Note: Descriptions are shown in the official language in which they were submitted.
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
ANTI-CCL2 AND ANTI-LOXL2 COMBINATION THERAPY FOR TREATMENT
OF SCLERODERMA
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims benefit under 35 USC 119(e) of U.S.
Provisional
Patent Application Serial No. 61/826,692 filed May 23, 2013, which application
is hereby
incorporated by reference in its entirety.
SEQUENCE LISTING
[0002] The present specification makes reference to a Sequence Listing
submitted
in electronic form as an ASCII.txt file named "2006685-0569 ST25" on May 23,
2014.
The .txt file was generated on May 12, 2014 and is 7 KB in size.
BACKGROUND
[0003] Systemic sclerosis (scleroderma) is a clinically heterogeneous
disorder of
the connective tissue, resulting in hardening and tightening of the skin. It
is an
autoimmune-type of disease characterized by immune activation, vascular
damage, and
fibrosis. Major organ-based complications involving the lungs, heart, kidneys,
and
gastrointestinal tract can contribute to mortality and morbidity. The
pathogenesis is
unknown.
[0004] The feature most commonly associated with scleroderma is
fibrosis¨a
buildup of collagen in the skin and organs. The buildup of collagen
contributes to
symptoms of the disorder, including hair loss, skin hardening and tightening,
skin
discoloration, joint pain, stiffness of fingers and joints, digestive tract
problems and
breathing complications (dry cough, shortness of breath, wheezing).
Scleroderma may be
classified into two major subgroups: limited cutaneous scleroderma and diffuse
cutaneous
scleroderma. In limited cutaneous scleroderma, fibrosis is mainly restricted
to the hands,
arms, and face. Diffuse cutaneous scleroderma is a rapidly progressing
disorder that
affects large areas of the skin and compromises one or more internal organs.
Patients with
limited cutaneous scleroderma have a relatively better long term prognosis
than patients
with diffuse cutaneous scleroderma. Widespread systemic scleroderma can damage
the
1
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
heart, kidney, lungs, or GI tract, which may cause death. Pulmonary fibrosis
is the most
common cause of death in patients with scleroderma.
[0005] Thus,
scleroderma is an extremely debilitating disease with potentially fatal
repercussions. There are about 50,000 patients in the US. The ratio of female
patients to
male patients is about 4:1. Current treatment methods are based only on
symptomatic
treatment and management of complications that arise through the course of the
disease
(e.g., corticosteroids, NSAIDs, and immune-suppressing medications such as
Metotrexate
and Cytoxan). There is no treatment shown to reverse or halt progression of
disease.
Therefore, there is a high unmet medical need for an effective treatment of
scleroderma.
SUMMARY OF THE INVENTION
[0006] The
present invention provides, among other things, improved methods and
compositions for effective treatment of scleroderma, in particular, based on
bi-specific
binding molecules, including, but not limited to, antibodies, fynomers,
aptamers, fusion
proteins, protein binding domains (e.g., those derived from receptors) that
can specifically
bind to lysyl oxidase-like-2 ("LOXL2") and C-C chemokine ligand-2 ("CCL2"),
and/or
combination therapy based on such molecules that specifically bind to LOXL2
and CCL2.
CCL2 is known to be a validated target for scleroderma. Several studies have
shown that
scleroderma fibroblasts display increased constitutive expression of CCL2 mRNA
and
protein. In scleroderma skin sections, expression of CCL2 was detected in
fibroblasts,
keratinocytes, and mononuclear cells, whereas it was undetectable in normal
skin (Galindo
et al., Arthritis Rheum. 2001 Jun; 44(6):1382-6; Distler et al., Arthritis
Rheum. 2001 Nov;
44(11):2665-78; Lioyd et al., Exp Med. 1997 Apr 7;185(7):1371-80; Yamamoto et
al., J
Dermatol Sci. 2001 Jun; 26(2):133-9; Denton et al.; Trends Immunol. 2005 Nov;
26(11):596-602. Epub 2005 Sep 15.). However, prior to the present invention,
no
effective treatment for scleroderma has been developed based on anti-CCL2
antibodies.
The present inventors observe that high levels of CCL2 in plasma sequester
anti-CCL2
antibodies injected intravenously, resulting in wasted anti-CCL2 antibodies
and ineffective
targeting of CCL2 in diseased tissues. To solve this problem, the present
inventors
contemplate the use of bi-specific molecules that allow sequestering anti-CCL2
activity in
diseased tissues with free anti-CCL2 arms that bind to tissue CCL2, which
provides tissue
specific targeting of CCL2. Thus, the present invention provides methods and
2
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
compositions that preferentially inhibit tissue CCL2 as opposed to plasma
CCL2, resulting
in highly effective treatment of scleroderma.
[0007] Thus, in one aspect, the present invention provides bi-specific
binding
molecules (e.g., bi-specific antibodies, fynomers, aptamers, fusion proteins,
or protein
binding domains) comprising a first antigen-binding site that specifically
binds to LOXL2
and a second antigen-binding site that specifically binds to CCL2.
[0008] In some embodiments, the first antigen-binding site specifically
binds to
LOXL2 with a binding affinity of 100 nM or greater (e.g., lOnM or greater, 1nM
or
greater, 500pM or greater, 100pM or greater, 50pM or greater, lOpM or greater,
1pM or
greater, 500fM or greater, 400fM or greater, 300fM or greater, 200fM or
greater, 100fM or
greater, 50fM or greater, 10fM or greater, or 1fM or greater).
[0009] In some embodiments, the second antigen-binding site specifically
binds to
CCL2 with a binding affinity of between about 500nM and 1fM (e.g., between
500nM and
10fM, between 500nM and 100fM, between 500nM and 1pM, between lOnM and 1fM,
between lOnM and 100fM, between lOnM and 1pM, between 1nM and 1fM, between
1nM and 100fM, between 1nM and 500fM, between 1nM and 1pM, between 1nM and
lOpM, between 1nM and 50pM, between 1nM and 100pM, between 1nM and 500pM). In
some embodiments, the second antigen-binding site specifically binds to CCL2
with a
binding affinity of greater than about 500nM (e.g., greater than about 500nM,
100nM,
lOnM, 1nM, 500pM, 100pM, 50pM, lOpM, 1pM, 500fM, 400fM, 300fM, 200fM, 100fM,
50fM, 10fM, 1fM).
[0010] In some embodiments, the first antigen-binding site comprises a
first full
length heavy chain and a first full length light chain. In some embodiments,
the first
antigen-binding site comprises a first Fab fragment. In some embodiments, the
first
antigen-binding site comprises a first single-chain variable fragments
(scFvs).
[0011] In some embodiments, the second antigen-binding site comprises a
second
full length heavy chain and a second full length light chain. In some
embodiments, the
second antigen-binding site comprises a second Fab fragment. In some
embodiments, the
second antigen-binding site comprises a second single-chain variable fragments
(scFvs).
[0012] In some embodiments, the first and second antigen-binding sites
are linked
by a peptide linker. In some embodiments, the peptide linker is > 5 (e.g., 6,
7, 8, 9, 10, 11,
3
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
12, 13, 14, 15, 20, 25 or more) amino acids long. In some embodiments, the
first and
second antigen binding sites are configured such that they form a single
polypeptide chain.
[0013] In some embodiments, the first and second antigen-binding sites
are
associated via chemical cross-linking.
[0014] In some embodiments, a bi-specific binding molecule according to
the
invention is a bi-specific antibody. In some embodiments, the bi-specific
antibody
comprises an Fc region.
[0015] In some embodiments, the bi-specific antibody is human. In some
embodiments, the bi-specific antibody is humanized.
[0016] In another aspect, the present invention provides pharmaceutical
compositions comprising the bi-specific binding molecule (e.g., a bi-specific
antibody,
fynomer, aptamer, fusion protein, protein binding domain) as described herein
and a
pharmaceutically acceptable carrier.
[0017] In further aspect, the present invention provides methods of
treating
scleroderma comprising administering to an individual who is suffering from or
susceptible to scleroderma a bi-specific binding molecule (e.g., a bi-specific
antibody,
fynomer, aptamer, fusion protein, protein binding domain) as described herein.
In some
embodiments, the bi-specific antibody is administered at a therapeutically
effective dose
and an administration interval such that at least one symptom or feature of
scleroderma on
a target tissue is reduced in intensity, severity, or frequency, or has
delayed onset.
[0018] In some embodiments, the at least one pathological feature of
scleroderma
is ameliorated, including but not limited to, endothelial-cell damage,
proliferation of basal-
lamina layers, perivascular mononuclear-cell infiltration, fibrosis,
derangement of
visceral-organ architecture, rarefaction of blood vessels, hypoxia, and
combination
thereof
[0019] In some embodiments, the target tissue is selected from the group
consisting of skin, blood vessels, lung, heart, kidney, gastrointestinal tract
(including
liver), musculoskeletal system and combinations thereof In some embodiments,
the target
tissue is lung. In some embodiments, the target tissue is heart.
4
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
[0020] In some embodiments, the individual is suffering from or
susceptible to
limited cutaneous scleroderma. In some embodiments, the individual is
suffering from or
susceptible to diffuse cutaneous scleroderma.
[0021] In some embodiments, the bi-specific antibody is administered
parenterally.
In some embodiments, the parenteral administration is selected from
intravenous,
intradermal, inhalation, transdermal (topical), subcutaneous, and/or
transmucosal
administration. In some embodiments, the parenteral administration is
intravenous
administration.
[0022] In some embodiments, the bi-specific antibody is administered
orally.
[0023] In certain embodiments, the bi-specific antibody is administered
bimonthly,
monthly, triweekly, biweekly, weekly, daily, or at variable intervals.
[0024] In some embodiments, the bi-specific antibody is co-administered
with one
or more anti-fibrotic or anti-inflammatory agents.
[0025] In another aspect, the present invention provides use of a bi-
specific
binding molecule as described herein in the manufacture of a medicament for
treatment of
scleroderma, wherein the treatment comprises administering to an individual
who is
suffering from or susceptible to scleroderma an effective amount of the bi-
specific
molecule, wherein the bi-specific binding molecule comprises a first antigen-
binding site
that specifically binds to LOXL2 and a second antigen-binding site that
specifically binds
to CCL2
[0026] In some embodiments, the first antigen-binding site specifically
binds to
LOXL2 with a binding affinity of 100 nM or greater (e.g., lOnM or greater, 1nM
or
greater, 500pM or greater, 100pM or greater, 50pM or greater, lOpM or greater,
1pM or
greater, 500fM or greater, 400fM or greater, 300fM or greater, 200fM or
greater, 100fM or
greater, 50fM or greater, 10fM or greater, or 1fM or greater).
[0027] In some embodiments, the second antigen-binding site specifically
binds to
CCL2 with a binding affinity of between about 500nM and 1fM (e.g., between
500nM and
10fM, between 500nM and 100fM, between 500nM and 1pM, between lOnM and 1fM,
between lOnM and 100fM, between lOnM and 1pM, between 1nM and 1fM, between
1nM and 100fM, between 1nM and 500fM, between 1nM and 1pM, between 1nM and
lOpM, between 1nM and 50pM, between 1nM and 100pM, between 1nM and 500pM). In
some embodiments, the second antigen-binding site specifically binds to CCL2
with a
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
binding affinity of greater than about 500nM (e.g., greater than about 500nM,
100nM,
lOnM, 1nM, 500pM, 100pM, 50pM, lOpM, 1pM, 500fM, 400fM, 300fM, 200fM, 100fM,
50fM, 10fM, 1fM).
[0028] In some embodiments, the first antigen-binding site comprises a
first full
length heavy chain and a first full length light chain. In some embodiments,
the first
antigen-binding site comprises a first Fab fragment. In some embodiments, the
first
antigen-binding site comprises a first single-chain variable fragments
(scFvs).
[0029] In some embodiments, the second antigen-binding site comprises a
second
full length heavy chain and a second full length light chain. In some
embodiments, the
second antigen-binding site comprises a second Fab fragment. In some
embodiments, the
second antigen-binding site comprises a second single-chain variable fragments
(scFvs).
[0030] In some embodiments, the first and second antigen-binding sites
are linked
by a peptide linker. In some embodiments, the peptide linker is > 5 (e.g., 6,
7, 8, 9, 10, 11,
12, 13, 14, 15, 20, 25 or more) amino acids long. In some embodiments, the
first and
second antigen binding sites are configured such that they form a single
polypeptide chain.
[0031] In some embodiments, the first and second antigen-binding sites
are
associated via chemical cross-linking.
[0032] In some embodiments, a bi-specific binding molecule according to
the
invention is a bi-specific antibody. In some embodiments, the bi-specific
antibody
comprises an Fc region.
[0033] In some embodiments, the bi-specific antibody is humanized.
[0034] In another aspect, the present invention provides a bi-specific
binding
molecule as described herein for use in a method of treating scleroderma
comprising a step
of administering an effective amount of the bi-specific binding molecule to a
subject who
is suffering from or susceptible to scleroderma, wherein the bi-specific
binding molecule
comprises a first antigen-binding site that specifically binds to LOXL2 and a
second
antigen-binding site that specifically binds to CCL2.
[0035] In some embodiments, the first antigen-binding site specifically
binds to
LOXL2 with a binding affinity of 100 nM or greater (e.g., lOnM or greater, 1nM
or
greater, 500pM or greater, 100pM or greater, 50pM or greater, lOpM or greater,
1pM or
6
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
greater, 500fM or greater, 400fM or greater, 300fM or greater, 200fM or
greater, 100fM or
greater, 50fM or greater, 10fM or greater, or 1fM or greater).
[0036] In some embodiments, the second antigen-binding site specifically
binds to
CCL2 with a binding affinity of between about 500nM and 1fM (e.g., between
500nM and
10fM, between 500nM and 100fM, between 500nM and 1pM, between lOnM and 1fM,
between lOnM and 100fM, between lOnM and 1pM, between 1nM and 1fM, between
1nM and 100fM, between 1nM and 500fM, between 1nM and 1pM, between 1nM and
lOpM, between 1nM and 50pM, between 1nM and 100pM, between 1nM and 500pM). In
some embodiments, the second antigen-binding site specifically binds to CCL2
with a
binding affinity of greater than about 500nM (e.g., greater than about 500nM,
100nM,
lOnM, 1nM, 500pM, 100pM, 50pM, lOpM, 1pM, 500fM, 400fM, 300fM, 200fM, 100fM,
50fM, 10fM, 1fM).
[0037] In some embodiments, the first antigen-binding site comprises a
first full
length heavy chain and a first full length light chain. In some embodiments,
the first
antigen-binding site comprises a first Fab fragment. In some embodiments, the
first
antigen-binding site comprises a first single-chain variable fragments
(scFvs).
[0038] In some embodiments, the second antigen-binding site comprises a
second
full length heavy chain and a second full length light chain. In some
embodiments, the
second antigen-binding site comprises a second Fab fragment. In some
embodiments, the
second antigen-binding site comprises a second single-chain variable fragments
(scFvs).
[0039] In some embodiments, the first and second antigen-binding sites
are linked
by a peptide linker. In some embodiments, the peptide linker is > 5 (e.g., 6,
7, 8, 9, 10, 11,
12, 13, 14, 15, 20, 25 or more) amino acids long. In some embodiments, the
first and
second antigen binding sites are configured such that they form a single
polypeptide chain.
[0040] In some embodiments, the first and second antigen-binding sites
are
associated via chemical cross-linking.
[0041] In some embodiments, a bi-specific binding molecule according to
the
invention is a bi-specific antibody. In some embodiments, the bi-specific
antibody
comprises an Fc region.
[0042] In some embodiments, the bi-specific antibody is human. In some
embodiments, the bi-specific antibody is humanized.
7
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
[0043] In yet another aspect, the present invention provides methods of
treating
fibrotic diseases, disorders or conditions comprising administering to an
individual who is
suffering from or susceptible to a fibrotic disease, disorder or condition a
bi-specific
binding molecule (e.g., a bi-specific antibody, fynomer, aptamer, fusion
protein, protein
binding domain) as described herein.
[0044] In another aspect, the present invention provides use of a bi-
specific
binding molecule as described herein in the manufacture of a medicament for
treatment of
fibrotic diseases, disorders or conditions, wherein the treatment comprises
administering
to an individual who is suffering from or susceptible to a fibrotic disease,
disorder or
condition the bi-specific binding molecule, wherein the bi-specific molecule
comprises a
first antigen-binding site that specifically binds to LOXL2 and a second
antigen-binding
site that specifically binds to CCL2.
[0045] In another aspect, the present invention provides a bi-specific
molecule for
use in a method of treating fibrotic diseases, disorders or conditions
comprising a step of
administering to an individual who is suffering from or susceptible to a
fibrotic disease,
disorder or condition the bi-specific binding molecule, wherein the bi-
specific molecule
comprises a first antigen-binding site that specifically binds to LOXL2 and a
second
antigen-binding site that specifically binds to CCL2.
[0046] In various embodiments, the fibrotic disease, disorder or
condition is
selected from the group consisting of skin fibrosis, kidney fibrosis, liver
fibrosis, lung
fibrosis, heart fibrosis, muscle fibrosis, and combination thereof
[0047] In another aspect, the present invention provides methods of
treating
inflammatory diseases, disorders or conditions comprising administering to an
individual
who is suffering from or susceptible to an inflammatory disease, disorder or
condition a
bi-specific binding molecule as described herein.
[0048] In another aspect, the present invention provides use of a bi-
specific
binding molecule as described herein in the manufacture of a medicament for
treatment of
inflammatory diseases, disorders or conditions, wherein the treatment
comprises
administering to an individual who is suffering from or susceptible to an
inflammatory
diseases, disorders or condition the bi-specific binding molecule, wherein the
bi-specific
molecule comprises a first antigen-binding site that specifically binds to
LOXL2 and a
second antigen-binding site that specifically binds to CCL2.
8
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
[0049] In another aspect, the present invention provides a bi-specific
molecule for
use in a method of treating fibrotic diseases, disorders or conditions
comprising a step of
administering to an individual who is suffering from or susceptible to a
fibrotic disease,
disorder or condition the bi-specific binding molecule, wherein the bi-
specific molecule
comprises a first antigen-binding site that specifically binds to LOXL2 and a
second
antigen-binding site that specifically binds to CCL2.
[0050] In various embodiments, the inflammatory disease, disorder or
condition is
selected from the group consisting of psoriasis, rheumatoid arthritis,
atherosclerosis,
epilepsy, Alzheimer's disease, obesity, lupus nephritis, general kidney
inflammation,
multiple sclerosis, Crohn's disease, asthma, discoid lupus erythematosus,
inflammatory
bowel disease, or systemic lupus erythematosus.
[0051] In another aspect, the present invention provides methods of
treating
scleroderma comprising administering to an individual who is suffering from or
susceptible to scleroderma an anti-CCL2 antibody, or fragment thereof, and an
anti-
LOXL2 antibody, or fragment thereof
[0052] In another aspect, the present invention provides use of an anti-
CCL2
antibody, or fragment thereof, and an anti-LOXL2 antibody, or fragment
thereof, in the
manufacture of a medicament for treatment of scleroderma, wherein the
treatment
comprises a step of administering the anti-CCL2 antibody, or fragment thereof,
and the
anti-LOXL2 antibody, or fragment thereof, to an individual who is suffering
from or
susceptible to scleroderma.
[0053] In another aspect, the present invention provides an anti-CCL2
antibody, or
fragment thereof, and an anti-LOXL2 antibody, or fragment thereof, for use in
a method of
treating scleroderma comprising a step of administering the anti-CCL2
antibody, or
fragment thereof, and the anti-LOXL2 antibody, or fragment thereof, to an
individual who
is suffering from or susceptible to scleroderma.
