Note: Descriptions are shown in the official language in which they were submitted.
NOVEL SELECTIVE PI3K DELTA AND/OR GAMMA PROTEIN KINASE
INHIBITORS
FIELD OF THE INVENTION
[01] The present invention provides novel compounds, useful as PI3K protein
kinase
modulators and in particular as P13K delta (6) and/or gamma (y) protein kinase
modulators,
methods of preparing them, pharmaceutical compositions containing them and
methods of
treatment, prevention and/or amelioration of PI3K kinase mediated diseases or
disorders with
them.
BACKGROUND OF THE INVENTION
[02] Phosphatidylinositol (hereinafter abbreviated as "PI") is one of a number
of
phospholipids found in cell membranes. In recent years it has become clear
that PI plays an
important role in intracellular signal transduction. Cell signaling via 3'-
phosphorylated
phosphoinositides has been implicated in a variety of cellular processes,
e.g., malignant
transformation, growth factor signaling, inflammation, and immunity (Rameh et
al. (1999)1
Biol Chem, 274:8347-8350). The enzyme responsible for generating these
phosphorylated
signaling products, phosphatidylinositol 3-kinase (also referred to as PI 3-
kinase or PI3K),
was originally identified as an activity associated with viral oncoproteins
and growth factor
receptor tyrosine kinases that phosphorylate phosphatidylinositol (PI) and its
phosphorylated
derivatives at the 3'-hydroxyl of the inositol ring (Panayotou et al. (1992)
Trends Cell Biol
2:358-60).
[03] The phosphoinositide 3-kinases (PI3Ks) are a family of enzymes that
regulate diverse
biological functions in every cell type by generating phosphoinositide second-
messenger
molecules. As the activity of these phosphoinositide second messengers is
determined by
their phosphorylation state, the kinases and phosphatises that act to modify
these lipids are
central to the correct execution of intracellular signaling events.
Phosphoinositide 3-kinases
(PI3K) phosphorylate lipids at the 3-hydroxyl residue of an inositol ring
(Whitman et al.
(1988) Nature, 332:664) to generate phosphorylated phospholipids (PIP3s) which
act as
second messengers recruiting kinases with lipid binding domains (including
plekstrin
homology (PH) regions), such as Akt and phosphoinositide-dependent kinase-1
(PDK1).
Binding of Akt to membrane PIP3s causes the translocation of Akt to the plasma
membrane,
bringing Akt into contact with PDK1, which is responsible for activating Akt.
The tumor-
104867946 v2
104867946 v2
Date Recue/Date Received 2020-12-07
suppressor phosphatase, PTEN, dephosphorylates PIP3 and therefore acts as a
negative
regulator of Akt activation. The P13-kinases Akt and PDK1 are important in the
regulation of
many cellular processes including cell cycle regulation, proliferation,
survival, apoptosis and
motility and are significant components of the molecular mechanisms of
diseases such as
cancer, diabetes and immune inflammation (Vivanco et al. (2002) Nature Rev.
Cancer 2:489;
Phillips etal. (1998) Cancer 83:41).
[04] The members of the class I family of PI3Ks are dimers of a regulatory and
a catalytic
subunit. The class I family consists of four isoforms, determined by the 110
kDa catalytic
subunits a, 13, y and 6. Engelman JA, Nat Rev Genet 2006;7:606-19; Carnero A,
Curr
Cancer Drug Targets 2008;8:187-98; Vanhaesebroeck B, Trends Biochem Sci
2005;30:194-
204. Class I can be subdivided into two subclasses: Ia, formed by the
combination of p110 a,
(3, and 6 and a regulatory subunit (p85, p55 or p50) and Ib, formed by p110 y
and p101
regulatory subunits.
[05] The four
class I PI3K isoforms differ significantly in their tissue distribution. PI3Ka
and PI3Kfl are ubiquitous and activated downstream of receptor tyrosine
kinases (RTK)
whereas PI3K 6 and PI3K y are primarily limited to hematopoietic and
endothelial cells , and
are activated downstream of RTKs, and G protein coupled receptors (GPCR),
respectively.
Mouse genetic studies have revealed that PI3Ka and PI3Kfl are essential for
normal
development, whereas loss of PI3K 6 and/or PI3K 7 yields viable offspring with
selective
immune deficits
[06] The expression pattern and functions of PI3K 6 and PI3K y have generated
much
interest in developing PI3K6/y inhibitors as agents for many diseases,
including rheumatoid
arthritis, allergies, asthma, chronic obstructive pulmonary disease and
multiple sclerosis
(Hirsch et al., Pharmacol. Ther. 118, 192-205 2008; Marone et al., Biochim.
Biophys.Acta.
1784, 159-185. 2008; Rommel et al. Nat. Rev. Immunol. 7, 191-201., 2007;
Ruckle et al.,
Nat. Rev. Drug Discov. 5, 903-918.2006). Studies using both pharmacologic and
genetic
methods have shown these two isoforms often demonstrate synergistic
interactions with each
other (Konrad et al., J. Biol.Chem. 283, 33296-33303. 2008; Laffargue et al.,
Immunity 16,
441-451.2002). In mast cells, for example, PI3K6 is essential for
degranulation in response to
IgE cross-linking of Fc-receptors ( Ali et al., J. Immunol. 180, 2538-2544.
2008), but PI3Ky
plays an important role in amplifying the response (Laffargue et al., Immunity
16, 441-451
2002). Similar effects have been seen in other cellular functions, including
lymphocyte
2
Date Recue/Date Received 2020-12-07
homing and the neutrophil respiratory burst where PI3K7 plays a critical role
and PI3K6
amplifies each process. The nonredundant but related roles of PI3K6 and PI3Ky
have made it
difficult to determine which of the two isoforms (alone or in combination) is
best targeted in a
particular inflammatory disorder. Studies using mice that lack PI3K6 and/or
PI3Ky or express
kinase-dead variants of PI3K6 and PI3Ky have been valuable tools in
understanding their
roles. For example, P131(.3 knockout mice demonstrated diminished neutrophil
chemotaxis,
diminished antibody production (both T cell dependent and independent) (Jou et
al., Mol.
Cell.Biol. 22, 8580-8591. 2002), and lower numbers of mature B cells (Clayton
et al., J. Exp.
Med. 196, 753-763. 2002; Jou et al., 2002), and a decrease in their
proliferation in response
to anti-IgM (Jou et al., 2002) . This phenotype was replicated in the PI3K6
kinase-dead
variant and with PI3K6 selective inhibitors along with decreased numbers of
and proliferation
of mast cells, and an attenuated allergic response. The PI3Ky knockout
contained higher
numbers of, but less responsive, neutrophils, lower numbers of and less
responsive
macrophages and dendritic cells displayed decreased mast cell degranulation
(Laffargue et
al., 2002), a higher ratio of CD4+ to CD8+ T cells), increased thymocyte
apoptosis,
diminished induction of CXCR3 on activated T cells and decreased cardiac
contractility.
This latter effect on cardiac tissue was a concern for chronic dosing of
patients with PI3Ky
inhibitors. However, this concern was largely mitigated when the PI3Ky kinase-
dead variant
(which better mimics inhibition of the kinase rather than loss of the protein)
showed similar
immune cell phenotypes, but importantly had no cardiac defects. The cardiac
effect was later
shown to be due to scaffolding effects rather than the catalytic activity of
PI3Ky. The dual
PI3K6 /PI3Ky knockout was viable but exhibited serious defects in T cell
development and
thymocyte survival. The PI3K7 knockout/ PI3K6 kinase-dead combination produced
a similar
phenotype suggesting that at least within the immune system, the role of PI3K6
is likely only
a catalytic one. Interpretation of studies using knockout and kinase-dead mice
can be
challenging because these models provide only a steady-state picture of the
immune system,
lack temporal and dose control, and do not permit a full understanding of how
a dynamic
immune response will react to reversible inhibition.Selective inhibitors with
varying profiles
(P131(.5, PI3Ky, and PI3K6/y) are necessary for studies of leukocyte signaling
in order to
assess the relative contributions of each PI3K to immune cell activation. (
see Olusegon et al.,
Chemistry & Biology 1,123-134 including the cited references threin)
[07] Dual inhibition of 6/7 is strongly implicated as an intervention strategy
in allergic and
non-allergic inflammation of the airways and other autoimmune diseases.
Scientific evidence
3
Date Recue/Date Received 2020-12-07
for PI3K-6 and y gamma involvement in various cellular processes underlying
asthma and
COPD stems from inhibitor studies and gene-targeting approaches. Also,
resistance to
conventional therapies such as corticosteroids in several COPD patients has
been attributed to
an up-regulation of the PI3K 6/y pathway. Disruption of PI3K- 6/y signalling
therefore
provides a novel strategy aimed at counteracting the immuno-inflammatory
response. Due to
the pivotal role played by PI3K- 6 and y in mediating inflammatory cell
functionality such as
leukocyte migration and activation, and mast cell degranulation, blocking
these isoforms may
also be an effective strategy for the treatment of rheumatoid arthritis as
well. Given the
established criticality of these isoforms in immune surveillance, inhibitors
specifically
targeting the delta and gamma isoforms would be expected to attenuate the
progression of
immune response encountered in airway inflammation and rheumatoid arthritis.
Given the
established criticality of these isoforms in immune surveillance, inhibitors
specifically
targeting the 6 and y isoforms would be expected to attenuate the progression
of immune
response encountered in airway inflammation and rheumatoid arthritis (William
et.al
Chemistry & Biology. 17:123-134,2010 and .Thompson, et al. Chemistry &
Biology. 17:101-
102. 2010)
1081 WO 2014/071109, WO 2014/071105, WO 2014/004470. WO 2011/008302, WO
2010/059593, US 2009/0312319, WO 2009088986A1 and WO 2009088990 disclose 2,3
disubstituted isoquinoline derivatives as Pi3K inhibitors and WO 2013147649,
WO
2013116562, WO 2013029116, WO 2012/118978, WO 2012/009452 and WO 2008/127226
disclose 2, 3 disubstitued quinazolin derivativatives as Pi3K inhibitors
1091 There is considerable evidence indicating that Class Ia PI3K enzymes
contribute to
tumourigenesis in a wide variety of human cancers, either directly or
indirectly (Vivanco and
Sawyers, Nature Reviews Cancer, 2002, 2, 489-501; Marone etal., Biochimica et
Biophysica
Acta 1784 (2008) 159-185). In particular, the p110 delta isoform has been
implicated in
biological functions related to immune-inflammatory diseases, including
signaling from the
B-cell receptor, T cell receptor, FcR signaling of mast cells and
monocyte/macrophage, and
osteoclast function/RANKL signaling (Deane J and Fruman D A (2004) Annu Rev.
Immunol.
2004. 22:563-98; Jams etal., The Journal of Immunology, 2008, 180: 739-746;
Marone R et
al., Biochim. Biophy. Acta 2007, 1784:159-185). Deletion of the PI3K delta
gene or selective
introduction of a catalytically inactive mutant of PI3K delta causes a nearly
complete ablation
of B cell proliferation and signaling, and impairment of signaling through T
cells as well.
4
Date Recue/Date Received 2020-12-07
[10] Reviews and studies regarding PI3K and related protein kinase pathways
have been
given by Pixu Liu et. al. (Nature Reviews Drug Discovery, 2009, 8, 627-644);
Nathan T. et.
al. (Mol Cancer Ther., 2009;8 (1) Jan., 2009); Romina Marone et, al.
(Biochimica et
Biophysica Acta 1784 (2008) 159-185) and B. Markman et. al. (Annals of
oncology Advance
access published August 2009). Similarly reviews and studies regarding role of
PI3K 6 and y
have been given by William et.al Chemistry & Biology. 17:123-134,2010 and
.Timothy et.al
J Med Chem. web publication 27th august 2012, Berndt et. al. Nature Chemical
Biology
(2010), 6(2), 117-124, Nature Chemical Biology (2010), 6(4), 306, and Nature
Chemical
Biology (2010), 6(3), 244, Williams et. al. Chemistry & Biology (Cambridge,
MA, United
States) (2010), 17(2), 123-134, Apsel, Beth et. al. Nature Chemical Biology
(2008), 4(11),
691-699 and Knight, Zachary A et al Cell (Cambridge, MA, United States)
(2006), 125(4),
733-747.
[11] There still remains an unmet and dire need for small molecule kinase
modulators in
order to regulate and/or modulate transduction of kinases, particularly PI3K,
for the treatment
of diseases and disorders associated with kinase-mediated events.
SUMMARY OF INVENTION
[12] The present invention is directed to compounds, which are useful as PI3K
protein
kinase modulators and in particular as PI3K 6 and/or 7 inhibitors.
[13] In one embodiment, the present invention relates to a compound of formula
(I):
R, ( )n
Rb
N N
N )1Rc
N
Cy
I-12N
(I)
or a tautomer thereof, N-oxide thereof, pharmaceutically acceptable ester
thereof, prodrug
thereof, or pharmaceutically acceptable salt thereof, wherein
Date Recue/Date Received 2020-12-07
each occurrence of R1 is independently H, substituted or unsubstituted alkyl,
substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl or
halogen;
X is CH or N;
each occurrence of R is independently H, substituted or unsubstituted alkyl,
or
halogen;
IV and Rb are independently selected from H and substituted or unsubstituted
alkyl;
RC is H, substituted or unsubstituted alkyl, -NH2 or halogen;
each occurrence of n is independently selected from 0, 1, 2, 3 and 4; and
Cy is selected from
R2 R2I R4,, 411, R2 711111
N V
N
R3 , R3 , R2 R4 and R4 R2 ;
wherein
R2 and R3 are independently selected from H, halogen, hydroxyl, substituted or
unsubstituted alkyl, substituted or unsubstituted alkoxy (e.g., alkyl
substituted with fluoro,
such as ¨OCHF2), CN, -NH-S02-R', -NO2, -NH2, -NH-C(0)-R', -C(0)-NH-R', -S02-R'
and
¨SO2NR'R';
each occurrence of R' is independently selected from H, hydroxy, halogen,
carboxyl,
cyano, nitro, oxo (=0), thio (=S), substituted or unsubstituted alkyl,
substituted or
unsubstituted alkoxy, substituted or unsubstituted alkenyl, substituted or
unsubstituted
alkynyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted
cycloalkenyl,
substituted or unsubstituted cycloalkylalkyl, substituted or unsubstituted
cycloalkenylalkyl,
substituted or unsubstituted heterocyclyl, substituted or unsubstituted
heterocycicyalkyl,
substituted or unsubstituted aryl, substituted or unsubstituted arylalkyl,
substituted or
unsubstituted heteroaryl, and substituted or unsubstituted heteroarylalkyl;
6
Date Recue/Date Received 2020-12-07
R4 is H or substituted or unsubstituted alkyl;
with the proviso that the compound is not selected from
1 (21/)-Is oquinol inone, 3- [ [4-amino-3 -(3-hydroxypheny1)-1H-pyrazolo [3,4-
a' pyrimi din-l-yl] methyl]-8-methyl-2-phenyl;
1 (21/)-Is oquinol inone, 3-[ [4-amino-3 -(3-hydroxypheny1)-1H-pyrazolo [3,4-
cfl pyrimi din-1 -yll methyl] -8-fluoro-2-(2-fluorophenyl);
B enzami de, 3 -[4-amino- 1 - [ [1,2-dihy dro-8-methy1-2-(2-methylpheny1)- 1 -
oxo-3-
is oquinolinyl] methy11-1H-pyrazolo [3,4-d] pyrimi ;
3- [4-amino-1 - [[1,2-dihy dro-8-methy1-2-(2-methyl pheny1)- 1 -oxo-3 -
is oquinolinyl] methy11-1H-pyrazolo[3,4-d] -N-methyl-Benzamide;
1 (21/)-Is oquinol inone, 3- [ [4-amino-3 -[4-(aminomethyl)phenyl] - 1H-py
razol o [3 ,4-
a' pyrimi din-l-yl] methyl] -8-methy1-2-(2-methylphenyl);
1 (2H)-Is oquinol inone, 3- [ [4-amino-3 -(3-hydroxypheny1)-1H-pyrazolo [3,4-
a' pyrimi din-l-yl] methyl] -2-(2-chloropheny1)-8-methyl;
1 (21/)-Is oquinol inone, 3- [ [4-amino-3 -(3-hydroxypheny1)-1H-pyrazolo [3 ,4-
a' pyrimi din-l-yl] methyl] -2-(2-ethylpheny1)-8-methyl ;
1 (2H)-Is o quinolinone, 3- [ [4-amino-3 -(3-hydroxypheny1)-1H-pyrazolo [3,4-
d] pyrimi din-1 -yl] methyl] -8-methy1-2-[2-(1-methylethyl)phenyll;
1 (21/)-Is oquinol inone, 3- [ [4-amino-3 -(3-hydroxypheny1)-1H-pyrazolo [3 ,4-
a' pyrimi din-l-yl] methyl] -8-fluoro-2-(2-methylphenyl);
1 (2H)-Is o quinolinone, 3- [ [4-amino-3 -(4-fluoro-3-hy droxypheny1)- 1H-py
razol o [3,4-
d] pyrimi din-l-yl] methyl] -8-fluoro-2-(2-methylphenyl);
1 (21/)-Is oquinol inone, 3- [ [4-amino-3 -(4-fluoro-3 -hy droxypheny1)-1H-py
razol o [3,4-
a' pyrimi din-l-yl] methyl] -2-(2-methylphenyl);
1 (21/)-Is oquinol inone, 3- [ [4-amino-3 -(4-chloro-3 -hy droxy pheny1)- 1H-
py razol o [3 ,4-
a' pyrimi din-l-yl] methyl] -2-(2-methylphenyl);
7
Date Recue/Date Received 2020-12-07
1 (21/)-Is oquinol inone, 3- [[4-amino-3 -(3 -hy droxy pheny1)- 1H-py razol o
113,4-
d pyrimi din-I -yl] methyl] -2 -(2-methylphenyl);
1 (21/)-Is oquinol inone, 3- [[4-amino-3 -(4-chloropheny1)-1H-pyrazolo [3,4-d]
py rimi din-
1 -yll methyl] -8-methy1-2-(2-methylphenyl);
1 (21/)-Is oquinol inone, 3- [[4-amino-3 -(3,4-dimethoxypheny1)-1H-pyrazolo
[3,4-
d pyrimi din-I -yl] methyl] -2 -(2-fluoropheny1)-8 -methyl;
1 (21/)-Is oquinol inone, 3- [[4-amino-3 -(4-hydroxypheny1)-1H-pyrazolo [3,4-
d pyrimi din-I -yl] methyl] -2 -(2-fluoropheny1)-8 -methyl;
1 (21/)-Is oquinol inone, 3- [[4-amino-3 -(4-methoxypheny1)-1H-pyrazolo [3,4-
d pyrimi din-I -yl] methyl] -8-methy1-2-(2-methylphenyl);
1 (2H)-Is oquinol inone, 3- [[4-amino-3 -(3,4-dimethoxypheny1)-1H-pyrazolo
[3,4-
d pyrimi din-I -yl] methyl] -8-methy1-2-(2-methylphenyl);
1 (21/)-Is oquinol inone, 3- [[4-amino-3 -(3 -fluoropheny1)- 1H-pyrazol o [3,4-
d] pyrimi din-
1 -yll methyl] -8-methy1-2-(2-methylphenyl);
1 (21/)-Is oquinol inone, 3- [[4-amino-3 -(3 -fluoropheny1)- 1H-pyrazol o [3,4-
d] pyrimi din-
1 -yll methyl] -2-(2-fluoropheny1)-8-methyl;
1 (21/)-Is oquinol inone, 3- [[4-amino-3 -(3 -chl oropheny1)- 1H-py razol o
[3,4-d] py rimi din-
1 -yll methyl] -8-methy1-2-(2-methylphenyl);
1 (21/)-Is oquinol inone, 3- [[4-amino-3 -(3 -chl oropheny1)- 1H-py razol o
[3,4-d] py rimi din-
1 -yll methyl] -2-(2-fluoropheny1)-8-methyl;
1 (21/)-Is oquinol inone, 3- [[4-amino-3 -(4-hydroxypheny1)-1H-pyrazolo [3,4-
d pyrimi din-I -yl] methyl] -8-methy1-2-(2-methylphenyl);
1 (2H)-Is o quinolinone, 3- [[4-amino-3 -(3 -fluoro-4-hy droxypheny1)- 1H-py
razol o [3,4-
d] pyrimi din-I -yl] methyl] -8-methy1-2-(2-methylphenyl);
1 (2H)-Is o quinolinone, 3- [[4-amino-3 -(3 -fluoro-4-hy droxypheny1)- 1H-py
razol o [3,4-
d] pyrimi din- 1-yl] methyl] -2 -(2-fluoropheny1)-8 -methyl;
8
Date Recue/Date Received 2020-12-07
1 (21/)-Is oquinol inone, 3- [ [4-amino-3 -(4-fluoropheny1)-1H-pyrazolo [3,4-
d] pyrimi din-
1 -yll methyl] -8-methy1-2-(2-methylphenyl);
1 (2H)-Is o quinolinone, 3 - [ [4-amino-3 -(3 -chl oro-4-hy droxypheny1)- 1H-
py razol o [3,4-
d] pyrimi din- 1 -yl] methyl] -2 -(2-fluoropheny1)-8 -methyl;
1 (21/)-Is oquinol inone, 3 - [ [4-amino-3 -(3 -chl oro-4-hy droxy pheny1)- 1H-
py razol o [3 ,4-
d pyrimi din-1 -yl] methyl] -8-methy1-2-(2-methylphenyl);
1 (21/)-Is oquinol inone, 3 - [ [4-amino-3 -(4-chloro-3 -hy droxy pheny1)- 1H-
py razol o [3 ,4-
d pyrimi din-1 -yl] methyl] -2 -(2-fluoropheny1)-8 -methyl;
1 (21/)-Is oquinol inone, 3 - [ [4-amino-3 -(4-chloro-3 -hy droxy pheny1)- 1H-
py razol o [3 ,4-
d pyrimi din-1 -yl] methyl] -8-methy1-2-(2-methylphenyl);
1 (2H)-Is oquinol inone, 3- [ [4-amino-3 -(4-chloro-3 -methoxypheny1)-1H-py
razol o [3 ,4-
d pyrimi din-1 -yl] methyl] -2 -(2-fluoropheny1)-8 -methyl;
1 (21/)-Is oquinol inone, 3 - [ [4-amino-3 -(4-chloro-3 -methoxypheny1)-1H-py
razol o [3 ,4-
d pyrimi din-1 -yl] methyl] -8-methy1-2-(2-methylphenyl);
1 (21/)-Is oquinol inone, 3 - [ [4-amino-3 -(4-fluoro-3 -hy droxypheny1)-1H-py
razol o [3,4-
d pyrimi din-1 -yl] methyl] -8-methy1-2-(2-methylphenyl);
1 (21/)-Is oquinol inone, 3 - [ [4-amino-3 -(3 -hy droxy pheny1)- 1H-py razol
o [3,4-
d pyrimi din-1 -yl] methyl] -2 -(2-fluoropheny1)-8 -methyl;
1 (21/)-Is oquinol inone, 3 - [ [4-amino-3 -(4-fluoro-3 -hy droxypheny1)-1H-py
razol o [3,4-
d pyrimi din-1 -yl] methyl] -2 -(2-fluoropheny1)-8 -methyl;
1 (21/)-Is oquinol inone, 3 - [ [4-amino-3 -(3 -methoxy pheny1)- 1H-py razol o
[3,4-
d pyrimi din-1 -yl] methyl] -8-methy1-2-(2-methylphenyl);
1 (21/)-Is oquinol inone, 3- [ [4-amino-3 -(3 -hy droxy pheny1)- 1H-py razol o
[3,4-
d pyrimi din-1 -yl] methyl] -8-methy1-2-(2-methylphenyl);
B enzenesulfonami de, 3- [4-amino-1 -[ [3,4-dihy dro-5 -methy1-3 -(2-methy
1pheny1)-4-
oxo-2-quinazolinyl] methyl] -1H-pyrazolo [3,4-d] py rimi din-3 -yll ;
9
Date Recue/Date Received 2020-12-07
Benzonitrile, 3-[4-amino-1-[[3,4-dihydro-5-methy1-3-(2-methylpheny1)-4-oxo-2-
quinazolinyllmethyll-1H-pyrazolo[3,4-dlpyrimidin-3-y11;
4(311)-Quinazolinone, 24[4-amino-3-(3-fluoro-4-methoxypheny1)-1H-pyrazolo[3,4-
d] pyrimi din-1 -yl] methyl] -5 -methyl-3 -(2-methylphenyl);
4(311)-Quinazolinone, 24[4-amino-3-(4-fluoro-3-hydroxypheny1)-1H-pyrazolo[3,4-
d] pyrimi din-1 -yl] methyl] -5 -methyl-3 -(2-methylphenyl);
4(311)-Quinazolinone, 24[4-amino-3-(4-chloro-3-hydroxypheny1)-1H-pyrazolo[3,4-
d] pyrimi din-1 -yl] methyl] -5 -methyl-3 -(2-methylphenyl);
4(311)-Quinazolinone, 2-[[4-amino-3-(4-methoxypheny1)-1H-pyrazolo[3,4-
d] pyrimi din-1 -yl] methyl] -5 -methyl-3 -(2-methylphenyl);
4(3H)-Quinazolinone, 24[4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo[3,4-
d] pyrimi din-1 -yl] methyl] -5 -methyl-3 -(2-methylphenyl);
4(311)-Quinazolinone, 2-[[4-amino-344-(phenylmethoxy)pheny11-1H-pyrazolo[3,4-
d] pyrimi din-1 -yl] methyl] -5 -methyl-3 -(2-methylphenyl);
4(311)-Quinazolinone, 24[4-amino-3-(4-phenoxypheny1)-1H-pyrazolo[3,4-
d] pyrimi din-1 -yl] methyl] -5 -methyl-3 -(2-methylphenyl);
4(311)-Quinazolinone, 2-[[4-amino-3-(3,4-dimethoxypheny1)-1H-pyrazolo[3,4-
d] pyrimi din-1 -yl] methyl] -5 -methyl-3 -(2-methylphenyl);
4(311)-Quinazolinone, 2-[[4-amino-3-(3-hydroxypheny1)-1H-pyrazolo[3,4-
d] pyrimi din-1 -yl] methyl] -3 -(2-chl oropheny1)-5 -methyl; and
4(311)-Quinazolinone, 2-[[4-amino-3-(3-hydroxypheny1)-1H-pyrazolo[3,4-
d] pyrimi din-1 -yl] methyl] -5 -methyl-3 -(2-methylpheny1).
