Note: Descriptions are shown in the official language in which they were submitted.
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 372
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 372
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE:
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
COMBINATION OF KINASE INHIBITORS AND USES THEREOF
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Patent Application No.
13/843,816, filed
on March 15, 2013, and U.S. Patent Application No. 14/099,644, filed on
December 6, 2013,
each incorporated herein by reference in its entirety.
BACKGROUND OF THE INVENTION
[0002] Kinase signaling pathways play a central role in numerous biological
processes.
Defects in various components of signal transduction pathways have been found
to account
for a vast number of diseases, including numerous forms of cancer,
inflammatory disorders,
metabolic disorders, vascular and neuronal diseases (Gaestel et al., Current
Medicinal
Chemistry (2007) 14:2214-2234). In recent years, kinases that are associated
with oncogenic
signaling pathways have emerged as important drug targets in the treatment of
various
diseases including many types of cancers.
[0003] The mammalian target of rapamycin (mTOR), also known as mechanistic
target of
rapamycin, is a serine/threonine protein kinase that regulates cell growth,
translational
control, angiogenesis and/or cell survival. mTOR is encoded by the FK506
binding protein
12-rapamycin associated protein 1 (FRAP1) gene. mTOR is the catalytic subunit
of two
complexes, mTORC1 and mTORC2. mTORC1 is composed of mTOR, regulatory
associated
protein of mTOR (Raptor), mammalian LST8/G-protein 13-subunit like protein
(mLST8/G13L), PRAS40, and DEPTOR. mTOR Complex 2 (mTORC2) is composed of
mTOR, rapamycin-insensitive companion of mTOR (Rictor), G13L, and mammalian
stress-
activated protein kinase interacting protein 1 (mSIN1).
[0004] Apart from their subunits, mTORC1 and mTORC2 are distinguished by their
differential sensitivities to rapamycin and its analogs (also known as
rapalogs). Rapamycin
binds to and allosterically inhibits mTORC1, but mTORC2 is generally rapamycin-
insensitive. As a result of this rapamycin-insensitive mTOR signaling mediated
by
mTORC2, cancer cells treated with rapamycin analogs usually display only
partial inhibition
of mTOR signaling, which can lead to enhanced survival and resistance to
rapamycin
treatment.
[0005] Another group of kinases involved in cellular functions that are
commonly
deregulated in diseases is the Phosphatidylinosito1-3-kinases (PI 3-kinases or
PI3Ks) family
of enzymes. These lipid kinases phosphorylate the 3-position hydroxyl group of
the inositol
-1-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
ring of phosphatidylinositol (PtdIns), activating signaling cascades
associated with such
processes as cell growth, proliferation, differentiation, motility, survival
and intracellular
trafficking. Disruption of these processes involving PI3K leads to many
diseases including
cancer, allergic contact dermatitis, rheumatoid arthritis, osteoarthritis,
inflammatory bowel
diseases, chronic obstructive pulmonary disorder, psoriasis, multiple
sclerosis, asthma,
disorders related to diabetic complications, and inflammatory complications of
the
cardiovascular system such as acute coronary syndrome.
[0006] The PI3K family comprises 15 kinases with distinct substrate
specificities, expression
patterns, and modes of regulation. The class I PI3Ks (p110a, p 11 op, p1106,
and p110y) are
typically activated by tyrosine kinases or G-protein coupled receptors to
generate
phosphatidylinosito1-3,4,5-trisphosphate (PIP3), which engages downstream
effectors such as
those in the Akt/PDK1 pathway, mTOR, the Tec family kinases, and the Rho
family
GTPases.
[0007] The alpha (a) isoform of type I PI3K has been implicated in a variety
of human
cancers. Angiogenesis has been shown to selectively require the a isoform of
PI3K in the
control of endothelial cell migration. (Graupera et al., Nature 2008;453;662-
6). Mutations in
the gene coding for PI3K a or mutations which lead to upregulation of PI3K a
are believed to
occur in many human cancers such as lung, stomach, endometrial, ovarian,
bladder, breast,
colon, brain and skin cancers. Often, mutations in the gene coding for PI3K a
are point
mutations clustered within several hotspots in helical and kinase domains,
such as E542K,
E545K, and H1047R. Many of these mutations have been shown to be oncogenic
gain-of-
function mutations. While other PI3K isoforms such as PI3K 6 or PI3K y are
expressed
primarily in hematopoietic cells, PI3K a, along with PI3K13, is expressed
constitutively.
[0008] The delta (6) isoform of class I PI3K has been implicated, in
particular, in a number
of diseases and biological processes. PI3K 6 is expressed primarily in
hematopoietic cells
including leukocytes such as T-cells, dendritic cells, neutrophils, mast
cells, B-cells, and
macrophages. PI3K 6 is integrally involved in mammalian immune system
functions such as
T-cell function, B-cell activation, mast cell activation, dendritic cell
function, and neutrophil
activity. Due to its integral role in immune system function, PI3K 6 is also
involved in a
number of diseases related to undesirable immune response such as allergic
reactions,
inflammatory diseases, inflammation mediated angiogenesis, rheumatoid
arthritis, auto-
immune diseases such as lupus, asthma, emphysema and other respiratory
diseases. Other
class I PI3K involved in immune system function includes PI3K y, which plays a
role in
-2-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
leukocyte signaling and has been implicated in inflammation, rheumatoid
arthritis, and
autoimmune diseases such as lupus.
[0009] PI3K 13 has been implicated primarily in various types of cancer
including PTEN-
negative cancer (Edgar et al. Cancer Research (2010) 70(3): 1 164-1 172), and
HER2-
overexpressing cancer such as breast cancer and ovarian cancer.
SUMMARY OF THE INVENTION
[0010] Due to the diverse essential functions of mTOR and PI3Ks, drugs that
bind to and
inhibit a broad range of kinase isoforms and complexes with low specificity
can lead to
deleterious side effects. For example, excessive inhibition of PI3K 13 may
lead to undesirable
effects on metabolic pathways and disruption of insulin signaling.
Alternatively, excessive
inhibition of PI3K 6 and/or PI3K y may disrupt or reduce immune function. The
present
disclosure provides an alternative approach that effectively targets disease-
related pathways,
while limiting undesirable side effects.
[0011] Accordingly, the invention provides a method for treating a disease
condition
associated with P13-kinase a and/or mTOR in a subject, comprising
administering to said
subject simultaneously or sequentially a therapeutically effective amount of a
combination of
a P13-kinase a inhibitor and an mTOR inhibitor, wherein the P13-kinase a
inhibitor exhibits
selective inhibition of P13-kinase a relative to one or more type I
phosphatidylinosito1-3-
kinases (P13-kinase) ascertained by an in vitro kinase assay, wherein the one
or more type I
P13-kinase is selected from the group consisting of P13-kinase 13, P13-kinase
y, and P13-kinase
6. In one aspect, the combination comprises a therapeutic effective amount of
a P13-kinase a
inhibitor and a therapeutic effective amount of an mTOR inhibitor. In another
aspect, the
combination comprises a synergistically effective therapeutic amount of P13-
kinase a
inhibitor and an mTOR inhibitor, wherein the P13-kinase a inhibitor and/or the
mTOR
inhibitor is present in a sub-therapeutic amount.
[0012] The invention also provides a method for treating a disease condition
associated with
P13-kinase a and/or mTOR in a subject, comprising administering to said
subject
simultaneously or sequentially a combination of (a) a therapeutically
effective amount of a
P13-kinase a inhibitor according to a first dosing regimen and (b) a
therapeutically effective
amount of an mTOR inhibitor according to a second dosing regimen, wherein the
P13-kinase
a inhibitor exhibits selective inhibition of P13-kinase a relative to one or
more type I
phosphatidylinosito1-3-kinases (P13-kinase) ascertained by an in vitro kinase
assay, wherein
the one or more type I P13-kinase is selected from the group consisting of P13-
kinase 13, P13-
-3-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
kinase y, and P13-kinase 6, wherein each dosing regimen independently
comprising repeating
cycles of a treatment period followed by a rest period, wherein at least one
dosing regimen
has one rest period of more than 0 day. In some methods, the first dosing
regimen and the
second dosing regimen are the same and are administered simultaneously. In
some methods,
the first dosing regimen and the second dosing regimen are different. In some
methods, the
first and/or the second dosing regimen independently comprises at least one
cycle of a
treatment period of at least 1 day followed by a rest period of at least 1
day. In some
methods, the first and/or the second dosing regimen independently comprises at
least one
cycle of a treatment period of 2, 3, 4, 5, 6 or 7 consecutive days followed by
a rest period of
at least 1 day. In some methods, the first and/or the second dosing regimen
independently
comprises at least one cycle of a treatment period of 2, 3, 4, 5, 6 or 7
consecutive days
followed by a rest period of at least 3, 4, or 5 consecutive days. In some
methods, the first
and/or the second dosing regimen independently comprises at least one cycle of
a treatment
period of at least 1 day followed by a rest period of 6 consecutive days. In
some methods, the
first and/or the second dosing regimen independently comprises at least one 7-
day cycle of a
treatment period of 3 consecutive days followed by a rest period of 4
consecutive days,
optionally the first dosing regimen and the second dosing regimen are the same
and are
administered simultaneously. In some methods, the first and/or the second
dosing regimen
independently comprises at least one 7-day cycle of a treatment period of 5
consecutive days
followed by a rest period of 2 consecutive days. In some methods, the first
and/or the second
dosing regimen independently comprises at least one 7-day cycle of a treatment
period of 1
consecutive days followed by a rest period of 6 consecutive days. In some
methods, the first
and/or the second dosing regimen independently comprises at least one 7-day
cycle
comprising at least 3 treatment periods on alternate days within the 7 days.
[0013] In some methods, the second dosing regimen has a rest period of 0 day.
In some
methods, the first dosing regimen has a rest period of 0 day. In some methods,
the first
dosing regimen has a rest period of 0 day, and the second dosing regimen
comprises at least
one 7-day cycle of a treatment period of 5 consecutive days followed by a rest
period of 2
consecutive days. In some methods, the first dosing regimen has a rest period
of 0 day, the
second dosing regimen comprises at least one 7-day cycle of a treatment period
of 1
consecutive days followed by a rest period of 6 consecutive days.
[0014] The invention also provides a method for treating a disease condition
associated with
P13-kinase a and/or mTOR in a subject, comprising administering to said
subject
simultaneously or sequentially a combination of (a) a therapeutically
effective amount of a
-4-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
P13-kinase a inhibitor and (b) a therapeutically effective amount of an mTOR
inhibitor,
wherein the P13 -kinase a inhibitor exhibits selective inhibition of P13 -
kinase a relative to one
or more type I phosphatidylinosito1-3-kinases (P13-kinase) ascertained by an
in vitro kinase
assay, wherein the one or more type I P13-kinase is selected from the group
consisting of P13-
kinase 13, P13-kinase y, and P13-kinase 6, wherein the clinical and
therapeutic effects of the
treatment of the disease condition continue for a durability of effect period
of at least as long
as the administration period. In some methods, the combination comprises a
synergistically
effective therapeutic amount of P13-kinase a inhibitor and an mTOR inhibitor,
wherein the
P13-kinase a inhibitor and/or the mTOR inhibitor is present in a sub-
therapeutic amount. In
some methods, the clinical and therapeutic effects are selected from the group
consisting of
sustained tumor regression, inhibited tumor re-growth, reduction of
proliferation, increased
apoptosis, or downregulation of activity of a target protein. In some methods,
the clinical and
therapeutic effects are sustained tumor regression and inhibited tumor re-
growth. In some
methods, the durability of effect period is at least 30 days. In some methods,
the durability of
effect period is at least 5 days. In some methods, the P13-kinase a inhibitor
is administered
according to a first intermittent dosing regimen comprising repeating cycles
of a treatment
period followed by a rest period. In some methods, the mTOR inhibitor is
administered
according to a second intermittent dosing regimen comprising repeating cycles
of a treatment
period followed by a rest period.
[0015] The invention also provides a method of treating a disease condition
associated with
P13-kinase a and/or mTOR in a subject, comprising administering to the subject
simultaneously or sequentially a combination of (a) a therapeutically
effective amount of a
P13-kinase a inhibitor and (b) a therapeutically effective amount of an mTOR
inhibitor
according to an intermittent regimen effective to achieve (a) higher
therapeutic efficacy, (b)
similar or better tolerability of the P13 -kinase a inhibitor and/or mTOR
inhibitor, and (c)
similar or smaller area under the curve, as compared to administering an
equivalent dose of
the P13-kinase a inhibitor and/or mTOR inhibitor once daily; wherein the P13-
kinase a
inhibitor exhibits selective inhibition of P13-kinase a relative to one or
more type I
phosphatidylinosito1-3-kinases (P13-kinase) ascertained by an in vitro kinase
assay, wherein
the one or more type I P13-kinase is selected from the group consisting of P13-
kinase 13, P13-
kinase y, and P13-kinase 6.
[0016] In some embodiments, the disease condition associated with P13-kinase a
and/or
mTOR can include but are not limited to a neoplastic condition, autoimmune
disease,
inflammatory disease, fibrotic disease and kidney disease. For example, the
neoplastic
-5-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
condition can be NSCLC, head and neck squamous cell carcinoma, pancreatic,
breast and
ovarian cancers, renal cell carcinoma, prostate cancer, neuroendocrine cancer,
endometrial
cancers, and other forms of cancer.
[0017] The invention further provides a method of inhibiting phosphorylation
of both Akt
(S473) and Akt (T308) in a cell, comprising contacting a cell with an
effective amount of a
P13-kinase a inhibitor and an mTOR inhibitor that selectively inhibits both
mTORC1 and
mTORC2 activity relative to one or more type I phosphatidylinosito1-3-kinases
(P13-kinase)
as ascertained by a cell-based assay or an in vitro kinase assay, wherein the
P13-kinase a
inhibitor exhibits selective inhibition of P13-kinase a relative to one or
more type I
phosphatidylinosito1-3-kinases (P13-kinase) ascertained by an in vitro kinase
assay, wherein
the one or more type I P13-kinase is selected from the group consisting of P13-
kinase 13, P13-
kinase y, and P13-kinase 6. In some embodiments, the P13-kinase a inhibitor
selectively
inhibits P13 -kinase a relative to all other type I phosphatidylinosito1-3-
kinases (P13 -kinase)
consisting of P13-kinase 13, P13-kinase y, and P13-kinase 6.
[0018] For instance, the P13-kinase a inhibitor utilized in the subject
methods inhibits P13-
kinase a with an IC50 value of about 500 nM or less, 400 nM or less, 300 nM or
less, 200 nM
or less, 100 nM or less, 10 nM or less, 1nM or less as ascertained in an in
vitro kinase assay.
In another instance, the P13 -kinase a inhibitor selectively inhibits P13 -
kinase a with an IC50
value that is at least 2, 5, 10, 50, 100, 1000 times less than its IC50 value
against one, two,
three or all other type I P13-kinases selected from the group consisting of
P13-kinase 13, P13-
kinase y, and P13-kinase 6. In some embodiments, the P13-kinase a inhibitor
selectively
inhibits P13-kinase a with an IC50 value that is less than about 200 nM, and
said IC50 value
is at least 2, 5 or 10 times less than its IC50 value against all other type I
P13-kinases selected
from the group consisting of P13-kinase 13, P13-kinase y, and P13-kinase 6.
[0019] In some embodiments, the P13 -kinase a inhibitor selectively inhibits
P13 -kinase a
and/or P13-kinase 0 with an IC50 value that is at least 5 times less than its
IC50 value against
P13-kinase y or P13-kinase 6. In yet other embodiments, the P13-kinase a
inhibitor selectively
inhibits P13-kinase a and/or P13-kinase 0 with an IC50 value that is at least
50 times less than
its IC50 value against P13-kinase y or P13-kinase 6. In still other
embodiments, the P13-kinase
a inhibitor selectively inhibits P13-kinase a with an IC50 value that is at
least 50 times less
than its IC50 value against P13-kinase y or P13-kinase 6.
[0020] In some embodiments of the methods of the invention, the mTOR inhibitor
binds to
and directly inhibits both mTORC1 and mTORC2. For example, the mTOR inhibitor
inhibits
both mTORC1 and mTORC2 with an IC50 value of about 500 nM or less, 400 nM or
less,
-6-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
300 nM or less, 200 nM or less, 100 nM or less, 50 nM or less, 10 nM or less,
or 1nM or less,
as ascertained in an in vitro kinase assay. In another embodiment, the mTOR
inhibitor
inhibits both mTORC1 and mTORC2 with an IC50 value of about 10 nM or less as
ascertained in an in vitro kinase assay, and the mTOR inhibitor is
substantially inactive
against one or more types I P13-kinases selected from the group consisting of
P13-kinase a,
P13-kinase 13, P13-kinase y, and P13-kinase 6. Alternatively, the mTOR
inhibitor inhibits both
mTORC1 and mTORC2 with an IC50 value of about 100 nM or less as ascertained in
an in
vitro kinase assay, and the IC50 value is at least 2, 5 or 10 times less than
its IC50 value
against all other type I P13-kinases selected from the group consisting of P13-
kinase a, P13-
kinase 13, P13-kinase y, and P13-kinase 6.
[0021] In some embodiments, the mTor inhibitor inhibits mTORC1 selectively.
For example,
the mTor inhibitor inhibits mTORC1 with an IC50 value of about 1000 nM or
less, 500 nM
or less, 100 nM or less, 50 nM or less, 10 nM or less, as ascertained in an in
vitro kinase. In
some embodiments, the mTor inhibitor is rapamycin or an analogue of rapamycin.
In other
embodiments, the mTor inhibitor is sirolimus (rapamycin), deforolimus
(AP23573, MK-
8669), everolimus (RAD-001), temsirolimus (CCI-779), zotarolimus (ABT-578), or
biolimus
A9 (umirolimus).
[0022] In some embodiments, the mTOR inhibitor is a compound of Formula I:
R31 R32
Ni/
M1
N
0 Taxi
X5, X3..
X4 X2
R1
Formula I
or a pharmaceutically acceptable salt thereof, wherein:
Xi is N or C-E1, X2 is N or C, X3 is N or C, X4 is C-R9 or N, X5 is N or C-E1,
X6 is C or N,
and X7 is C or N; and wherein no more than two nitrogen ring atoms are
adjacent;
Ri is H, ¨L-Ci_ioalkyl, -L-C3_8cycloalkyl, -L-Ci_ioalkyl -C3_8cycloalkyl, -L-
aryl, -L-
heteroaryl, -L-Ci_ioalkylaryl, -L- Ci_ioalkylhetaryl, -L-
Ci_ioalkylheterocylyl, -L-C2_ioalkenyl,
-L-C2_10alkynyl, -L-C2_ioalkenyl-C3_8cycloalkyl, -L-C2_10alkynyl-
C3_8cycloalkyl, -L-
heteroalkyl, -L-heteroalkylaryl, -L-heteroalkylheteroaryl, -L-heteroalkyl-
heterocyclyl, -L-
-7-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
heteroalkyl-C3_8cycloalkyl, -L-heteroaralkyl, or -L-heterocyclyl, each of
which is
unsubstituted or is substituted by one or more independent R3;
L is absent, -(C=0)-, -C(=0)0-, -C(=0) N(R31)-5-S-, -S(0)-5 -S(0)2-, -
S(0)2N(R31)-5 or -
N(R31)-;
El and E2 are independently -(Wi)j -R4;
M1 is a 5, 6, 7, 8, 9, or-10 membered ring system, wherein the ring system is
monocyclic or
bicyclic, substituted with R5 and additionally optionally substituted with one
or more -(W2)k
-R2;
each k is 0 or 1;
j in El or j in E25 is independently 0 or 1;
W1 is -0-, - -S(0)0_2-5-C(0)-,-C(0)N(R7)-, -N(R7)C(0)-5 -N(R7)S(0)-5-
N(R7)S(0)2-5 -C(0)0-5 -CH(R7)N(C(0)0R8)-5 -CH(R7)N(C(0)R8)-5 -CH(R7)N(S02R8)-5
-CH(R7)N(R8)-5 -CH(R7)C(0)N(R8)-5 -CH(R7)N(R8)C(0)-5 -CH(R7)N(R8)S(0)-5 or -
CH(R7)N(R8)S(0)2-;
W2 is -0-, - -S(0)0_2-5-C(0)-5-C(0)N(R7)-5 -N(R7)C(0)-5 -N(R7)C(0)N(R8)-5-
N(R7)S(0)-5 -N(R7)S(0)2-5-C(0)0-5 -CH(R7)N(C(0)0R8)-5 -CH(R7)N(C(0)R8)-5 -
CH(R7)N(S02R8)-5 -CH(R7)N(R8)-5 -CH(R7)C(0)N(R8)-5 -CH(R7)N(R8)C(0)-5 -
CH(R7)N(R8)S(0)-5 or -CH(R7)N(R8)S(0)2-;
R2 is hydrogen, halogen, -OH, -R315 -CF3, -0CF35 -0R315 -NR31R325 -NR34R355 -
C(0)R315 -
CO2R315 -C(=0)NR31R325 -C(=0)NR34R355 -NO2, -CN, -S(0)0_2R315 -SO2NR31R325 -
S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R335 -NR31S(0)0_2R325
-
C(=S)0R315 -C(=0)SR315 -NR31C(=NR32)NR33R325 -NR31C(=NR32)0R335 -
NR31C(=NR32)SR335 -0C(=0)0R335 -0C(=0)NR31R325 -0C(=0)SR315 -SC(=0)0R315 -
P(0)0R310R32, -SC(=0)NR31R32 , aryl (e.g. bicyclic aryl, unsubstituted aryl,
or substituted
monocyclic aryl), hetaryl, C iioalkyl, C3_8cycloalkyl, Ci_malkyl-C 3 _8
cycloalkyl, C 3 _8 cycloalkyl
-Ci_i0alkyl, C3_8 cyclo alkyl -C2_10alkenyl, C 3 _8 eycloalkyl- C2_10alkynyl,
C2_10alkenyl,
C2_10alkynyl, Ci_malkylaryl (e.g. C240alkyl-monocyclic aryl, Ci_malkyl-
substituted
monocyclic aryl, or Ci_malkylbicycloary1), Ci_malkylhetaryl,
Ci_malkylheterocyclyl, C2
1 alkenyl, C2_1 alkynyl, C 2_1 alkenyl -Ci_i 0 a 1 ky 1 , C2_1 alkynyl -Ci_i 0
a 1 ky 1 , C2_1 alkenylaryl, C2
loalkenylhetaryl, C2_10alkenylheteroalkyl, C2_10alkenylheterocycicyl,
C2_10alkenyl-C3_
8cycloalkyl, C2_10alkynylaryl, C2_10alkynylhetaryl, C2_ioalkynylheteroalkyl,
C2
malkynylheterocylyl, C2_10alkynyl-C3_8cycloalkenyl, Ci_ioalkoxy C iioaIkyl,
Ci_ioalkoxy-C2_
ioalkenyl, Ci_loalkoxy-C2_10alkynyl, heterocyclyl, heteroalkyl, heterocyclyl -
C iioalkyl,
heterocyclyl-C240alkenyl, heterocyclyl-C240alkynyl, aryl- Ci_i0alkyl (e.g.
monocyclic aryl-C2
-8-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
malkyl, substituted monocyclic aryl- Ci_malkyl, or bicycloaryl--Ci_malkyl),
aryl-C2_10alkenyl,
aryl-C2_ioalkynyl, aryl-heterocyclyl, hetaryl-Ci_i0alkyl, hetaryl-
C2_10alkenyl, hetaryl-C2-
ioalkynyl, hetaryl-C3_8cycloalkyl, hetaryl-heteroalkyl, or hetaryl-
heterocyclyl, wherein each
of said bicyclic aryl or heteroaryl moiety is unsubstituted, or wherein each
of bicyclic aryl,
heteroaryl moiety or monocyclic aryl moiety is substituted with one or more
independent
alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl,
arylalkyl, heteroaryl,
heteroarylalkyl, halo, -OH, -R31, -CF3, -0CF35 -0R315 -NR31R325 -NR34R355 -
C(0)R315 -
CO2R315 -C(=0)NR31R325 -C(=0)NR34R355 -NO2, -CN, -S(0)0_2R31, -SO2NR31R32, -
SO2NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R33, -NR31S(0)0_2R32,
-
C(=S)0R31, -C(=0)SR31, -NR31C(=NR32)NR33R32, -NR31C(=NR32)0R33, -
NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -0C(=0)SR31, -SC(=0)0R31, -
P(0)0R310R32, or-SC(=0)NR31R32, and wherein each of said alkyl, cycloalkyl,
heterocyclyl,
or heteroalkyl moiety is unsubstituted or is substituted with one or more
alkyl, heteroalkyl,
alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl,
heteroarylalkyl,
halo, -OH, -R31, -CF3, -0CF3, -0R31, -0-aryl, -NR31R32, -NR34R35 ,-C(0)R31, -
CO2R31, -
C(=0)NR34R35, or -C(=0)NR31R32;
R3 and R4 are independently hydrogen, halogen, -OH, -R31, -CF3, -0CF3, -0R31, -
NR31R32,
-NR34R35, -C(0)R31, -0O2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN, -
S(0)0_2R31,
-SO2NR31R32, -S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R33, -
NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -NR31C(=NR32)NR33R32, -
NR31C(=NR32)0R33,
-NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -0C(=0)SR31, -SC(=0)0R31, -
P(0)0R310R32, -SC(=0)NR31R32 , aryl, hetaryl, Ci_Ltalkyl, Ci_malkyl,
C3_8cycloalkyl, Ci-
ioalkyl-C3-8cycloalkyl, C3_8cycloalkyl -Ci_ioalkyl, C3_8cycloalkyl -
C2_10alkenyl, C3_
8eyeloalkyl- C2_10alkynyl, Ci_i0alkyl- C2_10alkenyl, Ci_ioalkyl- C2_10alkynyl,
Ci_i0alkylaryl, C1-
malkylhetaryl, Ci_loalkylheterocyclyl, C2_ioalkenyl, C2_ioalkynyl,
C2_10alkenyl -Ci_ioalkyl, C2_
10alkynyl -Ci_i0alkyl, C2_10alkenylaryl, C2_10alkenylhetaryl,
C2_10alkenylheteroalkyl, C2-
10alkenylheterocycicyl, C2-10alkenyl-C3-8cycloalkyl, C2_10alkynyl-
C3_8cycloalkyl, C2-
lOalkynylaryl, C2_10alkynylhetaryl, C2_10alkynylheteroalkyl,
C2_10alkynylheterocylyl, C2_
malkYnyl-C3-8cycloalkenyl, Ci_malkoxy Ci_malkyl, Ci_malkoxy-C2_10alkenyl,
Ci_loalkoxy-C2-
10alkynyl, heterocyclyl, heterocyclyl -Ci_i0alkyl, heterocyclyl-C2_10alkenyl,
heterocyclyl-C2_
loalkynY15 aryl- Ci_malkyl, aryl-C2_10alkenyl, aryl-C2_10alkynyl, aryl-
heterocyclyl, hetaryl-C1-
ioalkyl, hetaryl-C2-10alkenyl, hetaryl-C2_ioalkynyl, hetaryl-C3_8cycloalkyl,
heteroalkyl,
hetaryl-heteroalkyl, or hetaryl-heterocyclyl, wherein each of said aryl or
heteroaryl moiety is
unsubstituted or is substituted with one or more independent halo, -OH, -R31, -
CF3, -0CF3,
-9-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
-0R31, -NR31R32, -NR34R35, -C(0)R31, -0O2R31, -C(=0)NR31R32, -C(=0)NR34R35, -
NO2, -
CN, -S(0)0_2R31, -SO2NR31R32, -S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -
NR31C(=0)NR32R33, -NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -
NR31C(=NR32)NR33R32,
-NR31C(=NR32)0R33, -NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -
OC(=0)SR31, -SC(=0)0R31, -P(0)0R310R32, or-SC(=0)NR31R32, and wherein each of
said
alkyl, cycloalkyl, heterocyclyl, or heteroalkyl moiety is unsubstituted or is
substituted with
one or more halo, -OH, -R31, -CF3, -0CF3, -0R31, -0-aryl, -NR31R32, -NR34R35 ,-
C(0)R31,
-0O2R31, -C(=0)NR34R35, or -C(=0)NR31R32;
R5 is hydrogen, halogen, -OH, -R31, -CF3, -0CF3, -0R31, -NR31R32, -NR34R35, -
C(0)R31, -
CO2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN, -S(0)0_2R31, -SO2NR31R32, -
S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R33, -NR31S(0)0_2R32,
-
C(=S)0R31, -C(=0)SR31, -NR31C(=NR32)NR33R32, -NR31C(=NR32)0R33, -
NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -0C(=0)SR31, -SC(=0)0R31, -
P(0)0R310R32,or -SC(=0)NR3 'R32;
each of R31, R32, and R33 is independently H or Ci_malkyl , wherein the
Ci_i0alkyl is
unsubstituted or is substituted with one or more aryl, heteroalkyl,
heterocyclyl, or hetaryl
group, wherein each of said aryl, heteroalkyl, heterocyclyl, or hetaryl group
is unsubstituted
or is substituted with one or more halo, -OH, - CiioaIkyl, -CF3, -0-aryl, -
0CF3, -0Ci_
ioalkyl, -NH2, - N(Ci_i0alkyl)(Ci_10alkyl), - NH(Ci_i0alkyl), - NH( aryl), -
NR34R35, -
C(0)(Ci_malkyl), -C(0)(Ci_malkyl-ary1), -C(0)(ary1),
-0O2-aryl, -C(=0)N(Ci_malkyl)( Ci_ioalkyl), -C(=0)NH( -C(=0)NR34R35, -
C(=0)NH2, -0CF3, -0(Ci_malkyl), -0-aryl, -N(ary1)( -
NO2, -CN, -S(0)0_2 C1-
ioalkyl, -S(0)0_2 Ci_malkylaryl, -S(0)0_2 aryl, -SO2N(ary1), -SO2
N(Ci_malkyl)( Ci_ioalkyl), -
SO2 NH(Ci_malkyl) or -S02NR34R35;
R34 and R35 in -NR34R35, -C(=0)NR34R35, or -S02NR34R35, are taken together
with the
nitrogen atom to which they are attached to form a 3-10 membered saturated or
unsaturated
ring; wherein said ring is independently unsubstituted or is substituted by
one or more -
NR31R32, hydroxyl, halogen, oxo, aryl, hetaryl, Ci_6alkyl, or 0-aryl, and
wherein said 3-10
membered saturated or unsaturated ring independently contains 0, 1, or 2 more
heteroatoms
in addition to the nitrogen atom;
each of R7 and R8 is independently hydrogen, Ci_ioalkyl, C2_10alkenyl, aryl,
heteroaryl,
heterocyclyl or C340cycloalkyl, each of which except for hydrogen is
unsubstituted or is
substituted by one or more independent R6;
-10-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
R6 is halo, -0R315 -SH, -NH25 -NR34R35, - NR31R325 -0O2R315 -0O2aryl, -
C(=0)NR31R325
C(=0)NR34R35 5 -NO2, -CN, -S(0) 0_2 Ci_i0alkyl, -S(0) 0_2aryl, -S02NR34R355 -
S02NR31R325
Ci_malkyl, C2_10alkenyl, C2_10alkynyl; aryl-Ci_malkyl, aryl-C240alkenyl, aryl-
C2_10alkynyl,
hetaryl-Ci_i0alkyl, hetaryl-C2_10alkenyl, hetaryl-C2_10alkynyl, wherein each
of said alkyl,
alkenyl, alkynyl, aryl, heteroalkyl, heterocyclyl, or hetaryl group is
unsubstituted or is
substituted with one or more independent halo, cyano, nitro, -0C1_10alkyl,
Ci_malkyl, C2-
malkenyl, C2_10alkynyl, haloCi_malkyl, haloC2_10alkenyl, haloC2_10alkynyl, -
COOH, -
C(=0)NR31R325 -C(0)NR34R35 5 -S02NR34R355 -SO2 NR31R325 -NR31R325 or -NR34R35;
and
R9 is H5 halo, -0R315 -SH, -NH25 -NR34R35 5 - NR31R325 -0O2R315 -0O2aryl, -
C(=0)NR31R325 C(=0)NR34R35 5 -NO2, -CN, -S(0)0_2 Ci_i0alkyl, -5(0) 0_2aryl, -
S02NR34R355 -S02NR31R32, Ci_malkyl, C2_10alkenyl, C2_10alkynyl; aryl-
Ci_malkyl, aryl-C2-
ioalkenyl, aryl-C2_ioalkynyl, hetaryl-Ci_i0alkyl, hetaryl-C2_10alkenyl,
hetaryl-C2_10alkynyl,
wherein each of said alkyl, alkenyl, alkynyl, aryl, heteroalkyl, heterocyclyl,
or hetaryl group
is unsubstituted or is substituted with one or more independent halo, cyano,
nitro, -0Ci-
ioalkyl, Ci_ioalkyl, C2_10alkenyl, C2_10alkynyl, haloCi_malkyl,
haloC2_10alkenyl, haloC2-
ioalkynyl, -COOH, -C(=0)NR31R325 -C(=0)NR34R35 5 -S02NR34R355 -SO2 NR31R325 -
NR31R325 or -NR34R35. In other embodiments, the mTOR inhibitor is a compound
of
o-_1(NH2
NH2 * N
N ---- \ _
Pl
N N
formula:
[0023] In other embodiments, the P13-kinase a inhibitor is a compound of
formula:
R1'
4'
/W
W3 w6'
1 0 0
W2.,:........ ......."......,.. ...õ----................-----
-:::,.....................õ,........õ vv '
war W6
1 0> _______ R2'
wc ................
Wd'
wa'
Formula II
or its pharmaceutically acceptable salts thereof, wherein:
W1' is N5 NR3'5 or CR3'; W2' is N5 NR4', CR4', or C=0; W3' is N5 NR5' or CR5';
W4' is
N5 wherein no more than two N atoms and no more than two C=0 groups are
adjacent;
-11-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
W5' is N;
V' is N or CR8';
Wa' and Wb' are independently N or CR9';
one of Wc and Wi' is N, and the other is 0, NR10', or S;
R1' and R2' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
R3' and R4' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
or R3' and R4' taken together form a cyclic moiety;
R5', R6' , R7' and R8' are independently hydrogen, alkyl, heteroalkyl,
alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
R9' is alkyl or halo; and
R10' is hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl,
aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy, heterocycloalkyloxy,
amido, amino, acyl,
acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano, hydroxy, nitro, phosphate,
urea,
carbonate, or NR'R" wherein R' and R" are taken together with nitrogen to form
a cyclic
moiety.
[0024] In still other embodiments, the P13-kinase a inhibitor is a compound of
formula:
R1.
W3: 7/L 6'
W201 OW N- R2'
µ
Wl'VV! wg 41 I
X
or its pharmaceutically acceptable salts thereof, where:
Xis 0 or S or N;
-12-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Wr is S, N, NR3' or CR3', W2' is N or CR4', W3' is S, N or CR5', W4' is N or
C, and
W7 is N or C, wherein no more than two N atoms and no more than two C=0 groups
are
adjacent;
W5' is N or CR7;
W6 is N or CR8';
R1' and R2' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
R3' and R4' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
or R3' and R4' taken together form a cyclic moiety; and
R5', R7 and R8' are independently hydrogen, alkyl, heteroalkyl, alkenyl,
alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety.
[0025] For any of the methods of the invention, the P13-kinase a inhibitor
and/or the mTOR
inhibitor can be administered parenterally, orally, intraperitoneally,
intravenously,
intraarterially, transdermally, intramuscularly, liposomally, via local
delivery by catheter or
stent, subcutaneously, intraadiposally, or intrathecally. In some embodiments,
the P13-kinase
a inhibitor and/or the mTOR inhibitor are co-administered to the subject in
the same
formulation. In other embodiments, the P13-kinase a inhibitor and/or the mTOR
inhibitor are
co-administered to the subject in different formulations.
[0026] The invention also provides a pharmaceutical composition comprising a
combination
of an amount of P13-kinase a inhibitor and an amount of mTOR inhibitor,
wherein said
combination provides a synergistic therapeutic effect in a subject in need
thereof. For
example, the pharmaceutical composition is formulated in an oral dosage. In
some
embodiments, at least one of the amounts is administered as a sub-therapeutic
amount. In
other embodiments, the pharmaceutical composition is formulated a tablet or a
capsule. For
-13-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
example, the P13-kinase a inhibitor and the mTOR inhibitor are packaged as
separate tablets.
In other embodiments, the P13-kinase a inhibitor and the mTOR inhibitor are
formulated as a
single oral dosage form.
[0027] The invention also provides a pharmaceutical kit comprising (i) a
number of daily
dosage units placed in a packaging unit and intended for administration for a
period or a
multiple of a period of at least 1 day, wherein the daily dosage units each
comprise (a) a
therapeutically effective amount of a P13-kinase a inhibitor and/or (b) a
therapeutically
effective amount of an mTOR inhibitor; wherein the daily dosage units
comprising the PI3-
kinase a inhibitor and/or mTOR inhibitor are effective for treating a disease
condition
associated with P13-kinase a and/or mTOR in a subject, and (ii) a number of
daily dosage
units containing no active agent placed in a packaging unit and intended for
administration
for a period or a multiple of a period of at least 1 day. In some kits, the
number of daily
dosage units comprising the P13-kinase a inhibitor and/or mTOR inhibitor is 2,
3, 4, 5, 6 or 7,
or multiple of 2, 3, 4, 5, 6 or 7, and wherein the number of daily dosage
units containing no
active agent is at least 1. In some kits, the number of daily dosage units
comprising the PI3-
kinase a inhibitor and/or mTOR inhibitor is 2, 3, 4, 5, 6 or 7, or multiple of
2, 3, 4, 5, 6 or 7,
and wherein the number of daily dosage units containing no active agent is at
least 3, 4, or 5
or multiple of 3, 4, or 5. In some kits, the number of daily dosage units
comprising the PI3-
kinase a inhibitor and/or mTOR inhibitor is at least 1, and wherein the number
of daily
dosage units containing no active agent is 6 or multiple of 6. In some kits,
the number of
daily dosage units comprising the P13-kinase a inhibitor and/or mTOR inhibitor
is 3, or
multiple of 3, and wherein the number of daily dosage units containing no
active agent is 4 or
multiple of 4. In some kits, the number of daily dosage units comprising the
P13-kinase a
inhibitor and/or mTOR inhibitor is 5, or multiple of 5, and wherein the number
of daily
dosage units containing no active agent is 2 or multiple of 2. In some kits,
the number of
daily dosage units comprising the P13-kinase a inhibitor and/or mTOR inhibitor
is 1, or
multiple of 1, and wherein the number of daily dosage units containing no
active agent is 6 or
multiple of 6.
[0028] The invention further provides a pharmaceutical kit effective for
treating a disease
condition associated with P13-kinase a and/or mTOR in a subject comprising (i)
a number of
daily dosage units placed in a packaging unit and intended for administration
for a period or a
multiple of a period of at least 1 day, wherein the daily dosage units each
comprise a
combination of (a) a therapeutically effective amount of a P13-kinase a
inhibitor and (b) a
therapeutically effective amount of an mTOR inhibitor; and (ii) a number of
daily dosage
-14-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
units placed in a packaging unit and intended for administration for a period
or a multiple of a
period of at least 1 day, wherein the daily dosage units each comprise a
therapeutically
effective amount of a P13-kinase a inhibitor. In some kits, the number of
daily dosage units
comprising the combination is 2, 3, 4, 5, 6 or 7, or multiple of 2, 3, 4, 5, 6
or 7, and wherein
the number of daily dosage units comprising P13-kinase a inhibitor only is at
least 1. In some
kits, the number of daily dosage units comprising the combination is 2, 3, 4,
5, 6 or 7, or
multiple of 2, 3, 4, 5, 6 or 7, and wherein the number of daily dosage units
comprising PI3-
kinase a inhibitor only is at least 3, 4, or 5 or multiple of 3, 4, or 5. In
some kits, the number
of daily dosage units comprising the combination is at least 1, and wherein
the number of
daily dosage units comprising PI3-kinase a inhibitor only is 6 or multiple of
6. In some kits,
the number of daily dosage units comprising the combination is 3, or multiple
of 3, and
wherein the number of daily dosage units comprising P13-kinase a inhibitor
only is 4 or
multiple of 4. In some kits, the number of daily dosage units comprising the
combination is
5, or multiple of 5, and wherein the number of daily dosage units containing
no active agent
is 2 or multiple of 2. In some kits, the number of daily dosage units
comprising the
combination is 1, or multiple of 1, and wherein the number of daily dosage
units containing
no active agent is 6 or multiple of 6.
[0029] The invention further provides a pharmaceutical kit effective for
treating a disease
condition associated with P13-kinase a and/or mTOR in a subject comprising (i)
a number of
daily dosage units placed in a packaging unit and intended for administration
for a period or a
multiple of a period of at least 1 day, wherein the daily dosage units each
comprise a
combination of (a) a therapeutically effective amount of a P13-kinase a
inhibitor and (b) a
therapeutically effective amount of an mTOR inhibitor; and (ii) a number of
daily dosage
units placed in a packaging unit and intended for administration for a period
or a multiple of a
period of at least 1 day, wherein the daily dosage units each comprise a
therapeutically
effective amount of an mTOR inhibitor.
[0030] The invention also provides a method comprising: (a) determining the
presence in a
subject of a mutation in P13-kinase a that is associated with a disease
condition mediated by
PI3-kinase a; and (b) administering to said subject a pharmaceutical
composition of the
invention. For example, the mutation is in the nucleotide sequence coding for
P13-kinase a.
Exemplary mutations can include without limitation, deletion, insertion,
translation, which
can result in point mutations, frame shifts, and/or translation of the nucleic
acid sequence
coding for P13-kinase a. In another example, the mutation is in the amino acid
sequence of
P13-kinase a.
-15-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[0031] In some embodiments of any of the methods of the invention, the subject
or cell
comprises a mutation in P13-kinase a which is associated with a disease
condition mediated
by P13-kinase a.
INCORPORATION BY REFERENCE
[0032] All publications, patents, and patent applications mentioned in this
specification are
herein incorporated by reference to the same extent as if each individual
publication, patent,
or patent application was specifically and individually indicated to be
incorporated by
reference.
BRIEF DESCRIPTION OF THE DRAWINGS
[0033] The novel features of the invention are set forth with particularity in
the appended
claims. A better understanding of the features and advantages of the present
invention will be
obtained by reference to the following detailed description that sets forth
illustrative
embodiments, in which the principles of the invention are utilized, and the
accompanying
drawings of which:
[0034] Figure 1 is a schematic illustration of multiple and distinct signaling
pathways that
are activated in human cancer.
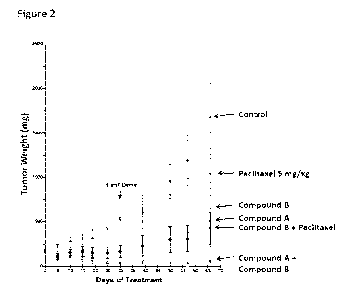
[0035] Figure 2 is a graph showing the synergistic effect of combined
treatment with a PI3-
kinase a inhibitor (Compound A) and an mTor inhibitor (Compound B) on tumor
weight in a
preclinical breast cancer model.
[0036] Figure 3 is a western blot depicting the synergistic effect of combined
treatment with
Compound A and Compound B in terms of downregulating Akt and S6
phosphorylation.
[0037] Figure 4 is a graph showing the synergistic effect of combined
treatment with
Compound A and rapamycin in terms of reducing tumor volume of a preclinical
breast cancer
model.
[0038] Figure 5 shows A) an illustration of the distinct signaling pathways
mediated by
mTORC1 and mTORC2 and B) a western blot depicting sensitivity of mTORC1-
dependent
NRDG1 phosphorylation to Compound B, but not rapamycin.
[0039] Figure 6 shows A) a graph depicting the selectivity of Compound B over
Compound
A in a PTEN-mutant negative control tumor model, B) a western blot depicting
selective
inhibition of Akt, S6, and 4EBP phosphorylation by Compound B and not Compound
A in
PTEN mutant cells, and C) a chart depicting the specificity of Compound A in
inhibiting
-16-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
PI3K a over mTOR and other PI3K isoforms, and the specificity of Compound B in
inhibiting mTOR over PI3K isoforms.
[0040] Figure 7 is a western blot depicting differential inhibition of Akt
phosphorylation at
serine 473 over threonine 308 by Compound B (top panel). Also shown is the
comparison of
Akt phosphorylation inhibition for Pan-PI3K inhibitor versus Compound B.
[0041] Figure 8 is a graph showing that Pan-PI3K inhibitor, but not Compound
A, blocks B
cell function in vivo. Mice were immunized with TNP-Ficoll and treated with 1)
vehicle; 2)
70 mg/kg GDC0941; 3) 30 mg/kg Compound A; 4) 60mg/kg Compound A; or 5)
120mg/kg
Compound A for 7 days. Antibody production was measured as a percentage of
control
group that were treated with vehicle.
[0042] Figure 9 shows, left panel, a graph showing reduction in tumor weight
of a breast
cancer model using 70 mg/kg Pan-PI3K inhibitor and 60 mg/kg compound A and,
right
panel, reduced presence of MZB cells in mouse spleen for 70 mg/kg Pan-PI3K
inhibitor
compared to 60 mg/kg Compound A.
[0043] Figure 10 illustrates the frequency of PI3K a mutation in various human
cancers.
[0044] Figure 11 is a western blot depicting inhibition of the PI3K pathway by
Compound A
in cell lines with elevated PI3K a activity. The left column shows data from
MDA-MB-361
breast cancer cells harboring PIK3CA mutation. The middle column shows data
from MDA-
MB-453 breast cancer cells harboring PIK3CA mutation. The right column shows
data from
SKBr3breast cancer cells harboring HER2 mutation.
[0045] Figure 12 shows A) a western blot showing inhibition of Akt
phosphorylation at
serine 473 by Compound A; and B) reduced inhibition of Akt phosphorylation at
serine 473
by Compound A in a PTEN-mutant cell line.
[0046] Figure 13 is a chart showing that Compound A preferentially inhibits
proliferation of
tumor cells harboring PI3K a mutations.
[0047] Figure 14 is an isobologram depicting the additive or synergistic
anticancer activity
achieved by a combination of Compound A and Compound B. The in vitro
combination
analysis demonstrated the additive or synergistic effects of the combination
across tumor
types or genetic types.
[0048] Figure 15 is a western blot depicting induction of cell apoptosis by
Compound A,
Compound B, or combination thereof in breast cell cancer cells. Cleaved PARP
is a
biomarker for apoptosis. The result show that a greater degree of apoptosis
and pathway
regulation (TORC1 and 2 substrates) can be induced by a combination of
compound A and
compound B as compared to single agents. The left column shows data from MDA-
MB-361
-17-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
breast cancer cells harboring PIK3CA and HER2 mutations. The right column
shows data
from HCC-1419 breast cancer cells harboring HER2 mutation.
[0049] Figure 16 shows that an intermittent dosing regimen of the combination
of
Compound A and Compound B leads to tumor growth inhibition similar to that
observed on
the QD schedule in vivo.
[0050] Figure 17 shows that the combination of Compound A and Compound B leads
to
increased durability of tumor control in vivo, and sustained tumor regression
whereas the
single agent control arms demonstrate re-growth.
[0051] Figure 18 shows that the combination of Compound A and Compound B leads
to
increased tumor growth inhibition in a PTEN null model, suggesting utility of
this
combination in diverse genotypes.
[0052] Figure 19 shows that the combination of Compound A and Compound B
demonstrates activity in a KRAS / PIK3CA colon model, whereas either single
agent is not
effective.
DETAILED DESCRIPTION OF THE INVENTION
[0053] Several aspects of the invention are described below with reference to
example
applications for illustration. It should be understood that numerous specific
details,
relationships, and methods are set forth to provide a full understanding of
the invention. One
having ordinary skill in the relevant art, however, will readily recognize
that the invention
can be practiced without one or more of the specific details or with other
methods. The
present invention is not limited by the illustrated ordering of acts or
events, as some acts may
occur in different orders and/or concurrently with other acts or events.
Furthermore, not all
illustrated acts or events are required to implement a methodology in
accordance with the
present invention.
[0054] The terminology used herein is for the purpose of describing particular
embodiments
only and is not intended to be limiting of the invention. As used herein, the
singular forms
"a", "an" and "the" are intended to include the plural forms as well, unless
the context clearly
indicates otherwise. Furthermore, to the extent that the terms "including",
"includes",
"having", "has", "with", or variants thereof are used in either the detailed
description and/or
the claims, such terms are intended to be inclusive in a manner similar to the
term
"comprising".
[0055] [0001] The term "about" or "approximately" means within an acceptable
error range
for the particular value as determined by one of ordinary skill in the art,
which will depend in
-18-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
part on how the value is measured or determined, i.e., the limitations of the
measurement
system. For example, "about" can mean within 1 or more than 1 standard
deviation, per the
practice in the art. Alternatively, "about" can mean a range of up to 20%,
preferably up to
10%, more preferably up to 5%, and more preferably still up to 1% of a given
value.
Alternatively, particularly with respect to biological systems or processes,
the term can mean
within an order of magnitude, preferably within 5-fold, and more preferably
within 2-fold, of
a value. Where particular values are described in the application and claims,
unless otherwise
stated the term "about" meaning within an acceptable error range for the
particular value
should be assumed.
[0056] "Treatment", "treating", "palliating" and "ameliorating", as used
herein, are used
interchangeably. These terms refer to an approach for obtaining beneficial or
desired results
including but not limited to therapeutic benefit and/or a prophylactic
benefit. By therapeutic
benefit is meant eradication or amelioration of the underlying disorder being
treated. Also, a
therapeutic benefit is achieved with the eradication or amelioration of one or
more of the
physiological symptoms associated with the underlying disorder such that an
improvement is
observed in the patient, notwithstanding that the patient may still be
afflicted with the
underlying disorder. For prophylactic benefit, the compositions may be
administered to a
patient at risk of developing a particular disease, or to a patient reporting
one or more of the
physiological symptoms of a disease, even though a diagnosis of this disease
may not have
been made.
[0057] As used herein, the term "neoplastic condition" refers to the presence
of cells
possessing abnormal growth characteristics, such as uncontrolled
proliferation, immortality,
metastatic potential, rapid growth and proliferation rate, perturbed oncogenic
signaling, and
certain characteristic morphological features. This includes the abnormal
growth of: (1)
tumor cells (tumors) that proliferate by expressing a mutated tyrosine kinase
or
overexpression of a receptor tyrosine kinase; (2) benign and malignant cells
of other
proliferative diseases in which aberrant tyrosine kinase activation occurs;
(3) any tumors that
proliferate by receptor tyrosine kinases; (4) any tumors that proliferate by
aberrant
serine/threonine kinase activation; and (5) benign and malignant cells of
other proliferative
diseases in which aberrant serine/threonine kinase activation occurs.
[0058] The term "effective amount" or "therapeutically effective amount"
refers to that
amount of an inhibitor described herein that is sufficient to effect the
intended application
including but not limited to disease treatment, as defined below. The
therapeutically
effective amount may vary depending upon the intended application (in vitro or
in vivo), or
-19-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
the subject and disease condition being treated, e.g., the weight and age of
the subject, the
severity of the disease condition, the manner of administration and the like,
which can readily
be determined by one of ordinary skill in the art. The term also applies to a
dose that will
induce a particular response in target cells, e.g., reduction of proliferation
or downregulation
of activity of a target protein. The specific dose will vary depending on the
particular
compounds chosen, the dosing regimen to be followed, whether it is
administered in
combination with other compounds, timing of administration, the tissue to
which it is
administered, and the physical delivery system in which it is carried.
[0059] A "sub-therapeutic amount" of an agent or therapy is an amount less
than the effective
amount for that agent or therapy, but when combined with an effective or sub-
therapeutic
amount of another agent or therapy can produce a result desired by the
physician, due to, for
example, synergy in the resulting efficacious effects, or reduced side
effects.
[0060] A "synergistically effective therapeutic amount" of an agent or therapy
is an amount
which, when combined with an effective or sub-therapeutic amount of another
agent or
therapy, produces a greater effect than when either of the two agents are
therapies are used
alone. In some embodiments, a synergistically effective therapeutic amount of
an agent or
therapy produces a greater effect when used in combination than the additive
effects of each
of the two agents or therapies when used alone.
[0061] As used herein, "agent" or "biologically active agent" refers to a
biological,
pharmaceutical, or chemical compound or other moiety. Non-limiting examples
include
simple or complex organic or inorganic molecule, a peptide, a protein, an
oligonucleotide, an
antibody, an antibody derivative, antibody fragment, a vitamin derivative, a
carbohydrate, a
toxin, or a chemotherapeutic compound. Various compounds can be synthesized,
for
example, small molecules and oligomers (e.g., oligopeptides and
oligonucleotides), and
synthetic organic compounds based on various core structures. In addition,
various natural
sources can provide compounds for screening, such as plant or animal extracts,
and the like.
A skilled artisan can readily recognize that there is no limit as to the
structural nature of the
agents of the present invention.
[0062] The term "agonist" as used herein refers to a compound having the
ability to initiate
or enhance a biological function of a target protein, whether by inhibiting
the activity or
expression of the target protein. Accordingly, the term "agonist" is defined
in the context of
the biological role of the target polypeptide. While preferred agonists herein
specifically
interact with (e.g., bind to) the target, compounds that initiate or enhance a
biological activity
of the target polypeptide by interacting with other members of the signal
transduction
-20-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
pathway of which the target polypeptide is a member are also specifically
included within
this definition.
[0063] The terms "antagonist" and "inhibitor" are used interchangeably, and
they refer to a
compound having the ability to inhibit a biological function of a target
protein, whether by
inhibiting the activity or expression of the target protein. Accordingly, the
terms "antagonist"
and "inhibitors" are defined in the context of the biological role of the
target protein. While
preferred antagonists herein specifically interact with (e.g., bind to) the
target, compounds
that inhibit a biological activity of the target protein by interacting with
other members of the
signal transduction pathway of which the target protein is a member are also
specifically
included within this definition. A preferred biological activity inhibited by
an antagonist is
associated with the development, growth, or spread of a tumor, or an undesired
immune
response as manifested in autoimmune disease.
[0064] The phrase "mTOR inhibitor that binds to and directly inhibits both
mTORC1 and
mTORC2 kinases" when used: herein refers to an mTOR inhibitor that interacts
with and
reduces the kinase activity of both mTORC1 and mTORC2 complexes.
[0065] An "anti-cancer agent", "anti-tumor agent" or "chemotherapeutic agent"
refers to any
agent useful in the treatment of a neoplastic condition. One class of anti-
cancer agents
comprises chemotherapeutic agents. "Chemotherapy" means the administration of
one or
more chemotherapeutic drugs and/or other agents to a cancer patient by various
methods,
including intravenous, oral, intramuscular, intraperitoneal, intravesical,
subcutaneous,
transdermal, buccal, or inhalation or in the form of a suppository.
[0066] The term "cell proliferation" refers to a phenomenon by which the cell
number has
changed as a result of division. This term also encompasses cell growth by
which the cell
morphology has changed (e.g., increased in size) consistent with a
proliferative signal.
[0067] The terms "co-administration," "administered in combination with," and
their
grammatical equivalents, encompass administration of two or more agents to an
animal so
that both agents and/or their metabolites are present in the animal at the
same time. Co-
administration includes simultaneous administration in separate compositions,
administration
at different times in separate compositions, or administration in a
composition in which both
agents are present. Co-administered agents may be in the same formulation. Co-
administered agents may also be in different formulations.
[0068] A "therapeutic effect," as used herein, encompasses a therapeutic
benefit and/or a
prophylactic benefit as described above. A prophylactic effect includes
delaying or
eliminating the appearance of a disease or condition, delaying or eliminating
the onset of
-21-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
symptoms of a disease or condition, slowing, halting, or reversing the
progression of a
disease or condition, or any combination thereof.
[0069] The term "pharmaceutically acceptable salt" refers to salts derived
from a variety of
organic and inorganic counter ions well known in the art. Pharmaceutically
acceptable acid
addition salts can be formed with inorganic acids and organic acids. Inorganic
acids from
which salts can be derived include, for example, hydrochloric acid,
hydrobromic acid,
sulfuric acid, nitric acid, phosphoric acid, and the like. Organic acids from
which salts can be
derived include, for example, acetic acid, propionic acid, glycolic acid,
pyruvic acid, oxalic
acid, maleic acid, malonic acid, succinic acid, fumaric acid, tartaric acid,
citric acid, benzoic
acid, cinnamic acid, mandelic acid, methanesulfonic acid, ethanesulfonic acid,
p-toluenesulfonic acid, salicylic acid, and the like. Pharmaceutically
acceptable base addition
salts can be formed with inorganic and organic bases. Inorganic bases from
which salts can
be derived include, for example, sodium, potassium, lithium, ammonium,
calcium,
magnesium, iron, zinc, copper, manganese, aluminum, and the like. Organic
bases from
which salts can be derived include, for example, primary, secondary, and
tertiary amines,
substituted amines including naturally occurring substituted amines, cyclic
amines, basic ion
exchange resins, and the like, specifically such as isopropylamine,
trimethylamine,
diethylamine, triethylamine, tripropylamine, and ethanolamine. In some
embodiments, the
pharmaceutically acceptable base addition salt is chosen from ammonium,
potassium,
sodium, calcium, and magnesium salts.
[0070] "Pharmaceutically acceptable carrier" or "pharmaceutically acceptable
excipient"
includes any and all solvents, dispersion media, coatings, antibacterial and
antifungal agents,
isotonic and absorption delaying agents and the like. The use of such media
and agents for
pharmaceutically active substances is well known in the art. Except insofar as
any
conventional media or agent is incompatible with the active ingredient, its
use in the
therapeutic compositions of the invention is contemplated. Supplementary
active ingredients
can also be incorporated into the compositions.
[0071] "Signal transduction" is a process during which stimulatory or
inhibitory signals are
transmitted into and within a cell to elicit an intracellular response. A
modulator of a signal
transduction pathway refers to a compound which modulates the activity of one
or more
cellular proteins mapped to the same specific signal transduction pathway. A
modulator may
augment (agonist) or suppress (antagonist) the activity of a signaling
molecule.
-22-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[0072] The term "selective inhibition" or "selectively inhibit" as applied to
a biologically
active agent refers to the agent's ability to selectively reduce the target
signaling activity as
compared to off-target signaling activity, via direct or interact interaction
with the target.
[0073] "Subject" refers to an animal, such as a mammal, for example a human.
The methods
described herein can be useful in both human therapeutics, pre-clinical, and
veterinary
applications. In some embodiments, the subject is a mammal, and in some
embodiments, the
subject is human.
[0074] The term "in vivo" refers to an event that takes place in a subject's
body.
[0075] The term "in vitro" refers to an event that takes places outside of a
subject's body.
For example, an in vitro assay encompasses any assay run outside of a subject
assay. In vitro
assays encompass cell-based assays in which cells alive or dead are employed.
In vitro
assays also encompass a cell-free assay in which no intact cells are employed.
[0076] When ranges are used herein for physical properties, such as molecular
weight, or
chemical properties, such as chemical formulae, all combinations and
subcombinations of
ranges and specific embodiments therein are intended to be included. The term
"about" when
referring to a number or a numerical range means that the number or numerical
range referred
to is an approximation within experimental variability (or within statistical
experimental
error), and thus the number or numerical range may vary from, for example,
between 1% and
15% of the stated number or numerical range. The term "comprising" (and
related terms such
as "comprise" or "comprises" or "having" or "including") includes those
embodiments, for
example, an embodiment of any composition of matter, composition, method, or
process, or
the like, that "consist of' or "consist essentially of' the described
features.
[0077] The following abbreviations and terms have the indicated meanings
throughout: PI3K
= Phosphoinositide-3-kinase; PI = phosphatidylinositol.
[0078] Unless otherwise stated, the connections of compound name moieties are
at the
rightmost recited moiety. That is, the substituent name starts with a terminal
moiety,
continues with any linking moieties, and ends with the linking moiety. For
example,
heteroarylthio C1_4 alkyl has a heteroaryl group connected through a thio
sulfur to a C1_4 alkyl
radical that connects to the chemical species bearing the substituent. This
condition does not
apply where a formula such as, for example "-L-C1_10 alkyl ¨ C3_8cycloalkyl"
is represented.
In such case, the terminal group is a C3_8cycloalkyl group attached to a
linking C1_10 alkyl
moiety which is attached to an element L, which is itself connected to the
chemical species
bearing the substituent.
-23-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[0079] "Alkyl" refers to a straight or branched hydrocarbon chain radical
consisting solely of
carbon and hydrogen atoms, containing no unsaturation, having from one to ten
carbon atoms
(e.g., C1-C10 alkyl). Whenever it appears herein, a numerical range such as "1
to 10" refers to
each integer in the given range; e.g., "1 to 10 carbon atoms" means that the
alkyl group may
consist of 1 carbon atom, 2 carbon atoms, 3 carbon atoms, etc., up to and
including 10 carbon
atoms, although the present definition also covers the occurrence of the term
"alkyl" where no
numerical range is designated. In some embodiments, it is a Ci-C4 alkyl group.
Typical alkyl
groups include, but are in no way limited to, methyl, ethyl, propyl,
isopropyl, n-butyl, iso-
butyl, sec-butyl isobutyl, tertiary butyl, pentyl, isopentyl, neopentyl,
hexyl, septyl, octyl,
nonyl, decyl, and the like. The alkyl is attached to the rest of the molecule
by a single bond,
for example, methyl (Me), ethyl (Et), n-propyl, 1-methylethyl (iso-propyl), n-
butyl, n-pentyl,
1,1-dimethylethyl (t-butyl), 3-methylhexyl, 2-methylhexyl, and the like.
Unless stated
otherwise specifically in the specification, an alkyl group is optionally
substituted by one or
more of substituents which independently are: alkyl, heteroalkyl, alkenyl,
alkynyl, cycloalkyl,
heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, hydroxy, halo,
cyano,
trifluoromethyl, trifluoromethoxy, nitro, trimethylsilanyl, -0Ra, -
SRa, -0C(0)-Ra, -N(Ra)2, -C(0)Ra, -C(0)0Ra, -0C(0)N(Ra)2, -C(0)N(Ra)2, -
N(Ra)C(0)
ORa, -N(Ra)C(0)Ra, -N(Ra)C(0)N(Ra)2, -N(Ra)C(NRa)N(Ra)2, -N(Ra)S(0)tRa (where
t is 1 or
2), -S(0)tORa (where t is 1 or 2), -S(0)tN(Ra)2 (where t is 1 or 2), or
P03(102 where each Ra
is independently hydrogen, alkyl, fluoroalkyl, carbocyclyl, carbocyclylalkyl,
aryl, aralkyl,
heterocycloalkyl, heterocycloalkylalkyl, heteroaryl or heteroarylalkyl.
[0080] The term "halo" or "halogen" refers to fluoro, chloro, bromo, or iodo.
[0081] The term "haloalkyl" refers to an alkyl group substituted with one or
more halo
groups, for example chloromethyl, 2-bromoethyl, 3-iodopropyl, trifluoromethyl,
perfluoropropyl, 8-chlorononyl, and the like.
[0082] "Acyl" refers to the groups (alkyl)-C(0)-, (aryl)-C(0)-, (heteroaryl)-
C(0)-,
(heteroalkyl)-C(0)-, and (heterocycloalkyl)-C(0)-, wherein the group is
attached to the
parent structure through the carbonyl functionality. In some embodiments, it
is a C1-C10 acyl
radical which refers to the total number of chain or ring atoms of the alkyl,
aryl, heteroaryl or
heterocycloalkyl portion of the acyloxy group plus the carbonyl carbon of
acyl, i.e. three
other ring or chain atoms plus carbonyl. If the R radical is heteroaryl or
heterocycloalkyl, the
hetero ring or chain atoms contribute to the total number of chain or ring
atoms. Unless
stated otherwise specifically in the specification, the "R" of an acyloxy
group is optionally
substituted by one or more substituents which independently are: alkyl,
heteroalkyl, alkenyl,
-24-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl,
heteroarylalkyl, hydroxy,
halo, cyano, trifluoromethyl, trifluoromethoxy, nitro, trimethylsilanyl, -0Ra,
SRa, -0C(0)-Ra, -N(Ra)2, -C(0)Ra, -C(0)0Ra, -0C(0)N(Ra)2, -C(0)N(Ra)2, -
N(Ra)C(0)0Ra, -N(Ra)C(0)Ra, - N(Ra)C(0)N(Ra)2, N(Ra)C(NRa)N(Ra)2, -N(Ra)S(0)Ra
(where t is 1 or 2), -S(0)tORa (where t is 1 or 2), -S(0)N(Ra)2 (where t is 1
or 2), or P03(Ra)2,
where each Ra is independently hydrogen, alkyl, fluoroalkyl, carbocyclyl,
carbocyclylalkyl,
aryl, aralkyl, heterocycloalkyl, heterocycloalkylalkyl, heteroaryl or
heteroarylalkyl.
[0083] "Cycloalkyl" refers to a monocyclic or polycyclic radical that contains
only carbon
and hydrogen, and may be saturated, or partially unsaturated. Cycloalkyl
groups include
groups having from 3 to 10 ring atoms (i.e., C2-C10 cycloalkyl). Whenever it
appears herein, a
numerical range such as "3 to 10" refers to each integer in the given range;
e.g., "3 to 10
carbon atoms" means that the cycloalkyl group may consist of 3 carbon atoms,
etc., up to and
including 10 carbon atoms. In some embodiments, it is a C3-C8 cycloalkyl
radical. In some
embodiments, it is a C3-05 cycloalkyl radical. Illustrative examples of
cycloalkyl groups
include, but are not limited to the following moieties: cyclopropyl,
cyclobutyl, cyclopentyl,
cyclopentenyl, cyclohexyl, cyclohexenyl, cycloseptyl, cyclooctyl, cyclononyl,
cyclodecyl,
norbornyl, and the like. Unless stated otherwise specifically in the
specification, a cycloalkyl
group is optionally substituted by one or more substituents which
independently are: alkyl,
heteroalkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl,
heteroaryl,
heteroarylalkyl, hydroxy, halo, cyano, trifluoromethyl, trifluoromethoxy,
nitro,
trimethylsilanyl, -0Ra, -
SRa, -0C(0)-Ra, -N(Ra)2, -C(0)Ra, -C(0)0Ra, -0C(0)N(Ra)2, -C(0)N(Ra)2, -
N(Ra)C(0)0Ra
, -N(Ra)C(0)Ra, - N(Ra)C(0)N(Ra)2, N(Ra)C(NRa)N(Ra)2, _N(Ra)S(0)Ra (where t is
1 or
2), -S(0)tORa (where t is 1 or 2), -S(0)N(Ra)2 (where t is 1 or 2), or
P03(Ra)2, where each Ra
is independently hydrogen, alkyl, fluoroalkyl, carbocyclyl, carbocyclylalkyl,
aryl, aralkyl,
heterocycloalkyl, heterocycloalkylalkyl, heteroaryl or heteroarylalkyl.
[0084] The term "Ci-ioalkyl ¨ C3-8cycloalkyl" is used to describe an alkyl
group, branched or
straight chain and containing 1 to 10 carbon atoms, attached to a linking
cycloalkyl group
which contains 3 to 8 carbons, such as for example, 2-methyl cyclopropyl, and
the like.
Either portion of the moiety is unsubstituted or substituted.
[0085] The term "bicycloalkyl" refers to a structure consisting of two
cycloalkyl moieties,
unsubstituted or substituted, that have two or more atoms in common. If the
cycloalkyl
moieties have exactly two atoms in common they are said to be "fused".
Examples include,
but are not limited to, bicyclo[3.1.0]hexyl, perhydronaphthyl, and the like.
If the cycloalkyl
-25-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
moieties have more than two atoms in common they are said to be "bridged".
Examples
include, but are not limited to, bicyclo[2.2.1]heptyl ("norbornyl"),
bicyclo[2.2.2]octyl, and
the like.
[0086] As used herein, the term "heteroatom" or "ring heteroatom" is meant to
include
oxygen (0), nitrogen (N), sulfur (S), phosphorus (P), and silicon (Si).
[0087] "Heteroalkyl", "heteroalkenyl" and "heteroalkynyl" include optionally
substituted
alkyl, alkenyl and alkynyl radicals and which have one or more skeletal chain
atoms selected
from an atom other than carbon, e.g., oxygen, nitrogen, sulfur, phosphorus or
combinations
thereof. A numerical range may be given, e.g., C1-C4 heteroalkyl which refers
to the chain
length in total, which in this example is 4 atoms long. For example, a
¨CH2OCH2CH3 radical
is referred to as a "C4" heteroalkyl, which includes the heteroatom center in
the atom chain
length description. Connection to the rest of the molecule may be through
either a heteroatom
or a carbon in the heteroalkyl chain. A heteroalkyl group may be substituted
with one or more
substituents which independently are: alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl,
heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, hydroxy, halo,
cyano, nitro, oxo,
thioxo, trimethylsilanyl, -0Ra, -
SW, -0C(0)-Ra, -N(W)2, -C(0)Ra, -C(0)0W, -C(0)N(Ra)2, -N(W)C(0)0Ra, -
N(W)C(0)Ra,
-N(Ra)S(0)tRa (where t is 1 or 2), -S(0)t0Ra (where t is 1 or 2), -S(0)N(W)2
(where t is 1 or
2), or P03(W)2, where each Ra is independently hydrogen, alkyl, fluoroalkyl,
carbocyclyl,
carbocyclylalkyl, aryl, aralkyl, heterocycloalkyl, heterocycloalkylalkyl,
heteroaryl or
heteroarylalkyl.
[0088] The term "heteroalkylaryl" refers to a heteroalkyl group as defined
above which is
attached to an aryl group, and may be attached at a terminal point or through
a branched
portion of the heteroalkyl, for example, an benzyloxymethyl moiety. Either
portion of the
moiety is unsubstituted or substituted.
[0089] The term "heteroalkylheteroaryl" refers likewise to a heteroalkyl group
which is
attached to a heteroaryl moiety, for example, an ethoxymethylpyridyl group.
Either portion
of the moiety is unsubstituted or substituted.
[0090] The term "heteroalkyl-heterocycly1" refers to a heteroalkyl group as
defined above,
which is attached to a heterocyclic group, for example, 4(3-aminopropy1)-N-
piperazinyl.
Either portion of the moiety is unsubstituted or substituted.
[0091] The term "heteroalkyl-C3_8cycloalkyl" refers to a heteroalkyl group as
defined above,
which is attached to a cyclic alkyl containing 3 to 8 carbons, for example, 1-
aminobuty1-4-
cyclohexyl. Either portion of the moiety is unsubstituted or substituted.
-26-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[0092] The term "heterobicycloalkyl" refers to a bicycloalkyl structure, which
is
unsubstituted or substituted, in which at least one carbon atom is replaced
with a heteroatom
independently selected from oxygen, nitrogen, and sulfur.
[0093] The term "heterospiroalkyl" refers to a spiroalkyl structure, which is
unsubstituted or
substituted, in which at least one carbon atom is replaced with a heteroatom
independently
selected from oxygen, nitrogen, and sulfur.
[0094] An "alkene" moiety refers to a group consisting of at least two carbon
atoms and at
least one carbon-carbon double bond, and an "alkyne" moiety refers to a group
consisting of
at least two carbon atoms and at least one carbon-carbon triple bond. The
alkyl moiety,
whether saturated or unsaturated, may be branched, straight chain, or cyclic.
[0095] "Alkenyl" refers to a straight or branched hydrocarbon chain radical
group consisting
solely of carbon and hydrogen atoms, containing at least one double bond, and
having from
two to ten carbon atoms (i.e., C2-C10 alkenyl). Whenever it appears herein, a
numerical range
such as "2 to 10" refers to each integer in the given range; e.g., "2 to 10
carbon atoms" means
that the alkenyl group may consist of 2 carbon atoms, 3 carbon atoms, etc., up
to and
including 10 carbon atoms. In certain embodiments, an alkenyl comprises two to
eight carbon
atoms. In other embodiments, an alkenyl comprises two to five carbon atoms
(e.g., C2-05
alkenyl). The alkenyl is attached to the rest of the molecule by a single
bond, for example,
ethenyl (i.e., vinyl), prop-l-enyl (i.e., allyl), but-l-enyl, pent-l-enyl,
penta-1,4-dienyl, and the
like. Unless stated otherwise specifically in the specification, an alkenyl
group is optionally
substituted by one or more substituents which independently are: alkyl,
heteroalkyl, alkenyl,
alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl,
heteroarylalkyl, hydroxy,
halo, cyano, trifluoromethyl, trifluoromethoxy, nitro, trimethylsilanyl, -OW, -
SRa, -0C(0)-Ra, -N(W)2, -C(0)Ra, -C(0)0W, -0C(0)N(W)2, -C(0)N(Ra)2, -
N(W)C(0)0Ra
, -N(Ra)C(0)Ra,
- N(W)C(0)N(W)2, N(Ra)C(NRa)N(W)2, -N(Ra)S(0)tRa (where t is 1 or 2), -
S(0)tORa
(where t is 1 or 2), -S(0)N(W)2 (where t is 1 or 2), or P03(W)2, where each Ra
is
independently hydrogen, alkyl, fluoroalkyl, carbocyclyl, carbocyclylalkyl,
aryl, aralkyl,
heterocycloalkyl, heterocycloalkylalkyl, heteroaryl or heteroarylalkyl.
[0096] The term "C2_10 alkenyl-heteroalkyl" refers to a group having an
alkenyl moiety,
containing 2 to 10 carbon atoms and is branched or straight chain, which is
attached to a
linking heteroalkyl group, such as, for example, allyloxy, and the like.
Either portion of the
moiety is unsubstituted or substituted.
-27-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[0097] The term "C2_10 alkynyl-heteroalkyl" refers to a group having an
alkynyl moiety,
which is unsubstituted or substituted, containing 2 to 10 carbon atoms and is
branched or
straight chain, which is attached to a linking heteroalkyl group, such as, for
example, 4-but-l-
ynoxy, and the like. Either portion of the moiety is unsubstituted or
substituted.
[0098] The term "haloalkenyl" refers to an alkenyl group substituted with one
or more halo
groups.
[0099] Unless otherwise specified, the term "cycloalkenyl" refers to a cyclic
aliphatic 3 to
8 membered ring structure, optionally substituted with alkyl, hydroxy and
halo, having 1 or 2
ethylenic bonds such as methylcyclopropenyl, trifluoromethylcyclopropenyl,
cyclopentenyl,
cyclohexenyl, 1,4-cyclohexadienyl, and the like.
[00100] "Alkynyl" refers to a straight or branched hydrocarbon chain radical
group
consisting solely of carbon and hydrogen atoms, containing at least one triple
bond, having
from two to ten carbon atoms (i.e., C2-C10 alkynyl). Whenever it appears
herein, a numerical
range such as "2 to 10" refers to each integer in the given range; e.g., "2 to
10 carbon atoms"
means that the alkynyl group may consist of 2 carbon atoms, 3 carbon atoms,
etc., up to and
including 10 carbon atoms. In certain embodiments, an alkynyl comprises two to
eight
carbon atoms. In other embodiments, an alkynyl has two to five carbon atoms
(e.g., C2-05
alkynyl). The alkynyl is attached to the rest of the molecule by a single
bond, for example,
ethynyl, propynyl, butynyl, pentynyl, hexynyl, and the like. Unless stated
otherwise
specifically in the specification, an alkynyl group is optionally substituted
by one or more
substituents which independently are: alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl,
heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, hydroxy, halo,
cyano,
trifluoromethyl, trifluoromethoxy, nitro, trimethylsilanyl, -OW,
SW, -0C(0)-Ra, -N(W)2, -C(0)Ra, -C(0)0W, -0C(0)N(W)2, -C(0)N(Ra)2, -
N(W)C(0)0Ra
, -N(Ra)C(0)Ra, - N(W)C(0)N(Ra)2, N(W)C(NW)N(Ra)2, -N(W)S(0)tRa (where t is 1
or
2), -S(0)OW (where t is 1 or 2), -S(0)N(W)2 (where t is 1 or 2), or P03(W)2,
where each Ra
is independently hydrogen, alkyl, fluoroalkyl, carbocyclyl, carbocyclylalkyl,
aryl, aralkyl,
heterocycloalkyl, heterocycloalkylalkyl, heteroaryl or heteroarylalkyl.
[00101] The term C2_10 alkynyl- C3_8 cycloalkyl refers to a group containing
an alkynyl
group, containing 2 to 10 carbons and branched or straight chain, which is
attached to a
linking cycloalkyl group containing 3 to 8 carbons, such as, for example 3-
prop-3-ynyl-
cyclopent-1yl, and the like. Either portion of the moiety is unsubstituted or
substituted.
[00102] The term "haloalkenyl" refers to an alkynyl group substituted with one
or more
independent halo groups.
-28-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00103] "Amino" or "amine" refers to a -N(Ra)2radical group, where each Ra is
independently hydrogen, alkyl, fluoroalkyl, carbocyclyl, carbocyclylalkyl,
aryl, aralkyl,
heterocycloalkyl, heterocycloalkylalkyl, heteroaryl or heteroarylalkyl, unless
stated otherwise
specifically in the specification. When a -N(Ra)2 group has two Ra other than
hydrogen they
can be combined with the nitrogen atom to form a 4-, 5-, 6-, or 7-membered
ring. For
example, -N(Ra)2 is meant to include, but not be limited to, 1-pyrrolidinyl
and 4-morpholinyl.
Unless stated otherwise specifically in the specification, an amino group is
optionally
substituted by one or more substituents which independently are: alkyl,
heteroalkyl, alkenyl,
alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl,
heteroarylalkyl, hydroxy,
halo, cyano, trifluoromethyl, trifluoromethoxy, nitro, trimethylsilanyl, -0Ra,
-
SRa, -0C(0)-Ra, -N(W)2, -C(0)Ra, -C(0)0W, -0C(0)N(W)2, -C(0)N(Ra)2, -
N(W)C(0)0Ra
, -N(Ra)C(0)Ra, -N(Ra)C(0)N(Ra)2, -N(Ra)C(NRa)N(Ra)2, _N(Ra)S(0)Ra (where t is
1 or
2), -S(0)tORa (where t is 1 or 2), -S(0)N(Ra)2 (where t is 1 or 2), or
P03(102, where each Ra
is independently hydrogen, alkyl, fluoroalkyl, carbocyclyl, carbocyclylalkyl,
aryl, aralkyl,
heterocycloalkyl, heterocycloalkylalkyl, heteroaryl or heteroarylalkyl and
each of these
moieties may be optionally substituted as defined herein.
[00104] "Amide" or "amido" refers to a chemical moiety with formula ¨C(0)N(R)2
or ¨
NHC(0)R, where R is selected from the group consisting of hydrogen, alkyl,
cycloalkyl, aryl,
heteroaryl (bonded through a ring carbon) and heteroalicyclic (bonded through
a ring
carbon), each of which moiety may itself be optionally substituted. In some
embodiments it is
a C1-C4 amido or amide radical, which includes the amide carbonyl in the total
number of
carbons in the radical. The R2' of - N(R)2 of the amide may optionally be
taken together with
the nitrogen to which it is attached to form a 4-, 5-, 6-, or 7-membered ring.
Unless stated
otherwise specifically in the specification, an amido group is optionally
substituted
independently by one or more of the substituents as described herein for
alkyl, cycloalkyl,
aryl, heteroaryl, or heterocycloalkyl. An amide may be an amino acid or a
peptide molecule
attached to a compound of Formula (I), thereby forming a prodrug. Any amine,
hydroxy, or
carboxyl side chain on the compounds described herein can be amidified. The
procedures and
specific groups to make such amides are known to those of skill in the art and
can readily be
found in reference sources such as Greene and Wuts, Protective Groups in
Organic Synthesis,
3rd Ed., John Wiley & Sons, New York, N.Y., 1999, which is incorporated herein
by
reference in its entirety.
[00105] "Aromatic" or "aryl" refers to an aromatic radical with six to ten
ring atoms (e.g.,
C6-C10 aromatic or C6-C10 aryl) which has at least one ring having a
conjugated pi electron
-29-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
system which is carbocyclic (e.g., phenyl, fluorenyl, and naphthyl). Bivalent
radicals formed
from substituted benzene derivatives and having the free valences at ring
atoms are named as
substituted phenylene radicals. Bivalent radicals derived from univalent
polycyclic
hydrocarbon radicals whose names end in "-y1" by removal of one hydrogen atom
from the
carbon atom with the free valence are named by adding "-idene" to the name of
the
corresponding univalent radical, e.g., a naphthyl group with two points of
attachment is
termed naphthylidene. Whenever it appears herein, a numerical range such as "6
to 10" refers
to each integer in the given range; e.g., "6 to 10 ring atoms" means that the
aryl group may
consist of 6 ring atoms, 7 ring atoms, etc., up to and including 10 ring
atoms. The term
includes monocyclic or fused-ring polycyclic (i.e., rings which share adjacent
pairs of ring
atoms) groups. Unless stated otherwise specifically in the specification, an
aryl moiety is
optionally substituted by one or more substituents which are independently:
alkyl,
heteroalkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl,
heteroaryl,
heteroarylalkyl, hydroxy, halo, cyano, trifluoromethyl, trifluoromethoxy,
nitro,
trimethylsilanyl, -0Ra, -SW, -0C(0)-Ra, -
N(W)2, -C(0)Ra, -C(0)0W, -0C(0)N(W)2, -C(0)N(W)2, -N(W)C(0)0Ra, -N(W)C(0)Ra, -
N(W)C(0)N(W)2, N(Ra)C(NRa)N(W)2, -N(Ra)S(0)tRa (where t is 1 or 2), -S(0)tORa
(where
t is 1 or 2), -S(0)tN(Ra)2 (where t is 1 or 2), or P03(W)2, where each Ra is
independently
hydrogen, alkyl, fluoroalkyl, carbocyclyl, carbocyclylalkyl, aryl, aralkyl,
heterocycloalkyl,
heterocycloalkylalkyl, heteroaryl or heteroarylalkyl.
[00106] "Heteroaryl" or, alternatively, "heteroaromatic" refers to a 5- to 18-
membered
aromatic radical (e.g., C5-C13 heteroaryl) that includes one or more ring
heteroatoms selected
from nitrogen, oxygen and sulfur, and which may be a monocyclic, bicyclic,
tricyclic or
tetracyclic ring system. Whenever it appears herein, a numerical range such as
"5 to 18"
refers to each integer in the given range; e.g., "5 to 18 ring atoms" means
that the heteroaryl
group may consist of 5 ring atoms, 6 ring atoms, etc., up to and including 18
ring atoms.
Bivalent radicals derived from univalent heteroaryl radicals whose names end
in "-y1" by
removal of one hydrogen atom from the atom with the free valence are named by
adding
"-idene" to the name of the corresponding univalent radical, e.g., a pyridyl
group with two
points of attachment is a pyridylidene. An N-containing "heteroaromatic" or
"heteroaryl"
moiety refers to an aromatic group in which at least one of the skeletal atoms
of the ring is a
nitrogen atom. The polycyclic heteroaryl group may be fused or non-fused. The
heteroatom(s) in the heteroaryl radical is optionally oxidized. One or more
nitrogen atoms, if
present, are optionally quaternized. The heteroaryl is attached to the rest of
the molecule
-30-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
through any atom of the ring(s). Examples of heteroaryls include, but are not
limited to,
azepinyl, acridinyl, benzimidazolyl, benzindolyl, 1,3-benzodioxolyl,
benzofuranyl,
benzoxazolyl, benzo[d]thiazolyl, benzothiadiazolyl, benzo [b][ 1,4]dioxepinyl,
benzo[b][1,4]oxazinyl, 1,4-benzodioxanyl, benzonaphthofuranyl, benzoxazolyl,
benzodioxolyl, benzodioxinyl, benzoxazolyl, benzopyranyl, benzopyranonyl,
benzofuranyl,
benzofuranonyl, benzofurazanyl, benzothiazolyl, benzothienyl
(benzothiophenyl),
benzothieno[3,2-d]pyrimidinyl, benzotriazolyl, benzo[4,6]imidazo[1,2-
a]pyridinyl,
carbazolyl, cinnolinyl, cyclopenta[d]pyrimidinyl,
6,7-dihydro-5H-cyclopenta[4,5]thieno[2,3-d]pyrimidinyl, 5,6-
dihydrobenzo[h]quinazolinyl,
5,6-dihydrobenzo[h]cinnolinyl, 6,7-dihydro-5H-benzo[6,7]cyclohepta[1,2-
c]pyridazinyl,
dibenzofuranyl, dibenzothiophenyl, furanyl, furazanyl, furanonyl, furo[3,2-
c]pyridinyl,
5,6,7,8,9,10-hexahydrocycloocta[d]pyrimidinyl,
5,6,7,8,9,10-hexahydrocycloocta[d]pyridazinyl, 5,6,7,8,9,10-
hexahydrocycloocta[d]pyridinyl,
isothiazolyl, imidazolyl, indazolyl, indolyl, indazolyl, isoindolyl,
indolinyl, isoindolinyl,
isoquinolyl, indolizinyl, isoxazolyl, 5,8-methano-5,6,7,8-
tetrahydroquinazolinyl,
naphthyridinyl, 1,6-naphthyridinonyl, oxadiazolyl, 2-oxoazepinyl, oxazolyl,
oxiranyl,
5,6,6a,7,8,9,10,10a-octahydrobenzo[h]quinazolinyl, 1-pheny1-1H-pyrrolyl,
phenazinyl,
phenothiazinyl, phenoxazinyl, phthalazinyl, pteridinyl, purinyl, pyranyl,
pyrrolyl, pyrazolyl,
pyrazolo[3,4-d]pyrimidinyl, pyridinyl, pyrido[3,2-d]pyrimidinyl, pyrido[3,4-
d]pyrimidinyl,
pyrazinyl, pyrimidinyl, pyridazinyl, pyrrolyl, quinazolinyl, quinoxalinyl,
quinolinyl,
isoquinolinyl, tetrahydroquinolinyl, 5,6,7,8-tetrahydroquinazolinyl,
5,6,7,8-tetrahydrobenzo[4,5]thieno[2,3-d]pyrimidinyl,
6,7,8,9-tetrahydro-5H-cyclohepta[4,5]thieno[2,3-d]pyrimidinyl,
5,6,7,8-tetrahydropyrido[4,5-c]pyridazinyl, thiazolyl, thiadiazolyl,
thiapyranyl, triazolyl,
tetrazolyl, triazinyl, thieno[2,3-d]pyrimidinyl, thieno[3,2-d]pyrimidinyl,
thieno[2,3-c]pyridinyl, and thiophenyl (i.e. thienyl). Unless stated otherwise
specifically in
the specification, a heteraryl moiety is optionally substituted by one or more
substituents
which are independently: alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl,
aryl, arylalkyl, heteroaryl, heteroarylalkyl, hydroxy, halo, cyano, nitro,
oxo, thioxo,
trimethylsilanyl, -0Ra, -
SW, -0C(0)-Ra, -N(W)2, -C(0)Ra, -C(0)0W, -C(0)N(Ra)2, -N(W)C(0)0Ra, -
N(W)C(0)Ra,
-N(Ra)S(0)tRa (where t is 1 or 2), -S(0)tORa (where t is 1 or 2), -S(0)N(W)2
(where t is 1 or
2), or P03(W)2, where each Ra is independently hydrogen, alkyl, fluoroalkyl,
carbocyclyl,
-31-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
carbocyclylalkyl, aryl, aralkyl, heterocycloalkyl, heterocycloalkylalkyl,
heteroaryl or
heteroarylalkyl.
