Note: Descriptions are shown in the official language in which they were submitted.
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
DIESTER-BASED BASE OIL BLENDS WITH IMPROVED COLD FLOW
PROPERTIES AND LOW NOACK
FIELD OF THE INVENTION
The present invention relates to base oil or base oil blends comprising a
diester with
suitable properties to improve the cold flow properties and lower the
volatility of a starting
base oil or starting base oil blend. The present invention relates to a
process for improving a
starting base oil or starting base oil blend by replacing portions of a
starting base oil or
starting base oil blend with a diester with suitable properties to improve the
cold flow
properties and lower the volatility relative to the starting base oil or
starting base oil blend.
BACKGROUND OF THE INVENTION
Esters have been used as lubricating oils for over 50 years. They are used in
a variety
of applications ranging from jet engines, refrigeration and motor oils. In
fact, esters were the
first synthetic crankcase motor oils in automotive applications. However,
esters gave way to
polyalphaolefins (PAOs) due to the lower cost of PAOs and their formulation
similarities to
mineral oils. In full synthetic motor oils, however, esters are almost always
used in
combination with PAOs to balance the effect on seals, additives solubility,
volatility
reduction, and energy efficiency improvement by enhanced lubricity. In this
aspect, novel
diester-based multi-grade engine oil compositions comprising PAOs have been
described in
commonly-assigned United States Patent Application Serial No. 12/548,191;
filed August 26,
2009.
Ester-based lubricants, in general, have excellent lubrication properties due
to the
polarity of the ester molecules of which they are comprised. The polar ester
groups of such
molecules adhere to positively-charged metal surfaces creating protective
films which slow
down the wear and tear of the metal surfaces. Such lubricants are less
volatile than the
traditional lubricants and tend to have much higher flash points and much
lower vapor
pressures. Ester lubricants are excellent solvents and dispersants, and can
readily solvate and
disperse the degradation by-products of oils. Therefore, they greatly reduce
sludge buildup.
While ester lubricants are stable to thermal and oxidative processes, the
ester functionalities
give microbes a handle to do their biodegrading more efficiently and more
effectively than
their mineral oil-based analogues. Therefore, there exists an opportunity to
employ an
alternative blending component that reduces volatility at a reduced cost and
with other
advantages not afforded with PAO.
1
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
In view of the foregoing, a simpler, more efficient method of generating
diester-based
multi-grade engine oils would be extremely useful, particularly wherein such
methods utilize
renewable raw materials in combination with converting low value Fischer-
Tropsch (FT)
olefins and alcohols to high value diester base oils.
Novel diester-based lubricant compositions and their corresponding syntheses
have
been described in commonly-assigned United States Patent No. US 7,871,967 B2;
issued
January 18, 2011. The synthetic routes described in this patent application
comprise and/or
generally proceed through the following sequence of reaction steps: (1)
epoxidation of an
olefin to form an epoxide; (2) conversion of the epoxide to form a diol; and
(3) esterification
of the diol to form a diester.
Moreover, novel diester-based lubricant compositions and their corresponding
syntheses have been described in commonly-assigned United States Patent No. US
7,867,959
B2; issued January 11, 2011. The synthetic routes described in this patent
application
comprise and/or generally proceed through the following sequence of reaction
steps: (1)
epoxidation of an olefin to form an epoxide; (2) directly esterifying the
epoxide with a
carboxylic acid to form a diester species.
The specifications for lubricating base oils and engine oils are calling for
products
with excellent low temperature properties, high oxidation stability, and low
volatility.
Currently, only a small fraction of the base oils manufactured today are able
to meet these
demanding specifications.
Accordingly, there is need for base oils and base oil blends, which have
improved low
volatility, excellent cold flow properties and improved fuel economy to meet
today's
stringent performance requirements.
SUMMARY OF THE INVENTION
In one embodiment, the present invention is directed to a base oil blend
comprising:
a) a diester component, comprising a quantity of at least one diester species
of Formula I:
2
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
0
R3 0
R2
R1
0 Rzt
0 I
wherein R1, R2, R3 and R4 are the same or independently selected from C2 to
C17
hydrocarbon groups; b) a second base oil, and wherein the second base oil is a
Group I base
oil, Group II base oil or Group III base oil.
In one embodiment, the present invention is directed to a process for
improving cold
flow properties and lowering Noack in a starting base oil or starting base oil
blend,
comprising: replacing between about 5 to 60 wt % of a starting base oil or
starting base oil
blend with an equal quantity of a base oil comprising: a) a diester component,
comprising a
quantity of at least one diester species of Formula I:
0
R3 0
............,\,................... R2
R1
O.... R
0 I
wherein R1, R2, R3 and R4 are the same or independently selected from C2 to
C17
hydrocarbon groups, and wherein the process affords an upgraded base oil
blend.
