Note: Descriptions are shown in the official language in which they were submitted.
- 1 -
CONCURRENT TREATMENT OF ORAL AND SYSTEMIC MALADIES
USING DIRECT CURRENT ELECTRICITY
Technical Field
The technical field relates to systems and methods for
conducting an electric current within a mouth of patient.
More particularly, the technical field relates to a
mouthpiece that includes electrodes for conducting an
electric current across gingival tissue.
Background
This invention relates to a method of concurrently
promoting general oral hygiene, treating periodontal
diseases such as gingivitis and periodontitis, killing oral
microbes including cavity-causing bacteria, reducing oral
biofilms, increasing blood flow in oral tissues, increasing
salivation, promoting gingival tissue regeneration,
fostering osteogenesis in the boney structures of the teeth,
mouth and related areas, treating systemic diseases
associated with oral bacteria, and treating other
Date Recue/Date Received 2020-07-13
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 2 -
periodontal and oral maladies through the non-invasive
application of weak direct current electricity to the
surfaces in the oral cavity, and it also relates to an
apparatus suitable for providing direct current
electricity for these therapeutic, prophylactic, and
regenerative effects_
Periodontal disease has been identified as a risk
factor for various systemic diseases by both dentists
and physicians. Included in these diseases are
cardiovascular disease, adverse pregnancy outcomes, and
diabetes with newfound evidence supporting its
association with pancreatic diseases and arthritis.
While many of the studies establish correlation between
the presence of periodontal disease and these systemic
conditions, causation, with most of these conditions,
is still a subject of ongoing research. A few of the
biological mechanisms which have been proposed as to
how oral bacteria stemming from periodontal disease can
cause systemic disease are as followed:
1. Direct effect of oral infections: Oral
microbes and their byproducts can gain systemic access
via die circulatory system through traveling through
compromised tissue and inflamed periodoncium in the
oral cavity. In gaining systemic access, oral microbes
have the potential to directly influence subolinical
mediators of various systemic diseases.
2. Inflammation: People with periodontal
disease have elevated levels of systemic inflammatory
markers due to The burden of increased levels of oral
bacteria. Treatment for periodontal disease has been
reported to decrease systemic inflammation levels.
3. Cross-reactivity: .. The ..
progression .. of
systemic diseases can be accelerated by the Immune
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 3 -
response to bacterial heat-shock proteins creating
antibodies that cross-react with innate heat shock
croteins expressed on cells of the damaged tissues.
Cardiovascular Disease
Studies investigating the potential association
between periodontal disease and cardiovascular
diseases, including atherosclerosis, coronary heart
disease, and stroke have found a significant positive
correlation between poor oral healLh and Lhe prevalence
of cardiovascular disease. while both diseases share
several common risk factors, recent studies suggest
that periodontitis may precede and therefore contribute
to atherosclerotic complications. In fact, meta-
analyses show that subjects suffering from
periodontitis experience an increased risk for
developing cardiovascular diseases.
While it has not been definitively shown it these
bacteria initiate ae,herosclerosis or rather invade an
already compromised artery, antibodies to periodontal
bacteria, Including Fuseobacterium nucleatum and
Streptococcus oralis, have been found in blood serum
and are associated with an increased risk of coronary
heart disease. A mouse study found that intravenous
inoculation with Porphyromonas gingivalis accelerated
atherosclerotic development. Fureher, following oral
inoculation, P. gingivalis DNA was found in the aortic
tissue of those infected mice that showed observable
signs of accelerated early atherosclerosis. Another
study has named F. nucleatum as a synergistic agent
with P. gingivalis. F. nucleatum enhances the ability
of P. gingivalis to invade host cells due to a
coaggregating effect between the two organisms. This is
significant as bacteria within the atheroma may lead to
CA 02907012 2015-09-15
WO 2014/149287
PCT/1JS2014/016710
- 4 -
the development of atherosclerotic plague. The evidence
thus far supports the idea that periodontitis leads to
systemic exposure to oral bacteria which serves as a
potential source of systemic inflammatory mediators,
cytokines produced in the infected periodontal tissues,
capable of ini,'-iating or worsening atherosclerosis and
coronary heart disease when they enter into the blood
stream. Clinical studies on periodontal disease have
also revealed a positive association with coronary
disease and emphasis is now being placed on
understanding the exact relation between periodontal
disease and atherosclerosis.
Pre-term Birth
Fusobaceterium nucleatum, one of ihe most
prevalent species of bacteria found in amniotic fluid
and placental infections thai cause preterm birth, is
also often named the sole infectious agent in preterm
labor with intact fetal membranes. F. nucleatum is also
highly associated with various types of periodontal
disease. During periodontal infection, when the oral
mucosa is injured and flamed and the quanitie3 of
periodontal pathogens increase dramatically, transient
levels of bacteria can appear in the blood leading to
selective colonization of undesired sites. One study
demonstrated that pregnant mice injected hematogenously
with F. nucleatum isolated from either amnioeic fluid
infection or an oral source resulted in fetal death.
Recen7_1y, a human seillbirth case was analyzed
and it was found that the F. nucleatum did indeed
originate from the mother's oral cavity, a fact that
had not yet been proven. It is likely that the F.
nucleatum translocated from Lhe mother's mouth via Lhe
blood stream where it was then able to cross the
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 5 -
endothelium to proliferate and colonize within the
fetal membranes, amniotic fluid and fefus whereupon its
presence lead to feral demise. In a mouse model,
hematogenous injection of F. nucleatum into pregnant
mice resulted in specific bacterial colonizat,ion in the
placenta causing localized inflammation_ F_ nucleatum
was completely cleared from the maternal circulation
after 24 hours of injection. However, once colonized in
Lhe immune privileged placenta, the bacteria
proliferated quickly and caused fetal death within 3
days. Chronic periodontal disease could mediate
infection through the translocation of periodontal
bacteria/inflammatory markers to the fetoplacental
unit.
Diabetes
Diabetes mellitus is an endocrine disease that
stems from genetic, environmental and behavioral risk
factors. For the past several decades, diabetes has
been considered a modifying factor for periodontal
disease with recent years suggesting a bidirectional
relationship between the two. Further, presence of
periodontal disease has been implicated as a risk for
diabetic complications, namely poor glycemic control.
Recent longitudinal and systemic studies have seen
periodontal disease correlated to higher risks of death
from ischemic heart disease, diabetic nephropathy, end-
stage renal disease and increased insulin resistance
compared to patients with mild or no periodontal
disease. In type II diabetes, insulin resistance is
linked to the actions of pro-inflammatory cytokines. It
is believed that periodontal disease leads to a
significantly higher amount of these serum markers of
inflammation, thus conferring insulin resistance. A
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 6 -
human study examining the bacterial content of adults
with and without type II diabetes found diabetic
patients had significantly more severe periodontitis
and higher levels of many oral bacteria, including
Streptococcus oralis.
Pyogenic Liver Abscess
F. nucleatum has recently been Implicated in
pyogenic liver abscess (PLA). Normally caused by
biliary tract pathology, diverLicular disease and bowel
malignancy, atrophic gastritis and cryptogenic liver
disease, PLA caused by F. nucleatum is very rare with
Escherichia coli, Klebsiella and Enterobacter being the
most commonly isolated microorganisms in the drained
abscesses. F. nucleatum was found in the liver abscess
with no other infectious source being found, except for
a dental extracoion. It is hypothesized That due to the
coaggregation properties of F. nucleatum, it is able to
transport and breach the mucosa of the colon and lead
to bacteremia which results in hepatic abscess.
