Note: Descriptions are shown in the official language in which they were submitted.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
FIBER-CONTAINING CARBOHYDRATE COMPOSITION
CROSS-REFERENCE TO RELATED APPLICATION
This application is related to and claims the benefit of U.S. Provisional
Application No. 61/784,286, entitled "FIBER-CONTAINING CARBOHYDRATE
COMPOSITION" filed March 14, 2013, the contents of which are incorporated
herein by
reference in their entirety and for all purposes.
BACKGROUND OF THE INVENTION
A variety of carbohydrates are used in food products, such as various sugars
and starches. Many of these carbohydrates are digested in the human stomach
and
small intestine. Dietary fiber in food products, in contrast, is generally not
digested in
the stomach or small intestine, but is potentially fermentable by
microorganisms in the
large intestine.
There is an interest in developing ingredients that are suitable for use in
food
products and that are either non-digestible or only digestible to a limited
extent, in
order to enhance the dietary fiber content or reduce the caloric content of
the food.
These modifications have certain health benefits.
There is a need for edible materials which have a reduced content of easily
digestible carbohydrates, and which can be used in place of, or in addition
to,
conventional carbohydrate products in foods.
SUMMARY OF THE INVENTION
One aspect of the invention is a process for making an oligosaccharide
composition. The process comprises producing an aqueous composition that
comprises
at least one oligosaccharide and at least one monosaccharide by
saccharification of
starch; membrane filtering the aqueous composition to form a monosaccharide-
rich
stream and an oligosaccharide-rich stream; and recovering the oligosaccharide-
rich
stream. In one embodiment of the invention, the oligosaccharide-rich stream is
slowly
digestible by the human digestive system. "Slowly digestible" as the term is
used
herein means that a substantial quantity (e.g., at least about 50% on a dry
solids
basis, and in some cases at least about 75%, or at least about 90%) of the
carbohydrates present in the stream are either not digested at all in the
human
stomach and small intestine, or are only digested to a limited extent. In
another
embodiment of the invention, the oligosaccharide-rich stream is resistant to
digestion
by the human digestive system.
Both in vitro and in vivo tests can be performed to estimate rate and extent
of
carbohydrate digestion in humans. The "Englyst Assay" is an in vitro enzyme
test that
can be used to estimate the amounts of a carbohydrate ingredient that are
rapidly
CA 02905812 2015-09-11
WO 2014/158777 PCT/U52014/020105
2
digestible, slowly digestible or resistant to digestion (European Journal of
Clinical
Nutrition (1992) Volume 46 (Suppl. 2), pages S33-S50). Thus, any reference
herein to
"at least about 50% by weight on a dry solids basis" of a material being
slowly
digestible, or to a material being "primarily slowly digestible," means that
the sum of
the percentages that are classified as slowly digestible or as resistant by
the Englyst
assay totals at least about 50%. Likewise, any reference herein to "at least
about 50%
by weight on a dry solids basis" of a material being digestion-resistant, or
to a material
being "primarily digestion-resistant," means that the percentage that is
classified as
resistant by the Englyst assay is at least about 50%.
In one embodiment of the process, the aqueous composition that is produced by
saccharification of starch, followed by isomerization, comprises dextrose,
fructose, and
a mixture of oligosaccharides. This aqueous composition can be nanofiltered to
separate it into the monosaccharide-rich permeate stream and the
oligosaccharide-rich
retentate stream. The oligosaccharide-rich stream can comprise at least about
50% by
weight oligosaccharides on a dry solids basis, or in some cases at least about
90%. In
certain embodiments of the process, the oligosaccharide-rich stream will still
comprise
a minor amount of dextrose and fructose. "A minor amount" is used herein to
mean
less than 50% by weight on a dry solids basis.
The process, 'can, in some embodiments, also include one or more of the
following steps: (1) contacting the oligosaccharide-rich stream with an
isomerization
enzyme, such that at least some of the dextrose is converted to fructose,
thereby
producing an isomerized oligosaccharide-rich stream; (2) membrane filtering
the
oligosaccharide-rich stream to produce a second monosaccharide-rich stream and
a
second oligosaccharide-rich stream that comprises more than about 90% by
weight
oligosaccharides on a dry solids basis as well as a minor amount of
monosaccharides;
(3) hydrogenating the oligosaccharide-rich stream to convert at least some of
the
monosaccharides therein to alcohols, thereby producing a hydrogenated
oligosaccharide-rich stream; (4) contacting the oligosaccharide-rich stream
with a
glucosidase enzyme to create a reversion product such that at least some of
any
residual monosaccharides present in the stream are covalently bonded to
oligosaccharides or other monosaccharides; and (5) reducing the color of the
oligosaccharide-rich stream by contacting it with activated carbon.
Another aspect of the invention is a process for preparing saccharide
oligomers.
The saccharide oligomer composition produced by some embodiments of this
process is
primarily digestion resistant. In other embodiment, the composition is
primarily slowly
digestible. The process uses an aqueous feed composition that comprises at
least one
monosaccharide or linear saccharide oligomer, and that has a solids
concentration of at
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
3
least about 70% by weight. The feed composition is heated to a temperature of
at
least about 400C, and is contacted with at least one catalyst that accelerates
the rate
of cleavage or formation of glucosyl bonds for a time sufficient to cause
formation of
non-linear saccharide oligomers. A product composition is produced that
contains a
higher concentration of non-linear saccharide oligomers than linear saccharide
oligomers.
In one embodiment of the process, the at least one catalyst is an enzyme that
accelerates the rate of cleavage or formation of glucosyl bonds. In
another
embodiment of the process, the at least one catalyst is an acid. In some
embodiments
of the process, acid and enzyme can be used in sequence, with the feed
composition
first being treated with enzyme and subsequently with acid, or vice versa.
Another aspect of the invention is an edible carbohydrate composition
(sometimes referred to herein as an oligosaccharide composition) that
comprises a
major amount of oligosaccharides on a dry solids basis, and that is slowly
digestible or
resistant to digestion by the human digestive system. This composition can be
produced by any of the above-described processes. "Major amount" is used
herein to
mean at least 50% by weight on a dry solids basis.
In one embodiment, the edible carbohydrate composition is produced by a
process in which the oligosaccharide rich stream has a solids content not less
than 70.0
percent mass/mass (m/m), and a reducing sugar content (dextrose equivalent),
expressed as D-glucose, that is not less than 20.0 percent m/m calculated on a
dry
basis. This embodiment of the composition can be classified as corn syrup
under food
labeling regulations. In another embodiment, the oligosaccharide rich stream
has a
solids content not less than 70.0 percent mass/mass (m/m), and reducing sugar
content (dextrose equivalent), expressed as D-glucose, less than 20.0 percent
m/m
calculated on a dry basis. This embodiment can be classified as maltodextrin
under
food labeling regulations.
Another aspect of the invention is an edible carbohydrate composition that
comprises a major amount on a dry solids basis (i.e., greater than 50% by
weight on a
dry solids basis) of linear and non-linear saccharide oligomers, wherein the
concentration of non-linear saccharide oligomers is greater than the
concentration of
linear saccharide oligomers. In some embodiments of the invention, the
concentration
of non-linear saccharide oligomers in the composition is at least twice as
high as the
concentration of linear saccharide oligomers.
Another embodiment is a carbohydrate composition that comprises linear and
non-linear saccharide oligomers, wherein the composition contains about 10-70%
by
weight fiber on a dry solids basis and has a dextrose equivalence of about 25-
65. In
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 4
some embodiments, the composition contains about 30-40% by weight fiber on a
dry
solids basis and has a caloric value of about 2.5-3.5 kcal/g.
The product of this embodiment can be prepared by a process that comprises:
heating an aqueous feed composition that comprises at least one monosaccharide
or
linear saccharide oligomer, and that has a solids concentration of at least
about 70%
by weight, to a temperature of at least about 400C; and contacting the feed
composition with at least one catalyst that accelerates the rate of cleavage
or formation
of glucosyl bonds for a time sufficient to cause formation of non-linear
saccharide
oligomers, wherein a product composition is produced that (a) contains about
10-70%
by weight fiber on a dry solids basis, and (b) has a dextrose equivalence of
about 25-
65. The at least one catalyst can be an acid, such as citric acid,
hydrochloric acid,
sulfuric acid, phosphoric acid, or a combination thereof. In one particular
embodiment,
the acid can be residual acid that is present in the feed composition from
previous
processing. In another embodiment, the at least one catalyst can be an enzyme
that
accelerates the rate of cleavage or formation of glucosyl bonds.
Alternatively, the
composition can be prepared by blending corn syrup (such as, for example, a
low sugar
syrup derived from corn) with a composition prepared by one or more of the
processes
described herein.
Another aspect of the invention is a method of preparing a food product. The
method comprises providing a food composition suitable for combination with a
carbohydrate material, and combining the food composition with an edible
carbohydrate composition that is slowly digestible or digestion-resistant, as
described
above.
Another aspect of the invention is a food product that comprises an edible
carbohydrate composition as described above. The food product can be, for
example, a
bread, cake, cookie, cracker, extruded snack, soup, frozen dessert, fried
food, pasta
product, potato product, rice product, corn product, wheat product, dairy
product,
yogurt, confectionary, hard candy, nutritional bar, breakfast cereal, or
beverage.
In one embodiment of the invention, the food product is selected from baked
foods, breakfast cereal, anhydrous coatings (e.g., ice cream compound coating,
chocolate), dairy products, confections, jams and jellies, beverages,
fillings, extruded
and sheeted snacks, gelatin desserts, snack bars, cheese and cheese sauces,
edible
and water-soluble films, soups, syrups, sauces, dressings, creamers, icings,
frostings,
glazes, pet food, tortillas, meat and fish, dried fruit, infant and toddler
food, and
batters and breadings. The edible carbohydrate composition, which is sometimes
referred to herein as an oligosaccharide composition, can be present in the
food
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
IV
product for one or more purposes, such as a complete or partial replacement
for
sweetener solids, or as a source of dietary fiber.
Another aspect of the invention is a method of controlling blood glucose in a
mammal suffering from diabetes. The method comprises feeding to the mammal a
5 __ food product as described above in various embodiments.
In another aspect of the invention, a carbohydrate composition useful as a
reduced calorie bulking agent and as an ingredient in food products is
provided which
comprises linear saccharide oligomers and non-linear saccharide oligomers, a
sugar
content of from about 5% to about 25% on a dry weight basis, a content of
higher
molecular weight polysaccharides sufficiently low such that the carbohydrate
composition has a viscosity of less than about 16,000 cP at 1000F and 75% dry
solids,
and from about 10% to about 70% fiber on a dry solids basis. The
aforementioned
carbohydrate composition may be prepared by a process comprising blending a
fiber-
containing syrup and a low sugar syrup. The fiber-containing syrup may be
prepared in
__ accordance with any of the above-mentioned processes. The low sugar syrup
may, for
example, have a total DP1 + DP2 content of from about 5% to about 30% by
weight on
a dry solids basis and little or no content of oligosaccharides having a DP
greater than
11 (e.g., not more than about 15% or not more than about 10% by weight on a
dry
solids basis of DP11+).
A still further aspect of the invention provides a carbohydrate composition
(useful as a reduced calorie bulking agent and food ingredient) which is a
blend of a
fiber-containing syrup and a low sugar syrup, wherein the fiber-containing
syrup is
comprised of linear and non-linear saccharide oligomers and contains from
about 10%
to about 80% by weight fiber on a dry solids basis and the low sugar syrup has
a sugar
__ content of from about 5% to about 30% (or from about 10% to about 25%) by
weight
on a dry solids basis and has a DP11+ content not greater than about 15% by
weight
on a dry solids basis. The fiber-containing syrup, provided that it possesses
the
aforementioned compositional characteristics, may be selected from any of the
substances elsewhere referred to herein as "oligosaccharide compositions,"
edible
__ carbohydrate compositions," "corn syrup fiber (CSF)," "oligomer-rich
syrups," "resistant
corn syrups (RCS)" and "digestion-resistant oligomer syrups" and may be
prepared in
accordance with any of the procedures described herein. In this embodiment,
the
fiber-containing syrup and the low sugar syrup may be present in proportions
effective
to impart to the carbohydrate composition a sugar content of from about 5% to
about
__ 25% on a dry solids basis, a content of higher molecular weight
polysaccharides
sufficiently low such that the carbohydrate composition has a viscosity of
less than
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 6 ¨
about 16,000 cP at 1000F and 75% dry solids, and a fiber content of from about
10%
to about 70% on a dry solids basis.
BRIEF DESCRIPTION OF THE DRAWINGS
Figure 1 is a process flow diagram of one embodiment of the present invention.
Figure 2 is a graph of the distribution of certain saccharides in three
dextrose
compositions used in Example 3.
Figure 3 is a graph of the distribution of certain saccharides in the starting
materials used in Example 4.
Figure 4 is a graph of the distribution of certain saccharides in the products
prepared by enzyme treatment in Example 4.
Figure 5 is a graph of the change over time in maltose and isomaltose
concentrations when a composition was treated with enzyme in Example 4.
Figure 6 is a graph of the change in maltose concentration and Figure 7 is a
graph of the change in isomaltose concentration when dextrose syrup was
treated with
different concentrations of enzyme in Example 4.
Figure 8 is a graph of the change over time in the concentrations of certain
saccharides when a composition was treated with enzyme in Example 4.
Figure 9 is a graph of the change over time in the concentrations of certain
saccharides when a diluted composition was treated with enzyme in Example 4.
Figure 10 is a graph of the effect of temperature on the formation of certain
saccharides as a result of enzyme treatment in Example 5.
Figure 11 is a graph of the effect of temperature on the formation of certain
saccharides as a result of another enzyme treatment in Example 5.
Figure 12 is a graph comparing the changes in saccharide distribution when a
composition was treated by acid or by enzyme in Example 6.
Figure 13 shows an analysis of a syrup treated with acid in Example 6.
Figure 14 shows a chromatographic analysis of a syrup treated with acid in
Example 6.
Figure 15 shows the change in blood glucose concentration in dogs after they
were fed either a composition of the present invention or a maltodextrin.
DESCRIPTION OF SPECIFIC EMBODIMENTS
One aspect of the present invention is a process for making a slowly
digestible
or digestion-resistant carbohydrate composition
(e.g., sacchari de oligomer
composition) that is suitable for use in foods.
Both in vitro and in vivo tests can be performed to estimate the rate and
extent
of carbohydrate digestion in humans. The "Englyst Assay" is an in vitro enzyme
test
that can be used to estimate the amounts of a carbohydrate ingredient that are
rapidly
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 7 ¨
digestible, slowly digestible or resistant to digestion (European Journal of
Clinical
Nutrition (1992) Volume 46 (Suppl. 2), pages S33-S50).
It should be understood that the term "food" is used in a broad sense herein
to
include a variety of substances that can be ingested by humans, such as
beverages and
medicinal capsules or tablets.
The terms "oligosaccharides" and "saccharide oligomers" are used herein to
refer to saccharides comprising at least two saccharide units, for example
saccharides
having a degree of polymerization ("DP") of about 2-30. For example, a
disaccharide
has a DP of 2.
The term, "viscosity", as used herein, refers to the resistance of a fluid to
flow.
The viscosity of a syrup is typically affected by temperature and solid
concentration.
Viscosity is expressed in terms of centipoise (cP) at a given temperature and
a given %
DS. Viscosity measurements were taken with a TA Instruments Advanced Rheometer
2000. The instrument was fitted with a concentric cylinder DIN 28mm diameter
bob
and aluminum cup, and all measurements were taken with a 5920 pm gap distance
at
a shear rate of 50 s-1. This shear rate was chosen to assure Newtonian flow
behaviour
with no apparent noise interference. Roughly 20 mL of sample was used for each
experimental run, enough to cover the upper surface of the bob without
overflowing
the cup. A cover for the cup was used during analysis to prevent moisture loss
at
higher sweep temperatures. The procedure consists of a conditioning step to
allow the
material to equilibrate at 20 C, a series of steady state flow measurements
that started
at 20 C and proceeded to 80 C (in increments of 20 C), and a post-experimental
cool
down step for safe handling.
"Glucose syrup" is any liquid starch hydrolysate of mono-, di-, and higher-
saccharides and can be made from any source of starch. The most common sources
of
glucose syrup are corn, wheat, tapioca and potatoes. According to the FDA
(21CFR184.1865), glucose syrup is obtained by partial hydrolysis of starch
with safe
and suitable acids or enzymes. Depending on the degree of hydrolysis, corn
syrup may
contain, in addition to glucose, maltose and higher saccharides. A "corn
syrup" is a
glucose syrup made from corn starch.
The functionality of a syrup depends on its composition. Historically,
Dextrose
Equivalence (DE) has been used to describe the composition of syrups. Dextrose
equivalence (DE) is a measure of the amount of reducing sugars present in a
syrup,
relative to glucose and expressed as a percentage on a dry basis. The DE
describes the
degree of conversion of starch to dextrose and glucose syrups contain a
minimum of
20% reducing sugars (DE>20). The DE gives an indication of the average degree
of
polymerization (DP) for starch sugars and the rule of thumb is DE x DP = 120.
Syrups
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
8
with different ranges of DE (20-38, 38-58, 58-73, >73) are commonly produced
and
sold by the sweetener industry.
The term "sugar," as used herein, is defined as the total of carbohydrates
with
DP1 and DP2 (DP1+2). The carbohydrate compositions of syrups typically range
from
15 to 99% by weight on a dry solids basis of total mono- and di-saccharides
(DP1+2),
with the most widely used syrups containing more than 25% total mono- and di-
saccharides. Syrups with less than 25% sugars are generally very viscous and
not very
sweet and their use is therefore somewhat limited in the food industry. The
high
viscosity of these low sugar syrups make it a challenge to use them due to
issues
around processing such as high resistance to pumping and high adhesiveness to
equipment. The conventional low sugar syrups are also more prone to microbial
contamination.
In some embodiments of the invention, the aqueous feed composition includes
at least one monosaccharide and at least one linear saccharide oligomer, and
may
contain several of each. In many cases, monosaccharides and oligosaccharides
will
make up at least about 70% by weight on a dry solids basis of the feed
composition. It
is generally helpful for the starting material to have as high a concentration
of
monosaccharides as possible, in order to maximize the yield of the desired
oligomers.
A high solids concentration tends to drive the equilibrium from hydrolysis
toward
condensation (reversion), thereby producing higher molecular weight products.
Therefore the water content of the starting material is preferably relatively
low. For
example, in certain embodiments, the feed composition comprises at least about
75%
dry solids by weight. ("Dry solids" is sometimes abbreviated herein as "ds.")
In some
cases, the feed composition comprises about 75 - 90% solids by weight, which
will
generally give the appearance of a viscous syrup or damp powder at room
temperature.
Examples of suitable starting materials include, but are not limited to,
syrups
made, by hydrolysis of starch, such as dextrose greens syrup (i.e., recycle
stream of
mother liquor from dextrose monohydrate crystallization), other dextrose
syrups, corn
syrup, and solutions of maltodextrin.
If the feed composition comprises maltodextrin, the process optionally can
also
include the steps of hydrolyzing the maltodextrin to form a hydrolyzed
saccharide
solution and concentrating the hydrolyzed saccharide solution to at least
about 70%
dry solids to form the feed composition. The concentrating and the contacting
of the
feed with the catalyst can occur simultaneously, or the concentrating can
occur prior to
contacting the feed composition with the catalyst.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
9
The feed composition is contacted with the at least one catalyst for a period
of
time that can vary. In some cases, the contacting period will be at least
about five
hours. In some embodiments of the invention, the feed composition is contacted
with
the at least one catalyst for about 15-100 hours. In other embodiments,
shorter
contacting times can be used with higher temperatures, in some cases even less
than
one hour.
In one embodiment of the invention, enzymatic reversion is used to produce
nonlinear oligosaccharides. The enzyme can be, for example, one that
accelerates the
rate of cleavage of alpha 1-2, 1-3, 1-4, or 1-6 glucosyl bonds to form
dextrose
residues. One suitable example is a glucoamylase enzyme composition, such as a
commercial enzyme composition that is denominated as a glucoamylase. It should
be
understood that such a composition can contain some quantity of enzymes other
than
pure glucoamylase, and it should not be assumed that it is in fact
glucoamylase itself
that catalyzes the desired production of nonlinear oligosaccharides.
Therefore, the feed composition can be contacted with glucoamylase or any
other enzyme that acts on dextrose polymers. The amount of enzyme can suitably
be
about 0.5 ¨ 2.5% by volume of the feed composition. In some embodiments of the
process, the feed composition is maintained at about 55 - 750C during the
contacting
with the enzyme, or in some cases about 60 - 65 C. At this temperature,
depending
on the water content, the material will become a liquid, or a mixture of
liquid and solid.
Optionally, the reaction mixture can be mixed or agitated to distribute the
enzyme.