[14] In another embodiment, the present invention relates to a compound of
formula (I):
Date Recue/Date Received 2020-12-07
0
(R)n (RI )n
Rb
\ N NIRc
N
Cy
H2N
(I)
or a tautomer thereof, N-oxide thereof, pharmaceutically acceptable ester
thereof, prodrug
thereof, or pharmaceutically acceptable salt thereof, wherein
each occurrence of Rl is independently H, substituted or unsubstituted alkyl,
substituted or unsubstituted alkenyl, substituted or unsubstituted alkynyl or
halogen;
X is CH or N;
each occurrence of R is independently H, substituted or unsubstituted alkyl,
or
halogen;
Ra and Rb are independently selected from H and substituted or unsubstituted
alkyl;
RC is H, substituted or unsubstituted alkyl, -NH2 or halogen;
each occurrence of n is independently selected from 0, 1, 2, 3 and 4; and
Cy is selected from
I X R4 40 R2 711111
N V
R2 R2 N \N
R3 R3 R2 R4 and R4 R2 ;
wherein
11
Date Recue/Date Received 2020-12-07
R2 and R3 are independently selected from H, halogen, hydroxyl, substituted or
unsubstituted alkyl, substituted or unsubstituted alkoxy (e.g., alkyl
substituted with fluoro,
such as ¨OCHF2), CN, -NO2, -
NH2, -NH-C(0)-R', -C(0)-NH-R', -S02-R' and
¨SO2NR'R';
each occurrence of R' is independently selected from H, hydroxy, halogen,
carboxyl,
cyano, nitro, oxo (=0), thio (=S), substituted or unsubstituted alkyl,
substituted or
unsubstituted alkoxy, substituted or unsubstituted alkenyl, substituted or
unsubstituted
alkynyl, substituted or unsubstituted cycloalkyl, substituted or unsubstituted
cycloalkenyl,
substituted or unsubstituted cycloalkylalkyl, substituted or unsubstituted
cycloalkenylalkyl,
substituted or unsubstituted heterocyclyl, substituted or unsubstituted
heterocycicyalkyl,
substituted or unsubstituted aryl, substituted or unsubstituted arylalkyl,
substituted or
unsubstituted heteroaryl, and substituted or unsubstituted heteroarylalkyl;
R4 is H or substituted or unsubstituted alkyl;
with the provisos that
R2
(i) when Ra
and Rb are H, X is CH, Cy is R3 and R3 is H, then R2 is not
-OH, F, Cl, -C(=0) NH2, or -OCH3;
R2
(ii) when Ra
and Rb are H, X is CH, Cy is R3 and R2 is H, then R3 is not
¨CH2-NH2, Cl, OH or -OCH3;
R2
(iii) when Ra and Rb are H, X is CH or N, Cy is R3
and R2 is OH, -O-CH3 or
F, then R3 is not F, Cl, OH or -O-CH3
12
Date Recue/Date Received 2020-12-07
R2
(iv) when Ra and Rb are H, X is N, Cy is R3
and R3 is H, then R2 is not -OH
or -SO2NH2; and
R2
(v) when Ra and Rb are H, X is N, Cy is R3
and R2 is H, then R3 is ¨OCH3, -
0-CH2-Ph, or -0-Ph.
[15] Yet another embodiment is a compound having the formula (I-A) or (I-B):
oTh
(R)n 0
(R1)n sjn
(R1)n
Ra
Rb
Rb
N N
Cy Cy
H2N H2N
(I-A) (I-B)
or a tautomer thereof, N-oxide thereof, pharmaceutically acceptable ester
thereof, prodrug
thereof, or pharmaceutically acceptable salt thereof,
wherein R1, R, n, Re', Rb, RC and Cy are as defined above in relation to the
compound
of formula (I).
[16] Yet another embodiment is a compound having the formula (I-A1), (I-A2),
(I-A3), (I-
A4), (I-A5), (I-B1), (I-B2), (I-B3), (I-B4) or (I-B5):
13
Date Recue/Date Received 2020-12-07
R
R R
0 4
R1 . 0 10 R1 0
N .
R1
N N
NRa Ra
Rb Rb
Rb
N_.--N
N__.--N
I I I
/ Cy y
CY CY
12 Rc Rc
------"N ------N ------N
H2N H2N H2N
(I-A1) (I-B1) (I-A2)
R
R1 0 = 0 .
0
RI
N N RI
N
Ra "" \/s...-Ra
Ra
Rb Rb
)
Rb
N----
N
....õ1õ..-N I /
/
Cy / )---Re CY
)------Re Cy
7--- RC
-----N ------N -----N
H2N H2N H2N
(I-B2) (I-A3) (I-B3)
R1 o
0
R1 0 =
N
N
Ra
Ns..-Ra
Rb
Rb N 'N
Cy Cy
Re
Re
-----N1 N
H2N H2N
14
Date Recue/Date Received 2020-12-07
(I¨A4) (I¨B4)
0
0
Ra
Rb
Rb
N_--N
/
/
CY CY
Rc Rc
H2N H2N
(I¨A5) (I¨B5)
or a tautomer thereof, N-oxide thereof, pharmaceutically acceptable ester
thereof, prodrug
thereof, or pharmaceutically acceptable salt thereof,
wherein R1, R, Re', Rb, RC and Cy are as defined above in relation to the
compound of
formula (I).
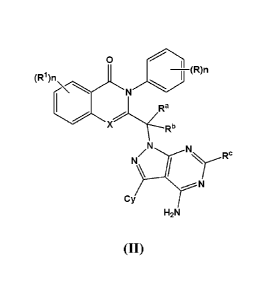
[17] Yet another embodiment is a compound having the formula (II):
I (R)n (RI )n
X Rb
N NIRc
N
Cy
H2N
(II)
or a tautomer thereof, N-oxide thereof, pharmaceutically acceptable ester
thereof, prodrug
thereof, or pharmaceutically acceptable salt thereof,
wherein R1, X, R, n, RC and Cy are as defined above in relation to the
compound of
formula (I); and
Ra and Rb are independently selected from H and substituted or unsubstituted
alkyl,
Date Recue/Date Received 2020-12-07
R2 4110
provided that when Cy is R3 ; at least one of Ra and Rb is not hydrogen.
[18] Yet another embodiment is a compound having the formula (II-1), (II-2),
(II-3), (II-
4) or (11-5):
___________________________ (R)n 0 ______ (R)n
(R1)n
(121)n
Ra
Ra
N N N N
N N
N N
I-12N I-12N
R2 R2 \ /
/
R3 R3
(II-1) (II-2)
o
(R)n
(R1)n
(R1)n
¨(R)n
N N
N N
N Rc
N
R4
\N H2N
R2 H2N
N
N
1R4
R2
(II-3) (II-4)
16
Date Recue/Date Received 2020-12-07
0
IR)n
(R1)n
N/)
Ra
N NIRa
N
H2N
1111
,N
R4
R2
(II-5)
or a tautomer thereof, N-oxide thereof, pharmaceutically acceptable ester
thereof, prodrug
thereof, or pharmaceutically acceptable salt thereof, wherein
Ra is substituted or unsubstituted alkyl; and
R1, X, R, n, Re, R2, R3 and R4 are as defined above in relation to the
compound
formula (I).
[19] Yet another embodiment is a compound having the formula (III):
(R1)fl IR)n
XRa
N NIRa
N
CY
H2N
or a tautomer thereof, N-oxide thereof, pharmaceutically acceptable ester
thereof, prodrug
thereof, or pharmaceutically acceptable salt thereof, wherein
Ra is substituted or unsubstituted alkyl; and
17
Date Recue/Date Received 2020-12-07
Rl, R, X, n, RC and Cy are as defined above in relation to the compound of
formula (I)
[20] Yet another embodiment is a compound having the formula (III-A) and (III-
B):
o (R)n __ R1 ____ 0 (R)n
R1
N N
(Ra )(Ra
X X
N N N N
N \ / )Rc N \ / )Rc
CY CY
H2N H2N
(III-A) (III-B)
or a tautomer thereof, N-oxide thereof, pharmaceutically acceptable ester
thereof, prodrug
thereof, or pharmaceutically acceptable salt thereof, wherein
Ra is substituted or unsubstituted alkyl;
Cy is selected from
F 0
o I
0O2
0 NHSMe
...õ_.....- HN //
N
S=0
I 0
0 FN 1
N 1
,--N
F
02N $1 H 2 N N \/ H N //C) HN // .. HN
//C) .. 0 F N
S=0 S=0 S=0
0 0
I I I
F ,
,
F 401 N/ \
HN, HNN N
,-NN
OH, -N , N N and I ;and
Rl, R, Re, X and Cy are as defined above in relation to the compound of
formula (I)
18
Date Recue/Date Received 2020-12-07
[21] A preferred embodiment is a compound having the formula (I), (I-A), (I-
A1), (I-A2),
(I-A3), (I-A4), (I-A5), (I-B), (I-B1), (I-B2), (I-B3), (I-B4), (I-B5), (II),
(II-1), (II-2), (II-3),
(II-4), (II-5) , (III), (III-A) or (III-B), wherein Cy is selected from
F 0
0
I 0 NHSO2Me
C) C) C) HN //
S=0
I 0
* ,/L
I * -
1 \
1 N
02N H2N F . N 1N H N , //C) HN //C1 HIµJ '/1
F 0 F
S-
F
-...õ.....--
S=0 -=0 S=0
I
0 0 I I
-=, -, F ,
,
0 N/ \
\
HN\ HNN ___.--N\ N
OH N N N or I
[22] Another preferred embodiment is a compound having the formula (I), (I-A),
(I-A1),
(I-A2), (I-A3), (I-A4), (I-A5), (I-B), (I-B1), (I-B2), (I-B3), (I-B4), (I-B5),
(II), (II-1), (II-2),
(II-3), (II-4), (II-5), (III), (III-A) and (III-B), wherein RC is H, F or NH2.
[23] Another preferred embodiment is a compound having the formula (I), (I-A),
(I-A1),
(I-A2), (I-A3), (I-A4), (I-A5), (I-B), (I-B1), (I-B2), (I-B3), (I-B4), (I-B5),
(II-1), (II-2), (II-
3), (II-4), (II-5), (III), (III-A) and (III-B)wherein Ra is H and Rb is H,
methyl or ethyl.
[24] Another preferred embodiment is a compound having the formula (I), (I-A),
(I-A1),
(I-A2), (I-A3), (I-A4), (I-A5), (I-B), (I-B1), (I-B2), (I-B3), (I-B4), (I-B5),
(II), (II-1), (II-2),
(II-3), (II-4), (II-5), (III), (III-A) and (HI-B) wherein Ra is H and Rh is
methyl.
[25] Another preferred embodiment is a compound having the formula (I), (I-
A), (I-A1),
(I-A2), (I-A5), (I-B), (I-B1), (I-B2), (I-B5), (II), (H-1), (II-2), (II-3),
(II-4), (II-5), (III),
(III-A) and (III-B), wherein R is H or halogen, preferably F.
19
Date Recue/Date Received 2020-12-07
[26] Another preferred embodiment is a compound having the formula (I), (I-A),
(I-A1),
(I-A2), (I-A3), (I-A4), (I-B), (I-B1), (I-B2), (I-B3), (I-B5), (II), (II-1),
(II-2), (II-3), (II-4),
(II-5), (III), (III-A) and (III-B) , wherein RI- is H or halogen, preferably
F.
[27] Another preferred embodiment is a compound having the formula (1), (1-A),
(1-B),
(II), (II-1), (II-2), (II-3), (II-4), (II-5), (III), (III-A) and (III-B)
wherein n is 0 or 1.
[28] Yet another embodiment is a compound having the formula (I), (II), (II-
1), (II-2), (II-
3), (II-4), (II-5), (III), (III-A) and (III-B) wherein X is CH.
[29] Yet another embodiment is a compound having the formula (I), (II), (II-
1), (II-2), (II-
3), (II-4), (II-5), (III), (III-A) and (III-B) wherein X is N.
[30] Compounds of the present invention include those recited below (and in
Table 1), and
pharmaceutically acceptable salts thereof. The present invention should not be
construed to be
limited to these specific compounds.
1. 3-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-dlpyrimi
din-1-
ypethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one;
2. 3-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-
dlpyrimidin-1-
ypethyl)-8-fluoro-2-phenylisoquinolin-1(2H)-one;
3. 2-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-
dlpyrimidin-1-
ypethyl)-5-fluoro-3-phenylquinazolin-4(3H)-one;
4. 2-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-
dlpyrimidin-1-
ypethyl)-6-fluoro-3-phenylquinazolin-4(3H)-one;
5. 3-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-
dlpyrimidin-1-
ypethyl)-2-phenylisoquinolin-1(2H)-one;
5a. (+)-3-(1 -(4-amino-3 -(3 -fluoro-4-i soprop oxy pheny1)-1H-py razol o
[3,4-di py rimi din-1-
yl)ethyl)-2-pheny s o quinolin-1(2H)-one;
5b. (-)-3-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo [3,4-di
pyrimidin-1 -
yl)ethyl)-2-phenylisoquinolin-1(2H)-one;
Date Recue/Date Received 2020-12-07
6. 2-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-
dlpyrimidin-
1-ypethyl)-3-phenylquinazolin-4(3H)-one;
6a. (+)-2-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-
d]pyrimidin-
1-ypethyl)-3-phenylquinazolin-4(3H)-one;
6b. (-)-2-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-
d]pyrimidin-
1-ypethyl)-3-phenylquinazolin-4(3H)-one;
7. 3-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo[3,4-
d]pyrimidin-1-
y1)ethyl)-2-phenylisoquinolin-1(2H)-one;
7a. (+)-3-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo[3,4-dlpyrimidin-
1-
y1) ethyl)-2-phenylisoquinolin-1(2H)-one;
7b. (-)-3-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo[3,4-dlpyrimidin-
1-
y1) ethyl)-2-phenylisoquinolin-1(2H)-one;
8. 2-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo[3,4-
d]pyrimidin-1-
ypethyl)-3-phenylquinazolin-4(3H)-one;
9. N-(5-(4-amino-1-(1-(1-oxo-2-pheny1-1,2-dihydroisoquinolin-3-
yl)ethyl)-1H-
pyrazolo[3,4-d]pyrimidin-3-y1)-2-methoxyphenyOmethanesulfonamide
9a. (+)-N-(5-(4-amino-1-(1-(1-oxo-2-pheny1-1,2-dihydroisoquinolin-3-ypethyl)-
1H-
pyrazolo[3,4-d]pyrimidin-3-y1)-2-methoxyphenyl)methanesulfonamide;
9b. (-)-N-(5-(4-amino-1-(1-(1-oxo-2-pheny1-1,2-dihydroisoquinolin-3-
ypethyl)-1H-
pyrazolo[3,4-d]pyrimidin-3-y1)-2-methoxyphenyl)methanesulfonamide;
10. 2-(1-(4-amino-3-(4-methoxy-3-nitropheny1)-1H-pyrazolo[3,4-
dlpyrimidin-1-
ypethyl)-3-phenylquinazolin-4(3H)-one;
11. 3-(1-(4-amino-3-(3-amino-4-methoxypheny1)-1H-pyrazolo[3,4-
dlpyrimidin-1-
ypethyl)-2-phenylisoquinolin-1(2H)-one;
12. N-(5-(4-amino-1-(1-(4-oxo-3-pheny1-3,4-dihydroquinazolin-2-ypethyl)-
1H-
pyrazolo[3,4-d]pyrimidin-3-y1)-2-methoxyphenyl)methanesulfonamide;
21
Date Recue/Date Received 2020-12-07
13. 2-(1 -(4-amino-3 -(3 -fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d]
pyrimi din- 1 -
yl) ethyl)-6-fluoro-3-phenylquinazolin-4(3H)-one;
14. 3 -(1 -(4-amino-3-(3 -fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d]
pyrimi din- 1 -
yl) ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one;
14a. (+)-3 -( 1 -(4-amino-3 -(3 -fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d]
py ri mi din-
1 -y1) ethyl)-8-chl oro-2-phenyl is o quinolin-1 (2H)-one;
1 4b . (-)-3-( 1-(4-amino-3 -(3 -fluoro-4-hy droxypheny1)-1H-pyrazolo [3,4-d]
py rimi din- 1 -
yl) ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one;
15. 3 -(1 -(4-amino-3-(3 -fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d]
pyrimi din- 1 -
yl)ethyl)- 8-fluoro-2-pheny s o quinolin- 1(2H)-one;
1 5a. (+)-3 -( 1 -(4-amino-3 -(3 -fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d]
py ri mi din-
1 -ypethyl)-8-fluoro-2-phenylisoquinolin-1 (2H)-one;
1 5 b. (-)-3-( 1-(4-amino-3 -(3 -fluoro-4-hy droxy pheny1)- 1H-pyrazol o [3,4-
d] py rimi din- 1 -
yl)ethyl)- 8-fluoro-2-pheny s o quinolin- 1(2H)-one;
16. 2-(1 -(4-amino-3 -(3 -fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d]
pyrimi din- 1 -
y Dethyl)-5 -fluoro-3 -phenylquinazolin-4(3H)-one;
16a. (+)-2-( 1 -(4-amino-3 -(3 -fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d] py
ri mi din-
1-y1) ethyl)-5-fluoro-3-phenylquinazolin-4(3H)-one;
16b. (-)-2-( 1-(4-amino-3 -(3 -fluoro-4-hy droxypheny1)-1H-pyrazolo [3,4-d] py
rimi din- 1 -
yl) ethyl)-5-fluoro-3-phenylquinazolin-4(3H)-one;
17. 3 -(1 -(4-amino-3 -(4-(difluoromethoxy)-3 -fluoropheny1)-1H-pyrazolo
[3,4-
d] pyrimi din- 1 -ypethyl)-8 -chloro-2-phenylisoquinolin-1 (2H)-one;
17a. (+)-3-(1 -(4-amino-3 -(4-(difluoromethoxy)-3 -fluoropheny1)- 1H-py razol
o [3,4-d]
pyrimi din- 1 -yl)ethyl)-8-chl oro-2-pheny s o quinolin- 1(2H)-one;
17b. (-)-3-(1 -(4-amino-3-(4-(difluoromethoxy)-3 -fluoropheny1)- 1H-pyrazol
o [3,4-d]
pyrimi din- 1 -yl)ethyl)-8-chl oro-2-pheny s o quinolin- 1(2H)-one;
22
Date Recue/Date Received 2020-12-07
18. 3-(1-(4-amino-3-(4-methoxy-3-nitropheny1)-1H-pyrazolo[3,4-dlpyrimidin-1-
ypethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one;
19. 3-(1-(4-amino-3-(3-amino-4-methoxypheny1)-1H-pyrazolo[3,4-d]pyrimidin-1-
ypethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one;
20. N-(5-(4-amino-1-(1-(8-chloro-l-oxo-2-pheny1-1,2-dihydroisoquinolin-3-
yl)ethyl)-1H-pyrazolo[3,4-d]pyrimidin-3-y1)-2-
methoxyphenyl)methanesulfonamide;
20a. (+)-N-(5-(4-amino-1-(1-(8-chloro-l-oxo-2-pheny1-1,2-dihydroisoquinolin-3-
y1)
ethyl)-1H-pyrazolo[3,4-d]pyrimidin-3-y1)-2-methoxyphenyl)methanesulfonamide;
20b. (-)-N-(5-(4-amino-1-(1-(8-chloro-1-oxo-2-pheny1-1,2-dihydroisoquinolin-
3-y1)
ethyl)-1H-pyrazolo[3,4-d]pyrimidin-3-y1)-2-methoxyphenyl)methanesulfonamide;
21. 3-(1-(4-amino-3-(3-methy1-1H-indazol-6-y1)-1H-pyrazolop,4-dlpyrimidin-1-
y1) ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one;
21a. (+)-3-(1-(4-amino-3-(3-methy1-1H-indazol-6-y1)-1H-pyrazolo[3,4-
d]pyrimidin-1-
y1) ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one;
21b. (-)-3-(1-(4-amino-3-(3-methy1-1H-indazol-6-y1)-1H-pyrazolo[3,4-
d]pyrimidin-1-
y1) ethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one;
22. 2- {144-Amino-3-(3-fluoro-4-isopropoxy-pheny1)-pyrazolo[3,4-d]pyrimidin-
1-
yl] -propy11-3-pheny1-3H-quinazolin-4-one;
and pharmaceutically acceptable salts thereof
TABLE 1
Ex Structure Ex Structure Ex Structure
23
Date Recue/Date Received 2020-12-07
1 ci 0 = 2 F 0 0 3 F 0 0
N N N
/ /
NY
F F F
H2N H2N H2N
O 0 0
4 0 0 5 0 5a o
F 0
N N S N
/
N
'N N
N N N N N
F F H2N H2N F H2N
0 0 0
5b o = 6 0 6a o
0
N N * N
N NH sµ
N N N N N
F F F
H2N H2N H2N
O 0 0
6b o 0 7 0 0 7a o 0
N N N
/
N
N N N N N N
F F F
H2N H2N H2N
O HO HO
)-
24
Date Recue/Date Received 2020-12-07
7b o 0 8 0 N0 9 0
N N
,N N N1 , N / ,
N N- ri N- "
\ / \\
F F
H2N H2N H2N
HO HO 0 NHSO2Me
\
9a EcfIIIto 0 9b o . 10 0 .