Osteomyelitis
Osteomyelitis is a bone infection caused by
bacteria, fungi or other germs. Commonly, bacteria
spreads to the bone from infected skin, muscles or
tendons and often time occur under a skin sore. The
infection can also start in another part of the body
and spread hematogenously. Occasionally Fusobacterium
species have been isolated from bone/joint infections
in the head and neck area and were associated with
chronic periodontitis. A recent study has reported a
case of osteomyelitis caused by F. nucleatum in
conjunction with muscle abscess. The patient had no
known predisposing factors and had no other infection
sources except a history of periodontal disease. It is
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 7 -
believed that due to the patient's poor oral hygiene,
F. nucleatum bacteremia may have developed and lead to
a hematogenous osteomyeli-tis of the lower leg.
Arthritis
Numerous clinical studies have suggested a
potential association between rheumatoid arthritis (RA)
and periodontal disease as several oral bacteria
species, such as P. gingivalis and Prevotella
intermedia, have been isolated from Lhe synovial fluid
of patients. Periodontal disease is thought to allow
bacteria to penetrate through the permeable pocket
epithelial in the oral cavity to reach the underlying
gingival connection tissue. From there, it may be
transported out into the bloodstream with the ability
to colonize elsewhere within the body. The oral
bacteria found in the synovial fluid of patients
suffering from RA has been attributed to synovial
inflammation favorably trapping oral bacteria DNA,
which suggests periodontal disease may have a
perpetuating effect on joint diseases. Therefore,
periodontitis may in fact be a factor leading to the
autoimmune inflammatory responses characteristic of RA.
Patients suffering from RA may also be at a higher risk
of developing periodontal disease thus suggesting a
bidirectional relationship between the two conditions.
One particular study examined the presence of bacterial
DNA in the synovial fluids of native and failed
prosthetic joints of Patients suffering from arthritis.
Out of the 5 paeients where bacterial DNA was found, F.
nucleatum was detected in 4 of these 5 patients. This
suggests that this bacterium can translocate from the
oral cavity to Lhe synovial fluid, as F. nucleatum was
also found in the patient's plague sample.
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 8 -
Oral Biofilm
Periodontitis, gingivitis, and caries
are infectious diseases of the oral cavity in
which oral biofilm plays a causative role. Biofilm
formation is also involved in the pathogenesis of
dental implant failures such as peri-implantitis,
denture stomatitis, and oral yeast infections such
as candidiasis. Oral biofilms begin with dental
pellicle formation on the teeth. This pellicle is
composed of salivary proteins that coat the
exposed surfaces of the teeth, primarily the
supra-gingival ones, to which the planktonic
bacteria begin to adhere. The aerobic bacteria,
including gram-positive cocci, such as S. rails,
are the early colonizers that begin forming the
initial biofilm colony, primarily through cellular
division of the adherent bacteria.
Once the initial colony has been
established, other co-aggregating bacteria
species, such as F. nucleatum, P. gingivalis, and
other gram-negative, anaerobic bacteria attach to
the previously formed colonies. As these colonies
mature, they grow to cover the sub-gingival
surfaces of the teeth and begin to induce
inflammation in the periodontium.
Summary of the Invention
One aspec,_ of die present invention is to
provide a method including the steps of oroviding a
mouthpiece having a first electrode and a second
electrode where the first and second electrodes having
opposite polarities and are coupled to a power source.
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 9 -
The method also includes positioning the mouthpiece
such that the first electrode is in electrical contact
with at least a portion of the patient's lingual
gingival tissue and the second electrode is in
electrical contact with at least a portion of the
patient's buccal gingival tissue Tfe method also
includes delivering current from the power source to
said gingival tissue according to a predetermined
treatment protocol.
According to another aspect of the present
invention the delivering step may further include
regulating the current delivered to Lhe gingival
tissue.
According to another aspect of the present
invention the delivering step may further include
administering the predeeermined treatment protocol to a
patient in a dental office.
According to another aspect of the present
invention the delivering step may further include
administering a predetermined treatment protocol to a
patient in a dental office prior to a dental procedure.
According to another aspect of the invention
the dental procedure may be cleaning or prophylaxis.
According to another aspect of the invention
the dental procedure may be scaling.
According to another aspect of The invention
the dental procedure may be root planing.
According to anoeher aspect of the invention
the current delivered to the gingival tissue is from 50
pA up to and including 500 pA.
According to another aspect of the present
invention the delivering step may further include
delivering current to the gingival tissue for a
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 10 -
duration of at least 10 minutes up to and including 30
minutes.
According to another aspect of the present
invention the delivering step may further include
administering the predetermined treatment protocol to a
patient in a dental office before or after a dental
procedure.
According to another aspect of the
invention, the mouthpiece may further include a third
electrode and a fourth electrode, the third and fourth
electrodes having opposite polarities and being coupled
to a power source. The positioning step may further
include positioning the mouthpiece such that the first
electrode is in electrical contact with at least a
portion of the patient's mandibular lingual gingival
tissue, the second electrode is in electrical contact
with at least a portion of the patient's mandibular
buccal gingival tissue, the third electrode is in
electrical contact with at least a portion of the
patient's maxillary lingual gingival tissue, and the
fourth electrode is in electrical contact with at least
a portion of Lhe patient's maxillary buccal gingival
tissue.
According to another aspect of the invention
the method may include reducing the amount of oral
biofilm in said patient.
According to another aspect of the invention
the method may include reducing oral bacterial to
prevent, treat and/or mitigate systemic disease.
According to another aspect of 7_,hc invention
the method may include reducing F. nucleatum to
prevent, treat and/or miLigate cardiovascular disease.
- 11 -
According to another aspect of the invention the method may
include reducing F. nucleatum to prevent still birth.
According to another aspect of the invention the method may
include reducing S. oralis to prevent, treat and/or mitigate
diabetes.
According to another aspect of the invention the method may
include reducing F. nucleatum to prevent, treat and/or mitigate
pyogenic liver abscess.
According to another aspect of the invention the method may
include reducing F. nucleatum to prevent, treat and/or mitigate
osteomyelitis.
According to another aspect of the invention the method may
include reducing F. nucleatum to prevent, treat and/or mitigate
arthritis.
Another aspect of the present invention is a method
including the step of providing a first mold, threading one or
more wires through the first mold, injecting a nonconductive
material into the first mold, curing the nonconductive material,
removing the partially molded mouthpiece from the first mold,
trimming the excess wire from the partially molded mouthpiece,
providing a second mold, placing the partially molded mouthpiece
within the second mold, injecting a conductive material into the
second mold, curing the conductive material, and removing a
finished mouthpiece from the second mold.
In accordance with another aspect, there is provided a
method for delivering an electric current in a mouth of a person,
the method comprising:
positioning a first electrode in electrical contact with at
least a portion of lingual gingival tissue of the mouth of the
person, and a second electrode in electrical contact with at
least a portion of buccal gingival tissue of the mouth of the
person;
Date Recue/Date Received 2021-06-09
- ha -
placing an electrically conductive medium between the first
electrode and the second electrode, the electrically conductive
medium including a teeth whitening agent; and
conducting a pulsed electrical current through the
electrically conductive medium and between the first electrode
and the second electrode and across gingival tissue defined
between the lingual gingival tissue and the buccal gingival
tissue according to a predetermined protocol.
