Note: Descriptions are shown in the official language in which they were submitted.
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
DETERMINATION OF JOINT CONDITION BASED ON VIBRATION ANALYSIS
[0001] This application claims the benefit of U.S. Patent Application
Serial
No. 13/841,632, filed March 15, 2013, which is a continuation-in-part of U.S.
Patent Application No. 13/196,701, filed August 2, 2011, claiming the benefit
of
PCT Patent Application No. PCT/US2010/022939, filed on February 2, 2010, and
being a continuation-in-part of U.S. Patent Application Serial No. 12/364,267,
filed on February 2, 2009, the disclosures of which are all incorporated by
reference herein in their entireties.
FIELD OF INVENTION
[0002] The present invention relates generally to systems and methods
for
characterizing a joint defect based on detected vibrations and acoustic
signatures, and more specifically, to assisting pre-operative diagnosis, intra-
operative implantation techniques, and post-operative evaluation of native,
injured, arthritic, and artificial joints using vibroarthrography.
BACKGROUND
[0003] Joint injuries are one of the most commonly reported
musculoskeletal problems. These injuries can occur due to various reasons. In
young adults, sports are a major cause of injuries. These injuries tend to be
mainly involve the soft tissue structures of the joint (e.g., meniscus and
cruciate
1
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
ligament injuries in the knee joint and labral injuries in the hip joint). In
older
subjects, arthritic degeneration (such as rheumatoid or osteoarthritis) of
joints
such as knees and hips is a common phenomenon, and may result from a variety
of traumatic causes. According to the Arthritis Foundation, arthritis-related
problems are second only to heart disease as the leading cause of work
disability. Mechanical loading, especially dynamic loading, is believed to
play a
major role in the degenerative process. This loading may result in bone to
bone
contact where the cushioning layers are damaged, thereby causing pain for the
patient. Osteoarthritis in particular can be extremely disabling, leading to
discomfort and often excruciating pain.
[0004] Depending on the type and nature of the joint damage, different
treatment modalities can be pursued. For soft-tissue damage, mainly meniscul
and ligament injuries, arthroscopic procedures can be implemented to determine
the nature of the injury as well as repair the damage caused by it. More
chronic
or severe joint damage, such as that caused by osteoarthritis, is typically
treated
in a stepwise treatment regime which includes pain relievers, NSAIDS, and
joint
visco supplementation. If these treatment methods fail, they may be followed
as
a last resort with artificial orthopedic implants, which are designed to
replace the
damaged articulating surfaces of the injured joint and thereby provide pain
relief.
Joint implants may allow a subject with severe osteoarthritis to return to a
normal
daily life. One exemplary type of joint implant procedure is known as a total
knee
arthroplasty.
[0005] Multiple artificial joint designs exist that seek to duplicate
the
geometry and behavior of a healthy knee joint. The differences in these
designs
are based on factors such as condylar geometry, bearing mobility, ligament
preservation vs. substitution, and fixation methods.
[0006] Irrespective of the type of injury sustained by an individual,
the
treatment modality typically includes three phases: (1) pre-operative
diagnosis of
the joint and selection of a treatment regime; (2) implementation of the
regime by
non-invasive physiotherapy and stabilization techniques or invasive surgery
(e.g.,
2
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
implantation of the replacement joint); and (3) post operative evaluation of
the
joint.
[0007] One of the major problems in determining knee joint conditions
caused by soft tissue damage or arthritis is the ability to detect the cause
of
abnormal joint conditions early. Use of X-rays, computer assisted tomography
(CAT) and magnetic resonance imaging (MRI) scans are limited to providing
information on defects that are gross in nature. In addition, in the case of
artificial joints, implants may include metal parts, thus MRI scans typically
cannot
be used post-operatively in implanted patients. Arthroscopic procedures can be
used to overcome the deficiencies in available imaging techniques. However,
arthroscopic procedures are semi-invasive and thus undesirable from a pre-
operative diagnostic stand point due to the need for surgery and the
corresponding expense and patient discomfort.
[0008] Therefore, there is a need for improved methods and systems for
determining the condition of joints and the effectiveness of implemented
treatment modalities without the use of ionizing radiation or invasive
procedures,
and that are adaptable for use during preoperative, perioperative, and
postoperative phases of the joint replacement process.
BRIEF DESCRIPTION OF THE DRAWINGS
[0009] The accompanying drawings, which are incorporated in and
constitute a part of this specification, illustrate embodiments of the
invention and,
together with a general description of the invention given below, serve to
explain
the principles of the invention.
[00010] FIG. 1 is high level view of a process for determining the
condition
of a joint.
[00011] FIG. 2 is perspective view of a knee of a patient including a
joint
monitoring apparatus in the form of a knee brace.
[00012] FIG. 3 is a schematic view of a joint monitoring apparatus of
FIG. 2.
[00013] FIG. 4 is a schematic view of an exemplary computing environment
for use with the joint monitoring apparatus of FIG. 3.
3
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
[00014] FIG. 5 is a diagrammatic view of a joint diagnostic system that
may
be hosted by the computing environment of FIG. 4.
[00015] Fig. 6 is a diagrammatic view of an evaluation sheet that may be
used to gather data in conjunction with the diagnostic system of FIG. 5.
[00016] FIG. 7 is a perspective view of a knee joint showing an exemplary
positioning of accelerometers for gathering vibration data.
[00017] FIG. 8 is a graphical view of an exemplary signal received from
one
of the accelerometers in FIG. 7 including a low-pass filtered portion and a
high-
pass filtered portion of the received signal.
[00018] FIGS. 9A-9C are schematic views of filters and spectral analysis
techniques for separating the low-pass and high-pass filtered portions of the
signal in FIG. 8.
[00019] FIG. 10 is a graphical view of exemplary vibrations received from
a
healthy joint and an injured joint.
[00020] FIG. 11 is a diagrammatic view of a vibration pattern classifier
that
may be implemented in the diagnostic system of FIG. 5.
[00021] FIGS. 12A-12E are graphical views illustrating results of
statistically
separating healthy joints from injured joints based on statistical features of
vibroarthrograms.
[00022] FIG. 13 is a schematic view of method of analyzing captured time-
domain signal using a Fourier transform and short-time Fourier transform.
[00023] FIG. 14A is a graphical view of a time-domain signal of a joint
vibration.
[00024] FIG. 14B is a graphical view of a short-time Fourier transformed
version of the time-domain signal in FIG 14A.
[00025] FIG. 15 is a diagrammatic view of a display that may be provided
by the diagnostic system of FIG. 5 including an image of a 3-D model of the
joint,
images showing contact areas between bones comprising the joint, and a
vibroarthrogram of the vibrations generated by the joint.
4
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
SUMMARY
[00026] In an embodiment of the invention, a method of determining a
condition of a joint is provided. The method includes receiving a first signal
indicative of a vibration generated by motion of the joint in a processor. The
method further includes generating a vibroarthrogram from the first signal and
extracting a first signal feature from the vibroarthrogram based on a first
statistical parameter of the vibroarthrogram. The first signal feature is
compared
to a plurality of signal features in a database, each of the plurality of
signal
features in the database being associated with at least one joint condition.
The
method further includes determining the condition of the joint based at least
in
part on a correspondence between the first signal feature and a signal feature
of
the plurality of signal features in the database.
[00027] In another embodiment of the invention, an additional method of
determining a condition of a joint is provided. The method includes receiving
a
first signal indicative of a vibration generated by motion of the joint in the
processor and generating a vibroarthrogram from the first signal. The method
further includes receiving a second signal indicative of a position of the
joint
during the motion of the joint in the processor, and determining an
orientation of
a 3-D model of the joint based at least in part on the second signal. The
method
synchronizes the first and second signals so that each point on the
vibroarthrogram is associated with a position of the joint, and displays a
first
image representing the orientation of the 3-D model of the joint, and a second
image representing the vibroarthrogram. The first and second images are
synchronized so that movement of the 3-D model corresponds to a position of a
sampling window in the vibroarthrogram.
[00028] In yet another embodiment of the invention, another method of
determining a condition of the joint is provided. The method includes
receiving a
first signal indicative of a vibration generated by a motion of the joint in
the
processor, generating a vibroarthrogram based on the first signal, and
extracting
a plurality of signal features from the vibroarthrogram, with each signal
feature
being based on a different statistical parameter. The method further includes
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
defining a plurality of feature vectors of the vibroarthrogram, each feature
vector
being based on one or more weighted signal features of the plurality of signal
features and being associated with at least one joint condition, and
determining a
score for each of the plurality of feature vectors based on the
vibroarthrogram.
The method further includes diagnosing the joint by selecting a joint
condition
associated with the feature vector having the highest score.
