Note: Descriptions are shown in the official language in which they were submitted.
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
SYSTEM AND METHOD FOR CHARACTERIZING
CIRCULATORY BLOOD FLOW
CLAIM OF PRIORITY
[0001] This application claims benefit of and priority to U.S. Patent
Application
Serial No. 13/839,534, entitled "System and Method for Characterizing
Circulatory Blood
Flow" and filed March 15, 2013, the disclosure of which is incorporated by
reference herein
in its entirety.
BACKGROUND
[0002] Circulatory blood flow delivers oxygen and nutrients to tissues
and organs
and removes toxins and wastes therefrom. Such delivery and removal is
essential to
maintaining cellular function and tissue and organ health. Broadly defined,
stress is the
aggregate impact of physical, cognitive, pathological, and environmental
factors to which an
organism must adapt in order to remain in a physiologically homeostatic state.
Adequate
circulatory blood volume must be maintained under varying forms and degrees of
stress, or
else homeostasis and adequacy of oxygenated blood flow delivery is
compromised.
Accordingly, in the healthy state, the autonomic nervous system continuously
adjusts
circulatory blood volume in order to meet these constantly changing demands.
In situations
where the ability to adjust circulatory blood volume is inadequate, the
delivery of oxygen and
nutrients to tissues and organs and the removal of toxins and wastes therefrom
is inadequate
to meet the cellular demands and, as a result, overall physiological function
is compromised.
[0003] Systems and methods for evaluating the condition of the
autoregulatory
components of the cardiovascular system are known in the art. Unfortunately,
while these
systems and methods are good predictors of the overall cardiovascular
condition resulting
from long-term pathological and age-related structural changes, they cannot
characterize the
functional adequacy of circulatory blood volume in the short-term. As such, in
the face of
stress, any resultant deficiencies in supplying the demands of the tissue and
organs is often
not detected until physiological function is so compromised that tissue and
organ dysfunction
become symptomatic and sustainability is at risk. Furthermore, while levels of
certain
metabolites are indicative of inadequate circulatory blood volume, such
metabolites are only
present after prolonged inadequate circulatory blood volume has occurred and
therefore
cannot characterize the functional adequacy of circulatory blood volume in the
pre-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
symptomatic stages to avoid a compromised physiological state that may be
irreversible.
Thus, there is a need for real-time systems and methods that characterize the
adequacy of
circulatory blood volume over contiguous, finite time intervals in order that
circulatory blood
volume may be assessed and any deficiencies in supply may be detected and
treated before
the patient's sustainability is at risk.
SUMMARY
[0004] In an embodiment, a computer-implemented method for
characterizing
circulatory blood volume is disclosed. The method has the steps of acquiring a
biological
signal from a sensor, wherein the biological signal emulates the arterial
pulse wave,
conditioning the biological signal to create a conditioned signal, processing
the conditioned
signal, and calculating a derived parameter from the conditioned signal. In
embodiments,
three derived parameters are extrapolated from the biological signal,
circulatory stress, which
reflects changes in a harmonic or the fundamental frequency of heart rate,
circulatory blood
volume, which reflects changes in the frequency strength (or amplitude) of the
unprocessed
biological signal, and the Pulse Volume Alteration (PVA) Index, which is a
ratio of the sum
of the strength of the heart rate harmonic frequencies (non-cardiac
contributions) within the
arterial pulse wave to the strength of the heart rate frequency (cardiac
contribution) which is
equivalent to the acoustical calculation referred to as the Total Harmonic
Distortion. Each
derived parameter is compared to a threshold value. The heart rate and
circulatory blood
volume threshold comparisons are assessed to determine an adequacy of
circulatory blood
volume. The PVA Index is assessed to measure the stiffness in the underlying
arterial
structure caused by either autonomic nervous system driven vascular changes or
from fluid
transfer into (volume loading) or out of the arterial tree (volume loss or
exanguination). In
embodiments, changes in the circulatory stress and circulatory blood volume
are extrapolated
from changes in the frequency and frequency strength, respectively, of the
arterial pulse wave
in order to characterize changes in circulatory blood volume over contiguous,
finite time
intervals. In embodiments, the assessment of circulatory blood volume is used
to manage a
patient's cardiovascular autoregulatory function or the adequacy of transfer
of fluids to and
from the circulatory system, with the ultimate goal of achieving a circulatory
blood volume
that adequately supplies the demands of the patient's tissues and organs. In
embodiments, the
PVA Index is a measure of arterial structural stiffness and is used to assess
the degree of fluid
loading or deficiency or the degree to which the sympathetic response has been
activated,
-2-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
which is the primary autoregulatory mechanism used to defend against
circulatory volume
changes.
[0005] In another embodiment, a system for characterizing circulatory
blood
volume is disclosed. The system has a processor that includes at least one
module configured
to process the biological signal and to calculate the derived parameters of
circulatory stress
and circulatory blood volume, and PVA Index therefrom. In embodiments, the
processor
includes a signal conditioning module configured to receive the biological
signal from the
sensor and to condition the biological signal. The processor also includes a
signal processing
module that is configured to process the biological signal to calculate the
derived parameters.
An analysis module is configured to assess the adequacy of a patient's
cardiovascular
autoregulatory function, the adequacy of transfer of fluids to and from the
circulatory system,
or the adequacy of the compensatory contributions by the vasculature with the
ultimate goal
of achieving a circulatory blood volume that meets the demands of the
patient's tissues and
organs.
[0006] In another embodiment, a computer-implemented apparatus for
assessing
circulatory blood volume is disclosed. The apparatus has means for acquiring
the biological
signal, means for conditioning the biological signal, means for processing the
conditioned
biological signal, and means for calculating the derived parameters
circulatory stress,
circulatory blood flow, and the PVA Index from the conditioned signal. The
apparatus
further includes means for comparing each derived parameter to a threshold
value and is used
to assess the adequacy of circulatory blood volume and the effectiveness of
the compensatory
mechanisms in so doing. In embodiments, changes in the circulatory stress and
circulatory
blood volume are extrapolated from changes in the frequency and frequency
strength,
respectively, of the arterial pulse wave in order to assess the adequacy of
the changes in
circulatory blood volume over contiguous, finite time intervals. In
embodiments, the PVA
Index is extrapolated from the Total Harmonic Distortion calculation
extrapolated from the
arterial pulse wave and is used to characterize changes in the vasculature
compliance to
assess the degree to which the sympathetic response has been activated. In
embodiments, the
assessment of circulatory blood volume is used to manage a patient's
cardiovascular
autoregulatory function or the adequacy of transfer of fluids to and from the
circulatory
system, with the ultimate goal of achieving a circulatory blood volume that
adequately
supplies the demands of the patient's tissues and organs.
-3-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
[0007] In another embodiment, a non-transitory computer-readable medium
having stored therein instructions which, when executed by a processor, causes
the processor
to acquire the biological signal from a sensor, wherein the biological signal
emulates the
arterial pulse wave, conditions the biological signal to create a conditioned
signal, processes
the conditioned signal, and calculates the derived parameters, circulatory
stress, circulatory
blood volume, and the PVA Index from the conditioned signal distortion. The
computer-
readable medium also has instructions stored therein to compare each derived
parameter to a
threshold value and to assess each derived parameter to determine an adequacy
of circulatory
blood volume and the effectiveness of the compensatory mechanisms. In
embodiments,
changes in the frequency (circulatory stress), frequency strength (circulatory
blood flow), and
the Total Harmonic Distortion (PVA Index) are extrapolated from changes in the
frequency,
changes in frequency strength, and a ratio of frequency strengths respectively
extrapolated
from the arterial pulse wave in order to assess the adequacy of an anesthetic
during surgical
care and its impact on adequacy of circulatory blood volume over contiguous,
finite time
intervals. In embodiments, changes in the frequency (circulatory stress),
frequency strength
(circulatory blood volume),and Total Harmonic Distortion measures (PVA Index)
are
extrapolated from changes in the frequency, changes in frequency strength, and
changes in
the ratio of frequency strengths respectively, of the arterial pulse wave in
order to assess the
appropriateness of antihypertensive medications and their impact on the
effectiveness of the
autoregulatory function in maintaining adequate circulatory blood volume over
contiguous,
finite time intervals. In embodiments, the assessment of circulatory blood
volume is used to
manage a patient's cardiovascular autoregulatory function or the adequacy of
transfer of
fluids to and from the circulatory system, with the ultimate goal of achieving
a circulatory
blood volume that adequately supplies the demands of the patient's tissues and
organs.
[0008] Those and other details, objects, and advantages of the present
invention
will be become better understood or apparent from the following description
and drawings
showing embodiments thereof.