N N N
N
N N- N NN - N
\ / \ / \ /
H2N H2N 02N H2N
0 NHSO2Me 0 NHSO2Me 0
\ \ \
11 o 0 12 o ei 13 o
F
N N N
/
N N
'N N 'N N ,N N
N N N
\ / \ / \ /
H2N H2N F H2N
NH2 0 NHSO2Me HO
\ \
14 oi 0 0 14a CI o 0 14b CI o 0
N N ij:iiiiN
----
r
N , ,N N N
N- N N- N
ri
/
¨N ¨N ¨N
F F F
H2N H2N H2N
HO HO HO
15 F 0 . 15a F 0 0 15b F 0 0
N N N
'N N N ,
N'N N N N- "
\ / \ / \ /
¨N ¨N ¨N
F F F
FN H2N H2N
HO HO HO
Date Recue/Date Received 2020-12-07
16 F 0 0 16a F 0 0 16b F 0 0
N rrN
N 'N N
N- = " N N
/ \ / \ / \
F F F
H2N H2N H2N
HO HO HO
17 a o 0 17a CI 0 0 17b CI o .
N N N
N N N
N- N N- N N-/ N
F H2 F F N H2N
H2N
0 0 0
)---F )----F )---F
F F F
18 a 0 0 19 a o 411 20 a 0 0111
N N N
'N N
N- = " N- ----N N
\ / \ / \ /
¨N ¨N ¨N
02N H2N H2N H2N Me02SHN H2N
0 0 0
\ \ \
20a ci o . 20b ci o 0 21 a o .
N N N
/
'N ,N N
'N
N N
\ / N N \ / \ / N
¨N ¨N ¨N
H2
Me02SHN N Me02SHN H2N HN H2N
N ,
0 0
\ \
26
Date Recue/Date Received 2020-12-07
21a o= 21b CiO 22 o
N N N
,N N ,N ,N
N N N N N
/ \ \
HN H2N H N H2N FHN
[31] Illustarative compounds of the present invention also include those
recited below (and
in Table 2), and pharmaceutically acceptable salts thereof Again, the present
invention
should not be construed to be limited to these specific compounds.
i. 2- {144-Amino-3-(3-fluoro-4-isopropoxy-pheny1)-pyrazolo[3,4-
d]pyrimidin-1-
y1]-ethy11-6-fluoro-3-(3-fluoro-pheny1)-3H-quinazolin-4-one;
2- {144-Amino-3-(3-fluoro-4-isopropoxy-pheny1)-pyrazolo[3,4-dlpyrimidin-1-
y1]-ethy11-5-fluoro-3-(3-fluoro-pheny1)-3H-quinazolin-4-one;
2- {144-Amino-3-(3-fluoro-4-isopropoxy-pheny1)-pyrazolo[3,4-dlpyrimidin-1-
yll-ethy11-3-(3-fluoro-pheny1)-3H-quinazolin-4-one;
iv. 2- {144-Amino-3-(3-fluoro-4-hydroxy-pheny1)-pyrazolo[3,4-d]pyrimidin-1-
y1]-ethy11-6-fluoro-3-(3-fluoro-pheny1)-3H-quinazolin-4-one;
v. 2- {144-Amino-3-(3-fluoro-4-hydroxy-pheny1)-pyrazolo[3,4-d]pyrimidin-1-
y1]-ethy11-5-fluoro-3-(3-fluoro-pheny1)-3H-quinazolin-4-one;
vi. 2- { 1 44-Amino-3-(3-fluoro-4-hydroxy-pheny1)-pyrazolo [3,4-d] py
rimidin- 1 -
yl] -ethyl1-3-(3-fluoro-pheny1)-3H-quinazolin-4-one;
vii. 3- {144-Amino-3-(3-fluoro-4-isopropoxy-pheny1)-pyrazolo[3,4-
dlpyrimidin-1-
y1]-ethy11-7-fluoro-2-(3-fluoro-pheny1)-2H-isoquinolin-1-one;
viii. 3- {144-Amino-3-(3-fluoro-4-isopropoxy-pheny1)-pyrazolo[3,4-
dlpyrimidin-1-
y1]-ethy11-8-fluoro-2-(3-fluoro-pheny1)-2H-isoquinolin-1-one;
ix. 3- 1144-Amino-3-(3-fluoro-4-isopropoxy-pheny1)-pyrazolo[3,4-d]pyrimidin-
1-
y1]-ethy11-7-fluoro-2-phenyl-2H-isoquinolin-l-one;
x. 3- {144-Amino-3-(3-fluoro-4-isopropoxy-pheny1)-pyrazolo[3,4-d]pyrimidin-
1-
yll-ethy11-2-(3-fluoro-pheny1)-2H-isoquinolin-1-one;
27
Date Recue/Date Received 2020-12-07
xi. 3-{1-{4-Amino-3-(3-fluoro-4-hydroxy-pheny1)-pyrazolo113,4-d]pyrimidin-1-
y1]-ethy1}-7-fluoro-2-(3-fluoro-pheny1)-2H-isoquinolin-1-one;
xii. 3-{1-[4-Amino-3-(3-fluoro-4-hydroxy-pheny1)-pyrazolo[3,4-d]pyrimidin-1-
y1]-ethy11-8-fluoro-2-(3-fluoro-pheny1)-2H-isoquinolin-1-one;
xiii. 3-{1-[4-Amino-3-(3-fluoro-4-hydroxy-pheny1)-pyrazolo[3,4-d]pyrimidin-
1-
y1]-ethy11-7-fluoro-2-phenyl-2H-isoquinolin-1-one;
xiv. 3-{1-[4-Amino-3-(3-fluoro-4-hydroxy-pheny1)-pyrazolo[3,4-d]pyrimidin-1-
y11-ethyll-2-(3-fluoro-pheny1)-2H-isoquinolin-1-one;
and pharmaceutically acceptable salts thereof
TABLE 2
Ex Structure Ex Structure Ex Structure Ex Structure
i F li F ill F
hi F
0 b F 0 40 0 0 0
0
F F
l
N N e N ILr N
IW ey
NY NY
,N .
,N . ,N N N ,N .
N\ / .'.
\ /
-N N\ / '"
')
F -N F -N
H2N F H2N
H2N H2N
0 0
)---- 0
)----- )----- HO
F F F F
V Vi Vii Viii
F 0 0 0 N 0
F N
0
0 F 0 0
N N
Ny
Ny /
,N ,N
N N ,N
,N m N N N
N " \ / \ /
\ / ¨N ¨N \ /
¨N F ¨N
F H2
F H2N N F NN
H2N 0
HO
HO --- 0
-----
28
Date Recue/Date Received 2020-12-07
ix 0 0 X F Xi F Xii F
F
N 0 a
F 0 a F 0 0
,N
N
N\ / ,N .
N .
/ ,N N N
-N N N \ /
F \ / \ /
-N
H2N -N -N
F
F
H2N
0 F H2N H2N
)----- 0 HO Ho
)-----
Xiii
FNal o '''LlIir XlV F
0 0
N
N .
,N .
\ / N .
F H2 \ /
N
-N
HO F H2N
HO
[32] Yet another embodiment is a compound having the formula (al), (a2), (c),
(c2) or (d):
(R)n (R)n. ^
(R)n , 0 41 0
0 , I
I (R1 ) (R1)n
N
(R1)131 \N
1 Ra I Ra
Rb Rb R-
OH CI 0Pg
(al) (a2) (c)
(R)n (R)n ^
0 Ai 0
(R1 )n, , I (R1) , I
SN N
I Ra 1 Ra
N N
Rb Rb
Lg OH
(c2) (d)
wherein
Ra and Rb are independently selected from H and substituted or unsubstituted
alkyl,
provided that at least one of Ra and Rb is not H;
29
Date Recue/Date Received 2020-12-07
Pg is protecting group;
Lg is leaving group; and
Rl, R, and n are are as defined above in relation to the compound of formula
(I).
[33] In another aspect, the present invention relates to method of preparing a
compound of
formula (I), (I-A), (I-A1), (I-A2), (I-A3), (I-A4), (I-A5), (I-B), (I-B1), (I-
B2), (I-B3), (I-B4),
(1-B5), (11), (11-1), (11-2), (11-3), (11-4), (11-5), (111), (111-A) and (11I-
B), comprising
converting a compound of formula (al), (a2), (c), (c2) or (d) to a compound of
formula (I),
(I-A), (I-A1), (I-A2), (I-A3), (I-A4), (I-B),
(I-B 1), (I-B2), (I-B3), (I-B4), (I-B5), (II),
(II-1), (II-2), (II-3), (II-4), (II-5), (III), (III-A) and (III-B), for
example, as described in
schemes 1 to 4 below.
[34] Yet another embodiment of the present invention is a method for
inhibiting PI3K
(such as PI3K 6 and/or y) in a patient comprising administering to the patient
an effective
amount of at least one compound of the present invention, for example, a
compound of
formula (I), (I-A), (I-A1), (I-A2), (I-A3), (I-A4), (I-A5), (I-B), (I-B1), (I-
B2), (I-B3), (I-B4),
(I-B5), (II), (II-1), (II-2), (II-3), (II-4), (II-5), (III), (III-A) and (III-
B), as defined above.
[35] Yet another embodiment of the present invention is a method for treating
an
inflammatory, autoimmune or proliferative disease via modulation of a protein
kinase (such
as Pi3K 6 and/or y kinase) comprising administering to a patient in need of
such treatment an
effective amount of at least one compound of the present invention. In one
embodiment, the
compound of the present invention inhibits the PI3K 6 protein kinase. In
another
embodiment, the compound of the present invention inhibits both the PI3K 6 and
y protein
kinas es .
[36] Yet another embodiment of the present invention is a method for treating
an
inflammatory, autoimmune or proliferative disease via modulation of a protein
kinase (such
as PI3K 6 and/or y kinase) comprising administering to a patient in need of
such treatment an
effective amount of at least one compound of the present invention, in
combination
(simultaneously or sequentially) with at least one other anti-inflammotory,
immunomodulator
or anti-cancer agent. In one embodiment, the compound of the present invention
inhibits the
PI3K 6 protein kinase. In another embodiment, the compound of the present
invention
inhibits both the PI3K 6 and y protein kinase.
Date Recue/Date Received 2020-12-07
[37] More particularly, the compounds of formula (I), (I-A), (I-A1), (I-A2),
(I-A3), (I-
A4), (I-A5), (I-B), (I-B1), (I-B2), (I-B3), (I-B4), (I-B5), (II), (II-1), (II-
2), (II-3), (II-4), (II-
5), (III), (III-A) and (III-B), and pharmaceutically acceptable esters or
salts thereof, can be
administered for the treatment, prevention and/or amelioration of PI3K and
related protein
kinase mediated diseases or disorders, in particular the amelioration of
diseases or disorders
mediated through PI3K 6 and/or y, including, but not limited to, inflammaotory
diseases or
disorders, autoimmune diseases or disorders, cancer, and other proliferative
diseases or
disorders.
[38] The compounds of the present invention are useful in the treatment of a
variety of
cancers, including, but not limited to, the following:
= carcinoma, including that of the bladder, breast, colon, kidney, liver,
lung, including
small cell lung cancer, esophagus, gall bladder, ovary, pancreas, stomach,
cervix, thyroid,
prostate, and skin, including squamous cell carcinoma;
= hematopoietic tumors of lymphoid lineage, including leukemia, acute
lymphocytic
leukemia, acute lymphoblastic leukemia, B-cell lymphoma, T-cell lymphoma,
Hodgkin's
lymphoma, non-Hodgkins lymphoma, hairy cell lymphoma and Burkett's lymphoma;
= hematopoietic tumors of myeloid lineage, including acute and chronic
myelogenous
leukemias, myelodysplastic syndrome and promyelocytic leukemia;
= tumors of mesenchymal origin, including fibrosarcoma and
rhabdomyosarcoma;
= tumors of the central and peripheral nervous system, including
astrocytoma,
neuroblastoma, glioma and schwannomas; and
= other tumors, including melanoma, seminoma, teratocarcinoma,
osteosarcoma,
xenoderoma pigmentosum, keratoctanthoma, thyroid follicular cancer and
Kaposi's sarcoma.
[39] Due to the key role of protein kinases in the regulation of cellular
proliferation in
general, the protein kinase inhibitors of the present invention may act as
reversible cytostatic
agents, which may be useful in the treatment of any disease process which
features abnormal
cellular proliferation, e.g., benign prostatic hyperplasia, familial
adenomatosis polyposis,
neuro-fibromatosis, atherosclerosis, pulmonary
fibrosis, arthritis, psoriasis,
glomerulonephritis, restenosis following angioplasty or vascular surgery,
hypertrophic scar
formation, inflammatory bowel disease, transplantation rejection, endotoxic
shock, and fungal
infections.
31
Date Recue/Date Received 2020-12-07
[40] The compounds of the present invention as modulators of apoptosis are
useful in the
treatment of cancer (including, but not limited to, those types mentioned
above), viral
infections (including, but not limited, to herpevirus, poxvirus, Epstein-Barr
virus, Sindbis
virus and adenovirus), prevention of AIDS development in HIV-infected
individuals,
autoimmune diseases (including, but not limited, to systemic lupus,
erythematosus,
autoimmune mediated glomerulonephritis, rheumatoid arthritis, psoriasis,
inflammatory
bowel disease, and autoimmune diabetes mellitus), neurodegenerative disorders
(including
but not limited to Alzheimer's disease, AIDS-related dementia, Parkinson's
disease,
amyotrophic lateral sclerosis, retinitis pigmentosa, spinal muscular atrophy
and cerebellar
degeneration), myelodysplastic syndromes, aplastic anemia, ischemic injury
associated with
myocardial infarctions, stroke and reperfusion injury, arrhythmia,
atherosclerosis, toxin-
induced or alcohol related liver diseases, hematological diseases (including
but not limited to
chronic anemia and aplastic anemia), degenerative diseases of the
musculoskeletal system
(including but not limited to osteoporosis and arthritis) aspirin-sensitive
rhinosinusitis, cystic
fibrosis, multiple sclerosis, kidney diseases and cancer pain.
[41] The compounds of present invention can modulate the level of cellular RNA
and
DNA synthesis. These agents are therefore useful in the treatment of viral
infections
(including, but not limited to, HIV, human papilloma virus, herpesvirus,
poxvirus, Epstein-
Barr virus, Sindbis virus and adenovirus).
[42] The compounds of the present invention are useful in the chemoprevention
of cancer.
Chemoprevention is defined as inhibiting the development of invasive cancer by
either
blocking the initiating mutagenic event or by blocking the progression of pre-
malignant cells
that have already suffered an insult or inhibiting tumor relapse. The
compounds described
herein are also useful in inhibiting tumor angiogenesis and metastasis. One
embodiment of
the invention is a method of inhibiting tumor angiogenesis or metastasis in a
patient in need
thereof comprising administering an effective amount of one or more compounds
of the
present invention.
[43] Another embodiment of the present invention is a method of treating an
immune
system-related disease (e.g., an autoimmune disease), a disease or disorder
involving
inflammation (e.g., asthma, chronic obstructive pulmonary disease, rheumatoid
arthritis,
inflammatory bowel disease, glomerulonephritis, neuroinflammatory diseases,
multiple
sclerosis, uveitis and disorders of the immune system), cancer or other
proliferative disease, a
32
Date Recue/Date Received 2020-12-07
hepatic disease or disorder, a renal disease or disorder. The method comprises
administering
an effective amount of one or more compounds of the present invention.
[44] Examples of immune disorders include, but are not limited to, psoriasis,
rheumatoid
arthritis, vasculitis, inflammatory bowel disease, dermatitis, osteoarthritis,
asthma,
inflammatory muscle disease, allergic rhinitis, vaginitis, interstitial
cystitis, scleroderma,
osteoporosis, eczema, allogeneic or xenogeneic transplantation (organ, bone
marrow, stem
cells and other cells and tissues) graft rejection, graft-versus-host disease,
lupus
erythematosus, inflammatory disease, type I diabetes, pulmonary fibrosis,
dermatomyositis,
Sjogren's syndrome, thyroiditis (e.g., Hashimoto's and autoimmune
thyroiditis), myasthenia
gravis, autoimmune hemolytic anemia, multiple sclerosis, cystic fibrosis,
chronic relapsing
hepatitis, primary biliary cirrhosis, allergic conjunctivitis and atopic
dermatitis.
[45] In one embodiment, the compounds described herein are used as
immunosuppresants
to prevent transplant graft rejections, allogeneic or xenogeneic
transplantation rejection
(organ, bone marrow, stem cells, other cells and tissues), and graft - versus -
host disease. In
other embodiments, transplant graft rejections result from tissue or organ
transplants. In
further embodiments, graft-versus-host disease results from bone marrow or
stem cell
transplantation. One embodiment is a method of preventing or decreasing the
risk of
transplant graft rejection, allogeneic or xenogeneic transplantation rejection
(organ, bone
marrow, stem cells, other cells and tissues), or graft - versus - host disease
comprising
administering an effective amount of one or more compounds of the present
invention.
[46] The compounds of the present invention may also be administered in
combination
(simultaneously or sequentially) with known anti-cancer treatments, such as
radiation therapy
or with cytostatic or cytotoxic anticancer agents, such as, for example, DNA
interactive
agents, such as cisplatin or doxorubicin; topoisomerase II inhibitors, such as
etoposide;
topoisomerase I inhibitors such as CPT-11 or topotecan; tubulin interacting
agents, such as
paclitaxel, docetaxel or the epothilones (for example ixabepilone), either
naturally occurring
or synthetic; hormonal agents, such as tamoxifen; thymidilate synthase
inhibitors, such as 5-
fluorouracil; and anti-metabolites, such as methotrexate, other tyrosine
kinase inhibitors such
as Iressa and OSI-774; angiogenesis inhibitors; EGF inhibitors; VEGF
inhibitors; CDK
inhibitors; SRC inhibitors; c-Kit inhibitors; Her1/2 inhibitors and monoclonal
antibodies
directed against growth factor receptors such as erbittix (EGF) and herceptin
(Her2), other
protein kinase modulators, and combinations thereof.