[00029] In yet another embodiment of the invention, a system for
determining a condition of the joint is provided. The system includes a
processor and a memory including program code. When executed by the
processor, the program code causes the processor to receive a first signal
indicative of a vibration generated by a motion of the joint, generate a
vibroarthrogram from the first signal, and extract a first signal feature from
the
vibroarthrogram based on a first statistical parameter of the vibroarthrogram.
The code may further cause the processor to compare the first signal feature
to a
plurality of signal features in a database, each of the plurality of signal
features in
the database being associated with at least one joint condition, and determine
the condition of the joint based at least in part on a correspondence between
the
first signal feature and a signal feature of the plurality of signal features
in the
database.
[00030] In yet another embodiment of the invention, another system for
determining a condition of a joint is provided. The system includes a
processor
and a memory including program code. When executed by the processor, the
code causes the processor to receive a first signal indicative of a vibration
generated by a motion of the joint, generate a vibroarthrogram based on the
first
signal, and extract a plurality of signal features from the vibroarthrogram,
each
signal feature being based on a different statistical parameter. The code
further
causes the processor to define a plurality of feature vectors of the
vibroarthrogram, each feature vector being based on one or more weighted
signal features of the plurality of signal features and being associated with
at
least one joint condition, determine a score for each of the plurality of
feature
6
CA 02906476 2015-09-14
WO 2014/150780
PCT/US2014/024205
vectors based on the vibroarthrogram, and diagnose the joint by selecting a
joint
condition associated with the feature vector having the highest score.
DETAILED DESCRIPTION
[00031] The present invention addresses the foregoing problems and other
shortcomings, drawbacks, and challenges of determining a condition of a joint.
The methods and systems described herein may be used preoperatively to
diagnose defects within the joint, perioperatively to adjust an artificial
joint or
repair soft tissue structures, and postoperatively to diagnose and monitor the
functions of the surgical procedures such as joint wear.
[00032]
Vibration and acoustic analysis of joints is based on the principle
that joints are functionally controlled by a mechanical system governed by
three
unique types of forces. These forces are: (1) active forces resulting from
motion,
such as those resulting from a muscle flexing or relaxing; (2) constraining
forces
that constrain motion, such as those resulting from ligaments being in
tension;
and (3) interaction forces that resist motion, such as those acting upon
bones. In
addition to these three types of forces, the soft tissue in the joint (e.g.,
the
cartilage and the meniscus in a knee) produce a dampening effect distributing
the compressive loads acting on the joint.
[00033] It has
been determined that an injury or defect to any one of the
joint ligaments or other soft-tissue structures may result in detectable
vibrations
and/or an acoustic pattern representative of the type of joint injury and/or
the
severity of the injury. These auditory and vibrational changes are produced as
the bones move in a distorted kinematic pattern and produce vibration and
acoustic signals when interacting with the defective/injured body structures.
Thus, the kinematics, acoustic signature and vibrations of injured joints may
differ
significantly from the look and vibration content of a properly balanced joint
moving through the same range and types of motion. Moreover, kinematic
patterns that are non-optimal due to a poorly fitted joint implant, or an
implant
that has experienced significant wear, may alter the vibrations and acoustics
produced by the joint.
7
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
[00034] Although embodiments of the invention are generally described
herein with respect to a knee joint for the sake of simplicity, those skilled
in the
art will recognize that the methods and systems described may also be used for
diagnosing and treating other types of joints without departing from the scope
of
embodiments of the invention. Moreover, embodiments of the invention may
apply to methods and systems used for the condition of joints in a veterinary
setting on non-human subjects, such as dogs, cats, race horses, farm and zoo
animals, or any other animal undergoing joint evaluation and/or treatment.
[00035] Referring now to FIG. 1, a high level overview of an exemplary
method 10 for determining a joint condition or type of joint injury in
accordance
with an embodiment of the invention is presented. In block 12, a 3-D model of
the joint is constructed. This 3-D model may be a patient specific model, and
may be generated by obtaining a plurality of raw RF signals using pulse echo
ultrasound acquisition methodologies. A bone contour may then be isolated in
each of the plurality of RF signals and transformed into a point cloud
representing the joint. The point clouds may then be used to optimize a 3-D
model of the bone such that the patient-specific model may be generated.
Methods of generating 3-D joint models and re-constructing joint cartilage are
described in U.S. Patent Application No. 13/758,151 filed on February 4, 2013
and entitled "METHOD AND APPARATUS FOR THREE DIMENSIONAL
RECONSTRUCTION OF A JOINT USING ULTRASOUND", the disclosure of
which is incorporated herein by reference in its entirety.
[00036] In block 14, acoustic vibrations are detected as the joint is
moved.
These vibrations may be detected using a suitable transducer, such as one or
more accelerometers coupled to the patient in proximity to the joint. As used
herein, the term "vibration" is intended to encompass any oscillatory,
periodic, or
random motion of particles of an elastic body or medium. "Vibrations" thus
include mechanical vibrations (such as those that may be produced by a moving
joint), acoustic energy (i.e., the sound produced by the joint), or any other
type of
time varying phenomena by which kinetic energy propagates through a medium
that is detectable by an accelerometer or other mechanical to electrical
energy
8
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
transducer. The signals generated by the transducers may be transmitted to a
computer either through a wired connection, or wirelessly.
[00037] In block 16, the movement of the joint is tracked. This tracking
may
be via signals received from one or more Inertial Measurement Units (IMUs)
attached to the patient, or some other suitable form of tracking, such as with
an
optical or electromagnetic tracking system. In any case, in block 18, the
motion
and joint vibration signals are received in the computer, which proceeds to
analyze the signals. The detected motion signals may be used to adjust the
orientation of the 3-D joint model and to determine the kinematics of the
joint.
The vibration signals may be used to generate a vibroarthrogram and/or a
corresponding acoustic or sound signature that may be listened to or analyzed
automatically. The kinematics and vibroarthrogram may then be synchronized to
each other and used to analyze the joint. This analysis may include displaying
images representing the orientation of the 3-D joint model, vibrations and the
accompanying sound generated by the joint as the joint is moved through a
range of motion. This analysis may also include grid wise graphical/visual
representations of the joint capsule condition recorded with a clinical
evaluation
sheet on said 3-D joint model that co-relate to the vibration and acoustic
analysis
pertaining to said joint, as will be described in more detail with respect to
FIG. 6.
In block 20, the condition of the joint may be determined automatically based
on
the aforementioned vibration analysis, acoustic analysis, kinematic analysis,
or a
combination of the three.
[00038] Referring now to FIG. 2, in accordance with an exemplary
embodiment of the invention, a patient leg 22 is shown including a shank 24
and
thigh 26 joined by a knee joint 28. A joint monitoring apparatus 30 is
depicted in
the form of a knee brace 32 for use in monitoring and tracking motion of the
knee
joint 28. The knee brace 32 may include a housing 34 that supports the joint
monitoring apparatus 30. The housing 34 may provide a location for one or more
inertial measurement unit ("IMU") sensors 36A, 36B, one or more vibration
sensors 38, one or more ultrasound transducers 40, and signal processing
circuitry 42 (FIG. 3) related to each of the IMU sensors 36, the vibration
sensors
9
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
38, and the ultrasound transducers 40. The housing 34 may also include at
least
one flexible segment 44 configured to secure the knee brace 32 to the leg 22.
The flexible segment 44 may include one or more layers of elastic material
having an intermediate layer (not shown) that is proximate to the patent's
skin
and that serves as an acoustic impedance matching layer. The one or more
layers of elastic material may thereby facilitate transmission of an
ultrasound
pulse into the knee joint 28 from the ultrasound transducers 40. The knee
brace
32 may also include elastic straps (not shown) with facing materials having
hooks
and loops (commonly known as VELCRO) for securing the brace 32 to the
patient.
[00039] Referring now to FIG. 3, a schematic of the joint monitoring
apparatus 30 is illustrated showing an inertial monitoring unit 48, a
vibration
detection module 50, and an ultrasound module 52 operatively coupled to a
computer 54. The inertial monitoring unit 48 may detect motion using the
inertial
monitoring sensors 36. As compared with position tracking systems that rely on
optical or electromagnetic localization, the inertial monitoring sensors 36 do
not
require external observation units. Rather, the inertial monitoring sensors 36
include a plurality of sensors that detect motion unilaterally, thereby
allowing the
inertial monitoring unit 48 to operate without the need for external reference
signals. The inertial monitoring sensors 36 in the exemplary embodiment
include, but are not limited to, one or more accelerometers 56, gyroscopes 58,
and magnetometers 60.
