3.4 Hz, 1H),
4.79 (tt, J = 6.9, 3.1 Hz, 1H),
NN 4.00 - 3.85 (m, 4H), 3.34 (dq, J
.. CNo) = 4.8, 2.6 Hz, 4H), 2.44 -2.16
(m, 4H), 1.92 (tdd, J = 16.4,
7.7, 2.8 Hz, 4H)
71
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
0 N-5 (CDC13) 6 8.68 (d, J = 1.9 Hz,
)
35 /0,0- =i H), 8.61 (d, J = 1.9 Hz, 1H),
6.99 - 6.85 (m, 2H), 4.70 (dq, J
372.23 = 7.3, 3.5 Hz, 1H), 4.05 - 3.84
\--0
N (m, 8H), 3.40 - 3.25 (m, 4H),
Co.) 2.19 - 1.93 (m, 6H), 1.77 - 1.64
(m, 2H)
(CDC13) 6 8.94 - 8.76 (m, 2H),
8.29 (d, J = 4.8 Hz, 2H), 7.67
N-I (d, J = 1.7 Hz, 1H), 6.53 (t, J =
ecro0 N 4.8 Hz, 1H), 6.37 (tt, J = 3.1,
1.5 Hz, 1H), 5.30 (d, J = 7.9
36 HN N Hz, 1H), 4.43 (q, J = 2.8 Hz,
404.2 Hz, 1H), 4.87 (dt, J = 7.5, 3.6
.L. ' N /
2H), 4.02 (t, J = 5.5 Hz, 3H),
L,1
0 2.68 (dqd, .1= 6.0, 3.4, 3.0, 1.8
Hz, 2H), 2.35 -2.11 (m, 2H),
2.07 - 1.84 (m, 6H)
(CDC13) 6 8.71 (d, J = 1.9 Hz,
N.1IN 0 42..b
HNea lir 1H), 8.63 (d, J = 1.9 Hz, 1H),
8.18 (dd, J = 3.7, 0.8 Hz, 2H),
7.01 - 6.85 (m, 2H), 5.37 - 5.20
425.25
37
-,, N (m, 1H), 4.79 (d, J = 5.5 Hz,
N ' N Co) 1H), 4.02 - 3.85 (m, 4H), 3.43 _
y 3.29 (m, 4H), 2.31 -2.15 (m,
F 2H), 2.02 - 1.85 (m, 6H)
(CDC13) 6 8.61 (d, J = 2.0 Hz,
1H), 8.55 (d, J = 1.9 Hz, 1H),
N 8.20 (d, J = 4.8 Hz, 2H), 6.87
I
.#00 0 N (d, J = 2.5 Hz, 1H), 6.81 (d, J =
2.5 Hz, 1H), 6.46 (t, J = 4.8 Hz,
38 HN 407.25 1H), 4.95 (s, 1H), 4.45 (tt, J =
),.. N 10.7, 3.6 Hz, 1H), 3.95 - 3.77
N ' N (m, 5H), 3.32 - 3.19 (m, 4H),
-.L.) Co) 2.34 - 2.10 (m, 4H), 1.82 (dt, J
= 12.9, 10.0 Hz, 2H), 1.45 -
1.20 (m, 2H)
72
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.72 (d, J = 1.9 Hz,
N 1H), 8.62 (d, J = 1.9 Hz, 1H),
0 gib. Hz, 1H), 6.92 (d, J = 2.5 Hz,
N 8.37 (s, 1H), 6.98 (d, J = 2.5
39 HViCi. 1.-P 441.28 1H), 6.36 (d, J = 1.0 Hz, 1H),
N 4.91 -4.76 (m, 1H), 4.00 - 3.88
N N CI (m, 4H), 3.45 - 3.24 (m, 4H),
k Co) 2.34 -2.17 (m, 2H), 2.03 - 1.84
(m, 6H)
(CDC13) 6 8.71 (d, J = 1.9 Hz,
N-7)
IN 1H), 8.61 (d, J = 1.9 Hz, 1H),
HN 0
Cr 0 7.15 (d, J = 9.3 Hz, 1H), 7.00 -
6.87 (m, 2H), 6.64 (d, J = 9.3
40 N 441.3 Hz, 1H), 4.89 - 4.76 (m, 2H),
--1\1 r , 4.11 - 4.03 (m, 1H), 4.00 - 3.83
(m, 4H), 3.40 - 3.24 (m, 4H),
I 0
2.23 (dq, J = 12.9, 6.3, 5.6 Hz,
CI
2H), 2.02 - 1.79 (m, 6H)
1\i'l (CDC13) 6 8.63 (d, J = 1.9 Hz,
I 1H), 8.55 (d, J = 1.9 Hz, 1H),
0 N
7.58 (d, J = 23.6 Hz, 2H), 6.89
(d, J = 2.4 Hz, 1H), 6.84 (d, J =
41 HNiCj. I. 421.43
2.5 Hz, 1H), 4.73 (s, 2H), 3.93
N
N - 3.72 (m, 5H), 3.34 - 3.18 (m,
NCH 4H),
( 0 ) 4H), 2.29 (s, 3H), 2.15 (m, 2H),
1.84 (m, 6H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
NII 1H), 8.63 (d, J = 1.9 Hz, 1H),
0 dal N 8.52 (d, J = 4.8 Hz, 2H), 7.01 -
6.87 (m, 3H), 5.17 (ddt, J = 8.7,
42 O'Cr 408.56 6.7, 3.4 Hz, I H), 4.76 - 4.58
rL= N (m, 1H), 4.00 - 3.87 (m, 4H),
N ' N 3.40 - 3.27 (m, 4H), 2.43 - 2.22
1,0 Co) (m, 4H), 2.05 - 1.87 (m, 2H),
1.86- 1.71 (m, 2H)
73
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.70 (d, J = 1.9 Hz,
1H), 8.61 (d, J = 1.9 Hz, 1H),
7.36 (s, 1H), 7.12 (d, J = 3.6
1\11
Hz, 1H), 6.95 (d, J = 2.5 Hz,
0 ii&i N 1H), 6.90 (d, J = 2.5 Hz, 1H),
412.48 6.48 (d' J = 3.6 Hz, 1H), 5.18
43 HNi(:).. WI
(d, J = 8.0 Hz, 1H), 4.79 (td, J
-=)N N S = 5.4, 2.7 Hz, 1H), 3.97 - 3.85
' N
(m, 4H), 3.70 (q, J = 6.8 Hz,
1H), 3.39 - 3.25 (m, 4H), 2.29 -
2.12 (m, 2H), 2.07 - 1.77 (m,
6H)
(CDC13) 6 8.71 (d, J = 2.0 Hz,
1H), 8.64 (d, J = 2.0 Hz, 1H),
NI 8.54 (d, J = 2.9 Hz, 1H), 8.47
...Øõ.0 "ii..1 N
(d, J = 3.0 Hz, 1H), 6.99 (d, J =
2.4 Hz, 1H), 6.92 (d, J = 2.5
HN 432.6 Hz, 1H), 5.81 (d, J = 8.3 Hz,
44
)N., N
N '" N 1H), 4.84 (dt, J = 5.3, 2.8 Hz,
y (o) 1H), 4.13 -4.05 (m, 1H), 4.00 -
3.84 (m, 4H), 3.43 - 3.30 (m,
CN 4H), 2.32 -2.17 (m, 2H), 2.02 -
1.85 (m, 6H)
N --I (CDC13) 6 8.70 (d, J = 2.0 Hz,
N 0 dil
HN=00# WI- 1H), 8.64 (d, J = 1.9 Hz, 1H),
8.29 (s, 2H), 6.98 (d, J = 2.5
Hz, 1H), 6.92 (d, J = 2.5 Hz,
.. N 485.26
N N
1H), 5.29 (d, J = 8.3 Hz, 1H),
4.81 (s, 1H), 4.04 - 3.84 (m,
y co) 4H), 3.42 -3.31 (m, 4H), 2.22
Br (s, 2H), 1.92 (d, J = 4.9 Hz, 6H)
(CDC13) 6 8.63 (d, J = 2.0 Hz,
1H), 8.52 (d, J = 4.8 Hz, 2H),
-4
H N I1
8.37 (d, J = 2.0 Hz, 1H), 6.92
N N
(t, J = 4.8 Hz, 1H), 6.62 (d, J =
2.4 Hz, 1H), 6.38 (d, J = 2.4
46 O'Cr* . 407.57
.1. N Hz, 1H), 6.16 (s, 1H), 5.27 (s,
NV N 1H), 4.06 - 3.78 (m, 4H), 3.64
(s, 1H), 3.48 - 3.20 (m, 4H),
2.14 (s, 2H), 2.04 - 1.80 (m,
4H)
74
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
N)I (CDC13) 6 8.66 (dd, J = 20.5,
47=
0 il
0=JO 1.0 N 1.9 Hz, 2H), 6.93 (dd, J= 17.3,
2.5 Hz, 2H), 4.87 - 4.65 (m,
372.16 1H), 4.04 - 3.83 (m, 4H), 3.72
0 r,u N (s, 3H), 3.46 - 3.22 (m, 4H),
`,..., i3 Co) 2.72 -2.40 (m, 1H), 2.35 - 1.99
(m, 4H), 1.99 - 1.51 (m, 4H)
(DMSO-d6) 6 12.13 (s, 1H),
N) 8.72 (d, J = 1.7 Hz, 1H), 8.58
0 0 N (d, J = 1.8 Hz, 1H), 7.13 (d, J =
2.1 Hz, 1H),6.83 (d, J = 2.0
0.Cf
48 358.64 Hz, 1H), 4.94 - 4.84 (m, 1H),
OH N 3.91 -3.68 (m, 4H), 3.51 -3.19
Co) (m, 4H), 2.47 - 2.33 (m, 1H),
2.04 - 1.82 (m, 4H), 1.82 - 1.60
(m, 4H)
(400 MHz, CDC13) 6 8.70 (d, J
N'''.1I = 1.9 Hz, 1H), 8.60 (d, J = 1.9
0 49 HNC'? ial N Hz, 1H), 7.99 (s, 1H), 6.96 (d, J
= 2.4 Hz, 1H), 6.89 (d, J = 2.3
WI 425.39 Hz, 1H), 6.20 (s, 1H), 5.19 (bs,
)- N 1H), 4.81 (bs, 1H), 3.96 - 3.84
Cci)
NV N (m, 4H), 3.40 - 3.27 (m, 4H),
F 2.29 -2.14 (m, 2H), 1.99 - 1.81
(m, 6H)
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.60 (d, J = 1.9
N-;..'.1
425.39
Hz, 1H), 8.30 (d, J = 2.1 Hz,
0
Cr lei 1H), 6.95 (d, J = 2.4 Hz, 1H),
50 HN
6.89 (d, J = 2.4 Hz, 1H), 5.84
(s, 1H), 5.42 (s, 1H), 4.81 (s,
N
es N 1H), 3.99
* o)
=. - 3.82 (m, 4H), 3.39 - 3.24 (m,
N F 4H), 2.31 -2.19 (m, 2H), 2.08 -
1.72 (m, 8H)
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.69 (d, J
N'.1I = 1.9 Hz, 1H), 8.60 (d, J = 1.9
0 rir, N Hz, 1H), 8.30 (d, J = 2.1 Hz,
1H), 6.95 (d, J = 2.4 Hz, 1H),
51 HNICI. I 425.33 6.89 (d, J = 2.4 Hz, 1H), 5.84
N (s, 1H), 5.42 (s, 1H), 4.81 (s,
--.N 1H), 3.99 - 3.82 (m, 4H), 3.39 -
F..,,, Co) 3.24 (m, 4H), 2.31 -2.19 (m,
2H), 2.08 - 1.72 (m, 8H)
(400 MHz, CDC13) 6 8.68 (d, J
NI = 1.4 Hz, 1H), 8.60 (d, J = 1.5
0 Ai N Hz, 1H), 6.93 (s, 1H), 6.90 (s,
1H), 6.28 (s, 1H), 5.06 (d, J =
52 HNII*C541 111 435.19 7.9 Hz, 1H), 4.77 (s,
1H), 4.06
NLN N (bs, 1H), 3.97 - 3.84 (m, 4H),
--
I II co) 3.38 - 3.25 (m, 4H), 2.27 (s,
......
H3C CH3 6H), 2.18 -2.09 (m, 2H), 1.94 -
1.83 (m, 7H)
(CDC13) 6.9 Hz, 1H), 8.28 (d, J
= 4.8 Hz, 2H), 6.85 (t, J = 1.9
N7-7)
I Hz, 2H), 6.51 (t, J = 4.8 Hz,
0
e[0. 0 N
433.25 1H), 5.29 (d, J = 6.3 Hz, 1H),
53 HN
4.80 (dq, J = 5.5, 2.8 Hz, 1H),
.1. 1\1.1 4.57 (d, J = 3.9 Hz, 2H), 4.02
NV N (t, J = 6.2 Hz, 1H), 3.54 - 3.43
(m, 2H), 3.19 (dd, J = 11.6, 2.6
0)
Hz, 2H), 2.27 - 2.14 (m, 2H),
2.08 - 1.85 (m, 10H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
1\1-1I 1H), 8.62 (d, J = 1.9 Hz, 1H),
0 AI N 7.45 (d, J = 7.8 Hz, 2H), 7.02 -
6.81 (m, 2H), 4.78 (ddd, J =
54 HNICI# WI 437.27 7.3, 5.6, 3.1 Hz, 1H), 4.66 (d, J
N
r.,N.. = 7.9 Hz, 1H), 4.01 - 3.78 (m,
N.k)L0,CH3 L
Ir.) 7H), 3.41 - 3.25 (m, 4H), 2.30 -
o) 2.08 (m, 2H), 1.94 (h, J = 8.8,
8.2 Hz, 6H)
76
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.73 (d, J = 2.0 Hz,
N".1
1 1H), 8.63 (d, J = 2.0 Hz, 1H),
HN 0
Cr 0 N 7.42 (d, J = 9.3 Hz, 1H), 6.96
(dd, J = 19.6, 2.5 Hz, 2H), 6.65
432.3 (d, J = 9.3 Hz, 1H), 5.40 (s,
55
N 1H), 4.86 (s, 1H), 3.94 (dd, J =
II 5.9, 3.8 Hz, 4H), 3.37 (dd, J =
6.0, 3.7 Hz, 4H), 2.27 (d, J =
12.7 Hz, 2H), 2.05 - 1.80
CN (m,6H)
(CDC13) 6 8.69 (d, J = 1.9 Hz,
1H), 8.61 (d, J = 1.9 Hz, 1H),
8.28 (d, J = 4.8 Hz, 2H), 6.98 -
N N
6.86 (m, 2H), 6.53 (t, J = 4.8
0 ii&h
Hz, 1H), 5.39 (s, 1H), 4.89 -
4.75 (m, 1H), 4.18 - 3.93 (m,
56 HN1j0# WI 421.47
N 2H), 3.93 - 3.72 (m, 2H), 3.70 -
N ' N 3.52 (m, 2H), 3.00 (td, J = 12.0,
1,)J )-= 3.5 Hz, 1H), 2.66 (dd, J = 12.1,
0 CH3 10.3 Hz, 1H), 2.21 (d, J = 8.9
Hz, 2H), 1.93 (d, J = 6.7 Hz,
6H), 1.30 (d, J = 6.2 Hz, 3H)
(CDC13) 6 8.69 (d, J = 1.9 Hz,
1H), 8.62 (d, J = 1.9 Hz, 1H),
NI 8.28 (d, J = 4.8 Hz, 2H), 7.02 -
0 ri&i N 6.86 (m, 2H), 6.53 (t, J = 4.8
Hz, 1H), 5.40 (s, 1H), 4.82 (d, J
57 HI\f"Cr 11" 437.49 = 5.8 Hz, 1H), 4.20 - 3.95 (m,
N
.).N N 2H), 3.94 - 3.53 (m, 6H), 3.03
' (td, J = 12.0, 3.5 Hz, 1H), 2.86
,I,...) o.)L,OH (dd, J = 12.1, 10.4 Hz, 1H),
2.20 (d, J = 9.2 Hz, 2H), 2.11 -
1.80(m, 7H)
N '-) (400 MHz, methanol-d4) 6 8.69
(d, J = 2.0 Hz, 1H), 8.56 (d, J =
0 ail
HNIC WI 2.0 Hz, 1H), 7.12 (d, J = 2.3
Hz, 1H), 6.89 (d, J = 2.4 Hz,
..L. N 467.16 1H), 5.36 (s, 1H), 3.97 (s,
1H),
58
N '. N C.o.) 3.91 -3.86 (m, 4H), 3.84 (s,
6H), 3.43 - 3.35 (m, 4H), 2.23 _
Y Y 2.10 (m, 2H), 2.00 - 1.81 (m,
CH3 CH3 6H)
77
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.70 (d, J
= 1.9 Hz, 1H), 8.61 (d, J = 1.9
N
I Hz, 1H), 8.36 (d, J = 1.8 Hz,
0 HNICiiiii
. WI N
1H), 7.54 (dd, J = 8.8, 2.2 Hz,
1H), 6.96 (d, J = 2.4 Hz, 1H),
I
59 431.19 6.90 (d, J = 2.4 Hz, 1H), 6.37
N
--LN (d, J = 8.7 Hz, 1H), 5.11 (s,
y Co) 1H), 4.80 (s, 1H), 3.94 (s, 1H),
3.94 - 3.79 (m, 4H), 3.38 -3.25
CN (m, 4H), 2.32 - 2.12 (m, 2H),
2.02 - 1.78 (m, 6H)
N ---.1 (CDC13) 6 8.71 (d, J = 1.9 Hz,
I 1H), 8.62 (d, J = 1.9 Hz, 1H),
HN00.0 waiih., N
7.06 - 6.88 (m, 3H), 6.80 (dd, J
= 9.4, 6.3 Hz, 1H), 4.97 - 4.71
60 425.23 (m, 2H), 4.14 (q, J = 7.1 Hz,
N
N 1H), 4.01 - 3.85 (m, 4H), 3.44 -
ii
=),.,N Co) 3.24 (m, 4H), 2.23 (d, J =
10.7
Hz, 2H), 1.96 (dt, J = 11.0, 7.6
F Hz, 6H)
(400 MHz, CDC13) 6 8.69 (d, J
1\nI = 1.8 Hz, 1H), 8.61 (d, J = 1.9
Hz, 1H), 6.94 (d, J = 2.3 Hz,
0 AI N
1H), 6.87 (s, 1H), 5.78 - 5.64
(m, 1H), 4.73 (s, 1H), 4.01 (s,
61 HNI.C# WI 397.15
1H), 3.97 - 3.78 (m, 4H), 3.43 -
N 3.18 (m, 4H), 2.26 -2.05 (m,
vA0 Co) 2H), 1.98 - 1.73 (m, 6H), 1.36 -
1.26 (m, 1H), 1.01 - 0.92 (m,
2H), 0.78 - 0.67 (m, 2H)
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.62 (d, J = 1.9
V-7 I -1 Hz, 1H), 6.95 (d, J = 2.3 Hz,
0
0 N 1H), 6.87 (d, J = 2.2 Hz, 1H),
62 HN
6.79 (d, J = 8.1 Hz, 1H), 4.73
427.23 (s, 1H), 4.34 (dd, J = 8.3, 5.9
N Hz, 1H), 4.04 - 3.84 (m, 7H),
a0 (o) 3.40 - 3.28 (m, 4H), 2.35 - 2.24
(m, 1H), 2.23 -2.11 (m, 2H),
2.09 - 1.98 (m, 1H), 1.97 - 1.76
(m, 8H)
78
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC11) 6 8.53 (s, 1H), 8.29 (d,
J = 4.8 Hz, 2H), 6.92 (d, J = 2.5
..--.1 Hz, 1H), 6.85 (d, J = 2.5 Hz,
1\11I CH3 1H), 6.52 (t, J = 4.8 Hz, 1H),
-
eio.õ.0 "at, N 5.25 (d, J = 8.3 Hz, 1H), 4.79
63 HN
(s, 1H), 4.07 (d, J = 20.4 Hz,
463.54
1H), 3.98 - 3.85 (m, 4H), 3.43 -
,-1. N 3.21 (m, 4H), 3.05 -2.83 (m,
N N 2H), 2.30 -2.14 (m, 1H), 2.03 -
,) Co) 1.71 (m, 7H), 1.45 (dq, J =
14.5, 7.3 Hz, 2H), 0.98 (t, J =
7.3 Hz, 3H)
(CDC13) 6 8.55 (s, 1H), 8.29 (d,
CH3 J = 1.5 Hz, 2H), 7.05 - 6.93 (m,
1H), 6.85 (d, .1= 2.5 Hz, 1H),
64 0 AI N
HN=Cr Mr 541.26 5.49 (d, J = 8.0 Hz, 1H), 4.80
(d, J = 5.8 Hz, 1H), 4.03 - 3.81
(m, 5H), 3.45 - 3.27 (m, 4H),
.-L N N N 2.98 (dd, J = 8.5, 7.0 Hz, 2H),
y co) 2.32 - 2.09 (m, 2H), 2.00 - 1.71
(m, 8H), 1.56 - 1.34 (m, 2H),
Br 0.98 (td, J = 7.3, 3.3 Hz, 3H)
N (CDC13) 6 8.76 - 8.66 (m, 3H),
ifor0 "Ail N 8.61 (d, J = 1.9 Hz, 1H), 6.99 -
6.87 (m, 2H), 5.71 (d, J= 8.1
HN Hz, 1H), 4.81 (s, 1H), 4.11 (s,
65 .-[.. NN
N 450.49
1H), 3.92 (t, J = 4.9 Hz, 4H),
--
Co) 3.34 (t, J = 4.9 Hz, 4H), 2.22
(d, J = 10.2 Hz, 2H), 2.02 -
ON H2 1.85 (m, 6H)
(CDC13) 6 8.77 (d, J = 2.3 Hz,
1H), 8.72 (d, J = 2.3 Hz, 1H),
N*-N1
1 7.12 (d, J = 2.4 Hz, 1H),7.02
N tir #0 i, (d, J = 2.4 Hz, 1H), 4.92 (s,
1H), 4.18 -4.08 (m, 2H), 3.93
HN.0, (t, J = 4.9 Hz, 4H), 3.85 - 3.76
495.23
(m, 2H), 3.60 (q, J = 7.0 Hz,
66
NV N CH3 (N'1
2H), 3.47 (t, J = 4.9 Hz, 4H),
Lk jj ]
0' 0 2.26 (d, J = 13.3 Hz, 2H), 2.07
(5,) (t, J = 10.5 Hz, 2H), 2.01 - 1.72
(m, 2H), 1.26 (t, J = 7.0 Hz,
4H)
79
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
N')I (CDC13) 6 8.71 (d, J = 2.0 Hz,
0 rial N 1H), 8.64 (d, J = 1.9 Hz, 1H),
8.02 (s, 1H), 6.94 (dd, J = 12.2,
HNICf lir 2.5 Hz, 2H), 5.32 (s, 2H), 4.87
67 .JN, N 450.3 (d, J = 8.1 Hz, 1H), 4.79 (s,
N ' N (o) 1H), 4.01 - 3.86 (m, 4H), 3.36
y(q, J = 5.4, 4.7 Hz, 4H), 2.83 (s,
6H), 2.19 (s, 2H), 1.92 (d, J =
H3C-N.-CH3 4.8 Hz, 6H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
N" 1H),
1H), 8.63 (d, J = 1.9 Hz, 1H),
0 Ali
HNea.. IIV 8.34 (s, 2H), 7.02 - 6.86 (m,
2H), 6.00 (tt, J = 3.0, 1.5 Hz,
1H), 5.41 - 5.21 (m, 1H), 4.81
68
N ' N N
Co) 489.24 (dt, J = 7.2, 3.6 Hz, 1H), 4.31
(q, J = 2.8 Hz, 2H), 4.03 (dd, J
= 7.8, 4.4 Hz, 1H), 4.00 - 3.82
L..,)
(m, 6H), 3.42 - 3.25 (m, 4H),
rk). 2.44 (tdd, J = 5.7, 2.9, 1.7 Hz,
L'IC) 2H), 2.22 (dq, J = 11.2, 6.6, 6.0
Hz, 2H), 2.02 - 1.83 (m, 6H)
NnI (CDC13) 6 9.05 (s, 1H), 8.88 -
0 ail N
HNiC) WI . 8.71 (m, 3H), 8.49 (s, 1H), 7.06
(d, J = 2.3 Hz, 1H), 6.96 (d, J =
2.3 Hz, 1H), 4.85 (s, 1H), 4.17
NN
69 ..L. N 451.21
(s, 1H), 3.93 (t, J = 4.8 Hz, 4H),
'
Co) 3.42 (t, J = 4.9 Hz, 4H), 2.25 -
2.10 (m, 2H), 1.95 (d, J = 11.9
HOk-0 Hz, 4H)
-
N'''iIN (methanol-d4) 6 8.69 (s, 1H),
0 All
HN.010" V) 8.56 (s, 1H), 7.20 (s, 1H), 7.10
(d, J = 2.2 Hz, 1H), 6.88 (d, J =
70 N''N 2.4 Hz, 1H), 4.93 (d, J = 14.3
j N
Co) 409.45 Hz, 2H), 3.95 - 3.76 (m, 7H),
y 3.42 - 3.32 (m, 4H), 3.09 (d, J =
7.3 Hz, 1H), 2.21 - 2.09 (m,
HN 2H), 1.85 (dd, J = 10.8, 5.6 Hz,
rN-CH3
6H)
-NI
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
NN')I (CDC13) 6 8.79 - 8.64 (m, 3H),
0 N 8.59 (d, J = 1.9 Hz, 1H), 6.99 -
6.88 (m, 2H), 6.19 (q, .1= 4.7
HN Cr *I Hz, 1H), 5.90 (d, J = 8.2 Hz,
71
N. N N 464.4 1H), 4.81 (dq, J = 5.3, 2.7 Hz,
.$) Co) 1H), 4.08 (qd, J = 8.2, 6.5, 2.3
Hz, 1H), 3.97 - 3.87 (m, 4H),
3.39 - 3.29 (m, 4H), 2.93 (d, J =
H(k-'0 4.8 Hz, 3H), 2.27 - 2.14 (m,
CH3 2H), 2.06 - 1.79 (m, 6H)
N (CDC13) 6 3.97 - 3.87 (m, 4H),
N I
I
i0,0 *I 3.39 - 3.29 (m, 4H), 3.10 (s,
6H), 2.22 (dt, J = 11.3, 5.1 Hz,
HN 2H), 1.94 (dd, J = 8.3, 3.9 Hz,
72 ..1.,
N '" N N 478.39 6H), 4.12 -4.01 (m, 1H), 8.70
(d, J = 1.9 Hz, 1H), 8.62 (d, J =
1.9 Hz, 1H), 8.46 (s, 2H), 7.00
- 6.87 (m, 2H), 5.57 (d, J = 8.1
H3C ..,,
'N 0 Hz, 1H), 4.81 (dq, J = 5.1, 2.4
1
CH3 Hz, 1H)
(400 MHz, CDC13) 6 8.69 (d, J
= 1.2 Hz, 1H), 8.57 (d, J = 21.7
1\1-1I Hz, 1H), 6.93 (d, J = 1.7 Hz,
0 46 N
1H), 6.86 (s, 1H), 5.75 (d, J =
7.4 Hz, 1H), 4.74 (s, 1H), 4.03
73 HN 11:1? IWP 427.2
- 3.87 (m, 8H), 3.81 (dd, J =
0.--co /NI 15.2, 7.5 Hz, 1H), 3.37 - 3.25
(m, 4H), 2.96 - 2.76 (m, 1H),
e 2.23 - 2.07 (m, 4H), 1.84 - 1.78
(m, 6H)
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.60 (d, J = 1.9
N-51I Hz, 1H), 6.94 (d, J = 2.4 Hz,
0 74 H N 'ID' II& N 1H), 6.86 (d, .1= 2.4 Hz, 1H),
5.42 (d, J = 7.9 Hz, 1H), 4.72
I.W. 411.25 (s, 1H), 3.98 (s, 1H), 3.95-
N
3.86 (m, 4H), 3.40 - 3.26 (m,
r 4H), 3.05 - 2.87 (m, 1H), 2.32 -
0 2.21 (m, 2H), 2.21 - 2.07 (m,
4H), 2.03 - 1.90 (m, 1H), 1.90 -
1.77 (m, 7H)
81
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
I (CDC13) 6 8.72 - 8.51 (m, 2H),
0
HNICT. 11" 7.01 (d, J = 2.5 Hz, 1H), 6.93
(d, J = 2.5 Hz, 1H), 4.84 (s,
1H), 4.40 (s, 2H), 3.99 (s, 1H),
N N
Co) 562.34 3.83 (dd, J = 5.9, 3.8 Hz, 4H),
3.65 (s, 2H), 3.35 (t, J = 4.9 Hz,
4H), 2.87 (s, 2H), 2.27 - 1.91
0 CH3 (m, 4H), 1.90 - 1.59 (m, 2H),
I<(-..1..1 0 H3C -3 1.41 (s, 9H)
(CDC13) 6 8.70 (t, J = 2.2 Hz,
1H), 8.63 (dd, J = 2.7, 1.9 Hz,
N-'1I 1H), 7.97 (s, 1H), 7.00 - 6.86
(m, 2H), 5.05 (d, J = 8.2 Hz,
0 N
HN lir 1H), 4.77 (s, 1H), 4.02 (s, 1H),
3.93 (t, J = 4.7 Hz, 4H), 3.86 (s,
76 462.23 1H), 3.35 (t, J = 4.8 Hz, 4H),
N N 3.17 (t, J = 6.0 Hz, 1H), 2.89
(o) (d, J = 5.5 Hz, 1H),2.71 (t, J =
6.0 Hz, 1H),2.21 (dd, J = 9.2,
4.9 Hz, 2H), 1.91 (d, J = 5.5
Hz, 3H), 1.75 (d, J = 5.6 Hz,
4H)
(400 MHz, CDC13) 6 8.68 (d, J
= 1.9 Hz, 1H), 8.63 - 8.54 (m,
I 1H), 6.93 (d, J = 2.4 Hz, 1H),
0 A.1
6.86 (d, J = 2.3 Hz, 1H), 5.76 -
77 H 11"
5.55 (m, 1H), 4.93 - 4.67 (m,
413.19
4H), 3.99 (d, J = 26.0 Hz, 1H),
o 1\1 3.95 - 3.81 (m, 4H), 3.77 - 3.55
õ
(m, 2H), 3.39 - 3.28 (m, 4H),
2.21 -2.09 (m, 2H), 1.90 - 1.75
(m, 6H)
I (400 MHz, CDC13) 6 8.69 (s,
0 AI 1H), 8.60 (s, 1H), 6.94 (s, 1H),
78 HNC'.
6.86 (s, 1H), 5.59 (s, 1H), 4.74
371.18 (s, 1H), 4.11 -3.81 (m, 5H),
3.42 - 3.23 (m, 4H), 2.28 - 2.09
0' CH3 (0) (m, 2H), 1.98 (s, 3H), 1.90 -
1.78 (m, 6H)
82
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.60 (d, J = 1.9
N')I Hz, 1H), 6.93 (d, J = 2.4 Hz,
0 liki 6.86 (d, J = 2.3 Hz, 1H), 5.52
N 1H),
79 HNICI. l'W-'' 385.18 (d, J = 7.3 Hz, 1H), 4.73
(s,
N 1H), 3.99 (s, 1H), 3.96 - 3.87
C 0--, ,
(m, 4H), 3.38 -
CH3 3.30 (m, 4H), 2.24 - 2.08 (m,
''I;)-
4H), 1.88- 1.79 (m, 6H), 1.16
(t, J = 7.6 Hz, 3H)
(400 MHz, CDC13) 6 8.69 (d, J
1\11I = 1.9 Hz, 1H), 8.60 (d, J = 1.9
Hz, 1H), 6.93 (d, J = 2.4 Hz,
0
sofOr la N
399.23 1H), 6.86 (d, J = 2.3 Hz, 1H),
80 HN
5.53 (d, J = 7.7 Hz, 1H), 4.73
--
r ,.. (s, 1H), 4.00 (s, 1H), 3.95 -
0 N
3.84 (m, 4H), 3.39 - 3.25 (m,
CH3
4H), 2.22 - 2.06 (m, 4H), 1.88 -
10'-
1.76 (m, 6H), 1.70 - 1.61 (m,
2H), 0.94 (t, J = 7.4 Hz, 3H)
(400 MHz, CDC13) 6 8.69 (d, J
N-7.I 1N = 1.9 Hz, 1H), 8.62 (d, J = 1.9
0
ICI Si Hz, 1H), 6.94 (d, J = 2.4 Hz,
81 HN
1H), 6.87 (d, J = 2.2 Hz, 1H),
401.21 6.65 (d, J = 8.5 Hz, 1H), 4.72
N (s, 1H), 4.06 (s, 1H), 3.96 -0
0 0 3.85 (m, 6H), 3.43 (s, 3H), 3.38
'CH3 0 - 3.28 (m, 4H), 2.24 - 2.09 (m,
2H), 1.95 - 1.76 (m, 6H)
(400 MHz, CDCI3) 6 8.68 (d, J
= 1.8 Hz, IH), 8.59 (d, J = 1.8
N-'51I Hz, 1H), 6.93 (d, J = 2.3 Hz,
0 di., N 1H), 6.86 (d, J = 2.0 Hz, 1H),
5.53 (d, J = 7.8 Hz, 1H), 4.72
82 HN)Cr. ill) 413.27 (s, 1H), 4.01 (s, 1H), 3.96
-
-J\
C31'. N
( ) 3.81 (m, 4H), 3.37 - 3.24 (m,
4H), 2.24 - 2.06 (m, 3H), 2.01
H3CCH3 0 (d, J = 7.0 Hz, 2H), 1.87 - 1.76
(m, 6H), 0.94 (d, J = 6.5 Hz,
6H)
83
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.62 (d, J
N--5.I = 1.9 Hz, 1H), 8.55 (d, J = 1.9
0 iii.h, N Hz, 1H), 6.87 (d, J = 2.4 Hz,
1H), 6.80 (d, J = 2.3 Hz, 1H),
83 HNiCr. ilij 413.23 5.62 (d, J = 7.7 Hz, 1H), 4.64
,k,r.....CH3 (s, 1H), 3.99 - 3.76 (m, 5H),
() CH3 rN 3.37 - 3.14 (m, 4H), 2.21 - 1.95
CH3 (31* (m, 2H), 1.87 - 1.63 (m, 6H),
1.13 (s, 9H)
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.61 (d, J = 1.9
N-'1I Hz, 1H), 8.19 (d, J = 4.8 Hz,
0 84 HNC" ma,h, N 1H), 6.95 (d, J = 2.3 Hz, 1H),
6.90 (d, J = 2.4 Hz, 1H), 6.70
lir 431.19 (dd, J = 5.1, 1.2 Hz, 1H),6.56
1\1 N (s, 1H), 4.87 (d, J = 7.6 Hz,
j-
, ( j 1H), 4.80 (s, 1H), 3.96 - 3.88
CN 0 (m, 4H), 3.85 (s, 1H), 3.38 -
3.28 (m, 4H), 2.28 -2.14 (m,
2H), 2.00 - 1.85 (m, 6H)
N-7)1 (CDC13) 6 8.67 (d, J = 2.0 Hz,
0 rial N
HNiadi IW 1H), 8.62 (d, J = 2.0 Hz, 1H),
7.05 - 6.95 (m, 2H), 6.72 (s,
1H), 4.88 (s, 1H), 3.98 - 3.82
85 ..1. r N 491.3 (m, 4H), 3.64 (t, J = 5.1 Hz,
N N 2H), 3.41 -3.31 (m, 4H), 3.24
y..NcH3 L ) (s, 3H), 3.09 (t, J = 5.2 Hz, 2H),
0
2.68 (s, 3H), 2.30 - 1.99 (m,
H3C-N-') 4H), 1.86 (d, J = 9.0 Hz, 4H)
(CDC13) 6 9.68 (s, 1H), 8.76 -1\1-I 8.58 (m, 2H), 7.00 (d, J = 2.5
ecr0 rail N Hz, 1H), 6.92 (d, J = 2.5 Hz,
1H), 6.41 (dd, J= 17.7, 11.1
HN 86 N N N 433.2 Hz, 1H), 5.65 (d, J = 17.7 Hz,
,i.
1H), 5.33 (d, .1= 11.1 Hz, IH),
'
(o) 4.85 (q, J = 3.6 Hz, 1H), 4.01
(s, 1H), 3.94 - 3.70 (m, 4H),
CF12 3.47 - 3.19 (m, 4H), 2.27 - 1.94
'
(m, 4H), 1.93 - 1.63 (m, 4H)
84
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.63 (d, J = 1.9 Hz,
1H), 8.57 (d, J = 1.9 Hz, 1H),
8.20 (dd, J = 5.0, 1.9 Hz, 1H),
N 7.57 (dd, J = 7.6, 1.9 Hz, 1H),
0
6.89 (d, J = 2.5 Hz, 1H), 6.85
87 HNC431.2 (d' J = 2.5 Hz, 1H), 6.51 (dd, J
1. = 7.6, 4.9 Hz, 1H), 5.12 (d, J =
N 7.7 Hz, 1H), 4.83 - 4.71 (m,
(o) OH), 4.24 - 4.03 (m, 1H), 3.92 -
3.77 (m, 4H), 3.35 - 3.19 (m,
4H), 2.28 -2.10 (m, 2H), 1.88
(td, J = 8.3, 6.8, 3.9 Hz, 6H)
(400 MHz, CDC13) 6 8.69 (s,
N 1H), 8.63 (s, 1H), 8.57 (s, 1H),
0N 8.30 - 8.12 (m, 2H), 7.85 (t, J =
7.1 Hz, 1H), 7.50- 7.37 (m,
88 HeCr 434.25 1H), 6.94 (s, 1H), 6.89 (s, 1H),
N 4.89 -4.63 (m, 1H), 4.35 -4.13
(I (m, 1H), 4.01 - 3.78 (m, 4H),
3.43 -3.19 (m, 4H), 2.37 -2.15
(m, 2H), 2.12 - 1.82 (m, 6H)
(400 MHz, CDC13) 6 8.99 (s,
1H), 8.73 (d, J = 3.4 Hz, 1H),
8.70 (d, J = 1.6 Hz, 1H), 8.60
(d, J = 1.7 Hz, 1H), 8.11 (d, J =
0
eCr
434.22 7.8 Hz, 1H), 7.40 (dd, J = 7.6,
89 HN
4.8 Hz, 1H), 6.95 (d, J = 2.1
Hz, 1H), 6.90 (s, 1H), 6.30 (d, J
0.1 (NI
= 7.6 Hz, 1H), 4.81 (s, 1H),
4.22 (s, 1H), 3.97 - 3.85 (m,
e 4H), 3.41 - 3.23 (m, 4H), 2.32 -
2.17 (m, 2H), 2.01 - 1.84 (m,
6H)
(400 MHz, CDC13) 6 8.75 (dd,
J = 4.4, 1.7 Hz, 2H), 8.70 (d, J
I = 1.9 Hz, 1H), 8.60 (d, J = 1.9
0 Hz, 1H), 7.62 (dd, J = 4.4, 1.7
=
90 HNe .
Hz, 2H), 6.95 (d, J = 2.4 Hz,
434.22 1H), 6.89 (d, J = 2.5 Hz, 1H),
N 6.33 (d, J = 8.2 Hz, 1H), 4.81
() (s, 1H), 4.27 - 4.16 (m, 1H),
3.96 - 3.87 (m, 4H), 3.38 -3.31
(m, 4H), 2.30 - 2.16 (m, 2H),
2.00- 1.86 (m, 6H)
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.63 - 8.58 (m,
1\r-7)I 1H), 7.77 (dd, J = 5.2, 3.2 Hz,
0 91 HNC" N 2H), 7.54 - 7.40 (m, 3H), 6.95
(d, J = 2.4 Hz, 1H), 6.89 (d, J =
. 433.21 2.4 Hz, 1H), 6.23 (d, J = 8.0
N Hz, 1H), 4.78 (s, 1H), 4.31 -
0 0 r -, 4.13 (m, 1H), 3.95 -3.87 (m,
L"C? 4H), 3.38 -3.27 (m, 4H), 2.31 -
2.17 (m, 2H), 1.97 - 1.85 (m,
6H)
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.62 (d, J = 1.9
1\nI Hz, 1H), 7.47 (d, J = 8.9 Hz,
ect 0 N 1H), 7.00 (d, J = 1.0 Hz, 1H),
6.95 (d, J = 0.7 Hz, 1H), 6.94
92 HN 437.24 (d, J = 2.5 Hz, 1H), 6.89 (d, J =
N 2.4 Hz, 1H), 4.81 (s, 1H), 4.07
0..).:"-'
iN Co) (d, J = 12.3 Hz, 4H), 3.98 -
,N
H3C 3.85 (m, 4H), 3.41 - 3.24 (m,
4H), 2.34 -2.12 (m, 2H), 2.06 -
1.83 (m, 6H)
(methanol-d4) 6 8.69 (d, J = 2.2
Hz, 1H), 8.59 (d, J = 2.2 Hz,
icr0 =N 1H), 8.53 (d, J = 4.9 Hz, 1H),
7.19 (d, J = 5.0 Hz, 1H),7.15
HN (d, J = 2.3 Hz, 1H), 6.87 (d, J =
451.21
93
.1. ,,N,,, 2.4 Hz, 1H), 4.95 (d, J = 7.7
N -- N Hz, 1H), 4.10 (s, 1H), 3.95 -1,..).1y0 Lcyj
3.82 (m, 4H), 3.47 - 3.37 (m,
OH 4H), 2.20 (d, J = 10.1 Hz, 2H),
2.04- 1.81 (m, 6H)
(CDC13) 6 8.68 (d, J = 2.0 Hz,
RP N% 1H), 8.60 (d, J = 1.9 Hz, 1H),
0 dik
HNea"' 8.29 (s, 2H), 6.98 - 6.87 (m,
2H), 5.36 (d, J = 8.1 Hz, 1H),
4.79 (q, J = 5.2, 4.0 Hz, 1H),
94
). N N N 437.44
4.52 (s, 2H), 4.01 (dd, J = 8.1,
"
Co) 4.3 Hz, 1H), 3.95 - 3.84 (m,
4H), 3.39 - 3.28 (m, 4H), 2.25 -
.N.OH 2.12 (m, 2H), 1.99 - 1.82 (m,
6H)
86
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.70 (d, J
= 1.7 Hz, 1H), 8.65 - 8.58 (m,
NI 1H), 7.80 (s, 1H), 7.22 (d, J =
0 d&h N
9.6 Hz, 1H), 7.15 (d, J = 7.6
Hz, 1H), 6.95 (d, J = 2.4 Hz,
95 HN"*Crs igr 424.21
1H), 6.89 (d, J = 2.4 Hz, 1H),
o (NI 4.80 (s, 1H), 4.24 - 4.09 (m,
1H), 3.98 - 3.85 (m, 4H), 3.40 -
3.29 (m, 4H), 2.29 -2.15 (m,
2H), 2.02 - 1.86 (m, 6H)
(400 MHz, CDC13) 6 8.89 (d, J
= 4.9 Hz, 2H), 8.69 (d, J = 1.9
N Hz, 1H), 8.62 (d, J = 1.9 Hz,
0
eCr 1H), 8.20 (d, J = 8.3 Hz, 1H),
7.45 (t, J = 4.9 Hz, 1H), 6.94
96 HN
435.19 (d, J = 2.4 Hz, 1H), 6.89 (d, J =
N 2.4 Hz, 1H), 4.75 (s, 1H), 4.39
() -4.21 (m, 1H), 4.00 - 3.84 (m,
4H), 3.41 - 3.21 (m, 4H), 2.29 -
2.13 (m, 2H), 2.12 - 1.87 (m,
6H)
(400 MHz, CDC13) 6 9.25 (d, J
= 1.4 Hz, 1H), 8.97 (d, J = 5.0
N =I Hz, 1H), 8.70 (d, J = 1.9 Hz,
0
eiCr 1H), 8.63 (d, J = 1.9 Hz, 1H),
97 HN
8.22 - 8.04 (m, 2H), 6.95 (d, J =
435.19 2.4 Hz, 1H), 6.89 (d, J = 2.4
(N,) Hz, 1H), 4.81 - 4.72 (m, 1H),
4.26 -4.15 (m, 1H), 3.96 - 3.86
Lo) (m, 4H), 3.38 - 3.29 (m, 4H),
2.28 -2.17 (m, 2H), 2.06 - 1.86
(m, 6H)
(400 MHz, CDC13) 6 9.33 (s,
1H), 9.12 (s, 2H), 8.70 (d, J =
1.9 Hz, 1H), 8.57 (d, J = 1.9
0
Hz, 1H), 6.95 (d, J = 2.4 Hz,
98 HNC'' 1H), 6.89 (d, J = 2.4 Hz, 1H),
435.19
6.44 (d, J = 7.8 Hz, 1H), 4.83
ON rN1 (s, 1H), 4.23 (qd, J = 9.2, 4.7
Hz, 1H), 3.96 - 3.87 (m, 4H),
3.38 -3.30 (m, 4H), 2.32 -2.18
(m, 2H), 2.01 - 1.88 (m, 6H)
87
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 9.29 (dd,
J = 5.0, 1.7 Hz, 1H), 8.70 (d, J
= 1.9 Hz, 1H), 8.64 (d, J = 1.9
Hz, 1H), 8.33 (dd, J = 8.4, 1.7
0 N
Hz, 2H), 7.67 (dd, J = 8.4, 5.0
Hz, 1H), 6.95 (d, J = 2.5 Hz,
99 HNIO. 435.19
1H), 6.91 (d, J = 2.5 Hz, 1H),
ON'N (NI 4.85 (s, 1H), 4.29 -4.13
(m,
1H), 3.98 - 3.85 (m, 4H), 3.40 -
Oj 3.26 (m, 4H), 2.32 -2.16 (m,
2H), 2.12 - 1.98 (m, 2H), 1.99 -
1.87 (m, 4H)
(400 MHz, CDC13) 6 11.83 (s,
1H), 8.71 (d, J = 1.9 Hz, 1H),
= I 8.59 (d, J = 1.9 Hz, 1H),
7.55
0
100 HNC( (d, J = 2.4 Hz, 1H), 7.05 (d, J =
8.5 Hz, 1H), 6.94 (d, J = 2.4
423.13 Hz, 1H), 6.87 (dd, J = 4.7, 2.3
Hz, 1H), 6.84 (d, J = 2.4 Hz,
o'"1:1111.NH 1H), 4.74 (s, 1H), 4.18 (s, 1H),
O 3.96 - 3.88 (m, 4H), 3.38 -3.29
(m, 4H), 2.17 -2.09 (m, 2H),
1.94 - 1.81 (m, 6H)
(400 MHz, CDC13) 6 8.70 (d, J
N-NiI = 1.9 Hz, 1H), 8.63 (d, J = 1.9
ici3O N Hz, 1H), 8.07 (s, 1H), 7.01 (d, J
= 8.3 Hz, 1H), 6.94 (d, J = 2.4
101 HN 438.18 Hz, 1H), 6.88 (d, J = 2.4 Hz,
O 0 (m, 1H), 3.98 - 3.84 (m,
4H),
1H), 4.76 (s, 1H), 4.23 - 4.09
N
Nz(
r
0)
3.40 - 3.26 (m, 4H), 2.48 (s,
CH3 3H), 2.28 -2.12 (m, 2H), 1.99 -
1.82 (m, 6H)
(CDC13) 6 8.71 (d, J = 1.9 Hz,
1H), 8.59 (d, J = 2.0 Hz, 1H),
I\1-NiI 6.96 (d, J = 2.5 Hz, 1H), 6.89
= N (d, J = 2.5 Hz, 1H), 4.73
(d, J =
5.7 Hz, 1H), 4.18 (d, J = 7.7
102 HNI!O 482.36 Hz, 1H), 3.93 (dd, J = 5.9, 3.8
Hz, 4H), 3.69 - 3.55 (m, 1H),
NN
0 (0
3.46 (d, J = 16.5 Hz, 1H), 3.41
C F3
- 3.28 (m, 4H), 3.08 (d, J = 16.4
HO
Hz, 1H), 2.17 (d, J = 11.6 Hz,
2H), 2.00 - 1.75 (m, 6H)
88
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
N-51I (CDC13) 6 8.72 (d, J = 2.0 Hz,
0 gal N 1H), 8.64 (d, J = 2.0 Hz, 1H),
6.99 (d, J = 2.5 Hz, 1H), 6.93
103 HNICr 1*-P 463.36 (d, J = 2.5 Hz, 1H), 5.74 (s,
1H), 4.80 (s, 1H), 4.02 - 3.87
tNH (N J (m, 4H), 3.36 (t, J = 4.9 Hz,
0 4H), 2.19 (s, 2H), 2.08 - 1.79
CF3 (m, 6H)
N (CDC13) 6 8.73 (s, 1H), 8.68 (d,
.11N J = 2.4 Hz, 1H), 6.99 (d, J = 2.4
0 ral
Hz, 1H), 6.85 (d, J = 2.4 Hz,
1H), 5.69 (d, J = 2.5 Hz, 1H),
104 H NIC:) . 14" .. 443.38
N 4.76 (s, 1H), 3.84 (dd, J = 5.9,
N v N 3.9 Hz, 4H), 3.38 (dd, J = 6.0,
F --F Co) 3.9 Hz, 4H), 2.15 (d, J = 11.1
- Hz, 2H), 1.96 - 1.67 (m, 6H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
N".I 1H), 8.59 (d, J = 1.9 Hz, 1H),
So N 8.30 (d, J = 1.3 Hz, 1H), 7.89
(d, J = 1.4 Hz, 1H), 6.93 (dd, J
HN = 18.4, 2.5 Hz, 2H), 5.52 (d, J
105 432.4
N = 7.8 Hz, 1H), 4.83 (tt, J = 4.8,
(-)--N
NI) Co) 2.7 Hz, 1H), 4.19 - 4.01 (m,
1H), 3.96 - 3.87 (m, 4H), 3.39 -
CN 3.26 (m, 4H), 2.31 -2.14 (m,
2H), 2.09 - 1.78 (m, 6H)
(400 MHz, CDC13) 6 8.71 (d, J
= 1.9 Hz, 1H), 8.64 (d, J = 1.9
N'..)I Hz, 1H), 7.88 (dd, J = 7.4, 2.1
0
=Cf 0 N Hz, 1H), 7.02 (dd, J =
11.9, 8.3
Hz, 1H), 6.97 (d, J = 2.4 Hz,
106 HN 465.14 1H), 6.92 (d, J = 2.4 Hz, 1H),
N 6.83 (dd, J = 12.4, 7.6 Hz, 1H),
0 C ) 4.78 (s, 1H), 4.25 (s, 1H), 4.03
F CH3 0 - 3.85 (m, 4H), 3.44 - 3.23 (m,
4H), 2.38 (s, 3H), 2.30 - 2.13
(m, 2H), 2.06 - 1.88 (m, 6H)
89
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.73 -
8.66 (m, 1H), 8.65 - 8.59 (m,
NI 1H), 8.05 (dd, J = 6.6, 2.8 Hz,
0 N 1H), 7.46 - 7.36 (m, 1H), 7.08
(dd, J= 11.1, 8.8 Hz, 1H), 6.95
107 HN'fC).# . 485.12 (d, J = 2.4 Hz, 1H), 6.89 (d, J -
N 2.4 Hz, 1H), 6.84 - 6.66 (m,
0 ( ) 1H), 4.76 (bs, 1H), 4.22 (bs,
F CI 0
1H), 3.96 - 3.87 (m, 4H), 3.37 -
3.28 (m, 4H), 2.28 -2.10 (m,
2H), 2.01 - 1.86 (m, 6H)
(CDC13) 6 8.69 (d, J = 1.9 Hz,
1H), 8.61 (d, J = 2.0 Hz, 1H),
Ø0 0 N 8.49 (d, J = 1.4 Hz, 1H), 7.77
(d, J = 1.4 Hz, 1H), 6.93 (dd, J
HN - 16.1, 2.5 Hz, 2H), 5.03 (d, J
108 -r.'N N 478.26 = 7.9 Hz, 1H), 4.81 (td, J = 5.3,
Nj Co) 2.6 Hz, 1H), 4.09 - 3.98 (m,
1H), 3.98 - 3.87 (m, 4H), 3.39 -
...,,, 0 11...CH3 3.26 (m, 4H), 3.19 (s, 3H), 3.12
(s, 3H), 2.22 (dt, J = 11.2, 4.9
CH3
Hz, 2H), 2.02 - 1.82 (m, 6H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
1H), 8.63 (d, J = 1.9 Hz, 1H),
NI 8.10 (d, J = 0.5 Hz, 2H), 6.97
cr0 go, N (d, J = 2.5 Hz, 1H), 6.92 (d, J =
2.5 Hz, 1H), 5.13 (d, J = 8.3
HN Hz, 1H), 4.80 (s, 1H), 4.06 -
109 1\1 N N 447.02
3.86 (m, 5H), 3.35 (dd, J = 5.9,
--
,13.1 Co) 3.8 Hz, 4H), 2.27 - 2.14 (m,
2H), 1.92 (d, J = 5.1 Hz, 6H),
1.79 - 1.44 (m, 6H), 1.28 (t, J =
7.1 Hz, 1H), 1.03 - 0.83 (m,
2H), 0.68 - 0.51 (m, 2H)
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.64 - 8.54 (m,
N ---.1I 1H), 6.94 (d, J = 2.4 Hz, 1H),
0 N
6.86 (d, J = 2.5 Hz, 1H), 6.06 -
5.78 (m, 1H), 4.79 - 4.68 (m,
110 HNeCri. 395.19
1H), 4.11 -3.96 (m, 1H), 3.96-
(D
(,)
, N 3.86 (m, 4H), 3.40 - 3.27 (m,
..,
CH3 Lo) 4H), 2.13 (dd, J = 11.0, 5.2 Hz,
2H), 1.94 (s, 3H), 1.90 - 1.78
(m, 6H)
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as (3 values
N".1I
O aili N
111 HNICI. 1*-P 421.19
'(:)=2
(400 MHz, CDC13) 6 8.70 (d, J
Nni = 1.9 Hz, 1H), 8.66 - 8.53 (m,
O N 3H), 7.93 (dd, J = 6.4, 5.0
Hz,
1H), 6.95 (d, J = 2.4 Hz, 1H),
112 HN'fCr . 452.18 6.90 (d, J = 2.4 Hz, 1H), 6.78
N (s, 1H), 4.78 (bs, 1H), 4.23 (bs,
()
1 1H), 3.97 - 3.85 (m, 4H), 3.38 -
F N (0') 3.27 (m, 4H), 2.30 - 2.15 (m,
2H), 2.02 - 1.86 (m, 6H)
N1-51I (400 MHz, CDC13) (3 8.70 (s,
icy s N 1H), 8.60 (s, 1H), 6.94 (s, 1H),
6.87 (s, 1H), 5.99 (d, J = 6.8
113 HN 439.24 Hz, 1H), 4.75 (bs, 1H), 4.03
(bs, 1H), 3.99 - 3.83 (m, 4H),
C;IH (NJ 3.43 - 3.21 (m, 4H), 2.33 (s,
H3c CH3
1H), 2.24 - 2.04 (m, 2H), 1.94 -
0
1.74 (m, 6H), 1.56 (s, 6H)
(400 MHz, CDC13) 6 8.70 (d, J
= 1.9 Hz, 1H), 8.63 (d, J = 1.9
N 'INI Hz, 1H), 8.40 (dt, J = 4.3, 1.3
O ail Hz, 1H),8.01 (d, J = 8.5
Hz,
1H), 7.58 - 7.52 (m, 1H), 7.51 -
114 HNiCja ill*r 452.15 7.46 (m, 1H), 6.95 (d, J = 2.4
N Hz, 1H), 6.90 (d, J = 2.4 Hz,
Co) 1H), 4.74 (s, 1H), 4.29 - 4.17
F-7- (m, 1H), 3.97 - 3.88 (m, 4H),
3.39 -3.30 (m, 4H), 2.25 -2.16
(m, 2H), 2.06 - 1.88 (m, 6H)
91
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.69 (d, J
= 1.8 Hz, 1H), 8.62 (d, J = 1.8
N-..I Hz, 1H), 8.09 (dd, J = 7.9, 6.1
0 115 HNead. . N Hz, 1H), 7.47 (d, J = 8.1 Hz,
1H), 7.13 (dd, J= 11.8, 7.9 Hz,
451.16 1H), 6.95 (d, J = 2.3 Hz, 1H),
N 6.90 (d, J = 2.3 Hz, 1H), 6.82
00 r) (s, 1H), 4.77 (s, 1H), 4.24 (s,
F 1H), 3.96 - 3.88 (m, 4H), 3.38 -
3.30 (m, 4H), 2.27 -2.14 (m,
2H), 2.01 - 1.87 (m, 6H)
N'(400 MHz, CDC13) 6 8.70 (d, J
vici3O 0 N = 1.9 Hz, 1H), 8.63 (d, J = 1.9
Hz, 1H), 7.83 (d, J = 2.7 Hz,
HN
1H), 7.19 - 6.93 (m, 4H), 6.89
116 473.17 (d, J = 2.4 Hz, 1H), 4.75 (s,
0-,..,--- rN 1H), 4.21 (s, 1H), 3.95 - 3.89
N-N (m, 4H), 3.38 - 3.30 (m, 4H),
>-F 2.26 1--F 2.26 -2.13 (m, 2H),
2.03 - 1.86
F (m, 6H)
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.62 (d, J = 1.9
NI Hz, IH), 7.35 (d, J = 2.2 Hz,
/or 0 N 1H), 6.99 (d, J = 8.2 Hz, 1H),
6.93 (d, J = 2.4 Hz, 1H), 6.88
117 HN 437.17 (d, J = 2.4 Hz, 1H), 6.78 (d, J =
1:::\-1- (N) 2.3 Hz, 1H), 4.73 (s, 1H), 4.26
- 4.14 (m, 1H), 3.96 - 3.87 (m,
N-N 0 7H), 3.36 - 3.29 (m, 4H), 2.23 -
µCH3
2.12 (m, 2H), 2.01 - 1.82 (m,
6H)
(400 MHz, CDC13) 6 10.00 (s,
NI 1H), 8.63 (d, .1= 1.9 Hz, 1H),
#0.0 0 N 8.54 (d, J = 1.9 Hz, 1H), 6.93
(s, 1H), 6.87 (d, J = 2.4 Hz,
118 HN 437.21 1H),6.81 (d, J = 2.4 Hz, 1H),
6.50 (s, 1H), 4.69 (s, 1H), 4.15
CH3 ) - 4.05 (m, 1H), 3.92 - 3.77 (m,
N-N 4H), 3.35 -3.19 (m, 4H), 2.27
0
sI-1 (d, J = 0.5 Hz, 3H), 2.18 -2.04
(m, 2H), 1.95 - 1.74 (m, 6H)
92
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
N)I (400 MHz, CDC13) 6 8.70 (s,
119 Head6 1H), 8.63 (s, 1H), 6.99 - 6.50
(m, 3H), 4.76 (d, J = 20.7 Hz,
N 451.25 1H), 4.19 (s, 1H), 4.13 - 3.74
(m, 7H), 3.41 - 3.21 (m, 4H),
2.37 -2.08 (m, 5H), 2.03 - 1.79
NN (m, 6H)
µCH3
(400 MHz, CDC13) 6 8.73 (d, J
N = 1.9 Hz, 1H), 8.58 (d, J = 1.9
Hz, 1H), 6.97 (d, J = 2.4 Hz,
1H), 6.93 (s, 1H), 6.88 (d, J =
120 HNe N 491.18 2.3 Hz, 1H), 6.53 (s, 1H), 4.79
(s, 1H), 4.24 -4.15 (m, 1H),
( 3.96 - 3.86 (m, 4H), 3.40 -3.27
HN¨N (m, 4H), 2.22 - 2.15 (m, 2H),
0
1.94- 1.82 (m, 6H)
(400 MHz, CDC13) 6 8.70 (d, J
= 1.9 Hz, 1H), 8.62 (d, J = 1.9
NI Hz, 1H), 6.95 (d, J = 2.4 Hz,
1H), 6.89 (d, J = 2.4 Hz, 1H),
121 HN
0.00 = 0 N
6.71 (d, J = 2.1 Hz, 1H), 6.54
436.18 (dd' J = 3.9, 1.7 Hz, 1H), 6.09
(dd, J = 3.9, 2.6 Hz, 1H), 5.94
(D z r (d, J = 8.1 Hz, 1H), 4.76 (s,
1H), 4.11 (s, 1H), 3.98 - 3.86
H3C
(m, 7H), 3.39 - 3.27 (m, 4H),
2.27 -2.14 (m, 2H), 1.95 - 1.83
(m, 6H)
(400 MHz, CDC13) 6 8.70 (s,
I 1H), 8.61 (s, 1H), 8.51 (s, 1H),
N
7.50 (s, 1H), 6.95 (s, 1H), 6.89
440.18 (s' 1H)' 6.16 (d, 1H), 4.80 (s,
122 HN 1H), 4.18 (s, 1H), 4.01 - 3.78
(m, 4H), 3.44 - 3.23 (m, 4H),
2.33 - 2.16 (m, 2H), 1.91 (d, J =
o¨N 25.1 Hz, 6H)
93
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.69 (d, J
= 1.8 Hz, 1H), 8.62 (d, J = 1.9
Hz, 1H), 7.50 (d, J = 1.2 Hz,
0 1H), 7.36 (d, J = 1.0 Hz, 1H),
7.16 (d, J = 7.9 Hz, 1H), 6.94
123 437.21 (d, J = 2.3 Hz, 1H), 6.88 (d, J
2.3 Hz, 1H), 4.78 (s, 1H), 4.20
- 4.09 (m, 1H), 3.95 - 3.87 (m,
4H), 3.73 (s, 3H), 3.38 - 3.28
(m, 4H), 2.25 - 2.14 (m, 2H),
2.00 - 1.82 (m, 6H)
(CDC13) 6 8.68 (d, J = 1.9 Hz,
N-7) 1H), 8.58 (d, J = 1.9 Hz, 1H),
I
0 7.85 - 7.72 (m, 2H), 6.95 (d, J =
2.5 Hz, 1H), 6.86 (d, J = 2.5
124 HNI"Cr. = N 473.2 Hz, 1H), 4.89 (d, J = 7.5 Hz,
0=N 1H), 4.71 (dq, J = 5.5, 2.7 Hz,
8rN-CH3 1H), 4.00 - 3.85 (m, 7H), 3.47 -
-NI 3.27 (m, 5H), 2.19 -2.07 (m,
2H), 1.97 - 1.65 (m, 6H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
1H), 8.55 (d, J = 1.9 Hz, 1H),
IN 7.46 (d, J = 2.0 Hz, 1H), 6.97
40, (d, J = 2.5 Hz, 1H), 6.81 (dd, J
= 22.5, 2.3 Hz, 2H), 5.65 - 5.56
125 HN 473.25 (m, 1H), 4.73 (p, J = 2.5 Hz,
0= 1H), 4.10 (s, 3H), 3.95 - 3.85
0
r N",
(m, 4H), 3.47 (d, J = 15.3 Hz,
N-N
H3C- 1H), 3.36 - 3.27 (m, 4H), 2.23 -
2.08 (m, 2H), 1.98 - 1.62 (m,
6H)
(CDC13) 6 8.68 (d, J = 1.9 Hz,
1H), 8.58 (d, J = 1.9 Hz, 1H),
7.41 (d, J = 2.3 Hz, 1H), 6.95
0N (d, J = 2.5 Hz, 1H), 6.86 (d, J --
2.5 Hz, 1H), 6.69 (d, J = 2.3
126 HN 473.25 Hz, 1H), 4.95 (d, J = 7.3 Hz,
N N 1H), 4.72 (h, J = 2.6 Hz, 1H),
8`.11-.27-cH3( 4.02 - 3.82 (m, 7H), 3.52 (d, J =
9.0 Hz, 1H), 3.37 - 3.27 (m,
4H), 2.12 (dt, J = 16.1, 5.7 Hz,
2H), 1.97 - 1.65 (m, 6H)
94
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC11) 6 8.62 (d, J = 1.9 Hz,
N''
I 1H), 8.54 (d, J = 1.9 Hz, 1H),
0 N 8.01 (s, 2H), 6.88 (s, 1H), 6.83
(d, J = 2.5 Hz, 1H), 4.93 (d, J =
HN' Si 8.1 Hz, 1H), 4.72 (d, J = 5.8
127 .). N N N 507.2
Hz, 1H), 4.53 (d, J = 6.0 Hz,
'."
