Note: Descriptions are shown in the official language in which they were submitted.
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
METHOD AND SYSTEM FOR THE TREATMENT OF PRODUCED WATER AND
FLUIDS WITH CHLORINE DIOXIDE FOR REUSE
FIELD OF THE INVENTION
[0001] The recited claims relate generally to methods and systems for
treating
produced water associated with gas and crude oil drilling, pumping and
production, including
but not limited to hydraulic fracturing. More particularly, the recited claims
relate to an
improved method and system of treating produced water, flowback water, source
water or
other industrial aqueous fluids in order to reduce contamination and bring the
treated water to
appropriate standards that allows the treated water to be reused for hydraulic
fracturing.
INTRODUCTION
[0002] In oil and gas production, tremendous amounts of water are used as
part of the
overall process. Of primary interest is the large amount of water that is used
to fracture oil or
gas wells to enhance the production from a given formation. The water that is
used in this
process must be free of contaminants that can impact the performance of the
fracking
process. In particular, hydrocarbon metals, inorganic contaminants or metal
ions, phosphates,
volatile organic compounds (VOCs), total dissolved solids (TDS) and other
contaminants can
impede the performance of the polymers used to reduce friction and/or to keep
sand in
suspension. Also of importance is the reduction or elimination of bacterial
contamination
that can reduce polymer performance and/or contaminate the producing
formation. For
example, bacterial contamination of the formation can cause plugging or loss
of production
and cause the formation of hydrogen sulfide which impacts the operability of
the well and the
value of the produced product. The present invention provides a process to
treat produced
water and achieve standards that will allow the treated produced water to be
reused in a
subsequent hydraulic fracturing process.
1
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[0003] Various methods and systems for the treatment of produced water
have been
explored and are known in the art. Examples of these technologies are reverse
osmosis,
microfiltration, electrocoagulation, and other technologies. These
technologies have severe
limitations regarding the variety of contaminants they can deal with in a
single step, and high
operating costs with relatively low throughput treatment rates. For example,
while reverse
osmosis (RO) is effective in eliminating ionic contaminants, hydrocarbon
contaminants can
severely plug and or damage the (RO) membrane making this technology
commercially
unrealistic. Similarly, the presence of certain cations and anions can cause
fouling, scaling,
or other forms of interference.
[0004] Wastewater associated with the production of crude oil, i.e.
oilfield water,
generally consists of two primary sources: flow-back water and produced water.
The reuse of
these waters is typically difficult due to high contaminant and bacterial
loading. More
specifically, oilfield water and fracturing fluids (or frac water) can be
contaminated with, for
example, bacteria, naturally-occurring organics in the formation, organic
treatment chemicals
(such as viscosifiers, emulsion stabilizers, etc), and production chemicals
(such as scale
reducers, friction reducers, anti-corrosive chemicals, pH modifiers, etc.),
and/or other
contaminants that result in a high percentage of TDS. The presence of these
contaminants
can interfere with later re-use of the water, storage and/or disposal (e.g.
injection into
disposal wells or sent to municipal treatment facilities).
[0005] Municipal treatment facilities are facing increasing regulatory
requirements
for wastewater associated with hydraulic fracturing and satisfying these
requirements is
costly. Similarly, to the extent contaminated frac and oilfield waters are
stored in oilfield
pits, open pools, or lagoons, high residual polymer levels and solids loading
within the pits
can contribute to high hydrogen sulfide production, causing safety and
environmental
concerns.
2
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[0006] More recently, producers are shifting to closed loop systems as
the preferred
method of handling flowback and produced waters (i.e. reusing these waters in
subsequent
operations). As such, the water used for hydraulic fracturing operations is
often a
combination of produced and/or flowback water, surface water and/or municipal
water (also
known as "commingled water"). Successful treatment of the contaminated
produced water in
storage pits and tanks allows commingled water to be made up of a larger
percentage of
produced water than it would otherwise, and in turn, provide for reduced
disposal costs, fresh
water costs, and lower water use/reuse concerns. Low cost, simple technologies
are desirable
so that small producers or isolated production areas can use the treatment
processes easily.
[0007] The process disclosed herein is a lower cost, unexpectedly
effective treatment
method that works well under difficult process conditions associated with
produced waters
from oil and gas drilling/production, for example, that contain high levels of
one or more of
calcium (Ca), magnesium (Mg), barium (Ba), iron (Ferrous, Fe2 ' or Ferric, Fe3
' ), manganese
(Mn), as well as hydrocarbons, sulfates (SO4), total organic carbon (TOC),
total dissolved
solids (TDS), volatile organic compounds (VOCs), and bacterial contamination.
[0008] The methods and system disclosed herein will reduce and/or
effectively
eliminate bacterial contamination, hydrocarbon metals, inorganic contaminants
or metal ions,
phosphates, VOCs, TSS, TDS and other contaminants from oil and gas wastewater
in order to
ultimately reduce the water footprint associated crude oil production, and
provide the ability
to reuse treated produced water as hydraulic fracturing fluid. More
specifically, the novel,
high-efficiency treatment method and system disclosed herein is extremely and
unexpectedly
effective in treating highly contaminated produced water by the removal and/or
reduction of
certain inorganic contaminants such as Ca, Mg, Na, Fe, Cl, Mn, TDS, CaC103,
SO4, Ba, as
well as hydrocarbons, biological contamination and other colloidal material.
Furthermore
3
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
through adjustment of physical and chemical process parameters, some
contaminants can be
selectively targeted at higher removal rates.
[0009] Chlorine dioxide's unique chemical and physical properties make it
ideal for
use in treatment of fracturing fluids. As an oxidant, it is able to penetrate
hydrocarbons and
break emulsions allowing for the separation and recovery of hydrocarbons, as
well as the
reduction and/or elimination of biological contamination. Because of its
specificity, its
oxidation power can be directed at contaminates such as sulfides and residual
polymers
without creation of undesirable by-products and, unlike bleach or chlorine,
chlorine dioxide
does not form chlorination by-products that can cause operational or
environmental concerns.
[0010] Chlorine dioxide (and/or chlorite), however, is heavily regulated
and caution is
necessary in its generation, handling and storage. Furthermore, it can be very
costly
depending on the chlorine dioxide demand of the wastewater and/or source
water. The
various embodiments of the invention use the oxidative power of chlorine
dioxide together
with oxygen (or air) to achieve unexpected results, unexpected increase in
efficiency, and
unexpected capacity for treatment for these waters that have not been achieved
prior to the
disclosure herein.
[0011] In addition, and according to some of the various embodiments of
the
invention, a combination of chlorine dioxide disinfection and oxygenation is
used to provide
a faster-acting treatment for wastewater. Such methods and systems result in
increased
chlorine dioxide capacity and increased efficiency in relation to volumes of
water treated,
which provides for reduced chemical usage, reduced energy, and reduced
effluent, which in
turn results in a reduced burden on the environment and reduced cost.
4
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[0012] In accordance with one or more of these embodiments, the use of
chlorine
dioxide in a closed loop system to treat tanks storing oilfield wastewater, or
produced water,
has the unexpected potential to reduce bacterial contamination, Ca, Mg, Na,
Fe, Cl, Mn, TDS,
CaC103, SO4, Ba, oil, grease, and combinations thereof, thus providing reduced
treatment
costs, fluid disposal costs and make-up water purchases (by allowing for
greater reuse of the
oilfield wastewater as hydraulic fracturing fluid), and reduced environmental
and safety
concerns. In alternative embodiments, chlorine dioxide can also be used for
the pretreatment
and disinfection of fracturing fluids prior to their use in crude oil
production and/or hydraulic
fracturing operations, including but not limited to surface water, produced
water, municipal
water, flowback water, or any combination thereof
[0013] Accordingly, it is desirable to provide methods and systems for
the treatment
of wastewater associated with gas and crude oil drilling, pumping and
production (i.e.
produced water) to alleviate the problems associated with existing treatments.
In particular, it
is desirable to provide methods and systems for improved treatment of produced
water for
reuse as fracturing fluids.
[0014] While certain aspects of conventional technologies have been
discussed to
facilitate disclosure of the invention, Applicants in no way disclaim these
technical aspects,
and it is contemplated that the claimed invention may encompass one or more of
the
conventional technical aspects discussed herein.
[0015] In this specification, where a document, act or item of knowledge
is referred to
or discussed, this reference or discussion is not an admission that the
document, act or item of
knowledge or any combination thereof was, at the priority date, publicly
available, known to
the public, part of common general knowledge, or otherwise constitutes prior
art under the
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
applicable statutory provisions; or is known to be relevant to an attempt to
solve any problem
with which this specification is concerned.
SUMMARY
[0016] In one aspect, the invention relates to a method comprising the
following steps
[0017] Step 1. Produced fluids are transferred into a vessel that allows
between 30
minutes and 60 minutes of residence time.
[0018] Step 2. Fluid is withdrawn from the first vessel via a pump pass
through a
venturi and returned to the first treatment vessel. Air is introduced into the
venturi to provide
for a finely divided and or dissolved airstream returning to the first vessel.
Also introduced in
the return is a solution of sodium chlorite or a combination of sodium
chlorite and sodium
hydroxide or those two chemicals as separate feeds. Alternatively, another
feedstock can be
added to facilitate the precipitation of a target compound. The supply stream
is drawn from a
level approximately 20% up from the bottom of the vessel. The discharge of the
fluid is
through a distribution line at the midline of the vessel. The first vessel has
provision for
skimming of hydrocarbons or other low specific gravity material, and provision
for removal
of high density solids.
[0019] Step 3. Fluid is discharged from the first vessel to the
centerline of a second
vessel. Chlorine dioxide gas is introduced and the transfer line. Chlorine
dioxide dosage is
sufficient to achieve a residual in the second vessel.
[0020] Step 4. The second vessel has a residence time of approximately 30
to 60
minutes. The second vessel has a underflow to the third vessel. The second
vessel has
provision for skimming of flocculate material that is of low specific gravity.
6
CA 02906186 2015-09-11
WO 2014/145825
PCT/US2014/030654
[0021] Step 5. The third vessel has a residence time of approximately 10
to 30
minutes. This vessel has an overflow to a third vessel or clear well. This
vessel also has
provision for removal of high density solids from the bottom of the vessel.
GLOSSARY
[0022] The following terms as used herein have the following meanings:
[0023] Demand or Chlorine Dioxide Demand - The amount of chlorine dioxide
(or
other oxidant) consumed by background, reactive impurities (both inorganic and
organic
materials) in a given sample of wastewater (i.e. oilfield water), fracturing
fluid, treatment or
other target fluids. Chlorine dioxide demand is determined by subtracting the
amount of
chlorine dioxide remaining after a specified time from the amount of chlorine
dioxide
initially added to a system.
[0024] Free Residual or Residual- The amount of chlorine dioxide (or
other
oxidant) present at a given time to react with biological species after
background
contaminants (or "demand") have been converted. In other words, the amount of
chlorine
dioxide (or other oxidant) available for bacterial control.
[0025] Biocide - chemical agent capable of killing living microorganisms,
often in a
selective way (also referred to as bactericides or antimicrobials).
[0026] Biological Contamination - any living microorganism or by-product
of a
living microorganism found in wastewater (i.e. oilfield water), fracturing
fluids, treatment
fluids, source water or other target fluids.
[0027] Biocidally-Effective Amount - An amount that will control, kill or
otherwise
reduce the bacterial or microbial content of the wastewater (i.e. oilfield
water), fracturing
fluids, treatment fluids, source water or other target fluids at issue.
7
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[0028] Well Fluid, Fracturing Fluid or Frac Fluid- Any fluid used in any
of the
drilling, completion, work over and production of subterranean oil and gas
wells. It generally
includes a source (or raw, or base) water feed (e.g. Frac Water) plus any
additives.
[0029] Frac Water - Raw water feed used in hydraulic fracturing process
from any
source, including but not limited to surface water, municipal water or treated
flowback or
produced water.
[0030] Produced Water - Water that is naturally occurring within a
subterranean
formation that is produced to the surface either as part of a hydraulic
fracturing or crude oil
operation
[0031] Flowback Water - Recovered fracturing fluids that flow back to the
surface
after being pumped down into a subterranean formation as part of a hydraulic
fracturing or
crude oil operation.
[0032] Oilfield Water - As used herein, includes production water,
flowback water
and other fluids that are the by-products of crude oil production, hydraulic
fracturing, or other
petroleum production processes.
[0033] Furthermore, as used herein, the words "comprise", "has," and
"include" and
all grammatical variations thereof are each intended to have an open, non-
limiting meaning
that does not exclude additional elements or parts of an assembly or
structural element.
[0034] The features of the present invention will be readily apparent to
those skilled
in the art upon a reading of the description of the embodiments that follows.
8
CA 02906186 2015-09-11
WO 2014/145825
PCT/US2014/030654
BRIEF DESCRIPTION OF THE DRAWINGS
[0035] The subject matter which is regarded as the invention is
particularly pointed
out and distinctly claimed in the claims at the conclusion of the
specification. The foregoing
and other features and advantages of the invention will be readily understood
from the
following detailed description of the preferred embodiments taken in
conjunction with the
accompanying drawings in which:
[0036] FIG. 1 is a schematic of a typical water treatment system in
accordance with
an exemplary embodiment of the invention.
[0037] FIG. 2 is a schematic diagram illustrating another embodiment of
the
invention.
[0038] FIG. 3 is a schematic diagram illustrating yet another embodiment
of the
invention.
[0039] FIG. 4 is a schematic diagram illustrating yet another embodiment
of the
invention.
[0040] FIG. 5 is a schematic diagram illustrating yet another embodiment
of the
invention.
[0041] FIG. 6 is a schematic diagram illustrating yet another embodiment
of the
invention.
[0042] FIG. 7 is a schematic diagram illustrating yet another embodiment
of the
invention.
[0043] FIG. 8 is a schematic diagram illustrating yet another embodiment
of the
invention.
