Note: Descriptions are shown in the official language in which they were submitted.
CA 02906596 2015-09-14
WO 2014/151766 PCT/US2014/026413
SCAFFOLD FOR SPRING LIGAMENT REPAIR
FIELD
[0001] The subject
disclosure is generally directed towards ligament
repair and/or augmentation, and particularly to an augment construct that is
configured to replace or augment a spring ligament in a foot.
BACKGROUND
[0002] This section
provides background information related to the
present disclosure which is not necessarily prior art.
[0003] A
spring ligament is an anatomical ligament that
interconnects various portions of a foot. Generally, the spring ligament may
interconnect at least a navicular and a calcaneus. Various branches of the
spring
ligament, however, may also extend to connect or interconnect the calcaneus
with
the first cuneiform.
[0004]
Injury to the spring ligament may lead to various issues, such
as flat-foot deformities. Injuries to the spring ligament may not generally be
diagnosed or repaired until various auxiliary issues present themselves, such
as
flat-foot deformities. Repairing a defect in the spring ligament, therefore,
may lead
to prevention of various afflictions.
SUMMARY
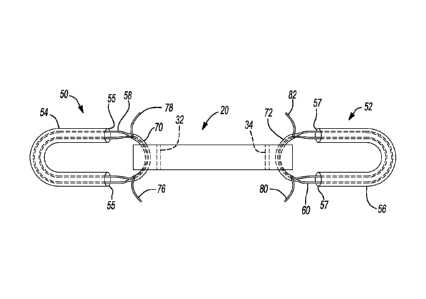
[0005] This
section provides a general summary of the disclosure,
and is not a comprehensive disclosure of its full scope or all of its
features.
[0006] A
ligament construct may be manufactured to repair or
replace a selected ligament. For example, a spring ligament replacement or
augment may include a synthetic scaffold that may be formed or manufactured to
generally replace the spring ligament. The synthetic scaffold may be formed of
polyester, polyethylene, resorbable materials, or combinations thereof. The
synthetic scaffold may be formed of a woven or braided polyester or
polyethylene
material that is formed into selected configuration or shape to replace the
spring
ligament.
1
CA 02906596 2015-09-14
WO 2014/151766 PCT/US2014/026413
[0007]
Selected suture anchoring systems and tensioning systems
may be used to fixedly connect the ligament augment to the anatomy. For
example, a JuggerknotTM soft anchor or suture anchor may be used to provide an
anchor adjacent to or inside of a bone member. Alternatively, a ToggleLocTm
hard
suture anchor may be used. A suture tensioning system, such as the Ziplooprm
suture tensioning system may be used to draw the scaffold into the bone, and
tension the augment relative to the bone while the JuggerknotTM anchor system
is
used to anchor the construct. It is understood, however, that the tensioning
system may be used in combination with any appropriate anchor, such as an
interference screw or other bone anchor system.
[0008] The
scaffold may be used to replace the spring ligament. For
example, a user may identify a spring ligament tear or removal from a bone
portion and identify a need for an augment or ligament replacement. The spring
ligament construct may then be fixed relative to the bone, such as with an
appropriate anchor and tensioning system. The anchors may include the
JuggerknotTM soft anchor system. The construct may then be maintained relative
to the bone for a procedure and/or fixed to the remaining portion of the
spring
ligament to assist in augmenting the remaining spring ligament portions.
[0009]
Further areas of applicability will become apparent from the
description provided herein. The description and specific examples in this
summary are intended for purposes of illustration only and are not intended to
limit
the scope of the present disclosure.
DRAWINGS
[0010] The drawings
described herein are for illustrative purposes
only of selected embodiments and not all possible implementations, and are not
intended to limit the scope of the present disclosure.
[0011] Fig.
1A is a top plan view of a ligament construct connected
to suture, according to various embodiments;
[0012] Fig. 1B is a
side plan view of the ligament construct of Fig. 1A
connected to suture;
[0013] Fig.
2A is a top plan view of a ligament construct according to
various embodiments;
2
CA 02906596 2015-09-14
WO 2014/151766 PCT/US2014/026413
[0014] Fig.
2B is a side plan view of the ligament construct of Fig. 2A
connected to suture;
[0015] Fig.
3 is a top plan view of a ligament construct according to
various embodiments;
[0016] Fig. 4 is a
detail view of a portion of a ligament construct
connected to a suture and an anchor, according to various embodiments;
[0017] Fig.
5 is a side plan view of bones of a human foot illustrating
a partial implantation of a ligament construct in a navicular bone;
[0018] Fig.
6 is a side plan view of bones of a human foot illustrating
a partial implantation of a ligament construct in a navicular bone and
calcaneus
bone;
[0019] Fig.
7A is a side plan view of bones of a human foot
illustrating an implantation and tensioning of a ligament construct in a
navicular
bone and calcaneus bone;
[0020] Fig. 7B is a
bottom plan view of bones of a human foot
illustrating an implantation and tensioning of a ligament construct in a
navicular
bone and calcaneus bone;
[0021] Fig.
8 is a side plan view of bones of a human foot illustrating
an implantation and tensioning of a ligament construct in a cuneiform, a
navicular
bone, and a calcaneus bone, according to various embodiments;
[0022] Fig.
9 is a top plan view of a ligament construct connected to
a suture, according to various embodiments;
[0023] Fig.
10 is a side plan view of bones of a human foot
illustrating an implantation and tensioning of a ligament construct in a
navicular
bone, talus bone, and calcaneus bone, according to various embodiments;
[0024] Fig.
