Note: Descriptions are shown in the official language in which they were submitted.
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
COMPOUNDS FOR TREATMENT OF
DRUG RESISTANT AND PERSISTENT TUBERCULOSIS
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This application claims the benefit of U.S. Application No. 61/827,539,
filed May 24,
2013, and U.S. Application No. 61/950,752, filed March 10, 2014, both of which
are hereby
incorporated by reference in their entirety.
BACKGROUND OF THE INVENTION
[0002] One of the greatest needs in global health is the development of new
drugs against
tuberculosis (TB) that shorten the duration of TB chemotherapy, and that are
potent against drug
resistant strains of Mtb for which current therapies are no longer effective
[Gandhi NR, et al.
(2010) HIV coinfection in multidrug- and extensively drug-resistant
tuberculosis results in high
early mortality. Am J Respir Crit Care Med 181:80-86]. TB is exceptional among
bacterial
infections in that even drug-susceptible strains are difficult to treat
rapidly and effectively. This
is in part due to the phenomenon of Mtb persistence, a state of phenotypic
drug-tolerance that is
attributed to a quiescent or non-replicating population of bacilli. Long
treatment regimes make
compliance problematic, and lead to the emergence of drug resistant mutants.
SUMMARY OF THE INVENTION
[0003] In one aspect, provided herein are compounds of Formula (I), a
pharmaceutically
acceptable salt, solvate, polymorph, prodrug, metabolite, N-oxide,
stereoisomer, or isomer
thereof:
Y2=-Y3
/ ,R1
YiN?-Z¨M
M11
N. 3
M2 R
A
Formula (I)
wherein:
M1 are each independently a bond, -C(=0)- or -S(=0)2-;
M2 is ¨(CH2)-, -C(=0)- or -S(=0)2-;
Z is a bond or NR2;
Yi is S, 0 or NR2;
Y2 is CR4 or N;
Y3 is CR5 or N;
-1-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
R1 is -0-(optionally substituted alkyl), -0-(alkenyl), -0-(alkynyl), -0-
(cycloalkyl),
-O-(heterocyclyl), -0-(optionally substituted aralkyl), -0-(optionally
substituted
heteroaralkyl), -0-(alkyl)-(alkoxy), -0-(alkyl)-(aralkoxy), -O-(alkyl)-
(heterocyclyl),
-0-(alkyl)-(COORa), -0-(alkyl)-(NR6R7), -NR6R7 or R8;
R2 and R3 are each independently selected from H, optionally substituted
alkyl, and
optionally substituted aryl; or R1 and R2 taken together form a heterocycle;
R4 is H, halogen, -CN, optionally substituted alkyl, optionally substituted
aryl, -RbCOORa or
-RbCH(COORa)2;
R5 is H, halogen, optionally substituted alkyl or cycloalkyl; or R4 and R5
taken together form
a carbocycle or an optionally substituted heterocycle;
R6 and R7 are each independently selected from H and optionally substituted
alkyl; or R6 and
R7 taken together form an optionally substituted heterocycle with the nitrogen
to which
they are attached;
R8 is optionally substituted alkyl, optionally substituted aryl, carbocyclyl,
optionally
substituted aralkyl, optionally substituted heteroaryl, optionally substituted
heterocyclyl, ¨
RbCOORa or ¨RbCONRaRa;
each Ra is independently selected from H and alkyl;
RD is a bond or alkylenyl;
Rc is a bond or alkenylenyl; and
A is optionally substituted aryl, optionally substituted heterocyclyl,
optionally substituted
heteroaryl, optionally substituted carbocyclyl, optionally substituted
aralkyl, optionally
substituted heteroaralkyl or ¨W-(optionally substituted heteroaryl).
[0004] In some embodiments of a compound of Formula (I), Y3 is CR5. In some
embodiments
of a compound of Formula (I), Y1 is S. In some embodiments of a compound of
Formula (I), Y2
is CR4. In some embodiments of a compound of Formula (I), each M1 is -C(=0)-.
In some
embodiments of a compound of Formula (I), Z is NR2. In some embodiments of a
compound of
Formula (I), M2 is
-C(=0)-. In some embodiments of a compound of Formula (I), R1 is -0-
(optionally substituted
alkyl); and R2 and R3 are both H.
[0005] In another aspect, provided herein are compounds of Formula (Ia), a
pharmaceutically
acceptable salt, solvate, polymorph, prodrug, metabolite, N-oxide,
stereoisomer, or isomer
thereof:
-2-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
R4
\, R2
1
Y1Ne'lc
0
0
Oy N-, R-' -4
A
(Ia)
wherein:
Yi is S or 0 or NR2;
Y3 is CR5 or N;
R1 is -0-(optionally substituted alkyl), -0-(alkenyl), -0-(alkynyl), -0-
(cycloalkyl), -0-
(heterocyclyl), -0-(optionally substituted aralkyl), -0-(optionally
substituted
heteroaralkyl), -0-(alkyl)-(alkoxy), -0-(alkyl)-(aralkoxy), -0-(alkyl)-
(heterocycly1),
-0-(a1ky1)-(C00Ra), -0-(alkyl)-(NR6R7), -NR6R7 or R8;
R2 and R3 are each independently selected from H, optionally substituted
alkyl, and
optionally substituted aryl; or R1 and R2 taken together form a heterocycle;
R4 is H, halogen, -CN, alkyl, aryl, -RbCOORa or -RbCH(C00Ra)2;
R5 is H, halogen, optionally substituted alkyl, or cycloalkyl; or R4 and R5
taken together
form a carbocycle or an optionally substituted heterocycle;
R6 and R7 are each independently selected from H and optionally substituted
alkyl; wherein
the optional substituent is halogen; or R6 and R7 taken together form an
optionally
substituted heterocycle with the nitrogen to which they are attached; wherein
the
optional substituent is halogen;
R8 is optionally substituted alkyl;
each Ra is independently selected from H and alkyl;
RD is a bond or alkylenyl;
Rc is a bond or alkenylenyl; and
A is optionally substituted aryl, optionally substituted heterocyclyl,
optionally substituted
heteroaryl, optionally substituted carbocyclyl, optionally substituted
aralkyl, optionally
substituted heteroaralkyl or ¨W-(optionally substituted heteroaryl).
[0006] In some embodiments of a compound of Formula (Ia), R1 is -0-(optionally
substituted
alkyl); wherein the optionally substituted alkyl is substituted with halogen.
In some
embodiments of a compound of Formula (Ia), R1 is -0-(cycloalkyl). In some
embodiments of a
compound of Formula (Ia), R1 is -0-(heterocycly1). In some embodiments of a
compound of
Formula (Ia), R1 is -0-(optionally substituted aralkyl) or -0-(optionally
substituted
-3-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
heteroaralkyl). In some embodiments of a compound of Formula (Ia), R1 is -O-
(alkyl)-
(heterocyclyl). In some embodiments of a compound of Formula (Ia), R4 and R5
taken together
form an optionally substituted heterocycle; wherein the optionally substituted
heterocycle is
substituted with a group selected from alkyl, aralkyl and
-S02Me. In some embodiments of a compound of Formula (Ia), R6 and R7 taken
together form
a heterocycle with the nitrogen to which they are attached; wherein the
heterocycle is selected
from piperidinyl and morpholinyl. In some embodiments of a compound of Formula
(Ia), n Y3 is
CR5. In some embodiments of a compound of Formula (Ia), Yi is S. In some
embodiments of a
compound of Formula (Ia), Y1 is S and Y3 is CH. In some embodiments of a
compound of
Formula (Ia), R4 is H. In some embodiments of a compound of Formula (Ia), R1
is
-0-(optionally substituted alkyl); and R2 and R3 are both H.
[0007] In some embodiments of a compound of Formulas (Ia), A is optionally
substituted aryl,
optionally substituted heterocyclyl, optionally substituted heteroaryl,
optionally substituted
carbocyclyl, optionally substituted aralkyl, optionally substituted
heteroaralkyl or -Rc-
(optionally substituted heteroaryl); and the optionally substituted aryl, the
optionally substituted
heterocyclyl, the optionally substituted heteroaryl, the optionally
substituted carbocyclyl, the
optionally substituted aralkyl and the optionally substituted heteroaralkyl
are substituted with 1-
6 R10; wherein each R1 is independently selected from H, halogen, -CN, -NO2, -
CF3, alkyl,
-SR6, -0R6, -NR6R7, -NR6C(=0)(alkyl), - NR6C(=0)(cycloalkyl), -
NR6C(=0)(heterocycly1),
-NR6C(=0)(ary1), -NR6C(=0)(heteroary1), -C(=0)NR6R7, -C(=0)NR6(cycloalkyl),
-C(=0)NR6(heterocycloalkyl), -C(=0)NR6(ary1), -C(=0)NR6(heteroary1), -
NR6C(=0)NR6R7,
-NR6C(=0)NR7(cycloalkyl), -NR6C(=0)NR7(heterocycloalkyl), -NR6C(=0)NR7(ary1),
-NR6C(=0)NR7(heteroary1), -NR6C(=0)0(alkyl), -NR6C(=0)0(cycloalkyl),
-NR6C(=0)0(heterocycloalkyl), -NR6C(=0)0(ary1), -NR6C(=0)0(heteroary1), -
NR6S02(alkyl),
-NR6S02(cycloalkyl), -NR6S02(heterocycloalkyl), -NR6S02(ary1), -
NR6S02(heteroary1),
-SO2NR6R7, -SO2NR6(cycloalkyl), -SO2NR6(heterocycloalkyl), -SR6, -S02R6, -
SO2NR6(ary1),
-SO2NR6(heteroary1), haloalkyl, aryl, heteroaryl, heterocyclyl and tetrazoyl.
[0008] In some embodiments of a compound of Formula (Ia), A is optionally
substituted aryl. In
some embodiments of a compound of Formula (Ia), A is optionally substituted
heteroaryl. In
some embodiments of a compound of Formula (Ia), A is selected from:
-4-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
Rio \\ 0 o io N
N/ R1 i
R Ir.\\. R r'Nµ N\µ`
I I I I Ki jk
--N
N , Rio" , N. , N , N.....,INI , R10 N
,
Rio
Riyy\
I10
N
1\1A. R
I N
i k ,
N.
Rio , Rio N , and N .In some embodiments of a compound
of
R10
/N\
1
Formula (Ia), A is: N . In some embodiments of a compound of Formula
(Ia), A is
X4 X3
\ X5 'X2
X
x6 ,
- T'L
X4. '\ X7 X7x 1
selected from: X5 X6 and ¨ ; wherein X1, X2, X3, X4, X5, X6, and X7
are
independently selected from N and CR10; and at least one of X1- X7 is N. In
some embodiments
N 401 \ e \
I , I
/
of a compound of Formula (Ia), A is selected from: ' N
N\
N\
.--
,
I\IN)1\ a 401 \ (N)%µ
I , \ I Rio \/ N N
N , / , Rio
, , ,
N Rio Rio N N \ I 41 0 I
/ 0 / /0
N
0 N
......A.
011114ININAP ANVW1/1. , and ¨ . In some
, , ,
X1
)( -X5
I' I
X3. 7)(
embodiments of a compound of Formula (Ia), A is selected from: X . ,
X1 x X5-._ X1x2
,x5 /x5 xl \ x/zi,if , XI 2
x5 xl \ ,5 xt
II Xi; I 1
X3' '
X4 X4 I
X\x4,- x3 X2 X -----% X3
,,,,.
¨ , and
¨
,X5--../L xi
X4' I 2
X 3X. wherein X1, X2, X3, X4, X5, X6, and X7 are independently selected
from N and
CR10; and Xis 0, S, or NR2. In some embodiments of a compound of Formula (Ia),
A is selected
-5-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
_________________ Rio N x2 Xl\
N -....õ xi
I R_7
I I I I io<
* Rio / I I
X3Sx3 i
'
Sx3'x2
from: x4 s , s-')(3' x2
/
Xi
N.õ,,. \ x2 , xi 1N-...,X1 x2
s_1X3 (
R10_ I I X2 N) N-.....õX '%\.
__________________________ 1 Rio_ I Rio_
*
13
0*----%3' X2 Ox3 /
....._. , 5 5 5
X1 , X1 1
N -....õ, x2 x2 ._,...--- N
,1"----5;717--- xi Ri Cl_ II_ II3 I R1 0 ______________ I
Rio- II ox..i xNi N.----%3' X2
0-------%3 X2 R6 , Re
......L., , 5
N=õ__,X1 x2N N.....õ.X1 2 Rio
......õ xi X , x' c__.,,
Rio_ Il Rio_ I I Ri o_ I I
N -----%3...."'Y N X3X2
R6 R6 Re
/ / ¨ , 5
R10
Ri _IRp¨
, X1 X1 Aµ xl
X2
I I _______ R1 R10 ir Rio i 1 X11 R'u ' I I
X4 Sx3' X2 S --%3/1 Sx3' X2
/ / / /
R1 R10 R10
,x1 ._..e.,.., -...õ.. R10
Xi
Ri / If X2 X1 _/ x2
I I 1 I I \ R10 Ri -h I I
R10 /
, *
X3 X3 0 ,4 X "x4*\0
,..2._ , XOx3' X2
5
R10 R10 R Ri
R-03õ............ / 6
X1 2 2, X-.,c 2, x1
X1)\
/ X1
io Rio / I) ) \ __ 1 -11 4¨i Ri R10-h II
R / I I
0"---.'- 3 ,...-
X3X4 /
/ X3 , X x4 ."..."-''N)
X3
_x2
0----'-'%3' X2 I'R6
...."'" ,
IR 5
Ro......._:)..,...T R1
2(1 x2
Ri /
N X3of
_....,,...
* R10 /
Xi
II R1 7 II N II 7 \
--..---- y
,.....¨.;,,,.., i
N e'--"'-%3_ X2 N -----r X3 \N"--x3' X-2 S
, 2
R.6 17Z R6 , IR \ N --- x3' X
/ / / /
,X1 _s Xi
X2 \ X2' ------ \
I I N I I
X3 *--,f N
X4
=^"'"' , and .---- . In some embodiments of a compound of Formula (Ia), A
is
R1 R1
R1
N R10 N N
N )---- N
SHI
R1 S Ri 0 s Ri 0j\ni S
selected from: R1 R1 0 R1 o , , ,
-6-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
R10 R' R'
R1 C....... R10
N S S
Ri.,N Rio R10
\ _ 0 / Rio_
101
I , )¨ I N Rio N
i
Rio 5 Rio'N .."-S , Rio Rio
,and. In
X2'.-X 1
1 /)¨ 1
NA -...õ3
some embodiments of a compound of Formula (Ia), A is selected from: R2 ,
X2-X1 X2:X1
II I I I
X3
"X , and X.--X3
. In some embodiments of a compound of Formula (Ia), A is selected
Rio Rio Rio Rio
Cl o
....:: l N =-----_
1 / I Rs N -N
I
1 I
N
from: -
Rio N 5 R2 NI " 5 Rio S 5 Rio N 5 R10"..N
5 R S
N-N i
R10 0 . In some embodiments of a compound of Formula (Ia), A is selected
from:
.,.. Xc
X2 x X3 X2N /., .....-Xi v /X2Al ' "----.X1\\ XX9N
....., , "--- 3 IN ,...-- ,_.., ...--X N 1 1
y-----:--x' / X7
X4
X5 X6 X5 6
rJ.'''.'' , aiwu=nme 11\3 X )(/6
5
...,,,,
ssii\X2 ' N ----Xi xN----XiX X2,
c N"--
\\X7 13 X7 I X7
Xzi. ,-\t/ X4 /-*'= /
X X4R X5 X6
X5 - X /xa 5 u , and ; wherein X1, X2, X3,
X4, X5,
,
X6, and X7 are independently selected from N and CR10. In some embodiments of
a compound
R'0 R'0 R'0
R'0
Ri..... Rir.....
---- -- 1 .--- -- i
m / 1
R1 OTh/ Rio'
Rio Rio Rio
of Formula (Ia), A is selected from: ,,
Rio Rio
Rio Rio Rio Rio Rio
Ri c< N rõ. --._ ----
. \
1
Njr'_ 1 Rio_ N
\ --..t 1 i 4 R10 ¨\ .--------1
R1 O N Th R1 N 0 N --1\rRi 0 Rio
Rio R1 5 Ri o Rio Rio Rio
, , ,
-7-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
R10 R10
R10
R1C<HN R1C<,... / R1Ø........ _ N
N 1
__two
I 1===-......0
RioN i R ..,----N
io-- - -
N
Rio--).--"N Rio'kr"--"N /
Rio ,,-""` Rio Rio R1 , Rio R1 ,
, ,
Rio Rio Rio Rio
RirN
it! Ri04.-: Rio_cji\I-N);\ Rio_c_____LN-N\
, N / 1 N Rio
Ri , r,,, N N^Rio N^Rio
Rio Rio , Rio , Rio
, ,
Rio
N Rio ....,N m
Riol\l"--N
Rio Rio , and R1 .
In some embodiments of a compound of Formula
R11
1
Ril N r \......- N_ 1 1\1 N
N
(Ia), A is selected from: X R11N-X ---X
, , ,
Rio Rio =,,..õ,õ
1 1
R 1:01
N..------X IN-'1 \ I N'"----c_Rio
111 ----X Ri1,,....õ-----x
Rio R11
Rio ii \
/õ.....--f_e_l
(---i_Rio \N 1 Rio IW
R11N----x , Rio
,and Rio wherein:X is 0, S, or NR2;
and R11 is H, alkyl, aryl, heteroaryl, -S02-(alkyl), -S02-(cycloalkyl), -S02-
(aryl),
-S02-(heteroary1), -S02-(heterocycloalkyl), -C(=0)0(alkyl), -
C(=0)0(cycloalkyl),
-C(=0)0(heterocycloalkyl), -C(=0)0(ary1), -C(=0)0(heteroary1), -C(=0)NR6R7,
-C(=0)NR6(cycloalkyl), -C(=0)NR6(heterocycloalkyl), -C(=0)NR6(ary1),
-C(=0)NR6(heteroary1), -C(=0)(alkyl), -C(=0)(cycloalkyl), -
C(=0)(heterocycloalkyl),
-C(=0)(ary1), or -C(=0)(heteroary1). In some embodiments of a compound of
Formula (Ia), A is
r...)\ H
N ---1 S 0 \ ..
110 1\1 Cr 1 <C) lel
\ ON
I \ I
selected from: S S , 0 )1
¨
, , , ,
-8-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
0 I \0 0
HNN)_ 0 ,N
HN
S
0 S , Crti¨
R10 R10
\
HNN\µ R10 \ R10
N
,
0 N R10 0 R10 0
0 R10 R10
,
=
0 \.,õ
aN,1 HN&N,1
0 s s , and N
[0009] In another aspect, provided herein are compounds of Formula (Ic), a
pharmaceutically
acceptable salt, solvate, polymorph, prodrug, metabolite, N-oxide,
stereoisomer, or isomer
thereof:
R4
___________________________________ õ R2
3 \
R8
0
Oy 1\1
A
(Ic)
wherein:
Yi is S or 0;
Y3 is CR5 or N;
R2 and R3 are each independently selected from H and optionally substituted
alkyl;
R4 is H, halogen, -CN, optionally substituted alkyl, or optionally substituted
aryl;
R5 is H, optionally substituted alkyl, or halogen;
R8 is optionally substituted alkyl, optionally substituted aryl, optionally
substituted aralkyl,
carbocyclyl, optionally substituted carbocyclylalkyl, optionally substituted
heteroaryl,
optionally substituted heteroarylalkyl, optionally substituted heterocyclyl,
optionally
substituted heterocyclylalkyl, ¨RbCOORa or ¨RbCONRaRa; or R2 and R8 taken
together
form an optionally substituted heterocycle with the nitrogen to which they are
attached;
each Ra is independently selected from H and alkyl;
RD is a bond or alkylenyl; and
-9-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
A is optionally substituted heteroaryl, optionally substituted aryl or
optionally substituted
heterocyclyl.
[0010] In some embodiments of a compound of Formula (Ic), R8 is optionally
substituted aryl;
wherein the optionally substituted aryl is substituted with halogen. In some
embodiments of a
compound of Formula (Ic), R8 is optionally substituted heteroaryl; wherein the
optionally
substituted heteroaryl is substituted with a group selected from alkyl, -0-
(alkyl) and -NR6R7. In
some embodiments of a compound of Formula (Ic), R8 isoptionally substituted
heterocyclyl;
wherein the optionally substituted heterocyclyl is substituted with alkyl. In
some embodiments
of a compound of Formula (Ic), R2 and R8 taken together form an optionally
substituted
heterocycle with the nitrogen to which they are attached.
[0011] In some embodiments of a compound of Formula (Ic), Y3 is CR5. In some
embodiments
of a compound of Formula (Ic), R5 is H. In some embodiments of a compound of
Formula (Ic),
Y1 is S. In some embodiments of a compound of Formula (Ic), R4 is H.
[0012] In some embodiments of a compound of Formula (Ic), wherein A is
optionally
substituted heteroaryl, optionally substituted aryl or optionally substituted
heterocyclyl; and the
optionally substituted aryl, the optionally substituted heterocyclyl, the
optionally substituted
heteroaryl are substituted with 1-6 R10; wherein each R1 is independently
selected from H,
halogen, -CN, -NO2, -CF3, alkyl, -SR6, -0R6, -NR6R7, -NR6C(=0)(alkyl), -
NR6C(=0)(cycloalkyl), -NR6C(=0)(heterocycly1), -NR6C(=0)(ary1), -
NR6C(=0)(heteroary1),
-C(=0)NR6R7,-C(=0)NR6(cycloalkyl), -C(=0)NR6(heterocycloalkyl), -
C(=0)NR6(ary1),
-C(=0)NR6(heteroary1), -NR6C(=0)NR6R7, -NR6C(=0)NR7(cycloalkyl),
-NR6C(=0)NR7(heterocycloalkyl), -NR6C(=0)NR7(ary1), -NR6C(=0)NR7(heteroary1),
-NR6C(=0)0(alkyl), -NR6C(=0)0(cycloalkyl), -NR6C(=0)0(heterocycloalkyl),
-NR6C(=0)0(ary1), -NR6C(=0)0(heteroary1), -NR6S02(alkyl), -NR6S02(cycloalkyl),
-NR6S02(heterocycloalkyl), -NR6S02(ary1), -NR6S02(heteroary1), -SO2NR6R7,
-SO2NR6(cycloalkyl), -SO2NR6(heterocycloalkyl), -SR6, -S02R6, -SO2NR6(ary1),
-SO2NR6(heteroary1), haloalkyl, aryl, heteroaryl, heterocyclyl and tetrazoyl.
R 1
)1/2.,
[0013] In some embodiments of a compound of Formula (Ic), A is selected from:
\N ,
-10-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
Rio
RR1\R1 \ Rio N \ R1 1
\
N ".. N N N
jj Ti - I I Ki j I
R10- N 5 NN
-..õ,..3,....- .., 5 Rio N 5 Rio 5
5
cN.;\ R
1 1i)k
N.
Rio N 5 N and . In some embodiments of a compound of Formula (Ic),
A is
Rio \
1
N . In some embodiments of a compound of Formula (Ic), A is selected from:
R1 R1 R1
Ri oR1 cy __ N
I
i
N Ri o NI N
)-"". N
0 )¨ I I , ,¨ I j N>¨ 1
\ 1 I ,¨ 1
N ----.
R' S Ri oThr'S Ri oys S
R' Ri o R' R'
5 5 5 5
R10 R10
R10
S \ SR10
R1C:.....N R10- . R10-
101
N Rio N
i
Rio r\j---S R10 Ri o if
5 and . In some embodiments
5
R10
R1
N
1.1 )¨ I
R' S
of a compound of Formula (Ic), A is R1 .
[0014] In another aspect, provided herein are compounds of Formula (Id), a
pharmaceutically
acceptable salt, solvate, polymorph, prodrug, metabolite, N-oxide,
stereoisomer, or isomer
thereof:
R4\
YliY3 RS' -R9
0
OyN.R3
A
(Id)
wherein:
-11-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
Y1 iS S or 0;
Y2 is CR5 or N;
R3 is H, haloalkyl or alkyl;
R4 is H, halogen, CN or optionally substituted alkyl;
R5 is H, optionally substituted alkyl or halogen;
R9 is optionally substituted alkyl, optionally substituted heteroaryl or
optionally substituted
aryl; and
A is optionally substituted heteroaryl, optionally substituted aryl, or
optionally substituted
heterocyclyl.
[0015] In another aspect, provided herein are compounds, or pharmaceutically
acceptable salts,
solvates, polymorphs, prodrugs, metabolites, N-oxides, stereoisomers, or
isomers thereof,
selected from the compounds of Table A.
[0016] In another aspect, provided herein are compounds selected from:
00
4 0 0 0
,
A ,
A
0 11 NH 0
NH 0 0 N OH
N N H
NH
Orl(!1 S HN 0 S
Orrm
N 0 0 0>
N 0 Ocy NH ...r
j 0
0 N-N F
TO-11 S F
and N ; or pharmaceutically acceptable salts, solvates,
polymorphs,
prodrugs, metabolites, N-oxides, stereoisomers, or isomers thereof.
[0017] In another aspect, provided herein are compounds of Formula (lIb), a
pharmaceutically
acceptable salt, solvate, polymorph, prodrug, metabolite, N-oxide,
stereoisomer, or isomer
thereof:
i
(
R KN R1
I 4)
n k R2
Y1 N....-R3
0\A
(Ith)
wherein:
Yi is N, CH, or CR4;
-12-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
R1 is -0-(optionally substituted alkyl), -0-(alkenyl), -0-(alkynyl), -0-
(cycloalkyl),
-O-(heterocyclyl), -0-(optionally substituted aralkyl), -0-(optionally
substituted
heteroaralkyl), -0-(alkyl)-(alkoxy), -0-(alkyl)-(aralkoxy), -O-(alkyl)-
(heterocyclyl),
-0-(alkyl)-(COORa), -0-(alkyl)-(NR6R7), -NR6R7 or R8;
R2 and R3 are each independently selected from H, optionally substituted
alkyl, and
optionally substituted aryl; or R1 and R2 taken together form a heterocycle;
each R4 is independently selected from halogen,-CN, optionally substituted
alkyl, optionally
substituted alkoxy, optionally substituted aryl, -RbCOORa , and
-RbCH(COORa)2;
R6 and R7 are each independently selected from H and optionally substituted
alkyl; or R6 and
R7 taken together form an optionally substituted heterocycle with the nitrogen
to which
they are attached;
R8 is optionally substituted alkyl, optionally substituted aryl, carbocyclyl,
optionally
substituted aralkyl, optionally substituted heteroaryl, or optionally
substituted
heterocyclyl;
each Ra is independently selected from H and alkyl;
RD is a bond or alkylenyl;
Rc is a bond or alkenylenyl;
n is 0, 1, 2, or 3; and
A is optionally substituted aryl, optionally substituted heterocyclyl,
optionally substituted
heteroaryl, optionally substituted carbocyclyl, optionally substituted
aralkyl, optionally
substituted heteroaralkyl or ¨W-(optionally substituted heteroaryl).
[0018] In some embodiments of a compound of Formula (lIb), Yi is N or CH. In
some
embodiments of a compound of Formula (lIb), Yi is N. In some embodiments of a
compound of
Formula (Ilb), R1 is -0-(optionally substituted alkyl); and R2 and R3 are both
H. In some
embodiments of a compound of Formula (lIb), A is optionally substituted aryl,
optionally
substituted heterocyclyl, optionally substituted heteroaryl, optionally
substituted carbocyclyl,
optionally substituted aralkyl, optionally substituted heteroaralkyl or ¨Rc-
(optionally substituted
heteroaryl); and the optionally substituted aryl, the optionally substituted
heterocyclyl, the
optionally substituted heteroaryl, the optionally substituted carbocyclyl, the
optionally
substituted aralkyl and the optionally substituted heteroaralkyl are
substituted with 1-6 R10;
wherein each R1 is independently selected from H, halogen, -CN,
-NO2, -CF3, alkyl, -SR6, -0R6, -NR6R7, -NR6C(=0)(alkyl), -
NR6C(=0)(cycloalkyl),
-NR6C(=0)(heterocycly1), -NR6C(=0)(ary1), -NR6C(=0)(heteroary1), -C(=0)NR6R7,
-C(=0)NR6(cycloalkyl), -C(=0)NR6(heterocycloalkyl), -C(=0)NR6(ary1),
-13-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
-C(=0)NR6(heteroary1), -NR6C(=0)NR6R7, -NR6C(=0)NR7(cycloalkyl),
-NR6C(=0)NR7(heterocycloalkyl), -NR6C(=0)NR7(ary1), -NR6C(=0)NR7(heteroary1),
-NR6C(=0)0(alkyl), -NR6C(=0)0(cycloalkyl), -NR6C(=0)0(heterocycloalkyl),
-NR6C(=0)0(ary1), -NR6C(=0)0(heteroary1), -NR6S02(alkyl), -NR6S02(cycloalkyl),
-NR6S02(heterocycloalkyl), -NR6S02(ary1), -NR6S02(heteroary1), -SO2NR6R7,
-SO2NR6(cycloalkyl), -SO2NR6(heterocycloalkyl), -SR6, -S02R6, -SO2NR6(ary1),
-SO2NR6(heteroary1), haloalkyl, aryl, heteroaryl, heterocyclyl and tetrazoyl.
[0019] In another aspect, provided herein are compounds of Formula (IIc), a
pharmaceutically
acceptable salt, solvate, polymorph, prodrug, metabolite, N-oxide,
stereoisomer, or isomer
thereof:
R12
(R4)nt
Y1 N--R3
0"\
A
(IIc)
wherein:
Yi is N, CH, or CR4;
R12 is -NR2R8 or -0R2;
R2 and R3 are each independently selected from H and optionally substituted
alkyl;
each R4 is independently selected from halogen, -CN, optionally substituted
alkyl, optionally
substituted alkoxy, and optionally substituted aryl;
R8 is optionally substituted alkyl, optionally substituted aryl, optionally
substituted aralkyl,
carbocyclyl, optionally substituted carbocyclylalkyl, optionally substituted
heteroaryl,
optionally substituted heteroarylalkyl, optionally substituted heterocyclyl,
optionally
substituted heterocyclylalkyl, ¨RbCOORa or ¨RbCONRaRa; or R2 and R8 taken
together
form an optionally substituted heterocycle with the nitrogen to which they are
attached;
each Ra is independently selected from H and alkyl;
Rb is a bond or alkylenyl;
n is 0, 1, 2, or 3; and
A is optionally substituted heteroaryl, optionally substituted aryl or
optionally substituted
heterocyclyl.
[0020] In some embodiments of a compound of Formula (IIc), Yi is N or CH. In
some
embodiments of a compound of Formula (IIc), Y1 is N. In some embodiments of a
compound of
-14-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
Formula (lie), A is optionally substituted heteroaryl, optionally substituted
aryl or optionally
substituted heterocyclyl; and the optionally substituted aryl, the optionally
substituted
heterocyclyl, the optionally substituted heteroaryl are substituted with 1-6
R10; wherein each R1
is independently selected from H, halogen, -CN, -NO2, -CF3, alkyl, -SR6, -0R6,
-NR6R7,
-NR6C(=0)(alkyl), - NR6C(=0)(cycloalkyl), -NR6C(=0)(heterocycly1), -
NR6C(=0)(ary1),
-NR6C(=0)(heteroary1), -C(=0)NR6R7, -C(=0)NR6(cycloalkyl), -
C(=0)NR6(heterocycloalkyl),
-C(=0)NR6(ary1), -C(=0)NR6(heteroary1), -NR6C(=0)NR6R7, -
NR6C(=0)NR7(cycloalkyl),
-NR6C(=0)NR7(heterocycloalkyl), -NR6C(=0)NR7(ary1), -NR6C(=0)NR7(heteroary1),
-NR6C(=0)0(alkyl), -NR6C(=0)0(cycloalkyl), -NR6C(=0)0(heterocycloalkyl),
-NR6C(=0)0(ary1), -NR6C(=0)0(heteroary1), -NR6S02(alkyl), -NR6S02(cycloalkyl),
-NR6S02(heterocycloalkyl), -NR6S02(ary1), -NR6S02(heteroary1), -SO2NR6R7,
-SO2NR6(cycloalkyl), -SO2NR6(heterocycloalkyl), -SR6, -S02R6, -SO2NR6(ary1),
-SO2NR6(heteroary1), haloalkyl, aryl, heteroaryl, heterocyclyl and tetrazoyl.
[0021] In some embodiments of a compound of Formula (lie), R8 is optionally
substituted alkyl,
optionally substituted aralkyl, carbocyclyl, optionally substituted
carbocyclylalkyl, optionally
substituted heteroarylalkyl, optionally substituted heterocyclyl, or
optionally substituted
heterocyclylalkyl.
[0022] Also provided herein is a pharmaceutical composition comprising a
compound of
Formulas (I), (Ia), (Ib), (Ic), (Id), (II), (Ha), (lIb), or (lie) or as
described above and below, or a
pharmaceutically acceptable salt, solvate, polymorph, prodrug, metabolite, N-
oxide,
stereoisomer, or isomer thereof, and a pharmaceutically acceptable excipient.
[0023] Further provided herein is a method to treat drug resistant and
persistent tuberculosis in a
mammal, the method comprising administering to the mammal a compound of
Formulas (I),
(Ia), (Ib), (Ic), (Id), (II), (Ha), (lIb), or (lie) or as described above and
below.
[0024] Also provided herein is a kit comprising: a biofilm formation media;
and instructions for
conducting a biofilm formation assay. The biofilm formation media may comprise
M63 salts
minimal medium. Also the biofilm formation media may comprise glucose,
casamino acid,
magnesium sulfate, calcium chloride, or any combination thereof. The kit may
further comprise
one or more agents. The one or more agents may comprise rifampicin (RIF),
TMC207,
isoniazid (NH), DMSO, or any combination thereof The one or more agents may be
a
chemical compound, protein, nucleic acid, or any combination thereof. The
protein may be an
antibody, enzyme, receptor, kinase, and/or proteinase. The one or more agents
may further be a
bactericide. The kit may further comprise one or more cells. The one or more
cells may be a
bacterial cell. The one or more cells may be a escherichia, staphylococcus,
and/or
pseudomonas. The one or more cells may also be a mycobacterium. The one or
more cells may
-15-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
be a Mycobacterium smegmatis cell. The instructions for conducting the biofilm
formation
assay comprise (i) instructions for culturing one or more cells; (ii)
instructions for contacting the
one or more cells with one or more agents; and (iii) instructions for assaying
the biofilm
formation of the one or more cells. The kit may further comprise one or more
plate readers.
The one or more plate readers may be a multilabel reader. The one or more
plate readers
comprises one or more detectors. The one or more detectors can enable
wavelength reading,
emission reading, barcode reading, or any combination thereof The one or more
plate readers
may be an EnVision Multilabel Reader.
[0025] Also provided here is a method comprising: a) culturing one or more
cells in a biofilm
formation media; b) contacting the one or more cells with one or more agents;
and c) assaying a
biofilm formation of the one or more cells. The method may further comprise
identifying the
one or more agents as a biofilm formation inhibitor based on the biofilm
formation assay. Also,
the method may further comprise identifying the one or more agents as a growth
inhibitor based
on the biofilm formation assay. The biofilm formation media may comprise M63
salts minimal
medium. The biofilm formation media may comprise glucose, casamino acid,
magnesium
sulfate, calcium chloride, or any combination thereof. The one or more agents
may comprise
rifampicin (RIF), TMC207, isoniazid (NH), DMSO, or any combination thereof The
one or
more agents may be a chemical compound, protein, nucleic acid, or any
combination thereof
The protein may be an antibody, enzyme, receptor, kinase, and/or proteinase.
The one or more
agents may also a bactericide. The one or more cells may be a bacterial cell.
The one or more
cells may be a escherichia, staphylococcus, and/or pseudomonas. The one or
more cells may be
a mycobacterium. The one or more cells may be a Mycobacterium smegmatis cell.
The
assaying of the biofilm formation may comprise one or more plate readers. The
one or more
plate readers may be a multilabel reader. The one or more plate readers may
comprise one or
more detectors. The one or more detectors may enable wavelength reading,
emission reading,
barcode reading, or any combination thereof The one or more plate readers may
be an
EnVision Multilabel Reader. The assaying of the biofilm formation may
comprise one or
more detectors for detecting a wavelength, emission, barcode, or any
combination thereof.
[0026] Further provided here is a method of treating a disease or condition in
a subject in need
thereof, the method comprising administering to the subject one or more
agents, wherein the one
or more agents are identified by the method for identifying the one or more
agents as a biofilm
formation inhibitor based on the biofilm formation assay. The disease or
condition may be a
pathogenic infection. The pathogenic infection may be a bacterial infection.
The bacterial
infection may be tuberculosis.
-16-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
[0027] Also provided herein is a composition comprising one or more agents
identified by the
method identified by the method for identifying the one or more agents as a
biofilm formation
inhibitor based on the biofilm formation assay. The composition may further
comprise an
excipient, adjuvant, buffer, oil, gel, solution, or any combination thereof.
[0028] Further provided herein is a system for identifying one or more agents
for treating a
bacterial infection in a subject in need thereof, the system comprising: a) a
biofilm formation
media; and b) a plate reader. The biofilm formation media may comprise M63
salts minimal
medium. The biofilm formation media comprises glucose, casamino acid,
magnesium sulfate,
calcium chloride, or any combination thereof. The sytem may further comprise
one or more
agents. The one or more agents may comprise rifampicin (RIF), TMC207,
isoniazid (NH),
DMSO, or any combination thereof The one or more agents may be a chemical
compound,
protein, nucleic acid, or any combination thereof The protein may be an
antibody, enzyme,
receptor, kinase, and/or proteinase. The one or more agents may be a
bactericide. The sytem
may further comprise one or more cells. The one or more cells may be a
bacterial cell. The one
or more cells may be a escherichia, staphylococcus, and/or pseudomonas. The
one or more cells
may be a mycobacterium. The one or more cells may be a Mycobacterium smegmatis
cell. The
one or more plate readers is a multilabel reader. The one or more plate
readers may comprise
one or more detectors. The one or more detectors may enable wavelength
reading, emission
reading, barcode reading, or any combination thereof. The one or more plate
readers may be an
EnVision Multilabel Reader.
INCORPORATION BY REFERENCE
[0029] All publications, patents, and patent applications mentioned in this
specification are
herein incorporated by reference to the same extent as if each individual
publication, patent, or
patent application was specifically and individually indicated to be
incorporated by reference.
BRIEF DESCRIPTION OF THE FIGURES
[0030] Figure 1 describes hit compounds from screen under biofilm culture
condition: (A)
chemical structures of active compounds; (B) TCA1 has selective activity
against mycobacteria;
(C) chemical structures of the affinity resin and the photo-affinity probe
used in pull-down
experiments.
[0031] Figure 2 describes in vitro activity of TCA1: (A) kill-kinetics of Mtb
by TCA1 (3.75
iug/m1) alone or in combination with NH (1 ug/m1) or RIF (2 ug/m1) in
comparison to RIF (2
iug/m1) and NH (1 ug/m1) alone in 7H9 medium; (B) activity of TCA1 (3.75
ug/m1) against a
RIF resistant Mtb strain and (C) NH resistant Mtb strain in 7H9 medium; (D)
kill-kinetics of a
XDR-TB strain by TCA1 (7.5 ug/m1) in 7H9 medium; (E) activity of TCA1 against
-17-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
non-replicating Mtb under nutrient starvation conditions; (F) qPCR analysis of
expression ratios
of selected genes from TCA1 (3.75 ug/m1) treated and untreated (0.1% DMSO)
Mtb.
[0032] Figure 3 describes in vivo efficacy of TCA1 in mouse models: (A) in an
acute Mtb
infection mouse model (2 week infection), followed by 4 weeks of drug
treatment, TCA1
showed significant bactericidal activity in lungs and (B) spleen both alone
(100 mg/kg) and in
combination (40 mg/kg) with NH (25 mg/kg) or RIF (10 mg/kg); The low activity
of RIF as a
mono-therapy in this model is consistent with what has been observed in a
previous study
[Makarov V, et al. (2009) Benzothiazinones kill Mycobacterium tuberculosis by
blocking
arabinan synthesis. Science 324:801-804]; (C) in a chronic TB infection mouse
model (4 week
low dose infection), followed by 4 weeks of drug treatment, TCA1 showed
activity in lungs and
(D) spleen both alone (100 mg/kg) and in combination (40 mg/kg) with NH (25
mg/kg) or RIF
(10 mg/kg) (p-value < 0.05). Mice were gavaged with TCA1 once per day and 5
days/week. RIF
and NH were administered in drinking water.
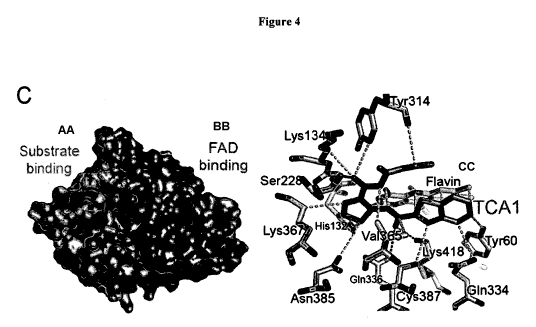
[0033] Figure 4 describes TCA1 is a DprEl inhibitor: (A) sequence alignment of
DprEl of M
smegatis and Mtb. A Y321C (Y314C in Mtb) mutation was identified in both M.
smegatis and
Mtb strains resistant to TCA1; (B) inhibition of DprEl by TCA1 in the cell-
free assay for DPA
production analyzed by TLC and autoradiography; M smegmatis membrane or cell
envelope
fractions were incubated with phospho-[14C]-ribose diphosphate and with 25
ug/m1 TCA1, or
BTZ043 (left) or TCA1 in a dose-response fashion (right). Both TCA1 and BTZ043
potently
inhibit conversion of the substrate, decaprenylphosphoryl ribose (DPR) to the
product,
decaprenylphosphoryl arabinose (DPA) by DprEl/DprE2 epimerase; (C) molecular
surface of
Mtb DprEl with the FAD domain in light blue and the substrate binding domain
in beige; the
surface areas in pale green and magenta indicate the positions of Cys387 and
Tyr314 (left);
non-covalent contacts between TCA1 and DprEl (right); residues within a 4 A-
radius of the
inhibitor (violet) are shown as sticks with FAD in yellow; dashed lines
indicate shortest contacts
(yellow = polar, orange = hydrophobic/van der Waals) between the residues and
the inhibitor;
Trp230, located within 4 A of the carbamate moiety of TCA1, has been omitted
for clarity.
[0034] Figure 5 describes TCA1 inhibits MoCo biosynthesis: (A) Mtb
overexpressing dprEl is
sensitive to TCA1 (7.5 1.1g/m1) under nutrient starvation conditions; (B) Mtb
overexpressing
dprEl conferred resistance to TCA1 (3.75 ug/m1) in 7H9 medium; in the
meantime, TCA1 acts
synergistically with NH (1 ug/m1) or RIF (2 ug/m1) against the same strain;
(C) - (F) MoCo
inhibition assay; HPLC profiles of MoCo form "A" dephospho standard and sample
extracted
from Mtb. Arrow indicates the position of MoCo form "A" dephospho; (C) MoCo
form "A"
dephospho standard from synthetic source; (D) extracted sample from Mtb in the
absence of
TCA1; (E) extracted sample from Mtb after treatment with TCA1 (7.5 ug/ml, 18
h); (F)
-18-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
extracted sample from Mtb in the absence of TCA1, spiked with a MoCo form "A"
dephospho
standard; (G) kill-kinetics of wild-type Mtb and Mtb overexpressing moeW by
7.5 g/m1TCA1
in media using nitrate as the only nitrogen source; (H) kill-kinetics of wild-
type Mtb and Mtb
overexpressing moeW by 7.5 g/m1TCA1 under nutrient starvation conditions.
[0035] Figure 6: In an acute TB infection mouse model (2 week infection),
followed by 4
weeks of gavage (once per day and 5 days /week), TCA1 showed bactericidal
activity both in
lungs (A) and spleen (B). The dose of TCA1, NH and RIF is 40 mg/kg, 25 mg/kg
and 10
mg/kg, respectively. RIF and NH were administered in drinking water.
[0036] Figure 7: DprEl is incubated with the drug of interest (different
concentration of TCA1)
for 15 min. BTZ-BODIPY is added and the sample is incubated for lh at 37 C.
Samples are then
analyzed by SDS-PAGE [Coomassie staining (top) and fluorescence scan
(bottom)]. Lane 1: 9
M DprEl, 20 M FAD, 20 M BTZ-BODIPY; Lane 2-8: 9 M DprEl, 20 M FAD, 20 M
BTZ-BODIPY, plus TCA1 (0, 50, 25, 12.5, 6.3, 3.1, 1.6 M).
[0037] Figure 8 describes chemical structure of Molybdenum cofactor.
[0038] Figure 9: In-gel fluorescence scanning. Probe-labeled MoeW was detected
by click
conjugation to a rohodmine-azide reporter tag, followed by SDS PAGE and in-gel
fluorescence
scanning. Lane 1&2, 3&4, 5&6, 7&8 are series of 2-fold dilution of the E. coli
lysate. Lane 1, 3,
5, and 7 are lysate of E. coli cells without IPTG induction. Lane 2, 4, 6, and
8 are lysate of E.
coli cells with IPTG induction. In lane 4, 6, and 8, a band with the size of
MoeW is present,
while it is absent in non-induced samples (pointed by red arrows). The band is
not very strong
because most of MoeW was found in inclusion body, not in soluble fraction,
when
overexpressed in E. coli.
DETAILED DESCRIPTION OF THE INVENTION
[0039] MDR (Multi-Drug-Resistant) and XDR (eXtensively Drug-Resistant) Mtb
strains are
becoming widespread resulting in high failure rates despite the use of second
and third line
antibiotics and longer treatment times (up to 2 years). A new drug in drug
regimen should
shorten chemotherapy and overcome the emergence of resistance to have a real
impact on TB.
[0040] Although numerous cell-based screens against Mtb have been performed,
to date most
screens are designed to identify molecules that are active against rapidly
growing mycobacteria
under growth-optimal laboratory conditions, and are inherently biased toward
identifying
bactericidal or bacteriostatic compounds against replicating Mtb [Pethe K, et
al. (2010) A
chemical genetic screen in Mycobacterium tuberculosis identifies carbon-source-
dependent
growth inhibitors devoid of in vivo efficacy. Nat Commun 1:57]. However, it is
becoming
apparent that the culture conditions used in a screen very much affect our
ability to identify
-19-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
inhibitors that will be active in vivo [(a) Pethe K, et al. (2010) A chemical
genetic screen in
Mycobacterium tuberculosis identifies carbon-source-dependent growth
inhibitors devoid of in
vivo efficacy. Nat Commun 1:57; (b) Stanley SA, et al. (2012) Identification
of novel inhibitors
of M. tuberculosis growth using whole cell based high-throughput screening.
ACS Chem Biol
7:1377-1384]. This issue is a particular concern in the development of drugs
targeting persistent
Mtb. Both target- and cell-based screens have been carried out under
conditions that are thought
to simulate those Mtb encounters during a chronic infection [(a) Mak PA, et
al. (2012) A
high-throughput screen to identify inhibitors of ATP homeostasis in non-
replicating
Mycobacterium tuberculosis. ACS Chem Biol 7:1190-1197; (b) Gold B, et al.
(2012)
Nonsteroidal anti-inflammatory drug sensitizes Mycobacterium tuberculosis to
endogenous and
exogenous antimicrobials. Proc Natl Acad Sci USA 109:16004-16011]. For
example, it has
been shown that oxygen deprivation or nutrient starvation in Mtb cultures
triggers metabolic
changes resulting in non-replicating, phenotypically drug resistant bacilli in
vitro [(a) Wayne LG
& Hayes LG (1996) An in vitro model for sequential study of shiftdown of
Mycobacterium
tuberculosis through two stages of nonreplicating persistence. Infect. Immun.
64:2062-2069; (b)
Wayne LG & Sohaskey CD (2001) Nonreplicating persistence of Mycobacterium
tuberculosis.
Annu Rev Microbiol 55:139-163]. Indeed, anaerobic Mtb cultures are resistant
to isoniazid
(NH) and partly resistant to rifampicin (RIF), but highly sensitive to
pyrazinamide (PZA)
[Mitchison DA & Coates AR (2004) Predictive in vitro models of the sterilizing
activity of
anti-tuberculosis drugs. Curr Pharm Des 10:3285-3295], underscoring the
differing drug
sensitivities of Mtb in different metabolic states. Given the lack of clear
consensus on cell
culture conditions that best reflect the in vivo biology of Mtb, a screen was
carried out based on
in vitro biofilm formation in the hope of identifying compounds with new
mechanism(s) of
action that may be effective against drug resistant and persistent Mtb. The
molecule TCA1,
which was identified through this screen, not only showed bactericidal
activity against both
replicating (wild type and drug-resistant) and non-replicating Mtb, but also
was efficacious in
acute and chronic Mtb infection mouse models, both alone and in combination
with NH or RIF.
Moreover, genetic and biochemical studies showed that TCA1 functions by
inhibiting two
distinct biosynthetic pathways with concomitant down-regulation of genes known
to be involved
in mycobacterial persistence.
A high throughput screen under biofilm culture conditions
[0041] Pathogenic Mtb is not conducive to high-throughput screens involving
automation as
these experiments would need to be carried out in a biosafety level-3
facility. However,
Mycobacterium smegmatis (M smegmatis), a saprophytic, non-pathogenic
mycobacteria that
also forms in vitro biofilms [Ojha A, et al. (2005) GroELl: a dedicated
chaperone involved in
-20-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
mycolic acid biosynthesis during biofilm formation in mycobacteria. Cell
123:861-873] which
induce drug-tolerance [Teng R & Dick T (2003) Isoniazid resistance of
exponentially growing
Mycobacterium smegmatis biofilm culture. FEMS Microbiol Lett 227:171-174], is
amenable to
high-throughput screening. Therefore, the primary cell-based screen was based
upon the
inhibition of biofilm formation in M. smegmatis. It was found that the in
vitro biofilm,
visualized as a pellicle that grows at the air-liquid interface, covered the
whole surface of the
well in a 384 well plate once formed, affording a high signal to noise ratio
for positive hits. A
diverse library of 70,000 heterocycles was screened (Supplemental information)
which afforded
17 compounds with minimum inhibitory concentrations (MIC50s) of less than 10
ILIM in a
biofilm inhibition assay. Two classes of compounds were identified: one class
inhibited the
growth of mycobacteria under biofilm culture conditions, while the second
class inhibited the
formation of biofilms without significant growth inhibition. These hit
compounds from the
primary screen were then tested for their ability to inhibit in vitro biofilm
growth in virulent Mtb
H37Rv using a scaled-up 24-well assay as previously described [Ojha AK, et al.
(2008) Growth
of Mycobacterium tuberculosis biofilms containing free mycolic acids and
harbouring
drug-tolerant bacteria. Mol Microbiol 69:164-174]. Two compounds, C7 and TCA1
were found
to also inhibit biofilm formation by Mtb H37Rv (Fig. 1A). TCA1, which
displayed potent
inhibitory activity against Mtb under both biofilm and planktonic culture
conditions, was
selected for further studies.
In vitro bactericidal activity
[0042] TCA1 showed selective inhibitory activity against bacterial growth - it
is inactive against
E. coli, S. aureus, and P. aeruginosa. The target for its bactericidal
activity may be specific to
the genus Mycobacterium (Fig. 1B). Interestingly, the activities of TCA1
against M. smegmatis,
M. bovis BCG, and Mtb were 20-150 fold higher in biofilm medium (MIC50 0.03
ug/ml, 0.04
iug/m1 and 0.01 ug/ml, respectively) than in 7H9 medium (MIC50 4.5 ug/ml, 3
ug/m1 and 0.19
iug/ml, respectively). This observation underscored the variable efficacy of a
drug in different
growth media [Stanley SA, et al. (2012) Identification of novel inhibitors of
M. tuberculosis
growth using whole cell based high-throughput screening. ACS Chem Biol 7:1377-
1384], which
in part may result from the expression of distinct target genes and metabolic
pathways. TCA1
was bactericidal with a MIC99 of 2.1 ug/m1 in solid medium. To evaluate the
bactericidal
activity of TCA1 against Mtb in comparison to the two frontline TB drugs, NH
and RIF, a 21-
day kinetic killing assay was performed using comparable levels of each of the
three drugs (20X
MIC50 of each of the three drugs). TCA1 was active by itself against
exponentially growing
virulent Mtb in 7H9 media, with a more than 3 log reduction in the number of
bacilli over a
treatment period of 21 days. Treatment with NH or RIF resulted in a comparable
drop in CFU
-21-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
over the first seven days of treatment, but the subsequent outgrowth of
bacilli detected in NH
and RIF treated cultures was absent in TCA1 treated cultures. Furthermore,
TCA1 in
combination with either RIF or NH was able to sterilize a Mtb culture in ¨ 3
weeks (Fig. 2A);
removal of drug after 3 weeks of combination drug treatment did not lead to
Mtb outgrowth.
[0043] The activity of TCA1 on drug resistant Mtb was also tested. RIF
resistance is a marker
for MDR-TB (90% of RIF resistant strains are also MDR) and typically requires
18-24 months
of treatment. TCA1 by itself was active against a clinical strain that is
resistant to RIF (due to a
mutation in rpoB) and, more importantly, in combination with NH, sterilized
the cultures within
one week (Fig. 2B). Removal of both drugs after 3 weeks of treatment did not
result in
outgrowth. TCA1 was also found to be bactericidal against a strain with a
mutation in katG
(resulting in resistance to NH) (Fig. 2C). Finally, TCA1 was tested against an
XDR-TB strain,
mc28013, which is resistant to 10 TB drugs, including all front line drugs.
TCA1 showed potent
bactericidal activity against the XDR-TB strain (5 log CFU reduction in 3
weeks) (Fig. 2D).
Given the lack of cross resistance to TCA1 in any of these drug-resistant
strains, TCA1 may
function by a distinct mechanism.
[0044] The activity of TCA1 was tested against non-replicating Mtb in a
nutrient starvation
assay, a widely used in vitro model of the Mtb dormancy phenotype [(a)
Gengenbacher M, Rao
SP, Pethe K, & Dick T (2010) Nutrient-starved, non-replicating Mycobacterium
tuberculosis
requires respiration, ATP synthase and isocitrate lyase for maintenance of ATP
homeostasis and
viability. Microbiology 156:81-87; (b) Betts JC, Lukey PT, Robb LC, McAdam RA,
& Duncan
K (2002) Evaluation of a nutrient starvation model of Mycobacterium
tuberculosis persistence
by gene and protein expression profiling. Mol Microbiol 43:717-731]. Under
these conditions,
Mtb enters a non-replicating state and has been shown to become tolerant to
drugs, without
acquiring heritable drug-resistance inducing mutations [Betts JC, Lukey PT,
Robb LC, McAdam
RA, & Duncan K (2002) Evaluation of a nutrient starvation model of
Mycobacterium
tuberculosis persistence by gene and protein expression profiling. Mol
Microbiol 43:717-731].
TCA1 showed bactericidal activity against non-replicating Mtb at a
concentration of 7.5 ug/m1
(40x MIC50 in 7H9 medium), reducing CFU by 3 logs in three weeks (Fig. 2E).
Under the same
assay conditions, RIF (40x MIC50 in 7H9 medium) showed less bactericidal
activity than TCA1.
The activity of TCA1 was also tested in an intra-macrophage cell culture
system to determine
whether it is active against intracellular mycobacteria, since in the mouse
model of infection and
in humans, Mtb is believed to reside mainly in macrophages. TCA1 was found to
be quite potent
in an intracellular CFU assay with a MIC50 of 0.6 ug/m1 [MIC50 (RIF) = 2.7
ug/ml, MIC50 (NH)
= 0.2 ug/m1]. Finally, TCA1 showed no cytotoxicity against five mammalian cell
lines (Huh7,
-22-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
293T, K562, HepG2, and Vero cells) at the highest concentration tested (100 M
for Vero cells
and 25 M for the others); and a hERG assay indicated that TCA1 had no
activity at 30 M).
TCA1 is efficacious in acute and chronic Mtb infection mouse models
[0045] The activity of TCA1 was examined in a mouse model of Mtb infection.
The physical
and pharmacokinetic (PK) characteristics of TCA1 were determined. It was
stable to proteolytic
activity in human or mouse plasma for up to 4 hours. Moreover, a GSH trapping
assay indicated
no GSH adduct was formed, and TCA1 had no inhibitory activity against four CYP
enzymes.
After intra-venous (IV) administration, TCA1 exhibited a low clearance (CL)
and steady-state
volume of distribution (Vss) with an elimination half life of 0.73 hour.
Following oral
administration of 20 and 50 mg/kg in solution formulation, TCA1 showed a high
Cmax (2122 and
5653 nM, respectively), moderate exposure with oral bioavailability ranging
from 19% - 46%
and a half life of 1.8 hours.
[0046] The in vivo efficacy experiments were initially performed in an acute
infection model
with a low dose of TCA1. BALB/c mice were infected with a low dose of Mtb
H37Rv (-200
bacilli). Two weeks after infection, mice were treated with TCA1 (40 mg/kg),
NH (25 mg/kg)
or RIF (10 mg/kg) for 4 weeks [dosed once per day, 5 days per week;]. The
doses of NH and
RIF were consistent with those published in the literature [Makarov V, et al.
(2009)
Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan
synthesis. Science
324:801-804]. After 4 weeks of treatment with TCA1 - the CFU dropped 0.5 log
in lung and 1.5
logs in spleen, which was comparable to the potency of RIF, but less than that
of NH. The gross
pathology and histopathology also showed significant improvement in both
tissues. The in vivo
efficacy of TCA1 (40 mg/kg) was also tested in combination with NH (25 mg/kg)
or RIF (10
mg/kg). In the acute infection model, TCAl+INH and TCAl+RIF showed nearly a 2
and 3 log
CFU reduction in lung, respectively, and a more than 3 log CFU reduction in
spleen (Fig. 3A
and 3B). There is a greater CFU drop in the lungs of mice treated by the
combination of TCA1
and NH relative to the combination of NH and RIF.
[0047] The compound was also tested in a mouse model of chronic TB infection.
Mice were
challenged with a low dose aerosol infection and treatment was initiated 4
weeks after infection.
Similar combination treatments were efficacious in the chronic infection model
as well (Fig. 3C
and 3D). Because the mice were able to tolerate 40 mg/kg TCA1, the dose was
increased to 100
mg/kg using a similar protocol as in the acute infection model. After 4 weeks,
the CFU dropped
nearly 2 log units in lungs and more than 3 log units in spleen, demonstrating
that the in vivo
bactericidal activity of TCA1 is dose-dependent (Fig. 3A and 3B). The mice
again showed no
obvious adverse effects or weight loss after 4 weeks of treatment. The
compound was tested in
the chronic infection model at 100 mg/kg. Again, TCA1 demonstrated efficacy in
both lung (1
-23-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
log CFU reduction) and spleen (1.4 log CFU reduction) (Fig. 3C and 3D). These
results
demonstrate that the in vitro efficacy of TCA1 is recapitulated in vivo. Thus,
in vitro
mycobacterial biofilm may be a useful phenotype to identify novel compounds
effective against
Mtb in vivo either alone or in combination with existing TB drugs.
Mechanism of action studies of TCA
[0048] To gain insight into the mechanism of action of TCA1, Mtb H37Rv was
treated with
TCA1 (3.75 ug/m1) in 7H9 media and carried out genome-wide transcriptional
analysis. Similar
to NH and ethambutol [Boshoff HI, et al. (2004) The transcriptional responses
of
Mycobacterium tuberculosis to inhibitors of metabolism: novel insights into
drug mechanisms of
action. J Biol Chem 279:40174-40184], cell wall and fatty acid biosynthetic
genes were affected
by TCA1 treatment, perhaps because TCA1 may interfere with these pathways.