[00040] In an exemplary embodiment of the invention, the inertial
monitoring sensors 36 may include an accelerometer 56 that is sensitive to
static
forces, i.e., an accelerometer configured to output a DC voltage in response
to
being subjected to a constant acceleration. Thus, the accelerometer 56 may be
sensitive to the constant force of gravity. The accelerometer 56 may also
include
a sensing axis so that the accelerometer 56 generates an output indicating a
force of 1 G when the accelerometer sensing axis is perpendicular to the force
of
gravity. As the accelerometer sensing axis is tilted, the force of gravity
acts at an
angle to the axis. In response to tilting the sensing axis, the output signal
may
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
decrease, indicating a lower sensed level of acceleration. This decrease may
continue until the accelerometer sensing axis is positioned parallel to the
force of
gravity, at which point the signal may reach an output level indicative of a
force of
0 G. Accordingly, the relationship between gravity and the accelerometer
sensing axis may be used to determine a tilt angle of the accelerometer 56
with
respect to the local gravitational field. In an alternative embodiment of the
invention, the accelerometer 56 may be a three axis accelerometer having three
orthogonal accelerometer sensing axes. In this embodiment, the accelerometer
56 may be configured to monitor the tilt angle for each of the three
accelerometer
sensing axes relative to the local gravitational field.
[00041] The gyroscope 58 may be configured to monitor an angular motion
of a gyroscopic sensing axis relative to a local IMU frame. To this end, the
gyroscope 58 may generate an output indicative of an angular velocity being
experienced by the gyroscopic sensing axis. Thus, a change in the angle of the
gyroscopic sensing axis relative to an initial orientation of the inertial
monitoring
unit 48 may be determined based on the output signal. This change in the angle
of the gyroscopic sensing axis may, in turn, be used to determine the angular
orientation of the inertial monitoring unit 48 and the orientation of the
brace 32 in
a known manner. That is, the gyroscope 58 generates an output relative to the
angular velocity experienced by the gyroscopic sensing axis. Thus,
repositioning
the gyroscopic sensing axis relative to an initial orientation may be
calculated in
accordance with the Newton's equations of angular motion:
L = f wAt = Li + wAt
where L is the angle of orientation, and Li is the orientation from previous
state. )
[00042] The magnetometer 60 may generate one or more output signals
indicative of the strength and/or orientation of a magnetic field relative to
the
magnetometer 60. The magnetometer 60 may thus be configured to serve as a
compass and/or magnetic field monitor that detects relative motion between a
magnetic sensing axis of the magnetometer 60 and a local magnetic field. The
outputs generated by the magnetometer 60 may thereby represent changes in a
11
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
magnetic field experienced on each magnetic sensing axis. In use, at least two
magnetic sensing axes may be used to determine an angle between the inertial
monitoring unit 48 and the axis of the magnetic field lines passing through
the
magnetometer 60. If one of the two magnetic sensing axes becomes insensitive
to the local magnetic field (e.g., one of the two magnetic sensing axes is
rotated
to a position that is orthogonal to the magnetic field), then a third magnetic
sensing axis may be used to determine the angle. In an alternative embodiment,
tilt angles may be determined from one or more output signals of the
accelerometers 56. These tilt angles may in turn be used to compensate for the
effects of tilting the magnetic sensing axis.
[00043] The inertial monitoring unit 48 may further include a power
module
62, an analog-to-digital converter (ADC) 64, a signal conditioning module 66,
a
multiplexer 68, a communication module 70, and a processor 72. The power
module 62 may include circuitry configured to provide power to the components
of the inertial monitoring unit 48, e.g., a +3.3 V and/or a +5 V direct
current (DC)
power source. The power module 62 may also provide a reference voltage to the
ADC 64.
[00044] The signal conditioning module 66 may couple the output of the
inertial monitoring sensors 36 to the ADC 64, and may be configured to reduce
noise in the signals provided to the processor 72 from the ADC 64 by
amplifying
the signals provided to the ADC 64. The level of the signals provided to the
ADC
64 may thereby be adjusted so that their amplitudes are within a desired
operating range of the ADC 64 input. To this end, the signal conditioning
module
66 may provide optimal input signals to the ADC 64 using one or more analog
circuits. For example, the signal conditioning module 66 may include a low
pass
filter, such as a passive low pass filter for reducing high frequency noise
from the
IMU sensor outputs and to prevent aliasing, and/or an amplifier to amplify the
sensor signal to be within a desired input range of the ADC 64.
[00045] The multiplexer 68 may include a plurality of inputs, with one
input
operatively coupled to each of the outputs of the inertial monitoring sensors
36.
The multiplexer may also include a single output operatively coupled to an
input
12
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
of the ADC 64. The multiplexer 68 may operate as a high frequency analog
switch that sequentially couples the signals at each of the plurality of
multiplexer
inputs to the multiplexer output. The multiplexer 68 may thereby serialize
signals
received on multiple inputs into a single time-division multiplexed output
that is
provided to the input of the ADC 64. In an exemplary embodiment of the
invention, the multiplexer 68 may multiplex 16 output signals from the
inertial
monitoring sensors 36 into one input that is coupled to the ADC 64. The output
generated by the multiplexer 68 may be converted into a corresponding digital
signal by the ADC 64. In an exemplary embodiment of the invention, the ADC 64
may include a high resolution converter that converts the analog input into a
digital signal having a resolution of 24 bits per sample. Alternatively, a
lower
resolution ADC 64 (e.g., a 16 bit converter) may be used to achieve a higher
processing speed and/or a greater sampling rate.
[00046] The communication module 70 may include a wireless
communication circuit that transmits the digital data generated by the ADC 64
to
the computer 54 over a wireless link. The communication module 70 may
operate, for example, on one of three frequency bands (e.g., 400MHz, 916MHz,
and 2.4GHz) approved by the Federal Communications Commission for
unlicensed medical and scientific applications. The communication module 70
may use any suitable wireless or wired communication protocol, such as IEEE
802.15.1 (Bluetoothe), X.25, IEEE 802.11 (WiFI), or a custom protocol such as
ultra-wideband (UWB) communication as appropriate depending on the
application, to encode the digital data for transmission to the computer 54.
Protocols may include signaling, authentication, communication with multiple
inertial monitoring units 48, and error detection and correction capabilities.
[00047] The processor 72 may be configured to operatively couple and
control the ADC 64, the multiplexer 68, and the communication module 70. The
processor 72 may acquire digital data from the output of the ADC 64, package
the data into data packets, and send the data packets to the communication
module 70 for transmission to the computer 54. In an embodiment of the
invention, the processor 72 may be a low power processor in order to minimize
13
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
power consumption of the joint monitoring apparatus 30. In an alternative
embodiment, the processor 72 may be a higher powered processor, such as a
digital signal processor (DSP) or application specific integrated circuit
(ASIC) so
that the processor 72 may be used to perform digital signal processing, such
as
data compression, prior to transmitting the data to the computer 54. Multiple
core or multiple processor architectures, and or a field programmable gate
array
(FPGA) may also be used.
[00048] Similarly as described above with respect to the inertial
monitoring
unit 48, the vibration detection module 50 may include one or more vibration
sensors 38, and signal processing circuitry 42 comprising a power module 73, a
signal conditioning module 74, which may include a charge amplifier (not
shown),
a multiplexer 76, an ADC 78, a processor 79, and a communication module 80.
The signal processing circuitry 42 of vibration detection module 50 may
operate
to provide signals generated by the vibration sensors 38 to the computer 54.
The
vibrations detected by the vibration sensors 38 may thereby be used to provide
insight into the condition of a patient's joint, such as the knee joint 28 in
FIG. 2.
To this end, the one or more vibration sensors 38 may be used to collect
vibrations generated by the joint. These vibrations may be used to generate a
vibration signature (i.e., a pattern or plot of vibration amplitude verses
time, or
vibroarthrogram) and an acoustic signature (i.e., an audio signal or pattern
that
may be listened to or analyzed via signal processing). These vibration and
acoustic signatures may characterize femur and tibia interaction (or other
bones
forming a joint, as the case may be) during patient activities. The vibration
and
acoustic signatures generated during knee motion may thereby be used to help
differentiate a healthy patient from an osteoarthritic patient. The vibration
and
acoustic signatures may also be used to determine various soft tissue defects
in
the joint, such as meniscul and ligament injuries, patellar
clunk/crepitus/chondromalacia etc. in the knee joint and the condition of the
labrum, and/or injuries to the hip joint ligaments in the hip joint. The
vibration and
acoustic signatures may further be used to determine abnormal conditions in
artificial implants, such as severe cam-post impact, condylar lift-off, and
14
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
unexpected wear patterns in the knee joint or total hip arthroplasty squeaking
and metal particle incursion in the hip joint. The observed vibration and its
accompanying sound may thus provide a useful indicator for diagnosing the
condition of the joint.