y0 co) 2H), 4.38 (d, J = 6.0 Hz, 2H),
0.CH3 3.89 -3.75 (m, 4H), 3.35 -3.13
(m, 4H), 2.10 (s, 3H), 1.95 -
1.72 (m, 6H), 1.36 (s, 3H)
N 1I (CDC13) 6 8.70 (d, J = 1.9 Hz,
-
.Ø4.0 is N 1H), 8.63 (d, J = 1.9 Hz, 1H),
8.56 (s, 2H), 7.04 - 6.87 (m,
HN 2H), 6.19 (d, J = 6.8 Hz, 1H),
128 .i.
N .' N N
Co) 477.2 5.15 (d, J= 8.2 Hz, 1H), 5.01
(d, J = 6.8 Hz, 1H), 4.81 (s,
L..,) 1H), 4.11 -3.85 (m, 6H), 3.43 -
3.22 (m, 4H), 2.21 (d, J = 9.5
Hz, 2H), 1.94 (t, J = 3.9 Hz,
0--CH3 6H), 1.36 (t, J = 7.1 Hz, 3H)
efaiso N1 (CDC13)
(CDC13) 6 8.60 (d, J = 1.9 Hz,
1H), 8.53 (d, J = 1.9 Hz, 1H),
HN 1110 2H), 5.08 - 4.89 (m, 2H), 4.89 -
7.81 (s, 2H), 6.91 - 6.75 (m,
129 N .1.N N 478.93 4.77 (m, 2H), 4.67 (ddd, J =
.'
y co) 8.2, 4.7, 1.9 Hz, 3H), 3.94 -
3.72 (m, 5H), 3.34 - 3.17 (m,
0.,...n 4H),2.21 - 2.00 (m, 2H), 1.91 -
1.69 (m, 6H)
(400 MHz, methanol-d4) 6 8.69
N (d, J = 2.0 Hz, 1H), 8.55 (d, J =
2.0 Hz, 1H), 7.10 (d, J = 2.3
0 N
Hz, 1H), 6.89 (d, J = 2.4 Hz,
1H), 4.85 -4.82 (m, 1H), 4.07
130 H NIC# 11$1 401.24
(q, J = 6.9 Hz, 2H), 3.95 - 3.81
0 0 (m, 4H), 3.64 - 3.53 (m, 1H),
H3C) 3.42 - 3.35 (m, 4H), 2.20 - 2.02
0
(m, 2H), 1.94 - 1.66 (m, 6H),
1.24 (t, J = 7.1 Hz, 3H)
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.73 -
NI 8.65 (m, 1H), 8.60 (d, J = 1.9
0 N Hz, 1H), 6.94 (d, J = 2.4 Hz,
1H), 6.87 (d, J = 2.3 Hz, 1H),
HN1Cr 4.90 -4.58 (m, 2H), 3.97 - 3.88
131
rN 429.21
(m, 4H), 3.83 (d, J = 6.3 Hz,
0 0
CH3 LO) 2H), 3.72 (s, 1H), 3.39 - 3.27
(m, 4H), 2.20 - 2.06 (m, 2H),
CH3 1.95 - 1.76 (m, 7H), 0.93 (d, J =
6.7 Hz, 6H)
(400 MHz, CDC13) 6 8.70 (d, J
N-NiI = 1.6 Hz, 1H), 8.62 (d, J = 1.7
Hz, 1H), 7.36 (t, J = 7.6 Hz,
iet
0
2H), 7.19 (t, J = 7.3 Hz, 1H),
HNo", 401 N
7.13 (d, J = 7.7 Hz, 2H), 6.96
132
-'. N 449.16 (s, 1H), 6.89 (s, 1H), 5.12
(d, J
0 0 CoD = 7.2 Hz, 1H), 4.77 (s, 1H),
3.98 - 3.85 (m, 4H), 3.79 (s,
11101 1H), 3.39 - 3.28 (m, 4H), 2.29 -
2.11 (m, 2H), 1.98- 1.79 (m,
6H)
NThI (CDC13) 6 8.71 (d, J = 1.9 Hz,
Ø0 1H), 8.63 (d, J = 1.9 Hz, 1H),
0 N
8.04 (s, 2H), 6.97 (d, J = 2.5
HN Hz, 1H), 6.92 (d, J = 2.7 Hz,
133 .1.
N -' N N 493.25 1H), 4.79 (s, 2H), 4.07 - 3.83
y co) (m, 6H), 3.42 - 3.25 (m, 4H),
2.29 - 2.02 (m, 2H), 1.92 (d, J =
0õõ.r...\ 4.6 Hz, 6H), 1.36- 1.12 (m,
3H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
W7)I 1H), 8.63 (d, J = 1.9 Hz, 1H),
icy() is N 8.07 (s, 2H), 6.97 (d, J = 2.5
Hz, 1H), 6.92 (d, J = 2.6 Hz,
HN 1H), 4.99 (d, J = 8.0 Hz, 1H),
134 N N N 437.3
4.81 (d, J = 5.9 Hz, 1H), 3.93
y Co) (dd, J = 6.0, 3.7 Hz, 5H), 3.81
(s, 3H), 3.45 - 3.24 (m, 4H),
0 rs,H3 2.21 (d, J = 8.6 Hz, 2H), 2.05 -
'
1.78 (m, 6H)
96
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC11) 6 8.70 (d, J = 1.9 Hz,
1H), 8.63 (d, J = 1.9 Hz, 1H),
0 N 8.19 (s, 2H), 6.96 (d, J = 2.5
HNIC Si
Hz, 1H), 6.92 (d, J = 2.5 Hz,
1H), 5.12 (d, J= 8.1 Hz, 1H),
S.
N 479.2 4.81 (d, J = 5.6 Hz, 1H), 4.09 -
135
N N 3.85 (m, 5H), 3.63 - 3.41 (m,
,Ij Co) 4H), 3.43 - 3.26 (m, 4H), 2.69
(t, J = 6.6 Hz, 2H), 2.33 -2.13
...,Ø H3 (m, 2H), 2.03 - 1.83 (m, 6H),
1.21 (t, J = 7.0 Hz, 3H)
(methanol-d4) 6 8.77 - 8.65 (m,
3H), 8.56 (d, J = 2.0 Hz, 1H),
HNocr0 0 N
7.11 (d, J = 2.4 Hz, 1H), 6.89
(d, J = 2.4 Hz, 1H), 4.92 (s,
136 .1.. N 494.24 1H), 4.02 (dd, J = 8.4, 4.3 Hz,
N ' N r N. 1H), 3.93 - 3.83 (m, 4H), 3.69
[0 (t, J = 5.7 Hz, 2H), 3.47 (1, J =
5.8 Hz, 2H), 3.42 - 3.33 (m,
.p.. .=-=,..,OH 5H), 2.26 -2.15 (m, 2H), 2.08 -
0 N
H 1.78 (m, 6H)
NI (CDC13) 6 8.73 - 8.58 (m, 4H),
oco0 ip N 7.01 - 6.87 (m, 2H), 6.12 (d, J =
7.9 Hz, 1H), 5.57 (d, J = 8.1
HN Hz, 1H), 4.80 (s, I H), 4.39 -
137 N .1.N N 522.23 4.28(m, 1H), 4.10 (d, J = 6.9
' .- N.
Hz, 1H), 3.97 - 3.87 (m, 4H),
LO o, j
1 CH3 3.54 - 3.29 (m, 9H), 2.21 (d, J =
0NO'CH3 9.9 Hz, 2H), 2.08 - 1.84 (m,
H 6H), 1.28 (d, J = 6.8 Hz, 3H)
(CDC11) 6 9.04 (d, J = 3.2 Hz,
1H), 8.97 (d, J = 3.3 Hz, 1H),
N -I 8.64 (d, J = 1.9 Hz, 1H), 8.55
HN
= 0,0 401N
(d, J = 1.9 Hz, 1H), 6.90 (d, J =
2.5 Hz, 1H), 6.84 (d, J = 2.5
138
-1, N 452.92 Hz, 1H), 6.03 (d, J = 8.2 Hz,
N ' N 1H), 4.77 (td, J = 5.1, 2.6 Hz,
y Co) 1H), 4.15 -4.07 (m, 1H), 3.92 -
3.79 (m, 4H), 3.35 -3.15 (m,
NO2 4H),2.25 -2.11 (m, 2H), 1.95 -
1.69 (m, 6H)
97
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.74 (d, J
N N = 1.9 Hz, 2H), 8.70 (d, J =
1.9
0 Hz, 1H), 8.03 (dd, J = 8.9, 2.0
Hz, 1H), 6.97 (d, J = 2.4 Hz,
FIN. Si 1H), 6.92 (d, J = 2.4 Hz, 1H),
139 450.17 6.37 (d, J = 8.9 Hz, 1H), 6.05
N
1\11 (s, 1H), 4.81 (s, 1H), 3.99 _
y Co) 3.88 (m, 4H), 3.84 (s, 1H), 3.39
-3.27 (m, 4H), 2.31- 2.17 (m,
00H 2H), 2.08 - 1.98 (m, 2H), 1.98 -
1.82 (m, 4H)
(400 MHz, CDC13) 6 8.70 (d, J
= 1.9 Hz, 1H), 8.61 (d, J = 1.9
NThi Hz, 1H), 8.53 (d, J = 2.1 Hz,
i0
1H), 7.88 (dd, J = 8.8, 2.4 Hz,
1H), 6.95 (d, J = 2.4 Hz, 1H),
HNcr 0 i\I 6.90 (d, J = 2.4 Hz, 1H), 6.39
140 N 449.19
Ni (d, J = 8.7 Hz, 1H), 5.61 (s,
y Co) 2H), 4.97 (d, J = 7.9 Hz, 1H),
4.80 (s, 1H), 4.02 - 3.82 (m,
0-N I-12 5H), 3.42 - 3.26 (m, 4H), 2.27 -
-'
2.14 (m, 2H), 2.00 - 1.81 (m,
6H)
N N
ocr,0 0 (CDC13) 6 8.70 (d, J = 1.9 Hz,
1H), 8.63 (d, .1= 1.9 Hz, 1H),
HN 8.09 (s, 2H), 7.01 - 6.86 (m,
141 .1.
N-' N N 492.17 2H), 4.88 - 4.75 (m, 1H), 4.08 _
y Co) 3.80 (m, 9H), 3.43 - 3.27 (m,
4H), 3.09 -2.96 (m, 4H), 2.31 -
2.15 (m, 2H), 2.05 - 1.81 (m,
6H)
0
N , (CDC13) 6 8.70 (d, J = 1.9
Hz,
N
Ø0 0 1H), 8.63 (d, J = 1.9 Hz, 1H),
8.14 (d, J = 0.8 Hz, 2H), 7.01 -
HN 6.90 (m, 2H), 4.81 (td, J = 5.6,
142
.i. N 421.2
2.7 Hz, 1H), 4.08 - 3.84 (m,
NV" N Co) 5H), 3.43 - 3.26 (m, 4H), 2.25 -
2.10 (m, 5H), 2.02 - 1.83 (m,
y
cH3 6H)
98
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.70 (d, J = 1.9 Hz,
1H), 8.63 (d, J = 1.9 Hz, 1H),
8.02 (s, 2H), 6.95 (dd, J = 11.8,
2.5 Hz, 2H), 4.81 (s, 2H), 4.08
-3.85 (m, 5H), 3.41 - 3.30 (m,
0
HNC" 4H), 2.20 (d, J = 10.1 Hz, 2H),
1.95 (d, J = 19.7 Hz, 6H), 1H
143 422.25 NMR (300 MHz, Methanol-d4)
N N C? 8.68 (d, J = 2.0 Hz, 1H), 8.56
o) (d, J = 2.1 Hz, 1H), 7.93 (s,
2H),7.11 (d, J = 2.5 Hz, 1H),
NH2 6.88 (d, J = 2.4 Hz, 1H), 3.96 -
3.71 (m, 5H), 3.37 (dd, J = 5.8,
3.9 Hz, 4H), 2.27 - 2.04 (m,
2H), 1.98 - 1.74 (m, 6H). [2]
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.61 (d, J = 1.9
N)I Hz, 1H), 8.47 (d, J = 2.1 Hz,
00.0 N 1H), 7.84 (dd, J = 8.7, 2.4 Hz,
1H), 6.95 (d, J = 2.4 Hz, 1H),
HN 6.90 (d, J = 2.4 Hz, 1H), 6.37
144 463.2 (d, J = 8.7 Hz, 1H), 5.94 (d, J
=
co) 3.9 Hz, 1H), 4.92 (d, J = 7.8
Hz, 1H), 4.79 (s, 1H), 3.98 -
0 N...CH3 3.84 (m, 5H), 3.39 - 3.26 (m,
4H), 2.98 (d, J = 4.8 Hz, 3H),
2.27 -2.12 (m, 2H), 1.97 - 1.83
(m, 6H)
N=I (400 MHz, CDC13) 6 8.69 (d, J
Ø0 N = 1.9 Hz, 1H), 8.61 (d, J = 1.9
Hz, 1H), 8.20 (s, 1H), 7.61 (d, J
HN = 8.2 Hz, 1H), 6.95 (d, J = 2.4
145 477.2 Hz, 1H), 6.91 (d, J = 2.4 Hz,
(o) 1H), 6.43 (d, .1= 7.9 Hz, 1H),
4.81 (s, 1H), 3.98 -3.81 (m,
5H), 3.40 - 3.27 (m, 4H), 3.09
0 N (s, 6H), 2.27 - 2.15 (m, 2H),
61-13 1.99 - 1.83 (m, 6H)
99
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC11) 6 8.76 (d, J = 2.0 Hz,
N .'1 1H),8.71 (d, J = 1.9 Hz, 1H),
0 8.63 (d, J = 1.9 Hz, 1H), 7.99
(dd, J = 8.8, 2.2 Hz, 1H), 6.97
H NCI 0 (d, J = 2.5 Hz, 1H), 6.92 (d, J =
N
146 464.17
N 2.5 Hz, 1H), 6.38 (d, J = 8.9
i
y =
co) Hz, 1H), 5.13 (s, 1H), 4.82 (s,
1H), 4.11 -3.73 (m, 8H), 3.44 -
0 0 ,...CH3 3.27 (m, 4H), 2.30 -2.17 (m,
2H), 2.07 - 1.79 (m, 6H)
(400 MHz, CDC13) 6 10.77 -
10.42 (m, 1H), 8.70 (d, J = 1.9
N ---7) Hz, 1H), 8.63 (d, J = 1.9 Hz,
ecr0 s k 1H), 7.30 (d, J = 8.2 Hz, 1H),
7.15 (d, J = 5.7 Hz, 2H), 6.95
147 HN 423.2 (d, J = 2.5 Hz, 1H), 6.90 (d, J
=
N
KN 2.4 Hz, 1H), 4.83 (s, 1H), 4.19
0 C-
HN--1 L0) - 4.05 (m, 1H), 3.99 - 3.84 (m,
4H), 3.40 -3.27 (m, 4H), 2.31 -
2.15 (m, 2H), 2.06 - 1.93 (m,
2H), 1.93 - 1.79 (m, 4H)
(DMSO-d6) 6 8.73 (d, J = 1.5
Hz, 3H), 8.59 (d, J = 1.9 Hz,
IN1 ..-
eCro 401 IN 1H), 8.12 (d, J = 8.2 Hz, 1H),
7.84 (d, J = 7.4 Hz, 1H), 7.14
(d, J = 2.4 Hz, 1H), 6.84 (d, J =
HN 2.3 Hz, 1H), 4.93 (s, 1H), 4.51
148 .1.
NV N N 534.28 (ddd, J = 13.3, 8.2, 5.2 Hz, 1H),
() 4.01 - 3.74 (m, 6H), 3.55 (q, J =
8.0 Hz, 1H), 3.38 - 3.27 (m,
,,c0
CD--N 4H), 2.33 -2.15 (m, 1H), 2.06
H CH3 (d, J = 11.1 Hz, 2H), 1.96-
1.67 (m, 8H), 1.02 (d, J = 6.3
Hz, 3H)
100
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(DMSO-d6) 6 8.76 - 8.67 (m,
NN) 3H), 8.59 (d, J = 1.9 Hz, 1H),
0 8.32 (d, J = 6.5 Hz, 1H), 7.84
(d, J = 7.3 Hz, 1H), 7.14 (d, J =
HNICX I* 2.5 Hz, 1H), 6.84 (d, J = 2.3
149 N N N 520.33 Hz, 1H), 4.93 (s, 1H), 4.49 -
--
,)0 C j 4.37 (m, 1H), 3.96 - 3.63 (m,
L.) 0 9H), 3.55 (dd, J = 8.9, 4.2 Hz,
0 N
1H), 3.38 - 3.27 (m, 4H), 2.23 -
H 2.00 (m, 4H), 1.95 - 1.71 (m,
6H)
(DMSO-d6) 6 8.76 - 8.67 (m,
ON -7) 3H), 8.59 (d, J = 1.9 Hz, 1H),
Ni
8.32 (d, J = 6.5 Hz, 1H), 7.84
(d, J = 7.3 Hz, 1H), 7.14 (d, J =
HN 2.5 Hz, 1H), 6.84 (d, J = 2.3
150 N 520.33 Hz, 1H), 4.93 (s, 1H), 4.49 -
NI-Li
4.37 (m, 1H), 3.96 - 3.63 (m,
j, /CO 9H), 3.55 (dd, J = 8.9, 4.2 Hz,
0 N' 1H), 3.38 - 3.27 (m, 4H), 2.23 -
H 2.00 (m, 4H), 1.95 - 1.71 (m,
6H)
NiI (CDC13) 6 8.70 (d, J = 1.9 Hz,
ificr0 0 N 1H), 8.64 (d, J = 2.0 Hz, 1H),
HN 2H), 4.82 (t, J = 6.7 Hz, 3H),
7.93 (s, 2H), 7.02 - 6.90 (m,
151 N ,-(N N 4.66 (t, J = 6.4 Hz, 2H), 4.14
y -- r ...
(q, J = 7.1 Hz, 1H), 4.08 - 3.89 t-o-- (m, 5H), 3.42 - 3.25 (m, 4H),
H3C-NI 2.80 (s, 3H), 2.22 (s, 2H), 2.05
U) - 1.79 (m, 6H)
N''")(CDC13) 8 8.70 (d, J = 1.9 Hz,
/00.0 O 1H), 8.64 (d, J = 1.9 Hz, 1H),
7.94 (s, 2H), 6.99 - 6.90 (m,
HN 2H), 4.82 (s, 1H), 4.05 - 3.87
152
NV N N 464.21 (m, 5H), 3.44 (q, J = 6.3 Hz,
y co) 1H), 3.39 - 3.26 (m, 4H), 2.23
(d, J = 12.4 Hz, 2H), 2.03 -
1-1Ny CH3 1.82 (m, 6H), 1.23 (d, J = 6.3
-
Hz, 6H)
CH3
101
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
N
(CDC13) 6 8.64 - 8.49 (m, 2H),
.)
6.93 (d, J = 3.9 Hz, 1H), 6.83
HNI 4. I N 0
(d, J = 2.4 Hz, 1H), 5.27 (d, J =
8.1 Hz, 1H), 4.94 (t, J = 1.9 Hz,
153
-I, N 483.1 2H), 4.81 (t, J = 1.9 Hz, 2H),
N ' N 4.72 (s, 1H), 3.96 (s, 1H), 3.89
,,L6 - 3.78 (m, 4H), 3.37 - 3.25 (m,
CI (o) 4H),2.11 (s, 2H), 1.82 (d, J =
0 5.1 Hz, 6H)
(CDC13) 6 8.68 (dd, J = 8.7, 2.0
N'I Hz, 1H), 8.56 (d, J = 2.0 Hz,
N
0 1H), 6.92 (d, J = 2.3 Hz, 1H),
6.84 (d, J = 2.4 Hz, 1H), 4.92
154 HNICr $1 483.1 (t, J = 2.7 Hz, 2H), 4.82 (t, J =
N --N
N 2.7 Hz, 2H), 4.75 (s, 1H), 3.94
I 0 ( ) - 3.81 (m, 4H), 3.35 - 3.15 (m,
CI N 0 4H), 2.14 (d, J = 12.0 Hz, 2H),
1.82 (d, J = 17.3 Hz, 6H)
1\11
N I .0,0 401 (CDC13) 6 8.71 (d, J = 1.9 Hz,
1H), 8.64 (d, J = 1.9 Hz, 1H),
HN 7.02 - 6.89 (m, 2H), 4.83 (s,
155 .L.
N ' N 478.3 1H), 4.08 -3.87 (m, 5H), 3.44 _
y cNo) 3.33 (m, 4H), 3.27 (d, J = 26.0
Hz, 4H), 2.23 (d, J = 9.8 Hz,
2H), 2.05 - 1.80 (m, 6H), 1.14
N
r (s, 6H)
CH3 CH3
(CDC13) 6 8.70 (d, J = 2.0 Hz,
N-51 1H), 8.63 (d, J = 1.9 Hz, 1H),
.000 401 N 7.72 (s, 2H), 6.96 (d, J = 2.5
Hz, 1H), 6.92 (d, J = 2.5 Hz,
HN 1H), 5.03 (s, 1H), 4.80 (q, J =
156 -1. N N N 462.23 4.4 Hz, 1H), 4.03 - 3.89 (m,
'
y co) 4H), 3.83 (t, J = 7.1 Hz, 4H),
3.44 - 3.29 (m, 4H), 2.41 (dq, J
N = 8.6, 7.1 Hz, 2H), 2.20 (q, J =
V 5.9 Hz, 2H), 1.99 - 1.81 (m,
6H)
102
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
N-5N)I
157 0
O0/1Cr lel N
433.2
HN N
le co)
(CDC13) 6 8.71 (d, J = 2.0 Hz,
N-
I 1H), 8.64 (d, J = 1.9 Hz, 1H),
0 N
8.41 (s, 2H), 6.97 (d, J = 2.4
Hz, 1H), 6.93 (d, J = 2.5 Hz,
HN'C'''' SI
1H), 5.32 (d, J= 8.1 Hz, 1H),
158
N N 4.82 (s, 1H), 4.07 (s, 1H),
4.00
y co) _ 3.88 (m, 4H), 3.43 - 3.29 (m,
4H), 2.22 (s, 2H), 2.06 - 1.81
OH (m, 6H)
N 159 l
0
OCr 0 434.24
HN C N
N o)
(400 MHz, CDC13) 6 8.69 (d, J
N"*N = 1.9 Hz, 1H), 8.61 (d, J = 1.9
sii
icy is N Hz, 1H), 8.05 (d, J = 2.2 Hz,
1H), 7.46 (dd, J = 8.5, 2.3 Hz,
HN 1H), 6.94 (d, J = 2.4 Hz, 1H),
160 N 436.2 6.90 (d, J = 2.4 Hz, IH), 6.39
I\1. (d, J = 8.6 Hz, 1H), 4.77 (s,
L.\,.e Co) 1H), 4.53 (s, 2H), 3.95 - 3.87
(m, 5H), 3.36 - 3.32 (m, 4H),
OH 2.25 -2.12 (m, 2H), 1.90 (d, J =
4.4 Hz, 6H)
103
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.69 (d, J = 1.9 Hz,
N "5".1I 1H), 8.61 (d, J = 1.9 Hz, 1H),
0 N 8.26 (s, 2H), 6.95 (d, J = 2.5
Hz, 1H), 6.90 (d, J = 2.6 Hz,
1H), 5.23 (d, J= 8.1 Hz, 1H),
161
N N N 451.28
4.80 (d, J = 5.8 Hz, 1H), 4.26
Co) (s, 2H), 4.03 (s, 1H), 3.97 -
3.85 (m, 4H), 3.44 - 3.21 (m,
0,CH3 7H), 2.32 - 2.09 (m, 2H), 2.06 -
1.70 (m, 6H)
(DMSO-d6) 6 8.81 - 8.64 (m,
3H), 8.58 (d, J = 1.9 Hz, 1H),
N '.1I 7.99 (d, J = 7.6 Hz, 1H), 7.76
HN...or 0 N
(d, J = 7.4 Hz, 1H), 7.14 (d, J =
2.5 Hz, 1H), 6.84 (d, J = 2.3
Hz, 1H), 4.92 (s, 1H), 3.98 -
162 .1. N 492.29
N -' N 3.84 (m, 1H), 4.05 (dq, J =
(o) 13.5, 6.7 Hz, 1H), 3.79 (d, J =
C H3 9.6 Hz, 4H), 3.32 (d, J = 8.2
)
0 N CH3 Hz, 4H), 2.06 (d, J = 11.8 Hz,
H 2H), 1.96 - 1.66 (m, 6H), 1.14
(d, J = 6.6 Hz, 6H)
N (CDC13) 6 8.69 (d, J = 1.9 Hz,
N
I
0 1H), 8.65 - 8.53 (m, 3H), 6.93
(dd, J = 14.6, 2.5 Hz, 2H), 5.48
HNØ 0 (d, J = 8.0 Hz, 1H), 4.81 (d, J =
163 .1. N 504.26 6.1 Hz, 1H), 4.19 -4.03 (m,
N -' N 1H), 3.92 (dd, J = 6.0, 3.7 Hz,
4H), 3.65 - 3.54 (m, 4H), 3.34
(dd, J = 5.9, 3.7 Hz, 4H), 2.27 -
0NO 2.15 (m, 2H), 2.08 - 1.81 (m,
10H)
(400 MHz, CDC13) 6 8.60 (d, J
= 1.9 Hz, 1H), 8.52 (d, J = 1.9
N i Hz, 1H), 8.01 (d, J = 2.1
Hz,
N jecr0 1 1H), 7.35 (dd, J = 8.8, 2.5 Hz,
1H), 6.86 (d, .1= 2.5 Hz, 1H),
HN 6.80 (d, J = 2.5 Hz, 1H), 6.20
164 484.12
N
N (d, J = 8.4 Hz, 1H), 4.68 (s,
y co) 1H), 4.47 (d, J = 8.0 Hz, 1H),
3.92 - 3.80 (m, 4H), 3.74 (s,
Br 1H), 3.34 - 3.14 (m, 4H), 2.18 -
2.02 (m, 2H), 1.94 - 1.67 (m,
6H)
104
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.63 (d, J = 1.9
NI Hz, 1H), 8.44 - 8.37 (m, 1H),
8.33 (d, J = 8.1 Hz, 1H), 7.58
(dd, J = 7.8, 0.9 Hz, 1H), 7.31
165 HN10" N 448.15 (dd' J = 7.7, 4.6 Hz, 1H), 6.95
(d, J = 2.4 Hz, 1H), 6.90 (d, J =
O 2.4 Hz, 1H), 4.75 (s, 1H), 4.25
- 4.10 (m, 1H), 3.97 - 3.85 (m,
02 4H), 3.41 - 3.26 (m, 4H), 2.75
(s, 3H), 2.29 - 2.13 (m, 2H),
2.06- 1.85 (m, 6H)
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.63 (d, J = 1.9
N Hz, 1H), 8.41 (d, J = 4.5 Hz,
oci,,0 N 1H), 8.21 (d, J = 8.0 Hz, 1H),
8.08 - 7.98 (m, 1H), 7.25 - 7.22
HN (m 1H) 6.95 (d, J = 2.5 Hz,
166
oN 448'19 1H'), 6.9'0 (d, J = 2.4 Hz, 1H),
(0) 4.76 (s, 1H), 4.27 -4.15 (m,
1H), 3.98 - 3.86 (m, 4H), 3.40 -
CH3 3.29 (m, 4H), 2.43 (s, 3H), 2.29
-2.16 (m, 2H), 2.07 - 1.86 (m,
6H)
(400 MHz, CDC13) 6 8.70 (d, J
= 1.9 Hz, 1H), 8.63 (d, J = 1.9
NI Hz, 1H), 8.37 (dd, J = 1.4, 0.7
Hz, 1H), 8.16 (d, J = 8.4 Hz,
1H), 8.09 (d, J = 8.0 Hz, 1H),
167 HN10. 7.66 - 7.61 (m" 1H) 6.95 (d, J =
N 448.19
2.4 Hz, 1H), 6.90 (d, J = 2.4
O (NJ Hz, 1H), 4.75 (s, 1H),
4.29 -
4.16 (m, 1H), 3.97 -3.86 (m,
CH3 0 4H), 3.39 - 3.29 (m, 4H), 2.41
(s, 3H), 2.28 -2.15 (m, 2H),
2.04 - 1.84 (m, 6H)
105
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.69 (d, J
= 1.8 Hz, 1H), 8.63 (d, J = 1.9
Hz, 1H), 8.39 (d, J = 1.6 Hz,
N-5')I 1H), 8.17 (d, J = 8.6 Hz, 1H),
0 8.11 (d, J = 8.0 Hz, 1H), 7.66
(dd, J = 7.9, 2.0 Hz, 1H), 6.95
HN'JC:re (d, J = 2.3 Hz, 1H), 6.90 (d, J =
168 462.16
LN N 2.4 Hz, 1H), 4.76 (s, 1H), 4.31
0CoJ -4.14 (m, 1H), 4.00 - 3.85 (m,
4H), 3.42 - 3.25 (m, 4H), 2.73
CH3 (q, J = 7.6 Hz, 2H), 2.30 -2.14
(m, 2H), 1.99 (ddd, J = 34.6,
19.6, 10.3 Hz, 7H), 1.29 (t, J =
7.6 Hz, 3H)
(400 MHz, CDC13) 6 8.70 (d, J
= 1.9 Hz, 1H), 8.64 (d, J = 1.9
Hz, 1H), 8.24 (d, J = 7.8 Hz,
1µ1-I 1H), 8.01 (d, J = 7.5 Hz, 1H),
0
169 HN1Cr 1.1 448.19 7.72 (t, J = 7.7 Hz, 1H),
7.27
(d, J = 6.6 Hz, 1H), 6.95 (d, J =
2.4 Hz, 1H), 6.90 (d, J = 2.4
CH N Hz, 1H), 4.82 - 4.68 (m, 1H),
CJ 4.27-4.13 - 4.13 (m, 1H), 3.98 - 3.86
O)
(m, 4H), 3.41 - 3.28 (m, 4H),
2.60 (s, 3H), 2.30 -2.18 (m,
2H), 2.09 - 1.88 (m, 6H)
(400 MHz, CDC13) 6 8.69 (d, J
= 1.8 Hz, 1H), 8.61 (d, J = 1.9
NI Hz, 1H), 7.90 (d, J = 7.2 Hz,
14V N 464.17 1H), 7.83 - 7.78 (m, 1H), 7.76 -
170 yid
7.69(m, 1H), 6.96 (d, J = 2.3
3
Hz, 1H), 6.94 - 6.86 (m, 2H),
N N ) 4.77 (s, 1H), 4.17 (s, 1H), 4.00
(s, 3H), 3.95 - 3.86 (m, 4H),
0
3.40 - 3.29 (m, 4H), 2.30 - 2.17
(m, 2H), 2.06 - 1.88 (m, 6H)
106
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.76 (dd,
J = 4.9, 0.8 Hz, 1H), 8.70 (d, J
1\n = 1.9 Hz, 1H), 8.62 (d, J = 1.9
NI Hz, 1H), 8.48 - 8.38 (m, 1H),
0
8.09 (d, J = 8.0 Hz, 1H), 7.67
(dd, J = 4.9, 1.6 Hz, 1H), 6.96
171 HNICr 11 459.17
(d, J = 2.4 Hz, 1H), 6.90 (d, J =
rN,1
u 2.4 Hz, 1H), 4.76 (s, 1H), 4.31
-4.14 (m, 1H), 4.01 - 3.83 (m,
4H), 3.43 -3.26 (m, 4H), 2.31 -
2.15 (m, 2H), 2.08 - 1.87 (m,
6H)
(400 MHz, CDC13) 6 8.70 (d, J
= 1.9 Hz, 1H), 8.64 (d, J = 1.9
I Hz, 1H), 8.11 (dd, J = 7.4, 1.4
HN0000 N Hz, 1H), 7.96 (dd, J = 15.6, 7.7
Hz, 1H), 7.82 (d, J = 7.7 Hz,
1H), 7.09 (dd, J = 8.1, 1.8 Hz,
452.22 172 N
1H), 6.96 (d, J = 2.4 Hz, 1H),
0 , r-1 6.91 (d, J = 2.4 Hz, 1H), 4.80
(s, 1H), 4.26 - 4.09 (m, 1H),
3.99 - 3.86 (m, 4H), 3.41 - 3.25
(m, 4H), 2.31 - 2.17 (m, 2H),
2.05 - 1.86 (m, 6H)
N)IN 0
173 HN**Cri 452.22
o
I (NLI
'109
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.62 (d, J = 1.9
N-7)I Hz, 1H), 8.30 (d, J = 2.1 Hz,
0N 1H), 7.80 (d, J = 7.9 Hz, 1H),
7.32 (ddd, J = 10.3, 8.1, 2.3 Hz,
174 HN1C" 116 470.22 1H), 6.95 (d, J = 2.4 Hz,
1H),
0 (N) 6.89 (d, J = 2.4 Hz, 1H), 4.74
(s, 1H), 4.27 -4.15 (m, 1H),
0 4.00 - 3.86 (m, 4H), 3.41 -3.27
(m, 4H), 2.27 - 2.13 (m, 2H),
2.08 - 1.86 (m, 6H)
107
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
N'5N10
175 HN".1 ' 450.13
`'
0)
(400 MHz, CDC13) 6 12.21 (s,
1H), 8.70 (d, J = 1.9 Hz, 1H),
8.63 (d, J = 1.9 Hz, 1H), 8.17
(d, J = 7.4 Hz, 1H), 8.07 (dd, J
0
= 4.2, 1.5 Hz, 1H), 7.33 (ddd, J
10.0, 8.5, 2.9 Hz, 2H), 6.97
176 HN.Cr 450.2
(d, J = 2.4 Hz, 1H), 6.90 (d, J -
N
o coj 2.5 Hz, 1H), 4.83 - 4.74 (m,
1H), 4.24 -4.11 (m, 1H), 3.99
3.86 (m, 4H), 3.41 - 3.26 (m,
4H), 2.30 -2.18 (m, 2H), 2.08 -
1.87 (m, 6H)
NI (400 MHz, CDC13) 6 11.13 (s,
1H), 8.71 (d, J = 1.9 Hz, 1H),
HNcr,,,0 N
8.59 (d, J = 1.9 Hz, 1H), 7.64 -
=
7.39 (m, 2H), 7.07 (d, J = 6.8
177 450.17 Hz, 1H), 7.02 - 6.79 (m, 3H),
0 , 4.76 (s, 1H), 4.32 -4.15 (m,
1H), 3.96 - 3.85 (m, 4H), 3.42 -
3.24 (m, 4H), 2.39 -2.14 (m,
OH 2H), 2.10 - 1.82 (m, 6H)
(400 MHz, CDC13) 6 8.70 (d, J
= 1.9 Hz, 1H), 8.64 (d, J = 1.9
Hz, 1H), 8.14 (dd, J = 7.6, 0.7
NI Hz, 1H), 7.91 (d, J = 8.1 Hz,
i0
1H),7.81 (t, J = 7.8 Hz, 1H),
HN(:), = N
7.46 (dd, J = 7.9, 0.7 Hz, 1H),
178 468.17 6.96 (d, J = 2.4 Hz, 1H),6.91
(d, J = 2.4 Hz, 1H), 4.80 (s,
Nyi 1H), 4.24 - 4.08 (m, 1H), 3.99 -
3.83 (m, 4H), 3.41 - 3.26 (m,
CI 4H), 2.33 -2.18 (m, 2H), 2.11 -
1.98 (m, 2H), 1.98 - 1.87 (m,
4H)
108
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.70 (d, J
= 1.9 Hz, 1H), 8.63 (d, J = 1.9
1\1.1
Hz, 1H), 8.13 (d, J = 7.7 Hz,
0
HNCi 1H), 8.02 (d, J = 7.8 Hz, 1H),
7.86 (t, J = 7.7 Hz, 1H), 7.48
Ifir
464.28 (d' J = 7.8 Hz, 1H), 6.96 (d, J --
2.4 Hz, 1H), 6.91 (d, J = 2.4
179
Hz, 1H), 4.85 (s, 2H), 4.79 (s,
1H), 4.28 -4.15 (m, 1H), 4.00 -
-.0H 3.87 (m, 4H), 3.41 - 3.27 (m,
4H), 2.88 (s, 1H), 2.33 - 2.16
(m, 2H), 2.07 - 1.88 (m, 6H)
(400 MHz, CDC13) 6 8.68 (d, J
1\11 = 1.9 Hz, 1H), 8.62 (d, J = 1.9
N
Hz, 1H), 8.16 (d, J = 7.8 Hz,
.0
1H), 7.56 (dd, J = 8.4, 7.3 Hz,
HN0,
1H), 7.43 (d, J = 6.7 Hz, 1H),
6.96 (d, J = 2.4 Hz, 1H),6.91
503.27 180
0 iN1 (d, J = 2.4 Hz, 1H), 6.50 (d, J =
7.8 Hz, 1H), 4.74 (s, 1H), 4.21
-4.07 (m, 1H), 3.97 - 3.86 (m,
4H), 3.54 - 3.48 (m, 4H), 3.40 -
3.29 (m, 4H), 2.26 -2.14 (m,
2H), 2.07 - 1.88 (m, 10H)
(400 MHz, CDC13) 6 8.91 (s,
2H), 8.71 (d, J = 1.9 Hz, 1H),
I 8.64 (d, J = 1.9 Hz, 1H), 8.30
N (dd, J = 7.7, 0.7 Hz, 1H),8.11
(t, J = 7.9 Hz, 1H), 7.74 (d, J =
HN 8.2 Hz, 1H), 7.57 (dd, J = 8.0,
181
0 iN1 501.25 0.7 Hz, 1H), 6.97 (d, J = 2.4
Hz, 1H), 6.92 (d, J = 2.4 Hz,
1H), 4.82 (s, 1H), 4.27 - 4.11
(m, 1H), 3.99 - 3.85 (m, 4H),
3.42 - 3.27 (m, 4H), 2.34 - 2.20
N-N
(m, 2H), 2.12 -2.00 (m, 2H),
2.00- 1.84 (m, 4H)
109
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.71 (d, J
N'5N) = 1.9 Hz, 1H), 8.63 (d, J = 1.9
182 H NfcIIII 0
140 N Hz, 1H), 8.15 (s, 1H), 7.43 (d, J
= 8.1 Hz, 1H), 6.97 (d, J = 2.3
424.21 Hz, 1H), 6.90 (d, J = 2.4 Hz,
1H), 4.82 (s, 1H), 4.24 - 4.13
rN (m, 1H), 3.98 - 3.86 (m, 4H),
N-NH 3.40 - 3.27 (m, 4H), 2.30 -2.16
L'O)
(m, 2H), 2.05 - 1.78 (m, 6H)
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.63 (d, J = 1.9
N'7)I Hz, 1H), 7.86 (d, J = 3.1 Hz,
183 HN 0
/CI 1110 1H), 7.57 (d, J = 3.1 Hz, 1H),
7.38 (d, J = 8.0 Hz, 1H), 6.95
440.12 (d, J = 2.4 Hz, 1H), 6.90 (d, J =
2.4 Hz, 1H), 4.79 (s, 1H), 4.25
N
- 4.09 (m, 1H), 3.97 - 3.88 (m,
4H), 3.39 - 3.29 (m, 4H), 2.28 -
2.17 (m, 2H), 2.07 - 1.96 (m,
2H), 1.96 - 1.85 (m, 4H)
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.62 (d, J = 1.9
Hz, 1H), 7.50 (d, J =
1.1 Hz, 1H), 7.28 (d, J = 8.3
0
Hz, 1H), 6.95 (d, J = 2.4 Hz,
1H), 6.89 (d, J = 2.4 Hz, 1H),
184 454.09
4.78 (s, 1H), 4.23 - 4.07 (m,
1H), 3.99 - 3.87 (m, 4H), 3.40 -
(--)Ys . -- CH3(
3.27 (m, 4H), 2.53 (d, J = 1.0
0 Hz, 3H), 2.28 - 2.14 (m, 2H),
2.04 - 1.95 (m, 2H), 1.95 - 1.84
(m, 4H)
(400 MHz, CDC13) 6 8.70 (t, J
= 5.6 Hz, 1H), 8.61 (dd, J =
N-7)1 12.5, 1.9 Hz, 1H), 7.35 (d, J =
0
Cr
454.13 8.0 Hz, 1H),7.11 (d, J = 0.9
Hz, 1H), 7.01 - 6.92 (m, 1H),
185 HN =6.89 (d, J = 2.4 Hz, 1H), 4.85 -
(
N 4.70(m, 1H), 4.26 - 4.11 (m,
0 1H), 3.90 (dd, J = 15.3, 10.4 'si Hz, 4H), 3.42 - 3.25 (m, 4H),
1:21)
CH3 2.49 (d, J = 0.9 Hz, 3H), 2.30 -
2.16 (m, 2H), 2.12 - 1.84 (m,
6H)
110
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC11) 6 8.71 (d, J = 1.9 Hz,
1H), 8.63 (d, J = 1.9 Hz, 1H),
N *-N
8.01 (d, J = 5.8 Hz, 1H), 7.00 -
0
6.86 (m, 2H), 6.03 (d, J = 5.8
Hz, 1H), 4.80 (d, J = 5.8 Hz,
186 HNICI . 437.2
), r N,1 1H), 4.07 (s, 1H), 3.97 - 3.83
N -- N (m, 7H), 3.41 - 3.28 (m, 4H),
-L...)L0_CH3 Lo) 2.20 (dd, J = 12.3, 5.6 Hz, 2H),
1.95 (dh, J = 11.8, 5.6, 4.7 Hz,
6H)
IV:71I (CDC13) 6 8.70 (d, J = 1.9 Hz,
1H), 8.63 (d, J = 1.9 Hz, 1H),
HN.0, 0 N 0
7.70 (s, 1H), 6.96 (q, J = 2.6
Hz, 2H), 5.75 (d, J = 6.5 Hz,
492.26 1H), 4.82 (s, 1H), 4.65 (s, 1H),
187
,I. N N -- N 3.93 (dd, J = 5.9, 3.8 Hz, 4H),
3.63 (s, 4H), 3.36 (dd, J = 6.1,
3.7 Hz, 4H), 2.14 (s, 2H), 1.95
(d, J = 31.2 Hz, 6H)
(400 MHz, CDC13) 6 9.02 (d, J
N-7) = 2.6 Hz, 1H), 8.70 (d, J = 1.9
Hz, 1H), 8.61 (d, J = 1.9 Hz,
HNiecf. 401 N 0
1H), 8.17 (dd, J = 9.3, 2.6 Hz,
1H), 6.97 (d, J = 2.4 Hz, 1H),
188 451.14 6.91 (d, J = 2.4 Hz, 1H), 6.36
N
N (d, J = 9.3 Hz, 1H), 5.37 (s,
y co) 1H), 4.82 (s, 1H), 4.04 (s, 1H),
3.96 - 3.87 (m, 4H), 3.40 -3.27
NO2 (m, 4H), 2.31 -2.18 (m, 2H),
2.03 - 1.84 (m, 6H)
(400 MHz, CDC13) 6 8.69 (d, J
N 'N = 1.6 Hz, 1H), 8.61 (d, J = 1.7
..0õ,0 401 Hz, 1H), 8.32 (s, 1H), 7.56 (d, J
= 6.7 Hz, 1H), 6.96 (d, J = 2.2
HN Hz, 1H), 6.90 (d, J = 2.3 Hz,
189 474.147
N 1H), 6.40 (d, J = 8.7 Hz, 1H),
Ni
y co) 4.96 (s, 1H), 4.80 (s, 1H), 4.01
- 3.84 (m, 5H), 3.42 - 3.24 (m,
CF3 4H), 2.21 (d, J = 8.4 Hz, 2H),
1.92 (d, J = 6.4 Hz, 6H)
111
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.52 (s, 1H), 8.29 (d,
H3 J = 4.8 Hz, 2H), 6.90 (d, J = 2.5
NC
Hz, 1H), 6.85 (d, J = 2.5 Hz,
0 N 1H), 6.52 (t, J = 4.8 Hz, 1H),
5.36 (s, 1H), 4.79 (dq, J = 5.6,
190 HN Cr ill 1 2.9 Hz, 1H), 4.04 (dp, J = 8.0,
..i. N N
N 3.8 Hz, 1H), 3.97 - 3.85 (m,
'
.)0) 4H), 3.38 - 3.26 (m, 4H), 2.70
(s, 3H), 2.29 - 2.11 (m, 2H),
2.00 - 1.78 (m, 6H)
OH 3 (CDC13) 6 8.52 (s, 1H), 8.29 (d,
J = 4.8 Hz, 2H), 6.90 (d, J = 2.5
NI Hz, 1H), 6.85 (d, J = 2.5 Hz,
,e0,,,,0 0 N 1H), 6.53 (t, J = 4.8 Hz, 1H),
5.42 (d, J = 7.9 Hz, 1H), 4.80
191 421.24
HN (dq, J = 5.9, 2.9 Hz, 1H), 4.11 -
.). N N N 3.98 (m, 1H), 3.91 (dd, J = 5.9,
' 3.8 Hz, 4H), 3.42 - 3.21 (m,
Co) 4H), 2.71 (s, 3H), 2.31 - 2.07
(m, 2H), 2.04 - 1.77 (m, 6H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
NI 1H), 8.63 (d, J = 1.9 Hz, 1H),
...000 s N 7.97 (s, 1H), 6.97 (d, J = 2.5
HN
Hz, 1H), 6.93 (d, J = 2.5 Hz,
1H), 5.36 (s, 1H), 4.80 (d, J =
192
./. N 463.2 6.2 Hz, 1H), 4.65 (d, J = 0.9
N ' N Hz, 2H), 4.02 (t, J = 5.8 Hz,
3H), 3.98 - 3.82 (m, 4H), 3.42 -
3.30 (m, 4H), 2.80 (t, J = 5.8
Hz, 2H), 2.29 - 2.11 (m, 2H),
2.03 - 1.77 (m, 7H)
NI
zr0 0 N (CDC13) 6 8.70 (d, J = 1.9 Hz,
1H), 8.63 (d, J = 1.9 Hz, 1H),
HN 7.95 (s, 2H), 7.05 - 6.88 (m,
.1. 193 N N N 2H), 4.92 (s, 1H), 4.79 (s, 1H),
524.21 '
y co) 3.93 (t, J = 4.8 Hz, 5H), 3.60 -
3.44 (m, 4H), 3.37 (d, J = 10.5
N Hz, 9H), 2.20 (d, J = 6.9 Hz,
r 2H), 1.91 (d, J = 4.6 Hz, 6H)
H3C,0
un3
112
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.70 (d, J = 1.9 Hz,
1H), 8.62 (d, J = 1.9 Hz, 1H),
N-1 8.28 (d, J = 0.9 Hz, 1H), 6.97
N 0 (d, J = 2.5 Hz, 1H), 6.91 (d, J =
2.5 Hz, 1H), 5.74 - 5.58 (m,
194 HNCf. 1161 437.23 1H), 5.16 (d, J = 8.0 Hz, 1H),
N 4.82 (dq, J = 5.4, 2.7 Hz, 1H),
4.06 - 3.85 (m, 7H), 3.70 (d, J =
N 0 3 ) 14.0 Hz, 1H), 3.42 - 3.28 (m,
4H), 2.30 -2.16 (m, 2H), 1.99 -
1.75 (m, 6H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
1H), 8.62 (d, J = 2.0 Hz, 1H),
0 7.43 (dd, J = 8.6, 7.2 Hz, 1H),
7.02 - 6.86 (m, 3H), 6.55 (dd, J
195 HNI"C"' = 431.22 = 8.6, 0.8 Hz, 1H), 4.80 (d, J =
6.6 Hz, 2H), 4.10 - 3.81 (m,
5H), 3.47 - 3.25 (m, 4H), 2.30
CN Co) 2.11 (m, 2H), 1.99- 1.81 (m,
6H)
(CDC13) 6 8.71 (d, J = 1.9 Hz,
1H), 8.63 (d, J = 1.9 Hz, 1H),
HN 0
,eC) 7.61 (d, J = 9.4 Hz, 1H), 7.41
(d, J = 7.2 Hz, 1H), 7.05 - 6.87
196 463.27 (m, 2H), 6.65 (s, 1H), 4.86 (s,
1H), 4.04 - 3.87 (m, 7H), 3.74
(N) (s, 1H), 3.43 - 3.27 (m, 4H),
CH3 0 2.21 (d, J = 10.8 Hz, 2H), 1.98
0 (d, J = 23.5 Hz, 6H)
(400 MHz, CDC13) 6 8.68 (d, J
N"I = 1.9 Hz, 1H), 8.61 (d, J = 1.9
õcr0 N Hz, 1H), 8.13 - 8.04 (m, 1H),
7.67 (d, J = 2.4 Hz, 1H), 6.99 -
HN 6.93 (m, 2H), 6.90 (d, J = 2.4
197 421.69
Hz, 1H), 6.33 (d, J = 8.7 Hz,
1H), 4.77 (s, 1H), 3.99 - 3.65
(m, 7H), 3.39 - 3.27 (m, 4H),
NH2 2.25 -2.10 (m, 2H), 1.96 - 1.82
(m, 6H)
113
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.69 (d, J
= 1.6 Hz, 1H), 8.61 (d, J = 1.7
Nj Hz, 1H), 8.57 (d, J = 2.1 Hz,
/00.0 01N 1H), 7.89 (dd, J = 8.9, 2.3 Hz,
1H), 6.95 (s, 1H), 6.90 (s, 1H),
HN
6.62 (d, J = 9.0 Hz, 1H), 5.99
198 N 532.11 (d, J = 7.7 Hz, 1H), 4.78 (s,
0 =k=-,p..,..,,N
1H), 4.18 (s, 1H), 4.03 - 3.81
I C) (m, 4H), 3.76 - 3.60 (m, 4H),
'CH3 3.42 - 3.25 (m, 4H), 2.62 - 2.44
(m, 4H), 2.36 (s, 3H), 2.29 -
2.14 (m, 2H), 1.99 - 1.84 (m,
6H)
NI (400 MHz, CDC13) 6 8.73 -
8.65 (m, 3H), 8.60 (d, J = 1.9
0 N
riCr 0 Hz, 1H), 6.95 (d, J = 2.4 Hz,
1H), 6.89 (d, J = 2.4 Hz, 1H),
HN 199 N 533.01 5.93 (d, J = 8.0 Hz, 1H), 4.79
0
-1 N C .)