In accordance with another aspect, there is provided a
method for increasing cellular permeability of bacteria in a
mouth of a person, the method comprising:
positioning a first electrode in electrical contact with at
least a portion of lingual gingival tissue of the mouth of the
person and a second electrode in electrical contact with at least
a portion of buccal gingival tissue of the mouth of the person,
the first electrode and the second electrode being coupled to a
power source; and
conducting an electrical current from the power source
between the first electrode and the second electrode and across
gingival tissue defined between the lingual gingival tissue and
the buccal gingival tissue according to a predetermined protocol.
In accordance with another aspect, there is provided a
system for delivering an electric current in a mouth of a person,
the system comprising:
a first electrode having a first polarity, the first
electrode being configured to be in electrical contact with at
least a portion of lingual gingival tissue of the mouth of the
person;
a second electrode having a second polarity opposite the
first polarity, the second electrode being configured to be in
electrical contact with at least a portion of buccal gingival
tissue of the mouth of the person; and
Date Recue/Date Received 2022-03-30
- llb -
a programmable power supply electrically coupled, through
conductors at least partially composed of an electrically
conductive polymer, to the first electrode and the second
electrode and configured for conducting a pulsed electrical
current between the first electrode while at the first polarity
and the second electrode while at the second polarity and across
gingival tissue defined between the lingual gingival tissue and
the buccal gingival tissue.
In accordance with another aspect, there is provided a use
of the system as described herein, for at least one of killing
oral microbes, increasing oral vasodilation, improving oral blood
circulation, teeth whitening, reversing oral bone resorption,
increasing oral osteogenesis, reducing gum tissue recession,
increasing gingival regeneration, and reducing oral malodor.
In accordance with another aspect, there is provided a use
of the system as described herein, for reducing a concentration of
bacteria in the mouth of the person.
In accordance with another aspect, there is provided a use
of the system as described herein, for increasing cellular
permeability of bacteria in the mouth of the person.
In accordance with another aspect, there is provided a use
of the system as described herein, for treating a systemic
disease.
In accordance with another aspect, there is provided a use
of a pair of electrodes and a power supply for conducting
electrical current between the pair of electrodes across gingival
tissue defined between lingual gingival tissue and buccal
gingival tissue.
Brief Description of the Drawings
Figure 1 demonstrates the overall structure of the first
embodiment of our invention, including a microcontrolled power
source, user input, user feedback and oral electrodes.
Figure 2 shows a top-down view of another
Date Recue/Date Received 2022-03-30
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 12 -
embodiment that includes a mouthpiece with two
continuous electrodes and associated conductors.
Figure 3 shows a perspective view of the
same embodiment of Figure 2, with two electrodes
embedded in a mouthpiece.
Figure 4 offers a top-down view of another
embodiment similar to Figure 2 chat includes a
mouthpiece, out with a plurality of discrete
electrodes.
Figure 5 provides a perspective view of an
additional embodiment similar to Figure 4 with a
plurality of electrodes thaL are electrically connected
by embedded conductors.
Figure 6 shows an additional embodiment of
with an analog power supply using a dual unit, three-
port switch.
Figure 7 is an exploded view of a first mold
for molding a mouthpiece according to the present
invention.
Figure 8 is a cross sectional view of a
first mold for molding a mouthpiece according to the
present invention.
Figure 9 is an exploded view of a first mold
for molding a mouthpiece according to the present
invention with a partially molded mouthpiece.
Figure 10 is an exploded view of a second
mold for molding a mouthpiece according to the present
invention with a partially molded mouthpiece.
Figure 11 is perspective view of a second
mold for molding a mouthpiece according to the present
invention with a partially molded mouthpiece.
Figure 12A is a cross section of ,he mold of
Figure 11 with a partially molded mouehpiece in its
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 13 -
unfilled state.
Figure 12B is a cross section of i_he mold of
Figure 11 with a partially molded mouThpiece in its
filled state.
Figure 13 is a perspective view of a
mouthpiece molded according to an embodiment of the
Present invention.
Detailed Description
Although Lhe disclosure hereof is detailed
and exact to enable chose skilled In the art to
practice the invention, the physical embodiments herein
disclosed merely exemplify the invention which may be
embodied in other specific structures. While the
preferred embodiment has been described, the details
may be changed wii,hout departing from the invention,
which is defined by the claims.
It is known in the art that oral bacteria
cannot survive when exposed to low-microampere direct
current electricity. This method of killing oral
bacteria and treating bacteria-caused conditions such
as gingivi-J,is has been demonstrated in Nachman, U.S.
Pat. No. 4,244,373 of Jan. 13, 1981 and in Detsch, U.S.
Patent 4,509,519 of Apr. 9, 1985. Killing oral bacteria
has i,he added benefit of preventing tooth decay and
dental caries, or cavities. Generally, tooth decay is
attributed to aerobic acid-producing bacteria whose
acid causes uncompensated demineralization of the
teeth. However, Nachman does not instruct optimal
approaches to reducing oral bacteria inducing aerobic
and anaerobic bacteria on a species-by-species level
and instead teaches a generic, untargeted treatment.
While researching the effect of direct
current electricity on the mouth, the applicants
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 14 -
discovered -chat by increasing the current level to the
approximate range of 50 to 250 microamperes, a direct
current elecirical treatment was able to deliver new
and unexpected therapeutic, prophylactic, and
regenerative benefits previously unknown in the art.
Specifically, by utill7ing a direct current
in the aforementioned range, not only did such a
treatment kill bacteria, but it was also found to kill
or disable viruses and fungus as well. Studies from Lhe
podiatric field have shown -chat higher current levels
than chose used in exiscing oral electrical treatments
are necessary LAD effectively treat fungal infections
("Low-Voltage Direct Current as a Fungicidal Agent for
Trcating Onychomycosis", Kalinowski, et al., Journal of
the American Podiairic Medical Associacion Vol. 94
No.6: 565-572,2004). By applying this knowledge of
increased current levels from research outside the art,
the applicants were able to add fungicidal and
viricidal benefits to a method already known to be
bactericidal. The applicants' studies have shown that
these mierobicidal properties begin to take effect
within approximately 5 and 15 minutes of treatment,
reducing both supra- and sub-gingival microbes.
In addiiion, the applicants clinical
research unexpectedly demonstrated that a direct
current in the approximate range of 50 to 250
microamperes was able to regenerate gingival tissues,
providing a non-surgical treatment alternative for
those with recessed gums. While the osteogenic
properties of electricity have been known in the art,
the connection between nonosseous tissue regeneration
and electricity were not well known in the art prior to
these experiments. The unique current range associated
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 15 -
with the method and apparatus of this invention is one
of a few effective methods in the dental field to
accomplish effective gingival tissue regeneration in a
non-surgical manner.
In further research, the applicants
conducted preciliniral testing that examined the effects
of direct current stimulation on three different oral
bacteria (F. nucleatum, S. oralis, P. gingivalis) in
both saline and saliva solu_ions. This testing varied
the current levels, inoculum size of bacteria, solution
medium, and treatment time to develop a optimal
treatment to reduce these three bacteria species
associated with both periodontal and systemic diseases.
The results of this testing yielded
unexpected results and showed that each different
bacterium had a different dose response to DC
stimulation. Through this testing, the applicants
identified treatment parameters that were able to kill
up to 100% of S. oralis, 99.1% of F. nucleatum, and
52.3% of P. gingivalis in a single treatment lasting
thirty minutes or less. This research yielded
specifications for DC-based treatments of targeted
pathogens that was previously unknown in the art. The
optimal treatment parameters discovered in this
research and described in this method can provide an
innovative way to reduce these three species of
bacteria, in both supra- and sub-gingival environments,
and thus prevent and/or treat their associated
complications including periodontal disease, biofilm
formation, as well as the systemic diseases correlated
to these oral pathogens.