BRIEF DESCRIPTION OF THE DRAWINGS
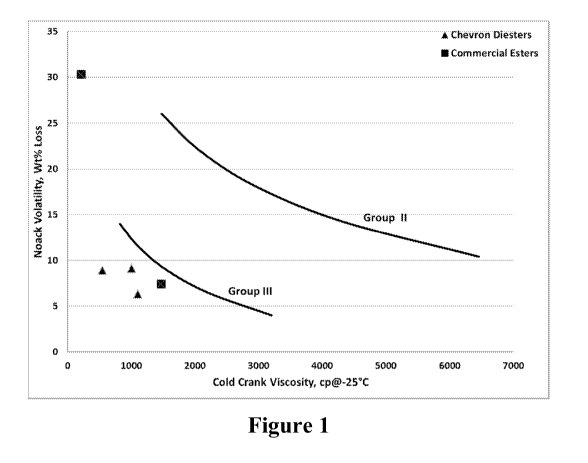
Figure 1 is a chart illustrates the Noack and CCS Viscosity of the diesters of
the
present invention as compared to the current commercial esters as presented in
Table 6 and
other Group II and III bases oils.
3
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
DETAILED DESCRIPTION OF THE INVENTION
In some embodiments, the present invention is directed to a base oil blend,
comprising: a) a diester component, comprising a quantity of at least one
diester species of
Formula I, wherein R1, R2, R3 and R4 are the same or independently selected
from C2 to C17
hydrocarbon groups; b) a second base oil; and wherein the second base oil is a
Group I base
oil, Group II base oil or Group III base oil.
In some embodiments, the present invention is directed to a base oil blend,
wherein
the diester component comprises a mixture of isomers where R1 and R2 are
different for each
isomer.
In some embodiments, the present invention is directed to a base oil blend,
further
comprising, a third base oil.
In some embodiments, the present invention is directed to a base oil blend,
wherein
the diester component has a Noack Volatility between about 6 and 10 wt%, a CCS
Viscosity
at ¨30 C between about 700 and 2000 cP, a pour point less than about -10 C, a
cloud point
less than about -10 C, a kinematic viscosity at 100 C between about 2.5 to 6.5
centistokes,
and a VI greater than about 110 and a BN Oxidator greater than about 20 hours.
In some embodiments, the present invention is directed to a base oil blend,
wherein
the diester component has a Noack Volatility between about 6 and 9 wt%, and a
CCS
Viscosity at ¨30 C between about 800 and 1900 cP.
In some embodiments, the present invention is directed to a base oil blend,
wherein
the diester component has a Noack Volatility between about 6 and 9 wt%, and a
CCS
Viscosity at ¨25 C between about 400 and 1250 cP.
In some embodiments, the present invention is directed to a base oil blend,
wherein
the second base oil is light neutral base oil, medium neutral base oil, Yubase
4, Yubase 6,
150R, 600R, 11ORLV, 220R or 100R.
In some embodiments, the present invention is directed to a base oil blend,
wherein
the third base oil is light neutral base oil, medium neutral base oil, Yubase
4, Yubase 6, 150R,
600R, 11ORLV, 220R or 100R.
In some embodiments, the present invention is directed to a base oil blend,
having: a)
a viscosity index between about 110 to 140; b) a Noack volatility of less than
about 16 wt %;
c) a Pour Point less than about -12 C; and d) a Cloud Point less than about -
12 C.
In some embodiments, the present invention is directed to a base oil blend,
wherein
the kinematic viscosity at a temperature of 100 C is between about 3 to 5.5
centistokes.
4
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
In some embodiments, the present invention is directed to a base oil blend,
wherein
the Pour Point and Cloud Point are independently about -20 C or lower.
In some embodiments, the present invention is directed to a base oil blend,
wherein
the CCS Viscosity at ¨30 C less than about 4,200 cP.
In some embodiments, the present invention is directed to a base oil blend,
wherein
the CCS Viscosity at ¨35 C less than about 3,400 cP.
In some embodiments, the present invention is directed to a base oil blend,
wherein
the CCS Viscosity at ¨25 C less than about 2,400 cP.
In some embodiments, the present invention is directed to a base oil blend,
wherein
the Noack Volatility less than about 15 wt%.
In some embodiments, the present invention is directed to a base oil blend,
wherein R1
and R2 of Formula I are selected to have a combined carbon number of from 6 to
16 and R3
and R4 are selected to have a combined carbon number of from 10 to 34.
In some embodiments, the present invention is directed to a base oil blend,
wherein R1
and R2 of Formula I are selected to have a combined carbon number of C16, C14
or C12 and R3
and R4 are independently selected from the group consisting of C12 and a
mixture of C6-C10.
In some embodiments, the present invention is directed to a base oil blend,
wherein
the diester component is derived from a C8 to C18 olefin and a C6 to C14
carboxylic acid.
In some embodiments, the present invention is directed to a base oil blend,
wherein
the diester component comprises quantities of at least two different diester
isomers.
In some embodiments, the present invention is directed to a base oil blend,
wherein
the diester component has a molecular mass that is from at least about 340
a.m.u. to at most
about 780 a.m.u.