1 ,I (s, 1H), 4.23 - 4.09 (m, 1H),
3.97 - 3.88 (m, 8H), 3.40 - 3.25
N---.'N--Th (m, 4H), 2.53 - 2.42 (m, 4H),
CH3 2.34 (s, 3H), 2.26 -2.18 (m,
2H), 1.97 - 1.83 (m, 6H)
N
(400 MHz, CDC13) 6 8.69 (d, J
ii\i
= 1.9 Hz, 1H), 8.61 (d, J = 1.9
0
"Cr 0 Hz, 1H), 7.51 - 7.42 (m, 2H),
6.98 - 6.85 (m, 3H), 6.07 (d, J =
HN 8.0 Hz, 1H), 4.77 (s, 1H), 4.25
200 N 549.17
0 C ) -4.11 (m, 1H), 3.99 - 3.85 (m,
4H), 3.40 - 3.27 (m, 4H), 3.26 -
3.14 (m, 4H), 2.67 -2.52 (m,
L ,
cH3 4H), 2.36 (s, 3H), 2.28 - 2.13
N
F
(m, 2H), 1.98 - 1.84 (m, 6H)
N1"1N (400 MHz, CDC13) 6 9.47 (s,
0 1H), 8.68 (d, J = 1.7 Hz, 1H),
r0". SI 8.56 (d, J = 1.7 Hz, 1H), 8.21 -
8.11 (m, 1H), 6.97 - 6.85 (m,
HN
201 N 549.1 4H), 4.82 (s, 1H), 4.23 - 4.07
0 (
3.38 - 3.29 (m, 4H), 3.03 (s,
F N 4H), 2.65 (s, 4H), 2.37 - 2.25
L,_,N'CH3 (m, 5H), 2.02 - 1.84 (m, 6H)
114
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.69 (d, J
= 1.8 Hz, 1H), 8.63 (d, J = 1.9
Hz, 1H), 8.27 (dd, J = 4.7, 1.9
NniN Hz, 1H), 8.19 (s, 1H), 8.11 (d, J
0
= 6.8 Hz, 1H), 6.95 (d, J = 2.4
Hz, 1H), 6.92 (d, J = 2.3 Hz,
202 0 HN'Cr* 1161 464.13
1H), 6.51 (dd, J = 7.4, 4.9 Hz,
N
r 1H), 4.76 (s, 1H), 4.30 (s, 1H),
4.02 - 3.90 (m, 4H), 3.88 (s,
3H), 3.41 - 3.26 (m, 4H), 2.29 -
2.11 (m, 2H), 2.11 -1.85 (m,
6H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
N".1I 1H), 8.60 (d, J = 1.9 Hz, 1H),
0 ra,h
8.15 (s, 1H), 6.93 (dd, J = 17.9,
2.5 Hz, 2H), 6.43 (d, J = 6.1
203 HNI 0 432.58
Hz, 1H), 5.20 (s, 1H), 4.82 (s,
1H), 4.00 - 3.82 (m, 4H), 3.44 -
-1\1*.CN Co) 3.25 (m, 4H), 2.23 (d, J = 11.2
Hz, 2H), 1.91 (s, 6H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
1H), 8.61 (d, J = 1.9 Hz, 1H),
I 8.09 (d, J = 6.0 Hz, 1H), 6.93
0 Ali (dd, J = 16.1, 2.5 Hz, 2H), 6.15
(d, J = 6.0 Hz, 1H), 5.10 (d, J =
204 HNC." lir 421.65 8.0 Hz, 1H), 4.81 (td, J = 5.5,
2.7 Hz, 1H), 3.97 - 3.87 (m,
fl4H), 3.49 (s, 1H), 3.39 - 3.27
CH3 0 (m, 4H), 2.49 (s, 3H), 2.27 -
2.14 (m, 2H), 2.03 - 1.80 (m,
6H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
N"--')I 1H), 8.61 (dd, J = 5.3, 1.9 Hz,
0 1H), 8.19 - 8.06 (m, 1H), 6.99 -
205 HNC'
6.84 (m, 2H), 5.34 - 5.21 (m,
436.63 1H), 4.76 (d, J = 9.7 Hz, 3H),
3.92 (t, J = 4.9 Hz, 4H), 3.81 (s,
N N 3
,CH 1H),3.33 (dd, J = 5.7, 4.1 Hz,
4H), 2.87 (d, J = 5.2 Hz, 3H),
0
2.19 (d, J = 8.5 Hz, 2H), 1.88
(dd, J = 13.3, 5.1 Hz, 6H)
115
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.68 (d, J
= 1.9 Hz, 1H), 8.61 (d, J = 1.9
N1 Hz, 1H), 7.82 (d, J = 2.8 Hz,
HNiC2X' 1110
0
1H), 7.09 (dd, J = 8.9, 3.0 Hz,
1H), 6.94 (d, J = 2.5 Hz, 1H),
6.90 (d' J = 2.5 Hz, 1H), 6.37
436.18 206 N (d, J = 8.8 Hz, 1H), 4.77 (s,
y
, 1H), 4.29 (bs, 1H), 3.98 - 3.87
0
(m, 4H), 3.85 - 3.79 (m, 1H),
0,0H3 3.77 (s, 3H), 3.41 - 3.24 (m,
4H), 2.27 -2.12 (m, 2H), 1.97 -
1.79 (m, 6H)
(CDC13) 6 8.71 (d, J = 2.0 Hz,
1H), 8.64 (d, J = 1.9 Hz, 1H),
N"I 8.44 (s, 1H), 6.98 (d, J = 2.5
0 42.11 N
Hz, 1H), 6.92 (d, J = 2.5 Hz,
1H), 6.82 (d, J = 4.7 Hz, 1H),
207 FINCI? II"
5.56 (d, J = 30.8 Hz, 1H), 4.83
N N-:-L-N (d, J = 5.2 Hz, 1H), 4.04 (s,
.,L..),., CN Co) 2H), 3.97 - 3.84 (m, 4H), 3.44 -
3.29 (m, 4H), 2.23 (d, J = 8.2
Hz, 2H), 2.02 - 1.77 (m, 6H)
N I (CDC13) 6 8.70 (d, J = 1.9 Hz,
N
0.0õ.0 0 1H), 8.64 (d, J= 1.9 Hz, 1H),
8.16 (s, 2H), 7.00 -6.84 (m,
HN 2H), 5.32 (s, 1H), 4.81 (s, 1H),
208 .1. N 435.6 4.03 (s, 1H), 3.96 - 3.85 (m,
N ' N 4H), 3.43 - 3.32 (m, 4H), 2.49
=L,,IJ Co) (q, J = 7.6 Hz, 2H), 2.21
(d, J =
8.8 Hz, 2H), 2.06 - 1.75 (m,
-.CH3 6H), 1.21 (t, J = 7.6 Hz, 3H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
N -1
I 1H), 8.63 (d, J = 1.9 Hz, 1H),
ecrO 0 N 8.18 (s, 2H), 7.01 -6.88 (m,
2H), 5.30 (d, J = 9.0 Hz, 1H),
HN 4.81 (d, J = 5.9 Hz, 1H), 4.02
209 .1. NN
N (s, 1H), 3.96 - 3.85 (m, 4H),
'
Co) 3.42 - 3.29 (m, 4H), 2.78 (p, J =
6.9 Hz, 1H), 2.31 -2.14 (m,
H3CCH3 2H), 2.01 - 1.83 (m, 6H), 1.25
(d, J = 6.9 Hz, 6H)
116
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, methanol-d4) 6 8.69
Nn (d, J = 2.0 Hz, 1H), 8.57 (d, J =
0 N 2.0 Hz, 1H), 7.92 (d, J = 6.2
Hz, 1H), 7.17 - 7.10 (m, 2H),
HNIC# Si 7.02 (d, J = 7.1 Hz, 1H), 6.90
210 450.17
N (d, J = 2.5 Hz, 1H), 4.93 (s,
I\1
rOH (o) 1H), 3.93 - 3.87 (m, 4H), 3.84 -
3.79 (m, 1H), 3.44 - 3.37 (m,
0 4H), 2.24 -2.15 (m, 2H), 1.97 -
1.82 (m, 6H)
Nn
0 N
0.9'0# 01
211 434.19
r.N
(31")
Nn
0 212 N
Oy'Cr Si
437.12
HN N N
T111 (a)
H3C'
Nn
0 N
Oyi:1T 0
213 434.15
HNY-' (N)
N
0
N --7)I (400 MHz, CDC13) 6 8.98 -
0 "Cr S N 8.83 (m, 1H), 8.79 - 8.67 (m,
1H), 8.64 - 8.50 (m, 1H), 8.35
HN (d, J = 9.2 Hz, 1H), 7.04 - 6.86
214 Ni N 474.12 (m, 2H), 6.75 (d, J= 9.0 Hz,
Ly Co) 2H), 4.78 (s, 1H), 4.00 - 3.79
(m, 5H), 3.42 - 3.21 (m, 4H),
N NH
2.17 - 2.11 (m, 2H), 2.02- 1.81
'
µN=NI (m, 6H)
117
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.70 (d, J = 1.9 Hz,
1H), 8.61 (d, J = 1.9 Hz, 1H),
1\11I 8.25 (d, J = 0.9 Hz, 1H), 6.93
(dd, J = 17.1, 2.5 Hz, 2H), 5.64
0
Cr
(d, J = 0.9 Hz, 1H), 5.00 (d, J =
215 HN
451.21 8.1 Hz, 1H)' 4.80 (dq, J = 5.5,
2.7 Hz, 1H), 4.33 (q, J = 7.1
Hz, 2H), 3.97 - 3.87 (m, 4H),
3.71 (s, 1H), 3.38 - 3.29 (m,
N 0 CH3 0 4H), 2.27 -2.14 (m, 2H), 2.03 -
1.80 (m, 6H), 1.37 (t, J = 7.1
Hz, 3H)
(CDC13) 6 8.69 (d, J = 1.9 Hz,
1H), 8.62 (d, J = 1.9 Hz, 1H),
6.95 (d, J = 2.5 Hz, 1H), 6.90
0
HN".-Cr (d, J = 2.5 Hz, 1H), 0.23 - 0.16
(m, OH), 4.93 (s, 1H), 4.76 (s,
216 481.26 1H), 4.19 -4.01 (m, 1H), 3.97 -
,
N 3.87 (m, 4H), 3.38 -3.29 (m,
H3CAyLS r,1\1..
,CH3 4H), 2.49 (s, 3H), 2.26 (s, 3H),
2.21 -2.10 (m, 2H), 2.05 (d, J =
CH3 1.8 Hz, 3H), 2.00 - 1.85 (m,
6H)
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.61 (d, J = 1.9
I Hz, 1H), 8.15 (d, J = 5.3 Hz,
0 Ali
HNJIIIIIIIIr
1H), 7.06 (dd, J = 5.4, 1.3 Hz,
1H), 7.00 (s, 1H), 6.95 (d, J
217 464.17 2.4 Hz, 1H), 6.91 (d, J = 2.4
Hz, 1H), 5.15 (s, 1H), 4.81 (d, J
= 2.5 Hz, 1H), 3.95 - 3.83 (m,
CH3 0 8H), 3.38 - 3.30 (m, 4H), 2.27 -
2.16 (m, 2H), 1.97 - 1.85 (m,
6H)
N-5)1 (CDC13) 6 8.70 (d, J = 2.0 Hz,
1H), 8.63 (d, J = 1.9 Hz, 1H),
HNicr.0 N
8.17 (d, J = 0.9 Hz, 1H), 7.02 -
=
6.85 (m, 2H), 5.40 (s, 1H), 5.05
218 (td, J = 1.8, 0.8 Hz, 2H), 4.91
N 4.73 (m, 3H), 4.05 (s, OH), 3.99
Co) - 3.85 (m, 4H), 3.43 - 3.27 (m,
4H), 2.30 - 2.12 (m, 2H), 2.02
1.82 (m, 6H)
118
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC11) 6 8.70 (d, J = 1.9 Hz,
V7)1 1H), 8.61 (d, J = 1.9 Hz, 1H),
0 Ati N
6.96 (d, J = 2.5 Hz, 1H), 6.89
(d, J = 2.5 Hz, 1H), 6.03 (s,
219 HNC'. WI 471.06
1H), 5.00 (s, 1H), 4.79 (s, 1H),
N 1\1 r,,i
3.92 (d, J = 9.3 Hz, 7H), 3.41 -
CIN0,0H3 La) 3.25 (m, 4H), 2.28 -2.13 (m,
2H), 1.92 (d, J = 18.6 Hz, 6H)
1\1..-.1I
O AI N (400 MHz, CDC13) 6 8.69
(s,
1H), 8.62 (s, 1H), 8.54 (s, 1H),
HNC( WI 8.21 (d, J = 4.9 Hz, 1H), 7.22 -
220 -%LN N 474.07 7.08 (m, 2H), 6.96 (s, 1H), 6.93
(s, 1H), 4.84 (s, 1H), 3.98 -
3.86 (m, 5H), 3.38 - 3.26 (m,
4H),2.31 - 2.19 (m, 2H), 2.03 -1\1' 0 1.86 (m, 6H)
i\l=i
(400 MHz, CDC13) 6 8.75 (d, J
= 1.9 Hz, 1H), 8.69 (d, J = 1.9
1\11I Hz, 1H), 8.61 (d, J = 1.9 Hz,
O dal N 1H), 8.39 (s, 1H), 8.05
(dd, J=
8.8, 2.3 Hz, 1H), 6.95 (d, J =
HNX). II" 2.4 Hz, 1H), 6.91 (d, J = 2.4
221 474.12
N''
1-0 1H), 5.23 (s, 1H), 4.88 - 4.71
Co)
I (m, 1H), 4.05 - 3.95 (m, 1H),
N--
N 3.95 -3.85 (m, 4H), 3.39 -3.26
(m, 4H), 2.28 - 2.16 (m, 2H),
2.00- 1.83 (m, 6H)
(CDC13) 6 8.63 (d, J = 1.9 Hz,
N--.I 1H), 8.53 (dd, J = 1.9, 0.6 Hz,
O idk N 1H),7.71 (d, J = 9.4 Hz,
1H),
6.89 (d, J = 2.4 Hz, 1H), 6.83
HN. C." II" (d, J = 2.5 Hz, 1H), 6.68 (d, J =
222 .), N N N 485.14 9.4 Hz, 1H), 5.33 (s, 1H),
4.76
L.. (o) (dq, J = 5.0, 2.5 Hz, 1H), 4.14
(s, 1H), 3.93 - 3.77 (m, 4H),
\----N1 3.28 (d, J = 5.1 Hz, 7H), 2.18
bH3 (dt, J = 13.4, 4.6 Hz, 2H), 2.02
- 1.75 (m, 6H)
119
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC11) 6 8.71 (d, J = 1.9 Hz,
NI 1H), 8.61 (d, J = 1.9 Hz, 1H),
0 223 HN1Cf 42.11 N 6.94 (dd, J = 15.2, 2.5 Hz, 2H),
6.07 (s, 1H), 4.82 (dt, J = 5.8,
II" 435.18 3.0 Hz, 1H), 4.02 - 3.83 (m,
r, N. 4H), 3.77 - 3.57 (m, 1H), 3.43 -
NF
L I
--N.,.,..CH3 L.,o) 3.28 (m, 4H), 2.52 (s, 3H), 2.37
(s, 3H), 2.30 -2.16 (m, 2H),
2.05 - 1.78 (m, 6H)
(CDC13) 6 8.72 (d, J = 1.9 Hz,
NII 1H), 8.63 (d, J = 1.9 Hz, 1H),
0
00. I. N 8.27 (s, 1H), 6.98 (d, J = 2.4
224 HN
Hz, 1H), 6.92 (d, J = 2.6 Hz,
475.02 1H), 6.44 (d, J = 6.0 Hz, 1H),
N" CH N 4.84 (s, 1H), 3.93 (dd, J = 6.0,
k=-.'", 3 )
js, I 3.7 Hz, 4H), 3.43 - 3.25 (m,
H3C"N CH3 co 4H), 2.23 (s, 2H), 2.08 - 1.83
(m, 6H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
1H), 8.62 (d, J = 1.9 Hz, 1H),
NiI 8.49 (d, J = 1.1 Hz, 1H), 6.93
0 At, N
(dd, J= 16.7, 2.5 Hz, 2H), 6.45
(d, J = 1.2 Hz, 1H), 5.01 (s,
225 HN*" . iii" 451.16
1H), 4.81 (s, 1H), 4.39 (d, J =
1\1":'= CH 3 r'N)
0.9 Hz, 2H), 3.97 - 3.87 (m,
4H), 3.49 (s, 3H), 3.39 - 3.29
.1\l'N-C1 0> (m, 4H), 2.22 (d, J = 9.4 Hz,
2H), 1.99 - 1.87 (m, 6H)
(CDC13) 6 8.70 (d, J = 2.0 Hz,
NiI 1H), 8.63 (d, J = 1.9 Hz, 1H),
6.95 (d, J =2.5 Hz, 1H), 6.91
o &hi N
(d, J = 2.5 Hz, 1H), 4.75 (d, J =
226 HNl
5.6 Hz, 1H), 4.66 (s, 1H), 4.32
ia iii.j 463.18
(s, 1H), 4.00 - 3.83 (m, 4H),
N
Co) 3.43 - 3.22 (m, 4H), 2.46 (d, J =
_.,L-, , 15.1 Hz, 5H), 2.36 (s, 3H), 2.19
F3C N- (q, J = 6.3, 3.9 Hz, 2H), 1.12
(t,
J = 7.6 Hz, 3H)
120
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.72 (d, J = 1.9 Hz,
1H), 8.64 (d, J = 1.9 Hz, 1H),
NN.)I 8.33 (d, J = 1.8 Hz, 1H), 7.04 -
0 227 HNIC). A ..,1 N 6.87 (m, 2H), 5.15 (s, 1H), 4.82
(dq, J = 5.2, 2.6 Hz, 1H), 4.24
II" 453.2 (dt, J = 8.3, 4.7 Hz, 1H), 4.03
-
N ,.N. 3.86 (m, 4H), 3.44 - 3.28 (m,
1 4H), 2.74 (qd, J = 7.6, 2.3 Hz,
H3C N CH3 '101' 2H), 2.24 (dq, J = 9.6, 4.6 Hz,
2H), 2.09 - 1.85 (m, 6H), 1.29
(t, J = 7.6 Hz, 3H)
N-IIN (CDC13) 6 8.60 (dd, J = 8.1, 1.9
H NeCi
0 46 Hz, 1H), 8.54 (d, J = 1.9 Hz,
1H), 8.00 (s, 1H), 6.92 - 6.75
11411 (m, 2H), 5.06 (d, J = 8.0 Hz,
228 N N 462.14 1H), 4.70 (s, 1H), 3.94 (s, 1H),
.
' I (0) 3.89 - 3.76 (m, 4H), 3.75 - 3.62
1\11,- (m, 4H), 3.34 - 3.17 (m, 4H),
0.:-....s 2.50 (s, 3H), 2.11 (s, 2H), 1.82
0li'CH3 (d, J = 5.0 Hz, 5H)
(400 MHz, CDC13) 6 8.70 (d, J
N% = 1.9 Hz, 1H), 8.62 (d, J = 1.9
0
f3? 0 Hz, 1H), 8.44 (d, J = 2.0 Hz,
HN
1H), 7.91 (d, .1= 8.8 Hz, 1H),
6.97 (d, J = 2.4 Hz, 1H), 6.92
229 N'i".'`i N 488.57 (d, J = 2.4 Hz, 1H), 6.60 (d, J =
it. co) 8.6 Hz, 1H), 5.52 (s, 1H), 4.83
(s, 1H), 4.18 (s, 3H), 4.00 -
NN -CH3
3.87 (m, 5H), 3.39 - 3.24 (m,
."
N=INI 4H), 2.31 -2.18 (m, 2H), 2.04-
1.87 (m, 6H)
N I
(400 MHz, CDC13) 6 8.81 (d, J
--5.
0 Aih, N = 1.8 Hz, 1H), 8.69 (d, J = 1.9
Hz, 1H), 8.61 (d, J = 1.9 Hz,
HNIXC:i. II" 1H), 8.13 (dd, J = 8.8, 2.2 Hz,
N 1H), 6.95 (d, J = 2.4 Hz, 1H),
230 N
(o) 488.57 6.92 (d, J = 2.4 Hz, 1H), 6.52
(d, J = 8.8 Hz, 1H), 4.82 (s,
1H), 4.36 (s, 3H), 3.96 - 3.85
N "N (m, 5H), 3.41 - 3.28 (m, 4H),
µ11-1\1 2.28 -2.17 (m, 2H), 2.06 - 1.83
NCH3 (m, 6H)
121
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC11) 6 8.70 (d, J = 1.9 Hz,
NI 1H), 8.62 (d, J = 2.0 Hz, 1H),
0
elivi4 I. N 7.70 (d, J = 3.4 Hz, 1H), 6.93
HN
(dd, J = 14.6, 2.5 Hz, 2H), 4.96
(dd, J = 17.8, 7.1 Hz, 2H), 4.76
231 .1.
N N N 510.2 (d, J = 6.2 Hz, 1H), 4.62 (ddt,
J
y., (a) = 12.6, 7.1, 3.5 Hz, 1H), 4.05-
NH
3.82 (m, 8H), 3.75 (dd, J = 9.4,
3.2 Hz, 1H), 3.40 - 3.27 (m,
F o
4H), 2.35 (ddt, J = 13.0, 8.2,
0 7.1 Hz, 1H), 2.25 -2.08 (m,
2H), 2.00 - 1.77 (m, 6H)
(CDC13) 6 8.61 (d, J = 1.9 Hz,
NI 1H), 8.54 (d, J = 1.9 Hz, 1H),
0 gal N 7.98 (s, 2H), 6.94 - 6.75 (m,
2H), 5.01 (s, 1H), 4.71 (d, J =
HNICI? 11" 5.9 Hz, 1H), 3.96 - 3.78 (m,
477.18 232 5H), 3.68 (d, J = 7.0 Hz, 2H),
NN C N o) 3.37 - 3.17 (m, 4H), 2.21 - 1.96
y (m, 2H), 1.92 - 1.75 (m, 6H),
1.25 - 1.06 (m, 1H), 0.66 - 0.44
(m, 2H), 0.34 - 0.18 (m, 2H)
NI (CDC13) 6 8.70 (d, J = 1.9 Hz,
0 nal N 1H), 8.63 (d, J = 1.9 Hz, 1H),
HNea WI
8.06 (s, 2H), 7.01 - 6.86 (m,
2H), 5.00 (d, J = 8.1 Hz, 1H),
.
233
.1. N 451.2 4.80 (d, J = 5.6 Hz, 1H), 4.10 -
N --/ N 3.82 (m, 7H), 3.42 - 3.26 (m,
y co) 5H), 2.20 (d, J = 8.2 Hz, 2H),
2.01 - 1.80 (m, 6H), 1.39 (t, J =
0CH3 7.0 Hz, 3H)
(CDC13) 6 8.72 (d, J = 1.9 Hz,
1H), 8.60 (t, J = 1.9 Hz, 2H),
0 Arb N
6.97 (d, J = 2.5 Hz, 1H), 6.91
(d, J = 2.5 Hz, 1H), 6.70 (d, J =
234 HNICI? 11" 432.17
1.2 Hz, 1H), 4.85 (d, J = 4.9
N
1\1 Hz, 1H), 3.98 - 3.84 (m, 4H),
k, Co) 3.42 - 3.26 (m, 4H), 2.33 - 2.17
N CN (m, 2H), 2.01 - 1.77 (m, 5H)
122
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
N)I (CDC13) 6 8.68 (d, J = 2.0 Hz,
O All N 1H), 8.59 (d, J = 1.9
Hz, 1H),
8.36 (s, 2H), 6.97 - 6.86 (m,
HNICI4 II"
235 N 2H), 5.53 (d, J = 8.1 Hz, 1H),
.1
4.96 - 4.72 (m, 3H), 4.09 - 3.86
'..N N 505.04 (o)
(m, 5H), 3.33 (dd, J = 5.8, 4.0
Hz, 4H), 2.28 - 2.10 (m, 2H),
H 0C F3 1.98 - 1.78 (m, 6H)
`v
N.1I (CDC13) 6 8.68 (d, J = 2.0 Hz,
O Ali N 1H), 8.59 (d, J = 1.9
Hz, 1H),
8.36 (s, 2H), 6.97 - 6.86 (m,
HNiCr lir 2H), 5.53 (d, J = 8.1 Hz, 1H),
236 .1. NN N 505.17
4.96 -4.72 (m, 3H), 4.09 - 3.86
' (oD
(m, 5H), 3.33 (dd, J = 5.8, 4.0
Hz, 4H), 2.28 - 2.10 (m, 2H),
HO..."C F3 1.98 - 1.78 (m, 6H)
N II (CDC13) 6 8.69 (d, J = 1.9
Hz,
O d" N 1H), 8.61 (d, J = 1.9 Hz,
1H),
8.31 (s, 2H), 6.99 -6.87 (m,
HNCra Sri 2H), 5.34 (d, J = 8.1 Hz, 1H),
237 .1. N 451.16 4.87 -4.73 (m, 2H), 4.06 -3.87
N ' N (m, 6H), 3.38 - 3.29 (m, 4H),
Co) 2.20 (q, J = 5.8 Hz, 2H), 2.04 -
1.84 (m, 6H), 1.51 (d, J = 6.5
HOCH3 Hz, 3H)
(CDC13) 6 8.71 (d, J = 1.9 Hz,
1H), 8.64 (d, J = 1.9 Hz, 1H),
N 8.15 (s, 2H), 6.99 -6.86 (m,
N 0 dik 2H),5.11 (td, J = 7.9, 7.4, 3.8
Hz, 1H), 4.82 - 4.55 (m, 3H),
1-11\119 µ II" 4.10 (dd, J = 4.1, 2.7 Hz, 2H),
238
NN
.1. N 492.98
3.93 (dd, J = 6.0, 3.7 Hz, 5H),
'
y co) 3.60 -3.43 (m, 1H), 3.36 (dd, J
= 6.0, 3.7 Hz, 4H), 2.85 - 2.61
OC2, (m, 1H), 2.30 - 2.10 (m, 2H),
1.92 (s, 6H), 1.24 (q, J = 6.9
Hz, 1H)
123
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
N''''N.I (CDC13) 6 8.61 (d, J = 1.9 Hz,
0 All N
1H), 8.54 (d, J = 1.9 Hz, 1H),
HNC? II"
7.95 (d, J = 13.9 Hz, 2H), 6.92
'
239 .).. N 507 - 6.72 (m, 2H), 4.85 - 4.64 (m,
N N 3H), 4.41 (t, J = 6.2 Hz, 2H),
y co) 3.83 (q, J = 6.7, 5.7 Hz, 6H),
3.33 -3.18 (m, 5H), 2.20 - 1.97
0.C\O (m, 1H), 1.82 (s, 7H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
N '1I 1H), 8.63 (d, J = 1.9 Hz, 1H),
O gil N 8.08 (s, 2H), 7.01 -
6.87 (m,
2H), 5.11 (d, J = 8.1 Hz, 1H),
HNC". II" 4.95 - 4.74 (m, 3H), 4.56 (t, J =
240 493.16
NN
Hz, 2H), 4.05 - 3.81 (m, 4H),
3.51 -3.29 (m, 5H), 2.21 (q, J =
0 6.4, 5.7 Hz, 2H), 2.03 - 1.80
(m, 8H)
(400 MHz, CDC13) 6 8.69 (d, J
N-'I = 1.9 Hz, 1H), 8.61 (d, J = 1.9
O All N Hz, 1H), 7.52 (d, J =
8.7 Hz,
NV .
1H), 6.96 (d, J = 2.5 Hz, 1H),
I.) 6.90 (d, J = 2.5 Hz, 1H), 6.23
241 445.54
N (d, J = 8.7 Hz, 1H), 5.09 (s,
,...r.ii,
C0
) 1H), 4.80 (s, 1H), 4.00 - 3.78
CH3
(m, 5H), 3.40 - 3.24 (m, 4H),
ON 2.56 (s, 3H), 2.29 -2.14 (m,
2H), 2.01 - 1.80 (m, 6H)
(400 MHz, CDC13) 6 8.70 (d, J
N- NI = 1.8 Hz, 1H), 8.62 (d, J = 1.9
HNICI.
O Ali Hz, 1H), 8.30 (d, J = 1.8
Hz,
1H), 7.37 (s, 1H), 6.96 (d, J --
IIW 2.3 Hz, 1H), 6.91 (d, J = 2.3
242 445.54
Nj.,...,CH3 (1\10) 1 Hz, 1H), 4.79 (s, 1H), 4.74 (s,
1H), 4.28 (s, 1H), 4.01 - 3.83
L. (m, 4H), 3.40 - 3.25 (m, 4H),
ON 2.30 - 2.17 (m, 2H), 2.11 (s,
3H), 1.99 - 1.87 (m, 6H)
124
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.69 (d, J = 2.0 Hz,
1
N
0 All 1H), 8.62 (d, J = 1.9 Hz, 1H),
5.36 (s, 1H), 4.03 (s, 1H), 1.57
HN1Cr# II" (s, 6H), 2.02 - 1.80 (m, 6H),
243 ..) N 465.2 8.42 (s, 2H), 6.99 - 6.87 (m,
N '.. N (o) 2H), 4.80 (s, 1H), 3.92 (dd, J =
....) 6.0, 3.7 Hz, 4H), 3.39 - 3.29
CH3 (m, 4H), 2.20 (d, J = 9.0 Hz,
HO CH3 2H)
N -I (CDC13) 6 8.98 - 8.86 (m, 2H),
0 Ali N 8.66 (dd, J = 23.8, 1.9 Hz, 2H),
8.44 (s, 1H), 7.00 - 6.88 (m,
H NI*113 . iiir 2H), 5.89 (d, J = 8.1 Hz, 1H),
244
N ' N N
475.15 4.83 (dq, J = 5.4, 2.6 Hz, 1H),
(a)
4.22 - 4.04 (m, 1H), 3.97 - 3.87
(m, 4H), 3.43 - 3.29 (m, 4H),
rkN 2.24 (td, J = 8.3, 7.4, 4.0 Hz, ,
0
\=Iµj 2H), 2.15 - 1.81 (m, 6H)
N (CDC13) 6 8.61 (d, J = 1.9 Hz,
I 1H), 8.53 (d, J = 1.9 Hz, 1H),
0 Ail N
6.84 (dd, J = 18.2, 2.5 Hz, 2H),
HNICI. Illri
5.26 (s, 1H), 4.78 (d, J = 8.0
245 467.14 Hz, 1H), 4.68 (d, J = 5.6 Hz,
r N,)
NI 1H), 3.82 (t, J = 4.0 Hz, 10H),
9)-k..Ni-0,CH3 L0) 3.30 -3.15 (m, 4H), 2.10 (q, J =
6.2, 5.7 Hz, 2H), 1.94 - 1.69
CH3 (m, 6H)
(CDC13) 6 8.61 (d, J = 1.9 Hz,
N'iI 1H), 8.54 (d, J = 1.9 Hz, 1H),
0 id& N 8.06 (d, J = 5.0 Hz, 1H), 6.94 -
6.78 (m, 2H), 6.32 (d, J = 5.0
246 HNia# II" 421.23 Hz, 1H), 5.09 (s, 1H), 4.70 (d, J
NN N = 6.0 Hz, 1H), 3.95 (s, 1H),
3.91 -3.77 (m, 5H), 3.37 -3.13
CH3 0 (m, 4H), 2.20 - 2.00 (m, 2H),
1.94- 1.71 (m, 6H)
125
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
N''I (CDC13) 6 8.71 (d, J = 1.9 Hz,
0 HNI.Ci* = N 1H), 8.64 (t, J = 2.4 Hz, 1H),
8.05 (s, 2H), 7.04 - 6.87 (m,
2H), 5.20 (s, 1H), 4.80 (s, 1H),
247 ..
N ' N N 465.1 4.30 (p, J = 6.1 Hz, 1H), 4.05 _
y Co) 3.81 (m, 4H), 3.44 - 3.27 (m,
4H), 2.22 (t, J = 7.3 Hz, 2H),
0..,,,CH3 1.93 (d, J = 4.6 Hz, 6H), 1.33
I (d, J = 6.1 Hz, 6H)
CH3
Nn
I (400 MHz, CDC13) 6 8.69 (d, J
0 N = 1.9 Hz, 1H), 8.66 (d, J = 1.9
Hz, 1H), 8.62 (d, J = 1.9 Hz,
HN1 ' . 1H), 8.05 (d, J = 8.5 Hz, 1H),
248
N 6.96 (d, J = 2.4 Hz, 1H), 6.92
N 'i
488.48
(d, J = 2.5 Hz, 1H), 6.51 (d, J =
8.7 Hz, 1H), 4.82 (s, 1H), 3.99
ON
-3.85 (m, 5H), 3.38 - 3.27 (m,
'
)=I 4H), 2.59 (s, 3H), 2.29 - 2.18
H3C (m, 2H), 2.01 - 1.84 (m, 6H)
(400 MHz, CDC13) 6 8.69 (d, J
Crio 11101NIN = 1.9 Hz, 1H), 8.61 (d, .1= 1.9
Hz, 1H), 8.45 (d, J = 1.9 Hz,
1H), 7.86 (d, J = 7.7 Hz, 1H),
HN 7.60 (d, J = 2.3 Hz, 1H), 6.96
249 I\I, N 472.54 (d, J = 2.4 Hz, 1H), 6.92 (d, J =
2.4 Hz, 1H), 6.50 (d, J = 2.3
Hz, 1H), 6.48 (d, J = 8.9 Hz,
1H), 4.81 (s, 1H), 3.95 - 3.85
, NH (m, 5H), 3.39 - 3.30 (m, 4H),
-IV 2.26 -2.17 (m, 2H), 1.99 - 1.84
(m, 6H)
126
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.69 (d, J
N-NiI = 1.9 Hz, 1H), 8.61 (d, J = 1.9
0 HN1 . All N Hz, 1H), 8.09 (d, J = 1.7 Hz,
1H), 7.56 - 7.45 (m, 2H), 6.96
II" (d, J = 2.4 Hz, 1H), 6.93 (d, J =
250 Ni N 486.5 2.4 Hz, 1H), 6.55 (d, J = 8.5
Hz, 1H), 6.25 (d, J = 1.9 Hz,
1H), 4.84 (s, 1H), 3.96 - 3.83
N -CH3
(m, 8H), 3.39 - 3.30 (m, 4H),
.,,, N, 2.28 -2.19 (m, 2H), 2.03 - 1.83
-
(m, 6H)
N-ThI (CDC13) 6 8.71 (d, J = 1.9 Hz,
o rail N 1H), 8.63 (d, J = 1.9 Hz, 1H),
HNeCi
8.18 (s, 2H), 7.04 -6.87 (m,
. lµF 2H), 5.21 (d, J = 8.2 Hz, 1H),
251 N--J.N N 4.80 (s, 1H), 4.2-4.0 (m, 3H),
' (o) 3.98 -3.85 (m, 4H), 3.61 -3.44
491.1
(m, 2H), 3.41 - 3.21 (m, 4H),
2.75 - 2.47 (m, 1H), 2.21 (d, J =
0 9.6 Hz, 2H), 1.92 (t, J = 6.4 Hz,
6H), 1.83- 1.66 (m, 4H)
0
(CDC13) 6 3.97 - 3.87 (m, 4H),
Nni 3.39 - 3.29 (m, 4H), 2.50 (s,
N 3H), 2.28 -2.16 (m, 2H), 2.02 -
1.89 (m, 6H), 4.26 -4.17 (m,
HNICI# lir 1H), 8.70 (d, J = 1.9 Hz, 1H),
252 .L,F N 471.1
N
8.63 (d, J = 1.9 Hz, 1H), 7.88
"- 1
S
.).. N Co) (d, J = 3.3 Hz, 1H), 6.93 (dd, J
= 18.7, 2.5 Hz, 2H), 5.10 (d, J
CH3 = 7.9 Hz, 1H), 4.79 (d, J = 3.7
Hz, 1H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
1H), 8.61 (d, .1= 2.0 Hz, 1H),
1\1.1I 8.38 (d, J= 1.1 Hz, 1H), 6.93
0 ig6 N (dd, J= 17.3, 2.5 Hz, 2H), 6.18
(d, J = 1.2 Hz, 1H), 5.03 (s,
253 H Nr.a# III" 453.19 1H), 4.81 (d, J = 6.1 Hz, 1H),
N N 3.97 - 3.87 (m, 4H), 3.83 (s,
-..j.-.
1H), 3.39 - 3.29 (m, 4H), 2.51
L.NkSCH3 ' LO'j (s, 3H), 2.21 (d, J = 8.8 Hz,
2H), 2.05 (s, OH), 1.91 (d, J =
10.6 Hz, 6H)
127
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.71 (d, J
= 1.9 Hz, 1H), 8.63 (d, J = 1.9
kiI Hz, 1H), 8.15 - 8.04 (m, 1H),
0 Ali N 7.46 - 7.39 (m, 1H), 6.97 (d, J =
2.5 Hz, 1H), 6.93 (d, J = 2.5
254 HNC'. MI 406.57 Hz, 1H), 6.60 - 6.52 (m, 1H),
1\1 N 6.41 (d, J = 8.4 Hz, 1H), 4.80
-jNi
Co) (s, 1H), 4.68 (s, 1H), 3.98 -
3.85 (m, 5H), 3.41 -3.30 (m,
4H), 2.27 -2.15 (m, 2H), 1.97 -
1.86 (m, 6H)
N-7)I (methanol-d4) 6 8.71 (dd, J =
0 46 N 7.5, 2.1 Hz, 1H), 8.57 (dd, J =
8.8, 2.1 Hz, 1H), 7.12 (d, J=
HN9.a 111" 2.5 Hz, 1H), 6.88 (d, J = 2.4
255 450.96
HN-1'.'-,
,-= Co) 4H), 3.45 - 3.35 (m, 4H), 3.19
0 N 0 (s, 3H), 2.25 - 2.01 (m, 2H),
613 2.00 - 1.80 (m, 6H)
(CDC13) 6 8.71 (d, J = 1.9 Hz,
N"I 1H), 8.64 (d, J = 1.9 Hz, 1H),
0 HNiCiii.,.
. I.) N
7.80(d, J= 1.5 Hz, 1H), 7.47
(d, J = 1.5 Hz, 1H), 7.02 - 6.86
i
256 420.1 (m, 2H), 4.80 (s, 1H), 4.27 (d,
J
N
= 8.3 Hz, 1H), 4.00 - 3.72 (m,
y co) 7H), 3.35 (dd, J = 6.0, 3.8 Hz,
4H), 2.23 (s, 2H), 2.02 - 1.80
CH3 (m, 6H)
NI (CDC13) 6 8.71 (d, J = 1.9 Hz,
N 0 AI 1H), 8.63 (dd, J = 1.9, 0.7 Hz,
HNICr
1H), 7.99 - 7.88 (m, 1H), 7.02 -
11" 6.87 (m, 2H), 6.34 (d, J = 8.4
257 N 437.1 Hz, 1H), 4.78 (s, 1H), 4.40 (d,
J
N Co) = 8.2 Hz, 1H), 4.03 - 3.75 (m,
5H), 3.35 (dd, J = 6.0, 3.7 Hz,
4H), 2.18 (s, 5H), 2.03 - 1.81
0'CH3 (m, 6H)
128
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
N.-5.)I
0 &.,i N (CDC13) 6 8.78 - 8.59 (m, 3H),
8.38 (s, 1H), 7.85 (s, 1H), 6.95
HNICXµ II" (d, J = 2.4 Hz, 1H), 6.91 (d, J =
2.3 Hz, 1H), 4.79 (s, 1H), 4.67
258 NH3 N 488.52
y Co) (d, J = 7.6 Hz, 1H), 4.32 (s,
1H), 3.99 - 3.82 (m, 4H), 3.40 -
3.27 (m, 4H), 2.33 -2.06 (m,
NO 5H), 2.03 - 1.87 (m, 6H)
N=i
(CDC13) 6 8.70 (d, J = 1.9 Hz,
0 a ..si N 1H), 8.62 (d, 1H), 8.41 (s, 1H),
8.04 - 7.93 (m, 1H), 6.96 (d, J =
HNCr II" 2.5 Hz, 1H), 6.91 (d, J = 2.1
259 N 488.48 Hz, 1H), 6.35 (d, J = 9.1 Hz,
''L'` .N.
:1 1H), 5.07 (s, 1H), 4.82 (s, 1H),
H3C N''0 4.02 - 3.79 (m, 5H), 3.41 - 3.27
(m, 4H), 2.83 - 2.72 (m, 3H),
N' 0 2.30 - 2.17 (m, 2H), 2.02- 1.80
N=i (m, 6H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
N 1H), 8.62 (d, J = 1.9 Hz, 1H),
N 0 mil 6.93 (dd, J = 14.5, 2.5 Hz, 2H),
6.79 (d, J = 9.4 Hz, 1H), 6.64
HNCid* WI (d, J = 9.4 Hz, 1H), 4.80 (d, J --
260 N 437.24 5.5 Hz, 1H), 4.27 (d, J = 7.5
N*C Hz, 1H), 4.14 (s, 1H), 4.02 (s,
I\1.( 3H), 3.98 - 3.88 (m, 4H), 3.42 -
3.29 (m, 4H), 2.31 -2.12 (m,
0 r_...i_,
..\, .3 2H), 1.95 (ddd, J = 17.2, 9.2,
6.0 Hz, 6H)
N*-')I (CDC13) 6 8.71 (d, J = 1.9 Hz,
N 0
=fCD? I. 1H), 8.64 (d, J = 1.9 Hz, 1H),
HN
8.20 (s, 2H), 7.04 - 6.84 (m,
2H), 5.16 (d, J = 8.0 Hz, 1H),
261 .1.
NV N N 477.3 4.80 (s, 1H), 4.19 - 3.85 (m,
LO Co) 7H), 3.66 (dd, J = 8.5, 7.1 Hz,
1H), 3.35 (dd, J = 5.9, 3.8 Hz,
4H), 3.24 (t, J = 7.5 Hz, 1H),
2.48 - 2.11 (m, 4H), 2.05 -1.82
C70 (m, 6H)
129
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.69 (d, J = 1.9 Hz,
N*-I 1H), 8.62 (d, J = 1.9 Hz, 1H),
0 Ail N 7.61 (d, J = 1.0 Hz, 1H), 6.98 -
HNC6.86 (m, 2H), 4.96 (s, 1H), 4.82
.... WI -4.71 (m, 1H), 4.63 - 4.56 (m,
262
N'-'1'N-'CH3 N 476.26 1H), 4.08 - 3.87 (m, 5H), 3.38 -
,.I. j. CoJ 3.29 (m, 4H), 2.80 (tdd, J = 6.8,
5.0,3.1 Hz, 1H),2.23 -2.11
HN N
A (m, 2H), 2.08 - 1.82 (m, 9H),
0.90 - 0.72 (m, 2H), 0.59 - 0.47
(m, 2H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
1H), 8.61 (d, J = 1.9 Hz, 1H),
N'7)I 8.11 (d, J = 0.9 Hz, 1H), 6.93
0 Awl N (dd, J = 15.5, 2.5 Hz, 2H), 5.30
(d, J = 1.0 Hz, 1H), 5.16 (s,
HN"Cf WI 1H), 4.95 (s, 1H), -0.17 - -0.23
263 N 480.22
LI\I (m, OH), 4.80 (s, 1H), 3.92 (dd,
HN.^:N) (o) J = 5.9, 3.7 Hz, 4H), 3.74 (s,
1H), 3.57 (dd, J = 5.6, 4.6 Hz,
2H), 3.50 - 3.29 (m, 9H), 2.20
..,..3
(d, J = 9.1 Hz, 2H), 1.93 (d, J =
13.1 Hz, 6H)
(CDC13) 6 8.69 (d, J = 1.9 Hz,
N ''N1I 1H), 8.62 (d, J = 2.0 Hz, 1H),
0 riik N 7.76 (d, J = 5.8 Hz, 1H), 7.02 -
6.86 (m, 2H), 5.66 (d, J = 5.9
HN C... WI Hz, 1H), 4.80 (d, J = 5.5 Hz,
264 N -1,N N 494.1 1H), 4.63 (s, 1H), 3.97 - 3.84
'
Co) (m, 5H), 1.30 - 1.20 (m, 1H),
HN 3.64 (s, 2H), 3.38 - 3.28 (m,
H3C4,..,OH 4H), 1.37 (s, 6H), 5.16 - 4.86
H3C (m, 1H), 2.24 - 2.13 (m, 2H),
1.96- 1.82 (m, 6H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
N ='iI 1H), 8.61 (d, J = 1.9 Hz, 1H),
8.47 (d, J= 1.1 Hz, 1H), 6.93
0
Cr 0 N
421.18 (dd, J = 16.7, 2.5 Hz, 2H), 6.17
265 HN
(t, J = 0.9 Hz, 1H), 4.95 (s, 1H),
4.81 (td, J = 5.3, 2.5 Hz, 1H),
r
N N 4.07 - 3.83 (m, 5H), 3.39 - 3.29
L I (m, 4H), 2.34 (s, 3H), 2.21 (dt,
'N'CH3 C)) J= 11.1, 5.1 Hz, 2H), 2.04 -
1.76 (m, 6H)
130
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC11) 6 11.49 (s, 1H), 8.69
NiI (d, J = 1.9 Hz, 1H), 8.59 (d, J =
0
r
1.9 Hz, 1H), 7.73 (d, J = 3.0
HNC 110 N
Hz, 1H), 7.03 - 6.89 (m, 2H),
266
.1. N 441.2 6.56 (d, J = 7.2 Hz, 1H), 4.82
N NH Co) (s, 1H), 3.91 (dd, J = 6.0, 3.8
Hz, 5H), 3.39 - 3.29 (m, 4H),
0 2.27 -2.14 (m, 2H), 2.07 - 1.81
F (m, 6H)
(CDC13) 6 8.70 (d, J = 1.9 Hz,
1H), 8.60 (d, J = 1.9 Hz, 1H),
N'I 8.03 (d, J = 6.1 Hz, 1H), 6.93
0 267 HNia." rial N (dd, J= 18.6, 2.5 Hz, 2H), 6.13
(d, J = 6.1 Hz, 1H), 5.09 (s,
11" 447.11 1H), 4.79 (s, 1H), 4.03 -3.78
!j N N (m, 5H), 3.34 (dd, J = 6.0, 3.8
L*.
Hz, 4H), 2.20 (d, J = 8.1 Hz,
2H), 1.96 (s, 7H), 1.10 (d, J =
2.8 Hz, 2H), 1.00 (d, J = 8.0
Hz, 2H)
(CDC13) 6 8.69 (d, J = 1.9 Hz,
N')I 1H), 8.60 (d, J= 1.9 Hz, 1H),
0 All N 7.95 (d, J = 5.8 Hz, 1H), 6.92
268 HNC?
(dd, J = 17.9, 2.5 Hz, 2H), 5.99
Ili) 437.19 (d, J = 5.9 Hz, 1H), 5.01 (s,
N
<N .1 1H), 4.79 (dt, J = 6.9, 3.4 Hz,
,=%"L 1H), 3.91 (d, J = 8.7 Hz, 8H),
:=,.NA.,0õ.CH3 L.,o) 3.43 - 3.25 (m, 4H), 2.32 -2.09
(m, 2H), 2.05 - 1.74 (m, 6H)
(CDC13) 6 8.69 (d, J = 1.9 Hz,
NI 1H), 8.62 (d, J = 1.9 Hz, 1H),
0 11 N 7.70 (d, J = 6.8 Hz, 1H), 6.92
(dd, J = 14.4, 2.5 Hz, 2H), 4.86
HNCra WI (d, J = 8.1 Hz, 1H), 4.75 (dt, J
269
NN .1. r,,N,) 468.13
= 8.6, 4.0 Hz, 1H), 3.97 - 3.87
-
y,N-CH3 La) (m, 5H), 3.38 - 3.28 (m, 4H),
3.14 (d, J = 2.2 Hz, 6H), 2.24 -
F 61-13 2.07 (m, 2H), 2.02 - 1.79 (m,
6H)
131
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
N-5..)I (CDC13) 6 8.71 (d, J = 1.9 Hz,
0 1H), 8.64 (d, J = 2.0 Hz, 1H),
HI\l'fa? N
8.15 (s, 2H), 7.01 -6.87 (m,
.. N 2H), 5.13 (d, J= 8.1 Hz, 1H),
N N 4.80 (s, 1H), 4.24 (s, 1H), 4.01
270 Lj Co) (s, 2H), 3.93 (dd, J = 6.0, 3.7
Hz, 4H), 3.35 (dd, J = 5.9, 3.9
Q CH3 Hz, 4H), 2.80 (t, J = 12.7 Hz,
2H),2.51 (t, J = 12.1 Hz, 1H),
2.21 (d, J = 8.3 Hz, 2H), 2.05 -
.,.. A-CH3
1.72 (m, 8H), 1.49 (s, 9H)
(CDC13) 6 8.62 (d, J = 1.9 Hz,
NN-II 1H), 8.55 (d, J = 1.9 Hz, 1H),
0 Ali N 8.09 (s, 2H), 6.93 - 6.78 (m,
2H), 5.03 (d, J = 8.1 Hz, 1H),
HNI9C. 111" 4.72 (d, J = 5.8 Hz, 1H), 4.02 -
.1. 271 I\1N N 3.75 (m, 5H), 3.34 - 3.21 (m,
.' 490.2
Co) 4H), 3.20 - 3.06 (m, 2H), 2.66
(td, J = 12.1, 2.5 Hz, 2H), 2.38
n (ddt, J = 12.2, 7.6, 3.8 Hz, 1H),
2.12 (d, J = 8.9 Hz, 2H), 1.96 -
N 1.65 (m, 10H), 1.53 (cid, J =
H
12.3, 3.9 Hz, 2H)
(CDC13) 6 8.69 (d, J = 1.9 Hz,
1H), 8.61 (d, J = 1.9 Hz, 1H),
NI 8.04 (d, J = 2.8 Hz, 1H), 7.94
0
ea' 0 N
406.53 (dd, J = 4.7, 1.3 Hz, 1H), 7.11
272 HN
(dd, J = 8.3, 4.7 Hz, 1H), 7.00 -
6.84 (m, 3H), 4.78 (s, 1H), 3.98
CN) - 3.88 (m, 4H), 3.85 (d, J = 8.1
o
Hz, 1H), 3.38 - 3.28 (m, 4H),
2.27 -2.15 (m, 2H), 1.97 - 1.84
(m, 6H)
132
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC1.3) 6 8.70 (d, J = 1.9 Hz,
1H), 8.61 (d, J = 1.9 Hz, 1H),
NI 8.18 (d, J = 6.3 Hz, 2H), 6.96
0 dal N
(d, J = 2.4 Hz, 1H), 6.90 (d, J =
2.4 Hz, 1H), 6.51 - 6.42 (m,
273 HNICI III 406.57
2H), 4.79 (s, 1H), 4.47 (d, J -
N 7.7 Hz, 1H), 3.98 - 3.86 (m,
.-/k-
Co) 4H), 3.56 (s, 1H), 3.39 - 3.26
-k-N.-
(m, 4H), 2.27 - 2.15 (m, 2H),
2.01 - 1.79 (m, 6H)
N-5N.NiI
0 iikb, N (CDC13) 6 8.71 (d, J = 1.9 Hz,
HNIC)." II" 8.18 (s, 2H), 7.03 - 6.86 (m,
1H), 8.63 (d, J = 1.9 Hz, 1H),
.L.. N N
N 2H), 5.11 (d, J= 8.1 Hz, 1H),
'
274 (a) 504.1 4.81 (d, J = 5.9 Hz, 1H), 4.12 -
L)
3.84 (m, 5H), 3.42 - 3.29 (m,
0 4H), 3.05 - 2.89 (m, 2H), 2.33
(s, 4H), 2.21 (d, J = 8.8 Hz,
li 2H),2.11 - 1.61 (m, 10H)
CH3
(CDC13) 6 8.62 (d, J = 1.9 Hz,
N.--'1I 1H), 8.54 (d, J = 1.9 Hz, 1H),
0 N 6.93 - 6.77 (m, 2H), 5.20 (s,
1H), 4.78 - 4.60 (m, 2H), 3.92 -
HN". ". 1.1 3.79 (m, 5H), 3.64 (s, OH), 3.51
275 N 519.2
N (t, J = 5.1 Hz, 4H), 3.36 - 3.16
C ) (m, 4H), 2.40 (t, J = 5.1 Hz,
H3C N N'''.1 0 4H), 2.27 (d, J = 4.8 Hz, 6H),
N
'CH3 2.16 - 2.02 (m, 2H), 1.81 (q, J =
8.1, 5.7 Hz, 6H)
(CDC13) 6 8.69 (d, J = 1.8 Hz,
1H), 8.61 (d, J = 1.9 Hz, 1H),
I 6.94 (d, J = 2.3 Hz, 1H), 6.90
.000 N
(d, J = 2.3 Hz, 1H), 5.87 (s,
1H), 5.13 (s, 1H), 4.76 (s, 1H),
276 HN 451.53
.1. 4.04 (s, 1H), 3.98 - 3.89 (m,
..N.,
N ' N 4H), 3.87 (s, 3H), 3.41 - 3.23
(m, 4H), 2.25 (s, 3H), 2.23 -
H3C 0- 0 2.09 (m, 2H), 1.99 - 1.80 (m,
6H)
133
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(CDC13) 6 8.71 (d, J = 1.9 Hz,
1H), 8.62 (d, J = 1.9 Hz, 1H),
N '-.1I 8.17 (d, J = 0.9 Hz, 1H), 6.94
0 dah N
(dd, J = 15.8, 2.5 Hz, 2H), 5.31
(s, 1H), 4.83 (dp, J = 4.6, 2.5
277 HNICI" WI 459.08
Hz, 1H), 4.22 (qt, J = 8.5, 4.9
T:
F N Hz, 1H), 4.03 - 3.87 (m, 4H), 1 r-'I
3.39 - 3.29 (m, 4H), 2.25 (td, J
N CI 0> = 7.9, 6.6, 3.9 Hz, 2H), 2.09 -
1.80 (m, 6H)
(400 MHz, CDC13) 6 8.83 (d, J
N%
= 2.2 Hz, 1H), 8.69 (d, J = 1.9
HNICf
Hz, 1H), 8.61 (d, J = 1.9 Hz,
1H), 8.02 (dd, J = 8.8, 2.3 Hz,
W 1H), 6.96 (d, J = 2.4 Hz, 1H),
N 6.91 (d, J = 2.5 Hz, 1H), 6.45
278 Ni
488.48
(d, J = 8.9 Hz, 1H), 5.12 (s,
1H),4.81 (d, J = 2.6 Hz, 1H),
ON
3.98 (s, 1H), 3.95 - 3.87 (m,
'
'N'=( 4H), 3.40 - 3.26 (m, 5H), 2.42
(s, 3H), 2.29 -2.16 (m, 2H),
CH3
1.99 - 1.87 (m, 6H)
(CDC13) 6 8.69 (d, J = 1.9 Hz,
NI 1H), 8.60 (d, J = 2.0 Hz, 1H),
N
0 0 7.21 - 7.11 (m, 1H), 7.00 - 6.85
279 HN
(m, 2H), 5.51 (s, 1H), 4.95 -
451.12 4.70 (m, 2H), 4.53 -4.34 (m,
N N 1H), 4.01 - 3.74 (m, 5H), 3.45 -
.=%'
3.25 (m, 4H), 2.25 - 2.09 (m,
2H), 2.03 - 1.73 (m, 6H), 1.39 -
1.25 (m, 3H)
(methanol-d4) 6 8.68 (d, J = 2.0
Hz, 1H), 8.54 (d, J = 2.0 Hz,
NeN
1H), 7.30 (d, J = 7.2 Hz, 1H),
0 1 i "
7.10 (d, J = 2.4 Hz, 1H), 6.87
(d, J = 2.4 Hz, 1H), 5.79 (d, J =
280 HNia" 11" 423.08
7.2 Hz, 1H), 4.13 (qd, J= 8.5,
N
-e N 6.7, 2.4 Hz, 1H), 3.87 (dd, J =
,k Co)
-. 5.9, 3.8 Hz, 4H), 3.42 - 3.32
N OH (m, 5H), 2.21 - 2.07 (m, 2H),
2.01 - 1.78 (m, 6H)
134
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
1H NMR (300 MHz, unless
Cmpd. ESMS
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.68 (d, J
= 1.9 Hz, 1H), 8.61 (d, J = 1.9
N-5'')I Hz, 1H), 8.18 (d, J = 2.2 Hz,
0 A .&.1 N 1H), 7.58 (dd, J = 8.7, 2.5 Hz,
1H), 6.95 (d, J = 2.5 Hz, 1H),
HNCI? 11" 6.91 (d, J = 2.4 Hz, 1H), 6.39
281 N 464.53
.--N (d, J = 8.8 Hz, 1H), 4.78 (s,
,e1 H3 (o) 1H), 4.69 (s, 1H), 3.96 - 3.83
(m, 5H), 3.49 (s, 1H), 3.38 -
HO CH3
C
3.28 (m, 4H), 2.26 - 2.13 (m,
2H), 1.95 - 1.84 (m, 6H), 1.56
(s, 6H)
(CDC13) 6 8.71 (d, J = 1.9 Hz,
N -4I 1H), 8.63 (d, J = 1.9 Hz, 1H),
0 diii N
7.93 - 7.74 (m, 2H), 7.04 - 6.82
HNC'. liffl
(m, 2H), 4.80 (s, 1H), 4.50 (d, J
282 421.2 = 8.1 Hz, 1H), 3.93 (dd, J =
N
r%kN 6.4, 3.4 Hz, 5H), 3.41 - 3.25
NI) Co) (m, 4H), 2.51 - 2.33 (m, 3H),
2.23 (s, 2H), 2.04 - 1.82 (m,
CH3 6H)
(CDC13) 6 8.62 (d, J = 1.9 Hz,
NI 1H), 8.55 (d, J = 1.9 Hz, 1H),
7.88 (dd, J = 5.3, 0.7 Hz, 1H),
0
420.2 6.95 - 6.79 (m, 2H), 6.32 (ddd,
283 HNCr I. N
J = 5.2, 1.5, 0.7 Hz, 1H), 6.12
(dt, J = 1.6, 0.8 Hz, 1H), 4.71
N
N''.1/N''
C j (d, J = 5.9 Hz, 1H), 4.40 (d, J =
8.2 Hz, 1H), 3.98 - 3.67 (m,
CH3 0 4H), 3.35 -3.17 (m, 4H), 2.15
(s, 4H), 1.83 (d, J = 5.2 Hz, 5H)
(CDC13) 6 8.62 (d, J = 1.9 Hz,
Nn 1H), 8.54 (d, J = 1.9 Hz, 1H),
0 284 HNCr dal N 6.86 (dd, J = 16.6, 2.5 Hz, 2H),
5.97 (d, J = 1.2 Hz, 1H), 5.02
IIP 426.1 (s, 1H), 4.71 (dt, J = 5.7, 3.0
,IN le S N Hz, 1H), 3.91 - 3.75 (m, 4H),
)-i (o) 3.63 -3.42 (m, 1H), 3.33 -3.17
(m, 4H), 2.21 - 2.03 (m, 5H),
H3C
1.94- 1.71 (m, 6H)
135
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
ESMS 1H NMR (300
MHz, unless
Cmpd.
Compound Structure indicated otherwise)
No. (M+H)
NMR peaks given as 6 values
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.61 (d, J = 1.9
Hz, 1H), 8.08 (d, J = 5.7 Hz,
1H), 6.96 (d, J = 2.4 Hz, 1H),
0
6.90 (d, J = 2.4 Hz, 1H), 6.30
285 HN.Cr (s, 1H), 6.28 (dd, J = 5.7, 2.2
420.57
Hz, 1H), 4.78 (s, 1H), 4.19 (d, J
= 7.7 Hz, 1H), 3.97 - 3.82 (m,
=k-N H3 0 4H), 3.53 (s, 1H), 3.40 -
3.24
O
(m, 4H), 2.41 (s, 3H), 2.27 -
2.11 (m, 2H), 1.92- 1.84 (m,
6H)
(400 MHz, CDC13) 6 8.69 (d, J
= 1.9 Hz, 1H), 8.60 (d, J = 1.9
NI Hz, 1H), 6.95 (d, J = 2.4 Hz,
286 HN
00.0 401 N
434.56 1H), 6.89 (d, J = 2.4 Hz, 1H),
6.15 (s, 2H), 4.77 (s, 1H), 4.12
(d, J = 7.8 Hz, 1H), 3.99 - 3.81
C (m, 4H), 3.53 (s, 1H), 3.40 -
H3C,kCH3 0 3.23 (m, 4H), 2.36 (d, J = 14.5
Hz, 6H), 2.25 - 2.14 (m, 2H),
1.89 (t, J = 7.8 Hz, 6H)
136
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Table 2.
Compound ESMS ITINMR
Compound Structure
No. (M+H)
1H NMR (300 MHz,
H Chloroform-d) 6 8.70 (d, J
=
WC N N4,31,
CH,
- 1
;,,,c,......._
0 (1d,
1.9 Hz, = , 11:3, 2 , 8.6.25 Hz, 2m
,J=1:9
Hz, 1H), 8.33 (s, 1H), 6.94
5.80 (d, J = 4.9 Hz, 1H), 5.42
287 0 N 478.3 (d, J = 8.1 Hz, 1H), 4.88
- 4.74
7 1 "=>)
1 (m, 1H), 4.08 (s, 2H), 3.99-
N t,,e) 3.86 (m, 4H), 3.50 (s, 3H),
3.42 - 3.28 (m, 4H), 3.01 (dd,
J = 18.4, 5.0 Hz, 3H), 2.54 (s,
3H), 2.28 - 2.08 (m, 2H), 2.03
- 1.79(m, 6H).
1H NMR (400 MHz, CDCI3) 6
H HC 8.68 (t, J = 5.2 Hz, 1H),
8.65
, N....õ,,,..."õ N 4,..,
I 0 (d, J = 1.7 Hz, 1H), 8.02
(d, J
= 5.9 Hz, 1H), 7.26 (d, J = 1.5
"to
0 Hz, 1H), 7.08 (d, J = 1.6
Hz,
404
1H), 7.00 (d, J = 1.6 Hz, 1H),
N
=-=,',,-,.. 6.07 (d, J = 6.0 Hz, 1H), 4.91
(s, 1H), 4.75 (s, 1H), 4.54 (t, J
288
le = 9.6 Hz, 2H), 3.03 (td, J
=
-....õ
0 9.8, 1.6 Hz, 2H), 2.42 (s,
3H),
2.21 -2.06 (m, 2H), 1.91 -
1.74(m, 7H).
H
_ 11 1H NMR (400 MHz, CDCI3) 6
8.79 - 8.71 (m, 2H), 8.03 (d, J
= 5.8 Hz, 1H), 7.39 (s, 1H),
6.38 (s, 1H), 6.08 (d, J = 5.9
289 404 Hz, 1H), 5.06 (s, 2H),
4.83 (d,
40 --) J = 29.0 Hz, 4H), 2.42 (s, 3H),
2.16 (d, J = 7.2 Hz, 2H), 1.88
N (dd, J = 21.2, 8.7 Hz, 6H),
0 I
\¨ 1.58 (s, 2H).
137
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H 1H NMR (400 MHz, CDCI3) 6
HG N N 8.62 (d, J = 1.9 Hz, 1H),
8.53
(d, J = 1.9 Hz, 1H), 8.03 (d, J
rik,...; = 5.9 Hz, 1H), 6.88 (td, J
=
0 19.2, 3.6 Hz, 2H), 6.07 (d, J =
6.0 Hz, 1H), 4.87 (s, 1H), 4.74
N
290 IP ') 435 (s, 1H), 4.06 - 3.93 (m,
1H),
3.80 - 3.69 (m, 2H), 3.58 -
3.38 (m, 2H), 2.94 (td, J =
11.9, 3.5 Hz, 1H), 2.65 - 2.52
0 ,(-1 (m, 1H), 2.42 (s, 3H), 2.13
(d,
J = 10.1 Hz, 2H), 1.81 (d, J =
CH, 9.7 Hz, 6H), 1.32 - 1.08
(m,
3H).
N H 1H NMR (400 MHz,
ei-Nzi, N Chloroform-d) 6 8.42 (d, J =
Hz, 1H), 8.04 (d, J = 4.8 Hz,
2H), 6.65 - 6.56 (m, 2H), 6.28
291 0 419.23 (t, J = 4.8 Hz, 1H),
4.99(d, J =
N [1] 8.1 Hz, 1H), 4.60 (d, J =
6.5
----' I '--",--1. Hz, 3H), 3.79 (dd, J = 8.2, 4.0
t Hz, OH), 3.62 - 3.38 (m, 4H),
riliN
14 3.17- 3.03 (m, 1H), 2.07 -
1.90 (m, 2H), 1.89 - 1.59 (m,
7H).
1H NMR (400 MHz, CDCI3) 6
H 8.83 (dd, J = 2.3, 0.8 Hz,
1H),
H ,C N N ,o.v. 8.77 - 8.68 (m, 2H), 8.54
(dd,
J = 4.8, 1.6 Hz, 1H), 7.95(d, J
= 6.0 Hz, 1H), 7.86 (ddd, J =
7.9, 2.4, 1.6 Hz, 1H), 7.76 (d,
292
NO IsiTh
,e)
N 413 J = 1.7 Hz, 1H), 7.31
(ddd, J =
7.9, 4.8, 0.8 Hz, 1H), 7.21 (d,
J = 1.6 Hz, 1H), 7.11 (d, J =
1 0.9 Hz, 1H), 6.00 (d, J =
6.0
....-' Hz, 1H), 4.80 (d, J = 27.2
Hz,
N 2H), 2.34 (s, 3H), 2.18 -
2.04
(m, 2H), 1.84 - 1.71 (m, 6H).
138
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No.
H 1H NMR (400 MHz, CDCI3) 6
H,C fµk, N 11.20(s, 1H), 8.74(t, J =
6.1
Hz, 1H), 8.71 (d, J = 1.8 Hz,
1H), 8.03(d, J = 5.9 Hz, 1H),
0
7.96 (s, 2H), 7.75 (t, J = 8.0
293 .N 402 Hz, 1H), 7.26 (d, J = 1.5
Hz,
1 1H), 6.08 (d, J = 6.0 Hz,
1H),
N/
\ .).3 4111 N 5.09(s, 1H), 4.82 (s, 1H),
11
3.77 (s, 1H), 2.43 (s, 3H),
N
2.24 - 2.08 (m, 2H), 1.93 -
H 1.76 (m, 6H).
H 1H NMR (300 MHz,
HC N N Chloroform-d) 6 8.10 (d, J
=
.'j, -i - r 0 6.0 Hz, 1H), 7.36 - 7.28 (m,
1H), 6.65 (d, J = 2.0 Hz, 1H),
6.13 (d, J = 6.0 Hz, 1H), 4.81
(d, J = 8.0 Hz, 1H), 4.00 - 3.80
294 410.35
._.....14 (m, 6H), 3.80 - 3.56 (m, 1H),
0 3.29 (dd, J = 5.8, 3.8 Hz,
4H),
..
(---.... -,... ..... --k, 3.02 - 2.84 (m, 2H), 2.48 (s,
0 3H), 2.36 (td, J = 11.4, 10.9,
2.5 Hz, 2H), 2.14 - 1.98 (m,
2H), 1.70- 1.46 (m, 2H).
1H NMR (300 MHz,
F CH, Chloroform-d) 6 8.98 - 8.78
H (m, 2H), 8.33 (d, J = 1.9 Hz,
...,N 1H), 7.67(d, J = 1.8 Hz, 1H),
i - 1 'N,......õ...-
7.30 (d, J = 1.8 Hz, 1H), 6.49 -
...--N 6.31 (m, 1H), 5.07 (d, J = 7.6
^
Hz, 1H), 4.87 (d, J = 5.5 Hz,
295 450.2
N 1H), 4.43 (q, J = 2.8 Hz,
2H),
1 I 4.24(s, 1H), 4.02(t, J =
5.4
NN,. ..." tie) Hz, 2H), 2.71 (dtd, J =
13.1,
7.7, 2.7 Hz, 4H), 2.26 (dt, J =
0 10.4, 5.3 Hz, 2H), 1.97 (d,
J =
5.3 Hz, 6H), 1.28 (t, J = 7.6
Hz, 3H).
139
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
Hat:.
1H NMR (300 MHz,
Chloroform-d) 6 8.79 - 8.67
), (11, 1H), 8.64(d, J = 1.9 Hz,
H 1H), 6.93 (dd, J = 13.0,
2.6
296 550 6 Hz, 2H), 4.77 (s, 1H),
3.94 (q,
¨\ ,_. 1 , ^b ...1
i -1 ..-&-414 J = 6.6, 5.0 Hz, 5H), 3.35 (t, J
...õ,.,,0
4.8 Hz, 4H), 2.16 (d, J =
f=--. Th..:014 µsil 33.6 Hz, 2H), 1.86 (mJ = 4.9
6H), 1.51 (q, J = 2.0, 1.6
o,,,.....)
H 1H NMR (400 MHz, CDCI3) 6
WC N N
8.60 (d, J = 1.9 Hz, 1H), 8.49
' (d, J = 1.9 Hz, 1H), 8.02
(d, J
....,-- = 6.0 Hz, 1H), 6.78 (dd, J =
0
10.1, 2.5 Hz, 2H), 6.08 (d, J =
6.0 Hz, 1H), 4.96 (s, 1H), 4.74
297 447
(d, J = 2.4 Hz, 1H), 4.49 (d, J
= 2.3 Hz, 2H), 3.41 (t, J = 5.4
SI It) Hz, 2H), 3.12 (dd, J = 11.6,
0 " 2.5 Hz, 2H), 2.43 (s, 3H), 2.14
4.7.
(dd, J = 9.6, 4.9 Hz, 2H), 1.98
- 1.68 (m, 10H).
H 1H NMR (300 MHz,
//4 N,,..,ci. Chloroform-d) 6 8.71 (d, J =
WC ¨N 1.9 Hz, 1H), 8.65(d, J = 1.9
Hz, 1H), 7.53 (dt, J = 8.1,1.0
1 459.4 Hz, 1H), 7.35 (ddd, J =
8.1,
6.8, 1.1 Hz, 1H), 7.20 (dt, J =
298 . 0
---(..,', 8.5, 0.9 Hz, 1H), 7.07 - 6.89
1, (m, 3H), 4.79 (td, J = 6.1, 3.1
Hz, 1H), 4.02 - 3.91 (m, 4H),
3.87 (s, 3H), 3.43 - 3.25 (m,
4H), 2.34 - 2.15 (m, 2H), 2.10
- 1.88 (m, 6H).
140
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
N 1H NMR (300 MHz,
Chloroform-d) 6 8.70 (d, J =
1.9 Hz, 1H), 8.63(d, J = 1.9
Ite tat¨ Hz, 1H), 7.02 - 6.89 (m,
2H),
¨ 6.57(d, J = 1.0 Hz, 1H),
4.87 -
299 CH ,õ:õ.õ-,L.se,,,S) 475.28 4.76
(m, 1H), 4.76 - 4.62 (m,
, -
1 i 1H), 4.09- 3.88 (m, 5H),
3.45
(N'hil%1 - 3.25 (m, 4H), 2.65 (d, J
= 0.8
Hz, 3H),2.56(s, 3H), 2.31 -
ON) 2.12 (m, 2H), 2.09- 1.81
(m,
6H).
1H NMR (400 MHz, CDCI3) 6
CH, 8.71 (d, J = 1.9 Hz, 1H),
8.61
1., -. (d, J = 1.9 Hz, 1H),
8.13(d, J
= 5.9 Hz, 1H), 6.94(d, J = 2.4
1 ',Nisi ",-, 0
Hz, 1H), 6.85 (d, J = 2.4 Hz,
1....,õ.õ--- N lor-Lif 1H), 6.13 (d, J = 6.0 Hz,
1H),
300 407.26 5.17(s, 1H), 4.50 (s,
1H),
H 4.29 (d, J = 6.7 Hz, 2H),
4.03 -
11:
1 . N N 3.82 (m, 4H), 3.45- 3.32 (m,
4H), 3.04 - 2.93 (m, 1H), 2.58
1 - 2.50 (m, 2H), 2.49 (s,
3H),
2.24 (ddd, J = 20.4, 10.3, 6.0
Hz, 2H).
1H NMR (400 MHz, CDCI3) 6
C H, 8.76 (s, 2H), 8.09 (d, J =
5.7
Hz, 1H), 6.94 (d, J = 2.3 Hz,
1 'N'I'l it----7-4' 0 1H), 6.80 (d, J = 2.2 Hz,
1H),
6.73 (s, 1H), 6.19 (d, J = 5.1
301 N 407.26
-1-..N.,91, Hz, 1H), 4.35(s, 1H), 4.17
(d,
H 40 "N) J = 3.9 Hz, 2H), 4.03- 3.77
(m, 4H), 3.45 - 3.21 (m, 4H),
2.85 (dd, J = 19.9, 9.1 Hz,
2H), 2.80 - 2.70 (m, 1H), 2.51
(s, 3H), 2.06 (dt, J = 12.7, 6.4
Hz, 2H).
141
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
1H NMR (400 MHz, CDCI3) 6
-1, 8.70(d, J = 1.9 Hz, 1H),
8.61
(d, J = 1.9 Hz, 1H), 8.13 (d, J
= 5.8 Hz, 1H), 6.92(d, J = 2.4
N, ..........,
H 0 1H), 6.18 (d, J
302 0
407.21 5.07 -4.97 (m, 1H), 4.91
(s,
1H), 3.99- 3.82 (m, 4H), 3.50
N (d, J = 5.5 Hz, 1H), 3.38 -
3.23
Milli 1
N 14 (m, 4H), 2.72 (dd, J = 8.1, 4.8
Hz, 1H), 2.65 (dt, J = 15.2, 7.7
Hz, 2H), 2.51 (s, 3H), 2.45
(ddd, J = 11.1, 6.8, 3.4 Hz,
2H).
1H NMR (400 MHz, CDCI3) 6
C H ,
8.71 (d, J = 1.9 Hz, 1H), 8.61
(d, J = 1.9 Hz, 1H), 8.10(d, J
= 5.8 Hz, 1H), 6.92(d, J = 2.4
L.,,,,,,....f.A.. N ..--.4.....õõrõµ
4.95 (s, 1H), 4.79 (p, J = 7.1
H
303 sµ*0 407.26
Hz, 1H), 3.98 - 3.83 (m, 4H),
N 3.45 (bt, 2H), 3.36 - 3.27
(m,
N µ'N"Iiill 14'''') 4H), 2.79 (dtd, J = 9.8, 7.2,
2.8 Hz, 2H), 2.48 (s, 3H), 2.38
(dt, J = 16.0, 8.0 Hz, 1H), 2.19
(ddd, J = 17.0, 9.7, 2.8 Hz,
2H).
1H NMR (400 MHz, CDCI3) 6
8.69 (d, J = 1.9 Hz, 1H), 8.61
(d, J = 1.9 Hz, 1H), 8.27 (d, J
1 jr--7'\*'µ\S'''N. 0 = 4.8 Hz, 2H), 6.93
(d, J = 2.4
N N
..,--- "S,$)
1H), 6.54 (t, J = 4.8 HH) z, 1,
304 H
1 393.21 5.36 (d, J = 7.2 Hz,
1H), 4.70 -
..--"..;)
N N 4.58 (m, 1H), 4.31 (d, J = 7.7
Hz, 2H), 4.01 - 3.86 (m, 4H),
3.44- 3.30 (m, 4H), 3.04 -
2.93 (m, 1H), 2.50 (ddd, J =
13.5, 7.8, 3.1 Hz, 2H), 2.31 -
2.19 (m, 2H).
142
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
1H NMR (400 MHz, CDCI3) 6
8.81 (d, J = 1.9 Hz, 1H), 8.74
(d, J = 1.9 Hz, 1H), 8.29 (d, J
r^7.-". 0 = 4.8 Hz, 2H), 6.93 (d, J =
2.4
1 Hz, 1H), 6.80 (d, J = 2.4
Hz,
,e-1----1
N N 1H), 6.64 (d, J = 8.5 Hz,
1H),
305 H
411 IlD 393.26 6.50 (t, J = 4.8 Hz, 1H),
4.60
(dq, J = 15.6, 7.6 Hz, 1H),
N I( 4.19 (d, J = 4.6 Hz, 2H), 4.01 -
0 ,....,..) 3.84 (m, 4H), 3.43 - 3.27 (m,
4H), 2.88 - 2.78 (m, 2H), 2.78
-2.68 (m, 1H), 2.08 - 1.97 (m,
2H).
..."'-'-=kw
1H NMR (400 MHz, CDCI3) 6
8.70(d, J = 1.9 Hz, 1H), 8.61
1 ,.......T_. (d, J = 1.9 Hz, 1H), 8.29
(d, J
',. = 4.8 Hz, 2H), 6.91 (d, J =
2.4
$47
0 Hz, 1H), 6.63 - 6.51 (m,
2H),
306 393.26 5.21 (s, 1H), 5.03 (p, J
= 6.6
ill& tiµN) Hz, 1H), 4.02 - 3.79 (m,
4H),
3.63 (dd, J = 7.3, 5.9 Hz, 2H),
Iti NIIIIIIP I( 3.42 - 3.19 (m, 4H), 2.79-
2.68 (m, 1H), 2.68 - 2.54 (m,
0) 2H), 2.52 - 2.40 (m, 2H).
1H NMR (400 MHz, CDCI3) 6
8.69(d, J = 1.8 Hz, 1H), 8.61
J = 1.8
rti 14 a, = 4.8 Hz, 2, H), 6.91 (d, J
=20
Hz, 1H), 6.68 (d, J = 2.1 Hz,
0 1H), 6.53 (t, J = 4.8 Hz,
1H),
307 393.26 5.19 (s, 1H), 4.76 (p, J
= 7.2
it.....)
...--) Hz, 1H), 3.99 - 3.79 (m,
4H),
3.56 (t, J = 6.4 Hz, 2H), 3.41 -
(----N I( 7.3 Hz, 2H), 2.39 (dt, J = 16.5,
0 ) 8.3 Hz, 1H), 2.19 (dd, J =
19.6, 9.7 Hz, 2H).
143
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
H 1H NMR (300 MHz,
HC N N Chloroform-d) 6 8.71 (d, J
=
1.9 Hz, 1H), 8.64 (d, J = 1.9
Hz, 1H), 7.79 (s, 1H), 7.23 (s,
X'"H" *CI* 0 1H), 6.95 (dd, J = 14.0,
2.5
Hz, 2H), 5.23 (d, J = 8.1 Hz,
308 478.64
0 NH
1H), 4.80 (s, 1H), 4.09 (s, 1H),
1 3.94 (dd, J = 6.1, 3.6 Hz,
4H),
N N 3.43 - 3.23 (m, 4H), 3.02
(d, J
= 5.1 Hz, 3H), 2.43 (s, 3H),
2.31 - 2.12 (m, 2H), 1.95 (p, J
= 10.0 Hz, 6H).
1H NMR (300 MHz,
N_ _N H Chloroform-d) 6 8.68 (d, J =
1.9 Hz, 1H), 8.54 (d, J = 1.9
'/'\)-- N'eki, Hz, 1H), 6.85 (s, 2H), 6.30
(s,
N
Nõ,f.,...j. 1H), 5.06 (d, J = 16.2 Hz,
1H),
4.85 (d, J = 6.5 Hz, 477.54 3H),=4.44 -
(3,
309
N.6. 4.30 (m, 2H), 4.14 (q, J
7.1
4\0
/ ...N Hz, 1H), 3.89 - 3.60 (m, 5H),
HC 3.50 (s, 3H), 3.35 (q, J = 7.2
,
,,,J-,....)
Hz, 1H), 2.49 (s, 3H), 2.24 (d,
I,5 0.-". J = 8.7 Hz, 2H), 2.08 (d, J
=
7.7 Hz, 2H), 1.92 (q, J = 9.6,
0
6.9 Hz, 6H), 1.28 (t, J = 7.1
Hz, 2H).
H
1H NMR (300 MHz, CDC13) 6
("X "IT'D 8.72(d, J = 1.8 Hz, 1H),
8.66
(d, J = 1.9 Hz, 1H), 7.35 (d, J
N N,N m'10 =4.3 Hz, 1H), 7.17(d, J
=4.8
310
H , C 40 461.26 rtN) 2.2 Hz, 2H), 4.92
(s, 1H), 4.35
(s, 1H), 4.01 - 3.84 (m, 4H),
...---'
N 3.48 - 3.26 (m, 4H), 2.72 (s,
3H), 2.36 - 2.21 (m, 2H), 2.13
0 õ....3 - 1.85 (m, 6H).
144
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H
0
=-..,.,. ,..,..
,N .,..--' ,-=
H,C 40
311 436.29
r----- N. N
H 1H NMR (300 MHz, CDCI3) 6
8.71 (d, J = 1.9 Hz, 1H), 8.65
(d, J = 1.9 Hz, 1H), 7.57 (d, J
( ,
= 3.2 Hz, 1H), 7.35 - 7.28 (m,
i 2H), 6.97 (d, J = 2.4 Hz,
1H),
312 CH, N...õ..1 437.33 6.91 (d, J = 2.4
Hz, 1H), 5.23
tr-) (s, 1H), 4.76 (s, 1H), 4.20
(s,
1H), 4.01 (s, 3H), 3.98 - 3.84
(m, 4H), 3.44 - 3.29 (m, 4H),
2.27 - 2.14 (m, 2H), 2.05 -
1.85 (m, 6H).
H 1H NMR (300 MHz, CDCI3) 6
N N 8.68 (dd, J = 21.2, 1.9 Hz,
i,,,,,,
( X,'r, %se 2H), 7.86 (s, 1H), 7.18 (d,
J =
4.9 Hz, 1H), 7.00 (d, J = 4.9
0
C , Hz, 1H), 6.96 (dd, J =
12.1,
313 14): 460.29
Hz, 1H), 4.80 (s, 1H), 4.30
(dd, J = 11.9, 6.0 Hz, 1H),
4.05 - 3.77 (m, 4H), 3.45 -0 3.14 (m, 4H), 2.75 (s, 3H),
2.37 - 2.14 (m, 2H), 2.14 -
1.87 (m, 6H).
145
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H
HC N 1H NMR (300 MHz, CDC13) 6
1 ,,,,)-'' 8.71 (d, J = 1.9 Hz, 1H),
8.63
(d, J = 1.9 Hz, 1H), 7.66(s,
HC 6 ,,,
0 1H), 6.94 (dd, J = 14.9,
2.4
Hz, 2H), 4.84 - 4.74 (m, 1H),
314 435.32
4.47(s, 1H), 4.02 - 3.87 (m,
4H), 3.87- 3.75 (m, 1H), 3.47
i------ N N - 3.21 (m, 4H), 2.38 (d, J = 4.0
0 ....õ...) Hz, 6H), 2.26 - 2.13 (m, 2H),
2.01 - 1.78(m, 6H).
- -, , , r 1 1 H NMR (300 MHz,
N m_ Chloroform-d) 6 8.68 (d, J
=
1.9 Hz, 1H), 8.59 -8.46 (m,
2H), 7.98 (s, 1H), 7.18 (s, 1H),
0 NH 0 6.85 (s, 2H), 4.85 (d, J =
6.6
315 N 476.23
400 ......_ ---n Hz, 2H), 3.90- 3.62 (m,
5H),
H,d
3.35 (d, J = 8.2 Hz, 1H), 3.03
(d, J = 5.1 Hz, 3H), 2.27 (s,
7 N 2H), 2.13- 1.80 (m, 7H),
10.58(s, 2H)
0
H
1H NMR (300 MHz,
CI:r
Chloroform-d) 6 8.14 (d, J =
6.2 Hz, 1H), 6.50 (d, J= 1.7
Olr'
i Hz, 1H), 6.43 (d, J = 1.6
Hz,
316 --R, CH, 411.34 1H), 6.21 (d, J =6.2 Hz,
1H),
0 4.98 (s, 1H), 4.01 - 3.81
(m,
(s-N ---/kif 5H), 3.37
2.20 (d, J= 9.1 Hz,
0i 3H), 2.05 - 1.71 (m, 6H).
146
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
o
1H NMR (300 MHz,
H,c,\N __
Chloroform-d) 6 8.68 (d, J =
H / 1/4/-J14 1.9 Hz, 1H), 8.62 - 8.41
(m,
---tsj 2H), 7.79 (s, 1H), 7.33 (d,
J =
4.9 Hz, 1H), 6.85 (q, J = 2.6
317 o 476.55 Hz, 3H), 4.85 (d, J =
6.3 Hz,
4H), 4.08 (s, OH), 3.88 - 3.58
41110 ----1 (m, 5H), 3.35 (q, J = 6.8
Hz,
1H), 3.03 (d, J = 5.1 Hz, 3H),
`.,.y N''..- 2.36 - 2.13 (m, 2H), 2.15 -
0 1.84 (m, 6H).
H 1H NMR (300 MHz,
irrA,___ ,4
tsi Chloroform-d) 6 8.68 (d, J
=
Is 4,
1.9 Hz, 1H), 8.56 (d, J = 1.9
Hz, 1H), 8.33 (d, J = 1.9 Hz,
F
\ 0 1H), 6.85 (s, 2H), 5.07 (d,
J =
318 465.3
8.1 Hz, 1H), 4.86 (d, J = 6.4
C H ,
NI Hz, 4H), 4.23 (s, 2H), 3.90
-
ISO '7.) 3.62 (m, 4H), 3.35 (q, J = 7.1
Hz, 1H), 2.73 (qd, J = 7.6, 2.3
7 N Hz, 2H), 2.27 (d, J = 10.1
Hz,
3H), 2.12- 1.82 (m, 6H), 1.28
(t, J = 7.6 Hz, 3H).
1H NMR (300 MHz,
H Chloroform-d) 6 8.83 (dt, J
=
6.4, 1.4 Hz, 2H), 8.51 (d, J =
4.7 Hz, 1H), 7.78 (s, 1H), 7.68
"Nr (d, J = 1.7 Hz, 1H), 7.34
(dd, J
0
= 4.9, 0.9 Hz, 1H), 6.38 (s,
319 0NH k
461.38
-,,, I.4.,
CH:: ...,.., ...-5;. ise.... Hz, 2H), 4.17-
3.94 (m, 3H),
3.03 (dd, J = 5.1, 1.0 Hz, 3H),
0
2.76 - 2.59 (m, 2H), 2.23 (d, J
= 12.7 Hz, 2H), 1.98 (d, J =
7.7 Hz, 6H).
147
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
1H NMR (300 MHz,
Chloroform-d) 6 8.70 (d, J =
0 iN 1.9 Hz, 1H), 8.62(d, J =
1.9
1
Hz, 1H), 8.10 (d, J = 6.0 Hz,
Ai r
1H), 6.97 (d, J = 2.4 Hz, 1H),
6.90 (d, J = 2.6 Hz, 1H), 6.15
320 ) 4111114
HN 451.44 (d, J = 6.0 Hz,
1H), 4.19 - 4.08
N (m, 1H), 3.94 - 3.69 (m, 5H),
,
131) ( -1 3.63 (t, J = 11.3 Hz, 2H), 3.04
.õ..1. 1 OH (td, J = 11.9, 3.5 Hz, 1H), 2.92
HC N 0 '''"*-`' -2.80 (m, 1H), 2.50 (s,
3H),
2.20 (d, J = 8.9 Hz, 2H), 1.90
(d, J = 5.6 Hz, 7H).
H 1H NMR (300 MHz,
N N44, Chloroform-d) 6 8.81 -8.67
r t
(m, 2H), 8.42 (s, 1H), 7.89 (s,
1H), 7.58(d, J = 1.8 Hz, 1H),
0
7.09 (s, 1H), 6.28 (dq, J = 3.0,
321 NH 14-,.. 461.63
0 -....õ --....õ
$ (q, J = 2.8 Hz, 2H), 3.92 (t, J =
CH, ..---...:--' 5.4 Hz, 2H), 2.93 (d, J = 5.1
-....õ N Hz, 3H), 2.67 - 2.48 (m,
2H),
0 2.18 (d, J = 11.3 Hz, 2H),
1.98
H 1H NMR (300 MHz,
, N
r.........7,-ic 10 Chloroform-d) 6 8.62 (d, J
=
1.9 Hz, 1H), 8.52 (d, J = 1.9
Hz, 1H), 6.85 (dd, J = 18.8,
'..0
2.5 Hz, 2H), 5.59 (d, J = 8.3
322 (N 4`,. 481.3 Hz, 1H), 5.24(s, 1H),
4.72 (s,
H 0
) 1H), 3.84 (t, J = 4.9 Hz, 5H),
3.66- 3.39 (m, 3H), 3.30 _
1,--------til N---- 3.19 (m, 5H), 2.78 (t, J =
7.0
0) Hz, 2H), 2.11 (s, 1H), 2.00
-
1.66 (m, 6H).
148
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No.
H
N r,õ1
(7. y
..A., ji, ,v,
H ,C N C H,C-,3 0
323 435.33
r------. ---- N4'*---
1H NMR (300 MHz, CDCI3) 6
KC 8.71 (d, J = 1.9 Hz, 1H),
8.63
1 H (d, J = 1.9 Hz, 1H), 7.79
(d, J
0
= 5.9 Hz, 1H), 6.97 (d, J = 2.4
..,......T.....-.),. N NO
1 i Hz, 1H), 6.91 (d, J = 2.4 Hz,
1H), 6.13 (dd, J = 5.9, 2.1 Hz,
324
4'10 450.35 1H), 5.87(d, J =2.0 Hz,
1H),
4.87 -4.70 (m, 1H), 4.32 (q, J
r------ II "2--)
N = 7.1 Hz, 2H), 4.21 (d, J =
7.8
Hz, 1H), 4.05 - 3.81 (m, 4H),
N
3.60 - 3.45 (m, 1H), 3.45 -0,,...) 3.24 (m, 4H), 2.33 - 2.10
(m,
2H), 2.02- 1.77 (m, 6H), 1.39
(t, J = 7.1 Hz, 3H). [2]
1H NMR (300 MHz, CDCI3) 6
H 11.03(s, 1H), 8.71 (d, J = 1.9
1444. Hz, 1H), 8.62 (d, J = 1.9
Hz,
1H), 7.09 (d, J = 7.1 Hz, 1H),
HN ,11 0
"0 6.97 (d, J = 2.4 Hz, 1H),
6.90
(d, J = 2.4 Hz, 1H), 5.67 (dd, J
325 0
422.34 = 7.2, 2.1 Hz, 1H), 5.58
(d, J =
I I 2.1 Hz, 1H), 4.84 - 4.75 (m,
....---'--- ---"" --,..:-,-) 1H), 4.41 (d, J = 7.6
Hz, 1H),
I N
N 4.02- 3.87 (m, 4H), 3.54 -
3.43 (m, 1H), 3.42 - 3.27 (m,
4H), 2.28 - 2.15 (m, 2H), 2.05
- 1.78(m, 6H).
149
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M+H)
H
õps.)..5õ.11/4,14,....
0.* 1H NMR (300 MHz,
H,C¨N 0 Chloroform-d) zh9,1oHlr F
izo ,f) c :1 r7Hm. )0-, :8) :6616.838.68( d ( d ,( jm7, 2Ji H. 9=) ,
HC 5.34 (s, 1H), 4.73 (d, J =
6.1
326
0 11-1 423.4
Hz, 1H), 3.91 (dd, J = 6.1, 3.7
Hz, 4H), 3.59 (s, 4H), 3.44 (s,
r--------N N''' 1H), 3.33 (t, J = 4.9 Hz,
4H),
2.17 (s, 5H), 1.86 (q, J = 9.1,
6.3 Hz, 6H).
1H NMR (300 MHz,
Chloroform-d) 6 8.70 (d, J =
H 2.0 Hz, 1H), 8.63(d, J =
1.9
r.....:NixN Hz, 1H), 8.00 (d, J = 1.3
Hz,
1H), 6.95 (d, J = 2.4 Hz, 1H),
N---õ,
F II 6.89 (d, J = 2.5 Hz, 1H),
4.88
(d, J = 8.2 Hz, 1H), 4.77 (s,
327 ., NH
,---* ....-J'Lli 523.35 1H), 4.63 (d, J = 9.0 Hz, 2H),
4.15 (s, 1H), 3.92 (t, J = 4.9
Hz, 4H), 3.39 - 3.26 (m, 4H),
N r N N 2.91 (td, J = 8.6, 3.9 Hz,
1H),
i
HC 0. 2.72 - 2.55 (m, 2H), 2.50 -
2.10 (m, 7H), 1.91 (d, J = 5.5
Hz, 6H), 1.72 (d, J = 4.7 Hz,
1H).
H 1H NMR (300 MHz,
N N
Chloroform-d) 6 8.81 - 8.59
H (m, 3H), 8.54 (d, J = 1.9
Hz,
H ,C, 14,i(L.Aal 1H), 6.92 - 6.73 (m, 2H),
5.50
# (d, J = 8.2 Hz, 1H), 4.72
(s,
328 CH, 0 4,..1, 521.69 1H), 4.01 (s, 2H), 3.84
(t, J =
li 4.9 Hz, 5H), 3.50 (q, J = 5.3
le"'N1111"1 Hz, 3H), 3.37- 3.17 (m, 5H),
2.62 (t, J = 5.6 Hz, 2H), 2.34
(s, 7H), 2.13 (d, J = 10.7 Hz,
2H), 1.87 (d, J = 22.1 Hz, 6H).
150
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
H
N, 1H NMR (300 MHz,
,N.....a Chloroform-d) 6 8.62 (t, J
=
H,C--N 1.7 Hz, 1H), 8.55 (t, J =
1.7
'''`-= 0 Hz, 1H), 8.37 (dt, J = 4.6,
1.5
329
ti.õ.4.õ..:õ...--- ' 46044 Hz, 1H), 7.78 (dt, J =
7.9, 1.5
Hz, 1H), 6.93 - 6.77 (m, 3H),
4.71 (d, J = 6.1 Hz, 1H), 3.96-
40 Nt')...,-'" 3.77 (m, 6H), 3.33- 3.18 (m,
4H), 2.23 - 2.07 (m, 2H), 2.01
- 1.77 (m, 6H).
H 1H NMR (300 MHz,
N Chloroform-d) 6 8.62 (d, J
=
/ttn )0 1.9 Hz, 1H), 8.53(d, J =
1.9
coco = ' 5 Hz, 1H), 8.10
(dd, J = 4 8.. ,
0, 1.5
Hz, 1H), 7.12 (dd, J = 8.1, 4.8
330 463.6
1110 l'4')
Hz, 1H), 6.95 - 6.76 (m, 2H),
5.39 (s, 1H), 4.74 (s, 1H),
3.96 - 3.75 (m, 5H), 3.26 (t, J
= 4.9 Hz, 4H), 2.16 (d, J =
12.8 Hz, 2H), 2.05 - 1.71 (m,
6H).
H
NI 1H NMR (300 MHz,
H2N1 40. Chloroform-d) 6 8.62 (d, J
=
1.9 Hz, 1H), 8.53 (d, J = 1.9
0 Hz, 1H), 7.28(d, J = 0.7 Hz,
0' 331 "
el 1H), 6.94 - 6.77 (m, 2H), 5.39
N..) 455.4
1H), 4.71 (s, 1H), 3.93 - 3.77
r-----N tr (m, 4H), 3.58 (s, 1H), 3.26
0 (dd, J = 6.0, 3.7 Hz, 4H),
2.11
(s, 2H), 1.97 - 1.66 (m, 6H).
151
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (MH-H)
332 H,C y0
465.36
CH,
0
H,C Nõ N,>4.
N C H, '1'10
333
4110 435.33
YTh
õ
KC 0
334 451.36
e
152
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
C H,
1;4140
C)
335 421.37
N
4110 14,)
C),,,,
H
itill =-$
/0
336 N 446.35
--.......
I
..õ.-- ....õ
a
H 1H NMR (300 MHz,
N' 1 Chloroform-d) 6 8.67 - 8.58
(m, 1H), 8.58 - 8.49 (m, 1H),
8.33 (s, 2H), 6.92 - 6.78 (m,
0
2H), 5.41 (d, J =8.1 Hz, 1H),
337 '0 .--kL\t 533.2 4.72 (s, 1H), 3.98
(d, J = 8.6
140 1 Hz, 1H), 3.84 (t, J = 4.8
Hz,
( -11, ,,,i 4H), 3.58 (s, 4H), 3.26 (t,
J =
4.9 Hz, 4H), 2.36 (t, J = 4.9
Hz, 4H), 2.25 (s, 3H), 2.13 (d,
J = 10.8 Hz, 2H), 1.84 (s,6H).
153
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (M-h1-1)
1H NMR (300 MHz, DMS0-
0 H d6) 6 8.72 (d, J = 1.9 Hz,
1H),
8.59 (d, J = 1.9 Hz, 1H), 7.17
(d, J = 2.5 Hz, 1H), 6.84 (d, J
= 2.4 Hz, 1H), 6.69 -6.61 (m,
1H), 6.50 (m, 2H), 4.94 (m,
338 Nõ....1 447.43
11. N-4) 1H), 4.50 (t, J = 8.7 Hz,
2H),
4.27 (d, J = 8.2 Hz, 1H), 3.79
(m, 4H), 3.45 (m, 1H), 3.32
1 (m, 4H), 3.13 (t, J = 8.7 Hz,
.,..,"
0 ,,,. 2H), 1.98 (m, 2H), 1.74 (m,
6H).
,r- 0 H 1H NMR (300 MHz, DMS0-
0 No..., d6) 6 8.72 (d, J = 1.9 Hz,
1H),
8.58 (d, J = 1.9 Hz, 1H), 7.14
/ (d, J = 2.5 Hz, 1H), 6.84(d, J
0 = 2.3 Hz, 1H), 6.64(t, J =
8.0
339 N 449.41 Hz, 1H), 6.36 (d, J =
8.2 Hz,
1H), 6.22 (dd, J = 7.7, 1.0 Hz,
(0 1H), 5.89 (s, 2H), 4.96 (m,
11 .....õ
(--,N1 N 2H), 3.79 (m, 4H), 3.51 (m,
1H), 3.32 (m, 4H), 2.03 (m,
2H), 1.75 (m, 6H).
H,C
N 1H NMR (300 MHz, DMSO-
N
H d6) 68.73 (d, J = 1.9 Hz,
1H),
0 N ---
...c--- .."1 8.59 (d, J = 1.9 Hz, 1H),
7.25
I (m, 1H), 7.14 (d, J = 2.4
Hz,
-,õ 1H), 6.89 - 6.78 (m, 3H), 5.47
340 '''',F's*0 474.48
iiii N (d, J = 7.7 Hz, 1H), 4.94
(m,
qpi i 1., ... ,) 1 H), 4.29 (s, 2H), 3.79
(m,
4H), 3.51 (m, 1H), 3.06 (s,
re.''''N N 3H), 2.09 (m, 2H), 1.81 (m,
0õ,,,,,) 6H).
154
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
H
N 1H NMR (300 MHz, DMSO-
d6) 68.72 (d, J = 1.9 Hz, 1H),
8.58 (d, J = 1.9 Hz, 1H), 8.17
N NO (s, 2H), 7.15(d, J = 2.4 Hz,
H,C 341 1H), 6.83 (d, J = 2.3 Hz, 1H),
CH, 465.57
6.06 (d, J = 7.8 Hz, 1H), 4.92
ip ) (m, 1H), 4.77 (s, 1H), 3.79
(m,
(N 4H), 3.50 (m, 1H), 3.30 (m,
) 4H), 2.02 (m, 2H), 1.80 (m,
6H), 1.42 (s, 6H).
H 1H NMR (400 MHz, CDCI3) 6
8.86 (d, J = 0.9 Hz, 1H), 8.84
(s, 1H), 8.16 (s, 1H), 7.73 -
7.63 (m, 2H), 7.17 (d, J = 7.0
342 364.27 Hz, 1H), 5.24 (d, J = 7.8 Hz,
fsr 1H), 5.03 (s, 2H), 4.83 (s,
2H),
4.79(s, 1H), 4.12 - 3.99 (m,
1H), 2.26 - 2.14 (m, 2H), 2.01
- 1.84(m, 6H).
1H NMR (300 MHz,
Chloroform-d) 6 8.70 (d, J =
H 2.0 Hz, 1H), 8.63(d, J =
1.9
N N,,,,,iciõ,
Hz, 1H), 8.00 (d, J = 1.3 Hz,
ccX
g 1 1H), 6.95 (d, J = 2.4 Hz,
1H),
6.89 (d, J = 2.5 Hz, 1H), 4.88
=F 0
(d, J = 8.2 Hz, 1H), 4.77 (s,
NH 523.44 1H), 4.63 (d, J = 9.0 Hz, 2H),
4.15 (s, 1H), 3.92 (t, J = 4.9
Hz, 4H), 3.39 - 3.26 (m, 4H),
, 1--------N N 2.91 (td, J = 8.6, 3.9 Hz, 1H),
HC 0 2.72 - 2.55 (m, 2H), 2.50 -
2.10 (m, 7H), 1.91 (d, J = 5.5
Hz, 6H), 1.72 (d, J = 4.7 Hz,
1H).
155
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
0
0
344 441.58
"'II
)
N
0,,õ..õ...)
a
-,N ) ).L a d16H)Nom9R.18(3(0d, J . 5 H
0MH1z.,DMz, 1H
S0-),
H
8.98 (t, 1H), 8.87 (d, J = 2.5
Hz, 1H), 8.76 - 8.69 (m, 2H),
0 8.57 (d, J = 1.9 Hz, 1H),
7.12
345 449.59
N (d, J = 2.6 Hz, 1H), 6.82(d, J
.õ...-- ......--- )
1 1 =2.3 Hz, 1H), 4.94 (m, 1H),
".,... ss...,. 3.78 (m, 4H), 3.27 (m, 6H),
N N 1.99 (m, 2H), 1.83 - 1.48
(m,
7H).
0
J.L 1H NMR (300 MHz, DMSO-
H,C N - d6) 68.71 (d, J = 1.9 Hz,
1H),
H 8.59(d, J = 1.9 Hz, 1H),
7.85
0 (t, 1H), 7.12 (d, J = 2.5
Hz,
346 385.56 1H), 6.83 (d, J = 2.4
Hz, 1H),
4.93 (m, 1H), 3.78 (m, 4H),
3.31 (m, 3H), 2.95 (t, J = 6.0
Hz, 2H), 2.03 - 1.90 (m, 2H),
1.80 (s, 3H), 1.52 (m, 7H).
156
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (MH-H)
N
I 1H NMR (300 MHz, DMSO-
8.59 (s, 1H), 7.60 (d, J = 2.3
d6) 68.72 (d, J = 1.9 Hz, 1H),
N '0
$ H
CH, Hz, 1H), 7.46 (s, 1H), 7.13
(s,
0 1H), 6.83 (s, 1H), 6.46 (s, 1H),
347 450.63
5.97(m, 1H), 4.96 (m 1H),
,N,,,,,,i
I) 3.87 - 3.70 (m, 7H), 3.30 (s,
4H), 2.95 (t, J = 5.7 Hz, 2H),
1.99(m, 2H), 1.62(t, J= 18.1
1..
Hz, 7H).
C.N 1H NMR (300 MHz, DMS0-
d6) 6 8.72 (d, J = 1.9 Hz, 1H),
8.60 (d, J = 1.9 Hz, 1H), 8.24
0
H (d, J = 4.7 Hz, 1H), 7.22
(t, J =
..**0 6.0 Hz, 1H), 7.11 (d, J =
2.6
348 421.6 Hz, 1H), 6.82 (d, J = 2.3
Hz,
=14,,,N1 1H), 6.51 (t, J = 4.7 Hz, 1H),
4.93 (m, 1H), 3.78 (m, 4H),
1-'7 3.31 m, 4H), 3.19 (t, J =
6.5
Hz, 2H), 1.97 (m, 2H), 1.55
(m, 7H).
H 1H NMR (300 MHz, DMS0-
KCY 0), N d6) 6 8.72 (d, J = 1.9 Hz,
1H),
CH
i 1 1
,
0 8.57 (d, J = 1.9 Hz, 1H), 7.59
. it-..õ ,( d 2, J.5=H5z, .91HHI,
61.H8)2, (7d, .1 4J ,_( d 2, J.3
349 464.58
Hz, 1H), 6.44 (d, J = 7.7 Hz,
, "..,
1H), 6.22 (dd, J = 5.8, 2.1 Hz,
i 1 1H), 5.80- 5.74 (m, 1H),
5.19
i''''\µ'N 'N'N - 5.1 1 (m, 1H), 4.92 (m,
1H),
0,,,õ..,,J 3.79 (m, 4H), 3.32 (m, 4H),
2.01 (m, 2H), 1.75 (m, 6H),
1.21 (d, J = 6.1 Hz, 6H).
157
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (M-h1-1)
0 1H NMR (300 MHz,
H
H,C, ,kõ..,,,,.N N...cA. Chloroform-d) 6 4.79 (s,
1H),
N ,,--`"' '''')'" 4.24 - 3.76 (m, 5H), 3.50 -
3.20 (m, 4H), 3.02 (d, J = 5.1
0 Hz, 3H), 2.20 (d, J = 12.8
Hz,
350 464.42 2H), 2.09 - 1.75 (m,
6H), 5.29
:FaC,P.N1 - 5.19 (m, 1H), 8.70(d, J = 1.9
1 11 Hz, 1H), 8.62(d, J = 1.9
Hz,
1H), 8.49 (d, J = 4.8 Hz, 1H),
7.77 (s, 1H), 7.32 (d, J = 4.9
Os...) Hz, 1H), 7.02 - 6.86 (m,
2H).
0 1H NMR (300 MHz,
H
WC
= "t:, Chloroform-d) 6
8.70 (d, J =
1.9 Hz, 1H), 8.62 (d, J = 2.0
1
Hz, 1H), 8.38 (d, J = 4.9 Hz,
0 1H), 6.93 (dd, J = 14.5,
2.4
351 478.38 Hz, 2H), 6.68 (d, J =
4.9 Hz,
1H), 5.28 (s, 1H), 4.79 (s, 1H),
1 Ã 4.22 - 3.86 (m, 5H), 3.34
(t, J
NN, N.,, ,..."
1,-----N N = 4.8 Hz, 4H), 3.17 -2.97
(m,
6H), 2.20 (s, 2H), 1.91 (d, J =
5.1 Hz, 6H).
H 1H NMR (300 MHz,
HC N N Chloroform-d) 6 8.91 - 8.75
1
. (m, 2H), 8.11 (d, J = 5.9
Hz,
1H), 7.68 (d, J = 1.8 Hz, 1H),
0 6.37 (tt, J = 3.0, 1.5 Hz,
1H),
352 '.;34) 418.5 6.16 (d, J = 6.0
Hz, 1H), 4.99
(s, 1H), 4.88(d, J = 6.0 Hz,
1H), 4.43 (q, J = 2.8 Hz, 2H),
i -- 4.02 (t, J = 5.4 Hz, 2H),
3.85
(s, 1H), 2.77 -2.64 (m, 2H),
0 2.51 (s, 3H), 2.23 (d, J =
13.2
Hz, 2H), 2.03 - 1.82 (m, 6H).
158
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
H 1H NMR (300 MHz,
HC N N Chloroform-d) 68.67 (d, J =
= 'to 1.9 Hz, 1H), 8.54
(d, J= 1.9
Hz, 1H),8.11 (d, J = 6.0 Hz,
1H), 6.85 (s, 2H), 6.16 (d, J =
353 433.62 6.0 Hz, 1H), 5.00 (s,
1H), 4.85
i1.71,44.)
(d, J = 6.5 Hz, 3H), 3.93 - 3.57
(m, 5H), 3.35 (q, J = 7.1 Hz,
7--A- N 1H), 2.62 (d, J = 8.0 Hz,
1H),
0 2.50 (s, 3H), 2.24 (d, J =
8.9
Hz, 2H), 2.01 - 1.81 (m, 6H).
H 1H NMR (300 MHz, DMS0-
1\144.
r\tr:)''''''' '==." d6) 68.73 (d, J = 1.9 Hz, 1H),
8.61 (d, J = 3.1 Hz, 1H), 8.58
(d, J = 2.0 Hz, 1H), 8.47 (d, J
N 0
= 6.2 Hz, 1H), 7.15(d, J = 2.4
354 407.56 Hz, 1H), 6.99 (d, J =
7.8 Hz,
410 1H), 6.83 (d, J = 2.3 Hz,
1H),
6.65(m, 1H), 4.94 (m, 1H),
r'fii N 3.79 (m, 4H), 3.55 (m, 1H),
0 ,,,,, 3.35 (m, 4H), 2.03 (m, 2H),
1.78 (m, 6H).
H 1H NMR (400 MHz, CDCI3) 6
, N
011
'1:" ft`i In 8.70 (d, J
(d, Hz, 1H), 8.17 (s,
\----A',..õõe=N .C:4 1H), 6.93 (d, J = 2.2 Hz,
1H),
6.71 (d, J = 2.2 Hz, 1H), 5.36
355 N)
...-.- 421.23 (d, J = 7.8
(m, 1H), 4.40 -4.28 (m, 1H),
r\NN 4.02- 3.82 (m, 4H), 3.43 -
3.27 (m, 4H), 3.26- 3.13 (m,
2H), 2.35 (dd, J = 19.4, 9.4
Hz, 2H).
159
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H 1H NMR (400 MHz, CDCI3) 6
N
r ..--# 44-c---\ 8.72(s, 1H), 8.62 (d, J =
1.5
1 i V.---'5 Hz, 1H), 8.50 (s, 1H), 7.74
(d,
J = 1.9 Hz, 1H), 6.95 (d, J =
0 2.0 Hz, 1H), 6.86 (d, J =
1.9
356 --- N 419.19 Hz, 1H), 6.73 (d, J =
2.1 Hz,
1--------N 'µ'''N 1H), 5.44 (d, J = 7.5 Hz,
1H),
4.82 - 4.72 (m, 1H), 4.72 -
4.61 (m, 1H), 4.00 - 3.86 (m,
0õ,....,$ 4H), 3.40- 3.32 (m, 4H),
3.32
- 3.22 (m, 2H), 2.53 -2.40 (m,
2H).
H 1H NMR (400 MHz, CDCI3) 6
HC ,,,,.....," N 8.71 (d, J = 1.9 Hz, 1H),
8.61
j (d, J = 1.9 Hz, 1H), 8.12
(d, J
= 5.9 Hz, 1H), 6.95(d, J = 2.4
Hz, 1H), 6.70 (d, J = 2.4 Hz,
0 N 1H), 6.13(d, J = 5.9 Hz, 1H),
357 393.39
....,' 11 5.06 (s, 1H), 4.72 (p, J = 6.9
Hz, 1H), 4.18 (s, 1H), 3.98-
'''11) 3.85 (m, 4H), 3.40 - 3.27 (m,
4H), 3.27 - 3.13 (m, 2H), 2.50
(s, 3H), 2.36 (ddd, J = 12.7,
10.0, 2.9 Hz, 2H).
1H NMR (300 MHz, DMSO-
H d6) 6 8.72 (d, J = 1.9 Hz,
1H),
8.57(d, J = 1.9 Hz, 1H), 8.22
1.1 r 1 1 (m, 1H), 8.00 (d, J = 2.7
Hz,
KC 'N
N ''--, 1H), 7.73 (d, J = 8.6 Hz,
1H),
- 4.0
b
7.15 (d, J = 2.3 Hz, 1H), 7.02
358 0 ....., t1,..,, 463.41 (dd, J = 8.7,
2.8 Hz, 1H), 6.83 (d, J = 2.4 Hz, 1H), 6.50(d, J
,...- = 7.6 Hz, 1H), 4.93(m, 1H),
N 3.79 (m, 4H), 3.52 (m, 1H),
3.34 (m, 4H), 2.76 (d, J = 4.9
Hz, 3H), 2.04 (m, 2H), 1.79
(m, 6H).
160
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
1H NMR (300 MHz,
Chloroform-d) 6 8.69 (d, J =
H 1.9 Hz, 1H), 8.62(d, J =
1.9
Hz, 1H), 7.99 (d, J = 1.3 Hz,
1:-)CN
1H), 6.92 (dd, J = 16.0, 2.5
Hz, 2H), 5.12 (d, J = 7.5 Hz,
r4400 1H), 476(d, J = 5.4 Hz,
1H),
359 0 0 NH )1) 537.71 4.69 - 4.54 (m, 1H),
4.15 (s,
---vi 1H), 4.01 - 3.82 (m, 4H),
3.72
N
t (ddd, J = 13.2, 7.6, 2.9 Hz,
WC i------N µN 1H), 3.44- 3.21 (m, 5H), 3.21
0 ,) - 2.99 (m, 1H), 2.46 (dq, J
=
....,,,..- 7.9, 3.9 Hz, 1H), 2.34 (s,
3H),
2.22 (t, J = 8.8 Hz, 3H), 1.86
(dd, J = 10.0, 4.8 Hz, 7H),
1.79 - 1.51 (m, 2H).
0 1H NMR (300 MHz,
H
H0.., ,
,A ,..r.,,''' ,r N, Chloroform-d) 6 8.69 (d, J
=
N -= C 1.9 Hz, 1H), 8.61 (d, J =
1.9
H I 1 Hz, 1H), 7.16 (d, J = 1.2
Hz,
\......,,...- --*
0 1H), 6.93 (dd, J = 17.1, 2.5
360 464.58 Hz, 2H), 5.38-5.21
(m,1H),
4.90 - 4.77 (m, 1H), 3.91 (dd,
11 J = 6.0, 3.7 Hz, 5H), 3.44 _
,,, -,....õ,
N N 3.26 (m, 4H), 3.00 (d, J = 5.1
0 .) Hz, 3H), 2.23(d, J = 11.4
Hz,
-....õ.. 2H), 2.04 - 1.76 (m, 6H).
0 1H NMR (300 MHz,
H
WC õ..uN Chloroform-d) 6 8.69 (d, J
=
.
11, 14',, 14 1 Hz, 1H), 8.38 (d, J = 4.9
Hz,
=-....,,-' 0 1H), 7.00 - 6.85 (m,
2H), 6.68
361 478.57 (d, J = 4.9 Hz, 1H),
5.29 (d, J
= 7.7 Hz, 1H), 4.79 (s, 1H),
1 : k 4.04 (s, 1H), 3.92 (dd, J =
6.0,
('N''''N'''N'') 3.8 Hz, 4H), 3.41 - 3.26 (m,
4H), 3.10 (s, 3H), 3.04 (s, 3H),
2.19 (d, J = 8.7 Hz, 2H).
161
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
H 1H NMR (300 MHz,
,.N N Chloroform-d) 6 8.69 (d, J =
1.9 Hz, 1H), 8.63 (t, J = 1.6
, Hz, 1H), 8.04 (s, 1H), 6.95
(d,
.41 1 j1 F ' 0 J = 2.3 Hz, 1H), 6.90 (d, J =
0
362 r, 0 512.55 2.7 Hz, 1H), 4.88 (d, J
= 8.3 )1 Hz, 1H), 4.80 (s, 1H), 4.48 (t,
) J = 5.9 Hz, 2H), 4.17 (s, 1H),
4.01 - 3.81 (m, 4H), 3.34(t, J
.H, 0,i -4.7 Hz, 4H), 2.73 (t, J =
5.9
Hz, 2H), 2.33 (d, J = 1.2 Hz,
8H), 2.07- 1.73 (m, 6H).
1H NMR (300 MHz,
H H Chloroform-d) 6 8.70 (d, J
=
(,,-,õ N if N 41/40.õ 1.9 Hz, 1H), 8.60(d, J =
1.9
Hz, 1H), 6.92 (dd, J = 21.1,
0 2.5 Hz, 2H), 4.74 (s,
1H),4.35
ite 0
(d, J = 7.9 Hz, 1H), 4.12 (d, J
363 --," )4'-, 469.62 = 8.0 Hz, 1H),
4.03 - 3.67 (m,
i 11 5H), 3.63- 3.50 (m, 1H),
3.47
'''.--,, "=--., ,.."' - 3.20(m, 4H), 2.77 (d, J =
I------N N 11.3 Hz, 2H), 2.28 (s, 3H),
2.22 - 2.04 (m, 4H), 1.90 (dd,
J = 38.6, 8.3 Hz, 8H), 1.45
(qd, J = 11.1, 3.8 Hz, 2H).
H H 1H NMR (300 MHz,
re......,i,Ny N.0,4, Chloroform-d) 6 8.70 (d, J =
1.9 Hz, 1H), 8.59 (d, J = 2.0
KC -1 0 Hz, 1H), 6.92 (dd, J =
21.8,
11 0 2.5 Hz, 2H), 4.75 (s, 1H),
4.47
364 0 r,,,,)--se,-,- 497.61 (dd, J = 28.7,
10.8 Hz, 2H),
4.19 (d, J = 7.7 Hz, 1H), 3.99 -
1 3.68 (m, 6H), 3.46 - 3.27 (m,
,,,N, ,-,,....,,.õ,="-z,., .....-
r N N 4H), 3.18(t, J = 11.7 Hz,
1H),
2.85 -2.64 (m, 1H), 2.11 (s,
5H), 1.83 (d, J = 4.9 Hz, 6H),
1.41 - 1.17 (m, 5H).
162
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
H 1H NMR (300 MHz,
F 0 Chloroform-d) 68.67 (dd, J =
21.6, 1.9 Hz, 2H), 8.01 (s,
1H), 6.94 (dd, J = 16.5, 2.5
Hz, 2H), 4.79 (s, 1H), 4.67 (s,
r,
0 }1) 495.54 2H), 4.18 (s, 2H), 3.93 (t, J =
4 1
r------. ---.. 4.8 Hz, 4H), 3.41 - 3.27
(m,
365 NH
6H), 2.20(d, J = 9.9 Hz, 2H),
1.93 (d, J = 5.5 Hz, 6H), 0.63 -
0.50 (m, 2H), 0.29 (t, J = 5.0
Hz, 2H).
H
N....,,,The, õ
F >r...1,- ,..z.z...F.Isi 0,0,0
F
366 F
ik,.. 475.56
i'N N
0 , ,,
H 1H NMR (400 MHz, CDCI3) 6
HC N N
- y.....--' '-',õ," 8.90 (dd, J = 7.2, 1.8 Hz,
2H),
I. 8.75 (dd, J = 4.5, 1.6 Hz,
2H),
8.10 (d, J = 6.0 Hz, 1H), 7.97
0
(d, J = 1.7 Hz, 1H), 7.62 (dd, J
367 ..j.-^.-,--"".;34) 413.34 = 4.5,
1.6 Hz, 2H), 7.38 (d, J =
1.7 Hz, 1H), 6.15(d, J = 6.0
Hz, 1H), 4.98 (s, 1H), 4.93 (s,
r1 1H), 3.86 (s, 1H), 2.49 (s,
3H),
2.34 - 2.18 (m, 2H), 1.96 (dd,
J = 20.7, 8.9 Hz, 6H).
163
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
..-,41.4.,,,,, 1H NMR (300 MHz, DMSO-
H,N d6) 6 8.73 (d, J = 2.0 Hz,
1H),
8.60(d, J = 2.0 Hz, 1H), 7.96
0 (s, 2H), 7.15 (d, J = 2.5
Hz,
368
116 4.-:). 343.48
r's-- 7 4H), 2.72 (t, J = 6.2 Hz,
2H),
2.65 (m, 1H), 2.01 (m, 2H),
0 õ_,......,õ,..-.
1.77 - 1.48 (m, 6H).
NH, 1H NMR (300 MHz,
Chloroform-d) 6 8.71 (d, J =
1.9 Hz, 1H), 8.62(d, J = 1.9
Hz, 1H), 7.71 (s, 1H), 6.94
--r-- 0 (dd, J = 14.6, 2.5 Hz, 2H),
369 436.5 5.09 (d, J = 7.7 Hz, 1H),
4.80
OOP
C H, .}4)
(d, J = 5.4 Hz, 1H), 4.29(s,
2H), 4.01 - 3.84 (m, 4H), 3.45
- 3.28 (m, 4H), 2.49 (s, 3H),
0 ..N.,õ......) 2.21 (q, J = 6.1 Hz, 2H),
1.95
(dt, J = 10.0, 4.1 Hz, 6H).
H 1H NMR (300 MHz,
HN2N Chloroform-d) 6 8.71 (t, J
=
1.9 Hz, 1H), 8.64 (dd, J = 3.9,
0 2.0 Hz, 1H), 8.11 (s, 1H),
7.01
- 6.90 (m, 2H), 4.75 (d, J =
370 448.25 20.3 Hz, 2H), 4.23 (s,
2H),
1-,,k 3.93 (t, J = 4.8 Hz, 4H), 3.68
N., g (t, J = 8.7 Hz, 2H), 3.42-
3.29
(m, 4H), 2.90 (t, J = 8.7 Hz,
2H), 2.34 - 2.12 (m, 2H), 2.11
- 1.83 (m, 6H).
164
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
1H NMR (300 MHz,
HNr
H Chloroform-d) 6 8.70 (d, J
= -6
1.9 Hz, 1H), 8.63 (d, J = 1.9
..,-." Hz, 1H), 7.79 (d, J = 5.6
Hz,
-.....õ IN 1H), 7.03 - 6.89 (m, 2H),
6.05
s*0 (d, J = 5.6 Hz, 1H), 4.77
(d, J
371 447.5 = 5.9 Hz, 1H), 4.21 (s,
1H),
3.93 (dd, J = 6.0, 3.7 Hz, 4H),
II 3.68 (t, J = 8.7 Hz, 2H), 3.36
(dd, J = 5.8, 4.0 Hz, 4H), 2.85
(t, J = 8.7 Hz, 2H), 2.20 (q, J =
12.1,9.5 Hz, 2H), 1.94 (t, J =
5.8 Hz, 6H).
H
CN
7r-- 4 0
4 ''''
N - 'N',;,N
''''0
372 446.44
-...,
1 11)
...,"*...,µ
H
HC 1H NMR (400 MHz, CDCI3) 6
, N N 44.
I . 8.71 (d, J = 1.8 Hz, 1H),
8.63
(d, J = 1.8 Hz, 1H), 7.70 (d, J
0 = 5.0 Hz, 2H), 6.97 (d, J =
1.6
ri
373 421.51
Hz, 1H), 6.92 (d, J = 2.1 Hz,
-c--....,,,A,..--1
1H), 4.81 (s, 1H), 4.65 (s, 1H),
3.99 - 3.85 (m, 5H), 3.40 -
3.30 (m, 4H), 2.37 (s, 3H),
2.28 - 2.16 (m, 2H), 2.01 -
1.82 (m, 6H).
165
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H
CI
N /0
374
ll'f) 441.45
r'-1\41 N
0,.....,,)
H 1H NMR (300 MHz,
N N CH, Chloroform-d) 6 8.81 -8.67
0...+6 -...õ,---.,,,, -,.....r.
, (m, 2H), 8.01 (d, J = 6.0 Hz,
0*Y. N''..'",-:.=-)4 1H), 7.46 (dd, J =
1.7, 0.7 Hz,
1H), 6.98 (d, J = 1.7 Hz, 1H),
375 420 57
6.06 (d, J = 6.0 Hz, 1H), 4.80
-e ...-' t4".=-= .
(d, J = 38.5 Hz, 2H), 4.13 -1 1 3.97 (m, 3H), 3.51
(td, J =
N,.,õ -....., ...õ,
r N 11.3, 3.5 Hz, 2H), 2.99 -
2.80
(m, 1H), 2.41 (s, 3H), 2.21 -
0 2.03 (m, 2H), 1.94 - 1.70
(m,
7H).
H 1 H NMR (300 MHz,
N N r.,...,)
xi .4 1/4 Chloroform-d) 68.69 (d, J =
I
. 1.9 Hz, 1H), 8.62(d, J =
1.9
0 Hz, 1H), 8.07 (s, 1H), 6.93
--I- F C---"
376 0 455.33 4(d.9d8'
''
.----11-)E*11 J- 4=.7171.0(m, 2,
2.5HH),4z, .211-17)(,s,
H,C
1H), 4.00 (s, 3H), 3.92 (dd, J
= 5.8, 3.9 Hz, 4H), 3.42 - 3.26
2H), 1.93 (dd, J = 6.1, 2.4 Hz,
6H).
166
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
H 1H NMR (300 MHz,
Chloroform-d) 68.69 (d, J =
fc - 1.9 Hz, 1H), 8.62 (d, J =
1.9
1 I
Hz, 1H), 7.95 (d, J = 1.2 Hz,
NN.,
CHl,, F Hz, 2H), 4.98 (s, 1H), 4.81
-
''.0 1H), 6.92 (dd, J = 16.9,
2.5
H LNH
,C ...-õ 1
377 512.36
HO õ 0 --Ill) 4.66 (m, 2H), 4.16 (s, 1H),
3.97 - 3.87 (m, 4H), 3.46 (d, J
= 6.1 Hz, 2H), 3.39 - 3.29 (m,
0,õ,..,....) 4H), 2.19 (d, J = 12.5 Hz,
2H),
1.91 (d, J = 5.4 Hz, 6H), 1.26
(s, 6H), 0.94 - 0.83 (m, 1H).
1H NMR (300 MHz,
H Chloroform-d) 6 8.69 (d, J
=
1.9 Hz, 1H), 8.62 (d, J = 1.9
Hz, 1H), 7.99 (d, J = 1.3 Hz,
1H), 6.92 (dd, J = 17.1, 2.5
Hz, 2H), 4.77 (s, 1H), 4.66 (d,
378 0,, NH
..-14 524.38 J = 8.5 Hz, 1H), 4.41 (d, J =
0 )
(------Ni----. 7.7 Hz, 1H), 4.23 - 4.10
(m,
2H), 4.05 - 3.87 (m, 6H), 3.54
(td, J = 11.7, 2.1 Hz, 2H), 3.39
-3.29 (m, 4H), 2.19 (d, J =
11.3 Hz, 2H), 2.08 - 1.85 (m,
8H), 1.64 - 1.44 (m, 2H).
H 1H NMR (300 MHz,
N NI
r y Chloroform-d) 6 8.69 (d, J
=
1.9 Hz, 1H), 8.62 (d, J = 1.9
Hz, 1H), 8.00 (d, J = 1.3 Hz,
1H), 6.95 (d, J = 2.5 Hz, 1H),
4
379 r,r, NH 10 ,)J., 510.38 6.89 (d, J = 2.5 Hz, 1H),
4.87 -
4.56 (m, 4H), 4.16 (s, 1H),
0 ---/ 4.04 - 3.65 (m, 8H), 3.46 _
r'N, N 3.26 (m, 4H), 2.43 - 2.26
(m,
1H), 2.20 (q, J = 6.6, 5.9 Hz,
2H), 1.90 (t, J = 5.5 Hz, 6H).
167
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M+H)
1H NMR (300 MHz,
Chloroform-d) 6 8.69 (d, J =
H 2.0 Hz, 1H), 8.62(d, J =
1.9
rjN N Hz, 1H), 8.00 - 7.91 (m, 1H),
40 6.92 (dd, J = 17.2, 2.5 Hz,
2H), 4.87 -4.64 (m, 2H), 4.59
1 (d, J = 5.7 Hz, 1H), 4.16 (ddt,
380 H 0 ,,-......N H
)1'1 498.36 J = 9.7, 6.8, 3.7 Hz,
2H), 4.01
- 3.85 (m, 4H), 3.75 (d, J =
CHõ (N11.2 Hz, 1H), 3.58 (dd, J =
N 10.9, 7.0 Hz, 1H), 3.48 -
3.23
(m, 4H), 2.36 -2.07 (m, 2H),
-,.....-
1.91 (d, J = 5.5 Hz, 6H), 1.28
(d, J = 6.8 Hz, 3H), 0.97 - 0.78
(m, 1H).
H 1H NMR (300 MHz,
N N Chloroform-d) 6 8.69 (d, J
=
P: 1 1.9 Hz, 1H), 8.62 (d, J =
1.9
NN..., Hz, 1H), 7.98 (d, J = 7.3
Hz,
F Q10 1H), 6.92 (dd, J = 16.7,
2.5
381 _...,,, NH N.,õ,i 523.39 Hz, 2H), 4.87 (d, J =
8.6 Hz,
.'N....)
s...,.,.. 1H), 4.77 (s, 2H), 4.61 (s,
2H),
40 ,.--eji
4.15 (s, 1H), 3.91 (dd, J = 5.9,
, r-----N N 3.8 Hz, 4H), 3.46 - 3.29
(m,
H,C 0) 4H), 2.99 - 2.77 (m, 2H),
2.74
-2.54 (m, 2H), 2.46 -2.10 (m,
6H), 1.91 (d, J = 5.5 Hz, 6H).
1H NMR (300 MHz,
H Chloroform-d) 6 8.69 (d, J
=
N N
.7:IX 1.9 Hz, 1H), 8.62 (d, J =
1.9
Hz, 1H), 8.00 (s, 1H), 6.92
(dd, J = 16.5, 2.5 Hz, 2H),
F 0
4.92 (s, 1H), 4.77 (s, 1H),
382 NH
)4) f 498.32 4.64 (d, J = 8.5 Hz,
1H), 4.16 (s, 1H), 4.03 - 3.82 (m, 4H),
3.65 (q, J = 5.8, 5.4 Hz, 2H),
3.56 (ddd, J = 5.6, 4.7, 1.0 Hz,
I
CH, 0 ,,1 2H), 3.48- 3.21 (m, 7H),
2.19
(d, J = 10.1 Hz, 2H), 1.91 (d, J
= 5.4 Hz, 6H).
168
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
HN2
--- N
1H NMR (300 MHz,
H
Chloroform-d) 6 8.69 (s, 1H),
8.62 (d, J = 1.9 Hz, 1H), 8.55
N.,x.r., (4 414CA (d, J = 1.9 Hz, 1H), 6.93 - 6.71
(m, 3H), 6.22 (dd, J = 3.6, 2.0
383 0 0 476.5 Hz, 1H), 5.01 (d, J = 8.1
Hz,
-..
C H, 1H), 4.71 (s, 1H), 4.34 (s, 1H),
, N 3.92 - 3.78 (m, 7H), 3.26
(dd,
j\\ J = 5.9, 3.9 Hz, 4H), 2.15
(d, J
= 10.8 Hz, 2H), 1.98 - 1.78
1H NMR (300 MHz,
14,0 ¨ N/R-1 Chloroform-d) 68.72 (d, J =
r,li4õ, 2.0 Hz, 1H), 8.65 (d, J =
1.9
t
N.,s. i
õ......., a Hz, 1H), 8.39 (s, 1H), 7.01 -
6.90 (m, 3H), 6.35 (d, J = 3.5
384 460.6
Hz, 1H), 5.05 (t, J = 8.6 Hz,
0
1H), 4.83(d, J = 5.6 Hz, 1H),
4.41 (s, 1H), 4.01 - 3.87 (m,
I
\, N
4H), 3.82 (d, J = 1.1 Hz, 3H),
1.---N'N"
0 j
N\,õ,.,..-- 6H).
1H NMR (300 MHz,
HN Chloroform-d) 6 8.72 (d, J =
H
)----- N
1.9 Hz, 1H), 8.65(d, J = 1.9
Hz, 1H), 8.03(d, J = 5.6 Hz,
NN / µ.
N.,..,..-- 1H), 7.11 (d, J =3.6 Hz,
1H),
7.03 - 6.91 (m, 2H), 6.41 (d, J
385 0 445.5
= 3.6 Hz, 1H), 6.28 (d, J = 5.7
Hz, 1H), 4.83 (s, 1H), 4.56 (d,
N.,õ
oh NI J = 8.0 Hz, 1H), 4.01 -3.88
r---,N,
,i 1H) (s, ,
2.28 (d,
0
Hz, 2H), 2.11 - 1.85 (m, 6H).
169
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
CH,
3 H 1H NMR (400 MHz, CDCI3) 6
,N Ni N H 8.70 (d, J = 1.9 Hz, 1H), 8.62
,C NT:::::-- ,
40 (d, J = 1.9 Hz, 1H), 7.49
(s,
1H), 6.95 (d, J = 2.4 Hz, 1H),
386 480A1
6.89 (d, J = 2.4 Hz, 1H), 5.25
t
CH, N (s, 1H), 4.72 (s, 1H), 4.18
(s,
'-.-. 1H), 3.96- 3.87 (m, 4H),
3.77
r-----N 14 (s, 3H), 3.39 - 3.30 (m,
4H),
3.10 (s, 6H), 2.21 -2.12 (m,
0, ) 2H), 2.02- 1.85 (m, 6H).
-........-
H H
N , CDCI3) 6
HC I( r---, 8.70(d, J = 1.9 Hz, 1H),
8.62
N-
i (d, J = 1.9 Hz, 1H), 7.49
(s,
-...
0 1/4....õ,..00'., 0
1H), 6.95 (d, J = 2.4 Hz, 1H),
387 C H,
..-'` 1 N=-=,,'I 466.4
(s, 1H), 4.72 (s, 1H), 4.18 (s,
1H), 3.96- 3.87 (m, 4H), 3.77
r----44 N--- (s, 3H), 3.39 - 3.30 (m,
4H),
3.10 (s, 6H), 2.21 -2.12 (m,
2H), 2.02- 1.85 (m, 6H).
H 1H NMR (400 MHz, CDCI3) 6
rN N r.,...,..) .. 8.69 (d, J = 1.9 Hz, 1H), 8.63
X 4µ. (d, J = 1.9 Hz, 1H), 8.04
(s,
1H), 6.94(d, J =2.4 Hz, 1H),
N'T 0 C=>µ.....0
t 6.89 (d, J = 2.4 Hz, 1H),
5.15
388 480.41 (d, J = 8.4 Hz, 1H),
4.75 (s,
r---N --- fr
2.25 - 2.13 (m, 2H), 2.00 -
1.83 (m, 6H).
170
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M-hH)
H 1H NMR (300 MHz,
C
1.9 Hz, 1H), 8.61 (d, J
Chloroform-d) 68.69 (d, J = = 1.9
Hz, 1H), 8.50(d, J = 1.1 Hz,
= 0 1H), 6.93 (dd, J =
16.5, 2.5
Hz, 2H), 6.49 (s, 1H), 4.90 (s,
389 40 .õ--,,.. 504.48
1H), 4.80 (s, 1H), 4.08 - 3.87
(m, 5H), 3.44 - 3.29 (m, 6H),
r-----N N 2.45 (t, J = 5.3 Hz, 4H),
2.26 -
2.14 (m, 2H), 1.90 (t, J = 6.5
Hz, 6H), 1.63 (d, J = 5.7 Hz,
4H), 1.53- 1.43 (m, 2H).
H 1H NMR (300 MHz,
H,C, ....---...._,...,N Chloroform-d) 6 8.69 (d, J =
NT T, ''''=
CH: tf ,--N 1.9 Hz, 1H), 8.61 (d, J = 1.9
Hz, 1H), 8.50 (d, J = 1.1 Hz,
0
1H), 6.93 (dd, J = 16.5, 2.5
390 --" NN,.."\I 464.62 Hz, 2H), 6.44
(d, J = 1.1 Hz,
1 1 1
1H), 4.93 (s, 1H), 4.80 (s, 1H),
3.97- 3.87 (m, 4H), 3.47 -
3.29 (m, 6H), 2.31 (s, 6H),
2.21 (d, J = 9.1 Hz, 2H), 1.89
(t, J = 6.9 Hz, 6H).
1H NMR (300 MHz,
0 Chloroform-d) 6 8.68 (d, J
=
ic H H
1.9 Hz, 1H), 8.58 (d, J = 1.9
1 Hz, 1H), 6.94 (d, J = 2.5
Hz,
1,1,0 .y.) 0 1H), 6.86 (d, J = 2.5 Hz, 1H),
'*0 4.81 -4.61 (m, 3H), 4.47
(td, J
391 500.63 = 8.7, 5.2 Hz, 1H), 3.98 - 3.88
CH,
...") (m, 4H), 3.85 - 3.66 (m, 4H),
3.40 - 3.25 (m, 4H), 2.24 _
r-----N N/ 2.07 (m, 2H), 1.93 - 1.74
(m,
6H), 1.71-1.64(m, 1H). 1.58 -
1.39 (m, 2H), 0.93 (t, J = 6.3
Hz, 6H).
171
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (M-h1-1)
CH, 0
''.., 1H NMR (300 MHz, DMSO-
H,C
H d6) 68.71 (d, J = 1.9 Hz,
1H),
8.58 (d, J = 1.9 Hz, 1H), 7.10
0 (d, J = 2.5 Hz, 1H), 6.82 (d, J
392 443.61
N = 2.6 Hz, 2H), 4.92 (m,
1H),
'-.-, 3.78 (m, 4H), 3.30 (m, 5H),
r---- ife) 2.84 (t, J = 6.0 Hz, 2H),
1.96 -
N
1.33 (m, 18H).
0,
-......,
H 1H NMR (300 MHz, DMSO-
W'' N4y.,....... d6) 6 8.73 (d, J = 1.9 Hz,
1H),
HO -,õ.. 1 ,
l'N'F'v0 8.58 (d, J = 1.9 Hz, 1H), 8.10
(d, J = 2.7 Hz, 1H), 7.97 (d, J
= 8.9 Hz, 1H), 7.38 (dd, J =
393 0 N 450.58 9.0, 2.7 Hz, 1H), 7.16
(d, J =
'''-)
11 2.4 Hz, 1H), 6.84 (d, J = 2.3
Hz, 1H), 4.96 (m, 1H), 3.79
r------N s--- -'1Cr- (m, 4H), 3.66 (m, 1H), 3.3
(m,
4H), 2.14- 1.95 (m, 2H), 1.81
(m, 5H).
CH, 1H NMR (300 MHz, DMS0-
i H
0 N d6) 6 8.72 (d, J = 1.9 Hz,
1H),
,.." 8.58 (d, J = 1.9 Hz, 1H), 7.54
i I 44\01,,N,
(d, J = 8.6 Hz, 1H), 7.14(d, J
0 N.õ
KC' 0 = 2.4 Hz, 1H), 6.83 (d, J =
2.3
394 493.61 Hz, 1H), 6.48 (d, J =
7.7 Hz,
0 N
---` ---' ''''.1 1H), 6.30 - 6.18 (m, 2H), 4.92
1 1 (m, 1H), 3.79 (m, 4H), 3.74
(s,
3H), 3.66 (s, 3H), 3.55 (m,
1H), 3.34 (m, 4H), 2.03 (m,
2H), 1.78 (m, 6H).
172
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
H 1H NMR (300 MHz,
HO N Is144,r,õ1 Chloroform-d) 6 8.61 (d, J
=
1.9 Hz, 1H), 8.53 (d, J = 2.0
X1 Hz, 1H), 7.13 (d, J = 5.3 Hz,
F C"--"`"'L* 0
1H), 6.84 (dd, J = 20.0, 2.4
395 441.2 Hz, 2H), 5.40 (d, J = 8.2
Hz,
....CLI...)44)
1H), 4.72 (s, 1H), 4.31 (d, J =
7.0 Hz, 1H), 3.83 (dd, J = 5.8,
r------N N'') 3.8 Hz, 4H), 3.26 (t, J =
4.9
0 ",) Hz, 4H), 2.14 (d, J = 12.3
Hz,
2H), 1.95- 1.68 (m, 6H).
CH, 1H NMR (300 MHz,
H Chloroform-d) 6 8.61 (d, J
=
1 1 0 1.9 Hz, 1H), 8.54(d, J = 1.9
Hz, 1H), 7.72 (d, J = 2.9 Hz,
1H), 6.87(d, J = 2.5 Hz, 1H),
H
396 455.2 6.81 (d, J = 2.5 Hz, 1H),
5.03
N
(d, J = 7.8 Hz, 1H), 4.71 (s,
i 1H), 4.16 (s, 2H), 3.83 (q, J =
r*N'Ill ....µ,.
3.9, 3.1 Hz, 7H), 3.32 - 3.21
(m, 4H), 2.13 (d, J = 11.2 Hz,
0 ,, 2H), 1.83 (t, J = 6.5 Hz,
6H).
H
142N N N 1H NMR (300 MHz,
...i,.õ -..õ,..
1. Chloroform-d) 6 8.70 (d, J =
1.9 Hz, 1H), 8.63(d, J = 1.9
0 Hz, 1H), 7.89(d, J = 5.8 Hz,
397 422.5
1H), 7.02 - 6.88 (m, 2H), 5.79
..--' '''-il.'-4,44 i
(d, J = 5.7 Hz, 1H), 4.80 (s,
1H), 4.61 (s, 2H), 4.08 - 3.88
(m, 5H), 3.35 (dd, J = 5.6, 4.1
0 ,.,.."...) Hz, 4H), 2.27 - 2.09 (m,
2H),
1.91 (d, J = 5.0 Hz, 6H).
173
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (M-h1-1)
C EA,
$ 1H NMR (300 MHz,
N Chloroform-d) 6 8.62 (d, J
=
H 1.9 Hz, 1H), 8.54 (d, J=
1.9
Hz, 1H), 8.38 (s, 1H), 6.94-
6.74 (m, 2H), 4.69 (s, 1H),
N
398 --....---' 0 476.3 4.34 (s, 1H), 4.25 (s,
1H),
3.84 (dd, J = 5.9, 3.8 Hz, 4H),
Sip ,,," ) 3.30 - 3.14 (m, 6H), 2.79 (d, J
= 5.7 Hz, 2H), 2.71 (d, J = 5.6
it õ,IN N Hz, 2H), 2.49 (s, 3H), 2.13
(m,
2H), 1.85 (d, J = 5.5 Hz, 6H).
CH, 1H NMR (300 MHz, CDCI3) 6
t
H,C
8.69 (d, J = 1.9 Hz, 1H), 8.61
N.,.
(d, J = 1.9 Hz, 1H), 8.08 (d, J
0 = 6.3 Hz, 1H), 6.96 (d, J =
2.5
'...6.0 Hz, 1H), 6.92 (d, J = 2.4 Hz,
399 435.35 1H), 6.23 (d, J = 6.3
Hz, 1H),
N
.....--' --- j 4.87 (s, 1H), 4.69 (s, 1H),
i 4.00- 3.83 (m, 4H), 3.45 -
r*N'Ill ....µ,. =-õ,,,tsi 3.24 (m, 4H), 2.98 (s, 3H),
2.50 (s, 3H), 2.45 - 2.30 (m,
0 õ,-, 2H), 1.92- 1.64 (m, 6H).
H
N. 1H NMR (300 MHz,
Chloroform-d) 6 8.71 (d, J =
H N ,..._,,,...N ===== 1.9 Hz, 1H), 8.62 (d, J =
1.9
i 0
Hz, 1H), 6.94 (dd, J = 19.8,
400 NH 452.3 2.4 Hz, 2H), 5.09 (s,
1H), 4.81
,
(s, 1H), 4.01 - 3.88 (m, 4H),
r.,....._ , .......õ ) 3.42 - 3.29 (m, 4H),
2.91 (d, J
= 4.8 Hz, 3H), 2.18 (m, 2H),
1.90 (s, 6H).
174
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H
01,,....:.,.,N N 1H NMR (300 MHz, CDCI3) 6
1 X 8.69(d, J = 1.9 Hz, 1H), 8.62
(d, J = 1.9 Hz, 1H), 7.51 (s,
0 0 1H), 6.95(d, J =2.4 Hz,
1H),
0
401 CH. " 6.89 (d, J = 2.4 Hz, 1H),
5.56
) 471.32 (d, J = 8.0 Hz, 1H), 4.75 (s,
1H), 4.25 (s, 1H), 3.96 - 3.89
(m, 4H), 3.88 (s, 3H), 3.47 -
3.23 (m, 4H), 2.29 - 2.06 (m,
2H), 2.06- 1.78 (m, 6H).
H
N 1H NMR (300 MHz, CDCI3) 6
X
44.04õ
N----, (d, J = 1.9 Hz, 1H), 8.14 (s,
0 0 1H), 6.96 (d, J = 2.4 Hz,
1H),
1 6.90 (d, J = 2.5 Hz, 1H),
5.52
47136 402 CO CH,
---14."`) .
(d, J = 8.6 Hz, 1H), 4.79 (s,
ji 1H), 4.18(s 1H), 3.96 - 3.89
(m, 4H), 3.89 (s, 3H), 3.41 -
3.27 (m, 4H), 2.31 - 2.14 (m,
0 ,,,,,....õ,,A 2H), 2.03- 1.83 (m, 6H).
H 1H NMR (300 MHz, CDCI3) 6
N
8.69(d, J = 1.9 Hz, 1H), 8.61
(d, J = 1.9 Hz, 1H), 6.95 (d, J
= 2.4 Hz, 1H), 6.89 (d, J = 2.5
9 o
Hz, 1H), 4.77 (s, 1H), 4.68 (d,
403 ,,,--'1'r'1µ) 463.4 J = 8.4 Hz,
1H), 4.58 (t, J =
1 9.1 Hz, 2H), 4.25 (s, 1H),
3.98
4-*--.... .....---(..--, ,..e.) - 3.84 (m, 4H), 3.40 - 3.27 (m,
( 14 -------N 4H), 3.19 (t, J = 9.0 Hz,
2H),
2.49(s, 3H), 2.25 - 2.10 (m,
2H), 2.00- 1.82 (m, 6H).
175
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (MH-H)
0
H
1H NMR (300 MHz, DMS0-
d6) 68.72 (d, J = 1.9 Hz, 1H),
1 11 1 8.57(d, J = 1.9 Hz, 1H),
7.69
0 (s, 1H), 7.15 (d, J = 2.5 Hz,
404 480.56 1H), 7.01 (s, 1H), 6.84
(d, J =
2.3 Hz, 1H), 4.94 (m, 1H),
1 11 3.75 (m, 8H), 3.34 (m, 4H),
2.05 (m, 2H), 1.86 - 1.70 (m,
6H).
0
H 1H NMR (300 MHz, DMSO-
H,C.õ - N41/4 d6) 68.72 (d, J = 1.9 Hz, 1H),
N Ow
H 8.57 (d, J = 1.9 Hz, 1H),
8.01
(m, 1H), 7.14 (s, 1H), 7.01 -
F 0 6.90 (m, 1H), 6.82 (m, 2H),
6.71 (m, 1H), 5.70 (d, J = 7.9
405 480.56
1 Ã Hz, 1H), 4.91 (m, 1H), 3.79
(m, 4H), 3.34 (m, 5H), 2.74 (d,
i-----N N J = 4.6 Hz, 3H), 2.02 (m,
2H),
1.76 (m, 6H).
1H NMR (300 MHz, DMS0-
KC, d6) 6 8.72 (d, J = 1.9 Hz, 1H),
NH 8.58(d, J = 1.9 Hz, 1H),
8.27
H
(d, J = 5.2 Hz, 1H), 7.46 (dd, J
0 -)1 i fµ'14sra, = 8.4, 7.1 Hz, 1H), 7.15 (d, J =
f 2.4 Hz, 1H), 7.10 (dd, J =
7.1,
--....,,,
0 0.9 Hz, 1H), 6.83 (d, J = 2.3
406 463.68
Hz, 1H), 6.75 (d, J = 7.8 Hz,
1H), 6.68 (dd, J = 8.4, 0.9 Hz,
40 .,õ ) 1H),4.91 (m, 1H), 4.14 (m,
('N N 1H), 3.80 (m, 4H), 3.35 (m,
0,,....4õ..-1 4H), 2.82 (d, J = 4.9 Hz,
3H),
2.03 (m, 2H), 1.93 - 1.73 (m,
6H).
176
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
0
H
KC, 0,40 1H NMR (300 MHz,
I 11 1 Chloroform-d) rotomers, 6
9.07 (m, 1H), 8.89 (m, 1H),
0 8.75 (m, 1H), 7.47 (m, 1H),
407 536.49
6.96 (s, 1H), 4.95 (m, 1H),
. CH,
b: 11 3.97 (m, 4H), 3.82 (m, 4H),
3.38 (s, 3H), 2.24 (m, 8H),
1.60 (s, 9H).
H 1H NMR (300 MHz, DMS0-
HC 11111 0 N d6) 6 8.72 (d, J = 1.9 Hz,
1H),
, ".* i---
8.57(d, J = 1.9 Hz, 1H), 7.13
(d, J = 2.4 Hz, 1H), 6.94(t, J =
8.0 Hz, 1H), 6.83 (d, J = 2.3
408 N 435.6 Hz, 1H), 6.26 - 6.14 (m,
2H),
11 6.10 -6.03 (m, 1H), 5.57 (d, J
= 7.8 Hz, 1H), 4.90 (m, 1H),
r-------N s-- ''N''''' 3.79 (m, 4H), 3.66 (s,
3H),
3.33 (m, 4H), 2.03 (m, 2H),
1.77 (m, 6H).
H 1 H NMR (300 MHz, CDCI3) 6
(N N 8.70(d, J = 1.9 Hz, 1H), 8.63
-3( ' CI (d, J = 1.9 Hz, 1H), 8.47(s,
N'---, 1H), 8.18 (s, 1H), 6.96 (d, J =
CI O
2.4 Hz, 1H),6.91 (d, J = 2.4
409 441.29 Hz, 1H), 5.47 (d, J =
7.6 Hz,
1 , 1H), 4.80 (s, 1H),4.31 -
4.19
r-Pli -....-_,-- 4,-...;,- (m, 1H), 4.02 -
3.79 (m, 4H),
3.48 - 3.24 (m, 4H), 2.32 -
2.17 (m, 2H), 2.09- 1.79 (m,
6H).
177
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
1H NMR (300 MHz,
Chloroform-d) 6 8.68 (d, J =
CL, H H
11 2.0 Hz, 1H), 8.59 (d, J =
1.9
Hz, 1H), 6.94(d, J = 2.5 Hz,
1H), 6.87 (d, J = 2.5 Hz, 1H),
4.73 (d, J = 5.5 Hz, 1H), 4.49 -
0 0
'4.0 4.19 (m, 2H), 4.03 - 3.87
(m,
410 468.38 4H), 3.81 (q, J = 6.6
Hz, 1H),
3.44 - 3.25 (m, 4H), 2.99 (t, J
''' 1)1) = 6.3 Hz, 2H), 2.13 (q, J =
7.5,
,---, "---, 5.0 Hz, 2H), 1.99 - 1.57
(m,
i N 11H), 1.42 (dqd, J = 10.0,
7.1,
6.7, 3.4 Hz, 1H), 1.20 (tt, J =
17.8, 10.5 Hz, 3H), 0.91 (q, J
= 12.1 Hz, 2H).
C H ,
t H 1 H NMR (300 MHz,
WC AlyN4" Chloroform-d) 6 8.69 (d, J
=
1.9 Hz, 1H),8.61 (d, J = 1.9
0 N*4* Hz, 1H), 6.94 (d, J = 2.5
Hz,
0 411 400.37 1H), 6.87 (d, J = 2.5
Hz, 1H),
4.70 (d, J = 2.6 Hz, 1H), 4.41
,...--' ----14
1 i (d, J = 7.7 Hz, 1H), 3.97 -
3.81
0) Hz, 2H), 1.98 - 1.71 (m,
6H).
0 I
H 1H NMR (300 MHz,
Nõri 1\10* Chloroform-d) 68.69 (d, J =
1.9 Hz, 1H), 8.60 (d, J = 1.9
o Hz, 1H), 6.90 (dd, J =
21.6,
0 2.5 Hz, 2H), 4.72 (s, 1H),
4.48
412 442.37
, (d, J = 7.9 Hz, 1H), 4.04 -
3.82
(m, 4H), 3.78 - 3.62 (m, 4H),
t
2.15 (s, 2H), 1.84 (d, J = 4.8
0,õ..,..J. Hz, 6H).
178
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H,C .....õ 1H NMR (300 MHz,
\ H
H,C Chloroform-d) 6 8.69 (d, J
=
-N N
0 1.9 Hz, 1H), 8.61 (d, J =
2.0
Hz, 1H), 6.94(d, J = 2.5 Hz,
1H), 6.87 (d, J -2.5 Hz, 1H),
413 428.37 4.71 (d, J = 5.4 Hz,
1H), 4.37
4011 )4"`Nt
1 (d, J = 7.9 Hz, 1H), 3.99 -
3.81
(m, 4H), 3.44 - 3.09 (m, 8H),
2.24 - 2.06 (m, 2H), 1.94 -
1.73(m, 6H), 1.15(t, J = 7.1
0 i Hz, 6H).
-....õ--
KC, õ--=õ. 1H NMR (300 MHz,
1 H Chloroform-d) 6 8.69 (d, J
=
[ N N 1.9 Hz, 1H), 8.60(d, J =
1.9
sir 0 Hz, 1H), 6.94 (d, J = 2.5 Hz,
0 1 Ni, 1H), 6.87 (d, J = 2.5 Hz,
1H),
0
414 455.42 4.72 (d, J = 5.6 Hz,
1H), 4.50
,, (d, J = 7.6 Hz, 1H), 4.02 -
3.80
(m, 4H), 3.49 - 3.18 (m, 8H),
2.41 (t, J = 5.1 Hz, 4H), 2.32
(-----N ii ) (s, 3H), 2.22 -2.05 (m,
2H),
1.95 - 1.75 (m, 6H).
H 1H NMR (300 MHz,
ON N
0 Ow Chloroform-d) 6 8.69 (d, J
=
1.9 Hz, 1H), 8.61 (d, J = 1.9
0 1H), 6.87 (d, J
415 426.52
4.70 (s, 1H), 4.24 (d, J = 7.9
Sp }4-71
r---------N \-ti-3 Hz, 1H), 4.02 - 3.82 (m,
5H),
344 - 3.23 (m, 8H), 2.61 (s,
179
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
a 1H NMR (300 MHz,
H Chloroform-d) 6 8.69 (d, J
=
N
'-ii '"TO 1.9 Hz, 1H), 8.61 (d, J =
1.9
Hz, 1H), 6.94 (d, J = 2.4 Hz,
0 -Nvo 1H), 6.87 (d, J = 2.5 Hz,
1H),
416 440.39 4.70 (d, J = 4.4 Hz,
1H), 4.48
)4,..,,,,, .....aii (d, J = 7.6 Hz, 1H), 4.03 -
3.80
(m, 5H), 3.49 (d, J = 4.2 Hz,
2H), 3.45 - 3.17 (m, 8H), 2.23
N 411111 ''14-'-- -2.04 (m, 2H), 2.02 - 1.73
(m,
6H), 1.56 - 1.49 (m, 4H).
H
N 1H NMR (300 MHz,
e 1
Chloroform-d) 6 8.81 - 8.55
N---, (m, 3H), 6.94 (dd, J =
17.2,
0
2.5 Hz, 2H), 6.65 (d, J = 1.1
417 ---$."`) 475.36 Hz, 1H), 4.84 (td, J
= 5.1, 2.4
F F
F Hz, 1H), 4.01 - 3.86 (m, 4H),
3.42 - 3.29 (m, 4H), 2.35 -
r-..N N 2.11 (m, 2H), 2.05 - 1.82
(m,
o)
6H).
........N n 1 H NMR (300 MHz,
Chloroform-d) 68.71 (d, J =
1.9 Hz, 1H), 8.63(d, J = 1.9
F>r,c,..... L 0, Hz, 1H), 8.49 (d, J = 4.9 Hz,
0
F 1H), 7.04 - 6.89 (m, 2H),
6.81
418 F e 475.4 (d, J = 4.9 Hz, 1H), 5.54
(d, J l )1)
1----N -II = 7.9 Hz, 1H), 4.80(d, J =
5.2
Hz, 1H), 4.17 - 3.98 (m, 1H),
3.98 - 3.87 (m, 4H), 3.42 -
3.31 (m, 4H), 2.33 - 2.12 (m,
2H), 2.06- 1.72 (m, 6H).
180
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M+H)
0
1 0
H
HO
N 1H NMR (300 MHz,
ti 4,
Chloroform-d) rotomers 6 8.82
(d, J = 2.9 Hz, 1H), 8.74 (m,
0 1H), 8.00 (m, 1H), 7.55 - 7.47
419 450.38
N) (m, 1H), 7.25 (m, 1H), 7.14
(m, 1H), 7.05 (m, 1H), 3.97
(m, 5H), 3.47 (m, 5H), 2.30 (s,
N N11 2H), 2.0 (m, 6H)..
0,,,....$
0
H
N, õ.".õ
HO 0 --.T.' -*-= 1H NMR (300 MHz, DMSO-
d6) 68.73 (d, J = 1.9 Hz, 1H),
F C.'"'".*0 8.58(d, J = 2.0 Hz, 1H),
7.27 -
6.96 (m, 4H), 6.83 (d, J = 2.3
;14 Is Hz, 1H), 4.92 (m, 1H), 3.85
-
420 467.48
3.74 (m, 4H), 3.45 (m, 1H),
r------N ..õ
3.34 (m, 4H), 2.05 (m, 2H),
1.76 (m, 6H).
0 ,J
-.......õ
CH: 0
H,C , .,:aH
WC
' N a 1H NMR (300 MHz, DMS0-
0 j,/ "" ,
d6) 68.93 (d, J = 1.8 Hz, 1H),
'N. 8.80 (d, J = 1.8 Hz, 1H),
7.20
F 0 (s, 1H), 7.13 -6.85 (m,
4H),
421 523.58
4.92 (m, 1H), 3.86 - 3.79 (m,
,....,-"'",..
1 1 4H), 3.32 (m, 5H), 2.09 (m,
2H), 1.90- 1.73 (m, 6H), 1.55
(s, 9H).
181
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1-H NMR
Compound Structure
N
1H NMR (300 MHz,
H Chloroform-d) 6 8.72 - 8.52
N
(m, 3H), 8.38 (s, 1H), 6.87
..---
(dd, J = 15.7, 2.5 Hz, 2H),
422 0 432.5
---....---' 5.50 (d, J = 7.9 Hz, 1H), 4.79
(p, J = 3.5 Hz, 1H), 4.20 (dp, J
= 14.0, 5.2, 4.7 Hz, 1H), 3.90-
S
,,,.....N, i1
3.76 (m, 4H), 3.32 - 3.22 (m,
1.--------,, mil t\i' 4H), 2.29 - 2.07 (m, 2H), 1.99
- 1.71 (m, 6H).
H 1H NMR (300 MHz,
H tirt N
,..---' Chloroform-d) 6 8.74 (d, J =
1.9 Hz, 1H), 8.65 (s, 1H), 8.01
(s, 1H), 7.05 -6.88 (m, 2H),
481.3 4.87 (s, 1H), 3.95 (d, J =
4.7
423 )
Cl .N Hz, 4H), 3.77 - 3.56 (m,
1H),
'11 3.37 (dd, J = 5.8, 3.8 Hz, 4H),
T --N---) 3.11 (qd, J = 7.4, 4.2 Hz,
1H),
2.28 (s, 2H), 1.98 (dd, J =
22.7, 9.4 Hz, 6H).
11 1H NMR (300 MHz,
HO -C-yi Chloroform-d) 6 8.62 (d, J
=
Ns., 419,N, 1.9 Hz, 1H), 8.52 (d, J =
1.9
-....õ..", 0 Hz, 1H), 8.46 - 8.35 (m, 1H),
6.84 (dd, J = 16.8, 2.5 Hz,
424 437.3 2H), 6.28 - 6.20 (m, 1H),
5.08
...,:oC)4)
(s, 1H), 4.72 (d, J = 5.2 Hz,
1H), 4.58 - 4.43 (m, 2H), 3.93
(------N --- -1, _ 3.77 (m, 4H), 3.34 - 3.19
(m,
0 I 4H), 2.14 (dd, J = 9.6, 5.4
Hz,
2H), 1.93 - 1.73 (m, 6H).
182
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M+H)
H H 1H NMR (300 MHz,
HC N N
Chloroform-d) 6 8.68 (d, J =
2.0 Hz, 1H), 8.59 (d, J = 2.0
CH: 0 Hz, 1H), 6.93 (d, J = 2.5
Hz,
µ.*0
1H), 6.86 (d, J = 2.5 Hz, 1H),
425 .,--)Ls= 428.46 4.72(s, 1H),
4.20 (s, 1H),
..,
....,,Ce s.õ. ''' i
4.11 (s, 1H), 3.98 - 3.85 (m,
4H), 3.78 (s, 1H), 3.40 - 3.24
r------N N (m, 4H), 2.14 (d, J = 9.2
Hz,
2H), 1.81 (t, J = 6.3 Hz, 6H),
1.33 (s, 9H).
H
e, N 1H NMR (300 MHz,
ts,---, C
0
8.90 (m, 1H), 8.79 - 8.64 (m,hloroform-d) 6 9.26 (m, 2H),
426 - -
499.35 2H), 7.22- 7.01 (m, 3H),
4.97
---
14.--,õ IN 4111 14) (m, 1H), 4.01 - 3.87 (m,
4H),
3.38 (m, 5H), 2.77 (s, 3H),
2.19 (m, 9H).
CH, 0,,,..,,)
H
1H NMR (300 MHz,
Chloroform-d) 68.94 -8.63
(m, 2H), 8.07 (d, J = 2.8 Hz,
N 0 1H), 7.45(d, J =8.7 Hz,
1H),
F F 7.14 -6.87 (m, 3H), 4.84
(m,
427 474.33
di 1**1 1H), 3.99 - 3.87 (m, 4H), 3.62
- 3.48(m, 1H), 3.35 (dt, J =
42.8, 4.8 Hz, 4H), 2.32 - 2.14
(m, 2H), 1.91 (d, J = 17.8 Hz,
6H).
183
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (M-h1-1)
H
y, 1H NMR (300 MHz,
F 1 Chloroform-d)6 8.81 -8.64
)1!'".14 0 (m, 2H), 8.20 (s, 2H), 7.08
(d,
F J = 2.4 Hz, 1H), 6.94(d, J
=
428 F Aga -Its 475.41 2.4 Hz, 1H), 4.90
-4.82 (m,
1H), 4.42 (m, 1H), 3.98 - 3.88
r-N N (m, 4H), 3.57 (m, 1H), 3.45 -
3.26 (m, 4H), 2.33 - 2.18 (m,
2H), 2.04- 1.82 (m, 6H).
H 1H NMR (300 MHz,
N Chloroform-d) 6 8.70 (d, J
=
t4---- t
I
'1/4 0 ''''' 0 2.0 Hz, 1H), 8.63 (d, J =
2.0
-....õ...
Hz, 1H), 8.42 (m, 1H), 8.16 -
8.08 (m, 1H), 7.20 (s, 1H),
429 F F 474.37 7.04 -6.98 (m, 1H), 6.94
(d, J
F ---tµ.--....-;%-
N i
= 2.4 Hz, 1H), 4.84 (m, 1H),
r
4.01 - 3.87 (m, 4H), 3.54 (m, 'N1 1H), 3.42 - 3.26 (m, 4H),
2.33
-2.19 (m, 2H), 2.08 - 1.80 (m,
6H).
H 1H NMR (300 MHz,
N N
..---- y .0 Chloroform-d) 6 8.69 (d, J =
.----.., sqt0 1.9 Hz, 1H), 8.62(d, J = 1.9
Hz, 1H), 8.15 (s, 2H), 7.03-
430 ,õ141,) 6.85 (m, 2H), 5.09 (d, J =
8.1
,...,cri, 533.47 Hz, 1H), 4.78 (s, 1H),
3.92
H,C I (dd, J = 5.9, 3.7 Hz, 5H),
3.40
- 3.24 (m, 4H), 2.71 - 2.33 (m,
8H), 2.30 (s, 3H), 2.20 (s, 2H),
1.91 (d, J = 5.0 Hz, 6H), 1.26
(d, J = 3.1 Hz, 4H).
184
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (M-h1-1)
H 1H NMR (300 MHz,
N ,N
4-1 41/4(21, Chloroform-d) 68.69 (d, J =
1.9 Hz, 1H), 8.62 (d, J = 1.9
HC Hz, 1H), 8.15 (s, 2H), 6.99
-
0
i 6.86 (m, 2H), 5.10 (d, J =
8.1
431 CH, ..,N 478.47 Hz, 1H), 4.79 (d, J =
5.9 Hz,
401 ..,..,
H), 4.09- 3.85 (m, 5H), 3.41
i
N - 3.25 (m, 4H), 2.68 - 2.51 (m,
2H), 2.52 - 2.37 (m, 2H), 2.28
0) (s, 6H), 2.19 (q, J = 6.2,
5.8
Hz, 2H), 2.02 - 1.81 (m, 6H).
H
1H NMR (300 MHz,
)4 syl N4µ Chloroform-d) 68.69 (d, J =
1.9 Hz, 1H), 8.62 (d, J = 1.9
CH C:10 Hz, 1H), 8.16 (s, 2H), 6.98
-
6.86 (m, 2H), 5.10 (d, J = 8.1
432
411 11''N 504.44
Hz, 1H), 4.78 (s, 1H), 4.08-
3.83 (m, 5H), 3.39 - 3.27 (m,
(NH N 4H), 2.60 (d, J = 16.9 Hz,
8H),
0,,,I 2.19 (d, J = 7.8 Hz, 2H),
2.01 -
1.74(m, 10H).
