Note: Descriptions are shown in the official language in which they were submitted.
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
PYROLYSIS SYSTEM AND METHOD FOR BIO-OIL COMPONENT
EXTRACTION
TECHNICAL FIELD
[0001] The disclosure herein relates to pyrolysis vapor condensation,
and more specifically to bio-oil component extraction in a pyrolysis
system.
BACKGROUND
[0002] Pyrolyzed vapors from "material" ("material" can consist of
and/or contain petroleum compounds, plastics, tires, biomass (both
vegetal and animal), solid wastes, extracts of liquid wastes, or a
combination thereof) can, when condensed completely, produce a liquid
known as bio-oil. The resulting raw bio-oil includes a high proportion of
water and organic acids, and other thermal decomposition products from
the pyrolized material. Raw bio-oil is often chemically unstable and
typically rapidly polymerizes. Moreover the energy content or energy
density of raw bio-oil is about half that of crude oil, due mostly from the
amount of water and polar species contained within. Raw bio-oil is
generally also very acidic and corrosive to some parts found in standard
motors and turbines. Raw bio-oil often cannot be blended directly with
other petroleum fuels due to its polarity as well as water content.
Upgrading and de-watering raw bio-oil has, to this point, been difficult
and expensive, making conventionally produced bio-oil economically
unattractive.
[0003] One method for processing bio-oil vapors obtained from a
slow pyrolysis process involves quenching the vapors with biodiesel in a
single-pass or stage. While this method may operate acceptably for some
situations, continually feeding pure biodiesel into a quenching vessel to
1
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
condense the bio-oil may prove costly for long-duration processes.
Further, significant volumes of biodiesel may prove impractical to employ
for such a system. In addition, a slow pyrolysis technique often produces
lower quantity bio-oil, therefore negatively affecting the economics of
such a system. Moreover, resulting bio-oil/biodiesel fuel mixtures
produced with bio-oil extracted via the single-pass process may have
problems passing fuel combustion standards, such as ASTM D975 or
D6751.
[0004] What is needed is a more economical and practical system
and method to extract bio-oil components from pyrolyzed material.
BRIEF DESCRIPTION OF THE DRAWINGS
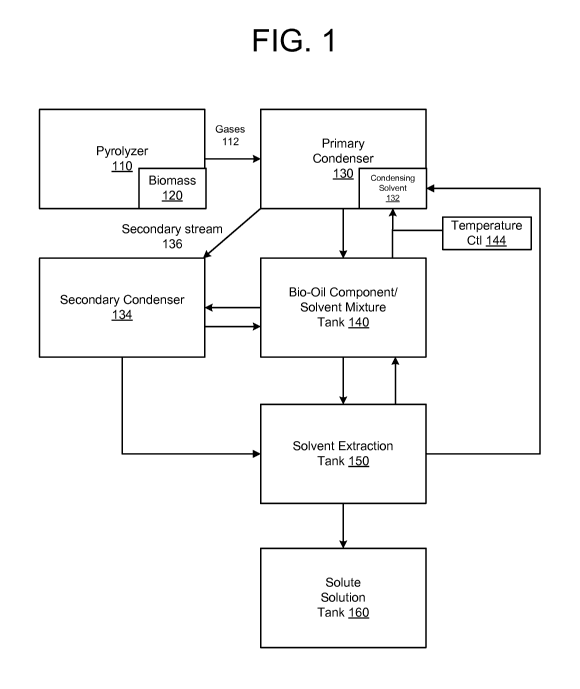
[0005] FIG. 1 illustrates a system for extracting bio-oil components
from pyrolyzed material.
[0006] FIG. 2 illustrates a method for condensing bio-oil components
from decomposed material fumes.
[0007] FIG. 3 illustrates further detail for one embodiment of the
condensing of FIG. 2.
[0008] FIG. 4 illustrates one example of a system that employs fast
pyrolysis for extracting bio-oil components from pyrolyzed material.
[0009] FIG. 5 illustrates one embodiment of a fast pyrolizer that may
be used in the system of FIG. 4.
[0010] FIGs. 6A - 6H illustrate various specific embodiments of an
elongated tubular housing capable of being used by the fast pyrolizer of
FIG. 5.
2
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
[0011] FIG. 7 illustrates a close-up view of section 5-5 of the fast
pyrolizer of FIG. 5 that employs an elevator according to one
embodiment.
[0012] FIG. 8 illustrates a close-up view of section 4-4 of the fast
pyrolizer of FIG. 5 that employs a heater according to one embodiment.
[0013] FIG. 9 illustrates a flow chart for one embodiment of a
method of fast pyrolysis.