In addiLion, scanning electron microscopy
(SEM) was conducted on F. nucleatum colonies before and
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 16 -
after at 30 minute treatment, according to the method
of this invention, to better understand the mechanism
by which the meehod according to this invention is able
to reduce bacterial levels. The SEM imagery suggested
that che method according to this invention interferes
with bacter al cellular division and can weaken the
outer envelope (cell membrane) resuleing in fragile
cellular structures that can easily break. It is
contemplated LhaL Lhis is phenomenon is an example of
electroporation, where the permeabilley of cellular
membranes may be affected by electrical stimulation
either temporarily or permanently. IL, Is further
contemplated that the electroporation caused by the
method according to this invention could play a role in
developing new therapies in molecular biology which
would take advantage of this cellular permeability and
introduce new material into the cells of oral (Pathogens
or oral tissues through mechanisms including, but not
limited to genetic material (transfection) such as DNA,
RNA, sRNA, siRNA, plasmids, etc. These effects would
prove a new tool in targeted gene therapies for oral
applications.
Specifically, the method according to the
present invention has been shown to reduce viable
colony forming units (CFU) in various oral bacteria.
Table 1 below shows the efficacy of
treatment according to the present invention at current
levels of 5C pA or 500 pA for 5, 10, 20 and 30 minute
durations for bacterial culeures ranging from 104 to 107
colony forming units (CFU) of Streptococcus rails in a
saline solution.
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 17 -
In Vif,ro Efficacy of Device Against
Streptococcus oralis in Saline
CPU pA 0 Min 5 Min 10 Min 20 Min 30 Min
50pA 1120 1080 600 320 280
10e4 500pA 1120 1200 800 240 0
50pA 10000 9600 8400 9200 7600
10e5 500pA 11600 10400 11200 10800 8400
50pA 80000 63200 52800 32400 24800
10e6 500pA 80800 70000 15200 14000 15600
50pA 1280000 1080000 1040000 800000 440000
10e7 500pA 1080000 520000 160000 120000 320000
Table 1
Table 2 below shows the efficacy of
treatment to the present invention at current levels of
50 pA or 500 pA for 5, 10, 20 and 30 minute durations
for bacterial cultures ranging from 104 to 10 CPU of
Streptococcus oralis in a saliva solution.
In Vitro Efficacy of Device Against
Streptococcus oralis in Saliva
CFU pA 0 Min 5 Min 10 Min 20 Min 30
Min
50pA 160 160 80 80 40
10e4 500pA 200 80 80 80 80
50pA 5600 5600 6800 5600 4000
10e5 500pA 8400 6800 7200 E400 2800
50pA 25600 25200 15200 17200 18400
10e6 500pA 23600 16800 15600 17600 15200
50pA 316000 284000 300000 27E000 220000
10e7 500pA 324000 328000 300000 292000 252000
Table 2
Table 3 below shows Lhe efficacy of
treatment to the present invention at curren:, levels of
50 pA or 500 pA for 5, 10, 20 and 30 minute durations
for bacterial cultures ranging for 104 and 10 CPU of
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 18 -
Fusobacterium nucleatun in a saline solution.
In Vitro Efficacy of Device Against
Fusobacterium nucleatum in Saline
CFU pA 0 Min 5 Min 10 Min 20 Min
30 Min
50pA 480 280 280, 120, 40
10e4 500pA 560 440 400 200 120
50pA 94000 91600 85600 70400 84400
10e6 500pA 46400 45600 27200
2000 400
Table 3
Table 4 below shows the efficacy of
treatment according to the present inven-L.ion at current
levels of 50 pA or 500 pA for 5, 10, 20 and 30 minute
durations for bacterial cultures ranging from 104 to 106
CFU of Fusobacterium nucleatum in saliva.
In Vitro Efficacy of Device Against
Fusobacterium nucleatum in Saliva
CFU PA 0 Min 5 Min 10 Min 20 Min
30 Min
50pA 1480 1480 1560 680 880
10e4 500pA 2360 2360 1720 1240 1080
50pA 19600 19600 15200 14400 14000
10e5 500pA 18000 17200 14400 11200 10800
50pA 348000 112000 120000 72000 68000
10e6 504A 156000 128000 124000 32000 28000
Table 4
Table 5 below shows the efficacy of
treatment to the present invention at current levels of
50 pA or 500 pA for 5, 10, 20 and 30 minute durations
for bacterial cultures ranging for 105 CFU of
Porphyromonas gingivalis in a saline soluLion.
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 19 -
In ViLro Efficacy of Device AgainsL
Porphyromonas gingivalis in Saline
CFU pA 0 Min 5 Min 10 Min 20 Min
30 Min
50pA 3440 2040 2720 1640 1640
10e4 500pA 2440 2120 2200 1880
1840
Table 5
Thus, this method and corresponding
apparatus are able to achieve multiple prophylactic,
therapeutic, and regenerative effects whose combination
was not previously known or available in the art.
Namely, these effects are: promotion of oral
osteogenesis, destruction or disabling of oral
microbes, gingival tissue regeneration, reduceion and
prevention of the formation of oral biofilms, caries
prevention, increased oral vasodilation and oral blood
flow, treatment of common oral conditions such as
gingivitis and periodontitis, treatment of systemic
diseases and condiLions correlated with oral pathogens,
and generally improved oral hygiene.
These effects are accomplished by the
delivery of direct current to the gingiva through a
plurality of electrodes in direct contact with the
lingual and buccal gingival surfaces. The electrodes
may be fashioned out of any electrically-conductive
material, including but not limited to metals such as
silver, seainless steel, copper, gold, platinum,
palladium, aluminum, an alloy thereof, electrically-
conductive nanoLubes, carbonized rubber, electrically-
conductive silicone, Or electrically-conductive
polymers. The electrodes may be composed of ehe same or
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 20 -
of differing materials. These electrodes fit snuggly
against the lingual and buccal sides of the gingiva and
make direct contact widh each side of the gingiva to
pass direct current electricity across the teeth and
neighboring gingival tissues.
The electrodes on each side (lingual or
buccal) of the gingiva are of the same polarity.
Electrodes on opposite sides of the gingiva are of the
opposite polariL.y. This allows the current to flow
across the teeth and gums to the electrodes positioned
on the transverse gingiva to complete the electrical
circuit. Put another way, all electrodes on the lingual
side of the gingiva will be completely anodic or
completely cathodic. All electrodes on the buccal
surfaces of the gingiva, transverse The lingual
surfaces of the gingiva, would have the opposite
polarity. The polarization of these electrodes may be
reversed during treatment or in between treatments.
The mandibular and maxillary gingiva each
have a set of a plurality of polarized electrodes as
previously described. This allows for treatment of both
the maxillary and mandibular periodontium either
simultaneously or in isolation. The maxillary and
mandibular sets of electrodes may be powered by two
different adjustable power supplies or by the same
adjustable power supply.
Electrical conductors than connect these
electrodes to an adjustable power supply. All of the
anodic electrodes will connect to the positive pole of
the power supply and all of the cathodic electrodes
will connect to the negative pole of the power supply.
