Note: Descriptions are shown in the official language in which they were submitted.
CA 02904989 2015-09-10
WO 2015/053739
PCT/US2013/063674
METHODS OF COMBINING MESENCHYMAL STEM CELLS AND
CARTILAGE CONTAINING ALLOGRAFTS, AND PRODUCTS OF
COMBINED M.ESENCHYMAL STEM CELLS AND CARTILAGE
CONTAINING ALLOGRAFTS
BACKGROUND OF THE INVENTION
100011 Regenerative medicine requires an abundant source of human adult stem
cells that
can be readily available at the point of care.
100021 Adipose-derived stem cells (ASCs), which can be obtained in large
quantities, have
been utilized as cellular therapy for the induction of bone formation in
tissue engineering
strategies.
100031 Allografts may be combined with stem cells. This requires a significant
amount of
tissue processing and cellular processing prior to seeding the allograft
substrate.
100041 Allografts seeded with living cells generally provide better surgical
results.
BRIEF SUMMARY OF THE INVENTION
100051 In an embodiment, there is provided a method of combining mesenchymal
stem
cells with an osteochondral allograft, the method comprising providing adipose
tissue having
the mesenchymal stem cells together with unwanted cells; digesting the adipose
tissue to
form a cell suspension having the mesenchymal stem cells and the unwanted
cells, e.g., a
stromal vascular fraction comprising a heterogenous population of mesenchymal
stem cells
and unwanted cells; adding the cell suspension with the mesenchymal stem cells
to seed the
osteochondral allograft so as to form a seeded osteochondral allograft; and
allowing the cell
1
CA 02904989 2015-09-3.0
WO 2015/053739
PCT/US2013/063674
suspension to adhere to the osteochondral allograft by incubating the seeded
osteochondral
allograft for a period of time to allow the mesenchymal stem cells to attach.
100061 In another embodiment, there is provided an allograft product including
a
combination of mesenchymal stem cells with an osteochondral allograft, and the
combination
manufactured by obtaining adipose tissue having the mesenchymal stem cells
together with
unwanted cells; digesting the adipose tissue to form a cell suspension having
the
mesenchymal stem. cells and the unwanted cells; adding the cell suspension
with the
mesenchymal stem cells to seed the osteochondral allograft so as to form a
seeded
osteochondral allograft; and allowing the cell suspension to adhere to the
seeded
osteochondral allograft for a period of time to allow the mesenchymal stem
cells to attach.
100071 In still another embodiment, there is provided a method of combining
mesenchymal stem cells with an osteochondral allograft, the method comprising
providing
adipose tissue having the mesenchymal stem cells together with unwanted cells;
digesting
the adipose tissue to form a cell suspension having the mesenchymal stem cells
and the
unwanted cells to acquire a stromal vascular fraction comprising a
heterogenous population
of mesenchymal stem cells and unwanted cells, wherein the digesting includes
making a
collagenase I solution, and filtering the solution through a 0.2 gm filter
unit, mixing the
adipose solution with the collagenase I solution, and adding the adipose
solution mixed with
the collagenase I solution to a shaker flask; placing the shaker with
continuous agitation at
about 75 RPM for about 45 to 60 minutes so as to provide the adipose tissue
with a visually
smooth appearance; aspirating a supernatant containing mature adipocytes so as
to provide a
pellet; adding the cell suspension with the mesenchymal stem. cells to seed
the osteochondral
allograft so as to form a seeded osteochondral allograft; and allowing the
cell suspension to
adhere to seeded osteochondral allograft by incubating the seeded
osteochondral allograft for
a period of time to allow the mesenchymal stem cells to attach.
100081 In yet another embodiment, there is provided an allow-aft product
including a
combination of mesenchymal stem cells with an osteochondral allograft, and the
combination
manufactured by obtaining adipose tissue having the mesenchymal stem cells
together with
unwanted cells; digesting the adipose tissue to form a cell suspension having
the
mesenchymal stem cells and the unwanted cells to acquire a stromal vascular
fraction, and
the digesting includes making a collagenase I solution, and filtering the
solution through a
0.2 gm filter unit, mixing the adipose solution with the collagenase I
solution, and adding the
2
CA 02904989 2015-09-10
WO 2015/053739
PCT/US2013/063674
adipose solution mixed with the collagenase I solution to a shaker flask;
placing the shaker
with continuous agitation at about 75 RPM for about 45 to 60 minutes so as to
provide the
adipose tissue with a visually smooth appearance; aspirating a supernatant
containing mature
adipocytes so as to provide a pellet; adding the cell suspension with the
mesenchymal stem
cells to seed the osteochondral allograft so as to form a seeded osteochondral
allograft; and
allowing the cell suspension to adhere to the osteochondral allograft for a
period of time to
allow the mesenchymal stem cells to attach.
100091 In an embodiment, there is provided a method of combining mesenchymal
stem
cells with decellularized, morselized cartilage, the method comprising
providing adipose
tissue having the mesenchymal stem cells together with unwanted cells;
digesting the adipose
tissue to form a cell suspension having the mesenchymal stem cells and the
unwanted cells,
e.g., a stromal vascular fraction comprising a heterogenous population of
mesenchymal stem
cells and unwanted cells; adding the cell suspension with the mesenchymal stem
cells (e.g.,
stromal vascular fraction) to seed the morselized cartilage so as to form
seeded morselized
cartilage; and allowing the cell suspension to adhere to the decellularized,
morselized
cartilage by incubating the seeded decellularized, morseling cartilage for a
period of time to
allow the mesenchymal stem cells to attach.