In some embodiments, the present invention is directed to a base oil blend,
wherein
the diester component is selected from the group consisting of decanoic acid 2-
decanoyloxy-
1-hexyl-octyl ester and its isomers, tetradecanoic acid- 1 -hexy1-2-
tetradecanoyloxy-octyl
esters and its isomers, dodecanoic acid 2-dodecanoyloxy-1-hexyl-octyl ester
and its isomers,
hexanoic acid 2-hexanoyloxy-1-hexy-octyl ester and its isomers, octanoic acid
2-
octanoyloxy- 1 -hexyl-octyl ester and its isomers, hexanoic acid 2-hexanoyloxy-
1-pentyl-
heptyl ester and isomers, octanoic acid 2-octanoyloxy- 1 -pentyl-heptyl ester
and isomers,
decanoic acid 2-decanoyloxy-1-pentyl-heptyl ester and isomers, dodecanoic acid-
2-
dodecanoyloxy-1-pentyl-heptyl ester and isomers, tetradecanoic acid 1-penty-2-
tetradecanoyloxy-heptyl ester and isomers, tetradecanoic acid 1-buty1-2-
tetradecanoyloxy-
hexy ester and isomers, dodecanoic acid- 1-buty1-2-dodecanoyloxy-hexyl ester
and isomers,
5
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
decanoic acid 1-butyl-2-decanoyloxy-hexyl ester and isomers, octanoic acid 1-
buty1-2-
octanoyloxy-hexyl ester and isomers, hexanoic acid 1-butyl-2-hexanoyloxy-hexyl
ester and
isomers, tetradecanoic acid 1-propy1-2-tetradecanoyloxy-pentyl ester and
isomers,
dodecanoic acid 2-dodecanoyloxy-1-propyl-pentyl ester and isomers, decanoic
acid 2-
decanoyloxy-l-propyl-pentyl ester and isomers, octanoic acid 2-octanoyloxy-1-
propyl-pentyl
ester and isomers, hexanoic acid 2-hexanoyloxy-1-propyl-pentyl ester and
isomers, and
mixtures thereof
In one embodiment, the present invention is directed to a process for
improving cold
flow properties and lowering Noack in a starting base oil or starting base oil
blend,
comprising: replacing between about 5 to 60 wt % of a starting base oil or
starting base oil
blend with an equal quantity of a base oil comprising: a) a diester component,
comprising a
quantity of at least one diester species of Formula I:
0
R3 0
R2
R1
0 Rzt
0 I
wherein R1, R2, R3 and R4 are the same or independently selected from C2 to
C17
hydrocarbon groups, and wherein the process affords an upgraded base oil
blend.
In some embodiments, the present invention is directed to a process for
improving
cold flow properties and lowering Noack in a starting base oil or starting
base oil blend,
wherein improving cold flow properties, further comprises lowering CCS VIS
(cold-cranking
simulator apparent viscosity) at ¨25 C, ¨30 C or ¨35 C, lowering pour point
and lowering
Noack.
In some embodiments, the present invention is directed to a process for
improving
cold flow properties and lowering Noack in a starting base oil or starting
base oil blend,
further comprising a process for lowering CCS Viscosity and lowering Noack in
a starting
base oil or starting base oil blend, comprising: replacing between about 5 to
60 wt % of a
6
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
starting base oil or starting base oil blend with an equal quantity of a base
oil comprising: a) a
diester component, comprising a quantity of at least one diester species of
Formula I:
0
R3 0
R2
R1
0
R4
0 I
wherein R1, R2, R3 and R4 are the same or independently selected from C2 to
C17
hydrocarbon groups, and wherein the process affords an upgraded base oil
blend.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, further
comprising a diester component that comprises a mixture of isomers where R1
and R2 are
independently different for each isomer.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, wherein the
base oil or base oil blend comprises a Group I base oil, Group II base oil or
Group III base
oil.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, wherein the
upgraded base oil blend comprises at least one of light neutral base oil,
medium neutral base
oil, Yubase 4, Yubase 6, 150R, 600R, 11ORLV, 100R and 220R.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, wherein the
diester component has a Noack Volatility between 6 and 10 wt%, a pour point
less than -
10 C, a cloud point less than -10 C, a kinematic viscosity at 100 C between
2.5 to 6.5
centistokes, a VI greater than 110 and a BN Oxidator greater than 20 hours.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, wherein the
kinematic viscosity of the upgraded base oil blend at a temperature of 100 C
is between 3 to
5.5 centistokes.
7
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, wherein the
upgraded base oil blend has a Pour Point and Cloud Point are independently -12
C or lower.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, wherein the
upgraded base oil blend has a CCS Viscosity at ¨30 C less than 4,200 cP.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, wherein the
upgraded base oil blend has a CCS Viscosity at ¨35 C less than 3,400 cP.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, wherein the
upgraded base oil blend has a CCS Viscosity at ¨25 C less than 2,400 cP.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, wherein the
upgraded base oil blend has a Noack Volatility less than 15 wt%.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, wherein R1 and
R2 of Formula I are selected to have a combined carbon number of from 6 to 16
and R3 and
R4 are selected to have a combined carbon number of from 10 to 34.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, wherein R1 and
R2 of Formula I are selected to have a combined carbon number of C16, C14 or
C12 and R3 and
R4 are independently selected from the group consisting of C12 and a mixture
of C6-C10.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, wherein the
diester component is derived from a C8 to C18 olefin and a C6 to C14
carboxylic acid.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, wherein the
upgraded base oil blend comprises quantities of at least two different diester
isomers.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, further
comprising an upgraded base oil blend with about a 2 to 35% lower Noack
Volatility as
compared to the starting base oil or starting base oil blend.
