Note: Descriptions are shown in the official language in which they were submitted.
COMPOSITION AND USE FOR TREATING CARDIAC FAILURE
BACKGROUND OF INVENTION
1. Field of Invention
The present invention relates to a composition and a method for inducing
haemoglobin
expression, mitochondrial biogenesis and autophagy in a subject.
2. Description of Related Art
Ischemia causes oxygen deprivation, cell injury and related organ
dysfunctions, such as
heart failure, stroke, chronic obstructive pulmonary disease, ischemic
retinopathy, liver injury,
and acute renal failure. Because mitochondrial dysfunction is a key factor in
organ ischemia
injury, upon loss of oxygen, mitochondrial oxidative phosphorylation rapidly
stops, with
resulting loss of the major source of ATP production for energy metabolism.
Erythropoietin (EPO) is essential for the regulation of the mass of
erythrocytes in response
to changes in tissue oxygenation during hypoxia and anaemia. The protective
effects of EPO
have been demonstrated in various tissues and experimental models of ischemia-
induced injury
and have been attributed to its effect on nonhaematopoietic metabolic
adaptation, inhibition of
apoptosis or stimulation of angiogenesis. Recently, EPO has been reported to
stimulate cardiac
mitochondrial proliferation through the activation of mitochondrial
biogenesis, which is
mediated by peroxisome proliferator-activated receptor coactivator 1-a (PGC-
1a), a key
regulator of cardiac bioenergetics. Clinically, EPO reverses cardiac
remodeling, improves
1
CA 2907965 2020-03-09
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
cardiac function, and enhances the exercise tolerance and quality of life of
patients by
inducing protective effects beyond the correction of anaemia. These findings
highlight
the possibility that EPO-mediated protection may depend on its modulatory
effects on
intracellular energetics.
Haemoglobin (Hb) is the main oxygen transporter in erythrocytes. Its main
form,
haemoglobin A, is a tetramer consisting of two a- and P-polypeptide chains,
each
carrying a heme group. Recently, Hb was unexpectedly found to be expressed in
many nonhaematopoietic cells, which may facilitate tissue oxygen transport or
increase cellular oxygenation to provide an intrinsic protective mechanism
against
hypoxic/ischemic injury.
Sleep has been implicated in the plastic cerebral changes that underlie
learning
and memory. Both rapid eye movement (REM) and non-REM sleep (NREM) play
important roles in memory. Behavioral observations in rats show that periods
of
learning are associated with subsequent increases in REM sleep, whereas REM
sleep
deprivation impairs memory of cognitive procedural or implicit types of
material
previously learned. NREM was found to be positively correlated with the
ability to
retain a word pair-association list which was a declarative memory. In
addition, the
transition from short-term to long-term memories by reactivation of sharp
wave-ripples in the hippocampus during NREM was important for memory
consolidation. It has also been demonstrated that inducing slow oscillation-
like
potential fields by transcranial application of oscillating potentials (0.75
Hz) during
early nocturnal NREM, enhances the retention of hippocampus-dependent
declarative
memories in healthy humans.
Patients with dementias, such as Alzheimer's disease (All), often have
nocturnally disrupted sleep. While the REM sleep in early-stage All patients
is
relatively unaffected by the disease process, later stages of Al) are marked
by
CA 02907965 2015-09-24
WO 2014/158165
PCT/1JS2013/034381
significant losses of REM sleep. These disruptions of nighttime sleep increase
in
magnitude with increasing severity of dementia. Memory loss is accompanied by
the
accumulation of oxidative damage to lipids, proteins, nucleic acids, and by
mitochondrial decay, all of which can disrupt neuronal function in aging and
disease.
Sleep deprivation (SD) also induced oxidative stress which resulted in memory
loss
and impaired mitochondrial activity. A study showed that 36h-SD in young
adults
results in neuropsychological results similar to those found in normal people
aged
approximately 60 years. Therefore, the regulation of mitochondrial function
and ROS
homeostasis may be useful as a therapeutic intervention in the oxidative
stress-related
memory loss.
Moreover, both EPO and the EPO receptor are expressed in neurons and
astrocytes, and EPO is produced primarily by astrocytes in the brain. EPO is
widely
used to enhance erythropoiesis in patients with anemia and recently has been
found to
have many non-haematopoietic beneficial effects, including cardioprotection
and
neuroprotection. An early clinical study has demonstrated cognitive
improvement
during EPO treatment among patients with chronic renal failure. Recently
studies
have shown that a high-dose EPO treatment improves hippocampal plasticity and
cognitive performance in patients suffering from neuropsychiatric diseases.
High-dose
EPO also enhances hippocampal long term potentiation by modulating plasticity,
synaptic connectivity and activity of memory-related neuronal networks and
improves
operant conditioning stability of cognitive performance in healthy mice.
It is hypothesized that EPO may play a pivotal role for phaimacological
applications in the treatment of SD-induced impairment of hippocampal learning
and
memory by modulating downstream mitochondria' regulator expression. Due to the
fact that EPO has limited clinical use because it cannot freely cross the
blood-brain
barrier (BBB), only systemic dosing of high-dose recombinant Epo (rEpo) would
3
result in neuroprotective activity.
Autophagy or "self digestion process" is an important physiological process
that targets
cytosolic components such as proteins, protein aggregates and organelles for
degradation in
lysosomes. The autophagic process is also essential for maintaining neuronal
homeostasis, and
its dysfunction has been directly linked to an increasing number of diseases.
In addition,
autophagy is directed to recycling intracellular nutrients in order to sustain
cell metabolism
during starvation, and eliminating damaged organelles and proteins that have
accumulated during
stress.
Defective autophagy is a major contributor to diseases which may be, but not
limited to,
neurodegeneration, liver disease, and cancer. A lot of human neurodegenerative
diseases are
associated with aberrant mutant and/ or polyubiquitinated protein accumulation
and excessive
neuronal cell death.
Polygonum multiflorurn Thunb is a Chinese medicine used for the treatment of
anaemia,
liver diseases, and other diseases commonly associated with aging. The present
invention
provides small molecular compounds isolated and identified from Polygonum
multiflorum Thunb.
These compounds have effects in experimental models of cardiovascular
diseases, cerebral
ischemia, Alzheimer's disease and inflammation diseases, and have antioxidant
and free
radical-scavenging properties. In addition, the present invention provides
therapeutic effects and
physiological mechanisms of such compounds in animal models.
SUMMARY OF INVENTION
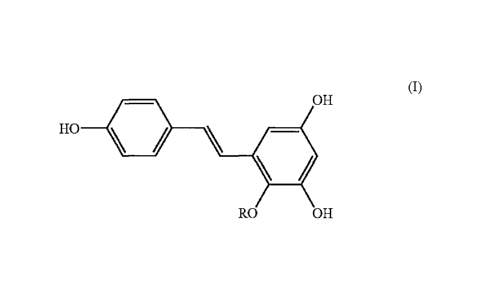
The present invention provides a compound represented by formula (I):
4
CA 2907965 2018-03-28
(I)
OH
HO \
RO OH
wherein R is a glycosyl group for use to treat cardiac failure. Also provided
is use of this
compound as the active ingredient for the preparation of a medicament for
treating cardiac
failure, and use of this compound for treating cardiac failure.
The glycosyl group may be one selected from the group consisting of
dihydroxyacetone,
glucose, galactose, glyceraldehyde, threose, xylose, mannose, ribose,
ribulose, tagatose, psicose,
fructose, sorbose, rhamnose, erythrose, erthrulose, arabinose, lyxose, allose,
altrose, gulose,
idose, talose, sucrose, lactose, maltose, lactulose, trehalose, cellobose,
isomaltotriose,
nigerotriose, maltotriose, melezitose, maltotriulose, raffinose, kestose and a
combination thereof.
In different aspects, the compound induces Hb-a, Hb-13, or dimeric Hb
expression in the
nonhaematopoietic cell of a subject, enhances erythropoietin-erythropoietin
receptor binding
affinity and also binds to the erythropoietin-bound erythropoietin receptor
complex. In addition,
the compound enhances endogenous EPO expression and stimulates Hb expression
in the
nonhaematopoietic cell.
The nonhaematopoietic cell is selected from the group consisting of a renal
cell, a
hepatocyte, a cardiomyocyte, a myoblast, a glial cell, a neuronal cell and a
retinal pigment
epithelium cell.
The present invention further provides a method for inducing erythropoietin
(EPO)-mediated haemoglobin (Hb) expression in a nonhaematopoietic cell of a
subject,
comprising administering to the subject a therapeutically effective amount of
the aforementioned
compound of formula (I). In accordance with the present invention, the subject
suffers a disease
or syndrome selected from the group consisting of hypoxia, anaemia, renal
ischemia, myocardial
ischemia, lung ischemia,
5
Date Recue/Date Received 2020-11-12
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
neurodegenerative disease, neuropsychiatric disease, age-related macular
degeneration (AMD)-related disease and a combination thereof.
The present invention further provides a composition for inducing
erythropoietin
(EPO)-mediated mitochondrial biogenesis in a nonhaematopoietic cell of a
subject,
comprising the aforementioned compound of formula (I) and a pharmaceutical
acceptable carrier.
In accordance with the present invention, the compound induces an increase of
a
mitochondrial number or PGC- 1 a expression for inducing the EPO-mediated
mitochondrial biogenesis, enhances erythropoietin-erythropoietin receptor
binding
affinity and also binds to the erythropoietin-bound erythropoietin receptor
complex.
In addition, the compound enhances endogenous EPO expression and stimulates Hb
expression in the nonhaematopoietic cell of the subject. The EPO-mediated
mitochondrial biogenesis is PGC-la-dependent.
The nonhaematopoietic cell is selected from the group consisting of a renal
cell,
a hepatocyte, a cardiomyocyte, a myoblast, a glial cell, a neuronal cell and a
retinal
pigment epithelium cell.
The present invention further provides a method for inducing erythropoietin
(EPO)-mediated mitochondria] biogenesis in a nonhaematopoietic cell of a
subject,
comprising administering to the subject a therapeutically effective amount of
the
aforementioned compound of foi mula (I). The compound induces an increase
of a
mitochondrial number or PGC- la expression for inducing the EPO-mediated
mitochondrial biogenesis.
The subject suffers a disease or syndrome selected from the group consisting
of
hypoxia, anaemia, ischemia-related disease, neurodegenerative disease,
neuropsychiatric disease, age-related macular degeneration (AMD)-related
disease,
cardiomyopathy, brain aging, chronic liver disease, multiple sclerosis, Pompe
disease,
6
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
hypertension, cardiac failure, obesity, diabetes mellitus, renal disease,
atherosclerosis,
aging, metabolic syndrome and a combination thereof.
The ischemia-related disease is one selected from the group consisting of
heart
ischemia, ischemic neurodegeneration, brain ischemia, myocardial ischemia,
limb
ischemia, cerebral ischemia, hepatic ischemia, retinal ischemia, stroke,
nephritic
ischemia, pulmonary ischemia, intestinal ischemia, cardiovascular ischemia,
renal
ischemia and kidney ischemia. The neurodegenerative disease is one selected
from the
group consisting of Alzheimer's disease, Parkinson's disease and Huntington's
disease.