The reaction mixture is maintained at the desired temperature for the time
necessary
to achieve the desired degree of reversion to non-linear oligomers. In
some
embodiments of the process, the feed composition is contacted with the enzyme
for
about 20-100 hours prior to inactivation of the enzyme, or in some cases, for
about 50-
100 hours prior to inactivation. Techniques for inactivating glucoamylase are
well
known in the field.
Alternatively, instead of inactivating the enzyme, it can be
separated by membrane filtration and recycled.
The resulting composition has a high concentration of non-linear
oligosaccharides, such as isomaltose. This product composition contains a
higher
concentration of non-linear saccharide oligomers than linear saccharide
oligomers. In
some cases, the concentration of non-linear saccharide oligomers in the final
composition is at least twice as high as the concentration of linear
saccharide
oligomers.
Gastrointestinal enzymes readily recognize and digest carbohydrates in which
the dextrose units are linked alpha (1->4) ("linear" linkages). Replacing
these linkages
with alternative linkages (alpha (1->3), alpha (1->6) ("non-linear" linkages)
or beta
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 10 -
linkages, for example) greatly reduces the ability of gastrointestinal enzymes
to digest
the carbohydrate. This will allow the carbohydrates to pass on into the small
intestines
largely unchanged.
In some cases, the product composition comprises a minor amount (i.e., less
than 50 wt % on a dry solids basis, and usually a much lower concentration) of
residual
monosaccharides. The process can include the additional step of removing at
least
some of the residual monosaccharides (and optionally other species as well)
from the
product composition by membrane filtration, chromatographic fractionation, or
digestion via fermentation. The separated monosaccharides can be combined with
-other process streams, for example for production of dextrose or corn syrup.
Alternatively, the separated monosaccharides can be recycled into the feed
composition.
Another embodiment of the invention is a process that involves acid reversion
of
monosaccharides. The starting material is the same as described above with
respect to
the enzyme version of the process. A variety of acids can be used, such as
hydrochloric acid, sulfuric acid, phosphoric acid, or a combination thereof.
In some
embodiments of the process, acid is added to the feed composition in an amount
sufficient to make the pH of the feed composition no greater than about 4, or
in some
cases, in an amount sufficient to make the pH of the feed composition about
1.0 - 2.5,
or about 1.5-2Ø In some embodiments, the solids concentration of the feed
composition is about 70-90%, the amount of acid added to the feed is about
0.05% -
0.25% (w/w) acid solids on syrup dry solids, and the feed composition is
maintained at
a temperature of about 70-900C during the contacting with the acid. As in the
enzyme
version of the process, the reaction conditions are maintained for a time
sufficient to
produce the desired oligomers, which in some embodiments of the process will
be
about 4-24 hours.
In one particular embodiment, the solids concentration of the feed composition
is at least about 80% by weight, acid is added to the feed composition in an
amount
sufficient to make the pH of the composition about 1.8, and the feed
composition is
maintained at a temperature of at least about 800C for about 4-24 hours after
it is
contacted with the acid.
In another particular embodiment, the solids concentration of the feed
composition is about 90-100% by weight, and the feed composition is maintained
at a
temperature of at least about 149 C (3000F) for about 0.1 - 15 minutes after
it is
contacted with the acid. The acid used to treat the feed can be a combination
of
phosphoric acid and hydrochloric acid (at the same concentrations discussed
above).
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 11 ¨
In one particular embodiment, the contacting of the feed composition with the
acid
takes place in a continuous pipe/flow through reactor.
By far the most plentiful glycosidic linkage in starch is the alpha-1,4
linkage,
and this is the linkage most commonly broken during acid hydrolysis of starch.
But
acid-catalyzed reversion (condensation) can take place between any two
hydroxyl
groups, and, given the large variety of combinations and geometries available,
the
probability of an alpha-1,4 linkage being formed is relatively small. The
human
digestive system contains alpha amylases which readily digest the alpha-1,4
linkages of
starch and corn syrups. Replacing these linkages with linkages unrecognized by
enzymes in the digestive system will allow the product to pass through to the
small
intestines largely unchanged.
The saccharide distributions resulting from acid treatment are believed to be
somewhat different than from enzyme treatment. It is believed that these acid-
catalyzed condensation products will be less recognizable by the enzymes in
the human
gut than enzyme-produced products, and therefore less digestible.
The acid treatment progresses differently than enzyme treatment. Enzymes
rapidly hydrolyze linear oligomers and slowly form non-linear oligomers,
whereas with
acid the reduction in linear oligomers and the increase in non-linear
oligomers occur at
comparable rates. Dextrose is formed rapidly by enzymatic hydrolysis of
oligomers,
and consumed slowly as non-linear condensation products are formed, whereas
with
acid dextrose concentrations increase slowly.
Optionally, enzymatic or acid reversion can be followed by hydrogenation. The
hydrogenated product should have lower caloric content than currently
available
hydrogenated starch hydrolysates. In one embodiment, the hydrogenation can be
used
to deodorize the product composition without substantially changing its
dextrose
equivalence (DE).
In one version of the process, enzyme and acid can be used sequentially, in
any
order. For example, the at least one catalyst used in the first treatment can
be
enzyme, and the product composition can be subsequently contacted with an acid
that
accelerates the rate of cleavage or formation of glucosyl bonds.
Alternatively, the at
least one catalyst used in the first treatment can be acid, and the product
composition
can be subsequently contacted with an enzyme that accelerates the rate of
cleavage or
formation of glucosyl bonds.
In an embodiment of the process in which acid treatment is used first,
followed
by an enzyme treatment, the acid can be phosphoric acid, hydrochloric acid, or
a
combination thereof. In this embodiment, after being contacted with the
enzyme, the
composition can be contacted with an ion exchange resin. After being contacted
with
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
12 ¨
the ion exchange resin, the concentration in the composition of saccharide
oligomers
having a degree of polymerization of at least three can be at least about 50%
by
weight on a dry solids basis.
The product composition produced by the treatment with acid, enzyme, or both,
has an increased concentration on a dry solids basis of non-linear saccharide
oligomers.
In some cases, the concentration of non-linear saccharide oligomers having a
degree of
polymerization of at least three (DP3+) in the product composition is at least
about
20%, at least about 25%, at least about 30%, or at least about 50% by weight
on a
dry solids basis. In some embodiments, the concentration of non-linear
saccharide
oligomers in the product composition is at least twice as high as the
concentration of
linear saccharide oligomers.
In one particular embodiment, the concentration of non-linear saccharide
oligomers in the product composition is at least about 90% by weight on a dry
solids
basis, and the concentration of isomaltose is at least about 70% by weight on
a dry
solids basis.
The product composition will often contain some quantity (typically less than
50% by weight on a dry solids basis, and often much less) of residual
monosaccharides. Optionally, at least some of the residual monosaccharides
(and
other species) can be separated from the oligomers (for example, by membrane
filtration, chromatographic separation, or digestion via fermentation) and the
monosaccharide stream can be recycled into the process feed. In this way,
simple
sugar syrups can be converted to high-value food additives.
The oligomer-rich syrup produced by the processes described herein can be
used in foods to increase dietary fiber. The
syrup contains naturally-occurring
oligosaccharides that have both low viscosity and low glycemic index. Many of
these
oligomers will comprise at least one non-alpha-1,4 linkage. They should be
highly
fermentable in the large intestine, which give them added health benefits as
prebiotics.
In some embodiments of the invention, at least about 50% by weight on a dry
solids
basis of the product composition is slowly digestible.
The beneficial effects of oligosaccharides as dietary fiber have been well
documented. Sugar oligomers that resist digestion in the small intestine but
are
fermentable in the large intestine have been shown to have several beneficial
effects,
such as reducing cholesterol, attenuating blood dextrose, and maintaining
gastrointestinal health.
In one embodiment, the product is a carbohydrate composition that comprises
linear and non-linear saccharide oligomers, contains about 10-70% by weight
fiber on a
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 13 ¨
dry solids basis, and has a dextrose equivalence (DE) of about 25-65. Fiber
content
can be measured by AOAC method 2001.03.
In this embodiment, the product can have an intermediate fiber content (i.e.,
higher than conventional corn syrup but lower than some of the compositions of
the
present invention that are described herein). When the feed is derived from
corn, the
product can be referred to as corn syrup fiber (CSF). In one embodiment, the
CSF
product contains about 30-40% by weight fiber on a dry solids basis and has a
caloric
value of about 2.5-3.5 kcal/g. We estimate that a 35% fiber CSF will result in
a caloric
content of 3 kcal/g, which is intermediate between a high fiber resistant corn
syrup at 2
kcal/g and typical digestible carbohydrate at 4 kcal/g. This represents a
caloric
reduction of 25% compared to traditional sugars and starches.
In one embodiment, the process for making this product comprises (1) heating
an aqueous feed composition that comprises at least one monosaccharide or
linear
saccharide oligomer, and that has a solids concentration of at least about 70%
by
weight, to a temperature of at least about 40 C, and (2) contacting the feed
composition with at least one catalyst that accelerates the rate of cleavage
or formation
of glucosyl bonds for a time sufficient to cause formation of non-linear
saccharide
oligomers, wherein a product composition is produced that (a) contains about
10-70%
by weight fiber on a dry solids basis, and (b) has a dextrose equivalence of
about 25-
65.
The catalyst can be acid (such as citric acid, hydrochloric acid, sulfuric
acid,
phosphoric acid, or a combination thereof), enzyme, or a combination of both
acid and
enzyme. The catalyst can be added during the process. Alternatively, in some
situations there can be sufficient residual catalyst (e.g., food grade acid)
present in the
feed as a result of previous processing, so that no further catalyst needs to
be added.
Thus, in one embodiment, the process to make CSF comprises a simple heating
step,
with optional addition of food grade acid. This process can be easily
implemented and
integrated into existing corn syrup refinery operations.
We expect that the fiber fraction of this "direct reversion" CSF product will
be
lower molecular weight, have less complicated branching and will be more
easily
fermentable by colonic microbiota than the fiber fraction in the higher fiber
resistant
corn syrup (RCS). The DE of the CSF product can be targeted to match the DE of
commercial corn syrup products. For example, CSF products with DE
approximately
equal to 26, 35, 43 and 63 would be matches for Staley 200, Staley 300,
Staley
1300 and Sweetosee 4300 traditional corn syrups, respectively.
Alternatively, the product can be prepared by blending conventional corn syrup
(having little or no fiber) with a resistant corn syrup (having a fiber
content of, for
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 14 ¨
example, about 70% or greater). Syrups that are derived from grains other than
corn
can also be used.
One embodiment of the invention provides a fiber-containing carbohydrate
composition having both a low sugar content and a low viscosity which is
useful as a
reduced calorie bulking agent. Such a carbohydrate composition may comprise
linear
saccharide oligomers and non-linear saccharide oligomers, a sugar content
(total DP1 +
DP2 content) of from about 5% to about 25% by weight on a dry solids basis, a
content of higher molecular weight polysaccharides sufficiently low such that
the
carbohydrate composition has a viscosity of less than about 16,000 cP at 1000F
and
75% dry solids, and from about 10% to about 70% fiber on a dry solids basis.
The
carbohydrate composition may contain about 25 to about 40% fiber on a dry
solids
basis. The dextrose equivalence (DE) of the carbohydrate composition may be
from
about 23 to about 30. The carbohydrate composition may, in one embodiment,
comprise about 10% to about 17% sugar (DP1 + DP2) on a dry solids basis. In
another embodiment, the carbohydrate composition may have a viscosity of less
than
about 7,000 cP at 1000F and 75% dry solids. The carbohydrate composition may,
in
certain embodiments of the invention, have a caloric value less than that of a
conventional corn syrup (i.e., a caloric value of less than about 4 kcal/g, as
determined
on a dry solids basis). The caloric value of the carbohydrate composition will
depend
upon its fiber content and may, for example, be at least about 10%, at least
about
20%, or at least about 30% lower than the caloric content of a conventional
corn
syrup. In one particular embodiment, the carbohydrate composition contains
about
30% to about 40% by weight fiber on a dry solids basis and has a caloric value
of from
about 2.5 to about 3.5 kcal/g. In still another embodiment, the
carbohydrate
composition may have a DE of from about 23 to about 30 and a viscosity of less
than
about 7,000 cP at 1000F and 75% dry solids and comprise about 25 to about 40%
fiber
on a dry solids basis and about 10% to about 17% sugar on a dry solids basis.
The aforementioned carbohydrate composition may be prepared by blending a
fiber-containing syrup and a low sugar syrup. Such carbohydrate compositions
typically will have dry solids contents of from about 65% to about 85% by
weight and
thus be in the form of syrups. However, the blend obtained by combining the
fiber-
containing syrup and the low sugar syrup may be dried to provide the
carbohydrate
composition in dry particulate form, for example.
The fiber-containing syrup may be comprised of linear and non-linear
saccharide
oligomers and contain from about 10% to about 80% fiber on a dry solids basis.
Fiber-
containing syrups suitable for such purpose may be made by any of the
processes
described herein. In particular, the fiber-containing syrup may be prepared by
a
CA 02905812 2015-09-11
WO 2014/158777 PCT/115201.1/020105
^, 15 ¨
process comprising heating an aqueous feed composition that comprises at least
one
monosaccharide or linear saccharide oligomer, and that has a solids
concentration of at
least about 70% by weight, to a temperature of at least about 400C
(alternatively, at
least about 600C, at least about 800C or at least about 1000C) and contacting
the feed
composition with at least one catalyst that accelerates the rate of cleavage
or formation
of glucosyl bonds for a time sufficient to cause formation of non-linear
saccharide
oligomers, wherein a product composition is produced that contains about 10%
to
about 70% by weight fiber on a dry solids basis and has a dextrose equivalence
(DE) of
from about 20 to about 35.
The low sugar syrup to be blended with the fiber-containing syrup may suitably
be any syrup having a content of mono- and disaccharides of not more than
about 30%
by weight on a dry solids basis and a relatively low content of higher
molecular weight
polysaccharides, such that the low sugar syrup has a favorably low viscosity.
In certain
embodiments of the present invention, the low sugar syrup is a syrup
comprising water
and saccharides, the saccharides having a saccharide distribution so as to
provide a
DP1 + DP2 content of about 10% to about 30% (or, alternatively, about 10% to
about
25%), a DP3-11 content of about 70% to about 90%, and a DP11+ content of 0% to
about 15%. Advantageously, the low sugar syrup has a viscosity of not more
than
about 16,000 cP at 1000F when the syrup has a dry solids content of 75% or not
more
than about 1500 poise at 200C when the syrup has a dry solids content of 80%.
In
other embodiments, the saccharides have a saccharide distribution so as to
provide a
DP11+ content of not more than 10% or not more than 5%. The low sugar syrup
may
have a DE of from about 20 to about 35. Typically, the low sugar syrup to be
blended
with the fiber-containing syrup has a dry solids content of from about 65% to
about
85% by weight.
In one particular embodiment, the low sugar syrup comprises water and
saccharides, the saccharides having a saccharide distribution of DP1 1-4%; DP2
10-
15%; DP3 9-13%; DP4 7-11%; DP5 6-10%; DP6 13-19%; DP7 12-17%; DP8 4-7%;
DP9 3-7%; DP10 2-6%; DP11 7-15%; DP11+ 0-4%, the total equaling 100%.
The low sugar syrup typically has a relatively low fiber content, e.g., a
fiber
content of less than about 10% or less than about 5% on a dry solids basis.
The low sugar syrup may be produced by contacting a starch or starchy material
such as corn starch with a first alpha amylase enzyme in an aqueous medium for
a
time effective to hydrolyze the starch or starchy material to provide a
reaction product
having a saccharide distribution having a DP1 + DP2 content of about 10% to
about
30% (or about 10% to about 25%), a DP3-11 content of about 70% to about 90%,
and
a DP11+ content of 0% to about 15%. The first alpha amylase enzyme may be a
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
16 -
polypeptide encoded by a nucleic acid having at least 70%, 71%, 72%, 73%, 74%,
75%, 76%, 77%, 78%, 79%, 80%, 81%, 82%, 83%, 84%, 85%, 86%, 87%, 88%,
89%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99%, or more, or complete
(100%) sequence identity to GenBank Accession No. AF504065 or an amino acid
sequence comprising an enzymatically active fragment of said polypeptide.
In one suitable method, a slurry of the starch or starchy material, aqueous
medium and first alpha amylase enzyme is initially jet cooked at a first
temperature of
from about 1000C (2120F) to about 1150C (239 OF) and then maintained at a
second
temperature of from about 800C (176 OF) to about 950C (203 OF) for a time
effective to
provide the desired reaction product. Preferably, the only type of enzyme used
in the
method of producing the low sugar syrup is alpha amylase, in particular an
alpha
amylase in accordance with the above-mentioned description.
Methods of preparing low sugar syrups suitable for use in the present
invention
are described, for example, in U.S. published Patent Application No.
2013/0197104,
incorporated herein by reference in its entirety for all purposes.
The fiber-containing syrup and the low sugar syrup are blended together in
amounts effective to yield a blended carbohydrate composition having the
desired
combination of characteristics, in particular, a desired sugar (DP1 + DP2)
content, a
desired fiber content and a desired viscosity. Increasing the proportion of
low sugar
syrup typically will lower the sugar content and viscosity of the resulting
blend, while
increasing the proportion of fiber-containing syrup typically will increase
the fiber
content of the blend. Generally speaking, however, the fiber-containing syrup
and the
low sugar syrup may be blended in a weight ratio of from about 10:90 to about
50:50
fiber-containing syrup:low sugar syrup.
The above-described product can be used as an ingredient in food products, as
explained in more detail in other parts of this patent application (the blend
or
admixture of fiber-containing syrup and low sugar syrup may be utilized in the
same
way and for the same applications as is described herein for the fiber-
containing syrup
by itself). This product can have one or more benefits. For example, it can
reduce the
caloric content and increase the dietary fiber content of corn syrup, it can
serve as a
"drop in" replacement for traditional corn syrup in foods, it can provide
appropriate
fiber loading in products that use high levels of corn syrup, and it can
provide a more
economical approach to fiber supplementation in food.
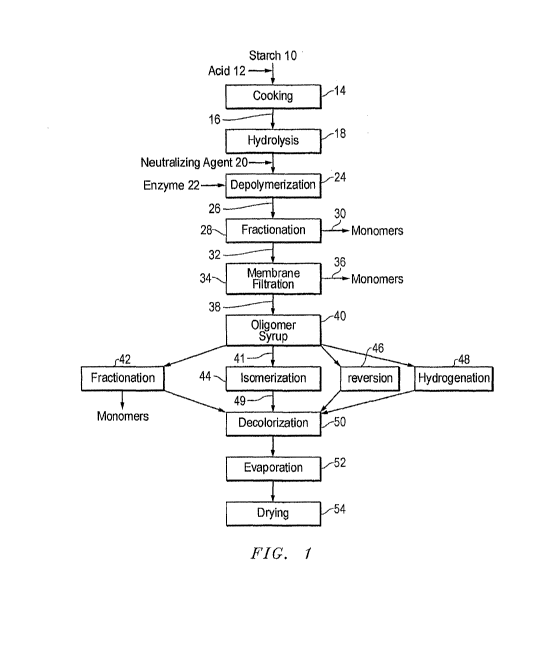
Figure 1 shows one embodiment of a process which can make use of the
reversion technique described above. The process can begin with a starch, for
example
a vegetable starch. Conventional corn starch is one suitable example. The
process will
generally operate more efficiently if the beginning starch has a relatively
high purity.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
In one embodiment, the high purity starch contains less than 0.5% protein on a
dry
solids basis. Although some of the following discussion focuses on corn, it
should be
understood that the present invention is also applicable to starches derived
from other
sources, such as potato and wheat, among others.
As shown in Figure 1, the starch 10 can have acid 12 added to it and can then
be gelatinized 14 in a starch cooker, for example in a jet cooker in which
starch
granules are contacted with steam. In one version of the process, the starch
slurry,
adjusted to a pH target of 3.5 by addition of sulfuric acid, is rapidly mixed
with steam
in a jet cooker and held at 149 to 152 C (300 to 305 F) for 4 minutes in a
tail line.
The gelatinized starch 16 is hydrolyzed 18 by exposure to acid at high
temperature
during jet cooking. The hydrolysis reduces the molecular weight of the starch
and
generates an increased percentage of monosaccharides and oligosaccharides in
the
composition. (As mentioned above, the term "oligosaccharides" is used herein
to refer
to saccharides comprising at least two saccharide units, for example
saccharides having
a degree of polymerization (DP) of about 2-30.) A neutralizing agent 20, such
as
sodium carbonate, can be added to stop the acid hydrolysis, and then the
composition
can be further depolymerized 24 by contacting it with a hydrolytic enzyme 22.