Unlike other
known TB drugs, 10 of the 86 genes differentially downregulated compared to
the DMSO
control are genes previously implicated in TB dormancy, stress response, and
RIF susceptibility.
These include rv3130c - rv3134c, fdxA, and hspX (members of the dos regulon),
cysD, and
rv3288c-rv3290c (members of the sigF regulon). The microarray results were
confirmed by
qPCR (Fig. 2F). Most of these genes are part of the dormancy regulon
controlled by dosR
[Voskuil MI, et al. (2003) Inhibition of respiration by nitric oxide induces a
Mycobacterium
tuberculosis dormancy program. J Exp Med 198:705-713], and are upregulated
under hypoxic
conditions or by nitric oxide exposure. For example, fdxA, a low-redox-
potential electron
carrier, was highly upregulated in Mtb under hypoxic conditions [Muttucumaru
DG, Roberts G,
Hinds J, Stabler RA, & Parish T (2004) Gene expression profile of
Mycobacterium tuberculosis
in a non-replicating state. Tuberculosis (Edinb) 84:239-246], but
significantly downregulated (>
20 fold) in response to TCA1 treatment. Likewise, rv3130c was induced (> 300
fold) under
multiple-stress conditions [Deb C, et al. (2009) A novel in vitro multiple-
stress dormancy model
for Mycobacterium tuberculosis generates a lipid-loaded, drug-tolerant,
dormant pathogen.
PLoS One 4:e6077], but was downregulated (>30 fold) by TCA1. This
downregulation of genes
involved in dormancy and drug tolerance appears to be unique to TCA1. As such,
TCA1 may
potentially sensitize Mtb to killing by antibiotics.
[0049] To further explore the mechanism of action of TCA1, a TCA1 resistant
mutant that
carries the cosmid (MSMEG 6379 - MSMEG 6384) was isolated by selection of M
smegmatis,
transformed with a genomic cosmid library and grown in biofilm formation
medium.
Overexpression of each gene in this cosmid revealed that MSMEG 6382, which is
homologous
to rv3790 in the Mtb genome, confered high-level resistance to TCA1 (>20x
MIC50) in both M.
smegmatis and Mtb. Spontaneous resistant mutants of M. smegmatis and Mtb were
isolated, even
though the spontaneous mutation rate to TCA1 resistance was extremely low (10-
8 - 10-9). Whole
-24-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
genome sequencing of the genomic DNA of the resistant mutants revealed that
they all have a
single point mutation resulting in the amino acid replacement Tyr321Cys in
MSMEG 6382 and
Tyr314Cys in rv3790 (Fig. 4A). rv3790, encodes DprEl, a component of the
essential
decaprenylphosphoryl-B-D-ribofuranose 2'-epimerase (DprEl/DprE2) required for
cell wall
arabinan biosynthesis. Indeed, TCA1 suppressed the activity of M. smegmatis
DprEl in
membrane and cell envelope enzymatic fractions in a dose-dependent manner
(Fig. 4B). DprEl
was previously identified as the target of the benzothiazinones (BTZ) and a
nitro-triazole
molecule [(a) Stanley SA, et al. (2012) Identification of novel inhibitors of
M tuberculosis
growth using whole cell based high-throughput screening. ACS Chem Biol 7:1377-
1384; (b)
Makarov V, et al. (2009) Benzothiazinones kill Mycobacterium tuberculosis by
blocking
arabinan synthesis. Science 324:801-804]. Both scaffolds contain active nitro-
moieties and are
believed to covalently modify Cys387 upon activation. A competitive binding
assay was
peformed using a fluorescently labeled BTZ analog. TCA1 potently competed with
BTZ in
binding to DprEl, indicating that the binding site of TCA1 may overlap with
BTZ (Fig. 7).
However, TCA1 does not have an active nitro-moiety and the Tyr314Cys mutant
strain that is
resistant to TCA1 is sensitive to BTZ. As such, the binding mechanism of TCA1
may be
different from these nitro-heterocycles.
[0050] To determine the molecular basis by which TCA1 inhibits DprEl, the
crystal structure of
the enzyme bound to TCA1 was determined. The overall structure of the DprEl-
TCA1 complex
was largely unaltered compared to that of the ligand-free protein with the
same crystal symmetry
[Batt SM, et al. (2012) Structural basis of inhibition of Mycobacterium
tuberculosis DprEl by
benzothiazinone inhibitors. Proc Natl Acad Sci U S A 109:11354-11359]. The
enzyme, which is
structurally related to the vanillyl-alcohol oxidase family of flavoproteins
[Mattevi A, Fraaije
MW, Coda A, & van Berkel WJ (1997) Crystallization and preliminary X-ray
analysis of the
flavoenzyme vanillyl-alcohol oxidase from Penicillium simplicissimum. Proteins
27:601-603],
consists of an FAD-binding and a substrate binding domain with the flavin
moiety of FAD
positioned at the interface between the two domains (Fig. 4C, left). The
substrate binding
domain includes two disordered loop regions (residues 269-283, 316-330) that
leave the active
site open and accessible for inhibitors. TCA1 was bound in the central cavity
of the enzyme,
adjacent to the isoalloxazine ring of FAD, in a boomerang-like conformation
with the thiophene
moiety inserted deeply into the bottom of the active site (Fig. 4C, right).
The benzothiazole ring
was oriented roughly parallel to the isoalloxazine of FAD. Non-covalent
interactions between
TCA1 and the enzyme were dominated by hydrophobic and van der Waals
interactions (Fig.
4C), with the flavin contributing a large fraction of the total contact
surface. Polar contacts were
sparse, but include the H-bonds between the carboxamido group and thiazole
nitrogen of TCA1
-25-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
and Nc of Lys418 (3.0 A and 3.1 A, respectively). The carbamate moiety made
van der Waals
interactions with the phenyl ring of Tyr314, consistent with the observation
that substituting this
tyrosine with cysteine renders DprEl insensitive to TCA1. Superimposition of
the structures of
DprEl bound to the BTZ analog (CT325) [Batt SM, et al. (2012) Structural basis
of inhibition of
Mycobacterium tuberculosis DprEl by benzothiazinone inhibitors. Proc Natl Acad
Sci U S A
109:11354-11359] and TCA1 showed that the binding sites of these two
inhibitors overlap
significantly.
[0051] Given the above results, DprEl may be a relevant target for the
bactericidal activity of
TCA1 against replicating bacteria, similar to BTZ. However, there are some
clear distinctions
between TCA1 and BTZ. First, BTZ is not active against non-replicating Mtb
[Makarov V, et al.
(2009) Benzothiazinones kill Mycobacterium tuberculosis by blocking arabinan
synthesis.
Science 324:801-804], while TCA1 is active against replicating and non-
replicating Mtb.
Secondly, the gene expression profiles of Mtb treated by two compounds are
also very different
- TCA1 downregulates persistence genes that are usually upregulated in Mtb
dormant models,
whereas BTZ does not [Makarov V, et al. (2009) Benzothiazinones kill
Mycobacterium
tuberculosis by blocking arabinan synthesis. Science 324:801-804]. The Mtb
strain
overexpressing DprEl is resistant to TCA1 in 7H9 medium, but still sensitive
to TCA1 in the
nutrient starvation model (Fig. 5A). Moreover, TCA1 still potentiates NH or
RIF on this DprEl
overexpressing strain (Fig. 5B). Thus, TCA1 could potentially act on an
additional
mycobacterial target.
[0052] Because TCA1 had diminished activity against the DprEl(Y314C) mutant in
normal
growth medium, it is possible that a second TCA1 target is not essential for
Mtb growth under
conditions of optimal growth. This makes the selection of relevant mutants
more difficult, and
therefore affinity-based methods were used to identify additional potential
targets. Among a
group of analogs of TCA1, it was found that a pyridyl analog, TCA17, had very
similar in vitro
activity to that of TCA1. TCA17 was immobilized on a resin through a linker
moiety (TCAP1;
Fig. 1C) and used in a pull-down experiment with cell lysates from Mtb. A 35
kDa band was
identified on an SDS-polyacrylamide gel electrophoresis gel (SDS-PAGE) after
silver staining
which disappeared in the presence of 50 uM TCA1 as competitor. Mass
spectrometry identified
the band as MoeW, a protein involved in the biosynthesis of the molybdenum
cofactor (MoCo)
(Fig. 8) with homologs in only a few bacterial genomes. To confirm the binding
of TCA1 to
MoeW, moeW was overexpressed in E. coli (which lacks a moeW gene homolog in
its genome)
and treated this strain with a photoaffinity probe analogous to TCA1 (TCAP2;
Fig. 1C) followed
by UV irradiation and cell lysis. As shown in Figure 9, a band with the size
of MoeW was
present on a SDS-PAGE gel for the sample from the moeW-induced strain and
absent in the
-26-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
un-induced control sample. These results demonstrate that TCA1 scaffold
directly binds to
MoeW.
[0053] MoeW is predicted to contain a FAD/NAD binding domain by protein
sequence analysis,
but its function has yet to be determined. The gene encoding MoeW is only
conserved in Mtb
and BCG, not in M. smegmatis or other mycobacterial species, although it is
homologous to
moeB, another gene involved in MoCo biosynthesis pathway and conserved in all
mycobacteria
species and many other bacteria [Williams MJ, Kana BD, & Mizrahi V (2011)
Functional
analysis of molybdopterin biosynthesis in mycobacteria identifies a fused
molybdopterin
synthase in Mycobacterium tuberculosis. J Bacteriol 193:98-106]. All
molybdenum-utilizing
enzymes identified to date contain MoCo. MoCo is essential for the nitrate
respiratory and
assimilatory function of Mtb nitro-reductase. Some of these nitro-reductases
have been found to
be involved in the response of Mtb to hypoxia and nitric oxide [(a) Williams
MJ, Kana BD, &
Mizrahi V (2011) Functional analysis of molybdopterin biosynthesis in
mycobacteria identifies a
fused molybdopterin synthase in Mycobacterium tuberculosis. J Bacteriol 193:98-
106; (b) Malm
S, et al. (2009) The roles of the nitrate reductase NarGHJI, the nitrite
reductase NirBD and the
response regulator GlnR in nitrate assimilation of Mycobacterium tuberculosis.
Microbiology
155:1332-1339]. To determine if TCA1 can block the biosynthesis of MoCo, cell
extracts were
analyzed from Mtb treated with TCA1 by detection of dephosphorylated MoCo form
"A" using
HPLC with fluorescence detection. Indeed, TCA1 (7.5 g/ml) completely
abolished the
formation of MoCo in Mtb (Fig. 5C-5F). It is known that MoCo is indispensable
for nitrate
assimilation by Mtb and thus essential for Mtb to survive in media which uses
nitrate as the only
nitrogen source (designated as nitrate media). An Mtb strain overexpressing
moeW was
generated, and it was found to confer resistance to TCA1 in nitrate media over
30 days of
treatment (Fig. 5G). These results clearly demonstrate that TCA1 asserts its
activity against Mtb
by inhibition of MoCo biosynthesis through interaction with MoeW. The Mtb
strain
overexpressing moeW conferred resistance to TCA1 in nutrient starvation model
over 21 days of
treatment as well (Fig. 5H), but the lower level of resistance to TCA1 in the
nutrient starvation
model than in nitrate media indicates that the mechanism of action of TCA1 may
be more
complicated under the former condition. Nevertheless, the biochemical and
genetic results
clearly demonstrate MoeW is a relevant target of TCA1.
[0054] A novel cell-based screen was developed involving the growth of
mycobacteria as an in
vitro biofilm (a pellicle). The natural mode of growth of Mtb in liquid
culture in the absence of
detergent is as a pellicle at the liquid-air interface. Indeed, BCG is grown
as a pellicle for
vaccine production. This assay allowed for the identification of a potent
inhibitor TCA1 against
both replicating and non-replicating Mtb as well as drug-resistant Mtb. TCA1
functions by a
-27-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
unique mechanism involving downregulation of persistence genes and inhibition
of both cell
wall and MoCo biosynthesis. Moreover, TCA1 showed excellent in vivo efficacy
in both acute
and chronic TB infection mouse models. This compound served as a lead for the
development of
a new class of drugs against persistent and drug resistant Mtb. Indeed,
compounds presented
herein were identified with activities under both aerobic and anaerobic
conditions. This work
underscores the power of cell-based phenotypic screens to uncover molecules
with novel
mechanisms of action that provide new approaches to the treatment of human
disease.
Definitions
[0055] In the following description, certain specific details are set forth in
order to provide a
thorough understanding of various embodiments. However, one skilled in the art
will
understand that the invention may be practiced without these details. In other
instances,
well-known structures have not been shown or described in detail to avoid
unnecessarily
obscuring descriptions of the embodiments. Unless the context requires
otherwise, throughout
the specification and claims which follow, the word "comprise" and variations
thereof, such as,
"comprises" and "comprising" are to be construed in an open, inclusive sense,
that is, as
"including, but not limited to." Further, headings provided herein are for
convenience only and
do not interpret the scope or meaning of the claimed invention.
[0056] Reference throughout this specification to "one embodiment" or "an
embodiment"
means that a particular feature, structure or characteristic described in
connection with the
embodiment is included in at least one embodiment. Thus, the appearances of
the phrases "in
one embodiment" or "in an embodiment" in various places throughout this
specification are not
necessarily all referring to the same embodiment. Furthermore, the particular
features,
structures, or characteristics may be combined in any suitable manner in one
or more
embodiments. Also, as used in this specification and the appended claims, the
singular forms
"a," "an," and "the" include plural referents unless the content clearly
dictates otherwise. It
should also be noted that the term "or" is generally employed in its sense
including "and/or"
unless the content clearly dictates otherwise.
[0057] The terms below, as used herein, have the following meanings, unless
indicated
otherwise:
[0058] "Amino" refers to the -NH2 radical.
[0059] "Cyano" or "nitrile" refers to the -CN radical.
[0060] "Hydroxy" or "hydroxyl" refers to the -OH radical.
[0061] "Nitro" refers to the -NO2 radical.
[0062] "Oxo" refers to the =0 substituent.
-28-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
[0063] "Oxime" refers to the =N-OH substituent.
[0064] "Thioxo" refers to the =S substituent.
[0065] "Alkyl" refers to a straight or branched hydrocarbon chain radical, has
from one to thirty
carbon atoms, and is attached to the rest of the molecule by a single bond.
Alkyls comprising
any number of carbon atoms from 1 to 30 are included. An alkyl comprising up
to 30 carbon
atoms is referred to as a Ci-C30 alkyl, likewise, for example, an alkyl
comprising up to 12 carbon
atoms is a C1-C12 alkyl. Alkyls (and other moieties defined herein) comprising
other numbers of
carbon atoms are represented similarly. Alkyl groups include, but are not
limited to, C1-C30
alkyl, Ci-C20 alkyl, C1-C15 alkyl, Ci-Cio alkyl, Ci-C8 alkyl, C1-C6 alkyl, C1-
C4 alkyl, C1-C3
alkyl, C1-C2 alkyl, C2-C8 alkyl, C3-C8 alkyl and C4-C8 alkyl. Representative
alkyl groups
include, but are not limited to, methyl, ethyl, n-propyl, 1-methylethyl (iso-
propyl), n-butyl,
i-butyl, s-butyl, n-pentyl, 1,1-dimethylethyl (t-butyl), 3-methylhexyl, 2-
methylhexyl, vinyl, allyl,
propynyl, and the like. Alkyl comprising unsaturations include alkenyl and
alkynyl groups.
Unless stated otherwise specifically in the specification, an alkyl group may
be optionally
substituted as described below.
[0066] "Alkylene", "alkylenyl" or "alkylene chain" refers to a straight or
branched divalent
hydrocarbon chain, as described for alkyl above. Unless stated otherwise
specifically in the
specification, an alkylene group may be optionally substituted as described
below.
[0067] "Alkoxy" refers to a radical of the formula -0Ra. where Ra is an alkyl
radical as defined.
Unless stated otherwise specifically in the specification, an alkoxy group may
be optionally
substituted as described below.
[0068] "Alkenyl" refers to a straight or branched hydrocarbon chain radical
group consisting
solely of carbon and hydrogen atoms, containing at least one carbon-carbon
double bond, and
having from two to twelve carbon atoms. In certain embodiments, an alkenyl
comprises two to
eight carbon atoms. In other embodiments, an alkenyl comprises two to four
carbon atoms. The
alkenyl is attached to the rest of the molecule by a single bond, for example,
ethenyl (i.e., vinyl),
prop-l-enyl (i.e., allyl), but-l-enyl, pent-l-enyl, penta-1,4-dienyl, and the
like. Unless stated
otherwise specifically in the specification, an alkenyl group may be
optionally substituted as
described below.
[0069] "Alkenylene" or "alkenylenyl" refers to a straight or branched
hydrocarbon chain
consisting solely of carbon and hydrogen atoms, containing at least one carbon-
carbon double
bond, and having from two to twelve carbon atoms, as described for alkenyl
above. Unless
stated otherwise specifically in the specification, an alkenylene group may be
optionally
substituted as described below.
-29-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
[0070] "Alkynyl" refers to a straight or branched hydrocarbon chain radical
group consisting
solely of carbon and hydrogen atoms, containing at least one carbon-carbon
triple bond, having
from two to twelve carbon atoms. In certain embodiments, an alkynyl comprises
two to eight
carbon atoms. In other embodiments, an alkynyl has two to four carbon atoms.
The alkynyl is
attached to the rest of the molecule by a single bond, for example, ethynyl,
propynyl, butynyl,
pentynyl, hexynyl, and the like. Unless stated otherwise specifically in the
specification, an
alkynyl group may be optionally substituted as described below.
[0071] "Aryl" refers to a radical derived from a hydrocarbon ring system
comprising hydrogen,
6 to 30 carbon atoms and at least one aromatic ring. The aryl radical may be a
monocyclic,
bicyclic, tricyclic or tetracyclic ring system, which may include fused or
bridged ring systems.
Aryl radicals include, but are not limited to, aryl radicals derived from the
hydrocarbon ring
systems of aceanthrylene, acenaphthylene, acephenanthrylene, anthracene,
azulene, benzene,
chrysene, fluoranthene, fluorene, as-indacene, s-indacene, indane, indene,
naphthalene,
phenalene, phenanthrene, pleiadene, pyrene, and triphenylene. Unless stated
otherwise
specifically in the specification, the term "aryl" or the prefix "ar-" (such
as in "aralkyl") is meant
to include aryl radicals that are optionally substituted.
[0072] "Aralkyl" refers to a radical of the formula ¨Rd-aryl where Rd is an
alkylene chain as
defined above, for example, methylene, ethylene, and the like. Unless stated
otherwise
specifically in the specification, the alkylene chain part of the aralkyl
radical is optionally
substituted as described above for an alkylene chain. Unless stated otherwise
specifically in the
specification, the aryl part of the aralkyl radical is optionally substituted
as described above for
an aryl group.
[0073] "Aralkoxy" refers to a radical bonded through an oxygen atom of the
formula -0-Rd-aryl
where Rd is an alkylene chain as defined above, for example, methylene,
ethylene, and the like.
Unless stated otherwise specifically in the specification, the alkylene chain
part of the aralkyl
radical is optionally substituted as described above for an alkylene chain.
Unless stated
otherwise specifically in the specification, the aryl part of the aralkyl
radical is optionally
substituted as described above for an aryl group.
[0074] "Cycloalkyl", "carbocycly1" or "carbocycle" refers to a stable, non-
aromatic, monocyclic
or polycyclic carbocyclic ring, which may include fused or bridged ring
systems, which is
saturated or unsaturated. Representative cycloalkyls or carbocycles include,
but are not limited
to, cycloalkyls having from three to fifteen carbon atoms, from three to ten
carbon atoms, from
three to eight carbon atoms, from three to six carbon atoms, from three to
five carbon atoms, or
three to four carbon atoms. Monocyclic cycloalkyls or carbocycles include, for
example,
cyclopropyl, cyclobutyl, cyclopentyl, cyclohexyl, cycloheptyl, and cyclooctyl.
Polycyclic
-30-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
cycloalkyls or carbocycles include, for example, adamantyl, norbornyl,
decalinyl,
bicyclo[3.3.0]octane, bicyclo[4.3.0]nonane, cis-decalin, trans-decalin,
bicyclo[2.1.1]hexane,
bicyclo[2.2.1]heptane, bicyclo[2.2.2]octane, bicyclo[3.2.2]nonane, and
bicyclo[3.3.2]decane,
and 7,7-dimethyl-bicyclo[2.2.1]heptanyl. Unless otherwise stated specifically
in the
specification, a cycloalkyl or carbocycle group may be optionally substituted.
Illustrative
examples of cycloalkyl groups include, but are not limited to, the following
moieties:
,
0
0., So, 4.0, fp., SS, and the like.
[0075] "Fused" refers to any ring structure described herein which is fused to
an existing ring
structure. When the fused ring is a heterocyclyl ring or a heteroaryl ring,
any carbon atom on
the existing ring structure which becomes part of the fused heterocyclyl ring
or the fused
heteroaryl ring may be replaced with a nitrogen atom.
[0076] "Halo" or "halogen" refers to bromo, chloro, fluoro or iodo.
[0077] "Haloalkyl" refers to an alkyl radical, as defined above, that is
substituted by one or
more halo radicals, as defined above, e.g., trifluoromethyl, difluoromethyl,
fluoromethyl,
trichloromethyl, 2,2,2-trifluoroethyl, 1,2-difluoroethyl, 3-bromo-2-
fluoropropyl,
1,2-dibromoethyl, and the like. Unless stated otherwise specifically in the
specification, a
haloalkyl group may be optionally substituted.
[0078] "Haloalkoxy" similarly refers to a radical of the formula -0Ra. where
Ra is a haloalkyl
radical as defined. Unless stated otherwise specifically in the specification,
a haloalkoxy group
may be optionally substituted as described below.
[0079] "Heterocycloalkyl" or "heterocyclyl" or "heterocyclic ring" or
"heterocycle" refers to a
stable 3- to 24-membered non-aromatic ring radical comprising 2 to 23 carbon
atoms and from
one to 8 heteroatoms selected from the group consisting of nitrogen, oxygen,
phosphorous and
sulfur. Unless stated otherwise specifically in the specification, the
heterocyclyl radical may be
a monocyclic, bicyclic, tricyclic or tetracyclic ring system, which may
include fused or bridged
ring systems; and the nitrogen, carbon or sulfur atoms in the heterocyclyl
radical may be
optionally oxidized; the nitrogen atom may be optionally quaternized; and the
heterocyclyl
radical may be partially or fully saturated. Examples of such heterocyclyl
radicals include, but
are not limited to, azetidinyl, dioxolanyl, thienyl[1,3]dithianyl,
decahydroisoquinolyl,
imidazolinyl, imidazolidinyl, isothiazolidinyl, isoxazolidinyl, morpholinyl,
octahydroindolyl,
octahydroisoindolyl, 2-oxopiperazinyl, 2-oxopiperidinyl, 2-oxopyrrolidinyl,
oxazolidinyl,
piperidinyl, piperazinyl, 4-piperidonyl, pyrrolidinyl, pyrazolidinyl,
quinuclidinyl, thiazolidinyl,
-31-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
tetrahydrofuryl, trithianyl, tetrahydropyranyl, thiomorpholinyl,
thiamorpholinyl,
1-oxo-thiomorpholinyl, 1,1-dioxo-thiomorpholinyl, 12-crown-4, 15-crown-5, 18-
crown-6,
21-crown-7, aza-18-crown-6, diaza-18-crown-6, aza-21-crown-7, and diaza-21-
crown-7. Unless
stated otherwise specifically in the specification, a heterocyclyl group may
be optionally
substituted. Illustrative examples of heterocycloalkyl groups, also referred
to as non-aromatic
heterocycles, include:
o
o o o o o o
A A N
LiS \ ____ ) N N aV a0 CL/0 a
S '
v N _____ z N __ c ___ 0) c 0 i .. N
> ) n
V )
0
0 lei
lel 0) , I. N , I. N , 0
,
0 0 , S . , S ,
0
,....."., rsõ..,-...õ. ,0, zs, H 0
N
N )C 0
L j ( j,), j
H H H H H
0
ii
NO= 0 , cc ,µ,....,,,,Ni ,
and the like. The term heterocycloalkyl also includes all
ring forms of the carbohydrates, including but not limited to the
monosaccharides, the
disaccharides and the oligosaccharides. Unless otherwise noted,
heterocycloalkyls have from 2
to 10 carbons in the ring. It is understood that when referring to the number
of carbon atoms in a
heterocycloalkyl, the number of carbon atoms in the heterocycloalkyl is not
the same as the total
number of atoms (including the heteroatoms) that make up the heterocycloalkyl
(i.e. skeletal
atoms of the heterocycloalkyl ring). Unless stated otherwise specifically in
the specification, a
heterocycloalkyl group may be optionally substituted.
[0080] "Heteroaryl÷ refers to a 5- to 14-membered ring system radical
comprising hydrogen
atoms, one to thirteen carbon atoms, one to six heteroatoms selected from the
group consisting
of nitrogen, oxygen, phosphorous and sulfur, and at least one aromatic ring.
For purposes of this
invention, the heteroaryl radical may be a monocyclic, bicyclic, tricyclic or
tetracyclic ring
system, which may include fused or bridged ring systems; and the nitrogen,
carbon or sulfur
atoms in the heteroaryl radical may be optionally oxidized; the nitrogen atom
may be optionally
quaternized. Examples include, but are not limited to, azepinyl, acridinyl,
benzimidazolyl,
benzothiazolyl, benzindolyl, benzodioxolyl, benzofuranyl, benzooxazolyl,
benzothiazolyl,
benzothiadiazolyl, benzo [b][1,4]dioxepinyl, 1,4-benzodioxanyl,
benzonaphthofuranyl,
-32-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
benzoxazolyl, benzodioxolyl, benzodioxinyl, benzopyranyl, benzopyranonyl,
benzofuranyl,
benzofuranonyl, benzothienyl (benzothiophenyl), benzotriazolyl,
benzo[4,6]imidazo[1,2-a]pyridinyl, carbazolyl, cinnolinyl, dibenzofuranyl,
dibenzothiophenyl,
furanyl, furanonyl, isothiazolyl, imidazolyl, indazolyl, indolyl, indazolyl,
isoindolyl, indolinyl,
isoindolinyl, isoquinolyl, indolizinyl, isoxazolyl, naphthyridinyl,
oxadiazolyl, 2-oxoazepinyl,
oxazolyl, oxiranyl, 1-oxidopyridinyl, 1-oxidopyrimidinyl, 1-oxidopyrazinyl, 1-
oxidopyridazinyl,
1-pheny1-1H-pyrrolyl, phenazinyl, phenothiazinyl, phenoxazinyl, phthalazinyl,
pteridinyl,
purinyl, pyrrolyl, pyrazolyl, pyridinyl, pyrazinyl, pyrimidinyl, pyridazinyl,
quinazolinyl,
quinoxalinyl, quinolinyl, quinuclidinyl, isoquinolinyl, tetrahydroquinolinyl,
thiazolyl,
thiadiazolyl, triazolyl, tetrazolyl, triazinyl, and thiophenyl (i.e.,
thienyl). Unless stated otherwise
specifically in the specification, a heteroaryl group may be optionally
substituted.
[0081] "Heteroarylalkyl" refers to a radical of the formula ¨Rd-heteroaryl,
where Rd is an
alkylene chain as defined above. If the heteroaryl is a nitrogen-containing
heteroaryl, the
heteroaryl is optionally attached to the alkyl radical at the nitrogen atom.
Unless stated
otherwise specifically in the specification, the alkylene chain of the
heteroarylalkyl radical is
optionally substituted as defined above for an alkylene chain. Unless stated
otherwise
specifically in the specification, the heteroaryl part of the heteroarylalkyl
radical is optionally
substituted as defined above for a heteroaryl group.
[0082] All the above groups may be either substituted or unsubstituted. The
term "substituted"
as used herein means any of the above groups (e.g, alkyl, alkylene, alkoxy,
aryl, cycloalkyl,
haloalkyl, heterocyclyl and/or heteroaryl) may be further functionalized
wherein at least one
hydrogen atom is replaced by a bond to a non-hydrogen atom substituent. Unless
stated
specifically in the specification, a substituted group may include one or more
substituents
selected from: oxo, amino, -CO2H, nitrile, nitro, hydroxyl, thiooxy, alkyl,
alkylene, alkoxy,
aryl, cycloalkyl, heterocyclyl, heteroaryl, dialkylamines, arylamines,
alkylarylamines,
diarylamines, trialkylammonium (-N 'R3), N-oxides, imides, and enamines; a
silicon atom in
groups such as trialkylsilyl groups, dialkylarylsilyl groups, alkyldiarylsilyl
groups, triarylsilyl
groups, perfluoroalkyl or perfluoroalkoxy, for example, trifluoromethyl or
trifluoromethoxy.
"Substituted" also means any of the above groups in which one or more hydrogen
atoms are
replaced by a higher-order bond (e.g., a double- or triple-bond) to a
heteroatom such as oxygen
in oxo, carbonyl, carboxyl, and ester groups; and nitrogen in groups such as
imines, oximes,
hydrazones, and nitriles. For example, "substituted" includes any of the above
groups in which
one or more hydrogen atoms are replaced with -NH2, -NRgC(=0)NRgRh, -
NRgC(=0)0Rh,
-NRgS02Rh, -0C(=0)NRgRh, -ORg, -SRg, -SORg, -SO2Rg, -0S02Rg, -S020Rg, =NSO2Rg,
and
-SO2NRgRh. In the foregoing, Rg and Rh are the same or different and
independently hydrogen,
-33-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
alkyl, alkoxy, alkylamino, thioalkyl, aryl, aralkyl, cycloalkyl,
cycloalkylalkyl, haloalkyl,
heterocyclyl, N-heterocyclyl, heterocyclylalkyl, heteroaryl, N-heteroaryl
and/or heteroarylalkyl.
In addition, each of the foregoing substituents may also be optionally
substituted with one or
more of the above substituents. Furthermore, any of the above groups may be
substituted to
include one or more internal oxygen, sulfur, or nitrogen atoms. For example,
an alkyl group
may be substituted with one or more internal oxygen atoms to form an ether or
polyether group.
Similarily, an alkyl group may be substituted with one or more internal sulfur
atoms to form a
thioether, disulfide, etc.
[0083] The term "optional" or "optionally" means that the subsequently
described event or
circumstance may or may not occur, and that the description includes instances
where said event
or circumstance occurs and instances in which it does not. For example,
"optionally substituted
alkyl" means either "alkyl" or "substituted alkyl" as defined above. Further,
an optionally
substituted group may be un-substituted (e.g., -CH2CH3), fully substituted
(e.g., -CF2CF3),
mono-substituted (e.g., -CH2CH2F) or substituted at a level anywhere in-
between fully
substituted and mono-substituted (e.g., -CH2CHF2, -CH2CF3, -CF2CH3, -CFHCHF2,
etc). It will
be understood by those skilled in the art with respect to any group containing
one or more
substituents that such groups are not intended to introduce any substitution
or substitution
patterns (e.g., substituted alkyl includes optionally substituted cycloalkyl
groups, which in turn
are defined as including optionally substituted alkyl groups, potentially ad
infinitum) that are
sterically impractical and/or synthetically non-feasible. Thus, any
substituents described should
generally be understood as having a maximum molecular weight of about 1,000
daltons, and
more typically, up to about 500 daltons.
[0084] "Pharmaceutically acceptable" refers to approved or approvable by a
regulatory agency
of the Federal or a state government or listed in the U.S. Pharmacopeia or
other generally
recognized pharmacopeia for use in animals, including humans.
[0085] "Pharmaceutically acceptable salt" refers to a salt of a compound that
is pharmaceutically
acceptable and that possesses the desired pharmacological activity of the
parent compound.
[0086] "Pharmaceutically acceptable excipient, carrier or adjuvant" refers to
an excipient, carrier
or adjuvant that may be administered to a subject, together with at least one
compound of the
present disclosure, and which does not destroy the pharmacological activity
thereof and is
nontoxic when administered in doses sufficient to deliver a therapeutic amount
of the
compound. "Pharmaceutically acceptable vehicle" refers to a diluent, adjuvant,
excipient, or
carrier with which at least one compound of the present disclosure is
administered.
-34-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
[0087] An "effective amount" or "therapeutically effective amount" refers to
an amount of a
compound administered to a mammalian subject, either as a single dose or as
part of a series of
doses, which is effective to produce a desired therapeutic effect.
[0088] "Treatment" of an individual (e.g. a mammal, such as a human) or a cell
is any type of
intervention used in an attempt to alter the natural course of the individual
or cell. In some
embodiments, treatment includes administration of a pharmaceutical
composition, subsequent to
the initiation of a pathologic event or contact with an etiologic agent and
includes stabilization
of the condition (e.g., condition does not worsen) or alleviation of the
condition. In other
embodiments, treatment also includes prophylactic treatment (e.g.,
administration of a
composition described herein when an individual is suspected to be suffering
from a bacterial
infection).
[0089] A "tautomer" refers to a proton shift from one atom of a molecule to
another atom of the
same molecule. The compounds presented herein may exist as tautomers.
Tautomers are
compounds that are interconvertible by migration of a hydrogen atom,
accompanied by a switch
of a single bond and adjacent double bond. In bonding arrangements where
tautomerization is
possible, a chemical equilibrium of the tautomers will exist. All tautomeric
forms of the
compounds disclosed herein are contemplated. The exact ratio of the tautomers
depends on
several factors, including temperature, solvent, and pH. Some examples of
tautomeric
interconversions include:
OH 0 \
- \ \
H H
0 OH N H2 NH
\N H2 \ N H \ N \ N
I
I H
N
rssc¨ N H
Nr--N
Ns Nr.,- Ns
N H
N ¨N' N¨N H N¨N' NN'
[0090] A "metabolite" of a compound disclosed herein is a derivative of that
compound that is
formed when the compound is metabolized. The term "active metabolite" refers
to a biologically
active derivative of a compound that is formed when the compound is
metabolized. The term
"metabolized," as used herein, refers to the sum of the processes (including,
but not limited to,
hydrolysis reactions and reactions catalyzed by enzymes, such as, oxidation
reactions) by which
a particular substance is changed by an organism. Thus, enzymes may produce
specific
structural alterations to a compound. For example, cytochrome P450 catalyzes a
variety of
oxidative and reductive reactions while uridine diphosphate glucuronyl
transferases catalyze the
transfer of an activated glucuronic-acid molecule to aromatic alcohols,
aliphatic alcohols,
-35-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
carboxylic acids, amines and free sulfhydryl groups. Further information on
metabolism may be
obtained from The Pharmacological Basis of Therapeutics, 9th Edition, McGraw-
Hill (1996).
Metabolites of the compounds disclosed herein can be identified either by
administration of
compounds to a host and analysis of tissue samples from the host, or by
incubation of
compounds with hepatic cells in vitro and analysis of the resulting compounds.
Both methods
are well known in the art. In some embodiments, metabolites of a compound are
formed by
oxidative processes and correspond to the corresponding hydroxy-containing
compound. In
some embodimets, a compound is metabolized to pharmacologically active
metabolites.
Compounds
[0091] Described herein are compounds that treat drug resistant and persistent
tuberculosis.
[0092] In one aspect, provided herein are compounds of Formula (I), a
pharmaceutically
acceptable salt, solvate, polymorph, prodrug, metabolite, N-oxide,
stereoisomer, or isomer
thereof:
Y27---Y3
Yi N?'Z M
M1 1
AN.. 3
1µ12 R
A
Formula (I)
wherein:
M1 are each independently a bond, -C(=0)- or
M2 is ¨(CH2)-, -C(=0)- or
Z is a bond or NR2;
Yi is S, 0, or NR2;
Y2 is CR4 or N;
Y3 is CR5 or N;
R1 is -0-(optionally substituted alkyl), -0-(alkenyl), -0-(alkynyl), -0-
(cycloalkyl),
-0-(heterocycly1), -0-(optionally substituted aralkyl), -0-(optionally
substituted
heteroaralkyl), -0-(alkyl)-(alkoxy), -0-(alkyl)-(aralkoxy), -0-(alkyl)-
(heterocycly1),
-0-(alkyl)-(C00Ra), -0-(alkyl)-(NR6R7), -NR6R7, or R8;
R2 and R3 are each independently selected from H, optionally substituted
alkyl, and
optionally substituted aryl; or R1 and R2 taken together form a heterocycle;
-36-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
R4 is H, halogen, -CN, optionally substituted alkyl, optionally substituted
aryl, -RbCOORa or
-RbCH(COORa)2;
R5 is H, halogen, optionally substituted alkyl or cycloalkyl; or R4 and R5
taken together form
a carbocycle or an optionally substituted heterocycle;
R6 and R7 are each independently selected from H and optionally substituted
alkyl; or R6 and
R7 taken together form an optionally substituted heterocycle with the nitrogen
to which
they are attached;
R8 is optionally substituted alkyl, optionally substituted aryl, carbocyclyl,
optionally
substituted aralkyl, optionally substituted heteroaryl, optionally substituted
heterocyclyl,
¨RbCOORa, or ¨RbCONRaRa;
each Ra is independently selected from H and alkyl;
Rb is a bond or alkylenyl;
Rc is a bond or alkenylenyl; and
A is optionally substituted aryl, optionally substituted heterocyclyl,
optionally substituted
heteroaryl, optionally substituted carbocyclyl, optionally substituted
aralkyl, optionally
substituted heteroaralkyl, or ¨Rc-(optionally substituted heteroaryl).
[0093] In some embodiments of a compound of Formula (I), Y3 is N. In some
embodiments of
a compound of Formula (I), Y3 is CR5.
[0094] In some embodiments of a compound of Formula (I), Yi is S. In some
embodiments of a
compound of Formula (I), Y1 is NR2.
[0095] In some embodiments of a compound of Formula (I), Y2 is CR4. In some
embodiments
of a compound of Formula (I), Y2 is N.
[0096] In some embodiments of a compound of Formula (I), Yi is 5; Y2 is CR4;
and Y3 is CR5.
In some embodiments of a compound of Formula (I), Yi is NR2; Y2 is N; and Y3
is CR5.
[0097] In some embodiments of a compound of Formula (I), each M1 is a bond. In
some
embodiments of a compound of Formula (I), each M1 is -C(=0)-. In some
embodiments of a
compound of Formula (I), each M1 is -S(=0)2-.
[0098] In some embodiments of a compound of Formula (I), -M1-Z-M1-R1 is ¨C(=0)-
R1. In
further embodiments of a compound of Formula (I), -M1-Z-M1-R1 is ¨C(=0)-0-
(optionally
substituted alkyl). In other embodiments of a compound of Formula (I), -M1-Z-
M1-R1 is ¨
C(=0)- NR6R7. In still other embodiments of a compound of Formula (I), -M1-Z-
M1-R1 is ¨
C(=0)-R8.
[0099] In some embodiments of a compound of Formula (I), Z is a bond. In some
embodiments
of a compound of Formula (I), Z is NR2. In some embodiments of a compound of
Formula (I),
Z is NR2 and each M1 is -C(=0)-.
-37-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
[00100] In some embodiments of a compound of Formula (I), M2 is -C(=0)-.
[00101] In some embodiments of a compound of Formula (I), M1 and M2 are both -
C(=0)-. In
some embodiments of a compound of Formula (I), M1 and M2 are both -C(=0)-; and
Z is NR2.
[00102] In some embodiments of a compound of Formula (I), R1 is -0-(optionally
substituted
alkyl),
-0-(alkenyl), -0-(alkynyl), -0-(cycloalkyl), -0-(heterocycly1), -0-(optionally
substituted
aralkyl),
-0-(optionally substituted heteroaralkyl), -0-(alkyl)-(alkoxy), -0-(alkyl)-
(aralkoxy),
-0-(alkyl)-(heterocycly1), -0-(alkyl)-(COORa), or -0-(alkyl)-(NR6R7). In some
embodiments of
a compound of Formula (I), R1 is -0-(optionally substituted alkyl). In some
embodiments of a
compound of Formula (I), R1 is -0-(alkeny1). In some embodiments of a compound
of Formula
(I), R1 is -0-(alkyny1). In some embodiments of a compound of Formula (I), R1
is -0-
(cycloalkyl). In some embodiments of a compound of Formula (I), R1 is -0-
(heterocycly1). In
some embodiments of a compound of Formula (I), R1 is -0-(optionally
substituted aralkyl). In
some embodiments of a compound of Formula (I), R1 is -0-(optionally
substituted
heteroaralkyl). In some embodiments of a compound of Formula (I), R1 is -0-
(alkyl)-(alkoxy).
In some embodiments of a compound of Formula (I), R1 is -0-(alkyl)-(aralkoxy).
In some
embodiments of a compound of Formula (I), R1 is
-0-(alkyl)-(heterocycly1). In some embodiments of a compound of Formula (I),
R1 is -0-(alkyl)-
(C0010. In some embodiments of a compound of Formula (I), R1 is -0-(alkyl)-
(NR6R7). In
some embodiments of a compound of Formula (I), R1 is -0-(optionally
substituted alkyl); and R2
and R3 are both H.
[00103] In some embodiments of a compound of Formula (I), R1 is -NR6R7. In
some
embodiments of a compound of Formula (I), R6 and R7 are each independently
selected from H
and optionally substituted alkyl. In some embodiments of a compound of Formula
(I), R6 and R7
are each independently selected from H and optionally substituted alkyl;
wherein the optional
substituent is halogen. In some embodiments of a compound of Formula (I), R6
and R7 are H. In
some embodiments of a compound of Formula (I), R6 and R7 are each optionally
substituted
alkyl. In some embodiments of a compound of Formula (I), R6 and R7 are each
optionally
substituted alkyl; wherein the optional substituent is halogen. In some
embodiments of a
compound of Formula (I), R6 and R7 taken together form an optionally
substituted heterocycle
with the nitrogen to which they are attached. In some embodiments of a
compound of Formula
(I), R6 and R7 taken together form an optionally substituted heterocycle with
the nitrogen to
which they are attached; wherein the optional substituent is halogen.
-38-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
[00104] In some embodiments of a compound of Formula (I), R1 is R8. In some
embodiments
of a compound of Formula (I), R8 is optionally substituted alkyl. In some
embodiments of a
compound of Formula (I), R8 is optionally substituted aryl. In some
embodiments of a
compound of Formula (I), R8 is carbocyclyl. In some embodiments of a compound
of Formula
(I), R8 is optionally substituted aralkyl. In some embodiments of a compound
of Formula (I), R8
is optionally substituted heteroaryl. In some embodiments of a compound of
Formula (I), R8 is
optionally substituted heterocyclyl. In some embodiments of a compound of
Formula (I), R8 is ¨
RbCOORa. In some embodiments of a compound of Formula (I), R8 is ¨RbCONRaRa.
[00105] In some embodiments of a compound of Formula (I), R2 and R3 are each
independently
selected from H, optionally substituted alkyl and optionally substituted aryl.
In some
embodiments of a compound of Formula (I), R2 and R3 are H. In some embodiments
of a
compound of Formula (I), R2 and R3 are each independently selected from H and
optionally
substituted alkyl. In some embodiments of a compound of Formula (I), R2 and R3
are optionally
substituted alkyl. In some embodiments of a compound of Formula (I), R2 and R3
are optionally
substituted aryl. In some embodiments of a compound of Formula (I), R2 and R3
are each
independently selected from optionally substituted alkyl and optionally
substituted aryl. In some
embodiments of a compound of Formula (I), R1 and R2 taken together form a
heterocycle.
[00106] In some embodiments of a compound of Formula (I), R4 is H. In some
embodiments of
a compound of Formula (I), R4 is halogen. In some embodiments of a compound of
Formula (I),
R4 is -CN. In some embodiments of a compound of Formula (I), R4 is optionally
substituted
alkyl. In some embodiments of a compound of Formula (I), R4 is optionally
substituted aryl. In
some embodiments of a compound of Formula (I), R4 is -RbCOORa. In some
embodiments of a
compound of Formula (I), R4 is -RbCH(C00102. In some embodiments of a compound
of
Formula (I), R4 is H, halogen, -CN, or optionally substituted alkyl. In some
embodiments of a
compound of Formula (I), R4 is H, halogen, -CN, optionally substituted alkyl,
or optionally
substituted aryl. In some embodiments of a compound of Formula (I), R4 is -
RbCOORa or -
RbCH(COORa)2.
[00107] In some embodiments of a compound of Formula (I), R5 is H, halogen,
optionally
substituted alkyl or cycloalkyl. In some embodiments of a compound of Formula
(I), R5 is H. In
some embodiments of a compound of Formula (I), R5 is halogen. In some
embodiments of a
compound of Formula (I), R5 is optionally substituted alkyl. In some
embodiments of a
compound of Formula (I), R5 is cycloalkyl. In some embodiments of a compound
of Formula
(I), R5 is H, halogen, or optionally substituted alkyl. In some embodiments of
a compound of
Formula (I), R5 is H, optionally substituted alkyl, or cycloalkyl. In some
embodiments of a
compound of Formula (I), R5 is H or alkyl. In some embodiments of a compound
of Formula (I),
-39-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
R5 is optionally substituted alkyl or cycloalkyl. In some embodiments of a
compound of
Formula (I), R4 and R5 taken together form a carbocycle or an optionally
substituted heterocycle.
[00108] In one aspect, provided herein are compounds of Formula (Ia), a
pharmaceutically
acceptable salt, solvate, polymorph, prodrug, metabolite, N-oxide,
stereoisomer, or isomer
thereof:
R4
R2
____1,13 \
Y1N1?-1,c N ¨1(R1
0
0
OyN-.... IR3
A
(Ia)
wherein:
Yi is S, 0, or NR2;
Y3 is CR5 or N;
R1 is -0-(optionally substituted alkyl), -0-(alkenyl), -0-(alkynyl), -0-
(cycloalkyl),
-0-(heterocycly1), -0-(optionally substituted aralkyl), -0-(optionally
substituted
heteroaralkyl), -0-(alkyl)-(alkoxy), -0-(alkyl)-(aralkoxy), -0-(alkyl)-
(heterocycly1),
-0-(alkyl)-(C00Ra), -0-(alkyl)-(NR6R7), -NR6R7 or R8;
R2 and R3 are each independently selected from H, optionally substituted
alkyl, and
optionally substituted aryl; or R1 and R2 taken together form a heterocycle;
R4 is H, halogen, -CN, optionally substituted alkyl, aryl, -RbCOORa or -
RbCH(C00Ra)2;
R5 is H, halogen, optionally substituted alkyl, or cycloalkyl; or R4 and R5
taken together
form a carbocycle or an optionally substituted heterocycle;
R6 and R7 are each independently selected from H and optionally substituted
alkyl; or R6 and
R7 taken together form an optionally substituted heterocycle with the nitrogen
to which
they are attached;
R8 is optionally substituted alkyl, optionally substituted aryl, carbocyclyl,
optionally
substituted aralkyl, optionally substituted heteroaryl, or optionally
substituted
heterocyclyl;
each Ra is independently selected from H and alkyl;
RD is a bond or alkylenyl;
Rc is a bond or alkenylenyl; and
-40-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
A is optionally substituted aryl, optionally substituted heterocyclyl,
optionally substituted
heteroaryl, optionally substituted carbocyclyl, optionally substituted
aralkyl, optionally
substituted heteroaralkyl or ¨Rc-(optionally substituted heteroaryl).
[00109] In some embodiments of a compound of Formula (Ia), R1 is -0-
(optionally substituted
alkyl), -0-(alkenyl), -0-(alkynyl), -0-(cycloalkyl), -O-(heterocyclyl), -0-
(optionally substituted
aralkyl),
-0-(optionally substituted heteroaralkyl), -0-(alkyl)-(alkoxy), -0-(alkyl)-
(aralkoxy),
-O-(alkyl)-(heterocyclyl), -0-(alkyl)-(C0010, or -0-(alkyl)-(NR6R7). In some
embodiments of
a compound of Formula (Ia), R1 is -0-(optionally substituted alkyl). In some
embodiments of a
compound of Formula (Ia), R1 is -0-(alkeny1). In some embodiments of a
compound of
Formula (Ia), R1 is -0-(alkyny1). In some embodiments of a compound of Formula
(Ia), R1 is -
0-(cycloalkyl). In some embodiments of a compound of Formula (Ia), R1 is -O-
(heterocyclyl).
In some embodiments of a compound of Formula (Ia), R1 is -0-(optionally
substituted aralkyl).
In some embodiments of a compound of Formula (Ia), R1 is -0-(optionally
substituted
heteroaralkyl). In some embodiments of a compound of Formula (Ia), R1 is -0-
(alkyl)-(alkoxy).
In some embodiments of a compound of Formula (Ia), R1 is -0-(alkyl)-
(aralkoxy). In some
embodiments of a compound of Formula (Ia), R1 is -O-(alkyl)-(heterocyclyl). In
some
embodiments of a compound of Formula (Ia), R1 is -0-(alkyl)-(COORa). In some
embodiments
of a compound of Formula (Ia), R1 is -0-(alkyl)-(NR6R7).
[00110] In some embodiments of a compound of Formula (Ia), R1 is -0-
(optionally substituted
alkyl); wherein the optionally substituted alkyl is substituted with halogen.
In further
embodiments of a compound of Formula (Ia), R1 is -0-(cycloalkyl). In still
further
embodiments of a compound of Formula (Ia), R1 is -0-(cyclobutyl), -0-
(cyclopentyl), or -0-
(cyclohexyl). In some embodiments of a compound of Formula (Ia), R1 is -0-
(heterocyclyl). In
further embodiments of a compound of Formula (Ia), R1 is -0-(optionally
substituted
piperidinyl), -0-(oxetanyl), or -0-(tetrahydrofuranyl), wherein the optionally
substituted
piperidinyl is substituted with ¨COCH3. In some embodiments of a compound of
Formula (Ia),
R1 is -0-(optionally substituted alkyl); and R2 and R3 are both H.
[00111] In some embodiments of a compound of Formula (Ia), R1 is -0-
(optionally substituted
aralkyl) or -0-(optionally substituted heteroaralkyl). In further embodiments
of a compound of
Formula (Ia), the optionally substituted aralkyl and the optionally
substituted heteroaralkyl are
substituted with a group selected from halogen, alkyl and ¨CF3.
[00112] In some embodiments of a compound of Formula (Ia), R1 is -0-(alkyl)-
(heterocyclyl).
In further embodiments of a compound of Formula (Ia), the heterocyclyl is
morpholinyl.
-41-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
[00113] In some embodiments of a compound of Formula (Ia), R1 is -NR6R7. In
some
embodiments of a compound of Formula (Ia), R6 and R7 are each independently
selected from H
and optionally substituted alkyl. In some embodiments of a compound of Formula
(Ia), R6 and
R7 are each independently selected from H and optionally substituted alkyl;
wherein the optional
substituent is halogen. In some embodiments of a compound of Formula (Ia), R6
and R7 are H. In
some embodiments of a compound of Formula (Ia), R6 and R7 are each optionally
substituted
alkyl. In some embodiments of a compound of Formula (Ia), R6 and R7 are each
optionally
substituted alkyl; wherein the optional substituent is halogen. In some
embodiments of a
compound of Formula (Ia), R6 and R7 taken together form an optionally
substituted heterocycle
with the nitrogen to which they are attached. In some embodiments of a
compound of Formula
(Ia), R6 and R7 taken together form an optionally substituted heterocycle with
the nitrogen to
which they are attached; wherein the optional substituent is halogen. In some
embodiments of a
compound of Formula (Ia), R6 and R7 taken together form a heterocycle with the
nitrogen to
which they are attached; wherein the heterocycle is selected from piperidinyl
and morpholinyl.
[00114] In some embodiments of a compound of Formula (Ia), R1 is R8. In some
embodiments
of a compound of Formula (Ia), R8 is optionally substituted alkyl. In some
embodiments of a
compound of Formula (Ia), R8 is optionally substituted aryl. In some
embodiments of a
compound of Formula (Ia), R8 is optionally substituted aryl; wherein the
optionally substituted
aryl is substituted with halogen. In some embodiments of a compound of Formula
(Ia), R8 is
carbocyclyl. In some embodiments of a compound of Formula (Ia), R8 is
optionally substituted
carbocyclylalkyl. In some embodiments of a compound of Formula (Ia), R8 is
optionally
substituted heteroaryl. In some embodiments of a compound of Formula (Ia), R8
is optionally
substituted heteroaryl; wherein the optionally substituted heteroaryl is
substituted with a group
selected from alkyl, -0-(alkyl) and -NR6R7. In some embodiments of a compound
of Formula
(Ia), R8 is optionally substituted heteroarylalkyl. In some embodiments of a
compound of
Formula (Ia), R8 is optionally substituted heterocyclyl. In some embodiments
of a compound of
Formula (Ia), R8 is optionally substituted heterocyclyl; wherein the
optionally substituted
heterocyclyl is substituted with alkyl. In some embodiments of a compound of
Formula (Ia), R2
and R8 taken together form an optionally substituted heterocycle with the
nitrogen to which they
are attached. In further embodiments of a compound of Formula (Ia), the
heterocycle is selected
from piperidinyl, morpholinyl, thiomorpholinyl, optionally substituted
diazepanyl and optionally
substituted piperizanyl; wherein the optionally substituted diazepanyl and the
optionally
substituted piperizanyl are substituted with a group selected from alkyl,
aryl, -COOtBu and ¨
SO2Me. In some embodiments of a compound of Formula (Ia), R8 is optionally
substituted
heterocyclylalkyl. In some embodiments of a compound of Formula (Ia), R2 and
R8 taken
-42-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
together form an optionally substituted heterocycle with the nitrogen to which
they are attached.
In some embodiments of a compound of Formula (Ia), R8 is optionally
substituted alkyl,
optionally substituted aryl, optionally substituted aralkyl, carbocyclyl,
optionally substituted
carbocyclylalkyl, optionally substituted heteroaryl, optionally substituted
heteroarylalkyl,
optionally substituted heterocyclyl, or optionally substituted
heterocyclylalkyl. In some
embodiments of a compound of Formula (Ia), R8 is optionally substituted alkyl,
carbocyclyl,
optionally substituted carbocyclylalkyl, optionally substituted heterocyclyl,
or optionally
substituted heterocyclylalkyl. In some embodiments of a compound of Formula
(Ia), R8 is
optionally substituted aryl, optionally substituted aralkyl, optionally
substituted heteroaryl, or
optionally substituted heteroarylalkyl. In some embodiments of a compound of
Formula (Ia), R8
is optionally substituted alkyl, optionally substituted aralkyl, carbocyclyl,
optionally substituted
carbocyclylalkyl, optionally substituted heteroarylalkyl, optionally
substituted heterocyclyl, or
optionally substituted heterocyclylalkyl.
[00115] In some embodiments of a compound of Formula (Ia), R2 and R3 are both
H. In some
embodiments of a compound of Formula (Ia), R2 and R3 are both optionally
substituted alkyl. In
some embodiments of a compound of Formula (Ia), R2 is hydrogen and R3 is
optionally
substituted alkyl. In some embodiments of a compound of Formula (Ia), R2 is
optionally
substituted alkyl and R3 is hydrogen. In some embodiments of a compound of
Formula (Ia), R2
is hydrogen and R3 is optionally substituted aryl. In some embodiments of a
compound of
Formula (Ia), R2 is optionally substituted aryl and R3 is hydrogen. In some
embodiments of a
compound of Formula (Ia), R2 is optionally substituted aryl and R3 is
optionally substituted
alkyl. In some embodiments of a compound of Formula (Ia), R2 is optionally
substituted alkyl
and R3 is optionally substituted aryl.
[00116] In some embodiments of a compound of Formula (Ia), R1 and R2 taken
together form a
heterocycle.
[00117] In some embodiments of a compound of Formula (Ia), R4 is H. In some
embodiments
of a compound of Formula (Ia), R4 is halogen. In some embodiments of a
compound of Formula
(Ia), R4 is -CN. In some embodiments of a compound of Formula (Ia), R4 is
optionally
substituted alkyl. In some embodiments of a compound of Formula (Ia), R4 is
aryl. In some
embodiments of a compound of Formula (Ia), R4 is -RbCOORa. In some embodiments
of a
compound of Formula (Ia), R4 is
-RbCH(COORa)2. In some embodiments of a compound of Formula (Ia), R4 is H,
halogen, -CN,
or optionally substituted alkyl. In some embodiments of a compound of Formula
(Ia), R4 is H,
halogen, -CN, optionally substituted alkyl, or aryl. In some embodiments of a
compound of
Formula (Ia), R4 is -RbCOORa or -RbCH(COORa)2.
-43-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
[00118] In some embodiments of a compound of Formula (Ia), R5 is H, halogen,
optionally
substituted alkyl or cycloalkyl. In some embodiments of a compound of Formula
(Ia), R5 is H. In
some embodiments of a compound of Formula (Ia), R5 is halogen. In some
embodiments of a
compound of Formula (Ia), R5 is optionally substituted alkyl. In some
embodiments of a
compound of Formula (Ia), R5 is cycloalkyl. In some embodiments of a compound
of Formula
(Ia), R5 is H, halogen, or optionally substituted alkyl. In some embodiments
of a compound of
Formula (Ia), R5 is H, optionally substituted alkyl, or cycloalkyl. In some
embodiments of a
compound of Formula (Ia), R5 is H or optionally substituted alkyl. In some
embodiments of a
compound of Formula (Ia), R5 is optionally substituted alkyl or cycloalkyl. In
some
embodiments of a compound of Formula (Ia), R4 and R5 taken together form a
carbocycle or an
optionally substituted heterocycle. In some embodiments of a compound of
Formula (Ia), R4 and
R5 taken together form an optionally substituted heterocycle; wherein the
optionally substituted
heterocycle is substituted with a group selected from alkyl, aralkyl and
¨S02Me.
[00119] In some embodiments of a compound of Formula (Ia), Y3 is N. In some
embodiments
of a compound of Formula (Ia), Y3 is CR5.
[00120] In some embodiments of a compound of Formula (Ia), Y1 is NR2. In some
embodiments of a compound of Formula (Ia), Y1 is 0. In some embodiments of a
compound of
Formula (Ia), Y1 is S. In some embodiments of a compound of Formula (Ia), Yi
is S and Y3 is
CH.
[00121] In some embodiments of a compound of Formula (I) or Formula (Ia), A is
optionally
substituted aryl, optionally substituted heterocyclyl, optionally substituted
heteroaryl, optionally
substituted carbocyclyl, optionally substituted aralkyl, optionally
substituted heteroaralkyl or ¨
Rc-(optionally substituted heteroaryl); and the optionally substituted aryl,
the optionally
substituted heterocyclyl, the optionally substituted heteroaryl, the
optionally substituted
carbocyclyl, the optionally substituted aralkyl and the optionally substituted
heteroaralkyl are
substituted with 1-6 R10; wherein each R1 is independently selected from H,
halogen, -CN, -
NO2, -CF3, alkyl, -SR6, -0R6, -NR6R7, -NR6C(=0)(alkyl), -
NR6C(=0)(cycloalkyl),
-NR6C(=0)(heterocycly1), -NR6C(=0)(ary1), -NR6C(=0)(heteroary1), -C(=0)NR6R7,
-C(=0)NR6(cycloalkyl), -C(=0)NR6(heterocycloalkyl), -C(=0)NR6(ary1),
-C(=0)NR6(heteroaryl) , -NR6C(=0)NR6R7, -NR6C(=0)NR7(cycloalkyl),
-NR6C(=0)NR7(heterocycloalkyl), -NR6C(=0)NR7(ary1), -NR6C(=0)NR7(heteroary1),
-NR6C(=0)0(alkyl), -NR6C(=0)0(cycloalkyl), -NR6C(=0)0(heterocycloalkyl),
-NR6C(=0)0(aryl), -NR6C(=0)0(heteroaryl), -NR6S02(alkyl), -NR6S02(cycloalkyl),
-NR6S02(heterocycloalkyl), -NR6S02(aryl), -NR6S02(heteroaryl), -SO2NR6R7,
-44-
CA 02911326 2015-10-30
WO 2014/190199
PCT/US2014/039227
-SO2NR6(cycloalkyl), -SO2NR6(heterocycloalkyl), -SR6, -S02R6, -SO2NR6(ary1),
-SO2NR6(heteroary1), haloalkyl, aryl, heteroaryl, heterocyclyl and tetrazoyl.