100101 In another embodiment, there is provided an allograft product including
a
combination of mesenchymal stem cells with decellularized, morselized
cartilage, and the
combination manufactured by obtaining adipose tissue having the mesenchymal
stem cells
together with unwanted cells; digesting the adipose tissue to form a cell
suspension having
the mesenchymal stem cells and the unwanted cells; adding the cell suspension
with the
mesenchymal stem cells (e.g., stromal vascular fraction) to seed the
morselized cartilage so as
to form seeded morselized cartilage; and allowing the cell suspension to
adhere to the
decellularized, morselized cartilage by incubating the seeded decellularized,
morselized
cartilage for a period of time to allow the mesenchymal stem cells to attach.
100111 In still another embodiment, there is provided a method of combining
mesenchymal
stem cells with decellularized, morselized cartilage, the method comprising
providing
adipose tissue having the mesenchymal stem cells together with unwanted cells;
digesting
the adipose tissue to form a cell suspension having the mesenchymal stem cells
and the
unwanted cells to acquire a stromal vascular fraction, wherein the stromal
vascular fraction
comprises a heterogenous population of mesenchymal stem cells and unwanted
cells, and
3
CA 02904989 2015-09-10
WO 2015/053739
PCT/US2013/063674
wherein the digesting includes making a collagenase I solution, and filtering
the solution
through a 0.2 pm filter unit, mixing the adipose solution with the collagenase
I solution, and
adding the adipose solution mixed with the collagenase I solution to a shaker
flask; placing
the shaker with continuous agitation at about 75 RPM for about 45 to 60
minutes so as to
provide the adipose tissue with a visually smooth appearance; aspirating a
supernatant
containing mature adipocytes so as to provide a pellet; adding the cell
suspension with the
mesenchymal stem cells to seed the morselized cartilage so as to form seeded
morselized
cartilage; and allowing the cell suspension to adhere to the decellularized,
morselized
cartilage for a period of time to allow the mesenchymal stem cells to attach.
100121 In yet another embodiment, there is provided an allograft product
including a
combination of mesenchymal stem cells with decellularized, morselized
cartilage, and the
combination manufactured by obtaining adipose tissue having the mesenchymal
stem cells
together with unwanted cells; digesting the adipose tissue to form a cell
suspension having
the mesenchymal stem cells and the unwanted cells to acquire a stomal vascular
fraction,
and the digesting includes making a collagenase I solution, and filtering the
solution through
a 0.2 jim filter unit, mixing the adipose solution with the collagenase I
solution, and adding
the adipose solution mixed with the collagenase I solution to a shaker flask;
placing the
shaker with continuous agitation at about 75 RPM for about 45 to 60 minutes so
as to provide
the adipose tissue with a visually smooth appearance; aspirating a supernatant
containing
mature adipocytes so as to provide a pellet; adding the cell suspension with
the mesenchymal
stem cells to seed the morselized cartilage so as to form seeded morselized
cartilage; and
allowing the cell suspension to adhere to the decellularized, morselized
cartilage for a period
of time to allow the mesenchymal stem cells to attach.
100131 In an embodiment, there is provided a method of combining mesenchymal
stem
cells with an osteochondral allograft, the method comprising obtaining the
mesenchymal
stem cells from adipose tissue of a cadaveric donor; obtaining the
osteochondral allograft
from the same cadaveric donor; adding the mesenchymal stem cells to seed the
osteochondral
allograft so as to form a seeded osteochondral allograft; and allowing the
cell suspension to
adhere to the osteochondral allograft for a period of time to allow the
mesenchymal stem
cells to attach.
100141 In another embodiment, there is provided an allograft product including
a
combination of mesenchymal stem cells with an osteochondral allograft, and the
combination
4
CA 02904989 2015-09-10
WO 2015/053739
PCT/US2013/063674
manufactured by combining mesenchymal stem cells with an osteochondral
allograft, the
method comprising providing the mesenchymal stem cells from adipose tissue of
a cadaveric
donor; providing the osteochondral allograft from the same cadaveric donor;
adding the
mesenchymal stem cells to seed the osteochondral allograft so as to form a
seeded
osteochondral allograft; and allowing the cell suspension to adhere to the
seeded
osteochondral allograft by incubating the seeded osteochondral allograft for a
period of time
to allow the mesenchymal stem cells to attach.
100151 in an embodiment, there is provided a method of combining mesenchymal
stem
cells with decellularized, morselized cartilage, the method comprising
obtaining the
mesenchymal stem cells from adipose tissue of a cadaveric donor; obtaining the
morselized
cartilage from the same cadaveric donor; adding the mesenchymal stem cells to
seed the
morselized cartilage so as to form a seeded osteochondral allograft; and
allowing the cell
suspension to adhere to the decellularized, morselized cartilage for a period
of time to allow
the mesenchymal stem cells to attach.
100161 in another embodiment, there is provided an allograft product including
a
combination of mesenchymal stem cells with decellularized, morselized
cartilage, and the
combination manufactured by providing the mesenchymal stem cells from adipose
tissue of a
cadaveric donor; providing the morselized cartilage from the same cadaveric
donor; adding
the mesenchymal stem cells to seed the morselized cartilage so as to form
seeded morselized
cartilage; and allowing the cell suspension to adhere to the decellularized,
morselized
cartilage for a period of time to allow the mesenchymal stem cells to attach.