Suitable
enzymes include alpha amylases such as Termamyl , which is available from
Novozymes. This
enzymatic hydrolysis further increases the percentage of
monosaccharides and oligosaccharides present in the composition. The overall
result of
the hydrolysis by acid and enzyme treatment is to saccharify the starch. The
saccharified composition can be isomerized to change the monosaccharide
profile, for
example to increase the concentration of fructose.
The saccharified composition 26 can then be purified, for example by
chromatographic fractionation 28. In one embodiment that employs a sequential
simulated moving bed (SSMB) chromatography procedure, a solution of mixed
saccharides is pumped through a column filled with resin beads. Depending on
the
chemical nature of the resin, some of the saccharides interact with the resin
more
strongly leading to a retarded flow through the resin compared to saccharides
that
interact with the resin more weakly. This fractionation can produce one stream
30 that
has a high content of monosaccharides, such as dextrose and fructose. High
fructose
corn syrup is an example of such a stream. The fractionation also produces a
raffinate
stream 32 (i.e., faster moving components through the resin bed) that has a
relatively
high concentration of oligosaccharides (e.g., about 5 - 15 % oligosaccharides
on a dry
solids basis (d.s.b.)) and also contains a smaller concentration of
monosaccharides
such as dextrose and fructose. Although the term "stream" is used herein to
describe
certain parts of the process, it should be understood that the process of the
present
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
18 ¨
invention is not limited to continuous operation. The process can also be
performed in
batch or semi-batch mode.
The raffinate 32 can be further fractionated by membrane filtration 34, for
example by nanofiltration, optionally with diafiltration. For example, these
filtration
steps can be performed using a Desal DK spiral wound nanofiltration cartridge
at about
500 psi of pressure and at 40 ¨ 60 degrees centigrade temperature. The
fractionation
described in step 34 could also be accomplished by sequential simulated moving
bed
chromatography (SSMB). The membrane filtration produces a permeate 36 (i.e.,
components that pass through the membrane) which comprises primarily
monosaccharides, and a retentate 38 (i.e., components rejected by the
membrane)
which comprises primarily oligosaccharides. ("Primarily" as used herein means
that the
composition contains more of the listed component than of any other component
on a
dry solids basis.) The permeate 36 can be combined with the monomer stream 30
(e.g., high fructose corn syrup). The permeate is a monosaccharide-rich stream
and
the retentate is an oligosaccharide-rich stream. In other words, the
nanofiltration
concentrates the oligosaccharides in the retentate and the monosaccharides in
the
permeate, relative to the nanofiltration feed.
The retentate 38, which can be described as an oligosaccharide syrup 40, can
have a sufficiently high content of oligosaccharides that are slowly
digestible (e.g., at
least about 50% by weight d.s.b., or in some cases at least about 90%) so that
it can
be dried or simply evaporated to a concentrated syrup and used as an
ingredient in
foods. However, in many cases, it will be useful to further process and purify
this
composition. Such purification can include one or more of the following
steps.
(Although Fig. 1 shows four such purification steps 42, 44, 46, and 48 as
alternatives,
it should be understood that two or more of these steps could be used in the
process.)
The oligomers syrup 40 can be subjected to another fractionation 42, such as a
membrane filtration, for example a second nanofiltration, in order to remove
at least
some of the residual monosaccharides, such as fructose and dextrose. Suitable
nanofiltration conditions and equipment are as described above. This
nanofiltration
produces a permeate, which is a second monosaccharide-rich stream, which can
be
combined with the monomer stream 30. Alternatively, the further fractionation
42
could be done by chromatographic separation, for example, by simulated mixed-
bed
chromatography.
The syrup 41 can be isomerized 44 by contacting it with an enzyme such as
dextrose isomerase. This will convert at least some of the residual dextrose
present
into fructose, which may be more valuable in certain situations.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 19 r=-=
As mentioned above, the syrup can be treated with an enzyme or acid to cause
reversion or repolymerization 46, in which at least some of the
monosaccharides that
are still present are covalently bonded to other monosaccharides or to
oligosaccharides,
thereby reducing the residual monomer content of the syrup even further.
Suitable
enzymes for use in this step include glucosidases, such as amylase,
glucoamylase,
transglucosidase, and pullulanase. Cellulase enzymes may produce valuable
reversion
products for some applications.
The syrup can be hydrogenated 48 to convert at least some of any residual
monosaccharides to the corresponding alcohols (e.g., to convert dextrose to
sorbitol).
When hydrogenation is included in the process, it will typically (but not
necessarily) be
the final purification step.
The purified oligomer syrup 49 produced by one or more of the above
purification steps can then be decolorized 50. Decolorization can be done by
treatment
with activated carbon followed by microfiltration, for example. In continuous
flow
systems, syrup streams can be pumped through columns filled with granular
activated
carbon to achieve decolorization. The decolorized oligomer syrup can then
be
evaporated 52, for example to about greater than about 70% dry solids (d.s.),
giving a
product that comprises a high content of oligosaccharides (e.g., greater than
90% by
wt d.s.b., and in some instances greater than 95%), and a correspondingly low
nnonosaccharide content. The product comprises a plurality of saccharides
which are
slowly or incompletely digested by humans, if not totally indigestible. These
sugars can
include isonnaltose, panose and branched oligomers having a degree of
polymerization
of four or greater.
The process conditions can be modified to recover the majority of the maltose
in
the feed either in the monomer-rich streams (30, 36) or in the oligomer
product
stream. For example, a nanofiltration membrane with a slightly more open pore
size,
such as Desal DL, operating at less than 500 psi pressure can be used to
increase the
amount of maltose in monomer-rich streams.
The product is suitable as an ingredient for foods, and is slowly digestible
or
resistant to digestion by the human digestive system. As mentioned above, some
components of the product can be substantially entirely indigestible in the
human
stomach and small intestine. Depending on the starch source used, the product
can be
classified in some embodiments as corn syrup or wheat syrup, as those terms
are used
in food labeling. In cases where more open pore sizes are used in
nanofiltration, a
higher molecular weight oligomer syrup product classified as a maltodextrin
can be
obtained.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 20 ¨
The oligosaccharide-containing syrup produced by the process (sometimes
referred to herein as a digestion-resistant syrup or a fiber-containing syrup)
can be
added to foods as replacement or supplement for conventional carbohydrates (in
one
embodiment of the invention, in admixture with a low sugar syrup). Thus,
another
aspect of the invention is a food product that comprises a carbohydrate
composition
that comprises a major amount on a dry solids basis of linear and non-linear
saccharide
oligomers, wherein the concentration of non-linear saccharide oligomers is
greater than
the concentration of linear saccharide oligomers. Specific examples of foods
in which
the digestion-resistant (fiber-containing) syrup (or its admixtures with a low
sugar
syrup) can be used include processed foods such as bread, cakes, cookies,
crackers,
extruded snacks, soups, frozen desserts, fried foods, pasta products, potato
products,
rice products, corn products, wheat products, dairy products, yogurts,
confectionaries,
hard candies, nutritional bars, breakfast cereals, and beverages. A food
product
containing the oligosaccharide syrup will have a lower glycemic response,
lower
glycemic index, and lower glycemic load than a similar food product in which a
conventional carbohydrate, such as corn starch, is used. Further, because at
least
some of the oligosaccharides are either only digested to a very limited extent
or are not
digested at all in the human stomach or small intestine, the caloric content
of the food
product is reduced. The syrup is also a source of soluble dietary fiber.
The digestion-resistant oligomer syrup described above (optionally in
admixture
with a low sugar syrup, as described elsewhere herein) can be used as an
ingredient in
food products as a syrup, or it can first be concentrated to form syrup
solids. In either
form, it can be used in a number of ways. As mentioned above, this syrup can
be
derived from various starch sources, such as corn. In some instances in this
patent,
the phrase "digestion-resistant corn syrup" or "resistant corn syrup"
(sometimes
abbreviated as "RCS") will be used, but it should be understood that the
invention is
not limited to syrups or syrup solids that are derived from corn.
The digestion-resistant oligomer syrup (in admixture with a low sugar syrup,
in
one embodiment of the invention) can be added to food products as a source of
soluble
fiber. It can increase the fiber content of food products without having a
negative
impact on flavor, mouth feel, or texture.
= The functionality of the digestion-resistant oligomer syrup (and its
admixtures
with the low sugar syrups described herein) is similar to corn syrup and
sugar, which
makes it suitable for complete or partial replacement of various nutritive
sweeteners in
food products. For example, the resistant syrup (and its admixtures with a low
sugar
syrup) can be used for total or partial replacement of sucrose, high fructose
corn syrup
(HFCS), fructose, dextrose, regular corn syrup, or corn syrup solids in food
products,
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 21 ¨
As one particular example, the digestion-resistant syrup or digestion-
resistant syrup
solids can be used to replace other sweetener solids on a 1:1 basis, up to a
complete
replacement of the sugar solids. At high sweetener solids replacement levels,
the
sweetness of the food product could be decreased, but mouth feel and flavor
release
would remain substantially the same, while sugar and calorie content would be
reduced. Also, the digestion-resistant syrup (and its admixtures with a low
sugar
syrup) could be used as a bulking agent, replacing fat, flour, or other
ingredients in a
food formula. Alternatively, the digestion-resistant syrup (or its admixtures
with a low
sugar syrup) can be used in food products in combination with sweeteners such
as
sucrose, HFCS, or fructose, resulting in no change in overall sweetness of the
food
product. As
another example, the digestion-resistant syrup can be used in food
products in combination with sucralose or other high intensity sweeteners
(including
both natural and synthetic high intensity sweeteners), which allows sweetener
replacement with no change in sweetness or mouth feel of the food product.
The digestion-resistant oligomer syrup (including as an admixture with a low
sugar syrup) can be used in food products in combination with resistant
starch,
polydextrose, or other fiber sources, to boost the fiber content of the food
product,
enhance physiological benefit from consumption of the product, reduce the
caloric
content, and/or enhance the nutritional profile of the product.
The digestion-resistant oligomer syrup (and its blends with a low sugar syrup)
can be used in food products in combination with bulking agents, such as sugar
alcohols or maltodextrins, to reduce caloric content and/or to enhance
nutritional
profile of the product. The syrup (and its admixtures with a low sugar syrup)
can also
be used as a partial replacement for fat in food products.
The digestion-resistant oligomer syrup (and its low sugar syrup admixtures)
can
be used in food products as a tenderizer or texturizer, to increase crispness
or snap, to
improve eye appeal, and/or to improve the rheology of dough, batter, or other
food
compositions. The syrup can also be used in food products as a humectant, to
increase
product shelf life, and/or to produce a softer, moister texture. It can also
be used in
food products to reduce water activity or to immobilize and manage water.
Additional
uses of the syrup include: to replace egg wash and/or to enhance the surface
sheen of
a food product, to alter flour starch gelatinization temperature, to modify
the texture of
the product, and to enhance browning of the product.
At least in some embodiments of the invention, the digestion-resistant
oligomer
syrup (and its admixtures with a low sugar syrup) has one or more of the
following
advantages: high solubility, which makes it relatively easy to incorporate
into food
compositions, such as batters and doughs; stability under elevated
temperatures
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
- 22 -
and/or acidic pH (some other soluble fibers, such as inulin, are not as
stable), lower
sweetness, clean flavor, and clear color. The properties of the syrup (and
those of its
admixtures with a low sugar syrup) allow food products in which it is used to
have a
clean label. In some embodiments of the invention, the digestion-resistant
oligomer
syrup contains about 2 calories per gram (d.s.b.), which can reduce the total
calorie
content of a food product.
The digestion-resistant oligomer syrup of the present invention (including
admixtures of the digestion-resistant oligomer syrup and a low sugar syrup, as
described elsewhere herein) can be used in a variety of types of food
products. One
type of food product in which the digestion-resistant (fiber-containing) syrup
and its
low sugar syrup admixtures can be very useful is bakery products (i.e., baked
foods),
such as cakes, brownies, cookies, cookie crisps, muffins, breads, and sweet
doughs.
Conventional bakery products can be relatively high in sugar and high in total
carbohydrates. The use of the digestion-resistant syrup and its low sugar
syrup
admixtures as an ingredient in bakery products can help lower the sugar and
carbohydrate levels, as well as reduce the total calories, while increasing
the fiber
content of the bakery product.
There are two main categories of bakery products: yeast-raised and chemically-
leavened. In yeast-raised products, like donuts, sweet doughs, and breads, the
digestion-resistant oligomer syrup (and its admixtures with low sugar syrup)
can be
used to replace sugars, but a small amount of sugar may still be desired due
to the
need for a fermentation substrate for the yeast or for crust browning.
Digestion-
resistant oligomer syrup solids (e.g., digestion-resistant corn syrup solids)
could be
added in a manner similar to nutritive dry sweeteners, with other dry
ingredients, and
would require no special handling. The resistant corn syrup can be added with
other
liquids (including a low sugar syrup) as a direct replacement for syrups or
liquid
sweeteners. The dough would then be processed under conditions commonly used
in
the baking industry including being mixed, fermented, divided, formed or
extruded into
loaves or shapes, proofed, and baked or fried. The product can be baked or
fried using
conditions similar to traditional products. Breads are commonly baked at
temperatures
ranging from 4200F to 5200F for 20 to 23 minutes and doughnuts can be fried at
temperatures ranging from 400 - 4150F, although other temperatures and times
could
also be used. High intensity sweeteners can be added to doughs as required to
obtain
optimum sweetness and flavor profile.
Chemically leavened products typically have more sugar and may contain have
a higher level of resistant corn syrup/solids. A finished cookie can contain
30% sugar,
which could be replaced, entirely or partially, with resistant corn
syrup/solids. These
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
products could have a pH of 4-9.5, for example. The moisture content can be
between
2-40%, for example.
The resistant corn syrup/solids is readily incorporated and may be added to
the
fat at the beginning of mixing during a creaming step or in a any method
similar to the
syrup or dry sweetener that it is being used to replace. The product would be
mixed
and then formed, for example by being sheeted, rotary cut, wire cut, or
through
another forming process. The products would then be baked under typical baking
conditions, for example at 200-4500F.
The resistant corn syrup/solids can also be used to form sugar glasses in the
amorphous state, to adhere particles to baked goods, and/or used to form a
film or
coating which enhances the appearance of a baked good. Resistant corn syrup
solids,
like other amorphous sugars, form glasses with heating and subsequent cooling
to a
temperature below their glass transition temperature.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup can be used (including as an admixture with a low sugar syrup) is
breakfast
cereal. For example, resistant corn syrup in accordance with the present
invention
could be used to replace all or part of the sugar in extruded cereal pieces
and/or in the
coating on the outside of those pieces. The coating is typically 30-60% of the
total
weight of the finished cereal piece. The syrup can be applied in a spray or
drizzled on,
for example. The formula for the coating can be as simple as a 75% solution of
resistant corn syrup. The resistant corn syrup could also be blended with
sugar at
various percentages, or with other sweeteners or polyols. The extra moisture
could
then be evaporated in a low heat oven. In an extruded piece, the resistant
corn syrup
solids could be added directly with the dry ingredients, or the syrup form
could be
metered into the extruder with water or separately. A small amount of water
could be
added in the extruder, and then it could pass through various zones ranging
from
1000F to 300 F. Optionally, other sources of fiber such as resistant starch
can be used
in the extruded piece. Using the resistant corn syrup would create a different
texture
than other fiber sources. Using it alone or in combination with other fibers
may alter
the texture to create product diversity.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with low sugar syrups) can be used is dairy
products.
Examples of dairy products in which it can be used include yogurt, yogurt
drinks, milk
drinks, flavored milks, smoothies, ice cream, shakes, cottage cheese, cottage
cheese
dressing, and dairy desserts, such as quarg and the whipped mousse-type
products.
This would include dairy products that are intended to be consumed directly
(e.g.,
packaged smoothies) as well as those that are intended to be blended with
other
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 24 ¨
ingredients (e.g., blended smoothie). It
can be used in pasteurized dairy products,
such as ones that are pasteurized at a temperature from 1600F to 285 F.
Complete
replacement of sugars in a dairy product is possible (which would be up to 24%
of the
total formula). The resistant corn syrup is generally stable at acid pH's (the
pH range
of dairy beverages typically would be 2-8).
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with low sugar syrups) can be used is confections.
Examples
of confections in which it can be used include hard candies, fondants, nougats
and
marshmallows, gelatin jelly candies or gummies, jellies, chocolate, licorice,
chewing
gum, caramels and toffees, chews, mints, tableted confections, and fruit
snacks. In
fruit snacks, the resistant corn syrup could be used in combination with fruit
juice. The
fruit juice would provide the majority of the sweetness, and the resistant
corn syrup
would reduce the total sugar content and add fiber. The syrup can be added to
the
initial candy slurry and heated to the finished solids content. The slurry
could be
heated from 200-3050F to achieve the finished solids content. Acid could be
added
before or after heating to give a finished pH of 2-7. The resistant corn syrup
could be
used as a replacement for 0-100% of the sugar and 1-100% of the corn syrup or
other
sweeteners present.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with low sugar syrups) can be used is jams and
jellies. Jams
and jellies are made from fruit. A jam contains fruit pieces, while jelly is
made from
fruit juice. The resistant corn syrup can be used in place of sugar or other
sweeteners
as follows: Weigh fruit and juice into a tank. Premix sugar, resistant corn
syrup and
pectin. Add the dry composition to the liquid and cook to a temperature of 214-
2200F.
Hot fill into jars and retort for 5-30 minutes.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with low sugar syrups) can be used is beverages.
Examples
of beverages in which it can be used include carbonated beverages, fruit
juices,
concentrated juice mixes (e.g., margarita mix), clear waters, and beverage dry
mixes.
The use of the resistant corn syrup of the present invention would in many
cases
overcome the clarity problems that result when other types of fiber are added
to
beverages. A complete replacement of sugars is possible (which could be, for
example,
up to 12% of the total formula). Because of the stability of the syrup at acid
pHs, it
could be used in beverages having pH ranging from 2-7, for example. The
resistant
corn syrup could be used in cold processed beverages and in pasteurized
beverages.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with low sugar syrups) can be used is high solids
fillings.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
rs, 25 ¨
Examples of high solids fillings in which it can be used include fillings in
snack bars,
toaster pastries, donuts, and cookies. The high solids filling could be an
acid/fruit filling
or a savory filling, for example. It could be added to products that would be
consumed
as is, or products that would undergo further processing, by a food processor
(additional baking) or by a consumer (bake stable filling). In some
embodiments of the
invention, the high solids fillings would have a solids concentration between
67-90%.
The solids could be entirely replaced with resistant corn syrup, or it could
be used for a
partial replacement of the other sweetener solids present (e.g., replacement
of current
solids from 5-100%). Typically fruit fillings would have a pH of 2-6, while
savory
fillings would be between 4-8 pH. Fillings could be prepared cold, or heated
at up to
250 F to evaporate to the desired finished solids content.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with low sugar syrups) can be used is extruded and
sheeted
snacks. Examples of extruded and sheeted snacks in which it can be used
include
puffed snacks, crackers, tortilla chips, and corn chips. In preparing an
extruded piece,
the resistant corn syrup/solids would be added directly with the dry products.
A small
amount of water would be added in the extruder, and then it would pass through
various zones ranging from 1000F to 3000F. This dry resistant corn
syrup/solids could
be added at levels from 0-50% of the dry products mixture. The liquid
resistant corn
syrup could also be added at one of the liquid ports along the extruder. The
product
would come out at either a low moisture content (5%) and then baked to remove
the
excess moisture, or at a slightly higher moisture content (10%) and then fried
to
remove moisture and cook out the product. Baking could be at temperatures up
to
500 F for 20 minutes. Baking would more typically be at 3500F for 10 minutes.
Frying
would typically be at 3500F for 2-5 minutes. In a sheeted snack, the resistant
corn
syrup solids could be used as a partial replacement of the other dry
ingredients (e.g.,
flour). It could be from 0-50% of the dry weight. The product would be dry
mixed,
and then water added to form cohesive dough. The product mix could have a pH
from
5 to 8. The dough would then be sheeted and cut and then baked or fried.
Baking
could be at temperatures up to 5000F for 20 minutes. Frying would typically be
at
3500F for 2-5 minutes. Another potential benefit from the use of the resistant
corn
syrup is a reduction of the fat content of fried snacks by as much as 15% when
it is
added as an internal ingredient or as a coating on the outside of a fried
food.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with low sugar syrups) can be used is gelatin
desserts. The
ingredients for gelatin desserts are often sold as a dry mix with gelatin as a
gelling
agent. The sugar solids could be replaced partially or entirely with resistant
corn syrup
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
26
solids in the dry mix. The dry mix can then be mixed with water and heated to
2120F
to dissolve the gelatin and then more water and/or fruit can be added to
complete the
gelatin dessert. The gelatin is then allowed to cool and set. Gelatin can also
be sold in
shelf stable packs. In that case the stabilizer is usually carrageenan-based.