33
Date Recue/Date Received 2020-12-07
[47] The compounds of the present invention may also be administered in
combination
(simultaneously or sequentially) with one or more steroidal anti-inflammatory
drugs, non-
steroidal anti-inflammatory drugs (NSAIDs) or immune selective anti-
inflammatory
serivatives (ImSAIDs), and combinations thereof
[48] In a further aspect, the present invention relates to a pharmaceutical
composition
comprising one or more compounds of the present invention (such as a compound
of formula
(I), (I-A), (I-A1), (I-A2), (I-A3), (I-A4), (I-A5), (I-B), (I-B1), (I-B2), (I-
B3), (I-B4), (I-B5),
(II), (II-1), (II-2), (II-3), (II-4), (II-5), (III), (III-A) or (III-B) and a
pharmaceutically
acceptable carrier. The pharmaceutical composition may further comprise one or
more of the
active ingredients identified above, such as, e.g., additional steroidal anti-
inflammatory drugs,
non-steroidal anti-inflammatory drugs (NSAIDs) or immune selective anti-
inflammatory
derivatives (ImSAIDs), anti-cancer agents, and combinations thereof
[49] In one
embodiment, the pharmaceutical composition comprises a therapeutically
effective amount of one or more compounds of formula (I), (I-A), (I-A1), (I-
A2), (I-A3), (I-
A4), (I-A5), (I-B), (I-B1), (I-B2), (I-B3), (I-B4), (I-B5), (II), (II-1), (II-
2), (II-3), (II-4) ,
(II-5) , (III), (III-A) or (III-B).
[50] Yet another embodiment is a method of treating leukemia in a patient in
need thereof
comprising administering a therapeutically effective amount of a compound of
the present
invention. For example, the compounds of the present invention is effective
for treating
autoimmune disorders such as asthma, COPD, rhematoid arthritis, psorias, lupus
and
experimental autoimmune encephalomyelitis (EAE).
[51] Yet another embodiment is a method of treating allergic rhinitis in a
patient in need
thereof comprising administering a therapeutically effective amount of a
compound of the
present invention.
[52] Yet another embodiment is a method of treating leukemia in a patient in
need thereof
comprising administering a therapeutically effective amount of a compound of
the present
invention. For example, the compounds of the present invention are effective
for treating
chronic lymphocytic leukemia (CLL), non-Hodgkin lymphoma (NHL), acute myeloid
leukemia (AML), multiple myeloma (MM), small lymphocytic lymphoma (SLL), and
indolent non-Hodgkin's lymphoma (I-NHL).
34
Date Recue/Date Received 2020-12-07
DETAILED DESCRIPTION OF THE INVENTION
[53] As used herein the following definitions shall apply unless otherwise
indicated.
Further many of the groups defined herein can be optionally substituted. The
listing of
substituents in the definition is exemplary and is not to be construed to
limit the substituents
defined elsewhere in the specification.
[54] The term "alkyl", unless otherwise specified, refers to a straight or
branched
hydrocarbon chain radical consisting solely of carbon and hydrogen atoms,
containing no
unsaturation, having from one to eight carbon atoms, and which is attached to
the rest of the
molecule by a single bond, e.g., methyl, ethyl, n-propyl, 1-methylethyl
(isopropyl), n-butyl, n-
pentyl, and 1,1-dimethylethyl (t-butyl). The term "(C1-6)alkyl" refers to an
alkyl group as
defined above having up to 6 carbon atoms.
[55] The term "alkenyl", unless otherwise specified, refers to an aliphatic
hydrocarbon
group containing a carbon-carbon double bond and which may be a straight or
branched or
branched chain having about 2 to about 10 carbon atoms, e.g., ethenyl, 1-
propenyl, 2-
propenyl (allyl), iso-propenyl, 2-methyl-1-propenyl, 1-butenyl, and 2-butenyl.
The term "(C2-
6)alkenyl" refers to an alkenyl group as defined above having up to 6 carbon
atoms.
[56] The term "alkynyl", unless otherwise specified, refers to a straight or
branched chain
hydrocarbyl radical having at least one carbon-carbon triple bond, and having
in the range of
2 to up to 12 carbon atoms (with radicals having in the range of 2 to up to 10
carbon atoms
presently being preferred) e.g., ethynyl, propynyl, and butnyl. The term
"(C2_6) alkynyl"
refers to an alkynyl group as defined above having up to 6 carbon atoms.
[57] The term "alkoxy" unless otherwise specified, denotes an alkyl,
cycloalkyl, or
cycloalkylalkyl group as defined herein attached via an oxygen linkage to the
rest of the
molecule. The term "substituted alkoxy" refers to an alkoxy group where the
alkyl
constituent is substituted (i.e., -0-(substituted alkyl) wherein the term
"substituted alkyl" is
the same as defined above for "alkyl". For example "alkoxy" refers to the
group -0-alkyl,
including from 1 to 8 carbon atoms of a straight, branched, cyclic
configuration and
combinations thereof attached to the parent structure through oxygen. Examples
include
methoxy, ethoxy, propoxy, isopropoxy, cyclopropyloxy, and cyclohexyloxy.
Date Recue/Date Received 2020-12-07
[58] The term "cycloalkyl", unless otherwise specified, denotes a non-aromatic
mono or
multicyclic ring system of about 3 to 12 carbon atoms such as cyclopropyl,
cyclobutyl,
cyclopentyl, and cyclohexyl. Examples of multicyclic cycloalkyl groups
include
perhydronaphthyl, adamantyl and norbomyl groups, bridged cyclic groups, and
sprirobicyclic
groups, e.g., sprio (4,4) non-2-yl. The term "(C3_8) cycloalkyl" refers to a
cycloalkyl group as
defined above having 3 to 8 carbon atoms.
[59] The term "cycloalkylalkyl", unless otherwise specified, refers to a
cyclic ring-
containing radical containing in the range of about 3 up to 8 carbon atoms
directly attached to
an alkyl group which are then attached to the main structure at any carbon
from the alkyl
group that results in the creation of a stable structure such as
cyclopropylmethyl,
cyclobutylethyl, and cyclopentylethyl.
[60] The term "cycloalkenyl", unless otherwise specified, refers to cyclic
ring-containing
radicals containing in the range of about 3 up to 8 carbon atoms with at least
one carbon-
carbon double bond such as cyclopropenyl, cyclobutenyl, and cyclopentenyl. The
term
"cycloalkenylalkyl" refers to a cycloalkenyl group directly attached to an
alkyl group which
are then attached to the main structure at any carbon from the alkyl group
that results in the
creation of a stable structure.
[61] The term "aryl", unless otherwise specified, refers to aromatic radicals
having in the
range of 6 up to 20 carbon atoms such as phenyl, naphthyl, tetrahydronaphthyl,
indanyl, and
biphenyl.
[62] The term "arylalkyl", unless otherwise specified, refers to an aryl group
as defined
above directly bonded to an alkyl group as defined above, e.g., -CH2C6H5 and -
C2H5C6H5.
[63] The term "heterocyclic ring", unless otherwise specified, refers to a non-
aromatic 3 to
15 member ring radical which consists of carbon atoms and at least one
heteroatom selected
from nitrogen, phosphorus, oxygen and sulfur. For purposes of this invention,
the
heterocyclic ring radical may be a mono-, bi-, tri- or tetracyclic ring
system, which may
include fused, bridged or spiro ring systems, and the nitrogen, phosphorus,
carbon, oxygen or
sulfur atoms in the heterocyclic ring radical may be optionally oxidized to
various oxidation
states. In addition, the nitrogen atom may be optionally quatemized. The
heterocyclic ring
radical may be attached to the main structure at any heteroatom or carbon atom
that results in
the creation of a stable structure.
36
Date Recue/Date Received 2020-12-07
[64] The term "heterocyclyl", unless otherwise specified, refers to a
heterocylic ring
radical as defined above. The heterocylcyl ring radical may be attached to the
main structure
at any heteroatom or carbon atom that results in the creation of a stable
structure.
[65] The term Theterocyclylalkyl", unless otherwise specified, refers to a
heterocylic ring
radical as defined above directly bonded to an alkyl group. The
heterocyclylalkyl radical may
be attached to the main structure at carbon atom in the alkyl group that
results in the creation
of a stable structure. Examples of such heterocycloalkyl radicals include, but
are not limited
to, dioxolanyl, thienyl[1,31dithianyl, decahydroisoquinolyl, imidazolinyl,
imidazolidinyl,
isothiazolidinyl, isoxazolidinyl, morpholinyl, octahydroindolyl,
octahydroisoindolyl, 2-
oxopiperazinyl, 2-oxopiperidinyl, 2-oxopyrrolidinyl, oxazolidinyl,
piperidinyl, piperazinyl, 4-
piperidonyl, pyrrolidinyl, pyrazolidinyl, quinuclidinyl, thiazolidinyl,
tetrahydrofuryl,
trithianyl, tetrahydropyranyl, thiomorpholinyl, thiamorpholinyl, 1-oxo-
thiomorpholinyl, and
1, 1 -di oxo-thi omorpholinyl.
[66] The term "heteroaryl", unless otherwise specified, refers to an
optionally substituted 5
to 14 member aromatic ring having one or more heteroatoms selected from N, 0,
and S as
ring atoms. The heteroaryl may be a mono-, bi- or tricyclic ring system.
Examples of such
"heterocyclic ring" or "heteroaryl" radicals include, but are not limited to,
oxazolyl, thiazolyl,
imidazolyl, pyrrolyl, furanyl, pyridinyl, pyrimidinyl, pyrazinyl,
benzofuranyl, indolyl,
benzothiazolyl, benzoxazolyl, carbazolyl, quinolyl, isoquinolyl, azetidinyl,
acridinyl,
benzodioxolyl, benzodioxanyl, benzofuranyl, carbazolyl, cinnolinyl,
dioxolanyl, indolizinyl,
naphthyridinyl, perhydroazepinyl, phenazinyl, phenothiazinyl, phenoxazinyl,
phthalazinyl,
pteridinyl, purinyl, quinazolinyl, quinoxalinyl, tetrazoyl,
tetrahydroisoquinolyl, piperidinyl,
piperazinyl, 2-oxopiperazinyl, 2-oxopiperidinyl, 2-oxopyrrolidinyl, 2-
oxoazepinyl, azepinyl,
4-piperidonyl, pyrrolidinyl, pyridazinyl, oxazolinyl, oxazolidinyl, triazolyl,
indanyl,
isoxazolyl, isoxazolidinyl, morpholinyl, thiazolinyl, thiazolidinyl,
isothiazolyl, quinuclidinyl,
isothiazolidinyl, isoindolyl, indolinyl, isoindolinyl, octahydroindolyl,
octahydroisoindolyl,
decahydroisoquinolyl, benzimidazolyl, thiadiazolyl, benzopyranyl,
tetrahydrofuryl,
tetrahydropyranyl, thienyl, benzothienyl, thiamorpholinyl, thiamorpholinyl
sulfoxide,
thiamorpholinyl sulfone, dioxaphospholanyl, oxadiazolyl, chromanyl, and
isochromanyl. The
heteroaryl ring radical may be attached to the main structure at any
heteroatom or carbon
atom that results in the creation of a stable structure. The term "substituted
heteroaryl" also
includes ring systems substituted with one or more oxide (-0-) substituents,
such as pyridinyl
N-oxides.
37
Date Recue/Date Received 2020-12-07
[67] The term "heteroarylalkyl", unless otherwise specified, refers to a
heteroaryl ring
radical as defined above directly bonded to an alkyl group. The
heteroarylalkyl radical may
be attached to the main structure at any carbon atom from alkyl group that
results in the
creation of a stable structure.
[68] The term "cyclic ring" refers to a cyclic ring containing 3 to 10 carbon
atoms.
[69] The term "substituted" unless otherwise specified, refers to substitution
with any one
or any combination of the following substituents which may be the same or
different and are
independently selected from hydrogen, hydroxy, halogen, carboxyl, cyano,
nitro, oxo (=0),
thio (=S), substituted or unsubstituted alkyl, substituted or unsubstituted
alkoxy, substituted
or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or
unsubstituted
aryl, substituted or unsubstituted arylalkyl, substituted or unsubstituted
cycloalkyl, substituted
or unsubstituted cycloalkylalkyl, substituted or unsubstituted cycloalkenyl,
substituted or
unsubstituted cycloalkenylalkyl, substituted or unsubstituted heteroaryl,
substituted or
unsubstituted heteroarylalkyl, substituted or unsubstituted heterocyclic ring,
substituted
heterocyclylalkyl ring, substituted or unsubstituted guanidine, ¨COOW, -C(0)W,
-C(S)W, -
C(0)NIURY, -C(0)0NWRY, -NRYW, -NWCONRYW, -N(Rx)SORY, -N(U)S02RY, -(=N-
N(W)RY), - NIUC(0)ORY, -NWRY, -NWC(0)RY-, -NIUC(S)RY -NIUC(S)NRYW, -SONIURY-,
-S02NWRY-,0Rx 01UC(0)NRYRz, -0WC(0)ORY-, -0C(0)Rx, -0C(0)NWRY, -
R"NRYC(0)W, -WOW, -1UC(0)0RY, -RxC(0)NRYW, -1UC(0)12', -Rx0C(0)RY, SRx, -
SOW% -S021U, and -0NO2, wherein Rx, RY and Rz in each of the above groups can
be
hydrogen, substituted or unsubstituted alkyl, substituted or unsubstituted
alkoxy, substituted
or unsubstituted alkenyl, substituted or unsubstituted alkynyl, substituted or
unsubstituted
aryl, substituted or unsubstituted arylalkyl, substituted or unsubstituted
cycloalkyl, substituted
or unsubstituted cycloalkylalkyl, substituted or unsubstituted cycloalkenyl,
substituted or
unsubstituted amino, substituted or unsubstituted heteroaryl, substituted or
unsubstituted
heteroarylalkyl, substituted or unsubstituted heterocyclic ring, or
substituted heterocyclylalkyl
ring, or any two of IV% RY and Rz may be joined to form a substituted or
unsubstituted
saturated or unsaturated 3-10 membered ring, which may optionally include
heteroatoms
which may be the same or different and are selected from 0, NIU (e.g., Rx can
be hydrogen or
C1_6 alkyl) or S. Substitution or the combinations of substituents envisioned
by this invention
are preferably those that result in the formation of a stable or chemically
feasible compound.
The term stable as used herein refers to the compounds or the structure that
are not
substantially altered when subjected to conditions to allow for their
production, detection and
38
Date Recue/Date Received 2020-12-07
preferably their recovery, purification and incorporation into a
pharmaceutical composition.
The substituents in the aforementioned "substituted" groups cannot be further
substituted. For
example, when the substituent on "substituted alkyl" is "substituted aryl",
the substituent on
"substituted aryl" cannot be "substituted alkenyl".
[70] The term
"halo", "halide", or, alternatively, "halogen" means fluoro, chloro, bromo or
iodo. The terms "haloalkyl," "haloalkenyl," "haloalkynyl" and "haloalkoxy"
include alkyl,
alkenyl, alkynyl and alkoxy structures that are substituted with one or more
halo groups or
with combinations thereof For example, the terms "fluoroalkyl" and
"fluoroalkoxy" include
haloalkyl and haloalkoxy groups, respectively, in which the halo is fluorine.
[71] The term "protecting group" or "PG" refers to a substituent that is
employed to block
or protect a particular functionality. Other functional groups on the compound
may remain
reactive. For example, an "amino-protecting group" is a substituent attached
to an amino
group that blocks or protects the amino functionality in the compound.
Suitable amino-
protecting groups include, but are not limited to, acetyl, trifluoroacetyl,
tert-butoxycarbonyl
(BOC), benzyloxycarbonyl (CBz) and 9-fluorenylmethylenoxycarbonyl (Fmoc).
Similarly, a
"hydroxy-protecting group" refers to a substituent of a hydroxy group that
blocks or protects
the hydroxy functionality. Suitable hydroxy-protecting groups include, but are
not limited to,
acetyl and silyl. A "carboxy-protecting group" refers to a substituent of the
carboxy group
that blocks or protects the carboxy functionality. Suitable carboxy-protecting
groups include,
but are not limited to, -CH2CH2S02Ph, cyanoethyl, 2-(trimethylsilyl)ethyl, 2-
(trimethylsilyl)ethoxymethyl, - 2-(p-toluenesulfonyl)ethyl, 2-(p-
nitrophenylsulfenyl)ethyl, 2-
(diphenylphosphino)-ethyl. and nitroethyl. For a general description of
protecting groups and
their use, see T. W. Greene, Protective Groups in Organic Synthesis, John
Wiley & Sons,
New York, 1991.
[72] Certain of the compounds described herein may contain one or more
asymmetric
centers and can thus give rise to enantiomers, diastereomers, and other
stereoisomeric forms
that can be defined, in terms of absolute stereochemistry, as (R)- or (S)-.
The present
chemical entities, pharmaceutical compositions and methods are meant to
include all such
possible isomers, including racemic mixtures, optically pure forms and
intermediate mixtures.
For the instance the non-limiting example of intermediate mixutures include a
mixture of
isomers in a ratio of 10:90, 13:87, 17:83, 20:80, or 22:78. Optically active
(R)- and (S)-
isomers can be prepared using chiral synthons or chiral reagents, or resolved
using
39
Date Recue/Date Received 2020-12-07
conventional techniques. When the compounds described herein contain olefinic
double
bonds or other centers of geometric asymmetry, and unless specified otherwise,
it is intended
that the compounds include both E and Z geometric isomers.
[73] The term "tautomers" refers to compounds, which are characterized by
relatively easy
interconversion of isomeric forms in equilibrium. These isomers are intended
to be covered
by this invention. "Tautomers" are structurally distinct isomers that
interconvert by
tautomerization. "Tautomerization" is a form of isomerization and includes
prototropic or
proton-shift tautomerization, which is considered a subset of acid-base
chemistry.
"Prototropic tautomerization" or "proton-shift tautomerization" involves the
migration of a
proton accompanied by changes in bond order, often the interchange of a single
bond with an
adjacent double bond. Where tautomerization is possible (e.g. in solution), a
chemical
equilibrium of tautomers can be reached. An example of tautomerization is keto-
enol
tautomerization. A specific example of keto-enol tautomerization is the
interconversion of
pentane-2,4-dione and 4-hydroxypent-3-en-2-one tautomers. Another example of
tautomerization is phenol-keto tautomerization. A specific example of phenol-
keto
tautomerization is the interconversion of pyridin-4-ol and pyridin-4(1H)-one
tautomers.
[74] A "leaving group or atom" is any group or atom that will, under the
reaction
conditions, cleave from the starting material, thus promoting reaction at a
specified site.
Suitable examples of such groups, unless otherwise specified, include halogen
atoms and
mesyloxy, p-nitrobenzensulphonyloxy and tosyloxy groups.
[75] The term "prodrug" refers to a compound, which is an inactive precursor
of a
compound that is converted into its active form in the body by normal
metabolic processes.
Prodrug design is discussed generally in Hardma, et al. (Eds.), Goodman and
Gilman's The
Pharmacological Basis of Therapeutics, 9th ed., pp. 11-16 (1996). A thorough
discussion is
provided in Higuchi, et al., Prodrugs as Novel Delivery Systems, Vol. 14, ASCD
Symposium
Series, and in Roche (ed.), Bioreversible Carriers in Drug Design, American
Pharmaceutical
Association and Pergamon Press (1987). To illustrate, prodrugs can be
converted into a
pharmacologically active form through hydrolysis of, for example, an ester or
amide linkage,
thereby introducing or exposing a functional group on the resultant product.
The prodrugs can
be designed to react with an endogenous compound to form a water-soluble
conjugate that
further enhances the pharmacological properties of the compound, for example,
increased
circulatory half-life. Alternatively, prodrugs can be designed to undergo
covalent
Date Recue/Date Received 2020-12-07
modification on a functional group with, for example, glucuronic acid,
sulfate, glutathione,
amino acids, or acetate. The resulting conjugate can be inactivated and
excreted in the urine,
or rendered more potent than the parent compound. High molecular weight
conjugates also
can be excreted into the bile, subjected to enzymatic cleavage, and released
back into the
circulation, thereby effectively increasing the biological half-life of the
originally
administered compound.
[76] The term "ester" refers to a compound, which is formed by reaction
between an acid
and an alcohol with elimination of water. An ester can be represented by the
general formula
RCOOR' (where R is a drug and R' is a chemical group).
[77] These prodrugs and esters are intended to be covered within the scope of
this
invention.
[78] Additionally the instant invention also includes the compounds which
differ only in
the presence of one or more isotopically enriched atoms for example
replacement of hydrogen
with deuterium or tritium, or the replacement of a carbon by 12C- or 14C-
enriched carbon.
[79] The compounds of the present invention may also contain unnatural
proportions of
atomic isotopes at one or more of atoms that constitute such compounds. For
example, the
compounds may be radiolabeled with radioactive isotopes, such as for example
tritium (3H),
iodine-125 (1251) or carbon-14 (14C). All isotopic variations of the compounds
of the present
invention, whether radioactive or not, are encompassed within the scope of the
present
invention.