11 is a side plan view of the ligament construct of Fig. 1A
connected to suture;
[0025] Fig.
12 is a top plan view of a ligament construct connected to
suture, according to various embodiments;
[0026] Fig. 13 is a
top plan view of a ligament construct connected to
suture, according to various embodiments; and
[0027] Fig.
14 is a side plan view of the ligament construct of Fig. 1A
connected to suture.
3
CA 02906596 2015-09-14
WO 2014/151766 PCT/US2014/026413
[0028]
Corresponding reference numerals indicate corresponding
parts throughout the several views of the drawings.
DETAILED DESCRIPTION
[0029]
Example embodiments will now be described more fully with
reference to the accompanying drawings.
[0030] With
reference to FIGS. 1A and 1B, a ligament augment or
replacement construct 20 is illustrated. The ligament construct 20 may be
formed
of selected materials, such as a woven or braided polyester or polyethylene
material. Additionally, various absorbable materials may be included or used
to
form the construct 20, such as polylactic acid or other selected materials.
The
construct 20 may be formed of a woven material and/or formed of a solid
material
of a selected type. The construct 20, according to various embodiments, may be
resilient with little to no elasticity. Thus, the length of the construct 20
may be
selected and maintained during implantation. Also, the construct 20,
therefore,
may be tensioned and maintain a tension after implantation.
[0031] The
construct 20 may include a selected length 22 that
extends between a first end 24 and a second end 26. The two ends 24, 26 may
be formed by folding terminal end regions 28 and 30 over and towards a center
of
the construct 20. The terminal ends 28, 30 may be folded onto a body portion
36
of the construct 20. The terminal ends 28, 30 may be stitched, sonically
welded,
or otherwise fixed with a mechanism 32, 34 to the main body portion 36 of the
construct. The folding over of the terminal ends 28, 30 may form passages,
also
referred to as eyelets, a loop, or open regions 40 and 42 near the ends 24, 26
of
the construct 20. The passages, as discussed herein, may be engaged or
coupled to sutures and/or anchor members.
[0032] The
main body portion, or selected portions of the construct
20, may have a thickness 44 based upon various characteristics of the
construct
20, such as strength, rigidity, elasticity, and the like. Moreover, it is
understood
that if the construct 20 is formed of a braided, woven, or other selected
material
material that the thickness 44 may decrease under a load between the two
eyelet
4
portions 40, 42. The thickness 44, however, may be defined by one or more
strands of a woven material
[00033] According to
various embodiments, the eyelets 40, 42 may
be formed around suture and one or more suture constructs including a first
suture
construct 50 and a second suture construct 52; and/or the suture constructs
50, 52
may be formed within the eyelets 40, 42 and around the construct 20. The
suture
constructs 50 and 52 may be separate, but substantially identical and
interconnected
with the separate eyelets 40, 42 for fixation of the construct 20 relative to
a selected
anatomy, as discussed further herein. The suture constructs 50, 52 may be
similar to
the suture constructs disclosed in U.S. Patent No. 8,118,836, issued February
21,
2012; U.S. Pat. App. Pub. No. 2011/0098727, published on April 28, 2011; or
U.S. Pat.
App. Pub. No. 2012/0095470, published on April 19, 2012.
[00034] Generally, the
suture constructs may include respective
anchors that may be formed as tubes 54, 56 through which a suture or selected
filament 58, 60 passes. The suture 58, 60, when acted upon by a user, may
cause the
tubes 54, 56 to form an anchoring mass as knots or soft anchors to hold the
construct
in a selected position. The anchoring mass can be formed on an exterior
surface of
20 a bone or
formed within a bone surface, as discussed further herein. The suture
anchors may include the ZiploopTM constructs to allow for slip tensioning of a
portion
positioned relative to the Ziploop suture construct and a Juggerknot TM anchor
portion
that may be formed by the respective tubes 54, 56. Each of the ZiploopTM
suture
construct and the JuggerknotTM soft anchor portion are sold by Biomet, Inc.
[00035] The sutures 58,
60 may further pass through a body or
passage portion 70, 72 where two suture ends of each suture construct 50, 52
extend
from the respective passage portions 70, 72. Each of the sutures 50, 52 has
the
respective passage portion 70, 72 and each defines a respective through-
passage
through the passage portions 70, 72. Each suture construct 50, 52 further has
two
ends 76, 78 and 80, 82, For each suture construct the first end 76, 80 is
passed into
the through-passage through a first aperture to form a first loop and exits
the through-
passage through a second aperture and the second end 78, 82 is passed into the
through-passage through a third aperture to form a
5
CA 2906596 2018-03-27
second loop and exits the through-passage through a fourth aperture. It is
understood, according to various embodiments, the first and third aperture may
be the
same aperture as may the second and fourth aperture. Thus, each passage
portion
may include 2, 3, or 4, or a selected number of apertures into the through-
passage of
the passage portion. Each of the two suture ends 76, 78 and 80, 82 may be
acted
upon by a user to collapse the respective anchors 50, 56 to form the soft
anchoring
masses and tension the construct 20 between two points of the anchors 54 and
56,
as discussed further herein.
[00036] The anchor
assemblies 50, 52 including the anchors 54, 56,
also referred to as tubes, which may generally include a collapsible tube that
is a
closed loop of suture material that is formed into the tube shape. According
to various
embodiments, the tube assembly 54, 56 may be slideable relative to the suture
loops
or filaments 58, 60. Briefly, although disclosed in the U.S. Patent No.
