Note: Descriptions are shown in the official language in which they were submitted.
CA 2912393 2017-05-23
REDUCING SUGAR-BASED SULFIDE SCAVENGERS AND METIIODS OF USE IN
SUBTERRANEAN OPERATIONS
BACKGROUND
[0001] This application claims priority to U.S. Patent Application Serial No.
13/927,714, filed June 26, 2013.
[0002] The present disclosure relates to fluids, additives, and methods for
use in
subterranean operations, and more specifically, to improved methods and
additives for
eliminating or reducing concentrations of hydrogen sulfide or soluble sulfide
ions for use in
subterranean formations and fluids.
[0003] Hydrocarbon producing wells may contain many different formation
liquids and gases such as methane, ethane, and other higher hydrocarbons, as
well as hydrogen
sulfide, water, and other compounds. In order to evaluate the commercial value
of a hydrocarbon
producing well, or as an aid in operations and well planning, it is often
useful to obtain
information by analyzing the component concentrations of the produced fluid
from a formation
or an individual well. Numerous systems have been developed to evaluate a
downhole fluid
composition and the relative component concentrations in the downhole fluid.
[0004] Hydrogen sulfide (H2S) is a very toxic, flammable, and pungent gas that
causes numerous problems in various aspects of the oil and gas industry. H2S
is extremely
corrosive to metal, which may damage or destroy tubing, casings, or other
types of well bore
equipment. H2S also presents health risks to operations personnel that may be
exposed to 112S
gas at a well site or in processing of well bore fluids. Severe iron sulfide
scaling may also choke
production, either in the production piping, perforations or within the
producing formation itself.
Thus, it is typically desirable to reduce or eliminate sulfides from
subterranean formations and
well bores, among other reasons, to control corrosion rates and to plan for
safe development and
production of the hydrocarbons.
[0005] The release of H2S gas can sometimes be controlled by maintaining the
pH
of the fluid containing H2S above 10. However, in many cases, it is not
practical or possible to
maintain this level pH in a fluid for extended periods of time. Sulfide
scavengers are often used
to react with H2S and convert it to a more inert form. Conventional 112S
scavengers include
certain aldehydes, certain amine-based chemicals, triazines, copper compounds,
hydrogen
peroxide, zinc compounds, and iron compounds. However, the reaction products
of many of
these compounds with H2S are poorly soluble in treatment fluids and/or fluids
in the well bore,
1
CA 02912393 2015-11-12
WO 2014/209639 PCT/US2014/042361
or may decompose, thereby releasing H2S. Moreover, many conventional sulfide
scavengers
themselves may have undesirable environmental and/or toxicity problems, and as
such may be
impractical to use or prohibited altogether in certain circumstances and/or
jurisdictions.
2
CA 02912393 2015-11-12
WO 2014/209639 PCT/11S2014/042361
SUMMARY
[0006] The present disclosure relates to fluids, additives, and methods for
use in
subterranean operations, and more specifically, to improved methods and
additives for
eliminating or reducing concentrations of hydrogen sulfide or soluble sulfide
ions for use in
subterranean formations and fluids.
[0007] In one embodiment, the present disclosure provides a method comprising:
providing a treatment fluid comprising a base liquid and a sulfide scavenging
additive
comprising one or more reducing sugars; introducing the treatment fluid into
at least a portion of
a subterranean formation; and allowing at least a portion of the sulfide
scavenging additive to
interact with hydrogen sulfide or sulfide ions present in the treatment fluid
to produce a
precipitate comprising one or more sulfur species.
[0008] In another embodiment, the present disclosure provides a method
comprising: providing a treatment fluid comprising a base liquid and a sulfide
scavenging
additive comprising one or more reducing sugars chelated with one or more
metal ions;
introducing the treatment fluid into at least a portion of a subterranean
formation; allowing at
least one of the metal ions to interact with hydrogen sulfide or sulfide ions
present in the
treatment fluid to produce a first product comprising one or more sulfur
species; and allowing
the reducing sugar to interact with hydrogen sulfide or sulfide ions present
in the treatment fluid
to produce a second product comprising one or more sulfur species.