100171 In one embodiment, there is disclosed a method of combining mesenchymal
stem
cells with cartilage (e.g., decellularized cartilage), the method comprising
providing the
mesenchymal stem cells from adipose tissue of a cadaveric donor; providing the
cartilage
from the same cadaveric donor; adding the mesenchymal stem cells to seed the
cartilage so as
to form a seeded cartilage; and allowing the cell suspension to adhere to the
mesenchymal
stem cells and the cartilage by incubating the seeded cartilage for a period
of time to allow
the mesenchymal stem cells to attach.
100181 In another embodiment, there is disclosed an allograft product
including a
combination of mesenchymal stem cells with cartilage (e.g., decellularized
cartilage), and the
combination manufactured by obtaining the mesenchymal stem cells from adipose
tissue of a
cadaveric donor; obtaining the cartilage from the same cadaveric donor; adding
the
CA 02904989 2015-09-3.0
WO 2015/053739
PCT/US2013/063674
mesenchymal stem cells to seed the cartilage so as to form a seeded cartilage;
and allowing
the cell suspension to adhere to the mesenchymal stem cells and the cartilage
for a period of
time to allow the mesenchymal stem cells to attach.
100191 Other embodiments are also disclosed.
BRIEF DESCRIPTION OF THE DRAWINGS
100201 Illustrative embodiments of the invention are illustrated in the
drawings, in which:
100211 FIGURE 1 illustrates a flow chart of an exemplary method of combining
mesenchymal stem cells with an osteochondral allograft;
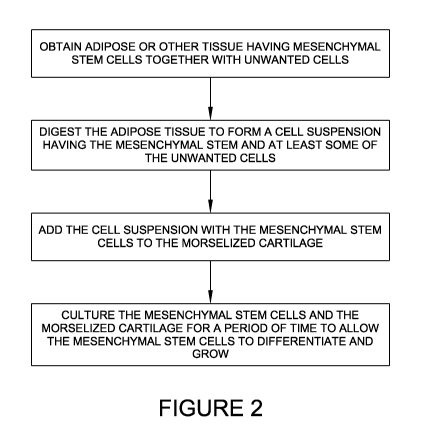
100221 FIGURE 2 illustrates a flow chart of an exemplary method of combining
mesenchymal stem cells with decellularized, morselized cartilage;
100231 FIGURE 3 illustrates an exemplary osteochondral allograft;
[0024] FIGURE 4 illustrates H&E staining of a cartilage control sample; and
[0025] FIGURE 5 illustrates H&E staining of adiposed-derived stem cells seeded
cartilage.
DETAILED DESCRIPTION OF THE 'INVENTION
[0026] Unless otherwise described, human adult stem cells are generally
referred to as
mesenchymal stem cells or MSCs. MSCs are pluripotent cells that have the
capacity to
differentiate in accordance with at least two discrete development pathways.
Adipose-
derived stem cells or ASCs are stem cells that are derived from adipose
tissue. Stromal
Vascular Fraction or SVF generally refers to the centrifuged cell pellet
obtained after
digestion of tissue containing MSCs. In some embodiments, the stromal vascular
fraction
comprises a heterogenous population of cells, including MSCs and unwanted
cells (non-
mesenchymal stem cells). In one embodiment, the SVF pellet may include
multiple types of
stem cells. These stem cells may include, for example, one or more of
hematopoietic stem
cells, epithelial stem cells, and mesenchymal stem cells. In an embodiment,
mesenchymal
stem cells are filtered from other stem cells by their adherence to an
osteochondral graft (or
cartilage or morselized cartilage), while the other stem cells (i.e., unwanted
cells) do not
adhere to the osteochondral graft (or cartilage or morselized cartilage).
Other cells that do
not adhere to the osteochondral graft (or cartilage or morselized cartilage)
may also be
included in these unwanted cells. In some embodiments, a stromal vascular
fraction (SVF)
6
CA 02904989 2015-09-10
WO 2015/053739
PCT/US2013/063674
that is seeded onto an allograft as described herein (e.g., an osteochondral
allograft, a
cartilage allograft, a morselized cartilage allograft, a decellularized
cartilage allograft, or a
decellularized morselized cartilage allograft) is directly seeded onto the
allograft without an
intermediate step of proliferating the mesenchymal stem cells. Methods of
obtaining a
stromal vascular fraction are described, e.g., in WO 2010/059565, incorporated
by reference
herein.
100271 In some embodiments, adipose derived stem cells are isolated from
cadavers and
characterized using flow cytometry and tri-lineage differentiation
(osteogenesis,
chondrogenesis and adipogenesis). The final product may be characterized using
histology
for microstructure and biochemical assays for cell count. This consistent cell-
based product
may be useful for osteochondral graft (or cartilage or morselized cartilage)
regeneration.
100281 Tissue engineering and regenerative medicine approaches offer great
promise to
regenerate bodily tissues. The most widely studied tissue engineering
approaches, which are
based on seeding and in vitro culturing of cells within the scaffold before
implantation, is the
cell source and the ability to control cell proliferation and differentiation.
Many researchers
have demonstrated that adipose tissue-derived stem cells (ASCs) possess
multiple
differentiation capacities. See, for example, the following, which are
incorporated by
reference:
Rada, T., R.L. Reis, and M.E. Gomes, Adipose Tissue-Derived Stern Cells and
Their
Application in Bone and Cartilage Tissue Engineering. Tissue Eng Part B Rev,
2009.
Ahn, H.H., et al., In vivo osteogenic differentiation of human adipose-derived
stem cells in an
injectable in situlbrining gel scaffirld. Tissue Eng Part A, 2009. 15(7): p.
1821-32.