As stated
above, resistant corn syrup can replace up to 100% of the other sweetener
solids. The
dry ingredients are mixed into the liquids and then pasteurized and put into
cups and
allowed to cool and set. The cups usually have a foil top.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with low sugar syrups) can be used is snack bars.
Examples
of snack bars in which it can be used include breakfast and meal replacement
bars,
nutrition bars, granola bars, protein bars, and cereal bars. It could be used
in any part
of the snack bars, such as in the high solids filling, the binding syrup or
the particulate
portion. A complete or partial replacement of sugar in the binding syrup is
possible
with the resistant corn syrup. The binding syrup is typically from 50-90%
solids and
applied at a ratio ranging from 10% binding syrup to 90% particulates, to 70%
binding
syrup to 30% particulates. The binding syrup is made by heating a solution of
sweeteners, bulking agents and other binders (like starch) to 160-2300F
(depending on
the finished solids needed in the syrup). The syrup is then mixed with the
particulates
to coat the particulates, providing a coating throughout the matrix. The
resistant corn
syrup could also be used in the particulates themselves. This could be an
extruded
piece, directly expanded or gun puffed. It could be used in combination with
another
grain ingredient, corn meal, rice flour or other similar ingredient.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with low sugar syrups) can be used is cheese, cheese
sauces, and other cheese products. Examples of cheese, cheese sauces, and
other
cheese products in which it can be used include lower milk solids cheese,
lower fat
cheese, and calorie reduced cheese. In block cheese, it can help to improve
the
melting characteristics, or to decrease the effect of the melt limitation
added by other
ingredients such as starch. It could also be used in cheese sauces, for
example as a
bulking agent, to replace fat, milk solids, or other typical bulking agents.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup/solids (and its admixtures with a low sugar syrup) can be used is films
that are
edible and/or water soluble. Examples of films in which it can be used include
films
that are used to enclose dry mixes for a variety of foods and beverages that
are
intended to be dissolved in water, or films that are used to deliver color or
flavors such
as a spice film that is added to a food after cooking while still hot. Other
film
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 27 ¨
applications include, but are not limited to, fruit and vegetable leathers,
and other
flexible films.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with a low sugar syrup) can be used is soups,
syrups,
sauces, and dressings. A typical dressing could be from 0-50% oil, with a pH
range of
2-7. It could be cold processed or heat processed. It would be mixed, and then
stabilizer would be added. The resistant corn syrup could easily be added in
liquid or
dry form with the other ingredients as needed. The dressing composition may
need to
be heated to activate the stabilizer. Typical heating conditions would be from
170-
2000F for 1-30 minutes. After cooling, the oil is added to make a pre-
emulsion. The
product is then emulsified using a homogenizer, colloid mill, or other high
shear
process.
Sauces can have from 0-10% oil and from 10-50% total solids, and can have a
pH from 2-8. Sauces can be cold processed or heat processed. The ingredients
are
mixed and then heat processed. The resistant corn syrup could easily be added
in
liquid or dry form with the other ingredients as needed. Typical heating would
be from
170-2000F for 1-30 minutes.
Soups are more typically 20-50% solids and in a more neutral pH range (4-8).
They can be a dry mix, to which the dry resistant corn syrup solids could be
added, or
a liquid soup which is canned and then retorted. In soups, resistant corn
syrup could
be used up to 50% solids, though a more typical usage would be to deliver 5 g
of
fiber/serving.
Syrups can incorporate the resistant corn syrup as up to a 100% replacement of
the sugar solids. Typically that would be 12-20% of the syrup on an as-is
basis. The
resistant corn syrup would be added with the water and then pasteurized and
hot filled
to make the product safe and shelf stable (typically 185 F for one minute
pasteurization).
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with a low sugar syrup) can be used is coffee
creamers.
Examples of coffee creamers in which it can be used include both liquid and
dry
creamers. A dry blended coffee creamer can be blended with commercial creamer
powders of the following fat types: soybean, coconut, palm, sunflower, or
canola oil, or
butterfat. These fats can be non-hydrogenated or hydrogenated. The resistant
corn
syrup solids can be added as a fiber source, optionally together with fructo-
oligosaccharides, polydextrose, inulin, maltodextrin, resistant starch,
sucrose, and/or
conventional corn syrup solids. The composition can also contain high
intensity
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 28 ¨
sweeteners, such as sucralose, acesulfame potassium, aspartame, or
combinations
thereof. These ingredients can be dry blended to produce the desired
composition.
A spray dried creamer powder is a combination of fat, protein and
carbohydrates, emulsifiers, emulsifying salts, sweeteners, and anti-caking
agents. The
fat source can be one or more of soybean, coconut, palm, sunflower, or canola
oil, or
butterfat. The protein can be sodium or calcium caseinates, milk proteins,
whey
proteins, wheat proteins, or soy proteins. The carbohydrate can be the
resistant corn
syrup alone or in combination with fructo-oligosaccharides, polydextrose,
inulin,
resistant starch, maltodextrin, sucrose, or corn syrup. The emulsifiers can be
mono-
and diglycerides, acetylated mono- and diglycerides, or propylene glycol
monoesters.
The salts can be trisodium citrate, monosodium phosphate, disodium phosphate,
trisodium phosphate, tetrasodium pyrophosphate, monopotassium phosphate,
and/or
dipotassium phosphate. The composition can also contain high intensity
sweeteners,
such as sucralose, acesulfame potassium, aspartame, or combinations thereof.
Suitable anti-caking agents include sodium silicoaluminates or silica
dioxides. The
products are combined in slurry, optionally homogenized, and spray dried in
either a
granular or agglomerated form.
Liquid coffee creamers are simply a homogenized and pasteurized emulsion of
fat (either dairy fat or hydrogenated vegetable oil), some milk solids or
caseinates,
corn syrup, and vanilla or other flavors, as well as a stabilizing blend. The
product is
usually pasteurized via HTST (high temperature short time) at 185 F for 30
seconds,
or UHT (ultra-high temperature), at 285 F for 4 seconds, and homogenized in a
two
stage homogenizer at 500-3000 psi first stage, and 200-1000 psi second stage.
The
coffee creamer is usually stabilized so that it does not break down when added
to the
coffee.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with a low sugar syrup) can be used is food coatings
such as
icings, frostings, and glazes. In icings and frostings, the resistant corn
syrup can be
used as a sweetener replacement (complete or partial) to lower caloric content
and
increase fiber content. Glazes are typically about 70-90% sugar, with most of
the rest
being water, and the resistant corn syrup can be used to entirely or partially
replace
the sugar. Frosting typically contains about 2-40% of a liquid/solid fat
combination,
about 20-75% sweetener solids, color, flavor, and water. The resistant corn
syrup can
be used to replace all or part of the sweetener solids, or as a bulking agent
in lower fat
systems.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with a low sugar syrup) can be used is pet food,
such as dry
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 29 ¨
or moist dog food. Pet foods are made in a variety of ways, such as extrusion,
forming, and formulating as gravies. The resistant corn syrup could be used at
levels
of 0-50% in each of these types.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with a low sugar syrup) can be used is tortillas,
which usually
contain flour and/or corn meal, fat, water, salt, and fumaric acid. The
resistant corn
syrup could be used to replace flour or fat. The ingredients are mixed and
then
sheeted or stamped and cooked. This addition could be used to add fiber or
extend the
shelf life.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with a low sugar syrup) can be used is fish and
meat.
Conventional corn syrup is already used in some meats, so the resistant corn
syrup can
be used as a partial or complete substitute. For example, the resistant corn
syrup
could be added to brine before it is vacuum tumbled or injected into the meat.
It could
be added with salt and phosphates, and optionally with water binding
ingredients such
as starch, carrageenan, or soy proteins. This would be used to add fiber, a
typical level
would be 5 g/serving which would allow a claim of excellent source of fiber.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with a low sugar syrup) can be used is dried
(infused) fruit.
Many kinds of dried fruit are only stable and palatable if they are infused
with sugar.
The resistant corn syrup can be substituted for all or part of the sugar. For
example,
the resistant corn syrup could be added to the brine used to infuse the fruit
before
drying. Stabilizing agents such as sulfates can be used in this brine as well.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with a low sugar syrup) can be used is infant and
toddler
food. The resistant corn syrup could be used as a replacement or a supplement
to one
or more conventional ingredients for such food. Because of its mild flavor and
clear
color, it could be added to a variety of baby foods to reduce sugar and
increase fiber
content.
Another type of food product in which the digestion-resistant (fiber-
containing)
syrup (and its admixtures with a low sugar syrup) can be used is batters and
breadings, such as the batters and breadings for meat. This could be done by
replacing
all or part of the dry components of the batter and/or breading (e.g., flour
type
ingredients) with the resistant corn syrup, or to use in combination with
addition to the
meat muscle or fried food itself. This could be used as a bulking agent, for
fiber
addition, or to reduce fat in the fried food.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 30 ¨
The process described herein takes advantage of a fraction of the saccharide
syrup (e.g., stream 26 in Fig. 1) that is resistant to saccharification. By
separating this
material as a purified product, it can be employed for its own useful
properties, rather
than being an undesirable by-product in syrups that are primarily
monosaccharides,
such as high fructose corn syrup. Removal of a greater percentage of the
oligosaccharides from the high fructose corn syrup allows that product to be
made
purer (i.e., with a higher concentration of dextrose and fructose) and thus
more
valuable.
Food products of the present invention can also be used to help control the
blood glucose concentration in mammals, such as humans, that suffer from
diabetes.
When the food product is consumed by the mammal, the slowly digestible and/or
digestion resistant components in the food product can cause a more moderate
relative
glycemic response in the bloodstream, which can be beneficial for diabetes
patients.
"Control" in this context should be understood as a relative term; i.e., the
glycemic
response can be improved relative to that occurring when the same mammal
consumes
a similar food product that does not contain such digestion-resistant and/or
slowly
digestible components, although the glycemic response may not necessarily be
equivalent to what would be observed in a mammal that does not suffer from
diabetes.
Certain embodiments of the invention can be further understood from the
following examples.
Example 1
Raffinate syrup was obtained from a plant in which corn starch was being
processed into high fructose corn syrup. The
raffinate was produced by a
chromatographic separation, and comprised primarily fructose and dextrose. The
raffinate was subjected to nanofiltration using a Desal DK1812C-31D
nanofiltration
cartridge at about 500 psi of pressure and at a temperature of 40-600C. The
retentate
from the nanofiltration was decolorized with activated charcoal, and then
evaporated to
approximately 80% dry solids. A saccharide analysis of the dry product was
performed
by HPAE-PAD chromatography, and the results are shown in Table 1.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨
31
Table
Table 1
Component Wt % d.s.b.
dextrose 38.9%
fructose 6.1%
isomaltose 14.3%
maltose 10.5%
maltotriose 0.3%
panose 9.5%
linear higher saccharides 0.0%
nonlinear higher 20.4%
saccharides
This material, termed Light Raffinate, was tested for digestibility using an
Englyst assay. About 600 mg of carbohydrate d.s.b. was added to 20 mL of 0.1 M
sodium acetate buffer in a test tube. The contents were mixed and then heated
to
about 92 C for 30 minutes, then cooled to 37 C. Then 5 mL of enzyme solution
was
added to the test tube and it was agitated by shaking in a water bath at 37
DC. Small
samples were removed at both 20 min and 120 min. The enzyme was inactivated,
the
samples were filtered and measured for digestibility using a glucose test from
YSI Inc.
A Heavy Raffinate, processed in a separate but similar nanofiltration
operation, was
also tested using the same assay. The Heavy Raffinate contained 25-35% dry
solids,
as opposed to 15-25% dry solids for the Light Raffinate, but both had
approximately
the same percentage of low molecular weight saccharides. A cooked potato
starch,
which had not been nanofiltered, was also tested as a comparison. The results
of the
digestibility assay and a saccharide analysis are shown in Table 2. Cooked
potato
starch is included in Table 2 for comparison. All percentages in Table 2 are
on a d.s.b.
Table 2
material % rapidly % slowly Wo resistant % mono- %
oligo-
digestible digestible saccharides saccharides
(by HPAE) (by HPAE)
Light 45 3 52 45 55
raffinate
Heavy 41 3 56 44 56
raffinate
Potato 78 11 11 44 56
starch
(cooked)
There was an excellent correlation between the percentage of oligosaccharides
in the material and the percentage of the material that was resistant to
digestion.
Example 2
About 1,025 L of raffinate syrup at 21.4% dry solids was obtained from a plant
in which corn starch was being processed into high fructose corn syrup. The
raffinate
=
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 32 ¨
was produced by a chromatographic separation, and comprised primarily fructose
and
dextrose. The raffinate was subjected to nanofiltration using two Desal
NF3840C-50D
nanofiltration cartridges at about 500 psi of pressure and at a temperature of
40-600C.
After the starting volume was reduced by about a factor of 20, the retentate
was
subjected to about 2 volumes of constant volume diafiltration using DI water.
After
diafiltration, 27.6 kg of retentate product (at 33.8% ds) was collected. This
material
was decolorized with activated carbon (0.5% by weight of syrup solids) by
stirring in a
refrigerator overnight. This slurry was sterilized by filtration through a
0.45 micron
hollow fiber filtration cartridge, and evaporated in parts to an average
concentration of
about 73% ds.
A saccharide analysis of the dry product was performed by HPAE-PAD
chromatography, and the results are shown in Table 3.
Table 3
Component Wt A) d.s.b.
dextrose 4.5%
fructose 0.9%
isomaltose 20.6%
maltose 23.5%
maltotriose 0.4%
panose 20.9%
linear higher saccharides 0.0%
nonlinear higher 29.1%
saccharides
Example 3 - Preparation of non-linear oligomers from dextrose by enzyme.
Concentrated dextrose syrups having solids concentrations of 74%, 79.5%, and
80% were prepared by (1) evaporating diluted syrup or (2) adding water to
dextrose
powder. Each dextrose/water mixture was placed in a suitable container and
heated to
600C in a water bath.
Glucoamylase enzyme (Dextrozyme or Spirizyme, from Novozynnes A/S) was
added to the syrup ¨ approximately 400 pl enzyme to 30 ml syrup. The syrup
container was capped, and then shaken vigorously to distribute the enzyme. The
syrup
was returned to the 600C water bath.
The change in sugar distribution was monitored over time by transferring 2-4
ml
syrup to a small glass vial, and heating it in a heated block to approximately
85-900C
to deactivate the enzyme.
The concentration of various sugar species was determined by High Performance
Anion Exchange with Pulsed Amperometric Detection, (HPAE-PAD). A Dionex ion
chromatograph, DX500, equipped with electrochemical detector and gradient
pump,
was used for the analyses. The sugars were separated on Dionex Carbopac PA1
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 33 (-
analytical and guard columns with gradient delivery of a sodium hydroxide and
sodium
acetate eluent. The sugars were detected using a gold electrode with a four-
potential
waveform. Samples were diluted with water and passed through Amicon Ultra-4
centrifugal filter devices before analysis.
Figure 2 illustrates the relative amounts of dextrose, isomaltose and "non-
linear
highers" (which in this figure refers to nonlinear oligomers having a degree
of
polymerization of four or more) in syrups of three different initial dextrose
compositions
treated with 1.3% vol/vol Dextrazyme, a commercial glucoamylase enzyme from
Novozymes, for 48 hrs at 600C. As syrup concentration increased, the amount of
monomeric dextrose, relative to other sugars, decreased, and the amount of non-
linear
higher oligomers increases.
Example 4 - Preparation of oligomer syrup from corn syrups.
Starting substrates were obtained having a range of extents of conversion,
from dextrose greens (95% dextrose) to lightly converted Staley 200 syrup (26
DE,
5% dextrose) and including high (34%) maltose syrup, Neto 7300. The specific
products used as starting materials in this example were Staley 200, Staley
300,
Staley 1300, Neto 7300, and Sweetose 4300 corn syrups, and Staleydex 3370
dextrose. Some of the characteristics of these materials are given in Table 4.
Table 4
Characteristics of starting syrups
Staley Staley Staley Neto Sweetose Staleydex
200 300 1300 7300 4300 3370
Degree of
very low low regular regular high
high
conversion
Type of acid- d acid acid- acid- acid-
conversion enzyme enzyme enzyme enzyme
Dextrose
equivalent (D.E.) 26 35 43 42 63 95
%
% dextrose 5 13 19 9 37 90
% maltose 8 10 14 34 29 4
% maltotriose 11 11 13 24 9 2
0/0 higher 76 66 54 33 25
saccharides
While many of the less-converted syrups have substantial quantities of
nonlinear
higher oligomers having a degree of polymerization of four or more (NL DP 4+),
they
also have substantial quantities of linear oligomers. Several of these syrups
contain
measurable linear oligomers up through DP 17. Figure 3 shows the initial
saccharide
distributions.
The enzymes used were Spirizyme Plus FG and Dextrozyme DX 1.5X
glucoamylases and Promozyme D2 pullulanase (supplied by Novozymes), CG 220
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 34 ¨
Cellulase and Transglucosidase L-500 (supplied by Genencor), Glucoamylase
GA150
(supplied by Sunson Industry Group), and Transglucosidase L (supplied by Bio-
Cat
Inc.).
The various corn syrups were adjusted to approximately 70% ds.
Approximately 3.3% (v/v) Spirizyme Plus FG Enzyme was added to each in 50 ml
tubes. The syrups were heated in 600C water baths for approximately 4 days.
The
enzyme was deactivated by heating the syrups to approximately 850C for 10 min.
Figure 4 shows the final saccharide distributions. All the syrups reached a
comparable
sugar distribution by the end of the four day treatment. After reversion, very
little
linear oligomers remained, and non-linear oligomer content had increased.
Several points should be noted. First, the reverted Staleydex 3370 syrup has
a somewhat higher dextrose content and lower content of non-linear oligomers
than
the other syrups. While all syrups were adjusted to approximately 70% ds
before
reversion, the less converted syrups, with low initial dextrose content,
consumed water
as the new distribution was established, and final concentrations were 4-9
percentage
points higher than the reverted 3370 syrup. (The hydrolysis of a single DP6
oligomer
of dextrose to six dextrose molecules, for example, consumes five water
molecules.)
As Table 5 shows, the water contents of the reverted syrups trend with the
dextrose
content, and trend inversely with the higher oligomer content.
Table 5
Concentrations After Reversion, %
Starting Syrup Water Dextrose NL DP4+
Staydex 3370 28 54 23
Sweetose 4300 25 49 27
Neto 7300 21 48 27
Staley 1300 24 48 27
Staley 300 19 47 27
Staley 200 20 46 28
Lower water content drives the equilibrium toward a higher concentration of
reversion products. If the water content had been adjusted so that final water
contents
had been identical, we believe the sugar distributions would also have been
identical.
Second, all syrups after reversion had much higher percentages of branched
oligomers at each degree of polymerization (DP) than linear oligomers. Compare
the
relative amount of maltose vs. isomaltose, panose vs. maltotriose, and NL DP4+
vs.
linear oligomers of DP4 and greater (of which there is virtually none
remaining after
reversion).
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 35 ¨
Figure 5 shows the change in maltose and isomaltose concentrations over time
when a concentrated dextrose syrup was treated with Spirizyme. It would appear
that
linear oligomers are the kinetic products while non-linear oligomers are the
thermodynamic products. That is, forming the linear dimer, maltose, from
dextrose is
a rapid and reversible reaction with low activation energy. Forming the non-
linear
dimer, isomaltose, is a slower reaction, and its reverse reaction has a high
activation
energy.
Figures 6 and 7 show the change in maltose and isomaltose concentrations over
time when 70% dextrose syrup is treated with different concentrations of
Spirizyme
enzyme at 600C.
In the treatment of Staley 1300 syrup with glucoamylase, the linear oligomers
of DP 3 and greater were rapidly consumed and converted to dextrose. The
concentration of these linear oligomers reached its equilibrium of about 1% of
total
sugars (at 70% syrup concentration, 0.13% Spirizyme and 60 C) within the first
few
hours of treatment. (See Figure 8.) Over a longer period, dextrose
concentration
slowly decreased, and the concentration of non-linear oligomers slowly
increased. The
change in concentration of maltose and isomaltose over time mirrors that seen
for
dextrose reversion (Figure 7).
Samples from the above experiments were heated above 85 C for 10-20
minutes to deactivate the enzymes before diluting for ion chromatography
analysis.
Had the samples been diluted in the presence of active enzyme, they might have
been
hydrolyzed back to dextrose.
Samples of the reverted syrups were diluted to 20% solids. A portion of each
was held in the presence of Spirizyme enzyme at 60 C and another portion of
each was
held in the presence of Spirizyme at 400C. The syrups were sampled over time,
and
the enzymes in each sample were deactivated as described above.