[0009] In another embodiment, the present disclosure provides a method of
treating a fluid comprising a first concentration of hydrogen sulfide or
sulfide ions, the method
comprising: adding a sulfide scavenging additive comprising one or more
reducing sugars to the
fluid; and allowing at least a portion of the sulfide scavenging additive to
interact with at least a
portion of the hydrogen sulfide or sulfide ions in the fluid to reduce the
concentration of
hydrogen sulfide or sulfide ions to a second concentration that is lower than
the first
concentration.
[0010] The features and advantages of the present disclosure will be readily
apparent to those skilled in the art. While numerous changes may be made by
those skilled in
the art, such changes are within the spirit of the disclosure.
3
CA 02912393 2015-11-12
WO 2014/209639 PCT/US2014/042361
BRIEF DESCRIPTION OF THE FIGURES
[0011] Some specific example embodiments of the disclosure may be understood
by referring, in part, to the following description and the accompanying
drawings.
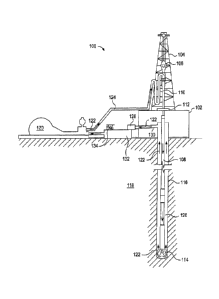
[0012] Figure 1 illustrates an example of a well bore drilling assembly that
may
be used in accordance with certain embodiments of the present disclosure.
[0013] While the present disclosure is susceptible to various modifications
and
alternative forms, specific example embodiments have been shown in the figures
and are herein
described in more detail. It should be understood, however, that the
description of specific
example embodiments is not intended to limit the invention to the particular
forms disclosed. On
the contrary, this disclosure is to cover all modifications and equivalents as
illustrated, in part, by
the appended claims.
4
CA 02912393 2015-11-12
WO 2014/209639 PCT/US2014/042361
DESCRIPTION OF PREFERRED EMBODIMENTS
[0014] The present disclosure relates to fluids, additives, and methods for
use in
subterranean operations, and more specifically, to improved methods and
additives for
eliminating or reducing concentrations of hydrogen sulfide or soluble sulfide
ions for use in
subterranean formations and fluids.
[0015] The fluids and sulfide scavenging additives of the present disclosure
generally comprise one or more reducing sugars. The term "reducing sugar" is
defined herein to
include any saccharide that includes an aldehyde functional group or can
isomerize to form an
aldehyde functional group in basic solution. In certain embodiments, the
fluids and sulfide
scavenging additives of the present disclosure may be substantially free of
compounds having
aldehyde functional group prior to placement in basic solution. In certain
embodiments, the
reducing sugar may be chelated with a metal ion, such as iron. When added to a
fluid
comprising a liquid, a chelated reducing sugar may interact with H2S and/or
sulfide ions present
in the fluid to produce one or more sulfur species (e.g., metal sulfides (such
as Fe2S3 and FeS)
and elemental sulfur), inter alia, in the form of a precipitate that can be
removed from the liquid.
In certain embodiments, this may be accomplished without further oxidization
of the metal ions.
[0016] Among the many potential advantages of the present disclosure, the
methods and compositions of the present disclosure may, among other things,
provide a means
of reducing or eliminating concentrations of hydrogen sulfide or soluble
sulfide ions in fluids
found and/or used in subterranean formations with significantly less risk of
environmental
damage and/or health and safety hazards. Such methods and compositions may be
more
compatible with regulatory requirements in various jurisdictions. The sulfide
scavenging
additives of the present disclosure comprising one or more reducing sugars may
be effective at a
wider range of pH levels (e.g., pH ranges above about 8) than other sulfide
scavenging additives
known in the art, and may reduce or eliminate concentrations of hydrogen
sulfide or soluble
sulfide ions in fluids more effectively. The methods and compositions of the
present disclosure
also may be more cost effective than other sulfide scavenging methods and
additives known in
the art.
[0017] The reducing sugars used in the methods, fluids, and sulfide scavenging
additives of the present disclosure may comprise any reducing sugar (or
combination thereof)
known in the art. Such reducing sugars may comprise manosaccharides,
disaccharides,
polysaccharides, and/or combinations thereof. Examples of reducing sugars that
may be suitable
5
CA 02912393 2015-11-12
WO 2014/209639 PCT/US2014/042361
for use in certain embodiments of the present disclosure include, but are not
limited to, glucose,
glucosamine, acetyl glucosamine, fructose, sucrose, lactose, maltose,
cellobiose, galactose,
mannose, ribose, ribulose, xylose, lyxose, rhamnose, arabinose, erytlarose,
and/or combinations
thereof. In certain embodiments, the reducing sugars used in the methods,
fluids, and sulfide
scavenging additives of the present disclosure may have a molecular weight of
from about 180
daltons to about 360 daltons. The reducing sugar optionally may be chelated
with any metal ion
known in the art, including but not limited to iron, zinc, copper, nickel,
manganese, and the like.