9
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[0044] FIG. 9 is an illustration of one embodiment of the invention.
DESCRIPTION OF THE ILLUSTRATIVE EMBODIMENTS
[0045] For the purposes of promoting an understanding of the principles
of the
invention, reference will now be made to the embodiments illustrated in the
drawings and
specific language will be used to describe the same. It will nevertheless be
understood that
no limitation of the scope of the invention is thereby intended, and any
alterations and further
modifications in the illustrated embodiments, and any further applications of
the principles of
the invention as illustrated therein as would normally occur to one skilled in
the art to which
the invention relates are contemplated an protected.
[0046] The terminology used herein is for the purpose of describing
particular
embodiments only and is not intended to be limiting of the invention. As used
herein, the
singular forms "a", "an" and "the" are intended to include the plural forms as
well, unless the
context clearly indicates otherwise. It will be further understood that the
terms "comprise"
(and any form of comprise, such as "comprises" and "comprising"), "have" (and
any form of
have, such as "has" and "having"), "include" (and any form of include, such as
"includes"
and "including"), and "contain" (and any form contain, such as "contains" and
"containing")
are open-ended linking verbs. As a result, a method or device that
"comprises", "has",
"includes" or "contains" one or more steps or elements possesses those one or
more steps or
elements, but is not limited to possessing only those one or more steps or
elements.
Likewise, a step of a method or an element of a device that "comprises",
"has", "includes" or
"contains" one or more features possesses those one or more features, but is
not limited to
possessing only those one or more features. Furthermore, a device or structure
that is
configured in a certain way is configured in at least that way, but may also
be configured in
ways that are not listed.
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[0047] Hydraulic fracturing and other oil field drilling and production
processes
require large quantities of water and, in turn, produce large quantities of
wastewater.
Additionally, many other types of industrial or commercial operations rely on
large quantities
of water and produce large quantities of wastewater, all of which needs to be
treated. These
industries include, but are not limited to, agriculture, chemical,
pharmaceutical, mining, metal
plating, textile, brewing, food and beverage processing, and semiconductor
industries. The
presence of biological contamination and other organic contaminants results in
decreased
efficiency and can cause damage (i.e. corrosion, blockages, growth of harmful
bacteria).
Similarly, waters that have high residual organic or biological contamination
are unsuitable
for use in oilfield operations and need to be treated prior to being injected
underground and
introduced into a subterranean formation.
[0048] In accordance with the embodiments of the invention, chlorine
dioxide can be
used to treat oilfield water (including production water, flow-back water and
surface water) in
order to reduce both the biological load and to aid in the breakdown of
residual organic
contamination in the water. For example, although not limiting, one or more
embodiments of
the present invention may be used for the treatment of produced or flowback
water prior to
disposal or reuse. Both produced and flowback water tend to have substantial
biological
contamination, as well as a high load of organic contaminants (such petroleum
hydrocarbons,
oil and grease, diesel-related organics, BTEX), polymers (such as
polyacrilamides), iron (Fe),
VOCs, inorganic transition metals or metal ions, suspended solids, and other
contaminants.
[0049] In an exemplary embodiment of the invention, the methods disclosed
herein
can be used in a continuous, closed-loop system to treat produced or flow-back
water before
the water is reused in a subsequent hydraulic fracturing operation. However,
and still in
accordance with the claimed invention, the methods can also be used as a
pretreatment for
11
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
frac water, including but not limited to a pretreatment "on the fly"; before
the water is
deposited in storage pits/tanks/lagoons; or as part of a wide variety of other
oilfield
production systems.
[0050] For example, one embodiment is a process for treating produced
water that
provides for a substantial reduction in bacterial contamination, hydrocarbon
metals, inorganic
contaminants or metal ions, phosphates, volatile organic compounds (VOCs),
total dissolved
solids (TDS) and other contaminants from oil and gas wastewater as compared to
conventional treatment methods, and allows the treated produced water to
achieve a final
residual of chlorine dioxide in the range of about 0.1 mg/1 and 50 mg/1, thus
making the
treated produced water suitable for re-use, for example, as a hydraulic
fracturing fluid
without the need for additional treatment technologies (such as RO systems).
More
specifically, the treatment system and method disclosed herein resulted in the
unexpected and
commercially unavailable reduction of metal ions including iron, calcium,
manganese,
magnesium and barium.
[0051] In certain embodiments, the amount of chlorine dioxide required to
treat the
produced water is substantially less than what would be expected by those of
ordinary skill in
the art and is substantially less as compared to what would be required if one
were to use
chlorine dioxide in isolation, thus also providing substantial financial and
economic benefits
for large scale commercial use.
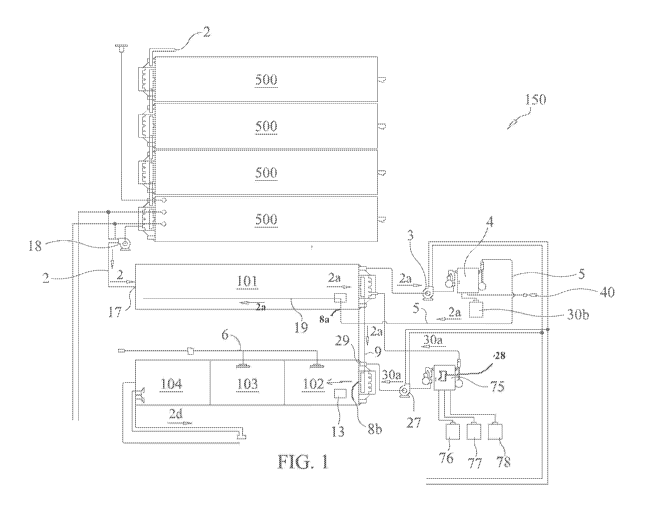
[0052] FIG 1 illustrates one embodiment of a closed loop treatment system
150 in
accordance with certain embodiments of the invention. In operation, and as
exemplified in
FIG. 1, raw produced fluid 2 is transferred into, or enters, a first treatment
vessel 101 through
treatment system inlet 17, via pump 18. Although not limiting, as shown in FIG
1, first
treatment vessel 101 is a frac tank located onsite at an oil and gas
production site. Prior to
12
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
transfer into first vessel 101, fluid 2 can be stored in one or more storage
frac tanks 500 (as
shown in FIG 1). In alternative embodiments, fluid 2 can be transferred
directly from, for
example, a truck, pipe, pit or well using various transfer methods and
apparatus known in the
art into first vessel 101.
[0053] In exemplary embodiments, although not required, raw produced
fluid 2 is a
highly contaminated wastewater stream from an oil and gas application
containing high levels
of contaminants selected from the group consisting of bacterial contamination,
Ca, Mg, Na,
Fe, Cl, Mn, TDS, CaC103, SO4, Ba, oil, grease, and combinations thereof In
certain
embodiments, fluid 2 undergoes initial hydrocarbon separation techniques prior
to being
transferred into first treatment vessel 101 using techniques known in the art.
Prior to entering
vessel 101, fluid 2 (e.g. raw, untreated produced water) has a first initial
demand 200, which
can be determined using tests known in the art.
[0054] For purposes of this disclosure, and referring to FIG 1, the
wastewater to be
treated, e.g. produced water, that is contained in vessel 101 is referred to
herein as fluid 2a.
First treatment vessel 101 provides fluid 2a with a residence time of about 15
minutes to 60
minutes. The total residence time required for fluid 2a will vary and depend
on the
characteristics of the fluid and other environmental factors. For example, in
some
embodiments the residency time will be 15, 18, 20, 22, 25, 27, 30, 35, 40, 45,
50, 55 or 60
minutes, including any and all ranges and subranges therein (e.g., 15 to 60
minutes, 18 to 55
minutes, 15 to 30 minutes, 20 to 30 minutes, 15 to 20 minutes, 20 to 25
minutes, 18 to 25
minutes, 25 to 50 minutes, etc.).
[0055] Once transferred into vessel 101, fluid 2a then is withdrawn from
first vessel
101 via a circulation pump 3, passed through a venturi 4, and returned to
first treatment
vessel 101. The supply stream for fluid 2a is drawn from a level approximately
20% up
from the bottom of first treatment vessel 101, all through transfer, or
distribution, line 5. As
13
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
fluid 2a is passed through venturi 4, oxidant 40, which in the embodiment
shown here is air,
is introduced into venturi 4 to provide for a finely divided or dissolved
stream of air in return
fluid 2a as it returns to first vessel 101. Air (or oxidant 40) is introduced
at a rate to avoid air
stripping so that the removal of volatile reductants (i.e. hydrogen sulfide)
is via oxidation,
rather than physical purging or stripping. By selecting an air flow rate that
prevents or avoids
off-gassing of hydrogen sulfide or other volatile compounds present in fluid
2a, the
reductants are oxidized in situ rather than purged by allowing the oxidant to
come into
contact with the sulfides and allow oxidation to occur. In the embodiment
disclosed in FIG.
1, oxidant 40 is air. However, in alternative embodiments, oxidant 40 can be
oxygen,
oxygen-enriched air, or any chemical oxygen source or combination that is
stable with
chlorite. Although not preferable, if ozone is used, it should not be used in
combination with
chlorine dioxide in the first step because ozone consumes chlorine dioxide.
Furthermore, in
alternate embodiments, oxidant 40 can be introduced into fluid 2a via means
other than
venturi 4, such as an injection boom, pressurized source, aerator, mechanical
agitation, air
sparger, diffuser, sprayer or other means for injecting and diffusing air
known in the art.
[0056] The amount of oxidant 40 dosage required will depend on the
characteristics
of fluid 2 (e.g. initial demand 200), the treatment system, and the intended
use or application,
together with other considerations known to those of ordinary skill in the
art. In exemplary
embodiments, oxidant 40 is added at an appropriate dosage and period of time
to achieve an
overall dosage ranging from about 20 mg/kg to about 2000 mg/kg of oxidant 40
to the total
volume of fluid 2a to be treated, with a more preferred dosage of about 20
mg/kg to about
1000 mg/kg of oxidant 40 to the total volume of fluid 2a to be treated.
[0057] For example, in some embodiments, the dosage comprises 20, 30, 50,
100,
250, 300, 500, 750, 800, 900, 1,000, 1,250, 1,500, 1,800, 2,000 mg/kg oxidant
to the total
volume of fluid to be treated, including any and all ranges and subranges
therein (e.g., 20 to
14
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
2000 mg/kg, 100 to 2000 mg/kg, 300 to 2000 mg/kg, 500 to 2000 mg/kg, 1000 to
2000
mg/kg, 20 to 1000 mg/kg, 50 to 1000 mg/kg, 100 to 1000 mg/kg, 500 to 1000
mg/kg, 20 to
500 mg/kg, etc.).
[0058] When fluid 2a is withdrawn from first vessel 101 via a circulation
pump 3,
and passed through a venturi 4, and before its return to first treatment
vessel 101, a solution
of sodium chlorite (chlorite 30b) is introduced either at the same time as
oxidant 40 or
substantially contemporaneously therewith. Alternatively, a combination of
sodium chlorite
(chlorite 30b) and sodium hydroxide (caustic 90) as a single feed or,
alternatively, sodium
chlorite (chlorite 30b) and sodium hydroxide (caustic 90) as two separate
feeds (not shown),
is introduced at the same time as the air, or substantially contemporaneously
therewith. In a
preferred embodiment, a 25% sodium chlorite solution, commercially available
as DiKlor0
from Sabre Oxidation Technologies, Inc. is used as chlorite 30b. However,
other available
sources of chlorite 30b are available and known to those of ordinary skill in
the art. In
embodiments of the invention, chlorite 30b is added at an appropriate dosage
and period of
time to achieve a dosage ranging from about 10 mg/1 to about 500 mg/lin fluid
2a to be
treated. For example, in some embodiments, the chlorite 30b dosage comprises
10, 20, 23,
27, 30, 50, 100, 120, 180, 230, 250, 300, 420, 500 mg/1, including any and all
ranges and
subranges therein (e.g., 10 to 500 mg/1, 25 to 500 mg/1, 25 to 300 mg/1, 10 to
300 mg/1, 10 to
100 mg/1, 25 to 200 mg/1, 50 to 500 mg/1, 50 to 420 mg/1, 23 to 420 mg/1, 27
to 420 mg/1, 23
to 300 mg/1, 27 to 300 mg/1, 23 to 230 mg/1, etc.).
[0059] Referring to FIG 1, after being dosed with a combination of air,
sodium
chlorite, and caustic (optional), returned fluid 2a is discharged back into
vessel 101 through
distribution line 5 and/or other distribution means known in the art for
uniformly distributing
fluid 2a within first vessel 101. In this particular embodiment, returned
fluid 2a is discharged
back into vessel 101 at the centerline or midline 8a (halfway up the vertical
height of fluid
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
volume contained in the vessel), or substantially about the midline, of first
vessel 101. A
number of different means can be used to distribute fluid 2a during this step,
including but
not limited to a submergible boom 19 (stationary or movable) with openings for
discharging
fluid 2a as shown in FIG 1, or other similar types of sparging or uniform
distribution
mechanisms known in the art of wastewater treatment.
[0060] The continuous circulation, or recycling, of fluid 2a from vessel
101 through
venturi 4 and then back into vessel 101 through distribution line 5 allows
fluid 2a to be
sufficiently diffused with oxidant 40 and chlorite 30b, ultimately insuring
that fluid 2a in first
treatment vessel 101 will be fully infused with and exposed to oxidant 40 and
chlorite 30b,
which are distributed substantially equally throughout vessel 101. Notably,
and
unexpectedly, this first stage, or phase, that combines oxidant 40 and
chlorite 30b (and
optionally caustic) results in a high reduction in the initial demand 200, or
high percentage of
contaminant reduction, as compared to oxidation/aeration with only air/oxygen.