Figure 9 shows the results. At 60 C, the concentration of nonlinear higher
oligomers (DP3 and greater) dropped to half within 3 hours and appeared to
plateau at
about 11.6% of total sugars by 7 hours. Lower temperature slowed hydrolysis.
As
Figure 9 shows, dextrose content increased as a result of hydrolysis. The rate
of
hydrolysis when two different glucoamylases (Spirizyme and Dextrozyme) were
used
was identical.
It appears from these experiments that the non-linear oligomers formed
through reversion are not immune to hydrolysis by glucoamylase enzymes (or
impurities therein). However, it appears that a portion of them is
resistant to
hydrolysis. At 20% ds the equilibrium between monomer and oligomer is well on
the
side of monomer. Yet 11.3 % DP4+ and 11.6% DP3+ remain after 7 hours at
optimum
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
- 36 -
temperature for glucoamylase activity. Compare this with the virtually
complete
conversion of linear oligomers to dextrose in the same time frame while at
much higher
solids (70% ds) and half the glucoamylase content, illustrated in Figure 8. It
would
appear that, while glucoamylase enzymes can hydrolyze non-linear oligomers,
the
hydrolysis is not rapid, and may not go to complete conversion. We propose
that the
digestive enzymes in the human gut will have similarly reduced activity
towards these
compounds.
Table =6 shows the change in concentration of all sugar species when reverted
syrup was diluted to 20% ds at 60 C in the presence of active Spirizyme enzyme
Table 6
hr % of total sugars
time
Glucose lsomaltose Maltose Panose Maltoriose L DP3+ NL DP3+ NL DP4+
0 46.7 16.8 4.5 2.4 0.3 1.0 30.5 28.1
1 58.6 18.1 2.0 0.6 0.1 0.6 20.1 19.5
2 64.0 17.0 2.3 0.5 0.1 0.5 15.3 14.9
3 68.6 15.3 2.1 0.4 0.1 0.4 12.8 12.4
4.75 69.6 14.7 2.1 0.3 0.1 0.5 12.2 11.9
7 72.3 13.0 1.9 0.3 0.1 0.5 11.6 11.3
("L DP3+" refers to linear oligomers having a degree of polymerization of
three or
more. "NL DP3" refers to nonlinear oligomers having a degree of polymerization
of
three or more. "NL DP4+" refers to
nonlinear oligomers having a degree of
polymerization of four or more.)
Regardless of starting sugar distribution or degree of conversion, all corn
syrups
tested were converted to a comparable sugar distribution by glucoamylase if
treated at
comparable syrup concentrations.
From these experiments, it appears that during the enzymatic reversion of corn
syrup, linear oligomers are rapidly hydrolyzed to dextrose. Over longer times
and at
high syrup concentrations the dextrose is consumed as non-linear oligomers are
formed. The production of non-linear oligomers is at least partially
reversible, as
evidenced by their hydrolysis by glucoamylase at lower syrup solids. Thus,
when the
reverted syrups are diluted before deactivating the glucoamylase, a portion
of, but
apparently not all of the oligomers are hydrolyzed back to dextrose monomer.
This
demonstrates that the formation of non-linear linkages by glucoamylase (or
perhaps
impurities it contains) is not entirely irreversible "mistakes" by the enzyme.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
- 37 -
Example 5 - Quality of Glucoamylases Impacts Reversion.
The amount of enzyme needed to effect the reversion is high relative to
typical
enzymatic processes. Approximately 1.5% v/v of commonly used glucoamylases
(for
example, Spirizyme Plus FG and Dextrozyme DX 1.5X, supplied by Novozymes) are
needed to reach 80% of equilibrium reversion in 24 hrs at 60-75 C. It should
be noted
that enzyme manufacturers have made great strides in reducing the tendency of
the
glucoamylase to form reversion products - improvements driven by the consumers
of
these enzymes - the manufacturers of corn syrup - for which reversion products
are a
bane. It is our belief that the enzymes from the 1950s would be much more
efficient
for forming these non-linear oligomer syrups than current glucoamylases.
Lending support to the concept that "impurities" still in these commercial
glucoamylases may be responsible for the reversion products in the experiments
reported here is the fact that, while Novozymes reports the optimum
temperature for
activity for both Spirizyme and Dextrozyme to be 59-61 C, the rate of
generation of
reversion products increases when temperature is increased from 60 to 65 C.
Figures
10 and 11 show the rate of formation of isomaltose and non-linear oligomers of
DP 3
and greater (NL DP3+), as a function of temperature, for Spirizyme and
Dextrozyme.
The substrate syrup was Staley 1300, and the amount of enzyme used was 2.7%
v/v.
Example 6 - Acid-catalyzed restructuring of corn syrup to form non-linear
oligomers.
Staley 1300 syrup was diluted 1:4 with deionized water to facilitate pH
determinations. The amount of acid (HCI or H2SO4) to drop syrup pH to the pH
target
was determined. In one experiment, 10% Krystar crystalline fructose was added
to
the syrup prior to acid treatment.
Staley 1300 syrup was heated to approximately 60 C in 50 ml screw-cap
centrifuge tubes in a shaking water bath. The pre-determined amount of acid
needed
to reach target pH was added to the syrup. The syrup tubes were shaken
vigorously to
uniformly distribute the acid. The tubes were returned to the water bath, and
bath
temperature adjusted as needed. Treatments were performed at 60, 70, and 80 C,
and at pHs of 1.2, 1.8 and 2.3. To monitor the progress of the reactions,
portions of
the syrup were removed from tubes and neutralized by adding a caustic
solution.
The caustic solutions were prepared such that a volume of caustic solution was
sufficient to neutralize an equal volume of acidified syrup. Approximately 80%
of this
volume was added all at once, which diluted the syrup sufficiently for pH
measurement.
Additional caustic solution was added dropwise until pH reached >5.0 (and
preferably
no greater than 6.5).
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 38 ¨
The syrup solutions were analyzed using ion chromatography. In addition to a
RSO Oligosaccharide column from Phenomenex, some samples were also analyzed
using a Dionex CarboPac PA200 column.
The first acid condensation reaction on Staley 1300 syrup was at pH 2.3 with
sulfuric acid, at 600C. The proportion of linear oligomers decreased, and non-
linear
oligomers increased.
Figure 12 compares the changes in sugar distributions in Staley 1300 syrup
caused by acid treatment and glucoamylase treatment (both at 600C). It can be
seen
that the processes proceed differently. Spirizyme glucoamylase consumes linear
oligomers very rapidly, generating dextrose. With
Staley 1300 syrup, the
concentration of linear oligomers of DP3 and greater drops from approximately
42% of
total sugars to its equilibrium value of approximately 1% within hours of
contact with
the enzyme. Over a longer period, a portion of the dextrose is converted to
non-linear
oligomers. The concentration of non-linear DP3 and higher (DP3+) increases
over
about 30 hours (under the conditions of this enzyme treatment).
In contrast, on contact with acid, linear oligomers are consumed and non-
linear
oligomers formed at comparable rates. Dextrose concentration increases very
slowly
over the course of the treatment.
In a parallel experiment, 10% dry fructose was added to Staley 1300 syrup, so
that the final syrup solids concentration was approximately 90%. It was
treated to the
same pH, temperature and time as the Staley 1300 syrup by itself. While the
Staley
1300 syrup developed color over the course of the treatment, the fructose-
containing
syrup turned coffee-colored almost immediately. IC analysis of samples pulled
from it
showed the rate of linear oligomers reduction, and non-linear oligomers
generation,
comparable to the acid-treated syrup by itself. Fructose content was not
significantly
altered.
A second round of acid treatments was conducted in which Staley 1300 syrup
was adjusted to 1.2 and 1.8 pH with HCI. Each pH treatment was run at
temperatures
of 700C and 800C. All syrups generated significant color over the course of
the
treatments. The extent of color increased with decreasing pH, increasing
temperature
and increasing time. At the extreme, darkly-colored insoluble components
formed.
As Figure 13 illustrates, the product of acid-treated syrup is a very broad
distribution of sugar oligomers. It
also shows a much higher concentration of
oligomers of DP3 than the enzyme reverted syrup. Also, the acid-treated syrup
contains sugars which do not appear in the enzyme-treated syrup. This is
expected
since the acid-catalyzed condensations can occur between any two hydroxyl
groups,
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
- 39 -
whereas enzymatic condensations are typically very specific in how two sugar
units are
joined together.
A Dionex CarboPac PA200 column was used for ion chromatographic separation
of the sugars. Figure 14 shows a chromatographic trace of an acid-treated
syrup
resolved by this column. It clearly shows four components in the DP2-3 range
that
elute separately from maltose, isomaltose, maltotriose and panose. (These four
all
elute before maltose.) It also shows a number of peaks for unidentified higher
oligomers.
Table 7 below shows changes in sugar distribution over time for these four
lower-pH, higher-temperature treatments, using the PA200 column. (The last
column
in the table shows the amount of the "unknown 1-4" peaks, and is not included
in the
NL DP3+).
Table 7
C hr % of total sugars
pH temp time color Glucose NL DP3+ L DP3+ NL
DP2-3?
1.8 70 0 white 22 23 42 0
1.8 70 4 white 27 27 28 1.7
1.8 70 8 white 28 29 25 2.8
1.8 70 24 white 34 30 13 7.3
1.8 70 48 tan 37 30 4.7 14
1.2 70 0 white 22 23 42 0
1.2 70 4 white 33 30 15 5.9
1.2 70 8 tan 36 30 6.6 12
1.2 70 24 tea 36 30 0.5 20
1.2 70 48 coffee 35 29 0.3 21
1.8 80 0 white 22 23 42 0
1.8 80 4 white 39 28 1.6 18
1.8 80 8 tan 36 29 0.7 21
1.8 80 24 tea 35 30 0.5 20
1.8 80 48 coffee 35 29 0.4 20
1.2 80 0 white 22 23 42 0
1.2 80 4 tan 29 33 18 4.5
1.2 80 8 tea 32 32 11 8.6
1.2 80 24 coffee+insol 37 31 0.5 18
1.2 80 48 coffee+insol 33 32 0.2 21
Example 7 - Enzyme Reversion - High Sugar
Approximately 35 gal of 43 DE corn syrup at 80% dry solids (Staley 1300) with
an additional 5 gal of deionized water was slowly agitated in a tank and
heated to a
temperature of 600C. About 1.6 gal of Spirizyme Plus FG enzyme was added to
the
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
40 ¨
syrup slowly and with good agitation. After 24 hours at 600C, the syrup was
heated to
850C and held for 20 minutes. The syrup was then diluted from 70% to 20% dry
solids
concentration by adding 100 gal water. The
sugar solution was subjected to
nanofiltration using a Desal NF3840C 30D nanofiltration cartridge at about 500
psi of
pressure and at a temperature of 55-600C. Fresh diafiltration water was added
to
maintain permeate flux in the range of 2 to 10 LMH. Filtration continued until
the
retentate contained less than 5% dextrose (d.s.b.) by combination of Karl
Fisher and
YSI dextrose analysis. The nanofiltration retentate was treated with 1%
activated
carbon on a dry solids basis. Next, the carbon was removed by filtration and
the
filtrate evaporated to 80.2% ds.
A saccharide analysis of the final product was performed by HPAE-PAD
chromatography, and the results are shown in Table 8.
Table 8
Component Wt % d.s.b.
dextrose 1.1%
fructose 0.1%
isomaltose 27.7%
maltose 5.2%
maltotriose 0.3%
panose 3.2%
linear higher saccharides 3.3%
nonlinear higher 59.1%
saccharides
("Higher saccharides" in the above table means oligomers having a DP of three
or
more.)
Example 8 - Enzyme Reversion - Low Sugar
Approximately 35 gal of 43 DE corn syrup at 80% dry solids (Staley 1300) with
an additional 5 gal of deionized water was slowly agitated in a tank and
heated to a
temperature of 600C. About 1.6 gal of Spirizyme Plus FG enzyme was added to
the
syrup slowly and with good agitation. After 24 hours at 600C, the syrup was
heated to
850C and held for 20 minutes. The syrup was then diluted from 70% to 20% dry
solids
concentration by adding 100 gal water. The sugar solution was subjected to
ultrafiltration using a Desal UF-1 3840C 50D ultrafiltration cartridge at
about 400 psi of
pressure and at a temperature of 55-600C. Fresh diafiltration water was added
to
maintain permeate flux in the range of 10 to 20 LMH. Filtration continued
until the
retentate contained less than 1% dextrose (d.s.b.) by combination of Karl
Fisher and
YSI dextrose analysis. The ultrafiltration retentate was treated with 1%
activated
carbon on a dry solids basis. Next, the carbon was removed by filtration and
the
filtrate evaporated to 73.4% ds.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 41 ¨
A saccharide analysis of the final product was performed by HPAE-PAD
chromatography, and the results are shown in Table 9.
Table 9
Component Wt % d.s.b.
dextrose 1.0%
fructose 0.1%
isomaltose 6.0%
maltose 7.5%
maltotriose 0.4%
panose 4.4%
linear higher saccharides 7.2%
nonlinear higher 73.3%
saccharides
Example 9 - Enzyme Reversion - High Isomaltose
The syrup from Example 7 was subjected to ultrafiltration using a Desal UF-1
3840C 50D ultrafiltration cartridge at about 400 psi of pressure and at a
temperature of
55-600C. The permeate from this operation was then subjected to nanofiltration
using
a Desal NF3840C 30D nanofiltration cartridge at about 500 psi of pressure and
at a
temperature of 55-600C. Fresh diafiltration water was added to maintain
permeate flux
in the range of 2 to 10 LMH. Filtration continued until the retentate
contained less than
5% dextrose (d.s.b.) by combination of Karl Fisher and YSI dextrose analysis.
The
nanofiltration retentate was treated with 1% activated carbon on a dry solids
basis.
Next, the carbon was removed by filtration and the filtrate evaporated to
90.2% ds.
A saccharide analysis of the final product was performed by HPAE-PAD
chromatography, and the results are shown in Table 10.
Table 10
Component Wt % d.s.b.
dextrose 2.8%
fructose 0.0%
isomaltose 70.8%
maltose 6.5%
maltotriose 0.1%
panose 0.6%
linear higher saccharides 0.0%
nonlinear higher 19.2%
saccharides
Example 10 - Acid Reversion - Moderately Resistant
Approximately 35 gal of 43 DE corn syrup at 80% dry solids (Staley 1300) was
slowly agitated in a tank and heated to a temperature of 800C. About 4.1 lb
37%
hydrochloric acid was added to the syrup slowly and with good agitation. The
reaction
was maintained at approximately 80% dry solids concentration, as measured by
Karl
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
^, 42 ¨
Fischer analysis through periodic additions of water. After 24 hours, heating
was
discontinued and approximately 35 gal of 0.35% sodium hydroxide solution was
added
slowly and with good agitation. Next, pH was adjusted to 5.0 and water was
added to
reach a final sugar concentration of 30% d.s. The sugar solution was subjected
to
ultrafiltration using a Desal UF-1 ultrafiltration cartridge at about 400 psi
of pressure
and at a temperature of 55-60C. Fresh diafiltration water was added to
maintain
permeate flux in the range of 10 to 20 LMH. Filtration continued until the
retentate
contained less than 5% dextrose (d.s.b.) by combination of Karl Fisher and YSI
dextrose analysis. The ultrafiltration retentate was treated with 2% activated
carbon
on a dry solids basis. Next, the carbon was removed by filtration and the
filtrate
evaporated to 71.5% ds.
A saccharide analysis of the final product was performed by HPAE-PAD
chromatography, and the results are shown in Table 11.
Table 11
Component Wt % d.s.b.
dextrose 6.4%
fructose 0.1%
isomaltose 1.6%
maltose 3.8%
maltotriose 4.3%
panose 3.8%
linear higher saccharides 25.6 /o
nonlinear higher 54.9%
saccharides
Example 11 - Acid Reversion followed by Hydrogenation
Approximately 35 gal of 63 DE corn syrup at 80% dry solids (SWEETOSE 4300)
was slowly agitated in a tank. Then 37% hydrochloric acid was added slowly
with good
agitation to give 0.25% (w/w) HCI with respect to syrup dry solids. The
mixture was
then heated to a temperature of 800C. The reaction was maintained at
approximately
80% dry solids concentration, as measured by Karl Fischer analysis through
periodic
additions of water. After 16 hours, heating was discontinued and pH was
adjusted to
4.5 using 0.35% sodium hydroxide solution. Additional water was added to reach
a
final sugar concentration of 30% d.s. The
sugar solution was subjected to
ultrafiltration using a Desal UF-1 ultrafiltration cartridge at about 400 psi
of pressure
and at a temperature of 55-600C. Fresh diafiltration water was added to
maintain
permeate flux in the range of 10 to 20 LMH. Ultrafiltration continued until
the retentate
contained less than 10% dextrose (d.s.b.) by combination of Karl Fisher and
YSI
dextrose analysis. The ultrafiltration retentate was subjected to
nanofiltration using a
Desal NF3840C 30D nanofiltration cartridge at about 500 psi of pressure and at
a
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
=-s, 43 ¨
temperature of 55-600C. Fresh diafiltration water was added to maintain
permeate flux
in the range of 2 to 10 LMH. Filtration continued until the retentate
contained less than
1% dextrose (d.s.b.) by combination of Karl Fisher and YSI dextrose analysis.
The
nanofiltration retentate was treated with 1% activated carbon on a dry solids
basis.
Next, the carbon was removed by filtration and the filtrate evaporated to
73.5% ds.
Dextrose Equivalence (DE) for this product was measured by AOAC method
920.51 (Lane Eynon) and was found to be 21 DE. A saccharide analysis of this
product
was performed by HPAE-PAD chromatography, and the results are shown in Table
12.
Table 12
Component Wt % d.s.b.
dextrose 1.4%
fructose 0.1%
isomaltose 0.0%
maltose 4.3%
sorbitol 0.0%
panose 6.3%
linear higher saccharides 12.6%
nonlinear higher 75.2%
saccharides
This product was further subjected to hydrogenation reaction conditions. About
1.5 kg of a 43% d.s. solution of the material described in Table 9 was
introduced into a
pressure reactor and 6.45 grams of 5% ruthenium on carbon catalyst was added
with
stirring to give 0.05% ruthenium (w/w) on syrup dry solids. The reactor was
closed,
purged with nitrogen gas, and then pressurized with hydrogen gas to a pressure
of 600
psi. The reactor was then heated to 120 C. This temperature and a hydrogen
pressure
of 600-650 psi was maintained for four hours. The reaction vessel was cooled,
carefully
vented and purged with nitrogen. The reaction product was then filtered
through
diatomaceous earth to give a clear colorless solution.
Dextrose Equivalence (DE) for this product was measured by AOAC method
920.51 (Lane Eynon) and was found to be 5 DE. A saccharide analysis of this
product
was performed by HPAE-PAD chromatography, and the results are shown in Table
13.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 44 ¨
Table 13
Component Wt % d.s.b.
dextrose 3.1%
fructose 0.2%
isomaltose 0.0%
maltose 5.9%
sorbitol 3.0%
panose 5.6%
linear higher saccharides 9.5%
nonlinear higher 72.7%
saccharides
Example 12 - Englyst Digestion Assay
The product materials from Examples 7, 8 and 10, were tested for digestibility
using an Englyst assay. About 600 mg of carbohydrate d.s.b. was added to 20 mL
of
0.1 M sodium acetate buffer in a test tube. The contents were mixed and then
heated
to about 92 PC for 30 minutes, then cooled to 37 PC. Then 5 mL of enzyme
solution
was added to the test tube and it was agitated by shaking in a water bath at
37 PC.
Small samples were removed at both 20 min and 120 min. The enzyme was
inactivated; the samples were filtered and measured for digestibility using a
dextrose
test from VS' Inc. A 10 DE maltodextrin (STAR-DRI 10), known to be very
digestible,
was also tested as a comparison. The results of the digestibility assay and a
saccharide
analysis are shown in Table 14. A 10 DE maltodextrin is included in Table 5
for
comparison. All percentages in Table 14 are on a d.s.b.
Table 14
material % rapidly % slowly % non-linear highers
digestible digestible resistant (by HPAE)
Example 7 4.2 10.2 85.6 59.1
Example 8 5.2 10.0 84.8 73.3
Example 10 24.8 5.5 69.8 54.9
10 DE maltodextrin 89.7 3.4 7.0 13.7
("Highers" in Table 14 refers to oligomers having a degree of polymerization
of three or
more.)
There was an excellent correlation (R2 = 0.95) between the percentage of non-
linear highers in the material and the percentage of the material that was
resistant to
digestion.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 45 ¨
Example 13 - Hard Candy, Lemon Flavored
980 grams (d.s.b.) of Example 7 (Enzyme Reversion - High Sugar) was added
to a pot and cooked on a stove to an internal temperature of 3000F. Next, 15
grams of
citric acid and 1.2 grams of sucralose were added with stirring. Then, yellow
color and
lemon flavor were added and the mixture was poured into candy moulds. The hard
candy was formed upon cooling to room temperature.