Chelated reducing sugars that may be suitable for use in certain embodiments
of the present
disclosure include, but are not limited to, ferric fructose, ferrous sucrose,
and the like.
[0018] The sulfide scavenging additives used in the present disclosure may
exhibit, among other features, an enhanced ability to scavenge sulfides as
compared to
conventional sulfide scavengers due, at least in part, to the manner in which
they react with
sulfides and other components of the fluid. In particular, sulfide scavenging
additives of the
present disclosure that comprise one or more metal ions chelated with reducing
sugars may
exhibit a dual sulfide scavenging mechanism wherein the metal ion and the
reducing sugar each
interact with hydrogen sulfide or sulfide ions to produce different products
that may be
precipitated or otherwise removed from of the fluid. The interaction of the
metal ions with
hydrogen sulfide or sulfide ions may proceed according to one or more
reactions similar those
discussed in paragraph [0015] below. The interaction of the reducing sugar
with hydrogen
sulfide or sulfide ions may involve the degradation of the reducing sugar and
the reaction of
those degradation products with hydrogen sulfide or sulfide ions.
Alternatively, the reducing
sugar may interact with hydrogen sulfide or sulfide ions to form an
intermediate sulfur-
containing compound, the sugar moiety in which may fragment to form other
sulfur containing
species. The chelation of the reducing sugar with the metal ion also may
inhibit the conversion
of the metal ion to a metal hydroxide, leaving the metal ion free to interact
with and/or scavenge
hydrogen sulfide or sulfide ions present in the fluid. However, the reaction
mechanisms
disclosed herein are provided only as non-limiting illustrations of how the
sulfide scavenging
additives of the present disclosure may react in certain embodiments, and are
not intended to
limit the scope of the claims.
[0019] One example of a sulfide scavenging additive that may be suitable for
use
in the present disclosure comprises ferric fructose. In those embodiments, the
iron (III) ions in
the ferric fructose may react with hydrogen sulfide to produce iron (III)
sulfide (Fe2S3).
Additionally, the iron (III) ions may be reduced by hydrogen sulfide to their
iron (II) oxidation
state, producing elemental sulfur (S ) (see Equation (1) below). The reduced
iron (II) ion may
6
CA 02912393 2015-11-12
WO 2014/209639 PCT/US2014/042361
react with additional hydrogen sulfides to produce iron (II) sulfide (FeS)
(see Equation (2)
below).
112S + 2Fe3+ - S + 2Fe2+ + 2H+ (1)
H2S + Fe2+ 4 FeS +2H+ (2)
In this process, the overall result for the ferric ion in this embodiment of
the present disclosure
may be expressed according to Equation (3) below:
2H2S + 2Fe3+ -> S + 2FeS + 4H+ (3)
The elemental sulfur and iron (II) sulfide may form a precipitate in the fluid
being treated, which
may be removed from the fluid. Similar reactions may occur using metallic ions
other than iron.
In embodiments where divalent metallic ions are used, elemental sulfur may not
be formed. In
addition to the reactions above, the fructose may interact with hydrogen
sulfide or sulfide ions to
produce various sulfur species that may precipitate or be removed from the
fluid being treated.
[0020] The reducing sugar (or chelated reducing sugar) may be added to or
included in a fluid in any concentration that effectively eliminates or
reduces by the desired
amount concentrations of II2S or sulfide ions that are present or expected to
be present in the
fluid. For example, the reducing sugar may be added in a stoichiometric amount
relative to the
estimated amount of H2S or sulfide ions in the fluid. In certain embodiments,
the reducing sugar
may be present a fluid in a concentration of about 0.1 to 5 pounds per barrel.
