Note: Descriptions are shown in the official language in which they were submitted.
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
USE OF NK-1 RECEPTOR ANTAGONIST SERLOPITANT IN PRURITUS
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] The present application claims priority to and the benefit of US Patent
Application No.
13/925,509 and US Provisional Patent Application No. 61/838,784, both filed on
June 24, 2013.
TECHNICAL FIELD
[0002] The invention relates to methods for treating acute or chronic pruritus
with an NK-1 receptor
antagonist. The invention further relates to pharmaceutical compositions
comprising an NK-1 receptor
antagonist.
BACKGROUND OF THE INVENTION
[0003] Pruritus, or itch, is an uncomfortable skin sensation that provokes a
desire to scratch. Although
itch may be acute, for example, from an insect sting, chronic pruritus
originates from many different
causes. It is a seriously debilitating condition, comparable to chronic pain,
which negatively impacts
quality of life.
[0004] Chronic pruritus affects millions of people worldwide, although solid
epidemiological data is very
limited. For example, one study reported that 8-10% of the population of Oslo
suffer from chronic
pruritus from all causes (F. Dalgard etal., J. Investig. DermatoL Symp. Proc.,
2004, 9(2):120-5).
Patients with certain diseases and conditions report high incidences of
chronic itch, including those with
psoriasis (78-84%), Hodgkin's disease (25-35%), dialysis patients (22%), and
polycythaemica vera
(48%) (M. Metz and S. Stander, CME DermatoL, 2008; 3(3):124-143). Chronic
pruritus is also a
prevalent symptom in cutaneous T-cell lymphoma (68-93%), a disease that
includes mycosis fungoides
and Sezary syndrome (N. Meyer etal., Acta Derm. VenereoL, 2010, 90:12-17).
Pruritus is the most
common dermatological complaint in elderly patients (S. Beauregard and B. A.
Gilchrest, Arch.
DermatoL, 1987, 123:1638-43). Itch is often the side effect of certain drugs,
such as EGF receptor
antagonists.
[0006] Antihistamines can sometimes effectively treat itch due to acute
urticaria, but many chronic
pruritic diseases respond poorly to conventional H1 receptor antagonists (Tey
H.L. and G. Yosipovitch;
Br. J. Dermatol., 2011, 166(1):5-17). In addition to marginal efficacy,
antihistamines can also cause
intolerable drowsiness. Other current therapies possess various limitations.
For example,
anticonvulsants such as gabapentin inhibit spinal mechanisms in the perception
of itch, but their use is
limited due to their slow onset of action (5-6 weeks) (Metz and Stander,
2008). Opiate receptor
1
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
antagonists such as naloxone, nalmefene, and naltrexone decreased pruritus
symptoms in patients
with liver and kidney disease, although significant central nervous and
gastrointestinal side effects
occurred (Metz and Stander, 2008; N. V. Bergasa et al., Hepatology, 2006,
44(5):1317-23).
[0006] Substance P, the endogenous ligand for the neurokinin-1 (NK-1)
receptor, is a significant
mediator of pruritus (T. Andoh et al., J. Pharmacol. Exp. Ther,1998, 286:1140-
5). Intradermal injection
of substance P elicits an itch sensation in human subjects, and an associated
itch response in mice.
The substance P-induced itch¨associated response in mice is not inhibited by
antihistamines (B.
Amatya et al., Skin PharmacoL PhysioL, 2010; 23:133-138; C. Weidner at al., J.
Invest. Dermatol.,
2000, 115:1015-1020). In an experiment designed to study the role of substance
P in pruritus, Ohmura
et al. reported that tachykinin NK-1 receptor antagonist, BlIF 1149 CL,
inhibited scratching behavior in a
picrylchloride-induced dermatitis model in NC/Nga mice (Eur. J. PhannacoL,
2004, 491:191-194; U.S.
Patent Application No. 2003/100565).
[0007] Aprepitant (Emend ), an NK-1 receptor antagonist, is approved by the
FDA for use in the
prevention of chemically induced nausea and vomiting (emesis) after
chemotherapy. Duval and
Dubertret first reported that oral aprepitant (80 mg daily) had utility in
treating pruritus in three patients
with Sezary syndrome (N. Engl. J. Med., 2009, 361(14):1415-6). Torres etal.
disclosed similar results
(J. Am. Acad. DermatoL, 2012; 66(1):e14-5). Stander et al. conducted a small,
open-label study which
demonstrated that aprepitant significantly decreased chronic pruritus caused
by conditions such as
atopic diathesis and prurigo nodularis. In this study, twenty previously
untreatable patients were given
a daily dose of 80 mg for 3 to 13 days. Eighty percent of the patients
experienced a considerable
reduction in itch intensity (S. Stander, et al., PLoS One, 2010, 5:6, e10968).
However, Wallengren
conducted a follow-up double-blind study based on Stander's work testing a
single dose of topical
aprepitant blended at a 5% concentration in a lipophilic vehicle in patients
suffering from chronic
pruritus of various etiologies. Although the drug was absorbed into the skin,
the patients' itch was not
alleviated (J. Wallengren, Arch. Dermatol., 2012, 148(8):957-9).
[0008] Although oral aprepitant is generally well-tolerated, it is extremely
expensive, limiting its use in
chronic pruritus (Tey, 2011). Further, aprepitant is a moderate inhibitor as
well as an inducer of
CYP3A4 and CYP2C9, indicating that drug-drug interactions with
chemotherapeutic agents and
corticosteroids must be considered (Torres, 2012). Mir and Coriat have
suggested that the risk of drug-
drug interactions with aprepitant is high because it can alter the activity of
cytochrome P450 3A4
isoform (CYP-3A4), an enzyme involved in the metabolism of a range of commonly
prescribed drugs,
including tyrosine-kinase inhibitors, either inducing or inhibiting the CYP-
3A4, depending on which
drugs are given concomitantly. Tyrosine-kinase inhibitors do not induce
frequent nausea and emesis;
2
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
therefore, clinical experience with concomitant administration of aprepitant
and these drugs is scarce.
Furthermore, the pharmacokinetics of tyrosine-kinase inhibitors varies widely
between patients, and
drug-drug interactions are common (O. Mir and R. Coriat, The Lancet, 2012,
13:964-965). Thus, the
need for additional, safe treatments for acute and chronic pruritus exists.
SUMMARY OF THE INVENTION
[0009] In one aspect, this invention provides a method of treating pruritus in
a patient in need of such
treatment comprising administering to said patient a therapeutically effective
amount of 3-
[(3aR,4R,5S,7aS)-5-R1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]-4-(4-
fluorophenyl)-1,3,3a,4,5,6,7,7a-
octahydroisoindo1-2-yl]cyclopent-2-en-1-one or a pharmaceutically acceptable
salt, solvate or
polymorph thereof. In one embodiment, the therapeutically effective amount
comprises a dosage of
0.10 mg, 0.15 mg, 0.20 mg, 0.25 mg, 0.5 mg, 0.75 mg, 1 mg, 2 mg, 2.5 mg, 3 mg,
4 mg, 5 mg, 6 mg, 7
mg, 8 mg, 9 mg, 10 mg, 15 mg, 20 mg, 25 mg, or 30 mg one or more times a day.
In another
embodiment, the therapeutically effective amount comprises a dosage of 0.25
mg, 1 mg, or 5 mg once
a day. In a further embodiment, the therapeutically effective amount comprises
a dosage of from about
0.1 mg to about 30 mg or from about 1 mg to about 7.5 mg. In another
embodiment, the therapeutically
effective amount is administered orally in the form of a tablet. In a further
embodiment, the
therapeutically effective amount is administered once a day at bedtime. In
another embodiment, the
therapeutically effective amount is administered once a day, once every other
day, once every third
day, once every fourth day, or once a week. In other embodiments, serlopitant
is administered under a
chronic dosing regimen. In some embodiments, a therapeutically effective
amount of serlopitant is
administered over a period of at least 2 weeks, 3 weeks, 1 month, 1.5 months,
2 months, 3 months,
4 months, 5 months, 6 months or longer.
[0010] In another aspect, this invention provides a method of treating
pruritus whereby 3-
[(3aR,4R,5S,7aS)-5-[(1R)-1-[3,5-bis(trifluoromethyl)phenyflethoxyl-4-(4-
fluoropheny1)-1,3,3a,4,5,6,7,7a-
octahydroisoindo1-2-yljcyclopent-2-en-1-one (serlopitant) or a
pharmaceutically acceptable salt, solvate
or polymorph thereof is administered to a patient in need of such treatment
according to a schedule,
wherein a least one loading dose is first administered, and, second, at least
one therapeutically effect
maintenance dose is administered. In one embodiment, the loading dose is five
times, four times, three
times, or two times the maintenance dose. In another embodiment, the loading
dose is three times the
maintenance dose. In a further embodiment, the loading dose is administered on
day 1 and the
maintenance dose is administered on day 2 and thereafter. In another
embodiment, the loading dose
and the maintenance dose are administered at bedtime. In another embodiment,
the method further
3
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
comprises administering a second loading dose prior to administering the
maintenance dose. In one
embodiment, the loading dose is three times the maintenance dose and the
second loading dose is two
times the maintenance dose. In a further embodiment, the therapeutically
effective maintenance dose
is 0.10 mg, 0.15 mg, 0.20 mg, 0.25 mg, 0.5 mg, 0.75 mg, 1 mg, 2 mg, 2.5 mg, 3
mg, 4 mg, 5 mg, 6 mg,
7 mg, 8 mg, 9 mg, 10 mg, 15 mg, 20 mg, 25 mg, or 30 mg administered one or
more times a day. In
another embodiment, the therapeutically effective maintenance dose comprises a
dosage of 0.25 mg, 1
mg, or 5 mg administered once a day. In a further embodiment, the
therapeutically effective
maintenance dose comprises a dosage from about 0.1 mg to about 30 mg or from
about 1 mg to about
7.5 mg. In another embodiment, the therapeutically effective maintenance dose
is administered once a
day, once every other day, once every third day, once every fourth day, or
once a week. In other
embodiments, serlopitant is administered under a chronic dosing regimen. In
some embodiments, a
therapeutically effective maintenance dose of serlopitant is administered over
a period of at least 2
weeks, 3 weeks, 1 month, 1.5 months, 2 months, 3 months, 4 months, 5 months, 6
months or longer.
In certain embodiments, serlopitant is administered orally.
[0011] In one aspect, this invention provides a pharmaceutical composition for
the treatment of pruritus
comprising 3-[(3aR,4R,5S,7aS)-5-[(1R)-143,5-bis(trif1uoromethyl)phenyl]ethoxy]-
4-(4-fluoropheny1)-
1,3,3a,4,5,8,7,7a-octahydroisoindol-2-yl]cyclopent-2-en-1-one or a
pharmaceutically acceptable salt,
solvate or polymorph thereof and a pharmaceutically acceptable carrier. In one
embodiment, the
pharmaceutical composition is formulated as a tablet comprising Compound 1 or
a pharmaceutically
acceptable salt, solvate or polymorph thereof and one or more diluents,
disintegrants, surfactants or
lubricants. In another embodiment, the composition comprises a capsule filled
with a solution
comprising Compound 1 or a pharmaceutically acceptable salt, solvate or
polymorph thereof and an
amphiphilic agent. In a further embodiment, the amphiphilic agent is a fatty
acid ester of glycerol,
propylene glycol or sorbitol. In another embodiment, the pharmaceutical
composition comprises 0.10
mg, 0.15 mg, 0.20 mg, 0.25 mg, 0.5 mg, 0.75 mg, 1 mg, 2 mg, 2.5 mg, 3 mg, 4
mg, 5 mg, 6 mg, 7 mg,
8 mg, 9 mg, 10 mg, 15 mg, 20 mg, 25 mg, or 30 mg of Compound 1 or a
pharmaceutically acceptable
salt, solvate or polymorph thereof. In another embodiment, the composition
comprises 0.25 mg, 1 mg,
or 5 mg of Compound 1 or a pharmaceutically acceptable salt, solvate or
polymorph thereof.