[0054] In some embodiments, the anti-CCL2 antibody, or fragment thereof,
and
the anti-LOXL2 antibody, or fragment thereof, are administered simultaneously.
In some
embodiments, the anti-CCL2 antibody, or fragment thereof, and the anti-LOXL2
antibody,
or fragment thereof, are administered sequentially.
[0055] In some embodiments, the anti-CCL2 antibody, or fragment thereof,
has a
binding affinity of 1nM or greater (e.g., 500pM or greater, 100pM or greater,
50pM or
9
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
greater, lOpM or greater, 1pM or greater, 500fM or greater, 400fM or greater,
300fM or
greater, 200fM or greater, 100fM or greater, 50fM or greater, 10fM or greater,
1fM or
greater).
[0056] In some embodiments, the anti-LOXL2 antibody, or fragment
thereof, has a
binding affinity of 1pM or greater (e.g., 500fM or greater, 400fM or greater,
300fM or
greater, 200fM or greater, 100fM or greater, 50fM or greater, 10fM or greater,
1fM or
greater).
[0057] In some embodiments, the anti-CCL2 antibody, or fragment thereof,
is
selected from the group consisting of intact IgG, F(ab')2, F(ab)2, Fab', Fab,
ScFvs,
diabodies, triabodies and tetrabodies.
[0058] In some embodiments, the anti-LOXL2 antibody, or fragment
thereof, is
selected from the group consisting of intact IgG, F(ab')2, F(ab)2, Fab', Fab,
ScFvs,
diabodies, triabodies and tetrabodies.
[0059] In some embodiments, one or both of the anti-CCL2 antibody, or
fragment
thereof, and the anti-LOXL2 antibody, or fragment thereof, are humanized.
[0060] In some embodiments, the anti-CCL2 antibody, or fragment thereof,
and
the anti-LOXL2 antibody, or fragment thereof, are administered via same
administration
route. In some embodiments, the anti-CCL2 antibody, or fragment thereof, and
the anti-
LOXL2 antibody, or fragment thereof, are administered via different
administration route.
[0061] In some embodiments, the anti-CCL2 antibody, or fragment, is
administered intravenously, intradermally, by inhalation, transdermally
(topically),
subcutaneously, transmucosally, and/or orally.
[0062] In some embodiments, the anti-CCL2 antibody, or fragment thereof,
is
administered bimonthly, monthly, triweekly, biweekly, weekly, daily, or at
variable
intervals.
[0063] In some embodiments, the anti-LOXL2 antibody, or fragment, is
administered intravenously, intradermally, by inhalation, transdermally
(topically),
subcutaneously, transmucosally, and/or orally.
[0064] In some embodiments, the anti-LOXL2 antibody, or fragment
thereof, is
administered bimonthly, monthly, triweekly, biweekly, weekly, daily, or at
variable
intervals.
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
[0065] In another aspect, the present invention provides methods of
treating
fibrotic diseases, disorders or conditions comprising administering to an
individual who is
suffering from or susceptible to a fibrotic disease, disorder or condition an
anti-CCL2
antibody, or fragment thereof, and an anti-LOXL2 antibody, or fragment thereof
[0066] In another aspect, the present invention provides use of an anti-
CCL2
antibody, or fragment thereof, and an anti-LOXL2 antibody, or fragment
thereof, in the
manufacture of a medicament for treatment of fibrotic diseases, disorders or
conditions,
wherein the treatment comprises a step of administering the anti-CCL2
antibody, or
fragment thereof, and the anti-LOXL2 antibody, or fragment thereof, to an
individual who
is suffering from or susceptible to a fibrotic disease, disorder or condition.
[0067] In another aspect, the present invention provides an anti-CCL2
antibody, or
fragment thereof, and an anti-LOXL2 antibody, or fragment thereof, for use in
a method of
treating fibrotic diseases, disorders or conditions comprising a step of
administering the
anti-CCL2 antibody, or fragment thereof, and the anti-LOXL2 antibody, or
fragment
thereof, to an individual who is suffering from or susceptible to a fibrotic
disease, disorder
or condition.
[0068] In another aspect, the present invention provides methods of
treating
inflammatory diseases, disorders or conditions comprising administering to an
individual
who is suffering from or susceptible to an inflammatory disease, disorder or
condition an
anti-CCL2 antibody, or fragment thereof, and an anti-LOXL2 antibody, or
fragment
thereof
[0069] In another aspect, the present invention provides use of an anti-
CCL2
antibody, or fragment thereof, and an anti-LOXL2 antibody, or fragment
thereof, in the
manufacture of a medicament for treatment of inflammatory diseases, disorders
or
conditions, wherein the treatment comprises a step of administering the anti-
CCL2
antibody, or fragment thereof, and the anti-LOXL2 antibody, or fragment
thereof, to an
individual who is suffering from or susceptible to an inflammatory disease,
disorder or
condition.
[0070] In another aspect, the present invention provides an anti-CCL2
antibody, or
fragment thereof, and an anti-LOXL2 antibody, or fragment thereof, for use in
a method of
treating inflammatory diseases, disorders or conditions comprising a step of
administering
the anti-CCL2 antibody, or fragment thereof, and the anti-LOXL2 antibody, or
fragment
11
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
thereof, to an individual who is suffering from or susceptible to an
inflammatory disease,
disorder or condition.
[0071] In another aspect, the present disclosure provides kits
comprising an anti-
CCL2 antibody, or fragment thereof, and an anti-LOXL2 antibody, or fragment
thereof
[0072] Other features, objects, and advantages of the present invention
are
apparent in the detailed description, drawings and claims that follow. It
should be
understood, however, that the detailed description, the drawings, and the
claims, while
indicating embodiments of the present invention, are given by way of
illustration only, not
limitation. Various changes and modifications within the scope of the
invention will
become apparent to those skilled in the art.
BRIEF DESCRIPTION OF THE DRAWING
[0073] The Drawing included herein, which is comprised of the following
Figures,
is for illustration purposes only not for limitation.
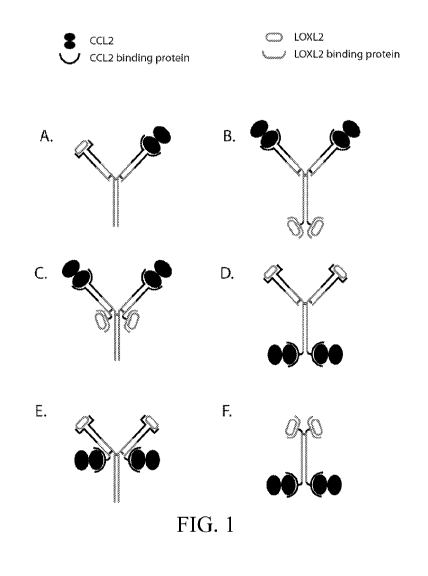
[0074] FIGs. 1A-1F illustrate diagrams depicting exemplary anti-CCL2 and
anti-
LOXL2 bi-specific antibodies.
[0075] FIG. 2 illustrates an exemplary diagram depicting the Modified
Rodnan
Skin Score. Locations on the body where skin fibrosis is assessed are
indicated.
[0076] FIG. 3 depicts an exemplary graph plotting serum and tissue
concentration
of CCL2 following equilibration.
[0077] FIG. 4 illustrates an exemplary diagram depicting CCL2 targeting
in
plasma and in diseased tissue.
[0078] FIG. 5 depicts an exemplary graph plotting concentration of CCL2
as a
function of days post treatment with either anti-CCL2 (Mono) or anti-
CCL2/LOXL2 (Bi),
illustrating preliminary bi-specific modeling results.
[0079] FIG. 6 shows the percentage of skin ulcers observed in C57BL/6
mice
treated with IgG, anti-CCL2 antibody, anti-LOXL2 antibody, or anti-CCL2 and
anti-
LOXL2 antibodies. PBS: negative control. BOTH: combination treatment with anti-
CCL2 antibody and anti-LOXL2 antibody.
[0080] FIG. 7 shows the fold-change in skin thickness observed in
C57BL/6 mice
treated with IgG, anti-CCL2 antibody, anti-LOXL2 antibody, or anti-CCL2 and
anti-
12
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
LOXL2 antibodies. PBS: negative control. BOTH: combination treatment with anti-
CCL2 antibody and anti-LOXL2 antibody.
[0081] FIG. 8 shows the Ashcroft score for lung tissue samples of
C57BL/6 mice
treated with IgG, anti-CCL2 antibody, anti-LOXL2 antibody, or anti-CCL2 and
anti-
LOXL2 antibodies. PBS: negative control. BOTH: combination treatment with anti-
CCL2 antibody and anti-LOXL2 antibody.
[0082] FIG. 9 shows Arginase 1 (Arg1)-staining in lung tissue samples of
C57BL/6 mice treated with IgG, anti-CCL2 antibody, anti-LOXL2 antibody, or
anti-CCL2
and anti-LOXL2 antibodies. PBS: negative control. BOTH: combination treatment
with
anti-CCL2 antibody and anti-LOXL2 antibody.
[0083] FIG. 10 shows a correlation plot of Arginase 1 (Arg1)-staining as
a
function of Ashcroft score in lung tissue samples of C57BL/6 mice treated with
IgG, anti-
CCL2 antibody, anti-LOXL2 antibody, or anti-CCL2 and anti-LOXL2 antibodies.
PBS:
negative control. BOTH: combination treatment with anti-CCL2 antibody and anti-
LOXL2 antibody.
[0084] FIG. 11 shows representative histological sections of lung tissue
samples
stained with Trichrome for various treatment groups of mice at 4X power. Top
row: PBS
(left), IgG (right). Middle row: anti-CCL2 (left), anti-LOXL2 (right). Bottom
row: anti-
CCL2 and anti-LOXL2.
[0085] FIG. 12 shows representative histological sections of lung tissue
samples
stained with Arginase 1 (Arg 1) for various treatment groups of mice. Top row:
PBS (left),
IgG (right). Middle row: anti-CCL2 (left), anti-LOXL2 (right). Bottom row:
anti-CCL2
and anti-LOXL2.
[0086] FIG. 13 shows representative histological sections of lung tissue
samples
stained with Arginase 1 (Argl) for IgG treatment groups at 20X (left) and 40X
(right)
power.
DEFINITIONS
[0087] In order for the present invention to be more readily understood,
certain
terms are first defined. Additional definitions for the following terms and
other terms are
set forth throughout the specification.
13
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
[0088] Affinity: As is known in the art, "affinity" is a measure of the
tightness
with which a particular ligand binds to (e.g., associates non-covalently with)
and/or the
rate or frequency with which it dissociates from, its partner. As is known in
the art, any of
a variety of technologies can be utilized to determine affinity. In many
embodiments,
affinity represents a measure of specific binding.
[0089] Affinity-matured (or affinity-matured antibody): As used herein,
refers to
an antibody with one or more alterations in one or more CDRs thereof which
result an
improvement in the affinity of the antibody for antigen, compared to a parent
antibody
which does not possess those alteration(s). In some embodiments, affinity
matured
antibodies will have nanomolar or even picomolar affinities for a target
antigen. Affinity
matured antibodies may be produced by any of a variety of procedures known in
the art.
Marks et al. BioTechnology 10:779-783 (1992) describes affinity maturation by
VH and
VL domain shuffling. Random mutagenesis of CDR and/or framework residues is
described by: Barbas et al. Proc Nat. Acad. Sci, USA 91:3809-3813 (1994);
Schier et al.
Gene 169:147-155 (1995); Yelton et al. J. Immunol. 155:1994-2004 (1995);
Jackson et al.,
J. Immunol. 154(7):3310-9 (1995); and Hawkins et al, J. Mol. Biol. 226:889-896
(1992).
[0090] Antibody: As used herein, the term "antibody" refers to a
polypeptide
consisting of one or more polypeptides substantially encoded by immunoglobulin
genes or
fragments of immunoglobulin genes. The recognized immunoglobulin genes include
the
kappa, lambda, alpha, gamma, delta, epsilon and mu constant region genes, as
well as
myriad immunoglobulin variable region genes. Light chains are typically
classified as
either kappa or lambda. Heavy chains are typically classified as gamma, mu,
alpha, delta,
or epsilon, which in turn define the immunoglobulin classes, IgG, IgM, IgA,
IgD and IgE,
respectively. A typical immunoglobulin (antibody) structural unit is known to
comprise a
tetramer. Each tetramer is composed of two identical pairs of polypeptide
chains, each
pair having one "light" (about 25 IcD) and one "heavy" chain (about 50-70
IcD). The N-
terminus of each chain defines a variable region of about 100 to 110 or more
amino acids
primarily responsible for antigen recognition. The terms "variable light
chain" (VL) and
"variable heavy chain" (VH) refer to these light and heavy chains
respectively. An
antibody can be specific for a particular antigen. The antibody or its antigen
can be either
an analyte or a binding partner. Antibodies exist as intact immunoglobulins or
as a
number of well-characterized fragments produced by digestion with various
peptidases.
Thus, for example, pepsin digests an antibody below the disulfide linkages in
the hinge
14
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
region to produce F(ab)'2, a dimer of Fab which itself is a light chain joined
to VH-CH1 by
a disulfide bond. The F(ab)'2 may be reduced under mild conditions to break
the disulfide
linkage in the hinge region thereby converting the (Fab')2 dimer into an Fab'
monomer.
The Fab' monomer is essentially an Fab with part of the hinge region (see,
Fundamental
Immunology, W. E. Paul, ed., Raven Press, N.Y. (1993), for a more detailed
description of
other antibody fragments). While various antibody fragments are defined in
terms of the
digestion of an intact antibody, one of ordinary skill in the art will
appreciate that such
Fab' fragments may be synthesized de novo either chemically or by utilizing
recombinant
DNA methodology. Thus, the term "antibody," as used herein also includes
antibody
fragments either produced by the modification of whole antibodies or
synthesized de novo
using recombinant DNA methodologies. In some embodiments, antibodies are
single
chain antibodies, such as single chain Fv (scFv) antibodies in which a
variable heavy and a
variable light chain are joined together (directly or through a peptide
linker) to form a
continuous polypeptide. A single chain Fv ("scFv") polypeptide is a covalently
linked
VH::VL heterodimer which may be expressed from a nucleic acid including VH-
and VL-
encoding sequences either joined directly or joined by a peptide-encoding
linker. (See,
e.g., Huston, et al. (1988) Proc. Nat. Acad. Sci. USA, 85:5879-5883, the
entire contents of
which are herein incorporated by reference.) A number of structures exist for
converting
the naturally aggregated, but chemically separated light and heavy polypeptide
chains
from an antibody V region into an scFv molecule which will fold into a three
dimensional
structure substantially similar to the structure of an antigen-binding site.
See, e.g. U.S.
Pat. Nos. 5,091,513 and 5,132,405 and 4,956,778.
[0091] Approximately: As used herein, the term "approximately" or
"about," as
applied to one or more values of interest, refers to a value that is similar
to a stated
reference value. In certain embodiments, the term "approximately" or "about"
refers to a
range of values that fall within 25%, 20%, 19%, 18%, 17%, 16%, 15%, 14%, 13%,
12%,
11%, 10%, 9%, 8%, 7%, 6%, 5%, 4%, 3%, 2%, 1%, or less in either direction
(greater
than or less than) of the stated reference value unless otherwise stated or
otherwise evident
from the context (except where such number would exceed 100% of a possible
value).
[0092] Binding agent: As used herein, the term "binding agent" includes
any
naturally occurring, synthetic or genetically engineered agent, such as
protein, that binds
an antigen or a target protein or peptide. "Binding agent" is also referred to
as "binding
protein." Binding agents can be derived from naturally occurring antibodies or
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
synthetically engineered. A binding protein or agent can function similarly to
an antibody
by binding to a specific antigen to form a complex and elicit a biological
response (e.g.,
agonize or antagonize a particular biological activity). Binding agents or
proteins can
include isolated fragments, "Fv" fragments consisting of the variable regions
of the heavy
and light chains of an antibody, recombinant single chain polypeptide
molecules in which
light and heavy chain variable regions are connected by a peptide linker
("ScFy proteins"),
and minimal recognition units consisting of the amino acid residues that mimic
the
hypervariable region. The term Binding Agent as used herein can also include
antibody
fragments either produced by the modification of whole antibodies or
synthesized de novo
using recombinant DNA methodologies. In some embodiments, antibodies are
single
chain antibodies, such as single chain Fy (scFv) antibodies in which a
variable heavy and a
variable light chain are joined together (directly or through a peptide
linker) to form a
continuous polypeptide. A single chain Fy ("scFv") polypeptide is a covalently
linked
VH::VL heterodimer which may be expressed from a nucleic acid including VH-
and VL-
encoding sequences either joined directly or joined by a peptide-encoding
linker. (See,
e.g., Huston, et al. (1988) Proc. Nat. Acad. Sci. USA, 85:5879-5883, the
entire contents of
which are herein incorporated by reference.) A number of structures exist for
converting
the naturally aggregated, but chemically separated light and heavy polypeptide
chains
from an antibody V region into an scFv molecule which will fold into a three
dimensional
structure substantially similar to the structure of an antigen-binding site.
See, e.g. U.S. Pat.
Nos. 5,091,513 and 5,132,405 and 4,956,778. In some embodiments, the term
Binding
Agent as used herein can also include antibody. See the definition of
Antibody.
[0093] Bi-specific: The term "bi-specific" as used herein refers to a
molecule
having two distinct binding specificities. Typically, a bi-specific binding
molecule
contains at least two antigen-binding sites, each of which specifically binds
to a different
antigen or epitope. A bi-specific molecule can be, for example, a bi-specific
antibody,
fynomer, aptamer, fusion protein, protein binding domain. As used herein, bi-
specific
molecules encompass molecules (e.g., antibodies, fynomers, aptamers, fusion
proteins,
protein binding domains or other binding agents) having higher valencies
(i.e., the ability
to bind more than two antigens, or epitopes), which are also referred to as
multispecific
molecules.
[0094] Bispecific antibody: The term "bispecific antibody" as used
herein, refers
to a bispecific binding molecule in which at least one, and typically both, of
the binding
16
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
moieties is or comprises an antibody component or fragment. A variety of
different bi-
specific antibody structures is known in the art. In some embodiments, each
binding
moiety in a bispecific antibody that is or comprises an antibody component or
fragment
includes VH and/or VL regions; in some such embodiments, the VH and/or VL
regions are
those found in a particular monoclonal antibody. In some embodiments, where
the
bispecific antibody contains two antibody component binding moieties, each
includes VH
and/or VL regions from different monoclonal antibodies.
[0095] Bispecific binding molecule: The term "bispecific binding
molecule" as
used herein, refers to a polypeptide with two discrete binding moieties, each
of which
binds with a distinct target. In some embodiments, a bispecific binding
molecule is a
single polypeptide; in some embodiments, a bispecific binding molecule is or
comprises a
plurality of peptides which, in some such embodiments may be covalently
associated with
one another, for example by cross-linking. In some embodiments, the two
binding
moieties of a bispecific binding molecule recognize different sites (e.g.,
epitopes) the same
target (e.g., antigen); in some embodiments, they recognize different targets.
In some
embodiments, a bispecific binding molecule is capable of binding
simultaneously to two
targets which are of different structure.
[0096] CDR: The term "CDR" as used herein, refers to a complementarity
determining region within an antibody variable region. There are three CDRs in
each of
the variable regions of the heavy chain and the light chain, which are
designated CDR1,
CDR2 and CDR3, for each of the variable regions. A "set of CDRs" or "CDR set"
refers
to a group of three or six CDRs that occur in either a single variable region
capable of
binding the antigen or the CDRs of cognate heavy and light chain variable
regions capable
of binding the antigen. Boundaries of CDRs have been defined differently
depending on
the system, of which several are known in the art (e.g., Kabat, Chothia,
etc.).