8,118,836, the
suture assemblies 50, 52 may include the suture portions 58, 60 that pass
through
openings 55, 57 of the respective tubes 54, 56. Alternatively, the suture
portions may
pass out an aperture that is not at the openings 55, 57 such that one or both
ends of
the tubes 54, 56 form flaccid or unbound ends.
[00037]
Generally, the suture portions 58, 60 may be single or
continuous suture filaments that extend through the respective tubes, 50, 56
in the
respective braided bodies 70, 72 of the suture assemblies 50, 52. The suture
assemblies 50, 52, therefore, may be manipulated by a user to generate a soft
anchor,
as discussed herein, by collapsing the respective tubes 50, 56, and tensioning
the
construct 20 relative to selected areas of a subject by moving the ends 76-82
to tension
the construct 20 relative to the respective anchors formed by the tubes 54,
56. The
anchors 54, 56 collapse under a force, such as a tensile force, provided by or
through
the respective suture portions 58, 60 to form soft or anchor masses in
respective areas,
as discussed further herein. The tubes 54, 56 may form the anchors by
collapsing the
tubes 54, 56 relative to a positive or adjacent surface of the tube 54, 56.
[00038] With reference to
FIGS. 2A and 28, a construct 100 is
illustrated. The construct 100 may be similar to the construct 20 in general
construction. For example, the construct 100 may be formed of a woven selected
6
CA 2906596 2018-03-27
material, such as polyester or polyethylene. Additionally, the construct 100
may be
formed from a selected material including bio-absorbable materials that may be
absorbed into a selected anatomy over time and allow for ingrowth of various
tissues to
replace portions of the construct 100.
[00039] The
construct 100, however, may also be formed in a
substantially elongated and rectangular construct including a length 102 from
a first
end 100a to a second end 100b of about 4 centimeters (cm) to about 10 cm, and
further including 4 cm to about 8 cm. Additionally, the construct 100 may
include a
width 104 that is about 0.5 cm to about 4 cm, including about 1-3 cm, and
further
including about 1 cm to about 2 cm. The construct 100, as illustrated in FIG.
28, may
further include a thickness 106 that is about 0.5 millimeters (mm) to about 4
mm, and
further including about 1 mm to about 3 mm.
[00040] The construct 100
may include one or more bores 110
formed from a first side to a second side through the thickness 106 of the
construct
100. The bores 110 may be formed in a selected configuration, such as near a
perimeter of the construct 100. The bores 110 are generally formed at or near
both of
the ends 100a, 100b of the construct 100. Thus, the ends 100a, 100b of the
construct
100 can be engaged and tensioned relative to selected anchoring regions, as
discussed herein. The bores 110 may allow for passage of selected anchor
assemblies, such as an anchor assembly 120, as discussed further herein. Any
appropriate anchoring assembly, such as the construct 50, however, may also be
used.
[00041] The
anchor assembly 120 may pass through one or more
of the throughbores 110 to allow for fixation of the construct 1 00 relative
to a selected
subject. The bores 110 may be throughbores formed through the construct 100
through various techniques such as puncturing, drilling, ultrasonic welding or
puncturing, or other appropriate mechanisms. Also, the through bores 110 may
be
formed between braided or woven fibers of the construct 100. Regardless, the
bores
110 may be throughbores formed through the entire thickness 106 of a construct
100 to
allow for passage of anchor assemblies 120.
[00042] The
anchor assembly 120 may be an anchor assembly
including an anchor assembly as disclosed in U.S. Patent 8,118,836. The anchor
assembly 120 may include various portions
7
CA 2906596 2018-03-27
similar to the anchor assemblies 50, 52 described above. Generally, the anchor
assembly 120 may include a first collapsible anchor 122 (also referred to as a
tube) and a second collapsible anchor 124 (also referred to as a tube) which
are
disposed about or around respective suture loops 126 and 128 that are a part
of a
single elongated suture of the anchor assembly 120. As tension is applied on
the
respective suture loops 126, 128, the anchors 122, 124 collapse and may form
anchoring masses relative to selected portions of a subject, similar to the
anchors 54,
56 discussed above. It is understood that the respective suture anchors 122,
124 may
be collapsed simultaneously and/or at staggered times, as selected and/or
desired
by a user.
[00043] The
suture loops 126, 128 are portions of a suture 130
that further include suture ends 132 and 134. The suture ends may be gasped or
manipulated by a user to collapse the tubes 122, 124 to form the soft anchors.
Additionally, the suture 130 may be passed through a passage portion 140
having a
through-passage as discussed above and as disclosed in U.S. Patent 8,118,836.
The
suture ends 132, 134 may be manipulated to move the passage portion 140
relative to
the tubes 122, 124. As discussed above, the movement of the passage portion
140
relative to the tubes 122, 124 may tension the construct 100 relative to the
tubes 122,
124 when the tubes are collapsed against a respective positive surface, such
as a
bone surface. Accordingly, the construct 100 may be tensioned relative to a
bone
surface as discussed further herein by pulling on the ends 132, 134 of the
suture loop
130.