[00122] In some embodiments of a compound of Formula (I) or Formula (Ia), A is
optionally
substituted aryl. In some embodiments of a compound of Formula (I) or Formula
(Ia), A is
optionally substituted heterocyclyl. In some embodiments of a compound of
Formula (I) or
Formula (Ia), A is optionally substituted carbocyclyl. In some embodiments of
a compound of
Formula (I) or Formula (Ia), A is optionally substituted aralkyl. In some
embodiments of a
compound of Formula (I) or Formula (Ia), A is optionally substituted
heteroaralkyl. In some
embodiments of a compound of Formula (I) or Formula (Ia), A is optionally
substituted ¨Rc-
(optionally substituted heteroaryl).
[00123] In some embodiments of a compound of Formula (I) or Formula (Ia), A is
optionally
substituted heteroaryl. In some embodiments of a compound of Formula (I) or
Formula (Ia), A is
R10
r.i\L N \ 0 o
R1 );\\. Ri \r\\. R10 N \
-....õ...õ,
........,õ,,r- N.
selected from:
N 5 Rio" \% 5 N 5 :1\1
N 5 \N
R10
Rlyy,,
1 R10
N N N 1
N.
Rio r\r 5 R10 5 Rio N."' N . In
some embodiments of a
, andR10
\r\N,
I
compound of Formula (I) or Formula (Ia), A is N . In some embodiments of a
)( x2 xl \
3
ii
)(4, '\ X7
compound of Formula (I) or Formula (Ia), A is selected from X5 X6 and
X4 X3
X5'X2
.. 1
X6, -rX1
X7
¨ , wherein X1, )(25 )(35 )(45 )(55 ¨65
A and X7 are independently selected from N and
CR10; and at least one of X1- X7 is N. In some embodiments of a compound of
Formula (I) or
N e \ I 1\ NN:\\
I I
Formula (Ia), A is selected from' , N /
5 5 5 5
N \
N\\* I e \ N
.\\ 1101 Rio 0 \
I
N N 5 N/ 5 R 1 0 5 N 5
-45-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
I \ 1 R1 R 1 o N N
I 1 0
I
401 101 / 110 / /
N
¨ 5 ¨.5 ¨ 5 and ¨ . In some embodiments of
X1X1 X
X2 N
X2 X5 s I I
I I
x5
X3, xL1/(
X3, 4----x
a compound of Formula (I) or Formula (Ia), A is selected from X
Ansewonv
X5 X1 \ x5 x1 \ ,5 xt
// ---.....-" ..y*. X5-.../ X1 x2
A ' X2 Xi; I 1
,,, =-...,õ,- õy- X5--.) xl
X4 I X \ I x2 4 I X -Th% X3 o
X4 I 1
X4 X3 X x3 i X X3 x2
.
^^^.A.^^ ,and
5 µr 55
wherein X1, X2, X3, X4, X5, X6, and X7 are independently selected from N and
CR10; and X is
0, S, or NR2. In some embodiments of a compound of Formula (I) or Formula
(Ia), A is selected
i ...,...x1;\ xi
N-......._õ:õ.,,
XI 12'x _______ 1 RioN IT R10- , * R10_ I I
NSx3 X2 Sx3 / Sx3' X2
from x4 s 5 5 5 5
N,,..X1 x2 , xi
1
Ri0¨ I I X2 N N-....,,X \%k N........X
s x2
.,....--- X3 H_ _______ I R10_ I R1c,_ , *
x2
x4 0 0 x3' (:)---x3 /
Xi , Xi Xi
N....õ..
NL x2 x2 .,,..---N
, ------",% xi R1 0_ II, Re
II,
____________________________________________ I R10_
R1 0_< I I oõ,---- Xi X'x,1%-......,.N
N ----%3 X2
5 reo.,~1 5 5 5
Xi Rlo
N...,,X1 x2 N-,,,...\ xi N-.../
X2 ,xi j
Rio_ , * Ri o_ I I Ri o_
N"---x3 / N...---%3' X2 N 113 X2 1
----- X ).1., _
ReRe Re
5 5
R10
R1 R1\ T
, Xl
X2 ______
\ X1
I I _______ R1 o R10 fr Ri0
, k
X4 S'-'---x3 /
x3
5 5 5 5
IR 1 R10 R10
X1Rl
_t x2-x 1 xi______
Rio r
x3 II \ __ x2- \ R10 Rio Rio 7 1,
S ------ x3 *....õ 1 I I
x4 0 X3 x4%--.-_. 0 0 )(3' x2 0 x3/
owvonv. 5 5 5 5 5
-46-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
R10 R10 R
R:p.,....' R1
2(1 2 2, X1 ,x1 Nf 6 X1 A
X1 R1 / i) )1(1 \ 1 )12 10 1 o_h
[r
Ri o / I I X3x4 / R R
0/ X3 X3)(4
x3
N
\
16 5 5 ' X2
...''''. R.6
.....~. 5 5
R1 R1
õ_........____ R1
x2
R1 /
* / X1 X X1
1X2 br\j \
I I Rl / II NI' 112 /N X1 \
Nx3 / R10 ---- y
-Si /-x3, x2 Nj x3 \ N .%3, X S
R.6 R6 R6 I R6 \N-----x3'' X2
5 5 .......... 5 5 5
,X1 _.õS X1
X2 .....- \ X2' C)\
I I i N I I N
and ,--- . In some embodiments of a compound of Formula (I)
or
R' R'
R'
N Rio N N
NN
10 , )¨ I
Rio S Rio'\r'S Riokr'S֬ I
Formula (Ia), A is selected from Rio
5 Ri 0
5 Rio
5
Rio Rio Rio
Ri R1 Rio
S=
\
I N )_ I Ric)N Rio S _ 0 Rio_
0
N (---,/S I , ,¨ I N Rio N
/
/
Rio 5 Ri OTh\r....S 5 Rio Rio
,and. In
some embodiments of a compound of Formula (I) or Formula (Ia), A is selected
from
_ y 1
X2 )(1 5 ;2X1 X2
I II\)¨ I 1
-N-. X X 3
5 X3-
R2 5 and X.--X3
. In some embodiments of a compound of
Rio Rio Rio
6_1 N .------
1 / I 1\ji I
Rio N R2 N
-,,
'N S
Formula (I) or Formula (Ia), A is selected from R 5 5R1 0 5
Rio
Ri 0
N..../ s
6_, 1
Rio N RiorN 5 R10 S 5 R io 0 . In some
embodiments of a
5
Xc N 1
I
) _____________________________________________________________ I
X4 X6
compound of Formula (I) or Formula (Ia), A is selected from X5
5
-47-
CA 02911326 2015-10-30
WO 2014/190199
PCT/US2014/039227
_)(2 X2
X 'N ' Xlµ X3 ' N ' X1\ ...,õX2, ,-Xi
I \ X7 I \ 1X7 X3 N \ N 1\
X4 --õ--( )(4 y---_--- x
\11,,X5X6 X4
X5 X6
e''''''' 5 w=wwww.
5 5 5
dwwwwv
X2, .....õ,....
X3 N'Xl\ X3 N"'-.
I \ X7 I X
X
, 7
4 /------------- / X4 /-_ 1
X5 X6
5 and X5 X6 ; wherein X15 )(25 )(35 )(45 )(55 X65 and X7 are
independently selected from N and CR10. In some embodiments of a compound of
Formula (I)
R1 R1 R1
Rlo
R1 R1I______
/ 1
R1 crN / 1 Ri m o"'N
i o R1 5 R1 o
or Formula Oa), A is selected from R 5
R10 R10
R10 R10 R1 R10 R1
R10::::.N );%\
-----
N I c N/ I Rio rx4IN o10
\ k 1
R1 OTh R10 R10 N - " rRi o
R1 R1 R 1 0 R1 R10 R10
5 5 5
R10 R10
R10
R1Cr:N R1N R10 ......N ,,11 N 1--%N
,
/ R10
_ 1 1
\ N 1 r N- 1
Ri or N -e¨ Ri o I I === N
"--)--'
Ri cr\r / Ri oJt
R1 or"''" 5 R10 R10 R1 5 R1 R1 5
5
R10 R10 R10 R10
R1r m , N
ll_ I Ri o_ N N-NN\L
Ri o_y R1
R1 CIN -1\I 1 N'Rio NRio NRio
R10 5 R10 5 R10 5 R10 5
R10
,NINµ Ri o N m
'"%=-=::- " 1
i---"1, 1
/)¨
N,...- N
Ri o Ri or 'm "'N
Rio Rio 5 and Rlo
. In some embodiments of a compound of Formula
R11
1
R11 ..___.N r\--1\L 1 N.,., N
N
I 1
--- /
i N_ v X /-----x
(I) or Formula Oa), A is selected from x 5 Ri 5
-48-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
/\---N Rlo Rlo .,,,,,,,
IR1 1
1 \ I 1:0 N-----c_Rio
R11 5 ...====)( 15 Rii N.,....õ..-----x
./---X
5 5
R' R11
R1 II \
....,,
N 1
r------cR10 I\J(..?¨ 1 Rio 101
R11 NX Rio ,and Rio ; wherein X is 0, S, or
NR2;
5
and R1 is H, alkyl, aryl, heteroaryl, -S02-(alkyl), -S02-(cycloalkyl), -S02-
(aryl),
-S02-(heteroary1), -S02-(heterocycloalkyl), -C(=0)0(alkyl), -
C(=0)0(cycloalkyl),
-C(=0)0(heterocycloalkyl), -C(=0)0(ary1), -C(=0)0(heteroary1), -C(=0)NR6R7,
-C(=0)NR6(cycloalkyl), -C(=0)NR6(heterocycloalkyl), -C(=0)NR6(ary1),
-C(=0)NR6(heteroary1), -C(=0)(alkyl), -C(=0)(cycloalkyl), -
C(=0)(heterocycloalkyl),
-C(=0)(ary1), or -C(=0)(heteroary1). In some embodiments of a compound of
Formula (I) or
/z------(. \
la N>,.ii Co_ 1 <0 is \
Formula (Ia), A is selected from S5 5 S 0
5
\ 0
H
ON 40 \ .. la 0 I N co -
=\S cq
,
JIN\ I HNaE ,_ I e:.--
S )1 - 0 5
5 5 5
R10 R10
io \R10 \
'Ntl R el \
-N 'Nµ ,N)\ I
Cu_S 1 H1\1 HN
5 (:) CON5 0 R10 0 R100 0
R10
5 I 5 R10 5 5
H
0 NQ
---.---
N 0 \
,.
I. aN, I 5 H N&N,- 1
cr,N---__ 0 \=
¨ 5 0 S 5 S ,and N
[00124] In one aspect, provided herein are compounds of Formula (Ib), a
pharmaceutically
acceptable salt, solvate, polymorph, prodrug, metabolite, N-oxide,
stereoisomer, or isomer
thereof:
R4
)----.--.
X
)......... 12
N
0 )r-R1
,--N, ,
R3 ' 0
A
Formula (Ib)
-49-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
wherein:
R1 is -0-(alkyl), -0-(haloalkyl), -0-(alkenyl), -0-(haloalkenyl), -0-
(alkynyl),
-0-(haloalkynyl), -0-(cycloalkyl), -0-(heterocycloalkyl), -0-(arylalkyl),
-0-(alkyl)-(alkoxy), -0-(alkyl)-(NR6R7), or -NR6R7;
R2 and R3 are independently selected from H, haloalkyl, and alkyl;
R4 is H, halogen, CN, or alkyl;
X is S or 0;
Y is CR5 or N;
R5 is H, alkyl, or halogen;
each R6 and R7 is independently selected from H and alkyl; or R6 and R7 taken
together
form a heterocycle with the nitrogen to which they are attached; and
A is heteroaryl, aryl, or heterocyclyl.
[00125] In some embodiments of a compound of Formula (Ib), Y is N. In some
embodiments
of a compound of Formula (Ib), Y is CR5.
[00126] In some embodiments of a compound of Formula (Ib), X is S. In some
embodiments
of a compound of Formula (Ib), Xis 0.
[00127] In some embodiments of a compound of Formula (Ib), A is heterocyclyl.
In some
embodiments of a compound of Formula (Ib), A is aryl. In some embodiments of a
compound
of Formula (Ib), A is heteroaryl.
[00128] In some embodiments of a compound of Formula (Ib),
X2 ----- R8TI N µzzz.
1 1 ___________________________ 1
3 ------._ 'TX1 A.
X el
>C2 x3-- >C4 R8 __
5
A is selected from: x4 B B
5
R8
N SR: 8_// N
R8) / .õ,. N .,............,..----,..
B
) 1 R '
.-----------\
N R8
B I .-----B 5 R8 5 R8
5 5
R8 R8 R8 78 R8
R8
_/ N N'R8 _t/ N µ R8------N
N R8 R8 NR8 N.-----R-. IR-
' ' 1
¨
---- ...õ..., ---- oN--.....¨/
---- ----.
R8 5 R85 R8 5 R8 R8 5
-50-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
R8 R8 R8 R8
N R8N RN N R8
4-1
R8 N R8
4-1 \ N4-1 N
N *--\r"
R8 R8 R8 5 R8 R8 5 R8 5 and
X2 X1 41.1.
>113
X x7
x6*- x6-- =
x15 x25 X35 X45 X5, X6, and X7 are independently selected from N and CR8;
each R8 is independently selected from H, halogen, CN, NO2, alkyl, -SR6, -0R6,-
NR6R7,
-NR6C(0)(alkyl), - NR6C(0)(cycloalkyl), -NR6C(0)(heterocycloalkyl),
-NR6C(0)(ary1), -NR6C(0)(heteroary1), -C(0)NR6R7, -C(0)NR6(cycloalkyl),
-C(0)NR6(heterocycloalkyl), -C(0)NR6(ary1), -C(0)NR6(heteroary1),
-NR6C(0)NR6R7, -NR6C(0)NR7(cycloalkyl), -NR6C(0)NR7(heterocycloalkyl),
-NR6C(0)NR7(ary1), -NR6C(0)NR7(heteroary1), -NR6C(0)0(alkyl),
-NR6C(0)0(cycloalkyl), -NR6C(0)0(heterocycloalkyl), -NR6C(0)0(ary1),
-NR6C(0)0(heteroary1), -NR6S02(alkyl),
-NR6S02(cycloalkyl),-NR6S02(heterocycloalkyl), -NR6S02(ary1),
-NR6S02(heteroary1), -SO2NR6R7, -SO2NR6(cycloalkyl), -
SO2NR6(heterocycloalkyl),
-SO2NR6(ary1), -SO2NR6(heteroary1), haloalkyl, aryl, and heteroaryl;
R9 is H, alkyl, aryl, heteroaryl, -S02-(alkyl), -S02-(cycloalkyl), -S02-
(aryl),
-S02-(heteroaryl), -S02-(heterocycloalkyl), -C(0)0(alkyl), -C(0)0(cycloalkyl),
-C(0)0(heterocycloalkyl), -C(0)0(ary1), -C(0)0(heteroary1), -C(0)NR6R7,
-C(0)NR6(cycloalkyl), -C(0)NR6(heterocycloalkyl), -C(0)NR6(ary1),
-C(0)NR6(heteroary1), -C(0)(alkyl), -C(0)(cycloalkyl), -
C(0)(heterocycloalkyl),
-C(0)(ary1), or -C(0)(heteroary1); and
B is 0, S, or NR2.
In further embodiments of a compound of Formula (Ib), X is S. In still further
embodiments of
a compound of Formula (Ib), X is S and Y is CH. In still further embodiments
of a compound of
Formula (Ib), X is S, Y is CH, and R4 is H.
-51-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
[00129] In some embodiments of a compound of Formula (Ib), A is selected from:
R8 R8 R8
R8 N R8./ N_....--N ) NI 8 N
1 I )------N 1 1 R----ie--_ 1
R8 SI S R8M------S R8K------S N ---,
R8R8 R8 R8
/ / / /
RI 8
R8...--- N
I ) __ 1
%."-----s
and R8N .
In further embodiments of a compound of Formula (Ib), X is S. In still
further embodiments of a compound of Formula (Ib), X is S and Y is CH.
[00130] In some embodiments of a compound of Formula (Ib), A is selected from:
R8,.............,-,...:õ,.......,...., R8......, \ R8.......õ...õ......-
õ, \ R8ZL.
1 1 1 I
N N / N
, N ,
N , and . In
further embodiments of a
,
compound of Formula (Ib), Xis S. In still further embodiments of a compound of
Formula (Ib),
Xis S and Y is CH.
[00131] In some embodiments of a compound of Formula (Ib), A is selected from:
R8 R8 R8 R8 R8
N )/8 \ _/ I\JNIµ N ---.m.----L......õ..A _.----"N
s.'").=-=A R8.----------.---N
R8 ¨c 1.õ.. \_ N R8 R8 ,
R8¨c...õ.: N R8 N R8 /
----- N-R8 NR8 R8 - --1
R8 R8 R8 R8 R8
R8
/ / / / /
R8R8 R8
R8 8 R6
N 1\N 1\.....--N R8.....õ¨,..-
--- \ ___.- N R8......,..-R R8 0 NI
R8 R8N-----N R8 N
R8N/N ------¨/ R8rN
R8 , , R8
,
R8 R8 R8 R8 R8 ,
and
,
,
3 x2õ xyl 4,,
X
II,
x, *- ,x7
-x5 x6 . In further embodiments of a compound of Formula (Ib), Xis S. In
still further
embodiments of a compound of Formula (Ib), X is S and Y is CH.
[00132] In some embodiments of a compound of Formula (Ib), R1 is -0-(alkyl), -
0-(haloalkyl),
-0-(alkenyl), -0-(haloalkenyl), -0-(alkynyl), -0-(haloalkynyl), -0-
(cycloalkyl),
-0-(heterocycloalkyl), -0-(arylalkyl), -0-(alkyl)-(alkoxy), or -0-(alkyl)-
(NR6R7). In some
embodiments of a compound of Formula (Ib), R1 is -0-(alkyl). In certain
embodiments of a
compound of Formula (Ib), R1 is ethoxy. In some embodiments of a compound of
Formula (Ib),
R1 is -0-(haloalkyl). In some embodiments of a compound of Formula (Ib), R1 is
-0-(alkeny1).
In some embodiments of a compound of Formula (Ib), R1 is -0-(haloalkeny1). In
some
-52-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
embodiments of a compound of Formula (Ib), R1 is -0-(alkyny1). In some
embodiments of a
compound of Formula (Ib), R1 is -0-(haloalkyny1). In some embodiments of a
compound of
Formula (Ib), R1 is -0-(cycloalkyl). In some embodiments of a compound of
Formula (Ib), R1 is
-0-(heterocycloalkyl). In some embodiments of a compound of Formula (Ib), R1
is
-0-(arylalkyl). In some embodiments of a compound of Formula (Ib), R1 is -0-
(alkyl)-(alkoxy).
In some embodiments of a compound of Formula (Ib), R1 is -0-(alkyl)-(NR6R7).
In further
embodiments of a compound of Formula (Ib), each R6 and R7 is independently
selected from H
and optionally substituted alkyl. In other embodiments of a compound of
Formula (Ib), R6 and
R7 taken together form a heterocycle with the nitrogen to which they are
attached.
[00133] In some embodiments of a compound of Formula (Ib), R1 is -NR6R7. In
further
embodiments of a compound of Formula (Ib), each R6 and R7 is independently
selected from H
and alkyl. In other embodiments of a compound of Formula (Ib), R6 and R7 taken
together form
a heterocycle with the nitrogen to which they are attached.
[00134] In some embodiments of a compound of Formula (Ib), R2 and R3 are both
hydrogen. In
some embodiments of a compound of Formula (Ib), R2 and R3 are both alkyl. In
some
embodiments of a compound of Formula (Ib), R2 is hydrogen and R3 is alkyl. In
some
embodiments of a compound of Formula (Ib), R2 is alkyl and R3 is hydrogen. In
some
embodiments of a compound of Formula (Ib), R2 is haloalkyl. In some
embodiments of a
compound of Formula (Ib), R3 is haloalkyl.
[00135] In one aspect, provided herein are compounds of Formula (Ic), a
pharmaceutically
acceptable salt, solvate, polymorph, prodrug, metabolite, N-oxide,
stereoisomer, or isomer
thereof:
R4
R2
Yir\---1N - R8
0
C) N R' ,
1
A
(Ic)
wherein:
Yi is S or 0;
Y3 is CR5 or N;
R2 and R3 are each independently selected from H and optionally substituted
alkyl;
R4 is H, halogen, -CN, optionally substituted alkyl, or optionally substituted
aryl;
-53-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
R5 is H, optionally substituted alkyl, or halogen;
R8 is optionally substituted alkyl, optionally substituted aryl, optionally
substituted
aralkyl, carbocyclyl, optionally substituted carbocyclylalkyl, optionally
substituted
heteroaryl, optionally substituted heteroarylalkyl, optionally substituted
heterocyclyl, optionally substituted heterocyclylalkyl, ¨RbCOORa or
¨RbCONRaRa;
or R2 and R8 taken together form an optionally substituted heterocycle with
the
nitrogen to which they are attached;
each Ra is independently selected from H and alkyl;
RD is a bond or alkylenyl; and
A is optionally substituted heteroaryl, optionally substituted aryl or
optionally
substituted heterocyclyl.
[00136] In some embodiments of a compound of Formula (Ic), R8 is optionally
substituted
alkyl. In some embodiments of a compound of Formula (Ic), R8 is optionally
substituted aryl. In
some embodiments of a compound of Formula (Ic), R8 is optionally substituted
aryl; wherein the
optionally substituted aryl is substituted with halogen. In some embodiments
of a compound of
Formula (Ic), R8 is carbocyclyl. In some embodiments of a compound of Formula
(Ic), R8 is
optionally substituted carbocyclylalkyl. In some embodiments of a compound of
Formula (Ic),
R8 is optionally substituted heteroaryl. In some embodiments of a compound of
Formula (Ic), R8
is optionally substituted heteroaryl; wherein the optionally substituted
heteroaryl is substituted
with a group selected from alkyl, -0-(alkyl) and -NR6R7. In some embodiments
of a compound
of Formula (Ic), R8 is optionally substituted heteroarylalkyl. In some
embodiments of a
compound of Formula (Ic), R8 is optionally substituted heterocyclyl. In some
embodiments of a
compound of Formula (Ic), R8 is optionally substituted heterocyclyl; wherein
the optionally
substituted heterocyclyl is substituted with alkyl. In some embodiments of a
compound of
Formula (Ic), R8 is optionally substituted heterocyclylalkyl. In some
embodiments of a
compound of Formula (Ic), R8 is
¨RbCOORa. In some embodiments of a compound of Formula (Ic), R8 is ¨RbCONRaRa.
In some
embodiments of a compound of Formula (Ic), R8 is optionally substituted alkyl,
optionally
substituted aryl, optionally substituted aralkyl, carbocyclyl, optionally
substituted
carbocyclylalkyl, optionally substituted heteroaryl, optionally substituted
heteroarylalkyl,
optionally substituted heterocyclyl, or optionally substituted
heterocyclylalkyl. In some
embodiments of a compound of Formula (Ic), R8 is optionally substituted alkyl,
carbocyclyl,
optionally substituted carbocyclylalkyl, optionally substituted heterocyclyl,
or optionally
substituted heterocyclylalkyl. In some embodiments of a compound of Formula
(Ic), R8 is
optionally substituted aryl, optionally substituted aralkyl, optionally
substituted heteroaryl, or
-54-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
optionally substituted heteroarylalkyl. In some embodiments of a compound of
Formula (Ic), R8
is optionally substituted alkyl, optionally substituted aralkyl, carbocyclyl,
optionally substituted
carbocyclylalkyl, optionally substituted heteroarylalkyl, optionally
substituted heterocyclyl, or
optionally substituted heterocyclylalkyl.
[00137] In some embodiments of a compound of Formula (Ic), R2 and R8 taken
together form
an optionally substituted heterocycle with the nitrogen to which they are
attached. In further
embodiments of a compound of Formula (Ic), the heterocycle is selected from
piperidinyl,
morpholinyl, thiomorpholinyl, optionally substituted diazepanyl and optionally
substituted
piperizanyl; wherein the optionally substituted diazepanyl and the optionally
substituted
piperizanyl are substituted with a group selected from alkyl, aryl, -COOtBu
and ¨S02Me. In
some embodiments of a compound of Formula (Ic), R2 and R8 taken together form
an optionally
substituted heterocycle with the nitrogen to which they are attached.
[00138] In some embodiments of a compound of Formula (Ic), Y3 is N. In some
embodiments
of a compound of Formula (Ic), Y3 is CR5.
[00139] In some embodiments of a compound of Formula (Ic), R5 is H. In some
embodiments
of a compound of Formula (Ic), R5 is optionally substituted alkyl. In some
embodiments of a
compound of Formula (Ic), R5 is halogen. In some embodiments of a compound of
Formula (Ic),
R5 is H or optionally substituted alkyl.
[00140] In some embodiments of a compound of Formula (Ic), Yi is 0. In some
embodiments
of a compound of Formula (Ic), Y1 is S. In some embodiments of a compound of
Formula (Ic),
Yi is S and Y3 is CH.
[00141] In some embodiments of a compound of Formula (Ic), R2 and R3 are H. In
some
embodiments of a compound of Formula (Ic), R2 and R3 are optionally
substituted alkyl. In some
embodiments of a compound of Formula (Ic), R2 is optionally substituted alkyl
and R3 is H. In
some embodiments of a compound of Formula (Ic), R2 is H and R3 is optionally
substituted
alkyl.
[00142] In some embodiments of a compound of Formula (Ic), R4 is H. In some
embodiments
of a compound of Formula (Ic), R4 is ¨CN. In some embodiments of a compound of
Formula
(Ic), R4 is optionally substituted alkyl. In some embodiments of a compound of
Formula (Ic), R4
is optionally substituted aryl. In some embodiments of a compound of Formula
(Ic), Yi is S, Y3
is CH, and R4 is H. In some embodiments of a compound of Formula (Ic), Yi is
S; Y3 is CH; R4
is H; and R2 and R3 are H.
[00143] In some embodiments of a compound of Formula (Ic), A is optionally
substituted
heteroaryl, optionally substituted aryl or optionally substituted
heterocyclyl; and the optionally
substituted aryl, the optionally substituted heterocyclyl, the optionally
substituted heteroaryl are
-55-
CA 02911326 2015-10-30
WO 2014/190199
PCT/US2014/039227
substituted with 1-6 R10; wherein each R1 is independently selected from H,
halogen, -CN, -
NO2,
-CF3, alkyl, -SR6, -0R6, -NR6R7, -NR6C(=0)(alkyl), - NR6C(=0)(cycloalkyl),
-NR6C(=0)(heterocycly1), -NR6C(=0)(ary1), -NR6C(=0)(heteroary1), -C(=0)NR6R7,
-C(=0)NR6(cycloalkyl), -C(=0)NR6(heterocycloalkyl), -C(=0)NR6(ary1),
-C(=0)NR6(heteroary1), -NR6C(=0)NR6R7, -NR6C(=0)NR7(cycloalkyl),
-NR6C(=0)NR7(heterocycloalkyl), -NR6C(=0)NR7(ary1), -NR6C(=0)NR7(heteroary1),
-NR6C(=0)0(alkyl), -NR6C(=0)0(cycloalkyl), -NR6C(=0)0(heterocycloalkyl),
-NR6C(=0)0(ary1), -NR6C(=0)0(heteroary1), -NR6S02(alkyl), -NR6S02(cycloalkyl),
-NR6S02(heterocycloalkyl), -NR6S02(ary1), -NR6S02(heteroary1), -SO2NR6R7,
-SO2NR6(cycloalkyl), -SO2NR6(heterocycloalkyl), -SR6, -S02R6, -SO2NR6(ary1),
-SO2NR6(heteroary1), haloalkyl, aryl, heteroaryl, heterocyclyl and tetrazoyl.
[00144] In some embodiments of a compound of Formula (Ic), A is selected from:
Rl N RI H\ RI 1\1 R1
R10" \% 5 N 5NN %
5 R N 5
R10
R1\tµ
N:'µµ R1
N
N-
R10 5 R113
N 5 and N . In
some embodiments of a compound of
R1
Formula (Ic), A is N .
[00145] In some embodiments of a compound of Formula (Ic), A is selected from:
R1 R1c) Rio
Rio D1O MN
1 )-1 jys,-1 I
Rio 01 S Rio'\r'S Rio
Rio Rio Rio Rio
-56-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
R10 R10
R10
R10
N R10_ Rb0-4.
)¨ I Rlo
Rlor\r"--S Rlo Rlo
, and .
In some embodiments
R1
R1
N\
R1 101
of a compound of Formula (Ic), A is R1
[00146] In one aspect, provided herein are compounds of Formula (Id), a
pharmaceutically
acceptable salt, solvate, polymorph, prodrug, metabolite, N-oxide,
stereoisomer, or isomer
thereof:
R4\
3 R9
0
OyN.R3
A
(Id)
wherein:
Y1 is S or 0;
Y3 is CR5 or N;
R3 is H, haloalkyl, or alkyl;
R4 is H, halogen, CN or optionally substituted alkyl;
R5 is H, optionally substituted alkyl, or halogen;
R9 is optionally substituted alkyl, optionally substituted heteroaryl or
optionally
substituted aryl; and
A is optionally substituted heteroaryl, optionally substituted aryl, or
optionally
substituted heterocyclyl.
[00147] In some embodiments of a compound of Formula (Id), Y3 is N. In some
embodiments
of a compound of Formula (Id), Y3 is CR5. In some embodiments of a compound of
Formula
(Id), Y3 iS CH.
-57-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
[00148] In some embodiments of a compound of Formula (Id), Yi is 0. In some
embodiments
of a compound of Formula (Id), Yi is S. In some embodiments of a compound of
Formula (Id),
Y1 is S and Y3 is CH.
[00149] In some embodiments of a compound of Formula (Id), R3 is H. In some
embodiments
of a compound of Formula (Id), R3 is haloalkyl. In some embodiments of a
compound of
Formula (Id), R3 is alkyl. In some embodiments of a compound of Formula (Id),
R3 is H or
alkyl. In some embodiments of a compound of Formula (Id), R3 is haloalkyl or
alkyl.
[00150] In some embodiments of a compound of Formula (Id), R4 is H. In some
embodiments
of a compound of Formula (Id), R4 is CN. In some embodiments of a compound of
Formula
(Id), R4 is optionally substituted alkyl. In some embodiments of a compound of
Formula (Id), Y1
is S, Y3 is CH, and R4 is H. In some embodiments of a compound of Formula
(Id), Y1 is S, Y3 is
CH, and R3 and R4 are H.
[00151] In some embodiments of a compound of Formula (Id), R5 is H. In some
embodiments
of a compound of Formula (Id), R5 is optionally substituted alkyl. In some
embodiments of a
compound of Formula (Id), R5 is halogen. In some embodiments of a compound of
Formula
(Id), R5 is H or optionally substituted alkyl. In some embodiments of a
compound of Formula
(Id), R5 is H or halogen. In some embodiments of a compound of Formula (Id),
R5 is optionally
substituted alkyl or halogen.
[00152] In some embodiments of a compound of Formula (Id), R9 is optionally
substituted
alkyl. In some embodiments of a compound of Formula (Id), R9 is optionally
substituted
heteroaryl. In some embodiments of a compound of Formula (Id), R9 is
optionally substituted
aryl.
[00153] In some embodiments of a compound of Formula (Id), A is optionally
substituted
heteroaryl. In some embodiments of a compound of Formula (Id), A is optionally
substituted
aryl. In some embodiments of a compound of Formula (Id), A is optionally
substituted
heterocyclyl.
[00154] In one aspect, provided herein are compounds of Formula (II), a
pharmaceutically
acceptable salt, solvate, polymorph, prodrug, metabolite, N-oxide,
stereoisomer, or isomer
thereof:
Mi, ....Mi.. 1
Z R
Ar
--N.,
M2 R3
I
A
-58-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
(II)
wherein:
Ar is optionally substituted aryl or optionally substituted heteroaryl;
M1 are each independently a bond, -C(=0)- or -S(=0)2-;
M2 is ¨(CH2)-, -C(=0)- or -S(=0)2-;
Z is a bond or NR2;
R1 is -0-(optionally substituted alkyl), -0-(alkenyl), -0-(alkynyl), -0-
(cycloalkyl),
-O-(heterocyclyl), -0-(optionally substituted aralkyl), -0-(optionally
substituted
heteroaralkyl), -0-(alkyl)-(alkoxy), -0-(alkyl)-(aralkoxy), -O-(alkyl)-
(heterocyclyl), -0-(alky1)-(COORa), -0-(alkyl)-(NR6R7), -NR6R7 or R8;
R2 and R3 are each independently selected from H, optionally substituted
alkyl, and
optionally substituted aryl; or R1 and R2 taken together form a heterocycle;
R6 and R7 are each independently selected from H and optionally substituted
alkyl; or
R6 and R7 taken together form an optionally substituted heterocycle with the
nitrogen to which they are attached;
R8 is optionally substituted alkyl, optionally substituted aryl, carbocyclyl,
optionally
substituted aralkyl, optionally substituted heteroaryl, optionally substituted
heterocyclyl, ¨RbCOORa or ¨RbCONRaRa;
each Ra is independently selected from H and alkyl;
RD is a bond or alkylenyl;
Rc is a bond or alkenylenyl; and
A is optionally substituted aryl, optionally substituted heterocyclyl,
optionally
substituted heteroaryl, optionally substituted carbocyclyl, optionally
substituted
aralkyl, optionally substituted heteroaralkyl or ¨Rc-(optionally substituted
heteroaryl).
[00155] In some embodiments of a compound of Formula (II), Ar is optionally
substituted aryl.
In some embodiments of a compound of Formula (II), Ar is phenyl. In some
embodiments of a
S.
compound of Formula (II), Ar is . or ,
. In some embodiments of a compound of
-59-
CA 02911326 2015-10-30
WO 2014/190199
PCT/US2014/039227
I'
Formula (II), Ar is sn,A, . In
some embodiments of a compound of Formula (II), Ar is
I>
[00156] In some embodiments of a compound of Formula (II), Ar is optionally
substituted
heteroaryl. In some embodiments of a compound of Formula (II), Ar is
pyridinyl. In some
N
I
\j_ss cv N..ss
embodiments of a compound of Formula (II), Ar is cs- 5 5 e 5 Or
N)t.
I,
1\1-1\cs
. In some embodiments of a compound of Formula (II), Ar is ir F. In some
N 2zz.
embodiments of a compound of Formula (II), Ar is csss . In some embodiments
of a
rµ
N,s
compound of Formula (II), Ar is gr . In some embodiments of a compound of
Formula
µ N)2L
I
I,
¨1
(II), Ar 1\1 . In some
embodiments of a compound of Formula (II), Ar is . 5
\
N
N
N
Or
) X3
/ X4
X3 x4c.ss y
[00157] In some embodiments of a compound of Formula (II), Ar is is"
Or vw1/4,-.=
wherein X15 )(25 X35 and X4 are independently selected from N and CR10; and
each R1 is
independently selected from H5 halogen, -CN, -NO2, -CF3, alkyl, -SR6, -OR65 -
NR6R75
-NR6C(=0)(alkyl), - NR6C(=0)(cycloalkyl), -NR6C(=0)(heterocycly1), -
NR6C(=0)(ary1),
-NR6C(=0)(heteroary1), -C(=0)NR6R75 -C(=0)NR6(cycloalkyl), -
C(=0)NR6(heterocycloalkyl),
-C(=0)NR6(ary1), -C(=0)NR6(heteroaryl) 5 -NR6C(=0)NR6R75 -
NR6C(=0)NR7(cycloalkyl),
-NR6C(=0)NR7(heterocycloalkyl), -NR6C(=0)NR7(ary1), -NR6C(=0)NR7(heteroary1),
-60-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
-NR6C(=0)0(alkyl), -NR6C(=0)0(cycloalkyl), -NR6C(=0)0(heterocycloalkyl),
-NR6C(=0)0(ary1), -NR6C(=0)0(heteroary1), -NR6S02(alkyl), -NR6S02(cycloalkyl),
-NR6S02(heterocycloalkyl), -NR6S02(ary1), -NR6S02(heteroary1), -SO2NR6R7,
-SO2NR6(cycloalkyl), -SO2NR6(heterocycloalkyl), -SR6, -S02R6, -SO2NR6(ary1),
-SO2NR6(heteroary1), haloalkyl, aryl, heteroaryl, heterocyclyl and tetrazoyl.
[00158] In some embodiments of a compound of Formula (II), each M1 is a bond.
In some
embodiments of a compound of Formula (II), each M1 is -C(=0)-. In some
embodiments of a
compound of Formula (II), each M1 is -S(=0)2-.
[00159] In some embodiments of a compound of Formula (II), Z is a bond. In
some
embodiments of a compound of Formula (II), Z is NR2. In some embodiments of a
compound
of Formula (II), Z is NR2 and each M1 is -C(=0)-.
[00160] In some embodiments of a compound of Formula (II), M2 is -C(=0)-.
[00161] In some embodiments of a compound of Formula (II), M1 and M2 are both -
C(=0)-. In
some embodiments of a compound of Formula (II), M1 and M2 are both -C(=0)-;
and Z is NR2.
[00162] In some embodiments of a compound of Formula (II), R1 is -0-
(optionally substituted
alkyl), -0-(alkenyl), -0-(alkynyl), -0-(cycloalkyl), -0-(heterocycly1), -0-
(optionally substituted
aralkyl), -0-(optionally substituted heteroaralkyl), -0-(alkyl)-(alkoxy), -0-
(alkyl)-(aralkoxy),
-0-(alkyl)-(heterocycly1), -0-(alkyl)-(C0010, or -0-(alkyl)-(NR6R7). In some
embodiments of
a compound of Formula (II), R1 is -0-(optionally substituted alkyl). In some
embodiments of a
compound of Formula (II), R1 is -0-(alkeny1). In some embodiments of a
compound of Formula
(II), R1 is -0-(alkyny1). In some embodiments of a compound of Formula (II),
R1 is -0-
(cycloalkyl). In some embodiments of a compound of Formula (II), R1 is -0-
(heterocycly1). In
some embodiments of a compound of Formula (II), R1 is -0-(optionally
substituted aralkyl). In
some embodiments of a compound of Formula (II), R1 is -0-(optionally
substituted
heteroaralkyl). In some embodiments of a compound of Formula (II), R1 is -0-
(alkyl)-(alkoxy).
In some embodiments of a compound of Formula (II), R1 is -0-(alkyl)-
(aralkoxy). In some
embodiments of a compound of Formula (II), R1 is -0-(alkyl)-(heterocycly1). In
some
embodiments of a compound of Formula (II), R1 is -0-(alkyl)-(C00Ra). In some
embodiments
of a compound of Formula (II), R1 is -0-(alkyl)-(NR6R7). In some embodiments
of a compound
of Formula (II), R1 is -0-(optionally substituted alkyl); and R2 and R3 are
both H.
[00163] In some embodiments of a compound of Formula (II), R1 is -NR6R7. In
some
embodiments of a compound of Formula (II), R6 and R7 are each independently
selected from H
and optionally substituted alkyl. In some embodiments of a compound of Formula
(II), R6 and
R7 are each independently selected from H and optionally substituted alkyl;
wherein the optional
substituent is halogen. In some embodiments of a compound of Formula (II), R6
and R7 are H. In
-61-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
some embodiments of a compound of Formula (II), R6 and R7 are each optionally
substituted
alkyl. In some embodiments of a compound of Formula (II), R6 and R7 are each
optionally
substituted alkyl; wherein the optional substituent is halogen. In some
embodiments of a
compound of Formula (II), R6 and R7 taken together form an optionally
substituted heterocycle
with the nitrogen to which they are attached. In some embodiments of a
compound of Formula
(II), R6 and R7 taken together form an optionally substituted heterocycle with
the nitrogen to
which they are attached; wherein the optional substituent is halogen.
[00164] In some embodiments of a compound of Formula (II), R1 is R8. In some
embodiments
of a compound of Formula (II), R8 is optionally substituted alkyl. In some
embodiments of a
compound of Formula (II), R8 is optionally substituted aryl. In some
embodiments of a
compound of Formula (II), R8 is carbocyclyl. In some embodiments of a compound
of Formula
(II), R8 is optionally substituted aralkyl. In some embodiments of a compound
of Formula (II),
R8 is optionally substituted heteroaryl. In some embodiments of a compound of
Formula (II), R8
is optionally substituted heterocyclyl. In some embodiments of a compound of
Formula (II), R8
is ¨RbCOORa. In some embodiments of a compound of Formula (II), R8 is
¨RbCONRaRa.
[00165] In some embodiments of a compound of Formula (II), R2 and R3 are each
independently selected from H, optionally substituted alkyl and optionally
substituted aryl. In
some embodiments of a compound of Formula (II), R2 and R3 are H. In some
embodiments of a
compound of Formula (II), R2 and R3 are each independently selected from H and
optionally
substituted alkyl. In some embodiments of a compound of Formula (II), R2 and
R3 are optionally
substituted alkyl. In some embodiments of a compound of Formula (II), R2 and
R3 are optionally
substituted aryl. In some embodiments of a compound of Formula (II), R2 and R3
are each
independently selected from optionally substituted alkyl and optionally
substituted aryl. In some
embodiments of a compound of Formula (II), R1 and R2 taken together form a
heterocycle.
[00166] In one aspect, provided herein are compounds of Formula (Ha), a
pharmaceutically
acceptable salt, solvate, polymorph, prodrug, metabolite, N-oxide,
stereoisomer, or isomer
thereof:
(R4),
Yi ''
Q z.--mi,
R1
---NA2
NA=
A
I
R3
(IIa)
wherein:
Yi is N, CH, or CR4;
-62-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
M1 are each independently a bond, -C(=0)- or -S(=0)2-;
M2 is ¨(CH2)-, -C(=0)- or -S(=0)2-;
Z is a bond or NR2;
R1 is -0-(optionally substituted alkyl), -0-(alkenyl), -0-(alkynyl), -0-
(cycloalkyl),
-O-(heterocyclyl), -0-(optionally substituted aralkyl), -0-(optionally
substituted
heteroaralkyl), -0-(alkyl)-(alkoxy), -0-(alkyl)-(aralkoxy), -O-(alkyl)-
(heterocyclyl), -0-(alkyl)-(COORa), -0-(alkyl)-(NR6R7), -NR6R7 or R8;
R2 and R3 are each independently selected from H, optionally substituted
alkyl, and
optionally substituted aryl; or R1 and R2 taken together form a heterocycle;
R4 is halogen,-CN, optionally substituted alkyl, optionally substituted
alkoxy,
optionally substituted aryl, -RbCOORa or -RbCH(COORa)2;
R6 and R7 are each independently selected from H and optionally substituted
alkyl; or
R6 and R7 taken together form an optionally substituted heterocycle with the
nitrogen to which they are attached;
R8 is optionally substituted alkyl, optionally substituted aryl, carbocyclyl,
optionally
substituted aralkyl, optionally substituted heteroaryl, optionally substituted
heterocyclyl, ¨RbCOORa or
¨RbCONRaRa;
each Ra is independently selected from H and alkyl;
Rb is a bond or alkylenyl;
Rc is a bond or alkenylenyl;
n is 0, 1, 2, or 3; and
A is optionally substituted aryl, optionally substituted heterocyclyl,
optionally
substituted heteroaryl, optionally substituted carbocyclyl, optionally
substituted
aralkyl, optionally substituted heteroaralkyl or ¨Rc-(optionally substituted
heteroaryl).
[00167] In some embodiments of a compound of Formula (Ha), Yi is N. In some
embodiments
of a compound of Formula (Ha), Y1 is CR4. In some embodiments of a compound of
Formula
(Ha), Yi is CH.
[00168] In some embodiments of a compound of Formula (Ha), each M1 is a bond.
In some
embodiments of a compound of Formula (Ha), each M1 is -C(=0)-. In some
embodiments of a
compound of Formula (Ha), each M1 is -S(=0)2-=
[00169] In some embodiments of a compound of Formula (Ha), Z is a bond. In
some
embodiments of a compound of Formula (Ha), Z is NR2. In some embodiments of a
compound
of Formula (Ha), Z is NR2 and each M1 is -C(=0)-.
-63-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
[00170] In some embodiments of a compound of Formula (Ha), -M1-Z-M1-R1 is
¨C(=0)-R1. In
further embodiments of a compound of Formula (Ha), -M1-Z-M1-R1 is ¨C(=0)-0-
(optionally
substituted alkyl). In other embodiments of a compound of Formula (Ha), -M1-Z-
M1-R1 is ¨
C(=0)- NR6R7. In still other embodiments of a compound of Formula (Ha), -M1-Z-
M1-R1 is ¨
C(=0)-R8.
[00171] In some embodiments of a compound of Formula (Ha), M2 is -C(=0)-.
[00172] In some embodiments of a compound of Formula (Ha), M1 and M2 are both -
C(=0)-.
In some embodiments of a compound of Formula (Ha), M1 and M2 are both -C(=0)-;
and Z is
NR2.
[00173] In some embodiments of a compound of Formula (Ha), R1 is -0-
(optionally substituted
alkyl), -0-(alkenyl), -0-(alkynyl), -0-(cycloalkyl), -0-(heterocycly1), -0-
(optionally substituted
aralkyl), -0-(optionally substituted heteroaralkyl), -0-(alkyl)-(alkoxy), -0-
(alkyl)-(aralkoxy),
-0-(alkyl)-(heterocycly1), -0-(alkyl)-(COORa), or -0-(alkyl)-(NR6R7). In some
embodiments of
a compound of Formula (Ha), R1 is -0-(optionally substituted alkyl). In some
embodiments of a
compound of Formula (Ha), R1 is -0-(alkeny1). In some embodiments of a
compound of
Formula (Ha), R1 is -0-(alkyny1). In some embodiments of a compound of Formula
(Ha), R1 is -
0-(cycloalkyl). In some embodiments of a compound of Formula (Ha), R1 is -0-
(heterocycly1).
In some embodiments of a compound of Formula (Ha), R1 is -0-(optionally
substituted aralkyl).
In some embodiments of a compound of Formula (Ha), R1 is -0-(optionally
substituted
heteroaralkyl). In some embodiments of a compound of Formula (Ha), R1 is -0-
(alkyl)-(alkoxy).
In some embodiments of a compound of Formula (Ha), R1 is -0-(alkyl)-
(aralkoxy). In some
embodiments of a compound of Formula (Ha), R1 is -0-(alkyl)-(heterocycly1). In
some
embodiments of a compound of Formula (Ha), R1 is -0-(alkyl)-(COORa). In some
embodiments
of a compound of Formula (Ha), R1 is -0-(alkyl)-(NR6R7). In some embodiments
of a compound
of Formula (Ha), R1 is -0-(optionally substituted alkyl); and R2 and R3 are
both H.
[00174] In some embodiments of a compound of Formula (Ha), R1 is -NR6R7. In
some
embodiments of a compound of Formula (Ha), R6 and R7 are each independently
selected from
H and optionally substituted alkyl. In some embodiments of a compound of
Formula (Ha), R6
and R7 are each independently selected from H and optionally substituted
alkyl; wherein the
optional substituent is halogen. In some embodiments of a compound of Formula
(Ha), R6 and
R7 are H. In some embodiments of a compound of Formula (Ha), R6 and R7 are
each optionally
substituted alkyl. In some embodiments of a compound of Formula (Ha), R6 and
R7 are each
optionally substituted alkyl; wherein the optional substituent is halogen. In
some embodiments
of a compound of Formula (Ha), R6 and R7 taken together form an optionally
substituted
heterocycle with the nitrogen to which they are attached. In some embodiments
of a compound
-64-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
of Formula (Ha), R6 and R7 taken together form an optionally substituted
heterocycle with the
nitrogen to which they are attached; wherein the optional substituent is
halogen.
[00175] In some embodiments of a compound of Formula (Ha), R1 is R8. In some
embodiments
of a compound of Formula (Ha), R8 is optionally substituted alkyl. In some
embodiments of a
compound of Formula (Ha), R8 is optionally substituted aryl. In some
embodiments of a
compound of Formula (Ha), R8 is carbocyclyl. In some embodiments of a compound
of Formula
(Ha), R8 is optionally substituted aralkyl. In some embodiments of a compound
of Formula (Ha),
R8 is optionally substituted heteroaryl. In some embodiments of a compound of
Formula (Ha),
R8 is optionally substituted heterocyclyl. In some embodiments of a compound
of Formula (Ha),
R8 is ¨RbCOORa. In some embodiments of a compound of Formula (Ha), R8 is
¨RbCONRaRa. In
some embodiments of a compound of Formula (Ha), R2 and R8 taken together form
an
optionally substituted heterocycle with the nitrogen to which they are
attached. In some
embodiments of a compound of Formula (Ha), R8 is optionally substituted alkyl,
optionally
substituted aryl, optionally substituted aralkyl, carbocyclyl, optionally
substituted
carbocyclylalkyl, optionally substituted heteroaryl, optionally substituted
heteroarylalkyl,
optionally substituted heterocyclyl, or optionally substituted
heterocyclylalkyl. In some
embodiments of a compound of Formula (Ha), R8 is optionally substituted alkyl,
carbocyclyl,
optionally substituted carbocyclylalkyl, optionally substituted heterocyclyl,
or optionally
substituted heterocyclylalkyl. In some embodiments of a compound of Formula
(Ha), R8 is
optionally substituted aryl, optionally substituted aralkyl, optionally
substituted heteroaryl, or
optionally substituted heteroarylalkyl. In some embodiments of a compound of
Formula (Ha), R8
is optionally substituted alkyl, optionally substituted aralkyl, carbocyclyl,
optionally substituted
carbocyclylalkyl, optionally substituted heteroarylalkyl, optionally
substituted heterocyclyl, or
optionally substituted heterocyclylalkyl.
[00176] In some embodiments of a compound of Formula (Ha), R2 and R3 are each
independently selected from H, optionally substituted alkyl, and optionally
substituted aryl. In
some embodiments of a compound of Formula (Ha), R2 and R3 are H. In some
embodiments of a
compound of Formula (Ha), R2 and R3 are each independently selected from H and
optionally
substituted alkyl. In some embodiments of a compound of Formula (Ha), R2 and
R3 are
optionally substituted alkyl. In some embodiments of a compound of Formula
(Ha), R2 and R3
are optionally substituted aryl. In some embodiments of a compound of Formula
(Ha), R2 and R3
are each independently selected from optionally substituted alkyl and
optionally substituted aryl.
In some embodiments of a compound of Formula (Ha), R1 and R2 taken together
form a
heterocycle.
-65-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
[00177] In some embodiments of a compound of Formula (Ha), R4 is halogen. In
some
embodiments of a compound of Formula (Ha), R4 is -CN. In some embodiments of a
compound
of Formula (Ha), R4 is optionally substituted alkyl. In some embodiments of a
compound of
Formula (Ha), R4 is optionally substituted alkoxy. In some embodiments of a
compound of
Formula (Ha), R4 is optionally substituted aryl. In some embodiments of a
compound of
Formula (Ha), R4 is
-RbCOORa. In some embodiments of a compound of Formula (Ha), R4 is -
RbCH(COORa)2. In
some embodiments of a compound of Formula (Ha), R4 is halogen, -CN, or
optionally
substituted alkyl. In some embodiments of a compound of Formula (Ha), R4 is
halogen, -CN,
optionally substituted alkyl, or optionally substituted aryl. In some
embodiments of a compound
of Formula (Ha), R4 is
-RbCOORa or -RbCH(COORa)2.
[00178] In one aspect, provided herein are compounds of Formula (Hb), a
pharmaceutically
acceptable salt, solvate, polymorph, prodrug, metabolite, N-oxide,
stereoisomer, or isomer
thereof:
>tNiR1
I
(R4)
n / R2
Y1 N--R3
(:).\
A
(Ith)
wherein:
Yi is N, CH, or CR4;
R1 is -0-(optionally substituted alkyl), -0-(alkenyl), -0-(alkynyl), -0-
(cycloalkyl),
-0-(heterocycly1), -0-(optionally substituted aralkyl), -0-(optionally
substituted
heteroaralkyl), -0-(alkyl)-(alkoxy), -0-(alkyl)-(aralkoxy), -0-(alkyl)-
(heterocycly1),
-0-(alkyl)-(COORa), -0-(alkyl)-(NR6R7), -NR6R7 or R8;
R2 and R3 are each independently selected from H, optionally substituted
alkyl, and
optionally substituted aryl; or R1 and R2 taken together form a heterocycle;
each R4 is independently selected from halogen,-CN, optionally substituted
alkyl,
optionally substituted alkoxy, optionally substituted aryl, -RbCOORa , and
-RbCH(COORa)2;
-66-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
R6 and R7 are each independently selected from H and optionally substituted
alkyl; or
R6 and R7 taken together form an optionally substituted heterocycle with the
nitrogen to which they are attached;
R8 is optionally substituted alkyl, optionally substituted aryl, carbocyclyl,
optionally
substituted aralkyl, optionally substituted heteroaryl, or optionally
substituted
heterocyclyl;
each Ra is independently selected from H and alkyl;
RD is a bond or alkylenyl;
Rc is a bond or alkenylenyl;
n is 0, 1, 2, or 3; and
A is optionally substituted aryl, optionally substituted heterocyclyl,
optionally
substituted heteroaryl, optionally substituted carbocyclyl, optionally
substituted
aralkyl, optionally substituted heteroaralkyl or ¨Rc-(optionally substituted
heteroaryl).
[00179] In some embodiments of a compound of Formula (lIb), Yi is N. In some
embodiments
of a compound of Formula (lIb), Y1 is N and n is 0. In some embodiments of a
compound of
Formula (IIb), Yi is N and n is 1. In some embodiments of a compound of
Formula (lIb), Y1 is
N and n is 2.
[00180] In some embodiments of a compound of Formula (lIb), Y1 is CR4. In some
embodiments of a compound of Formula (lIb), Yi is CH. In some embodiments of a
compound
of Formula (IIb), Y1 is CH and n is 0. In some embodiments of a compound of
Formula (lIb), Yi
is CH and n is 1. In some embodiments of a compound of Formula (IIb), Yi is CH
and n is 2.
[00181] In some embodiments of a compound of Formula (lIb), R1 is -0-
(optionally substituted
alkyl), -0-(alkenyl), -0-(alkynyl), -0-(cycloalkyl), -O-(heterocyclyl), -0-
(optionally substituted
aralkyl), -0-(optionally substituted heteroaralkyl), -0-(alkyl)-(alkoxy), -0-
(alkyl)-(aralkoxy),
-O-(alkyl)-(heterocyclyl), -0-(alkyl)-(COORa), or -0-(alkyl)-(NR6R7). In some
embodiments of
a compound of Formula (IIb), R1 is -0-(optionally substituted alkyl). In some
embodiments of a
compound of Formula (lIb), R1 is -0-(alkeny1). In some embodiments of a
compound of
Formula (IIb), R1 is -0-(alkyny1). In some embodiments of a compound of
Formula (lIb), R1 is -
0-(cycloalkyl). In some embodiments of a compound of Formula (lIb), R1 is -O-
(heterocyclyl).
In some embodiments of a compound of Formula (lIb), R1 is -0-(optionally
substituted aralkyl).
In some embodiments of a compound of Formula (lIb), R1 is -0-(optionally
substituted
heteroaralkyl). In some embodiments of a compound of Formula (lIb), R1 is -0-
(alkyl)-(alkoxy).
In some embodiments of a compound of Formula (lIb), R1 is -0-(alkyl)-
(aralkoxy). In some
embodiments of a compound of Formula (lIb), R1 is -O-(alkyl)-(heterocyclyl).
In some
-67-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
embodiments of a compound of Formula (JIb), R1 is -0-(a1ky1)-(COORa). In some
embodiments
of a compound of Formula (JIb), R1 is -0-(alkyl)-(NR6R7).
[00182] In some embodiments of a compound of Formula (lIb), R1 is -0-
(optionally substituted
alkyl); wherein the optionally substituted alkyl is substituted with halogen.
In further
embodiments of a compound of Formula (lIb), R1 is -0-(cycloalkyl). In still
further
embodiments of a compound of Formula (lIb), R1 is -0-(cyclobutyl), -0-
(cyclopentyl), or -0-
(cyclohexyl). In some embodiments of a compound of Formula (IIb), R1 is -0-
(heterocycly1). In
further embodiments of a compound of Formula (lIb), R1 is -0-(optionally
substituted
piperidinyl), -0-(oxetanyl), or -0-(tetrahydrofuranyl), wherein the optionally
substituted
piperidinyl is substituted with ¨COCH3. In some embodiments of a compound of
Formula (JIb),
R1 is -0-(optionally substituted alkyl); and R2 and R3 are both H.
[00183] In some embodiments of a compound of Formula (JIb), R1 is -0-
(optionally substituted
aralkyl) or -0-(optionally substituted heteroaralkyl). In further embodiments
of a compound of
Formula (IIb), the optionally substituted aralkyl and the optionally
substituted heteroaralkyl are
substituted with a group selected from halogen, alkyl and ¨CF3.
[00184] In some embodiments of a compound of Formula (JIb), R1 is -0-(alkyl)-
(heterocycly1).
In further embodiments of a compound of Formula (JIb), the heterocyclyl is
morpholinyl.
[00185] In some embodiments of a compound of Formula (JIb), R1 is -NR6R7. In
some
embodiments of a compound of Formula (JIb), R6 and R7 are each independently
selected from
H and optionally substituted alkyl. In some embodiments of a compound of
Formula (JIb), R6
and R7 are each independently selected from H and optionally substituted
alkyl; wherein the
optional substituent is halogen. In some embodiments of a compound of Formula
(JIb), R6 and
R7 are H. In some embodiments of a compound of Formula (JIb), R6 and R7 are
each optionally
substituted alkyl. In some embodiments of a compound of Formula (JIb), R6 and
R7 are each
optionally substituted alkyl; wherein the optional substituent is halogen. In
some embodiments
of a compound of Formula (JIb), R6 and R7 taken together form an optionally
substituted
heterocycle with the nitrogen to which they are attached. In some embodiments
of a compound
of Formula (IIb), R6 and R7 taken together form an optionally substituted
heterocycle with the
nitrogen to which they are attached; wherein the optional substituent is
halogen. In some
embodiments of a compound of Formula (JIb), R6 and R7 taken together form a
heterocycle with
the nitrogen to which they are attached; wherein the heterocycle is selected
from piperidinyl and
morpholinyl.
[00186] In some embodiments of a compound of Formula (JIb), R1 is R8. In some
embodiments
of a compound of Formula (JIb), R8 is optionally substituted alkyl. In some
embodiments of a
compound of Formula (JIb), R8 is optionally substituted aryl. In some
embodiments of a
-68-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
compound of Formula (JIb), R8 is optionally substituted aryl; wherein the
optionally substituted
aryl is substituted with halogen. In some embodiments of a compound of Formula
(JIb), R8 is
carbocyclyl. In some embodiments of a compound of Formula (JIb), R8 is
optionally substituted
carbocyclylalkyl. In some embodiments of a compound of Formula (Ilb), R8 is
optionally
substituted heteroaryl. In some embodiments of a compound of Formula (JIb), R8
is optionally
substituted heteroaryl; wherein the optionally substituted heteroaryl is
substituted with a group
selected from alkyl, -0-(alkyl) and -NR6R7. In some embodiments of a compound
of Formula
(JIb), R8 is optionally substituted heteroarylalkyl. In some embodiments of a
compound of
Formula (Ilb), R8 is optionally substituted heterocyclyl. In some embodiments
of a compound of
Formula (Ilb), R8 is optionally substituted heterocyclyl; wherein the
optionally substituted
heterocyclyl is substituted with alkyl. In some embodiments of a compound of
Formula (JIb), R2
and R8 taken together form an optionally substituted heterocycle with the
nitrogen to which they
are attached. In further embodiments of a compound of Formula (JIb), the
heterocycle is selected
from piperidinyl, morpholinyl, thiomorpholinyl, optionally substituted
diazepanyl and optionally
substituted piperizanyl; wherein the optionally substituted diazepanyl and the
optionally
substituted piperizanyl are substituted with a group selected from alkyl,
aryl, -COOtBu and ¨
SO2Me. In some embodiments of a compound of Formula (JIb), R8 is optionally
substituted
heterocyclylalkyl. In some embodiments of a compound of Formula (JIb), R2 and
R8 taken
together form an optionally substituted heterocycle with the nitrogen to which
they are attached.