[0097] Chimeric: A "chimeric" antibody as used herein, is a recombinant
protein
that contains the variable domains including the complementarity-determining
regions
(CDRs) of an antibody derived from one species, preferably a rodent antibody,
while the
constant domains of the antibody molecule is derived from those of a human
antibody. For
veterinary applications, the constant domains of the chimeric antibody may be
derived
from that of other species, such as a cat or dog.
17
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
[0098] Combination: The term "in combination" as used herein, refers to
the use
of more than one prophylactic and/or therapeutic agents (e.g., an anti-CCL2
antibody and
an anti-LOXL2 antibody). The use of the term "in combination" does not
restrict the order
in which prophylactic and/or therapeutic agents are administered to a subject
with a
disorder. A first prophylactic or therapeutic agent (e.g., an anti-CCL2
antibody) can be
administered prior to (e.g., 5 minutes, 15 minutes, 30 minutes, 45 minutes, 1
hour, 2 hours,
4 hours, 6 hours, 12 hours, 24 hours, 48 hours, 72 hours, 96 hours, 1 week, 2
weeks, 3
weeks, 4 weeks, 5 weeks, 6 weeks, 8 weeks, or 12 weeks before), concomitantly
with, or
subsequent to (e.g., 5 minutes, 15 minutes, 30 minutes, 45 minutes, 1 hour, 2
hours, 4
hours, 6 hours, 12 hours, 24 hours, 48 hours, 72 hours, 96 hours, 1 week, 2
weeks, 3
weeks, 4 weeks, 5 weeks, 6 weeks, 8 weeks, or 12 weeks after) the
administration of a
second prophylactic or therapeutic agent (e.g., an anti-LOXL2 antibody) to a
subject with
a disorder.
[0099] Compound and Agent: The terms "compound" and "agent" are used
herein
interchangeably. They refer to any naturally occurring or non-naturally
occurring (i.e.,
synthetic or recombinant) molecule, such as a biological macromolecule (e.g.,
nucleic
acid, polypeptide or protein), organic or inorganic molecule, or an extract
made from
biological materials such as bacteria, plants, fungi, or animal (particularly
mammalian,
including human) cells or tissues. The compound may be a single molecule or a
mixture
or complex of at least two molecules.
[0100] Comparable: The term "comparable" as used herein, refers to
describe two
(or more) sets of conditions or circumstances that are sufficiently similar to
one another to
permit comparison of results obtained or phenomena observed. In some
embodiments,
comparable sets of conditions or circumstances are characterized by a
plurality of
substantially identical features and one or a small number of varied features.
Those of
ordinary skill in the art will appreciate that sets of conditions are
comparable to one
another when characterized by a sufficient number and type of substantially
identical
features to warrant a reasonable conclusion that differences in results
obtained or
phenomena observed under the different sets of conditions or circumstances are
caused by
or indicative of the variation in those features that are varied.
[0101] Control: As used herein, the term "control" has its art-
understood meaning
of being a standard against which results are compared. Typically, controls
are used to
augment integrity in experiments by isolating variables in order to make a
conclusion
18
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
about such variables. In some embodiments, a control is a reaction or assay
that is
performed simultaneously with a test reaction or assay to provide a
comparator. In one
experiment, the "test" (i.e., the variable being tested) is applied. In the
second experiment,
the "control," the variable being tested is not applied. In some embodiments,
a control is a
historical control (i.e., of a test or assay performed previously, or an
amount or result that
is previously known). In some embodiments, a control is or comprises a printed
or
otherwise saved record. A control may be a positive control or a negative
control.
[0102] Dosing regimen: A "dosing regimen" (or "therapeutic regimen"), as
that
term is used herein, is a set of unit doses (typically more than one) that are
administered
individually to a subject, typically separated by periods of time. In some
embodiments, a
given therapeutic agent has a recommended dosing regimen, which may involve
one or
more doses. In some embodiments, a dosing regimen comprises a plurality of
doses each
of which are separated from one another by a time period of the same length;
in some
embodiments, a dosing regimen comprises a plurality of doses and at least two
different
time periods separating individual doses.
[0103] Diagnosis: As used herein, the term "diagnosis" refers to a
process aimed
at determining if an individual is afflicted with a disease or ailment. In the
context of the
present invention, "diagnosis of scleroderma" refers to a process aimed at one
or more of:
determining if an individual is afflicted with scleroderma, identifying a
scleroderma
subtype (i.e., diffuse or limited cutaneous scleroderma), and determining the
severity of
the disease.
[0104] Effective amount: As used herein, the term "effective amount"
refers to an
amount of a compound or agent that is sufficient to fulfill its intended
purpose(s). In the
context of the present invention, the purpose(s) may be, for example: to
modulate the
cause or symptoms of scleroderma; and/or to delay or prevent the onset of
scleroderma;
and/or to slow down or stop the progression, aggravation, or deterioration of
the symptoms
of scleroderma; and/or to alleviate one or more symptoms associated with
scleroderma;
and/or to bring about amelioration of the symptoms of scleroderma, and/or to
cure
scleroderma.
[0105] Framework or framework region: As used herein, refers to the
sequences
of a variable region minus the CDRs. Because a CDR sequence can be determined
by
different systems, likewise a framework sequence is subject to correspondingly
different
19
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
interpretations. The six CDRs divide the framework regions on the heavy and
light chains
into four sub-regions (FR1, FR2, FR3 and FR4) on each chain, in which CDR1 is
positioned between FR1 and FR2, CDR2 between FR2 and FR3, and CDR3 between FR3
and FR4. Without specifying the particular sub-regions as FR1, FR2, FR3 or
FR4, a
framework region, as referred by others, represents the combined FRs within
the variable
region of a single, naturally occurring immunoglobulin chain. As used herein,
a FR
represents one of the four sub-regions, FR1, for example, represents the first
framework
region closest to the amino terminal end of the variable region and 5' with
respect to
CDR1, and FRs represents two or more of the sub-regions constituting a
framework
region.
[0106] Human antibody: As used herein, is intended to include antibodies
having
variable and constant regions generated (or assembled) from human
immunoglobulin
sequences. In some embodiments, antibodies (or antibody components) may be
considered to be "human" even though their amino acid sequences include
residues or
elements not encoded by human germline immunoglobulin sequences (e.g., include
sequence variations, for example that may (originally) have been introduced by
random or
site-specific mutagenesis in vitro or by somatic mutation in vivo), for
example in one or
more CDRs and in particular CDR3.
[0107] Humanized: As is known in the art, the term "humanized" is
commonly
used to refer to antibodies (or antibody components) whose amino acid sequence
includes
VH and VL region sequences from a reference antibody raised in a non-human
species
(e.g., a mouse), but also includes modifications in those sequences relative
to the reference
antibody intended to render them more "human-like", i.e., more similar to
human germline
variable sequences. In some embodiments, a "humanized" antibody (or antibody
component) is one that immunospecifically binds to an antigen of interest and
that has a
framework (FR) region having substantially the amino acid sequence as that of
a human
antibody, and a complementary determining region (CDR) having substantially
the amino
acid sequence as that of a non-human antibody. A humanized antibody comprises
substantially all of at least one, and typically two, variable domains (Fab,
Fab', F(ab')2,
FabC, Fv) in which all or substantially all of the CDR regions correspond to
those of a
non-human immunoglobulin (i.e., donor immunoglobulin) and all or substantially
all of
the framework regions are those of a human immunoglobulin consensus sequence.
In
some embodiments, a humanized antibody also comprises at least a portion of an
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
immunoglobulin constant region (Fc), typically that of a human immunoglobulin
constant
region. In some embodiments, a humanized antibody contains both the light
chain as well
as at least the variable domain of a heavy chain. The antibody also may
include a CHi,
hinge, CH2, CH3, and, optionally, a CH4 region of a heavy chain constant
region. In some
embodiments, a humanized antibody only contains a humanized VL region. In some
embodiments, a humanized antibody only contains a humanized VH region. In some
certain embodiments, a humanized antibody contains humanized VH and VL
regions.
[0108] Fynomers: As used herein, the term "fynomers" refers to a class
of
binding proteins derived from the Src homology (SH3) domain of the human Fyn
kinase,
which is a human protein composed of 63 amino acid residues (D. Grabulovski et
al. J.
Biol. Chem. 282, 3196-3204 (2007)). Fynomers can bind to target molecules with
the
same affinity and specificity as antibodies. It can be produced in bacteria
with high yields.
Moreover, several Fynomers can be linked to yield a protein with multiple
binding
specificities
[0109] Improve, increase, or reduce: As used herein, the terms
"improve,"
"increase" or "reduce," or grammatical equivalents, indicate values that are
relative to a
baseline measurement, such as a measurement in the same individual prior to
initiation of
the treatment described herein, or a measurement in a control individual (or
multiple
control individuals) in the absence of the treatment described herein. A
"control
individual" is an individual afflicted with the same type and approximately
the same
severity of scleroderma as the individual being treated, who is about the same
age as the
individual being treated (to ensure that the stages of the disease in the
treated individual
and the control individual(s) are comparable).
[0110] Kit: As used herein, the term "kit" refers to any delivery system
for
delivering materials. Such delivery systems may include systems that allow for
the
storage, transport, or delivery of various diagnostic or therapeutic reagents
(e.g.,
oligonucleotides, enzymes, etc. in the appropriate containers) and/or
supporting materials
(e.g., buffers, written instructions for performing the assay etc.) from one
location to
another. For example, kits include one or more enclosures (e.g., boxes)
containing the
relevant reaction reagents and/or supporting materials. As used herein, the
term
"fragmented kit" refers to a delivery systems comprising two or more separate
containers
that each contain a subportion of the total kit components. The containers may
be
delivered to the intended recipient together or separately. For example, a
first container
21
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
may contain an enzyme for use in an assay, while a second container contains
oligonucleotides. The term "fragmented kit" is intended to encompass kits
containing
Analyte Specific Reagents (ASR's) regulated under section 520(e) of the
Federal Food,
Drug, and Cosmetic Act, but are not limited thereto. Indeed, any delivery
system
comprising two or more separate containers that each contain a subportion of
the total kit
components are included in the term "fragmented kit." In contrast, a "combined
kit"
refers to a delivery system containing all of the components in a single
container (e.g., in a
single box housing each of the desired components). The term "kit" includes
both
fragmented and combined kits.
[0111] Normal: As used herein, the term "normal," when used to modify
the term
"individual" or "subject" refers to an individual or group of individuals who
does not have
a particular disease or condition and is also not a carrier of the disease or
condition. The
term "normal" is also used herein to qualify a biological specimen or sample
isolated from
a normal or wild-type individual or subject, for example, a "normal biological
sample."
[0112] Nucleic Acid: As used herein the term "nucleic acid" refers to an
oligonucleotide, nucleotide or polynucleotide, and fragments or portions
thereof, and to
DNA or RNA of genomic or synthetic origin that may be single or double
stranded, and
represents the sense or antisense strand.
[0113] Nucleic Acid Molecule: The terms "nucleic acid molecule" and
"polynucleotide" are used herein interchangeably. They refer to a
deoxyribonucleotide or
ribonucleotide polymer in either single- or double-stranded form, and unless
otherwise
stated, encompass known analogs of natural nucleotides that can function in a
similar
manner as naturally occurring nucleotides. The terms encompasses nucleic acid-
like
structures with synthetic backbones, as well as amplification products.
[0114] Protein: In general, a "protein" is a polypeptide (i.e., a string
of at least
two amino acids linked to one another by peptide bonds). Proteins may include
moieties
other than amino acids (e.g., may be glycoproteins) and/or may be otherwise
processed or
modified. Those of ordinary skill in the art will appreciate that a "protein"
can be a
complete polypeptide chain as produced by a cell (with or without a signal
sequence), or
can be a functional portion thereof Those of ordinary skill will further
appreciate that a
protein can sometimes include more than one polypeptide chain, for example
linked by
one or more disulfide bonds or associated by other means.
22
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
[0115] Sample: As used herein, the term "sample" encompasses any sample
obtained from a biological source. The terms "biological sample" and "sample"
are used
interchangeably. A biological sample can, by way of non-limiting example,
include skin
tissue, liver tissue, kidney tissue, lung tissue, cerebrospinal fluid (CSF),
blood, amniotic
fluid, sera, urine, feces, epidermal sample, skin sample, cheek swab, sperm,
amniotic fluid,
cultured cells, bone marrow sample and/or chorionic villi. Cell cultures of
any biological
samples can also be used as biological samples. A biological sample can also
be, e.g., a
sample obtained from any organ or tissue (including a biopsy or autopsy
specimen), can
comprise cells (whether primary cells or cultured cells), medium conditioned
by any cell,
tissue or organ, tissue culture. In some embodiments, biological samples
suitable for the
invention are samples which have been processed to release or otherwise make
available a
nucleic acid for detection as described herein. Fixed or frozen tissues also
may be used.
[0116] Subject: As used herein, the term "subject" refers to a human or
any non-
human animal (e.g., mouse, rat, rabbit, dog, cat, cattle, swine, sheep, horse
or primate). A
human includes pre- and post-natal forms. In many embodiments, a subject is a
human
being. A subject can be a patient, which refers to a human presenting to a
medical
provider for diagnosis or treatment of a disease. The term "subject" is used
herein
interchangeably with "individual" or "patient." A subject can be afflicted
with or is
susceptible to a disease or disorder but may or may not display symptoms of
the disease or
disorder.
[0117] Suffering from: An individual who is "suffering from" a disease,
disorder,
and/or condition (e.g., scleroderma) has been diagnosed with or displays one
or more
symptoms of the disease, disorder, and/or condition.
[0118] Susceptible to: An individual who is "susceptible to" a disease,
disorder,
and/or condition has not been diagnosed with and/or may not exhibit symptoms
of the
disease, disorder, and/or condition. In some embodiments, an individual who is
susceptible to a disease, disorder, and/or condition (for example,
scleroderma) may be
characterized by one or more of the following: (1) a genetic mutation
associated with
development of the disease, disorder, and/or condition; (2) a genetic
polymorphism
associated with development of the disease, disorder, and/or condition; (3)
increased
and/or decreased expression and/or activity of a protein associated with the
disease,
disorder, and/or condition; (4) habits and/or lifestyles associated with
development of the
disease, disorder, and/or condition; (5) a family history of the disease,
disorder, and/or
23
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
condition; (6) reaction to certain bacteria or viruses; (7) exposure to
certain chemicals. In
some embodiments, an individual who is susceptible to a disease, disorder,
and/or
condition will develop the disease, disorder, and/or condition. In some
embodiments, an
individual who is susceptible to a disease, disorder, and/or condition will
not develop the
disease, disorder, and/or condition.
[0119] Treatment: As used herein, the term "treatment" (also "treat" or
"treating") refers to any administration of a therapeutic molecule (e.g., bi-
specific anti-
CCL2/LOXL antibody or simultaneous or sequential co-administration of an anti-
CCL2
monoclonal antibody or antigen binding fragment thereof and an anti-LOXL2
monoclonal
antibody or antigen binding fragment thereof) that partially or completely
alleviates,
ameliorates, relieves, inhibits, delays onset of, reduces severity of and/or
reduces
incidence of one or more symptoms or features of a particular disease,
disorder, and/or
condition (e.g., scleroderma, fibrosis or inflammation). Such treatment may be
of a
subject who does not exhibit signs of the relevant disease, disorder and/or
condition and/or
of a subject who exhibits only early signs of the disease, disorder, and/or
condition.
Alternatively or additionally, such treatment may be of a subject who exhibits
one or more
established signs of the relevant disease, disorder and/or condition.
DETAILED DESCRIPTION OF THE INVENTION
[0120] The present invention provides, among other things, bi-specific
molecules,
including including, but not limited to, antibodies, fynomers, aptamers,
fusion proteins,
protein binding domains (e.g., those derived from receptors) against CCL2 and
LOXL2
and uses thereof, in particular, for treatment of scleroderma and related
fibrotic and/or
inflammatory diseases, disorders and conditions. In some embodiments, the
present
invention further provides methods and compositions for treatment of
scleroderma and
related fibrotic and/or inflammatory diseases, disorders and conditions based
on the
combination of mono-specific anti-CCL2 and anti-LOXL2 molecules (e.g.,
antibodies).
[0121] The present invention is, in part, based on the unique insights
observed by
the present inventors, that is, bi-specific molecules, including antibodies or
fusion
proteins, allow tissue specific targeting of CCL2 without wasting anti-CCL2
molecules
such as antibodies in plasma, resulting in highly effective treatment of
scleroderma.
Embodiments of the invention include bi-specific antibodies that bind to both
CCL2 and
LOXL2. Bi-specific antibodies capable of binding to CCL2 and LOXL2 are
particularly
24
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
advantageous in that they possess a unique tissue selectivity profile and have
the potential
to arrest and clear development of scleroderma, fibrosis and inflammation. As
LOXL2 is
an important enzyme for the development of connective tissue, anti-LOXL2
binding
activity may be used to preferentially target or identify tissues with
relatively large
amounts of connective tissue. Likewise, anti-LOXL2 binding activity may be
used to
target or identify tissues with aberrant connective tissue formation; e.g., as
may be
observed in scleroderma. Moreover, an anti-LOXL2 antibody provides synergistic
therapeutic benefit in that inhibition or neutralization of LOXL2 reduces
amine oxidase
activity afflicted tissues, thus blocking an initiating step in the formation
of connective
tissue. The therapeutic benefit and tissue specificity of LOXL2 antibodies can
be
combined with the therapeutic efficacy of a neutralizing anti-CCL2 monoclonal
antibody
to synergistically target inflammation and reduce fibrotic formation. This
synergistic
targeting is particularly important in the treatment of more advanced cases of
scleroderma
because the LOX binding allows anti-CCL2 antibodies to be sequestered and
compensates
for decreased permeability due to fibrosis.
[0122] Various aspects of the invention are described in detail in the
following
sections. The use of sections is not meant to limit the invention. Each
section can apply
to any aspect of the invention. In this application, the use of "or" means
"and/or" unless
stated otherwise.
Scleroderma
[0123] Scleroderma, or systemic sclerosis, is generally considered a
chronic
systemic autoimmune disease characterized, among other things, fibrosis or
hardening,
vascular alterations, and autoantibodies. Without wishing to be bound by
theory, it is
thought that scleroderma is caused by a hyperactive autoimmune response
trapped in a
reinforcing amplification loop. For example, scleroderma is histologically
characterized
by inflammatory infiltrates of mononuclear cells, which in turn activate and
are associated
with increased collagen synthesis in the surrounding fibroblasts. In
particular, activated
macrophages produce TGF-beta and PDGF, which activate fibroblasts in the
affected
areas to produce high amounts of collagen.
[0124] T cells also appear to play a role in the disease process through
activation
of macrophages and the direct release of inflammatory pro-fibrogenic
cytokines. In
addition to collagen, the activated fibroblasts appear to secrete factors that
recruit
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
additional inflammatory cells to the affected areas, which release cytokines,
which recruit
further cytokine-releasing inflammatory cells, thereby leading to unregulated
inflammation and tissue fibrosis.