The present invention further provides a method for inducing autophagy in a
subject having an autophagy defect, comprising administering to the subject a
therapeutically effective amount of the aforementioned compound of formula
(I),
wherein the autophagy enhances clearance of protein aggregates in the subject.
The autophagy defect is in a cell expressing the protein aggregates in the
subject,
wherein the protein aggregate is an aggregate selected from the group
consisting of
hungtingtin, amyloid 1 (Ap), a-synuclein, tau, superoxide dismutase 1 (SOD1),
variants and mutated forms thereof, and a combination thereof. The cell of the
subject
is a neuronal cell or a glial cell.
The autophagy defect is one disease selected from the group consisting of
neurodegenerative disease, retinal disease, Crohn 's disease, aging, cardiac
hypertrophy, chronic heart failure, tuberculosis, chronic obstructive
pulmonary
disease (COPD), cystic fibrosis, hepatic steatosis, polycystic kidney disease,
renal
failure, muscle atrophy, Paget's disease of bone, inclusion body myopathy,
fronto-temporal dementia, glomerular disease, metabolic disease, glycogen
storage
disease type II, inflammatory bowel disease, and Pompe disease. The
neurodegenerative disease is one selected from the group consisting of
Huntington's
7
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
disease, Alzheimer's disease, Parkinson's disease, amyotrophic lateral
sclerosis (ALS)
and insomnia.
The present invention further provides a composition for inducing autophagy in
a
subject having an autophagy defect. The composition comprises the
aforementioned
compound of formula (I) and a pharmaceutical acceptable carrier.
In addition, the invention provides a method for preventing memory loss in a
subject, comprising administering to the subject a therapeutically effective
amount of
the aforementioned compound of formula (I). The compound induces
erythropoietin
(EPO) to activate the autophagy in the subject.
The autophagy enhances protein clearance in the subject.
The autophagy defect is a neurodegenerative disease selected from the group
consisting of Huntington's disease, Alzheimer's disease, Parkinson's disease
and
insomnia.
BRIEF DESCRIPTION OF THE DRAWINGS
The patent or application file contains at least one drawing executed in
color.
Copies of this patent or patent application publication with color drawing(s)
will be
provided by the Office upon request and payment of the necessary fee.
FIG. IA to FIG. IB show EH-201 characterization. (A) HPLC profile of EH-201.
Mightysil RP-18 column (4.6 x 250 mm i.d.. 5 pm) was used at flow rate of 0.8
ml/min with Me0II/II20 (20/80, v/v) gradient to 100% Me0II in 60 minutes in
the
detection wavelength of 280 and 300 nm. (B) Positive ion mode LC-APC1/MS/MS of
EH-201.
FIG. 2A to FIG. 2J show that EH-201 is a potent inducer of EPO expression. (A)
8
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
The chemical structure of EH-201. (B, C) The EH-201-treated kidney slices and
hepatocytes were analyzed for EPO expression by Q-PCR and Western blotting.
(D, E)
Primary mice cardiomyocytes and (E, G) C2C12 myotubes were treated with EH-
201,
and the effects on EPO and EPOR expression were analyzed by QPCR and Western
blotting. (H) The bone marrow cells were incubated with EH-201 for 48 h, and
the
expression of EPO was detected by Q-PCR. (I) The bone marrow cells were
incubated
with EH-201, and the colonies were counted on day 9 for burst-forming
units-erythroid (BFU-E). (J) The quantification of the differentiated
erythroid
progenitors was performed using a haemoglobin colorimetric assay. The control
represents vehicle treatment. The values are presented as the means SEM (n=6
for
each). *P < 0.01, *P <0.05 versus control, Student's t-test.
FIG. 3A to FIG. 3G show that the induction of mitochondrial biogenesis by
EH-201 is mediated by EPO. (A, B) EH-201-treated kidney slices and primary
cardiomyocytes and (C, D) EH-201-treated hepatoeytes and C2C12 myotubes with
or
without the neutralizing EPO antibody (nEPO-ab, 1iag/m1) were analyzed for PGC-
1ce
expression by QPCR (n=6) and Western blotting (n=4), citrate synthase activity
(n=3),
and mtDNA copy number (n=6) and via the MitoTracker assay (n=6). The control
represents vehicle treatment. (E, F and G) rhEP0 was given to kidney slices,
hepatocytes and C2C12 myotubes. The mitochondria] activity was determined by
PGC- 1 a Q-PCR (n=6), citrate synthase activity (n=3), mtDNA copy number
(n=6),
and MitoTracker assays (n=6). The control represents vehicle treatment. PGC-la
siRNA-transfected C2C12 myotubes were treated with rhEPO (n=6). The control
represents the scrambled siRNA treatment. The values are presented as the
means SEM. < 0.01, *P < 0.05 versus untreated control, n.s., not
significant,
Student's t-test.
FIG. 4A to FIG. 41 show that the induction of haemoglobin expression in
9
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
nonhaematopoietic cells by EH-201 is mediated by EPO. (A) Cultured C2C12
myotubes under normoxia or hypoxia (5% 07) for 24 hours were analyzed for the
expression of haemoglobin-alpha (Hb-a) and -beta (Hb-I3) by R f-PCR, followed
by
1.5% agarose gel electrophoresis. (B) The rhEPO-treated C2C12 myotubes with or
.. without PGC- la siRNA transfection were analyzed for haemoglobin expression
by
Q-PCR (n=6). The control represents the scrambled siRNA treatment. (C, D) The
rhEPO-treated kidney slices and hepatocytes were analyzed for haemoglobin
expression by Q-PCR (n=6). The control represents vehicle treatment. (E, F)
EH-201-treated kidney slices and primary mice cardiomyocytes and (G, H)
EH-201-treated hepatocytes and C2C12 myotubes with or without the neutralizing
EPO antibody (nEPO-ab, 11.1g/m1) were analyzed for haemoglobin expression by
Q-PCR (n=6). The values are represented as means SEM. **P < 0.01, *P < 0.05
versus untreated control, #P<0.05 versus rhEPO treated control (50 ng/ml). (I)
The
effects of rhEPO and EH-201 on the proliferation of TF-1 cells were determined
by a
.. trypan blue dye exclusion assay (upper part of FIG. 41, n=6). The rhEPO (2
rig/ml)
and EH-201 cotreated TF-1 cells were incubated with or without the
neutralizing
antibody (nEPOR-ab, 0.5 ug/m1; nEPO-ab, 1ug/m1) for a 48 hour proliferation
assay
(lower part of FIG. 41, n=6). The values are represented as means SEM. **P
<0.01,
*P < 0.05 versus control (upper part of FIG. 41) or rhEPO alone (lower part of
FIG.
41), P<0.01 versus rhEPO+ EH-201 25 M, Student's t-test.
FIG. 5A to FIG. 5G show that EH-201 increases endurance performance and
activation of mitochondrial activity and haemoglobin expression in mice. (A)
The
endurance of normal mice was measured with the rotarod exercise under normoxic
or
hypoxic (8% 02) conditions (ND: normal diet). (B) The effect of EH-201 on
plasma
RBC numbers and haemoglobin levels. (C, D) EPO mRNA expression in the kidney
and liver of mice was measured by Q-PCR after 3 days of EH-201 administration.
CA 02907965 2015-09-24
WO 2014/158165
PCT/1JS2013/034381
The serum levels of EPO were determined by ELISA. (E, F) Isolated myocardium
tissues after 3 days of EH-201 administration were analyzed for haemoglobin
expression by Q-PCR, and the mitochondrial biogenesis was determined by mtDNA
copy number. (G) The effects of EH-201 treatment on ventricular haemoglobin
(Hb)
expression were quantified by TMBZ staining in SDS-PAGE (left part of FIG.
5G).
The quantification of Hb expression (tetramer and dimer, right part of FIG.
5G). The
values are represented as the means SEM (n= 5 animals each group). P < 0.01.
*P
<0.05 versus the ND group; 4*P <0.01, #1) <0.05 versus the day 7 ND group by
one-way ANOVA with Tukey's posthoc test.
FIG. 6A to FIG. 6H show that EH-201 has therapeutic effects on cardiac
dysfunction in doxorubicin (Dox)-induced cardionayopathy in mice. (A) The
survival
rate was analyzed using the Kaplan-Meier method (detailed treatment protocol
in
Materials and Methods). The normal (N) group represents saline injection. (B)
The
effect of EH-201 treatment on mice performing the hypoxic rotarod endurance
test
two weeks after Dox injection. (C, D) The effect of EH-201 on cardiac
abnormality
and functionality was characterized by ECG and echocardiography. EF, ejection
fraction; FS, fractional shortening; LVIDshl, left ventricular internal
diameter at
systole/diastole. (E) Representative photomicrographs of left ventricular
sections of
mouse hearts stained with haematoxylin-eosin and Masson's trichrome (left part
of
FIG. 6E, bars=10 um). The blue staining indicates fibrosis, and quantification
of the
interstitial fibrosis was performed (right part of FIG. 6E). (F) Isolated
myocardium
tissues after 2 weeks of Dox were analyzed for haemoglobin expression by Q-PCR
and (C) a TMBZ stain of each myocardium lysate of the treatment groups in
SDS-PAGE was performed, with (H) quantitative values. The values are
represented
as the means SEM (n=5-6 animals each group). **P < 0.01, *I' < 0.05 versus Dox
group by one-way ANOVA with Tukey's posthoc test.
11
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
FIG. 7A to FIG. 711 show that EH-201 accelerates the recovery from anaemia
and renal function in cisplatin-induced nephropathy in mice. (A) Schematic
diagram
protocol. (B) The time course kinetics of the RBC numbers in the peripheral
blood. (C)
The time course kinetics of the blood urea nitrogen (BUN) values after
cisplatin
injection. (D) The functional recovery of the kidneys of mice treated with EH-
201 on
day 28. (E) The haematoxylin-eosin stain of kidney sections after EH-201
administration on day 28 (bars=100 (F, G) The EPO expression in the kidney
and liver on day 28 was detemiined by Q-PCR. (H) The numbers of BFU-E colonies
in the isolated bone marrow cells from the treated mice on day 28. The values
are
represented as the means SEM (n=5-6 animals each group). " P < . 1 versus with
normal group; **P<0.01, *P<0.05 versus control group by one-way ANOVA with
Tukey' s posthoc test.
FIG. 8 shows that EH-201 induces Sirtl expression. Sirtl protein expression in
the lysates of the EH-201-treated kidney slices and hepatocytes were analyzed
by
Western blotting (n=4). The control represents vehicle treatment. The values
are
represented as the means SEM. **P<0.01, *P<0.05 compared with control.
FIG. 9 shows ribbon diagrams of the computational docking results for EH-201
on EPO/EPOR complex. Docking calculations were carried out using DockingServer
on EPO complexed with extracellular domain of EPOR protein model (PDB entry
code lcn4). The carbon backbone (green color) with balls and sticks indicated
the
ligand molecule EH-201, the helix (red color, left part of FIG. 9) indicated
the helix A
of EPO, and the loop (gray color, right part of FIG. 9) indicated the loop 5
of EPOR.