[00044] With
reference to FIG. 3, a construct 170, according to
various embodiments, is illustrated. The construct 170 may include various
dimensions,
such as a length 172 that may be about 3 cm to about 10 cm, including about 4
cm to
about 8 cm. The construct 170 may further include a height 174 that includes a
selected height of about 1 cm to about 4 cm, including about 2-3 cm. The
height 174
may include a height or a length of arm portions 176 and 178 that extend from
a main
body portion 180 of the construct 170. The main body portion 180, however, may
include an end 182 that may include a height 184 having a dimension of about
0.5 cm
to about 2 cm, and further including a height of about 1 cm.
8
CA 2906596 2018-03-27
[00045] The
construct 170 may be similar in construction to the
constructs 20 and 100, as discussed above. Accordingly, the construct 170 can
include any selected portion to interconnect with a suture and/or anchor
assembly. For
example, an eyelet can be formed near the terminal ends of the construct
and/or
throughbores may be formed through the construct 170. For example, a suture
construct 52a that includes an anchor 56a can be coupled to an eyelet formed
by
folding over a terminal end and fixing it with a fixing structure 183 near the
first end
182. The suture construct can be similar or identical to the suture construct
521. It
is understood that each arm 176, 178 can include a fold formed eyelet and one
or
more throughbores and individual coupled suture constructs, according to
various
embodiments.
[00046] The
construct 170, however, may include the single end
182 and the two arms 176 and 178 extending from an area at the second end of
the
construct 170. As discussed herein, the first end 182 may engage a first bone
member or portion while the two arms 176 and 178 may engage separate bone
members or constructs as discussed herein. Thus, at each arm 176, 178 and the
first
end 182 a suture construct including an anchor portion can be connected.
Fixation
of the construct 172 to selected bone portions may include various suture
anchors and .
assemblies 50, 52 and 120 as discussed above. Accordingly, the construct 170
may
be fixed relative to a selected portion of a subject similar to the fixation
or anchoring
mechanisms as discussed above.
[00047] With
reference to FIGS. 1A-1B, discussed above, and
additional reference to FIG. 4, the construct 20, or construct according to
various
embodiments as discussed above, may be engaged or associated with an anchor
assembly 190. The anchor assembly 190 may include a fixation member 192, such
as a Toggleloom' anchor, sold by Biomet, Inc., that is rigid. The rigid
fixation member
192 may include that illustrated and disclosed in U.S. Patent Application
Publication
No. 2012/0116452 and U.S. Patent No. 7,695,503. In addition, the anchor 192
may be
interconnected or associated with a suture assembly 202, similar to the suture
assemblies 50, 52 discussed above. The suture assembly 202 may include a
passage
portion 204 to which a continuous suture 206 is passed. The continuous suture
206
may include a continuous loop of suture that passes through the passage
portion
204. The
9
CA 2906596 2018-03-27
CA 02906596 2015-09-14
WO 2014/151766 PCT/US2014/026413
passage portion 204 may be formed through the anchor 192, as illustrated in
FIG.
4.
[0048] The
construct 20 may also be formed around the filament or
loop portions of the suture portion 208 to engage the construct 20 relative to
the
anchor 192. The construct 20 may have the eyelet 42 formed by suturing or
interconnecting an end 30 of the construct 20 with a suture portion 34. The
construct 20, therefore, may be held relative to the anchor 192 with the
suture
assembly 202. Generally, the anchor 192 may include an elongated arm portion
220 and an eyelet or interconnection portion 222 to engage the suture assembly
202.
[0049]
Accordingly, it is understood that the construct, according to
various embodiments including the construct 20, the construct 100, and the
construct 170, may be interconnected with a selected anchor or connecting
portion. The connecting portion may include soft anchors, such as those
illustrated with the suture assembly 50 or the suture anchor assembly 120.
Additionally, a hard or rigid fixation member may include the fixation member
192
illustrated in FIG. 4.
[0050] The
ligament construct, according to various embodiments as
discussed above, may be provided relative to a selected portion of a subject,
such
as a foot 300 of a subject, illustrated in FIG. 5. The foot 300 of the subject
may
include various anatomical bone portions, such as those generally known in the
art. For example, the foot 300 may include phalange members 302, metatarsal
members 304, a medial (first tarsal) cuneiform 306, a navicular 308, a talus
310,
and a calcaneus 312. The foot 300 may be connected or articulated with other
boney portions, such as a tibia and a fibula, as is generally understood in
the art.
[0051] With
additional reference to FIG. 5, the various boney
portions of the foot 300 may be interconnected with a construct, including the
various embodiments of a construct as discussed above. For
exemplary
purposes, the construct 20 is illustrated and discussed. It is understood,
however,
that the construct 100 may be used relative to the foot 300 including the
suture
assembly 50, 52 and/or the anchor suture assembly 120, as discussed above.
Nevertheless, for illustration of a selected procedure to replace a spring
ligament
CA 02906596 2015-09-14
WO 2014/151766 PCT/US2014/026413
or augment a spring ligament in a foot 300, the construct 20 is discussed
further
herein.
[0052] As
illustrated in FIG. 5, the navicular 308 may be configured
to include a throughbore 320 through which the anchor 54 may be passed and
collapsed upon an exterior bone surface of the navicular 308 to form the
anchoring mass or portion 54', such as a soft anchor, relative to the
navicular
bone 308. The anchor portion 54' may be defined as a large or anchoring mass
formed near the bone surface. A locking profile of the tube 54 may allow the
anchoring mass to be formed by the application of a tensile force with the
suture
58. The bore 320 may be formed to allow for passage and receipt of at least a
portion of the construct 20 into a portion of the bore 320. The drawing or
tensioning of the construct 20 into the bore 320 may be performed with the
suture
assembly 52 by drawing or tensioning the suture ends 76, 78 and drawing the
construct 20 into the bore 320. The formation of the bore 320 and tensioning
of
the construct 20 into the bore 320 will be discussed further in relation to
the
calcaneus 312 in a bore 340 formed therein, discussed further herein.