For
example, the reduction of initial demand 200 in treatment vessel 101 can be as
much as 70-
80% during phase 1 of the disclosed process. In certain embodiments, and as
shown in FIG
1, treatment system 150 can be set up so that chlorine dioxide (30a) can be
introduced into
fluid 2a instead of, or in combination with, chlorite 30b during Phase 1.
[0061] In one or more embodiments, first treatment vessel 101 has
skimming means 6
for removing hydrocarbons or other low specific gravity material that has
risen to the top of
fluid 2a. Skimming means 6 are well known in the art of wastewater treatment,
and include
oil skimmers, (e.g. a paddle type), conveying belt, dissolved air flotation or
other equipment
known in the art to remove lighter solids, hydrocarbons or floc from the
surface of a
treatment tank. In certain embodiments, the amount of hydrocarbon removed at
this phase
can be in the range of about 1-2% of fluid 2a.
16
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[0062] In one or more embodiments, first treatment vessel 101 also has a
separation
system (or removal means) 7 for removing precipitated or settled high density
solids from the
bottom of treatment vessel 101. Separation system, or removal means, 7 include
mechanical
equipment known in the art of wastewater treatment that can removed settled
solids from the
bottom of a treatment tank, and can include, for example, an auger or scraper
mechanism. In
one exemplary embodiment, the auger or other scraping mechanism sits at the
bottom of the
vessel 101, pulls the settled solids to center of the tank, where these is a
pit, and the solids are
pumped out of vessel 101 through the pit.
[0063] In the second phase of the embodiments of the invention claimed
herein, and
as shown in FIG.1, using pump 27, treated fluid 2a is pulled or discharged
from first
treatment vessel 101 to the centerline 8b of a second treatment vessel 102 via
transfer line 9.
Chlorine dioxide gas is introduced into fluid 2a at injection port 29 via
transfer line 9 and
prior to distribution into second vessel 102 as fluid 2b. The chlorine dioxide
dosage
introduced into fluid 2a is sufficient to achieve a chlorine dioxide residual
in fluid 2b within
second treatment vessel 102 and is dependent on the amount of biological
contamination in
the fluid. In some embodiments, the chlorine dioxide 30a dosage comprises
about 10 mg/1 to
about 500 mg/1, for example, 10, 20, 25, 47, 50, 100, 120, 150, 210, 230, 250,
300, 335, 350,
400, 500 mg/1, including any and all ranges and subranges therein (e.g., 10 to
500 mg/1, 10 to
335 mg/1, 25 to 500 mg/1, 25 to 335 mg/1, 25 to 230 mg/1, 45 to 335 mg/1, 45
to 500 mg/1, 47
to 335 mg/1, 50 to 400 mg/1, 20 to 230 mg/1, 20 to 47 mg/1, 10 to 50 mg/1,
etc.).
[0064] In certain embodiments, the amount of biological contamination can
be
determined or monitored prior to introducing raw fluid 2 into treatment vessel
101,
immediately upon withdrawing fluid 2a from treatment vessel 101, or both. For
example,
one or more samples from frac storage tanks 500, inlet 17, vessel 101,
distribution line 5, or
some combination of the same, may be removed and tested for microbial
contamination. In
17
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
alternative embodiments, in-line monitoring equipment may be coupled at
various places
along the treatment system to allow for continuous monitoring of biological
contamination.
Testing can be accomplished by test known to those skilled in the art for
determining
biological demand and/or microbial kill.
[0065] The target residual concentration of chlorine dioxide in the
treated fluid 2b
depends on the intended storage period prior to reuse as, for example, a
hydraulic fluid. For
example, for immediate use as frac water in a hydraulic fracturing system, the
desired
chlorine dioxide residual of fluid 2b is between about 0.1 mg/1 and about 20
mg/1, preferably
between about 0.5 mg/1 and about 5 mg/l. If, on the other hand, fluid 2b is to
be stored for
several days or more before being reused as a hydraulic fluid, the target
residual
concentration of chlorine dioxide should be between about 5 mg/1 and about 50
mg/1,
preferably between about 20 mg/1 and about 50 mg/l. One of ordinary skill in
the art can
calculate and determine the required target residual, and thus dosage
required, depending on
the characteristics of fluid 2b, intended storage time, intended use and other
factors.
[0066] For example, in some embodiments, the target residual comprises
0.1 mg/1, 0.2
mg/1, 0.3 mg/1, 0.5 mg/1, 1.0 mg/1, 1.5 mg/1, 3 mg/1, 5 mg/1, 10 mg/1, 15
mg/1, 20 mg/1, 25
mg/1, 40 mg/1, or 50 mg/1, including any and all ranges and subranges therein
(e.g., 0.1 to 50
mg/1, 0.1 to 20 mg/1, 0.1 to 10 mg/1, 0.1 to 5 mg/1, 0.1 to 2 mg/1, 0.5 to 20
mg/1, 0.5 to 10
mg/1, 0.5 to 5 mg/1, 1.0 to 20 mg/1, 1.0 to 10 mg/1, 5 to 10 mg/1, 5 to 40
mg/1, 5 to 50 mg/1, 10
to 50 mg/1, 20 to 50 mg/1, etc.).
[0067] Any appropriate method of producing chlorine dioxide known in the
art may
be used to generate chlorine dioxide suitable for use in the present
invention. In general,
chlorine dioxide solutions can be produced by treatment of chlorite salt
solutions (e.g.
NaC102) with an acid solution to produce acidic solutions that contain C102,
which can be
then be flushed as a gas into water to produce aqueous C102. Other precursors
such as sodium
18
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
chlorate can also be used. For example, in a preferred embodiment, the present
invention
provides a process that comprises producing chlorine dioxide by using an
apparatus such as a
chlorine dioxide generator, e.g. as disclosed and claimed in U.S. Pat. No.
6,468,479, the
disclosure of which is incorporated herein by reference. The chlorine dioxide
is generated
either directly as a gas, or preferably as an aqueous (or other suitable
liquid carrier) chlorine
dioxide mixture. The generator is preferably run using an excess of sodium
chlorite to reduce
the possibility of generating chlorine gas as an impurity.
[0068] Referring to FIG 1, chlorine dioxide generator 75 is placed in
line with the
treatment system, and the chlorine dioxide precursors (hydrochloric acid,
sodium chlorite,
and sodium hypochlorite are stored in tanks 76, 77 and 78, respectively.
Venturi 28 has a
water stream flowing there through which establishes a vacuum and draws the
chlorine
dioxide from the reaction column of the generator into the water stream to
form aqueous
chlorine dioxide.
[0069] In an exemplary embodiment, second treatment vessel 102 has a
residence
time for fluid 2b of approximately 10 to 30 minutes, although reaction time
will vary
depending on the nature of fluid 2b. For example, in some embodiments the
residency time
will be 10, 13, 15, 18, 20, 22, 25, 27 or 30 minutes, including any and all
ranges and
subranges therein (e.g., 10 to 30 minutes, 12 to 30 minutes, 15 to 30 minutes,
20 to 30
minutes, 10 to 20 minutes, 10 to 25 minutes, 15 to 25 minutes, etc.). Second
vessel 102 has
underflow means 13 to a third treatment vessel 103. Underflow means 13 can
include a
conduit, pipe, transfer line, or any other means for allowing fluid 2b located
at the bottom of
vessel 102 to flow into vessel 103 that are well known in the art for
wastewater treatment
systems. In an exemplary embodiment, second vessel 102 also comprises skimming
means
14 (not shown) to remove light flocculate material, or other materials with
low specific
19
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
gravity, off the surface or top of second treatment vessel 102. As used
herein, examples of
skimming means 14 are well known in the art and may include, for example oil
skimmers,
(e.g. a paddle type), conveying belt, dissolved air flotation or other
equipment known in the
art to remove lighter solids, hydrocarbons or floc from the surface of a
treatment tank.
[0070] Third treatment vessel 103 contains fluid 2c. Vessel 103 has a
residence time
of approximately 10 to 30 minutes, although reaction time will vary depending
on the nature
of fluid 2c. For example, in some embodiments the residency time will be 10,
13, 15, 18, 20,
22, 25, 27 or 30 minutes, including any and all ranges and subranges therein
(e.g., 10 to 30
minutes, 12 to 30 minutes, 15 to 30 minutes, 20 to 30 minutes, 10 to 20
minutes, 10 to 25
minutes, 15 to 25 minutes, etc.).
[0071] Third vessel 103 has overflow means 15 (not shown) to a fourth
vessel 104 or
clear well. As used herein, examples of overflow means 15 are well known in
the art and
may include, for example, a conduit, pipe, transfer line, or any other means
for allowing fluid
2c to flow from the top of vessel 103 into vessel 104 that are well known in
the art for
wastewater treatment systems. Third vessel 103 also has means for removal of
high density
solids from treated fluid 2d from the bottom of vessels 103, examples of which
are well
known in the art and may include, for example, scrapers or augers at the
bottom of the tank.
[0072] In the embodiment shown in FIG 1, treatment vessels 101-104 are
frac tanks
located on site at a oil and gas production site, and the disclosed and
claimed produced water
treatment system is integrated with a closed loop system already in place at
the site. In
alternate embodiments, the above method and system can be modified yet still
fall within and
be consistent within the invention disclosed herein. For example and more
generally, in
alternate embodiments exemplified generally in FIG. 2, a closed loop treatment
system 150 in
accordance with the claims of the invention is shown. In FIG 2, closed loop
treatment system
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
150 comprises a venturi 20, a fluid stream 100 (e.g. wastewater 15) to be
treated,
treatment/storage vessel or system 50, chlorine oxide 30 and oxidant 40.
[0073] Fluid stream 100 comprises wastewater 15, for example, a
wastewater fluid
stream from an oil and gas site. For example, in FIG 1, wastewater 15 is raw
produced water
2. A combination of a chlorine oxide 30 and an oxidant 40 is introduced into a
fluid stream
100. Chlorine oxide 30 preferably comprises chlorite (30b), but it can also
include chlorine
dioxide (30a), or a combination of chlorite (30b) and chlorine dioxide (30a),
which are
introduced into stream 100. For example, as was disclosed in connection with
FIG.1,
sodium chlorite (i.e. chlorite 30b) can be introduced via eduction using a
venturi 20 (or
venturi 4, if referring to FIG 1, wherein venturi 20 is part of fluid stream
100 being treated, or
other means well known in the art. In alternate embodiments, one of ordinary
skill in the art
could use techniques known in the art for introducing, mixing and/or diffusing
chemicals
within a treatment system, either directly in the tank or in a fluid flow
line, including but not
limited to inductor pumps, high pressure injectors, pumps, flow lines,
conduits, mixers,
spargers or a combination of the same.
[0074] In exemplary embodiments, oxidant 40 can be air, oxygen, oxygen-
enriched
air, or any chemical oxygen source or combination that is stable with chlorine
dioxide (30a)
and/or chlorite (30b), or some combination of the same. And, in one or more
embodiments
of the invention, oxidant 40 is introduced via direct injection into the
wastewater in fine
bubbles (i.e. air sparging, a pressurized source, an aerator, mechanical
agitation, a diffuser,
spraying, or eduction via venturi 20 (4, 28) (see, e.g., FIGs 1-9). If ozone
is used as oxidant
40, it should not be used in combination with chlorine dioxide in the first
step because ozone
consumes chlorine dioxide. Rather, chlorine dioxide should only be used in the
second
phase, or step, and only after the ozone residual in the waste water, or
aqueous volume, is
depleted or close to depletion.
21
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[0075] In accordance with exemplary embodiments of the invention as
applied to the
oilfield and fraccing industry, storage/treatment vessel (or system) 50
contains wastewater
15, which is supplied by a source 10. Source 10 comprises a source of produced
water. In
alternate embodiments, source 10 comprises flowback water, surface water,
municipal water,
frac water, wastewater, or any combination thereof One of ordinary skill in
the art will also
recognize that wastewater 15 can be any water or target aqueous fluid that is
contaminated
(for example, with organics and/or microorganisms) and is being recycled or
treated for
reuse, storage and/or discharge back into the environment, regardless of
industry. In one or
more embodiments, the oxidant demand of the contaminants in wastewater 15,
prior to
treatment, is from about 30 mg/1 to about 5000 mg/1, preferably from about 50
mg/1 to about
500 mg/l. The oxidant demand comprises reducing agents including, but not
limited to,
reduced sulfur compounds, biomass and other biological by-products, and
reduced metals
including but not limited to iron (Fe) II.
[0076] For example, in some embodiments, the oxidant demand comprises 30,
50,
100, 250, 300, 500, 750, 800, 900, 1,000, 1,250, 1,500, 2,000, 3,000, 4,000,
and 5,000 mg/1,
including any and all ranges and subranges therein (e.g., 30 to 5000 mg/1, 100
to 2000 mg/1,
300 to 2000 mg/1, 500 to 2000 mg/1, 1000 to 2000 mg/1, 30 to 1000 mg/1, 50 to
1000 mg/1,
100 to 1000 mg/1, 500 to 5000 mg/1, 50 to 500 mg/1, etc.).
[0077] In certain embodiments, venturi 20 is used to both generate and
introduce
chlorine oxide 30 (i.e. chlorine dioxide (30a), and/or a combination of
chlorine dioxide (30a)
and chlorite (Mb)) into fluid stream 100 and, additionally, to then introduce
oxidant 40. In
other embodiments, separate venturis are used (e.g. venturi 4 & 28 in FIG 1).
In a preferred
embodiment, a drive fluid 33 for venturi 20 comes directly from storage vessel
50. Vessel 50
contains wastewater 15, i.e. the wastewater to be treated, or a combination of
treated
22
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
wastewater (or other target fluid) and the wastewater to be treated. One of
ordinary skill in
the art will recognize, however, that drive fluid 33 can come from any
available water source
placed in line with system 150.