In some embodiments of a compound of Formula (JIb), R8 is optionally
substituted alkyl,
optionally substituted aryl, optionally substituted aralkyl, carbocyclyl,
optionally substituted
carbocyclylalkyl, optionally substituted heteroaryl, optionally substituted
heteroarylalkyl,
optionally substituted heterocyclyl, or optionally substituted
heterocyclylalkyl. In some
embodiments of a compound of Formula (JIb), R8 is optionally substituted
alkyl, carbocyclyl,
optionally substituted carbocyclylalkyl, optionally substituted heterocyclyl,
or optionally
substituted heterocyclylalkyl. In some embodiments of a compound of Formula
(JIb), R8 is
optionally substituted aryl, optionally substituted aralkyl, optionally
substituted heteroaryl, or
optionally substituted heteroarylalkyl. In some embodiments of a compound of
Formula (JIb),
R8 is optionally substituted alkyl, optionally substituted aralkyl,
carbocyclyl, optionally
substituted carbocyclylalkyl, optionally substituted heteroarylalkyl,
optionally substituted
heterocyclyl, or optionally substituted heterocyclylalkyl.
[00187] In some embodiments of a compound of Formula (JIb), R4 is halogen. In
some
embodiments of a compound of Formula (JIb), R4 is -CN. In some embodiments of
a compound
of Formula (Ilb), R4 is optionally substituted alkyl. In some embodiments of a
compound of
Formula (JIb), R4 is optionally substituted alkoxy. In some embodiments of a
compound of
-69-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
Formula (Ilb), R4 is optionally substituted aryl. In some embodiments of a
compound of
Formula (Ilb), R4 is
-RbCOORa. In some embodiments of a compound of Formula (JIb), R4 is -
RbCH(COORa)2. In
some embodiments of a compound of Formula (JIb), R4 is halogen, -CN, or
optionally
substituted alkyl. In some embodiments of a compound of Formula (Ilb), R4 is
halogen, -CN,
optionally substituted alkyl, or optionally substituted aryl. In some
embodiments of a compound
of Formula (Ilb), R4 is
-RbCOORa or -RbCH(COORa)2.
[00188] In some embodiments of a compound of Formula (lIb), Yi is N; n is 1;
and R4 is
halogen,
-CN, or optionally substituted alkyl. In some embodiments of a compound of
Formula (lIb), Y1
is CH; n is 1; and R4 is halogen, -CN, or optionally substituted alkyl.
[00189] In some embodiments of a compound of Formulas (II), (ha), or (Ilb), A
is optionally
substituted aryl, optionally substituted heterocyclyl, optionally substituted
heteroaryl, optionally
substituted carbocyclyl, optionally substituted aralkyl, optionally
substituted heteroaralkyl or -
Rc-(optionally substituted heteroaryl); and the optionally substituted aryl,
the optionally
substituted heterocyclyl, the optionally substituted heteroaryl, the
optionally substituted
carbocyclyl, the optionally substituted aralkyl and the optionally substituted
heteroaralkyl are
substituted with 1-6 R10; wherein each R1 is independently selected from H,
halogen, -CN, -
NO2, -CF3, alkyl, -SR6, -0R6, -NR6R7, -NR6C(=0)(alkyl), -
NR6C(=0)(cycloalkyl),
-NR6C(=0)(heterocycly1), -NR6C(=0)(ary1), -NR6C(=0)(heteroary1), -C(=0)NR6R7,
-C(=0)NR6(cycloalkyl), -C(=0)NR6(heterocycloalkyl), -C(=0)NR6(ary1),
-C(=0)NR6(heteroaryl) , -NR6C(=0)NR6R7, -NR6C(=0)NR7(cycloalkyl),
-NR6C(=0)NR7(heterocycloalkyl), -NR6C(=0)NR7(ary1), -NR6C(=0)NR7(heteroary1),
-NR6C(=0)0(alkyl), -NR6C(=0)0(cycloalkyl), -NR6C(=0)0(heterocycloalkyl),
-NR6C(=0)0(ary1), -NR6C(=0)0(heteroary1), -NR6S02(alkyl), -NR6S02(cycloalkyl),
-NR6S02(heterocycloalkyl), -NR6S02(ary1), -NR6S02(heteroary1), -SO2NR6R7,
-SO2NR6(cycloalkyl), -SO2NR6(heterocycloalkyl), -SR6, -S02R6, -SO2NR6(ary1),
-SO2NR6(heteroary1), haloalkyl, aryl, heteroaryl, heterocyclyl and tetrazoyl.
[00190] In some embodiments of a compound of Formulas (II), (Ha), or (JIb), A
is optionally
substituted aryl. In some embodiments of a compound of Formulas (II), (Ha), or
(JIb), A is
optionally substituted heterocyclyl. In some embodiments of a compound of
Formulas (II), (Ha),
or (JIb), A is optionally substituted carbocyclyl. In some embodiments of a
compound of
Formulas (II), (Ha), or (JIb), A is optionally substituted aralkyl. In some
embodiments of a
compound of Formulas (II), (Ha), or (JIb), A is optionally substituted
heteroaralkyl. In some
-70-
CA 02911326 2015-10-30
WO 2014/190199
PCT/US2014/039227
embodiments of a compound of Formulas (II), (Ha), or (JIb), A is optionally
substituted ¨Rc-
(optionally substituted heteroaryl).
[00191] In some embodiments of a compound of Formulas (II), (ha), or (Ilb), A
is optionally
substituted heteroaryl. In some embodiments of a compound of Formulas (II),
(Ha), or (lIb), A is
R10
N .\ Rio \ )( Rio T \ Rio N \ 1
I I
selected from:
N 5 Rio" 5 N 5 :NI
N 5 \N 5
Rio
R1*\
I
N l N.)\\ R1 1\
N A
j Y
%
N-
R' Rio N R10 5 R in ¨ N 5 and N
. In some embodiments of a
Rio
N'1/2k
I
compound of Formulas (II), (Ha), or (JIb), A is N . In some embodiments
of a
x2 xi \
X3
1 1
compound of Formulas (II), (Ha), or (JIb), A is selected from X5 X6 and
X4 X3
X5'X2
.. 1
X6, -X1
X7
¨ , wherein X1, )(25 )(35 )(45 )(55 ¨65
A and X7 are independently selected from N and
CR10; and at least one of X1- X7 is N. In some embodiments of a compound of
Formulas (II),
N 01 \ I \ NN)'%k
I , 401 I
\.\./
(H N /
a), or (Ilb), A is selected from ' 5 5 5
N \
N)%k \ N ;N\ \ 1 I el 1101
Ri o 401
I
N5 N 5 N 5 R 1 0 N 5
5
N Rl Rio N N
I 01 401 / 401 / I el
N
AINFINAIM 5 PAIVNIWY. . .......... 5 and ¨ . In
some embodiments of
,X1 X1 x
X2 ,
X X5 , II X5
2
II
,¨I X3.
X4 \
a compound of Formulas (II), (Ha), or Mb), A is selected from Xt 5
-71-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
X5 Xi \ x5 xl \ , A5 xt
// "-------- X
X2
,,, =-=.,.....-- ...),./ X4
X4 x\x4 ,-I x3 X2 X4 I 1
\ X /t X3 X2 X x3 oisi 5 and
A X X3 X2 .
====,,^^
5 5 5
wherein X1, X2, X3, X4, X5, X6, and X7 are independently selected from N and
CR10; and X is
0, S, or NR2. In some embodiments of a compound of Formulas (II), (Ha), or
(Hb), A is selected
x2-x1xi
.-----N N.....,..Xl=-=\ N,...........,/, x2 N-........ xi
I I \> 1 R10_ I
I Rici- , * Rio_ I I
X3---.....
from Sx3' X2 S"-----x3' X2
x4 s Sx3 /
5 5 5 5
N....555X1x2 , xi
1N.......X1 x2
R10_ I I X2 .----"Nj N-....,, X ).\.
IRI ci_ 1 RI o_ 11
Ox3//
5 5 5 5
Xi , Xi Xi\,
N-...õ.. x2 x2 _.,....--N N--
X1
N-------jL-' )(1 R1 0- II, II, I R1 CI_
R1 0- I I oõ..-- Xi X') x7--...,.N/
N -----%3 X2
0..----%3 X2 i'R6 Re
5 .........1 5 5 5
Xi
N-..,...X1\ x2 N -,,... xi N-.,
,X, 2 ,x1 R10, j
Ri c)_
, 11 Ri o_ 11 RI o_ I I 2 \
N-----x3N,-%3 x3 ),I,
X ) __ 1
Re Re Re xo, ...;,-......_
R10
R1 IR1x T
, Xl...____
X2 \
I I _______ R1 0 R1 0_t¨' ir Ri0 / i Xi
,i0
x3 ,....s s--x3- X2 SX3' x2
X4 S'-'---x3 /
5 5 5 5
R1 R10 R10
X1Rl
Rio_t -If x2-x
X3 I I Rio Rio / R10 / II
s------r - - )(3 *--....... 1 I I
X3 *-----..f, Clx3' X2
x4 0 0 x3/
\ x4 l_J
........ 5 5 5 5 5
R10 R10 R
R:0
X1
.......... r R1
R10
X1 2 2, , x1 N X1 A,
Xi / I) \ __ 1 )1 4_Rio R1o_h IT
Rio , II ox3 x3x,4NI,R6 X3 xi
\
Ox3 X2 Nx3_ X2
0,===''' 5 R6
5 5 5 5
R1 R1 R1
___.......X1 2
R1 / X R10 /
I 1 I I Ri / I I N1// I I /N,.....,..../
Xri \
N......õ. ,,,
X3 X2 NI ---'--1 X3 5 R N X3
\NX3_ x2 s\-----õ,---, X2
R6 R6
5 5 R6 5 5
-72-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
x2 ------ \x2' ------C)\
I I I I
e x3 x4,,fN
,
....õ 5 and ---- . In some embodiments of a compound of Formulas (II),
(Ha),
R' R'
R'
N Ri o N N
N )'N 1
1.1 , )¨ I
Rio S Ri o'\r".-S
Ri o S
kr'
or (Hb), A is selected from Rio
RI o
5 Rio
5
Rio Rio Rio
Ri c....., wo
N S \ S Rio
I )¨ I Ri c:<)_____N Rio_ 0 Rio_
101 /
S
N r----/ I , ,¨ I N Rio N
i
Ri o5 Ri o'-N------ 5
S Rio Ri o
,and . In
some embodiments of a compound of Formulas (II), (Ha), or (Hb), A is selected
from
x-
2 -_-.X1
'
I X2
I
,N1---. 3 X --- 3
R2 X 5 x3...X 5 and X . In some embodiments of a
compound of
Rio Rio Rio
,OL:-.-1
N - r, 1
Rio N 5 R2 IN ,Rio S
5
Formulas (II), (Ha), or Mb), A is selected from
Rio
6_1
Rs N-N I
Rio N Rio N 5 R 1 0 S 5 Rio 0 . In some
embodiments of a
5
,...... X2 , _......x
Xc N 1
I
X4 .-----'-...----1
compound of Formulas (II), (Ha), or (Hb), A is selected from X5 '6
5
v /,õ,..X2 .11/4 1 /Xi
"3 IN \
I X3- ,..,...X2N \ iic, ....--Xi
. X2. ..---X
I \ X7 \,X7 N 1\
X4 X4 ----- .--:---x'6
\,X7 \ X7
X5 ----, i
Ni,X X6 X4 X6
--:---..."---: /
0..0". X5
¨I 5
5 5 5 5
. . . r. n n A = =
) X X
2 , ====.õ,..
X3' N" 1\ X3 N----
I \ X7 I X7
X4 --:=====.-- .. .."---v/ X4 ----,...---, /
X5 "6
, and X5 X6 ; wherein X1, )(25
)(35 )(45 )(55 X65 and X7 are
independently selected from N and CR10. In some embodiments of a compound of
Formulas
-73-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
R10 R10 R10
R10
R1C<)_ R10_
--"*" ----- 1 ,="" ---- i
R10 N / 1 Ri or
Rio Rio , Rio
(II), (Ha), or Mb), A is selected from R ,
Rio Rio
Rio Rio Rio Rio Rio
RiN \
1 r\V / I RioN R14---
RioN*---e¨ ' RiojN ' N----Rio N-NRio
Rio Rio Rio Rio Rio , Rio ,
, ,
Rio
Rio Rio
RiN Ri 4:),.....*N / R1......;:xN...r. .
NN
s...tRio
m I \ NIN 1
RiorN i RioN RicO r\r Rio,e¨
Rio ,,.-"' Rio Rio R1 , Rio Rio ,
, ,
Rio Rio Rio Rio
RicrN , N\
it i RioIN Rio_y Rio_c_L
f., ,N
IRi- N N^Rio N^Rio
Rio Rio , Rio , Rio
, ,
Rio
Ro ......N N
,N1( i
1)\. 1
N,..õ-N 4 n
Rio--)----N-N
Rio Rio Ri o
, and . In some embodiments of a compound of Formulas
R11
1
RilN ..___.N r\õ..--N1 1\1_N
(II), (Ha), or Mb), A is selected from X R11 N x
, , ,
/\--N Rio Rio =,,µõ,,,,
I 1 r\,-.,_ IR11
N R1
..----X NI I \ I Nc_ io
Rii N X
X R
111 , X , , ,
Rio R11
Rio N\
0 rc_Rio N 1 Rio
R11 N /---)( Rio
,and Rio ; wherein X is 0, S, or
NR2;
,
and R1 is H, alkyl, aryl, heteroaryl, -S02-(alkyl), -S02-(cycloalkyl), -S02-
(aryl),
-S02-(heteroary1), -S02-(heterocycloalkyl), -C(=0)0(alkyl), -
C(=0)0(cycloalkyl),
-C(=0)0(heterocycloalkyl), -C(=0)0(ary1), -C(=0)0(heteroary1), -C(=0)NR6R7,
-74-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
-C(=0)NR6(cycloalkyl), -C(=0)NR6(heterocycloalkyl), -C(=0)NR6(ary1),
-C(=0)NR6(heteroary1), -C(=0)(alkyl), -C(=0)(cycloalkyl), -
C(=0)(heterocycloalkyl),
-C(=0)(ary1), or -C(=0)(heteroary1). In some embodiments of a compound of
Formulas (II),
/.\\
\
0N I y----- N Cr:3H < 401
(Ha), or (IIb), A is selected from S 5 S0
5
H \ 0 0 S
ON 40 \ N ..___ 1 SI 0 I
H N
1 NH LAS I /
S N 0
5 5 5
R10 R10
R µ R10 \
''stl 0 , ,
HN
1 S l , 1 0 H).\1
/ R10 10
5 o 0 N
1, I Rio 0 R 0 R10
5 5 5 5
H
0 Ny0
0 0,),
5 S , 5 HN&N, \
A\1 0',1\1
..=,..... 5 0 aN S 5 and N01 .
[00192] In one aspect, provided herein are compounds of Formula (IIc), a
pharmaceutically
acceptable salt, solvate, polymorph, prodrug, metabolite, N-oxide,
stereoisomer, or isomer
thereof:
L;R12
(R4)
Y1 N--"R3
(:)\
A
MO
wherein:
Yi is N, CH, or CR4;
R12 is -NR2R8 or -0R2;
R2 and R3 are each independently selected from H and optionally substituted
alkyl;
each R4 is independently selected from halogen, -CN, optionally substituted
alkyl,
optionally substituted alkoxy, and optionally substituted aryl;
R8 is optionally substituted alkyl, optionally substituted aryl, optionally
substituted
aralkyl, carbocyclyl, optionally substituted carbocyclylalkyl, optionally
substituted
heteroaryl, optionally substituted heteroarylalkyl, optionally substituted
-75-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
heterocyclyl, optionally substituted heterocyclylalkyl, ¨RbCOORa or
¨RbCONRaRa;
or R2 and R8 taken together form an optionally substituted heterocycle with
the
nitrogen to which they are attached;
each Ra is independently selected from H and alkyl;
RD is a bond or alkylenyl;
n is 0, 1, 2, or 3; and
A is optionally substituted heteroaryl, optionally substituted aryl or
optionally
substituted heterocyclyl.
[00193] In some embodiments of a compound of Formula (IIc), Yi is N. In some
embodiments
of a compound of Formula (IIc), Yi is N and n is 0. In some embodiments of a
compound of
Formula (IIc), Y1 is N and n is 1. In some embodiments of a compound of
Formula (IIc), Yi is N
and n is 2.
[00194] In some embodiments of a compound of Formula (IIc), Yi is CR4. In some
embodiments of a compound of Formula (IIc), Y1 is CH. In some embodiments of a
compound
of Formula (IIc), Y1 is CH and n is 0. In some embodiments of a compound of
Formula (IIc), Y1
is CH and n is 1. In some embodiments of a compound of Formula (IIc), Y1 is CH
and n is 2.
[00195] In some embodiments of a compound of Formula (IIc), R2 and R3 are H.
In some
embodiments of a compound of Formula (IIc), R2 and R3 are each independently
optionally
substituted alkyl. In some embodiments of a compound of Formula (IIc), R2 and
R3 are the same
and optionally substituted alkyl. In some embodiments of a compound of Formula
(IIc), R2 is H
and R3 is optionally substituted alkyl. In some embodiments of a compound of
Formula (IIc), R2
is optionally substituted alkyl and R3 is H.
[00196] In some embodiments of a compound of Formula (IIc), R12 is -NR2R8. In
some
embodiments of a compound of Formula (IIc), R12 is -0R2. In some embodiments
of a
compound of Formula (IIc), R12 is -0R2 and R2 is optionally substituted alkyl.
[00197] In some embodiments of a compound of Formula (IIc), R8 is optionally
substituted
alkyl. In some embodiments of a compound of Formula (IIc), R8 is optionally
substituted aryl. In
some embodiments of a compound of Formula (IIc), R8 isoptionally substituted
aryl; wherein
the optionally substituted aryl is substituted with halogen. In some
embodiments of a compound
of Formula (IIc), R8 is carbocyclyl. In some embodiments of a compound of
Formula (IIc), R8 is
optionally substituted carbocyclylalkyl. In some embodiments of a compound of
Formula (IIc),
R8 isoptionally substituted heteroaryl. In some embodiments of a compound of
Formula (IIc),
R8 isoptionally substituted heteroaryl; wherein the optionally substituted
heteroaryl is
substituted with a group selected from alkyl, -0-(alkyl) and -NR6R7. In some
embodiments of a
compound of Formula (IIc), R8 isoptionally substituted heteroarylalkyl. In
some embodiments
-76-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
of a compound of Formula (IIc), R8 is optionally substituted heterocyclyl. In
some embodiments
of a compound of Formula (IIc), R8 is optionally substituted heterocyclyl;
wherein the optionally
substituted heterocyclyl is substituted with alkyl. In some embodiments of a
compound of
Formula (IIc), R2 and R8 taken together form an optionally substituted
heterocycle with the
nitrogen to which they are attached. In further embodiments of a compound of
Formula (IIc), the
heterocycle is selected from piperidinyl, morpholinyl, thiomorpholinyl,
optionally substituted
diazepanyl and optionally substituted piperizanyl; wherein the optionally
substituted diazepanyl
and the optionally substituted piperizanyl are substituted with a group
selected from alkyl, aryl, -
COOtBu and ¨S02Me. In some embodiments of a compound of Formula (IIc), R8 is
optionally
substituted heterocyclylalkyl. In some embodiments of a compound of Formula
(IIc), R8 is ¨
RbCOORa. In some embodiments of a compound of Formula (IIc), R8 is ¨RbCONRaRa.
In some
embodiments of a compound of Formula (IIc), R2 and R8 taken together form an
optionally
substituted heterocycle with the nitrogen to which they are attached. In some
embodiments of a
compound of Formula (IIc), R8 is optionally substituted alkyl, optionally
substituted aryl,
optionally substituted aralkyl, carbocyclyl, optionally substituted
carbocyclylalkyl, optionally
substituted heteroaryl, optionally substituted heteroarylalkyl, optionally
substituted heterocyclyl,
or optionally substituted heterocyclylalkyl. In some embodiments of a compound
of Formula
(IIc), R8 is optionally substituted alkyl, carbocyclyl, optionally substituted
carbocyclylalkyl,
optionally substituted heterocyclyl, or optionally substituted
heterocyclylalkyl. In some
embodiments of a compound of Formula (IIc), R8 is optionally substituted aryl,
optionally
substituted aralkyl, optionally substituted heteroaryl, or optionally
substituted heteroarylalkyl. In
some embodiments of a compound of Formula (IIc), R8 is optionally substituted
alkyl, optionally
substituted aralkyl, carbocyclyl, optionally substituted carbocyclylalkyl,
optionally substituted
heteroarylalkyl, optionally substituted heterocyclyl, or optionally
substituted heterocyclylalkyl.
[00198] In some embodiments of a compound of Formula (IIc), A is optionally
substituted
heteroaryl. In some embodiments of a compound of Formula (IIc), A is
optionally substituted
aryl. In some embodiments of a compound of Formula (IIc), A is optionally
substituted
heterocyclyl. In some embodiments of a compound of Formula (IIc), A is
optionally substituted
heteroaryl, optionally substituted aryl or optionally substituted
heterocyclyl; and the optionally
substituted aryl, the optionally substituted heterocyclyl, the optionally
substituted heteroaryl are
substituted with 1-6 R10; wherein each R1 is independently selected from H,
halogen, -CN,
-NO2, -CF3, alkyl, -SR6, -0R6, -NR6R7, -NR6C(=0)(alkyl), -
NR6C(=0)(cycloalkyl),
-NR6C(=0)(heterocycly1), -NR6C(=0)(ary1), -NR6C(=0)(heteroary1), -C(=0)NR6R7,
-C(=0)NR6(cycloalkyl), -C(=0)NR6(heterocycloalkyl), -C(=0)NR6(ary1),
-C(=0)NR6(heteroary1), -NR6C(=0)NR6R7, -NR6C(=0)NR7(cycloalkyl),
-77-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
-NR6C(=0)NR7(heterocycloalkyl), -NR6C(=0)NR7(ary1), -NR6C(=0)NR7(heteroary1),
-NR6C(=0)0(alkyl), -NR6C(=0)0(cycloalkyl), -NR6C(=0)0(heterocycloalkyl),
-NR6C(=0)0(ary1), -NR6C(=0)0(heteroary1), -NR6S02(alkyl), -NR6S02(cycloalkyl),
-NR6S02(heterocycloalkyl), -NR6S02(ary1), -NR6S02(heteroary1), -SO2NR6R75
-SO2NR6(cycloalkyl), -SO2NR6(heterocycloalkyl), -SR6, -S02R65 -SO2NR6(ary1),
-SO2NR6(heteroary1), haloalkyl, aryl, heteroaryl, heterocyclyl and tetrazoyl.
[00199] In some embodiments of a compound of Formula (IIc), A is selected
from:
Rlo \
N \i, \ R1 Rlo \ Rio N \
µ
, K ,
11
\N 5 R10" \% 5 N 5 NN
.\õ..51.,..'11 5 R' N 5
R10
R1W\
1 N\. R1
N N \
1 k 1
N.
R10 5 R10 N. N 5 and . In
some embodiments of a compound of
R1-\
Formula (IIc), A is N .
[00200] In some embodiments of a compound of Formula (IIc), A is selected
from:
R' R' R'
Ri oRiyiN
N Rio N IN ,
'
-..,,..- õ....:,õõ N 1\1 i
Rio S Rio'\r'S R1 OS NLr----. S
Rio Ri o Rio Rio
5 5 5 5
Rio Rio
R1
S \ SRio
IR1 N R10¨ R10¨
0
N (00 Rio N
if
i
Rior\J"--"S Rio Ri o
5 and . In some embodiments
5
of a
Rio
Rio
N\ 1
Rio 1101 2¨
compound of Formula (IIc), A is Rio .
[00201] In some embodiments of a compound of Formula (IIc), R4 is halogen. In
some
embodiments of a compound of Formula (IIc), R4 is -CN. In some embodiments of
a compound
of Formula (IIc), R4 is optionally substituted alkyl. In some embodiments of a
compound of
Formula (IIc), R4 is optionally substituted alkoxy. In some embodiments of a
compound of
-78-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
Formula (lie), R4 is optionally substituted aryl. In some embodiments of a
compound of
Formula (lie), R4 is halogen,
-CN, or optionally substituted alkyl. In some embodiments of a compound of
Formula (lic), R4
is halogen, -CN, alkyl, or aryl.
[00202] In one aspect, provided herein are compounds, or pharmaceutically
acceptable salts,
solvates, polymorphs, prodrugs, metabolites, N-oxides, stereoisomers, or
isomers thereof,
0 0
00
0 N AO
H NH Nj-LO
NH 0 0 H
oNH NH
0cS)1 0S HN 0
N 0 0>
N 0 N 0
selected from:
0
CO2LC)
N N H
0
NU 0 ,and I U
Preparation of Compounds
[00203] Described herein are compounds of Formulas (I), (Ia), (Ib), (Ic),
(Id), (II), (Ha), (lib),
and (lie) that treat drug resistant and persistent tuberculosis, and processes
for their preparation.
Also described herein are pharmaceutically acceptable salts, pharmaceutically
acceptable
solvates, pharmaceutically active metabolites, and pharmaceutically acceptable
prodrugs of such
compounds. Pharmaceutical compositions comprising at least one such compound
or a
pharmaceutically acceptable salt, pharmaceutically acceptable solvate,
pharmaceutically active
metabolite or pharmaceutically acceptable prodrug of such compound, and a
pharmaceutically
acceptable excipient are also provided.
[00204] Compounds of of Formulas (I), (Ia), (Ib), (Ic), (Id), (II), (Ha),
(lib), and (lie) may be
synthesized using standard synthetic reactions known to those of skill in the
art or using methods
known in the art. The reactions can be employed in a linear sequence to
provide the compounds
or they may be used to synthesize fragments which are subsequently joined by
the methods
known in the art.
[00205] The starting material used for the synthesis of the compounds
described herein may be
synthesized or can be obtained from commercial sources, such as, but not
limited to, Aldrich
Chemical Co. (Milwaukee, Wisconsin), Bachem (Torrance, California), or Sigma
Chemical Co.
-79-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
(St. Louis, Mo.). The compounds described herein, and other related compounds
having
different substituents can be synthesized using techniques and materials known
to those of skill
in the art, such as described, for example, in March, ADVANCED ORGANIC
CHEMISTRY 4th Ed.,
(Wiley 1992); Carey and Sundberg, ADVANCED ORGANIC CHEMISTRY 4th Ed., Vols. A
and B
(Plenum 2000, 2001); Green and Wuts, PROTECTIVE GROUPS IN ORGANIC SYNTHESIS
3rd Ed.,
(Wiley 1999); Fieser and Fieser's Reagents for Organic Synthesis, Volumes 1-17
(John Wiley
and Sons, 1991); Rodd's Chemistry of Carbon Compounds, Volumes 1-5 and
Supplementals
(Elsevier Science Publishers, 1989); Organic Reactions, Volumes 1-40 (John
Wiley and Sons,
1991); and Larock's Comprehensive Organic Transformations (VCH Publishers
Inc., 1989). (all
of which are incorporated by reference in their entirety). Other methods for
the synthesis of
compounds described herein may be found in International Patent Publication
No. WO
01/01982901, Arnold et al. Bioorganic & Medicinal Chemistry Letters 10 (2000)
2167-2170;
Burchat et at. Bioorganic & Medicinal Chemistry Letters 12 (2002) 1687-1690.
General
methods for the preparation of compound as disclosed herein may be derived
from known
reactions in the field, and the reactions may be modified by the use of
appropriate reagents and
conditions, as would be recognized by the skilled person, for the introduction
of the various
moieties found in the formulae as provided herein.
[00206] The products of the reactions may be isolated and purified, if
desired, using
conventional techniques, including, but not limited to, filtration,
distillation, crystallization,
chromatography and the like. Such materials may be characterized using
conventional means,
including physical constants and spectral data.
[00207] Compounds described herein may be prepared as a single isomer or a
mixture of
isomers.
Further Forms of Compounds Disclosed Herein
Isomers
[00208] Furthermore, in some embodiments, the compounds described herein exist
as
geometric isomers. In some embodiments, the compounds described herein possess
one or more
double bonds. The compounds presented herein include all cis, trans, syn,
anti, entgegen (E),
and zusammen (Z) isomers as well as the corresponding mixtures thereof. In
some situations,
compounds exist as tautomers. The compounds described herein include all
possible tautomers
within the formulas described herein. In some situations, the compounds
described herein
possess one or more chiral centers and each center exists in the R
configuration, or S
confirguration. The compounds described herein include all diastereomeric,
enantiomeric, and
epimeric forms as well as the corresponding mixtures thereof. In additional
embodiments of the
-80-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
compounds and methods provided herein, mixtures of enantiomers and/or
diastereoisomers,
resulting from a single preparative step, combination, or interconversion are
useful for the
applications described herein. In some embodiments, the compounds described
herein are
prepared as their individual stereoisomers by reacting a racemic mixture of
the compound with
an optically active resolving agent to form a pair of diastereoisomeric
compounds, separating the
diastereomers and recovering the optically pure enantiomers. In some
embodiments, dissociable
complexes are preferred (e.g., crystalline diastereomeric salts). In some
embodiments, the
diastereomers have distinct physical properties (e.g., melting points, boiling
points, solubilities,
reactivity, etc.) and are separated by taking advantage of these
dissimilarities. In some
embodiments, the diastereomers are separated by chiral chromatography, or
preferably, by
separation/resolution techniques based upon differences in solubility. In some
embodiments, the
optically pure enantiomer is then recovered, along with the resolving agent,
by any practical
means that would not result in racemization.
Labeled compounds
[00209] In some embodiments, the compounds described herein exist in their
isotopically-labeled forms. In some embodiments, the methods disclosed herein
include methods
of treating diseases by administering such isotopically-labeled compounds. In
some
embodiments, th emethods disclosed herein include methods of treating diseases
by
administering such isotopically-labeled compounds as pharmaceutical
compositions. Thus, in
some embodiments, the compounds disclosed herein include isotopically-labeled
compounds,
which are identical to those recited herein, but for the fact that one or more
atoms are replaced
by an atom having an atomic mass or mass number different from the atomic mass
or mass
number usually found in nature. Examples of isotopes that can be incorporated
into compounds
of the invention include isotopes of hydrogen, carbon, nitrogen, oxygen,
phosphorous, sulfur,
fluorine and chloride, such as 2H5 3H5 13C5 14C, '5N,1805 1705 31P5 32P5 35s5
5 18¨F and 36C1,
respectively. Compounds described herein, and the metabolites,
pharmaceutically acceptable
salts, esters, prodrugs, solvate, hydrates or derivatives thereof which
contain the aforementioned
isotopes and/or other isotopes of other atoms are within the scope of this
invention. Certain
isotopically-labeled compounds, for example those into which radioactive
isotopes such as 3H
and 14C are incorporated, are useful in drug and/or substrate tissue
distribution assays. Tritiated,
i. e., 3H and carbon-14, i. e., 14C, isotopes are particularly preferred for
their ease of preparation
and detectability. Further, substitution with heavy isotopes such as
deuterium, i.e., 2H, produces
certain therapeutic advantages resulting from greater metabolic stability, for
example increased
in vivo half-life or reduced dosage requirements. In some embodiments, the
isotopically labeled
-81-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
compounds, pharmaceutically acceptable salt, ester, prodrug, solvate, hydrate
or derivative
thereof is prepared by any suitable method.
[00210] In some embodiments, the compounds described herein are labeled by
other means,
including, but not limited to, the use of chromophores or fluorescent
moieties, bioluminescent
labels, or chemiluminescent labels.
Pharmaceutically acceptable salts
[00211] In some embodiments, the compounds described herein exist as their
pharmaceutically
acceptable salts. In some embodiments, the methods disclosed herein include
methods of
treating diseases by administering such pharmaceutically acceptable salts. In
some
embodiments, the methods disclosed herein include methods of treating diseases
by
administering such pharmaceutically acceptable salts as pharmaceutical
compositions.
[00212] In some embodiments, the compounds described herein possess acidic or
basic groups
and therefore react with any of a number of inorganic or organic bases, and
inorganic and
organic acids, to form a pharmaceutically acceptable salt. In some
embodiments, these salts are
prepared in situ during the final isolation and purification of the compounds
of the invention, or
by separately reacting a purified compound in its free form with a suitable
acid or base, and
isolating the salt thus formed.
[00213] Examples of pharmaceutically acceptable salts include those salts
prepared by reaction
of the compounds described herein with a mineral, organic acid or inorganic
base, such salts
including, acetate, acrylate, adipate, alginate, aspartate, benzoate,
benzenesulfonate, bisulfate,
bisulfite, bromide, butyrate, butyn-1,4-dioate, camphorate, camphorsulfonate,
caproate,
caprylate, chlorobenzoate, chloride, citrate, cyclopentanepropionate,
decanoate, digluconate,
dihydrogenphosphate, dinitrobenzoate, dodecylsulfate, ethanesulfonate,
formate, fumarate,
glucoheptanoate, glycerophosphate, glycolate, hemisulfate, heptanoate,
hexanoate,
hexyne-1,6-dioate, hydroxybenzoate, y-hydroxybutyrate, hydrochloride,
hydrobromide,
hydroiodide, 2-hydroxyethanesulfonate, iodide, isobutyrate, lactate, maleate,
malonate,
methanesulfonate, mandelate metaphosphate, methanesulfonate, methoxybenzoate,
methylbenzoate, monohydrogenphosphate, 1-napthalenesulfonate, 2-
napthalenesulfonate,
nicotinate, nitrate, palmoate, pectinate, persulfate, 3-phenylpropionate,
phosphate, picrate,
pivalate, propionate, pyrosulfate, pyrophosphate, propiolate, phthalate,
phenylacetate,
phenylbutyrate, propanesulfonate, salicylate, succinate, sulfate, sulfite,
succinate, suberate,
sebacate, sulfonate, tartrate, thiocyanate, tosylate undeconate and
xylenesulfonate.
[00214] Further, the compounds described herein can be prepared as
pharmaceutically
acceptable salts formed by reacting the free base form of the compound with a
pharmaceutically
acceptable inorganic or organic acid, including, but not limited to, inorganic
acids such as
-82-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
hydrochloric acid, hydrobromic acid, sulfuric acid, nitric acid, phosphoric
acid metaphosphoric
acid, and the like; and organic acids such as acetic acid, propionic acid,
hexanoic acid,
cyclopentanepropionic acid, glycolic acid, pyruvic acid, lactic acid, malonic
acid, succinic acid,
malic acid, maleic acid, fumaric acid, p-toluenesulfonic acid, tartaric acid,
trifluoroacetic acid,
citric acid, benzoic acid, 3-(4-hydroxybenzoyl)benzoic acid, cinnamic acid,
mandelic acid,
arylsulfonic acid, methanesulfonic acid, ethanesulfonic acid, 1,2-
ethanedisulfonic acid,
2-hydroxyethanesulfonic acid, benzenesulfonic acid, 2-naphthalenesulfonic
acid,
4-methylbicyclo-[2.2.2]oct-2-ene-1-carboxylic acid, glucoheptonic acid,
4,4'-methylenebis-(3-hydroxy-2-ene-1 -carboxylic acid), 3-phenylpropionic
acid,
trimethylacetic acid, tertiary butylacetic acid, lauryl sulfuric acid,
gluconic acid, glutamic acid,
hydroxynaphthoic acid, salicylic acid, stearic acid and muconic acid. In some
embodiments,
other acids, such as oxalic, while not in themselves pharmaceutically
acceptable, are employed
in the preparation of salts useful as intermediates in obtaining the compounds
of the invention
and their pharmaceutically acceptable acid addition salts.
[00215] In some embodiments, those compounds described herein which comprise a
free acid
group react with a suitable base, such as the hydroxide, carbonate,
bicarbonate, sulfate, of a
pharmaceutically acceptable metal cation, with ammonia, or with a
pharmaceutically acceptable
organic primary, secondary, tertiary, or quaternary amine. Representative
salts include the alkali
or alkaline earth salts, like lithium, sodium, potassium, calcium, and
magnesium, and aluminum
salts and the like. Illustrative examples of bases include sodium hydroxide,
potassium
hydroxide, choline hydroxide, sodium carbonate, N'(Ci_4 alky1)4, and the like.
[00216] Representative organic amines useful for the formation of base
addition salts include
ethylamine, diethylamine, ethylenediamine, ethanolamine, diethanolamine,
piperazine and the
like. It should be understood that the compounds described herein also include
the
quaternization of any basic nitrogen-containing groups they contain. In some
embodiments,
water or oil-soluble or dispersible products are obtained by such
quaternization.
Solvates
[00217] In some embodiments, the compounds described herein exist as solvates.
The invention
provides for methods of treating diseases by administering such solvates. The
invention further
provides for methods of treating diseases by administering such solvates as
pharmaceutical
compositions.
[00218] Solvates contain either stoichiometric or non-stoichiometric amounts
of a solvent, and,
in some embodiments, are formed during the process of crystallization with
pharmaceutically
acceptable solvents such as water, ethanol, and the like. Hydrates are formed
when the solvent is
water, or alcoholates are formed when the solvent is alcohol. Solvates of the
compounds
-83-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
described herein can be conveniently prepared or formed during the processes
described herein.
By way of example only, hydrates of the compounds described herein can be
conveniently
prepared by recrystallization from an aqueous/organic solvent mixture, using
organic solvents
including, but not limited to, dioxane, tetrahydrofuran or methanol. In
addition, the compounds
provided herein can exist in unsolvated as well as solvated forms. In general,
the solvated forms
are considered equivalent to the unsolvated forms for the purposes of the
compounds and
methods provided herein.
Polymorphs
[00219] In some embodiments, the compounds described herein exist as
polymorphs. The
invention provides for methods of treating diseases by administering such
polymorphs. The
invention further provides for methods of treating diseases by administering
such polymorphs as
pharmaceutical compositions.
[00220] Thus, the compounds described herein include all their crystalline
forms, known as
polymorphs. Polymorphs include the different crystal packing arrangements of
the same
elemental composition of a compound. In certain instances, polymorphs have
different X-ray
diffraction patterns, infrared spectra, melting points, density, hardness,
crystal shape, optical and
electrical properties, stability, and solubility. In certain instances,
various factors such as the
recrystallization solvent, rate of crystallization, and storage temperature
cause a single crystal
form to dominate.
Prodrugs
[00221] In some embodiments, the compounds described herein exist in prodrug
form. The
invention provides for methods of treating diseases by administering such
prodrugs. The
invention further provides for methods of treating diseases by administering
such prodrugs as
pharmaceutical compositions.
[00222] Prodrugs are generally drug precursors that, following administration
to an individual
and subsequent absorption, are converted to an active, or a more active
species via some
process, such as conversion by a metabolic pathway. Some prodrugs have a
chemical group
present on the prodrug that renders it less active and/or confers solubility
or some other property
to the drug. Once the chemical group has been cleaved and/or modified from the
prodrug the
active drug is generated. Prodrugs are often useful because, in some
situations, they are easier to
administer than the parent drug. They are, for instance, bioavailable by oral
administration
whereas the parent is not. In certain insatnces, the prodrug also has improved
solubility in
pharmaceutical compositions over the parent drug. An example, without
limitation, of a prodrug
would be a compound as described herein which is administered as an ester (the
"prodrug") to
-84-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
facilitate transmittal across a cell membrane where water solubility is
detrimental to mobility but
which then is metabolically hydrolyzed to the carboxylic acid, the active
entity, once inside the
cell where water-solubility is beneficial. A further example of a prodrug
might be a short peptide
(polyamino acid) bonded to an acid group where the peptide is metabolized to
reveal the active
moiety. (See for example Bundgaard, "Design and Application of Prodrugs" in A
Textbook of
Drug Design and Development, Krosgaard-Larsen and Bundgaard, Ed., 1991,
Chapter 5,
113-191, which is incorporated herein by reference).
[00223] In some embodiments, prodrugs are designed as reversible drug
derivatives, for use as
modifiers to enhance drug transport to site-specific tissues. The design of
prodrugs to date has
been to increase the effective water solubility of the therapeutic compound
for targeting to
regions where water is the principal solvent.
[00224] Additionally, prodrug derivatives of compounds described herein can be
prepared by
methods described herein are otherwise known in the art (for further details
see Saulnier et al.,
Bioorganic and Medicinal Chemistry Letters, 1994, 4, 1985). By way of example
only,
appropriate prodrugs can be prepared by reacting a non-derivatized compound
with a suitable
carbamylating agent, such as, but not limited to, 1,1-
acyloxyalkylcarbanochloridate,
para-nitrophenyl carbonate, or the like. Prodrug forms of the herein described
compounds,
wherein the prodrug is metabolized in vivo to produce a derivative as set
forth herein are
included within the scope of the claims. Indeed, some of the herein-described
compounds are
prodrugs for another derivative or active compound.
[00225] In some embodiments, prodrugs include compounds wherein an amino acid
residue, or
a polypeptide chain of two or more (e. g., two, three or four) amino acid
residues is covalently
joined through an amide or ester bond to a free amino, hydroxy or carboxylic
acid group of
compounds of the present invention. The amino acid residues include but are
not limited to the
20 naturally occurring amino acids and also includes 4-hydroxyproline,
hydroxylysine,
demosine, isodemosine, 3-methylhistidine, norvaline, beta-alanine, gamma-
aminobutyric acid,
cirtulline, homocysteine, homoserine, ornithine and methionine sulfone. In
other embodiments,
prodrugs include compounds wherein a nucleic acid residue, or an
oligonucleotide of two or
more (e. g., two, three or four) nucleic acid residues is covalently joined to
a compound of the
present invention.
[00226] Pharmaceutically acceptable prodrugs of the compounds described herein
also include,
but are not limited to, esters, carbonates, thiocarbonates, N-acyl
derivatives, N-acyloxyalkyl
derivatives, quaternary derivatives of tertiary amines, N-Mannich bases,
Schiff bases, amino
acid conjugates, phosphate esters, metal salts and sulfonate esters. Compounds
having free
amino, amido, hydroxy or carboxylic groups can be converted into prodrugs. For
instance, free
-85-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
carboxyl groups can be derivatized as amides or alkyl esters. In certain
instances, all of these
prodrug moieties incorporate groups including but not limited to ether, amine
and carboxylic
acid functionalities.
[00227] Hydroxy prodrugs include esters, such as though not limited to,
acyloxyalkyl (e.g.
acyloxymethyl, acyloxyethyl) esters, alkoxycarbonyloxyalkyl esters, alkyl
esters, aryl esters,
phosphate esters, sulfonate esters, sulfate esters and disulfide containing
esters; ethers, amides,
carbamates, hemisuccinates, dimethylaminoacetates and
phosphoryloxymethyloxycarbonyls, as
outlined in Advanced Drug Delivery Reviews 1996, 19, 115.
[00228] Amine derived prodrugs include, but are not limited to the following
groups and
combinations of groups:
RR
-NAR -NA0- -NAS-R -NA0- -NAS-R -1\ILOAR
-1\1).0 -NSAR -NOR -NSAR
N
R' S
A , ,R A ,R
-N 0 SR -N 0 0
as well as sulfonamides and phosphonamides.
[00229] In certain instances, sites on any aromatic ring portions are
susceptible to various
metabolic reactions, therefore incorporation of appropriate substituents on
the aromatic ring
structures, can reduce, minimize or eliminate this metabolic pathway.
Metabolites
[00230] In some embodiments, compounds of Formulas (I), (Ia), (Ib), (Ic),
(Id), (II), (IIa), (IIb),
and (IIc) are susceptible to various metabolic reactions. Therefore, in some
embodiments,
incorporation of appropriate substituents into the structure will reduce,
minimize, or eliminate a
metabolic pathway. In specific embodiments, the appropriate substituent to
decrease or
eliminate the susceptibility of an aromatic ring to metabolic reactions is, by
way of example
only, a halogen, or an alkyl group.
[00231] In additional or further embodiments, the compounds of Formulas (I),
(Ia), (Ib), (Ic),
(Id), (II), (Ha), (lIb), and (IIc) described herein are metabolized upon
administration to an
organism in need to produce a metabolite that is then used to produce a
desired effect, including
a desired therapeutic effect.
-86-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
Pharmaceutical Compositions/Formulations
[00232] In another aspect, provided herein are pharmaceutical composition
comprising a
compound of Formulas (I), (Ia), (Ib), (Ic), (Id), (II), (Ha), (lIb), or (IIc)
as described herein, or a
pharmaceutically acceptable salt, polymorph, solvate, prodrug, N-oxide,
stereoisomer, or isomer
thereof, and a pharmaceutically acceptable excipient.
[00233] In some embodiments, the compounds described herein are formulated
into
pharmaceutical compositions. Pharmaceutical compositions are formulated in a
conventional
manner using one or more pharmaceutically acceptable inactive ingredients that
facilitate
processing of the active compounds into preparations that can be used
pharmaceutically. Proper
formulation is dependent upon the route of administration chosen. A summary of
pharmaceutical
compositions described herein can be found, for example, in Remington: The
Science and
Practice of Pharmacy, Nineteenth Ed (Easton, Pa.: Mack Publishing Company,
1995); Hoover,
John E., Remington's Pharmaceutical Sciences, Mack Publishing Co., Easton,
Pennsylvania
1975; Liberman, H.A. and Lachman, L., Eds., Pharmaceutical Dosage Forms,
Marcel Decker,
New York, N.Y., 1980; and Pharmaceutical Dosage Forms and Drug Delivery
Systems, Seventh
Ed. (Lippincott Williams & Wilkins1999), herein incorporated by reference for
such disclosure.
[00234] Provided herein are pharmaceutical compositions that include a
compound of Formulas
(I), (Ia), (Ib), (Ic), (Id), (II), (Ha), (lIb), or (IIc) and at least one
pharmaceutically acceptable
inactive ingredient. In some embodiments, the compounds described herein are
administered as
pharmaceutical compositions in which a compound of Formulas (I), (Ia), (Ib),
(Ic), (Id), (II),
(Ha), (lIb), or (IIc) is mixed with other active ingredients, as in
combination therapy. In other
embodiments, the pharmaceutical compositions include other medicinal or
pharmaceutical
agents, carriers, adjuvants, preserving, stabilizing, wetting or emulsifying
agents, solution
promoters, salts for regulating the osmotic pressure, and/or buffers. In yet
other embodiments,
the pharmaceutical compositions include other therapeutically valuable
substances.
[00235] A pharmaceutical composition, as used herein, refers to a mixture of a
compound of
Formulas (I), (Ia), (Ib), (Ic), (Id), (II), (Ha), (lIb), or (IIc) with other
chemical components (i.e.
pharmaceutically acceptable inactive ingredients), such as carriers,
excipients, binders, filling
agents, suspending agents, flavoring agents, sweetening agents, disintegrating
agents, dispersing
agents, surfactants, lubricants, colorants, diluents, solubilizers, moistening
agents, plasticizers,
stabilizers, penetration enhancers, wetting agents, anti-foaming agents,
antioxidants,
preservatives, or one or more combination thereof The pharmaceutical
composition facilitates
administration of the compound to an organism. In practicing the methods of
treatment or use
provided herein, therapeutically effective amounts of compounds described
herein are
administered in a pharmaceutical composition to a mammal having a disease,
disorder, or
-87-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
condition to be treated. In some embodiments, the mammal is a human. A
therapeutically
effective amount can vary widely depending on the severity of the disease, the
age and relative
health of the subject, the potency of the compound used and other factors. The
compounds can
be used singly or in combination with one or more therapeutic agents as
components of
mixtures.
[00236] The pharmaceutical formulations described herein are administered to a
subject by
appropriate administration routes, including but not limited to, oral,
parenteral (e.g., intravenous,
subcutaneous, intramuscular), intranasal, buccal, topical, rectal, or
transdermal administration
routes. The pharmaceutical formulations described herein include, but are not
limited to,
aqueous liquid dispersions, liquids, gels, syrups, elixirs, slurries,
suspensions, self-emulsifying
dispersions, solid solutions, liposomal dispersions, aerosols, solid oral
dosage forms, powders,
immediate release formulations, controlled release formulations, fast melt
formulations, tablets,
capsules, pills, powders, dragees, effervescent formulations, lyophilized
formulations, delayed
release formulations, extended release formulations, pulsatile release
formulations,
multiparticulate formulations, and mixed immediate and controlled release
formulations.
[00237] Pharmaceutical compositions including a compound of Formulas (I),
(Ia), (Ib), (Ic),
(Id), (II), (Ha), (JIb), or (IIc) are manufactured in a conventional manner,
such as, by way of
example only, by means of conventional mixing, dissolving, granulating, dragee-
making,
levigating, emulsifying, encapsulating, entrapping or compression processes.
[00238] The pharmaceutical compositions will include at least one compound of
Formulas (I),
(Ia), (Ib), (Ic), (Id), (II), (Ha), (JIb), or (IIc) as an active ingredient in
free-acid or free-base form,
or in a pharmaceutically acceptable salt form. In addition, the methods and
pharmaceutical
compositions described herein include the use of N-oxides (if appropriate),
crystalline forms,
amorphous phases, as well as active metabolites of these compounds having the
same type of
activity. In some embodiments, compounds described herein exist in unsolvated
form or in
solvated forms with pharmaceutically acceptable solvents such as water,
ethanol, and the like.
The solvated forms of the compounds presented herein are also considered to be
disclosed
herein.
[00239] Pharmaceutical preparations for oral use are obtained by mixing one or
more solid
excipient with one or more of the compounds described herein, optionally
grinding the resulting
mixture, and processing the mixture of granules, after adding suitable
auxiliaries, if desired, to
obtain tablets or dragee cores. Suitable excipients include, for example,
fillers such as sugars,
including lactose, sucrose, mannitol, or sorbitol; cellulose preparations such
as, for example,
maize starch, wheat starch, rice starch, potato starch, gelatin, gum
tragacanth, methylcellulose,
microcrystalline cellulose, hydroxypropylmethylcellulose, sodium
carboxymethylcellulose; or
-88-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
others such as: polyvinylpyrrolidone (PVP or povidone) or calcium phosphate.
If desired,
disintegrating agents are added, such as the cross-linked croscarmellose
sodium,
polyvinylpyrrolidone, agar, or alginic acid or a salt thereof such as sodium
alginate. In some
embodiments, dyestuffs or pigments are added to the tablets or dragee coatings
for identification
or to characterize different combinations of active compound doses.
[00240] Pharmaceutical preparations that are administered orally include push-
fit capsules
made of gelatin, as well as soft, sealed capsules made of gelatin and a
plasticizer, such as
glycerol or sorbitol. The push-fit capsules contain the active ingredients in
admixture with filler
such as lactose, binders such as starches, and/or lubricants such as talc or
magnesium stearate
and, optionally, stabilizers. In soft capsules, the active compounds are
dissolved or suspended in
suitable liquids, such as fatty oils, liquid paraffin, or liquid polyethylene
glycols. In some
embodiments, stabilizers are added.
[00241] In certain embodiments, delivery systems for pharmaceutical compounds
may be
employed, such as, for example, liposomes and emulsions. In certain
embodiments,
compositions provided herein can also include an mucoadhesive polymer,
selected from among,
for example, carboxymethylcellulose, carbomer (acrylic acid polymer),
poly(methylmethacrylate), polyacrylamide, polycarbophil, acrylic acid/butyl
acrylate
copolymer, sodium alginate and dextran.
Combination Treatment
[00242] The compounds according to Formulas (I), (Ia), (Ib), (Ic), (Id), (II),
(IIa), (lIb), and
(IIc) may be used in combination with one or more additional antibiotic
agents. The antibiotic
agent may be selected from an aminoglycoside, ansamycin, carbacephem,
carbapenem,
cephalosporin, glycopeptide, lincosamide, lipopeptide, macrolide, monobactam,
nitrofurans,
penicillin, polypeptide, quinolone, sulfonamide, or tetracycline antibiotic.
Examples of
antibiotic agents include, but are not limited to, Aminoglycoside derivatives
like amikacin,
gentamicin, kanamycin, neomycin, netilmicin, tobramicin, paromomycin;
Ansamycin
derivatives like geldanamycin, herbimycin; Carbacephem derivatives like
loracarbef,
Carbapenem derivatives like ertapenem, doripenem, imipenem, meropenem;
Cephalosporin
derivatives like cefadroxil, cefazolin, cefalotin, cefalexin, cefaclor,
cefamandole, cefoxitin,
cefprozil, cefuroxime, cefixime, cefdinir, cefditoren, cefoperazone,
cefotaxime, cefpodoxime,
ceftazidime, ceftibuten, ceftizoxime, ceftriaxone, cefepime, ceftobiprole;
Glycopeptide
derivatives like teicoplanin, vancomycin, telavancin; Lincosamides like
clindamycin,
lincomycin; Lipopeptide derivatives like daptomycin; Macrolide derivatives
like azithromycin,
clarithromycin, dirithromycin, erythromycin, roxithromycin, troleandomycin;
telithreomycin,
-89-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
spectinomycin; Monobactam derivatives like aztreonam; Nitrofuran derivatives
like
furazolidone, nitrofurantoin; Penicillin derivatives like amoxicillin,
ampicillin, azlocillin,
carbinicillin, cloxacillin, dicloxacillin, flucloxacillin, mezlocillin,
methicillin, nafcillin, oxacillin,
penicillin G, penicillin V, piperacillin, temocillin, ticarcillin; Penicillin
combinations like
amoxicillin/clavulanate, ampicillin/sulbactam, piperacillin/tazobactam,
ticarcillin/clavulanate;
Polypeptide derivatives like bacitracin, colistin, polymyxin B; Quinolone
derivatives like
ciprofloxacin, enoxacin, gatifloxacin, levofloxacin, lomefloxacin,
moxifloxacin, nalidixic acid,
norfloxacin, ofloxacin, trovafloxacin, grepafloxacin, sparfloxacin,
temafloxacin; Sulfonamide
derivatives like mafenide, sulfonamidochrysoidine, sulfacetamide,
sulfadiazine, silver
sulfadiazine, sulfamethoxazole, sulfanilimide, sulfasalazine, sulfisoxazole,
trimethoprim,
trimethoprim/sulfamethoxazole; Tetracyclin derivatives like demeclocycline,
doxycycline,
minocycline, oxytetracycline, tetracycline; Derivatives against mycobacteria
like clofazimine,
dapsone, capreomycin, cycloserine, ethambutol, ethioamide, isoniazid,
pyrazinamide, rifampin,
refampicin, rifabutin, rifapentine, streptomycin; or other antibiotic agents
like arsphenamine,
chloramphenicol, fosfomycin, fusidic acid, linezolid, metronidazole,
mupirocin, platensimycin,
quinupristin/dalfopristin, rifaximin, thiampheniol, tigecycline, tinidazole.
In preferred
embodiments, the antibiotic agent is useful in the treatment of tuberculosis
and/or infection with
Mycobacterium tuberculosis and may be selected from with rifampicin, TMC207,
or isoniazid.
Administration of Pharmaceutical Composition
[00243] Suitable routes of administration include, but are not limited to,
oral, intravenous,
rectal, aerosol, parenteral, ophthalmic, pulmonary, transmucosal, transdermal,
vaginal, otic,
nasal, and topical administration. In addition, by way of example only,
parenteral delivery
includes intramuscular, subcutaneous, intravenous, intramedullary injections,
as well as
intrathecal, direct intraventricular, intraperitoneal, intralymphatic, and
intranasal injections.
[00244] In some embodiments, compounds of Formulas (I), (Ia), (Ib), (Ic),
(Id), (II), (Ha), (lIb),
and (IIc) and compositions thereof are administered in any suitable manner.
The manner of
administration can be chosen based on, for example, whether local or systemic
treatment is
desired, and on the area to be treated. For example, the compositions can be
administered orally,
parenterally (e.g., intravenous, subcutaneous, intraperitoneal, or
intramuscular injection), by
inhalation, extracorporeally, topically (including transdermally,
ophthalmically, vaginally,
rectally, intranasally) or the like.
[00245] Parenteral administration of the composition, if used, is generally
characterized by
injection. Injectables can be prepared in conventional forms, either as liquid
solutions or
suspensions, solid forms suitable for solution of suspension in liquid prior
to injection, or as
-90-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
emulsions. A more recently revised approach for parenteral administration
involves use of a
slow release or sustained release system such that a constant dosage is
maintained.
Methods
[00246] Further provided herein is a method to treat drug resistant and
persistent tuberculosis in
a mammal, the method comprising administering to the mammal a compound of
Formulas (I),
(Ia), (Ib), (Ic), (Id), (II), (Ha), (JIb), or (IIc) or as described above and
below. In some
embodiments, the method further comprises administering an additional
antibiotic agent.
EXAMPLES
List of abbreviations
[00247] As used above, and throughout the description of the
invention,
the following abbreviations, unless otherwise indicated, shall be understood
to have the
following meanings:
ACN acetonitrile
Bn benzyl
BOC or Boc tert-butyl carbamate
BOP benzotriazol-1-yl-oxytris (dimethylamino) phosphonium
t-Bu tert-butyl
Cbz benzyl carbamate
Cy Cyclohexyl
DBU 1,8-Diazabicyclo[5.4.0]undec-7-ene
DCC dicyclohexylcarbodiimide
DCM dichloromethane (CH2Cl2)
DIC 1,3-diisopropylcarbodiimide
DEAD diethyl azodicarboxylate
DIAD diisopropyl azodicarboxylate
DIEA diisopropylethylamine
DMAP 4-(N,N-dimethylamino)pyridine
DMP reagent Dess-Martin Periodinane reagent
DMF dimethylformamide
DMA N,N-Dimethylacetamide
DME 1,2-Dimethoxy-ethane
DMSO dimethylsulfoxide
Dppf 1,1'-Bis(diphenylphosphino)ferrocene
-91-
CA 02911326 2015-10-30
WO 2014/190199
PCT/US2014/039227
EDCI 1-ethy1-3-(3-dimethylaminopropyl) carbodiimide HC1
eq equivalent(s)
Et ethyl
Et20 diethyl ether
Et0H ethanol
Et0Ac ethyl acetate
HOAt 1-hydroxy-7-azabenzotriazole
HOBT 1-hydroxybenztriazole
HOSu N-hydroxysuccinamide
HPLC high performance liquid chromatography
LAH lithium aluminum anhydride
Me methyl
Mel methyliodide
Me0H methanol
MOMC1 methoxymethylchloride
MOM methoxymethyl
MS mass spectroscopy
NMP N-methyl-pyrrolidin-2-one
NMR nuclear magnetic resonance
PyBOP benzotriazole-1-yl-oxytris-pyrrolidino-phosphonium
Hexafluorophosphate
SPHOS 2-Dicyclohexylphosphino-2',6'-dimethoxybiphenyl
TBD 1,5,7-triazabicyclo[4.4.0]-dec-5-ene
RP-HPLC reverse phase-high pressure liquid chromatography
TBS tert-butyldimethylsilyl
TBSC1 tert-butyldimethylsilyl chloride
TBTU 0-(Benzotriazol-1-y1)-N,N,N',N'-tetramethyluronium
TEOC 2-Trimethylsilylethyl Carbamate
TFA trifluoroacetic acid
Tf20 triflate anhydride
TMG 1,1,3,3-Tetramethylguanidine
THF tetrahydrofuran
THP tetrahydropyran
TLC thin layer chromatography
XPHOS 2-Dicyclohexylphosphino-2',4',6'-triisopropylbiphenyl
-92-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
General Examples for the Preparation of Compounds of the Invention
[00248] The starting materials and intermediates for the compounds of this
invention may be
prepared by the application or adaptation of the methods described below,
their obvious
chemical equivalents, or, for example, as described in literature such as The
Science of
Synthesis, Volumes 1-8. Editors E. M. Carreira et al. Thieme publishers (2001-
2008). Details of
reagent and reaction options are also available by structure and reaction
searches using
commercial computer search engines such as Scifinder (www.cas.org) or Reaxys
(www.reaxys.com).