[00107] The terms "aryl-alkyl", "arylalkyl" and "aralkyl" are used to describe
a group
wherein the alkyl chain can be branched or straight chain forming a linking
portion with the
terminal aryl, as defined above, of the aryl-alkyl moiety. Examples of aryl-
alkyl groups
include, but are not limited to, optionally substituted benzyl, phenethyl,
phenpropyl and
phenbutyl such as 4-chlorobenzyl, 2,4-dibromobenzyl, 2-methylbenzyl, 2-(3-
fluorophenyl)ethyl, 2-(4-methylphenyl)ethyl, 2-(4-
(trifluoromethyl)phenyl)ethyl, 2-(2-
methoxyphenyl)ethyl, 2-(3-nitrophenyl)ethyl, 2-(2,4-dichlorophenyl)ethyl,
243,5-
dimethoxyphenyl)ethyl, 3-phenylpropyl, 3-(3-chlorophenyl)propyl, 3-(2-
methylphenyl)propyl, 3-(4-methoxyphenyl)propyl, 3-(4-
(trifluoromethyl)phenyl)propyl, 3-
(2,4-dichlorophenyl)propyl, 4-phenylbutyl, 4-(4-chlorophenyl)butyl, 4-(2-
methylphenyl)butyl, 4-(2,4-dichlorophenyl)butyl, 4-(2-methoxphenyl)butyl, and
10-
phenyldecyl. Either portion of the moiety is unsubstituted or substituted.
[00108] The term "Ci_ioalkylaryl" as used herein refers to an alkyl group, as
defined above,
containing 1 to 10 carbon atoms, branched or unbranched, wherein the aryl
group replaces
one hydrogen on the alkyl group, for example, 3-phenylpropyl. Either portion
of the moiety
is unsubstituted or substituted.
[00109] The term C2_10 alkyl monocycloaryl" refers to a group containing a
terminal alkyl
group, branched or straight chain and containing 2 to 10 atoms attached to a
linking aryl
group which has only one ring, such as for example, 2-phenyl ethyl. Either
portion of the
moiety is unsubstituted or substituted.
[00110] The term "Ci_io alkyl bicycloaryl" refers to a group containing a
terminal alkyl
group, branched or straight chain and containing 2 to 10 atoms attached to a
linking aryl
group which is bicyclic, such as for example, 2-(1-naphthyl)- ethyl. Either
portion of the
moiety is unsubstituted or substituted.
[00111] The terms "aryl-cycloalkyl" and "arylcycloalkyl" are used to describe
a group
wherein the terminal aryl group is attached to a cycloalkyl group, for example
phenylcyclopentyl and the like. Either portion of the moiety is unsubstituted
or substituted.
[00112] The terms "heteroaryl-C3_8cycloalkyl" and "heteroaryl- C3_8cycloalkyl
" are used to
describe a group wherein the terminal heteroaryl group is attached to a
cycloalkyl group,
which contains 3 to 8 carbons, for example pyrid-2-yl-cyclopentyl and the
like. Either portion
of the moiety is unsubstituted or substituted.
-32-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00113] The term "heteroaryl- heteroalkyl" refers to a group wherein the
terminal heteroaryl
group is attached to a linking heteroalkyl group, such as for example, pyrid-2-
y1
methylenoxy, and the like. Either portion of the moiety is unsubstituted or
substituted.
[00114] The terms "aryl-alkenyl", "arylalkenyl" and "aralkenyl" are used to
describe a
group wherein the alkenyl chain can be branched or straight chain forming a
linking portion
of the aralkenyl moiety with the terminal aryl portion, as defined above, for
example styryl
(2-phenylvinyl), phenpropenyl, and the like. Either portion of the moiety is
unsubstituted or
substituted.
[00115] The term "aryl -C2-ioalkenyl" means an arylalkenyl as described above
wherein the
alkenyl moiety contains 2 to 10 carbon atoms such as for example, styryl (2-
phenylvinyl),
and the like. Either portion of the moiety is unsubstituted or substituted.
[00116] The term "C2-ioalkenyl-aryl" is used to describe a group wherein the
terminal
alkenyl group, which contains 2 to 10 carbon atoms and can be branched or
straight chain, is
attached to the aryl moiety which forms the linking portion of the alkenyl-
aryl moiety, such
as for example, 3-propenyl- naphth-l-yl, and the like. Either portion of the
moiety is
unsubstituted or substituted.
[00117] The terms "aryl-alkynyl", "arylalkynyl" and "aralkynyl" are used to
describe a
group wherein the alkynyl chain can be branched or straight chain forming a
linking portion
of the aryl-alkynyl moiety with the terminal aryl portion, as defined above,
for example 3-
phenyl-1-propynyl, and the like. Either portion of the moiety is unsubstituted
or substituted.
[00118] The term "aryl- C2-ioalkynyl" means an arylalkynyl as described above
wherein the
alkynyl moiety contains two to ten carbons, such as, for example 3-phenyl-1-
propynyl, and
the like . Either portion of the moiety is unsubstituted or substituted.
[00119] The term "C2-ioalkynyl- aryl" means a group containing an alkynyl
moiety attached
to an aryl linking group, both as defined above, wherein the alkynyl moiety
contains two to
ten carbons, such as, for example 3-propynyl-naphth-1-yl. Either portion of
the moiety is
unsubstituted or substituted.
[00120] The terms "aryl-oxy", "aryloxy" and "aroxy" are used to describe a
terminal aryl
group attached to a linking oxygen atom. Typical aryl-oxy groups include
phenoxy, 3,4-
dichlorophenoxy, and the like. Either portion of the moiety is unsubstituted
or substituted.
[00121] The terms "aryl-oxyalkyl", "aryloxyalkyl" and "aroxyalkyl" are used to
describe a
group wherein an alkyl group is substituted with a terminal aryl-oxy group,
for example
pentafluorophenoxymethyl and the like. Either portion of the moiety is
unsubstituted or
substituted.
-33-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00122] The term "Ci_loalkoxy-Ci_loalkyl" refers to a group wherein an alkoxy
group,
containing 1 to 10 carbon atoms and an oxygen atom within the branching or
straight chain,
is attached to a linking alkyl group, branched or straight chain which
contains 1 to 10 carbon
atoms, such as, for example methoxypropyl, and the like. Either portion of the
moiety is
unsubstituted or substituted.
[00123] The term "Ci_loalkoxy-C2_10alkenyl" refers to a group wherein an
alkoxy group,
containing 1 to 10 carbon atoms and an oxygen atom within the branching or
straight chain,
is attached to a linking alkenyl group, branched or straight chain which
contains 1 to 10
carbon atoms, such as, for example 3-methoxybut-2-en-1-yl, and the like.
Either portion of
the moiety is unsubstituted or substituted.
[00124] The term "Ci_loalkoxy-C2_10alkynyl" refers to a group wherein an
alkoxy group,
containing 1 to 10 carbon atoms and an oxygen atom within the branching or
straight chain,
is attached to a linking alkynyl group, branched or straight chain which
contains 1 to 10
carbon atoms, such as, for example 3-methoxybut-2-in-1-yl, and the like.
Either portion of
the moiety is unsubstituted or substituted.
[00125] The term "heterocycloalkenyl" refers to a cycloalkenyl structure,
which is
unsubstituted or substituted in which at least one carbon atom is replaced
with a heteroatom
selected from oxygen, nitrogen, and sulfur.
[00126] The terms "heteroaryl-oxy", "heteroaryl-oxy", "heteroaryloxy",
"heteroaryloxy",
"hetaroxy" and "heteroaroxy" are used to describe a terminal heteroaryl group,
which is
unsubstituted or substituted, attached to a linking oxygen atom. Typical
heteroaryl-oxy
groups include 4,6-dimethoxypyrimidin-2-yloxy and the like.
[00127] The terms "heteroarylalkyl", "heteroarylalkyl", "heteroaryl-alkyl",
"heteroaryl-
alkyl", "hetaralkyl" and "heteroaralkyl" are used to describe a group wherein
the alkyl chain
can be branched or straight chain forming a linking portion of the
heteroaralkyl moiety with
the terminal heteroaryl portion, as defined above, for example 3-furylmethyl,
thenyl, furfuryl,
and the like. Either portion of the moiety is unsubstituted or substituted.
[00128] The term "heteroaryl-Ci_ioalkyl" is used to describe a heteroaryl
alkyl group as
described above where the alkyl group contains 1 to 10 carbon atoms. Either
portion of the
moiety is unsubstituted or substituted.
[00129] The term "Ci_ioalkyl-heteroaryl" is used to describe a alkyl attached
to a hetaryl
group as described above where the alkyl group contains 1 to 10 carbon atoms.
Either portion
of the moiety is unsubstituted or substituted.
-34-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00130] The terms "heteroarylalkenyl", "heteroarylalkenyl", "heteroaryl-
alkenyl",
"heteroaryl-alkenyl", "hetaralkenyl" and "heteroaralkenyl" are used to
describe a
heteroarylalkenyl group wherein the alkenyl chain can be branched or straight
chain forming
a linking portion of the heteroaralkenyl moiety with the terminal heteroaryl
portion, as
defined above, for example 3-(4-pyridy1)-1-propenyl. Either portion of the
moiety is
unsubstituted or substituted.
[00131] The term "heteroaryl- C2-ioalkenyl" group is used to describe a group
as described
above wherein the alkenyl group contains 2 to 10 carbon atoms. Either portion
of the moiety
is unsubstituted or substituted.
[00132] The term "C2-ioalkenyl- heteroaryl" is used to describe a group
containing an
alkenyl group, which is branched or straight chain and contains 2 to 10 carbon
atoms, and is
attached to a linking heteroaryl group, such as, for example 2-styry1-4-
pyridyl, and the like.
Either portion of the moiety is unsubstituted or substituted.
[00133] The terms "heteroarylalkynyl", "heteroarylalkynyl", "heteroaryl-
alkynyl",
"heteroaryl-alkynyl", "hetaralkynyl" and "heteroaralkynyl" are used to
describe a group
wherein the alkynyl chain can be branched or straight chain forming a linking
portion of the
heteroaralkynyl moiety with the heteroaryl portion, as defined above, for
example 4-(2-
thieny1)-1-butynyl, and the like. Either portion of the moiety is
unsubstituted or substituted.
[00134] The term "heteroaryl- C2-ioalkynyl" is used to describe a
heteroarylalkynyl group as
described above wherein the alkynyl group contains 2 to 10 carbon atoms.
Either portion of
the moiety is unsubstituted or substituted.
[00135] The term "C2-ioalkynyl- heteroaryl" is used to describe a group
containing an
alkynyl group which contains 2 to 10 carbon atoms and is branched or straight
chain, which
is attached to a linking heteroaryl group such as, for example, 4(but-1-ynyl)
thien-2-yl, and
the like. Either portion of the moiety is unsubstituted or substituted.
[00136] The term "heterocyclyl" refers to a four-, five-, six-, or seven-
membered ring
containing one, two, three or four heteroatoms independently selected from
nitrogen, oxygen
and sulfur. The four-membered ring has zero double bonds, the five-membered
ring has zero
to two double bonds, and the six- and seven-membered rings have zero to three
double bonds.
The term "heterocyclyl" also includes bicyclic groups in which the
heterocyclyl ring is fused
to another monocyclic heterocyclyl group, or a four- to seven-membered
aromatic or
nonaromatic carbocyclic ring. The heterocyclyl group can be attached to the
parent molecular
moiety through any carbon atom or nitrogen atom in the group.
-35-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00137] "Heterocycloalkyl" refers to a stable 3- to 18-membered non-aromatic
ring radical
that comprises two to twelve carbon atoms and from one to six heteroatoms
selected from
nitrogen, oxygen and sulfur. Whenever it appears herein, a numerical range
such as "3 to 18"
refers to each integer in the given range; e.g., "3 to 18 ring atoms" means
that the
heterocycloalkyl group may consist of 3 ring atoms, 4 ring atoms, etc., up to
and including 18
ring atoms. In some embodiments, it is a C5-C10 heterocycloalkyl. In some
embodiments, it
is a C4-Ci0heterocycloalkyl. In some embodiments, it is a C3-
Ci0heterocycloalkyl. Unless
stated otherwise specifically in the specification, the heterocycloalkyl
radical is a monocyclic,
bicyclic, tricyclic or tetracyclic ring system, which may include fused or
bridged ring
systems. The heteroatoms in the heterocycloalkyl radical may be optionally
oxidized. One
or more nitrogen atoms, if present, are optionally quaternized. The
heterocycloalkyl radical
is partially or fully saturated. The heterocycloalkyl may be attached to the
rest of the
molecule through any atom of the ring(s). Examples of such heterocycloalkyl
radicals
include, but are not limited to, dioxolanyl, thienyl[1,3]dithianyl,
decahydroisoquinolyl,
imidazolinyl, imidazolidinyl, isothiazolidinyl, isoxazolidinyl, morpholinyl,
octahydroindolyl,
octahydroisoindolyl, 2-oxopiperazinyl, 2-oxopiperidinyl, 2-oxopyrrolidinyl,
oxazolidinyl,
piperidinyl, piperazinyl, 4-piperidonyl, pyrrolidinyl, pyrazolidinyl,
quinuclidinyl,
thiazolidinyl, tetrahydrofuryl, trithianyl, tetrahydropyranyl,
thiomorpholinyl,
thiamorpholinyl, 1-oxo-thiomorpholinyl, and 1,1-dioxo-thiomorpholinyl. Unless
stated
otherwise specifically in the specification, a heterocycloalkyl moiety is
optionally substituted
by one or more substituents which independently are: alkyl, heteroalkyl,
alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
hydroxy, halo,
cyano, nitro, oxo, thioxo, trimethylsilanyl, -OW,
SW, -0C(0)-Ra, -N(Ra)2, -C(0)Ra, -C(0)0W, -C(0)N(W)2, -N(W)C(0)0W, -N(W)C(0)
Ra, -N(Ra)S(0)Ra (where t is 1 or 2), -S(0)tORa (where t is 1 or 2), -
S(0)N(Ra)2 (where t is 1
or 2), or P03(W)2, where each Ra is independently hydrogen, alkyl,
fluoroalkyl, carbocyclyl,
carbocyclylalkyl, aryl, aralkyl, heterocycloalkyl, heteroaryl or
heteroarylalkyl.
[00138] "Heterocycloalkyl" also includes bicyclic ring systems wherein one non-
aromatic
ring, usually with 3 to 7 ring atoms, contains at least 2 carbon atoms in
addition to 1-3
heteroatoms independently selected from oxygen, sulfur, and nitrogen, as well
as
combinations comprising at least one of the foregoing heteroatoms; and the
other ring,
usually with 3 to 7 ring atoms, optionally contains 1-3 heteroatoms
independently selected
from oxygen, sulfur, and nitrogen and is not aromatic.
-36-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00139] The terms "heterocyclylalkyl", "heterocyclyl-alkyl", "hetcyclylalkyl",
and
"hetcyclyl-alkyl" are used to describe a group wherein the alkyl chain can be
branched or
straight chain forming a linking portion of the heterocyclylalkyl moiety with
the terminal
heterocyclyl portion, as defined above, for example 3-piperidinylmethyl and
the like. The
term "heterocycloalkylene" refers to the divalent derivative of
heterocycloalkyl.
[00140] The term "Ci_ioalkyl-heterocycyl" refers to a group as defined above
where the
alkyl moiety contains 1 to 10 carbon atoms. Either portion of the moiety is
unsubstituted or
substituted.
[00141] The term "heterocyclyl- Ci_ioalkyl" refers to a group containing a
terminal
heterocyclic group attached to a linking alkyl group which contains 1 to 10
carbons and is
branched or straight chain, such as, for example, 4-morpholinyl ethyl, and the
like. Either
portion of the moiety is unsubstituted or substituted.
[00142] The terms "heterocyclylalkenyl", "heterocyclyl-alkenyl",
"hetcyclylalkenyl" and
"hetcyclyl-alkenyl" are used to describe a group wherein the alkenyl chain can
be branched
or straight chain forming a linking portion of the heterocyclylalkenyl moiety
with the
terminal heterocyclyl portion, as defined above, for example 2-morpholiny1-1-
propenyl and
the like. The term "heterocycloalkenylene" refers to the divalent derivative
of
heterocyclylalkenyl. Either portion of the moiety is unsubstituted or
substituted.
[00143] The term "heterocycyl- C2_10 alkenyl" refers to a group as defined
above where the
alkenyl group contains 2 to 10 carbon atoms and is branched or straight chain,
such as, for
example, 4-(N-piperaziny1)-but-2-en-1 -yl, and the like. Either portion of the
moiety is
unsubstituted or substituted.
[00144] The terms "heterocyclylalkynyl", "heterocyclyl-alkynyl",
"hetcyclylalkynyl" and
"hetcyclyl-alkynyl" are used to describe a group wherein the alkynyl chain can
be branched
or straight chain forming a linking portion of the heterocyclylalkynyl moiety
with the
terminal heterocyclyl portion, as defined above, for example 2-pyrrolidiny1-1-
butynyl and the
like. Either portion of the moiety is unsubstituted or substituted.
[00145] The term "heterocycyl- C2_10 alkynyl" refers to a group as defined
above where the
alkynyl group contains 2 to 10 carbon atoms and is branched or straight chain,
such as, for
example, 4-(N-piperaziny1)-but-2-yn-1-yl, and the like.
[00146] The term "aryl- heterocycyl" refers to a group containing a terminal
aryl group
attached to a linking heterocyclic group, such as for example, N4-(4-phenyl)-
piperazinyl,
and the like. Either portion of the moiety is unsubstituted or substituted.
-37-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00147] The term "heteroaryl- heterocycyl" refers to a group containing a
terminal
heteroaryl group attached to a linking heterocyclic group, such as for
example, N4-(4-
pyridy1)- piperazinyl, and the like. Either portion of the moiety is
unsubstituted or substituted.
[00148] The term "carboxylalkyl" refers to a terminal carboxyl (-COOH) group
attached to
branched or straight chain alkyl groups as defined above.
[00149] The term "carboxylalkenyl" refers to a terminal carboxyl (-COOH) group
attached
to branched or straight chain alkenyl groups as defined above.
[00150] The term "carboxylalkynyl" refers to a terminal carboxyl (-COOH) group
attached
to branched or straight chain alkynyl groups as defined above.
[00151] The term "carboxylcycloalkyl" refers to a terminal carboxyl (-COOH)
group
attached to a cyclic aliphatic ring structure as defined above.
[00152] The term "carboxylcycloalkenyl" refers to a terminal carboxyl (-COOH)
group
attached to a cyclic aliphatic ring structure having ethylenic bonds as
defined above.
[00153] The terms "cycloalkylalkyl" and "cycloalkyl-alkyl" refer to a terminal
cycloalkyl
group as defined above attached to an alkyl group, for example
cyclopropylmethyl,
cyclohexylethyl, and the like. Either portion of the moiety is unsubstituted
or substituted.
[00154] The terms "cycloalkylalkenyl" and "cycloalkyl-alkenyl" refer to a
terminal
cycloalkyl group as defined above attached to an alkenyl group, for example
cyclohexylvinyl,
cycloheptylallyl, and the like. Either portion of the moiety is unsubstituted
or substituted.
[00155] The terms "cycloalkylalkynyl" and "cycloalkyl-alkynyl" refer to a
terminal
cycloalkyl group as defined above attached to an alkynyl group, for example
cyclopropylpropargyl, 4-cyclopenty1-2-butynyl, and the like. Either portion of
the moiety is
unsubstituted or substituted.
[00156] The terms "cycloalkenylalkyl" and "cycloalkenyl-alkyl" refer to a
terminal
cycloalkenyl group as defined above attached to an alkyl group, for example 2-
(cyclopenten-
1 -yl)ethyl and the like. Either portion of the moiety is unsubstituted or
substituted.
[00157] The terms "cycloalkenylalkenyl" and "cycloalkenyl-alkenyl" refer to
terminal a
cycloalkenyl group as defined above attached to an alkenyl group, for example
1-
(cyclohexen-3-yl)ally1 and the like.
[00158] The terms "cycloalkenylalkynyl" and "cycloalkenyl-alkynyl" refer to
terminal a
cycloalkenyl group as defined above attached to an alkynyl group, for example
1-
(cyclohexen-3-yl)propargyl and the like. Either portion of the moiety is
unsubstituted or
substituted.
-38-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00159] The term "alkoxy" refers to the group -0-alkyl, including from 1 to 8
carbon atoms
of a straight, branched, cyclic configuration and combinations thereof
attached to the parent
structure through an oxygen. Examples include methoxy, ethoxy, propoxy,
isopropoxy,
cyclopropyloxy, cyclohexyloxy and the like. "Lower alkoxy" refers to alkoxy
groups
containing one to six carbons. In some embodiments, C1-C4 alkyl, is an alkyl
group which
encompasses both straight and branched chain alkyls of from 1 to 4 carbon
atoms.
[00160] The term "haloalkoxy" refers to an alkoxy group substituted with one
or more halo
groups, for example chloromethoxy, trifluoromethoxy, difluoromethoxy,
perfluoroisobutoxy,
and the like.
[00161] The term "alkoxyalkoxyalkyl" refers to an alkyl group substituted with
an alkoxy
moiety which is in turn is substituted with a second alkoxy moiety, for
example
methoxymethoxymethyl, isopropoxymethoxyethyl, and the like. This moiety is
substituted
with further substituents or not substituted with other substituents.
[00162] The term "alkylthio" includes both branched and straight chain alkyl
groups
attached to a linking sulfur atom, for example methylthio and the like.
[00163] The term "alkoxyalkyl" refers to an alkyl group substituted with an
alkoxy group,
for example isopropoxymethyl and the like. Either portion of the moiety is
unsubstituted or
substituted.
[00164] The term "alkoxyalkenyl" refers to an alkenyl group substituted with
an alkoxy
group, for example 3-methoxyally1 and the like. Either portion of the moiety
is unsubstituted
or substituted.
[00165] The term "alkoxyalkynyl" refers to an alkynyl group substituted with
an alkoxy
group, for example 3-methoxypropargyl and the like. Either portion of the
moiety is
unsubstituted or substituted.
[00166] The term "C2_10alkeny1C3_8cycloalkyl" refers to an alkenyl group as
defined above
substituted with a three to eight membered cycloalkyl group, for example, 4-
(cyclopropyl) -2-
butenyl and the like. Either portion of the moiety is unsubstituted or
substituted.
[00167] The term "C2_10alkyny1C3_8cycloalkyl" refers to an alkynyl group as
defined above
substituted with a three to eight membered cycloalkyl group, for example, 4-
(cyclopropyl) -2-
butynyl and the like. Either portion of the moiety is unsubstituted or
substituted.
[00168] The term "heterocyclyl-Ci_ioalkyl" refers to a heterocyclic group as
defined above
substituted with an alkyl group as defined above having 1 to 10 carbons, for
example, 4-(N-
methyl)-piperazinyl, and the like. Either portion of the moiety is
unsubstituted or substituted.
-39-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00169] The term "heterocyclyl-C2_10alkenyl" refers to a heterocyclic group as
defined
above, substituted with an alkenyl group as defined above, having 2to 10
carbons, for
example, 4-(N-ally1) piperazinyl, and the like. Moieties wherein the
heterocyclic group is
substituted on a carbon atom with an alkenyl group are also included. Either
portion of the
moiety is unsubstituted or substituted.
[00170] The term "heterocyclyl-C2_10alkynyl" refers to a heterocyclic group as
defined
above, substituted with an alkynyl group as defined above, having 2 to 10
carbons, for
example, 4-(N-propargyl) piperazinyl, and the like. Moieties wherein the
heterocyclic group
is substituted on a carbon atom with an alkenyl group are also included.
Either portion of the
moiety is unsubstituted or substituted.
[00171] The term "oxo" refers to an oxygen that is double bonded to a carbon
atom. One in
the art understands that an "oxo" requires a second bond from the atom to
which the oxo is
attached. Accordingly, it is understood that oxo cannot be substituted onto an
aryl or
heteroaryl ring, unless it forms part of the aromatic system as a tautomer.
[00172] The term "oligomer" refers to a low-molecular weight polymer, whose
number
average molecular weight is typically less than about 5000 g/mol, and whose
degree of
polymerization (average number of monomer units per chain) is greater than one
and
typically equal to or less than about 50.
[00173] "Sulfonamidyl" or "sulfonamido" refers to a ¨S(=0)2-NR'R' radical,
where each R'
is selected independently from the group consisting of hydrogen, alkyl,
cycloalkyl, aryl,
heteroaryl (bonded through a ring carbon) and heteroalicyclic (bonded through
a ring
carbon). The R' groups in ¨NR'R' of the ¨S(=0)2-NR'R' radical may be taken
together with
the nitrogen to which it is attached to form a 4-, 5-, 6-, or 7-membered ring.
A sulfonamido
group is optionally substituted by one or more of the substituents described
for alkyl,
cycloalkyl, aryl, heteroaryl respectively.
[00174] Compounds described can contain one or more asymmetric centers and may
thus
give rise to diastereomers and optical isomers. The present invention includes
all such
possible diastereomers as well as their racemic mixtures, their substantially
pure resolved
enantiomers, all possible geometric isomers, and pharmaceutically acceptable
salts thereof.
Compounds may be shown without a definitive stereochemistry at certain
positions. The
present invention includes all stereoisomers of the disclosed compounds and
pharmaceutically acceptable salts thereof. Further, mixtures of stereoisomers
as well as
isolated specific stereoisomers are also included. During the course of the
synthetic
procedures used to prepare such compounds, or in using racemization or
epimerization
-40-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
procedures known to those skilled in the art, the products of such procedures
can be a mixture
of stereoisomers.
[00175] The present invention includes all manner of rotamers and
conformationally
restricted states of an inhibitor of the invention.
[00176] Substituents for alkyl, heteroalkyl, cycloalkyl, heterocycloalkyl
monovalent and
divalent derivative radicals (including those groups often referred to as
alkylene, alkenyl,
heteroalkylene, heteroalkenyl, alkynyl, cycloalkyl, heterocycloalkyl,
cycloalkenyl, and
heterocycloalkenyl) can be one or more of a variety of groups selected from,
but not limited
to: alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl,
arylalkyl,
heteroaryl, heteroarylalkyl, -OR', =0, =NR', =N-OR', -NR'R", -SR', -halogen, -
SiR'R"R'", -0C(0)R', -C(0)R', -CO2R',-C(0)NR'R", -0C(0)NR'R", -
NR"C(0)R', -NR'-C(0)NR"R'", -NR"C(0)OR', -NR-C(NR'R")=NR", -S(0)R', -S(0)2R', -
S(0)2NR'R", -NRSO2R', -CN and -NO2 in a number ranging from zero to (2m'+1),
where m'
is the total number of carbon atoms in such radical. R', R", R" and R" each
preferably
independently refer to hydrogen, substituted or unsubstituted heteroalkyl,
substituted or
unsubstituted cycloalkyl, substituted or unsubstituted heterocycloalkyl,
substituted or
unsubstituted aryl (e.g., aryl substituted with 1-3 halogens), substituted or
unsubstituted alkyl,
alkoxy or thioalkoxy groups, or arylalkyl groups. When an inhibitor of the
invention includes
more than one R group, for example, each of the R groups is independently
selected as are
each R', R", R" and R" groups when more than one of these groups is present.
[00177] When R' and R" or R" and R" are attached to the same nitrogen atom,
they can be
combined with the nitrogen atom to form a 4-, 5-, 6-, or 7-membered ring. For
example, -NR'R" is meant to include, but not be limited to, 1-pyrrolidinyl, 4
piperazinyl, and
4-morpholinyl. From the above discussion of substituents, one of skill in the
art will
understand that the term "alkyl" is meant to include groups including carbon
atoms bound to
groups other than hydrogen groups, such as haloalkyl (e.g., -CF3 and -CH2CF3)
and acyl
(e.g., -C(0)CH3, -C(0)CF 3, -C(0)CH2OCH3, and the like).
[00178] Similar to the substituents described for alkyl radicals above,
exemplary substituents
for aryl and heteroaryl groups ( as well as their divalent derivatives) are
varied and are
selected from, for example: halogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl,
heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, -OR', -NR'R", -
SR', -halogen, -
SiR'R"R'", -0C(0)R', -C(0)R', -CO2R', -C(0)NR'R", -
OC(0)NR'R", -NR"C(0)R', -NR'-C(0)NR"R'", -NR"C(0)OR', -NR-
C(NR'R"R")=NR", -NR-C(NR'R")=NR", -S(0)R', -S(0)2R', -S(0)2NR'R", -NRSO2R', -
CN
-41-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
and ¨NO2, -R', -N3, -CH(Ph)2, fluoro(Ci-C4)alkoxo, and fluoro(Ci-C4)alkyl, in
a number
ranging from zero to the total number of open valences on aromatic ring
system; and where
R', R", R" and R" are preferably independently selected from hydrogen,
substituted or
unsubstituted alkyl, substituted or unsubstituted heteroalkyl, substituted or
unsubstituted
cycloalkyl, substituted or unsubstituted heterocycloalkyl, substituted or
unsubstituted aryl and
substituted or unsubstituted heteroaryl. When an inhibitor of the invention
includes more
than one R group, for example, each of the R groups is independently selected
as are each R',
R", R" and R" groups when more than one of these groups is present.
[00179] As used herein, 0-2 in the context of -S(0)(0_2)- are integers of 0,
1, and 2.
[00180] Two of the substituents on adjacent atoms of aryl or heteroaryl ring
may optionally
form a ring of the formula -T-C(0)-(CRR)q-U-, wherein T and U are
independently ¨NR-
, -0-, -CRR'- or a single bond, and q is an integer of from 0 to 3.
Alternatively, two of the
substituents on adjacent atoms of aryl or heteroaryl ring may optionally be
replaced with a
substituent of the formula -A-(CH2),-B-, wherein A and B are independently
¨CRR'-, -0-
, -NR-, -S-, -S(0)-, -S(0)2-, -S(0)2NR'- or a single bond, and r is an integer
of from 1 to 4.
One of the single bonds of the new ring so formed may optionally be replaced
with a double
bond. Alternatively, two of the substituents on adjacent atoms of aryl or
heteroaryl ring may
optionally be replaced with a substituent of the formula -(CRR')s-X'-(C"R")d-,
where s and d
are independently integers of from 0 to 3, and X' is ¨0-, -NR'-, -S-, -S(0)-, -
S(0)2-,
or -S(0)2NR'-. The substituents R, R', R" and R" are preferably independently
selected from
hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted
cycloalkyl,
substituted or unsubstituted heterocycloalkyl, substituted or unsubstituted
aryl, and
substituted or unsubstituted heteroaryl.
[00181] Unless otherwise stated, structures depicted herein are also meant to
include
compounds which differ only in the presence of one or more isotopically
enriched atoms. For
example, compounds having the present structures except for the replacement of
a hydrogen
by a deuterium or tritium, or the replacement of a carbon by 13C- or 14C-
enriched carbon are
within the scope of this invention.
[00182] The compounds of the present invention may also contain unnatural
proportions of
atomic isotopes at one or more of atoms that constitute such compounds. For
example, the
compounds may be radiolabeled with radioactive isotopes, such as for example
tritium (3H),
iodine-125 (1251) or carbon-14 (4C). All isotopic variations of the compounds
of the present
invention, whether radioactive or not, are encompassed within the scope of the
present
invention.
-42-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00183] The term "treatment period" as used herein is defined as the time
period in which a
subject is administered the daily doses of the pharmaceutical composition
according to a
dosing regimen. The term "resting period" refers to a period of time during
which a subject is
not administered a pharmaceutical composition according to a dosing regimen.
For example,
if the pharmaceutical composition has been given on a daily basis, there would
be rest period
if the daily administration is discontinued, e.g., for some number of days or
weeks. If a dose
is administered on a different schedule a rest period would occur where that
dosing is
discontinued for some time. In some dosing regimens, a rest period occurs
where the
concentration of the pharmaceutical composition is maintained at a sub-
therapeutic level.
Preferably, during the rest period, the plasma concentration of the
pharmaceutical
composition is maintained at sub-therapeutic level. A combination therapy can
have different
dosing regimens for different compounds, e.g., one dosing regimen for a first
compound and
another dosing regimen for a second compound. Under such a combination
therapy, a subject
can undergo a rest period with respect to the first compound, at the same time
undergoes a
treatment period with respect to the second compound. In some combination
therapy, a rest
period refers to a period of time during which a subject is not administered
any
pharmaceutical composition.
[00184] The term "intermittent dosing regimen" refers to a dosing regimen that
comprises
administering a pharmaceutical composition, followed by a rest period.
[00185] The term "durability of effect" refers to the continuation of at least
one of the
clinical and/or therapeutic effects of the PI3-kinase a inhibitor and/or mTOR
inhibitor after
discontinuing the administration thereof. Such continuation of the clinical
and therapeutic
effects can last for a period of time at least as long as the administration
period of the PI3-
kinase a inhibitor and/or mTOR inhibitor. The term "durability of effect
period" refers to the
period beginning immediately following the administration period. This period
occurring
after the administration period relates is characterized by no P13-kinase a
inhibitor or mTOR
inhibitor being administered, but where the therapeutic and clinical effects
of the inhibitor
administration still continue. Depending on the dosage as well as the length
of the
administration, the durability of effect period lasts at least as long as the
administration
period, but can last up to about 5 or more times the length of the
administration period. In
some regimens, the durability of effect period is at least 5, 10, 20, 30 days.
In some regimens,
the durability of effect period is at least a month, three months, six months,
or a year.
[00186] The present invention provides methods for treating treating a disease
condition
associated with P13-kinase a and/or mTOR, in particular neoplastic condition,
autoimmune
-43-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
disease, inflammatory disease, fibrotic disease and kidney disease, which
provide for
durability of effect periods which are at least as long as the administration
period of PI3-
kinase a inhibitor or mTOR inhibitor. The clinical and therapeutic effects
which are extended
from the administration period into the durability of effect period can
include sustained tumor
regression, inhibited tumor re-growth, reduction of proliferation, increased
apoptosis, or
downregulation of activity of a target protein, or combinations thereof An
administration
period refers to the period of time in which a dosing regimen (e.g., an
intermittent dosing
regimen) is administered to a subject. The administration period can be from
about 1 to
about 52 weeks, or from about 4 to about 24 weeks, or from about 6 to about 12
weeks, or
about 8 weeks, or about 4 weeks. The administration period can include one or
more
treatment periods and one or more rest periods. With each administration
period there is a
corresponding durability of effect period which lasts at least as long as the
administration
period.
Methods
[00187] In one aspect, the present invention provides a method for treating a
disease
condition associated with P13-kinase a and/or mTOR in a subject. The method
typically
comprises administering to a subject simultaneously or sequentially a
therapeutically
effective amount of a combination of a P13-kinase a inhibitor and an mTOR
inhibitor.
[00188] As used herein, a therapeutically effective amount of a combination of
a P13-kinase
a inhibitor and an mTOR inhibitor refers to a combination of a PI3-kinase a
inhibitor and an
mTOR inhibitor, wherein the combination is sufficient to effect the intended
application
including but not limited to disease treatment, as defined herein. Encompassed
in this subject
method is the use of therapeutically effective amount of a PI3-kinase a
inhibitor and/or an
mTOR inhibitor in combination to effect such treatment. Also contemplated in
the subject
methods is the use of a sub-therapeutic amount of a P13-kinase a inhibitor
and/or an mTOR
inhibitor in the combination for treating an intended disease condition. The
individual
inhibitors, though present in sub-therapeutic amounts, synergistically yield
an efficacious
effect and/or reduced a side effect in an intended application.
[00189] Accordingly, in a separate but related aspect, the present invention
provides for a
method for treating a disease condition associated with P13-kinase a and/or
mTOR in a
subject, comprising administering to the subject simultaneously or
sequentially a
synergistically effective therapeutic amount of a combination of a P13-kinase
a inhibitor and
an mTOR inhibitor.
-44-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00190] The therapeutically effective amount of the subject combination of
compounds may
vary depending upon the intended application (in vitro or in vivo), or the
subject and disease
condition being treated, e.g., the weight and age of the subject, the severity
of the disease
condition, the manner of administration and the like, which can readily be
determined by one
of ordinary skill in the art. The term also applies to a dose that will induce
a particular
response in target cells, e.g., reduction of proliferation or downregulation
of activity of a
target protein. The specific dose will vary depending on the particular
compounds chosen,
the dosing regimen to be followed, whether it is administered in combination
with other
compounds, timing of administration, the tissue to which it is administered,
and the physical
delivery system in which it is carried.
[00191] The P13-kinase a inhibitor utilized in the subject methods typically
exhibits
selective inhibition of P13-kinase a relative to one or more type I
phosphatidylinosito1-3-
kinases (P13-kinase) including, e.g., P13-kinase 13, P13-kinase y, and P13-
kinase 6.
[00192] Selective inhibition of P13-kinase a can be ascertained by an in vitro
or an in vivo
method. Any assay known in the art may be used, including without limitation,
immunoassays, immunoprecipitation, fluorescence or cell-based assays. In some
embodiments, an in vitro assay is used to determine selective inhibition of
P13-kinase a by an
assay which measures the activity of the PI3Ka protein relative to the
activity of another PI3-
kinase such as P13-kinase 13, P13-kinase y, and P13-kinase 6. For example, a
time resolved
FRET assay that indirectly measures PIP3 product formed by the activity of a
P13-K may be
used to determine an IC50 value for a test compound for P13-kinase a and/or
any of the other
PI3-kinases.
[00193] As used herein, the term "IC50" refers to the half maximal inhibitory
concentration
of an inhibitor in inhibiting biological or biochemical function. This
quantitative measure
indicates how much of a particular inhibitor is needed to inhibit a given
biological process (or
component of a process, i.e. an enzyme, cell, cell receptor or microorganism)
by half. In other
words, it is the half maximal (50%) inhibitory concentration (IC) of a
substance (50% IC, or
IC50). EC50 refers to the plasma concentration required for obtaining 50% of a
maximum
effect in vivo.
[00194] Determination of IC50 can be made by determining and constructing a
dose-
response curve and examining the effect of different concentrations of an
inhibitor on
reversing agonist activity. In vitro assays that are useful in making these
determinations are
referred to as "in vitro kinase assays."
-45-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00195] In some embodiments, an in vitro kinase assay includes the use of
labeled ATP as a
phosphate donor, and following the kinase reaction the substrate peptide is
captured on an
appropriate filter. Unreacted labeled ATP and metabolites are resolved from
the radioactive
peptide substrate by various techniques, involving trichloroacetic acid
precipitation and
extensive washing. Addition of several positively charged residues allows
capture on
phosphocellulose paper followed by washing. Radioactivity incorporated into
the substrate
peptide is detected by scintillation counting. This assay is relatively
simple, reasonably
sensitive, and the peptide substrate can be adjusted both in terms of sequence
and
concentration to meet the assay requirements.
[00196] Other exemplary kinase assays are detailed in U.S. Pat. No. 5,759,787
and US
Application Ser. No. 12/728,926, both of which are incorporated herein by
reference.
[00197] In other embodiments, a cell-based assay is used to ascertain
selective inhibition of
P13-kinase a. For example, an inhibitor can be shown to be selective for P13-
kinase a if it
selectively downregulates P13-kinase signal transduction in cells that express
P13-kinase a,
preferably in cells that exhibit abnormally high level or activity of P13-
kinase a. A variety of
cells having P13-kinase a mutations and hence exhibiting such P13-kinase a
abnormalities are
known in the art. Non-limiting examples of cell lines harboring such mutations
include those
that carry point mutations, deletions, substitutions, or translation of
nucleic acid sequence of
the P13-kinase a gene. Examples of such cell lines include but are not limited
to BT20
(H1047R mutation), MCF-7 (E545K mutation), MDA-MB-361 (E545K mutation), MDA-
MB-453 (H1047R mutation), T47D (H1047R mutation), Hec-1A (G1049R mutation) and
HCT-116 (H1047R mutation). Other cell lines having mutations in the PI3Ka
protein may be
used, such as cells harboring mutations in the p85, C2, helical or kinase
domains.
[00198] In addition, inhibition of P13-kinase a activity can be determined by
a reduction in
signal transduction of the P13-kinase a pathway. A wide variety of readouts
can be utilized to
establish a reduction of the output of such signaling pathway. Some non-
limiting exemplary
readouts include (1) a decrease in phosphorylation of Akt at residues,
including but not
limited to S473 and T308; (2) a decrease in activation of Akt as evidenced by
a reduction of
phosphorylation of Akt substrates including but not limited to Fox01/03a
T24/32, GSK3a/I3
S21/9, and TSC2 T1462; (3) a decrease in phosphorylation of signaling
molecules
downstream of P13-kinase a, including but not limited to ribosomal S6
S240/244, 70S6K
T389, and 4EBP1 T37/46; (4) inhibition of proliferation of cells including but
not limited to
normal or neoplastic cells, mouse embryonic fibroblasts, leukemic blast cells,
cancer stem
cells, and cells that mediate autoimmune reactions; (5) induction of apoptosis
of cells or cell
-46-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
cycle arrest; (6) reduction of cell chemotaxis; and (7) an increase in binding
of 4EBP1 to
eIF4E. The term "eIF4E" refers to a 24-kD eukaryotic translation initiation
factor involved
in directing ribosomes to the cap structure of mRNAs, having human gene locus
4q21-q25.
[00199] In some embodiments, the P13-kinase a inhibitor selectively inhibits
P13-kinase a
relative to one, two or three other type I phosphatidylinosito1-3-kinases (P13-
kinases)
consisting of P13-kinase 13, P13-kinase y, and P13-kinase 6. In other
embodiments, some of the
subject inhibitors selectively inhibit P13-kinase a and P13-kinase y as
compared to the rest of
the type I PI3-kinases. In yet other embodiments, some of the subject
inhibitors selectively
inhibit P13-kinase a and P13-kinase 0 as compared to the rest of the type I
P13-kinases. In
still yet other embodiments, some of the subject inhibitors selectively
inhibit PI3-kinase a
and P13-kinase 6 as compared to the rest of the type I P13-kinases.
[00200] In some embodiments, the subject methods utilizes a PI3-kinase a
inhibitor with an
IC50 value of about or less than a predetermined value, as ascertained in an
in vitro kinase
assay. In some embodiments, the PI3-kinase a inhibitor inhibits PI3-kinase a
with an 150
value of about 1 nM or less, 2 nM or less, 5 nM or less, 7 nM or less, 10 nM
or less, 20 nM or
less, 30 nM or less, 40 nM or less, 50 nM or less, 60 nM or less, 70 nM or
less, 80 nM or less,
90 nM or less, 100 nM or less, 120 nM or less, 140 nM or less, 150 nM or less,
160 nM or
less, 170 nM or less, 180 nM or less, 190 nM or less, 200 nM or less, 225 nM
or less, 250 nM
or less, 275 nM or less, 300 nM or less, 325 nM or less, 350 nM or less, 375
nM or less, 400
nM or less, 425 nM or less, 450 nM or less, 475 nM or less, 500 nM or less,
550 nM or less,
600 nM or less, 650 nM or less, 700 nM or less, 750 nM or less, 800 nM or
less, 850 nM or
less, 900 nM or less, 950 nM or less, 1 M or less, 1.2 M or less, 1.3 M or
less, 1.4 M or
less, 1.5 M or less, 1.6 M or less, 1.7 M or less, 1.8 M or less, 1.9 M
or less, 2 M or
less, 5 M or less, 10 M or less, 15 M or less, 20 M or less, 25 M or
less, 30 M or less,
40 M or less, 50 M, 60 M, 70 M, 80 M, 90 M, 100 M, 200 M, 300 M, 400
M,
or 500 M, or less.
[00201] In some embodiments, the P13-kinase a inhibitor selectively inhibits
P13-kinase a
with an IC50 value that is at least 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25,
30, 35, 40, 45, 50, 100,
or 1000 times less than its IC50 value against one, two, or three other type I
P13-kinase(s)
selected from the group consisting of P13-kinase 13, P13-kinase y, and P13-
kinase 6.
[00202] In some embodiments, the P13-kinase a inhibitor selectively inhibits
P13-kinase a
with an IC50 value that is less than about 1 nM, 2 nM, 5 nM, 7 nM, 10 nM, 20
nM, 30 nM,
40 nM, 50 nM, 60 nM, 70 nM, 80 nM, 90 nM, 100 nM, 120 nM, 140 nM, 150 nM, 160
nM,
170 nM, 180 nM, 190 nM, 200 nM, 225 nM, 250 nM, 275 nM, 300 nM, 325 nM, 350
nM,
-47-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
375 nM, 400 nM, 425 nM, 450 nM, 475 nM, 500 nM, 550 nM, 600 nM, 650 nM, 700
nM,
750 nM, 800 nM, 850 nM, 900 nM, 950 nM, 1 M, 1.2 M, 1.3 M, 1.4 M, 1.5 M,
1.6
M, 1.7 M, 1.8 M, 1.9 M, 2 M, 5 M, 10 M, 15 M, 20 M, 25 M, 30 M, 40
M,
50 M, 60 M, 70 M, 80 M, 90 M, 100 M, 200 M, 300 M, 400 M, or 500 M,
and
said IC50 value is at least 2, 3, 4, 5, 6, 7, 8, 9, 10, 15, 20, 25, 30, 35,
40, 45, 50, 100, or 1000
times less than its IC50 value against one, two or three other type I P13-
kinases selected from
the group consisting of P13 -kinase 13, P13 -kinase y, and P13 -kinase 6. In
some embodiments,
the P13-kinase a inhibitor inhibits P13-kinase a with an IC50 value of about
100 nM or less as
ascertained in an in vitro kinase assay.
[00203] In some instances, the P13-kinase a inhibitor inhibits P13-kinase a
with an IC50
value of about 200 nM or less as ascertained in an in vitro kinase assay and
the IC50 value is
at least 5, 10, 15, 20, 25, 50, 100, or 1000 times less than its IC50 value
against all other type
I P13-kinases selected from the group consisting of P13-kinase 13, P13-kinase
y, and P13-kinase
6. In some embodiments, the P13-kinase a inhibitor selectively inhibits P13-
kinase a with an
IC50 value that is less than about 100 nM, and said IC50 value is at 5, 10,
15, 20, 25, 50, or
100, 1000 times less than its IC50 value against all other type I P13-kinases
selected from the
group consisting of P13-kinase 13, P13-kinase y, and P13-kinase 6.
[00204] In some instances, the P13-kinase a inhibitor inhibits P13-kinase a
with an IC50
value of about 50 nM or less as ascertained in an in vitro kinase assay and
the IC50 value is at
least 5, 10, 15, 20, 25, 50, 100, or 1000 times less than its IC50 value
against all other type I
P13-kinases selected from the group consisting of P13-kinase 13, P13-kinase y,
and P13-kinase
6.
[00205] In some embodiments, the P13-kinase a inhibitor selectively inhibits
P13-kinase a
with an IC50 value that is less than about 20 nM, and said IC50 value is at 5,
10, 15, 20, 25,
50, or 100, 1000 times less than its IC50 value against all other type I P13-
kinases selected
from the group consisting of P13-kinase 13, P13-kinase y, and P13-kinase 6.
[00206] In some embodiments, the P13-kinase a inhibitor selectively inhibits
P13-kinase a
with an IC50 value that is less than about 20 nM, and said IC50 value is at 5,
10, 15, 20, 25,
50, or 100, 1000 times less than its IC50 value against all other type I P13-
kinases selected
from the group consisting of P13-kinase 13, P13-kinase y, and P13-kinase 6.
[00207] In some instances, the P13-kinase a inhibitor inhibits P13-kinase a
with an IC50
value of about 20 nM or less as ascertained in an in vitro kinase assay and
the IC50 value is at
least 100 times less than its IC50 value against all other type I P13-kinases
selected from the
group consisting of P13-kinase 13, P13-kinase y, and P13-kinase 6.
-48-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00208] Alternatively, the P13-kinase a inhibitor inhibits P13-kinase a with
an EC50 value of
about 10 ILIM or less, 5 M or less, 2.5 ILIM or less,1 M or less, 500nM or
less, 100 nM or less,
75 nM or less, 50 nM or less, 25 nM or less, 10 nM or less, 5 nM or less, 1 nM
or less, 500
pM or less, or 100 pM or less as ascertained in an in vitro kinase assay.
[00209] In some embodiments, the P13-kinase a inhibitor selectively inhibits
P13-kinase a
with an EC50 value that is at least 5, 10, 15, 20, 25, 50, 100, or 1000 times
less than its EC50
value against one, two or three other type I P13-kinases selected from the
group consisting of
P13-kinase 13, P13-kinase y, and P13-kinase 6.
[00210] In some embodiments, the P13-kinase a inhibitor inhibits P13-kinase a
with an EC50
value of about 10 ILIM or less, 5 M or less, 2.5 ILIM or less,1 M or less,
500nM or less, 100
nM or less, 75 nM or less, 50 nM or less, 25 nM or less, 10 nM or less, 5 nM
or less, 1 nM or
less, 500 pM or less, or 100 pM or less as ascertained in an in vitro kinase
assay, and such
EC50 value is at least 5, 10, 15, 20, 25, 50, or 100, 1000 times less than its
EC50 value
against one, two or three other type I P13-kinases selected from the group
consisting of P13-
kinase 13, P13-kinase y, and P13-kinase 6.
[00211] The mTOR inhibitor utilized in the subject methods is typically highly
selective for
the target molecule. In one aspect, the mTOR inhibitor binds to and directly
inhibits both
mTORC1 and mTORC2. Such ability can be ascertained using any method known in
the art
or described herein. For example, inhibition of mTorC1 and/or mTorC2 activity
can be
determined by a reduction in signal transduction of the PI3K/Akt/mTor pathway.
A wide
variety of readouts can be utilized to establish a reduction of the output of
such signaling
pathway. Some non-limiting exemplary readouts include (1) a decrease in
phosphorylation
of Akt at residues, including but not limited to S473 and T308; (2) a decrease
in activation of
Akt as evidenced by a reduction of phosphorylation of Akt substrates including
but not
limited to Fox01/03a T24/32, GSK3a/13 S21/9, and TSC2 T1462; (3) a decrease in
phosphorylation of signaling molecules downstream of mTor, including but not
limited to
ribosomal S6 S240/244, 70S6K T389, and 4EBP1 T37/46; (4) inhibition of
proliferation of
cells including but not limited to normal or neoplastic cells, mouse embryonic
fibroblasts,
leukemic blast cells, cancer stem cells, and cells that mediate autoimmune
reactions; (5)
induction of apoptosis of cells or cell cycle arrest; (6) reduction of cell
chemotaxis; and (7) an
increase in binding of 4EBP1 to eIF4E.
[00212] mTor exists in two types of complexes, mTorC1 containing the raptor
subunit and
mTorC2 containing rictor. As known in the art, "rictor" refers to a cell
growth regulatory
protein having human gene locus 5p13.1. These complexes are regulated
differently and
-49-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
have a different spectrum of substrates. For instance, mTorC1 phosphorylates
S6 kinase
(S6K) and 4EBP1, promoting increased translation and ribosome biogenesis to
facilitate cell
growth and cell cycle progression. S6K also acts in a feedback pathway to
attenuate
PI3K/Akt activation. Thus, inhibition of mTorC1 (e.g. by a biologically active
agent as
discussed herein) results in activation of 4EBP1, resulting in inhibition of
(e.g. a decrease in)
RNA translation.
[00213] mTorC2 is generally insensitive to rapamycin and selective inhibitors
and is thought
to modulate growth factor signaling by phosphorylating the C-terminal
hydrophobic motif of
some AGC kinases such as Akt. In many cellular contexts, mTorC2 is required
for
phosphorylation of the S473 site of Akt. Thus, mTorC1 activity is partly
controlled by Akt
whereas Akt itself is partly controlled by mTorC2.
[00214] Growth factor stimulation of PI3K causes activation of Akt by
phosphorylation at
the two key sites, S473 and T308. It has been reported that full activation of
Akt requires
phosphorylation of both S473 and T308Active. Akt promotes cell survival and
proliferation
in many ways including suppressing apoptosis, promoting glucose uptake, and
modifying
cellular metabolism. Of the two phosphorylation sites on Akt, activation loop
phosphorylation at T308, mediated by PDK1, is believed to be indispensable for
kinase
activity, while hydrophobic motif phosphorylation at S473 enhances Akt kinase
activity.
[00215] Selective mTor inhibition may also be determined by expression levels
of the mTor
genes, its downstream signaling genes (for example by RT-PCR), or expression
levels of the
proteins (for example by immunocytochemistry, immunohistochemistry, Western
blots) as
compared to other P13-kinases or protein kinases.
[00216] Cell-based assays for establishing selective inhibition of mTorC1
and/or mTorC2
can take a variety of formats. This generally will depend on the biological
activity and/or the
signal transduction readout that is under investigation. For example, the
ability of the agent
to inhibit mTorC1 and/or mTorC2 to phosphorylate the downstream substrate(s)
can be
determined by various types of kinase assays known in the art. Representative
assays include
but are not limited to immunoblotting and immunoprecipitation with antibodies
such as anti-
phosphotyrosine, anti-phosphoserine or anti-phosphothreonine antibodies that
recognize
phosphorylated proteins. Alternatively, antibodies that specifically recognize
a particular
phosphorylated form of a kinase substrate (e.g. anti-phospho AKT S473 or anti-
phospho
AKT T308) can be used. In addition, kinase activity can be detected by high
throughput
chemiluminescent assays such as AlphaScreenTM (available from Perkin Elmer)
and eTagTm
assay (Chan-Hui, et al. (2003) Clinical Immunology 111: 162-174). In another
aspect, single
-50-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
cell assays such as flow cytometry as described in the Phosflow experiment can
be used to
measure phosphorylation of multiple downstream mTOR substrates in mixed cell
populations.
[00217] One advantage of the immunoblotting and Phosflow methods is that the
phosphorylation of multiple kinase substrates can be measured simultaneously.
This provides
the advantage that efficacy and selectivity can be measured at the same time.
For example,
cells may be contacted with an mTOR inhibitor at various concentrations and
the
phosphorylation levels of substrates of both mTOR and other kinases can be
measured. In
one aspect, a large number of kinase substrates are assayed in what is termed
a
"comprehensive kinase survey." Selective mTOR inhibitors are expected to
inhibit
phosphorylation of mTOR substrates without inhibiting phosphorylation of the
substrates of
other kinases. Alternatively, selective mTOR inhibitors may inhibit
phosphorylation of
substrates of other kinases through anticipated or unanticipated mechanisms
such as feedback
loops or redundancy.
[00218] Effect of inhibition of mTorC1 and/or mTorC2, or P13-kinase a can be
established
by cell colony formation assay or other forms of cell proliferation assay. A
wide range of cell
proliferation assays are available in the art, and many of which are available
as kits. Non-
limiting examples of cell proliferation assays include testing for tritiated
thymidine uptake
assays, BrdU (5'-bromo-2'-deoxyuridine) uptake (kit marketed by Calbiochem),
MTS uptake
(kit marketed by Promega), MTT uptake (kit marketed by Cayman Chemical),
CyQUANTO
dye uptake (marketed by Invitrogen).
[00219] Apoptosis and cell cycle arrest analysis can be performed with any
methods
exemplified herein as well other methods known in the art. Many different
methods have
been devised to detect apoptosis. Exemplary assays include but are not limited
to the
TUNEL (TdT-mediated dUTP Nick-End Labeling) analysis, ISEL (in situ end
labeling), and
DNA laddering analysis for the detection of fragmentation of DNA in
populations of cells or
in individual cells, Annexin-V analysis that measures alterations in plasma
membranes,
detection of apoptosis related proteins such p53 and Fas.
[00220] A cell-based assay typically proceeds with exposing the target cells
(e.g., in a
culture medium) to a test compound which is a potential mTorC1 and/or mTorC2
selective
inhibitor, or a P13-kinase a inhibitor and then assaying for readout under
investigation.
Depending on the nature of the candidate mTor inhibitors or P13-kinase a
inhibitors, they can
directly be added to the cells or in conjunction with carriers. For instance,
when the agent is
nucleic acid, it can be added to the cell culture by methods well known in the
art, which
-51-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
include without limitation calcium phosphate precipitation, microinjection or
electroporation.
Alternatively, the nucleic acid can be incorporated into an expression or
insertion vector for
incorporation into the cells. Vectors that contain both a promoter and a
cloning site into
which a polynucleotide can be operatively linked are well known in the art.
Such vectors are
capable of transcribing RNA in vitro or in vitro, and are commercially
available from sources
such as Stratagene (La Jolla, CA) and Promega Biotech (Madison, WI). In order
to optimize
expression and/or in vitro transcription, it may be necessary to remove, add
or alter 5' and/or
3' untranslated portions of the clones to eliminate extra, potential
inappropriate alternative
translation initiation codons or other sequences that may interfere with or
reduce expression,
either at the level of transcription or translation. Alternatively, consensus
ribosome binding
sites can be inserted immediately 5' of the start codon to enhance expression.
Examples of
vectors are viruses, such as baculovirus and retrovirus, bacteriophage,
adenovirus, adeno-
associated virus, cosmid, plasmid, fungal vectors and other recombination
vehicles typically
used in the art which have been described for expression in a variety of
eukaryotic and
prokaryotic hosts, and may be used for gene therapy as well as for simple
protein expression.
Among these are several non-viral vectors, including DNA/liposome complexes,
and targeted
viral protein DNA complexes. To enhance delivery to a cell, the nucleic acid
or proteins of
this invention can be conjugated to antibodies or binding fragments thereof
which bind cell
surface antigens. Liposomes that also comprise a targeting antibody or
fragment thereof can
be used in the methods of this invention. Other biologically acceptable
carriers can be
utilized, including those described in, for example, REMINGTON'S
PHARMACEUTICAL
SCIENCES, 19th Ed. (2000), in conjunction with the subject compounds.
[00221] The subject agents can also be utilized to inhibit phosphorylation of
both Akt (S473)
and Akt (T308) in a cell. Accordingly, the present invention provides a method
comprises
the step of contacting a cell with an effective amount of such biologically
active agent such
that Akt phosphorylation at residues S473 and T308 is simultaneously
inhibited. In one
aspect, the biologically active agent inhibits phosphorylation of S473 of Akt
more effectively
than phosphorylation of T308 of Akt when tested at a comparable molar
concentration,
preferably at an identical molar concentration.
[00222] Inhibition of Akt phosphorylation can be determined using any methods
known in
the art or described herein. Representative assays include but are not limited
to
immunoblotting and immunoprecipitation with antibodies such as anti-
phosphotyrosine
antibodies that recognize the specific phosphorylated proteins. Cell-based
ELISA kit
-52-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
quantifies the amount of activated (phosphorylated at S473) Akt relative to
total Akt protein
is also available (SuperArray Biosciences).
[00223] In practicing the subject methods, any cells that express P13-kinase
a, mTorCl,
mTorC2 and/or Akt can be utilized. Non-limiting examples of specific cell
types whose
proliferation can be inhibited include fibroblast, cells of skeletal tissue
(bone and cartilage),
cells of epithelial tissues (e.g. liver, lung, breast, skin, bladder and
kidney), cardiac and
smooth muscle cells, neural cells (glia and neurones), endocrine cells
(adrenal, pituitary,
pancreatic islet cells), melanocytes, and many different types of haemopoietic
cells (e.g., cells
of B-cell or T-cell lineage, and their corresponding stem cells,
lymphoblasts). Also of
interest are cells exhibiting a neoplastic propensity or phenotype. Of
particular interest is the
type of cells that differentially expresses (over-expresses or under-
expresses) a disease-
causing gene. The types of diseases involving abnormal functioning of genes
include but are
not limited to autoimmune diseases, cancer, obesity, hypertension, diabetes,
neuronal and/or
muscular degenerative diseases, cardiac diseases, endocrine disorders, and any
combinations
thereof.
[00224] In some embodiments, the mTOR inhibitor inhibits both mTORC1 and
mTORC2
with an IC50 value of about 1 nM, 2 nM, 5 nM, 7 nM, 10 nM, 20 nM, 30 nM, 40
nM, 50 nM,
60 nM, 70 nM, 80 nM, 90 nM, 100 nM, 120 nM, 140 nM, 150 nM, 160 nM, 170 nM,
180
nM, 190 nM, 200 nM, 225 nM, 250 nM, 275 nM, 300 nM, 325 nM, 350 nM, 375 nM,
400
nM, 425 nM, 450 nM, 475 nM, 500 nM, 550 nM, 600 nM, 650 nM, 700 nM, 750 nM,
800
nM, 850 nM, 900 nM, 950 nM, 1 M, 1.2 M, 1.3 M, 1.4 M, 1.5 M, 1.6 M, 1.7
M, 1.8
M, 1.9 M, 2 M, 5 M, 10 M, 15 M, 20 M, 25 M, 30 M, 40 M, 50 M, 60 M,
70
M, 80 M, 90 M, 100 M, 200 M, 300 M, 400 M, or 500 M or less as
ascertained in
an in vitro kinase assay, and said IC50 value is at least 2, 3, 4, 5, 6, 7, 8,
9, 10, 15, 20, 25, 30,
35, 40, 45, 50, 100, or 1000 times less than its IC50 value against all other
type I P13-kinases
selected from the group consisting of P13-kinase a, P13-kinase 13, P13-kinase
y, and P13-
kinase 6. For example, the mTOR inhibitor inhibits both mTORC1 and mTORC2 with
an
IC50 value of about 200, 100, 75, 50, 25, 10, 5, 1 or 0.5 nM or less as
ascertained in an in
vitro kinase assay. In one instance, the mTOR inhibitor inhibits both mTORC1
and mTORC2
with an IC50 value of about 100nM or less as ascertained in an in vitro kinase
assay.
Alternatively, the mTOR inhibitor inhibits both mTORC1 and mTORC2 with an IC50
value
of about 10 nM or less as ascertained in an in vitro kinase assay.
[00225] In some embodiments, the present invention provides the use of an mTOR
inhibitor,
wherein the mTOR inhibitor directly binds to and inhibits both mTORC1 and
mTORC2 with
-53-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
an IC50 value of about or less than a predetermined value, as ascertained in
an in vitro kinase
assay. In some embodiments, the mTOR inhibitor inhibits both mTORC1 and mTORC2
with
an IC50 value of about 1 nM or less, 2 nM or less, 5 nM or less, 7 nM or less,
10 nM or less,
20 nM or less, 30 nM or less, 40 nM or less, 50 nM or less, 60 nM or less, 70
nM or less, 80
nM or less, 90 nM or less, 100 nM or less, 120 nM or less, 140 nM or less, 150
nM or less,
160 nM or less, 170 nM or less, 180 nM or less, 190 nM or less, 200 nM or
less, 225 nM or
less, 250 nM or less, 275 nM or less, 300 nM or less, 325 nM or less, 350 nM
or less, 375 nM
or less, 400 nM or less, 425 nM or less, 450 nM or less, 475 nM or less, 500
nM or less, 550
nM or less, 600 nM or less, 650 nM or less, 700 nM or less, 750 nM or less,
800 nM or less,
850 nM or less, 900 nM or less, 950 nM or less, 1 ILIM or less, 1.2 ILIM or
less, 1.3 ILIM or less,
1.4 ILIM or less, 1.5 ILIM or less, 1.6 ILIM or less, 1.7 ILIM or less, 1.8
ILIM or less, 1.9 ILIM or less,
2 ILIM or less, 5 ILIM or less, 10 ILIM or less, 15 ILIM or less, 20 ILIM or
less, 25 ILIM or less, 30
ILIM or less, 40 ILIM or less, 50 ILIM or less, 60 ILIM or less, 70 ILIM or
less, 80 ILIM or less, 90
ILIM or less, 100 ILIM or less, 200 ILIM or less, 300 ILIM or less, 400 ILIM
or less, or 500 ILIM or
less.
[00226] In some embodiments, the mTOR inhibitor inhibits both mTORC1 and
mTORC2
with an IC50 value of about 1 nM or less, 2 nM or less, 5 nM or less, 7 nM or
less, 10 nM or
less, 20 nM or less, 30 nM or less, 40 nM or less, 50 nM or less, 60 nM or
less, 70 nM or less,
80 nM or less, 90 nM or less, 100 nM or less, 120 nM or less, 140 nM or less,
150 nM or less,
160 nM or less, 170 nM or less, 180 nM or less, 190 nM or less, 200 nM or
less, 225 nM or
less, 250 nM or less, 275 nM or less, 300 nM or less, 325 nM or less, 350 nM
or less, 375 nM
or less, 400 nM or less, 425 nM or less, 450 nM or less, 475 nM or less, 500
nM or less, 550
nM or less, 600 nM or less, 650 nM or less, 700 nM or less, 750 nM or less,
800 nM or less,
850 nM or less, 900 nM or less, 950 nM or less, 1 ILIM or less, 1.2 ILIM or
less, 1.3 ILIM or less,
1.4 ILIM or less, 1.5 ILIM or less, 1.6 ILIM or less, 1.7 ILIM or less, 1.8
ILIM or less, 1.9 ILIM or less,
2 ILIM or less, 5 ILIM or less, 10 ILIM or less, 15 ILIM or less, 20 ILIM or
less, 25 ILIM or less, 30
ILIM or less, 40 ILIM or less, 50 ILIM or less, 60 ILIM or less, 70 ILIM or
less, 80 ILIM or less, 90
ILIM or less, 100 ILIM or less, 200 ILIM or less, 300 ILIM or less, 400 ILIM
or less, or 500 ILIM or
less, and the mTOR inhibitor is substantially inactive against one or more
types I P13-kinases
selected from the group consisting of P13-kinase a, P13-kinase 13, P13-kinase
y, and P13-
kinase 6. In some embodiments, the mTOR inhibitor inhibits both mTORC1 and
mTORC2
with an IC50 value of about 10 nM or less as ascertained in an in vitro kinase
assay, and the
mTOR inhibitor is substantially inactive against one or more types I P13-
kinases selected
from the group consisting of P13-kinase a, P13-kinase 13, P13-kinase y, and
P13-kinase 6.
-54-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00227] As used herein, the terms "substantially inactive" refers to an
inhibitor that inhibits
the activity of its target by less than approximately 1%, 5%, 10%, 15% or 20%
of its maximal
activity in the absence of the inhibitor, as determined by an in vitro
enzymatic assay (e.g. in
vitro kinase assay).
[00228] In other embodiments, the mTOR inhibitor inhibits both mTORC1 and
mTORC2
with an IC50 value of about 1000, 500, 100, 75, 50, 25, 10, 5, 1, or 0.5 nM or
less as
ascertained in an in vitro kinase assay, and said IC50 value is at least 2, 5,
10, 15, 20, 50, 100
or 100 times less than its IC50 value against all other type I P13-kinases
selected from the
group consisting of P13-kinase a, P13-kinase 13, P13-kinase y, and P13-kinase
6. For example,
the mTOR inhibitor inhibits both mTORC1 and mTORC2 with an IC50 value of about
100
nM or less as ascertained in an in vitro kinase assay, and said IC50 value is
at least 5 times
less than its IC50 value against all other type I P13-kinases selected from
the group consisting
of P13-kinase a, P13-kinase 13, P13-kinase y, and P13-kinase 6.
[00229] In some embodiments, the mTOR inhibitor inhibits both mTORC1 and
mTORC2
with an IC50 value of about 100 nM or less as ascertained in an in vitro
kinase assay, and
said IC50 value is at least 5 times less than its IC50 value against all other
type I P13-kinases
selected from the group consisting of P13-kinase a, P13-kinase 13, P13-kinase
y, and P13-
kinase 6.
[00230] In some embodiments, the mTOR inhibitor utilized in the subject
methods inhibits
one of mTORC1 and mTORC2 selectively with an IC50 value of about 1000, 500,
100, 75,
50, 25, 10, 5, 1, or 0.5 nM or less as ascertained in an in vitro kinase. For
example, the
mTOR inhibitor utilized in the subject methods inhibits mTORC1 selectively
with an IC50
value of about 1000, 500, 100, 75, 50, 25, 10, 5, 1, or 0.5 nM or less as
ascertained in an in
vitro kinase. For example, rapamycin and rapamycin derivatives or analogues
have been
shown to primarily inhibit mTORC1 and not mTORC2. Suitable mTORC1 inhibitors
compounds include, for example, sirolimus (rapamycin), deforolimus (AP23573,
MK-8669),
everolimus (RAD-001), temsirolimus (CCI-779), zotarolimus (ABT-578), and
biolimus A9
(umirolimus).
[00231] P13 -kinase a inhibitors or mTOR inhibitors suitable for use in the
subject methods
can be selected from a variety types of molecules. For example, an inhibitor
can be
biological or chemical compound such as a simple or complex organic or
inorganic molecule,
peptide, peptide mimetic, protein (e.g. antibody), liposome, or a
polynucleotide (e.g. small
interfering RNA, microRNA, anti-sense, aptamer, ribozyme, or triple helix).
Some
-55-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
exemplary classes of chemical compounds suitable for use in the subject
methods are detailed
in the sections below.
[00232] The advantages of selective inhibition of a cellular target as a way
of treating a
disease condition mediated by such target are manifold. Because healthy cells
depend on the
signaling pathways that are activated in cancers for survival, inhibition of
these pathways
during cancer treatment can cause harmful side effects. In order for a method
of treating
cancer to be successful without causing excessive damage to healthy cells, a
very high degree
of specificity in targeting the aberrant signaling component or components is
desirable.
Moreover, cancer cells may depend on overactive signaling for their survival
(known as the
oncogene addiction hypothesis). In this way, cancer cells are frequently
observed to adapt to
drug inhibition of an aberrant signaling component by selecting for mutations
in the same
pathway that overcome the effect of the drug. Therefore, cancer therapies may
be more
successful in overcoming the problem of drug resistance if they target a
signaling pathway as
a whole, or target more than one component within a signaling pathway.
[00233] Without being bound by theory, selective inhibition of P13-kinase a
provides a more
targeted treatment to a disease condition mediated by P13-kinase without
disrupting one or
more pathways that are implicated by one or more other type I
phosphatidylinosito1-3-
kinases, namely P13-kinase 13, P13-kinase y, and P13-kinase 6.
[00234] Some signaling pathways that contain PI3K and mTOR are illustrated in
Figure 1.
One major downstream effector of PI3K and mTOR signaling is the Akt
serine/threonine
kinase. Akt possesses a protein domain known as a PH domain, or Pleckstrin
Homology
domain, which binds to phosphoinositides with high affinity. In the case of
the PH domain of
Akt, it binds either PIP3 (phosphatidylinositol (3,4,5)-trisphosphate,
PtdIns(3,4,5)P3) or PIP2
(phosphatidylinositol (3,4)-bisphosphate, PtdIns(3,4)P2). PI3K phosphorylates
PIP2 in
response to signals from chemical messengers, such as ligand binding to G
protein-coupled
receptors or receptor tyrosine kinases. Phosphorylation by PI3K converts PIP2
to PIP3,
recruiting Akt to the cell membrane where it is phosphorylated at serine 473
(S473) by
mTORC2. Phosphorylation of Akt at another site, threonine 308 (T308), is not
directly
dependent on mTORC2, but requires PI3K activity. Therefore, PI3K activity
towards Akt
can be isolated from mTOR activity by examining Akt threonine 308
phosphorylation status
in cells lacking mTORC2 activity.
[00235] The subject methods are useful for treating a disease condition
associated with PI3-
kinase a and/or mTOR. Any disease condition that results directly or
indirectly from an
-56-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
abnormal activity or expression level of P13-kinase a and/or mTOR can be an
intended
disease condition.
[00236] A vast diversity of disease conditions associated with P13-kinase a
and/or mTOR
have been reported. P13-kinase a has been implicated, for example, in a
variety of human
cancers. Angiogenesis has been shown to selectively require the a isoform of
PI3K in the
control of endothelial cell migration. (Graupera et al, Nature 2008;453;662-
6). Mutations in
the gene coding for PI3K a or mutations which lead to upregulation of PI3K a
are believed to
occur in many human cancers such as lung, stomach, endometrial, ovarian,
bladder, breast,
colon, brain and skin cancers. Often, mutations in the gene coding for PI3K a
are point
mutations clustered within several hotspots in helical and kinase domains,
such as E542K,
E545K, and H1047R. Many of these mutations have been shown to be oncogenic
gain-of-
function mutations. Because of the high rate of PI3K a mutations, targeting of
this pathway
provides valuable therapeutic opportunities. While other PI3K isoforms such as
PI3K 6 or
PI3K y are expressed primarily in hematopoietic cells, PI3K a, along with
PI3K13, is
expressed constitutively.
[00237] Disease conditions associated with P13-kinase a and/or mTOR can also
be
characterized by abnormally high level of activity and/or expression of
downstream
messengers of P13-kinase a. For example, proteins or messengers such as PIP2,
PIP3, PDK,
Akt, PTEN, PRAS40, GSK-313, p21, p27 may be present in abnormal amounts which
can be
identified by any assays known in the art.
[00238] Deregulation of the mTOR pathway is emerging as a common theme in
diverse
human diseases and as a consequence drugs that target mTOR have therapeutic
value. The
diseases associated with deregulation of mTORC1 include, but are not limited
to, tuberous
sclerosis complex (TSC) and lymphangioleiomyomatosis (LAM), both of which are
caused
by mutations in TSC1 or TSC2 tumor suppressors. Patients with TSC develop
benign tumors
that when present in brain, however, can cause seizures, mental retardation
and death. LAM
is a serious lung disease. Inhibition of mTORC1 may help patients with Peutz-
Jeghers
cancer-prone syndrome caused by the LKB 1 mutation. mTORC1 may also have role
in the
genesis of sporadic cancers. Inactivation of several tumor suppressors, in
particular PTEN,
p53, VHL and NF1, has been linked to mTORC1 activation. Rapamycin and its
analogues
(e.g. CCI-779, RAD001 and AP23573) inhibit TORC1 and have shown moderate anti-
cancer
activity in phase II clinical trials. However, due to the negative signal from
S6K1 to the
insulin/PI3K/Akt pathway, it is important to note that inhibitors of mTORC1,
like rapalogs,
can activate PKB/Akt. If this effect persists with chronic rap amycin
treatment, it may provide
-57-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
cancer cells with an increased survival signal that may be clinically
undesirable. The
PI3K/Akt pathway is activated in many cancers. Activated Akt regulates cell
survival, cell
proliferation and metabolism by phosphorylating proteins such as BAD, FOXO, NF-
KB,
p21Cipl, p27Kipl, GSK3I3 and others. Aid might also promote cell growth by
phosphorylating TSC2. Akt activation probably promotes cellular transformation
and
resistance to apoptosis by collectively promoting growth, proliferation and
survival, while
inhibiting apoptotic pathways. The combination of an inhibitor of mTORC 1 and
mTORC2
and a P13 -kinase a inhibitor is beneficial for treatment of tumors with
elevated Akt
phosphorylation, and should down-regulate cell growth, cell survival and cell
proliferation.
[00239] Where desired, the subject to be treated is tested prior to treatment
using a
diagnostic assay to determine the sensitivity of tumor cells to a P13 Ku
kinase inhibitor. Any
method known in the art that can determine the sensitivity of the tumor cells
of a subject to a
PI3Ka kinase inhibitor can be employed. Where the subject is tested prior to
treatment using
a diagnostic assay to determine the sensitivity of tumor cells to an P13 Ku
kinase inhibitor, in
one embodiment, when the subject is identified as one whose tumor cells are
predicted to
have low sensitivity to an PI3Ka kinase inhibitor as a single agent, are
likely to display
enhanced sensitivity in the presence of an mTOR inhibitor, or vice versa, when
the subject is
administered, simultaneously or sequentially, a therapeutically effective
amount of a
combination of an PI3Ka kinase inhibitor and an mTOR inhibitor. In another
embodiment,
when the subject is identified as one whose tumor cells are predicted to have
high sensitivity
to an PI3Ka kinase inhibitor as a single agent, but may also display enhanced
sensitivity in
the presence of an mTOR inhibitor based on the results described herein, the
subject is
administered, simultaneously or sequentially, a therapeutically effective
amount of a
combination of an PI3Ka kinase inhibitor and an mTOR inhibitor. In these
methods one or
more additional anti-cancer agents or treatments can be co-administered
simultaneously or
sequentially with the P13 Ku kinase inhibitor and mTOR inhibitor, as judged to
be appropriate
by the administering physician given the prediction of the likely
responsiveness of the subject
to the combination of PI3Ka kinase inhibitor and mTOR inhibitor, in
combination with any
additional circumstances pertaining to the individual subject.
[00240] Accordingly, in some embodiments, the present invention provides for a
method
comprising: (a) determining the presence in a subject of a mutation in P13-
kinase a that is
associated with a disease condition mediated by P13-kinase a; and (b)
administering to said
subject a pharmaceutical composition of the invention.
-58-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00241] In yet another embodiment, the present invention provides for a method
of
inhibiting phosphorylation of both Akt (S473) and Akt (T308) in a cell,
comprising
contacting a cell with an effective amount of a P13-kinase a inhibitor and an
mTOR
inhibitor, biologically active agent that selectively inhibits both mTORC1 and
mTORC2
activity relative to one or more type I phosphatidylinosito1-3-kinases (P13-
kinase) as
ascertained by a cell-based assay or an in vitro kinase assay, wherein the P13-
kinase a
inhibitor exhibits selective inhibition of P13-kinase a relative to one or
more type I
phosphatidylinosito1-3-kinases (P13-kinase) ascertained by an in vitro kinase
assay, wherein
the one or more type I P13-kinase is selected from the group consisting of P13-
kinase 13, P13-
kinase y, and P13-kinase 6.
[00242] The data presented in the Examples herein below demonstrate that the
anti-tumor
effects of a combination of an mTOR inhibitor and PI3K a inhibitor are
superior to the anti-
tumor effects of either inhibitor by itself, and co-administration of an mTOR
inhibitor with a
PI3K a inhibitor can be effective for treatment of a neoplastic condition
associated with P13-
kinase a and/or mTOR. Non-limiting examples of such conditions include but are
not limited
to Acanthoma, Acinic cell carcinoma, Acoustic neuroma, Acral lentiginous
melanoma,
Acrospiroma, Acute eosinophilic leukemia, Acute lymphoblastic leukemia, Acute
megakaryoblastic leukemia, Acute monocytic leukemia, Acute myeloblastic
leukemia with
maturation, Acute myeloid dendritic cell leukemia, Acute myeloid leukemia,
Acute
promyelocytic leukemia, Adamantinoma, Adeno carcinoma, Adenoid cystic
carcinoma,
Adenoma, Adenomatoid odontogenic tumor, Adrenocortical carcinoma, Adult T-cell
leukemia, Aggressive NK-cell leukemia, AIDS-Related Cancers, AIDS-related
lymphoma,
Alveolar soft part sarcoma, Ameloblastic fibroma, Anal cancer, Anaplastic
large cell
lymphoma, Anaplastic thyroid cancer, Angioimmunoblastic T-cell lymphoma,
Angiomyolipoma, Angiosarcoma, Appendix cancer, Astrocytoma, Atypical teratoid
rhabdoid
tumor, Basal cell carcinoma, Basal-like carcinoma, B-cell leukemia, B-cell
lymphoma,
Bellini duct carcinoma, Biliary tract cancer, Bladder cancer, Blastoma, Bone
Cancer, Bone
tumor, Brain Stem Glioma, Brain Tumor, Breast Cancer, Brenner tumor, Bronchial
Tumor,
Bronchioloalveolar carcinoma, Brown tumor, Burkitt's lymphoma, Cancer of
Unknown
Primary Site, Carcinoid Tumor, Carcinoma, Carcinoma in situ, Carcinoma of the
penis,
Carcinoma of Unknown Primary Site, Carcinosarcoma, Castleman's Disease,
Central
Nervous System Embryonal Tumor, Cerebellar Astrocytoma, Cerebral Astrocytoma,
Cervical
Cancer, Cholangiocarcinoma, Chondroma, Chondrosarcoma, Chordoma,
Choriocarcinoma,
Choroid plexus papilloma, Chronic Lymphocytic Leukemia, Chronic monocytic
leukemia,
-59-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Chronic myelogenous leukemia, Chronic Myeloproliferative Disorder, Chronic
neutrophilic
leukemia, Clear-cell tumor, Colon Cancer, Colorectal cancer,
Craniopharyngioma, Cutaneous
T-cell lymphoma, Degos disease, Dermatofibrosarcoma protuberans, Dermoid cyst,
Desmoplastic small round cell tumor, Diffuse large B cell lymphoma,
Dysembryoplastic
neuroepithelial tumor, Embryonal carcinoma, Endodermal sinus tumor,
Endometrial cancer,
Endometrial Uterine Cancer, Endometrioid tumor, Enteropathy-associated T-cell
lymphoma,
Ependymoblastoma, Ependymoma, Epithelioid sarcoma, Erythroleukemia, Esophageal
cancer, Esthesioneuroblastoma, Ewing Family of Tumor, Ewing Family Sarcoma,
Ewing's
sarcoma, Extracranial Germ Cell Tumor, Extragonadal Germ Cell Tumor,
Extrahepatic Bile
Duct Cancer, Extramammary Paget's disease, Fallopian tube cancer, Fetus in
fetu, Fibroma,
Fibrosarcoma, Follicular lymphoma, Follicular thyroid cancer, Gallbladder
Cancer,
Gallbladder cancer, Ganglioglioma, Ganglioneuroma, Gastric Cancer, Gastric
lymphoma,
Gastrointestinal cancer, Gastrointestinal Carcinoid Tumor, Gastrointestinal
Stromal Tumor,
Gastrointestinal stromal tumor, Germ cell tumor, Germinoma, Gestational
choriocarcinoma,
Gestational Trophoblastic Tumor, Giant cell tumor of bone, Glioblastoma
multiforme,
Glioma, Gliomatosis cerebri, Glomus tumor, Glucagonoma, Gonadoblastoma,
Granulosa cell
tumor, Hairy Cell Leukemia, Hairy cell leukemia, Head and Neck Cancer, Head
and neck
cancer, Heart cancer, Hemangioblastoma, Hemangiopericytoma, Hemangiosarcoma,
Hematological malignancy, Hepatocellular carcinoma, Hepatosplenic T-cell
lymphoma,
Hereditary breast-ovarian cancer syndrome, Hodgkin Lymphoma, Hodgkin's
lymphoma,
Hypopharyngeal Cancer, Hypothalamic Glioma, Inflammatory breast cancer,
Intraocular
Melanoma, Islet cell carcinoma, Islet Cell Tumor, Juvenile myelomonocytic
leukemia,
Kaposi Sarcoma, Kaposi's sarcoma, Kidney Cancer, Klatskin tumor, Krukenberg
tumor,
Laryngeal Cancer, Laryngeal cancer, Lentigo maligna melanoma, Leukemia,
Leukemia, Lip
and Oral Cavity Cancer, Liposarcoma, Lung cancer, Luteoma, Lymphangioma,
Lymphangiosarcoma, Lymphoepithelioma, Lymphoid leukemia, Lymphoma,
Macroglobulinemia, Malignant Fibrous Histiocytoma, Malignant fibrous
histiocytoma,
Malignant Fibrous Histiocytoma of Bone, Malignant Glioma, Malignant
Mesothelioma,
Malignant peripheral nerve sheath tumor, Malignant rhabdoid tumor, Malignant
triton tumor,
MALT lymphoma, Mantle cell lymphoma, Mast cell leukemia, Mediastinal germ cell
tumor,
Mediastinal tumor, Medullary thyroid cancer, Medulloblastoma,
Medulloepithelioma,
Melanoma, Meningioma, Merkel Cell Carcinoma, Mesothelioma, Metastatic Squamous
Neck
Cancer with Occult Primary, Metastatic urothelial carcinoma, Mixed Mullerian
tumor,
Monocytic leukemia, Mouth Cancer, Mucinous tumor, Multiple Endocrine Neoplasia
-60-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Syndrome, Multiple myeloma, Mycosis Fungoides, Myelodysplastic Disease,
Myelodysplastic Syndromes, Myeloid leukemia, Myeloid sarcoma,
Myeloproliferative
Disease, Myxoma, Nasal Cavity Cancer, Nasopharyngeal Cancer, Nasopharyngeal
carcinoma, Neoplasm, Neurinoma, Neuroblastoma, Neurofibroma, Neuroma, Nodular
melanoma, Non-Hodgkin Lymphoma, Non-Hodgkin lymphoma, Nonmelanoma Skin Cancer,
Non-Small Cell Lung Cancer, Ocular oncology, Oligoastrocytoma,
Oligodendroglioma,
Oncocytoma, Optic nerve sheath meningioma, Oral Cancer, Oral cancer,
Oropharyngeal
Cancer, Osteosarcoma, Ovarian Cancer, Ovarian cancer, Ovarian Epithelial
Cancer, Ovarian
Germ Cell Tumor, Ovarian Low Malignant Potential Tumor, Paget's disease of the
breast,
Pancoast tumor, Pancreatic Cancer, Pancreatic cancer, Papillary thyroid
cancer,
Papillomatosis, Paraganglioma, Paranasal Sinus Cancer, Parathyroid Cancer,
Penile Cancer,
Perivascular epithelioid cell tumor, Pharyngeal Cancer, Pheochromocytoma,
Pineal
Parenchymal Tumor of Intermediate Differentiation, Pineoblastoma, Pituicytoma,
Pituitary
adenoma, Pituitary tumor, Plasma Cell Neoplasm, Pleuropulmonary blastoma,
Polyembryoma, Precursor T-lymphoblastic lymphoma, Primary central nervous
system
lymphoma, Primary effusion lymphoma, Primary Hepatocellular Cancer, Primary
Liver
Cancer, Primary peritoneal cancer, Primitive neuroectodermal tumor, Prostate
cancer,
Pseudomyxoma peritonei, Rectal Cancer, Renal cell carcinoma, Respiratory Tract
Carcinoma
Involving the NUT Gene on Chromosome 15, Retinoblastoma, Rhabdomyoma,
Rhabdomyosarcoma, Richter's transformation, Sacrococcygeal teratoma, Salivary
Gland
Cancer, Sarcoma, Schwannomatosis, Sebaceous gland carcinoma, Secondary
neoplasm,
Seminoma, Serous tumor, Sertoli-Leydig cell tumor, Sex cord-stromal tumor,
Sezary
Syndrome, Signet ring cell carcinoma, Skin Cancer, Small blue round cell
tumor, Small cell
carcinoma, Small Cell Lung Cancer, Small cell lymphoma, Small intestine
cancer, Soft tissue
sarcoma, Somatostatinoma, Soot wart, Spinal Cord Tumor, Spinal tumor, Splenic
marginal
zone lymphoma, Squamous cell carcinoma, Stomach cancer, Superficial spreading
melanoma, Supratentorial Primitive Neuroectodermal Tumor, Surface epithelial-
stromal
tumor, Synovial sarcoma, T-cell acute lymphoblastic leukemia, T-cell large
granular
lymphocyte leukemia, T-cell leukemia, T-cell lymphoma, T-cell prolymphocytic
leukemia,
Teratoma, Terminal lymphatic cancer, Testicular cancer, Thecoma, Throat
Cancer, Thymic
Carcinoma, Thymoma, Thyroid cancer, Transitional Cell Cancer of Renal Pelvis
and Ureter,
Transitional cell carcinoma, Urachal cancer, Urethral cancer, Urogenital
neoplasm, Uterine
sarcoma, Uveal melanoma, Vaginal Cancer, Verner Morrison syndrome, Verrucous
-61-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
carcinoma, Visual Pathway Glioma, Vulvar Cancer, Waldenstrom's
macroglobulinemia,
Warthin's tumor, Wilms' tumor, or any combination thereof
[00243] In other embodiments, the methods of using a PI3Ka inhibitor and an
mTOR
inhibitor described herein are applied to the treatment of heart conditions
including
atherosclerosis, heart hypertrophy, cardiac myocyte dysfunction, elevated
blood pressure and
vasoconstriction. The invention also relates to a method of treating diseases
related to
vasculogenesis or angiogenesis in a mammal that comprises administering to
said mammal a
therapeutically effective amount of a PI3Ka inhibitor and an mTOR inhibitor of
the present
invention, or any pharmaceutically acceptable salt, ester, prodrug, solvate,
hydrate or
derivative thereof.