[0078] As shown in FIGs 2-8, storage/treatment system 50 comprises a
single tank or
vessel. However, in accordance with the invention, vessel system 50 comprises
multiple
tanks, pits or ponds, or any other storage means (e.g. reservoir, container,
or lagoon) that
stores, holds, transports or contains wastewater 15 from source 10. Vessel
system 50 may
comprise one or more treatment tanks, vessels, containers or other wastewater
treatment
systems suitable for treating wastewater 15. For example, as described in
connection with
FIG.1, vessel 50 actually comprises four treatment vessels 101-104 and storage
tanks 500.
[0079] In embodiments of the invention, chlorine oxide 30 and oxidant 40
are applied
at such a rate that the removal of volatile reductants (i.e. hydrogen sulfide)
is via oxidation,
rather than physical purging or stripping. By selecting an air flow rate that
prevents or avoids
off-gassing of the hydrogen sulfide (or other volatile reductants) present in
wastewater 15, the
reductants are oxidized in situ rather than purged. The goal is to add oxidant
40 to the fluid at
a flow rate that brings it into contact with the sulfides to allow oxidation
to occur. Thus, a
flow rate that results in the addition of air being violent, and thus
stripping the sulfides before
they can oxidize, should be avoided. The volume of fluid to be treated in
vessel system 50
will directly affect the range of flow rates that can be used to avoid off-
gassing/purging and,
thus, the appropriate range is widespread. For example, a small tank would
require a much
lower air flow rate than a deep pond. However, one of ordinary skill in the
art will be able to
determine the appropriate flow rate to avoid purging, or stripping, of the
volatiles, depending
on the volume, depth and/or size of vessel system 50 (or fluid to be treated),
the treatment
system as a whole, and oxidant demand.
23
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[0080] In FIG. 3, a diffuser 70 is used to introduce oxidant 40. In one
or
embodiments, and as shown in FIG. 4, oxidant 40 is added directly to
wastewater 15 via a
pressurized source 80 near the bottom of vessel 50 and mechanical action can
thereby be used
to enhance mixing of wastewater 15 within vessel 50. If a single point
introduction method is
used, it is preferred that the injector be movable throughout the horizontal
plane of vessel 50
(not shown).
[0081] In one or more of the embodiments disclosed herein, chlorine oxide
30 and
oxidant 40 are introduced into wastewater 15 as follows. Initially, chlorine
oxide 30 is
introduced for a sufficient amount of time and at a sufficient dosage to
reduce the chlorine
dioxide demand of the wastewater 15 in the range of about 10 percent to about
20 percent,
including all ranges and subranges therein (e.g. 12 percent, 15 percent, etc).
The amount of
time and dosage required will depend on the characteristics of wastewater 15
(e.g. chlorine
dioxide demand), the treatment system, and the intended use or application. In
one or more
embodiments, during this initial (or first) stage of treatment, chlorine oxide
30 comprises
chlorite (30b) only. In embodiments where chlorine oxide 30 comprises chlorite
(30b) only,
the step of introducing oxidant 40 may be (and, in many instances, is
preferred to be)
performed soon after, simultaneously, substantially simultaneously, or
substantially
contemporaneously therewith (See, e.g. FIG 1, description for FIG 1 and
examples herein).
In embodiments of the invention, chlorite 30b is added at an appropriate
dosage and period of
time to achieve a dosage ranging from about 10 mg/1 to about 500 mg/lin fluid
2a to be
treated. For example, in some embodiments, the chlorite 30b dosage comprises
10, 20, 23,
27, 30, 50, 100, 120, 180, 230, 250, 300, 420, 500 mg/1, including any and all
ranges and
subranges therein (e.g., 10 to 500 mg/1, 25 to 500 mg/1, 25 to 300 mg/1, 10 to
300 mg/1, 10 to
100 mg/1, 25 to 200 mg/1, 50 to 500 mg/1, 50 to 420 mg/1, 23 to 420 mg/1, 27
to 420 mg/1, 23
to 300 mg/1, 27 to 300 mg/1, 23 to 230 mg/1, etc.).
24
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[0082] In alternate embodiments, chlorine oxide 30 may be chlorine
dioxide (30a),
chlorite (30b) or a combination thereof Because chlorine dioxide reacts as a
free radical
and, therefore, reacts almost instantaneously, chlorine dioxide cannot be
added at high rates
or concentrations at the same time as when a large volume of oxidant 40 is
being added.
Therefore, if chlorine oxide 30 comprises chlorine dioxide (30a) during this
initial step (or a
combination of chlorite (30b) and chlorine dioxide (30a)), oxidant 40 cannot
be added at the
same time until all of the chlorine dioxide (30a) has converted to chlorite
(30b) or, if
performed simultaneously, oxidant 40 must be added at a rate low enough to
make sure any
chlorine dioxide is not stripped, or purged, from wastewater 15 before it
disperses through the
fluid body.
[0083] Furthermore, in certain embodiments, caustic 90 (not shown) can be
added
either prior to treatment with chlorine oxide 30, or concurrently therewith,
to raise the pH of
wastewater 15 to about 7 - 10. For example, in the embodiment described with
FIG 1,
sodium hydroxide (caustic 90) is added to achieve a pH of about 8.5. By
introducing a higher
pH for wastewater 15, contaminant metals (for example, iron (Fe)) will drop
out of solution
and the formation of certain metal complexes that tend to form in low pH will
be avoided. In
still other embodiments anticipated within the scope of this invention, the
first step of adding
chlorine oxide 30 can be skipped, depending on the chlorine dioxide demand and
the
application/system at hand.
[0084] Oxidant 40 is introduced into wastewater 15 either prior to
treatment with
chlorine oxide 30, or substantially concurrently therewith. Oxidant 40 is
added at an
appropriate dosage and period of time to achieve an overall dosage ranging
from about 20
mg/kg to about 2000 mg/kg of oxidant 40 to the total volume of wastewater 15
to be treated,
including all ranges and subranges therein, with a more preferred dosage of
about 20 mg/kg
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
to about 1000 mg/kg of oxidant 40 to the total volume of fluid to be treated.
Again, the
amount of time and dosage required will depend on the characteristics of
wastewater 15 (e.g.
chlorine dioxide demand), the treatment system, and the intended use or
application, together
with other mechanical considerations known to those of ordinary skill in the
art. In one or
more embodiments, the application of oxidant 40 consumes, in total, from about
10 percent to
about 90 percent of the total chlorine dioxide demand, preferably from about
60 percent to
about 90 percent of the chlorine dioxide demand. For example, in some
embodiments the
oxidant will consume 10, 20, 25, 40, 50, 60, 65, 70, 80, or 90 percent of the
chlorine dioxide
demand, including any and all ranges and subranges therein (e.g., 10 to 25
percent, 10 to 70
percent, 15 to 90 percent, 20 to 80 percent, 50 to 85 percent, 60 to 80
percent, 60 to 90
percent, etc.).
[0085] The step of introducing oxidant 40 can be performed substantially
contemporaneous with, simultaneously with, or immediate after the first step
of adding
chlorine oxide 30, in particular when chlorine oxide 30 is chlorite (30b)
only, during the first
treatment step. As discussed earlier, the synergistic impact of oxidant and
chlorine oxide can
reduce the initial demand by as much as 70-80%, or more.
[0086] In the next (or second treatment) step, chlorine oxide 30 is
introduced at a
dosage sufficient to achieve a target C102 residual in the fluid, which is
based upon the
oxidant demand in the fluid. In FIG 1, for example, after sodium chlorite,
air, and caustic are
introduced in Step 1 of the treatment process into treatment vessel 101 for a
period of time
(i.e. about 15 to about 60 minutes), chlorine dioxide is introduced in the
second step into
second treatment vessel 102 until a target residual of chlorine dioxide is
reached. However,
in alternative embodiments, during this second step chlorine oxide 30 may
comprise one or
26
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
more of chlorine dioxide (30a), chlorite (30b) or a combination thereof It can
be, but does
not have to be, the same chlorine oxide that was used in the first initial
step.
[0087] The target residual concentration of chlorine dioxide in the
treated fluid or
wastewater depends on the intended storage period prior to reuse as, for
example, a hydraulic
fluid. For example (and as shown in FIGs 7-9), for immediate use as frac water
in a
hydraulic fracturing system, the desired chlorine dioxide residual of fluid
200 exiting the
system is between about 0.1 mg/1 and about 20 mg/1, preferably between about
0.5 mg/1 and
about 5 mg/1, and including any and all ranges and subranges therein (See Ilt
64-65). By way
of further example (see, e.g. FIGs 2-6), if the treated fluid is to be stored
in vessel 50 for
several days or more, the target residual concentration of chlorine dioxide
should be between
about 5 mg/1 and about 50 mg/1, preferably between about 20 mg/1 and about 50
mg/1, and
including any and all ranges and subranges therein (See Ilt 64-65). As
discussed above, in
exemplary embodiments, chlorine oxide 30 comprises only chlorine dioxide (30a)
during the
last stage of the treatment process. During this treatment step, chlorine
oxide 30 (in the form
of chlorine dioxide (30a)) and oxidant 40 cannot be added to stream 100 at the
same time.
[0088] In one or more embodiments of the invention, the total treatment
time required
for wastewater 15 to achieve oxidation and/or disinfection is less than 24
hours, preferably
less than 8 hours, if storage/treatment system or vessel 50 comprises a tank,
pit, pond, or
lagoon. In still other embodiments, the total treatment time required for
wastewater 15 to
achieve oxidation and/or disinfection is less than about 60 minutes, and
preferably less than
about 15 minutes, if system/vessel 50 is a pipeline, or a combination pipeline
and a tank, such
as would be used for "on the fly" operations out in the field (see, e.g., FIGs
1, 6-8), when
there is a limited residency time and treated fluid 200 is to be used
immediately.
27
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[0089] Referring to the embodiments shown in FIGs 2-5, various
embodiments of
treatment system 150 are shown. In FIG.3, air injection is used to introduce
oxidant 40 into
vessel 50 via diffuser 70 and a pressurized source 71. In FIG. 4, a chemical
tank and
pressurized source 80 is used to introduce oxidant 40. In FIG. 5, diffuser 70
and pressurized
source 71, placed in-line, are used to introduce oxidant 40 into stream 100.
In certain
embodiments, mechanical agitator 92 is used. Referring to the embodiments
shown in FIGs
6-8 (and also FIG 1), various embodiments of treatment system 250 are shown.
Specifically,
in these embodiments, treatment system 150 comprises an "in-line" treatment
system 250, for
example, a frac-on-the-fly treatment system or any other industrial water
treatment system
that is placed in-line for immediate use. In FIG. 6, oxidant 40 is introduced
in-line via
venturi 20 from chemical source 72. In FIG. 7, a chemical tank 80 is used in-
line to
introduce oxidant 40 into stream 100. In FIG. 8, air injection is used to
introduce oxidant 40
into stream 100 via diffuser 70 and a pressurized source 71. Although not
shown, treatment
system 150/250 may also include a source, a means for introducing, caustic 90
as well as
other wastewater treatment methods known in the art, such as mechanical
agitators, overflow
systems, defoaming agents, and electronic sensors and monitoring devices.
[0090] As discussed above, any appropriate method of producing chlorine
dioxide
known in the art may be used to generate chlorine dioxide suitable for use in
the present
invention. In general, chlorine dioxide solutions can be produced by treatment
of chlorite salt
solutions (e.g. NaC102) with an acid solution to produce acidic solutions that
contain C102,
which can be then be flushed as a gas into water to produce aqueous C102.
Other precursors
such as sodium chlorate can also be used.
[0091] Several chemical means of generating chlorine dioxide and their
corresponding chlorine dioxide precursor chemicals are known in the art, and
the choice of
28
CA 02906186 2015-09-11
WO 2014/145825
PCT/US2014/030654
suitable means and chemicals is within the abilities of those skilled in the
art. Exemplary
chemical means of generating chlorine dioxide are disclosed in U.S. Pat. Nos.
4,689,169
(Mason et al.), 5,204,081 (Mason et al.), 5,227,306 (Eltomi et al.), 5,258,171
(Eltomi et al.),
5,965,004 (Cowley et al.), and 6,645,457 (Mason et al.) the disclosures of
which are
incorporated herein by reference.
[0092] In preferred embodiments, the chlorine dioxide should be of the
highest
possible purity. More specifically, chlorine gas should be present in the
introduced chlorine
dioxide gas at a level less than about 5%, preferably less than about 0.5%.
For example, in a
preferred embodiment, the present invention provides a process that comprises
producing
chlorine dioxide by using an apparatus such as a chlorine dioxide generator,
e.g. as disclosed
and claimed in U.S. Pat. No. 6,468,479, the disclosure of which is
incorporated herein by
reference. The chlorine dioxide is generated either directly as a gas, or
preferably as an
aqueous (or other suitable liquid carrier) chlorine dioxide mixture. The
generator is
preferably run using an excess of sodium chlorite to reduce the possibility of
generating
chlorine gas as an impurity. Other generally accepted methods for generating
chlorine
dioxide can be found in, for example, U.S. Patent Pub. No. 2006/0068029 (U.S.
Pat. App.
No. 11/131021), the disclosure of which is incorporated herein by reference.
Furthermore,
the generator preferably uses wastewater 15 as the drive fluid for generating
chlorine dioxide
and brings chlorine dioxide gas into contact with wastewater 15 under a vacuum
pressure
such that the chlorine dioxide gas is drawn into wastewater 15 to form a
chlorine dioxide
aqueous solution.
[0093] In certain embodiments, the fluid to be treated is circulated
through a closed-
loop system and treated in situ in accordance with the methods and systems
disclosed herein,
in repetition, until the contaminants are oxidized and the appropriate
residual of chlorine
29
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
dioxide is achieved prior to discharge from system 150. In still other
embodiments, after
treatment with chlorine oxide 30 and second oxidant 40, the treated fluids are
allowed to
stand in vessel 50 for an appropriate period of time to allow the solids to
settle and free oil to
be skimmed prior to reuse or discharge using wastewater treatment methods
known in the art.