Example 14 - Jelly Candy, Grape Flavored
840 grams of Example 8 (Enzyme Reversion - Low Sugar) was added to a
mixing bowl. Purple color and grape flavor was added to taste. Next, 160 grams
of
MiraThik 468 instant starch was added in portions with moderately vigorous
mixing.
The jelly candy was formed after cooling to room temperature over 20 minutes.
Example 15 - Yogurt
900 grams of milk (2% fat) was added to a pot on a stove. Next 80 grams
(d.s.b.) of Example 10 (Acid Reversion - Moderately Resistant) was added with
stirring.
Then the mixture was heated to a target temperature of 1500F. As the mixture
was
heating, 20 grams of Rezista 682 starch was added in portions with mixing.
After the
mixture reached an internal temperature of 1500F, it was held for five
minutes, then
passed through a two stage homogenizer (1500/500 psi). The product was next
pasteurized at 1900F for 5 minutes. Then the mixture was cooled to 900F and
inoculated with active yogurt cultures. The incubation was allowed to continue
until the
yogurt reached a pH of 4.5, then it was refrigerated prior to consumption.
Example 16
The following general procedures were used to prepare samples of digestion-
resistant corn syrups in accordance with the present invention. In the
preparation of
some low sugar samples, nanofiltration was run to less than 1% dextrose,
instead of
5% as described in the general procedures below.
Sample 1 - Oligomer Syrup from HFCS Raffinate
1. Transfer mixed raffinate from high fructose corn syrup (HFCS) process to
filtration unit and concentrate volume by 10x to 30x with Desal UF-1 membrane.
Note:
this step is optional, depending on the final DP2 target.
2. Switch filtration membrane to nanofiltration (Desal NF3840C 30D "DL").
Add fresh diafiltration water at a rate to maintain permeate flux in the range
of 2 to 10
LMH. Continue until retentate contains less than 5% dextrose (d.s.b.) by
combination
of Karl Fisher and YSI dextrose analysis.
3. Collect retentate product and add 1% activated carbon on a dry solids
basis. Refrigerate.
4. Remove carbon by filtration and evaporate filtrate to >70% ds.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 46 ¨
Sample 2 - Oligomer Syrup from Dextrose Greens
1. Transfer diluted dextrose greens (at 20-30% ds) to filtration unit and
concentrate volume by 10x to 30x with Desal UF-1 membrane. Note: this step is
optional, depending on the final DP2 target.
2. Switch filtration membrane to nanofiltration (Desal NF3840C 30D "DL").
Add fresh diafiltration water at a rate to maintain permeate flux in the range
of 2 to 10
LMH. Continue until retentate contains less than 5% dextrose (d.s.b.) by
combination
of Karl Fisher and YSI dextrose analysis.
3. Collect retentate product and add 1% activated carbon on a dry solids
basis. Refrigerate.
4. Remove carbon by filtration and evaporate filtrate to >70% ds.
Sample 3 - Enzymatic Reversion of STALEY 1300 Corn Syrup to form >25%
non-linear oligomers of dextrose
1. Pump 35 gal Staley 1300 syrup and 5 gal water to tank. Start agitator
and begin heating.
2. Heat syrup to 600C and confirm that temperature has stabilized at 600C
+/-5C.
3. Add 1.6 gal (6.1 liter) Spirizyme Plus FG enzyme to the syrup.
4. Hold at 600C +/- 5C for 24 hr.
5. At the end of the 600C/24 hr hold, heat syrup to 85-900C. Once syrup
temperature has stabilized above 850C, hold for 20 min.
6. Turn off heat to tank.
7. Dilute syrup from 70% to 20% solids by adding 100 gal water (140 gal
total).
8. Transfer to filtration unit and concentrate volume by 10x to 30x with
Desal UF-1 membrane.
9. Switch filtration membrane to nanofiltration (Desal NF3840C 30D "DL").
Add fresh diafiltration water at a rate to maintain permeate flux in the range
of 2 to 10
LMH. Continue until retentate contains less than 1% dextrose (d.s.b.) by
combination
of Karl Fisher and YSI dextrose analysis.
10. Collect retentate product and add 1% activated carbon on a dry solids
basis. Refrigerate.
11. Remove carbon by filtration and evaporate filtrate to >70% ds.
Sample 4 - Acid-catalyzed restructuring of Tate & Lyle SWEETOSE 4300 Corn
Syrup
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 47 ¨
1. Pump 35 gal SWEETOSE 4300 syrup to tank. Start agitator and begin
heating to 800C.
2. Add ¨2.8 lb 37% hydrochloric acid to the syrup slowly and with good
agitation (calculated to give 0.25% HO dry solids on syrup dry solids in the
reaction,
based on assumption that 4300 syrup density is 11.9 lb/gal).
3. Hold at 80% ds +/- 5%. Remove a reaction sample every two hours and
dilute with an equal weight of DI water. Run Karl Fischer on diluted sample.
If less than
40% ds do nothing. If greater than 40% ds, add 4 lb DI water for every 100 lb
of initial
reaction contents for every 1% ds over 40% ds.
4. In addition to
the above samples for Karl Fischer, collect samples to be
used for monitoring the progress of the reaction. Remove these at the
following
intervals following acid addition: 2 hr, 4 hr, 8 hr, and 16 hr. After each
sampling,
move quickly to adjust the pH of the sample by adding an equal weight of 0.35%
NaOH
solution, mix well, and measure pH. Adjust sample pH as needed to bring to 5.0-
6.5.
5. At the end of
the 800C/16 hr hold, discontinue heating. Add 0.35%
caustic solution, slowly and with good agitation until pH is stable in the
range of 4.5-
5.5.
6. Add dilution
water, if needed, to reach a final solids concentration of 30%
d.s.
7. Transfer to
filtration unit and concentrate volume by 10x to 30x with
Desal UF-1 membrane. Note: this step is optional, depending on the final DP2
target.
8. Switch filtration membrane to nanofiltration (Desal NF3840C 30D "DL").
Add fresh diafiltration water at a rate to maintain permeate flux in the range
of 2 to 10
LMH. Continue until retentate contains less than 5% dextrose (d.s.b.) by
combination
of Karl Fisher and YSI dextrose analysis.
9. Collect retentate product and add 1% activated carbon on a dry solids
basis. Refrigerate.
10. Remove carbon by filtration and evaporate filtrate to >70% ds.
Sample 5 - Phosphoric and Hydrochloric Acid-catalyzed restructuring of
SWEETOSE 4300 Corn Syrup
1. Pump 35 gal SWEETOSE 4300 syrup to tank. Start agitator and begin
heating to 800C.
2. Add ,-0.35 lb 75% phosphoric acid to the syrup slowly and with good
agitation. Then add 0.10 lb 37% hydrochloric acid to the syrup slowly and with
good
agitation (calculated to give 0.08% H3PO4 and 100 ppm HCI dry solids on syrup
dry
solids in the reaction, based on assumption that 4300 syrup density is 11.9
lb/gal).
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 48
3. Hold at 80% ds +/- 5%. Remove a reaction sample every two hours
and
dilute with an equal weight of DI water. Run Karl Fischer on diluted sample.
If less than
40% ds, do nothing. If greater than 40% ds, add 4 lb DI water for every 100 lb
of
initial reaction contents for every 1% ds over 40% ds.
4. In addition to the above samples for Karl Fischer, collect samples to be
used for monitoring the progress of the reaction. Remove these at the
following
intervals following acid addition: 2 hr, 4 hr, 8 hr, and 16 hr. After each
sampling,
move quickly to adjust the pH of the sample by adding an equal weight of 0.35%
NaOH
solution, mix well, and measure pH. Adjust sample pH as needed to bring to 5.0-
6.5.
5. At the end of the 800C/16 hr hold, .discontinue heating. Add 0.35%
caustic solution, slowly and with good agitation until pH is stable in the
range of 4.5-
5.5.
6. Add dilution water, if needed, to reach a final sugar
concentration of 30%
d.s.
7. Transfer to filtration unit and concentrate volume by 10x to 30x with
Desal UF-1 membrane. Note: this step is optional, depending on the final DP2
target.
8. Switch filtration membrane to nanofiltration (Desal NF3840C 30D "DL").
Add fresh diafiltration water at a rate to maintain permeate flux in the range
of 2 to 10
LMH. Continue until retentate contains less than 5% dextrose (d.s.b.) by
combination
of Karl Fisher and YSI dextrose analysis.
9. Collect retentate product and add 1% activated carbon on a dry solids
basis. Refrigerate.
10. Remove carbon by filtration and evaporate filtrate to >70% ds.
Sample 6 - Acid-catalyzed restructuring of Tate and Lyle STALEY 1300 Corn
Syrup
1. Pump 35 gal SWEETOSE 1300 syrup to tank. Start agitator and begin heating
to 800C.
2. Add ,.2.8 lb 37% hydrochloric acid to the syrup slowly and with good
agitation
(calculated to give 0.25% HO dry solids on syrup dry solids in the reaction,
based on assumption that 4300 syrup density is 11.9 lb/gal).
3. Hold at 80% ds +/- 5%. Remove a reaction sample every 2 hours and dilute
with an equal weight of DI water. Run Karl Fischer on diluted sample. If less
than 40% ds do nothing. If greater than 40% ds add 4 lb DI water for every 100
lb of initial reaction contents for every 1% ds over 40% ds.
4. In addition to the above samples for Karl Fischer, collect samples to be
used for
monitoring the progress of the reaction. Remove these at the following
intervals
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 49 ¨
following acid addition: 2 hr, 4 hr, 8 hr, and 16 hr. After each sampling,
move
quickly to adjust the pH of the sample by adding an equal weight of 0.35%
NaOH solution, mix well, and measure pH. Adjust sample pH as needed to bring
to 5.0-6.5.
5. At the end of the 800C/16 hr hold, discontinue heating. Add 0.35% caustic
solution, slowly and with good agitation until pH is stable in the range of
4.5-
5.5.
6. Add dilution water, if needed, to reach a final solids concentration of 30%
d.s.
7. Transfer to filtration skid and concentrate volume by 10x to 30x with Desal
UF-1
membrane. Note: this step is optional, depending on the final DP2 target.
8. Switch filtration membrane to nanofiltration (Desal NF3840C 30D "DL"). Add
fresh diafiltration water at a rate to maintain permeate flux in the range of
2 to
10 LMH. Continue until retentate contains less than 5% dextrose (d.s.b.) by
combination of Karl Fisher and YSI dextrose analysis.
9. Collect retentate product and add 1% activated carbon on a dry solids
basis.
Refrigerate.
10. Remove carbon by filtration and evaporate filtrate to >70% ds.
Some of the syrups prepared by these methods were used in the subsequent
examples, where they are referenced by sample number.
Example 17
A breakfast cereal comprising an oligosaccharide composition according to the
present invention can be prepared as described below. The cereal comprises an
extruded portion and a coating placed on the extruded portion. The composition
of the
extruded portion can be as follows (by weight percent):
Corn Meal 54.80
Whole Wheat Flour 25.19
Resistant Corn Syrup Solids (Sample 5) 13.51
Whole Oat Flour 5.00
Vitamin blend 0.50
Salt 1.00
Total 100.0
The extruded portion is prepared using the following steps: Uniformly mix
ingredients together in a mixer/blender. Feed the dry blend and water to
achieve
target extrusion moisture. Use typical extrusion and drying conditions. Cool
and
package.
The coating composition is a 75% solids solution of 50% sugar, 50% resistant
corn syrup. It is prepared using the following steps: Place spray gun in
convection
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 50 ¨
oven at 250 F to preheat. Weigh out approximately 100 g of cereal and place
into
tumbler that has been first coated with oil based release agent. Blend the dry
ingredients (75% total dry solids) in kettle. Add water and mix. Heat the
syrup to
approximately 230 F (rapid boil). Weight out the desired amount of syrup
needed to
give the correct ratio of cereal:coating to achieve the appropriate ratio
(approximately
45-50% coating by final weight of the cereal). Pour the syrup into the pre-
heated
spray gun and attach the air line hose to the spray gun. As the cereal is
tumbling,
spray the syrup onto the cereal until all of the syrup has been applied. After
desired
amount of coating is applied, allow coated cereal to tumble in enrobing drum
for three
minutes to insure an even coating. Pour the coated cereal out onto a baking
sheet that
has been sprayed with release agent. Dry the cereal in convection oven at 250
F for
six minutes or until cereal appears dry. Stir halfway through drying to
prevent cereal
from sticking to the pan and clumping of the cereal. After drying, allow
cereal to cool
for five minutes. After cooling, weigh the cereal to determine the percent
coating.
Package cereal in plastic storage bag.
Example 18
Yogurt comprising an oligosaccharide composition according to the present
invention was prepared.
The ingredients were:
2% milk 3614
Non fat dry milk (NFDM) 133
Resistant corn syrup (Sample 5) 200
Rezista 682 starch 53
Total weight: 4000 g
The yogurt was prepared using the following steps: Disperse dry ingredients
into
liquid ingredients using a pump and funnel or liquefier. Preheat to 1500F.
Homogenize
at 1500/500 psi using a two stage homogenizer. Pasteurize at 1900F for 5
minutes.
Cool to 900F and add culture. Culture to finished pH 4.4. Stir the product and
begin to
cool to stop active culture growth. Package and cool.
Example 19
A yogurt drink comprising an oligosaccharide composition according to the
present invention was prepared.
The ingredients were:
Skim Milk 94.21
Whey protein concentrate 1.2
Resistant corn syrup (Sample 5) 4.25
Stabilizer blend 0.442
CA 02905812 2015-09-11
W02014/158777 PCT/US2014/020105
¨ 51 ¨
Sucralose solution 0.008
Total 100.0
The yogurt drink was prepared using the following steps: Add dry ingredients
to
liquid using a pump and funnel or liquefier. Preheat to 1500F. Homogenize at
1500/500 psi using a two stage homogenizer. Pasteurize at 1900F for 5 minutes.
Cool
to 900F and add culture. Culture to finished pH 4.4. Break, package and cool.
Example 20
A frozen novelty comprising an oligosaccharide composition according to the
present invention can be prepared as described below.
The ingredients are:
Ingredients ok
Butterfat 1.20%
Milk solids, nonfat 11.75%
(MSNF)
Sucrose 10.70%
Resistant corn 6.70%
syrup (Sample 5)
Whey Protein 34 2.00%
Polydextrose 4,10%
Stabilizer Blend 0.67%
Total Solids 37.12%
Weight per Gallon 9.63 lbs
The frozen novelty can be prepared using the following steps: Standardize
cream, milk and nonfat dry milk to desired butterfat and milk-solids, nonfat
(MSNF)
level. Add the stabilizer to liquid sugar using moderate agitation to ensure
proper
dispersion. Blend milk and liquid sugar portions thoroughly in batch tank.
Incorporate
milk fat solids portion with mix and use low agitation to minimize air
incorporation.
Pasteurize at 185 oF for 30 seconds or the equivalent time and temperature.
Homogenize using a two stage homogenizer at 2500 psi double stage (2000 and
500
psi, first and second stage respectively). Cool mix to 34-38 OF and hold for a
minimum
of four hours for aging. (Overnight aging is preferred).
Example 21
A sugarless ice cream comprising an oligosaccharide composition according to
the present invention was prepared.
The ingredients were:
Butterfat 7 - 12%
Milk Solids Non-fat 10 - 12%
Resistant corn syrup (Sample 5) 12-15%
maltodextrin 3-5%
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 52 ¨
sucralose 0.0085% - 0.012%
vitamin A palmitate 0.009%,
Stabilizer blend 0.40 - 0.50%
The sugarless ice cream was prepared using the following steps: Stabilizer
blend, sucralose, vitamin A and maltodextrin are mixed in skim milk under
shear.
Resistant corn syrup is added to the mixture under shear. Cream (butterfat) is
then
added slowly to avoid churning and aeration. The ice cream is then pasteurized
and
homogenized at 175 F for 30 seconds, 2500 psi 2 stage, respectively. The mix
is
refrigerated overnight (35-400F) and then processed to a frozen state using a
continuous freezing system.
Example 22
Marshmallows comprising an oligosaccharide composition according to the
present invention were prepared.
The ingredients were prepared in three separate parts:
Part A
Gelatin 250 Bloom 22.5
Cold Water 44.5
Part B
Resistant corn syrup (Sample 5, 71%) 337.5
Part C
Hystar Maltitol Syrup 585.5
Total 990g
The marshmallows were prepared using the following steps: Mix ingredients in
Part A (gelatin into water). Preheat resistant corn syrup to 1350F. Heat
maltitol syrup
to 2000F. Combine Parts B and C and cool to 1450F. Melt Part A in microwave
for 30
seconds to dissolve gelatin. Add Part A to other parts and whip mixture with a
wire
whisk in a Hobart mixer until a 0.5 density is reached. Fill marshmallow into
pastry
bags and deposit into starch molds.
Example 23
A hard candy comprising an oligosaccharide composition according to the
present invention was prepared.
The ingredients were:
Sugar 42.0
Resistant Corn Syrup (Sample 4) 43.7
Water 14.3
Total 100.0
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 53 ¨
The hard candy was prepared using the following steps: Mix sugar and resistant
corn syrup with water. Heat to ca. 138 C with Bosch cooker and vacuum for two
minutes to 129 C. Add citric acid (18 g for 3 kg product), and flavor.
Deposit or form
the sweets.
Example 24
A gelatin jelly candy comprising an oligosaccharide composition according to
the
present invention was prepared.
The ingredients were:
Sugar 35.2
Resistant corn syrup (Sample 5, 71%) 36.6
Water 12.3
Gelatin 6.6
Water 9.3
Total 100.0
The gelatin jelly candy was prepared using the following steps: Mix gelatin
and
water and keep at 70 C. Mix sugar, resistant corn syrup, and water. Heat until
solids
reach 89% (approximately 120 C). Cool down to 90 C. Add gelatin solution. Add
citric acid solution 50% (18g/1000g), and flavor and color to suit. Deposit in
molding
starch and dry at ambient conditions to a weight percentage of dry solids (ds)
of 81 -
82%.
Example 25
A jam comprising an oligosaccharide composition according to the present
invention was prepared.
The ingredients were:
Water 36.5
Apricots 32.8
Resistant corn syrup (Sample 5, 71%) 15.5
Maltodextrin 10.2
Pectin (low methoxy) 4.58
Xanthan Gum 0.10
Citric Acid 0.15
Sucralose 0.06
Potassium Sorbate 0.10
Calcium Chloride Ø01
Total 100.0
The jam was prepared using the following steps: Mix dry ingredients. Add dry
ingredients to liquid ingredients and fruit. Heat to 2200F. Put into
containers and cool.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 54 ¨
Example 26
A sweetened children's beverage comprising an oligosaccharide composition
according to the present invention was prepared.
The ingredients were:
Water 86.35
Citric Acid 0.15
Strawberry flavor 0.10
Resistant corn syrup (Sample 5, 73.4%) 13.3
Color (#40, 10%) 0.10
Sucralose 0.004
The drink was prepared using the following steps: Add ingredients slowly into
the water using a mixer. Heat drink to 180 F. Immediately hot fill into
bottles. Place
bottles in a water bath to cool.
Example 27
An orange flavored juice soda beverage comprising an oligosaccharide
composition according to the present invention was prepared.
The ingredients were:
Ingredient
Potassium citrate 0.0200
Acid (citric, malic) 0.2000
RCS (Sample 5, 71% ds) 1.8750
High intensity sweeteners 0.015
(sucralose, Ace-K)
5% Clarified Val 03 Conc., 60.56 Brix 1.0177
Red #40 0.0009
Yellow #5 0.0044
Orange flavor 0.1218
Filtered water 96.7452
100
The orange juice soda was prepared using the following steps: Dry blend the
potassium citrate, acids, resistant corn syrup, and high intensity sweeteners.
Blend
orange juice concentrate, Red #40, Yellow #5, orange flavor and the blend from
the
previous step into the water. Carbonate to desired volume of CO2 (2-4).
Example 28
A savory high solids filling comprising an oligosaccharide composition
according
to the present invention was prepared.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 55 ¨
The ingredients were:
Ingredients Amount (g)
Tate and Lyle texturizing 18.6
blend'
Canola Oil 34
Cheese Flavors 28
Resistant corn syrup 17
solids (Sample 5)
Salt 1.3
Jalapeno Flavors 0.75
Lactic Acid 0.2
Citric Acid 0.15
TOTAL 100
1 Blend of food starch modified, wheat protein, and maltodextrin.
Ingredients were incorporated into the product mixture in the following order:
(1) Canola oil, (2) Flavors, Citric Acid, Lactic Acid and Salt, (3) resistant
corn syrup,
and (4) Tate and Lyle Texturizing Blend.