8
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, further
comprising an upgraded base oil blend with about a 2 to 35% lower CCS
Viscosity at ¨35 or
CCS Viscosity at ¨30 as compared to the starting base oil or starting base
oil blend.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, wherein the
diester component has a molecular mass that is from at least about 340 a.m.u.
to at most
about 780 a.m.u.
In some embodiments, the present invention is directed to a process for
lowering CCS
Viscosity and lowering Noack in a starting base oil or starting base oil
blend, wherein the
diester component is selected from the group consisting of decanoic acid 2-
decanoyloxy- 1-
hexyl-octyl ester and its isomers, tetradecanoic acid- 1-hexy1-2-
tetradecanoyloxy-octyl esters
and its isomers, dodecanoic acid 2-dodecanoyloxy-l-hexyl-octyl ester and its
isomers,
hexanoic acid 2-hexanoyloxy-l-hexy-octyl ester and its isomers, octanoic acid
2-
octanoyloxy- 1 -hexyl-octyl ester and its isomers, hexanoic acid 2-hexanoyloxy-
l-pentyl-
heptyl ester and isomers, octanoic acid 2-octanoyloxy- 1 -pentyl-heptyl ester
and isomers,
decanoic acid 2-decanoyloxy-l-pentyl-heptyl ester and isomers, dodecanoic acid-
2-
dodecanoyloxy-l-pentyl-heptyl ester and isomers, tetradecanoic acid 1-penty-2-
tetradecanoyloxy-heptyl ester and isomers, tetradecanoic acid 1-buty1-2-
tetradecanoyloxy-
hexy ester and isomers, dodecanoic acid- 1-buty1-2-dodecanoyloxy-hexyl ester
and isomers,
decanoic acid 1-butyl-2-decanoyloxy-hexyl ester and isomers, octanoic acid 1-
buty1-2-
octanoyloxy-hexyl ester and isomers, hexanoic acid 1-butyl-2-hexanoyloxy-hexyl
ester and
isomers, tetradecanoic acid 1-propy1-2-tetradecanoyloxy-pentyl ester and
isomers,
dodecanoic acid 2-dodecanoyloxy-l-propyl-pentyl ester and isomers, decanoic
acid 2-
decanoyloxy-l-propyl-pentyl ester and isomers, octanoic acid 2-octanoyloxy-l-
propyl-pentyl
ester and isomers, hexanoic acid 2-hexanoyloxy-l-propyl-pentyl ester and
isomers, and
mixtures thereof
Definitions and Terms
The following terms will be used throughout the specification and will have
the
following meanings unless otherwise indicated.
The phrase "Group I Base Oil" contain less than 90 percent saturates and/or
greater
than 0.03 percent sulfur and have a viscosity index greater than or equal to
80 and less than
9
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
120 using the ASTM methods specified in Table E-1 of American Petroleum
Institute
Publication 1509.
The term "Group II Base Oil" refers to a base oil which contains greater than
or equal
to 90% saturates and less than or equal to 0.03% sulfur and has a viscosity
index greater than
or equal to 80 and less than 120 using the ASTM methods specified in Table E-1
of
American Petroleum Institute Publication 1509.
The term "Group II+ Base Oil" refers to a Group II base oil having a viscosity
index
greater than or equal to 110 and less than 120.
The term "Group III Base Oil" refers to a base oil which contains greater than
or
equal to 90% saturates and less than or equal to 0.03% sulfur and has a
viscosity index
greater than or equal to 120 using the ASTM methods specified in Table E-1 of
American
Petroleum Institute Publication 1509.
The term "Fischer-Tropsch derived" means that the product, fraction, or feed
originates from or is produced at some stage by a Fischer-Tropsch process.
The term "petroleum derived" means that the product, fraction, or feed
originates
from the vapor overhead streams from distilling petroleum crude and the
residual fuels that
are the non-vaporizable remaining portion. A source of the petroleum derived
product,
fraction, or feed can be from a gas field condensate.
The term "multi-grade engine oil" refers to an engine oil that has
viscosity/temperature characteristics which fall within the limits of two
different SAE
numbers in SAE J300. The present invention is directed to the discovery that
multi-grade
engine oils meeting the specifications under SAE J300 as revised 2009,
including the MRV
viscosity specifications, may be prepared from Fischer-Tropsch base oils
having a defined
cycloparaffin functionality when they are blended with a pour point depressing
base oil
blending component and an additive package.
The term "light neutral base oil" refers to a base oil with a boiling range
from about
700 F to about 800 F, a kinematic viscosity at 100 C from 4 cSt to about 5
cSt.