In still other embodiments, the fluid treated is used (or reused) immediately
after treatment
for subsequent crude oil, hydraulic fracturing, or other industrial
applications. FIG 9 is an
schematic iillustration of another embodiment of the invention.
[0094] It should be understood that the treatment system and method
disclosed herein
may be coupled, upstream or downstream, to treatment units or systems already
in place for
treating or transferring produced water. For example, the treatment system
claimed herein
can be added into a system already located at an oil and gas production site.
Additionally, in
exemplary embodiments and referring to FIG 1 as one example, the concentration
of
biological contaminants, particulate size, volatile compounds, TDS, and
inorganics may be
monitored as the treated fluid 2d exits vessel 104 and/or the treatment
system. Monitoring
can be continuous or periodic. If the fluid exiting the treatment system is
not within a
predetermined acceptable range, the fluid may be recycled back into the
treatment system
and/or the amount of oxidant and/or chlorine dioxide introduced into the
treatment system
may be modified. Similarly, in some embodiments, all or a portion of the
stream flowing out
of the treatment system may be recycled through the treatment system via one
or more
recycle lines (not shown). Recycling the fluid stream through the system for a
number of
passes may allow for significant reduction of the concentration of
contaminants. Referring
again to FIG 1 as one example, in some embodiments a portion of the stream
exiting the
system may be mixed with a portion of the stream entering the treatment system
inlet 17.
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[0095] Although the Examples and descriptions above discuss what is, in
essence, a
closed loop treatment system, the systems and methods disclosed herein and
claimed could
also be utilized for a frac "on the fly" system and method, wherein the
treated water would be
used immediately and/or shortly after being treated for fracturing (See FIGs 6-
8). For
example, in one embodiment, the frac water to be injected into the
subterranean formation
would be treated using the methods disclosed herein out in the oilfield, ahead
of the well
head. For this system, you would continuously be filling vessel 50 (e.g.
onsite frac tanks,
located at the frac site/oilfield) with source water that needs to be treated
prior to introduction
into the well. The water could comprise surface water, municipal water,
produced water,
flow back water, or any combination of the above ("commingled water").
[0096] Furthermore, in alternative embodiments of the invention, the
system or
process disclosed herein may be combined with one or more traditional or
nontraditional
biocides, either oxidizing or non-oxidizing, to achieve a synergistic biocidal
effect.
Additionally, in alternative embodiments, one of ordinary skill in the art
will readily
appreciate that additional treatment processes known in the art can be
incorporated in line or
elsewhere in the system (either prior to treatment in accordance with this
invention, or
subsequent thereto) in either batch or continuous operation. By way of example
only, and not
meant to be limiting, treatment processes to remove oil and/or solids can be
incorporated into
the system, or if foaming occurs, one might incorporate a chlorine dioxide
compatible
defoamer. Similarly, in certain embodiments, the method and system disclosed
herein can be
added to, or retrofitted into, a preexisting recycling or treatment system,
and it can be
conducted continuously in-line or in selected quantities in a batch process.
One of ordinary
skill in the art will also readily appreciate that in one or more embodiments,
appropriate
measurement and monitoring apparatus and/or equipment may be incorporated into
the
method and system disclosed herein.
31
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[0097] In the embodiments disclosed herein, one of ordinary skill in the
art will
appreciate that chlorine dioxide residual can be determined and/or calculated
using Method
4500-C102 E Amperometric Method II described in Standard Methods the Analysis
of Water
and Wastewater, or via modified versions of the same, wherein Standard Method
4500-C102
E Amperometric Method II uses the following calculations:
C102 (mg/L) = 1.25 x (B-D) x 0.00564 x 13,490 / 200
Chlorite (mg/L) = D x 0.00564 x 16,863 / 200
Chlorine (mg/L) = [A ¨ (B ¨ D) / 4] x 0.00564 x 35,453 / 200,
where Titration A titrates the chlorine and one-fifth of the available
chlorine dioxide,
Titration B titrates four-fifths of the chlorine dioxide and chlorite,
Titration C titrates the non-
volatilized chlorine (nitrogen gas purges the sample of the chlorine dioxide),
but is not used
in any calculation, and Titration D titrates the chlorite. In still other
embodiments, chlorine
dioxide residual can be determined spectrometrically or by measurement of
oxidation
reduction potential (ORP), each of which are incorporated herein, or via
modified versions of
the same.
CLAUSES
[0098] Embodiments of the present invention, include but are not limited
to, the
following:
1. A method for treating an aqueous system, comprising:
providing an aqueous fluid volume having an initial oxidant demand;
introducing an oxidant into the aqueous volume at a flow rate that avoids off-
gassing
of volatile reductants from the aqueous volume, wherein said oxidant is
selected from
oxygen, air, oxygen-enriched air, and a combination of the same;
32
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
combining the aqueous volume and oxidant for a minimum residence time
sufficient
to lower the initial oxidant demand to a reduced oxidant demand;
providing at least one chlorine oxide; and
combining the aqueous volume and a quantity of at least one chlorine oxide in
an
amount sufficient to eliminate the reduced oxidant demand, wherein said at
least one chlorine
oxide is selected from chlorine dioxide, chlorite, and a combination of the
same.
2. The method of claim 1 wherein the step of combining the aqueous fluid
volume and a
quantity of at least one chlorine oxide comprises the steps of:
a) introducing a first chlorine oxide into the aqueous volume either prior to
or
substantially contemporaneous with the step of introducing the oxidant,
wherein said first
chlorine oxide comprises sodium chlorite; and
b) introducing a second chlorine oxide into the aqueous volume after the step
of
combining the aqueous volume and oxidant for said minimum residence time,
wherein said
second chlorine oxide comprises chlorine dioxide, chlorite, or a combination
of the same.
3. The method according to claim 2 wherein sodium hydroxide is introduced
into the
aqueous volume prior to the step of introducing the oxidant, either in
combination with the
sodium chlorite or as a separate feed substantially contemporaneously
therewith.
4. The method according to any of claims 1-3 comprising the additional step
of
measuring and maintaining a chlorine dioxide residual of at least about 0.1
mg/1 in the
aqueous volume after treatment.
5. The method of claim 4 wherein the chlorine dioxide residual is in the
range of about
0.1 mg/1 to about 50 mg/1 in the aqueous volume.
6. The method according to any of claims 2-5 comprising the additional step
of allowing
solids formed in the aqueous volume to settle or rise and separating the
solids from the
33
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
wastewater contained therein prior to the step of introducing the second
chlorine oxide into
the aqueous volume.
7. The method according to any of claims 1-6 wherein the source of the
aqueous volume
is chosen from the group consisting of an aqueous fluid stream, a vessel,
tank, pit, lagoon, or
pond for storing waste water, a water treatment plant, a hydraulic fracturing
tank, or a piece
of equipment, pipeline or vessel used for hydraulic fracturing or crude oil
production.
8. The method according to any of claims 1-6 wherein the aqueous system is
chosen
from the group consisting of hydraulic fracturing, crude oil production, water
distribution
systems, fluid transporting pipelines, wastewater treatment facilities,
storage tanks, food and
beverage processing lines, machining coolant or metalworking fluid (MWF)
systems, coal
and mineral slurries, metal leaching fluids, acid mine drainage, or any
aqueous system
contaminated by biological species or sulfur compounds.
9. The method according to any of claims 1-8 comprising the step of
generating a
chlorine dioxide aqueous solution using a chlorine dioxide generator that
brings chlorine
dioxide gas into contact with a portion of the aqueous volume to be treated
under a vacuum
pressure such that the chlorine dioxide gas is drawn into a portion of the
aqueous volume to
be treated to form the chlorine dioxide aqueous solution.
10. The method according to any of claims 1-9 wherein the aqueous system
comprises
one or more separation apparatus for removing precipitated contaminants from
the aqueous
volume either during, before or after treatment.
11. The method according to any of claims 1-10 that reduces, inactivates,
destroys,
eliminates or removes from the aqueous volume contaminants selected from the
group
consisting of Ca, Mg, Na, Fe, Cl, Mn, CaC103, SO4, Ba, hydrocarbons, total
dissolved
solids, biological contamination, and combinations thereof
34
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
12. A method for reducing, inactivating, destroying, removing, or
eliminating from an
aqueous fluid contaminants selected from the group consisting of Ca, Mg, Na,
Fe, Cl, Mn,
CaC103, SO4, Ba, hydrocarbons, total dissolved solids, biological
contamination, and
combinations thereof, comprising the steps of introducing an oxidant and
introducing at least
one chlorine oxide, wherein said oxidant is selected from the group consisting
of oxygen, air,
oxygen-enriched air, and combinations thereof and said at least one chlorine
oxide is selected
from the group consisting of chlorine dioxide, chlorite, and combinations
thereof
13. The method of claim 12 wherein the step of introducing the at least one
chlorine oxide
comprises the steps of:
a) introducing a first chlorine oxide into the aqueous fluid either prior to
or
substantially contemporaneous with the step of introducing the oxidant,
wherein said first
chlorine oxide comprises sodium chlorite; and
b) introducing a second chlorine oxide into the aqueous fluid after the step
of
combining the aqueous fluid and oxidant, wherein said second chlorine oxide
comprises
chlorine dioxide, chlorite, or a combination of the same.
14. The method according to claim 13 wherein sodium hydroxide is introduced
into the
aqueous fluid prior to the step of introducing the oxidant, either in
combination with the
sodium chlorite or as a separate feed substantially contemporaneously
therewith.
15. The method according to either of claims 13-14 wherein the second
chloride oxide is
an aqueous chlorine dioxide solution.
16. The method according to claim 15 further comprising the step of
generating the
chlorine dioxide aqueous solution by using a portion of the aqueous fluid to
be treated.
17. The method of according to any of claims 12-16 wherein the oxidant is
introduced at
a flow rate that avoids off-gassing of volatile reductants from the aqueous
fluid.
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
18. The method according to any of claims 12-17 comprising the additional
step of
measuring and maintaining a chlorine dioxide residual in the range of about
0.1 mg/1 to about
50 mg/1 in the aqueous fluid after treatment.
19. The method according to any of claims 12-18 wherein the source of the
aqueous fluid
stream or volume is chosen from the group consisting of a vessel, tank, pit,
lagoon, or pond
for storing waste water, a water treatment plant, a hydraulic fracturing tank,
or a piece of
equipment, pipeline or vessel used for hydraulic fracturing or crude oil
production.
20. A method for treating an aqueous system, comprising:
introducing an oxidant into an aqueous volume at a flow rate that avoids off-
gassing
of volatile reductants from the aqueous volume prior to introducing chlorine
dioxide into the
volume, wherein said oxidant is selected from the group consisting of oxygen,
air, oxygen-
enriched air, and combinations thereof, and wherein the oxidant provides
synergistic
oxidation activity in the presence of the chlorine dioxide such that the
chlorine dioxide is
introduced at substantially reduced amounts as compared to a predetermined
chlorine dioxide
demand.
21. A method according to claim 20 comprising the additional step of
introducing sodium
chlorite into said volume prior to the step of introducing the oxidant.
22. A method for treating an aqueous system, comprising:
providing an aqueous volume having an initial oxidant demand;
introducing an oxidant into the aqueous volume, wherein said oxidant comprises
oxygen, air, oxygen-enriched air, or a combination of the same, at a flow rate
that avoids off-
gassing of volatile reductants from the aqueous volume;
introducing sodium chlorite into the aqueous volume;
combining the aqueous volume, oxidant and sodium chlorite, thereby lowering
the
initial oxidant demand to a reduced oxidant demand;
36
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
introducing a separate feed of chlorine dioxide into the aqueous volume in an
amount
sufficient to eliminate the reduced oxidant demand and provide a chlorine
dioxide residual of
at least about 0.1 mg/1 in the aqueous volume.
23. A method for treating an aqueous system, comprising:
providing an aqueous volume having an initial oxidant demand;
introducing an oxidant into the aqueous volume at a flow rate that avoids off-
gassing
of volatile reductants from the aqueous volume, wherein said oxidant comprises
oxygen, air,
oxygen-enriched air, ozone or a combination of the same;
combining the aqueous volume and oxidant for a minimum residence time
sufficient
to lower the initial oxidant demand to a reduced oxidant demand;
providing at least one chlorine oxide; and
combining the aqueous volume and a quantity of at least one chlorine oxide in
an
amount sufficient to eliminate the reduced oxidant demand, wherein said at
least one chlorine
oxide comprises chlorine dioxide, chlorite, or a combination of the same,
provided that if the
at least one chlorine oxide comprises chlorine dioxide than said aqueous
volume does not
comprise an ozone residual.
24. A method for treating wastewater comprising the following steps:
introducing a wastewater stream to a fluid treatment system, the fluid
treatment
system comprising at least one first treatment cell, at least one second
treatment cell, and at
least one third treatment cell;
flowing the wastewater stream through at least one first fluid flow line to
the at least
one first treatment cell; withdrawing at least a portion of the wastewater
from the at least one
first treatment cell through at least one second fluid flow line;
introducing air into the withdrawn portion of the wastewater through at least
one in-
line air treatment point within the at least one second fluid flow line under
conditions
37
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
sufficient to produce thorough mixing of the air within the wastewater, and
introducing
sodium chlorite into the withdrawn portion of the wastewater through at least
one in-line
sodium chlorite treatment point within the at least one second fluid flow line
to produce
treated wastewater;
recirculating the treated wastewater back to the at least one first treatment
cell;
allowing solids formed in the treated wastewater to settle or rise in the at
least one
first treatment cell and separating the solids from the treated wastewater
contained therein;
discharging the treated wastewater from the at least one first treatment cell
to the at
least one second treatment cell through at least one third fluid flow line,
wherein chlorine
dioxide gas is introduced into the treated wastewater through at least one in-
line treatment
point within the at least one third fluid flow line at a determined rate
sufficient to provide a
chlorine dioxide residual within the treated wastewater before discharge into
at least one
second treatment cell;
allowing solids formed in the treated wastewater to settle or rise in the at
least one
second treatment cell and separating the solids from the wastewater contained
therein;
flowing the wastewater stream from the at least one second treatment cell to
at least
one first underflow outlet that discharges into the at least one third
treatment cell;
allowing solids formed in the wastewater to settle or rise in the at least one
second
treatment cell and separating the solids from the wastewater contained
therein;
and flowing the wastewater stream from the at least one third treatment cell
to at least
one first overflow outlet in fluid communication with a fourth treatment cell,
vessel or clear
well.