[0053] With
a continuing reference to FIG. 5, the calcaneus 312 may
have a bore 340 formed therein including a plurality of diameters. For
example, a
first or small drill/pin 342 may be passed through or drilled through the
calcaneus
312 to form a small bore or first bore passage 344. A second or larger drill
bit 349
(the larger drill bit 349 may be cannulated) may be passed over the small bit
342
to form a second or larger bore 352. Accordingly, the bore 340 formed through
the calcaneus 312 may include a first diameter bore 344 and a second diameter
bore 352. The bore 320 formed in the navicular 308 may include a similar large
diameter bore portion and a small diameter bore portion. The large diameter
bore
portion may receive a portion of the construct 20 while the small diameter
bore
portion may allow for passage of the suture construct 50, 52 to allow for
movement of the respective tubes 54, 56 to a second portion of the respective
bone to form the soft anchor relative thereto. Moreover, a shoulder or ledge
320a,
340a is formed within the respective bores 320, 340 due to the different
diameters.
[0054] With
reference to FIG. 6, the anchor assembly, including the
suture anchor assembly 52 that is connected to an end of the construct 20, may
11
CA 02906596 2015-09-14
WO 2014/151766 PCT/US2014/026413
be passed through the bore 340 formed to the calcaneus 312, or selected bone
portion. The suture assembly 52 and/or only the anchor 56 alone may be passed
through the bore 340. An appropriate passing member may interconnect with the
anchor 56 and/or the suture ends 80, 82 to push or pull the suture assembly
through the bore 340 formed to the calcaneus 312. The construct 20
interconnected with the suture anchor assembly 52, however, may be moved
relative to the calcaneus 312 to allow for passage of the suture anchor
assembly
52 through the bore 340 formed through the calcaneus 312.
[0055] As
illustrated in FIG. 6, the construct 20 may be pulled into
the bore 320 formed in the navicular 308 and the anchoring portion 54' may be
formed relative to the navicular 308 by tensioning the anchor 54 relative to
the
navicular 308, as is generally understood in the art. The suture assembly 52
may
then be moved through the bore 340 formed through the calcaneus 312, as
illustrated in FIG. 6. It is understood, however, that the suture assembly 52
may
be first passed through the calcaneus 312 prior to passage of the suture
assembly
50 through the navicular 308. The order of placing the anchors relative to
individual bone portions is exemplary, and not intended to be limiting. Aafter
forming the bore 340 through the calcaneus 312, the suture assembly 52 may be
passed through the calcaneus 312. According to various embodiments, the
anchors, including the soft anchors, can be formed on the surface of the bone
portions at all selected anchoring points. Then each end of the construct can
be
alternatingly tightened to substantially ensure uniform tensioning of the
construct
20.
[0056] The
passage of the suture assembly 52 through the
calcaneus 312 may generally be in the direction of arrow 313. The passage of
the
suture assembly 52 may be according to appropriate mechanisms, such as pulling
the suture assembly 52 through the bore 340 or pushing the suture assembly 52
through the bore 340. Generally, however, the anchor 56 may be passed through
a distal cortical bone portion 391a, as illustrated in FIG. 7A, and a second
cortical
bone portion 391b, is also illustrated in FIG. 7A. The bore 340 may further be
formed through both of the cortical bone portions 391a and 391b and further
through the cancellous bone portion 389, therebetween.
12
CA 02906596 2015-09-14
WO 2014/151766 PCT/US2014/026413
[0057]
Accordingly, a soft anchor may be formed by the anchor 56
on an exterior distal surface of the cortical bone portion of the calcaneus
312. The
anchor 56 may be formed by drawing the construct 20 into the large bore
portion
352, by manipulating the ends of the suture filaments 80, 82, to form the soft
anchor 56'. The construct 20 may then be tensioned and drawn into the bore
340.
[0058] With
continuing reference FIGS. 5 and 6, and additional
reference to FIGS. 7A and 7B, the construct 20 may be tensioned into the bore
320 formed within the navicular 308 and the bore 340 formed in the calcaneus
312. The bore 320 in the navicular 308 may include a large bore portion 320a
and
a small bore portion 320b similar to the large bore 352 and the small bore
portion
344 formed through the calcaneus 312. The large bore portion, of both the
navicular 308 and the calcaneus 312, may include a diameter 352a such as about
4 mm to about 6 mm. The small bore portion of both the calcaneus 312 and the
navicular 308 may include a diameter 344a of about 2 mm to about 4 mm. It is
understood, however, that the bore formed through the respective bone portions
may be any appropriate diameter to receive the constructs positioned within
the
respective bore portions and interact with the anchor members positioned
relative
to the bone portions.
[0059] The
anchor portion 54' formed by the tube 54 and anchor
portion 56' formed by the tube 56 may be formed on an exterior surface of the
bone portions 308, 312 such as on an exterior surface of the cortical bone
thereof.