Part A: Thiophen-2-amine starting materials:
Starting material (2):
[00249] The following final compounds utilized (1) in the final stage of
synthesis: 1, 43, 54-61,
63-97.
IS OH
NCI(
0 H2N HO O 0 0
S HN¨µ
NC
J(N)L0 0
OH S
POCI3, Py, RT morpholine, DMF 0
A B NH2
[00250] To a solution of A (120 g, 1410 mmol), ethyl carbamate (108 g, 706
mmol) in dry
toluene (500 mL) at 0 C under nitrogen was slowly added DMF (29 ml), then
POC13 (66 ml, 730
mmol). Reaction mixture was heated at 70 C for 1.5 h, monitored by TLC. After
cooling to
room temperature, solvent and POC13 were removed, and reaction quenched with
ice water (100
mL). Precipitate was filtered to give B (210 g, 95%) as white solid.
[00251] To a solution of B (210 g, 1345 mmol), 1,4-dithiane-2,5-diol (108 g,
706 mmol) in
DMF (1300 mL) at 0 C under nitrogen was added morpholine (117 g, 1345 mmol).
Reaction
mixture was heated at 65 C for 3 h, monitored by TLC. Solvent was removed in
vacuo, and the
mixture poured into ice water (500m1), extracted with Et0Ac, washed with water
and brine and
concentrated. The residue was recrystallized from Et0Ac and hexane to give (1)
as brown solid.
Starting material (2):
[00252] The following compounds utilized (2) in the final stage of synthesis:
41.
s OH
0
0¨(
NCJ( 0 0
OH HO S 0
H2N 0 NCJ( A0
N TEA, Me0H, 40 C, 1.5h 0
POCI3, DMF, toluene, 75 C, NH2 2
1.5h
A
[00253] To a solution of A (4.2 g, 48 mmol), isopropyl carbamate (A) (5.0 g,
48 mmol) in dry
toluene (18 mL) at 0 C under nitrogen was added DMF (1.2 ml), then POC13 (2.4
ml, 26 mmol).
Reaction mixture was heated at 75 C for 1.5 h, monitored by TLC. After cooling
to room
-93-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
temperature, solvent and POC13 were removed, and reaction quenched with ice
water (50 mL).
White solid was filtered to give B (3.5 g, 42%).
[00254] To a solution of B (3.5 g, 21 mmol), 1,4-dithiane-2,5-diol (1.7 g, 11
mmol) in Me0H
(100 mL) at 0 C under nitrogen was added EtN3 (2.3 g, 23 mmol). Reaction
mixture was heated
at 40 C for 1.5 h, monitored by TLC. Solvent was removed in vacuo, and the
reaction dissolved
in a mixture of solvent (i-PrOH:DCM=1:4) and washed with Sat. aq. NH4C1.
Organic layer was
concentrated. Crude material was purified by column chromatography (silica
gel, Et0Ac:PE =
1:2-1:1) to give (2) (3.5g, 75%) as a yellow solid.
Starting material (3):
[00255] The following compounds utilized (3) in the final stage of synthesis:
37.
0
HO 0 0
0 (C0C OH 1)2
NCJ( N C A
F NCJ(NA0F DCE
DCM
A
ISyOH HIVCrS)
TEA, MeON
0¨ \
HN¨µ
0
o
NH2 3
[00256] To a solution of A (1.0 g, 11.9 mmol) in dry DCE (10 mL) at 0 C under
nitrogen was
added oxalyl chloride (1.5 ml, 17.3 mmol). Reaction mixture was heated at 75 C
for 1.5 h to
give B, monitored by TLC. After cooling to 0 C, 2-fluoroethanol (4.7 ml, 55.6
mmol) was
added, and the resulting dark brown suspension stirred at room temperature for
30 min.
Afterwards, reaction was quenched with water, dissolved in solvent mixture
(i-PrOH:DCM=1:4), and filtered. Filtration cake was washed with a mixture of
solvent
(i-PrOH:DCM=1:4), and the filtrate washed with water and brine, then dried and
concentrated to
give crude C. To a solution of C and 1,4-dithiane-2,5-diol (0.24 g, 1.61 mmol)
in Me0H (15
mL) at 0 C under nitrogen was added EtN3 (0.4 ml, 3.2 mmol). Reaction mixture
was heated at
40 C for 1.5 h, monitored by TLC. Solvent was removed in vacuo, and the
reaction dissolved in
a mixture of solvent (i-PrOH:DCM=1:4) and washed with Sat. aq. NH4C1. Organic
layer was
concentrated. Crude material was purified by column chromatography (silica
gel, Et0Ac:
PE=1:2) to give 3 (210 mg, 8%, over three steps).
Starting material (4):
[00257] Starting material (4) synthesized in an analogous manner to (3),
substituting
2-fluoroethanol with methoxyethanol:
-94-
CA 02911326 2015-10-30
WO 2014/190199
PCT/US2014/039227
0 0
A 0
el)rii 0
S
NH2 4
Starting material (5):
[00258] The following compounds utilized (5) in the final stage of synthesis:
62.
o o o o
0 A o o
H2N 0 H
S)(e.
NC NC 8 N
j= N AV \
OH -VI'
POCI3, Py, RT H morpholine, DMF S
NH2
A B 5
[00259] To a solution of A (120 g, 1410 mmol), ethyl carbamate (108 g, 706
mmol) in dry
toluene (500 mL) at 0 C under nitrogen was added DMF (29 ml), then POC13 (66
ml, 730
mmol). Reaction mixture was heated at 70 C for 1.5 h, monitored by TLC. After
cooling to
room temperature, solvent and POC13 were removed, and reaction quenched with
ice water (100
mL). White solid was collected to give B (210 g, 95%).
[00260] To a solution of B (25 g, 160 mmol), propionaldehyde (9.3 g, 160 mmol)
and Sg (5.1 g,
160 mmol) in DMF (30 mL) at 0 C under nitrogen was added morpholine (7.9 g, 90
mmol).
Reaction mixture was heated at 50 C for 30 min, monitored by TLC. Solvent was
removed in
vacuo, and the mixture poured into ice water (50m1), extracted with Et0Ac,
washed with water
and brine and concentrated. Crude material was purified by column
chromatography (silica gel,
Et0Ac:PE = 1:1) to give 5 (6.8g, 18%) as a yellow solid.
Part B: Carboxylic Acid startinz materials:
[00261] A majority of the carboxylic acid starting materials were purchased by
Bioduro. The
reaction schemes of starting materials synthesized in Bioduro laboratories are
as follows:
Starting material (1):
[00262] The following final compounds utilized (1) in the final stage of
synthesis: 1, 18, 37, 41.
1.I ______________________________ HO S
0 N THF/Me0H )11. 0 N
A 1
Method A
[00263] To a solution of A (10 g, 48 mmol) in a mixture of solvent
(THF:Me0H=1:1,v/v) (50
mL) at 0 C under nitrogen was added aq.LiOH (72m1, 2M, 145 mmol). Reaction
mixture was
stirred at room temperature for 2-12h, monitored by TLC. Mixture was acidified
with aq.HC1
(6M) to pH=3-4, and precipitate filtered to give 1 (5 g, 58%) as a white
solid.
-95-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
Method B
[00264] To a solution of A (10 g, 48 mmol) in THF (50 mL) at 0 C under
nitrogen was added
aq.NaOH (72m1, 2M, 145 mmol). Reaction mixture was stirred at room temperature
for
30-120min, monitored by TLC. Mixture was acidified with aq.HC1 (6M) to pH=3-4,
and
precipitate filtered to give 1 (7 g, 81%) as a white solid.
Starting material (2):
[00265] The following final compounds utilized (2) in the final stage of
synthesis: 83.
0
c,)y0, 0
NH2 11_ A
N N s o
e\ Lawesson reagent
I 0
a 11)
N Oi THF, Et3N, 0 C, RT N CI
Toluene, reflux N 0¨µ
\
A B C
1
LiOH, THF/H20
N s 0
OX H
2
[00266] To a solution of A (30 g, 234 mmol) and Et3N (52 g, 516 mmol) in dry
THF (400 mL)
at 0 C under nitrogen was added ethyl 2-chloro-2-oxoacetate (31 ml, 281 mmol)
dropwise.
Reaction was stirred at room temperature for 4 h, monitored by TLC. Solvent
was removed in
vacuo, and the mixture poured into ice water (50m1), extracted with Et0Ac,
washed with water
and brine and concentrated. The crude material was purified by column
chromatography (silica
gel, Et0Ac:PE = 1:1) to give B (40g, 75%) as a white solid.
[00267] A mixture of B (5 g, 22 mmol) and Lawesson's reagent (7.1 g, 18 mmol)
in toluene (40
ml) was heated at 110 C under nitrogen for 2 h, monitored by TLC. The mixture
was dissolved
in Et0Ac, and filtered. Filtration cake was washed with Et0Ac, and the
filtrate extracted with
Et0Ac, washed with water and brine, dried and concentrated. The crude material
was purified
by column chromatography (silica gel, Et0Ac:PE = 1:4-1:2-1:1). Product was
recrystalliazed
with Et0Ac and hexane to give pure C (1.2 g, 26%) as a yellow solid and a
crude target product
(3.1g, 60% of purity) as a yellow solid.
[00268] To a solution of C (1.2 g, 5.7 mmol) in a mixture of solvent
(THF:Me0H=1:1,v/v) (50
mL) at 0 C under nitrogen was added aq.LiOH (7.2m1, 2M, 14.2 mmol). Reaction
was stirred at
room temperature for 2-12h, monitored by TLC. The mixture was acidified with
aq.HC1 (6M) to
pH=3-4, and precipitate collected to give 2 (0.6 g, 58%) as a white solid.
-96-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
Additional synthetic information:
[00269] The following compounds were prepared using the identical reaction
with the
following starting materials:
Target compound number Starting material (A) structure
NH2
Na
82 I
/
ci
Starting material (3):
[00270] The following final compounds utilized (3) in the final stage of
synthesis: 85
NHBoc NHBoc N... _-
\ N--:--\
n-BuLi, S8 HSHCOOH Mel
____________________________________________ > 6/s
,...
o THE, -78 C to RT.' 'oN Reflux N / DMF, 80 C
/16/s
0 D
A B C
NaBH4, H20/Me0H
RT
0\\¨
OH
n-BuLi, CO2 S
iso/S
THE, -78 C to RT N E
/ /
3
[00271] To a stirred solution of A (10 g, 51 mmol) in anhydrous THF (50 ml)
was added
n-BuLi (2.5 M in hexane, 52 ml, 130 mmol) dropwise at -78 C under nitrogen.
Reaction mixture
was warmed to 0 C, and stirred at 0 C for 2 h, then recooled to -78 C. To the
mixture was added
Sg in one portion. Reaction warmed to room temperature and stirred for 1 h,
monitored by TLC.
Water was added and reaction acidified with aq.HC1 (2M) to pH=3-4, and
extracted with DCM,
washed with water and brine, then dried and concentrated. Crude material was
purified by
column chromatography (silica gel, Et0APE = 1:3-1:2-1:1) to give a B (5.5 g,
47%) as a yellow
solid.
[00272] A solution of B (5.5 g, 24 mmol) in HCOOH (45 ml) was heated to 100 C
under
nitrogen for 4 h, monitored by TLC. The mixture was concentrated and to the
residue was added
aq. NaOH (5M) at 0 C to basify to pH=7-8. Mixture was extracted with Et0Ac,
washed with
water and brine, then dried and concentrated. The crude material was purified
by column
chromatography (silica gel, Et0Ac:PE=1:1) to give a C (2.5 g, 76%) as a yellow
solid.
[00273] To a solution of C (2.5 g, 18 mmol) in DMF (6.0 ml) was added methyl
iodide (2.4 ml,
36 mmol) under nitrogen. Reaction was heated to 80 C in a sealed tube for 1 h,
monitored by
-97-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
TLC. The mixture was concentrated under reduced pressure and the white solid
collected to give
D (5.4 g, 100% crude).
[00274] To a solution of D (5.4 g, 18 mmol) in Me0H (20 ml) was added sodium
borohydride
(2.4 g, 64 mmol) at 0 C. Mixture was stirred at room temperature for 1 h,
monitored by TLC.
Reaction was concentrated under reduced pressure. Sat. aq. NaHCO3 and Et0Ac
were added
and organic layer extracted with Et0Ac, washed with water and brine, then
dried and
concentrated. Crude material was purified by column chromatography (silica
gel,
Et0Ac:PE=1:2 Et0Ac:Me0H=30:1) to give E (2.4 g, 85%, over two steps) as a
yellow oil.
[00275] To a stirred solution of E (0.73 g, 4.7 mmol) in anhydrous THF (15 ml)
was added
n-BuLi (2.5 M in hexane, 2.3 ml, 5.7 mmol) dropwise at -78 C under nitrogen.
Reaction mixture
was stirred at -78 C for 40min, monitored by TLC (quenched with DMF before TLC
). After
release of CO2 gas for 20 min (temperature was quickly increased to -50 C and
recooled to
-78 C), mixture was warmed to room temperature and stirred for 20 min, and
concentrated in
vacuo. Residue washed with hexane to give 3 (0.6 g, 64%) as a yellow solid.
Starting material (4):
[00276] The following final compounds utilized (4) in the final stage of
synthesis: 57.
0
>1)( S
ci Et u
aNH 3N 1. n-BuLi 2.5M, THF
a. 0 2. TITD, THF as0Npr2
,... , . õL C
N N -50-0 C N NH
N H
2 DCM, 0 C
B
A Oi<
10% NaOH, Me0H
1 R
E
$
I.
aSCNPr2
90% S
5M HCI ii
Reflux
c.----xS -...¨
N N + Et3NNHCOCH3 CH3COCI
, 20 C I1 SCNPr2
N
N NH2
G S
1
KMn04, H20
Reflux
aSCNPr2
10%
N N(COCH3)2
F D
c,,
cS OH
N N 0
4
[00277] To a solution of A (11 g, 116 mmol) in dry DCM (100 ml) was added Et3N
(14.7 g,
145 mmol) and a solution of pivaloyl chloride (15.3 g, 128 mmol) in dry DCM
(20 ml) at 0 C.
-98-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
Reaction mixture was stirred at 0 C for lh, then warmed to room temperature
and stirred for 12
h under nitrogen, monitored by TLC. Cold water (50 ml) was added, and the
mixture extracted
with DCM, washed with Sat. aq. NaHCO3, water and brine, then dried and
concentrated. The
crude material was purified by column chromatography (silica gel,
Et0Ac:PE=1:2) to give B
(12 g, 84%) as a white solid.
[00278] To a stirred solution B (2 g, 11 mmol) in anhydrous THF (10 ml) was
added n-BuLi
(2.5 M in hexane, 9.6 ml, 24 mmol) dropwise at -50 C under nitrogen. Reaction
mixture was
stirred at 0 C for 4 h and recooled to -50 C. A solution of TITD (4.2 g, 12
mmol) in dry THF (5
ml) was added slowly at -50 C. Mixture was warmed to room temperature and
stirred for 45
min. Ether (10 ml) was added and the solution washed with water and brine,
then concentrated
in vacuo. Residue washed with Et0Ac to give C (1.3 g, 33%) as a white solid.
[00279] A mixture of C (1.3 g, 3.68 mmol) and Na0H/Me0H (10%wt, 40 ml) was
stirred at
room temperature for 12 h under nitrogen, monitored by TLC. Filtration of
resulting suspension
gave D (0.7 g, 71%).
[00280] To a stirred solution of D (0.7 g, 2.6 mmol) in anhydrous DCM (15 ml)
was added
Et3N (1.5 ml) dropwise at 0 C under nitrogen, followed by appropriate acid
chloride (8.6 mmol).
Reaction mixture was stirred at room temperature for 12 h. Mixture was poured
into cold water
(20 ml) and extracted with DCM, washed with Sat. aq. NaHCO3, water and brine,
then dried and
concentrated. The crude material was purified by column chromatography (silica
gel,
Et0Ac:PE=1:2) to give a mixture of E and F (0.8 g, 90%) as a yellow oil.
[00281] A mixture of E and F (0.8 g) and aq. HC1 (5M, 30 ml) was heated to 100
C for 5 h
under nitrogen, monitored by TLC. The cooled solution was washed with ether
(80 ml), and
basified with NaOH (2M) to pH=7-8 at 0 C. Mixture was poured into cold water
(20 ml) and
extracted with DCM, washed with Sat. aq. NaHCO3, water and brine, then dried
and
concentrated to give G (310 mg, 80%).
[00282] To a stirred solution of G (310 mg, 2.07 mmol) in water (20 ml) was
added KMnat
(490 mg, 3.1 mmol). Reaction mixture was stirred at 100 C for 17 h. The
precipitate was
removed and filtrate mixed with water and DCM. Aqueous layer was acidified
with aq. HC1
(6M) to pH=5-6, and concentrated in vacuo. The residue was washed with water
and ether to
give 4 (98 mg, 26%) as a white solid.
Building block for starting materials (5) and (6):
[00283] The following starting material (F) is common to the reagent syntheses
(5) and (6) for
compounds 58 and 59 respectively.
-99-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
Br SH SH
NO2NO2 s NH2
Na2S Zn, AcOH
, 0,
DMF, RT THF, 50 C
Br Br Br
A B C
1 Triphosgen
AcOH, 120 C
H
H
NC s N Zn(CN)2, Pd(cIPOCl2 Br
N
0
S 120 C S
E D
1 TBAB
NC 0 N
)¨Br
S
F
[00284] To a solution of A (20 g, 71 mmol) in DMF (200 ml) was added Na2S (20
g, 320
mmol). Reaction mixture was stirred at room temperature for 16 h under
nitrogen, monitored by
TLC. Solution was mixed with water (200 ml), acidified with aq. HC1 (3 M) to
pH=3-4. Yellow
precipitate was collected to give B (14 g, 84%).
[00285] Zn (70 g, 1077 mmol) was added to a solution of B (28 g, 120 mmol) in
AcOH (500
m1). Reaction mixture was stirred at 65 C for 15 h under nitrogen to give C,
monitored by TLC.
The mixture was cooled to room temperature, and triphosgene (25 g, 84 mmol)
added slowly at
0 C. Mixture was refluxed for 18 h, monitored by TLC. Solution was then
concentrated and
hydrolyzed with water. Yellow solid was collected to give D (20 g, 73%, over
two steps).
[00286] To a solution of D (5 g, 22 mmol) in DMF (15 ml) was added Zn(CN)2 (5
g, 43 mmol),
Pd(dppf)C12 (1.5 g, 2.2 mmol) and Pd2(dba)3 (0.6 g, 1.1 mmol). Reaction
mixture was stirred at
120 C for 4 h under nitrogen, monitored by TLC. The mixture was extracted with
Et0Ac,
washed with water and brine, then dried and concentrated. Crude material was
purified by
column chromatography (silica gel, Et0Ac:PE=1:4) to give E (1.0 g, 26%) as a
yellow solid.
[00287] A mixture of E (0.7 g, 4.0 mmol), Bu4NBr (TBAB, 1.54 g, 4.8 mmol) and
P205 (1.36
g, 9.5 mmol) in dry toluene (15 ml) was heated at 100 C for 3 h under
nitrogen, monitored by
TLC. Mixture was extracted with Et0Ac, washed with water and brine and then
dried and
-100-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
concentrated. The crude material was purified by column chromatography (silica
gel,
Et0Ac:PE=1:5) to give F (0.4 g, 42%) as a yellow solid.
Starting material (5):
[00288] The following final compounds utilized (5) in the final stage of
synthesis: 58.
(o
NC N HN) K2CO3
NC 0
DMF, RT 0 lµ 141\__ ii¨\0 Con.
HCI
HO [10 N /¨µ b
S si¨ \__/ 100 C /
S
F G 5
[00289] To a solution of F (0.2 g, 0.84 mmol) in dry DMF (10 ml) was added
morpholine (0.1
g, 1.0 mmol) and K2CO3 (0.3 g, 2.1 mmol). Reaction mixture was stirred at room
temperature
for 12 h under nitrogen, monitored by TLC. Mixture was extracted with Et0Ac,
washed with
water and brine, then dried and concentrated. The crude material was purified
by column
chromatography (silica gel, Et0Ac:PE=1:4) to give G (0.11 g, 53%) as a yellow
solid.
[00290] A mixture of G (0.11 g, 0.45 mmol) and Con. HC1 (4 ml) was heated at
100 C for 3 h
under nitrogen, monitored by TLC. The mixture was concentrated under reduced
pressure to
give 5 (0.11 g, 100%).
Starting material (6):
[00291] The following final compounds utilized (6) in the final stage of
synthesis: 59.
NH
NC S N,¨Br /Nj DIPEA
S
NC C. HCI 0
101 iLir¨\N_ on S
HO 0 N,N/¨\
¨ N¨
DMF, RT I \--/ 100 C \__/
F G 6
[00292] To a solution of F (0.2 g, 0.84 mmol) in dry DMF (10 ml) was added
1-methylpiperazine (0.1 g, 1.0 mmol) and DIPEA (0.4 g, 3.0 mmol). Reaction
mixture was
stirred at room temperature for 12 h under nitrogen, monitored by TLC. Mixture
was extracted
with Et0Ac, washed with water and brine then dried and concentrated. The crude
material was
purified by column chromatography (silica gel, Et0Ac:PE=1:4) to give G (0.15
g, 69%) as a
yellow solid.
[00293] A mixture of G (0.15 g, 0.58 mmol) and Con. HC1 (5 ml) was heated at
100 C for 3 h
under nitrogen, monitored by TLC. Mixture was concentrated under reduced
pressure to give 6
(0.15 g, 100%).
Part C: Condensation of carboxylic acid and thiphen-2-amine:
Method A:
[00294] The following compounds were synthesized via Method A: 1, 18, 37, 41,
54-61, 63,
65, 66, 69-74, 77-79, 80, 82-92, 94-97.
-101-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
[00295] Example Scheme: Compound 82
N H ) C S 2 0-\ 0-µ 0 \ 0/N H
\ C H B
N N 0 H S t
A C
/
N N
[00296] To a solution of carboxylic acid (50 mg, 0.277 mmol) in DCM (0.5 mL)
at 0 C under
nitrogen was added ethyl 2-aminothiophene-3-carbonylcarbamate (59 mg, 0.227
mmol), Et3N
(84 mg, 0.831 mmol) and T3P (50%wt in EA, 0.3 ml, 0.554 mmol). Reaction
mixture was
stirred at 25 C for 2-12 h, monitored by TLC. The mixture was poured into cold
water (10 ml)
and extracted with DCM (20m1), washed with water and brine and then dried and
concentrated.
The crude material was purified by column chromatography (silica gel,
Et0Ac:PE=1:2) to give
C (10 mg, 10%) as a yellow solid.
Additional synthetic information:
[00297] The following compounds were prepared using the identical reaction
with the
following starting materials:
Target compound Thiophen starting material Carboxylic acid starting
number structure material
ETrz.$)
1
0
7rts.$)
18 4, I /
bi)......(OH
0131r141 S
0
0 0
H
;
o
F yM I )
37 r...... / * N.....(OH
s
0 0 0
H2N s
41 ,c)1411.rt., *
I 011 0 S
0
H2N
S N
54 ol.ii yrt) il _ acs 0 Nss X --
--f
N
OH
0 0
H2N 0
S
H I i
HNN OH
55 ONIP
II I H
0 0 S 0
-102-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
Target compound Thiophen starting material
Carboxylic acid starting
number structure material
H2N
),Na N OH
56 H I
I )¨µ
S 0
0 0
H2N s
clxN N OH
57 ,yyt 1 H
S 0
O 0
H2N s 0
58 ,yyt 0 /¨\ N I* OH
N-
0 0 \¨/ S
H2Ns 0
59 cilrOlr /--\
;41 0 OH
¨N N¨<
O 0
y NH I
H2N.1ixs) 0 _lay
60 ....,..,õ.0
/
>iii--N I
/ OH
H
O 0 0
H2N s
0 30 r.
I
61 ,yyt õOA N / OH
H
O 0 0
H2N s 0
63 ,yyt HO (6 N
====
..-""
0 0
H2N s
65 cilrOlr 5 HO,,,c,i ...
==".. ...L..)_N'''N
O 0 N
0
H2N s 0
66 ,..,..,õ.0y NH I
/
/ OH
O 0
0
H2N
I
69 H I
O 0 0
H2N 0 41) 0
/
70 ...,..,õ.o Ir.)
y NH I
OH
O 0 0
-1 03 -
CA 02911326 2015-10-30
WO 2014/190199
PCT/US2014/039227
Target compound Thiophen starting material
Carboxylic acid starting
number structure material
HETrt...) 1400
71 ...OyN I / \ OH
0
O 0 0
0
Trr....) N
72 H I ,
...,....õOyN / HO)Lr
HN 40
O 0
CI
N
H2N 1N\
73 ...,....õoy Nil Ir..../
O 0 CO2H
H2N N'
74H I ,
...OyN / HO11..1-
O 0 0
e,.
H2N NS
77
, N 1
yµN-
O 0 CO2H
.O HO2CIN
Tr
S )
78 H I ,
%,,..,...yNz /
N N
\=/
0 0
Tro
...,....õOyN / S OH
C(
-........õN N 0
O 0
H2N 0
S
80 oy Myt)/
,
---4 \
O 0 N-4
H2N S
N S OH
83
0:
y
- N 0
O 0
H2N
S OH
84 o r.liy.)/
, HNS
I%........õ, y
N 0
O 0
H2N
s OH
85 yt>
\Ay Ill
OE
N 0
O 0
-104-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
Target compound Thiophen starting material
Carboxylic acid starting
number structure material
86
Trr.,..s.) o
r.11 I / N )cH
Oy
I I
0 0 N /
0
H2N s
HO)L( N
87 1::=li 141 irti N *
O 0
W
:2N 0 S =
I S/
88 \
Oy
HO
O 0 CI
T N
rr.....s.)
89
o y
O 0 OH
Trz.$) 0 S *
90r41 I /
o y \
HO
O 0
0
Trz.$)
91XY(1 OH
OyI
O 0 0 NN'
H
Trzs) 0
92HO)LC0 0
Oy
O 0 0
Trz.$)
oy
HO S%1\
o o
Trz.$) s 0H
95 0 1-io
o y y
o o o
Trzs) s OH
96 r.11 I / % CC H
o y
. e N 0
O 0 CV \
Trzs) SOH
97I i %
Oy
*
0 0
Method B.
-105-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
[00298] The following compounds were synthesized via Method B: 43, 58, 64, 67,
68, 81, 93.
[00299] Example Scheme: Compound 58
R4.,...HN4 s / 0
0 0
124
s / 0
0 HN-
NH2
HO (40/ N /¨\ B 0-\
________________________________________ 0 NH 0-\
A C
* N
s_k
NTh
c,0
[00300] To a solution of carboxylic acid (0.11 g, 0.448 mmol) and B (0.1 g,
0.448 mmol) in
pyridine (2 mL) at -30 C-0 C under nitrogen was added POC13 (0.3 m1). Reaction
was stirred at
-30 C-0 C-RT for 20-60 min, monitored by TLC. Mixture was poured into cold
water (10 ml)
and extracted with DCM (20m1), washed with Sat. aq. NaHCO3, water and brine,
then dried and
concentrated. Crude material was purified by column chromatography (silica
gel,
Et0Ac:PE=1:4) to give C (0.1 g, 48%) as a yellow solid.
Additional synthetic information:
[00301] The following compounds were prepared using the identical reaction
with the
following starting materials
Target compound Thiophene starting material
Carboxylic acid starting material
number structure
o
Trr......) HO (10
43 Oy NH I /
S
0 0
Nr-:--/
64 HO N
HNrr......$) 0
).LC
Oy NH I / H
O 0 0
,Trz.$) 00 0
67 oyNil I / OH
O 0 0
Trz.$) s 0
68 oyNil I / OH
0
O 0 0
75 *
Trr......) OH
,0
Oy NH I / \\ 0
N
O 0
-106-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
Target compound Thiophene starting material
Carboxylic acid starting material
number structure
Trz.$) o
81 Oy Li I / HO))
0 0 N
)4
HNr......$)
93 oy1.1 I /
HO N
0 0
Method C.
[00302] The following compounds were synthesized via Method C: 62, 76.
[00303] Generalized Example Scheme: Compound 76
0
0
0
s24 N
siR4 _ H4
HN
NH2 0¨\ 0 NH 0¨\
B
isa
A C
\ /
[00304] To a solution of carboxylic acid (1.1 g, 7 mmol) in dry DCM (10 mL) at
0 C under
nitrogen was added B (1.5 mg, 7 mmol), Et3N (2.1 g, 21 mmol), EDCI (4.7 g,
23.8 mmol),
HOBt (2.8 g, 21 mmol) and DMAP (150 mg, catalytic). Reaction mixture was
stirred at 25 C for
2-12 h, monitored by TLC. Mixture was poured into cold water (10 ml) and
extracted with DCM
(20m1), washed with water and brine, then dried and concentrated. Crude
material was purified
by column chromatography (silica gel, Et0Ac:PE=1:2) to give C (751 mg, 30%) as
a yellow
solid.
Additional synthetic information:
[00305] The following compounds were prepared using the identical reaction
with the
following starting materials
Target compound Thiophene starting material Carboxylic acid starting
number structure material
H2N N
Hyt>
62 _o N S # OH
v y
0 0 o
[00306] Additional compounds were prepared using similar protocols as
described above.
These compounds are listed in Table A.
-107-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
Biological Examples
EXAMPLE I: High throughput screen for inhibitors of biofilm formation
inhibition
[00307] A diverse chemical library (-70,000 compounds) was used for the
primary screen. This
in-house compound library was created based on a chemoinformatic analysis of
scaffold
chemical diversity, historical proprietary screen hit rates (> 300 Mio data
points from the HTS
database) and commercial availability. 105/m1M smegmatis cells were plated in
384 well plates
in biofilm formation medium (M63 salts minimal medium supplemented with 2%
glucose, 0.5%
Casamino Acids, 1 mM MgSO4, and 0.7 mM CaC12). RIF and TMC207 were used as
positive
controls, and DMSO (0.1%) as a negative control. Cells were treated with 10
ILIM compound,
incubated for 3 days, and the OD of each well was determined with an EnVision
Multilabel
Reader. The average Z' and coefficient values are 0.512 and 8.7%,
respectively. A high
stringency cutoff (3-fold inhibition) was used to pick hits that are most
likely growth inhibitors
(hit rate 0.03%), and a low stringency cutoff (2-fold inhibition) to include
hits that inhibit
biofilm formation without significant growth inhibition (hit rate 0.17%).
EXAMPLE II: In vitro activity assays
[00308] For kinetic killing assays, exponentially growing cultures of
mycobacteria were diluted
in fresh media to an 0D600 of 0.1 -0.2. Various drugs were added to the
culture at the indicated
concentrations. The number of colony forming units (CFU) at the start of the
experiment was
estimated by plating appropriate dilutions of the culture onto 7H10 agar
plates. The effect of
drug was monitored by plating for CFU at the indicated time points. All
experiments were
carried out in triplicate. MICs were determined by a turbidity assay.
Threefold serial dilutions in
DMSO were prepared for each compound. Mtb cultures (OD = 0.04) were incubated
with
compounds at 37 C for 5 days and 0D600 was determined with an Envision plate
reader. All
experiments were carried out in duplicate. For assays under starvation
conditions, a log-phase
growing Mtb culture was centrifuged and the cell pellet was washed twice with
PBS,
resuspended in PBS with Tyloxapol (0.05%) (OD = 0.3), and incubated with DMSO,
TCA1 (7.5
iug/mL), and RIF (2 iug/mL). All experiments were carried out in triplicate.
For intracellular
macrophage assays, J744.1 murine macrophage cells were infected with Mtb at a
MOI of 1:3
and incubated for 2 hours at 37 C. After washing the cell monolayer three
times, 20 [iM
amikacin was added and the culture was incubated for an additional 2 hours to
kill remaining
extracellular bacteria. Infected cells were then incubated in the presence of
serial dilutions of
compounds for 5 days. Cells were washed three times and lysed in each well;
the lysate was
transferred to a 96 well plate for serial dilution, and then plated on 7H11
agar medium for CFU
assays. All experiments were carried out in triplicate.
-108-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
EXAMPLE III: In vivo efficacy experiments
[00309] Six- to eight-week-old-female BALB/c mice (US National Cancer
Institute) were
infected via aerosol with a low dose (approximately 50 bacilli) of Mtb H37Rv.
Infection dose
was verified by plating the inoculum and the whole lung homogenates onto 7H10
plates at 24
hrs post-infection. Treatment of BALB/c mice began at either 2 weeks or 4
weeks post-infection
with RIF (10 mg/kg) and NH (25 mg/kg) administered ad libitum in drinking
water (changed
once every two days). TCA1 was administered by oral gavage, once daily for 5
days per week at
a dosage of either 40 mg/kg or 100 mg/kg for the indicated durations. At
predetermined time
points, or at humane end-points, animals were heavily sedated, euthanized and
tissues collected
for culture and pathology. Treatment efficacy was assessed on the basis of CFU
in the lungs and
spleen of treated mice compared to untreated controls and bacterial burden in
these organs prior
to treatment start. Organs were homogenized in PBS containing Tween-80 (0.05%)
and various
dilutions were placed on 7H10 plates. Plates were incubated at 37 C for three
weeks and CFU
on the various plates recorded. All animal experimental protocols were
approved by the Animal
Care and Usage Committee of AECOM.
EXAMPLE IV: Genome-wide transcriptional analysis
[00310] Triplicate 10 ml cultures of mycobacteria were grown to log phase for
transcriptional
profiling of planktonic cells or for three weeks in pellicle media for
transcriptional profiling of
pellicle cells. For TCA1 treatment, log phase cultures were treated with 3.75
ug/m1 TCA1 or
DMSO vehicle for 12 hours. Cells were harvested, washed, and re-suspended in 1
ml RNA
Protect reagent (Qiagen) and incubated 4 h at room temperature (21 C). All
transcriptional
profiling procedures, including RNA extraction, DNase treatment, cDNA
synthesis, labeling,
microarray hybridization, washing, scanning, and data analysis were performed
as previously
described [Vilcheze C, Weinrick B, Wong KW, Chen B, & Jacobs WR, Jr. (2010)
NAD+
auxotrophy is bacteriocidal for the tubercle bacilli. Mol Microbiol 76:365-
377]. Microarray data
have been deposited in the US National Center for Biotechnology Information
Gene Expression
Omnibus (GEO series accession number G5E37392). For qPCR experiments, diluted
cDNA was
used as a template at 50 ng per reaction for real time PCR reactions
containing primer sets
designed by Primer 3 and SYBR Green PCR Master Mix (Applied Biosystems) in
accordance
with the manufacturers' instructions. These reactions were carried out on an
ABI 9700HT
real-time PCR cycler (Applied Biosystems).
EXAMPLE V: DprEl competition assay
[00311] DprEl was incubated with serial dilutions of TCA1 for 15 min. BTZ-
BODIPY was
added and the sample incubated for 1 h at 37 C. BTZ-BODIPY is a fluorescent
BTZ derivative
-109-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
which reacts in the presence of farnesylphosphoribose (FPR) with DprEl forming
a covalent
bond. Samples are then analyzed by SDS-PAGE (fluorescence and Coomassie
staining).
EXAMPLE VI: Crystallization and structure determination
[00312] Mycobacterium tuberculosis DprEl (Rv3790) was prepared for
crystallization [Batt
SM, et al. (2012) Structural basis of inhibition of Mycobacterium tuberculosis
DprEl by
benzothiazinone inhibitors. Proc Natl Acad Sci U S A 109:11354-11359]. Prior
to setting up
crystallization experiments, the TCA1 inhibitor (in DMSO) was incubated with
concentrated
protein (¨ 35 mg/ml) for 30 min at a molar ratio of 3 TCA1: 1 DprEl. Crystals
were grown by
sitting drop vapor diffusion and appeared over a reservoir consisting of 40 -
43% (w/v)
polypropylene glycol 400 and 0.1 M imidazole, pH 7Ø Crystals were mounted
into nylon loops
directly from the drop and frozen in liquid nitrogen. X-ray diffraction data
to 2.6 A resolution
were recorded on beamline 102 of the Diamond Light Source. The crystals were
in space group
P21, with two molecules of the complex in the crystallographic asymmetric
unit. Initial phases
were obtained by molecular replacement (PHASER) [McCoy AJ, et al. (2007)
Phaser
crystallographic software. J Appl Crystallogr 40:658-674], using the apo
structure of DprEl
(PDB entry 4FDP) [Batt SM, et al. (2012) Structural basis of inhibition of
Mycobacterium
tuberculosis DprEl by benzothiazinone inhibitors. Proc Natl Acad Sci U S A
109:11354-11359]
as a search model. Following refinement of the molecular replacement solution,
density for
TCA1 was clearly visible in the active sites of the two crystallographically
distinct copies of
DprEl. Density shape and stereochemical constraints allowed us to
unequivocally place the
inhibitor in the active site of DprEl. Model rebuilding and structure
refinement (COOT)
[Emsley P, Lohkamp B, Scott WG, & Cowtan K (2010) Features and development of
Coot. Acta
Crystallogr D Biol Crystallogr 66:486-501], REFMAC5 [Murshudov GN, et al.
(2011)
REFMAC5 for the refinement of macromolecular crystal structures. Acta
Crystallogr D Biol
Crystallogr 67:355-367], PHENIX.REFINE [Adams PD, et al. (2010) PHENIX: a
comprehensive Python-based system for macromolecular structure solution. Acta
Crystallogr D
Biol Crystallogr 66:213-221] led to final R-factors of 23.7% and 17.6% for the
test and working
sets, respectively.
EXAMPLE VII: Affinity-based proteomics and photo-affinity labeling
[00313] H37Ra cells were lysed with homogenization buffer [60 mM13-
glycerophosphate, 15
mM p-nitrophenyl phosphate, 25 mM Mops (pH 7.2), 15 mM MgC12, 1 mM DTT,
protease
inhibitors, and 0.5% Nonidet P-40]. Cell lysates were centrifuged at 16,000 x
g for 20 min at
4 C and the supernatant was collected. Total protein concentration in the
supernatant was
determined by a BCA protein assay kit (Pierce). The lysates (1 mg) were then
added to the
-110-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
affinity resin (30 1) and the loading buffer [50 mM Tris=HC1 (pH 7.4), 5 mM
NaF, 250 mM
NaC1, 5 mM EDTA, 5 mM EGTA, protease inhibitors, 0.1% Nonidet P-40] was added
to a final
volume of 1 ml (for the competition experiment, TCA1 was added to a final
concentration of 50
M). After rotating at 4 C for 1 h, the mixture was centrifuged at 16,000 x g
for 1 min at 4 C,
and the supernatant was removed. The affinity resin was then washed for 5
times with cold
loading buffer and eluted by boiling with Laemmli sample buffer (Invitrogen)
at 95 C for 3 min.
Samples were loaded and separated on a 4-20% Tris-glycine gel (Invitrogen).
The gel band was
extracted and analyzed by proteomics. For the photo-affinity experiments, E.
coli cells
overexpressing MoeW and native cells were lysed and the photo-affinity probe
was added to cell
lysates (1 mg) in 50 L PBS and incubated for 2 h at room temperature followed
by UV
irradiation with a UV lamp for 20 min. The reaction mixtures were then
subjected to
click-chemistry with rhodamine-azide (100 M) and incubated for 2 h at room
temperature with
gentle mixing. The reactions were terminated by the addition of pre-chilled
acetone (0.5 mL),
placed at -20 C for 30 min and centrifuged at 16,000 x g for 10 min at 4 C to
precipitate
proteins. The pellet was washed two times with 200 L of pre-chilled methanol,
resuspended in
25 lut lxstandard reducing SDS-loading buffer and heated for 10 min at 95 C;
samples were
loaded for separation by SDS-PAGE, then visualized by in-gel fluorescent
scanning.
EXAMPLE VIII: In vitro assays in nitrate-only media
[00314] An Mtb culture was re-suspended under nitrogen-limiting conditions
[Malm S, et al.
(2009) The roles of the nitrate reductase NarGHJI, the nitrite reductase NirBD
and the response
regulator GlnR in nitrate assimilation of Mycobacterium tuberculosis.
Microbiology
155:1332-1339] (a basal medium [1 L of the basal medium contains 1 g KH2PO4,
2.5 g
Na2HPO4, 2 g K2504 and 2 ml of trace elements; 1 L of trace elements contained
40 mg ZnC12,
200 mg FeC13.6H20, 10 mg CuC12.4H20, 10 mg MnC12.4H20, 10 mg Na2B407.10H20 and
10
mg (NH4)6Mo7024.4H20)] supplemented with NaNO3 as sole source of nitrogen, 0.5
mM
MgC12, 0.5 mM CaC12, 10 % ADS, 0.2 % glycerol and 0.05 % Tween 80) and
incubated for
24 hours. 7.5 g/m1 of TCA1 was then added to the culture and incubated for 30
days. CFU
assay was used to determine the bacterial viability at each time point.
[00315] All experiments were carried out in triplicate.
EXAMPLE IX: MoCo inhibition assay
[00316] The synthesis of MoCo form "A" dephospho was carried out according to
the
procedures described. The 1H-NMR spectrum matches what has been reported in
the literature
[(a) Taylor EC, Ray PS, & Darwish IS (1989) Studies on the Molybdenum
Cofactor.
Determination of the Structure and Absolute Configuration of Form A. J Am Chem
Soc
-111-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
111:7664-7665; (b) Mohr D, Kazimierczuk Z, & Pfleiderer W (1992) Pteridines.
Part XCVII.
Synthesis and properties of 6-thioxanthopterin and 7-thioisoxanthopterin.
Hely. Chim. Acta.
75:2317-2326]. Conversion of all sources of molybdopterin to Form "A"
dephospho was
performed by following the methods previously reported with slight
modifications [(a) Williams
MJ, Kana BD, & Mizrahi V (2011) Functional analysis of molybdopterin
biosynthesis in
mycobacteria identifies a fused molybdopterin synthase in Mycobacterium
tuberculosis. J
Bacteriol 193:98-106; (b) Johnson ME & Rajagopalan KV (1987) Involvement of
chlA, E, M,
and N loci in Escherichia coli molybdopterin biosynthesis. J Bacteriol 169:117-
125]. 100 ml of
Mtb culture was harvested and the pellet was re-suspended in extraction
solution (2 mL, 10 mM
sodium ascorbate). The cells were lysed and centrifuged at 16,000 x g; the
supernatant was
collected and treated with acidic iodine solution at 95 C for 25 min and the
excess iodine was
removed by adding sodium ascorbate. After centrifugation, the solution was
neutralized with
ammonium hydroxide, then concentrated and dephosphorylated using calf
intestinal phosphatase
(NEB) at 37 C for 3h. HPLC analysis was performed using Agilent C18 column
(150 x 4.6 mm,
10- m of particle size) with gradient elution by buffer A (50 mM ammonium
acetate) and buffer
B (Me0H) (97% A to 93% A in 14 min and 97% B wash from 15 min to 22 min).
Fluorescence
detection was at 370/450 nm.
EXAMPLE X: Cell-free assay for DPA biosynthesis
Preparation of the mycobacterial membrane and cell envelope enzymatic
fraction.
[00317] About 2 g cell pellet of Mycobacterium smegmatis mc2155 grown in the
LB medium
(Invitrogen) supplemented with 0.05 % Tween 80, was washed with Buffer A [50
mM MOPS
(pH 7.9), 5 mM 2-mercaptoethanol and 10 mM MgC12] and suspended in 10 ml of
the same
buffer. The cells were disintegrated by probe sonication performed in 30 s
pulses with 90 s
cooling pauses, repeated 20 times. The sonicate was centrifuged at 15,600 x g
for 20 min at 4 C
and the resulting pellet was used for preparation of the cell envelope
enzymatic fraction, as
described [Mikusova K, et al. (2005) Decaprenylphosphoryl arabinofuranose, the
donor of the
D-arabinofuranosyl residues of mycobacterial arabinan, is formed via a two-
step epimerization
of decaprenylphosphoryl ribose. J Bacteriol 187:8020-8025] with minor
modifications. Briefly,
the pellet was homogenized with Buffer A to the volume 4 ml of the final
suspension, to which 6
ml of Percoll (GE Healthcare) was added and the mixture was centrifuged at
15,600 x g for
60 min at 4 C. White upper band was collected and Percoll was removed from the
sample by
repeated washings with Buffer A and centrifugations at 15,600 x g for 20 min
at 4 C. The final
pellet was resuspended in 400[L1 of Buffer A resulting in the sample with the
protein
concentration of 6.8 mg/ml, which was used as the source of the cell envelope
enzyme in the cell
-112-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
free reactions. Membrane fraction with protein concentration of 49 mg/ml was
prepared by
centrifugation of 15,600 x g supernatant of the sonicate at 100,000 x g, as
described [Mikusova
K, et al. (2005) Decaprenylphosphoryl arabinofuranose, the donor of the D-
arabinofuranosyl
residues of mycobacterial arabinan, is formed via a two-step epimerization of
decaprenylphosphoryl ribose. J Bacteriol 187:8020-8025].
Composition and analysis of the cell free reactions
[00318] The reaction mixtures contained 75,000 dpm of phospho-[14C]-ribose
diphosphate
[Scherman MS, et al. (1996) Polyprenylphosphate-pentoses in mycobacteria are
synthesized
from 5-phosphoribose pyrophosphate. J Biol Chem 271:29652-29658], 0.1 mM NADH,
3.125%
DMSO, 500 [tg of membrane protein or 200 [tg of the cell envelope protein and
Buffer A in the
final volume of 80 pl. TCA1 and BTZ043 dissolved in DMSO were added to the
reaction
mixtures in the final concentration of 25 [tg/ml. For dose-dependence
experiment TCA1 was
added in the final concentrations 1, 3, 6, 12 and 25 jig/m1 in the reaction
mixtures. After 1 h
incubation at 37 C, the reactions were stopped by the addition of 1.5 ml of
CHC13/CH3OH (2:1).
After 20 min extraction of the reaction products at RT, 170 pi of Buffer A was
added, the tubes
were thoroughly mixed and then briefly centrifuged at 3,000 x g to achieve
separation of two
phases of the mixture [Folch J, Lees M, & Sloane Stanley GH (1957) A simple
method for the
isolation and purification of total lipides from animal tissues. J Biol Chem
226:497-509]. Upper,
aqueous phase containing unreacted radiolabelled substrate was discarded;
bottom, organic
phase was transferred to the new tube and dried under the stream of N2 at RT.
The organic
extract was dissolved in 40 pi of CHC13/CH3OH/H20/conc. NH4OH (65:25:3.6:0.5)
and
analysed by TLC on aluminium-backed silica gel plates (F2545 Merck) in
CHC13/CH3OH/1M
CH3COONH4/conc. NH4OH/H20 (180:140:9:9:23). Radiolabeled compounds were
visualized
by autoradiography [BioMax MR film (Kodak)].
EXAMPLE XI: Biological Assay and Analytical Data
[00319] Table A shows the biological data for compounds 1-460 as well as
analytical data
(NMR and/or MS) for selected compounds.
[00320] The MIC of compounds of Formulas (I), (Ia), (Ib), (Ic), (Id), (II),
(Ha), (IIb), and (IIc)
was determined in a microplate alamar blue assay (MABA; listed as "I" in Table
A) or turbidity
(listed as "II" in Table A) assay. The MIC of compounds of Formulas (I), (Ia),
(Ib), (Ic), (Id),
(II), (Ha), (JIb), and (IIc) in the assay was graded as A: <1 [tg /mL; B: 1-3
1..tg /mL; and C: >31..tg
/mL.
[00321] The compounds of Formulas (I), (Ia), (Ib), (Ic), (Id), (II), (Ha),
(JIb), and (IIc) were
also evaluated under starvation conditions in a low-oxygen-recovery assay
(LORA; listed as
-113-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
"III" in Table A). The MIC of compounds of Formulas (I), (Ia), (Ib), (Ic),
(Id), (II), (Ha), (Ilb),
and (lic) in the assay was graded as A: <5 [ig /mL; B: 5-25 [ig /mL; and C:
>25 tg /mL.
[00322] The compounds of Formulas (I), (Ia), (Ib), (Ic), (Id), (II), (Ha),
(Hb), and (He) were
further evaluated in cytotoxicity assays using either Vero cells (listed as
"IV" in Table A) or
Huh7 cells (listed as "V" in Table A). The LC50 was graded as A: >50 tg /mL;
B: 50-25 tg
/mL; and C: <25 [ig /mL.
Table A.
Cpd Structure I II III IV V LCMS and/or
NMR Data
ID
BBC A AESMS M/Z=376 (M+1)
1 N S 0
I-H NMR (400 MHz, CD30D) 8.24 (d, J =
Sji)r.NH 0 0 8.2 Hz, 1H), 8.16 (d, J = 8.0
Hz, 1H),
0 7.68 (t, J = 7.6 Hz, 1H), 7.62
(t, J = 7.6
Hz, 1H), 7.56 (d, J = 5.9 Hz, 1H), 7.10
(d, J = 5.9 Hz, 1H), 4.33 (q, J = 7.1 Hz,
2H), 1.39 (t, J = 7.1 Hz, 3H).
2
HN&ID irs
S N
0
3 0 3.1 A
_
N NH
HN H to
4 H B A
0 r
0 o HN 0
H S
A
0
o
co.e
NH
0
o s
6 A
* s ii,cr:LsNH
N.r14
0 s
-114-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
7 00 ________ A
eyLNAO
es.NH, _NH $
o NH"
i u-,µ
0 N, 0
8 o 4* F B
cy..NH
\ /
0 0 HN 0
A
- 0 Ni.L6
H -- -
9 o B
o 0 HN
0)k141)L6a *
HN.r j
0
S/ A A B A
II
N--1(1N
H ¨ 0
S
11 0 B A
O 0 HNy ....N
opikisl)C6a
H , s' 1
--
j.....0
B A
12
As-N
O _.\p
H
N-N
-MAN HN-1.
...õ S
13 _14
O HN_p A A
O -NH
(:)--
..., s
14 A ES MS M/Z=390 (M+1)
IIP N S "----- II;11 0 I-H NMR (400 MHz, CDCI3) 8.08
(d, J =
SNH 0 0 8.2 Hz, 1H), 8.02 (d, J = 8.2
Hz, 1H),
7.54 (t, J = 8.2 Hz, 1H), 7.48 (t, J = 8.2
0
Hz, 1H), 7.06 (s, 1H), 4.18 (q, J = 7.1
Hz, 2H), 2.35 (s, 3H), 1.25 (t, J = 7.2
Hz, 3H).
ci A I-H NMR (400 MHz, CDCI3) 13.088 (br,
IPN s:-.11õ 'nily 0 1H), 10.837 (br, 1H), 8.326
(d, J = 10
-.' Hz, 1H), 7.880 (s, 1H), 7.691 (m, 2H),
s--kr. NH0 0 4.241 (m, 2H), 2.285 (s, 3H), 1.301 (t, J
o
= 7.2 Hz, 3H).
-115-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
16 F A B 'H NMR (400 MHz, DMSO-d6)
10.762
# N S ----Thri.N1 0 (s, 1H), 8.304 (d, J = 8 Hz,
2H), 7.693-
7.646 (m, 2H), 7.495 (s, 1H), 4.217 (q,
SNH 0 0 J = 7.0 Hz, 2H), 1.285 (t, J =
7.1 Hz,
0
3H); I-9F NMR (376 MHz, DMSO-d6) 5 -
139.886 (s)
17 F H F,F A C 'H NMR (400 MHz, CD30D) 8.224
(d, J
0...ic
s)7.114/..11 F = 7.9 Hz, 1H), 8.148 (d, J = 7.4 Hz, 1H),
0 NH -
n 0 7.667-7.607 (m, 2H), 7.166 (d,
J = 3.6
,,
'IN Hz, 1H), 4.777 (q, J = 8.7 Hz,
2H); '9FN' S NMR (376 MHz, CD30D)
0 -74.961 (s), -140.976 (s)
18 ilk .-- H A C A ES-MS M/Z=406.1 (M+1)
mirt N - -- N 0,. ..,
lr 0 I-H NMR (400 MHz, DMSO-d6)
8.303
S-Irs.NH 0 0
(d, J = 9.3 Hz, 2H), 7.784 (d, J = 6.0 Hz,
o
1H), 7.770 (m, 2H), 7.188 (d, J = 5.9
Hz, 2H), 4.295 (t, J = 4.5 Hz, 2H), 3.602
(t, J = 4.5 Hz, 2H), 3.290 (s, 3H).
19 A I-H NMR (500 MHz, CDCI3) 8.354
(d, J =
II 8.5 Hz, 1H), 8.079 (d, J = 8.0 Hz, 1H),
o*NH 0 0
7.9 (s, 1H), 7.170 (d, J = 6.0 Hz, 1H),
N--.% 7.044 (d, J = 5.5 Hz, 1H), 4.377 (d, J
=
ir 6.5 Hz, 2H), 1.822-1.792 (m, 1H),
1.557-1.527 (m, 1H), 1.1 (t, J = 6.8 Hz,
3H).
20 0 A C ES-MS M/Z=394 (M+1)
0 HNJCITS
I-H NMR (301 MHz, CDCI3) 12.99 (s,
0
F )L14?Le/S N *
1H), 8.23 (d, J=8.1Hz, 1H), 8.15 (s, 1H),
F F 7.58 (m, 2H), 7.16 (d, J=6.0
Hz, 1H),
6.97 (d, J=6.0 Hz, 1H), 4.77 (m, 1H),
4.58 (m, 2H), 4.47 (m, 1H).
21 0 A
ii
NYt \ lk ikl 0
S H
0 NA 0
H
22 11_A I-H NMR (500 MHz, DMSO-d6)
10.89 (s,
NH 1H), 10.57 (s, 1H), 9.04 (s,
1H), 8.84 (s,
H 0 11_0 1H), 8.63 (s, 1H), 7.79 (d, J
= 10 Hz,
0 \-
--N 1H), 7.18 (d, J = 5 Hz, 1H),
4.28 (q, J =
Hz, 2H), 2.20 (s, 3H), 1.35 (t, J = 10
Hz, 3H).
23 0 A I-H NMR (400 MHz, DMSO-d6)
10.572
0 HNI-Is (s, 1H), 8.312-8.282 (m, 2H),
7.741 (d,
0
0)LN)L6 N * J = 6.0 Hz, 1H), 7.696 (m,
2H), 7.177
H (d, J = 5.9 Hz, 1H), 1.507 (s,
9H).
-116-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
24 0 A C I-H NMR (400 MHz, DMS0- d6)
8.301
0 HNiLr)41 (d, J = 8.7 Hz, 2H), 7.769 (d,
J = 6.0 Hz,
0 t)
F),0)LN)L6 S WI 1H), 7.696 (m, 2H), 7.204 (d, J = 6.0
H Hz, 1H), 5.557-5.523 (m, 1H),
1.460 (d,
F F
J = 6.6 Hz, 3H).
25 o A B I-H NMR (400 MHz, DMS0- d6)
8.301
0 HNjLr:-N (d, J = 8.7 Hz, 2H), 7.769 (d,
J = 6.0 Hz,
F 0 S *
F-1,L it.. N X-els 1H), 7.696 (m, 2H), 7.204 (d, J = 6.0
0 ru H Hz, 1H), 5.557-5.523 (m, 1H), 1.460 (d,
J = 6.6 Hz, 3H).
26 0 A A I-H NMR (400 MHz, DMSO-d6)
10.819
O 0 HN (s, 1H), 8.940 (s, 1H),
8.344 (t, J = 1.2
spAN)L6c * Hz, 1H), 8.034 (d, J = 1.2 Hz, 1H),
0
Nz..-/ 7.718 (d, J = 6.0 Hz, 1H), 7.087 (d, J =
5.9 Hz, 1H), 4.207 (q, J = 7.1 Hz, 1H),
1.275 (t, J = 7.1 Hz, 1H).
27 o A C I-H NMR (500 MHz, DMSO-d6)
10.84 (s,
o 0 HN 1H), 7.78 (d, J = 5 Hz,
1H), 7.58 (d, J =
OANjL6 * 10 Hz, 1H), 7.48 (s, 1H), 7.23 (d, J = 10
H , S
O/0 Hz, 1H), 7.22 (d, J = 5 Hz, 1H), 6.25
..
(s,2H), 4.28 (q, J = 5 Hz, 1H), 1.35 (t, J
= 5 Hz, 1H).
29
It 0 A C B 'H NMR (500 MHz, DMSO-d6)
10.20 (s,
HN Njb....H 1H), 8.63 (s, 1H), 8.62 (s, 1H), 7.78
(d, /-/ J = 5.0 Hz, 1H), 7.56-7.54 (m, 1H), 7.16
F 0 =
(d, J = 5.0 Hz, 1H), 4.78-4.65 (m, 2H),
4.49-4.40 (m, 2H), 1.28 (s, 9H).
30 Q
O A A 'H NMR (400 MHz, DMS0-
d6) 10.784
(s, 1H), 8.306-8.284 (m, 2H), 7.761 (d,
N J = 6.0 Hz, 1H), 7.695 (m,
7H), 7.186
S S (d, J = 5.8 Hz, 1H), 4.740-4.707 (m,
Ir 1H), 1.908-1.091 (m, 10H).
31 0 N *
A A I-H NMR (400 MHz, DMS0- d6)
10.758
HN S (s, 1H), 8.296 (d, J = 7.8 Hz,
1H), 7.751
0
O..4 (d, J = 6.0 Hz, 1H), 7.694 (m, 7H),
7.183 (d, J = 5.8 Hz, 1H), 7.740-7.704
1.-NH
0-0 (m, 1H), 5.145-5.130 (m, 1H),
1.969-
1.584 (m, 8H).
32
C C A 'H NMR (400 MHz, DMSO-d6)
10.159
>co
(br, 1H), 8.581 (br, 2H), 7.721(br, 1H),
HN
N) 7.519(br, 1H), 7.127(br, 1H),
4.28 (m,
2H), 3.565 (m, 4H), 2.493-2.475 (m,
i-Nr-\0 6H buried inside the DMSO peak),
OZ-'
NH 0-1 \-/ 1.250 (s, 9H).
T.,54N_µ
0
0
-117-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
33
_Ft 0 A C A 'H NMR (500 MHz, DMSO-d6) 10.89 (s,
HN
1H), 9.65 (s, 1H), 8.58 (d, J = 5 Hz, 1H),
H b i__ 11
0- 0 , i "1,4)
-/ 0 ' N r 8.39 (s, 1H), 7.80 (d, J = 10
Hz, 1H),
C5 7.46 (t, J = 5 Hz, 1H), 7.20 (d, J = 5 Hz,
0 1H), 4.28 (q, J = 5 Hz, 1H),
3.68-3.66
(m, 4H), 3.57-3.55 (m, 4H), 1.35 (t, J =
Hz, 1H).
34 0 C B C I-H NMR (500 MHz, CD30D) 8.30-
8.28
o'' 1 0 0 HN-11)...S (m, 2H), 7.74-7.61 (m, 3H),
7.17 (m,
C.'NOANj6 NI
H -- S 4 1H), 5.29 (m, 1H), 3.77 (m,
4H), 2.73-
2.57 (m, 6H), 1.44 (d, J = 5.0 Hz, 3H).