[80] Pharmaceutically acceptable salts forming part of this invention include
salts derived
from inorganic bases such as Li, Na, K, Ca, Mg, Fe, Cu, Zn, and Mn; salts of
organic bases
such as N,N'-diacetylethylenediamine, glucamine, triethylamine, choline,
hydroxide,
dicyclohexylamine, metformin, benzylamine, trialkylamine, and thiamine; chiral
bases such
as alkylphenylamine, glycinol, and phenyl glycinol; salts of natural amino
acids such as
glycine, alanine, valine, leucine, isoleucine, norleucine, tyrosine, cystine,
cysteine,
methionine, proline, hydroxy proline, histidine, omithine, lysine, arginine,
and serine;
quaternary ammonium salts of the compounds of invention with alkyl halides,
alkyl sulphates
such as MeI and (Me)2SO4; non-natural amino acids such as D-isomers or
substituted amino
acids; guanidine; and substituted guanidine wherein the substituents are
selected from nitro,
amino, alkyl. alkenyl, alkynyl, ammonium or substituted ammonium salts and
aluminum
salts. Salts may include acid addition salts where appropriate which may be
sulphates,
nitrates, phosphates, perchlorates, borates, hydrohalides, acetates,
tartrates, maleates, citrates,
41
Date Recue/Date Received 2020-12-07
fumarates, succinates, palmoates, methanesulphonates, benzoates, salicylates,
benzenesulfonates, ascorbates, glycerophosphates, and ketoglutarates.
[81] When ranges are used herein for physical properties, such as molecular
weight, or
chemical properties, such as chemical formulae, all combinations and
subcombinations of
ranges and specific embodiments therein are intended to be included. The term
"about" when
referring to a number or a numerical range means that the number or numerical
range referred
to is an approximation within experimental variability (or within statistical
experimental
error), and thus the number or numerical range may vary from, for example,
between 1% and
15% of the stated number or numerical range. The term "comprising" (and
related terms such
as "comprise" or "comprises" or "having" or "including") includes those
embodiments, for
example, an embodiment of any composition of matter, composition, method, or
process, or
the like, that "consist of" or "consist essentially of" the described
features.
[82] The following abbreviations and terms have the indicated meanings
throughout: PI3-
K = Phosphoinositide 3-kinase; PI = phosphatidylinositol; AIDS = Acquired
Immuno
Deficiency Syndrome; HIV = Human Immunodeficiency Virus; Mel = Methyl Iodide;
ND:
Not determined.
[83] Abbreviations used herein have their conventional meaning within the
chemical and
biological arts.
[84] The term "cell proliferation" refers to a phenomenon by which the cell
number has
changed as a result of division. This term also encompasses cell growth by
which the cell
morphology has changed (e.g., increased in size) consistent with a
proliferative signal.
[85] The terms "co-administration," "administered in combination with," and
their
grammatical equivalents, as used herein, encompass administration of two or
more agents to
an animal so that both agents and/or their metabolites are present in the
animal at the same
time. Co-administration includes simultaneous administration in separate
compositions,
administration at different times in separate compositions, or administration
in a composition
in which both agents are present.
[86] The term "effective amount" or "therapeutically effective amount" refers
to that
amount of a compound described herein that is sufficient to effect the
intended application
including but not limited to disease treatment, as defined below. The
therapeutically effective
amount may vary depending upon the intended application (in vitro or in vivo),
or the subject
and disease condition being treated, e.g., the weight and age of the subject,
the severity of the
disease condition, the manner of administration and the like, which can
readily be determined
by one of ordinary skill in the art. The term also applies to a dose that will
induce a particular
42
Date Recue/Date Received 2020-12-07
response in target cells, e.g. reduction of platelet adhesion and/or cell
migration. The specific
dose will vary depending on the particular compounds chosen, the dosing
regimen to be
followed, whether it is administered in combination with other compounds,
timing of
administration, the tissue to which it is administered, and the physical
delivery system in
which it is carried.
[87] As used herein, "treatment," "treating," or "ameliorating" are used
interchangeably.
These terms refers to an approach for obtaining beneficial or desired results
including but, not
limited to, therapeutic benefit and/or a prophylactic benefit. By therapeutic
benefit is meant
eradication or amelioration of the underlying disorder being treated. Also, a
therapeutic
benefit is achieved with the eradication or amelioration of one or more of the
physiological
symptoms associated with the underlying disorder such that an improvement is
observed in
the patient, notwithstanding that the patient may still be afflicted with the
underlying disorder.
For prophylactic benefit, the compositions may be administered to a patient at
risk of
developing a particular disease, or to a patient reporting one or more of the
physiological
symptoms of a disease, even though a diagnosis of this disease may not have
been made.
[88] A "therapeutic effect," as that term is used herein, encompasses a
therapeutic benefit
and/or a prophylactic benefit as described above. A prophylactic effect
includes delaying or
eliminating the appearance of a disease or condition, delaying or eliminating
the onset of
symptoms of a disease or condition, slowing, halting, or reversing the
progression of a disease
or condition, or any combination thereof
[89] The term "subject" or "patient" refers to an animal (e.g., a dog, cat,
horse, or pig),
such as a mammal, for example a human. The methods described herein can be
useful in both
human therapeutics and veterinary applications. In some embodiments, the
patient is a
mammal, and in some embodiments, the patient is human.
[90] "Radiation therapy" means exposing a patient, using routine methods and
compositions known to the practitioner, to radiation emitters such as alpha-
particle emitting
radionuclides (e.g., actinium and thorium radionuclides), low linear energy
transfer (LET)
radiation emitters (i.e. beta emitters), conversion electron emitters (e.g.
strontium-89 and
samarium- 153-EDTMP), or high-energy radiation, including, without limitation,
x-rays,
gamma rays, and neutrons.
[91] "Signal transduction" is a process during which stimulatory or
inhibitory signals are
transmitted into and within a cell to elicit an intracellular response. A
modulator of a signal
transduction pathway refers to a compound which modulates the activity of one
or more
43
Date Recue/Date Received 2020-12-07
cellular proteins mapped to the same specific signal transduction pathway. A
modulator may
augment (agonist) or suppress (antagonist) the activity of a signaling
molecule.
[92] The term "selective inhibition" or "selectively inhibit" as applied to
a biologically
active agent refers to the agent's ability to selectively reduce the target
signaling activity as
compared to off-target signaling activity, via direct or indirect interaction
with the target.
[93] The term "pharmaceutically acceptable carrier" or "pharmaceutically
acceptable
excipient" includes, but is not limited to, any and all solvents, dispersion
media, coatings,
antibacterial and antifungal agents, isotonic and absorption delaying agents,
one or more
suitable diluents, fillers, salts, disintegrants, binders, lubricants,
glidants, wetting agents,
controlled release matrices, colorants/flavoring, carriers, excipients,
buffers, stabilizers,
solubilizers, and combinations thereof Except insofar as any conventional
media or agent is
incompatible with the active ingredient, its use in the therapeutic
compositions of the
invention is contemplated. Supplementary active ingredients can also be
incorporated into the
compositions.
[94] In certain embodiments, one or more of the compounds described herein
bind
specifically to a PI3 kinase or a protein kinase selected from the group
consisting of mTor,
DNA-dependent protein kinase (Pubmed protein accession number (PPAN)
AAA79184), AbI
tyrosine kinase (CAA52387), Bcr-Abl, hemopoietic cell kinase (PPAN CAI19695),
Src
(PPAN CAA24495), vascular endothelial growth factor receptor 2 (PPAN
ABB82619),
epidermal growth factor receptor (PPAN AG43241), EPH receptor B4 (PPAN
EAL23820),
stem cell factor receptor (PPAN AAF22141), Tyrosine-protein kinase receptor
TIE-2 (PPAN
Q02858), fms-related tyrosine kinase 3 (PPAN NP 004110), platelet-derived
growth factor
receptor alpha (PPAN NP 990080), RET (PPAN CAA73131), and any other related
protein
kinases, as well as any functional mutants thereof
[95] In other embodiments, the ICso of a compound described herein for pi 10a,
pi 10(3, pi
by, or pi 106 is less than about 1 1,04, less than about 100 nM, less than
about 50 nM, less
than about 10 nM, less than 1 nM or less than about 0.5nM. In some
embodiments, the ICso of
a compound described herein for mTor is less than about 1 1,04, less than
about 100 nM, less
than about 50 nM, less than about 10 nM, less than 1 nM or less than about
0.5nM. In some
other embodiments, one or more of the compounds described herein exhibit dual
binding
specificity and are capable of inhibiting a PI3 kinase (e.g., a class I PI3
kinase) as well as a
protein kinase (e.g., mTor) with an ICso value less than about 1 less
than about 100 nM,
less than about 50 nM, less than about 10 nM, less than 1 nM or less than
about 0.5 nM.
44
Date Recue/Date Received 2020-12-07
[96] In additional embodiments, the compounds of the present invention exhibit
one or
more functional characteristics disclosed herein. For example, one or more of
the compounds
described herein bind specifically to a PI3 kinase. In some embodiments, the
IC50 of a
compound described herein for pi 10a, pi 10(3, pi by, or pi 106 is less than
about 1 [iM, less
than about 100 nM, less than about 50 nM, less than about 10 nM, less than
about 1 nM, less
than about 0.5nM, less than about 100pM, or less than about 50 pM.
[97] In other embodiments, the compounds of the present invention selectively
inhibit one
or more members of type I or class I phosphatidylinositol 3-kinases (P13-
kinase) with an IC50
value of about 100 nM or less, about 50 nM or less, about 10 nM or less, about
5 nM or less,
about 100 pM or less, about 10 pM or less, or about 1 pM or less as measured
in an in vitro
kinase assay.
[98] In yet another aspect, an inhibitor that selectively inhibits one or more
members of
type I P13-kinases, or an inhibitor that selectively inhibits one or more type
I P13-kinase
mediated signaling pathways, alternatively can be understood to refer to a
compound that
exhibits a 50% inhibitory concentration (IC50) with respect to a given type I
P13-kinase, that
is at least 10-fold lower, at least 20-fold lower, at least 50-fold lower, at
least 100-fold lower,
at least 1000-fold lower than the inhibitor's ICso with respect to the rest of
the other type I PI3
-kinases.
[99] As used herein, the term "dual P13-kinase 6 / y inhibitor" and "dual P13-
kinase 6 / y
selective inhibitor" refers to a compound that inhibits the activity of both
the P13-kinase 6 and
y isozyme more effectively than other isozymes of the PI3K family. A dual P13-
kinase 6 / y
inhibitor is therefore more selective for P13-kinase 6 and y than conventional
PI3K inhibitors
such as CAL-130, wortmannin and LY294002, which are nonselective PI3K
inhibitors.
[100] Inhibition of P13-kinase 6 and y may be of therapeutic benefit in
treatment of various
conditions, e.g., conditions characterized by an inflammatory response
including, but not
limited to, autoimmune diseases, allergic diseases, and arthritic diseases.
Importantly,
inhibition of P13-kinase 6 and y function does not appear to affect biological
functions such as
viability and fertility.
[101] "Inflammatory response" as used herein is characterized by redness,
heat, swelling
and pain (i.e., inflammation) and typically involves tissue injury or
destruction. An
inflammatory response is usually a localized, protective response elicited by
injury or
destruction of tissues, which serves to destroy, dilute or wall off
(sequester) both the injurious
agent and the injured tissue. Inflammatory responses are notably associated
with the influx of
leukocytes and/or leukocyte (e.g., neutrophil) chemotaxis. Inflammatory
responses may result
Date Recue/Date Received 2020-12-07
from infection with pathogenic organisms and viruses, noninfectious means such
as trauma or
reperfusion following myocardial infarction or stroke, immune responses to
foreign antigens,
and autoimmune diseases. Inflammatory responses amenable to treatment with the
methods
and compounds according to the invention encompass conditions associated with
reactions of
the specific defense system as well as conditions associated with reactions of
the non-specific
defense system.
[102] The therapeutic methods of the invention include methods for the
amelioration of
conditions associated with inflammatory cell activation. "Inflammatory cell
activation" refers
to the induction by a stimulus (including but not limited to, cytokines,
antigens or auto-
antibodies) of a proliferative cellular response, the production of soluble
mediators (including
but not limited to cytokines, oxygen radicals, enzymes, prostanoids, or
vasoactive amines), or
cell surface expression of new or increased numbers of mediators (including
but not limited
to, major histocompatibility antigens or cell adhesion molecules) in
inflammatory cells
(including but not limited to monocytes, macrophages, T lymphocytes, B
lymphocytes,
granulocytes (polymorphonuclear leukocytes including neutrophils, basophils,
and
eosinophils) mast cells, dendritic cells, Langerhans cells, and endothelial
cells). It will be
appreciated by persons skilled in the art that the activation of one or a
combination of these
phenotypes in these cells can contribute to the initiation, perpetuation, or
exacerbation of an
inflammatory condition.
[103] "Autoimmune disease" as used herein refers to any group of disorders in
which tissue
injury is associated with humoral or cell-mediated responses to the body's own
constituents.
[104] "Transplant rejection" as used herein refers-to any immune response
directed against
grafted tissue (including organs or cells (e.g., bone marrow), characterized
by a loss of
function of the grafted and surrounding tissues, pain, swelling, leukocytosis,
and
thrombocytopenia).
[105] "Allergic disease" as used herein refers to any symptoms, tissue damage,
or loss of
tissue function resulting from allergy.
[106] "Arthritic disease" as used herein refers to any disease that is
characterized by
inflammatory lesions of the joints attributable to a variety of etiologies.
[107] "Dermatitis" as used herein refers to any of a large family of diseases
of the skin that
are characterized by inflammation of the skin attributable to a variety of
etiologies.
[108] As previously described, the term "dual P13-kinase 6 / y selective
inhibitor" generally
refers to a compound that inhibits the activity of the P13-kinase 6 and y
isozyme more
effectively than other isozymes of the PI3K family. The relative efficacies of
compounds as
46
Date Recue/Date Received 2020-12-07
inhibitors of an enzyme activity (or other biological activity) can be
established by
determining the concentrations at which each compound inhibits the activity to
a predefined
extent and then comparing the results. Typically, the preferred determination
is the
concentration that inhibits 50% of the activity in a biochemical assay, i.e.,
the 50% inhibitory
concentration or "ICso". ICso determinations can be accomplished using
conventional
techniques known in the art. In general, an ICso can be determined by
measuring the activity
of a given enzyme in the presence of a range of concentrations of the
inhibitor under study.
The experimentally obtained values of enzyme activity then are plotted against
the inhibitor
concentrations used. The concentration of the inhibitor that shows 50% enzyme
activity (as
compared to the activity in the absence of any inhibitor) is taken as the ICso
value.
Analogously, other inhibitory concentrations can be defined through
appropriate
determinations of activity. For example, in some settings it can be desirable
to establish a
90% inhibitory concentration, i.e., IC9o, etc.
[109] Accordingly, a dual P13-kinase 6 / y selective inhibitor alternatively
can be understood
to refer to a compound that exhibits a 50% inhibitory concentration (IC50)
with respect to P13-
kinase 6 and y, that is at least 10-fold lower, at least 20-fold lower, or at
least 30-fold lower
than the IC50 value with respect to any or all of the other class I PI3K
family members. In an
alternative embodiment of the invention, the term dual P13-kinase 6 / y
selective inhibitor can
be understood to refer to a compound that exhibits an ICso with respect to P13-
kinase 6 and y
that is at least 30-fold lower, at least 50-fold lower, at least 100-fold
lower, at least 200-fold
lower, or at least 500-fold lower than the ICso with respect to any or all of
the other PI3K
class I family members. A dual P13-kinase 6 / y selective inhibitor is
typically administered in
an amount such that it selectively inhibits both P13-kinase 6 and y activity,
as described
above.
[110] In certain embodiments, the compounds of the present invention exhibit
P13-kinase 6
and y inhibition almost equally (¨ 1:1) or at a maximum ratio of 1:5, i.e.,
the compound the of
the present invention exhibit almost equal ICso values for both P13-kinase 6
and y enzyme,
or at most a 3 to 8 fold difference between the two.
11111 The methods of the invention may be applied to cell populations in vivo
or ex vivo. "In
vivo" means within a living individual, as within an animal or human or in a
subject's body. In
this context, the methods of the invention may be used therapeutically or
prophylactically in
an individual. "Ex vivo" or "in vitro" means outside of a living individual.
Examples of ex
vivo cell populations include in vitro cell cultures and biological samples
including but not
limited to fluid or tissue samples obtained from individuals. Such samples may
be obtained
47
Date Recue/Date Received 2020-12-07
by methods known in the art. Exemplary biological fluid samples include blood,
cerebrospinal fluid, urine, and saliva. Exemplary tissue samples include
tumors and biopsies
thereof In this context, the invention may be used for a variety of purposes,
including
therapeutic and experimental purposes. For example, the invention may be used
ex vivo or in
vitro to determine the optimal schedule and/or dosing of administration of a
P13-kinase 6
selective inhibitor for a given indication, cell type, individual, and other
parameters.
Information gleaned from such use may be used for experimental or diagnostic
purposes or in
the clinic to set protocols for in vivo treatment. Other ex vivo uses for
which the invention
may be suited are described below or will become apparent to those skilled in
the art.
Pharmaceutical Compositions
[112] The invention provides a pharmaceutical composition comprising one or
more
compounds of the present invention and one or more pharmaceutically acceptable
carriers or
excipients. In one embodiment, the pharmaceutical composition includes a
therapeutically
effective amount of a compound of the present invention. The pharmaceutical
composition
may include one or more additional active ingredients as described herein.
[01] The pharmaceutical carriers and/or excipients may be selected from
diluents, fillers,
salts, disintegrants, binders, lubricants, glidants, wetting agents,
controlled release matrices,
colorants, flavorings, buffers, stabilizers; solubilizers, and combinations
thereof
[113] In one embodiment, the pharmaceutical compositons described herein
contain from
about 0.1 mg to about 1,000 mg, such as from about 1 mg to about 1,000 mg or
from about 20
mg to about 800 mg or 50 mg to about 600 mg or 50 mg to about 600 mg of one or
more
compounds of the present invention. 100 mg to about 400 mg of one or more
compounds of
the present invention.
[114] The pharmaceutical compositions of the present invention can be
administered alone
or in combination with one or more other active agents. Where desired, the
subject
compounds and other agent(s) may be mixed into a preparation or both
components may be
formulated into separate preparations to use them in combination separately or
at the same
time.
[115] The compounds and pharmaceutical compositions of the present invention
can be
administered by any route that enables delivery of the compounds to the site
of action, such as
orally, intranasally, topically (e.g., transdermally), intraduodenally,
parenterally (including
intravenously, intraarterially, intramuscularally, intravascularally,
intraperitoneally or by
48
Date Recue/Date Received 2020-12-07
injection or infusion), intradermally, by intramammary, intrathecally,
intraocularly,
retrobulbarly, intrapulmonary (e.g., aerosolized drugs) or subcutaneously
(including depot
administration for long term release e.g., embedded-under the-splenic capsule,
brain, or in the
cornea), sublingually, anally, rectally, vaginally, or by surgical
implantation (e.g., embedded
under the splenic capsule, brain, or in the cornea).
[116] The compositions can be administered in solid, semi-solid, liquid or
gaseous form, or
may be in dried powder, such as lyophilized form. The pharmaceutical
compositions can be
packaged in forms convenient for delivery, including, for example, solid
dosage forms such
as capsules, sachets, cachets, gelatins, papers, tablets, suppositories,
pellets, pills, troches, and
lozenges. The type of packaging will generally depend on the desired route of
administration.
Implantable sustained release formulations are also contemplated, as are
transdermal
formulations.
Method of Treatment
[117] The amount of the compound to be administered is dependent on the
subject (e.g.,
mammal) being treated, the severity of the disorder or condition, the rate of
administration,
the disposition of the compound and the discretion of the prescribing
physician. However, an
effective dosage is in the range of about 0.001 to about 100 mg/kg body weight
per day,
preferably about 1 to about 35 mg/kg/day, in single or divided doses. For a 70
kg human, this
would amount to about 0.05 to 7 g/day, preferably about 0.05 to about 2.5
g/day An effective
amount of a compound of the invention may be administered in either single or
multiple
doses (e.g., twice or three times a day).
[118] The present invention also provides methods of using the compounds or
pharmaceutical compositions of the present invention to treat disease
conditions, including
but not limited to diseases associated with malfunctioning of one or more
types of PI3 kinase.
A detailed description of conditions and disorders mediated by PI3K 6 and/or y
kinase
activity is set forth in, e.g., WO 2001/81346, US 2005/043239, WO 2011/055215
and WO
2012/151525.
[119] The treatment methods provided herein comprise administering to the
subject a
therapeutically effective amount of a compound of the invention. In one
embodiment, the
present invention provides a method of treating an inflammation disorder,
including
autoimmune diseases in a mammal. The method comprises administering to said
mammal a
49
Date Recue/Date Received 2020-12-07
therapeutically effective amount of a compound of the present invention, or a
pharmaceutically acceptable salt, ester, prodrug, solvate, hydrate or
derivative thereof
[120] It will be appreciated that the treatment methods of the invention are
useful in the
fields of human medicine and veterinary medicine. Thus, the individual to be
treated may be a
mammal, preferably human, or other animals. For veterinary purposes,
individuals include
but are not limited to farm animals including cows, sheep, pigs, horses, and
goats; companion
animals such as dogs and cats; exotic and/or zoo animals; laboratory animals
including mice,
rats, rabbits, guinea pigs, and hamsters; and poultry such as chickens,
turkeys, ducks, and
geese.
[121] In some embodiments, the method of treating inflammatory or autoimmune
diseases
comprises administering to a subject (e.g. a mammal) a therapeutically
effective amount of
one or more compounds of the present invention that selectively inhibit PI3K-6
and/or PI3K-y
as compared to all other type I PI3 kinases. Such selective inhibition of PI3K-
6 and/or PI3K-
y may be advantageous for treating any of the diseases or conditions described
herein. For
example, selective inhibition of PI3K- 6 may inhibit inflammatory responses
associated with
inflammatory diseases, autoimmune disease, or diseases related to an
undesirable immune
response including but not limited to asthma, emphysema, allergy, dermatitis,
rhuematoid
arthritis, psoriasis, lupus erythematosus, or graft versus host disease.
