Note: Descriptions are shown in the official language in which they were submitted.
CA 02915026 2015-11-19
METHOD OF PRODUCING WELD JOINT
[Technical Field of the Invention]
[0001]
The present invention relates to a method of producing a weld joint having
weld
metal which has high hardness and excellent abrasion resistance and does not
easily
cause cold cracking when a high-hardness steel plate which has excellent
abrasion
resistance and is used in the field of construction machines and industrial
machines is
welded.
[Related Art]
[0002]
In many cases, a steel plate used in a construction machine for mine
excavation
or civil engineering work needs to be replaced due to wear. In order to
lengthen the
service life of the steel plate, an abrasion resistant steel to increase the
hardness of the
steel plate is used. The hardness of the steel plate may vary depending on the
use
environment or purpose, and in general, abrasion resistant steel plates in the
HB400 grade
(from HB360 to HB440 in terms of Brinell hardness standard value, and from
HV380 to
HV469 in terms of Vickers hardness standard value), in the HB450 grade (from
HB410 to
HB490 in terms of Brinell hardness standard value, and from HV435 to HV533 in
terms
of Vickers hardness standard value), in the HB500 grade (from HB450 to HB550
in terms
of Brinell hardness standard value, and from HV478 to HV585 in terms of
Vickers
hardness standard value), and in the HB600 grade (from HB550 to HB650 in terms
of
Brinell hardness standard value, and from HV585 to HV693 in telins of Vickers
hardness
standard value) are widely used.
- 1 -
CA 02915026 2015-11-19
[0003]
Most types of abrasion resistant steel are welded, and weld metals may also
require abrasion resistance close to base metals (abrasion resistant steel).
In order to
increase the abrasion resistance of the weld metal, there is also a need to
increase the
hardness thereof. However, when the hardness of the weld metal is increased,
cold
cracking caused by hydrogen that infiltrates during welding is very likely to
occur.
Furthermore, since abrasion resistant steel having a high hardness is used as
the base
metal, an increase in the binding force is also a cause of the easy occurrence
of cold
cracking.
[0004]
In order to avoid such cold cracking, preheating is generally performed before
welding. However, the hardness of the abrasion resistant steel is more easily
reduced by
heating than typical steel and thus a high preheating temperature need not be
employed.
It is preferable that the hardness of the weld metal be at the same level as
that of
the base metal. For example, in a case where the abrasion resistant steel in
the HB400
grade or HB500 grade is used as the base metal, it is preferable that the
hardness of the
weld metal be at least HV337 (HB320) or higher, or HV380 (HB360) or higher if
possible.
[0005]
In addition, the hardness in the vicinity of the surface is important for a
weld
metal zone from the viewpoint of abrasion resistance. During multi-layer
welding, weld
metal for a lower layer is re-heated in a subsequent pass and thus the
hardness thereof is
slightly reduced. However, weld metal for the uppermost layer in the case of
multi-
layer welding or weld metal in a case of single pass welding may have
sufficient hardness
in the vicinity of the surface of the weld metal.
- 2 -
CA 02915026 2015-11-19
Accordingly, it is thought that a welding method of forming weld metal which
has a surface hardness of HV337 or higher and HV533 or lower and sufficient
abrasion
resistance and does not cause cold cracking even when preheating is not
performed, or a
welding method of foiming weld metal which has a surface hardness of HV380 or
higher
and HV533 or lower and sufficient abrasion resistance and does not cause cold
cracking
even when preheating is not performed, is extremely useful in a weld joint
which uses an
abrasion resistant steel having a surface hardness of HV380 or higher and
HV693 or
lower as the base metal.
[0006]
As a technique for suppressing cold cracking caused by hydrogen which occurs
in high-strength weld metal, for example, methods of Patent Documents 1 to 5
are
proposed.
In Patent Document 1, the occurrence of cold cracking is prevented by allowing
retained austenite in a steel plate used for a high-strength line pipe or the
like to function
as a hydrogen-trapping site. In Patent Document 2, the occurrence of cold
cracking is
also prevented by allowing oxides in a steel plate used for a high-strength
line pipe or the
like to function as a hydrogen-trapping site.
[0007]
Patent Document 3 discloses a technique for preventing the occurrence of cold
cracking by allowing Mo carbides in steel having a tensile strength of 800 MPa
to 1150
MPa to function as a trapping site. Patent Document 4 discloses a technique
for
improving the cold cracking resistance of steel having a tensile strength of
880 MPa to
1180 MPa by appropriately mixing Mg with the covered material of a shielded
metal arc
welding material and thus reducing the amount of diffusible hydrogen in weld
metal
immediately after welding to about 3.0 m1/100 g to 4.0 m1/100 g. Patent
Document 5
- 3 -
CA 02915026 2015-11-19
discloses a technique for suppressing cold cracking by limiting the amount of
hydrogen
contained in a flux-cored wire for gas-shielded arc welding.
The techniques are applied to base metals and weld metals having a strength of
lower than 1200 MPa and are not techniques capable of improving the cold
cracking
properties of weld metal having a hardness of HV380 (about 1200 MPa in terms
of
tensile strength) and abrasion resistance.
[0008]
Moreover, in general, when an austenitic stainless steel welding material is
used,
the infiltration of hydrogen into weld metal is significantly reduced and thus
sensitivity to
cold cracking can also be reduced. In addition, since the material has an
austenite
structure, cracking due to reduced ductility is less likely to occur. However,
the weld
metal which uses the austenitic stainless steel welding material cannot easily
increase
strength, that is, hardness, and thus abrasion resistance cannot be expected.
[0009]
Accordingly, there is a demand for forming, in a weld joint which uses an
abrasion resistant steel having a high hardness of HV380 or higher and HV693
or lower
as the base metal, weld metal which has a surface hardness of HV337 or higher
and
HV533 or lower and excellent abrasion resistance and does not easily cause
cold cracking,
or weld metal which has a surface hardness of HV380 or higher and HV533 or
lower and
excellent abrasion resistance and does not easily cause cold cracking through
gas-
shielded arc welding.
[Prior Art Document]
[Patent Document]
[0010]
[Patent Document 1] Japanese Unexamined Patent Application, First
Publication No. 2012-176434
- 4 -
CA 02915026 2015-11-19
[Patent Document 2] Japanese Unexamined Patent Application, First
Publication No. 2012-218034
[Patent Document 3] Japanese Unexamined Patent Application, First
Publication No. 2005-40816
[Patent Document 4] Japanese Unexamined Patent Application, First
Publication No. H11-147196
[Patent Document 5] Japanese Unexamined Patent Application, First
Publication No. 2009-255168
[Disclosure of the Invention]
[Problems to be Solved by the Invention]
[0011]
An object of the present invention is to provide a method of producing a weld
joint which uses a high-hardness steel plate having a high C content and a
surface
hardness of HV380 or higher and HV693 or lower as a base metal, and has weld
metal
which has a surface hardness of HV337 or higher and HV533 or lower and
excellent
abrasion resistance and does not easily cause cold cracking, or weld metal
which has a
surface hardness of HV380 or higher and HV533 or lower and excellent abrasion
resistance and does not easily cause cold cracking.
[Means for Solving the Problem]
[0012]
For abrasion resistant steel according to the related art, a preheating
temperature
during welding was important to prevent cold cracking. Accordingly, in
general,
welding was performed using a welding material for mild steel by setting a
preheating
temperature as the top priority. Therefore, the hardness of the weld metal
zone was low
and wear was very likely to occur. This is thought of as a problem. In the
present
- 5 -
CA 02915026 2015-11-19
invention, it is newly found that, when the hardness of the weld metal zone is
increased
on the contrary, cracking is very likely to occur not in the heat-affected
zone of the base
metal but in the weld metal itself. Therefore, the relationship between the
CEN of the
weld metal and cracking is examined, and then an appropriate range of the CEN
of the
weld metal is obtained.
[0013]
Cold cracking that occurs in the weld metal during welding is affected by the
strength of the weld metal, a joint-restricting force, and the amount of
diffusible hydrogen
in the weld metal. The inventors examined various methods to reliably suppress
cold
cracking using high-hardness weld metal having a surface hardness of HV337 or
higher
and HV533 or lower, or high-hardness weld metal having a surface hardness of
HV380 or
higher and HV533 or lower. As a result, it was concluded that the most
reliable method
is to sufficiently reduce the amount of diffusible hydrogen in the weld metal
and to set a
CEN specified with alloy components in the weld metal to be 0.20 mass% to 0.58
mass%.
[0014]
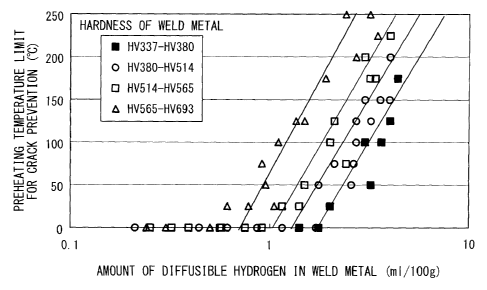
FIG. 1 shows results of a y-groove weld-cracking test specified in JIS Z 3158
performed on various welding materials which varied in steel plates and flux
compositions under various conditions. Various weld metals in which the
hardnesses of
the weld metals vary and the amounts of diffusible hydrogen in the weld metals
vary are
produced, and preheating temperature limits at which the occurrence of
cracking is
suppressed are obtained. In FIG. 1, the relationship between the amount of
diffusible
hydrogen in the weld metal and the preheating temperature limit at which the
occurrence
of cracking is suppressed is plotted according to the hardness levels of the
weld metals.
Here, as a cold-cracking test, a test based on JIS Z 3158 (method of y-groove
weld-cracking test in 1993) was performed at room temperature (25 C), and the
absence
of cracking in surfaces and sections is evaluated as passing. A test for
measuring the
- 6 -
CA 02915026 2015-11-19
amount of diffusible hydrogen was performed according to a gas chromatography
method
based on JIS Z 3118 (method for measurement of amount of hydrogen evolved from
steel
welds in 2007).
[0015]
As illustrated in FIG. 1, when the amount of diffusible hydrogen in the weld
metal immediately after welding is lower than 1.0 m1/100 g, the preheating
temperature
limit for crack prevention at low temperature does not significantly depend on
the
hardness of the weld metal. Therefore, by allowing the amount of diffusible
hydrogen
to be lower than 1.0 m1/100 g, the sensitivity of the weld metal having a
hardness of
HV337 or higher and HV533 or lower and the weld metal having a hardness of
HV380 or
higher and HV533 or lower to cold cracking can be significantly reduced.
[0016]
However, reducing the amount of diffusible hydrogen in the weld metal
immediately after welding to such a level is not easily performed in the
related art. The
inventors repeated various examinations, and newly found that the amount of
diffusible
hydrogen in weld metal can be stably reduced to a level which is not easily
achieved in
the related art by improving the flux composition of a flux-cored wire.
Specifically, it is
found that by allowing a certain amount of fluorides including CaF2 to be
contained in
the flux components, adjusting the amount of oxides, and allowing the mixing
ratios of
fluorides and oxides to be in predetermined ranges, the amount of diffusible
hydrogen in
the weld metal can be stably suppressed to be lower than 1.0 m1/100 g.
[0017]
The sensitivity of the weld metal to cold cracking significantly depends on
the
hardness of the weld metal and is also affected by alloy elements. The
inventors
examined the relationship between various alloy compositions and the
sensitivity of cold
cracking (cracking suppression preheating temperature) for weld metals having
a
- 7 -
CA 02915026 2015-11-19
hardness of HV337 or higher and HV533 or lower and weld metals having a
hardness of
HV380 or higher and HV533 or lower. As a cold-cracking test, a test based on
JIS Z
3158 (method of y-groove weld-cracking test in 1993) was performed at varying
preheating temperatures, and the lowest preheating temperature at which cold
cracking
did not occur is referred to as a preheating temperature limit for crack
prevention.
During welding, flux-cored weld wires of the present invention described below
are used,
and all of the amounts of diffusible hydrogen in the weld metals are lower
than
1.0 m1/100 g.
[0018]
As a result, as shown in FIG. 2, it is found that when a CEN calculated by
Expression 1 (refer to Welding book selections 10. "Welding of iron and steel
materials"
published by Sanpo Publications Incorporated. (1999), p.163) is 0.58 mass% or
lower, the
preheating temperature limit for crack prevention can be equal to or lower
than room
temperature (25 C), and the occurrence of cold cracking can be suppressed
without
preheating.
CEN=[C]+(0.75+0.25 xtanh(20 x([C]-
0.12)))x ([Si]/24+[Mn1/6+[Cu]/15+[Ni]/20+([Cr]+[MoHNb]+[V])/5+5 x [B])
...(Expression 1)
Here, elements with [] represent the amounts (mass%) of the corresponding
elements. In a case where there are no added elements, [] is substituted with
zero.
[0019]
The present invention has been made based on the findings, and the summary is
as follows.
[0020]
(1) According to a first aspect of the invention, a method is provided of
producing a weld joint by performing a gas-shielded arc welding, using a flux-
cored wire
- 8 -
CA 02915026 2015-11-19
filled with flux into a steel sheath, on any one of a steel plate having a
Vickers hardness
HV of 380 or higher and 514 or lower, a plate thickness of 20 mm to 100 mm, a
C
content of 0.120 mass% to 0.300 mass%, and a CEN calculated by the following
Expression 1 of 0.20 mass% to 0.75 mass%, a steel plate having a Vickers
hardness HV
of higher than 514 and 565 or lower, a plate thickness of 12 mm to 100 mm, a C
content
of 0.120 mass% to 0.300 mass%, and a CEN calculated by the following
Expression 1 of
0.20 mass% to 0.75 mass%, and a steel plate having a Vickers hardness HV of
higher
than 565 and 693 or lower, a plate thickness of 6 mm to 12 mm, a C content of
0.350
mass% to 0.450 mass%, and a CEN calculated by the following Expression 1 of
0.20
mass% to 0.85 mass%, the method including:
(a) during the gas-shielded arc welding, not performing a preheating operation
in
a case where a temperature of the steel plate is 10 C or higher, and in a case
where the
temperature of the steel plate is lower than 10 C, performing the preheating
operation so
that the temperature of the steel plate is 10 C or higher,
(b) wherein the flux-cored wire contains one or more of CaF2, BaF2, SrF2, and
MgF2, and when a sum of amounts thereof is a, the a with respect to a total
mass of the
flux-cored wire is 3.3% to 8.0% in terms of mass%,
the flux-cored wire contains one or more of Ti oxides, Si oxides, Mg oxides,
and
Al oxides, and when a sum of amounts thereof is [3, the r3 with respect to the
total mass of
the flux-cored wire is 0.10% to 1.50% in terms of mass%,
a sum of amounts of CaCO3, BaCO3, SrCO3, and MgCO3 with respect to the
total mass of the flux-cored wire is lower than 0.60% in terms of mass%,
an amount of an iron powder in the flux with respect to the total mass of the
flux-cored wire is lower than 10.0% in terms of mass%,
a ratio of the amount of CaF2 to the a is 0.90 or higher,
a ratio of the a to the 13 is 3.0 or higher and 80.0 or lower,
- 9 -
CA 02915026 2015-11-19
an amount of CaO with respect to the total mass of the flux-cored wire is
lower
than 0.20% in terms of mass%,
the flux-cored wire includes chemical components excluding metal fluorides,
metal oxides, and metal carbonates, with respect to the total mass of the flux-
cored wire,
in terms of mass%:
C: 0.010% to lower than 0.060%;
Si: 0.05% to 1.80%;
Mn: 0.50% to 4.00%;
P: 0.050% or lower;
S: 0.020% or lower;
Al: 0.005% to 0.150%;
Cu: 0% to 0.75%;
Ni: 0% to lower than 1.00%;
Cr: 0% to 3.50%;
Mo: 0% to 1.50%;
Ti: 0% to 0.150%;
Nb: 0% to 0.15%;
V: 0% to 0.45%;
B: 0% to 0.0500%;
Mg: 0% to 2.0%;
Ca: 0% to 2.0%;
REM: 0% to 0.0150%; and
the remainder: Fe and impurities,
(c) wherein a weld metal of the weld joint includes as a chemical composition,
in terms of mass%:
C: 0.100% to 0.170%;
- 10 -
CA 02915026 2015-11-19
Si: 0.05% to 0.80%;
Mn: 0.20% to 2.50%;
Al: 0.0050% to 0.1000%;
P: 0.050% or lower;
S: 0.020% or lower;
N: 0.015% or lower;
Cu: 0% to 0.50%;
Ni: 0% to lower than 0.70%;
Cr: 0% to 2.50%;
Mo: 0% to 1.00%;
Ti: 0% to 0.100%;
Nb: 0% to 0.100%;
V: 0% to 0.30%;
B: 0% to 0.0100%;
0: 0% to 0.100%;
Mg: 0% to 0.100%;
Ca: 0% to 0.100%;
REM: 0% to 0.0100%; and
the remainder: Fe and impurities,
a CEN of the weld metal calculated by the following Expression 1 is 0.20
mass% to 0.58 mass%,
an average Vickers hardness HV of the weld metal measured at 1 mm inward
from a surface of the weld metal is 337 to 440, and
all of (a) to (c) are satisfied.
- 11 -
CA 02915026 2015-11-19
CEN=[C]+(0.75+0.25 xtanh(20 x ([C]-
0.12))) aSil/24+[Mn]/6+[Cu]/15+[Ni]/20+([Cr]+[MoHNbHVD/5+5 X [B])
...(Expression 1)
where elements with [] represent the amounts (mass%) of the corresponding
elements.