BRIEF DESCRIPTION OF THE DRAWINGS
[0009] Various embodiments of the present invention are described herein
by way
of example in conjunction with the following figures, wherein:
[0010] Fig. 1 illustrates embodiments of use of the inventive system and
method.
-4-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
[0011] Fig. 2 illustrates an embodiment of the arterial pulse wave (A)
and the
corresponding frequency strength (amplitude) (B) and frequency (C).
[0012] Fig. 3 illustrates (A) an embodiment of the biological signal
super-
imposed on the arterial pulse wave and (B) graphical depictions of the derived
parameters
circulatory stress (top), which reflects a harmonic of heart rate, and
circulatory blood flow
(middle), which reflects the amplitude of the unprocessed biological signal.
An embodiment
of the automated event monitoring system display is also illustrated (bottom).
The top,
middle, and bottom panels in (B) are vertically aligned in time. The
biological signal and
arterial pulse wave were recorded in a patient undergoing dialysis treatment.
[0013] Fig. 4 illustrates embodiments of an arterial pulse waves
acquired from a
sensor placed on a healthy subject's forehead. Panel A illustrates the
arterial pulse wave at
rest and Panel B illustrates the power spectrum of the arterial pulse wave
illustrated in Panel
A. Panel C illustrates the arterial pulse wave during simulated blood loss
created by placing
the subject in a lower body negative pressure chamber and Panel D illustrates
the power
spectrum of the arterial pulse wave illustrated in Panel C.
[0014] Fig. 5 illustrates a flowchart of various embodiments of a method
for
characterizing circulatory stress and circulatory blood volume.
[0015] Fig. 6 illustrates a flowchart of various embodiments of the step
of
conditioning a biological signal.
[0016] Figs. 7-9 illustrate flowcharts of various embodiments of the
steps of
calculating and analyzing the conditioned biological signal.
[0017] Fig. 10 illustrates a schematic of a biological signal broken
down into a
time window.
[0018] Fig. 11 illustrates the changes in frequency (A) and frequency
strength (B)
of the fundamental frequency for the biological signal and its component
harmonics recorded
in a patient exposed to a lower body negative pressure chamber.
[0019] Figs. 12-16 illustrate various embodiments of systems for
characterizing
circulatory blood volume.
-5-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
[0020] Fig. 17 illustrates the pulsatile and non-pulsatile portions of a
photo-optic
signal.
[0021] Fig. 18 illustrates an embodiment of the system used in
conjunction with
other sensors to characterize circulatory blood volume.
[0022] Figs. 19-22 illustrate various examples of data collected using
embodiments of the systems and methods.
[0023] Fig. 23 illustrates a flowchart of an embodiment of a process for
calculating and analyzing the conditioned biological signal.
[0024] Figs. 24 and 25 illustrate examples of data collected using
embodiments
of the systems and methods.
DETAILED DESCRIPTION
[0025] As used herein, "arterial pulse wave" means the pressure wave
that results
from the ejection of blood from the left ventricle of the heart during systole
and the aggregate
of vascular effects on the pressure wave.
[0026] A system and method is described herein to extract morphology-
related
features of the arterial pulse wave using frequency domain-based techniques
that are captured
in response to a stress condition. One or more features are then used to
assess the short-term
functional adequacy of circulatory blood volume to adapt to the stress
condition. The
inventive system and method can be used to assess the aggregate of
cardiovascular adaptive
mechanisms that contribute to maintaining adequate circulatory blood volume
referred to as
the cardiovascular autoregulatory system. The inventive system and method can
also be used
to assess specific autoregulatory components by isolating specific arterial
pulse wave
morphology features. Given that these frequency-based measures represent an
aggregate of
physiological effects, various embodiments may use ratios, summations, or
other mathematic
manipulations of changing frequencies, frequency strengths (amplitude), and/or
other features
resulting from the power spectrum analysis in order to isolate a
cardiovascular autoregulatory
component of interest. Other embodiments include ratios, summations, and
mathematical
formulae wherein weighted variables for elements resulting from either or both
frequency and
time domain analyses are combined.
-6-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
[0027] The system may be used for various clinical applications,
embodiments of
which are illustrated in Figure 1. In various embodiments, the system may be
used to assess
the appropriateness of the functional health of the cardiovascular
autoregulatory mechanisms
by employing a controlled stress condition and assessing the adequacy of the
response. A
standardized stress maneuver such as a sit-to-stand orthostatic test may be
used for this
purpose (Fig. 1A). In other embodiments, the system may be used to assess
whether the
functional health of the cardiovascular system is degrading, such as by
assessing the response
to a standardized stress test when performed repeatedly over a period of time.
A trend
indicating a decrease in autoregulatory function indicates, in a chronic heart
failure patient,
that the cardiac muscle is degrading and the patient is in a decompensating
condition (Fig.
1B). In various other embodiments, the system may also be used to assess
whether a patient
has an intolerant circulatory volume condition, such as by using the system to
monitor
stability for an end-stage renal disease patient undergoing controlled fluid
removal during a
dialysis treatment performed over time (1C). In this instance, the system may
be used to
predict a hypotensive event arising from the induced hypovolemic state (i.e.,
resulting from
fluid removal) (1D). In other embodiments, the system may be used to assess
the effect of
pharmaceuticals or anesthetics on the autoregulatory function (1E).
[0028] Embodiments of the present invention utilize a biological signal
that
emulates the arterial pulse wave. The arterial pulse waveform morphology, an
example of
which is shown in Fig. 2A, represents a composite of frequencies of varying
strengths. The
system and method provide a more quantitative means by which to characterize
the
morphology changes that may otherwise be limited to a qualitative measure when
capturing
time-series based changes to the arterial pulse wave. The utility of the
invention is illustrated
in Fig. 4, which shows the arterial pulse wave in a patient at rest (Panel A)
and the
corresponding power spectrum (Panel B). During dialysis treatment, there is a
change in the
arterial pulse wave (Panel C) and a corresponding change in the power spectrum
(Panel D)
which represents the resulting changes from fluid removal during dialysis. In
an
embodiment, assessing specific arterial pulse frequency-based changes in
response to an
ongoing or created stress provides a method for assessing short-term
cardiovascular
functional changes and related cardiovascular functional conditions by
evaluating the
frequency changes associated with the heart rate, referred to herein as
circulatory stress and
depicted in Fig. 2B, and the frequency strength of the unprocessed biological
signal, referred
to as circulatory blood volume and depicted in Fig. 2C.
-7-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
[0029] In various embodiments, use of frequency-based mathematical
calculations
such as summations or ratios are used to determine the degree to which a
specific derived
cardiovascular parameter contributes to the cardiovascular condition. The
system and
method are an alternative to more conventional systems and methods which
measure arterial
pulse wave frequency changes in the steady state to quantify long-term
cardiovascular
structural changes that result from aging or chronic pathological conditions.
[0030] In various embodiments, normalization of the derived parameter is
needed
to generalize measured changes to accommodate differences in cardiovascular
efficiencies
and for physiological properties related to the signal transducer employed. In
various
embodiments, when a photo-optic signal is employed, normalization is performed
by
capturing a baseline value for the derived parameters occurring during a
steady state
condition and providing measures in terms of percentage of change from this
baseline value.
In addition to normalizing for varying cardiovascular efficiencies, percentage
of change
enables normalization for changes in photo-optic signal attenuation due to
varying levels of
melanin in the skin.
[0031] Use of such a biological signal acquired from a non-invasive
sensor
presents fewer risks to the patient, in embodiments is less sensitive to
motion and noise, and
enables broad use, including use outside of a clinical setting, such as in the
home, on an
athletic field, etc. Use of changes for a specified frequency domain enable
removal of
undesirable physiological artifacts such as those from respiration or the
nervous system and
environmental artifacts such as from motion, noise, and electrical sources.
[0032] In various embodiments the systems and methods of the present
invention
extrapolate changes in two derived parameters, the frequency strength and
frequency change
of the biological signal over contiguous, finite time intervals, referred to
herein as circulatory
blood flow and circulatory stress, respectively, in order to characterize
changes in circulatory
blood volume and circulatory stress, respectively, over time. Averaging values
in this way
over contiguous time windows provides an additional filtering method for
example, to reduce
the effects of motion, noise, and the modulation effects of respiration on the
circulatory
volume (frequency strength) parameter.