DETAILED DESCRIPTION
[0014] Examples of systems and methods are described below that
provide for bio-oil component extraction from a material. In one
embodiment, a system is described that includes a pyrolyzer and a
primary condenser. The primary condenser is coupled to the pyrolyzer
and includes an input to receive pyrolytic vapors from the pyrolyzer and a
solvent. The condenser is further configured to condense the pyrolytic
vapors by contacting the pyrolytic vapors with the solvent to form a
condensed liquid that exits the primary condenser via an output. A
capture vessel receives the condensed liquid from the condenser output.
A recirculator couples the capture vessel to the primary condenser input
and is configured to receive the condensed liquid from the primary
condenser, and to provide at least a portion of the condensed liquid as
part of or all of the solvent in the primary condenser.
[0015] Examples further provide for a method of extracting bio-oil
components from vapors comprising: (a) pyrolyzing a material; (b)
condensing a first amount of bio-oil component vapors produced by
pyrolyzing the material with a solvent to produce a condensed liquid; and
(c) recirculating at least a portion of the condensed liquid to condense a
second amount of bio-oil component vapors.
3
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
[0016] In a specific example, a non-polar high boiling point solvent is
used to quench bio-oil components from a material or waste material
pyrolysis vapor stream. The resulting liquid is returned to the quenching
zone to quench more pyrolysis vapors and load the solvent with more bio-
oil components. During the quenching process, an injection rate and
temperature of the quenching solution are controlled to obtain a particular
quantity and quality of the resulting loaded solution. Moreover, in some
embodiments, chemical species such as acetone, acetaldehyde, water and
acetic acid may be separated in situ by controlling the temperature.
[0017] In another example, a bio-oil component solution is further
concentrated by extracting the solvent mixed with bio-oil components and
returning the solvent to the quenching system loop. A small proportion of
solvent may be preserved to improve some characteristics like viscosity
and solubility, for example, of the final liquid.
[0018] In a third example, a liquid is produced from pyrolysis vapors
which can be used directly in conjunction with a wide variety of fuels.
[0019] SYSTEM DESCRIPTION
[0020] Although illustrative embodiments are described in detail
herein with reference to the accompanying drawings, variations to specific
embodiments and details are encompassed by this disclosure. It is
intended that the scope of embodiments described herein be defined by
claims and their equivalents. Furthermore, it is contemplated that a
particular feature described, either individually or as part of an
embodiment, can be combined with other individually described features,
or parts of other embodiments.
[0021] FIG. 1 illustrates a system, generally designated 100, for
extracting bio-oil components from pyrolyzed material. The system 100
includes a pyrolizer 110 where a material 120 is exposed to heat with
4
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
little or no oxygen present. Embodiments recognize that fast and flash
pyrolysis (e.g. greater than 1000 'DC/min heating rates) may provide, for
example, generation of higher liquid yields of better quality of bio-oil
component/solvent solution 140. The material 120 fed to the pyrolizer can
consist of and/or contain petroleum compounds, plastics, tires, biomass
(both vegetal and animal), solid wastes, extracts of liquid wastes, or a
combination thereof, and the like.
[0022] Gases 112 generated by the pyrolysis of the material 120 are
directed from the pyrolyzer 110 to an input of a primary condenser 130.
The condenser causes bio-oil component vapor to condense to a liquid
form of bio-oil components. In one embodiment, the primary condenser
takes the form of a quenching chamber. Other embodiments may employ
non-quenching techniques. In a specific quenching embodiment, a second
input to the condenser receives a condensing solvent 132. The solvent is
generally sprayed onto the gases (pyrolysis vapors) to form a bio-oil
component/solvent mixture that is stored in a bio-oil component/solvent
mixture tank 140.
[0023] Further referring to FIG. 1, for one embodiment, a recirculator
142 couples the inlet to the condenser 130 to an outlet of the mixture
tank 140 to feed at least a portion of the bio-oil component/solvent
mixture back to the condenser 130. The fed back mixture is then used to
quench additional bio-oil component vapors as more fully explained
below. In some embodiments, a temperature controller 144 may be
employed to control the temperature of the mixture going into the
condenser to extract an optimal percentage of bio-oil components from
the vapor stream.
[0024] With continued reference to FIG. 1, bio-oil component vapors
that fail to condense in the primary condenser 130 may be directed to an
input of a secondary condenser 134 along a secondary path 136. A
condensing process similar to that of the primary condenser 130 is carried
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
out in the secondary condenser 134. A resulting liquid bio-oil
component/solvent mixture from the secondary condenser is fed from an
outlet to the mixture tank 140. A secondary recirculator extends from the
mixture tank 140 back to the secondary condenser 134 to feed the bio-oil
component/solvent mixture as the quenching agent in the secondary
condenser.