Anghileri, E., et al., Neuronal differentiation potential of human adipose-
derived
mesenchymal stem cells. Stem Cells Dev, 2008. 17(5): p. 909-16.
Arnalich-Montiel, F., et al., Adipose-derived stem cells are a source for cell
therapy of the
corneal stroma. Stem Cells, 2008. 26(2): p. 570-9.
Bunnell, B.A., et al., Adipose-derived stem cells: isolation, expansion and
difierentiation.
Methods, 2008. 45(2): p. 115-20.
Chen, R.B., et al., [Differentiation of rat adipose-derived stern cells into
smooth-muscle-like
cells in vitro]. Zhonghua Nan Ke Xue, 2009. 15(5): p. 425-30.
7
CA 02904989 2015-09-10
WO 2015/053739
PCT/US2013/063674
Cheng, N.C., et al., Chondrogenic differentiation of adipose-derived adult
stem cells by a
porous scaffold derived from native articular cartilage extracellular matrix.
Tissue Eng Part
A, 2009. 15(2): p. 231-41.
Cui, L., et al., Repair of cranial bone defects with adipose derived stem
cells and coral
scqffold in a canine model. Biomaterials, 2007. 28(36): p. 5477-86.
de Girolamo, L., et al., Osteogenk differentiation of human adipose-derived
stem cells:
comparison of two different inductive media. J Tissue Eng Regen Med, 2007.
1(2): p. 154-7.
Elabd, C., et al., Human adipose tissue-derived multzpotent stem cells
differentiate in vitro
and in vivo into osteocyte-like cells. Biochem Biophys Res Commun, 2007.
361(2): p. 342-8.
Flynn, L., et al., Adipose tissue engineering with naturally derived scqfiblds
and adipose-
derived stem cells. Biomaterials, 2007. 28(26): p. 3834-42.
Flynn, L.E., et al., Proliferation and differentiation of adipose-derived stem
cells on naturally
derived scaffolds. Biomaterials, 2008. 29(12): p. 1862-71.
Fraser, j.K., et al., Adipose-derived stem cells. Methods Mol Biol, 2008. 449:
p. 59-67.
Gimble, J. and F. Guilak, Adipose-derived adult stem cells: isolation,
characterization, and
differentiation potential. Cytotherapy, 2003. 5(5): p. 362-9.
Gimble, J.M. and F. Guilak, Differentiation potential of adipose derived adult
stem (ADAS)
cells. CurrTop Dev Bioi, 2003. 58: p. 137-60.
Jin, X.B., et al., Tissue engineered cartilage from h'TGF beta2 transduced
human adipose
derived stem cells seeded in PLGA/alginate compound in vitro and in vivo. .1
Biomed Mater
Res A, 2008. 86(4): p. 1077-87.
Kakudo, N., et al., Bone tissue engineering using human adipose-derived stem
cells and
honeycomb collagen scqffold. J Biomed Mater Res A, 2008. 84(1): p. 191-7.
Kim, H.J. and GI. lm, Chondrogenic differentiation of adipose tissue-derived
mesenchymal
stem cells: greater doses of growth factor are necessary. J Orthop Res, 2009.
27(5): p. 612-9.
Kingham, P.J., et al., Adipose-derived stem cells differentiate into a Schwann
cell phenotype
and promote neurite outgrowth in vitro. Exp Neural, 2007. 207(2): p. 267-74.
8
CA 02904989 2015-09-3.0
WO 2015/053739
PCT/US2013/063674
Mehlhom, AT., et al., Chondrogenesis of adipose-derived adult stem cells in a
poly-lactide-
co-glycolide scafibld. Tissue Eng Part A, 2009. 15(5): p. 1159-67.
Merceron, C., et al., Adipose-derived mesenchymal stem cells and biomaterials
fbr cartilage
tissue engineering. Joint Bone Spine, 2008. 75(6): p. 672-4.
Mischen, B.T., et al., Metabolic andfunctional characterization of human
adipose-derived
stem cells in tissue engineering. Plast Reconstr Surg, 2008. 122(3): p. 725-
38.
Mizuno, H., Adipose-derived stem cells for tissue repair and regeneration: ten
years of
research and a literature review. J Nippon Med Sch, 2009. 76(2): p. 56-66.
Tapp, H., et al., Adipose-Derived Stem Cells: Characterization and Current
Application in
Orthopaedic Tissue Repair. Exp Bioi Med (Maywood), 2008.
Tapp, H., et al., Adipose-derived stem cells: characterization and current
application in
orthopaedic tissue repair. Exp Bioi Med (Maywood), 2009. 234(1): p. 1-9.
van Dijk, A., et al., Diffrrentiation of human adipose-derived stem cells
towards
cardiomyocytes is facilitated by laminin. Cell Tissue Res, 2008. 334(3): p.
457-67.
Wei, Y., et al., A novel injectable scaffold for cartilage tissue engineering
using adipose-
derived adult stem cells. .1. Orthop Res, 2008. 26(1): p. 27-33. Wei, Y., et
al., Adipose-derived
stem cells and chondrogenesis. Cytotherapy, 2007. 9(8): p. 712-6.
Zhang, Y.S., et at., [Adipose tissue engineering with human adipose-derived
stem cells and
fibrin glue injectable scaffold]. Zhonghua Vi Xue Za Zhi, 2008. 88(38): p.
2705-9.
100291 Additionally, adipose tissue is probably the most abundant and
accessible source of
adult stem cells. Adipose tissue derived stem cells have great potential for
tissue
regeneration. Nevertheless, ASCs and bone marrow-derived stem cells (BMSCs)
are
remarkably similar with respect to growth and morphology, displaying
fibroblastic
characteristics, with abundant endoplasmic reticulum and large nucleus
relative to the
cytoplasmic volume. See, for example, the following, which are incorporated by
reference:
Gimble, J. and F. Guilak, Adipose-derived adult stem cells: isolation,
characterization, and
differentiation potential. Cytotherapy, 2003. 5(5): p. 362-9.
Gimble, J.M. and F. Guilak, Dfferentiation potential of adipose derived adult
stem (ADAS)
cells. Curr Top Dev Bioi, 2003. 58: p. 137-60.
9
CA 02904989 2015-09-10
WO 2015/053739
PCT/US2013/063674
Stem, B.M., et al., Multipotential differentiation of adipose tissue-derived
stem cells. Keio
Med, 2005. 54(3): p. 132-41.
De Ugarte, D.A., et al., Comparison of multi-lineage cells from human adipose
tissue and
bone marrow. Cells Tissues Organs, 2003. 174(3): p. 101-9.
Hayashi, 0., et al., Comparison of osteogenic ability of rat mesenchymal stem
cells from bone
marrow, periosteum, and adipose tissue. Calcif Tissue Int, 2008. 82(3): p. 238-
47.
Kim, Y., et al., Direct comparison of human mesenchymal stem cells derived
from adipose
tissues and bone marrow in mediating neovascularization in response to
vascular ischemia.
Cell Physiol Biochem, 2007. 20(6): p. 867-76.
Lin, L., et al., Comparison of osteogenic potentials qfBii4P4 transduced stem
cells from
autologous bone marrow andAt tissue in a rabbit model of calvarial defects.
Calcif Tissue
Int, 2009. 85(1): p. 55-65.
Niemeyer, P., et al., Comparison of immunological properties of bone marrow
stromal cells
and adipose tissue-derived stem cells before and Oster osteogenic
differentiation in vitro.
Tissue Eng, 2007. 13(1): p. 111-21.
Noel, D., et al., Cell specific differences between human adipose-derived and
mesenchymal-
stromal cells despite similar differentiation potentials. Exp Cell Res, 2008.
314(7): p. 1575-
84.
Yoo, K.H., et al., Comparison of immunomodulatoty properties of mesenchymal
stem cells
derived from adult human tissues. Cell lmmunol, 2009.
Yoshimura, H., et al., Comparison of rat mesenchymal stem cells derived from
bone marrow,
synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res, 2007.
327(3): p. 449-62.
100301 FIGURE 1 is a flow chart of a process for combining an osteochonclral
allograft
with stem cells. In an embodiment, a stromal vascular fraction (e.g., a
heterogenous
population of cells comprising mesenchymal stem cells and unwanted cells) may
be used to
seed the allograft. It should be apparent from the present disclosure that the
term "seed"
relates to addition and placement of the stem cells within, or at least in
attachment to, the
allograft, but is not limited to a specific process.
100311 In an exemplary embodiment, a method of combining mesenchymal stem
cells with
an osteochondral allograft is provided. In some embodiments, the method is an
ex vivo
CA 02904989 2015-09-10
WO 2015/053739
PCT/US2013/063674
method. The method may include obtaining adipose tissue having the mesenchymal
stem
cells together with unwanted cells. Unwanted cells may include hematopoietic
stem cells and
other stromal cells. The method may further include digesting the adipose
tissue to form a
cell suspension having the mesenchymal stem cells and at least some or all of
the unwanted
cells (e.g., a stromal vascular fraction). In another embodiment, this
digestion step may be
followed by negatively depleting some of the unwanted cells and other
constituents to
concentrate mesenchymal stem cells.
100321 Next, the method includes adding the cell suspension with the
mesenchymal stem
cells to seed the osteochondral allograft. This may be followed by allowing
the cell
suspension to adhere to the osteochondral allograft (e.g., by incubating the
seeded allograft
under appropriate incubation conditions) for a period of time to allow the
mesenchymal stem
cells to attach. In order to provide a desired product, the method may include
rinsing the
seeded osteochondral allograft to remove the unwanted cells from the seeded
ostechondral
allograft.
100331 In one embodiment, an allograft product may include a combination of
mesenchymal stem cells with an osteochondral allograft such that the
combination is
manufactured by the above exemplary embodiment.
100341 In an embodiment, the adipose tissue may be obtained or recovered from
a
cadaveric donor. A typical donor yields 2 liters of adipose containing 18
million MSCs. In
one embodiment, an osteochondral allograft may be from the same cadaveric
donor as the
adipose tissue. In another embodiment, the adipose tissue may be obtained from
a patient
that will be undergoing the cartilage or osteochondral
replacement/regeneration surgery. In
addition, both the osteochondral graft (or cartilage or morselized cartilage)
and the adipose
tissue may be obtained from the same cadaveric donor. Adipose cells may be
removed using
liposuction. Other sources, and combination of sources, of adipose tissue,
other tissues, and
osteochondral allografts may be utilized.
100351 Optionally, the adipose tissue may be washed prior to or during
digestion. Washing
may include using a thermal shaker at 75 RPM at 37 C for at least 10 minutes.
Washing the
adipose tissue may include washing with a volume of PBS substantially equal to
the adipose
tissue. In an embodiment, washing the adipose tissue includes washing with the
PBS with
1% penicillin and streptomycin at about 37 C.
11
CA 02904989 2015-09-3.0
WO 2015/053739
PCT/US2013/063674
10036] For example, washing the adipose tissue may include agitating the
tissue and
allowing phase separation for about 3 to 5 minutes. This may be followed by
aspirating off a
supernatant solution. The washing may include repeating washing the adipose
tissue multiple
times until a clear infranatant solution is obtained. In one embodiment,
washing the adipose
tissue may include washing with a volume of growth media substantially equal
to the adipose
tissue.
100371 FIGURE 2 is a flow chart of a process for combining morselized
cartilage (e.g.,
decellularized morselized cartilage) with stem cells. In an embodiment, a
stromal vascular
fraction (e.g., a heterogenous population of cells comprising mesenchymal stem
cells and
unwanted cells) may be used to seed the allograft.
100381 In another exemplary embodiment, a method of combining mesenchymal
stem. cells
with decellularized, morselized cartilage is provided. In some embodiments,
the method is an
ex vivo method. The method may include obtaining adipose tissue having the
mesenchymal
stem cells together with unwanted cells. Unwanted cells may include
hematopoietic stem
cells and other stromal cells. The method may further include digesting the
adipose-derived
tissue to form a cell suspension having the mesenchymal stem cells and the
unwanted cells
(e.g., stromal vascular fraction). In another embodiment, this digesting step
may be followed
by naturally selecting MSCs and depleting some of the unwanted cells and other
constituents
to concentrate mesenchymal stem cells.
100391 Next, the method includes adding the cell suspension with the
mesenchymal stem
cells (e.g., the stromal vascular fraction) to the morselized cartilage. This
may be followed
by allowing the cell suspension to adhere to the mesenchymal stem cells and
the morselized
cartilage (e.g., by incubating the seeded morselized cartilage under
appropriate incubation
conditions) for a period of time to allow the mesenchymal stem cells to
attach. In order to
provide a desired product, the method may include rinsing the seeded
morselized cartilage to
remove the unwanted cells from the seeded morselized cartilage.
100401 In one embodiment, an allograft product may include a combination of
mesenchymal stem cells with decellularized, morselized cartilage such that the
combination
is manufactured by the above exemplary embodiment.
100411 In an embodiment, the adipose tissue may be obtained from a cadaveric
donor. A
typical donor yields 2 liters of adipose containing 18 million MSCs. In one
embodiment,
morselized cartilage may be from the same cadaveric donor as the adipose
tissue. In another
12
CA 02904989 2015-09-10
WO 2015/053739
PCT/US2013/063674
embodiment, the adipose tissue may be obtained from a patient. In addition,
both the
osteochondral graft or cartilage or morselized cartilage) and the adipose
tissue may be
obtained from the same cadaveric donor. Adipose cells may be removed using
liposuction.
Other sources, and combination of sources, of adipose tissue, other tissues,
and morselized
cartilage may be utilized.
100421 Optionally, the adipose tissue may be washed prior to or during
digestion. Washing
may include using a thermal shaker at 75 RPM at 37 C for at least 10 minutes.
Washing the
adipose tissue may include washing with a volume of PBS substantially equal to
the adipose
tissue. In an embodiment, washing the adipose tissue includes washing with the
PBS with
1% penicillin and streptomycin at about 37 C.
[0043] For example, washing the adipose tissue may include agitating the
tissue and
allowing phase separation for about 3 to 5 minutes. This may be followed by
aspirating off a
supernatant solution. The washing may include repeating washing the adipose
tissue multiple
times until a clear infranatant solution is obtained. In one embodiment,
washing the adipose
tissue may include washing with a volume of growth media substantially equal
to the adipose
tissue.
[0044] Digesting the cell suspension may include making a collagenase I
solution, and
filtering the solution through a 0.2 gm filter unit, mixing the adipose tissue
with the
collagenase I solution, and adding the cell suspension mixed with the
collagenase 1 solution
to a shaker flask. Digesting the cell suspension may further include placing
the shaker with
continuous agitation at about 75 RPM for about 45 to 60 minutes so as to
provide the adipose
tissue with a visually smooth appearance.
100451 Digesting the cell suspension may further include aspirating
supernatant containing
mature adipocytes so as to provide a pellet, which may be referred to as a
stromal vascular
fraction. (See, for example, FIGURE 2.) Prior to seeding, a lab sponge or
other mechanism
may be used to pat dry cells from the pellet.
[0046] In various embodiments, adding the cell suspension with the mesenchymal
stem
cells to the osteochondral allograft or the morselized cartilage (e.g.,
decellularized morselized
cartilage) may include using a cell pellet for seeding onto the osteochondral
graft (or cartilage
or morselized cartilage, e.g., decellularized cartilage or decellularized
morselized cartilage).
The cell suspension is allowed to adhere to seeded allografts for a period of
time to allow the
mesenchymal stem cells to attach to the osteochondral allograft or the
morselized cartilage.
13
CA 02904989 2015-09-10
WO 2015/053739
PCT/US2013/063674
In some embodiments, the seeded osteochondral graft (or seeded cartilage or
seeded
morselized cartilage, e.g., seeded decellularized cartilage or seeded
decellularized morselized
cartilage) is incubated for a period of time under appropriate incubation
conditions. In some
embodiments, the seeded osteochondral graft (or seeded cartilage or seeded
morselized
cartilage, e.g., seeded decellularized cartilage or seeded decellularized
morselized cartilage)
is incubated in a humidified incubator. In some embodiments, the seeded
osteochondral graft
(or seeded cartilage or seeded morselized cartilage, e.g., seeded
decellularized cartilage or
seeded decellularized morselized cartilage) is incubated in the presence of
culture medium
and/or one or more growth factors. In some embodiments, following incubation,
the seeded
osteochondral graft (or seeded cartilage or seeded morselized cartilage, e.g.,
seeded
decellularized cartilage or seeded decellularized morselized cartilage) is
rinsed to remove
unwanted cells from the allograft. After the rinsing step, a lab sponge or
other mechanism
may be used to pat dry the cells.
100471 In various embodiments, the method may include placing the
osteochondral graft
(or cartilage or morselized cartilage, e.g., decellularized cartilage or
decellularized morselized
cartilage) into a cryopreservation media after rinsing the osteochondral
allograft or the
morselized cartilage. This cryopreservation media may be provided to store the
final
products. For example, the method may include maintaining the osteochondral
allograft or
the morselized cartilage into a frozen state after rinsing the osteochondral
allograft or the
morselized cartilage to store the final products. The frozen state may be at
about negative
80 C.
100481 In another embodiment, Ficoll density solution may be utilized. For
example,
negatively depleting the concentration of the mesenchymal stem cells may
include adding a
volume of PBS and a volume of Ficoll density solution to the adipose solution.
The volume
of PBS may be 5 ml and the volume of Ficoll density solution may be 25 ml with
a density of
1.073 g/ml. Negatively depleting the concentration of the mesenchymal stem
cells may also
include centrifuging the adipose solution at about 1160 g for about 30 minutes
at about room
temperature. In one embodiment, the method may include stopping the
centrifuging the
adipose solution without using a brake.
100491 Negatively depleting the concentration of the mesenchymal stem cells is
optional
and may next include collecting an upper layer and an interface containing
nucleated cells,
and discarding a lower layer of red cells and cell debris. Negatively
depleting the
14
CA 02904989 2015-09-10
WO 2015/053739
PCT/US2013/063674
concentration of the mesenchymal stem cells may also include adding a volume
of D-PBS of
about twice an amount of the upper layer of nucleated cells, and inverting a
container
containing the cells to wash the collected cells. Negatively depleting the
concentration of the
mesenchymal stem cells may include centrifuging the collected cells to pellet
the collected
cells using the break during deceleration.
100501 In an embodiment, negatively depleting the concentration of the
mesenchymal stem
cells may further include centrifuging the collected cells at about 900 g for
about 5 minutes at
about room temperature. Negatively depleting some of the unwanted cells may
include
discarding a supernatant after centrifuging the collected cells, and
resuspending the collected
cells in a growth medium.
100511 Previous methods used autogenous osteochondral grafts, wherein a raft
from one
area of a donor knee was transplanted to same donor knee, but to an area that
was damaged.
However, this method causes trauma to the patient and creates a new area that
is damaged.
Allografts are currently used that prevent the trauma caused by autografts.
Non-processed
osteochondral allografts suffer from being immune reactive. Processed
osteochondral
allografts suffer from either having no viable cells, reduced viability, or
fully differentiated
cells that are not capable of undergoing regeneration. Thus, there is a need
to provide a
cartilage graft that contains viable MSCs to recapitulate the regenerative
cascade.
100521 The surface of cartilage, by its very nature, is not adherent to cells.
The
mesenchymal stem cells are anchorage dependent, but this has been defined in
the art as
being adherent to tissue culture plastic, not to a biological tissue like
cartilage. Surprisingly,
the methods provided herein permit viable MSCs that bind to cartilage.
100531 The methods provided herein describe the allograft processing that
allows MSCs to
adhere to the non-synthetic osteochondral or cartilage (e.g., decellularized
cartilage) scaffolds
described herein. The method in the example demonstrates a blending and
processing
method that removes cells from the cartilage graft such that viable MSCs can
adhere.
100541 The mesenchymal stem cells are non-immunogenic and regenerate cartilage
of the
osteochondral allograft or the morselized cartilage. The unwanted cells are
generally
anchorage independent. This means that the unwanted cells generally do not
adhere to the
osteochondral allograft or the morselized cartilage. The unwanted cells may be
immunogenic. For cell purification during a rinse, mesenchymal stem cells
adhere to the
osteochondral allograft or the morselized cartilage while unwanted cells, such
as
CA 02904989 2015-09-3.0
WO 2015/053739
PCT/US2013/063674
hematopoietic stem cells, are rinsed away leaving a substantially uniform
population of
mesenchymal stem cells on the osteochondral graft (or cartilage or morselized
cartilage).
100551 The ability to mineralize the extra.cellular matrix and to generate
cartilage is not
unique to MSCs. In fact, ASCs possess a similar ability to differentiate into
chondrocytes
under similar conditions. Human ASCs offer a unique advantage in contrast to
other cell
sources. The multipotent characteristics of ASCs, as wells as their abundance
in the human
body, make these cells a desirable source in tissue engineering applications.
100561 In addition, this method and combination product involve processing
that does not
alter the relevant biological characteristics of the tissue. Processing of the
adipose/stem cells
may involve the use of antibiotics, cell media, collagenase. None of these
affects the relevant
biological characteristics of the stem cells. The relevant biological
characteristics of these
mesenchymal stem cells are centered on renewal and repair. The processing of
the stem cells
does not alter the cell's ability to continue to differentiate and repair.
100571 In the absence of stimulation or environmental cues, mesenchymal stem
cells
(MSCs) remain undifferentiated and maintain their potential to form tissue
such as bone,
cartilage, fat, and muscle. Upon attachment to an osteoconductive matrix, MSCs
have been
shown to differentiate along the osteoblastic lineage in vivo. See, for
example, the following,
which are incorporated by reference:
Arinzeh T.L., Peter S.J., Archambault M.P., van den Bos C., Gordon S., Kraus
K., Smith A.,
Kadiyala S. Allogeneic mesenchymal stem cells regenerate bone in a critical
sized canine
segmental defect. J Bone Joint Surg Am. 2003; 85-A:1927-35.