As discussed
below, an initial amount of the sulfide scavenging additives of the present
disclosure comprising
one or more reducing sugars may be added to a fluid, and subsequently,
additional amounts of
the sulfide scavenging additives of the present disclosure may be added to the
same fluid. This
technique may be used, among other purposes, to increase and/or maintain a
concentration of the
reducing sugar that is sufficient to effectively eliminate or reduce by the
desired amount
concentrations of 112S or sulfide ions in the fluid throughout the course of a
given operation.
[0021] The additives of the present disclosure may be used in conjunction with
any fluid, which may include, but are not limited to, treatment fluids
introduced into a
subterranean formation as well as fluids found in a subterranean formation
(e.g., formation
water, hydrocarbon fluids, etc.) and/or any combination thereof. The treatment
fluids and
formation fluids in the present disclosure generally comprise a base liquid,
which may comprise
any liquid known in the art, such as aqueous liquids, non-aqueous liquids, or
any mixture
thereof. Where the base liquid comprises an aqueous liquid, it may comprise
fresh water, salt
water (e.g., water containing one or more salts dissolved therein), brine
(e.g., saturated salt
water), or seawater. Generally, the water can be from any source, provided
that it does not
contain compounds that adversely affect other components of the fluid. Where
the base liquid
7
CA 02912393 2015-11-12
WO 2014/209639 PCT/US2014/042361
comprises a non-aqueous liquid, it may comprise any number of organic liquids.
Examples of
suitable organic liquids include, but are not limited to, mineral oils,
synthetic oils, esters, and the
like. In certain embodiments, the treatment fluids and/or formation fluids in
the present
disclosure may comprise emulsions (including invert emulsions), suspensions,
gels, foams, or
other mixtures of liquids with solids and/or gases.
[0022] The fluids used in the present disclosure optionally may comprise any
number of additional additives, including, but not limited to, salts,
surfactants, acids, fluid loss
control additives, gas, nitrogen, carbon dioxide, surface modifying agents,
tackifying agents,
foamers, corrosion inhibitors, scale inhibitors, catalysts, clay control
agents, biocides, friction
reducers, antifoam agents, bridging agents, dispersants, flocculants,
additional H2S scavengers,
CO2 scavengers, oxygen scavengers, lubricants, viscosifiers, breakers,
weighting agents, relative
permeability modifiers, resins, particulate materials (e.g., proppant
particulates), wetting agents,
coating enhancement agents, and the like. A person skilled in the art, with
the benefit of this
disclosure, will recognize the types of additives that may be included in the
fluids of the present
disclosure for a particular application.
[0023] The methods, fluids, and/or additives of the present disclosure may be
used during or in conjunction with any subterranean operation wherein a fluid
is used or treated.
In certain embodiments, the methods, fluids, and/or additives of the present
disclosure may be
used in the course of drilling operations. In these embodiments, the methods,
fluids, and/or
additives of the present disclosure may be used to reduce or eliminate
concentrations of H2S
from a drilling fluid used in drilling a well or borehole, for example, in a
hydrocarbon-bearing
subterranean formation where H2S is often encountered. Other suitable
subterranean operations
may include, but are not limited to, preflush treatments, afterflush
treatments, hydraulic
fracturing treatments, sand control treatments (e.g., gravel packing),
acidizing treatments (e.g.,
matrix acidizing or fracture acidizing), "frac-pack" treatments, well bore
clean-out treatments,
and other operations where a treatment fluid may be useful. Such treatment
fluids may include,
but are not limited to, drilling fluids, preflush fluids, afterflush fluids,
fracturing fluids, acidizing
fluids, gravel packing fluids, packer fluids, spacer fluids, and the like.