[0012] In another aspect, this invention provides a method of treating acute
or chronic pruritus in a
patient in need of such treatment comprising administering to said patient a
therapeutically effective
amount of a pharmaceutical composition comprising 3-[(3aR,4R,5S,7aS)-5-[(1R)-
143,5-
bis(trifluoromethyl)phenyl]ethoxy]-4-(4-fluorophenyl)-1,3,3a,4,5,6,7,7a-
octahydroisoindol-2-yl]cyclopent-
2-en-1-one or a pharmaceutically acceptable salt, solvate or polymorph thereof
and a pharmaceutically
4
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
acceptable carrier. In one embodiment, the method involves treatment with a
pharmaceutical
composition formulated as a tablet comprising Compound 1 or a pharmaceutically
acceptable salt,
solvate or polymorph thereof and one or more diluents, disintegrants,
surfactants or lubricants. In
another embodiment, the method involves administration of a composition
comprising a capsule filled
with a solution comprising Compound 1 or a pharmaceutically acceptable salt,
solvate or polymorph
thereof and an amphiphilic agent. In a further embodiment, the amphiphilic
agent is a fatty acid ester of
glycerol, propylene glycol or sorbitol. In another embodiment, the method
involves treatment with a
pharmaceutical composition comprising 0.10 mg, 0.15 mg, 0.20 mg, 0.25 mg, 0.5
mg, 0.75 mg, 1 mg, 2
mg, 2.5 mg, 3 mg, 4 mg, 5 mg, 6 mg, 7 mg, 8 mg, 9 mg, 10 mg, 15 mg, 20 mg, 25
mg, or 30 mg of
Compound 1 or a pharmaceutically acceptable salt, solvate or polymorph
thereof. In another
embodiment, the composition comprises 0.25 mg, 1 mg, or 5 mg of Compound 1 or
a pharmaceutically
acceptable salt, solvate or polymorph thereof.
[0013] In a further embodiment, a pruritus-associated condition is treated by
administration of
serlopitant (Compound 1) and an additional antipruritic agent. In a still
further embodiment, a sleep
problem or disorder is treated by administration of serlopitant, optionally in
combination with an
additional sleep-aiding agent.
[0014] Other objects of the invention may be apparent to one skilled in the
art upon reading the
following specification and claims.
BRIEF DESCRIPTION OF THE DRAWINGS
[0015] The novel features of the invention are set forth with particularity in
the appended claims. A
better understanding of the features and advantages of the present invention
will be obtained by
reference to the following detailed description that sets forth illustrative
embodiments, in which the
principles of the invention are utilized, and the accompanying drawings of
which:
[0016] FIG.1 depicts a synthetic scheme for serlopitant, Compound 1.
[0017] FIG. 2 illustrates a Franz diffusion cell for studying skin permeation
of a drug in vitro.
[0018] FIG. 3 shows the cumulative release of serlopitant from topical
formulations B and C into the
receptor chamber of a Franz diffusion cell at various time points in an in
vitro study of skin permeation.
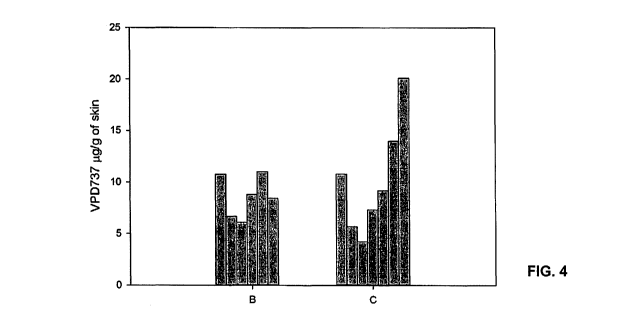
[0019] FIG. 4 shows the amount of serlopitant (called "VPD737") retained in
the skin at the end of the
Franz diffusion cell study. Each bar represents ug of serlopitant/g of skin in
250 um skin layers. For
each of topical formulations B and C, the bars from left to right represent
the amount of serlopitant
retained in skin layers from the stratum comeum to the dermis.
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
DETAILED DESCRIPTION OF THE INVENTION
[0020] Unless defined otherwise, all technical and scientific terms used
herein have the same meaning
as commonly understood by one of ordinary skill in the art to which this
application belongs. It must be
noted that as used herein and in the appended claims, the singular forms "a",
"and", and "the" include
plural referents unless the context clearly dictates otherwise.
[0021] Reference will now be made in detail to certain preferred methods of
treatment, compounds and
methods of administering these compounds. The invention is not limited to
those preferred compounds
and methods, but rather is defined by the claim(s) issuing herefrom.
Introduction
[0022] Serlopitant is a neurokinin-1 (NK-1) receptor antagonist. The present
invention provides a
method for treating chronic pruritus and related conditions using serlopitant
or a pharmaceutically
acceptable salt or hydrate thereof. Chemically, the generic name serlopitant
refers to the compound of
Compound 1:
CF3
N
cH3 1111
0
Compound 1
The I.U.P.A.C. name for the compound is 3-[(3aR,4R,5S,7aS)-5-[(1R)-143,5-
bis(trifluoromethyl)phenyllethoxy]-4-(4-fluoropheny1)-1,3,3a,4,5,6,7,7a-
octahydroisoindol-2-yllcyclopent-
2-en-1-one. Alternatively, Compound 1 may be named 3-[(3aR,4R,5S,7aS)-5-{(1R)-
143,5-
bis(trifluoromethyl)phenyl]ethoxy}-4-(4-fluorophenypoctahydro-2H-isoindol-2-
yl]cyclopent-2-en-1-one.
For purposes of the present invention, it is understood that any of these
designations for Compound 1
may be interchangeably used and have the same meaning. It is further
understood that the invention
also encompasses the racemic form of serlopitant (Compound 1).
[0023] Serlopitant has previously been disclosed as a neurokinin-1 (NK-1)
receptor antagonist, an
inhibitor of tachykinin and, in particular, of substance P (J. Jiang, etal.,
J. Med. Chem., 2009, 52:3039-
3046)). Neurokinin receptors are part of the larger family of G-protein
coupled receptors that elicit
6
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
many of their effects via activation of the inositol phosphate signal
transduction pathway. NK-1
receptors are present in both the central and peripheral nervous system and in
vascular endothelial
cells, muscle and cells of the immune system. Compound 11$ unusually selective
(>39,000 fold) for
the cloned human NK-1 receptor over the cloned human NK-2 and NK-3 receptors,
as demonstrated
using Chinese hamster ovary cells stably expressing the respective receptors
(Jiang et aL,2009). Jiang
et al. showed that serlopitant binds to the human NK-1 receptor with a Kd of
46 pM and that it displaces
substance P binding at the same receptor with an IC50 of 61 pM.
[0024] Compound 1 is a weak reversible inhibitor of human CYP-3A4, 2C8, 2C9,
2C19, 2D6, and 1A2
enzymes, the IC50 values of which are 39, 58, 30, 29, 35, and >100 IAA,
respectively. Serlopitant did
not significantly induce CYP-3A4 mRNA in three individual preparations of
human hepatocytes. These
data suggest that serlopitant will have minimal drug-drug interaction
liability in humans and that any
drug-drug interactions will be reduced in comparison with other NK-1 receptor
antagonists. Although
broad-based counter-screening of serlopitant in more than 145 assays
identified a number of weak
activities between 1 and 10 pM, no assays for which IC50 <11.1M were observed.
Therefore, off-target
activities were more than 20000-fold less potent than hNK-1 activity (Jiang et
aL, 2009).
[0026] It has been suggested serlopitant and its analogs would be useful in
the prevention and
treatment of a variety of clinical conditions characterized by the presence of
an excess of tachykinin, in
particular substance P, activity. Serlopitant has been disclosed as a
treatment for emesis and for
urinary incontinence (U.S. Patent Nos. US 7,217,731, US 7,345,083, US
7,544,815, US 7,645,790, and
US 7,893,091, the disclosures of which are herein incorporated by reference;
U.S. Published
Application Nos. US 2009/0270477, US 2010/0113469, and US 2010/0209496, the
disclosures of
which are herein incorporated by reference; and PCT Publication WO
2007/146224, the disclosure of
which is herein incorporated by reference).
[0026] The safety and tolerability of serlopitant have been evaluated in
several human clinical trials for
the treatment or prevention of with overactive bladder (OAB). In one
investigation, a total of 557
patients with OAB were randomized into this double-blind, placebo-controlled
and active- controlled
(tolterodine), dose-ranging study. Serlopitant at 0.25 and 4 mg daily
significantly reduced the number
of daily nnicturitions compared with placebo. There were no drug-related
serious adverse experiences
and the drug was generally well tolerated. However, serlopitant did not show a
dose response
relationship with micturition frequency, and did not significantly influence
the secondary efficacy end
points of urinary urgency, urge incontinence and total incontinence.
Tolterodine was numerically more
effective than serlopitant at all efficacy end points and statistically
significantly more effective than
placebo. Serlopitant was not associated with the adverse experience of dry
mouth common in patients
receiving tolterodine, a muscarinic antagonist. (See: Frenki, T. L. et al., J.
Urology, 2009, 181(4),
7
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
Suppl. S, p. 676; Frenkl, T. L. etal., NeurouroL Uroclyn., 2009, 28(2)143-144;
Frenkl, T. L. et al.,
European Urology Supplements, 2009, 8(4):134; Frenkl, Tara L, etal., J.
Urology, 2010, 184(2):616-
622.)
Chemical Description of Serlopitant
[0027] The term "pharmaceutically acceptable salts" refers to salts prepared
from pharmaceutically
acceptable non-toxic bases or acids including inorganic or organic bases and
inorganic or organic
acids. Salts derived from inorganic bases include aluminum, ammonium, calcium,
copper, ferric,
ferrous, lithium, magnesium, manganic salts, manganous, potassium, sodium,
zinc, and the like.
Particularly preferred are the ammonium, calcium, magnesium, potassium, and
sodium salts. Salts in
the solid form may exist in more than one crystal structure, and may also be
in the form of hydrates.
Salts derived from pharmaceutically acceptable organic non-toxic bases include
salts of primary,
secondary, and tertiary amines, substituted amines including naturally
occurring substituted amines,
cyclic amines, and basic ion exchange resins, such as arginine, betaine,
caffeine, choline, N,Nt-
dibenzylethylene-diamine, diethylamine, 2-diethylaminoethanol, 2-dimethylamino-
ethanol,
ethanolamine, ethylenediamine, N-ethyl-morpholine, N-ethylpiperidine,
glucamine, glucosamine,
histidine, hydrabamine, isopropylamine, lysine, methylglucamine, morpholine,
piperazine, piperidine,
polyamine resins, procaine, purines, theobromine, triethylamine,
trimethylamine, tripropylamine,
tromethamine, and the like. When the compound of the present invention is
basic, salts may be
prepared from pharmaceutically acceptable non-toxic acids, including inorganic
and organic acids.
Such acids include acetic, benzenesulfonic, benzoic, cam phorsulfonic, citric,
ethanesulfonic, fumaric,
gluconic, glutamic, hydrobromic, hydrochloric, isethionic, lactic, maleic,
malic, mandelic, ethanesulfonic,
mucic, nitric, pannoic, pantothenic, phosphoric, succinic, sulfuric, tartaric,
p-toluenesulfonic acid, and the
like. Particularly preferred are citric, hydrobromic, hydrochloric, maleic,
phosphoric, sulfuric, fumaric,
and tartaric acids. It will be understood that, as used herein, references to
the compounds of the
present invention are meant to also include the pharmaceutically acceptable
salts.
[0028] The term "solvate" refers to an aggregate that consists of a solute ion
or molecule with one or
more solvent molecules. "Solvates" include hydrates, that is, aggregates of a
compound of interest with
water. It will be understood that, as used herein, references to the compounds
of the present invention
are meant to also include the solvates.