Selective inhibition of
PI3K-6 may further provide for a reduction in the inflammatory or undesirable
immune
response without a concomittant reduction in the ability to reduce a
bacterial, viral, and/or
fungal infection. Selective inhibition of both PI3K- 6 and PI3K-y may be
advantageous for
inhibiting the inflammatory response in the subject to a greater degree than
that would be
provided for by inhibitors that selectively inhibit PI3K -6 or PI3K-y alone.
In one aspect, one
or more of the subject methods are effective in reducing antigen specific
antibody production
in vivo by about 2-fold, 3-fold, 4-fold, 5-fold, 7.5-fold, 10-fold, 25-fold,
50-fold, 100-fold,
250-fold, 500-fold, 750-fold, or about 1000-fold or more. In another aspect,
one or more of
the subject methods are effective in reducing antigen specific IgG3 and/or
IgGM production
in vivo by about 2-fold, 3-fold, 4-fold, 5-fold, 7.5-fold, 10-fold, 25-fold,
50-fold, 100- fold,
250-fold, 500-fold, 750-fold, or about 1000-fold or more.
[122] In other embodiments, the present invention provides methods of using
the
compounds or pharmaceutical compositions to treat respiratory diseases
including but not
limited to diseases affecting the lobes of lung, pleural cavity, bronchial
tubes, trachea, upper
Date Recue/Date Received 2020-12-07
respiratory tract, or the nerves and muscle for breathing. For example,
methods are provided
to treat obstructive pulmonary disease. Chronic obstructive pulmonary disease
(COPD) is an
umbrella term for a group of respiratory tract diseases that are characterized
by airflow
obstruction or limitation. Conditions included in this umbrella term are:
chronic bronchitis,
emphysema, and bronchi ectasi s
[123] In another embodiment, the compounds described herein are used for the
treatment of
asthma. Also, the compounds or pharmaceutical compositions described herein
may be used
for the treatment of endotoxemia and sepsis. In one embodiment, the compounds
or
pharmaceutical compositions described herein are used to for the treatment of
rheumatoid
arthritis (RA). In yet another embodiment, the compounds or pharmaceutical
compositions
described herein is used for the treatment of contact or atopic dermatitis.
Contact dermatitis
includes irritant dermatitis, phototoxic dermatitis, allergic dermatitis,
photoallergic dermatitis,
contact urticaria, systemic contact-type dermatitis and the like. Irritant
dermatitis can occur
when too much of a substance is used on the skin of when the skin is sensitive
to certain
substance. Atopic dermatitis, sometimes called eczema, is a kind of
dermatitis, an atopic skin
disease.
[124] The invention also relates to a method of treating a hyperproliferative
disorder in a
mammal that comprises administering to said mammal a therapeutically effective
amount of a
compound of the present invention, or a pharmaceutically acceptable salt,
ester, prodrug,
solvate, hydrate or derivative thereof In some embodiments, said method
relates to the
treatment of cancer such as acute myeloid leukemia, thymus, brain, lung,
squamous cell, skin,
eye, retinoblastoma, intraocular melanoma, oral cavity and oropharyngeal,
bladder, gastric,
stomach, pancreatic, bladder, breast, cervical, head, neck, renal, kidney,
liver, ovarian,
prostate, colorectal, esophageal, testicular, gynecological, thyroid, CNS,
PNS, AIDS-related
(e.g. Lymphoma and Kaposi's Sarcoma) or viral-induced cancer. In some
embodiments, said
method relates to the treatment of a non-cancerous hyperproliferative disorder
such as benign
hyperplasia of the skin (e. g., psoriasis), restenosis, or prostate (e.g.,
benign prostatic
hypertrophy (BPH)).
[125] The invention also relates to a method of treating diseases related to
vasculogenesis or
angiogenesis in a mammal that comprises administering to said mammal a
therapeutically
effective amount of a compound of the present invention, or a pharmaceutically
acceptable
salt, ester, prodrug, solvate, hydrate or derivative thereof In some
embodiments, said method
51
Date Recue/Date Received 2020-12-07
is for treating a disease selected from the group consisting of tumor
angiogenesis, chronic
inflammatory disease such as rheumatoid arthritis, atherosclerosis,
inflammatory bowel
disease, skin diseases such as psoriasis, eczema, and scleroderma, diabetes,
diabetic
retinopathy, retinopathy of prematurity, age-related macular degeneration,
hemangioma,
glioma, melanoma, Kaposi's sarcoma and ovarian, breast, lung, pancreatic,
prostate, colon
and epidermoid cancer.
[126] Patients that can be treated with compounds of the present invention, or
pharmaceutically acceptable salt, ester, prodrug, solvate, hydrate or
derivative of said
compounds, according to the methods of this invention include, for example,
patients that
have been diagnosed as having psoriasis; restenosis; atherosclerosis; BPH;
breast cancer such
as a ductal carcinoma in duct tissue in a mammary gland, medullary carcinomas,
colloid
carcinomas, tubular carcinomas, and inflammatory breast cancer; ovarian
cancer, including
epithelial ovarian tumors such as adenocarcinoma in the ovary and an
adenocarcinoma that
has migrated from the ovary into the abdominal cavity; uterine cancer;
cervical cancer such as
adenocarcinoma in the cervix epithelial including squamous cell carcinoma and
adenocarcinomas; prostate cancer, such as a prostate cancer selected from the
following: an
adenocarcinoma or an adenocarinoma that has migrated to the bone; pancreatic
cancer such as
epitheliod carcinoma in the pancreatic duct tissue and an adenocarcinoma in a
pancreatic
duct; bladder cancer such as a transitional cell carcinoma in urinary bladder,
urothelial
carcinomas (transitional cell carcinomas), tumors in the urothelial cells that
line the bladder,
squamous cell carcinomas, adenocarcinomas, and small cell cancers; leukemia
such as acute
myeloid leukemia (AML), acute lymphocytic leukemia, chronic lymphocytic
leukemia,
chronic myeloid leukemia, hairy cell leukemia, myelodysplasia,
myeloproliferative disorders,
acute myelogenous leukemia (AML), chronic myelogenous leukemia (CML),
mastocytosis,
chronic lymphocytic leukemia (CLL), multiple myeloma (MM), and myelodysplastic
syndrome (MDS); bone cancer; lung cancer such as non-small cell lung cancer
(NSCLC),
which is divided into squamous cell carcinomas, adenocarcinomas, and large
cell
undifferentiated carcinomas, and small cell lung cancer; skin cancer such as
basal cell
carcinoma, melanoma, squamous cell carcinoma and actinic keratosis, which is a
skin
condition that sometimes develops into squamous cell carcinoma; eye
retinoblastoma;
cutaneous or intraocular (eye) melanoma; primary liver cancer (cancer that
begins in the
liver); kidney cancer; thyroid cancer such as papillary, follicular, medullary
and anaplastic;
AIDS-related lymphoma such as diffuse large B-cell lymphoma, B-cell
immunoblastic
52
Date Recue/Date Received 2020-12-07
lymphoma and small non-cleaved cell lymphoma; Kaposi's Sarcoma; viral-induced
cancers
including hepatitis B virus (HBV), hepatitis C virus (HCV), and hepatocellular
carcinoma;
human lymphotropic virus-type 1 (HTLV-I) and adult T-cell leukemia/lymphoma;
and human
papilloma virus (HPV) and cervical cancer; central nervous system cancers
(CNS) such as
primary brain tumor, which includes gliomas (astrocytoma, anaplastic
astrocytoma, or
glioblastoma multiforme), Oligodendroglioma, Ependymoma, Meningioma, Lymphoma,
Schwannoma, and Medulloblastoma; peripheral nervous system (PNS) cancers such
as
acoustic neuromas and malignant peripheral nerve sheath tumor (MPNST)
including
neurofibromas and schwannomas, malignant fibrous cytoma, malignant fibrous
histiocytoma,
malignant meningioma, malignant mesothelioma, and malignant mixed Mifflerian
tumor; oral
cavity and oropharyngeal cancer such as, hypopharyngeal cancer, laryngeal
cancer,
nasopharyngeal cancer, and oropharyngeal cancer; stomach cancer such as
lymphomas,
gastric stromal tumors, and carcinoid tumors; testicular cancer such as germ
cell tumors
(GCTs), which include seminomas and nonseminomas, and gonadal stromal tumors,
which
include Leydig cell tumors and Sertoli cell tumors; thymus cancer such as to
thymomas,
thymic carcinomas, Hodgkin disease, non-Hodgkin lymphomas carcinoids or
carcinoid
tumors; rectal cancer; and colon cancer.
[127] In another aspect, the present invention provides methods of disrupting
the function of
a leukocyte or disrupting a function of an osteoclast. The method includes
contacting the
leukocyte or the osteoclast with a function disrupting amount of a compound of
the invention.
[128] In another aspect of the present invention, methods are provided for
treating
ophthalmic disease by administering one or more of the subject compounds or
pharmaceutical
compositions to the eye of a subject.
[129] In some embodiments, the kinase is a lipid kinase or a protein kinase.
In some
embodiments, the kinase is selected from the group consisting of PI3 kinase
including
different isorforms such as PI3 kinase a, PI3 kinase 13, PI3 kinase y, PI3
kinase 6; DNA-PK;
mTor; AbI, VEGFR, Ephrin receptor B4 (EphB4); TEK receptor tyrosine kinase
(HE2);
FMS-related tyrosine kinase 3 (FLT-3); Platelet derived growth factor receptor
(PDGFR);
RET; ATM; ATR; hSmg-1; Hck; Src; Epidermal growth factor receptor (EGFR); KIT;
Inulsin Receptor (IR) and IGFR.
[130] The invention further provides methods of modulating PI3 kinase activity
by
contacting a PI3 kinase with an amount of a compound of the invention
sufficient to modulate
53
Date Recue/Date Received 2020-12-07
the activity of the PI3 kinase. Modulate can be inhibiting or activating PI3
kinase activity. In
some embodiments, the invention provides methods of inhibiting PI3 kinase
activity by
contacting a PI3 kinase with an amount of a compound of the invention
sufficient to inhibit
the activity of the PI3 kinase. In some embodiments, the invention provides
methods of
inhibiting PI3 kinase activity. Such inhibition can take place in solution, in
a cell expressing
one or more PI3 kinases, in a tissue comprising a cell expressing one or more
PI3 kinases, or
in an organism expressing one or more PI3 kinases. In some embodiments, the
invention
provides methods of inhibiting PI3 kinase activity in an animal (including
mammal such as
humans) by contacting said animal with an amount of a compound of the
invention sufficient
to inhibit the activity of the PI3 kinase in said animal.
[131] The following general methodology described herein provides the manner
and process
of making and using the compound of the present invention and are illustrative
rather than
limiting. Further modification of provided methodology and additionally new
methods may
also be devised in order to achieve and serve the purpose of the invention.
Accordingly, it
should be understood that there may be other embodiments which fall within the
spirit and
scope of the invention as defined by the specification hereto.
[132] Illustartive compounds of the present invention include those specified
above in
Tables 1 and 2, and pharmaceutically acceptable salts thereof The present
invention should
not be construed to be limited to them.
General Methods of Preparation of Compounds of the Invention
[133] The compounds of the present invention may be prepared by using the
methods (with
or without modifications) as disclosed in International Patent Publication
Nos. WO
2008/127226, WO 2009/088986, WO 2011/055215 and WO 2012/151525.
[134] Unless otherwise indicated, the variables (e.g., R, Ra, Rb,
Re, n and Cy) when
used in the below formulae are to be understood to present those groups
described above in
relation to the formulas above, such as formula (I). These methods can
similarly be applied to
other compounds of formula as provided herein above with or without
modification.
[135] Scheme 1: This scheme provides a method for the preparation of a
compound of
formula (I) wherein X is N, R is H, alkyl or halogen, Pg is protecting group
and all the other
54
Date Recue/Date Received 2020-12-07
variables such as R, R2, R3, Ra, Rb, Re, X and n are the same as described
above in relation
to formula (I)
Scheme-1
(R)n (R)n (R)n
I (Ri) 0 0
n I n
(R1) deprotection (R1 ) I
I
0 -I yH<RaI õRa
H2 N ci)<OPg
(a) (c) Rb H op OH Rb
Rb Ra 0Pg / N t-Re
(b) H2N¨N
R2 4t
mitsunobu reaction
R3 H2N
mitsunobu
reaction kg)
(R)n (R)n
0 %1 0
(R1)n
jS)LNI (R1)n
jL IN
xH<Ra I õ a
suzuki coupling R
Rb Rb
N\ R3_b_BpH (e) 'NN,--N Re
OH
--N
Cy (0
H2N
H2N
(I)
The compound of the formula (a) can be reacted with the compound of formula
(b) to form a
compound of formula (c), wherein Pg is a suitable protecting group. The
compound of
formula (c) can be de-protected to yield the compound of formula (d). The
compound of
formula (d) can be coupled with a compound of formula (g) under Mitsunobu
reaction
conditions to form a compound of formula (e), for example, in the presence of
a dialkyl
azodicarboxylate and a triaryl phosphine. The compound of formula (e) can be
coupled with a
compound of formula (f) under Suzuki reaction conditions, for example, in the
presence of a
suitable base and a Palladium catalyst such as Pd(dppf)2C12.CH2C12, to afford
the desired
compound of formula (I).
Alternatively, the compound of formula (d) can be coupled with a compound of
formula (h)
to form the desired compound of formula (I) under Mitsunobu reaction
conditions, for
example, in the presence of a dialkyl azodicarboxylate and a triaryl
phosphine.
[136] Scheme 2: This scheme provides a method for the preparation of a
compound of
formula (I) wherein X is CH, R is H, alkyl or halogen, and all the other
variables such as W,
R2, R3, W, Rb, Re and n are the same as described above in relation to formula
(I).
Date Recue/Date Received 2020-12-07
[137] The compound of formula (al) can be coupled with a compound of formula
(g) to
form a compound of formula (el) under Mitsunobu reaction conditions, for
example, in the
presence of a dialkyl azodicarboxylate and a triaryl phosphine. The compound
of formula
(el) can be coupled with a compound of formula (f) to form the desired
compound of formula
(I) under Suzuki reaction conditions, for example, in the presence of a
suitable base and a
Palladium catalyst such as Pd(dppO2C12.CH2C12.
Scheme-2
N N
(R), N j (R),
0 -N 0
I H2N
(121).7\N
Ra (g)
(a1) R
mitsunobu
b Rb
reaction (c1)
OH )Ersil N mitsunobu smuki
R2 H2N ¨N
y-IR reaction coupling
-N H2N
R2
R3 (h)
(R),
0
(R1) II (1)
xyRa
Rb
,N
N) _________________________________
¨
Cy N
H2N
(I)
Alternatively, the compound of formula (al) can be coupled with a compound of
formula (h)
to form the desired compound of formula (I) under Mitsunobu reaction
conditions, for
example, in the presence of a dialkyl azodicarboxylate and a triaryl
phosphine.
[138] Scheme 3: This scheme provides a method for the preparation of a
compound of
formula (I) wherein X is N, R is H, alkyl or halogen, G is OH or Cl, Lg is
Leaving Group and
all the other variables such as R2, R3, Re', Rb, RC and n are the same as
described above in
relation to formula (I)
Scheme-3
56
Date Recue/Date Received 2020-12-07
0 (R)fl
(R)n (R)n (R1)n
0 0
Ra
11
(R in )
(R1)j\N Rb
0
NH2 (c2) Imsfi41 (e) NcN
Rb
Rb Ra Lg I NH2
--N
(g)
G is OH or Cl H2N
(b1) N c R2
OH
-N
R2 iy 2N R3 B.OH
(0 R3 (h) suzuki coupling
0 (R)%rrj
(R1)n
I
eyRa
Rb
Cy --N
H2N
(I)
The compound of the formula (a) can be reacted with the compound of formula
(bl) to form
a compound of formula (c2), wherein Lg is Leaving group, such as a halogen
group. The
compound of formula (c2) can be alkylated with a compound of formula (g) in
the presence
of a suitable base to form the compound of formula (e) which can be coupled
with compound
of formula (I) under Suzuki reaction conditions, for example, in the presence
of a suitable
base and a Palladium catalyst such as Pd(dppf)2C12.CH2C12, to afford the
desired compound
of formula (I)
Alternatively, the compound of formula (c2) can be alkylated with a compound
of formula
(h) in the presence of a suitable base to form the desired compound of formula
(I).
[139] Scheme 4: This scheme provides a method for the preparation of a
compound of
formula (I) wherein X is CH, R is H, alkyl or halogen, and all the other
variables such as Rl,
R2, R3, Re', Rb, RC and n are same as described above in relation to formula
(I).
Scheme-4
57
Date Recue/Date Received 2020-12-07
(R)n
0
0 0 (R1)n
(On I (R1)n I
T/\N/';,/ Ra
Ra ¨"'" Ra H
(cl) ,N
Rb
\%\/\Z___/ Rb NtirirNZR
Rb N
(al) la-2)
CI I NH2 N\ /
OH (g)
--N
N N c
N R2
-41 R3-b¨ H2N
R2 =FI2N (h)
(f)
R3 suzuki
(R)n coupling
0
(R1)n
j
Ra
N
N \
H2N
(1)
The compound of the formula (al) can be transformed to a compound of the
formula (a2).
The compound of formula (a2) can be alkylated with a compound of formula (g)
in the
presence of a suitable base to form the compound of formula (el) which can be
coupled with
a compound of formula (f) under Suzuki reaction conditions, for example, in
the presence of a
suitable base and a Palladium catalyst such as Pd(dppf)2C12.CH2C12, to afford
the desired
compound of formula (I).
Alternatively, the compound of formula (a2) can be alkylated with a compound
of formula
(h) in the presence of a suitable base to form the desired compound of formula
(I).
[140] Similar methodologies with certain modifications as known to those
skilled in the art
can be used to synthesize compounds of the formula (I), (I-A), (I-A1), (I-A2),
(I-A3), (I-A4),
(I-A5), (I-B), (I-B1), (I-B2), (I-B3), (I-B4), (I-B5), (II), (II-1), (II-2),
(II-3), (II-4), (II-5),
(III), (III-A) and (III-B), wherein all the variables are to be understood to
present those
groups described above in relation to these formulas using suitable
intermediates and
reagents.
Experimental
58
Date Recue/Date Received 2020-12-07
[141] The examples and preparations provided below further illustrate and
exemplify the
compounds of the present invention and methods of preparing such compounds. It
is to be
understood that the scope of the present invention is not limited in any way
by the scope of
the following examples and preparations. In the following examples molecules
with a single
chiral center, unless otherwise noted, exist as a racemic mixture. Those
molecules with two or
more chiral centers, unless otherwise noted, exist as a racemic mixture of
diastereomers.
Single enantiomers/diastereomers may be obtained by methods known to those
skilled in the
art.
[142] As used herein the Superscript 1 refers to International Patent
Application No.
PCT/IB2010/002804 (WO 2011/055215) and Superscript 2 refers to International
Patent
Application No. PCT/US2012/036594 (WO 2012/151525).
Intermediates
Table 3
CI 0 0 CI 0 el F i 0 F 0 F 0
NKrN ''''-'' 'ftr- ).-)1'N N
I 1 H
/ / ..--- ----
NH
OH CI OH CI YO
0i
Intermediate 1 Intermediate 2 Intermediate 3 Intermediate 4
Intermediate 5
F 0 0 0 0 0 40 0 40 0 40
F F
N N N N N
H H
/
N NH N NH
CI CI CI
0 YO
CI a
Intermediate 6 Intermediate 8 Intermediate 9
Intermediate 7 Intermediate 10
0 o 0
. 0, 0 0 o 40/ 0 0
N N N N
H
N N . NH N
)
Cl N- ,= \ N- N -----N 0 CI
¨N
I ) Y CI
H2N ¨N
Intermediate 11 i Intermediate 15
H2N
Intermediate 12 Intermediate 14
59
Date Recue/Date Received 2020-12-07
Intermediate 13
Intermediate 1: 8-chloro-3-(1-hydroxyethyl)-2-phenylisoquinolin-1(2H)-one: To
3-(1-
aminoethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one (7.0 g, 23.46 mmol) , Hcl
(7.13 ml) was
added and cooled to 0 C. To this mixture solution of sodium nitrite (4.85 g,
70.39 mmol)
dissolved in water (135 ml) was added and stirred at RT for 30 min. The
reaction mixture was
heated to 135 C for 4h. The reaction mixture was basified with saturated
sodium bicarbonate
solution, extracted with ethyl acetate, dried over sodium sulphate and
concentrated under
reduced pressure. The crude product was column chromatographed with ethyl
acetate :
petroleum ether to afford the desired compound as brown solid (3.70 g, 53%)
which was
used as such in next step.
Intermediate 2: 8-chloro-3-(1-chloroethyl)-2-phenylis oquinolin-1(2H)-one: To
a cooled
solution of intermediate 1 (0.500 g, 1.67 mmol) in dichloromethane (10 ml) and
triethylamine
(0.50 ml, 5.01 mmol), methanesulphonyl chloride (0.30 ml, 3.34 mmol) was added
stirred at
room temperature for 24h. The reaction mass was quenched with water, extracted
with
dichloromehane, dried over sodium sulphate and concentrated. The crude product
was
column chromatographed with ethyl acetate : petroleum ether to afford the
desired compound
as brown solid (0.200 g, 37%). Mass: 318.0(W).
Intermediate 3: 8-fluoro-3-(1-hydroxyethyl)-2-phenylis oquinolin-1(2H)-one:
The title
compound was obtained as yellow solid (0.940 g, 100%) by using a procedure
that is similar
to the one described for intermediate 1 from 3-(1-aminoethyl)-8-fluoro-2-
phenylisoquinolin-
1(2H)-one (0.880 g, 3.11 mmol) , 6N HC1 (12 ml) sodium nitrite (0.335 g, 4.86
mmol) which
was used as such in next step.