The predictive interaction residues including PR0144, 0u:1475
PRO149, Met150, and
1'11R151 are located in loop 5 of EPOR, which is important for EPO binding.
FIG. 10A to FIG. 10C show that EH-201-induced EPO production does not
12
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
involve Hif-a activation. (A) The hypoxia response element (HRE)-driven
luciferase
reporter (Luci) transfected HEK 293 cells were incubated with EH-201 under
normoxia or hypoxia (5% 02, as the positive control) for 24 hours. The plasmid
for
13-Galactosidase (13-Gal) was used as a transfection control, and the pGL3-v
served as
.. a vector control. Similar results were observed in three additional
independent
experiments. (B) The VEGF expression of the EH-201-treated hepatocytes were
analyzed by Q-PCR (n=3). Hypoxia condition served as a positive control. (C)
The
Hif-2a protein expression levels in the nuclear lysates of the EH-201-treated
kidney
slices were analyzed by Western blotting (H: 5% 02 hypoxia as a positive
control).
The control represents vehicle treatment. The values are represented as the
means SEM. **P < 0.01, *P < 0.05 compared with normoxia, Student's t-test.
FIG. 11A and FIG. 11B show that EH-201 increases mitochondrial function and
biogenesis in the liver and skeletal muscle. (A, B) Isolated liver and
skeletal muscle
tissues after 14 days of EH-201 administration were analyzed for the
mitochonthial
activity by PGC-1 a Q-PCR, citrate synthase activity and intDNA copy number.
The
values are represented as the means SEM (n= 5 animals each group). **P < 0.01,
*P
< 0.05 versus the ND group; "P <0.01, 41) < 0.05 versus the day 7 ND group by
one-way ANOVA with Tukey's posthoc test.
FIG. 12A and FIG. 12B show that EH-201 has therapeutic effects on cardiac
dysfunction in doxorubicin (Dox)-induced cardiomyopathy in mice. (A) The
effect of
EH-201 on the body weight of mice two weeks after Dox injection. (B) The
effect of
EII-201 on cardiac function was characterized by ECG, heart rate presented as
the
beat per second (bps). The values are represented as the means SEM (n=5-6
animals
each group). **P < 0.01, *P < 0.05 versus Dox group by one-way ANOVA with
Tukey's posthoc test.
FIG. 13 A to FIG. 13 F show that EH-201 stimulats EPO expression in primary
13
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
astrocytes and PC12 neuronal cells. (A) Structure of EH-201. (B, C) Real time
PCR
shows that EH-201 treatment for 24 hours increase EPO mRNA in astrocytes and
PC12 neuronal cells. The expression of GAPDH was used as an internal control.
(D)
Western blotting shows that EH-201 treatment for 24 hours increase EPO protein
expression in astrocytes and PC12 neuronal cells. The results are expressed as
the
relative index of untreated controls SD of at least three independent
measurements.
*P < 0.05, **P < 0.01 compared to untreated controls by one-way ANOVA followed
by Tukey's multiple comparison test. (E) Real time PCR shows that EPO
treatment
for 24 hours does not increase Hb-a mRNA in astrocytes and PC12 neuronal
cells. (F)
Real time PCR shows that EH-201 treatment for 24 hours does not increase Hb-a
mRNA in astrocytes and PC12 neuronal cells. The expression of GAPDH was used
as
an internal control. The results are expressed as the relative index of
untreated
controls SD of at least three independent measurements. * P < 0.05, ** P <
0.01
compared to untreated controls by one-way ANOVA followed by Tukey's multiple
comparison test.
FIG. 14A to FIG. 14F show that EH-201, a neuronal EPO inducer, stimulates the
expression of the mitochondrial regulator (PGC- in, Hb-B) and an antioxidant
gene
(HO- I) in primary astrocytes and PC12 neuronal cells. (A) Real time PCR shows
that
EPO or EH-201 treatment for 24 h increase PGC- I a, (B) Hb-B and (C) HO-1 mRNA
expression in primary astrocytes. (D) Real time PCR shows that EPO or EH-201
treatment for 24 h increase PGC- a, (E) Hb-P and (F) HO-1 mRNA expression in
PC12 neuronal cells. The expression of GAPDII was used as an internal control.
The
results are expressed as the relative index of untreated controls SD from at
least
three independent measurements. *1' < 0.05, "I' < 0.01 by one-way ANOVA
followed by Tukey's multiple comparison test.
FIG. 15A to FIG. 15H show that EH-201 increases mitochondrial activity,
14
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
decreases intracellular ROS and attenuates 11202-induced cell toxicity in
primary
astrocytes and PC12 neuronal cells. (A, E) Different forms of 1-lb (monomer:
16kD,
dimer: 32kD, tetramer: 641(D) expression identify by Hb-I3 Ab in primary
astrocytes
and PC12 neuronal cells treated with EH-201. The results are expressed as the
relative
expression of untreated controls SD from at least three independent
measurements.
*P < 0.05, **P < 0.01 by Student's t-test. (B, F) Succinate dehydrogenase
activity of
astrocytes and PC12 cells treated with EPO or EH-201 at 24 hour is determined
using
the MTT reduction assay (n=8) and is expressed relative to the respective
control
conditions (without treatment at 24 hour). The values are the means SD (n=8).
*P <
0.05, *13 < 0.01 compared to untreated controls. (C, G) Astrocytes and PC12
cells
treated with EPO or EH-201 for 24 hours are exposed to 100 1.04 H202 for 6
hours.
Intracellular ROS formation is measured using the DCFH-DA assay. The graph
shows
results in relative fluorescence units (RFU). The values are the means SD
(n=8). "P
< 0.01 compared to untreated controls; *P < 0.05, **P < 0.01 compared to H202
controls. Scale bar: 50 pm. (D, H) Astrocytes and PC12 cells treated with EPO
or
EH-201 for 24 hours are exposed to 500 [tM H202 for 6 hours. Cytotoxicity is
analyzed with trypan blue. The values are the means SD (n=3). "P < 0.01
compared
to untreated controls: *P < 0.05, **P < 0.01 compared to H202 controls using
Student's t-test.
FIG. 16A to FIG. 16F show that EPO is required for the neuroprotective effects
of EH-201 in astrocytes and PC12 neuronal cells. (A, D) Co-incubation of EH-
201
with an anti-EPO antibody results in the loss of EII-201-induced increase in
succinate
dehydrogenase activity, as assessed by the MTT reduction assay (n=8) in
astrocytes
and PC12 cells. *13 <0.05, **P < 0.01 compared to controls. (B, E) Co-
incubation of
EH-201 with an anti-EPO antibody for 24 hours results in the loss of
EH-201-mediated reduced ROS generation induced by H202, as assessed by the
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
DCFH-DA assay (n=8), and (C, E) the reduced 11202-mediated cytotoxicity as
assessed by trypan blue staining (n=3), *P <0.05, **P <0.01 compared to 11202,
" P
<0.01 compared to control using Student's t-test.
FIG. 17 A to FIG. 17 G show that effects of EH-201 in a mouse model of sleep
deprivation-induced memory loss. (A) Procedure of EH-201 treatment in
sleep-deprived (SD) mice. (B) Real time PCR and (C) western blot analysis of
EPO
expression in mouse hippocampus from each group. '1'p < 0.01 statistically
significant compared with the SD group; "p < 0.01, statistically significant
compared
with the control groups. (D) Real time PCR analysis of Hb13, PGC- 1 a and HO-1
expression levels in mouse hippocampus (n=6) *p < 0.05, **p < 0.01
statistically
significant compared with the SD group and #p < 0.05, 4* p < 0.01
statistically
significant compared with the control by one-way ANOVA followed by Tukey's
multiple comparison test. (E) The MTT assay is used as a marker for
mitochondria'
activity. The values depict mitochondria' function after sleep deprivation of
untreated
control mice and EH-201-treated mice. *p < 0.05. **p < 0.01 statistically
significant
compared with the SD group; "p <0.01, statistically significant compared with
the
control groups by one-way ANOVA followed by Tukey's multiple comparison test.
(F) Acquisition of step-through passive avoidance during 5 successive training
trials
in mice treated with or without EH-201. EH-201 treatment does not affect
learning
ability in mice. (G) Acquisition of step-through passive avoidance during 3
successive
testing trials in mice treated with or without EH-201. *p < 0.05, **p < 0.01
statistically significant compared with the SD group; "p < 0.01, statistically
significant compared with the control groups by one-way ANOVA followed by
Tukey's multiple comparison test.
FIG. 18 shows EH-201 induction of cellular EPO expression level in mice RPE
cells. C57mice RPE cells were incubated with 0.4, 2, 10 jig/m1 EH-201 in DMEM
16
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
supplemented with 10% FCS. The cultures were incubated at 37 t for 24 hours.
After incubation period, whole cell lysates were prepared with lysis buffer.
Total cell
lysates were prepared and subjected to western blot analysis to detect the
level of
endogenous EPO. GAPDH was used as a loading control. Bars represent mean SD
(n=3 different experiments; **p<0.01,***p<0.001).
FIG. 19A to FIG. 19D show induction of autophagy by EH-201. Primary mice
hepatocytes were treated with EH-201 at different doses (0.6, 2.5, 10 and 40
it g/ml),
rapamycin (autophagy activator,Rm, 50 nM) or 3-methyladenine (3MA, 10 mM) for
24 hours (A and B). The primary mice hepatocyte cultures under starvation
(sty) acted
as autophagy activation control (A, B). These treated cells were stained with
monodansylcadayerine (MDC) followed by fluorescent microscopy examination
(scale bars: 50 mm); and the fluorescent intensity was measured in
spectroflurometer
(B). Western immunoblotting was performed with hepatocyte lysate to study the
expression of autophagic marker proteins LC3 using LC3 antibody (C, D). Kidney
slices treated with EH-201 for 18 hours were used to study the effects of EH-
201 on
autophagy induction: analysis of autophagy induction was done by analyzing
western
blot against LC3. Quantification of LC3-II/LC3-I was performed using the
iminunoreactiye bands with ImageQuant imaging software (A mersham
Biosciences).
Data are expressed as mean SEM. **P<0.01, *P<0.05 compared with control.
FIG. 20A and FIG 20B show that EH-201 induced autophagic activation is
through hepatocyte growth factor (HGE) induction. Hepatocytes were treated
with
EI1-201 at different doses (0.6, 2.5, 10 and 40 mg/ml) for 24 hours. rhEPO
represented recombinent human FPO; rmIIGF represented recombinant murine
heaptocyte growth factor and nEPO-ab represented neutralizing FPO antibody.
DETAILED DESCRIPTION OF PREFERRED EMBODIMENTS
17
CA 02907965 2015-09-24
WO 2014/158165
PCT/1JS2013/034381
'Me following specific examples are used for illustrating the present
invention. A
person skilled in the art can easily conceive the other advantages and effects
of the
present invention. The present invention can also be implemented by different
specific
cases be enacted or application, the details of the instructions can also be
based on
different perspectives and applications in various modifications and changes
do not
depart from the spirit of the creation.