[0029] The term "polymorph" refers to a crystalline form of a compound that
can crystallize in different
forms. The invention also encompasses polymorphs of serlopitant. Examples of
polymorphs of
serlopitant include without limitation anhydrous crystalline Forms I and II of
free base serlopitant as
8
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
disclosed in US Pat. App. Pub. No. 2009/0270477 to Kuethe et al. Form I is
characterized by diffraction
peaks obtained from X-ray powder diffraction pattern corresponding to d-
spacings of 10.4, 9.9, 9.2, 5.5,
5.0, 4.1, 3.9, 3.6 and 3.5 angstroms. Form 11 is characterized by diffraction
peaks obtained from X-ray
powder diffraction pattern corresponding to d-spacings of 7.7, 5.3, 4.9, 4.8,
4.6, 4.2, 3.9, 3.8 and 2.8
angstroms. US 2009/0270477 is incorporated herein by reference in its
entirety.
[0030] Chemical Synthesis. Serlopitant may be prepared as described by Jiang
et al. (J. Med. Chem.
2009, 52:3039-3046), which is herein incorporated by reference in its
entirety. Alternatively, the
method of Kuethe et al., as described in U.S. Patent No. 7,544,815, or Bunda
et aL, as described in
U.S. Patent No. 7,217,731, both of which are herein incorporated by reference
in their entirety, may be
used.
[0031] The method of Kuethe of al. is depicted in Figure 1. Briefly,
commercially available 4-
fluorophenylacetic acid (1) (Sigma-Aldrich Co. LLC, St. Louis, MO) is reacted
with thionyl chloride in
DMF/toluene to yield acid chloride (1). The acid chloride (1) is then reacted
with the hydrochloride salt
of the Weinreb amine (CH3NHOCH3.1-1C1) in the presence of sodium hydroxide to
give 2-(4-
fluoropheny1)-N-methoxy-N-methylacetamide (4). A vinyl Grignard reaction
converts (4) to 1-(4-
fluorophenyl)but-3-en-2-one (I). TES dienyl ether (6) is produced from the
reaction of ( ) with
chlorotriethylsilane (TESCI) in the presence of iPr2NEt2.
[0032] Commercially available fumaryl chloride and two equivalents of (-)-
menthol (both Sigma-Aldrich)
are reacted to yield di+)-menthylfumarate (Z). A DieIs-Alder reaction between
(g) and (Z) produces
(g). Any E-isomer of the diene (<5%) that is present does not react in the
DieIs-Alder reaction.
Deprotection and epimerization of ( ) in acid gives W. The desilylation of (q)
initially gave a mixture of
2,3-cis- and 2,3-trans-ketones, which, driven by crystallization of desired
(2), isomerized to the
predominantly trans compound. Reduction of (2) with lithium tri-t-butoxy
aluminum hydride (Li(t-
Bu0)3A1H), followed by lithium aluminum hydride (LAIN, produces trio l (io),
which is then protected
with n-propyl sulfonyl chloride (nPrSO2C12) to give (11).
[0033] S-BTBA ((S)-143,5-bis(trifluoromethyl)] phenylethanol)) ea is reacted
with trichloroacetonitrile
(Sigma-Aldrich) in the presence of base 1,8-diazabicycloundec-7-ene (DBU) to
produce imidate (11).
HBF4 is used to catalyze the reaction of (j) with (11) to yield ether (14).
Treatment with allylamine
and bis-propylsulfonate cyclizes (14) to allylamine-protected pyrrolidine (1
). Removal of the allyl
protecting group with thiosalicylic acid and 1,4-bis(diphenylphosphino)butane
(dppb), followed by
bis(dibenzylideneacetone)palladium (Pd2(dba)3) and isolation with acetic acid
gives crystalline (n).
Finally, (j1) is reacted with 1,3-cyclopentanedione (Sigma-Aldrich) in
isopropyl alcohol to give
9
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
Compound 1. Compound 1 is a white to off-white powder. It is freely soluble in
methanol, soluble
in ethanol, slightly soluble in isopropyl acetate, sparingly soluble in
isopropyl alcohol, ethyl acetate,
and acetonitrile, and insoluble in water.
Pharmaceutical Compositions
[0034] Compositions containing serlopitant or a pharmaceutically acceptable
salt, solvate or polymorph
thereof as the active ingredient may be advantageously used to treat chronic
pruritus. While it is
possible for serlopitant or a pharmaceutically acceptable salt, solvate or
polymorph thereof to be
administered alone, it is preferable to present it as a formulation. The
compositions, or dosage forms,
may be administered or applied singly, or in combination with other agents.
The formulations may also
deliver serlopitant to a patient in combination with another pharmaceutically
active agent.
[0035] The term "composition" as used herein is intended to encompass a
product comprising
specified ingredients in predetermined amounts or proportions, as well as any
product which results,
directly or indirectly, from combination of the specified ingredients in the
specified amounts. This term
in relation to pharmaceutical compositions is intended to encompass a product
comprising one or more
active ingredients, and an optional pharmaceutically acceptable carrier
comprising inert ingredients, as
well as any product which results, directly or indirectly, from combination,
complexation or aggregation
of any two or more of the ingredients, or from dissociation of one or more of
the ingredients, or from
other types of reactions or interactions of one or more of the ingredients. In
general, pharmaceutical
compositions are prepared by uniformly and intimately bringing the active
ingredient into association
with a liquid carrier or a finely divided solid carrier or both, and then, if
necessary, shaping the product
into the desired formulation. In the pharmaceutical composition the active
object compound is included
in an amount sufficient to produce the desired effect upon the process or
condition of diseases.
Accordingly, the pharmaceutical compositions of the present invention
encompass any composition
made by admixing a compound of the present invention and a pharmaceutically
acceptable carrier.
Said compositions are prepared according to conventional mixing, granulating,
or coating methods,
respectively, and contain about 0.1 to 75%, preferably about 1 to 50%, of the
active ingredient.
[0036] By "pharmaceutically acceptable" it is meant the carrier, diluent or
excipient must be compatible
with the other ingredients of the formulation and not deleterious to the
recipient thereof. Pharmaceutical
compositions intended for oral use may be prepared according to any method
known to the art for the
manufacture of pharmaceutical compositions and such compositions may contain
one or more agents
selected from the group consisting of sweetening agents, flavoring agents,
coloring agents and
preserving agents in order to provide pharmaceutically elegant and palatable
preparations.
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
[0037] Tablets contain the active ingredient in admixture with non-toxic
pharmaceutically acceptable
excipients which are suitable for the manufacture of tablets. These excipients
may be for example,
inert diluents, such as calcium carbonate, sodium carbonate, lactose, calcium
phosphate or sodium
phosphate; granulating and disintegrating agents, for example, cornstarch, or
alginic acid; binding
agents, for example starch, gelatin or acacia, and lubricating agents, for
example magnesium stearate,
stearic acid or talc. The tablets may be uncoated or they may be coated by
known techniques to delay
disintegration and absorption in the gastrointestinal tract and thereby
provide a sustained action over a
longer period. A tablet may be made by compressing or molding the active
ingredient optionally with
one or more pharmaceutically acceptable ingredients. Compressed tablets may be
prepared by
compressing, in a suitable machine, the active ingredient in a free-flowing
form such as a powder or
granules, optionally mixed with a binder, lubricant, inert diluent, surface
active, or dispensing agent.
Molded tablets may be made by molding, in a suitable machine, a mixture of the
powdered active
ingredient and a suitable carrier moistened with an inert liquid diluent.
[0038] Compositions for oral use may also be presented as hard gelatin
capsules wherein the active
ingredient is mixed with an inert solid diluent, for example, calcium
carbonate, calcium phosphate or
kaolin, or as soft gelatin capsules wherein the active ingredient is mixed
with water or an oil medium,
for example peanut oil, liquid paraffin, or olive oil. In particular, a
pharmaceutical composition of the
present invention may comprise a liquid-filled capsule dosage form in which
the active ingredient is in
solution in certain combinations of liquid and semi-solid excipients. In one
embodiment, the invention is
directed to a solution comprising the active agent 3-[(3aR,4R,5S,7aS)-5-{(1R)-
143,5-
bis(trifluoromethyl)phenyl]ethoxy)-4-(4-fluoropheny1)-octahydro-2H-isoindol-2-
yllcyclopent-2-en-1-one
(Compound 1) or a pharmaceutically acceptable salt, solvate or polymorph
thereof, and an amphiphilic
agent, said amphiphilic agent being a fatty acid ester of glycerol, propylene
glycol or sorbitol, as
described in U.S. Published Application No. 2010/0209496 (Dakou et al.), which
is herein incorporated
by reference in its entirety. Preferably, the amphiphilic agent consists
essentially of mono- and di-
glycerides of C8 to C12 saturated fatty acids and mixtures thereof.
[0039] Compositions for oral administration may also be formulated as aqueous
suspensions
containing the active ingredient in admixture with excipients suitable for the
manufacture of aqueous
suspensions. Oily suspensions may be formulated by suspending the active
ingredient in a suitable oil.
Oil-in-water emulsions may also be employed. Dispersible powders and granules
suitable for
preparation of an aqueous suspension by the addition of water provide the
active ingredient in
admixture with a dispersing or wetting agent, suspending agent arid one or
more preservatives.
11
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
[0040] The active ingredient of the present invention may be administered in
an oral sustained release
formulation. "Sustained release" refers to release of an active agent from a
dosage form at a rate
effective to achieve a therapeutic amount of the agent, or active metabolite
thereof, in the systemic
blood circulation over a prolonged period of time relative to that achieved by
oral administration of a
conventional formulation of the agent. Release of the agent occurs over an
extended period of hours,
for example, over a period of at least 6 hours, over a period of at least 8
hours, over a period of at least
12 hours, or over a period of at least 24 hours.
[0041] Suitable topical formulations and dosage forms include ointments,
creams, gels, lotions, pastes,
and the like, as described in Remington: The Science and Practice of Pharmacy
(21st Edition,
University of the Sciences in Philadelphia, 2005). Ointments are semi-solid
preparations that are
typically based on petrolatum or other petroleum derivatives. The specific
ointment base to be used, as
will be appreciated by those skilled in the art, is one that will provide for
optimum drug delivery, and,
preferably, will provide for other desired characteristics as well, e.g.,
emolliency or the like. Creams are
viscous liquids or semisolid emulsions, either oil-in-water or water-in-oil.
Cream bases are water-
washable, and contain an oil phase, an emulsifier and an aqueous phase. The
oil phase, also called
the "internal" phase, is generally comprised of petrolatum and a fatty alcohol
such as cetyl or stearyl
alcohol. The aqueous phase usually, although not necessarily, exceeds the oil
phase in volume, and
generally contains a humectant. The emulsifier in a cream formulation is
generally a nonionic, anionic,
cationic or amphoteric surfactant. Gels are semisolid, suspension-type
systems. Single-phase gels
contain organic macromolecules (polymers) distributed substantially uniformly
throughout the carrier
liquid, which is typically aqueous, but also, preferably, contain an alcohol
such as ethanol or
isopropanol and, optionally, an oil. In order to prepare a uniform gel,
dispersing agents such as
alcohol or glycerin can be added, or the gelling agent can be dispersed by
trituration, mechanical
mixing or stirring, or combinations thereof. Lotions are preparations to be
applied to the skin surface
without friction, and are typically liquid or semiliquid preparations in which
solid particles, including the
active agent, are present in a water or alcohol base. Lotions are usually
suspensions of finely divided
solids and will typically contain suspending agents to produce better
dispersions as well as compounds
useful for localizing and holding the active agent in contact with the skin.
Pastes are semisolid dosage
forms in which the active agent is suspended in a suitable base. Depending on
the nature of the base,
pastes are divided between fatty pastes or those made from single-phase
aqueous gels.
[0042] Various additives, known to those skilled in the art, may be included
in the topical formulations.