[00049] One exemplary vibration sensor 38 is a dynamic accelerometer,
which is a type of accelerometer configured to detect rapid changes in
acceleration, such as may be associated with vibrations generated by a moving
joint. In an embodiment of the invention, the joint monitoring apparatus 30
may
be configured so that the vibration sensor 38 is detachable to allow
positioning of
the sensor 38 in proximity to a desired portion of the joint. As best shown in
FIG. 1, the detachable vibration sensor 38 may be placed near the knee joint
28
and secured with adhesives to monitor vibration while the knee joint 28 is in
motion. As compared to static accelerometers, dynamic accelerometers are not
necessarily sensitive to a static accelerative force, such as gravity.
However, in
an alternative embodiment of the invention, accelerometers having a wide
frequency range may be used to detect both patient motion and joint vibration,
so
that both these signals are provided to the computer 54 from a single
accelerometer. The power module 73 may include, for example, a +3.3 V DC
source, a +5 V DC source, and power source having an output voltage between
+18 V and +30 V (e.g., +24 V) DC. The power module may also provide a
precision voltage reference to the ADC 78
[00050] The signal generated by the vibration sensors 38 may be
processed by the signal conditioning module 74 before entering the multiplexer
76 and ADC 78, similarly as described above with reference to the inertial
monitoring sensors 36. As compared to the ADC 64 of the inertial monitoring
unit
48, the ADC 78 of vibration detection module 50 may have a higher sample rate
to capture the higher frequency signals generated by the vibration sensors 38
(e.g., a sample rate above the Nyquist rate for the desired bandwidth of the
vibration sensor output signals). To this end, the ADC 78 of vibration
detection
module 50 may be selected to trade resolution for a higher sample rate. The
digital data output by the ADC 78 may be coupled to the communication module
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
80 for processing and transmission to the computer 54 similarly as described
above with respect to the inertial monitoring unit 48.
[00051] The processor 79 may be configured to control the components of
the vibration detection module 50, as well as receive the digitized output
signal
from the ADC 78, package the received data into data packets, and send the
data packets to the communication module 80 for transmission to the computer
54. Similarly as discussed with respect to inertial monitoring unit 48, the
processor 79 may be any suitable processor, such as a low power processor in
order to minimize power consumption of the joint monitoring apparatus 30. In
an
alternative embodiment, the processor 79 may be a higher powered processor,
such as a digital signal processor (DSP) or application specific integrated
circuit
(ASIC) so that the processor 72 may be used to perform digital signal
processing, such as data compression, prior to transmitting the data to the
computer 54. Multiple core or multiple processor architectures, and or a field
programmable gate array (FPGA) may also be used.
[00052] The communication module 80 may include a wireless
communication circuit that transmits the digital data generated by the ADC 78
to
the computer 54 over a wireless link. The communication module 80 may
operate, for example, on one of three frequency bands (e.g., 400MHz, 916MHz,
and 2.4GHz) approved by the Federal Communications Commission for
unlicensed medical and scientific applications. The communication module 80
may use any suitable wireless or wired communication protocol, such as
Bluetoothe, X.25, WiFI, or a custom protocol such UWB communication as
appropriate depending on the application, to encode the digital data for
transmission to the computer 54. Protocols may include signaling,
authentication, communication with multiple vibration detection modules, and
error detection and correction capabilities.
[00053] The ultrasound module 52 may include one or more ultrasound
transducers 40, a power module 82, a high voltage multiplexer 84, a signal
conditioning module 86, a multi-channel variable gain amplifier (VGA) 88, an
ADC 90, a processor 92, and a communication module 94. The ultrasound
16
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
transducers 40 may include a plurality of pulse echo mode ultrasound
transducers arranged in the flexible segment 44 of knee brace 32. Each
ultrasound transducer 40 may be comprised of a piezoelectric crystal
configured
to emit an ultrasound pulse in response to an electrical signal. The
ultrasound
pulse may be transmitted from the ultrasound transducer 40 through the skin
and
soft tissues of the patient. When the ultrasound pulse reaches a boundary
between tissues having different acoustic impedance properties, such as an
interface between bone and a soft-tissue, an echo is generated and reflected
back to the ultrasound transducer 40. The time delay between an initial echo
(i.e., the echo generated by the interface between the flexible segment 44 of
knee brace 32 and the skin) and an echo generated by the bone-tissue interface
may be used to determine a distance between the ultrasound transducer 40 and
the bone. By including one or more ultrasound transducers 40 in the brace 32,
the relative motions between the knee brace 32 and the patient's bones may be
determined as is described in greater detail in U.S. Application Pub. No.
2012/0029345, filed on August 2, 2011 and entitled "NONINVASIVE
DIAGNOSTIC SYSTEM", the disclosure of which is incorporated herein by
reference in its entirety.
[00054] In addition to providing power to the ultrasound module 52, the
power module 82 may include a high voltage pulse generator configured to
excite
the ultrasound transducers 40 with ultrasound bursts via the high voltage
multiplexer 84. To this end, the high voltage multiplexer 84 may include an
analog switch configured to selectively couple the high voltage output of the
high
voltage pulse generator to one or more of the plurality of ultrasound
transducers
40.
[00055] The signal conditioning module 86 may be coupled to (or include)
the multi-channel VGA 88, which may provide a time-based variable gain control
over the received echo signals generated by the ultrasound transducers.
Normally the transmitted ultrasound pulse and the returning echo are
attenuated
by soft tissue as each signal propagates through the human body. Accordingly,
after the ultrasound transducer 40 emits the ultrasound pulse, the amplitude
of
17
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
the pulse is attenuated as the signal passes through the patient. Thus, echo
signals originating from deep within the patient tend to have lower amplitude
than
those originating from close to the surface due to their longer propagation
path.
A received echo signal that initially has sufficient amplitude to be encoded
by the
ADC 90 may therefore fade into the background noise by the end of the
ultrasound scanning or receiving period. To address this issue, the VGA 88 may
be configured to dynamically increase the gain applied to the received echo
signal over the receiving period to compensate for this varying attenuation.
The
gain may also be varied across the inputs to the VGA 88 so that the gain may
be
adjusted independently for each ultrasound transducer 40 coupled to the VGA
88. The VGA 88 may thereby improve the reliability and quality of the echo
signal conversion by the ADC 90 as compared to systems lacking this dynamic
gain feature.
[00056] The ADC 90 of ultrasound module 52 may be similar to the ADCs
64, 78 of inertial monitoring unit 48 and vibration detection module 50.
However,
because the ADC 90 is responsible for converting the echo signal of an
ultrasound pulse into to a digital signal, the ADC 90 may require a higher
sampling frequency than either the ADC 64 of inertial monitoring unit 48 or
the
ADC 78 of vibration detection module 50. This higher conversion rate may be
required because the bandwidth of the ultrasound pulse is significantly higher
than signals generated by either the inertial monitoring sensors 36 or
vibration
sensors 38. In any case, the output signal generated by the ADC 90 may include
an array of digital data points, or samples representing the analog echo
signal
similarly as described above with respect to the other ADCs 64, 78.
[00057] The processor 92 may be configured to control the components of
the ultrasound module 52, as well as receive the digitized output signal from
the
ADC 90, package the received data into data packets, and send the data packets
to the communication module 94 for transmission to the computer 54. Similarly
as discussed with respect to inertial monitoring unit 48, the processor 92 of
ultrasound module 52 may be any suitable processor. In an embodiment of the
invention, the processor 92 may be a DSP, ASIC, multiple core processor,
and/or
18
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
may include multiple processors configured to process the digital signal
generated from the ultrasound transducers 40 into physical units indicative of
the
distance between the ultrasound transducer 40 and the bone surface. Signal
processing may thereby be performed in the ultrasound module 52 prior to
transmission of the processed data to the computer 54. This processing may
reduce the amount of data that must be transmitted to, and the processing load
on, the computer 54. In any case, and similarly as described above with
respect
to the inertial monitoring unit 48, the communication module 94 may include a
wireless communication circuit that transmits the digital data generated by
the
ADC 90 and/or processor 92 to the computer 54 over a wireless link.
[00058] The modules 48, 50, 52 may receive power from batteries
incorporated into the housing of the knee brace 32. In an alternative
embodiment, the modules 48, 50, 52 may receive power from an external power
source 96 coupled to the brace 32 via a power line 98. Using an external power
source 96 may reduce the size and weight of the joint monitoring apparatus 30
as well as allow the use of higher performance circuitry.