[00244] In some embodiments, said method is for treating a disease selected
from the group
consisting of tumor angiogenesis, chronic inflammatory disease such as
rheumatoid arthritis,
atherosclerosis, inflammatory bowel disease, skin diseases such as psoriasis,
eczema, and
scleroderma, diabetes, diabetic retinopathy, retinopathy of prematurity, age-
related macular
degeneration, hemangioma, glioma, melanoma, Kaposi's sarcoma and ovarian,
breast, lung,
pancreatic, prostate, colon and epidermoid cancer.
[00245] In some embodiments, the invention provides for the use of a P13 Ku
inhibitor and
an mTOR inhibitor for treating a disease condition associated with P13-kinase
a and/or
mTOR, including, but not limited to, conditions related to an undesirable,
over-active,
harmful or deleterious immune response in a mammal, collectively termed
"autoimmune
disease." Autoimmune disorders include, but are not limited to, Crohn's
disease, ulcerative
colitis, psoriasis, psoriatic arthritis, juvenile arthritis and ankylosing
spondilitis, Other non-
limiting examples of autoimmune disorders include autoimmune diabetes,
multiple sclerosis,
systemic lupus erythematosus (SLE), rheumatoid spondylitis, gouty arthritis,
allergy,
autoimmune uveitis, nephrotic syndrome, multisystem autoimmune diseases,
autoimmune
hearing loss, adult respiratory distress syndrome, shock lung, chronic
pulmonary
inflammatory disease, pulmonary sarcoidosis, pulmonary fibrosis, silicosis,
idiopathic
interstitial lung disease, chronic obstructive pulmonary disease, asthma,
restenosis,
spondyloarthropathies, Reiter's syndrome, autoimmune hepatitis, inflammatory
skin
disorders, vasculitis oflarge vessels, medium vessels or small vessels,
endometriosis,
prostatitis and Sjogren's syndrome. Undesirable immune response can also be
associated
with or result in, e.g., asthma, emphysema, bronchitis, psoriasis, allergy,
anaphylaxsis, auto-
immune diseases, rheumatoid arthritis, graft versus host disease,
transplantation rejection,
lung injuries, and lupus erythematosus. The pharmaceutical compositions of the
present
-62-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
invention can be used to treat other respiratory diseases including but not
limited to diseases
affecting the lobes of lung, pleural cavity, bronchial tubes, trachea, upper
respiratory tract, or
the nerves and muscle for breathing. The compositions of the invention can be
further used to
treat multiorgan failure.
[00246] The invention also provides methods of using a P13 Ku inhibitor and an
mTOR
inhibitor for the treatment of liver diseases (including diabetes),
pancreatitis or kidney disease
(including proliferative glomerulonephritis and diabetes- induced renal
disease) or pain in a
mammal.
[00247] The invention also provides a method of using a PI3Ka inhibitor and an
mTOR
inhibitor for the treatment of sperm motility. The invention further provides
a method of
using a PI3Ka inhibitor and an mTOR inhibitor for the treatment of
neurological or
neurodegenerative diseases including, but not limited to, Alzheimer's disease,
Huntington's
disease, CNS trauma, and stroke.
[00248] The invention further provides a method of using a P13 Ku inhibitor
and an mTOR
inhibitor for the prevention of blastocyte implantation in a mammal.
[00249] The invention also relates to a method of using a PI3Ka inhibitor and
an mTOR
inhibitor for treating a disease related to vasculogenesis or angiogenesis in
a mammal which
can manifest as tumor angiogenesis, chronic inflammatory disease such as
rheumatoid
arthritis, inflammatory bowel disease, atherosclerosis, skin diseases such as
psoriasis,
eczema, and scleroderma, diabetes, diabetic retinopathy, retinopathy of
prematurity, age-
related macular degeneration, hemangioma, glioma, melanoma, Kaposi's sarcoma
and
ovarian, breast, lung, pancreatic, prostate, colon and epidermoid cancer.
[00250] The invention further provides a method of using a P13 Ku inhibitor
and an mTOR
inhibitor for the treatment of disorders involving platelet aggregation or
platelet adhesion,
including but not limited to Bernard-Soulier syndrome, Glanzmann's
thrombasthenia, Scott's
syndrome, von Willebrand disease, Hermansky-Pudlak Syndrome, and Gray platelet
syndrome.
[00251] In some embodiments, methods of using a PI3Ka inhibitor and an mTOR
inhibitor
are provided for treating a disease which is skeletal muscle atrophy, skeletal
muscle
hypertrophy, leukocyte recruitment in cancer tissue, invasion metastasis,
melanoma, Kaposi's
sarcoma, acute and chronic bacterial and viral infections, sepsis, glomerulo
sclerosis,
glomerulo, nephritis, or progressive renal fibrosis.
[00252] Certain embodiments contemplate a human subject such as a subject that
has been
diagnosed as having or being at risk for developing or acquiring a disease
condition
-63-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
associated with P13-kinase a and/or mTOR. Certain other embodiments
contemplate a non-
human subject, for example a non-human primate such as a macaque, chimpanzee,
gorilla,
vervet, orangutan, baboon or other non-human primate, including such non-human
subjects
that can be known to the art as preclinical models, including preclinical
models for
inflammatory disorders. Certain other embodiments contemplate a non-human
subject that is
a mammal, for example, a mouse, rat, rabbit, pig, sheep, horse, bovine, goat,
gerbil, hamster,
guinea pig or other mammal. There are also contemplated other embodiments in
which the
subject or biological source can be a non-mammalian vertebrate, for example,
another higher
vertebrate, or an avian, amphibian or reptilian species, or another subject or
biological source.
In certain embodiments of the present invention, a transgenic animal is
utilized. A transgenic
animal is a non-human animal in which one or more of the cells of the animal
includes a
nucleic acid that is non-endogenous (i.e., heterologous) and is present as an
extrachromosomal element in a portion of its cell or stably integrated into
its germ line DNA
(i.e., in the genomic sequence of most or all of its cells).
Exemplary mTor Inhibitor Compounds
[00253] In one aspect, the invention provides a compound which is an inhibitor
of mTor of
the Formula I:
R31 R32
Ni/
M1
N
0 Taxi
X5, X3..
X4 X2
R1
Formula I
or a pharmaceutically acceptable salt thereof, wherein:
Xi is N or C-E1, X2 is N or C, X3 is N or C, X4 is C-R9 or N, X5 is N or C-E1,
X6 is C or N,
and X7 is C or N; and wherein no more than two nitrogen ring atoms are
adjacent;
Ri is H, ¨L-Ci_ioalkyl, -L-C3_8cycloalkyl, -L-Ci_ioalkyl -C3_8cycloalkyl, -L-
aryl, -L-
hetero aryl, -L-C iioalkylaryl, -L- C i_ioalkylhetaryl, -L- C
iioalkylheterocylyl, -L-C2_101kenyl,
-L-C2_10alkynyl, -L-C 2_1 Oalkenyl-C 3_8cycloalkyl, -L-C 2_1 oalkynyl-C
3_8cycloalkyl, -L-
heteroalkyl, -L-heteroalkylaryl, -L-heteroalkylheteroaryl, -L-heteroalkyl-
heterocylyl, -L-
heteroalkyl-C3_8cycloalkyl, -L-aralkyl, -L-heteroaralkyl, or -L-heterocyclyl,
each of which is
unsubstituted or is substituted by one or more independent R3;
-64-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
L is absent, -(C=0)-, -C(=0)0-, -C(=0) N(R31)-5-S-, -S(0)-, -S(0)2-, -
S(0)2N(R31)-, or -
N(R31)-;
E1 and E2 are independently -(Wi)j -R4;
M1 is a 5, 6, 7, 8, 9, or-10 membered ring system, wherein the ring system is
monocyclic or
bicyclic, substituted with R5 and additionally optionally substituted with one
or more -(W2)k
-R2;
each k is 0 or 1;
j in E1 or j in E2, is independently 0 or 1;
W1 is -0-, -NR7-, -S(0)0_2-5-C(0)-5-C(0)N(R7)-, -N(W)C(0)-, -N(W)S(0)-,-
N(W)S(0)2-, -C(0)0-, -CH(R7)N(C(0)0R8)-, -CH(R7)N(C(0)R8)-, -CH(R7)N(S02R8)-,
-CH(R7)N(R8)-, -CH(R7)C(0)N(R8)-, -CH(R7)N(R8)C(0)-, -CH(R7)N(R8)S(0)-, or -
CH(R7)N(R8)S(0)2-;
W2 is -0-, -NR7-, -S(0)0_2-5-C(0)-5-C(0)N(R7)-, -N(R7)C(0)-, -N(R7)C(0)N(R8)-,-
N(R7)S(0)-, -N(R7)S(0)2-,-C(0)0-, -CH(R7)N(C(0)0R8)-, -CH(R7)N(C(0)R8)-, -
CH(R7)N(S02R8)-, -CH(R7)N(R8)-, -CH(R7)C(0)N(R8)-, -CH(R7)N(R8)C(0)-, -
CH(R7)N(R8)S(0)-, or -CH(R7)N(R8)S(0)2-;
R2 is hydrogen, halogen, -OH, -R31, -CF3, -0CF3, -0R31, -NR31R32, -NR34R35, -
C(0)R31, -
CO2R31, -C(=0)NR31R325 -C(=0)NR34R355 -NO2, -CN, -S(0)0_2R31, -SO2NR31R325 -
S02NR34R355 -NR31C(=0)R325 -NR31C(=0)0R325 -NR31C(=0)NR32R335 -NR31S(0)0_2R325
-
C(=S)0R315 -C(=0)SR315 -NR31C(=NR32)NR33R325 -NR31C(=NR32)0R335 -
NR31C(=NR32)SR335 -0C(=0)0R335 -0C(=0)NR31R325 -0C(=0)SR315 -SC(=0)0R315 -
P(0)0R310R325 -SC(=0)NR31R32 5 aryl (e.g. bicyclic aryl, unsubstituted aryl,
or substituted
monocyclic aryl), hetaryl, C iioalkyl, C3_8cycloalkyl, Ci_malkyl-
C3_8cycloalkyl, C3_8cycloalkyl
-Ci_ioalkyl, C3_8cycloalkyl -C2_10alkenyl, C3_8cycloalkyl- C2_10alkynyl,
C2_10alkenyl,
C2_10alkynyl, Ci_malkylaryl (e.g. C240alkyl-monocyclic aryl, Ci_malkyl-
substituted
monocyclic aryl, or Ci_malkylbicycloary1), Ci_ioalkylhetaryl,
Ci_ioalkylheterocyclyl, C2_
1 alkenyl, C2_1 alkynyl, C2_1 alkenyl -Ci_i 0 a 1 ky 1 , C2_1 alkynyl -Ci_i 0
a 1 ky 1 , C2_1 alkenylaryl, C2
loalkenylhetaryl, C2_10alkenylheteroalkyl, C2-loalkenylheterocycicyl,
C2_10alkenyl-C3_
8cycloalkyl, C2_10alkynylaryl, C2_10alkynylhetaryl, C2_ioalkynylheteroalkyl,
C2
ioalkynylheterocylyl, C2_10alkynyl-C3_8cycloalkenyl, Ci_malkoxy C iioaIkyl, C
ioalkenyl, Ci_loalkoxy-C2_10alkynyl, heterocyclyl, heteroalkyl, heterocyclyl -
C iioalkyl,
heterocyclyl-C240alkenyl, heterocyclyl-C240alkynyl, aryl- Ci_i0alkyl (e.g.
monocyclic aryl-C2
ioalkyl, substituted monocyclic aryl- Ci_malkyl, or bicycloaryl--Ci_malkyl),
aryl-C2_10alkenyl,
aryl-C2_10alkynyl, aryl-heterocyclyl, hetaryl-Ci_i0alkyl, hetaryl-
C2_10alkenyl, hetaryl-C 2-
-65-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
malkynyl, hetaryl-C3_8cycloalkyl, hetaryl-heteroalkyl, or hetaryl-
heterocyclyl, wherein each
of said bicyclic aryl or heteroaryl moiety is unsubstituted, or wherein each
of bicyclic aryl,
heteroaryl moiety or monocyclic aryl moiety is substituted with one or more
independent
alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl,
arylalkyl, heteroaryl,
heteroarylalkyl, halo, -OH, -R315 -CF3, -0CF35 -0R315 -NR31R325 -NR34R355 -
C(0)R315 -
CO2R315 -C(=0)NR31R325 -C(=0)NR34R355 -NO2, -CN, -S(0)0_2R315 -SO2NR31R325 -
SO2NR34R355 -NR31C(=0)R325 -NR31C(=0)0R325 -NR31C(=0)NR32R335 -NR31S(0)0_2R325
-
C(=S)0R315 -C(=0)SR315 -NR31C(=NR32)NR33R325 -NR31C(=NR32)0R335 -
NR31C(=NR32)SR335 -0C(=0)0R335 -0C(=0)NR31R325 -0C(=0)SR315 -SC(=0)0R315 -
P(0)0R310R325 or-SC(=0)NR31R325 and wherein each of said alkyl, cycloalkyl,
heterocyclyl,
or heteroalkyl moiety is unsubstituted or is substituted with one or more
alkyl, heteroalkyl,
alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl,
heteroarylalkyl,
halo, -OH, -R315 -CF3, -0CF35 -0R315 -0-aryl, -NR31R325 -NR34R35 5-C(0)R315 -
CO2R315 -
C(=0)NR34R355 or -C(=0)NR31R32;
R3 and R4 are independently hydrogen, halogen, -OH, -R315 -CF3, -0CF35 -0R315 -
NR31R325
-NR34R355 -C(0)R315 -0O2R315 -C(=0)NR31R325 -C(=0)NR34R355 -NO2, -CN, -
S(0)0_2R315
-SO2NR31R325 -S02NR34R355 -NR31C(=0)R325 -NR31C(=0)0R325 -NR31C(=0)NR32R335 -
NR31S(0)0_2R325 -C(=S)0R315 -C(=0)SR315 -NR31C(=NR32)NR33R325 -
NR31C(=NR32)0R335
-NR31C(=NR32)SR335 -0C(=0)0R335 -0C(=0)NR31R325 -0C(=0)SR315 -SC(=0)0R315 -
P(0)0R310R325 -SC(=0)NR31R32 5 aryl, hetaryl, Ci_Ltalkyl, Ci_ioalkyl,
C3_8cycloalkyl, Ci-
ioalkyl-C3-8cycloalkyl, C3_8cycloalkyl -Ci_iOalkyl, C3_8cycloalkyl -C2_ 1 0
alkenyl, C3_
8cYcloalkyl- C2_10alkynyl, Ci_ioalkyl- C2_10alkenyl, Ci_ioalkyl- C2_10alkynyl,
Ci_ioalkylaryl, C1-
malkylhetaryl, Ci_loalkylheterocyclyl, C2_10alkenyl, C2_10alkynyl,
C2_10alkenyl -Ci_ioalkyl, C2-
10alkynyl -Ci_ioalkyl, C2_10alkenylaryl, C2_10alkenylhetaryl,
C2_10alkenylheteroalkyl, C2-
10alkenylheterocycicyl, C2_10alkenyl-C3-8cycloalkyl, C2_ioalkynyl-
C3_8cycloalkyl, C2-
ioalkynylaryl, C2_10alkynylhetaryl, C2_ioalkynylheteroalkyl,
C2_10alkynylheterocylyl, C2_
malkynyl-C3_8cycloalkenyl, Ci_ioalkoxy Ci_ioalkyl, Ci_loalkoxy-C2_10alkenyl,
Ci_loalkoxy-C2-
10alkynyl, heterocyclyl, heterocyclyl -Ci_ioalkyl, heterocyclyl-C2_10alkenyl,
heterocyclyl-C2_
loalkYnY15 aryl- Ci_ioalkyl, aryl-C2_10alkenyl, aryl-C2_10alkynyl, aryl-
heterocyclyl, hetaryl-C1-
ioalkyl, hetaryl-C2_10alkenyl, hetaryl-C2_ioalkynyl, hetaryl-C3_8cycloalkyl,
heteroalkyl,
hetaryl-heteroalkyl, or hetaryl-heterocyclyl, wherein each of said aryl or
heteroaryl moiety is
unsubstituted or is substituted with one or more independent halo, -OH, -R315 -
CF3, -0CF35
-0R315 -NR31R325 -NR34R355 -C(0)R315 -0O2R315 -C(=0)NR31R325 -C(=0)NR34R355 -
NO2, -
CN, -S(0)0_2R315 -SO2NR31R325 -S02NR34R355 -NR31C(=0)R325 -NR31C(=0)0R325 -
-66-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
NR31C(=0)NR32R33, -NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -
NR31C(=NR32)NR33R32,
-NR31C(=NR32)0R33, -NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -
0C(=0)SR31, -SC(=0)0R31, -P(0)0R310R32, or-SC(=0)NR31R32, and wherein each of
said
alkyl, cycloalkyl, heterocyclyl, or heteroalkyl moiety is unsubstituted or is
substituted with
one or more halo, -OH, -R31, -CF3, -0CF3, -0R31, -0-aryl, -NR31R32, -NR34R35 ,-
C(0)R31,
-CO2R31, -C(=0)NR34R35, or -C(=0)NR31R32;
R5 is hydrogen, halogen, -OH, -R31, -CF3, -0CF3, -0R31, -NR31R32, -NR34R35, -
C(0)R31, -
CO2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN, -S(0)0_2R31, -SO2NR31R32, -
S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R33, -NR31S(0)0_2R32,
-
C(=S)0R31, -C(=0)SR31, -NR31C(=NR32)NR33R32, -NR31C(=NR32)0R33, -
NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -0C(=0)SR31, -SC(=0)0R31, -
P(0)0R310R32,or -SC(=0)NR31R32;
each of R31, R32, and R33 is independently H or Ci_malkyl , wherein the
Ci_i0alkyl is
unsubstituted or is substituted with one or more aryl, heteroalkyl,
heterocyclyl, or hetaryl
group, wherein each of said aryl, heteroalkyl, heterocyclyl, or hetaryl group
is unsubstituted
or is substituted with one or more halo, -OH, - Ci_malkyl, -CF3, -0-aryl, -
0CF3, -0C1_
ioalkyl, -NH2, - N(Ci_i0alkyl)(Ci_10alkyl), - NH(Ci_i0alkyl), - NH( aryl), -
NR34R35, -
C(0)(Ci_malkyl), -C(0)(Ci_malkyl-ary1), -C(0)(ary1), -0O2-Ci_malkyl, -0O2-
Ci_malkylaryl,
-0O2-aryl, -C(=0)N(Ci_malkyl)( Ci_ioalkyl), -C(=0)NH( Ci_malkyl), -
C(0)NR34R35, -
C(=0)NH2, -0CF3, -0(Ci_malkyl), -0-aryl, -N(ary1)( Ci_ioalkyl), -NO2, -CN, -
S(0)0_2 Ci-
ioalkyl, -S(0)0_2 Ci_malkylaryl, -S(0)0_2 aryl, -SO2N(ary1), -SO2
N(Ci_malkyl)( Ci_malkyl), -
SO2 NH(Ci_malkyl) or -S02NR34R35;
R34 and R35 in -NR34R35, -C(=0)NR34R35, or -S02NR34R35, are taken together
with the
nitrogen atom to which they are attached to form a 3-10 membered saturated or
unsaturated
ring; wherein said ring is independently unsubstituted or is substituted by
one or more -
NR31R32, hydroxyl, halogen, oxo, aryl, hetaryl, Ci_6alkyl, or 0-aryl, and
wherein said 3-10
membered saturated or unsaturated ring independently contains 0, 1, or 2 more
heteroatoms
in addition to the nitrogen atom;
each of R7 and R8 is independently hydrogen, Ci_malkyl, C2_10alkenyl, aryl,
heteroaryl,
heterocyclyl or C340cycloalkyl, each of which except for hydrogen is
unsubstituted or is
substituted by one or more independent R6;
R6 is halo, -0R31, -SH, -NH2, -NR34R35, - NR31R32, -0O2R31, -0O2aryl, -
C(0)NR31R32,
C(=0)NR34R35 , -NO2, -CN, -S(0) 0_2 Ci_i0alkyl, -5(0) 0_2aryl, -502NR34R35, -
502NR31R32,
Ci_malkyl, C2_10alkenyl, C2_10alkynyl; aryl-Ci_malkyl, aryl-C240alkenyl, aryl-
C2_10alkynyl,
-67-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
hetaryl-Ci_i0alkyl, hetaryl-C 2_10 alkenyl, hetaryl-C 2_1 0 alkynyl, wherein
each of said alkyl,
alkenyl, alkynyl, aryl, heteroalkyl, heterocyclyl, or hetaryl group is
unsubstituted or is
substituted with one or more independent halo, cyano, nitro, -0Ci_i0alkyl,
Ci_malkyl, C 2-
malkenyl, C2_10alkynyl, haloCi_malkyl, haloC2_10alkenyl, haloC2_10alkynyl, -
COOH, -
C(=0)NR31R32, -C(=0)NR34R" , -S02NR34R35, -SO2 NR31R32, -NR31R32, or -NR34R35;
and
R9 is H, halo, -0R31, -SH, -NH2, -NR34R35 , - NR31R32, -0O2R31, -0O2aryl, -
C(=0)NR31R32, C(=0)NR34R35 , -NO2, -CN, -S(0)0-2 Ci_i0alkyl, -S(0) 0_2aryl, -
S02NR34R35, -S02NR31R32, Ci_malkyl, C2_10alkenyl, C2_10alkynyl; aryl-
Ci_malkyl, aryl-C 2-
1 0 alkenyl, aryl-C 2_1 0 alkynyl, hetaryl-Ci_ioalkyl, hetaryl-C 2_1 0
alkenyl, hetaryl-C 2_1 0 alkynyl,
wherein each of said alkyl, alkenyl, alkynyl, aryl, heteroalkyl, heterocyclyl,
or hetaryl group
is unsubstituted or is substituted with one or more independent halo, cyano,
nitro, -0C1-
ioalkyl, Ci_ioalkyl, C2_10alkenyl, C2_10alkynyl, haloCi_malkyl,
haloC2_10alkenyl, haloC2-
ioalkynyl, -COOH, -C(=0)NR31R32, -C(=0)NR34R35 , -S02NR34R35, -SO2 NR31R32, -
NR31R32, or -NR34R35.
[00254] M1 is a 5, 6, 7, 8, 9, or-10 membered ring system, wherein the ring
system is
monocyclic or bicyclic. The monocyclic M1 ring is unsubstituted or substituted
with one or
more R5 substituents (including 0, 1, 2, 3, 4, or 5 R5 substituents). In some
embodiments, the
monocyclic M1 ring is aromatic (including phenyl) or heteroaromatic (including
but not
limited to pyridinyl, pyrrolyl, imidazolyl, thiazolyl, or pyrimidinyl) . The
monocyclic M1 ring
may be a 5 or 6 membered ring (including but not limited to pyridinyl,
pyrrolyl, imidazolyl,
thiazolyl, or pyrimidinyl). In some embodiments, M2 is a five membered
heteroaromatic
group with one heteroatom, wherein the heteroatom is N, S, or 0. In another
embodiment,
M2 is a five membered heteroaromatic group with two heteroatoms, wherein the
heteroatoms
are nitrogen and oxygen or nitrogen and sulfur.
[00255] The bicyclic M1 ring is unsubstituted or substituted with one or more
R5 substituents
(including 0, 1, 2, 3, 4, 5, 6 or 7 R5 substituents). Bicyclic M1 ring is a 7,
8, 9, or 10
membered aromatic or heteroaromatic. Examples of an aromatic bicyclic M1 ring
include
naphthyl. In other embodiments the bicyclic M1 ring is heteroaromatic and
includes but is
not limited to benzothiazolyl, quinolinyl, quinazolinyl, benzoxazolyl, and
benzimidazolyl.
[00256] The invention also provides compounds wherein M1 is a moiety having a
structure
of Formula M1 -A or Formula M1 -B:
-68-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
uti \/\/2'%/1/ ----W4 W
2, ___ \A/8
v vi1 ' v v 3 \ W ' W
W5 11 3 \\
, W9
..-....%---... /
µ444- W6
-- 7 W7 W10
Formula M 1 -A Formula Ml-B
wherein Wi, W2, and W7 are independently N or C-R5; W4 and Wio are
independently N-R5,
0, or S; W6 and Wg are independently N or C-R5; W5 and W9 are independently N
or C-R2;
and W3 is C or N, provided no more than two N and/or N-R5 are adjacent and no
two 0 or S
are adjacent.
[00257] In some embodiments of the invention, the M1 moiety of Formula Ml-A is
a moiety
of Formula M 1 -A 1 , Formula M 1 -A2, Formula M 1 -A3 , or Formula M 1 -A4 :
R5
R5
R5 W4W 5
R5 40 w4
R5 40w4v -,..õ--....--- ¨4
W5 5
// W _________________________________________ R2 R2 ,zz(-----
-w
= w6
R5 R5 R5 R5
Formula Ml-Al Formula Ml-A2 Formula M 1 -A3 Formula M 1 -A4
wherein W4 is N-R5, 0, or S; W6 is N or C-R5 and W5 is N or C-R2.
[00258] Some nonlimiting examples of the M1 moiety of Formula Ml-A include:
R2 R2 ---R2
S----(w2)k fw2)k tw2)k
(R5)n, \ \ N N (R5)n, \ \ (R5)n, \ 1
N
wherein R5 is ¨(Wl)k ¨R53 or R55; each k is independently 0 or 1, n is 0, 1,
2, or 3, and ¨
(Wl)k ¨R53 and R55 are as defined above.
[00259] In other embodiments of the invention, the M1 moiety of Formula Ml-B
is a moiety
of Formula M 1 -B 1 , Formula M 1 -B2, Formula M 1 -B3 , or Formula M 1 -B4 :
R5
R5
R¨W8 R5 40 W
R
- 8 - N..,..,.\.:,.....,--W R5 8 40 w81
'W5
/ 'W
v .5 _________________________________________ R2 R2
=
R5 R5 R5 R5
Formula M 1 -B 1 Formula M 1 -B2 Formula Ml-B3
Formula Ml-B4
wherein Wio is N-R5, 0, or S, Wg is N or C-R5, and W5 is N or C-R2.
-69-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00260] Some nonlimiting examples of the M1 moiety of Formula M1 -B include:
,w2 ) .
' N_-_<` (w2),
<` ' N.--<` tw2
N- \,
... '
(R5)n= S (R5)n= 0 (R5)nfa NH
wherein R'5 is ¨(Wl)k ¨R53 or R55; k is 0 or 1, n i sO, 1, 2, or 3, and ¨(Wl)k
¨R53 and R55 are
as defined above.
[00261] The invention also provides compounds wherein M1 is a moiety having a
structure
of Formula M1 -C or Formula Mi-D:
,(
Wi 1 W12 W12
wi 13
\ / W \
I '713
W16 -;14
'5/. ---->W14
W15 W15
Formula M 1 -C Formula M1 -D
wherein W125 W135 W145 and Wi5 are independently N or C-R5; Wii and Wig are
independently N-R5, 0, or S; Wi6 and Wi7 are independently N or C-R5; provided
no more
than two N are adjacent.
[00262] In other embodiments of the invention, the Mi moiety of Formula M1 -C
or Formula
M1 -D is a moiety of Formula M1 -C1 or Formula M1 -D1 :
R5 R5
71 8 R5 .i W11 4110 R5
Wx
R5 W16 R5
R5 R5
=
Formula Mi-Di Formula M 1 -C 1
wherein Wii and W18 are N-R5, 0, or S; and Wi6 and W17 are N or C-R5.
[00263] Some nonlimiting examples of the Mi moiety of Formula M1 -C and
Formula M1 -D
include:
H
/S N
H 0
-70-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
wherein R'5 is ¨(Wl)k -R53 or R55; k is 0 or 1, and ¨(Wl)k -R53 and R55 are as
defined above.
[00264] The invention also provides compounds wherein Mi is a moiety having a
structure
of Formula Ml-E:
.....õ.õ-X13õ.........A14.
X12 ' -X, 15
I
,2zz,
^11 ^17
Formula Ml -E
wherein Xii, X125 X135 X14, X155 X16, and X17 are independently N, or C-R5;
provided that no
more than two N are adjacent.
[00265] In some embodiments of the invention, the Mi moiety having a structure
of Formula
Ml-E, is a moiety having a structure of Formula MI-El, Ml-E2, Ml-E3, Ml-E4, Ml-
E5,
Ml-E6, Ml-E7, or Ml-E8:
R5
R5 N. N, R5 R NN R5 R5 40 Nr R5 R5
1 1 R5 40N R5
N %%.'\----R5 "2E. N
R5
R5 R5 R5 R5 R5 R5 R5 R5
Formula M 1 -E 1 Formula Ml -E2
Formula Ml -E3 Formula Ml -E4
R5
R5
R5 R5 NN R5
R5 N, R5 R5 N, R5
N N p5
, -
1 \ N-R5 1
...-7.,,5
N L'
R5 R5 R5 R5
Formula M 1 -E5 Formula M 1 -E6 Formula M 1 -E7
Formula M 1 -E8
-71-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00266] In some embodiments of the invention, the M1 moiety having a structure
of
Formula Ml-E, is a moiety having a structure:
R5X13Xizi. R5
1 T
;2z2./ X16
R5 R5 .
[00267] Some nonlimiting examples of the M1 moiety of Formula Ml-E include:
R5
R5
R'\11
( \ __________________________ /
0 /7
)2- and 4;
wherein R'5 is ¨(Wl)k ¨R53 or R55; k is 0 or 1, n i sO, 1, 2, or 3, and ¨(Wl)k
¨R53 or R55 are as
defined above. In some embodiments, k is 0, and R5 is R53.
[00268] In some embodiments, R53 is hydrogen, unsubstituted or substituted Ci-
Cioalkyl
(which includes but is not limited to -CH3, -CH2CH3, n-propyl, isopropyl, n-
butyl, tert-
butyl, sec-butyl, pentyl, hexyl, and heptyl),or unsubstituted or substituted
C3-C8cycloalkyl
(which includes but is not limited to cyclopropyl, cyclobutyl, cyclopentyl,
and cyclohexyl). In
other embodiments, R53 is monocyclic or bicyclic aryl, wherein the R53 aryl is
unsubstituted
or substituted. Some examples of aryl include but are not limited to phenyl,
naphthyl or
fluorenyl. In some other embodiments, R53 is unsubstituted or substituted
heteroaryl,
including but not limited to monocyclic and bicyclic heteroaryl. Monocyclic
heteroaryl
R53includes but is not limited to pyrrolyl, thienyl, furyl, pyridinyl,
pyranyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazolyl. Bicyclic heteroaryl R53 includes but is
not limited to
benzothiophenyl, benzofuryl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl, pyrazolopyrimidinyl,
and purinyl.
Additionally, R53 may be alkylcycloalkyl (including but not limited to
cyclopropylethyl,
cyclopentylethyl, and cyclobutylpropyl), -alkylaryl (including but not limited
to benzyl,
phenylethyl, and phenylnaphthyl), ¨ alkylhetaryl (including but not limited to
pyridinylmethyl, pyrrolylethyl, and imidazolylpropyl) ,or ¨alkylheterocyclyl (
non-limiting
examples are morpholinylmethyl, 1-piperazinylmethyl, and azetidinylpropyl).
For each of
alkylcycloalkyl, alkylaryl , alkylhetaryl, or ¨alkylheterocyclyl, the moiety
is connected to M1
through the alkyl portion of the moiety In other embodiments, R53 is
unsubstituted or
substituted C2-Cioalkenyl (including but not limited to alkenyl such as, for
example, vinyl,
-72-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
allyl, 1-methyl propen-l-yl, butenyl, or pentenyl) or unsubstituted or
substituted alkynyl
(including but not limited to unsubstituted or substituted C2-Ci0alkynyl such
as acetylenyl,
propargyl, butynyl, or pentynyl).
[00269] Further embodiments provide R53 wherein R53 is alkenylaryl,
alkenylheteroaryl,
alkenylheteroalkyl, or alkenylheterocyclyl, wherein each of alkenyl, aryl,
heteroaryl,
heteroalkyl, and heterocyclyl is as described herein and wherein the
alkenylaryl,
alkenylhetaryl, alkenylheteroalkyl, or alkenylheterocycicyl moiety is attached
to M1 through
the alkenyl. Some nonlimiting examples in include styryl, 3-pyridinylallyl, 2-
methoxyethoxyvinyl, and 3-morpholinlylally1 In other embodiments, R53 is
¨alkynylaryl, ¨
alkynylhetaryl, ¨alkynylheteroalkyl, ¨alkynylheterocylyl, ¨alkynylcycloalkyl,
or ¨alkyny1C3_
8cycloalkenyl, wherein each of alkynyl, aryl, heteroaryl, heteroalkyl, and
heterocyclyl is as
described herein and wherein the alkynylaryl, alkynylhetaryl,
alkynylheteroalkyl, or
alkynylheterocyclyl moiety is attached to M1 through the alkynyl.
Alternatively, R53 is ¨
alkoxyalkyl, ¨alkoxyalkenyl, or ¨alkoxyalkynyl, wherein each of alkoxy, alkyl,
alkenyl, and
alkynyl is as described herein and wherein the ¨alkoxyalkyl, ¨alkoxyalkenyl,
or ¨
alkoxyalkynyl moiety is attached to M1 through the alkoxy. In yet other
embodiments, R53 is
¨heterocyclylalkyl, ¨heterocyclylalkenyl, or ¨heterocyclylalkynyl, wherein the
heterocyclyl,
alkyl, alkenyl, or alkynyl is as described herein and wherein the
¨heterocyclylalkyl,¨
heterocyclylalkenyl, or ¨heterocyclylalkynyl is attached to M1 through the
heterocyclyl
portion of the moiety. Further, R53 may be aryl¨alkenyl, aryl¨alkynyl, or aryl-
heterocyclyl,
wherein the aryl, alkenyl, alkynyl, or heterocyclyl is as described herein and
wherein the
aryl¨alkenyl, aryl¨alkynyl, or aryl-heterocyclyl moiety is attached to M1
through the aryl
portion of the moiety. In some other embodiments, R53 is heteroaryl ¨ alkyl,
heteroaryl ¨
alkenyl, heteroaryl ¨alkynyl, heteroaryl ¨cycloalkyl, heteroaryl ¨heteroalkyl,
or heteroaryl ¨
heterocyclyl, wherein each of heteroaryl, alkyl, alkenyl, alkynyl, cycloalkyl,
heteroalkyl, and
heterocyclyl is as described herein and wherein the heteroaryl ¨ alkyl,
heteroaryl ¨alkenyl,
heteroaryl ¨alkynyl, heteroaryl ¨cycloalkyl, heteroaryl ¨heteroalkyl, or
heteroaryl ¨
heterocyclyl moiety is attached to M1 through the heteroaryl portion of the
moiety.
[00270] For each of the aryl or heteroaryl moieties forming part or all of
R53, the aryl or
heteroaryl is unsubstituted or is substituted with one or more independent
halo, ¨OH, ¨R31, ¨
CF3, ¨0CF3, ¨0R31, ¨NR31R32, ¨NR34R35, ¨C(0)R31, ¨0O2R31, ¨C(=0)NR31R32, ¨
C(=0)NNR34R35, -NO2, ¨CN, ¨S(0)0_2R31, ¨SO2NR31R32, ¨502NR34R35, -
NR31C(=0)R32, ¨
NR31C(=0)0R32, ¨NR31C(=0)NR32R33, ¨NR31S(0)0_2R32, ¨C(=S)0R31, ¨C(=0)5R31, ¨
NR31C(=
NR32)NR33R32, ¨NR31C(=NR32)0R33, ¨NR31C(=NR32)SR33, ¨0C(=0)0R33, ¨
-73-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
OC(=0)NR"R32, -0C(=0)SR3i, -SQ=0)0R3i, -P(0)0R3iOR32, or-SC(=0)NR3iR32
substituents. Additionally, each of the alkyl, cycloalkyl, heterocyclyl, or
heteroalkyl moieties
forming part of all of R53 is unsubstituted or substituted with one or more
halo, -OH, -R31, -
CF3, -0CF3, -0R31, -0-aryl, -NR31R32, -NR34R35 ,-C(0)R31, -CO2R31, -
C(=0)NNR34R35,
or -C(=0)NR31R32 substituents.
[00271] In other embodiments, R5 is -W1 -R53. In some embodiments, R5 is -
0R53,
including but not limited to -0-alkyl (including but not limited to methoxy or
ethoxy), -0-
aryl ( including but not limited to phenoxy), -0-heteroaryl (including but not
limited to
pyridinoxy) and -0-heterocycloxy( including but not limited to 4-N-
piperidinoxy). In some
embodiments R5 is -NR6R53 including but not limited to anilinyl, diethylamino,
and 4-N-
piperidinylamino. In yet other embodiments R5 is -S(0)0_2R53, including but
not limited to
phenylsulfonyl and pyridinylsulfonyl. The invention also provides compounds
wherein R5
is-C(0) (including but not limited to acetyl, benzoyl, and pyridinoyl) or -
C(0)0 R53 (
including but not limited to carboxyethyl, and carboxybenzyl). In other
embodiments, R5 is -
C(0)N(R6)R53 (including but not limited to C(0)NH(cyclopropyl) and
C(0)N(Me)(pheny1))
or -CH(R6)N(R7)R53 (including but not limited to -CH2-NH-pyrrolidinyl, CH2-
NHcyclopropyl, and CH2-aniliny1). Alternatively, R5 is -N(R6)C(0)R53
(including but not
limited to -NHC(0)phenyl, -NHC(0)cyclopentyl, and to -NHC(0)piperidinyl) or -
N(R6)S(0)2 R53 ( including but not limited to -NHS(0)2phenyl, -
NHS(0)2piperazinyl, and -
NHS(0)2methyl. Additionally, R5 is-N(R6)S(0) R53, -CH(R6)N(C(0)0R7) R53, -
CH(R7)N(C(0)R7) R53,-CH(R6)N(S02R7) R53, -CH(R6)N(R7) R53, -CH(R6)C(0)N(R7)
R53, -
CH(R6)N(R7)C(0) R53, -CH(R6)N(R7)S(0) R53, or -CH(R6)N(R7)S(0)2 R53.
[00272] Alternatively, R5 is R55. R55 is halo, -OH, -NO2, -CF3, -0CF3, or -CN.
In some
other embodiments, R55 is -R31, -0R31(including but not limited to methoxy,
ethoxy, and
butoxy) -C(0)R31 (non-limiting examples include acetyl, propionyl, and
pentanoyl), or -
CO2R31( including but not limited to carboxymethyl, carboxyethyl and
carboxypropyl). In
further embodinents, R55 is -NR31R32,-C(=0)NR31R32, -SO2NR31R32, or -S(0)0
2R31. In
other embodiments, R55 is-NR34R35 or -SO2 NR34R35, wherein R34R35 are taken
together with
the nitrogen to which R34R35 are attached to form a cyclic moiety. The cyclic
moiety so
formed may be unsubstituted or substituted, wherein the substituents are
selected from the
group consisting of alkyl, -C(0)alkyl, -S(0)2alkyl, and -S(0)2aryl . Examples
include but are
not limited to morpholinyl, piperazinyl, or -S02-(4-N-methyl-piperazin-1-yl.
Additionally,
R55 is -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R33, -NR31S(0)0_2R32, -
C(=S)0R31, -C(=0)SR31, -NR31C(=NR32)NR33R32, -NR31C(=NR32)0R33, -
-74-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -C(=0)NNR34R35, -0C(=0)SR31, -
SC(=0)0R31, ¨P(0)0R310R32, or¨SC(=0)NR31R32; . In yet another embodiment, R55
is -0-
aryl, including but not limited to phenoxy, and naphthyloxy.
[00273] The invention further provides a compound which is an mTor inhibitor,
wherein the
compound has the Formula I-A:
R31 R32
/
M1
N
Xi
, v
E2 XX34 "2
R1
Formula I-A
[00274] or a pharmaceutically acceptable salt thereof, wherein:
[00275] X1 is N or C-E1, X2 is N, X3 is C, and X4 is C-R9 or N; or X1 is N or
C-E1, X2 is C,
X3 is N, and X4 is C-R9 or N;
[00276] R1 is ¨H, ¨L-Ci_ioalkyl, -L-C3_8cycloalkyl, -L-Ci_ioalkyl -
C3_8cycloalkyl, -L- aryl, -
L-heteroaryl, -L-Ci_ioalkylaryl, -L- Ci_malkylheteroaryl, -L- C
i_ioalkylheterocyclyl, -L-C2-
ioalkenY15 -L-C2_10alkynyl, -L-C2_10alkenyl-C3_8cycloalkyl, -L-C2_10alkynyl-
C3_8cycloalkyl, -L-
heteroalkyl, -L-heteroalkylaryl, -L-heteroalkylheteroaryl, -L-heteroalkyl-
heterocyclyl, -L-
heteroalkyl-C3_8cycloalkyl, -L-aralkyl, -L-heteroaralkyl, or -L-heterocyclyl,
each of which is
unsubstituted or is substituted by one or more independent R3;
[00277] L is absent, -(C=0)-5 -C(=0)0-5 -C(=0) N(R31)-5-S-5 -S(0)-5 -S(0)2-5 -
S(0)2N(R31)-5
or -N(R31)-;
[00278] M1 is a moiety having the structure of Formula Ml-Fl or Ml-F2:
R2 R2
(w2)k (N2)k
/
cyN
-R5
1 R5
=
Or
Formula M 1 -F 1 Formula Ml -F2
[00279] k is 0 or 1;
[00280] E1 and E2 are independently ¨(W1)j -R4;
-75-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00281] j, in each instance (i.e., in E1 or j in E2), is independently 0 or 1
[00282] W1 is -0-, -NW-, -S(0)0_2-,-C(0)-,-C(0)N(R7)-, -N(R7)C(0)-, -N(R7)S(0)-
, -
N(R7)S(0)2-5 -C(0)0-5 -CH(R7)N(C(0)0R8)-5 -CH(R7)N(C(0)R8)-5 -CH(R7)N(S02R8)-5
-CH(R7)N(R8)-5 -CH(R7)C(0)N(R8)-, -CH(R7)N(R8)C(0)-, -CH(R7)N(R8)S(0)-5 or -
CH(R7)N(R8)S(0)2-;
[00283] W2 is -0-, -NR7-, -S(0)0_2-,-C(0)-,-C(0)N(R7)-, -N(R7)C(0)-, -
N(R7)C(0)N(R8)-5-N(R7)S(0)-5 -N(R7)S(0)2-5-C(0)0-5 -CH(R7)N(C(0)0R8)-5 -
CH(R7)N(C(0)R8)-5 -CH(R7)N(S02R8)-5 -CH(R7)N(R8)-5 -CH(R7)C(0)N(R8)-5 -
CH(R7)N(R8)C(0)-5 -CH(R7)N(R8)S(0)-5 or -CH(R7)N(R8)S(0)2-;
[00284] R2 is hydrogen, halogen, -OH, -R315 -CF3, -0CF35 -0R315 -NR31R325 -
NR34R355 -
C(0)R315 -0O2R315 -C(=0)NR31R325 -C(=0)NR34R355 -NO2, -CN, -S(0)0_2R315 -
SO2NR31R325 -S02NR34R355 -NR31C(=0)R325 -NR31C(=0)0R325 -NR31C(=0)NR32R335 -
NR31S(0)0_2R325 -C(=S)0R315 -C(=0)SR315 -NR31C(=NR32)NR33R325 -
NR31C(=NR32)0R335
-NR31C(=NR32)SR335 -0C(=0)0R335 -0C(=0)NR31R325 -0C(=0)SR315 -SC(=0)0R315 -
P(0)0R310R325 -SC(=0)NR31R32 5 aryl (e.g. bicyclic aryl, unsubstituted aryl,
or substituted
monocyclic aryl), heteroaryl, Ci_ioalkyl, C 3 _8 cycloalkyl, Ci_ioalkyl-C 3 _8
cycloalkyl, C3 _
8 cycloalkyl -Ci_malkyl, C 3 _8 cycloalkyl -C2_10alkenyl, C3_8 cycloalkyl-
C2_10alkynyl, Ci_malkyl-
C2_10alkenyl, Ci_malkyl- C2_10alkynyl, Ci_malkylaryl (e.g. C240alkyl-
monocyclic aryl, C1-
malkyl-substituted monocyclic aryl, or Ci_i0alkylbicycloary1),
Ci_malkylheteroaryl, C1_
ioalkylheterocyclyl, C2_10alkenyl, C2_10alkynyl, C2_10alkenyl -Ci_i0alkyl, C2-
loalkynyl -C1-
ioalkyl, C2_10alkenylaryl, C2_10alkenylheteroaryl, C2_10alkenylheteroalkyl, C
2-
loalkenylheterocycicyl, C2_10 alkellyl- C3 _8 cycloalkyl, C2_10alkynylaryl,
C2_10alkynylheteroaryl,
C240alkynylheteroalkyl, C240alkynylheterocyclyl, C2_10alkynyl-C3 _8
cycloalkenyl, Ci_malkoxy
Ci_malkyl, Ci_malkoxy-C2_10alkenyl, Ci_loalkoxy-C2_10alkynyl, heterocyclyl,
heteroalkyl,
heterocycly1 -Ci_malkyl, heterocyclyl-C240alkenyl, heterocyclyl-C240alkynyl,
aryl- Ci_malkyl
(e.g. monocyclic aryl-C2_10alkyl, substituted monocyclic aryl- Ci_i0alkyl, or
bicycloaryl--C1_
ioalkyl), aryl-C2_10alkenyl, aryl-C2_10alkynyl, aryl-heterocyclyl, heteroaryl-
Ci_i0alkyl,
heteroaryl-C2_10alkenyl, heteroaryl-C2_10alkynyl, heteroaryl-C 3 _8
cycloalkyl, heteroaryl-
heteroalkyl, or heteroaryl-heterocyclyl, wherein each of said bicyclic aryl or
heteroaryl
moiety is unsubstituted, or wherein each of bicyclic aryl, heteroaryl moiety
or monocyclic
aryl moiety is substituted with one or more independent alkyl, heteroalkyl,
alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
halo, -OH, -R315 -
CF35 -0CF35 -0R315 -NR31R325 -NR34R355 -C(0)R315 -0O2R315 -C(=0)NR31R325 -
C(=0)NR34R355 -NO2, -CN, -S(0)0_2R315 -SO2NR31R325 -S02NR34R355 -NR31C(=0)R325
-
-76-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
NR31C(=0)0R32, -NR31C(=0)NR32R33, -NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -
NR31C(=NR32)NR33R32, -NR31C(=NR32)0R33, -NR31C(=NR32)SR33, -0C(=0)0R33, -
OC(=0)NR31R325 -0C(=0)SR315 -SQ=0)0R315 -P(0)0R310R325 or-SC(=0)NR31R32, and
wherein each of said alkyl, cycloalkyl, heterocyclyl, or heteroalkyl moiety is
unsubstituted or
is substituted with one or more alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl,
heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, halo, -OH, -
R31, -CF3, -0CF3,
-0R31, -0-aryl, -NR31R32, -NR34R35 ,-C(0)R31, -CO2R31, -C(=0)NR34R35, or -
C(=0)NR31R32;
[00285] R3 and R4 are independently hydrogen, halogen, -OH, -R31, -CF3, -0CF3,
-0R31, -
NR31R32, -NR34R35, -C(0)R31, -CO2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN,
-
S(0)0_2R31, -SO2NR31R32, -S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -
NR31C(=0)NR32R33, -NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -
NR31C(=NR32)NR33R32,
-NR31C(=NR32)0R33, -NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -
OC(=0)SR31, -SC(=0)0R31, -P(0)0R310R32, -SC(=0)NR31R32, aryl, heteroaryl,
Ci_4alkyl,
Ci_ioalkyl, C3_8cycloalkyl, Ci_ioalkyl-C3-8cycloalkyl, C3_8cycloalkyl -
Ci_ioalkyl, C3_
8eyeloalkyl -C2_10alkenyl, C3_8cycloalkyl- C2_10alkynyl, Ci_ioalkyl-
C2_10alkenyl, Ci_ioalkyl- C2-
ioalkYnyl, Ci_ioalkylaryl, Ci_ioalkylheteroaryl, Ci_loalkylheterocyclyl,
C2_10alkenY15 C2-
alkynyl, C2_10 alkenyl -Ci_ioalkyl, C2_10 alkynyl -Ci_ioalkyl, C2_10
alkenylaryl, C2-
10 alkenylheteroaryl, C2_10 alkenylheteroalkyl, C2-10 alkenylheterocycicyl,
C2_10 alkenyl-C3_
8 cycloalkyl, C2_10 alkynyl-C3_8 cyclo alkyl, C2_10 alkynylaryl, C2_10
alkynylheteroaryl, C2_
malkynylheteroalkyl, C240alkynylheterocyclyl, C2_10alkynyl-C3_8cycloalkenyl,
Ci_ioalkoxy C1_
malkyl, Ci_loalkoxy-C2_10alkenyl, Ci_loalkoxy-C2_10alkynyl, heterocyclyl,
heterocyclyl -Ci_
malkyl, heterocyclyl-C240alkenyl, heterocyclyl-C240alkynyl, aryl- Ci_ioalkyl,
aryl-C2-
ioalkenyl, aryl-C2_10 alkynyl, aryl-heterocyclyl, heteroaryl-Ci_ioalkyl,
heteroaryl-C2_10 alkenyl,
heteroaryl-C240alkynyl, heteroaryl-C3_8cycloalkyl, heteroalkyl, heteroaryl-
heteroalkyl, or
heteroaryl-heterocyclyl, wherein each of said aryl or heteroaryl moiety is
unsubstituted or is
substituted with one or more independent halo, -OH, -R31, -CF3, -0CF3, -0R31, -
NR31R32, -
NR34R35, -C(0)R31, -0O2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN, -
S(0)0_2R31, -
SO2NR31R32, -S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R33, -
NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -NR31C(=NR32)NR33R32, -
NR31C(=NR32)0R33,
-NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -0C(=0)SR31, -SC(=0)0R31, -
P(0)0R310R32, or-SC(=0)NR31R32, and wherein each of said alkyl, cycloalkyl,
heterocyclyl,
or heteroalkyl moiety is unsubstituted or is substituted with one or more
halo, -OH, -R31, -
-77-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
CF3, -0CF3, -0R31, -0-aryl, -NR31R32, -NR34R35 ,-C(0)R31, -0O2R31, -
C(=0)NR34R35, or
-C(=0)NR31R32;
[00286] R5 is hydrogen, halogen, -OH, -R31, -CF3, -0CF3, -0R31, -NR31R32, -
NR34R35, -
C(0)R31, -0O2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN, -S(0)0_2R31, -
SO2NR31R32, -S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R33, -
NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -NR31C(=NR32)NR33R32, -
NR31C(=NR32)0R33,
-NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -0C(=0)SR31, -SC(=0)0R31, -
P(0)0R310R32,or -SC(=0)NR31R32;
[00287] R31, R32, and R33, in each instance, are independently H or Ci_ioalkyl
, wherein the
Ci_malkyl is unsubstituted or is substituted with one or more aryl,
heteroalkyl, heterocyclyl,
or heteroaryl group, wherein each of said aryl, heteroalkyl, heterocyclyl, or
heteroaryl group
is unsubstituted or is substituted with one or more halo, -OH, - Ci_malkyl, -
CF3, -0-aryl, -
OCF3, -0C1_10alkyl, -NH2, - N(Ci_malkyl)(Ci_loalkyl), - NH(Ci_ioalkyl), - NH(
aryl), -
NR34R35, -C(0)(Ci_malkyl), -C(0)(Ci_malkyl-ary1), -C(0)(ary1), -0O2-Ci_malkyl,
-0O2-C1-
malkylaryl, -0O2-aryl, -C(=0)N(Ci_malkyl)( Ci_malkyl), -C(0)NH( Ci_malkyl), -
C(0)NR34R35, -C(0)NH2, -0CF3, -0(Ci_ioalkyl), -0-aryl, -N(ary1)( Ci-malkyl), -
NO2, -
CN, -S(0)0_2 Ci_malkyl, -S(0)0_2 Ci_malkylaryl, -S(0)0_2 aryl, -SO2N(ary1), -
SO2 N(Ci-
malkyl)( Ci_malkyl), -SO2 NH(Ci_malkyl) or -S02NR34R35;
[00288] R34 and R35 in -NR34R35, -C(=0)NR34R35, or -S02NR34R35, are taken
together with
the nitrogen atom to which they are attached to form a 3-10 membered saturated
or
unsaturated ring; wherein said ring is independently unsubstituted or is
substituted by one or
more -NR31R32, hydroxyl, halogen, oxo, aryl, heteroaryl, Ci_6alkyl, or 0-aryl,
and wherein
said 3-10 membered saturated or unsaturated ring independently contains 0, 1,
or 2 more
heteroatoms in addition to the nitrogen atom;
[00289] R7 and R8 are each independently hydrogen, Ci_malkyl, C2_10alkenyl,
aryl,
heteroaryl, heterocyclyl or C3_10cycloalkyl, each of which except for hydrogen
is
unsubstituted or is substituted by one or more independent R6;
[00290] R6 is halo, -0R31, -SH, -NH2, -NR34R35 , -NR31R32, -0O2R31, -0O2aryl, -
C(=0)NR31R32, C(=0)NR34R35 , -NO2, -CN, -S(0)0_2 Ci_i0alkyl, -5(0) 0_2aryl, -
S02NR34R35, -S02NR31R32, Ci_ioalkyl, C2_10alkenyl, C2_10alkynyl; aryl-
Ci_ioalkyl, aryl-C2
loalkenyl, aryl-C 2_ loalkynyl, heteroaryl-Ci_i0alkyl, heteroaryl-C 2_
ioalkenyl, heteroaryl-C2
malkynyl, wherein each of said alkyl, alkenyl, alkynyl, aryl, heteroalkyl,
heterocyclyl, or
heteroaryl group is unsubstituted or is substituted with one or more
independent halo, cyano,
nitro, -0Ci_ioalkyl, Ci_ioalkyl, C2_10alkenyl, C2_10alkynyl, haloCi_ioalkyl,
haloC2_10alkenyl,
-78-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
haloC2_10alkynyl, ¨COOH, ¨C(=0)NR31R32, ¨C(=0)NR34R35 , ¨S02NR34R35, ¨SO2
NR31R32,
-NR31R32, or ¨NR34R35 ; and
[00291] R9 is H, halo, ¨0R31, ¨SH, -NH2, ¨NR34R35 , ¨ NR31R32, ¨0O2R31,
¨0O2aryl, ¨
C(=0)NR31R32, C(=0)NR34R35 , ¨NO2, ¨CN, ¨S(0)0-2 Ci_i0alkyl, ¨5(0) 0_2aryl, ¨
S02NR34R35, ¨S02NR31R32, Ci_malkyl, C2_10alkenyl, C2_10alkynyl; aryl-
Ci_malkyl, aryl-C 2-
1 0 alkenyl, aryl-C 2_ 1 0 alkynyl, heteroaryl-Ci_i0alkyl, heteroaryl-C 2_ 1 0
alkenyl, heteroaryl-C 2-
malkynyl, wherein each of said alkyl, alkenyl, alkynyl, aryl, heteroalkyl,
heterocyclyl, or
heteroaryl group is unsubstituted or is substituted with one or more
independent halo, cyano,
nitro, ¨0Ci_i0alkyl, Ci_malkyl, C2_10alkenyl, C2_10alkynyl, haloCi_malkyl,
haloC2_10alkenyl,
haloC2_10alkynyl, ¨COOH, ¨C(=0)NR31R32, ¨C(=0)NR34R35 , ¨S02NR34R35, ¨SO2
NR31R32,
-NR31R32, or ¨NR34R35.
[00292] In some embodiments, X4 is C-R9.
[00293] The invention also provides an inhibitor as defined above, wherein the
compound is
of Formula I:
R31 R32
\ /
N
M1
N y X 1
............ . . . . = -3 - i
E2 N "2
\
Ri
Formula I-B
or a pharmaceutically acceptable salt thereof, and wherein the substituents
are as defined
above.
[00294] In various embodiments the compound of Formula I-B or its
pharmaceutically
acceptable salt thereof, is a compound having the structure of Formula I-B1 or
Formula I-B2:
R31 R32
\ / R31 R32
N \ /
M1 N
Mi
N"-------K N
0 0/X1 0 NO Xi
.......--,, ....,..----..v/
E2 N .----- X2 E2 N "2
\ µ
R1 Ri
Formula I-B1 Formula I-B2
or a pharmaceutically acceptable salt thereof
-79-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00295] In various embodiments of Formula I-B1, Xi is N and X2 is N. In other
embodiments, Xi is C-E1 and X2 is N. In yet other embodiments, X1 is NH and X2
is C. In
further embodiments, Xi is CH-E1 and X2 is C.
[00296] In various embodiments of Formula I-B2, Xi is N and X2 is C. In
further
embodiments, Xi is C-E1 and X2 is C.
[00297] In various embodiments, Xi is C¨(W1)j -R4, where j is 0.
[00298] In another embodiment, X1 is CH. In yet another embodiment, Xi is C-
halogen,
where halogen is Cl, F, Br, or I.
[00299] In various embodiments of X1, it is C ¨(W1)j ¨R4. In various
embodiments of X1, j is
1, and W1 is ¨0¨. In various embodiments of Xi, j is 1, and W1 is ¨NR7-. In
various
embodiments of Xi, j is 1, and W1 is ¨NH-. In various embodiments of Xi, j is
1, and W1 is
¨S(0)0_2¨. In various embodiments of Xi, j is 1, and W1 is ¨C(0)¨. In various
embodiments of X1, j is 1, and W1 is ¨C(0)N(R7)¨. In various embodiments of
X1, j is 1,
and W1 is ¨N(R7)C(0)¨. In various embodiments of Xi, j is 1, and W1 is
¨N(R7)S(0)¨. In
various embodiments of Xi, j is 1, and W1 is ¨N(R7)S(0)2¨. In various
embodiments of Xi,
is 1, and W1 is ¨C(0)0¨. In various embodiments of Xi, j is 1, and W1 is
CH(R7)N(C(0)0R8)¨. In various embodiments of X1, j is 1, and W1 is
¨CH(R7)N(C(0)R8)¨.
In various embodiments of Xi, j is 1, and W1 is ¨CH(R7)N(S02R8)¨. In various
embodiments
of Xi, j is 1, and W1 is ¨CH(R7)N(R8)¨. In various embodiments of Xi, j is 1,
and W1 is ¨
CH(R7)C(0)N(R8)¨. In various embodiments of Xi, j is 1, and W1 is
¨CH(R7)N(R8)C(0)¨.
In various embodiments of X1, j is 1, and W1 is ¨CH(R7)N(R8)S(0)¨. In various
embodiments of X1, j is 1, and W1 is ¨CH(R7)N(R8)S(0)2¨.
[00300] In another embodiment, X1 is CH2. In yet another embodiment, X1 is CH-
halogen,
where halogen is Cl, F, Br, or I.
[00301] In another embodiment, X1 is N.
[00302] In various embodiments, X2 is N. In other embodiments, X2 is C.
[00303] In various embodiments, E2 is ¨(Wi)j -R4, where j is 0.
[00304] In another embodiment, E2 is CH. In yet another embodiment, E2 is C-
halogen,
where halogen is Cl, F, Br, or I.
[00305] In various embodiments of E2, it is ¨(W1)j ¨R4. In various embodiments
of E2, j is 1,
and W1 is ¨0¨. In various embodiments of E2, j is 1, and W1 is ¨NR7-. In
various
embodiments of E2, j is 1, and W1 is ¨NH-. In various embodiments of E2, j is
1, and W1 is
¨S(0)0_2¨. In various embodiments of E2, j is 1, and W1 is ¨C(0)¨. In various
embodiments of E2, j is 1, and W1 is ¨C(0)N(R7)¨. In various embodiments of
E2, j is 1, and
-80-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
W1 is ¨N(R7)C(0)¨. In various embodiments of E2, j is 1, and W1 is
¨N(R7)S(0)¨. In
various embodiments of E2, j is 1, and W1 is ¨N(R7)S(0)2¨. In various
embodiments of E2, j
is 1, and W1 is ¨C(0)0¨. In various embodiments of E2, j is 1, and W1 is
CH(R7)N(C(0)0R8)¨. In various embodiments of E2, j is 1, and W1 is
¨CH(R7)N(C(0)R8)¨.
In various embodiments of E2, j is 1, and W1 is ¨CH(R7)N(S02R8)¨. In various
embodiments
of E2, j is 1, and W1 is ¨CH(R7)N(R8)¨. In various embodiments of E2, j is 1,
and W1 is ¨
CH(R7)C(0)N(R8)¨. In various embodiments of E2, j is 1, and W1 is
¨CH(R7)N(R8)C(0)¨.
In various embodiments of E2, j is 1, and W1 is ¨CH(R7)N(R8)S(0)¨. In various
embodiments of E2, j is 1, and W1 is ¨CH(R7)N(R8)S(0)2¨.
[00306] In various embodiments when M1 is a moiety of Formula Ml-F1, M1 is
benzoxazolyl substituted with ¨(W2)k-R2. In some embodiments, M1 is a
benzoxazolyl
substituted at the 2-position with ¨(W2)j ¨R2. In some embodiments, M1 is
either a 5-
benzoxazolyl or a 6- benzoxazolyl moiety, optionally substituted at the 2-
position with ¨(W2)j
¨R2. Exemplary Formula Ml-Fl M1 moieties include but are not limited to the
following:
__-R2
õ---- R2
0 ----I (W2 )k (w2) k 333 o( (w2)k
R5----71_, \ N i..--S.- N
I-
5 5
R2
\
...--= R2 (w2) k
(w2)k
II
A R5 \ I
p2
R 0
'
533 v-tfli, 5 vlilf
5 5
R2
(W2 )k
I
/----
R5
S'SS ,and R5 ,_s=
c) .
-81-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00307] In various embodiments when M1 is a moiety of Formula M1-F2, Formula
M1-F2 is
an aza-substituted benzoxazolyl moiety having a structure of one of the
following formulae:
,- R2 .. R2
(w2)k (w2)k ¨I'
(w2).
p
R5
>R11
Pr
GL \---1 R5
G. 5 7- 5 C-1 5
....... R2 R2
..--='
oN2). (w2)k (w2)k
0 --- l' 0 ----. 0 ----
Nir---õ,µNI
Al
t N cµ11\1
.._ -;....f- R5 O5
NA1- R5
R
(22. ,or
5
õ.õ... R2
(W2) k
(-?..? .1\1"-j R5
[00308] Exemplary Formula M1-F2 M1 moieties include but are not limited to the
following:
(w2)k
0---/ t w 2 \
li CR2 ___5( µ 1 k
N R5/
\
R5 it- N
11
N
5 5
R2
2 \
0N2)k
R5¨ CA N
R5-- oiv2
R2 R5¨ '-- No R5- 2 , R2
0 )k 0 ON )k
.SS5 5 111-1'. 5 fIll' , 5 and
5
R2
\
(W2)k
- N
\
R5_01 0
OS .
-82-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00309] In various embodiments of Mi, k is O. In other embodiments of Mi, k is
1, and W2
is selected from one of the following: -0-, -NR7-, -S(0)0_2-, -C(0)-, -
C(0)N(R7)-, -
N(R7)C(0)-, or -N(R7)C(0)N(R8)-. In yet another embodiment of Mi, k is 1, and
W2 is -
N(R7)S(0)-, -N(R7)S(0)2-, -C(0)0-, -CH(R7)N(C(0)0R8)-, -CH(R7)N(C(0)R8)-, or -
CH(R7)N(S02R8)-. In a further embodiment of Mi, k is 1, and W2 is -CH(R7)N(R8)-
, -
CH(R7)C(0)N(R8)-, -CH(R7)N(R8)C(0)-, or -CH(R7)N(R8)S(0)-. In yet another
embodiment of Mi, k is 1, and W2 is -CH(R7)N(R8)S(0)2-.
[00310] The invention provides an inhibitor of mTor which is a compound of
Formula I-C
or Formula I-D:
R2
(N2)k
R2
R31 R32
o
(vv2)k
\ /
R31 /R32 fN Nfj*R5
N R5
N (Th
N0 , lx
U
E2 N X\2 E2 N X2
Ri
Formula I-C Formula I-D
or a pharmaceutically acceptable salt thereof, wherein X1 is N or C-E1, X2 is
N, and X3 is C;
or Xi is N or C-E1, X2 is C, and X3 is N;
[00311] Ri is -H, -L-Ci_10alkyl, -L-C3_8cycloalkyl, -L- Ci_10alkyl -
C3_8cycloalkyl, -L- aryl, -
L-heteroaryl, -L-C1_10alkylaryl, -L- Ci_malkylheteroaryl, -L-
Ci_malkylheterocyclyl, -L-C2-
ioalkenYl, -L-C2_10alkynyl, -L-C2_10alkenyl-C3_8cycloalkyl, -L-C2_10alkynyl-
C3_8cycloalkyl, -L-
heteroalkyl, -L-heteroalkylaryl, -L-heteroalkylheteroaryl, -L-heteroalkyl-
heterocyclyl, -L-
heteroalkyl-C3_8cycloalkyl, -L-aralkyl, -L-heteroaralkyl, or -L-heterocyclyl,
each of which is
unsubstituted or is substituted by one or more independent R3;
[00312] L is absent, -(C=0)-, -C(=0)0-, -C(=0) N(R31)-,-S-, -S(0)-, -S(0)2-, -
S(0)2N(R31)-,
or -N(R31)-;
[00313] E1 and E2 are independently -(Wi)j -R4;
[00314] j in E1 or j in E2, is independently 0 or 1;
[00315] W1 is -0-, -S(0)0_2-,-C(0)-,-C(0)N(R7)-, -N(R7)C(0)-, -N(R7)S(0)-,
-
N(R7)S(0)2-, -C(0)0-, -CH(R7)N(C(0)0R8)-, -CH(R7)N(C(0)R8)-, -CH(R7)N(S02R8)-,
-83-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
-CH(R7)N(R8)-, -CH(R7)C(0)N(R8)-, -CH(R7)N(R8)C(0)-, -CH(R7)N(R8)S(0)-, or -
CH(R7)N(R8)S(0)2-;
[00316] W2 is -0-, -NR7-, -S(0)0_2-,-C(0)-,-C(0)N(R7)-, -N(R7)C(0)-, -
N(R7)C(0)N(R8)-, -N(R7)S(0)-, -N(R7)S(0)2-, -C(0)0-, -CH(R7)N(C(0)0R8)-, -
CH(R7)N(C(0)R8)-, -CH(R7)N(S02R8)-, -CH(R7)N(R8)-, -CH(R7)C(0)N(R8)-, -
CH(R7)N(R8)C(0)-, -CH(R7)N(R8)S(0)-, or -CH(R7)N(R8)S(0)2-;
[00317] k is 0 or 1;
[00318] R2 is hydrogen, halogen, -OH, -R31, -CF3, -0CF3, -0R31, -NR31R32, -
NR34R35, -
C(0)R31, -0O2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN, -S(0)0_2R31, -
SO2NR31R32, -S02NR34R355 -NR31C(=0)R325 -NR31C(=0)0R325 -NR31C(=0)NR32R335 -
NR31S(0)0_2R325 -C(=S)0R315 -C(=0)SR315 -NR31C(=NR32)NR33R325 -
NR31C(=NR32)0R335
-NR31C(=NR32)SR335 -0C(=0)0R335 -0C(=0)NR31R325 -0C(=0)SR315 -SC(=0)0R315 -
P(0)0R310R325 -SC(=0)NR31R32 5 aryl (e.g. bicyclic aryl, unsubstituted aryl,
or substituted
monocyclic aryl), heteroaryl, Ci_malkyl, C3_8 cycloalkyl, Ci_malkyl-
C3_8cycloalkyl, C3_
8 cycloalkyl -Ci_ioalkyl, C3_8 cycloalkyl -C2_10alkenyl, C3_8cycloalkyl-
C2_10alkynyl, Ci_loalkyl-
C2_10alkenyl, Ci_ioalkyl- C2_10alkynyl, Ci_malkylaryl (e.g. C240alkyl-
monocyclic aryl, C1-
malkyl-substituted monocyclic aryl, or Ci_i0alkylbicycloary1),
Ci_malkylheteroaryl, C1_
ioalkylheteroeyelyl, C2_10alkenyl, C2_10alkynyl, C2_10alkenyl -Ci_i0alkyl,
C2_10alkynY1 -Ci-
ioalkyl, C2_10alkenylaryl, C2_10alkenylheteroaryl, C2_10alkenylheteroalkyl, C
2-
loalkenylheterocycicyl, C2_10alkenyl-C3_8cycloalkyl, C2_10alkynylaryl,
C2_10alkynylheteroaryl,
C240alkynylheteroalkyl, C240alkynylheterocyclyl, C2_10alkynyl-
C3_8cycloalkenyl, Ci_malkoxy
Ci_malkyl, Ci_malkoxy-C2_10alkenyl, Ci_malkoxy-C2_10alkynyl, heterocyclyl -
Ci_malkyl,
heterocyclyl-C240alkenyl, heterocyclyl-C240alkynyl, aryl- Ci_i0alkyl (e.g.
monocyclic aryl-C2
malkyl, substituted monocyclic aryl- Ci_malkyl, or bicycloaryl--Ci_ioalkyl),
aryl-C2_10alkenyl,
aryl-C2_10alkynyl, aryl-heterocyclyl, heteroaryl-Ci_i0alkyl, heteroaryl-
C2_10alkenyl,
heteroaryl-C2_10alkynyl, heteroaryl-C3_8cycloalkyl, heteroaryl-heteroalkyl, or
heteroaryl-
heterocyclyl, wherein each of said bicyclic aryl or heteroaryl moiety is
unsubstituted, or
wherein each of bicyclic aryl, heteroaryl moiety or monocyclic aryl moiety is
substituted with
one or more independent alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl,
aryl, arylalkyl, heteroaryl, heteroarylalkyl, halo, -OH, -R31, -CF3, -OC F35 -
0R315 -NR31R325
-NR34R355 -C(0)R315 -0O2R315 -C(=0)NR31R325 -C(=0)NR34R355 -NO2, -CN, -
S(0)0_2R31,
-SO2NR31R325 -S02NR34R355 -NR31C(=0)R325 -NR31C(=0)0R325 -NR31C(=0)NR32R335 -
NR31S(0)0_2R325 -C(=S)0R315 -C(=0)SR315 -NR31C(=NR32)NR33R325 -
NR31C(=NR32)0R335
-NR31C(=NR32)SR335 -0C(=0)0R335 -0C(=0)NR31R325 -0C(=0)SR315 -SC(=0)0R315 -
-84-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
P(0)0R310R32, or-SC(=0)NR31R32 s, and wherein each of said alkyl, cycloalkyl,
heterocyclyl, or heteroalkyl moiety is unsubstituted or is substituted with
one or more alkyl,
heteroalkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl,
heteroaryl,
heteroarylalkyl, halo, -OH, -R31, -CF3, -0CF3, -0R31, -0-aryl, -NR31R32, -
NR34R35 ,-
C(0)R31, -CO2R31, -C(=0)NR34R35, or -C(=0)NR31R32;
[00319] R3 and R4 are independently hydrogen, halogen, -OH, -R31, -CF3, -0CF3,
-0R31, -
NR31R32, -NR34R35, -C(0)R31, -CO2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN,
-
S(0)0_2R31, -SO2NR31R32, -S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -
NR31C(=0)NR32R33, -NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -
NR31C(=NR32)NR33R32,
-NR31C(=NR32)0R33, -NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -
OC(=0)SR31, -SC(=0)0R31, -P(0)0R310R32, -SC(=0)NR31R32, aryl, heteroaryl,
Ci_malkyl,
C3_8cycloalkyl, Ci_malkyl-C 3 _8 cycloalkyl, C3_8cycloalkyl -Ci_ioalkyl, C 3
_8 cycloalkyl -C 2-
malkenyl, C3_8 cycloalkyl- C2_10alkynYl, Ci_malkyl- C2_10alkenyl, Ci_malkyl-
C2_10alkynYl, Ci-
loalkYlarY1, Ci_malkylheteroaryl, Ci_malkylheterocyclyl, C2_10alkenyl,
C2_10alkynyl, C 2-
loalkenyl -Ci-ioalkyl, C2_10alkynyl -Ci_i0alkyl, C2_10alkenylaryl,
C2_10alkenylheteroaryl, C 2-
loalkenylheteroalkyl, C2-loalkenylheterocycicyl, C2_10alkenyl-C 3_8
cycloalkyl, C2_10alkynylaryl,
C240alkynylheteroaryl, C240alkynylheteroalkyl, C240alkynylheterocyclyl,
C2_10alkynyl-C3_
8cycloalkenyl, Ci_malkoxy Ci_malkyl, Ci_loalkoxy-C2_10alkenyl, Ci_loalkoxy-
C2_10alkynyl,
heterocyclyl -Ci_malkyl, heterocyclyl-C240alkenyl, heterocyclyl-C240alkynyl,
aryl- Ci_malkyl,
aryl-C2_10alkenyl, aryl-C 2_10alkynyl, aryl-heterocyclyl, heteroaryl-
Ci_i0alkyl, heteroaryl-C2
loalkenyl, heteroaryl-C240alkynyl, heteroaryl-C3_8cycloalkyl, heteroaryl-
heteroalkyl, or
heteroaryl-heterocyclyl, wherein each of said aryl or heteroaryl moiety is
unsubstituted or is
substituted with one or more independent alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl,
heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, halo, -OH, -
R31, -CF3, -0CF3,
-0R31, -NR31R32, -NR34R35, -C(0)R31, -0O2R31, -C(=0)NR31R32, -C(=0)NR34R35, -
NO2, -
CN, -S(0)0_2R31, -SO2NR31R32, -S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -
NR31C(=0)NR32R33, -NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -
NR31C(=NR32)NR33R32,
-NR31C(=NR32)0R33, -NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -
OC(=0)SR31, -SC(=0)0R31, -P(0)0R310R32, or-SC(=0)NR31R32, and wherein each of
said
alkyl, cycloalkyl, heterocyclyl, or heteroalkyl moiety is unsubstituted or is
substituted with
one or more alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl, aryl, arylalkyl,
heteroaryl, heteroarylalkyl, halo, -OH, -R31, -CF3, -0CF3, -0R31, -0-aryl, -
NR31R32, -
NR34R35 ,-C(0)R31, -0O2R31, -C(=0)NR34R35, or -C(=0)NR31R32;
-85-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00320] R5 is hydrogen, halogen, -OH, -R31, -CF3, -0CF3, -0R31, -NR31R32, -
NR34R35, -
C(0)R31, -0O2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN, -S(0)0_2R31, -
SO2NR31R32, -SO2NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R33, -
NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -NR31C(=NR32)NR33R32, -
NR31C(=NR32)0R33,
-NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -0C(=0)SR31, -SC(=0)0R31, -
P(0)0R310R32,or -SC(=0)NR31R32;
[00321] R31, R32, and R33, in each instance, are independently H or Ci_ioalkyl
, wherein the
Ci_malkyl is unsubstituted or is substituted with one or more aryl,
heteroalkyl, heterocyclyl,
or heteroaryl group, wherein each of said aryl, heteroalkyl, heterocyclyl, or
heteroaryl group
is unsubstituted or is substituted with one or more halo, -OH, - Ci_ioalkyl, -
CF3, -0-aryl, -
OCF3, -0C1_10alkyl, -NH2, - N(Ci_loalkyl)(Ci_malkyl), - NH(Ci_malkyl), - NH(
aryl), -
NR34R35, -C(0)(Ci_ioalkyl), -C(0)(Ci_malkyl-ary1), -C(0)(ary1), -0O2-
Ci_loalkyl, -0O2-C1-
malkylaryl, -0O2-aryl, -C(=0)N(Ci_malkyl)( Ci_malkyl), -C(0)NH( Ci_ioalkyl), -
C(=0)NR34R35, -C(=0)NH2, -0CF3, -0(Ci_ioalkyl), -0-aryl, -N(ary1)( Ci_malkyl),
-NO2, -
CN, -S(0)0_2 Ci_malkyl, -S(0)0_2 Ci_malkylaryl, -S(0)0_2 aryl, -SO2N(ary1), -
SO2 N(Ci-
malkyl)( Ci_ioalkyl), -SO2 NH(Ci_malkyl) or -SO2NR34R35;
[00322] R34 and R35 in -NR34R35, -C(=0)NR34R35, or -SO2NR34R35, are taken
together with
the nitrogen atom to which they are attached to form a 3-10 membered saturated
or
unsaturated ring; wherein said ring is independently unsubstituted or is
substituted by one or
more -NR31R32, hydroxyl, halogen, oxo, aryl, heteroaryl, Ci_olkyl, or 0-aryl,
and wherein
said 3-10 membered saturated or unsaturated ring independently contains 0, 1,
or 2 more
heteroatoms in addition to the nitrogen atom; and
[00323] R7 and R8 are each independently hydrogen, Ci_malkyl, C2_10alkenyl,
aryl,
heteroaryl, heterocyclyl or C3_10cycloalkyl, each of which except for hydrogen
is
unsubstituted or is substituted by one or more independent R6; and R6 is halo,
-0R31, -SH,
NH2, -NR34R35, - NR31R32, -0O2R31, -0O2aryl, -C(=0)NR31R32, C(=0) NR34R35 , -
NO2, -
CN, -S(0)0_2 Ci_ioalkyl, -5(0) 0_2aryl, -S02NR34R35, -S02NR31R32, Ci_i0alkyl,
C2_10alkenyl,
or C2_10alkynyl; or R6 is aryl-Ci_malkyl, aryl-C2_10alkenyl, aryl-
C2_10alkynyl, heteroaryl-C1-
malkyl, heteroaryl-C2_10alkenyl, heteroaryl-C2_10alkynyl, each of which is
unsubstituted or is
substituted with one or more independent halo, cyano, nitro, -0Ci_ioalkyl,
Ci_malkyl, C2-
malkenyl, C2_10alkynyl, haloCi_malkyl, haloC2_10alkenyl, haloC2_10alkynyl, -
COOH, -
C(0)NR31R32, -C(=0) NR34R35 , -S02NR34R35, -SO2 NR31R32, -NR31R32, or -
NR34R35.
[00324] In various embodiments of the compound of Formula I-C, the compound
has a
structure of Formula I-C 1 or Formula I-C2:
-86-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
R2
R2
ON 2
--7 (w2)k
1\
R31 R32 \ N R31 R32 \ N
------- R5 R5
N, iX
)0 0 0 0
N v'
E2 N Xx2 E2 N 'µ2
R1
R1
Formula I-C1 Formula I-C2
or a pharmaceutically acceptable salt thereof
[00325] In some embodiments of Formula I-C1, X1 is N and X2 is N. In other
embodiments,
Xi is C-E1 and X2 is N. In yet other embodiments, Xi is NH and X2 is C. In
further
embodiments, X1 is CH-E1 and X2 is C.
[00326] In several embodiments of Formula I-C2, Xi is N and X2 is C. In yet
other
embodiments, Xi is NH and X2 is C. In further embodiments, X1 is CH-E1 and X2
is C.
[00327] In various embodiments of the compound of Formula I-D, the compound
has a
structure of Formula I-D1 or Formula I-D2:
R2 R2
(N2)k (A/2)k
o 0-<
R31 R32 / R31 R32
/
R5 0 R5
N 0 0
N 0 0 Xi
/
E` N X2 E2 N ^2
R1 Ri
Formula I-D1 Formula I-D2
or a pharmaceutically acceptable salt thereof
[00328] In some embodiments of Formula 1-Di, Xi is N and X2 is N. In other
embodiments,
Xi is C-E1 and X2 is N. In yet other embodiments, X1 is NH and X2 is C. In
further
embodiments, X1 is CH-E1 and X2 is C.
[00329] In several embodiments of Formula I-D2, Xi is N and X2 is C. In
further
embodiments, Xi is C-Eland X2 is C.
[00330] In various embodiments, X1 is C¨(Wi)j -R4, where j is 0.
[00331] In another embodiment, X1 is CH. In yet another embodiment, Xi is C-
halogen,
where halogen is Cl, F, Br, or I.
-87-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00332] In various embodiments of X1, it is C ¨(W1)j ¨R4. In various
embodiments of X1, j is
1, and W1 is ¨0¨. In various embodiments of Xi, j is 1, and W1 is ¨NR7-. In
various
embodiments of Xi, j is 1, and W1 is ¨NH-. In various embodiments of Xi, j is
1, and W1 is
¨S(0)0_2¨. In various embodiments of Xi, j is 1, and W1 is ¨C(0)¨. In various
embodiments of X1, j is 1, and W1 is ¨C(0)N(R7)¨. In various embodiments of
X1, j is 1,
and W1 is ¨N(R7)C(0)¨. In various embodiments of Xi, j is 1, and W1 is
¨N(R7)S(0)¨. In
various embodiments of Xi, j is 1, and W1 is ¨N(R7)S(0)2¨. In various
embodiments of Xi,
is 1, and W1 is ¨C(0)0¨. In various embodiments of Xi, j is 1, and W1 is
CH(R7)N(C(0)0R8)¨. In various embodiments of X1, j is 1, and W1 is
¨CH(R7)N(C(0)R8)¨.
In various embodiments of Xi, j is 1, and W1 is ¨CH(R7)N(S02R8)¨. In various
embodiments
of Xi, j is 1, and W1 is ¨CH(R7)N(R8)¨. In various embodiments of Xi, j is 1,
and W1 is ¨
CH(R7)C(0)N(R8)¨. In various embodiments of Xi, j is 1, and W1 is
¨CH(R7)N(R8)C(0)¨.
In various embodiments of X1, j is 1, and W1 is ¨CH(R7)N(R8)S(0)¨. In various
embodiments of X1, j is 1, and W1 is ¨CH(R7)N(R8)S(0)2¨.
[00333] In various embodiments, X1 is CH¨(W1)j -R4, where j is 0.
[00334] In another embodiment, X1 is CH2. In yet another embodiment, X1 is CH-
halogen,
where halogen is Cl, F, Br, or I.
[00335] In various embodiments of X1, it is CH ¨(W1)j ¨R4. In various
embodiments of X1,
is 1, and W1 is ¨0¨. In various embodiments of Xi, j is 1, and W1 is ¨NR7-. In
various
embodiments of Xi, j is 1, and W1 is ¨NH-. In various embodiments of Xi, j is
1, and W1 is
¨S(0)0_2¨. In various embodiments of Xi, j is 1, and W1 is ¨C(0)¨. In various
embodiments of X1, j is 1, and W1 is ¨C(0)N(R7)¨. In various embodiments of
X1, j is 1,
and W1 is ¨N(R7)C(0)¨. In various embodiments of Xi, j is 1, and W1 is
¨N(R7)S(0)¨. In
various embodiments of Xi, j is 1, and W1 is ¨N(R7)S(0)2¨. In various
embodiments of Xi,
is 1, and W1 is ¨C(0)0¨. In various embodiments of Xi, j is 1, and W1 is
CH(R7)N(C(0)0R8)¨. In various embodiments of X1, j is 1, and W1 is
¨CH(R7)N(C(0)R8)¨.
In various embodiments of Xi, j is 1, and W1 is ¨CH(R7)N(S02R8)¨. In various
embodiments
of Xi, j is 1, and W1 is ¨CH(R7)N(R8)¨. In various embodiments of Xi, j is 1,
and W1 is ¨
CH(R7)C(0)N(R8)¨. In various embodiments of X1, j is 1, and W1 is
¨CH(R7)N(R8)C(0)¨.
In various embodiments of X1, j is 1, and W1 is ¨CH(R7)N(R8)S(0)¨. In various
embodiments of Xi, j is 1, and W1 is ¨CH(R7)N(R8)S(0)2¨.
[00336] In another embodiment, X1 is N.
[00337] In various embodiments, X2 is N. In other embodiments, X2 is C.
[00338] In various embodiments, E2 is ¨(W1)j -R4, where j is 0.
-88-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00339] In another embodiment, E2 is CH. In yet another embodiment, E2 is C-
halogen,
where halogen is Cl, F, Br, or I.
[00340] In various embodiments of E2, it is ¨(Wi)j ¨R4. In various embodiments
of E2, j is 1,
and W1 is ¨0¨. In various embodiments of E2, j is 1, and W1 is ¨NR7-. In
various
embodiments of E2, j is 1, and W1 is ¨NH-. In various embodiments of E2, j is
1, and W1 is
¨S(0)0_2¨. In various embodiments of E2, j is 1, and W1 is ¨C(0)¨. In various
embodiments of E2, j is 1, and W1 is ¨C(0)N(R7)¨. In various embodiments of
E2, j is 1, and
W1 is ¨N(R7)C(0)¨. In various embodiments of E2, j is 1, and W1 is
¨N(R7)S(0)¨. In
various embodiments of E2, j is 1, and W1 is ¨N(R7)S(0)2¨. In various
embodiments of E2, j
is 1, and W1 is ¨C(0)0¨. In various embodiments of E2, j is 1, and W1 is
CH(R7)N(C(0)0R8)¨. In various embodiments of E2, j is 1, and W1 is
¨CH(R7)N(C(0)R8)¨.
In various embodiments of E2, j is 1, and W1 is ¨CH(R7)N(S02R8)¨. In various
embodiments
of E2, j is 1, and W1 is ¨CH(R7)N(R8)¨. In various embodiments of E2, j is 1,
and W1 is ¨
CH(R7)C(0)N(R8)¨. In various embodiments of E2, j is 1, and W1 is
¨CH(R7)N(R8)C(0)¨.
In various embodiments of E2, j is 1, and W1 is ¨CH(R7)N(R8)S(0)¨. In various
embodiments of E2, j is 1, and W1 is ¨CH(R7)N(R8)S(0)2¨.
[00341] In various embodiments, k is 0. In other embodiments, k is 1 and W2 is
¨0¨. In
another embodiment, k is 1 and W2 is ¨NR7¨. In yet another embodiment of, k is
1, and W2
is ¨S(0)0_2¨. In another embodiment of, k is 1 and W2 is ¨C(0)¨. In a further
embodiment, k
is 1 and W2 is ¨C(0)N(R7)¨. In another embodiment, k is 1, and W2 is
¨N(R7)C(0)¨. In
another embodiment, k is 1 and W2 is ¨N(R7)C(0)N(R8)¨. In yet another
embodiment, k is 1
and W2 is ¨N(R7)S(0)¨. In still yet another embodiment, k is 1 and W2 is
¨N(R7)S(0)2¨. In
a further embodiment, k is 1 and W2 is ¨C(0)0¨. In another embodiment, k is 1
and W2 is ¨
CH(R7)N(C(0)0R8)¨. In another embodiment, k is 1 and W2 is ¨CH(R7)N(C(0)R8)¨.
In
another embodiment, k is 1 and W2 is ¨CH(R7)N(S02R8)¨. In a further
embodiment, k is 1
and W2 is ¨CH(R7)N(R8)¨. In another embodiment, k is 1 and W2 is
¨CH(R7)C(0)N(R8)¨. In
yet another embodiment, k is 1 and W2 is ¨CH(R7)N(R8)C(0)¨. In another
embodiment, k is
1 and W2 is ¨CH(R7)N(R8)S(0)¨. In yet another embodiment, k is 1 and W2 is ¨
CH(R7)N(R8)S(0)2¨.