For example, solvents, including relatively small amounts of alcohol, may be
used to solubilize certain
drug substances. Other optional additives include pacifiers, antioxidants,
fragrance, colorant, gelling
12
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
agents, thickening agents, stabilizers, surfactants and the like. Other agents
may also be added, such
as antimicrobial agents, to prevent spoilage upon storage, i.e., to inhibit
growth of microbes such as
yeasts and molds. For those drugs having an unusually low rate of permeation
through the skin or
mucosal tissue, it may be desirable to include a permeation enhancer in the
formulation. The
formulation may also contain irritation-mitigating additives to minimize or
eliminate the possibility of skin
irritation or skin damage resulting from the drug, the enhancer, or other
components of the dosage
form. The formulations may also contain ether physiologically acceptable
excipients or other minor
additives, such as fragrances, dyes, emulsifiers, buffers, cooling agents
(e.g. menthol), antibiotics,
stabilizers or the like. In some instances, one component may serve more than
one function.
[0043] The concentration of the active agent in a topical formulation can vary
a great deal, and will
depend on a variety of factors, including the disease or condition to be
treated, the nature and activity
of the active agent, the desired effect, possible adverse reactions, the
ability and speed of the active
agent to reach its intended target, and other factors within the particular
knowledge of the patient and
physician. The formulations will typically contain on the order of about 0.1
wt `)/0 to 50 wt % active
agent, preferably about 0.1 wt % to 5 wt % active agent, optimally about 5 wt
% to 20 wt % active
agent.
[0044] In some embodiments, a topical dosage form of serlopitant is formulated
as a buccal or
sublingual tablet or pill. Advantages of a buccal or sublingual tablet or pill
include avoidance of first-
pass metabolism and circumvention of gastrointestinal absorption. In addition
to a therapeutically
effective amount of serlopitant, the buccal or sublingual tablet or pill can
contain suitable excipients,
including without limitation any combination of fillers and diluents (e.g.,
mannitol and sorbitol), binding
agents (e.g., sodium carbonate), wetting agents (e.g., sodium carbonate),
disintegrants (e.g.,
crospovidone and croscarmellose sodium), lubricants (e.g., silicon dioxide
including colloidal silicon
dioxide) and sodium stearyl fumarate), stabilizers (e.g., sodium bicarbonate),
flavoring agents (e.g.,
spearmint flavor), sweetening agents (e.g., sucralose), and coloring agents
(e.g., yellow iron oxide).
The buccal or sublingual tablet or pill containing serlopitant can be used to
treat, e.g., any pruritus-
associated condition described herein.
[0045] The pharmaceutical compositions of the present invention may be
formulated as a depot
formulation for administration via intramuscular or subcutaneous injection.
Depot formulations are
efficient, well-tolerated, sustained or delayed release compositions of the
active ingredient that are
therapeutically effective for a number of weeks, such as at least one week, at
least two weeks, at least
three weeks, at least four weeks, at least five weeks, or at least six weeks
or more. In addition to the
active agent, additional ingredients may be used in the depot formulations of
the present invention
13
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
including surfactants, solubilizers, emulsifiers, preservatives, isotonicity
agents, dispersing agents,
wetting agents, fillers, solvents, buffers, stabilizers, lubricants, and
thickening agents. A combination of
additional ingredients may also be used. The amount of the active ingredient
in a depot formulation will
depend upon the severity of the pruritus being treated.
[0046] The compositions of the present invention may be presented in unit
dosage form and may be
prepared by any of the methods well known in the art of pharmacy. The term
"unit dosage form" is
taken to mean a single dose wherein all active and inactive ingredients are
combined in a suitable
system, such that the patient or person administering the drug to the patient
can open a single
container or package with the entire dose contained therein, and does not have
to mix any components
together from two or more containers or packages. Typical examples of unit
dosage forms are tablets
or capsules for oral administration. These examples of unit dosage forms is
not intended to be limiting
in any way, but merely to represent typical examples in the pharmacy arts of
unit dosage forms.
[0047] The compositions of the present invention may also be presented as a
kit, whereby two or more
components, which may be active or inactive ingredients, carriers, diluents,
and the like, are provided
with instructions for preparation of the actual dosage form by the patient or
person administering the
drug to the patient. Such kits may be provided with all necessary materials
and ingredients contained
therein, or they may contain instructions for using or making materials or
components that must be
obtained independently by the patient or person administering the drug to the
patient.
Topical Compositions Comprising Serlopitant
[0048] Topical formulations for application to the skin or mucosa can be
useful for treatment of
conditions of the upper skin or mucosal layers and for transdermal or
transmucosal administration of an
active agent to the local tissue underlying the skin or mucosa and, if
desired, into the blood for systemic
distribution. Advantages of topical administration can include avoidance of
first-pass metabolism,
circumvention of gastrointestinal absorption, delivery of an active agent with
a relatively short biological
half-life, more controlled release of the active agent, administration of a
more uniform plasma dosing of
the active agent, and improvement in user compliance.
[0049] In general and in addition to the disclosure on topical formulations
described elsewhere herein,
compositions suitable for topical administration include without limitation
liquid or semi-liquid
preparations such as sprays, gels, liniments, lotions, oil-in-water or water-
in-oil emulsions such as
creams, foams, ointments and pastes, and solutions or suspensions such as
drops (e.g., eye drops,
nose drops and ear drops). In some embodiments, a topical composition
comprises an active agent
dissolved, dispersed or suspended in a carrier. The carrier can be in the form
of, e.g., a solution, a
14
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
suspension, an emulsion, an ointment or a gel base, and can contain, e.g.,
petrolatum, lanolin, a wax
(e.g., bee wax), mineral oil, a long-chain alcohol, polyethylene glycol or
polypropylene glycol, a diluent
(e.g., water and/or an alcohol [e.g., ethanol or propylene glycol]), an
emulsifier, a stabilizer or a
thickening agent, or a combination thereof. A topical composition can include,
or a topical formulation
can be administered by means of, e.g., a transdermal patch, a microneedle
patch or an iontophoresis
device. A transdermal patch can contain, e.g., a microporous membrane made of
a suitable material
(e.g., cellulose nitrate or acetate, propylene or a polycarbonate), a skin
adhesive and backing material.
A topical composition can deliver the active agent transdermally (including
percutaneously and
transmucosally) via a concentration gradient or an active mechanism (e.g.,
ionospheres).
[0050] Representative kinds of topical compositions are described below for
purposes of illustration.
I. Topical Compositions Comprising a Permeation Enhancer
[0051] In some embodiments, a topical composition comprises serlopitant and a
permeation enhancer.
The composition can optionally contain an additional therapeutic agent. In
certain embodiments, the
composition contains serlopitant in free base form.
[0052] The permeation enhancer increases the permeability of the skin or
mucosa to the therapeutic
agent(s). In certain embodiments, the permeation enhancer is N-lauroyl
sarcosine, sodium octyl
sulfate, methyl laurate, isopropyl myristate, oleic acid, glyceryl oleate or
sodium lauryl sulfoacetate, or a
combination thereof. In certain embodiments, the composition contains on a
weight/volume (w/v) basis
the permeation enhancer in an amount of about 1-20%, 1-15%, 1-10% or 1-5%. To
enhance further
the ability of the therapeutic agent(s) to penetrate the skin or mucosa, the
composition can also contain
a surfactant, an azone-fike compound, an alcohol, a fatty acid or ester, or an
aliphatic thiol.
[0063] The composition can further contain one or more additional excipients.
Suitable excipients
include without limitation solubilizers (e.g., C2-C8 alcohols), moisturizers
or humectants (e.g., glycerol
[glycerin], propylene glycol, amino acids and derivatives thereof, polyamino
acids and derivatives
thereof, and pyrrolidone carboxylic acids and salts and derivatives thereof),
surfactants (e.g., sodium
laureth sulfate and sorbitan monolaurate), emulsifiers (e.g., cety) alcohol
and stearyl alcohol),
thickeners (e.g., methyl cellulose, ethyl cellulose, hydroxymethyl cellulose,
hydroxypropyl cellulose,
polyvinylpyrrolidone, polyvinyl alcohol and acrylic polymers), and formulation
bases or carriers (e.g.,
polyethylene glycol as an ointment base). As a non-limiting example, the base
or carrier of the
composition can contain ethanol, propylene glycol and polyethylene glycol
(e.g., PEG 300), and
optionally an aqueous liquid (e.g., isotonic phosphate-buffered saline).
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
[0054] The topical composition can have any suitable dosage form, such as a
solution (e.g., eye drop,
nose drop or ear drop), a suspension, an emulsion, a cream, a lotion, a gel,
an ointment, a paste, a
jelly, a foam, a shampoo, or a spray. In some embodiments, the composition is
applied to the skin or
mucosa covering a surface area of about 10-800 cm2, 10-400 cm2 or 10-200 cm2.
The composition can
deliver the therapeutic agent(s) to the skin or mucosa or the underlying
tissue. The composition can
also be formulated for transdermal administration of the therapeutic agent(s)
to the systemic circulation,
e.g., as a transdemial patch or a microneedle patch.
H. Topical Compositions Comprisinu a Permeation Enhancer and a Volatile Liquid
[0055] In further embodiments, a topical composition comprises serlopitant, a
permeation enhancer
and a volatile liquid. The composition can optionally contain an additional
therapeutic agent. In certain
embodiments, the composition contains serlopitant in free base form.
[0056] The permeation enhancer increases the permeability of the skin or
mucosa to the therapeutic
agent(s). In some embodiments, the permeation enhancer is selected from the
group consisting of C8
-
C18 alkyl aminobenzoates (e.g., CerCis alkyl p-aminobenzoates), C5-C15 alkyl
dimethylaminobenzoates
(e.g., CErCig alkyl p-dimethylaminobenzoates), CB-Cis alkyl cinnamates, C5-C,5
alkyl
methoxycinnamates (e.g., C8-C18 alkyl p-methoxycinnamates), and C5-C15 alkyl
salicylates. In certain
embodiments, the permeation enhancer is octyl salicylate, octyl p-
dimethylaminobenzoate or octyl p-
methoxycinnamate, or a combination thereof.
[0057] The volatile liquid can be any volatile, skin- or mucosa-tolerant
solvent. In certain embodiments,
the volatile liquid is a C2-05 alcohol or an aqueous solution thereof, such as
ethanol or isopropanol or
an aqueous solution thereof. An aerosol propellant (e.g., dimethyl ether) can
be considered as a
volatile liquid. In some embodiments, the volatile liquid functions as a
carrier or vehicle of the
composition.
[0058] The composition can optionally contain a thickening agent. Non-limiting
examples of thickening
agents include cellulosic thickening agents (e.g., ethyl cellulose,
hydroxypropyl cellulose and
hydroxypropyl methylcellulose), povidone, polyacrylic acids/polyacrylates
(e.g., Carbopol polymers),
Sepigel (polyacrylamidefisoparaffinfiaureth-7), and the Gantrez series of
polymethyl vinyl
ether/maleic anhydride copolymers (e.g., butyl ester of PMV/MA copolymer
Gantrez A-425).
[0059] In some embodiments, the composition contains on a weight basis about
0.5-10%, 0.5-5% or 1-
5% of serlopitant, about 1-20%, 1-15% or 1-10% of the permeation enhancer, and
about 40-98%, 45-
95%, 50-90% or 60-80% of the volatile liquid. In further embodiments, the
composition optionally
16
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
contains on a weight basis about 1-40%, 1-30%, 1-20% or 5-20% water and/or
about 0.1-15%, 0.5-10%
or 1-5% of a thickening agent.