[0125] Typically, monocytes/macrophages and T cells increase in both
numbers
and activation in the circulation and tissues of scleroderma patients. Tissue
accumulation
is both a cause and effect of microvascular injury, which is one of the early
events in the
pathogenesis of scleroderma. The microvascular injury is characterized by
endothelial-
cell damage, the proliferation of basal-lamina layers, occasional entrapment
of peripheral-
blood mononuclear cells in the vessel wall, and initial perivascular
mononuclear-cell
infiltrates. As the inflammatory cascades worsen, it is dominated by fibrosis,
derangement
of visceral organ architecture, rarefaction of blood vessels, and
consequently, hypoxia.
All of these factors and the continual recruitment of monocytes contributes to
the
maintenance of fibrosis
[0126] In some embodiments, scleroderma is also considered a connective
tissue
disease generally characterized with an excessive accumulation of
Extracellular Matrix
proteins in the skin and internal organs, vascular injury, and immunological
abnormalities.
[0127] Many of the clinical manifestations of the disease are thought to
involve a
misregulation of vascular remodeling. One of the earliest symptoms of
scleroderma is
microvascular injury. This microvascular injury is thought to cause increased
endothelial
cell activation. Activated endothelial cells are believed to express adhesion
molecules
resulting in altered capillary permeability allowing migration of inflammatory
cells
through the endothelium and entrapment in the vessel wall. The immune
activation is
thought to contribute to sustained endothelial activation, which results in
the breakdown of
endothelial cells. This process is believed to contribute to the loss of
elasticity and
narrowing of the vessels commonly observed in scleroderma patients.
Furthermore, it is
thought that microvascular injury contributes to perivascular infiltrates of
mononuclear
cells in the dermis which is thought to contribute to the activation of
fibroblasts and may
of the associated hallmark symptoms of scleroderma.
[0128] Many of the clinical manifestations of the disease are generally
thought to
involve the misregulation of fibroblasts. The main function of fibroblasts is
to maintain
the structural integrity of connective tissues by continuously secreting
precursors of the
extracellular matrix. Fibroblasts provide a structural framework (stroma) for
many tissues,
26
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
play an important role in wound healing and are the most common cells of
connective
tissue in animals. Fibroblasts are morphologically heterogeneous with diverse
appearances depending on their location and activity.
[0129] There are two major forms of scleroderma: limited systemic
sclerosis/scleroderma and diffuse systemic sclerosis/scleroderma. In limited
cutaneous
scleroderma, the fibrosis of the skin is generally confined to the area
proximal to the
elbow. Patients with limited cutaneous scleroderma generally experience
vascular
impairment. Cutaneous and organ fibrosis generally progresses slowly in
patients with
limited scleroderma. Patients with diffuse scleroderma generally experience
fibrosis of
skin and organs that progresses more rapidly than in limited scleroderma
and/or
widespread inflammation and/or more severe internal organ involvement than is
seen in
limited scleroderma.
[0130] It is generally thought that interstitial lung disease, resulting
in pulmonary
fibrosis, is the leading cause of scleroderma related deaths (Ludwicka-
Bradley, A., et al.
Coagulation and autoimmunity in scleroderma interstitial lung disease. Semin
Arthritis
Rheum, 41(2), 212-22, 2011). Further complications resulting in scleroderma-
related
deaths include but are not limited to cancer, heart failure, pulmonary
hypertension, kidney
failure, and malabsorption, or any combination thereof
[0131] Scleroderma is most commonly diagnosed by inspection of skin
symptoms.
Tests to diagnosis include but are not limited to visual and/or manual
inspection of the
skin, blood pressure testing, chest x-ray, lung CT, echocardiogram,
urinalysis, skin biopsy,
and blood tests including antinuclear antibody testing, antitopoisomerase
antibody testing,
anticentromere antibody testing, anti-U3 antibody testing, anti-RNA antibody
testing,
other types of antibody testing, erythrocyte sedimentation rate, and
rheumatoid factor.
Bi-specific Anti-CCL2 and Anti-LOXL2 Molecules
[0132] The present invention provides methods and compositions for
treating
scleroderma, and related fibrotic and/or inflammatory diseases, disorders and
conditions
based on administration of molecules that bind both CCL2 and LOXL2, in
particular, bi-
specific anti-CCL2 and LOXL2 molecules. In some embodiments, bi-specific
molecules
are nucleic acids, such as, bi-specific nucleic acid aptamers. In some
embodiments, bi-
specific molecules are proteins, such as, bi-specific fusion proteins, protein
aptamers, and
protein binding domains. In some embodiments, bi-specific molecules comprise
bi-
27
CA 02912443 2015-11-12
WO 2014/190316 PCT/US2014/039437
specific fynomers. In some embodiments, bi-specific molecules comprise bi-
specific
antibodies. In some embodiments, a bi-specific antibody suitable for the
present invention
includes a first antigen-binding site that specifically binds to LOXL2 and a
second
antigen-binding site that specifically binds to CCL2 (see FIG. 1).
CCL2
[0133] CCL2 is a
chemokine produced by a variety of cell types. It is also known
as monocyte chemoattractant protein-1 (MCP-1). CCL2 is known to be a potent
attractant
for many cell types of the immune system, including but not limited to
monocytes, CD4
and CD8 memory T lymphocytes and NK cells (Carulli, M. et al. Can CCL2 serum
levels
be used in risk stratification or to monitor treatment response in systemic
sclerosis? Ann
Rheum Dis, 67, 105-109, 2008, Yamamoto , T. Scleroderma ¨ Pathophysiology. Eur
J
Dermatol, 19 (1), 14-24). CCL2 has been shown to promote leukocyte migration
across
endothelial monolayers, suggesting a role in the promotion of perivascular
infiltrates of
mononuclear cells (Id.). CCL2 has also been shown to promote activation of
fibroblasts
and to upregulate Collagen type I mRNA expression in rat fibroblasts in vitro.
Elevated
CCL2 levels have been shown in patients with scleroderma and also in animal
models of
scleroderma (Id.). Specifically, increased CCL2 expression levels have been
shown in
scleroderma skin and increased CCL2 RNA and protein has been shown in
scleroderma
fibroblasts (Id.).
[0134] Human
CCL2 is an 8.6 kDa protein containing 76 amino acid residues, the
amino acid sequence of which is shown in Table 1. It is expressed by a variety
of cell
types, including monocytes, vascular endothelial cells, smooth muscle cells,
certain
epithelial cells, among others and binds its receptor CCR2. CCL2 belongs to
the family of
the CC chemokines which contains two cysteine residues that are adjacent (the
adjacent
cysteine residues underlined in Table 1).
TABLE 1
Human CCL2
MKVSAALLCLLLIAATFIPQGLAQPDAINAPVTCCYNFTN
Protein Sequence
RKISVQRLASYRRITSSKCPKEAVIFKTIVAKEICADPKQK
(GeneBank: WVQDSMDHLDKQTQTPKT (SEQ ID NO: 1)
NP 002973)
28
CA 02912443 2015-11-12
WO 2014/190316 PCT/US2014/039437
[0135] CCL2 has also been purified, characterized, cloned and sequenced
from
non-human sources and can be recombinantly produced or chemically synthesized.
As
used herein, the term CCL2 encompasses any CCL2 proteins naturally-occurring
in other
species including, but not limited to, mouse, rats, primates, pigs, chickens,
dogs, goats,
sheeps, horses, camels, llama, to name but a few, and any recombinant or
synthetic CCL2
that is substantially homologous or identical to human CCL2. In some
embodiments, a
CCL2 protein as used herein has a sequence at least 50%, 55%, 60%, 65%, 70%,
75%,
80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% or more homologous
to SEQ ID NO: 1. In some embodiments, a CCL2 protein as used herein has a
sequence at
least 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%,
96%, 97%, 98%, 99% or more identical to SEQ ID NO: 1. Typically, a CCL2
protein
substantially homologous or identical to human CCL2 also retains substantial
activity of
human CCL2.
[0136] Any of the above described CCL2 proteins can be used to generate
and
identify mono-specific and/or bi-specific antibodies that specifically bind to
CCL2. See
the Anti-CCL2 Antibodies and Bi-specific Anti-CCL2 and LOXL2 Antibodies
sections
below.
LOXL2
[0137] LOXL2 is a member of the lysyl oxidase family of copper-dependent
amine
oxidases. Without wishing to be bound by theory, it is thought that LOXL2
catalyzes the
covalent cross-link of the component side chains of collagen and those of
elastin, thus
stabilizing these proteins in the extracellular matrix (ECM). The polypeptide
sequence of
human LOXL2 is well characterized, as shown in Table 2.
TABLE 2
Human LOXL2 MEGYVEVKEGKTWKQICDKHWTAKNSRVVCGMFGFPG
Protein Sequence ERTYNTKVYKMFASRRKQRYWPF SMD CT GTEAHIS S CK
(GeneBank: LGPQVSLDPMKNVTCENGQPAVVSCVPGQVFSPDGPSRF
AAD34343) RKAYKPEQPLVRLRGGAYIGEGRVEVLKNGEWGTVCDD
KWDLVSASVVCRELGFGSAKEAVTGSRLGQGIGPIHLNEI
Q CT GNEKSIID CKFNAE S Q GCNHEEDAGVRCNTPAMGLQ
KKLRLNGGRNPYEGRVEVLVERNGSLVWGMVCGQNWG
IVEAMVVCRQLGLGFASNAFQETWYWHGDVNSNKVVM
SGVKCSGTELSLAHCRHDGEDVACPQGGVQYGAGVACS
ETAPDLVLNAEMVQQTTYLEDRPMFMLQCAMEENCLSA
29
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
SAAQTDPTTGYRRLLRFSSQIHNNGQSDFRPKNGRHAWI
WHDCHRHYHSMEVFTHYDLLNLNGTKVAEGQKASFCL
EDTECEGDIQKNYECANFGDQGITMGCWDMYRHDIDCQ
WVDITDVPPGDYLFQVVINPNFEVAESDYSNNIMKCRSR
YDGHRIWMYNSHIGGSFSEETEKKFEHFSGLLNNQLSPPV
KKPAWSTPVFRPHHIFHGTSPQQLSLNECHVPSPSPAPTLS
RPLQLCLSSGGKGPSHHSWGAAT (SEQ ID NO: 2)
[0138] LOXL2 has also been purified, characterized, cloned and sequenced
from
non-human sources and can be recombinantly produced or chemically synthesized.
As
used herein, the term LOXL2 encompasses any LOXL2 proteins naturally-occurring
in
other species including, but not limited to, mouse, rats, primates, pigs,
chickens, dogs,
goats, sheeps, horses, camels, llama, to name but a few, and any recombinant
or synthetic
LOXL2 that is substantially homologous or identical to human LOXL2. In some
embodiments, a LOXL2 protein as used herein has a sequence at least 50%, 55%,
60%,
65%, 70%, 75%, 80%, 85%, 90%, 91%, 92%, 93%, 94%, 95%, 96%, 97%, 98%, 99% or
more homologous to SEQ ID NO:2. In some embodiments, a LOXL2 protein as used
herein has a sequence at least 50%, 55%, 60%, 65%, 70%, 75%, 80%, 85%, 90%,
91%,
92%, 93%, 94%, 95%, 96%, 97%, V76 /o -no z,
99% or more identical to SEQ ID NO:2.
Typically, a LOXL2 protein substantially homologous or identical to human
LOXL2 also
retains substantial activity of human LOXL2.
[0139] Any of the above described LOXL2 proteins can be used to generate
and
identify desired mono-specific and/or bi-specific antibodies that specifically
bind to
LOXL2. See the Anti-LOXL2 Antibodies and Bi-specific Anti-CCL2 and LOXL2
Antibodies sections below.
[0140] "Percent (%) amino acid sequence identity" with respect to the
CCL2 and
LOXL2 sequences identified herein is defined as the percentage of amino acid
residues in
a candidate sequence that are identical with the amino acid residues in the
CCL2 or
LOXL2 sequence, after aligning the sequences and introducing gaps, if
necessary, to
achieve the maximum percent sequence identity, and not considering any
conservative
substitutions as part of the sequence identity. Alignment for purposes of
determining
percent amino acid sequence identity can be achieved in various ways that are
within the
skill in the art, for instance, using publicly available computer software
such as BLAST,
ALIGN or Megalign (DNASTAR) software. Those skilled in the art can determine
appropriate parameters for measuring alignment, including any algorithms
needed to
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
achieve maximal alignment over the full length of the sequences being
compared.
Preferably, the WU-BLAST-2 software is used to determine amino acid sequence
identity
(Altschul et al., Methods in Enzymology 266, 460-480 (1996);
http://blast.wustl/edu/blast/README.html). WU-BLAST-2 uses several search
parameters, most of which are set to the default values. The adjustable
parameters are set
with the following values: overlap span=1, overlap fraction=0.125, world
threshold
(T)=11. HSP score (S) and HSP S2 parameters are dynamic values and are
established by
the program itself, depending upon the composition of the particular sequence,
however,
the minimum values may be adjusted and are set as indicated above.
Anti-CCL2 and Anti-LOXL2 Mono-specific Antibodies
[0141] CCL2 and LOXL2 proteins described herein, or fragments thereof,
can be
used to generate antibodies by methods well known to those of skill in the
art. As used
herein, anti-CCL2 mono-specific antibodies include any antibodies or fragments
of
antibodies that bind specifically to any epitopes of CCL2 and anti-LOXL2 mono-
specific
antibodies include any antibodies or fragments of antibodies that bind
specifically to any
epitopes of LOXL2. As used herein, the term "antibodies" is intended to
include
immunoglobulins and fragments thereof which are specifically reactive to the
designated
protein or peptide, or fragments thereof For example, the term "antibodies"
includes
intact monoclonal antibodies, polyclonal antibodies, single domain antibodies
(e.g., shark
single domain antibodies (e.g., IgNAR or fragments thereof)), and antibody
fragments so
long as they exhibit the desired biological activity. Suitable antibodies also
include, but
are not limited to, human antibodies, primatized antibodies, chimeric
antibodies, bi-
specific antibodies, humanized antibodies, conjugated antibodies (i.e.,
antibodies
conjugated or fused to other proteins, radiolabels, cytotoxins), Small Modular
ImmunoPharmaceuticals ("SMIPsTm"), and antibody fragments.
[0142] As used herein, an "antibody fragment" includes a portion of an
intact
antibody, such as, for example, the antigen-binding or variable region of an
antibody.
Examples of antibody fragments include Fab, Fab', F(ab')2, and Fy fragments;
triabodies;
tetrabodies; linear antibodies; single-chain antibody molecules. The term
"antibody
fragment" also includes any synthetic or genetically engineered protein that
acts like an
antibody by binding to a specific antigen to form a complex. For example,
antibody
fragments include isolated fragments, "Fv" fragments, consisting of the
variable regions of
31
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
the heavy and light chains, recombinant single chain polypeptide molecules in
which light
and heavy chain variable regions are connected by a peptide linker ("ScFy
proteins"), and
minimal recognition units consisting of the amino acid residues that mimic the
hypervariable region.
[0143] Mono-specific antibodies can be generated using methods well
known in
the art. For example, protocols for antibody production are described by
Harlow and
Lane, Antibodies: A Laboratory Manual, (1988). Typically, antibodies can be
generated
in mouse, rat, guinea pig, hamster, camel, llama, shark, or other appropriate
host.
Alternatively, antibodies may be made in chickens, producing IgY molecules
(Schade et
al., (1996) ALTEX13(5):80-85). In some embodiments, antibodies suitable for
the present
invention are subhuman primate antibodies. For example, general techniques for
raising
therapeutically useful antibodies in baboons may be found, for example, in
Goldenberg et
al., international patent publication No. WO 91/11465 (1991), and in Losman et
al., Int. J.
Cancer 46: 310 (1990). In some embodiments, monoclonal antibodies may be
prepared
using hybridoma methods (Milstein and Cuello, (1983) Nature 305(5934):537-
40.). In
some embodiments, monoclonal antibodies may also be made by recombinant
methods
(U.S. Pat. No. 4,166,452, 1979).
[0144] Many of the difficulties associated with generating monoclonal
antibodies
by B-cell immortalization can be overcome by engineering and expressing
antibody
fragments in E. coli, using phage display. To ensure the recovery of high
affinity,
monoclonal antibodies a combinatorial immunoglobulin library must typically
contain a
large repertoire size. A typical strategy utilizes mRNA obtained from
lymphocytes or
spleen cells of immunized mice to synthesize cDNA using reverse transcriptase.
The
heavy- and light-chain genes are amplified separately by PCR and ligated into
phage
cloning vectors. Two different libraries are produced, one containing the
heavy-chain
genes and one containing the light-chain genes. Phage DNA is isolated from
each library,
and the heavy- and light-chain sequences are ligated together and packaged to
form a
combinatorial library. Each phage contains a random pair of heavy- and light-
chain
cDNAs and upon infection of E. coli directs the expression of the antibody
chains in
infected cells. To identify an antibody that recognizes the antigen of
interest, the phage
library is plated, and the antibody molecules present in the plaques are
transferred to
filters. The filters are incubated with radioactively labeled antigen and then
washed to
remove excess unbound ligand. A radioactive spot on the autoradiogram
identifies a
32
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
plaque that contains an antibody that binds the antigen. Cloning and
expression vectors
that are useful for producing a human immunoglobulin phage library can be
obtained, for
example, from STRATAGENE Cloning Systems (La Jolla, Calif.).
[0145] A similar strategy can be employed to obtain high-affinity scFv.
See, e.g.,
Vaughn et al., Nat. Biotechnol., 14: 309 314 (1996). An scFv library with a
large
repertoire can be constructed by isolating V-genes from non-immunized human
donors
using PCR primers corresponding to all known VH, Vk and V), gene families.
Following
amplification, the Vk and V), pools are combined to form one pool. These
fragments are
ligated into a phagemid vector. The scFv linker, (G1y4, Ser)3, is then ligated
into the
phagemid upstream of the VL fragment. The VH and linker-VL fragments are
amplified
and assembled on the JH region. The resulting VH-linker-VL fragments are
ligated into a
phagemid vector. The phagemid library can be panned using filters, as
described above,
or using immunotubes (Nunc; Maxisorp). Similar results can be achieved by
constructing
a combinatorial immunoglobulin library from lymphocytes or spleen cells of
immunized
rabbits and by expressing the scFv constructs in P. pastoris. See, e.g.,
Ridder et al.,
Biotechnology, 13: 255 260 (1995). Additionally, following isolation of an
appropriate
scFv, antibody fragments with higher binding affinities and slower
dissociation rates can
be obtained through affinity maturation processes such as CDR3 mutagenesis and
chain
shuffling. See, e.g., Jackson et al., Br. J. Cancer, 78: 181 188 (1998);
Osbourn et al.,
Immunotechnology, 2: 181 196 (1996).
[0146] Another form of an antibody fragment is a peptide coding for a
single
CDR. CDR peptides ("minimal recognition units") can be obtained by
constructing genes
encoding the CDR of an antibody of interest. Such genes are prepared, for
example, by
using the polymerase chain reaction to synthesize the variable region from RNA
of
antibody-producing cells. See, for example, Larrick et al., Methods: A
Companion to
Methods in Enzymology 2:106 (1991); Courtenay-Luck, "Genetic Manipulation of
Monoclonal Antibodies," in MONOCLONAL ANTIBODIES: PRODUCTION,
ENGINEERING AND CLINICAL APPLICATION, Ritter et al. (eds.), pages 166 179
(Cambridge University Press 1995); and Ward et al., "Genetic Manipulation and
Expression of Antibodies," in MONOCLONAL ANTIBODIES: PRINCIPLES AND
APPLICATIONS, Birch et al., (eds.), pages 137 185 (Wiley-Liss, Inc. 1995).