Bruder S.P., Kurth A.A., Shea M., Hayes W.C., Jaiswal N., Kadiyala S. Bone
regeneration
by implantation of purified, culture-expanded human mesenchymal stem cells, J
Orthop Res.
1998; 16:155-62.
100581 Referring to FIGURE 3, and in an embodiment, there is illustrated an
osteochondral
allografi 10, which may include cartilage 15 and bone 20 from a cadaver.
Osteochondral
allografl may be placed in the area of a knee 25 or other joint where
cartilage is missing.
This technique may be used where there is a large area of cartilage that is
missing or if there
both bone and cartilage are missing. The donor allograft must be tested for
contamination,
which may include bacteria, hepatitis, and HIV. Having a single donor for both
the
16
CA 02904989 2015-09-10
WO 2015/053739 PCT/US2013/063674
osteochondral allograft and adipose-derived mesenchymal stem cells may reduce
testing
burdens and minimize other potential issues.
EXAMPLE
100591 The following example is offered to illustrate, but not to limit, the
claimed
invention.
Cartilage Combined with Adipose-derived Stem Cells
100601 The objective was to determine whether adipose derived stem cells
adhere to
processed and ground articular cartilage.
100611 ASCs adhere to cartilage, and promotcartilage repair and regeneration.
100621 Experiment Design:
Cartilage with Cartilage w/o ASCs only Medium only
ASCs ASCs
n=3,36h n=3,36h n=3, 36h n=3,36h
incubation incubation incubation incubation
100631 Materials and Methods:
100641 Sample Preparation: Cartilage pieces previously shaved from knee
articulating
surface and frozen at -80 C were thawed and blended (Waring Blender) for
approximately 2
minutes on "Hi" (22,000 rpms) while submerged in PBS. Resulting particles were
approximately lmm x 2-3mm x imm. The particles were then rinsed and drained in
a sieve
and were separated into six 5m1 samples and placed into a 6-well plate. Prior
to seeding,
cartilage samples were patted dry with sterile gauze. Three wells containing
cartilage were
each seeded with 200111 cell suspension. The other three wells containing
cartilage only were
left as unseeded controls. An empty 6-well plate was seeded in the same
fashion with three
wells receiving cells and three wells without cells. The wells were incubated
for an hour at
37 C and 5% CO2 in a humidified incubator, then submerged in 5m1DMEM-
F12/10%FBS/1%PSA and incubated for 36 hrs. All the samples in the 6-well
plates were
tested using CCK-8 assay for cell counts and the cartilage samples were
collected for
histology.
100651 Cell count: CCK-8 Assay
100661 Cell Counting Kit-8 (CCK-8, Dojindo Molecular Technologies, Maryland)
allows
sensitive colorimetric assays thr the determination of the number of viable
cells in cell
17
CA 02904989 2015-09-10
WO 2015/053739 PCT/US2013/063674
proliferation assays. The amount of the formazan dye generated by the activity
of
dehydrogenases in cells is directly proportional to the number of living
cells. The samples
were rinsed with PBS and then patted dry. Growth medium and CCK-8 solution
were added
into wells at a ratio of 10:1 cultured at 37 C for 2 hours and evaluated in a
plate reader with
excitation set to 460 nm and emission set to 650 nm. The results were
interpolated from a
standard curve based on ASCs only (passage=1).
100671 Histology
100681 The cartilage samples were fixed in 10% neutral buffered fonnalin
(Sigma, St.
Louis, MO) for 48 h, put in a processor (Citadel 2000; Thermo Shandon,
Pittsburgh, PA)
overnight, and embedded in paraffin. Sections were cut to 51.tm and mounted
onto glass
slides and stained with hematoxylin and eosin (H&E). Conventional light
microscopy was
used to analyze sections for matrix and cell morphology.
100691 Results
100701 Cell Counts: The number of cells on cartilage was significantly
different from
ASCs-only controls which were cultured in the 6 well plates.
ASCs + Cartilage Cartilage only Medium Only
Number of 4,665 0 0
, Viable Cells
100711 FIGURE 4 illustrates H&E staining of cartilage control (10X
magnification). Note
that there were no live cells in the voids of the ground cartilage matrix.
100721 FIGURE 5 illustrates H&E staining of ASCs seeded cartilage (10X
magnification).
Note the live cell nuclei in the voids.
100731 In the cartilage only control, there were no live cells, only the dead
cell debris was
discovered. The cells seemed to be all dead and left the voids behind. in the
ASCs seeded
cartilage, it seemed that all the seeded cells repopulated the voids left by
pre-existing cells
from the cartilage. There were no live cells on the cartilage surface that
lacked decellularized
zones.
100741 Conclusions
100751 ASCs did not adhere to the cartilage matrix, however, they repopulated
in the voids
left from pre-existing cartilage cells.
18
CA 02904989 2015-09-10
WO 2015/053739
PCT/US2013/063674
100761 Although the foregoing invention has been described in some detail by
way of
illustration and example for purposes of clarity of understanding, one of
skill in the art will
appreciate that certain changes and modifications may be practiced within the
scope of the
appended claims, in addition, each reference provided herein in incorporated
by reference in
its entirety to the same extent as if each reference was individually
incorporated by reference.
19