The term "medium neutral base oil" refers to a base oil with a boiling range
from
about 800 F to about 900 F, a kinematic viscosity at 100 C from 5 cSt to about
8 cSt.
Highly paraffinic wax means a wax having a high content of n-paraffins,
generally
greater than 40 wt %, but can be greater than 50 wt %, or even greater than 75
wt %, and less
than 100 wt % or 99 wt %. Examples of highly paraffinic waxes include slack
waxes, deoiled
slack waxes, refined foots oils, waxy lubricant raffinates, n-paraffin waxes,
NAO waxes,
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
waxes produced in chemical plant processes, deoiled petroleum derived waxes,
microcrystalline waxes, Fischer-Tropsch waxes, and mixtures thereof
The term "derived from highly paraffinic wax" means that the product,
fraction, or
feed originates from or is produced at some stage by from a highly paraffinic
wax.
Aromatics means any hydrocarbonaceous compounds that contain at least one
group
of atoms that share an uninterrupted cloud of delocalized electrons, where the
number of
delocalized electrons in the group of atoms corresponds to a solution to the
Huckel rule of 4n
+ 2 (e.g., n = 1 for 6 electrons, etc.). Representative examples include, but
are not limited to,
benzene, biphenyl, naphthalene, and the like.
Molecules with cycloparaffinic functionality mean any molecule that is, or
contains as
one or more substituents, a monocyclic or a fused multicyclic saturated
hydrocarbon group.
The cycloparaffinic group can be optionally substituted with one or more, such
as one to
three, substituents. Representative examples include, but are not limited to,
cyclopropyl,
cyclobutyl, cyclohexyl, cyclopentyl, cycloheptyl, decahydronaphthalene,
octahydropentalene,
(pentadecan-6-yl)cyclohexane, 3,7,10-tricyclohexylpentadecane, dec ahydro-1 -
(pentadec an-6-
yl)naphthalene, and the like.
Molecules with monocycloparaffinic functionality mean any molecule that is a
monocyclic saturated hydrocarbon group of three to seven ring carbons or any
molecule that
is substituted with a single monocyclic saturated hydrocarbon group of three
to seven ring
carbons. The cycloparaffinic group can be optionally substituted with one or
more, such as
one to three, substituents. Representative examples include, but are not
limited to,
cyclopropyl, cyclobutyl, cyclohexyl, cyclopentyl, cycloheptyl, (pentadecan-6-
yl)cyclohexane,
and the like.
Molecules with multicycloparaffinic functionality mean any molecule that is a
fused
multicyclic saturated hydrocarbon ring group of two or more fused rings, any
molecule that is
substituted with one or more fused multicyclic saturated hydrocarbon ring
groups of two or
more fused rings, or any molecule that is substituted with more than one
monocyclic
saturated hydrocarbon group of three to seven ring carbons. The fused
multicyclic saturated
hydrocarbon ring group often is of two fused rings. The cycloparaffinic group
can be
optionally substituted with one or more, such as one to three, substituents.
Representative
examples include, but are not limited to, decahydronaphthalene,
octahydropentalene, 3,7,10-
tricyclohexylpentadecane, decahydro-1-(pentadecan-6-yl)naphthalene, and the
like.
Brookfield Viscosity: ASTM D2983-04a is used to determine the low-shear-rate
viscosity of automotive fluid lubricants at low temperatures. The low-
temperature, low-shear-
11
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
rate viscosity of automatic transmission fluids, gear oils, torque and tractor
fluids, and
industrial and automotive hydraulic oils are frequently specified by
Brookfield viscosities.
Kinematic viscosity is a measurement of the resistance to flow of a fluid
under
gravity. Many base oils, lubricant compositions made from them, and the
correct operation
of equipment depends upon the appropriate viscosity of the fluid being used.
Kinematic
viscosity is determined by ASTM D445-06. The results are reported in mm2/s.
Viscosity index (VI) is an empirical, unitless number indicating the effect of
temperature change on the kinematic viscosity of the oil. Viscosity index is
determined by
ASTM D2270-04.
Pour point is a measurement of the temperature at which a sample of base oil
will
begin to flow under carefully controlled conditions. Pour point can be
determined as
described in ASTM D5950-02. The results are reported in degrees Celsius. Many
commercial
base oils have specifications for pour point. When base oils have low pour
points, the base
oils are also likely to have other good low temperature properties, such as
low cloud point,
low cold filter plugging point, and low temperature cranking viscosity.
Noack volatility is usually tested according to ASTM D5800-05 Procedure B. A
more
convenient method for calculating Noack volatility and one which correlates
well with
ASTM D5800-05 is by using a thermogravimetric analyzer (TGA) test by ASTM
D6375-05.
TGA Noack volatility is used throughout the present disclosure unless
otherwise stated.
The base oils of the lubricant composition as disclosed herein also have
excellent
viscometric properties under low temperature and high shear, making them very
useful in
multi-grade engine oils. The cold-cranking simulator apparent viscosity (CCS
VIS) is a test
used to measure the viscometric properties of base oils under low temperature
and high shear.