[0024] The reducing sugar may be provided in an additive in a solid form,
liquid
form (e.g., in solution of water or another solvent), or a combination
thereof. The sulfide
scavenging additives of the present disclosure may be added to a fluid by any
means known in
the art. The additive may be added to the fluid, for example, in the mud pit
before the fluid has
circulated or before the fluid contains any detectable amount of sulphur or
H2S, as a prophylactic
measure against any H2S the fluid may encounter downhole. In certain
embodiments, the
8
CA 02912393 2015-11-12
WO 2014/209639 PCT/US2014/042361
additive may be added after the fluid has been circulating downhole and has
already encountered
sulphur or H2S and contains same. In certain embodiments, the amount of the
additive added to
the fluid may be controlled and/or varied during the course of an operation
based on, among
other things, the amount of sulfur or H2S detected in fluids exiting the well
bore. In these
embodiments, any system or technique capable of monitoring or detecting sulfur
or H2S content
in fluids exiting the well bore may be used. Moreover, the sulfide scavenging
additives of the
present disclosure may be added to a fluid in multiple portions that are added
to the fluid at
separate intervals over a period of time. For example, a first amount of a
scavenging additive of
the present disclosure may be added to a fluid at one point in time in the
course of a particular
operation. At a subsequent point during that operation, an elevated amount of
sulfur or H2S may
be detected exiting the well bore, at which point a second amount of a
scavenging additive of the
present disclosure may be added to the fluid based at least in part on the
amount of sulfur or H2S
detected.
[0025] The exemplary fluids and additives disclosed herein may directly or
indirectly affect one or more components or pieces of equipment associated
with the preparation,
delivery, recapture, recycling, reuse, and/or disposal of the disclosed fluids
and additives. For
example, and with reference to FIG. 1, the disclosed fluids and additives may
directly or
indirectly affect one or more components or pieces of equipment associated
with an exemplary
wellbore drilling assembly 100, according to one or more embodiments. It
should be noted that
while FIG. 1 generally depicts a land-based drilling assembly, those skilled
in the art will readily
recognize that the principles described herein are equally applicable to
subsea drilling operations
that employ floating or sea-based platforms and rigs, without departing from
the scope of the
disclosure.
[0026] As illustrated, the drilling assembly 100 may include a drilling
platform
102 that supports a derrick 104 having a traveling block 106 for raising and
lowering a drill
string 108. The drill string 108 may include, but is not limited to, drill
pipe and coiled tubing, as
generally known to those skilled in the art. A kelly 110 supports the drill
string 108 as it is
lowered through a rotary table 112. A drill bit 114 is attached to the distal
end of the drill string
108 and is driven either by a downhole motor and/or via rotation of the drill
string 108 from the
well surface. As the bit 114 rotates, it creates a borehole 116 that
penetrates various
subterranean formations 118.
[0027] A pump 120 (e.g., a mud pump) circulates drilling fluid 122 through a
feed pipe 124 and to the kelly 110, which conveys the drilling fluid 122
downhole through the
interior of the drill string 108 and through one or more orifices in the drill
bit 114. The drilling
9
CA 02912393 2015-11-12
WO 2014/209639 PCT/US2014/042361
fluid 122 is then circulated back to the surface via an annulus 126 defined
between the drill
string 108 and the walls of the borehole 116. At the surface, the recirculated
or spent drilling
fluid 122 exits the annulus 126 and may be conveyed to one or more fluid
processing unit(s) 128
via an interconnecting flow line 130. After passing through the fluid
processing unit(s) 128, a
"cleaned" drilling fluid 122 is deposited into a nearby retention pit 132
(i.e., a mud pit). While
illustrated as being arranged at the outlet of the wellbore 116 via the
annulus 126, those skilled in
the art will readily appreciate that the fluid processing unit(s) 128 may be
arranged at any other
location in the drilling assembly 100 to facilitate its proper function,
without departing from the
scope of the scope of the disclosure.
[0028] One or more of the disclosed additives may be added to the drilling
fluid
122 via a mixing hopper 134 communicably coupled to or otherwise in fluid
communication
with the retention pit 132. The mixing hopper 134 may include, but is not
limited to, mixers and
related mixing equipment known to those skilled in the art. In other
embodiments, however, the
disclosed additives may be added to the drilling fluid 122 at any other
location in the drilling
assembly 100. In at least one embodiment, for example, there could be more
than one retention
pit 132, such as multiple retention pits 132 in series. Moreover, the
retention pit 132 may be
representative of one or more fluid storage facilities and/or units where the
disclosed additives
may be stored, reconditioned, and/or regulated until added to the drilling
fluid 122.
[0029] As mentioned above, the disclosed fluids and additives may directly or
indirectly affect the components and equipment of the drilling assembly 100.