-89-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00342] The invention also provides a compound which is an mTor inhibitor of
Formula I-E:
R31 R32
/
Mi
N
X3
R9 Ri
Formula I-E
[00343] or a pharmaceutically acceptable salt thereof, wherein: X1 is N or C-
El, X2 is N, and
X3 is C; or Xi is N or C-El, X2 is C, and X3 is N;
[00344] Ri is ¨H, ¨L-Ci_ioalkyl, -L-C3_8cycloalkyl, -L-Ci_ioalkyl -
C3_8cycloalkyl, -L- aryl, -
L-heteroaryl, -L-Ci_ioalkylaryl, -L- Ci_ioalkylheteroaryl, -L-
Ci_ioalkylheterocyclyl, -L-C2-
ioalkenYl, -L-C2_malkynyl, -L-C2_10alkenyl-C3_8cycloalkyl, -L-C2_101kynyl-
C3_8cycloalkyl, -L-
heteroalkyl, -L-heteroalkylaryl, -L-heteroalkylheteroaryl, -L-heteroalkyl-
heterocyclyl, -L-
heteroalkyl-C3_8cycloalkyl, -L-aralkyl, -L-heteroaralkyl, or -L-heterocyclyl,
each of which is
unsubstituted or is substituted by one or more independent R3;
[00345] L is absent, -(C=0)-, -C(=0)0-, -C(=0) N(R31)-,-S-, -S(0)-, -S(0)2-, -
S(0)2N(R31)-,
or
[00346] Mi is a moiety having the structure of Formula Ml-Fl or Formula Ml-F2:
R2 R2
(N2)k (N2)k
0 /
cyN
-R5
111 R5
Or '1111-
Formula M 1 -F 1 Formula Ml -F2
[00347] k is 0 or 1;
[00348] El and E2 are independently ¨(Wi)j -R4;
[00349] j in El or j in E2, is independently 0 or 1;
[00350] Wi is ¨0¨, ¨NR7¨, ¨S(0)0_2¨,¨C(0)¨,¨C(0)N(R7)¨, ¨N(R7)C(0)¨,
¨N(R7)S(0)¨,¨
N(R7)S(0)2¨, ¨C(0)0¨, ¨CH(R7)N(C(0)0R8)¨, ¨CH(R7)N(C(0)R8)¨, ¨CH(R7)N(S02R8)¨,
-90-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
-CH(R7)N(R8)-, -CH(R7)C(0)N(R8)-, -CH(R7)N(R8)C(0)-, -CH(R7)N(R8)S(0)-, or -
CH(R7)N(R8)S(0)2-;
[00351] W2 is -0-, -NR7-, -S(0)0_2-,-C(0)-,-C(0)N(R7)-, -N(R7)C(0)-, -
N(R7)C(0)N(R8)-,-N(R7)S(0)-, -N(R7)S(0)2-,-C(0)0-, -CH(R7)N(C(0)0R8)-, -
CH(R7)N(C(0)R8)-, -CH(R7)N(S02R8)-, -CH(R7)N(R8)-, -CH(R7)C(0)N(R8)-, -
CH(R7)N(R8)C(0)-, -CH(R7)N(R8)S(0)-, or -CH(R7)N(R8)S(0)2-;
[00352] R2 is hydrogen, halogen, -OH, -R31, -CF3, -0CF3, -0R31, -NR31R32, -
NR34R35, -
C(0)R31, -0O2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN, -S(0)0_2R31, -
SO2NR31R32, -S02NR34R355 -NR31C(=0)R325 -NR31C(=0)0R325 -NR31C(=0)NR32R335 -
NR31S(0)0_2R325 -C(=S)0R315 -C(=0)SR315 -NR31C(=NR32)NR33R325 -
NR31C(=NR32)0R335
-NR31C(=NR32)SR335 -0C(=0)0R335 -0C(=0)NR31R325 -0C(=0)SR315 -SC(=0)0R315 -
P(0)0R310R325 -SC(=0)NR31R32 5 aryl (e.g. bicyclic aryl, unsubstituted aryl,
or substituted
monocyclic aryl), heteroaryl, Ci_malkyl, C3_8 cycloalkyl, Ci_malkyl-
C3_8cycloalkyl, C3_
8 cycloalkyl -Ci_malkyl, C3_8 cycloalkyl -C2_10alkenyl, C3_8cycloalkyl-
C2_10alkynyl, Ci_malkyl-
C2_10alkenyl, Ci_ioalkyl- C2_10alkynyl, Ci_malkylaryl (e.g. C240alkyl-
monocyclic aryl, C1-
malkyl-substituted monocyclic aryl, or Ci_i0alkylbicycloary1),
Ci_malkylheteroaryl, C1_
loalkylheterocyclyl, C2_10alkenyl, C2_10alkynyl, C2_10alkenyl -Ci_i0alkyl,
C2_10alkynY1 -Ci-
ioalkyl, C2_10alkenylaryl, C2_10alkenylheteroaryl, C2_10alkenylheteroalkyl, C
2-
loalkenylheterocycicyl, C2_10alkenyl-C3_8cycloalkyl, C2_10alkynylaryl,
C2_10alkynylheteroaryl,
C240alkynylheteroalkyl, C240alkynylheterocyclyl, C2_10alkynyl-
C3_8cycloalkenyl, Ci_malkoxy
Ci_malkyl, Ci_malkoxy-C2_10alkenyl, Ci_malkoxy-C2_10alkynyl, heterocyclyl -
Ci_malkyl,
heterocyclyl-C240alkenyl, heterocyclyl-C240alkynyl, aryl- Ci_i0alkyl (e.g.
monocyclic aryl-C2
malkyl, substituted monocyclic aryl- Ci_malkyl, or bicycloaryl--Ci_ioalkyl),
aryl-C2_10alkenyl,
aryl-C2_10alkynyl, aryl-heterocyclyl, heteroaryl-Ci_i0alkyl, heteroaryl-
C2_10alkenyl,
heteroaryl-C2_10alkynyl, heteroaryl-C3_8cycloalkyl, heteroaryl-heteroalkyl, or
heteroaryl-
heterocyclyl, wherein each of said bicyclic aryl or heteroaryl moiety is
unsubstituted, or
wherein each of bicyclic aryl, heteroaryl moiety or monocyclic aryl moiety is
substituted with
one or more independent alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl,
aryl, arylalkyl, heteroaryl, heteroarylalkyl, halo, -OH, -R31, -CF3, -OC F35 -
0R315 -NR31R325
-NR34R355 -C(0)R315 -0O2R315 -C(=0)NR31R325 -C(=0)NR34R355 -NO2, -CN, -
S(0)0_2R31,
-SO2NR31R325 -S02NR34R355 -NR31C(=0)R325 -NR31C(=0)0R325 -NR31C(=0)NR32R335 -
NR31S(0)0_2R325 -C(=S)0R315 -C(=0)SR315 -NR31C(=NR32)NR33R325 -
NR31C(=NR32)0R335
-NR31C(=NR32)SR335 -0C(=0)0R335 -0C(=0)NR31R325 -0C(=0)SR315 -SC(=0)0R315 -
P(0)0R310R325 or-SC(=0)NR31R32, and wherein each of said alkyl, cycloalkyl,
heterocyclyl,
-91-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
or heteroalkyl moiety is unsubstituted or is substituted with one or more
alkyl, heteroalkyl,
alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl,
heteroarylalkyl,
halo, -OH, -R31, -CF3, -0CF3, -0R31, -0-aryl, -NR31R32, -NR34R35 ,-C(0)R31, -
CO2R31, -
C(=0)NR34R35, or -C(=0)NR31R32;
1003531 R3 and R4 are independently hydrogen, halogen, -OH, -R31, -CF3, -0CF3,
-0R31, -
NR31R32, -NR34R35, -C(0)R31, -CO2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN,
-
S(0)0_2R31, -SO2NR31R32, -S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -
NR31C(=0)NR32R33, -NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -
NR31C(=NR32)NR33R32,
-NR31C(=NR32)0R33, -NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -
OC(=0)SR31, -SC(=0)0R31, -P(0)0R310R32, -SC(=0)NR31R32 , aryl, heteroaryl,
Ci_4alkyl,
C3_8cycloalkyl, Ci_ioalkyl-C3 -8 cycloalkyl, C 3 _8 cycloalkyl C3
8 eyeloalkyl -C2_10alkenyl, C 3 _8 cycloalkyl- C2_10alkynyl,
C2_10alkenyl, Ci_ioalkyl- C 2-
ioalkYnyl, C iioaIkylaryl, Ci_i0alkylheteroaryl, Ci_malkylheterocyclyl,
C2_10alkenY15 C 2-
1 0 alkynyl, C2_10 alkenyl -Ci_i0alkyl, C2_10 alkynyl -Ci_i0alkyl, C2_10
alkenylaryl, C 2-
1 0 alkenylheteroaryl, C2_10 alkenylheteroalkyl, C2-10 alkenylheterocycicyl,
C2_10 alkenyl-C 3_
8 cycloalkyl, C2_10 alkynyl-C 3_8 cyclo alkyl, C2_10 alkynylaryl, C2_10
alkynylheteroaryl, C2
malkynylheteroalkyl, C240alkynylheterocyclyl, C2_10alkynyl-C3_8cycloalkenyl,
Ci_malkoxy C1_
ioalkyl, Ci_loalkoxy-C2_10alkenyl, Ci_malkoxy-C2_10alkynyl, heterocyclyl,
heterocyclyl -C1_
heterocyclyl-C240alkenyl, heterocyclyl-C240alkynyl, aryl- Ci_malkyl, aryl-C 2-
0 alkenyl, aryl-C 2_1 0 alkynyl, aryl-heterocyclyl, heteroaryl-Ci_i0alkyl,
heteroaryl-C 2_1 0 alkenyl,
heteroaryl-C240alkynyl, heteroaryl-C3_8cycloalkyl, heteroalkyl, heteroaryl-
heteroalkyl, or
heteroaryl-heterocyclyl, wherein each of said aryl or heteroaryl moiety is
unsubstituted or is
substituted with one or more independent halo, -OH, -R31, -CF3, -0CF3, -0R31, -
NR31R32, -
NR34R35, -C(0)R31, -0O2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN, -
S(0)0_2R31, -
SO2NR31R32, -S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R33, -
NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -NR31C(=NR32)NR33R32, -
NR31C(=NR32)0R33,
-NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -0C(=0)SR31, -SC(=0)0R31, -
P(0)0R310R32, or-SC(=0)NR31R32, and wherein each of said alkyl, cycloalkyl,
heterocyclyl,
or heteroalkyl moiety is unsubstituted or is substituted with one or more
halo, -OH, -R31, -
CF3, -0CF3, -0R31, -0-aryl, -NR31R32, -NR34R35 ,-C(0)R31, -0O2R31, -
C(=0)NR34R35, or
-C(=0)NR31R32;
[00354] R5 is hydrogen, halogen, -OH, -R31, -CF3, -0CF3, -0R31, -NR31R32, -
NR34R35, -
C(0)R31, -0O2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN, -S(0)0_2R31, -
SO2NR3 IR32, -S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R33, -
-92-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -NR31C(=NR32)NR33R32, -
NR31C(=NR32)0R33,
-NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -0C(=0)SR31, -SC(=0)0R31, -
P(0)0R310R32,or -SC(=0)NR31R32;
[00355] R31, R32, and R33, in each instance, are independently H or Ci_ioalkyl
, wherein the
Ci_malkyl is unsubstituted or is substituted with one or more aryl,
heteroalkyl, heterocyclyl,
or heteroaryl group wherein each of said aryl, heteroalkyl, heterocyclyl, or
heteroaryl group is
unsubstituted or is substituted with one or more halo, -OH, - Ci_malkyl, -CF3,
-0-aryl, -
0CF3, -0C1_10alkyl, -NH2, - N(Ci_malkyl)(Ci_malkyl), - NH(Ci_malkyl), - NH(
aryl), -
NR34R35, -C(0)(Ci_malkyl), -C(0)(Ci_malkyl-ary1), -C(0)(ary1), -0O2-Ci_malkyl,
-0O2-C1-
malkylaryl, -0O2-aryl, -C(=0)N(Ci_i0alkyl)( Ci_malkyl), -C(0)NH( Ci_malkyl), -
C(0)NR34R35, -C(0)NH2, -0CF3, -0(Ci_ioalkyl), -0-aryl, -N(ary1)( Ci-malkyl), -
NO2, -
CN, -S(0)0_2 Ci_malkyl, -S(0)0_2 Ci_malkylaryl, -S(0)0_2 aryl, -SO2N(ary1), -
SO2 N(Ci-
malkyl)( Ci_malkyl), -SO2 NH(Ci_malkyl) or -S02NR34R35;
[00356] R34 and R35 in -NR34R35, -C(=0)NR34R35, or -S02NR34R35, are taken
together with
the nitrogen atom to which they are attached to form a 3-10 membered saturated
or
unsaturated ring; wherein said ring is independently unsubstituted or is
substituted by one or
more -NR31R32, hydroxyl, halogen, oxo, aryl, heteroaryl, Ci_6alkyl, or 0-aryl,
and wherein
said 3-10 membered saturated or unsaturated ring independently contains 0, 1,
or 2 more
heteroatoms in addition to the nitrogen atom;
[00357] R7 and R8 are each independently hydrogen, Ci_malkyl, C2_10alkenyl,
aryl,
heteroaryl, heterocyclyl or C3_10cycloalkyl, each of which except for hydrogen
is
unsubstituted or is substituted by one or more independent R6;
[00358] R6 is halo, -0R31, -SH, -NH2, -NR34R35 , -NR31R32, -CO2R31, -0O2aryl, -
C(0)NR31R32, C(0)NR34R35 , -NO2, -CN, -S(0)0-2 Ci_i0alkyl, -5(0) 0_2aryl, -
SO2NR34R35, -SO2NR31R32, Ci_malkyl, C2_10alkenyl, C2_10alkynyl; aryl-
Ci_malkyl, aryl-C 2-
1 oalkenyl, aryl-C 2_ 1 oalkynyl, heteroaryl-Ci_i0alkyl, heteroaryl-C 2_ 1
oalkenyl, heteroaryl-C 2-
malkynyl, wherein each of said alkyl, alkenyl, alkynyl, aryl, heteroalkyl,
heterocyclyl, or
heteroaryl group is unsubstituted or is substituted with one or more
independent halo, cyano,
nitro, -0Ci_walkyl, Ci_malkyl, C2_malkenyl, C2_malkynyl, haloCi_malkyl,
haloC2_malkenyl,
haloC2_10alkynyl, -COOH, -C(=0)NR31R32, -C(0)NR34R35 , -SO2NR34R35, -SO2
NR31R32,
-NR31R32, or -NR34R35; and
[00359] R9 is H, halo, -0R31, -SH, -NH2, -NR34R35 , - NR31R32, -0O2R31, -
0O2aryl, -
C(=0)NR31R32, C(=0)NR34R35 , -NO2, -CN, -S(0)0_2 Ci_i0alkyl, -5(0) 0_2aryl, -
SO2NR34R35, -S02NR31R32, Ci_malkyl, C2_10alkenyl, C2_10alkynyl; aryl-
Ci_malkyl, aryl-C 2-
-93 -
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
io alkenyl, aryl-C2_10alkynyl, heteroaryl-Ci_i0alkyl, heteroaryl-C2_10alkenyl,
heteroaryl-C2-
ioalkynyl, wherein each of said alkyl, alkenyl, alkynyl, aryl, heteroalkyl,
heterocyclyl, or
heteroaryl group is unsubstituted or is substituted with one or more
independent halo, cyano,
nitro, ¨0C1_10alkyl, C iioaIkyl, C2_10alkenyl, C2_10alkynyl, haloC iioalkyl,
haloC2_10alkenyl,
haloC2_10alkynyl, ¨COOH, _c(="R"R32, ¨C(=0)NR34R" , ¨S02NR34R35, ¨SO2 NR"R32,
-NR3'R32, or ¨NR"R".
[00360] In various embodiments of the compound of Formula I-E, the compound
has a
structure of Formula I-E1 or Formula I-E2:
R31 R32
R31 0432
/r
M1 M
N N
0 0 Xi 0 0 Xi
E2 X2 E2 N
X2
R9 R1 R9
Formula I-E1 Formula I-E2
or a pharmaceutically acceptable salt thereof
[00361] In some embodiments of Formula I-El, X1 is N and X2 is N. In other
embodiments,
Xi is C-E1 and X2 is N. In yet other embodiments, Xi is NH and X2 is C. In
further
embodiments, X1 is CH-E1 and X2 is C.
[00362] In several embodiments of Formula I-E2, X1 is N and X2 is C. In
further
embodiments, Xi is C-E1 and X2 is C.
[00363] In various embodiments, Xi is C¨(Wi)j -R4, where j is 0.
[00364] In another embodiment, Xi is CH. In yet another embodiment, Xi is C-
halogen,
where halogen is Cl, F, Br, or I.
[00365] In various embodiments of Xi, it is C ¨(Wi)j ¨R4. In various
embodiments of Xi, j is
1, and W1 is ¨0¨. In various embodiments of Xi, j is 1, and W1 is ¨NR7-. In
various
embodiments of Xi, j is 1, and W1 is ¨NH-. In various embodiments of Xi, j is
1, and W1 is
¨S(0)0_2¨. In various embodiments of Xi, j is 1, and W1 is ¨C(0)¨. In various
embodiments of Xi, j is 1, and W1 is ¨C(0)N(R7)¨. In various embodiments of
Xi, j is 1,
and W1 is ¨N(R7)C(0)¨. In various embodiments of Xi, j is 1, and W1 is
¨N(R7)S(0)¨. In
various embodiments of Xi, j is 1, and W1 is ¨N(R7)S(0)2¨. In various
embodiments of Xi,
is 1, and W1 is ¨C(0)0¨. In various embodiments of Xi, j is 1, and W1 is
CH(R7)N(C(0)0R8)¨. In various embodiments of Xi, j is 1, and W1 is
¨CH(R7)N(C(0)R8)¨.
-94-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
In various embodiments of Xi, j is 1, and W1 is ¨CH(R7)N(S02R8)¨. In various
embodiments
of Xi, j is 1, and W1 is ¨CH(R7)N(R8)¨. In various embodiments of Xi, j is 1,
and W1 is ¨
CH(R7)C(0)N(R8)¨. In various embodiments of Xi, j is 1, and W1 is
¨CH(R7)N(R8)C(0)¨.
In various embodiments of Xi, j is 1, and W1 is ¨CH(R7)N(R8)S(0)¨. In various
embodiments of X1, j is 1, and W1 is ¨CH(R7)N(R8)S(0)2¨.
[00366] In another embodiment, X1 is N.
[00367] In various embodiments, X2 is N. In other embodiments, X2 is C.
[00368] In various embodiments, E2 is ¨(Wi)j -R4, where j is 0.
[00369] In another embodiment, E2 is CH. In yet another embodiment, E2 is C-
halogen,
where halogen is Cl, F, Br, or I.
[00370] In various embodiments of E2, it is ¨(Wi)j ¨R4. In various embodiments
of E2, j is 1,
and W1 is ¨0¨. In various embodiments of E2, j is 1, and W1 is ¨NR7-. In
various
embodiments of E2, j is 1, and W1 is ¨NH-. In various embodiments of E2, j is
1, and W1 is
¨S(0)0_2¨. In various embodiments of E2, j is 1, and W1 is ¨C(0)¨. In various
embodiments of E2, j is 1, and W1 is ¨C(0)N(R7)¨. In various embodiments of
E2, j is 1, and
W1 is ¨N(R7)C(0)¨. In various embodiments of E2, j is 1, and W1 is
¨N(R7)S(0)¨. In
various embodiments of E2, j is 1, and W1 is ¨N(R7)S(0)2¨. In various
embodiments of E2, j
is 1, and W1 is ¨C(0)0¨. In various embodiments of E2, j is 1, and W1 is
CH(R7)N(C(0)0R8)¨. In various embodiments of E2, j is 1, and W1 is
¨CH(R7)N(C(0)R8)¨.
In various embodiments of E2, j is 1, and W1 is ¨CH(R7)N(S02R8)¨. In various
embodiments
of E2, j is 1, and W1 is ¨CH(R7)N(R8)¨. In various embodiments of E2, j is 1,
and W1 is ¨
CH(R7)C(0)N(R8)¨. In various embodiments of E2, j is 1, and W1 is
¨CH(R7)N(R8)C(0)¨.
In various embodiments of E2, j is 1, and W1 is ¨CH(R7)N(R8)S(0)¨. In various
embodiments of E2, j is 1, and W1 is ¨CH(R7)N(R8)S(0)2¨.
[00371] In various embodiments when M1 is a moiety of Formula I-El, M1 is
benzoxazolyl
substituted with ¨(W2)k-R2. In some embodiments, M1 is a benzoxazolyl moiety,
substituted
at the 2-position with ¨(W2)k-R2. In some embodiments, M1 is either a 5-
benzoxazolyl or a
6- benzoxazolyl moiety, optionally substituted with ¨(W2)k-R2. Exemplary
Formula I-E1 M1
moieties include but are not limited to the following:
-95-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
-- R2
__-R2 (w2)k
- R2 oN2)k0 --/
(W2)k
O---(
(w2)k R2 -cs____3:::,-( \\
0-1(
R5---../._ \ N i \ N R5---;!. \ N
IR5/\ \ \II\I
R5 SS'S
5 5
R2 R2 5
\ \
0N2)k (w2)k
0-- N
o-- m ---11 I - N
r R2
/ \ / \ 0 // \ (W2rk R2 \ I?)
R5/----
R5
vliv v1,1, -R5 ssy
5 5 ,and .
[00372] In various embodiments when M1 is a moiety of Formula I-E2, Formula I-
E2 is an
aza-substituted benzoxazolyl moiety having a structure of one of the following
formulae:
....., R2 _. R2
2 R2
(w2)k k ---
(W \
(w2)
0 -- 0 ---
11 0R1 0._...µ "
N N N
AR5
La c? N 5 GA:
5 .2- 5 5
........ R2 R2 R2
...--- ..---
(My (w2)k (vv2)k
O7 I` 0 ---. 0 ----
o
ON N cµ11\1
R5 I\PI;1- R5
L'ill LL \--N R5
Laa ,or
õ.õ..- R2
(w2)k
0 ---
N -Ui
'1.7 .1\1"-j R5
[00373] Exemplary Formula I-E2 M1 moieties include but are not limited to the
following:
-96-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
R2
o
2 R2
)k tw 2 \
\Al2)k R2 5'53 (:)(k ik
R5
N
R5J.:26õ.cm N 0 N
(2?,
R5/
5 5
R2 R2
"2)k
(W 2) k
0 0 N
0 =-= N
R5 --- N
0)1,, R2 No R5 2 R2
R5-- 0 oiv2)k R5¨ 0 OA/ k
.SS5 5 '111-tr 5 and
R2
(W2)k
- N
R5 _C)
[00374] In various embodiments of Mi, k is O. In other embodiments of Mi, k is
1 and W2 is
¨0¨. In another embodiment of Mi, k is 1 and W2 is ¨NR7¨. In yet another
embodiment of
M1, k is 1 and W2 is ¨S(0)0_2¨. In another embodiment of M1, k is 1 and W2 is
¨C(0)¨. In a
further embodiment of M1, k is 1 and W2 is ¨C(0)N(R7)¨. In another embodiment
of M1, k is
1 and W2 is ¨N(R7)C(0)¨. In another embodiment, k is 1 and W2 is
¨N(R7)C(0)N(R8)¨. In
yet another embodiment of Mi, k is 1 and W2 is ¨N(R7)S(0)¨. In still yet
another
embodiment of M1, k is 1 and W2 is ¨N(R7)S(0)2¨. In a further embodiment of
M1, k is 1
and W2 is ¨C(0)0¨. In another embodiment of M1, k is 1 and W2 is
¨CH(R7)N(C(0)0R8)¨.
In another embodiment of Mi, k is 1 and W2 is ¨CH(R7)N(C(0)R8)¨. In another
embodiment
of M1, k is 1 and W2 is ¨CH(R7)N(S02R8)¨. In a further embodiment of M1, k is
1 and W2 is
¨CH(R7)N(R8)¨. In another embodiment of M1, k is 1 and W2 is
¨CH(R7)C(0)N(R8)¨. In yet
another embodiment of M1, k is 1 and W2 is ¨CH(R7)N(R8)C(0)¨. In another
embodiment of
M1, k is 1 and W2 is ¨CH(R7)N(R8)S(0)¨. In yet another embodiment of M1, k is
1 and W2 is
¨CH(R7)N(R8)S(0)2¨.
[00375] Additional embodiments of compounds of Formula I, including I-A, I-B,
I-C, I-D, I-
E and others are described below.
[00376] In various embodiments of compounds of Formula I, L is absent. In
another
embodiment, L is ¨(C=0)-. In another embodiment, L is -C(=0)0-. In a further
embodiment,
L is -C(=0) NR31-. In yet another embodiment, L is -S-. In one embodiment, L
is -S(0)-. In
-97-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
another embodiment, L is -S(0)2-. In yet another embodiment, L is -S(0)2NR31-.
In another
embodiment, L is -NR31- .
[00377] In various embodiments of compounds of Formula I, R1 is ¨L-Ci_ioalkyl,
which is
unsubstituted. In another embodiment, R1 is ¨L-Ci_ioalkyl, which is
substituted by one or
more independent R3. In yet another embodiment, R1 is ¨L- unsubstituted
Ci_ioalkyl, where L
is absent. In another embodiment, R1 is ¨L-Ci_ioalkyl, which is substituted by
one or more
independent R3, and L is absent.
[00378] In various embodiments of compounds of Formula I, R1 is -L-
C3_8cycloalkyl, which
is unsubstituted. In another embodiment, R1 is L-C3_8cycloalkyl, which is
substituted by one
or more independent R3. In yet another embodiment, R1 is -L-C3_8cycloalkyl,
which is
unsubstituted, and L is absent. In a further embodiment, R1 is -L-
C3_8cycloalkyl which is
substituted by one or more independent R3, and L is absent.
[00379] In various embodiments of compounds of Formula I, R1 is H.
[00380] In various embodiments of compounds of Formula I, R1 is -L- aryl,
which is
unsubstituted. In another embodiment, R1 is -L- aryl, which is substituted by
one or more
independent R3. In another embodiment, R1 is -L- aryl which is unsubstituted,
and L is
absent. In yet another embodiment, R1 is -L- aryl, which is substituted by one
or more
independent R3, and L is absent.
[00381] In various embodiments of compounds of Formula I, R1 is -L-heteroaryl,
which is
unsubstituted. In another embodiment, R1 is -L-heteroaryl, which is
substituted by one or
more independent R3. In a further embodiment, R1 is -L-heteroaryl which is
unsubstituted
and L is absent. In yet another embodiment, R1 is -L- heteroaryl, which is
substituted by one
or more independent R3, and L is absent.
[00382] In various embodiments of compounds of Formula I, R1 is - L-
Ci_ioalkyl -C3_
8cycloalkyl, which is unsubstituted. In another embodiment, R1 is - L-
Ci_ioalkyl -C3_
8cycloalkyl, which is substituted by one or more independent R3. In a further
embodiment,
R1 is - L- Ci_ioalkyl -C3_8cycloalkyl which is unsubstituted and L is absent.
In yet another
embodiment, R1 is - L- Ci_ioalkyl -C3_8cycloalkyl, which is substituted by one
or more
independent R3, and L is absent.
[00383] In various embodiments of compounds of Formula I, R1 is - L-
Ci_ioalkylaryl, which
is unsubstituted. In another embodiment, R1 is - L-Ci_ioalkylaryl, which is
substituted by one
or more independent R3. In a further embodiment, R1 is - L-Ci_ioalkylaryl
which is
unsubstituted and L is absent. In yet another embodiment, R1 is - L-
Ci_ioalkylaryl, which is
substituted by one or more independent R3, where L is absent.
-98-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00384] In various embodiments of compounds of Formula I, R1 is -L-
Ci_malkylheteroaryl,
which is unsubstituted. In another embodiment, R1 is -L- Ci_malkylheteroaryl,
which is
substituted by one or more independent R3. In a further embodiment, R1 is -L-
C1_
malkylheteroaryl which is unsubstituted and L is absent. In yet another
embodiment, R1 is -L-
C i_malkylheteroaryl, which is substituted by one or more independent R3,
where L is absent.
[00385] In various embodiments of compounds of Formula I, R1 is -L- Ci-
malkylheterocyclyl, which is unsubstituted. In another embodiment, R1 is -L-
C1_
malkylheterocyclyl, which is substituted by one or more independent R3. In a
further
embodiment, R1 is -L- Ci_loalkylheterocycly1 which is unsubstituted and L is
absent. In yet
another embodiment, R1 is -L- Ci_loalkylheterocyclyl, which is substituted by
one or more
independent R3, where L is absent.
[00386] In various embodiments of compounds of Formula I, R1 is -L-
C2_10alkenyl, which is
unsubstituted. In another embodiment, R1 is -L-C2_10alkenyl which is
substituted by one or
more independent R3. In a further embodiment, R1 is -L-C2_10alkenyl which is
unsubstituted
and L is absent. In yet another embodiment, R1 is -L-C2_10alkenyl, which is
substituted by
one or more independent R3, where L is absent.
[00387] In various embodiments of compounds of Formula I, R1 is -L-
C2_10alkynyl, which is
unsubstituted. In another embodiment, R1 is -L-C2_10alkynyl which is
substituted by one or
more independent R3. In a further embodiment, R1 is -L-C2_10alkynyl which is
unsubstituted
and L is absent. In yet another embodiment, R1 is -L-C2_10alkynyl, which is
substituted by
one or more independent R3, where L is absent.
[00388] In various embodiments of compounds of Formula I, R1 is -L-
C2_10alkenyl-C3_
8cycloalkyl, which is unsubstituted. In another embodiment, R1 is -L-
C2_10alkenyl-C3_
8cycloalkyl which is substituted by one or more independent R3. In a further
embodiment, R1
is -L-C2_10alkenyl-C3_8cycloalkyl which is unsubstituted and L is absent. In
yet another
embodiment, R1 is -L-C2_10alkenyl-C3_8cycloalkyl, which is substituted by one
or more
independent R3, where L is absent.
[00389] In various embodiments of compounds of Formula I, R1 is -L-
C2_10alkynyl-C3_
8cycloalkyl, which is unsubstituted. In another embodiment, R1 is -L-
C2_10alkynyl-C3_
8cycloalkyl which is substituted by one or more independent R3. In a further
embodiment, R1
is -L-C2_10alkynyl-C3_8cycloalkyl which is unsubstituted and L is absent. In
yet another
embodiment, R1 is -L-C2_10alkynyl-C3_8cycloalkyl, which is substituted by one
or more
independent R3, where L is absent.
-99-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00390] In various embodiments of compounds of Formula I, R1 is -L-
C2_10alkynyl-C3_
8cycloalkyl, which is unsubstituted. In another embodiment, R1 is -L-
C2_10alkynyl-C3_
8cycloalkyl which is substituted by one or more independent R3. In a further
embodiment, R1
is -L-C2_10alkynyl-C3_8cycloalkyl which is unsubstituted and L is absent. In
yet another
embodiment, R1 is -L-C2_10alkynyl-C3_8cycloalkyl, which is substituted by one
or more
independent R3, where L is absent.
[00391] In various embodiments of compounds of Formula I, R1 is -L-
heteroalkyl, which is
unsubstituted. In another embodiment, R1 is -L-heteroalkyl which is
substituted by one or
more independent R3. In a further embodiment, R1 is -L-heteroalkyl which is
unsubstituted
and L is absent. In yet another embodiment, R1 is -L-heteroalkyl, which is
substituted by one
or more independent R3, where L is absent.
[00392] In various embodiments of compounds of Formula I, R1 is -L-
heteroalkylaryl, which
is unsubstituted. In another embodiment, R1 is -L-heteroalkylaryl which is
substituted by one
or more independent R3. In a further embodiment, R1 is -L-heteroalkylaryl
which is
unsubstituted and L is absent. In yet another embodiment, R1 is -L-
heteroalkylaryl, which is
substituted by one or more independent R3, where L is absent.
[00393] In various embodiments of compounds of Formula I, R1 is -L-
heteroalkylheteroaryl,
which is unsubstituted. In another embodiment, R1 is -L-heteroalkylheteroaryl,
which is
substituted by one or more independent R3. In a further embodiment, R1 is -L-
heteroalkylheteroaryl which is unsubstituted and L is absent. In yet another
embodiment, R1
is -L-heteroalkylheteroaryl, which is substituted by one or more independent
R3, where L is
absent.
[00394] In various embodiments of compounds of Formula, R1 is -L-heteroalkyl-
heterocyclyl, which is unsubstituted. In another embodiment, R1 is -L-
heteroalkyl-
heterocyclyl, which is substituted by one or more independent R3. In a further
embodiment,
R1 is -L-heteroalkyl-heterocyclyl which is unsubstituted, and L is absent. In
yet another
embodiment, R1 is -L-heteroalkyl-heterocyclyl, which is substituted by one or
more
independent R3, where L is absent.
[00395] In various embodiments of compounds of Formula I, R1 is -L-heteroalkyl-
C3_
8cycloalkyl, which is unsubstituted. In another embodiment, R1 is -L-
heteroalkyl-C3_
8cycloalkyl, which is substituted by one or more independent R3. In a further
embodiment,
R1 is -L-heteroalkyl-C3_8cycloalkyl which is unsubstituted and L is absent. In
yet another
embodiment, R1 is -L-heteroalkyl-C3_8cycloalkyl, which is substituted by one
or more
independent R3, where L is absent.
-100-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00396] In various embodiments of compounds of Formula I, R1 is -L-aralkyl,
which is
unsubstituted. In another embodiment, R1 is -L-aralkyl, which is substituted
by one or more
independent R3. In a further embodiment, R1 is -L-aralkyl which is
unsubstituted. In yet
another embodiment, R1 is -L-aralkyl, which is substituted by one or more
independent R3,
where L is absent.
[00397] In various embodiments of compounds of Formula I, R1 is -L-
heteroaralkyl, which
is unsubstituted. In another embodiment, R1 is -L-heteroaralkyl, which is
substituted by one
or more independent R3. In a further embodiment, R1 is -L-heteroaralkyl which
is
unsubstituted and L is absent. In yet another embodiment, R1 is -L-
heteroaralkyl, which is
substituted by one or more independent R3, where L is absent.
[00398] In various embodiments of compounds of Formula I, R1 is -L-
heterocyclyl, which is
unsubstituted. In another embodiment, R1 is -L-heterocyclyl, which is
substituted by one or
more independent R3. In a further embodiment, R1 is -L-heterocyclyl which is
unsubstituted
and L is absent. In yet another embodiment, R1 is -L- heterocyclyl, which is
substituted by
one or more independent R3, where L is absent.
[00399] In various embodiments of compounds of Formula I, R1 is a substituent
as shown
below:
3-553
\ .r5s3 ssy .r-rs \
C H 3 \ \77 I
/ \NH2 r\NH2
C H 3
rric s:Nsb A..
/
\.CN itt,CN L 1 NH NH
NH
.pr-ri .Prjj
N H 53-5j Sjjj\ _____ =S`PC;! .rrjjv., xrjj
.prrj
C U
II...
HO HO
sssc iss4 isrrS'S.S.3
\ SSS$ S.533
.145
\
441* = 0
---, ..--
N
H N
H
-101-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
((,)) II.:Is Ø/ll, WIN VUllll
SjSc
,
NH (NI) OH OH CONHMe NHAc
H
0
...,
.n.nr, =rWs
( N ----- \N/
0
\--..N) N'OH N'OMe /0 OH (N---\
)
\--N \-- 1\1---2-\
Me 0
Me
=A/V`
(N ONI
sss:
N H \ N-OH N-0Me
---Ir
...-N
is?
sisix___,
/ .0H /\ 'OH 44 'OH ------\CM
OH /\ IIH2 NFI2
6
.-...k......
,CH3 ........ x
0 0 I * aNi aNr,
N
rsi lei rsi I N
H3C
N
A .
[00400] In various embodiments of compounds of Formula I, R2 is hydrogen. In
another
embodiment, R2 is halogen. In another embodiment, R2 is ¨OH. In another
embodiment, R2
is ¨R31. In another embodiment, R2 is ¨CF3. In another embodiment, R2 is
¨0CF3. In
another embodiment, R2 is ¨0R31. In another embodiment, R2 is ¨NR31R32. In
another
embodiment, R2 is ¨NR34R35. In another embodiment, R2 is ¨C(0)R31. In another
embodiment, R2 is ¨0O2R31. In another embodiment, R2 is ¨C(=0)NR31R32. In
another
embodiment, R2 is ¨C(=0)NR34R35. In another embodiment, R2 is -NO2. In another
embodiment, R2 is ¨CN. In another embodiment, R2 is ¨S(0)0_2R3* In another
embodiment,
R2 is ¨S02NR31R32. In another embodiment, R2 is ¨S02NR34R35. In another
embodiment, R2
is -NR31C(=0)R32. In another embodiment, R2 is ¨NR31C(=0)0R32. In another
embodiment,
R2 is ¨NR31C(=0)NR32R33. In another embodiment, R2 is ¨NR31S(0)0 2R32. In
another
embodiment, R2 is ¨C(=S)0R31. In another embodiment, R2 is ¨C(=0)SR31. In
another
embodiment, R2 is ¨NR31C(=NR32)NR33R32. In another embodiment, R2 is ¨
-102-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
NR31C(=NR32)0R33. In another embodiment, R2 is -NR31C(=NR32)SR33. In another
embodiment, R2 is -0C(=0)0R33. In another embodiment, R2 is -0C(=0)NR31R32. In
another embodiment, R2 is -0C(=0)SR31. In another embodiment, R2 is -
SC(=0)0R31. In
another embodiment, R2 is -P(0)0R310R32. In another embodiment, R2 is -
SC(=0)NR31R32.
In another embodiment, R2 is monocyclic aryl. In another embodiment, R2 is
bicyclic aryl.
In another embodiment, R2 is substituted monocyclic aryl. In another
embodiment, R2 is
heteroaryl. In another embodiment, R2 is Ci_4alkyl. In another embodiment, R2
is Ci_ioalkyl.
In another embodiment, R2 is C3_8cycloalkyl. In another embodiment, R2 is
C3_8cycloalkyl-
Ci_ioalkyl. In another embodiment, R2 is Ci_ioalkyl -C3_8cycloalkyl. In
another embodiment,
R2 is Ci_malkyl-monocyclic aryl. In another embodiment, R2 is C240alkyl-
monocyclic aryl.
In another embodiment, R2 is monocyclic aryl- C2_10alkyl. In another
embodiment, R2 is C1_
malkyl-bicyclic aryl. In another embodiment, R2 is bicyclic aryl- Ci_ioalkyl.
In another
embodiment, R2 is - Ci_ioalkylheteroaryl. In another embodiment, R2 is - C1-
malkylheterocyclyl. In another embodiment, R2 is -C2_10alkenyl. In another
embodiment, R2
is -C2_10alkynyl. In another embodiment, R2 is C240alkenylaryl. In another
embodiment, R2 is
C240alkenylheteroaryl. In another embodiment, R2 is C240alkenylheteroalkyl. In
another
embodiment, R2 is C240alkenylheterocycicyl. In another embodiment, R2 is -
C2_10alkynylaryl.
In another embodiment, R2 is -C240alkynylheteroaryl. In another embodiment, R2
is -C2_
malkynylheteroalkyl. In another embodiment, R2 is -C240alkynylheterocyclyl. In
another
embodiment, R2 is -C2_10alkyny1C3_8cycloalkyl. In another embodiment, R2 is -
C2_
loalkYny1C3_8cycloalkenyl. In another embodiment, R2 is - Ci_loalkoxy-
Ci_loalkyl. In another
embodiment, R2 is - Ci_loalkoxy-C2_10alkenyl. In another embodiment, R2 is -
Ci_loalkoxy-
C2_10alkynyl. In another embodiment, R2 is -heterocyclyl Ci_ioalkyl. In
another embodiment,
R2 is heterocycly1C240alkenyl. In another embodiment, R2 is
heterocycly1C240alkynyl. In
another embodiment, R2 is aryl-C2_10alkyl. In another embodiment, R2 is aryl-
Ci_ioalkyl. In
another embodiment, R2is aryl-C2_10alkenyl. In another embodiment, R2 is aryl-
C2_10alkynyl.
In another embodiment, R2 is aryl-heterocyclyl. In another embodiment, R2 is
heteroaryl- C1_
malkyl. In another embodiment, R2 is heteroaryl-C240alkenyl. In another
embodiment, R2 is
heteroaryl-C240alkynyl. . In another embodiment, R2 is heteroaryl-
C3_8cycloalkyl. In
another embodiment, R2 is heteroaryl- heteroalkyl. In another embodiment, R2
is heteroaryl-
heterocyclyl.
[00401] In various embodiments of compounds of Formula I, when R2 is bicyclic
aryl,
monocyclic aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, heterocyclyl,
heteroalkyl, C2-
malkenyl, C2_ioalkynyl, monocyclic aryl-C2_ioalkyl, heterocyclyl Ci_ioalkyl,
or C3_8cycloalkyl-
-1 03 -
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Ci_ioalkyl, it is unsubstituted. In various embodiments, when R2 is bicyclic
aryl, monocyclic
aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_
malkynyl, monocyclic aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl, or
C3_8cycloalkyl- Ci_ioalkyl, it
is substituted with one or more independent halo. In another embodiment, when
R2 is
bicyclic aryl, monocyclic aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl,
heterocyclyl,
heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or
C3_8cycloalkyl- Ci_ioalkyl, it is substituted with one or more independent -
OH. In another
embodiment, when R2 is bicyclic aryl, monocyclic aryl, heteroaryl, Ci_ioalkyl,
C3_8cycloalkyl,
heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-
C2_ioalkyl, heterocyclyl
Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is substituted with one or more
independent -R31.
In another embodiment, when R2 is bicyclic aryl, monocyclic aryl, heteroaryl,
Ci_ioalkyl, C3_
8cycloalkyl, heterocyclyl, heteroalkyl, C2_ioalkenyl, C2_10alkynyl, monocyclic
aryl-C2_ioalkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is substituted with
one or more
independent -CF3. In another embodiment, when R2 is bicyclic aryl, monocyclic
aryl,
heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_10alkynyl,
monocyclic aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl-
Ci_ioalkyl, it is
substituted with one or more independent -0CF. In another embodiment, when R2
is bicyclic
aryl, monocyclic aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, heterocyclyl,
heteroalkyl, C2_
malkenyl, C2_10alkynyl, monocyclic aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl,
or C3_8cycloalkyl-
Ci_ioalkyl, it is substituted with one or more independent -0R31. In another
embodiment,
when R2 is bicyclic aryl, monocyclic aryl, heteroaryl, Ci_ioalkyl,
C3_8cycloalkyl, heterocyclyl,
heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or
C3_8cycloalkyl- Ci_ioalkyl, it is substituted with one or more independent -
NR31R32. In
another embodiment, when R2 is bicyclic aryl, monocyclic aryl, heteroaryl,
Ci_ioalkyl, C3_
8cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic
aryl-C2_ioalkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is substituted with
one or more
independent -NR34R35. In another embodiment, when R4 is bicyclic aryl,
monocyclic aryl,
heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_10alkynyl,
monocyclic aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl-
Ci_ioalkyl, it is
substituted with one or more independent -C(0)R31. In another embodiment, when
R2 is
bicyclic aryl, monocyclic aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl,
heterocyclyl,
heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or
C3_8cycloalkyl- Ci_ioalkyl, it is substituted with one or more independent -
0O2R31. In
another embodiment, when R2 is bicyclic aryl, monocyclic aryl, heteroaryl,
Ci_ioalkyl, C3_
-104-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
8cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic
aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_malkyl, it is substituted with
one or more
independent -C(=0)NR31R32. In another embodiment, when R2 is bicyclic aryl,
monocyclic
aryl, heteroaryl, Ci_malkyl, C3_8cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_
malkynyl, monocyclic aryl-C2_10alkyl, heterocyclyl Ci_malkyl, or
C3_8cycloalkyl- Ci_ioalkyl, it
is substituted with one or more independent -C(=0)NR34R35. In another
embodiment, when
R2 is bicyclic aryl, monocyclic aryl, heteroaryl, Ci_malkyl, C3_8cycloalkyl,
heterocyclyl,
heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-C2_10alkyl,
heterocyclyl Ci_malkyl, or
C3_8cycloalkyl- Ci_malkyl, it is substituted with one or more independent -
NO2. In another
embodiment, when R2 is bicyclic aryl, monocyclic aryl, heteroaryl, Ci_ioalkyl,
C3_8cycloalkyl,
heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-
C2_10alkyl, heterocyclyl
Ci_malkyl, or C3_8cycloalkyl- Ci_malkyl, it is substituted with one or more
independent -CN.
In another embodiment, when R2 is bicyclic aryl, monocyclic aryl, heteroaryl,
Ci_malkyl, C3_
8cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic
aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_malkyl, it is substituted with
one or more
independent -S(0)0_2R31. In another embodiment, when R2 is bicyclic aryl,
monocyclic aryl,
heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_10alkynyl,
monocyclic aryl-C2_10alkyl, heterocyclyl Ci_malkyl, or C3_8cycloalkyl-
Ci_i0alkyl, it is
substituted with one or more independent -S02NR31R32. In another embodiment,
when R2 is
bicyclic aryl, monocyclic aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl,
heterocyclyl,
heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-C2_10alkyl,
heterocyclyl Ci_malkyl, or
C3_8cycloalkyl- Ci_malkyl, it is substituted with one or more independent -
S02NR34R35. In
another embodiment, when R2 is bicyclic aryl, monocyclic aryl, heteroaryl,
C1_10alkyl, C3_
8cycloalkyl, heterocyclyl, heteroalkyl, C2_ioalkenyl, C2_10alkynyl, monocyclic
aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_malkyl, it is substituted with
one or more
independent NR31C(=0)R32. In another embodiment, when R2 is bicyclic aryl,
monocyclic
aryl, heteroaryl, Ci_malkyl, C3_8cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_
malkynyl, monocyclic aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl, or
C3_8cycloalkyl- Ci_ioalkyl, it
is substituted with one or more independent -NR31C(=0)0R32. In another
embodiment, when
R2 is bicyclic aryl, monocyclic aryl, heteroaryl, Ci_malkyl, C3_8cycloalkyl,
heterocyclyl,
heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-C2_10alkyl,
heterocyclyl Ci_malkyl, or
C3_8cycloalkyl- Ci_malkyl, it is substituted with one or more independent -
NR31C(=0)NR32R33. In another embodiment, when R2 is bicyclic aryl, monocyclic
aryl,
heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_10alkynyl,
-105-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
monocyclic aryl-C2_10alkyl, heterocyclyl Ci_malkyl, or C3_8cycloalkyl-
Ci_i0alkyl, it is
substituted with one or more independent -NR31S(0)0_2R32. In another
embodiment, when R2
is bicyclic aryl, monocyclic aryl, heteroaryl, Ci_malkyl, C3_8cycloalkyl,
heterocyclyl,
heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-C2_10alkyl,
heterocyclyl Ci_malkyl, or
C3_8cycloalkyl- Ci_malkyl, it is substituted with one or more independent -
C(=S)0R31. In
another embodiment, when R2 is bicyclic aryl, monocyclic aryl, heteroaryl,
Ci_ioalkyl, C3_
8cycloalkyl, heterocyclyl, heteroalkyl, C2_ioalkenyl, C2_10alkynyl, monocyclic
aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_malkyl, it is substituted with
one or more
independent -C(=0)SR31. In another embodiment, when R2 is bicyclic aryl,
monocyclic aryl,
heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_10alkynyl,
monocyclic aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl-
Ci_i0alkyl, it is
substituted with one or more independent -NR31C(=NR32)NR33R32. In another
embodiment,
when R2 is bicyclic aryl, monocyclic aryl, heteroaryl, Ci_malkyl,
C3_8cycloalkyl, heterocyclyl,
heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-C2_10alkyl,
heterocyclyl Ci_malkyl, or
C3_8cycloalkyl- Ci_malkyl, it is substituted with one or more independent , -
NR31C(=NR32)0R33. In another embodiment, when R2 is bicyclic aryl, monocyclic
aryl,
heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_10alkynyl,
monocyclic aryl-C2_10alkyl, heterocyclyl Ci_malkyl, or C3_8cycloalkyl-
Ci_i0alkyl, it is
substituted with one or more independent -NR31C(=NR32)SR33. In another
embodiment,
when R2 is bicyclic aryl, monocyclic aryl, heteroaryl, Ci_malkyl,
C3_8cycloalkyl, heterocyclyl,
heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-C2_10alkyl,
heterocyclyl Ci_malkyl, or
C3_8cycloalkyl- Ci_malkyl, it is substituted with one or more independent -
0C(=0)0R33. In
another embodiment, when R2 is bicyclic aryl, monocyclic aryl, heteroaryl,
Ci_ioalkyl, C3_
8cycloalkyl, heterocyclyl, heteroalkyl, C2_ioalkenyl, C2_10alkynyl, monocyclic
aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_malkyl, it is substituted with
one or more
independent -0C(=0)NR31R32. In another embodiment, when R2 is bicyclic aryl,
monocyclic aryl, heteroaryl, Ci_malkyl, C3_8cycloalkyl, heterocyclyl,
heteroalkyl, C2-
malkenyl, C2_ioalkynyl, monocyclic aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl,
or C 3 _8 cycloalkyl-
C i_ioalkyl, it is substituted with one or more independent -0C(=0)SR31. In
another
embodiment, when R2 is bicyclic aryl, monocyclic aryl, heteroaryl, Ci_ioalkyl,
C3_8cycloalkyl,
heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-C 2_1 0
alkyl, heterocyclyl
Ci_malkyl, or C3_8cycloalkyl- Ci_malkyl, it is substituted with one or more
independent -
SC(=0)0R31. In another embodiment, when R2 is bicyclic aryl, monocyclic aryl,
heteroaryl,
Ci_malkyl, C3_8 cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl,
C2_10alkynyl, monocyclic
-106-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is
substituted with one
or more independent -P(0)0R310R32. In another embodiment, when R2 is bicyclic
aryl,
monocyclic aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, heterocyclyl,
heteroalkyl, C2-
malkenyl, C2_10alkynyl, monocyclic aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl,
or C3_8cycloalkyl-
Ci_ioalkyl, it is substituted with one or more independent -SC(=0)NR31R32. In
another
embodiment, when R2 is bicyclic aryl, monocyclic aryl, heteroaryl, Ci_ioalkyl,
C3_8cycloalkyl,
heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-
C2_10alkyl, heterocyclyl
Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is substituted with one or more
independent alkyl.
In another embodiment, when R2 is bicyclic aryl, monocyclic aryl, heteroaryl,
Ci_ioalkyl, C3_
8cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic
aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is substituted with
one or more
independent heteroalkyl. In another embodiment, when R2 is bicyclic aryl,
monocyclic aryl,
heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_10alkynyl,
monocyclic aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl-
Ci_ioalkyl, it is
substituted with one or more independent alkenyl. In another embodiment, when
R2 is
bicyclic aryl, monocyclic aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl,
heterocyclyl,
heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or
C3_8cycloalkyl- Ci_ioalkyl, it is substituted with one or more independent
alkynyl. In another
embodiment, when R2 is bicyclic aryl, monocyclic aryl, heteroaryl, Ci_ioalkyl,
C3_8cycloalkyl,
heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-
C2_10alkyl, heterocyclyl
Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is substituted with one or more
independent
cycloalkyl. In another embodiment, when R2 is bicyclic aryl, monocyclic aryl,
heteroaryl, C1_
malkyl, C3_8cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl,
monocyclic aryl-
C2_10alkyl, heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is
substituted with one or
more independent heterocycloalkyl. In another embodiment, when R2 is bicyclic
aryl,
monocyclic aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, heterocyclyl,
heteroalkyl, C2-
malkenyl, C2_10alkynyl, monocyclic aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl,
or C3_8cycloalkyl-
Ci_ioalkyl, it is substituted with one or more independent aryl. In another
embodiment, when
R2 is bicyclic aryl, monocyclic aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl,
heterocyclyl,
heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or
C3_8cycloalkyl- Ci_ioalkyl, it is substituted with one or more independent
arylalkyl. In another
embodiment, when R2 is bicyclic aryl, monocyclic aryl, heteroaryl, Ci_ioalkyl,
C3_8cycloalkyl,
heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl, monocyclic aryl-
C2_ioalkyl, heterocyclyl
Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is substituted with one or more
independent
-107-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
heteroaryl. In another embodiment, when R2 is bicyclic aryl, monocyclic aryl,
heteroaryl, C1_
C3_8cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl,
monocyclic aryl-
C2_malkyl, heterocyclyl Ci_malkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is
substituted with one or
more independent heteroarylalkyl.
[00402] In various embodiments of compounds of Formula I, R3 is hydrogen. In
another
embodiment, R3 is halogen. In another embodiment, R3 is -OH. In another
embodiment, R3
is -R31. In another embodiment, R3 is -CF3. In another embodiment, R3 is -
0CF3. In
another embodiment, R3 is -0R31. In another embodiment, R3 is -NR31R32. In
another
embodiment, R3 is -NR34R35. In another embodiment, R3 is -C(0)R31. In another
embodiment, R3 is -0O2R31. In another embodiment, R3 is -C(=0)NR31R32. In
another
embodiment, R3 is -C(=0)NR34R35. In another embodiment, R3 is -NO2. In another
embodiment, R3 is -CN. In another embodiment, R3 is -S(0)0_2R3. In another
embodiment,
R3 is -S02NR31R32. In another embodiment, R3 is -S02NR34R35. In another
embodiment, R3
is -NR31C(=0)R32. In another embodiment, R3 is -NR31C(=0)0R32. In another
embodiment,
R3 is -NR31C(=0)NR32R33. In another embodiment, R3 is -NR31S(0)0 2R32. In
another
embodiment, R3 is -C(=S)0R31. In another embodiment, R3 is -C(=0)SR31. In
another
embodiment, R3 is -NR31C(=NR32)NR33R32. In another embodiment, R3 is -
NR31C(=NR32)0R33. In another embodiment, R3 is -NR31C(=NR32)SR33. In another
embodiment, R3 is -0C(=0)0R33. In another embodiment, R3 is -0C(=0)NR31R32. In
another embodiment, R3 is -0C(=0)SR31. In another embodiment, R3 is -
SC(=0)0R31. In
another embodiment, R3 is -P(0)0R310R32. In another embodiment, R3 is -
SC(=0)NR31R32.
In another embodiment, R3 is aryl. In another embodiment, R2 is heteroaryl. In
another
embodiment, R3 is Ci_4alkyl. In another embodiment, R3 is Ci_ioalkyl. In
another
embodiment, R3 is C3_8cycloalkyl. In another embodiment, R3 is C3_8cycloalkyl-
Ci_ioalkyl.
In another embodiment, R3 is - Ci_ioalkyl -C3_8cycloalkyl. In another
embodiment, R3 is C2_
malkyl-monocyclic aryl. In another embodiment, R3 is monocyclic aryl-
C2_10alkyl. In another
embodiment, R3 is Ci_malkyl-bicyclicaryl. In another embodiment, R3 is
bicyclic aryl- C1_
malkyl. In another embodiment, R3 is Ci_malkylheteroaryl. In another
embodiment, R3 is C1_
malkylheterocyclyl. In another embodiment, R3 is C2_10alkenyl. In another
embodiment, R3 is
C2_10alkynyl. In another embodiment, R3 is C240alkenylaryl. In another
embodiment, R3 is C2_
malkenylheteroaryl. In another embodiment, R3 is C2_10.1kenylheteroalkyl. In
another
embodiment, R3 is C2_malkenylheterocycicyl. In another embodiment, R3 is -
C2_10alkynylaryl.
In another embodiment, R3 is -C240alkynylheteroaryl. In another embodiment,
R3is -C2_
malkynylheteroalkyl. In another embodiment, R3 is C240alkynylheterocyclyl. In
another
-108-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
embodiment, R3 is ¨C2_10alkyny1C3_8cycloalkyl. In another embodiment, R3 is
C2_
loalkYny1C3-8cycloalkenyl. In another embodiment, R3 is ¨ Ci_loalkoxy-
Ci_loalkyl. In another
embodiment, R3 is Ci_loalkoxy-C2_10alkenyl. In another embodiment, R3 is ¨
Ci_ioalkoxy-C2-
ioalkynyl. In another embodiment, R3is heterocyclyl- Ci_ioalkyl. In another
embodiment, R3
is ¨heterocycly1C240alkenyl. In another embodiment, R3 is heterocyclyl-
C240alkynyl. In
another embodiment, R3 is aryl¨Ci_ioalkyl. In another embodiment, R3 is
aryl¨C2_10alkenyl.
In another embodiment, R3 is aryl¨C2_10alkynyl. In another embodiment, R3 is
aryl-
heterocyclyl. In another embodiment, R3 is heteroaryl¨ Ci_ioalkyl. In another
embodiment,
R3 is heteroaryl¨C240alkenyl. In another embodiment, R3 is
heteroaryl¨C240alkynyl. . In
another embodiment, R3 is heteroaryl¨ C3_8cycloalkyl. In another embodiment,
R3 is
heteroaryl¨ heteroalkyl. In another embodiment, R3 is heteroaryl¨
heterocyclyl.
[00403] In various embodiments of compounds of Formula I, when R3 is aryl,
heteroaryl, C1_
C3_8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl
Ci_ioalkyl, or
heteroalkyl, it is unsubstituted. In another embodiment, when R3 is aryl,
heteroaryl, C1_
C3_8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl
Ci_ioalkyl, or
heteroalkyl, it is substituted with one or more independent halo. In another
embodiment,
when R3 is aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, C3_8cycloalkyl-
Ci_ioalkyl,
heterocyclyl, heterocyclyl Ci_ioalkyl, or heteroalkyl, it is substituted with
one or more
independent ¨OH. In another embodiment, when R3 is aryl, heteroaryl,
Ci_ioalkyl, C3_
8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl
Ci_ioalkyl, or heteroalkyl, it
is substituted with one or more independent ¨R31. In another embodiment, when
R3 is aryl,
heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl,
heterocyclyl, heterocyclyl
Ci_ioalkyl, or heteroalkyl, it is substituted with one or more independent
¨CF3. In another
embodiment, when R3 is aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl,
C3_8cycloalkyl- C1_
heterocyclyl, heterocyclyl Ci_ioalkyl, or heteroalkyl, it is substituted with
one or more
independent ¨0CF. In another embodiment, when R3 is aryl, heteroaryl,
Ci_ioalkyl, C3_
8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl
Ci_ioalkyl, or heteroalkyl, it
is substituted with one or more independent ¨0R31. In another embodiment, when
R3 is aryl,
heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl,
heterocyclyl, heterocyclyl
Ci_ioalkyl, or heteroalkyl, it is substituted with one or more independent
¨NR31R32. In another
embodiment, when R3 is aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl,
C3_8cycloalkyl- C1_
heterocyclyl, heterocyclyl Ci_ioalkyl, or heteroalkyl, it is substituted with
one or more
independent ¨NR34R35. In another embodiment, when R3 is aryl, heteroaryl,
Ci_ioalkyl, C3_
8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl
Ci_ioalkyl, or heteroalkyl, it
-109-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
is substituted with one or more independent ¨C(0)R31. In another embodiment,
when R3 is
aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, C3_8cycloalkyl- Ci_malkyl,
heterocyclyl,
heterocyclyl Ci_malkyl, or heteroalkyl, it is substituted with one or more
independent ¨
CO2R31. In another embodiment, when R3 is aryl, heteroaryl, Ci_ioalkyl,
C3_8cycloalkyl, C3
8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl Ci_malkyl, or heteroalkyl,
it is substituted
with one or more independent ¨C(=0)NR31R32. In another embodiment, when R3 is
aryl,
heteroaryl, Ci_malkyl, cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl,
C2_10alkynyl, aryl-
C2_10alkyl, heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_malkyl, it is
substituted with one or
more independent ¨C(=0)NR34R35. In another embodiment, when R3 is aryl,
heteroaryl, Ci_
ioalkyl, C3_8cycloalkyl, C3_8cycloalkyl- Ci_malkyl, heterocyclyl, heterocyclyl
Ci_malkyl, or
heteroalkyl, it is substituted with one or more independent -NO2. In another
embodiment,
when R3 is aryl, heteroaryl, Ci_ioalkyl, C 3 _8 cycloalkyl, C 3 _8 cycloalkyl-
Ci_malkyl,
heterocyclyl, heterocyclyl Ci_malkyl, or heteroalkyl, it is substituted with
one or more
independent ¨CN. In another embodiment, when R3 is aryl, heteroaryl,
Ci_malkyl, C3
8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl Ci_malkyl,
or heteroalkyl, it
is substituted with one or more independent ¨S(0)0_2R31. In another
embodiment, when R3 is
aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, C3_8cycloalkyl- Ci_malkyl,
heterocyclyl,
heterocyclyl Ci_malkyl, or heteroalkyl, it is substituted with one or more
independent ¨
S02NR31R32. In another embodiment, when R3 is aryl, heteroaryl, Ci_malkyl,
C3_8cycloalkyl,
C3_8cycloalkyl- Ci_malkyl, heterocyclyl, heterocyclyl Ci_malkyl, or
heteroalkyl, it is
substituted with one or more independent ¨S02NR34R35. In another embodiment,
when R3 is
aryl, heteroaryl, Ci_malkyl, cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_10alkynyl,
aryl-C2_10alkyl, heterocyclyl Ci_i0alkyl, or C3_8cycloalkyl- Ci_i0alkyl, it is
substituted with one
or more independent NR31C(=0)R32. In another embodiment, when R3 is aryl,
heteroaryl, Ci_
ioalkyl, cycloalkyl, heterocyclyl, heteroalkyl, C2_ioalkenyl, C2_ioalkynyl,
aryl-C 2_ioalkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_malkyl, it is substituted with
one or more
independent ¨NR31C(=0)0R32. In another embodiment, when R3 is aryl,
heteroaryl, Ci_
ioalkyl, cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl,
aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_malkyl, it is substituted with
one or more
independent ¨NR31C(=0)NR32R33. In another embodiment, when R3 is aryl,
heteroaryl, C1_
ioalkyl, cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl,
aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is substituted with
one or more
independent ¨NR31S(0)0_2R32. In another embodiment, when R3 is aryl,
heteroaryl, C1_
ioalkyl, cycloalkyl, heterocyclyl, heteroalkyl, C2_ioalkenyl, C2_ioalkynyl,
aryl-C 2_ioalkyl,
-110-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is substituted with
one or more
independent ¨C(=S)0R31. In another embodiment, when R3 is aryl, heteroaryl,
Ci_ioalkyl,
cycloalkyl, heterocyclyl, heteroalkyl, C2_ioalkenyl, C2_10alkynyl, aryl-
C2_10alkyl, heterocyclyl
Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is substituted with one or more
independent ¨
C(=0)SR31. In another embodiment, when R3 is aryl, heteroaryl, Ci_ioalkyl,
C3_8cycloalkyl,
C3_8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl Ci_ioalkyl, or
heteroalkyl, it is
substituted with one or more independent ¨NR31C(=NR32)NR33R32. In another
embodiment,
when R3 is aryl, heteroaryl, Ci_ioalkyl, cycloalkyl, heterocyclyl,
heteroalkyl, C2_10alkenyl, C2_
loalkYnYl, aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl-
Ci_ioalkyl, it is
substituted with one or more independent¨NR31C(=NR32)0R33. In another
embodiment,
when R3 is aryl, heteroaryl, Ci_ioalkyl, cycloalkyl, heterocyclyl,
heteroalkyl, C2_10alkenyl, C2_
loalkynyl, aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl-
Ci_ioalkyl, it is
substituted with one or more independent ¨NR31C(=NR32)SR33. In another
embodiment,
when R3 is aryl, heteroaryl, Ci_ioalkyl, cycloalkyl, heterocyclyl,
heteroalkyl, C2_10alkenyl, C2_
loalkynyl, aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl-
Ci_ioalkyl, it is
substituted with one or more independent ¨0C(=0)0R33. In another embodiment,
when R3
is aryl, heteroaryl, Ci_ioalkyl, cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_10alkynyl,
aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is
substituted with one
or more independent ¨0C(=0)NR31R32. In another embodiment, when R3 is aryl,
heteroaryl,
Ci_ioalkyl, cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl,
aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is substituted with
one or more
independent ¨0C(=0)SR31. In another embodiment, when R3 is aryl, heteroaryl,
Ci_ioalkyl,
cycloalkyl, heterocyclyl, heteroalkyl, C2_ioalkenyl, C2_10alkynyl, aryl-
C2_10alkyl, heterocyclyl
Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is substituted with one or more
independent ¨
SC(=0)0R31. In another embodiment, when R3 is aryl, heteroaryl, Ci_ioalkyl,
C3_8cycloalkyl,
C3_8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl Ci_ioalkyl, or
heteroalkyl, it is
substituted with one or more independent ¨P(0)0R310R32. In another embodiment,
when R3
is aryl, heteroaryl, Ci_ioalkyl, cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_10alkynyl,
aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is
substituted with one
or more independent ¨SC(=0)NR31R32.
[00404] In various embodiments of compounds of Formula I, R4 is hydrogen. In
another
embodiment, R4 is halogen. In another embodiment, R4 is ¨OH. In another
embodiment, R4
is ¨R31. In another embodiment, R4 is ¨CF3. In another embodiment, R4 is
¨0CF3. In
another embodiment, R4 is ¨0R31. In another embodiment, R4 is ¨NR31R32. In
another
-111-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
embodiment, R4 is -NR34R35. In another embodiment, R4 is -C(0)R31. In another
embodiment, R4 is -0O2R31. In another embodiment, R4 is -C(=0)NR31R32. In
another
embodiment, R4 is -C(=0)NR34R35. In another embodiment, R4 is -NO2. In another
embodiment, R4 is -CN. In another embodiment, R4 is -S(0)0_2R3. In another
embodiment,
R4 is -S02NR
31R32. In another embodiment, R4 is -S02NR34R35. In another embodiment, R4
is -NR31C(=0)R32. In another embodiment, R4 is -NR31C(=0)0R32. In another
embodiment,
R4 is -NR31C(=0)NR32R33. In another embodiment, R4 is -NR31S(0)0 2R32. In
another
embodiment, R4 is -C(=S)0R31. In another embodiment, R4 is -C(=0)SR31. In
another
embodiment, R4 is -NR31C(=NR32)NR33R32. In another embodiment, R4 is -
NR31C(=NR32)0R33. In another embodiment, R4 is -NR31C(=NR32)SR33. In another
embodiment, R4 is -0C(=0)0R33. In another embodiment, R4 is -0C(=0)NR31R32. In
another embodiment, R4 is -0C(=0)SR31. In another embodiment, R4 is -
SC(=0)0R31. In
another embodiment, R4 is -P(0 )0R31 OR32 . In another embodiment, R4 is -
SC(=0)NR31R32.
In another embodiment, R4 is aryl. In another embodiment, R4 is heteroaryl. In
another
embodiment, R4 is Ci_4alkyl. In another embodiment, R4 is Ci_ioalkyl. In
another
embodiment, R4 is C3_8cycloalkyl. In another embodiment, R4 is Ci_ioalkyl -
C3_8cycloalkyl.
In another embodiment, R4 is Ci_ioalkylaryl. In another embodiment, R4 is Ci_
malkylheteroaryl. In another embodiment, R4 is Ci_loalkylheterocyclyl. In
another
embodiment, R4 is C2_malkenyl. In another embodiment, R4 is C2_101kynyl. In
another
embodiment, R4 is C2_101kynyl- C3_8cycloalkyl. R4 is C2_10alkenyl-
C3_8cycloalkyl. In another
embodiment, R4 is C2_101kenylaryl. In another embodiment, R4 is C2_101kenyl-
heteroaryl. In
another embodiment, R4 is C2401kenylheteroalkyl. In another embodiment, R4 is
C2-
malkenylheterocycicyl. In another embodiment, R4 is -C2_101kynylaryl. In
another
embodiment, R4 is C2_101kynylheteroaryl. In another embodiment, R4 is C2_
malkynylheteroalkyl. In another embodiment, R4 is C240alkynylheterocyclyl. In
another
embodiment, R4 is C2_10alkyny1C3_8cycloalkyl. In another embodiment, R4 is
heterocyclyl C1_
malkyl. In another embodiment, R4 is heterocycly1C2_10.1kenyl. In another
embodiment, R4 is
heterocyclyl-C240alkynyl. In another embodiment, R4 is aryl- Ci_ioalkyl. In
another
embodiment, R4 is aryl-C2_101kenyl. In another embodiment, R4 is aryl-
C2_101kynyl. In
another embodiment, R4 is aryl-heterocyclyl. In another embodiment, R4 is
heteroaryl- Ci_
malkyl. In another embodiment, R4 is heteroaryl-C2401kenyl. In another
embodiment, R4 is
heteroaryl-C2_ioalkynyl. In another embodiment, R4 is C3_8cycloalkyl-
Ci_malkyl. In another
embodiment, R4 is C3_8cycloalkyl- C2_10alkenyl. In another embodiment, R4 is
C3_8cycloalkyl-
C2_ioalkynyl.
-112-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00405] In various embodiments of compounds of Formula I, when R4 is aryl,
heteroaryl, C1_
C3_8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl
Ci_ioalkyl, or
heteroalkyl, it is unsubstituted. In another embodiment, when R4 is aryl,
heteroaryl, C1_
C3_8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl
Ci_ioalkyl, or
heteroalkyl, it is substituted with one or more independent halo. In another
embodiment,
when R4 is aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, C3_8cycloalkyl-
Ci_ioalkyl,
heterocyclyl, heterocyclyl Ci_ioalkyl, or heteroalkyl, it is substituted with
one or more
independent ¨OH. In another embodiment, when R4 is aryl, heteroaryl,
Ci_ioalkyl, C3_
8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl
Ci_ioalkyl, or heteroalkyl, it
is substituted with one or more independent ¨R31. In another embodiment, when
R4 is aryl,
heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl,
heterocyclyl, heterocyclyl
Ci_ioalkyl, or heteroalkyl, it is substituted with one or more independent
¨CF3. In another
embodiment, when R4 is aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl,
C3_8cycloalkyl- C1_
heterocyclyl, heterocyclyl Ci_ioalkyl, or heteroalkyl, it is substituted with
one or more
independent ¨OCF. In another embodiment, when R4 is aryl, heteroaryl,
Ci_ioalkyl, C3_
8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl
Ci_ioalkyl, or heteroalkyl, it
is substituted with one or more independent ¨0R31. In another embodiment, when
R4 is aryl,
heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl,
heterocyclyl, heterocyclyl
Ci_ioalkyl, or heteroalkyl, it is substituted with one or more independent
¨NR31R32. In another
embodiment, when R4 is aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl,
C3_8cycloalkyl- C1_
heterocyclyl, heterocyclyl Ci_ioalkyl, or heteroalkyl, it is substituted with
one or more
independent ¨NR34R35. In another embodiment, when R4 is aryl, heteroaryl,
Ci_ioalkyl, C3_
8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl
Ci_ioalkyl, or heteroalkyl, it
is substituted with one or more independent ¨C(0)R31. In another embodiment,
when R4 is
aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl,
heterocyclyl,
heterocyclyl Ci_ioalkyl, or heteroalkyl, it is substituted with one or more
independent ¨
CO2R31. In another embodiment, when R4 is aryl, heteroaryl, Ci_ioalkyl,
C3_8cycloalkyl, C3_
8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl Ci_ioalkyl, or
heteroalkyl, it is substituted
with one or more independent ¨C(=0)NR31R32. In another embodiment, when R4 is
aryl,
heteroaryl, Ci_ioalkyl, cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl,
C2_10alkynyl, aryl-
C2_malkyl, heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is
substituted with one or
more independent ¨C(=0)NR34R35. In another embodiment, when R4 is aryl,
heteroaryl, C1_
C3_8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl
Ci_ioalkyl, or
heteroalkyl, it is substituted with one or more independent -NO2. In another
embodiment,
-113-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
when R4 is aryl, heteroaryl, Ci_malkyl, C 3 _8 cycloalkyl, C 3 _8 cycloalkyl-
Ci_malkyl,
heterocyclyl, heterocyclyl Ci_malkyl, or heteroalkyl, it is substituted with
one or more
independent ¨CN. In another embodiment, when R4 is aryl, heteroaryl,
Ci_ioalkyl, C3
8cycloalkyl, C3_8cycloalkyl- Ci_ioalkyl, heterocyclyl, heterocyclyl Ci_malkyl,
or heteroalkyl, it
is substituted with one or more independent ¨S(0)0_2R31. In another
embodiment, when R4 is
aryl, heteroaryl, Ci_ioalkyl, C3_8cycloalkyl, C3_8cycloalkyl- Ci_malkyl,
heterocyclyl,
heterocyclyl Ci_malkyl, or heteroalkyl, it is substituted with one or more
independent ¨
S02NR31R32. In another embodiment, when R4 is aryl, heteroaryl, Ci_malkyl,
C3_8cycloalkyl,
C3_8cycloalkyl- Ci_malkyl, heterocyclyl, heterocyclyl Ci_malkyl, or
heteroalkyl, it is
substituted with one or more independent ¨S02NR34R35. In another embodiment,
when R4 is
aryl, heteroaryl, Ci_malkyl, cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_10alkynyl,
aryl-C2_10alkyl, heterocyclyl Ci_i0alkyl, or C3_8cycloalkyl- Ci_i0alkyl, it is
substituted with one
or more independent NR31C(=0)R32. In another embodiment, when R4 is aryl,
heteroaryl, C1_
ioalkyl, cycloalkyl, heterocyclyl, heteroalkyl, C2_ioalkenyl, C2_ioalkynyl,
aryl-C2_ioalkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is substituted with
one or more
independent ¨NR31C(=0)0R32. In another embodiment, when R4 is aryl,
heteroaryl, Ci_
ioalkyl, cycloalkyl, heterocyclyl, heteroalkyl, C2_ioalkenyl, C2_ioalkynyl,
aryl-C2_ioalkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_malkyl, it is substituted with
one or more
independent ¨NR31C(=0)NR32R33. In another embodiment, when R4 is aryl,
heteroaryl, C1_
ioalkyl, cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl,
aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_malkyl, it is substituted with
one or more
independent ¨NR31S(0)0_2R32. In another embodiment, when R4 is aryl,
heteroaryl, Ci_
ioalkyl, cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl,
aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_ioalkyl, it is substituted with
one or more
independent ¨C(=S)0R31. In another embodiment, when R4 is aryl, heteroaryl,
Ci_malkyl,
cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl, aryl-
C2_ioalkyl, heterocyclyl
Ci_malkyl, or C3_8cycloalkyl- Ci_malkyl, it is substituted with one or more
independent ¨
C(=0)SR31. In another embodiment, when R4 is aryl, heteroaryl, Ci_malkyl,
C3_8cycloalkyl,
C3_8cycloalkyl- Ci_malkyl, heterocyclyl, heterocyclyl Ci_malkyl, or
heteroalkyl, it is
substituted with one or more independent ¨NR31C(=NR32)NR33R32. In another
embodiment,
when R4 is aryl, heteroaryl, Ci_ioalkyl, cycloalkyl, heterocyclyl,
heteroalkyl, C2_10alkenyl, C2
loalkynyl, aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl, or C 3_8 cycloalkyl-
Ci_ioalkyl, it is
substituted with one or more independent , ¨NR31C(=NR32)0R33. In another
embodiment,
when R4 is aryl, heteroaryl, Ci_malkyl, cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2
-114-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
loalkYnYl, aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl-
Ci_malkyl, it is
substituted with one or more independent -NR31C(=NR32)SR33. In another
embodiment,
when R4 is aryl, heteroaryl, Ci_malkyl, cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2
loalkynyl, aryl-C2_10alkyl, heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl-
Ci_malkyl, it is
substituted with one or more independent -0C(=0)0R33. In another embodiment,
when R4
is aryl, heteroaryl, Ci_malkyl, cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_10alkynyl,
aryl-C2_10alkyl, heterocyclyl Ci_i0alkyl, or C3_8cycloalkyl- Ci_i0alkyl, it is
substituted with one
or more independent -0C(=0)NR31R32. In another embodiment, when R4 is aryl,
heteroaryl,
Ci_malkyl, cycloalkyl, heterocyclyl, heteroalkyl, C2_10alkenyl, C2_10alkynyl,
aryl-C2_10alkyl,
heterocyclyl Ci_ioalkyl, or C3_8cycloalkyl- Ci_malkyl, it is substituted with
one or more
independent -0C(=0)SR31. In another embodiment, when R4 is aryl, heteroaryl,
Ci_malkyl,
cycloalkyl, heterocyclyl, heteroalkyl, C2_10 alkenyl, C2_1 0 alkynyl, aryl-C
2_1 0 alkyl, heterocyclyl
Ci_malkyl, or C3_8cycloalkyl- Ci_malkyl, it is substituted with one or more
independent -
SC(=0)0R31. In another embodiment, when R4 is aryl, heteroaryl, Ci_malkyl,
C3_8cycloalkyl,
C3_8cycloalkyl- Ci_malkyl, heterocyclyl, heterocyclyl Ci_malkyl, or
heteroalkyl, it is
substituted with one or more independent -P(0)0R310R32. In another embodiment,
when R4
is aryl, heteroaryl, Ci_malkyl, cycloalkyl, heterocyclyl, heteroalkyl,
C2_10alkenyl, C2_10alkynyl,
aryl-C2_10alkyl, heterocyclyl Ci_malkyl, or C3_8cycloalkyl- Ci_malkyl, it is
substituted with one
or more independent -SC(=0)NR31R32.
[00406] In various embodiments of compounds of Formula I, R5 is hydrogen. In
another
embodiment, R5 is halogen. In another embodiment, R5 is -OH. In another
embodiment, R5
is -R31. In another embodiment, R5 is -CF3. In another embodiment, R5 is -
0CF3. In
another embodiment, R5 is -0R31. In another embodiment, R5 is -NR31R32. In
another
embodiment, R5 is -NR34R35. In another embodiment, R5 is -C(0)R31. In another
embodiment, R5 is -0O2R31. In another embodiment, R5 is -C(=0)NR31R32. In
another
embodiment, R5 is -C(=0)NR34R35. In another embodiment, R5 is -NO2. In another
embodiment, R5 is -CN. In another embodiment, R5 is -S(0)0_2R31. In another
embodiment,
R5 is -S02NR31R32. In another embodiment, R5 is -S02NR34R35. In another
embodiment, R5
is -NR31C(=0)R32. In another embodiment, R5 is -NR31C(=0)0R32. In another
embodiment,
R5 is -NR31C(=0)NR32R33. In another embodiment, R5 is -NR31S(0)0 2R32. In
another
embodiment, R5 is -C(=S)0R31. In another embodiment, R5 is -C(=0)SR31. In
another
embodiment, R5 is -NR31C(=NR32)NR33R32. In another embodiment, R5 is -
NR31C(=NR32)0R33. In another embodiment, R5 is -NR31C(=NR32)SR33. In another
embodiment, R5 is -0C(=0)0R33. In another embodiment, R5 is -0C(=0)NR31R32. In
-115-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
another embodiment, R5 is -0C(=0)SR31. In another embodiment, R5 is -
SC(=0)0R31. In
another embodiment, R5 is -P(0)0R310R32. In another embodiment, R5 is or -
SC(=0)NR31R32.
[00407] In various embodiments of compounds of Formula I, R7 is hydrogen. In
another
embodiment, R7 is unsubstituted Ci_i0alkyl. In another embodiment, R7 is
unsbustituted C2_
malkenyl. In another embodiment, R7 is unsubstituted aryl. In another
embodiment, R7 is
unsubstituted heteroaryl. In another embodiment, R7 is unsubstituted
heterocyclyl. In another
embodiment, R7 is unsubstituted C3_10cycloalkyl. In another embodiment, R7 is
Ci_malkyl
substituted by one or more independent R6. In another embodiment, R7 is
C2_10alkenyl
substituted by one or more independent R6. In another embodiment, R7 is aryl
substituted by
one or more independent R6. In another embodiment, R7 is heteroaryl
substituted by one or
more independent R6. In another embodiment, R7 is heterocyclyl substituted by
one or more
independent R6. In another embodiment, R7 is C3_10cycloalkyl substituted by
one or more
independent R6.
[00408] In various embodiments of compounds of Formula I, R8 is hydrogen. In
another
embodiment, R8 is unsubstituted Ci_i0alkyl. In another embodiment, R8 is
unsubstituted C2_
malkenyl. In another embodiment, R8 is unsubstituted aryl. In another
embodiment, R8 is
unsubstituted heteroaryl. In another embodiment, R8 is unsubstituted
heterocyclyl. In another
embodiment, R8 is unsubstituted C3_10cycloalkyl. In another embodiment, R8 is
Ci_malkyl
substituted by one or more independent R6. In another embodiment, R8 is
C2_10alkenyl
substituted by one or more independent R6. In another embodiment, R8 is aryl
substituted by
one or more independent R6. In another embodiment, R8 is heteroaryl
substituted by one or
more independent R6. In another embodiment, R8 is heterocyclyl substituted by
one or more
independent R6. In another embodiment, R8is C3_10cycloalkyl substituted by one
or more
independent R6.
[00409] In various embodiments of compounds of Formula I, R6 is halo, in
another
embodiment, R6 is-OR31. In another embodiment, R6 is -SH. In another
embodiment, R6 is
NH2. In another embodiment, R6 is -NR34R35. In another embodiment, R6 is -
NR31R32. In
another embodiment, R6 is -0O2R31. In another embodiment, R6 is -0O2aryl. In
another
embodiment, R6 is -C(=0)NR31R32. In another embodiment, R6 is C(=0) NR34R35.
In
another embodiment, R6 is -NO2. In another embodiment, R6 is -CN. In another
embodiment, R6 is -S(0)0-2 Ci_malkyl. In another embodiment, R6 is -S(0)
0_2aryl. In
another embodiment, R6 is -S02NR34R35. In another embodiment, R6 is -
SO2NR31R32. In
another embodiment, R6 is Ci_malkyl. In another embodiment, R6 is
C2_10alkenyl. In another
-116-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
embodiment, R6 is C2_10alkynyl. In another embodiment, R6 is unsubstituted
aryl-Ci_ioalkyl.
In another embodiment, R6 is unsubstituted aryl-C2_10alkenyl. In another
embodiment, R6 is
unsubstituted aryl-C2_10alkynyl. In another embodiment, R6 is unsubstituted
heteroaryl-C1_
ioalkyl. In another embodiment, R6 is unsubstituted heteroaryl-C240alkenyl. In
another
embodiment, R6 is aryl-Ci_ioalkyl, aryl-C 2_ ioalkenyl, aryl-C 2_10 alkynyl,
heteroaryl-Ci_ioalkyl,
or heteroaryl-C240alkenyl substituted by one or more independent halo. In
another
embodiment, R6 is aryl-Ci_ioalkyl, aryl-C 2_ ioalkenyl, aryl-C 2_ ioalkynyl,
heteroaryl-Ci_ioalkyl,
or heteroaryl-C240alkenyl substituted by one or more independent cyano. In
another
embodiment, R6 is aryl-Ci_ioalkyl, aryl-C 2_10 alkenyl, aryl-C 2_ ioalkynyl,
heteroaryl-Ci_ioalkyl,
or heteroaryl-C240alkenyl substituted by one or more independent nitro. In
another
embodiment, R6 is aryl-Ci_ioalkyl, aryl-C 2_10 alkenyl, aryl-C 2_ ioalkynyl,
heteroaryl-Ci_ioalkyl,
or heteroaryl-C240alkenyl substituted by one or more independent ¨0Ci_ioalkyl.
In another
embodiment, R6 is aryl-Ci_ioalkyl, aryl-C 2_ ioalkenyl, aryl-C 2_ ioalkynyl,
heteroaryl-Ci_ioalkyl,
or heteroaryl-C240alkenyl substituted by one or more independent -Ci_ioalkyl.
In another
embodiment, R6 is aryl-Ci_ioalkyl, aryl-C 2_10 alkenyl, aryl-C 2_10 alkynyl,
heteroaryl-Ci_ioalkyl,
or heteroaryl-C240alkenyl substituted by one or more independent -
C2_10alkenyl. In another
embodiment, R6 is aryl-Ci_ioalkyl, aryl-C 2_10 alkenyl, aryl-C 2_10 alkynyl,
heteroaryl-Ci_ioalkyl,
or heteroaryl-C240alkenyl substituted by one or more independent -
C2_10alkynyl. In another
embodiment, R6 is aryl-Ci_ioalkyl, aryl-C 2_10 alkenyl, aryl-C 2_10 alkynyl,
heteroaryl-Ci_ioalkyl,
or heteroaryl-C240alkenyl substituted by one or more independent
¨(halo)Ci_ioalkyl. In
another embodiment, R6 is aryl-Ci_ioalkyl, aryl-C 2_10 alkenyl, aryl-C 2_10
alkynyl, heteroaryl-C1_
ioalkyl, or heteroaryl-C240alkenyl substituted by one or more independent ¨
(halo)C2_
ioalkenyl. In another embodiment, R6 is aryl-Ci_ioalkyl, aryl-C 2_10 alkenyl,
aryl-C 2_10 alkynyl,
heteroaryl-Ci_ioalkyl, or heteroaryl-C240alkenyl substituted by one or more
independent ¨
(halo)C 2_10 alkynyl. In another embodiment, R6 is aryl-Ci_ioalkyl, aryl-C
2_10 alkenyl, aryl-C2
1 oalkynyl, heteroaryl-Ci_ioalkyl, or heteroaryl-C2_10alkenyl substituted by
one or more
independent ¨COOH. In another embodiment, R6 is aryl-Ci_ioalkyl, aryl-
C2_10alkenyl, aryl-
C2_10alkynyl, heteroaryl-C i_ioalkyl, or heteroaryl-C240alkenyl substituted by
one or more
independent ¨C(=0)NR31R32. In another embodiment, R6 is aryl-Ci_ioalkyl, aryl-
C2-
ioalkenyl, aryl-C2_10alkynyl, heteroaryl-C i_ioalkyl, or heteroaryl-
C240alkenyl substituted by
one or more independent ¨C(=0) NR34R35. In another embodiment, R6 is aryl-
Ci_ioalkyl,
aryl-C 2_10 alkenyl, aryl-C 2-10 alkynyl, heteroaryl-Ci_ioalkyl, or heteroaryl-
C 2_10 alkenyl
substituted by one or more independent ¨S02NR34R35. In another embodiment, R6
is aryl-C1_
ioalkyl, aryl-C2_10alkenyl, aryl-C2_10alkynyl, heteroaryl-C i_ioalkyl, or
heteroaryl-C240alkenyl
-117-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
substituted by one or more independent ¨SO2 NR31R32. In another embodiment, R6
is aryl-
Ci_malkyl, aryl-C240alkenyl, aryl-C2_10alkynyl, heteroaryl-Ci_ioalkyl, or
heteroaryl-C 2_
malkenyl substituted by one or more independent -NR31R32. In another
embodiment, R6 is
aryl-Ci_i0alkyl, aryl-C 2- loalkenyl, aryl-C 2- loalkynyl, heteroaryl-
Ci_i0alkyl, or heteroaryl-C 2-
ioalkenyl substituted by one or more independent ¨NR34R35.
[00410] In various embodiments of compounds of Formula I, R9 is H. In another
embodiment, R9 is halo. In another embodiment, R9 is¨OR31. In another
embodiment, R9 is ¨
SH. In another embodiment, R9 is NH2. In another embodiment, R9 is ¨NR34R35.