H 1H NMR (300 MHz,
N N Chloroform-d) 68.69 (d, J =
1.9 Hz, 1H), 8.62 (d, J = 1.9
.0
Hz, 1H), 8.15 (s, 2H), 6.99 -
.õ...---...N 0 6.85 (m, 2H), 5.09 (d, J =
8.1
Hz, 1H), 4.78 (s, 1H), 4.06 -
433 518.72
3.85 (m, 5H), 3.40- 3.27 (m,
4H), 2.67 - 2.55 (m, 2H), 2.47
(NI? N (d, J = 9.1 Hz, 6H), 2.19
(d, J
= 9.6 Hz, 2H), 1.91 (d, J = 4.8
0 )
Hz, 6H), 1.62 (d, J = 5.5 Hz,
6H).
185
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M+H)
H 1H NMR (300 MHz,
a Chloroform-d) 6 8.69 (d, J
=
1.9 Hz, 1H), 8.62 (d, J = 1.9
Na a Hz, 1H), 8.16 (s, 2H), 6.99-
0,N
434 # 2 6.84 (m, 2H), 5.11 (d, J =
8.1
520 .47 Hz, 1H), 4.78 (s, 1H), 4.05
-
3.87 (m, 5H), 3.78 - 3.66 (m,
N 4H), 3.40 - 3.27 (m, 4H),
2.69
-2.43 (m, 8H), 2.19 (d, J = 8.2
Hz, 2H), 1.91 (d, J = 4.8 Hz,
6H).
H 1H NMR (400 MHz, CDCI3) 6
H,C N. 8.70 (d, J = 1.9 Hz, 1H),
8.62
'µIN' (d, J = 1.9 Hz, 1H), 7.68
(d, J
= 2.1 Hz, 1H), 6.96 (d, J = 2.4
II
,, 0 0 Hz, 1H), 6.91 (d, J = 2.4
Hz,
435 N 461.37 1H), 6.76 (d, J = 2.2
Hz, 1H), .,....,' ) 5.21 (d, J = 8.4 Hz, 1H), 4.86 -
4.76 (m, 1H), 4.45 - 4.33 (m,
1H), 3.98- 3.84 (m, 4H), 3.39
- 3.25 (m, 4H), 2.59 (s, 3H),
2.31 - 2.16 (m, 2H), 2.06 -
1.89 (m, 6H).
H
HC N N 1H NMR (400 MHz, CDCI3) 6
8.70 (d, J = 1.9 Hz, 1H), 8.61
(d, J = 1.9 Hz, 1H), 6.95 (d, J
= 2.4 Hz, 1H), 6.90 (d, J = 2.4
436 CH,
'34 1 449.44 Hz, 1H), 4.88 - 4.70 (m,
2H),
4.32 (s, 1H), 3.98- 3.85 (m,
4H), 3.41 - 3.27 (m, 4H), 2.53
(s, 3H), 2.40 (s, 3H), 2.26 -0 2.14 (m, 2H), 1.99 (s, 3H),
1.94 - 1.88 (m, 6H).
186
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
F
1H NMR (300 MHz,
H Chloroform-d) 6 8.72 (d, J
=
HN N
.,---- L.
Hz, 1H), 8.31 (s, 1H), 7.02 -
6.88 (m, 2H), 6.77 (d, J = 2.6
0
437 464.2 Hz, 1H), 5.42 (d, J = 8.1
Hz,
N 41111.,,,_ 1H), 4.90 - 4.78 (m, 1H),4.42
r 11 (d, J = 7.8 Hz, 1H), 3.99 - 3.85
r----- (m, 4H), 3.43 - 3.27 (m, 4H),
N N
2.37 - 2.13 (m, 2H), 2.10 -0,,.....) 1.88 (m, 6H).
1H NMR (300 MHz,
0
H Chloroform-d) 6 8.72 (d, J
=
eLs....r. 144,a 2.0 Hz, 1H), 8.64 (d, J=
1.9
Hz, 1H), 8.28 (s, 1H), 7.72 (s,
1H), 6.94 (dd, J = 16.4, 2.5
438 437.3 Hz, 2H), 5.45 (d, J = 8.2
Hz,
01H), 4.76 (d, J = 6.6 Hz, 1H),
4.26 (s, 1H), 4.01 - 3.85 (m,
(-----N 11 ---' 7H), 3.44- 3.28 (m, 4H), 2.30
- 2.11 (m, 2H), 2.08 - 1.82 (m,
0,,,,,....) 6H).
H
N N 1H NMR (400 MHz, CDCI3) 6
H=C N'rj'-'- 4.1Ø 8.71 (d, J = 1.8 Hz, 1H),
8.63
. J ' (d, J = 1.8 Hz, 1H), 8.14
(d, J
0 = 5.9 Hz, 1H), 6.98 (d, J =
2.4
Hz, 1H), 6.17(d, J = 6.0 Hz,
439 435.44
34) 1H), 5.05 (s, 1H), 4.01 - 3.70
(m, 5H), 3.42 - 3.25 (m, 4H),
2.77 (q, J = 7.6 Hz, 2H), 2.32 -
0 -1 2.16 (m, 2H), 2.04- 1.80
(m,
6H), 1.32 (t, J = 7.6 Hz, 3H).
187
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M+H)
H 1H NMR (300 MHz,
Chloroform-d) 6 8.70 (d, J =
1.9 Hz, 1H), 8.60 (d, J = 1.9
,
t
Hz, 1H), 7.81 (d, J = 6.3 Hz,
N---,, NO 1H), 6.96 (d, J = 2.4 Hz,
1H),
6.90 (d, J = 2.6 Hz, 1H), 6.24
1101 ) 436.22
(d, J = 6.2 Hz, 1H), 5.92 (s,
440
1H), 4.80 (m, 1H), 3.98 (s,
br 3H), 3.95- 3.87 (m, 4H), 3.54
,,,,,i (m, 1H), 3.38 - 3.28 (m,
4H),
2.23 (m, 2H), 2.00 - 1.81 (m,
6H).
C H.
H
N 4,cip 1H NMR (300 MHz,
N----'' ,
1 Chloroform-d) rotomers, 6
8.80 (d, 1H), 8.73 (d, 1H),
0 7.73(s, 1H), 7.16 - 6.99
(m,
441 434.35
C H, 3H), 4.91 (m, 1H), 3.95 (m,
OOP -j4)
4H), 3.55 (m, 1H), 3.37 (m,
4H), 2.73 (s, 3H), 2.41 (s, 3H),
2.29 (m, 2H), 2.13 (m, 6H).
CH: 1H NMR (300 MHz,
H
N Chloroform-d) 6 8.70 (d, J
=
1.9 Hz, 1H), 8.62 (d, J = 1.9
I Hz, 1H), 7.89 (dd, J = 5.0, 1.4
--õ,
0 Hz, 1H), 7.19 - 7.10 (m,
1H),
442 420.4 7.03 - 6.95 (m, 2H), 6.92
(d, J
N
,,--- '''.--- = 2.6 Hz, 1H), 4.80 (m, 1H),
I, 3.96 - 3.87 (m, 4H), 3.79 (m,
r---'-fii --k------ 1H), 3.51 (m, 1H), 3.38 -
3.29
(m, 4H), 2.51 (m, 3H), 2.25
(m, 2H), 2.00 - 1.87 (m, 6H).
188
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M+H)
H 1H NMR (300 MHz,
HaNya
Chloroform-d) 6 8.69 (d, J =
1 1.9 Hz, 1H),8.61 (d, J = 1.9
-....., . Hz, 1H), 8.01 (s, 1H), 7.89 (d,
C H ,
, 0
J = 4.8 Hz, 1H), 7.03 (d, J =
443 420.36 4.8 Hz, 1H), 6.96 (d, J
= 2.4
Hz, 1H), 6.91 (d, J = 2.5 Hz,
r----N, ---- --k--) 1H), 4.78 (m, 1H), 3.98 -
3.86
(m, 4H), 3.61 (m, 2H), 3.39 -
0 õ....,..,) 3.28 (m, 4H), 2.30 - 2.15
(m,
5H), 1.93 (m, 6H).
H 1H NMR (300 MHz,
Chloroform-d) 68.69 (d, J =
1.9 Hz, 1H), 8.60 (d, J = 1.9
Hz, 1H), 8.03 (dd, J = 2.7, 0.9
Hz, 1H), 7.12 - 7.00 (m, 2H),
444 ..--14--) 420.28 6.95 (d, J =
2.5 Hz, 1H), 6.90
(d, J = 2.5 Hz, 1H), 4.79 (m,
1H), 4.08- 3.87 (m, 5H), 3.49
N
re'N'lli (m, 1H), 3.38 - 3.28 (m,
4H),
2.53 (s, 3H), 2.21 (m, 2H),
1.84 (d, J = 6.8 Hz, 6H).
KC,
0
H 1H NMR (300 MHz,
1",..j. N 4/a0 Chloroform-d) 6 8.65 (m,
1H),
8.40 (m, 1H), 7.50 (dd, J =
4.5, 2.2 Hz, 1H), 7.03 (s, 1H),
445 436.43 6.92 (s, 1H), 6.80 (m,
2H),
õ--" 4.76 (m, 1H), 4.08 (s, 3H),
114V ) 3.93 (m, 5H), 3.49 (m, 1H),
3.42 - 3.30 (m, 4H), 2.20 (m,
2H), 1.92 (m, 6H).
0,...,....)
189
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (M-h11)
1...,;i. ri 4.0 1H NMR (300 MHz,
Chloroform-d) 68.69 (d, J =
1.9 Hz, 1H), 8.60 (d, J = 1.9
--õ,õ Hz, 1H), 7.71 (d, J = 2.4
Hz,
0 1H), 7.65(d, J =2.4 Hz,
1H),
446 .-Aa
ftõ,) 436.17 6.96 (d, J = 2.4 Hz,
1H), 6.90
H,C ,...,,s1 (d, J = 2.4 Hz, 1H), 6.55
(t, J =
1 1 2.4 Hz, 1H), 4.79 (m, 1H),
N N 4.13(m, 1H), 3.92 (m, 4H),
0 õ,..õ-1 3.85 (s, 3H), 3.48 (m, 1H),
3.38 - 3.29 (m, 4H), 2.22 (m,
2H), 1.98- 1.88 (m, 6H).
H
., , 1H NMR (300 MHz,
41::::j
Chloroform-d) 68.69 (d, J =
s.*0 1.9 Hz, 1H), 8.60 (d, J =
2.0
Hz, 1H), 7.70 (s, 1H), 7.15 (m,
1H), 6.96 (d, J = 2.4 Hz, 1H),
447 CH 436.34 436.34
6.92 (m, 1H), 6.64 (d, J = 8.8
0 Hz, 1H), 4.80 (m, 1H), 3.90
N
r'lli (m, 7H), 3.45 - 3.26 (m,
5H),
2.24 - 2.12 (m, 2H), 1.98 -
1.81 (m, 6H).
CH
H 1H NMR (300 MHz,
y Chloroform-d) 6 8.78 (dd, J
=
4.0, 2.0 Hz, 1H), 8.66 (d, J =
tCsql 1 C-')-* 2.1 Hz, 1H), 8.55 (s, 1H),
8.06
0 (s, 1H), 7.02(d, J = 2.5
Hz,
448 421.39
N 1H), 6.96 (d, J = 2.5 Hz,
1H),
,,--," -"---
I: 4.88 (m, 1H), 3.93 (m, 4H),
3.55 (m, 1H), 3.40 (m, 4H),
N --k------ 3.29 (m, 1H), 2.58 (s,
3H),
2.28 (m, 2H), 2.07 (m, 6H).
190
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (M+H)
CH,
H
1H NMR (300 MHz,
N-----
1
Chloroform-d) rotomers, 6
8.85 (d, 1H), 8.67 d, 1H), 7.92
449 435.35 (d, 1H), 7.07 - 6.96 (m,
2H),
N 4.91 (m, 1H),4.01 - 3.88 (m,
Ai ,---' 1 4H), 3.53 - 3.24 (m, 5H), 2.87
(s, 3H), 2.64 (s, 3H), 2.04 -
r'l? N4111" NI'.-- 1.76 (m, 6H).
H
N 1H NMR (300 MHz,
Chloroform-d) 6 8.69 (d, J =
1.9 Hz, 1H), 8.61 (d, J = 1.9
Hz, 1H), 7.21 (d, J = 9.2 Hz,
s
450 421.36 1H), 6.99 - 6.87 (m, 3H),
4.87 o ----- (m, 1H), 3.98 - 3.82 (m, 5H),
3.39 - 3.29 (m, 4H), 2.51 (s, N''14 N 3H), 2.30 - 2.22 (m, 2H), 2.11
- 1.84 (m, 6H).
H
N*11:1 1H NMR (300 MHz,
Chloroform-d) rotomers, 6
8.92 (d, J = 1.8 Hz, 1H), 8.77
HõC N 0
(d, J = 1.8 Hz, 1H), 8.30 (m,
451 LL'.;34) 421.56 2H), 7.02 - 6.90
(m, 2H), 4.86
(m, 1H), 3.94 (m, 4H), 3.52 -
,...,-",..'=-, "--14 3.26 (m, 5H), 2.78 (s, 3H),
i I'l 2.26 (d, J = 11.8 Hz, 2H),
1.90
(m, 6H).
191
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (MH-H)
H 1H NMR (400 MHz, CDCI3) 6
HC N N CJ 8.69 (d, J = 1.9 Hz, 1H),
8.61
- NI
1 *I (d, J = 1.9 Hz, 1H),
6.95(d, J
= 2.4 Hz, 1H), 6.89(d, J = 2.4
Hz, 1H), 5.01 (s, 2H), 4.91 (t,
452 0
LI1'," 463.35 J = 2.5 Hz, 2H), 4.79
(s, 1H),
....,,, ., ...õ.x )
4.49(s, 1H), 4.16 (s, 1H),
C1
3.99 - 3.82 (m, 4H), 3.40 _
r------N N'') 3.25 (m, 4H), 2.53 (s, 3H),
2.29 - 2.15 (m, 2H), 1.96 -
1.80 (m, 6H).
?K H 1H NMR (300 MHz, CDCI3) 6
H,C-.NyjljN4 8.69 (s, 1H), 8.62 (s, 1H),
7.89 (d, J = 5.9 Hz, 1H), 6.94
RN.,
L......*0 (s, 1H), 6.89 (s, 1H), 5.64
(d,
453 450.43 J = 6.0 Hz, 1H), 4.87 -
4.66
N.õ,..õ1
,,,) (m, 2H), 3.98 - 3.83 (m,
4H),
3.44 - 3.24 (m, 4H), 3.13 (s,
N 6H), 2.26 - 2.10 (m, 2H),
2.04
- 1.75 (m, 6H).
H H 1 H NMR (300 MHz, CDCI3) 6
N
=C' , N , 8.61 (d, J = 1.8 Hz,
1H), 8.52
(d, J = 1.9 Hz, 1H), 7.69 (s,
N,.) 1H), 6.86 (d, J = 2.4 Hz,
1H),
0
6.80 (d, J = 2.2 Hz, 1H), 5.62
454 4....,-,-..., 1 11....1 436.34 (d, J
= 6.0 Hz, 1H), 4.83 (s,
1H), 4.69 (s, 1H), 3.91 - 3.74
(m, 4H), 3.36 - 3.10 (m, 4H),
2.86 (d, J = 5.0 Hz, 3H), 2.22 -
0 I 2.03 (m, 2H), 1.91 - 1.72
(m,
6H).
192
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1HH NMR
Compound Structure
H 1H NMR (300 MHz, CDCI3) 6
N N 8.69(d, J = 1.9 Hz, 1H), 8.61
.....-Cli ,,,,i . (d, J = 1.9 Hz, 1H), 8.05
(d, J
= 5.2 Hz, 1H), 6.94 (d, J = 2.5
-1w0 Hz, 1H), 6.89 (d, J = 2.5 Hz,
455 }111 447.41 1H), 6.39 (d, J = 5.2
Hz, 1H),
5.13 (bd, 1H), 4.76 (s, 1H),
õ.....
r
'1\1'.." 4.10 - 3.96 (m, 1H), 3.95-
7 3.83 (m, 4H), 3.40 - 3.24 (m,
4H), 2.26 - 2.09 (m, 2H), 1.97
0 ,..-, - 1.76 (m, 7H), 1.10 - 1.00
(m,
2H), 1.00 - 0.91 (m, 2H).
H 1H NMR (300 MHz, CDCI3) 6
N 8.72(d, J = 1.9 Hz, 1H),
8.64
e , (d, J = 1.9 Hz, 1H), 8.23
(d, J
N----, = 1.8 Hz, 1H), 6.97 (d, J =2.5
F 0 Hz, 1H), 6.92 (d, J = 2.4
Hz,
456 --""."`) 465.29 1H), 5.04(d, J = 7.6
Hz, 1H),
4.81 (s, 1H), 4.21 (s, 1H),
4.02- 3.82 (m, 4H), 3.44 -1'''''fli N 3.26 (m, 4H), 2.33 -
2.10 (m,
3H), 2.06- 1.82 (m, 6H), 1.19
- 1.11 (m, 2H), 1.06 - 0.97 (m,
2H).
1H NMR (300 MHz, CDCI3) 6
8.69 (d, J = 1.9 Hz, 1H), 8.62
H (d, J = 1.9 Hz, 1H),8.21
(d, J
N N = 51H NMR (300 MHz,
....-- .,...' I'''
'µµt 0 8.21 (d, J = 5.1 Hz, 1H), 6.95
C).
(d, J = 2.5 Hz, 1H), 6.90 (d, J
457 437.38
HO -..-- 11 14.-='..-1 = 2.5 Hz, 1H), 6.45 (d, J
= 5.1
Hz, 1H), 5.33 (d, J = 7.6 Hz,
1------N N 1H), 4.85 - 4.74 (m, 1H),
4.56
0õ (s, 2H), 4.12 - 4.00 (m,
1H),
3.95 - 3.86 (m, 4H), 3.66 (s,
1H), 3.39- 3.27 (m, 4H), 2.18
(dd, J = 14.1, 8.4 Hz, 2H),
2.01 - 1.83 (m, 6H).
193
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M-I-H)
H 1H NMR (300 MHz, CDCI3) 6
1
N N 8.97 (s, 2H), 8.69 (d, J =
1.9
Hz, 1H), 8.62 (d, J = 1.9 Hz,
1H), 6.96 (d, J = 2.4 Hz, 1H),
N/ 6.91 (d, J = 2.4 Hz, 1H),
5.54
.s. 1,4
458 N - N 489.41 (d, J = 8.0 Hz, 1H),
4.81 (s,
14,d . ) 1H), 4.38 (s, 3H), 4.16 -
4.05
(m, 1H), 4.00 - 3.81 (m, 4H),
rw N* 3.42 - 3.23 (m, 4H), 2.29 -
o) 2.15 (m, 2H), 2.05- 1.83 (m,
6H).
,x,tiyttl
NT
1H NMR (300 MHz, CDCI3) 6
8.80 -8.64 (m, 3H), 8.62 (d, J
N .. RI L,..,õ.õ---,,,0 -- = 1.9
Hz, 1H), 6.97 (d, J = 2.4
Hz, 1H), 6.91 (d, J = 2.4 Hz,
459 `I) -N, N, 489.41 1H), 5.69 (d, J = 7.6
Hz, 1H),
...," 4.82 (s, 1H), 4.18 (s, 3H),
CH,
1
..) 4.15 - 4.06 (m, 1H),4.01 _
r-----N -- t4--. 3.83 (m, 4H), 3.44 - 3.24
(m,
O ) 4H), 2.32 - 2.18 (m,
2H), 2.07
- 1.84 (m, 6H).
H
1H NMR (300 MHz, CDCI3) 6
9.02 (s, 2H), 8.84 (d, J = 2.0
N \=-. 'PI -,,,..$,N, -- Hz, 1H), 8.66
(d, J = 2.0 Hz,
0 1H), 7.01 (d, J =2.3 Hz,
1H),
460 - NH 475.37 6.94 (d, J = 2.4 Hz,
1H), 5.90
,---. i ) (d, J = 7.1 Hz, 1H), 4.74
(s,
1H), 4.13 (s, 1H), 4.04 - 3.88
(-----N N (m, 4H), 3.45 - 3.32 (m,
4H),
2.06 (d, J = 7.5 Hz, 2H), 1.96 -
---...--- 1.84 (m, 6H).
194
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H 1H NMR (300 MHz, CDCI3) 6
N N
8.72 (d, J = 1.9 Hz, 1H), 8.64
(d, J = 1.9 Hz, 1H), 8.59(s,
1H), 8.54 (bd, 1H), 7.37 (d, J
= 4.9 Hz, 1H), 6.97 (d, J = 2.5
461 475.19 Hz, 1H), 6.93 (d, J = 2.1 Hz,
0
s, i
,! 40 il 1H), 5.55 (bd, 1H), 4.83
(s,
N--
1H), 4.14 (s, 1H), 4.00 - 3.89
r'''''',;, N ''''' (m, 4H), 3.42 - 3.29 (m,
4H),
2.30 - 2.18 (m, 2H), 1.96 (s,
6H).
H 1H NMR (300 MHz, CDCI3) 6
8.69 (d, J = 2.5 Hz, 3H), 8.62
H
C.4 (d, J = 1.9 Hz, 1H), 7.62
(d, J
N = 2.3 Hz, 1H), 6.95 (d, J =
2.4
0
Hz, 1H), 6.91 (d, J = 2.4 Hz,
462 N 473.39 1H), 6.52 (d, J = 2.1
Hz, 1H),
5.37 (d, J = 7.9 Hz, 1H), 4.86 -
4.75(m, 1H), 4.14 - 4.01 (m,
1H), 3.99- 3.83 (m, 4H), 3.41
6.-) - 3.26 (m, 4H), 2.28 -2.15
(m,
2H), 2.01 - 1.86 (m, 6H).
H 1H NMR (400 MHz, CDCI3) 6
HC N.,...., N
8.69 (d, J = 1.9 Hz, 1H), 8.62
(d, J = 1.9 Hz, 1H), 6.95 (d, J
= 2.4 Hz, 1H), 6.90(d, J = 2.4
F 0
Hz, 1H), 4.95 (d, J ¨7.1 Hz,
463 CH 453.4 453.4 1H), 4.78 (s, 1H),
4.23 (d, J =
3.8 Hz, 1H), 3.99 - 3.82 (m,
4-*--,_ ,...,--"-",õ"-,,, "--14,,-J 4H), 3.42-
3.25 (m, 4H), 2.45
i Ni (d, J = 0.6 Hz, 3H), 2.30
(d, J
0 ,.,.,..õ,õ.) = 2.8 Hz, 3H), 2.26 - 2.14
(m,
2H), 1.99- 1.86 (m, 6H).
195
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H 1H NMR (300 MHz, CDCI3) 6
8.69(d, J = 1.9 Hz, 1H), 8.62
1,4''''''N N4tICIv (d, J = 1.9 Hz, 1H),
8.22(s,
1H), 6.94 (d, J = 2.4 Hz, 1H),
0 0
6.89(d, J = 2.4 Hz, 1H), 4.78
464 / 449.35 (d, J = 7.8 Hz, 2H),
4.62 (t, J =
,..=,' 1 1µ--1
9.1 Hz, 2H), 4.23 (s, 1H), 3.97
rml= N õ...:õ..,1
- 3.85 (m, 4H), 3.40 - 3.30 (m,
4H), 3.25 (t, J = 9.1 Hz, 2H),
0 ..õ....õ) 2.27 - 2.11 (m, 2H), 2.01 -
1.82 (m, 6H).
H 1H NMR (300 MHz,
r4----' I
1 ll'a 0 cl.h9loHrzo,folrz1) 6 8.70
8.61(d (d, J =
,j.1.9
H1Hz,),17H.7),87(.s8,61(Hd),,J6=.926.70H, jz,,
--.õ
C H ,
---14."`) 420.19 2.4 Hz, 1H), 6.90 (d, J
= 2.5
465
Hz, 1H), 6.78 (t, J = 2.1 Hz,
1H), 4.78 (m, 1H), 4.00 - 3.86
(m, 4H), 3.50 (s, 1H), 3.38 -
3.28 (m, 4H), 2.28 (s, 3H),
2.25 - 2.14 (m, 2H), 1.95 -
1.85 (m, 6H).
H
N 04,
IV' 1H NMR (300 MHz,
1 Chloroform-d) 6 8.69 (m,
1H),
-.õ..õ 8.60 (d, J = 1.9 Hz, 1H),
8.20
0
(m, 1h), 7.93 (s, 1H), 6.97 -
466 e NH, )1) 461.33 6.89 (m, 3H), 4.77 (m,
1H), l
1----N --ii 3.92 m, 4H), 3.49 (m, 1H),
3.34 (m, 4H), 2.20 (m, 2H),
1.88 (d, J = 4.1 Hz, 6H), 1.70
0,,........, (m, 4H).
196
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No.
H 1H NMR (400 MHz, CDCI3) 6
N N 8.68 (d, J = 1.9 Hz, 1H),
8.61
cli: *0 (d, J = 1.9 Hz, 1H),
7.95(s,
'**,0 1H), 6.94 (d, J = 2.5 Hz,
1H),
H,C
6.90 (d, J = 2.5 Hz, 1H), 5.00
467 CH 435.35 435.35 (d, J = 7.9 Hz,
1H), 4.83 - 4.74
....,,Ce
(m, 1H), 4.07 - 3.96 (m, 1H),
3.96 - 3.87 (m, 4H), 3.38 -
r14 N 3.28 (m, 4H), 2.29 (s, 3H),
2.22 - 2.12 (m, 2H), 2.07 (s,
3H), 1.93- 1.85 (m, 6H).
1H NMR (300 MHz,
H Chloroform-d) 6 8.70 (d, J
=
N 1.9 Hz, 1H), 8.62(d, J =
1.9
14,' i *s.Cir
Hz, 1H), 7.91 (d, J = 2.7 Hz,
11H)): 7.11 d
7.73 ((dd,
d j , 8.6 0.7
Hz'
0 J : , 8.6, 2.8 Hz,
H
0
468
OH , 478.21 1H), 6.96 (d, J = 2.4
Hz, 1H),
6.91 (d, J = 2.5 Hz, 1H), 5.11 -
1 1 5.02 (m, 2H), 4.85 - 4.75
(m,
1H), 4.75 -4.67 (m, 2H), 3.98
, - 3.86 (m, 4H), 3.54 (s,
1H),
3.40- 3.27 (m, 4H), 2.31 -
2.15 (m, 2H), 2.00- 1.83 (m,
6H).
H 1H NMR (400 MHz, CDCI3) 6
N 8.69(d, J = 1.9 Hz, 1H),
8.62
(d, J = 1.9 Hz, 1H), 8.03 (s,
F 1H), 6.95 (d, J = 2.4 Hz,
1H),
0
6.89 (d, J = 2.4 Hz, 1H), 4.77
469 NH --34 454.3 (s, 1H), 4.67 (s, 1H),
4.60 (s,
H:C''' ...,," 1 '.
1 1H), 4.16 (s, 1H), 4.00 - 3.82
(m, 4H), 3.41 - 3.25 (m, 4H),
r N
N 3.04 (d, J = 5.0 Hz, 3H), 2.28 -
2.11 (m, 2H), 1.99 - 1.84 (m,
6H).
197
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
1H NMR (400 MHz, CDCI3) 6
H 8.69(d, J = 1.9 Hz, 1H),
8.63
(d, J = 1.9 Hz, 1H), 7.88 (d, J
1 = 5.5 Hz, 1H), 7.61 (d, J = 2.1
--a, Hz, 1H), 6.94 (d, J = 2.4
Hz,
0 0
1H), 6.91 (d, J =2.4 Hz, 1H),
470 ./
}111 446.38 6.84 (d, J = 5.5 Hz, 1H), 6.70
(d, J = 2.1 Hz, 1H), 4.92(s,
r'1\1'.." 1H), 4.82 - 4.74 (m, 1H),
4.44 _ 4.28 (m, 1H), 3.95 - 3.86 (m,
4H), 3.39- 3.26 (m, 4H), 2.28
-2.17 (m, 2H), 2.06 - 1.92 (m,
6H).
CH, 1H NMR (300 MHz,
rj,,y. la Chloroform-d) 6 8.62 (d, J
=
1.9 Hz, 1H), 8.54 (d, J = 2.0
Hz, 1H), 8.42 (s, 1H), 7.91 (d,
--,...---- 0 J = 13.5 Hz, 1H), 6.93 - 6.78
471 421.35 (m, 2H), 4.71 (s, 1H),
4.57 (d,
II. --")
J = 7.9 Hz, 1H), 4.22 (s, 1H),
3.91 - 3.78 (m, 4H), 3.33 -
3.19 (m, 4H), 2.16 (d, J = 6.6
Hz, 2H), 1.96 (s, 3H), 1.91 -
1.77 (m, 6H).
1H NMR (300 MHz,
KC 0 ,
Chloroform-d) 68.62 (d, J =
H 1.9 Hz, 1H), 8.54 (d, J =
1.9
Hz, 1H), 8.10 (d, J = 0.8 Hz,
1H), 6.92 - 6.77 (m, 2H), 5.36
NN., a (dd, J = 7.9, 0.9 Hz, 1H),
4.71
472 534.2 (q, J = 5.2, 4.2 Hz, 2H),
3.97 -
3.69 (m, 7H), 3.58 - 3.37 (m,
)1) 4H), 3.30 - 3.03 (m, 6H),
2.22
(FNN N -2.04 (m, 2H), 1.93 - 1.73 (m,
6H), 1.52 (dtd, J = 12.8, 8.9,
0 J 3.8 Hz, 2H), 1.28 - 1.16
(m,
=,,,
3H).
198
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
1H NMR (300 MHz,
H Chloroform-d) 6 8.70 (d, J
=
H;C-9,.õ,,,õ N,,,,,,....---õ,_ 2.0 Hz, 1H), 8.62(d, J = 1.9
...-," i ,
N=N., iJ.,,,,,o Hz, 1H), 8.16 (s, 1H), 6.92
(dd, J = 15.4, 2.5 Hz, 2H),
4.77 (d, J = 5.4 Hz, 1H), 4.25 -
)
473 462.3
V 4.07 (m, 2H), 3.98 - 3.86
(m,
1 5H), 3.51 (dd, J = 9.3, 8.2 Hz,
r-,-,N
0) 2.19 (d, J = 7.1 Hz, 2H),
1.91
(t, J = 4.0 Hz, 6H).
H 1H NMR (400 MHz, CDCI3) 6
N 8.69(d, J = 1.9 Hz, 1H),
8.61
rj 44.04õ (d, J = 1.9 Hz, 1H), 7.99
(d, J
ts,----, = 1.5 Hz, 1H), 6.94 (d, J
=2.4
F 0 Hz, 1H), 6.89 (d, J = 2.4
Hz,
( N 523.31 1H), 4.82(d, J = 5.5 Hz,
1H),
474 l }4) r------N il ---ti 4.77 (s, 1H),
4.15 (s, 1H),
3.96- 3.85 (m, 4H), 3.76 -
N)
3.65 (m, 4H), 3.38 - 3.26 (m,
4H), 2.59 - 2.45 (m, 4H), 2.34
CH, 0, (s, 3H), 2.27 - 2.13 (m,
2H),
1.96- 1.85(m, 6H).
H 1H NMR (400 MHz, CDCI3) 6
risk, 14,0 8.69 (d, J = 1.9 Hz, 1H),
8.60
(d, J = 1.9 Hz, 1H), 8.52(s,
1H), 6.95 (d, J = 2.4 Hz, 1H),
0
6.89 (d, J = 2.4 Hz, 1H), 5.01
475 0 ---. ,--""'",,,, 449.21 (d, J = 2.6
Hz, 2H), 4.98 - 4.89
I (m, 2H), 4.80 (s, 1H), 4.54 (s,
-......,,õN -,...h.õ.... 1H), 4.22 (s, 1H), 3.95 - 3.86
CL,-1 2.29 - 2.16 (m, 2H), 1.98 -
1.84 (m, 6H).
199
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H
" 1H NMR (400 MHz, CDCI3) 6
8.70(d, J = 1.9 Hz, 1H), 8.63
-..õ.. L (d, J = 1.9 Hz, 1H), 8.03 (d, J
F '0 = 1.7 Hz, 1H), 6.97 (d, J =
2.5
476 CH, 40 N...., 439.4 Hz, 1H), 6.92 (d,
J = 2.5 Hz, õ.,..-- -.... 1H), 5.18 - 5.04 (m, 1H), 4.80
1 (s, 1H), 3.99 - 3.90 (m, 5H),
r------N N 3.40 - 3.32 (m, 4H), 2.36
(d, J
0 .õ,...õõ...i = 2.4 Hz, 3H), 2.23 - 2.15
(m,
2H), 1.95 - 1.85 (m, 6H).
H 1H NMR (400 MHz, CDCI3) 6
8.70(d, J = 1.9 Hz, 1H), 8.62
tsc-X (d, J = 1.9 Hz, 1H), 8.26 (d, J
F '..'" 0 = 1.7 Hz, 1H), 6.95 (d, J = 2.4
Hz, 1H), 6.90 (d, J = 2.4 Hz,
477 CH,
..-----"."`) 439.36 1H), 5.07 (d, J = 7.2
Hz, 1H),
4.80(s, 1H), 4.30 - 4.13 (m,
1H), 3.98- 3.83 (m, 4H), 3.38
fil N - 3.26 (m, 4H), 2.36 (d, J
= 2.8
Hz, 3H), 2.29 - 2.16 (m, 2H),
1.97 - 1.85 (m, 6H).
2H 1H NMR (400 MHz, CDCI3) 6
8.64 (t, J = 9.6 Hz, 1H), 8.53
21.4..,õ6,..r,õ..,õõ
(d, J = 1.9 Hz, 1H), 8.02(d, J
I = 5.9 Hz, 1H), 6.89 (d, J = 2.4
tI Hz, 1H), 6.83(d, J = 2.4
Hz,
478 N 424 1H), 6.07 (d, J = 6.0 Hz,
1H),
4.91 (s, 1H), 4.73 (s, 1H),
I 3.83 (dd, J = 17.8, 12.9 Hz,
5H), 3.25 (dd, J = 17.8, 13.0
Hz, 4H), 2.14 (dd, J = 8.9, 5.0
Hz, 2H), 1.91 - 1.76 (m, 6H).
200
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M+H)
1H NMR (300 MHz,
H
0/1?,,,,si...õ Chloroform-d) 6 8.62 (d, J
=
`,,, ..õ--. N 4=04, 1.9 Hz, 1H), 8.53 (d, J =
1.9
Hz, 1H), 8.15 (s, 1H), 6.84
0 (dd, J = 17.3, 2.5 Hz, 2H),
479 449.3 4.70 (s, 1H), 4.55 (dd, J
= 9.2,
)4 3 8.3 Hz, 2H), 4.36 (s, 1H),
4.16
(s, 1H), 3.92 - 3.76 (m, 4H),
(M4 II 3.35 - 3.15 (m, 4H), 3.03 -
2.83 (m, 2H), 2.14 (s, 2H),
1.84 (d, J = 5.4 Hz, 6H).
H 1H NMR (300 MHz,
t9
Cr> H Chloroform-d) 6 8.70 (d, J
=
2.0 Hz, 1H),8.61 (d, J = 1.9
Hz, 1H), 8.20 (d, J = 0.9 Hz,
o ......ry.....---, N.,...a 1H), 6.93 (dd, J = 17.6,
2.5
T N'N, LI Hz, 2H), 5.65 (d, J = 0.9
Hz,
480 -.......e- 0 478.26 1H), 5.54- 5.38 (m, 1H),
4.97
(d, J = 8.0 Hz, 1H), 4.80 (s,
.---- ...,14 1H), 4.04- 3.85 (m, 5H), 3.81
- 3.62 (m, 3H), 3.41 - 3.24 (m,
1 N 4H), 2.62 (s, 1H), 2.31 -
2.11
0 i (m, 2H), 1.90 (dd, J = 8.0,
3.5
-,..õ---
Hz, 6H).
H 1H NMR (300 MHz,
r..
N N Chloroform-d) 68.69 (d, J =
....(--:,
1.9 Hz, 1H), 8.62 (d, J = 1.9
Hz, 1H), 8.18 (s, 2H), 6.99 -
6.87 (m, 2H), 5.16 (d, J = 8.1
,
,
481 451.41 Hz, 1H), 4.78 (s, 1H),
4.03 -
I 3.87 (m, 5H), 3.80 (t, J =
6.5
Hz, 2H), 3.39 - 3.29 (m, 4H),
0µ,,,,,,,) 2.67 (t, J = 6.5 Hz, 2H),
2.26 -
2.14 (m, 2H), 1.90 (t, J = 6.5
Hz, 6H).
201
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
1 H 1H NMR (400 MHz, CDCI3) 6
HU, )1.N , 8.68 (d, J = 1.9 Hz, 1H),
8.61
(d, J = 1.9 Hz, 1H), 7.65 (d, J
= 3.6 Hz, 1H), 6.94 (d, J = 2.4
F '0 482 454.35 Hz, 1H), 6.90 (d, J = 2.4 Hz,
iso 14,, 1H), 5.14 (s, 1H), 4.90 (s,
1H),
4.76 (s, 1H), 4.01 - 3.87 (m,
5H), 3.39- 3.31 (m, 4H), 3.01
(d, J = 5.0 Hz, 3H), 2.23 - 2.12
(m, 2H), 1.99 - 1.81 (m, 6H).
ir'..,,,N 1H NMR (300 MHz, CDCI3):
ppm 1.81 - 1.98 (m, 6 H), 2.11
N 0
,,,,, N=v"--'N'f CH. -2.23 (m, 2 H), 2.29
(s, 6 H),
I "1õ, 3.29 - 3.38 (m, 6 H), 3.89 -
483 -*"'" N '-'-N,'"Nõ..,''N 464.3 3.94 (m, 4 H), 3.98 -4.08
(m,
C H:
H 1 H), 4.75 - 4.82 (m, 1 H),
(NN, 5.21 (d, J = 7.9 Hz, 1 H),
6.61
(d, J = 5.1 Hz, 1 H), 6.90 (d, J
= 2.3 Hz, 1 H)
CO-"'
1H NMR (300 MHz, CDCI3):
1 ppm 1.79- 1.98(m, 6 H),
2.13
Pi CI 44, 4,,,,,,,,,,. r"NFCH,
PI -2.26 (m, 2 H), 2.34 (s, 3 H),
484
2.46 -2.78 (m, 8 H), 3.28-
\,...) 519.3 3.38 (m, 4 H), 3.60 (s, 2
H),
"=\, ..eiN/N
N N
H 3.78 - 3.98 (m, 5 H), 4.76 -
N
4.85 (m, 1 H), 5.10 (br s, 1 H),
6.18 (d, J = 6.0 Hz, 1 H), 6.90
N. ...--- (d, J = 2.4 H
0
202
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M-I-H)
H
ei,,,,,,,,,,,ctõ 1H NMR (400 MHz, CDCI3) 6
8.68 (d, J = 1.9 Hz, 1H), 8.62
el.'"---'' 0 (d, J = 1.9 Hz, 1H), 8.20
(s,
2H), 6.96 (d, J = 2.3 Hz, 1H),
485
010 4:-) 441.29 6.90 (d, J = 2.4 Hz,
1H), 5.27
(d, J = 7.4 Hz, 1H), 4.79 (s,
1H), 4.01 - 3.84 (m, 5H), 3.40
r"--H- N - 3.25 (m, 4H), 2.25 -2.12
(m,
0.,....,,) 2H), 1.97 - 1.82 (m, 6H).
H 1H NMR (400 MHz, CDCI3) 6
N 8.62 (d, J = 1.9 Hz, 1H),
8.54
rj 44.04õ (d, J = 1.9 Hz, 1H), 8.49
(s,
1H), 8.08 (d, J = 6.0 Hz, 1H),
0 6.89 (d, J = 2.5 Hz, 1H), 6.83
(d, J = 2.5 Hz, 1H), 6.24 (dd, J
486 407
= 6.0, 1.1 Hz, 1H), 4.88 (s,
1H), 4.78 - 4.67 (m, 1H), 3.87
- 3.82 (m, 4H), 3.25 (dd, J =
13.6, 8.8 Hz, 4H), 2.15 (dd, J
= 8.8, 5.1 Hz, 2H), 1.90- 1.78
(m, 6H).
H 1H NMR (400 MHz, CDCI3) 6
N N õ...,II
8.69 (d, J = 1.9 Hz, 1H), 8.61
r. 1 (d, J = 1.9 Hz, 1H), 8.45
(s,
"Nr 1H), 6.94 (d, J = 2.4 Hz,
1H),
0 6.87 (t, J = 9.8 Hz, 1H), 4.77
(s, 1H), 4.49(d, J = 8.0 Hz,
487 447.37
1H), 4.28 (s, 1H), 3.96 - 3.86
I: (m, 4H), 3.40 - 3.28 (m, 4H),
r,..,.õN -....,,,N -,....,õ,-.. 2.95 - 2.85
(m, 2H), 2.73 -
2.61 (m, 2H), 2.28 - 2.17 (m,
0,,,....) 2H), 2.17 - 2.06 (m, 2H),
1.94
- 1.89 (m, 6H).
203
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (MH-H)
H 1H NMR (400 MHz, CDCI3) 6
N N 8.70 (d, J = 1.9 Hz, 1H),
8.62
_10
14:: 41CL 447.37 (d, J = 1.9 Hz, 1H), 8.48
(s,
1H), 7.73 (d, J = 2.1 Hz, 1H),
488
6.96 (d, J = 2.4 Hz, 1H), 6.91
(d, J = 2.4 Hz, 1H), 6.85 (d, J
,..=,' }1) = 2.1 Hz, 1H), 5.29 (d, J =
8.3
Hz, 1H), 4.88 - 4.75 (m, 1H),
r------N, ---- --k-) 4.46 -4.33 (m, 1H), 3.95 -
3.87 (m, 4H), 3.38 - 3.30 (m,
0 õ...,......õ) 4H), 2.31 -2.19 (m, 2H),
2.06
- 1.91 (m, 6H).
?K 1H NMR (400 MHz, CDCI3) 6
H 8.68(d, J = 1.9 Hz, 1H),
8.61
'Nif r----'s (d, J = 1.9 Hz, 1H),
7.85(d, J
= 6.1 Hz, 1H), 6.94(d, J = 2.4
'4 t''.'*0 Hz, 1H), 6.90 (d, J = 2.4
Hz,
489 450.34 1H), 5.81 (d, J = 6.1
Hz, 1H),
N.,,,..õ1
4.92 (s, 1H), 4.75 (s, 1H),
4.02 (s, 1H), 3.97- 3.86 (m,
ri N 4H), 3.32 (dd, J = 17.6,
12.8
Hz, 4H), 3.04 (s, 6H), 2.18
0,,,..) (dd, J = 24.2, 17.3 Hz,
2H).
H 1H NMR (400 MHz, CDCI3) 6
HC N N
--,,,,- C 8.70 (d, J = 1.9 Hz, 1H), 8.62
1: *IC) 0 (d, J = 1.9 Hz, 1H), 7.87
(d, J
H = 0.7 Hz, 1H), 6.95(d, J =
2.4
Hz, 1H), 6.90 (d, J = 2.4 Hz,
490 ---"'" '''-il"'N,'", i 435.35 1H),
4.78 (s, 1H), 4.67 (s, 1H),
4.41 -4.28 (m, 1H), 3.96 -
õ---"-","--=i? 3.88 (m, 4H), 3.38 - 3.30 (m,
4H), 2.52 (s, 3H), 2.28 -2.16
0 ,.,...,,,,,) (m, 2H), 2.00 (s, 3H), 1.98
-
1.90 (m, 6H).
204
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H
1H NMR (400 MHz, CDC13) 6
IN...,.õ.yi 8.70(d, J = 1.7 Hz, 1H),
8.61
. NCH 0 (d, J = 1.7 Hz, 1H), 8.42
(s,
1H), 6.95 (s, 1H), 6.90 (s, 1H),
491 C H, 435.35 4.79 (s, 2H), 4.28 (s,
1H),
,..=,' }1)
3.98 - 3.86 (m, 4H), 3.40 _
0) 3H), 1.99 - 1.89 (m, 6H).
H 1H NMR (300 MHz,
(.2_, ti,4. Chloroform-d) 6 8.71 (d, J
=
1.9 Hz, 1H), 8.63 (d, J = 1.9
N----,.õ 1 Hz, 1H), 8.20(d, J = 0.8
Hz,
0 1H), 6.97 (d, J = 2.4 Hz,
1H),
410
N.,,,,
492 (i 6.92 (d, J = 2.5 Hz, 1H),
5.47
506.2 --,S."11 (d, J = 1.0 Hz, 1H), 4.76
(d, J
cy
)
= 23.9 Hz, 2H), 4.12 - 3.75 r------N ---..--) (m, 7H), 3.41 -
3.28 (m, 4H),
3.20 (ddd, J = 13.2, 9.6, 3.2
OH 0,,J Hz, 2H), 2.20 (d, J = 6.9
Hz,
2H), 1.91 (d, J = 5.2 Hz, 6H).
1H NMR (400 MHz, DMSO) 6
H 8.72(d, J = 1.9 Hz, 1H),
8.57
(d, J = 1.9 Hz,
1H), 7.14 (d, J =
iss)
its, 1 '
'''; 0 2.4 Hz, 1H), 6.83 (d, J =
2.3
Hz, 1H), 6.15 (d, J = 5.8 Hz,
1 1H), 4.92(s, 1H), 4.26(t, J
=
493 534
) 6.1 Hz, 2H), 4.09 - 3.89
(m,
1H), 3.83- 3.72 (m, 4H), 3.34
(d, J = 7.7 Hz, 4H), 2.57 (t, J =
6.1 Hz, 2H), 2.36 (d, J = 24.2
Hz, 4H), 2.01 (d, J = 17.5 Hz,
2H), 1.76 (d, J = 4.8 Hz, 6H),
1.52- 1.35(m, 6H).
205
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (MH-H)
H 1H NMR (300 MHz,
HC N N,,) Chloroform-d) 6 8.62 (d, J
=
1.9 Hz, 1H), 8.54 (d, J = 1.9
Hz, 1H), 7.82 (d, J = 3.4 Hz,
1H), 6.88 (d, J = 2.5 Hz, 1H),
6.82 (d, J = 2.5 Hz, 1H), 4.96
494 .,--)L-, 439.23
(d, J = 8.0 Hz, 1H), 4.72 (d, J
....,,,Ce ...õ.x i
= 3.1 Hz, 1H), 4.18 (s, 1H),
r------N N''' 3.90 - 3.78 (m, 4H), 3.35 -
0 ",,,J 3.19 (m, 4H), 2.41 (d, J =
0.9
Hz, 3H), 2.13 (q, J = 6.4 Hz,
2H), 1.84 (t, J = 6.3 Hz, 6H).
1H NMR (300 MHz, CDCI3):
1(-4,11
N ' ppm 1.37- 1.47 (m, 2 H),
1.51
- 1.60 (m, 4 H), 1.82 - 1.99
(m, 6 H), 2.14 - 2.24 (m, 2 H),
N N 504.3 2.30 - 2.41 (m, 4 H),
3.29 (s, 2
H H), 3.30- 3.35 (m, 4 H),
3.89 -
N
3.93 (m, 4 H), 3.96 -4.06 (m,
1 H), 4.75 - 4.81 (m, 1 H),
Ns. 5.18 (d, J =
CF
1H NMR (300 MHz, CDCI3):
fr--- ppm 1.38- 1.49 (m, 2 H),
1.56
N C14IF"Ni :CI (N- - 1.64 (m, 4 H), 1.79 -
1.97
496
(m, 6 H), 2.15 - 2.26 (m, 2 H),
INA \--11,-\,Nsõ,7
N 504.3 2.45 -2.57 (m, 4 H), 3.28
-
, H 3.38 (m, 4 H), 3.53 (s, 2
H),
h
3.68 - 3.99 (m, 5 H), 4.76 -
4.85 (m, 1 H), 5.11 (br s, 1 H),
6.18 (d, J = 6.0
206
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M-I-H)
H
NLN 1H NMR (300 MHz,
'-=,:i 40..
E
0 Chloroform-d) 6 8.71 (d, J
=
1.9 Hz, 1H), 8.63 (d, J = 1.9
Hz, 1H), 8.22 (s, 2H), 7.01 -
497 N
( ) ---- 14µ'N
519.2 6.85 (m, 2H), 5.21 (d, J =
8.1
Hz, 1H), 4.79 (d, J = 5.3 Hz,
1H), 4.11 - 3.87 (m, 5H), 3.35
N re.....õ. ,...._ ...li
(q, J = 3.4 Hz, 6H), 2.46 (s,
6H), 2.29 (s, 3H), 2.19 (s, 3H),
CH, 0,,,,i 2.01 - 1.83 (m, 6H).
1H NMR (300 MHz,
H Chloroform-d) 6 8.72 (d, J =
N 1.9 Hz, 1H), 8.63 (d, J = 1.9
cr., -...õ..., Hz, 1H), 8.23 (dd, J = 7.6,
0.8
Hz, 1H), 7.85 (d, J = 2.2 Hz,
0 1H), 6.97 (d, J = 2.5 Hz,
1H),
498 ,,,` ,-P1 446.1 6.92 (d, J = 2.5 Hz,
1H), 6.12
(dd, J = 2.2, 0.8 Hz, 1H), 6.00
(d, J = 7.6 Hz, 1H),4.91 (d, J
N--,...õ --.,..
r... _.,
N- = 7.9 Hz, 1H), 4.81 (s,
1H),
4.20 (s, 1H), 4.01 - 3.88 (m,
4H), 3.42 - 3.28 (m, 4H), 2.22
(s, 2H), 1.97 (dd, J = 7.8, 5.5
Hz, 6H).
1H NMR (300 MHz,
, ON Chloroform-d) 6 8.70 (d, J
=
J 1.9 Hz, 1H), 8.61 (d, J =
1.9
Hz, 1H), 8.19 (d, J = 0.8 Hz,
1H), 6.93 (dd, J = 16.4, 2.5
rõ,-õõeti ,..,õ..r......,......Tõ.N.,...)
Hz, 2H), 5.42 (d, J = 1.0 Hz,
0
499 4,1,,,,,1 ti,.., IN 535.24 1H), 4.87 -
4.67 (m, 2H), 4.01
\,--' - 3.77 (m, 5H), 3.61 (dt, J
=
N 27.3, 5.1 Hz, 6H), 3.41 -
3.26
0,..... '11
r.N 14- (m, 4H), 2.73 (t, J = 5.3
Hz,
1H), 2.59 (ddd, J = 6.2, 5.0,
3.7 Hz, 6H), 2.19 (d, J = 6.6
Hz, 2H), 1.89 (t, J = 5.2 Hz,
6H).
207
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (M-J-1)
H 1H NMR (300 MHz,
HC ..õ--,N,õ1 Chloroform-d) 68.64 (d, J =
1.9 Hz, 1H), 8.55 (d, J = 1.9
" \ L..''''L* 0 Hz, 1H), 8.21 (s, 1H), 6.88
500
/ (dd, J = 16.1, 2.5 Hz, 2H),
&
461.24 6.16 (d, J = 8.3 Hz, 1H),
5.98
...;i4)
(s, 1H), 4.80 (d, J = 2.9 Hz,
1H), 3.90- 3.77 (m, 4H), 3.72
r------N N-3 _ 3.51 (m, 1H), 3.33 - 3.20
(m,
4H), 2.51 (s, 3H), 2.34 - 1.66
(m, 8H).
H H 1H NMR (300 MHz,
Chloroform-d) 6 8.63 (d, J =
1.9 Hz, 1H), 8.55 (d, J = 1.9
Hz, 1H), 6.95 (dd, J = 3.6, 2.3
, Hz, 1H), 6.86 (dd, J = 13.7,
501 CI 1.11 480.15 2.4 Hz, 2H), 6.30 (dd, J
= 3.6,
) '
....-..ii 2.0 Hz, 1H), 5.22 (m, 1H),
4.74 (s, 1H), 4.30 (s, 1H),
3.91 - 3.79 (m, 4H), 3.35 -
3.19 (m, 4H), 2.15 (m, 2H),
2.03 - 1.76 (m, 6H).
0
\>....).õ*y., 1H NMR (300 MHz,
H Chloroform-d) 6 8.63 (d, J
=
HN N
1.9 Hz, 1H), 8.54 (d, J = 2.0
Hz, 1H), 8.25 (s, 1H), 6.88 (d,
0 J = 2.4 Hz, 1H), 6.82 (d, J =
502 462.19
2.5 Hz, 1H), 4.73 (s, 1H), 4.54
N 4111 --.3
N (s, 1H), 4.12 (s, 1H), 3.84
(dd,
J = 6.0, 3.7 Hz, 4H), 3.37 -
(N
3.19 (m, 5H), 2.17 (d, J = 10.1
Hz, 2H), 1.92 - 1.71 (m, 6H).
208
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M-I-H)
rsN 1H NMR (300 MHz, CDCI3):
ppm 1.79 fi 1.99 (m, 6 H),
.0
l''LICC 1/40 (7'14 ro 2.15 - 2.26 (m, 2 H), 2.54
-
1 1 : ...),,,, 2.65 (m, 4 H), 3.29 - 3.37
(m,
506.3 4 H), 3.58 (s, 2 H), 3.74 -
3.83
503 ,N.,,,,,-,..., ,,,,, ANs, ,....N,$)
N N
H (m, 5 H), 3.88 - 3.95 (m, 4
H),
(NN. 4.75 -4.85 (m, 1 H), 5.11 (br.
1 s, 1 H), 6.19 (d, J = 5.9 Hz, 1
2 H), 6.90 (d, J
0
1H NMR (300 MHz, CDCI3):
1(-4,11
14 IN, 0 ppm 1.40- 1.49 (m, 2 H),
1.55
17N\ - 1.65 (m, 4 H), 1.84 - 1.96
504 L.4."' a1/40.** ek:).-...,,N.Ny-
N N (m, 6 H), 2.12 - 2.23 (m, 2
H),
504.3 2.43 (br. s, 4 H), 3.30-
3.40
H (m, 6 H), 3.88 - 3.95 (m, 4
H),
N 3.97 -4.08 (m, 1 H), 4.75 -
4.82 (m, 1 H), 5.17 (d, J = 7.9
Ns. Hz, 1 H), 6.68
1H NMR (300 MHz, CDCI3):
tJ ppm 1.83- 1.96(m, 6 H),
2.13
, 0,õ.., N.,,, r.,.....,0
-2.23 (m, 2 H), 2.53 (br. s, 4
N L, H), 3.30 - 3.36 (m, 4 H),
3.42
505 1 21 IN),,,, ...-- -sT42-\,..M.õ..)
506.3 (s, 2 H), 3.72 - 3.78 (m, 4 H),
H 3.88 - 3.95 (m, 4 H), 4.03
(br.
N s, 1 H), 4.75 - 4.82 (m, 1
H),
1 5.21 (d, J = 8.0 Hz, 1 H), 6.67
--s. -) (d, J = 5)
Os
209
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M+H)
11-41
1H NMR (300 MHz, DMSO-
d6): ppm 1.71 - 1.85 (m, 6 H),
506 1.1 40*N 1 :\> 1.98 - 2.10 (m, 2 H), 3.33-
3.38 (m, 4 H), 3.74 - 3.91 (m,
446.3 5 H), 4.87 - 4.96 (m, 1 H),
H H 6.02 (d, J = 7.2 Hz, 1 H),
6.51
N (s, 1 H), 6.83 (d, J = 1.9
Hz, 1
C ) H), 7.14 (d, J = 1.9 Hz, 1
H),
7.94 (s, 1 H), 8.3
0
1H NMR (300 MHz, CDCI3):
1(-4,11
ppm 1.39 - 1.48 (m, 2 H), 1.54
-1.69 (m, 4 H), 1.83 - 1.98
(m, 8 H), 2.12 - 2.24 (m, 2 H),
507 ,,, =,,,, N.