[0025] The bio-oil component/solvent mixture tank 140 may
maintain a consistent volume, and includes a third outlet that feeds a
solvent extraction tank or vessel 150. As more fully explained below, the
solvent component of the bio-oil component/solvent mixture may be
separated from the mixture, and returned to the mixture tank 140. The
solvent extracted from the mixture can also be returned to the line going
from the bio-oil component/solvent mixture tank to the condenser 130.
The resulting bio-oil component liquid may then be fed to a solute
solution tank 160, where further purification or refining may take place.
[0026] For some embodiments, the characteristics of the condensing
solvent can be selected to improve the component separation of the
pyrolytic gases 112. For example, the solvent polarity may provide better
separation of chemicals of interest, and as such may be selected based on
the intended end use. For example, in the case of a fuel compatible
mixture, a non-polar or substantially non-polar solvent may be used to
capture non-polar chemical species from the bio-oil components which are
miscible in standard petroleum fuels.
[0027] Polar solvents can also be used as the condensing solvent. For
example, use of a polar solvent as condensing solvent can cause polar
compounds to be trapped, causing the non-polar species to separate in a
different layer from the polar solvent. The non-polar species can then be
separated. Ionic solvents can also be used and similarly removed,
recycled and reused.
6
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
[0028] The primary condenser 130 may further be injected with
reagents, such as, for example, steam, hydrogen, or other catalysts. The
reagents can be injected into the condenser 130 or blended with the
condensing solvent when applicable. The heat present in the pyrolysis
vapors or condenser 130 can then be utilized to activate a chemical
reaction.
[0029] The boiling and melting points of the solvent can also be
varied. In an example, the solvent can be selected to have a melting point
lower than that of room temperature to avoid mechanical issues, such as
clogging of the condensation and transfer systems. The solvent may also
be selected to have a low melting point to avoid freezing during normal
ambient storage. Additionally, the boiling point of the solvent can be
selected based on the use of the condenser 130 and solvent, for purpose
of condensation. The solvent can further be selected to have a minimum
of decomposition during condensation.
[0030] The solvent can be selected to have a boiling temperature low
enough to be distilled under normal or reduced pressure while
maintaining captured bio-oil components. For example the solvent can be
selected from the following chemical groups; alkanes, alkenes, aromatics,
alcohols, ketones, aldehydes, fatty acids, fatty esters, triglycerides,
esters, their derivatives, and a combination thereof. The solvent can also
include a pure solvent mixture. More complex mixtures like biodiesel,
vegetable oil, motor oil, and hydrocarbon distillation cuts can also be
used. Alternatively the solvent can also be ionic liquids some of which can
be recycled via atmospheric or vacuum distillation.
[0031] After gases 112 are quenched by condensing solvent, a bio-oil
component/solvent solution is formed and contained by the bio-oil
component/solvent solution mixture tank 140. The bio-oil
component/solvent solution includes components from the gases 112,
particularly bio-oil components. The bio-oil component/solvent solution
7
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
can be captured for a maximum recycling yield as well as minimizing the
losses downstream and avoid contamination in the rest of the system.
[0032] The removal of heat by condensation is obtained when the
heat of the gases 112 is transferred to the solvent. For example this can
be accomplished by rapidly contacting the pyrolysis gases 112 with the
solvent in the primary condenser 130. In such examples the solvent can
be sprayed in the direct path of the pyrolysis gases in a quenching
process. In another example, the solvent may be introduced as a falling
film with the gases 112.
[0033] As noted above, in the example of FIG. 1, the bio-oil
component/solvent solution contained in the bio-oil component/solvent
solution mixture tank 140 is further used as the condensing solvent. To
do this, the bio-oil component/solvent solution is directed back to the
primary condenser 130 via the recirculator 142 as the condensing solvent
for further condensation. Examples provide for the system to be operated,
among other possibilities, as a batch or a continuous process. In a batch
process, the bio-oil component/solvent solution mixture tank 140 is filled
with the pure solvent to a level corresponding to the fraction of solvent in
the final bio-oil component/solvent solution mixture. A portion of the
solvent is transferred to the primary condenser 130 to condense a first
portion of bio-oil components. The resulting bio-oil component/solvent
solution is continually transferred back to the primary condenser 130 until
the liquid level in the bio-oil component/solvent solution mixture tank has
reached the filled mark, giving a final bio-oil component/solvent solution
mixture with an optimum bio-oil component/solvent ratio. The recirculator
is stopped and the final bio-oil component/solvent solution is entirely
transferred to solvent extraction tank 150. In a continuous process, the
bio-oil component/solvent solution may be slowly bled to the solvent
extraction tank 150 while fresh or recycled condensing solvent is mixed
with the bio-oil component/solvent solution, and this mixture is then
introduced to the condensation system. For one embodiment, during a
8
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
continuous process, a volume level and concentration of the mixture is
kept constant.
[0034] Embodiments recognize that, after condensation by
condensing solvent, desirable components (e.g. bio-oil components) or
undesirable components (e.g. impurities) may still be present in gaseous,
liquid or other forms. As such, the embodiment of FIG. 1 includes the
secondary condenser 134 to receive a secondary stream from the primary
condenser 130 for further condensation. Small quantities of the solvent
may be present in the secondary stream 136 where, for example, a
solvent has a relatively high boiling point. The solvent can be separated
or extracted from the secondary condenser 134 by the solvent extraction
system and then returned to the quenching process.
[0035] By adjusting the exit temperature of the primary condenser
130 it is possible to selectively extract bio-oil components from the bio-oil
component/solvent solution. For example, by controlling a gas outlet exit
temperature of the primary condenser 130 to about 125 degrees C, it is
possible to remove the acetic acid, water, methanol, and all other light
chemical species having a boiling point inferior to the set temperature.
This results in an anhydrous bio-oil component/solvent solution containing
little organic acids which can be stripped during the solvent recycling
step.
[0036] When the bio-oil component/solvent solution is directed into
the solvent extractor 150, the condensing solvent can be removed by
heating and condensing the vapors either by atmospheric or reduced
pressure distillation, evaporation, and flash evaporation, or other
methods. The bio-oil component/solvent solution can be cooled or the
heat absorbed from the primary condenser 130 can be used beneficially
to help in solvent extraction 150. The solvent is then usually, but not
necessarily, purified further before being sent back to the primary
condenser 130. Although the solvent can be extracted in its totality, the
9
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
resulting bio-oil components solution can also contain a fraction of the
condensing solvent in order to improve its physicochemical
characteristics, like viscosity. Alternatively, another solvent can also be
added to improve the characteristics of the bio-oil component solution.
This resulting bio-oil component solution or concentrate is chemically and
physically stable and can be stored, blended or further processed while
maintaining chemical properties.
[0037] METHOD DESCRIPTION
[0038] FIG. 2 illustrates a method for using a solvent to obtain bio-oil
components from thermally decomposed material fumes. Reference is
made to the embodiment of FIG. 1 in describing elements of FIG. 2.
[0039] At (210), a material is thermally decomposed to produce
vapors. As described regarding FIG. 1, the vapors may include
components which, when condensed (e.g. quenched), produce bio-oil
components. For example, with reference to FIG. 1, a pyrolyzer may be
used to decompose the material in the absence of oxygen to produce the
vapors. In an example, the vapors produced in (210) can be obtained by
heating the material (e.g. by exposure to a heating rate of 10,000
degrees Celsius/minute) without oxygen so that the material decomposes,
producing gases.
[0040] At (220), the vapors are provided to a condenser, such as a
quenching reactor. The quenching reactor cools the gases from (210) by,
for example, exposure to a quenching solution. Examples of quenching
reactors include a condenser, such as described in FIG. 1, provided with a
solvent. Among other forms, the quenching solution may be a pure
solvent (e.g. substantially of a single kind of compound), a mixture of
different compounds, or a loaded solvent (e.g. including having been
exposed to, and loaded, with bio-oil components as more fully described
below in (230) and (240)).
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
[0041] Further referring to FIG. 2, after being provided to the
quenching reactor, the heated vapors are quenched at (230) by exposure
to the quenching solution, and the quenching solution is loaded with bio-
oil components from the heated vapors at (240). The quenching solution,
material and condenser may be selected or configured so that particular
components are loaded into the solvent. For example, aspects of the
steps described above at (210) through (230) can be varied for
production of a particular solvent at (240). By way of example, at (210)
the injection rate of the solvent and temperature of the quenching
solution may be manipulated by the temperature controller to control the
quantity and quality of the resulting loaded solution. Moreover, the
temperature can be controlled to separate undesired chemical species.
[0042] At (250) the loaded solvent having bio-oil components is
recirculated to further quench vapors. Examples provide for (230)-(250)
to be performed, among other possibilities, as a batch or a continuous
process. In a batch process, once a target concentration of chemical
species is attained, the loaded solvent is transferred to the solvent
extraction tank or system. In a continuous process, the loaded solvent is
slowly bled to the solvent extraction tank or system while fresh or
recycled solvent is mixed with the loaded solvent, and this mixture is then
introduced to the quenching system. During a continuous process a level
and concentration of the mixture is kept constant.
[0043] The solvent can then be separated from the bio-oil
components for reuse, and for use of the bio-oil components, at (260).
[0044] FIG. 3 illustrates further detail for one specific quenching
method corresponding to the condensing step 230 described above in
FIG. 2. The quenching process involves introducing bio-oil component
gases into a quenching reactor, at 302, at a temperature selected
between 350-750 degrees. Solvent may then be introduced into the
reactor, at 304, at a temperature that may be based on a temperature of
11
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
captured bio-oil components from the quenching process, more fully
explained below. The solvent may then be sprayed or otherwise rapidly
drawn into contact with the bio-oil component vapor, at 306. The
resulting exchange of heat results in the condensation of a large portion
of the bio-oil component vapor to bio-oil components liquid.
[0045] Further referring to FIG. 3, once the bio-oil components
condense into liquid, it is then captured, at 308, and the resulting
temperature monitored, at 310. For some embodiments, a resulting
temperature of about 125 degrees C results in an optimal extraction of
desired bio-oil components liquid from the vapor. A determination is
carried out, at 312, as to whether the captured liquid is approximately
125 degrees C. If so, then no temperature adjustments are carried out on
newly fed solvent into the quenching reactor. Should the temperature not
be approximately 125 degrees C, then a temperature adjustment is
made, at 314, to increase the temperature of the solvent (if the resulting
captured liquid is less than 125 degrees) or reduce the temperature of the
solvent (if the resulting captured liquid is higher than 125 degrees C).
This temperature control mechanism optimizes the volume and quality of
bio-oil components liquid extracted during each quenching operation.
[0046] EXAMPLE
[0047] For one specific example of system operation consistent with
the disclosure above, a material in the form of waste wood was directed
into a flash pyrolysis oven where it was rapidly heated at a rate in excess
of 10,000 'DC/min up to about 500-550 C. The pyrolysis gases generated
were rapidly removed and separated from hot biochars and directed,
through a heated duct kept near 500 C, to the quencher. There, the
pyrolysis vapors were sprayed-in-flight with a relatively cold mixture of
condensed/quenched bio-oil components in undecane. The
condensed/quenched resulting liquid dropped into the primary quencher
12
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
tank and was kept at about 125 0C, while the unquenched chemical
species having a boiling point inferior to 125 0C went through the
quencher tank to exit to a secondary quencher/condenser for collection.
From the secondary quencher/condenser, the non-condensable gases
were directed to a thermal oxidizer, returned to the process for heat
generation, for the generation of other chemicals from catalysts, used
elsewhere in the plant operation or transported off plant for other usage.
The resulting concentration in the primary quencher tank was maintained
at about 50% bio-oil components/undecane.
[0048] At the same rate that the 50% bio-oil components/undecane
solution was removed from the quencher tank, pure undecane was mixed
with the 50% bio-oil components/undecane before introduction to the
quenching zone. The liquid level in the quencher tank was kept constant.
The 50% bio-oil components/undecane solution removed from the
quenching tank was directed to the solvent extraction system while
keeping it at 125 0C. The solvent extraction system was maintained at a
constant temperature for the undecane to evaporate at about 190-2000C.
Alternatively, a vacuum system could be used to extract the undecane
and possibly use less energy than normal distillation at ambient pressure.
The totality of the undecane was extracted. The resulting bio-oil
components were cooled and stored for future blending or transformation.
The undecane extracted could be further purified and then stored or
returned for further quenching.
[0049] In such an embodiment, undecane is a pure solvent so its
extraction can be done at a single temperature which is better for process
control. When the process uses a pure solvent, no residues are left to
accumulate in the system and the final product.
[0050] The utilization of an alkane as a co-solvent precipitates
chemical species responsible for unacceptable levels of micro-carbon
residues in the final blend. Moreover, in cases where a small quantity of
13
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
solvent is still present in the final product, the gel point of undecane is
significantly low (-25 0C) to make the product compatible in environments
with sub-zero temperatures.
[0051] The system, apparatus, and methods described above lend
themselves well to extracting a purified form of bio-oil components liquid
that may be mixed with, for example, diesel at fairly high mixture
percentages. This is due in large part on the purity of the bio-oil
components via the system and methods described herein, which results
in lower residues forming during combustion of a purified bio-oil
component/diesel fuel mixture. As a result, diesel mixed with a high
percentage of bio-oil components may pass standards mandated by diesel
fuel standards such as ASTM D975.
[0052] FIG. 4 illustrates a system, generally designated 400, that
employs fast pyrolysis in an application for extracting bio-oil components.
It is but one example of an application for fast pyrolysis. The system 400
includes a pyrolizer 402 where material is exposed to heat with little or no
oxygen present. The pyrolized material is then fed to a condenser 404
where, for example, bio oil may be condensed from the gases generated
by the pyrolizer. An oil extractor 406 may then extract the condensed bio
oil for use as a fuel. The material fed to the pyrolizer 402 may contain
petroleum compounds, plastics, tires, biomass (both vegetal and animal),
solid wastes, extracts of liquid wastes, or a combination thereof, and the
like. The material is usually solid, but can also be or contain liquids.
[0053] One specific embodiment of a pyrolysis reactor, or pyrolizer,
generally designated 500, is shown in FIG. 5. The pyrolizer includes an
elongated hollow tube or reactor 502 formed of metal with a feed inlet
504 and an outlet 506. To minimize complexity, the interior of the tube
forms an unobstructed flow path, and includes no moving parts. The flow
path includes at least one interior surface 508 that forms a contact
surface for material progressing through the tube.
14
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
[0054] The elongated hollow tube, or reactor 502, may be formed
from different alloys of stainless steel to avoid oxidation. However, a
proper selection will often depend on the mechanical, electrical and
magnetic properties of the metal. Carbon steel can also be used. Although
the corrosion resistance of carbon steel is much less than that of stainless
steel, considering that the inside of the reactor is usually not exposed to
oxygen, and also its price, electrical properties and magnetic properties,
standard carbon steel may be very attractive to use for an economical
reactor. The magnetic properties are important depending on the
selection of the heat generation device, as is explained below. Aluminum
and aluminum alloys can also be used as building materials for the
reactor. Any metal cladding can also be used for improved passivation to
the harsh conditions the reactor can be subjected to. Electro-deposition,
anodizing are also other methods to passivate the metal on its surface to
avoid oxidation or reduction of the ramp reactor. These coating
techniques can be very attractive to keep the costs low while still using
the core material's characteristics.
[0055] Various alternative embodiments for the shape of the
elongated reactor 502 are shown in FIGs 6A - 6H. The reactor could
basically have any cross-sectional shape, but those offering the best
material-surface contact are those with a flat bottom. This optimizes the
conduction mode of heating. Moreover, the opposite wall of the contact
surface must also not be placed too far from the material falling through
it, in order to take advantage of radiation heating. Square, rectangular, or
half-circle reactors are preferable. However, other cross-section
configurations could also be used, like triangular or trapezoidal. It is also
possible to transit to other cross-sectional shapes and thicknesses along
the tube length.
[0056] In its most straightforward form, the reactor is a straight
tubular element from the feed inlet to the outlet, and shown in FIG. 6A, at
602. In this case, and if the coefficient of friction is neglected (since the
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
material is degassing rapidly), the material entering the tube will have a
constant acceleration. In other words, the speed of the material sliding
through the reactor will constantly increase, until it exits the outlet.
[0057] FIG. 6B illustrates an alternative tube construction that
maintains the rectangular cross section, but curves the tube, at 604. This
results in the flowing material decreasing its acceleration, but at a
constant speed. FIG. 6C illustrates an embodiment where the tube curves
laterally back and forth (zigzagged), at 606, to increase path and
residence time, and also to increase the mixing of the material falling
through the flow path. To further mix the material as it flows through the
tube, plural fixed transverse mixing elements 608 may be employed
throughout the length of the tube, as shown in FIG. 6D.
[0058] For many applications, a straight elongated hollow tube works
well for its straightforward nature and robustness during operation.
However, in some situations, space is limited. FIG.s 6E - 6G illustrate
tube constructions that employ a coiled configuration to minimize space,
yet maximize surface area contact for pyrolysis. It is possible to twist the
tube while maintaining the optimum free-sliding angle for the material to
flow through to be optimally thermally treated. The general material
properties will help determine the slopes (elevation angle of tube) for
optimum spread and speed for thermal treatment.
[0059] FIG. 6E shows a high coil tube, at 610. For this configuration,
gravity is still the main drive force to move the material through the
length of the tube. In FIG. 6F, at 612, a more compact form of the coiled
tube is shown that cannot rely on gravity alone to move the material. For
such a construction, a mechanical device such as a vibration mechanism
or pressure device may be employed to cooperate with gravity in moving
the material through the flow path.
[0060] For relatively long residence times, the tube may be relatively
flat, such as that shown in FIG. 6G, at 614. For even longer residence
16
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
times, it is possible to force the substance to be treated upwards the
reactor ramp. In such case, when a vibration device is attached to the
reactor, a mesh or rough reactor floor will help prevent the substance
from flowing back downward to the inlet. Moreover, such a compact coiled
reactor can alternatively use an open top, or U-shaped ramp, to rapidly
remove the gases generated during thermal treatment. In this case, an
outer shell surrounding the whole coil may be used. However, the
radiative mode of heat transfer can still be used but only when the coil is
with a very low profile. The heat from the coil floor above will serve to
heat the material by radiation. Alternatively, an enclosed coil could also
have a series of holes along its side walls to rapidly remove the gases in
the same manner as the topless ramp. In this later design detail, as
shown in FIG. 6H, an outer shell 616 may be utilized to contain the gases.
[0061] Other variations in the reactor shape are possible. Because
the organic particles lose weight and volume during their thermal
treatment, in regards to the optimization of the conduction mode of heat
transfer as well as optimization of the heating source, it is possible that
the reactor width could be reduced along the path of the material falling
through. Furthermore, the width reduction would also reduce the overall
weight and cost of the reactor. The reactor ramp may also be
constructed of separate longitudinal elements joined together, instead of
one large tube. In some cases, the joining mechanism is preferred to be
non-electrically conductive, with a non-electrically conductive joint.
[0062] Referring back to FIG. 5, the elongated hollow tube is oriented
such that the feed inlet 504 is elevated relative to the outlet 506. Where
the relative elevation is at or greater than a critical free-sliding angle,
the
force of gravity directs the material downwardly through the tube. In
some situations, the relative elevation angle may be less than the critical
free-sliding angle. In such circumstances, an additional driving force such
as the vibration or pressure device noted above may be used to assist
gravity in drawing the material through the tube. Generally speaking, the
17
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
critical free-sliding angle depends on the characteristics of the material,
its density, its weight, particle size, etc. Injection of high velocity oxygen-
less gas would help move the organic material through the reactor but
would also disturb the material bed and most likely lift if from the bottom,
thus breaking the heat conduction efficiency. For this reason, a
mechanical means, such as through vibration, to move the material along
the reactor is preferred.
[0063] Further referring to FIG. 5, and in particular section 4-4
(shown close-up in FIG. 7), the angle of the reactor can be fixed for a
given process but the system can also incorporate mechanical elements
permitting for the reactor angle to be changed for optimization of the
process. To allow for adjusting the elevation angle, a pivot 702 may be
employed for raising and lowering the tube. While FIG. 7 shows a half-
pivot, which enables for easy removal of the ramp, various other shapes
may also be employed. A support (not shown) at the other end of the
tube keeps the feed inlet at the desired height.
[0064] FIG. 8 illustrates a close-up view of section 5-5 of FIG. 5, and
shows one specific embodiment of a heater 802 that employs strip heater
elements 804 that are held against the periphery of the reactor by a
removable clip 806. Instead of a removable clip, permanent mounting
materials may be used to secure the heater elements to the tube. As seen
in FIG. 5, multiple heaters are distributed along the length of the reactor
to optimize the heating. Heating rods, strips, or other types of Joule or
infrared heaters can be attached or be part of the reactor faces. As a
minimum, only the contact (bottom) face should be heated. With proper
insulation the other faces could reach a temperature sufficient enough to
help the fast heating process. Ultimately, all faces should be heated in
this fashion for optimal heat transfer to the substance flowing through the
reactor. Another advantage of such system is the possibility of heating
different zones to different temperatures.
18
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
[0065] As alternatives to the strip heaters described above, various
other heating methods could be used to heat the reactor. For example,
gas burners are maybe the most well developed methods for heating
processes. However, their efficiencies are not as good as some other
methods. The efficiency can be improved when integrated with other
processes from a pyrolysis plant, like using syngas from pyrolysis. In
order to use gas burners with the present ramp reactor, it will be
important to use a shell on the ramp to contain the combustion gases,
such as that shown in FIG. 6H. A layer of high efficiency thermal insulator
significantly reduces heat loss. The burners can be installed inside the
outer shell of the ramp or produced separately in a burner box. The hot
combustion gases can then be directed inside the ramp reactor outer shell
to heat the ramp uniformly.
[0066] Heat transfer fluid (i.e. air, combustion gases, syngas,
thermal oil, ionic or liquid salts, fluidized solid particulates, etc.) can be
heated remotely using gas burners or via electrical heating and
subsequently transferred to a shell built around the reactor where the
heat will be transferred to all faces of the reactor. The fluid may be
returned to the heating box to be reheated or discarded appropriately.
[0067] The reactor ramp could also be heated directly using the Joule
heating effect by an electrical current passing through it. In this case, the
ramp should be completely isolated electrically from all other equipment
attached to it, including sensors. When the ramp includes more than one
electrically insulated section, it is possible to heat each section
independently to different temperatures.
[0068] Induction heating can also be used to heat the reactor. A
single induction coil can be placed around a straight reactor. It is also
possible to use multiple coils. The multiple coils can be controlled
individually by one or more induction generators. A single induction
generator can also be used in a switching mode using an internal or
19
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
external switcher to alternatively turn on and off each coil. In this
manner, a smaller induction generator can be used to heat a very long
section of reactor. Two spiral induction coils can also be used to heat a
spiral reactor. A series of spiral reactors can be heated by a series of
spiral coils. As is often the case, standard water cooled induction coils
must be thermally insulated from the heated ramp as not to cool down
the ramp reactor. However, it is also possible to use wire coils, but in this
case there would be advantages to include the heat generated by the
current going through the wires by installing them in close physical
proximity to the ramp element, inside the insulation layer.
[0069] In the case of induction heating, a ferro-magnetic
construction material for the ramp also offers an added advantage of
adding magnetic and electrical hysteresis effects to the standard Eddie
current induction heating, increasing the overall induction effect which
results in a more efficient heating of the ramp reactor. Moreover, in the
case of pyrolysis of material, given a high enough induction current, it is
also possible to turn the charred layer on the material being pyrolyzed
into a heating device. It is known that graphite like material can heat up
when submitted to an electrical induction field. Induction heating can also
be used to generate heat directly in the bulk of the material particles
being pyrolyzed, always in close proximity to the unpyrolyzed material,
inducing a very high heating rate, but also high liquid yields. This latest
phenomenon can also be extended to other applications, including
catalysis, cracking, etc.
[0070] The rate of heating is important for complete thermal
treatment within the time of flight inside the reactor, as well as obtaining
maximum liquid yield. Although a single method for heating the reactor
ramp could be used, it could be advantageous to use different heating
devices to heat different zones along the path of the ramp reactor. The
following is simply one example but many cross features can be used
consistent with this idea. A thin section for the first section could be used
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
along with induction heating to have a very rapid heat transfer/generation
as fresh and relatively cold material comes in through the ramp reactor
entrance, the ramp could then transition to a thicker construction material
and be heated using electrical heating strips. The heat generation/transfer
in that reactor zone does not need rapid response but a sustained
temperature since the material was already preheated in the first zone.
These changes in thickness and heating zones can help maximize the
thermal treatment efficiencies while reducing equipment and operation
costs.
[0071] The fast thermal treatment apparatus described herein can be
used for many different applications, including thermal treatment of
solids, liquids and gases. It can be used for drying or evaporation. It can
be used as a fast chemical reactor. It can also be used for fast pyrolysis
and gasification. It can also be used in many different applications where
a control of the atmosphere is necessary.
[0072] FIG. 9 illustrates high-level steps for a method of pyrolizing a
material. Reference is made to the embodiment of FIG. 5 in describing
elements of FIG. 9.
[0073] At 902, a fast pyrolysis reactor is provided that includes an
feed inlet, an outlet, and internal walls. The reactor inlet is oriented to a
non-vertical elevation with respect to the outlet for gravity feed flow, at
904. A user may then feed material into the reactor inlet, at 906. As the
material progresses through the reactor, it is heated via direct heat
transfer between the material and at least one of the internal walls, at
908. The resulting pyrolized material and gases may then be further
processed, depending on the application, at 910.
[0074] Those skilled in the art will appreciate the benefits and
advantages afforded by the embodiments disclosed herein. By providing a
recirculator to recycle a bio-oil component solvent mixture in a
21
CA 02913180 2015-11-20
WO 2014/193997
PCT/US2014/039853
condensing process as well as extracting and recycling the solvent,
significant logistical and cost savings may be realized in the extraction of
bio-oil components in a pyrolysis system. Further, by controlling the
temperature of the solvent based on a desired end-temperature, an
optimal extraction during condensation may be attained.
[0075] It is
contemplated for examples described herein to extend to
individual elements and concepts described herein, independently of other
concepts, ideas or system, as well as for examples to include
combinations of elements recited anywhere in this application. Although
examples are described in detail herein with reference to the
accompanying drawings, it is to be understood that the invention is not
limited to those precise examples. As such, many modifications and
variations will be apparent to practitioners skilled in this art. Accordingly,
it is intended that the scope of the invention be defined by the following
claims and their equivalents. Furthermore, it is contemplated that a
particular feature described either individually or as part of an example
can be combined with other individually described features, or parts of
other examples, even if the other features and examples make no
mentioned of the particular feature. Thus, the absence of describing
combinations should not preclude the inventor from claiming rights to
such combinations.
22