[0060] For purposes of illustration, in certain embodiments a topical spray
composition contains about
0.5-5% w/v of serlopitant, about 2-10% w/v of octyl salicylate or octyl p-
methyoxycinnamate, and about
95% aqueous ethanol as the carrier. In further embodiments, a topic gel
composition comprises about
0.5-5% w/v of serlopitant, about 1-10% w/v of octyl salicylate or octyl p-
methyoxycinnamate, about 0.5-
5% w/v of a Carbopole polyacrylic acid, and about 70% aqueous ethanol as the
carrier, and optionally
about 1-10% w/v of a basic solution (e.g., 0.1 N NaOH). In additional
embodiments, a topical lotion
composition contains about 0.5-5% w/v of serlopitant, about 1-10% w/v of octyl
salicylate or octyl p-
methyoxycinnamate, about 1-5% w/v of ethyl cellulose or hydroxypropyl
cellulose, and about 90%
aqueous ethanol as the carrier.
[0061] The composition can further comprise other excipients, such as a
compounding agent (e.g.,
paraffin oil, silicone oil, a vegetable oil, or a fatty ester such as
isopropyl myristate), a diluent, a co-
solvent (e.g., acetone or a glycol ether such as diethylene glycol monoethyl
ether), an emulsifier, a
surfactant (e.g., an ethoxylated fatty alcohol, glycerol mono stearate or a
phosphate ester), a stabiliser,
an antioxidant or a preservative (e.g., a hydroxybenzoate ester), or a
combination thereof. For
example, a co-solvent and/or a surfactant can be used to maintain the
therapeutic agent(s) in solution
or suspension at the desired concentration.
[0062] The topical composition can have any suitable dosage form, such as a
cream, a lotion, a gel, an
ointment, a mousse, a spray or aerosol, or any transdermal device (e.g., a
patch) that administers a
drug by absorption through the skin or mucosa. In some embodiments, the
topical composition is
applied to the skin or mucosa covering a surface area of about 10-800 cm2, 10-
400 cm2 or 10-200 cm2.
Ill. Topical Compositions Comprising a Permeation Enhancer and Another
Excipient
[0063] in yet further embodiments, a topical composition comprises
serlopitant, a permeation
enhancer, and at least one of a lipophilic solvent, a formulation base and a
thickener. In some
embodiments, the composition contains a lipophilic solvent and a formulation
base, or the same
substance can function as both a lipophilic solvent and a formulation base. In
further embodiments, the
composition contains a lipophilic solvent, a formulation base and a thickener.
The composition can
optionally comprise an additional therapeutic agent. In certain embodiments,
the composition contains
serlopitant in free base form.
(0064] The permeation enhancer increases the permeability of the skin or
mucosa to the therapeutic
agent(s). Non-limiting examples of permeation enhancers include dimethyl
sulfoxide (DMSO),
17
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
decylmethylsulfoxide, laurocapram, pyrrolidones (e.g., 2-pyrrolidone and N-
methyl-2-pyrrolidine),
surfactants, alcohols (e.g., oleyl alcohol), polyethylene glycol (e.g., PEG
400), diethylene glycol
monoethyl ether, oleic acid, and fatty acid esters (e.g., isopropyl myristate,
methyl laurate, glycerol
monooleate, and propylene glycol monooleate).
[0066] Non-limiting examples of liphophilic solvents include lipophilic
alcohols (e.g., hexylene glycol,
octyldodecanol, oleyl alcohol and stearyl alcohol), polyethylene glycol (e.g.,
PEG 100, PEG 300,
PEG 400 and PEG 3350), diethylene glycol monoethyl ether, polysorbates (e.g.,
Tween 20 to 80),
Labrasol , fatty acid esters (e.g., isopropyl myristate and diisopropyl
adipate), diethyl sebacate,
propylene glycol monocaprylate, propylene glycol laurate, mono- and di-
glycerides (e.g., Capmut
MCM), medium-chain triglycerides, caprylic/capric triglyceride, glyceryl
monocaprylate, glyceryl mono-
oleate, glyceryl mono-linoleate, glycerol oleate/propylene glycol, mineral
oil, and vegetable oils.
[0066] A liphophilic solvent may also function as a formulation base or
carrier. For example,
polyethylene glycol (e.g., from PEG 100 to PEG 3500, such as PEG 300, PEG 400
and PEG 3350) can
function as a liphophilic solvent and a formulation base.
[0067] The composition can also contain a hydrophilic solvent, such as a C1-05
alcohol (e.g., ethanol,
isopropanol, glycerol, propylene glycol and 1,2-pentanediol) and/or water.
[0068] The composition can contain a thickener to increase the viscosity
and/or the physical stability of
the composition. Examples of thickeners include without limitation glycerol,
stearyl alcohol, and
polymers (e.g., polydimethylsiloxane [dimethicone] and Carbopol polymers).
[0069] In some embodiments, the composition further contains an antioxidant.
Non-limiting examples
of antioxidants include butylated hydroxyanisole (BHA), butylated
hydroxytoluene (BHT), tocopherols
(e.g., Vitamin E and esters thereof), flavinoids, glutathione, ascorbic acid
and esters thereof, DMSO,
and chelating agents (e.g., EDTA and citric acid).
[0070] In certain embodiments, the topical composition comprises on a w/w
basis about 0.5-10% or 1-
5% of serlopitant, about 2-30% or 5-20% of a permeation enhancer, about 20-80%
or 30-70% of a
lipophilic solvent that may also function as a formulation base, about 0.1-10%
or 1-7.5% of a thickener,
and about 0.01-2% or 0.05-1% of an antioxidant. As a non-limiting example, a
topical composition can
contain serlopitant, PEG 400 and/or PEG 3350 as lipophilic solvent(s) and
formulation base(s),
diethylene glycol monoethyl ether, oleyl alcohol and/or isopropyl myristate as
permeation enhancer(s),
stearyl alcohol as a thickener, and BHT as an antioxidant.
18
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
[0071] The topical composition can have any suitable dosage form, such as a
cream, a lotion, a gel, an
ointment, a jelly, a paste, or any transdermal device (e.g., a patch) that
administers a drug by
absorption through the skin or mucosa.
IV. Topical Compositions Comprising a Permeation Enhancer and an Adhesive
[0072] In additional embodiments, a topical composition comprises serlopitant,
a permeation enhancer
and an adhesive. The composition can optionally contain an additional
therapeutic agent. In certain
embodiments, the composition contains serlopitant in free base form.
[0073] The permeation enhancer increases the permeability of the skin or
mucosa to the therapeutic
agent(s). The permeation enhancer can be, e.g., a fatty acid ester having a
fatty acyl chain length of
C8-C20 or C12-C18 and a C1-C8 or C2-C4 alcohol component (e.g., isopropanol).
In certain embodiments,
the permeation enhancer is isopropyl myristate or isopropyl palmitate. In some
embodiments, the
permeation enhancer is in an amount of about 0.1-20%, 0,5-15%, 1-15%, 2-12% or
4-10% by weight of
the composition or the skin-contacting layer of a transdermal patch.
[0074] The adhesive maintains contact of the topical composition to the skin
or mucosa. Non-limiting
examples of adhesives include acrylics/acrylates (e.g., polyacrylates,
including polyalkyl acrylates and
Duro-Take polyacrylates), polyvinyl acetate, ethylenevinylacetate copolymers,
polysiloxanes,
polyurethanes, plasticized polyether block amide copolymers, natural and
synthetic rubbers, plasticized
styrene-butadiene rubber block copolymers (e.g., Duro-Take 87-6173), and
mixtures thereof.
[0075] The topical composition can comprise one or more additional excipients.
The additional
excipient(s) can be, e.g., a diluent, an emollient, a plasticizer, or an agent
that reduces irritation to the
skin or mucosa, or a combination thereof.
[0076] In certain embodiments, the topical composition prior to application to
the skin or mucosa is
substantially free of water, tetraglycol (glycofurol) and/or a hydrophilic
organic solvent (e.g., a C1-C8
alcohol).
[0077] The composition can administer the therapeutic agent(s) transdermally
(including
percutaneously and transmucosally) through a body surface or membrane such as
intact unbroken skin
or intact unbroken mucosal tissue into the systemic circulation.
[0078] In some embodiments, the topical composition is in the form of a
transdermal patch for
application to the skin or mucosa. The patch has a skin- or mucosa-contacting
layer ("skin-contacting
layer" for simplicity) laminated or otherwise attached to a support layer. The
skin-contacting layer can
19
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
be covered by a removable release liner before use to protect the skin-
contacting surface and to keep it
clean until it is applied to the skin or mucosa.
[0079] The support layer of the patch acts as a support for the skin-
contacting layer and as a barrier
that prevents loss of the therapeutic agent(s) in the skin-contacting layer to
the environment. The
material of the support layer is compatible with the therapeutic agent(s), the
permeation enhancer and
the adhesive, and is minimally permeable to the components of the patch. The
support layer can be
opaque to protect the components of the patch from degradation via exposure to
ultraviolet light. The
support layer is also capable of binding to and supporting the adhesive layer,
yet is sufficiently pliable to
accommodate the movements of the subject using the patch. The material of the
support layer can be,
e.g., a metal foil, a metalized polyfoil, or a composite foil or film
containing a polymer (e.g., a polyester
[such as polyester terephthalate] or aluminized polyester, polyethylene,
polypropylene,
polytetrafluoroethylene, a polyethylene methyl methacrylate block copolymer, a
polyether block amide
copolymer, a polyurethane, polyvinylidene chloride, nylon, a silicone
elastomer, rubber-based
polyisobutylene, styrene, or a styrene-butadiene or styrene-isoprene
copolymer). The release liner can
be made of the same material as the support layer, or can be a film coated
with an appropriate release
surface.
Pruritus
[0080] Pruritus is a physiological perception within the sensory neuronal
network in the skin which,
along with pain and physical or mechanical stimuli, can serve as a warning
system against potential
bodily threats. Itching is an unpleasant sensation that can lead to
scratching, but is independent of
pain. The International Federation for the Study of Itch (IFSI) defines
chronic pruritus (as opposed to
acute pruritus) as itching that lasting six weeks or longer (S. StAnder et
al., Acta Dorm. Venerea, 2007,
87(4)291-4). Several factors in and on the skin can activate the sensory nerve
fibers or modulate their
activity and thus trigger, suppress, or exacerbate itching. Physical stimuli
such as cold and heat
modulate the perception of itching; painful heat and cold can significantly
diminish it, while moderate
cold intensifies it (Valet et al., J. Invest. DermatoL, 2008,128(2):426-33.).
Mechanical factors such as
rubbing or scratching the skin can briefly suppress itching by activating
nerve fibers that selectively
activate and de-activate certain areas of the brain (Yosipovitch et al, J.
Invest. DermatoL, 2008,
128(7):1806--11).
[0081] Chronic pruritus can seriously diminish the quality of life in its
sufferers as it can be intractable
and incapacitating. It is a seriously debilitating condition, comparable to
chronic pain, which can lead to
frustration, desperation and depression. Moreover, chronic scratching often
produces open skin
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
lesions, subject to primary or secondary infection, scarring and potential
disfigurement. Chronic
pruritus is often an indication of underlying disease and is always present in
diseases such as urticaria
and atopic dermatitis. Diagnosis of the underlying disease is desirable and
clinical presentation, patient
history, and patient self-evaluation form important parts of such diagnosis.