Example 29
A high solids fruit filling comprising an oligosaccharide composition
according to
the present invention was prepared.
The ingredients were:
Part A
Isosweet 5500 H
FCS 21
Mirathik 603 (food-
modified starch) 6
Part B
resistant corn syrup
(Sample 6) 70.88
water 1.55
nat. and art. rasp. flavor
256639 (tastemaker) 0.3
Part C
malic acid 0.1
citric acid 0.1
red color 09310 (WJ) 0.06
blue color 09918 (WJ) 0.01
100
The jam was prepared using the following steps: Place Part A ISOSWEET 5500
in a Hobart mixer. Slowly add Mirathik 603 while mixing for 1.5 minutes. Add
Part B
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 56 ¨
resistant corn syrup, flavor, and water. Blend until uniform (1 minute). Allow
to rest
for about three minutes until mixture becomes thick. Preblend Part C
ingredients and
add to the mixture. Blend until uniform. Allow filling to set 24 hours to
achieve full
viscosity.
Example 30
A sheeted cracker comprising an oligosaccharide composition according to the
present invention was prepared.
The ingredients were:
Flour 70.949
Resistant corn syrup solids (Sample 5) 17.00
Shortening 10.0
Sucralose 0.001
Sodium bicarbonate 0.70
Salt 0.50
Monocalcium phosphate 0.85
Total 100.00
Amount of water 30
The sheeted cracker was prepared using the following steps: Mix dough until
all
ingredients are wetted and dough is pliable. Sheet dough to 1.1 mm. Cut
pieces.
Bake in convection oven (low fan) at 3500F for five minutes.
Example 31
An expanded extruded snack comprising an oligosaccharide composition
according to the present invention was prepared.
The ingredients were:
Corn flour 75.00
Resistant corn syrup solids (Sample 5) 23.50
Salt 1.50
Total 100.0
The expanded extruded snack was prepared using the following steps: Mix dry
ingredients. Feed dry ingredients into the extruder. Extrude into proper
shapes. Dry
for 10 minutes to 1% finished moisture content.
Example 32
Tortilla chips comprising an oligosaccharide composition according to the
present invention was prepared.
The ingredients were:
Corn Chip #8 flour 23.5
Tortilla Chip #1 flour 24.0
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 57 ¨
Resistant corn syrup (Sample 5) 2.50
water 40.0
Total 100.0
The tortilla chips were prepared using the following steps: Make a 1:1 mixture
of Tortilla Chip #1 flour and Corn Chip #8 flour. Mix on low speed for one
minute in
Hobart mixer. Add resistant corn syrup and mix on low for one minute. With the
mixer
still running on low speed, slowly add room temperature water in a stream to
the dry
mixture. Once all the water is added, increase mixer speed and mix for three
minutes.
Cover dough and let sit for 30 minutes in a plastic beaker. Sheet the dough
using a
Rondo sheeter, and gradually roll dough to have about a 1.3 mm thickness
(testing
thickness by using micrometer). Use the Rondo sheeter, cut the dough using the
cutter by placing the dough horizontally. Fry for approximately 1:45 to 2
minutes
(until chips appear golden brown and bubbling was almost ceased) in a fryer
pre-
heated to 375 F. While chips are frying use a metal spatula to stir the chips
so they
are constantly being submerged on both sides (to help even fat absorption).
Remove
from fryer and let chips drain for four minutes by hanging the basket. Pour
chips onto
a cloth towel and let sit for six minutes. Bag, seal, and label the tortilla
chips in a
plastic bag.
Example 33
A gelatin dessert dry mix comprising an oligosaccharide composition according
to the present invention was prepared.
The ingredients were:
Resistant corn syrup solids (Sample 5) 88.66
Gelatin 250 bloom 9.00
Adipic acid 0.90
Fumaric acid 0.60
Strawberry flavor 0.50
Disodium phosphate 0.20
Color (red #40) 0.14
Sucralose 0.03
The gelatin dessert dry mix was prepared using the following steps: Mix dry
ingredients. Weigh 85.1 g of dry mix and add to 226.8 g of water at 2120F.
Dissolve
completely. Add 226.8 g of cold water and mix thoroughly. Refrigerate at least
four
hours.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 58 ¨
Example 34
A snack bar comprising an oligosaccharide composition according to the present
invention was prepared, comprising a high solids filling, a binding syrup, and
an
extruded piece.
The ingredients for the high solids filling were:
Part A
Resistant corn syrup (Sample 6) 21.00
MiraThik 603 starch 6.00
Part B
Resistant corn syrup (sample 6) 80.88
Water 1.55
Raspberry flavor 0.30
Part C
Malic Acid 0.10
Citric Acid 0.10
Red Color 0.06
Blue color 0.01
Total 100.00
The high solids filling was prepared using the following steps: Place part A,
comprising resistant corn syrup, in a mixer. Slowly add Mirathik 603 while
mixing on
a slow speed for 1.5 minutes. Add part B (resistant corn syrup, flavor, water)
and
blend until uniform (one minute on low speed). Allow to rest for about 3
minutes until
mixture becomes thick. Preblend part C ingredients and add to mixture. Blend
until
uniform (allow filling to set 24 hours to achieve full viscosity.).
The ingredients for the binding syrup were:
Resistant corn syrup (Sample 2) 67.7
Glycerine 10.7
StaSlim 150 starch 13.3
Shortening 7.5
Salt 0.8
Total 100.0
The binding syrup was prepared using the following steps: Combine and heat to
1720F. Add to cereal/granola pieces and combine to coat pieces evenly. Combine
at a
ratio of 54% syrup, 46% cereal.
The ingredients for the extruded piece were:
Corn meal 55.30
Whole wheat flour 25.19
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 59 ¨
Resistant corn syrup (Sample 2) 13.51
Whole oat flour 5.00
Salt 1.00
Total 100.0
The extruded piece was prepared using the following steps: Uniformly mix
ingredients together in a mixer/blender. Feed the dry blend and water to
achieve
target extrusion moisture. Use typical extrusion and drying conditions. Cool
and
package.
Binding syrup is mixed to coat extruded piece or other particulate and mixture
is
sheeted or formed and cut to appropriate size. High solids filling typically
would be
added between the two sheets of binder/particulate mixture.
Example 35
A spice cake comprising an oligosaccharide composition according to the
present
invention was prepared.
The ingredients were:
Ingredient %
water 40.67
Purasnow cake flour 21.56
sorbitol 17.70
RCS solids (Sample 5) 8.85
Mira-Thik 603 food starch modified 1.00
Core M90 (maltodextrin, sucralose) 0.25
EC-25 emulsifier 2.65
Provon 190 whey protein isolate 1.25
HiJel S food starch - modified 0.99
dry egg whites 0.99
salt 0.79
GMS 90 emulsifier 0.59
Baking soda 0.56
Pan 0 Lite 0.45
dry vanilla 1011320 0.40
Dicalcium phosphate dihydrate 0.34
cinnamon 0.29
Sodium propionate 0.21
nutmeg 0.17
xanthan gum 0.12
Durafax 60 emulsifier 0.10
ground cloves 0.07
100
The spice cake was prepared using the following steps:
Dry Mix Procedure:
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
60 ¨
Place RCS, Mira-Thik 603, Core M90, and sorbitol into mixer bowl. Melt EC-25
in microwave taking care not to get it too hot. (Do not melt GMS 90 or Durfax
60).
Add EC-25, mix 5 minutes on speed 1, scraping bowl as needed. Add Durfax 60
while
mixing 1 minute on speed 1, scraping bowl as needed. Add GMS 90 while mixing 1
minute on speed 1, scraping bowl as needed. Run dry mix through a food
processor
for 2 minutes, scraping after each minute. Transfer dry mix back to mixing
bowl. Sift
remaining dry ingredients and add slowly (1 large spoonful at a time) to
sorbitol
mixture while the mixer is running. Mix for a total of 5 minutes on speed 1.
Water Mixing Procedure:
Place dry mix in bowl. Slowly add water while mixing 30 seconds on speed 1.
Scrape bowl. Mix 31/2 minutes on speed 2, scraping bowl as needed. Spray edges
of 8-
inch layer cake pan with non-stick spray cooking oil and use a circular
parchment paper
to line each pan. Pour 450 g batter into each cake pan. Bake at 350 F for 37
minutes
or until done.
Example 36
A cheese sauce comprising an oligosaccharide composition according to the
present invention was prepared.
The ingredients were:
Cheddar 23.41
Butter 5.88
Water 50.50
Sweet Whey 5.44
Disodium phosphate (DSP) 0.73
Trisodium phosphate (TSP) 0.16
Sodium Citrate 0.36
Salt 0.78
MaxiGel 420 starch 2.73
RCS (Sample 5) 9.09
Total 100.0
The cheese sauce was prepared using the following steps: Mix all ingredients.
Heat to 2000F under constant agitation. Hot
fill the cheese sauce into jars or
containers and seal with lid or closure. Cool to 400F.
Example 37
= A block of imitation mozzarella cheese comprising an oligosaccharide
composition according to the present invention was prepared.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 61 ¨
The ingredients were:
Weight
Percent in g
Rennet Casein 19.494 974.70
Sorbic Acid 0.2964 14.82
Whey Powder 1.4288 71.44
Soybean Oil 20.121 1006.05
Salt 2.0007 100.04
Sodium Citrate 2.09 104.50
Lactic Acid (liquid) 1.2692 63.46
StaSlim 151 starch 3.42 171.00
Resistant Corn Syrup
(Sample 5, 71% ds) 4.75 237.50
Trisodium phosphate
(TSP) 0.76 38.00
Water 44.3699 2218.50
Total 55.6301 2781.51
The cheese was prepared using the following steps: Add water, sodium citrate,
casein, and soybean oil (120 g). Blend for five min. Add remaining soybean
oil. Add
sorbic acid, salt, starch, resistant corn syrup. Then add whey and lactic
acid. Blend for
five min. Add remaining ingredients. Cook to 1850F.
Example 38
An edible film comprising an oligosaccharide composition according to the
present invention was prepared. Without being bound by theory, it is believed
that the
oligosaccharide composition served as a plasticizer in the edible film.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 62 ¨
The ingredients were:
SOLIDS: Grams
Pullulan (PI-20) 21.252
Star-Dri 1005A
maltodextrin 1.65
RCS (Sample 5,
71% solids) 3.3
Polysorbate 80 0.165
Na Benzoate 0.033 _
TOTAL 26.4
FILM:
Grams
Solids 26.4
Water 83.6_
TOTAL 110
color/flavor mix 22
The edible film was prepared using the following steps:
Dispersion of Ingredients
Mix pullulan and maltodextrin in a beaker with a whisk. Mix water, polysorbate
80, sodium benzoate, and resistant corn syrup (RCS) in a separate beaker. Use
a
Servodyne Mixer Head model 50003-30 to further mix the wet ingredients. Start
with
RPM at 700. Slowly add in the dry flavor mix. When all the lumps are gone,
slowly
add in the pullulan mixture. Adjust the RPM as necessary when the mixture
thickens
(up to 1,000 RPM). When all the dry ingredients are
in, stop the mixer and scrape the
sides of the beaker. Turn up the mixer to 1,000 RPM and mix for 2 more
minutes.
Pour 50 g into centrifuge tubes. Centrifuge for 10 minutes to remove air.
Filming Procedure
Films were drawn using a Gardco adjustable drawdown set at 0.045 in. These
drawdowns were adjusted to the proper thickness using
feeler gauge blades. Films
were drawn onto Mylar with the use of a vacuum plate. The films were dried in
an
environmental chamber at 650C and 25% RH for two hours. They were cured in the
environmental chamber at 250C and 28% RH overnight. The dried films were
packaged into plastic bags.
Example 39
A low fat pound cake comprising an oligosaccharide composition according to
the present invention was prepared.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 63 ¨
The ingredients were:
Ingredient
Part A
Cake flour 28.81
RCS Solids (Sample 5) 26
Water 16.27
GMS-90 Emulsifier 5.92
dextrose 4.17
Non-fat dry milk, high
heat 1.6
STA-SLIM 150 starch 1.29
STA-SLIM 142 starch 0.64
Salt 0.63
Leavening acid, Pan-0-
Lite 0.5
Baking soda 0.5
Vanilla Flavor #464174 0.45
Annatto color 0.1
Xanthan 0.09
Part B
Liquid egg whites 8.4
Water 4.63
100
The pound cake was prepared using the following steps: Blend dry ingredients
of Part A in a Hobart mixer at speed 1. Add GMS-90 emulsifier and blend for 2
minutes
(speed 1). Add water and Annatto color and blend 4 minutes (speed 2). Scrape
bowl
and paddle after 2 minutes of mixing and at end of mixing. Mix Part B
ingredients
together. Add in 1/3 of the Part B egg white/water mixture to Part A and blend
for 1
minute (Speed 2). Scrape bowl and paddle after mixing. Repeat first step for
Part B
= twice to incorporate remaining 2/3 of egg white/water mixture. Pour 200
grams of
batter into a loaf pan pre-coated with non-stick spray. Bake at 350 F for 30
minutes.
Example 40
Oatmeal chocolate chip raisin cookies having polyol levels and comprising an
oligosaccharide composition according to the present invention were prepared.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
64 -
The ingredients were:
Formula
Ingredients Percent Baker's
Percent
Vream Rite Shortening 12.50 50.40
BAKERY REBALANCE 706 (Tate & Lyle) 9.00 36.29
STA-LITE III polydextrose (Tate & Lyle) 5.00 20.16
Sorbitol (Sorbogem fines) 3.00 12.10
NutraFloraC) scFOSC) (Fructo
Oligosaccharide) 4.50 18.15
Resistant Corn Syrup (Sample 4) 3.00 12.10
Salt 0.50 2.02
Cinnamon 0.30 1.21
Cinnamon flavor 0.25 1.01
Oatmeal cookie flavor 0.25 1.01
Vanilla flavor 0.25 1.01
Dry egg 0.90 3.63
Water 9.00 36.29
Glycerine 1.25 5.04
Pastry flour 24.80 100.00
Quick rolled oats 12.40 50.00
Baking soda 0.40 1.61
Pan-O-Lite 0.20 0.81
Chopped walnuts 6.00 24.19
Raisins 6.50 26.21
Total 100.00 403.23
The oatmeal raisin cookies were prepared using the following steps: Mix
shortening and flavors in a N-50 Hobart mixer at speed 1 for 30 seconds. Add
the
remaining stage 1 ingredients. Mix at speed 1 for 1 min. Scrape the sides of
the bowl.
Mix at speed 2 for 1 min. Add the stage 2 ingredients. Mix at speed 1 for 1
min.
Scrape the sides of the bowl. Mix at speed 2 for 1 min. Add the stage 3
ingredients.
Mix at speed 1 for 1 min 30 sec. Scrape the sides of the bowl. Repeat mix at
speed 1
for 1 min 30 sec. Add the stage 4 ingredients. Mix at speed 1 for 15 sec.
Weigh 30 g
dough piece onto a parchment with double-lined baking pans. Bake 12 cookies in
convection oven at 375 F for 11 min.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 65 ¨
Example 41
Soft chocolate cookies comprising an oligosaccharide composition according to
the present invention were prepared.
The ingredients were:
Ingredient %
Flour, pastry 28.70
Resistant corn syrup solids (Sample
5) 22.20
Butter 20.40
RCS (Sample 5, 71% ds) 10.90
Eggs, whole 9.10
Natural cocoa N-11-N 3.60
Lightly alkalized cocoa D-11-A 2.00
Instant TENDER-3EL C food starch
modified 1.90
Vanilla flavor 0.46
Salt 0.44
Baking soda 0.30
100.00
The cookies were prepared using the following steps: Blend sugar/RCS Solids,
butter, and RCS (71% ds) in Hobart mixing bowl on speed 1. Add egg. Dry blend
remaining ingredients and add to this mixture. Bake at 350 F for 15 minutes.
Example 42
A maple syrup comprising an oligosaccharide composition according to the
present invention was prepared.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 66 ¨
The ingredients were:
Water 80.132
Resistant Corn Syrup Solids (Sample 5) 17.00
Cellulose Gum 1.00
Maple Flavor 0.45
Salt 0.45
SPLENDA sucralose 0.35
Guar Gum 0.28
Phosphoric Acid (85%) 0.15
Caramel Color 0.13
Sodium Hexameta Phosphate 0.05
Butter Flavor 0.008
Total 100.00
The maple syrup was prepared using the following steps: Add sucralose,
preservatives, salt, flavoring, and color to water using slow speed in
standard mixer.
Slowly add gums to mixture, allowing to hydrate 20-25 minutes. Blend in
resistant
corn syrup solids, while heating to 1850F. Hold for one minute. Remove heat
and add
acid. Fill containers at 180-1850F and invert for one minute. Cool to 750F.
Example 43
A barbeque sauce comprising an oligosaccharide composition according to the
present invention was prepared.
The ingredients were:
Part A
Tomato Paste 27.23
Water 14.7
Apple Cider Vinegar 15.13
Resistant corn syrup (Sample 5, 71%) 33.73
Molasses 5.04
Liquid Hickory Smoke 0.30
Caramel Color 0.21
Part B
Salt 2.02
Spice Blend 1.65
Sucralose 0.014
CA 02905812 2015-09-11
WO 2014/158777 PCT/1JS2014/020105
¨ 67 ¨
The barbeque sauce was prepared using the following steps: Heat Part A
ingredients on to 1900F. Add dry ingredients to part A and heat for 15 minutes
at
2000F. Hot fill containers and cool.
Example 44
A French dressing comprising an oligosaccharide composition according to the
present invention was prepared.
The ingredients were:
Soybean oil 9.00
Resistant corn syrup (Sample 5, 71%) 47.57
Vinegar, 120 grain 12.00
Water 18.59
Tomato Paste 7.00
Salt 2.00
MiraThik 603food starch modified 2.00
Polysorbate 60 0.20
Onion Powder 0.18
Garlic Powder 0.15
Xanthan Gum 0.10
Sorbic Acid 0.10
Oleoresin Paprika 0.10
EDTA 0.01
Total 100.0
The French dressing was prepared using the following steps: Place water and
resistant corn syrup in container. Dry mix onion, salt, garlic, sorbic acid,
and EDTA and
add to water mixture. Slurry starch and xanthan gum in small amount of oil,
add to
water mixture, and mix for five minutes to allow starch to hydrate. Add tomato
paste
and paprika. Add vinegar. Melt polysorbate 60 and add to mixture slowly. Add
remaining oil and mix five minutes.
Process through a colloid mill at 0.26" (2
revolutions).
Example 45
A cream of chicken soup concentrate comprising an oligosaccharide composition
according to the present invention was prepared.
The ingredients were:
Water 65.65
Chicken Bouillon 11.30
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
68 ¨
Resistant Corn Syrup Solids (Sample 5) 11.00
Half & half 5.60
Rezista Starch 3.10
Titanium Dioxide 1.00
Salt 0.50
Sugar 0.16
Spices 0.69
Xanthan Gum 0.10
Total 100.00
The cream of chicken soup concentrate was prepared using the following steps:
Mix dry ingredients. Mix liquid ingredients for 3-5 minutes. Add dry
ingredients slowly
using lightning mixer on medium speed. Mix 3-5 minutes ensuring even
dispersion.
Heat to 1900F without stirring. Hold for 5 minutes. Fill
hot intb cans, seal
immediately. Retort at 2500F for 40 minutes. Cool cans to room temperature. To
serve, add one can soup to equal volume of 2% milk. Mix well. Heat to simmer
(approximately 10 minutes). Serve hot.
Example 46
A ketchup comprising an oligosaccharide composition according to the present
invention was prepared.
The ingredients were:
Tomato Paste 37.54
Resistant Corn Syrup Solids (Sample 5) 12.01
Water 41.37
Vinegar 120 grain 7.01
Garlic Powder 0.02
Onion Powder 0.03
Smoke Flavor 0.001
Salt 2.00
Sucralose (dry) 0.02
The ketchup was prepared using the following steps: Dry mix spices, RCS,
sucralose and salt. Mix water, vinegar, and dry mix using a lightening mixer.
Add
smoke flavor to wet mix. Blend tomato paste and 1/4 of the wet mix (water,
vinegar,
and dry mix) in a Hobart mixer with paddle attachment on speed 1 for 2
minutes.
Blend in the remainder of the wet mix on speed 1 for 1 minute. Stop and scrape
the
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 69 ¨
bowl well. Continue blending on speed 1 for 1 minute. Heat ketchup to 105 C
and
hold for 15 seconds. Cool to 80 C. Homogenize using Panda homogenizer at
150/50
bars. Immediately package in glass jars.
Example 47
A beef-flavored gravy mix comprising an oligosaccharide composition according
to the present invention was prepared.