[0147] In some embodiments, antibodies suitable for the invention may
include
humanized or human antibodies. Humanized forms of non-human antibodies are
chimeric
33
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
Igs, Ig chains or fragments (such as Fv, Fab, Fab', F(ab')2 or other antigen-
binding
subsequences of Abs) that contain minimal sequence derived from non-human Ig.
Generally, a humanized antibody has one or more amino acid residues introduced
from a
non-human source. These non-human amino acid residues are often referred to as
"import" residues, which are typically taken from an "import" variable domain.
Humanization is accomplished by substituting rodent complementarity
determining
regions (CDRs) or CDR sequences for the corresponding sequences of a human
antibody
(Riechmann et al., Nature 332(6162):323-7, 1988; Verhoeyen et al., Science.
239(4847):1534-6, 1988.). Such "humanized" antibodies are chimeric Abs (U.S.
Pat. No.
4,816,567, 1989), wherein substantially less than an intact human variable
domain has
been substituted by the corresponding sequence from a non-human species. In
some
embodiments, humanized antibodies are typically human antibodies in which some
CDR
residues and possibly some FR residues are substituted by residues from
analogous sites in
rodent Abs. Humanized antibodies include human Igs (recipient antibody) in
which
residues from a CDR of the recipient are replaced by residues from a CDR of a
non-
human species (donor antibody) such as mouse, rat or rabbit, having the
desired
specificity, affinity and capacity. In some instances, corresponding non-human
residues
replace Fy framework residues of the human Ig. Humanized antibodies may
comprise
residues that are found neither in the recipient antibody nor in the imported
CDR or
framework sequences. In general, the humanized antibody comprises
substantially all of
at least one, and typically two, variable domains, in which most if not all of
the CDR
regions correspond to those of a non-human Ig and most if not all of the FR
regions are
those of a human Ig consensus sequence. The humanized antibody optimally also
comprises at least a portion of an Ig constant region (Fc), typically that of
a human Ig
(Riechmann et al., Nature 332(6162):323-7, 1988; Verhoeyen et al., Science.
239(4847):1534-6, 1988.).
[0148] Human
antibodies can also be produced using various techniques, including
phage display libraries (Hoogenboom et al., Mol Immunol. (1991) 28(9):1027-37;
Marks
et al., J Mol Biol. (1991) 222(3):581-97) and the preparation of human
monoclonal
antibodies (Reisfeld and Sell, 1985, Cancer Surv. 4(1):271-90). Similarly,
introducing
human Ig genes into transgenic animals in which the endogenous Ig genes have
been
partially or completely inactivated can be exploited to synthesize human
antibodies. Upon
challenge, human antibody production is observed, which closely resembles that
seen in
34
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
humans in all respects, including gene rearrangement, assembly, and antibody
repertoire
(Fishwild et al., High-avidity human IgG kappa monoclonal antibodies from a
novel strain
of minilocus transgenic mice, Nat Biotechnol. 1996 July; 14(7):845-51; Lonberg
et al.,
Antigen-specific human antibodies from mice comprising four distinct genetic
modifications, Nature 1994 April 28;368(6474):856-9; Lonberg and Huszar, Human
antibodies from transgenic mice, Int. Rev. Immunol. 1995;13(1):65-93; Marks et
al., By-
passing immunization: building high affinity human antibodies by chain
shuffling.
Biotechnology (N Y). 1992 July; 10(7):779-83).
[0149] In some embodiments, a mono-specific anti-CCL2 antibody or
fragment
thereof suitable for the present invention has a binding affinity of or
greater than
approximately 500nM, 100nM, lOnM, 1nM, 500pM, 100pM, 50pM, lOpM, 1pM, 500fM,
400fM, 300fM, 200fM, 100fM, 50fM, 10fM, 1fM. In some embodiments, a mono-
specific anti-CCL2 antibody or fragment thereof suitable for the present
invention has a
binding affinity ranging between approximately 500nM and 1fM, between 500nM
and
10fM, between 500nM and 100fM, between 500nM and 1pM, between lOnM and 1fM,
between lOnM and 100fM, between lOnM and 1pM, between 1nM and 1fM, between
1nM and 100fM, between 1nM and 500fM, between 1nM and 1pM, between 1nM and
lOpM, between 1nM and 50pM, between 1nM and 100pM, between 1nM and 500pM.
[0150] In some embodiments, a mono-specific anti-LOXL2 antibody or
fragment
thereof suitable for the present invention has a binding affinity of or
greater than
approximately lOnM, 1nM, 500pM, 100pM, 50pM, lOpM, 1pM, 500fM, 400fM, 300fM,
200fM, 100fM, 50fM, 10fM, 1fM. In some embodiments, a mono-specific anti-LOXL2
antibody or fragment thereof suitable for the present invention has a binding
affinity
ranging between approximately lOnM and 1fM, between lOnM and 100fM, between
lOnM and 1pM, between 1nM and 1fM, between 1nM and 100fM, between 1nM and
500fM, between 1nM and 1pM, between 1nM and lOpM, between 1nM and 50pM,
between 1nM and 100pM, between 1nM and 500pM.
Bi-specifie Anti-CCL2 and Anti-LOXL2 Antibodies and Fusion Proteins
[0151] In some embodiments, the present invention provides bi-specific
anti-CCL2
and anti-LOXL2 antibodies and/or fusion proteins. As used herein, the term "bi-
specific
antibodies or fusion proteins" encompasses any antibodies, fusion proteins, or
fragments
thereof, that contain at least two antigen-binding sites or antigen-binding
arms with
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
distinct specificities. For example, a bi-specific anti-CCL2 and anti-LOXL2
antibody or
fusion protein suitable for the present invention contains at least a first
antigen-binding site
or arm that specifically binds to LOXL2 and at least a second antigen-binding
site or arm
that specifically binds to CCL2.
[0152] Each individual antigen-binding sites or arms of a bi-specific
antibody may
have desired binding affinity against its specific binding target (e.g., CCL2
or LOXL2). In
some embodiments, an antigen-binding site or arm specifically binds to CCL2
with a
binding affinity of or greater than approximately 500nM, 100nM, lOnM, 1nM,
500pM,
100pM, 50pM, lOpM, 1pM, 500fM, 400fM, 300fM, 200fM, 100fM, 50fM, 10fM, 1fM.
In some embodiments, an antigen-binding site or arm specifically binds to CCL2
with a
binding affinity ranging between approximately 500nM and 1fM, between 500nM
and
10fM, between 500nM and 100fM, between 500nM and 1pM, between lOnM and 1fM,
between lOnM and 100fM, between lOnM and 1pM, between 1nM and 1fM, between
1nM and 100fM, between 1nM and 500fM, between 1nM and 1pM, between 1nM and
lOpM, between 1nM and 50pM, between 1nM and 100pM, between 1nM and 500pM. In
some embodiments, an antigen-binding site or arm specifically binds to LOXL2
with a
binding affinity of or greater than approximately lOnM, 1nM, 500pM, 100pM,
50pM,
lOpM, 1pM, 500fM, 400fM, 300fM, 200fM, 100fM, 50fM, 10fM, 1fM. In some
embodiments, an antigen-binding site or arm specifically binds to LOXL2 with a
binding
affinity ranging between approximately lOnM and 1fM, between lOnM and 100fM,
between lOnM and 1pM, between 1nM and 1fM, between 1nM and 100fM, between 1nM
and 500fM, between 1nM and 1pM, between 1nM and lOpM, between 1nM and 50pM,
between 1nM and 100pM, between 1nM and 500pM. The present invention
encompasses
bi-specific antibodies with combinations of the anti-CCL2 and anti-LOXLs
antigen
binding sites or arms with any of the above described binding affinities. In
particular, a
bi-specific antibody may contain a first antigen-binding site or arm that
specifically binds
to LOXL2 with a binding affinity of 1pM or greater and a second antigen-
binding site or
arm that binds to CCL2 with a binding affinity ranging between 500nM and 1fM.
In
particular, a bi-specific antibody may contain a first antigen-binding site or
arm that
specifically binds to LOXL2 with a binding affinity of 1pM or greater and a
second
antigen-binding site or arm that binds to CCL2 with a binding affinity greater
than 1pM.
[0153] Each antigen-binding site of a bi-specific antibody can be
independently a
complete antigen-binding arm including a full length heavy chain and a full
length light
36
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
chain, an Fab fragment, a single-chain variable fragments (scFvs), or other
forms of
antibody fragments. In some embodiments, desired antigen-binding sites or arms
may be
prepared from mono-specific antibodies against CCL2 and LOXL2 produced using
the
techniques described above and then associating such desired anti-CCL2 and
anti-LOXL2
antigen-binding sites or arms to produce desired bi-specific antibodies. For
example, a
desired anti-CCL2 or anti-LOXL2 antigen¨binding site or arm may be isolated,
separated
or enzymatically digested from mono-specific monoclonal antibodies described
above.
Antigen-binding sites or arms of a bi-specific antibody can be arranged in
various
configurations that allow the two sites or arms to associate while retaining
their antigen
binding ability.
[0154] Suitable bi-specific antibodies or fusion proteins can be in
various bi-
specific antibody formats including, but not limited to, quadromas, chemical
heteroconjugates, recombinant constructs using selected heterodimerization
domains and
recombinant constructs of minimal size consisting of two minimal antigen-
binding sites.
In general, quadromas look like monoclonal antibodies but have two different
antigen-
binding arms. Their classical way of production is based on the somatic fusion
of two
different hybridoma (fused between a tumor cell and an antibody-making normal
cell)
cells, each producing a unique monoclonal antibody (e.g., anti-CCL2 or anti-
LOXL2
monoclonal antibody). Bi-specific antibodies with desired antigen-binding arms
(e.g.,
anti-CCL2 and anti-LOXL2) can be produced by random pairing of two different
antibody
heavy and light chains. Various preferential pairing methods are available to
reduce
mispaired by-products and increase bi-specific antibody yield. For example, a
murine and
a rat hybridoma cell line can be fused that express monoclonal antibodies of
particular IgG
subclasses preferentially pairing with each other. Additionally, the
preferential pairing of
two different antibody heavy chains can be achieved by certain mutations in
the CH3-
domain of human IgGl, so-called "knobs-into-holes" strategy.
[0155] Quadroma format typically contains an Fc region, which can
interact with
Fc receptors. Therefore, bi-specific antibodies with an Fc part are also
referred to as
trispecific antibodies. In some embodiments, the Fc part of a quadroma can be
enzymatic
removed, resulting in bi-specific F(ab')2 (the two antigen binding arms of an
antibody
chemically linked through (a) disulfide bond(s)). In addition, two antigen-
binding sites or
arms can be linked with thioether bonding or through one or more functional
groups on the
antibody or fragment including amine, carboxyl, phenyl, thiol, or hydroxyl
groups.
37
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
[0156] In some embodiments, a bi-specific antibody can be produced by
chemical
coupling of two different monoclonal antibodies or antibody fragments with,
e.g., a
hetero-bifunctional crosslinker. For example, two different Fab's (monovalent
antigen
binding arm(s) of an antibody) can be chemically crosslinked at their hinge
cysteine
residues in a site-directed way. Examples of chemicals appropriate for
chemical
crosslinking or coupling include but are not limited to N-succinimidy1-3-(2-
pyridyldithio)propionate) (SPDP), 1-Ethy1-3-(3-
dimethylaminopropyl)carbodiimide
hydrochloride (EDC), 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB), o-phenylene
dimaleimide, carbodiimides, diisocyanates, diazobenzenes, hexamethylene
diamines,
dimaleimide, glutaraldehyde, 4-succinimidyl-oxycarbonyl-.alpha.-methyl
.alpha.(2-
pyridylthio)toluene (SMPT), N-succinimidyl-S acetyl-thioacetate (SATA), and
combinations thereof
[0157] In some embodiments, certain protein domains that naturally form
heterodimers are used to construct heterodimeric bi-specific antibodies suited
for large-
scale expression. One example is the leucine zipper domains of transcription
factors Fos
and Jun which can be fused to the carboxy-terminus of two different Fab's or
single-chain
(sc) FIT (fragment of the variable region) antibody fragments. In some
embodiments,
antibody constant region domains CI( and CH1 can be used instead of Fos and
Jun
dimerization domains for expression of the bi-specific antibody in bacteria
such as E. co/i.
In certain embodiments, the two antigen-binding sites or arms can be
associated via GST
(glutathione S-transferase) fusion proteins, or a dimerization motifs thereof,
PDZ
dimerization domains, FK-506 BP (binding protein) or dimerization motifs
thereof, natural
or artificial helix-turn-helix dimerization domains (e.g., p53), Protein A or
its dimerization
domain, domain B, among others. In certain embodiments, two antigen-binding
sites or
arms may be associated via interaction with an exogenous component. For
example, the
two antigen-binding sites or arms may contain avidin motifs and both interact
with added
biotin.
[0158] In some embodiments, bi-specific antibodies include so called
diabodies
and tandem single-chain FIT constructs. Typically, these forms of bi-specific
antibodies
are made up from two different antigen-binding sites with minimal additional
protein
sequences acting as linker sequences. Each antigen binding site uses the
minimal VH and
VL domains from two antibody heavy and light chains, respectively. In
diabodies, the VL
domain of one antigen binding site is connected by a short peptide linker with
the VH
38
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
domain of the other antigen binding site and vice versa. Bi-specific
antibodies in the
tandem scFy format typically include two different VL/VH pairs connected by a
flexible
peptide linker on a single protein chain. In some embodiments, tandem scFy
constructs
can be expressed in mammalian host cells which are capable of properly folding
the four
consecutively aligned antibody V regions. The fully functional bi-specific
tandem single-
chain antibodies are secreted into the cell culture supernatant and can be
efficiently
purified by affinity chromatography via a poly-histidine tag followed by size
exclusion
chromatography. Suitable peptide linkers may include any sequence that does
not
interfere with the conformation of the antigen binding sites or arms. In
certain
embodiments, a suitable peptide linker is 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 or
more amino acids
long.
[0159] Antibodies produced by various methods described herein typically
contain
both homospecific and bi-specific molecules. Methods to assay for the presence
of bi-
specific monoclonals are known in the art, including bridge ELISA assays (see,
e.g.,
Suresh et al. (1986) Proc. Natl. Acad. Sci. USA 83, 7989-93; Koolwijk et al.
(1988)
Hybridoma 7, 217-225; and De Lau et al. (1989) J. Immunol. 149, 1840-46).
Double-
antigen ELISA may be employed if sufficient quantities of the respective
antigens are
available.
[0160] The particular methods of bi-specific antibody preparation
described above
occasional result in the formation of monospecific as well as bi-specific
antibodies (e.g.,
following procedures of chemical coupling). When this occurs, the desired bi-
specific
antibodies can be separated from the monospecific ones by any of a variety of
procedures
which allow differentiation between the two forms. Such procedures include but
are not
limited to passive elution from preparative, non-denaturing acrylamide gels or
various
conventional chromatographic techniques, e.g., anion-exchange, HPLC, or
thiophilic
adsorption chromatography (see,e.g., Kreutz et al. (1998). J. Chromatography
14, 161-
170). Additionally, each of the antigen-binding sites or arms may be tagged
with a
different tag, and doubly tagged, bi-specific antibodies are separated from
singly tagged
monospecific antibodies by dual affinity chromatography.
[0161] Additional methods of generating, purifying and characterizing bi-
specific
antibodies are known in the art; for example, as disclosed in U.S. Pat. Nos.
5,601,819;
6,004,555, 5,762,930; 6,060,285; 6,010,902; 5,959,083; 5,807,706, and U.S.
Patent
Publication No. 2002/0025317, each of which is incorporated by reference
herein.
39
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
[0162] Desired bi-specific anti-CCL2 and anti-LOXL2 antibodies can be
further
modified to produce chimeric, humanized, or fully human bi-specific antibodies
using
standard methods known in the art including various methods described herein.
Treatment of Seleroderma and Related Diseases, Disorders or Conditions
[0163] Bi-specific and/or mono-specific anti-CCL2 and anti-LOXL2
molecules
(e.g., antibodies, fynomers, aptamers, fusion proteins, or protein binding
domains)
described herein may be used to effectively treat individuals suffering from
or susceptible
to scleroderma or related fibrotic, inflammatory diseases, disorders or
conditions. The
terms, "treat" or "treatment," as used herein, refers to amelioration of one
or more
symptoms, prevention or delay of the onset of one or more symptoms, and/or
lessening of
the severity or frequency of one or more symptoms of the relevant disease,
disorder or
condition.
[0164] Various molecules of the invention may be administered alone or
in
combination. In some embodiments, a method of treatment according to the
present
invention involves administering a bi-specific molecule described herein into
a subject in
need of treatment. In some embodiments, a method of treatment according to the
present
invention involves administering an anti-CCL2 and an anti-LOXL2 mono-specific
antibodies, or fragments thereof, described herein into a subject in need of
treatment.
Anti-CCL2 and anti-LOXL2 mono-specific antibodies, or fragments thereof, can
be
administered simultaneously or sequentially, via same or different
administration routes.
[0165] In some embodiments, molecules described herein may be
administered
alone or in conjunction with other therapeutic agents, such as those that are
useful in
treating fibrotic or inflammatory diseases, disorders or conditions. Such
therapeutic
agents include, but are not limited to, corticosteroids, NSAIDs, immune-
suppressing drugs
(e.g., Metotrexate and Cytoxan), small molecule immunomodulators, interferon
receptor
antibodies, anti-fibrotic drugs including D-penicillamine, colchicine, PUVA,
relaxin, and
cyclosporine and anti-TGFbeta treatments, and endothelin receptor antagonists.
[0166] In some embodiments, molecules described herein can be
administered
using conventional doses and delivery methods, such as those described for
other,
comparable therapeutic agents. Dosages to be administered can be determined by
conventional procedures known to those of skill in the art. See, e.g., The
Pharmacological
Basis of Therapeutics, Goodman and Gilman, eds., Macmillan Publishing Co., New
York.
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
In general, effective dosages are those which are large enough to produce the
desired
effect, e.g., neutralizing CCL2 and/or LOXL2 and/or blocking the binding of
CCL2 and/or
LOXL2 to their cognate receptors. The dosage should not be so large as to
cause adverse
side effects, such as unwanted cross-reactions, anaphylactic reactions, and
the like.
Factors to be considered include the activity of the specific antibody/agent
involved, its
metabolic stability and length of action, mode and time of administration,
drug
combination, rate of excretion, and the age, body weight, general health, sex,
diet, and
severity of the particular disease-states of the host undergoing therapy.
[0167] Molecules described herein can be administered in any dosing
regimen that
is therapeutically effective. In some embodiments, anti-CCL2/LOXL2 bi-specific
or
mono-specific antibodies are administered at bimonthly, monthly, triweekly,
biweekly,
weekly, daily, or at variable intervals.
[0168] Molecules described herein can be administered using any method
of
administration including parenteral and non-parenteral routes of
administration.
Parenteral routes include, e.g., intravenous, intraarterial, intraportal,
intramuscular,
subcutaneous, intraperitoneal, intraspinal, intrathecal,
intracerebroventricular, intracranial,
intrapleural or other routes of injection. Non-parenteral routes include,
e.g., oral, nasal,
transdermal, pulmonary, rectal, buccal, vaginal, ocular. Administration may
also be by
continuous infusion, local administration, sustained release from implants
(gels,
membranes or the like), and/or intravenous injection. When a combination of
anti-CCL2
and anti-LOXL2 antibodies are used, anti-CCL2 and anti-LOXL2 antibodies can be
administered via the same administration route or via different administration
routes.