[0082] According to Arbeitsgemeinschaft der Wissenschaftlichen Medizinischen
Fachgesellschaften
(AWMF) (Association of the Scientific Medical Societies of Germany)
guidelines, diseases and
disorders with chronic pruritus as a symptom may be classified by whether the
skin is inflamed or not
inflamed (S. Stander, Clin. Exp. Dertnatol., 2006,31(6):762-7). The IFS(
further characterizes pruritus
as dermatologic, systemic, neurogenic, psychogenic, mixed and other. Chronic
pruritus on non-
inflamed skin may result from dermatological diseases, including atopic
diathesis, asteatosis, porphyria,
suburticarial stages of solar injury, cholinergic, adrenergic urticaria,
initial stage of mastocytosis, bullous
pemphigdd, and Duhring's disease (dermatitis herpetiformis); from endocrine
and metabolic disorders,
such as chronic renal insufficiency and the dialysis needed treat it,
hepatopathies with cholestasis,
diabetes mellitus, malabsorption disorders, anorexia, gluten-enteropathies,
hyperthyroidism,
hypothyroidism, hyperparathyroidism, and perimenopausal pruritus; from
infections including HIV
infection, parasites, Helicobacter pylori, and helminth-related; from
hemotological and
lymphoproliferative diseases such as iron deficiency, polycythaemica vera,
hypereosinophilia
syndrome, myelodysplastic syndrome, Hodgkin's disease, non-Hodgkin's lymphoma,
plasmocytoma,
and systemic mastocytosis; from solid malignant tumors including cervical,
breast, prostate or large
intestinal cancer, and carcinoid tumors; from neurological disorders such as
brachioradial pruritus,
notalgia paraesthetica, post-zoster neuralgia, vulvodynia, neuropathies of
various origin, multiple
sclerosis, tumors, abscesses, underperfusion, infarctions involving the
CNS/spinal cord; from
psychogenic disorders such as depression, schizophrenia, and tactile
hallucinations; and from
intrahepatic cholestasis in pregnant women (pruritus gravidarum).
100831 Chronic pruritus on inflamed skin may be observed in patients with
inflammatory skin disease
including, but not limited to, atopic dermatitis, allergic, irritant contact
dermatitis, exsiccation dermatitis,
nummular and dyshidrotic dermatitis, lichen planus, lichen sclerosus et
atrophicus, polymorphous light
eruption psoriasis, Grover's disease, mucinosis, mastocytosis, and urticaria;
infectious skin diseases
such as mycoses, bacterial and viral infections, scabies, pediculosis, insect
bites, and folliculitides;
autoimmune skin diseases including Bullous skin disorders, especially
dermatitis herpetiformis
(Duhring's disease), and bullous pemphigoid; genodermatoses such as Darier's
disease, and Halley-
Halley disease; pregnancy-related skin diseases including polymorphic eruption
of pregnancy (PEP,
21
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
formerly known as PUPPP), atopic eruption of pregnancy, and pemphigoid
gestation's; and neoplasias
such as cutaneous T-cell lymphoma (especially the erythrodermic form).
[0084] Prurigo nodularis (PN), or nodular prurigo, is a particularly severe
form of chronic itching that
may treated by methods and compositions of the present invention.
Characterized by itchy, excoriated,
lichenified papules and nodules, PN can occur at any age, but most often
presents in middle-aged and
elderly patients on their arms and legs (E. Weisshaar and S. Stander, Acta
Derm. Venereol., 2012,
92:532-533). The etiology of PN is unknown, but it usually occurs in patients
with a personal or family
history of atopic dermatitis, and often with concomitant medical conditions
such as hepatic or renal
function, local trauma or insult to the skin, infection, and HIV or other
immunodeficiencies. PN may
result in permanent changes to the skin, including nodular lichenification,
hyperkeratosis,
hyperpigmentation, and skin thickening.
Combination Therapies with Serlopitant and Other Antipruritic Agents
[0085] Serlopitant, alone or in combination with one or more additional
antipruritic agents, can be used
to treat pruritus (including acute and chronic pruritus) associated with any
condition. The itch sensation
can originate, e.g., in the peripheral nervous system (e.g., dermal or
neuropathic itch) or in the central
nervous system (e.g., neuropathic, neurogenic or psychogenic itch).
[0086] Examples of pruritus-associated conditions include without limitation
those described elsewhere
herein and the following:
dermatological disorders and conditions (including inflammatory and non-
inflammatory skin
conditions), including but not limited to adult blaschkitis, amyloidoses
(e.g., primary cutaneous
amyloidosis [including macular amyloidosis, lichen amyloidosis and nodular
amyloidosis]), burns (e.g.,
chemical burns and sunburn), dermatitis {e.g., atopic dermatitis, contact
dermatitis (including allergic
contact dermatitis, irritant contact dermatitis arid photodermatitis), eczema
(e.g., autosensitization
dermatitis, dermatitis herpetiformis [Duhring's disease], discoid eczema,
dyshidrosis [pompholyx], hand
eczema, id reaction [generalized eczema], nummular eczema, stasis dermatitis
[gravitational eczema],
venous eczema and xerotic eczema), pustular dermatitis (e.g., eosinophilic
pustular folliculitis [Ofuji's
disease], reactive arthritis [Reiter's disease] and subcorneal pustular
dermatosis [Sneddon-Wilkinson
disease]), and seborrheic dermatitis (e.g., infantile seborrheic dermatitis,
Leiner's disease and pityriasis
simplex capillitii [dandruff])}, erythroderma (exfoliative dermatitis),
folliculitis, pseudofolliculitis barbae
(barber's itch), hidradenitis suppurativa, ichthyoses (e.g., ichthyosis
vulgaris, congenital ichthyosis,
epidermolytic hyperkeratosis and lamellar ichthyosis), lichen planus (e.g.,
cutaneous lichen planus and
oral lichen planus), lichen sclerosis (e.g., lichen sclerosis et atrophicus of
the vulva), lichen simplex
22
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
(e.g., lichen simplex chronicus [neurodermatitisp, linear IgA bullous
dermatosis (linear IgA dermatosis),
lupus erythematosus (e.g., cutaneous lupus erythematosus, discoid lupus
erythematosus and systemic
lupus erythematosus), miliaria (sweat rash), palmoplantar keratoderma (e.g.,
punctate palmoplantar
keratoderma), pityriasis (e.g., pityriasis amiantacea, pityriasis lichenoides
[including pityriasis
lichenoides chronica and pityriasis lichenoides et varioliformis acuta],
pityriasis rosea, pityriasis rubra
pilaris [Devergie's disease] and pityriasis versicolor), prurigo (e.g.,
actinic prurigo, Besnier's prurigo,
prurigo nodularis, prurigo pigmentosa and prurigo simplex), pruritus ani,
pruritus scroti, pruritus vulvae,
psoriasis (e.g., erythrodermic psoriasis, Guttate psoriasis [eruptive
psoriasis], psoriasis vulgaris [chronic
stationary psoriasis], pustular psoriasis, and pustulosis palmaris et
plantaris), parapsoriasis (e.g., large
plaque parapsoriasis and small plaque parapsoriasis [chronic superficial
dermatitis]), puncta pruritica
(itchy points), rashes (e.g., intertrigo and perioral dermatitis), rosacea,
urticaria (e.g., contact urticaria
[including hives] and idiopathic urticaria), vitiligo, xerosis (dry skin),
chapped skin (e.g., chapped feet),
scalp pruritus, scab healing, scar development, and development of moles,
pimples and ingrown hair;
medical disorders and conditions (including peripheral and systemic
disorders), including but not
limited to atopic diathesis, autoimmune disorders (e.g., celiac disease,
dermatomyositis, Graves'
disease, pemphigoid [e.g., bullous pemphigoid], scleroderma and SjElgren's
syndrome), blood
disorders (e.g., anemia [e.g., iron deficiency anemia and sickle cell anemia],
hypercalcemia,
myeiodysplastic syndromes and polycythemia [e.g., polycythemia vera]),
Creutzfeldt-Jakob disease
(e.g., prion pruritus), diabetes mellitus, genetic diseases (e.g., AlegiIle
syndrome, Darier's disease,
epidermolysis bullosa, Hailey-Hailey disease and SjOgren-Larsson syndrome),
Grover's disease,
HIV/AIDS, kidney disorders (e.g., diabetic nephropathy, glomerulonephritis,
chronic kidney disease,
end-stage kidney disease and chronic kidney failure), uraemia (e.g., uremic
pruritus [renal pruritus]),
liver diseases (e.g., cirrhosis [e.g., primary biliary cirrhosis], hepatitis
[including hepatitis A, B, C, D and
E and their chronic conditions], and liver failure), cholestasis (e.g.,
cholestatic pruritus), jaundice (e.g.,
biliary pruritus), lymphadenopathy (e.g., enlarged lymph nodes), mast cell
diseases (e.g., mast cell
activation syndrome and mastocytosis), multiple sclerosis, neuropathies (e.g.,
peripheral neuropathy
[e.g., brachioradial pruritus, notalgia paresthetica, polyneuropathy and small
fiber peripheral
neuropathyl), nerve irritation, pinched nerves, parathyroid disorders (e.g.,
hyperparathyroidism and
hypoparathyroidism), thyroid disorders (e.g., hyperthyroidism, hypothyroidism
and myxedema), stroke,
cancers {e.g., carcinoid syndrome, leukemia (e.g., leukemia cutis and
lymphatic leukemia), lymphomas
(e.g., Hodgkin's disease and non-Hodgkin lymphomas [e.g., cutaneous B-cell
lymphoma and
cutaneous T-cell lymphoma (including mycosis fungoides and Sezary's
disease)]), Kaposi's sarcoma,
multiple myeloma and skin cancers}, tumors (e.g., brain tumor, plasmacytoma,
and solid tumors of the
cervix, colon and prostate), paraneoplastic pruritus, psychiatric disorders
(e.g., stress, anxiety
23
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
disorders, delusional parasitosis, depression, obsessive-compulsive disorders
[e.g., neurotic
excoriation], and tactile hallucinations), aging (e.g., senile pruritus) and
changes in hormonal balances
associated with aging (e.g., perimenopause and menopause);
infections and infestations, including but not limited to cercarial dermatitis
(swimmer's itch),
insect bites and stings (e.g., by ants, bees, chiggers, fleas, lice [including
body lice, head lice and pubic
lice], mites, mosquitos, spiders, ticks and wasps), scabies, bacterial
infections (e.g., abscess, dermatitis
gangrenosa, ecthyma, erythrasma, impetigo and Lyme disease), fungal infections
(e.g., candidiasis,
dermatophytosis, tinea corporis [ringworm of the body], tinea cruris [jock
itch] and tinea pedis [athlete's
foot]), viral infections {e.g., herpes (including herpes zoster [shingles] and
post-herpetic itch), measles,
parvovirus infections (e.g., parvovirus B19), varicella (chickenpox) and
Yellow fever), and worm
infections {e.g., helminths (e.g., helminthiasis [helminthosis]), hookworms
(e.g., cutaneous larva
migrans), Onchocerca worms (e.g., onchocerciasis [river blindness)), pinworms,
roundworms (e.g.,
filariasis and trichinosis) and Schistosoma worms (e.g., schistosomiasis));
reactions to allergens and irritants, including but not limited to allergic
rhinitis (e.g., pollinosis
[including hay fever)), asthma, animal allergens (e.g., cat dander and dog
dander), chemical allergens
(e.g., acids [e.g., abietic acid and sorbic acid], cosmetics, detergents,
dyes, fabric softeners, fungicides,
hydroxyethyl starch and latex), food allergens (e.g., milk proteins, peanuts,
tree nuts, seafood, spices,
preservatives [e.g., nitrates], vitamins [e.g., vitamins A and B), alcohol,
caffeine and monosodium
glutamate), metal and metal salt allergens (e.g., chromium, cobalt, gold and
nickel and salts thereof),
plant allergens (e.g., Balsam of Peru and urushiol [e.g., in poison ivy,
poison oak and poison sumac]),
chemical irritants (e.g., acids, alkalis, metalworking fluids, solvents,
surfactants, detergents, soaps,
cleaning products, cosmetics, perfumes, deodorants, antiperspirants, food
flavorings, spices,
preservatives [e.g., formaldehyde and parabens], monomers and polymers [e.g.,
acrylics, epoxy resins,
ethylene oxide, latex and lacquers], and oils [e.g., kerosene)), fabrics
(e.g., wool), plant irritants (e.g.,
alkyl resorcinols [e.g., in Grevillea banksii, Grevillea "Robyn Gordon" and
Gingko biloba]), and physical
irritants (e.g., water (e.g., aquadynia and aquagenic pruritus), low humidity
from air conditioning, and
cold temperature);
pruritus caused by drugs/medication, including but not limited to chloroquine,
hydroxyethyl
cellulose, hydroxyethyl starch, angiotensin-converting enzyme inhibitors,
xanthine oxidase inhibitors
(e.g., allopurinol), antibiotics (e.g., isoniazid, neomycin, penicillin,
sulfonamides and vancomycin),
antifungals (e.g., fiuconazole, griseofulvin, itraconazole and ketoconazole),
neuroleptics/antipsychotics
(e.g., phenothiazines), antiarrhythmic drugs (e.g., amiodarone and quinidine),
chemotherapeutic drugs,
diuretic drugs (e.g., hydrochlorothiazide), statins (e.g., simvastatin), and
drugs (e.g., opioids) that
activate the histamine H1 receptor or trigger histamine release; and
24
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
conditions related to pregnancy, including but not limited to gestational
pemphigoid, impetigo
herpetiformis, intrahepatic cholestasis of pregnancy (pruritus gravidarum),
polymorphic eruption of
pregnancy, prurigo of pregnancy, pruritic folliculitis of pregnancy, and
pruritic urticarial papules and
plaques of pregnancy.