N N 548.4 2.33 -2.47 (m, 6 H), 3.31
-
I H I 3.35 (m, 4 H), 3.89 - 3.99
(m,
N N
7 H), 4.74 - 4.81 (m, 1 H),
4.95 (d, J = 8.2 Hz, 1 H), 6.90
(d, J = 2.3 Hz, 1
0"
"N
1 1 1H NMR (300 MHz, CDCI3):
,... ...., , ppm 1.82- 1.98(m, 8 H),
2.13
-2.23 (m, 2 H), 2.29 (s, 3 H),
N 'Nisi 2.35 -2.62 (m, 10 H), 3.29 -
508 I 563.3 3.37 (m, 4 H), 3.87 -
3.94 (m,
, H
N 5 H), 3.97 (t, J = 6.3 Hz, 2 H),
(I's- 4.74 -4.81 (m, 1 H), 4.96 (d, J
= 8.1 Hz, 1 H), 6.90 (d, J = 2.3
O N Hz, 1 H
i
CH,
210
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 11-1 NMR
Compound Structure
No. (M-h1-1)
tr" 1H NMR (300 MHz, CDCI3)
ppm 1.77- 1.96 (m, 10 H),
N 151'''''Nt 14,4\''010 2.11 -2.25 (m, 2 H), 2.57-
i ' 1 2.65 (m, 4 H), 2.86 (t, J = 5.7
509 520.3 Hz, 2 H), 3.31 - 3.35 (m,
4 H),
H 3.87 - 3.98 (m, 5 H), 4.05
(t, J
....A., = 5.7 Hz, 2 H), 4.74 -4.81
(m,
1 H), 4.98 (d, J = 8.2 Hz, 1 H),
6.90 (d, J =
0"
H 1H NMR (300 MHz,
N Chloroform-d) 6 8.95 (d, J
=
ri'-'41 "CD 1.3 Hz, 1H), 8.72 (d, J =
1.9
N Hz, 1H), 8.64 (d, J = 1.9
Hz,
.,=,, o 'tw
N\ r 1H), 8.14 (s, 1H), 6.95
(dd, J
= 16.0, 2.5 Hz, 2H), 6.36 (d, J
510
" ) 447.16
= 1.3 Hz, 1H), 5.11 (d, J = 7.8
Hz, 1H), 4.83 (dd, J = 5.5, 2.9
r"----N=
--"11 Hz, 1H), 4.01 - 3.84 (m,
4H),
,J 3.73- 3.55 (m, 1H), 3.40 -
3.30 (m, 4H), 2.34 - 2.21 (m,
2H), 2.08- 1.83 (m, 6H).
1H NMR (300 MHz,
F
H Chloroform-d) 6 8.63 (d, J
=
1....,...õ.N
1.9 Hz, 1H), 8.55 (d, J = 1.9
,y),-,i,NeicLo
Hz, 1H), 7.93 (d, J = 1.7 Hz,
1H), 6.85 (dd, J = 16.5, 2.5
Hz, 2H), 4.74 (ddd, J = 21.7,
511 510.2 7.5, 3.9 Hz, 2H), 4.09
(p, J =
0 )4 ) 7.7, 7.2 Hz, 1H), 3.92 - 3.79
(m, 4H), 3.71 (dd, J = 5.7, 3.7
r---,N --1,--) Hz, 4H), 3.57 (dd, J = 5.7,
3.7
Hz, 4H), 3.34 - 3.20 (m, 4H),
0..N.3 2.15 (td, J = 11.0, 10.0,
6.2
Hz, 2H), 1.94 - 1.79 (m, 6H).
211
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
CH, p 1H NMR (300 MHz,
3 1 H Chloroform-d) 6 8.71 (d, J
=
1.9 Hz, 1H), 8.64 (d, J = 1.9
Hz, 1H), 7.99 (d, J = 1.8 Hz,
1H), 7.00 - 6.85 (m, 2H), 4.77
512 468.27 (d, J = 6.6 Hz, 2H),
4.14 (q, J
N = 7.2 Hz, 1H), 4.03 - 3.87 (m,
...---- ) 4H), 3.78- 3.64 (m, 1H), 3.43
(\141 ..\-N Hz, 6H), 2.98 -
0, ) 2.20(d, J = 11.9 Hz, 2H),
1.94
-.......- (p, J = 5.8, 5.3 Hz, 6H).
1H NMR (300 MHz,
H Chloroform-d) 6 10.85 (d, J
=
3.0 Hz, 1H), 8.66(d, J = 1.9
i 1 Hz, 1H), 8.40 (d, J = 2.0
Hz,
NH 0 1H), 7.22(t, J = 2.9 Hz,
1H),
6.85 (d, J = 2.4 Hz, 1H), 6.75
-..,....
513 N 480.1 (d, J = 2.4 Hz, 1H), 6.36
(dd, J
I = 3.1, 1.9 Hz, 1H), 5.86
(d, J =
l ' -V-.V-..,.., õ,....-
7.8 Hz, 1H), 4.59 (s, 1H),4.25
I N
(s, 1H), 3.82 (dd, J = 6.0, 3.7
0,,õ.....,, Hz, 4H), 3.24 (dd, J = 6.0,
3.8
Hz, 4H), 1.71 (s, 11H), 1.51 (t,
J = 10.1 Hz, 2H).
H 1H NMR (300 MHz,
N,_ <i N4sici Chloroform-d) 6 10.96 (s,
1H), x: Jr: 8.64(d, J = 1.9 Hz, 1H), 8.40
(d, J = 1.9 Hz, 1H), 6.84 (d, J
N N "N*0 = 2.4 Hz, 1H), 6.75 (d, J =
2.4
H
514 Ci
----"'")1 480.1 Hz, 1H), 6.34 (dd, J = 3.1, 1.9
11110 -...õ, j,.1 Hz,
1H), 4.59 (s, 1H), 4.23 (td, J =
r'll N 8.8, 8.3, 4.2 Hz, 1H), 3.89
-0....4 3.74 (m, 4H), 3.30 - 3.03 (m,
4H), 1.89- 1.61 (m, 6H), 1.61
- 1.39 (m, 2H).
212
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
1H NMR (300 MHz,
--
H Chloroform-d) 6 9.53 (s,
1H),
N.,,,(91H
8.71 (d, J = 2.0 Hz, 1H), 8.64
Cjitc õ....-- ,
(d, J = 1.9 Hz, 1H), 8.35 (s,
1H), 7.06 (dd, J = 3.6, 2.1 Hz,
1H), 7.00 - 6.85 (m, 2H), 6.39
)
515 446.42
(dd, J = 3.6, 1.8 Hz, 1H), 5.14 1) (d, J = 8.3 Hz, 1H), 4.82
(s,
1H), 4.40 (d, J = 8.0 Hz, 1H),
4.01 - 3.81 (m, 4H), 3.44 -
o) 3.25 (m, 4H), 2.26 (d, J = 9.8
Hz, 2H), 2.08 - 1.86 (m, 6H).
1H NMR (300 MHz,
N Chloroform-d) 6 12.29(s,
1H),
8.71 (d, J = 1.9 Hz, 1H), 8.64
-..õ...0,- (d, J = 1.9 Hz, 1H),
8.43(s,
04e 1H), 7.95 (s, 1H), 7.00 -
6.89
516 447.32
(m, 2H), 6.03 (s, 1H), 4.86 (s,
1H), 4.42 (s, 1H), 3.92 (dd, J
'7 Niq" -le' = 6.0, 3.7 Hz, 4H), 3.40 - 3.30
r-
(m, 4H), 2.27 (d, J = 10.1 Hz,
O , 2H), 2.07- 1.88 (m,
6H).
H 1H NMR (300 MHz,
Chloroform-d) 6 11.28(s, 1H),
8.75 (d, J = 1.9 Hz, 1H), 8.53
(d, J = 2.0 Hz, 1H), 8.47 (s,
, L
ri 1H), 7.34 (s, 1H), 6.93 (d,
J =
0 2.3 Hz, 1H), 6.82 (d, J =
2.5
517 446.33 Hz, 1H), 1.93 - 1.71 (m, 6H),
0
.N 6.54 (d, J = 3.0 Hz, 1H),
5.57
...-'" I
(d, J = 7.9 Hz, 1H), 4.64 (s,
N1 N-) 1H), 4.33(s, 1H), 3.90(t, J =
4.8 Hz, 4H), 3.31 (t, J = 4.9
O.--, Hz, 4H), 1.80-1.68 (m, 6H),
1.54 (d, J = 9.2 Hz, 2H).
213
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
H 1H NMR (300 MHz,
Chloroform-d) 6 8.63 (d, J =
1 õ..õ.1õ. I... 1.9 Hz, 1H), 8.55 (d, J =
1.9
Hz, 1H), 8.32 (s, 1H), 7.42 (d,
0
J = 2.5 Hz, 1H), 6.88 (d, J =
/ 2.5 Hz, 1H), 6.83 (d, J =
2.5
518 0 447.2
,..=,' }1 ) Hz, 1H), 6.58 (d, J = 2.5 Hz,
1H), 5.07 (s, 1H), 4.74 (s, 1H),
r----N, ------ --k---' 4.26 (s, 1H), 3.84 (dd, J
= 6.0,
3.7 Hz, 4H), 3.42 - 3.10 (m,
0.,....õ) 4H), 2.18 (d, J = 10.2 Hz,
2H),
2.06- 1.70(m, 6H).
H 1H NMR (300 MHz,
yttyN Chloroform-d) 68.61 (d, J =
1.9 Hz, 1H), 8.54(d, J = 1.9
=. N
6H.z791(Hm): 28H.0)7, 5(70, 42H(d),, 6J.9,38-.1 ,
0
Hz, 1H), 4.70 (s, 1H), 4.02 -
519 _.," N is Ik.,.. 543.21
3.76 (m, 5H), 3.34 - 3.20 (m,
4H), 3.02 - 2.87 (m, 2H), 2.66
N (t, J = 6.9 Hz, 2H), 2.53 -
2.42
N'sfr.
0,\,) (m, 2H), 2.35 - 2.22 (m, 1H),
2.10 (td, J = 11.6, 3.0 Hz, 3H),
1.90- 1.46(m, 11H).
H 1H NMR (300 MHz,
N Chloroform-d) 6 8.61 (d, J =
2.0 Hz, 1H), 8.56 (d, J = 0.6
Hz, 2H), 8.53 (d, J = 1.9 Hz,
'...0 1H), 6.86(d, J = 2.5 Hz,
1H),
520 455.17
N....1 4.67 (d, J = 6.1 Hz, 1H), 3.89-
)
3.81 (m, 4H), 3.79 (d, J = 0.7
Hz, 2H), 3.31 - 3.17 (m, 4H),
N-"-
2.60 (s, 1H), 2.20 - 2.03 (m,
0 2H), 1.79 - 1.59 (m, 6H).
214
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (M-h1-1)
H
F N N 1H NMR (300 MHz,
Chloroform-d) 6 8.74 (d, J =
14.'1' 'IC 44-ta. 1.9 Hz, 1H), 8.66 (d, J =
2.0
0 Hz, 1H), 7.93 (s, 1H), 6.99
(d,
521 N -2/ 465.16 J = 2.4 Hz, 1H), 6.93
(d, J =
H
00 }4) 2.5 Hz, 1H), 6.20 (d, J =
8.5
Hz, 1H), 4.87 (s, 1H), 4.36 (s,
1H), 3.94 (dd, J = 5.9, 3.8 Hz,
) 4H), 3.42 - 3.32 (m, 4H),
2.26
(s, 2H), 1.93 (s, 6H).
H 1H NMR (300 MHz,
N N ec) Chloroform-d) 6 8.70 (d, J
=
svo 1.9 Hz, 1H), 8.63 (d, J =
1.9
Hz, 1H), 8.17 (s, 2H), 7.01 -
6.89 (m, 2H), 5.12 (d, J = 8.1
522 ,.....N ,..\ µ 552.21 Hz, 1H), 4.80 (s, 1H),
4.09-
1 3.86 (m, 5H), 3.63 (t, J =
7.1
...,.. Hz, 2H), 3.40 - 3.29 (m,
4H),
e CI ,,-
3.06 (d, J = 11.3 Hz, 2H), 2.79
1
(t, J = 7.1 Hz, 2H), 2.39-2.15
(m, 4H), 2.02 - 1.53 (m, 10H).
H
N N act. 1H NMR (300 MHz,
r '7 Yt Chloroform-d) 6 8.71 (d, J
=
V4 1.9 Hz, 1H), 8.63 (d, J =
1.9
0 Hz, 1H), 8.18 (s, 2H), 7.02
-
1
523 r,N 518.2 6.86 (m, 2H), 5.12 (d, J
= 8.1
'Le-MN'," ,..õ.$ Hz, 1H), 4.80 (s, 1H), 4.09
-
3.87 (m, 5H), 3.42 - 3.29 (m,
CH, ,".... ....-õ,...)õ,
r N 4H), 3.12 (d, J = 11.2 Hz,
2H),
2.60 - 1.54 (m, 17H), 1.15 (t, J
= 7.2 Hz, 3H).
215
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H,N
1H NMR (400 MHz, CDCI3) 6
8.69(d, J = 1.9 Hz, 1H), 8.61
0 (d, J = 1.9 Hz, 1H), 6.91
(d, J
= 2.5 Hz, 1H), 6.61 (d, J = 2.5
N Hz, 1H), 5.19- 5.05(m, 1H),
524 301.24
4111 hf"'N
3.97 - 3.85 (m, 5H), 3.37 -
I
3.29 (m, 4H), 2.71 (ddd, J =
2H).
0,....)
H
1H NMR (400 MHz, CDCI3) 6
8.71 (d, J = 1.9 Hz, 1H), 8.63
0 (d, J = 1.9 Hz, 1H), 8.31 (s,
1H), 8.30(s, 1H), 6.93(d, J =
2.4 Hz, 1H), 6.64 -6.54 (m,
Nõ... 2H), 5.38 (d, J = 5.1 Hz,
1H),
525 N....z.... 379.26
5.17 - 5.07 (m, 1H), 4.68 -
OS
4.56 (m, 1H), 3.95 - 3.85 (m,
4H), 3.35 - 3.27 (m, 4H), 2.94
N e (ddd, J = 13.4, 8.2, 5.1
Hz,
2H), 2.59 (ddd, J = 13.9, 7.0,
0 ,,s.õ.....) 4.5 Hz, 2H).
iFkl'i i 1 1H NMR (300 MHz, CDCI3):
ppm 1.80 - 2.07 (m, 8 H), 2.11
N 0r 0
ail 44.ssi N-LIT µ.1
''',-)s""N". 44 - 2.25 (m, 2 H), 2.39 - 2.76
(m, 6 H), 3.27 - 3.40 (m, 4 H),
526 N.
550.3 3.70 - 3.84 (m, 4 H), 3.87 -
i H i 3.96 (m, 5 H), 3.99 (t, J =
6.2
N N
,., N. r ..... Hz, 2 H), 4.73 -4.82 (m, 1
H),
4.97 (d, J = 8.1 Hz, 1 H), 6.90
.....a..-- (d, J = 2.4 H
CO'
216
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M-hH)
r---,N 1H NMR (300 MHz, CDCI3):
I 1 ppm 1.39- 1.48 (m, 2 H),
1.56
N o
- 1.67 (m, 4 H), 1.78 - 1.96
(m, 6 H), 2.14 - 2.25 (m, 2 H),
527 400-"'"N), 534.3 2.44 - 2.52 (m, 4 H),
2.72 (t, J
I H i = 6.0 Hz, 2 H), 3.31 - 3.35
(m,
N N
c ) c 4 H), 3.63- 3.75 (m, 1 H),
3.90 - 3.93 (m, 4 H), 4.41 (t, J
= 6.0 Hz, 2
0
1H NMR (300 MHz,
H Chloroform-d) 6 8.70 (d, J
=
N N
...-- y 1.9 Hz, 1H), 8.63 (d, J =
1.9
Hz, 1H), 8.31 (s, 2H), 7.02 -
6.86 (m, 2H), 5.52 (d, J = 8.0
Hz, 1H), 4.81 (dt, J = 6.8, 3.4
528 , i"... ,..N -1 490.2 Hz, 1H),
4.04 (td, J = 8.0, 4.0
\...-/ Hz, 1H), 3.97 - 3.86 (m,
4H),
N"-- 3.64 (s, 2H), 3.43 - 3.27
(m,
4H), 2.75 (t, J = 5.4 Hz, 4H),
2.32 - 2.15 (m, 2H), 2.07 (s,
2H), 1.92 (tt, J = 6.7, 3.6 Hz,
8H).
H 1 H NMR (300 MHz,
N õTo...4p0 Chloroform-d) 6 8.70 (d, J
=
1.9 Hz, 1H), 8.63(d, J = 1.9
Hz, 1H), 8.22 (s, 2H), 7.02 -
I 6.86 (m, 2H), 5.21 (d, J =
8.1
529 ..-N, HC C H 464.23 Hz, 1H), 4.81
(dt, J = 7.5, 3.7
,, 4r? )4 )
Hz, 1H), 4.04 (d, J = 6.9 Hz,
1H), 3.96 - 3.82 (m, 4H), 3.41
- 3.30 (m, 4H), 3.27 (s, 2H),
0 I 2.24 (m, 8H), 2.07 - 1.77
(m,
',...,,"
6H).
217
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M+H)
H 1H NMR (300 MHz,
N N Chloroform-d) 6 8.61 (d, J
=
EL
0 1.9 Hz, 1H), 8.53 (d, J =
1.9
Hz, 1H), 8.13 (s, 2H), 6.87 (d,
J = 2.5 Hz, 1H), 6.83 (d, J =
530 N
( ) ---- 14µ'N
506.25 2.5 Hz, 1H), 5.10 (d, J =
8.1
Hz, 1H), 4.71 (s, 1H), 3.94 (s,
1H), 3.88- 3.78 (m, 4H), 3.67
-3.56 (m, 4H), 3.32 -3.17 (m,
) 6H), 2.41 -2.26 (m, 4H),
2.12
(d, J = 8.7 Hz, 2H), 1.92 - 1.76
(m, 6H).
1H NMR (300 MHz,
H Chloroform-d) 6 8.71 (d, J
=
rjN 1.9 Hz, 1H), 8.64(d, J =
1.9
44.04õ Hz, 1H), 8.39 (dd, J = 2.7,
0.5
N----õ, Hz, 1H), 8.04 (d, J = 3.4 Hz,
F 0
1H), 6.97 (d, J = 2.5 Hz, 1H),
531 ---14."`) 425.19 6.92 (d, J =
2.5 Hz, 1H), 5.15
(d, J = 8.1 Hz, 1H), 4.83 (dq, J
= 5.1, 2.6 Hz, 1H), 4.25 (dd, J
= 8.1, 4.6 Hz, 1H), 3.98- 3.88
0 ,õ,,,A (m, 4H), 3.39 - 3.28 (m,
4H),
2.34 - 2.17 (m, 2H), 2.03 -
1.84 (m, 6H).
H 1H NMR (400 MHz, CDCI3) 6
CI,N.N
8.69 (d, J = 1.9 Hz, 1H), 8.61
(d, J = 1.9 Hz, 1H), 8.13(d, J
= 4.8 Hz, 1H), 6.96 (d, J = 2.4
0
Hz, 1H), 6.90 (d, J = 2.5 Hz,
532 rillh "-- 441.29 1H), 6.54 (d, J
= 5.2 Hz, 1H),
5.36 (s, 1H), 4.79 (s, 1H),
4.03(s, 1H), 3.97 - 3.84 (m,
4H), 3.41 - 3.23 (m, 4H), 2.26
- 2.12 (m, 2H), 1.98 - 1.81 (m,
6H).
218
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
H 1H NMR (400 MHz, CDCI3) 6
8.69 (d, J = 1.9 Hz, 1H), 8.60
(d, J = 1.9 Hz, 1H), 7.99 (s,
1H), 6.96 (d, J = 2.4 Hz, 1H),
0
6.90 (d, J = 2.4 Hz, 1H), 6.22
533
4111 ll'f) 441.25 (d, J = 5.9 Hz, 1H), 5.22
(s,
1H), 4.81 (s, 1H), 4.03 (dt, J =
12.1,6.2 Hz, 1H), 3.94 - 3.86
r'-14- N (m, 4H), 3.40 - 3.26 (m,
4H),
0.,....,,) 2.27 - 2.13 (m, 2H), 1.90
(t, J
= 17.4 Hz, 6H).
o,1
H 1H NMR (400 MHz, CDCI3) 6
8.69(s, 1H),8.61 (s, 1H),
1 7.89 (d, J = 5.5 Hz, 1H),
6.95
(s, 1H), 6.89 (s, 1H), 5.71 (d,
0
534 492.39 J = 5.8 Hz, 1H), 4.76
(s, 2H),
N
0 4.01 - 3.81 (m, 5H), 3.74
(s,
8H), 3.41 - 3.26 (m, 4H), 2.26
_ 2.12 (m, 2H), 1.98 - 1.81 (m,
6H).
0,,....)
1H NMR (400 MHz, CDCI3) 6
H,C, õ---,,,,i
N H 8.69(d, J = 1.9 Hz, 1H),
8.61
(d, J = 1.9 Hz, 1H), 7.88 (d, J
= 5.7 Hz, 1H), 6.95 (d, J = 2.4
,,µ 1 Hz, 1H), 6.89 (d, J = 2.4
Hz,
0 1H), 5.67(d, J = 5.8 Hz,
1H),
535 505.35
4.84 -4.63 (m, 2H), 3.99 -
3.83 (m, 5H), 3.82 - 3.73 (m,
4H), 3.37- 3.27 (m, 4H), 2.49
(---.. N - 2.40 (m, 4H), 2.33 (s,
3H),
2.23 - 2.11 (m, 2H), 1.96 -
1.82 (m, 6H).
219
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
1H NMR (300 MHz,
i A H Chloroform-d) 6 8.73 (d, J
=
1.9 Hz, 1H), 8.65 (d, J = 1.9
I 1 Hz, 1H), 8.30 (s, 1H), 6.97
536 (dd, J = 15.8, 2.5 Hz, 2H),
0 6.23 (d, J = 8.2 Hz, 1H),
6.07
461.2
CH: (s, 1H), 4.90 (dt, J = 5.1,
2.4
'''''D
r=''''' N N''' Hz, 1H), 3.99 - 3.88 (m,
4H),
41110
3.72 (dq, J = 9.2, 4.7 Hz, 1H),
3.40 - 3.29 (m, 4H), 2.60 (s,
o) 3H), 2.34 (dt, J = 14.1,
5.2 Hz,
2H), 2.23- 1.79 (m, 6H).
H 1H NMR (400 MHz, CDCI3) 6
8.69(d, J = 1.7 Hz, 1H), 8.60
(fix Nõ.ta
(d, J = 1.7 Hz, 1H), 8.18(s,
1H), 6.95 (d, J = 2.2 Hz, 1H),
0 6.90 (s, 1H), 5.42 (s, 1H),
537 N
505.35
----"."`)
4.78 (s, 1H), 4.71 (d, J = 8.3
Hz, 1H), 3.98 - 3.87 (m, 4H),
3.83 (s, 1H), 3.65- 3.48 (m,
(N) i'''N N 4H), 3.41 - 3.24 (m, 4H),
2.54
- 2.42 (m, 4H), 2.33 (s, 3H),
CH, 0, 2.25 - 2.11 (m, 2H), 1.97 -
1.82 (m, 6H).
H 1H NMR (300 MHz,
CI N Chloroform-d) 68.74 (d, J =
1.9 Hz, 1H),8.61 (d, J = 1.9
Hz, 1H), 7.57 (d, J = 1.6 Hz,
\II j 0
1H), 7.38 (dd, J = 1.7, 0.8 Hz,
538 dall "-- 480.19 1H), 7.05 - 6.88 (m,
3H), 5.52
(d, J = 8.1 Hz, 1H), 4.87 (d, J
r''N'T 4111" re = 4.0 Hz, 1H), 4.47 - 4.31
(m,
1H), 3.98- 3.88 (m, 4H), 3.42
0...õ..,,,, - 3.30 (m, 4H), 2.35 - 2.21
(m,
2H), 2.16- 1.88 (m, 6H).
220
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M-I-H)
rekti ,,i 1H NMR (300 MHz, CDCI3):
d 0 ppm 1.76- 1.97(m, 6 H),
2.15
,..,N,. - 2.26 (m, 2 H), 3.31 -
3.35
539 441a
(m, 4 H), 3.43 (s, 3 H), 3.61 -
1110 481.3 3.72 (m, 3 H), 3.90 -
3.93 (m,
H 4 H), 4.45 - 4.48 (m, 2 H),
N 0' 4.76 -4.82 (m, 1 H), 4.94
(d, J
---- `N, CH:
= 7.1 Hz, 1 H), 5.73 (s, 1 H),
6.89 (d, J = 2.
re'k=-,N 1H NMR (300 MHz, CDCI3):
14 01/40... ppm 1.82- 1.99(m, 6 H),
2.13
.,
-2.25 (m, 2 H), 3.29 - 3.38
lb 14-, 1 ----k>
(m, 4 H), 3.73 (br. s, 1 H),
540 N N 445.3 3.84 - 3.98 (m, 4 H),
4.45 (br.
H H s, 1 H), 4.74 - 4.84 (m, 1
H),
N 6.31 (s, 1 H), 6.43 -6.47
(m, 1
C ) H), 6.91 (d, J = 2.3 Hz, 1
H),
6.93 - 6.99 (m
0
1H NMR (300 MHz, CDCI3):
ppm 1.40- 1.48 (m, 2 H), 1.56
N s 0 ym ,, - 1.67 (m, 4 H), 1.84 -
1.99
1 (m, 6 H), 2.11 - 2.24 (m, 2
H),
541 INVLNA\11 'OreNi 534.4 2.45 -2.52 (m, 4 H), 2.72
(t, J
, H = 6.2 Hz, 2 H), 3.32 - 3.35
(m,
h 4 H), 3.90 - 3.93 (m, 4 H),
CI;1 3.96 -4.08 (m, 1 H), 4.40 (t, J
= 6.2 Hz, 2
221
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M-I-H)
1H NMR (300 MHz,
i A H Chloroform-d) 6 8.63 (d, J
=
N,,r, N404, 1.9 Hz, 1H), 8.56 (d, J =
1.9
Hz, 1H), 8.18 (s, 1H), 7.83 (d,
C.,,,,,,......ij J = 6.3 Hz, 1H), 6.96 - 6.80
0
542 447.25 (m, 3H), 6.23 (d, J =
8.2 Hz,
1H), 4.77 (dd, J = 5.8, 3.3 Hz,
1H), 4.23 (dq, J = 8.6, 4.3 Hz,
N
1H), 3.89- 3.75 (m, 4H), 3.35
- 3.20 (m, 4H), 2.20 (d, J =
12.8 Hz, 2H), 2.10- 1.79 (m,
6H).
H
1H NMR (300 MHz,
c.,..,-00 Chloroform-d) 6 8.53 (s,
1H),
8.18 (s, 2H), 6.96 - 6.80 (m,
2H), 5.14 (d, J = 8.1 Hz, 1H),
518.2 4.78 (s, 1H), 4.08- 3.86
(m,
H,C'N.NV 5H), 3.33 (dd, J = 6.0, 3.8
Hz,
4H), 3.07 - 2.94 (m, 2H), 2.71
7NN I( CH, (s, 3H), 2.35 (s, 3H),
2.26 -
1.97 (m, 4H), 1.99 - 1.57 (m,
01,,,,i 11H).
H
1H NMR (300 MHz,
Chloroform-d) 6 8.61 (s, 1H),
',......, 1;1 =\._ ,---=õ, 8.18 (s, 2H), 7.02 - 6.87
(m,
.." 0 2H), 5.12 (d, J = 8.2 Hz,
1H),
4.80 (s, 1H), 4.05- 3.87 (m,
544
H,CõN tigiu N\yCH: 518.2
5H), 3.38- 3.27 (m, 4H), 3.01
(d, J = 11.4 Hz, 3H), 2.74 (s,
(Nµ14 411111 el 3H), 2.36 (s, 3H), 2.20 (m,
2H), 2.06 (m,2H), 2.00 - 1.58
(m, 11H).
222
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
i H
H' C ¨ N..e, N
, 40 1H NMR (300 MHz,
-- Chloroform-d) 6 8.73 (d, J
=
Nt*, if 2.0 Hz, 1H), 8.64(d, J =
2.0
Hz, 1H), 8.39 (s, 1H), 7.92 (s,
545 N 461.24 1H), 7.05 - 6.89 (m,
2H), 4.88
(s, 1H), 4.06 (s, 3H), 3.94 (dd,
J = 6.0, 3.8 Hz, 4H), 3.36 (t, J
= 4.9 Hz, 5H), 2.29 (s, 2H),
1.98 (d, J = 35.4 Hz, 6H).
izr-N H
,+='''' 1 r i:).õ
U.'s, ,ii ' 1H NMR (300 MHz,
Chloroform-d) ó&62 (d, J =
1.9 Hz, 1H), 8.55 (d, J = 1.9
-=.,.,e--- 0 Hz, 1H), 8.32 (s, 1H), 7.66
(s,
546 461.24 1H), 6.92 - 6.81 (m,
2H), 4.79
,fis'il (s, 1H), 3.87 - 3.80 (m, 4H),
3.75 (s, 3H), 3.31 - 3.22 (m,
N'e 4H), 2.18 (d, J = 12.7 Hz,
2H),
2.08 - 1.75 (m, 6H).
0-.......)
1H NMR (400 MHz, CDCI3) 6
H 8.70(d, J = 1.9 Hz, 1H),
8.63
N N (d, J = 1.9 Hz, 1H),
7.90(d, J
Cri 0
= 6.0 Hz, 1H), 6.96(d, J = 2.4
"No Hz, 1H), 6.92 (d, J = 2.3
Hz,
0
1H), 5.89 (d, J = 6.1 Hz, 1H),
547 14
..,,--- }4) 505.4 4.96(s, 1H), 4.77 (s,
1H),
4.08- 3.98 (m, 1H), 3.96 -
N r'N, 4H), 3.43 - 3.27 (m, 4H),
2.53
t
C H , a -. -2.42 (m, 4H), 2.35 (s,
3H),
2.25 - 2.13 (m, 2H), 2.00 -
1.85 (m, 6H).
223
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
H 1H NMR (400 MHz, CDCI3) 6
N N ci
-T- ...,..-ri ab k..0
(d, J = 1.9 Hz, 1H), 7.93 (d, J
= 5.9 Hz, 1H), 6.96 (d, J = 2.3
--a,
0 Hz, 1H), 6.91 (d, J = 2.3
Hz,
C
N }1,,,,
1H), 5.87 (d, J =6.1 Hz, 1H),
548 ) 492.39
4.97 (s, 1H), 4.77 (s, 1H),
4.02 (s, 1H), 3.97- 3.88 (m,
4H), 3.83 - 3.70 (m, 4H), 3.63
- 3.52 (m, 4H), 3.39 - 3.27 (m,
0 ,,, 4H), 2.25 - 2.12 (m, 2H),
2.01
- 1.85 (m, 6H).
1H NMR (300 MHz,
H,C,N Chloroform-d) 6 8.70 (d, J
=
1
,
H
,)'-r-N.N., 1.9 Hz, 1H), 8.62 (d, J =
1.9
Hz, 1H), 8.18 (d, J = 5.1 Hz,
1H), 6.98 - 6.89 (m, 2H), 6.42
(d, J = 5.1 Hz, 1H), 5.18 (d, J
^- 0
549 504.27 = 8.0 Hz, 1H), 4.79 (dt,
J =
õ-- =y-"*"."---, 7.9, 3.7 Hz, 1H),4.11 -3.87
1 (m, 5H), 3.42 - 3.25 (m, 4H),
(NN NN =-=\ife- 3.00 (dq, J = 9.9, 3.3
Hz, 2H),
2.63 - 2.35 (m, 5H), 2.27 -
2.04 (m, 4H), 1.97 - 1.82 (m,
9H).
1H NMR (300 MHz,
H Chloroform-d) 6 8.70 (d, J
=
N 14
r-F "y=
CH: L 1.9 Hz, 1H), 8.63(d, J =
1.9
Hz, 1H), 8.16 (s, 2H), 7.05 -
6.83 (m, 2H), 5.14 (d, J = 8.1
0
Hz, 1H), 4.79 (d, J = 5.3 Hz,
550 HN r,--N-,, 546.31
5H), 3.41
/
1 - 3.28 (m, 4H), 2.87 (tt, J =
HC CH, ,".... ....-,, ....-= .. 12.8, 3.2 Hz,
1H), 2.20 (q, J =
r N N 6.2 Hz, 2H), 1.91 (p, J =
3.7,
o 2.8 Hz, 6H), 1.73 (dd, J =
..,
13.1, 3.2 Hz, 2H), 1.29(d, J =
24.9 Hz, 12H).
224
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M+H)
H NMR (300 MHz,
HN Chloroform-d) 6 8.71 (d, J
=
H 1.9 Hz, 1H), 8.64 (d, J =
1.9
Hz, 1H), 8.20 (d, J = 5.1 Hz,
1H), 7.01 -6.89 (m, 2H), 6.42
. (d, J = 5.1 Hz, 1H), 5.15 (d, J
551 490.28 = 7.9 Hz, 1H), 4.80 (s,
1H),
4.05 (s, 1H), 3.97- 3.88 (m,
1 4H), 3.43 - 3.31 (m, 4H), 3.24
,,,,,. `=-= `Nij F''' (d, J = 12.3 Hz, 2H), 2.84 -
1- N
2.69 (m, 2H), 2.59 (m, 1H),
2.20 (m, 2H), 1.92 (t, J = 8.2
Hz, 6H).
H H
1H NMR (300 MHz,
Chloroform-d) 6 8.61 (d, J =
2.0 Hz, 1H), 8.53 (d, J = 1.9
0 Hz, 1H), 7.85 (s, 1H), 6.94 -
6.73 (m, 2H), 5.33 (d, J = 7.9
552 ," s-eti`N 462.2
Hz, 1H), 4.69 (s, 1H), 4.00 -
1 3.78 (m, 5H), 3.38 (d, J = 1.0
Hz, 2H), 3.33 - 3.20 (m, 4H),
2.19- 1.99 (m, 2H), 1.83 (h, J
= 5.6 Hz, 6H).
?H 1H NMR (300 MHz,
H
Chloroform-d) 6 8.71 (d, J =
1.9 Hz, 1H), 8.63(d, J = 1.9
I Hz, 1H), 6.93 (dd, J =
17.8,
L\-''''*0 2.5 Hz, 2H), 5.07 (s, 1H), 4.78
480.19 (d, J = 6.2 Hz, 1H), 4.68
(d, J
553
6 N
'."CH, ---...-, ---' ) = 7.9 Hz, 1H), 4.03 -
3.90 (m,
I 4H), 3.87 (s, 3H), 3.71 (s, 1H),
3.42 - 3.28 (m, 4H), 3.13 (s,
6H), 2.27 - 2.00 (m, 2H), 2.00
- 1.79 (m, 6H).
225
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H 1H NMR (300 MHz,
iiisit:NX,i, N , Chloroform-d) 6 8.70 (d, J
=
1.9 Hz, 1H), 8.63 (d, J = 1.9
0 Hz, 1H), 8.04 (s, 1H), 5.04 (d,
J = 8.2 Hz, 1H), 4.79 (s, 1H),
554
4111 )1) 462.2 4.09 - 3.90 (m, 5H), 3.85 (s,
2H), 3.42- 3.31 (m, 4H), 3.11
(t, J = 5.8 Hz, 2H), 2.63 (t, J =
rN N 5.8 Hz, 2H), 2.19 (d, J =
8.1
Hz, 2H), 1.91 (d, J = 5.5 Hz,
6H).
Co
õ_ 1H NMR (400 MHz, CDCI3) 6
H NIL
8.68 (d, J = 1.9 Hz, 1H),8.61
, r
ta (d, J = 1.9 Hz,
= 2.4 Hz, 1H), 6.87 (d, J = 2.4
Hz, 1H), 5.47 (s, 1H), 5.30 (s,
555 010357.15
1H), 4.50 (s, 1H), 4.00 - 3.84
' 14'
N --- (m, 4H), 3.40 - 3.27 (m, 4H),
2.45 - 2.33 (m, 2H), 2.33 -
N
2.18 (m, 1H), 2.15 - 2.00 (m,
0.,,,,...õ..) 2H), 1.74 (d, J = 5.4 Hz, 4H).
H 1H NMR (300 MHz,
H,C, ,^,N N Chloroform-d) 6 8.61 (d, J
=
N- -y-,-- -ii: , 1.9 Hz, 1H), 8.53 (d, J =
1.9
L.,......,,, Hz, 1H), 7.97 (s, 1H), 6.92
-
0 6.77 (m, 2H), 4.95 (d, J = 8.1
Hz, 1H), 4.76 - 4.64 (m, 1H),
556 =:,.,"".., 476.23
3.97 - 3.75 (m, 5H), 3.34 (s,
i 1 2H), 3.30 - 3.17 (m, 4H),
2.60
r-----N '-'1N'^' (dq, J = 9.7, 5.3, 4.8 Hz,
4H),
2.37 (s, 3H), 2.10 (td, J =
10.8, 10.3, 6.1 Hz, 2H), 1.90 -
1.62 (m, 6H).
226
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
CH 1H NMR (300 MHz,
; 0
H:C i,, A Chloroform-d) 6 8.71 (d, J
=
WC
2.0 Hz, 1H), 8.63 (d, J = 1.9
0 N
H Hz, 1H), 8.20 (d, J = 5.1
Hz,
KID,. 1H), 7.01 -6.89 (m, 2H),
6.40
N (d, J = 5.1 Hz, 1H),
5.20(s,
557 0 590.2 1H), 4.81 (d, J = 6.1 Hz,
1H),
4.23 (s, 2H), 4.04 (s, 1H),
tyPN, 3.97 - 3.89 (m, 4H), 3.42 -
I .." 11 3.28 (m, 4H), 2.82 (t, J =
12.8
`-..,-.4k\
N N Hz, 2H), 2.67 - 2.44 (m,
1H),
0,) 2.27 - 2.11 (m, 2H), 1.98 -
1.87 (m, 8H), 1.49 (s, 9H).
111r\44 0 1H NMR (300 MHz, CDCI3):
ppm 1.84- 1.99(m 6 H), 2.11
110 10
Ni, NH -2.25 (m, 2 H), 3.32 - 3.36
(m, 4 H), 3.43 (s, 3 H), 3.70 -
558 481.3 3.73 (m, 2 H), 3.90 -
3.93 (m,
I 4 H), 3.96 - 4.07 (m, 1 H),
N 4.41 -4.44 (m, 2 H), 4.72 -
C )
f t. 4.80 (m, 1 H), 5.10 (br. s,
1
H), 6.06 (d, J = 5.6
,
1H NMR (300 MHz, CDCI3):
1,) ip ,,,,r,"...1 14,õõ), 0 ppm 1.84 -
1.98 (m, 6 H), 2.13
-2.23 (m, 2 H), 2.32 (s, 6 H),
2.68 (t, J = 5.8 Hz, 2 H), 3.30-
494.3
559 IN,,,-1,94, riSN, "1'N "NI
N N 0 3.37 (m, 4 H), 3.88 - 3.95
(m,
H 4 H), 3.98 - 4.08 (m, 1 H),
N WC"\CH N 4.37 (t, J = 5.8 Hz, 2 H), 4.73 -
..." N...
= 4.80 (m, 1 H), 5.05 (d, J = 7.5
Hz, 1 H)
227
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M-I-H)
r.--N 1H NMR (300 MHz, CDCI3):
I 1 ppm 1.83- 2.00 (m, 6 H),
2.10
N . , - 2.25 (m, 2 H), 2.52 -
2.59
1 (m, 4 H), 2.76 (t, J = 5.8
Hz, 2
560 N '/1%! 0.-"'"NN 536.3 H), 3.31 -3.38 (m,
4 H), 3.70 -
I H I 3.77 (m, 4 H), 3.89 - 3.95
(m,
N N
( ) r o 4 H), 4.03 (br. s, 1 H),
4.41 (t,
J = 5.8 Hz, 2 H), 4.72 -4.80
(m, 1 H),
O tN
njr"N 1H NMR (300 MHz, CDCI3):
14 ppm 1.78- 1.94 (m, 6 H),
2.14
0.1/41Cip Nz,-----14 -2.25 (m, 2 H), 2.32 (s, 6
H),
i 2.67 (t, J = 5.5 Hz, 2 H),
3.28 -
561 ....-- .--L,' ,,,-.),,,,
N 0""*NI 494.3 3.38 (m, 4 H), 3.66 (br. s, 1
H H), 3.84- 3.98 (m, 4 H), 4.39
N HC,,NCH
, (t, J = 5.5 Hz, 2 H), 4.79 (br. s,
C1
--õ, ,- ,
1 H), 4.90 (d, J = 7.2 Hz, 1 H),
5.73
LO'"
"N
I 1 1H NMR (300 MHz, CDCI3):
1%1 0
õ.,....õ 40, N71-,µ-1,1 ppm 1.81 - 1.94 (m, 6 H), 2.14
/*õ."EN -2.24 (m, 2 H), 2.28 (s, 3 H),
562
H
2.47 (br. s, 4 H), 2.59 (br. s, 4
N O"-N\I 549.3 H), 2.76 (t, J = 5.8 Hz, 2 H),
,
N 3.30 - 3.37 (m, 4 H), 3.69 (br.
r '---- s, 1 H), 3.88 - 3.95 (m, 4
H),
4.42 (t, J = 5.8 Hz, 2 H), 4.75 -
O N 4.83
i
CH,
228
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
H 1H NMR (400 MHz, CDCI3) 6
Nõ N 8.68(d, J = 1.9 Hz, 1H), 8.61
(d, J = 1.9 Hz, 1H), 8.48 (d, J
= 0.6 Hz, 1H), 7.75 (d, J = 2.2
0 Hz, 1H), 6.94 (d, J = 2.4
Hz,
1H), 6.92 - 6.88 (m, 1H), 6.67
563 1.4
1001
(d, J = 1.4 Hz, 1H), 5.22 (t, J =
9.5 Hz, 1H), 4.79 (s, 1H), 4.06
1.-------N N (s, 1H), 4.00 - 3.84 (m, 4H),
0..õ,..) 3.40 - 3.24 (m, 4H), 2.16 (t, J
= 21.2 Hz, 2H), 1.98 - 1.85
(m, 6H).
1H NMR (300 MHz,
CH, 0
14,0õj i H
N N Chloroform-d) 6 8.70 (d, J =
1.9 Hz, 1H), 8.62 (d, J = 1.9
H;C"..NO"-\ 1 a xi k n
N. Hz, 1H), 8.07 (s, 1H), 6.99 -
6.84 (m, 2H), 5.10 (d, J = 8.1
Hz, 1H), 4.78 (d, J = 5.7 Hz,
564 562.25
N 1H), 4.42 (s, 2H), 4.10 -
3.85
311 (m, 5H), 3.64 (t, J = 5.6
Hz,
r'''NN Nisr 2H), 3.40 - 3.29 (m, 4H), 2.65
(dt, J = 6.2, 2.8 Hz, 2H), 2.19
0 (q, J = 6.0 Hz, 2H), 1.98 - 1.85
\...." (m, 6H), 1.49 (s, 9H).
0
H 1H NMR (300 MHz,
KC, ,ic.,...N N Chloroform-d) 68.55 (d, J =
1 z k y i 4 1 1.9 Hz, 1H), 8.47(d, J = 1.9
565
Hz, 1H), 6.93 (s, 1H), 6.86-
'*0
479.1
6.72 (m, 2H), 5.25 (s, 1H),
CH, 4.66(s, 1H), 4.05 - 3.90
(m,
1H), 3.86- 3.71 (m, 7H), 3.30
r- 3.09 (m, 4H), 2.27 (d, J = 2.2 N 1.414.) Hz, 3H), 2.04 (d, J = 9.2
Hz,
2H), 1.75 (d, J = 4.8 Hz, 6H).
229
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
H
1H NMR (300 MHz,
r 1
Chloroform-d) 6 8.71 (d, J =
N NO, 0 2.0 Hz, 2H), 8.63 (d, J =
1.9
Hz, 1H), 7.42 (s, 1H), 7.01 -
566 N 475.15 6.86 (m, 2H), 5.64 (d, J
= 8.2
- 0 ^ g -
\I qi = =i- Hz, 1H), 4.83 (s, 1H), 3.92
(dd, J = 6.0, 3.7 Hz, 4H), 3.34
N (dd, J = 5.8, 3.9 Hz, 4H),
2.11
- 1.78 (m, 6H).
H 1H NMR (300 MHz,
N N Chloroform-d) 6 8.70 (d, J
=
1.9 Hz, 1H), 8.63 (d, J = 1.9
H.,0 s.*0 Hz, 1H), 7.74 (s, 1H), 7.00
-
6.89 (m, 2H), 4.91 (s, 1H),
1
567 N 504.1 4.76 (d, J = 6.1 Hz, 1H),
4.04 _
r ..,....
I 4111 "hi 3.93 (m, 5H), 3.43 - 3.28
(m,
4H), 2.26 - 2.14 (m, 2H), 2.08
c------- rs-N, (d, J = 0.8 Hz, 3H), 2.00 -
1.92
(m, 6H), 1.69(d, J = 21.2 Hz,
10H).
1H NMR (300 MHz,
H Chloroform-d) 6 8.70 (d, J
=
N N .....-,...*IkU 1.9 Hz, 1H), 8.63 (d, J =
2.0
(Nr,CIN/ Hz, 1H), 8.22 (s, 2H), 7.04-
Nip 6.88 (m, 2H), 5.14 (d, J = 8.1
0
Hz, 1H), 4.80 (d, J = 5.5 Hz,
568 ,,N-/ 490.23 1H), 4.09 - 3.85 (m,
5H), 3.41
1-1C - 3.31 (m, 4H), 3.29 - 3.13 (m,
,
r-----N -1,, - 1H), 3.04 - 2.92 (m, 1H),
2.81
(q, J = 8.1 Hz, 1H), 2.68 (dt, J
= 9.2, 4.6 Hz, 1H), 2.52 - 2.26
(m, 5H), 2.24 -2.13 (m, 2H),
2.02 - 1.78 (m, 7H).
230
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (MH-H)
H 1H NMR (300 MHz,
Chloroform-d)6 8.61 (d, J =
1.9 Hz, 1H), 8.54(d, J = 1.9
Hz, 1H), 8.10 (s, 2H), 6.93 -
6.76 (m, 2H), 5.07 (d, J = 8.1
569 N /L )1i 476.06 hil
Hz,),14104:638(8d,(mJ =, 51H5).83H3z5,
H
1
- 3.20 (m, 4H), 3.18 -2.92 (m,
3H), 2.70 (dd, J = 10.7, 8.4
Hz, 1H), 2.24 - 2.02 (m, 3H),
1.94 - 1.62 (m, 7H).
H
"tsiN") 1H NMR (300 MHz,
Chloroform-d) 6 8.71 (d, J =
2.2 Hz, 1H), 8.63(d, J = 2.1
Hz, 1H), 8.19 (s, 2H), 7.03-
570 576.2 6.86 (m, 2H), 4.10 - 3.86
(m,
5H), 3.46 - 3.28 (m, 4H), 3.24
- 3.13 (m, 1H), 2.35 - 2.16 (m,
1H), 2.05- 1.84 (m, 8H), 1.81
- 1.66 (m, 1H), 1.55 - 1.41
HC (m,11H).
,
H 1H NMR (400 MHz, CDCI3) 6
N
41:21. 8.69 (d, J = 1.9 Hz, 1H),
8.61
1 (d, J = 1.9 Hz, 1H), 7.88
(d, J
H,C ',.,. = 2.4 Hz, 1H), 6.96 (d, J = 2.5
0
Hz, 1H), 6.90 (d, J = 2.4 Hz,
H,C
571 0- N 476.31 1H), 6.76 (s, 1H), 4.98
(s, 2H),
...-,
i 4.78 (s, 1H), 4.00- 3.86
(m,
4H), 3.83 (s, 1H), 3.39 - 3.27
(m, 4H), 2.21 (d, J = 9.6 Hz,
$
2H), 1.89 (dd, J = 13.3, 8.6
Hz, 6H), 1.50 (s, 6H).
231
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
r.----s...:,......N
14 04, 1 H NMR (300 MHz, CDCI3):
ill
NH ppm 1.82 - 1.99 (m, 6 H),
2.14
- 2.28 (m, 2 H), 3.32 - 3.35
(m, 4 H), 3.80 - 3.88 (m, 1 H),
572 N 472.2 3.90 - 3.93 (m, 4 H),
4.70 -
C ) --,,-- r
4.83 (m, 2 H), 6.37 (d, J = 8.8
Hz, 1 H), 6.38 (t, J = 73.8 Hz,
0
1 H), 6.90 (d, J = 2.3 Hz, 1 H),
F 0 6.95 (d, J =
'1--
F
r-------,,,..N
14 .0 1H NMR (300 MHz, CDCI3):
ppm 1.52 (s, 6 H), 1.73 (br s,
1 1 1 H), 1.85 - 1.96 (m, 6 H),
N H 2.11 - 2.25 (m, 2 H), 3.30-
573 464.3 3.36 (m, 4 H), 3.88 -4.00
(m,
H), 4.55 (d, J = 8.1 Hz, 1 H),
4.74 -4.81 (m, 1 H), 6.52 (s, 1
I
Co--- Hs: CH. 1.4 Hz, 1
1-40
H
N 1H NMR (400 MHz, CDCI3) 6
:C ,L.,:- 1 o 8.69(d, J = 1.9 Hz,
1H), 8.61
H
-0 N (d, J = 1.9 Hz, 1H), 7.99
(s,
4....,-;., )4 NI 62H.8)9,
1
r'N'II 60.9, 5J (=d 2, J. 4= Hz,2 . 41HHI, 41 H. 7)8,
574 437.42
(s, 1H), 4.01 - 3.84 (m, 7H),
.23.41 (s, 2H), 3.37- 3.27 (m,
4H), 2.26 - 2.14 (m, 2H), 1.93
0 i - 1.81 (m, 6H).
232
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (MH-H)
H
1H NMR (400 MHz, CDCI3) 6
8.69 (d, J = 1.9 Hz, 1H), 8.62
1 (d, J = 1.9 Hz, 1H), 8.57
(s,
N 0 1H), 8.13 (s, 2H), 6.98 (d,
J =
2
575 407.35 .4 Hz, 1H), 6.91 (d, J =
2.5
,--- ,--14-11 Hz, 1H), 4.86 - 4.73 (m,
1H),
3.95 - 3.88 (m, 4H), 3.56 _
r-----., ---- ---k--) 3.49 (m, 1H), 3.39 - 3.31
(m,
4H), 2.30 - 2.18 (m, 2H), 1.97
0.,,....õ) - 1.84 (m, 6H).
H
N N 1 H NMR (300 MHz,
.....- -ir-
(..C.,1
--. Chloroform-d) 6 8.71 (d, J
=
2.0 Hz, 1H), 8.63 (d, J = 1.9
Hz, 1H), 8.13 (s, 1H), 7.01 -
576 OH CH, 450.13 6.87 (m, 2H), 4.81 (s,
1H),
so ----- 4.57 (s, 2H), 4.06 (s, 1H),
3.99 - 3.87 (m, 4H), 3.35 (dd,
.--;:--
r'lli N J = 5.9, 3.8 Hz, 4H), 2.45
(s,
3H), 2.20 (d, J = 8.0 Hz, 2H),
1.93 (d, J = 5.1 Hz, 6H).
H 1H NMR (300 MHz,
N N õop Chloroform-d) 6 8.71 (d, J
=
r.c...- -r-
1.9 Hz, 1H), 8.63 (d, J = 2.0
Hz, 1H), 8.02 (d, J = 1.4 Hz,
N 0
1H), 7.87 (d, J = 1.5 Hz, 1H),
577 OH
'').----'1"-34 1 437.25 6.94 (dd, J = 16.2, 2.5 Hz,
2H), 4.89 -4.71 (m, 2H), 4.65
(s, 2H), 4.06 - 3.85 (m, 4H),
3.40 - 3.27 (m, 4H), 2.23 (dt, J
o) = 11.5, 5.5 Hz, 2H), 2.04 -
1.80 (m, 6H).
233
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M-I-H)
H
14,C,N N .0
1H NMR (300 MHz,
Chloroform-d) 6 8.71 (d, J =
0
.---- '**
0 2.0 Hz, 1H), 8.63 (d, J =
1.9
578 437.2
Hz, 1H), 7.01 -6.88 (m, 2H),
". 6.87 - 6.66 (m, 2H), 3.94
(t, J
= 4.9 Hz, 4H), 3.65 (s, 3H),
r-----N N 3.42 - 3.30 (m, 4H), 2.19 (s,
2H), 1.91 (d, J = 4.9 Hz, 6H).