Erythropoietin is abbreviated as EPO in this specification and drawings.
Example 1 extraction, isolation and characterization of EH-201
EH-201, 2,3,5,4'-tetrahydroxystilbene-2-o-beta-d-glucoside (hereinafter
referred
to as EH-201)(FIG. 2A) was extracted and purified to 99.2% purity. The dried
and
milled roots of Polygonum multiflorum Thunb. was extracted with 40% ethanol
and
then evaporated to form syrup. In order to enrich the target components, the
extract
was diluted twice with 15% ethanol, loaded on a Diaion HP-20 resin column and
then
eluted with sequential 20%, 40%, and 70% ethanol, respectively. The effluent
of 40%
ethanol was collected and evaporated. The 40% ethanol effluent was then
redissolved
in 10% ethanol by sonication and partitioned with ethyl acetate of equal
volume five
times successively. The residue of ethyl acetate was then passed through a
Sephadex
LH-20 column eluting with methanol. A pale yellow compound, EH-201, was
obtained. The overall yield is about 0.5 Vcc from the crude, dried, milled
roots of
Polygonum multiflorum Thunb. to final compound EH-201 in pure form (99.2%).
For
future clinical test purpose, the crystallization of this compound was further
performed. The 30% aqueous-ethanolic solution of EI1-201 was then placed into
the
-20 C refrigerator overnight then placed into 4 C refrigerator. An acicular
crystal was
obtained several days later.
The chemical identity of EH-201 was confirmed by LC/MS/MS, UV, 1H-NMR
18
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
and proton-decoupled 13C-NMR data WIG. 1 and Table 1). and 1H-NMR and
proton-decoupled "C-NMR data sets using a Bruker AVIII-500 NMR spectrometer.
'the proton and carbon chemical shifts of EH-201 are listed in fable 1. The
LCMS
data of the purified EH-201 was performed with a Bruker LC/MS/MS spectrometer
Esquire 2000 in APCI (Atmospheric Pressure Chemical Ionization) mode with
positive ion polarity, using a gradient of HPLC grade water and methanol over
60
minutes with a reverse phase C18 column (FIG. 1A). The LCMS data is exhibited
in
FIG. 1B showing the correct mass of EH-201 at m/z 407Ø The EH-201 ion at m/z
407.0 is further subjected to MS/MS analysis where only the 407.0 ion was
isolated
and fragmented. The resulting daughter ion at m/z 245.1 is consistent with the
EH-201 loses its sugar moiety. Therefore, the compound was identified as
2,3,5,4'-tetrahydroxystilbene-2-o-beta-d-glucoside (TSG or THSG) (FIG. 2A).
19
Table 1
Table 1 Proton (500 MHz) and carbon (125 MHz) chemical shifts* of EH-201
Carbon 61.1 dc
1 133.8
2 138.0
3 152.2
4 6.57 (d, ./ 2.75 Hz) 103.7
5 156,1
6 6.21 d.J2.75 114 102.8
1' 131.0
2%6' 7,41 8.6 Hz) 129.4
3%5' 6.72 (dd, .1= 8.6, 2.6 Hz) 116.6
4' 158.5
a 7.67 (d,./" 16.5 Hz, Innis) 121.9
6.88 (d, I = 16.45 111z, trans) 130.2
1" 446 (d. 7.9 Hz) 108.3
2- 3.23- 3.75 (n) 75.6
3.23- 3.75 (m) 78.1
4" 3.23- 3.75 (m) 70.9
5" 3.23- 3,75 (m) 78.3
6" 33.23- 3.75 (m) 61.2
*All NMR spectra were recorded at 300 K and reference to the methanol solvent
peak at 3.31
ppm for proton and 49.15 ppm for carbon resonances.
Example 2 activation of mitochondria] function and haemoglobin expression in
nonhaematopoietic cells by the compound of the present invention
This example describes various assays that are useful in evaluating the
activation
of mitochondria] function and haemoglobin expression in nonhaematopoietic
cells by
the compound of the present invention. The compound of the present invention
is
Date Recue/Date Received 2021-04-29
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
prepared according to the methods provided in Example 1. The potency of this
compound is evaluated using a series of activity assays and these assays are
further
described in detail below.
1. Animals
Eight-to-ten-week-old specific pathogen-free C57BL/6J male mice (20-25 g),
obtained from the National Laboratory Animal Centre (Taiwan) were housed 5-6
per
cage at a constant temperature of 22 2'C and fcd standard laboratory chow
(PMI,
Brentwood, MO, USA) and water ad libitum under a 12 hour dark/light cycle. The
experimental protocol was approved by the Animal Research Committee of
National
Yang-Ming University (Guide for Animal Experiments, National Yang-Ming
University). All efforts were made to minimize animal suffering, to reduce the
number of animals used and to utilize alternatives to in vivo techniques, if
available.
All studies involving animals were reported in accordance with the ARRIVE
guidelines for reporting experiments involving animals.
2. Cell culture and treatment
The C2C12 myoblast, HEK293, and TF-1 cells were purchased from
Bioresources Collection and Research Centre (BCRC, Hsinchu, Taiwan). The C2C12
myoblasts were differentiated to rnyotubes and were treated with drugs for 24
hours.
Ex vivo 250 um-thick kidney slices were prepared from eight-to-ten-week-old
C57B1161 mice as previously described. The slices were treated with drugs in
the
gassed media (DMEM/F12 buffered with 15 mM HEPES and 20 mM sodium
bicarbonate) in an atmospheric chamber at 37 C with 50% 02: 5% CO2: 45% N2 for
18 hours. Mouse primary hepatocytes were isolated and purified from
eight-to-ten-week-old C57BL/6.1 mice as previously described and plated onto
1%
gelatin-coated microplates in DMEM supplemented with 10% PBS (Gibco, Germany).
After the hepatocytes had attached, fresh medium containing drugs was added
for 24
21
hours. Neonatal C57BL/6J mouse cardiomyocyte cultures were prepared from
post-natal one day-old C57BL/6J mice obtained from the Animal Centre at the
National Yang-Ming University as described previously, and the isolated
ventricular
cells were resuspended in 10% FCS-containing M199 medium (Gibco, Germany).
The cardiomyocytes were incubated in a humidified atmosphere at 37 C with 5%
CO2
on plates precoated with 1% gelatin. The subconfluence of spontaneously
beating
cells was achieved after 48 hours of culture, after which treatments with
various drugs
were performed for 24 hours. The bone marrow progenitor cell cultures for the
colony-forming assay and the haemoglobin colorimetric assay were prepared as
previously described. In the knockdown experiment, the C2C12 myotubes were
transfected with scrambled or PGC-1 a-specific siRNA (Table 2) using the
Lipofectamine 2000 reagent, according to the manufacturer's instructions
(Invitrogen).
These cultured cells were treated with rhEPO (recombinant human
erythropoietin,
Roche, Germany) or EH-201 or were co-incubated with EPO neutralizing antibody
(R&D, MN) for the indicated time periods. Thereafter, the drug treated cell
and tissue
lysates were collected and homogenized to examine the specific expression of
mRNA
and protein, as well as their mitochondrial activity.
Table 2
'LW 2 $0.1Licricc,, r I IL. Ygne uscd for ifficm. \ . anti I IP FF
INitate. Str41,60ce
GANN{ WIY4CATTO1 CIAA rliC4(.1 A-3?
N: -011 VGAGI FA( II E ;14't
EPO Pik! 51.AATOCIA< i < Amaii A ( p(
./41. I V: 1,C( (, A V 1-(.;
"[ ( iG ............................. VG( i.Ailt(i(.60c6.A.6( = 1-3'
At SUI3A(f. 6AtiAM.VtitiA6CtiOC (:" (.'=( 6 E-31
POC-10 FW: =1-1 1=3"
REM: 5. I t I ( .ic _______________________________ rum lUr ,(
22
Date Recue/Date Received 2021-04-29
Kb-at FW. :7:- 1, [GM creTTCCt ( At"CACCAAG-3"
: 5',(411.6( ( A Ark rt-A(
rAxicA4
Feb+ Fv..: '- 1-c 1: I rUAGANutit_Tc A. I ( (
= 14:
Rii'5"1ariCCCITIGAti( i=C =I C Ati1IuA,3"
1113S. rRNA FW: c ,CAACIEK AAAG A.1 \.( ; -
(0011041A) RI V: !;'.-i utrix 1 1. rire
HK2 FVV45 .a.=,[ = 1.,( c.= [ (.= [ CPI rrr I riTa'
-(c (=.( IC
VEUF FVfi i( \c. i4. '( a( if. I
REV:1% ru.
P0C-1,1 s.i RNA -tot( =6t. 1.(. ..t I IIi I _t
Ant ,r-( I.H,A(,(AKAAL( ((ri 1.11):3'
HIE y.txt m iraCT011 Cc = ( = I CarriC7iairCK rA
(.61.0( it cc,k(..
FW, trivant REV. 11 , 'L1E110115.
3. Real-time PCR
The total RNA was extracted using the TRIzol reagent (Invitrogen) and was
reverse transcribed by M-MLV Reverse Transcriptase (Promega). The EPO, EPOR,
PGC-la, Hb-a, Hb-13, and GAPDH mRNA expression were quantified by quantitative
real-time PCR (Q-PCR) with an ABI 7500 sequence detector (Applied Biosystems)
using SYBR Green Master MixR (ABI-7500).
The relative mRNA expression levels were determined using the TTCt method,
with GAPDH as the endogenous control. The primers used are listed in Table 2.
4. Western blot
The total protein (50 1.tg) was separated by 12% SDS-PAGE, transferred onto
PVDF membranes, and probed with antibodies against EPO, PGC-1 a, GAPDH,
23
Date Recue/Date Received 2021-04-29
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
PCNA (from Santa Cruz, CA), Sirtl (Millipore, Billerica), or 1-Iif-2ct (Novus
Biologicals, Littleton). Following incubation with the appropriate horseradish
peroxidase-conjugated secondary antibody, the signals were visualized by ECL
detection, according to the manufacturer's protocol (Perkin-Elmer).
5. Quantification of the mtDNA copy number
The total cellular DNA was purified using a conventional phenol-chloroform
method, and the mtDNA copy number was measured, as previously described.
6. The MitoTracker assay
The mitochondrial content was assessed by the MitoTracker microplate assay.
The treated cells were loaded with 0.1 i.tM green fluorescent MitoTracker-
Green
(MTG, Invitrogen) for 60 minutes at 37 C. The intracellular MTG content was
measured by fluorescence photometry (Theimo Scientific Inc.). Subsequently,
the
fixed cells were labeled with H33342 to assess the cell density. The
MTG/H33258
fluorescence ratios were calculated.
7. Measurement of citrate synthase activity
The citrate synthase activity was measured in tissue lysates. The changes in
absorbance at 412 nun were measured, and the activity was expressed as p.mol/
min/
mg protein.