[0033] Fig. 3A illustrates an arterial pulse wave acquired from a photo-
optic
sensor placed on the forehead of an end-stage renal failure patient undergoing
dialysis
-8-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
treatment. The derived parameter circulatory blood flow, which represents
changes in the
frequency strength of the heart rate frequency or any of the heart rate
frequency harmonics,
captured from the same patient using the system and method is shown
superimposed on the
arterial pulse wave, demonstrating that the acquired biological signal
correlates with a filtered
amplitude of the arterial pulse wave. Fig. 3B graphically depicts circulatory
stress (top) and
circulatory blood volume (middle) over time and are derived from the
biological signal
similar to Fig. 3A. This figure illustrates how two components derived from
the arterial pulse
wave, referred to herein as derived parameters, can be used to assess
circulatory blood
volume adequacy. One derived parameter, circulatory stress, is illustrated in
the top panel
and is a functional indicator of the current adequacy of the supply of
circulatory blood
volume to satisfy physiological demand. Another derived parameter, circulatory
blood
volume, is illustrated in the middle panel and indicates changes in the
frequency strength of
the unprocessed biological signal. In use, the derived parameters are
calculated as a
percentage change from a steady state baseline value in order to normalize for
varying types
of physiologies and photo-optic measurement differences, such as variations
that result from
different patient skin types. In Fig. 3B, the bottom panel illustrates an
embodiment of the
automated event monitoring system display which includes various levels of an
alarm
depending upon the percent change of one or both of the derived parameters
(i.e., an event)
that is activated when specific threshold values are met, indicative of either
predictive or
correlative cardiovascular volume insufficiency states. In effect, percent
change in the
derived parameters circulatory stress (top) and circulatory blood volume
(middle) are used
together to characterize the adequacy of circulatory blood volume. The
circulatory stress in
effect is used to calibrate circulatory blood volume to indicate the values
that are either
predictive or correlative for when cardiovascular volume insufficiency (e.g.
hypovolemia)
exists. The top, middle, and bottom panels in Fig. 3B are aligned vertically
in time.
[0034] In various embodiments, the systems and methods may be used to
assess
the adequacy of circulatory blood volume. In embodiments, the assessment of
adequacy of
circulatory blood volume may be used to manage a patient's cardiovascular
autoregulatory
function or the adequacy of transfer of fluids to and from the circulatory
system, each of
which can impact the adequacy of circulatory blood volume, with the ultimate
goal of
achieving a circulatory blood volume that adequately supplies the demands of
the patient's
tissues and organs.
-9-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
[0035] Fig. 5 illustrates a flowchart of various embodiments of a method
of
acquiring derived parameters. In embodiments described herein, the derived
parameters
described are circulatory blood volume, circulatory stress, and the PVA Index.
The skilled
artisan will recognize that the system and method may be used to calculate any
derived
parameter that correlates with the arterial pulse wave. As described below,
changes in the
derived parameters over contiguous, finite time intervals may be used to
characterize changes
in circulatory blood volume, to assess adequacy of circulatory blood volume,
and to provide a
clinical management tool. As illustrated in Fig. 5, in embodiments, the
present invention may
be utilized in a method for managing a patient's health, such as the
effectiveness of the
cardiovascular autoregulatory function to compensate for changes in
circulatory demand, the
adequacy of circulatory blood volume, and the adequacy of the transfer of
fluids to and from
the circulatory system, the effect of pharmaceuticals such as hypertensive
medications on the
autoregulatory function, the effect of anesthetics on the autoregulatory
function, the effects of
environmental factors such as heat exhaustion on the autoregulatory function,
the effect of
cardiac functional health on the autoregulatory function, the effect of the
vascular
compensatory mechanisms on the autoregulatory function, the effect of adequate
fluid
resuscitation on the autoregulatory function, each of which can impact the
adequacy of
circulatory blood volume. The use of the present invention in methods of
managing a
patient's health have, in embodiments, the goal of achieving a circulatory
blood volume that
adequately supplies the demands of the patient's tissues and organs.
[0036] The steps illustrated in Fig. 5 may be performed in any order. At
step 1, a
biological signal is acquired from a sensor 10. As described below, the sensor
10 may be any
invasive or non-invasive device that includes circuitry to acquire the
biological signal.
Examples of biological signals are provided in Table 2, below. In a preferred
embodiment,
the sensor 10 is a photo-optic sensor positioned on a patient's forehead. Such
placement
eliminates potential noise from respiration, movement, and the like and
undesirable arterial
transmission artifacts that occur when the sensor is placed at a distal
location such as the
finger.
[0037] At step 12, the acquired signal is transmitted from the sensor 10
to a
processor via a wireline or wireless connection. In some embodiments, the
acquired signal is
stored to memory 70 at step 24, as described below.
-10-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
[0038] At step 14, post-acquisition conditioning of the biological
signal is
performed. The post-acquisition conditioning may be specific to the sensor 10.
In various
embodiments, post-acquisition conditioning of the acquired biological signal
includes any of
a variety of steps implemented in circuitry, firmware, software, or any
combination thereof to
improve signal quality and sensitivity such as by normalizing variances,
translating the signal
to a form that is compatible with other elements of the system, etc. In
embodiments, post-
acquisition conditioning includes filtering the biological signal to remove
noise, such as
electrical noise, amplifying the biological signal, or converting the
biological signal from an
analog to a digital waveform. See Fig. 6, described below.
[0039] At step 16, the derived parameters, circulatory blood volume,
circulatory
stress, and the PVA Index are calculated and the circulatory blood volume and
circulatory
stress values normalized using the conditioned biological signal. In various
embodiments,
calculation of the derived parameters includes any of a variety of steps
implemented in
circuitry, firmware, software, or any combination thereof. See Figs. 7-8,
described below.
Optionally, the derived parameters are stored in a memory 70 at step 24,
described below.
[0040] Optionally, at step 18, the derived parameters are analyzed in
order to
assess the adequacy of circulatory blood volume. In various embodiments,
analysis of the
derived parameters includes any of a variety of steps implemented in
circuitry, firmware,
software, or any combination thereof. See Fig. 9, described below.
[0041] Optionally, at step 20, an output 60 such as that illustrated in
Fig. 3B is
generated to an output device that is in communication, via a wireline or a
wireless
connection, with the processor 90. Examples of output 60 include a graphical
depiction of
the derived parameters, an audio alarm that warns of an impending event, a
communication to
a caregiver or clinician that summarizes the assessment, etc. Optionally,
output 60 is stored
in a memory 70 at step 24, described below.
[0042] Optionally, at step 22, at least one of the derived parameters or
the output
is used to manage a patient's cardiovascular autoregulatory function. In
embodiments,
management has the ultimate goal of achieving a circulatory blood volume that
adequately
supplies the demands of the patient's tissues and organs.
[0043] Optionally, at step 24, the derived parameters and/or output are
stored in a
memory 70 such as a database or a computer readable medium. In various
embodiments, the
-11-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
derived parameters are stored in memory 70 together with a time stamp that
identifies the
time at which the derived parameter was calculated. In other embodiments, the
derived
parameters are stored in memory 70 together with a marker that identifies the
stressor that
was occurring at the point at which the derived parameter was calculated and
may be used,
for example, to create patterns of behavior to classify types of patients, as
described below.
For example, in a dialysis setting, derived parameters are stored in
conjunction with a
description that includes specifics of the stress applied, such as the volume
of fluid removed.
The data may be stored at step 24 locally or remotely. In various embodiments,
the derived
parameters and associated time stamp or stress measure are stored in
conjunction with other
patient-specific data, such as patent demographic parameters, patient co-
morbidities, patient
medications, and the like, in order to facilitate categorizing particular
patterns of derived
parameter responses to stress based upon these patient-specific data. In
various
embodiments, these patient classifications could be used to identify optimal
treatment or
intervention strategies for each patient classification. See Fig. 9, described
below.
[0044] Referring again to Fig. 5, in various embodiments, a second
sensor 10' is
operated in parallel with the first sensor 10. At step l', a second signal is
acquired from the
second sensor 10'. The second signal is processed in steps 12', 14', 16', and
18' as described
above in steps 12, 14, 16, and 18. As illustrated in Fig. 5, the second signal
may be processed
by a second processor 90' that is collocated with the first processor 90 or
the second signal
may be processed by a second processor 90' that operates in parallel with the
first processor
90. The second processor 90' includes at least one module 20', 30', 40' that
processes and
analyzes the second signal to generate an output 60.
[0045] Various embodiments of steps 14, 14', 16, 16', and 18, 18' are
set forth in
the flow charts illustrated in Figs. 6-9. The embodiments illustrated in Figs.
6-9 show the
steps for calculating and normalizing the derived parameters, circulatory
blood volume and
circulatory stress, from a biological signal acquired from a photo-optic
sensor and, in
particular, a near infrared photo-optic device (frequency range of about 770-
910 run) such
that density changes in both oxygenated and deoxygenated hemoglobin are
acquired while
light absorption by water is not acquired. Use of a photo-optic sensor is for
illustration only
and one skilled in the art will appreciate that any sensor that records a
biological signal may
be used for the inventive method and system.