35 (0\
r A C C I-H NMR (500 MHz, CD30D) 8.30
(d, J =
10.0 Hz, 1H), 8.23 (d, J = 10.0 Hz, 1H),
0 0 7.75-7.63 (m, 3H), 7.18 (d, J
= 5.0 Hz,
\r.
HN 1H), 5.66-5.57 (m, 1H), 5.04
(m, 2H),
X4.84-4.82 (m, 2H).
NH S
S e4 (10
C) N
36 o B C A I-H NMR (400 MHz, CD30D) 8.976
(d, J
0 0 HNj ..1*i.....\..õ = 1.2 Hz, 1H), 8.876 (d, J =
1.2 Hz, 1H),
.N3j.kNjL6s N , I a X
05 . 7.506 (d, J = 5.9 Hz, 1H), 7.043 (d, J =
5.9 Hz, 1H), 4.283 (q, J = 7.2 Hz, 1H),
1.335 (t, J = 7.2 Hz, 1H).
37 * srH
pi A C A ES-MS M/Z=394.0 (M+1)
, Nõnraõ.......",F
I-H NMR (400 MHz, CD30D) 8.218 (d, J
NH 0 0
= 8.2 Hz, 1H), 8.143 (d, J = 7.8 Hz, 1H),
o
7.599 (m, 2H), 7.562 (d, J = 5.9 Hz,
1H), 7.080 (d, J = 6.0 Hz, 1H), 4.765-
4.626 (m, 1H), 4.534-4.441 (m, 2H).
A A ES-MS Mg=461(M+1)
38 ir N s -.?rPl 'N
ic I-H NMR (400 MHz, CD30D) 8.185
(d, J
S'I'r NH 0 0 c,0
= 8.2 Hz, 1H), 8.117 (d, J = 7.6 Hz, 1H),
o
7.64 (m, 2H), 7.508 (d, J = 5.9 Hz, 1H),
7.066 (d, J = 6.4 Hz, 1H), 4.404 (t, J =
5.6 Hz, 2H), 3.731-3.719 (m, 4H),
2.777 (t, J = 5.6 Hz, 2H), 2.619 (m, 4H).
39 1P, SrH C A 'H NMR (500 MHz, CD30D) 8.243-
N
8.181 (m, 2H), 7.710-7.644 (m, 2H),
S'ir..NH 0 0 I
7.566 (m, 1H), 7.108 (m, 1H), 4.530
o
(m, 2H), 3.034 (m, 2H), 2.608 (s, 6H).
40 lip, s?yj 0 j A C I-H NMR (400 MHz, CD30D)
8.218 (d, J
N
= 7.9 Hz, 1H), 8.149 (d, J = 7.2 Hz, 1H),
Sir. NH 0 IC F 7.660 (m, 2H), 7.559 (d, J =
5.9 Hz,
0
1H), 7.080 (d, J = 5.9 Hz, 1H), 6.170
(tt, J = 54.7 and 3.6 Hz, 1H), 4.470 (td,
J = 14.2 and 3.6 Hz, 1H); I-9F NMR (376
MHz, CD30D) -127.492 (s).
-118-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
41 A C C ES-MS M/Z=390.1 (M+1)
s?r1-411 0
II I-H NMR (500 MHz, DMSO-d6)
10.863
'.NH 0 0
(s, 1H), 8.381 (d, J = 8.4 Hz, 2H), 7.844
Ikis (d, J = 5.9 Hz, 1H), 7.771-
7.725 (m,
* 2H), 7.262 (d, J = 5.7 Hz,
1H), 5.084-
5.033 (m, 1H), 1.380 (d, J = 6.2 Hz,
6H).
42 s .?y",11 0 A C 'H NMR (500 MHz, DMSO-d6)
10.839
ir
0 (s, 1H), 9.632 (s, 1H), 8.855
(s, 1H),
NH 0 0
8.326 (d, J = 8.6 Hz, 1H), 8.070 (d, J =
s * 8.7 Hz, 1H), 7.742 (d, J = 5.9
Hz, 1H),
17-N 7.111 (d, J = 5.9 Hz, 1H),
4.225 (q, J =
7.1 Hz, 1H), 1.292 (t, J = 7.1 Hz, 1H).
43
' A B A ES-MS M/Z=376.0 (M+1)
s .? ill 0
1r I-H NMR (400 MHz, DMSO-d6)
10.825
0 NH 0 0
(s, 1H), 9.572 (s, 1H), 8.604 (d, J = 1.4
I* Hz, 1H), 8.438 (d, J = 8.5 Hz,
1H),
8.026 (dd, J = 8.4 and 1.6 Hz, 1H),
s.., 7.729 (d, J = 6.0 Hz, 1H),
7.097 (d, J =
5.9 Hz, 1H), 4.213 (q, J = 7.1 Hz, 1H),
1.279 (t, J = 7.1 Hz, 1H).
44 talk- s B A
4111r/ l -0" Ny0,..Ø0
S---rNH 0 0
0
* C A I-H NMR (400 MHz, DMSO-d6)
10.132
s (s, 1H), 8.304-8.267 (m, 2H), 7.686-
...el
7.633 (m, 2H), 7.595 (d, J = 5.9 Hz,
0-Nii o o
syA . 1H), 7.216 (d, J = 5.8 Hz,
1H), 2.974 (s,
rii y 6H).
46 a r A C A I-H NMR (400 MHz, CDCI3) 9.785
(br,
72..., ... j
HN-bN 1H), 8.829 (s, 1H), 8.479 (d,
J = 5.1 Hz,
\ s 1H), 7.945 (br, 1H), 7.580 (d,
J = 5.3
Hz, 1H), 7.085 (d, J = 6.0 Hz, 1H),
4.359-4.306 (m, 2H), 3.85 (br, 4H),
3.288 (br, 2H), 2.717 (br, 4H), 1.368 (t,
J = 7.1 Hz, 3H).
47 0 rµs
p.. A C A 'H NMR (500 MHz, Acetone-d6)
9.692
N rtiNµ " (br, 1H), 8.801 (br, 1H),
8.590-8.517
53- (m, 2H), 7.697 (d, J = 6.0 Hz,
1H),
j0 HN 0 7.480 (m, 1H), 7.088 (d, J =
5.5 Hz,
`c3riiiLds 1H), 4.254-4.226 (m, 2H),
4.146 (m,
4H), 3.250-3.241 (m, 4H), 1.292 (t, J =
7.0 Hz, 3H).
-119-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
48 0 r`o A C A 'H NMR (400 MHz, CDCI3)
8.524 (s,
)41 rtiNN' 1H), 8.366 (t, J = 4.5 Hz, 1H), 8.258
ipow (br, 1H), 7.483 (dd, J = 5.2 and 1.6 Hz,
0 0 HN 0 1H), 7.474 (br, 1H), 7.480 (m, 1H),
F.........,....11,..11.t.
`' ri -s 7.122 (d, J = 5.9 Hz, 1H),
6.871 (d, J =
6.0 Hz, 1H), 4.756-4.473 (m, 4H),
3.785-3.761 (m, 4H), 3.566-3.554 (m,
4H).
49 HCI HCI B C A I-H NMR (400 MHz, CD30D) 8.50
(br,
0 1H), 8.34 (br, 1H), 7.57 (br,
1H), 7.54
N *(2i'
(d, J = 4 Hz, 1H), 7.06 (d, J = 4 Hz, 1H),
- .
y0 r 4.2 (m, 2H), 4.29 (q, J = 8 Hz, 2H),
o HN
3.58-3.30 (m, 6H buried inside the
o
OAN solvent peak), 2.98 (s, 3H),
1.36 (t, J =
ii)6S
8 Hz, 3H).
50 0 A C B 'H NMR (400 MHz, CDCI3) 8.55
(s, 1H),
NN * 8.38 (t, J = 4 Hz, 1H), 7.89 (br, 1H),
- .
v- 7.50 (dd, J = 8 and 4 Hz, 1H), 7.36 (br,
o 0 HN 1H), 7.06 (d, J = 4 Hz, 1H), 6.92 (d, J =
o
0j.LNjL6s 4 Hz, 1H), 4.33 (q, J = 8 and
4 Hz, 2H),
3.68 (m, 4H), 2.11-2.04 (m, 4H), 1.37
(t, J = 8 Hz, 3H).
51A C B 'H NMR (400 MHz, CDCI3) 8.60
(s, 1H),
cr NH ...D
N 0 Tpir 8.34 (t, J = 4 Hz, 1H), 7.96 (br, 1H),
7.46-7.45 (m, 2H), 7.06 (d, J = 4 Hz,
0 0 HN40 1H), 6.88 (d, J = 4 Hz, 1H),
4.33 (q, J =
8 Hz, 2H), 3.53-3.51 (m, 4H), 1.66-1.64
(m, 6H), 1.37 (t, J = 8 Hz, 3H).
52
_p 0 A A
HN 11-1S-N
0-<µ 0 s
* CI
53
It 0 A A C B
HN 14.11St:N
0-µk 0 s
¨/ 0
F
54 NC?1 µ A C ES-MS M/Z=402 (M+1)
I-H NMR (301 MHz, CDCI3) 13.11 (s,
' ,
P12---S731)3-NH 1H), 8.94 (s, 1H), 8.77 (s, 1H), 7.93 (s,
o - 1H), 7.12 (d, J=6.0 Hz,
1H), 7.01 (d,
s ,
J=6.0 Hz, 1H), 4.36 (q, J = 7.1 Hz, 2H),
1.38 (t, J = 7.1 Hz, 3H).
-120-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
55 ictillc: C C ES-MS M/Z=395 (M+1)
I-H NMR (301 MHz, DMSO-d6) 12.92
/ N ==== N,(:),,
Fl (s, 1H), 10.85 (s, 1H), 8.23 (s, 1H), 7.73
SNH 0 0 (d, J = 5.8 Hz, 1H), 7.19 (d, J = 5.8 Hz,
0
1H), 4.21 (q, J = 7.1 Hz, 2H), 3.56 (dd, J
= 6.7, 2.5 Hz, 2H), 3.15 (t, J = 7.0 Hz,
2H), 1.28 (t, J = 7.1 Hz, 3H).
56 ), A C ES-MS M/Z=423 (M+1)
N4s
o, 'H NMR (301 MHz, CD30D) 7.49
(d,
J=6.3Hz, 1H), 7.01 (d, J=6.3Hz, 1H),
14=-7_,M)3¨NH
4.28 (q, J = 7.1 Hz, 2H), 3.88 (s, 2H),
S
0
3.04 (s, 3H), 2.99 (s, 2H), 1.36 (m, 3H),
1.22 (d, J = 6.5 Hz, 6H).
A C ES-MS M/Z=377 (M+1)
N , S S ==== N ON I-H NMR (301 MHz, DMSO-
d6) 13.18
N:::".NH 0 0 (s, 1H), 10.88 (s, 1H), 8.90 (dd, J = 4.5,
o 1.5 Hz, 1H), 8.81 (dd, J=8.1,
1.5 Hz,
1H), 7.79 (d, J=5.7 Hz, 1H), 7.67 (dd,
J=8.1, 4.5 Hz, 1H), 7.22 (d, J=5.7 Hz,
1H), 4.26 (q, J = 6.9 Hz, 2H), 1.30 (t,
J=7.2 Hz, 3H)
58 o s)./r A B A ES-MS M/Z=461 (M+1)
iri NH 'H NMR (400 MHz, CDCI3) 12.58
(s,
('N-k
N 40 grON_
1H), 8.28 (s, 1H), 7.92 (s, 1H), 7.78 (d,
s
0,....., - J = 22.6 Hz, 2H), 7.08 (s,
1H), 6.90 (s,
1H), 4.36 (d, J = 6.7 Hz, 2H), 3.88 (s,
4H), 3.71 (s, 4H), 1.28 (s, 3H).
59 o s)_i A B A ES-MS M/Z=474 (M+1)
tir NH 'H NMR (301 MHz, CDCI3) 12.54 (s,
r'\NA
N 4 0 'ON_
1H), 8.21 (s, 1H), 7.89 (s, 1H), 7.73
S
...141,...../ (dd, J = 22.2, 8.1 Hz, 2H),
7.03 (d, J =
6.0 Hz, 1H), 6.85 (d, J = 5.9 Hz, 1H),
4.32 (q, J = 7.1 Hz, 2H), 3.83 ¨ 3.69 (m,
4H), 2.75 ¨ 2.62 (m, 4H), 2.42 (s, 3H),
1.36 (t, J = 7.1 Hz, 3H).
60 A A C A A ES-MS M/Z=419 (M+1)
Vo OA 'H NMR (301 MHz, DMSO-d6)
12.57
0 0 HN (s, 1H), 10.83 (s, 1H), 10.16 (s, 1H),
OANjL6' 8.60 (d, J = 4.9 Hz, 2H), 7.73 (d, J = 6.0
Hz, 1H), 7.53 (s, 1H), 7.13 (d, J = 5.9
Hz, 1H), 4.21 (d, J = 7.1 Hz, 2H), 1.33 ¨
1.20 (m, 12H).
61 0 / B A B A B ES-MS M/Z=393 (M+1)
N 0
I-H NMR (301 MHz, DMSO-d6) 12.31
-.....NH
\ I (s, 1H), 10.82 (s, 1H), 8.75 (d, J = 4.8
Hz, 2H), 7.97 (d, J = 5.3 Hz, 2H), 7.72
0 0 HN 0V (d, J = 6.0 Hz, 1H), 7.12 (d, J = 5.7
Hz,
/N:6
H ..... ¨ 1H), 4.21 (dd, J = 14.1, 7.0
Hz, 2H),
2.86 (s, 3H), 1.29 (t, J = 7.1 Hz, 3H).
-121-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
62 sc-*--N B C B ES-MS M/Z=390 (M+1)
* sr.-- 1-41T0 I-H NMR (301 MHz, CDCI3) 12.60 (s,
1H), 9.10 (s, 1H), 8.84 (s, 1H), 8.09 (s,
NH 0 0
1H), 7.78 (s, 1H), 6.68 (s, 1H), 4.32 (q,
o
J = 7.1 Hz, 2H), 2.45 (s, 3H), 1.37 (t, J =
7.1 Hz, 3H).
63 HIrqs A B A ES-MS M/Z=370 (M+1)
0,N **--
11 0
0 0 HN 'H NMR (301 MHz, DMSO-d6)
12.85
(s, 1H), 10.85 (s, 1H), 9.06 (dd, J = 4.1,
1.7 Hz, 1H), 8.59 (s, 1H), 8.52 (d, J =
I
/ 8.7 Hz, 1H), 8.24 (d, J = 8.5 Hz, 1H),
8.12 (d, J = 1.7 Hz, 1H), 7.75 (d, J = 6.0
Hz, 1H), 7.70 (dd, J = 8.3, 4.2 Hz, 1H),
7.13 (d, J = 5.9 Hz, 1H), 4.23 (q, J = 7.1
Hz, 2H), 1.30 (t, J = 7.1 Hz, 3H).
64 0 A A A ES-MS M/Z=339 (M+1)
0 0 HN% I-H NMR (301 MHz, DMSO-d6)
12.59
OAN)6* (s, 1H), 11.48 (s, 1H), 10.70
(s, 1H),
H , S
0 7.68 (d, J = 6.0 Hz, 1H), 7.04 (d, J = 6.0
Hz, 1H), 4.20 (q, J = 6.9 Hz, 2H), 2.86
(t, J = 8.4 Hz, 2H), 2.49 (t, J = 8.4 Hz,
2H), 1.27 (t, J = 7.1 Hz, 3H).
_r_z A A A ES-MS M/Z=388 (M+1)
0
N v.,
1
0 0 'H NMR (301 MHz, CDCI3) 12.37
(s,
4
1H), 8.76 (s, 1H), 7.86 (s, 1H), 7.07 (d,
¨/ o NN ..._
--)-N J = 6.0 Hz, 1H), 6.93 (d, J = 6.0 Hz, 1H),
6.60 (s, 1H), 4.33 (q, J = 7.2 Hz, 2H),
3.17 (s, 3H), 2.58 (s, 3H), 1.37 (t, J =
7.1 Hz, 3H).
66 A A A ES-MS M/Z=403 (M+1)
'H NMR (301 MHz, CDCI3) 12.26 (s,
N NH
-- H 1H), 7.92 (s, 1H), 7.36 (s, 1H), 7.26 (s,
4 0 0 -0
0 \¨ 1H), 7.02 (d, J=6.0 Hz, 1H),
6.90 (s,
0 2H), 6.84 (d, J=6.0 Hz, 1H),
5.18 (s,
/
1H), 4.33 (q, J = 7.1 Hz, 2H), 3.89 (s,
3H), 1.37 (t, J = 7.1 Hz, 3H).
67 ilk) A A A ES-MS M/Z=373 (M+1)
0 WI I-H NMR (301 MHz, DMSO-d6) 12.19
-- (s, 1H), 10.77 (s, 1H), 7.69 (d, J=5.7 Hz,
0 0 HN 0 1H), 7.41 (t, J = 6.0 Hz, 2H), 7.31 (t,
(3ANi'L .-6 J=6.0 Hz, 1H), 7.04 (m, 2H), 6.90 (d,
J=5.7 Hz, 1H), 5.07 (s, 1H), 4.21 (q, J =
6.9 Hz, 2H), 1.29 (t, J = 7.1 Hz, 3H).
-122-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
68 1 A A A ES-MS M/Z=403 (M+1)
0 tau
W 0 I-H NMR (301 MHz, CDCI3) 12.28 (s,
1H), 7.96 (s, 1H), 7.34 (d, J=6.0 Hz,
NH 0 0
1H), 7.04 (d, J=6.0 Hz, 1H), 6.81 (m,
0 j
o
4H), 5.05 (s, 2H), 4.32 (q, J = 7.1 Hz,
Y
SNA
-- H 2H), 3.79 (s, 3H), 1.38 (t, J
= 7.1 Hz,
3H).
69 C
--
ii 9-Thr _ A
"` NN NH 0 Nlr
'...-r A C ES-MS M/Z=371 (M+1)
I-H NMR (301 MHz, DMSO-d6) 10.77
o
(s, 1H), 9.26 (q, J=1.8 Hz, 1H), 8.80 (d,
0
J=8.4 Hz, 1H), 8.65 d, J=6.0 Hz,
1H),8.41 (d, J=8.4 Hz, 1H), 7.80 (m,
2H), 7.16 (d, J=6.0 Hz, 1H), 4.26 (q, J =
6.9 Hz, 2H), 1.31 (t, J = 7.1 Hz, 3H).
70 ---o , C C A ES-MS M/Z=405 (M+1)
= o o >
o ,-o I-H NMR (301 MHz, CDCI3) 11.91
(s,
Lid-NH 1H), 7.84 (s, 1H), 7.00 (m, 1H), 6.85 (d,
o J=6.0 Hz, 1H), 6.51 (d, J=6.0 Hz, 1H),
6.45 (m, 1H), 4.52 (s, 1H), 4.35 (q, J =
7.1 Hz, 2H), 4.31 (m, 1H), 3.77 (s, 3H),
3.11 (m, 3H), 1.38 (t, J = 7.1 Hz, 3H).
71 p( 0 B A A ES-MS M/Z=405 (M+1)
HN_ N I-H NMR (301 MHz, DMSO-d6) 5 11.81
oz:1- 0 H * 0= (s, 1H), 10.70 (s, 1H), 7.60
(d, J=6.0 Hz,
o
1H), 6.99 (d, J=6.0 Hz, 1H), 6.70 (m,
3H), 4.38 (q, J=3.0 Hz, 1H), 4.22 (q, J =
7.1 Hz, 2H), 4.16 (m, 1H), 3.68 (s, 3H),
3.05 (m, 2H), 1.37 (t, J = 7.1 Hz, 3H).
72 o 3/_ NH B C B A A ES-MS M/Z=393 (M+1)
'H NMR (301 MHz, DMSO-d6) 13.03
HN-Th.11
N µ (s, 1H), 10.80 (s, 1H), 7.92 (m, 1H),
ci = o ¨
7.74 (d, J=6.0 Hz, 1H), 7.59 (d, J=6.0
Hz, 1H), 7.39 (m, 1H), 7.14 (d, J=6.0
Hz, 1H), 4.22 (q, J = 7.1 Hz, 2H), 1.30
(t, i = 7.1 Hz, 3H).
73 _rz0 B C A ES-MS M/Z=359 (M+1)
I-H NMR (301 MHz, CD30D) 9.54 (s,
HN N-1..(
N 1H), 8.41 (m, 2H), 8.14 (m, 2H), 7.54
¨/ 0 (d, J=6.3Hz, 1H), 7.05 (d,
J=6.3Hz, 1H),
N
4.30 (q, J = 7.1 Hz, 2H), 1.37 (t, J = 7.1
Hz, 3H).
74 0 3/
N___ _ NH C C B ES-MS M/Z=325 (M+1)
'H NMR (301 MHz, DMSO-d6) 12.82
Lii.`11 ¨
(s, 1H), 10.84 (s, 1H), 7.75 (d, J = 5.9
0 ¨ Hz, 1H), 7.19 (d, J = 5.2 Hz,
1H), 4.21
(q, J = 7.1 Hz, 2H), 2.75 (s, 3H), 1.28 (t,
J = 7.1 Hz, 3H).
-123-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
75 N/1-0 C C A ES-MS M/Z=360 (M+1)
___________
* s? (1-4111(0 I-H NMR (301 MHz, CDCI3) 12.68 (s,
1H), 8.24 (m, 4H), 7.07 (d, J=6.0 Hz,
NH 0 0
o 1H), 6.90 (d, J=6.0 Hz, 1H), 4.33 (q, J =
7.1 Hz, 2H), 1.37 (t, J = 7.1 Hz, 3H).
76 o B C A A A ES-MS M/Z=359 (M+1)
'H NMR (301 MHz, DMSO-d6) 12.89
oiN 11 HIN-j-\.. N
liCs '1(.3 (s, 1H), 10.74 (s, 1H), 8.64
(m, 2H),
7.77 (m, 2H), 7.43 (m, 1H), 7.07 (m,
2H), 4.23 (d, J = 6.9 Hz, 2H), 1.29 (t, J =
7.1 Hz, 3H).
77_p 0 B C A ES-MS M/Z=360 (M+1)
HN
'H NMR (400 MHz, DMSO-d6) 13.06 (s,
ril ....N .
0¨ 0 N , 1H), 10.84 (s, 1H), 8.66 ¨8.45
(m, 1H),
,¨µ)
N 8.40 (dd, J = 9.5, 0.8 Hz, 1H), 8.04 (d, J
= 1.3 Hz, 1H), 7.88 (d, J = 9.5 Hz, 1H),
7.76 (d, J = 5.9 Hz, 1H), 7.17 (dd, J =
5.9, 0.8 Hz, 1H), 4.23 (q, J = 7.1 Hz,
2H), 1.31 (t, J = 7.1 Hz, 3H).
78 o C C ES-MS M/Z=361 (M+1)
o o HNikr.-\ I-H NMR (301 MHz, DMSO-d6)
12.96
oAN)L6 N Ns-x (s, 1H), 10.78 (s, 1H), 9.34
(s, 1H), 8.79
4:--N (s, 1H), 8.72 ¨8.62 (m, 1H), 8.03 (d, J
= 4.6 Hz, 1H), 7.73 (d, J = 5.7 Hz, 1H),
7.10 (d, J = 5.4 Hz, 1H), 4.22 (q, J = 7.1
Hz, 2H), 1.30 (t, J = 7.1 Hz, 3H).
79 riNJ B C ES-MS M/Z=409 (M+1)
I-H NMR (301 MHz, CDCI3) 12.71 (s,
I-14z---N 1H), 7.82 (s, 1H), 7.03 (d, J
= 5.3 Hz,
1H), 6.90 (d, J = 5.9 Hz, 1H), 4.33 (d, J
s = 6.6 Hz, 2H), 3.77 (s, 2H),
3.02 (s, 2H),
2.84 (s, 2H), 2.69 (d, J = 6.9 Hz, 2H),
1.37 (t, J = 6.9 Hz, 3H), 1.22 (t, J = 7.0
Hz, 3H).
80 B C ES-MS M/Z=360 (M+1)
...,N I-H NMR (301 MHz, DMSO-d6)
12.40
o o HN
(s, 1H), 10.80 (s, 1H), 9.72 (s, 1H), 8.80
0
(s, 1H), 7.70 (s, 1H), 7.35 (d, J = 22.1
/oAN)L6s
H Hz, 2H), 7.19¨ 6.91 (m, 2H), 4.21 (d, J
--
= 6.9 Hz, 2H), 1.34¨ 1.24 (m, 6H).
81 Hirqs A C ES-MS M/Z=360 (M+1)
ON ---
11 'H NMR (301 MHz, CD30D) 9.55
(s,
o 0 HN1
1H), 9.09 (s, 1H), 8.38 (m, 1H), 7.49
C.1 (m, 1H), 6.97(m, 1H), 6.82 (m,
1H),
nmv N. 4.36 (q, J = 7.1 Hz, 2H), 1.39
(t, J = 7.1
-..
Hz, 3H).
-124-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
82 o A A A ES-MS M/Z=377 (M+1)
O 0 HNJY-S 'H NMR (301 MHz, CDCI3) 13.06 (s,
N
A I......\
- o vi)L6s 1H), 9.55 (s, 1H), 8.63 (d, J
= 5.6 Hz,
1H), 7.92 (d, J = 5.5 Hz, 1H), 7.08 (d, J
= 5.8 Hz, 1H), 6.97 (d, J = 5.9 Hz, 1H),
4.33 (q, J = 7.1 Hz, 2H), 1.36 (t, J = 7.1
Hz, 3H).
83 o A A A ES-MS M/Z=377 (M+1)
O 0 HNJYS I-H NMR (301 MHz,
CDCI3) 13.02 (s,
oAN)L6e Nioj
% 1H), 8.70 (d, J = 4.0 Hz, 1H), 8.47 (d, J
, = 8.3 Hz, 1H), 7.96 (s, 1H),
7.53 (dd, J =
8.0, 4.6 Hz, 1H), 7.11 (d, J = 6.0 Hz,
1H), 6.95 (d, J = 5.9 Hz, 1H), 4.31 (dd, J
= 14.1, 7.0 Hz, 2H), 1.34 (t, J = 7.0 Hz,
3H).
84 o B C A ES-MS M/Z=381 (M+1)
o yLE- ...1.....bNH 'H NMR (301 MHz, DMSO-d6)
12.84
oArisii -- s Ni / (s, 1H), 10.83 (s, 1H), 9.26
(s, 1H),
7.74 (d, J=6.0 Hz, 1H), 7.16 (d, J=6.0
Hz, 1H), 4.56 (s, 2H), 4.20 (q, J=7.1 Hz,
2H),3.55 (s, 2H), 3.17 (s, 2H), 1.28 (t,
J=7.2 Hz, 3H)
85 ,cN-' B A A ES-MS M/Z=395 (M+1)
¨\ ,o
s \ I-H NMR (301 MHz, CD30D) 8.21
(s,
HN/(.
r71.)-117N 2H), 7.50 (d, J = 5.9 Hz, 1H), 7.03 (d, J
--- o = 5.9 Hz, 1H), 4.29 (q, J =
7.1 Hz, 2H),
\ S
4.06 (s, 2H), 3.19 (s, 4H), 2.78 (s, 3H),
1.36 (t, J = 7.1 Hz, 3H).
86 o B B A ES-MS M/Z=321 (M+1)
o 0 HN""1 I-H NMR (301 MHz,
DMSO-d6) 12.61
(s, 1H), 10.86 (s, 1H), 9.60 (s, 1H), 9.55
(d, J = 4.7 Hz, 1H), 8.07 (dd, J = 5.4, 2.3
Hz, 1H), 7.71 (d, J = 6.0 Hz, 1H), 7.17
(d, J = 5.8 Hz, 1H), 4.21 (q, J = 7.1 Hz,
2H), 1.28 (t, J = 7.0 Hz, 3H).
87 o A A A ES-MS M/Z=371 (M+1)
O 0 HNJ'Y I-H NMR (301 MHz,
DMSO-d6) 13.36
OAN)L6S !k\ r IN (s, 1H), 10.81 (s, 1H), 9.60
(s, 1H), 8.24
(d, J = 7.3 Hz, 2H), 8.13 -7.97 (m, 2H),
7.76 (d, J = 5.9 Hz, 1H), 7.17 (d, J = 5.8
Hz, 1H), 4.23 (dd, J = 14.1, 7.1 Hz, 2H),
1.31 (t, J = 7.1 Hz, 3H).
-125-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
88 Hlrqs A B A ES-MS M/Z=409 (M+1)
=====
11 0 I-H NMR (301 MHz, DMSO-d6)
12.89
0 0 HN
(s, 1H), 10.82 (s, 1H), 8.18 (d, J = 6.9
Ci / S Hz, 1H), 8.01 (d, J = 9.1 Hz, 1H), 7.74
* (d, J = 6.0 Hz, 1H), 7.65 (t,
J = 2.9 Hz,
2H), 7.16 (d, J = 6.0 Hz, 1H), 4.21 (dd, J
= 14.1, 7.1 Hz, 2H), 1.29 (t, J = 7.1 Hz,
3H).
89 B B A ES-MS M/Z=370 (M+1)
0õN ====
n 0 I-H NMR (301 MHz, DMSO-d6)
12.73
0 0 HN
(s, 1H), 10.84 (s, 1H), 9.05 (d, J = 2.6
III-- Hz, 1H), 8.65 (s, 1H), 8.22
(d, J = 3.5
Hz, 2H), 7.74 (d, J = 6.0 Hz, 1H), 7.67
%
N / (dd, J = 8.3, 4.2 Hz, 1H),
7.11 (d, J = 5.8
Hz, 1H), 4.22 (q, J = 7.1 Hz, 2H), 1.29
(t, J = 7.1 Hz, 3H).
90 0 A A B ES-MS M/Z=375 (M+1)
o 0 HN S I-H NMR (301 MHz, DMSO-
d6) 12.62
OAN)L6 I
H , S ilp (s, 1H), 10.83 (s, 1H), 8.19
(s, 1H), 8.13
(t, J = 7.7 Hz, 2H), 7.73 (d, J = 6.0 Hz,
1H), 7.52 (dd, J = 9.3, 7.3 Hz, 2H), 7.11
(d, J = 6.0 Hz, 1H), 4.22 (q, J = 7.1 Hz,
2H), 1.29 (t, J = 7.1 Hz, 3H).
91 o B A A ES-MS M/Z=337 (M+1)
o 0 HN%1 'H NMR (301 MHz,
DMSO-d6) 13.77
0A) \-- sitIH
- NL6 s (s, 1H), 12.82 (s, 1H), 10.76
(s, 1H),
H 0 7.94 (d, J = 9.9 Hz, 1H), 7.71
(d, J = 5.7
Hz, 1H), 7.09 (d, J=6.0 Hz, 1H), 7.00 (d,
J=9.9 Hz, 1H), 4.20 (q, J = 6.9 Hz, 2H),
1.27 (t, J = 7.1 Hz, 3H).
92
_pNA-0 0 C B B ES-MS M/Z=377 (M+1)
HN
'H NMR (301 MHz, CD30D) 7.44 (d, J =
(3- 0 5.9 Hz, 1H), 7.27 (d, J = 6.4
Hz, 1H),
-/ o o . 6.97 (d, J = 5.9 Hz, 1H), 6.93
- 6.83 (m,
3H), 4.46 (dd, J = 10.8, 4.3 Hz, 2H),
4.28 (q, J = 7.1 Hz, 2H), 1.35 (t, J = 7.1
Hz, 3H).
93 o A A A ES-MS M/Z=360 (M+1)
o 0 HN'icr--\ I-H NMR (301
MHz, DMSO-d6) 12.92
OAN)L ...6s N , N-N (s, 1H), 10.74 (s, 1H), 8.96 (s, 1H), 8.67
H
crz..." (d, J = 2.9 Hz, 1H), 8.39 (d,
J = 9.4 Hz,
1H), 7.72 (d, J = 5.9 Hz, 1H), 7.41 (dd, J
= 9.4, 4.4 Hz, 1H), 7.08 (d, J = 5.8 Hz,
1H), 4.22 (dd, J = 14.1, 7.0 Hz, 2H),
1.30 (t, J = 7.1 Hz, 3H).
-126-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
94 A C A ES-MS M/Z=391 (M+1)
I-H NMR (301 MHz, DMSO-d6) 13.07
sjr..1,1113-mi (s, 1H), 10.87 (s, 1H), 9.45 (s, 1H), 8.17
0 (s, 1H), 7.77(d, J =6.0 Hz,
1H), 7.20 (d,
J = 5.9 Hz, 1H), 4.23 (q, J = 7.1 Hz, 2H),
1.38 (t, J = 7.1 Hz, 3H).
A C ES-MS M/Z=439 (M+1)
o-141 o I-H NMR (301 MHz, CDCI3) 12.75
(s,
/ s
1H), 7.81 (s, 1H), 7.04 (d, J = 5.9 Hz,
143
-NH
1H), 6.91 (d, J = 5.9 Hz, 1H), 4.77 (s,
o
S / 2H), 4.34 (q, J = 6.9 Hz, 2H), 3.83 (t, J =
6.6 Hz, 2H), 3.77 (s, 3H), 2.99 (d, J =
6.9 Hz, 2H), 1.38 (t, J = 6.9 Hz, 3H).
960 / A C ES-MS M/Z=459 (M+1)
;Ns*
0, p I-H NMR (301 MHz, DMSO-d6) 12.85
(s, 1H), 10.80 (s, 1H), 7.74 (d, J = 5.9
--
N -...S Hz, 1H), 7.15 (d, J = 5.9 Hz,
1H), 4.55
(s, 2H), 4.22 (q, J = 6.9 Hz, 2H), 3.58 (s,
4:fr- NH 0 0 1
syA ) 2H), 3.31 (s, 2H), 3.05 (s, 3H), 1.28 (t,
J
N 0 , 6.9 Hz, 3H).
, H
A C A ES-MS M/Z=457 (M+1)
N \Z-11 I t
04 o H 'H NMR (301 MHz, CD30D) 7.52
(s,
-µ eCj
FIN-e,..(i44:-"N 1H), 7.49 (m, 2H), 7.07 (m, 3H), 6.87
- o (m, 1H), 4.47 (s, 2H), 4.29
(q, J = 6.9
\ s
Hz, 2H), 3.69 (s, 2H), 3.11 (s, 2H), 1.37
(t, J = 6.9 Hz, 3H).
98 ( B
0,
,,,...:r,a,N.,..A.
iC),1 YHi
\
N,N
A A B ES-MS Mg=389.0(M+1)
0
0 ,-0 'H NMR: 13.1 (s, 1H), 10.98
(s, 1H),
9.7 (s, 1H), 8.7 (d, J = 5.6 Hz), 8.47 (d,
k----6.8 Hz, 1H), 7.8 (d, J = 6.0 Hz, 1H), 7.2
NH
NH (d, 6.0 Hz, 1H), 6.0 (m, 1H),
5.4 (m,
S
0 1H), 5.30 (m, 1H), 4.7 (m, 2H)
\ / X
N ,N
100 0 A C B ES-MS Mg=386.9(M+1)
0 ,-0 I-H NMR: 13.08 (s, 1H), 11.10
(s, 1H),
k-----NH NH 9.62 (s, 1H), 8.71 (d, J= 5.6
Hz, 1H),
8.39 (dd, J= 0.8 Hz, 1H), 7.80 (d, J = 6
Hz, 1H), 7.25 (d, J = 6 Hz, 1H), 4.88 (d,
J =2.4 Hz, 2H), 3.68 (t, J= 2.4 Hz, 1H)
N
-N
-127-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
101 = A B A ES-MS Mg=439.1(M+1)
0 'H NMR: 13.11 (s, 1H), 11.0
(s, 1H),
0 Y0
NH 9.62 (s, 1H), 8.7 (d, J = 8 Hz, 1H), 8.40
(d, J = 4 Hz, 1H), 7.79 (d, 8 Hz, 1H),
k------NH 7.45 (m, 5H), 7.2 (d, J = 4 Hz, 1H), 5.27
S S...\/ \ (s, 2H)
N--....1,11
102 2 A A A ES-MS Mg=418.9(M+1)
O I-H NMR: 13.3 (s, 1H), 10.19 (s, 1H),
O YO 9.99 (m, 1H), 9.11 (m,
2H), 7.76 (d, J=
NH 4Hz, 1H), 7.27 (d, J = 8 Hz, 1H), 5.43
kNH (m, 1H), 3.89 (m, 4H), 1.5 (m, 2H) s s
0.__OX
N --N
103 \cf A C A ES-MS Mg=406.9(M+1)
O /¨/ I-H NMR (400 MHz, DMSO-
d6) 13.13 (s,
O ,-0 1H), 11.02 (s, 1H),
9.74 (d, J = 0.9 Hz,
NH 1H), 8.74 (d, J = 5.7 Hz, 1H), 8.51 (dd, J
I' = 5.6, 0.9 Hz, 1H), 7.82 (d, J
= 5.9 Hz,
NH 1H), 7.24 (d, J = 5.9 Hz, 1H), 4.37 ¨
4.23 (m, 2H), 3.71¨ 3.50 (m, 2H), 3.30
N--.....111 (s, 3H).
104 Me A A C ES-MS M/Z=409 (M+1)
0 )--Me I-H NMR (400 MHz, CD30D) 9.51 (s,
0 YO 1H), 8.66 (s, 1H), 8.32 (d, J
= 5.7 Hz,
NH
1H), 7.19 (d, J = 3.5 Hz, 1H), 5.10 (m,
I 1H), 1.36 (dd, J = 18.2, 6.3 Hz, 6H).
F Sz!..
Of/ ---\ / \
N --N
105 0 0 C ES-MS M/Z=374.1 (M+1)
I-H NMR (400 MHz, DMSO-d6) 8.216-
NeNAo,
H 8.142 (m, 2H), 7.660-7.590 (m,
3 H),
/N NH 4.091-4.078 (m, 2H), 3.601 (s, 3H),
0 ._N 1.064-1.030 (t, J = 6.8 Hz,
3H).
-
s 0
-128-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
106 0 0 C I-H NMR (400 MHz, DMSO-d6)
10.251
ItifL.,A, (s, 1H), 8.846 (s, 1H), 8.369-8.335 (m,
<0 II m 2 H), 7.756-7.672 (m, 2H), 4.238 (q, J =
S
NH 6.8 Hz, 2H), 1.292 (t, J = 7.2
Hz, 3H).
0'...-N
sO
U
107 0 0 C ES-MS Mg=374.0(M+1)
N-) 'H NMR (400 MHz, DMSO-d6)
8.313-
C)
0 (\ s
) 8.252 (m, 2H), 7.705-7.627 (m,
2H),
3 HINq
7.290 (d, J = 5.8 Hz, 1H), 7.130 (d, J =
5.8 Hz, 1H), 4.456 (t, J = 7.7 Hz, 2H),
4.114 (t, J = 7.8 Hz, 2H).
N 0
108 0 0, 0 C I-H NMR (400 MHz, CD30D) 8.264-
NS' 8.242 (m, 1H), 8.128-8.105 (m,
1H),
,c1()Liti- 'co 7.635-7.615 (m, 2H), 7.412 (d,
J = 5.7
S
NH Hz, 1H), 6.907 (d, J = 5.7 Hz,
1H),
(:) S 4.290 (q, J = 7.1 Hz, 2H), 1.354 (t, J =
...-
NOD r-- 7.1 Hz, 2H).
109 0 0 C ES-MS Mg=375.1(M+1)
S0)1.. ....---...õ I-H NMR (400 MHz, DMSO-d6) 10.472
11 (s, 1H), 8.538 (m, 1H), 8.314-8.263 (m,
NH 2H), 7.858 (d, J = 5.9 Hz,
1H), 7.698
ON
N (m, 2H), 7.207 (d, J = 6.0 Hz,
1H),
.)..-
s0 3.282 (2H buried inside the
H20
U peak), 1.162 (t, J = 7.2 Hz, 3H).
110 NicRI,N C ES-MS Mg=402(M+1)
I-H NMR (500 MHz, DMSO-d6) 8.398-
0 8.352 (m, 2H), 8.1 (br, 1H), 7.788-
HN1.
7.734(m, 2H), 7.350 (br, 1H), 2.755 (s,
3H).
S HN 0
0 0> S
N 0
-129-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
111 0 0 C ES-MS Mg=377.1(M+1)
___________
(Z)L11A 'H NMR (400 MHz, DMSO-d6) 10.724
(s, 1H), 10.663 (s, 1H), 8.623 (d, J = 5.5
S
NH Hz, 1H), 8.392 (d, J = 2.0 Hz,
1H),
O)15,1 7.819 (d, J = 2.2 Hz, 1H), 7.700 (d, J =
5.9 Hz, 1H), 7.091, (d, J = 5.9 Hz, 1H),
4.195 (q, J = 7.1 Hz, 2H), 2.119 (s, 3H),
HNr0 1.267 (t, J = 7.1 Hz, 3H).
112 0 0 C C A ES-MS M/Z=370 (M+1)
A)LVIA 'H NMR (301 MHz, DMSO-d6) 15.79
(s, 1H), 10.64 (s, 1H), 9.15 (d, J = 2.5
S
NH Hz, 1H), 8.70 (dd, J = 28.3,
7.9 Hz, 2H),
O 8.34 (d, J = 8.0 Hz, 1H), 7.95 ¨7.74 (m,
0 2H), 7.63 (d, J = 5.9 Hz, 1H),
7.07 (d, J
NO , 6.0 Hz, 1H), 4.21 (q, J =
7.0 Hz, 2H),
1.30 (t, J = 7.1 Hz, 3H).
113 0 0 C C A ES-MS M/Z=371 (M+1)
eljr-i)L 'H NMR (301 MHz, DMSO-d6) 13.49
(s, 1H), 10.80 (s, 1H), 9.59 (s, 1H), 8.99
S
NH ¨8.83 (m, 2H), 8.44 (d, J =
8.5 Hz, 1H),
O 8.03 (d, J = 6.0 Hz, 1H), 7.75 (d, J = 6.0
NO Hz, 1H), 7.17 (d, J = 5.9 Hz,
1H), 4.23
(d, J = 7.1 Hz, 2H), 1.29 (dd, J = 17.8,
ON 10.7 Hz, 3H).
114 0 0 C C A ES-MS M/Z=400 (M+1)
1()Ill)L 'H NMR (301 MHz, CD30D) 13.49 (s,
1H), 10.77 (s, 1H), 8.25 (d, J = 8.2 Hz,
S
NH 1H), 8.11 (d, J = 8.1 Hz, 1H),
7.91 (d, J
0
1 = 7.0 Hz, 1H), 7.74 (dd, J =
6.8, 3.5 Hz,
0
NO 2H), 7.14 (d, J = 5.9 Hz, 1H),
4.24 (dd, J
= 15.3, 8.2 Hz, 5H), 1.32 (t, J = 7.1 Hz,
0 3H).
115 0 0 C C A ES-MS M/Z=386 (M+1)
irl)L 'H NMR (301 MHz, DMSO-d6) 12.13
(d, J = 11.5 Hz, 1H), 10.80 (s, 1H), 7.82
S
NH (d, J = 7.4 Hz, 1H), 7.68 (d,
J = 5.9 Hz,
O 0 1H), 7.62 ¨7.50 (m, 1H), 7.39
(d, J =
ON 8.0 Hz, 1H), 7.27 ¨7.12 (m,
1H), 7.01
(d, J = 8.6 Hz, 1H), 6.90 (d, J = 1.7 Hz,
HO 1H), 4.18 (q, J = 7.1 Hz, 2H),
1.25 (dd, J
= 11.8, 4.7 Hz, 3H).
-130-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
116 0 0 C C A ES-MS M/Z=370 (M+1)
als-IA 'H NMR (301 MHz, DMSO-d6) 13.48 (s,
1H), 10.74 (s, 1H), 9.52-7.10 (m, 8H),
S
NH 4.23 (dd, J = 14.1, 7.1 Hz,
2H), 1.29 (t,
O N J = 7.1 Hz, 3H).
0
0
117 0 0 C C C ES-MS M/Z=438 (M+1)
cll()11)L 'H NMR (400 MHz, DMSO-d6) 12.32 (s,
1H), 10.86 (s, 1H), 8.36 (m, 3H), 8.01
S
NH (m, 2H), 7.71 (d, J=6.0 Hz,
1H), 7.25 (d,
O 0 J=6.0 Hz, 1H), 4.20 (q, J =
7.1 Hz, 2H),
ON 1.28 (t, J = 7.1 Hz, 3H).
F F
F
118 0 0 C B A I-H NMR (500 MHz, CD30D) 9.025
(d, J
als-IA = 4.8 Hz, 1H), 7.973 (d, J = 4.9 Hz, 1H),
7.617 (d, J = 5.9 Hz, 1H), 7.153 (d, J =
S
NH 6.0 Hz, 1H), 4.391 (q, J = 7.1
Hz, 1H),
O),Th 1.451 (t, J = 7.2 Hz, 1H).
Ho
119 0 0 C C A ES-MS M/Z=373 (M+1)
ar-I)L 'H NMR (301 MHz, CDCI3) 12.43 (s,
1H), 7.96 (s, 1H), 7.76 (m, 2H), 7.07
S
NH (m, 1H), 7.06 (m, 1H), 6.85
(m, 1H),
O 4.33 (q,J = 7.1 Hz, 2H), 2.84 (m, 3H),
0 1.83 (m, 5H), 1.37 (t, J = 7.1
Hz, 3H).
120 0 0 C C A ES-MS M/Z=373 (M+1)
11)L13 'H NMR (301 MHz, CD30D+CDC13)
8.13 (m, 1H), 7.61 (s, 1H), 7.42 (m,
S
NH 2H), 7.13 (d, J = 5.9 Hz, 1H),
6.86 (d, J
O N = 5.2 Hz, 1H), 4.28 (q, J
= 7.1 Hz, 2H),
CYNH 2.49 (s, 3H), 1.35 (t, J = 7.1
Hz, 3H).
0
-131-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
121 0 0 C C A ES-MS M/Z=388 (M+1)
___________
0')L 'H NMR (301 MHz, DMSO-d6)
12.87
(s, 1H), 10.74 (s, 1H), 7.71 (d, J=6.0Hz,
S
NH 1H), 7.13 (m, 3H), 4.21 (q, J
= 7.2 Hz,
ON 2H), 2.80 (s, 3H), 2.49 (s,
3H), 1.29 (t, J
= 7.2 Hz, 3H).
..J
N
122 0 0 C C A ES-MS M/Z=384 (M+1)
A)1-1A 'H NMR (301 MHz, CDCI3) 12.25
(s,
1H), 8.36 (m, 1H), 8.07 (m, 2H), 7.74
S
NH (m, 1H), 7.58 (m, 2H), 7.11
(m, 1H),
0 0 6.93 (m, 1H), 4.29 (q, J = 7.1
Hz, 2H),
ON 2.81 (s, 3H), 1.31 (t, J = 7.1
Hz, 3H).
123 o o C B A ES-MS M/Z=400 (M+1)
I-H NMR (301 MHz, DMSO-d6) 12.38
0 Ho (s, 1H), 10.82 (s, 1H), 8.95
(d, J =4.5
s
N Hz, 1H), 8.09-7.71 (m, 5H),
7.17 (d, J =
H 5.7 Hz, 1H), 4.18 (dd, J =
14.1, 7.1 Hz,
\ 0 2H), 3.90 (s, 3H), 1.26 (t, J
= 7.1 Hz,
U N 3H).
124 0 0 C A A ES-MS M/Z=384 (M+1)
Clerli)L I-H NMR (301 MHz, DMSO-d6)
12.81 (s,
1H), 10.75 (s, 1H), 8.92 (d, J=7.0 Hz,
S
NH 1H), 8.83 (s, 1H), 8.17 (t,
J=9.0 Hz, 1H),
7.71 (d, J=6.0 Hz, 1H), 7.23 (t, J=7.0
ol\i'l Hz, 1H), 7.10 (d, J=5.7 Hz,
1H), 4.22 (q,
Np J = 7.1 Hz, 2H), 1.30 (t, J =
7.1 Hz, 3H).
N
125 0 0 C B A ES-MS M/Z=361 (M+1)
all-IA I-H NMR (301 MHz, DMSO-d6)
13.11 (s,
1H), 10.80 (s, 1H), 9.58 (m, 1H), 9.06
S
NH (m, 1H), 7.76 (m, 1H), 7.55
(m, 1H),
7.16 (m, 1H), 4.22 (q, J = 7.1 Hz, 2H),
ON
D-- 1.30 (t, J = 7.1 Hz, 3H).
N
'NON
-132-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
126 0 0 C C C ES-MS M/Z=433 (M+1)
Lls-1)L I-H NMR (301 MHz, DMSO-d6)
13.37 (s,
1
1H), 10.84 (s, 1H), 9.46 (s, 1H), 7.77
S
mu
ru--, ci (d, J=6.0Hz, 1H), 7.21 (d,
J=5.7 Hz, 1H),
O 4.21 (q,J = 7.2 Hz, 2H), 3.65 (s, 3H),
NON 1.29 (t, J = 7.2 Hz, 3H).
sz-_-0
I
127 0 0 C C A ES-MS M/Z=351 (M+1)
Lrs-1)L I-H NMR (301 MHz, CDCI3) 13.08
(s,
i
1H), 8.98 (s, 1H), 8.25 (d, J=6.0 Hz,
S
NH 1H), 7.91 (d, J=6.0Hz, 1H),
7.07 (d,
O-N J=6.0 Hz, 1H), 6.90 (d, J=6.0 Hz, 1H),
r-N 4.33 (q,J = 7.1 Hz, 2H), 4.07
(s, 3H),
N_N.A
1.37 (t, J = 7.1 Hz, 3H).
0
I
128 N C C A ES-MS M/Z=384 (M+1)
II I-H NMR (301 MHz, DMSO-d6)
12.92
(s, 1H), 10.75 (s, 1H), 8.36 (m, 2H),
0 7.70 (m, 2H), 7.07 (m, 1H),
4.22 (q, J =
(J,N 7.1 Hz, 2H), 1.29 (t, J = 7.1
Hz, 3H).
O N
H
NH
Sc_o
y
0 0
129 0 0 C C A ES-MS M/Z=337 (M+1)
cleill)L 'H NMR (301 MHz, DMSO-d6) 5
13.03
(s, 1H), 10.80 (s, 1H), 8.00-7.14 (m,
S
NH 5H), 4.22 (q, J = 6.9 Hz, 2H),
1.29 (t, J =
O), 7.1 Hz, 3H).
cal
N
OH
130 0 0 C C A ES-MS M/Z=400 (M+1)
11)Lc' 'H NMR (301 MHz, DMSO-d6)
12.65
(s, 1H), 10.74 (s, 1H), 8.01 (m, 2H),
S
NH 7.70 (d, J=6.0 Hz, 1H), 7.61
(m, 3H),
o_7.07 (d, J=6.0 Hz, 1H), 4.22 (q, J = 7.0
plo
N Hz, 2H), 2.75 (s, 3H), 1.31
(t, J = 7.1
Hz, 3H).
0
-133-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
131 0 0 C B C ES-MS M/Z=394 (M+1)
I-H NMR (301 MHz, DMSO-d6) 12.87 (s,
1H), 10.83 (s, 1H), 8.95 (s, 1H), 7.75
S
NH (d, J=6.0 Hz, 1H), 7.19 (d,
J=6.0 Hz,
ONra 1H), 4.21 (q, J = 7.1 Hz, 2H), 1.29 (t, J
s
= 7.1 Hz, 3H).
N(
A-F
F F
132 0 0 C B A ES-MS M/Z=361 (M+1)
01)L 'H NMR (301 MHz, DMSO-d6) 7.83 (m,
2H), 7.47 (m, 1H), 6.90 (m, 2H), 4.87
S
NH (m, 2H), 4.67 (m, 2H), 4.29
(q, J = 7.1
O Hz, 2H), 1.36 (t, J = 7.1 Hz,
3H).
0
0
133 0 0 C C A ES-MS M/Z=403 (M+1)
leL rI-41A I-H NMR (301 MHz, DMSO-d6) 8.70
(m, 2H), 8.14 (m, 2H), 7.72 (d, J=5.7
S
NH Hz, 1H), 7.60 (m, 1H), 7.10
(d, J=5.7Hz,
O)_,(( 1H), 4.23 (q, J = 7.1 Hz, 2H), 1.31 (t, J
s
= 7.1 Hz, 3H).
N
NO
134 0 0 C C A ES-MS M/Z=393 (M+1)
01)L 'H NMR (301 MHz, CD30D) 8.21 (m,
1H), 7.58 (m, 1H), 7.41 (d, J=6.0 Hz,
S
NH 1H), 7.23 (m, 1H), 6.85 (d,
J=6.0 Hz,
O N 1H), 4.29 (q, J = 7.1 Hz,
2H), 1.35 (t, J
CYNH = 7.1 Hz, 3H).
0
CI
135 0 0 C C A ES-MS M/Z=417 (M+1)
I-H NMR (301 MHz, DMSO-d6) 12.32 (s,
1H), 10.82 (s, 1H), 8.75 (m, 2H), 7.96
S
NH (m, 2H), 7.72 (m, 1H), 7.11
(m, 1H),
1:/_..r- 4.21 (q,J = 7.1 Hz, 2H), 2.86
(s, 3H),
S.}1,.14._ 1.28 (t, J = 7.1 Hz, 3H).
CO
N
-134-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
136 0 0 C C A ES-MS M/Z=417 (M+1)
___________
cZ)11)L 'H NMR (301 MHz, DMSO-d6) 12.32 (s,
1H), 10.83 (s, 1H), 9.21 (m, 1H), 8.75
S
NH (m, 1H), 8.41 (m, 1H), 7.73
(m, 1H),
O)_c¨) N 7.59 (m, 1H), 7.12 (m, 1H), 4.22 (q, J =
7.1 Hz, 2H), 2.86 (s, 3H), 1.28 (t, J =
S 6
7.1 Hz, 3H).
137 0 0 C C A ES-MS M/Z=408 (M+1)
als-1)L 'H NMR (301 MHz, CDCI3) 12.21 (s,
1H), 7.89 (s, 1H), 6.94 (m, 2H), 4.31 (q,
S F F
IH 7.1 Hz, 2H),
2.80 (s, 3H), 1.35 (t, J =
7 .1
\
138 0 0 C C A ES-MS M/Z=400 (M+1)
1()11)L 'H NMR (301 MHz, DMSO-d6) 12.75
(s, 1H), 10.80 (s, 1H), 8.05 (d, J = 6.0
S
NH Hz, 2H), 7.74-7.63 (m, 4H),
7.12 (m,
CI").7c¨DIN 1H), 4.21 (m, 2H), 2.51 (s, 3H), 1.29
(m, 3H).
N,N=
0
139 0 0 C C A ES-MS M/Z=370 (M+1)
,c11()11)Lc) 'H NMR (301 MHz, CDCI3) 12.42 (s,
1H), 9.22-7.83 (m, 9H), 4.30 (dd, J =
S
NH 14.1, 7.1 Hz, 2H), 1.26 (t, J
= 7.1 Hz,
O 3H).
0
ON
140 0 0 C B A ES-MS M/Z=350 (M+1)
,c11()Isi)L 'H NMR (301 MHz, DMSO-d6) 14.13
(s, 1H), 8.33 (m, 1H), 8.16 (m, 1H),
S
NH 7.08 (m, 1H), 6.46 (d, J = 5.7
Hz, 1H),
O 6.32 (d, J = 5.7 Hz, 1H), 4.17 (dd, J =
0 14.1, 7.1 Hz, 2H), 3.51 (s, 3H), 1.29 (t,
N 0 J = 7.1 Hz, 3H).
I
-135-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
141 0 C C A ES-MS M/Z=387 (M+1)
)L NH 'H NMR (301 MHz, CD30D)
7.47(d,
NO J=6.0 Hz, 1H), 7.35-7.19 (m,
2H), 7.04-
0
0 6.93 (m, 3H), 4.27 (q, J = 7.2 Hz, 2H),
NH 1.34 (t, J = 7.2 Hz, 3H).
cSc ri 0
y
0 0
142 0 0 C C B ES-MS M/Z=359 (M+1)
Cl'()L1-41)L I-H NMR (301 MHz, DMSO-d6) 12.80 (s,
1H), 10.74 (s, 1H), 8.64 (m, 2H), 7.81-
S
NH 7.70 (m, 2H), 7.43 (m, 1H), 7.06 (m,
2H), 4.23 (d, J = 6.9 Hz, 2H), 1.29 (m,
=13¨(3\N
a 3H).
143 0 0 C B A ES-MS M/Z=374 (M+1)
0')L 'H NMR (301 MHz, DMSO-d6) 12.29 (s,
1H), 10.63 (s, 1H), 7.61 (d, J = 5.7 Hz,
S
NH 1H), 7.01-6.52 (m, 6H), 4.15 (dd, J =
0 14.1, 7.1 Hz, 2H), 2.27 (m,
1H), 2.24-
1.35 (m, 4H), 1.29 (t, J = 7.1 Hz, 3H).
HN
0
144 0 0 C C A ES-MS M/Z=394 (M+1)
'CZ)L 'H NMR (301 MHz, CDCI3) 12.74(s,
1H), 8.49 (s, 1H), 7.97 (m, 1H), 7.09
S
NH (m, 1H), 6.92 (d, J=5.7Hz, 1H), 4.32 (q,
C:1")S J = 7.1 Hz, 2H), 1.29 (t, J =
7.1 Hz, 3H).
N3._
, F
F F
145 0 0 C C A ES-MS M/Z=370 (M+1)
0)L 'H NMR (301 MHz, DMSO-d6) 12.73 (s,
1H), 10.85 (s, 1H), 9.47(s, 1H), 8.66-
S
NH 8.08 (m, 5H), 7.74 (m, 1H), 7.14 (m,
0 1H), 4.22 (q, J = 7.1 Hz, 2H),
1.29 (t, J =
0 7.1 Hz, 3H).
ON
-136-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
146 0 0 C C A ES-MS M/Z=390 (M+1)
___________
hhslIA I-H NMR (301 MHz, DMSO-d6) 11.47 (s,
1H), 10.68 (s, 1H), 9.54 (s, 1H), 8.64
S
NH (d, J = 4.8 Hz, 1H), 8.36 (d,
J = 4.5 Hz,
O 1H), 8.01 (t, J = 4.5 Hz, 1H), 6.77 (d, J =
O 5.7 Hz, 1H), 4.17 (dd, J = 14.1, 7.0 Hz,
2H), 2.24 (s, 3H), 1.26 (t, J = 7.1 Hz,
OS
N--../ 3H).
147 0 0 C C A ES-MS M/Z=337 (M+1)
(Z)l-sill 'H NMR (301 MHz, DMSO-d6) 13.12 (s,
1H), 10.77 (s, 1H), 8.50 (m, 1H), 7.74
S
NH (m, 1H), 7.16 (m, 1H), 6.96
(m, 1H),
O)4 4.20 (dd, J = 14.1, 7.1 Hz, 2H), 1.28 (t,
J = 7.1 Hz, 3H).
HO
148 0 0 C
al-s11A
S
NH
0
O,/a
,S
0-- W09/ X
149 0 0 C
()L1-'11AC)
S
NH
Opir,i
(3
1
150 0 0 C
Clel-s11)L
S
NH
0
O1,0
,
HN 01 '
-137-
CA 02911326 2015-10-30
WO 2014/190199
PCT/US2014/039227
151 0
0--
NH
0
0 OH
S ON
152 0 <JLNII
0--
NH
0
N -NM
153 o
NH
ON9:),N
HN /0
,µSi
Cs/ 0
154 )0.L
vi 0
HN
0
NH
0
0
¨138¨
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
155 0 __ 0
0 C
)L
S
NH
0
.s1
0
156
0 C
0
,S,/
0' 15
HN S
&C)
N
0 NH
157 0 0 C
,cleisiA(3
S
NH
0
0
N F
F
158 0 0 C
leL1-'11)L13
S
NH
0
Nra\ril
Sõ
159 0 0 C
ell-1)L
S
NH
0
ON
0 NH
-139-
CA 02911326 2015-10-30
WO 2014/190199
PCT/US2014/039227
160 C
..-----A
N
QN<
HN---.0
S HNO---
0 0> S'
N 0
161 0 0 C
S
NH
0,--S
Nc?