The ingredients were:
Water 90.17
Perma-Flo Starch 3.58
Beef Flavors 3.25
Resistant Corn Syrup solids (Sample 5) 10.00
Sugar 0.43
Sweet Dairy Whey 0.42
Caramel Color 0.09
Spices 0.03
Total 100.0
The beef-flavored gravy mix was prepared using the following steps: Blend dry
ingredients and TALO TF-55 flavoring (all ingredients except water) until
uniformly
blended. Using a wire whisk, disperse this dry mix into cold water. Cook with
agitation
to 190 F. Hold mixture at 190 F with agitation for 10 minutes.
Example 48
A dry blended coffee creamer comprising an oligosaccharide composition
according to the present invention was prepared.
The ingredients were:
Commercial creamer powder (Jerzee blend 220077) 21.8
Resistant Corn Syrup solids (Sample 5) 78.2
The dry blended coffee creamer was prepared using the following steps: the
ingredients are blended, scaled and screened through a 10 mesh screen into a
tumble
blender vessel, ribbon blender, or paddle blender. The formulation is blended
from 10
to 25 minutes and packaged. Silica dioxide or sodium silicoaluminate can be
added as
anti-caking agents if required.
Example 49 =
A soy-based dry coffee creamer powder slurry comprising an oligosaccharide
composition according to the present invention was prepared.
The ingredients were:
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 70 ¨
% Dry
pounds solids comp formula
Hydrogenated
Soybean oil 1050F 65 65 50.67% 23.50% 48.64
Sodium Caseinate 4.1 3.895 3.04% 1.48% 3.07
Resistant corn syrup
solids (Sample 5) 61.47 58.4 45.52% 22.23% 46.00
Alphadign 70K Mono-
and diglycerides 0.5 0.5 0.39% 0.18% 0.37
BFP 75K mono- and
diglycerides 0.5 0.5 0.39% 0.18% 0.37
water 145 0 0.00% 52.43% 108.50
276.57 128.3 100.00% 100.00% 206.95
Yield @ 40/0
Total solids 46.39% moisture 100.00
The water is added to a batch tank and is heated to 120 to 140 F. The sodium
caseinate is added to the water and allowed to hydrate for 10 to 30 minutes.
The
mono and diglycerides can be melted into the hydrogenated soybean oil or
melted
separately. Once the sodium caseinate has been hydrated the soybean oil and
mono
and diglycerides are added to the batch tank. The mixture is well blended. The
remaining resistant corn syrup is added to the batch tank and the mixture is
heated to
170 F, homogenized via double stage homogenization (if required) and held for
30
minutes. The product is then ready to be spray dried with a inlet temperature
of 350
to 500 F and an exhaust temperature of 150 to 200 F. An optional fluid bed
dryer
can be used. Sodium silicoaluminate or silica dioxide could also be included
for anti-
caking purposes. Phosphate salts and/or anti-caking agents could also be
included.
Example 50
A coconut-based coffee creamer powder slurry for spray-drying, comprising an
oligosaccharide composition according to the present invention was prepared.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 71 ¨
The ingredients were:
% dry
Ingredients pounds solids comp % form
Hydrogenated
Coconut Oil 92 32 32 48.51% 21.67% 46.57
Sodium Caseinate 3.2 3.04 4.61% 2.17% 4.66
Resistant corn syrup
solids (Sample 5) 31 29.45 44.65% 20.99% 45.12
Dipotassium
Phosphate 0.4 0.392 0.59% 0.27%
0.58
Distilled mono- and
diglycerides 1.08 1.08 1.64% 0.73% 1.57
water 80 0 0.00% 54.17% 116.43
147.68 65.96 100.00% 100.00% 214.93
Yield @ 4%
Total solids 44.67% moisture 100.00
A coconut-based coffee creamer powder was prepared using the following steps:
The water is added to a batch tank and is heated to 120 to 140 F. The sodium
caseinate is added to the water and allowed to hydrate for 10 to 30 minutes.
The
mono and diglycerides can be melted into the hydrogenated coconut oil or
melted
separately. Once the sodium caseinate has been hydrated, the coconut oil and
mono
and diglycerides are added to the batch tank. The mixture is well blended. The
remaining ingredients resistant corn syrup and dipotassium phosphate are added
to the
batch tank and the mixture is heated to 170 F, homogenized via double stage
homogenization (if required), and held for 30 minutes. The product is then
ready to be
spray dried with a inlet temperature of 350 to 500 F and an exhaust
temperature of
150 to 200 F. An optional fluid bed dryer can be used. Sodium silicoaluminate
or
silica dioxide could also be included for anti-caking purposes.
Example 51
An ice cream coating and/or compound coating can be prepared using resistant
corn syrup solids to lower or eliminate sugar content thereby reducing overall
calories.
Fiber content can be significantly enhanced in comparison to a typical
coating, (e.g.,
this illustration has 33 grams/100 grams versus a comparable control at 5
gram/100
grams of coating).
=
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 72 ¨
Ingredients percentage
Resistant corn syrup solids (sample 40.5
5)
Vegetable Shortening (92 coconut) 45.0
Cocoa powder 10/12 (fat) 14.0
Lecithin 0.45
sucralose 0.05
total 100.00
The ice cream coating and/or compound coating can be prepared using the
following steps: Grind corn syrup solids to a particle size between 5-125
microns,
average near 30-40 micron. Sieve solids to achieve desired particles. Combine
cocoa
powder and sucralose with corn syrup solids. Melt shortening and combine with
lecithin.
While mixing the blended dry ingredients, add the melted shortening/lecithin
combination, scraping the bowl regularly. Apply to frozen novelties, baked
goods, etc.
as desired.
Example 52
Two samples of resistant corn syrup (RCS) were prepared as in Sample 5 of
Example 16 above, one of which had a lower monosaccharide content. ("LS" in
the
following description refers to "low sugar.") The wt% d.s.b. of
monosaccharides,
disaccharides, trisaccharides, and tetra- and higher order saccharides were as
follows:
Formulation DP1 DP2 DP3 DP4+
RCS 12.5 4.7 4.1 78.7
RCS LS 1.6 4.6 4.6 89.2
Samples of the two resistant corn syrups and maltodextrin were fed to dogs.
Blood samples were taken from the dogs at intervals after the feeding to
determine the
glycemic response. The changes in blood glucose concentrations over time are
shown
in Figure 15, and are summarized in the table below.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
73 ¨
Item Maltodextrin RCS RCS LS SEM
5 5 5
Time to glucose peak, min 30 18 18 4.9
Incremental area under the
curve for glucose 155.1d 37.7b 73.9' 12.9
Relative glycemic response 100.0d 24.5b 50.1` 7.8
ab Means in the same row with different superscripts are different (P < 0.05).
SEM = standard error of the mean.
Example 53
Six samples of resistant corn syrup were prepared as in Sample 5 of Example 16
above. Each sample was a 72% ds syrup, with the balance being water. The
samples
contained essentially no fat, protein, or ash. The six samples were:
RCS GR1 (RCS, 72% ds syrup 70% fiber, 15% sugar) ("sugar" in these samples
refers to the total of mono- and disaccharides)
RCS GR2 (RCS LS, 72% ds syrup 80% fiber, 5% sugar)
RCS GR3 (RCS with 50% fructose, 72% ds syrup)
RCS GR4 (RCS with 50% sorbitol, 72% ds syrup)
RCS GR5 (RCS LS with 25% fructose, 72% ds syrup)
RCS GR6 (RCS LS, with 25% sorbitol, 72% ds syrup)
Samples containing 25 g (dsb) of the syrup were prepared as follows. 2.838 kg
of filtered water was added to a jug containing a pre-weighed quantity of RCS.
The lid
was placed on the jug, and it was then mixed thoroughly by shaking and
swirling until
all syrup was dissolved. 12 oz. (350g) of this solution contained 25 g of the
test
carbohydrate on a dry solids basis.
The control solution was prepared by mixing 25 g anhydrous glucose with 300
mL of water.
The samples were administered to 10 healthy human subjects. The
characteristics of the subjects were: 5 male, 5 female; age, 35 10 y; body
mass
index, 24.0 3.8 kg/m2. Each subject undertook nine tests on separate days
which
included the six test foods and on three occasions the standard glucose drink
containing 25 g of available carbohydrate. Blood glucose was measured fasting
and at
15, 30, 45, 60, 90 and 120 minutes after eating. Incremental areas under the
blood
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
- 74 -
glucose response curves (iAUC) were calculated. Each
subject's iAUC after
consumption of each test food was expressed as a percentage of the mean iAUC
of the
three glucose controls taken by the same subject. The incremental areas under
the
curve and relative glycemic response (RGR) of the products were:
iAUC RGR
Glucose (25 g) 124.4 13.5a 100a
RCS GR1 38.5 4.6b 32.6 3.8b
RCS GR2 25.6 3.7b 23.2 4.6b
RCS GR3 30.1 4.4b 26.2 4.2b
RCS GR4 17.4 4.1b 15.3 3.6c
RCS GR5 27.6 4.0b 25.4 4.3b
RCS GR6 20.9 4.0b 18.2 3.5C
Values with different superscripts differ significantly (p<0.001). There were
no
statistically significant differences in palatability ratings between any of
the foods.
Example 54
Sweetose 4300 corn syrup (81% ds) was evaporated to less than 6% moisture
content by passing it through a hot oil jacketed paddle mixer at a rate of 77
kg/h. The
paddle mixer rotor speed was typically set for 300 to 600 rpm and the oil
jacket
temperature was varied from 150 C to 2 0 5 C. In some of the tests phosphoric
acid
was added at a rate to give from 0.1% to 0.4% phosphoric acid solids on corn
syrup
solids. In some of the tests hydrochloric acid was added at 25 ppm, in place
of or in
addition to the phosphoric acid.
Product collected from these tests (25 mg) was dissolved in 4 mL of pH 4.0
buffer and incubated with 100 microliters of a 10 mg/mL amyloglucosidase
enzyme
(Amyloglucoxidase Sigma Catalog #A-7255) solution for 2 hours at 45 C. An
aliquot
from this incubation was treated with a small quantity of ion exchange resin
and
filtered (0.45 microns) prior to saccharide distribution analysis by liquid
chromatography. From this analysis, the weight percent of carbohydrate found
to exist
as trisaccharides and higher was quantified as digestion resistant
carbohydrate and is
labeled as % fiber in the table below:
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 75 ¨
Sample name Temp C %H3PO4 HCI ppm %fiber
run 1 194 0.2% 43
run 2 195 0.2% 25 52
run 3 193 0.4% 25 62
run 4 203 0.4% 25 68
run 5 180 0.2% 27
run 6 181 0.4% 37
run 7 181 0.4% 25 33
polydextrose control 82
A laboratory sample of polydextrose was used as a control for this test, and
showed a level of approximately 82% fiber.
Example 55
Sweetose 4300 corn syrup (81% ds) was evaporated to less than 3% moisture
content by passing it through a hot oil jacketed paddle mixer at a rate of 77
kg/h. The
paddle mixer rotor speed was typically set for 800 rpm and the oil jacket
temperature
was set to 210 C. In some of the tests phosphoric acid was added at a rate to
give
from 0.1% to 0.4% phosphoric acid solids on corn syrup solids. In some of the
tests
hydrochloric acid was added at 25 or 50 ppm, in place of or in addition to the
phosphoric acid.
Product collected from these tests (25 mg) was dissolved in 4 mL of pH 4.0
buffer and incubated with 100 microliters of a 10 mg/mL amyloglucosidase
enzyme
(Amyloglucoxidase Sigma Catalog #A-7255) solution for 2 hours at 45 C. An
aliquot
from this incubation was treated with a small quantity of ion exchange resin
and
filtered (0.45 microns) prior to saccharide distribution analysis by liquid
chromatography. From this analysis, the weight percent of carbohydrate found
to exist
as trisaccharides and higher was quantified as digestion resistant
carbohydrate and is
labeled as %fiber in the table below:
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 76
Sample name Temp C %H3PO4 H01 ppm %fiber
run 2-1 210 0.0% 11
run 2-2 210 0.2% 79
run 2-3 210 0.0% 12
run 2-4 210 0.1% 43
run 2-5 210 0.1% 51
run 2-6 210 0.2% 61
run 2-7 210 0.3% 84
run 2-8 210 0.2% 25 79
run 2-9 210 0.0% 11
run 2-10 210 0.1% 43
run 2-11 210 0.1% 25 57
run 2-12 210 0.2% 53
run 2-13 210 0.2% 25 62
run 2-14 210 0.4% 56
run 2-15 210 0.4% 25 55
run 2-16 210 0.4% 50 62
run 2-17 210 0.0% 50 65
run 2-18 210 0.0% 50 59
polydextrose control 82
A laboratory sample of polydextrose was used as a control for this test, and
showed a level of approximately 82% fiber.
Example 56
500 grams of Staley 300 corn syrup (80.0 AD ds, 35 DE, 0% fiber, 4 kcal/g)
was thoroughly blended with 500 grams of resistant corn syrup (69.0 A) ds, 21
DE,
71% fiber, 2 kcal/g) to give 1 kg of corn syrup fiber (74.5 % ds, 28 DE, 35%
fiber, 3
kcal/g).
Example 57
500 grams of Staley 1300 corn syrup (80.3 A) ds, 43 DE, 0% fiber, 4 kcal/g)
was thoroughly blended with 500 grams of resistant corn syrup (69.0 % ds, 21
DE,
71% fiber, 2 kcal/g) to give 1 kg of corn syrup fiber (74.7 % ds, 32 DE, 35%
fiber, 3
kcal/g).
Example 58
500 grams of Staley 4300 corn syrup (81.6 % ds, 63 DE, 0% fiber, 4 kcal/g)
was thoroughly blended with 500 grams of resistant corn syrup (69.0 % ds, 21
DE,
71% fiber, 2 kcal/g) to give 1 kg of corn syrup fiber (75.3 % ds, 42 DE, 35%
fiber, 3
kcal/g).
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 77 ¨
Example 59
500 grams of Staleydex 130 syrup (70.5 % ds, 99 DE, 0% fiber, 4 kcal/g)
were thoroughly blended with 500 grams of resistant corn syrup (69.0 % ds, 21
DE,
71% fiber, 2 kcal/g) to give 1 kg of corn syrup fiber (69.8 % ds, 60 DE, 35%
fiber, 3
kcal/g).
Example 60: Preparation of Fiber-Containing Syrup/Low Sugar Syrup Blend
A fiber-containing syrup composition was blended with a low sugar syrup using
a conventional laboratory overhead mixer. The two components were weighed out
using a standard laboratory scale and the blended products were prepared by
mixing
the two components for about 30 minutes at medium speed using an overhead
laboratory mixer with a metal blade attachment. The weight ratios of the two
components that were used are shown in Table 15. The viscosities and sugar
contents
(DP1 + DP2) of the blends are also shown in Table 15.
Table 15. Composition of different blend samples
Fiber Containing Low Sugar 0/0DP1+2
Viscosity (cP)
Sample# Syrup (wt /0) Syrup (wt /0) (dsb)
15-1 100 0 1300 18.2
15-2 50 50 4100 16.4
15-3 40 60 11200 16.0
15-4 30 70 8200 15.6
15-5 20 80 11700 15.2
15-6 15 85 14400 15.0
15-7 10 90 15100 14.8
15-8 5 95 19200 14.7
15-9 0 100 16500 14.5
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 78 ¨
Example 61: Preparation of a cereal bar using a fiber-containing, low sugar,
low-
viscosity syrup
Processing Procedures:
1. Mix oats, rice crisps and dried cranberries in a Hobart bowl for 30 seconds
at
speed 1 with a paddle attachment. (spray bowl with non-stick spray to reduce
sticking when syrup is added)
2. Heat a blended syrup (i.e., a blend of a fiber-containing syrup and a low
sugar
syrup), water and glycerin to 140 F (60 C).
3. Slowly add pre-blended dries of KRYSTAR8 300 crystalline fructose, sucrose
and
STAR-DRI 200 corn syrup solids to liquid mixture. Mix continuously to obtain
a
binding syrup as a smooth and homogeneous slurry.
4. Disperse lecithin in canola oil and add to the slurry with continuous
mixing.
5. Add flavor and mix thoroughly for 30 seconds.
6. Transfer the product from step 5 to the Hobart bowl containing dry
particulates
and mix at speed 1 with the paddle attachment for 30 seconds or until the
binding syrup is uniformly dispersed around dry particulates.
7. Transfer coated particulates on a flat metal pan (sprayed with non-stick
spray)
and compress evenly to 1/2" thickness with the help of a rolling pin.
8. Cover compressed slab and let cool for 2 hours at room temperature, or 30
minutes in a refrigerator.
9. Cut into desired shape and size, package into film
10.A 500 gram batch, 8"x11" will make 30 - 2"x1" bars.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 79 ¨
Bar
Ingredients %
Binding Syrup
Blended Syrup 25.02
Glycerin 3.50
KRYSTAR 300 crystalline fructose (Tate & Lyle) 3.50
Granulated sucrose 1.00
STAR-DRI8 200 corn syrup solids (Tate & Lyle) 1.50
Water 0.50
Salt, granular 0.37
Oil Phase
Canola oil 3.20
Lecithin 0.20
Vanilla flavor 0.20
Granola Particulates
Coated oats, 1011 42.80
Rice crisps, #13 10.11
Dried cranberries, chopped 1/8" slices 8.10
Total 100.00
Formula % (by weight):
Granola Particulates 61.01%
Binding Syrup 38.99%
Example 62: Preparation of a fruit filling using a blended syrup (fiber-
containing syrup
+ low sugar syrup)
1. Prepare and set aside: Dry blend color and citric acid into MIRA-GEL 463.
Add dry blend to 240 gm portion of HFCS, blend.
Add remaining 540 gm HFCS and flavor, blend.
2. Weigh blended syrup to be tested into pot. Blend in the REZISTA starch.
Add cherries,
mix well.
Cook to 80% solids (220 F) at 220-240 F induction heater setting.
Heat and mix constantly, scraping bottom on pot to prevent burn-on until 80
Brix.
3. Add remaining 540 gm HFCS and cherry flavor, blend.
4. Add mixture to hot slurry, add this in stages. Blend well.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
,=-= 80
5. Product will thicken, clear and reduce in temperature. Hot pack. Target -79
Brix.
CHERRY FRUIT PREP
30x Ingredient
INGREDIENT Grams Grams % Solids %
Blended Syrup 53.75 1612.50 40.15 51.732%
REZISTA starch 2.00 60.00 1.925%
Chopped frozen cherries 20.00 600.00 19.249%
FD&C red #40 granular 0.01 0.30 0.010%
MIRA-GEL 463 modified starch 2.00 60.00 1.925%
Citric Acid 0.11 3.30 0.106%
ISOSWEET 5500 (HFCS, 77%
TS) 8.00 240.00 6.16 7.700%
ISOSWEET 5500 (HFCS, 77%
TS) 18.00 540.00 13.86 17.324%
Nat Black Cherry Flavor TW-
045-108-7 0.03 0.90 0.029%
TOTAL GRAMS 3117.00 100.000%
Example 63: Preparation of a fruit snack using a blended syrup (fiber-
containing syrup
+ low sugar syrup)
1. Dry blend sucrose and starches in a separate container and transfer into
a cooking kettle
containing specified amount of water.
2. Transfer blended syrup into the kettle while mixing.
3. Mix thoroughly at <1500F (650C) to obtain a homogeneous slurry without
lumps.
4. Cook slurry to 2100F (990C) with continuous agitation. Add fruit juice
concentrate.
5. Adjust steam back pressure valve to 70 psi and set temperature to 285 OF
(141 0C).
6. Open cooking kettle valve, start pump and run precooked slurry through
jet cooker at 285 OF
(141 0C).
7. Collect a portion of jet cooked slurry in a stainless steel container
for addition of color,
flavors and acidulants.
8. Add citric acid, flavor and color as specified and mix thoroughly.
9. Deposit slurry into starch moulds with the help of metal funnel
depositors.
CA 02905812 2015-09-11
WO 2014/158777 PCT/US2014/020105
¨ 81 ¨
11. Dry jelly candies for 48 hours and collect samples after 84-86 Brix is
reached.
12. Cool and package.
Ingredients %
Blended Syrup 51.18
Apple Juice Concentrate 9.00
Pear Juice Concentrate 9.00
Sucrose 14.58
Water 5.24
Confectioners G 6.60
Mira-Set 285 starch 4.40
TOTAL 100.00
Target Brix of finished product 84
The preceding description of specific embodiments of the invention is not
intended to be a list of every possible embodiment of the invention. Persons
skilled in
the art will recognize that other embodiments would be within the scope of the
following claims. For example, certain specific slowly digestible or digestion
resistant
compositions are used as ingredients in food products in some of the above
examples.
It should be recognized that other slowly digestible or digestion resistant
compositions
of the present invention could be used instead in those same food products,
although
the exact characteristics of the food product may vary to some degree
depending on
the exact nature of the ingredients used. Many other modifications could also
be made
to the specific examples herein.