The anchor portions 54', 56' can fix or anchor the construct 20 relative to
the bone
portions. The anchor portions 54', 56' may be defined as a large or anchoring
mass formed near the bone surface. A locking profile of the tubes 54, 56 may
allow the anchoring mass to be formed by the application of a tensile force
with
the suture 58, 60. The construct 20 may then be tensioned relative to the
anchor
portions 54', 56' to form a selectively tensioned construct 20 relative to the
two
bones of the navicular 308 and the calcaneus 312 to augment or replace the
spring ligament.
[0060] The soft
anchors 54', 56' however, may be formed within the
respective bone portions 308 and 312 relative to a cancellous bone portions
389
and 367 to engage the proximal cortical bone portions 391b and 369 of the
respective bone members 308 and 312. Accordingly, the soft anchor portions
54',
13
56' need not be formed on the exterior surface of the respective bone member
308 and
312 to anchor the construct 20 relative to the respective bone portions 308
and 312.
Rather, the bone anchors or construct anchors may be formed within the bone
surfaces
to provide for fixation of the construct 20 relative to the respective bone
members 308
and 312. Formation of an anchor assembly within the bone member may be formed
as
discussed above and with an anchor assembly as disclosed in U.S. Patent
Application
2011/0098727, published April 28, 2011. The formation of the soft anchor 54',
56' may
be substantially similar, save that the soft anchor is formed within the
tunnel 340 and
320 of the respective bone members.
[00061] The construct 20 may be positioned relative to
respective bone portions, such as the calcaneus 312 and navicular 308, as
discussed
above and illustrated in various figures, including FIGS. 6-78. The construct
100 may
be similarly positioned between two selected bone portions, such as the
calcaneus 312
and navicular 308. According to various embodiments, the construct 170 may be
interconnected with two or more bones portions of the foot 300, as illustrated
in FIG. 8.
The construct 170, including the main body portion 180, the arm member 178,
and the
arm member 176 may be connected or positioned relative to the navicular 308
and the
first cuneiform 306. Additionally, the end of 182 of the main body 180 may be
positioned relative to the calcaneus 312 of the foot 300.
[00062] The fixation of the construct 170 relative to the
respective bone portions may be with the suture anchor construct, such as the
suture
anchor construct 52. Accordingly, each of the ends of the construct 170 may be
coupled to bone portions with a suture anchor such as the suture anchor at 56a
relative
to the first cuneiform, a suture anchor 56b relative to the navicular 308, and
a suture
anchor 56c relative to the calcaneus 312. It is understood, however, that the
various
anchor of fixation members may be positioned relative to the respective boney
members, including the first cuneiform 306, navicular 308, and the calcaneus
312, to fix
the construct 170 relative thereto. For example, the suture anchor assembly
120 may
be interconnected with the construct 170 to anchor relative to the selected
bone
portions in addition to the anchor fixation member 190, as illustrated in FIG.
4.
14
CA 2906596 2018-03-27
CA 02906596 2015-09-14
WO 2014/151766 PCT/US2014/026413
[0063]
Accordingly, a ligament construct may be provided to
augment and/or replace the spring ligament. The ligament construct, therefore,
can interconnect various portions of the foot 300, including the calcaneus 312
and
the navicular 308. In various embodiments, the first cuneiform 306 may also be
interconnected relative to the navicular 308 and the calcaneus 312. The
interconnection of the respective boney portions of the foot 300 may be
provided
to augment or replace the spring ligament of the foot 300. The spring ligament
to
the foot 300 may be further augmented by interconnecting the respective
construct, such as the construct 20 or the construct 170, with the spring
ligament.
The interconnection of the construct relative to the spring ligament may be
provided by further suturing the construct to the spring ligament. For
example, the
construct may be sutured directly to the spring ligament by generally known
suture
techniques such as threading or knotting the appropriate construct to a
portion of
the spring ligament that is remaining in the foot 300. For example, the spring
ligament may be interconnected by the navicular bone 308 portion and the
augment may be sutured to the spring ligament that remains connected at the
bone portion. Accordingly, it is understood that the construct, as discussed
above, may be provided to replace or augment the spring ligament in the foot
300.
Moreover, the construct may be interconnected with a respective bone portion
of
the foot 300, according to various embodiments, including those discussed
above.
The construct, such as the construct 20, the construct 100, or the construct
170
may be formed through various weaving and molding techniques to provide an
appropriate construct to replace or augment the spring ligament in the foot
300.
[0064] With
reference to FIGS. 9 and 10, a deltoid ligament in the
foot 300 can interconnect a tibia 321 relative to the foot 300. The deltoid
ligament
can interconnect the tibia 321 relative to the calcaneus 312, the navicular
308,
and a talus 322. The tibia 321, the talus 322, the navicular 308 and the
calcaneus
312 can be interconnected with a construct 350, as illustrated in FIG. 9. The
ligament construct 350 can include construct bodies 350a and 350b that are
formed similar to the ligament construct 20, as discussed above. Accordingly,
the
construct bodies 350a and 350b can be formed as substantially elongated
members extending between first and second respective ends and can be formed
of a woven polymer material and/or a solid material.
CA 02906596 2015-09-14
WO 2014/151766 PCT/US2014/026413
[0065]
According to various embodiments, the first construct body
350a can extend from a first end 360 that has a first eyelet formed by folding
over
and fixing (e.g. via suturing or sonically welding) with a fixing member or
system
362 a terminal end of the main body to an internal position of the body 350a.