The adjustable power supply is capable of delivering a
stable, direct curren-z in the approximate range of 1 to
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 21 -
500 microamperes. The preferred current setting for
most treatments is in the approximate range of 50 to
250 microamperes.
In order to increase conductivity in the
tissues adjacent to the electrodes, an ionic or
colloidal liquid or gel may be used as a conductive
medium to decrease electrical resistance in the mouth.
This medium would be placed along any desired areas of
desired electrical contact, such as the Leeth, gums, or
surrounding oral tissues. Examples of such a medium
would include, but not be limited to, colloidal silver
gel, liquid colloidal silver, colloidal copper gel,
liquid colloidal copper, colloidal gold gel, liquid
colloidal gold, saline gel, liquid saline or any
combination thereof.
Colloidal silver, in whole or in
combination, has great promise not only in increasing
electrical current flow, but also in offering
additional bactericidal benefits. Colloidal silver, in
concentrations as little as five parts per million, is
known to be bactericidal by inhibiting a bacterium's
production of adenosine triphosphate.
This conductive medium may also contain
dietary supplements including, but not limited to, oil
of oregano. Oil of oregano is believed to have many
health benefits and may also be microbicidal. Such
microbicidal properties would be effective In treating
common oral infections and diseases as well as aiding
in preventative oral care.
This conductive medium may also contain
teeth whitening agents. This would allow for the
addition of teeth whiLening to the list of benefits
offered by an embodiment of this invention. A whitening
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 22 -
agent that is catalyzed by direct current electricity
could be included and may even offer reduced teeth
whitening treatment times when compared with
nonelectrically-catalyzed whitening agents.
Artificial or natural flavorings may also be
added to this conductive medium to offer a more
appealing taste to the user, similar to the method of
flavoring dental fluoride treatments. This flavoring
would mask any unpleasant tastes from the ingredients
of the conductive medium or as well as any taste of the
mouthpiece or electrodes themselves.
Figure 1 shows one embodiment of a treatment
apparatus according to this invention. A user input
device 120 is connected to a microcontrolled dircct
current power supply 110. This input device 120 may
include, but not be limited to potentiometer dials,
push buttons, switches, toggles, etc. User input device
120 allows the patient to control various aspects of
the treatment including but not limited to power on or
off, output current levels, treatment program
selection, treatment duration, treatment reminders,
polarity, etc. Input device 120 may also be used to run
pre-programmed treatment regimens as described by this
method targeted at specific pathogens, including but
not limited to, S. oralas, P. gingivalis, and F.
nucleatum. Microcontrolled power supply 110 reads the
state of input device 120 and adjusts thc outcut
current to compensate for the continually varying
resistance across cathodic electrodes 140 and anodic
electrodes 150.
An optional user feedback device 130 is show
in Figure 1 connected to microcontrolled power supply
110. Feedback device 130 may contain various methods
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 23 -
and devices capable of relaying treatment information
to the user. Feedback device 130 could include, but not
be limited to an LCD display, LCD matrix display, color
LCD displays, indicator LEDs, LED bar graphs, LED
segment displays, OLED displays, audio speakers,
vibrating devices, or any combination thereof. Feedback
device 130 offers the user informa-eion including, but
is not limited to, output current level, treatment time
elapsed, treatment Lime remaining, date, Lime of day,
battery power level, treatment reminder indicators or
sound alarms, recharging indicators, etc. Feedback
device 130 also provides information regarding any
state change from input device 120. This allows the
user to receive information on how his/her input is
affecting the treatment. Feedback device 130 is not
required for the operation of this embodiment of the
treatment apparatus and may be omitted.
Microcontrolled power supply 110 contains a
microcontroller 112 and a direct current power source
116. Microcontroller 112 is electrically connected to
input device 120 and is capable of reading the device's
state(s). Microcontroller 112, upon reading these
state(s), is able to dynamically adjust the output of
power source 116. This allows the user to control the
level of current generated by the power source 116.
Microcontroller 112 is also connected to an optional
user feedback device 130. Microcontroller 112 is able
to output information related to the treatment
duration, current timer status, curreneõ levels, and
other information to feedback device 130.
Microcontroller 112 also has timing capabilities,
represented by Limer 114, that allow for limiLing
treatment time based on some predetermined treatment
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 24 -
duration. Timer 114 is also used to output the elapsed
treatment time to feedback device 130, if present. The
user is able to input desired treatment parameters such
as treatment duration, treatment current levels, etc.
to microcontroller 112 by way of input device 120.
The programmable nature of microcontroller
112 allows for advanced functionality not present in
other oral electrical treatment devices. For example,
the software on microcontroller 112 could be programmed
to run a predetermined treatment regimen. This
treatment regimen could include but not be limited to
such factors as: treatment duration, targeted pathogen,
treatment current levels, treatment time-of-day,
treatment reminders, etc. This treatment regimen could
also be programmed by a dental professional by way of
input device 120 so that a patient's treatment may be
simplified and guaranteed to follow set parameters.
Cathodic electrodes 140 are connected to the
positive pole of power source 116 and anodic electrodes
150 are connected to the negative pole of power source
116. These electrodes are placed in direct contact with
the gingiva, mounted transversely from one another.
This allows a current flow from cathodic electrodes 140
to the gingival tissues, surrounding teeth, honey
structures, and connected mouth tissues to anodic
electrodes 150 mounted on the transverse gingiva and
then back to power supply 110, forming a complete
circuit.
Power source 116 may be any known device
capable of delivering an adjustable direct electrical
current. This includes, but is not limited to
disposable batL.eries, rechargeable batteries, Ac-DC
power converter, etc. Microcontroller 112 is able to
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 25 -
regulate the current output of power source 116 by a
known method of electrical current control. Power
supply 116 is capable of delivering a direct current of
between 1 and 500 microamperes, with an approximate
range of 50 to 250 microamperes used for most
treatments_ Microcontrollpr 112 is also able to reverse
the polarity of the cathodic electrodes 140 and anodic
electrodes 150 by controlling ihe output of power
source 116. This allows for dynamic changing of
electrode polarity during treatment. microcontroller
112 is also programmable to allow for pulsed
application of direct current across the gingiva.
Figure 2 shows a top-down view of another
embodiment of the treatment apparatus. In this
embodiment, a mouthpiece unit 200, known in the art,
has two electrodes attached to or embedded in it, and
is worn in the mouth. A single lingual gingiva
electrode 210 fits snugly against 7_he lingual gingival
tissue of the mouth. A single buccal gingiva electrode
220 is attachcd to or embedded in mouthpiece 200 so
that it is transverse from ehe lingual gingiva
electrode 210 and fits snugly againsL the buccal
gingival tissues of the mouth. Two electrical
conductors 230 connect electrodes 210 and 220 to an
adjustable current power supply, of whose embodiment
may be similar to that of 110 or that of Figure 3.
Electrical conductors 230 arc insulated so thai a short
circuit does not occur inside or outside of the mouth.
Electrical conductors 230 are shown as attached to the
anterior of mouthpiece 200, out may be electrically
connected to electrodes 210 and 220 at any point along
mouthpiece 200, so long as electrical conductors 230
are not attached to the same electrode. Electrical
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 26 -
conductors 230 may also be partially or wholly composed
of an electrically conductive polymer.
Figure 3 shows a perspective view of the
same type of embodiment shown in Figure 2. A mouthpiece
300 has a lingual gingiva electrode 310 and an buccal
gingiva electrode 320 attached to or embedded it
Electrodes 310 and 320 span the lingual and buccal
gingival surfaces of the mouth, respectively. A set of
embedded electrical conductors 340 are connected to
electrodes 310 and 320 on one end and on the other end
to a set of conductors to the power supply 330.