H
N N,1/4, 1H NMR (400 MHz, CDCI3) 6
8.68 (s, 1H), 8.61 (s, 1H),
IN 8.07 (s, 2H), 6.94 (s, 1H),
!IµI '
''''' ..e0 6.90 (s, 1H), 4.96 (d, J =
8.1
579 õ,N) 505.53 Hz, 1H), 4.78 (s, 1H),
4.02-
H,C 3.81 (m, 5H), 3.47 - 3.19
(m,
N'''.1 5H), 3.19 - 2.83 (m, 4H),
2.76
-2.52 (m, 3H), 2.37 (s, 3H),
2.26 - 2.11 (m, 2H), 2.03 -
1.82 (m, 6H).
r------
a
illi ,,, ,, 1 ppm 1.84- 2.17 (m, 6 H), 2.23
-2.35 (m, 2 H), 3.31 - 3.38
580 1H NMR (300 MHz, CDCI3):
(m, 4 H), 3.63 n 3.75 (m, 1 H),
N 445.3 3.87 - 3.96 (m, 4 H), 4.82 -
H 4.89 (m, 1 H), 5.88 (d, J = 7.2
N Hz, 1 H), 6.11 (d, J = 7.8
Hz,
L.....,
1 H), 6.44 (d, J = 2.2 Hz, 1 H),
6.90 fi 6.95 (
0
234
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
1H NMR (300 MHz, CDCI3):
iki 0 r W. N ppm 1.85- 2.18 (m, 6 H),
2.24
581 1.1 4'a N
--,
N 1 -2.36 (m, 2 H), 3.31 -3.39
(m, 4 H), 3.64 - 3.77 (m, 1 H),
446.3 3.88 - 3.97 (m, 4 H), 4.82 -
H 4.89 (m, 1 H), 5.91 (d, J = 7.9
r
N Hz, 1 H), 6.12 (d, J = 7.9 Hz, .. I H),
6.93 (d, J = 2.4 Hz, 1 H),
C6.98 (d, J = 0 '''.1
ir'..,:ti 1H NMR (300 MHz, CDCI3):
1 ppm 1.83ñ 1.96 (m, 6 H),
N 0
1 "N. INVA". 2.14 -2.26 (m, 2 H), 330-
i . ,
1.= 3.36 (m, 4 , ,
582 ...,"
N FIN"--- \C H: 450.3 3.87 li
3.95H)3.4 H)
(m, 5 (s,
H 2 H), 4.59 - 4.89 (m, 2 H),
6.38 (s, 1 H), 6.48 (d, J = 5.2
Hz, 1 H), 6.90 (d, J = 2.4 Hz,
1 H), 6.95 (d, J = 2.4
1\"0
r----''''*----,Ni
a o ass
NH 1H NMR (300 MHz, CDCI3):
ppm 1.84- 2.00 (m, 6 H), 2.09
-2.24 (m, 2 H), 3.30 - 3.37
(m, 4 H), 3.64 - 3.74 (m, 1 H),
583 466.3 3.78 (s, 3 H), 3.89 -
3.95 (m, 7
N
- H), 4.71 -4.79 (m, 1 H), 5.88
1 (d, J = 8.3 Hz, 1 H), 6.90
(d, J
HC,. **---, CH, =2.4 Hz, 1 H), 6.95(d, J
=2.4
0 '0
0
-"'
235
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 11-1 NMR
Compound Structure
rt-----,N 1H NMR (300 MHz, CDCI3):
ppm 1.86- 2.04 (m, 6 H), 2.12
a 0
584 0 NH OH - 2.26 (m, 2 H), 3.28 -
3.39
(m, 4 H), 3.88 - 3.96 (m, 4 H),
436.3 4.26 (br s, 1 H), 4.62 (s,
2 H),
I 4.69 -4.78 (m, 1 H), 5.56 (br
N s, 1 H), 6.50 (t, J = 5.3 Hz, 1
C ) ,-----
N i
I
--,, H), 6.90 (d, J = 1.9 Hz, 1
H),
6.93 (d, J
0
õ.õ
4 ' t
11 I _1 1H NMR (300 MHz, CDCI3):
L
ppm 1.81 - 1.96 (m, 6 H), 2.11
-r --
ra , ''---- '444 ai
..i. -2.25 (m, 2 H), 2.56 (br s,
4
H), 2.76 (t, J = 5.3 Hz, 2 H),
585 t i
.z..., 536.3 3.30 - 3.36 (m, 4 H),
3.74 (t, J
0 = = 4.4 Hz, 4 H), 3.88 -
3.96 (m,
0 5 H), 4.06 (t, J = 5.3 Hz, 2 H),
--.1
1-. 4.73 -4.81 (m, 1 H), 4.99
(d, J
i.1-----N,, =7.8
cm.4
r-------
a 1H NMR (300 MHz, CDCI3):
ppm 1.88 li 2.01 (m, 6 H),
w, w....õ.õ,..- ,
2.10 (s, 3 H), 2.15 - 2.25 (m, 2
H), 3.30- 3.37 (m, 4 H), 3.89
586 N -,,,, 1
420.3 ri 3.95 (m, 4 H), 4.25 (br
s, 2
H H), 4.71 -4.78 (m, 1 H),
6.51
N CH, (dd, J = 6.8, 5.4 Hz, 1 H), 6.90
r ,....,
(d, J = 2.3 Hz, 1 H), 6.94 (d, J
= 2.3 Hz, 1
C0
236
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
F
F F 1H NMR (300 MHz, CDCI3):
r44 --..----
ppm 1.82 - 1.98 (m, 6 H), 2.12
-2.26 (m, 2 H), 3.25 - 3.43
(m, 4 H), 3.82 - 3.98 (m, 5 H),
587 474.2 4.80 (br s, 2 H), 6.52
(d, J =
N 8.4 Hz, 1 H), 6.87 -6.93
(m, 2
H
....N H), 6.95- 7.00 (m, 1 H),
7.50
(t, J = 7.9 Hz, 1 H), 8.63 (d, J
= 1.2 Hz, 1
0
CH,
L. 1H NMR (300 MHz, CDCI3):
0 ppm 1.38 (t, J = 7.0 Hz, 3 H),
I) 0 1.83 - 2.00 (m, 6 H), 2.11-
411) * No
'===N,--' ``'.- 1 .. 2.24 (m, 2 H), 3.30 - 3.37 (m,
588 0
450.3 4 H), 3.75 - 3.85 (m, 1 H),
3.89 - 3.94 (m, 4 H), 4.25 (q, J
H = 7.0 Hz, 2 H), 4.42 -4.54
(m,
N
C ) 1 H), 4.72 -4.79 (m, 1 H),
5.94 (d, J = 7.7 H
0
(.....44 1H NMR (300 MHz, CDCI3):
EJ 0 0 ppm 1.86- 1.99(m, 6 H),
2.16
Nn 4,.. -2.27 (m, 2 H), 3.31 - 3.36
(m, 4 H), 3.88 - 3.95 (m, 5 H),
F
589 N 474.2 4.77 -4.84 (m, 1 H), 4.90
(br
H ''''-''''.7Th<F s, 1 H), 6.55 (s, 1 H),
6.72 (d,
N F J = 5.0 Hz, 1 H), 6.91 (d,
J =
11 ) 2.4 Hz, 1 H), 6.95 (d, J = 2.4
Hz, 1 H),8.
0
237
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (MH-H)
(0
1H NMR (300 MHz, CDCI3):
1-----Ni N ppm 1.82- 1.98(m, 6 H),
2.10
-2.22 (m, 2 H), 3.31 - 3.35
,....õ , (m, 4 H), 3.41 - 3.45 (m, 4 H),
590 1 491.3 3.69 - 3.82 (m, 5 H),
3.90 -
3.93 (m, 4 H), 4.43 (d, J = 7.7
N
H Hz, 1 H), 4.70 -4.77 (m, 1
H),
N 5.81 (d, J = 8.0 Hz, 1 H), 5.90
(d, J = 8.0 H
1.-----, 1H NMR (300 MHz, CDCI3):
ti 0 ppm 1.88- 2.00 (m, 6 H),
2.13
= 410 -2.28 (m, 2 H), 3.29
- 3.38
591 'N.
1 (m, 4 H), 3.88 - 3.95 (m, 4 H),
µ----
N 424.2 4.21 (br s, 1 H), 4.62 -
4.84
H (m, 2 H), 6.45 -6.54 (m, 1
H),
N F 6.90 (d, J = 2.4 Hz, 1
H), 6.95
C
0
(d, J = 2.4 Hz, 1 H), 7.07 -
7.18(m, 1 H),
C
r------ 1H NMR (300 MHz, CDCI3):
a 04, ppm 1.84- 2.03 (m, 6 H),
2.11
592
-Ow õ..L,3 -2.24 (m, 2 H), 3.30 - 3.37
i (m, 4 H), 3.84 (s, 3 H), 3.89 -
N -.õ
436.3 3.95 (m, 4 H), 4.17 -4.29
(m,
H 1 H), 4.67 - 4.76 (m, 1 H),
N 0 5.08 (d, J = 7.4 Hz, 1
H), 6.50
L
....,
14,W'
(dd, J = 7.3, 5.2 Hz, 1 H), 6.82
(d, J = 7.4 Hz
238
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
rrN 1H NMR (300 MHz, CDCI3):
a ppm 1.86- 2.00 (m, 6 H),
2.13
-2.26 (m, 2 H), 3.30 - 3.38
.....---
I (m, 4 H), 3.88 - 3.97 (m, 4
H),
593 "=== õ---Lk,,,,,,,, -'''
N . 474.2 4.22 - 4.35 (m, 1 H),
4.73 -
H . 4.80 (m, 1 H), 4.89 -4.99
(m,
1 H), 6.60 (dd, J = 7.1, 5.1 Hz,
C F F
F 1 H), 6.91 (d, J = 2.3 Hz, 1 H),
) 6.96 (d, J =
0
i---, 1H NMR (300 MHz, CDCI3):
4 ,,....õ. 040 2.2.1gF ppm 1.85-
2.00 (m, 6 H), 2.11
- 2.28 (m, 2 H), 3.29 - 3.39
I I (m, 4 H), 3.87 - 3.97 (m, 4
H),
594 =-=,.. Nr '''-
N 442.2 4.06 -4.18 (m, 1 H), 4.56
(d, J
H = 7.6 Hz, 1 H), 4.73 -4.81
(m,
II F 1 H), 6.90 (d, J = 2.4 Hz,
1 H),
( ) 6.95 (d, J = 2.4 Hz, 1 H),
7.05
(ddd, J =
0
1-44 1H NMR (300 MHz, CDCI3):
ppm 1.88- 2.02 (m, 6 H), 2.08
C H s., ,,,,Q.õ ,
(s, 3 H), 2.15 (s, 3 H), 2.14-
õ
1 2.24 (m, 2 H), 3.31 - 3.36
(m,
595 N 434.3 4 H), 3.89 - 3.95 (m, 4
H),
H 4.03 - 4.16 (m, 1 H), 4.23
(br
N CH., s, 1 H), 4.69 -4.77 (m, 1
H),
C ) 6.90 (d, J = 2.4 Hz, 1 H),
6.94
(d, J = 2.4 Hz
0
239
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
0 H 1H NMR (300 MHz,
`-- N Chloroform-d) 6 8.70 (d, J
=
Et 2.0 Hz, 1H), 8.63 (d, J =
2.0
0)...7.õ,y-N 4.(11 Hz, 1H), 6.99 (d, J = 2.4 Hz,
1H), 6.92 (t, J = 2.8 Hz, 1H),
596 HC 0 477.68 6.08 (d, J = 1.1 Hz,
1H), 5.21
(s, 1H), 4.81 (s, 2H), 3.98 -1116,µ N 3.87 (m, 4H), 3.43 -
3.30 (m,
(---NN -W.¨ .._õ...) 4H), 2.51 (d, J = 1.0
Hz, 3H),
0, if N 2.19 (s, 2H), 2.01 - 1.77
(m,
-,,---- 6H).
H 1H NMR (300 MHz,
HC ,N N Chloroform-d) 6 8.71 (d, J
=
1.9 Hz, 1H), 8.63(d, J = 1.9
Kt's, Hz, 1H), 7.07 - 6.87 (m,
2H),
s'w0
6.02 (s, 1H), 4.83 (d, J = 5.5
597 I 'µ` CH, ,--14"--- 449.23 -- Hz, 1H), 4.03 -
3.81 (m, 5H), .- -- I -- 3.44 - 3.25 (m, 4H), 2.72 -
2.57 (m, 1H), 2.51 (s, 3H),
rs-Ni N 2.29 - 2.11 (m, 2H), 2.05 -
0 1.72 (m, 6H), 1.27 (t, J = 7.6
Hz, 3H).
H
N
1 1H NMR (300 MHz,
: Chloroform-d) 6 8.61 (d, J =
,
0 1.9 Hz, 1H), 8.54 (d, J =
1.9
N N
1 Hz, 1H), 6.93 - 6.72 (m,
3H),
598 CH, 450.23 6.55(s, 1H),4.71 (s,
1H),
4.01 (s, 1H), 3.92- 3.76 (m,
4H), 3.34 - 3.18 (m, 4H), 2.98
r-------N-'--- l'E's) (s, 6H), 2.07 (d, J = 24.1
Hz,
0 I 2H), 1.87 (d, J = 9.2 Hz,
6H).
240
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
H
1H NMR (300 MHz,
Chloroform-d) 6 8.62 (d, J =
----" 1.9 Hz, 1H), 8.54 (t, J = 2.4
0 0
Hz, 1H), 6.85 (dd, J = 18.9,
599 CH, iiii l'k,s,õ 437.21 2.4 Hz, 2H),
6.63 - 6.50 (m,
1H), 4.68 (s, 1H), 3.92 - 3.76
(m, 4H), 3.66 (s, 1H), 3.32 _
r------N 411111911111'- N 3.17 (m, 4H), 2.16- 1.99
(m,
0.õ,..õ.õ...i 5H), 1.92 - 1.72 (m, 6H).
H
HO N N 41:) 1H NMR (300 MHz,
-ir- Chloroform-d) 6 8.71 (d, J
=
Kt's, iµl 2.0 Hz, 1H), 8.62(d, J =
2.0
1 ''.0 Hz, 1H), 7.00 - 6.85 (m, 2H),
600 N 464.26 5.44 (d, J = 8.5 Hz,
1H),4.78
õ....- ---,.. (s, 1H), 3.93 (t, J = 4.8 Hz,
I 4H), 3.35 (t, J = 4.9 Hz, 4H),
r'lli =-õ, ,..õ..-
N 3.06 - 2.91 (m, 1H), 2.15
(d, J
= 26.6 Hz, 2H), 1.89 (s, 6H),
1.30 -0.94 (m, 4H).
1H NMR (300 MHz,
H Chloroform-d) 6 8.70 (d, J
=
1
1.9 Hz, 1H), 8.62 (d, J = 1.9 4-i1-.4 1 N'1/40 Hz, 1H), 7.06 (d, J = 9.1
Hz,
,../ =,õ, 1H), 6.98 - 6.86 (m, 2H),
6.58
0
(d, J = 9.1 Hz, 1H),4.81 (dq, J
601 461.32 = 5.5, 2.7 Hz, 1H), 4.52
(s,
.....1 )4)
1H), 4.17(d, J = 5.0 Hz, 1H),
4.01 - 3.87 (m, 4H), 3.67 (dq,
I NI N J = 8.9, 8.1 Hz, 1H), 3.43 -
3.30 (m, 4H), 2.47 - 2.29 (m,
4H), 2.22 (dt, J = 11.9, 5.5 Hz,
2H), 2.05- 1.82 (m, 6H).
241
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
H 1H NMR (300 MHz,
H , C i N .0 Chloroform-d) 6 8.70 (d, J
=
1.9 Hz, 1H), 8.62(d, J = 1.9
N'=--, 1 '**,0 Hz, 1H), 7.04 - 6.92 (m,
2H),
4.82 (d, J = 3.0 Hz, 1H), 3.93
602 _N,
)).4"'", 464.1 (dd, J = 5.9, 3.8 Hz, 4H), 3.65
....--C:-.LL L (m, 4H), 3.08 (s, 6H), 2.39 (s,
r14. N'''. 3H), 2.21 (d, J = 14.4 Hz,
2H),
1.92 (dt, J = 17.0, 10.7 Hz,
6H).
CH,
H
N 1H NMR (300 MHz,
14; c.,-..r,..-y-N41/4.,,,,"----. Chloroform-d) 6 8.70 (d, J
=
1.9 Hz, 1H), 8.62 (d, J = 1.9
--..õ,...,- Hz, 1H), 8.18 (s, 1H), 7.02
-
603 450.1 6.86 (m, 2H), 4.81 (s,
2H),
N.,õ.
4.00 - 3.76 (m, 5H), 3.42 -
3.26 (m, 4H), 3.07 (s, 6H),
2.20 (q, J = 6.3, 5.8 Hz, 2H),
2.01 -1.79 (m, 6H).
H
0 1H NMR (300 MHz,
y
Chloroform-d) 6 8.62 (d, J =
T
1.9 Hz, 1H), 8.53 (d, J = 1.9
0
Hz, 1H), 6.87 (d, J = 2.5 Hz,
N.
,. . . . ,:e.., ,1 i k i N 4.
1H), 6.81 (d,
604 CH, 452.1
") 3.94 - 3.72 (m,
7H), 3.25 (dd, J = 5.8, 3.8 Hz,
4H), 2.29 (d, J = 10.4 Hz, 3H),
0 -1 2.21 -2.02 (m, 2H), 1.93 -
1.71 (m, 6H).
242
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS NMR
Compound Structure
No.
1.1
N N
ye-
605
0
ler 1 482.3
10,.<71/4)1
1H NMR (300 MHz,
H N Chloroform-d)6 9.41 (s,
1H),
8.20 (dd, J = 4.8, 2.4 Hz, 2H),
6.98 - 6.77 (m, 3H), 6.44 (dt, J
606 450.17 = 7.5, 4.8 Hz, 2H),
4.71 (s,
0
2H), 3.83 (dd, J = 5.7, 4.0 Hz,
6H), 3.27 (dt, J = 30.0, 4.9 Hz,
Nft, 5H), 2.13 (s, 2H), 2.00 -
1.68
(m, 6H).
1H NMR (300 MHz,
Chloroform-d) 6 8.71 Jd, J =
= 1.9
Hz, 1H), 7.79(d, J = 2.9 Hz,
0 1H), 6.93 (dd, J = 17.8,
2.5
607 1AC08 Hz, 2H), 5.19 - 5.07
(m, 1H),
4.80 (dt, J = 6.7, 3.4 Hz, 1H),
4.32 (q, J = 7.1 Hz, 3H), 3.98 -
rs'\N 3.87 (m, 4H), 3.41 - 3.29
(m,
4H), 2.30 - 2.13 (m, 2H), 2.03
- 1.84(m, 6H), 1.41 (t, J = 7.1
Hz, 3H).
243
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M-I-H)
H
N N 1H NMR (300 MHz,
HC y-- i, 4)--0 Chloroform-d) 6 8.71 (d, J
=
N, 1.9 Hz, 1H), 8.63 (d, J =
2.0
0 Hz, 1H), 6.94 (dd, J =
18.9,
1
2.5 Hz, 2H), 5.42 (d, J = 8.4
608 0 1101468.63
'
) Hz, 1H), 4.79 (s, 1H), 4.22-
4.06 (m, 1H), 4.03 - 3.89 (m,
10H), 3.42 - 3.22 (m, 4H),
,,,,,i 2.19 (m, 2H), 2.01 - 1.79
(m,
6H).
H
,0 Nylk14,
1H NMR (300 MHz,
-*.,..... 'tea Chloroform-d) 6 8.72 (d, J =
ftisl c.,, 1.9 Hz, 1H), 8.63 (d, J =
1.9
' Hz, 1H), 7.03 - 6.84 (m, 2H),
609 CI
1 ''',.... N'I 471.87 5.66 (d, J = 9.1 Hz,
1H), 4.82
(s, 1H), 4.09 - 3.84 (m, 7H),
3.35 (dd, J = 5.5, 3.6 Hz, 4H),
2.20 (s, 2H), 1.91 (d, J = 6.4
Hz, 6H).
1\1:317''-o 1H NMR (300 MHz,
Chloroform-d) 6 8.69 (d, J =
0..Ø 00 1\1 1.9 Hz, 1H),8.61 (d, J =
1.9
Hz, 1H), 8.51 (s, 2H), 6.95 (d,
0-- J =2.5 Hz, 1H), 6.91 (d, J
=
HN
610 ,---1-... N 479.2 2.6 Hz, 1H), 5.39 (d, J =
8.1
(Ti Hz, 1H), 4.88 (d, J = 1.8
Hz,
4H), 4.80 (d, J = 6.2 Hz, 1H),
-....., t
4.13- 3.98 (m, 1H), 3.96 -
OH 3.89 (m, 4H), 3.38 - 3.30
(m,
4H), 3.06 (s, 1H), 2.28 -2.11
0 (m, 2H), 1.98 - 1.84 (m,
6H).
244
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M+H)
1H NMR (300 MHz,
r........,TA0 14 Chloroform-d) 6 8.67 (d, J
=
1.9 Hz, 1H), 8.60 (d, J = 1.9
Hz, 1H), 6.91 (dd, J = 16.1,
611
HO 344.19
, 2.5 Hz, 2H), 4.81 (q, J =
3.6,
''. '-')
2.4 Hz, 1H), 4.03 - 3.75 (m,
r
N 4H), 3.59 (d, J = 2.9 Hz, 2H),
3.45 - 3.28 (m, 4H), 2.20 (dt, J
1---,
= 9.5, 4.6 Hz, 2H), 1.74 - 1.23
..----
0 (m, 7H) 1.41 (s, 1H).
ir-...,,,,i 1H NMR (300 MHz, CDCI3):
ppm 1.83- 1.96 (m, 6 H), 2.14
N 0
, ...v\,,,,
-2.24 (m, 2 H), 3.31 - 3.35
612
1 1 ), 1: (m, 4 H), 3.44 (s, 3 H), 3.69 -
---,
N 'N'N" ''.0 481.3 3.72 (m, 2 H),
3.89 - 3.95 (m,
H , 5 H), 4.06 - 4.09 (m, 2 H),
H C ,
, 4.74 -4.83 (m, 1 H), 5.04-
(
NN5.15 (m, 1 H), 6.90 (d, J = 2.4
Hz, 1 H), 6.94 (d,
CO-"'
1H NMR (300 MHz, CDCI3):
1J ppm 1.39- 1.75 (m, 6 H),
1.82 - 1.95 (m, 6 H), 2.11 -
1 1 2.24 (m, 2 H), 2.37 - 2.63 (m,
613 534.3 4 H), 2.67 - 2.90 (m, 2
H),
H N 3.33 (t, J = 4.7 Hz, 4 H), 3.87-
3.97 (m, 5 H), 4.01 -4.20 (m,
2 H), 4.74 - 4.82 (m, 1 H),
Nõos, 4.98 (d, J = 7.9 Hz, 1
245
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M-I-H)
fN F 1H NMR (300 MHz, CDCI3):
a riiih . ppm 1.83- 1.95(m, 6 H),
2.12
614 111111P N N:13 -2.26 (m, 2 H), 3.30 - 3.40
(m, 4 H), 3.85 - 3.96 (m, 5 H),
424.2 4.55 -4.68 (m, 1 H), 4.74 -
H 4.82 (m, 1 H), 6.10 (dd, J
=
N 7.7, 1.8 Hz, 1 H), 6.18 (dd, J =
C ) 8.0, 1.8 Hz, 1 H), 6.90 (d, J =
1.8 Hz, 1 H),6
0
1H NMR (300 MHz, CDCI3):
NI ' 'iIIJ0,0 N"õ, i ppm 1.82- 2.10 (m, 7 H),
2.14
- 2.29 (m, 2 H), 3.26 - 3.40
(m, 4 H), 3.82 - 4.05 (m, 5 H),
615 ',if N N., 0H 436.3 4.64 (s, 2 H), 4.75 -
4.84 (m, 1
i H H), 4.85 - 5.13 (m, 1
H),6.46
C
(s, 1 H), 6.51 (d, J = 5.3 Hz, 1
H), 6.90 (d, J = 1.7 Hz, 1 H),
6.94 (d,
1,.,0')
H,C, 0 1H NMR (300 MHz,
H Chloroform-d) 6 8.71 (d, J
=
1.9 Hz, 1H), 8.63 (d, J = 1.9
1 Hz, 1H), 6.95 (dd, J = 16.6,
2.5 Hz, 2H), 6.30 (s, 1H), 5.02
616 T
,
465.1 (s, 1H), 4.82 (s, 1H), 4.38
(d,
CH, J = 0.9 Hz, 2H), 3.99 -
3.83
r----- Illi '4)
(m, 5H), 3.39 - 3.24 (m, 4H),
2.49 (s, 3H), 2.33 - 2.11 (m,
N N
2H), 1.91 (q, J = 9.0, 6.9 Hz,
0i 6H).
246
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
-
1H NMR (300 MHz,
NyN Chloroform-d) 6 8.63 (s, 1H),
8.20 (d, J = 4.8 Hz, 2H), 6.90
14 N ,.Ø4.
(dd, J = 16.2, 2.5 Hz, 2H),
617 4371
6.44 (t, J = 4.8 Hz, 1H), 5.15
.
0 (d, J = 8.1 Hz, 1H), 4.90
(d, J
1,1 '''' = 3.9 Hz, 2H), 4.79 -4.64
(m,
illi õ.... 0 H 1H), 3.99- 3.71 (m, 6H),
3.32
----- r
7 - 3.19 (m, 4H), 2.21 - 2.04 (m, ---"- N '
2H), 1.95- 1.70 (m, 6H).
o........õ..--
H 1H NMR (300 MHz,
HC 1.,:;7...y N Chloroform-d) 6 8.63 (d, J =
W., 1.9 Hz, 1H), 8.55(d, J =
1.9
:
Hz, 1H), 6.86 (dd, J = 16.5,
2.5 Hz, 2H), 5.94 (s, 1H), 4.73
618 XL
463.13 (s, 1H), 3.85 (dd, J = 5.9,
3.7
à IP ) Hz, 4H), 3.35 - 3.20 (m,
4H),
CH, 2.67 -2.51 (m, 2H), 2.26
(s,
r-----N Kr 3H), 2.09 (d, J = 36.8 Hz,
3H),
1.94 - 1.59 (m, 6H), 0.91 (t, J
= 7.4 Hz, 3H).
1H NMR (300 MHz,
LI Chloroform-d) 6 8.77 (s, 1H),
-'1-- 8.29 (d, J = 4.7 Hz, 2H),
7.02
(d, J = 2.5 Hz, 1H), 6.97 (d, J
= 2.5 Hz, 1H), 6.53 (t, J = 4.8
619 N,,,,, Hz, 1H), 5.16 (dd, J =
16.9,
N 7.5 Hz, 2H), 4.81 (s, 1H),
3.93
-,.._,..-1,
(t, J = 4.8 Hz, 5H), 3.34(t, J =
1 4.9 Hz, 4H), 2.27(d J =
21.1
Hz, 2H), 2.06 - 1.76 (m, 6H),
o ,.......) 1.66 (d, J = 6.6 Hz, 3H).
247
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (MH-H)
r-....,-----1
1 H NMR (300 MHz,
''-i- Chloroform-d) 6 10.21 (s, 1H),
9.26 (s, 1H), 8.30 (d, J = 4.8
H N 4,61:::).
Hz, 2H), 7.09 - 6.88 (m, 2H),
6.54 (t, J = 4.8 Hz, 1H), 4.89
620 435.1
O o (d q, J = .5 H
, J =64.9, z2; Hz,1H5), .9
31H9 -.8
), 43.04
(ci 7
(m, 5H), 3.55 - 3.39 (m, 5H),
2.35 - 2.17 (m, 3H), 2.09 -
( -)
--- 19 LIIIIIIIAII" #411*-; "
1.82 (m, 6H).
0
H 1H NMR (400 MHz, CDCI3) 6
8.68 (d, J = 1.7 Hz, 1H), 8.61
(d, J = 1.7 Hz, 1H), 7.95 (s,
1H), 6.94 (d, J = 2.1 Hz, 1H),
6.90 (s, 1H), 5.12 (d, J = 7.5
621 1.11--IC') 449.55 Hz, 1H), 4.77
(s, 1H), 4.60 (t,
J = 8.5 Hz, 2H), 4.00 (s, 1H),
-,,,, .) 3.94 - 3.84 (m, 4H), 3.38 -
r'slki N 3.27 (m, 4H), 3.11 (t, J =
8.3
Hz, 2H), 2.25 - 2.10 (m, 2H),
1.97 - 1.82 (m, 6H).
1H NMR (300 MHz, CDCI3):
d0 r ppm 1.81 -1.98 (m, 6 H),
2.13
0 4-1:11, N----- / -2.25 (m, 2 H), 3.29 -
3.38
(m, 4 H), 3.75 - 3.85 (m, 1 H),
622 N 424.2 3.88 - 3.96 (m, 4 H),
4.56 -
H 4.71 (m, 1 H), 4.75 -4.83
(m,
N 1 H), 6.36 (dd, J = 9.1, 3.3 Hz,
C ) 1 H), 6.90 (d, J = 1.9 Hz,
1 H),
6.95 (d, J =
0
248
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
rõ
a 01 0 CH, 1H NMR (300 MHz, CDCI3):
ppm 1.80- 1.98(m, 6 H), 2.13
-2.23 (m, 2 H), 2.38 (s, 3 H),
3.25 - 3.37 (m, 4 H), 3.62-
623 N ''..O''''' 420.3 3.77 (m, 1 H), 3.88 -
3.97 (m,
H 4 H), 4.76 - 4.83 (m, 1 H),
N 6.23 (d, J = 7.9 Hz, 1 H),
6.43
C ) (d, J = 7.3 Hz, 1 H), 6.91
(d, J
= 2.5 Hz, 1 H)
njr'N 1H NMR (300 MHz, CDCI3):
ppm 1.78- 2.01 (m, 6 H), 2.11
0,1/41Cip
-2.24 (m, 2 H), 2.33 (s, 6 H),
2.38 -2.45 (m, 1 H), 2.69 (t, J
624 "N 0-1...\- ," \-.N.-CH' 494.3 = 5.2
Hz, 2 H), 3.26 - 3.42 (m,
N N
H I 4 H), 3.84 - 3.98 (m, 4 H),
N OH, 4.01 (t, J = 5.2 Hz, 2 H), 4.72 _
C4.83 -,
4.83 (m, 1 H), 4.97 (d, J = 7.5
Hz, 1 H)
LO'"
1H NMR (300 MHz, CDCI3):
14 0 ppm 1.81 - 1.95 (m, 6 H),
2.11 - 2.26 (m, 2 H), 3.31 -I 1 ,,,,_i 11 ' 3.35 (m, 4 H),
3.44 (s, 3 H),
625 ..,, .,".'
N - s.':`----) 0 480.3 3.69 - 3.73 (m, 2 H),
3.76 -
H i 3.84 (m, 1 H), 3.89 - 3.93
(m,
eõ...N CH, 4 H), 4.04 - 4.08 (m, 2 H),
1 ) 4.44 (br s, 1 H), 4.74 -
4.81
(m, 1 H), 6.37 (d, J = 8.9
0
249
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M-I-H)
1H NMR (300 MHz, CDCI3):
"N I .114 ppm 1.81 - 1.98 (m, 6 H), 2.12
0
NV 1 NN-F. -2.25 (m, 2 H), 2.37 - 2.48
40 'laN ',,ta (m, 3 H), 2.58 -2.77 (m, 4
H),
626 504.3 3.04 - 3.16 (m, 4 H),
3.28 -
H 3.37 (m, 4 H), 3.77 - 3.85
(m,
N 1 H), 3.87- 3.97 (m, 4 H),
r -....
4.32 -4.51 (m, 1 H), 4.73-
4.83 (m, 1 H), 6.38 (
L'o'-'
1H NMR (300 MHz, CDCI3):
ppm 1.81 -2.03 (m, 7 H), 2.12
-2.28 (m, 2 H), 3.28 - 3.39
l' (m, 4 H), 3.81 (s, 3 H),
3.87 -
' ..õ, --,µ....Lcõ..1,,CH,
627 N 0 436.3 3.98(m, 5 H), 4.76 -4.84
(m,
i H 1 H), 5.87 (d, J = 1.5 Hz, 1 H),
(1,1,1 6.21 (dd, J = 5.9, 1.5 Hz,
1 H),
6.91 (d, J = 1.7 Hz, 1 H), 6.95
(d, J = 1
1,...0'-'1
H
N Nay,Th 1H NMR (300 MHz,
õS Chloroform-d) 6 8.71 (d, J
=
KC S 1.9 Hz, 1H), 8.63 (d, J =
1.9
Hz, 1H), 6.94 (dd, J = 14.6,
628 458.05 2.5 Hz, 2H), 5.57 (s,
1H), 4.86
-4.72 (m, 1H), 4.02 - 3.86 (m,
4H), 3.50- 3.26 (m, 5H), 2.65
1------N -- -lc) (s, 3H), 2.32 - 2.06 (m,
2H),
0.,..õ) 2.06 - 1.73 (m, 6H).
250
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M+H)
H
di Nt 1H NMR (300 MHz, CDCI3) 6
,,,.,:j 1 8.62 (s, 1H), 8.54 (s, 1H),
8.15 - 7.93 (m, 1H), 7.40 (d, J
KC' 0 = 8.0 Hz, 1H), 6.88 (s,
1H),
629 518.4
6.84 (s, 1H), 6.34 (d, J = 8.6
FIN'F
..::-)4 \',1 Hz, 1H), 4.83 - 4.56 (m, 2H),
F 1 11 4.40 -4.22 (m, 1H), 4.00 -
)
3.62 (m, 5H), 3.47 - 3.04 (m,
) 7H), 2.24 - 2.02 (m, 2H),
1.96
- 1.70 (m, 6H).
H 1H NMR (400 MHz, CDCI3) 6
8.68 (d, J = 1.8 Hz, 1H), 8.59
i (d, J = 1.8 Hz, 1H), 8.06
(s,
0 1H), 7.55(d, J =8.3 Hz,
1H),
6.94 (d, J = 2.3 Hz, 1H), 6.89
630 504.22 (d, J = 2.2 Hz, 1H),6.41
(d, J
F 1 F ..-'` .,,,-.' -) = 8.7 Hz, 1H), 4.88 (dd, J =
F 11 13.4, 6.7 Hz, 1H), 4.85 - 4.71
(m, 2H), 3.98 - 3.85 (m, 5H),
3.39 - 3.28 (m, 4H), 2.25 -
2.12 (m, 2H), 1.94 - 1.82 (m,
6H).
H 1H NMR (400 MHz, CDCI3) 6
0 N N 8.68 (d, J = 1.7 Hz, 1H), 8.61
(d, J = 1.8 Hz, 1H),7.01 (d, J
= 8.4 Hz, 1H), 6.94(d, J = 2.3
0 0 Hz, 1H), 6.89 (s, 1H), 5.96 (d,
J = 8.4 Hz, 1H), 4.75 (s, 1H),
631 464.53
4.43 -4.35 (m, 2H), 4.22 (s,
1H), 4.19 - 4.10 (m, 2H), 3.98
r-----N -- -le) -3.86 (m, 4H), 3.82 (s,
1H),
3.36 - 3.28 (m, 4H), 2.23 -
2.08 (m, 2H), 1.94 - 1.79 (m,
6H).
251
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H 1H NMR (300 MHz,
N N Chloroform-d) 6 8.70 (d, J
=
, µ 1.9 Hz, 1H), 8.63 (d, J =
1.9
Hz, 1H), 7.98 (s, 1H), 7.02 -
,N..,,,,,,--
H,C 0 6.85 (m, 2H), 5.04 (d, J =
8.2
Hz, 1H), 4.79 (s, 1H), 4.02 (s,
632 ;(;)4 \1 476A4
1H), 3.96 - 3.87 (m, 4H), 3.40
1 11 - 3.25 (m, 4H), 2.82 (t, J
= 5.9
Hz, 2H), 2.73 (t, J = 5.8 Hz,
2H), 2.48 (s, 3H), 2.19 (d, J =
8.5 Hz, 2H), 1.91 (d, J = 5.3
Hz, 5H).
1H NMR (300 MHz,
CH, Chloroform-d) 6 8.70 (d, J
=
L
1.9 Hz, 1H), 8.63 (d, J = 1.9 i", Hz, 1H), 8.18 (d, J = 5.1
Hz,
1 1H), 7.01 -6.88 (m, 2H),
6.42
(d, J = 5.1 Hz, 1H), 5.15 (d, J
0 633 4352 = 8.0 Hz, 1H), 4.79 (d, J
= 5.7
.
N Hz, 1H), 4.17 - 4.00 (m,
1H),
A i Fri 1 , ...;,.
3.98 - 3.83 (m, 4H), 3.67 (td, J
4111111 = 6.7, 4.0 Hz, 1H), 3.42 -
3.31
(m, 4H), 2.59 (q, J = 7.6 Hz,
0 ) 2H), 2.20 (q, J = 6.1 Hz,
2H),
2.03 - 1.86 (m, 6H), 1.26 (t, J
= 7.6 Hz, 3H).
1H NMR (300 MHz,
CH, Chloroform-d) 6 8.71 (d, J
=
H 1.9 Hz, 1H), 8.63 (d, J =
1.9
N
H,, C --Is* - - N T 1 : - ( * 0 Hz, 1H), 8.19 (d, J = 5.1
Hz,
1H), 7.01 -6.88 (m, 2H), 6.42
Is{ -*0 (d, J = 5.2 Hz, 1H),
5.14(d, J
634 N 449.2 = 7.9 Hz, 1H), 4.80 (dt,
J =
''''-)
7.8, 3.8 Hz, 1H), 4.06 (d, J =
4.1 Hz, OH), 3.99 - 3.85 (m,
4H), 3.46- 3.24 (m, 4H), 2.78
(hept, J = 7.0 Hz, 1H), 2.34 -
2.11 (m, 2H), 2.04 - 1.81 (m,
7H), 1.25 (d, J = 7.0 Hz, 6H).
252
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H 1H NMR (300 MHz,
H,C S N Nt
. ` =Nr;FT Chloroform-d) 68.63 (d, J =
i 1 i 1.9 Hz, 1H), 8.54 (d, J= 1.9
Ø0 Hz, 1H), 6.85 (dd, J =
17.6,
2.5 Hz, 2H), 5.86 - 5.70 (m,
635 CH, .\., 11,,, 481.05 1H), 4.89 -
4.67 (m, 2H), 3.94
11 ' - 3.77 (m, 5H), 3.34 - 3.20
(m,
, õ...,
4H), 3.02 (q, J = 7.3 Hz, 2H),
r---N N 2.27 - 2.04 (m, 5H), 1.93 -
0) 1.63 (m, 6H), 1.30 (t, J = 7.3
Hz, 3H).
H 1H NMR (300 MHz,
N Chloroform-d) 6 8.63 (d, J =
0
2.5 Hz, 2H), 5.85 (s, 1H), 4.75
636 CH, N,....1 461.1 (d, J = 5.5 Hz, 1H),
3.96 - 3.76
AP
(m, 4H), 3.34 - 3.19 (m, 4H),
2.41 (s, 3H), 2.13 (d, J = 8.5
...---,... ---;-`)
r N N Hz, 2H), 1.82 (q, J = 10.2, 8.8
:;, Hz, 6H), 1.58 - 1.33 (m,
3H),
1.03 - 0.86 (m, 3H).
Hs c H
:i N 1H NMR (300 MHz,
...µ -f N Chloroform-d) 6 8.70 (d, J =
1.9 Hz, 1H), 8.62(d, J = 1.9
1114 *0 Hz, 1H), 7.02 - 6.82 (m, 2H),
5.41 (d, J = 0.8 Hz, 1H), 4.76
637 -.0 409.2
(td, J = 5.9, 3.0 Hz, 1H), 4.03 -
NTh 3.84 (m, 4H), 3.49 (q, J =
5.8
. -...1) Hz, 1H), 3.40 - 3.25 (m,
5H),
2.33 - 2.10 (m, 4H), 2.00-
N
1.77 (m, 7H).
253
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M+H)
r-N4,1 1H NMR (300 MHz, CDCI3):
1+ 0 0 ppm 1.82- 1.95 (m, 6 H),
2.14
638
-2.26 (m, 2 H), 3.31 - 3.35
le 0, ti,,N,V I NV10 (m, 4 H), 3.77 - 3.85 (m, 1
H),
N 478.3 3.90 - 3.93 (m, 4 H),
4.71 -
1 H 4.80 (m, 3 H), 4.92 (t, J = 6.6
N Hz, 2 H), 5.10 (quint., J = 5.6
( ) Hz, 1 H), 6.39 (d, J = 8.9
Hz,
1 H), 6.90 (d,
0
1H NMR (300 MHz, CDCI3):
NI 1 0 0 1.80 - 1.95 (m, 6 H), 2.10-
639
41/40, N,..-= , =,.., 2.25 (m, 2 H), 2.52 -2.64 (m,
. õ0" 4 H), 2.77 (t, J = 5.6 Hz,
2 H),
N ..\'' s\N"-N1 535.3 3.33 (t, J = 4.7 Hz,
4 H), 3.74
i
. H (t, J = 4.7 Hz, 4 H), 3.70-
3.85
(m, 1 H), 3.91 (t, J = 4.7 Hz, 4
H), 4.06 (t, J = 5.6 Hz, 2 H),
4.30 -1r41 1H NMR (300 MHz, CDCI3):
ppm 1.40- 1.50 (m, 2 H), 1.56
- 1.70 (m, 4 H), 1.84 - 1.94
1 1 (m, 6 H), 2.11 - 2.23 (m, 2 H),
640 N., Nõ..."sviN "N.- ',.. 7N.
N 533.4 2.46 -2.58 (m, 4 H), 2.71
-
H N 2.80 (m, 2 H), 3.33 (t, J = 4.7
Hz, 4 H), 3.76 - 3.84 (m, 1 H),
3.91 (t, J = 4.7 Hz, 4 H), 4.07
(t, J = 5.7 H
254
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
H
H,CN... 1H NMR (400 MHz, CDCI3) 6
i 8.73 -8.65 (m, 1H), 8.60
(d, J
CH, = 1.8 Hz, 1H), 6.93(d, J = 2.4
0 Hz, 1H), 6.89(d, J = 2.3
Hz,
641 371.56
1H), 4.75 (s, 1H), 3.95 - 3.87
.....--" $ 11:3 (m, 4H), 3.39 - 3.28 (m, 4H),
i õ...... 3.05 - 2.93 (m, 1H), 2.82 _
2.69 (m, 1H), 2.24 - 2.08 (m,
2H), 1.80- 1.71 (m, 6H), 1.07
0õ........õ...-. (d, J = 6.2 Hz, 6H).
H 1H NMR (400 MHz, CDCI3) 6
F...).4...7,.......õ,N404õ 8.70(d, J = 1.9 Hz, 1H),
8.61
1 (d, J = 1.9 Hz, 1H), 7.78 (d, J
= 5.8 Hz, 1H), 6.96(d, J = 2.4
0 Hz, 1H), 6.90(d, J = 2.4 Hz,
1H), 6.36 - 6.26 (m, 1H), 5.99
642 go 424.53 (d, J = 1.9 Hz, 1H),
4.79 (d, J
=2.4 Hz, 1H), 4.47(d J = 7.1
Hz, 1H), 3.99 - 3.85 (m, 4H),
3.56- 3.49 (m, 1H), 3.39 -
3.26 (m, 4H), 2.28 - 2.16 (m,
2H), 1.98- 1.80 (m, 6H).
1H NMR (300 MHz,
Chloroform-d) 6 8.70 (d, J =
`Ne'iN'N-J4
0 1.9 Hz, 1H), 8.61 (d, J =
1.9
Hz, 1H), 8.57 - 8.45 (m, 1H),
7.81 (d, J = 8.4 Hz, 1H), 7.18
(d, J = 1.2 Hz, 1H), 6.93 (dd, J
HN
643 492.2 = 16.8, 2.5 Hz, 2H), 5.48
(s,
N 1H), 4.83 (dp, J = 4.5, 2.4
Hz,
I-01'N'
H
1H), 4.23 (dp, J = 8.3, 6.6 Hz,
,
KC N ..y *=--. ) 1H), 4.00- 3.85 (m, 4H),
3.42
T li N 0 - 3.23 (m, 4H), 2.35 -2.15
(m,
CH, 0 2H), 1.97- 1.76 (m, 6H), 1.26
(d, J = 6.6 Hz, 6H).
255
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
1H NMR (300 MHz,
Chloroform-d) 6 8.69 (d, J =
(** O. 14 1.9 Hz, 1H), 8.61 (d, J =
1.9
MODHz, 1H), 6.93 (dd, J = 17.5,
HN
ve 2.5 Hz, 2H), 5.47 (s, 1H),
4.90
*
644 465.16
r.N., 2H), 3.92 (dd, J = 5.9, 3.7
Hz,
r 4H), 3.60(s, 1H), 3.38 -
3.28
....e,
(m, 4H), 2.40 (s, 3H), 2.19 (d,
0 N CH. LO)
J = 9.4 Hz, 2H), 1.97- 1.80
J (m, 6H), 1.36 (t, J = 7.1
Hz,
H,C.- 3H), 4.83 -4.76 (m, 1H).
H 1H NMR (400 MHz, CDCI3) 6
0117- N 8.68(d, J = 1.8 Hz, 1H), 8.60
40 (d, J = 1.9 Hz, 1H), 6.94 (d, J
= 2.4 Hz, 1H), 6.87(d, J = 2.4
0
Hz, 1H), 4.83 (t, J = 6.9 Hz,
645 385.51 2H), 4.76 (s, 1H), 4.45
(t, J =
Oil 6.6 Hz, 2H), 4.14 -4.05 (m,
,
N
.. õ' l:i
-- 1H), 3.94- 3.88 (m, 4H),
3.35
r. tf
- 3.30 (m, 4H), 2.65 (s, 1H),
2.24 - 2.15 (m, 2H), 1.75 -
1.64 (m, 6H).
H
,N
1H NMR (300 MHz,
H,C --47:--, T . Chloroform-d) 6 8.71 (d, J =
\ N 1.9 Hz, 1H), 8.57(d, J =
1.9
C 14: 0
Hz, 1H), 7.01 -6.84 (m, 2H),
646 ,....õ.1 õ..14-... 423.16 6.18 (d,
J = 1.4 Hz, 1H), 4.80
1: (s, 1H), 4.43 (s, 1H), 3.93
(t, J
N 'N' = 4.9 Hz, 5H), 3.48 (s,
3H),
3.43- 3.25 (m, 5H), 2.38 -
0
1.86 (m, 9H).
,,,,
256
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
H 1H NMR (400 MHz, CDCI3) 6
N 8.67(d, J = 1.9 Hz, 1H),
8.59
T 461:a (d, J = 1.9 Hz, 1H), 6.94
(d, J
0
= 2.5 Hz, 1H), 6.88 (d, J = 2.5
0 Hz, 1H), 4.75 (s, 1H), 4.03-
647 413.59
3.94 (m, 2H), 3.94 - 3.83 (m,
,..=,' 1 "ti--1 4H), 3.41 (td, J = 11.7,
2.1 Hz,
2H), 3.36- 3.25 (m, 4H), 2.89
r-N= ....,, õ.......)
14 -2.77 (m, 2H), 2.24 -2.13
(m,
2H), 1.85- 1.68 (m, 8H), 1.40
0 õ....,....) (ddd, J = 16.2, 12.3, 4.4
Hz,
3H).
H 1H NMR (400 MHz, CDCI3) 6
8.69(d, J = 1.9 Hz, 1H), 8.62
(d, J = 1.9 Hz, 1H), 7.17 (dd, J
= 8.5, 7.4 Hz, 2H), 6.95 (d, J =
2.5 Hz, 1H), 6.90 (d, J = 2.4
648 14) 405.58 Hz, 1H), 6.69 (t, J =
7.3 Hz,
1H), 6.63 (d, J = 7.9 Hz, 2H),
1H), 3.40 - 3.30
(m, 4H), 2.24 - 2.11 (m, 2H),
1.94 - 1.84 (m, 6H).
H 1H NMR (300 MHz,
N...)õ.. Chloroform-d) 6 8.69 (d, J
=
1.9 Hz, 1H), 8.62(d, J = 1.9
L
Hz, 1H), 7.10 (d, J = 2.3 Hz,
0 1H), 6.99 - 6.81 (m, 2H),
5.52
a
649 1 14=,....õ..,. 409.17 (d, J = 2.3
Hz, 1H), 4.76 (t, J =
5.9 Hz, 1H), 4.06- 3.86(m, , 5H), 3.72 (s, 3H), 3.49 (t,
J =
5.3 Hz, 1H), 3.40- 3.18 (m,
4H), 2.19 (dq, J = 12.9, 7.1,
0 ,,- 6.7 Hz, 2H), 1.90 (dd, J =
9.4,
5.2 Hz, 7H).
257
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
1H NMR (300 MHz,
0 Chloroform-d) 6 8.70 (d, J
=
1.9 Hz, 1H), 8.62 (d, J = 1.9
....t
Hz, 1H), 8.47 - 8.36 (m, 1H),
H N 6.96 (d, J = 2.5 Hz, 1H),
6.90
650 447.2 (d, J = 2.5 Hz, 1H), 6.18 (d, J
I
N = 1.2 Hz, 1H), 4.95 -4.73
(m,
2H), 4.04 - 3.78 (m, 5H), 3.44
_ 3.24 (m, 4H), 2.30 -2.12 (m,
2H), 2.03- 1.70 (m, 7H), 1.17
- 0.80 (m, 4H).
1H NMR (300 MHz,
Chloroform-d) 6 8.70 (d, J =
0 IN 1.9 Hz, 1H),8.61 (d, J = 1.9
Hz, 1H), 8.50(d, J = 1.1 Hz,
...,,-
1 1H), 6.93 (dd, J = 15.9, 2.5
MI ..Ø64* -...õ Hz, 2H), 6.17 (d, J = 1.1
Hz,
651 435.18 1H), 4.98 (d, J = 8.1
Hz, 1H),
N 4.86 - 4.75 (m, 1H), 3.97 -
C ) 3.87 (m, 4H), 3.39 - 3.29 (m,
4H), 2.61 (q, J = 7.6 Hz, 2H),
0 2.21 (dt, J = 11.2, 5.0 Hz,
2H),
CH, 2.04 - 1.82 (m, 6H), 1.25
(t, J
= 7.6 Hz, 3H).
1H NMR (300 MHz,
Chloroform-d) 6 8.70 (d, J =
0 ).<1 1.9 Hz, 1H),8.61 (d, J =
1.9
Hz, 1H), 8.52 (d, J = 1.1 Hz,
.1....." I.
1H), 6.93 (dd, J = 16.0, 2.5
Hhe
Hz, 2H), 6.19 - 6.12 (m, 1H), r-C.-,---}
652 449.23 4.91 (d, J = 8.0 Hz,
1H), 4.81
N, (d, J = 5.4 Hz, 1H), 4.13 -
3.77
...---' 1.4 (m, 5H), 3.40 - 3.29 (m,
4H),
H,C ,...-CL C ,,, 2.80 (hept, J = 6.8 Hz,
1H),
N 0 2.27 - 2.00 (m, 2H), 1.99 -
CH, 1.83 (m, 6H), 1.40 - 1.12
(m,
6H).
258
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 11-1 NMR
Compound Structure
14 11
1 '
C)41k 0 401 *1 1H NMR (300 MHz, Methanol-
d4/ CDCI3) 6 8.82 - 8.68 (m,
2H), 8.61 (d, J = 0.8 Hz, 1H),
7.38 (t, J = 0.8 Hz, 1H), 7.18
HH
653 451.07 (s, 1H), 5.11 - 4.99 (m,
1H),
N , 4.47 - 4.31 (m, 1H), 3.99 -
141---- 1 3.88 (m, 4H), 3.74 - 3.58 (m,
1-..,' 0 H L,) 4H), 2.37 -6H). 2.23 (m,
2H), 2.13
N
0
d 0.,c), 1H NMR (300 MHz, CDCI3):
ppm 1.82 - 1.95 (m, 6 H), 2.11
-2.24 (m, 2 H), 2.32 (s, 6 H),
N 2.68 (t, J = 5.7 Hz, 2 H),
3.29 -
654
H'slia.
N,,,
N ,-- 493.3 3.37 (m, 4 H), 3.76 -
3.86 (m,
c
1 H), 3.88 - 3.95 (m, 4 H),
0
rj 4.00 (t, J = 5.7 Hz, 2 H), 4.29
0
(d, J = 8.2 Hz, 1 H), 4.70 -
4.83 (m, 1 H)
H,C CH,
a :7, .m. ,,
- 41
, 1H NMR (300 MHz, CDCI3):
11
ppm 1.81 - 1.97 (m, 6 H), 2.10
- 2.25 (m, 2 H), 2.33 (s, 3H),
F-E -T,- -----
655 1 1 ti,,,,,,, .....,0 2.42 -2.83 (m, 8 H),
2.78 (t, J
548.3 = 5.6 Hz, 2 H), 3.28 - 3.39
(m,
j
....- 4 H), 3.75 - 3.85 (m, 1 H),
1 3.86 - 3.97 (m, 4 H), 4.05
(t, J
L 1 = 5.6 Hz, 2 H), 4.29 (t, J
= 8.4
Hz, 1 H)
slf.'-
C 14,
259
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS III NMR
Compound Structure
No. (MH-H)
H 1H NMR (300 MHz, DMSO-
d6) 68.64 (d, J = 1.9 Hz, 1H),
Cr N iC1.4,
8.47 (d, J = 1.9 Hz, 1H), 8.25
(d, J = 4.8 Hz, 2H), 7.15 (d, J
0 = 7.3 Hz, 1H), 6.59 -6.47
(m,
656 ..--"' ..--)4') 393.1 2H), 6.31 (d, J =
2.2 Hz, 1H),
5.80 - 5.68 (m, 1H), 4.85 (s,
s....,.. =-=.., ...) 2H), 4.62 (d, J = 5.3
Hz, OH),
gii re 4.24 (t, J = 7.4 Hz, 2H),
3.83
(s, 1H), 3.71 (dd, J = 8.4, 4.7
HO Hz, 2H), 2.14 - 1.97 (m,
2H),
1.79 (d, J = 19.4 Hz, 5H).
0H,
i
N
r>.41y .1/4a 1H NMR (300 MHz,
Chloroform-d) 6 8.59 (d, J =
1,
1.9 Hz, 1H), 8.50(d, J = 1.9
0 Hz, 1H), 8.11 (s, 2H), 6.91
-
657 518.32 6.70 (m, 2H), 4.66 (s,
1H),
,N
KC.
3.92 - 3.56 (m, 7H), 3.45 (d, J
i = 4.3 Hz, 3H), 3.31 -3.18 (m,
6H), 2.08 (s, 2H), 1.84 (d, J =
38.5 Hz, 8H).
fill 1H NMR (300 MHz, CDCI3):
ppm 1.37 (t, J = 7.0 HZ, 3 H),
N 0hhII1., ...."..... ,,,0,,, C H,
N., -y -, 1.84- 1.94(m, 6 H), 2.10-
2.25 (m, 2 H), 3.29 - 3.38 (m,
658 1 t 7 `',N.,--N, N µ,,c71 450.3 4 H), 3.75- 3.85
(m, 1 H),
H 3.88 - 3.95 (m, 4 H), 3.97
(q, J
N = 7.0 Hz, 2 H), 4.22 -4.34
(m,
C ) 1 H), 4.73 -4.80 (m, 1 H),
6.36 (d, J = 9.0 H
0
260
CA 02904641 2015-09-08
WO 2014/159690 PCT/US2014/024767
Compound ESMS 1H NMR
Compound Structure
No. (M+H)
1H NMR (300 MHz,
. Chloroform-d) 6 8.69 (d, J =
1.9 Hz, 1H), 8.60 (d, J = 1.9
Hz, 1H), 6.92 (dd, J = 19.6,
Mel Ill
659 451.34 2.5 Hz, 2H), 5.25 (s,
1H), 4.76
L.(q, J = 8.3, 7.2 Hz, 2H), 3.97
C ) 3.87 (m, 4H), 3.58 - 3.28
(m,
L. 8H), 2.40 (s, 3H), 2.24 -
2.13
0 N CH, 0 (m, 2H), 2.07 - 1.78 (m,
6H).
CH,
Biological assay of compounds of the invention
DNA-PK Inhibition Assay
[00166] Compounds were screened for their ability to inhibit DNA-PK kinase
using a
standard radiometric assay. Briefly, in this kinase assay the transfer of the
terminal
33P-phosphate in 33P-ATP to a peptide substrate is interrogated. The assay was
carried out
in 384-well plates to a final volume of 50 [IL per well containing
approximately 6 nM
DNA-PK, 50 mM HEPES (pH 7.5), 10 mM MgCl2, 25 mM NaC1, 0.01% BSA, 1 mM
DTT, 10 laginaL sheared double-stranded DNA (obtained from Sigma), 0.8 mgimL
DNA-
PK peptide (Glu-Pro-Pro-Leu-Ser-Gln-Glu-Ala-Phe-Ala-Asp-Leu-Trp-Lys-Lys- Lys,
obtained from American Peptide), and 100 itiM ATP. Accordingly, compounds of
the
invention were dissolved in DMSO to make 10 mM initial stock solutions. Serial
dilutions in DMSO were then made to obtain the final solutions for the assay.
A 0.75 1_,
aliquot of DMSO or inhibitor in DMSO was added to each well, followed by the
addition
of ATP substrate solution containing "P-ATP (obtained from Perkin Elmer). The
reaction was started by the addition of DNA-PK, peptide and ds-DNA. After 45
min, the
reaction was quenched with 25 pI of 5% phosphoric acid. The reaction mixture
was
transferred to MultiScreen HTS 384-well PH plates (obtained from Millipore),
allowed to
bind for one hour, and washed three times with 1% phosphoric acid. Following
the
addition of 50 pI of Ultima GoldTM high efficiency scintillant (obtained from
Perkin
Elmer), the samples were counted in a Packard TopCount NXT Microplate
Scintillation
and Luminescence Counter (Packard BioScience). The Ki values were calculated
using
261
CA 02904641 2015-09-08
WO 2014/159690
PCT/US2014/024767
Microsoft Excel Solver macros to fit the data to the kinetic model for
competitive tight-
binding inhibition.
[00167] Each of compounds 1-291, 295-331, 333-367, 369-523, 525-640, 642-644,
646, and 648-659 has a Ki of less than 1.0 micromolar for the inhibition of
DNA-PK.
Each of compounds 3, 6-14, 16-18, 23-34, 36-37, 39-41, 43-46, 49-72, 74-76,
78, 84-101,
103-123, 127-200, 202-291, 295-299, 305, 307-331, 333-341, 343, 347-366, 369-
374,
376-391, 393-519, 521-523, 526-554, 556-610, 612-640, 642-644, 646, 648-655
and 657-
659 has a Ki of less than 0.10 micromolar for the inhibition of DNA-PK.
[00168] Although the foregoing invention has been described in some detail by
way of
illustration and example for purposes of clarity of understanding, it will be
readily
apparent to those of ordinary skill in the art in light of the teachings of
this invention that
certain changes and modifications may be made thereto without departing from
the spirit
or scope of the appended claims.
262