In another
embodiment, R9 is ¨ NR31R32. In another embodiment, R9 is ¨0O2R31. In another
embodiment, R9 is ¨0O2aryl. In another embodiment, R9 is ¨C(=0)NR31R32. In
another
embodiment, R9 is C(=0) NR34R35. In another embodiment, R9 is ¨NO2. In another
embodiment, R9 is ¨CN. In another embodiment, R9 is ¨S(0)0-2 Ci_malkyl. In
another
embodiment, R9 is ¨5(0)0 2aryl. In another embodiment, R9 is ¨S02NR34R35. In
another
embodiment, R9 is ¨SO2NR31R32. In another embodiment, R9 is Ci_malkyl. In
another
embodiment, R9 is C2_10alkenyl. In another embodiment, R9 is C2_10alkynyl. In
another
embodiment, R9 is unsubstituted aryl-Ci_malkyl. In another embodiment, R9 is
unsubstituted
aryl-C2_10alkenyl. In another embodiment, R9 is unsubstituted aryl-
C2_10alkynyl. In another
embodiment, R9 is unsubstituted heteroaryl-Ci_malkyl. In another embodiment,
R9 is
unsubstituted heteroaryl-C240alkenyl. In another embodiment, R9 is aryl-
Ci_i0alkyl, aryl-C2-
ioalkenyl, aryl-C2_10alkynyl, heteroaryl-Ci_ioalkyl, or heteroaryl-C240alkenyl
substituted by
one or more independent halo. In another embodiment, R9 is aryl-Ci_i0alkyl,
aryl-C2-
ioalkenyl, aryl-C2_10alkynyl, heteroaryl-Ci_malkyl, or heteroaryl-C240alkenyl
substituted by
one or more independent cyano. In another embodiment, R9 is aryl-Ci_malkyl,
aryl-C2-
ioalkenyl, aryl-C2_10alkynyl, heteroaryl-Ci_ioalkyl, or heteroaryl-C240alkenyl
substituted by
one or more independent nitro. In another embodiment, R9 is aryl-Ci_malkyl,
aryl-C2-
ioalkenyl, aryl-C2_10alkynyl, heteroaryl-Ci_malkyl, or heteroaryl-C240alkenyl
substituted by
one or more independent ¨0C1_10alkyl. In another embodiment, R9 is aryl-
Ci_malkyl, aryl-C2_
malkenyl, aryl-C2_10alkynyl, heteroaryl-Ci_ioalkyl, or heteroaryl-C240alkenyl
substituted by
one or more independent -Ci_i0alkyl. In another embodiment, R9 is aryl-
Ci_malkyl, aryl-C2_
malkenyl, aryl-C2_10alkynyl, heteroaryl-Ci_malkyl, or heteroaryl-C240alkenyl
substituted by
one or more independent - C2_10alkenyl. In another embodiment, R9 is aryl-
Ci_i0alkyl, aryl-
C 2_ ioalkenyl, aryl-C 2- loalkynyl, heteroaryl-Ci_ioalkyl, or heteroaryl-C 2-
loalkenyl substituted by
one or more independent - C2_10alkynyl. In another embodiment, R9 is aryl-
Ci_i0alkyl, aryl-
C 2_10 alkenyl, aryl-C 2_ loalkynyl, heteroaryl-Ci_ioalkyl, or heteroaryl-C 2_
loalkenyl substituted by
-118-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
one or more independent -(halo)Ci_malkyl. In another embodiment, R9 is aryl-
Ci_malkyl,
aryl-C2_10alkenyl, aryl-C2_10alkynyl, heteroaryl-Ci_ioalkyl, or heteroaryl-
C2_10alkenyl
substituted by one or more independent - (halo)C2_10alkenyl. In another
embodiment, R9 is
aryl-Ci_i0alkyl, aryl-C2-loalkenyl, aryl-C2-loalkynyl, heteroaryl-Ci_i0alkyl,
or heteroaryl-C 2-
malkenyl substituted by one or more independent - (halo)C2_10alkynyl. In
another
embodiment, R9 is aryl-Ci_i0alkyl, aryl-C2_10alkenyl, aryl-C2_10alkynyl,
heteroaryl-Ci_ioalkyl,
or heteroaryl-C240alkenyl substituted by one or more independent -COOH. In
another
embodiment, R9 is aryl-Ci_i0alkyl, aryl-C2_ioalkenyl, aryl-C2_10alkynyl,
heteroaryl-Ci_i0alkyl,
or heteroaryl-C240alkenyl substituted by one or more independent -
C(=0)NR31R32. In
another embodiment, R9 is aryl-Ci_i0alkyl, aryl-C2_10alkenyl, aryl-
C2_10alkynyl, heteroaryl-C1_
ioalkyl, or heteroaryl-C240alkenyl substituted by one or more independent -
C(=0) NR34R35.
In another embodiment, R9 is aryl-Ci_malkyl, aryl-C2_10alkenyl, aryl-
C2_10alkynyl, heteroaryl-
Ci_malkyl, or heteroaryl-C240alkenyl substituted by one or more independent -
S02NR34R35.
In another embodiment, R9 is aryl-Ci_ioalkyl, aryl-C2_10alkenyl, aryl-
C2_10alkynyl, heteroaryl-
Ci_i0alkyl, or heteroaryl-C2_10alkenyl substituted by one or more independent -
SO2 NR31R32 .
In another embodiment, R9 is aryl-Ci_malkyl, aryl-C2_10alkenyl, aryl-
C2_10alkynyl, heteroaryl-
Ci_malkyl, or heteroaryl-C240alkenyl substituted by one or more independent -
NR31R32. In
another embodiment, R9 is aryl-Ci_i0alkyl, aryl-C2_10alkenyl, aryl-
C2_10alkynyl, heteroaryl-C1_
ioalkyl, or heteroaryl-C240alkenyl substituted by one or more independent -
NR34R35.
[00411] In various embodiments of compounds of Formula I, R31is H. In some
embodiments, R31is unsubstituted Ci_malkyl. In some embodiments, R31is
substituted C1-
malkyl. In some embodiments, R31is Ci_malkyl substituted with one or more
aryl. In some
embodiments, R31is Ci_malkyl substituted with one or more heteroalkyl. In some
embodiments, R31is Ci_malkyl substituted with one or more heterocyclyl. In
some
embodiments, R31is Ci_malkyl substituted with one or more heteroaryl. In some
embodiments,
when R31is Ci_malkyl substituted with one or more aryl, each of said aryl
substituents is
unsubstituted or substituted with one or more halo, -OH, - Ci_malkyl, -CF3, -0-
aryl, -0CF3,
-0C1_10alkyl, -NH2, - N(Ci_loalkyl)(Ci_malkyl), - NH(Ci_malkyl), - NH( aryl), -
NR34R35, -
C(0)(Ci_ioalkyl), -C(0)(Ci_malkyl-ary1), -C(0)(ary1), -0O2-Ci_malkyl, -0O2-
Ci_malkylaryl,
-0O2-aryl, -C(=0)N(Ci_ioalkyl)( Ci_ioalkyl), -C(=0)NH( Ci_malkyl), -
C(=0)NR34R35, -
C(=0)NH2, -0CF3, -0(Ci_malkyl), -0-aryl, -N(ary1)( Ci_ioalkyl), -NO2, -CN, -
S(0)0_2 Ci-
ioalkyl, -S(0)0_2 Ci_malkylaryl, -S(0)0_2 aryl, -SO2N(ary1), -SO2
N(Ci_malkyl)( Ci_ioalkyl), -
SO2 NH(Ci_malkyl) or -S02NR34R35. In some embodiments, when R31is Ci_malkyl
substituted with one or more heteroalkyl, each of said heteroalkyl group is
unsubstituted or
-119-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
substituted with one or more halo, -OH, - Ci_malkyl, -CF3, -0-aryl, -OCF3, -
0C1_10alkyl, -
NH2, - N(Ci_i0alkyl)(Ci_10alkyl), - NH(Ci_i0alkyl), - NH( aryl), -NR34R35, -
C(0)(Ci_i0alkyl),
-C(0)(Ci_malkyl-aryl), -C(0)(ary1), -0O2-Ci_i0alkyl, -0O2-Ci_i0alkylaryl, -0O2-
aryl, -
C(=0)N(Ci_malkyl)( Ci_malkyl), -C(=0)NH( Ci_malkyl), -C(=0)NR34R35, -C(=0)NH2,
-
OCF3, -0(Ci_ioalkyl), -0-aryl, -N(ary1)( Ci_malkyl), -NO2, -CN, -S(0)0_2
Ci_i0alkyl, -
S(0)0_2 Ci_i0alkylaryl, -S(0)0_2 aryl, -SO2N(ary1), -SO2 N(Ci_i0alkyl)(
Ci_ioalkyl), -502
NH(Ci_malkyl) or -SO2NR34R35 substituents. In some embodiments, when R31is
Ci_malkyl
substituted with one or more heterocyclyl, each of said heterocyclyl group is
unsubstituted or
substituted with one or more halo, -OH, - Ci_malkyl, -CF3, -0-aryl, -OCF3, -
0C1_10alkyl, -
NH2, - N(Ci_i0alkyl)(Ci_10alkyl), - NH(Ci_i0alkyl), - NH( aryl), -NR34R35, -
C(0)(Ci_i0alkyl),
-C(0)(Ci_malkyl-aryl), -C(0)(ary1), -0O2-Ci_i0alkyl, -0O2-Ci_i0alkylaryl, -0O2-
aryl, -
C(=0)N(Ci_malkyl)( Ci_malkyl), -C(=0)NH( Ci_malkyl), -C(=0)NR34R35, -C(0)NH2, -
OCF3, -0(Ci_ioalkyl), -0-aryl, -N(ary1)( Ci_malkyl), -NO2, -CN, -S(0)0_2
Ci_i0alkyl, -
S(0)0_2 Ci_i0alkylaryl, -S(0)0_2 aryl, -SO2N(ary1), -502N(Ci_i0alkyl)(
Ci_ioalkyl), -SO2
NH(Ci_malkyl) or -5O2NR34R35. In some embodiments, when R31is Ci_malkyl
substituted
with one or more heteroaryl, each of said heteroaryl group is unsubstituted or
substituted with
one or more halo, -OH, - Ci_i0alkyl, -CF3, -0-aryl, -OCF3, -0Ci_i0alkyl, -NH2,
- N(Ci_
ioalkyl)(Ci_ioalkyl), - NH(Ci_i0alkyl), - NH( aryl), -NR34R35, -
C(0)(Ci_i0alkyl), -C(0)(C1-
loalkyl-ary1), -C(0)(ary1), -0O2-Ci_walkyl, -0O2-Ci_malkylaryl, -0O2-aryl, -
C(=0)N(C1-
malkyl)( Ci_malkyl), -C(=0)NH( Ci_malkyl), -C(=0)NR34R35, -C(0)NH2, -OCF3, -
0(C1_
ioalkyl), -0-aryl, -N(ary1)( Ci_malkyl), -NO2, -CN, -S(0)0_2 Ci_malkyl, -
S(0)0_2 Ci-
malkylaryl, -S(0)0_2 aryl, -502N(ary1), -SO2 N(Ci_malkyl)( Ci_malkyl), -SO2
NH(Ci_malkyl)
or -502NR34R35. In some embodiments, when R31is substituted Ci_i0alkyl, it is
substituted by
a combination of aryl, heteroalkyl, heterocyclyl, or heteroaryl groups.
[00412] In various embodiments of compounds of Formula I, R32 is H. In some
embodiments, R32 is unsubstituted Ci_i0alkyl. In some embodiments, R32 is
substituted C1_
malkyl. In some embodiments, R32 is Ci_malkyl substituted with one or more
aryl. In some
embodiments, R32 is Ci_malkyl substituted with one or more heteroalkyl. In
some
embodiments, R32 is Ci_malkyl substituted with one or more heterocyclyl. In
some
embodiments, R32 is Ci_malkyl substituted with one or more heteroaryl. In some
embodiments, when R32 is Ci_malkyl substituted with one or more aryl, each of
said aryl
group is unsubstituted or substituted with one or more halo, -OH, - Ci_malkyl,
-CF3, -0-aryl,
-OCF3, -0C1_10alkyl, -NH2, - N(Ci_ioalkyl)(Ci_ioalkyl), - NH(Ci_malkyl), - NH(
aryl), -
NR34R35, -C(0)(Ci_malkyl), -C(0)(Ci_malkyl-ary1), -C(0)(ary1), -0O2-Ci_malkyl,
-0O2-C1-
-120-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
ioalkylaryl, -0O2-aryl, -C(=0)N(Ci_malkyl)( Ci_malkyl), -C(0)NH( Ci_malkyl), -
C(=0)NR34R35, -C(=0)NH2, -0CF3, -0(Ci_i0alkyl), -0-aryl, -N(ary1)( Ci_malkyl),
-NO2, -
CN, -S(0)0_2 Ci_malkyl, -S(0)0_2 Ci_malkylaryl, -S(0)0_2 aryl, -SO2N(ary1), -
SO2 N(Ci-
ioalkyl)( Ci_malkyl), -SO2 NH(Ci_malkyl) or -S02NR34R35. In some embodiments,
when R32
is Ci_malkyl substituted with one or more heteroalkyl, each of said
heteroalkyl group is
unsubstituted or substituted with one or more halo, -OH, - Ci_malkyl, -CF3, -0-
aryl, -0CF3,
-0C1_10alkyl, -NH2, - N(Ci_loalkyl)(Ci_malkyl), - NH(Ci_ioalkyl), - NH( aryl),
-NR34R35, -
C(0)(Ci_malkyl), -C(0)(Ci_malkyl-ary1), -C(0)(ary1), -0O2-Ci_loalkyl, -0O2-
Ci_malkylaryl,
-0O2-aryl, -C(=0)N(Ci_malkyl)( Ci_loalkyl), -C(=0)NH( Ci_malkyl), -
C(=0)NR34R35, -
C(=0)NH2, -0CF3, -0(Ci_malkyl), -0-aryl, -N(ary1)( Ci_malkyl), -NO2, -CN, -
S(0)0_2 C1-
ioalkyl, -S(0)0_2 Ci_malkylaryl, -S(0)0_2 aryl, -SO2N(ary1), -SO2
N(Ci_ioalkyl)( Ci_ioalkyl), -
SO2 NH(Ci_malkyl) or -S02NR34R35. In some embodiments, when R32 is Ci_i0alkyl
substituted with one or more heterocyclyl, each of said heterocyclyl group is
unsubstituted or
substituted with one or more halo, -OH, - Ci_ioalkyl, -CF3, -0-aryl, -0CF3, -
0Ci_ioalkyl, -
NH2, - N(Ci_i0alkyl)(Ci_10alkyl), - NH(Ci_i0alkyl), - NH( aryl), -NR34R35, -
C(0)(Ci_i0alkyl),
-C(0)(Ci_malkyl-aryl), -C(0)(ary1), -0O2-Ci_i0alkyl, -0O2-Ci_i0alkylaryl, -0O2-
aryl, -
C(=0)N(Ci_ioalkyl)( Ci_malkyl), -C(=0)NH( Ci_malkyl), -C(=0)NR34R35, -
C(=0)NH2, -
OCF3, -0(Ci_ioalkyl), -0-aryl, -N(ary1)( Ci_malkyl), -NO2, -CN, -S(0)0_2
Ci_i0alkyl, -
S(0)0_2 Ci_i0alkylaryl, -S(0)0_2 aryl, -502N(ary1), -502N(Ci_i0alkyl)(
Ci_ioalkyl), -SO2
NH(Ci_malkyl) or -502NR34R35. In some embodiments, when R32 is Ci_i0alkyl
substituted
with one or more heteroaryl, each of said heteroaryl group is unsubstituted or
substituted with
one or more halo, -OH, - Ci_ioalkyl, -CF3, -0-aryl, -0CF3, -0Ci_i0alkyl, -NH2,
- N(Ci_
ioalkyl)(Ci_ioalkyl), - NH(Ci_i0alkyl), - NH( aryl), -NR34R35, -
C(0)(Ci_i0alkyl), -C(0)(C1-
loalkyl-ary1), -C(0)(ary1), -0O2-Ci_loalkyl, -0O2-Ci_malkylaryl, -0O2-aryl, -
C(=0)N(C1-
ioalkyl)( Ci_ioalkyl), -C(=0)NH( Ci_ioalkyl), -C(=0)NR34R35, -C(=0)NH2, -0CF3,
-0(C1_
ioalkyl), -0-aryl, -N(ary1)( Ci_malkyl), -NO2, -CN, -S(0)0_2 Ci_malkyl, -
S(0)0_2 Ci-
malkylaryl, -S(0)0_2 aryl, -502N(ary1), -SO2 N(Ci_malkyl)( Ci_malkyl), -SO2
NH(Ci_malkyl)
or -502NR34R35. In some embodiments, when R32is substituted Ci_i0alkyl, it is
substituted by
a combination of aryl, heteroalkyl, heterocyclyl, or heteroaryl groups.
[00413] In various embodiments of compounds of Formula I, R33 is unsubstituted
Ci_i0alkyl.
In some embodiments, R33 is substituted Ci_malkyl. In some embodiments, R33 is
Ci_malkyl
substituted with one or more aryl. In some embodiments, R33 is Ci_i0alkyl
substituted with
one or more heteroalkyl. In some embodiments, R33 is Ci_malkyl substituted
with one or
more heterocyclyl. In some embodiments, R33 is Ci_i0alkyl substituted with one
or more
-121-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
heteroaryl. In some embodiments, when R33 is Ci_malkyl substituted with one or
more aryl,
each of said aryl group is unsubstituted or substituted with one or more halo,
-OH, - C1_
malkyl, -CF3, -0-aryl, -0CF3, -0Ci_ioalkyl, -NH2, - N(Ci_malkyl)(Ci_malkyl), -
NH(Ci-
ioalkyl), - NH( aryl), -NR34R35, -C(0)(Ci_ioalkyl), -C(0)(Ci_i0alkyl-aryl), -
C(0)(ary1), -
CO2-Ci_loalkyl, -0O2-Ci_malkylaryl, -0O2-aryl, -C(=0)N(Ci_malkyl)( Ci_malkyl),
-
C(0)NH( Ci_malkyl), -C(=0)NR34R35, -C(=0)NH2, -0CF3, -0(Ci_malkyl), -0-aryl, -
N(ary1)( Ci_ioalkyl), -NO2, -CN, -S(0)0_2 Ci_i0alkyl, -S(0)0_2 Ci_i0alkylaryl,
-S(0)0_2 aryl,
-SO2N(ary1), -SO2 N(Ci_malkyl)( Ci_ioalkyl), -SO2 NH(Ci_malkyl) or -
SO2NR34R35. In some
embodiments, when R33 is Ci_malkyl substituted with one or more heteroalkyl,
each of said
heteroalkyl group is unsubstituted or substituted with one or more halo, -OH, -
Ci_malkyl, -
CF3, -0-aryl, -0CF3, -0C1_10alkyl, -NH2, - N(Ci_malkyl)(Ci_malkyl), -
NH(Ci_i0alkyl), -
NH( aryl), -NR34R35, -C(0)(Ci_ioalkyl), -C(0)(Ci_malkyl-aryl), -C(0)(ary1), -
0O2-C1-
10alkyl, -0O2-Ci_malkylaryl, -0O2-aryl, -C(=0)N(Ci_malkyl)( Ci_malkyl), -
C(0)NH( Ci_
loalkyl), -C(=0)NR34R35, -C(=0)NH2, -0CF3, -0(Ci_ioalkyl), -0-aryl, -N(ary1)(
Ci_malkyl),
-NO2, -CN, -S(0)0_2 Ci_malkyl, -S(0)0_2 Ci_malkylaryl, -S(0)0_2 aryl, -
SO2N(ary1), -SO2
N(Ci_i0alkyl)( Ci_malkyl), -SO2 NH(Ci_i0alkyl) or -S02NR34R35. In some
embodiments,
when R33 is Ci_i0alkyl substituted with one or more heterocyclyl, each of said
heterocyclyl
group is unsubstituted or substituted with one or more halo, -OH, - Ci_malkyl,
-CF3, -0-aryl,
-0CF3, -0C1_10alkyl, -NH2, - N(Ci_ioalkyl)(Ci_ioalkyl), - NH(Ci_malkyl), - NH(
aryl), -
NR34R35, -C(0)(Ci_ioalkyl), -C(0)(Ci_malkyl-ary1), -C(0)(ary1), -0O2-
Ci_malkyl, -0O2-C1-
ioalkylaryl, -0O2-aryl, -C(=0)N(Ci_malkyl)( Ci_malkyl), -C(0)NH( Ci_malkyl), -
C(=0)NR34R35, -C(=0)NH2, -0CF3, -0(Ci_i0alkyl), -0-aryl, -N(ary1)( Ci_malkyl),
-NO2, -
CN, -S(0)0_2 Ci_malkyl, -S(0)0_2 Ci_malkylaryl, -S(0)0_2 aryl, -SO2N(ary1), -
SO2 N(Ci-
ioalkyl)( Ci_malkyl), -502NH(Ci_malkyl) or -S02NR34R35. In some embodiments,
when R33
is Ci_malkyl substituted with one or more heteroaryl, each of said heteroaryl
group is
unsubstituted or substituted with one or more halo, -OH, - Ci_malkyl, -CF3, -0-
aryl, -0CF3,
-0C1_10alkyl, -NH2, - N(Ci_loalkyl)(Ci_malkyl), - NH(Ci_malkyl), - NH( aryl), -
NR34R35, -
C(0)(Ci_malkyl), -C(0)(Ci_malkyl-ary1), -C(0)(ary1), -0O2-Ci_malkyl, -0O2-
Ci_malkylaryl,
-0O2-aryl, -C(=0)N(Ci_malkyl)( Ci_ioalkyl), -C(=0)NH( Ci_malkyl), -
C(0)NR34R35, -
C(=0)NH2, -0CF3, -0(Ci_malkyl), -0-aryl, -N(ary1)( Ci_malkyl), -NO2, -CN, -
S(0)0_2 C1-
malkyl, -S(0)0_2 Ci_malkylaryl, -S(0)0_2 aryl, -502N(ary1), -502N(Ci_malkyl)(
Ci_ioalkyl), -
SO2 NH(Ci_malkyl) or -502NR34R35. In some embodiments, when R33 is substituted
C1-
ioalkyl, it is substituted by a combination of aryl, heteroalkyl,
heterocyclyl, or heteroaryl
groups.
-122-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00414] In various embodiments of compounds of Formula I, R34 and R35 in
¨NR34R35, ¨
C(=0)NR34R35, or ¨S02NR34R35, are taken together with the nitrogen atom to
which they are
attached to form a 3-10 membered saturated or unsaturated ring; wherein said
ring is
independently unsubstituted or is substituted by one or more ¨NR31R32,
hydroxyl, halogen,
oxo, aryl, heteroaryl, Ci_6alkyl, or 0-aryl, and wherein said 3-10 membered
saturated or
unsaturated ring independently contains 0, 1, or 2 more hetero atoms in
addition to the
nitrogen.
[00415] In some embodiments, the R34 and R35 in ¨NR34R35, ¨C(0)NR34R35, or ¨
S02NR34R35, are taken together with the nitrogen atom to which they are
attached to form:
.,;,
N
"v iv N ¨nr 7
c , c N
N N
...-- -.. N NI
0
" ) )
, 0 N '0 ' HO ' N '
C H 3 600 Et
Tv I NI I
N N N OH
...-- -,..
..-- --... N _..-CH3 C j , C ) L y ,or N y n,r, Li ' N
1
OPO3H2
\Jr L I 31-12 0 CH3
[00416] In another embodiment, Xi is C¨NH2.
[00417] In various embodiments, Xi is C--NH -R4,where ¨NH-R4 is:
H 2N '-- HN-µ HN"1.- HN A
HN A
HN"
HN -'-
, 1
CH3 ,
'
I , '
I
N N
N 41)
Co) cH3 N
N )
C Co) N
HN-1.-N.\,-
H HN)': \(..
HN "IC- HN HN HNN HN - HN"1"-
-
N
I. NN
rf`rµr
\/ N OH Co) C )
N
I
-123-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
HN-
, or =
\N OH
c\N
[00418] In one embodiment, the invention provides an inhibitor of Formula I-C1
where R5 is
H. In another embodiment, the invention provides an inhibitor of Formula I-C2
where R5 is
H.
[00419] In some embodiments, the invention provides an inhibitor of Formula I-
C1 a:
--- R2
(W2)k
H H N
\ /=
0 0/X1
E2 N Xµ2
Formula I-C 1 a
[00420] or a pharmaceutically acceptable salt thereof wherein:
[00421] E2 is -H;
[00422] X1 and X2 are N;
[00423] R1 is -L-Ci_malkyl, -L-C3_8cycloalkyl, -L- Ci_malkylheterocyclyl, or -
L-heterocyclyl,
each of which is unsubstituted or is substituted by one or more independent
R3;
[00424] L is absent, -(C=0)-, -C(=0)0-, -C(=0) N(R31)-,-S-, -S(0)-, -S(0)2-, -
S(0)2N(R31)-,
or -N(R31)-;
[00425] R3 is hydrogen, -OH, -0R31, -NR31R32, -C(0)R31, -C(=0)NR31R32, -
C(=0)NR34R35, aryl, heteroaryl, Ci_4alkyl, CiioaIkyl, C3_8cycloalkyl, or
heterocyclyl, wherein
each of said aryl or heteroaryl moiety is unsubstituted or is substituted with
one or more
independent alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl, aryl, arylalkyl,
heteroaryl, heteroarylalkyl, halo, -OH, -R31, -CF3, -0CF3, -0R31, -NR31R32, -
NR34R35, -
C(0)R31, -0O2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN, -S(0)0_2R31, -
SO2NR31R32, -SO2NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R33, -
NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -NR31C(=NR32)NR33R32, -
NR31C(=NR32)0R33,
-NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -0C(=0)SR31, -SC(=0)0R31, -
P(0)0R310R32, or-SC(=0)NR31R32, and wherein each of said alkyl, cycloalkyl, or
heterocyclyl moiety is unsubstituted or is substituted with one or more alkyl,
heteroalkyl,
alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl,
heteroarylalkyl,
-124-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
halo, -OH, -R31, -CF3, -0CF3, -0R31, -0-aryl, -NR31R32, -NR34R35 ,-C(0)R31, -
0O2R31, -
C(=0)NR34R35, or -C(=0)NR31R32;
[00426] -(W2)k- is -NH-, -N(H)C(0)- or
1004271 R2 is hydrogen, halogen, -0R31, -NR31R32, -NR34R35, -C(0)R31, -CO2R31,
-
C(=0)NR31R32, -C(0)NR34R35 5-S (0)0_2R31, -SO2NR31R32, -SO2NR34R35, bicyclic
aryl,
substituted monocyclic aryl, heteroaryl, Ci_malkyl, C3_8cycloalkyl, Ci_malkyl-
C3_8cycloalkyl,
C3_8cycloalkyl- Ci_malkyl, C3_8 cycloalkyl- C2_10alkenyl, C3_8cycloalkyl-
C2_10alkynyl, C 2-
malkyl-monocyclic aryl, monocyclic aryl-C2_10alkyl, Ci_malkylbicycloaryl,
bicycloaryl--C1_
malkyl, substituted Ci_ioalkylaryl, substituted aryl-Ci_ioalkyl,
Ci_ioalkylheteroaryl, C1_
ioalkylheterocyclyl, C2_10alkenyl, C2_10alkynyl, C2_10alkenylaryl,
C2_10alkenylheterearyl, C 2-
malkenylheteroalkyl, C240alkenylheteroeyeleyl, C2_10alkynylaryl,
C240alkynylheteroaryl, C2_
10alkynylheteroalkyl, C2_10alkynylheterocyclyl, C2_10alkenyl-C3_8cycloalkyl,
C2_10alkynyl-C3_
8cYcloalkenyl, Ci_ioalkoxy Ci_malkyl, Ci_malkoxyC2_10alkenyl,
Ci_malkoxyC2_10alkynyl,
heterocyclyl, heterocyclyl Ci_malkyl, heterocycly1C240alkenyl, heterocyclyl-
C240alkynyl,
aryl-C2_10alkenyl, aryl-C2-loalkynyl, aryl-heterocyclyl, heteroaryl-
Ci_i0alkyl, heteroaryl-C 2-
ioalkenyl, heteroaryl-C240alkynyl, heteroaryl-C3_8cycloalkyl, heteroaryl-
heteroalkyl, or
heteroaryl-heterocyclyl, wherein each of said bicyclic aryl or heteroaryl
moiety is
unsubstituted, or wherein each of bicyclic aryl, heteroaryl moiety or
monocyclic aryl moiety
is substituted with one or more independent halo, -OH, -R31, -CF3, -0CF3, -
0R31, -
NR31R32, -NR34R35, -C(0)R31, -CO2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN,
-
S(0)0_2R31, -SO2NR31R32, -S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -
NR31C(=0)NR32R33, -NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -
NR31C(=NR32)NR33R32,
-NR31C(=NR32)0R33, -NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R325 -
OC (=0)SR31, -SC(=0)0R31, -P(0)0R310R32, or-SC(=0)NR31R32, and wherein each of
said
alkyl, cycloalkyl, heterocyclyl, or heteroalkyl moiety is unsubstituted or is
substituted with
one or more halo, -OH, -R31, -CF3, -0CF3, -0R31, -0-aryl, -NR31R32, -NR34R35 ,-
C(0)R31,
-0O2R31, -C(=0)NR34R35, or -C(=0)NR31R32;
[00428] R31, R32, and R33, in each instance, are independently H or
Ci_ioalkyl, wherein the
Ci_malkyl is unsubstituted; and
[00429] R34 and R35 in -NR34R35, -C(=0)NR34R35, or -S02NR34R35, are taken
together with
the nitrogen atom to which they are attached to form a 3-10 membered saturated
or
unsaturated ring; wherein said ring is independently unsubstituted or is
substituted by one or
more -NR31R32, hydroxyl, halogen, oxo, aryl, heteroaryl, Ci_6alkyl, or 0-aryl,
and wherein
-125-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
said 3-10 membered saturated or unsaturated ring independently contains 0, 1,
or 2 more
heteroatoms in addition to the nitrogen.
[00430] In another aspect, an inhibitor of Formula I-C1 is a compound of
Formula I-C1 a:
--- R2
0--V (W2)k
\ \
H\ /
N H lit N
N
0 0/X1
E2 N X\2
Ri
Formula I-C lb
[00431] or a pharmaceutically acceptable salt thereof, wherein: E2 is -H; X1
is CH and X2 is
N;
[00432] R1 is -L-Ci_i0alkyl, -L-C3_8cycloalkyl, -L- Ci_i0alkylheterocyclyl, or
-L-heterocyclyl,
each of which is unsubstituted or is substituted by one or more independent
R3;
[00433] L is absent, -(C=0)-, -C(=0)0-, -C(=0) N(R31)-,-S-, -S(0)-, -S(0)2-, -
S(0)2N(R31)-,
or -N(R31)-;
[00434] R3 is hydrogen, -OH, -0R31, -NR31R32, -C(0)R31, -C(0)NR31R32, -
C(=0)NR34R35, aryl, heteroaryl, Ci_4alkyl, Ci_malkyl, C3_8cycloalkyl, or
heterocyclyl, wherein
each of said aryl or heteroaryl moiety is unsubstituted or is substituted with
one or more
independent alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl, aryl, arylalkyl,
heteroaryl, heteroarylalkyl, halo, -OH, -R31, -CF3, -0CF3, -0R31, -NR31R32, -
NR34R35, -
C(0)R31, -CO2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN, -S(0)0_2R31, -
SO2NR31R32, -S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R33, -
NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -NR31C(=NR32)NR33R32, -
NR31C(=NR32)0R33,
-NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -0C(=0)SR31, -SC(=0)0R31, -
P(0)0R310R32, or-SC(=0)NR31R32, and wherein each of said alkyl, cycloalkyl, or
heterocyclyl moiety is unsubstituted or is substituted with one or more alkyl,
heteroalkyl,
alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl,
heteroarylalkyl,
halo, -OH, -R31, -CF3, -0CF3, -0R31, -0-aryl, -NR31R32, -NR34R35 ,-C(0)R31, -
0O2R31, -
C(=0)NR34R35, or -C(=0)NR31R32;
[00435] -(W2)k- is -NH-, -N(H)C(0)- or
1004361 R2 is hydrogen, halogen, -0R31, -NR31R32, -NR34R35, -C(0)R31, -0O2R31,
-
C(=0)NR31R32, -C(=0)NR34R35,-S(0)0_2R31, -SO2NR31R32, -S02NR34R35, bicyclic
aryl,
substituted monocyclic aryl, heteroaryl, Ci_malkyl, C3_8cycloalkyl, Ci_malkyl-
C3_8cycloalkyl,
-126-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
C3_8 cycloalkyl- Ci_malkyl, C240alkyl-monocyclic aryl, monocyclic aryl-
C2_10alkyl, C1_
malkylbicycloaryl, bicycloaryl--Ci_malkyl, substituted Ci_i0alkylaryl,
substituted aryl-C1_
malkyl, Ci_malkylheteroaryl, Ci_malkylheterocyclyl, C2_10alkenyl,
C2_10alkynyl, heterocyclyl,
heterocyclyl Ci_malkyl, heterocyclyl-C240alkenyl, heterocyclyl-C240alkynyl,
aryl-
heterocyclyl, heteroaryl-Ci_malkyl, heteroaryl-heteroalkyl, or heteroaryl-
heterocyclyl,
wherein each of said bicyclic aryl or heteroaryl moiety is unsubstituted, or
wherein each of
bicyclic aryl, heteroaryl moiety or monocyclic aryl moiety is substituted with
one or more
independent halo, -OH, -R31, -CF3, -0CF3, -0R31, -NR31R32, -NR34R35, -C(0)R31,
-
CO2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN, -S(0)0_2R31, -SO2NR31R32, -
S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R33, -NR31S(0)0_2R32,
-
C(=S)0R31, -C(=0)SR31, -NR31C(=NR32)NR33R32, -NR31C(=NR32)0R33, -
NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -0C(=0)SR31, -SC(=0)0R31, -
P(0)0R310R32, or-SC(=0)NR31R32, and wherein each of said alkyl, cycloalkyl,
heterocyclyl,
or heteroalkyl moiety is unsubstituted or is substituted with one or more
halo, -OH, -R31, -
CF3, -0CF3, -0R31, -0-aryl, -NR31R32, -NR34R35 ,-C(0)R31, -0O2R31, -
C(=0)NR34R35, or
-C(=0)NR31R32;
[00437] R31, R32, and R33, in each instance, are independently H or
Ci_ioalkyl, wherein the
Ci_malkyl is unsubstituted; and
[00438] R34 and R35 in -NR34R35, -C(=0)NR34R35, or -S02NR34R35, are taken
together with
the nitrogen atom to which they are attached to form a 3-10 membered saturated
or
unsaturated ring; wherein said ring is independently unsubstituted or is
substituted by one or
more -NR31R32, hydroxyl, halogen, oxo, aryl, heteroaryl, Ci_6alkyl, or 0-aryl,
and wherein
said 3-10 membered saturated or unsaturated ring independently contains 0, 1,
or 2 more
heteroatoms in addition to the nitrogen.
[00439] The invention further provides a compound which is an mTor inhibitor,
wherein the
compound has the Formula I-A:
R31 R32
\ /
N
M1
N(
0 OX1
- v /
E2 xX34 "2
\
R1
Formula I-A
-127-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00440] or a pharmaceutically acceptable salt thereof, wherein:
[00441] Xi is N or C-El, X2 is N, X3 is C, and X4 is C-R9 or N; or X1 is N or
C-El, X2 is C
X3 is N, and X4 is C-R9 or N;
[00442] Ri is ¨H, ¨L-Ci_ioalkyl, -L-C3_8cycloalkyl, -L-Ci_ioalkyl -
C3_8cycloalkyl, -L- aryl, -
L-heteroaryl, -L-Ci_ioalkylaryl, -L- Ci_malkylheteroaryl, -L-
Ci_loalkylheterocyclyl, -L-C 2-
ioalkenYl, -L-C2_10alkynyl, -L-C2_10alkenyl-C3_8cycloalkyl, -L-C2_10alkynyl-
C3_8cycloalkyl, -L-
heteroalkyl, -L-heteroalkylaryl, -L-heteroalkylheteroaryl, -L-heteroalkyl-
heterocyclyl, -L-
heteroalkyl-C3_8cycloalkyl, -L-aralkyl, -L-heteroaralkyl, or -L-heterocyclyl,
each of which is
unsubstituted or is substituted by one or more independent R3;
[00443] L is absent, -(C=0)-5 -C(=0)0-5 -C(=0) N(R31)-5-S-5 -S(0)-5 -S(0)2-5 -
S(0)2N(R31)-5
or
[00444] Mi is benzothiazolyl substituted with ¨(W2)k ¨R2;
[00445] k is 0 or 1;
[00446] El and E2 are independently ¨(Wi)j -R4;
[00447] j, in each instance (i.e., in El or j in E2), is independently 0 or 1
[00448] Wi is ¨0¨, - ¨S(0)0_2¨,¨C(0)¨,¨C(0)N(R7)¨, ¨N(R7)C(0)¨,
¨N(R7)S(0)¨, ¨
N(R7)S(0)2¨, ¨C(0)0-5 ¨CH(R7)N(C(0)0R8)-5 ¨CH(R7)N(C(0)R8)¨, ¨CH(R7)N(S02R8)-5
¨CH(R7)N(R8)¨, ¨CH(R7)C(0)N(R8)¨, -CH(R7)N(R8)C(0)¨, ¨CH(R7)N(R8)S(0)-5 or ¨
CH(R7)N(R8)S(0)2¨;
[00449] W2 is ¨0¨, - ¨S(0)0_2¨,¨C(0)¨,¨C(0)N(R7)¨, ¨N(R7)C(0)¨, ¨
N(R7)C(0)N(R8)-5¨N(R7)S(0)-5 ¨N(R7)S(0)2-5¨C(0)0-5 ¨CH(R7)N(C(0)0R8)-5 ¨
CH(R7)N(C(0)R8)-5 ¨CH(R7)N(S02R8)-5 ¨CH(R7)N(R8)-5 ¨CH(R7)C(0)N(R8)-5 ¨
CH(R7)N(R8)C(0)-5 ¨CH(R7)N(R8)S(0)-5 or ¨CH(R7)N(R8)S(0)2¨;
[00450] R2 is hydrogen, halogen, ¨OH, ¨R315 ¨CF3, ¨0CF35 ¨0R315 ¨NR31R325
¨NR34R355 ¨
C(0)R315 ¨CO2R315 ¨C(=0)NR31R325 ¨C(=0)NR34R355 -NO2, ¨CN, ¨S(0)0_2R315 ¨
SO2NR31R325 ¨S02NR34R355 -NR31C(=0)R325 ¨NR31C(=0)0R325 ¨NR31C(=0)NR32R335 ¨
NR31S(0)0_2R325 ¨C(=S)0R315 ¨C(=0)SR315 ¨NR31C(=NR32)NR33R325
¨NR31C(=NR32)0R335
¨NR31C(=NR32)SR335 ¨0C(=0)0R335 ¨0C(=0)NR31R325 ¨0C(=0)SR315 ¨SC(=0)0R315 ¨
P(0)0R310R325 ¨SC(=0)NR31R32 5 aryl (e.g. bicyclic aryl, unsubstituted aryl,
or substituted
monocyclic aryl), heteroaryl, Ci_ioalkyl, C3 _8 cycloalkyl, Ci_ioalkyl¨C 3 _8
cycloalkyl, C3 _
8 cycloalkyl -Ci_ioalkyl, C3 _8 cycloalkyl -C2_10alkenyl, C3_8cycloalkyl-
C2_10alkynyl, Ci_loalkyl-
C2_10alkenyl, Ci_ioalkyl- C2_10alkynyl, Ci_ioalkylaryl (e.g. C240alkyl-
monocyclic aryl, Ci-
malkyl-substituted monocyclic aryl, or Ci_loalkylbicycloary1),
Ci_malkylheteroaryl, Ci_
ioalkylheterocyclyl, C2_10alkenyl, C2_10alkynyl, C2_10alkenyl -Ci_ioalkyl,
C2_10alkynY1 -Ci-
-128-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
ioalkyl, C2_10alkenylaryl, C2_10alkenylheteroaryl, C2_10alkenylheteroalkyl, C
2-
loalkenylheterocycicyl, C2_10 alkellyl- C3 _8 cycloalkyl, C2_10alkynylaryl,
C2_10alkynylheteroaryl,
C240a1kyny1heteroa1ky1, C240alkynylheterocyclyl, C2_10alkynyl-
C3_8cycloalkenyl, Ci_malkoxy
Ci_malkyl, Ci_loalkoxy-C2_10alkenyl, Ci_loalkoxy-C2_10alkynyl, heterocyclyl,
heteroalkyl,
heterocyclyl -Ci_ioalkyl, heterocyclyl-C240alkenyl, heterocyclyl-C240alkynyl,
aryl- Ci_ioalkyl
(e.g. monocyclic aryl-C2_10alkyl, substituted monocyclic aryl- Ci_i0alkyl, or
bicycloaryl--C1_
ioalkyl), aryl-C2_10alkenyl, aryl-C2_10alkynyl, aryl-heterocyclyl, heteroaryl-
Ci_i0alkyl,
heteroaryl-C 2_1 0 alkenyl, heteroaryl-C 2_1 0 alkynyl, heteroaryl-C 3 _8
cycloalkyl, heteroaryl-
heteroalkyl, or heteroaryl-heterocyclyl, wherein each of said bicyclic aryl or
heteroaryl
moiety is unsubstituted, or wherein each of bicyclic aryl, heteroaryl moiety
or monocyclic
aryl moiety is substituted with one or more independent alkyl, heteroalkyl,
alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
halo, -OH, -R31, -
CF3, -0CF3, -0R31, -NR31R32, -NR34R35, -C(0)R31, -0O2R31, -C(=0)NR31R32, -
C(=0)NR34R35, -NO2, -CN, -S(0)0_2R31, -SO2NR31R32, -S02NR34R35, -NR31C(=0)R32,
-
NR31C(=0)0R32, -NR31C(=0)NR32R33, -NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -
NR31C(=NR32)NR33R32, -NR31C(=NR32)0R33, -NR31C(=NR32)SR33, -0C(=0)0R33, -
OC(=0)NR31R32, -0C(=0)SR31, -SC(=0)0R31, -P(0)0R310R32, or-SC(=0)NR31R32, and
wherein each of said alkyl, cycloalkyl, heterocyclyl, or heteroalkyl moiety is
unsubstituted or
is substituted with one or more alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl,
heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, halo, -OH, -
R31, -CF3, -0CF3,
-0R31, -0-aryl, -NR31R32, -NR34R35 ,-C(0)R31, -0O2R31, -C(=0)NR34R35, or -
C(=0)NR31R32;
[00451] R3 and R4 are independently hydrogen, halogen, -OH, -R31, -CF3, -0CF3,
-0R31, -
NR31R32, -NR34R35, -C(0)R31, -0O2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN,
-
S(0)0_2R31, -SO2NR31R32, -S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -
NR31C(=0)NR32R33, -NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -
NR31C(=NR32)NR33R32,
-NR31C(=NR32)0R33, -NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -
OC(=0)SR31, -SC(=0)0R31, -P(0)0R310R32, -SC(=0)NR31R32, aryl, heteroaryl,
Ci_Ltalkyl,
Ci_malkyl, C3_8cycloalkyl, Ci_ioalkyl-C3_8cycloalkyl, C3_8cycloalkyl -
Ci_malkyl, C3
8 cycloalkyl -C2_10alkenyl, C 3 _8 cycloalkyl- C2_10alkynyl, Ci_malkyl-
C2_10alkenyl, Ci_ioalkyl- C 2-
ioalkynyl, Ci_malkylaryl, Ci_i0alkylheteroaryl, Ci_malkylheterocyclyl,
C2_ioalkenyl, C 2-
1 0 alkynyl, C2_10 alkenyl -Ci_i0alkyl, C2_10 alkynyl -Ci_i0alkyl, C2_10
alkenylaryl, C 2-
1 0 alkenylheteroaryl, C2_1 0 alkenylheteroalkyl, C2_1 0 alkenylheterocyolcyl,
C2_10 alkenyl-C 3_
8 cycloalkyl, C2_10 alkynyl-C 3_8 cycloalkyl, C2_1 0 alkynylaryl, C2_1 0
alkynylheteroaryl, C2
-129-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
malkynylheteroalkyl, C240alkynylheterocyclyl, C2_10alkynyl-C3_8cycloalkenyl,
Ci_malkoxy C1_
ioalkyl, Ci_loalkoxy-C2_10alkenyl, Ci_malkoxy-C2_10alkynyl, heterocyclyl,
heterocyclyl -C1_
malkyl, heterocyclyl-C240alkenyl, heterocyc1y1-C240alkyny1, aryl- Ci_malkyl,
aryl-C 2-
10alkenyl, aryl-C2_10alkynyl, aryl-heterocyclyl, heteroaryl-Ci_i0alkyl,
heteroaryl-C2_10alkenyl,
heteroaryl-C240alkynyl, heteroaryl-C3_8cycloalkyl, heteroalkyl, heteroaryl-
heteroalkyl, or
heteroaryl-heterocyclyl, wherein each of said aryl or heteroaryl moiety is
unsubstituted or is
substituted with one or more independent halo, -OH, -R31, -CF3, -0CF3, -0R31, -
NR31R32, -
NR34R35, -C(0)R31, -CO2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN, -
S(0)0_2R31, -
SO2NR31R32, -SO2NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R33, -
NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -NR31C(=NR32)NR33R32, -
NR31C(=NR32)0R33,
-NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -0C(=0)SR31, -SC(=0)0R31, -
P(0)0R310R32, or-SC(=0)NR31R32, and wherein each of said alkyl, cycloalkyl,
heterocyclyl,
or heteroalkyl moiety is unsubstituted or is substituted with one or more
halo, -OH, -R31, -
CF3, -0CF3, -0R31, -0-aryl, -NR31R32, -NR34R35 ,-C(0)R31, -CO2R31, -
C(=0)NR34R35, or
-C(=0)NR31R32;
[00452] R5 is hydrogen, halogen, -OH, -R31, -CF3, -0CF3, -0R31, -NR31R32, -
NR34R35, -
C(0)R31, -0O2R31, -C(=0)NR31R32, -C(=0)NR34R35, -NO2, -CN, -S(0)0_2R31, -
SO2NR31R32, -S02NR34R35, -NR31C(=0)R32, -NR31C(=0)0R32, -NR31C(=0)NR32R33, -
NR31S(0)0_2R32, -C(=S)0R31, -C(=0)SR31, -NR31C(=NR32)NR33R32, -
NR31C(=NR32)0R33,
-NR31C(=NR32)SR33, -0C(=0)0R33, -0C(=0)NR31R32, -0C(=0)SR31, -SC(=0)0R31, -
P(0)0R310R32,or -SC(=0)NR31R32;
[00453] R31, R32, and R33, in each instance, are independently H or Ci_ioalkyl
, wherein the
Ci_malkyl is unsubstituted or is substituted with one or more aryl,
heteroalkyl, heterocyclyl,
or heteroaryl group, wherein each of said aryl, heteroalkyl, heterocyclyl, or
heteroaryl group
is unsubstituted or is substituted with one or more halo, -OH, - Ci_ioalkyl, -
CF3, -0-aryl, -
OCF3, -0C1_10alkyl, -NH2, - N(Ci_loalkyl)(Ci_malkyl), - NH(Ci_ioalkyl), - NH(
aryl), -
NR34R35, -C(0)(Ci_ioalkyl), -C(0)(Ci_malkyl-ary1), -C(0)(ary1), -0O2-
Ci_loalkyl, -0O2-C1-
malkylaryl, -0O2-aryl, -C(=0)N(Ci_malkyl)( Ci_malkyl), -C(0)NH( Ci_malkyl), -
C(=0)NR34R35, -C(=0)NH2, -0CF3, -0(Ci_i0alkyl), -0-aryl, -N(ary1)( Ci_malkyl),
-NO2, -
CN, -S(0)0_2 Ci_malkyl, -S(0)0_2 Ci_malkylaryl, -S(0)0_2 aryl, -SO2N(ary1), -
SO2 N(Ci-
malkyl)( Ci_ioalkyl), -SO2 NH(Ci_malkyl) or -S02NR34R35;
[00454] R34 and R35 in -NR34R35, -C(=0)NR34R35, or -S02NR34R35, are taken
together with
the nitrogen atom to which they are attached to form a 3-10 membered saturated
or
unsaturated ring; wherein said ring is independently unsubstituted or is
substituted by one or
-130-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
more -NR31R32, hydroxyl, halogen, oxo, aryl, heteroaryl, Ci_6alkyl, or 0-aryl,
and wherein
said 3-10 membered saturated or unsaturated ring independently contains 0, 1,
or 2 more
heteroatoms in addition to the nitrogen atom;
[00455] R7 and R8 are each independently hydrogen, Ci_malkyl, C2_10alkenyl,
aryl,
heteroaryl, heterocyclyl or C3_10cycloalkyl, each of which except for hydrogen
is
unsubstituted or is substituted by one or more independent R6;
[00456] R6 is halo, -0R31, -SH, -NH2, -NR34R35 , -NR31R32, -0O2R31, -0O2aryl, -
C(0)NR31R32, C(0)NR34R35 , -NO2, -CN, -S(0)0-2 Ci_i0alkyl, -S(0) 0_2aryl, -
S02NR34R35, -S02NR31R32, Ciioalkyl, C2_10alkenyl, C2_10alkynyl; aryl-
C2
loalkenyl, aryl-C2_10alkynyl, heteroaryl-Ci_i0alkyl, heteroaryl-C2_10alkenyl,
heteroaryl-C 2-
ioalkynyl, wherein each of said alkyl, alkenyl, alkynyl, aryl, heteroalkyl,
heterocyclyl, or
heteroaryl group is unsubstituted or is substituted with one or more
independent halo, cyano,
nitro, -0Ci_i0alkyl, C iioaIkyl, C2_10alkenyl, C2_10alkynyl, haloC iioalkyl,
haloC2_10alkenyl,
haloC2_10alkynyl, -COOH, -C(=0)NR3 'R32, -C(0)NR34R35 , -S02NR34R35, -SO2 NR3
'R32,
-NR31R32, or -NR34R35 ; and
[00457] R9 is H, halo, -0R31, -SH, -NH2, -NR34R35 , - NR31R32, -0O2R31, -
0O2aryl, -
C(=0)NR31R32, C(=0)NR34R35 , -NO2, -CN, -S(0)0_2 Ci_i0alkyl, -5(0) 0_2aryl, -
S02NR34R35, -S02NR31R32, Ciioalkyl, C2_10alkenyl, C2_10alkynyl; aryl-C
2-
loalkenyl, aryl-C2_10alkynyl, heteroaryl-Ci_i0alkyl, heteroaryl-C2_10alkenyl,
heteroaryl-C2
ioalkynyl, wherein each of said alkyl, alkenyl, alkynyl, aryl, heteroalkyl,
heterocyclyl, or
heteroaryl group is unsubstituted or is substituted with one or more
independent halo, cyano,
nitro, -0Ci_i0alkyl, C iioaIkyl, C2_10alkenyl, C2_10alkynyl, haloC iioalkyl,
haloC2_10alkenyl,
haloC2_10alkynyl, -COOH, -C(=0)NR31R32, -C(0)NR34R35 , -S02NR34R35, -SO2
NR31R32,
-NR31R32, or -NR34R35.
[00458] In some embodiments, X4 is C-R9.
[00459] The invention also provides an inhibitor as defined above, wherein the
compound is
of Formula I-B:
R31 .32
/r
1\41
N
0 0/1
'
E2 N A2
Ri
Formula I-B
-131-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
or a pharmaceutically acceptable salt thereof, and wherein the substituents
are as defined
above.
[00460] In various embodiments the compound of Formula I-B or its
pharmaceutically
acceptable salt thereof, is an inhibitor having the structure of Formula I-B1
or Formula I-B2:
R31 R32
/ R31 R32
/
M1
Mi
N
0 Xi N
0 m 0 Xi
E2 NX2
x2
Formula I-B1 Formula I-B2
or a pharmaceutically acceptable salt thereof
[00461] In various embodiments of Formula I-B1, X1 is N and X2 is N. In other
embodiments, X1 is C-E1 and X2 is N. In yet other embodiments, Xi is NH and X2
is C. In
further embodiments, X1 is CH-E1 and X2 is C.
[00462] In various embodiments of Formula I-B2, X1 is N and X2 is C. In
further
embodiments, Xi is C-E1 and X2 is C.
[00463] In various embodiments, X1 is C¨(W1)j -R4, where j is 0.
[00464] In another embodiment, X1 is CH. In yet another embodiment, Xi is C-
halogen,
where halogen is Cl, F, Br, or I.
[00465] In various embodiments of X1, it is C ¨(W1)j ¨R4. In various
embodiments of X1, j is
1, and W1 is ¨0¨. In various embodiments of Xi, j is 1, and W1 is ¨NR7-. In
various
embodiments of Xi, j is 1, and W1 is ¨NH-. In various embodiments of Xi, j is
1, and W1 is
¨S(0)0_2¨. In various embodiments of Xi, j is 1, and W1 is ¨C(0)¨. In various
embodiments of X1, j is 1, and W1 is ¨C(0)N(R7)¨. In various embodiments of
X1, j is 1,
and W1 is ¨N(R7)C(0)¨. In various embodiments of Xi, j is 1, and W1 is
¨N(R7)S(0)¨. In
various embodiments of Xi, j is 1, and W1 is ¨N(R7)S(0)2¨. In various
embodiments of Xi, .1
is 1, and W1 is ¨C(0)0¨. In various embodiments of Xi, j is 1, and W1 is
CH(R7)N(C(0)0R8)¨. In various embodiments of X1, j is 1, and W1 is
¨CH(R7)N(C(0)R8)¨.
In various embodiments of Xi, j is 1, and W1 is ¨CH(R7)N(S02R8)¨. In various
embodiments
of Xi, j is 1, and W1 is ¨CH(R7)N(R8)¨. In various embodiments of Xi, j is 1,
and W1 is ¨
CH(R7)C(0)N(R8)¨. In various embodiments of X1, j is 1, and W1 is
¨CH(R7)N(R8)C(0)¨.
In various embodiments of X1, j is 1, and W1 is ¨CH(R7)N(R8)S(0)¨. In various
embodiments of Xi, j is 1, and W1 is ¨CH(R7)N(R8)S(0)2¨.
-132-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00466] In another embodiment, Xi is CH2. In yet another embodiment, X1 is CH-
halogen,
where halogen is Cl, F, Br, or I.
[00467] In another embodiment, Xi is N.
[00468] In various embodiments, X2 is N. In other embodiments, X2 is C.
[00469] In various embodiments, E2 is -(W1)j -R4, where j is 0.
[00470] In another embodiment, E2 is CH. In yet another embodiment, E2 is C-
halogen,
where halogen is Cl, F, Br, or I.
[00471] In various embodiments of E2, it is -(Wi)j -R4. In various embodiments
of E2, j is 1,
and W1 is -0-. In various embodiments of E2, j is 1, and W1 is -NR7-. In
various
embodiments of E2, j is 1, and W1 is -NH-. In various embodiments of E2, j is
1, and W1 is
-S(0)0_2-. In various embodiments of E2, j is 1, and W1 is -C(0)-. In various
embodiments of E2, j is 1, and W1 is -C(0)N(R7)-. In various embodiments of
E2, j is 1, and
W1 is -N(R7)C(0)-. In various embodiments of E2, j is 1, and W1 is -N(R7)S(0)-
. In
various embodiments of E2, j is 1, and W1 is -N(R7)S(0)2-. In various
embodiments of E2, j
is 1, and W1 is -C(0)0-. In various embodiments of E2, j is 1, and W1 is
CH(R7)N(C(0)0R8)-. In various embodiments of E2, j is 1, and W1 is -
CH(R7)N(C(0)R8)-.
In various embodiments of E2, j is 1, and W1 is -CH(R7)N(S02R8)-. In various
embodiments
of E2, j is 1, and W1 is -CH(R7)N(R8)-. In various embodiments of E2, j is 1,
and W1 is -
CH(R7)C(0)N(R8)-. In various embodiments of E2, j is 1, and W1 is -
CH(R7)N(R8)C(0)-.
In various embodiments of E2, j is 1, and W1 is -CH(R7)N(R8)S(0)-. In various
embodiments of E2, j is 1, and W1 is -CH(R7)N(R8)S(0)2-.
[00472] In various embodiments of Formula I-A, I-B, I-B1 and I-B2, Mi is:
(w2)k-R2
(VV2)k -R2 (W2)k -R2
N N
).5.3
,
or- 14 I or 1 Or
[00473] In some embodiments of the invention, Mi is benzothiazolyl substituted
with -(W2)k
-R2. W2 can be -0-, -S(0)0_2-(including but not limited to -S-, -S(0)-, and -
S(0)2-),-
C(0)- , or -C(0)0-. In other embodiments, W1 is -NR6- or -CH(R6)N(R7)-,
wherein R6
and R7 are each independently hydrogen, unsubstituted or substituted Ci-
Cioalkyl (which
includes but is not limited to -CH3, -CH2CH3, n-propyl, isopropyl, n- butyl,
tert- butyl, sec-
butyl, pentyl, hexyl, and heptyl), unsubstituted or substituted C2-Cioalkenyl
(including but not
-133-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
limited to alkenyl such as, for example, vinyl, allyl, 1-methyl propen-l-yl,
butenyl, or
pentenyl). Additionally when W2 is ¨NR6¨ or ¨CH(R6)N(R7)¨, R6 and R7 are each
independently unsubstituted or substituted aryl (including phenyl and
naphthyl). In yet other
embodiments, when W2 is ¨NR6¨ or ¨CH(R6)N(R7)¨, R6 and R7 are each
independently
heteroaryl, wherein the heteroaryl is unsubstituted or substituted. R6 and R7
heteroaryl is
monocyclic heteroaryl, and includes but is not limited to imidazolyl,
pyrrolyl, oxazolyl,
thiazolyl, and pyridinyl. In some other embodiments, when W2 is ¨NR6¨ or
¨CH(R6)N(R7)¨,
R6 and R7 are each independently unsubstituted or substituted heterocyclyl
(which includes
but is not limited to pyrrolidinyl, tetrahydrofuranyl, piperidinyl,
tetrahydropyranyl,
thiazolidinyl, imidazolidinyl, morpholinyl, and piperazinyl) or unsubstituted
or substituted
C3_8cycloalkyl (including but not limited to cyclopropyl, cyclobutyl, and
cyclopentyl). Non
limiting exemplary W2 include ¨NH-, -N(cyclopropyl), and ¨N(4-N-piperidiny1).
[00474] For example, exemplary mTor inhibitors of the invention have the
Formulas:
)R2 2R2
)k N--((W )k
S
NH2 NH2 =
LO 0,N OO
N N N N
IR\ 1 R1
R2 R2
tw)k (10)k
= N =N
NH2 NH2
0 N 0
N N N N
R1 R1
Reaction Schemes ¨ mTor inhibitor compounds
[00475] The mTor inhibitor compounds disclosed herein may be prepared by the
routes
described below. Materials used herein are either commercially available or
prepared by
synthetic methods generally known in the art. These schemes are not limited to
the
compounds listed or by any particular substituents employed for illustrative
purposes.
Numbering does not necessarily correspond to that of claims or other tables.
-134-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
Scheme A
NH2 N H2 1
NIS
NCrN NOON H2 ......jr . Ri -Lg
H2 N FNI 160 5h, 90% N N 80 C, 16 h, 90% N
N Base
H H
A-1 A-2 A-3
NH2 1 NH2 Ar
ArB(01-02
=x."µ
1 J\I _NI..- Nj14
I ,N
Suz uki Coupl ing
N N N N
Ri Ri
A-4 A-5
[00476] In one embodiment, compounds are synthesized by condensing a
functionalized
heterocycle A-1 with formamide, to provide a pyrazolopyrimidine A-2. The
pyrazolopyrimidine is treated with N-iodosuccinimide, which introduces an iodo
substituent
in the pyrazole ring as in A-3. The R1 substituent is introduced by reacting
the
pyrazolopyrimidine A3 with a compound of Formula Ri-Lg in the presence of a
base such as
potassium carbonate to produce a compound of Formula A-4. Other bases that are
suitable
for use in this step include but are not limited to sodium hydride and
potassium t- butoxide.
The compound of Formula Ri-Lg has a moiety R1 as defined for R1 of a compound
of
Formula I-A, and wherein ¨Lg is an appropriate leaving group such as halide
(including
bromo, iodo, and chloro), tosylate, or other suitable leaving group,
[00477] The substituents corresponding to M1 are thereafter introduced by
reacting aryl or
heteroaryl boronic acids with the compound of Formula A-4 to obtain compound A-
5.
Scheme A-1
N H2 1
5Fi,..
Ri OH
l
I ,N
N N PPh3, DIAD N NI
H
Ri
A-3
A-4
[00478] Alternatively, Mitsunobu chemistry can be used to obtain alkylated
pyrazolopyrimidine A-4, as shown in Scheme A-1. Iodopyrazolopyrimidine A-3 is
reacted
with a suitable alcohol, in the presence of triphenylphosphine and
diisopropylazodicarboxylate (DIAD) to produce pyrazolopyrimidine A-4.
-135-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Scheme B
R31\ ,R32
R31\ ,R32
1
GO
zs
Oy30_ ,X1
B¨M ________________________________________________ y 0/X1
X2
GO/ NrX2
R1 R1
Formula A Formula B Formula C
[00479] The compounds of the invention may be synthesized via a reaction
scheme
represented generally in Scheme B. The synthesis proceeds via coupling a
compound of
Formula A with a compound of Formula B to yield a compound of Formula C. The
coupling
step is typically catalyzed by using, e.g., a palladium catalyst, including
but not limited to
palladium tetrakis (triphenylphosphine). The coupling is generally performed
in the presence
of a suitable base, a nonlimiting example being sodium carbonate. One example
of a suitable
solvent for the reaction is aqueous dioxane.
[00480] A compound of Formula A for use in Scheme B has a structure of Formula
A,
wherein T1 is triflate or halo (including bromo, chloro, and iodo), and
wherein R1, Xi, X25
X3, R31 and R32 are defined as for a compound of Formula I-A. For boronic
acids and acid
derivatives as depicted in Formula B, M is either M1 or M2. Mi is defined as
for a compound
of Formula I-A. For example, M1 can be a 5- benzoxazolyl or a 6- benzoxazolyl
moiety,
including but not limited to those M1 moieties disclosed herein. M2 is a
moiety which is
synthetically transformed to form M1, after the M2 moiety has been coupled to
the bicyclic
core of the compound of Formula A.
[00481] For a compound of Formula B, G is hydrogen or RG1, wherein RG1 is
alkyl, alkenyl,
or aryl. Alternatively, B(OG)2 is taken together to form a 5- or 6- membered
cyclic moiety.
In some embodiments, the compound of Formula B is a compound having a
structure of
Formula E:
GO
GO-1 * N
0 H
Formula E
GO
Bf
[00482] wherein G is H or RG1; RG1 is alkyl, alkenyl, or aryl. Alternatively,
GO forms
a 5- or 6- membered cyclic moiety; and R2 is a RG2 moiety, wherein the RG2
moiety is H,
acyl, or an amino protecting group including but not limited to tert-butyl
carbamate (Boc),
-136-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
carboxybenzyl (Cbz), benzyl (Bz), fluorenylmethyloxycarbonyl (FMOC), p-
methoxybenzyl
(PMB), and the like.
Scheme C
RG10\ HO
\
B¨M B¨M
T2¨ M ¨0-- / -1, /
RG10 HO
Formula D Formula B' Formula B"
[00483] In some embodiments, a compound of Formula B is a compound of Formula
B',
wherein G is RGi. or a compound of Formula B", wherein G is hydrogen. Scheme C
depicts
an exemplary scheme for synthesizing a compound of Formula B' or, optionally,
Formula B"
for use in Reaction Scheme C. This reaction proceeds via reacting a compound
of Formula D
with a trialkyl borate or a boronic acid derivative to produce a compound of
Formula B'. The
reaction is typically run a solvent such as dioxane or tetrahydrofuran. The
trialkyl borate
includes but is not limited to triisopropyl borate and the boronic acid
derivative includes but
is not limited to bis(pinacolato)diboron.
[00484] When the reaction is performed with trialkyl borate, a base such as n-
butyl lithium
is first added to the compound of Formula D to generate an anion, prior to the
addition of the
borate. When the reaction is performed with a boronic acid derivative such as
bis(pinacolato)diboron, a palladium catalyst and a base is used. Typical
palladium catalysts
include but are not limited to palladium chloride
(diphenylphosphino)ferrocene. A suitable
base includes but is not limited to potassium acetate.
[00485] A compound of Formula D for use in Scheme C is a compound wherein T2
is halo
or another leaving group, and M is as defined above in Scheme B. The compound
of
Formula B' may further be converted to a compound of Formula B" by treatment
with an acid
such as hydrochloric acid.
[00486] In one embodiment of a compound of Formula B, B', B", or E, the G
groups are
hydrogen. In another of a compound of Formula B, B', B", or E, the G groups
are RGi.
[00487] In some embodiments, no further synthetic transformation of M1 moiety
is
performed after the coupling reaction when, e.g. M1 is 2- N-acetyl-benzoxazol-
5-yl.
[00488] Some exemplary compounds of Formula B that can be synthesized via
Scheme C
include but are not limited to compounds of the following formulae:
-137-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
7 1\1
)¨NHCOCH3 0, ..t0:B 411
N
\-----10 40 0 --t.
0
N N 0 1
N NH2 0,B
>5--0 /
NHCOCH3
H
H-7
F-7 G-6 1-4
i`1)-NH2 4
0_ * NCrNF12
G-8 HOB* N5-NH2
C) __
I ¨
I
OH 0
G-7 G-9
o--13 401 ,'N
\ N
of
o/
NHCOC H3
J-4 K-6 L-6
0 Y
HO-
NNHCOCH3 HO, . HO\ /,'\ io OisN
0
HO B HO-.B
OH N N N NH2 I NHCOCH3
H OH
H-7-B
F-7-B G-6-B 1-4-B
HROH
I HN -.4 OH
I HN-CH3
HOB 0 0,
HO--B 0 HO--B 0
\ N
N \ N
o/
o/
NHCOCH3
J-4-B K-6-B L-6-B
[00489] In other embodiments of the invention, a compound of Formula E is
synthesized
from a compound of Formula F, as shown in Scheme C-1:
Scheme C-1
GO
T
I* shi¨RGI
2 ¨,..- GO" # Il_N_RG2
0 H
Formula F Formula E
[00490] Scheme C-1 depicts an exemplary scheme for synthesizing a compound of
Formula
E. This reaction proceeds via reacting a compound of Formula F with a trialkyl
borate or a
boronic acid derivative to produce a compound of Formula E. The conditions of
the reaction
are as described above in Scheme C.
[00491] A compound of Formula F for use in Scheme C-1 is a compound wherein T2
is halo
(including Br, Cl, and I) or another leaving group ( including but not limited
to triflate,
tosylate, and mesylate), and the Gp moiety is H, acyl, or an amino protecting
group including
-138-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
but not limited to tert-butyl carbamate (Boc), carboxybenzyl (Cbz), benzyl
(Bz),
fluorenylmethyloxycarbonyl (FMOC), p-methoxybenzyl (PMB), and the like.
[00492] The compound of Formula E, wherein G is alkyl, may further be
converted to a
compound of Formula E, wherein G is hydrogen, by treatment with an acid such
as
hydrochloric acid
[00493] Where desired, deprotection of a substituent (e.g., removal of Boc
protection from
an amino substituent) on the benzoxazolyl moiety (i.e. M1 of Formula C) is
performed after
coupling the compound of Formula B to the compound of Formula A.
[00494] Some exemplary compounds with such protecting groups, include but are
not
limited to compounds of the following formulae:
----- q 1:61 11)-NJO
HQ 0 0 0 0-y OH )\
-
HUB fa 1\1,)_N-Ac4 ? la to 0_
* 1\12¨NHIQ
/:)\---1 0 H
Or HO¨B
\
OH 1-----
[00495] An exemplary transformation of M2 to M1 can be carried out via Scheme
D as
shown below.
Scheme D
OH OH OH
R31\ ,R32
B(OH)2 j
1101 R31 \ ,R32 fik R31 \ ,R32 ik= NO2
N ri N N
N
Step 1 _____________________________________
N X2 \---/õ.X=.1'Xi __ Step 2 - N
px30,xi Step 3
\ N X2
1 N X2
\
Ri Ri Ri
Formula 3-1 Formula 3-2 Formula 3-3 Formula 3-4
OH
0---(NH2
il
. N
R31\ ,R32 4.0 NH2 R31 \ ,R32
N N
IKDI Step 4 __ N
lqx ,X1
3-v N 3-X2
N "2
\ \
Ri R1
Formula 3-5 Formula 3-6
-139-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00496] In Step 1, a compound of Formula 3-1 is reacted with boronic acid 3-2,
in the
presence of palladium tetrakis (triphenylphosphine) and a suitable base, such
as sodium
carbonate in an aqueous/ organic solvent mixture to produce a compound of
Formula 3-3. In
Step 2, the compound of Formula 3-3 is reacted with about 2 equivalents of
nitric acid in
acetic acid as solvent to produce a compound of Formula 3-4. Two alternative
transformations may be used to effect the next transformation of Step 3. In
the first method,
the compound of Formula 3-4 is treated with sodium dithionite and sodium
hydroxide in
water to produce a compound of Formula 3-5. Alternatively, the compound of
Formula 3-4 is
reduced using palladium on carbon in a suitable solvent under a hydrogen
atmosphere to
yield a compound of Formula 3-5.
[00497] In Step 4, compound 3-5 is reacted with about 1.2 equivalents of
cyanogen bromide
in a solvent such as methanol/tetrahydrofuran mixture to produce a compound of
Formula 3-
6. The compound of Formula 3-6 may be further transformed by other
substitution or
derivatization.
[00498] A compound of Formula 3-1 useful in the method of Scheme D is a
compound
having a structure of Formula 3-1, wherein wherein T1 is triflate or halo
(including bromo,
chloro, and iodo), and wherein R1, Xi, X2, X3, R31 and R32 are defined as for
a compound of
Formula I-A.
[00499] Exemplary compounds having a pyrazolopyrimidine core can be
synthesized via
Scheme E.
Scheme E
NH2 i..-(1-1
NTi S
1'..'N Ri Tx
6 I , DMF 1 ,N
N
N
H 80 C N H K2CO3, DMF
A-2 Step 1 4-1 Step 2
NH2 T1 NI-12 m
1\1 NXN -µ1 GO \ Pd(PPh3)4 le, N*1-x-µ
1 ,N +
GrM Sat'd Na2CO3, I ,N
N
Dioxane, reflux N
R1 R1
4-2 Formula B Step 3 Formula C
[00500] In Step 1 of Scheme E, compound A-2 in dimethylformamide (DMF), is
reacted
with an N- halosuccinimide (NTiS) at about 80 C, to provide compound 4-1,
where T1 is
iodo or bromo. In Step 2, compound 4-1 in DMF is reacted with a compound R1 T,
in the
presence of potassium carbonate, to provide compound 4-2. In Step 4, compound
4-2 is
-140-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
coupled with a compound of Formula B using palladium catalysis such as
palladium tetrakis
(triphenylphosphine) , and in the presence of sodium carbonate, to yield a
pyrazolopyrimidine
compound as shown.
[00501] A compound of Formula RiTõ suitable for use in Reaction Scheme E is
the
compound wherein R1 is cycloalkyl or alkyl and Tx is halo (including bromo,
iodo, or chloro)
or a leaving group, including but not limited to mesylate or tosylate.
[00502] Reaction Schemes F-M illustrate methods of synthesis of borane
reagents useful in
preparing intermediates of use in synthesis of the compounds of the invention
as described in
Reaction Schemes A, B, and E above, to introduce M1 substituents.
Reaction Scheme F
S
OH OH OH OH ).L
40 NH2
NO2 0 NH 1 HNO3 / H2SO4 01 2 SnCi2.2H20 NH4SCN
____________________________________ ...
0 C 0.5 h Et0H H20/reflux
Br Br 75 C, 2h Br Overnight Br
F-1 F-2 F-3 F-4
0 0
NH2 ¨0B-BQ¨k NH
¨ 0 0---µ
PbO/Me0H 0"-i CH3000I NH /0
Overnight _______ 0 N
N ___________________________________ 0--\(
.... .
pyridine 0 N _______ 0
Reflux PdC12dPPf
KOAc B,
Br
Br 1,4-Dioxane 0, 0
80 C, 5h
F-5 F-6 F-7
-141-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Reaction Scheme G
0 OH OTs NH2 I
). NaBH4 TsCI , >\ Cs2CO3 N ---).-4
N
,-. ,-.
v v Et3N/DCM
DMF 1.-. le.-'1\1
THF/Me0H .
G-2 G-3 G-4 a
G-1
0
0 (:) NH2
NH ____________ B-B
0--µ '0 0 0----=\(
N ________________________________ N
0 PdC12dPPf 0. 010)
KOAc B,
Br 1,4-Dioxane 0- 0
80 C, 5h V¨L
G-5 G-6
Reaction Scheme H
OH OH OH H
OH
0 HNO3/H2SO4 0 NO2 SnC12.2H20 0 NH2 NH4SCN 0 NyNH2
0 C, 0.5h Br Et0H Br H20/reflux Br N1
Br
75 C, 2h Overnight
H-1 H-2 H-3 H-4
0,\
Y-
0 HN
NH20 )--
=N
13-130(
2
0¨ :
\( NH 0
PbO/Me0H N cH3000i
0- 7O( 0
110
__________ . 0 _______________ . N .
Reflux, 3h Pyridine dppfPdC12
lei KOAc
Br B,
0 C, 1h
Br 1,4-Dioxane 0- 0
5h
H-5 H-6 H-7
-142-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Reaction Scheme I
0
NOH
Br is CN _________________________________ is 01,N CH3COCI
i.-
_____________________________________________________________ ,....
F t-BuOK, DMF Br TEA
RT, 3h NH2 0.5h
1-1 1-2
0.....c
i
____ B -B 00, 01,N 7-d \c, 01 1 N
Br /KOAc/DME NH000H3
'Y
dppfPdC12
NH000H3 1-0
Reflux, 2h
1-3 1-4
Reaction Scheme J
0
(:)F1
N Br 0 o cH3coci
0 CN
______________________________ ,..- ,....
Br F t-BuOK,DMF IN TEA
RT, 3h NH2
0.5h
J-1 J-2
Br 0
IN ;
___________________________________________ 1 N
NHCOCH3 dppfPdC12/KOAc/DME
NHCOCH3
Reflux, 2h
J-3 J-4
-143-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Reaction Scheme K
CI
NH2OH HCI NCS/DMF
Br Na0H(aq)/Et0H Br Br
40 , 60 C, 1h
0 _____________________ N ________________________ 0 ,N
. I ..
I
OH OH
F RT, Over night F F
K-1 K-2 K-3
H2N/A HNA
HN___.4 --C)sB-B/
Br C)/
Br DBU/THF /-0"0--
0
__________ ,... 0 ...,,,
_____________________________________ ,.. ,N
OH Seal-tube
Ether F /
150 C 0 dppfPdC12/KOAc/DME
RT, overnight
Overnight Reflux, 2h
K-4 K-5
1-1N---4
)-11
0 0 ,
N
0
K-6
Reaction Scheme L
CI
NH2OH HCI
BrNa0H(aq)/Et0H Br NCS/DMF Br
is
40 N
O RT, overnight 40 N 60 C, 1h
OH
F FOH F
L-1 L-2 L-3
HN--_¨ 0, ,c) ¨ /
B-13.
O
HN /
H2N/ --
DBU/THF --0/ N
Br
____________ 0 N ____________________ Br
..
OH . "N _________________ .
Seal-tube
H20 F 150 C 0/ clopfPdC12/KOAc/DME
RT, overnight Reflux 2h
Overnight
L-4 L-5
HN--
)--.1
0 0 ,
N
0
L-6
-144-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Reaction Scheme M
bis(pinacolato)diboron,
PdC12(dppf), KOAc
1,4-dioxane, 110 C
-NH 40
N HCI OH C)>NH2
BrCN, Me0H =Q , OH B
2 Step 2
Br NH2 35 oc Br
2. 6N HC1, 80 C OH
M-1 Stepl M-2
Step 3 G-6-B
Reaction Scheme N
R31\ ,R32G9
R31 \ ,R32
I ---OG
NO yO,X1 Ti¨M ___
0 Xi
X2
NX3,
X2
Ri
N-1 N-2 C R1
[00503] In an alternative method of synthesis, a compound of Formula N-1 and a
compound
of N-2 are coupled to produce a compound of Formula C. The coupling step is
typically
catalyzed by using, e.g., a palladium catalyst, including but not limited to
palladium tetrakis
(triphenylphosphine). The coupling is generally performed in the presence of a
suitable base,
a nonlimiting example being sodium carbonate. One example of a suitable
solvent for the
reaction is aqueous dioxane.
[00504] A compound of Formula N-1 for use in Scheme N has a structure of
Formula N-1,
wherein G is hydrogen or RGi, wherein RGi is alkyl, alkenyl, or aryl.
Alternatively, B(OG)2
of the compound of Formula N-1 is taken together to form a 5- or 6- membered
cyclic
moiety. R1, Xi, X2, X3,R31 and R32 of the compound of Formula N-1 are defined
as for a
compound of Formula I-A.
[00505] A compound of Formula N-2 for use in Scheme N has a structure of
Formula N-2
wherein T1 is triflate or halo (including bromo, chloro, and iodo). M of the
compound of
Formula N-2 is either M1 or M2. Mi is defined as for a compound of Formula I.
For
example, M1 can be a 5- benzoxazolyl or a 6- benzoxazolyl moiety, including
but not limited
to those M1 moieties disclosed herein. M2 is a moiety which is synthetically
transformed to
-145-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
form M1, after the M2 moiety has been coupled to the bicyclic core of the
compound of
Formula N-1.
Scheme N-1
R31\ ,R32 R31\ ,R32G?
N T2 N B---OG
NC-- N----L'.-----1\
ex39,X1 e x39 /Xi
N X2 N X2
\ \
RI RI
Formula N-3 Formula N-1
[00506] A compound of Formula N-1 may be synthesized as shown in Scheme N-1. A
compound of Formula N-1 is reacted with a trialkyl borate or a boronic acid
derivative to
produce a compound of Formula N-1. The reaction is typically run a solvent
such as dioxane
or tetrahydrofuran. The trialkyl borate includes but is not limited to
triisopropyl borate and
the boronic acid derivative includes but is not limited to
bis(pinacolato)diboron.
[00507] When the reaction is performed with trialkyl borate, a base such as n-
butyl lithium
is first added to the compound of Formula N-3 to generate an anion, prior to
the addition of
the borate. When the reaction is performed with a boronic acid derivative such
as
bis(pinacolato)diboron, a palladium catalyst and a base is used. Typical
palladium catalysts
include but is not limited to palladium chloride
(diphenylphosphino)ferrocene). A suitable
base includes but is not limited to potassium acetate.
[00508] A compound of Formula N-3 suitable for use in Scheme N-1 is a compound
wherein T2 is halo or another leaving group such as mesylate, tosylate, or
triflate. X1, X25 X35
R1, R31, and R32 of the compound of Formula N-3 is as defined for a compound
of Formula I-
A.
[00509] In some embodiments of the invention, a compound of Formula A, B, B',
B", C, C",
D, E, E", 3-1, 3-2, 3-3, 3-4, 3-5, 3-6, N-1", N-3", 3-1", 3-3", 3-4", 3-5", 3-
6", N-1", or N-3"
is provided as its salt, including but not limited to hydrochloride, acetate,
formate, nitrate,
sulfate, and boronate.
[00510] In some embodiments of the invention, a palladium compound, including
but not
limited to palladium chloride (diphenylphosphino)ferrocene) and palladium
tetrakis
(triphenylphosphine), is used in the synthesis of a compound of Formula A, B,
B', B", C, C",
D, E, E", 3-1, 3-2, 3-3, 3-4, 3-5, 3-6, N-1", N-3", 3-1", 3-3", 3-4", 3-5", 3-
6", N-1", or N-3" .
When a palladium compound is present in the synthesis of a compound of Formula
A, B, B',
B", C, C", D, E, E", 3-1, 3-2, 3-3, 3-4, 3-5, 3-6, N-1", N-3", 3-1", 3-3", 3-
4", 3-5", 3-6", N-
-146-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
1", or N-3" ,it is present in an amount ranging from about 0.005 molar
equivalents to about
0.5 molar equivalents, from about 0.05 molar equivalents to about 0.20 molar
equivalents,
from about 0.05 molar equivalents to about 0.25 molar equivalents, from about
0.07 molar
equivalents to about 0.15 molar equivalents, or about 0.8 molar equivalents to
about 0.1
molar equivalents of the compound of Formula A, B, B', B", C, D, E, 3-1, 3-2,
3-3, 3-4, 3-5,
3-6, N-1, or N-3. I n some embodiments, a palladium compound, including but
not limited to
palladium chloride (diphenylphosphino)ferrocene) and palladium tetrakis
(triphenylphosphine) is present in the synthesis of a compound of Formula A,
B, B', B", C,
C", D, E, E", 3-1, 3-2, 3-3, 3-4, 3-5, 3-6, N-1", N-3", 3-1", 3-3", 3-4", 3-
5", 3-6", N-1", or N-
3" in about 0.07, about 0.08, about 0.09, about 0.10, about 0.11, about 0.12,
about 0.13,
about 0.14, or about 0.15 molar equivalents of a starting material of Formula
A, B, B', B", C,
C", D, E, E", 3-1, 3-2, 3-3, 3-4, 3-5, 3-6, N-1", N-3", 3-1", 3-3", 3-4", 3-
5", 3-6", N-1", or N-
3" that is used to synthesize a compound of Formula A, B, B', B", C, C", D, E,
E", 3-1, 3-2,
3-3, 3-4, 3-5, 3-6, N-1", N-3", 3-1", 3-3", 3-4", 3-5", 3-6", N-1", or N-3" .
[00511] In some embodiments of the above reaction schemes B, D, E, N or N-1,
another
embodiment of the compounds of Formula A, C, 3-1, 3-3, 3-4, 3-5, 3-6, A-2, 4-
1, 4-2, N-1
and N-3 is as shown in Schemes B'. D'. E', N' or N-1' below. In these
alternative syntheses,
producing a compound of Formula C, 3-1, 3-3, 3-4, 3-5, 3-6, A-2, 4-1, 4-2, N-1
or N-3, use
compounds that comprise an amino moiety having a RG2 moiety present during one
or more
of the synthetic steps, wherein RG2 is an amino protecting group including but
not limited to
tert-butyl carbamate (Boc), carboxybenzyl (Cbz), benzyl (Bz),
fluorenylmethyloxycarbonyl
(FMOC), p-methoxybenzyl (PMB), and the like. These compounds include a
compound of
Formula A", C", 3-1", 3-3", 3-4", 3-5", 3-6", A-2", 4-1", 4-2", N-1" or N-3".
[00512] The RG2 moiety is removed, using suitable methods, at any point
desired,
whereupon the compound of Formula C, 3-1, 3-3, 3-4, 3-5, 3-6, A-2, 4-1, 4-2, N-
1 or N-3 has
a R31 hydrogen replacing the RG2 moiety on the amino moiety. This
transformation is
specifically illustrated for the conversion of a compound of Formula C" to a
compound of C (
i.e., as in Step 4 of Scheme E') and for the conversion of a compound of
Formula 3-6" to a
compound of Formula 3-6 ( i.e., as in Step 5 of Scheme D'). This illustration
is in no way
limiting as to the choice of steps wherein a compound comprising a NR31RG2
moiety may be
converted to a compound comprising a NR31R32 moiety wherein the R32 moiety is
hydrogen.
Scheme B'
-147-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
R3Z ,RG2 R. ,RG2 D31 R32
T, .... .
GO
\
__L I\V1)1(1 ________ M1
r\ei\ Xi +
L /Xi
N X2 GO Xi
N 2 N x2
\ \
R1 R1 \
A' B C" C R1
Scheme D'
OH OH
R31\ ,RG2.
-(1 R31 \ ,RG24. R31 \DP ,. ,G2 NO2
N N
N
e,x92 .._i,x
N Xi
stept N rTh )(n
..?,X1 Step 2 ' N
pxo,xi Step 3 "
\ N X2 N 3-X2
\ \
Ri Ri Ri
Formula 3-1' Formula 3-2" Formula 3-3" Formula 3-4"
OH 0---7,N H2
,4. I I I I
R31 ,RG2401 R31\ N
RG2
NH2 N
R31\ ,R32 fi
N N N
N n
_____________________________________________________ n 0
Step 4 X1 Step 5 Xi
=-=j,X3- / -1,X3- i \-.-1)(3- i
N X2 N X2 N X2
\ \ \
R1 R1 Ri
Formula 3-5" Formula 3-6" Formula 3-6
Scheme E
RG2
NH RG2 \
RG2,
'
..11H x. -(1- 1 NHTi
erN N / \ Ri Tx N LIN ssss.(
\ GO\
/BM
N N I
H Step 1 N N
n Step 2 N GORi
Formula A-2" Formula 4-1" Formula 4-2" Formula B
RG2
NH M NH2 m
N .X....µ
N.CI.--41 N
-No.-
Step 3 N 1 Step 4 N N
%
R1 R1
Formula C" Formula C
-148-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Scheme N' and N-1"
R31\ , RG2
R31\ ,RG2G? R31 \ ,RG2 R31\
,R32
N
N 1 i B---OG
1 /T2 N N
yi y i
1\1
Xi
[ -0
N
"2 \ \ N )(3v-/2 N)(3-iµµ,/2 N
)(3.-iµv
2
H
\ /x
\
\
Ri H
Ri Ri
Ri
Formula N-1" Formula N-3" Formula D Formula C" Formula C
[00513] Additionally, the invention encompasses methods of synthesis of the
compounds of
A, B, B', B", C, E, 3-1, 3-2, 3-3, 3-4, 3-5, 3-6, N-1 or N-3, wherein one or
more of M, M1, or
R1 has a protecting group present during one or more steps of the synthesis.
Protecting
groups suitable for use for a M, M1, or R1 moiety are well known in the art,
as well as the
methods of incorporation and removal, and the reagents suitable for such
transformations.
[00514] Compounds of the invention where X4 is C-R9 may be prepared by methods
analogous to the ones described in the Schemes illustrated above.
Reaction Schemes 0, P and Q illustrate methods of synthesis of borane reagents
useful in
preparing intermediates of use in synthesis of the compounds of the invention
as described in
Reaction Schemes 1 and 2 above, to introduce benzothiazolyl substituents.
Scheme 0
0 N 0 N SI N
HNO3 SnCl2
' ____________________ ,..
S 02N S H2N S
0-1 0-2 0-3
Si N 0 N
NaNO2/H+ BuLi
,..-
CuBr2 Br S B(01P03 (H0)2B S
0-4 0-5
A compound of Formula 0-1 is treated with, for example, nitric acid to produce
a compound
of Formula 0-2. The compound of Formula 0-2 is treated with a reducing agent
such as
stannous chloride to produce a compound of Formula 0-3. The compound of 0-3 is
treated
with sodium nitrate in acid and cupric bromide to produce a compound of
Formula 0-4. The
compound of 0-4 is treated a base such as butyl lithium and boron tris-
isopropoxide to
produce a compound of Formula 0-5.
-149-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Scheme P
Br NH2 _______ KSCN , Br el s, CH3COCI Br is s N
HAc
Br2 DMAP
P-1 P-2 P-3
)0
:013 0B 401 s
P-4 '
/ ¨NHAc
PdC12dppf
P-5
A compound of Formula P-1 is treated with, for example, potassium thiocyanate
and bromine
in acetic acid to produce a compound of Formula P-2. The compound of Formula P-
2 is
treated with an acetylating reagent such as acetyl chloride to produce a
compound of Formula
P-3. The compound of P-3 is reacted with, for example, bis(pinacolato)diboron
(compound
P-4) in the presence of a catalyst such as palladium chloride to produce a
compound of
Formula P-5.
Scheme Q
Pd2(dba)3
2-Dicyclohexylphosphino-
2',4',6'-triisopropylbiphenyl
Br s 0 0
)¨NH2 _______________ Br S S/)¨ NH N\H
=/)--NH
Pyridine A3-B
RI overnight 0 p-4 02
P-2 41
CIC(0)NHCH3
KOAc
1,4-Dioxane
reflux overnight
[00515] The compound of Formula P-2 is reacted with, for example, methyl
carbamic acid
chloride to produce a compound of Formula Q-1. The compound of Formula Q-1 is
reacted
with bis(pinacolato)diboron (compound P-4) in the presence of a catalyst such
as Pd2(dba)3
2-chlorohexylphosphino-2, 4, 6-triisopropylbiphenyl, a base such as potassium
acetate, to
produce the compound of Formula Q-2.