25. The method of claim 24 wherein sodium hydroxide is introduced into the
wastewater
through the at least one second fluid flow line either in combination with the
sodium chlorite
or as a separate feed substantially contemporaneously therewith.
38
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
26. The method according to any of claims 24-25 wherein the at least one
first treatment
cell provides a first residence time of between about 15 minutes to about 60
minutes.
27. The method according to any of claims 24-26 wherein the oxidant is
introduced at a
flow rate that avoids off-gassing of volatile reductants from the wastewater.
28. The method according to any of claims 24-27 wherein the at least one
first treatment
cell contains one or more separation apparatus for removing precipitated
contaminants from
the at least one first treatment cell.
29. The method according to any of claims 24-27 wherein the at least one
first treatment
cell contains one or more skimming apparatus for removing contaminants from
the surface of
the wastewater contained in the at least one first treatment cell.
30. The method of according to any of claims 24-29 wherein the at least one
second
treatment cell contains one or more skimming apparatus for removing
contaminants from the
surface of the wastewater contained in the at least one second treatment cell.
31. The method according to any of claims 24-30 wherein the at least one
second
treatment cell provides a second residence time of between about 10 minutes to
about 30
minutes.
32. The method according to any of claims 24-31 wherein the at least one
third treatment
cell provides a third residence time of between about 10 minutes to about 30
minutes.
33. The method according to any of claims 24-32 wherein the at least one
third treatment
cell contains a separation apparatus for removing precipitated contaminants
from the at least
one third treatment cell.
34. The method according to any of claims 24-33 wherein wastewater is
withdrawn from
the first treatment cell through the at least one second fluid flow line via a
pump, passed
through at least one venturi and returned to the first treatment cell.
39
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
35. The method according to any of claims 24-34 wherein air is introduced
into the
second fluid flow line via the venturi.
36. The method of claim 35 wherein a solution of sodium chlorite, a
combination of
sodium chlorite and sodium hydroxide, or those two chemicals as separate feeds
are
introduced into the second fluid flow line via the at least one venturi.
37. The method according to any of claims 24-36 wherein the wastewater
stream entering
the fluid treatment system comprises an initial oxidant demand, and said
treated wastewater
entering the at least one second treatment cell has a reduced oxidant demand.
38. The method according to any of claims 24-37 wherein the portion of
wastewater
withdrawn from the first treatment cell through at least one second fluid flow
line is
withdrawn from a level approximately 20% up from the bottom the at least one
first treatment
cell.
39. The method according to any of claims 24-38 wherein the treated
wastewater is
discharged from the at least one second fluid flow line at substantially the
midline of the at
least one first treatment cell.
40. The method according to any of claims 24-39 that removes contaminants
selected
from the group consisting of Ca, Mg, Na, Fe, Cl, Mn, CaC103, SO4, Ba,
hydrocarbons, total
dissolved solids, and biological contamination.
41. The method of claim 40 wherein the calcium and magnesium content,
stated as
CaCo3, of the treated fluid is reduced to less than about 2,200 mg/L.
42. The method of claim 40 wherein calcium and magnesium content, stated as
CaCo3, of
the treated fluid is reduced by up to 93%.
43. The method according to any of claims 24-39 wherein said method
reduces,
inactivates, destroys, or eliminates sulfur compounds, bacteria or a
combination thereof from
the aqueous volume.
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
44. The method according to any of claims 24-43 wherein the wastewater
stream is
produced water from an oil and gas production site.
45. The method according to any of claims 24-44 wherein the chlorine
dioxide residual is
in the range of about 0.1 mg/1 to about 50 mg/1 in the treated wastewater in
the second
treatment cell.
46. The method according to any of claims 24-45 wherein a source of the
wastewater
stream is chosen from the group consisting of an aqueous fluid stream, vessel,
tank, pit,
lagoon, or pond for storing waste water, a water treatment plant, a hydraulic
fracturing tank,
or a piece of equipment, pipeline or vessel used for hydraulic fracturing or
crude oil
production.
47. The method according to any of claims 24-46 wherein first treatment
cell is a vessel
chosen from the group consisting of a storage tank, hydraulic fracturing tank,
or other onsite
vessel used for hydraulic fracturing or crude oil production.
48. The method according to any of claims 24-47 wherein second and third
treatment cell
are located within a single vessel chosen from the group consisting of a
storage tank,
hydraulic fracturing tank, or other onsite vessel used for hydraulic
fracturing or crude oil
production.
49. The method according to any of claims 24-48 comprising the step of
generating a
chlorine dioxide aqueous solution using a chlorine dioxide generator that
brings chlorine
dioxide gas into contact with a portion of the wastewater stream to be treated
under a vacuum
pressure such that the chlorine dioxide gas is drawn into a portion of the
wastewater stream to
form the chlorine dioxide aqueous solution.
50. A fluid treatment system that performs the method according to any of
claims 24-49.
51. A fluid treatment system for in-line use at a hydrocarbon producing
well site
comprising:
41
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
at least one first treatment cell, at least one second treatment cell, and at
least one
third treatment cell;
at least one first fluid flow line for flowing a wastewater stream with an
initial oxidant
demand into the at least one first treatment cell, said at least one first
treatment cell having at
least one outlet in fluid communication therewith;
at least one second fluid flow line for circulating wastewater from the at
least one first
treatment cell, said at least one first treatment cell having at least one
outlet and at least one
inlet in fluid communication therewith;
at least one first eductor, disposed in fluid communication with the at least
one second
fluid flow line and at least one oxidant source, for introducing at least one
oxidant into the at
least one second fluid flow line in controlled quantities and at a controlled
flow rate;
at least one second eductor, disposed in fluid communication with the at least
one
second fluid flow line and at least one chlorine oxide source, for introducing
at least one first
chlorine oxide into the at least one second fluid flow line in controlled
quantities;
at least one third fluid flow line for transferring treated wastewater from
the at least
one first treatment cell to the at least one second treatment cell, wherein
said at least one third
fluid flow line is in fluid communication with at least one outlet of said at
least one first
treatment cell and at least one inlet of said at least one second treatment
cell;
at least one third eductor disposed in fluid communication with the at least
one third
fluid flow line and at least one chlorine dioxide source for introducing
chlorine dioxide into
the at least one third fluid flow line in controlled quantities and at a
controlled flow rate; and
wherein first treatment cell comprises at least one skimming apparatus, at
least one
separation apparatus for removing precipitated contaminants from the at least
one first
treatment cell, and a residence time of between about 15 minutes to about 60
minutes;
42
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
wherein said second treatment cell comprises at least one skimming apparatus,
at least
one underflow outlet that discharges into the at least one third treatment
cell, and a residence
time of about 10 minutes to about 30 minutes;
and wherein said third treatment cell comprises at least one separation
apparatus for
removing precipitated contaminants from the at least one third treatment cell,
at least one
overflow outlet, and a residence time of about 10 minutes to about 30 minutes.
52. The system of claim 51 wherein the first eductor and second eductor are
combined
and configured to introduce the at least one oxidant prior to or
simultaneously with
introducing the at least one chlorine oxide.
53. The system of claim 52 wherein said oxidant is selected from the group
comprising
oxygen, air, oxygen-enriched air, and combinations thereof
54. The system according to any of claims 52-53 wherein said at least one
first chlorine
oxide comprises an aqueous solution of sodium chlorite.
55. The system according to any of claims 52-54 wherein the controlled flow
rate of the
oxidant avoids off-gassing of volatile reductants from the wastewater.
56. The system according to any of claims 52-55 wherein the first treatment
cell is a
vessel, tank, or a piece of equipment or vessel used for hydraulic fracturing
or crude oil
production.
57. The aqueous system according to any of claims 52-56 wherein the
chlorine dioxide
residual in the treated wastewater in second treatment cell is in the range of
about 0.1 mg/1 to
about 50 mg/l.
58. The aqueous system according to any of claims 52-57 wherein the first,
second and
third eductors are a venturi.
59. A method for treating wastewater comprising the follow steps:
43
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
introducing a wastewater stream to a fluid treatment system, the fluid
treatment
system comprising at least one first treatment cell, at least one second
treatment cell, and at
least one third treatment cell;
flowing the wastewater stream through at least one first fluid flow line to
the at least
one first treatment cell; withdrawing at least a portion of the wastewater
from the at least one
first treatment cell through at least one second fluid flow line;
introducing an oxidant into the withdrawn portion of the wastewater through at
least
one in-line oxidant treatment point within the first fluid flow line under
conditions sufficient
to produce thorough mixing of the oxidant within the wastewater and at a
controlled flow rate
to avoid off-gassing of volatile reductants from the wastewater, wherein said
oxidant is
selected from the group consisting of oxygen, air, oxygen-enriched air, and
combinations
thereof, and introducing at least one chlorine oxide into the withdrawn
portion of the
wastewater through at least one in-line chlorine oxide treatment point within
the at least one
first fluid flow line to produce treated wastewater;
recirculating the treated wastewater back to the at least one first treatment
cell,
allowing solids formed in the treated wastewater to settle or rise in the at
least one first
treatment cell, and separating the solids from the treated wastewater
contained therein;
discharging the treated wastewater from the at least one first treatment cell
to the at
least one second treatment cell through at least one third fluid flow line,
wherein chlorine
dioxide gas is introduced into the treated wastewater through at least one in-
line treatment
point within the at least one second fluid flow line at a determined rate
sufficient to provide a
chlorine dioxide residual within the treated wastewater before discharge into
at least one
second treatment cell;
allowing solids formed in the treated wastewater to settle or rise in the at
least one
second treatment cell and separating the solids from the wastewater contained
therein;
44
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
flowing the wastewater stream from the at least one second treatment cell to
at least
one first underflow outlet that discharges into the at least one third
treatment cell; allowing
solids formed in the wastewater to settle or rise in the at least one second
treatment cell and
separating the solids from the wastewater contained therein;
flowing the wastewater stream from the at least one third treatment cell to at
least one
first overflow outlet into at least one fourth treatment cell, vessel or clear
well.
EXAMPLES
[0099] To facilitate a better understanding of the present invention, the
following
examples of embodiments in accordance with the invention are given. It should
be
understood, however, that no limitation of the scope of the invention is
intended, and the
following examples should not be read to limit or define the scope of the
invention.
[00100] In the following examples, the effect of chlorine dioxide on
oilfield
wastewater, with and without oxygen treatment, was studied.
[00101] Example 1: The following experiment was conducted to determine how
significantly the addition of air/oxygen affects chlorine dioxide (and/or
chlorite) treatment of
a sample of oilfield wastewater. The experimental results demonstrate that the
combination of
air/oxygen with chlorine dioxide or chlorite has an unexpected, beneficial
result of
substantially reducing the oxidant dosage required for oxidation of sulfides
present in oilfield
wastewater. Additionally, the combination of air/oxygen with chlorine dioxide
unexpectedly
achieves bacterial kill at significantly reduced dosages. In contrast,
air/oxygen addition alone
is not sufficient over a reasonable period of time to remove sulfides from
wastewater or to
kill bacteria present therein, and the addition of alternative oxidants (i.e.
nitrogen) do not
have the same synergistic effect.
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[00102] For each of experiments 1(A) ¨ l(G) below, a sample of water was
used that
contains 10 percent solids with 110 mg/1 of sulfide in the aqueous phase and
has a pH of 8.2.
The solids consist of biomass, inorganic material, hydrocarbon, and insoluble
sulfides at a
concentration of 82.5 mg/kg. Sulfide reducing and general aerobic bacteria
were cultured
from the sample, demonstrating growth over 106 cfu/ml. The sample (solution
and solids)
have a black coloration.
[00103] First, a series of experimental controls were conducted as
follows:
[00104] Control A. A 200 ml portion of the sample was treated with 335
mg/1 chlorine
dioxide over a 15 minute period while stirring to achieve a trace (< 1.0 mg/1)
residual of
chlorine dioxide in solution. The sample quickly turns from a black coloration
to a
brown/orange with the insoluble solids settling quickly and an iron type floc
forming. There
was also a slight sheen of hydrocarbon on the surface of the treated sample.
No further
change in appearance of the treated fluid was observed over 5 minutes. The
solids (sludge)
and fluid were analyzed for sulfide content using a Garret Gas Train. No
detectable sulfides
were found in the solids or fluids. Sulfur reducing and general aerobic
bacteria were cultured
from the sample, demonstrating no bacterial growth.
[00105] Control B. A 200 ml portion of the sample was treated with 230
mg/1 chlorine
dioxide over a five minute period while stirring. The sample quickly turns
from a black
coloration to a grey brown/orange with the insoluble solids settling quickly
and an iron type
floc forming. No further change in appearance of the treated fluid was
observed over 5
minutes. The solids (sludge) and fluid were analyzed for sulfide content using
a Garret Gas
Train. There was 31 mg/1 and 51 m/kg found in the fluid and sludge,
respectively. No
chlorine dioxide residual was present. Sulfur reducing and general aerobic
bacteria were
cultured from the sample, demonstrating bacterial growth over 106 cfu/ml.
46
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[00106] Control C. A 200 ml portion of the sample was treated with 420
mg/1 of
chlorite (560 mg/1 as sodium chlorite) while stirring. The sample turns from a
black
coloration to a brown/orange with the insoluble solids settling and an iron
type floc forming
over a ten minute period. There was also a slight sheen of hydrocarbon on the
surface of the
treated sample. No further change in appearance of the treated fluid was
observed after 10
minutes. The solids and fluid were analyzed for sulfide content using a Garret
Gas Train. No
detectable sulfides were found in the fluids, however the solids contain
approximately 15
mg/1 sulfide. Sulfur reducing and general aerobic bacteria were cultured from
the sample,
demonstrating growth over 106 cfu/ml.