Intermediate 4: 3-(1-chloroethyl)-8-fluoro-2-phenylisoquinolin-1(2H)-one: The
title
compound was obtained as yellow solid (0.586 g, 58%) by using a procedure that
is similar to
the one described for intermediate 2 from intermediate 3 (0.940 g, 3.31 mmol)
,
dichloromethane (20 ml) and triethylamine (1.30 ml, 9.95 mmol),
methanesulphonyl chloride
(0.51 ml, 6.63 mmol) which was used as such in next step.
Intermediate 5: 2-(2-chloropropanamido)-6-fluoro-N-phenylbenzamide: To a
solution of
2-amino-6-fluoro-N-phenylbenzamide (1.50g, 6.51 mmol) in dichloromethane (20
ml) and N-
Date Recue/Date Received 2020-12-07
diisopropylethylamine (1.00 g, 7.81 mmol) cooled to 0 C, 2-chloropropionyl
chloride (0.75
ml, 7.81 mmol) was added drop wise. After lh, the reaction mixture was
quenched with
water, extracted with dichloromethane. The organic layer was washed with
saturated sodium
bicarbonate solution, brine solution, dried over sodium sulphate and
concentrated. The crude
product was column chromatographed with ethyl acetate : petroleum ether to
afford the
desired compound as off-white solid (1.60 g, 77%).1-H-NMR (6 ppm, CDC13, 400
MHz):
11.86 (s, 1H), 8.45 (d, J = 8.4Hz, 1H), 8.37 (d, J= 12.8 Hz, 1H), 7.63 (m,
2H), 7.49-7.38 (m,
3H), 7.23 (t, J= 7.5Hz, 1H), 6.97 (dd, J= 11.7,8.4Hz, 1H), 4.53 (q, J = 7.0Hz,
1H), 1.81 (d, J
= 7.0Hz, 3H).
Intermediate 6: 2-(1-chloroethyl)-5-fluoro-3-phenylquinazolin-4(3H)-one:
To
intermediate 5 (1.60g, 4.98 mmol), P0C13 (13.3 ml) was added and heated to 125
C for 12h.
The reaction mixture was quenched into crushed ice, neutralised with saturated
sodium
bicarbonate solution and extracted with ethyl acetate. The organic layer was
dried over
sodium sulphate and concentrated. The crude product was column chromatographed
with
ethyl acetate: petroleum ether to afford the desired compound as brown solid
(0.85 g, 57%).
Mass: 303.0(W).
Intermediate 7: 2-(2-chloropropanamido)-6-fluoro-N-phenylbenzamide: The title
compound was obtained as brown solid (2.60 g, 80%) by using a procedure that
is similar to
the one described for intermediate 5 from 2-amino-5-fluoro-N-phenylbenzamide
(2.50g,
10.07 mmol) , dichloromethane (20 m1). N-diisopropylethylamine (2.10 g, 12.08
mmol) and
2-chloropropionyl chloride (1.17 ml, 12.08 mmol). 1-H-NMR (6 ppm, CDC13, 400
MHz):
11.23 (s, 1H), 8.45 (dd, J= 9.2,5.1Hz, 1H), 7.96 (s, 1H), 7.66 (m, 2H), 7.43
(m, 2H), 7.35
(dd, J = 12.0,9.1Hz, 1H), 7.23 (m, 2H), 4.51 (q, J = 7.0Hz, 1H), 1.81 (d, J=
7.0Hz, 3H).
Intermediate 8: 2-(1-chloroethyl)-6-fluoro-3-phenylquinazolin-4(3H)-one: The
title
compound was obtained as yellow solid (0.398 g, 35%) by using a procedure that
is similar to
the one described for intermediate 6 from intermediate 7 (1.20 g, 3.74 mmol)
and P0C13 (10
m1). Mass: 303.1(W).
Intermediate 9: 3-(1-chloroethyl)-2-phenylisoquinolin-1(2H)-one: The title
compound was
obtained as yellow solid (1.00 g, 58%) by using a procedure that is similar to
the one
described for intermediate 1 from 3-(1-aminoethyl)-2-phenylisoquinolin-1(2H)-
one (1.60 g,
6.05 mmol) , 6N HC1 (19.2 ml) sodium nitrite (0.650 g, 9.44 mmol). Mass:
284.2(W).
61
Date Recue/Date Received 2020-12-07
Intermediate 10: 2-(2-chloropropanamido)-N-phenylbenzamide: The title compound
was
obtained as yellow solid (2.10 g, 98%) by using a procedure that is similar to
the one
described for intermediate 5 from 2-amino-N-phenylbenzamide (1.50 g, 7.06
mmol) ,
dichloromethane (15 ml), N-diisopropylethylamine (1.09 g, 8.48 mmol) and 2-
chloropropionyl chloride (1.76 g, 8.48 mmol) which was used as such in next
step.
Intermediate 11: 2-(1-chloroethyl)-3-phenylquinazolin-4(3H)-one: The title
compound
was obtained as off-white solid (1.00 g, 53%) by using a procedure that is
similar to the one
described for intermediate 6 from intermediate 10 (2.00 g, 6.60 mmol) and
POC13 (16 m1).
Mass: 284.9(M).
Intermediate 12: 3-(1-(4- amino-3-iod o- 1H- pyrazolo [3,4-d] pyrimidin-
1-yl)ethyl)-2-
phenylis oquin olin-1(2H)- one : To a solution of 3-iodo-1H-pyrazolo[3,4-
d]pyrimidin-4-
amine1 (0.45 g, 1.76 mmol) in DMF ( 7.5 ml), potassium carbonate (0.48 g, 3.52
mmol) was
added and stirred at room temperature for lh. The reaction mixture was cooled
to 0 C,
Intermediate 9 (0.65 g, 2.29 mmol) was added and stirred at room temperature
for 12h. The
reaction mixture was quenched with water extracted with ethyl acetate. The
organic layer was
dried over sodium sulphate and concentrated under reduced pressure. The crude
product was
purified by column chromatography with ethyl acetate: petroleum ether to
afford the title
compound as a brown solid (0.046 g, 39%). Mass: 508.9(W).
Intermediate 13: 3-(1-(4-amino-3-iodo-1H-pyrazolo[3,4-d] pyrimidin-1-
ypethyl)-8-
chloro-2-phenylisoquinolin-1(2H)-one: The title compound was obtained as brown
solid
(0.500 g, 53%) by using a procedure that is similar to the one described for
intermediate 12
from 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine (0.45 g, 1.72 mmol), potassium
carbonate
(0.476 g, 3.44 mmol), DMF (7.3 ml) and intermediate 2 (0.713g, 2.24 mmol)
which was used
as such in next step.
Intermediate 14: 2-(2-chlorobutanamido)-N-phenylbenzamide: To a solution of 2-
amino-
N-phenylbenzamide (2.0 g, 9.42 mmol) in dichloromethane (40 ml), 2-
chlorobutyric acid
(1.26 g, 10.36 mmol), triethylamine (14.81 ml, 101 mmol), and 4-
Dimethylaminopyridine
(0.230 g, 1.88 mmol) were added followed by dicyclohexylcarbodiimide (3.88 g,
18.84
mmol). After 12h at room temperature, the solid precipitated was filtered and
the solution was
concentrated. The crude product was column chromatographed with ethyl acetate:
petroleum
ether to afford the desired compound as off-white solid (2.00 g, 67%). 11-1-
NMR (6 ppm,
62
Date Recue/Date Received 2020-12-07
DMSO-d6, 400 MHz): 11.06 (s, 1H), 10.41 (s, 1H), 8.21 (d, J = 8.2Hz, 1H), 7.83
(d, J = 7.8
Hz, 1H), 7.69 (m, 2H), 7.57 (m, 1H), 7.37 (t, J= 7.9Hz, 2H), 7.30 (t, J=
7.6Hz, 1H), 7.14 (t,
J= 7.4Hz, 1H), 4.64 (dd, J= 7.3, 5.6Hz, 1H), 2.07-1.86 (m, 2H), 0.98 (d, J =
7.3Hz, 3H).
Intermediate 15: 2-(1-chloropropy1)-3-phenylquinazolin-4(3H)-one: To
intermediate 14
(1.00 g, 3.15 mmol), POC13 (15 ml) was added and heated to 125 C for 48h. The
reaction
mixture was quenched into crushed ice, neutralised with saturated sodium
bicarbonate
solution and extracted with ethyl acetate. The organic layer was dried over
sodium sulphate
and concentrated. The crude product was column chromatographed with ethyl
acetate :
petroleum ether to afford the desired compound as yellow solid (0.200 g, 21%).
11-1-NMR (6
ppm, DMSO-d6, 400 MHz): 8.16 (dd, J = 7.9, 1.0 Hz, 1H), 7.92 (dt, J = 8.3,1.5
Hz, 1H),
7.78 d, J = 7.7 Hz, 1H), 7.62-7.44 (m, 6H), 4.64 (dd, J = 7.6, 6.7 Hz, 1H),
2.38 (m, 1H), 2.09
(m, 1H), 0.91 (d, J = 7.3Hz, 3H).
Example 1
3-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-1-
ypethyl)-
8-chloro-2-phenylisoquinolin-1(2H)-one
3-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-d] pyrimi din-1 -
ypethyl)-8-
chl oro-2-phenyli s oquinolin-1(2H)-one: To a solution of 3-(3-fluoro-4-
isopropoxypheny1)-1H-
pyrazolo[3,4-d]pyrimidin-4-aminel ( 0.091 g, 0.314 mmol) in DMF ( 2 ml),
potassium
carbonate (0.087 g, 0.628 mmol) was added and stirred at room temperature for
30min.. The
reaction mixture was cooled to 0 C, intermediate 2 (0.200 g, 0.628 mmol) was
added and
stirred at 90 C for 3h. The reaction mixture was quenched with water and the
precipitate
formed was filtered and dried under vacuum. The crude product was purified by
column
chromatography with ethyl acetate: petroleum ether to afford the title
compound as off-white
solid (0.030 g, 17%). MP: 164-166 C. Mass: 569.7 (M+).
Example 2
3-(1-(4-amino-3-(3-flu o ro-4-is op ropoxypheny1)-1H-pyrazolo [3,4-d]
pyrimidin-1-ypethyl)-
8-fluoro-2-phenylisoquinolin-1(2H)-one
3-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-d] pyrimi din-1 -
ypethyl)-8-
fluoro-2-phenylisoquinolin-1(2H)-one : The title compound was obtained as
brown solid
(0.025 g, 13%) by using a procedure that was similar to the one described for
example 1
63
Date Recue/Date Received 2020-12-07
from 3-(3 -fluoro-4-is oprop oxypheny1)-1H-py raz ol o [3,4-d] py rimi din-4-
amine ( 0.099 g,
0.346 mmol), DMF ( 2.2 ml), potassium carbonate (0.096 g, 0.693 mmol) and
intermediate 4
(0.156 g, 0.519 mmol). MP: 264-266 C. Mass: 553.1 (M++1).
Example 3
2-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-1-
ypethyl)-
5-fluoro-3-phenylquinazolin-4(3H)-one
2-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-d] pyrimi din-1 -
ypethyl)-5-
fluoro-3-phenylquinazolin-4(3H)-one: The title compound was obtained as brown
solid
(0.050 g, 26%) by using a procedure that was similar to the one described for
example 1
from 3-(3 -fluoro-4-is oprop oxypheny1)-1H-py raz ol o [3,4-d] py rimi din-4-
aminel ( 0.099 g,
0.346 mmol), DMF ( 2.2 ml), potassium carbonate (0.096 g, 0.693 mmol) and
intermediate 6
(0.157 g, 0.519 mmol). MP: 251-153 C. Mass: 553.8 (Mt).
Example 4
2-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-1-
ypethyl)-
6-fluoro-3-phenylquinazolin-4(3H)-one
2-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-d] pyrimi din-1 -
ypethyl)-6-
fluoro-3-phenylquinazolin-4(3H)-one : The title compound was obtained as brown
solid
(0.080 g, 42%) by using a procedure that was similar to the one described for
example 1
from 3-(3 -fluoro-4-is oprop oxypheny1)-1H-py raz ol o [3,4-d] py rimi din-4-
aminel ( 0.099 g,
0.346 mmol), DMF ( 2.2 ml), potassium carbonate (0.096 g, 0.693 mmol) and
intermediate 8
(0.210 g, 0.693 mmol). MP: 215-218 C. Mass: 553.9 (Mt).
Example 5
3-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo [3,4-d] pyrimidin- 1-
yl)ethyl)-
2-phenylisoquinolin-1(2H)-one
3-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-d] pyrimi din-1 -
ypethyl)-2-
phenylis oquinolin-1(2H)-one: The title compound was obtained as off-white
solid (0.109 g,
39%) by using a procedure that was similar to the one described for example 1
from 3-(3-
fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-d]pyrimidin-4-aminel ( 0.150 g,
0.522 mmol),
64
Date Recue/Date Received 2020-12-07
DMF ( 3.5 ml), potassium carbonate (0.144 g, 1.044 mmol) and intermediate 9
(0.296 g,
1.044 mmol). MP: 264-267 C. Mass: 555.0 (Mt).
Example 5a and 5b
(+)-3-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo [3,4-d]
pyrimidin-1-
ypethyl)-2-phenylisoquinolin-1(2H)-one
and (+3-(1-(4-
amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-
1-ypethyl)-2-phenylisoquinolin-1(2H)-one
The two enantiomerically pure isomers were separated by preparative SFC
conditions from 3-
(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo [3,4-cl] pyrimidin-1-
yl)ethyl)-2-
phenylisoquinolin-1(2H)-one (0.500 g) on a CHIRALPAK AS-H column (250 x 20 mm;
5[1m) using methanol: CO2 (40:60) as the mobile phase at a flow rate of 80g /
min.
Example 5a: Brown solid (0.438 g). e.e. 100%. Rt: 2.29 min. Mass: 535.2(W).
MP: 231-
233 C.
Example 5b: Brown solid (0.439 g). e.e. 100%. Rt: 3.99 min. Mass: 535.2(W).
MP: 232-
235 C.
Example 6
2-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-1-
yl)ethyl)-
3-phenylquinazolin-4(3H)-one
2-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-d] pyrimi din-1 -
ypethyl)-3-
phenylquinazolin-4(3H)-one : The title compound was obtained as brown solid
(0.200 g,
64%) by using a procedure that was similar to the one described for example 1
from 3-(3-
fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-d]pyrimidin-4-aminel ( 0.168 g,
0.584 mmol),
DMF ( 2.0 ml), potassium carbonate (0.121 g, 0.877 mmol) and intermediate 11
(0.248 g,
0.877 mmol). MP: 236-239 C. Mass: 536.1 (Mt).
Example 6a and 6b
(+)-2-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo [3,4-d]
pyrimidin-1-
yl)ethyl)-3-phenylquinazolin-4(3H)-one
Date Recue/Date Received 2020-12-07
and (-)-2-(1-(4-amino-3-(3-fluoro-4-isop ropoxypheny1)-1H-pyrazolo 13,4-d]
pyrimidin-
1-ypethyl)-3-phenylquinazolin-4(3H)-one
The two enantiomerically pure isomers were separated by preparative SFC
conditions from 2-
(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo[3,4-dlpyrimidin-1-
yeethyl)-3-
phenylquinazolin-4(3H)-one (0.500 g) on a CHIRALPAK AS-H column (250 x 20 mm;
51.1m) using methanol: CO2 (55:45) as the mobile phase at a flow rate of 60g /
min.
Example 6a: Brown solid (0.519 g). e.e. 100%. Rt: 2.41 min. Mass: 536.2 (M+).
MP: 189-
192 C.
Example 6b: Brown solid (0.490 g). e.e. 99.62%. Rt: 4.10 min. Mass: 536.2(M).
MP: 192-
195 C.
Example 7
3-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-1-
ypethyl)-2-
phenylisoquinolin-1(2H)-one
3 -(1-(4-amino-3-(3 -fluoro-4-hy droxy pheny1)-1H-pyrazol o [3,4-d] pyrimidin-
l-y pethyl)-2-
phenyl isoquinolin-1(2H)-one: To Example 5 (0.060 g, 0.112 mmol) in
dichloromethane (10
ml) cooled to 0 C, boron tribromide (1M in dichloromethane, 0.64 ml) was added
drop wise
and stirred for lh. The reaction mixture was quenched with 2N HC1 solution,
extracted with
dichloromethane, dried over sodium sulphate and concentrated under reduced
pressure. The
crude product was purified by column chromatography with ethyl acetate:
petroleum ether to
afford the title compound as a off-white solid (0.025 g, 45%). MP: 237-2390C.
Mass: 492.8
(M+).
Example 7a and 7b
(+)-3-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-
1-
ypethyl)-2-phenylis oquinolin-1(2H)-one
and (-)-3-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d]
pyrimidin- 1-
ypethyl)-2-phenylis oquinolin-1(2H)-one
The two enantiomerically pure isomers were separated by preparative SFC
conditions from 3-
(1-(4-amino-3-(3-fluoro-4-hy droxypheny1)-1H-py razol o [3,4-d] pyrimidin- 1 -
ypethyl)-2-
66
Date Recue/Date Received 2020-12-07
phenylisoquinolin-1(2H)-one (0.500 g) on a CHIRALPAK AS-H column (250 x 20 mm;
5[4m) using methanol: CO2 (55:45) as the mobile phase at a flow rate of 60g /
min.
Example 7a: Brown solid (0.428 g). e.e. 100%. Rt: 2.25 min. Mass: 493.2(W).
MP: 229-
231 C.
Example 7b: Brown solid (0.347 g). e.e. 99.48%. Rt: 4.11 min. Mass: 493.2(W).
MP: 230-
232 C.
Example 8
2-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-1-
yDethyl)-3-
phenylquinazolin-4(3H)-one
2-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d] py rimi din-1-y
pethyl)-3-
phenylquinazolin-4(3H)-one: The title compound was obtained as brown solid
(0.035 g,
40%) by using a procedure that was similar to the one described for example 7
from example
6 ( 0.095 g, 0.177 mmol), dichloromethane (5.0 ml) and boron tribromide (1M in
dichloromethane, 1.0 m1). MP: 230-233 C. Mass: 494.1 (W+1).
Example 9
N-(5-(4-amino- 1-(1-(1-oxo-2-phenyl- 1,2-dihyd rois oquinolin-3-yl)ethyl)-1H-
pyrazolo [3,4-
d] pyrimidin-3-y1)-2-methoxyphenyOmethanesulfonamide
N-(5-(4-amino-1 -(1 -(1 -oxo-2-pheny1-1,2-dihy droi s o quinolin-3 -ypethyl)-
1H-py razol o [3,4-
d]pyrimidin-3-y1)-2-methoxyphenyl)methanesulfonamide : To a solution of
intermediate 12
(0.200 g, 0.393 mmol ), in DME (2 mL), and water (0.7 mL),N-(2-methoxy-5-
(4,4,5,5-
tetramethy1-1,3,2-dioxaborolan-2-yl)phenyl)methane sulfonamide2 (0.157 g,
0.590 mmol)
and sodium carbonate (0.083 g, 0.786 mmol ) were added and the system was
degassed for 5
min. and bis(diphenylphosphino)ferrocene]dichloropalladium(II) (0.064 g, 0.078
mmol) was
added under nitrogen atmosphere and the mixture was heated to 100 C at a
microwave
reactor for 15 min. The reaction mixture was celiteTM filtered, diluted with
ethyl acetate, dried
over Na2SO4 and concentrated. The crude product was column chromatographed
with ethyl
acetate : petroleum ether to afford the desired compound as brown solid (0.033
g, 15%).
MP: 260-263 C. Mass : 582.1 (W+1).
67
Date Recue/Date Received 2020-12-07
Example 9a and 9b
(+)-N-(5-(4-amino-1-(1-(1-oxo-2-pheny1-1,2-dihydroisoquinolin-3-ypethyl)-1H-
pyrazolo [3,4-d] pyrimidin-3-y1)-2-methoxyphenyl)methanesulfonamide
and (-)-N-(5-(4-amino-1-(1-(1-oxo-2-pheny1-1,2-dihydrois oquinolin-3-
ypethyl)-1H-
pyrazolo [3,4-d] pyrimidin-3-y1)-2-methoxyphenyl)methanesulfonamide
The two enantiomerically pure isomers were separated by preparative SFC
conditions from
N-(5-(4-amino-1 -(1 -(1 -oxo-2-pheny1-1,2-dihy drois o quinolin-3 -ypethyl)-1H-
py razol o [3,4-
dlpyrimidin-3-y1)-2-methoxyphenyl)methanesulfonamide (0.500 g) on a CHIRALPAK
AS-
H column (250 x 20 mm; 5[tm) using methanol : CO2 (40:60) as the mobile phase
at a flow
rate of 60g / min.
Example 9a: Brown solid (0.149 g). e.e. 99.55%. Rt: 2.06 min. Mass: 582.2(W).
MP: 165-
168 C.
Example 9b: Brown solid (0.212 g). e.e. 99.79%. Rt: 3.67 min. Mass: 582.2(W).
MP: 158-
162 C.
Example 10
2-(1-(4-amino-3-(4-methoxy-3-nitropheny1)-1H-pyrazolo13,4-d[pyrimidin-1-
ypethyl)-3-
phenylquinazolin-4(3H)-one
2-(1-(4-amino-3-(4-methoxy-3-nitropheny1)-1H-pyrazolo[3,4-dlpyrimidin-1-
ypethyl)-3-
phenylquinazolin-4(3H)-one : The title compound was obtained as brown solid
(1.05 g,
70%) by using a procedure that was similar to the one described for example 1
from 3-(4-
methoxy-3-nitropheny1)-1H-pyrazolo[3,4-d]pyrimidin-4-amine ( 0.610 g, 2.15
mmol), DMF (
9.6 ml), potassium carbonate (0.594 g, 4.30 mmol) and intermediate 11 (0.800
g, 2.79 mmol)
Which was used as such in next step.