The test method to determine CCS VIS is ASTM D5293-02. Results are reported in
mPa.s.
CCS VIS has been found to correlate with low temperature engine cranking.
Specifications
for maximum CCS VIS are defined for automotive engine oils by SAE J300,
revised in 2009.
The maximum CCS VIS for a OW SAE Viscosity Grade engine oil is 6200 mPa.s at -
35 C.
The phrase "improving cold flow properties" refers to one or more of lowering
CCS
VIS (cold-cranking simulator apparent viscosity) at ¨25 C, ¨30 C or ¨35 C,
lowering pour
point and lowering Noack.
The Mini-Rotary Viscometer (MRV) test, ASTM D4684-07, which is related to the
mechanism of pumpability, is a low shear rate measurement. Slow sample cooling
rate is the
method's key feature. A sample is pretreated to have a specified thermal
history which
includes warming, slow cooling, and soaking cycles. The MRV measures an
apparent yield
12
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
stress, which, if greater than a threshold value, indicates a potential air-
binding pumping
failure problem. Above a certain viscosity (currently defined as 60,000 mPa.s
by SAE J300
2009), the oil may be subject to pumpability failure by a mechanism called
"flow limited"
behavior. An SAE OW oil, for example, is required to have a maximum viscosity
of 60,000
mPa.s at -40 C with no yield stress. This method also measures an apparent
viscosity under
shear rates of 1 to 50 s-1.
High temperature high shear rate viscosity (HTHS) is a measure of a fluid's
resistance
to flow under conditions resembling highly-loaded journal bearings in fired
internal
combustion engines, typically 1 million s-1 at 150 C. HTHS is a better
indication of how an
engine operates at high temperature with a given lubricant than the kinematic
low shear rate
viscosities at 100 C. The HTHS value directly correlates to the oil film
thickness in a
bearing. SAE J300 2009 contains the current specifications for HTHS measured
by ASTM
D4683, ASTM D4741, or ASTM D5481. An SAE 20 viscosity grade engine oil, for
example,
is required to have a minimum HTHS of 2.6 mPa.s.
Scanning Brookfield Viscosity: ASTM D5133-05 is used to measure the low
temperature, low shear rate, viscosity/temperature dependence of engine oils.
The low
temperature, low shear viscometric behavior of an engine oil determines
whether the oil will
flow to the sump inlet screen, then to the oil pump, then to the sites in the
engine requiring
lubrication in sufficient quantity to prevent engine damage immediately or
ultimately after
cold temperature starting. ASTM D5133-05, the Scanning Brookfield Viscosity
technique,
measures the Brookfield viscosity of a sample as it is cooled at a constant
rate of 1 C/hour.
Like the MRV, ASTM D5133-05 is intended to relate to the pumpability of an oil
at low
temperatures. The test reports the gelation point, defined as the temperature
at which the
sample reaches 30,000 mPa.s. The gelation index is also reported, and is
defined as the
largest rate of change of viscosity increase from -5 C to the lowest test
temperature. The
latest API SM/ILSAC GF-4 specifications for passenger car engine oils require
a maximum
gelation index of 12.
"Lubricants," as defined herein, are substances (usually a fluid under
operating
conditions) introduced between two moving surfaces so to reduce the friction
and wear
between them. Base oils used as motor oils are generally classified by the
American
Petroleum Institute as being mineral oils (Group I, II, and III) or synthetic
oils (Group IV and
V). See American Petroleum Institute (API) Publication Number 1509.
13
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
"Pour point," as defined herein, represents the lowest temperature at which a
fluid
will pour or flow. See, e.g., ASTM International Standard Test Methods D 5950-
96, D 6892-
03, and D 97.
"Cloud point," as defined herein, represents the temperature at which a fluid
begins to
phase separate due to crystal formation. See, e.g., ASTM Standard Test Methods
D 5773-95,
D 2500, D 5551, and D 5771.
"Centistoke," abbreviated "cSt," is a unit for kinematic viscosity of a fluid
(e.g., a
lubricant), wherein 1 centistoke equals 1 millimeter squared per second (1 cSt
= 1 mm2/s).
See, e.g., ASTM Standard Guide and Test Methods D 2270-04, D 445-06, D 6074,
and D
m 2983.
With respect to describing molecules and/or molecular fragments herein, "R.,"
where
"n" is an index, refers to a hydrocarbon group, wherein the molecules and/or
molecular
fragments can be linear and/or branched.
As defined herein, "C.," where "n" is an integer, describes a hydrocarbon
molecule or
fragment (e.g., an alkyl group) wherein "n" denotes the number of carbon atoms
in the
fragment or molecule.
The prefix "bio," as used herein, refers to an association with a renewable
resource of
biological origin, such as resource generally being exclusive of fossil fuels.
The term "internal olefin," as used herein, refers to an olefin (i.e., an
alkene) having a
non-terminal carbon-carbon double bond (C=C). This is in contrast to "a-
olefins" which do
bear a terminal carbon-carbon double bond.