[00107] Control D. A 200 ml portion of the sample was sparged with air
through a
fine diffuser stone at a rate of 2 SLPM for 30 minutes. Over the 30-min
period, the sample
turns from a black coloration to a grey coloration. The solids (sludge) and
fluid were
analyzed for sulfide content using a Garret Gas Train. The fluid contains 60
mg/1 sulfide and
the solids contain 75 mg/1 sulfide. Sulfur reducing and general aerobic
bacteria were cultured
from the sample, demonstrating growth over 106 cfu/ml.
[00108] Sparging experiments were then conducted in three systems (air-
chlorine
dioxide, nitrogen-chlorine dioxide, and air-chlorite) as follows:
[00109] Experiment E. A 200 ml portion of the sample was sparged with air
through a
fine diffuser stone at a rate of 2 SLPM for four (4) minutes. Initiated
concurrently, a dose of
230 mg/1 chlorine dioxide was added over a five (5) minute period, with the
last minute of
dosing being added without air sparging. In this example, C102 is added at a
low enough rate
with a volume and flow rate of air that does not strip the chlorine dioxide
before it reacts.
The sample quickly turns from a black coloration to a brown/orange with the
insoluble solids
settling quickly and an iron type floc forming upon the cessation of sparging.
No further
47
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
change in appearance of the treated fluid was observed over five (5) minutes.
The solids
(sludge) and fluid were analyzed for sulfide content using a Garret Gas Train.
There were no
detectable sulfides in the solids or fluid. Sulfur reducing and general
aerobic bacteria were
cultured from the sample, demonstrating no bacterial growth.
[00110] Experiment F. A 200 ml portion of the sample was sparged with
nitrogen
through a fine diffuser stone at a rate of 2 SLPM for four (4) minutes.
Initiated concurrently,
a dose of 230 mg/1 chlorine dioxide was added over a five (5) minute period,
with the last
minute of dosing being added without nitrogen sparging. The sample quickly
turns from a
black coloration to a brown/orange with the insoluble solids settling quickly
and an iron type
floc forming upon the cessation of sparging. No further change in appearance
of the treated
fluid was observed over 5 minutes. The solids (sludge) and fluid were analyzed
for sulfide
content using a Garret Gas Train. There were 7 mg/1 and 160 mg/1 sulfides
remaining in the
fluid and the solids, respectively. Sulfur reducing and general aerobic
bacteria were cultured
from the sample, demonstrating over 106 bacterial growth.
[00111] Experiment G. A 200 ml portion of the sample was sparged with air
through
a fine diffuser stone at a rate of 2 SLPM for 15 minutes. Initiated
concurrently, a dose of 300
mg/1 of chlorite (402 mg/1 as sodium chlorite) was added over a five (5)
minute period. The
sample turns from a black coloration to a brown/orange with the insoluble
solids settling
quickly and an iron type floc forming upon the cessation of sparging. No
further change in
appearance of the treated fluid was observed over 15 minutes. The solids
(sludge) and fluid
were analyzed for sulfide content using a Garret Gas Train. There were no
detectable sulfides
in the solids or fluid. Sulfur reducing and general aerobic bacteria were
cultured from the
sample, demonstrating bacterial growth over 106 cfu/ml.
48
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[00112] In the following examples, the unexpected, synergistic effect of
treating a
storage tank with oilfield wastewater with a treatment of chlorine dioxide and
oxygen in a
closed loop system was studied. Sparging experiments were conducted on two
systems (air-
chlorine dioxide only, and air-chlorite-chlorine dioxide) as follows:
[00113] Example 2: A tank containing about 30,000 barrels (bbl) of
produced fresh
and flow back water was analyzed and found to contain 16,000 mg/1 TDS, over
106cfu/m1
bacteria, and 40 mg/1 sulfides in the homogenized fluid at a pH of 7.8. The
chlorine dioxide
demand of the fluid to be treated was determined to be 180 mg/l. The amount of
50% sodium
hydroxide required to maintain the pH was determined to be 630 gallons.
[00114] The tank was rigged to a chlorine dioxide generator (see, e.g.
U.S. Patent No.
6,468,479). Although not limiting, one example of generator would be a Sabre
BB series
portable DiKlor0 generation system with a maximum capacity of 24,000 lbs. per
day
continuous production. This system is self-contained and has a distribution
system that
allows it to circulate fluids in the tank. More specifically, a drive fluid
stream was withdrawn
from the tank and circulated through a chlorine dioxide generator by means of
a centrifugal
pump at a rate of 320 gallons per minute. The generator is arranged so that
the suction for the
drive fluid stream is pulled from the lowest end of the tank, and the
discharge solution
containing chlorine dioxide and/or air was returned to the tank and discharge
to the bottom of
the tank via a movable injection boom. (See FIG. 5 & 8). The injection boom
was
continuously moved around the tank at a rate of 50 feet per minute.
[00115] Sodium hydroxide was added to the tank with enough sodium chlorite
to
absorb approximately 10 percent of the theoretical chlorine dioxide demand. In
this specific
example, and in accordance with calculations readily known in the art, the
amount of sodium
chlorite required to absorb 10% of the chlorine dioxide demand was a dosage of
49
CA 02906186 2015-09-11
WO 2014/145825
PCT/US2014/030654
approximately 23 mg/1 chlorite. The sodium hydroxide and chlorite were added
over a sixty
minute period with air at a rate of 125 SCFM. In this embodiment, air was
introduced via a
venturi. At the end of the 60-minute period, the injection of air is
discontinued, and chlorine
dioxide demand was retested and found to be 27 mg/l. Chlorine dioxide then was
introduced
via a venturi at an appropriate rate to achieve a dosage of 47 mg/1 over a 30
minute period.
No air was introduced during the chlorine dioxide step.
[00116] The resulting fluid was clear with orange/brown sediment and had a
thin layer
of floc on top that was determined to be 98% inorganic material and 2%
hydrocarbons. 8
mg/1 chlorine dioxide was found as a residual in the fluid. The fluid, sludge,
and floc were
analyzed by garret gas train and determined to contain no sulfides. No
bacterial growth was
found by culture analysis. The fluid was analyzed to determine suitability for
"gelling" for
fracturing use. The fluid gelled and cross linked without difficulty. This
method resulted in a
75% reduction in the amount of chlorine dioxide required to achieve the target
chlorine
dioxide residual and no bacterial grown.
[00117] Example 3: A tank containing about 30,000 bbl of produced fresh
and flow
back water was analyzed and found to contain 16,000 mg/1 TDS, over 106 cfu/ml
bacteria,
and 40 mg/1 sulfides in the homogenized fluid at a pH of 7.8. The chlorine
dioxide demand
of the fluid was determined to be 180 mg/l. The tank was rigged to a chlorine
dioxide
generator where the suction for a drive fluid stream is pulled from the lowest
end of the tank,
and the discharge solution containing chlorine dioxide and/or air was returned
to the tank and
discharge to the bottom of the tank via a movable injection boom. The
injection boom was
continuously moved around the tank at a rate of 50 feet per minute. The fluid
was withdrawn
from the tank and circulated through the chlorine dioxide generator by means
of a centrifugal
pump at a rate of 320 gallons per minute.
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[00118] In this example, chlorite was not added directly to the system as
sodium
chlorite. Instead, chlorine dioxide was added to the tank initially (which
converted to
chlorite), followed by air and then a second dosage of chlorine dioxide as set
forth below.
More specifically, 1) from time zero (0) and over the first 10 minutes,
chlorine dioxide was
added to provide 20% of the total dosage; 2) from minute 10 through minute 30,
the solution
was circulated; 3) from minute 30 to minute 60, air was added; and 3) from
minute 60
through minute 80, the remaining 80% of the chlorine dioxide was introduced
into the tank.
In total, the tank was treated with 110 mg/1 chlorine dioxide over an
aggregate (but,
nonconsecutive) 50-minute period. In regards to step 2, one of ordinary skill
in the art will
recognize that, when a big tank is used, one has to be careful not to get
localized "hot spots"
and allow the chlorine dioxide to disperse a bit.
[00119] In step 3, air was added in isolation through a venturi at a rate
of 100 SCFM to
tank 250 from minute 30 to minute 60. In alternate embodiments, one could add
low dosages
of C102 with air, depending on the size and depth of the vessel, as well as
the flow rate.
Sodium hydroxide was added concurrently to maintain stable pH. The fluid was
analyzed
post treatment. The resulting fluid was clear with orange/brown sediment and
had a thin layer
of floc on top that was determined to be 98% inorganic material and 2%
hydrocarbons. 12
mg/1 chlorine dioxide was found as a residual in the fluid. The fluid, sludge,
and floc were
analyzed by garret gas train and determined to contain no sulfides. No
bacterial growth was
found by culture analysis. The fluid was analyzed to determine suitability for
"gelling" for
fracturing use. The fluid gelled and cross linked without difficulty. This
method resulted in
about a 40% reduction in the amount of chlorine dioxide required to achieve a
target chlorine
dioxide residual and no bacterial grown.
51
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[00120] Example 4: A tank contained 4200 gallons of produced water. The
homogenized fluid was analyzed and found to contain 23,000 mg/1 TDS, over 104
cfu/ml
bacteria, and 175 mg/1 sulfides with a pH of 7.8. The chlorine dioxide demand
of the fluid
was determined to be 580 mg/l. The tank was rigged to a chlorine dioxide
generator where
the suction for the drive fluid stream is pulled from the lowest end of the
tank, and the
discharge solution containing chlorine dioxide and/or air via a perforated
pipe along the
length of the bottom of the tank. The fluid is withdrawn from the tank and
circulated through
the chlorine dioxide generator by means of a centrifugal pump at a rate of 320
gallons per
minute.
[00121] As in Example 3, chlorine dioxide was added to the tank initially
via a venturi
320, followed by air and then a second dosage of chlorine dioxide as set forth
below. More
specifically, 1) from time zero (0) and over the first minute, chlorine
dioxide was added to
provide 20% of the total dosage; and then 2) from minute six (6) through
minute ten (10), the
remaining 80% of the chlorine dioxide was introduced into the tank. In total,
the tank was
treated with 310 mg/1 chlorine dioxide over an aggregate (but, nonconsecutive)
5-minute
period. Air was added through a venturi at a rate of 50 SCFM to the tank from
minute one
(1) to minute six (6). Sodium hydroxide was added concurrently to maintain
stable pH. The
fluid was analyzed post treatment. The fluid was clear with orange/brown
sediment and had a
thin layer of floc on top that was determined to be 96% inorganic material and
4%
hydrocarbons. 7 mg/1 chlorine dioxide was found as a residual in the fluid.
The fluid, sludge,
and floc were analyzed by garret gas train and determined to contain no
sulfides. No bacterial
growth was found by culture analysis. This method resulted in about a 47%
reduction in the
amount of chlorine dioxide required to achieve a target chlorine dioxide
residual and no
bacterial grown.
52
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
[00122] Example 5: A tank contained 4200 gallons of produced water. The
homogenized fluid was analyzed and found to contain 23,000 mg/1 TDS, over 104
cfu/ml
bacteria, and 175 mg/1 sulfides with a pH of 7.8. The chlorine dioxide demand
of the fluid
was determined to be 580 mg/l. The tank was rigged to a chlorine dioxide
generator where
the suction for the drive fluid stream is pulled from the lowest end of the
tank, and the
discharge solution containing chlorine dioxide and/or air via a perforated
pipe along the
length of the bottom of the tank. The fluid is withdrawn from the tank and
circulated through
the chlorine dioxide generator by means of a centrifugal pump at a rate of 320
gallons per
minute.
[00123] In this example, chlorite was introduced directly at a rate to
achieve a dosage
of 120 mg/1 over the first minute. Chlorine dioxide also was added at a rate
to achieve a
dosage of 210 mg/1 over an aggregate five (5) minute period. Specifically,
chlorine dioxide
was added from time zero (0) to minute one (1), and then again from minute six
(6) to minute
ten (10). Air was added through a venturi at a rate of 50 SCFM to the tank
from minute zero
to minute nine. Sodium hydroxide was added concurrently to maintain stable pH.
The fluid
was analyzed post treatment.
[00124] The treated fluid was clear with orange/brown sediment and had a
thin layer of
floc on top that was determined to be 96% inorganic material and 4%
hydrocarbons. 7 mg/1
chlorine dioxide was found as a residual in the fluid. The fluid, sludge, and
floc were
analyzed by garret gas train and determined to contain no sulfides. No
bacterial growth was
found by culture analysis. This method resulted in about a 43% reduction in
the amount of
chlorine oxides required to achieve a target chlorine dioxide residual and no
bacterial grown.
[00125] The following experimental results demonstrate that the
combination of
oxidant 40 (air/oxygen) with chlorite (30b), followed by introduction of
chlorine dioxide
53
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
(30a), has an unexpected, beneficial result of substantially reducing unwanted
contaminants
in oilfield wastewater (or produced water), including Ca, Mg, Na, Fe, Cl, Mn,
TDS, CaC103,
SO4, Ba, oil, grease, as well as bacterial contamination, such that the
treated fluids are
suitable for reuse as fracturing fluids.
[00126] Example 6: A treatment train is arranged for a typical produced
fluid (2) from
northern Texas production. Approximately six barrels per minute of fluid is
transferred via a
circulation pump (3) to a 500 barrel frac tank (first frac tank 101) at a
steady flow rate.