162 0 0 C
0A
S
NH
0
0
0 NH
SH
163 0 0 C
1-slIA
S
NH
0
..NP:Drki
H2N
164 0 0 C
01)L
S
NH
0.Vsry
-140-
CA 02911326 2015-10-30
WO 2014/190199
PCT/US2014/039227
165 0 0 C
'Cl1()I-'11A
S
NH
I:))/)
NO
166 0 0 C
elt11)L0
S
NH
0
NO
167 0 0 C
0)L
S
NH
OD),
168 0 0 C
S
NH
0
.V)N
,NN
169 0/Th 0 C
.---S H N 0
C
U 0> µ S I
\ /--- N 0
170 0 0 C
S
NH
0
It)DN
-141-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
171 0 0 _______________ C
0A
S
NH
0
0
172 0 0 C
()L1-41A
S
NH
0
0
F
173 0 0 C
()Lr-IA
s
NH F
0
F
OX
174 0 0 C
0A
S
NH
0
0
0
i
175 0 0 C
C11()11)L0
S
NH F
0
0
F
-142-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
176 0 0 _______________ C
(11()L11)L
s
NH F
0
0
F
F
177 0 0 C
s
NH
ID-z)
178 0 0 C
al-sil)LC)
S
NH
0
ON
F F
F
179
H2N.? C
S HN ce--_---1)
oo>
N -
0
180 0 C
CIN-J15
HN S
& 3
N
0 NH
-143-
CA 02911326 2015-10-30
WO 2014/190199
PCT/US2014/039227
181 0
HN S
&C)
N
ONH
182 CI
irff
HN S
&C)
0NH
183 0 S
0 N=-= 0
HN
184 0 S-\
YI))*Lrm
H -
0 N==-. 0-":"
185
HN/L0
NH
oScOH
Ny
0 0
186 0
C ( 0
HN-1K
0
-144-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
187 0 0 C
A
0 11:11
ocrik1H NH
N 0
188
C
HN/L0
0$D11)41
NH
S
0 H
NO
0 0
189 0 0 C
eisl'A '
S
0ivrkIH Nr
H
0
190 o N-N F C
II N S F
H
0 N-
191
0 C
HN---.0
S HNl)
0 0> S
N 0
192 I C
Isl_Ni
01-RN
S HN
0 0> µ ti-N
N 0
-145-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
193 0 __ 0
A)LitiA
NH
0
N9-31N
HN 0
FX-F
194
,0
,s,
0,
HN S
&$3
N
0NH
195 0 0
NH
0
OiTh
196 0 0
0A
NH
0
00
CI
197 0 0
01A
NH
oo
-146-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
198
g c __________________________________________
0 N3 H EiN 0
0AN 'rN 0
H
0 S
199 0 0 C
C11()I-41A
S
NH
0
0
0 NH
N-_c
200 0 0 C
IcZ).L N AV
H
S NH
0
0
0
0
201 0 0 C
eljti)L
S
NH
0 N
CYN--
0
202 0 0 c
031A
S
N N
HØ__
No N
FO
F
F
-147-
CA 02911326 2015-10-30
WO 2014/190199
PCT/US2014/039227
203 0 0
NH
0
0
0
0
204 0 0
NH
0
0
NRN
A-F
F F
2050 S
0 Ng.
0 ;
206 o o
CeNAO 0
S NH H
0Nr-N
so.
207 0 0
S NH
0
0
NNN
-148-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
208 0 0 C C A ES-MS Mg= 372.9(M+1)
___________
eil)L I-H NMR (400 MHz, CD30D) 1.36 (s, 3
H) 2.51 (s, 3 H) 4.22 - 4.37 (m, 2 H)
S
NH 7.03 - 7.14 (m, 1 H) 7.50 -
7.60 (m, 1
ONH) 7.81 - 7.89 (m, 1 H)
7.91 - 8.02 (m, 1 H) 8.62 - 8.71 (m, 1
N4 H) 8.83 - 8.91 (m, 1 H)
209 0 0 A ES-MS Mg= 361.9 (M+1)
I-H NMR (400 MHz, CD30D) 1.33-1.37
(t,3H,J=6.8Hz), 4.24-4.29 (m,2H),4.96
S
N\
H r-r- (m,2H),6.37-6.38 (m,1H),7.24-
7.25
ril-i(Th (m,1H),7.41-7.45 (m,1H),7.51-
..) 7.55(m,1H),7.96-7.98 (m,2H)
210 H2N _Nii A ES-MS Mg= 343.9 (M+1)
1-CYN I-H NMR (400 MHz, DMSO-d6)
7.20-
S
HNtOo 7.22 (m,1H),7.34-7.37
(m,3H),7.63-
7.70 (t,2H),8.25-8.31 (t,2H),12.26-
0 0> µ -<S: 12.29 (m,1H).
N 0
211 No:k Asi A ES-MS Mg= 371.9 (M+1)
TCYN 'H NMR (400 MHz, CDCI3) 1.60-
1.64
HN. (t,3H,J=6.8Hz),4.87-4.93 (m,2H),7.00-
õ(
7.01 (m,1H),7.40-7.41 (m,2H),7.54-
S HN45
0 0> µ S 7.60 (m,2H),7.99-8.04(m,2H)
N 0
212 0 0 ES-MS Mg= 459 (M+1)
Clerl-s11A I-H NMR (400 MHz, DMSO-d6) 1.29 (t,
J=7.03 Hz, 3 H) 3.00 (s, 3 H) 3.08 (br.
S
NH s., 2 H) 3.62 - 3.65 (m, 2 H)
4.18 -4.25
O.-N (m, 2 H) 4.64 - 4.70 (m, 2 H) 7.12 -
7.18 (m, 1 H) 7.72 - 7.77 (m, 1 H)
Sgo
10.81 (s, 1 H) 12.80 - 12.87 (m, 1 H)
N
'so
0--c
213 0 0 A ES-MS Mg= 521.1(M+1)
WLI1A 'H NMR (400 MHz, CD30D) 1.33 (br.
s., 3 H) 4.25 - 4.32 (m, 2 H) 4.38 - 4.45
S
NH (m, 2 H) 4.51 -4.58 (m, 2 H)
4.81 -
0 )...-N 4.85 (m, 2 H) 4.93 - 4.96(m, 2 H) 7.55
S 0 (d, J=2.21 Hz, 7 H) 8.13 (d, J=7.28 Hz,
2H)
-149-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
214 0 0 A ES-MS Mg= 369.9(M+1)
0 NAO I-H NMR (400 MHz, CD30D)
H 1.38-1.34(t,3H,7.2Hz), 4.34-
NH 4.29(q,2H,J=7.2Hz), 7.35-
Oc!, 7.3(t,1H,J=6.8Hz),7.61-
N 0 7.60(t,1H,J=7.2Hz),7.68-7.66(m,2H),
7.86-7.83(d,1H,J=9.6Hz), 8.15 - 8.13
(d, 1H,J=7.6Hz), 8.27-8.25(d,
1H,J=8.0Hz)8.65-8.63 (d, 1H,J=8.4Hz)
4 A ES-MS Mg= 280.1(M+1)
215
I-H NMR (400 MHz,DMSO-d6) 7.453-
NH 7.759 (m, 5H), 7.881-7.900 (m, 1H),
0 8.205-8.281 (m, 2H), 11.331
(s, 1H)
S HN 0
00>
N 0
216 0 0 A ES-MS Mg= 325.9 de-carbamate
A
N 0 - 'H NMR (400 MHz, CDCI3) 12.82-13.04
N_____<(
H (m, 1H), 8.16-8.21 (m, 1H), 7.93-7.99
S
NH (m, 1H), 7.47-7.59 (m, 2H),
4.42-4.49
0)..-N (m, 2H), 2.57 (s, 3H),
S 0 1.47 (d, J=7.09 Hz, 3H)
217 0 0 A C A ES-MS Mg= 380(M+1)
I-H NMR (400 MHz, CDCI3) 1.35 (t,
C11()L11:11)LC) J=7.09 Hz, 3 H) 1.88 (br. s., 4 H) 2.87
S
NH (br. s., 4 H) 4.24 - 4.40 (m,
1 H) 6.82 -
0 ....-N 6.92 (m, 1 H) 6.96 -7.09 (m, 1 H) 7.74 -
Sgo 7.90 (m, 1 H) 12.60 - 12.75
(m, 1 H).
218 0 0 ES-MS Mg=360.0 (M+1)
cl()LitiA
s
NH
ON
HN 4-3
N-"j
219 0 0 ES-MS Mg=452.2 (M+1)
0 0 IIA '''
s
NH
1:1,,...N
St>
-150-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
220 0 0 ES-MS M/Z=391.1 (M+1) ________
01A
S
NH
0.--S
Ng6
N
221 0 0 ES-MS M/Z=391.1 (M+1)
CZ)L1-41)L
S
NH
Ncti,
222 N ES-MS M/Z=416.1 (M+1)
N 0
coPs N
NH
Ny
0 0
223 0 0 ES-MS M/Z=409.1 (M+1)
al-s11)L
S
NH
Ngo
N
\
224 0 0 A ES-MS M/Z=391 (M+1)
,c11()11)Ltz) 'H NMR (400 MHz,DMSO-d6) 1.28-
1.31(t,3H,J=7.2Hz), 2.68(s,3H),4.20-
S
NH 4.25(m,2H), 7.19-7.21
(d,1H,J=6.0Hz),
0...-N 7.62-7.64(d,1H,J=8.4Hz),7.76-7.78
SPq/ (d,1H,J=5.6Hz),8.61-8.63(d, 1H,
J=8.8Hz),8.60(s,1H),10.86
N (s,1H),13.05(s,1H)
-151-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
225 ----N7.Th 0 B ES-MS M/Z= 387.0(M+1)
L./N-.... I-H NMR (400 MHz,DMSO-d6) 2.70
-
2.84 (m, 3 H) 3.09 - 3.21 (m, 2 H) 3.43
S HN (C.--) -3.49 (m, 2 H)
3.52 - 3.57 (m, 2 H)
0 0> µ S'
4.23 - 4.48 (m, 2 H)7.17 - 7.22 (m,1 H)
N 0
7.26 - 7.31 (m, 1 H) 7.59 - 7.75 (m, 2
H) 8.21 - 8.37 (m, 2 H) 12.00 - 12.08
(m, 1 H)
226 H A ES-MS M/Z= 372.9(M+1)
ON
isl--/ 'H NMR (400 MHz,DMSO-d6) 8.17-
8.33 (m, 2H), 7.59-7.68 (m, 3H), 7.28-
0,_,
013\ HN _5 s
2 7.45 (m, 3H), 6.49-6.61 (m,
1H), 3.58-
3.75 (m, 2H), 3.15-3.25 (m, 2H)
N 0
227 0 A ES-MS M/Z= 473.1(M+1)
)LN/o 'H NMR (400 MHz,DMSO-d6) 1.34 -
7 0 v......../N-.?
1.44 (m, 9 H) 3.40 - 3.45 (m, 4 H) 3.57
S HN 0 - 3.63 (m, 4 H) 7.11 - 7.15
(m, 1 H)
...----
0 0> µ S 7.20 - 7.25 (m, 1 H) 7.60 - 7.68 (m,2 H)
N 0 8.20 - 8.28 (m, 2 H)
228 (-NH C ES-MS M/Z= 372.9(M+1)
NJ 1H NMR (400 MHz,DMSO-d6) 3.19 -
$::
0 r_iN g
U1 3 3.25 (m, 4 H) 3.83 - 3.89 (m,
4 H) 7.19
- 7.22 (m, 1 H) 7.26 - 7.29 (m, 1 H)
S \ HN 7.62 - 7.72 (m, 2 H) 8.24 -
8.33 (m,2 H)
9.51 - 9.61 (m, 2 H) 11.97 - 12.01 (m,
N 0
1H)
229 0 A ES-MS M/Z= 473(M+1)
ii
---S-Nr--A 0 1H NMR (400 MHz,DMSO-d6) 2.87 (s, 3
d v.,..,./N -.....
H) 3.18 - 3.25 (m, 4 H) 3.68 - 3.76 (m,
4 H) 7.11 - 7.16 (m, 1 H) 7.23 - 7.28
S HN
0 0> µ S (m, 1 H) 7.59 - 7.69 (m, 2 H)8.21 -
8.30
N 0 (m, 2 H) 12.01 - 12.05 (m, 1 H)
230 0 0 A ES-MS M/Z= 480(M+23)
I-H NMR (400 MHz, CDCI3) 1.38-
(1()11:11)LC) 1.35(t,3H,J=7.2Hz),3.25-
S
NH 3.22(m,2H),2.87(s,3H),3.65-
0
3.62(m,2H),4.36-
Os
4.30(q,2H,J=7.2Hz),4.56(s,2H),6.87-
6.85(d,1H,J=5.6Hz),7.04-
7.03(d,1H,J=6.0Hz),7.87(s,1H),7.26(s,1
N
H),12.31(s,1H),8.0(s,1H),12.31 (5,1H)
0-4:---
I
-152-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
231 0 0 C ES-MS M/Z= 378.9(M+1)
CD1r1-41)L I-H NMR (400 MHz, CD30D) 1.37-
1.33(t,3H,J=6.8Hz),1.86-
S
NH
1.85(m,4H),2.93(m,2H),2.81(m,2H),4.
28-4.27(q,2H,J=6.8Hz), 6.95-
o 6)1s 6.93(d,1H,6.0Hz),7.48-
7.46(d,1H,J=6.0Hz),7.96(s,1H).
232 0 0 A ES-MS M/Z=458.1(M+1)
CZ)L11-'11)LCI 'H NMR (400 MHz,DMSO-d6) 1.27-
1.24(t,3H,J=7.6Hz),
S
NH 3.0(m,2H),2.94(s,3H),4.20-
0 S
4.17(q,2H,J=7.6Hz),4.37(s,2H),7.05-
0 7.03(d,1H,J=6.0Hz), 7.63(s,1H),7.69-
7.67 (d,1H,J=6.0Hz), 10.77(s,1H),
N 12.38(s,1H).
0-:S\--
233 0 0 A ES-MS M/Z=459 (M+1)
'Cl1()11-41)L I-H NMR (400 MHz,DMSO-d6) 1.23 -
1.29 (m, 3 H) 1.99 - 2.07 (m, 2 H) 2.87
S
NH - 2.92 (m, 2 H) 3.21 (s, 3 H)
3.75 - 3.80
0.-N (m, 2 H) 4.15 -4.22 (m, 2 H)
7.08 - 7.11 (m, 1 H) 7.68 - 7.71 (m, 1
Sgo
H) 10.74 - 10.77 (m, 1 H) 12.70 - 12.73
13, N (m, 1 H)
N e,
/'o
234 A ES-MS M/Z= 375.9(M+1)
so0 I-H NMR (400 MHz,DMSO-d6) 3.67 (s, 3
H) 4.06 -4.11 (m, 2 H) 7.19 - 7.23 (m,
0)---N
1 H) 7.53 - 7.56 (m, 1 H) 7.59 - 7.69
NH (m, 2 H) 8.23 - 8.30 (m, 2
H)8.98 - 9.05
,c_o. rri 0
(m, 1 H) 13.36 - 13.40 (m, 1 H)
0
0
235 A ES-MS M/Z= 397(M+23)
so0 I-H NMR (400 MHz,DMSO-d6) 2.61 -
2.65 (m, 3 H) 3.87 - 3.92 (m, 2 H) 7.19
0)----N
- 7.25 (m, 1 H) 7.57 - 7.61 (m, 1 H)
NH 7.62 - 7.71 (m, 2 H) 7.92 -
7.98 (m,1 H)
Scc,5) 0
8.26 - 8.33 (m, 2 H) 8.84 - 8.91 (m, 1
N
H H) 13.51 - 13.55 (m, 1 H)
0
-153-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
236 A ES-MS M/Z= 404.0(M+1)
so0 I-H NMR (400 MHz,DMSO-d6) 1.20 (s, 3
H) 2.60 - 2.66 (m, 2 H) 3.50 - 3.61 (m,
0)--"N
2 H) 4.04 - 4.14 (m, 2 H) 7.16 - 7.22
NH
grrS iNisci (m, 1 H) 7.48 - 7.55 (m, 1
H)7.61 - 7.73
(m, 2 H) 8.24 - 8.35 (m, 2 H) 8.61 -
0
8.68 (m, 1 H) 13.53 - 13.64 (m, 1 H)
0
237 0 0 B ES-MS M/Z= 403.9(M+23)
I-H NMR (400 MHz,DMSO-d6) 1.75 -
1.85 (m, 2 H) 2.35 - 2.41 (m, 2 H) 3.33
NH - 3.40 (m, 2 H) 3.57 (s, 3 H)
7.14 -7.19
C:0 S (m, 1 H) 7.50 - 7.55 (m, 1
H)7.58 - 7.69
-Nr-
N 0(m, 2 H) 8.26 (s, 2 H) 8.51 - 8.58 (m, 1
H) 13.56 - 13.62 (m, 1 H)
238 0 0 B ES-MS M/Z= 406(M+17)
0')L 'H NMR (400 MHz,DMSO-d6) 1.3-
1.27(t,3H,J=6.8Hz),4.23-
NH
4.18(q,2H,J=6.8Hz),5.16(s,1H),7.03-
C) 6.98(m,3H),7.22-
7.20(m,1H),7.36-
7.34(d,2H,J=7.2Hz),7.62-
SoN 7.61(d,1H,J=6.0Hz),11.0(s,1H),10.74(s,
1H),12.12(s,1H).
0
239 0 0 A ES-MS M/Z= 379(M+1)
C11()11-'11)LC) I-H NMR (400 MHz, CDCI3) 12.275 (s,
1H), 7.856 (s, 1H), 7.511 (s, 1H),
S
NH 7.009-6.994(d, 1H), 6.827-
6.812 (d,
Oszi-t), 1H), 4.348-4.295 (m, 2H),
2.822-2.794
(m, 2H), 2.655-2.627 (m, 2H), 1.863-
1.806 (m, 4H), 1.373-1.337 (m, 3H)
240 0 0 A ES-MS M/Z= 402M+1)
1-41)L I-H NMR (400 MHz,DMSO-d6) 1.29-
1.26(t,3H,J=7.2Hz),4.21-
S
NH 4.19(q,2H,J=7.2Hz),7.15-
0.....-S
7.13(d,1H,J=6.0Hz),7.42(m,1H),7.53-
NO 7.50(m,2H),7.73-
7.72(d,2H,J=6.4Hz),8.06-
8.05(d,1H,J=7.2Hz),8.60(s,1H),10.80(s,
0 1H),13.01(s,1H).
-154-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
241 0 0 A ES-MS Mg= 416(M+1)
'H NMR (400 MHz,DMSO-d6) 1.31-
C'eLiti)L 1.27(t,3H,J=7.2Hz),
2.75(s,3H),4.25-
S
NH 4.20(q,2H, J=7.2Hz),7.10-
7.08(d,1H,
0 J=6.0Hz), 7.74-7.73(d,1H,
0 J=6.0Hz),8.01-8.00(d,2H,
J=8.4Hz),
8.19-8.16(m,3H), 10.81 (s,1H),
NOS 12.64(s,1H).
\
242 0 0 C A ES-MS Mg=454 (M+23)
0
NAO
0 H
S
NH
S 0
243 0 0 ES-MS Mg=381.0 (M+1)
1-41)L
S
NH
0
S 0S
244 0 0 C C ES-MS Mg=383.0 (M+1)
Clel-'11)L
S
NH
Oi!,
0
0
245C C
0 H
S
NH
0),-N
S 0
-155-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
246 0 0 ES-MS M/Z=462 (M+1) __________
(Z)(11A''' 'H NMR (400 MHz,DMSO-d6) 1.21 -
1.37 (m, 3 H) 3.86 - 3.97 (m, 4 H) 4.02
S
NH -4.11 (m, 4 H) 4.16 -4.26 (m,
2 H)
0)_--N 6.90 - 7.06 (m, 1 H) 7.10 -
7.20 (m, 1
H) 7.69 - 7.87 (m, 1 H) 8.25 - 8.40 (m,
ScdN
1 H) 10.70 - 10.86 (m, 1 H) 13.24 -
13.41 (m, 1 H)
247 0 0 A B B ES-MS Mg= 462(M+1)
0A 'H NMR (400 MHz,DMSO-d6) 1.21 -
1.37 (m, 3 H) 3.86 - 3.97 (m, 4 H) 4.02
S
j)k ..1F ___=1N N (---, -4.11 (m, 4 H) 4.16 -4.26 (m,
2 H)
6.90 - 7.06 (m, 1 H) 7.10 - 7.20 (m,1 H)
0
7.69 - 7.87 (m, 1 H) 8.25 - 8.40 (m, 1
SCOI H) 10.70 - 10.86 (m, 1 H) 13.24 - 13.41
N (m, 1 H).
248 0 0 A C A ES-MS Mg= 462(M+1)
I-H NMR (400 MHz,DMSO-d6) 1.252-
1.287 (m, 3H), 3.748 (s, 0.674H),
S
l'ilHr.....) 3.851 (m, 4H), 4.061 (s,
0.720H),
0
4.171-4.224 (m, 6H), 7.116-7.131(d, J
- )---N N = 6.0 Hz 1H 7.297-7.309 d, J =
4.8
, ), (
SgtD(N Hz,1H), 7.740-7.753 (d, J =
5.2 Hz, 1H),
8.094-8.107 (d, J = 5.2 Hz,1H), 10.757
(s, 1H) ,13.348 (5, 1H).
249 0 0 C C ES-MS M/Z=321 (M+1)
CZ)Lr1-41)() 'H NMR (400 MHz, DMSO-d6) 13.55 (s,
1H), 10.82 (s, 1H), 9.54 (dd, J = 5.1, 1.7
S
NH Hz, 1H), 8.40 (dd, J = 8.5,
1.7 Hz, 1H),
0 rsioD 8.04 (dd, J = 8.5, 5.0 Hz,
1H), 7.77 (d, J
= 5.9 Hz, 1H), 7.18 (d, J = 5.9 Hz, 1H),
14 4.23 (q, J = 7.1 Hz, 2H), 1.30 (t, J = 7.1
Hz, 3H).
250 0 0 A B ES-MS Mg=360(M+1)
0)L 'H NMR (400 MHz, DMSO-d6) 12.88 (s,
1H), 10.81 (s, 1H), 9.40 (dt, J = 6.9, 1.3
S
NH Hz, 1H), 8.73 (dd, J = 4.0,
1.7 Hz, 1H),
0.5...... 7.75 (d, J = 5.9 Hz, 1H), 7.48
-7.23 (m,
2H), 7.23 -7.08 (m, 1H), 4.23 (q, J =
N,NON 7.1 Hz, 2H), 1.30 (t, J = 7.1 Hz, 3H).
-156-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
251 0 0 A B ES-MS M/Z=359 (M+1)
I-H NMR (400 MHz, DMSO-d6) 12.66 (s,
Clerl-1)L 1H), 10.86 (s, 1H), 8.92 (d, J = 7.3 Hz,
S
NH 1H), 8.37 (d, J = 1.8 Hz, 1H),
8.19 (d, J
0 = 2.4 Hz, 1H), 7.75 (d, J =
5.9 Hz, 1H),
ON 7.28 (dd, J = 7.3, 2.1 Hz, 1H), 7.13 (d, J
= 5.9 Hz, 1H), 7.03 (d, J = 2.1 Hz, 1H),
aN 4.23 (q, J = 7.1 Hz, 2H), 1.30 (t, J = 7.1
Hz, 3H).
252 0 0 C C ES-MS M/Z=360 (M+1)
I-H NMR (400 MHz, DMSO-d6) 13.24 (s,
0-'11)L 1H), 10.83 (s, 1H), 9.44 - 9.37 (m, 1H),
S
NH 8.48 (d, J = 2.5 Hz, 1H), 7.80
-7.67 (m,
())
NON 2H), 7.17 (d, J = 6.0 Hz, 1H),
7.12 (dd, J
= 2.4, 1.0 Hz, 1H), 4.23 (q, J = 7.1 Hz,
P;r4 2H), 1.31 (t, J = 7.1 Hz, 3H).
253 0 0 C C ES-MS M/Z=359 (M+1)
I-H NMR (400 MHz, DMSO-d6) 12.86 (s,
eL11-41)L 1H), 10.66 (s, 1H), 9.41 (dd, J = 7.0, 1.6
S
NH Hz, 1H), 8.93 (dd, J = 4.2,
1.7 Hz, 1H),
o_ 8.79
8.79 (s, 1H), 7.64 (d, J = 5.9 Hz, 1H),
7.38 (dd, J = 7.0, 4.2 Hz, 1H), 7.03 (dd,
J = 5.9, 0.8 Hz, 1H), 4.22 (q, J = 7.1 Hz,
V 2H), 1.30 (t, J = 7.1 Hz, 3H).
254 C C A ES-MS M/Z=401.1 (M+1)
0
-N N..., I-H NMR (400 MHz, CD30D) 2.24 -
2.35
(m, 2 H) 2.98 (s, 3 H) 3.42 (d, J=6.36
S HN4-d Hz, 1 H) 3.50 - 3.61 (m, 2 H)
3.67 -
0 0> µ S' 3.78 (m, 2 H) 3.83 - 3.92 (m,
1 H) 3.94
N 0
- 4.01 (m, 1 H) 4.24 -4.33 (m, 1 H)
7.10 - 7.15 (m, 1 H) 7.16 - 7.22 (m, 1
H) 7.53 - 7.65 (m, 2 H) 8.08 - 8.18 (m,
2H)
255 p c C A ES-MS M/Z=401 (M+1)
r, 'H NMR (400 MHz,DMSO-d6) 1.79 _
N--/ 2.10 (m, 4 H) 2.72 (d, J=3.91 Hz, 2 H)
/\--s HNO1 3.00 - 3.16 (m, 2 H) 3.20 -
3.40 (m, 3
H) 4.07 -4.20 (m, 1 H) 7.14 - 7.22 (m,
0 0> µ S 1 H) 7.51 - 7.75 (m, 3 H) 8.18
- 8.30
0 (m, 2 H) 8.53 - 8.62 (m, 1 H) 10.30 -
10.43 (m, 1 H) 13.56 (s, 1 H)
-157-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
256 \ C B C ES-MS M/Z=413.1 (M+1)
/4--.\
I-H NMR (400 MHz,DMSO-d6) 0.75 -
0.83 (m, 2 H) 1.02 - 1.13 (m, 2 H) 2.69
\----( 0
HN-- - 2.93 (m, 2 H) 3.42 - 3.58
(m, 4 H)
4.25 -4.43 (m, 2 H) 7.15 - 7.21 (m,1 H)
S HN4--) 7.24 - 7.30 (m, 1 H) 7.58 -
7.70 (m, 2
00> µ S' H) 8.19 - 8.30 (m, 2 H) 12.03
(s, 1 H)
N 0
257 /"."-Nr----A 0 C B B ES-MS M/Z=401 (M+1)
\_..../N I-H NMR (400 MHz,DMSO-d6) 1.18
-
1.27 (m, 1 H) 3.04 - 3.18 (m, 1 H) 3.47
0 S HN 1 -3.54 (m, 1 H) 4.27 -4.41 (m, 1 H)
0> µ S 7.14 - 7.21 (m, 1 H) 7.24-
7.29 (m, 1
N 0
H) 7.59 - 7.70 (m, 1 H) 8.19 - 8.25 (m,
1 H) 8.25 - 8.30 (m, 1 H) 10.17 - 10.31
(m, 1 H) 12.02 (s, 1 H)
258 0 C C A ES-MS M/Z=449.2 (M+1)
I-H NMR (400 MHz,DMSO-d6) 3.21 -
CA
NJ 3.29 (m, 4 H) 3.71 - 3.85 (m, 4 H) 6.77
HNO- ID - 6.84 (m, 1 H) 6.94 - 7.01
(m, 2 H)
S
7.13 - 7.30 (m, 4 H) 7.58 - 7.70 (m, 2
....,..., H) 8.19 - 8.30 (m, 2 H) 12.04 -
12.11
U 0> µ C
\--N 0 (M, 1 H)
259 H C C A ES-MS M/Z=387 (M-101)
(N---\
I-H NMR (400 MHz,DMSO-d6) 1.39 (s, 9
H) 1.75 - 1.82 (m, 2 H) 2.78 - 2.90 (m,
\----( 0
HN-- 2 H) 3.92 -4.02 (m, 2 H) 4.02 -
4.20
(m, 2 H) 7.14- 7.19 (m, 1 H) 7.56 -
S HN 7.69 (m, 3 H) 8.21 -
8.31 (m, 3 H)
0 0> S 13.59 - 13.63 (m, 1 H).
N 0
260 C C B ES-MS M/Z=386.1 (M+1)
----CN--f I-H NMR (400 MHz,DMSO-d6) 0.90 (d,
J=6.11 Hz, 3 H) 1.09 - 1.21 (m, 2 H)
S HN.'d 1.59 - 1.72 (m, 3 H)
2.89 - 3.02 (m, 2
00 µ S'
N
H) 4.14 -4.26 (m, 2 H) 7.09 (s, 1H)
0
7.20 - 7.24 (m, 1 H) 7.59 - 7.69 (m, 2
H) 8.20 - 8.30 (m, 2 H) 12.04 (s, 1 H)
261 C'S C C A ES-MS M/Z=390 (M+1)
N--) I-H NMR (400 MHz,DMSO-d6) 2.67
-0 2.76 (m, 4 H) 3.78 - 3.88 (m, 4 H) 7.07
- 7.12 (m, 1 H) 7.21 -7.26 (m, 1 H)
S HN 0 7.58 - 7.70 (m, 2 H) 8.20 -
8.30 (m, 2
0 0> µ -c
H) 11.93 (s, 1 H)
N 0
-158-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
262 0 0 B B B ES-MS M/Z=475.2 (M+1)
C11()11-'11)L I-H NMR (400 MHz,DMSO-d6) 1.29 (t,
J=6.97 Hz, 3 H) 2.43 - 2.46 (m, 7 H)
S /
NH c-N 3.39 - 3.45 (m, 4 H) 4.16
(d,J=6.85 Hz,
O N N) 2 H) 6.60 - 6.69 (m, 1
H) 7.16 (d,J=5.14
Hz, 1 H) 7.33 (d, J=3.67 Hz, 1 H) 7.98 -
SgbN
8.08 (m, 1 H) 12.96 (br. s., 1 H)
263 0 0 C C A ES-MS M/Z=323 (M+1)
eLrl-'11ACI I-H NMR (400 MHz,DMSO-d6) 1.25 (t,
J=7.09 Hz, 3 H) 3.88 (s, 3 H) 4.18 (d,
S
NH J=7.09 Hz, 2 H) 6.98 - 7.05
(m, 1 H)
ONc-Ni 7.64 (br. s., 2 H) 7.95 (s, 1
H) 10.74 (s,
0> 1 H) 12.18 - 12.25 (m, 1 H)
N
264 0 0 A B C ES-MS M/Z=370 (M+1)
I-H NMR (400 MHz, DMSO-d6) 12.72 (s,
1H), 10.88 (s, 1H), 9.36 (d, J = 2.3 Hz,
S
NH 1H), 8.98 (d, J = 2.3 Hz, 1H),
8.27 (dd, J
0 = 8.2, 1.4 Hz, 1H), 8.16 (dd,
J = 8.3, 1.1
ON Hz, 1H), 7.96 (ddd, J = 8.4,
6.9, 1.5 Hz,
1H), 7.84 - 7.71 (m, 2H), 7.15 (d, J =
0 5.9 Hz, 1H), 4.23 (q, J = 7.1
Hz, 2H),
1.30 (t, J = 7.1 Hz, 3H).
265 0 0 C C B ES-MS M/Z=359 (M+1)
0)L 'H NMR (400 MHz, DMSO-d6) 12.27 (s,
1H), 10.78 (s, 1H), 9.04 - 8.85 (m, 1H),
S
NH 8.52 (s, 1H), 8.36 -8.22 (m,
1H), 7.76
O -7.65 (m, 1H), 7.32 -7.17 (m, 1H),
0,N 7.03 (d, J = 5.9 Hz, 1H), 6.12
(s, 1H),
ON 4.23 (q, J = 7.1 Hz, 2H), 1.30
(t, J = 7.1
Hz, 3H).
266 0 0 A C B ES-MS M/Z=437(M+1)
'CZ)1-41)LC) 'H NMR (400 MHz, DMSO-d6) 12.46 (s,
1H), 10.87 (s, 1H), 9.68 (d, J = 2.1 Hz,
S
NH 1H), 8.99 (d, J = 2.1 Hz, 1H),
8.64 (s,
O1H), 7.70 (d, J = 5.9 Hz, 1H), 7.15 (d, J
l= 5.9 Hz, 1H), 4.23 (q, J = 7.1 Hz, 2H),
IslICY-Br 1.29 (t, J = 7.1 Hz, 3H).
N
-159-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
267 0 0 A C A ES-MS M/Z=411(M+1)
01A 'H NMR (400 MHz,DMSO-d6) 1.28-
1.31 (t,3H,J=7.2Hz), 4.20-
S
NH 4.25(q,2H,J=7.2Hz),7.22-7.24
ONr...-N (d,1H,J=6.0Hz),7.78-7.79(d,1H,
J=5.6Hz),8.52(s,1H),9.45(s,1H),10.89(s
SPON
,1H),13.10(s,1H)
CI
268 0 0 A C A ES-MS M/Z=420 (M+1)
CeL11-41A 'H NMR (400 MHz,DMSO-d6) 1.27 (t,
J=7.06 Hz, 3 H) 3.59 (s, 6 H) 4.13 -4.27
S
NH (m, 2 H) 6.88 (d, J=6.62 Hz, 1
H) 7.16
0...i.....(. \ (d, J=5.95 Hz, 1 H) 7.75 (d,
J=5.95 Hz,
.5 µN___
1 H) 8.32 (d, J=6.62 Hz, 1 H) 10.80 (s, 1
s---C-3 H) 13.20 (s, 1 H)
N
269 0 0 A B A ES-MS Mg= 475.1(M+1)
LII-'11)L I-H NMR (400 MHz, CD30D) 1.35 (s, 3
H) 3.08 (s, 3 H) 3.47 - 3.61 (m, 2 H)
S /
NH /----N 3.71 - 3.87 (m, 2 H) 4.04 -
4.18 (m, 2
NO j\N H) 4.22 -4.38 (m, 2 H)5.27 -
5.51 (m, 2
H) 7.00 - 7.13 (m, 1 H) 7.16 - 7.28 (m,
SPI 1 H) 7.47 - 7.64 (m, 1 H) 8.35
- 8.49
N (m, 1 H).
270 0 0 A C A ES-MS Mg= 420(M+1)
'Cl1()L11:11A I-H NMR (400 MHz, CDCI3) 1.31
(0=7.21 Hz, 3 H) 3.58 (s, 6 H) 4.26
S
NH (d,J=7.09 Hz, 2 H) 6.84 - 6.89
(m, 1 H)
\
0 N____ 7.02 (dd,J=9.29, 5.62 Hz, 2 H)
7.77 -
,
7.85 (m, 1 H) 8.01 - 8.07 (m, 1 H)
SOCI'l 12.97 - 13.04 (m, 1 H)
271 0 0 ES-MS Mg= 373.9(M+1)
I-H NMR (400 MHz,DMSO-d6) 0.945-
0.983 (m, 3H), 1.610-1.665 (m, 2H),
HN S 2.772 (s, 2H), 7.201-7.213 (m,
1H),
N 0 7.501-7.630 (m, 2H), 7.793-
7.810 (d,
-...*
c--Os 1H), 8.401-8.491 (m, 2H),
10.822 (s,
U 1H), 13.052 (s, 1H)
-160-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
272 cii?)0=L 0 ES-MS Mg= 430(M+1)
NAO" I-H NMR (400 MHz,DMSO-d6) 11.93-
0 H 12.05 (m, 1H), 10.37-10.46 (m,
1H),
S
NH 8.19-8.35 (m, 2H), 7.58-7.75 (m, 2H),
CY )_.-N 4.17 (s, 2H), 2.70 (br. s.,
5H), 1.67-
S 0
1.84
(m, 3H), 1.24 (s, 3H)
n. ES-MS Mg= 521.1(M+1)
273
'H NMR (400 MHz,DMSO-d6) 1.26 (br.
Ojil s., 3 H) 3.00 - 3.08 (m, 1 H)
3.16 - 3.23
NH (m, 1 H) 3.56 - 3.76 (m, 2 H) 4.19
0 so H
NI.r0 (d,J=5.52 Hz, 2 H) 4.29 - 4.52 (m, 4 H)
N
0 0 7.52 (d,J=3.51 Hz, 3 H) 7.56 - 7.61 (m,
2 H) 7.63 - 7.75 (m, 2 H) 8.19 - 8.35
(m, 2 H) 10.67 - 10.77 (m, 1 H) 12.08
(br. s., 1 H)
274 0 0 B C C ES-MS Mg=477.9 (M+23)
erl-'11)
NH
4::(Nr-N
SC)rm
275 0 0 C C B ES-MS Mg=456.9(M+1)
QeLm)Lolal
S ,"F
NH
ONF.-N
StN
Nq C C B ES-MS Mg=449 (M+1)
276
OS I-H NMR (400 MHz, CDCI3) 10.84
(s,
N--cr.0 1H), 9.61 (s, 1H), 8.70 (d, J
= 5.5 Hz,
HN 0 / 1H), 8.39 (dd, J = 5.5, 1.0 Hz, 1H), 7.64
s
H 0 0 (s, 1H), 4.22 (q, J = 7.1 Hz, 2H), 3.92 (s,
ON
II 2H), 3.68 (s, 3H), 1.29 (t, J
= 7.1 Hz,
0 0 3H).
277 0 0 ES-MS Mg= 373(M+1)
01A 'H NMR (400 MHz,DMSO-d6) 1.27-
1.24(t,3H,J=6.8Hz), 2.84(s,3H),4.21-
NH 4.15(q,2H, J=6.8Hz),7.04-
7.03(d,1H,
01)-d' J=6.0Hz),7.18-7.15(t,1H, J=6.8),7.6-
N
O'
7.5(m,1H),7.70-7.67(m,2H),9.45-
9.43(d,1H, J=6.8Hz), 10.73(s,1H),
12.04(s,1H).
-161-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
278 s 0 C C A ES-MS M/Z=549 (M+1)
aN>4NH I-H NMR (400 MHz,DMSO-d6)
13.04 (s,
1H), 10.80 (s, 1H), 9.66 (s, 1H), 8.72
U 0 (d, J = 5.6 Hz, 1H), 8.43 (d, J = 5.6 Hz,
HN--- 1H), 7.62 (s, 1H), 4.22 (q, J
= 7.1 Hz,
4:11r.r() 1:3___\
2H), 4.18 -4.05 (m, 4H), 3.84 (t, J =
0 0 7.5 Hz, 1H), 3.26 (d, J = 7.5
Hz, 2H),
1.29 (t, J = 7.1 Hz, 3H), 1.16 (t, J = 7.1
Hz, 6H).
279 0 0 C C B ES-MS M/Z=449 (M+1)
A
I-H NMR (400 MHz,DMSO-d6) 13.28 (s,
H 0
HN s 0--\ 1H), 11.14 (s, 1H), 9.65 (s,
1H), 8.72
(d, J = 5.6 Hz, 1H), 8.65 (s, 1H), 8.43
NION......0 (d, J = 5.6 Hz, 1H), 4.32 (q, J = 7.1 Hz,
D9S 2H), 4.23 (q, J = 7.1 Hz, 2H), 1.33 (t, J =
6.9 Hz, 3H), 1.29 (t, J = 7.1 Hz, 3H).
280 0 0 C C B ES-MS M/Z=454 (M+1)
A
S N
NH
4:3\r-N
SgibN
281 0 0 A C B ES-MS M/Z=404 (M+1)
r-N)-LO I-H NMR: 7.40 (d, J = 8.8 Hz,
1H), 7.34
H (d, J= 8 Hz, 1H), 6.8 (m, 1H), 6.28 (d, J=
S
NH 6Hz, 1H), 3.23 (d, J = 6.8 Hz,
2H), 1.2
0r.N (m, 1H), 0.2 (d, J =6.8 Hz,
6H)
N-
s 0
282 0 0 C C B ES-MS M/Z=404.7 (M+1)
ICZ)I-silAC)
S
NH
ON
SPICoN
283 0 0 B C B ES-MS M/Z=388.0 (M+1)
els-1A
s
NH
0
ilig?N
-162-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
284 o o C C B ES-MS Mg=439.8(M+1)
(Z)(111)
S F
NH
O&
,N6
285 0 0 C C C ES-MS Mg=440.0(M+1)
,c).LNAO
H 0
S NH F
0
(Q14(1
isligN
286 0 0 B B C ES-MS Mg=445.9(M+1)
A
el).LN 0
H 0
S NH F
ONr-N
s 0
287 0 0 B B B ES-MS Mg=457.0(M+1)
A
el)Lisl 0
H 0
S NH F
O),.-N
StN
288C B C ES-MS Mg=506.9(M+1)
S091--
O)---14
F
F
NH
ti oF 0
Y
00
289 0 0 C C C ES-MS Mg=490.0(M+1)
A
IcZ).LN 0
H 0
S F
NH F
F
0
INC2)N143)1
-163-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
290 0 0 C C A ES-MS Mg= 373(M+1)
11-'11A I-H NMR (400 MHz, CD30D) 1.36 (t,
J=7.06 Hz, 3 H) 2.93 (s, 3 H) 4.31 (d,
S
NH J=7.28 Hz, 2 H) 7.07 - 7.16
(m, 1 H)
7.39 - 7.48 (m, 1 H) 7.53 -7.61 (m, 1 H)
7.79 - 7.88 (m, 1 H) 7.98 - 8.09 (m, 1
N 0 H) 8.85 - 8.95 (m, 1 H)
291 C C A ES-MS Mg= 418 (M+1)
N2 'H NMR (400 MHz,DMSO-d6) 3.34 -
3.40 (m, 2 H) 3.66 - 3.77 (m, 4 H) 3.79
H0 - 3.90 (m, 2 H) 4.08 - 4.16
(m, 2 H)
0 4.78 - 4.85 (m, 2 H) 7.06 - 7.14 (m, 1
H) 7.40 - 7.48 (m, 1 H) 7.58 - 7.73 (m,
S HN 15- 2 H) 8.11 - 8.26 (m,
2 H)
0 0> µ S
N 0
292 o ,os C C A ES-MS Mg= 414.9(M+1)
0C3)1-Vril I-H NMR (400 MHz,DMSO-d6) 1.32-
s
0 TH 1.28(t,3H,J=6.8Hz),4.25-
4.20(q,2H,J=6.8Hz),7.07-
e'o 7.06(d,1H,J=5.6),7.53-
7.51(m,1H),7.61-7.59(m,1H), 7.72-
7.71(d,1H,J=6.0Hz),8.13-
8.11(d,1H,J=8.0Hz),8.24-
8.22(d,1H,J=8.0Hz),9.17(s,1H),10.75(s,
1H),12.78(s,1H).
293 0 0 A A C ES-MS Mg=388.0(M+1)
NAO 1HNMR: 13.07 (s, 1H), 10.95 (s, 1H),
H 8.32 (dd, J = 9.2 Hz, 2H),
7.79 (d, J =
S
NH 8.0 Hz, 1H), 7.69 (m, 2H),
7.22 (d, J =
0NS 6Hz, 1H), 6.03 (ddt, J= 17.2,
5.2, 4.8
r.-
N 0Hz, 1H), 5.45 (dq, J= 17.2, 1.6 Hz, 1H),
5.31 (dq, J = 17.2, 1.6, 1H), 4.72 (dt, J
= 5.6, 1.2 Hz, 2H)
294 C C B ES-MS Mg=505.7(M+1)
s00
Co)---N
F
F
NH
till oF 0
Y
00
-164-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
295 N C B C ES-MS Mg=506.6(M+1)
sP
0/L-N
F
F
NH
tYcrsii oF 0
00
296 0 0 C B C ES-MS Mg=422.7(M+1)
Lirs-1)L
s
NH F
0 F
0
F
F
F
297 0 0 A A C ES-MS Mg=394(M+24)
0A
S
NH
0
NCrs(1
IsligN
298 0 0 A A C ES-MS Mg=374(M+1)
Clerl-1)LC) I-H NMR (400 MHz, DMSO-d6)
12.49 (s,
1H), 10.81 (s, 1H), 9.64 (d, J = 2.1 Hz,
S
NH 1H), 8.93 (d, J = 2.2 Hz, 1H),
8.48 (d, J
0 = 2.3 Hz, 1H), 7.70 (d, J =
5.9 Hz, 1H),
10,1i 7.14 (d, J = 5.9 Hz, 1H), 6.94
(d, J = 2.3
islig Hz, 1H), 4.99 (m, 1H), 1.31
(d, J = 6.2
N Hz, 6H).
299 0 0 A C C ES-MS Mg=466(M+1)
()L1-411)L43 I-H NMR (400 MHz, CDCI3) 12.78
(s,
S 1H), 9.43 (s, 1H), 9.12 (s,
1H), 8.60 (s,
NH 1H), 8.17 ¨7.80 (m, 3H), 7.23
¨ 6.83
0N (m, 4H), 4.39 (m, 2H), 3.89
(s, 3H),
(C)/141 1.41 (t, J = 7.0 Hz, 3H).
1110 0 O\
N''
-165-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
300 0 1 C C B ES-MS Mg=443.9(M+1)
0 0
S NH
0
ONN
No
301 0 0 C C B ES-MS Mg=439.9(M+1)
a-L11)Le,3
S N
NH
O--S
Ngb
N
302 0 9 c c C ES-MS Mg=447.69(M+1)
Qem)co,
S
NH
O0
NOS
0
303 0 0 A C C ES-MS Mg=407.9(M+1)
I-H NMR: 13.05 (s, 1H), 11.08 (s, 1H),
).1,1A0
H 8.33 (dt, J = 8 Hz, 4 Hz, 2H), 7.71 (m,
A
S NH 3H), 7.22 (d, J = 4Hz, 1H),
4.88 (d, J =
2.4 Hz, 2H), 3.68 (t, J =4 Hz, 1H)
0Nr-S
N 0
304
n B C B ES-MS M/Z=506.9 (M+1)
,C
0 0 C'
A)LAN
FH VHF<F
S NH
O.-N
SitN
-166-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
0 B C C ES-MS Mg=467 (M+1)
305 0 0
'H NMR (400 MHz,DMSO-d6) 13.09 (s,
Ae 1H), 10.98 (s, 1H), 9.57 (s,
1H), 8.70
(d, J = 5.5 Hz, 1H), 8.38 (d, J = 5.5 Hz, Lr1-411 CI 1H), 7.81 (d, J =
5.9 Hz, 1H), 7.49 -
NH 7.38 (m, 5H), 7.37- 7.30 (m,
1H), 7.25
(d, J = 6.0 Hz, 1H), 5.69 (t, J = 6.8 Hz,
Ng) 1H), 2.04- 1.81 (m, 2H), 0.93
(t, J =
7.3 Hz, 3H).
N
0 C C C ES-MS Mg=472 (M+1)
306
I-H NMR (400 MHz,DMSO-d6) 12.41 (s,
00
A 1H), 10.94 (s, 1H), 9.63 (d, J
= 2.2 Hz,
1H), 8.92 (d, J = 2.2 Hz, 1H), 8.47 (d, J
0 Cs = 2.3 Hz, 1H), 7.70 (d, J = 5.9 Hz, 1H),
NH 7.48 -7.36 (m, 4H), 7.36- 7.27
(m,
0
N 1H), 7.16 (d, J = 5.9 Hz, 1H),
6.93 (d, J
= 2.3 Hz, 1H), 5.69 (t, J = 6.8 Hz, 1H),
Zc51 1.89 (ddq, J = 28.0, 13.9, 6.9
Hz, 2H),
0.91 (t, J = 7.3 Hz, 3H).
0 C B C ES-MS Mg=488.9 (M+1)
307
I-H NMR (400 MHz,DMSO-d6) 13.05 (s,
0 0
A1H), 10.95 (s, 1H), 8.39 -8.14 (m, 2H),
7.79 (d, J = 5.9 Hz, 1H), 7.73 -7.60 (m,
Cs 2H), 7.47 -7.38 (m, 4H), 7.36 -7.31
NH (m, 1H), 7.23 (dd, J = 8.3,
6.7 Hz, 2H),
0:0s 7.15 (dd, J = 15.1, 7.1 Hz,
1H), 5.69
N 0 (dd, J = 7.5, 6.1 Hz, 1H),
2.01 - 1.82
(m, 2H), 0.92 (t, J = 7.3 Hz, 3H).
308 Ng, C C C ES-MS Mg=434.9 (M+1)
OS I-H NMR (400 MHz,DMSO-d6)
13.06 (s,
N--1Nr.0 1H), 10.84 (s, 1H), 9.61 (s,
1H), 8.70
HN 0 (d, J = 5.5 Hz, 1H), 8.39 (d,
J = 5.5 Hz,
S
H 0 OH 1H), 7.62 (s, 1H), 4.22 (q, J
= 7.1 Hz,
ON
II 2H), 1.29 (t, J = 7.1 Hz, 3H).
00
309 0 0 B B C ES-MS Mg= 363(M+1)
I-H NMR (400 MHz, CD30D) 1.35 (s, 3
C11()11-41)L H) 1.94 - 2.29 (m, 4 H) 2.98 - 3.19 (m,
S
NH 2 H) 4.19 -4.38 (m, 4 H) 6.91 -
7.18
0__--.\ (m, 1 H) 7.30 - 7.68 (m, 1H)
8.25 (s, 1
H)
N910
-167-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
310 0 0 C C A ES-MS Mg= 358(M+1)
11-'11)L I-H NMR (400 MHz,DMSO-d6)
1.28-1.24(t,3H,J=7.2Hz),4.21-
S
NH 4.16(q,2H,J=7.2Hz),6.68-
O 6.67(m,1H),6.81-6.78(m,2H),7.00-
0 6.99(d,1H,J=6.0Hz),7.55-
NO 7.53(d,1H,J=9.2Hz),7.69-
7.67(d,1H,J=6.0Hz),8.17(s,1H),8.38-
8.36(d,1H,J=6.8Hz),
10.74(s,1H),12.35(s,1H).
311 0 0 A C A ES-MS Mg= 462.1(M+1)
Cl()11-41)L I-H NMR (400 MHz,DMSO-d6) 1.36
(t,J=7.06 Hz, 3 H) 3.67 (br. s., 4 H) 3.80
S
NH (d,J=4.85 Hz, 4 H) 4.30
(q,J=7.06 Hz, 2
O,..-N H) 7.25 (d,J=5.73 Hz, 1 H) 7.65 (s, 1 H)
7.79 - 7.89 (m, 1 H) 9.22 (s, 1 H) 10.88
SPON
- 10.97 (m, 1 H) 12.99 - 13.10(m, 1 H)
/N---\
\-01
312 0 0 C C B ES-MS Mg=391 (M+1)
'CZ)L11-'11ACI 'H NMR (400 MHz,DMSO-d6) 12.38 (s,
1H), 11.60 (s, 1H), 10.67 (s, 1H), 7.94
S
NH (d, J = 7.8 Hz, 1H), 7.69 (s,
1H), 7.63
ONH (d, J = 6.0 Hz, 1H), 7.42 (ddd, J = 8.3,
ec 7.3, 1.3 Hz, 1H), 7.28 (td, J
= 7.6, 1.2
.0N Hz, 1H), 6.99 (d, J = 6.0 Hz, 1H), 4.21
(q, J = 7.1 Hz, 2H), 1.29 (t, J = 7.1 Hz,
0 3H).
313 0 C C B ES-MS Mg=346 (M+1)
N N-N I-H NMR (400 MHz,DMSO-d6)
13.00 (s,
H Qg 1H), 10.79 (s, 1H), 8.53
(s, 1H), 8.35
0 N
NH (d, J = 9.6 Hz, 1H), 8.01 (s,
1H), 7.84
CAco (d, J = 9.6 Hz, 1H), 7.72 (d,
J = 6.0 Hz,
/ 1H), 7.13 (d, J = 6.0 Hz, 1H),
3.98 (s,
3H).
314 \ 0 C C B ES-MS Mg=363 (M+1)
0.--f
0 I-H NMR (400 MHz, CD30D) 9.59
(s,
HN
HN__ 1H), 8.70 (d, J = 5.8 Hz, 1H), 8.44 (d, J
= 5.8 Hz, 1H), 7.57 (d, J = 5.9 Hz, 1H),
NoS 0
co> µ s, 7.14 (d, J = 5.9 Hz, 1H), 3.87 (s,
3H).
N 0
-168-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
315 0 0 A C B ES-MS Mg=445(M+1) ____________
CeLitiA
s
NH
0
NCII'(1
N ,,r--\w
N \---/
316 0 0 B C B ES-MS Mg=392(M+1)
F---N)L 'H NMR (400 MHz, CD30D) 9.46 (s,
H 1H), 8.98 (d, J = 1.8 Hz, 1H), 8.36 (d, J
S
NH = 3.4 Hz, 1H), 7.54 (d, J =
6.0 Hz, 1H),
N
O 7.04 (d, J = 6.1 Hz, 1H), 6.13 (s, 1H),
o- 7.04
5.09 (m, 1H), 1.39 (d, J = 6.2 Hz, 6H).
r'llg)N
317 0 0 A C C ES-MS Mg=373(M+1)
I-H NMR (400 MHz, CDCI3) 10.80 (s,
1H), 8.92 (dd, J = 7.3, 1.0 Hz, 1H), 8.48
S
NH -8.31 (m, 1H), 8.18 (d, J =
2.3 Hz, 1H),
0 7.74 (d, J = 5.9 Hz, 1H), 7.27
(dd, J =
ON 7.3, 2.1 Hz, 1H), 7.11 (d, J =
5.9 Hz,
1H), 7.02 (dd, J = 2.3, 0.9 Hz, 1H), 4.94
CYN (m, 1H), 1.30 (d, J = 6.3 Hz,
6H).
318 0 0 A C B ES-MS Mg=395(M+1)
F--)LN)L 'H NMR (400 MHz, CDCI3) 13.05 (s,
H 1H), 8.77 (dd, J = 4.6, 1.5 Hz, 1H), 8.55
S
NH (dd, J = 8.3, 1.6 Hz, 1H),
7.74 (s, 1H),
O.-N 7.61 (dd, J = 8.4, 4.6 Hz, 1H), 6.57 (d, J
= 2.7 Hz, 1H), 4.37 (q, J = 7.2 Hz, 2H),
Sgb 1.40 (t, J = 7.1 Hz, 3H).
N
319 0 0 F A C C ES-MS Mg=443.9(M+1)
A )<F
I-H NMR: 13.06 (s, 1H), 11.03 (s, 1H),
1
ICZ)1-411 F
8.32 (dt, J = 7.6, 1.6 Hz, 2H), 7.71 (m,
NH 3H), 7.22 (d, J = 6 Hz, 1H),
4.43 (t, J= 6
Hz, 2H), 2.78 (m, 2H)
0.--S
N 0
-169-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
320 0 0 F A C A ES-MS Mg=444.9(M+1)
0 F
NH
Co.Nr-S
Ngb
N
321 A C A ES-MS Mg=401.9(M+1)
'H NMR: 13.09 (s, 1H), 10.89 (s, 1H),
9 0
0 8.32 (dd, J = 1.6, 0.8 Hz, 2H), 7.71 (m,
HN1., 3H), 7.21 (d, J = 6 Hz, 1H), 5.01 (q, J =
7.2 Hz, 1H), 2.37 (m, 2H), 2.14 (m,
S HN 0 2H), 1.06 (m, 2H)
0 0>¨µ S
N 0
322 0 0 F C C C ES-MS Mg=428.0(M+1)
A )<F
0 F
NH
0
NDI!(1
isligN
323 0 0 B C ES-MS Mg=373(M+1)
'CZ)L11-'11)L 'H NMR (400 MHz, CDCI3) 12.88
(s,
1H), 8.33 (s, 1H), 8.27 (d, J = 7.0 Hz,
S
NH 1H), 7.96 (s, 1H), 7.88 (d, J
= 9.3 Hz,
(3 1H), 7.40 (t, J = 8.0 Hz, 1H),
7.12 (dd, J
*.N
= 5.9, 2.6 Hz, 1H), 6.99 (d, J = 6.8 Hz,
Nb 1H), 6.89 (d, J = 5.9 Hz, 1H),
5.13 (m,
1H), 1.37 (d, J = 6.3 Hz, 6H).
324 7 0 A C ES-MS Mg=386.0(M+1)
I-H NMR: 12.47 (s, 1H), 10.87 (s, 1H),
N
0 N
r-ikl.
H _4-.,-)/ 9.64 (d, J= 2.4 Hz, 1H), 8.93
(d, J = 2
NH Hz, 1H), 8.48 (d, J = 2Hz, 1H), 7.70 (d, J
0=\c) = 6 Hz, 1H), 7.14 (d, J = 5.6
Hz, 1H),
d 6.94 (d, J = 2Hz, 1H), 5.00 (p, J = 7.2
Hz, 1H), 2.35 (m, 2H, 2.12 (m, 2H),
1.70 (m, 2H)
-170-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
325 0 0 A C ES-MS M/Z=445.0(M+1)
___________
0 A F I-H NMR: 13.03 (s, 1H), 11.29 (s, 1H),
<F 9.66 (d, J = 0.8 Hz, 1H), 8.72 (d, J = 5.6
NH Hz, 1H), 8.44 (dd, J= 5.6, 0.8 Hz, 1H),
(:) ...s 7.81 (d, J = 6 Hz, 1H), 7.25 (d, J = 5.6
.
Ngb Hz, 1H) 5.57 (hept, J = 6.8 Hz, 1H),
1.48 (d, J = 6.8 Hz, 3H)
N
326 0 0 C C ES-MS M/Z=427.9(M+1)
0A F<F
F
NH
0
Ols('
g?