[00059] One or more of the communication modules 70, 80, 94 may be
incorporated into the housing of the knee brace 32. In an alternative
embodiment, to reduce the size and weight of the joint monitoring apparatus
30,
one or more of the communication modules 70, 80, 94 may also be external to
the knee brace 32, and may communicate with the electronic components of the
modules 48, 50, 52 wirelessly or via one or more wires tethering the one or
more
communication modules 70, 80, 94 to the knee brace 32. In embodiments
having external communication modules, the communication modules may be
integrated into the external power source 96. In embodiments including
communication modules 70, 80, 94 employing wireless communication links, the
communication modules 70, 80, 94 may operate, for example, on one of three
different bandwidths (e.g., 400MHz, 916MHz, and 2.4GHz) that are approved by
the Federal Communications Commission for medical and scientific applications.
As discussed with respect to the communication module 70 of inertial
monitoring
unit 48, the communication modules 70, 80, 94 may use any suitable wireless or
19
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
wired communication protocol, such as IEEE 802.15.1 (Bluetoothe), X.25, IEEE
802.11 (WiFI), or a proprietary protocol as appropriate depending on the
application, to encode the digital data for transmission to the computer 54.
Protocols may include signaling, authentication, communication with multiple
inertial monitoring units 48, and error detection and correction capabilities.
[00060] In an embodiment of the invention, the accelerometer 56, the
gyroscope 58, and the magnetometer 60 may be separated into distinct sensor
circuit layouts to increase the modularity and customizability of the inertial
monitoring unit 48. Furthermore, the sensitivities of the inertial monitoring
sensors 36 in the inertial monitoring unit 48 may be designed to perform
within a
finite sensitivity range and boundary conditions. For example, the gyroscope
58
may have a sensitivity rating selected to accommodate an expected maximum
measurable angular motion for a particular application. Because each motion
performed by the joint under study has a different kinematic characteristic
(for
example, the shank 24 has far less motion during a rising motion from a chair
as
compared with walking), selecting the components of the inertial monitoring
unit
48 in accordance with a selected capability for a particular motion may
optimize
the performance of the joint monitoring apparatus 30.
[00061] Moreover, segmenting the circuit layouts allows for greater
adaptability of the inertial monitoring unit 48 for use in analyzing the
motion of
another portion of the patient's body. That is, while the illustrative
embodiment is
specifically drawn to the knee joint, other joints (such as the hip, the
shoulder,
and the spine) may exhibit significantly different kinematics as compared with
the
knee. The modular design of the inertial monitoring unit 48 and the inertial
monitoring sensors 36 provides for a quick and easy adjustment of the joint
monitoring apparatus 30 by enabling the switching or exchange of one
component for another having a different selected sensitivity range that is
better
suited for evaluating the joint or movement in question.
[00062] Additionally, while the illustrative embodiment of the present
invention is specifically described as including one accelerometer 56, one
gyroscope 58, and one magnetometer 60, those having ordinary skill in the art
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
will understand that the inertial monitoring unit 48 may have other
combinations
and numbers of inertial monitoring sensors 36. Thus, inertial monitoring units
48
in accordance various embodiments of the present invention may include any
combination of components, including, for example, two accelerometers 56, two
gyroscopes 58 each with a different operational dynamic range, and one
magnetometer 60. The selection of components may be based, in part, on the
particular need or preference of the evaluating physician, the joint to be
evaluated, the range of motion of the patient, the expected rate of motion
(slow
versus fast movement or rotation), and/or the range of motion permitted in the
evaluation setting (examination room versus surgical table). Apparatuses,
systems and methods for monitoring a joint are also described in concurrently
filed U.S. Patent Application entitled "MOTION TRACKING SYSTEM WITH
INERTIAL-BASED SENSING UNITS", Attorney Docket No. JVUE-6CIP1, the
disclosure of which is incorporated herein by reference in its entirety.
[00063] Referring now to FIG. 4, the computer 54 may include a processor
110, memory 112, an input/output (I/O) interface 114, a mass storage device
116, and a user interface 118. The computer 54 may be considered to represent
any suitable type of computer, computing system, server, disk array, or
programmable devices such as a handheld device, a networked device, or an
embedded device, etc. The computer 54 may be in communication with one or
more networked computers 120 via one or more networks 122, such as a cluster
or other distributed computing system, through the I/O interface 114.
[00064] The processor 110 may include one or more devices selected from
microprocessors, micro-controllers, digital signal processors, microcomputers,
central processing units, field programmable gate arrays, programmable logic
devices, state machines, logic circuits, analog circuits, digital circuits, or
any
other devices that manipulate signals (analog or digital) based on operational
instructions that are stored in the memory 112. Memory 112 may be a single
memory device or a plurality of memory devices including but not limited to
read-
only memory (ROM), random access memory (RAM), volatile memory, non-
volatile memory, static random access memory (SRAM), dynamic random
21
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
access memory (DRAM), flash memory, or cache memory. Memory 112 may
also include a mass storage device such as a hard drive, optical drive, tape
drive,
non-volatile solid state device, or any other device capable of storing
digital
information.
[00065] The processor 110 may operate under the control of an operating
system 124 that resides in memory 112. The operating system 124 may manage
computer resources so that computer program code embodied as one or more
computer software applications, such as an application 126 residing in memory
112 may have instructions executed by the processor 110. In an alternative
embodiment, the processor 110 may execute the applications 126 directly, in
which case the operating system 124 may be omitted.
[00066] The mass storage device116 typically includes at least one hard
disk drive and may be located externally to the computer 54, such as in a
separate enclosure or in one or more networked computers 120, one or more
networked storage devices 128 (including, for example, a tape or optical
drive),
and/or one or more other networked devices (including, for example, a server).
The mass storage device 116 may also host one or more databases 130.
[00067] The user interface 118 may be operatively coupled to the
processor
110 of computer 54 in a known manner to allow a system operator to interact
directly with the computer 54. The user interface 118 may include output
devices
such as video and/or alphanumeric displays, a touch screen, a speaker, and any
other suitable audio and visual indicators capable of providing information to
the
system operator. The user interface 118 may also include input devices and
controls such as an alphanumeric keyboard, a pointing device, keypads,
pushbuttons, control knobs, microphones, etc., capable of accepting commands
or input from the operator and transmitting the entered input to the processor
110.
[00068] Those skilled in the art will recognize that the computing
environment illustrated in FIG. 4 is not intended to limit the present
invention. In
addition, various program code described herein may be identified based upon
the application or software component within which it is implemented in a
specific
22
CA 02906476 2015-09-14
WO 2014/150780
PCT/US2014/024205
embodiment of the invention. However, it should be appreciated that any
particular program nomenclature that follows is used merely for convenience,
and thus the invention should not be limited to use solely in any specific
application identified and/or implied by such nomenclature. It should be
further
appreciated that the various features, applications, and devices disclosed
herein
may also be used alone or in any combination. Moreover, given the typically
endless number of ways in which computer programs may be organized into
routines, procedures, methods, modules, objects, and the like, as well as the
various ways in which program functionality may be allocated among various
software layers that are resident within a typical computing system (e.g.,
operating systems, libraries, APIs, applications, applets, etc.), and/or
across one
or more hardware platforms, it should be appreciated that the invention is not
limited to the specific organization and allocation of program or hardware
functionality described herein.
[00069]
Referring now to FIG. 5, an exemplary diagnostic system 140 is
presented in accordance with an embodiment of the invention. The diagnostic
system 140 may include the brace 32, a diagnosis and data visualization module
142, and an ultrasound probe 144 for imaging the joint 28 during diagnosis (if
necessary) and to register the bones in the joint 28 to the 3-D model in
computer
54. The inertial monitoring unit 48, ultrasound module 52, and ultrasound
probe
144 may collectively provide joint kinematics tracking information to the
diagnosis
and data visualization module 142. To this end, the ultrasound probe 144 may
include an ultrasound module 139 that obtains ultrasound data from the
patient,
and a location tracking module 141 that provides probe location data to the
diagnosis and data visualization module 142. The diagnosis and data
visualization module 142 may in turn manipulate the patient-specific 3-D model
based on the received data to provide kinematic data to the system user via a
display module 149. The diagnosis and data visualization module 142 may also
receive vibration data from the vibration detection module 50 while the joint
28 is
in motion.
23
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
[00070] The diagnosis and visualization module 142 may include program
code executed on the computer 54 in the form of one or more applications 126,
databases 130, and/or modules. These applications, databases, and modules
may include a 3-D modeling module 143, a vibration and acoustic analysis
module 145, a feature vector module 146, a classification module 147, a
diagnosis module 148, and the aforementioned display module 149. The 3-D
modeling module 143 may build and access an atlas or database of 3-D joint
models, and may receive and process joint position data from the brace 32 to
synchronize a selected 3-D joint model with the position of the actual patient
joint. The 3-D modeling module 143 may also generate kinematic data for use
by the diagnosis module 148.