[00516] Some illustrative compounds of the invention which are mTor inhibitors
are
described below. The compounds of the invention are not limited in any way to
the
compounds illustrated herein.
-150-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
'
v
0õ/
h ¨7< if
NH2 J
ti ,F12 i)
N" ).---- . N ------
), .1___ /Xi
-)'' --'------C/X1- H
H N. N\ H N \
Ri Ri
Subclass la Subclass lb
v oyv
NH2 0
NH2
N
N
---- \
4.,Xi X1.___Ei
,---= t
CH3 N
CH3N ----C/
N
\ \
R1 R1
Subclass 2a Subclass 2b
v v
0
cy
N ef IN
NH2 NH2
N '-'--- \ N ------
N
CH3 N/X1 CH3):- Xi¨ H-, -----,./ N '
\ \
CH3 R1 CH3 Ri
Subclass 3a Subclass 3b
v
0 I/
/
411 IN /, __ II
NH2 NH2 __ ,
N--,\N ---- ---
1 X1X1- H
0 N N\
Ri a-N ---:-----'C/
1
Ri
Subclass 4a Subclass 4b
v
0 0
.,7, v
___________________________ /
)----- N
NH2 __ / NH2\¨
N '--, \ N --" -----
I Xi X1-H
.,-;,-,---___ /
ip -N--------C/
-- N N\
1
\ / Hi R1
Subclass 5a Subclass 5b
-151-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
0 cõ ,V
NH ID N
NI-12
0 N
N- ---
NI ."--, \ xl
N- ,i4 ,X1-__H
'
RI RI
. 41
Subclass 6a Subclass 6b
V
( 7
CL_/
NH2 li
I ii N
NH,
N-- Il----( .
,x1 N -' ---
NI' N\ -----
N Xi¨N
Ri
Ri
N N
Subclass 7a Subclass 7b
v
(:)_ /
/ 7 V
NH2 _______________________ /
NH
N - I'--- \
I Xi N ---- ---
X1¨H
---' N
\ \
\ / N Ri R1
Subclass 8a Subclass 8b
NyV Nyv
NH2 NH2
N ''''-i \ N ---- ----
)1, Xi
/ N/ õ,...1....,, ...... /Xi¨H
H N H N C
\ \
R1 R1
Subclass 9a Subclass 9b
N N
410 yv
yv
, 0 . 0
NH2 NH2
N ..-==== \ N ==-- ---
.... Xi¨H
/ / ..õ1-..,.., ¨iiii. /
H3C N N\ H3C N C
R1 \
Ri
Subclass 10a Subclass 10b
NyV N yv
NH2 NH2
N ''=== \ N '' ---
H3C \ .õ, ../ ,X1¨H
X1 H3C,, .... ----/
CH N N CH N '
/ \
/ H3C \
H3C Ri Ri
Subclass 1 1 a Subclass 1 lb
-152-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
V
N
1µ1/V
/ =
NH 0
2 41 0
N2**-- NH2 114
---=
XI-H
e s
..... ,,,
N - Ciri''N C
\
61 Ri
Subclass 12a Subclass 12b
v
N N v
8
NH2 0,
NH2 41,
N N --"*. ..---
I Xi Xi-H
---, /
100 N li 40, N C\ 1
Subclass 13a Subclass 13b
N/V
V
N
NH2 410# 0
NH2 4i 0
-"*. ----
N \ N
L1 C
---.. N N1X1\
Ri
Ri
X1-1-1
Subclass 14a Subclass 14b
V
NI.,../ N
NH 4.= 0
NH
N2".. ..---
N '2. \ x,
I X1-1-I
....' / ==., ---- /
N N N C
\ \
R1 Ri
41k .
Subclass 15a Subclass 15b
N /V N V
7
NH2 = NH 0,
N \ \ x, N ..... -----
SN
1
XI-H
N N
Subclass 16a Subclass 16b
[00517] Illustrative compounds of the invention include those of subclass la,
lb, 2a, 2b, 3a,
3b, 4a, 4b, 5a, 5b, 6a, 6b, 7a, 7b, 8a, 8b, 9a, 9b, 10a, 10b, 11a, 11b, 12a,
12b, 13a, 13b, 14a,
14b, 15a, 15b, 16a, or 16b, where the substituents Ri, Xi, and V are as
described below.
-153-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00518] In some embodiments, when Ri is H and Xi is CH, V is phenylamino,
benzyl,
phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is H and Xi is N, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is CH3
and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when
Ri is CH3 and Xi is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt,
NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
embodiments, when Ri is Et and Xi is CH, V is phenylamino, benzyl, phenyl,
NHMe, NH2,
NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In
other embodiments, when Ri is Et and Xi is N, V is phenylamino, benzyl,
phenyl, NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me. In other embodiments, when Ri is iPr and X1 is CH, V is phenylamino,
benzyl,
phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is iPr and Xi is N, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In one embodiment, Ri is iPr, X1 is N,
and
V is NH2. In another embodiment, Ri is iPr, Xi is N, and V is NHCOMe. In other
embodiments, when Ri is cyclobutyl and Xi is CH, V is phenylamino, benzyl,
phenyl,
NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me. In other embodiments, when Ri is cyclobutyl and X1 is N, V is
phenylamino,
benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is cyclopentyl and Xi is CH,
V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is
cyclopentyl and Xi is N V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt,
NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
embodiments, when Ri is phenyl and Xi is CH, V is phenylamino, benzyl, phenyl,
NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me. In other embodiments, when Ri is phenyl and Xi is N, V is
phenylamino, benzyl,
phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is pyridin-2-y1 and Xi is
CH, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
-154-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is pyridin-
2-y1 and X1 is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
embodiments, when Ri is N-methylaminocyclohex-4-y1 and Xi is CH, V is
phenylamino,
benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is N-methylaminocyclohex-4-
y1
and Xi is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri
is N-methylpiperidin-4-y1 and Xi is CH, V is phenylamino, benzyl, phenyl,
NHMe, NH2,
NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In
other embodiments, when Ri is N-methylpiperidin-4-y1 and Xi is N, V is
phenylamino,
benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is N-methylaminocyclobut-3-
y1
and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri
is N-methylaminocyclobut-3-y1 and Xi is N, V is phenylamino, benzyl, phenyl,
NHMe, NH2,
NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In
other embodiments, when Ri is tert-butyl and X1 is CH, V is phenylamino,
benzyl, phenyl,
NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me. In other embodiments, when Ri is tert-butyl and Xi is N, V is
phenylamino,
benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is 1-cyano-but-4-y1 and X1
is CH,
V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is 1-
cyano-but-4-y1 and Xi is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt,
NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
embodiments, when Ri is 1-cyano-prop-3-y1 and Xi is CH, V is phenylamino,
benzyl, phenyl,
NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me. In other embodiments, when Ri is 1-cyano-prop-3-y1 and Xi is N, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is 3-
azetidinyl and Xi is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt,
NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
embodiments, when Ri is 3-azetidinyl and Xi is N, V is phenylamino, benzyl,
phenyl,
-155-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me.
[00519] In other embodiments, when Ri is C2--- and Xi is CH, V is phenylamino,
benzyl,
phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is C7--- and Xi is N, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is
C/NH and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
embodiments, when Ri is C/NH and Xi is N, V is phenylamino, benzyl, phenyl,
NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me. In other embodiments, when Ri isc/NH and Xi is CH, V is phenylamino,
benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is Q\IFI and Xi is N, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is
/ 'NH2 and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
embodiments, when Ri is /--NNI-I2 and Xi is N, V is phenylamino, benzyl,
phenyl, NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me. In other embodiments, when Ri is r-NNFI2 and X1 is CH, V is
phenylamino,
benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is ("NH2 and Xi is N, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
-156-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
C?
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is N"
and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri
isNH and Xi is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
C?
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
embodiments, when Ri is HO and Xi is CH, V is phenylamino, benzyl, phenyl,
NHMe, NH2,
NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In
other embodiments, when Ri is !Tic)) and X1 is N, V is phenylamino, benzyl,
phenyl, NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
/
NHSO2Me. In other embodiments, when Ri is Ho and Xi is CH, V is phenylamino,
benzyl,
phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
/
,....
CONHMe, or NHSO2Me. In other embodiments, when Ri is HO and X1 is N, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
-4..
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is Co
and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri
-4,
is 00 and X1 is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
--\
embodiments, when Ri is 0 and
X1 is CH, V is phenylamino, benzyl, phenyl, NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
.,,ri
--\
NHSO2Me. In other embodiments, when Ri is 0
and Xi is N, V is phenylamino, benzyl,
-157-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
,ro
bCONHMe, or NHSO2Me. In other embodiments, when Ri is and Xi
is CH, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
bNHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is
and Xi is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when
,
bR1 is and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt,
NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
bembodiments, when Ri is and Xi is N, V is phenylamino, benzyl, phenyl,
NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
./
C
NHSO2Me. In other embodiments, when Ri is N and X1 is CH, V is phenylamino,
benzyl,
phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
n
CONHMe, or NHSO2Me. In other embodiments, when Ri is N and X1 is N, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
S
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is I
and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri
srs'
is and X1
is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
embodiments, when Ri is -2µ---\ and Xi is CH, V is phenylamino, benzyl,
phenyl, NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
-158-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
NHSO2Me. In other embodiments, when Ri is ¨\--\ and X1 is N, V is phenylamino,
benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
S\
CONHMe, or NHSO2Me. In other embodiments, when Ri is )---- and X1 is CH, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
ssc
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is )----
and Xi is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri
/
is 40
and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
/
embodiments, when Ri is . and
Xi is N, V is phenylamino, benzyl, phenyl, NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
\ fai
NHSO2Me. In other embodiments, when Ri is le
and X1 is CH, V is phenylamino,
benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
\ Ai
CONHMe, or NHSO2Me. In other embodiments, when Ri is 1Ir
and X1 is N, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
C
N
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is H
and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri
C
N
is H and X1 is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
-159-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
,.-'_
embodiments, when Ri is aH and Xi is CH, V is phenylamino, benzyl, phenyl,
NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
õ
NHSO2Me. In other embodiments, when Ri is 6 NH and Xi is N, V is phenylamino,
benzyl,
phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is NH and X1 is CH, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
¨
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is NH
and X1 is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when
.?.
c.N)
Ri is o and Xi is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt,
NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
rN)
embodiments, when Ri is Co and X1 is N, V is phenylamino, benzyl, phenyl,
NHMe, NH2,
NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In
other embodiments, when Ri is OH and Xi is CH, V is phenylamino, benzyl,
phenyl, NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
,..:
NHSO2Me. In other embodiments, when Ri is OH and X1 is N, V is phenylamino,
benzyl,
phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
-160-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
WV,
CONHMe, or NHSO2Me. In other embodiments, when Ri is OH and Xi is CH, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when R1 is OH
and Xi is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when
A-1 Is CONHMe and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt,
NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
embodiments, when Ri is CONHMe and X1 is N, V is phenylamino, benzyl, phenyl,
NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me. In other embodiments, when Ri is NHAc and X1 is CH, V is phenylamino,
benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is NHAc and X1 is N, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is Kfl'e
and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when R1
is 'Me and X1 is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt,
NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
-161-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
embodiments, when Ri is N'OH and Xi is CH, V is phenylamino, benzyl, phenyl,
NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
41.
NHSO2Me. In other embodiments, when Ri is N'OH and Xi is N, V is phenylamino,
benzyl,
phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is Nome and Xi is CH, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is N'OMe
and X1 is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when
Ri is o and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt,
NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
embodiments, when Ri is o and Xi is N, V is phenylamino, benzyl, phenyl, NHMe,
NH2,
NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In
other embodiments, when Ri is /c) and Xi is CH, V is phenylamino, benzyl,
phenyl,
NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me. In other embodiments, when Ri is /c) and X1 is N, V is phenylamino,
benzyl,
phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is OH and X1 is CH, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
-162-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
a
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is (..31d
and X1 is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when
¨
CN:N)
Ri is me and Xi is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt,
NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
¨
C.N..,)
embodiments, when Ri is me and X1 is N, V is phenylamino, benzyl, phenyl,
NHMe, NH2,
NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In
other embodiments, when Ri is (1) and Xi is CH, V is phenylamino, benzyl,
phenyl,
NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me. In other embodiments, when Ri is 0 and Xi is N, V is phenylamino,
benzyl,
phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is ..1-( and Xi is CH, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is Cirri
and X1 is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when
am
Ri is H and Xi is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt,
NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
-163-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
'b,
embodiments, when Ri is k' and Xi is N, V is phenylamino, benzyl, phenyl,
NHMe, NH2,
NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In
o
N
other embodiments, when Ri is )---- and Xi is CH, V is phenylamino, benzyl,
phenyl,
NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
o
NHSO2Me. In other embodiments, when Ri is1)--j -- and X1 is N, V is
phenylamino, benzyl,
phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is N-OH and X1 is CH, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is
N-OH and Xi is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
embodiments, when Ri is N'OMe and Xi is CH, V is phenylamino, benzyl, phenyl,
NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me. In other embodiments, when Ri is Nome and X1 is N, V is phenylamino,
benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
1N
CONHMe, or NHSO2Me. In other embodiments, when Ri is 0 and Xi is CH, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
-164-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
1N
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is Lo
and X1 is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when
-1-1
ON
Ri is C,0 and Xi is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt,
NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
-ii
ceN"")
embodiments, when Ri is C,0 and X1 is N, V is phenylamino, benzyl, phenyl,
NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me.
r's---\
[00520] In other embodiments, when Ri is :. OH and X1 is CH, V is phenylamino,
benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is OH and Xi is N, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is
/ µOH
and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
embodiments, when Ri is / 'OH and Xi is N, V is phenylamino, benzyl, phenyl,
NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me. In other embodiments, when Ri is /\ OH and X1 is CH, V is
phenylamino,
benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
IrSc_.....
CONHMe, or NHSO2Me. In other embodiments, when Ri is /\ 'OH and X1 is N, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is
4-\ H
and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
-165-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
ss',.._.....\
embodiments, when Ri is 4...\ 'OH and Xi is N, V is phenylamino, benzyl,
phenyl, NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
/
------\0H
NHSO2Me. In other embodiments, when Ri is OH
and Xi is CH, V is phenylamino,
benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
rrsj
-----\OH
CONHMe, or NHSO2Me. In other embodiments, when Ri is OH and Xi is N, V
is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is
isc...\
/\ NH2 and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
SrSc.,
embodiments, when Ri is i\ NH2 and Xi is N, V is phenylamino, benzyl, phenyl,
NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
Allik
NHSO2Me. In other embodiments, when Ri is NH2 and Xi is CH, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is
4 'NH2
and Xi is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me.
snnAn.n.
[00521] In other embodiments, when Ri is 0 and X1 is CH, V is phenylamino,
benzyl,
phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is IS and Xi is N, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
-166-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
I
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is -1s1
and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe,
NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when
Ri is and Xi
is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
140
embodiments, when Ri is H3c and Xi is CH, V is phenylamino, benzyl, phenyl,
NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me. In other embodiments, when Ri is H3c and Xi is N, V is phenylamino,
benzyl,
phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
cl/cH3
I
CONHMe, or NHSO2Me. In other embodiments, when Ri is r%1 and Xi
is CH, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is
ozCH3
N and X1 is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
./)
embodiments, when Ri is N and X1 is CH, V is phenylamino, benzyl, phenyl,
NHMe, NH2,
NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In
other embodiments, when Ri is N and Xi is N, V is phenylamino, benzyl, phenyl,
NHMe,
NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me. In other embodiments, when Ri is / and Xi is CH, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is
-167-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
and X1 is N, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
embodiments, when Ri is and
Xi is CH, V is phenylamino, benzyl, phenyl,
NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me. In other embodiments, when Ri is;VN7..:----=-4 and Xi is N, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is
N*
and Xi is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH,
NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
7____/N =
embodiments, when Ri is and Xi is N, V is phenylamino, benzyl, phenyl,
NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
N_CIN
NHSO2Me. In other embodiments, when Ri is and
Xi is CH, V is phenylamino,
benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe,
CONHMe, or NHSO2Me. In other embodiments, when Ri is and
Xi is N, V is
phenylamino, benzyl, phenyl, NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt,
NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other embodiments, when Ri is
N¨CN-H
and X1 is CH, V is phenylamino, benzyl, phenyl, NHMe, NH2, NHEt,
NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or NHSO2Me. In other
N-C/N-11
embodiments, when Ri is and Xi is N, V is phenylamino, benzyl,
phenyl,
NHMe, NH2, NHEt, NHCOH, NHCOMe, NHCOEt, NHC0iPr, NHCOOMe, CONHMe, or
NHSO2Me.
[00522] In the noted embodiments, pyridin-2-y1 is N-
methylaminocyclohex-4-y1 is
NHCH,YrTh
N-methylpiperidin-4-y1 is "1-
ci,õ and N-methylaminocyclobut-3-y1 is I--NHCH,.
-168-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
[00523] Illustrative compounds of the invention include those of subclass la,
lb, 2a, 2b, 3a,
3b, 4a, 4b, 5a, 5b, 6a, 6b, 7a, 7b, 8a, 8b, 9a, 9b, 10a, 10b, 11a, 11b, 12a,
12b, 13a, 13b, 14a,
14b, 15a, 15b, 16a, or 16b, where the substituents Ri, Xi, and V are as
described below. In
some embodiments, when Ri is H and X1 is CH, V is cyclopropanecarboxamido,
cyclopropylamino, morpholinoethylamino, hydroxyethylamino, or N-morpholino. In
other
embodiments, when Ri is H and Xi is N, V is cyclopropanecarboxamido,
cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In some embodiments,
when
Ri is CH3 and Xi is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is CH3 and Xi is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In some embodiments,
when
Ri is Et and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is Et and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In some embodiments,
when
Ri is iPr and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is iPr and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In some embodiments,
when
Ri is cyclobutyl and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is cyclobutyl and Xi is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In some embodiments,
when
Ri is cyclopentyl and X1 is CH, V is cyclopropanecarboxamido,
cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is cyclopentyl and Xi is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In some embodiments,
when
Ri is phenyl and Xi is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is phenyl and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In some embodiments,
when
Ri is pyridin-2-y1 and X1 is CH, V is cyclopropanecarboxamido,
cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is pyridin-2-y1 and X1 is N, V is cyclopropanecarboxamido,
cyclopropylamino,
-169-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In some embodiments,
when
Ri is N-methylaminocyclohex-4-y1 and Xi is CH, V is cyclopropanecarboxamido,
cyclopropylamino, morpholinoethylamino, hydroxyethylamino, or N-morpholino. In
other
embodiments, when Ri is N-methylaminocyclohex-4-y1 and Xi is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
or N-morpholino. In some embodiments, when Ri is N-methylpiperidin-4-y1 and X1
is CH, V
is cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
or N-morpholino. In other embodiments, when Ri is N-methylpiperidin-4-y1 and
Xi is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
or N-morpholino. In some embodiments, when Ri is N-methylaminocyclobut-3-y1
and X1 is
CH, V is cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino, or N-morpholino. In other embodiments, when Ri is N-
methylaminocyclobut-3-y1 and X1 is N, V is cyclopropanecarboxamido,
cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is tert-butyl and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is tert-butyl and Xi is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is 1-cyano-but-4-y1 and Xi is CH, V is cyclopropanecarboxamido,
cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is 1-cyano-but-4-y1 and X1 is N, V is cyclopropanecarboxamido,
cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is 1-cyano-prop-3-y1 and Xi is CH, V is cyclopropanecarboxamido,
cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is 1-cyano-prop-3-y1 and X1 is N, V is cyclopropanecarboxamido,
cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is 3-azetidinyl and X1 is CH, V is cyclopropanecarboxamido,
cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is 3-azetidinyl and X1 is N, V is cyclopropanecarboxamido,
cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
-170-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
R1 is )---and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is L/NH and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
R1 is /1\1E1 and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
R1 is L,NH and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is L,NH and Xi is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
/44
Ri is F NH2 and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is TµNH2 and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is r\NH2 and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is nNH2 and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is NH and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is NH and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
-171-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
---
R1 iS HO and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
.,,=')
---
Ri is HO and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
/
Ri is HO and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
/
Ri is HO and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is 0 and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
-4.
Ri is CO and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
--\.,,ri
Ri is 0 and Xi is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is 0 and Xi is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
b
.,.,..
R1 is and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
bR1 is and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
-172-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
,
bR1 is and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
."
bR1 is and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
.er')
n
R1 15 N and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
..I)
r,
R1 is N and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
/
R1 is 1and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
/
1
R1 is and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
¨\\R1 is and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
¨\\R1 is and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
/\
R1 is )----- and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
./\
R1 is )----- and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
-173-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
/
R1 is la and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
so'
Ri is . and Xi is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
\
Ri is * and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
\
Ri is * and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
0
N
R1 is H and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
.,5
0
N
R1 is H and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
/
Ri is NH and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
/
Ri is NH and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is NH and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
-174-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
R1 is NH and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
(NI)
R1 is o and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
(N)
R1 is o and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
R1 is OH and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
R1 is OH and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
R1 is OH and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
R1 is OH and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
R1 is CONHMe and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
R1 IS CONHMe and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
-175-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is NHAc and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is NHAc and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is Me and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is Me and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is N-OH and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
41.
Ri is 1\1'0H and Xi is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is N'OMe and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is WOMe and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is o and Xi is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
-176-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
R1 is 'o' and Xi is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
1\1
Ri is /c) and Xi is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
N
Ri is /c) and Xi is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
a
R1 is 5H and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
a
Ri is 5H and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
7ds?
C")
R1 is Ne and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
..s?,
C---)
R1 is iinle and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
..,..E.1)
R1 is Cr\-1-0-.) and X1 is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
R1 is Cr\-1-0-.) and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
-177-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
lir
Ri is CN and Xi is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
N-,1.--
R1 is ciN and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
am
R1 is H and Xi is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
"-------)
R1 is iNi and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
o
N
Ri is )--- and Xi is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
o
N
Ri is )-- and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
R1 is N-OH and Xi is CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
R1 is N'OH and X1 is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
Ri is N'OMe and Xi is CH, V is cyclopropanecarboxamido, cyclopropylamino,
-178-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
'11?'
Ri is N'OMe and Xi is N, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
4
(N1
R1 is 0 and Xi is CH,
V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
4
(N
R1 is 0 and X1 is N, V
is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
-i
A
Ri is O 0 and X1 is
CH, V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino. In other
embodiments, when
-1-
ON
Ri is c.0 and Xi is N,
V is cyclopropanecarboxamido, cyclopropylamino,
morpholinoethylamino, hydroxyethylamino, or N-morpholino.
[00524] In other embodiments, when Ri is :-= OH and X1 is CH, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
.4
-----\
or N-morpholino. In other embodiments, when Ri is OH and Xi is N,
V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
rr?
or N-morpholino. In other embodiments, when Ri is )0H and Xi is CH, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
rrsc____ \
or N-morpholino. In other embodiments, when Ri is / 'OH and X1 is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
or N-morpholino. In other embodiments, when Ri is \-----\OH and
X1 is CH, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
-179-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
)or N-morpholino. In other embodiments, when Ri is OH and Xi is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
/
or N-morpholino. In other embodiments, when Ri is .-----\OH and X1 is CH, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
sss"
or N-morpholino. In other embodiments, when Ri is =I\OH and Xi is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
rre
.--01-1
or N-morpholino. In other embodiments, when Ri is OH and X1 is CH, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
ire
rOH
or N-morpholino. In other embodiments, when Ri is OH and Xi is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
"3
or N-morpholino. In other embodiments, when Ri is )\--\NI-12 and Xi is CH, V
is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
"4
or N-morpholino. In other embodiments, when Ri is )C1\1112 and Xi is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
/
or N-morpholino In other embodiments, when Ri is ''----\ NH2 and X1 is CH, V
is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
/
or N-morpholino. In other embodiments, when Ri is '--..----.N N H2 and X1 is
N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
or N-morpholino.
-180-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
[00525] In other embodiments, when Ri is and X1 is CH, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
~Ann.
or N-morpholino. In other embodiments, when Ri is and Xi is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
or N-morpholino. In other embodiments, when Ri is N and Xi is CH, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
or N-morpholino. In other embodiments, when Ri is N and X1 is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
140
or N-morpholino. In other embodiments, when Ri is H3c and Xi is CH, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
or N-morpholino. In other embodiments, when Ri is H3c and X1 is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
CH
I 3
or N-morpholino. In other embodiments, when Ri is N and Xi is CH, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
cH3
I
or N-morpholino. In other embodiments, when Ri is N and Xi is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
y
or N-morpholino. In other embodiments, when Ri is N and Xi is CH, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
or N-morpholino. In other embodiments, when Ri is N and Xi is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
-181-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
'Nin7-
or N-morpholino. In other embodiments, when Ri is / and Xi is CH, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
Nn'7
or N-morpholino. In other embodiments, when Ri is / and Xi is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
or N-morpholino. In other embodiments, when Ri isJVN..-7-----=-4 and X1 is CH,
V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
or N-morpholino. In other embodiments, when Ri isJVN::::-------=---4 and Xi is
N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
N git
or N-morpholino. In other embodiments, when Ri is -%".-... and Xi is CH, V
is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
N .
7----1
or N-morpholino. In other embodiments, when Ri is X and Xi is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
N¨C/N
\ /
or N-morpholino. In other embodiments, when Ri is "C"---/ and Xi is CH, V
is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
N -C/N
/"------/
or N-morpholino. In other embodiments, when Ri is "i", and X1 is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
N¨CN-H
r¨/
or N-morpholino. In other embodiments, when Ri is )"- and Xi
is CH, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
CN¨H
N
r-----/
or N-morpholino. In other embodiments, when Ri is i'- and X1
is N, V is
cyclopropanecarboxamido, cyclopropylamino, morpholinoethylamino,
hydroxyethylamino,
or N-morpholino.
-182-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
)1---V.
[00526] In the noted embodiments, cyclopropanecarboxamido is
cyclopropylamino is H 2-morpholinoethylamino is (J----\N--) ,
hydroxyethylamino is
(----0
\
and N-morpholino is
Table 1. Biological activity of several illustrative mTor inhibitor compounds
of the invention.
mTOR PI3K a PI3K 0 PI3K y PI3K 6
PC3
Structure IC50 (nM) IC50 (nM) IC50 (nM) IC50 (nM)
EC50
IC50 (nM)
(nM)
1 0-_(NH2 ++++ +++ ++ ++++
+++ ++++
NH2 4 * N
N .---- \
k n,
'"
N N
).----
2 0INHcocH3
++++ ++ + +++ +++
+++
NH2 *
N 'N \ N
kN.' N.
)...-
3 N1NHcocH3 ++ + ++ ++ ++
NH2 *
N ."N \ N
N N
kc .
4 Nr...õrNFI2 +++ ++ ++ +++
+++ ++
NH2 .
N \
''
n,
k , '
N N
2--
5NH2 ++++ +++ ++ ++++ +++
++++
01
NH2* "
N N
0
601NH2 ++++ ++ + ++ +++
+++
NH2*
N N
0
¨183-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
7 0____.(NH2 ++++ +++ ++ ++ +++
++
\\
N
NH2*
N'' \N
k -=
N-
id
01
.----
o
8 o_iNH2 ++++ +++ + +++ +++ ++++
\\
N
NH2 .
N" \
k -
N N
)----
901NH2 ++++ ++ + +++ +++ ++++
NH2 =
IsC
10 o-N
1 ++
+
= NH2
NH2
N ..", \ N
kr\J- '
N)s__._
11 o
HN)1----- +++
+
¨Ne
NH2*
N \
k ,N
N N
/\-----
12 H2N +++
+
-1
0
NH2 =
N \
k ,N
N N
)-----
13 HN ++ ++ +++ +++
--1
0
NH2 *
N
_., ,N
N
)----
-184-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
14
HNP' ++ ++ +++ ++
-.1
N = 0
H2
N \ N
k ,=
N N\_
7.----
15 0-N + + + +
NH2 1111, II1H
N \
u , )4
N)
----
160-N + + ++ +
411 NH
NH2
A
N "==== \
N N
h
17 0-N
I + + + +
. N
NH2 H2
N \
It'- - NIN
No)
Q
18 o-N
1 + + + +
NH2 * NH2
N "==== ,N
k - =
N N
(1:1-0)
19 O-N ++ + + +
iii6 1
N
NH21111-F
N"-"N I
N N
2----
20 014 ++ ++ + ++
NH2 * N
N µN 0 01
N N
/\--
21 01"2 +++ + + + +
NH2* N
N .."- \
N
,
1 ,
N N,
2.----
-185-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
220lNH2 ++++ ++++ ++ +++ +++
++
AL N
NH2 W-
N \
k
N
- Isi
N 1...._....(...D
D D
D D D
23 olNH2 ++++ ++ + ++ ++
411L N
NH2 WV
N \
k , p
N " OH
2¨/
2401NH2 + + + +
IIlk N
NH2 W.
N \
k - Nil
N ..._/0..../
/ 10
25 0INH2 +++ ++ ++++ +++
AL N
NH2 NM
N \
krsj N'N
0
26 0INH2 ++++ +++ ++++ +++
jilk N
NH2 W.
N\
U )4
Thq N
OH
27 0lNH2 ++ + + +++
NH2 fe N
N \ N
k 14 /' OH
[00527] Table 1 shows the biological activity in mTOR and PI3K kinase assays
of several
compounds of the invention. The scale utilized in Table 1 is as follows:
++++less than
100nM; +++ less than 1.0 M; ++ less than 10 M; and + greater than 10 M.
[00528] In other embodiments, the present invention provides the following
compounds:
-186-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
0
HN"---
H2N HNP.
¨N ¨N
-1
e 0
NH2 . 6 NH2 NH2
NH2 * N
N \ N N ..", \ N --=== \
k - = k , , N k ,N N = 00
N I
N N kv N N\ N N\ N,
Nv
/----- 7.---- 7----- 7----
HN/
----Nµi oINH2
0 \ 1
NH2 * . NH . NH NH ik N
NH2 I NH2 1 2
N -", \ NN N \ N N \ N
k -= k - =
N Nv N Nµ N N\._ N l'i.._..../OH
O-N O-N
I I
. NH2 * NH2
NH2 NH2
0-NNH2
N \ N "==== \ 1 01
k ..., ,
N N NH2*
N
N N
NH2* N
0)
( \ 00
I N-
N QN--.\ N
, N 1 , ,
N
(---0) 7--- /-----
0---/NH2
---( NH2 1\ 0-----(NH2
0
1 = N II 0...
NH2 . * N
N NH2 ...( IINH2
NH2 iik N
N \
NH2
N \ kNNm N \N
N' N ' ---
N "\
7---- U
0 )----- ---m' \
N"
[00529] Any of the compounds shown above may show a biological activity in an
mTOR or
PI3K inhibition assay of between about 0.5nM and 25 [iM (IC50).
[00530] Additional compounds which are mTor inhibitors of the invention are
shown in
Table 2.
-187-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00531] Table 2. In vitro ICso values for Illustrative mTor Inhibitor
Compounds of the
Invention.
# mTORC
PI3K PI3K PI3K y PI3K 6 PC3
Structure ICso a P ic50 ICso prolif-
(nM) ICso IC50 (nM) (nM) eration
(nM) (nM) (nM)
HO
#
++++
NH2 \ NH + + ++ ++ +++
1
N \ N
k'
N N\
/-----
F
O*
2 'NANH \ NH + - - - - -
I
N \
k N
= -
N N\_
7-----
F
*
++ + _ - - _
3 NH2 \ NH
N\
k , ,N
N N\
7-----
NC
4 *+ + -
NH2 \ NH
N \N
k -=
N Nv
7-----
Me0
0+ +
NH2 \ NH
+
N \
kN
- =
N Nv
7.----
-188-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
# mTORC PI3K
PI3K PI3K y P131( 6 PC3
Structure IC50 a R IC50 IC50 prolif-
(nM) IC50 IC50 (nM) (nM) eration
(nM) (nM) (nM)
#
6 NH2 \ N
N \N + + +
'
N N\
7.----
t.
NH2 \ NH
7 +++ + +
N \N
N'
N N\
7.---
OMe
S
8 NH2 \ NH
+ + +
N
k N
N N\
C's
NH2 \ NH
9 ++++ + +
N \N
'
N N\
7-----
# OMe
NH2 \ NH
N --"-- \ +++++ + + + + +
k ,N
N N\
*OH
NH2 \ NH
11 ++++++ + + ++ ++ ++++
N \ N
k - = +
N N\
7---
-189-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
# mTORC PI3K
PI3K PI3K y PI3K 6 PC3
Structure IC50 a R IC50 IC50 prolif-
(nM) IC50 IC50 (nM) (nM) eration
(nM) (nM) (nM)
HO
4
12 NH2 NH ++++++ + + ++ + ++++
N \
,N
N N).......õ
c--/
Me0
0 CI
NH2 \NH
13 + + +
N '', \ N
kr( NI
h
Me0
* F
NH2 \ NH
14 + + -
N \
k , ,N
N N
h
HO
0 CI
NH2 \ NH
15N \
k ,N ++++++ + + ++++
++++ ++++
N N
h +
HO
#
16 F ++++++ +
+ ++ +++ ++
NH2 \ NH
+
N \
ki( N'N
h
CI
#
17 NH2 \ NH + + +
N \ N
'
N N
h
-190-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mTORC PI3K PI3K PI3K y PI3K 6 PC3
Structure ICso a ic50 ic50 prolif-
(nM) IC50 IC50 (nM) (nM) eration
(nM) (nM) (nM)
Me0
N
18 NH2 \ NH
N
klsr
Me0
/
19 NH2 \ NH
N N
- =
N N
NH2 \ NH
N=-====
N
OH
NH2 \ NH
21 N\ N ++++ ++ ++ ++
kisr
HO
CI
22NH2 \ NH ++++++ - ++
Nii N
\
N
0 NH2
23
NH2 \ NH
N
ii N
/¨
-191-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
# mTORC PI3K
PI3K PI3K y PI3K 6 PC3
Structure IC50 a R IC50 IC50 prolif-
(nM) IC50 IC50 (nM) (nM) eration
(nM) (nM) (nM)
CN
*
NH2 \ NH
24 + + +
N \
U ,N
N N
h
0
NH2
*
25 NH2 NH ++ + +
N \ii N
'
N Nv
f"---
HO
*
26 NH2 NH
++++++ + + ++ +++ ++
N .."- \
,N
N l'S__\
U
HO
*
27+++++ ++
NH2 \ NH
N \N
'
N N
b
HO
0
28 NH2 NH ++ + + - +
+
N .--- \
I ,rsi
-4(
0--/
--0
*
NH2 \ NH
29 +
N\N
ki,r NI
)-----
-192-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mTORC PI3K PI3K PI3K y PI3K 6 PC3
Structure ICso a ic50 Ic50 prolif-
(nM) IC50 1050 (nM) (nM) eration
(nM) (nM) (nM)
HO
30 NH2 NH +++++
N
ii N
\¨N1
0
HO
NH2 \ NH
31 N +++++ ++
kr%r N'N
HO
CI
32 NH2 NH ++
N
ii N
N N\
HO
NH2 \ NH
NH \ N
33 ++
cNj
0
HO
NH2 \ NH
34
N \ N
krsr N
Co)
-193-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
# mTORC PI3K
PI3K PI3K y PI3K 6 PC3
Structure IC50 a R IC50 IC50 prolif-
(nM) IC50 IC50 (nM) (nM) eration
(nM) (nM) (nM)
HO
0
35 NH2 NH + + _ + + _
N .."-- \ N
kN N'
HN
4111\ OH
NH2 \ NH
36++++++ + - +++ ++ +++
N .."=- \
k N
, =
N N)........,
ci
HO 41110
37 NH2 \ NH + ++ _ ++ ++ _
N \
k N
=
N N\
7.---
HO
*
38 NH2 \ NH ++ +
- + + +
N \ N
k , =
N N C
\
\
N
CI *OH
NH2 \ NH
39 ++++++ + -
+ + +
N \
k N
, =
N N\_
/-
-194-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
# mTORC PI3K
PI3K PI3K y PI3K 6 PC3
Structure IC50 a R IC50 IC50 prolif-
(nM) IC50 IC50 (nM) (nM) eration
(nM) (nM) (nM)
HO
di
40 +++ + _ + + +
NH2 \ NH
N \ N
N
k - =
!qv
-----\
*OH
NH2 \ NH
41 ++++++ + +
++++ + +
N \
k , N
N N' 1
U
N
0
HO
42 ++++++ + + -
+++ +
NH2 \ NH +
N \ N
k - =
N Ny._...1
S--... )
0
HO
43 .+ + + _ + _
NH2 \ NH
N \ N
kisr ,=
----N10
H
-195-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
# mTORC PI3K PI3K PI3K y PI3K 6 PC3
Structure IC50 a R IC50 IC50 prolif-
(nM) IC50 IC50 (nM) (nM) eration
(nM) (nM) (nM)
HO
=
44 NH2 \ NH
+++ + + ¨ + ¨
N \ N
'
N?
IN¨(
µ--N
HO
410
+
45 NH2 \ NH
N \N
I / N'
)-
46 HO
F$
_
NH2 \ NH
N ii \ N
fsr Niv._
/¨
47 HO
F
. _
NH2 NH
Nii \ N
Isr It
/¨
-196-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
# mTORC PI3K PI3K PI3K y PI3K 6 PC3
Structure IC50 a R IC50 IC50 prolif-
(nM) IC50 IC50 (nM) (nM) eration
(nM) (nM) (nM)
48 CI 0 ++++ + + + +
NH2 \ NH
N \
k,N
N N \
\-- )
0
49 HO ++++++ + + ++ ++
414
NH2 \ NH
N \
k , p
N r`i)
(N.--)
\--0
50 ++++ + + ++ ++
= CI
NH2 \ NH
N \ N
=
N N\._
7-----
51 ++++ + + ++ ++
= CI
NH2 \ NH
N\ N
k - =
N )......%
0
-197-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
# mTORC PI3K PI3K PI3K y PI3K 6 PC3
Structure IC50 a R IC50 IC50 prolif-
(nM) IC50 IC50 (nM) (nM) eration
(nM) (nM) (nM)
52 HO ++ + + + ++
#
NH2 \ NH
N \ N
k - =
N
(N---)
\---N
Me
53 HO +++ + + + _
411
NH2 \ NH
N \ N
k - =
N 14)
0
54 HO +++++ + + + -
41
NH2 NH
N \ N
kikr Isi).
OH
55 HO ++ + + + _
ilt
NH2 \ NH
N \ N
N y
le
)\
-198-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mTORC PI3K PI3K PI3K y PI3K 3 PC3
Structure IC50 a IC50 IC50 prolif-
(nM) IC50 IC50 (nM) (nM) eration
(nM) (nM) (nM)
56 HO
di CI
NH2 \ NH
N
ii N
N N
57 HO +++++
NH2 \ NH
N
ii ,N
N
0
58 HO
NH2 \ NH
Nii \ N
N
C
59
NH2 \ NH
N
u.N
N
-199-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
# mTORC PI3K PI3K PI3K y P131( 6 PC3
Structure IC50 a R IC50 IC50 prolif-
(nM) IC50 1050 (nM) (nM) eration
(nM) (nM) (nM)
60 OH +++ + + +++ -
4
NH2 \ NH
N "N
kN N=
)\
(:1
61 ci 4+++++ + + + +
/
o
NH2 \ NH
N "N
k ,
N )ThN
S--.. )
0
62 ++++++ 4 + + + +++ 111 OH +
NH2 \ NH
N "N
k - =
N N)Th
U0
63 ++++++ ++ + +++++ +++++
0 0/
+
NH2 \ NH
N "=-= "N
k - =
N 1`1).____I
U0
64 +++++ * OH + + ++ ++
NH2 \ NH
N "N
k - =
N )....%
UN
)----
-200-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
# mTORC PI3K PI3K PI3K y PI3K 6 PC3
Structure IC50 a R IC50 IC50 prolif-
(nM) IC50 IC50 (nM) (nM) eration
(nM) (nM) (nM)
65 OH ++++++ ++++ + +++++ +++++
0
NH2 \ NH
N '''=-= \
k µ/%1
N Nivm
UN
o"-----
66 + + + + +
$ 0/
NH2 \ NH
N \ N
k /kr N__
¨Th
.."N)
0"
67 o/ + + + + +
Sc'
NH2 \ NH
N \ N
ktsr N)Th.
U0
68 HO ++++++ ++ + ++++ +++++
0 +
CI
NH2 \ NH
N \
k N
, =
N N x
S--- )0
-20 1-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mTORC PI3K PI3K PI3K y PI3K 6 PC3
Structure IC50 a IC50 IC50 prolif-
(nM) IC50 IC50 (nM) (nM) eration
(nM) (nM) (nM)
69 ci * ++++++ ++
OH
NH2 \ NH
Nii \ N
O
)
70 HO ++++++ ++ +++ +++++
CI
NH2 \ NH
N \N
kisr
0
71 CI +++
OH
NH2 \ NH
N
ii N
N N
[00532] In Table 2 above, a +++++++ indicates an IC50 of 5 nM or less; a
++++++ indicates
an IC50 of 10 nM or less; a +++++ indicates an IC50 of 25nM or less; an ++++
indicates an
IC50 of 50nm or less, a +++ indicates an IC50 of 100nM or less, a ++ indicates
an IC50 of
500nM or less, and a + indicates an IC50 of more than 500nM.
-202-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Exemplary PI3Ka Inhibitor Compounds
[00533] In one aspect, the present invention provides a PI3Ka inhibitor which
is a
compound of Formula I:
R1'
/W
W3 wu
1 0 0
W2,.......... ........"...........
.......======.,,........õ............õwd
w1' W6
1 0> _______ R2'
wb'
Wd'
wa'
Formula II
or its pharmaceutically acceptable salts thereof, wherein:
W1' is N, NR3', or CR3'; W2' is N, NR4', CR4', or C=0; W3' is N, NR5' or CR5';
W4' is
N, wherein no more than two N atoms and no more than two C=0 groups are
adjacent;
W5' is N;
W6' is N or CR8';
Wa and Wb' are independently N or CR9';
one of Wc' and Wi' is N, and the other is 0, NR10', or S;
R1' and R2' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
R3' and R4' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
or R3' and R4' taken together form a cyclic moiety;
R5', R6' , R7' and R8' are independently hydrogen, alkyl, heteroalkyl,
alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
-203-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
R9' is alkyl or halo; and
R10' is hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl,
aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy, heterocycloalkyloxy,
amido, amino, acyl,
acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano, hydroxy, nitro, phosphate,
urea,
carbonate, or NR'R" wherein R' and R" are taken together with nitrogen to form
a cyclic
moiety.
[00534] For example, the present invention provides a P13 Ku inhibitor which
is a compound
of Formula I:
R1'
WO 0 W N:-:::( R2'
W`
W 1 W5
X
\ /
Formula II
or its pharmaceutically acceptable salts thereof, wherein:
Xis 0 or S or N;
W1' is N, NR3', CR3', or C=0, W2' is N, NR4', CR4', or C=0, W3' is N, NR5' or
CR5',
W4' is N, C=0 or CR6', wherein no more than two N atoms and no more than two
CO groups
are adjacent;
W5' is N or CR7';
W6' is N or CR8';
R1' and R2' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
R3' and R4' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
or R3' and R4' taken together form a cyclic moiety; and
-204-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
R5', R6' ,R7 and R8' are independently hydrogen, alkyl, heteroalkyl, alkenyl,
alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety.
[00535] In some embodiments, the compound of Formula II exists as a tautomer,
and such
tautomers are contemplated by the present invention.
[00536] In some embodiments, the compound of Formula II has the formula:
Ow
1, 5. 40
W W
X
[00537] For example, a compound of Formula II is:
6.
vY0 Ow
R2
0
[00538] In some embodiments of the compound of Formula II, Wi' is CR3', W2' is
CR4', W3'
is CR5', W4' is N, W5' is CR7', and W6' is CR8'; W1' is N, W2' is CR4', W3' is
CR5', W4' is N, W5'
is CR7', and W6 is CR8'; or W1' is CR3', W2' is N, W3' is CR5', W4' is N, W5'
is CR7', and W6' is
CR8'. Formulas for such embodiments are shown below:
R1' R1' R1'
N Rs'
2
0 0 ______________ R 0 0 N-
R2 1 0 0 __ R2
R4' N N
R3' R7' R3' R7'
R1' R1' R1'
N RN N R8'
2
R 2
0 0 0 0 _______________ NyR2 1 0 0 N R
I N
R3. R7. N.
[00539] In some embodiments, X is 0. In other embodiments, X is S.
[00540] In some embodiments, R1' is hydrogen. In other embodiments, R1' is
alkyl,
heteroalkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl,
heteroaryl,
heteroarylalkyl, alkoxy, heterocycloalkyloxy, amido, amino, acyl, acyloxy,
alkoxycarbonyl,
-205-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
sulfonamido, halo, cyano, hydroxy, nitro, phosphate, urea, carbonate, or
NR'R", wherein R'
and R" are taken together with nitrogen to form a cyclic moiety.
[00541] In some embodiments, R2' is hydrogen. In other embodiments, R2' is,
for example,
unsubstituted or substituted alkyl (including but not limited to CH3, -CH2CH3,
n-propyl,
isopropyl, n- butyl, ten'- butyl, sec-butyl, pentyl, hexyl, and heptyl). In
other embodiments,
R2' is unsubstituted or substituted alkenyl (including but not limited to
unsubstituted or
substituted C2-05alkenyl such as, for example, vinyl, allyl, 1-methyl propen-l-
yl, butenyl, or
pentenyl) or unsubstituted or substituted alkynyl (including but not limited
to unsubstituted or
substituted C2-05alkynyl such as acetylenyl, propargyl, butynyl, or pentynyl).
Alternatively,
R2' is unsubstituted or substituted aryl (including but not limited to
monocyclic or bicyclic
aryl) or unsubstituted or substituted arylalkyl (including but not limited to
monocyclic or
bicyclic aryl linked to alkyl wherein alkyl includes but is not limited to
CH3, -CH2CH3, n-
propyl, isopropyl, n- butyl, sec-butyl, and pentyl). In some other
embodiments, R2' is
unsubstituted or substituted heteroaryl, including but not limited to
monocyclic and bicyclic
heteroaryl. Monocyclic heteroaryl R2' includes but is not limited to pyrrolyl,
thienyl, furyl,
pyridinyl, pyranyl, pyrimidinyl, pyrazinyl, pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and
oxazolyl. Bicyclic heteroaryl R2' includes but is not limited to
benzothiophenyl, benzofuryl,
indolyl, quinolinyl, isoquinolinyl, benzimidazolyl, benzoxazolyl,
benzothiazolyl,
quinazolinyl, azaindolyl, pyrazolopyrimidinyl, purinyl, pyrrolo [1, 2-
b]pyridazinyl,
pyrrolopyrimidinyl, indazolyl, pyrazolylpyridinyl, imidazo[1, 2-a]pyridinyl,
and pyrrolo[1, 2-
f] [l, 2, 4]triazinyl. The present invention also provides compounds wherein
R2' is
unsubstituted or substituted heteroarylalkyl, including but not limited to
monocyclic and
bicyclic heteroaryl as described above, that are linked to alkyl, which in
turn includes but is
not limited to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, sec-butyl, and
pentyl. In some
embodiments, R2' is unsubstituted or substituted cycloalkyl (including but not
limited to
cyclopropyl, cyclobutyl, and cyclopentyl) or unsubstituted or substituted
heteroalkyl (non-
limiting examples include ethoxymethyl, methoxymethyl, and
diethylaminomethyl). In some
further embodiments, R2' is unsubstituted or substituted heterocycloalkyl
which includes but
is not limited to pyrrolidinyl, tetrahydrofuranyl, piperidinyl,
tetrahydropyranyl, thiazolidinyl,
imidazolidinyl, morpholinyl, and piperazinyl. In yet other embodiments of the
compounds of
Formula II, R2' is unsubstituted or substituted alkoxy including but not
limited to Ci-C4alkoxy
such as methoxy, ethoxy, propoxy or butoxy. R2' can also be unsubstituted or
substituted
heterocycloalkyloxy, including but not limited to 4-NH piperidin-l-yl-oxy, 4-
methyl
piperidin-l-yl-oxy, 4-ethyl piperidin-l-yl-oxy, 4-isopropyl- piperidin-l-yl-
oxy, and
-206-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
pyrrolidin-3-yl-oxy. In other embodiments, R2' is unsubstituted or substituted
amino, wherein
the substituted amino includes but is not limited to dimethylamino,
diethylamino, di-
isopropyl amino, N-methyl N-ethyl amino, and dibutylamino. In some
embodiments, R2' is
unsubstituted or substituted acyl, unsubstituted or substituted acyloxy,
unsubstituted or
substituted Ci-C4acyloxy, unsubstituted or substituted alkoxycarbonyl,
unsubstituted or
substituted amido, or unsubstituted or substituted sulfonamido. In other
embodiments, R2' is
halo, which is ¨I, -F, -Cl, or -Br. In some embodiments, R2' is selected from
the group
consisting of cyano, hydroxy, nitro, phosphate, urea, and carbonate. Also
contemplated are
R2' being -CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, ten'- butyl, sec-
butyl, pentyl, hexyl,
heptyl, -OCH3, -OCH2CH3, or -CF3.
[00542] In some embodiments of the compound of Formula II, Wi' is CR3. R3' can
be, for
example, hydrogen, unsubstituted or substituted alkyl (including but not
limited to CH3, -
CH2CH3, n-propyl, isopropyl, n- butyl, tert- butyl, sec-butyl, pentyl, hexyl,
and heptyl). In
other embodiments, R3' is unsubstituted or substituted alkenyl (including but
not limited to
unsubstituted or substituted C2-05alkenyl such as, for example, vinyl, allyl,
1-methyl propen-
l-yl, butenyl, or pentenyl) or unsubstituted or substituted alkynyl (including
but not limited to
unsubstituted or substituted C2-05alkynyl such as acetylenyl, propargyl,
butynyl, or
pentynyl). Alternatively, R3' is unsubstituted or substituted aryl (including
but not limited to
monocyclic or bicyclic aryl) or unsubstituted or substituted arylalkyl
(including but not
limited to monocyclic or bicyclic aryl linked to alkyl wherein alkyl includes
but is not limited
to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, sec-butyl, and pentyl). In
some other
embodiments, R3' is unsubstituted or substituted heteroaryl, including but not
limited to
monocyclic and bicyclic heteroaryl. Monocyclic heteroaryl R3' includes but is
not limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazolyl. Bicyclic heteroaryl R3' includes but is
not limited to
benzothiophenyl, benzofuryl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl, pyrazolopyrimidinyl,
purinyl, pyrrolo
[1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl, pyrazolylpyridinyl,
imidazo[1, 2-
a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl. The present invention also
provides
compounds of Formula II wherein R3' is unsubstituted or substituted
heteroarylalkyl,
including but not limited to monocyclic and bicyclic heteroaryl as described
above, that are
linked to alkyl, which in turn includes but is not limited to CH3, -CH2CH3, n-
propyl,
isopropyl, n- butyl, sec-butyl, and pentyl. In some embodiments, R3' is
unsubstituted or
substituted cycloalkyl (including but not limited to cyclopropyl, cyclobutyl,
and cyclopentyl)
-207-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
or unsubstituted or substituted heteroalkyl (non-limiting examples include
ethoxymethyl,
methoxymethyl, and diethylaminomethyl). In some further embodiments, R3' is
unsubstituted
or substituted heterocycloalkyl which includes but is not limited to
pyrrolidinyl,
tetrahydrofuranyl, piperidinyl, tetrahydropyranyl, thiazolidinyl,
imidazolidinyl, morpholinyl,
and piperazinyl. In yet other embodiments of the compounds of Formula II, R3'
is
unsubstituted or substituted alkoxy including but not limited to Ci-C4alkoxy
such as
methoxy, ethoxy, propoxy or butoxy. R3' can also be unsubstituted or
substituted
heterocycloalkyloxy, including but not limited to 4-NH piperidin-l-yl-oxy, 4-
methyl
piperidin-l-yl-oxy, 4-ethyl piperidin-l-yl-oxy, 4-isopropyl- piperidin-l-yl-
oxy, and
pyrrolidin-3-yl-oxy. In other embodiments, R3' is unsubstituted or substituted
amino, wherein
the substituted amino includes but is not limited to dimethylamino,
diethylamino, di-
isopropyl amino, N-methyl N-ethyl amino, and dibutylamino. In some
embodiments, R3' is
unsubstituted or substituted acyl, unsubstituted or substituted acyloxy,
unsubstituted or
substituted Ci-C4acyloxy, unsubstituted or substituted alkoxycarbonyl,
unsubstituted or
substituted amido, or unsubstituted or substituted sulfonamido. In other
embodiments, R3' is
halo, which is ¨I, -F, -Cl, or -Br. In some embodiments, R3' is selected from
the group
consisting of cyano, hydroxy, nitro, phosphate, urea, and carbonate. Also
contemplated are
R3' being -CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, ten'- butyl, sec-
butyl, pentyl, hexyl,
heptyl, -OCH3, -OCH2CH3, or -CF3.
[00543] R3' of the compounds of Formula II can also be NR'R" wherein R' and R"
are taken
together with the nitrogen to form a cyclic moiety having from 3 to 8 ring
atoms. The cyclic
moiety so formed may further include one or more heteroatoms which are
selected from the
group consisting of S, 0, and N. The cyclic moiety so formed is unsubstituted
or substituted,
including but not limited to morpholinyl, azetidinyl, pyrrolidinyl,
piperidinyl, piperazinyl,
isothiazolidinyl 1,2, dioxide, and thiomorpholinyl. Further non-limiting
exemplary cyclic
moieites are the following:
H3
"-NO -1-N( __ )0 A-N,)
g ,
0 0
0 0-el -CH.1
1-:/\34/ )(1\0 N.N,)
0 ,
-208-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00544] The invention also provides compounds of Formula II, wherein when R3'
is a
member of the group consisting of alkyl, alkenyl, alkynyl, cycloalkyl,
heteroalkyl,
heterocycloalkyl, heterocycloalkyloxy, aryl, arylalkyl, heteroaryl,
heteroarylalkyl, acyl,
alkoxy, amido, amino, sulfonamido, acyloxy, alkoxycarbonyl, and NR'R" (wherein
R' and R"
are taken together with nitrogen to form a cyclic moiety), then R3' is
optionally substituted
with one or more of the following substituents: alkyl, alkenyl, alkynyl,
cycloalkyl,
heteroalkyl, heterocycloalkyl, heterocycloalkyloxy, aryl, arylalkyl,
heteroaryl,
heteroarylalkyl, acyl, heterocycloalkyloxy, alkoxy, amido, amino, sulfonamido,
acyloxy,
alkoxycarbonyl, halo, cyano, hydroxy, nitro, phosphate, urea, carbonate, or
NR'R" wherein R'
and R" are taken together with nitrogen to form a cyclic moiety. Each of the
above
substituents may be further substituted with one or more substituents chosen
from the group
consisting of alkyl, alkoxy, amido, amino, sulfonamido, acyloxy,
alkoxycarbonyl, halo,
cyano, hydroxy, nitro, oxo, phosphate, urea, and carbonate.
[00545] For example, the invention provides compounds wherein when R3' is
alkyl, the alkyl
is substituted with NR'R" wherein R' and R" are taken together with the
nitrogen to form a
cyclic moiety. The cyclic moiety so formed can be unsubstituted or
substituted. Non-limiting
exemplary cyclic moieties includes but are not limited to morpholinyl,
azetidinyl,
pyrrolidinyl, piperidinyl, piperazinyl, and thiomorpholinyl. In other examples
of the
compounds of Formula II, when R3' is alkyl, the alkyl is substituted with
heterocycloalkyl,
which includes oxetanyl, azetidinyl, tetrahydrofuranyl, pyrrolyl,
tetrahydropyranyl,
piperidinyl, morpholinyl, and piperazinyl. All of the above listed
heterocycloaklyl
substituents can be unsubstituted or substituted.
[00546] In yet other examples of the compounds of Formula II, when R3' is
alkyl, the alkyl is
substituted with a 5, 6, 7, 8, 9, or 10 membered monocyclic or bicyclic
heteroaryl, which is
unsubstituted or substituted. The monocyclic heteroaryl includes but is not
limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazolyl. The bicyclic heteroaryl includes but is
not limited to
benzothiophenyl, benzofuryl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl, pyrazolopyrimidinyl,
purinyl, pyrrolo
[1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl, pyrazolylpyridinyl,
imidazo[1, 2-
a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl.
[00547] In other embodiments of the compound of Formula II, R3' is ¨NHR3u, -
N(CH3)R3u, -
N(CH2CH3)R3u, -N(CH(CH3)2)R3u, or ¨0R3, wherein R3" is unsubstituted or
substituted
heterocycloalkyl (nonlimiting examples thereof include 4-NH piperidin-l-yl, 4-
methyl
-209-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
piperidin-l-yl, 4-ethyl piperidin-l-yl, 4-isopropyl- piperidin-l-yl, and
pyrrolidin-3-y1),
unsubstituted or substituted monocyclic aryl, or unsubstituted or substituted
monocyclic
heteroaryl (including but not limited to pyrrolyl, thienyl, furyl, pyridinyl,
pyranyl,
pyrimidinyl, pyrazinyl, pyridazinyl, imidazolyl, thiazolyl, pyrazolyl, and
oxazolyl). In one
example, R3' is ¨0-aryl, i.e. phenoxy. In another example, R3' is ¨0-(4-
methyl)piperidin-1-y1
or ¨0-(4-isopropyl)piperidin-1-yl.
[00548] In some embodiments of the compound of Formula II, R3' is one of the
following
moieties:
CH3
..------N¨cH3 I .NH
/N-CH
\
0 10 0---\) o--) CH 3 0--)
I I 1 , - I -
vv
i i
5 I 5 5 5
/
CH3
,.........",õNõ,...CH3
el +N
)>. 1-N/CH3
kN
vLnA,
5 / 5 -CH(CH3)2 5 H 5
CH3 5 5
CH3
I
--N' CH3 0 H3C õ...,..õ....,,r.---,........õ , H3C 0
\
_\-
, 1 0 -N,
/----CH3 Lz,f HN--",:-. --
CH---- 0
,, N ),-------,,,,..õ,õ.N.,õ..õ,õ..õ.
1,17/,.
H3C 5 i 0
5 'µ2. 5 '2' 5 5
CICN
40 el
_______________________________________________________ H3C
NcH' H C
3 \
0 N A¨ / \ NO \\N ____ ( __ >
>.--",õ,_,,,=N..õ,õ,õ--- / \ __ /
N
. Me0 0
N
le I /N ,S 'N)1------ H C\
3 10I
/.1\1
0 o 101 1 (:)) N
I , `,.1.--"N 1 1
wv
I 5 5 5 5 5
(s
...\--N,)
cH2cH3
N (N\/
,
,.....,. õ......,CN 0
)----
01 iN N CN N
õ.õ--,.. ..-------z
/\)
c'z''?
5
5 5 5 5
-210-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
0
_ ,OH SO2Me
N.------,- N
SO2Me
N
CI) O 0.
1 I
5 5
_\--N
_ ,OH
õõ-----., N ..- ----,
I Sµ
I /2-N
5)Z'c
I
0 NI'
/ ---\ 't=tt.7-- N ?
5 Or
5 5 5 5
, S N
I -N1_ j
N
=
0 N-Ac /'N-S02Me
/N--SO2Me 0
0
VVI/ JUN"
I I
5 5 -I. 5 -2' 5 -I' 5
0 ..,....... / __ 0
\ __
N,CH 3 N,Ac
N....*CF13
N
ri r-1N r-7 r¨jN
_..c) ',;__.0 µ,; --= 0
N,S02Me ,Ac
N,S02Me
---"'N--CH3
N
1---/ N _________ N
H N ---\)
--c)
vt?a= 'Iv'
5 5 5 5
ra -== CH 3
ral --Ac
0 N --Ac
H11---\) I-111--\) `...-NH !2.24-- NH
µfltrt 5 VIµ 5 2 5 .
rao ro
rN_cH3
(N-AC
rN)
NH HN H N HN ____ j-- N J-N
HN_IN
(.2227----1
/
i
5 v"(-, /
4/1,14 vIA,
5 5 5
0 \ p H I H 0 I 10I I/1,
\,S--..1
"Zz.. S -5, , Sµ '2?.. S , V N '/S \ µt2(
X 0
0 -N i, s> ,/ - // \-
0 05 0 05 0 5 0/µ 0
5 \ > 5 5 5
-211-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
I r:sf CN g
µ) 101
0, /N ¨\ _______________________________________ N /--\ 40 CN
0
Cr __,-., \
u CH3 i? 0/ \CH 3
5 5 5 .
[00549] In some embodiments of the compound of Formula II, Wr is NR3'5 wherein
R3' is
hydrogen, unsubstituted or substituted Ci-Cioalkyl (which includes but is not
limited to -CH3,
-CH2CH3, n-propyl, isopropyl, n- butyl, tert- butyl, sec-butyl, pentyl, hexyl,
and heptyl), or
unsubstituted or substituted C3-C7cycloalkyl (which includes but is not
limited to
cyclopropyl, cyclobutyl, cyclopentyl, and cyclohexyl). In other embodiments of
the
compound of Formula II, R3' is unsubstituted or substituted heterocycloalkyl
(which includes
but is not limited to oxetanyl, tetrahydrofuranyl, pyrrolidinyl,
tetrahydropyranyl, piperidinyl,
and piperazinyl), or unsubstituted or substituted C2-Cioheteroalkyl (which
includes but is not
limited to methoxyethoxy, methoxymethyl, and diethylaminoethyl).
Alternatively, R3' is
unsubstituted or substituted monocyclic heteroaryl (which includes but is not
limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazoly1) or unsubstituted or substituted monocyclic
aryl.
[00550] In still other embodiments, Wr is C=0.
[00551] In some embodiments of the compound of Formula II, W2' is CR4'. R4'
can be, for
example, hydrogen, or unsubstituted or substituted alkyl (including but not
limited to CH3, -
CH2CH3, n-propyl, isopropyl, n- butyl, tert- butyl, sec-butyl, pentyl, hexyl,
and heptyl). In
other embodiments, R4' is unsubstituted or substituted alkenyl (including but
not limited to
unsubstituted or substituted C2-05alkenyl such as, for example, vinyl, allyl,
1-methyl propen-
1-yl, butenyl, or pentenyl) or unsubstituted or substituted alkynyl (including
but not limited to
unsubstituted or substituted C2-05alkynyl such as acetylenyl, propargyl,
butynyl, or
pentynyl). Alternatively, R4' is unsubstituted or substituted aryl (including
but not limited to
monocyclic or bicyclic aryl) or unsubstituted or substituted arylalkyl
(including but not
limited to monocyclic or bicyclic aryl linked to alkyl wherein alkyl includes
but is not limited
to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, sec-butyl, and pentyl). In
some other
embodiments, R4' is unsubstituted or substituted heteroaryl, including but not
limited to
monocyclic and bicyclic heteroaryl. Monocyclic heteroaryl R4' includes but is
not limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazolyl. Bicyclic heteroaryl R4' includes but is
not limited to
benzothiophenyl, benzofuryl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl, pyrazolopyrimidinyl,
purinyl, pyrrolo
-212-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl, pyrazolylpyridinyl,
imidazo[1, 2-
a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl.
[00552] The present invention also provides compounds of Formula II wherein
R4' is
unsubstituted or substituted heteroarylalkyl, including but not limited to
monocyclic and
bicyclic heteroaryl as described above, that are linked to alkyl, which in
turn includes but is
not limited to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, sec-butyl, and
pentyl. In some
embodiments, R4' is unsubstituted or substituted cycloalkyl (including but not
limited to
cyclopropyl, cyclobutyl, and cyclopentyl) or unsubstituted or substituted
heteroalkyl (non-
limiting examples include ethoxymethyl, methoxymethyl, and
diethylaminomethyl). In some
further embodiments, R4' is unsubstituted or substituted heterocycloalkyl
which includes but
is not limited to pyrrolidinyl, tetrahydrofuranyl, piperidinyl,
tetrahydropyranyl, thiazolidinyl,
imidazolidinyl, morpholinyl, and piperazinyl. In yet other embodiments of the
compounds of
Formula II, R4' is unsubstituted or substituted alkoxy including but not
limited to Ci-C4alkoxy
such as methoxy, ethoxy, propoxy or butoxy. R4' can also be unsubstituted or
substituted
heterocycloalkyloxy, including but not limited to 4-NH piperidin-l-yl-oxy, 4-
methyl
piperidin-l-yl-oxy, 4-ethyl piperidin-l-yl-oxy, 4-isopropyl- piperidin-l-yl-
oxy, and
pyrrolidin-3-yl-oxy. In other embodiments, R4' is unsubstituted or substituted
amino, wherein
the substituted amino includes but is not limited to dimethylamino,
diethylamino, di-
isopropyl amino, N-methyl N-ethyl amino, and dibutylamino. In some
embodiments, R4' is
unsubstituted or substituted acyl, unsubstituted or substituted acyloxy,
unsubstituted or
substituted Ci-C4acyloxy, unsubstituted or substituted alkoxycarbonyl,
unsubstituted or
substituted amido, or unsubstituted or substituted sulfonamido. In some
embodiments, R4' is
halo, which is ¨I, -F, -Cl, or -Br. In some embodiments, R4' is selected from
the group
consisting of cyano, hydroxy, nitro, phosphate, urea, or carbonate. Also
contemplated are R4'
being -CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, ten'- butyl, sec-butyl,
pentyl, hexyl,
heptyl, -OCH3, -OCH2CH3, or -CF3.
[00553] R4' of the compounds of Formula II, can also be NR'R" wherein R' and
R" are taken
together with the nitrogen to form a cyclic moiety having from 3 to 8 ring
atoms. The cyclic
moiety so formed may further include one or more heteroatoms which are
selected from the
group consisting of S, 0, and N. The cyclic moiety so formed is unsubstituted
or substituted,
including but not limited to morpholinyl, azetidinyl, pyrrolidinyl,
piperidinyl, piperazinyl,
isothiazolidinyl 1,2, dioxide, and thiomorpholinyl. Further non-limiting
exemplary cyclic
moieties are the following :
-213-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
CH3
/
"¨NO 0
0 0
0, 0 rw ki\
¨CH3
A¨Nlr N
;0)
0
[00554] The invention also provides compounds of Formula II, wherein when R4'
is a
member of the group consisting of alkyl, alkenyl, alkynyl, cycloalkyl,
heteroalkyl,
heterocycloalkyl, heterocycloalkyloxy, aryl, arylalkyl, heteroaryl,
heteroarylalkyl, acyl,
alkoxy, amido, amino, sulfonamido, acyloxy, alkoxycarbonyl, and NR'R" (wherein
R' and R"
are taken together with nitrogen to form a cyclic moiety), then R4' is
optionally substituted
with one or more of the following substituents: alkyl, alkenyl, alkynyl,
cycloalkyl,
heteroalkyl, heterocycloalkyl, heterocycloalkyloxy, aryl, arylalkyl,
heteroaryl,
heteroarylalkyl, acyl, alkoxy, amido, amino, sulfonamido, acyloxy,
alkoxycarbonyl, halo,
cyano, hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R"
are taken
together with nitrogen to form a cyclic moiety. Each of the above substituents
may be further
substituted with one or more substituents chosen from the group consisting of
alkyl, alkoxy,
amido, amino, sulfonamido, acyloxy, alkoxycarbonyl, halo, cyano, hydroxy,
nitro, oxo,
phosphate, urea, and carbonate.
[00555] For example, the invention provides compounds wherein when R4' is
alkyl, the alkyl
is substituted with NR'R" wherein R' and R" are taken together with the
nitrogen to form a
cyclic moiety. The cyclic moiety so formed can be unsubstituted or
substituted. Non-limiting
exemplary cyclic moieties includes but are not limited to morpholinyl,
azetidinyl,
pyrrolidinyl, piperidinyl, piperazinyl, isothiazolidinyl 1,2, dioxide, and
thiomorpholinyl. In
other examples of the compounds of Formula II, when R4' is alkyl, the alkyl is
substituted
with heterocycloalkyl, which includes oxetanyl, azetidinyl, tetrahydrofuranyl,
pyrrolyl,
tetrahydropyranyl, piperidinyl, morpholinyl, and piperazinyl. All of the above
listed
heterocycloaklyl substituents can be unsubstituted or substituted.
[00556] In yet other examples of the compounds of Formula II, when R4' is
alkyl, the alkyl is
substituted with a 5, 6, 7, 8, 9, or 10 membered monocyclic or bicyclic
heteroaryl, which is
unsubstituted or substituted. The monocyclic heteroaryl includes but is not
limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazolyl. The bicyclic heteroaryl includes but is
not limited
-214-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
benzothiophenyl, benzofuryl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl, pyrazolopyrimidinyl,
purinyl, pyrrolo
[1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl, pyrazolylpyridinyl,
imidazo[1, 2-
a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl.
[00557] In some embodiments of the compound of Formula II, W2' is NR4',
wherein R4' is
hydrogen, unsubstituted or substituted Ci-Cioalkyl (which includes but is not
limited to -CH3,
-CH2CH3, n-propyl, isopropyl, n- butyl, tert- butyl, sec-butyl, pentyl, hexyl,
and heptyl), or
unsubstituted or substituted C3-C7cycloalkyl (which includes but is not
limited to
cyclopropyl, cyclobutyl, cyclopentyl, and cyclohexyl). In other embodiments of
the
compound of Formula II, R4' is unsubstituted or substituted heterocycloalkyl
(which includes
but is not limited to oxetanyl, tetrahydrofuranyl, pyrrolidinyl,
tetrahydropyranyl, piperidinyl,
and piperazinyl), or unsubstituted or substituted C2-Cioheteroalkyl (which
includes but is not
limited to methoxyethoxy, methoxymethyl, and diethylaminoethyl).
Alternatively, R4' is
unsubstituted or substituted monocyclic heteroaryl (which includes but is not
limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazoly1) or unsubstituted or substituted monocyclic
aryl.
[00558] In some embodiments R3' and R4' taken together form a cyclic moiety.
Such a
moiety may have, for example, from 3 to 8 ring atoms. The cyclic moiety so
formed may
further include one or more heteroatoms which are selected from the group
consisting of S,
0, and N. The cyclic moiety so formed is unsubstituted or substituted. In some
embodiments,
the substituent is Ci-Cioalkyl (which includes but is not limited to -CH3, -
CH2CH3, n-propyl,
isopropyl, n- butyl, ten'- butyl, sec-butyl, pentyl, hexyl, and heptyl), or C3-
C7cycloalkyl
(which includes but is not limited to cyclopropyl, cyclobutyl, cyclopentyl,
and cyclohexyl);
heterocycloalkyl (which includes but is not limited to oxetanyl,
tetrahydrofuranyl,
pyrrolidinyl, tetrahydropyranyl, piperidinyl, and piperazinyl), C2-
Cioheteroalkyl (which
includes but is not limited to methoxyethoxy, methoxymethyl, and
diethylaminoethyl);
monocyclic heteroaryl (which includes but is not limited to pyrrolyl, thienyl,
furyl, pyridinyl,
pyranyl, pyrimidinyl, pyrazinyl, pyridazinyl, imidazolyl, thiazolyl,
pyrazolyl, and oxazoly1)
or unsubstituted or substituted monocyclic aryl. The cyclic moiety may have
one or more
substituents, which may be the same or different.
[00559] In some embodiments, the cyclic moiety formed by R3' and R4' is
substituted with at
least one of the following substituents:
-215-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
CH3
..."---N.-CH3 I
/N-CH .NH
\
0 10 0.---) 0-----) 1¨NO p ________ <
1 1 O , ' 1 '1' W J1J1J I
, ,
OW
I , I , 1 71-1,
,
CH3 CH3
I\ICH3
1401 +N/ /
)>. 1¨N\
kN
-0-1(0-13)2 , H , CH3
, ,
CH3
I
CH-
i/ CH3
¨N 0 H C
3 ,..,....,.._õ,;; H3C 0
\
, 1 0
/CH---_cH3 µf A N,
H N - - - - .N - -" -. , z z N 1 1 . ,(:)
H
0
3C
CI 0 CN
VcH' H C I. I-13C\ __ ( \
3 \ / \ N 0
0 N A¨N ______________
>..-N..,,,_____,
________________________________________________ /
, , , ,
0
'N Me0 )1--
I N ,S - N H3C\ 01 1\l/N
0
, I (:) ) N
1 o I.
,
"-- N 1 1
1 ,
, , , ,
('S
A-N,)
cH2cH3
N r, 0
el ,, --N-----/CN
CN
N-,/ /\.) ,.....--.N.---,/
A cj -µr
, , , , ,
0
õ
,...--.N.-11--.. .õ..---.N OH N
so2Me
N
C)) o
C Y-)
1 \(\/\/
I
-216-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
_ ,OH
õõ---,,N,..----.
I N
5-V 0----N
...
/--L
_\- N N 2 I
`7/,,,---N
, Or
5 5 5
--S /N
I/)¨NJ
N
=
0 /N-Ac
N-S02Me
/r\j.--S02Me 0
0.---) 0"--) µ-cr)
1 1
..rv,.. y (?2:-0
5 I
1 5 4" 5 -1"
0 ...........'''' N .---C H3 / o
\ __
N,CH3 N,Ac
N
r---I
rp ry rp
5 e-- 5 2 5 2 5 2
N,S02Me .Ac
N,S02Me
N
rj ri N
HN---\)
_-o
1 r-
5 5 5 5
rerAc
ra --CH3
0 /N-Ac
HN--\) 1-111--\) (.224--NH !?.a.--NH
rap (-or N_cH3 (N-AC
NH HN --
r-N)
/ µ
..._ IN r-N J-N ,----1
/ HN
i HN--j HN
/
kn.4,1
5 5 5 5
0µ 0
\4/ H I H
µ 0 -1\1/\
'
60 0cro µVP
5 5 , , , , o la
5
1 (3.f. CN g
N N
0. / ¨\_
. ¨\_ /--\ CN
µa. X 401 A OH
17 101 > N 0
0 `'? 'L 1401
0 CH3 0/ \CH3 \--/
5 5 5 5 .
[00560] In some embodiments of the compound of Formula II, W3' is CR5'. R5'
can be, for
example, hydrogen, or unsubstituted or substituted alkyl (including but not
limited to CH3, -
CH2CH3, n-propyl, isopropyl, n- butyl, tert- butyl, sec-butyl, pentyl, hexyl,
and heptyl). In
-217-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
one embodiment, R5' is H. In other embodiments, R5' is unsubstituted or
substituted alkenyl
(including but not limited to unsubstituted or substituted C2-05alkenyl such
as, for example,
vinyl, allyl, 1-methyl propen-l-yl, butenyl, or pentenyl) or unsubstituted or
substituted
alkynyl (including but not limited to unsubstituted or substituted C2-
05alkynyl such as
acetylenyl, propargyl, butynyl, or pentynyl). Alternatively, R5' is
unsubstituted or substituted
aryl (including but not limited to monocyclic or bicyclic aryl) or
unsubstituted or substituted
arylalkyl (including but not limited to monocyclic or bicyclic aryl linked to
alkyl wherein
alkyl includes but is not limited to CH3, -CH2CH3, n-propyl, isopropyl, n-
butyl, sec-butyl,
and pentyl). In some other embodiments, R5' is unsubstituted or substituted
heteroaryl,
including but not limited to monocyclic and bicyclic heteroaryl. Monocyclic
heteroaryl R5'
includes but is not limited to pyrrolyl, thienyl, furyl, pyridinyl, pyranyl,
pyrimidinyl,
pyrazinyl, pyridazinyl, imidazolyl, thiazolyl, pyrazolyl, and oxazolyl.
Bicyclic heteroaryl R5'
includes but is not limited to benzothiophenyl, benzofuryl, indolyl,
quinolinyl, isoquinolinyl,
benzimidazolyl, benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl,
pyrazolopyrimidinyl,
purinyl, pyrrolo [1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl,
pyrazolylpyridinyl,
imidazo[1, 2-a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl.
[00561] In some embodiments of the compound of Formula II, W3' is N or NR5',
wherein R5'
is hydrogen, unsubstituted or substituted Ci-Cioalkyl (which includes but is
not limited to -
CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, tert- butyl, sec-butyl, pentyl,
hexyl, and heptyl),
or unsubstituted or substituted C3-C7cycloalkyl (which includes but is not
limited to
cyclopropyl, cyclobutyl, cyclopentyl, and cyclohexyl). In other embodiments of
the
compound of Formula II, R5' is unsubstituted or substituted heterocycloalkyl
(which includes
but is not limited to oxetanyl, tetrahydrofuranyl, pyrrolidinyl,
tetrahydropyranyl, piperidinyl,
and piperazinyl), or unsubstituted or substituted C2-Cioheteroalkyl (which
includes but is not
limited to methoxyethoxy, methoxymethyl, and diethylaminoethyl).
Alternatively, R5' is
unsubstituted or substituted monocyclic heteroaryl (which includes but is not
limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazoly1) or unsubstituted or substituted monocyclic
aryl.
[00562] In some embodiments of the compound of Formula II, W4' is CR6. R6 can
be, for
example, hydrogen, or unsubstituted or substituted alkyl (including but not
limited to CH3, -
CH2CH3, n-propyl, isopropyl, n- butyl, tert- butyl, sec-butyl, pentyl, hexyl,
and heptyl). In
one embodiment, R6 is H. In other embodiments, R6 is unsubstituted or
substituted alkenyl
(including but not limited to unsubstituted or substituted C2-05alkenyl such
as, for example,
vinyl, allyl, 1-methyl propen-l-yl, butenyl, or pentenyl) or unsubstituted or
substituted
-218-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
alkynyl (including but not limited to unsubstituted or substituted C2-
05alkynyl such as
acetylenyl, propargyl, butynyl, or pentynyl). Alternatively, R6 is
unsubstituted or substituted
aryl (including but not limited to monocyclic or bicyclic aryl) or
unsubstituted or substituted
arylalkyl (including but not limited to monocyclic or bicyclic aryl linked to
alkyl wherein
alkyl includes but is not limited to CH3, -CH2CH3, n-propyl, isopropyl, n-
butyl, sec-butyl,
and pentyl). In some other embodiments, R6 is unsubstituted or substituted
heteroaryl,
including but not limited to monocyclic and bicyclic heteroaryl. Monocyclic
heteroaryl R6
includes but is not limited to pyrrolyl, thienyl, furyl, pyridinyl, pyranyl,
pyrimidinyl,
pyrazinyl, pyridazinyl, imidazolyl, thiazolyl, pyrazolyl, and oxazolyl.
Bicyclic heteroaryl R6
includes but is not limited to benzothiophenyl, benzofuryl, indolyl,
quinolinyl, isoquinolinyl,
benzimidazolyl, benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl,
pyrazolopyrimidinyl,
purinyl, pyrrolo [1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl,
pyrazolylpyridinyl,
imidazo[1, 2-a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl.
[00563] In some embodiments of the compound of Formula II, W4' is N or NR6,
wherein R6
is hydrogen, unsubstituted or substituted Ci-Cioalkyl (which includes but is
not limited to -
CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, tert- butyl, sec-butyl, pentyl,
hexyl, and heptyl),
or unsubstituted or substituted C3-C7cycloalkyl (which includes but is not
limited to
cyclopropyl, cyclobutyl, cyclopentyl, and cyclohexyl). In other embodiments of
the
compound of Formula II, R6 is unsubstituted or substituted heterocycloalkyl
(which includes
but is not limited to oxetanyl, tetrahydrofuranyl, pyrrolidinyl,
tetrahydropyranyl, piperidinyl,
and piperazinyl), or unsubstituted or substituted C2-Cioheteroalkyl (which
includes but is not
limited to methoxyethoxy, methoxymethyl, and diethylaminoethyl).
Alternatively, R6 is
unsubstituted or substituted monocyclic heteroaryl (which includes but is not
limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazoly1) or unsubstituted or substituted monocyclic
aryl.
[00564] In other embodiments, W4' is C=0.
[00565] In some embodiments of the compound of Formula II, W5' is N. In other
embodiments of the compound of Formula II, W5' is CR7. R7 can be, for example,
hydrogen,
or unsubstituted or substituted alkyl (including but not limited to CH3, -
CH2CH3, n-propyl,
isopropyl, n- butyl, ten'- butyl, sec-butyl, pentyl, hexyl, and heptyl). In
one embodiment, R7
is H. In other embodiments, R7 is unsubstituted or substituted alkenyl
(including but not
limited to unsubstituted or substituted C2-05alkenyl such as, for example,
vinyl, allyl, 1-
methyl propen-l-yl, butenyl, or pentenyl) or unsubstituted or substituted
alkynyl (including
but not limited to unsubstituted or substituted C2-05alkynyl such as
acetylenyl, propargyl,
-219-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
butynyl, or pentynyl). Alternatively, R7 is unsubstituted or substituted aryl
(including but not
limited to monocyclic or bicyclic aryl) or unsubstituted or substituted
arylalkyl (including but
not limited to monocyclic or bicyclic aryl linked to alkyl wherein alkyl
includes but is not
limited to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, sec-butyl, and
pentyl). In some other
embodiments, R7 is unsubstituted or substituted heteroaryl, including but not
limited to
monocyclic and bicyclic heteroaryl. Monocyclic heteroaryl R7 includes but is
not limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazolyl. Bicyclic heteroaryl R7 includes but is not
limited to
benzothiophenyl, benzofuryl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl, pyrazolopyrimidinyl,
purinyl, pyrrolo
[1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl, pyrazolylpyridinyl,
imidazo[1, 2-
a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl.
[00566] In some embodiments of the compound of Formula II, W6' is N. In other
embodiments of the compound of Formula II, W6' is CR8'. R8' can be, for
example, hydrogen,
or unsubstituted or substituted alkyl (including but not limited to CH3, -
CH2CH3, n-propyl,
isopropyl, n- butyl, ten'- butyl, sec-butyl, pentyl, hexyl, and heptyl). In
one embodiment, R8'
is H. In other embodiments, R8' is unsubstituted or substituted alkenyl
(including but not
limited to unsubstituted or substituted C2-05alkenyl such as, for example,
vinyl, allyl, 1-
methyl propen-l-yl, butenyl, or pentenyl) or unsubstituted or substituted
alkynyl (including
but not limited to unsubstituted or substituted C2-05alkynyl such as
acetylenyl, propargyl,
butynyl, or pentynyl). Alternatively, R8' is unsubstituted or substituted aryl
(including but not
limited to monocyclic or bicyclic aryl) or unsubstituted or substituted
arylalkyl (including but
not limited to monocyclic or bicyclic aryl linked to alkyl wherein alkyl
includes but is not
limited to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, sec-butyl, and
pentyl). In some other
embodiments, R8' is unsubstituted or substituted heteroaryl, including but not
limited to
monocyclic and bicyclic heteroaryl. Monocyclic heteroaryl R8' includes but is
not limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazolyl. Bicyclic heteroaryl R8' includes but is
not limited to
benzothiophenyl, benzofuryl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl, pyrazolopyrimidinyl,
purinyl, pyrrolo
[1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl, pyrazolylpyridinyl,
imidazo[1, 2-
a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl.
-220-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
[00567] In some embodiments, the compound of Formula II has the formula:
R1'
N
w3 -"====-='--' w6'
1 0 0
Wc............,. ..,..,..0=-==,..........._====,õ../...,...,...,.......õwc'
1 0> _______ R2'
wb' ,......2,...........
Wd'
w a'
[00568] In other embodiments, the compound of Formula II is:
R1'
N
w3
1 0 0
W2..:,.,., ..........,-,.....,... .........."...... ,we
N N
1 0> _______ R2'
wb'....;...!.............
Wd'
w a'
[00569] For example, the compound of Formula II is:
R1 R1'
3./.. N..,..,.....õ,,,,..
6
W
I 0 W
I 0 0w
w 0 w 2 ,......õ,_
N W2 N
WI N N N =Oe> R2
III> __ R2.
0 Or 0
[00570] In some embodiments, the compound of Formula II is:
R1'
R1' N
N
ro o
w2 ro o
W2 N
N
N N
N
410> _________________________ R2' 0 1111> R2'
0 Or 0 .
-221-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00571] In another aspect, the invention provides compounds of Subformula Ha.
R1'
R5 N' Rs
00
N
R4'
R3 N
401 0> __________________________________________________ Rz
'
Subformula ha
[00572] In one embodiment, R1', R3', R4', R5', and R8' are hydrogen. In
another embodiment,
R1', R3', R5', and R8' are hydrogen and R4' is alkyl, heteroalkyl, alkenyl,
alkynyl, cycloalkyl,
heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy,
heterocycloalkyloxy,
amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano,
hydroxy, nitro,
phosphate, urea, carbonate, or NR'R" wherein R' and R" are taken together with
nitrogen to
form a cyclic moiety. R4' can be, for example, hydrogen, unsubstituted or
substituted alkyl
(including but not limited to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl,
tert- butyl, sec-
butyl, pentyl, hexyl, and heptyl). In other embodiments, R4' is unsubstituted
or substituted
alkenyl (including but not limited to unsubstituted or substituted C2-
05alkenyl such as, for
example, vinyl, allyl, 1-methyl propen-l-yl, butenyl, or pentenyl) or
unsubstituted or
substituted alkynyl (including but not limited to unsubstituted or substituted
C2-05alkynyl
such as acetylenyl, propargyl, butynyl, or pentynyl). Alternatively, R4' is
unsubstituted or
substituted aryl (including but not limited to monocyclic or bicyclic aryl) or
unsubstituted or
substituted arylalkyl (including but not limited to monocyclic or bicyclic
aryl linked to alkyl
wherein alkyl includes but is not limited to CH3, -CH2CH3, n-propyl,
isopropyl, n- butyl, sec-
butyl, and pentyl). In some other embodiments, R4' is unsubstituted or
substituted heteroaryl,
including but not limited to monocyclic and bicyclic heteroaryl. Monocyclic
heteroaryl R4'
includes but is not limited to pyrrolyl, thienyl, furyl, pyridinyl, pyranyl,
pyrimidinyl,
pyrazinyl, pyridazinyl, imidazolyl, thiazolyl, pyrazolyl, and oxazolyl.
Bicyclic heteroaryl R4'
includes but is not limited to benzothiophenyl, benzofuryl, indolyl,
quinolinyl, isoquinolinyl,
benzimidazolyl, benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl,
pyrazolopyrimidinyl,
purinyl, pyrrolo [1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl,
pyrazolylpyridinyl,
imidazo[1, 2-a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl.
-222-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
R1' R1'
2R5 N
00 _______________ N-y R 00 NR 2
R
RT RT
Subformula ha' Subformula lib'
[00573] In another aspect, the invention provides compounds of Subformula Ha'
and lib',
where W1' is CR3', W2 is CR4', W3' is CR5', W4' is N, W5' is CR7, and W6' is
CR8'. In one
embodiment, R1', R3', R4', R5', R7 and R8' are hydrogen. In another
embodiment, R1', R4', R5',
R7 and R8' are hydrogen and R3' is alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl,
heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy,
heterocycloalkyloxy,
amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano,
hydroxy, nitro,
phosphate, urea, carbonate, or NR'R" wherein R' and R" are taken together with
nitrogen to
form a cyclic moiety. R3' can be, for example, hydrogen, unsubstituted or
substituted alkyl
(including but not limited to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl,
tert- butyl, sec-
butyl, pentyl, hexyl, and heptyl). In other embodiments, R3' is unsubstituted
or substituted
alkenyl (including but not limited to unsubstituted or substituted C2-
05alkenyl such as, for
example, vinyl, allyl, 1-methyl propen-l-yl, butenyl, or pentenyl) or
unsubstituted or
substituted alkynyl (including but not limited to unsubstituted or substituted
C2-05alkynyl
such as acetylenyl, propargyl, butynyl, or pentynyl). Alternatively, R3' is
unsubstituted or
substituted aryl (including but not limited to monocyclic or bicyclic aryl) or
unsubstituted or
substituted arylalkyl (including but not limited to monocyclic or bicyclic
aryl linked to alkyl
wherein alkyl includes but is not limited to CH3, -CH2CH3, n-propyl,
isopropyl, n- butyl, sec-
butyl, and pentyl). In some other embodiments, R3' is unsubstituted or
substituted heteroaryl,
including but not limited to monocyclic and bicyclic heteroaryl. Monocyclic
heteroaryl R3'
includes but is not limited to pyrrolyl, thienyl, furyl, pyridinyl, pyranyl,
pyrimidinyl,
pyrazinyl, pyridazinyl, imidazolyl, thiazolyl, pyrazolyl, and oxazolyl.