N
327 0 0 B C C ES-MS M/Z=802.8(2M+1)
0)(
S
NH
0
C/1'(1
iii1g)N
328 0 0 A C C ES-MS M/Z=419.0(M+1)
I-H NMR: 13.14 (s, 1H), 10.86 (s, 1H),
9.64 (d, J = 0.8 Hz, 1H), 8.72 (d, J = 5.6
S NH Hz, 1H), 8.43 (dd, J= 6, 1.2
Hz, 1H),
0" S 7.81 (d, J = 6 Hz, 1H), 7.24
(d, J = 5.6
Hz, 1H), 4.74 (m, 1H), 1.65 (m, 4H),
Ngb 0.93 (t, J = 7.2 Hz, 6H)
N
329 0 0 A C C ES-MS M/Z=376(M+1)
01A 'H NMR (400 MHz, CD30D) 1.36
(s, 3
H) 4.21 -4.37 (m, 2 H) 7.03 - 7.13 (m,
S
NH 1 H) 7.53 - 7.62 (m, 1 H) 8.43
- 8.50
0 (m, 1 H) 8.51 - 8.58 (m, 1H)
8.66 - 8.76
S 0 (m, 1 H) 9.64 - 9.75 (m, 1 H)
N
-171-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
330 0 0 A B C ES-MS M/Z=428.9 (M+23)
I-H NMR (400 MHz, DMSO-d6) 1.17 -
1.32 (m, 6 H) 4.89 - 5.00 (m, 1 H) 7.06
S
NH - 7.14 (m, 1 H) 7.28 -7.38 (m,
1 H)
O S 7.51 - 7.60 (m, 1 H) 7.65 -
7.72 (m, 1
Oc-- H) 7.92 - 8.00 (m, 1 H) 8.09 - 8.15 (m,
U 1 H) 10.75 - 10.83 (m, 1 H) 12.60 -
12.63 (m, 1 H)
F
331 0 0 A B C ES-MS M/Z=414.8 (M+23)
11-'11)L I-H NMR (400 MHz, DMSO-d6) 1.22 -
1.30 (m, 3 H) 4.15 - 4.24 (m, 2 H) 7.08
S
NH - 7.13 (m, 1 H) 7.28 -7.36 (m,
1 H)
O F 7.53 - 7.60 (m, 1 H) 7.67
- 7.71 (m, 1
sOr- H) 7.93 - 7.99 (m, 1 H) 8.11 (s, 1 H)
..} 10.82 - 10.86 (m, 1 H) 12.59 - 12.63
(m, 1 H)
332 0 0 A C A ES-MS Mg= 417.1(M+41)
CeL11-41ACI 'H NMR (400 MHz, DMSO-d6) 1.26
(t,J=7.04 Hz, 3 H) 4.14 - 4.25 (m, 2 H)
S
NH 7.11 (d,J=5.67 Hz, 1 H) 7.50 -
7.60 (m,
O 1 H) 7.67 - 7.76 (m, 1 H) 8.19 (s, 1 H)
S (-) 8.50 - 8.59 (m, 1 H) 8.67 - 8.75 (m, 1
N\--/ H) 10.79 - 10.89 (m, 1 H) 12.61 -
12.68(m, 1 H)
333 0 0 A B B ES-MS Mg= 409(M+1)
01)L 'H NMR (400 MHz, DMSO-d6) 1.24 -
1.29 (m, 3 H) 4.14 - 4.26 (m, 2 H) 7.10
S
NH (d,J=5.87 Hz, 1 H) 7.53 (s, 1
H) 7.71
O (d,J=5.87 Hz, 1 H) 8.11 - 8.16 (m, 1 H)
800
8.17 - 8.20 (m, 1 H) 8.26 - 8.31 (m, 1
.._) H) 10.77 - 10.87 (m, 1 H) 12.56 -
12.64(m, 1 H)
CI
334 0 0 A C A ES-MS Mg= 376.1(M+1)
Clell-'11)LC) I-H NMR (400 MHz, DMSO-d6) 1.26 (s,
3 H) 4.15 -4.25 (m, 2 H) 7.11 - 7.19
S
NH (m, 1 H) 7.70 - 7.77 (m, 1 H)
8.48 -
O 8.55 (m, 1 H) 8.59 - 8.66 (m, 1 H) 8.68
- 8.75 (m, 1 H) 9.61 - 9.69 (m, 1 H)
10.84 - 10.93 (m, 1 H) 12.69 - 12.78
(m, 1 H)
-172-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
335 0 0 ES-MS Mg= 476.1(M+1)
11-41)LC) I-H NMR (400 MHz, DMSO-d6)
1.28
(d,J=5.87 Hz, 6 H) 3.88 (br. s., 4 H)
S
NH 4.18 (br. s., 4 H) 4.92 - 5.04
(m, 1 H)
it:),..-S 7.10 - 7.19 (m, 1 H) 7.54 - 7.63 (m, 1
H) 7.73 - 7.81 (m, 1 H) 8.05 - 8.15 (m,
NTO 1 H) 10.70 - 10.80 (m, 1 H)
13.33 -
N 13.43 (m,1 H)
(1
----N
0-
336 0 0 C C B ES-MS Mg=387(M+1)
C11()L11-41)L
S
NH
C640
N
337 0 0 A C B ES-MS Mg=395(M+1)
F--- L 'H NMR (400 MHz, CDCI3) 13.13
(s,
N)L
H 1H), 9.62 (d, J = 0.9 Hz, 1H),
8.76 (d, J
S
NH = 5.8 Hz, 1H), 8.13 (d, J =
5.8 Hz, 1H),
0 ,--N 7.76 (s, 1H), 6.60 (d, J = 2.7
Hz, 1H),
So N 4.38 (q, J = 7.1 Hz, 2H), 1.41
(t, J = 7.1
Hz, 3H).
338 0 0 A C B ES-MS Mg=377(M+1)
F--- L 'H NMR (400 MHz, DMSO-d6)
12.79 (s,
N)L
H 1H), 10.76 (d, J = 7.1 Hz,
1H), 8.41 (dt,
S
NH J = 6.9, 1.2 Hz, 1H), 7.75 -
7.68 (m,
1H), 7.50 -7.39 (m, 2H), 7.17 -7.08
(m, 2H), 4.24 - 4.18 (m, 2H), 1.30 (t, J
= 7.2 Hz, 3H).
339 0 0 A C B ES-MS Mg=391(M+1)
FN)L 'H NMR (400 MHz, DMSO-d6) 12.80 (s,
H 1H), 10.70 (d, J = 9.2 Hz,
1H), 8.41 (dt,
S
NH J = 7.0, 1.2 Hz, 1H), 7.82 -
7.72 (m,
o 1H), 7.51 -7.39 (m, 2H), 7.19 -
7.05
cci3i
(m, 2H), 4.90 (m, H), 1.31 d, J = 6.3 Hz,
r,b), 6H).
-173-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
340 0 0 A C B ES-MS Mg=374(M+1)
I-H NMR (400 MHz, CD30D) 9.07 (ddd,
1-41)L J = 7.2, 1.7, 0.9 Hz, 1H),
8.65 (dd, J =
S
NH 4.1, 1.7 Hz, 1H), 7.53 (d, J =
5.9 Hz,
Ork(1 1H), 7.27 (d, J = 0.9 Hz, 1H), 7.21 (dd, J
= 7.1, 4.1 Hz, 1H), 7.03 (d, J = 5.9 Hz,
N----)/ 1H), 5.10 (m, 1H), 1.38 (d, J = 6.3 Hz,
6H).
341 0 0 A C B ES-MS Mg=378(M+1)
F--A)LNA
H
S
NH
0
N(Q!'('
ilig)N
342 0 0 A B B ES-MS Mg=408(M+1)
F---A)LNA 'H NMR (400 MHz, CDCI3) 13.03 (s,
H 1H), 8.27 (ddd, J = 8.3, 1.3, 0.7 Hz,
S
NH 1H), 8.01 (ddd, J = 8.0, 1.3,
0.6 Hz,
0 --S 1H), 7.75 (s, 1H), 7.59 (dddd,
J = 26.5,
)
N rTh 8.3, 7.2, 1.3 Hz, 2H), 6.60 (dd, J = 2.8,
0.8 Hz, 1H), 5.14 (hept, J = 6.2 Hz, 1H),
1.37 (d, J = 6.3 Hz, 6H).
343 0 0 A C C ES-MS Mg=377(M+1)
F--(CZ)LNA 'H NMR (400 MHz, DMSO-d6) 5 12.64
H (s, 1H), 10.87 (s, 1H), 8.82 (dt, J = 7.4,
S
NH 1.2 Hz, 1H), 8.55 -8.27 (m,
2H), 7.74
0 (d, J = 5.9 Hz, 1H), 7.27 (dd,
J = 7.4, 2.0
ON Hz, 1H), 7.14 (d, J = 5.9 Hz, 1H), 4.23
(q, J = 7.1 Hz, 2H), 1.30 (t, J = 7.1 Hz,
aN 3H).
344 0 0 A C B ES-MS Mg=409(M+1)
F--LNA I-H NMR (400 MHz, CDCI3) 13.07 (s,
H 1H), 8.77 (dd, J = 4.6, 1.6 Hz, 1H), 8.54
S
NH (dd, J = 8.4, 1.6 Hz, 1H),
7.68 (s, 1H),
0)--S 7.61 (dd, J = 8.3, 4.6 Hz, 1H), 6.58 (d, J
Ngt.), = 2.7 Hz, 1H), 5.15 (p, J = 6.2 Hz, 1H),
1.38 (d, J = 6.3 Hz, 6H).
-174-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
345 0 0 A C B ES-MS Mg=392(M+1)
F-LNA I-H NMR (400 MHz, CDCI3) 12.86 (s,
H 1H), 9.05 -8.77 (m, 1H), 8.77 -8.50
S
NH (m, 1H), 7.74 (s, 1H), 7.36
(d, J = 0.8
OiCi< Hz, 1H), 7.03 (dd, J = 7.1, 4.0 Hz, 1H),
6.57 (d, J = 2.8 Hz, 1H), 5.13 (p, J = 6.2
Hz, 1H), 1.36 (d, J = 6.2 Hz, 6H).
346 0 0 B C C ES-MS Mg=391(M+1)
F-LN)L 'H NMR (400 MHz, CDCI3) 12.72 (s,
H 1H), 8.47 -8.39 (m, 1H), 8.33 (dd, J =
S
NH 2.0, 1.0 Hz, 1H), 7.94 (d, J =
3.7 Hz,
O 1H), 7.90 (s, 1H), 7.35 (dd, J = 7.4, 2.1
ON Hz, 1H), 7.11 (d, J = 5.9 Hz,
1H), 6.96
(d, J = 5.9 Hz, 1H), 5.16 (p, J = 6.2 Hz,
CYN 1H), 1.39 (d, J = 6.2 Hz, 6H).
347 \ 0 C C B ES-MS M/Z=345 (M+1)
0.--f
O I-H NMR (400 MHz, DMSO-d6) 12.85 (s,
N
HN 1H), 10.66 (s, 1H), 8.75 -
8.50 (m, 2H),
7.78 (d, J = 9.2 Hz, 1H), 7.69 (d, J = 5.9
tyg? µ
HN 0__
Hz, 1H), 7.41 (dd, J = 9.3, 6.8Hz, 1H),
S
N 0 7.10 -7.05 (m, 2H), 3.82 (s,
3H).
348 C C B ES-MS M/Z=399 (M+1)
'H NMR (400 MHz, DMSO-d6) 12.87 (s,
R
o -...
o 1H), 10.67 (s, 1H), 8.76 -8.50 (m, 2H),
o
HN 7.80 (dd, J = 9.2 Hz, 1H),
7.71 (d, J =
5.9 Hz, 1H), 7.43 (dd, J = 9.3, 6.8 Hz,
tYNHN Q_ 1H), 7.11 -7.04 (m, 2H), 5.23 -
5.13
0> µ S (m, 1H), 1.97- 1.88
(m, 2H), 1.81 -
0 1.69 (m, 4H), 1.60 (m, 2H).
349 0 0 C C B ES-MS M/Z=429 (M+1)
NAN F I-H NMR (400 MHz, CDCI3) 12.89
(s,
.sa---A H HIF<F 1H), 9.10 (s, 1H), 8.43 (s,
1H), 8.32 (d,
NH J = 8.2 Hz, 1H), 8.05 (d, J =
8.0 Hz, 1H),
0 N 7.69 -7.52 (m, 2H), 7.23 (d, J
= 6.0 Hz,
...-
S0r 1H), 7.02 (d, J = 5.9 Hz, 1H),
4.18 -
...J 4.07 (m, 2H).
350 0 0 C C B ES-MS M/Z=359.0 (M+1)
CZArl-ill)L
S
NH
0
0
aNH
N
-175-
CA 02911326 2015-10-30
WO 2014/190199
PCT/US2014/039227
351 0 0 C C B ES-MS M/Z=372.9 (M+1)
Cl()L11-41A
S
NH
0
0
aNH
N
352 0 1 A C B ES-MS M/Z=395.0 (M+23)
C11()L11-41
S
NH
ON
353
arThN
._)
353 0 0 B C B ES-MS M/Z=359.9 (M+1)
01A
S
NH
0
ON
0
354 0 0 C C B ES-MS M/Z=374.0 (M+1)
A)1
)LC)
S
NH
0
ON
0
355 0 0 C C B ES-MS M/Z=359.0 (M+1)
(11()L11A
S
NH
0
ON
HN
-176-
CA 02911326 2015-10-30
WO 2014/190199
PCT/US2014/039227
356 0 0 C C B ES-MS M/Z=346.0 (M+1)
Clk)L1-41)LC)
S
NH
O i
ON
357 0 0 ES-MS M/Z=360.0 (M+1)
C11()L111A
S
NH
O i
ON
358 0 0 C C B ES-MS M/Z=360.0 (M+1)
0-41)L
S
NH
O N
CYNH
ON
359 0 0 C C B ES-MS M/Z=374.0 (M+1)
Clel-'11)LC)
S
NH
O N
CYNH
ON
360 0 0 ES-MS Mg=360.0(M+1)
OAC)
S
NH
O&
aNH
N
-177-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
361 0 0 ES-MS Mg=374.0(M+1)
____________
0)L
S
NH
0
ON
ONH
N
362 0 0 ES-MS Mg=385.0(M+1)
0)LC)
S
NH
0-N
NON
0
363 0 0 C C B ES-MS Mg=360.0(M+1)
111)L0
S
NH
0
0
ONH
N-r,µ
364 0 0 B C B ES-MS M/2=374.0 (M+1)
Ciell)L13
s
NH
0
0
ONH
N-N'
365 0 1 A C C ES-MS Mg=401.9(M+1)
CIJLI-sil
S
NH
0,.._N
s 0
-178-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
366 0 0 A B C ES-MS Mg=416.0(M+1)
el-41)L
S
NH
II
0.___S
N0r-
367 _ /70 C C B ES-MS M/Z=474 (M+1)
-7
r.c:2
o -..0
HN---0
S HN45
,0 co > µ s
N 0
368 N 5?DIS 0 C C B ES-MS M/Z=457 (M+1)
0 r- I OM- N
NH
0\o
(-)
N
C:$=\
3694 A C B ES-MS M/Z=482.9 (M+1)
N I-H NMR (400 MHz, DMSO-d6)
13.12 (s,
1H), 11.02 (s, 1H), 9.59 (d, J = 1.0 Hz,
s_ NH 1H), 8.70 (d, J = 5.6 Hz, 1H),
8.40 (dd, J
= 5.6, 1.0 Hz, 1H), 7.81 (d, J = 5.9 Hz,
.(.7--..5.1.(1=1
o o o.,..õ.---, ...---.õ---õ,
0
1H), 7.36 (s, 2H), 7.35 (d, J = 3.5 Hz,
y -,.....\-_- n
_.%
2H), 7.34 - 7.25 (m, 2H), 7.23 (d, J =
5.9 Hz, 1H), 4.56 (s, 2H), 4.40 -4.31
(m, 2H), 3.76- 3.71 (m, 2H).
370 z91 A C B ES-MS Mg=466(M+1)
o /GIN
s NH
calf:4
y 0 0
n
0 0 \ -......-
-179-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
371 A B C ES-MS Mg=482(M+1)
0.)N00 I-H NMR (400 MHz, DMSO-d6) 13.10 (s,
--"--S
1H), 11.00 (s, 1H), 8.44 - 8.20 (m, 2H),
7.81 (d, J = 5.9 Hz, 1H), 7.74 -7.62 (m,
,cii4iriiil 0
2H), 7.40 -7.33 (m, 4H), 7.32 -7.25
0 I ,,...._... (m, 1H), 7.21 (dd, J = 5.9,
0.8 Hz, 1H),
4.57 (s, 2H), 4.41 -4.30 (m, 2H), 3.81
-3.66 (m, 2H).
372 0 0 A C B ES-MS Mg=390(M+1)
(ii-a)Liri)L00
S
NH
0
-01s(1
rillg)N
373 0 0 C C B ES-MS Mg=389(M+1)
0)LC)A
S
NH
ONr--.\
Ng0
374
Np A C B ES-MS Mg=465(M+1)
1::4/CC2
.c.4llirirkiy0,0 0
--õ,õ---
375 _ nO C C B ES-MS Mg=456(M+1)
7
rk(1.7
o.-._0
0
tYc5>1 ir
N 0
-180-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
376 0 0 C C C ES-MS Mg=391(M+1)
I-H NMR (400 MHz, DMSO-d6) 11.46 (s,
laj)L 1H), 9.50 (s, 1H), 8.67 (s,
1H), 8.35 (d,
S
NH J = 4.7 Hz, 1H), 8.32 (d, J =
5.5 Hz, 1H),
O.,-S 7.35 (d, J = 5.9 Hz, 1H), 6.96
(dd, J =
Ngb 5.8, 0.8 Hz, 1H), 4.20 (q, J =
7.1 Hz,
2H), 2.76 (d, J = 4.5 Hz, 3H), 1.26 (t, J =
N 7.1 Hz, 3H).
377 0 0 C C B ES-MS Mg=373(M+1)
NH'
S
O\cN
Nb
378 0 0 C C B ES-MS Mg=375.9(M+1)
C11()L1-'11)L
S
NH
O N
Q's
0
379 0 0 C C B ES-MS Mg=398.8(M+1)
elliA
S
NH
0
ON
HN0
R o A C C ES-MS M/Z=417 (M+1)
380
I-H NMR (400 MHz, CD30D) 9.52 (s,
0--f
0 1H), 8.66 (d, J = 5.7 Hz, 1H), 8.35 (d, J
HN = 5.7 Hz, 1H), 7.59 (d, J =
5.9 Hz, 1H),
7.12 (d, J = 5.9 Hz, 1H), 5.34 - 5.23 (m,
NoS HN 0 1H), 2.05 - 1.94 (m, 2H), 1.93
- 1.78
:0> µ s, (m, 4H), 1.70 (m, 2H).
N 0
-181-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
381 C C C ES-MS M/Z=413 (M+1)
'H NMR (400 MHz, DMSO-d6) 12.87 (s,
Q 0 1H), 10.68 (s, 1H), 8.76 ¨8.54
(m, 2H),
0¨f
0 7.80 (dd, J = 9.2, 1.1 Hz,
1H), 7.72 (d, J
HN,._
= 5.9 Hz, 1H), 7.43 (ddd, J = 9.3, 6.8,
nN HN 1.3 Hz, 1H), 7.12 ¨7.03 (m, 2H), 4.81 rg?
µ
q
s ¨4.64 (m, 1H), 1.93 (m, 1H), 1.75 (d, J
N 0 = 10.5 Hz, 2H), 1.60 ¨ 1.18 (m, 7H).
382 A C C ES-MS M/Z=413 (M+1)
'H NMR (400 MHz, DMSO-d6) 12.87 (s,
C? 0 1H), 10.68 (s, 1H), 8.76 ¨8.54
(m, 2H),
0¨f
HN---.0 7.80 (dd, J = 9.2, 1.1 Hz,
1H), 7.72 (d, J
= 5.9 Hz, 1H), 7.43 (ddd, J = 9.3, 6.8,
1.3 Hz, 1H), 7.12 ¨7.03 (m, 2H), 4.81
OtS> El\N4)2'S ¨4.64 (m, 1H), 1.93 (m, 1H),
1.75 (d, J
N/----1k1 0 = 10.5 Hz, 2H), 1.60 ¨ 1.18
(m, 7H).
383 \ 0 C C C ES-MS M/Z=358 (M+1)
/N¨f 0
'H NMR (400 MHz, DMSO-d6) 12.88 (s,
HN__._ 1H), 10.02 (s, 1H), 8.65 (m,
2H), 7.76
(d, J = 9.2 Hz, 1H), 7.55 (d, J = 6.0 Hz,
tYNHN Q_
CD1 µ S 1H), 7.43 (m, 1H), 7.10 (d, J = 5.7 Hz,
0 1H), 7.06 (m, 1H), 2.99 (s, 6H).
384 \ 0 C C C ES-MS M/Z=376 (M+1)
/N¨f 0
'H NMR (400 MHz, DMSO-d6) 13.21 (s,
HN-.-- 1H), 10.19 (s, 1H), 9.57 (s,
1H), 8.69
(d, J = 5.5 Hz, 1H), 8.37 (d, J = 5.6 Hz,
S (0--
NoHN :0> µ s, 1H), 7.63 (d, J = 6.2 Hz, 1H), 7.26 (d, J
N 0 = 6.0 Hz, 1H), 3.00(s, 6H).
385 0/Th 0 C C C ES-MS M/Z=417 (M+1)
v._..../N¨f
HN-0 'H NMR (400 MHz, DMSO-d6)
13.15 (s,
1H), 10.23 (s, 1H), 8.38 ¨8.24 (m, 2H),
7.74 ¨ 7.65 (m, 2H), 7.63 (d, J = 5.9 Hz,
S HN415' 1H), 7.24 (d, J = 5.8 Hz, 1H), 3.68-3.62
0 0> µ S (m, 4H), 3.54¨ 3.47 (m, 4H).
N 0
386 0/ 0 C C C ES-MS M/Z=418 (M+1)
0 I-H NMR (400 MHz, DMSO-d6)
13.18
HNI__. (s, 1H), 10.26 (s, 1H), 9.57
(s, 1H), 8.70
(d, J = 5.5 Hz, 1H), 8.37 (d, J = 5.5 Hz,
S HN 0
c 1H), 7.64 (d, J = 5.9 Hz, 1H),
7.27 (d, J
co> µ s,
= 5.8 Hz, 1H), 3.69 ¨ 3.60 (m, 4H),
N 0
3.51 (m, 4H).
-182-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
387 0 0 B C C ES-MS Mg=378(M+1)
F.----(C)LN)L I-H NMR (400 MHz, CDCI3) 13.20
(s,
H 1H), 8.25 (d, J = 9.5 Hz, 1H),
8.02 (d, J
S
NH = 9.7 Hz, 1H), 7.91 (s, 1H),
7.64 (d, J =
0 i) 6.7 Hz, 1H), 7.13 (s, 1H),
7.01 (d, J =
6.1 Hz, 1H), 4.38 (q, J = 7.1 Hz, 2H),
'N
LgN 1.40 (t, J = 7.2 Hz, 3H).
388
NnO A C C ES-MS Mg=479(M+1)
(:) )------s
S
NH
H
0 0 Lo
389 0 0 B C C ES-MS Mg=395(M+1)
F__ <1O 'H NMR (400 MHz, DMSO-d6)
13.17 (s,
LNA
H 1H), 10.80 (s, 1H), 8.91 (dd,
J = 4.5, 1.7
S
NH Hz, 1H), 8.82 (d, J = 8.2 Hz,
1H), 7.67
C:1)1N (dd, J = 8.2, 4.5 Hz, 1H),
7.52 (s, 1H),
4.24 (q, J = 7.1 Hz, 2H), 1.41 (t, J = 7.2
sgb, Hz, 3H).
390 0 0 A C C ES-MS Mg=391(M+1)
1()1-411ACI I-H NMR (400 MHz, CDCI3) 13.11
(s,
1H), 8.93 (dd, J = 4.5, 1.7 Hz, 1H), 8.41
S
NH (dd, J = 8.1, 1.7 Hz, 1H),
7.81 (s, 1H),
43...-S 7.50 (dd, J = 8.2, 4.5 Hz,
1H), 7.13 (dd,
J = 5.9, 0.8 Hz, 1H), 7.01 (dd, J = 5.9,
Ngb 0.8 Hz, 1H), 5.16 (h, J = 6.2
Hz, 1H),
N 1.39 (d, J = 6.3 Hz, 6H).
391 0 0 A C C ES-MS Mg=409(M+1)
F----1(-NA 'H NMR (400 MHz, DMSO-d6) 13.17 (s,
H 1H), 10.75 (s, 1H), 8.92 (dd,
J = 4.4, 1.6
S
NH Hz, 1H), 8.84 (s, 1H), 7.68
(dd, J = 8.2,
0.-S 4.5 Hz, 1H), 7.53 (s, 1H),
5.00 (p, J =
6.3 Hz, 1H), 1.32 (d, J = 6.2 Hz, 6H).
Ngb
N
392 o01 - B C B ES-MS Mg=548(M+1)
rt
0,.._
I-H NMR (400 MHz, CDCI3) 12.94 (s,
K5
NH N 1H), 8.78 (s, 1H), 8.67 - 8.51
(m, 1H),
s i-cCi Lti 0 8.18 - 8.08 (m, 2H), 7.42 (dd,
J = 8.9,
rii ir,
2.1 Hz, 1H), 7.15 (d, J = 5.9 Hz, 1H),
6.95 (d, J = 5.8 Hz, 1H), 5.37 (s, 1H),
4.36 (q, J = 7.1 Hz, 2H), 4.01 (d, J = 5.3
Hz, 2H), 1.52 (s, 9H), 1.39 (t, J = 7.1
Hz, 3H).
-183-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
393 0 0 C C C ES-MS Mg=448(M+1)
I-H NMR (400 MHz, CD30D) 8.65 (d, J =
C11()L11:11)L 2.1 Hz, 1H), 8.15 (d, J = 8.9 Hz, 1H),
S
NH 7.61 (dd, J = 8.8, 2.0 Hz,
1H), 7.56 (d, J
ONN = 5.9 Hz, 1H), 7.09 (d, J =
5.9 Hz, 1H),
..-
S004.33 (q, J = 7.1 Hz, 2H), 3.96 (s, 2H),
1.38 (t, J = 7.1 Hz, 3H).
ONH
H2N
394 0 0 A C C ES-MS Mg=376.0(M+1)
OAC)
S
NH
0
ON
0
S
395 0 0 A C C ES-MS Mg=389.9(M+1)
I-H NMR: 12.68 (s, 1H), 10.81 (s, 1H),
Cl()11-41)L 9.17 (d, J = 2Hz, 1H), 9.09 (d, J = 3.6
S
NH Hz, 1H), 8.46 (d, J= 5.46 Hz,
1H), 7.74
0 (m, 2H), 7.12 (d, J = 5.6 hz,
1H), 4.99
ON (hept, J = 6.4 Hz, 1H), 1.31
(d, J = 6.4
Hz, 6H)
0
S
396 0 0 A C C ES-MS Mg=360.0(M+1)
I-H NMR: 13.10 (s, 1H), 10.89 (s, 1H),
A)(11A`) 8.07 (d, J = 7.6 Hz, 1H), 7.97 (d, J = 8
S
NH Hz, 1H), 7.80 (d, J = 6 Hz,
1H), 7.61 (m,
0.. N 2H), 7.22 (d, J = 6 Hz, 1H),
4.24 (q, J =
....-
00(Th 6.8 Hz, 2H), 1.31 (t, J= 6.8 Hz, 3H)
...}
397 0 0 A C C ES-MS Mg=373.9(M+1)
I-H NMR: 13.10 (s, 1H), 10.84 (s, 1H),
C11()11-41AC) 8.07 (d, J = 7.6 Hz, 1H), 7.97 (d, J = 8
S
NH Hz, 1H), 7.80 (d, J = 5.6 Hz,
1H), 7.61
0. (dtd, J = 28, 8.4, 1.2 Hz,
2H), 7.21 (d, J
N rTh = 6 Hz, 1H), 5.00 (hept, J = 6.4, 1H),
..) 6.12 (d, J = 8.4, 6.4, 1.2 Hz)
-184-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
398 0 0 A B C ES-MS Mg=371.0(M+1)
Clk)L11-41)LC) I-H NMR: 12.87 (s, 1H), 10.88
(s, 1H),
9.11 (q, J = 1.6 Hz, 2H), 8.66 (t, J = 4
S
NH Hz, 1H), 8.35 (d, J =1 .2,
2H), 7.77 (d, J
0 = 6Hz, 1H), 7.16 (d, J = 5.6
Hz, 1H),
0 4.24 (q, J = 7.2 Hz, 2H), 1.30
(t, J = 6.8
Hz, 3H)
NDN
399 0 0 A C C ES-MS Mg=385.0(M+1)
ClIerl-41)L I-H NMR : 12.88 (s, 1H), 10.83
(s, 1H),
9.12 (q, J = 1.6 Hz, 2H), 8.67 (t, J = 1.2
S
NH Hz, 1H), 8.35 (d, J = 0.8 Hz,
2H), 7.77
O (d, J = 6Hz, 1H), 7.15 (d, J = 6 Hz, 1H),
0 4.99 (hept, J = 6.4 Hz, 1H),
1.31 (d, J =
6.4 Hz, 6H)
N
ND
400 0 0 A C C ES-MS Mg=386.9(M+1)
r1-41)L
S
NH
O 0
00
0
401 0 0 A C C ES-MS Mg=401.0(M+1)
Cle11-1)LC) I-H NMR : 13.05 (s, 1H), 10.86
(s, 1H),
8.11 (dd, J = 7.6, 1.6 Hz, 1H), 7.98
S
NH (ddd, J = 7.6, 2, 1.6 Hz, 1H),
7.64 (m,
O 0 2H), 7.22 (d, J = 5.6 Hz,
1H), 7.01 (s,
00 1H), 5.00 (hept, J = 6 Hz,
1H), 1.32 (d, J
= 6 Hz, 6H)
0
402 0 0 A C C ES-MS Mg=360.9(M+1)
S
NH
0
0
ON
N-d
-185-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
403 0 0 A C C ES-MS Mg=375.0(M+1)
arl-s11A I-H NMR: 12.65 (s, 1H), 10.84 (s, 1H),
8.71 (t, J = 0.8 Hz, 1H), 8.31 (d, J = 10
S
NH Hz, 1H), 7.99 (d, J = 10 Hz,
1H), 7.74
0 (d, J = 6 Hz, 1H), 7.17 (d, J
= 6 Hz, 1H),
0 4.99 (hept, J = 6.4 Hz, 1H),
1.31 (d, J =
6.4 Hz, 6H)
0,N
N0-
404 0 0 A B C ES-MS Mg=417.0(M+1)
1()11-41A
S
NH
0
/ 0
0
O\
405 N B C C ES-MS Mg=383.9(M+1)
I-H NMR (400 MHz,DMSO-d6) 12.92 (s,
1H), 10.78 (s, 1H), 8.83 (s, 1H), 8.79
(dd, J = 7.2, 1.0 Hz, 1H), 8.67 -8.63
(m, 1H), 7.73 (d, J = 6.0 Hz, 1H), 7.36
(dd, J = 7.1, 1.6 Hz, 1H), 7.11 (d, J = 5.8
NH Hz, 1H), 4.23 (q, J = 7.1 Hz,
2H), 1.30
tir,ii 0
(t, J = 7.1 Hz, 3H).
y
0 0
406 0 0 A C C ES-MS Mg=430.8(M+1)
1()A F I-H NMR (400 MHz,DMSO-d6) 13.03 (s,
11 F<F 1H), 11.33 (s, 1H), 9.60 (s, 1H), 8.71
NH (d, J = 5.5 Hz, 1H), 8.39 (d, J = 5.5 Hz,
1H), 7.81 (d, J = 5.8 Hz, 1H), 7.26 (d, J
0,...-N
Sa N = 5.9 Hz, 1H), 4.92 (q, J = 9.1 Hz, 2H).
407 0 0 A B C ES-MS Mg= 393.1(M+1)
(11()11-'11)LCI I-H NMR (400 MHz,DMSO-d6) 1.26 (br.
s., 3 H) 4.12 -4.26 (m, 2 H) 7.06 - 7.15
S
NH (m, 1 H) 7.39 - 7.47 (m, 1 H)
7.48 -
0 7.58 (m, 1 H) 7.67 - 7.74 (m,
1 H) 7.95
S (--) - 8.05 (m, 1 H) 8.22 - 8.30 (m, 1 H)
_..) 10.76 - 10.92 (m, 1 H) 12.55 - 12.72
(m, 1 H)
F
-186-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
408 0 0 A B C ES-MS Mg= 407.1(M+1)
O'A'"' 'H NMR (400 MHz,DMSO-d6) 1.27
(d,J=5.87 Hz, 6 H) 4.95 (br. s., 1 H)
S
NH 7.05 - 7.16 (m, 1 H) 7.38 -
7.57 (m, 2
0 S F H) 7.70 (d,J=5.48 Hz, 1 H)
7.95 - 8.06
(i) (m, 1 H) 8.22 - 8.31 (m, 1 H) 10.73 -
U 10.85 (m, 1 H) 12.55 - 12.69 (m, 1 H)
409 0 0 A A B ES-MS Mg= 466.1(M+41)
C11()11-'11A I-H NMR (400 MHz,DMSO-d6) 1.28 -
1.36 (m, 3 H) 2.65 (s, 3 H) 4.09 - 4.21
S
NH (m, 2 H) 6.63 - 6.73 (m, 1 H)
7.13 -
0.-S 7.21 (m, 1 H) 8.61 - 8.70 (m,
1 H)
13.04- 13.13 (m, 1 H)
Ngrp__
CI
410 0 0 A B C ES-MS Mg= 389.9(M+1)
C11()11-41)L I-H NMR (400 MHz,DMSO-d6) 1.29
(d,J=6.26 Hz, 6 H) 4.92 - 5.01 (m, 1 H)
S
NH 7.14 - 7.21 (m, 1 H) 7.72 -
7.78 (m, 1
0 H) 8.39 - 8.43 (m, 1 H) 8.44 -
8.48(m, 1
0 H) 8.70 - 8.76 (m, 1 H) 9.70
(s, 1 H)
10.83 - 10.90 (m, 1 H) 12.76 - 12.84
ON
S.--/ (m, 1 H)
\/- A B B ES-MS Mg= 528(M+23)
411
I-H NMR (400 MHz,DMSO-d6) 13.09(s,
0
1:3 1H), 10.85 (s, 1H), 8.70 (s,
1H), 8.52 (s,
HN 1H), 7.75 (d, J = 5.87 Hz,
1H), 7.56-
N--- 7.63 (m, 1H), 7.19 (d, J = 5.48 Hz, 1H),
4.35 (d, J = 5.48 Hz, 2H), 4.16-4.25 (m,
c; 2H), 1.32-1.45 (m, 6H), 1.27
(t, J =
0 N 7.04 Hz,3H).
0"---S
NH
Sc;l.rH 0
Ny
0 0
-187-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
412 0 0 A C A ES-MS Mg= 406.1(M+1)
I-H NMR (400 MHz,DMSO-d6) 1.29 _
0')L 1.38 (m, 3 H) 3.96 (s, 3 H) 4.30 (m, 2
S
NH H) 7.24 - 7.26 (m, 1 H) 7.35
(d,J=8.40
(3 N Hz, 1 H) 7.83(d,J=6.0 Hz, 1 H)
7.93 (s, 1
)_-
S r- H) 8.26 (d,J=8.8 Hz, 1 H)
10.92 (s, 1 H)
..) 13.07 (s, 1 H)
0
/
413 0 0 A C A ES-MS Mg= 439.9(M+23)
I-H NMR (400 MHz,DMSO-d6) 1.29 (t,
0)L J=7.04 Hz, 3 H) 1.44 (d, J=7.04 Hz, 6 H)
S
NH 3.44 - 3.53 (m, 1 H) 4.15 -
4.28 (m, 2
O H) 7.11 (d, J=5.87 Hz, 1
H)7.69 - 7.80
0 (m, 1 H) 7.89 - 8.03 (m, 1 H)
8.28 -
8.37 (m, 1 H) 8.42 - 8.52 (m, 1 H)
NOS 10.86 (s, 1 H) 12.69 - 12.84 (m, 1 H).
.-_5........
414 0 0 A C A ES-MS Mg= 437.8(M+23)
I-H NMR (400 MHz,DMSO-d6) 1.17 -
11-41)L 1.41 (m, 7 H) 2.55 - 2.64 (m, 1 H) 4.22
S
NH (d, J=7.04 Hz, 2 H) 7.10 (d,
J=5.48 Hz,
O 1 H) 7.74 (d, J=5.87 Hz, 1
H)7.92 (s, 1
0 H) 8.22 - 8.30 (m, 1 H) 8.34
(s, 1 H)
10.86 (s, 1 H) 12.62 - 12.81 (m, 1 H).
OS
415 0 0 C C A ES-MS Mg= 431.9(M+1)
I-H NMR (400 MHz,DMSO-d6) 1.26 (t,
0-4IA J=7.04 Hz, 3 H) 1.47 (s, 9 H) 4.11 -4.30
S
NH (m, 2 H) 7.08 (d, J=5.48 Hz, 1
H) 7.71
O (d, J=5.87 Hz, 1 H) 7.94 (d,J=7.43 Hz, 1
0 H) 8.30 (d, J=8.22 Hz, 1 H)
8.43 (s, 1 H)
10.73 - 10.93 (m, 1 H) 12.70 - 12.79
OS (m, 1 H).
14.-........
-188-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
416 0 0 B C A ES-MS Mg= 427.9(M+23)
C11()LII)L I-H NMR (400 MHz,DMSO-d6) 13.16 (s,
1H), 10.89(s, 1H), 8.93 (d, J = 5.2 Hz,
S
NH 2H), 8.67(s, 3H), 7.79 (d, J =
6 Hz, 1H),
O,--S 7.22 (d, J = 6 Hz, 1H),
4.31(d, J = 5.6
Ngqir-- Hz, 2H), 4.22 (m, 2H), 1.30 (t, J = 7.2
..J Hz, 3H).
H2N
417 0 0 ES-MS Mg= 390(M+1)
CZ)11-'11)L() 'H NMR (400 MHz,DMSO-d6) 1.18 -
1.34 (m, 3 H) 2.30 - 2.46 (m, 3 H) 4.20
S
NH (q,J=7.04 Hz, 2 H) 7.12 - 7.19
(m, 1 H)
O__-N 7.49 (d,J=8.61 Hz, 1 H) 7.74(d,J=5.87
Or Hz, 1 H) 8.06 (s, 1 H) 8.16 (d,J=8.22 Hz,
S
..J 1 H) 10.84 (s, 1 H) 13.02 (s, 1 H)
418 / ES-MS Mg= 450.1(M+1)
0
I-H NMR (400 MHz,DMSO-d6) 1.14 -
1.40 (m, 3 H) 3.29 (s, 3 H) 3.60 - 3.76
0 (m, 2 H) 4.05 - 4.44 (m, 4 H)
7.06 -
7.35 (m, 2 H) 7.65 - 7.90 (m, 2 H)8.15
s00 (d,J=9.00 Hz, 1 H) 10.83 (s, 1 H) 12.87
- 13.15 (m, 1 H)
O)--141
NH
ti.rH 0
Ny
0 0
419 0 0 ES-MS M/Z= 389.1(M+1)
C11()LII)L 1-1-1NMR (400 MHz,DMSO-d6) 12.59 (s,
1H), 10.83 (s, 1H), 7.90- 8.09 (m, 3H),
S
NH 7.71 (d, J = 4 Hz, 1H),
7.36(d, J = 8 Hz,
O S 1H), 7.09(d, J = 4 Hz, 1H),
4.17-4.21(m,
Oc-- 2H), 2.42 (s, 3H), 1.26 (t, J = 6 Hz, 3H).
U
-189-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
420 0 0 ES-MS Mg= 417.2(M+1)
-4IA I-H NMR (400 MHz,DMSO-d6) 12.67(s,
1H), 10.86 (s, 1H), 8.83 (s, 1H),8.22-
0
NH 8.33 (m,2H), 8.02 (d, J = 8
Hz, 1H),
O S 7.72(d, J = 8 Hz, 1H), 7.11(d,
J = 8 Hz,
0c-- 1H), 4.17-4.23(dd, J = 8, 16 Hz, 2H),
U 2.65(s, 3H), 1.27(t, J = 6 Hz, 3H).
0
421 / ES-MS Mg= 485.9(M+23)
C)o I-H NMR (400 MHz,DMSO-d6)
13.08 (s,
HN 1H), 10.85 (s, 1H), 8.71 (s, 1H), 8.54(s,
1H), 7.82-7.90 (m, 1H), 7.75(d, J = 6.0
Hz, 1H), 7.17 (d, J = 5.6 Hz, 1H), 4.40
N--- (d, J = 5.6Hz, 2H), 4.20 (dd, J = 6.8 Hz,
2H), 3.55(s, 3H), 1.27 (t, J = 8.8 Hz,
O)---()S N
3H).
NH
,c0ii 0
Ny
0 0
422 0 0 ES-MS Mg= 447.9(M+1)
0:11)L 11-INMR (400 MHz,DMSO-d6) 13.09 (s,
1 H), 10.86 (s, 1 H), 8.71 (s, 1H), 8.54
S
NH (d, J = 6.4Hzõ 2 H), 7.74 -
7.78 (m, 1
ONr.-S H),7.17 - 7.21(m, 1 H), 4.45 - 4.48(d, J
= 5.6 Hz, 2H), 4.17 - 4.23(m, 2 H), 1.89
Ngtrk?ir
_.} (s, 3 H)1.25 - 1.29(t, J = 6.8 Hz, 3H)
HN
/0
423 0 0 ES-MS Mg= 416.1(M+1)
(11()1-411ACI I-H NMR (400 MHz, CDCI3) 1.31 - 1.41
(m, 3 H) 1.50 (s, 9 H) 4.23 -4.42 (m, 2
S
NH H) 6.72 - 6.92 (m, 1 H) 6.99 -
7.09 (m,
O 1 H) 7.51 - 7.66 (m,1 H) 7.76 - 7.88 (m,
0 1 H) 7.98 - 8.12 (m, 1 H) 8.36
- 8.54
(m, 1 H) 12.51 - 12.83 (m, 1 H).
Is1.-.
0.......
-190-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
424 0 0 ES-MS Mg= 399.9(M+1)
()L11:11A(3 I-H NMR (400 MHz, CDCI3) 1.16 (d,
J=5.48 Hz, 2 H) 1.21 - 1.38 (m, 5 H)
S
NH 2.09 - 2.24 (m, 1 H) 4.28 (d,
J=7.04 Hz,
0 2 H) 6.82 (d, J=5.87Hz, 1 H)
6.99 (d,
0 J=5.87 Hz, 1 H) 7.38 - 7.53
(m, 1 H)
7.74 - 7.87 (m, 1 H) 7.87 - 7.99 (m, 1
0
NI. H) 8.23 - 8.39 (m, 1 H) 12.13 - 12.88
(m, 1 H).
425 0 0 ES-MS Mg= 401.9(M+1)
I-H NMR (400 MHz, CDCI3) 1.36 (s, 2 H)
'Cl1()L1:11)L(3 1.47 (d, J=6.65 Hz, 6 H) 3.15 - 3.38 (m,
S
NH 1 H) 4.15 - 4.44 (m, 1 H) 6.88
(s, 1 H)
0 7.04 (d, J=5.87Hz, 1 H) 7.58
(s, 1 H)
0 7.83 - 7.92 (m, 1 H) 7.95 -
8.08 (m, 1
H) 8.33 - 8.50 (m, 1 H) 12.54 - 12.77
0
N (m, 1 H).
--...5.__
426 0 0 ES-MS Mg= 395.9(M+23)
I-H NMR (400 MHz, CDCI3) 1.31 (s, 3 H)
0')L 2.63 (s, 3 H) 4.28 (d, J=7.04 Hz, 2 H)
S
NH 6.80 - 6.85 (m, 1 H) 6.97 -
7.01 (m, 1
0 H) 7.48 - 7.55 (m, 1H) 7.79 -
7.84 (m, 1
0 H) 7.93 - 8.01 (m, 1 H) 8.32 -
8.38 (m,
1 H) 12.53 - 12.60 (m, 1 H).
0
N--c
427 0 0 ES-MS Mg= 473.2(M+1)
I-H NMR (400 MHz,DMSO-d6) 0.91
A)L11:11A (d,J=6.65 Hz, 3 H) 1.26 (0=7.04 Hz, 5
S
NH H) 1.56 (br. s., 1 H) 1.68
(d,J=12.52 Hz,
O N 2 H) 2.82 (0=11.54 Hz, 2
H)3.87
r.-
(d,J=12.52 Hz, 2 H) 4.19 (q,J=7.04 Hz,
S r
2 H) 7.13 (d,J=5.48 Hz, 1 H) 7.36
(d,J=7.43 Hz, 1 H) 7.63 (s, 1 H) 7.72
N (d,J=5.87 Hz, 1 H) 8.03 (d,J=9.39Hz, 1
----
\----c
H) 10.82 (s, 1 H) 12.83 - 12.98 (m, 1 H)
-f
HN.--? B C A ES-MS Mg=416 (M+1)
428 CN
'H NMR (400 MHz,DMSO-d6) 13.23 (s,
1H), 10.18 (s, 1H), 9.59 (s, 1H), 8.70
S 7 (d, J = 5.5 Hz, 1H), 8.40 (d,
J = 5.6 Hz,
C> 43:
0 , s 1H), 7.64 (d, J = 5.9 Hz, 1H),
7.26 (d, J
= 5.8 Hz, 1H), 3.68 (m, 4H), 1.70 -
N ..,..,...,,.,....,N 0
1.47 (m, 6H).
-191-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
429 C C A ES-MS M/Z=373 (M+1)
I-H NMR (400 MHz,DMSO-d6) 12.28 (s,
R o
o 1H), 8.33 (d, J = 7.8Hz, 1H), 8.26 (d, J =
8.5 Hz, OH), 7.70 (dddd, J = 20.5, 8.3,
S HN 1 7.2, 1.3 Hz, 2H), 7.29 (d, J =
5.7 Hz,
0 0> S 1H), 7.22 (d, J = 5.7 Hz, 1H), 5.49 ¨
N 0 5.36 (m, 1H), 1.99 (m,
1H), 1.95 ¨ 1.77
(m, 3H), 1.67 (m, 1H).
430 C C A ES-MS M/Z=387 (M+1)
'H NMR (400 MHz,DMSO-d6) 12.37 (s,
(I? 0 1H), 8.33 (d, J = 7.4 Hz, 1H), 8.26 (d, J
0
= 7.5 Hz, 1H), 7.71 (dt, J = 13.8, 7.5 Hz,
S HN 1 2H), 7.31 (d, J = 5.7 Hz, 1H),
7.23 (d, J
0 (3> S' = 5.7 Hz, 1H), 5.06 (tt, J = 8.7, 3.8 Hz,
N 0 1H), 2.04¨ 1.88 (m, 2H),
1.86 ¨ 1.71
(m, 2H), 1.62 (m, 3H), 1.49 ¨ 1.31 (m,
3H).
431 A C B ES-MS M/Z=421 (M+1)
so91 I-H NMR (400 MHz,DMSO-d6) 13.08 (s,
1H), 11.24 (s, 1H), 9.60 (s, 2H), 8.71
0.)--"N
(d, J = 5.8 Hz, 1H), 8.39 (d, J = 5.7 Hz,
NH
0 1H), 7.82 (d, J = 6.0 Hz, 1H),
7.26 (d, J
clrisiiyo ii
-20 = 5.8 Hz, 1H), 4.85 (s, 2H),
3.74 (s, 3H).
O0
432 \ 0 C C B ES-MS M/Z=320 (M+1)
01_
'H NMR (400 MHz,DMSO-d6) 12.48 (s,
1H), 9.60 (s, 1H), 8.71 (d, J = 5.6 Hz,
S 0
O HN
D> ¨c 1H), 8.41 (d, J = 5.6, 1H),
7.33 (d, J =
N 0 5.7 Hz, 1H), 7.26 (d, J = 5.7 Hz, 1H),
3.94 (s, 3H).
433 N B C B ES-MS M/Z=398 (M+1)
II-H NMR (400 MHz,DMSO-d6) 12.91 (s,
1H), 10.72 (s, 1H), 9.41 (d, J = 1.6 Hz,
1H), 8.70 (s, 1H), 7.99 (d, J = 9.5 Hz,
1H), 7.73 (d, J = 6.0 Hz, 1H), 7.70 (dd, J
= 9.5, 1.7 Hz, 1H), 7.10 (d, J = 5.9 Hz,
NH 1H), 6.54 (s, OH), 4.98 (p, J
= 6.3 Hz,
1H), 1.30 (d, J = 6.2 Hz, 6H).
y--.....----
O 0
-192-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
434 0 0 C C B ES-MS M/Z=430.9 (M+1)
cll()A F I-H NMR (400 MHz,DMSO-d6)
13.09 (s,
11 F`F 1H), 11.31 (s, 1H), 8.92 (dd, J = 4.5, 1.7
NH Hz, 1H), 8.84 (dd, J = 8.2,
1.7 Hz, 1H),
(:)._-N7.82 (d, J = 5.9 Hz, 1H), 7.69 (dd, J =
8.2, 4.5 Hz, 1H), 7.26 (d, J = 5.8 Hz,
SPOI 1H), 4.93 (q, J = 9.1 Hz, 2H).
435 OS C C B ES-MS M/Z=387.9 (M+1)
N.....,(
HN---0 'H NMR (400 MHz,DMSO-d6) 13.18
(s,
1H), 9.66 (s, 1H), 8.73 (d, J = 5.6 Hz,
1H), 8.47 (s, 1H), 7.98 (d, J = 5.7 Hz,
NoSHN-o- 1H), 7.62 (d, J = 3.6 Hz, 1H), 7.38 (d, J
co> µ s- , 3.6 Hz, 1H), 7.30 (d, J = 5.8 Hz, 1H).
N 0
436 0 0 C C B ES-MS Mg=407(M+1)
I-H NMR (400 MHz,DMSO-d6) 13.18 (s,
.) H 1H), 11.00 (s, 1H), 8.92 (dd,
J = 4.6, 1.7
S
NH Hz, 1H), 8.83 (dd, J = 8.2,
1.7 Hz, 1H),
1::) 7.82 (d, J = 5.9 Hz, 1H), 7.68
(dd, J =
Nr_.-N
8.2, 4.6 Hz, 1H), 7.23 (d, J = 5.9 Hz,
SPC 1H), 4.37 -4.22 (m, 2H), 3.70 - 3.52
(m, 2H), 3.30 (s, 3H).
g=482.9(M+1)
N-jcbD A B C ES-MS M 'H NMR (400 MHz,DMSO-
d6) 13.18 (s,
437
o.o
)----s
1H), 11.00 (s, 1H), 8.92 (dd, J = 4.6, 1.7
Hz, 1H), 8.83 (dd, J = 8.2, 1.7 Hz, 1H),
c4iiiri4ilroo
7.81 (d, J = 5.9 Hz, 1H), 7.68 (dd, J =
o o 0 8.2, 4.5 Hz, 1H), 7.36 (s,
2H), 7.35 (d, J
= 1.9 Hz, 2H), 7.34 - 7.25 (m, 1H),
7.23 (d, J = 5.8 Hz, 1H), 4.55 (s, 2H),
4.40 -4.31 (m, 2H), 3.76- 3.70 (m,
2H).
438 0 0 A C B ES-MS Mg=436.9(M+1)
0')L 'H NMR (400 MHz,DMSO-d6) 12.93
(s,
1H), 10.79 (s, 1H), 9.39 (t, J = 1.4 Hz,
S
NH 1H), 8.88 (s, 1H), 8.04 (d, J
= 9.6 Hz,
0.Ncci5,1 1H), 7.82 (dd, J = 9.6, 1.9 Hz, 1H), 7.74
(d, J = 5.9 Hz, 1H), 7.11 (d, J = 5.8 Hz,
NO 1H), 4.23 (q, J = 7.1 Hz, 2H), 1.30 (t, J =
7.1 Hz, 3H).
0"-S\--0
-193-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
0 0 C C B ES-MS M/Z=450.9(M+1)
439 c=LNA0 I-H NMR (400 MHz,DMSO-d6)
12.93 (s,
H 1H), 10.72 (s, 1H), 9.48 -9.28 (m, 1H),
S
NH 8.87 (s, 1H), 8.03 (d, J = 9.5
Hz, 1H),
0c131 7.81 (dd, J = 9.6, 1.9 Hz, 1H), 7.73 (d, J
= 6.0 Hz, 1H), 7.10 (d, J = 5.8 Hz, 1H),
No 4.98 (p, J = 6.4 Hz, 1H), 3.36
(s, 3H),
1.31 (d, J = 6.3 Hz, 6H).
0"-S\--
440 C C B ES-MS M/Z=382.9(M+1)
N\_____ JON
A o 'H NMR (400 MHz,DMSO-d6) 13.30 (s,
1H), 11.10 (s, 1H), 9.64 (s, 1H), 9.45
HN__
41 N (d, J = 1.5 Hz, 1H), 8.71 (d, J = 5.6 Hz,
q
1H), 8.57 -8.52 (m, 1H), 8.49 (d, J =
S
NO S >
2.6 Hz, 1H), 8.42 (d, J = 5.6 Hz, 1H),
....,.........--N 0 8.00 (d, J = 5.9 Hz, 1H), 7.30
(d, J = 5.8
Hz, 1H).
441
ON A C B ES-MS M/Z=382.9(M+1)
I-H NMR (400 MHz,DMSO-d6) 13.37 (s,
N---(
0
HN 1H), 11.06 (s, 1H), 9.57 (s,
1H), 8.82
(d, J = 4.8 Hz, 2H), 8.69 (d, J = 5.5 Hz,
s HN 1 1H), 8.37 (d, J = 5.3 Hz, 1H),
8.16 (s,
NOµ s, 2H), 7.95 (s, 1H), 7.91 (d, J
= 6.0 Hz,
N 0 1H), 7.36 (t, J = 4.9 Hz, 1H),
7.27 (d, J =
6.0 Hz, 1H).
442 B C B ES-MS M/Z=382.9(M+1)
014(14 I-H NMR (400 MHz,DMSO-d6)
13.26 (s,
0 1H), 11.42 (s, 1H), 9.56 (s, 1H), 9.09
HN
HN
(dd, J = 4.7, 1.5 Hz, 1H), 8.69 (d, J = 5.6
Hz, 1H), 8.42 (dd, J = 9.0, 1.4 Hz, 1H),
S --1.----D
Noco> µ s, 8.40 -8.37 (m, 1H), 8.05 (d, J
= 5.9 Hz,
N 0 1H), 7.83 (dd, J = 8.9, 4.7
Hz, 1H), 7.31
(d, J = 5.9 Hz, 1H).
443 0 0 A C ES MS M/Z=391 (M+1)
0A I-H NMR (400 MHz, CD30D) 9.59
(d, J =
0.9 Hz, 1H), 8.69 (d, J = 5.9 Hz, 1H),
S
NH 8.46 (dd, J = 5.8, 0.9 Hz, 1H), 7.21 (d, J
0=Nr.-N = 1.2 Hz, 1H), 4.32 (q, J =
7.2 Hz, 2H),
2.48 (d, J = 1.2 Hz, 3H), 1.39 (t, J = 7.1
sgibN Hz, 3H).
444 \ 0 B C ES MS M/Z=314 (M+1)
:1 'H NMR (400 MHz, CD30D) 8.63
(dd, J
S 11) = 4.9, 1.9 Hz, 1H),
8.54 (dd, J = 7.9, 1.9
Hz, 1H), 8.29 - 8.25 (m, 1H), 8.17 (dd,
N 0 J = 8.0, 1.3Hz, 1H), 7.66
(ddd, J = 8.3,
7.2, 1.3 Hz, 2H), 7.36 (d, J = 7.9 Hz,
1H), 4.07 (s, 3H).
-194-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
445 0 0 A C ES-MS Mg=411(M+1)
A I-H NMR (400 MHz, CD30D) 9.43
(d, J =
C I 1C----Z)L
H 0.9 Hz, 1H), 8.56 (d, J = 5.7 Hz, 1H),
S
NH 8.26 (dd, J = 5.7, 1.0 Hz, 1H), 7.44 (s,
ONi._N 1H), 4.20 (q, J = 7.1 Hz, 2H),
1.27 (t, J =
S N 7.1 Hz, 3H).
446 0 0 A B ES MS M/Z=391 (M+1)
I-H NMR (400 MHz, CD30D) 8.86 (dd, J
= 4.6, 1.6 Hz, 1H), 8.71 (dd, J = 8.2, 1.6
S
NH Hz, 1H), 7.64 (dd, J = 8.2,
4.6 Hz, 1H),
0Nr.-N 7.19 (s, 1H), 4.31 (q, J = 7.1 Hz, 2H),
2.48 (s, 3H), 1.38 (t, J = 7.1 Hz, 3H).
Sgb,
447 0 0 B C ES-MS M/Z=417.9 (M+1)
Cl'el:11A
S
NH
0
ON
0
CI
448 0 0 A B ES-MS M/Z=376.9 (M+1)
()Ii'lA
s
NH
0
0
NaN
S
449 0 0 1 A B ES-MS M/Z=390.9 (M+1)
A
S
NH
0
0
0 N
N-s,
-195-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
450 0 0 C C ES-MS M/Z=404 (M+1)
____________
0111
S
NH
0
ON
0
CI
451 0 0 A B ES-MS M/Z=360 (M+1)
0A
S
NH
OV-)N)
N-N
452 0 0 1 A C ES-MS M/Z=374 (M+1)
A
NH
0
XP-)Ni
\
N-N
453 NO c C ES-MS Mg=447.9(M+1)
N 'H NMR (400 MHz,DMSO-d6) 13.44
(s,
CYNH 1H), 11.08 (s, 1H), 9.57 (s,
1H), 8.84
(d, J = 6.6 Hz, 1H), 8.70 (d, J = 5.5 Hz,
HN,,s.0
1H), 8.39 (dd, J = 5.6, 1.0 Hz, 1H), 8.19
(s, 2H), 7.89 (s, 1H), 7.38 (s, 1H), 7.31
NebS) 11(\N (d, J = 5.8 Hz, 1H).
454
NC C ES-MS Mg=381.9(M+1)
I-H NMR (400 MHz,DMSO-d6) 13.38 (s,
HN._.0 1H), 10.79 (s, 1H), 9.59 (s,
1H), 8.70
(d, J = 5.5 Hz, 1H), 8.48 -8.43 (m, 1H),
8.39 (d, J = 5.5 Hz, 1H), 8.20 (d, J = 8.4
Ncl> "<\144Is Hz, 1H), 8.01 (d, J = 5.9 Hz,
1H), 7.97 -
---N 0 7.88 (m, 1H), 7.28 (d, J = 6.2
Hz, 1H),
7.26 - 7.23 (m, 1H).
-196-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
455 C C ES-MS M/Z=388.9(M+1)
I-H NMR (400 MHz,DMSO-d6) 13.75 (s,
HN-- 1H), 9.71 (s, 1H), 8.74 (d, J
= 5.7 Hz,
'7 1H), 8.71 (d, J = 5.9 Hz, 1H),
8.51 (d, J
r-s> "(5--
Nd-- \ S = 5.7 Hz, 1H), 7.65 (d, J =
5.8 Hz, 1H),
/--N 0 7.24 (d, J = 5.8 Hz, 1H), 4.06
-4.00 (m,
1H), 3.81 (dt, J = 8.1, 6.5 Hz, 1H), 3.71
-3.60 (m, 1H), 3.39 (dd, J = 6.2, 4.7
Hz, 2H), 2.03 - 1.90 (m, 1H), 1.90 -
1.77 (m, 2H), 1.61 (ddt, J = 11.6, 8.2,
6.6 Hz, 1H).
456 )1\ B C ES-MS M/Z=371.9(M+1)
0 I-H NMR (400 MHz,DMSO-d6)
13.79 (s,
HN OH), 9.61 (s, 1H), 8.71 (d, J
= 5.6 Hz,
1H), 8.43 (dd, J = 5.5, 1.0 Hz, 1H), 8.04
erS)¨µ
HN q
N S (s, 1H), 7.68 (s, 1H), 7.31
(d, J = 1.2 Hz,
1H), 7.26 (d, J = 5.8 Hz, 1H).
/N 0
457 0 0 A B ES-MS M/Z=391 (M+1)
Crilli
S
NH
ONI...._N
Sgtisirm
..}
458 0 0 C C ES-MS M/Z=377 (M+1)
c,eirlA I-H NMR (400 MHz,DMSO-d6)
13.14 (s,
1H), 9.32 (s, 1H), 8.64 (d, J = 5.6 Hz,
S
NH 1H), 8.02 (d, J = 5.5 Hz, 1H),
7.20 (d, J
O)1N = 5.8 Hz, 1H), 6.72 (d, J = 5.9 Hz, 1H),
s4.20(q, J = 7.1 Hz, 2H), 1.36 (t, J = 7.1
Hz, 3H).
N
459 0 0 ES-MS M/Z=413 (M+1)
A F
1-11 F<F
NH
ONcc1311
Nb
-197-
CA 02911326 2015-10-30
WO 2014/190199 PCT/US2014/039227
460 N ES-MS M/Z=398 (M+1)
Ii
1.
NO
0 N
...c)
s NH
1:11 0
y
0 0
-198-