-12-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
[0046] In an embodiment, the biological signal is a photo-optic signal
that
measures changes in absorption of light that result from changes in blood
density that occurs
as the arterial pulse wave is generated (see, e.g., Figs. 2, 3). The resulting
waveform acquired
by the photo-optic device indicates the amount of light attenuated as light is
transmitted
through the blood. Fig. 17 illustrates all physiological components that
attenuate the photo-
optic signal as a result of absorption of the signal. The inventive system and
method filter out
all causes of light attenuation except the pulsatile portion of the signal
because only the
pulsatile portion indicates changes related to the arterial pulse wave. In
various embodiment,
the valley in the photo-optic signal occurs when the arterial pulse wave is at
its peak (because
light transmission decreases as the pulse wave is generated by systole and
therefore the
volume of circulating blood moving through the tissue increases) and the peak
in the photo-
optic signal occurs when the arterial pulse wave is at its valley (because
light transmission
increases as the pulse wave subsides and therefore the volume of circulating
blood moving
through the tissue decreases). In other embodiments, the biological signal
could be a photo-
optic signal that correlates to the changes in the strength of the reflection
of the light resultant
of changes in blood density in the optical path.
[0047] The flow chart illustrated in Fig. 6 sets forth various
embodiments of step
14. In embodiments, steps 141 through 144 are implemented in the signal
conditioning
module 30. The steps described herein may be performed in any order. In
embodiments, the
voltage that comes from the photo-optic device is small. Therefore, optionally
at step 141,
the biological or photo-optic signal is amplified. At step 142, the signal is
convened from
analog to digital. Optionally, at step 143, the signal is inverted so that the
peak in the photo-
optic signal occurs when the arterial pulse wave is at its peak and the valley
in the photo-
optic signal occurs when the arterial pulse wave is at its valley. At step
144, the converted,
inverted (i.e., conditioned) signal is transmitted, via a wireline or wireless
communication, to
the signal processing module.
[0048] The flow charts illustrated in Figs. 7-8 set forth various
embodiments of
step 16, shown in Fig. 5. The steps described herein may be performed in any
order.
Referring specifically to Fig. 7, in embodiments, steps 161 through 174 are
implemented in
the signal processing module 30. At step 161, the conditioned signal is
received. At step
162, a window size N is used to break the conditioned signal into contiguous
windows of data
over discrete time intervals between time ti and time t2, each referred to
herein as a time
-13-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
window. Window size N is used at step 170, described below. An example of a
time
window is illustrated in Fig. 10. In various embodiments, the conditioned
signal is broken
into time windows each having a duration ranging from about 3 seconds to about
15 seconds,
and in one embodiment each having a duration of about 10 seconds. Time windows
of about
seconds each represent about ten cardiac cycles in a patient whose heart rate
is about 60
beats per minute.
[0049] At step 163, for each time window, a spectrum analysis is
performed on
the conditioned signal that separates the conditioned signal into the
fundamental and
harmonic frequency bands. Step 163 may utilize any separation technique,
algorithm, or the
like known to those skilled in the art. In various embodiments, a Fast-Fourier
Transform
(FFT) algorithm is applied to the conditioned signal in each time window and
separates the
conditioned signal into the fundamental and harmonic frequency bands which
comprise the
conditioned signal. In various embodiments, a wavelet transformation is
applied to the
conditioned signal in each time window to separate the conditioned signal into
the
fundamental and harmonic frequency bands which comprise the conditioned
signal.
Examples of the fundamental and the first five harmonic frequency bands for
the photo-optic
signal are illustrated in Fig. 11. The fundamental signal A is depicted in
solid line and the
harmonics are identified as lines B1 through B5. The fundamental A or any of
the harmonics
B1 thru B5 may be used in embodiments for the calculation for circulatory
stress or
frequency shifts. Changes over time in frequencies and frequency strength over
time are
illustrated in Figs. 11A and 11B, respectively. In the illustration shown in
Fig. 11, the
biological signal was acquired during changes in simulated blood loss created
by decreases in
pressure in a lower body negative pressure chamber.
[0050] At step 164, the component(s) of the signal are selected. In the
embodiment shown in Fig. 11, the second harmonic (B2) is selected when the
assessment is
limited to the absolute value of the frequency because it may be more
sensitive to centralized
blood loss but is not limited by the condition of the patient's blood vessels
(such as in a co-
morbidity state). In contrast, the higher harmonics (i.e., B3-B5) are more
sensitive to the
condition of the patient's cardiovascular health. For example, an elderly
patient with stiff
blood vessels will have fewer harmonic frequencies than a younger subject with
more supple
blood vessels. In embodiments, selection of the most reliable harmonic is
determined by
patient population, resolution of the sensor used to acquire the biological
signal, the stress
-14-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
employed, and other such variables. In embodiments, the system and method are
used to
determine the most reliable harmonic over various populations. In embodiments
where the
assessment is based upon percentage of change measures, either the fundamental
frequency
or any of the harmonics will produce the same quantitative results assuming
the quality of
each of the harmonics are the same.
[0051] At step 165, the selected harmonic(s), B2 and the fundamental
signal in the
embodiment shown, are maintained and the other harmonics are removed.
[0052] At step 166, a linear continuous-time filter is applied to smooth
the
selected harmonic B2 and the fundamental signal A and to generate a filtered,
conditioned
harmonic B2 and fundamental signal A at step 167. In various embodiments, a
Butterworth
Filter, implemented with a polynomial transfer function, is applied to the
second harmonic
and the fundamental photo-optic signal. Those skilled in the art will
understand, however,
that other filters may also be applied, including for example, Chebyshev,
Bessel, Elliptical
filters, custom low pass filter modules, and techniques using moving
averagers.
[0053] At step 168, the method determines if a baseline has been set. If
no
baseline has been set, an embodiment of a baseline calculation is illustrated
at steps 170
through 173, although those skilled in the art will understand that any method
of identifying a
baseline may be used herein. If a baseline has been set, then the derived
parameters are
calculated and analyzed as set forth in Fig. 9, described below.
[0054] The flow chart illustrated in Fig. 23 sets forth another
embodiment of the
calculation that is performed in Fig. 7. The steps described herein may be
performed in any
order. The embodiment illustrated in Fig. 23 employs a total harmonic
distortion (THD)
calculation to measure a third derived parameter referred to as the Pulse
Volume Alterations
(PVA) Index. The measure may be used to assess the degree of arterial
compliance
activation when placing the sensor on a large artery or degree of activation
of arteriole
constriction or tone when placing the sensor on a capillary bed. In other
embodiments, this
parameter may be used to indirectly assess the compliance related effects on a
large artery
resultant of changes to the effective circulatory volume resultant of
vasoconstriction, the
transfer of fluid into or out of the arterial tree, or the effects of
anesthetics or medications on
the cardiovascular compensatory mechanisms.
-15-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
[0055] The equation for calculating the PVA Index provides a ratio of
the sum of
the strengths of the heart rate related harmonic frequencies to the strength
of the primary
harmonic or fundamental frequency for the heart rate. When used to assess
acoustical
properties, the greater the numerator or summed harmonic strength, the greater
the amount of
acoustical distortion present. In the case of an arterial pulse wave,
frequency decomposition
reveals a primary harmonic or heat rate frequency and additional lesser
strength harmonics
that are integer multiples of the fundamental pulse wave frequency. It is the
combination of
these frequencies and their specific strengths that can be used to
characterize the pulse wave
morphology. De-activation of the sympathetic nervous system results in
increases in the
compliance of the arterial wall of the large arteries. Increases in arterial
compliance results in
the increase in the summed harmonics or integer multiple frequencies of the
heart rate of the
arterial pulse wave in proportion to the frequency strength of the fundamental
heart rate
frequency and a larger PVA Index. In the acoustical context, when a guitar
string becomes
more taut, the aggregate strength of the harmonic frequencies decreases in
proportion the
fundamental frequencies. Similarly, when the sympathetic nervous system is
activated, the
walls of the large arteries become less compliant, causing a decrease in the
summed
frequency strengths of the integer multiple frequencies of the heart rate or
its harmonics.
[0056] An increase in the activation of the sympathetic nervous system
results in
an increase in the arterial tone or vascular constriction of the small
arteries or arterioles.
When the sensor is placed over a small arterial bed such as when placed on the
finger, an
increase in the arterial tone will result in a decrease in total harmonic
distortion percentage.
Alterations in the strength of the harmonics can also be attributed to long
term structural
changes that affect vascular stiffness in addition to short term autonomic
nervous driven
changes.
[0057] Referring specifically to Fig. 23, in embodiments, steps 1161
through are
implemented in the signal processing module 30. At step 1161, the conditioned
signal is
received. At step 1162, a window size N is used to break the conditioned
signal into
contiguous windows of data over discrete time intervals between time ti and
time t2, each
referred to herein as a time window. Window size N is used at step 170,
described below.
An example of a time window is illustrated in Fig. 10. In various embodiments,
the
conditioned signal is broken into time windows each having a duration ranging
from about 3
seconds to about 15 seconds, and in one embodiment each having a duration of
about 10
-16-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
seconds. Time windows of about 10 seconds each represent about ten cardiac
cycles in a
patient whose heart rate is about 60 beats per minute.
[0058] At step 1163, for each time window, a spectrum analysis is
performed on
the conditioned signal that separates the conditioned signal into the
fundamental and
harmonic frequency bands. Step 1163 may utilize any separation technique,
algorithm, or the
like known to those skilled in the art. In various embodiments, a Fast-Fourier
Transform
(FFT) algorithm is applied to the conditioned signal in each time window and
separates the
conditioned signal into the fundamental and harmonic frequency bands which
comprise the
conditioned signal. In various embodiments, a wavelet transformation is
applied to the
conditioned signal in each time window to separate the conditioned signal into
the
fundamental and harmonic frequency bands which comprise the conditioned
signal.
Examples of the fundamental and the first five harmonic frequency bands for
the photo-optic
signal are illustrated in Fig. 11. The fundamental signal A is depicted in
solid line and the
harmonics are identified as lines B1 through B5. Changes over time in the
shift of the
frequencies and in the frequency strength over time are illustrated in
Figs.11A and 11B,
respectively. In the illustration shown in Fig. 11, the biological signal was
acquired during
changes in simulated blood loss created by decreases in pressure in a lower
body negative
pressure chamber.
[0059] At step 1164, the components (i.e., the harmonics) of the signal
are
selected using, for example, the process described herein in connection with
Fig. 7. At step
1165, the selected harmonics are maintained and other harmonics are removed.
[0060] At step 1166, a linear continuous-time filter is applied to
smooth the
selected harmonics. In various embodiments, a Butterworth Filter, implemented
with a
polynomial transfer function, is applied to the harmonics and the fundamental
photo-optic
signal. Those skilled in the art will understand, however, that other filters
may also be
applied, including for example, Chebyshev, Bessel, Elliptical filters, custom
low pass filter
modules, and techniques using moving averages. At step 1168, the strongest
harmonic (B 1)
is selected from the remaining harmonics for the denominator of the total
harmonic distortion
calculation of step 1172. At step 1170 the second strongest and all other
harmonics in the
remaining harmonics are selected for the numerator of the total harmonic
distortion
calculation of step 1172. At step 1172 the total harmonic distortion
calculation is performed
and the process advances to "C" on Fig. 9.
-17-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
[0061] Referring now to Fig. 8, a filtered conditioned signal is
continuously
inputted at step 169 such that baseline is continuously recalculated as the
filtered conditioned
signal is received until baseline criteria are met. As shown, at step 170, a
set of N time
windows is selected for use in steps 171 through 173, described below. In an
embodiment,
the set of time windows N is 4 to 10 time windows. In a preferred embodiment,
the set of
time windows N is 6 time windows.
[0062] At step 171, the signal variance is calculated for each of the
filtered
conditioned signals received at step 169 in each window comprising the set of
N time
windows selected at step 170. In an embodiment, signal variance is the slope
of the filtered
conditioned signal received at step 169 in each of the N time windows. In
another
embodiment, signal variance is the percent change in the signal strength of
the filtered
conditioned signal received at step 169 in each of the N time windows, where
strength is
calculated according to the following Equation 1:
the root mean square (rms) of the peak voltage for one pulse
wave in the photo-optic signal, where the root mean square is
obtained by multiplying the peak voltage by 0.707.
In an example, in each of the N windows, the signal variance of the harmonic
2B is
calculated as the slope of the harmonic frequency is calculated and the signal
variance of the
fundamental signal A is calculated as the percent change in the strength of
the fundamental
signal.
[0063] At step 172, the calculations from step 171 are compared to a pre-
determined baseline criteria. If the calculations from step 171 meet the
baseline criteria, then
the baseline is set at step 173. In a preferred embodiment, if the slope of
the harmonic
frequency 2B over each of the N time windows is less than 0.1 and the percent
change in the
strength of the fundamental signal A is less than 10%, then the set of N time
windows may be
used as a baseline.
[0064] At step 24, the baseline is stored in a memory 70 such as a
database or a
computer readable medium.
[0065] If the calculations from step 171 do not meet the baseline
criteria, then a
moving window technique is applied to the signal(s) A and 2B at step 174 such
that the set of
-18-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
time windows is moved forward by one time window and steps 170 through 172 are
repeated
until a baseline is set at step 173.
[0066] The flow chart illustrated in Fig. 9 sets forth various
embodiments of step
18. In optional embodiments, steps 181 through 186 are implemented in the
analysis module
40.
[0067] As illustrated in Fig. 9, in embodiments, the present invention
may be
utilized in a method for assessing the adequacy of circulatory blood volume.
In the
embodiment illustrated in Fig. 9, the analysis module 40 receives, via a
wireline or a wireless
connection, a filtered conditioned signal is continuously inputted at step 169
such that the
filtered conditioned signal is continuously used to calculate the derived
parameter(s) percent
change from baseline as the filtered conditioned signal is received. At step
181, the filtered
conditioned signal is received for analysis. At step 182, the derived
parameter(s) percent
change(s) from baseline are calculated. In an embodiment, the percent changes
of the
frequency and frequency strength parameters are calculated by dividing the
respective
derived parameter by the corresponding baseline for that derived parameter
that was set in
step 173. In an embodiment, a harmonic frequency value 2B is used as the
derived
parameter, circulatory stress, and Equation 1 is used to calculate the derived
parameter,
circulatory blood volume.
[0068] Optionally, at step 183, the pattern of at least one of the
derived
parameters over time is compared to a library of patterns of that derived
parameter over time,
where the library of patterns is stored in memory. The comparison at step 183
can be used to
identify abnormal physiological conditions to which standard rules of
autoregulatory volume
adequacy cannot be applied, such as for example, where the patient has an
arrhythmia, is
taking medications that alter the autoregulatory function, or has other
conditions that impact
autoregulatory function. In embodiments, the patterns are stored in a look-up
table. In
embodiments, the library of patterns is a collection of previously recorded
and stored derived
parameters recorded from patients with known abnormal physiological
conditions. In other
embodiments, the library of patterns includes other external measurements such
as blood
pressure, oxygen saturation, core temperature, electrocardiology, skin
temperature and the
like. If at step 183, the derived parameter matches one of the patterns in the
library of
patterns, then the patient is classified into an outlier patient population
and the threshold
value, described at step 185 below, does not apply, and instructions are
implemented to
-19-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
initiate an action at step 186. Optionally, an output 60 is generated at step
20. Optionally,
the output and/or the action are stored in memory 70 at step 24.
[0069] If the patient is not in an outlier patient population, then at
step 185, each
derived parameter calculated at step 182 is compared to a threshold value,
where the
threshold value is a pre-determined value that represents a specific condition
or level of
circulatory blood volume adequacy. In embodiments, the threshold value is user-
specified or
has been clinically validated in a specific patient population. If the derived
parameter meets
the threshold value, then at step 186 instructions are implemented to initiate
an action
instructions are implemented to initiate an action. Optionally, an output 60
is generated at
step 20. Optionally, the output and/or the action are stored in memory 70 at
step 24.
[0070] Optionally, the filtered signal is continually received at step
169 and steps
through 186 and steps 20, 24 are repeated, as depicted in Fig. 9.
[0071] Examples of actions at step 186 include activation of an alarm
that
indicates a prediction that the patient is pre-symptomatic to an inadequate
circulatory volume
condition, or activation of an instruction to implement treatment to improve
the patient's
circulatory volume condition.
[0072] If the threshold is not met at step 185, then monitoring of the
patient
continues by repeating steps 169, 181 through 185. Even where the criteria are
met,
optionally, monitoring of the patient may continue by repeating steps 169, 181
through 185.
[0073] An example of a look-up table for the circulatory volume and
circulatory
stress parameters used at step 186 is shown in Table 2. As illustrated: (i) if
a patient's
circulatory stress value is 10% and the maintained circulatory blood flow is
+/- 10%, then the
patient is at an "Alarm Level 1" and data are plotted on a trend graph and an
alarm panel 1
light is lit; (ii) if a patient's circulatory stress value is 15% and the
maintained circulatory
blood volume is +/- 10%, then the patient is at an "Alarm Level 2" and data
are plotted on a
trend graph and an alarm level 2 panel light is lit; (iii) if a patient's
circulatory stress value is
20% and the maintained circulatory blood volume is +/- 10%, then the patient
is at an "Alarm
Level 3" and data are plotted on a trend graph, an alarm level 3 panel light
is lit, and an audio
alarm is sounded; and (iv) if a patient's circulatory stress value is greater
than or equal to
25% and the maintained circulatory blood volume is +/- 10%, then the patient
is at an "Alarm
Level 4" and data are plotted on a trend graph, an alarm level 4 panel light
is lit, and a high
-20-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
level alarm is sounded. In another embodiment, a look-up table would include a
value of
approximately 40% as normal range for the PVA Index for a healthy individual
with ranges
below 40% to indicate a sympathetically activated, increased effective
circulatory blood
volume, or fluid overload condition. Similarly, a value greater than 40% would
indicate a
parasympathetically activated, a decreased effective circulatory volume, or a
circulatory
volume insufficiency condition.