For example, the
disclosed fluids and additives may directly or indirectly affect the fluid
processing unit(s) 128
which may include, but is not limited to, one or more of a shaker (e.g., shale
shaker), a
centrifuge, a hydrocyclone, a separator (including magnetic and electrical
separators), a desilter,
a desander, a separator, a filter (e.g., diatomaceous earth filters), a heat
exchanger, any fluid
reclamation equipment, The fluid processing unit(s) 128 may further include
one or more
sensors, gauges, pumps, compressors, and the like used store, monitor,
regulate, and/or
recondition the exemplary fluids and additives.
[0030] The disclosed fluids and additives may directly or indirectly affect
the
pump 120, which representatively includes any conduits, pipelines, trucks,
tubulars, and/or pipes
used to fluidically convey the fluids and additives downhole, any pumps,
compressors, or motors
(e.g., topside or downhole) used to drive the fluids and additives into
motion, any valves or
related joints used to regulate the pressure or flow rate of the fluids and
additives, and any
sensors (i e , pressure, temperature, flow rate, etc.), gauges, and/or
combinations thereof, and the
CA 02912393 2015-11-12
WO 2014/209639 PCT/US2014/042361
like. The disclosed fluids and additives may also directly or indirectly
affect the mixing hopper
134 and the retention pit 132 and their assorted variations.
[0031] The disclosed fluids and additives may also directly or indirectly
affect the
various downhole equipment and tools that may come into contact with the
fluids and additives
such as, but not limited to, the drill string 108, any floats, drill collars,
mud motors, downhole
motors and/or pumps associated with the drill string 108, and any MWD/LWD
tools and related
telemetry equipment, sensors or distributed sensors associated with the drill
string 108. The
disclosed fluids and additives may also directly or indirectly affect any
downhole heat
exchangers, valves and corresponding actuation devices, tool seals, packers
and other wellbore
isolation devices or components, and the like associated with the wellbore
116. The disclosed
fluids and additives may also directly or indirectly affect the drill bit 114,
which may include,
but is not limited to, roller cone bits, PDC bits, natural diamond bits, any
hole openers, reamers,
coring bits, etc.
[0032] While not specifically illustrated herein, the disclosed fluids and
additives
may also directly or indirectly affect any transport or delivery equipment
used to convey the
fluids and additives to the drilling assembly 100 such as, for example, any
transport vessels,
conduits, pipelines, trucks, tubulars, and/or pipes used to fluidically move
the fluids and
additives from one location to another, any pumps, compressors, or motors used
to drive the
fluids and additives into motion, any valves or related joints used to
regulate the pressure or flow
rate of the fluids and additives, and any sensors (i.e., pressure and
temperature), gauges, and/or
combinations thereof, and the like.
[0033] Therefore, the present disclosure is well adapted to attain the ends
and
advantages mentioned as well as those that are inherent therein. The
particular embodiments
disclosed above are illustrative only, as the present disclosure may be
modified and practiced in
different but equivalent manners apparent to those skilled in the art having
the benefit of the
teachings herein. Furthermore, no limitations are intended to the details of
construction or
design herein shown, other than as described in the claims below. It is
therefore evident that the
particular illustrative embodiments disclosed above may be altered or modified
and all such
variations are considered within the scope and spirit of the present
disclosure. While
compositions and methods are described in terms of "comprising," "containing,"
or "including"
various components or steps, the compositions and methods can also "consist
essentially of' or
"consist of' the various components and steps. All numbers and ranges
disclosed above may
vary by some amount. Whenever a numerical range with a lower limit and an
upper limit is
disclosed, any number and any included range falling within the range is
specifically disclosed.
11
CA 2912393 2017-05-23
In particular, every range of values (of the form, "from about a to about
or, equivalently,
"from approximately a to b," or, equivalently, "from approximately a-b")
disclosed herein is to
be understood to set forth every number and range encompassed within the
broader range of
values. Also, the terms in the claims have their plain, ordinary meaning
unless otherwise
explicitly and clearly defined by the patentee. Moreover, the indefinite
articles "a" or "an", as
used in the claims, are defined herein to mean one or more than one of the
element that it
introduces. If there is any conflict in the usages of a word or term in this
specification and one or
more patent or other documents that may be referred to herein, the definitions
that are consistent
with this specification should be adopted.
12