Scleroderma
[0169] In some embodiments, methods and compositions described herein
can be
used to treat a subject who is suffering or susceptible to all forms of
scleroderma,
including, the limited systemic sclerosis/scleroderma, the diffuse systemic
sclerosis/scleroderma, and other forms of scleroderma. Limited systemic
sclerosis/scleroderma typically involves cutaneous manifestations that mainly
affect the
hands, arms and face. It is also known as CREST syndrome in reference to the
following
complications: Calcinosis, Raynaud's phenomenon, Esophageal dysfunction,
Sclerodactyly, and Telangiectasias. Additionally, pulmonary arterial
hypertension may
occur in up to one-third of patients, and is the most serious complication for
this form of
41
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
scleroderma. Diffuse systemic sclerosis/scleroderma is rapidly progressing and
affects a
large area of the skin and one or more internal organs, frequently the
kidneys, esophagus,
heart and lungs. Other forms of scleroderma include systemic sine scleroderma,
which
lacks skin changes, but has systemic manifestations, and two localized forms
which affect
the skin, but not the internal organs: morphea and linear scleroderma.
[0170] In some embodiments, treatment refers to partially or completely
alleviation, amelioration, relief, inhibition, delaying onset, reducing
severity and/or
incidence of one or more symptoms associated with scleroderma, including but
not limited
to, endothelial-cell damage, proliferation of basal-lamina layers,
perivascular
mononuclear-cell infiltration, fibrosis, derangement of visceral-organ
architecture,
rarefaction of blood vessels, hypoxia, swelling of the fingers, dorsa, and
forearms,
sensations of coldness in the extremities, digital ulcers, elongation of nail
folds, pitted
bleeding of the nails, pitting scars on the nails, pulmonary hypertension,
skin fibrosis, hair
loss, skin tightness, skin hardness, hyperpigmentation, hypopigmentation,
itching of the
skin, carpal tunnel syndrome, muscle weakness, joint pain, joint stiffness,
kidney fibrosis,
esophageal fibrosis, mouth fibrosis, heart fibrosis, and lung fibrosis, liver
fibrosis, muscle
fibrosis, dry cough, shortness of breath, difficulty breathing, alveolitis,
pneumonia,
wheezing, bloating after meals, constipation, diarrhea, difficulty swallowing,
gastric antral
vascular ectasia, esophageal reflux, heartburn, fecal incontinence, flat white
patches in the
mouth, loss of attached gingival mucosa, gingival recession, diffuse widening
of the
periodontal ligament, dysphagia, inelasticity of the mouth, resorption of
posterior ramus of
the mandible, coronoid process, and condyle, cancer, heart failure, pulmonary
hypertension, kidney failure, malabsorption, or any combination thereof, as
compared to
an untreated control or the pre-treatment state.
[0171] In some embodiments, treatment refers to partially or completely
alleviation, amelioration, relief, inhibition, delaying onset, reducing
severity and/or
incidence of fibrosis. As used herein, the term "fibrosis" refers to the
formation of an
excess fibrous connective tissue in an organ or tissue. Without wishing to be
bound by
particular theory, it is thought that fibrosis may be caused by activation of
certain
fibroblast. Different subtypes of fibroblasts are known to perform diverse
functions, even
within a single tissue. For example, papillary fibroblasts of the upper layers
of the skin
produce thin collagen bundles and have a high rate of proliferation, whereas
reticular
fibroblasts from the deeper dermal layer of the skin produce thick collagen
bundles and
42
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
abundant versican, and promote rapid lattice contraction. Fibroblasts can be
in a quiescent
state or at varying stages of activation. During normal cellular function,
fibroblasts
become activated, for example, in response to injury to facilitate wound
healing.
Activated fibroblasts produce increased components of the extracellular
matrix, including
collagen and collagen modifying enzymes. In individuals with scleroderma, an
increase in
fibroblast activation is generally observed, accompanied by an overproduction
of the
ECM. This overproduction of the ECM is generally believed to cause fibrosis,
the
formation of excess fibrous connective tissue in an organ or tissue, that is a
characteristic
of scleroderma.
[0172] In some embodiments, treatment refers to partially or completely
alleviation, amelioration, relief, inhibition, delaying onset, reducing
severity and/or
incidence of fibrosis in skin, kidney, liver, lung and/or oesophagus.
[0173] In some embodiments, treatment results in partially or completely
alleviation, amelioration, relief, inhibition, delaying onset, reducing
severity and/or
incidence of skin fibrosis. Typically, skin fibrosis is associated with skin
thickening,
hardening, or formation of scars (e.g., keloid or burn scar, etc.). In some
embodiments,
skin fibrosis is assessed by Modified Rodnan Skin Score. For example, as
illustrated in
FIG. 2 uninvolved skin is given a score 0; mild thickening is given a score 1;
moderate
thickening is given a score 2; and severe thickening is given a score 3. In
some
embodiments, treatment results in a reduction of Modified Rodnan Skin Score by
more
than 10%, more than 15%, more than 20%, more than 25%, more than 30%, more
than
35%, more than 40%, more than 45%, more then 50%, more than 55%, more than
60%,
more than 65%, more than 70%, more than 75%, more than 80%, more than 85%,
more
than 90%, more than 95%, or more, as compared to the pre-treatment state. In
some
embodiments, treatment results in substantial elimination of skin fibrosis.
[0174] Without wishing to be bound by theory, it is also thought that
activation of
fibroblasts in scleroderma patients may be caused by the activation of the
immune
response by the production of cytokines. Examples of cytokines include but are
not
limited to TGF-P, CCL2, CTGF, ET-1, Fibroblast Growth Factor, IL-1, IL-4, IL-
6, IL-12,
IL-13, IL-17, MCP-1, MCP-3, and PDGF. Cytokines can be produced by pro-
inflammatory cells of the immune system, for example activated T-cells,
monocytes, or
macrophages or, alternatively, cytokines can be produced by epithelial cells.
One factor
contributing to the activation of fibroblasts may be perivascular infiltrates
of mononuclear
43
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
cells in the dermis associated with increased capillary permeability.
Alternative or
additional means of fibroblast activation include interaction with the
extracellular matrix
and/or mechanical tension. Thus, in some embodiments, treatment of scleroderma
patients
according to the present invention results in reduction of the production of
one or more
pro-inflammatory cytokines, such as those described herein. In some
embodiments,
treatment results in a reduction of a pro-inflammatory cytokine (e.g., TGF-P,
CCL2,
CTGF, ET-1, Fibroblast Growth Factor, IL-1, IL-4, IL-6, IL-12, IL-13, IL-17,
MCP-1,
MCP-3, and/or PDGF) by more than 10%, more than 15%, more than 20%, more than
25%, more than 30%, more than 35%, more than 40%, more than 45%, more then
50%,
more than 55%, more than 60%, more than 65%, more than 70%, more than 75%,
more
than 80%, more than 85%, more than 90%, more than 95%, or more, as compared to
the
pre-treatment state. Various methods for determining the level of cytokines
are known in
the art and can be used to practice the present invention.
[0175] In some embodiments, treatment results in reduced CCL2 serum
levels. In
some embodiments, treatment results in a reduction of CCL2 serum levels by
more than
10%, more than 15%, more than 20%, more than 25%, more than 30%, more than
35%,
more than 40%, more than 45%, more then 50%, more than 55%, more than 60%,
more
than 65%, more than 70%, more than 75%, more than 80%, more than 85%, more
than
90%, more than 95%, or more, as compared to the pre-treatment state. In some
embodiments, treatment results in a CCL2 serum level of less than about 800
pg/ml, 700
pg/ml, 600 pg/ml, 500 pg/ml, 400 pg/ml, 350 pg/ml, 300 pg/ml, 250 pg/ml, 200
pg/ml,
150 pg/ml, or 100 pg/ml. In some embodiments, treatment results in a CCL2
serum level
comparable to that of a healthy control of substantially same age or
developmental stage.
Fibrotic diseases, disorders or conditions
[0176] In addition to Scleroderma, methods and compositions according to
the
present invention can be used to treat fibrotic diseases, disorders or
conditions in general
including, but not limited to, multifocal fibrosclerosis, sclerodermatous
graft-vs-host-
disease, nephrogenic systemic fibrosis, organ specific fibrosis, and the like.
Illustrative
organ specific fibrotic disorders include, but are not limited to, pulmonary
fibrosis,
pulmonary hypertension, cystic fibrosis, asthma, chronic obstructive pulmonary
disease,
liver fibrosis, kidney fibrosis, NASH, and the like. Many fibrotic diseases,
disorders or
conditions have disordered and/or exaggerated deposition of extracellular
matrix in
44
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
affected tissues. Fibrosis may be associated with inflammation, occur as a
symptom of
underlying disease, and/or caused by surgical procedure or wound healing
process.
Unchecked fibrosis can result in destruction of the architecture of the
underlying organ or
tissue, commonly referred to as scarring.
[0177] NASH is usually a silent disease with few or no symptoms.
Patients
generally feel well in the early stages and only begin to have symptoms¨such
as fatigue,
weight loss, and weakness¨once the disease is more advanced or cirrhosis
develops. The
progression of NASH can take years, even decades. The process can stop and, in
some
cases may even begin to reverse on its own without specific therapy. Or NASH
can slowly
worsen, causing scarring or fibrosis to appear and accumulate in the liver. As
fibrosis
worsens, cirrhosis develops in which the liver becomes seriously scarred,
hardened, and
unable to function normally. Not every person with NASH develops cirrhosis,
but once
serious scarring or cirrhosis is present, few treatments can halt the
progression. A person
with cirrhosis experiences fluid retention, muscle wasting, bleeding from the
intestines,
and liver failure. Liver transplantation is the only treatment for advanced
cirrhosis with
liver failure, and transplantation is increasingly performed in people with
NASH. NASH
ranks as one of the major causes of cirrhosis in America, behind hepatitis C
and alcoholic
liver disease.
[0178] Kidney (renal) fibrosis results from excessive formation of
fibrous
connective tissue in the kidney. Kidney fibrosis causes significant morbidity
and mortality
and leads to a need for dialysis or kidney transplantation. Fibrosis can occur
in either the
filtering or reabsorptive component of the nephron, the functional unit of the
kidney. A
number of factors may contribute to kidney scarring, particularly derangements
of
physiology involved in the autoregulation of glomerular filtration. This in
turn leads to
replacement of normal structures with accumulated extracellular matrix. A
spectrum of
changes in the physiology of individual cells leads to the production of
numerous peptide
and non-peptide fibrogens that stimulate alterations in the balance between
extracellular
matrix synthesis and degradation to favor scarring.
Inflammatory diseases, disorders or conditions
[0179] In some embodiments, methods and compositions according to the
present
invention are used to treat inflammatory diseases, disorders or conditions
including, but
not limited to: Systemic Inflammatory Response (SIRS); Alzheimer's Disease
(and
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
associated conditions and symptoms including: chronic neuroinflammation, glial
activation; increased microglia; neuritic plaque formation; and response to
therapy);
Amyotropic Lateral Sclerosis (ALS), arthritis (and associated conditions and
symptoms
including, but not limited to: acute joint inflammation, antigen-induced
arthritis, arthritis
associated with chronic lymphocytic thyroiditis, collagen-induced arthritis,
juvenile
arthritis; rheumatoid arthritis, osteoarthritis, prognosis and streptococcus-
induced arthritis,
spondyloarthopathies, gouty arthritis), asthma (and associated conditions and
symptoms,
including: bronchial asthma; chronic obstructive airway disease; chronic
obstructive
pulmonary disease, juvenile asthma and occupational asthma); cardiovascular
diseases
(and associated conditions and symptoms, including atherosclerosis; autoimmune
myocarditis, chronic cardiac hypoxia, congestive heart failure, coronary
artery disease,
cardiomyopathy and cardiac cell dysfunction, including: aortic smooth muscle
cell
activation; cardiac cell apoptosis; and immunomodulation of cardiac cell
function;
diabetes and associated conditions and symptoms, including autoimmune
diabetes, insulin-
dependent (Type 1) diabetes, diabetic periodontitis, diabetic retinopathy, and
diabetic
nephropathy); gastrointestinal inflammations (and related conditions and
symptoms,
including celiac disease, associated osteopenia, chronic colitis, Crohn's
disease,
inflammatory bowel disease and ulcerative colitis); gastric ulcers; hepatic
inflammations
such as viral and other types of hepatitis, cholesterol gallstones and hepatic
fibrosis, HIV
infection (and associated conditions and symptoms, including degenerative
responses,
neurodegenerative responses, and HIV associated Hodgkin's Disease), Kawasaki's
Syndrome (and associated diseases and conditions, including mucocutaneous
lymph node
syndrome, cervical lymphadenopathy, coronary artery lesions, edema, fever,
increased
leukocytes, mild anemia, skin peeling, rash, conjunctiva redness,
thrombocytosis; multiple
sclerosis, nephropathies (and associated diseases and conditions, including
diabetic
nephropathy, endstage renal disease, acute and chronic glomerulonephritis,
acute and
chronic interstitial nephritis, lupus nephritis, Goodpasture's syndrome,
hemodialysis
survival and renal ischemic reperfusion injury), neurodegenerative diseases
(and
associated diseases and conditions, including acute neurodegeneration,
induction of IL-1
in aging and neurodegenerative disease, IL-1 induced plasticity of
hypothalamic neurons
and chronic stress hyperresponsiveness), opthalmopathies (and associated
diseases and
conditions, including diabetic retinopathy, Graves' opthalmopathy, and
uveitis,
osteoporosis (and associated diseases and conditions, including alveolar,
femoral, radial,
vertebral or wrist bone loss or fracture incidence, postmenopausal bone loss,
mass,
46
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
fracture incidence or rate of bone loss), otitis media (adult or pediatric),
pancreatitis or
pancreatic acinitis, periodontal disease (and associated diseases and
conditions, including
adult, early onset and diabetic); pulmonary diseases, including chronic lung
disease,
chronic sinusitis, hyaline membrane disease, hypoxia and pulmonary disease in
SIDS;
restenosis of coronary or other vascular grafts; rheumatism including
rheumatoid arthritis,
rheumatic Aschoff bodies, rheumatic diseases and rheumatic myocarditis;
thyroiditis
including chronic lymphocytic thyroiditis; urinary tract infections including
chronic
prostatitis, chronic pelvic pain syndrome and urolithiasis. Immunological
disorders,
including autoimmune diseases, such as alopecia aerata, autoimmune
myocarditis, Graves'
disease, Graves opthalmopathy, lichen sclerosis, multiple sclerosis,
psoriasis, systemic
lupus erythematosus, systemic sclerosis, thyroid diseases (e.g. goiter and
struma
lymphomatosa (Hashimoto's thyroiditis, lymphadenoid goiter), sleep disorders
and chronic
fatigue syndrome and obesity (non-diabetic or associated with diabetes).
Resistance to
infectious diseases, such as Leishmaniasis, Leprosy, Lyme Disease, Lyme
Carditis,
malaria, cerebral malaria, meningitis, tubulointerstitial nephritis associated
with malaria),
which are caused by bacteria, viruses (e.g. cytomegalovirus, encephalitis,
Epstein-Barr
Virus, Human Immunodeficiency Virus, Influenza Virus) or protozoans (e.g.,
Plasmodium
falciparum, trypanosomes). Response to trauma, including cerebral trauma
(including
strokes and ischemias, encephalitis, encephalopathies, epilepsy, perinatal
brain injury,
prolonged febrile seizures, SIDS and subarachnoid hemorrhage), low birth
weight (e.g.
cerebral palsy), lung injury (acute hemorrhagic lung injury, Goodpasture's
syndrome,
acute ischemic reperfusion), myocardial dysfunction, caused by occupational
and
environmental pollutants (e.g. susceptibility to toxic oil syndrome
silicosis), radiation
trauma, and efficiency of wound healing responses (e.g. burn or thermal
wounds, chronic
wounds, surgical wounds and spinal cord injuries). Hormonal regulation
including
fertility/fecundity, likelihood of a pregnancy, incidence of preterm labor,
prenatal and
neonatal complications including preterm low birth weight, cerebral palsy,
septicemia,
hypothyroidism, oxygen dependence, cranial abnormality, early onset menopause.
A
subject's response to transplant (rejection or acceptance), acute phase
response (e.g. febrile
response), general inflammatory response, acute respiratory distress response,
acute
systemic inflammatory response, wound healing, adhesion, immunoinflammatory
response, neuroendocrine response, fever development and resistance, acute-
phase
response, stress response, disease susceptibility, repetitive motion stress,
tennis elbow, and
pain management and response.
47
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
Biomarkers or Indicators for Patient Stratification, Treatment Monitoring
and/or
Optimization
[0180] In some embodiments, methods and compositions based on anti-
CCL2/LOXL2 bi-specific or mono-specific molecules (e.g., antibodies, fynomers,
aptamers, fusion proteins, or protein binding domains) described herein can be
used with
biomarkers for patient stratification, treatment monitoring and/or
optimization. In some
embodiments, suitable biomarkers are differentially expressed biomarkers. As
used
herein, the term "differentially expressed biomarker" refers to a biomarker
whose level of
expression is different in a subject (or a population of subjects) afflicted
with scleroderma
relative to its level of expression in a healthy or normal subject (or a
population of healthy
or normal subjects). The term also encompasses a biomarker whose level of
expression is
different for a different disease subtype (i.e., Limited cutaneous or diffuse
cutaneous).
The term further encompasses a biomarker whose level of expression is
different at
different stages of the disease (e.g., mild or early scleroderma, severe or
late scleroderma).
Differential expression includes quantitative, as well as qualitative,
differences in the
temporal or cellular expression pattern of the biomarker. As described in
greater details
below, a differentially expressed biomarker, alone or in combination with
other
differentially expressed biomarkers, is useful in a variety of different
applications in
diagnostic, staging, therapeutic, drug development and related areas. The
expression
patterns of the differentially expressed biomarkers disclosed herein can be
described as a
fingerprint or a signature of scleroderma, scleroderma subtype, scleroderma
stage and
scleroderma disease severity and/or progression. They can be used as a point
of reference
to compare and characterize unknown samples and samples for which further
information
is sought. The term "decreased level of expression", as used herein, refers to
a decrease in
expression of at least 10% or more, for example, 20%, 30%, 4u,-so z/0,
or 50%, 60%, 70%,
80%, 90% or more, or a decrease in expression of greater than 1-fold, 2-fold,
3-fold, 4-
fold, 5-fold, 10-fold, 50-fold, 100-fold or more as measured by one or more
methods
described herein. The term "increased level of expression", as used herein,
refers to an
increase in expression of at least 10% or more, for example, 20%, 30%, 40%, or
50%,
60%, 70%, ro , ,
u /0 90% or more or an increase in expression of greater than 1-fold, 2-fold,
3-fold, 4-fold, 5-fold, 10-fold, 50-fold, 100-fold or more as measured by one
or more
methods, such as method described herein.
48
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
Skin gene expression analysis
[0181] Various methods for identifying differentially expressed
biomarkers in
scleroderma patients are known in the art and can be used to practice the
present
invention. For example, skin gene expression analysis can be a powerful tool
for
subsetting patients, identifying protein biomarkers and indicators of
responsive patient
subsets. In some embodiments, genes that are differentially regulated in
patients with
scleroderma can be identified by comparing transcriptional profiles of skin
samples of
healthy individuals with those having scleroderma. Further, gene transcripts
that associate
with severity of disease can be identified by including scleroderma patients
at various
stages of degree progression. Transcriptional profiles can be analyzed by
microarray
analysis, as has been described, for example, by Milano et al. in "Molecular
Subsets in the
Gene Expression Signatures of Scleroderma Skin" (PLOS One, 3:7, 1-18, 2008),
the
entirety of which is herein incorporated by reference. For example, microarray
analysis
can be performed on skin samples (e.g., forearm and back samples) from
patients with
diffuse scleroderma, limited scleroderma, morphea (a disease similar to
scleroderma with
no internal organ involvement) and healthy controls. To identify genes most
highly
associated with scleroderma, the genes that are most internally consistent
between
replicates and sample sites, while being the most variable between
individuals, are
selected for further analysis. Cluster analysis based on differential gene
expression
correlated with severity of scleroderma can be used to select genes affected
by
scleroderma.