Table 1. Event Alarms.
Circulatory Maintained Action
Stress Circulatory Blood
Flow
Alarm Level 1 10% +/- 10% Plot data value on trend
graph and light alarm level 1
panel light
Alarm Level 2 15% +/- 10% Plot data value on trend
graph and light alarm level 2
panel light
Alarm Level 3 20% +/- 10% Plot data value on trend
graph and light alarm level 3
panel light, make light blink,
and initiate low level audio
alarm
Alarm Level 4 25% +/- 10% Plot data value on trend
graph and light alarm level 4
panel light, make light blink,
and initiate high level audio
alarm
[0074] In another embodiment, step 183 is used to classify patients with
similar
physiological responses. A classification may indicate a group of patients
with similar
comorbidities and/or demographics that exhibit a similar physiological
response to a form of
stress. In this embodiment, step 186 is a look-up table is used to identify a
standardized
evidence-based intervention or treatment protocol such as for hemodialysis
applicable to this
patient classification.
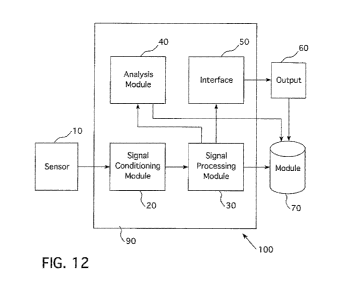
[0075] Figs. 12-16 illustrate various embodiments of a system 100 in which
embodiments of the present invention may be used. Various embodiments of a
system 100
for characterizing circulatory blood volume include a first sensor 10 that
acquires a first
signal, a first processor 90 that includes at least one module 20, 30, 40 for
processing and
analyzing the first signal, and an interface 50 that generates an output 60.
In the embodiment
-21-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
illustrated in Fig. 12, the sensor 10 is in communication with, via a wireline
or wireless
connection, a first processor 90 that is external to the sensor 10 and that
includes at least one
module 20, 30, 40 that processes and analyzes the signal to generate an output
60.
[0076] As described in greater detail below, the first sensor 10 may be
any
invasive or non-invasive device that includes circuitry to acquire a
biological signal.
[0077] Although Fig. 12 illustrates the case of a first processor 90, it
can be
understood that in various embodiments, the system may include one or more
second
processors 90', as illustrated in Figs. 14-16. As illustrated in Fig. 16, the
second processor
90' may be collocated with the first processor 90. As illustrated in Figs. 12-
13, 16, the
second processor 90' may be external to the first processor 90 and optionally
may be located
within the first sensor 10, and may include at least one module 30 configured
for post-
acquisition processing of the first signal and that communicates, via a
wireline or wireless
connection, with the first processor 90 for further processing of the signal
prior to generation
of an output 60.
[0078] Although Fig. 12 illustrates the case of a first sensor 10, it
can be
understood that the system 100 may include at least one second sensor 10'
configured to
record at least one second signal, as shown in Figs. 13, 14, 16. In various
embodiments, as
illustrated, the second sensor 10' communicates with the first processor 90,
via a wireline or
wireless connection, to transmit the second signal to the first processor 90
for post-acquisition
processing and analysis by modules 20, 30, 40 collocated therein. In various
embodiments,
the second sensor 10' may include a second processor 90' that includes at
least one module
20', 30', 40' configured to process the acquired signal.
[0079] In various embodiments, at least one module 20, 30, 40 is in
communication via, for example, wireline or wireless connections, with a
graphic interface
50.
[0080] The system further includes a memory 70, such as a database or a
computer readable medium. An output device 60 is in communication with the
processor.
[0081] Table 1 provides a list of examples of sensors 10, 10' and the
primary
signal captured from each. This list is exemplary only and is not intended to
be inclusive.
-22-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
Table 2. Primary Sensors and Primary Signals
Primary Sensor Primary Signal Acquired
Photo-optic sensor (transmissive) Blood density
Photo-optic sensor (reflective) Blood density
Pressure transducer Pulse pressure
Tonometry device Vascular palpation
Strain gauge Vessel circumference
Ultrasound device Vessel diameter
Electrical impedance Fluid electrical conductivity
Radar device Cardiac pulses
[0082] In various embodiments, the primary sensor 10 is a photo-optic
sensor that
acquires a photo-optic signal as described above. The photo-optic sensor may
acquire the
signal at a wavelength at which density changes reflect changes in density of
both oxygenated
and deoxygenated blood. In embodiments, the photo-optic sensor acquires the
signal at
wavelengths between about 700 nm and about 950 nm.
[0083] The photo-optic sensor may be either transmissive or reflective.
In various
embodiments, the photo-optic sensor is a reflective photo-optic sensor. The
pulsatile and
non-pulsatile portions of the photo-optic signal are illustrated in Fig. 17.
The transmitter and
the receiver are separated by a distance. In embodiments, the reflective photo-
optic sensor is
positioned on a patient's forehead or the like. In other various embodiments,
the photo-optic
sensor is a transmissive photo-optic sensor. In embodiments, the transmissive
photo-optic
sensor is positioned on a patient's finger or the like and light is
transmitted through the finger
or the like to a receiver on the other side of the finger.
[0084] In various embodiments, the primary sensor 10 is a pressure
transducer
that acquires a pulse pressure signal that indicates pulsatile changes in
total blood volume. In
embodiments, the pressure transducer is non-invasive. In other embodiments,
the pressure
transducer receives the pulse pressure signal from an arterial pressure line
implanted in an
artery.
[0085] In various embodiments, the primary sensor 10 is a tonometry
device that
acquires a signal that measures changes in vascular tension or pressure that
result from
-23-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
changes in blood density that occur as the pulse wave travels through the
arterial bed. In
embodiments, tissue is applanated to obtain the vascular pressure change.
[0086] In various embodiments, the primary sensor 10 is a strain gauge
that
acquires a signal that measures changes in the circumference of an extremity
that result from
changes in blood density that occur as the pulse wave travels through the
arterial bed.
[0087] In various embodiments, the primary sensor 10 is an ultrasound
device that
acquires a signal that measures changes in the diameter of a blood vessel that
result from
changes in blood density that occur as the pulse wave travels through the
arterial bed.
[0088] In various embodiments, the primary sensor 10 is an electrical
impedance
device that acquires a signal that measures changes in electrical conductivity
of the blood that
result from changes in blood density that occur as the pulse wave travels
through the arterial
bed.
[0089] In various embodiments, the primary sensor 10 is a radar device
that
acquires a signal that measures changes in contraction of the cardiac muscle
during a cardiac
cycle.
[0090] In embodiments, the system includes at least one secondary sensor
10', as
illustrated in Figs. 13, 14, 16. Secondary sensor 10' may be any invasive or
non-invasive
device that includes circuitry to acquire a secondary signal. Secondary sensor
10' includes a
controller and circuitry configured to acquire a secondary signal that denotes
the initiation
and termination of an event. In embodiments, use of secondary sensor 10' in
conjunction
with the primary sensor 10 enables circulatory blood volume to be
characterized before,
during and after an event. In embodiments, secondary sensor 10' is an
accelerometer that
measures a patient's axial changes such as when a patient goes from a supine
to a sitting to a
standing position. A system such as the one shown in Fig. 16 is useful in a
clinical setting
where multiple patients are being monitored. A primary sensor 10 is attached
to each patient
and records a biological signal. Each primary sensor 10 is in communication
with, via a
wireline or wireless connection, a first processor 90 that is external to the
sensor 10 and that
includes at least one module 20, 30, that processes the signal. In an
alternate embodiment,
the primary sensor 10 is in communication with, via a wireline or wireless
connection, a
second processor 90' that includes at least one module 20', 30' that processes
the signal. In
this embodiment, the second processor 90' is configured to receive signals
from each primary
-24-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
sensor 10, each recording a signal from a different patient, and to process
each signal and
generate an output 70 that is useful to the clinician monitoring the
circulatory blood volume
of each of these patients.
[0091] In other embodiments such as the system shown in Fig. 18, sensor
10
includes circuitry to acquire a secondary signal that measures secondary
parameters such as,
for example, oxygen saturation, heart rate, or core body temperature (not
shown). The
processor 90 includes modules 20, 30 to condition and process the biological
signal and the
analysis module 40 analyzes the biological signal and secondary parameters to
evaluate
circulatory blood volume and to generate an output 70.
[0092] In another embodiment, secondary sensor 10' is a thermocouple
used to
measure changes in cutaneous circulatory blood volume in order to remove the
cutaneous
contribution to the frequency strength measure that subsumes contributions
from both the
cutaneous and subcutaneous circulatory blood volume when a photo-optical
sensor is placed
on the skin. In another embodiment, the secondary sensor 10' is a thermocouple
used to
measure changes in cutaneous circulatory blood flow to calibrate the reduction
or increase in
autoregulatory capacity resultant of the diversion or decrease of circulatory
blood volume to
the skin due to thermal regulation.
[0093] In various embodiments, the secondary sensor 10' is an
electrodermal
sensor that provides a qualitative measure of cognitive stress that may be
used to calibrate the
impact that cognitive stress has on the patient's autoregulatory capacity to
maintain
homeostasis.
[0094] Various embodiments of the present invention may be implemented
on
non-transitory computer-readable media. The terms "computer-readable medium"
and
"computer-readable media" in the plural as used herein may include, for
example, magnetic
and optical memory devices such as diskettes, compact discs of both read-only
and writeable
varieties, optical disk drives, hard disk drives, and the like, all of which
may store non-
transitory signals. A computer-readable medium may also include memory storage
that can
be physical, virtual, permanent, temporary, semi-permanent and/or semi-
temporary.
-25-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
EXAMPLES
[0095] The following examples illustrate several embodiments of the
claimed
chromatography column. These examples should not be construed as limiting.
[0096] Figs. 19-22, 24, and 25 illustrate data collected using systems
and methods
embodying the present invention.
Example 1
[0097] The combination of the derived parameters circulatory stress and
circulatory blood volume can be used to predict and recognize circulatory
blood volume
adequacy. In Example 1, a Lower Body Negative Pressure Chamber was used to
simulate
circulatory blood volume loss. A human patient was placed into a sealed
pressure chamber
that comes up to just below the rib cage. A vacuum was used to decrease
chamber pressure
having the effect of sequestering blood to the feet and pulling it out of
circulation. As shown
in Fig. 19A, pressure in the chamber was held at zero mm Hg, then was
decreased in 5 steps
of -10 mm Hg each and held for 3 minutes per step, and then was returned back
to zero mm
Hg. Sensor 10 was positioned on the patient's forehead to record the
biological signal from
which the derived parameters, circulatory stress and circulatory blood volume
values were
calculated according to the method shown in Figs. 5-9, described above.
Percent change in
the derived parameters were plotted over time, as shown in Fig. 19B. Fig. 19C
shows the
systolic blood pressure of the patient, recorded using a Finapres. All graphs
shown in Figs.
19A-C are aligned vertically in time.
[0098] Periods 1, 2, and 3 are depicted in Fig. 19. Referring to Fig.
19B, during
Period 1 , the subject's compensatory mechanisms were adequately adapting to
the reduction
in circulatory volume as indicated by the minimal percentage change in
circulatory stress.
However, also in Period 1, there was a substantial decrease in the percentage
change in
circulatory blood volume, indicating a poor compensatory capacity during this
initial low
stress period of the test. As pressure in the chamber was further decreased,
the percentage
change in circulatory stress began to increase, indicating the beginning of
inadequate
compensation corresponding with a further decline in the percentage change in
circulatory
blood volume being maintained by the autoregulatory mechanisms.
-26-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
[0099] During Period 2, the sharp rise in the percentage change in
circulatory
stress indicates a more pronounced compensatory inadequacy to accommodate the
continued
loss of blood volume from the circulatory system. During this same period, the
percentage
change in circulatory blood volume was not decreasing at the same rate as the
percentage
change in circulatory stress was increasing. This pattern indicates that the
subject has little
remaining stress capacity tolerance. In this example, in each of Periods 1-3,
the derived
parameters indicate an ensuing hypovolemic event and related compensatory
inadequacy
while symptomatic measures such as blood pressure have not yet changed. With
less
capacity to tolerate this simulated volume loss, indicated by a small
percentage change in
circulatory blood volume during Period 3, circulatory stress rapidly
increases, indicating a
failing cardiovascular autoregulatory function. This conclusion is reinforced
by the severe
drop in systolic pressure (Fig. 19C) during Period 3.
Example 2
[0100] The derived parameters circulatory stress and circulatory blood
volume
can be used to indicate pre-symptomatic and symptomatic conditions of
circulatory blood
volume inadequacy. In practice, the conditions that the derived parameters can
be used to
recognize are equivalent to recognizing when the patient has become intolerant
to the stress
of fluid removal during dialysis treatment. In example 2, data were captured
from an end-
stage renal failure patient undergoing stress from accumulated fluid removal
during
hemodialysis employed as kidney replacement therapy. The treatment period was
approximately 4 hours long and performed about three times per week. Fig. 20A
illustrates
the points in time at which event alarms from the device were activated. Fig.
20B illustrates
the percentage change in circulatory stress and circulatory blood volume over
the time of the
therapy. Fig. 20C illustrates systolic blood pressure captured from a blood
pressure cuff
placed on the brachial artery of the arm and recorded every ¨10 minutes
throughout the
treatment. All graphs vertically aligned in time.
[0101] The accumulation of fluid in the circulatory system causes
increased blood
pressure and has a pronounced load on the cardiovascular function. As fluid is
removed from
the circulatory system, where 8% of the total body fluid resides, the load on
the
cardiovascular function is greatly reduced. This is demonstrated by the rapid
increase in
circulatory blood volume and the decrease in circulatory stress during Period
1, illustrated in
-27-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
Fig. 20B. Hypertension, indicated by systolic blood pressure in Period 1 (Fig.
20C) also is
reduced.
[0102] As the therapy progresses, the loss in circulatory volume causes
accumulated plasma water to be drawn into the arterial tree from the
interstitial and cellular
compartments. If the rate of fluid removal exceeds the vascular refill rate, a
hypovolemic
condition then ensues. If this condition exceeds the cardiovascular
compensatory
mechanisms, the patient can undergo an acute hypovolemic event resulting from
inadequate
tissue and organ circulating blood volume. A hypovolemic progression is
illustrated in
Period 2 (Fig. 20B) where the circulatory blood volume rapidly decreases,
suggesting a fluid
removal rate that exceeded the refill rate. Compensatory inadequacy to
accommodate the
continued decrease in circulatory volume as the fluid removal continues is
indicated by the
sudden rise in circulatory stress during Period 2. Autoregulatory capacity to
tolerate the
current circulatory stress is denoted by the severe drop in circulatory blood
volume during
Period 2. Use of the inventive system and method, which identify these changes
in the
derived parameters, trigger an event alarm (Fig. 20A) well in advance of the
time at which
the drop in systolic blood pressure occurs indicating autoregulatory failure.
[0103] During Period 3, the fluid removal rate has been reduced and
eventually
stopped at the end of period 3. This corresponds to the decreased percentage
change in
circulatory stress value and restoration of the circulatory blood volume,
indicating that the
fluid refill combined with autoregulatory mechanisms have adequately addressed
the
impaired circulatory blood volume that occurred during Period 2. Again, this
observation is
reinforced by the restoration of the systolic blood pressure during Period 3.
[0104] Patterns of response based on circulatory stress and circulatory
blood
volume can be used to recognize specific pathologies and to assess
cardiovascular functional
health. When a patient having compromised cardiovascular function undergoes
therapy, the
derived parameters may be used to identify a dosage endpoint. The data shown
in Fig. 21
were collected from a patient having both end-stage renal failure and right-
sided heart failure
and who underwent hemodialysis therapy lasting approximately 4 hours. Heart
failure refers
to a condition where the heart muscle becomes progressively weakened resulting
in a
degraded cardiovascular compensatory capacity. When fluid is accumulated in an
end-stage
renal failure patient who also suffers from heart failure, the weakened heart
has difficulty
-28-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
pumping volume against the increased load from the accumulated fluid at the
output of the
left ventricle.
[0105] As the heart failure patient attempts to adapt to the stress from
the
hemodialysis treatment, the weakened heart confronted with hypertension from
the
accumulated circulatory volume has difficulty adapting to this stress and the
percentage
change in circulatory blood volume immediately drops during Period 1 (Fig.