Approximately four barrels per minute of fluid is withdrawn from a level 2
feet above the
bottom of the first frac tank (101). This fluid (2a) is passed through a
venturi (4) where air
(40), sodium chlorite (30b) and sodium hydroxide (90) are added to the fluid
stream. The
fluid stream (2a) that reenters the first frac tank (101) containing the air
(4), chlorite (30b)
and caustic (90) is introduced along the centerline (8a) of the tank (101)
through a
distribution bar (19) running the length of the tank (101). For this example,
chlorite (30b) is
introduced to achieve a dosage of 50 mg/1 and oxidant (40) is added to achieve
a dosage of
400 mg/l. A skimmer (6) is placed into the top of the first frac tank (101) to
recover floating
material.
[00127] Fluid (2a) is withdrawn to a second frac tank (102) via a
discharge
approximately 2 feet from the bottom of the first frac tank (101). The fluid
(2a) is introduced
into the second frac tank (102) at approximately the 2 foot level. Chlorine
dioxide (30a) is
injected into the fluid stream between the first and second frac tanks (101,
102). For this
example, chlorine dioxide (30a) is added to achieve a dosage of 25 mg/l. Fluid
(2b) from the
second frac tank (102) under flows to the third frac tank (103). Fluid (2c)
from the third frac
tank (103) overflows to the clear well (104).
54
CA 02906186 2015-09-11
WO 2014/145825
PCT/US2014/030654
[00128] In this example, the produced fluid (2) that flowed into the first
frac tank (101)
originally contained approximately 1% by volume petroleum hydrocarbon. In the
first frac
tank (101) the free hydrocarbon was predominantly recovered at the surface. In
the second
frac tank (102), iron sulfide (FeS) that was oxidized by the chlorine dioxide
reacted to form
iron three hydroxide. The low density solids formed by this reaction and
additional
hydrocarbons further liberated were recovered by a surface skim of the second
frac tank. The
high density solids that were formed as part of this process dropped to the
bottom of the
common second and third tanks. The third tank (103) overflowed into a clear
well (104). The
treated fluid (2d) was pumped out of the clear well (104) for further storage.
Treated fluid
has a chlorine dioxide residual of 20 mg/l. Tables 1A-1C denotes the results
obtained by the
above method and system.
Table lA
Field Trial H2S Ca ++ % Mg++ A Na+
% Ba+
Sample Description pH Mg/1 Mg/I Red Mg/I Red Mg/I
Red j \1g/1
FLOW LINE
RAW WATER
FROM TANK 6.87 4,230 44,580
IN 23.00 12,363 72.00
FARM
TREATED
7.80
WITH AIR VESSEL! 0.00 1,801 85% 688 84%
17,270 61% 1.10
AND DIKLOR
FLOW LINE
TREATED FROM VESSEL
7.77 0.00 1,777 86% 134 97% 14,353 68% 0.82
WITH C102 1 TO 2 after
CI02
TANK 2 WEIR VESSEL 2 6.91 0.00 1,044 92% 210 95%
8,826 80% 0.77
TANK 2 WEIR VESSEL 3 6.88 0.00 1,401 89%
333 92% 11,657 74% 0.68
TANK 2 WEIR VESSEL 4 6.94 0.00 1,209 90% 319 92%
8,929 80% 0.65
TREATED FLOW LINE
TO STORAGE 6.82 0.00 1,022 92% 303 93%
8,753 80% 0.73
WATER OUT
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
Table 1B
Field Trial Mn++ Sr+ OH- CO3-2 11CO2- SO4-2 CI
% Fe000
Sample Description Mg/I Mg/I Mg/ Mg/IMg/I
Mg/I Mg/I Mg/L Red
FLOW LINE
RAW WATER
FROM TANK 7.22 0.00 0.00 97.76 96.32
IN
158.00 278.00 84,264
FARM
TREATED
WITH AIR VESSEL! 1.80 22.20 0.00 0.00 391.04 75.20
32,411 62% 5.15 95%
AND DIKLOR
FLOW LINE
TREATED FROM VESSEL
0.35 1.30 0.00 36.00 293.28 70.10 26,254 69% 0.54 99%
WITH C102 I TO 2 after
CI02
TANK 2 WEIR VESSEL 2 0.33 1.10 0.00 0.00
366.60 65.30 17,458 79% 0.77 99%
TANK 2 WEIR VESSEL 3 0.15 0.68 0.00 0.00
342.16 68.20 16,183 81% 0.77 99%
TANK 2 WEIR VESSEL 4 0.18 0.50 0.00 0.00
317.22 59.30 17,854 79% 0.91 99%
TREATED FLOW LINE
0.26 0.54 0.00 0.00 366.60 64.23 17,814 79% 0.68 99%
WATER OUT TO STORAGE
Table 1C
TDS % %
Sample Mg/I Red
Red SpG
Description TPH
FLOW LINE
RAW WATER
FROM TANK 142,563 9,875.00 1.097
IN
FARM
TREATED
WITH AIR AND VESSEL! 52,874 63% 2,642.00 73% 1.035
DIKLOR
FLOW LINE
TREATED
FROM VESSEL I 38,256 73% 2,355.00 76%
1.038
WITH C102
TO 2 after CI02
TANK 2 WEIR VESSEL 2 32,154 77% 35.00 100%
1.022
TANK 2 WEIR VESSEL 3 34,327 76% 22.63 100%
1.025
TANK 2 WEIR VESSEL 4 32,456 77% 18.15 100%
1.025
TREATED FLOW LINE TO
33,529 76% 11.20 100% 1.020
WATER OUT STORAGE
[00129] Example 7: A treatment train is arranged for a typical produced
fluid from
Permian Basin production. Approximately six barrels per minute of fluid is
transferred via a
pump to a 500 barrel frac treatment tank (101) at a steady flow rate from a
bank of six frac
storage tanks (500). The six storage frac tanks (500) are filled by truck from
the field.
Approximately four barrels per minute of fluid is withdrawn from a level 2
feet above the
bottom of the first frac treatment tank (101). This fluid (2a) is passed
through a venturi (4),
where air (40), sodium chlorite (30b) and sodium hydroxide (90) are added to
the fluid
56
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
stream. The fluid stream that reenters the first frac treatment tank, and
which contains air
(40), chlorite (30b) and caustic (90), is introduced along the centerline of
the first frac tank
(101) through a distribution bar (19) running the length of the tank (101).
For this example,
chlorite (30b) is introduced to achieve a dosage of 50 mg/1 and oxidant (40)
is added to
achieve a dosage of 400 mg/1 (e.g. 2200 mg/1 air). A skimmer (6) is placed
into the top of the
tank (101) to recover floating material.
[00130] Fluid is withdrawn to a second frac tank (102) via a discharge
approximately 2
feet from the bottom of the first tank (101) and is introduced into the second
tank (102) at
approximately the 2 foot level. Chlorine dioxide (30a) is injected into the
fluid stream
between the first and second tanks (101, 102). For this example, chlorine
dioxide (30a) is
added to achieve a dosage of 25 mg/l. Fluid (2b) from the second tank (102)
under flows to
the third tank (103). Fluid (2c) from the third tank (103) overflows to the
clear well (104).
[00131] In the first tank (101), the free hydrocarbon was predominantly
recovered at
the surface. In the second tank (102), iron sulfide oxidized by the chlorine
dioxide reacted to
form iron three hydroxide. The low density solids that were formed by this
reaction and the
hydrocarbon that was liberated was recovered by a surface skim of this tank.
The high
density solids that were formed dropped to the bottom of the second and third
cells/tanks..
The third cell/tank (103) overflowed into a clear well (104). Fluid (2d) was
pumped out of
the clear well (104) for further storage. Treated fluid has a chlorine dioxide
residual of 20
mg/l.
[00132] In this example, the inlet fluid measured 60 ppm by volume of
petroleum
hydrocarbon in grab samples. This measurement was inaccurate, however, due to
the nature
of the truck transfer. Some trucks that came in had no visible hydrocarbon,
while others
contained several percent due to slugs of hydrocarbon that went into the
system. A large
57
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
amount of hydrocarbon was recovered from the first and second tanks by
skimming,
representing 1.72% of the total fluid feed. There was no visible hydrocarbon
present in the
third frac tank and the fluid grab samples in the discharge from the last tank
showed an
average of 45 ppm total hydrocarbon.
[00133] Tables 2A-2C denotes the results obtained by this treatment.
Table 2A
Field Trial H2S Ca ++ % Mg++ % Na+
% Ba+
Sample
Description pH Mg/I Mg/I Red Mg/I Red Mg/I Red Mg/I
FLOW LINE
RAW WATER
FROM TANK 6.66 356 12,800 42.80
IN 31.00 1,153
FARM
TREATED
WITH AIR VESSEL! 7.75 0.00 652 43% 385
-8% 12,952 -1% 1.02
AND DIKLOR
FLOW LINE
TREATED FROM VESSEL
7.70 0.00 680 41% 288 19% 11,383 11% 0.77
WITH C102 1 to 2 after
CI02
TANK 2 WEIR VESSEL 2 6.88 0.00 754 35% 272
24% 8,562 33% 0.36
TANK 2 WEIR VESSEL 3 6.58 0.00 432 63% 254
29% 9,256 28% 0.33
TANK 2 WEIR VESSEL 4 6.64 0.00 258 78% 248
30% 8,255 36% 0.41
TREATED Flow Line to
6.61 0.00 322 72% 252 29% 8,356 35% 0.26
WATER OUT Storage
Table 2B
Mn ++ Sr + OH- CO3-2 11CO2- SO4-2 CI % Feoon %
Sample Description Mg/I Mg/I Mg/I Mg/I Mg/I Mg/I Mg/L Red Red Red
FLOW
LINE
RAW
FROM 115 0.00 0.00 115.20 321.00 30,153
WATER IN 44.80. 77.50
TANK
FARM
TREATED
WITH AIR
VESSEL I 135 0.00 13.00 432.00 82.60
31,070 -3% 71%
AND 11.30. 22.10
DIKLOR
FLOW
LINE
TREATED
FROM
WITH 0.26 2.52 0.00 42.00 277.30 78.10 28,453 6% 4.50 94%
VESSEL I
C102
to 2 after
CI02
TANK 2
VESSEL 2 0.21 1.15 0.00 0.00 250.60 69.30
27,854 8% 6.20 92%
WEIR
TANK 2
VESSEL 3 0.22 0.88 0.00 0.00 262.00
69.69 26,510 12% 82%
WEIR 13.90
TANK 2
VESSEL 4 0.26 0.68 0.00 0.00 320.80 62.10
24,123 20% 4.20 95%
WEIR
TREATED
Flow Line to
WATER 0.18 0.57 0.00 0.00 311.10 64.00 22,110 27% 3.90 95%
Storage
OUT
58
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
Table 2C
TDS % %
Sample Mg/1 Red
Red SpG
Description TPH
F
RAW WATER LOW LINE
FROM TANK 44,200 17,450.00 1.031
IN
FARM
TREATED
WITH AIR AND VESSEL! 46,501 -5% 2,638.00 85% 1.033
DIKLOR
TREATED FLOW LINE
FROM VESSEL 1 42,150 5% 2,254.00 87% 1.029
WITH C102
to 2 after CIO2
TANK 2 WEIR VESSEL 2 38,273 13% 54.20 100% 1.024
TANK 2 WEIR VESSEL 3 36,504 17% 34.20 100% 1.015
TANK 2 WEIR VESSEL 4 35,888 19% 38.70 100% 1.017
TREATED Flow Line to
36,210 18% 45.20 100% 1.018
WATER OUT Storage __
[00134] The process of the present invention is unexpectedly and
economically
effective at removing contaminants from highly contaminated, hydrocarbon-
bearing
produced water streams. The reduction of most of the contaminants is in the
range of 75 -
98% as shown in Tables 1 and 2. As such, the treated water is acceptable to be
used as a
hydraulic fracturing fluid without further treatment, in most cases, or as a
feed stream that
can be further treated (if necessary) for disposal to the environment. The
removal of a high
level of contaminants, such as Ca, Mg, Na, Fe, Cl, Mn, TDS, CaC103, SO4, Ba,
hydrocarbons
and biological contamination, as achieved by the claimed process is highly
desirable for
treating the large quantities of produced water contaminated waste streams
created by oil
field applications. The process also beneficially, and significantly, avoids
off-gassing of
harmful, regulated volatile compounds such as hydrogen sulfide.
[00135] While the preferred application for the method and system
disclosed herein is
in the oil field applications, such as petroleum wells, downhole formations,
and industrial and
petroleum process water, additional industrial applications include, but are
not limited to,
cooling water systems, mineral process waters, geothermal wells, paper mill
digesters,
59
CA 02906186 2015-09-11
WO 2014/145825 PCT/US2014/030654
washers, bleach plants, stock chests, and white water systems, black liquor
evaporators in the
pulp industry, continuous casting processes in the metallurgical industry, air
conditioning and
refrigeration systems, water reclamation systems, water purification systems,
membrane
filtration systems, food processing streams (meat, vegetable, sugar cane,
poultry, fruit and
soybean); and waste treatment systems as well as clarifiers, municipal sewage
treatment,
municipal water systems, potable water systems, aquifers, and water tanks.
Furthermore, for
purposes of this disclosure, aqueous volume, wastewater stream, wastewater,
aqueous fluid
volume, and aqueous fluid stream are all considered to be the fluid to be
treated by the
method and system disclosed herein.
[00136] Various embodiments and modifications of this invention have been
described
in the foregoing description. Such embodiments and modifications are
illustrative only and
are not to be taken as limiting in any way the scope of the invention, which
is defined by the
following claims. Other variations of what has been described also fall within
the scope of
the invention, and the present invention may be modified and practices in
different but
equivalent manners apparent to those skilled in the art having the benefit of
the teachings
herein. Furthermore, no limitations are intended to the details of
construction or design
herein shown, other than as described in the claims below. All numbers and
ranges disclosed
above may vary by some amount. Also, the terms in the claims shall have their
plain,
ordinary meaning unless otherwise explicitly and clearly defined by the
patentee. Subject
matter incorporated by reference is not considered to be an alternative to any
claim
limitations, unless otherwise explicitly indicated.
****