Conductors 340 are embedded in the mouthpiece material
and are electrically insulated. Conductors 330 then
connect to the positive and negative poles of a direct
current power source, similar to that of 110 or Figure
6.
Figure 4 presents another embodiment similar
in nature to that, of Figures 2 and 3. In this
embodiment, electrode sets are attached to or embedded
in a mouehpiece unit 400. A set of lingual gingiva
electrodes 410 are affixed to or embedded in mouthpiece
400. Electrode set 410 comprises a plurality of
discrete lingual gingiva electrodes 4102, which are
electrically connected by embedded electrical
conductors 4104. Conductors 4104 are insulated and are
embedded in or attached to mouthpiece 400. Likewise, a
set of buccal gingiva electrodes 420 are affixed to or
embedded in mouthpiece 400 transverse of electrode set
410. Electrode set 420 comprises a plurality of
discrete lingual gingiva electrodes 4202, which are
electrically connected by embedded electrical
conductors 4204. Conductors 4204 are insulated and are
embedded in or attached to mouthpiece 400. This
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 27 -
embodiment allows for multiple, discrete points of
electrical contact within the mouth. In Figure 4,
conductors to the power supply 430 are shown as
attached to the posterior electrodes in mouthpiece 400.
However, conductors 430 may be electrically connected
to any point of electrode sets 410 and 420, so long as
conductors 430 are not connected to the same electrode
set. Conductors 430 then connect to the positive and
negative poles of a direct current power source,
similar to that of 110 or Eigure 6.
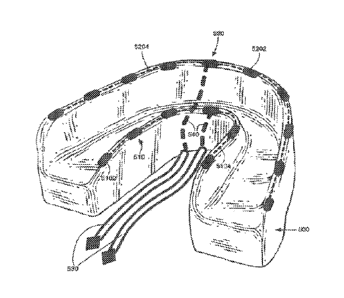
Figure 5 offers a perspective view of an
embodiment similar to Figure 4. A lingual gingiva
electrode set 510 and a buccal gingiva electrode set
520 are attached to or embedded in a mouthpiece 500.
Electrode set 510 comprises a plurality of lingual
gingiva electrodes 5102 that are electrically connected
by embedded electrical conductors 5104. Conductors 5104
are electrically insulated and are embedded in or
attached to mouthpiece 500. Similarly, electrode set
520 comprises a plurality of buccal gingiva electrodes
5202 that are electrically connected by embedded
electrical conductors 5204. Conductors 5204 are
electrically insulated and are embedded in or attached
to mouthpiece 500. Electrode set 520 is mounted
transverse of electrode set 510 to allow direct current
to flow across the tissue of the teeth and gums.
Electrode sets 510 and 520 are connected to conductors
to the power supply 530 by way of embedded electrical
conductors 540. Conductors 540 are electrically-
insulated and are embedded in mouthpiece 500.
Conductors 530 then connect to t,he positive and
negative poles of a direct current power source,
similar to that of 110 or Figure 6.
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 28 -
Figure 6 presents another embodiment of an
adjustable direct current power source used to supply
direct current to a plurali-sy of oral electrodes. This
particular circuit design is capable of delivering a
steady current regardless of moderate fluctuations in
the resistance between the electrodes. The circuit uses
a 9-volt power supply 640, which could be a disposable
battery, a rechargeable battery, an AC-to-DC converter,
or any other suilable 9-volt power source. A dual uniL,
three-port switch 610 is used to select the current
level in the circuit. The three options of the switch
circuit are power off 614, 100pA 612, or 200pA 616.
Switch option 614 simply does not complete a circuit,
preventing current from flowing. Swi-:ch option 612
comprises a 332kQ resistor 618 in series with a 2 volt
LED 620. These two components are in parallel with a
48.7kQ resistor 622 to provide a 100 microamp current.
The Switch option 616 comprises a 665kg2 resistor 624 in
series wich a 2 volt LED 626. These two components are
in parallel with a 97.6kL2 resistor 628 to provide a 200
microamp current. Cathodic upper mouth electrodes 630
and cathodic lower mouth electrodes 632 are in parallel
with each other and are electrically connected to the
output of switch 610. Electrical current then travels
from power supply 640 to these electrodes, through the
gingival tissues of the mouth to anodic upper mouth
electrodes 636 and anodic lower mouth electrodes 634
and finally back to Power supply 640. This circuit
design will allow moderate and reasonable fluctuations
in the resistance across the electrodes and prevent
over driving the circuit should the resistance in the
mouth vary.
In another embodiment of this invention or
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 29 -
in combination with those previously described, an
ionic or colloidal medium in the form of a liquid or a
gel may be used to decrease electrical resisance in
the mouth and to facilitate a more even current
distribution across oral electrodes. Any combination of
one or more ionic or colloidal compounds may be used.
Examples of such a medium would include, but not be
limited to, colloidal silver gel, liquid colloidal
silver, colloidal copper gel, liquid colloidal copper,
colloidal gold gel, liquid colloidal gold, saline gel,
liquid saline or any combination thereof. Artificial or
natural flavorings may be added to this medium to offer
a more appealing taste to the user. The medium may also
contain dietary supplements including, but not limited
to, oil of oregano. This medium may also contain teeth-
whitening chemical agents. A whitening agent thae, is
catalyzed by the direct current would be most effective
in this ionic or colloidal medium.
In yet another embodiment, microcontrolled
power supply 110 would be miniaturized and be
physically atached to or embedded in a mouthpiece
similar to 200, 300, 400, or 500. This would allow for
an all-in-one unit that would fit inside the user's
mouth. In this embodiment, power source 116 would have
to be of small physical size. One of many possible
options is a watch-type battery or other small,
portable power source. This circuitry would then be
encased in a waterproof manner in the material of the
mouthpiece itself. Input device 120 and feedback device
130 would be waterproofed and protected from any kind
of electrical shorting, as well.
Thus the reader will see that at least one
embodiment addresses a desired need in the oral hygiene
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 30 -
and dental fields to concurrently treat common oral
diseases and conditions in a more effective, less
invasive, and less expensive manner. These embodiments
promote general oral hygiene, reduce oral biofilm,
treat periodontal diseases such as gingivitis and
periodontitis, kill oral microbes including bacteria
and thus preventing cavities and tooth decay, increase
vasodilation and blood flow in oral tissues, promote
gingival Lissue regeneraLion, foster osteogenesis in
the boney structures of the teeth, mouth, and related
areas, treat systemic diseases related to oral
pathogens, and treat other periodontal and oral
maladies through the non-invasive application of weak
direct current electricity to the surfaces in the oral
cavity.
While our above descriptions contain many
specificities, these should not be construed as
limitations on the invention, but rather as an
exemplification of several preferred embodiments
thereof. Many other variations are possible. For
example, electrodes may be attached directly to the
gingiva without the use of a mouthpiece, perhaps using
an electrically-conductive paste. Or electrodes may be
placed in contact with tissues neighboring the gingiva,
such as the teeth or tissues of the cheek, instead of
directly on the gingiva to accomplish the same result.
Another example would be replacing LEDs 626 and 620
from Figure 6 with standard diodes to achieve the same
resultant circuit. Overall, the circuitry from Figures
1 and 6 could be altered in many ways to deliver the
same electrical current to ,the oral electrodes.