21A). However,
during Period 1, circulatory stress remains in a steady state, indicating the
patient is not in
danger of an acute hypovolemic condition. As soon as an adequate amount of the
accumulated fluid and its corresponding load on the heart has been reduced,
the percentage
change in circulatory blood volume dramatically increases during Period 2
(Fig. 20A) as does
the systolic blood pressure (Fig. 20B) as the pumping function of the heart is
restored. There
is a steady increase in the percentage change in circulatory stress throughout
the remainder of
the treatment but due to the low level of this stress. There is no
compensatory inadequacy
portrayed as shown by the modest increase in the percentage change in
circulatory stress.
[0106] The ability to provide a non-invasive, low risk methodology to
recognize
heart failure behavior is very valuable. The only alternative means to
recognize
hemodynamic behavior for heart failure is by measuring the ejection fraction
of the heart by
inserting a Swan Ganz catheter in one of the heart chambers. Recognition in
changes of
cardiovascular autoregulation due to the decline of the heart function in
heart failure patients
is referred to as decompensating heart failure and leads to poor circulatory
volume adequacy
and failing organ and tissue functions. Use of this technology to recognize
cardiovascular
autoregulatory changes when challenged by a standardized stress such as a
sitting-to-standing
maneuver, lying-to-sitting, or passive leg raise maneuver is valuable. In one
embodiment, a
standardized test such as a passive leg raise maneuver, can be used to
assesses the preload
and afterload dependency on the cardiac function resultant of the stress from
the leg raise.
Similarly, once the cardiovascular system has adapted to this increased
transient thoracic
volume, a leg lower can be used to assess the autoregulatory or compensatory
capacity of the
cardiovascular function. In another embodiment, collecting responses to a
physical maneuver
over time can be used to identify early signs of autoregulatory changes such
as cardiovascular
decompensation. This can be used to determine whether the current heart
function was
functioning adequately to support a normal level of physical stress
experienced during
independent living. Given that patient observation is not required to perform
a standardized
-29-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
stress test and that values from the test may be obtained remotely, use of
this device and
method provide a pre-symptom, sensitive, and pathology-specific test to
recognize and
manage chronic heart failure patients remotely as part of a telemedicine
communications
configuration.
Example 4
[0107] The inventive system and method, in combination with a stress
such as
dialysis treatment or a standardized physical maneuver, may be used to assess
and manage
the appropriateness of the measured autoregulatory response. This technique
may be used to
assess changes in the functional performance of the autoregulatory mechanisms
and/or to
manage pharmaceuticals that are used to treat hypertension and other
cardiovascular diseases
or dysfunctions that often have an effect on autoregulatory function, thereby
altering the
compensatory mechanisms.
[0108] Fig. 22 illustrates data collected over approximately a four hour
time
period from a hypertensive end-stage renal failure patient undergoing
hemodialysis treatment.
Graphs illustrated in Fig. 22A show the patient's response to fluid removal
while under the
influence of a high dosage of beta-blockers, which had a blunting effect on
the autoregulatory
response. There was little change in the percentage change in circulatory
stress throughout
the four hour treatment even though the patient was experiencing nausea and
light-
headedness, symptoms of inadequate circulatory blood volume and
autoregulation. Blood
pressure (Fig. 22A) remained relatively stable throughout the treatment.
Additionally, when
the patient stood at the conclusion of the treatment (designated by the *),
blood pressure
dropped, also indicative of a poor autoregulatory function.
[0109] Data illustrated in Fig. 22B were captured during a follow-on
dialysis
treatment several days later after the beta-blocker dosage was reduced in
half. The
percentage change in circulatory stress is more dynamic and responsive
throughout the
treatment. Similarly, the percentage change in circulatory blood flow is also
more dynamic
indicating a more responsive autoregulation of the circulatory blood volume.
Examples 5 and 6
[0110] Figs. 24 and 25 illustrate examples of data collected using a
total
harmonic distortion as described in connection with Fig. 23. The example
illustrated in Fig.
-30-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
24 is an example where embodiments of the present invention are used to manage
anesthetic
and fluid for resuscitation or during acute care. The illustrated example is a
porcine example
that demonstrates the effects of exsanguination. The total harmonic distortion
(THD) p
waveform portrays alterations in the frequency strength of the harmonics in
proportion to
frequency strength of the fundamental heart rate frequency resultant of the
effects of
compliance changes in internal branch of the carotid artery on the forehead
where the sensor
is placed. An initial increase in the THD ratio portrays initial increase in
compliance at the
start of the bleed followed by an decrease in compliance to adapt to blood
loss. As illustrated
in Fig. 24, the PVA Index shown as the total harmonic distortion wave form
portrays the
effects of increases or decreases in overall circulating blood volume, the
effects of circulatory
blood volume viscosity or oxygen carrying capacity resultant of transfusion of
blood
additives such as Hextend that can thin the blood and/or reduce the hematocrit
density
resulting in stiffening of the arterial tree to compensate for these changes
that then results in
decreasing the harmonics of the cardiac pulse wave.
[0111] At minute 260 in Fig. 24, the effects of the anesthesia wear off
and the pig
becomes more sensitive to cutting of tissue (see increase in effective
volume). As another
embodiment, the recognition of increased sympathetic responses during surgery
can be used
as feedback to titrate the appropriate level of anesthetic. In this example,
the anesthesia is
increased to reduce the effects, but the anesthesia dilates the blood vessels
and blunts the
nervous system. This results in the effective volume being reduced primarily
due to the
dilation effect. Lactate ringers were then administered to bring the fluid
levels up to level
prior to the additional anesthesia being administered.
[0112] The pulse oximetry in Fig. 24 indicates the amount of oxygenation
at any
moment in the blood. The pulse wave form is a measure of the density of total
red blood
cells (oxygenated and deoxygenated hemoglobin) in the underlying arterial bed
from the
changes in absorption of the near infrared frequency. As illustrated in Fig.
24, the relative
changes of the red blood cells from a baseline value in the first 7 minutes
indicates that, when
red blood cells are diluted through transfusion, the effective circulatory
volume (or pulse
strength) signal indicates how well the cardiovascular system is able to
maintain a constant
perfusion of red blood cells in the tissue. Such a scenario comes at the cost
of increasing
heart rate in order to circulate the diluted red blood cells faster.
-31-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
[0113] Fig. 25 illustrates another porcine example. Data has indicated
that a
normal circulatory volume level is represented by a PVA Index portrayed as the
total
harmonic distortion measure of approximately 40%, and thus such a measure may
be used to
recognize whether an individual is either volume loaded or volume deficient
(e.g.
dehydrated). As shown in Fig. 25, the total harmonic distortion level begins
at a value of
40%. As described herein, the total harmonic distortion value can show
decreases due to
blood loss as illustrated in Fig. 25 where the total harmonic distortion value
increases as the
subject pig is initially bled from the 10 minute mark to the 20 minute mark.
The PVA Index
(total harmonic distortion value) can also show the effects of the
compensatory mechanisms
of vasoconstriction of the small arteries indirectly even when placed on top
of a large artery
resultant in the pulse strength alterations resultant in a systemic change in
circulatory blood
volume when peripheral vasoconstriction occurs. In this example, even though
the subject
pig in Fig. 25 is still being bled at the same rate, when the total harmonic
distortion value
approaches minute 25 it begins to decrease, indicating that the walls of this
large artery are
becoming more stiff (less compliant), a compensatory related change. The
circulatory
volume change is further confirmed by the decrease in the relative measure of
circulatory
volume shown in this figure as "frequency strength".
[0114] After the bleed, the PVA Index (total harmonic distortion value)
reaches a
new steady state but circulatory volume increases as fluid is transferred from
other
compartments to compensate for the blood loss. Given that only 5-10% of the
volume of the
body is in the arterial tree, one defensive mechanisms is to transfer volume
over time from
venous reserves or diversion from the circulatory flow from the organs or
tissues into the
arterial tree to defend against arterial volume loss.
[0115] A clinician thus may see the dynamics related to defensive
mechanisms
when looking at the PVA Index. When viewed in conjunction with the circulatory
volume
(amplitude), a clinician may see the effectiveness of these mechanisms in
restoring or
compensating for circulatory volume loss.
[0116] The frequency signal in Fig. 25 represents changes in heart rate
of the
subject pig. Changes indicate circulatory stress and the signal may be used to
indicate the
severity of the volume deficiency challenge on the cardiovascular compensatory
mechanisms,
thus providing a more complete picture of how much difficulty the patient is
having with
circulatory changes at any point in time.
-32-
CA 02904682 2015-09-08
WO 2014/143962
PCT/US2014/028167
[0117] While
several embodiments of the invention have been described, it should
be apparent that various modifications, alterations, and adaptations to those
embodiments
may occur to persons skilled in the art with the attainment of some or all of
the advantages of
the present invention. It is therefore intended to cover all such
modifications, alterations, and
adaptations without departing from the scope and spirit of the present
invention.
-33-