Example 11
3-(1-(4-amino-3-(3-amino-4-methoxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-1-
ypethyl)-2-
phenylisoquinolin-1(2H)-one
68
Date Recue/Date Received 2020-12-07
3 -(1-(4-amino-3-(3 -amino-4-methoxypheny1)-1H-pyrazol o [3,4-d] py rimi din-1-
y Dethyl)-2-
phenylis oquinolin-1(2H)-one : To a solution of example 10 (1.00 g, 1.98 mmol)
in ethanol
(20 ml), Raney Ni (0.50 g) was added and hydrogeneated at 40psi for 12h. The
reaction
mixture was passed through celiteTM pad and concentrated. The crude product
was column
chromatographed with ethyl acetate: petroleum ether to afford the final
product as a yellow
solid (0.54 g, 57%) which was used as such in next step.
Example 12
N-(5-(4-amino-1-(1-(4-oxo-3-pheny1-3,4-dihydroquinazolin-2-yl)ethyl)-1H-
pyrazolo [3,4-
d] pyrimidin-3-y1)-2-methoxyphenyOmethanesulfonamide
N-(5-(4-amino-1 -(1 -(4-oxo-3 -phenyl-3,4-dihy dro quinazolin-2-y 1)ethyl)-1H-
py razol o [3,4-
d]pyrimidin-3-y1)-2-methoxyphenyl)methanesulfonamide : To a solution of
example 11
(0.260 g, 0.515 mmol) in dichloromethane cooled to 0 C, pyridine (0.08 ml,
1.03 mmol) was
added and stirred for 10 min. Methanesulphonyl chloride (0.039 ml, 0.515 mmol)
was added
stirred for 30 min. The reaction mixture was quenched with water, extracted
with
dichloromethane and dried over sodium sulphate. . The crude product was column
chromatographed with methanol : dichloromethane to afford the title compound
as a yellow
solid (0.080 g, 26%). Mass : 583.0 (Mt).
Example 13
2-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-1-
ypethyl)-6-
fluoro-3-phenylquinazolin-4(3H)-one
2-(1-(4-amino-3-(3-fluoro-4-hy droxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-l-
ypethyl)-6-
fluoro-3-phenylquinazolin-4(3H)-one : The title compound was obtained as
yellow solid
(0.026 g, 14%) by using a procedure that was similar to the one described for
example 7
from example 4 ( 0.200 g, 0.360 mmol), dichloromethane (10.0 ml) and boron
tribromide
(1M in dichloromethane, 2.05 m1). MP: 231-232 C. Mass: 511.8 (Mt).
Example 14
3-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-1-
ypethyl)-8-
chloro-2-phenylisoquinolin-1(2H)-one
69
Date Recue/Date Received 2020-12-07
3 -(1-(4-amino-3-(3 -fluoro-4-hy droxy pheny1)-1H-pyrazol o [3,4-d] py rimi
din-1-y Dethyl)-8-
chloro-2-phenylis oquinolin-1(2H)-one : The title compound was obtained as
yellow solid
(0.032 g, 29%) by using a procedure that was similar to the one described for
example 7
from example 1 ( 0.120 g, 0.210 mmol), dichloromethane (6.0 ml) and boron
tribromide (1M
in dichloromethane, 1.18 m1). MP: 218-219 C. Mass: 526.6 (Mt).
Example 14a and 14b
(+)-3-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-1-
ypethyl)-8-chloro-2-phenylis oquinolin-1(2H)-one
and (+3-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d]
pyrimidin-1-
ypethyl)-8-chloro-2-phenylis oquinolin-1(2H)-one
The two enantiomerically pure isomers were separated by preparative SFC
conditions from 3-
(1-(4-amino-3-(3-fluoro-4-hy droxypheny1)-1H-py razol o [3,4-d] pyrimi din-1 -
ypethyl)-8-
chloro-2-phenylisoquinolin-1(2H)-one (0.500 g) on a CHIRALPAK AS-H column (250
x 20
mm; 5[1m) using methanol: CO2 (45:55) as the mobile phase at a flow rate of
80g / min.
Example 14a: Brown solid (0.349 g). e.e. 97.07%. Rt: 2.51 min. Mass: 527.1(M
). MP:215-
219 C.
Example 14b: Brown solid (0.026 g). e.e. 95.21%. Rt: 3.82 min. Mass: 527.1(M
). MP: 215-
219 C.
Example 15
3-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-1-
ypethyl)-8-
fluoro-2-phenylis o quinolin-1(2H)- one
3 -(1-(4-amino-3-(3 -fluoro-4-hy droxy pheny1)-1H-pyrazol o [3,4-d] py rimi
din-1-y pethyl)-8-
fluoro-2-phenylisoquinolin-1(2H)-one: The title compound was obtained as
yellow solid
(0.066 g, 47%) by using a procedure that was similar to the one described for
example 7
from example 2 ( 0.150 g, 0.271 mmol), dichloromethane (7.5 ml) and boron
tribromide (1M
in dichloromethane, 1.55 m1). MP: 228-229 C. Mass: 511.0 (Mt).
Example 15a and 15b
Date Recue/Date Received 2020-12-07
(+)-3-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-1-
ypethyl)-8-fluoro-2-phenylisoquinolin-1(2H)-one
and (+3-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d]
pyrimidin-1-
yl)ethyl)-8-fluoro-2-phenylisoquinolin-1(2H)-one
The two enantiomerically pure isomers were separated by preparative SFC
conditions from 3-
(1-(4-amino-3-(3-fluoro-4-hy droxypheny1)-1H-py razol o [3,4-d] pyrimi din-1 -
ypethyl)-8-
fluoro-2-phenylisoquinolin-1(2H)-one (0.500 g) on a CHIRALPAK AS-H column (250
x 20
mm; 51.1m) using methanol: CO2 (45:55) as the mobile phase at a flow rate of
80g / min.
Example 15a: Off-white solid (0.480 g). e.e. 100%. Rt: 2.91 min. Mass:
511.1(Mt). MP:
268-270 C.
Example 15b: Off-white solid (0.453 g). e.e. 99.9%. Rt: 5.12 min. Mass:
511.1(Mt). MP:
258-260 C.
Example 16
2-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-1-
ypethyl)-5-
fluoro-3-phenylquinazolin-4(3H)-one
2-(1-(4-amino-3-(3 -fluoro-4-hy droxy pheny1)-1H-pyrazol o [3,4-d] py rimi din-
1-y Dethyl)-5-
fluoro-3-phenylquinazolin-4(3H)-one : The title compound was obtained as brown
solid
(0.050 g, 27%) by using a procedure that was similar to the one described for
example 7
from example 3 ( 0.200 g, 0.360 mmol), dichloromethane (10.0 ml) and boron
tribromide
(1M in dichloromethane, 2.05 m1). MP: 212-214 C. Mass: 512.0 (Mt).
Example 16a and 16b
(+)-2-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-1-
ypethyl)-5-fluoro-3-phenylquinazolin-4(3H)-one
and (+2-(1-(4-amino-3-(3-fluoro-4-hydroxypheny1)-1H-pyrazolo [3,4-d]
pyrimidin-1-
ypethyl)-5-fluoro-3-phenylquinazolin-4(3H)-one
The two enantiomerically pure isomers were separated by preparative SFC
conditions from 2-
(1-(4-amino-3-(3-fluoro-4-hy droxypheny1)-1H-py razol o [3,4-d] pyrimi din-1 -
ypethyl)-5 -
71
Date Recue/Date Received 2020-12-07
fluoro-3-phenylquinazolin-4(3H)-one (0.500 g) on a CHIRALPAK AS-H column (250
x 20
mm; 5 m) using methanol: CO2 (45:55) as the mobile phase at a flow rate of 80g
/ min.
Example 16a: Off-white solid (0.458 g). e.e. 99.88%. Rt: 2.34 min. Mass:
512.2(W).
MP:268-270 C.
Example 16b: Off-white solid (0.469 g). e.e. 99.05%. Rt: 3.92 min. Mass:
512.2(W). MP:
269-271 C.
Example 17
3-(1-(4-amino-3-(4-(difluoromethoxy)-3-fluoropheny1)-1H-pyrazolo [3,4-d]
pyrimi din-1-
ypethyl)-8-chlo ro-2-phenylis oquinolin-1(2H)-one
3 -(1-(4-amino-3-(4-(difluoromethoxy)-3 -fl uoropheny1)-1H-py razol o [3,4-d]
pyrimi din-1-
ypethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one : The title compound was
obtained as off-
white solid (0.020 g, 7%) by using a procedure that was similar to the one
described for
example 1 from 3 -(4-(difluoromethoxy)-3 -fluoropheny1)-1H-pyrazol o [3,4-d]
pyrimi
amine' ( 0.150 g, 0.507 mmol), DMF ( 1.5 ml), potassium carbonate (0.140 g,
1.015 mmol)
and intermediate 2 (0.323 g, 1.015 mmol). MP: 160-162 C. Mass: 576.7 (Mt).
Example 17a and 17b
(+)-3-(1-(4-amino-3-(4-(difluoromethoxy)-3-fluoropheny1)-1H-pyrazolo [3,4-
d] pyrimidin-1-ypethyl)-8-chloro-2-phenylis oquinolin-1(2H)-one
and (+3-(1-(4-amino-3-(4-(difluoromethoxy)-3-fluoropheny1)-1H-pyrazolo [3,4-
d] pyrimidin-1-ypethyl)-8-chloro-2-phenylis oquinolin-1(2H)-one
The two enantiomerically pure isomers were separated by preparative SFC
conditions from 3-
(1-(4-amino-3-(4-(difluoromethoxy)-3-fluoropheny1)-1H-py razol o [3,4-d]
pyrimi din-1-
ypethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one (0.500 g) on a CHIRALPAK AS-H
column (250 x 20 mm; 5vim) using methanol: CO2 (50:50) as the mobile phase at
a flow rate
of 70g / min.
Example 17a: Browne solid (0.500 g). e.e. 99.25%. Rt: 2.35 min. Mass:
577.2(W).
MP:180-184 C.
72
Date Recue/Date Received 2020-12-07
Example 17b: Brown solid (0.422 g). e.e. 100%. Rt: 3.87 min. Mass: 577.2(W).
MP: 181-
185 C.
Example 18
3-(1-(4-amino-3-(4-methoxy-3-nitropheny1)-1H-pyrazolo13,4-d]pyrimidin-1-
ypethyl)-8-
chloro-2-phenylisoquinolin-1(2H)-one
3 -(1-(4-amino-3-(4-methoxy-3-nitropheny1)-1H-py razol o [3,4-d] py ri mi din-
1-y pethyl)-8-
chloro-2-phenylis oquinolin-1(2H)-one: The title compound was obtained as
brown solid
(0.270 g, 54%) by using a procedure that was similar to the one described for
example 1
from 3-(4-methoxy-3-nitropheny1)-1H-pyrazolo[3,4-dlpyrimidin-4-amine ( 0.250
g, 0.876
mmol), DMF ( 2.5 ml), potassium carbonate (0.241 g, 1.75 mmol) and
intermediate 2 (0.557
g, 1.75 mmol) Which was used as such in next step.
Example 19
3-(1-(4-amino-3-(3-amino-4-methoxypheny1)-1H-pyrazolo [3,4-d] pyrimidin-1-
ypethyl)-8-
chloro-2-phenylisoquinolin-1(2H)-one
3 -(1-(4-amino-3-(3 -amino-4-methoxypheny1)-1H-pyrazol o [3,4-d] py rimi din-1-
y pethyl)-8-
chloro-2-phenylis oquinolin-1(2H)-one : The title compound was obtained as
brown solid
(0.150 g, 58%) by using a procedure that was similar to the one described for
example 11
from example 18 (0.270 g, 0.476 mmol), ethanol (5 ml), Raney Ni (0.135 g)
which was used
as such in next step.
Example 20
N-(5-(4-amino-1-(1-(8-chloro-1-oxo-2-pheny1-1,2-dihyd roisoquinolin-3-ypethyl)-
1H-
pyrazolo [3,4-d] pyrimidin-3-y1)-2-methoxyphenyl)methanesulfonamide
N-(5-(4-amino-1 -(1 -(8-chl oro-l-oxo-2-pheny1-1,2-dihy droisoquinolin-3-
ypethyl)-1H-
pyrazolo [3,4-d] py rimi din-3 -y1)-2-methoxy pheny pmethanesulfonami de : The
title compound
was obtained as off-white solid (0.020 g, 13%) by using a procedure that was
similar to the
one described for example 12 from example 19 (0.135 g, 0.251 mmol), pyridine
(0.10 ml,
0.484 mmol), dichloromethane (2 ml) and methanesulphonyl chloride (0.10 ml,
0.252
mmol).Mass: 616.0 (M+).
73
Date Recue/Date Received 2020-12-07
Example 20a and 20b
(+)-N-(5-(4-amino-1-(1-(8-chloro-1-oxo-2-pheny1-1,2-dihydroisoquinolin-3-
ypethyl)-1H-
pyrazolo [3,4-d] pyrimidin-3-y1)-2-methoxyphenyl)methanesulfonamide
and (-)-N-(5-(4-amino-1-(1-(8-chloro-1-oxo-2-pheny1-1,2-dihydroisoquinolin-3-
ypethyl)-1H-pyrazolo [3,4-d] pyrimidin-3-y1)-2-
methoxyphenyl)methanesulfonamide
The two enantiomerically pure isomers were separated by preparative SFC
conditions from
N-(5-(4-amino-1 -(1 -(8-chl oro-l-oxo-2-pheny1-1,2-dihy droi s o quinolin-3-y
pethyl)-1H-
pyrazolo[3,4-d]pyrimidin-3-y1)-2-methoxyphenyl)methanesulfonamide (0.500 g) on
a
CHIRALPAK AS-H column (250 x 20 mm; 5i.tm) using methanol : CO2 (45:55) as the
mobile phase at a flow rate of 80g / min.
Example 20a: Brown solid (0.397 g). e.e. 98.54%. Rt: 2.26 min. Mass: 616.2(W).
MP:178-
181 C.
Example 20b: Brown solid (0.252 g). e.e. 98.77%. Rt: 3.18 min. Mass: 616.2(W).
MP: 180-
183 C.
Example 21
3-(1-(4-amino-3-(3-methy1-1H-ind azol-6-y1)-1H-pyrazolo [3,4-d] pyrimidin-1-
ypethyl)-8-
chloro-2-phenylisoquinolin-1(2H)-one
3-(1-(4-amino-3-(3-methy1-1H-indazol-6-y1)-1H-pyrazolo [3,4-d] pyrimidin-1 -
ypethyl)-8-
chloro-2-phenylis oquinolin-1(2H)-one : The title compound was obtained as
brown solid
(0.020 g, 10 %) by using a procedure that was similar to the one described for
example 9
from intermediate 13 (0.200 g, 0.368 mmol ), DME (2.5 ml), water (1.0 ml),
tert-butyl 3-
methy1-6-(4,4,5,5-tetramethy1-1,3,2-dioxaborolan-2-y1)-1H-indazole-1-
carboxylate (0.198 g,
0.552 mmol) , sodium carbonate (0.078 g, 0.736 mmol ) and
bis(diphenylphosphino)ferroceneldichloropalladium(II) (0.060 g, 0.073 mmol )
under
microwave irradiation (microwave power = 100W, temperature = 100 C) for 45
min. MP:
289-291 C. Mass : 546.9 (Mt).
Example 21a and 21b
74
Date Recue/Date Received 2020-12-07
(+)-3-(1-(4-amino-3-(3-methy1-1H-indazol-6-y1)-1H-pyrazolo13,4-d]pyrimidin-1-
ypethyl)-8-chloro-2-phenylisoquinolin-1(2H)-one
and (-)-3-(1-(4-amin o-3-(3-methy1-1H-ind azol-6-y1)-1H-pyrazolo [3,4-d]
pyrimidin-1-
ypethyl)-8-chloro-2-phenylis oquinolin-1(2H)-one
The two enantiomerically pure isomers were separated by preparative SFC
conditions from
3-(1-(4-amino-3-(3-methy1-1H-indazol-6-y1)-1H-pyrazolo [3,4-d] pyrimidin-1 -
ypethyl)-8-
chloro-2-phenylis oquinolin-1(2H)-one (0.500 g) on a CHIRALPAK AS-H column
(250 x 20
mm; 51.1m) using methanol: CO2 (35:65) as the mobile phase at a flow rate of
80g / min.
Example 21a: Brown solid (0.327 g). e.e. 98.29%. Rt: 4.26 min. Mass: 547.2(W).
MP:230-
233 C.
Example 21b: Brown solid (0.599 g). e.e. 95.03%. Rt: 5.44 min. Mass: 547.2(W).
MP: 225-
227 C.
Example 22
2-{ 1-14-Amin o-3-(3-fluo ro-4-is op ro p oxy-pheny1)-pyrazol o [3,4-d]
pyrimidin-l-y1]-
propy1}-3-pheny1-3H-quinazolin-4-one
2-(1-(4-amino-3-(3-fluoro-4-isopropoxypheny1)-1H-pyrazolo13,4-d] pyrimi din-1 -
yl)propy1)-3-
phenylquinazolin-4(3H)-one: To a solution of 3-(3-fluoro-4-isopropoxypheny1)-
1H-
pyrazolo[3,4-d]pyrimidin-4-aminel ( 0.115 g, 0.402 mmol) in DMF ( 2 ml),
potassium
carbonate (0.084 g, 0.604 mmol) was added and stirred at room temperature for
30min. The
intermediate 15 (0.180 g, 0.604 mmol) was added and stirred at 90 C for 12h.
The reaction
mixture was quenched with water and the precipitate formed was filtered and
dried under
vacuum. The crude product was purified by column chromatography with methanol:
dichloromethane ether to afford the title compound as brown solid (0.070 g,
17%). MP: 261-
263 C. Mass: 550.6 (M++1).
BIOLOGICAL ASSAY
[143] The pharmacological properties of the compounds of this invention may be
confirmed
by a number of pharmacological assays. The pharmacological assays which can be
been
Date Recue/Date Received 2020-12-07
carried out with the compounds according to the invention and/or their
pharmaceutically
acceptable salts is exemplified below.
[144] Assay 1: Fluorescent determination of PI3Kinase enzyme activity
Phosphoinositide 3 kinases (PI3K) belong to a class of lipid kinases that play
a critical role in
the regulation of several key cellular processes. The PI3K are capable of
phosphorylating the
3-hydroxy position of phosphoinositols thereby generating second messengers
involved in
downstream signalling events. The homogenous time resolved fluorescence (HTRF)
assay
allows detection of 3,4,5-triphosphate (PIP3) formed as a result of
phosphorylation of
phosphotidylinositol 4,5-biphosphate (PIP2) by PI3K isoforms such as a, (3, y
or 6.
PI3K isoform activity for a, (3, y or 6 is to be determined using a PI3K human
HTRFTm Assay
Kit (Millipore, Billerica, MA) with modifications. All incubations were
carried out at room
temperature. Briefly, 0.5 1 of 40X inhibitor (in 100% DMSO) or 100% DMSO were
added
to each well of a 384-well black plate (Greiner Bio-One, Monroe, NC)
containing 14.5 ill lx
reaction buffer /PIP2 (10 mM MgCl2, 5 mM DTT, 1.38 uM PIP2) mix with or
without
enzyme and incubated for 10 min. After the initial incubation, 5 ttl/well of
400 tIM ATP was
added and incubated for an additional 30 minutes. Reaction was terminated by
adding 5
ill/well stop solution (Millipore, Billerica, MA). Five microliters of
detection mix (Millipore,
Billerica, MA) were then added to each well and was incubated for 6-18 h in
the dark. HRTF
ratio was measured on a microplate reader (BMG Labtech., Germany) at an
excitation
wavelength of 337 nm and emission wavelengths of 665 and 620 nm with an
integration time
of 400 pec. Data was analyzed using Graphpad Prism (Graphpad software; San
Diego CA)
for IC50 determination.). The % inhibition for PI3K isoforms as a, (3, y or 6
for the compounds
of the invention are as provided below.
Example % Inhibition @
P1310 PI3ky PI3ka PI3143
100/300 nM IC50 100/300 nM IC50 1 uM 1 uM
1 El El
2 El E2
3 El E2
4 El
A El E2
5a A 3.05 B 22.18 El
5b El
6 A El El
6a A 2.74 C 15.01 El
6b El
76
Date Recue/Date Received 2020-12-07
7 C - D E2 D
7a B 13.33 B 57.73 El D
7b C - D El D
8 D - E - El D
9 C - D D C
9a A 16.22 C 203.3 El D
9b C - E El E2
12 E - D - El D
13 D - D - El E2
14 B - A - D B
14a A 4.2 A 19.54 El E2
14b B - D El D
15 C - C El D
15a A 9.99 C 52.04 El E2
15b D - E El El
16 C - C El B
16a A 33.26 D 121.6 El E2
16b D - E El El
17 A - A - D C
17a D - E - El El
17b A 45.61 A 47.26 El D
20 A B C D
20a B 76.41 D 141.6 El E2
20b D - E El D
21 A - B - D B
21a A - B - El El
21b A - C El E2
underlined values are for compounds tested at 300 nM.;% inhibition: A
represents >80-<100%; B represents
>60-<80%; C represents >40-<60%; D is represents >20-<40%, E is represents 0-
<20%, E2 is represents
>10-<20%, and El is represents 0-<10%.
[145] Although the invention herein has been described with reference to
particular
embodiments, it is to be understood that these embodiments are merely
illustrative of the
principles and applications of the present invention. It is therefore to be
understood that
numerous modifications may be made to the illustrative embodiments and that
other
arrangements may be devised without departing from the spirit and scope of the
present
invention as described above. It is intended that the appended claims define
the scope of the
invention and that methods and structures within the scope of these claims and
their
equivalents be covered thereby.
77
Date Recue/Date Received 2020-12-07