[00071] The vibration and acoustic analysis and feature vector modules
145, 146 receive and analyze vibration data from the brace 32. These modules
may also provide data to the classification module 147 regarding statistical
features of a vibroarthrogram and the acoustic signature generated from the
vibration data. The classification module 147 may, in turn, determine a level
of
correlation or correspondence between the statistical features of the
generated
vibroarthrogram acoustic signature, and statistical features of
vibroarthrograms
acoustic signatures indicative of known joint conditions, which may be stored
in a
database of vibroarthrograms acoustic signatures. The classification module
147
may also determine scores for each of a plurality of feature vectors based on
the
statistical features of the vibroarthrograms.
[00072] The diagnosis module 148 is configured to identify a condition of
the knee joint 28 based on the output of the classification module 147. The
diagnosis module 148 may also mathematically describe the relative motion of
the bones in the knee joint 28 as such motion is tracked by the 3-D modeling
module 143. The kinematics of the knee may then be correlated with a database
of mathematical descriptions of joint motion that includes descriptions of
healthy
and clinically undesirable joint motion to identify possible conditions in the
knee
28.
24
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
[00073] During a dynamic activity, the interaction between the moving
articulating surfaces of the joint may induce vibrations of the bones. In a
healthy
joint, the articulating surfaces are smooth and the vibration is minimal. But
as the
cartilage degenerates or there is injury to other soft tissue structures,
vibrations
increase, and may become audible. Loss of cartilage is a natural process of
aging but may not necessarily be severe enough to cause pain. However, if the
cartilage deteriorates due to arthritis, its loss is accelerated and most
often
causes unbearable pain that limits the mobility of afflicted patients. In case
of a
complete loss of articular cartilage, the raw bone surfaces interact with each
other directly so that the joint produces an identifiable vibration and/or
acoustic
signatures. In the case of soft tissue damage, the injury may result in the
change
of the vibration and acoustic signatures in a unique injury specific manner.
This
unique injury specific change may then be detected through the changes in the
vibroarthogram and/or the acoustics produced by the vibration signals when
compared to other healthy patterns.
[00074] To create a vibroarthrography database 130 of joint vibrations,
vibration data may be collected from a first plurality of test subjects having
healthy knees and a second plurality of test subjects suffering from a joint
condition, such as knee arthritis or other soft tissue and/or ligament
injuries. To
further augment the vibroartrography database, during a subsequent joint
replacement procedure or investigative arthroscopic procedure on test subjects
selected from the second plurality of patients, the surgeon may examine the
condition of the articular cartilage and soft tissue structures of the joint
and
record an assessment of the joint condition. To this end, the surgeon's
observations may be recorded on an intrasurgical evaluation sheet during a
joint
procedure to provide the information about the location and severity of the
articular cartilage damage. Other factors that might affect the vibrations
generated by the joint, such as ligament deficiency or meniscus injuries may
also
be examined and described.
[00075] Referring now to FIG. 6, in an exemplary embodiment of the
invention, an intrasugical evaluation sheet 150 for a knee joint may include a
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
section 152 for recording articular cartilage condition that includes five
numerical
classifications. These classifications may be assessed for each of a plurality
of
regions 154a-154u of the medial and lateral patella 156, distal femur 158, and
tibial plateau 160. The classifications may include, for example: (0) normal;
(1)
minor changes; (2) abnormal; (3) severe cartilage loss; and (4) raw bone. The
evaluation sheet 150 may also include a section 162 for recording ligament
conditions for the Anterior Cruciate Ligament (ACL), Posterior Cruciate
Ligament
(PCL), Medial Collateral Ligament (MCL), Lateral Collateral Ligament (LCL) and
patellar ligament as being either: (1) intact; (2) attenuated; or (3)
absent/disrupted. The evaluation sheet may further include a section 164 for
recording the condition of the medial meniscus and lateral meniscus as one of:
(1) intact; (2) having an anterior tear; (3) having a posterior tear; (4)
having been
subject to a partial anterior meniscectomy; (5) having been subject to a
partial
posterior meniscectomy; or (6) absent.
[00076] The vibroarthrography database 130 may be augmented by
associating the surgical observations entered on the evaluation sheet 150 with
the corresponding recorded joint vibration and acoustic signatures for the
joint in
question. This information may then be converted to grid based coded
graphic/visual display and provide input to the display module 149 of the
diagnostic system 140. Embodiments of the invention may thereby provide
insight into the exact condition of the cartilage and the amount of damage at
every compartment of the joint, as well as any other factors passably altering
the
vibration pattern, such as any ligament deficiency or meniscal injuries. The
visual display may further provide information on the exact interacting
locations
(and their condition as recorded on the evaluation sheet of FIG. 6) of the
femur/tibia/patella that interact to produce a specific vibroathrographic
pattern.
Having this information may facilitate correlating the condition of the joint
with the
detected vibration data, and the vibration and acoustic signatures generated
there from. Thus, vibroarthrography as used herein may provide an additional
source of data that can be collected non-invasively under in-vivo conditions,
to
enhance the diagnostic capabilities of the diagnostic system 140.
26
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
[00077] Referring now to FIG. 7, and in accordance with an embodiment of
the invention, vibration data may be collected using a plurality (e.g., four)
tri-axial
accelerometers 168-171 each having a sensitivity of about 100 mV/g, a
measurement range of about 50 g, and a frequency response of about 0.5 to 5
kHz. One such accelerometer suitable for collecting vibration data is a model
356Al2 accelerometer available from PCB Piezotronics Inc. of Depew, NY. The
accelerometers 168-171 may be included in an embodiment of the knee brace
32, or may be attached to the surface of the skin at the lateral femoral
epicondyle
(accelerometer 168), medial femoral epicondyle (accelerometer 169), the tibial
tuberocity (accelerometer 170), and the patella (accelerometer 171)
respectively
using any suitable means, such as elastic wrap and/or hypoallergenic adhesive
tape 172.
[00078] As previously described, the accelerometers are coupled to the
signal conditioning module 74, which may have a gain of about 10 dB and
include a low-pass filter with a cut-off frequency of about 4700Hz. One
suitable
device for providing the signal conditioning module 74 is a Model 4820 signal
conditioner available from PCB Piezotronics Inc., Depew, NY. The conditioned
signal is then coupled to the ADC 78. The ADC 78 may be a multi-channel
analog-to-digital converter having a 14 bit resolution and 150-200 kHz
waveform
recording capability. One suitable device for providing ADC 78 is a Model
DI-720, available from DATAQ Instruments Inc. of Akron, OH. As the joint 28 is
moved to produce vibrations, the movement may be tracked using the inertial
monitoring unit 48 so that the vibration signal data can be synchronized to
the
joint position as the vibration signal data is generated and stored in memory
112.
[00079] In an embodiment of the invention, the accelerometers 168-171
may have sufficient bandwidth so that the signals generated reflect
acceleration
resulting from both movement of the joint 28 as well as from vibrations of the
bones caused by the movement. The accelerometers 168-171 may thereby
provide signals for use by both the inertial monitoring unit 48 and vibration
detection module 50. To this end, the raw accelerometer signals may be
separated into motion and vibration components. This separation may be
27
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
achieved by passing the output of the accelerometers 168-171 through one or
more low-pass and/or high-pass filters. One suitable filter may be an Infinite
Impulse Response (IIR) Butterworth filter configured to have a signal
attenuation
of 80dB at a cut-off frequency of 20Hz. The raw accelerometer signals may be
separated by such a filter into a low-frequency band containing motion
information, and a high-frequency band containing vibration information.
[00080] Referring now to FIGS. 8 and 9A-9C, a series of graphs are
presented each illustrating a plot of an exemplary signal, or a portion
thereof,
received from one of the accelerometers 168-171. Graph 172 includes a plot
174 representing a raw signal received from the accelerometer via the signal
conditioner. This signal may have been filtered through a low pass filter
having,
for example, a cut-off frequency of about 4700-5000 Hz to reduce noise and
prevent aliasing. Graph 176 includes a plot 178 representing a low-pass
filtered
portion of the raw signal of plot 174 that includes a motion component of the
raw
vibration signal. Graph 180 includes a plot 182 representing a high pass
filtered
portion of the raw signal of plot 174 that includes the vibration portion of
the raw
signal of plot 174. As can be seen from the plots 174, 176, 178, a low pass
filter,
such as the filter 183 shown in FIG. 9A, or some other type of signal
processing
such as shown in FIGS. 9B and 90, may be used to separate the low frequency
portion of the accelerometer output signal from the high frequency portion of
the
signal.