[0087] One or more additional antipruritic agents can optionally be used in
combination with serlopitant
to treat pruritus (including acute and chronic pruritus). Examples of
antipruritic agents include without
limitation:
antihistamines, including but not limited to antihistamines that inhibit
action at the histamine Hi
receptor (e.g., acrivastine, antazoline, azelastine, bilastine,
brompheniramine, buclizine,
bromodiphenhydramine, carbinoxamine, cetirizine, chlorpromazine, cyclizine,
chlorpheniramine,
chlorodiphenhydramine, clemastine, cyproheptadine, desloratadine,
dexbrompheniramine,
dexchiorpheniramine, dimenhydrinate, dimetindene, diphenhydramine, doxepin,
doxylamine, ebastine,
embramine, fexofenadine, hydroxyzine, levocetirizine, loratadine, meclozine,
mepyramine, mirtazapine,
olopatadine, orphenadrine, phenindamine, pheniramine, phenyltoloxamine,
promethazine, pyrilamine,
quetiapine, rupatadine, tripelennamine and triprolidine), and antihistamines
that inhibit action at the
histamine H4 receptor (e.g., thioperamide, JNJ 7777120 and VUF-6002), and
analogs and derivatives
thereof;
serotonin receptor antagonists, including but not limited to 5-HT2 antagonists
(e.g., clozapine,
cyproheptadine, ketanserin, pizotifen and quetiapine) and 5-HT3 antagonists
(e.g., alosetron,
cilansetron, dolasetron, granisetron, ondansetron, palonosetron and
tropisetron), and analogs and
derivatives thereof;
neurokinin-1 (NK-1) receptor antagonists, including but not limited to
aprepitant, casopitant
(GW679769), dapitant, ezlopitant, fosaprepitant, lanepitant (LY-303870),
maropitant, netupitant,
nolpitant, orvepitant, rolapitant, vestipitant, vofopitant, AV-818, BIIF
1149CL, CPI 22,721, DNK-333,
GSK-424887, L-733060, L-759274, LY-686017, M516102 and TA-5538, and analogs
and derivatives
thereof;
opioid receptor antagonists, including but not limited to butorphanol,
cyprodime, levallorphan
(lorfan or naloxiphan), nalbuphine, nalorphine (lethidrone or nalline),
naloxone, naloxol, nalmefene,
naltrexone (e.g., naltrexone 1% cream) and naltrexol, and analogs and
derivatives thereof;
opioid receptor agonists, including but not limited to selective kappa plaid
receptor agonists
(e.g., asimadoline, bremazocine, dynorphin, enadoline, ketazocine,
nalfurafine, salvinorin A, 2-
methoxymethyl salvinorin B, 2-ethoxymethyl salvinorin B, 2-fluoroethoxymethyl
salvinorin B,
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
spiradoline, tifluadom, BRL-52537, FE 200665, GR-89696, HZ-2, ICI-199,441, ICI-
204,448, LPK-26, U-
50488 and U-69,593), and analogs and derivatives thereof;
Janus kinase (JAK) inhibitors, including but not limited to JAK1 inhibitors
(e.g., GLPG0634 and
GSK2586184), JAK2 inhibitors (e.g., lestaurtinib, pacritinib, CYT387 and
TG101348), JAK1/JAK2
inhibitors (e.g., baricitinib and ruxolitinib), and JAK3 inhibitors (e.g.,
tofacitinib), and analogs and
derivatives thereof;
immunomodulators and immunosuppressants, including but not limited to
thalidomide,
antimetabolites (e.g., antifolates such as methotrexate), and calcineurin
inhibitors (e.g., ciclosporin
[cyclosporinj, pimecrolimus and tacrolimus), and analogs and derivatives
thereof;
antidepressants, including but not limited to tricyclic antidepressants (e.g.,
amitriptyline,
amitriptylinoxide, amoxapine, dosulepin [dothiepin], doxepin and melitracen),
tetracyclic
antidepressants (e.g., amoxapine, maprotiline, mazindol, mianserin,
mirtazapine and setiptiline),
selective serotonin reuptake inhibitors (SSRls, e.g., citalopram, dapoxetine,
escitalopram, fluoxetine,
fluvoxamine, paroxetine and sertraline), and serotonin-norepinephrine reuptake
inhibitors (SNRIs, e.g.,
bicifadine, duloxetine, milnacipran, levomilnacipran, sibutramine,
venlafaxine, desvenlafaxine and SEP-
227162), and analogs and derivatives thereof;
anticonvulsants, including but not limited to carbamazepine, gabapentin,
pregabalin, and
valproic acid and salts thereof (e.g., sodium valproate), and analogs and
derivatives thereof;
corticosteroids, including but not limited to hydrocortisone types (e.g.,
cortisone and derivatives
thereof [e.g., cortisone acetate], hydrocortisone and derivatives thereof
[e.g., hydrocortisone acetate,
hydrocortisone-17-aceponate, hydrocortisone-17-buteprate, hydrocortisone-17-
butyrate and
hydrocortisone-17-valerate], prednisolone, methylprednisolone and derivatives
thereof [e.g.,
methylprednisolone aceponate], prednisone, and tixocortol and derivatives
thereof [e.g., tixocortol
pivalate]), betamethasone types (e.g., betamethasone and derivatives thereof
[e.g., betamethasone
dipropionate, betamethasone sodium phosphate and betamethasone valerate],
dexamethasone and
derivatives thereof [e.g., dexamethasone sodium phosphate], and fluocortolone
and derivatives thereof
[e.g., fluocortolone caproate and fluocortolone pivalate]), halogenated
steroids (e.g., alclometasone and
derivatives thereof [e.g., alclometasone dipropionate], beclometasone and
derivatives thereof [e.g.,
beclometasone dipropionate], clobetasol and derivatives thereof [e.g.,
clobetasol-17-propionatel,
clobetasone and derivatives thereof [e.g., clobetasone-17-butyrate],
desoximetasone and derivatives
thereof [e.g., desoximetasone acetate], diflorasone and derivatives thereof
[e.g., dMorasone diacetate],
diflucortolone and derivatives thereof [e.g., diflucortolone valerate],
fluprednidene and derivatives
thereof [e.g., fluprednidene acetate], fluticasone and derivatives thereof
[e.g., fluticasone propionate],
halobetasol [ulobetasol] and derivatives thereof [e.g., halobetasol
proprionate], halometasone and
26
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
derivatives thereof [e.g., halometasone acetate], and mometasone and
derivatives thereof [e.g.,
mometasone furoate]), acetonides and related substances (e.g., amcinonide,
budesonide, ciclesonide,
desonide, fiuocinonide, fiuocinolone acetonide, flurandrenolide [
flurandrenolone or fludroxycortide],
halcinonide, triamcinolone acetonide and triamcinolone alcohol), and
carbonates (e.g., prednicarbate),
and analogs and derivatives thereof;
local anesthetics, including but not limited to amides (e.g., articaine,
bupivacaine, cinchocaine
[dibucaine], etidocaine, levobupivacaine, lidocaine [e.g., lidocaine 2.5-5%
cream], prilocaine [e.g.,
prilocaine 2.5% cream], EMLA [lidocaine 2.5%/prilocaine 2.5% cream],
mepivacaine, ropivacaine and
trimecaine), esters (e.g., benzocaine, chloroprocaine, cocaine,
cyclomethycaine, dimethocaine
[larocaine], piperocaine, procaine [novocaine], proparacaine, propoxycaine,
stovaine and tetracaine
[amethocaine]), ethers (e.g., polidocanol [e.g., polidocanol 3% foam] and
pramocaine [pramoxine] (e.g.,
pramoxine 1% cream% and naturally derived local anesthetics (e.g., cocaine,
eugenol, menthol,
saxitoxin, neosaxitoxin and tetrodotoxin), and analogs and derivatives
thereof;
counterirritants and cooling agents, including but not limited to capsaicin,
camphor, mint oil,
menthol (e.g., menthol 1-3% cream), and phenol (e.g., in calamine lotion), and
analogs and derivatives
thereof;
moisturizers, including but not limited to aqueous moisturizers, low pH
moisturizers containing
an acid (e.g., lactic acid), and moisturizers containing a humectant that
attracts and retains water (e.g.,
glycerol, sorbitol, lactate, urea, and hyaluronic acid and salts thereof), an
occlusive that prevents
evaporation {e.g., oils (e.g., mineral oil and silicone oil [e.g.,
dimethicone]) and petroleum jelly
(petrolatum)}, and/or an emollient that provides partial hydration and
occlusion (e.g., oils, waxes [e.g.,
lanolin and paraffin], lipids [e.g., phospholipids, ceramides, triglycerides,
glycol stearate, glyceryl
stearate, fatty acids and squalene], and sterols [e.g., cholesterol and
phytosterol]), and analogs and
derivatives thereof; and
other kinds of antipruritic agents, including but not limited to S-adenosyl
methionine, botulinum
toxin (e.g., botulinum toxin types A and 6), vitamin D and analogs and
derivatives thereof (e.g., calcitriol
and calcipotriol [calcipotriene]), non-steroidal anti-inflammatory drugs
(NSAIDs, e.g., aspirin),
cannabinoid receptor agonists (e.g., C62 agonists, such as
palmitoylethanolamide), inhibitors of
cytokines (e.g., antibodies to interleukins, such as IL-31), antagonists of
the prostaglandin D2 receptor
(D131) and/or the chemoattractant receptor homologous molecule expressed on
TH2 cells (CRTH2)
(e.g., TS-022), phosphodiesterase (PDE) inhibitors (e.g., PDE4 inhibitors,
such as apremilast),
protease-activated receptor 2 (PAR2) antagonists (e.g., GB83), transient
receptor potential vanilloid
(TRPV) antagonists (e.g., TRPV1 antagonists, such as capsazepine and SB-
705498), inhibitors of
neurotrophic tyrosine kinase receptors (e.g., TrkA inhibitors, such as CT327),
antimicrobials (including
27
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
antibiotics, antifungals, antivirals and antiparasitics, such as crotamiton
and rifampin trifampicinj), bile
absorption-reducing or bile sequestering agents (e.g., ursodeoxycholic acid
[ursodiol]), ultraviolet
radiation (e.g., ultraviolet A and B), and therapeutic agents that treat the
underlying causes of the
pruritus-associated conditions, and analogs and derivatives thereof.
[0088] If desired (e.g., for relief from pruritus during the day), a non-
sedating antipruritic agent can be
used. For example, second-generation and third-generation antihistamines are
designed to be non-
sedating, or less sedating than first-generation antihistamines. Non-limiting
examples of second-
generation and third-generation antihistamines include acrivastine,
astemizole, azelastine, bepotastine,
bilastine, cetirizine, levocetirizine, ebastine, fexofenadine, ketotifen,
levocabastine, loratadine,
desloratadine, mizolastine, olopatadine, quifenadine, rupatadine and
terfenadine.