In some cases dental procedures can break up
oral bacterial colonies found in biofilms and introduce
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 31 -
bacteria into ehe bloodstream causing bacteremia and
other infections. It is further contemplated thae it
may be desirable to utilize a mouthpiece according to
the present invention immediately prior to performing a
dental procedure. The mouehpiece according to this
invention may be used by the patient either at home or
in the deneal office. In this manner, the living
bacteria in the patient's mouth, both supra- and sub-
gingival, can be reduced prior to the procedure and Lhe
risk of bacteremia and. other infections will be
reduced. For example, and not by way of limitation, a
mouthpiece according to the present invention may be
utilized prior to a dental nrophylaxis or a scaling and
root planning procedure in a dental office to reduce
the risks of ineroducing bacteria into the patient's
blood stream. Such a pre-procedural ereaement would he
used for approximately 10 to 20 minute with a current
level ranging from 50pA to 500pA and would be timed to
conclude immediately before the procedure.
A mouthpiece according to the present
invention may also be utilized following a clinical
procedure as prevenrion for infections, for scenarios
including but not limited to post-extraction or post-
implantation infection preveneion. Such a post-
procedural treatment would last for approximately 10 to
20 minutes with a currene ranging from 50pA to 500pA.
This procedure may then be repeated at home by the
eatient one or more times a week until the risk of
infection has passed.
Prevention of Systemic Disease
It is contemplated chat a mouthpiece
according to Lhe present invention may be used to
prevent or treat systemic diseases as will be outlined
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 32 -
in more detail below. The method according to the
present invention has been shown to be effective in
reducing the amount of oral bacteria, specifically F.
nucleatum, P. gingivalis, and S. oralis.
1. Cardiovascular disease
Tt is contemplated that use of a mouthpiece
according to the present invention may be used to
reduce microbial burdens caused by the translocation of
oral bacteria, including but not limited to S. oralis,
P. gingivalls, and nucleatum, from the gingival
tissues to the rest of the body and also decrease the
amount of inflammatory mediators produced by oral
bacteria. Further, by reducing F. nucleatum, it is
contemplated that the ability of P. gingivalis to
invade host cells will be lessened and thus diminishing
the development of bacteremia that has been linked with
the initiation/worsening of atherosclerosis and
coronary heart disease.
It is contemplated chat a mouthpiece
according to the present invention may be used
according to a predetermined treatment regimen to
prevent, treat and/or mitigate cardiovascular disease.
In the predetermined treatment regimen, the patient
will wear a mouthpiece according to the present
invention for a predetermined amount of time at a
Predetermined current level and at predetermined time
intervals. It is further contemplated that the specific
treatment regimen may be determined based on the
bacterial levels present in a patient. According to one
embodiment of the invention, the treatment regimen
would consist of a patient wearing a mouthpiece
according to the present invention for 20 minutes once
per day at a current level of 500pA. For acute
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 33 -
cardiovascular conditions, this -_reatment may continue
on a daily basis until the conditions is resolved. For
chronic cardiovascular disease, this treatment may be
repeated a few times a week on a continuing basis.
2. Still Birth
Tt is further contemplated that a treatment
with a mouthpiece according to the present invention
according to a predetermined treatment protocol would
reduce the oral population of F. nucleatum associated
with periodontal disease and thus prevent, treat and/or
mitigate still birth. In turn, this reduction would
lessen the likelihood of F. nucleatum translocating
from the oral cavity into the bloodstream, where it
could then migrate into thc placenta and colonize. It
is contemplated that a mouthpiece according to the
present invention may be used according to a
predetermined treatment regimen to prevent still birth.
In the predetermined treatment regimen, .the patient
will wear a mouthpiece according to the present
invention for a predetermined amount of time at a
predetermined current level and at predetermined time
intervals. It is further contemplated that Lhe specific
treatment regimen may be determined based on the
bacterial levels present in a patient. According to one
embodiment of the invention, the treatment regimen
would consist of a patient wearing a mouthpiece
according to the present invention for 20 minutes once
Per day at a current level of 500pA for -the duration of
the pregnancy. The treatmeno parameters outlined above
have been demonstrated to be highly efficient at
reducing levels of S. oralis and F. nucleatum at
inoculation sizes of 10 colony-forming units (CFU).
3. Diabetes
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 34 -
It is contemplated that a mouthpiece
according to the present invention according to a
predetermined treatment protocol may be used to
prevent, treat and/or mitigate diabetes by causing a
reduction of S. rails in the oral cavity and
consequently reduce the amount of serum markers of
inflammation produced by bacterial infections. In the
predetermined treatment regimen, the patient will wear
a mouthpiece according to the present invention for a
predetermined amount of time at a predetermined current
level and at predetermined time intervals. It is
further contemplated bhat the specific treatment
regimen may be determined based on the bacterial levels
present in a patient. According to one embodiment of
the invention, the treatment regimen would consist of a
patient wearing a mouthpiece according to the present
invention for 20 minutes once per day at a current
level of 500pA to effectively reduce oral levels of S.
oralis that in turn will lower the amount of systemic
inflammatory markers. This treatment may be repeated
multiple times a week on an ongoing basis to help
reduce inflammatory markers.
4. Pyogenic Liver Abscess
It is contemplated that a mouthpiece
according to the present invention according to a
predetermined treatment protocol may be used to
prevent, treat and/or mitigate pyogcnic liver abscess
by causing a reduction of F. nucleatum. Specifically,
it is contemplated that treatment with a mouthpiece
according to the present invention would reduce
bacterial levels and may stop F. nucleatum and other
oral bacteria species from traveling to the liver and
reduce overall bacteremia. In the predetermined
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 35 -
treatment regimen, the patient will wear a mouthciece
according to the present invention for a predetermined
amount of time at a predetermined current level and at
predetermined time intervals. It is further
contemplated that the specific treatment regimen may be
determined based on the bacterial levels present in a
patient. According to one embodiment of the invention,
the treatment regimen would consist of a patient
wearing a mouthpiece according to the presenL invention
for 20 minutes once per day at a current level of 500pA
to effectively reduce oral levels of F. nucleatum which
may prevent any bacteria from being transporLed from
the oral cavity systemically. This treatment may be
repeated multiple times per week until the abscess is
reduced.
5. Osteomyelitis
It is contemplated that a mouthpiece
according to the present invention according to a
predetermined treatment protocol may be used to
prevent, treat and/or mitigate osteomyelitis by causing
a reduction of F. nucleatum. In the predetermined
treatment regimen, the patient will wear a mouthpiece
according to the present invention for a predetermined
amount of time at a predetermined current level and at
predetermined time intervals. It is further
contemplated that the specific treatment regimen may be
determined based on the bacterial levels present in a
Patient. According to one embodiment of the invention,
the treatment regimen would consist of a patient
wearing a mouthpiece according to the present invention
for 20 minutes per treatment at a current level of
500A to effectively reduce oral levels of F. nucleatum
bacteria and prevent any bacteria from being
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 36 -
transported from the oral cavity systemically. This
treatment may be used in conjunction with or separate
from standard antibiotic-based treatments for
osteomyelitis. When used in conjunction with
antibiotics, treatment will normally continue for
approximately 29 to 42 days_ When used separately from
antibiotics, this treatment may be used once a day for
a few months for acute conditions, or a few times a
week on a continuing basis for chronic condiLions.