Bicyclic heteroaryl R3'
includes but is not limited to benzothiophenyl, benzofuryl, indolyl,
quinolinyl, isoquinolinyl,
benzimidazolyl, benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl,
pyrazolopyrimidinyl,
purinyl, pyrrolo [1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl,
pyrazolylpyridinyl,
imidazo[1, 2-a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl. The present
invention also
provides compounds of Formula II wherein R3' is unsubstituted or substituted
heteroarylalkyl,
including but not limited to monocyclic and bicyclic heteroaryl as described
above, that are
linked to alkyl, which in turn includes but is not limited to CH3, -CH2CH3, n-
propyl,
isopropyl, n- butyl, sec-butyl, and pentyl. In some embodiments, R3' is
unsubstituted or
-223-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
substituted cycloalkyl (including but not limited to cyclopropyl, cyclobutyl,
and cyclopentyl)
or unsubstituted or substituted heteroalkyl (non-limiting examples include
ethoxymethyl,
methoxymethyl, and diethylaminomethyl). In some further embodiments, R3' is
unsubstituted
or substituted heterocycloalkyl which includes but is not limited to
pyrrolidinyl,
tetrahydrofuranyl, piperidinyl, tetrahydropyranyl, thiazolidinyl,
imidazolidinyl, morpholinyl,
and piperazinyl. In yet other embodiments of the compounds of Formula II, R3'
is
unsubstituted or substituted alkoxy including but not limited to Ci-C4alkoxy
such as
methoxy, ethoxy, propoxy or butoxy. R3' can also be unsubstituted or
substituted
heterocycloalkyloxy, including but not limited to 4-NH piperidin-l-yl-oxy, 4-
methyl
piperidin-l-yl-oxy, 4-ethyl piperidin-l-yl-oxy, 4-isopropyl- piperidin-l-yl-
oxy, and
pyrrolidin-3-yl-oxy. In other embodiments, R3' is unsubstituted or substituted
amino, wherein
the substituted amino includes but is not limited to dimethylamino,
diethylamino, di-
isopropyl amino, N-methyl N-ethyl amino, and dibutylamino. In some
embodiments, R3' is
unsubstituted or substituted acyl, unsubstituted or substituted acyloxy,
unsubstituted or
substituted Ci-C4acyloxy, unsubstituted or substituted alkoxycarbonyl,
unsubstituted or
substituted amido, or unsubstituted or substituted sulfonamido. In other
embodiments, R3' is
halo, which is ¨I, -F, -Cl, or -Br. In some embodiments, R3' is selected from
the group
consisting of cyano, hydroxy, nitro, phosphate, urea, and carbonate. Also
contemplated are
R3' being -CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, ten'- butyl, sec-
butyl, pentyl, hexyl,
heptyl, -OCH3, -OCH2CH3, or -CF3. In some embodiments R3' can also be NR'R"
wherein R'
and R" are taken together with the nitrogen to form a cyclic moiety having
from 3 to 8 ring
atoms. The cyclic moiety so formed may further include one or more heteroatoms
which are
selected from the group consisting of S, 0, and N. The cyclic moiety so formed
is
unsubstituted or substituted, including but not limited to morpholinyl,
azetidinyl, pyrrolidinyl,
piperidinyl, piperazinyl, isothiazolidinyl 1,2, dioxide, and thiomorpholinyl.
Further non-
limiting exemplary cyclic moieites are the following:
cH 3
"¨NO ¨1¨N( __ )0 A¨N,)
g ,
0 0
0 0 -el ¨CH.1
N.N,)
0 ,
-224-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00574] In another aspect, the invention provides compounds of Subformula IIb:
R1'
R5' N Rg
00
R`v N N
N 0 0>
0 ____________________________________________________ R2
Subformula I%
[00575] In one embodiment, R1', R4', R5' and R8' are hydrogen. In another
embodiment, R1',
R5', and R8' are hydrogen and R4' is alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl,
heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy,
heterocycloalkyloxy,
amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano,
hydroxy, nitro,
phosphate, urea, carbonate, or NR'R" wherein R' and R" are taken together with
nitrogen to
form a cyclic moiety. R4' can be, for example, hydrogen, unsubstituted or
substituted alkyl
(including but not limited to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl,
tert- butyl, sec-
butyl, pentyl, hexyl, and heptyl). In other embodiments, R4' is unsubstituted
or substituted
alkenyl (including but not limited to unsubstituted or substituted C2-
05alkenyl such as, for
example, vinyl, allyl, 1-methyl propen-l-yl, butenyl, or pentenyl) or
unsubstituted or
substituted alkynyl (including but not limited to unsubstituted or substituted
C2-05alkynyl
such as acetylenyl, propargyl, butynyl, or pentynyl). Alternatively, R4' is
unsubstituted or
substituted aryl (including but not limited to monocyclic or bicyclic aryl) or
unsubstituted or
substituted arylalkyl (including but not limited to monocyclic or bicyclic
aryl linked to alkyl
wherein alkyl includes but is not limited to CH3, -CH2CH3, n-propyl,
isopropyl, n- butyl, sec-
butyl, and pentyl). In some other embodiments, R4' is unsubstituted or
substituted heteroaryl,
including but not limited to monocyclic and bicyclic heteroaryl. Monocyclic
heteroaryl R4'
includes but is not limited to pyrrolyl, thienyl, furyl, pyridinyl, pyranyl,
pyrimidinyl,
pyrazinyl, pyridazinyl, imidazolyl, thiazolyl, pyrazolyl, and oxazolyl.
Bicyclic heteroaryl R4'
includes but is not limited to benzothiophenyl, benzofuryl, indolyl,
quinolinyl, isoquinolinyl,
benzimidazolyl, benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl,
pyrazolopyrimidinyl,
purinyl, pyrrolo [1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl,
pyrazolylpyridinyl,
imidazo[1, 2-a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl.
[00576] In another aspect, the invention provides compounds of Subformula IIc
and lid,
where W1' is N, W2' is CR4', W3' is CR5', W4' is N, W5' is CR7', and W6' is
CR8':
-225-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
R1' R1'
R N R8 2R5 N
00 _______________ R 00
/-(NR2
r
Subformula lie Subformula lid
[00577] In one embodiment, R1', R4', R5', R7' and R8' are hydrogen. In another
embodiment,
R1', R5', R7' and R8' are hydrogen and R4' is alkyl, heteroalkyl, alkenyl,
alkynyl, cycloalkyl,
heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy,
heterocycloalkyloxy,
amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano,
hydroxy, nitro,
phosphate, urea, carbonate, or NR'R" wherein R' and R" are taken together with
nitrogen to
form a cyclic moiety. R4' can be, for example, hydrogen, unsubstituted or
substituted alkyl
(including but not limited to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl,
tert- butyl, sec-
butyl, pentyl, hexyl, and heptyl). In other embodiments, R4' is unsubstituted
or substituted
alkenyl (including but not limited to unsubstituted or substituted C2-
05alkenyl such as, for
example, vinyl, allyl, 1-methyl propen-l-yl, butenyl, or pentenyl) or
unsubstituted or
substituted alkynyl (including but not limited to unsubstituted or substituted
C2-05alkynyl
such as acetylenyl, propargyl, butynyl, or pentynyl). Alternatively, R4' is
unsubstituted or
substituted aryl (including but not limited to monocyclic or bicyclic aryl) or
unsubstituted or
substituted arylalkyl (including but not limited to monocyclic or bicyclic
aryl linked to alkyl
wherein alkyl includes but is not limited to CH3, -CH2CH3, n-propyl,
isopropyl, n- butyl, sec-
butyl, and pentyl). In some other embodiments, R4' is unsubstituted or
substituted heteroaryl,
including but not limited to monocyclic and bicyclic heteroaryl. Monocyclic
heteroaryl R4'
includes but is not limited to pyrrolyl, thienyl, furyl, pyridinyl, pyranyl,
pyrimidinyl,
pyrazinyl, pyridazinyl, imidazolyl, thiazolyl, pyrazolyl, and oxazolyl.
Bicyclic heteroaryl R4'
includes but is not limited to benzothiophenyl, benzofuryl, indolyl,
quinolinyl, isoquinolinyl,
benzimidazolyl, benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl,
pyrazolopyrimidinyl,
purinyl, pyrrolo [1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl,
pyrazolylpyridinyl,
imidazo[1, 2-a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl. The present
invention also
provides compounds of Formula II wherein R4' is unsubstituted or substituted
heteroarylalkyl,
including but not limited to monocyclic and bicyclic heteroaryl as described
above, that are
linked to alkyl, which in turn includes but is not limited to CH3, -CH2CH3, n-
propyl,
isopropyl, n- butyl, sec-butyl, and pentyl. In some embodiments, R4' is
unsubstituted or
substituted cycloalkyl (including but not limited to cyclopropyl, cyclobutyl,
and cyclopentyl)
or unsubstituted or substituted heteroalkyl (non-limiting examples include
ethoxymethyl,
-226-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
methoxymethyl, and diethylaminomethyl). In some further embodiments, R4' is
unsubstituted
or substituted heterocycloalkyl which includes but is not limited to
pyrrolidinyl,
tetrahydrofuranyl, piperidinyl, tetrahydropyranyl, thiazolidinyl,
imidazolidinyl, morpholinyl,
and piperazinyl. In yet other embodiments of the compounds of Formula II, R4'
is
unsubstituted or substituted alkoxy including but not limited to Ci-C4alkoxy
such as
methoxy, ethoxy, propoxy or butoxy. R3' can also be unsubstituted or
substituted
heterocycloalkyloxy, including but not limited to 4-NH piperidin-l-yl-oxy, 4-
methyl
piperidin-l-yl-oxy, 4-ethyl piperidin-l-yl-oxy, 4-isopropyl- piperidin-l-yl-
oxy, and
pyrrolidin-3-yl-oxy. In other embodiments, R4' is unsubstituted or substituted
amino, wherein
the substituted amino includes but is not limited to dimethylamino,
diethylamino, di-
isopropyl amino, N-methyl N-ethyl amino, and dibutylamino. In some
embodiments, R4' is
unsubstituted or substituted acyl, unsubstituted or substituted acyloxy,
unsubstituted or
substituted Ci-C4acyloxy, unsubstituted or substituted alkoxycarbonyl,
unsubstituted or
substituted amido, or unsubstituted or substituted sulfonamido. In other
embodiments, R4' is
halo, which is ¨I, -F, -Cl, or -Br. In some embodiments, R4' is selected from
the group
consisting of cyano, hydroxy, nitro, phosphate, urea, and carbonate. Also
contemplated are
R4' being -CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, ten'- butyl, sec-
butyl, pentyl, hexyl,
heptyl, -OCH3, -OCH2CH3, or -CF3. In some embodiments R4' can also be NR'R"
wherein R'
and R" are taken together with the nitrogen to form a cyclic moiety having
from 3 to 8 ring
atoms. The cyclic moiety so formed may further include one or more heteroatoms
which are
selected from the group consisting of S, 0, and N. The cyclic moiety so formed
is
unsubstituted or substituted, including but not limited to morpholinyl,
azetidinyl, pyrrolidinyl,
piperidinyl, piperazinyl, isothiazolidinyl 1,2, dioxide, and thiomorpholinyl.
Further non-
limiting exemplary cyclic moieites are the following:
c H3
1-NO
Ã-_a, N N __N\ /0
0 0
o p
r N )---- r,,,,,k.\_cH3
A¨ N
s
j
¨N `zzeL) µz., N 0
0 , , , `'= .
-227-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00578] In another aspect, the invention provides compounds of Subformula lie
and IIf,
where W1' is CR3', W2' is N, W3' is CR5', W4' is N, W5' is CR7', and W6' is
CR8':
R1' R1'
R5' NR8'
YO 0 _____________ NrR2 1 0 0
Rr / XR3 Rr
Subformula lie Subformula IIf
[00579] In one embodiment, R1', R3', R5', R7' and R8' are hydrogen. In another
embodiment,
R1', R5', R7' and R8' are hydrogen and R3' is alkyl, heteroalkyl, alkenyl,
alkynyl, cycloalkyl,
heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy,
heterocycloalkyloxy,
amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano,
hydroxy, nitro,
phosphate, urea, carbonate, or NR'R" wherein R' and R" are taken together with
nitrogen to
form a cyclic moiety. R3' can be, for example, hydrogen, unsubstituted or
substituted alkyl
(including but not limited to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl,
tert- butyl, sec-
butyl, pentyl, hexyl, and heptyl). In other embodiments, R3' is unsubstituted
or substituted
alkenyl (including but not limited to unsubstituted or substituted C2-
05alkenyl such as, for
example, vinyl, allyl, 1-methyl propen-l-yl, butenyl, or pentenyl) or
unsubstituted or
substituted alkynyl (including but not limited to unsubstituted or substituted
C2-05alkynyl
such as acetylenyl, propargyl, butynyl, or pentynyl). Alternatively, R3' is
unsubstituted or
substituted aryl (including but not limited to monocyclic or bicyclic aryl) or
unsubstituted or
substituted arylalkyl (including but not limited to monocyclic or bicyclic
aryl linked to alkyl
wherein alkyl includes but is not limited to CH3, -CH2CH3, n-propyl,
isopropyl, n- butyl, sec-
butyl, and pentyl). In some other embodiments, R3' is unsubstituted or
substituted heteroaryl,
including but not limited to monocyclic and bicyclic heteroaryl. Monocyclic
heteroaryl R3'
includes but is not limited to pyrrolyl, thienyl, furyl, pyridinyl, pyranyl,
pyrimidinyl,
pyrazinyl, pyridazinyl, imidazolyl, thiazolyl, pyrazolyl, and oxazolyl.
Bicyclic heteroaryl R3'
includes but is not limited to benzothiophenyl, benzofuryl, indolyl,
quinolinyl, isoquinolinyl,
benzimidazolyl, benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl,
pyrazolopyrimidinyl,
purinyl, pyrrolo [1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl,
pyrazolylpyridinyl,
imidazo[1, 2-a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl. The present
invention also
provides compounds of Formula II wherein R3' is unsubstituted or substituted
heteroarylalkyl,
including but not limited to monocyclic and bicyclic heteroaryl as described
above, that are
linked to alkyl, which in turn includes but is not limited to CH3, -CH2CH3, n-
propyl,
isopropyl, n- butyl, sec-butyl, and pentyl. In some embodiments, R3' is
unsubstituted or
-228-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
substituted cycloalkyl (including but not limited to cyclopropyl, cyclobutyl,
and cyclopentyl)
or unsubstituted or substituted heteroalkyl (non-limiting examples include
ethoxymethyl,
methoxymethyl, and diethylaminomethyl). In some further embodiments, R3' is
unsubstituted
or substituted heterocycloalkyl which includes but is not limited to
pyrrolidinyl,
tetrahydrofuranyl, piperidinyl, tetrahydropyranyl, thiazolidinyl,
imidazolidinyl, morpholinyl,
and piperazinyl. In yet other embodiments of the compounds of Formula II, R3'
is
unsubstituted or substituted alkoxy including but not limited to Ci-C4alkoxy
such as
methoxy, ethoxy, propoxy or butoxy. R3' can also be unsubstituted or
substituted
heterocycloalkyloxy, including but not limited to 4-NH piperidin-l-yl-oxy, 4-
methyl
piperidin-l-yl-oxy, 4-ethyl piperidin-l-yl-oxy, 4-isopropyl- piperidin-l-yl-
oxy, and
pyrrolidin-3-yl-oxy. In other embodiments, R3' is unsubstituted or substituted
amino, wherein
the substituted amino includes but is not limited to dimethylamino,
diethylamino, di-
isopropyl amino, N-methyl N-ethyl amino, and dibutylamino. In some
embodiments, R3' is
unsubstituted or substituted acyl, unsubstituted or substituted acyloxy,
unsubstituted or
substituted Ci-C4acyloxy, unsubstituted or substituted alkoxycarbonyl,
unsubstituted or
substituted amido, or unsubstituted or substituted sulfonamido. In other
embodiments, R3' is
halo, which is ¨I, -F, -Cl, or -Br. In some embodiments, R3' is selected from
the group
consisting of cyano, hydroxy, nitro, phosphate, urea, and carbonate. Also
contemplated are
R3' being -CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, ten'- butyl, sec-
butyl, pentyl, hexyl,
heptyl, -OCH3, -OCH2CH3, or -CF3. In some embodiments R3' can also be NR'R"
wherein R'
and R" are taken together with the nitrogen to form a cyclic moiety having
from 3 to 8 ring
atoms. The cyclic moiety so formed may further include one or more heteroatoms
which are
selected from the group consisting of S, 0, and N. The cyclic moiety so formed
is
unsubstituted or substituted, including but not limited to morpholinyl,
azetidinyl, pyrrolidinyl,
piperidinyl, piperazinyl, isothiazolidinyl 1,2, dioxide, and thiomorpholinyl.
Further non-
limiting exemplary cyclic moieites are the following:
cH 3
"¨NO ¨1¨N( __ )0 A¨N,)
g ,
0 0
0 0 -el ¨CH.1
N.N,)
0 ,
-229-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00580] In some embodiments, the substituents R3', R4', R5', R6' or R8' may be
any of the
substituents shown in Table 1:
Table 1. R3', R4', R5', R6', R8' moieties of the compounds of Formula II, each
independently
includes but is not limited to the following:
Sub- R Sub- R Sub- R
class class class
# # #
R-1 R-2N¨CH 3 R-3 cH3
I
N-CH
0"---) /
\
0 I. 1 _......) CH3
I urt./v 0
alai' I I
VVV
I I
R-4 /NH R-5 R-6
0
,s/ <
R-7 NcH3 R-8
I. R-9 -CH(CH3)2
/
R-10I R-11 /CH 3 R-12 cH3
1¨N/CH3
1¨NCH
) . \C H3 1-N\
H3C CH--CH3
/
R-13 R-14 hl3c\ R-15 o
0 , 1
HN----N )7z.õ.---
.........,.....õ,, N ..õ........,,,õ.--
R-16 H3C . R-17 CI0 R-18
0 0
R-19 . CN R-20 / \ R-21 H3c\ ( \
H3c\ 1_,, / / 0 \N 0
\
N
/
R-22-- R-23 Me0 . R-24 ,S
0 I / N I
`,),N
0
1 0
_1
l'Ir
R-25 0 R-26 ,_, N R-27 N
.......-..N.11--- . .3._r, \ Si /.N la I ;N
N
1
. ¨\
-230-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
R-28 cH2cH3 R-29_ ,CN R-30 _
,ON
,..----.N, ---- .õ----.N.---_,
0 /\.) 0')
kj \C
R-31 0 R-32 0 R-33
_ PH
õõ......., ,
0
3r 0)
I I
R-34 _ pH R-35 SO2Me R-36 ,------.
2Me
.õ....-,. N
/\) C)
\--
R-37 , R-38 , R-39 ,s
I I I
0---- e N '11,7"---N
----V
R-40 H R-41
S R-42 ,--S
\----
`11,ZN 1
R-43
R-44 R-45 rs
0
R-46 rN R-47 //\1--S02Me R-48 o
_\--N o----)
I
,,õ o¨ \)
I
aVV
I I
R-49 o R-50A
N- c R-51m so m
..- 2 e
ko
R-52
0 R-53 /Th\i-cH3 R-54 N,Ac
N
N
ri
,32c0
µ.-0
R-55 / o
\ R-56 N,CH3 R-57 N,S02Me
N
rj N N
--0
--0
-231-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
R-58 ,S02Me R-59 ,Ac R-60
HIJ---\)
K) siY
ri
ri N
.Vo
L''';'1,
R-61 .------ N -CH3 R-62 0 R-63
ral-Ac
Hl--\) Hl."\)
4Yt dr. !224--NH
R-64 N - C H 3 R-65
ra¨CH3 R-66 (N3F111
N
"--\) r)
µ111. HN---1
/
virL,
R-67 0 R-68 ro R-69 (N-AC
rN,)
N HHN
HN'j >
i
vIrt,
/
\R-70 R-71 R-72 0õ0
,/)
JD
'3?z. = 1-N
HN
/
R-73 H R-74 I R-75
..v N,/s(
EN el
0/ NO 0/ NO µ' ;S
,
00
R-76 R-77 H R-78 I
N el N,
d,
,Sõ0 40
d \ 0
R-79 ,i-F R-80 ON R-81
N-\
;
(:)S /1\1-\_OH (:) S / \- /--
\
,\ 2=? . , N 0
0"CH3 \-
0/ CH3
[00581] In another aspect, the invention provides a PI3Ka inhibitor which is a
compound of
Formula III:
R1'
W3: 7 '
6
\f\i(DWI OW
I\1-õ,r R2'
1. W4
W 'wg . X
or its pharmaceutically acceptable salts thereof, where:
Xis 0 or S or N;
-232-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Wr is S, N, NR3' or CR3', W2' is N or CR4', W3' is S, N or CR5', W4' is N or
C, and
W7' is N or C, wherein no more than two N atoms and no more than two C=0
groups are
adjacent;
W5' is N or CR7';
W6' is N or CR8';
R1' and R2' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
R3' and R4' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
or R3' and R4' taken together form a cyclic moiety; and
R5', R7' and R8' are independently hydrogen, alkyl, heteroalkyl, alkenyl,
alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety.
[00582] In some embodiments, the compound of Formula III exists as a tautomer,
and such
tautomers are contemplated by the present invention.
[00583] In some embodiments, the PI3Ka inhibitor is a compound of Formula III
which has
the Formula:
R1'
W3L-/ 6
K20 Dw
wi. W.. N
R2'
0 .
Formula III
[00584] In yet other embodiments, W1' is CR3', W2' is CR4', W3' is N, W4' is
N, W5' is CR7',
and W6 is CR8'. In other embodiments, W1' is CR3', W2' is CR4', W3' is N, W4'
is N, W5' is
CR7', and W6' is CR8'. In other embodiments, W1' is CR3', W2' is CR4', W3' is
N, W4' is N, W5'
-233 -
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
is N, and Wc is CR8'. In still other embodiments, W1' is NR3', W2' is CR4', W3
is N, W4' is C,
W5' is CR7', and Wc is CR8'. In other embodiments, W1' is S, W2' is CR4', W3'
is N, W4' is C,
W5' is CR7', and Wc is CR8'. In other embodiments, W1' is CR3', W2' is CR4',
W3' is S, W4' is C,
W5'' is N, and Wc is N.
[00585] In other embodiments, an inhibitor of Formula III is a compound
according to one
of the formulas:
irl 0R1 R1
R4 c6.1 R2 R4 _____________________ c-1:10/18R2 R4-<N00
,..,,.(
R8
N
NR2
NT x
R3 R7 \\ ___________________________________________________
R
RI 1 Ri RI 1
R8 N .,.,r R 8 ----> \ / R8
N N
R4 ____________ C,)' NT,C __ Nty R2 R4 q:2 N 0 .c__I R2R2
R7
Ri
Ri
R4 ____________ ?..0 ______ N R2 R4 T._ _______ cyR2 R4 N----rC:L. j
IN N:-Z:r R2
R3 7 \\
R3 R3 R7 R
Rl
Ri
R4 ____________ ?p _______________ -rR2R4 cc,),IN0 p-R2R4 91.1(_,¨<NYR2
R3 _________________ / R3 R7 R3 R7
R1 R1
R8R8
N
2 N
/ N R2
NQ 0 N R
-----Zr NQ N 0 y
ilk 0 )----- = X
R3 R7 R3 R7 5
wherein for each of the above formulas, each respective R variable includes a
'prime' (').
[00586] In some embodiments, X is 0. In other embodiments, X is S.
[00587] In some embodiments, R1' is hydrogen. In other embodiments, R1' is
alkyl,
heteroalkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl,
heteroaryl,
heteroarylalkyl, alkoxy, heterocycloalkyloxy, amido, amino, acyl, acyloxy,
alkoxycarbonyl,
sulfonamido, halo, cyano, hydroxy, nitro, phosphate, urea, carbonate, or
NR'R", wherein R'
and R" are taken together with nitrogen to form a cyclic moiety.
[00588] In some embodiments, R2' is hydrogen. In other embodiments, R2' is,
for example,
unsubstituted or substituted alkyl (including but not limited to CH3, -CH2CH3,
n-propyl,
isopropyl, n- butyl, ten'- butyl, sec-butyl, pentyl, hexyl, and heptyl). In
other embodiments,
-234-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
R2' is unsubstituted or substituted alkenyl (including but not limited to
unsubstituted or
substituted C2-05alkenyl such as, for example, vinyl, allyl, 1-methyl propen-l-
yl, butenyl, or
pentenyl) or unsubstituted or substituted alkynyl (including but not limited
to unsubstituted or
substituted C2-05alkynyl such as acetylenyl, propargyl, butynyl, or pentynyl).
Alternatively,
R2' is unsubstituted or substituted aryl (including but not limited to
monocyclic or bicyclic
aryl) or unsubstituted or substituted arylalkyl (including but not limited to
monocyclic or
bicyclic aryl linked to alkyl wherein alkyl includes but is not limited to
CH3, -CH2CH3, n-
propyl, isopropyl, n- butyl, sec-butyl, and pentyl). In some other
embodiments, R2' is
unsubstituted or substituted heteroaryl, including but not limited to
monocyclic and bicyclic
heteroaryl. Monocyclic heteroaryl R2' includes but is not limited to pyrrolyl,
thienyl, furyl,
pyridinyl, pyranyl, pyrimidinyl, pyrazinyl, pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and
oxazolyl. Bicyclic heteroaryl R2' includes but is not limited to
benzothiophenyl, benzofuryl,
indolyl, quinolinyl, isoquinolinyl, benzimidazolyl, benzoxazolyl,
benzothiazolyl,
quinazolinyl, azaindolyl, pyrazolopyrimidinyl, purinyl, pyrrolo [1, 2-
b]pyridazinyl,
pyrrolopyrimidinyl, indazolyl, pyrazolylpyridinyl, imidazo[1, 2-a]pyridinyl,
and pyrrolo[1, 2-
f] [l, 2, 4]triazinyl. The present invention also provides compounds wherein
R2' is
unsubstituted or substituted heteroarylalkyl, including but not limited to
monocyclic and
bicyclic heteroaryl as described above, that are linked to alkyl, which in
turn includes but is
not limited to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, sec-butyl, and
pentyl. In some
embodiments, R2' is unsubstituted or substituted cycloalkyl (including but not
limited to
cyclopropyl, cyclobutyl, and cyclopentyl) or unsubstituted or substituted
heteroalkyl (non-
limiting examples include ethoxymethyl, methoxymethyl, and
diethylaminomethyl). In some
further embodiments, R2' is unsubstituted or substituted heterocycloalkyl
which includes but
is not limited to pyrrolidinyl, tetrahydrofuranyl, piperidinyl,
tetrahydropyranyl, thiazolidinyl,
imidazolidinyl, morpholinyl, and piperazinyl. In yet other embodiments of the
compounds of
Formula III, R2' is unsubstituted or substituted alkoxy including but not
limited to C1-
C4alkoxy such as methoxy, ethoxy, propoxy or butoxy. R2' can also be
unsubstituted or
substituted heterocycloalkyloxy, including but not limited to 4-NH piperidin-l-
yl-oxy, 4-
methyl piperidin-l-yl-oxy, 4-ethyl piperidin-l-yl-oxy, 4-isopropyl- piperidin-
l-yl-oxy, and
pyrrolidin-3-yl-oxy. In other embodiments, R2' is unsubstituted or substituted
amino, wherein
the substituted amino includes but is not limited to dimethylamino,
diethylamino, di-
isopropyl amino, N-methyl N-ethyl amino, and dibutylamino. In some
embodiments, R2' is
unsubstituted or substituted acyl, unsubstituted or substituted acyloxy,
unsubstituted or
substituted Ci-C4acyloxy, unsubstituted or substituted alkoxycarbonyl,
unsubstituted or
-235-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
substituted amido, or unsubstituted or substituted sulfonamido. In other
embodiments, R2' is
halo, which is ¨I, -F, -Cl, or -Br. In some embodiments, R2' is selected from
the group
consisting of cyano, hydroxy, nitro, phosphate, urea, and carbonate. Also
contemplated are
R2' being -CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, ten'- butyl, sec-
butyl, pentyl, hexyl,
heptyl, -OCH3, -OCH2CH3, or -CF3.
[00589] In some embodiments of the compound of Formula III, Wi' is CR3. R3'
can be, for
example, hydrogen, unsubstituted or substituted alkyl (including but not
limited to CH3, -
CH2CH3, n-propyl, isopropyl, n- butyl, tert- butyl, sec-butyl, pentyl, hexyl,
and heptyl). In
other embodiments, R3' is unsubstituted or substituted alkenyl (including but
not limited to
unsubstituted or substituted C2-05alkenyl such as, for example, vinyl, allyl,
1-methyl propen-
l-yl, butenyl, or pentenyl) or unsubstituted or substituted alkynyl (including
but not limited to
unsubstituted or substituted C2-05alkynyl such as acetylenyl, propargyl,
butynyl, or
pentynyl). Alternatively, R3' is unsubstituted or substituted aryl (including
but not limited to
monocyclic or bicyclic aryl) or unsubstituted or substituted arylalkyl
(including but not
limited to monocyclic or bicyclic aryl linked to alkyl wherein alkyl includes
but is not limited
to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, sec-butyl, and pentyl). In
some other
embodiments, R3' is unsubstituted or substituted heteroaryl, including but not
limited to
monocyclic and bicyclic heteroaryl. Monocyclic heteroaryl R3' includes but is
not limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazolyl. Bicyclic heteroaryl R3' includes but is
not limited to
benzothiophenyl, benzofuryl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl, pyrazolopyrimidinyl,
purinyl, pyrrolo
[1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl, pyrazolylpyridinyl,
imidazo[1, 2-
a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl. The present invention also
provides
compounds of Formula III wherein R3' is unsubstituted or substituted
heteroarylalkyl,
including but not limited to monocyclic and bicyclic heteroaryl as described
above, that are
linked to alkyl, which in turn includes but is not limited to CH3, -CH2CH3, n-
propyl,
isopropyl, n- butyl, sec-butyl, and pentyl. In some embodiments, R3' is
unsubstituted or
substituted cycloalkyl (including but not limited to cyclopropyl, cyclobutyl,
and cyclopentyl)
or unsubstituted or substituted heteroalkyl (non-limiting examples include
ethoxymethyl,
methoxymethyl, and diethylaminomethyl). In some further embodiments, R3' is
unsubstituted
or substituted heterocycloalkyl which includes but is not limited to
pyrrolidinyl,
tetrahydrofuranyl, piperidinyl, tetrahydropyranyl, thiazolidinyl,
imidazolidinyl, morpholinyl,
and piperazinyl. In yet other embodiments of the compounds of Formula III, R3'
is
-236-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
unsubstituted or substituted alkoxy including but not limited to Ci-C4alkoxy
such as
methoxy, ethoxy, propoxy or butoxy. R3' can also be unsubstituted or
substituted
heterocycloalkyloxy, including but not limited to 4-NH piperidin-l-yl-oxy, 4-
methyl
piperidin-l-yl-oxy, 4-ethyl piperidin-l-yl-oxy, 4-isopropyl- piperidin-l-yl-
oxy, and
pyrrolidin-3-yl-oxy. In other embodiments, R3' is unsubstituted or substituted
amino, wherein
the substituted amino includes but is not limited to dimethylamino,
diethylamino, di-
isopropyl amino, N-methyl N-ethyl amino, and dibutylamino. In some
embodiments, R3' is
unsubstituted or substituted acyl, unsubstituted or substituted acyloxy,
unsubstituted or
substituted Ci-C4acyloxy, unsubstituted or substituted alkoxycarbonyl,
unsubstituted or
substituted amido, or unsubstituted or substituted sulfonamido. In other
embodiments, R3' is
halo, which is ¨I, -F, -Cl, or -Br. In some embodiments, R3' is selected from
the group
consisting of cyano, hydroxy, nitro, phosphate, urea, and carbonate. Also
contemplated are
R3' being -CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, ten'- butyl, sec-
butyl, pentyl, hexyl,
heptyl, -OCH3, -OCH2CH3, or -CF3.
[00590] R3' of the compounds of Formula III, can also be NR'R" wherein R' and
R" are
taken together with the nitrogen to form a cyclic moiety having from 3 to 8
ring atoms. The
cyclic moiety so formed may further include one or more heteroatoms which are
selected
from the group consisting of S, 0, and N. The cyclic moiety so formed is
unsubstituted or
substituted, including but not limited to morpholinyl, azetidinyl,
pyrrolidinyl, piperidinyl,
piperazinyl, isothiazolidinyl 1,2, dioxide, and thiomorpholinyl. Further non-
limiting
exemplary cyclic moieites are the following:
c H3
"-NO
/0\ ¨N
0 0
N
0 rN)----.. -SH ¨CH3
..\....õ.1( ,,,
_N,\3s ,NL) rN\\
N1,)
[00591] The invention also provides compounds of Formula III, wherein when R3'
is a
member of the group consisting of alkyl, alkenyl, alkynyl, cycloalkyl,
heteroalkyl,
heterocycloalkyl, heterocycloalkyloxy, aryl, arylalkyl, heteroaryl,
heteroarylalkyl, acyl,
alkoxy, amido, amino, sulfonamido, acyloxy, alkoxycarbonyl, and NR'R" (wherein
R' and R"
are taken together with nitrogen to form a cyclic moiety), then R3' is
optionally substituted
with one or more of the following substituents: alkyl, alkenyl, alkynyl,
cycloalkyl,
-237-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
heteroalkyl, heterocycloalkyl, heterocycloalkyloxy, aryl, arylalkyl,
heteroaryl,
heteroarylalkyl, acyl, heterocycloalkyloxy, alkoxy, amido, amino, sulfonamido,
acyloxy,
alkoxycarbonyl, halo, cyano, hydroxy, nitro, phosphate, urea, carbonate, or
NR'R" wherein R'
and R" are taken together with nitrogen to form a cyclic moiety. Each of the
above
substituents may be further substituted with one or more substituents chosen
from the group
consisting of alkyl, alkoxy, amido, amino, sulfonamido, acyloxy,
alkoxycarbonyl, halo,
cyano, hydroxy, nitro, oxo, phosphate, urea, and carbonate.
[00592] For example, the invention provides compounds wherein when R3' is
alkyl, the alkyl
is substituted with NR'R" wherein R' and R" are taken together with the
nitrogen to form a
cyclic moiety. The cyclic moiety so formed can be unsubstituted or
substituted. Non-limiting
exemplary cyclic moieties includes but are not limited to morpholinyl,
azetidinyl,
pyrrolidinyl, piperidinyl, piperazinyl, and thiomorpholinyl. In other examples
of the
compounds of Formula III, when R3' is alkyl, the alkyl is substituted with
heterocycloalkyl,
which includes oxetanyl, azetidinyl, tetrahydrofuranyl, pyrrolyl,
tetrahydropyranyl,
piperidinyl, morpholinyl, and piperazinyl. All of the above listed
heterocycloaklyl
substituents can be unsubstituted or substituted.
[00593] In yet other examples of the compounds of Formula III, when R3' is
alkyl, the alkyl
is substituted with a 5, 6, 7, 8, 9, or 10 membered monocyclic or bicyclic
heteroaryl, which is
unsubstituted or substituted. The monocyclic heteroaryl includes but is not
limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazolyl. The bicyclic heteroaryl includes but is
not limited to
benzothiophenyl, benzofuryl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl, pyrazolopyrimidinyl,
purinyl, pyrrolo
[1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl, pyrazolylpyridinyl,
imidazo[1, 2-
a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl.
[00594] In other embodiments of the compound of Formula III, R3' is ¨NHR3u, -
N(CH3)R3u,
-N(CH2CH3)R3u, -N(CH(CH3)2)R3u, or ¨0R3, wherein R3" is unsubstituted or
substituted
heterocycloalkyl (nonlimiting examples thereof include 4-NH piperidin-l-yl, 4-
methyl
piperidin-l-yl, 4-ethyl piperidin-l-yl, 4-isopropyl- piperidin-l-yl, and
pyrrolidin-3-y1),
unsubstituted or substituted monocyclic aryl, or unsubstituted or substituted
monocyclic
heteroaryl (including but not limited to pyrrolyl, thienyl, furyl, pyridinyl,
pyranyl,
pyrimidinyl, pyrazinyl, pyridazinyl, imidazolyl, thiazolyl, pyrazolyl, and
oxazolyl). In one
example, R3' is ¨0-aryl, i.e. phenoxy. In another example, R3' is ¨0-(4-
methyl)piperidin-1-y1
or ¨0-(4-isopropyl)piperidin-1-yl.
-238-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00595] In some embodiments of the compound of Formula III, R3' is one of the
following
moieties:
CH3
N¨.CH3I NH
.--""-N-CH
0 1.-) 0----\) \CH3 0----..,..) +NO 2 __________ <
1 I I
VV d 5 d'
O5JV
I N,
I 5 I 5 5 5
CH3
I\ICF13
I. 1-N"
)>. +N\/CH3
kN
5 / 5 -CH(CH3)2 5 H CH3
5 5 5
CH3
I
CH--....
+NI/ CH3
0 H3C õ.õ..õ,,,..... H3C el
\
, 1 0 A __ N
CH ,-,u
/ -"L.! 13 ,õ..4,5- HN---- 0
,!,,,, N )7,----....,N..........õ.õ--.
L.L.t;t.
H3C 5 I 0
5 CL 5 Is 5 5
CICN
I. .
_____________________________________________________________ I-13C\ / \0
1\1cH' H C / \ N __ \ /
3 \
-1-N
0 N 0 XI
),....^..,,õ..N- / \ __ /
0
N. Me0
,I ,N --S N-- H3C\ =i
NIN
0 0 el I 0- N
I 5 5 5 5 5
rs
A¨N,)
cH2cH3
N(1 _CN
N.-------, 0
ei /.N CN N).1-
----
rN___./ ,)
,)
A, V
5 1r
5 5 5 5
0
_SO2ME
õ......./S0 2 ME
C)) (:)') 9
i
5 1 5 \ 5 = 5
A--N,
-239-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
_OH
õ,---,,N,- ---,
I , S
I N
,-V 0"---Ni
/ .--\-- \Z---"N 2 I
---N
Or
5 5 5 5
.--S /1\1
I ¨N__ j
N
0 /N-Ac
N-S02Me
/r\j.---S02Me 0
1 1
1
5 I
N .---C H3 / __ 0
\
N,CH3 N,Ac
N
r---iN r-jN
5 -? 5 2 5
N, _________ N
S02Me Ac
,
N,S02Me
.---"-N---CH3
N
N
HN --\)
_-o
'7a= .b-L,L
5 5 5 5
rerAc
ra ....CH3
0 N-Ac
HN ---- \) HN--\) (..--NH V-NH
ro ro
r N_cH3
(N-AC
j---N)
/ µ
NH HN HN HN
j--N.) r-N
HN
/ --j
kn.4õ
5 5 5 5
0
H I H H
\\4/ I.
,,,,,N /s
0
\. 101 ¨1\1/\3
/ 05 0 05 0/ 5 0/ '0 6 0 IW 5
5 5 5
I C3' CN g
0
> _N CN
µL X 101 S\ OH µ) 101 0
0/ \ \__/
5 ..
0 Si
0 CH3 5 CH3
-240-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00596] In some embodiments of the compound of Formula III, Wr is NR3',
wherein R3' is
hydrogen, unsubstituted or substituted Ci-Cioalkyl (which includes but is not
limited to -CH3,
-CH2CH3, n-propyl, isopropyl, n- butyl, tert- butyl, sec-butyl, pentyl, hexyl,
and heptyl), or
unsubstituted or substituted C3-C7cycloalkyl (which includes but is not
limited to
cyclopropyl, cyclobutyl, cyclopentyl, and cyclohexyl). In other embodiments of
the
compound of Formula III, R3' is unsubstituted or substituted heterocycloalkyl
(which includes
but is not limited to oxetanyl, tetrahydrofuranyl, pyrrolidinyl,
tetrahydropyranyl, piperidinyl,
and piperazinyl), or unsubstituted or substituted C2-Cioheteroalkyl (which
includes but is not
limited to methoxyethoxy, methoxymethyl, and diethylaminoethyl).
Alternatively, R3' is
unsubstituted or substituted monocyclic heteroaryl (which includes but is not
limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazoly1) or unsubstituted or substituted monocyclic
aryl.
[00597] In other embodiments, Wi' is N. In still other embodiments, W1' is S.
[00598] In some embodiments of the compound of Formula III, W2' is CR4'. R4'
can be, for
example, hydrogen, or unsubstituted or substituted alkyl (including but not
limited to CH3, -
CH2CH3, n-propyl, isopropyl, n- butyl, tert- butyl, sec-butyl, pentyl, hexyl,
and heptyl). In
other embodiments, R4' is unsubstituted or substituted alkenyl (including but
not limited to
unsubstituted or substituted C2-05alkenyl such as, for example, vinyl, allyl,
1-methyl propen-
l-yl, butenyl, or pentenyl) or unsubstituted or substituted alkynyl (including
but not limited to
unsubstituted or substituted C2-05alkynyl such as acetylenyl, propargyl,
butynyl, or
pentynyl). Alternatively, R4' is unsubstituted or substituted aryl (including
but not limited to
monocyclic or bicyclic aryl) or unsubstituted or substituted arylalkyl
(including but not
limited to monocyclic or bicyclic aryl linked to alkyl wherein alkyl includes
but is not limited
to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, sec-butyl, and pentyl). In
some other
embodiments, R4' is unsubstituted or substituted heteroaryl, including but not
limited to
monocyclic and bicyclic heteroaryl. Monocyclic heteroaryl R4' includes but is
not limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazolyl. Bicyclic heteroaryl R4' includes but is
not limited to
benzothiophenyl, benzofuryl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl, pyrazolopyrimidinyl,
purinyl, pyrrolo
[1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl, pyrazolylpyridinyl,
imidazo[1, 2-
a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl.
[00599] The present invention also provides compounds of Formula III wherein
R4' is
unsubstituted or substituted heteroarylalkyl, including but not limited to
monocyclic and
-241-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
bicyclic heteroaryl as described above, that are linked to alkyl, which in
turn includes but is
not limited to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, sec-butyl, and
pentyl. In some
embodiments, R4' is unsubstituted or substituted cycloalkyl (including but not
limited to
cyclopropyl, cyclobutyl, and cyclopentyl) or unsubstituted or substituted
heteroalkyl (non-
limiting examples include ethoxymethyl, methoxymethyl, and
diethylaminomethyl). In some
further embodiments, R4' is unsubstituted or substituted heterocycloalkyl
which includes but
is not limited to pyrrolidinyl, tetrahydrofuranyl, piperidinyl,
tetrahydropyranyl, thiazolidinyl,
imidazolidinyl, morpholinyl, and piperazinyl. In yet other embodiments of the
compounds of
Formula III, R4' is unsubstituted or substituted alkoxy including but not
limited to C1-
C4alkoxy such as methoxy, ethoxy, propoxy or butoxy. R4' can also be
unsubstituted or
substituted heterocycloalkyloxy, including but not limited to 4-NH piperidin-l-
yl-oxy, 4-
methyl piperidin-l-yl-oxy, 4-ethyl piperidin-l-yl-oxy, 4-isopropyl- piperidin-
l-yl-oxy, and
pyrrolidin-3-yl-oxy. In other embodiments, R4' is unsubstituted or substituted
amino, wherein
the substituted amino includes but is not limited to dimethylamino,
diethylamino, di-
isopropyl amino, N-methyl N-ethyl amino, and dibutylamino. In some
embodiments, R4' is
unsubstituted or substituted acyl, unsubstituted or substituted acyloxy,
unsubstituted or
substituted Ci-C4acyloxy, unsubstituted or substituted alkoxycarbonyl,
unsubstituted or
substituted amido, or unsubstituted or substituted sulfonamido. In some
embodiments, R4' is
halo, which is ¨I, -F, -Cl, or -Br. In some embodiments, R4' is selected from
the group
consisting of cyano, hydroxy, nitro, phosphate, urea, or carbonate. Also
contemplated are R4'
being -CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, ten'- butyl, sec-butyl,
pentyl, hexyl,
heptyl, -OCH3, -OCH2CH3, or -CF3.
[00600] R4' of the compounds of Formula III, can also be NR'R" wherein R' and
R" are
taken together with the nitrogen to form a cyclic moiety having from 3 to 8
ring atoms. The
cyclic moiety so formed may further include one or more heteroatoms which are
selected
from the group consisting of S, 0, and N. The cyclic moiety so formed is
unsubstituted or
substituted, including but not limited to morpholinyl, azetidinyl,
pyrrolidinyl, piperidinyl,
piperazinyl, isothiazolidinyl 1,2, dioxide, and thiomorpholinyl. Further non-
limiting
exemplary cyclic moieties are the following:
-242-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
CH3
/
"¨NO 0
0 0
0, 0 rw ki\
¨CH3
A¨Nlr N
;0)
0
[00601] The invention also provides compounds of Formula III, wherein when R4'
is a
member of the group consisting of alkyl, alkenyl, alkynyl, cycloalkyl,
heteroalkyl,
heterocycloalkyl, heterocycloalkyloxy, aryl, arylalkyl, heteroaryl,
heteroarylalkyl, acyl,
alkoxy, amido, amino, sulfonamido, acyloxy, alkoxycarbonyl, and NR'R" (wherein
R' and R"
are taken together with nitrogen to form a cyclic moiety), then R4' is
optionally substituted
with one or more of the following substituents: alkyl, alkenyl, alkynyl,
cycloalkyl,
heteroalkyl, heterocycloalkyl, heterocycloalkyloxy, aryl, arylalkyl,
heteroaryl,
heteroarylalkyl, acyl, alkoxy, amido, amino, sulfonamido, acyloxy,
alkoxycarbonyl, halo,
cyano, hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R"
are taken
together with nitrogen to form a cyclic moiety. Each of the above substituents
may be further
substituted with one or more substituents chosen from the group consisting of
alkyl, alkoxy,
amido, amino, sulfonamido, acyloxy, alkoxycarbonyl, halo, cyano, hydroxy,
nitro, oxo,
phosphate, urea, and carbonate.
[00602] For example, the invention provides compounds wherein when R4' is
alkyl, the alkyl
is substituted with NR'R" wherein R' and R" are taken together with the
nitrogen to form a
cyclic moiety. The cyclic moiety so formed can be unsubstituted or
substituted. Non-limiting
exemplary cyclic moieties includes but are not limited to morpholinyl,
azetidinyl,
pyrrolidinyl, piperidinyl, piperazinyl, isothiazolidinyl 1, 2, dioxide, and
thiomorpholinyl. In
other examples of the compounds of Formula III, when R4' is alkyl, the alkyl
is substituted
with heterocycloalkyl, which includes oxetanyl, azetidinyl, tetrahydrofuranyl,
pyrrolyl,
tetrahydropyranyl, piperidinyl, morpholinyl, and piperazinyl. All of the above
listed
heterocycloaklyl substituents can be unsubstituted or substituted.
[00603] In yet other examples of the compounds of Formula III, when R4' is
alkyl, the alkyl
is substituted with a 5, 6, 7, 8, 9, or 10 membered monocyclic or bicyclic
heteroaryl, which is
unsubstituted or substituted. The monocyclic heteroaryl includes but is not
limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazolyl. The bicyclic heteroaryl includes but is
not limited
-243-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
benzothiophenyl, benzofuryl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl, pyrazolopyrimidinyl,
purinyl, pyrrolo
[1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl, pyrazolylpyridinyl,
imidazo[1, 2-
a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl. In some embodiments of the
compound of
Formula III, W2' is N.
[00604] In some embodiments R3' and R4' taken together form a cyclic moiety.
Such a
moiety may have, for example, from 3 to 8 ring atoms. The cyclic moiety so
formed may
further include one or more heteroatoms which are selected from the group
consisting of S,
0, and N. The cyclic moiety so formed is unsubstituted or substituted. In some
embodiments,
the substituent is Ci-Cioalkyl (which includes but is not limited to -CH3, -
CH2CH3, n-propyl,
isopropyl, n- butyl, ten'- butyl, sec-butyl, pentyl, hexyl, and heptyl), or C3-
C7cycloalkyl
(which includes but is not limited to cyclopropyl, cyclobutyl, cyclopentyl,
and cyclohexyl);
heterocycloalkyl (which includes but is not limited to oxetanyl,
tetrahydrofuranyl,
pyrrolidinyl, tetrahydropyranyl, piperidinyl, and piperazinyl), C2-
Cioheteroalkyl (which
includes but is not limited to methoxyethoxy, methoxymethyl, and
diethylaminoethyl);
monocyclic heteroaryl (which includes but is not limited to pyrrolyl, thienyl,
furyl, pyridinyl,
pyranyl, pyrimidinyl, pyrazinyl, pyridazinyl, imidazolyl, thiazolyl,
pyrazolyl, and oxazoly1)
or unsubstituted or substituted monocyclic aryl. The cyclic moiety may have
one or more
substituents, which may be the same or different.
[00605] In some embodiments, the cyclic moiety formed by R3' and R4' is
substituted with at
least one of the following substituents:
TH3
/
..--"-N¨cH3 NCH NH
\
0 10 0----\) _ ___õ,...) CH3 0 --.) 1¨NO p ________ <
1 1 Y 5
OW ~I VW
5 , 5 5 5
I
i <CH3
-
õ.............N.,,CH3 . 1-N/CH3
kN
5 / 5 -CH(CH3)2 5 H, CH3
5
CH3
I
1-N
H3c 0
0
_\ _____________________________________________________________ Niii
/ ------CH3 µ3,f H N ------ --".-- 0
H 3 C
4,,,, N :3,..----...........2A ..õ...... õ1.17/,
5 / 0
5 'Z'
G, 40 40 CN
,..".....õ ,,....CH3 HI3C \
\ ( ____________________________________________________________
--- - N H 3C / \ N 0
Y y
\ --N /
0 N ______________ \ c ",õ,_...õ.= N ..,,õ...--
/ \ /0
5
-244-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
0
M Me0 N,
N I , N S )1.---- I-1 c
. .3_\ =i /.
1\1N
0 0 el I (:) N
1 ,
. , , , , ,
rs
A--N
cH2cH3
N N , 0
(----N\
, ---.,
el ,N CN .õ..---... )1-----
.
N
......---. ..-------../
rN____./ N
/\)
'z''? V ''c 0)
, 3r
, , , ,
0
_OH _ ,S02ME
.õ---- . .õõ----... ...--,,- .õ----. -- --_,
N
N N1.1---.. ,..._..../S02ME
C))
9
, C)) 0\1
i 1 \ ,i
, ,
A-- N
,
_ pH
......--..
N
I , S
I N ---S
,-\z. ------.. -.-
0 N
\Z---N ? I
"21,N
,or
S /N
I ¨Nv.... j
0 N-Ac'N-S02Me
/r\j,¨S02Me 0
0----\.) 10---) ',__-o)
1 1 "?.?-0 "?r-0
LAIN1 JUN"
I I
5 -I. 5 -2" 5
0 - / __ 0
N,CH3 N,Ac
NCH 3
\
N
ri rj ri rij
5 7-- 5 .2 5 .? 5 =2
N,S02Me ,Ac
N,S02Me
----N--CH3
N
r-1 N N
HN--\)
--0
vt7a. / Vift
5 5 5 5
-245-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
rej-CH3 rel-Ac
0 /N-Ac
IdN--\) 1-IN--\) !tr4
dr. 5 4'11. 5 5 V .
0 ro
r N_cH3 r,N.Ac
r-N _PN
-NH HN---j
/ HN---1
i HN--j
i HN
/
vl.n.,.t
5 5
0 0
H N I I-1
I H 0
\\41
N el ! 's 0
µ 0 -1\1/\3 µ,. N0 .../ µ,
0 ,, s 0..; s µ
05 oi \ /,
5 5 5 5 5`z2z 0 i'W
5
I ,:cf CN g
N ,/s \ i& 0\OH
0N-\ N ,--,0 io CN
'
0 c) / \ tw 5 0/ \ 50H3 \--/5cH3 / \c \
5 -`? *I .
[00606] In some embodiments of the compound of Formula III, W3' is CR5'. R5'
can be, for
example, hydrogen, or unsubstituted or substituted alkyl (including but not
limited to CH3, -
CH2CH3, n-propyl, isopropyl, n- butyl, tert- butyl, sec-butyl, pentyl, hexyl,
and heptyl). In
one embodiment, R5' is H. In other embodiments, R5' is unsubstituted or
substituted alkenyl
(including but not limited to unsubstituted or substituted C2-05alkenyl such
as, for example,
vinyl, allyl, 1-methyl propen-l-yl, butenyl, or pentenyl) or unsubstituted or
substituted
alkynyl (including but not limited to unsubstituted or substituted C2-
05alkynyl such as
acetylenyl, propargyl, butynyl, or pentynyl). Alternatively, R5' is
unsubstituted or substituted
aryl (including but not limited to monocyclic or bicyclic aryl) or
unsubstituted or substituted
arylalkyl (including but not limited to monocyclic or bicyclic aryl linked to
alkyl wherein
alkyl includes but is not limited to CH3, -CH2CH3, n-propyl, isopropyl, n-
butyl, sec-butyl,
and pentyl). In some other embodiments, R5' is unsubstituted or substituted
heteroaryl,
including but not limited to monocyclic and bicyclic heteroaryl. Monocyclic
heteroaryl R5'
includes but is not limited to pyrrolyl, thienyl, furyl, pyridinyl, pyranyl,
pyrimidinyl,
pyrazinyl, pyridazinyl, imidazolyl, thiazolyl, pyrazolyl, and oxazolyl.
Bicyclic heteroaryl R5'
includes but is not limited to benzothiophenyl, benzofuryl, indolyl,
quinolinyl, isoquinolinyl,
benzimidazolyl, benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl,
pyrazolopyrimidinyl,
purinyl, pyrrolo [1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl,
pyrazolylpyridinyl,
imidazo[1, 2-a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl. In some
embodiments of the
compound of Formula III, W3' is N. In other embodiments, W3' is S.
-246-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00607] In some embodiments of the compound of Formula III, W4' is C. In other
embodiments, W4' is N.
[00608] In some embodiments of the compound of Formula III, W5' is N. In other
embodiments of the compound of Formula III, W5' is CR7. R7 can be, for
example, hydrogen,
or unsubstituted or substituted alkyl (including but not limited to CH3, -
CH2CH3, n-propyl,
isopropyl, n- butyl, ten'- butyl, sec-butyl, pentyl, hexyl, and heptyl). In
one embodiment, R7
is H. In other embodiments, R7 is unsubstituted or substituted alkenyl
(including but not
limited to unsubstituted or substituted C2-05alkenyl such as, for example,
vinyl, allyl, 1-
methyl propen-l-yl, butenyl, or pentenyl) or unsubstituted or substituted
alkynyl (including
but not limited to unsubstituted or substituted C2-05alkynyl such as
acetylenyl, propargyl,
butynyl, or pentynyl). Alternatively, R7 is unsubstituted or substituted aryl
(including but not
limited to monocyclic or bicyclic aryl) or unsubstituted or substituted
arylalkyl (including but
not limited to monocyclic or bicyclic aryl linked to alkyl wherein alkyl
includes but is not
limited to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, sec-butyl, and
pentyl). In some other
embodiments, R7 is unsubstituted or substituted heteroaryl, including but not
limited to
monocyclic and bicyclic heteroaryl. Monocyclic heteroaryl R7 includes but is
not limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazolyl. Bicyclic heteroaryl R7 includes but is not
limited to
benzothiophenyl, benzofuryl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl, pyrazolopyrimidinyl,
purinyl, pyrrolo
[1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl, pyrazolylpyridinyl,
imidazo[1, 2-
a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl.
[00609] In some embodiments of the compound of Formula III, W6' is N. In other
embodiments of the compound of Formula III, W6' is CR8'. R8' can be, for
example, hydrogen,
or unsubstituted or substituted alkyl (including but not limited to CH3, -
CH2CH3, n-propyl,
isopropyl, n- butyl, ten'- butyl, sec-butyl, pentyl, hexyl, and heptyl). In
one embodiment, R8'
is H. In other embodiments, R8' is unsubstituted or substituted alkenyl
(including but not
limited to unsubstituted or substituted C2-05alkenyl such as, for example,
vinyl, allyl, 1-
methyl propen-l-yl, butenyl, or pentenyl) or unsubstituted or substituted
alkynyl (including
but not limited to unsubstituted or substituted C2-05alkynyl such as
acetylenyl, propargyl,
butynyl, or pentynyl). Alternatively, R8' is unsubstituted or substituted aryl
(including but not
limited to monocyclic or bicyclic aryl) or unsubstituted or substituted
arylalkyl (including but
not limited to monocyclic or bicyclic aryl linked to alkyl wherein alkyl
includes but is not
limited to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, sec-butyl, and
pentyl). In some other
-247-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
embodiments, R8' is unsubstituted or substituted heteroaryl, including but not
limited to
monocyclic and bicyclic heteroaryl. Monocyclic heteroaryl R8' includes but is
not limited to
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazolyl. Bicyclic heteroaryl R8' includes but is
not limited to
benzothiophenyl, benzofuryl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl, pyrazolopyrimidinyl,
purinyl, pyrrolo
[1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl, pyrazolylpyridinyl,
imidazo[1, 2-
a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl.
[00610] In some embodiments of the compound of Formula III, W7' is C. In other
embodiments, W7' is N.
[00611] The invention also provides compounds of Formula III which are defined
as defined
by the following subclasses:
R1
R8 R8
R4 _________________ ?1\ ri_jR2 R= 4
N,
z x 0
R3 R3
Subclass Ma Subclass Mb
R1 R1
R4 0 11__yR2 R= 4 __ c)N 0 __ R2
( 0
R R7 ________________________________ R3 R7 \µ
Subclass Mc Subclass IIId
R8
N ,/R8 NR
R4¨ 0 CD ________________________ R4¨(0
0
R3 R7 R3 R7 ___
Subclass Me Subclass IIIf
)R1 R1
N- \/ISfr3
R4 _________________ ?P ;IR2 R= 4 __ ?I\LI 0 ___ N R2
x \
R3 R3
Subclass Mg Subclass IIIh
-248-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Ri R1
NN N1C3N
R4 _________________ ?._N 1 iii-yR2 R4 __ 19,N 1 l_cr R2
R3 z X
R7 R3 z 0
R7 ______________________________________________
Subclass IIIi Subclass IIIj
R8
R4 _________________ ?
N.---C\_lYR2 R4 _______________________ 70NR8z 1\i'
i____YR2
R3 z X
R3 0
R7 R7 5
Subclass IIIk Subclass 1111
wherein for each of the above formulas, each respective R variable includes a
'prime' (').
[00612] In some embodiments of compounds of Subclasses Ma - Illj, R1' is
hydrogen. In
other embodiments of compounds of Subclasses Ma - 1111, R2' is NH2 of
NHCO(alkyl). In
other embodiments of compounds of Subclasses Ma - 1111, R4' is hydrogen. In
other
embodiments of compounds of Subclasses Inc - IIIf and IIIi - 1111, R7' is
hydrogen. In other
embodiments of compounds of Subclasses Illa - IIIh and IIIk - 1111, R8' is
hydrogen.
[00613] In some embodiments of compounds of Subclasses Ma through 1111, R3' is
alkyl,
heteroalkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl,
heteroaryl,
heteroarylalkyl, alkoxy, heterocycloalkyloxy, amido, amino, acyl, acyloxy,
alkoxycarbonyl,
sulfonamido, halo, cyano, hydroxy, nitro, phosphate, urea, carbonate, or NR'R"
wherein R'
and R" are taken together with nitrogen to form a cyclic moiety. R3' can be,
for example,
hydrogen, unsubstituted or substituted alkyl (including but not limited to
CH3, -CH2CH3, n-
propyl, isopropyl, n- butyl, ten'- butyl, sec-butyl, pentyl, hexyl, and
heptyl). In other
embodiments, R3' is unsubstituted or substituted alkenyl (including but not
limited to
unsubstituted or substituted C2-05alkenyl such as, for example, vinyl, allyl,
1-methyl propen-
l-yl, butenyl, or pentenyl) or unsubstituted or substituted alkynyl (including
but not limited to
unsubstituted or substituted C2-05alkynyl such as acetylenyl, propargyl,
butynyl, or
pentynyl). Alternatively, R3' is unsubstituted or substituted aryl (including
but not limited to
monocyclic or bicyclic aryl) or unsubstituted or substituted arylalkyl
(including but not
limited to monocyclic or bicyclic aryl linked to alkyl wherein alkyl includes
but is not limited
to CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, sec-butyl, and pentyl). In
some other
embodiments, R3' is unsubstituted or substituted heteroaryl, including but not
limited to
monocyclic and bicyclic heteroaryl. Monocyclic heteroaryl R3' includes but is
not limited to
-249-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
pyrrolyl, thienyl, furyl, pyridinyl, pyranyl, pyrimidinyl, pyrazinyl,
pyridazinyl, imidazolyl,
thiazolyl, pyrazolyl, and oxazolyl. Bicyclic heteroaryl R3' includes but is
not limited to
benzothiophenyl, benzofuryl, indolyl, quinolinyl, isoquinolinyl,
benzimidazolyl,
benzoxazolyl, benzothiazolyl, quinazolinyl, azaindolyl, pyrazolopyrimidinyl,
purinyl, pyrrolo
[1, 2-b]pyridazinyl, pyrrolopyrimidinyl, indazolyl, pyrazolylpyridinyl,
imidazo[1, 2-
a]pyridinyl, and pyrrolo[1, 2-f][1, 2, 4]triazinyl. The present invention also
provides
compounds of Formula II wherein R3' is unsubstituted or substituted
heteroarylalkyl,
including but not limited to monocyclic and bicyclic heteroaryl as described
above, that are
linked to alkyl, which in turn includes but is not limited to CH3, -CH2CH3, n-
propyl,
isopropyl, n- butyl, sec-butyl, and pentyl. In some embodiments, R3' is
unsubstituted or
substituted cycloalkyl (including but not limited to cyclopropyl, cyclobutyl,
and cyclopentyl)
or unsubstituted or substituted heteroalkyl (non-limiting examples include
ethoxymethyl,
methoxymethyl, and diethylaminomethyl). In some further embodiments, R3' is
unsubstituted
or substituted heterocycloalkyl which includes but is not limited to
pyrrolidinyl,
tetrahydrofuranyl, piperidinyl, tetrahydropyranyl, thiazolidinyl,
imidazolidinyl, morpholinyl,
and piperazinyl. In yet other embodiments of the compounds of Formula II, R3'
is
unsubstituted or substituted alkoxy including but not limited to Ci-C4alkoxy
such as
methoxy, ethoxy, propoxy or butoxy. R3' can also be unsubstituted or
substituted
heterocycloalkyloxy, including but not limited to 4-NH piperidin-l-yl-oxy, 4-
methyl
piperidin-l-yl-oxy, 4-ethyl piperidin-l-yl-oxy, 4-isopropyl- piperidin-l-yl-
oxy, and
pyrrolidin-3-yl-oxy. In other embodiments, R3' is unsubstituted or substituted
amino, wherein
the substituted amino includes but is not limited to dimethylamino,
diethylamino, di-
isopropyl amino, N-methyl N-ethyl amino, and dibutylamino. In some
embodiments, R3' is
unsubstituted or substituted acyl, unsubstituted or substituted acyloxy,
unsubstituted or
substituted Ci-C4acyloxy, unsubstituted or substituted alkoxycarbonyl,
unsubstituted or
substituted amido, or unsubstituted or substituted sulfonamido. In other
embodiments, R3' is
halo, which is ¨I, -F, -Cl, or -Br. In some embodiments, R3' is selected from
the group
consisting of cyano, hydroxy, nitro, phosphate, urea, and carbonate. Also
contemplated are
R3' being -CH3, -CH2CH3, n-propyl, isopropyl, n- butyl, ten'- butyl, sec-
butyl, pentyl, hexyl,
heptyl, -OCH3, -OCH2CH3, or -CF3. In some embodiments R3' can also be NR'R"
wherein R'
and R" are taken together with the nitrogen to form a cyclic moiety having
from 3 to 8 ring
atoms. The cyclic moiety so formed may further include one or more heteroatoms
which are
selected from the group consisting of S, 0, and N. The cyclic moiety so formed
is
unsubstituted or substituted, including but not limited to morpholinyl,
azetidinyl, pyrrolidinyl,
-250-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
piperidinyl, piperazinyl, isothiazolidinyl 1,2, dioxide, and thiomorpholinyl.
Further non-
limiting exemplary cyclic moieites are the following:
c1H3
"-NO jN
\ _______________________________ /0 __V-N/
2' ,
0 0
0, 0 -C1-1/
A-NI( .a
`VI\L)
\.N,)
0 ,
[00614] The invention further provides a PI3Ka inhibitor which is a compound
of Formula
IV:
R1'
YV2.
R7X wi'
or its pharmaceutically acceptable salts thereof, wherein
W1' is CR3', W2' is C-benzoxazolyl substituted with R2' and W3' is S;
W1' is CR3', W2' is C-benzoxazolyl substituted with R2' and W3' is CR5';
W1' is N or NR3', W2' is CR4', and W3' is C-benzoxazolyl substituted with R2;
W1' is CR3', W2' is CR4', and W3' is C-benzoxazolyl substituted with R2; or
W1' is N or NR3', W2' is NR4', and W3' is C-benzoxazolyl substituted with R2;
X is N;
R1' and R2' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
R3' and R4' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
-251-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
R5', R6' ,R7 and R8' are independently hydrogen, alkyl, heteroalkyl, alkenyl,
alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety.
[00615] In some embodiments of the compound of Formula IV, the compound is:
0-..r R2'
R1 10 N
R6'
0 0 YV2.
RT X W1
and wherein Wi' is CR3' or NR3' and W2' is CR4'.
[00616] In another aspect, the invention provides a PI3Ka inhibitor which is a
compound of
Formula V:
R1'
\Ai
W3 wu
1 0 0
w2,.......... ...../.............
....././"...õ.............õ..............wd
w1' W6
1 0> _______ R2'
wb'
Wd'
wa'
Formula V
or its pharmaceutically acceptable salts thereof, wherein:
W1' is N, NR3', CR3', or C=0; W2' is N, NR4', CR4', or C=0; W3' is N, NR5' or
CR5';
W4' is N, C=0 or CR6', wherein no more than two N atoms and no more than two
C=0 groups
are adjacent;
W5' is N or CR7';
W6' is N or CR8';
Wa and Wb' are independently N or CR9';
one of Wc' and Wi' is N, and the other is 0, NR10', or S;
-252-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
R1' and R2' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
R3' and R4' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
or R3' and R4' taken together form a cyclic moiety;
R5', R6' , R7' and R8' are independently hydrogen, alkyl, heteroalkyl,
alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
R9' is alkyl or halo; and
R10' is hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl,
aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy, heterocycloalkyloxy,
amido, amino, acyl,
acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano, hydroxy, nitro, phosphate,
urea,
carbonate, or NR'R" wherein R' and R" are taken together with nitrogen to form
a cyclic
moiety.
[00617] In some embodiments of the compound of Formula IV, Wi' is CR3', W2' is
CR4', W3'
is CR5', V' is N, W5' is CR7', and W6' is CR8'; W1' is N, W2'' is CR4', W3' is
CR5', W4' is N, W5'
is CR7', and V' is CR8'; or W1' is CR3', V' is N, W3' is CR5', V' is N, W5' is
CR7', and W6' is
CR8'. In some embodiments of the compound of Formula IV, Wb' is N. In other
embodiments,
Wa' is CR9' and R9' is alkyl.
-253-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00618] The invention also provides a PI3Ka inhibitor which is a compound of
Formula VI:
R1'
W7W6'
"c2. 0
/ ac
Wu
0> ______________________________________________________ RZ
wb'
wd'
wa'
Formula VI
or its pharmaceutically acceptable salts thereof, wherein
W1' is S, N, NR3' or CR3', W2' is N or CR4', W3' is S, N or CR5', W4' is N or
C, and
W.7' is N or C, wherein no more than two N atoms and no more than two C=0
groups are
adjacent;
W5' is N or CR7';
W6' is N or CR8';
Wa and Wb' are independently N or CR9';
one of Wc' and is N, and the other is 0, NR10', or S;
R1' and R2' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
R3' and R4' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
or R3' and R4' taken together form a cyclic moiety;
R5', R7' and R8' are independently hydrogen, alkyl, heteroalkyl, alkenyl,
alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
-254-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
R9' is alkyl or halo; and
R10' is hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl,
aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy, heterocycloalkyloxy,
amido, amino, acyl,
acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano, hydroxy, nitro, phosphate,
urea,
carbonate, or NR'R" wherein R' and R" are taken together with nitrogen to form
a cyclic
moiety.
[00619] In some embodiments of the compound of Formula VI, Wi' is CR3', W2' is
CR4', W3'
is N, W4' is N, W5' is CR7', and W6' is CR8'. In other embodiments, W1' is
CR3', W2' is CR4',
W3' is N, W4 is N, W5' is CR7', and W6' is CR8'. In other embodiments, W1' is
CR3', W2' is
CR4', W3' is N, W4' is N, W5' is N, and W6' is CR8'. In still other
embodiments, W1' is NR3', W2'
is CR4', W3' is N, W4' is C, W5' is CR7', and W6' is CR8'. In other
embodiments, W1' is S, W2' is
CR4', W3' is N, W4' is C, W5' is CR7', and W6' is CR8'. In other embodiments,
W1' is CR3', W2'
is CR4', W3' is S, W4' is C, W5' is N, and W6' is N.
[00620] In some embodiments of the compound of Formula VI, Wb' is N. In other
embodiments, Wa' is CR9' and R9' is alkyl.
[00621] The invention further provides PI3Ka inhibitors which are compounds of
Formula
VI-A and VI-B:
RI RI
N, /N
N
is N
VV1 ' 40 2. W 1
R
R2.
0 Or 0
Formula VI-A Formula VI-B
or its pharmaceutically acceptable salts thereof, wherein
W1' is CR3';
R1' and R2' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
and R3' is hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy,
heterocycloalkyloxy,
amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano,
hydroxy, nitro,
-255-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
phosphate, urea, carbonate, or NR'R" wherein R' and R" are taken together with
nitrogen to
form a cyclic moiety.
[00622] Also provided herein are PI3Ka inhibitors which are compounds of
Formula VI-C
and VI-D:
R1' R1'
Wi w5' Wi
R2' 0> __ R2'
Vv \/1' d'
wa' v v
Or W
Formula VI-C Formula VI-D
or its pharmaceutically acceptable salts thereof, wherein
W1' is CR3';
W5' is N or CR7';
Wa and Wb' are independently N or CR9';
one of Wc' and is N, and the other is 0, NR10', or S;
R1' and R2' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
R3' is hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl,
aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy, heterocycloalkyloxy,
amido, amino, acyl,
acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano, hydroxy, nitro, phosphate,
urea,
carbonate, or NR'R" wherein R' and R" are taken together with nitrogen to form
a cyclic
moiety;
R7' is hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl,
aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy, heterocycloalkyloxy,
amido, amino, acyl,
acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano, hydroxy, nitro, phosphate,
urea,
carbonate, or NR'R" wherein R' and R" are taken together with nitrogen to form
a cyclic
moiety;
R9' is alkyl or halo; and
R10' is hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl,
aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy, heterocycloalkyloxy,
amido, amino, acyl,
acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano, hydroxy, nitro, phosphate,
urea,
-256-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
carbonate, or NR'R" wherein R' and R" are taken together with nitrogen to form
a cyclic
moiety.
[00623] In some embodiments of the compound of Formula V-C or V-D, Wb' is N.
In other
embodiments, Wa is CR9' and R9' is alkyl.
[00624] Also provided herein is a PI3Ka inhibitor which is a compound of
Formula VII:
RI
/N----
w2'
wa'
\\\A/1'-¨R1(:).
1 .,.
-:/.'"------D11'
N 's
or its pharmaceutically acceptable salts thereof, wherein
W1' is CR3'; W2' is CR4';
Wa' is CH or N;
R1' is hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl,
aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy, heterocycloalkyloxy,
amido, amino, acyl,
acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano, hydroxy, nitro, phosphate,
urea,
carbonate, or NR'R" wherein R' and R" are taken together with nitrogen to form
a cyclic
moiety;
R3' is alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl, heterocycloalkyl,
aryl,
arylalkyl, heteroarylalkyl, alkoxy, heterocycloalkyloxy, amido, amino, acyl,
acyloxy,
alkoxycarbonyl, sulfonamido, halo, cyano, hydroxy, nitro, phosphate, urea,
carbonate, or
NR'R" wherein R' and R" are taken together with nitrogen to form a cyclic
moiety;
R4' is hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl,
aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy, heterocycloalkyloxy,
amido, amino, acyl,
acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano, hydroxy, nitro, phosphate,
urea,
carbonate, or NR'R" wherein R' and R" are taken together with nitrogen to form
a cyclic
moiety;
or R3' and R4' taken together form a cyclic moiety; and
R10' and R11' are independently hydrogen, alkyl, heteroalkyl, alkenyl,
alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety.
-257-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00625] The invention further provides a PI3Ka inhibitor which is a compound
of Formula
VIII:
z,N ,.....õ¨ X4
x1 x C\1\ic'
X2 3 x5
I 0> __ NR1'R2
\A/I d'
wa W
or a pharmaceutically acceptable salt thereof, wherein
Xi is CR3', NR3', or S;
X2 is CR4', NW', CR4' ¨CR5', or CR4' ¨NW';
X3 and X4 are independently C or N;
X5 is CR6', NR6'5 or S;
X4 is CR75 NR75 CR7' ¨CR8', or CR7' ¨NR8;
Wa' and Wb' are independently N or CR9';
one of Wc' and Wi' is N5 and the other is 0, NR10', or S;
R1' and R2' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
R3' and R4' are independently hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl,
cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl, heteroarylalkyl,
alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
or R3' and R4' taken together form a cyclic moiety;
R5', R6' 5 R7', and R8' are independently hydrogen, alkyl, heteroalkyl,
alkenyl,
alkynyl, cycloalkyl, heterocycloalkyl, aryl, arylalkyl, heteroaryl,
heteroarylalkyl, alkoxy,
heterocycloalkyloxy, amido, amino, acyl, acyloxy, alkoxycarbonyl, sulfonamido,
halo, cyano,
hydroxy, nitro, phosphate, urea, carbonate, or NR'R" wherein R' and R" are
taken together
with nitrogen to form a cyclic moiety;
R9' is alkyl or halo; and
R10' is hydrogen, alkyl, heteroalkyl, alkenyl, alkynyl, cycloalkyl,
heterocycloalkyl,
aryl, arylalkyl, heteroaryl, heteroarylalkyl, alkoxy, heterocycloalkyloxy,
amido, amino, acyl,
-258-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
acyloxy, alkoxycarbonyl, sulfonamido, halo, cyano, hydroxy, nitro, phosphate,
urea,
carbonate, or NR'R" wherein R' and R" are taken together with nitrogen to form
a cyclic
moiety.
[00627] In some embodiments of the compound of Formula VIII, Wb' is N. In
other
embodiments, Wa' is CR9' and R9' is alkyl.
[00628] In some embodiments, the mTor inhibitor is a compound as described in
U.S. Patent
Nos. 7,651,687 or 7,585,868; or as described International Patent Applications
WO
2007/079164, WO 2007/061737, WO 2007/106503, WO 2007/134828 or W02011/025889
which are hereby incorporated by reference in their entirety.
[00629] In other embodiments, the mTor inhibitor is NVP-BEZ235 (Novartis),
BGT226
(Novartis), XL765 (Sanofi-Aventis, Exelixis), GDC0980 (Genentech), SF1126
(Semafore),
PKI587 (Wyeth), PF04691502 (Pfizer), or G5K2126458 (GlaxoSmithKline). In still
other
embodiments, the mTor inhibitor is CC223 (Celgene), 0SI027 (OSI
Pharmaceuticals),
AZD8055 (Astra Zeneca), AZD2014 (Astra Zeneca), or Palomid 529 (Paloma
Pharmaceuticals).
[00630] Structures of exemplary mTor inhibitors are shown below:
mTor inhibitor Structure
NVP-BEZ235
N
1
N
XL765
0
cvd/
/77..õ_N
A
NH
HN¨ H7,, ,I¨NH (
6
o
-259-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
GDC0980 WO 0
N
\(,
N
SF1126
0,
0 -1,
FIN
07,r0
fiAN
µYa
1111,
PK1587
Piirz.
. t.
ti
PF04691502
z=Ir
t.
GSK2126458
0 N
-
CC N. tar
-260-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
OSI027
^ :
N
/
N /^ =N
AZD8055
1
rs"-- N
HONNI
N-Th
P529 0
Reaction Schemes - PI3Ka Inhibitor Compounds
[00631] In general, compounds of the invention may be prepared by the
following reaction
scheme:
Scheme A':
R1 OG R2 R1"
6.
GO ( W \IY) 0 W __
WI 3bWC:0 V16' WV W w5 __
W'
W w / WIT
Formula B W W
Formula A Formula C
[00632] For example, compounds of the invention may be prepared by the
following
reaction schemes:
Scheme A":
R1'
R1' OG
,N,,,r R2
3,vv4 6. \ 3.&õ, 6.
2.
V73, 011 ______________________ GO OVr
W1r5 W w5r-L ¨
W W
Formula B
Formula A Formula C
-261-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Scheme B':
OG R2
1\1-..y- Fr
vA,3' B
GO * X 6'
A2027 w6' V\420V(40 NR2
40
W1 W Ti w5 X
Formula B
Formula A Formula C
[00632] The compounds of the invention may be synthesized via a reaction
scheme
represented generally in Schemes A', A" and B'. The synthesis proceeds via
coupling a
compound of Formula A with a compound of Formula B to yield a compound of
Formula C.
The coupling step is typically catalyzed by using a palladium catalyst,
including but not
limited to palladium tetrakis (triphenylphosphine). The coupling is generally
performed in
the presence of a suitable base, a nonlimiting example being sodium carbonate.
One example
of a suitable solvent for the reaction is aqueous dioxane.
[00633] A compound of Formula A for use in Scheme A' or A" has a structure of
Formula A,
wherein T1 is halo including bromo, chloro, fluoro, and iodo, and wherein the
remaining
substituents are defined for Formulas I and II of compounds of the invention.
For boronic
acids and acid derivatives as depicted in Formula B, X is either 0 or S, and
the benzoxazole
or benzothiazole moiety can be attached at the 4-, 5-, 6- or 7- position.
[00634] For a compound of Formula B, G is hydrogen or RGi, wherein RGi is
alkyl, alkenyl,
or aryl. Alternatively, B(OG)2 is taken together to form a 5- or 6- membered
cyclic moiety.
In some embodiments, the compound of Formula B is a compound having a
structure of
Formula E:
GO
GO
*N
0 H
Formula E
[00635] wherein G is H or RGi; RGi is alkyl, alkenyl, or aryl. Alternatively,
B(OG)2 is taken
together to form a 5- or 6- membered cyclic moiety; and RG2 is H, tert- butyl
carbamate, or
acyl.
Scheme C':
RGiO\ HO
B¨M B¨M
T2 ¨M
RG10 HO
Formula D Formula B' Formula B"
-262-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
[00636] Scheme C' depicts an exemplary scheme for synthesizing a compound of
Formula
B' or, optionally, Formula B" for use in Reaction Scheme C'. M is a
heterocyclic moiety such
as a benzoxazolyl or benzothiazolyl moiety as described by Formula B. This
reaction
proceeds via reacting a compound of Formula D with a trialkyl borate or a
boronic acid
derivative to produce a compound of Formula B'. The trialkyl borate includes
but is not
limited to triisopropyl borate and the boronic acid derivative includes but is
not limited to
bis(pinacolato)diboron. The reaction typically is run in the presence of a
base, a nonlimiting
example being potassium acetate. The reaction may be run in a solvent such as
dioxane or
tetrahydrofuran.
[00637] A compound of Formula D for use in Scheme C' is a compound wherein T2
is halo
or another leaving group, and M is as defined above. The compound of Formula
B' may
further be converted to a compound of Formula B" by treatment with an acid
such as
hydrochloric acid.
[00638] Some exemplary compounds of Formula B that can be synthesized via
Scheme C'
include but are not limited to compounds of the following formulae:
0
7 NNHCOCH3 0
. 0 Os
N
c10 0 -t0:13 = 0 0 -----. 0,B 41*
0
) c
N N -,
N NH2 0,B
1---O
NHCOCH3
H
H-7 F-7 G-6 1-4
I\1)¨N H2 4r, 1\1)-1\11-12
0--B 1110 0 Ll--- y IPS 0 1>c I\I N H2-O cl
1 HOB*
I
OH 0
G-7 G-8 G-9
1---R )--? HN ________________ 4 )----o
1 HN-CH3
B 40 0--B
o-B 10 ,'N 0--
\ N \ N
o/ 0 oi
NHCOC H3
J-4 K-6 L-6
0 r\INHCOCH3 HO\ HO\ 0 0;N
HO - Y 0
Ho'B 41
HOB
OH N N N NH2 I NHCOCH3
H OH
H-7-B F-7-B G-6-B 1-4-B
-263-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
FIR OH
I HN --4 OH
I HN -CH3
HO-B 0 Ck 1N OH B le \ OH
B lei \ N
oi
N
0
NHCOCH3
J-4-B K-6-B L-6-B
[00639] Where desired, deprotection of a substituent (e.g., removal of Boc
protection from
an amino substituent) on the benzoxazolyl moiety (i.e. M1 of Formula C) is
performed after
coupling the compound of Formula B to the compound of Formula A.
[00640] Some exemplary compounds with such protecting groups, include but are
not
limited to compounds of the following formulae:
¨p
HO 0 0 0
j--- 0 Y 0 H 2\
HO
-I 01 11)¨NJO CIYI 401 NiJ0- (:),_
5
101 N)-N-1
0-p
HOB * 1)-1\11-119.1_
I I ,or OH .
[00641] The following Reaction Schemes illustrate the preparation of several
compounds of
the invention.
-264-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Scheme D': Synthesis of 5-(7-(3-(4-isopropylpiperazin-l-yDazetidin-l-y1)-1,5-
naphthyridin-2-
Abenzo[d]
oxazol-2-amine
N
!
Br2 , Na0Ac !N m-CPBA
I
\rsr AcOH, RT to 90 C Br N Br N-
DCM 1
C-1 C-2 C-30
N
TsCI,K2CO3 N-. 4-Methoxybenzylchloride !
I
DCM, I-120 I , NaH, DMF
_________________________________________________ D. Br N 0
-I" Br N /0
RT H RT overnight
C-4
C-5 . e
OH
/*
I
N HCI N N
H !
I 303 N
!
I
Pc12(dba)3,Xantphos C.ININ 0
Et3 N, DM SO NNON 0
Cs2CO3, dioxane , HO ________________________ k
*reflux RT 0.----j
0 110
C-6 C-7 0
rNH
),N
I
I TFA
NaHB(0Ac)3, r . AcOH N O C...pNr$0
-31.. N H
DCM, reflux r le---I * reflux
N
C-8 e IN C-9
HO
L
P
-OH N
Isl. N
I
POCI3 .iC.N--.'' N*-C I (1 .2eg.) C.IN N
-b... ___________________________________________ Ii. rN
0
reflux 1 h rrs,
Pd(PPh3)4, Na2CO3 N.,..)
--=---(
)N 72 N-
C-10 dioxane/H20 NH2
-265-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Scheme E': Synthesis of 2-amino-1-(4-(6-(2-aminobenzo[d]oxazol-5-y1)-1,5-
naphthyridin-3-y1)
piperazin-l-y1) -2-methylpropan-1-one
0
H2N Ao ,N HCI(con)
I
Boc, I _________________ 7.-
Br INI Pd2(dba)3, Xantphos N
H
e Me0H H2NINI-
C-2
CS2C037dioxane 0-12 0-13
reflux 16h
NaN037HCI(con) INI m-CPBA, CHCI3 INI TsCI, K2CO3
I it DCM,H20
KI, H20 lit INO
RT, 2h
I H
O-14 D-1? RT 0-16
r NH
,N) INI
POC ___________ rN Boc I3 I HCI
/Me0H
xi- rNNCI __
INCIa-
reflux lh Pd2(dba)3, Xantphos N) RT 2h
O-17 Cs2C037dioxane Boo 0-18
reflux overnight
HO
lo l'ELOH
00H N
INI INI
H2N___Zi
I Boc,N I so
rNNCI H ). ON N---NCI DA/DM. I M rn
I- ukr- I- 1 13147 via2,7%.3
HINI) EDCI 7 HOBt __________________________________ a
0-19 0-20
dioxane/H20
Et3N 7 DCM Boc,N
RT
H reflux 2h
N N
I I õ
rN , Isr 10
N HCI /Me0H rN N- 0
O)
Boc,N 14----=( RT 2h
H2N< N----:--(
H NH2 43 NH2
0-21
-266-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Scheme F': Synthesis of 5-(3-morpholinopyrido[2,3-b]pyrazin-6-
yl)benzo[d]oxazol-2-amine
H 0
NE12Raney nickel Me0H NH2 Ethyl bromoacetate Nj-ci
....,... ,/,.. .0
CI N N - RT 24h CI N NH2 DMF, K2CO3
...õ...., .. .-.-..,.
CI N NH2
8 RT to 60 C
E-23 E-24 E-25
H
/1%1
NaH, 1,4-dioxane 1 Mn02, 1,4-dioxane POCI3
______________ a 1
CI NN ¨) - CI -NN0
reflux H reflux 1h H reflux 3h
E-26 E-27
HO
6-OH N
1 I
rsi morpholine, Et3N r.N H2N--- 11106 1
2
I õ N N N
0 1
_......._ ..-)..--..... .-- ,...-õ1 p.
CINNCI CI¨N N N 0
DCML,...,,,0 Pd(PPh3)4 0
E-28 E-29 Na2CO3 )----=-N 2
dioxane/H20 H2N
Scheme G': Synthesis of 5-(6-morpholino-1,5-naphthyridin-3-yl)benzo[d]oxazol-2-
amine
N N morpholine
! POCI3 !
I _________________________________________________________ W.
Br N 0 reflux
BrNCI sealed tube
H 140 C overnight
C-4 F-31
HO
b-OH
N
H2N__ * N
N 0 (1.2eq.) \
! 1
I _3...
BrNN Pd(PPh3)4 Na2CO3
01 ic N
0
dioxane/H20 0
).--L---N
73
F-32 reflux 2h H2N
-267-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
Reaction Scheme H':
.......Q40B
N N
0
0 \>-NH2 cN ..-
N
H2N,0, Cl"--.'CHO N..,.... 0 ...