[0021]
(2) According to a second aspect of the invention, a method is provided of
producing a weld joint by performing a gas-shielded arc welding, using a flux-
cored wire
filled with flux into a steel sheath, on any one of a steel plate having a
Vickers hardness
HV of 380 or higher and 514 or lower, a plate thickness of 20 mm to 100 mm, a
C
content of 0.120 mass% to 0.300 mass%, and a CEN calculated by the following
Expression 1 of 0.20 mass% to 0.75 mass%, a steel plate having a Vickers
hardness HV
of higher than 514 and 565 or lower, a plate thickness of 12 mm to 100 mm, a C
content
of 0.120 mass% to 0.300 mass%, and a CEN calculated by the following
Expression 1 of
0.20 mass% to 0.75 mass%, and a steel plate having a Vickers hardness HV of
higher
than 565 and 693 or lower, a plate thickness of 6 mm to 12 mm, a C content of
0.350
mass% to 0.450 mass%, and a CEN calculated by the following Expression 1 of
0.20
mass% to 0.85 mass%, the method including:
(a) during the gas-shielded arc welding, not performing a preheating operation
in
a case where a temperature of the steel plate is 10 C or higher, and in a case
where the
temperature of the steel plate is lower than 10 C, performing the preheating
operation so
that the temperature of the steel plate is 10 C or higher,
(b) wherein the flux-cored wire contains one or more of CaF2, BaF2, SrF2, and
MgF2, and when a sum of amounts thereof is a, the a with respect to a total
mass of the
flux-cored wire is 3.3% to 8.0% in terms of mass%,
- 12 -
CA 02915026 2015-11-19
the flux-cored wire contains one or more of Ti oxides, Si oxides, Mg oxides,
and
Al oxides, and when a sum of amounts thereof is P., the i3 with respect to the
total mass of
the flux-cored wire is 0.10% to 1.50% in terms of mass%,
a sum of amounts of CaCO3, BaCO3, SrCO3, and MgCO3 with respect to the
total mass of the flux-cored wire is lower than 0.60% in temis of mass%,
an amount of an iron powder in the flux with respect to the total mass of the
flux-cored wire is lower than 10.0% in terms of mass%,
a ratio of the amount of CaF2 to the a is 0.90 or higher,
a ratio of the a to the 13 is 3.0 or higher and 80.0 or lower,
an amount of CaO with respect to the total mass of the flux-cored wire is
lower
than 0.20% in terms of mass%,
the flux-cored wire includes chemical components excluding metal fluorides,
metal oxides, and metal carbonates, with respect to the total mass of the flux-
cored wire,
in terms of mass%:
C: 0.060% to 0.350%;
Si: 0.05% to 1.80%;
Mn: 0.50% to 4.00%;
P: 0.050% or lower;
S: 0.020% or lower;
Al: 0.005% to 0.150%;
Cu: 0% to 0.75%;
Ni: 0% to lower than 1.00%;
Cr: 0% to 3.50%;
Mo: 0% to 1.50%;
Ti: 0% to 0.150%;
Nb: 0% to 0.15%;
- 13 -
CA 02915026 2015-11-19
V: 0% to 0.45%;
B: 0% to 0.0500%;
Mg: 0% to 2.0%;
Ca: 0% to 2.0%;
REM: 0% to 0.0150%; and
the remainder: Fe and impurities,
(c) wherein a weld metal of the weld joint includes as a chemical composition,
in terms of mass%:
C: 0.120% to 0.250%;
Si: 0.05% to 0.80%;
Mn: 0.20% to 2.50%;
Al: 0.0050% to 0.1000%;
P: 0.050% or lower;
S: 0.020% or lower;
N: 0.015% or lower;
Cu: 0% to 0.50%;
Ni: 0% to lower than 0.70%;
Cr: 0% to 2.50%;
Mo: 0% to 1.00%;
Ti: 0% to 0.100%;
Nb: 0% to 0.100%;
V: 0% to 0.30%;
B: 0% to 0.0100%;
0: 0% to 0.100%;
Mg: 0% to 0.100%;
Ca: 0% to 0.100%;
- 14 -
CA 02915026 2015-11-19
REM: 0% to 0.0100%;
the remainder: Fe and impurities,
a CEN of the weld metal calculated by the following Expression 1 is 0.20
mass% to 0.58 mass%,
an average Vickers hardness HV of the weld metal measured at 1 mm inward
from a surface of the weld metal is 380 to 533, and
all of (a) to (c) are satisfied.
CENICH0.75+0.25 xtanh(20 x ([C] -
0.12))) x ([Si]/24+[Mn]/6+[Cu]/15+[Ni]/20+([Cr]+[Mo]+[Nb]+[V])/5+5 x [B])
...(Expression 1)
where elements with [] represent the amounts (mass%) of the corresponding
elements.
(3) According to a third aspect of the invention, a method is provided of
producing a weld joint by performing a gas-shielded arc welding, using a flux-
cored wire
filled with flux into a steel sheath, on any one of a steel plate having a
Vickers hardness
HV of higher than 565 and 693 or lower, a plate thickness of 12 mm to 20 mm, a
C
content of 0.350 mass% to 0.450 mass%, and a CEN calculated by the following
Expression 2 of 0.20 mass% to 0.85 mass%, and a steel plate having a Vickers
hardness
HV of higher than 565 and 693 or lower, a plate thickness of greater than 20
mm to
50 mm or smaller, a C content of 0.350 mass% to 0.450 mass%, and a CEN
calculated by
the following Expression 2 of 0.20 mass% to 0.85 mass%, the method including:
(a) during the gas-shielded arc welding, performing a preheating operation so
that a temperature of the steel plate is 100 C or higher in a case where the
plate thickness
of the steel plate is 20 mm or smaller, and in a case where the plate
thickness of the steel
plate is greater than 20 mm, performing the preheating operation so that the
temperature
of the steel plate is 150 C or higher,
- 15 -
CA 02915026 2015-11-19
(b) wherein the flux-cored wire contains one or more of CaF2, BaF2, SrF2, and
MgF2, and when a sum of amounts thereof is a, the a with respect to a total
mass of the
flux-cored wire is 3.3% to 8.0% in terms of mass%,
the flux-cored wire contains one or more of Ti oxides, Si oxides, Mg oxides,
and
Al oxides, and when a sum of amounts thereof is [3, the 13 with respect to the
total mass of
the flux-cored wire is 0.10% to 1.50% in terms of mass%,
a sum of amounts of CaCO3, BaCO3, SrCO3, and MgCO3 with respect to the
total mass of the flux-cored wire is lower than 0.60% in terms of mass%,
an amount of an iron powder in the flux with respect to the total mass of the
flux-cored wire is lower than 10.0% in terms of mass%,
a ratio of the amount of CaF2 to the a is 0.90 or higher,
a ratio of the a to the 13 is 3.0 or higher and 80.0 or lower,
an amount of CaO with respect to the total mass of the flux-cored wire is
lower
than 0.20% in terms of mass%,
the flux-cored wire includes chemical components excluding metal fluorides,
metal oxides, and metal carbonates, with respect to the total mass of the flux-
cored wire,
in terms of mass%:
C: 0.060% to 0.350%;
Si: 0.05% to 1.80%;
Mn: 0.50% to 4.00%;
P: 0.050% or lower;
S: 0.020% or lower;
Al: 0.005% to 0.150%;
Cu: 0% to 0.75%;
Ni: 0% to lower than 1.00%;
Cr: 0% to 3.50%;
- 16 -
CA 02915026 2015-11-19
MO: 0% to 1.50%;
Ti: 0% to 0.150%;
Nb: 0% to 0.15%;
V: 0% to 0.45%;
B: 0% to 0.0500%;
Mg: 0% to 2.0%;
Ca: 0% to 2.0%;
REM: 0% to 0.0150%;
the remainder: Fe and impurities,
(c) wherein a weld metal of the weld joint includes as a chemical composition,
in terms of mass%:
C: 0.120% to 0.250%;
Si: 0.05% to 0.80%;
Mn: 0.20% to 2.50%;
Al: 0.0050% to 0.1000%;
P: 0.050% or lower;
S: 0.020% or lower;
N: 0.015% or lower;
Cu: 0% to 0.50%;
Ni: 0% to lower than 0.70%;
Cr: 0% to 2.50%;
Mo: 0% to 1.00%;
Ti: 0% to 0.100%;
Nb: 0% to 0.100%;
V: 0% to 0.30%;
B: 0% to 0.0100%;
- 17 -
CA 02915026 2015-11-19
0: 0% to 0.100%;
Mg: 0% to 0.100%;
Ca: 0% to 0.100%;
REM: 0% to 0.0100%; and
the remainder: Fe and impurities,
a CEN of the weld metal calculated by the following Expression 2 is 0.20
mass% to 0.58 mass%,
an average Vickers hardness HV of the weld metal measured at 1 mm inward
from a surface of the weld metal is 380 to 533, and
all of (a) to (c) are satisfied.
CENICH0.75+0.25 xtanh(20x ([C] -
0.12))) x ([Si1/24+[Mn]/6+[Cu]/15+[Ni]/20+([Cr]+[MoHNbHVD/5+5 x [131)
...(Expression 2)
where elements with [] represent the amounts (mass%) of the corresponding
elements.
[0022]
(4) In the method of producing a weld joint described in (1) to (3), the
amount of
CaO in the flux-cored wire may be 0.15% or lower in terms of mass% with
respect to the
total mass of the flux-cored wire.
[0023]
(5) In the method of producing a weld joint described in any of (1) to (4),
the
flux-cored wire may include the chemical components excluding the metal
fluorides,
metal oxides, and metal carbonates, with respect to the total mass of the flux-
cored wire,
in terms of mass%:
Ni: 0% to 0.1%.
- 18 -
CA 02915026 2015-11-19
[0024]
(6) In the method of producing a weld joint described in any of (1) to (5),
the
flux-cored wire may include the chemical components excluding the metal
fluorides,
metal oxides, and metal carbonates, with respect to the total mass of the flux-
cored wire,
in terms of mass%:
Cu: 0% to 0.50%;
Cr: 0% to 1.00%;
Mo: 0% to 0.50%;
Ti: 0% to 0.050%; and
Nb: 0% to 0.05%.
[0025]
(7) In the method of producing a weld joint described in any of (1) to (6),
the
steel sheath of the flux-cored wire may have a slit-like gap.
(8) In the method of producing a weld joint described in any of (1) to (6),
the
steel sheath of the flux-cored wire may not have a slit-like gap.
[0026]
(9) In the method of producing a weld joint described in any of (1) to (8), a
perfluoropolyether oil may be applied to a surface of the flux-cored wire.
[Effects of the Invention]
[0027]
According to the aspects of the present invention, a weld joint which uses a
high-hardness steel plate having a high C content and a surface hardness of
HV380 or
higher and HV693 or lower as a base metal, and has weld metal which has a
surface
hardness of HV320 or higher and HV533 or lower and excellent abrasion
resistance and
does not easily cause cold cracking, or weld metal which has a surface
hardness of
- 19 -
CA 02915026 2015-11-19
HV380 or higher and HV533 or lower and excellent abrasion resistance and does
not
easily cause cold cracking can be obtained.
[Brief Description of the Drawings]
[0028]
FIG. 1 is a diagram showing the relationship between the hardness of a base
metal, the amount of diffusible hydrogen in weld metal, and a preheating
temperature
limit for crack prevention.
FIG. 2 is a diagram showing the relationship between a CEN and a preheating
temperature limit for crack prevention in weld metal having an amount of
diffusible
hydrogen of lower than 1.0 m1/100 g among weld metals having a hardness of
HV337 or
higher and HV533 or lower.
FIG. 3A is a view showing a cut section of a wire.
FIG. 3B is a view showing a cut section of a wire.
FIG. 3C is a view showing a cut section of a wire.
[Embodiments of the Invention]
[0029]
Regarding a weld joint which uses a high-hardness steel plate as a base metal,
the inventors found that when the amount of diffusible hydrogen in weld metal
immediately after welding is lower than 1.0 m1/100 g as described above, a
preheating
temperature limit for crack prevention at low temperature does not
significantly depend
on the hardness of the weld metal and the sensitivity of weld metal having a
hardness of
HV337 or higher and HV533 or lower and weld metal having a hardness of HV380
or
higher and HV533 or lower to cold cracking can be significantly reduced.
- 20 -
CA 02915026 2015-11-19
[0030]
Furthermore, in order to allow the amount of diffusible hydrogen in the weld
metal immediately after welding to be lower than 1.0 m1/100 g, the inventors
repeated
examination by varying the combination of flux components of a flux-cored wire
and the
mixing ratios thereof.
As a result, it is found that fluorides including CaF2 are particularly
effective in
reducing the amount of hydrogen, the amount of diffusible hydrogen in the weld
metal
can be significantly reduced by allowing a certain amount of fluorides to be
contained in
the flux components, and the amount of diffusible hydrogen can be stably
suppressed to
be lower than 1.0 m1/100 g by adjusting the amount of oxides and allowing the
mixing
ratios of fluorides and oxides to be in predetermined ranges.
[0031]
The present invention has been made based on the examinations. Hereinafter,
an aspect of a method of producing a weld joint according to an embodiment
will be
described.
The present invention is for a weld joint which is formed by using a high-
hardness thick steel plate that is widely used as an abrasion resistant steel
plate, has a C
content of 0.12% to 0.45% in terms of mass%, and a hardness of HV380 or higher
and
HV693 or lower as a base metal, and performing a gas-shielded arc welding
using the
steel plate.
In the present invention, weld metal has a chemical composition in (1) or (2)
described above.
Hereinafter, the reasons that the chemical composition of the weld metal is
limited will be described. In the following description, "%" means "mass%" if
not
particularly specified.
- 21 -
CA 02915026 2015-11-19
[0032]
(C: 0.100% to 0.250%)
C is an element which most affects the hardness of the weld metal. When the
hardness of the base metal is HV380 or higher, it is preferable that the
surface hardness of
the weld metal be at least HV337 or higher in order to ensure a certain degree
of abrasion
resistance for the weld metal. For this, the C content of the weld metal needs
to be
0.100% or higher. In addition, when the hardness of the base metal is HV380 or
higher,
it is preferable that the surface hardness of the weld metal be also HV380 or
higher in
order to ensure a similar degree of abrasion resistance to that of the base
metal. In a
case where the surface hardness of the weld metal needs to be HV380 or higher,
the C
content of the weld metal needs to be 0.120% or higher. However, when the C
content
is higher than 0.250%, the hardness of the weld metal becomes higher than
HV533 and
thus the toughness of the weld metal may be reduced. Therefore, the upper
limit of the
C content is 0.250%. In addition, typically, the C content of the weld metal
of a weld
joint made by using a flux-cored wire having a C content of 0.010% to less
than 0.060%,
which will be described later, is 0.100% to 0.170%. In order to allow the base
metal to
stably obtain a hardness of HV380 or higher, the lower limit of the C content
may be
0.130% or 0.140%. In addition, in order to allow the weld metal to stably
obtain
toughness, the upper limit of the C content may be 0.230% or 0.210%.
[0033]
(Si: 0.05% to 0.80%)
Si is a deoxidizing element and reduces the 0 content of the weld metal, and
thus a certain amount of Si is added to the flux in order to enhance
cleanliness.
Therefore, the Si content in the weld metal is also 0.05% or higher. As
necessary, the
lower limit of the Si content may be 0.10%, 0.15%, or 0.20%. When Si is
contained in a
proportion of higher than 0.80%, the toughness of the weld metal may be
deteriorated,
- 22 -
CA 02915026 2015-11-19
and thus 0.80% is the upper limit of the Si content. In order to improve the
toughness of
the weld metal, the upper limit of the Si content may be 0.70%, 0.65%, 0.60%,
or 0.50%.
[0034]
(Mn: 0.20% to 2.50%)
Mn forms MnS and thus has an effect of suppressing grain boundary
embrittlement due to S, and thus at least 0.20% or higher of Mn is contained
in the weld
metal. In addition, Mn is an element which ensures the hardenability of the
weld metal
and is thus effective in increasing strength. Therefore, in order to stably
obtain hardness,
0.50% or higher of Mn is preferably contained. In order to enhance the
hardness of the
weld metal, the lower limit of the Mn content may be 0.60%, 0.70%, 0.80%, or
0.90%.
On the other hand, when Mn is contained in a proportion of higher than 2.50%,
sensitivity to grain boundary embrittlement is increased, and thus the
toughness of the
weld metal is deteriorated. Therefore, 2.50% is the upper limit of the Mn
content. In
order to improve the toughness of the weld metal, the upper limit of the Mn
content may
be limited to 2.30%, 2.10%, 1.90%, 1.70%, or 1.50%.
[0035]
(Al: 0.0050% to 0.1000%)
Al is a deoxidizing element and like Si, reduces the 0 content of the weld
metal,
and thus has an effect of enhancing the cleanliness of the weld metal.
Therefore, a
certain amount of Al needs to be added to the flux. Typically, 0.0050% or
higher Al is
contained in the weld metal of the weld joint made by using the flux-cored
wire
according to this embodiment. When the Al content is lower than 0.0050%, there
is
concern that the low temperature toughness of the weld metal may be degraded.
On the
other hand, when Al is contained in a proportion of higher than 0.1000%, Al
forms
nitrides or oxides and thus deteriorates the toughness of the weld metal.
Therefore,
0.1000% is the upper limit of the Al content. In order to improve the
toughness of the
- 23 -
CA 02915026 2015-11-19
weld metal, the upper limit of the Al content may be limited to 0.0900%,
0.0800%,
0.0700%, or 0.0600%.
[0036]
(P: 0.050% or lower)
P is an impurity element and deteriorates toughness. Therefore, the P content
needs to be reduced as much as possible. However, as a range in which an
adverse
effect of P on toughness is acceptable, the P content of the weld metal is
limited to
0.050% or lower. As necessary, the upper limit of the P content may be limited
to
0.030%, 0.0250%, 0.0200%, or 0.0150%. The lower limit of the P content does
not
need to be limited. The lower limit of the P content is 0%.
[0037]
(S: 0.020% or lower)
S is an impurity element, and when an excessive amount of S is present in the
weld metal, both toughness and ductility are deteriorated, and thus it is
preferable that the
S content be excessively reduced. As a range in which an adverse effect of S
on
toughness and ductility is acceptable, the S content of the weld metal is
limited to 0.020%
or lower. As necessary, the upper limit of the S content may be limited to
0.015%,
0.010%, 0.008%, or 0.006%. The lower limit of the S content does not need to
be
limited. The lower limit of the S content is 0%.
[0038]
(N: 0.015% or lower)
N is unavoidably contained in the weld metal. However, when the N content is
higher than 0.015%, coarse AIN or BN is formed and thus toughness is reduced.
As the
upper limit at which the effect of N on the weld metal is acceptable, the N
content is
limited to 0.015% or lower. As necessary, the upper limit of the N content may
be
- 24 -
CA 02915026 2015-11-19
limited to 0.010%, 0.008%, or 0.006%. The lower limit of the N content does
not need
to be limited. The lower limit of the N content is 0%.
[0039]
(0: 0% to 0.100%)
0 is unavoidably contained in the weld metal. However, as a range in which an
adverse effect of 0 on toughness and ductility is acceptable, the 0 content of
the weld
metal is limited to 0.100% or lower. As necessary, the upper limit of the 0
content may
be 0.080%, 0.060%, 0.050%, or 0.040%. The lower limit of the 0 content does
not
need to be limited. The lower limit of the 0 content is 0%.
[0040]
(Cu: 0% to 0.50%)
Cu can enhance the strength and toughness of the weld metal and thus can be
contained as a selective element. However, when the Cu content is higher than
0.50%,
toughness may be reduced. Therefore, the Cu content of the weld metal is 0.50%
or
lower. As necessary, the upper limit of the Cu content may be 0.40% or 0.30%.
The
lower limit of the Cu content may not be limited. Therefore, the lower limit
of the Cu
content is 0%. On the other hand, in order to sufficiently obtain a
strengthening effect,
0.10% or higher of Cu may be contained in the weld metal. As a method of
including
Cu in the weld metal, there is a method of adding Cu to the coating of the
surface of the
sheath of the wire or the flux as a single element or an alloy element, and
the like.
[0041]
(Ni: 0% to lower than 0.70%)
Ni is considered as an element effective in enhancing toughness and can be
contained as a selective element. However, in a case where the C content is
high, the
effect of Ni is limited, and since Ni is an expensive element, the Ni content
in the weld
metal is lower than 0.70%. As necessary, the upper limit of the Ni content may
be
- 25 -
CA 02915026 2015-11-19
0.60%, 0.40%, or 0.20%. The lower limit of the Ni content may not be limited.
Therefore, the lower limit of the Ni content is 0%. On the other hand, in
order to
sufficiently obtain a toughness enhancing effect, 0.05% or higher of Ni may be
contained
in the weld metal.
[0042]
(Cr: 0% to 2.50%)
Cr is an element which increases hardenability and is effective in enhancing
the
hardness of the weld metal, and thus can be contained as a selective element.
However,
when Cr is excessively contained in a proportion of higher than 2.50%,
toughness may be
reduced. Therefore, 2.50% is the upper limit of the Cr content. As necessary,
the
upper limit of the Cr content may be 1.50%, 1.00%, 0.70%, or 0.40%. The lower
limit
of the Cr content may not be limited. Therefore, the lower limit of the Cr
content is 0%.
On the other hand, in a case of adding Cr for the purpose of enhancing the
hardness of the
weld metal, in order to obtain the effect, 0.10% or higher of Cr may be
contained.
[0043]
(Mo: 0% to 1.00%)
Mo is an element which increases hardenability and is effective in enhancing
the
hardness of the weld metal, and thus can be contained as a selective element.
However,
when Mo is excessively contained in a proportion of higher than 1.00%,
toughness may
be reduced. Therefore, 1.00% is the upper limit of the Mo content. As
necessary, the
upper limit of the Mo content may be 0.70%, 0.60%, 0.40%, or 0.20%. The lower
limit
of the Mo content may not be limited. Therefore, the lower limit of the Mo
content is
0%. On the other hand, in a case of adding Mo for the purpose of
enhancing the
hardness, in order to obtain the effect, 0.05% or higher of Mo may be
contained.
- 26 -
CA 02915026 2015-11-19
[0044]
(Ti: 0% to 0.100%)
Ti is, like Al, effective as a deoxidizing element, has an effect of reducing
the 0
content of the weld metal, and thus can be contained as a selective element.
In addition,
Ti is also effective in fixing solid-soluted N and relaxing an adverse effect
on toughness.
However, when the Ti content in the weld metal becomes higher than 0.100% and
is thus
excessive, a possibility of toughness deterioration due to the formation of
coarse oxides
and toughness deterioration due to excessive precipitation strengthening is
increased.
Therefore, the upper limit of the Ti content is 0.100%. As necessary, the
upper limit of
the Ti content may be 0.080%, 0.050%, 0.030%, or 0.020%. The lower limit of
the Ti
content may not be limited. Therefore, the lower limit of the Ti content is
0%. For the
purpose of improving toughness, 0.010% or higher of Ti may be contained.
[0045]
(Nb: 0% to 0.100%)
Nb is solid-soluted in the weld metal metal and has an effect of enhancing the
hardness of the weld metal, and thus can be contained as a selective element.
However,
when Nb is contained in a proportion of higher than 0.100%, Nb is excessively
contained
in the weld metal, forms coarse precipitates, and thus deteriorates toughness,
which is not
preferable. Therefore, the upper limit of the Nb content is 0.100%. As
necessary, the
upper limit of the Nb content may be 0.080%, 0.050%, 0.030%, or 0.020%. The
lower
limit of the Nb content may not be limited. Therefore, the lower limit of the
Nb content
is 0%. For the purpose of enhancing the hardness of the weld metal, 0.010% or
higher
of Nb may be contained.
- 27 -
CA 02915026 2015-11-19
[0046]
(V: 0% to 0.30%)
V is an element which increases hardenability and is effective in enhancing
the
hardness of the weld metal, and thus can be contained as a selective element.
However,
when V is excessively contained in a proportion of higher than 0.30%,
toughness may be
reduced. Therefore, the upper limit of the V content is 0.30%. As necessary,
the upper
limit of the V content may be 0.25%, 0.20%, or 0.15%. The lower limit of the V
content
may not be limited. Therefore, the lower limit of the V content is 0%. For the
purpose
of enhancing the hardness of the weld metal, 0.01% or higher of V may be
contained.
[0047]
(B: 0% to 0.0100%)
When an appropriate amount of B is contained in the weld metal, B is bonded to
solid-soluted N and forms BN, and thus has an effect of reducing an adverse
effect of the
solid-soluted N on toughness. In addition, B increases hardenability and
contributes to
the enhancement of strength, and thus can be contained as a selective element.
In order
to obtain this effect, 0.0003% or higher of B may be contained. On the other
hand,
when the B content is higher than 0.0100%, B is excessively contained in the
weld metal,
forms coarse BN or B compounds such as Fe23(C, B)6, and thus deteriorates
toughness,
which is not preferable. Therefore, the upper limit of the B content in a case
of
including B is 0.0100%. As necessary, the upper limit of the B content may be
0.0080%,
0.0060%, 0.0040%, or 0.0020%. The lower limit of the B content does not need
to be
limited, and the lower limit of the B content is 0%.
[0048]
(Mg: 0% to 0.100%)
The lower limit of the Mg content does not need to be limited, and the lower
limit of the Mg content is 0%. However, Mg is a strong deoxidizing element and
thus
- 28 -
CA 02915026 2015-11-19
reduces the 0 content in the weld metal, and 0.001% or higher of Mg may be
contained
in order to enhance the ductility and toughness of the weld metal. However,
when the
Mg content in the weld metal is higher than 0.100%, a reduction in the
toughness due to
the formation of coarse oxides in the weld metal cannot be neglected.
Therefore, even
in a case of including Mg, the Mg content is 0.100% or lower. As necessary,
the upper
limit of the Mg content may be 0.0080%, 0.0060%, 0.0040%, or 0.0020%.
[0049]
(Ca: 0% to 0.100%)
(REM: 0% to 0.0100%)
The lower limits of the amounts of Ca and REM do not need to be limited, and
the lower limits of the amounts of Ca and REM are 0%. However, both of Ca and
REM
change the structure of sulfides in the weld metal to refine the sizes of
sulfides and oxides
and are thus effective in enhancing ductility and toughness, and thus 0.002%
or higher of
Ca and 0.0002% or higher of REM may be contained. On the other hand, when Ca
and
REM are excessively contained, sulfides and oxides are coarsened and cause the
deterioration of ductility and toughness. Therefore, in a case of including Ca
and REM,
the upper limits of the Ca and REM contents are respectively 0.100% and
0.0100%.
[0050]
In the weld metal having the above chemical composition, the remainder
containing iron (Fe) as its primary component may also contain impurities that
are
incorporated during the production process and the like in a range in which
the
characteristics of the weld joint according to this embodiment are not
impeded.
[0051]
(CEN: 0.20 mass% to 0.58 mass%)
As illustrated in FIG. 2, regarding the weld metal having a hardness of HV380
or higher and HV533 or lower, when the amount of diffusible hydrogen in the
weld metal
- 29 -
CA 02915026 2015-11-19
is lower than 1.0 m1/100 g, by allowing a CEN calculated by Expression 1 to be
0.58
mass% or lower, the preheating temperature limit for crack prevention can be
25 C or
lower in a y-groove weld-cracking test according to JIS Z 3158, and thus
welding can be
performed substantially without preheating.
Here, in order to reliably prevent weld cracking, the upper limit of the CEN
may
be 0.55 mass%, 0.53 mass%, 0.50 mass%, 0.47 mass%, or 0.45 mass%. In order to
allow the hardness of the weld metal to be HV380 or higher, the lower limit of
the CEN
is 0.20 mass%. When the hardness of the weld metal is high, abrasion
resistance is
enhanced. Therefore, the lower limit of the CEN may be 0.24 mass%, 0.28 mass%,
0.30
mass%, or 0.32 mass%.
(a) A base metal in which the Vickers hardness HV of the base metal is HV380
or higher and HV514 or lower (corresponding to HB360 or higher and HB475 or
lower),
the plate thickness of the base metal is 20 mm to 100 mm, the C content of the
base metal
is 0.120% to 0.300%, and the CEN calculated by Expression 1 is 0.20 mass% to
0.75
mass%.
(b) A base metal in which the Vickers hardness HV of the base metal is higher
than HV514 and equal to or lower than HV565 (corresponding to higher than
HB475 and
equal to or lower than HB530), the plate thickness of the base metal is 12 mm
to 100 mm,
the C content of the base metal is 0.120% to 0.300%, and the CEN calculated by
Expression 1 is 0.20 mass% to 0.75 mass%.
(c) A base metal in which the Vickers hardness HV of the base metal is higher
than HV565 and equal to or lower than HV693 (corresponding to higher than
HB530 and
equal to or lower than HB650), the plate thickness of the base metal is 6 mm
to 12 mm,
the C content of the base metal is 0.350% to 0.450%, and the CEN calculated by
Expression 1 is 0.20 mass% to 0.85 mass%.
- 30 -
CA 02915026 2015-11-19
Regarding the base metal which satisfies any one of (a) to (c) described
above,
in a case where the temperature of the base metal is 10 C or higher during gas-
shielded
arc welding, there is no need to perform a preheating operation during the
welding.
However, in a case where the temperature of the base metal is lower than 10 C,
a
preheating operation needs to be performed so that the temperature of the base
metal
becomes 10 C or higher. That is, only in the case where the temperature of the
base
metal (steel plate) is lower than 10 C, the preheating operation needs to be
performed so
that the temperature of the base metal (steel plate) becomes 10 C or higher.
The upper
limit of the temperature (preheating temperature) of the base metal does not
need to be
particularly determined and may be lower than 75 C or lower than 50 C.
(d) A base metal in which the Vickers hardness HV of the base metal is higher
than HV565 and equal to or lower than HV693 (corresponding to higher than
HB530 and
equal to or lower than HB650), the plate thickness of the base metal is 12 mm
to 20 mm,
the C content of the base metal is 0.350% to 0.450%, and the CEN calculated by
Expression 1 is 0.20 mass% to 0.85 mass%.
(e) A base metal in which the Vickers hardness HV of the base metal is higher
than HV565 and equal to or lower than HV693 (corresponding to higher than
HB530 and
equal to or lower than HB650), the plate thickness of the base metal is 20 mm
to 50 mm,
the C content of the base metal is 0.350% to 0.450%, and the CEN calculated by
Expression 1 is 0.20 mass% to 0.85 mass%.
Regarding the base metal which satisfies (d) or (e) described above, in a case
where the plate thickness of the base metal is 20 mm or smaller during gas-
shielded arc
welding, preheating is performed to heat the base metal to 100 C or higher. In
a case
where the plate thickness of the base metal is greater than 20 mm, preheating
is
performed to heat the base metal to 150 C or higher. The upper limit of the
temperature
(preheating temperature) of the base metal does not need to be particularly
determined
- 31 -
CA 02915026 2015-11-19
and may be lower than 175 C or lower than 150 C. In order to achieve a Vickers
hardness of HV380 or higher, the CEN is allowed to be 0.20 mass%.
CEN=[C]+(0.75+0.25 xtanh(20 x ([C] -
0.12))) x ([Si]/24+[Mn]/6+[Cu]/15+[Ni]/20+([Cr]+[MoHNbHVD/5+5 X [B])
...(Expression 1)
Here, elements with [] represent the amounts (mass%) of the corresponding
elements.
[0052]
In Expression 1, regarding elements that are not contained, [] corresponding
to
the elements is substituted with zero. This calculation method is common to
the base
metal (steel plate) and the weld metal.
[0053]
In the present invention, an average Vickers hardness of the weld metal
measured at 1 mm inward from the surface thereof is HV337 or higher and HV533
or
lower, or HV380 or higher and HV533 or lower. In the present invention, the
amount of
diffusible hydrogen of the weld metal immediately after welding is lower than
1.0 m1/100
g.
When the hardness measured at a position 1 mm inward from the surface is
HV337 or higher and HV533 or lower, an abrasion resistance requirement which
is
necessary for the weld metal is satisfied. When the hardness is lower than
HV337,
abrasion resistance is insufficient. When the hardness is higher than HV533,
cold
cracking is likely to occur.
To measure the hardness, the section of the weld metal is cut in a direction
perpendicular to the welding direction and polished to acquire a sample, the
Vickers
hardnesses of 10 points of the sample at a position 1 mm inward from the
surface of the
- 32 -
CA 02915026 2015-11-19
weld metal are measured, and the average value thereof is calculated to obtain
the
hardness.
[0054]
Regarding the amount of diffusible hydrogen in the weld metal immediately
after welding, as described above with reference to FIG. 1, when the amount of
diffusible
hydrogen is lower than 1.0 m1/100 g, the preheating temperature limit for
crack
prevention at low temperature does not significantly depend on the hardness of
the weld
metal, and the sensitivity of weld metal having a hardness of HV337 or higher
and
HV533 or lower and weld metal having a hardness of HV380 or higher and HV533
or
lower to cold cracking can be significantly reduced.
The amount of diffusible hydrogen is measured by a gas chromatography
method based on JIS Z 3118 (method for measurement of amount of hydrogen
evolved
from steel welds in 2007).
In addition, the hydrogen diffusion speed is relatively fast at room
temperature,
and thus the amount of diffusible hydrogen of the weld metal needs to be
measured
immediately after welding. Therefore, the amount of diffusible hydrogen cannot
be
accurately measured unless it is measured immediately after welding.
[0055]
In order to produce a weld joint having the weld metal described above, high-
hardness thick steel plates to be welded are used as the base metal, and two
plates of the
base metal are set on welding positions to form a groove therebetween, gas-
shielded arc
welding is performed thereon by using a flux-cored weld wire to generate weld
metal
between the plates of the base metal, such that a weld joint formed of the
weld metal and
the steel plates for the base metal on both sides of the weld metal is formed.
Hereinafter, the steel plate, the flux-cored weld wire, and welding conditions
used to form the weld metal will be described.
- 33 -
CA 02915026 2015-11-19
[0056]
As the steel plate for the base metal, a high-hardness thick steel plate
having a C
content of 0.120% or higher and 0.450% or lower in terms of mass% and a
hardness of
HV380 or higher and HV693 or lower is employed.
Regarding the plate thickness of the steel plate to be used, a steel plate
having a
thickness of 6 mm or greater and 100 mm or smaller, generally called a thick
plate, is
employed.
The steel plate that satisfies such conditions is widely used where abrasion
resistance is necessary, such as a machine for civil engineering and
construction work,
and the chemical composition thereof is not particularly limited except for
the C content.
However, as an example, steel includes as a chemical composition:
C: 0.120% to 3.000%, Si: 0.10% to 0.55%, Mn: 0.20% to 2.00%, Al: 0.01% to
0.10%, P: 0.020% or lower, S: 0.015% or lower, Cu: 0.50% or lower, Ni: 1.00%
or lower,
Cr: 1.20% or lower, Mo: 0.60% or lower, Nb: 0.05% or lower, V: 0.10% or lower,
and B:
0.0050% or lower. In addition, steel in which the CEN calculated by Expression
1 is
0.20 mass% to 0.85 mass% is employed.
The upper limit of the CEN is 0.85 mass% so as not to cause weld cracking in
the heat-affected zone (HAZ) of the base metal. In order to more reliably
prevent weld
cracking in the HAZ, the upper limit of the CEN may be 0.80 mass%, 0.75 mass%,
0.73
mass%, 0.70 mass%, 0.68 mass%, 0.65 mass%, 0.63 mass%, or 0.60 mass%. In order
to allow the hardness of the base metal to be HV380 or higher, the lower limit
of the CEN
is 0.20 mass%. In order to increase the hardness of the base metal, the lower
limit of the
CEN may be 0.24 mass%, 0.28 mass%, 0.30 mass%, 0.32 mass%, 0.35 mass%, or 0.38
mass%. The CEN of a steel plate in which the hardness of the base metal is
HV565 or
lower does not generally exceed 0.75 mass%. Therefore, the upper limit of the
CEN of
the steel plate in which the hardness of the base metal is HV565 or lower is
0.75 mass%.
- 34 -
CA 02915026 2015-11-19
As a method of measuring the hardness of the base metal, a method of
measuring the Vickers hardnesses of five or more points at a position 1 mm
inward from
the surface of the section of the base metal in the plate thickness direction
and obtaining
the average value thereof is employed.
[0057]
Subsequently, regarding the flux-cored weld wire to be used, the flux
components and alloy components thereof will be separately described. The
amounts of
the components in the description of the flux-cored weld wire represent mass%
with
respect to the total mass of the flux-cored weld wire.
Initially, the flux components inserted into a steel sheath of the wire will
be
described.
[0058]
By including a predetermined amount of one type or two or more types of metal
fluorides including CaF2, BaF2, SrF2, and MgF2 and one type or two or more
types of
metal oxides including Ti oxides (for example, Ti02), Si oxides (for example,
Si02), Mg
oxides (for example, MgO), and Al oxides (for example, A1203) in the weld wire
and by
allowing the ratios of the fluorides and the oxides to be in a predetermined
range, the
amount of diffusible hydrogen in the weld metal is stably lower than 1.0
m1/100 g.
Requirements for obtaining this effect are, when the total amount of CaF2,
BaF2,
SrF2, and MgF2 being contained is a, to allow the total amount a with respect
to the total
mass of the flux-cored wire in terms of mass% to be 3.3% or higher and 8.0% or
lower,
when the total amount of the contained Ti oxides, Si oxides, Mg oxides, and Al
oxides is
[3, to allow the total amount 13 with respect to the total mass of the flux-
cored wire in
terms of mass% to be 0.10% or higher and 1.50% or lower, to allow the ratio of
the CaF2
content to the a to be 0.90 or higher, and to allow the ratio ([total amount
a]/[total amount
13]) of the total amount a to the total amount 13 to be 3.0 or higher and 80.0
or lower.
- 35 -
CA 02915026 2015-11-19
[0059]
When the total amount a of the contained metal fluorides is lower than 3.3%,
the
amount of diffusible hydrogen in the weld metal cannot be stably lower than
1.0 m1/100 g.
In order to further reduce the amount of diffusible hydrogen in the weld
metal, the lower
limit of the total amount a may be 3.5%, 3.7%, or 3.9%. When the total amount
a is
higher than 8.0%, welding fumes or slag is excessively formed, and thus
welding
workability is significantly degraded, which is not preferable. In order to
avoid the
excessive generation of welding fumes or slag, the upper limit of the total
amount a may
be 7.5%, 7.0%, 6.5%, 6.0%, or 5.7%. When the total amount 13 of the contained
metal
oxides is lower than 0.10%, the shape of welding beads may be deteriorated.
When the
total amount 13 is higher than 1.50%, toughness may be degraded. In order to
enhance
the shape of the welding beads, the lower limit of the total amount 13 may be
0.20%,
0.30%, 0.40%, or 0.50%. In order to improve toughness, the upper limit of the
total
amount 13 may be 1.30%, 1.20%, 1.10%, 1.00%, 0.90%, or 0.80%.
Furthermore, when the ratio of the total amount a to the total amount p is
lower
than 3.0, the amount of diffusible hydrogen in the weld metal may not be
stably lower
than 1.0 m1/100 g. When the ratio thereof is higher than 80.0, welding fumes
or slag is
excessively generated, and thus welding workability is significantly degraded,
which is
not preferable. In order to further reduce the amount of diffusible hydrogen
in the weld
metal, the lower limit of the ratio ([total amount a]/[total amount p]) may be
3.2, 3.5, 3.7,
or 4Ø In order to avoid the excessive generation of welding fumes or slag,
the upper
limit of the ratio ([total amount a]/[total amount Pp may be 40.0, 30.0, 20.0,
15.0, or 13Ø
In a case where the ratio of the CaF2 content to the a is lower than 0.90, the
amount of
diffusible hydrogen in the weld metal may not be lower than 1.0 m1/100 g. This
is
because CaF2 has the greatest effect in reducing the amount of diffusible
hydrogen among
the metal fluorides. A situation in which the ratio of the CaF2 content to the
a is
- 36 -
CA 02915026 2015-11-19
maximized means a case where no metal fluorides other than CaF2 are contained
in the
flux. Therefore, the upper limit of the ratio of the CaF, content to the a is
1Ø
[0060]
Accordingly, the total amount a. of the contained metal fluorides, the total
amount 13 of the metal oxides, and the ratio of the total amount a of the
metal fluorides to
the total amount 13 of the metal oxides are limited as described above.
In addition, the total amount 13 is the content in the flux-cored wire, and
the
content is obtained by also adding metal fluorides contained in a binder
(water glass
primarily containing Si02) used to granulate the flux and the like.
[0061]
To the flux-cored weld wire according to this embodiment, one type or two or
more types of metal carbonates including CaCO3, BaCO3, SrCO3, and MgCO3 may
further be added for the purpose of enhancing an arc stabilizing effect and
concentration
of arc. However, when one type or two or more types of the metal carbonates
are added
in a proportion of 0.60% or higher, concentration of arc is too strong, and
thus the amount
of generated spatter is increased. Furthermore, the amount of oxygen in the
weld metal
is increased. Therefore, in a case of including the metal carbonates, the sum
of the
amounts of the metal carbonates is lower than 0.60%. The lower limit of the
sum of the
amounts of the metal carbonates is 0%. In order to suppress the amount of
generated
spatter, the upper limit thereof may be 0.50%, 0.40%, 0.20%, or 0.10%.
[0062]
The reason that the metal fluorides reduce the amount of diffusible hydrogen
is
not necessarily clear. However, it is thought that the metal fluorides are
decomposed by
welding arc and the generated fluorine is bonded to hydrogen and scatters in
the air as 14F
gas, or hydrogen is fixed to the weld metal as HF as it is.
- 37 -
CA 02915026 2015-11-19
[0063]
In the present invention, it is preferable that CaO not be added to the flux.
Therefore, the lower limit of the CaO content is 0%. However, there may be
cases
where CaO is contained in the raw material of the flux. In this case, the CaO
content is
limited to be lower than 0.20%. The CaO content is preferably 0.15% or lower
or
0.10% or lower. When the CaO content is limited to be lower than 0.20%,
effects
according to the method of producing a weld joint according to this embodiment
are
obtained. CaO comes into contact with the air and changes to CaOH. Therefore,
there
is a possibility that CaO may increase the amount of diffusible hydrogen in
the weld
metal.
[0064]
The amounts of alloy elements in the flux-cored wire excluding the metal
fluorides, metal oxides, and metal carbonates are limited as follows.
[0065]
(C: 0.010% to 0.350% in a case where the average Vickers hardness HV of the
weld metal measured at 1 mm inward from the surface is 337 to 440, and 0.060%
to
0.350% in a case where the average Vickers hardness HV of the weld metal
measured at
1 mm inward from the surface is 380 to 533)
When the C content in the flux-cored wire is lower than 0.010%, the C content
of the weld metal becomes lower than 0.100%, and thus the hardness of the weld
metal
becomes lower than HV337. Therefore, the C content in the flux-cored wire is
0.010%
or higher. When the C content in the flux-cored wire is lower than 0.060%, the
C
content of the weld metal becomes lower than 0.120%, and thus the hardness of
the weld
metal becomes lower than HV380. Therefore, in order to allow the hardness of
the weld
metal to be HV380, the C content in the flux-cored wire is 0.060% or higher.
In order to
enhance the hardness of the weld metal, the lower limit of the C content may
be 0.020%
- 38 -
CA 02915026 2015-11-19
or 0.030%. In order to further enhance the hardness of the weld metal, the
lower limit
of the C content may be 0.070%, 0.080%, 0.090%, 0.100%, or 0.110%. When the C
content in the flux-cored wire is higher than 0.350%, the C content of the
weld metal
becomes higher than 0.250%. Therefore, the C content in the flux-cored wire is
0.350%
or lower. In order to improve the cold cracking resistance of the weld metal,
the upper
limit of the C content may be 0.300%, 0.250%, 0.180%, 0.170%, or 0.160%.
[0066]
(Si: 0.05% to 1.80%)
When the Si content in the flux-cored wire is lower than 0.05%, the Si content
of
the weld metal becomes lower than 0.05%. Therefore, the Si content in the flux-
cored
wire is 0.05% or higher. In order to reduce the 0 content in the weld metal,
the lower
limit of the Si content may be 0.10%, 0.20%, 0.30%, or 0.40%. When the Si
content in
the flux-cored wire is higher than 1.80%, the Si content of the weld metal
becomes higher
than 0.80% even when oxidative consumption is considered. Therefore, the Si
content
in the flux-cored wire is 1.80% or lower. In order to improve the toughness of
the weld
metal, the upper limit of the Si content may be 1.50%, 1.20%, 1.00%, 0.80%, or
0.60%.
[0067]
(Mn: 0.50% to 4.00%)
When the Mn content in the flux-cored wire is lower than 0.50%, the Mn content
of the weld metal becomes lower than 0.20%. Therefore, the Mn content in the
flux-
cored wire is 0.50% or higher. In order to enhance the hardness of the weld
metal, the
lower limit of the Mn content may be 0.70%, 0.80%, 0.90%, 1.00%, or 1.10%.
When
the Mn content in the flux-cored wire is higher than 4.00%, the Mn content of
the weld
metal becomes higher than 2.50% even when oxidative consumption is considered.
Therefore, the Mn content in the flux-cored wire is 4.00% or lower. In order
to improve
- 39 -
CA 02915026 2015-11-19
the toughness of the weld metal, the upper limit of the Mn content may be
3.00%, 2.50%,
2.20%, 2.00%, or 1.80%.
[0068]
(P: 0.050% or lower)
When the P content in the flux-cored wire is higher than 0.050%, the P content
of the weld metal may become higher than 0.050%. Therefore, the P content in
the flux-
cored wire is 0.050% or lower. As necessary, the upper limit of the P content
may be
limited to 0.030%, 0.025%, 0.020%, or 0.015%. The lower limit of the P content
does
not need to be limited. The lower limit of the P content is 0%.
[0069]
(S: 0.020% or lower)
When the S content in the flux-cored wire is higher than 0.020%, the S content
of the weld metal may become higher than 0.020%. Therefore, the S content in
the
flux-cored wire is 0.020% or lower. As necessary, the upper limit of the S
content may
be limited to 0.015%, 0.010%, 0.008%, or 0.006%. The lower limit of the S
content
does not need to be limited. The lower limit of the S content is 0%.
[0070]
(Al: 0.005% to 0.150%)
When the Al content in the flux-cored wire is lower than 0.005%, the Al
content
of the weld metal becomes lower than 0.005%. Therefore, the Al content in the
flux-
cored wire is 0.005% or higher. In order to further reduce the 0 content in
the weld
metal, the lower limit of the Al content may be 0.007%, 0.010%, or 0.012%.
When the
Al content in the flux-cored wire is higher than 0.150%, the Al content of the
weld metal
may become higher than 0.100%. Therefore, the Al content in the flux-cored
wire is
0.150% or lower. In order to improve the toughness of the weld metal, the
upper limit
of the Al content may be limited to 0.090%, 0.070%, 0.050%, or 0.040%.
- 40 -
CA 02915026 2015-11-19
[0071]
(Cu: 0% to equal to or lower than 0.75%)
When the Cu content in the flux-cored wire is higher than 0.75%, the Cu
content
of the weld metal becomes higher than 0.50%. Therefore, the Cu content in the
flux-
cored wire is 0.75% or lower. In order to further reduce the Cu content of the
weld
metal, the Cu content may be 0.50% or lower. As necessary, the upper limit of
the Cu
content may be 0.40% or 0.30%. The lower limit of the Cu content may not be
limited.
Therefore, the lower limit of the Cu content is 0%. On the other hand, in
order to
enhance the hardness of the weld metal, 0.10% or higher of Cu may be contained
in the
weld metal.
[0072]
(Ni: 0% to lower than 1.00%)
When the Ni content in the flux-cored wire is 1.00% or higher, the Ni content
of
the weld metal becomes 0.70% or higher, and the alloy cost of the wire is
increased.
Therefore, the Ni content in the flux-cored wire is lower than 1.00%. In order
to
prevent solidification cracking of the weld metal, the upper limit of the Ni
content may be
0.50%, 0.40%, 0.30%, 0.20%, or 0.10%. The lower limit of the Ni content may
not be
limited. Therefore, the lower limit of the Ni content is 0%.
[0073]
(Cr: 0% to 3.50%)
When the Cr content in the flux-cored wire is higher than 3.50%, the Cr
content
of the weld metal becomes higher than 2.50%. Therefore, the Cr content in the
flux-
cored wire is 3.50% or lower. As necessary, the upper limit of the Cr content
may be
1.50%, 1.00%, 0.50%, or 0.10%. The lower limit of the Cr content may not be
limited.
Therefore, the lower limit of the Cr content is 0%. On the other hand, in a
case of
- 41 -
CA 02915026 2015-11-19
adding Cr for the purpose of enhancing the hardness of the weld metal, in
order to obtain
the effect, 0.05% or higher of Cr may be contained.
[0074]
(Mo: 0% to 1.50%)
When the Mo content in the flux-cored wire is higher than 1.50%, the Mo
content of the weld metal becomes higher than 1.00%. Therefore, the Mo content
in the
flux-cored wire is 1.50% or lower. In order to enhance toughness, the upper
limit of the
Mo content may be 0.70%, 0.50%, 0.30%, or 0.20%. The lower limit of the Mo
content
may not be limited. Therefore, the lower limit of the Mo content is 0%. On the
other
hand, in a case of adding Mo for the purpose of enhancing the hardness of the
weld metal,
in order to obtain the effect, 0.05% or higher of Mo may be contained.
[0075]
(Ti: 0% to 0.150%)
When the Ti content in the flux-cored wire is higher than 0.150%, the Ti
content
of the weld metal becomes higher than 0.100%. Therefore, the Ti content in the
flux-
cored wire is 0.150% or lower. In order to enhance toughness, the upper limit
of the Ti
content may be 0.100%, 0.080%, or 0.050%. The lower limit of the Ti content
may not
be limited. Therefore, the lower limit of the Ti content is 0%. For the
purpose of
enhancing toughness, 0.010% or higher of Ti may be contained.
[0076]
(Nb: 0% to 0.15%)
When the Nb content in the flux-cored wire is higher than 0.15%, the Nb
content
of the weld metal becomes higher than 0.10%. Therefore, the Nb content in the
flux-
cored wire is 0.15% or lower. In order to enhance toughness, the upper limit
of the Nb
content may be 0.10%, 0.08%, or 0.05%. The lower limit of the Nb content may
not be
- 42 -
CA 02915026 2015-11-19
limited. Therefore, the lower limit of the Nb content is 0%. For the purpose
of
enhancing the hardness of the weld metal, 0.01% or higher of Nb may be
contained.
[0077]
(V: 0% to 0.45%)
When the V content in the flux-cored wire is higher than 0.45%, the V content
of
the weld metal becomes higher than 0.30%. Therefore, the V content in the flux-
cored
wire is 0.45% or lower. In order to enhance toughness, the upper limit of the
V content
may be 0.25%, 0.20%, or 0.15%. The lower limit of the V content may not be
limited.
Therefore, the lower limit of the V content is 0%. For the purpose of
enhancing the
hardness of the weld metal, 0.01% or higher of V may be contained.
[0078]
(B: 0% to 0.0500%)
When the B content in the flux-cored wire is higher than 0.0500%, the B
content
of the weld metal becomes higher than 0.0100%. Therefore, the B content in the
flux-
cored wire is 0.0500% or lower. In order to enhance toughness, the upper limit
of the B
content may be 0.0400%, 0.0200%, 0.0100%, or 0.0050%. The lower limit of the B
content does not need to be limited, and the lower limit of the B content is
0%.
[0079]
(Mg: 0% to 2.0%)
When the Mg content in the flux-cored wire is higher than 2.0%, the Mg content
of the weld metal becomes higher than 0.10%. Therefore, the Mg content in the
flux-
cored wire is 2.0% or lower. In order to enhance the toughness and ductility
of the weld
metal, the upper limit of the Mg content may be 1.5%, 1.0%, 0.4%, or 0.2%. The
lower
limit of the Mg content does not need to be limited, and the lower limit of
the Mg content
is 0%.
- 43 -
CA 02915026 2015-11-19
[0080]
(Ca: 0% to 2.0%)
When the Ca content in the flux-cored wire is higher than 2.0%, the Ca content
of the weld metal becomes higher than 0.10%. Therefore, the Ca content in the
flux-
cored wire is 2.0% or lower. In order to enhance the toughness and ductility
of the weld
metal, the upper limit of the Ca content may be 1.5%, 1.0%, 0.5%, or 0.3%. The
lower
limit of the Ca content does not need to be limited, and the lower limit of
the Ca content
is 0%.
[0081]
(REM: 0% to 0.0150%)
When the REM content in the flux-cored wire is higher than 0.0150%, the REM
content of the weld metal becomes higher than 0.0100%. Therefore, the REM
content
in the flux-cored wire is 0.0150% or lower. In order to enhance the toughness
and
ductility of the weld metal, the upper limit of the REM content may be
0.0100%,
0.0050%, or 0.0030%. The lower limit of the REM content does not need to be
limited,
and the lower limit of the REM content is 0%.
[0082]
The reason that the chemical composition of the flux-cored wire according to
this embodiment is limited has been described above. Regarding the other
chemical
composition of the alloys of the remainder, the remainder primarily containing
Fe may
also contain impurities that are incorporated during the production process
and the like in
a range in which the characteristics of the weld joint according to this
embodiment are
not impeded. The Fe component contains Fe in the steel sheath, and Fe in iron
powder
and alloy components added to the flux. The iron powder content in the flux is
lower
than 10.0% in terms of mass% with respect to the total mass of the flux-cored
wire.
When the iron powder content is increased, there may be a case where the
amount of
- 44 -
CA 02915026 2015-11-19
oxygen is also increased. As necessary, the iron powder content may be lower
than
5.0% or lower than 1.0%. Since the iron powder does not need to be contained,
the
lower limit of the iron powder content is 0%.
[0083]
Subsequently, the morphology of the flux-cored wire will be described.
The flux-cored wire is primarily divided into a seamless wire (that is, a wire
in
which the seams of the steel sheath are welded to each other) in which slit-
like seams are
not formed in the steel sheath, and a seamed wire in which the seams of the
steel sheath
have a slit-like gap. The present invention may employ any sectional
structure.
However, in order to suppress the cold cracking of the weld metal, a wire
without slit-like
seams (seamless wire) is preferable.
[0084]
Hydrogen infiltrated into the weld zone during welding is diffused into the
weld
metal and the steel side, is accumulated to a stress concentration zone, and
acts as a cause
of the occurrence of cold cracking. As the hydrogen source, moisture held in
the
welding material, moisture incorporated from the air, rust or scales adhered
to the surface
of the steel, and the like are mentioned. However, during welding in which the
cleanliness of the weld zone and shielding gas conditions are sufficiently
managed,
hydrogen contained in the wire primarily in the form of moisture becomes the
main cause
of diffusible hydrogen that is present in the weld joint.
[0085]
Therefore, it is preferable that a (seamless) pipe without slit-like seams be
used
as the steel sheath to suppress the infiltration of hydrogen in the air from
the steel sheath
to the flux until the wire is used after being produced. In a case where a
(seamed) pipe
with slit-like seams is used as the steel sheath, moisture in the air easily
infiltrates into the
flux from the slit-like seams (seamed portion) of the sheath. Therefore, when
such a
- 45 -
CA 02915026 2015-11-19
pipe is used as it is, the infiltration of the hydrogen source such as
moisture cannot be
prevented. Therefore, in a case where a time period from production to use is
long, it is
preferable that the entire wire be vacuum-packed or be stored in a container
that can be
maintained in a dry state.
In addition, in order to enhance the transportation performance of the wire,
there
may be a case where lubricating oil is applied to the surface of the wire.
From the
viewpoint of reducing the amount of diffusible hydrogen, as the lubricating
oil applied to
the surface of the wire, oil that does not contain hydrogen such as
perfluoropolyether
(PFPE) oil is preferable.
[0086]
The flux-cored wire used in the present invention can be produced in the same
production process as that of a typical method of producing a flux-cored wire.
That is, first, a steel strip which is to become the sheath, and a flux in
which
metal fluorides, alloy components, metal oxides, metal carbonates, and an arc
stabilizer
are mixed to have predetermined contents are prepared. While the steel strip
is
transported in the longitudinal direction thereof, the steel strip is formed
into an open
pipe (U-shape) by a forming roll to be used as the steel sheath, the flux is
supplied from
the opening of the open pipe during the formation, and the edge faces of the
opening that
oppose each other are subjected to butt seam welding. A seamless pipe obtained
by the
welding is drawn, and is subjected to annealing during the drawing or after
the
completion of the drawing process, thereby obtaining a (seamless) wire having
a desired
wire diameter without slit-like seams. In addition, a (seamed) wire having
slit-like
seams is obtained by supplying a flux from the opening of the open pipe to be
formed as
a seamed pipe that is not subjected to seam welding, and drawing the pipe. A
cut
section of the wire without slit-like gaps, which is made by butt seam
welding, is
illustrated in FIG. 3A. When the section is polished and etched, welding
traces are
- 46 -
CA 02915026 2015-11-19
observed. However, when the section is not etched, welding traces are not
observed.
Therefore, the section may be called "seamless". On p.111 of "New Edition of
Introduction to Welding and Joining Techniques" (2008) edited by "the Japan
Welding
Society" and published by Sanpo Publications Incorporated, a seamless type is
described.
As illustrated in FIG. 3B, when brazing is performed after butting is
performed, or as
illustrated in FIG. 3C, when brazing is performed after caulking is performed,
wires
without slit-like gaps can also be obtained. In FIGS. 3B and 3C, the wires
that are not
subjected to brazing and are used as they are become wires having slit-like
gaps.
[0087]
In the present invention, gas-shielded arc welding as multi-layer welding is
performed on the steel plate by using the flux-cored wire that satisfies the
above-
described conditions to form weld metal that satisfies the above-described
conditions,
thereby accomplishing the object. The gas-shielded arc welding method is not
particularly limited, and a typically used method can be employed. For
example, as the
shielding gas, as well as 100% CO2 gas, a mixed gas of 3 vol% to 20 vol% of
CO2 gas
and Ar gas, or the like can be used. The flow rate of shielding gas may be
under typical
conditions, that is, about 15 L/min to 30 L/min.
In addition, regarding welding conditions such as current, voltage, and the
like,
for example, a current of 200 A to 350 A, a voltage of 25 V to 35 V, and the
like may be
employed. The welding rate may be controlled to allow a weld heat input to be
10
kJ/cm to 50 kJ/cm.
[0088]
The shape of the produced weld joint is determined depending on the
application
or the like and is not particularly limited. Weld joints in which a groove is
formed, such
as a typical butt joint, a corner joint, and a T joint may be applied.
Therefore, the shape
of the steel plate to be welded may be formed so that at least a portion
thereof where the
- 47 -
CA 02915026 2015-11-19
weld joint is formed is a plate shape, and the shape may not entirely have the
plate shape.
For example, shaped steel may also be included. In addition, the steel plate
is not
limited to various steel plates, and a single steel plate may be formed into a
predetermined shape such as a pipe shape. However, a butt weld joint may also
be
employed.
[Examples]
[0089]
Next, the applicability and effects of the weld joint according to this
embodiment will be described with reference to Examples.
Steel plates having components shown in Table 1 were used as base metals. In
addition, as backing metals for welding, the same steel plates as the base
metals were
used.
While a steel strip was transported in the longitudinal direction thereof, the
steel
strip was formed into an open pipe by a forming roll, a flux was supplied from
the
opening of the open pipe during the formation, and the edge faces of the
opening that
opposed each other were subjected to butt seam welding, thereby forming a pipe
without
slit-like seams. During drawing work of a wire of the formed pipe, annealing
was
performed, thereby producing a flux-cored wire having a final wire diameter of
(1)1.2mm.
In addition, some of the steel plates were formed into pipes having slit-like
seams that
were not subjected to seam welding, and the pipes were drawn, thereby
producing flux-
cored wires having a wire diameter of (1)1.2 mm. In the case of the wire
having slit-like
gaps, the entire wire was vacuum-packed and stored in a container so as to be
maintained
in a dry state, until welding is performed.
The chemical components of the produced flux-cored wire were analyzed as
follows. First, the filling flux was extracted from the flux-cored wire, and
the flux-
cored wire was separated into the steel sheath and the flux. The chemical
components
- 48 -
CA 02915026 2015-11-19
of the steel sheath were obtained by measuring the content of each of metal
components
through chemical analysis. The chemical components of the flux were performed
in the
following order. First, the constituent materials and components of the flux
were
subjected to quantitative evaluation by X-ray diffractometry and fluorescent X-
ray
spectroscopy. Thereafter, the flux was separated into a slag content and an
alloy content
by using a separation method such as flotation or magnetic separation, and the
chemical
components thereof were analyzed by performing chemical analysis, gas
analysis, or the
like. The chemical compositions of the produced flux-cored wires are shown in
Tables
2-1-1 to 2-2, and Tables 3-1-1 to 3-2.
[0090]
The base metals were allowed to abut each other with a root gap of 16 mm and a
groove angle of 20 by using the flux-cored wire, and were welded by using the
backing
metal under the welding conditions shown in Tables 4-1-1 to 4-2-3. On the
surfaces of
the groove surface of the base metal and the backing metal, buttering of two
or more
layers and an excess weld metal height of 3 mm or higher was perfoimed by
using the
tested flux-cored wire.
Here, as Ti oxides, Si oxides, Mg oxides, and Al oxides, Ti02, SiO2, MgO, and
A1203 were respectively used. In Tables 2-2 to 2-4, the metal carbonates
include CaCO3,
BaCO3, SrCO3, and MgCO3.
[0091]
The analysis results of the chemical compositions of the obtained weld metals
are shown in Tables 5-1-1, 5-1-2, 5-2-1, 5-2-2, 5-2-4, and 5-2-5. A sample of
a polished
section of the weld metal, which is perpendicular to the welding direction,
was acquired,
and the Vickers hardnesses of 10 points of the sample at a position 1 mm
inward from the
surface of the weld metal were measured, and were converted into Brinell
hardnesses
using the hardness conversion table from SAE J417 (1983). In addition, a No. 4
Charpy
- 49 -
CA 02915026 2015-11-19
test piece (2 mm V-notch) based on JIS Z3111 (2005) was acquired, and the
Charpy
absorbed energy of the weld metal at -40 C was measured. A -40 C absorbed
energy of
27 J or higher was evaluated as passing.
The obtained results of the hardnesses and the Charpy test are shown in Tables
5-1-3, 5-2-3, and 5-2-6.
[0092]
In addition, a cold-cracking test and a diffusible hydrogen amount-measuring
test were performed on each of the weld joints obtained under the same welding
conditions. As the cold-cracking test, a test based on JIS Z 3158 (method of y-
groove
weld-cracking test in 1993) was performed at room temperature (25 C), and the
absence
of cracking in surfaces and sections was evaluated as passing. The diffusible
hydrogen
amount-measuring test was performed according to a gas chromatography method
based
on JIS Z 3118 (method for measurement of amount of hydrogen evolved from steel
welds
in 2007). An amount of diffusible hydrogen of lower than 1.0 m1/100 g was
evaluated
as passing.
The results are shown in Tables 5-1-3, 5-2-3, and 5-2-6.
[0093]
During welding, a significant level of the generation of fumes or slag was
evaluated as poor welding workability. A low level of the generation of fumes
or slag
was evaluated as good welding workability. The results are shown in Tables 5-1-
3, 5-2-
3, and 5-2-6.
[0094]
As shown in the test results of Table 5-1-3, the weld metals of Examples 1 to
54
which are examples of the present invention were excellent in all of hardness,
toughness,
cold cracking resistance, and welding workability and thus passed the tests.
- 50 -
CA 02915026 2015-11-19
On the other hand, as shown in the test results of Tables 5-2-3 to 5-2-6, the
weld
metals of Comparative Examples 101 to 165 did not satisfy the requirements
specified in
the present invention and at least one of hardness, toughness, cold cracking
resistance,
and welding workability did not pass the tests. The underlined numbers in
Comparative
Examples of Tables 5-2-1 to 5-2-6 represent outside of the ranges of the
present invention.
- 51 -
'
, =-,= ,---,
Plate
Vickers 11) c>
Chen- ca components of steel plate for base metal [rrassn3:,
CEN cr µc)
thickness hardness
, -
r
I [ mass% ) F '-'
C Si Mn P 5 Al NI V Cu Cr Mo Ti
Nb B [aim] ....F1v1 -
0.267 0.25 1 11 0.007 0.004 0.04 0.53 0
012 001 0.0014 32 521 0.58
0.287 0.38 1.18 0.012 0.006 0.06 0.47 0.25 002 0.0012
25 551 0.65
0.165 0.52 1 35 0.013 0.006 0.02 , ,
0.55 0.32 0 012 0.0014 50 434 , 0.56
0.188 0.27 097 , 0.008 0.005 0.03 ,
0.84 0 012 , 0.0009 22 448 0.52
0.244 0.26 067 0.012 , 0.003 0.03 0.54 0.08 , 0.23
0.47 0 018 002 0.0013 32 505 0.53
0.215 0.31 1 87 0.012 0.003
0.04 25 498 0.54
0.378 0.21 056 , 0.011 0.004 0.04
0.34 , 0 012 0.0014 16 581 0.56
0.392 0.22 054 0.012 , 0.006 0.03 , 0.41 0
013 0 0013 32 594 0.58
,
: 0.385 0.27 067 0.011 0.005 0.05
0.37 0.011 0 0010 11 601 0.59
.
.
0
0
=.>
====
..
====
0
I
=.>
0
U.S
-
N.>
=.>
0
.
.
====
1
=
..
..
=
..
====
a Composit.on of flux :mass% in wire3
- FT-- ea c)
CP =0
2
; , pt., - a"..ount a j.., ICaF2J/
o Class.fication Note Metal fl,inride , Metal oxide
ek
C -0 WV:al
^
,. 3,14 o =I., . ,=,,, ..,, :IDtal amount 5. :Iota: Meant al i
CF, BaF2. SrF2 j Me;a4.Att 2 T l02 SO2 Mg0 A1203 CaO amo,,.4 8 a===
1 Example 37 37 015 0.52 002
067 , 55 100 -1
. -
2 .,, r )(ample 36 36 , 015 004
015 , 013 240 100
, 3 Example , 7 5 04 7.9 , 025 0.22 e, ,
0.05 0.47 16.8 0.95
.
,
4 Example PFPEI 's appl:ed* 63 6.3 0.31 009 ,
0.01 0.40 15,8 1.00 ,
-- .
Example , 67 , 6.7 024 0.15 0.05
0.39, 1.2, 17.2, 1.00,
6 Example , Searree** 43 , 4.3 051 023 003
074 58 100
-4
1
7 Example 41 0.3 44 027 0.82 0.09
1.04 4.2 093
- - , . .
.._ -
8 Example , 7 l , 7.1 0.33 , 0.04
0,33 2.7 21.5 1.00
,
.
_ 9 Example , 6 16.1 029 0.38 0.02
0.67 9,1 1.00
. . .
Example . 57 , 5.2 017 0.18 005 0.04
0.35 0.17 14.9 1.00 0
. , -
11 Example 76 03 7.9 027 029 , 005
056 141 , 096 e,
h)
=
, ,i,
12 Example PFPE =r. aepf ed.' 41 j 03 4.4 , 029 i 001
029- 15.2 093 ..
cn
e,
, h)
. 13 , Example 45 0.2 , 4.7 , 0.27 0.33 l
0.05 0.60 7.8 0.96 a,
(..A õ. 14 Example , Seamoc** 35 0.2 3.7 0
18 I 0.05 0.18' 20.6 0.95 h)
e
1..
Example l, 34 3.4 0.42 0.62 0.09
1.04 3.3 1.00 '
..
õ 16 , Example 62, , 62 , 044 004
0.44 28 14.1 100 '
..
-. . . .1.
, 17_ Example 4 1 4 1 037 0.27 0.02
064 6.4_ 100 ,
, ,
18 Example 65- 6.5 0.11 0.24 , I 0.12
0.35 0.08 18.6 1.00
-
19 Example , 787.8 0 27 0.21
I 0.05 0.48 ,
,
16.3 1.00
Example PrPE :s aepfeexr 64 . 6.4 0310.03 014 0.01
0.48 13.3 1,00
21 Example , 48 4 8 0.21 0 21 0
05 0 42 114w 1.00.,
.
.
22 õ Example , Searnea** 54 , 03 , 02, 59 015 1 003 ,
015 , 393 092
, 23 Example 38 , , 0.3 , 4.1 0.52 0.41
, 0.09, 0.93 , , 4.4 0.93 .
24 Example , 48 , 4.8 0.21 1 0.04
0.21 4.2 22.9 1.00
_ ,,
Example , 35 õ 3.5 0.35 0.32 0.05
0.67 5.2_, 1.00
, - , . . -
26 Example , 3 9 3 9 0 13 0 2.4 0.01
0 37 0.11 10.5 1 00
.
. .
27 Example , 51 CS 5.6 033 022 0)2
005 __________________ 067 32 84 0 91
--..,
* PFPE: Perfluoropolvether oil
0. Seamed: the steel sheath having a shape wIth a slit-Me gap
i a Compostiort of flux
7rnass'l n w rdl
I Zd
Classi
7 , :Total amcdrt tx1 ' :Carif.'
2 ficatiun Note Meta l fluoride , Metal
ox:e o
, c
Meta'
[Total rre'_,rt $1 [7otal aTio..rt ct
Car 2 BaF2 Str2 Me, 1 !-a TiO Si02 MgO A1203 Ca0 I" -t:
;' :Y.-we
at-io.mt a
rt.:i.rt .,6 'z. s
78 Examp c ?f P1 s applied*_ 4.2 , , 4.2
032 0.21 0.14 013 0.67 0.13 63 1.00 I 0
.
. Is-)
129 t: xa TWe 6.7 03 _ 65 041 009 0.41
159 095 ,
1 30 _ Examp:e Seamed** 11 03 80 013
_ 004 013 615 096
' 31 Examp'e 4.1 4.1 0.33 062 0.15 032 1.10
37 1.00 1
.
! 32 Example 6.3 63 032 ' , 004 0.32 2.9
197 1.00
i 33 Example 3.7 3.7 0.18 0.27 _ 005 0.45
8.2 _ 1.00
4
I 34 Example 5.9- 5.9 0.12 0.18 , 0 01 0 30
011 10.7 1 00 '
_
_
_
35 Example 72 02 04 78 031 - 031
005 062 126 0 97 1
i 36 Example PEPE ,s =lied* 6.3 63 _ _ 021
_0.12 0.18 001 0.51_ 124 1.00 i
i 37 Examp'c 4.64 6 0.13 0 02 0.13
354 1.00 '
_ .
_
' 38 Examp'e Scamca** 5.4 0.3 0.1 , 5.8 0.21 _0.25
004 0.46 12 6 0.93
0
i 39 Example 4.7 _ , 4.2 029 0.81 008 1.10 _
3.8 1.00 o
i
40
140 Examfre 52 _ s7 022 003 016 025 18
208 100. i =D
1-.
s
====
41 E.'xample 5.2 5.2 0.06 0.09 r 0 12 0.15
34 7 1,00
(jI 4
Ow
4=
5.3 5 3 0 24 0 24 0 08 0.48 113 1.00 1 42
Examp.c
, _
o
= 1 43 1:.xarrryc 4.6 4.6 0.24 0.72 006
0.96 48 1.00 - ow
=
i 44 Example 4.4 4.4 0.23 004 0.23 1.5
191 1.00, 1-
=-=
i=
=-=
Example 47 42 027 0 21 001 043
08 1.00:
46
# - _
I 46 Example 4.1 4.1 025 008 0.25 2.5
16.4 1.00 i
i
t 47 Example 4.3 43 ' 0.35 004 0.35
123 1.001,
' 48 Example 4.1 4.1 023 0.02 _ 0.23
3.2_ 178 1.00
4
49 Example PEPE :s applied* 3.7_ 37 , 0.16 0.21
0.04 0.37 _ 0.15 _ 100 1.00
Example 4 14 1 055 008 0 55 1 5
/ 5 1 00 !
_ -
51 Examp'e 3.3 03 _ 3.6 0.12 _ , 008 0.12
30.0 0.92 '
52 Example 3.9 , 39 0.42 004 0.42 3.2
93 1.00.
53 Example 3.6 3.6 0.13 _ 0.39 006 0.52
69 1.00 1
_ _ i
54 Example 4.2 4 2 0 17 0.21 0 06 0 38
11 1 1.00 i
i _ .
* PEP{, perft,o=opolyether cil
** Seamed the steel sheath having a shape with a sk-Ase gap
6
Corrpositior or fIJ X ! ria s s 'I in wire] '-
OD
C>
ot.
a-: al'. (c4t-,7.: = cr ,c)
o Clasr.4',.eation
No 11 a1 rlos,
Noe Meta fuoride Metal
oxide r -g 1./e;,1 o A
;.. r
0a1 Ual Sr 1 0
Total 3rflt%gal ety.e..int. 2 ]
. v.a. :7 -2 F-2 MgF2
'.11.4 .07 5,7 Mg120,i ,' , , a ..* 0 Ce.:c-..Z.: t=.)
i
Cao ,..3:r.o.,=.. t.,4
Ca,4 \)
, ' ,.... 4
I 01 , CO" 7....41'31 Ve7 Exa-np e 42 , 42
012 035 0 030 47 89 1 00
102 Ce=-..o/vat ve 1 xanip e 4? 47 077 071 006
338 11 1 100
. . . .
! 103 Cofncsa-at ve E:Y3'11f) e 39 39 0'S 019 009
334 115 1 00
104 Cc -:.-a-at ve rxa'np c
1
105 Cc=-ca-atvc Exa7np c rcaxanp c 5.1 5.1 0.11 0.41 0
12 0 52 9.8 1 .
107,Cer.za-at ve Exanip c 5.7
3.8
45 =-= 5.7 0.18 0.32
3.8 0,13 0.24
45 0'Q 034 ' 0 01 0
50
0 13 , 0
37 '
;
0 15 0
44 . 11.4
10.3
,
107 , 1.00
1.00
106 GeIwt vc E
c-.3
100
, - . .
1108 Co=-=.sarat ve Eia-to e 41 41 016 024 004
040 103 100
109 Cortoa,at ye Flea-no * 42 42 009 0.25 007
034 124 1 DO
110 Cernowat ve Exarnp!e 41 , 47 074 025 004
039 10 S 1 00
111 Cerna,at vc rca-no e 4.7 4.1 0.12 0.22
0.03 , 0.34 13.8 1.00
t112 Cc--;arat vc Exenp 4 4.1 , 4.1 0.08 0.32 005
, 0.40 10.3 1.30 0
. , 2
113 Coricealvc "..: xamp c 5.5 , , 5.5 , 0.14 0.21
0.02 035 15.7 1.30 µD
..
e.
114 Comoraratvc Exanto e 4 7 4 7 0 15 0 15 004
330 157 103 ei
0
I
115 Corloarat ve E xarnp e 52 5 2 0 15 0 12
0.06 0 21, 193 1 30 ow
t...ri
IO
(-A 116 Comosrat ve F1(3"10 * 4 5 4 5 009 0
79 005 0 3811 8 1 CO
,
e.
. . .
. ei
I . I I 7 Cer-4-,a,at ve Exa-lp c 41 4/
012 0.I5 006 021 1 / 4 1 00 =
e.
e.
118 Ce.e-cacat ye rxeip.c 3.8 3.8 0.09 0.19 002
028 13.6 1.00 =
e.
.-
µD
119 Coreat vc Examp'c 5.4 5.4 0.15 0.21 012
036, 15.0 1.00
.
.
120 Conowal vc t....xamp c 5.3 , 5.3 0.11 0.15 ,
001 026 20.4 1.00
0
.
1'21 Ce-lowat ve EY/a-rip e 55 5.5 01? 015
001 327 20.4 , 100
1
. .
122 Co....0a,at ve ba'ip e 45 , 45 012 027 013
039 115 100
. .--
...
173 Coreta,at ve Exaelp e 3.33 3 0 11 1 35 0
15 1 57 7 1 103
,
, .
.
124 Cora-at ye Exarrip e 2 2 2 2 0 12 021 , 0
12 , 0 39 , 5 6 1 00
-
125 Coroarat ve rcamp c 8.9 ; 8.9 0.12 006 017
74.7 1.00
1126 Ccr-Ta-at vc Exarne c 7.5 , 7.5 0.02 0.05 004
007, 107.1 1.00
,
127 Cc,=np.zrat vc Examp c
i 3.8 , 3.8 0.07 , 0 030 07
õ
, 54.3 1,00
.,128 CorTa.at ye Examp 4? 42 016 027 034043 .
98 1.00 ,
,
129 Cema-al ve Exarip e 44 44 0.25 004 025 0 R
11 6 1 00
,
130 Cernevat ye Exarno e 42 42 0 ' 2 319 007
031 11 1 135 IOU
;131 Cernoarat vc Exa-rip c 34 C2 03
03 42 076 018 005 034 _ 124 081
=
.--1
,--.
Wire Wire coalawlents (mass 0
:armotat on 1) A) 0
Clasziificat:ori No.
_
Alloy comporteritti o- metal deox,oic ri,g corwonents
C Si M., P S Al Ni V C...1 Cr Mu Ti Nb B MK Ca REM (0)
1 Example 0 082 027 156 O011 0
004 0 017 0th 032 019 0 015 001 1
- 7
2 Example 0.242 . 0.39
, 0.54 , 3.010 0 006 0 007 . 027 0.21 0 012 0.0007
3 Example 0.112 0.54 1.73
0.012 0 005 0 011 0.45 , 0.44 0.0029 0.2
4 Example 0 131 0.48 277
0 010 0 003 0 012 ,
'
, Example 0 145 0.34 1.25 0.008 0
003 0 024 097 027 1,12 0 014 0.0033 . 04
6 Example 0 123 0,38 1.24
0.008 0 005 0 005 0.27 028 0.33 0 011 0.2
7 Example 0 105 OP. I 15 0 008 0
004 0008 032 039 025 0 0043
S Example 0.137 0.41
1.37 0.014 , 0 006 0 006 0.25 0.0018 .
,
. 9 Example 0.091 0.09
1.84 0.016 0 005 0 010 . 0.15 025 0.45 0.12
E.xample 0 241 0.12 124 0.015 0 005 , 0 006
071 , 021 0 024 002 0.0025 _
,
1 ' Example 0 121 0.34 0.95
0.014 0 007 0 005 022 0.43 0 025 0.0024 0.2 .
12 Example 0 094 0.41 1.34
0.012 0 006 0 023 0.87 ., 056 0.32 0.25 0 023 , 04
0
13 Example 0115 0.52
125 , 0 009 0 001 0 121 , 031 0 020 05 0
h)
0
14 Example 0.185 0.55 1.08
0.015 0.012 0 014 022 0.74 0 015 0.004/
"
tx
I
c,
Example 0 084 0.56 1.22 0.008 0 007 0 015
0 32 0.75 0.25 0.0054
ow
c.."
,
c"
16 Example 0 125 051 154
0 013 0 006 0 032 _ 0.23 0
022 h)
0
,
I 1/ _. Example 0.156 012 , 134 0 011 0 004 0
012 025 1.12 0 12, 0 054 002 0 0040
00
, 0
, 18 Example 0.284 0.34 1.25
0.013 0 005 0 012 0.22 024 0.18 0
0
19 , Example 0 164
043 2 1 1 , 0 007 11004 , 0
062 ,C.
1
Example 0 125 0.39 1.51 0 016 0 008 0
045 089 054 044 002 05 0
2' Exampl..- 0.166 , 0.09
1.93 0.013 0 007 0.025 , 038 0.48 , 0.35 0.0021 4
' 27 Example 0 139 0.18 155 0 015 0
005 0 015 027 011 0 011 00077 07
23 Example 0 129 034 , 1.52 0 011 0 003 0
008 025 0 57 , 017 , 0 085 0 6
.s
. 24 Example 0.141 , 0.28 0.84
0.009 0.005 0 008 0.18 036 , 0.27 0 072 0.5
, Example 0 125 05? 134 0 011 0 004 001? 021 117
020
,
26 Exampie 0 139 037 175 0 013 0
005 0 012 034 029 003 0.0037
27 Example 0.262 0.41 , 1.02 0.007 0 004
0.062 0,07 065 .
28 Example 0.165 , 0.28
1.51 0.016 0 008 0.052 _0.70 0.42 0.32 0 022 0.0015 0.0023 '
.- .
; 29 Example 0 134 0.41 1.98 0 015
0 007 0 026 026 048 ,, 0 3 ,
. =
: 30 Example 0.150 0.38 1.55
_ 0.015 0 004 0 016 0 22 _ 0.31_ 003 0.0032 ,.
An.lotation 1- remainder re and irnotni0es
Wire corn *orients Ernass% (anrot.al.-on 1)
oh)
--
W recr o
,C assfica: on Al oy componentsor metal
deox,o17 ng cornoonentr.
NoFr C)
G Si Mn P s Al N V C 1 C> Mo Ti No
B EWE KV (,)
31 WW1= 0 121 0.12 1.78
0.011 0 004 0 009 0.31 024 0.39 IIIIII
37 Example 0 145 018 184 COI/ 0 003 0 015
Emi 071 03! 0 018 0 0041 03 1:)
' 33 Example 0 146 07! 137 0 012 0004 0 011
035 051 075 IIIIIIIIIIII
34 11052111 0 134 0.33 Emi 0.013 0 005 0 013 11111 019 EN
0012 0.0032 02
35 MEM 0.098 0.4' 2,0 0.009 0
004 0062022 0 011 0 03 0.0033
' 36 Examp:e 0 158 0.39 1 5 0 016 0
008 0 311 092INE 056 02! MIMI
37 Exam lo 0.245 0.1? 1.98 0.014 0.003 0 025
0 17 0.48 0 024 0.0032 EMI
38 Wenn 0.155 0.19 NEI 0.014 0 005 0 015 .111 021
0.45 0.015 0.0021 1111 0.0032
39 Examp:e 0.099 0.21 1.52 0 018 0 003 0 012
0.44 0.18 0.52 0.25 NE
40 1111=311 0.138 0.24 1.78 0.016 0 004 0 008
0.31 0.37 0 0' 8 0.0029 02
1 41 111ffirra 0 215 0.15 1.35 0.018 0 004 0 012
42 MM. 0164 018 145 0015
0003 001/ 032 111111 0 013 00030
1111.11111 0
e
43 Exarnp e. 0,125 0.24 1.35 0.012 0 003
0 021 024 0.52 0.21 I h)
%I
I..
I 44 WM= 0.133 0.22 1.41 0.013 0.005 0 024
033 1 u=
0
h)
45 Minn 08/9 024 184 3013 0004 0015 02.2
IIIIIIIIII . a,
h)
..4 . 46 IMMO 0 081 022
1,18 0011 0005 0022 031 MINI .
.-
0
I '7 MEMEMI 0 077 0.34 1.75 0.009 0 003 0.032
029 =
1-
48 1111fefflra 0 039 045 1,64 0 011 000? 0 034
035 0.22
.).-
49 1111M21 0 034 038 155 0 012 0005 0 051 055
11111111111 0 012 0 0041 111111
50 larinn 0 045 0.5' 1.86
0.007 0.007 0 034 MIII RINI
51 IIIMMI 0849 EEE 1.24 0.013 0 004 0 029 078 025 NM 0.34
MINI
52 111M1111 0 035 037 1 35
0 010 0 006 0 034
53 IIMS111 0.022 0.45 1.26 0.014 0 005 0 038
0.36 IIIIII 0.3
54 ExarnpIe 0 051 038 162 C011 0005
0021 0 16
Annol'ation 1- remainder Fe aid impurtles
_
_______________________________________________________________________________
___________________________
'-i
W;re compone=qs :.rrass%1 annotation 1)
co .-i 0
Wi= e
cr
Ciassicicauor At:0,y
___________________________ compw,ents or metal deoxidtzing components o
No.
(7 '
C . St Mn . P S Al Ni V Cu . Cr &Jo Ti NO
. 8 Mg Ca NEM ta
101 Compa-at yr. E-xampe 0 361 025
1 56 0 008 0 005 0 025 021 1&)
102 Compaiatve Examo`e Q004 036 1.64 0.012 0.005
0.023 0.15
103 Compa-ativr Example 0 128 0 04 ' 52 0
014 0.007 0.024 0.17 . . .
104 Cempa-ative Examole 0 174 ..91 1.54 0.009
0.006 0.024 028
105 Compaiiative Example 0 127 035 018 0 015
0008 0.023 021
.,
106 Compa-auve Example 0 121 041
4,.,65 0.008 0.004 0.012 016 .
107 Conlpaiatve Example 0 124 034
_____________________________________ 151 0,072 0.003 0 013 011
108 Compwatvc Examp]e 0 135 0_33 1.55 0.008
, 0,035 0.075 0.20
,
109 Compaiative ExamPie 0 131 035
_____________________________________ 1.48 0.013 0.005 0,01;14 016
110 Compa-at ve Example 0 131 033 147 0 012 ,
0 008 0 161 038 . . .
111 Compaiative 7 )(ample 0 126 045 1.67 0.012 0.005
0.012 . 0_ .,,V 030 ,
117 Comparative Example 0 134 035 152
0 011 0 006 0 013 L61 0
113 Compaiative rxame4 0 090 0 38 1.43
0.009 0.64:13 0.015 , 0.21 3./1
ei
h)
114 Compa-ative Example 0 154 042 1 46
0.008 0.005 0,016 0_25 L 5.
...
L.
,
, c=
=
1 I5 Compaiatve Example 0 111 033
1.51 0.009 0.004 0,019 0.210.2 79 h)
OW
.
1 .
LA
oe 116 Cornpa-ative Examp'e 0 098 038
155 0012 0.005 0 011 020 018
ei
8 117 Compwative Examole 0 125 0.37 1.42
0 012 0.005 , 0.012 , .
0.160.97 ui
=
.
. ...
118 Compaiiative Example 0 128 03.
141 0 013 0 007 0 021 019 ei
=
119 Compa,ative Examo!e 0 134 038 1.31 0.011 0.006
0.015 0.16
120 Compa-ativc ExamVe 0.121 041
1.41 0.012 0.006 0.010 0.24 2...3
121 Compa-atve ExamP'e 0 115 0.32 1.44
0.011 0.006 0.010 0.19 p,18
122 Compaeatve Example 0 214 0.52
1.35 0.009 0.007 0.121 , 0 16 1.20 0.38
123 Compa-ative Example 0 195 051 157
0 015 0 012 0 014 0.19 038 .
124 Compa-ative Exampic 0 182 053 1.59 0.008
0.00/ 0.015 0.16 0. /5 0.25
,
=
125 Compa-atve Examale 0 132 055
_____________________________________ 1 61 0.012 0.005 0.018 0.18
126 Compa-atve ExamP:t 0 146 057
_____________________________________ 1.63 0.009 0.007 , 0.017 0.14
127 Compa-atve Examp!e 0 138 049 1.56 0.008 0
006 , 0 016 003 = . .
128 Compa-ative Examp!e 0 127 , 034 1.54 0.012 0.006
0.015 0.17
.
. , _________________ .
129 Compa-atve Examipe., 0 172 , 033 146 0 001 0 007
0015,0 18
,
-
130 'Compa-ative Examp1e 0 115 032
_____________________________________ 1.54 0.012 0.005 0.016 019
, 131 Compa-ative Examo1e 0.121 034 1.52
0.001 _ 0.005 0.015 _ 0.17
-
_______________________________________________________________________________
_______
Annotation I: remaincier: Fe and impurities
' ' - T
.'".-
Welding conditions
6 c , _
- i rzi) i-
No Steel plate
Classrfication z ?).z Temperature
.:1; Intcrpass Gas flow (7 t=-)
t.) ,3 rz Current Voltage Welding rate Heat input , . õ .
=N temperature
No. i._ xi .....f
temperature Shield rig ga:µ rate
4="
LA] :Vi Ecm/rnin: ikdlcm) s'ec' ""! Z; after
preheatinp
:c1
71.-/mtil
i-
,
Example 1 . 1 1 280 30 30 16.8 12 No -
150 or lower Ar-20W02 25 =¨,
Example 2 2 1 . 280 30 . 30 16.8 14 No
- 150 or lower Ar-7MCO2 25
rxamole 3 . 3 1 . 280 . 30 30 16.8 17 No -
150 or lower Ar-20µ002 . 25
Example 4 4 1 280 . 30 30 , 16.8 12 No ,
- 150 or lower Ar- 20W07 25
Exarnple 5 . 5 1 280 30 30 168 21 No -
150 or lower Ar 204402 25
Example 6 6 1 280 30 30 16.8 21 No -
ISO or lower Ar-20t002 25
Example 7 7 1 280 30 , 30 16.8 14 No -
150 or lower. 100%002 25
Example 8 . 8 1 , 280 30 30 . 16.8
19 No - , ISO or lower 100%002 25
Example 9 9 2 . 280 30 30 16.8 15 .
No - 150 or tower At -204402 25
Exarnp]e 10 ... 10 2 . 280 30 30 , 16,8 20 No -
150 or lower Ar 20'402 25 0
ei
Example 11 11 2 280 30 30 168 15 . No .
- 150 or ;owe?. Ar 204402 25 "
io
Example 12 12 2 280 30 . 30 16.8 12 No ,
- 150 or lower Ai-20402 25 =.."
ci
I
h)
Ow
vl Example 13 13 2 280 30 30 16.8 11 No -
150 or lower, Ar.-20ACO2 25 =0
vzi
ei
Example 14 14 2 280 30 . 30 16.8 19 No -
150 or lower , Ar-20%C.02 , 25 =.."
I
I
Example 15 , 15 2 . 280 30 30 16.8 0 No
25 .150 or lower 100%002 25 ...'"
=
Example 16 16 2 . 280 30 30 . 16.8 24 No -
150 or lower 100%002 25
Example 17 117 3 280 30 30 168 21 No -
150 or lower Ar 20%002 25
Example 18 18 3 280 30 30 16.8 20 No -
150 or lower Ar-20%CO2 25
Example 19 19 3 . 280 30 30 16.8 21 No -
150 or lower Ar-20%CO2 25
Example 20 . 20 3 280 30 30 16.8 19 No -
150 or lower Ar-20µ002 25
Example 21 . 21 3 280 30 . 30 16.8 18 . No -
150 or lower, Ar-70%002 _. 25
Example 22 22 3 280 30 30 168 21 No -
. 150 or lower Ar-20%CO2 . 25
Example 23 , 23 3 280 30 30 16.8 20 No -
150 or lower 100'402 25
Example 24 24 3 280 30 30 16.8 19 No -
150 or lower 100%002 25 .
Example 25 25 4 280 30 30 16.8 17 No -
150 or lower Ar-20%002 25 i
. Example 26 . 26 4 . 280 30 30 16.8 22 No
- .150 or lower . Ar-201402 25
Example 21 2/ 4 _., 280 30 30 16.8 25 No -
150 or lower. Ar-20%CO2 25 i
V.'elnirg condit=ons
go =-=
6 .,õ ..0
Steel olate
Clar.s..r:cetion z g'.7- :...
1 emperature ....
Intorpass Gas flow (7> ,I.._,,'
u f3 ;;:, Current Voltage Welciirlg rate Heat input
t .t. .1 late 't!!
temperature temperature Sr:ield rig gas rate
IA `,.V.; lem;rnini 1*j:cm.'
9 ' V' -9 ' -i; af ter vet-mat:rig
c_ I e7C; [Limin] i '--
ri :G
. ,
Examp'e 2828 4 280 30 30 16.8 19 No -
150 or ,owee. Ar--20µCO7 25
.
Example 29 29 4 280 30 30 168 2 Yes 19
150 or !owe, Ar-20µ,CO2 25
,
Example 30 30 4 280 30 30 16.8 17 No . -
150 or lower Ar 23W07 25
,
Example 31 . 31 4 . 280 30 30 168 20 No -
150 or lower 100%CO2 25
Example 32 , 37 4 280 30 30 16.8 18 No -
150 or !ower 100%C07 25 i
Example 33 33 5 280 30 30 . 16.8 22 No -
150 or lower Ar-70NCO2 25
Example 34 34 5 280 30 30 . 16.8 20 No . -
150 or lower Ar-70CC* , 25 i
l .
Example 35 35 5 280 30 30 16.8 18 No , -
150 or ower Ar-20W02 25
-.
Example 36 36 5 280 30 30 168 21 No . -
150 cr lower Ar 20%CO2 25
, ,
0
E..xample 37 , 37 5 280 30 30 . 168 18 No -
150 or Power Ar 20v.:07 25 ! 0
.)
Example 38 . 38 5 280 30 30 16.8 23 No -
150 or lower Ar-20%002 25 .
,.."
i
Example 39 . 39 5 280 30 30 16.8 21 No . -
150 or iowe, 100%CO2 25
.)
,
0\ Example 40 40 5 280 30 30 16.8 24 No -
150 or lower 100%CO2 25 ^)
0
c> .
, Example 41 ; 41 6 280 . 30 30 16.8 , 17 No
. - 150 or lower Ar- 2;Y:teCO2 . 25 ,.."
,
..
Example 42 4 42 6 280 30 30 168 20 No -
150 or lower Ar-23'CO2 25
Example 43 43 6 280 30 30 168 21 No -
150 or lower Ar 20W0, 75 7
Exarrp'e 44 44 6 280 30 . 30 16.8 18 No
- 150 or lower Ar 20%:,--02 25
Example 45 . 45 1 280 30 30 16.8 100 Yes
102 150 or lower Ar-2.0%CO2 25
Example 46 46 8 280 30 30 , 16.8 150 . Yes
163 150 or lower Ar-20%CO2 25 '
Example 47_4. 47 9 4 280 , 30 30 16.8 22 No
- 150 or lower At 2a-ACO2 , 25
- .
. Exarrp'e 48 48 1 280 30 30 168 15 No -
150 or lower Ar-20V.102 25
, .
Example 49 . 49 2 280 30 30 16.8 18 No -
150 or lower Ar 20`te02 25
'
Example 50 .. 50 5 280 30 30 16.8 17 No -
150 or lower Ar 20%C07 25 :
Example 51 51 6 280 30 30 16.8 . 20 No -
150 or lower Ar-20007 25 1
r ,
Example 52 52 7 280 30 30 . 16.8 15 . Yes
105 150 or lower Ar-23%002 25 i
. Example 53 4 53 . 8 280 30 30 . 16.8 12 . Yes
152 150 or lower Ar-20tC07 25 :
Example 54 4. 54 1 280 4_. 30 30 168 21 No
- 150 or lower Ar-20%CO2 ...._ 25 _..41
i .--)
Welding cond tions
- A) ,--, 0
0 o
z c.: Z tt
Stet' plate
Classification 14 ...... ,, Heat = Temperature
-wo In t erpass Gas flow ' FD- -1=`
2 kxurrent Voltage Weidtng rate mput f , 1 plate z
temperature 4=.
i No.
? :Al I Vi [cm,min] Ikslicrr: c
s'cww.c -g after preheating
[ CI tempo. ature Sh:elaing gas rate
[I../minj
t..)
cr:- L'GJ
,
Cemparatve Example 101, '01 1 280 30 30 16.8 17 No -
150 or lower A, 20%CO2 25 w--,
:Comparat ve Example 102 '02 1 280 30 ,.., 30 16.8 20
No - 150 or lower A- 20%CO2 25
Comparat ve Example 7 03 103 1 280 30 30 168 1 15 No -
150 or lower A-20%CO2 25
Cemparative Example 104 104 1 280 30 30 168 19 No -
150 or lower Ae-201C.0:e 25
.Compa-at ve Example 105 '05 1 280 30 30 , 168 20
No - 150 or lower A--201.1CO2 25
-
:Cerriparat ve Example 106 106 1 280 30 30 16.8 23
No - 150 or lower Arr 20SCO2 25
Comparative Example 107 107 1 280 30 30 168 21 No -
150 or lower Ar- 20%002 75
iComparat ve Example 308 108 1 280 30 30 16.8 15 No -
150 or lower A- -20A=CO7 25
:Comparative Example 109 109 1 280 30 30 16.8 18 No -
, 150 or lower A--70%CO2 25
=
.Comparatve Example 11C 110 1 280 30 30 168 , 21 No -
150 or lower A-20%CO; 25 0
.,Comparat.ve Example 111 111 1 280 30 30 i68 , 20
No - 150 or lower A-- 204,CO; 25 0
h)
,C.
:Compa-at ve Example 112 112 1 280 30 30 168 19 No -
150 or lower kr. 7011CO2 25 "
ow
ew
1 ECcmparat-ve Example 113 113 1 280 30 30 , 16.8
21 No - , 150 or lower Ar- 200O3 25
"
ow
0\ :Comparat ve Example 114 114 1 280 30 30 168 17
No - 150 or lower A'-20%CO2 25 I "
ew
wwww
Comparative Example 1lb 1 1 5 1 280 30 30 16.8 16
No - 150 or lower A- 201402 25
=
=wwww
Comparative Example 116 116 1 280 30 , 30 16.8 24 No -
150 or lower Ar-20%CO2 , 25 i wwww.
wwww
Compa-at ve Example 117 117 1 280 30 30 168 21 No -
150 Of lower A, 70\CO2 25 i wo
i
.Cemparat ve Example 118 118 1 780 30 30 , 168 73
No - 150 or lower A- 20%002 25 i
Comparat;ve Example 119 119 1 280 30 30 16.8 18 No -
150 or lower A- 20%CO2 25
Comparative Example 123; 120 I 280 30 30 __ 168 16 No -
lb or lower A- 20%CO2 ,. 25_
-
-
- - -
Welding condtions
O
6 0" 0
Z tt Z
Steel plate
Class 9cation ,n - T,-reperature
-.!"7. Intorpass Gas flow F. (-A
fu ec e$ CJrrent Volters Welding rate Pleat input .:
iv tempera:Lire
No .6, to -;=-= - at stek
pate ::2 temoerture Shielding gas rate
"r 4 [A]
o- c. [V: l ern /min] kJ/em'',
L after
preheat:rig
i*Ci
:C.: [L=jmin, 1:.)
,
N
Compare' ve Example 121 '21 1 280 30 30 168 20 No -
150 or lower Ar-70%CO2 25
,
Compare( ve E.rxemp e 122 '22 1 280 30 30 168 20
No - 150 or lower A-- 201iCO2 25
Compare' vc Examp,c 123,173 1 280 30 30 16.8 21 No -
150 o.. lower A- 20SCO2 25
Ce-parat ve Example 124 124 1 280 30 30 16.8 , 18 , No
- 150 or lower Ar 20%CO2 25
.Comparat ve Examp t 125 '25 1 , 280 30 30 16.8 21 No -
150 or lower Ar-20%C07 25
Cernparat ve Example 126 126 1 280 30 30 16.8 20 No -
150 or lower A-- 20%CO2 25
Compare( ye Exarrip:e 127 127 1 , 280 30 30 168 18 , No -
150 or lower A--20t>CO2 25
,
Comparat ye Examp'e 128 129 1 , 280 30 30 16,8 18 No -
150 or lower Ay-20%032 25
Comparat vc Examp'e 129 130 1 280 30 , 30 16.8 71 No -
150 or lower A- -20'SCO2 25
Comparat ve Example 133 131 1 280 30 30 16.8 18 No -
150 or lower Ar 20%C07 25 0
Comparat ve Eon-plc 131 132 1 280 30 30 16.8 19 No -
150 or lower Ar 204402 25 0
= h)
Comparat ve Examwe 132 /01 7 280 30 30 16.8 15 Yes
101 150 or lower Ar-200O2 25 wi
1-.
' .C. o rn p a r a t ve Examp'e 133 '07 7 280 30 , 30 168 ,
1:3 Yes 102 150 or lower Ar-20%CO2 25
e
6)
aw
CIN ,Comparat-ve Exempt 134 103 7 280 30 30 16 8 15
Yes 105 150 or lower Ar-20SCO2 25 6)
Iv -
e
!Comparat ve Example 135 104 7 280 30 30 168 16 Yes
102 150 or lower Aii--20SCO7 25 0"
a
I
1-.
Corrparat ve Example 136 105 7 280 30 30 16.8 I. Yes
110 150 or lower Ar 20SCC2 , 25
=
1-.
Comparat ve Examp'c 137 106 / , 280 30 30 16.8 16 Yes
102 150 or lower A- 20%C07 25 wi
Corriparat ye Examp:c 138 10/, 7 280 30 , 30 16.8 15
Yes , 110 150 or lower A--20402 25
1Comparat ve Example 139 108 7 280 30 30 16.8 , 22
Yes 105 ,150 or lower A--201,CO2 25
Comparat ve Example 143 109 7 280 30 30 16.8 18 Yes
102 150 or lower A,-20%CO2 25
¨ - - = -
¨ -
=
. _ _
Weldrts, conclIt'ors
H 0
o o
Z t.., z mp ..4
cr c,
Class,fication T
, eerature
.:-. Steel plate Interpass Gas flow
LAirrent Votage Welding rate Heat mput f . , . t 2
temperature
t em,nate
r
e
- She d
il -1 ,o or , rgas rate
-1=,
'Al fV7 f cm/triril i'lru.li
err' "e'-j.) 3 f! t a't.er prehcatlig
E , :C.
ri
7.C. C.1 (1 /m
.
in.
Y
(...)
Compardt ye Example 141 110 7 280 30 30 _ 168 15
Yes 101 150 or lower Ar 20's007 25
Comparat ye Example 142 I 1 1 / 280 30 1 30 16.8 16
Yes 100 150 or lower Ar 20SCO2 25
Co-rp?.rat ve Example 143 112 1 280 30 30 16.8 16
Yes 103 150 or lower A-- 20%CC2 , 25
Cemparat ye Example 144 113 / 280 30 , 30 , 16.8
15 Yes 104 150 or lower A- 20%CO2 25
Corrparat ye Example 145 114 7 280 30 30 I 6 8 15 Yes
101 150 or lower Ar-201402 , 25
- .
Comparat vr Examp'c 146 115 7 280 30 1 30 i 168 19
Yes 102 150 or lower A- 200O2 25
. .
Comparat ye Fxample 147 116 7 280 30 30 168 16 Yes
103 150 or lower Ar--20%002 25
Comparat ve Example 148 117 7 280 30 30 16.8 20 Yes
104 150 or lower A, 20SC07 25
,
Comparat ye Example 149,118 7 280 30 30 , 16.8 21
Yes '03 150 or lower A---200O2 25
,Comparat ve Example 150 119 7 280 30 30 16.8 , 14 Yes
102 150 or lower Ar--20',CO2 25
0
Comparat ve Example 151 120 7 280 30 30 , 16.8 16
Yes 104 150 or lower Ar-20,.0O2 , 25 e.
h)
Comparat ve Example 152 121 7 280 30 30 168 19 Yes
103 150 or lower Ar-205,C0/ 25 .:.
1..
cx
,= Co-paratve Examplet 153 122 7 , 280 30 30 1 168
15 Yes 104 150 or lower A:- 2000
%2
25 0
h)
OW
ON Comparat ye Example 154 173 7 280 30 . 30 16.8
11 Yes 105 150 or lower A- 20%002 25
h)
t....)e.
Compare re Example 155 124 / 280 30 30 16.8 , 24 Yes
102 150 or lower Ar-20CC7 25 61
==
'Comparat ye Example 156 125 7 280 30 30 16.8 16 Yes
100 150 or lower A 20%002 25 1-
1..
=
Comparat ye Example 157,126._ 7 280 , 30 30 16.8 12 Yes
102 , 150 or lower A-20%C04 25
.:.
C.emparat ye Example 158 127 7 280 30 , 30 168 26 Yes
103 150 or lower , A,--200O2 25
Comparat ye Example 159 128 7 280 30 . 30 i 168 16
Yes 101 150 or lower , Ar---20%CO2 25
Comparat ye Example 160 129 7 280 30 3016.8 16 Yes
105 150 or lower Ar 20%007 25
- .
Ce-paratve Example 161 130 7 280 30 30 16.8 , 20 Yes
109 150 or lower Ar 20%CO2 25
Comparat ve fixamp`c 162 131 7 280 30 , 30 .1 16.8
15 Yes 102 150 or lower Ar-20%CO2 25
Compatat ve Examp`e 163 45 7 280 30 30 I 16.8 21 No
- 150 or lower A--20%CO2 25
Comparat ye Example 164 46 8 280 30 30t 16 8 17 Yes
51 150 or lower A,-20',CO2 25
Cemparat ye Exa-rple 165 45 1 280 30 I
30 16 8 150 or lower A---20NCO 25
-
-
CA 02915026 2015-11-19
[0107]
[Table 5-1-1]
õ
C!assification Weld metal components tmass%., (annotation 1)
No C Si Mn P S Al
Ni V Cu Cr
Example 1 0 120 035 104 0 009 0 004 00210
012 038
; Example 2 0.221 0.23 053 0.008 0.005
0.0139 0.17 0.15
; Example 3 ; 0.137 0.37 1.12 0.010 0.004
0.0270 0.29 0.16
Examp'e 4 0.149 0.37 1.56 0.008 0.003 0.0621
0.14
' Example 5 0 161 026 088
0 007 0 003 0 0370 056 018 089 ,
Example 6 0 145 039 088 0 007 0 004
0 0270 017 018 017
Example 7 0.136 , 0.43 , 0.83 , 0.001
0.004 0.0139 0.21 0.42
Example 8 , 0.154 0.35 0.94 0.011 0.005 0.0270
0.16
Example 9 0.138 0.24 1 20 0.013 0.005 0.0270 0.09
0.15 0.43
Example 10 0 234 019 089 0 013 0.005 0.0430 014 012
Example 11 0 150 033 074 0 012 0 006
0 0270 015 014
Example 12 0.138 0.36 094 0.011 0.005 0.0670 0.55
0.35 0,35
, Example 13 0.14/ 0.40 0.90 0.009
0.006 0.0656 022 , 0.11 ,
Example 14 0.192 , 0.33 , 0.81 0.013 0.009 0.0228
0.14 0,12
Example 15 , 0 138 054 , 088 0 008
0.006 0 0470 019 063 1
' Example 16 0 149 046 105 0 011 0 005
0 0370 012
, Example 17 0.137 0.52 0.98 0.011
0.004 0.0155 0.15 0.87
. Example 18 0.206 0.35 0.94 0.012 0.005 0.0270 ,
0.13 0.14 0.18
Example 19 0.147 0.31 1.34 0.008 0.004 0.0338
0.20 ,
. Example 20 0 124 039 106 0 013 0.007 0
0975 051 033 047
Example 21 0 149 023 128 0 012 0 006 0 0202
025 048 '
Example 22 0.133 0.20 1.08 0.013 0.005 , 0.0270 ,
0.15 0.19
Example 23 0.128 0.40 1.07 0.011 0.004 0.0140 0
16 0.53 ,
; Example 24 , 0.137 , 0.31 0/5 0.009 0.005 0.0140
0.10 022 0.19 ,
Example 25 0 131 040 090 0 009
0.004 0.0167 0 19 0.97 ,
Example 26 0.129 0.28 0.85 0.010 0.005 , 0,0270
021 0.23
Example 27 0.224 0.32 0.73 0.007 0.004 , 0.0670 0.04
0.41 0.21 i
Example 28 , 0.153 0.29 0.99 0.012 0.008 0.07/0
0.45 0.49
Example 29 0.134 0.38 1.23 0.012 , 0.006 0.0225
0.16 0.56
Example 30 0 145 023 , 101 0 013 , 0 004
0 0183 014 020,
; Example 31 , 0 120 031 113 0.009 0.004
0 0370 0.20 015 023
' Examole 32 0 138 024 116 0 013 0.003
0 0179 016 021
. Example 33 0.156 0,46 084 0.011 0.003 0.02/0,
0.14 0.02 0.22 0.4/ :
Example 34 , 0.152 , 0.26 081 0.012 0.004 0.0115 0.16 0.03 ,
012 0.14
4_ Example 35 0.127 , 034 , 116 0009 , 003 0 0366 015 002, 012 015
Example 36 0.168, 0.30 091 0,013 0.008 0.0970 061 0.02
039 013
Examole 37 O222 0.11 , 114 0 012 0 003 00271 014
003 , Oil 045 1
Example 38 0.164 0.21 0.93 0.012 0.004 0.0182 0.16 0.03
0.13 0.15
" Example 39 0.123 0.44 0.91 0.015 0.003 0.0171 0.15
0,29 0.10 0.46
Example 40 0.155 0.24 1.04 0.013 0.003 0.0155 0.14 0.04
0,17 0.13
. Example 41 0.189 0.16 109 0 015 0.004 0 0112
Example 42 0 154 0.? 114 0 013 0.003 0 0133 015
Examole 43 0.134 0.41 1.09 0.011 0.003
0.0149 0 14 0.36
Example 44 0.131 0.24 1.12 0.011 0.004 0.0162
0.19
L xamnle 45 , 0.149 022 101 0.011 0.004
0.0125 , 0.14 0.11 ,
Example 46 0 152 073 097 0 010 0 005
0 0140 , 019 013
7 Example 47 0 158 033 098 0 009 0 003
, 0 0209 , 0 19 0 12
1 Example 48 0.114 0.33 1.12 0.009 0.002
0.0187 0.22 0,18
Example 49 0.111 0.36 1.15 0.011 0.005 , 0.0306
0.44 0.16
Example 50 0.117 0.38 1.38 0.008 0,005 0.0187 0.14 0.02
007 0.16
Example 51 0.106 079 098 0 011 0.003 0018? 061
019
Example 52 0 144 031 094 0 009 0 005 0 0203 ,
012
Example 53 0.125 , 0.28 0.62 0.012 0.005 0.0202
0.15
Example 54 0.109 _ 0.30 1 07_ 0 009 0.004 0 0200
__. 0 11:0.13
Annotator, 1: remainder Fe and imputes
- 64 -
CA 02915026 2015-11-19
[0108] .
[Table 5-1-2]
Classification Weld metal components [mass 4: (annotation 1)
No Mo Ti No B N Mg Ca REM 0 CEN
. Example 1 0.13 0 020 0 009 0.0004 0.005 0.022
035
. Example 2 0.12 0 019
0.004 0.0007 0 006, 0.031 ; 0.39
Example 3 030 0 026 0 005 0 0018 0 005 0 011 0 033
041
Example 4 0 003 0.003 0 0004 000/ 0 027 042
Example 5 0 029 0.006 0.0021 0.009 0.009 0.029 052
Example 6 0.22 0.004 0.004 0.0004 0.012 0.019 0.037
040
Example 7 0.17 0 023 0.003 0.0026 0.005 0.032 039
Example 8 016 000) 0 004 0 0006 0 008 0.0017 0 029
037
Example 9 0.16 0 029 0 005 0 0003 0 005 0 033 044
' Example 10 0.22 0 008 0.018 0.0016 0 004 0.034
048
Example 11 0.37 0 033 0.006 0.0015 0 005 0.017 0.030
038
E xample 12 0.24 0 006 0.004 0.0005 0
006 0.007 0.009 0.030 0 43 t,
. , 4
Example 13 007 0 005 0 005 0 006 0C34 0 027 034
i
; Example 14 058 0 007 0 007 00027 0 005 0.029
050 l
Example 15 0.25 0 037 0.005 0.0003 0.00/ 0.0021 0.036 ,
044
Examp:e 16 0.23 0 011 0.006 0.0004 0.008 0.031 0 38
Example 17 0.19 0 051 0.012 0.0023 0.00/ 0.032 049
Example 18 023 0 013 0.0004 0 009 0 032 049
.;; Examole 19 0.12 0 027 0 0006 0 004 0 034 ; 041
= Example 20 0.11 0 003 0.012 , 0.0004 0.005
0.012 0.028 040
Example 21 0.33 0 002 0.0014 0.005 0.030 051
Example 22 0.18 , 0 019 0.0012 0 006 0.023 0.029 036
Example 23 022 0 069 , 0.0004 0 007 0 018 0 027
041
Example 24 0 27 0 017 0.0005 0 0.028 036 005
0 021
. ,
Example 25 0.14 0 036 0.004 ' 0.033 045
Example 26 0.20 0 002 0.019 0.0019
0.006 , 0.031 034
,
Example 27 0 033 0.005 , 0.012 0.030 043
,
Example 28 0.24 0 007 0.0010 0 009 0 007 0 0014 0 032 047
,
Example 29 0 002 0.004 0 015 0.028 041
. ,
Example 30 0.22 0.019 0.0019 0.008 0.031
0.39
Example 31 0.29 0 034 0.006 0.008 0.025 039 ,
, Example 32 0.21 0 006 0.0024 0.007 , 0.015
0.024 040 :
= f. xample 33 017 0 020 0 006 0 007 003)
044 i
,
,
- Example 34 0 017 0 007 0.0020
0006*0.029 034 l
Example 35 0 036 0 024 0 0018 0 001 00)8 0 033 , 034
j
Example 36 0.14 0 003 0.008 0 004 0.001 0.03/
043 I
E )(amp:a 31 0 021 0.007 0.0019 0 007 , 0.029 0.035
054 1
Example 38 0.30 0 007 0.006 0.0014 0.004 0.014 0.0014
0.032 0.43 .
Example 39 0.17 0 029 0.008 0.005 0 038 041 i
,
Example 40 02) 0 006 0 006 0 0018 0 008 0 003 003)
047 1
Example 41 0.006 0.024 037 1
Example 42 0 003 0.0016 0.004 0.018 0.035 035 ;
Example 43 0.14 0.006 0.031 0.39 l
,
..
Example 44 0 007 0 028 031 1
,
..
_
, -xample 45 0 00 / 0 0005 0 006 0 032 034 1
, ,..,
Example 46 0 005 0.0006 0 005 0.031 034 1
Example 47 0 007 0.0*07 0 005 , 0.024 0.36 I
. Example 48 0.16 0.004 0.035
0.32 I
Example 49 007 0 005 0 006 , 0 033 031 1
Example 50 0 006 0004, 003) 033 1
Example 51 0.27 0 006 0.032 03) I
Example 52 0 005 0.033 031 1
... Example 53 , 0.28 , 0.005 , 0.015 0.038
, 031 I
,
Example 54 0 018 0 004 _____________ ' 0 032 0 26 !
.... __________________________________ _ - _ .. . _ .
Annotator) 1: remainder: Fe arid anpurities
- 65 -
CA 02915026 2015-11-19
[0109]
[Table 5-1-3]
_ , ________________________________________________ ----,
iV ckers -40.0 Charpy Diffusible
Classification Vtielding XS 2 3158 1
hardness absorbed hydrogen
No.kabilitY - 8 crackin te..t I
thy. energy Ed] [911/100g] 1A1c)!
, Examp c 1 385 72 0 7 Good No cracking
Examp o 2 500 32 0.3 Good ., No cracking,
Exarripe 3 406 52 0.6 Good No cracking,
F xarnp' A 4 418 61 0.8 Good No cracking I
. Exarrip'e 5 437 44 05 Good No cracking I
. Examp e 6 411 52 0 3 Good No cracking I
Ixamp c 7 403 58 0.4 Good No cracking i
-
Examp'e 8 421 64 0.9 Good , No cracking 1
. Examp e 9 409 81 0.5 Good No cracking I
Example 10 504 64 0 4I
Good No cracking ,
- Example 11 418 62 0,7 Good No cracking =i
Example 12 407 60 . 0.5 Good , No crackins 1
, Example 13 410 55 0.4 , Good , No cracking 1
Example 14 472 31 , 0.5 Good No cracking i
t. xample IS 411 49 0.7 Good , No cracking I
Example 16 417 50 03 Good No cracking
Example 17 414 - 59 0 4 Good No cracking I
. , I
Example 18 483 42 0.6 Good No cracking i
Example 19 , 41/ 69 0.7 Good , No cracking
_ Example 20 391 8o 0.3 Good No cracking!
. ,
Example 21 428 63 08 Good No cracking
_ .
Example 22 399 88 0 4 Good No cracking I
- i
Example 23 399 81 0.5 Good No cracking 1
Example 24 402 /2 0.51
Good No cracking
Example 25 406 65 0./ Good No cracking I
Example 26 393 49 0.6 Good No c=acking 1
, Example 27 497 55 06 Good No cracking I
, Example 28 427 61 09 Good No cracking i
, Lxample 29 404 58 0.4 1 Good No cracking I
Example 30 413 75 0.3 Good No cracking I
Example 31 38/ 62 0.4 Good No cracking
Example 32 40/ 50 0.6 Good No cracking I
Example 33 427 49 04 Good No cracking 1
Example 34 413 , 55 0.7 Good , No cracking 1
Exarnple 35 388 67 , 0.3 Good No cracking_t
Example 36 436 /5 0.4 Good No cracking
E x a rr p! e 37 503 50 0 7 Good No cracking i
Example __ 38 435 61 0 3 Good No cracking l
- . I
= Example 39 390 45 04 Good No cracking I
Lxample 40 425 62 0.7 Good No cracking i
_E.' Xafrpie 41 456 49 0.6 Good No cracking !
F_xample 42 418 62 0.4 Good No cracking I
Example 43 401 56 0.3 Good No cracking I
Example 44 399 63 05 Good No cracking
-
--
I
Example 45 450 47 0.5 Good No cracking
Example 46 444 42 0.6 Good No cracking 1
Example 41 459 50 0.6 i
Good No cracking .
- 1
Example 48 38375 0.4 Good No cracking 1
.I
--
Example 49 391 81 , 06 , Good No cracking i
Example 50 354 76 0.5 Good No cracking j
Example 51 3/2 83 , 0.6 Good No cracking I
Example 52 361 17 0.7 Good No cracking !
Example 53 345 79 0.4 Good No cracking ,
_Example 54 352 65 0.5 Good No cracking 1
- 66 -
CA 02915026 2015-11-19
[0110]
[Table 5-2-1]
Classification Web ci metal components rmass%1 (annotation 1)
No C Si Mn P S Al Ni V , Cu Cr
1Comparative Example 101 Q281 0.27 1.04 0.007 0.004 0.0300 0.13
0.15
Comparative Example 102 0,091 0.29 1.05 0.008 0.005 0.0200 0.12
0.14 ,
'Comparative Example 103 0.151 9.0a 0.78 0.007 0.003 0.0500 0.15 0.12
iComparative Example 104 0.178 211 1.03 0.008 0.005 0.0800 0.17
0.17
Comparative Example 105 0.133, 0.25 , 0.006 , 0.004 , 0.0400 0.17
0.11
1Comparative Example 106 0.152 0.37 2.58 0.007 0.004 0.0200 0.11 0.18
Comparative Example 107 0.145 0.32 1,01 2,02 0.003 0.0200 0,10
0.11
!Comparative Example 108 0.153 0.28 1.03 0.007 0923 0.0300 0,12 ,
0.12
iComparative Example 109 0.145 0.24 0.98 , 0.003, 0.003 0.0005 ,
0.15 , 0.12 ,
!Comparative Example 110 0.146 0.24 0.89 0.004 0.005 0.1400 0.16
0,13 ,
'Comparative Example 111 0.146 0.33 1.09 0.010 0.004 0.0200 0.36
, 0,16 0.15 ,
!Comparative Example 112 0.151 0.32 1.02 0.009 0.005 0.0200 QM
0.11 ,
!Comparative Example 113 0.122 0.30 0.97 0.008 0.003 0.0300 , 0.12
2wIti
Corrparative Example 114 0.163 0.29 0.99 0.007 0.004 0.0200 0.13
0.16
Comparative Example 115 0.134 0.24 1.01 0.003 0.004 0.0200 0.13
0.14
1Comparative Example 116 0 137 0.32 1.03 0.010 0.004 0.0300 0.14
0.15
Comparative Example 117 0.145 0.27 0.97 0.010 0.004 0.0400 0.10
0.93
!Comparative Example 118 0.147 0.23 0.98 0.005 0.003 0.0300 0.16
0.24
!Comparative Example 119 0.155 0.30 0.94 0.009 0.005 0.0200 0,11 0.65
!Comparative Example 120 0.147 0.29 0.96 0.010 0.005 0.0300 0.13 0.21
'Comparative Example 121 0.141 0.25 0.98 0.009 0.005 0.0200 0.12
0.14
iComparative Example 122 0.199 0.34 0.99 0.008 0.005 0.0500 0.10 0,93
!Comparative Example 123 0.184 0.37 1.04 0.012 0.009 0.0200 0.12
0.15
iComparative Example 124 0.175 0.58 1.02 0.007 0.005 0.0200 0.11
0.65
iComparative Example 125 0.151 0.37 1.07 0.005 0.002 0.0300 0.10
0.15
'Comparative Example 126 0.159 0.34 1,07 0.007 0.003 0.0200 0.09
0,14
Comparative Example 127 0.154 0.29 1.05 0.006 0.004 0.0200 0.01
0.13 ,
-Comparative Example 128 0.148 0.22 1.02 0.005 0.002 0.0300 0.15
, 0.13
!Comparative Example 129 0.137 0.24 1.04 0.005 0.003 0.0400 0.14
0.10
,Comparative Example 130 0.138 0.22 1.01 0.004 0.003 0.0300 0.17
0.17
[Comparative Example 131 0.134 0.23 1.05 0.005 0.003 0.0400 0.18
0.12
!Comparative Example 132 0.305 0.16 0.94 0.008 0.004 0.0166 0.14
0.09
!Comparative Example 133 0.115 0.22 0.97 0 009 0.003 0.0300 0.10
0.04
Annotation 1: remainder: Fe ard impurities
- 67 -
CA 02915026 2015-11-19
[0111]
[Table 5-2-2]
Classification Weld metal components [massq annotation 1)
No. Mo Ti Nb B N Mg Ca REM 0 CEN
Comparative Example 101, 0.013 0.005 0.022
0.50
Comparative Example 102 0.016 0.004 ,
0.031 0.23
Comparative Example 103 0.020 , 0.004
0.021 0.30,
Comparative Example 104 0.019 , 0.005
0.019 0.42
Comparative Example 105 0,1_90 0.005 0.027
9_,,I8
Comparative Example 106 0.012 0.003 0.028
059
Comparative Example 107 0.011 0.004 0.025
0.33 ,
Comparative Example 108 0.017 0.005 0.029
0.35 ,
,
Comparative Example 109 0.018 0.004 0.032
0.32
Comparative Example 110 0.020 0.003 0.026
0.32
Comparative Example 111 0.013 0.005,
0.028 0.41
Comparative Example 112 0.009 0.003 0.033
0.37
,
Comparative Example 113 0.030 , 0.005 ,
0.031 0..65 i
Comparative Example 114 1,11. 0.016 , 0.004 0.034 0.57
Comparative Example 115 0.1.10 , 0.004
0.030 0.31
Comparative Example 116 0.020 0.11.4 0.006 0.028
0.34
CornparatIve Example 117. 0.013 0,036 0.005
0.028 0.62
Comparative Example 118, 0.018 0,024 0.022
0.35
,
Comparative Example 119 0.073 0.006 0,190
0.030 0.43
Comparative Example 120 0.014 0.004 0.180 0.029
0.34
Comparative Example 121 0.015 0.003 , 0,021.
0.032 0.32
Comparative Example 122 0.26 0.013 0.005 0.031
0.6...1 ,
Comparat;ve Example 123 0.24 0.014 , 0.004. 0.032
0.45 i
Comparative Example 124 0.16 0.015 0.006 0.029 0.52
I
,õ..
Comparatve Example 125, 0.006 0.005 0.030
0.36
Comparative Example 126 0.008 0.005 0.037
0.37
!Comparative Example 127 , 0.005 , 0.004,
0.027 0.35
1Comparative Example 128 0.018 0.004 0_121
0.34
!Comparative Example 129, 0.007 0.006 0,114
0.31
!Comparative Example 130 0.013 0.005 0.108
0.32
Comparative Example 131 0.015 0.006 0.038
0.31
lComparative Example 132, 0 012 . 0.007 .
0.031 0.49
,
pomparative Example 133_ 0.013 _ 0.005_
0.027 0.25.
Annotation 1: remainder: Fe and impurities
- 68 -
CA 02915026 2015-11-19
[0112]
[Table 5-2-3]
Classification
Vickers --40*C Charpy DiffusibIe
Welding J1S Z 3158
. hardness absorbed hydrogen
No. [Hy] energy [J] [m1/104 workability cracking test
7
Comparative Example 101 ala 12 0.9 Good Cracking occured1
, 1
Comparative Example 102 32,5 69 0.6 Good No cracking
Comparative Example 103 449 15 0.6 Good No cracking
Comparative Example 104 424 a 0.7 Good No cracking
' Comparative Example 105 440 a 0.7 Good No cracking
Comparative Example 106 406 11 0.8 Good Crack nz occvredl
,,. Comparative Example 107 391 , 15 0.7 Good No cracking
Comparative Example 108 399 a 0.8 Good No cracking
i
Comparative Example 109 407 isi 0.6 Good No cracking
Comparative Example 110 395 a 0.7 Good No cracking
Comparative Example I 1 1 393 .Li/ 0.5 Good , No cracking ,
, Comparative Example 112 , 397 2.4 0.8 Good No
cracking I
Comparative Example 113 427 11 0.7 Good Cracknz occurredi
Comparative Example 114 441 , 12 0.7 Good Crack az
occyred!
Comparative Example 115 382 14 0.6 Good No cracking 1
Comparative Example 116 381 II 0.6 Good No cracking I
,
Comparative Example 117 402 n 0.9 Good Cracknz oc,ctirredi
Comparative Example 118 428 2.4 0.8 Good No cracking
Comparative Example 119 409 1.2 0.7 Good No cracking
Comparative Example 120 393 ira 0.6 Good No cracking
Comparative Example 121 386 15 0.6 Good No cracking i
Comparative Example 122 467 29 0.9 Good Crack nz occt.rred!
Comparative Example 123 439 34 La Good Cracknz occurre41
Comparative Example 124 435 . 35 _La , Good cra.sknz
occtxrecli
Comparative Example 125 398 33 0.8 2221 No cracking I
Comparative Example 126 407 36 0.9 Poor No cracking
Comparative Example 127 401 30 0.8 E2217 No cracking 1
Comparative Example 128 418 .12 0.9 Poor No cracking
Comparative Example 129 428 1.5 0.9 Poor No cracking
Comparative Example 130 430 Ili 0.9 Ei291 No cracking
Comparative Example 131 425 , 30 ik- ps, or gisuLngszg.uj1
Comparative Example 132 551 18 0.7 Good CdsuLtjujugszzgsli
Comparative Example 133 342 57 0.6 Good No cracking
- 69 -
CA 02915026 2015-11-19
[0113]
[Table 5-2-4]
Classificaton Weld metal components [massq (annotation 1)
No. C Si Mn P S Al Ni V Cu Cr
Comparative Example 134 0.175 0.04 0.92 0.012 0.006 0.0162 0.11 0.09
Comparative Example 135 0 205 0.98 0,93 0 008 0.003 0.0162 0.18 0.07
Comparative Example 136 0.175 0.21 018 0.011 0.003 0.0158 0.14 0.05
Comparatve Example 137 0 171 0.23 254 0.008 0 004 0.0300 0.10 0,09
Comparatve Example 138 0.1/3 0.20 0.89 0.056 0.003 0.0117 0.11 0.07
Comparatve Example 139 0 180 0.20 092 0.008 0 027 0.0166 0.13 009
Comparative Example 140 0.177 0.21 0.89 0.008 0.004 0.0040 0.10 0.08
Comparative Example 141 0.177 0,20 089 0.011 0,004 p; 1 top 0.12 0.09
Comparative Example 142 0.174 0.25 1.00 0.007 0.004 0.0300 0.38 0.20
0.07
i Comparative Example 143 0.179 0.21 0.92 0.005 0.003 0.0200 0..55 0.09
Comparative Example 144 0.151 0.22 0.87 0.009 0.003 0.0300 0.14 2.64
Comparative Example 145 0.192 0.24 0.88 0.006 0.004 0.0129 0.16 0.10
Comparatve Example 146 0.164 0.20 0.91 0.009 0.004 0.0300 0.14 0.09
Comparative Example 147 0.156 0.22 0.93 0.007 0.004 0.0108 0.13 0.07
Comparative Example 148 0.173 0.23 0.96 0.011 0.004 0.0300 0.10 0.09
Comparative Example 149 0.175 0.19 0.86 0.008 0.006 0.0150 0.12 0.10
Comparative Example 150 0,179 0.22 0.84 0.009 0.003 0.0300 0.10 0.08
Comparatve Example 151 0.171 0.23 0.87 0.005 0.005 0.0104 0.16 0.09
. Comparatve Example 152 0 167 0.19 087 0.008 0.005 0.0400 0.12 0.07
Comparative bample 153 0.231 0.29 0.83 0.001 0.006 0.0562 0.10 0.91
Comparative Example 154 0 219 , 0.28 0.94 0 008 0.004 0.0400 0.12 0.09
Comparatve Example 155 0.210 0.29 0.97 0.007 0.006 0.0125 0.10 0.60
Comparative Example 156 0.178 0.31 0.96 0_009 0.004 0.0300 012 0.09
Comparatve Example 157 0.187 0.31 0.97 0.008 0.006 0.0200 0.09 0.11
Comparatve Example 158 0 182 0.27 0.94 0 008 0.005 0.0300 0.02 0.08
i Comparatve Example 159 0.175 0.22 0.93 0.007 0.005 0.0300 0.11 ,
0.09
I Comparative Example 160 0.172 0.20 0.86 0.003 0.002 0.0200 0.12 0.08
Comparative Example 161 0.167 0.19 0.93 0.005 0.004 0.0129 0.12 0.10
Comparatve Example 162 0 171 0.20 0.92 0.004 0.004 0.0125 0.11 0.09
Comparatve Example 163 0.148 0.23 0.99 0.009 0.003 0.0200 0.13 0.09
Comparatve Example 164 0.153 0.24 0.99 0.008 0.005 0.0200 0.15 0.11
Comparatve Example 165 0.149 0.25 1.06 0.007 0.004 0.0200 0.01 0.10
Annotation I: remaincier: Fe and impurities
- 70 -
CA 02915026 2015-11-19
[0114]
[Table 5-2-5]
1- - -
: Classification Wed metal
components Imasstol ;annotation 1)
_
No. Mo Ti Nb
B N Mg Ca REM 0 CEN
i
i
Comparative Example 134 0.014 0.004 , 0.033 035
Comparative Example 135 0.013 0.004 0.033 042
Comparative Example 136 0.015 , 0.005 , 0.026
023
1 Comparative Example 137 0.016 0.006 0.035 060
E Comparative Example 138 0.012 0.004 0.027 034
. ,
i Comparative Exampie 139 0.014 0.004 0.04 036
,
I Corrparative Example 143 0.012 0.006 0.038
0.35
1.
! Comparative Example 141 , 0 014 0 005 0.033
035
Comparative Example 142 0.009 0.007 0.027 044
Comparative Example 143 0.011 0.005 , 0.029 039 .
,
, Comparative Example 144 , 0.010 , 0.003 , 0.028
9.76
1 Comparative Example 145 1.17 0.014 0.005, 0.034 P.60
, Comparative Example 146 0.111 _ 0.004, 0.035
0.34
, -
1 Comparative Example 14/ 0.012 0.11õ7 0.004 0.039
035
Comparative Example 148 0.014 0.072 0.005 , 0.034 0.46
[ Comparative Examp'e 149 0.013 0.925, 0.041
0.35
1
i Comparative Example 150 0.011 0.006 0.18 0.035
0.34
I Comparative Example 151 0.009 0.004 0.19 0.042 0.34
i
i Comparative Exampie 152 0.012 0.003 0.016 0.037 033
I
i Comparative Example 153 0.269 0.011 0.006 0.036 062
I Comparative Example 154 0.269 0.010 0.005 0.041 0.46
i
I Comparative Example 155 0.111 0.012 0.006 0.038 054
,
r Comparative Example 156 0 011 0.004 , 0.033
037
1
Comparative Example 157 0.013 0.005 0.035 0.38
1 Comparative Example 158 0.005 0.006 0.033 036
: Comparative Example 159 0.005 0.004 0,109
0.35
,
i Comparative Example 160 0 012 0.005 Qj_12
0.34
Comparative Example 161 0.011 0.004 i ..il : 035
Comparative Example 162 , 0.007 0.006 0.037 0.35
1 Comparative Example 163 0.008 , 0.0005 0.006
0.032 0.34
... , _
1 Comparative Examp'e 164 0.010 , 0.0007 0.006
0.031 , 034
LComparative Example 165 0.009 * 0.0007 0.005 _ .
a035 0.34 ,
_
Annotation 1: remainder: re and impurities
- 71 -
CA 02915026 2015-11-19
[0115]
[Table 5-2-6]
Vickers -40`t Charpy Diffusible
Classification Welding JIS Z 3158
hardness absorbed hydrogen
No. workability cracking
test
clivi energy IJi im 1 /100g1
Comparative Example 134 462 14 0.8 Good 4 No cracking
Comparative Example 135 430 17 0.7 Good No cracking
Comparative Example 136 462 LI 0.6 Good No cracking
Comparative Example 137 419 12 0.7 Good Crackinz
occurred
Comparative Example 138 395 .12 0.8 Good No cracking
Comparative Example 139 412 .1..;/ 0.7 Good No cracking
Comparative Example 140 413 14 0.7 Good No cracking
Comparative Example 141 404 12 0.7 Good No cracking
Comparative Example 142 405 I/ 0.8 Good No cracking
Comparative Example 143 400 22 0.7 Good No cracking
Comparative Example 144 428 15 0.7 Good Cracking
occurred,
Comparative Example 145 434 12 0.8 Good Cracking
occurred I
Comparative Example 146 410 n 0.7 Good No cracking ,
Comparative Examole 147 403 14 0.7 Good No cracking I
,
Comparative Example 148 422 11 0.8 Good
Cracking.occurres1 I
Comparative Example 149 426 1.9 0.6 Good No cracking I
Comparative Example 150 427 11 0.7 Good No cracking I
Comparative Example 151 412 ii 0.6 Good No cracking I
Comparative Example 152 409 ik 0.7 Good No cracking
Comparative Example 153 462 31 0.9 Good Crack:az
ot:cirret:i i
Comparative Example 154 437 33 2_1 Good Crackinz
occurred i
Comparative Example 155 450 37 2.0 Good , Crackles
aeciored '
,
Comparative Example 156 399 31 0.7 2221 No cracking
Comparative Example 157 412 40 0.7 P90c. No cracking
,
Comparative Example 158 408 29 0.6 Poor No cracking
Comparative Example 159 426 2 0.7 Poor No cracking
Comparative Example 160 439 14 0.8 Emu No cracking
Comparative Example 161 441 12 0.7 Poor No cracking !
Comparative Example 162 436 30 19. Poor Crackinf
occurred I
Comparative Example 163 469 32 0.8 Good Cracking
occurred 1
Comparative Example 164 464 28 0.9 Good Cracking
occurred i
Comparative Example 165 459 32 0.9 Good
Ciacking.occiered I
[Industrial Applicability]
[0116]
According to the present invention, in a weld joint which uses a high-hardness
steel plate having a high C content and a surface hardness of HV380 or higher
and
I-IV693 or lower as a base metal, weld metal which has a surface hardness of
HV337 or
- 72 -
CA 02915026 2015-11-19
higher and HV533 or lower and excellent abrasion resistance or weld metal
which has a
surface hardness of HV380 or higher and HV533 or lower and excellent abrasion
resistance can be obtained without the occurrence of cold cracking even when
preheating
is not performed. Therefore, welding efficiency can be significantly enhanced,
and thus
such a weld joint is extremely valuable in the industrial field.
- 73 -