The phrase an "upgraded base oil blend" refers to a base oil or base oil blend
that has
had a portion replaced with a diester herein to afford the upgraded base oil
blend having
improved cold flow properties and lower Noack compared to the starting base
oil or base oil
blend.
14
CA 02910832 2015-10-28
WO 2014/179723 PCT/US2014/036628
The terms Yubase 4 and Yubase 6 are base oils defined as presented in Table 1
shown
below.
Table 1
Property Test Method YUBASE 4 YUBASE 6
Appearance Visual Bright & Clear Bright & Clear
Specific Gravity, @15/4 C ASTM D 1298 0.8338 0.8423
Kinematic Viscosity, @40 C ASTM D 445 19.57 36.82
Kinematic Viscosity, @100 C ASTM D 445 4.23 6.52
Viscosity Index ASTM D 2270 122 131
Noack Volatility, wt% DIN 51581 15 7
Flash Point, C ASTM D 92 230 240
Pour Point, C ASTM D 97 -15 -15
Color ASTM D 1500 L0.5 L0.5
Con. Carbon Residue, wt% ASTM D 189 <0.01 <0.01
Copper Corrosion ASTM D 130 1-a 1-a
Sulfur, ppm ASTM D 2622 <10 <10
Total Acid No., mgKOH/g ASTM D 664 0.01 0.01
The terms "100R, 150R, 220R, 600R and 11ORLV" are base oils defined as
presented
in Table 2 shown below.
Table 2
Property ASTM Method 100R 150R 220R 600R 11ORLV
API Base Oil Category API 1509 E 1.3 II II II II II(+)
Bright Bright Bright Bright Bright
and and and and and
Appearance SM 360-99 Clear Clear Clear Clear
Clear
Color ASTM D 1500 L0.5 L0.5 L0.5 L0.5
L0.5
API Gravity, deg. ASTM D 4052 34.4 33.4 31.9 31.2
35.4
Density, lb/gal ASTM D 4052 7.1 7.15 7.22 7.28 7.06
Density, kg/1 ASTM D 4052 0.853 0.858 0.867 0.874
0.848
Specific Gravity, @
60 F/60 F ASTM D 4052 0.853 0.858 0.867 0.874
0.848
Viscosity @ 40 C, cSt ASTM D 445 20.3 30.9 43.7 108
21.1
Viscosity @ 100 C, cSt ASTM D 445 4.1 5.3 6.6 12.2 4.4
Viscosity @ 100 F,
SUS ASTM D 2161 107 153 214 590 113
Viscosity Index ASTM D 2270 102 107 102 103 118
CCS @ -20 C, cP ASTM D 5293 N/A 1750 3400 N/A 822
CCS @ -25 C, cP ASTM D 5293 1400 2660 5600 N/A
1350
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
CCS @ -30 C, cP ASTM D 5293 2650 5070 N/A N/A 2450
ASTM D
Pour Point, C 5950/1C -15 -15 -13 -17 -15
Flash Point, COC, C ASTM D 92 206 227 230 270 216
Volatility, wt. %
distilled at
700 F/371 C ASTM D 2887 13 N/A N/A N/A N/A
Evaporative Loss, ASTM D5800
NOACK, wt% (B) 26 14 10 2 16
ASTM D 6304-
Water, ppm 98 <50 <50 <50 <50 <50
Sulfur, ppm ICP/XRF <10 <10 <10 <10 <6
Saturates, HPLC wt. % Chevron >99 >99 >99 >99 >99
Aromatics, HPLC wt.
% Chevron <1 <1 <1 <1 <1
Unless otherwise indicated herein, scientific and technical terms used in
connection
with the present invention shall have the meanings that are commonly
understood by those of
ordinary skill in the art. Further, unless otherwise required by context,
singular terms shall
include pluralities and plural terms shall include the singular. More
specifically, as used in
this specification and the appended claims, the singular forms "a", "an" and
"the" include
plural referents unless the context clearly dictates otherwise. Thus, for
example, reference to
"a fatty acid" includes a plurality of fatty acids, and the like. In addition,
ranges provided in
the specification and appended claims include both end points and all points
between the end
points. Therefore, a range of 2.0 to 3.0 includes 2.0, 3.0 and all points
between 2.0 and 3Ø
Furthermore, all numbers expressing quantities, percentages or proportions,
and other
numerical values used in the specification and claims, are to be understood as
being modified
in all instances by the term "about". As used herein, the term "include" and
its grammatical
variants are intended to be non-limiting, such that recitation of items in a
list is not to the
exclusion of other like items that can be substituted or added to the listed
items. As used
herein, the term "comprising" means including elements or steps that are
identified following
that term, but any such elements or steps are not exhaustive, and an
embodiment can include
other elements or steps.
EXAMPLES
The following examples are provided to demonstrate particular embodiments of
the
present invention. It should be appreciated by those of skill in the art that
the methods
disclosed in the examples which follow merely represent exemplary embodiments
of the
16
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
present invention. However, those of skill in the art should, in light of the
present disclosure,
appreciate that many changes can be made in the specific embodiments described
and still
obtain a like or similar result without departing from the spirit and scope of
the present
invention.