[00081] The low pass filter 183 removes low frequency motion components
from the raw signal, thereby yielding a vibroarthrogram suitable for further
analysis by the diagnosis and visualization module 142. The resulting
vibroarthrogram may also be also converted into audible form and correlated
with
the motion of the 3-D model for display to the user. Persons having ordinary
skill
in the art will understand that other signal analysis techniques may also be
used
to separate the portions of the accelerometer output signals containing motion
information from those portions containing vibration information. These
techniques may include analysis such as: (1) model based analysis techniques
that compute the vibroarthrogram spectrum by comparing the input signal to
28
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
filtered white noise 181; (2) multiple signal classification (e.g., the MUSIC
algorithm) 185 which estimates the frequency content of a signal using an
eigenspace method; or (3) traditional spectral analysis, such as with fast
Fourier
transforms 187 as is known in the art of signal processing.
[00082] To reduce the subjectivity of diagnoses based on simply listening
to
the noise emitted by a moving joint, the diagnosis module 148 uses numerical
methods to identify possible injuries. These methods may identify joint
condition
based on the digital signature of the vibration, and may use pattern
recognition
techniques. Methods may include time-domain analysis or frequency domain
analysis. However, the detected vibration signal pattern can be affected by a
number of factors including but not limited to: (1) the severity of the joint
degeneration; (2) the thickness of the subcutaneous tissue present between the
articulating bone and the accelerometer due to the damping effect of the soft
tissue; (3) the location of the accelerometer relative to the underlying
bones; (4)
the type of activity requiring joint motion (e.g., load bearing or free
movement);
(5) the speed of the activity (faster movements result in higher
accelerations);
and (6) the direction in which the acceleration is being measured.
[00083] Some of these parameters may be controlled to at least some
extent (e.g. the speed of the activity), and others may be optimized
(direction and
location of the accelerometer placement). However, the anatomical diversity
and
various stages of arthritis may cause significant variations in observed
vibration
patterns. These variations, in turn, must be accounted for by the diagnosis
and
visualization module 142 in order to provide accurate diagnosis of joint
conditions.
[00084] Referring now to FIG. 10, a graph 184 illustrates an exemplary
plot
186 of a vibroarthrogram obtained for a healthy subject, and a graph 188
illustrates an exemplary plot 190 of a vibroarthrogram obtained from a subject
diagnosed with patellofemoral joint arthritis. As can be seen from the plots
188,
190, the magnitude of the vibration caused by joint movement tends to be
higher
for a degenerated knee as compared to a healthy knee. Analysis of the plots,
or
vibroarthrograms 186, 190 may include calculating statistical parameters of
the
29
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
original and rectified vibroarthrograms. These statistical parameters may
include, but are not limited to, mean, variance, standard deviation, skewness,
kurtosis, signal envelope integral, signal envelope integral as a function of
duration, as well as 90th, 95, 97th and 99th quantiles. These statistical
parameters may in turn be used to examine features that could be included in a
feature vector of the signals. A desirable feature for a statistical parameter
is that
the statistical parameter provides separation between vibroarthrograms from
healthy joints and vibroarthrograms from injured joints. Statistical
parameters
that have this feature may provide the highest success rate for classifying
joint
conditions. Although embodiments of the invention are generally described
herein with respect to a few statistical parameters, those skilled in the art
will
recognize that the methods and systems described may also be used with the
analysis of other statistical parameters (e.g., entropy, complexity) without
departing from the scope of embodiments of the invention.
[00085] By way of example, it has been determined that the mean and
standard deviations of rectified vibroarthrograms are higher for arthritic
than for
healthy subjects. Another statistical parameter that may provide separation
between vibroarthrograms of injured and healthy joints is the 99th quantile.
Once
the statistical parameters that provide separation between vibroarthrograms
produced by various joint conditions are identified, the parameters may be
used
to define a feature vector. The feature vector may classify vibroarthrograms
by
combining the separations provided by multiple statistical parameters into a
composite separation, thereby providing improved diagnosis as compared to
relying on a single statistical parameter.
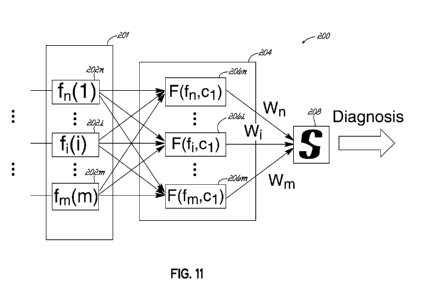
[00086] Referring now to FIG. 11, a pattern classifier 200 in accordance
with an embodiment of the invention includes a set of statistical parameters
that
are determined by a signal features module 201. The signal features module
201 may include functions 202n-202m that calculate one or more signal features
such as the mean ( ), standard deviation (a), complexity (FF), skewness (S),
kurtosis (K), and/or entropy (H) of a vibroarthrogram which may be calculated
using the equations below:
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
= xP (x) (1)
a = ,\11+/Eliv-1(xi ¨ (2)
Nts, r" /qv
FF .............................
ai (3)
in3
S= ____________________________________
)
(4)
ni4
K ¨ ___________________________________ , 3
(5)
I.- 1
H = ¨ p,(r1) log, p, (x/)]
1,0 (6)
[00087] The outputs of the statistical parameter functions 202n-202m may
be provided to a radial basis function network 204 that includes a plurality
of
feature vectors 206n-206m. The feature vectors 206n-206m may in turn
selectively weigh and combine selected outputs of the statistical parameter
functions 202n-202m to calculate a relative value or likelihood that the
vibroarthrogram being analyzed corresponds to a joint having a particular
condition. That is, each feature vector may include one or more weighted
signal
features of the vibroarthrogram, and based on these weighted signal features,
produce a score that may be compared to scores of other feature vectors. Each
feature vector may thereby provide a score indicative of a level of
correspondence between the statistical features of the vibroarthrogram and a
condition of the joint. The scores of the feature vectors 206n-206m are
provided
to a selector 208 that determines a diagnosis based on the scores.
[00088] In an exemplary embodiment of the invention, based on the
selected signal features included in the feature vector, the pattern
classifier 200
classifies the pattern of the vibroarthrographic signal to the defective
condition of
31
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
the joint in question To this end, the pattern classifier 200 may use a
minimum-
error-rate classification to identify the group to which the
vibroarthrographic
signal belongs. This classification can be achieved by the use of the
discriminant
functions:
gi(x) = ln p(xlcoi)+1nP(coi) (7)
which, assuming that the densities p(x100 are multivariate normal, becomes:
1 ,
g1(x) =1(x¨ /./1)t F1 (8)
du )--dln 2z ¨ ¨1nIE ¨ ln P(coi) (8)
2 2 2
To classify the vibroarthrogram as either arthritic or healthy, the prior
probabilities
for both categories in this embodiment may be assumed to be equal, e.g.,
P(00= P(0)2)=-1
2 (9)
[00089] Referring now to FIG. 12A, a scatter plot is presented showing
mean values of vibroarthrograms for 18 healthy knees and 18 arthritic knees.
As
can be seen, the rectified vibroarthrograms for the arthritic knees tend to
have
higher mean values than the vibroarthrograms for the healthy knees. Thus, in
an
embodiment of the invention, the mean of the rectified signal may be used as a
single feature to design a dichotomizer that classifies the vibroarthrograms.
For
example, drawing a horizontal line across the plot at a mean of approximately
0.003 provides reasonable separation of the arthritic vibroarthrograms from
the
healthy vibroarthrograms. Experimental results indicate that the expected
success rate obtained using the mean of the rectified vibroarthrogram as a
this
single feature classifier is about 73%.
[00090] Referring now to FIG. 12B, a scatter plot is presented showing
standard deviation values of vibroarthrograms for the 18 healthy knees and 18
arthritic knees. As can be seen, the arthritic knees also tend to have higher
standard deviation values than the healthy knees. In an alternative embodiment
of the invention in which the, a second feature, standard deviation, is
included in
the discriminant function a second feature, experimental. Experimental results
indicate that the expected success rate of this alternative embodiment
increased
to about 81%.
32
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
[00091] Referring now to FIG. 120, a scatter plot is presented showing
99th
quintile values of vibroarthrograms for the 18 healthy knees and 18 arthritic
knees. As can be seen, the arthritic knees also tend to have higher 99th
quintile
values than the healthy knees, indicating that the 99th quintile may also be a
useful feature to improve separation between vibroarthrograms for healthy
joints
and injured joints.