8. TF-1 cell proliferation assay
Cells of the tEPO-sensitive cell line TF-lwere seeded in 96-well microplates
at a
cell density of 1 x 105 cells/ml in RPMI 1640 medium with 2% FBS, and the
cells
were treated with rhEPO and EH-201 with or without EPOR neutralizing antibody
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
(Santa Cruz) for 48 hours. The cell numbers were determined by a trypan blue
dye
exclusion assay.
9. Rotarod endurance assessment
Before being divided into treatment groups, eight-to-ten-week-old C57B1/6J
male mice were trained on a rotarod apparatus (14 rpm) for a maximum of 10
minutes
for each of 3 consecutive training sessions per day for 3 days, and the
animals that did
not master this task were excluded from the experiments. After training, the
qualified
mice were randomly divided into EH-201-treating groups (10, 30 or 90 mg/kg per
day,
n=5 for each group) for seven days. On the testing day, each mouse was
subjected to
three trials on the rotarod at 22 rpm under a normoxic or hypoxic (8% 07)
atmosphere.
The endurance performance was measured over time until the mice suffered from
exhaustion and fell off of the rotarod. The maximum trial length was 60
minutes, and
there was a 30-minute rest period between each trial.
10. EPO ELISA
The serum EPO concentrations were analyzed using an ELISA kit specific for
mouse EPO (R&D, MN), according to the manufacturer's instructions.
11. Doxorubi ci n-induced c ardiomyopathy
Cardiomyopathy was induced in eight-to-ten-week-old C57B1/6.1 male mice by a
single intraperitoneal (i.p.) injection of 15 mg/kg doxorubicin-IIC1 (Sigma-
Aldrich),
and the normal group was injected with saline (n=6). Seven days after the
injection,
the presence of doxorubicin-induced cardiomyopathy was confirmed with
electrocardiogram by observing a prolonged S-'I' interval. An average eighty
percent
of injected mice were successful induced (27/34), and the ineffective mice
were
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
excluded from the EH-201 treating experiments. The cardiomyopathic mice were
randomly divided into 4 cohorts comprising the control (Dox, n=9) and three
EH-201-treating groups (n=6 for each group) for an additional week. EH-201 was
administered orally by mixing it into the feed. The Dox group was fed a normal
diet
and EH-201-treating groups were fed nomial diet containing different doses of
EH-201 (10, 30 or 90 mg/kg per day). One week later, the mice were subjected
to the
rotarod endurance test, echocardiography and electrocardiogram. The mice were
killed after electrocardiogram, and the isolated hearts were subjected to
histological
examination and haemoglobin analysis.
12. Haemoglobin staining
The staining for haemoglobin in the isolated myocardium tissue lysates was
perfoimed with tetramethylbenzidine (TMBZ, Sigma-Aldrich), following
nonreducing SDS-PAGE. The photography and scanning of the gels was performed
using a Typhoon Triorm imager (GE Healthcare). The TMBZ stain was removed from
the gels by the addition of a 70 niM sodium sulfite solution. Thereafter, 30%
isopropanol was used to replace the sodium sulfite, and then the gels were
stained
with Coomassie blue for analysis of the protein loading control.
.. 13. Echocardiography and Electrocardiogram
The mice from all treatment groups were anaesthetized with isoflumne
(0.75-1.5% inhalation), and echocardiographic measurements were taken in M-
mode
in triplicates for each mouse using an ATL IIDI 5000 ultrasound system
(Philips
Medical Systems). To assess the electrocardiogram (ECG) parameters, three
electrodes were utilized. The ECG tracings from lead I were recorded by means
of an
electrocardiograph connected to subcutaneous needle electrodes in the
26
CA 02907965 2015-09-24
WO 2014/158165
PCT/ES2013/034381
isoflurane-anaesthetized mouse. All probes were connected to an amplifier and
digital
converter for signal recording at the 100-mV range with low-pass 1 kHz and
high-pass 1 kHz filters. An acquisition data system with Lab VIEW software
(National
instruments, Inc.) was used to record and analyze the ECG signals.
14. Cisplatin-induced nephropathy
Forty eight-to-ten-week-old C57B1/6J male mice were i.p. injected with three
doses of cisplatin (Sigma-Aldrich), following the scheme of 7, 6, and 6 mg/kg
body
weight, at 4-day intervals, and the normal group (n=6) was injected with
saline (FIG.
6A). On day 13, the collected serum samples were assayed for the urea nitrogen
content (BUN). Mice with BUN values greater than 100 mg/ dL were chosen for
the
experiment. An average seventy percent of injected mice were successful
induced
renal dysfunction (26/40), and the ineffective mice were excluded from the EH-
201
treating experiments. The mice were subsequently divided randomly into 4
cohorts
comprising the control (Ctrl, n=8) and three EH-201-treated groups (n=6 for
each
group) for an additional 2 weeks. Blood samples from all the mice were
collected
every 5 days. The RBC numbers were determined from the complete blood cell
count
using a Sysinex Kx-21 hematology analyzer (Sysmex America), and the serum BUN
levels were determined through the urease GI,DH method using a commercial kit
(Urea FS, DiaSys, Germany).
15. Bone marrow progenitor cell colony-forming assay
The bone marrow cell suspensions were isolated and cultured from the femurs of
six-week-old C57BL/6J male mice (National Laboratory Animal Centre, Taiwan)
for
assaying burst-forming units-erythroid (13FU-E). All cells were cultured in
MEM-alpha medium containing 15% FES (Gibco, Germany), 1% bovine serum
27
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
albumin, 0.8% methylcellulose, 0.1 mM 2-mercaptoethanol (Sigma-Aldrich), 2
U/m1
EPO (Roche, Geimany), and 10 ng/ml IL-3 (Sigma-Aldrich). The colonies (> 50
cells)
were counted on day 9 for BETI-E using an inverted microscope.
16. Haemoglobin colorimetric assay
For the detection of differentiated erythroid progenitors, the isolated bone
marrow progenitor cells were cultured in the presence of thc drug treatments
in
MEM-alpha medium containing 1% bovine serum albumin, 7.5 i.tM
2-mercaptoethanol, 1.4 mM L-glutamate (Sigma-Aldrich), 5 jiM FeCl3
(Sigma-Aldrich), and 25 [mil/mil EPO for 96 hours. Thereafter, the extracted
haemoglobin was mixed with the 2,7-diaminofluorene (DAF, Sigma-Aldrich)
working solution. The change in absorbance at 610 nm was continuously
monitored at
25 C for one minute. The initial rate of the reaction was measured, and the
amount of
Hb in the samples was determined from the Hb standard curve.
17. Luciferase reporter assay
HEK293 cells were transfected with a luciferase reporter plasmid (pGL3,
Promega) containing four repeats of the minimal hypoxia response elements
(HRE)
from the EPO gene. The transfected cells were incubated with EH-201 under
normoxia for 24 hours. The cells were kept under mimetic hypoxic (75 mM CoCl2)
or
hypoxic conditions (5% 02) as a positive control of Hif-la activity. After the
treatments, the cell lysates were harvested, and the luciferase expression was
measured by the Dual-Luciferase Reporter Assay System (Promega).
18. Histological analysis
The heart and kidney tissues were fixed with 10% formalin for paraffin
28
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
embedding. Paraffin sections (cross-section for the heart) of 5 gm thickness
were
prepared for the H&E and Masson's trichrome staining protocols. For the
analysis of
myocardial fibrosis, 6 random photomicrographs were taken in the viable
myocardium at a 400x magnification for each animal. The extent of fibrosis in
these
photomicrographs was quantified by a blinded observer using the ImageJ program
from NII-1.
19. Isolation retinal pigment epithelial cells sheets from mice and cell
culture
Intact eyes were removed quickly from 6-8 week old C57/BL6 mice (National
Laboratory Animal Center, Taiwan R.O.C.) and stored in ice cold PBS, which
contained: 8.0 g/L NaC1, 0.2 g/ L KC1, 0.8 g/L KJ-2[1)4., and 1.15 g/L
NaII2PO4. Eyes
were washed twice in growth medium (GM) consisting of Dulbecco's modified
eagle's medium (DMEM) containing high glucose, 10% FBS, 1%
penicillin/streptomycin, 2.5m ML-Glutamine and 1% non-essential amino acids.
After
washing, the eyes were then transferred into fresh PBS for dissection. Using
microdissection scissors and an upright dissection microscope, a circular
incision was
made around the ora serrata of each eye. The posterior eyecup containing the
neural
retina and the lens were placed in fresh GM medium and incubated for 20
minutes at
37 C in 5% CO2 incubator to facilitate separation of the Retinal Pigment
Epithelial
(RPE) cell sheets from the neural retina. After removal of the RPE sheets from
the
neural retina, intact sheets of RPE cells were peeled and collected in an
eppendorf
tube. RPE cells were centrifuged at 1500 rpm for 5 minutes and resuspended in
GM
medium. The cell suspension (0.5m1) was added to a 12-well plate. Cells were
cultured at 37 C in 5% CO2 for 10 days, with a change of medium (GM) every
other
day. After 10 days the cells were washed with EDrIA and then trypsinized for 4
minutes to detach the cells. The cells were collected in a tube, centrifuged
at 1000 rpm
29
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
for 5 minutes and resuspended in DMEM, 10% FES, PEN/strep. I-glutamine, sodium
bicarbonate. The cells were plated in 6 cm dish until they reached confluence,
at
which time they were trypsinized and grown in a larger dish.
C57mice RPE cells were incubated with 0.4, 2, 10 jig/m1 EH-201 in DMEM
supplemented with 10% FCS. The cultures were incubated at 37 t for 24 hours.
After incubation period, whole cell lysates were prepared with lysis buffer.
Total cell
lysates were prepared and subjected to western blot analysis to detect the
level of
endogenous EPO. GAPDH was used as a loading control.
20. Statistics
All results are expressed as the mean SEM. The statistical analysis was
perfoimed using Student's t-test. One-way ANOVA was used to examine the
differences across the animal experimental groups. The posthoc differences
between
the means of the experimental groups were determined via Tukey's test. P <
0.05 was
considered significant.
20. Results
(1) EH-201 is a potent EPO inducer
To determine whether EH-201 has the ability to induce EPO expression, kidney
slices and hepatocytes were treated with EH-201 ex vivo. EH-201 was observed
to
dramatically induce FPO mRNA and protein expression in a concentration-
dependent
manner in the kidney slices and hepatocytes (FIGS. 2B and 2C). According to
the
gene expression pattern of EPO in human tissues in the publicly available
database
created by Su, AI, et al., the EPO transcript is expressed at a surprisingly
high level
in human cardiomyocytes. Therefore, whether EH-201 can also induce EPO
expression in neonatal mice cardionnyocytes and C2C12 myocytes was also
tested. It
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
was observed that E11-201 concentration-dependently induced the expression of
EPO
and EPO receptor (EPOR) in the primary cardiomyocytes and C2C12 myocytes
(FIGS. 2D to 2G). Because the bone marrow progenitor cells can express FPO to
mediate hematopoiesis, bone marrow cells were cultured with EH-201 to examine
its
effect on erythropoiesis. The expression of EPO mRNA was increased in the bone
marrow cells exposed to EH-201 (FIG. 2H). EH-201 significantly increased the
number of BFLT-E colonies (FIG. 21) and Hb expression in a concentration-
dependent
manner (FIG. 2J). Accordingly, EH-201 is an EPO inducer.
.. (2) The induction of mitochondrial biogenesis by EH-201 is mediated by EPO
To determine whether EH-201 influences mitochondrial biogenesis, a series of
experiments were performed to test the effects of the EPO inducer in
nonhaematopoietic cells. In the EH-201-treated kidney slices, the activity of
the
mitochondrial marker enzyme citrate synthase increased in a concentration-
dependent
manner, and a dramatic increase in the mitochondrial copy number and PGC-1a
expression was also observed (FIG. 3A). The stimulatory effects of EH-201 on
mitochondrial biogenesis were also observed with hepatocytes, cardiomyocytes,
and
C2C12 myocytes (FIGS. 3B to 3D). However, neutralizing-EPO antibody treatment
abolished the effects of EH-201-induced mitochondrial biogenesis (FIGS. 3C and
3D),
whereas EPO treatment increased PGC-la expression and mitochondrial biogenesis
(FIGS. 3E to 3G). It was next examined whether these effects were mediated by
a
PGC- 1 a-dependent pathway using PGC- la-specific siRNA-transfected C2C12
myocytes. The PGC-la siRNA resulted in a 44% reduction in PGC-la mRNA
expression and a concomitant failure of EPO to induce mitochondrial biogenesis
(FIG.
3(i), which indicated that the activation of mitochondrial biogenesis by FPO
is
PGC-1a-dependent. Additionally, because the mammalian sirtuin (Sirtl)
regulates
31
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
mitochondrial function and biogenesis in the skeletal muscles and liver along
with
PGC-la, Sirtl expression was investigated and it was observed that EH-201
treatment
increased Sirtl expression (FIG. 8), which indicates that EH-201's induction
of
EPO-mediated mitochondrial activity might occur through the Sirtl/PGC-la
pathway.
Therefore, the increase in PGC-1 a due to EH-201 is dependent on the induction
of
mitochondrial biogenesis in nonhaematopoietic cells by increased EPO levels.
(3) The induction of haemoglobin expression in nonhaematopoietic cells by EH-
201
is mediated by EPO
It was further determined whether the expression of haemoglobin (Hb) was
regulated by hypoxia inducible EPO signaling in nonhaematopoietic cells. In
vitro
experiments were perfouned by incubating C2C12 cells in the absence and
presence
of hypoxic conditions. The exposure of the C2C12 myoblasts to hypoxia resulted
in a
noticeable increase in the expression of Hb-a and Hb-13 (FIG. 4A). In the EPO-
treated
C2C12 myocytes, the induction of Hb-a expression was more susceptible to
treatment
than that of Hb-I3 (FIG. 4B). In addition, the expression of both Hb-a and Hb-
I3 was
increased in a concentration-dependent manner in the EPO-treated kidney
slices,
whereas only the expression of Hb-13 was susceptible to induction in the EPO-
treated
hepatocytes (FIGS. 4C and 4D). The expression of Hb subunits was significantly
increased in EH-201-treated nonhaematopoietic cells (FIGS. 4E to 4H), and this
increase was abolished by concomitant neutralizing-EPO antibody treatment
(FIGS.
4G and 411). Studies were also conducted to determine the role of EPO
signaling in
the induction of Hb expression by PGC-la siRNA. It was observed that the
reduction
of PGC-la expression in C2C12 myocytes led to a decrease in the expression of
both
Hb-a and Hb-13 mRNA and also resulted in a decrease in the inducing effect of
EPO
on Hb-a (FIG. 4B). Hence, the regulation of Hb expression in nonhaematopoietic
32
CA 02907965 2015-09-24
WO 2014/158165
PCT/1JS2013/034381
cells occurs through both EPO mediated PGC-1a-dependent and PGC-1a-independent
pathways. These results show that EPO-mediated signaling is required for EH-
201's
induction of haemoglobin expression in nonhaematopoietic cells.
(4) EH-201 as an enhancer of EPO to EPOR binding instead of involving Hif-a
activation
To examine the mechanism behind EH-201's activity, computational docking
methods were carried out to predict the binding of EH-201 to EPOR. It was
found that
EH-201 binds preferentially to the EPO-bound EPOR complex (EPO/EPOR) rather
than the EPO-free naive EPOR (estimated total intermolecular energy -7.48
kcal/ mol
and -6.30 kcal/mol, respectively). Autodock identified more than two preformed
binding sites in the EPO/EPOR complex for EH-201 with negative favorable
binding
free energy, and the predicted interaction residues on EPOR (Tvlet150, Thr151,
FIG. 9)
involved the hot-spot residues located in loop 5. Because EPO autocrine
activity also
plays an important role in EPOR activation and the regulation of EPO
production, the
hypothesis that EH-201 may act as binding enhancer of EPO to EPOR, thus
enhancing the EPOR activation was tested. A TF-1 cell (EPOR positive)
proliferation
assay was performed to address the EPO biological activity. It was observed
that
rhEPO induced the proliferation of TF-1 cells concentration-dependently,
whereas, in
the absence of rhEPO, EH-201 alone was unable to induce cell proliferation
(FIG. 41).
In the presence of even very low concentrations of rhEPO, e.g., 2 ng/ml, EH-
201
significantly induced TF-1 cell proliferation in a concentration dependent
manner.
The addition of neutralizing EPO or neutralizing EPOR antibodies both
significantly
reduced 'IT-1 cell proliferation (FIG. 41). These data indicate that EPO is
required for
the activity of EH-201 and that EPO/EPOR complex may be the target of EH-201,
which serves as an enhancer of EPO and EPOR binding. It was also investigated
33
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
whether EH-201-induced expression of EPO involves the activation of the
hypoxia-inducible factor (Hit), as EPO expression is regulated by Hif. As
shown in
FIG. 10A, using a hypoxia response element driven luciferase reporter to
assess the
activation of Hif-la, EH-201 treatment did not activate the promoter activity.
Furthermore, Hif- 1 a targeted vascular endothelial growth factor (VEGF)
expression
was upregulated during hypoxia, whereas EH-201 did not alter the VEGF
expression
(FIG. 10B). EH-201 treatment also did not stabilize the Hif-2a protein levels
(FIG.
10C). These findings indicate that the induction of EPO by EH-201 is not due
to the
activation of Hif-la or Hif-2a.
(5) EH-201 administration enhances the endurance performance of mice
Given EH-201's EPO-inducing effect, whether EH-201 could enhance endurance
perfoithance in mice undergoing hypoxic stress was tested. Notably, the
administration of EH-201 for 3 days increased the run time to exhaustion under
both
normoxia and hypoxia in a dose-dependent manner (FIG. 5A), with a further
enhancement at 7 days. However, there was only a slight increase in RBC counts
and
Hb content in the peripheral blood (FIG. 5B), which indicated that EH-201
increased
the RBC numbers by inducing an increase in the endogenous EPO levels (FIG.
SD),
as confii __ Hied by the induction of the production of renal and hepatic EPO
(FIG. SC).
The expression of Hb-a and Hb-I3 in the myocardium of the EH-201-treated mice
was
significantly increased (FIG. SF), as confilmed by an increase in Hb protein
expression observed with TMBZ staining (FIG. SG). High doses of EI1-201 also
induced cardiac mitochondrial biogenesis (FIG. 5E). Furthermore, EI1-201
treatment
resulted in significantly increased PGC-1 a expression and mitochondria
content and
activity in the liver and skeletal muscles (FIGS. 11A and 11B). These results
show
that EH-201 treatment dramatically enhances the endurance performance and
hypoxic
34
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
tolerance of the mice via the induction of increased endogenous ETU expression
and
the stimulation of mitochondria' biogenesis and Hb expression in
nonhaematopoietic
tissues.
(6) Therapeutic effect of EH-201 on established doxorubicin-induced
cardiomyopathy
To assess the therapeutic potential of EH-201 in myocardial ischemia, a
doxorubicin (Dox)-induced cardiomyopathy model was used. One week after Dox
injection, the cardiomyopathic mice, as identified by ECG measurements, were
started on EH-201 treatment for seven days to examine EH-201's therapeutic
effects.
The survival rates of the EH-201-freated groups were seen to improve, and the
high-dose group remained alive until the end of the study period (FIG. 6A).
Following
the hypoxic rotarod endurance tests, although none of the groups recovered
from the
initial changes in body weight (FIG. 12A), the endurance performance activity
of the
EH-201-treated groups was found to be robustly increased (especially for the
30 and
90 mg/kg doses), whereas that of the Dox group was significantly reduced (FIG.
6B).
Myocardium injury was measured by ECG up to 2 weeks following the injection of
Dox, and these ECG parameters were significantly abnormal, which reflected the
extensive cardiac damage caused by Dox (FIG. 6C). Seven days after the
administration of EH-201, these ECG signs were significantly recovered in the
mice
treated with EH-201 (30, 90 mg/kg), which indicated an improvement in cardiac
activity (FIGS. 6C and 12B). Echocardiography performed 2 weeks after Dox
administration demonstrated that mice receiving Dox alone had significant
cardiac
functional deterioration, as characterized by decreased ejection fractions and
fractional shortening. Mice receiving EH-201 (30, 90 mg/kg) treatment had
significantly greater ejection fractions and fractional shortening, by
comparison (FIG.
6D). However, there were no significant differences in the left ventricular
diameters at
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
the systole and diastole between the groups. 'I here results indicate that
treatment with
EH-201 significantly mitigated the Dox-induced impairment of cardiac function.
In
addition, the Dox-damaged hearts presented with cytoplasmic vacuolization,
myofibrillar loss, and developed myocardial fibrosis, which were ameliorated
by
EH-201 treatment (FIG. 6E). The image quantification results indicated that
Dox
increased the area of fibrosis in the ventricular endomysium, compared with
nomml
mice (normal, 1.71 0.18% versus Dox, 8.31% 0.94%, (FIG. 6E), whereas fibrosis
was almost absent in the mice treated with medium to high doses of EH-201.
also It
was also observed that Hb expression in the isolated myocardium of the
EH-201-treated mice was upregulated (FIG. 6F, 30, 90 mg/kg) and Hb dimer forms
increased (FIGS. 6G and 6H). Taken together, these data show that EH-201 has
therapeutic effects, improving the cardiac function and ischemic tolerance of
the
Dox-induced cardiomyopathic mice.
(7) EH-201 ameliorates anaemia and renal function in cisplatin-induced
nephropathy
Since acute kidney injury may result from renal ischemia caused by the use of
nephrotoxic agents, to examine the effect of EH-201-induced EPO production on
the
anaemia with renal insufficiency, an established cisplatin-induced nephropathy
mouse
model was adopted (FIG. 7A). Significant anaemia from day 10 and impaired
renal
function from day 13 after the first injection of cisplatin was observed (FIG.
7B and
7C). Notably, the administration of 30 and 90 mg/kg of EH-201 for 2 weeks (on
day
28. FIG. 7B) led to an almost complete recovery of anaemia. Moreover, the BIJN
levels of the EI1-201 30 and 90 mg/kg treatment groups were also significantly
recovered (FIG. 7D). The histochemical examination revealed renal
tubuloepithelial
necrosis, vacuolation, and desquamation from day 13; however, treatment with
EH-201 significantly attenuated this renal damage (FIG. 7E). In addition, a
significant
36
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
increase in EPO in the kidneys of the anaemic mice, and EH-201 treatment did
not
lead to any further increases were observed (FIG. 7F), a finding which may due
to the
compensatory effect of the remaining functional kidney cells and the recovered
renal
function generated by EH-201 relieving the hypoxic stress on the kidney. The
EH-201
30 and 90 mg/kg treatments induced significant recovery of the hepatic EPO
expression (FIG. 7G). Furthermore, EH-201 administration also activated the
erythroid progenitor cells in the bone marrow (FIG. 7H). Collectively, these
findings
show that EH-201 improved the recovery from cisplatin-induced anaemia and
renal
dysfunction by inducing the production of EPO.
(8) EH-201 increases a cellular EPO expression level in mice RPE cells
FIG. 18 shows EH-201 induction of cellular EPO expression level in mice RPE
cells. C57mice RPE cells were incubated with 0.4, 2, 10 ug/m1 EH-201 in DMEM
supplemented with 10% FCS. The cultures were incubated at 37 C for 24 hours.
After
incubation period, whole cell lysates were prepared with lysis buffer. Total
cell lysates
were prepared and subjected to western blot analysis to detect the level of
endogenous
EPO. GAPDH was used as a loading control. Bars represent mean SD (n=3
different
experi ments ; **p<0. 01,***p<0.001).
Example 3 activating mitochondri a] function and haemoglobin expression in
neuronal
cells by the compound of the present invention
This example describes various assays that are useful in evaluating the
activation
of mitochondrial function and haemoglobin expression in neuronal cells by the
compound of the present invention. The compound of the present invention is
prepared according to the methods provided in Example 1. The potency of this
compound is evaluated using a series of activity assays and these assays are
further
37
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
described in detail below.
1. Cell culture
Astrocyte-enriched cultures were prepared from one-day-old C57BL/6,1 mice
obtained from the Animal Center at the National Yang Ming University as
described
below. Briefly, cortical tissue was digested with trypsin, and the resultant
dissociated
cells were suspended in DMEM containing 10% FBS and incubated in 100-mm
culture dishes. After 3 days in culture, the media was replaced with fresh 10%
FBS/DMEM, and the cells were maintained at 37 C for an additional 3 days. The
cells were dissociated with trypsin, suspended in 10% FBS/DMEM and incubated
in a
10-cm dish for 7-8 days prior to use. Cells prepared by this method consisted
of
approximately 90-95% astrocytes as determined by immunochemical staining with
an
antibody against glial fibrillary acidic protein (GFAP), a specific marker for
astrocytes. Rat PC12 neuronal cells were maintained in RPMI 1640.
2. RNA isolation and real time PCR
RNA was prepared using RNA-BeeTM RNA isolation reagent (Tel-test,
Friendswood, TX). An aliquot of 5 !_ig total RNA was incubated with AMV-RT
(Promega) to produce the cDNA for the RT-PCR analysis of the expression levels
of
I3-actin, NGF and PGC-la using the ABI Prism 7700 Sequence Detection System
and
the SYBR Green Master Mix kit (Applied Biosystems, Foster City, CA). The
expression level of mouse I3-actin was used as an internal reference. Relative
gene
expression levels were calculated with the 2¨AACT method. Fragments (100-250
bp)
were amplified using specific primers for each gene. The following primers
were used:
EPO (5'- AAT (JGA GUT GGA AGA ACA GG-3' and 5'- ACC CGA AtiC AGT
GAA GIG A-3'), Hb-13 (5'- 'I'GA TUC TGA GAA UGC TUC TGT CTC TG-3') and
(5'-GTG CCC TTG AGG C l'G TCC AAG TGA-3'), PGC- 1 a (5'- AGC CG'I GAC
38
CA 02907965 2015-09-24
WO 2014/158165
PCT/1JS2013/034381
CAC TGA CAA CGA 0-3') and (5'-GCT GCA 'IOU 'ITC TGA (J'1'0 CTA AG-3'),
110-1(5'- CGC CEP CCT OCT CAA CAT '1'-3') and (5-TUT 0TT CCT CTO TCA
GCA ICA C-3') and GAPDH (5'- TCT TCA CCA CCA TOG AGA AG-3' and 5'-
ACC AAA OTT GTC ATG GAT GAC-3').
3. Western blot
Cell and brain tissue lysates were prepared using a radioimmunoprecipitation
assay lysis buffer. Approximately 20 14 of protein was loaded, and western
blot
analysis was performed using a monoclonal mouse antibody against EPO (1:500;
sc-7956, Santa Cruz, California, USA), Hb-I3 (1:500; sc-31116, Santa Cruz,
California,
USA) and an anti-GAPDH antibody (1:10,000; ab9385, Abeam, Cambridge, UK) that
was used as a loading control. A horseradish peroxidase-conjugated anti-IgG
secondary antibody was used for enhanced chemiluminescence detection
(Amersham,
Buckinghamshire, UK).
4. Succinate dehydrogenase assay
Astrocytes or PC12 neuronal cells were plated at 104 cells per well in 96-well
plates. Twenty-four hours later, the cells were incubated with or without EPO
or
EH-201-containing media (100 ul per well) for 48 hours. Succinate
dehydrogenase
activity was determined by the MTT reduction assay. The activity was
noinialized to
the cellular protein level (measured with a BioRad protein kit), and changes
in
absorbance were measured using a microplate reader (PerkinElmer Life Sciences
Wallac Victor2). Activity was expressed relative to the control condition.
5. Intracellular reactive oxygen species generation
Astrocytes and PC12 neuronal cells were treated with EPO or EH-201 for 24
39
CA 02907965 2015-09-24
WO 2014/158165
PCT/1JS2013/034381
hours. The culture medium was replaced with 100 uM1420), and cells were
incubated
for 6 hours (astrocytes) or 30 minutes (PC12 cells). Reactive oxygen species
(ROS)
production in cells was then measured using 2',7'-dichlorofluorescin diacetate
(DCFH-DA; Molecular Probes, Eugene, OR, USA). DCFH-DA accumulates in cells
and is hydrolyzed by cytoplasmic esterases to become 2',7'-dichlorofluorescin.
2',7'-Dichlorofluorescin is oxidized by H202 to give a fluorescent product,
2',7' -dichlorofluoresecin. Briefly, cultures in 96-well plates were washed
with
DMEM containing 1% FCS and loaded with 50 MM DCFH-DA for 30 minutes at 37
C. Wells were then washed twice with Kreb's buffer, and the cells were
solubilized
.. with 0.1 N NaOH in 50% methanol. The wells were vortexed for 10 minutes,
and
2'-7'-dichlorofluorescein (DCF) fluorescence was either observed under
fluorescence
microscopy or measured in a microplate reader (PerkinElmer Life Sciences
Wallac
Victor2).
.. 6. H202 induced cytotoxicity in astrocytes and PC12 neuronal cells
Astrocytes and PC12 neuronal cells were treated with EPO or EH-201 for 24
hours. Astrocyte culture medium was replaced with 500 IttM H202, and the cells
were
incubated for 6 hours. PC12 cell culture medium was replaced with 250 [tM
H202,
and the cells were incubated for 4 hours. Cell viability was determined by the
exclusion of trypan blue as assessed by light microscopy.
7. Sleep deprivation procedure
Forty 12-week-old C57B1/61 adult male mice were obtained from the National
Laboratory Animal Center (Taipei, Taiwan). Mice were housed at a constant
temperature and supplied with laboratory chow (PML Brentwood, MO, USA) and
water ad libitum. 'Me experimental procedure was approved by the Animal
Research
CA 02907965 2015-09-24
WO 2014/158165
PCT/1JS2013/034381
Committee of National Yang-Ming University. The animals were deprived of sleep
(SD) or maintained in their home cages (control group) in the same room.
Briefly,
C57BL/6J male mice (7 weeks of age) were housed on a 12 hours/12 hours
light/dark
schedule with lights on at AM 6:00 and were handled for 7 days. The mice were
sleep-deprived in their home cages for 5 hours by gentle handling beginning at
AM
6:00 or left undisturbed (non-sleep-deprived mice). Mice were fed with normal
diet or
normal diet containing different concentrations of EH-201 (50, 100 or 200
mg/kg per
day) for 3 days prior to sleep deprivation.
8. Passive avoidance task
Passive avoidance experiments were conduced as previously described with
minor modifications. A two-way shuttle-box with a guillotine door placed
between
the modular testing chambers was employed. One chamber was illuminated with a
40
W bulb while the other remained in the dark. In the training session, the
animals were
individually placed in the illuminated chamber that faced away from the
guillotine
door. When the animal entered the darkened chamber, the door was silently
lowered
and a 0.5 mA foot shock was applied for 2 seconds through the grid floor. In
the test
sessions, the animals were again placed in the illuminated chamber, but no
foot shock
was applied. Latency to step through was recorded in each session.
9. Statistical analysis
All results are expressed as the mean and standard deviation (SD). The
significance of the differences of the means between more than two groups was
determined using a one-way analysis of variance (ANOVA) followed by "fukey's
post-hoc test. The Student's t-test was employed for the statistical
comparison of
paired samples. A P value of < 0.05 was considered statistically significant.
41
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
10. Results
(1) EH-201 induced neuronal EPO and elevated expression of EPO in primary
astrocytes and PC12 neuronal cells
EH-201, a neuronal EPO inducer, elevated the expression of EPO in primary
astrocytes and PC12 neuronal cells. Because exogenous EPO cannot cross the
blood-brain barrier, its clinical use is limited. Thus, the effect of an
endogenous
neuronal EPO inducer, EH-201, was tested. The structure of EH-201 is shown in
FIG.
13A. After EH-201 treatment, astrocytes demonstrated a dose-dependent increase
in
EPO mRNA expression, as measured by real time PCR analysis (FIG. 13B). EH-201
treatment also up-regulated EPO mRNA expression in PC12 neuronal cells (FIG.
13C). The intracellular EPO protein expression in astrocytes and PC12 cells
was
up-regulated during EH-201 treatment (FIG. 13D).
(2) EH-201 elevated the expression of mitochondrial regulator PGC-1 a and Hb
in
primary astrocytes and PC12 neuronal cells
After EPO or EH-201 treatment for 24 hours, cellular mRNA was extracted to
determine EPO-mediated gene expression. Real time PCR revealed elevated
expression of PGC-la and Hb-3 mRNA expression; HO-1, a known antioxidant gene
up-regulated by EPO, was also induced during EH-201 treatment, both in
astrocytes
(FIG. 14A to FIG. 14C) and in PC12 neuronal cells (FIG. 14D to FIG. 14F); Hb-a
expression, however, was not significantly changed (FIG. 13E to FIG. 13F).