[0182] It has been reported that differentially expressed exemplary
genes in
scleroderma can be clustered into 6 groups. The first group includes
immunoglobulin
genes expressed highly in a subset of patients with diffuse scleroderma and in
patients
with morphea, including but not limited to CCR2, CCL4, and IGLL1. The second
group
includes proliferation signature, including genes that are expressed only when
the cell is
dividing. Genes showing increased expression in this cluster include the cell-
cycle
regulated genes such as CKS1B, CDKS2, CDC2, MCM8 and E2F7. The existence of a
proliferation signature is consistent with reports from skin biopsies
demonstrating that
cells of diffuse scleroderma tissue undergoing increased proliferation. The
third group
includes collagen and extracelluar matrix components, including but not
limited to
COL5A2, COL8A1, COL10A1, COL12A1. The fourth group includes genes typically
associated with the presence of T-lymphocyes and macrophages, which are
similarly
49
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
expressed to the third group and include PTPRC, which is required for T-cell
activation, as
well as CD2 and CDW52, that are expressed on the surface of T lymphocytes. The
fifth
group includes genes showing low expression in diffuse scleroderma. These
genes show
higher expression levels in other biopsies and include WIF1, Tetranectin,
IGFBP6, and
IGFBP5, among others. The final group is a heterogeneous gene expression
cluster that is
high in limited scleroderma and a subset of diffuse scleroderma, including but
not limited
to, UTS2R, GALR3, PARD6G, PSEN1, PHOX2A, CENTG3, HCN4, KLF16, and
GPR15G. Additional differentially expressed exemplary genes are described in
Milano et
al. in "Molecular Subsets in the Gene Expression Signatures of Scleroderma
Skin" (PLOS
One, 3:7, 1-18, 2008), the entirety of which is herein incorporated by
reference.
Multi-gene signature as surrogate markers
[0183] Combinations of genes may be used as biomarkers. Exemplary
methods
for biomarker identification is provided in, for example, Farina et al., in "A
Four-Gene
Biomarker Predicts Skin Disease in Patients with Diffuse Cutaneous Systemic
Sclerosis"
(Arthritis Rheum. 62(2), 580-588, 2010), the entirety of which is incorporated
herein by
reference. Starting with targets such as TGFB and interferon known to be
regulated in
scleroderma, Farina identified a four-gene biomarker, including the genes
CTGF, THS1,
COL4, and PAIl. The transcription of these four genes in combination was found
to be
highly correlated with Modified Rodnan Skin Score (mRSS) and highly predictive
of
diffuse scleroderma.
[0184] mRSS is used as one clinical marker of scleroderma. Typically,
mRRS is
assigned as shown in FIG. 2: uninvolved skin is assigned a score 0; mild
thickening is
given a score 1; moderate thickening is given a score 2; and severe thickening
is given a
score 3. Typically, a total mRSS score ranging from 0-51 can be determined
based on a
grading of 0-3 at 17 skin areas of a patient. mRSS can be used as indicators
for diagnosis
and monitoring treatment alone or in combination with other biomarkers..
[0185] Similar strategy can be used to identify and validate potential
signature
biomarkers for scleroderma. Specifically, gene transcripts identified as
positively or
negatively regulated in scleroderma are tested alone or in combination to
identify
biomarkers comprised of gene transcript(s) or combinations of gene transcripts
that are
most highly correlated with clinical markers of scleroderma. In addition to
mRSS, other
clinical markers can be used, such as the HAQ - DI, DLCO, or FVC.
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
CCL2 levels
[0186] CCL2 levels, for example, CCL2 serum levels, can be used as
biomarker or
indicators for determining disease severity, organ involvement, selecting
appropriate
treatment, monitoring disease progression and patient response. To determine
CCL2
levels as biomarkers or indicators, CCL2 levels in the serum of patients at a
variety of
stages of scleroderma and unaffected individuals are determined. This can be
done by
assaying CCL2 protein levels in serum by, e.g., ELISA, and correlated with
skin and other
organ (e.g., lung, liver, kidney, oesophagus) involvement. Exemplary methods
are
described in Carulli et al. Ann Rheum Dis. 67:105-109, 2008.
[0187] CCL2 levels present in skin, such as from a biopsy, and/or serum
can also
be correlated with mRSS or other clinical markers, such as the Health
Assessment
Questionnaire (HAQ - DI), Diffusing capacity of the lung for carbon monoxide
(DLCO),
or Forced Vital Capacity (FVC).
[0188] Various biomarkers can be used alone or in combination, or
alternatively,
together with clinical diagnostic markers, such as mRSS, to stratify patients
based on
severity of scleroderma, selecting proper therapy or dosing regimen,
evaluating the
effectiveness of a therapy, monitoring responsiveness to therapy, prognosis
for disease
course, and measurement of disease progression in a subject. Typically, in
such methods,
levels of suitable biomarkers (e.g., such as those selected from various
differentially
expressed genes described herein and other known markers such as CCL2 levels)
determined for a biological sample obtained from the subject from one or more
time points
are compared to the levels from the subject from one or more other time
points. For
example, biomarker levels may be measured before or at the beginning of a
treatment
course. Biomarker levels may be measured at one or more time points throughout
the
course of treatment and compared with the level before the treatment or from
an earlier
time point of a treatment course. Identification or selection of appropriate
treatment,
determining if a patient has positive response to a treatment and/or
optimization of the
treatment can be determined based on the evaluation of biomarkers.
51
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
Pharmaceutical Compositions
[0189] The present invention also provides compositions comprising one
or more
provided molecules (e.g., antibodies, fynomers, aptamers, fusion proteins,
protein binding
domains). In some embodiments the present invention provides at least one
molecule and
at least one pharmaceutically acceptable excipient. Such pharmaceutical
compositions
may optionally comprise and/or be administered in combination with one or more
additional therapeutically active substances. In some embodiments, provided
pharmaceutical compositions are useful in medicine. In some embodiments,
provided
pharmaceutical compositions are useful as prophylactic agents (i.e., vaccines)
in the
treatment or prevention of scleroderma or of negative ramifications associated
or
correlated with scleroderma. In some embodiments, provided pharmaceutical
compositions are useful in therapeutic applications, for example in
individuals suffering
from or susceptible to scleroderma. In some embodiments, pharmaceutical
compositions
are formulated for administration to humans.
[0190] For example, pharmaceutical compositions provided here may be
provided
in a sterile injectable form (e.g., a form that is suitable for subcutaneous
injection or
intravenous infusion). For example, in some embodiments, pharmaceutical
compositions
are provided in a liquid dosage form that is suitable for injection. In some
embodiments,
pharmaceutical compositions are provided as powders (e.g., lyophilized and/or
sterilized),
optionally under vacuum, which are reconstituted with an aqueous diluent
(e.g., water,
buffer, salt solution, etc.) prior to injection. In some embodiments,
pharmaceutical
compositions are diluted and/or reconstituted in water, sodium chloride
solution, sodium
acetate solution, benzyl alcohol solution, phosphate buffered saline, etc. In
some
embodiments, powder should be mixed gently with the aqueous diluent (e.g., not
shaken).
[0191] In some embodiments, provided pharmaceutical compositions
comprise
one or more pharmaceutically acceptable excipients (e.g., preservative, inert
diluent,
dispersing agent, surface active agent and/or emulsifier, buffering agent,
etc.). In some
embodiments, pharmaceutical compositions comprise one or more preservatives.
In some
embodiments, pharmaceutical compositions comprise no preservative.
[0192] In some embodiments, pharmaceutical compositions are provided in
a form
that can be refrigerated and/or frozen. In some embodiments, pharmaceutical
compositions are provided in a form that cannot be refrigerated and/or frozen.
In some
52
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
embodiments, reconstituted solutions and/or liquid dosage forms may be stored
for a
certain period of time after reconstitution (e.g., 2 hours, 12 hours, 24
hours, 2 days, 5 days,
7 days, 10 days, 2 weeks, a month, two months, or longer). In some
embodiments, storage
of antibody compositions for longer than the specified time results in
molecular
degradation.
[0193] Liquid dosage forms and/or reconstituted solutions may comprise
particulate matter and/or discoloration prior to administration. In some
embodiments, a
solution should not be used if discolored or cloudy and/or if particulate
matter remains
after filtration.
[0194] Compositions of the pharmaceutical compositions described herein
may be
prepared by any method known or hereafter developed in the art of
pharmacology. In
some embodiments, such preparatory methods include the step of bringing active
ingredient into association with one or more excipients and/or one or more
other accessory
ingredients, and then, if necessary and/or desirable, shaping and/or packaging
the product
into a desired single- or multi-dose unit.
[0195] A pharmaceutical composition in accordance with the invention may
be
prepared, packaged, and/or sold in bulk, as a single unit dose, and/or as a
plurality of
single unit doses. As used herein, a "unit dose" is discrete amount of the
pharmaceutical
composition comprising a predetermined amount of the active ingredient. The
amount of
the active ingredient is generally equal to a dose which would be administered
to a subject
and/or a convenient fraction of such a dose such as, for example, one-half or
one-third of
such a dose.
[0196] Relative amounts of active ingredient, pharmaceutically
acceptable
excipient, and/or any additional ingredients in a pharmaceutical composition
in accordance
with the invention may vary, depending upon the identity, size, and/or
condition of the
subject treated and/or depending upon the route by which the composition is to
be
administered. By way of example, the composition may comprise between 0.1% and
100% (w/w) active ingredient.
[0197] Pharmaceutical compositions of the present invention may
additionally
comprise a pharmaceutically acceptable excipient, which, as used herein, may
be or
comprise solvents, dispersion media, diluents, or other liquid vehicles,
dispersion or
suspension aids, surface active agents, isotonic agents, thickening or
emulsifying agents,
53
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
preservatives, solid binders, lubricants and the like, as suited to the
particular dosage form
desired. Remington's The Science and Practice of Pharmacy, 21st Edition, A. R.
Gennaro,
(Lippincott, Williams & Wilkins, Baltimore, MD, 2006) discloses various
excipients used
in formulating pharmaceutical compositions and known techniques for the
preparation
thereof Except insofar as any conventional excipient medium is incompatible
with a
substance or its derivatives, such as by producing any undesirable biological
effect or
otherwise interacting in a deleterious manner with any other component(s) of
the
pharmaceutical composition, its use is contemplated to be within the scope of
this
invention.
EXAMPLES
[0198] The present invention will be further illustrated by the
following non-
limiting examples. These Examples are set forth to aid in the understanding of
the
invention but are not intended to, and should not be construed to, limit its
scope in any
way. The Examples do not include detailed descriptions of conventional methods
that
would be well known to those of ordinary skill in the art. Unless indicated
otherwise,
parts are parts by weight, molecular weight is average molecular weight,
temperature is
indicated in Celsius, and pressure is at or near atmospheric.
Example 1. Preparation of Bi-specific Anti-CCL2/LOXL2 Antibodies
[0199] This example illustrates preparation of bi-specific anti-
CCL2/LOXL2
antibodies. As described above, various methods are available to generate and
select bi-
specific antibodies with desired specificities and binding affinities.
[0200] In this particular example, the bi-specific antibody is composed
of a
complete antigen-binding arm against CCL2 and a complete antigen-binding arm
against
LOLX2. Specifically, a mouse cell line producing a humanized CCL2-specific
monoclonal antibody is fused to a rat cell line producing a humanized LOXL2-
specific
monoclonal antibody to produce a quadroma. Supernatants of quadroma cells are
tested
for binding to target cells by FACS analysis. Antibodies are purified from
quadroma cell
culture by protein A affinity followed by ion exchange chromatography.
54
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
Example 2. Dose range testing
[0201] This example illustrates a dose response study designed to
evaluate
effective dose ranges of bi-specific anti-CCL2 and anti-LOXL2 antibody for
treatment of
scleroderma.
[0202] A bleomycin induced scleroderma mouse model is used in this
example.
Typically, fibrosis is induced in mice by repeated subcutaneous injection of
bleomycin,
polyinosinic-polycytidylic acidand/or LPS into the dorsal skin. Specifically,
osmotic
pumps (7-day) containing either bleomycin at concentration of 10-110 p.g and
up to 200
p.g, LPS at a concentration of 300 p.g, polycytidylic acid at a concentration
of 100 p.g or
PBS alone are implanted subcutaneously into groups of 10 B6 mice. In this
mouse model,
histopathological changes in the skin closely resembles that seen in
scleroderma. Early
mononuclear cell accumulation and upregulated TGF-P and chemokine expression
is
followed by dermal fibrosis characterized by thick collagen bundles and
accumulation of
activated fibroblasts. Mice also manifest evidence of pulmonary and renal
fibrosis.
[0203] Dose(s) of bi-specific CCL2/LOXL2 antibody or a control antibody
escalating concentrations are administered into the mice via intraperitoneal
injection.
Example 3. In vivo efficacy of bi-specific anti-CCL2/LOXL2 antibody
[0204] This example illustrates a study designed to evaluate the effect
of treatment
with anti-CCL2/LOXL2 antibodies on inflammation and fibrosis in the bleomycin
mouse
model for scleroderma.
[0205] 7 or 28-day osmotic pumps containing either PBS alone or 10-110
p.g and
up to 200 p.g bleomycin in PBS will be implanted subcutaneously into B6 mice.
Every
two days, mice will be treated via intraperitoneal injection with anti-
CCL2/LOXL2 bi-
specific antibody at suitable concentrations, as determined in example 2, or
with a control
antibody.
[0206] After 7 days, in the case of a 7 day osmotic pump, or 28 days, in
the case of
a 28 day osmotic pump, skin and lung tissue will be harvested for
transcriptional and
histological analysis. Levels of CCL2 protein in tissue samples is measured by
ELISA.
For transcriptional analysis, RNA is extracted from skin tissue and the
isolated RNA is
subject to and semi-quantitative or quantitative reverse transcriptase-PCR
using techniques
commonly known in the art. Levels of TGFP gene expression and gene expression
levels
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
of pro-inflammatory genes, including but not limited to PAIL COMP, COLlal,
F4/80,
IL-6, and TNFa is measured using commercially available primers (TaqMan ). For
histological analysis, skin fibrosis is analyzed by microscopic examination of
tissue
sections stained with hematoxylin and eosin (H&E). The use of H&E staining to
visualize
tissue morphology is well known in the art. Immunohistochemistry is used to
quantify
monocyte infiltration by microscopic examination of tissue sections probed
with the
monocyte specific anti¨F4/80 antibody using techniques well known in the art.
[0207] It is anticipated that treatment with anti-CCL2/LOXL2 antibody
will reduce
infiltration of monocytes and macrophages, will reduce inflammatory gene
expression
(ex., IL-6, TNFa), and will decrease TGFP¨induced marker gene expression. This
is
expected to result in a general decrease in fibrosis.
Example 4. Therapeutic modeling
[0208] This example illustrates a model of CCL2 production and turnover
in
various tissues and plasma to predict tissue target levels. The illustrated
model represents
an extreme presentation of high CCL2 levels.
[0209] Typically, CCL2 is produced in disease tissues and secreted into
plasma.
In healthy individuals, CCL2 synthesis in skin is low or undetectable. CCL2
synthesis
increases with involvement of total skin in both non-affected and affected
skin, leading to
increased serum CCL2 levels. Serum CCL2 levels further increase with organ
involvement. Typically, healthy individuals have an average serum CCL2 level
of less
than about 100 pg/ml. Individuals having so called Raynaud's phenomenon has
slightly
increased average serum CCL2 levels. Patients suffering from sclerosis
typically have an
average serum CCL2 level of about 250 pg/ml. Patients suffering from limited
cutaneous
systemic sclerosis typically have an average serum CCL2 level of about 250
pg/ml.
Patients suffering from diffuse cutaneous systemic sclerosis typically have an
average
serum CCL2 level of about 380 pg/ml. Patients suffering from limited cutaneous
systemic
sclerosis typically have an average serum CCL2 level of about 250 pg/ml.
[0210] The molecular weight of CCL2 is about 8.6kDa, which is much
smaller
than the glomerular filtration threshold of about 50 kDa, resulting in rapid
kidney
clearance. CCL2 is internalized by active receptor mediated internalization.
Typical kd
for CCL2 to bind its receptor CCR2 is about 60pM- 2nM. CCR2 is primarily
present on
56
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
lymphoid-origin cells and lymphatic endothelium. It is contemplated that
scleroderma
causes increased vascular permeability early in disease progression, which
permits
substantial equilibration of CCL2 and any therapeutic antibodies between
interstitium and
serum. Therefore, serum half-life of CCL2 is about 10 minutes based on data
from mice
and rabbits. It is expected that CCL2 serum half-life in humans is similar.
Relatively
permeable tissue allows CCL2 reach equilibration from tissue to serum (half-
max)
quickly, for example in about 2 hours. In some cases, serum CCL2 level may
reach 1000
pg/ml (¨ 75pM) with whole skin involvement but without organ involvement. A
target
profile showing serum and tissue CCL2 equilibration is shown in FIG. 3, which
predicts
the desired amount of antibodies need to neutralize 3nM of tissue CCL2 and
compete it off
its receptor.
[0211] Monoclonal antibodies injected intravenously typically binds CCL2
in
plasma and forms a complex before they reach diseased tissues, resulting in
wasted
monoclonal antibodies. Bi-specific mAbs allow us to sequester mAb in diseased
tissue
with a "free" anti-CCL2 arm to bind to tissue CCL2, which provides tissue
specific
targeting of CCL2 (See FIG. 4). We can design the affinity for CCL2 such that
it does not
bind to serum CCL2 but binds tissue CCL2. Furthermore, we can also compete
with the
60pM affinity for CCR2 by increasing dose. Thus, this approach allows us
preferentially
inhibit tissue CCL2 as opposed to plasma CCL2, resulting in highly effective
treatment of
scleroderma.
[0212] A preliminary set of results from modeling bi-specific binding
proteins are
shown in FIG. 5 based on the following assumptions: Anti-LOXL2 arm has a kd of
1pM
or better; anti-CCL2 arm has a kd ranging between 500pM and 1nM; and LOXL2
bound
mAb is not internalized or degraded.
Example 5: Clinical design
[0213] Based upon the success of animal treatments, Phase I-III dose
ranging and
single dose studies of anti-CCL2/LOXL2 bi-specific antibody detailed in Tables
3-7 are
designed in healthy individuals and individuals with different stages of
scleroderma to
evaluate the safety, tolerability, efficacy, and pharmacokinetics of anti-
CCL2/LOXL2.
[0214] A primary objective of Human Clinical Trial 1 includes
determining the
safety of 4 dose levels of anti-CCL2/LOXL2 antibody administered in healthy
individuals.
Secondary objectives include evaluating the pharmacokinetics of 4 different
dose levels of
57
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
anti-CCL2/LOXL2 antibody administered in healthy individuals. A detailed
protocol
synopsis of this clinical trial is shown in Table 3.