I ______________________ 2
111
Br Et0H, reflux, 6 h Br Pd(PPh3)43Na2CO3
dioxane I H20 0
Reflux, 2 h N-,----.(
NH2
HO
B ---
NBS, DMF N-
HO- 0====. N-
, N / 411,
________________ ,.... y 4 4 1 / N 0
RT, 5 h 0
N'-js,
',... NH2
Br
N=1\ Pd(PPh3)4, Na2CO3
I
NH2 dioxane / H20
Reflux, 2 h N
Reaction Scheme I':
00
)0......, 14,..-.. Na0H(4N), Et0H
Bry-1 CI \ i!, 50 C, 24 h N.......,
."--;-----.'Br
Et0H, seal-tube ¨.--N ,Br
N NH2 200 C, 24 h 00
COH
H
N
0 CN)
ii N.,.....õ
CICI
B r DCM -:--- Br
x
100 C, 0.5 h 0 CI RT, 10 min 0
N--"\
c____ /
N \
OH
HOB,
14)-NH2 N....
0
N
Pd(PPh3)4, Na2CO3 ..-N /
s
dioxane / H20 0
r N---\ 0
100 C, 1 h
N \
-268-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
Reaction Scheme J':
0 0 Et20 00
CI -'o- + 11-0----- + Na-0------ -).- 11,,.0
RT /3 days
CI
Brn
N1 e..
N- NH2
0
Et0H Br 3)r0 NaOH (4N), Et0H IN r OH
\-- - __
80 C, 2 h 50 C, 24 h
Br
0 0
H
N
0CN)
N N__.--0,--
CI-SII-C1 i
0 DCM
N r Br
CI a
100 C, 2 h RT, 10 min
Br 0
70B toN,_NH2 N..._ ,..õ
0
Pd(PPh3)4, Na2CO3
dioxane / H20 0 _NEI2
100 C, 1h
Reaction Scheme K':
00
Na0H(4N), Et0H
D 0
CI N-----1N 50 C, 24 h N.....-..N
Brisl,,,,..
Et0H \ N
Ikl-NH2 ) Br ________ r o OH
150 C, 24 0
0----\
H
0 N
N.,......N Co) Ikl,......N
CI CI DCM
)---N
---- Br
____________________ D. ---- Br a
100 C, 0.5 h 0 RT, 10 min 0
CI N¨)
..-c,
OH
HO b
SO N,-NH2
r N
0 \ N / N
Pd(PPh3)4, Na2CO3
dioxane / H20 0 40 _NEI2
i,... 0
10000, 1 h NTh
__.-cl
-269-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Reaction Scheme L':
l'I
so NH2
H2N 0
Cl"----CHO Isk-, 0-B .
)
N1
Et0H, reflux, 6 h
µ14--- -N CI
Pd(PPh3)4, Na2CO3
CI dioxane / H20
Relux, 2 h
H9
N
N N _ HOB
t\-_, \
N=N--- NBS, DMF $ N
::.
01/ RT, 5 h r
Br N,,
4101a-
Pd(PPh3)4, Na2CO3
--z_-0 0 dioxane / H20
N
Isl-=< Relux, 2 h
NH2 NH2
--...
N-
',....ljsl-N/ 11
0
lei\NH2
i \
I
Isr
Reaction Scheme M':
00
u u
Th0'.Na0H(4N), Et0H
N., N --
CI ,14-"-<"1 50 C, 24 h
Et0H CI ________ N.,....õ..
CI
...-
r yo....-.N.,N-;..,-..,CI
-,N 80 C, 24 h
H2NN 0
0-\ 0
OH
H
N
9 (N) N.........r:-..õ
N.....-....(:-..õ
I
...-N,
CIS'CI
DCM N ci
0
100 C, 0.5 h 0 RT, 10 min
N\
OH
HOB
01 N)-NH2 N.., ..õ
0
...--N,N N
Pd(PPh3)4, Na2CO3
dioxane / H20 0 0 ¨NH2
..- N--"N 0
100 C, 1 h
N \
-270-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Reaction Scheme N':
o
CI N........ N....-...
,KCI
Et0H, sealed-tube,¨S_A!j , NIS, DMF 3..... \ Ill ,
1 11,1 'N CI 80 C, 5 h N CI
H2N N-- 200 C, 24h
I
--7? (:)2 401 N"-NH2
HO,B_OH
N,...-..., N.,
N \ N 0
Pd(PPh3)4, Na2CO3L-J Pd(PPh3)4, Na2CO3 \ N,N-- 0 N
dioxane / H20 , dioxane / H20 ¨NH2
a
0
100 C, 1h \ / 100 C, 1h \ /
N N
Reaction Scheme 0':
ci
H 0
N ,0
r.-....0 r
N Bt H2N Ise N N,....-..
\ N,
140 N:N lz----0 0> H ZnBr2, DCE N CI
)....- )....-
Ethanol, RT, 24 h Bt N¨
H 60 C, 4 h
0 0
A)
o 01 N)¨NH2 N__
Pd(PPh3)4, Na2CO3 N
dioxane / H20 (--Nj 101 ¨NH2
).- 0
100 C, 1h
o
Reaction Scheme P':
o yo
0S N
,w rNH2 NN
( ,
CI 4014 N Pd(PPh3)4, Na2D03
14,,,NH2 1
I 1 Et0H
l INI-1.1 dioxane / H20
=
BrN 70 C, 24h Br ( 100 C, 24h 0
14,-----(
NH2
HO B OH
NN, a N1N,
N
NIS/DMF
5,-N Pd(PPh3)4, Na2CO3 \ N -_- N
= I
el dioxane / H20 0 ¨NH2
RT, 1h 0
0 100 C, 1h \ /
N=--( N
NH2
-271-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
Reaction Scheme Q':
0-13 N
H CO2Et
..-N
) POCI Isl-"I'l, 0
õ....../1 ;N ¨''' HO) ______________________________________ s -
Pd(PPh3)43 Na2CO3
NH2 dioxane / H20
Reflux, 2 h
HO
HO'Bri,
N,N
I .41
c.....1.õ ,.- NBS, DMF /N-N
N
RT, 2 h N 0
Pd(PP113)45 Na2CO3
0 Br dioxane / Hp
np-----( 0 Reflux, 2 h
NH2 Nr-X
NH2
c....-:--N 0
i \ 0
N¨ Isr----K
NH2
Reaction Scheme R':
H2N
0
is NO2 _ 0 NO2 401 NH2
,,.. Fe, NH4CI _,,..
Br NH Br NH
Br F K2CO3 Me0H, H20 (111)
DMF, 80 C, 2 h 0 RT, 24 h
0
0
,
_________________________________________ 0 Nj¨NH2 N
HCOOH ____________________________________ Isi 0
_________________________________________ Br 0 0
_________________________________________ o- N N
reflux, 24 h N IS) 0,_NH2
a pd(pPh3)4, Na2CO3
dioxane / H20
Reflux, 2 h
Reaction Scheme S':
H2N soi NH2
0 NO2
0 NO2 00 Br NH Fe, NH4CI
Me0H, H2O v Br NH
Br F K2CO3, DMF,),.... RT, 24 h ----L,
80 C, 2 h yield:56%
yield:92% -,..c).--=
0
1110N¨NH2 N 40
N 0
HCOOH 0
> ________________________________________ V N N
reflux, 24 h N Br 0 ¨NH2
(---5 Pd(PPh3)4., Na2CO3
dioxane/ H2O
Relux, 2 h 0
yield:68%
0
0 Yield: 61%
-272-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Reaction Scheme T':
0 0 c H3N H2, Et0H 0
IS OH 9 100 C, 0.5 h RI, 10 min
+ S, ___________________________________ a CI 0
_____________________________________________________ .
I 11 0
Br NH2 CI' CI H
H2N Br 2N Br
H
0 i*. NO2 N
0 0 CN)
CI "1(0 4"
I I
DMAP, DCM N 0 ______________ :t
RT, 2 h a 1
CN Br 150 C, 12 h l'..
POC3
CI N Br DCM, RT, 10 min
H
rso oN j)_N H2 0
0
HO y.., 410
,14,...
N 401
________________________________________ - r----N--c-N N
i-----N N Br Pd(PPh3)4, Na2CO3,¨NH2
..-= 0
dioxane I H20
Reflux, 2 h
Reaction Scheme U':
0
NH2 FIN-'NH2 AcOH eõN N
II POCI3 r -0
________________________ I. N õAP ¨1.- N ----
Br COOH 120 C, 5 h Br reflux, 3 h Br
OH CI
OH
CC,N
M .... 1110
0- N ,N
B
I Br
N N / 0 ¨NH2 II .'11110
0 N ..--- N
_)õ. r
NaH r............0 0 0,¨NH2
Pd(PPh3)4, Na2CO3 r......,0
THF, RI, 0.5 h .._,N..,.õ.., dioxane / H20
Reflux, 2 h ...--N--..../
Reaction Scheme V':
0
H(0N
NH2 40
o 0 Br2, AcOH
yr x.
RT, 0.5 h HON Br
0 NH2 Et0H, reflux, 24 h i-ION
Ts0 0-13 0N
N H2
...".., N 0
DMF, K2CO3
0X N Br Pd(PPh3)4, Na2CO3
80 C, 2 h dioxane / H20
Reflux, 2 h
r N
0
i
N
l:YL:N
-273-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
Reaction Scheme W':
POC13/PCI5 o__====_.
L
N 40 reflux, 2 h N / .NH
0
r-N N Br
He-% CIN el Br _______________________________ Br 150 C, 3 h
0)
____\,CN)
0 B
I. r%i¨NH2 rN
0 I.
> r N N N
Pd(PPh3)4, Na2CO3 0õ) el --NH2
0
dioxane / H20
Ref lux, 2 h
Reaction Scheme XI:
N
r el NBoc Br N 0 6N HCI, Et0H
>, l.
N /RI, 24 h
Br DMF, K2CO3 BocN 0
OH 80 C, 2 h
CH20, H20, DCM
I N
Br
. 0
r el AcOH
N NaB(0Ac)3H
N N
r.
Br _________________________________ ).-
N
RT, 24 h 0
HN 0
0,B N iiµl
0¨N H2
ri 10
N
0
N 0 0
Pd(PPh3)4, Na2CO3
dioxane / H20
Reflux, 2 h
-274-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Reaction Scheme Y':
Bu3Sris
N Pd(OAc)2, PPh3 N
a 0s04, Na104 N
0
C 0 _____________________
,
,..., ..*....,,,,,,,.
dioxane Br
CI N Br N Br THE, H20 ' QN
reflux, 24 h rt, 5 h
0
N
0
Ozone, monopersulfate SOCl2 O
)" _________________________________________ ir-
DMF, rt, 2 h O N Br N Br
Cl
OH
0
N 1.I ¨NH2 N
0
0 0
Morpholine
J. (N Br 3, ON
DCM, rt, 1 h
N Pd(PPh3)4, Na2CO3 N 0
Co) dioxane / H20
N1
Relux, 2 h ( )
NH2
Reaction Scheme Z':
H CN
Br 0 0)ki
Cs2CO3, DMF dowtherm 140
________________________ r r
NH2 RT, 24 h Br 0 I
260 C, 4 h NC Br
OH
POCI3 N
0
_____________________ r ________________________ >
100 C, 3 h NC Br Zn, HCI, Me0H NRT, 45 min I.
NC Br
CI
OH
HOB
N)-NH2
to
N
0 0
Pd(PPh3)4, Na2CO3 I
dioxane / H203. N
NC
¨NH
100 C, 1 h SI 0 2
-275-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Reaction scheme AA':
CN CN CN
Mel, DCM
0
ri m 0 NaOH, H20
_________________________ i.-
ri mi. SnCl2, EA
Ethyl acetate
RT, 24 h
=-=2.. =-=2.. reflux, 2.5 h H2N
HN-OH
0 0 /=1 0
BrH Br 02N Br _
110 OH 20, HCI OH
0 . ,,.. OH
NH2
RT, 24 h NH2 HCI RT ,24 h NN02
H
CN
OHPOCI3 Cl
0
KOAc, Ac20 H2N
120 C, 2 h Br NO2 reflux, 45 min Br s NO2
).- _________________________________ x ____________________ ii,
HOAc, reflux, 2 h
N N
THF, Methanol NC 0 0 NO2
NC 0 0
Raney Ni/H2
NH )L
NH RT, 24 h CVO
_______________________________ f Br
Br NO22 ________
2 0 \
= Et2N, DCM
N 0 C, 1 h
N
NC 0I,0 1%14 CN
0 B 0 N . . N
0
/¨NH2 1/0
I
), N
Br NH pd(pPh3)4, Na2CO3 0
/10 DMF /Et0H / H20 N
N Reflux, 2 h 0
Reaction Scheme AB':
HN-N
F 0,13 N \
NH2
NH2NH2.F120 HN-N 110 0:¨NH2 .
*I CN \
_________________________ ),- NH2 _____________ >
Br Ethanol, reflux, 12 h 1 10
Pd(PPh3)4, Na2CO3
Br dioxane / H20 0
Reflux, 2 h N-------(
NH2
-276-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Reaction Scheme AC':
00
o )\ i( CI CI
CI CI
).--S DCM, DMF
,.. .....) n-BuLi, 12, THF
....-S )... I
40 C, 2 h -N -78 C, 1 h Nj---i¨
N
H
HO.B.0H
>Y0
I N oB)-NH 2 N ky-NH2 01 l'i
0 1 \ 'II
Pd(PPh3)4, Na2CO3 Pd(PPh3)4, Na2CO3 S
dioxane / H20 dioxane / H20
100 C, 1 h 100 C, 1 h I
N
Reaction Scheme AD':
Cl
mCPBA
DME, heptane POCI3 1 \
_________________________ ).- -... --;:-.... )...
RT, 2.5 h NN 85 C, 4h N----N
H ii HH
0
CI 1 CI 1
NIS, DMF (Boc)20, DMAP, DCM
-.... \
RT, 2h -.....,,, RT, 20 min
N 1`, N.....N
H
Boc
HO B OH
1 1%1 0
OB 0 r'l_NH2 CI =(3¨NH2
a 00 -NFI2
0 N N N
Pd(PPh3)4, Na2CO3
Pd(PPh3)4, Na2CO3
dioxane / H20 .. 1 \ dioxane / H20 1 \
100 C, 1h lc N 100 C, lh __ >
N N
Boc H
Reaction Scheme AE':
o
01 11¨NH2
A0-13
. -60
Br ao N
...- H2N4
S¨NH2 ________________ N
0 Pd(PPh3)4, Na2CO3 0 IS¨NH2
DMF /Et0H / H20 0
Reflux, 2 h
-277-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Reaction Scheme AF':
o
0 40
7 N,_NH2
..:13,
0
N
NH2N-- 1401
_____________________________________ ...
H2N¨ S N
S l Br Pd(PPh3)4, Na2CO3 1401
DMF /Et0H / H20 0
Reflux, 2 h
Reaction Scheme AG':
N NaOH N SOCl2
\ 10 Reflux, 24 h x
\ el _________ >
Reflux, 2 hours
NC Br HOOC Br
N (Orsi
40)
H N 0
= c.-
CIOC Br Et3N, DCM
1.........õ.N \ Br
RT , 20min
0
o-B 0 N\
NH2 N
0 00 0
..
N
Pd(PPh3)4, Na2CO3 N
dioxane / H20 0 0 -'-NH2
Ref lux, 2 h o
Reaction Scheme AH':
6 ,13
0
- 0 1S¨NH2
N N
0
\ SO 40) N
NC Br NC
CI Pd(PPh3)4, Na2CO3
dioxane / H20 CI 0
Relux, 2 h
H9
N
HOB 0N NC N
______________________ ),- 1101 ¨NH2
0
Pd(PPh3)4, Na2CO3 I
dioxane / H20 N
Relux, 2 h
-278-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
Reaction Scheme AI':
Et0 0
s=-=;!*
Br
N,.....õõ..0,....7.,,,r0
...,
__________________________ IP- CO ..,.. OP 10% NaOH
Br reflux, 1.5 h ..,N
" 0 Br
Dowtherm Et
NH2 reflux, 0.5 h OEt OH OH OH
FIS)
õ0
N N
HOI3
.... POCI3 ,, 0 , .... N
Dowthermreflux, "===., OP Br
0.5 h reflux, 1.5 h Br Pd(PPh3)4, Na2CO3
OH CI dioxane / H20
Reflux, 2 h
HO
)
HO,B N
.... 40
N 0 S¨NHAc
..... 0
N
SI
p--
Br
Pd(PPh3)4, Na2CO3 / 1
dioxane / H20 I NI
..., i S
I N
Reflux, 2 h ...
NH
N
0
N
HCI, Me0H
reflux, 0.5h
.1
¨I N
S--1(
N
NH2
Reaction Scheme AJ':
N 9
N
--- Op HN, 0,B.õ) ,¨NH2
Br 01 0
_____________________________ s- r
Br dioxane N
CI 120 C, 12h C ) Pd(PPh3)4, Na2CO3
dioxane / H20
N Reflux, 2 h
I
N
.... 0
N 110/
0
N
I NH2
-279-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
Table 3 shows exemplary PI3Ka inhibitors of the invention.
Table 3. In Vitro IC50 data for selected compounds of the invention. The
following symbols
are used: + (greater than 10 microMolar), ++ (less than 10 microMolar), +++
(less than 1
microMolar), and ++++ (less than 100 nM).
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
1 Is'
Calcd
I ,
0 Oi_l,
I el
388.1
N Foun
d:
389.0
[M+
+++ ++++ +++ ++++ ++++ +++ +++
H] '
2 IN;(2. Calcd
_
0 >-NH
I el
396.1
N 0
Foun
d:
397.0
[M+
+ +++ + + +++ ++
H] '
1
I, Nõ
Calcd
'
I. . srb¨NH2
354.0
N 9
Foun
d:
355.0
[M+
+++ ++++ + +++ +++
H] '
4 l'C
Calcd
I ,
0 NH,
,- 140 o
338.1
N 2
Foun
d:
339.0
[M+
+++ ++++ ++++ ++++ ++++ ++++
H] '
< N,
j
I Calcd
N, NH2
WI
I Ol
348.1
N 4
+++ +++ + ++ +++
Foun
-280-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
d:
349.0
[M+
H] '
6 1 l'; Calcd
40 /-NI-1;
/ N 327.1
N--
1
Foun
d:
328.0
[M+
++ ++++ ++ +++ ++++ ++ +++
H] '
N li CN Calcd
NI 0
474.1
0 014 H2
8
Foun
d:
475.0
M+H
++++ ++++ ++++ ++++ ++++ ++++ t-
8 N Calcd
I N
140 I ,li 349.1
, lel
N NH2 3
Foun
d:
350.0
[M+
+ ++ + +++
H] '
,-, 14 Calcd
" I 0 N2rNõ,,,H2
349.1
N 3
Foun
d:
350.0
[M+
+ +++ ++ +++
H] '
-281-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
11 Calcd
,N el ,1-NH2
/ N , 328.1
N-- 1
Foun
d:
329.0
[M+
+++ ++++ +++ ++++ +++ +++
H] '
11 . Calcd
101 "S¨NH2
iii.. N
326.1
N iiir
2
Foun
d:
327.0
[M+
+ ++++ ++ +++ +
H] '
12 N
Calcd
I,
s...---
am ;
N
1 &
WI
NH2 354.0
9
N
Foun
d:
355.0
[M+
+ + + +
H] '
13 N- Calcd
I
s
1 0 140 ;14 354.0
N 9
NH2
Foun
d:
355.0
[M+
++++ +++ +++ +++
H] '
14 N
Calcd
I
N,I,NH2
344.0
I s/ .
14 9
Foun
d:
345.0
[M+
++ + + ++
H] '
-282-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
15 N El2N Calcd
1 ;
40 338.1
I.
N 2
Foun
d:
339.0
[M+
++++ ++++ ++++ +++ +++
H] '
16 N0--N Calcd
I
\
NH2
338.1
N 2
Foun
d:
339.0
[M+
++++ ++ ++ +++ +++
H] '
17N 2 H N
\ Calcd
1 sr¨N
.... 0
354.0
1401 9
N
Foun
d:
355.0
[M+
++++ +++ +++ ++++
H] '
18 ", "2" N Calcd
1 b
...-- 411
338.1
2
N
Foun
d:
339.0
[M+
++ + + ++
H] - Calcd
'
19 IN
,-- N (:)¨
.,., _3
I.379.1
N 4
Foun
d:
380.0
[M+
+ + + +
H] '
-283-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
20 1'1;0 N Calcd
ilo 0,_NH;
327.1
\N-NH
1
Foun
d:
328.0
[M+
++++ ++++ ++++
H] '
21 1 "AO Calcd
11N
N0 0,¨NH2
C ) 359.1
N
1 7
Foun
d:
360.0
[M+
+++ ++ ++ ++
H] '
22 t; 0 Calcd
N 10 ,DN-NH2
C ) 347.1
O 4
Foun
d:
348.0
[M+
+++ ++++ ++++ ++++ ++++
H] '
23 ,Ir",-. Calcd
N 1100
orS¨N H2
C ) 360.1
N 7
1
Foun
d:
361.0
[M+
++++ +++ ++ ++++
H] '
24 4 I;
N 0 Calcd
1 YNH2
FIN,
361.1
,(1:i 5
Foun
d:
362.0
[M+
++++ +++ ++++ ++++
H] '
-284-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
25 oINH2
N
1 . N
1 \
N N ++ + + ++
H
26 1'1;0
Calcd
so 0IS-N H2
341.1
\IµJ-N
3
Foun
d:
342.2
[M+
+++ ++++ ++++ ++++
H] '
27 IN;0
Calcd
0 oN,¨NH;
374.1
N
C ) 7
0
Foun
d:
375.2
[M+
+++ ++ ++++
H] '
28 1'1;0
Calcd
so oNis,H:
N
C )
346.1
0 4
Foun
d:
347.2
[M+
++++ +++ ++++
H] '
29 (N,&
Calcd
N /NIP
N 101 0N¨NH2
361.1
OH
Foun
d:
362
.0
[M+
++++ ++++ ++++
H] '
-285-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
30 1N-.
Calcd
,
to oN_NH2
436.2
N NrTh 0
,1\1,
Foun
d:
437.2
[M+
++++ ++++ ++++
H] '
31 1 N; 0
Calcd
N
so 0,_N H2
..,.... NI
338.1
2
Foun
d:
339.2
[M+
++++ ++++ ++++
H] '
32 IN; 0 N
Calcd
HN, 0 0¨Nii2
360.1
6
Foun
d:
361.2
[M+
++ + ++
H] '
33 I NA01 s
Calcd
Ni_NE,2
HN...., =0
389.1
LI\J 9
Foun
d:
390.2
[M+
++++ +++ +++
H] '
34Nic N"; 0
Calcd
0 0 (:)¨NH
375.7
ON,
Foun
d:
376.0
[M+
++++ +++ ++++
H] '
-286-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
35 e...N,.....ikõ
Calcd
r1 /WI
r\i
HN'CI0
o ¨NH
374.1
N,
9
Foun
d:
375.0
[M+
++ ++++ ++++ ++++ ++
H] '
36 N,rmi2 Calcd
N = ID
KIN \
0
375.1
7
NO
Foun
d:
376.0
[M+
++ ++++ ++ + ++
H] '
37 õc,,,,.:N _...,
Calcd
04
327.1
1
Foun
d:
328.0
[M+
++++ ++++ ++++
H] '
38 \N; ,
ri
c...,
40 (;-__NH, Calcd
N_N
316.1
H 1
Foun
d:
317.0
[M+
++++ ++++ ++++
H] '
39 Isk, ....õ
Calcd
ir:...-=
io >¨N H2
314.1
2
Foun
d:
315.0
[M+
++++ ++++ ++++
H] '
-287-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50 pro lifer pro lifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
40 CI ;
Calcd
ioNi____NH2
0
250.0
9
Foun
d:
251.0
[M+
++ ++ + ++
H] '
41 \N-N
I____
Calcd
/ \ I. ---NH2
N,
330.1
N
I
2
Foun
d:
331.0
[M+
++ ++++ +++ +++ ++
H] '
42 NJ__ ..õ,
Calcd
N
\ r,
425.2
0 0
Foun
d:
426.0
[M+
+ ++++ +++ +++ ++
H] '
8._
43 \N-N N
40 (:)_Nii2
. /
N
Cr)
++++ ++++ ++++
44 N ..,
Calcd
...-N ,===
0 0 0N-NH,
No
376.1
6
Foun
d:
377.0
[M+
++ ++++ ++ ++
H] '
-288-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
45 \N-
Calcd
N
0
NH 0>__ NH;
406.1
8
CN)
Foun
0
d:
407.0
[M+
++++ ++++ ++++
H]
46 N;61
Calcd
NC 0N)_NH2
I
363.1
1
Foun
d:
364.0
[M+
++++ ++++ ++++ ++++
H]
47N--Calcd
so o
oN,NH2
362.1
4
o
Foun
d:
363.0
[M+
++++ ++++ +++ +++ +++ +++
H]
48 <N N
Calcd
0 is 0,-NH2
334.1
4
Foun
d:
335.0
[M+
++
H]
49 ,CN N
Calcd
0 N c--NH2
362.1
0
4
Foun
d:
363.0
[M+
++ ++++ ++ +++ ++
H]
-289-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
50 % N
0 c?--NH,
Calcd
382.1
N,,.(0 2
NH2
Foun
d:
383.0
[M+
+ ++++ ++ ++++ ++
H] '
51 ¨
NI¨ iv NrH2
Calcd
...,. 114 /
412.1
i
6
N Foun
C )
0 d:
413.0
[M+
++++ +++ +++ +++
H] '
52 N__ ¨ A-..... N?:NH2
Calcd
11
/
...,.. 1 lip)
412.1
I,
N N 1
6
Foun
d:
413.0
[M+
++ ++++ ++++ ++++
H] '
53
N- ¨ ___ Nz-NH2
Calcd
ip-(..,...(õN/
N 335.1
C )
0 4
Foun
d:
336.0
[M+
++ + + +
H] '
54 N__ --- Aiask N2-NH2
Calcd
L1N
ON
363.1
3
Foun
d:
364.2
[M+
++ ++++ ++ ++ ++ ++
H] '
-290-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
55 N,NH2
-(1)
Calcd
N e *
\N \
342.1
r_c
2 o
Foun
d:
363.0
[M+
+ +++ + +
H] '
56 N- ---, 414
Calcd
342.1
I
N 2
Foun
d:
343.0
[M+
+++ ++++ +++ + ++++ ++
H] '
57 N_ --/ * Nz_NH2
Calcd
N
336.1
( ) 3
0
Foun
d:
337.0
[M+
+++ +++ + ++
H] '
58 N-
Calcd
--1 * N2,-NH2
'4---N
328.1
\ NI
1
Foun
d:
329.0
[M+
++++ ++++ ++++ ++++
H] '
59 N- --/ * %.rNH2
Calcd
"X"--N
317.1
\
N-NH 0
Foun
d:
318.0
[M+
++++ ++++ +++ ++++
H] '
-29 1-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
60 c 10 N
Calcd
NH =0,NH2
a
402.1
8
Foun
d:
403.0
[M+
+++ ++++ ++ +++ ++++
H] '
61 Nz---r",
Calcd
8-N / 0
328.1
N N-----X
1
NH2
Foun
d:
329.0
[M+
+ + + +
H] '
\8..j,
62 /NI-N
Calcd
=N N / 0 328.1
N.----(
1
NH2
Foun
d:
329.0
[M+
++++ ++++ ++++ ++++ ++++
H] '
63
= 4*
(' IN
Calcd
N H2
7---( 0
`
362.1
0--/
4
Foun
d:
363.2
[M+
++ + + +
H] '
64 <101
Calcd
0 1101
N,----(
347.1
NH,
4
Foun
d:
348.0
[M+
++ ++++ +++ + ++++ ++
H] '
-292-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
65 _CN*
Calcd
(NN 40
360.1
NH2
7
Foun
d:
361.2
[M+
++++ ++++ ++ +++ ++
H]
66 N = 0
Calcd
N
N
403.1
c)
6
Foun
d:
404.2
[M+
++
H]
67
= :LNH
Calcd
N
0 2
439.1
o=7:0
3
Foun
d:
440.0
[M+
+++
H]
68
Calcd
1.1 o 328.1
(N)
NH2
Foun
d:
329.0
[M+
++++ ++ ++ +++
H]
69
Calcd
HoN 0
N=-( 317.1
0
NH
Foun
d:
318.0
[M+
+++ ++ ++ +++
H]
-293-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
70 N.
I
Calcd
W
IW
354.0
s
I 9
. N=-----(
N
NI-12 Foun
d:
355.0
[M+
++++ ++++ ++++ ++++
H] '
71N ,
Calcd
1, INV 0
N ..-
N------( 339.1
NH2 1
Foun
d:
340.0
[M+
++++ ++ +++ ++++
H] '
72 rN 6
Calcd
ON
40 , 278.0
H
isr---( 8
NH2
Foun
d:
279.0
[M+
+++ ++++ +++ + ++++
H] '
73 rN 1 0
Calcd
0- 0 40
rN
N-,--K 389.1
NH2
9
Foun
d:
390.2
[M+
+++ ++ + +
H] '
74 rN 1 0
Calcd
a 0 I0
403.2
NH
0
Foun
d:
404.2
[M+
++ ++++ ++ + ++ ++
H] '
-294-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
75 rN
Calcd
HNra 0
361.1
NH,
Foun
d:
362.0
[M+
++++ ++ ++
H]
76 N
I .1
-N
Calcd
LNJ SO
339.1
NH2 1
Foun
d:
340.0
[M+
++ ++++ + +++ ++
H]
77
Calcd
NC
011
320.0
CI
NH2 5
Foun
d:
321.0
[M+
+++
H]
78N
Calcd
'1=7NX 10
N=(
NH2
328.1
1
Foun
d:
329.0
[M+
++++ +++ +++ ++++
H]
79 0
Calcd
Nril,N 140 so
,r11,)
390.1
NH
8
Foun
d:
391.2
[M+
++
H]
-295-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
Calcd
1 101
0 N 0
377.1
N--r-( 5
NH2
Foun
d:
378.0
[M+
+++ + + +
H] '
81N
X 101
Calcd
aHN N 0
N.--(o
374.1
N 9
1 NH2
Foun
d:
375.2
[M+
++++ +++ ++++ +++
H] '
82 ,cN 161
Calcd
HN N ...lr"- 10
aN.-X(3
402.1
A, 0 NH2 8
Foun
d:
403.2
[M+
++++ ++ ++++ ++++
H] '
83 N,... ..,
Calcd
).--N /
0
NH
a 5
NH2 IW N o
377.1
-=( o Foun
d:
378.0
[M+
++++ ++ ++++ ++++
H] '
84 ,, it * 0
Calcd
-="--N N'-.1.'NH2
(--)
388.2
N
---- 0
Foun
d:
389.2
[M+
++++ ++++ +++ ++++ ++
H] '
-296-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
85 Alt *NNH 0
Calcd
388.1
6
Foun
d:
389.0
[M+
++++ ++++ ++++ ++++
H]
86
Calcd
N\ -NH2
424.1
3
Foun
d:
425.0
[M+
++++ +++ +++ ++++
H]
87 r140
Calcd
HN so
438.1
N'<H2
,s.
0 ro
Foun
d:
439.0
[M+
++++ +++ ++++ ++++
H]
88 AI itNNH 0
Calcd
346.1
5
Foun
d:
347.2
[M+
++++ ++++ ++ +++ ++
H]
89ri:10
Calcd
HN
NH2 428.2
N---
0
tD,7
Foun
d:
429.2
[M+
++++ ++ ++++ ++++
H]
-297-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
90 N4, it 0
Calcd
N NH
452.1
6
0.7,0
Foun
d:
453.0
[M+
++++ +++ ++ +++ +++
H]
91 N-
Calcd
0
390.1
N
8
NH
Foun
d:
391.0
[M+
H]
92 _C--N
Calcd
110
377.1
Niz.õ(0
NH2
Foun
d:
378.0
[M+
H]
93
101
Calcd
NC
40 o
286.0
NH2 9
Foun
d:
287.0
[M+
++ +++ + +++
H]
94 0
Calcd
te-LNH,
-N
342.1
NN
Foun
d:
343.0
[M+
++++ +++ ++ ++++
H]
-298-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
95 1.N1401
Calcd
HN N
438.1
N=---(C)
NH2 5
Foun
d:
439.0
[M+
++++ ++ ++++ ++++
H]
96
,C
Calcd
HN N
40 o
361.1
0
NH: 5
Foun
d:
362.0
[M+
++++ ++ +++ ++++ +++
H]
97
Calcd
0
40 ( o
374.1
0
NH2 4
Foun
d:
375.0
[M+
++
H]
98 XN 101
Calcd
cNj N 410
395.1
4
NH2 1
Foun
d:
396.0
[M+
++++ + ++++
H]
99
,Ci
Calcd
(N N
40 0
361.1
OH
NH: 5
Foun
d:
362.2
[M+
++++ ++ +++ +++ ++
H]
-299-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
100 N
,C lel
Calcd
(
N) N
0 0
390.1
N N=<-
H NH2 8
OH
Foun
d:
391.0
[M+
++++ ++++ ++ +++ +++
H]'
101 rN*
Calcd
IA W
HN
402.2
IW 0
a Nr-=( 2
NH2
Foun
N
d:
403.0
[M+
++++ ++ +++ ++ ++
H]'
102 le I.
Calcd
HN
420.1
ISI o
0 1%1=X
NH2 7
Foun
CN d:
421.0
[M+
++++ +++ +++ ++++
H]'
103 isr I.
Calcd
HN
445.2
a . o
Nr------(
NH2 2
Foun
N 1
ON d:
446.0
[M+
++++ +++ +++ +++ ++
H]'
104 '1
Calcd
N 6
377.1
or,Q 7.....(0
6
NH2
Foun
d:
378.0
[M+
++ + + ++
H]'
-300-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
105 N
Calcd
N
364.1
or,o)
3
NH2
Foun
d:
365.0
[M+
+++ ++ +++
H]
106 N
Calcd
40
391.1
N'-( 6
NH2
Foun
d:
392.0
[M+
++ ++
H]
107 C---N
Calcd
101
411.1
10=oN 0
II NH
Foun
d:
412.0
[M+
+++ + ++
H]
108
Calcd
N ioN
372.1
NH2 3
OH
Foun
d:
373.2
[M+
++++ ++ ++++ +++
H]
109 N
Calcd
N
00
399.1
0
(o)0
NH2
Foun
d:
400.0
[M+
H]
-30 1-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
110 N-
Calcd
0=10
412.1
CNJ N( 3
NH2
Foun
d:
413.0
[M+
H]
111 N-
Calcd
0
362.1
1(.1-NH N----X 5
NH2
Foun
d:
363.0
[M+
++++ ++
H]
112
N Calcd
440.1
NI-12 3
o Foun
d:
441.0
[M+
++++ + ++ +++ +++
H]
113
N Calcd
o 404.1
C-N1N< 6
0H2 Foun
d:
405.2
[M+
++++ + +++ ++
H]
114 ,CN
Calcd
(1,1,1 N
N 363.1
s_i(
NH2
2
Foun
d:
364.0
[M+
++++ + +++
H]
-302-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
115 N 16
Calcd
cNi N -"r"' 0
NH2 376.1
1
Foun
d:
377.0
[M+
++++ ++++ + ++ ++
H] '
116 ,CN la
Calcd
...W' so
363.1
NH2 2
Foun
d:
364.0
[M+
++++ +++ + +++ +++
H] '
117 N 161
Calcd
rNN ...W'' 0
LO) 0-1(11
347.1
NH2 4
Foun
d:
348.2
[M+
++++ +++ + +++ ++
H] '
118 ,CN la
Calcd
(NJ N 'W.' so
N 0-1(11
NH2 360.1
1
7
Foun
d:
361.0
[M+
+++ ++++ + ++
H] '
119 N
,C lel Calcd
(N) N 0
418.1
I W----eN FI2 8
Foun
d:
419.0
[M+
++ ++++ ++ ++ ++ ++
H] '
-303-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
120 ,c-N la
Calcd
C:
417.1
40 0
417.1
N N-,---(--
rErsl NH2 9
0
Foun
d:
418.0
[M+
++++ +++ ++ + +++
H] '
121 N
,C I.
Calcd
N N
( ) 40 o
443.2
O31 NH,
1
Foun
d:
444.2
[M+
+ ++++ ++++ ++++ ++++ +++
H] '
122 N-.1.----
Calcd
).:---N /
aO N IW
n 364.1
3
Ni-CNH2
Foun
d:
365.0
[M+
+ + + +
H] '
123 N-....(:-...N
Calcd
....--N /
Oir n
377.1
Q "- 6
--NH2
Foun
d:
378.2
[M+
++ + + +
H] '
124 ''-:N
Calcd
Q 363.1 = N
0_2(N
3
NH2
Foun
d:
364.0
[M+
+++ +++ + +++
H] '
-304-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
125 N
Calcd
io
379.1
N¨\
s-1(11 1
NH2
Foun
d:
380.0
[M+
+++ + ++ +++
H]
126 CLN
Calcd
O
379.1
s
1
NH2
Foun
d:
380.0
[M+
+++ ++ +++
H]
127 ,CN
Calcd
N N
C
376.1
NH2 5
Foun
d:
377.2
[M+
+++ ++++ + ++
H]
128
,C
Calcd
HN N
375.1
7
NH2
OH
Foun
d:
376.2
[M+
++++ ++ ++++ ++++
H]
129 (0)
Calcd
/4arks..N
N 4.N`)-N1d, 529.1
0"lz7'
6
Foun
d:
530.0
[M+
+++ ++++ +++ +++ ++++ +++
H]
-305-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
130 CD
Calcd
01It
587.1
4
Foun
d:
588.0
[M+
H]
131 XN
Calcd
NH 374.1
9
Foun
d:
375.2
[M+
+++ +++ +++ +++
H]
132
le
Calcd
NH 375.1
7
Foun
d:
376.2
[M+
+++ + ++
H]
133 XN 161
Calcd
HNN Olj
390.1
C NH2 8
0
Foun
d:
390.2
++++ +++ +++ +++ ++ [M]
134 ,cN 161
Calcd
cNj N
417.1
(:)yNH2 9
NH2
Foun
d:
418.0
[M+
++++ +++ +++ ++ +++
H]
-306-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
135 ,C:N 161
Calcd
N N
C 101 o
360.1
N 0
NH2 3
Foun
d:
361.0
[M+
++++ +++ + +++ +++
H]
136 x-:
Calcd
(NN
374.1
NH2 9
Foun
d:
375.2
[M+
+++ +++ + ++
H]
137 rN
Calcd
N,
416.2
0
NH2
Foun
d:
417.2
[M+
++++ +++ +++ +++ +++
H]
138 e
Calcd
N,
HN
457.2
o
NH 2
Foun
0
d:
458.2
[M+
++++ +++ ++++ +++
H]
139
,C
Calcd
cNj N
431.2
oLL NH2 1
Foun
d:
432.2
[M+
++++ +++ +++ +++ +++
H]
-307-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
140 N
Calcd
390.1
o
( )N 8
NH2
Foun
d:
391.2
[M+
+++ ++
H]
141 N
Calcd
O 390.1
___xo
N N 8
\-- NH
Foun
d:
391.2
[M+
+++ + ++
H]
142 N
Calcd
40
390.1
14_10
Ci-NH 8
Foun
d:
391.2
[M+
++
H]
143 C--N
Calcd
391.1
= HN 0
Nz---(NH2 6
OH
Foun
d:
392.2
[M+
++++ ++ ++++ ++++ +++
H]
144 N-
Calcd
O 392.1
4
NH2
Foun
d:
393.0
[M+
+++ ++
H]
-308-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
145 4i5N
Calcd
o 40
376.1
Qo-z(N 6
N \
NH
Foun
d:
377.2
[M+
+++ ++ + ++
H] '
146 "---
Calcd
O ir
0 392.1 4 s_ j(N
4
NH
Foun
d:
393.0
[M+
++ ++ + +
H] '
147 <L
Calcd
o
418.1
N--(-
8
NH NH2
o.K
Foun
d:
419.2
[M+
+++ ++ ++ ++
H] '
148 (L:14
Calcd
ol,Q40 406.1
N 8
r.x0
N
ZOH H2
Foun
d:
407.2
[M+
++++ + + ++ ++
H] '
149 ,cN 16
Calcd
=0 ),360.1
NH
N--,---
NH2 7
Foun
d:
361.0
[M+
++++ ++++ ++ +++ +
H] '
-309-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
150 (
N
401
Calcd
N
424.1
CNH 6
0
Foun
d:
425.0
[M+
+++ +
H]
151 ,cN=
Calcd
N
388.2
NH2 0
Foun
d:
389.0
[M+
++++ ++ +++ ++ ++
H]
152 ,CN 161
Calcd
(NJ N is
374.1
Nr--<
NH2 9
Foun
d:
375.0
[M+
++++ ++++ ++ +++ ++
H]
,C
Calcd
153
(Nj N 1110
400.2
NH2 0
Foun
d:
401.0
[M+
++++ ++++ +++ +++ +++
H]
154 ,CN 161
Calcd
cNj N el
403.1
NJ
NH2 8
Foun
d:
404.0
[M+
++++ +++ ++ ++++ +++
H]
-310-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
155 e
Calcd
388.2
0
NH2
Foun
d:
389.0
[M+
++++ ++++ +++ ++++
H]
156 XN 101
Calcd
o cNj N
443.2
()C-\N NH2 1
Foun
d:
444.0
[M+
++++ +++ +++ +++ +++
H]
157 0 CN 101
Calcd
rN
C D 1\1,--(
388.1
NH2 6
Foun
d:
389.0
[M+
++
H]
158
Calcd
oN N
C375.1
0
NH2 3
Foun
d:
376.0
[M+
++
H]
159 <-2N
Calcd
o.NH I I e
390.1
8
NH2
Foun
d:
391.0
[M+
++++ +++ ++++ +++ ++
H]
-311-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
160 <'
Calcd
0
404.2
or,_____I Nrrs(ON
0
---- H2
Foun
d:
405.0
[M+
++++ ++ + + ++
H] '
161 CL-N
Calcd
O I. a
e 416.2
N ::___
0
'2 NH2
Foun
d:
417.0
[M+
+++ ++ ++ ++
H] '
162 <i
Calcd
o
IQ . o 377.1
N--(---
OH NH2
Foun
d:
378.0
[M+
+++ + + ++
H] '
163 N-
Calcd
O ir 446.2
IQ Nr_xo
1
NH2
0)
Foun
d:
447.0
[M+
+++ ++ ++ ++
H] '
164 <L
Calcd
o
0 377.1
N'" N,.(0
NH 5
NH2
Foun
d:
378.0
[M+
+++ +++ + +
H] '
-312-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
165 N
Calcd
N
0 IW 0
N--
404.2
C-5
N-=(-
NH2 0
Foun
d:
405.0
[M+
+++ ++ ++ +++
H]
166
Calcd
N N so
404.2
( NH2 0
0
Foun
d:
405.2
[M+
+++ ++ ++
H]
Calcd
167 iso,N
N
424.1
6
( NH2
O Foun
d:
425.0
[M+
++
H]
168 ,CN =
Calcd
rH1 N 410
360.1
NH2
NH2 7
Foun
d:
361.0
[M+
++++ +++ ++ ++
H]
169
Calcd
N N 40
430.1
ON N
L) NH2 8
Foun
d:
431.0
[M+
++ ++++ +++ +++ +++ +++
H]
-313-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
170N
C . Calcd
YN N 0
N=---) 443.2
0 N-Th 1
NI-12
Foun
d:
444.2
[M+
++ ++++ +++ +++ ++ +++
H] '
171 XN &
Calcd
rhs1 N 411111"
40 n
N=---(-- 430.2
N 1
C0 ) NH2
Foun
d:
431.0
[M+
+ ++++ +++ +++ +++ +++
H] '
172 ,CN 10 rH a
Calcd 1 N --.W.--
1
n
N----(- 388.2
NH2 0
Foun
d:
389.0
[M+
++++ ++++ ++++ +++
H] '
173 ,CN a
Calcd
COL.
361.1
0
361.1 N.----(C)
NH2 5
Foun
d:
362.0
[M+
++++ +++ ++ ++++ ++
H] '
174 ,cN 0
Calcd
CNN--N 40 n
342.1
N(
NH2 2
Foun
d:
343.0
[M+
++++ +++ ++ ++++ ++
H] '
-314-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
175 ,CN
Calcd
N
40 n
345.1
NH2 6
Foun
d:
346.0
[M+
++++ + ++
H]
176
Calcd
cN) N
40 n
331.1
NH2 4
Foun
d:
332.0
[M+
++++ +++ +++ ++++ +++
H]
177
.0 40
Calcd
vCNN N
343.1
NH2 2
Foun
d:
343.0
++++ + +++ [M]
178N Calcd
N N
C ) s 40 n
360.1
NH2 7
Foun
d:
361.0
[M+
++++ ++++ +++ +++
H]
179 ,cN
Calcd
N
LNkr 101 n
360.1
NH2 7
Foun
d:
361.0
[M+
++++ +++ ++ ++ ++
H]
-315-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
180 N
Calcd
is
338.1
NH2 2
Foun
d:
339.0
[M+
+++ +
H]
181 ,CN
Calcd
HN N 011
o
466.1
8
NH2
Foun
.s.
0-1-0 d:
467.0
[M+
++++ ++ +++ ++++ ++
H]
182 ,c"
Calcd
(Nj N is
_y<
402.2
NH2 2
Foun
d:
403.2
[M+
++++ +++ +++ +++ ++
H]
183 t"
Calcd
ro o
306.1
1
NH2
Foun
d:
307.0
[M+
++++ ++ ++ ++++ ++
H]
184 r" 40
Calcd
40
HO
322.1
NH2 1
Foun
d:
323.0
[M+
++++ ++ +++ ++
H]
-316-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
185 _C-,"
Calcd
N
353.1
NH2 3
Foun
d:
354.0
[M+
+++ +
H]
186 N-
Calcd
347.1
o
4
NH2
Foun
d:
348.0
[M+
++++ ++ +++ ++++ ++
H]
187 N
Calcd
NH
333.1
o
2
NH2
Foun
d:
334.0
[M+
++++ ++ +++ ++++ +++
H]
188 4i:14
Calcd
NH
321.1
o
Nr-X 2
NH2
Foun
d:
322.0
[M+
++++ +++ +++ ++++ +++
H]
189 N
Calcd
0
NH o
337.1
NH2 2
OH
Foun
d:
338.0
[M+
++++ +++ +++ +++ +++
H]
-317-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
190 N-
Calcd
0
NH o
370.1
2
NH2
Foun
d:
371.0
[M+
++++ + +++ ++++
H]
191 N-
Calcd
0
418.2
ON 1
Nfr- NH2
Foun
d:
419.0
[M+
++++ ++ ++ ++
H]
192 XN 101
Calcd
cNj N
403.1
oLNH2 N
8
Foun
d:
404.0
[M+
++++ +++ +++ +++
H]
193N =
Calcd
cNj N
404.1
011 o' NH2 6
Foun
d:
405.0
[M+
++++ +++ ++ ++++
H]
194AIN
Calcd
N
101 n
No
353.1
NH2 3
Foun
d:
354.0
[M+
++++ + ++++
H]
-318-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
195 N
jN 40
Calcd
N' I 101
339.1
N.---(
NH2 1
Foun
d:
340.0
[M+
+++ ++ + ++++
H] '
196 N-
Calcd
O ir
361.1
ON N4 5
NH2
Foun
d:
362.0
[M+
+++ ++ + ++
H] '
197 C--N
Calcd
o 333.1
No I. o
N.------K 2
NH2
Foun
d:
334.0
[M+
++++ ++ ++ ++++
H] '
198 <111;
Calcd
o 40
468.1
Noi N,_____(0
6
Z NH2
Foun
_o
d:
6 '
469.0
[M+
++++ ++ ++ +++
H] '
199 N-
Calcd
o 482.1
NH ir 0
N"----X 7
01 NH2
Z
Foun
d:
sl...:o
cr
483.0
[M+
++++ ++ ++++ ++++
H] '
-319-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
200 C--N Calcd
o 349.1
1,4 ISI o
N",---( 2
OH NH2
Foun
d:
350.0
[M+
++++ ++ ++ ++++ ++
H] '
201 C-N
Calcd
c). 0
377.1
isl-- N____x0
----c) NH2
Foun
d:
378.0
[M+
++++ ++ + ++ ++
H] '
202 C--N Calcd
o 363.1
¨.\ ISI_o
/"--OH N--< 3
NH2
Foun
d:
364.0
[M+
++++ ++ ++ +++ +++
H] '
203 s"---
Calcd
o
NH o 391.1
N-----( 8
NH2
''Ikl
Foun
1
d:
392.0
[M+
++++ ++++ +++ ++++
H] '
204 4_1;
Calcd
o
IQ 1.1 o 376.1
147,---(
6
N
NH2 H2
Foun
d:
377.0
[M+
++++ +++ + +
H] '
-320-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
205
Calcd
12:7,1, 0 w
o
332.1
NH2 3
Foun
d:
333.0
[M+
++++ +
H]
206 rN
Calcd
o 0 10
3201.
NH2 3
Foun
d:
321.0
[M+
++++ + +++
H]
207
.0 40 Calcd
N N is
417.1
NI-12 9
Foun
d:
418.0
[M+
++++ ++++ +++ ++++ +++
H]
208 ,CN
Calcd
(Nj N io
417.1
0;NH2 N'eNH2 9
Foun
d:
418.0
[M+
++++ +++ +++ ++ ++
H]
209 x,N =
Calcd
o 347.1
OH
NH2 4
Foun
d:
348.0
[M+
++++ +++ ++ ++++ ++
H]
-32 1-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
210 c"
Calcd
rN
N1,N
NH2
NH2
355.1
2
Foun
d:
356.0
[M+
+++ +
H]
211 e
Calcd
HN= N 110
403.1
8
NH2
Thq
Foun
O d:
404.0
[M+
++++ ++++ ++++ ++++ +++
H]
212 e
Calcd
HN= N
375.1
NH2 8
Foun
d:
376.0
[M+
++++ +++ +++ +++ +++
H]
213
.0 40
Calcd
cNj N 40
431.2
ONH2 N(
NH2 1
Foun
d:
432.0
[M+
++++ +++ +++ +++
H]
214
Calcd
ON So
374.1
NH2 5
Foun
d:
375.0
[M+
++++ ++++ ++ +++
H]
-322-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
215 ,CN 6
Calcd
N N
ONA N-4)
400.1
H NH2 6
Foun
d:
401.0
[M+
++++ +++ ++ +++
H] '
216 ,cN a Calcd
N N till
? 333.1
OH N.----e
NH2 2
Foun
d:
334.0
[M+
++++ +++ ++ ++++
H] '
217 ,c" a
Calcd
431.2
c;-----"-- Ns'eNH2 1
Foun
d:
432.0
[M+
++++ +++ +++ +++
H] '
218 N
? 1.1 Calcd
HN N .
N=---(
376.2
rN1
NH2 0
Foun
d:
377.2
[M+
+++ +++ +++ ++
H] '
219 N 6
Calcd
HN
? 374.1
po N.,---(
NH2 9
Foun
d:
375.0
[M+
+++ +++ +++ +++
H] '
-323-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
220 ,cN 6
Calcd
HN N
N.----K
NH2
390.2
2
)
Foun
d:
391.2
[M+
++++ +++ ++++ +++
H] '
221 ,cN 6
Calcd
HN N -41r....- 0
.---.K 388.2
N
9
NH2 0
Foun
d:
389.0
[M+
++++ +++ ++++ ++++
H] '
222 ,cN al
Calcd
HN N -4r...-- .
e404.2
N.----e
N-Th
NH2 0
,(:)
Foun
d:
405.0
[M+
++++ +++ ++++ +++
H] '
223 ,cN al
Calcd
H2
425.2
N u, N.--ze
NH2 0
cv Foun
d:
426.0
[M+
++++ + + +++
H] '
224 (" .
Calcd
LNI 0
) N.----e
NH2
377.1
9
Foun
d:
378.0
[M+
++ + + +
H] '
-324-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
225 ,c"
Calcd
CNj N 40
443.2
NH2 1
Foun
d:
444.0
[M+
++++ ++++ ++++ ++++
H]
226 ,c" =
Calcd
(Nj N
429.1
0..xNH2
NH2 9
Foun
d:
430.2
[M+
+++ + ++ ++
H]
227
Calcd
o (Hsi N 40
443.2
NH2 4
C
Foun
d:
444.0
[M+
++++ +++ ++++ ++
H]
228 ,CN
Calcd
N
347.1
OH Nr-,K
NI-12 4
Foun
d:
348.0
[M+
H]
229 N'N, N
Calcd
( is
.3- =
328.1
1
Foun
d:
329.0
[M+
+++ +++ + ++
H]
-325-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
230 N.,N,
Calcd
110 c:?-N H2
0
N
328.1
1
Foun
d:
329.0
[M+
++ +++ ++ +++
H] '
231 0 10 N>-NH2
Calcd
-&-4 40
N
+++ 324.0
7
Foun
d:
325.0
[M+
++ ++++ +++ ++++ ++++ ++
H] '
23240 (31--NH,
Calcd
H2N---eN =40
282.0
6
Foun
d:
283.0
[M+
++ +++ +++ ++ ++++
H] '
233 0
Calcd
N .'", N
FI2NN
227.0
8
Foun
d:
228.0
[M+
+ + + + ++
H] '
234 p .1 (:),? - - N H 2
Calcd
---FCIN-c4 N
+++ 307.1
1
Foun
d:
+++ ++++ +++ ++++ ++++ ++++
308.0
-326-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
[M+
H] '
235 1)1 \ 401 N---NH2
Calcd
):L S
H
274.0
Foun
d:
275.0
[M+
++ ++ + ++ ++
H] '
236 N__ NH2 a 0
Calcd
Fini 0 v
265.1
0
Foun
d:
266.0
[M+
+ + + +
H] '
237--
Calcd
(:
H2N-4-1 .N H
YN 2
N-
285.1
2
Foun
d:
286.0
[M+
+++ ++ ++ ++
H] '
238 c\./N
N ,r, H2
Calcd
H2N---( N\ *O
N--
257.0
9
Foun
d:
258.0
[M+
+++ +++ ++ +++
H] '
239 )--CF,
Calcd
0
N H
H2N---e-N\ *T N 2
N--
339.0
9
Foun
d:
++ +++ ++ ++
340.0
[M+
-327-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
H]
240 H2N-i
Calcd
Q)-NH2
266.0
8
Foun
d:
267.0
[M+
++ +++ ++ ++++
H]
241 H2N-i
Calcd
N%H;
266.0
8
Foun
d:
267.0
[M+
++ +++ +++ ++++
H]
242 N,orNN2
Calcd
jN-tit
324.0
N
7
Foun
d:
325.0
[M+
++++ ++++ + ++++
H]
243 NTNN2
Calcd
282.0
H2N2N 6
Foun
d:
283.0
[M+
+++ ++++ + ++++
H]
244Calcd
45,01y, NH2
N
308.0
9
3Lrei,c)
Foun
d:
309.0
[M+
H]
-328-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
245 11.0iNH2
Calcd
2
1-
65.1N /
H2N 0
Foun
d:
+++ ++ +++
266.0
246 H2
Calcd
266.0
9 8
H2N)'N
Foun
d:
267.0
[M+
H]
247
Calcd
265.1
H2N 0
Foun
d:
266.2
[M+
++ ++
H]
248 S¨N1-1;
Calcd
N
o
282.0
H2N 6
++
Foun
d:
283.0
[M+
++ +++ ++ +++
H]
249 S¨NFI
Calcd
so N
282.0
H2N 6
Foun
d:
283.0
[M+
++++ ++++ +++ ++++
H]
-329-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
250 1401 H
Calcd
N
324.0
H2N 7
Foun
d:
325.0
[M+
++++
H]
251 140 S¨NH
Calcd
N-
N(1-
324.0
H2N 7
Foun
d:
325.0
[M+
++++ ++++ ++++ ++++
H]
252 N,(o
coN,
N-4.2
++ ++ ++
253 N,C:10
0 0
++ ++ ++
254 e
Calcd
vN 0 w
318.1
NH2 1
Foun
d:
319.0
[M+
+++ + +++
H]
255 t"
Calcd
( o o
375.1
7
NH2
Foun
d:
376.0
[M+
++ ++ ++ ++
H]
-330-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
256 r"
Calcd
a 0 =
346.1
NH2 4
Foun
d:
347.0
[M+
++
H]
257
.0
Calcd
ON
'rNH2
374.1
NH2 5
Foun
d:
375.0
[M+
++++ + ++
H]
258 ,c"
Calcd
ccNNH2-r-t.
-
390.1
o
NH2 4
Foun
d:
391.0
[M+
++++ ++++ + ++++ +++
H]
259 N
Calcd
N io0
H2N,ro
390.1
NH2 4
0
Foun
d:
391.0
[M+
+++ + ++
H]
260 N
Calcd
N so0
390.1
8µ. NH2 4
Foun
d:
391.0
[M+
+++ + ++
H]
-331-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
261 xN =
Calcd
N N
( 0
386.1
N=c
NH2 9
Foun
d:
387.0
[M+
++++ +++ + +++ ++
H]
262 XN
410as6
Calcd
N N
347.1
c
OH N=
NH2 4
Foun
d:
348.0
[M+
++ ++++ +++ + +++ ++
263 N-
Calcd
402.1
ir 0
C N=K 8
NH2
Foun
d:
403.0
[M+
H]
++++ ++ ++ ++ ++
264 N-
Calcd
0
363.1
N-' o
OH N- 3
NH2
Foun
d:
364.0
[M+
++++ ++ ++ +++ +++
H]
265 N
Calcd
363.1 N-' o
c,/"OH N=K 3
NH2
Foun
d:
364.0
[M+
++++ ++ ++ ++++ +++
H]
-332-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
266 ''
Calcd
oN=
o 418.1
? N-----(
8
NH2
N
Foun
o) d:
419.2
[M+
++++ ++ +++ +++ ++
H] '
267N
X 40 Calcd
YN N 0
Y N---= 443.2
0 N
1
NH NH2
Foun
d:
444.2
[M+
++ ++++ ++ +++ +++ +++
H] '
268N
C 101 Calcd
N N ip
o 443.2
OYN '" N'XN 1
L.. uNH ..2
Foun
d:
444.2
[M+
++++ ++ ++ +++ +++
H] '
269 N
,C: 40 Calcd
N N 0
C )
445.2
r;, 1 N.-----(
(:).-------k- NH2 2
NH2
Foun
d:
446.2
[M+
++++ +++ +++ ++ ++
H] '
270 N
. Calcd
N N 0
C )
445.2
N-4
0 NH2 2
NH2
Foun
d:
446.2
[M+
++ ++++ +++ +++ +++ +++
H] '
-333-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
271 r" s
Calcd
0 0
O N.---(
389.1
NH2 9
Foun
d:
390.2
[M+
+++ ++ ++ +
H] '
272 r" .
Calcd
f 0 0
0
336.1
1 N.--=-e
NH2 2
Foun
d:
337.0
[M+
+++ ++ + ++
H] '
273 N 6
Calcd
N N -4r".- 0
?
402.1
N N.-e--
C ) NH2 8
0
Foun
d:
403.0
[M+
+++ ++++ + + ++ +++
H] '
274 N 6
Calcd
?
415.2
N N-,---e
C ) NH2 1
N
I
Foun
d:
416.2
[M+
++ ++++ ++ ++ ++ +++
H] '
275N
,C 110
Calcd
HN N lip
?
N W-r-
e 403.2
( ) NH2 1
N
1
Foun
d:
404.0
[M+
++++ +++ +++ +++ +++
H] '
-334-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
276
Calcd
N
469.2
NH2 2
Foun
d:
470.2
[M+
++ ++++ ++ +++ +++ +++
H]
277 ,c"
Calcd
N N
C
374.1
N 0
NH2 5
Foun
d:
375.0
[M+
++ ++++ +++ ++ +++ +++
H]
278 ,c"
Calcd
cNj N
457.2
OL N-4NH2 2
Foun
d:
458.2
[M+
++++ +++ ++ +++ +++
H]
279 ,c"
Calcd
cN) N 40
459.2
N4NH2 4
Foun
d:
460.2
[M+
++++ ++ ++ ++ +++
H]
280 r" oo
Calcd
f 0 =
C )
404.2
NH2 0
Foun
d:
405.2
[M+
++
H]
-335-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
281 :N
Calcd
Jo SI
391.2
1N1
NH2 0
Foun
d:
392.2
[M+
+++ + ++
H]
282 rs
Calcd
ip oN_NH2
364.1
3
Foun
d:
365.0
[M+
H]
283 =
Calcd
N N tip
O 457.1
ON
NH2 9
O/ Foun
NH2
d:
458.0
[M+
++++ + +++ ++
H]
284
Calcd
so N N
457.1
O No N
NH2 9
NH
Foun
2
d:
458.0
[M+
++++ ++ ++ +++ ++
H]
285
Calcd
N N
471.2
ON :H2
4
Foun
d:
472.2
[M+
++++ ++ +++ +++ +++
H]
-336-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
286 ,c" 6
Calcd
(Nj N --"r/- 0
N 0
443.2
0.....<5.NH2 N(
NH2
1
Foun
d:
444.2
[M+
++++ +++ +++ ++++ +++
H] '
287 f 6
Calcd
N N
483.2
05?'N IsF----eNH2
,NH 4
Foun
d:
484.2
[M+
++++ ++ +++ +++ ++
H] '
288 _'-__-
\ N /
Calcd
NH
420.1
Ci N--,---(
9
NH2
N\)
Foun
d:
421.2
[M+
+++ +++ +++ ++ ++
H] '
289 r" 0
Calcd
NI 0 40
( ) N---=e
391.1
0 NH2 6
Foun
d:
392.0
[M+
+++ ++ + +++
H] '
290 ICN 40
Calcd
0 1.1
N N.--e
405.1
( ) NH2 8
0
Foun
d:
406.2
[M+
+++ + + +
H] '
-337-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
291 r" =
Calcd
ri 0 I0
N N=--(C)
418.2
C ) NH2 1
N
I
Foun
d:
419.0
[M+
+++ + + +
H] '
292 r" 0
Calcd
N
349.1
1 N.--e
NH2 5
Foun
d:
350.0
[M+
++ + + +
H] '
293 N-
Calcd
....-N ..., io
0
NH
404.2
N-----X
0
NH2
Foun
od:
405.0
[M+
'
++++ +++ ++++ +++ +++ H]
294 <
Calcd
o=4NH 410 ,
384.1
N--,--(
NH2 3
===I,õ-N
Foun
d:
385.0
[M+
++++ + ++ ++++ ++
H] '
295 N-
Calcd
0
NH
392.2
? N-----X
NH2 0
rN.1
Foun
d:
393.0
[M+
+++ +++ +++ +++
H] '
-338-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
296 N
Calcd
oNH 1$1
o 390.1
NH2 8
(Ni
Foun
d:
391.0
[M+
+++ ++ +++
H]
297 N
Calcd
441.1
N=K 9
NH2
Foun
144 d:
442.0
[M+
+++ + ++
H]
298 XN 40
Calcd
N N
)
433.1
OYOH NH2
9
NH2
Foun
d:
434.0
[M+
++ ++++ +++ ++ ++ ++
H]
299 x-,N =
Calcd
o cN1 N
433.1
= OH NH2
9
NH2
Foun
d:
434.0
[M+
++++ +++ ++++ ++++ +++
H]
300NP---
Calcd
328.1
o
1
NH2
Foun
d:
329.0
[M+
++ ++
H]
-339-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
30114\L- N
Calcd
-6- 041 328.1
N 0 1
NH2
Foun
d:
329.0
[M+
++ ++ + +
H] '
302 N-
Calcd
.,-N ...,
o
NH s 0
406.2
N------( 1
NH2
NJ
Foun
--/ d:
407.2
[M+
+++ ++ ++++ ++
H] '
303 (L-N
Calcd
C)N 40 õ
431.2
? N------(
1
NH2
N
Foun
C )
N d:
1
432.2
[M+
'
++ ++++ + ++ + +++ H]
304 t---N
Calcd
o 40
404.2
QN 14=--) 0
N--. NH2
/
Foun
d:
405.2
[M+
+++ + + +
H] '
305 N,\N---N
Calcd
o):N
No
348.1
o-A 3
NH2
Foun
d:
349.0
[M+
++ + + +
H] '
-340-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
306 N.
Calcd
NH
1.1
419.2
isl'..:3NH2 1
cN)
Foun
N d:
1
420.0
[M+
++++ +++ +++ +++
H] '
307 _CNN*
Calcd
HO 10
376.1
NH,
Foun
d:
377.0
[M+
+++ +++ + +
H] '
308 NN =
Calcd
HO 1101
s-tI 376.1
NH2
5
Foun
d:
377.0
[M+
+++ +++ + ++
H] '
309 ,(N1401
Calcd
HO 10
--'(N 360.1
NH2
7
Foun
d:
361.0
[M+
+++ +++ + ++
H] '
310 N
XN
: I.
Calcd
FIN,) 110
360.1
0),N
H2N 7
Foun
d:
361.2
[M+
+ + + +
H] '
-341-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
311Calcd
H2N-ZN * jc('
386.1
H2N
2
Foun
d:
387.0
[M+
+++ +
H]
312 Calcd
H2N-ZN 11 /s-\N jc
288.0
7
Foun
d:
289.0
[M+
+++ ++ +++ ++++
H]
313 ,CN la
Calcd
N N
441.2
(NNj
NH2 3
Foun
d:
442.2
[M+
++ ++++ ++ ++ ++
H]
314 x:N
Calcd
so N N
386.1
NH2 9
Foun
d:
387.2
[M+
H]
+++ ++ ++ ++
315 la
Calcd
N N
402.1
NH2 8
Ho
Foun
d:
403.2
[M+
+++ ++++ ++ ++ ++
H]
-342-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
316 N
Calcd
0
381.1
HO NH2 4
Foun
d:
382.0
[M+
+++ ++ ++
H]
317
Calcd
odr.i 1101
363.1
ro N.4)
3
NH2
Foun
d:
364.0
[M+
H]
318 ,CN 161
Calcd
o N N 100
383.1
NH2 5
Foun
d:
384.2
[M+
+++ ++++ ++ +++
H]
319 ,CN 161
Calcd
o N N is
383.1
<1111
NH2 5
Foun
d:
384.2
[M+
++++ +++ ++ +++
H]
320 XN161
Calcd
N N
397.1
NH2 7
Foun
d:
398.0
[M+
++++ ++ ++ ++
H]
-343-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
321 ,CNI401
Calcd
HNJI N
sN ¨
376.1
NH2
Foun
d:
377.0
[M+
H]
322 la
Calcd
NN
(I)
NH2
402.1
8
Hcf
Foun
d:
403.2
[M+
H]
++++ +++ ++ +++
323 x: la
Calcd
N N
429.1
C 1 NH2 9
N 0
Foun
d:
430.2
[M+
++++ ++ ++ +++
H]
324
X 140 Calcd
N N
388.2
rN1
NH2 0
Foun
d:
389.2
[M+
++++ ++ ++ +++
H]
325 XN IS
Calcd
N N
415.1
N=-X
C 1 NH2 8
N 0
Foun
d:
416.2
[M+
++++ +++ ++ ++++
H]
-344-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50 pro lifer pro lifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
326 cr'Jia
Calcd
<;5 N
401.2
NH2 0
Foun
d:
402.2
[M+
++++ +++ +++ +++
H]
327 x,"
Calcd
<15 N so
443.2
cN)
NH2 1
Foun
d:
444.2
[M+
++++ ++ +++ +++
H]
328
140 Calcd
<15 N /110
N=. 479.1
(N) NH2 7
-s'
Foun
0-1-0
d:
480.2
[M+
H]
++++ ++ ++ ++
329 ,cN la
Calcd
<15 N so
429.2
(Nj
NH2 3
Foun
d:
430.2
[M+
++++ +++ +++ ++
H]
330 0
C Calcd
N 478.2
I ,N
No 1
Foun
H2N
oN' d:
479.2
[M+
+++ +++ ++
H]
-345-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
331 r la
Calcd
N N
443.2
crq
NI-12 4
Foun
d:
444.2
[M+
++++ ++ ++ ++
H]
332 XN 101
Calcd
(NJ N
374.1
NH2 9
Foun
d:
375.2
[M+
+++ ++++ ++ +++
H]
333 la
Calcd
=N N
445.2
Nr=e
C NH2 2
Foun
d:
OH
446.2
[M+
H]
++++ ++ +++ ++ +++
334
101 Calcd
o N N so
450.1
NH2 5
Foun
d:
451.0
[M+
++++ ++ ++ ++ +++
H]
335 ,cNIO
Calcd
N N
455.2
C NH2 4
Foun
d:
456.2
[M+
++++ ++ ++ +++
H]
-346-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
336 ,CN 161
Calcd
so r-11
374.1
NH2 9
Foun
d:
375.2
[M+
+++ +++ ++
H]
337
Calcd
N
411.1
Nji
NH2 8
Foun
d:
412.2
[M+
++++ + +++
H]
338 ,CN la
Calcd
rhq N 110
425.2
(14 NH2 0
Foun
d:
426.2
[M+
++++ ++ ++ ++ ++
H]
339 ,cN la
Calcd
N N
o
397.1
iirsA
NH2 7
Foun
d:
398.2
[M+
++++ +++ +++ +++
H]
340 '1.2
Calcd
N
0 n
319.1
NH2 1
Foun
d:
320.2
[M+
+++ + +++
H]
-347-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
341
101
Calcd
N N
416.2
(H1
NH2 0
Foun
OH
d:
417.2
[M+
+++ + ++ ++ ++
H]
342
Calcd
N
360.1
7
o-z(N
Foun
NH2
d:
361.2
[M+
H]
343
.(N
Calcd
N
Flis0
7 360.1
o
Foun
NH2
d:
361.2
[M+
++
H]
344 ,cNia
Calcd
N
425.2
NH2 0
Foun
d:
426.2
[M+
++++ ++ +++ ++
H]
345 la
Calcd
N so
411.1
NH2 8
Foun
d:
412.2
[M+
++ ++++ +++ +++ +++
H]
-348-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
346 '1.-14
Calcd
347.1
0
No 7,0
NH2 4
Foun
d:
348.2
[M+
++++ ++ +++
H]
347 3sh-N
Calcd
363.1
Nr_xo
3
NH2
Foun
d:
364.2
[M+
+++ ++ +++
H]
348
Calcd
N'jrN 420.2
Isr 0
Foun
d:
H2N
421.2
[M+
+++ +++ ++++ ++ +++
H]
349 N-
=
ON 0
Calcd
11N
NH2
377.1
5
Foun
d:
378.2
[M+
H]
350
I el
Calcd
N N
415.2
N
C NH2 1
Foun
d:
416.2
[M+
++++ ++ ++
H]
-349-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3 T47D Mass
Structure RC a IC50 IC50 prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
351 (NN0
Calcd
H op
374.1
NH2
9
Foun
d:
375.0
[M+
H]
++ +++ +
352 N_ ¨
Calcd
/
N-Th N NH2 377.1
Foun
d:
378.2
[M+
H]
353 ,c"
Calcd
N N Oi
416.2
N-=-(
C NH2 0
0
Foun
d:
417.2
[M+
++
H]
354 la
Calcd
N N
"--=
397.1
N<NH2 7
Foun
d:
398.0
[M+
++ ++++ ++ ++ ++ +++
H]
355
,C Calcd
N N
e
415.2
N=
C NH2 1
Foun
d:
416.2
[M+
++++ ++ +++ ++ +++
H]
-350-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
356 1/1-N
Calcd
/ o
327.1
1
NH2
Foun
d:
328.2
[M+
++++ ++++ ++++ ++++ +++
H]
357
Calcd
N-N
377.1
Isr"\ 5
NH2
Foun
d:
378.2
[M+
+++ ++ ++ +++
H]
358
Calcd
N N
CI
436.1
C ) NH2 4
0
Foun
d:
437.0
[M+
++++ +++ +++ +++
H]
359
N
Calcd
CI
o
s397.0
w.....x0
NH2 9
Foun
d:
398.0
[M+
++++ +++ ++ ++++
H]
360 N
Calcd
F
0
381.1
NH2 2
Foun
d:
382.0
[M+
++++ +++ ++ ++++ H]
-35 1-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
361 XN 101 F
Calcd
N N
420.1
C NH2 7
O Foun
d:
421.0
[M+
++++ +++ ++ +++
H]
362
,C I el Calcd
=N N
416.2
N -4)
C NH2 0
O Foun
d:
417.0
[M+
H]
363
101
NN
C
NH2
364 5:-
N
Calcd
o
377.1
N<
0
NH2 5
Foun
d:
378.0
[M+
+++ ++ +++
H]
365
I el Calcd
N N =
416.2
C NH2 0
O Foun
d:
417.0
[M+
++ ++ ++ ++
H]
366
X I 401 Calcd
so N N
360.1
NH2 7
++++ ++ ++
Foun
-352-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
d:
361.0
[M+
H] '
367 ,cN la
Calcd
?
332.1
NH2 Nr,--(
NH2 4
Foun
d:
333.0
[M+
+++ +++ ++ +++
H] '
368
Calcd
N-N -....
i
--- 7 0
361.1
o
No 1,1,e 5
NH2
Foun
d:
362.0
[M+
++++ ++ +++ +++
H] '
369 .'1,-:
\
Calcd
N7
o IW
10) N,...<0
377.1
NH2 5
Foun
d:
378.0
[M+
H] '
+ + + +
370 ,N- CI
Calcd
0 IW
a
O
397.0
NH2N.õ...x
9
Foun
d:
398.0
[M+
++ + + +
H] '
371 N
101 c' Calcd
N N ?
=0 436.1
N N=e
C ) NH2 4
+++ ++ + +
0
Foun
-353-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50 prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
d:
437.0
[M+
H]
372 N F
Calcd
N
0 IW
o
381.1
"NH2 2
Foun
d:
382.2
[M+
+++ ++ ++ ++
H]
373
101F Calcd
<*l N=N4)
420.1
C NH2 7
0
Foun
d:
421.2
[M+
++++ ++ ++ ++
H]
374 ,c"
Calcd
NN
374.1
NH2 9
Foun
d:
375.2
[M+
+++ ++++ ++
H]
375 ,c"=Calcd
N -"r"'
416.2
NTh
) NH2 0
Foun
d:
417.2
[M+
+++ ++ +++ +++
H]
376 ,cN =
Calcd
CI) Nso
N 416.2
) NH2 0
0
Foun
++++ ++ ++ ++++
d:
-354-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
417.2
[M+
H] '
377 x-,N 6
Calcd
r N -"r"-- (10
507.2
N--,--<
N)
NH2 1
(
N
Foun
i
.s. d
0-1-0 :
508.2
[M+
++ ++++ ++ ++ +++
H] '
378 x-: c.> 6
Calcd N ''r'.-- =0 374.1
,i1- N----e
NH2 9
Foun
d:
375.2
[M+
H] '
+++ ++++ ++++ ++ ++
379 N
C10
Calcd
cr) N 000
429.2
N<
C.-N) NH2 3
\
Foun
d:
430.2
[M+
++ ++++ +++ ++ +
H] '
380 3'N
Calcd
o 376.1
noi Nr_xo
6
NH
Foun
d:
377.0
[M+
+++ ++ + ++
H] '
381 x-: a
Calcd
-4r"...- so
429.2
NTh N--(
-,o
C.-N) NH2 3
Foun
\ ++++ +++ ++ ++
d:
-355-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
430.2
[M+
H] '
382 ,c" cs? ft
Calcd N --.1r/- =0 374.1
N-... N---,-(0
NH2 9
Foun
d:
375.2
0
[M+
++++ +++ ++ +++
H] '
383 ,CN ft
Calcd
el 0 416.2
N N.,-(
C ) NH2 0
O Foun
d:
417.2
[M+
+++ + + +
H] '
384 ,cN ft
Calcd
NN(J -r--- 0
414.1
ti CF3 N.,..._(C)
NH2 4
Foun
d:
415.2
[M+
+++ ++++ +++ ++ +++
H] '
385 la
Calcd
ciLN 46" so
N359.1
I N----X
NH2 7
Foun
d:
360.2
[M+
++++ ++ + +
H] '
386 N ft
Calcd
N -"Ir'''N=so
?
415.2
C ) NH2 1
N
H Foun
++++ + +
d:
-356-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
416.2
[M+
H]
387 ,c" a
Calcd
(Nj N sim
404.2
NH, 0
Foun
d:
405.2
[M+
H]
++++ +++ + +++
388 ,c"
Calcd
N N
o
429.2
C NH2 3
Foun
d:
430.2
[M+
++++ ++++ +++ +++ +++
H]
389
Calcd
0..N) W =
I
N-='(
360.1
NH2
3
Foun
d:
361.0
[M+
+++ + ++
H]
390 N,CNN
Calcd
N=-X =
374.1
NH,
Foun
d:
375.2
[M+
++ ) )
H]
391 ,cNN
Calcd
0.1) 4111"
=
N=X
NH2
388.1
6
++
Foun
d:
-357-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
389.2
[M+
H]
392 ,c:NN
Calcd
4111"
=
N=X
NH2
402.1
8
Foun
d:
403.2
[M+
H]
393
Calcd
ON = 0
NH2
414.1
8
Foun
d:
415.2
[M+
++
H]
394Calcd
N \
383.1
NH2 5
Foun
d:
384.2
[M+
++++ ++++ +++ ++++
H]
395 Calcd
CI) N
428.1
NH2 6
Foun
d:
429.2
[M+
++++ + ++++
H]
396 N
Calcd
C
359.1
NH2 7
Foun
d:
++++ ++++ + ++
360.2
-358-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM) (nM)* (nM) ation
[M+
H]
397
Calcd
N-11
341.1
/ o 3
NH2
Foun
d:
342.2
[M+
++++ +++ +++ ++++
H]
398 3'N
Calcd
446.2
IQ Nr,---( 1
NH2
çi Foun
d:
447.2
[M+
+++ ++ ++ ++
H]
399 .N S
Calcd
I /
= 365.1
3
N,N,0
Foun
H2N d:
366.2
[M+
H]
400 C.;
Calcd
I
364.1
Q N--K 3
NH2
Foun
d:
365.2
[M+
++++ ++ ++
H]
401 HN
Calcd
F
325.1
NH2 0
Foun
d:
++
326.0
-359-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
[M+
H]
402 N ¨/
Calcd
,
/s
345.0
7
N
Foun
H2
d:
346.0
[M+
H]
403 H2NxN
Calcd
CNj N 411"
375.1
NH2 8
Foun
d:
376.2
[M+
++
H]
404 H2NxN
Calcd
=N N
417.1
N..(0
( NH2 9
Foun
d:
418.2
[M+
H]
405 N
Calcd
N
0
441.1
1
,NH
0=-;
Foun
d:
442.0
[M+
H]
406
N
Calcd
377.1
N<0
/NH
Foun
d:
++ ++
378.2
-360-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
[M+
H]
407 N-
Calcd
v
O
439.1
Nzxo
6
NH
Foun
d:
440.2
[M+
++
H]
408 N
Calcd
N v
0
460.2
N,(0
2
NH
Foun
d:
461.2
[M+
H]
409 N-
Calcd
O
403.1
6
NH
<f
Foun
d:
404.2
[M+
++
H]
410
Calcd
NC
3
0
aN NH2
391.1
3
Foun
d:
392.0
[M+
++
H]
411 M N
Calcd
04
N- NH2 F3
436.1
Foun
d:
437.2
++++ ++ ++ +++
[M+
-361-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
H]
412 <4---11,
oCalcd
N Ni
364.1
NH2 3
Foun
d:
365.0
[M+
++
H]
413
1 =Calcd
(14 N
Lle,1YF =
396.1
NH2 5
Foun
d:
397.2
[M+
++++ +++ ++ +++
H]
414
Calcd
NH2
0
aN
353.1
Foun
d:
354.2
[M+
H]
++
415
Calcd
0
NThNC1
357.1
0
Foun
d:
358.0
[M+
++
H]
416 isir 0-4,0
Calcd
CNH
431.1
N 9
3
Foun
d:
++++ ++ ++ +++
432.0
[M+
-362-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a ic50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
H]
417 F
Calcd
F*
529.1
NH 2
0
= N 0\
Foun
d:
530.0
[M+
+++ ++++ ++++ ++++ ++++
H]
418
c4,0 Calcd
NH
= N
CI 435.0
8
Foun
d:
436.0
[M+
++++ ++++ ++++ ++++ ++++
H]
419 F
Calcd
F*
0=Sz.-0 533.0
IVH 7
O=
= N CI
Foun
c-o d:
534.0
[M+
+++ ++++ ++++ ++++ ++++
H]
420
49 Calcd
NH
0
493.1
= N
0\ 4
Foun
d:
494.2
[M+
++++ + +++ +++
H]
421 F
Calcd
511.1
NH 3
0 I
NM N
Foun
d:
512.0
[M+
++++ +++ ++++ ++++
H]
-363-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
422
4fik
Calcd
F
511.1
N 3
Foun
d:
512.0
[M+
++++ ++ +++ ++++
H]
423 0-F
Calcd
511.1
NJ' 9
3
Foun
d:
512.0
[M+
++++ +++ ++++ ++++
H]
424
Calcd
-2., or
463.5
1
N
Foun
d:
464.0
[M+
+++ + ++ +++
H]
425 F
Calcd
4
</\,1 F
99.4
9
N
Foun
d:
500.0
[M+
++++ + +++ ++++
H]
426 io2
Calcd
so=N
H2N
307.1
1
Foun
d:
308.0
[M+
H]
-364-
CA 02906542 2015-09-14
WO 2014/151147
PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
427FN1
Calcd
N' 0 N
H2N 0 0-Ni H2
265.2
7
Foun
d:
266.0
[M+
+ + + +
H] '
428 H op c)-NH2
Calcd
= N
H2N
265.1
0
Foun
d:
266.0
[M+
++ ++ + +++
H] '
429 [j I* (:)-NH2
Calcd
N', 01 "
cHN
385.1
Ne0
Foun
d:
386.2
[M+
H] '
+ + + +
430 H 140 c)-NH2
Calcd
N
HN
307.1
1¨ 1
Foun
d:
308.0
[M+
++ ++ + ++
H] '
431IN1
Calcd
N
HN liel c?-NH2
/¨
307.1
1
Foun
d:
308.0
[M+
+ + + +
H] '
-365-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
432 H2N4
Calcd
284.0
NH2 7
Foun
d:
285.0
[M+
++ +++ + ++++
H]
433 H2N4' 1101
Calcd
n
284.0
NH2 7
Foun
d:
285.2
[M+
+++ +++ ++ ++++
H]
434 iC)N4
Calcd
wip F
o 342.0
NH2 6
Foun
d:
343.0
[M+
++++ ++++ ++++ ++++
H]
435 -1:4
Calcd
wp F
o 342.0
6
NH2
Foun
d:
343.0
[M+
++ ++ ++++
H]
436 H2N4IS ,
Calcd
n
300.0
NH2 5
Foun
d:
301.1
[M+
++++ ++++ +++ ++++
H]
-366-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
437 H2N¨e = F
Calcd
300.0
NH2 5
Foun
d:
301.2
[M+
++ +++ + +++
H]
438 F
Calcd
=õ,
,====
381.1
Nr..xo 2
NH2 Foun
d:
382.2
[M+
++
H]
439 F
Calcd
381.1
0
0_1(NN 2
H2 Foun
d:
382.2
[M+
++
H]
440 N 9
Calcd
8 11
462.1
4
Foun
d:
463.0
[M+
H]
441
'12N 4)
10 811 F
442 ;4---
Calcd
so tar
No) 400.1
2
-367-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
Foun
d:
401.0
[M+
H]'
443 N H o
Calcd
N , II
O 101 g
400.1
2
Foun
d:
401.2
[M+
H]'
444 e--N 9
Calcd
0Z 110
?
492.1
Foun
d:
493.0
[M+
++
H]'
445 1;1- 9
Calcd
O 0 H
?430.1
3
Foun
d:
431.0
[M+
H]'
446 INIO
Calcd
C 0 SI
376.1
NH2 6
Foun
d:
377.2
[M+
+++ + H]'
447 00
Calcd
= Hois-1
a N
463.1
3
++
Foun
-368-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
d:
464.0
[M+
H]
448
Calcd
0 IW
377.4
ro Nz.xo
0
NH2
Foun
d:
378.2
[M+
+++ + ++
H]
449
Calcd
377.1
NH2 Foun
d:
378.2
[M+
H]
+++ +++ + ++++
450 N- 9
Calcd
0
Th INC) I 0 H
N
401.1
2
Foun
d:
402.2
[M+
++
H]
451N H
Calcd
o1iXc
N CI WI
497.9
5
Foun
d:
498.0
++++ +++ ++++ ++++ +++
[M]
452 F
Calcd
04 -
aL10
N CI
515.0
8
Foun
++++ +++ ++++ ++++ ++
d:
-369-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50
prolifer prolifer Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
516.0
[M+
H]
453
Calcd
O
g
c_cf
462.1
4
Foun
d:
463.0
[M+
++ ++
H]
454 (LN F
Calcd
04 PI 8
F
498.1
2
Foun
d:
499.0
[M+
++ ++ ++
H]
455 ç õ0
Calcd
0g-10
?
492.1
Foun
d:
493.2
[M+
++ ++
H]
456 (1-_-r, H
Calcd
04= NO
CC
?430.1
3
Foun
d:
431.0
[M+
H]
457 <-2N
F
Calcd
,
N CI 41111blIP
515.0
8
Foun
d:
++++ ++++ ++++ ++++ +++
516.0
-370-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a R IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
[M+
H] '
458 '17 , 14, ,c)
Calcd
No N CI Mr
F
515.0
8
Foun
d:
516.0
[M+
++++ ++++ ++++ ++++ +++
H] '
459 1 : , i
r, , ,c, Calcd
O 0 .r 0
a CI
496.1
0
Foun
d:
497.0
[M+
+++ + + +++
H] '
460 (1::,, ', 0, )0
Calcd
(:) 10 F
ON CI
434.0
8
Foun
d:
435.0
[M+
++ + + +
H] '
461 (L,,, , klõ,0
Calcd
,, W 0
0 - F F
532.0
8
Foun
d:
533.0
[M+
+++ ++ ++ +++
H] '
462nN:iiIõo
Calcd
O- 1 , 0 g-T)
a I N--
494.1
4
Foun
d:
++++ ++++ ++++ ++++ +++
495.0
-371-
CA 02906542 2015-09-14
WO 2014/151147 PCT/US2014/025090
mT0 PI3K PI3K PI3K 6 PI3K y PC3
T47D Mass
Structure RC a IC50 IC50 prolifer prolifer
Chara
IC50 IC50 IC50 (nM) (nM) ation ation cteriz
(nM) (nM) (nM)
(nM)* (nM) ation
[M+
H]'
463 N-
Calcd
377.1
o
NH2
6
Foun
d:
378.2
[M+
++++ ++ ++ +++
H]'
464 N-
Calcd
377.1
o
5
NH2
/0
Foun
d:
378.2
[M+
++++ + +++ ++
H]'
465 N
Calcd
40
432.1
9
NH2
ENo
Foun
d:
433.2
[M+
++++ + +++
H]'
466 N-
Calcd
0o
432.1
9N
9
NH2
cNo.)
Foun
d:
433.2
[M+
+++ ++ ++ +++
H]'
467 (12-N
Calcd
o 40
363.1
3
NH2
Foun
d:
364.0
++++ ++ ++ ++++ +++
[M+
-372-
DEMANDE OU BREVET VOLUMINEUX
LA PRESENTE PARTIE DE CETTE DEMANDE OU CE BREVET COMPREND
PLUS D'UN TOME.
CECI EST LE TOME 1 DE 2
CONTENANT LES PAGES 1 A 372
NOTE : Pour les tomes additionels, veuillez contacter le Bureau canadien des
brevets
JUMBO APPLICATIONS/PATENTS
THIS SECTION OF THE APPLICATION/PATENT CONTAINS MORE THAN ONE
VOLUME
THIS IS VOLUME 1 OF 2
CONTAINING PAGES 1 TO 372
NOTE: For additional volumes, please contact the Canadian Patent Office
NOM DU FICHIER / FILE NAME:
NOTE POUR LE TOME / VOLUME NOTE: