Note: Descriptions are shown in the official language in which they were submitted.
CA 2,908,295
Blakes Ref.: 12770/00001
1 METHOD FOR PURIFYING TERIPARATIDE
2 The present invention relates to a novel method for purifying
teriparatide, a therapeutically active
3 polypeptide fragment of full-length human parathyroid hormone. The method
is based on ion
4 exchange chromatography and is effective in separating the
therapeutically active polypeptide
from undesirable variants, such as truncated polypeptides. The method of the
invention can be
6 used at a preparative scale which allows it to be implemented into the
production process for
7 teriparatide. Accordingly, the invention also provides a method for the
production of teriparatide
8 which includes a step in which teriparatide is purified by the novel ion
exchange chromatography
9 method of the invention.
BACKGROUND OF THE INVENTION
11 Parathyroid hormone (PTH) is a polypeptide hormone which is naturally
produced in the
12 parathyroid of mammals. The human polypeptide consists of 84 amino acids
and is involved in
13 the regulation of the calcium concentration in blood plasma. If the
calcium level drops below a
14 threshold level, PTH is secreted by cells of the parathyroid glands into
the blood and induces a
release of calcium from bone tissue. At the same time, PTH supports calcium
absorption from the
16 small intestine and enhances calcium reabsorption from the primary
urine, thereby suppressing
17 calcium loss via the kidneys. Due to its calcium releasing effects, an
excessive amount of PTH in
18 the blood as commonly observed in primary and secondary
hyperparathyroidism has been found
19 to be associated with reduced bone density and bone atrophy
(osteoporosis). Considering the
physiological effects of PTH, it appears odd that the hormone nevertheless
proved useful in the
21 therapy of osteoporosis.
22 However, animal studies in rats for the first time revealed that a short-
term exposure to PTH
23 supports bone formation due to transient activation of osteoclasts,
whereas a sustained exposure
24 ultimately results in bone atrophy. Subsequent clinical studies in
humans using the
pharmaceutically active fragment PTH1-34 of human PTH confirmed that the
fragment can be
26 used for treating osteoporosis. PTH1-34 is a polypeptide having a
molecular mass of 4.7 kDa that
27 consists of the first 34 amino acids of the human PTH hormone. It was
approved for osteoporosis
28 therapy in 2002 under the product name "teriparatide". Teriparatide is
sold by Lilly Pharma under
29 the trade name Forteo (in the US) and Forsteo (in Europe).
CPST Doc: 375089.2 1
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 At present, PTH1-34 for therapeutic use is produced by heterologous
expression in bacterial host
2 cells and subsequent purification of the pharmacological active
polypeptide. However, it has been
3 .. found that all known fragments and variants of PTH1-34 occur during the
production process
4 demonstrate a reduced potency compared to PTH1-34. In addition a negative
effect on overall
purity is observed. For example, a number of fragments derived from PTH1-34
were identified in
6 .. the final batch obtained after heterologous expression, including amongst
others PTH1-30, PTH2-
7 34, PTH3-34 and PTH4-34. Moreover, chemical modifications of certain
amino acids of PTH1-34,
8 e.g. oxidation of methionine residues or deamidation of asparagine
residues, give rise to
9 additional variants.
However, as homogeneity of a product intended to be used as a therapeutic is
of utmost
11 importance for safety reasons, any kind of truncated or chemically
modified form of PTH1-34 in
12 the final product is clearly undesirable. Accordingly, current
production processes normally
13 include one or more chromatography steps that aim at the purification of
the PTH1-34 polypeptide.
14 However, it has been found to be problematic to separate PTH1-34 from
some of its variants and
fragments without significant product loss. In particular, it was observed
that the fragment PTH2-
16 .. 34 shows essentially the same retention properties in ion exchange
chromatography as PTH1-
17 34. Consequently, the PTH2-34 fragment is co-eluted with PTH1-34 which
makes PTH1-34
18 purification challenging. Moreover, alternative chromatographic
techniques, e.g. reversed phase
19 chromatography, were tested but did not show any potential for a
preparative separation of
truncated and full-length teriparatide.
21 In light of the above, new methods are needed which are effective in
separating PTH1-34 from its
22 fragments and chemically modified variants. The method of the present
invention provides for the
23 .. effective purification of the PTH1-34 polypeptide by cation exchange
chromatography using
24 .. inversely directed gradients of pH and ionic strength. As discussed in
more detail below, the
method is particularly suitable for separating PTH1-34 from PTH2-34.
26 .. BRIEF DESCRIPTION OF THE FIGURES
27 Fig. 1 shows the primary and secondary structure of the parathyroid
hormone fragment PTH1-34.
28 Fig. la shows the amino acid sequence and the distribution of charges in
PTH1-34. Fig. lb shows
29 the secondary structure of PTH1-34.
CPST Doc: 375089.2 2
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 Fig. 2 shows the attempt to separate PTH1-34 from PTH2-34 by cation
exchange chromatography
2 at analytical scale using a salt gradient. Column volume (CV): 1 mL.
Resin: FractogelTM S03- (S).
3 Eluent A: 20 mM KH2PO4, pH 6.5. Eluent B: 20 mM KH2PO4, 500 mM KCI, pH
6.5. Loading: 1
4 mg/mL resin. Gradient 0-100% eluent B in 60 CV. Flow rate: 120 cm/h.
Fig. 3 shows the chromatographic separation of PTH1-34 and PTH2-34 using a
cation exchange
6 column with a column volume of 29 mL. Eluent A: 20 mM Tris, 20 mM Bis-
Tris, pH 6.5. Eluent B:
7 20 mM Tris, 20 mM Bis-Tris, 4 mM KCI, pH 8.2. Loading: 4.5 mg/mL resin.
Step elution with 13.5
8 column volumes of 100% elution buffer. Resin: Fractogel S03- (S). Flow
rate: 150 cm/h.
9 Fig. 4 shows the chromatographic separation of PTH1-34 and PTH2-34 at
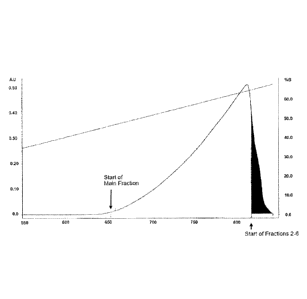
preparative scale using
a cation exchange column with a column volume of 26.3 L. Eluent A: 20 mM Tris,
20 mM
11 potassium dihydrogen phosphate, pH 6.5. Eluent B: 20 mM Tris, 16 mM
dipotassium hydrogen
12 phosphate, 4 mM potassium dihydrogen phosphate, pH 8.9. Loading: 3.5 g/L
resin. Resin:
13 Fractogel 503- (S). Flow rate: 100 cm/h.
14 Fig. 5 shows the results of the reversed-phase ultra high performance
liquid chromatography (RP-
uHPLC) analysis of fractions collected from the cation exchange chromatography
run depicted in
16 Figure 4.
17 DETAILED DESCRIPTION OF THE INVENTION
18 The present invention is based on the surprising insight that
teriparatide (also referred to herein
19 as PTH1-34) can be effectively purified in an ion exchange
chromatography process by increasing
the pH and simultaneously decreasing the ionic strength in the fluid used for
eluting the
21 polypeptide from the cation exchange material. The use of such inversely
directed gradients has
22 not been disclosed in the prior art. Instead, it was basic knowledge in
the field of ion exchange
23 chromatography that polypeptides, which have bound to an ion exchanger,
can be eluted by
24 increasing the ionic strength in the eluent, e.g., by increasing the
salt concentration in the eluent
(S. Yamamoto, K. Nakanishi, R. Matsuno (1988), "Ion-Exchange Chromatography of
Proteins",
26 Chromatographic Science Series, Vol. 43, Marcel Dekker, New York; C. T.
Mant et al. (2007),
27 "HPLC Analysis and Purification of Peptides", Methods in Molecular
Biology, Vol. 386, 3-55).
28 Decreasing the ionic strength in the eluent is not a usual step in ion
exchange chromatography.
29 It is therefore surprising that a dual gradient as applied in the method
of the present invention is
suitable for providing highly purified PTH1-34.
CPST Doc: 375089.2 3
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 In a first aspect, the present invention therefore relates to a method
for purifying PTH1-34 by ion
2 exchange chromatography, said method comprising the steps of:
3 (a) contacting a fluid containing PTH1-34 with a cation exchange
material under conditions
4 that allow for the reversible binding of PTH1-34 to said cation exchange
material;
(b) optionally washing the cation exchange material to remove unbound
material;
6 (c) eluting PTH1-34 by increasing the pH and decreasing the ionic
strength.
7 PTH1-34 and impurities derived thereof
8 The present method has been found to be effective for purifying
therapeutically active PTH1-34
9 which is present in a fluid from any undesired PTH1-34 variants and
fragments which might also
be present in said fluid. These fragments may include, for example, truncated
forms of PTH1-34
11 which can be regarded as product related impurities resulting from the
polypeptide production
12 process. For example, the most common way of producing PTH1-34 includes
heterologous
13 expression of the polypeptide in E. coil host cells. It was found that
heterologous expression of
14 PTH1-34 may give rise to N-terminally and C-terminally truncated forms
of PTH1-34 which are
unacceptable and have to be removed from the product for safety reasons.
16 In particular, it has been observed that PTH2-34, a truncated form of
PTH1-34 which lacks the N-
17 terminal serine residue, is regularly present after heterologous
expression. The overall amount of
18 the truncated PTH2-34 in the product solution obtained is up to 1%
(w/w). Other possible
19 truncated forms of PTH1-34 might lack the first 2 or 3 amino acids at
the N-terminus. These
variants are referred to herein as PTH3-34 and PTH4-34, respectively. In a
preferred embodiment
21 of the invention, the ion exchange chromatography method provided herein
is applied for the
22 purpose of removing one or more of the N-terminally truncated fragments
PTH2-34, PTH3-34 and
23 PTH4-34. In a particular preferred embodiment, the claimed method is
directed to the removal of
24 the truncated fragment PTH2-34.
Apart from N-terminally truncated fragments of PTH1-34, the fluid to be
subjected to the
26 purification method of the present invention may also contain C-
terminally truncated forms of
27 PTH1-34. For example, fragments which lack 1, 2, 3 or 4 of the amino
acids located at the C-
28 terminus in PTH1-34 may occur. Therefore, in another preferred
embodiment, the ion exchange
CPST Doc: 375089.2 4
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 chromatography method provided herein is applied for the purpose of
removing one or more of
2 the C-terminally truncated polypeptides PTH1-33, PTH1-32, PTH1-31 and
PTH1-30. In a
3 particularly preferred embodiment, the claimed method is applied for
removing PTH1-30.
4 In a still further embodiment of the present invention, the new method is
carried out for the purpose
of removing chemically modified variants of PTH1-34 comprising modifications
at one or more
6 amino acid side chains. These variants may arise as product related
impurities in the production
7 of PTH1-34. A number of variants of PTH1-34 have been identified which
differ from the original
8 polypeptide in that certain amino acid residues have been oxidized,
deaminated and/or modified
9 by succinimide formation. For example, a variant has been identified
which has a deaminated
asparagine residue in position 16 of PTH1-34. Another variant has oxidized
methionine residues
11 in position 8 and/or 18 of PTH1-34. Like with the truncated fragments,
it is required for safety
12 reasons to remove these variants from the final PTH1-34 product.
13 The PTH1-34 to be purified with the method of the invention preferably
comprises or consists of
14 the amino acid sequence set forth in SEQ ID NO:1. The polypeptide
depicted in SEQ ID NO:1
corresponds to the 34 N-terminal amino acids of the naturally occurring human
parathyroid
16 hormone. For comparison, the amino acid sequence of the full-length
human parathyroid hormone
17 is shown in SEQ ID NO:2. Figure la shows the distribution of the charged
amino acids within the
18 PTH1-34 sequence of SEQ ID NO:1. Under physiological conditions, the
PTH1-34 sequence of
19 SEQ ID NO:1 comprises two alpha-helical regions that are connected via a
loop region (see
Figure 1 b). No tertiary structure had been observed for the molecule under
physiological
21 conditions.
22 Also encompassed by the term "PTH1-34" are homologs of the sequence set
forth in SEQ ID
23 NO:1. As used herein, homologs of the PTH1-34 of SEQ ID NO:1 are
polypeptides which differ
24 from the polypeptide in SEQ ID NO:1 in a limited number of amino acids,
e.g. in 1, 2, 3, 4, 5, 6, 7,
8, 9, 10, 11, 12 or more amino acids. Preferably, the homolog differs in no
more than 15 amino
26 acid from the polypeptide depicted in SEQ ID NO:l. For example, the
polypeptide to be purified
27 by the method of the invention can be a polypeptide which differs from
PTH1-34 as shown in SEQ
28 ID NO:1 in that no more than 1, 2, 3,4, or 5 amino acids have been
substituted by other amino
29 acids. Where an amino acid substitution is made, the substitution
preferably is a conservative
amino acid substitution, i.e. a substitution of one amino acid by another
amino acid of similar
31 polarity which can act as a functional equivalent. Preferably, the amino
acid used as a substitute
CPST Doc: 375089.2 5
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 is selected from the same group as the amino acid residue to be
substituted. For example, a
2 hydrophobic residue can be substituted with another hydrophobic residue,
or a polar residue can
3 be substituted with another polar residue having the same charge.
Functionally homologous
4 amino acids which may be used for a conservative substitution comprise,
for example, non-polar
amino acids such as glycine, valine, alanine, isoleucine, leucine, methionine,
proline,
6 phenylalanine, and tryptophan. Examples of uncharged polar amino acids
comprise serine,
7 threonine, glutamine, asparagine, tyrosine and cysteine. Examples of
charged polar (basic) amino
8 acids comprise histidine, arginine and lysine. Examples of charged polar
(acidic) amino acids
9 comprise aspartic acid and glutamic acid.
It is particularly preferred that the PTH1-34 homolog which may comprise 1, 2,
3, 4, or 5 amino
11 acid substitutions compared with the PTH1-34 of SEQ ID NO:1 comprises no
chemical modifica-
12 tions, such as oxidations, deamidations, cyclizations, and the like. It
is furthermore preferred that
13 the homolog comprises or consists of 34 amino acids.
14 According to the invention, the PTH1-34 polypeptide which is to be
subjected to the method of
the invention can be derived from any suitable method which is known in the
art for producing
16 polypeptides. The polypeptide may have been prepared both by ribosomal
and non-ribosomal
17 methods. For example, the PTH1-34 may have been chemically synthesized
by solid phase or
18 liquid phase methods. Protocols for solution-phase chemical synthesis of
peptides have been
19 described (see, e.g., Andersson et al., Biopolymers 55:227-250, 2000).
For solid phase synthesis
the basic technique described by Merrifield (J. Am. Chem. Soc., 1964, 85, 2149-
2154) may have
21 been used. In that approach, the growing peptide is anchored on an
insoluble resin, and unreacted
22 soluble reagents are removed by filtration or washing steps without
manipulative losses. The solid
23 phase synthesis technique of Merrifield has been improved in the last
decade and nowadays
24 allows the synthesis of polypeptides with up to 50 amino acids. The
chemical synthesis of PTH1-
34 fragments was disclosed, e.g. in the US patents 4,427,827 and US 4,105,602.
26 Alternatively, the PTH1-34 polypeptide to be purified according to the
invention can be a
27 recombinantly produced PTH1-34, i.e. the polypeptide has been produced
by biotechnological
28 processes that involve genetically modified cells or organisms.
Typically, a nucleotide sequence
29 encoding the PTH1-34 polypeptide is cloned into a suitable vector which
provides for the express-
ion or overexpression of the polypeptide in a host cell. The vector is
introduced into a prokaryotic
31 or eukaryotic host cell, and the cell is cultured under conditions
suitable for the expression of the
CPST Doc: 375089.2 6
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 polypeptide. Normally, the polypeptide to be expressed will be under the
control of an inducible
2 promoter so that expression may be initiated by the addition of an
inducer, e.g. IPTG, to the
3 culture medium. After incubation of the cells to allow expression, the
cells are usually harvested
4 and homogenized to release the recombinant PTH1-34 polypeptide. Finally,
the polypeptide is
recovered by one or more purification steps. In accordance with the present
invention, it is
6 particularly preferred that the PTH1-34 polypeptide is a polypeptide that
has been recombinantly
7 produced by heterologous expression in host cells.
8 To simplify its subsequent enrichment and purification, the recombinant
PTH1-34 may be
9 prepared in form of a fusion polypeptide. As used herein, a fusion
polypeptide refers to a fusion
of the amino acid sequence of PTH1-34 to a second amino acid sequence. The
second amino
11 acid sequence may be, for example, an affinity tag, i.e. an amino acid
sequence which is either
12 N-terminally or C-terminally fused to the PTH1-34 polypeptide, and which
has a strong affinity to
13 another compound or material, thereby allowing enrichment and/or
purification of the fusion
14 polypeptide as a whole. The affinity tag may be, for example, a poly-
histidine sequence
comprising 6-12 histidines which specifically interact with a nickel ion
chelate matrix or alternative
16 IMAC resins. Alternatively, the affinity tag may be a glutathione-S-
transferase which allows the
17 purification via a glutathione matrix. Further affinity tags are well-
known in the art. An affinity tag
18 sequence can be removed from the PTH1-34 sequence after purification
with the affinity matrix,
19 for example, by providing a proteolytic cleavage site between PTH1-34
and the affinity tag.
The recombinant PTH1-34 may be expressed in high amounts in the bacterial host
cells so that
21 inclusion bodies are formed in which the recombinant polypeptides are
present in partially folded
22 form and aggregate through non-covalent hydrophobic or ionic
interactions. Where the method
23 for producing the recombinant PTH1-34 provides the polypeptide in
inclusion bodies, it will
24 preferably also include a step in which the polypeptides in the
inclusion bodies are solubilised and
refolded. Methods for solubilisation and refolding of polypeptides from
inclusion bodies are well
26 known in the art.
27 Chromatographic device and cation exchange material
28 The present method is preferably carried out by use of a FPLC (fast
protein liquid
29 chromatography) device, i.e. an automated device which comprises a
column which includes the
cation exchange material as the stationary phase, one or more pumps which
provide a pressure
31 that is sufficient for causing the mobile phase (i.e. the buffers used
for binding, washing and
CPST Doc: 375089.2 7
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 elution) to flow through the cation exchange material, an apparatus which
collects the fractions
2 eluted from the column, and a detector that is capable of detecting the
PTH1-34 which is eluted
3 from the column. Detection can be achieved by measuring the absorption at
a wavelength of e.g.
4 280 nm. In addition, the changes in pH and conductivity can also be
monitored in the fractions. A
considerable number of automated FPLC devices which are suitable for cation
exchange
6 chromatography are commercially available from different manufacturers.
Non-limiting examples
7 for such chromatographic devices include the AKTApurifier, AKTAavant,
AKTApilot,
8 AKTAexplorer or AKTAprocess systems (GE Healthcare, the K-Prime 40 systems
(Merck
9 Millipore), the NCG systems (Bio-Rad), and similar devices. The use of
these automated devices
is, however, not mandatory for carrying out the method of the present
invention.
11 The method of the invention can make use of any cation exchange material
that has been
12 described in the art as being useful for the purification of
polypeptides. As used herein, the cation
13 exchange material is an insoluble polymeric matrix which comprises on
its surface negatively
14 charged functional groups that are capable of attracting cations or
positively charged molecules.
The cation exchange material can be in the form of porous beads having, e.g.,
a size of between
16 10-100 pm, preferably 20-80 pm. A number of different cation exchange
materials are known in
17 the art, including cross-linked natural polymers, organic polymers and
inorganic materials. While
18 carboxymethyl groups are commonly used in weak cation exchangers, strong
cation exchangers
19 often include sulfopropyl groups. When performing the method of the
present invention, it is
preferred that the cation exchanger is present in the form of a packed column
suitable for use in
21 an automated FPLC device, such as the Akta Purifier 100 FPLC System.
22 In another preferred embodiment of the invention the cation exchange
material used in the
23 purification method is a strong cation exchanger which comprises
sulfoethyl, sulfopropyl,
24 sulfobutyl and/or sulfoisobutyl as functional groups. Cation exchanger
materials which comprise
a mixture of one or more of the above groups may likewise be used in the
method of the invention.
26 Further, the cation exchange material can be based on any common
polymeric material which
27 has been described for use in this particular type of chromatography.
For example, the polymeric
28 cation exchange material can comprise cross-linked
polystyroldivinylbenzol, polymethacrylate,
29 styrene-divinylbenzene, cross-linked Agarose, silica-based matrices,
polyvinyl ether, cellulose,
and dextran.
CPST Doc: 375089.2 8
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 Cation exchange materials or columns containing these materials can be
purchased from different
2 providers and include, e.g. the Unosphere S 70 resin (Biorad, Munich,
Germany), the Nuvia TM S
3 resin (Biorad, Munich, Germany), the Poros HS20 or HS50 resin (Life
Technologies, Darmstadt,
4 Germany), the Source 15S or 30S resin (GE Healthcare, Freiburg, Germany),
the Polysulphoethyl
A resin (PolyLC, Columbia, USA), the Ceramic HyperD F(S) resin (Pall,
Dreieich, Deutschland),
6 the BakerbondTM PolyCSX-35 Bioseparation Media (Avantor Performance
Materials, Center
7 Valley, USA), the Eshmuno S and CPX resins (Merck Millipore, Darmstadt,
Germany), the
8 Toyopearl resins SP-650M and GigaCap S-650M (Tosoh Bioscience LLC, King
of Prussia, USA)
9 and other cation exchange stationary phases regularly used for purifying
proteins.
The use of one of the following resins is particularly preferred in the method
of the invention: the
11 Eshmuno S resin, the Eshmuno CPX resin, the Poros HS20 resin, and the
Toyopearl Tm
12 GigaCapTM S-650M resin. A further particularly preferred cation exchange
resin material for use
13 in the method of the invention is the Fractogele resin developed by
Merck Millipore. The
14 Fractogele resin uses the so-called "tentacle technology" which means
that the surface of the
resin beads comprises long polymer chains which provide for an improved
accessibility of the
16 proteins to be purified to the functional groups of the resin material.
The method of the invention
17 is preferably carried out with the Fractogele resin EMD SE Hicap (M),
the Fractogele resin EMD
18 503- (S), or the Fractogele resin EMD 503- (M). Use of the Fractogele
resin EMD 503- (S) is
19 particularly preferred according to the invention.
The cation exchange columns used in the purification method disclosed herein
can be of any size.
21 Given that the method of the invention aims at the purification of PTH1-
34 from production
22 batches, the use of preparative columns which allow for the binding of
high amounts of PTH1-34
23 is particularly preferred. Preparative columns having a bed volume of 1-
100 L can be used,
24 whereas bed volumes of 10-50 L are particularly preferred, for example
45 L. For example, the
preparative columns used in the purification method of the present invention
can have a bed
26 volume of at least 1 L, at least 2 L, at least 3 L, at least 4 L, at
least 5 L, at least 10 L, at least 20
27 L, at least 25 L, or more. Usually, preparative columns useful in the
method of the invention will
28 have a diameter of between 1-100 cm, whereas a diameter of between 20-80
cm, or 40-60 cm is
29 particularly preferred.
Analytical columns having bed volumes of 1-5 mL may be used for further
optimizing the
31 conditions of the cation exchange purification method of the invention,
for example, to adjust the
CPST Doc: 375089.2 9
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 method to other polypeptides. It should, however, be noted that
conditions that were found highly
2 useful for an analytical column might not equally be transferable to the
conditions on a preparative
3 column (see Examples below).
4 Sample and column preparation
The method of the invention uses as a starting material a fluid that contains
PTH1-34. The fluid
6 can be the supernatant of a cell culture that was used for heterologous
expression of the
7 polypeptide. For example, it is possible to subject the supernatant
comprising the soluble PTH1-
8 34 polypeptides and other soluble components of the host cells directly
to the ion exchange
9 chromatography method of the invention, either with or without prior
dilution. It is, however,
preferred that before applying the purification method of the invention the
supernatant obtained
11 from cell culture is further processed, e.g. by centrifugation,
filtration, size-exclusion chromato-
12 graphy, affinity chromatography, RP-HPLC, and the like, to remove
undesired substances, e.g.
13 polynucleotides and/or polysaccharides, to cleave off an affinity tag or
another peptide or
14 polypeptide that is fused to PTH1-34, and/or to enrich the PTH1-34
polypeptide. The fluid to be
used in the method of the invention preferably comprises the PTH1-34
polypeptide in substantially
16 enriched form which means that at least 50% of the polypeptide compounds
dissolved in the fluid
17 is PTH1-34.
18 In one embodiment, the fluid used as a starting material in the method
of the invention is obtained
19 from a prior reversed phase chromatography run, i.e. the fluid is the
eluate of reversed phase
chromatography. This eluate will contain significant amounts of organic
solvents, such as
21 acetonitrile, and it might be necessary to dilute it with a suitable
buffer before applying it to the
22 cation exchange material. Conveniently, the buffer used for dilution can
be the same buffer that
23 was used for equilibrating the cation exchange material. The eluate from
the reversed phase
24 chromatography can be diluted by factor 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or
more. A dilution by factor 2-
4 has been proven particularly useful. Where dilution is not possible, e.g.,
due to an excessive
26 amount of acetonitrile in the fluid, a complete buffer exchange may be
performed, which means
27 that PTH1-34 is transferred from the reversed phase eluate to a buffer
that is adjusted to
28 subsequent ion exchange chromatography. A buffer exchange can be easily
accomplished, e.g.
29 by tangential flow filtration, size exclusion chromatography,
diafiltration or dialysis. It is preferred
that PTH1-34 is transferred to the buffer which is used during binding of the
polypeptide to the
31 cation exchange material (referred to in the following as "binding
buffer").
CPST Doc: 375089.2 10
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 Before application of the fluid containing the PTH1-34 polypeptide to the
cation exchange
2 material, the cation exchange material will usually be equilibrated by
washing the column with 1-
3 50, preferably 1-25, and more preferably 1-10 column volumes of an
equilibration buffer to provide
4 conditions that promote binding of the polypeptide to the cation
exchanger. Preferably the
equilibration buffer and the binding buffer are identical. After
equilibration, the fluid containing the
6 PTH1-34 polypeptide is contacted with the cation exchange material.
7 Contacting PTH1-34 with the cation exchange material
8 According to step (a) of the above method, the fluid containing PTH1-34
is contacted with the
9 cation exchange material under conditions that provide for the binding of
the polypeptide to the
polymeric matrix of the cation exchanger. This means that the conditions in
terms of temperature,
11 pressure and pH are such that PTH1-34 can displace the cations that have
bound to the cation
12 exchange material after equilibration. In particular, the binding of the
polypeptide to the cation
13 exchange material is facilitated by selection of an appropriate pH of
the binding buffer.
14 For identifying a suitable pH that allows binding of the PTH1-34 to a
particular cation exchange
material, the polypeptide can be contacted with the cation exchanger of choice
under different pH
16 conditions while detecting the amount of the polypeptide in the flow-
through. In this way, a suitable
17 pH range for binding of the polypeptide to the cation exchanger can be
readily determined. It is
18 preferred according to the invention that the pH is selected such that
the PTH1-34 is present in
19 the form of a positively charged polypeptide which supports its binding
to the cation exchanger.
To provide positively charged PTH1-34 polypeptide molecules the pH of the
buffer used in the
21 binding step should preferably be below the isoelectric point of the
polypeptide. The PTH1-34 set
22 forth in SEQ ID NO:1 has an isoelectric point of 8.3. Thus, where it is
intended to purify the PTH1-
23 34 of SEQ ID NO:1 with the method of the present invention, the pH in
the binding step should
24 be selected to be below 8.3.
Preferably, the binding buffer used for application of the PTH1-34 polypeptide
of SEQ ID NO:1 to
26 the cation exchange material will be adjusted to a pH of 8.0 or less. It
will be particularly preferred
27 that the pH of the binding buffer will be at least 0.5 pH units, more
preferably at least 1.0, at least
28 1.5, or at least 2.0 pH units below the isoelectric point of the
polypeptide to be purified. Where the
29 method of the invention is performed with the PTH1-34 set forth in SEQ
ID NO:1, binding of the
polypeptide to the cation exchange material is preferably performed in binding
buffer having a pH
31 below 7.8, below 7.3, below 6.8, and more preferably below 6.3, or even
below 6Ø For example,
CPST Doc: 375089.2 11
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 the PTH1-34 of SEQ ID NO:1 is bound to the cation exchange material using
binding buffer at a
2 pH of between 6.0 and 8.0, preferably at a pH of between 6.5 and 7.5, and
more preferably at a
3 .. pH of between 6.8 and 7.2. In cases where a homolog of PTH1-34 is used
which differs from the
4 PTH1-34 in SEQ ID NO:1 by 1, 2 or 3 amino acid substitutions, it may be
possible that the
.. isoelectric point of said modified polypeptide is slightly different. The
skilled person will be readily
6 able to determine the isoelectric point of the homolog by use of routine
methods well known in
7 the art.
8 The present invention is amongst others based on the insight that
chromatographic separation of
9 .. PTH1-34 from its fragments and variants can be improved by eluting PTH1-
34 from the cation
exchange material by increasing the pH and simultaneously lowering ionic
strength in the mobile
11 phase. This can be conveniently be accomplished by using an eluent
which, compared to the
12 binding or washing buffer, has a higher pH and a lower conductivity.
Thus, the binding buffer used
13 .. for binding PTH1-34 to the cation exchange resin in the method of the
invention will exhibit an
14 .. ionic strength which is higher than that of the eluent. This is unusual
for cation exchange
chromatography, because the ionic strength in the eluent is normally gradually
increased to
16 support displacement of polypeptides from a cation exchanger. The
conductivity (as expressed in
17 .. mS/cm) of the buffers used in the ion exchange chromatography process is
a suitable measure
18 that reflects ionic strength of said buffer. Accordingly, the terms
"conductivity" and "ionic strength"
19 .. are used interchangeably herein.
.. It is preferred that the binding buffer has a conductivity that is at least
30% higher, at least 50%
21 higher, at least 75% higher, at least 100% higher, at least 150% higher,
at least 200% higher, or
22 .. at least 300% higher than the conductivity of the corresponding eluent.
The conductivity of the
23 binding buffer will preferably be in the range 1.5-3.0 mS/cm, for
example, more than 1.5 mS/cm,
24 more than 1.6 mS/cm, more than 1.7 mS/cm, more than 1.8 mS/cm, more than
1.9 mS/cm, more
than 2.0 mS/cm, more than 2.1 mS/cm, more than 2.2 mS/cm, more than 2.3 mS/cm,
more than
26 .. 2.4 mS/cm, more than 2.5 mS/cm, more than 2.6 mS/cm, more than 2.7
mS/cm, more than 2.8
27 mS/cm, or more than 2.9 mS/cm. Preferably, the conductivity of the
binding buffer will be at least
28 0.5 mS/cm higher than the conductivity of the corresponding eluent, more
preferably at least 0.6
29 mS/cm, at least 0.7 mS/cm, at least 0.8 mS/cm, at least 0.9 mS/cm, at
least 1.0 mS/cm, at least
1.1 mS/cm, at least 1.2 mS/cm, at least 1.3 mS/cm, at least 1.4 mS/cm, at
least 1.5 mS/cm, at
31 .. least 1.6 mS/cm, at least 1.7 mS/cm, at least 1.8 mS/cm, at least 1.9
mS/cm, or at least 2.0
32 mS/cm.
CPST Doc: 375089.2 12
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 In a preferred aspect, the conductivity of the binding buffer is in the
range between 2.0 and 3.0
2 mS/cm, and the conductivity of the eluent is in the range between 0.5 and
1.5 mS/cm. In an even
3 more preferred aspect, the conductivity of the binding buffer is in the
range between 2.5 and 2.8
4 mS/cm, and the conductivity of the eluent is in the range between 1.0 and
1.3 mS/cm.
Several buffers can be used in the method of the present invention. It is
preferred according to
6 the invention that the binding buffer and the elution buffer are prepared
with the same buffer
7 substance. The buffer compound used for preparing the binding buffer and
the elution buffer is
8 selected such that its pH range covers the isoelectric point of the PTH1-
34 polypeptide, i.e. the
9 pK, value of the buffer substance is not more than one pH unit higher or
lower than the isolelectric
point of the PTH1-34 polypeptide. Where the PTH1-34 polypeptide to be purified
is the
11 polypeptide of SEQ ID NO:1, the buffer will be selected such that its pH
range covers pH 8.3, i.e.
12 the buffering compound has a pK, of between 7.3 and 9.3. A pH range in
the area of the isoelectric
13 point of the PTH1-34 has the particular advantage that the preparation
of the binding buffer, which
14 preferably has a pH of below 7.5, and more preferably a pH around 6.5,
will require considerable
amounts of acid, such as HCI, for pH adjustment. The addition of HCI or other
acids to the binding
16 buffer will increase the ionic strength of the binding buffer relative
to the elution buffer, the latter
17 of which has a higher pH and therefore requires only slight or no pH
adjustment.
18 Suitable buffer substances having a pK, in the range of 8.3 include
19 Tris(hydroxymethyl)aminomethane (referred to as "Tris" herein),
triethanolamine, and borate
buffer. Additional buffers that have been found to be suitable for carrying
out the method of the
21 invention include a barbital buffer having a pH in the range of, e.g.,
6.8-9.2, and a glycylglycine
22 buffer having a pH in the range of, e.g., 7.3-9.3. The use of Tris
buffer is preferred herein, because
23 it was unexpectedly found to result in a particularly good separation of
PTH1-34. The use of Tris
24 buffer was not an obvious measure in the method of the invention,
because Tris is commonly
recommended in the literature for use in anion exchange chromatography. Tris
is a cationic buffer
26 which is in principle able to bind to the functional groups of the
cation exchange resin, thereby
27 disturbing reproducible separation. In the method of the present
invention, however, it was
28 observed that the use of Tris does not negatively influence the
purification of PTH1-34 via cation
29 exchange chromatography.
The buffering compound will be present in the binding buffer in an amount of 1-
200 mM, preferably
31 10-100 mM, and more preferably 20-80 mM. Where Tris is used in the
binding buffer, the preferred
CPST Doc: 375089.2 13
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 concentration will be in range of 20-40 mM, wherein 20 mM is particularly
preferred. Additional
2 salts, such as NaCI or KH2PO4, may be used as additives in amounts of 1-
100 mM, preferably
3 20-80 mM, to adjust the conductivity of the buffer where necessary. Other
buffering compounds,
4 such as Bis-Tris may also be added to the Tris buffer. Non-limiting
examples for a Tris-based
binding buffer which is suitable to be used in the purification of the PTH1-34
polypeptide of SEQ
6 ID NO:1 include:
7 20 mM Tris, pH 6.5-7.5
8 30 mM Tris, pH 6.5-7.5
9 40 mM Tris, pH 6.5-7.5
20 mM Tris, 20 mM KH2PO4, pH 6.5-7.5
11 30 mM Tris, 30 mM KH2PO4, pH 6.5-7.5
12 40 mM Tris, 40 mM KH2PO4, pH 6.5-7.5
13 20 mM Tris, 20 mM Bis-Tris, pH 6.5-7.5
14 30 mM Tris, 30 mM Bis-Tris, pH 6.5-7.5
40 mM Tris, 40 mM Bis-Tris, pH 6.5-7.5
16 Based on his experience and the information and the particular examples
provided herein, the
17 skilled person will be readily able to prepare additional binding
buffers that are suitable for being
18 used in the purification process of the present invention.
19 The contacting of the PTH1-34 polypeptide with the cation exchange
material can be performed
in different ways, depending on the particular equipment which is used for
carrying out the method
21 of the invention. Where an FPLC device is used for the purification
process of the invention, the
22 PTH1-34 polypeptide can be contacted with the cation exchange material
conveniently by
23 injecting the PTH1-34 containing fluid (either diluted with binding
buffer or not) into an injection
24 loop which is then introduced into the flow of the binding buffer. The
flow rate during binding will
be in the range of 50-400 cm/h, preferably 100-300 cm/h, more preferably 150-
200 cm/h.
26 When using preparative columns having a having a bed volume size of 1-50
L, the PTH1-34 will
27 be applied to the column such that the overall protein load is in the
range of 2.0-4.5 g/L. In
28 approaches using columns having a bed volume size of 10-250 mL, the
overall protein load is in
29 the range of 10-20 mg/ mL. When using analytical columns having a bed
volume size of less than
CPST Doc: 375089.2 14
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 10 mL, e.g., 5 mL or 1 mL, the overall protein load will normally be in
the range of 0.1-5 mg/ mL,
2 e.g. about 1 mg/ mL.
3 Washing the cation exchange resin
4 After protein binding the cation exchange material is optionally washed
to remove unbound
material. For this purpose, the cation exchange material is preferably washed
with several column
6 volumes of washing buffer. According to the invention, the washing buffer
may be identical to the
7 binding buffer in terms of its pH or ionic strength. The washing buffer
may contain additional
8 compounds not included in the binding buffer. In any case, however, the
pH and ionic strength of
9 the washing buffer should be adjusted to avoid any premature elution of
the PTH1-34 polypeptide
from the cation exchange material.
11 Depending on the size of the column used in the method of the invention,
it will be useful to wash
12 the column after polypeptide binding with 1-25 column volumes,
preferably 1-15 column volumes,
13 and more preferably 1-10 column volumes. In an even more preferable
embodiment of the
14 invention, the cation exchange resin is washed after PTH1-34 binding
until the detected UV
absorption, pH and conductivity signals remain constant.
16 Eluting the PTH1-34 polvpeptide
17 After PTH1-34 binding to the cation exchange material and (where
applicable) washing said
18 material, the PTH1-34 polypeptide is eluted. According to the invention,
elution is effected by
19 increasing the pH and decreasing the ionic strength. Compared with the
binding buffer, the eluent
will thus have a higher pH which is close to the isoelectric point of the PTH1-
34 polypeptide. By
21 increasing the pH, the overall positive charge of the PTH1-34
polypeptide is reduced and the
22 polypeptide will ultimately be released from the cation exchange
material. Preferably, the eluent
23 is an elution buffer, i.e. it comprises at least one buffering compound.
24 The increase in pH can be accomplished in different ways. For example, a
gradient elution can
be conducted in which the eluent is gradually mixed with the binding or
washing buffer. In this
26 way, the pH of the mobile phase flowing over the cation exchange
material gradually increases
27 so that different polypeptides, which have bound to the cation exchange
material, are released in
28 accordance with their interaction strength. The gradient used can be a
convex, concave or linear
29 gradient, but linear gradients are particularly preferred. The steepness
of the gradient can be
varied as needed. Normally, linear gradients with a steepness of 1-5% elution
buffer per column
CPST Doc: 375089.2 15
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 volume are useful for carrying out the method of the invention.
Alternatively, an isocratic step
2 elution can be performed by switching from binding or wash buffer
directly to 100% eluent without
3 any gradient. In an isocratic step elution, the composition of the eluent
remains constant during
4 elution, because the eluent is not gradually mixed with binding or
washing buffer in the course of
elution.
6 The eluent used for releasing the PTH1-34 polypeptide from the cation
exchange material will be
7 adjusted to a pH of 8.0 or higher. It will be preferred that the pH of
the eluent will be 0.1 pH units,
8 0.2 pH units, or 0.3 pH units higher or lower than the isoelectric point
of the PTH1-34 polypeptide
9 to be purified. Where the method of the invention is performed with the
PTH1-34 of SEQ ID NO:1,
the eluent preferably has a pH of at least 8.0 or more, at least 8.1 or more,
at least 8.2 or more,
11 at least 8.3 or more, at least 8.4 or more, at least 8.5 or more, or at
least 9.0 or more. In cases
12 where a homolog of PTH1-34 is used which differs from the PTH1-34 in SEQ
ID NO:1 by 1, 2 or
13 3 amino acid substitutions, it may be possible that the isoelectric
point of said modified polypeptide
14 is slightly different. Therefore, the optimum pH to be used during the
elution step might slightly
vary from that determined for the PTH1-34 of SEQ ID NO:1.
16 As stated above in the context with the binding buffer, the eluent used
for elution of PTH1-34 will
17 have an ionic strength which is significantly lower than that of the
binding and/or washing buffer.
18 Preferably, the eluent will have a conductivity in the range 0.1-1.4
mS/cm, for example, less than
19 1.4 mS/cm, less than 1.3 mS/cm, less than 1.2 mS/cm, less than 1.1
mS/cm, less than 1.0 mS/cm,
less than 0.9 mS/cm, less than 0.8 mS/cm, less than 0.7 mS/cm, less than 0.6
mS/cm, less than
21 0.5 mS/cm, less than 0.4 mS/cm, less than 0.3 mS/cm, or less than 0.2
mS/cm. In another aspect,
22 the conductivity of the eluent is in the range between 0.2 and 1.4
mS/cm, preferably between 0.5
23 and 1.3 mS/cm.
24 The buffer used for preparing the eluent will preferably be the same as
the one used for binding
and/or washing buffer, wherein Tris, triethanolamine, and borate buffer are
preferred for use. The
26 use of Tris buffer is particularly preferred.
27 The buffering substance will be present in the eluent in an amount of 1-
200 mM, preferably 10-
28 100 mM, and more preferably 20-80 mM. Where Tris is used in the eluent,
the preferred
29 concentration will be in range of 20-40 mM. An amount of 20 mM Tris in
the eluent is particularly
preferred. Additional salts, such as NaCI or KH2PO4, may be used as additives
in amounts of 1-
CPST Doc: 375089.2 16
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 100 mM, preferably 20-80 mM. Other buffering compounds, such as Bis-Tris
may also be added
2 to the Tris buffer. Non-limiting examples for a Tris-based eluent which
is suitable to be used in
3 the purification of the PTH1-34 polypeptide of SEQ ID NO:1 include:
4 20 mM Tris, pH 9.0
30 mM Tris, pH 9.0
6 40 mM Tris, pH 9.0
7 20 mM Tris, 3 mM KH2PO4, 13 mM K2HPO4, pH 9.0
8 30 mM Tris, 4 mM KH2PO4, 14 mM K2HPO4, pH 9.0
9 40 mM Tris, 5 mM KH2PO4, 15 mM K2HPO4, pH 9.0
20 mM Tris, 20 mM Bis-Tris, 5 mM KCI, pH 8.2
11 20 mM Tris, 20 mM Bis-Tris, 5 mM KCI, pH 8.3
12 20 mM Tris, 20 mM Bis-Tris, 5 mM KCI, pH 9.0
13 20 mM Tris, 20 mM Bis-Tris, 5 mM KCI, pH 10.0
14 The elution will be performed by applying 10-50 column volumes to the
cation exchange material.
If a linear gradient from 0-100% eluent is used, the gradient may be applied
in 10-50 column
16 volumes. Gradient volumes of 10-20 column volumes are expected to
provide the best
17 compromise between peak dilution and resolution.
18 Preferred modes for carrying out the purification
19 The purification method of the invention is able to separate
therapeutically active PTH1-34 from
chemically modified by-products and fragments. It is preferred that the eluate
obtained in step (c)
21 contains less than 5% impurities, i.e. proteinaceous compounds which are
not unmodified PTH1-
22 34. Specifically, it is preferred that the eluate obtained in step (c)
contains less than 5% (w/w) of
23 the PTH2-34 fragment and/or less than 5% deamidated PTH1-34. Even more
preferably, the
24 eluate obtained in step (c) contains less than 0.5% (w/w) of the PTH2-34
fragment and/or less
than 0.5% deamidated PTH1-34. In a most preferred embodiment of the method,
the eluate
26 obtained in step (c) contains no detectable PTH2-34.
27 According to a particularly preferred embodiment of the invention, the
PTH1-34 to be purified is
28 the polypeptide depicted in SEQ ID NO:1, and the purification method is
performed with a buffer
29 system comprising eluent A: 20 mM Tris (with or without 20mM Bis-Tris),
pH 6.5-7.0, and eluent
CPST Doc: 375089.2 17
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 B: 20 mM Tris (with or without 20mM Bis-Tris), pH 8.0-10Ø It is
moreover preferred to initiate
2 elution by performing a step elution with 75-100% eluent B.
3 According to the invention, it is possible to add further compounds to
eluent A or B that may prove
4 advantageous for a number of embodiments of the invention. For example,
the elution buffer may
comprise 250 mM sucrose, Tween 20 (1%), urea (1-2 M) and/or arginine (50-100
mM).
6 Preparation of the PTH1-34 polypeptide
7 The purification method described above can be part of a process for the
preparation of PTH1-
8 34. This means that the purification method of the present invention can
be implemented into a
9 .. manufacturing process which includes the heterologous expression of the
polypeptide and
several downstream processing steps which are directed to the refolding and
purification of the
11 .. polypeptide.
12 .. Thus, in another aspect, the present invention also relates to a method
for preparing PTH1-34, in
13 .. particular the PTH1-34 of SEQ ID NO:1, comprising the steps of
14 (a) recombinantly expressing PTH1-34 in a host cell;
(b) optionally disrupting the host cells to release the recombinantly
expressed PTH1-34;
16 (c) purifying the PTH1-34 by a purification method as described in
more detail above.
17 .. In step (a) of the above manufacturing method, the PTH1-34, or a homolog
thereof as described
18 elsewhere herein, is recombinantly expressed in a host cell, such as a
prokaryotic or eukaryotic
19 host cell. In a preferred embodiment of the manufacturing method of the
invention, the PTH1-34
is expressed in bacterial host cells, e.g. in bacteria of the genus
Escherichia. The manufacturing
21 .. method may use, for example, common bacterial strains of E. coli for
expression of high amounts
22 .. of the PTH1-34 polypeptide.
23 After expression of the polypeptide in the cells, it may be necessary to
disrupt the cells to release
24 the recombinant polypeptide from the cytoplasm. Alternatively, where the
polypeptide is secreted
.. by the host strain in the supernatant, e.g. as a result of the use of
appropriate signal peptides, it
26 will not be necessary to disrupt the cells. Instead, the recombinant
polypeptide can be directly
27 obtained from the culture supernatant. The recombinant polypeptide can
be further processed,
28 e.g. by removal of the signal peptide.
CPST Doc: 375089.2 18
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 In step (c) of the above manufacturing method, the PTH1-34 is purified by
employing the cation
2 exchange chromatography method referred to above.
3 Preferably, the method for preparing PTH1-34 also comprises one or more
additional
4 chromatography steps, such as reversed phase chromatography, which are
performed prior to or
subsequent to step (c).
CPST Doc: 375089.2 19
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 EXAMPLES
2 Example 1: Purification of PTH1-34 on an analytical column
3 The conditions for separating PTH1-34 from the N-terminally truncated
PTH2-34 were analyzed
4 on an analytic Fractogel S03- (S) cation exchange column with a volume of
1 mL (diameter 0.8
mm, height: 20 mm). Eluate from a reversed phase chromatography containing
approximately 0.6
6 mg/ mL PTH1-34 was applied to the column at a load of approximately 1 mg/
mL of the resin.
7 Binding of the polypeptides to the column was performed in Eluent A (also
used as binding buffer)
8 consisting of 20 mM KH2PO4, pH 6.5. Elution was performed by use of
Eluent B consisting of 20
9 mM KH2PO4, 500 mM KCI, pH 6.5. The eluent was applied in a linear
gradient of 0-100% eluent
in 60 column volumes. The flow rate was 120 cm/h. Fractions of 1 mL were
collected. For each
11 of the collected samples, the extinction at 220 nm was measured.
12 The result of the chromatography is depicted in Figure 2. It can be seen
that PTH1-34 and PTH2-
13 34 co-eluted in the same peak. It was evidently not possible under the
conditions applied to
14 separate PTH1-34 and PTH2-34 to a sufficient extent. There was no
binding selectivity of either
PTH1-34 or PTH2-34 to the Fractogel S03- (S) cation exchange column.
Accordingly, efforts were
16 made to provide an improved method for purifying PTH1-34 and obtaining
increased yields.
17 Example 2: Purification of PTH1-34 on a 29 mL column
18 To define robust and reliable scale up conditions, a number of different
conditions were tested
19 using a Fractogel S03- (S) cation exchange column with a volume of 29
mL. Improvements were
observed when using an increasing pH instead of an increasing salt
concentration for elution. To
21 provide for a low ionic strength during elution, the buffers were
prepared such that eluent A (which
22 corresponds to the binding buffer) has a high ionic strength and eluent
B has a significantly lower
23 ionic strength. Tris buffer was found to be particularly useful for this
approach. The following
24 conditions were tested:
CPST Doc: 375089.2 20
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
Gradient Loading Step Yield
ro [nigicm2 ro]
Process run Buffer Conditions BICV]
and nig/rnL
Resin)
Fluent A 20 mM Tris 20 mM
Bis-Tris pH 65
2;7
Cond; 2.7 matm
P225
lEluent B: 20 mriA Tris 20 mM (40% wash; 40- 60/6 76
100% Bis Tris 5 mM KCI pH 10.0 elution)
Gond_ 0.8 mS/cm
lEluent A 20 rail Tris 20 mM
Bis-Tris pH 6;5
2_5
Gond_ 2.7 mS/cm
P226 65 /4_5 73
lEluent B: 20 mM Iris 20 mM (45% wash; 45-
100% Bis Tris 5 mM KCI pH 10.0 elution)
Cond_ 0.8 mS/cm
lEluent A 20 mM Tris 20 mM
Bis-Tris pH 65
P242
Cond_ 2.7 mS/cm (0% wash 75%
65 /4_5 92
lEluent B; 20 rail Tris 20 mM elution)
Bis Tris 5 mM KCI pH 10.0
Cond. 0.8 mS/cm
lEluent A 20 rail Tris 20 mM
Bis-Tris pH 6;5
'Gond_ 2.7 mS/cm (0% wash; 100%
P243 65 /4;5 88
lEluent B: 20 rail Tris 20 mM elution)
Bis Tris 4 mM KCI pH 8_3
Cond. 1.3 mS/cm
lEluent A 20 rail Tris 20 mM
Bis-Tris pH 6;5
P250 _ Gond 2.7 mS/cm (0% wash 100%
Fluent B: 20 mM Tris 20 mM elution)
Bis Iris 4 mM KCI pH 82
Cond. 1.4 mS/cm
lEluent A 20 rail Tris 20 mM
Bis-Tris pH 6;5
Cond. 2.7 mS/cm (0% wash 100%
P251 65 /4;5 75
Fluent B: 20 rail Tris 20 mM elution)
Bis Tris 4 mM KCI pH 8.1
Cond. 1.3 mS/cm
1
2 Eluate from a reversed phase RP chromatography containing approximately
0.6 mg/mL PTH1 -34
3 was diluted by factor 2 to reduce the acetonitrile content. The RP el
uate was applied to the column
4 at loads of 4.5-6 mg/mL resin. The column was washed with 3 column
volumes binding buffer
5 (eluent A). Elution from the resin was effected either with isocratic
elution or gradient elution.
CPST Doc: 375089.2 21
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 Fractions of 10 mL were collected. For each of the collected samples, the
extinction at 220 nm
2 was measured.
3 As can be seen from the above table, PTH1-34 yield was significantly
increased by elution using
4 a pH increase/ionic strength decrease. Figure 3 exemplarily shows the
result of process run P250.
It can be seen that the modified elution profile has the effect that PTH2-34
is eluted as a separate
6 peak which allows for its separation from the PTH1-34 product.
7 Example 3: Purification of PTH1-34 on a 26.3 L column
8 The method of the invention was tested at preparative scale using a
Fractogel S03- (S) cation
9 exchange column having a volume of 26.3 L (column diameter: 450 mm,
column height 166 mm).
The PTH1-34 preparation used in this chromatographic step was recombinantly
expressed PTH1-
11 34 that had previously been subjected to reversed-phase (RP)
chromatography. The PTH1-34
12 preparation obtained from the RP chromatography step contained
approximately 1.2 % PTH2-34.
13 To reduce the acetonitrile concentration, the main fraction obtained
from RP chromatography was
14 diluted 1:2 with Eluent A (20 mM Tris, 20 mM potassium dihydrogen
phosphate, pH 6.5,
conductivity about 4.1 mS/cm) and loaded onto the column. The column was
washed with an
16 initial gradient ranging from 0-35% Eluent B (20 mM Tris, 16 mM
dipotassium hydrogen
17 phosphate, 4 mM potassium dihydrogen phosphate, pH 8.9, conductivity
about 3.5 mS/cm) in one
18 column volume and, in a subsequent step, at 35% Eluent B for 10 column
volumes. The flow rate
19 was adjusted to 100 cm/h. The column was loaded with 3.5 g/L of the
polypeptide. Elution was
performed with a gradient from 35-100% Eluent B in 22 column volumes.
21 The PTH1-34 product was found to elute in the slowly ascending peak
shown in Figure 4, while
22 PTH2-34 eluted in the steeply descending peak area (i.e., the area shown
in black in Figure 4).
23 Fractions of the peak were collected for subsequent analysis by ultra
high performance liquid
24 chromatography (RP-uH PLC). A first fraction ("fraction 1" comprising
100.7 L) was collected until
start of the main fraction. Following the further processed main fraction,
further fractions were
26 collected starting from peak maximum. These fractions ("fractions 2-6"),
which comprised different
27 volumes (fraction 2: 1.4 L, fraction 3: 3.7 L, fraction 4: 8.1 L,
fraction 5: 3.6 L and fraction 6: 9.7
28 L), were collected from the descending peak area.
CPST Doc: 375089.2 22
Date recue / Date received 2021-11-09
CA 2,908,295
Blakes Ref.: 12770/00001
1 The results of the RP-uHPLC are shown in Figure 5. It can be seen there
that the initial PTH1-34
2 preparation obtained from RP chromatography contained about 1.2% PTH2-34,
while fraction 1
3 collected after cation exchange chromatography contained only about 0.1%
of the truncated
4 polypeptide. Almost the complete PTH2-34 eluted in fractions 3-6, i.e. in
the descending peak
area. A total of 62.9 g PTH1-34 was loaded to the cation exchange column. The
chromatographic
6 process yielded 54.0 g PTH1-34, which represents a recovery of 86%.
7 From these results, it can therefore be concluded that the method of the
present invention is highly
8 effective for separating PTH1-34 from PTH2-34 at preparative scale
without any significant loss
9 of PTH1-34. The method is therefore suitable for being used in the large-
scale production of
PTH1-34.
CPST Doc: 375089.2 23
Date recue / Date received 2021-11-09