Positioned through the eyelet at the first end 360 can be a first suture
construct
364. The first suture construct 364 can be similar to the suture construct 50
discussed above, and will not be described here in detail. Nevertheless, the
suture construct 364 can include a collapsible tube 366 that is formed around
a
suture 368 that passes through a passage portion 370 with first and second
suture
ends 372 and 374 extending therefrom. As discussed above, the suture construct
364 can be positioned relative to a bone portion, as discussed further herein,
to
allow the collapsible tube 366 to form an anchoring mass by drawing or pulling
on
the suture end 372 and 374.
[0066] The
first construct body 350a may extend from the first end
360 to a second end 380 that also has an eyelet that may be formed by fixing a
terminal end at the second end 380 with a fixation portion 382. A second
suture
construct 390 can also be passed through the eyelet that includes a
collapsible
tube 394 formed around a suture 396. The suture 396 can have a passage
portion 398 through which the suture passes and a first end 400 and a second
end 402 extends therefrom. Again, the suture construct 390 can be used to
tension and anchor the first body 350a relative to selected boney portions, as
discussed further herein.
[0067]
Additionally, passed or formed through the eyelet at the
second end 380 can be the second construct body 350b. The second construct
body 350b can extend from a first end 420 that forms an eyelet by having a
terminal end portion fixed a distance from the terminal end with a fixation
mechanism 422. Passed through the eyelet formed at the first end 420 can be a
third suture construct 430, similar to the suture constructs 364 and 390.
Accordingly, the suture construct 430 can include a collapsible tube 432 that
is
formed around a suture 434 that passes through a passage body or portion 436
with a first terminal end 438 and a second terminal end 440 extending
therefrom.
Again, the suture construct 430 can be used to fix or anchor the second body
16
CA 02906596 2015-09-14
WO 2014/151766 PCT/US2014/026413
portion 350b by forming the collapsible tube 432 into an anchor mass by
pulling
on the terminal suture ends 438 and 440.
[0068] The
second construct body 350b may extend from the first
end 420 to a second end 450 that may also include a second eyelet formed by
fixing a terminal end a distance from the terminal end with a fixation portion
452.
A fourth suture construct 460 can also be passed through the eyelet formed at
the
second end 450. The fourth suture construct 460 can include a collapsible tube
462 that is formed over a suture 464. The suture 464 can include a passage
portion 466 from which extends a first terminal suture end 468 and a second
terminal suture end 470. Therefore, the fourth suture construct 460 can also
be
used to form an anchoring mass by drawing the terminal suture ends 468 and 470
as discussed above.
[0069] The
augment or replacement construct 350 can be used to
interconnect at least a tibia 321, talus 322, navicular 308, and calcaneus 312
by
positioning the respective suture constructs, 390, 430, 460, and 364 in bone
tunnels formed in the respective bone portions. Without repeating the
procedure
discussed above for forming the bone tunnels, bone tunnels can be formed in
and/or through each of the bone portions similar to a manner as discussed
above.
Accordingly, a first tunnel 500 can be formed in the tibia 321 and the
collapsible
tube 394 can be formed into the anchoring mass 394' on an exterior surface of
the
tibia 321. A portion of the construct 350 can then be tensioned or drawn into
the
tunnel 500, as discussed above. A second tunnel 520 can be formed through the
talus 322 and the collapsible tube 432 can be formed into the anchoring mass
432' on an exterior surface of the talus 322. A portion of the construct 350
can be
tensioned into the tunnel 520, as discussed above. A third tunnel 530 can be
formed in the calcaneus 312 and an anchor mass 366' can be formed from the
collapsible tube 366 and the construct can be tensioned into or onto the
calcaneus
312, as discussed above.
[0070] A
fourth tunnel 540 can be formed into the interior talus or,
alternatively, into the navicular 308 (shown in phantom at 540'). It is
understood,
however, that if the alternative tunnel 540' is formed in the navicular 308
the
tunnel 540 may also be formed in an anterior portion of the talus 322 to allow
connection of the navicular 308 and the talus 322. The suture construct can be
17
CA 02906596 2015-09-14
WO 2014/151766 PCT/US2014/026413
similar to the anchor 120, illustrated in FIG. 2B, to allow for
interconnection with a
tunnel formed in the anterior portion of the talus 322. Accordingly, it is
understood
that the construct 350 can be provided to interconnect a plurality of bone
portions
of the foot 300 including interconnection with a leg bone portion, such as the
tibia
321. Moreover, it is understood that the suture constructs can be provided in
various configurations to allow for fixing of the ligament or tendon construct
350 to
the respective bone portions.
[0071] With
reference to FIGS. 11 and 12, a ligament augment or
replacement construct 600 is illustrated. The ligament construct 600 can be
substantially similar to those discussed above, such as the construct 20
illustrated
in FIGS. 1A and 1B. For example, the ligament construct 600 can be formed of
various materials, such as polylactic acid and/or other absorbable materials.
The
construct 600 can be formed as a woven, braided, or a solid material.
Moreover,
one or more of a terminal end 602 of the ligament construct 600 can be folded
inward or inboard toward a center of the ligament construct 600. Thus, the
terminal end 602 is folded upon the construct 600 to form one or more folded
portions or regions 603. The construct 600, therefore, can have a portion that
is
folded upon itself, as in the folded region 603. The folded region 603 can
include
a first loop region or eyelet 610 and an overlap region 612.
[0072] The folded
portion, or at least a portion thereof, can be
adhered in place with welding points 604 and 606 and/or stitching or suturing.
The folding over of the terminal end 602 onto the body of the ligament
construct
600 can form the first loop region or eyelet 610. Formed in the overlap region
612
of the terminal end 602 onto the ligament construct 600 can be a bore eyelet
614.