TABLE 3: Human Clinical Trial 1
Phase Phase 1
# of Trials 1
Patient Population Healthy volunteers
Trial Design and Endpoints Single dose, dose escalation
Primary: Safety
Secondary: PK
# of Subjects 4 dose groups
n=4 each
16 subjects total
Trial Length (FPI to LPV) 0.5 years
¨ 6 weeks to dose
¨ 15 weeks follow up for PK
Comments Single Phase 1 unit
[0215] A primary objective of Human Clinical Trial 2 includes
determining the
safety of 4 dose levels of anti-CCL2/LOXL2 antibody administered in
individuals with
early symptoms of scleroderma. Secondary objectives include (1) to determine
the
pharmacokinetics of 4 different dose levels of anti-CCL2/LOXL2 antibody
administered
in individuals with early symptoms of scleroderma (2) to determine the
pharmacodynamic
(PD) response of individuals with early symptoms of scleroderma to 4 different
dose
levels of anti-CCL2/LOXL2 antibody by assaying gene expression in sequential
skin
biopsies and (3) to determine the clinical response of individuals with early
symptoms of
scleroderma to 4 different dose levels of anti-CCL2/LOXL2 antibody as measured
by the
Modified Rodnan Skin Score (mRSS). A detailed protocol synopsis of this
clinical trial is
shown in Table 4.
TABLE 4: Human Clinical Trial 2
Phase Phase 1/2
# of Trials 1
Patient Population Early (<2 yrs since non- Raynaud's Phenomenon
(RP) symptom onset) diffuse SSc
mRSS > 15
Trial Design and Endpoints Multiple Dose Escalation
58
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
Double-blind placebo-controlled
Treatment duration: 6 months
4 Dose levels
Primary: Safety
Secondary: PK
PD response (sequential skin biopsy gene
expression ¨ baseline, 4 wks, 6 months)
Clinical response (mRSS)
# of Subjects 4 dose groups
n = 10 each (8 active / 2 placebo)
40 subjects total
Trial Length (FPI to LPV) 1.5 years
Comments Up to 8 sites to recruit within 1 yr
[0216] A primary objective of Human Clinical Trial 3 includes
determining the
efficacy of a single dose level of anti-CCL2/LOXL2 antibody administered in
individuals
with early symptoms of scleroderma as measured by the Modified Rodnan Skin
Score
(mRSS). Secondary objectives include (1) determining the efficacy of a single
dose level
of anti-CCL2/LOXL2 antibody administered in individuals with early symptoms of
scleroderma as measured by the Health Assessment Questionnaire ¨ Disability
Index
(HAQ - DI) and (2) determining the efficacy of a single dose level of anti-
CCL2/LOXL2
antibody administered in individuals with early symptoms of scleroderma as
measured by
organ specific assessments. A detailed protocol synopsis of this clinical
trial is shown in
Table 5.
TABLE 5: Human Clinical Trial 3
Phase Phase 2
# of Trials 1
Patient Population Early (<2 yrs since non- Raynaud's Phenomenon
(RP) symptom onset) diffuse SSc
mRSS > 15
Trial Design and Endpoints 1 dose level
Double-blind Placebo Controlled Parallel Group
Treatment duration 6 months
Open-label extension
Primary: mRSS
Secondary: HAQ DI, organ-specific assessments
# of Subjects 2:1 randomization
120 subjects total
Trial Length (FPI to LPV) 1.5 years
Comments Up to 20 sites to recruit within 1 yr
59
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
[0217] A primary objective of Human Clinical Trial 4 includes
determining the
efficacy relative to oral cyclophosphamide of a single dose level of anti-
CCL2/LOXL2
antibody administered in individuals with limited or diffuse scleroderma with
lung disease
as measured by Forced Vital Capacity (FVC). Secondary objectives include (1)
determining the efficacy relative to oral cyclophosphamide of a single dose
level of anti-
CCL2/LOXL2 antibody administered in individuals with limited or diffuse
scleroderma
with lung disease as measured by the HAQ - DI, (2) determining the efficacy
relative to
oral cyclophosphamide of a single dose level of anti-CCL2/LOXL2 antibody
administered
in individuals with limited or diffuse scleroderma with lung disease as
measured by the
mRSS, and (3) determining the efficacy relative to oral cyclophosphamide of a
single dose
level of anti-CCL2/LOXL2 antibody administered in individuals with limited or
diffuse
scleroderma with lung disease as measured by diffusing capacity of the lung
for carbon
monoxide (DLCO). A detailed protocol synopsis of this clinical trial is shown
in Table 6.
TABLE 6: Human Clinical Trial 4
Phase Phase 2
# of Trials 1
Patient Population Limited or Diffuse SSc with lung disease:
Active alveolitis by HRCT
<7 yrs since non-RP symptom onset
FVC <85%>45% predicted
Trial Design and Endpoints 1 dose level
Double-blind Controlled Parallel Group
Comparator: SoC (oral cyclophosphamide)
Treatment duration 12 months
Open-label extension
Primary: FVC
Secondary: DLCO, HAQ DI, mRSS
# of Subjects 2:1 randomization
120 subjects total
Trial Length (FPI to LPV) 1.5 years
Comments Up to 10 sites to recruit within 6 months
[0218] Objective of Human Clinical Trial 5 include (1) determining the
efficacy
relative to oral cyclophosphamide of a single dose level of anti-CCL2/LOXL2
antibody
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
administered in individuals with early symptoms of scleroderma and/or limited
or diffuse
scleroderma with lung disease as measured by Forced Vital Capacity (FVC), (2)
determining the efficacy relative to oral cyclophosphamide of a single dose
level of anti-
CCL2/LOXL2 antibody administered in individuals with early symptoms of
scleroderma
and/or limited or diffuse scleroderma with lung disease as measured by the HAQ
- DI, (3)
determining the efficacy relative to oral cyclophosphamide of a single dose
level of anti-
CCL2/LOXL2 antibody administered in individuals with early symptoms of
scleroderma
and/or limited or diffuse scleroderma with lung disease as measured by mRSS,
and (4)
determining the efficacy relative to oral cyclophosphamide of a single dose
level of anti-
CCL2/LOXL2 antibody administered in individuals with early symptoms of
scleroderma
and/or limited or diffuse scleroderma with lung disease as measured by DLCO. A
detailed
protocol synopsis of this clinical trial is shown in Table 7.
TABLE 7: Human Clinical Trial 5
Phase Phase 3
# of Trials 1 each
Trial Design and Endpoints Single dose level, double-blind head-to- head
comparison
with SoC in either or both early dSSc or SSc Lung
Disease, depending on outcome of Phase 2s
Endpoints as in Phase 2
# of Subjects 120 patients each
Trial Length (FPI to LPV) 2.0 years
0.5 to 1 year enrollment period
Comments Treatment duration 12 months
[0219] Patients exhibiting early symptoms of scleroderma treated with
anti-
CCL2/LOXL2 antibody are expected to demonstrate significant improvement of
symptoms as measured by the mRSS and HAQ - DI. Patients with limited or
diffuse
scleroderma with lung disease treated with anti-CCL2/LOXL2 antibody are
expected to
demonstrate significant improvement of symptoms as measured by the mRSS , HAQ -
DI,
and FVC. Anti-CCL2/LOXL2 antibody is expected to be more effective than
cyclophosphamide in treatment of patients either with early symptoms of
scleroderma or
with limited or diffuse scleroderma with lung disease as measured by mRSS ,
HAQ - DI,
and/or FVC.
61
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
Example 6: In vivo efficacy of anti-CCL2 and anti-LOXL2 Combination Therapy in
Bleomycin-induced Fibrosis
[0220] This example describes the effect of treatment of inflammation
and fibrosis
with a combination of monospecific anti-CCL2 and anti-LOXL2 antibodies in an
animal
model of scleroderma over a two-week time course. The evaluation of
monotherapy using
either an anti-CCL2 or an anti-LOXL2 antibody and combination therapy (both
anti-CCL2
and anti-LOXL2 antibodies) in a murine model of fibrosis was performed. A
chronic
bleomycin 14-day mini-osmotic subcutaneous pump was used with skin and lung
fibrosis
as outcomes for drug efficacy. As shown below, combination therapy with both
antibodies demonstrated a significant effect in both skin and lung fibrosis.
[0221] Briefly, a bleomycin 14-day pump murine SSc-skin and lung
fibrosis model
was used to test the efficacy of the drugs. Groups (n=5; 8-10 weeks) of female
C57BL/6
mice were exposed subcutaneously to bleomycin (90 U/Kg) or PBS (n=3 mice) via
osmotic pump for 7 days with skin and lungs harvested on day 14. Bleomycin
exposed
mice were treated intraperitoneally with anti-CCL2 (dose 2 mg/Kg/2x/week),
anti-LOXL2
(dose 15 mg/Kg/2x/week) or IgG-control (dose 17 mg/Kg/2x/week) twice a week
starting
on the day of the pump insertion until day 14. Treatment groups are set forth
in Table 8.
TABLE 8
Treatment
Description
Group
PBS mini-osmotic pumps with PBS only
IgG mini-osmotic pumps with bleomycin, IgG/IP/2x/week
anti-CCL2 mini-osmotic pumps with bleomycin, anti-CCL2/IP/2x/week
anti-LOXL2 mini-osmotic pumps with bleomycin, anti-LOXL2/IP/2x/week
mini-osmotic pumps with bleomycin, anti-CCL2 and anti-
Both
LOXL2/IP/2x/week
Outcomes measures for cutaneous fibrosis
[0222] To determine drug efficacy in dermal fibrosis, skin was analyzed
by
hematoxylin-eosin (H&E) and collagen deposition by Masson's trichrome
staining. In
addition, the presence of ulcers was assessed clinically and the skin
thickness was
62
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
measured by the maximal distance between the epidermal-dermal junction and the
dermal-
subcutaneous fat junction in four different skin sections from each mouse
using Olympus
DP70 camera and OLYMPUS Micro Suite Basic software. Tissue sections were
analyzed by a blinded investigator.
Outcome measures for lung fibrosis
[0223] To determine drug efficacy in lung fibrosis, histology and gene
expression
were analyzed. Insufflated lungs were fixed in formalin, embedded in paraffin,
and
stained with H&E, Masson's trichrome staining, and Arginase-1. The Aschroft
score
(Ashcroft et al., 1988,1 Clin. Pathol. 41:467-470) was blindly analyzed in all
groups after
Masson's staining. Arginase-1 staining was also blindly scored from zero to
four in at
least four sections per slides. The average of Aschroft and Arginase-1 scores
in each
mouse was used as the final score.
Statistical analysis
[0224] Comparison of gene expressions, histological analysis of specific
staining
and lung score was analyzed by One-Way ANOVA and Bonferroni's multiple
comparison
post-tests. Two-group comparisons was analyzed by Student T-test. Microan-ay
analysis
followed standard false discovery rate (FDR) of less than 10%, comparing
treated samples
with controls. P-values less than or equal to 0.05 were considered
statistically significant.
Results
Cutaneous fibrosis
[0225] The surgical procedure to implant the mini-osmotic pumps was
overall well
tolerated in mice. One mouse in the PBS treatment group died after 18 hours,
which was
likely attributed to anesthesia. FIG. 6 shows the percentage of skin ulcers
observed in
each treatment group. FIG. 7 shows the thickness of skin tissue samples
measured for
each treatment group.
[0226] As shown in FIG. 6, none of the mice treated with PBS developed
skin
ulcers. In contrast, all the mice exposed to bleomycin and treated with IgG
(control) or
anti-CCL2 developed skin ulcers, while only two mice exposed to bleomycin and
treated
63
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
with anti-LOXL2 developed skin ulcers. In the combination treatment group,
none of the
mice developed skin ulcers.
[0227] As shown in FIG. 7, skin thickness was strongly suppressed in
both mono-
and combination therapy (anti-CCL2, anti-LOX2, Both) groups as compared to
control
(IgG). The average fold-change for each group is set forth in Table 9.
TABLE 9
Average fold-change
Treatment group
skin thickness
PBS 1.07
IgG 1.64
anti-CCL2 1.30
anti-LOXL2 1.34
Both 1.21 (p<0.001)
Lung fibrosis
[0228] Lung tissue samples were scored using the Ashcroft method (as
described
above; FIG. 8). The Aschroft score was blindly analyzed in the lungs of all
five treatment
groups (Table 8). The average of the Aschroft score for each treatment group
is set forth
in Table 10. Using the ANOVA analysis for all treatment groups, only the
combination
treatment group (Both) was statistically reduced as compared to control (IgG;
p<0.01). A
Student T-test comparing control (IgG) and anti-CCL2 groups was not
significant,
whereas control as compared to anti-LOXL2 treatment group showed a trend
(p=0.07).
Control versus combination treatment group was reduced (p< 0.01).
TABLE 10
Treatment group Average Aschroft Score
PBS 1.5
IgG 5.76
anti-CCL2 4.87
anti-LOXL2 3.38
Both 2.64 (p<0.01)
64
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
Arginase-1 lung staining
[0229] Staining for Arginase 1 (Argl), a specific marker for macrophage
activation, was also performed on lung tissue samples for each treatment group
(FIG. 9).
The inventors have previously observed that the bleomycin chronic model
deomstrates a
strong activation of macrophages in the lungs based on CD163 staining, which
is almost
abolished in CCL2-deficient mice. Therefore, since the peak of cell influx
into the lungs
is known to happen on day-14 of the bleomycin model, analysis of CD163+
expression in
lung cells from all bleomycin-exposed groups after treatments was performed.
Strong
expression of CD163+ was confirmed in the lungs after exposure to bleomycin.
[0230] Arginase-1 expression was blindly quantified in at least four
sections in the
lungs of each mouse. The average Arginase-1 score for each treatment group is
set forth
in Table 11. Arginase-1 staining was strongly correlated with Aschroft score
(FIG. 10).
FIG. 11 shows histological sections of lung tissue samples stained with
Trichrome. FIGs.
12 and 13 shows histological sections of lung tissue samples stained with
Arginase 1
(Argl).
TABLE 10
Treatment group Average Argl Score
PBS 0
IgG 2.82
anti-CCL2 3.32
anti-LOXL2 3.05
Both 2.01 (p=0.06)
[0231] As shown in this example, all mice in the IgG and anti-CCL2
treatment
groups contained skin ulcers. However, only 2 of 5 mice in the anti-LOXL2
treatment
group contained skin ulcers. Interestingly, the treatment group that received
both anti-
CCL2 and anti-LOXL2 antibodies did not show any ulcers in the harvested skin
tissue (see
FIG. 6). Analysis of variance (ANOVA) confirmed that the reduction in skin
thickness for
both mono-treatment (either anti-CCL2 or anti-LOXL2 alone) and combination
treatment
(anti-CCL2 and anti-LOXL2 together) was significant as compared to the IgG
treatment
group (see FIG. 7). Therefore, treatment of fibrotic disease by combination
therapy with
anti-CCL2 and anti-LOXL2 antibodies was effective to stop the formation of
skin ulcers,
and effective to reduce the thickening of skin tissue, in mice treated with
bleomycin.
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
[0232] Further, ANOVA also confirmed that combination therapy with anti-
CCL2
and anti-LOXL2 antibodies significantly reduced the degree of fibrosis in lung
tissue
samples (Aschroft score of about 2) as compared to the IgG treatment group
(Aschroft
score of about 6) (see FIG. 8). Combination therapy with anti-CCL2 and anti-
LOXL2
antibodies demonstrated the lowest level of macrophage activation in lungs of
all four
treatment groups (Argl-staining, see FIG. 9).
[0233] Taken together, these data demonstrate that treatment with anti-
CCL2, anti-
LOXL2 alone or in combination in the bleomycin 14-day murine model showed
reduction
of cutaneous and/or lung fibrosis with the strongest effect in the skin and in
the lungs
observed when treating with an anti-LOXL2 antibody or a combination of anti-
CCL2 and
anti-LOXL2 antibodies. Thus, anti-CCL2 and anti-LOXL2 antibodies can be
administered in combination to effectively treat and/or ameliorate one or more
symptoms
of a fibrotic or related inflammatory disease (e.g., scleroderma), disorder or
condition.
Equivalents and Scope
[0234] Those skilled in the art will recognize, or be able to ascertain
using no more
than routine experimentation, many equivalents to the specific embodiments of
the
invention described herein. The scope of the present invention is not intended
to be
limited to the above Description, but rather is as set forth in the appended
claims.
[0235] In the claims articles such as "a", "an" and "the" may mean one
or more
than one unless indicated to the contrary or otherwise evident from the
context. Thus, for
example, reference to "an antibody" includes a plurality of such antibodies,
and reference
to "the cell" includes reference to one or more cells known to those skilled
in the art, and
so forth. Claims or descriptions that include "or" between one or more members
of a
group are considered satisfied if one, more than one, or all of the group
members are
present in, employed in, or otherwise relevant to a given product or process
unless
indicated to the contrary or otherwise evident from the context. The invention
includes
embodiments in which exactly one member of the group is present in, employed
in, or
otherwise relevant to a given product or process. The invention includes
embodiments in
which more than one, or all of the group members are presenting, employed in,
or
otherwise relevant to a given product or process. Furthermore, it is to be
understood that
the invention encompasses all variations, combinations, and permutations in
which one or
more limitation, elements, clauses, descriptive terms, etc., from one or more
of the listed
66
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
claims is introduced into another claim. For example, any claim that is
dependent on
another claim can be modified to include one or more limitations found in any
other claim
that is dependent on the same base claim. Furthermore, where the claims recite
a
composition, it is to be understood that methods of using the composition for
anyone of
the purposes disclosed herein are included, and methods of making the
composition
according to any of the methods of making disclosed herein or other methods
known in the
art are included, unless otherwise indicated or unless it would be evident to
one of
ordinary skill in the art that a contradiction or inconsistency would arise.
[0236] Where elements are presented as lists, e.g., in Markush group
format, it is
to be understood that each subgroup of the elements is also disclosed, and any
element(s)
can be removed from the group. It should be understood that, in general, where
the
invention, or aspects of the invention, is/are referred to as comprising
particular elements,
features, etc., certain embodiments of the invention or aspects of the
invention consist, or
consist essentially of, such elements, features, etc. For purposes of
simplicity those
embodiments have not been specifically set forth in haec verba herein. It is
noted that the
term "comprising" is intended to be open and permits the inclusion of
additional elements
or steps.
[0237] Where ranges are given, endpoints are included. Furthermore, it
is to be
understood that unless otherwise indicated or otherwise evident from the
context and
understand of one of ordinary skill in the art, values that are expressed as
ranges can
assume any specific value or sub-range within the state ranges in different
embodiments of
the invention, to the tenth of the unit of the lower limit of the range,
unless the context
clearly dictates otherwise.
[0238] In addition, it is to be understood that any particular
embodiment of the
present invention that falls within the prior art may be explicitly excluded
from any one or
more of the claims. Since such embodiments are deemed to be known to one of
ordinary
skill in the art, they may be excluded even if the exclusion is not set forth
explicitly herein.
Any particular embodiment of the compositions of the invention (e.g., any HCV
genotype/subtype, any HCV antibody, any epitope, any pharmaceutical
composition, any
method of administration, any therapeutic application, etc.) can be excluded
from any one
or more claims, for any reason, whether or not related to the existence of
prior art.
67
CA 02912443 2015-11-12
WO 2014/190316
PCT/US2014/039437
[0239] The publications discussed above and throughout the text are
provided
solely for their disclosure prior to the filing date of the present
application. Nothing herein
is to be construed as an admission that the inventors are not entitled to
antedate such
disclosure by virtue of prior disclosure.
Other Embodiments
[0240] Those of ordinary skill in the art will readily appreciate that
the foregoing
represents merely certain preferred embodiments of the invention. Various
changes and
modifications to the procedures and compositions described above can be made
without
departing from the spirit or scope of the present invention, as set forth in
the following
claims.
68