6. Arthritis
It is contemplated chat a mouthpiece
according to the present invention according to a
predetermined treatment protocol may be used to
prevent, treat and/or mitigate arthritis by causing a
reduction of F. nucleatum. In the predetermined
treatment regimen, the patient will wear a mouthPiece
according to the present invention for a predetermined
amount of time at a predetermined current_ level and at
predetermined time intervals. It is further
contemplated that the specific treatment regimen may be
determined based on the bacterial levels present in a
patient. According to one embodiment of the invention,
tho treatment regimen would consist of a patient
wearing a mouthpiece according to the present invention
for 20 minutes once per day at a current level of 500p
to effectively reduce oral levels of F. nucleatum
bacteria and prevent any bacteria from being
transported from the oral cavity and translocating to
the synovial fluid and reducing the associated
inflammation. This treatment may be repeated multiple
times per week on a continual basis for this type of
chronic condi Lion.
Reducing Blofilm and Preventing Bio film Formation
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 37 -
It is contemplated chat a mouthpiece
according to the present invention according to a
predetermined treatment protocol may be used to
prevent, treat and/or mitigate oral biofilm by causing
a reduction of F. nucleatum, P. gingivalis, and/or S.
oralis, all of which are involved in oral biofilm
formation. In The predetermined treatmenc regimen, the
patient will wear a mouthpiece according to the present
invention for a predetermined amounL of Lime at a
Predetermined curreni level and at predetermined time
intervals. It is further contemplated that the specific
treatment regimen may be determined based on the
bacterial levels of specific bacterial species present
in a patient. According to one embodiment of the
invention, the treatment regimen would consist of a
patient wearing a mouthpiece according to the present
invention for 20 minutes once per day at a current
level of 500pA to effectively reduce oral levels of F.
nucleatum bacteria to prevent further biofilm formation
caused by F. nucleatum and to reduce the viability of
existing biofilm colonies of F. nucleatum.
According to another embodiment of this
invention, the treatment regimen would consist of a
patient wearing a mouthpiece according to the present
invention for 20 minutes once per day at a current
level of 50pA to effectively reduce oral levels of P.
gingivalis bacteria to prevent further biofilm
formation caused by P. gingivalis and to reduce the
viability of existing biofilm colonies of P.
gingivalis.
Furthermore, according to another embodiment
of his invention, the L.reatment_ regimen would consist
of a patient wearing a mouthpiece according to the
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 38 -
present invention for 20 minutes once per day at a
current level of 500pA to effectively reduce oral
levels of S. oralis bacteria to prevene further biofilm
formation caused by S. oralis and to reduce the
viability of existing biofilm colonies of S. oralis.
These treatments for binfilm reduction and
prevention may be repeated on a daily basis for a three
to six weeks for acute biofilm-based issues or may be
repeated once or more per week on a continuing basis
for chronic blofilm issues.
Method of Manufacture
A mouthpiece according to the present
invention may be formed using any method and means
known in the art. In one embodiment of such a method, a
first mold 700 is provided. The first mold 700
preferably includes a top portion 710 and a bottom
portion 720. Each portion of the first mold 700
includes a sealing means for sealing the first mold
700. In the illustrated embodimen: the sealing means
take the form of a cap 730A,7303. Each cap 730A,730B
preferably has a first channel 740 and a second channel
750 therethrough. The first mold 700 preferably
includes one or more fill ports 760 and one or more
vents 770.
The pieces of the first mold 700 are
preferably cleaned. A plurality of wires
780A,780B,780C,780D are then prepared and treaded
through the first mold 700. In the preferred
embodiment, the four wires 780A,780B,780C,780D are
threaded through the first mold 700 as shown in Figure
8. Preferably a first and a second wire 780A,780B are
Areaded Lhrough the top cap 730A and through ihe first
mold 700 and a third and a fourth wire 780C,780D arE:
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 39 -
threaded through the bottom cap 730 B and through the
first mold 700. Preferably the first wire 780A extends
through the first channel 740 in the top cap 730A and
the second wire 780 B extends through the second
channel 750 in the top cap 730A. Similarly, preferably,
tho third wire 780C extends through tho first channel
740 in the bottom cap 730B and the fourth wire 780D
extends through the second channel 750 in the bottom
cap 730B. The first mold 700 is then closed. A non-
conductive material is then injected through one or
more fill ports 760 in the first mold 700. The first
mold 700 cavity is filled when material is coming out
of all vents 770 in the first mold 700. The non-
conductive material may be a thermoplastic, a
thermoplastic elastomer, a thermoset polymer, a room
temperature vulcanizing elastomer, or other polymer.
When the non-conductive material is curcd,
the first mold 700 is preferably opened and the
partially formed mouthpiece 790 is removed from the
first mold 700. The plurality of wires 780A,7805,
780C,780D are now encapsulated by the non-conductive
material. Preferably a wire 780A,7808,780C,780D Is
located in each of the four exposed channels 800 of the
mouthpiece 790. Preferably the first wire 780A Is
located in the inner upper channel 800A, the second
wire 780B is located in the outer upper channel 800B,
the third wire 780C is located in the inner lower
channel 800C (not shown), and the fourth wire 780D is
located in the outer lower channel 800D (not shown).
The excess wire is then preferably trimmed from the
mouthpiece 790 and the remaining wire
780A,780B,780C,7801D is preferably inserted fully into
its associated channel 800A,800B,800C,8000.
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 40 -
A second mold 810 is preferably provided.
The second mold preferably includes a top portion 810A
and a boteom portion 810B. In the illustrated
embodiment, each of thc top and bottom portions of the'
second mold 810 preferably includes a mold base
820A,8202, a center piece 830A,8302, a first insert
840A,8400, and a second insert 850A,850B. The pieces of
the second mold 850 are preferably designed to silos
Lhe channels 800A,80013,800C,800D of the mouthpiece 790
to be filled with an electrically-conductive material.
The second mold 810 preferably includes one or more
fill ports 760 for filling the mold cavities. As there
are four channels 800A,800B,8000,8000 to be filled, the
preferred embodiment includes four fill ports 760.
Further, the second mold 810 preferably includes one or
more vents 770. In the illustrated embodiment each
cavity includes its own vent. 770.
Preferably the pieces of the second mold 810
are cleaned and prepared. The second mold 810 is then
assembled with the mouth piece 790 as shown in Figures
10-12B. Preferably, the bottom portion 8100 of the
second mold 810 is assembled first, wiLh the mouthpiece
790. The top portion 810A of the second mold 810 is
then assembled. A conductive material is then inserted
into each of the fill ports 760. The cavities are
filled when material is coming out of all vents 770 in
the second mold 810. The conductive material is
preferably a thermoseeting elastomer, but may also be a
thermoplastic, a thermoplastic elastomer, or other
polymer.
After the conductive material is cured, the
second mold 810 is opened and Lhe finished mouthpiece
760 is removed. Preferably, the top half 810A of the
CA 02907012 2015-09-15
WO 2014/149287
PCT/US2014/016710
- 41 -
second mold 810 is removed first. The first 840A and
second inserts 850A are preferably removed first. The
center piece 830A can then be removed. The bottom half
810B of -,ihe second mold may then be removed, again
first removing the first 840B and second inserts 850B
and then the center piece 830B.
The foregoing is considered as illustrative
only of the principles of 7_,he inven7_ion. Furthermore,
since numerous modifications and changes will readily
occur to those skilled in the art, it is not desired to
limit the Invention to the exact construction and
operation shown and described. While the preferred
embodiment has been described, the details may be
changed without departing from the invention, which is
defined by the claims.