(3) EH-201 increased mitochondrial activity and attenuated oxidative stress in
primary astrocytes and PC12 neuronal cells
Because PGC- in and Hb are known as mitochondrial regulators, it was analyzed
42
CA 02907965 2015-09-24
WO 2014/158165
PCT/1JS2013/034381
which form of Hb was regulated by EH-201. FIG. 15A and E showed that EH-201
increased all three foinis of Hb expression in astrocytes and PC12 cells.
Next,
mitochondrial activity in cells treated with or without EPO or EH-201 was
measured
by the MTT assay. FIG. 15B and F showed that EPO or EH-201 induced
mitochondrial activity in both astrocytes and PC12 cells. It was examined
whether
EPO or EH-201-mediated up-regulation of these genes attenuates oxidative
stress
induced by FI202 in astrocytes and PC12 cells. It was estimated ROS generation
in the
cultured cells after exposure to H202 using an oxidative probe, CM-H2DCFDA.
EPO
and EH-201 treatment decreased intracellular ROS in astrocytes (FIG. 15C) and
PC12
cells (FIG. 150). EPO and EH-201 also decreased cell toxicity in cells exposed
to
H202, indicating that the mitochondrial regulation and ROS homeostasis effect
of
EPO is biologically important (FIG. 15D and 15H).
(4) EPO is required for EH-201-mediated increased mitochondria' activity and
attenuation of oxidative stress in primary astrocytes and PC12 neuronal cells
It was evaluated whether the increased mitochondria' activity and the
reduction
in H202-induced ROS generation and cytotoxicity following treatment with EH-
201
in astrocytes and PC12 cells were dependent on EPO. The increased
mitochondria]
activity observed with EH-201 treatment was blocked in the presence of an anti-
EPO
antibody as measured by the MTT assay (FIG. 16A) compared to cells treated
with
EH-201 alone. The reduction of ROS generation induced by H202 in astrocytes
and
PC12 cells treated with EI1-201 was abolished when cells were co-incubated
with an
anti-EPO antibody (FIG. 1611). The anti-EPO antibody also inhibited the
EH-201-mediated reduction in 14202-inducted cytotoxicity (FIG. 16C).
(5) Effects of EH-201 in a mouse model of sleep deprivation-induced memory
loss
43
CA 02907965 2015-09-24
WO 2014/158165
PCT/1JS2013/034381
It was evaluated the neuroprotective effect of EH-201 on memory by using a SD
model. the experimental procedure is outlined in FIG. 17A. It was analyzed the
EPO
expression in the hippocampus from each treated animal. Real time PCR and
western
blotting showed an increase in EPO expression in animals fed with EH-201 (FIG.
17B
and 17C). Hb[3, HO-1 and PGC-la mRNA expression in the hippocampus was
analyzed by real time PCR (FIG. 17D). It was further evaluated the
mitochondrial
succinate dehydrogenase activity using the MTT assay (FIG. 17E). In the
passive
avoidance test, animals fed EH-201 for three days did not exhibit a difference
in the
ability to learn (FIG. 17F). However, there was a significant improvement in
memory
performance in EH-201-fed mice after SD in the passive avoidance test (FIG.
17G).
The gene expression changes and the increased mitochondrial activity in
hippocampus
from animals fed with EH-201, especially at doses of 100 and 200 mg/kg,
correlated
with the passive avoidance test.
Example 4 inducing autophagy by the compound of the present invention
This example describes various assays that are useful in evaluating the
inducing
autophagy by the compound of the present invention. The compound of the
present
invention is prepared according to the methods provided in Example 1. The
potency
of this compound is evaluated using a series of activity assays and these
assays are
further described in detail below.
1. POS Phagocytosis Assays
After the treatments indicated above, cells were treated with FITC-OS (1x107
OS/well) and incubated at 37'C for 4 hours. Untreated cells were used to
obtain
baseline fluorescence. The cells were washed four times with (FBSS) to remove
excess POS. Finally, EBSS was added to each well, at 100 1.d/well, and the
analysis of
44
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
mean FITC-OS fluorescence was achieved by a fluorometer, which quantified the
FITC-OS fluorescence at excitation 485 nm and emission 535 nm. "[hereafter,
fluoro-quenching dye was added per well, at 25 gllwell, and the dye was
incubated at
37 C for 30 minutes; the dye was quantified by fluorometer analysis of
fluorescence
(excitation, 485 nm: emission, 535 nm).
2. Western blot analysis
After the indicated treatments, cells were washed twice with ice-cold PBS, and
were lyzed in extraction buffer (1M Tris, pH 6.8, 10% SDS, 1M DTT). 10-1514 of
total protein was separated by SDS-PAGE, and analyzed by immunoblotting using
chemiluminescence. The primary antibodies used were LC3B antibody (Gene Tex,
USA, 1:1000), EPO (GAPDH (Santa Cruz, CA, USA,1:1000) or GAPDH (Santa Cruz,
CA, USA,1:1000), peroxidase-conjugated anti-mouse IgG or peroxidase-conjugated
anti-rabbit IgG. (Santa Cruz, CA, USA, 1:1000). The intensity of protein bands
was
quantified using image j software and the ratio of specific band to control
was
analyzed.
3. Labeling of autophagic vacuoles with monodansylcadaverine
Monodansylcadaverine (MDC) is a spontaneously fluorescent dye that can be
incorporated selectively into autophagosomes and autolysosomes. Cells were
incubated with 0.05 mM MDC in PBS at 37 C for 1 hour. After incubation, cells
were
washed two times with PBS and immediately analyzed by fluorescence microscopy
(excitation: 380-420 nm, barrier filter 450 nm).
4. Cell culture and treatment
The culture of murine kidney slices and primary mice hepatocytes have
CA 02907965 2015-09-24
WO 2014/158165
PCMJS2013/034381
described previously. These cultures were treated with EH-201 at different
doses (0.6,
2.5, 10 and 40 mg/ml), autophagy activator raparnycin (Rm, 50 nM) or autophagy
inhibitor 3-methyladenine (3MA, 10 mM) for 24 hours. The hepatocyte culture
under
starvation (sty) was autophagy activation control.
5. Results
(1) FIG. 19A to FIG 19D show induction of autophagy by EH-201.
(2) As shown in FIG 20A and FIG 20B, EH-201 induced autophagic activation is
through hepatocyte growth factor (HGF) induction.
The foregoing descriptions are only illustrative of the features and functions
of
the present invention but are not intended to restrict the scope of the
present invention.
It is apparent to those skilled in the art that all equivalent modifications
and variations
made in the foregoing descriptions according to the spirit and principle in
the
disclosure of the present invention should fall within the scope of the
appended claims.
46
CA 02907965 2015-12-23
SEQUENCE LISTING TN ELECTRONIC FORM
In accordance with Section 111(1) of the Patent Rules, this
description contains a sequence listing in electronic form in ASCIT
text format (file: 96200-2T SEQ 23-DEC-15 v1.txt).
A copy of the sequence listing in electronic form is available from
the Canadian Intellectual Property Office.
The sequences in the sequence listing in electronic form are
reproduced in the following table.
SEQUENCE TABLE
<110> Wu, Sophia Shu Fen
Wu, Rong-Tsun
<120> COMPOSITION AND METHOD FOR INDUCING EPO-MEDIATED HAEMOGLOBIN
EXPRESSION AND MITOCHONDRIAL BIOGENESIS IN NONHAEMATOPOIETIC CELL
<130> 96200-2T
<140> CA 2,907,965
<141> 2013-03-28
<160> 27
<170> PatentIn version 3.5
<210> 1
<211> 20
<212> DNA
<213> Mus musculus
<400> 1
tggcattgtg gaagggctca 20
<210> 2
<211> 20
<212> DNA
<213> Mus musculus
<400> 2
ggaagaatgg gagttgctgt 20
<210> 3
<211> 20
<212> DNA
<213> Moe musculus
46a
CA 02907965 2015-12-23
<400> 3
aatggaggtg gaagaacagg 20
<210> 4
<211> 19
<212> DNA
<213> Mus musculus
<400> 4
acccgaagca gtgodgtgd 19
<210> 5
<211> 21
<212> DNA
<213> Mus musculus
<400> 5
tctgggagga agcggcgagc t 21
<210> 6
<211> 22
<212> DNA
<213> Mus musculus
<400> 6
gaggagaacc ggacgcctcc gt 22
<210> 7
<211> 20
<212> DNA
<213> Mus musculus
<400> 7
cgccttcttg ctcttccttt 20
<210> 8
<211> 21
<212> DNA
<213> Mus musculus
<400> 8
tctgcctctc tatctgtttg g 21
<210> 9
<211> 27
<212> DNA
<213> Mus musculus
<400> 9
atgtttgcta gcttccccac caccaag 27
46b
CA 02907965 2015-12-23
<210> 10
<211> 24
<212> DNA
<213> Mus musculus
<400> 10
ggtggctagc caaggtcacc agca 24
<210> 11
<211> 26
<212> DNA
<213> Mus musculus
<400> 11
tgatgctgag aaggctgctg tctctg 26
<210> 12
<211> 24
<212> DNA
<213> Mus musculus
<400> 12
gtgcccttga ggctgtccaa gtga 24
<210> 13
<211> 22
<212> DNA
<213> Mus musculus
<400> 13
ccgcaaggga aagatgaaag ac 22
<210> 14
<211> 20
<212> DNA
<213> Mus musculus
<400> 14
tcgtttggtt tcggggtttc 20
<210> 15
<211> 24
<212> DNA
<213> Mus musculus
<400> 15
gccagcctct cctgatttta gtgt 24
<210> 16
<211> 24
46c
CA 02907965 2015-12-23
<212> DNA
<213> Mus musculus
<400> 16
gggaacacaa aagacctctt ctgg 24
<210> 17
<211> 24
<212> DNA
<213> Mus musculus
<400> 17
gcaacragagc gggctgcctc gcag 24
<210> 18
<211> 24
<212> DNA
<213> Mus musculus
<400> 18
acttgatcac ttcatgggac ttct 24
<210> 19
<211> 21
<212> RNA
<213> Mus musculus
<400> 19
gacggauugc ccucauuugu u 21
<210> 20
<211> 21
<212> RNA
<213> Mus musculus
<400> 20
caaaugaggg caauccgucu u 21
<210> 21
<211> 51
<212> DNA
<213> Mus musculus
<400> 21
ccctacgtgc Lgtccctacg tgctgtccct acgtgctgtc ccacgtgctg t 51
<210> 22
<211> 22
<212> DNA
<213> Mus musculus
46d
CA 02907965 2015-12-23
<400> 22
agccgtgacc actgacaacg ag 22
<210> 23
<211> 23
<212> DNA
<213> Mus musculus
<400> 23
gctqcatqqt tctgagtqct aag 23
<210> 24
<211> 19
<212> DNA
<213> Mus musculus
<400> 24
cgccttcctg ctcaacatt 19
<210> 25
<211> 22
<212> DNA
<213> Mus musculus
<400> 25
tgtgttcctc tgtcagcatc ac 22
<210> 26
<211> 20
<212> DNA
<213> Mus musculus
<400> 26
tcttcaccac catggagaag 20
<210> 27
<211> 21
<212> DNA
<213> Mus musculus
<400> 27
accaaagttg tcatqgatga c 21
46e