[00092] FIG. 12D presents a 3-D graph showing the results from applying
the discriminant function of equation (8) when applied to a group of test
subjects
including 15 subjects having healthy knees and 20 subjects suffering from
arthritis. These patients were recruited from a group of candidates for the
primary total knee replacement procedure. The plot shows subjects that were
diagnosed as having healthy knees as O's and those diagnosed as having
arthritic knees as X's. Misdiagnosed subjects have a box around the character.
That is, a boxed X is someone diagnosed as having an arthritic knee by the
discriminant function but that was found to have a healthy knee during a
subsequent total knee replacement procedure. Likewise, a boxed 0 represents
a subject diagnosed as having a healthy knee that was later determined to have
an arthritic knee. As can be seen, the correct diagnosis was returned in 28
out of
the 35 cases, for a success rate of about 80%. FIG. 12E presents a 3-D graph
showing results obtained using a discriminant function including 33 features.
In
this embodiment, the success rate was about 95%. Thus, proper selection and
weighting of statistical features of vibroarthrograms can provide accurate
diagnoses of joint conditions in a non-invasive manner that does not require
exposing the patient to ionizing radiation.
[00093] In an embodiment of the invention, the feature vectors 206n-206m
may be provided to the diagnosis and visualization module 142 and be included
the feature vector set. This may enable the diagnosis and visualization module
142 to utilize other feature vectors provided by the ultrasound module 52 and
inertial monitoring unit 48 along with feature vectors 206n-206m to form a
complete feature vector set. This feature vector set may then be used by the
classification and diagnosis modules 147, 148 to provide a diagnosis.
33
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
[00094] In an embodiment of the invention, Fourier transforms 187 may be
used to process vibration data. A Fourier transform is a mathematical
operation
that transforms a signal from the time-domain to the frequency domain. The
Fourier transform views the frequency content of an interval of the signal as
a
whole. That is, it looks at the time interval being transformed, and converts
that
portion of the signal into the frequency domain so that the frequency content
of
the interval is revealed. Thus, Fourier transforms switch the dimension of
time
with the dimension of frequency for the analyzed interval.
[00095] Referring to FIG. 13, the vibration content of the signals from
accelerometers 168-171 may be filtered to remove the motion component of the
vibration and an interval of the filtered signal defined. This defined
interval may
provide a captured time-domain signal 230 that is provided to a Fourier
transform
module 232. The Fourier transform module 232 may use a Discrete Fourier
Transform (DFT) to convert the captured time-domain signal 230 from the time
to
the frequency domain. The resulting captured frequency-domain signal 234 may
then be used to analyze the propertied of the captured time-domain signal 230.
A DFT is a Fourier transform applied to a sampled (i.e., discrete) signal
rather
than a continuous signals. DFT's are thus well adapted for processing digital
signals. A Fast Fourier Transform (FFT) is a simplified version of the DFT
that
may be applied to digitized intervals or sample periods when the number of
samples in the sample period is a power of two. An FFT computation takes
approximately N * log2(N) operations, whereas a DFT takes approximately N2
operations, so the FFT is significantly faster.
[00096] As a result of applying a Fourier transform to convert the
captured
time-domain signal 230 to the captured frequency-domain signal 234, the time
information is obscured. However, the vibration data collected by the
vibration
detection module 50 of joint monitoring apparatus 30 is part of a larger set
of
data (i.e., the position data captured by inertial tracking and ultrasound
modules
48, 52) that characterizes the joint. Because this additional data is in the
time-
domain, it may be difficult to correlate a specific characteristic the
captured
frequency-domain signal 234 with an occurrence of a particular event
identified
34
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
by the time-domain signals generated by inertial tracking and ultrasound
modules
48, 52. The vibration signals collected by the vibration detection module 50
may
also contain numerous non-stationary or transitory characteristics, i.e.,
abrupt
changes, beginnings and ends of events. These characteristics may be a
valuable part of the signal since they may indicate the occurrence of certain
events of interest. However, these events are typically not identified or
detected
by a Fourier analysis of the time-domain signal.
[00097] In order to address this short coming of Fourier transforms, the
captured time-domain signal 230 may be processed by a short-time Fourier
transform module 236, which use a windowing technique to analyze the vibration
signals and output a short-time frequency domain signal 238. To this end, the
short-term Fourier transform module applies a Fourier transform over several
small sections of the signal so that both time and frequency information is
captured. This type of analysis is called the short-time Fourier Transform
(STFT)
analysis.
[00098] Referring now to FIGS. 14A and 14B, a graph 240 illustrates a
exemplary captured time-domain signal 242. A corresponding spectrogram 246
may be generated from the time-domain signal 240 using an STFT. The
spectrogram 244 reveals the portion of the captured time-domain signal's
energy
with respect to frequency as the captured time-domain signal 240 changes over
time. Since the spectrogram 244 reveals both time information and information
about the frequency content of the signal 242, the spectrogram 242 may be used
to filter and analyze vibration signals in both time and frequency
simultaneously.
A feature vector containing the frequency of a vibration signal occurring at a
particular time period may then be employed as an input to the radial basis
function network 204 for further analysis or input as part of feature vector
set
provided to the diagnosis and visualization module 142 by the feature vector
module 146.
[00099] Referring now to FIG. 15, the diagnostic system 140 may be
configured to display diagnostic information page 210 that includes an image
212
representing one or more views of the 3-D model of the patient's joint,
contact
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
location maps of the distal femur 214 and patella 216, and a vibroarthrogram
218
including a sampling window 220. The contact location maps 214, 216 may
include an end view 222 of the distal femur, and an end view 224 of the
patella.
At different positions of the joint model, respective contact locations 226,
228
may change in size and color to indicate areas of pressure or contact between
the bones of the joint. This diagnosis can be extended to include the tibio-
femoral joint interaction. The image 212, contact location maps 214, 216 and
vibroarthrogram 218 may also be synchronized with respect to joint position.
The
contact location maps 214, 216 may also include visual feedback of the
condition
of the joint based on surgical observations entered into the database 130 from
one or more evaluation sheets 150. The visually observed condition of the
joint
may thereby be correlated with respect to the vibration signal in the sampling
window 220.
[000100] Upon playback, this synchronization may cause the sampling
window 220 to move across the vibroarthrogram 218 in synchronization with
movement of the 3-d model image 212 and changes to the contact location maps
214, 216. The system user may also be able to examine specific positions of
the
joint by dragging the sampling window 220 across the vibroarthrogram 218. The
diagnostic information page 210 may thereby provide a "movie" that allows the
system user to simultaneously observe the kinematics of the joint (as shown by
the motion of the 3-D model in image 212), with the corresponding contact
between the joint components, and the joint vibrations being produced. The
in-vivo kinematics and predicted contact location map correlated with the
vibration data may bring insight into not only the severity of the articular
cartilage
degeneration, but also to other defects as determined by the cartilage and
meniscus damage map generated based on the surgical observations entered
into the database 130. The location of the contact between the interacting
surfaces, when co-related with the joint condition of the corresponding
location
may provide insight in to the nature of the vibration signal in sampling
window
220 that co-relates to a certain defect. The contact location maps 214, 216
may
provide information on the location of the contact between two bones. The grid
36
CA 02906476 2015-09-14
WO 2014/150780 PCT/US2014/024205
based visual representation of the evaluation sheet may provide information on
the condition of the joint at the location predicted by the contact location
maps
214, 216. And the vibration signal in the sampling window 220 may provide
information on the exact vibration pattern corresponding to the degenerative
condition of the joint being analyzed. Thus, the diagnostic information page
210
may provide the joint information in a unique and comprehensive manner that is
invaluable for treatment planning.
[000101] The above described vibroarthrography based diagnostic system
may be used for pre-operative diagnosis to help determine a proper course of
treatment. The system is also well adapted for use perioperatively, and could
be
used, for example, to assist surgeons with fine tuning a joint implant to
ensure
that the implant is properly sized and positioned within the patient prior to
closing
the joint. Moreover, the system may also be useful for post-operative
evaluation
of implant wear and for early identification of abnormal wear or conditions.
The
information provided by the diagnostic system may also provide users with
immediate feedback on the effectiveness of joint injections (e.g., Visco
supplementation) and other treatments. Thus, the diagnostic system may be
helpful for surgeons planning joint replacement procedures, and may eliminate
the need for imaging modalities involving harmful radiation.
[000102] While the invention has been illustrated by a description of
various
embodiments, and while these embodiments have been described in
considerable detail, it is not the intention of the applicant to restrict or
in any way
limit the scope of the appended claims to such detail. Additional advantages
and
modifications will readily appear to those skilled in the art. Accordingly,
departures may be made from such details without departing from the spirit or
scope of applicant's general inventive concept.
[000103] What is claimed is:
37