The bore eyelet 614 can be formed through both a main portion 618 of the
ligament construct and the overlap portion 620 that extends from the terminal
end
602. Accordingly, the bore eyelet 614 can be formed through two layers of the
ligament construct 600.
[0073] As
illustrated in FIG. 12, a second terminal end 630 can also
be folded over on the ligament construct to form a second overlap region 632
similar to the first overlap regions 612. A second throughbore eyelet 634 can
also
be formed through the overlap region 632, such as with sonic welding or
boring.
Thus, both ends of the construct 600 may have bores formed therethrough.
18
CA 02906596 2015-09-14
WO 2014/151766 PCT/US2014/026413
[0074] A
suture construct or at least a portion thereof, such as the
suture construct 50, may be passed through or formed through the first bore
614.
The second suture construct 52 can be passed through the second bore 634.
Accordingly, the ligament construct 600 can be provided for use in a procedure
similar to the construct 20, discussed above. The suture constructs 50 and 52,
however, can pass through bores 614 and 634 formed through overlap regions
612 and 632 rather than through the eyelet 600 formed through the construct
ends
602, 630 or by the overlap of the suture ligament construct 600.
[0075] By
providing the bores 614 and 634 through an overlap
region, the withdrawal or pull-out strength may be increased by providing a
greater mass and width of the ligament construct 600 to be pulled through. The
eyelets 614 and 634 may be formed at any appropriate selected location
relative
to the ligament construct 600 and the fold-over eyelet 610 and need not be
formed
at a terminal end of the overlapped ligament construct 600.
[0076] With reference
to FIGS. 13 and 14, a ligament construct 650
is illustrated. The ligament construct 650 can extend from a first end to a
second
end, similar to the ligament constructs discussed above, including those
illustrated
in FIGS. 1A and 1B. Each end of the ligament construct can include at least
one
eyelet 652 similar to the eyelets 40 and 42 of the ligament construct 20. The
eyelet 652 can be formed similar to the eyelet as discussed above, such as
overlapping or folding over a terminal end 654 of the suture construct 650 and
fixing it with a weld or suture element 656. The eyelet 652 of the ligament
construct 650 can be rounded or curved relative to a width 650w of the
ligament
construct 650.
[0077] A first edge
660 can be folded or rolled towards an axial
center line or longitudinal axis 662 of the ligament construct 650. A second
edge
664 can also be folded or rolled towards the central axis 662. The first edge
660
and the second edge 664 can be exterior lateral or longitudinal edges. Each of
the edges 660, 664 can be folded towards a central longitudinal axis 662 of
the
construct 650, as discussed further herein. Accordingly, the folded or rolled
dimension relative to the fold width 650w at the rolled region can be less
than the
width 650w and can include a width 670. The width 670 can be less than the
fold
width 650w as defined in phantom 672 in FIG. 13.
19
CA 02906596 2015-09-14
WO 2014/151766 PCT/US2014/026413
[0078] The
suture construct 50 can be passed or formed through the
eyelet 652 similar to the suture construct 50, 52 discussed regarding FIG. 1A.
It is
further understood that the ligament construct 650 can include a second end
and
may have a similar eyelet 652 or an eyelet similar to those discussed above. A
second suture construct can be passed through the second eyelet of the
ligament
construct 650 in a manner similar to that described for the construct 350.
[0079] The
end of the ligament construct 650 including the eyelet
652 can be passed through a bore, such as a bore in a bone, as illustrated
above
in FIG. 6. The eyelet 652 formed by folding the edges 660 and 664 can reduce a
width of the eyelet region 652 to be less than a width 650w of the ligament
construct 650. Accordingly, the eyelet portion 652 can be efficiently inserted
into
a bore formed in a bone. Additionally, rolling or folding the edges over can
provide a smoother and/or contoured edge to minimize contact with a bore and
ease passage through a bore. The rolled and folded edges can be maintained in
their rolled and folded configuration by a fixation member, such as a suture
680 or
other appropriate adhesive. The eyelet 652 can therefore, be formed with the
ligament construct 650 for positioning relative to and/or passing through
bores
formed in boney portions.
[0080] It is
understood that the various embodiments of the construct
including the eyelets discussed above can be formed in substantially similar
manner and interchanged for various purposes. The construct can be used for
various purposes, including ligament and/or tendon repair, augmentation, or
replacement. Moreover, a selected eyelet can be formed in an appropriate
manner for selected procedures, as is understood by one skilled in the art.
Moreover, forming the eyelets in various manners can be used for productions,
efficiencies and/or costs and specific procedures.
[0081] The
foregoing description of the embodiments has been
provided for purposes of illustration and description. It is not intended to
be
exhaustive or to limit the disclosure. Individual elements or features of a
particular
embodiment are generally not limited to that particular embodiment, but, where
applicable, are interchangeable and may be used in a selected embodiment, even
if not specifically shown or described. The same may also be varied in many
ways. Such variations are not to be regarded as a departure from the
disclosure,
CA 02906596 2015-09-14
WO 2014/151766 PCT/US2014/026413
and all such modifications are intended to be included within the scope of the
disclosure. The terminology used herein is for the purpose of describing
particular
example embodiments only and is not intended to be limiting. As used herein,
the
singular forms "a," "an," and "the" may be intended to include the plural
forms as
well, unless the context clearly indicates otherwise.
21