EXAMPLE 1
This example serves to illustrate the base oil blends with and without the
diesters of
the present invention with the analytics presented in Table 3 below.
The diester-free base oil blend was prepared by mixing 82.67 wt% Yubase 4 and
17.33 wt% Yubase 6. The base oil component with diester was prepared by mixing
69.7 wt%
Yubase 4, 13.8 wt% Yubase 6, and 16.5 wt% diester of Formula I wherein R1 and
R2 are
combined to have a carbon number of C12 and R3 and R4 are both C12. Both
samples were
submitted for standard base oil testing, including API gravity, viscosity at
40 C and 100 C,
Viscosity Index, pour point, cloud point, Noack volatility, cold cranking
viscosity, and
Bromine number.
Table 3
Base Oil Blend Example 1
Diester A, wt% 0 16.5
Yubase 4, wt% 82.67 69.7
Yubase 6, wt% 17.33 13.8
Properties
API 42.5 36.2
Vis @ 100 C, cSt 4.515 4.523
VI 130 132
Pour point, C -14 -17
Cloud point, C -10 -12
Noack, wt% 12.65 12.46
CCS @ -35 C, cP 3225 2928
Bromine number 0.1 0.08
Example 2
This example serves to illustrate the base oil blends with the diesters of the
present
invention, a single comparative without diester and a second comparative with
a
commercially available ester (i.e., Esterex A51) with the analytics presented
in Table 4
below. Diester A is a diester of Formula I, wherein R1 and R2 are combined to
have a carbon
number of C12 and R3 and R4 are both C12. Diester B2 is a diester of Formula
I, wherein R1
17
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
and R2 are combined to have a carbon number of C12 and R3 and R4 are both
independently
C6-C10. The examples in Table 3 were prepared in a similar manner as those of
Example 1
herein.
Table 4
Composition, wt% B0B02958 B0B02959 B0B02960 B0B02961
110RLV 52.43 59.31 59.25 59.16
100R 13.01
220R 34.56 35.55 35.51 35.46
Diester A 5.14
Diester B2 5.24
Esterex A51 5.37
BOB Properties, Calculated
KV100, centistokes 4.988 5.07 5.044 5.103
KV40, centistokes 26.76 27.06 27.03 27.46
VI 112 115 114 115
CCS, cP @ -25 C 2137 2103 2120 2164
CCS, cP @ -30 C 3929 3840 3880 3961
Noack volatility, % wt loss 14.8
BOB Properties, Observed
KV100, centistokes 4.971 5.002 4.975 5.047
KV40, centistokes 26.51 26.35 26.27 26.73
VI 113 117 115 117
CCS, cP @ -25 C 2165 1952 1972 2084
CCS, cP @ -30 C 3912 3513 3529 3751
Noack, wt% 14.2 13.4 13.9 13.1
10
18
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
Example 3
This example serves to illustrate the diesters prepared and their respective
properties as presented in Table 5.
Table 5
Starting Materials Properties
No. Olefins Fatty Cloud Pour Viscosity Viscosity Viscosity Oxidator
NOACK,
Acids Point Point (40 C) (100 C) Index BN
wt% loss
A C14 C12 -28 -27 19.5cSt 4.76cSt 176 26 hrs
8.9
131 C14 C6(high)- -69 -66 16.41cSt 3.68cSt 109 19.3hrs -
C10
C14 C6(low)- -60 -60 19.47cSt 4.191cSt 120
26.25hrs 9.1
B2
C10
C C16 C12 -18 -19 24.44cSt 5.218cSt 152 38 hrs
E C18 C6-C10 -24 -26 20.4 cSt 4.5 cSt 137 25.5
D1 C16 C6(high)- -51 -51 17.90cSt 4.015cSt 124
C10
C16 C6(low)- -51 -53 21.54cSt 4.545cSt 128 26
hrs 6.3
D2
C10
Example 4
This example serves to illustrate the Noack and CCS Viscosity of the diesters
of
the present invention as compared to the current commercial esters and other
Group II and III
bases oils as presented in Table 6 and Figure 1.
Table 6
Sample CCS (-25) CCS (-30) Noack KV100
Diester A 542 812 8.9 4.76
Diester B2 1002 1717 9.1 4.19
Diester D2 1104 1875 6.3 4.545
Esterex A51 1468 2487 7.4 5.4
Esterex A32 212 319 30.3 2.8
19
CA 02910832 2015-10-28
WO 2014/179723
PCT/US2014/036628
All patents, patent applications and publications are herein incorporated by
reference
to the same extent as if each individual patent, patent application or
publication was
specifically and individually indicated to be incorporated by reference.
The present invention if not to be limited in scope by the specific
embodiments
described herein, which are intended as single illustrations of individual
aspects of the
invention, and functionally equivalent methods and components are within the
scope of the
invention. Indeed, various modifications of the invention, in addition to
those shown and
described herein will become apparent to those skilled in the art from the
foregoing
description and accompanying drawings. Such modifications are intended to fall
within the
to scope of the appended claims.