[0089] In some embodiments, a corticosteroid of moderate or medium potency is
used in combination
with serlopitant to treat a pruritus-associated condition. Examples of
corticosteroids having moderate
or medium potency include Groups III, IV and V corticosteroids under the 7-
group US classification
system and Class H corticosteroids under the 4-class European classification
system, including without
limitation amcinonide 0.1% (e.g., cream), betamethasone dipropionate 0.05%
(e.g., Diprosonee
cream/ointment), betamethasone valerate 0.1% (e.g., cream/ointment),
clobetasone butyrate 0.05%
(e.g., Eumovate cream), desonide 0,05% (e.g., Tridesilon cream/ointment and
DesOwen
cream/ointment), fiuocinolone acetonide 0.01-0.2% (e.g., Synetar
cream/ointment and Synemole
cream), flurandrenolide 0.05% (e.g., Cordran tape), fluticasone propionate
0.005% (e.g., Cutivate
ointment), fluticasone propionate 0.05% (e.g., Cutivate cream), halometasone
0.05% (e.g., cream),
hydrocortisone butyrate 0.1% (e.g., Locoid cream/ointment), hydrocortisone
valerate 0.2% (e.g.,
Westcort cream/ointment), mometasone furoate 0.1% (e.g., Elocon
cream/ointment), triamcinolone
acetonide 0.025-0.5% (e.g., Aristocort cream/ointment, Kenacomb
cream/ointment, Kenalog
cream and Viaderm KC cream/ointment), and triamcinolone diacetate 0.5% (e.g.,
cream/ointment).
[0090] The optional additional antipruritic agent(s) can be administered to a
subject suffering from
pruritus concurrently with (e.g., in the same composition as serlopitant or in
separate compositions) or
sequentially to (before or after) administration of serlopitant. Serlopitant
and the optional additional
antipruritic agent(s) independently can be administered in any suitable mode,
including without
limitation orally, topically (e.g., dermally/epicutaneously, transdermally,
mucosally, transmucosally,
intranasally [e.g., by nasal spray or drop], opthalmically [e.g., by eye
drop], pulmonarily [e.g., by
inhalation], bucally, sublingually, rectally and vaginally), by injection or
infusion (e.g., parenterally,
including intramuscularly, subcutaneously, intradermally,
intravenously/intravascularly, and
intrathecally), and by implantation (e.g., subcutaneously and
intramuscularly). In some embodiments,
28
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
an antipruritic agent is administered topically (e.g., dermally) if the
pruritus is localized, and is
administered systemically (e.g., orally or intravenously) if the pruritus is
widespread (generalized) or
has a systemic cause. In certain embodiments, serlopitant and/or the optional
additional antipruritic
agent(s) are administered orally. In other embodiments, serlopitant and/or the
optional additional
antipruritic agent(s) are administered topically (e.g., dermally, mucosally,
bucally or sublingually).
[00911 Serlopitant and the optional additional antipruritic agent(s)
independently can be administered in
any suitable frequency, including without limitation daily (one, two, three or
more times per day), every
two days, twice weekly, thrice weekly, weekly, every two weeks, every three
weeks, monthly, every two
months and every three months. The dosing frequency can depend on, e.g., the
mode of
administration chosen. For example, a dermal formulation of serlopitant,
and/or that of the optional
additional antipruritic agent(s), can be applied to the skin of a subject two,
three or four times a day. In
some embodiments, serlopitant is administered under a chronic dosing regimen.
In certain
embodiments, serlopitant is administered over a period of at least 2 weeks, 3
weeks, 1 month, 1.5
months, 2 months, 3 months, 4 months, 5 months, 6 months or longer.
[00921 Examples of topical dosage forms include without limitation creams,
ointments, gels, liniments,
lotions, suppositories (e.g., rectal and vaginal suppositories), buccal and
sublingual tablets and pills,
sprays (e.g., dermal and nasal sprays), and drops (e.g., eye, nose and ear
drops). Non-limiting
examples of oral dosage forms include solid dosage forms (e.g., cachets,
capsules and tablets) and
liquid dosage forms (e.g., solutions or suspensions in an aqueous liquid
and/or a non-aqueous liquid,
and oil-in-water liquid emulsions or water-in-oil liquid emulsions). In a non-
limiting example of a
formulation for injection, the formulation is in the form of a solution and
comprises an antipruritic agent
(e.g., a local anesthetic), a vehicle (e.g., a water-based vehicle or sterile
water), a buffer, a reducing
agent/antioxidant (e.g., sodium metabisulfite if epinephrine is used as a
vasoconstrictor) and a
preservative (e.g., methylparaben), and optionally a vasoconstrictor (e.g.,
epinephrine) to increase the
duration of the pharmacological effect of the antipruritic agent by
constricting the blood vessels, thereby
concentrating the antipruritic agent for an extended duration and increasing
the maximum dose of the
antipruritic agent.
[00931 Table 4 provides non-limiting examples of combination therapies
employing serlopitant and one
or more additional antipruritic agents for the treatment of pruritus
associated with various conditions.
Table 4 may also show other therapeutic agents used to treat the underlying
causes of the conditions.
29
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
Table 4
Agents in Addition to Serlopitant Conditions
Corticosteroid Skin inflammation, chapped skin,
atopic
dermatitis, contact dermatitis, eczema,
seborrheic dermatitis, erythroderma, lichen
planus, lichen simplex chronicus, lichen
sclerosis, lupus erythematosus, psoriasis,
rashes, scabies and bums (e.g., sunburn)
Antihistamine (e.g., doxepin for topical use, and Urticaria, allergy-based
pruritus, localized
sedating diphenhydramine or non-sedating pruritus (e.g., insect bites and
stings) and
cetirizine for oral use) generalized pruritus (e.g.,
chickenpox)
Local anesthetic + optional counterirritant/ Localized pruritus (e.g.,
insect bites and stings),
cooling agent and mild to moderate pruritus
Counterirritant (e.g., capsaicin) Chronic localized pruritus (e.g.,
notalgia
paresthetica and prurigo nodularis)
Moisturizer &/or calamine Allergic rashes (e.g., poison
ivy/oak and
urticaria), burns (e.g., sunburn), and insect bites
and stings
Moisturizer + optional counterirritant/cooling Atopic dermatitis, contact
dermatitis, eczema,
agent seborrheic dermatitis, ichthyosis,
psoriasis and
xerosis
Immunomodulator (e.g., tacrolimus) + optional Atopic dermatitis
corticosteroid
JAK inhibitor (e.g., tofacitinib) or PDE inhibitor Psoriasis
(e.g., apremilast) or vitamin D (e.g., calcipotriol)
TrkA inhibitor (e.g., CT327) Atopic dermatitis, psoriasis and
cutaneous T-cell
lymphoma
JAK inhibitor (e.g., ruxolitinib) Anemia, peripheral neuropathy and
polycythemia
vera
Aspirin (topical) Lichen simplex chronicus
Tricyclic antidepressant (e.g., doxepin) Chronic severe pruritus
Opioid receptor antagonist (e.g., naloxone) Intractable pruritus of renal
and cholestatic
diseases
1) Ultraviolet B phototherapy + erythropoietin; or Chronic renal disease
2) Cholestyramine + opioid receptor antagonist
(e.g., naltrexone) + activated charcoal; or
3) Thalidomide
1) Ion-exchange resin (e.g., cholestyramine) + Cholestasis
opioid receptor antagonist (e.g., naloxone); or
2) SSRI, S-adenosyl methionine, rifampicin &/or
ursodeoxycholic acid; or
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
3) Cholestyramine + opioid receptor antagonist
(e.g., nalmefene) + serotonin antagonist (e.g.,
ondansetron) + ursodeoxycholic acid +
rifampicin + optional bright-light therapy; or
4) Ultraviolet B + cannabinoid (e.g., dronabinol)
1) Counterirritant (e.g., capsaidn) + ultraviolet B Uremia (uremic
pruritus)
phototherapy + optional activated charcoal +
optional low pH moisturizer; or
2) Kappa opioid receptor agonist (e.g.,
nalfurafine) + optional ultraviolet B
Ultraviolet B phototherapy Aquagenic dermatitis, atopic
dermatitis,
HIV/AIDS and prurigo nodularis
Ultraviolet A phototherapy + psoralen Eczema, psoriasis, vitiligo and
cutaneous T-cell
lymphoma
1) Ultraviolet A phototherapy + psoralen; or Polycythemia vera
2) SSRI (e.g., paroxetine), aspirin 8,/or interferon
alpha
Serotonin receptor antagonist (e.g., ondansetron) Spinal opioid-induced
pruritus
(concurrent with opioid) + opioid receptor
antagonist (e.g., nalbuphine) (concurrent with
opioid)
Antipsychotic (e.g., pimozide) + SSRI (e.g., Pruritic psychiatric disorders
(e.g., neurotic
fluvoxamine) excoriation)
Use of Serlooitant as a Sleep Aid
[0094] The invention also encompasses the use of serlopitant as a sleep aid.
Accordingly, the
invention provides a method of aiding sleep, comprising administering to a
subject suffering from a
sleep problem or disorder an effective amount of serlopitant or a
pharmaceutically acceptable salt,
solvate or polymorph thereof. An additional sleep-aiding agent optionally can
also be administered to
the subject.
[0096] Serlopitant can aid sleep in subjects who suffer from a sleep disorder
or a sleep problem in
general. As a sleep aid, serlopitant may have a sedative effect (reducing
irritability, anxiety or
excitement) and/or a hypnotic effect (inducing, sustaining and/or lengthening
sleep).
[0096] Examples of sleep disorders that serlopitant can potentially alleviate
include without limitation
insomnia (including primary and secondary insomnia, and transient, acute and
chronic insomnia);
sleeping sickness (African trypanosomiasis); circadian rhythm sleep disorders
(e.g., advanced sleep
phase disorder tASPD], delayed sleep phase disorder [DSPD1, irregular sleep
wake rhythm, non-24
hour sleep-wake disorder, jet lag and shift work sleep disorder [SWSIA);
parasomnias (e.g., bruxism,
31
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
rapid eye movement sleep behavior disorder [RBD], periodic limb movement
disorder [PLMD or
nocturnal myoclonus], restless legs syndrome [RLS], sleep paralysis, exploding
head syndrome, sleep
terror [night terror or Pavor nocturnus], nocturia, nocturnal eating syndrome,
sleep talking [somniloquy],
sleepwalking [somnambulism] and somniphobia); and breathing-related sleep
disorders (e.g., sleep
apnea [including central, obstructive and mixed sleep apnea], hypopnea
syndrome, sleep-related
hypoventilation, snoring and upper airway resistance syndrome).
[0097] For use as a sleep aid, serlopitant is administered when the subject
desires to sleep (e.g., at
night or around bedtime). An effective amount of serlopitant is administered
to aid sleep. The effective
amount may depend on various factors, including the mode of administration;
the age, body weight,
general health, sex and diet of the subject; the severity of the sleep
problem; and the response of the
subject to the treatment. In certain embodiments, the dose of serlopitant as a
sleep aid is about 0.1-
500 mg, or about 0.25-400 mg, or about 0.5-300 mg, or about 1-200 mg, or about
2.5-100 mg, or about
5-50 mg, or as deemed appropriate by the treating physician. A single dose or
multiple doses of
serlopitant can be administered to aid sleep. In further embodiments, the
dosage of serlopitant to aid
sleep is about 0.01- 10 mg/kg, 0.025- 7.5 mg/kg, 0.05-5 mg/kg, 0.075-2.5 mg/kg
or 0.1-1 mg/kg body
weight, or as deemed appropriate by the treating physician.
[0098] Serlopitant can be administered via any suitable route. Potential
routes of administration of
serlopitant include without limitation oral, parenteral (including
intramuscular, subcutaneous,
intradermal, intravenous, intraarterial, intramedullary and intrathecal),
intraperitoneal, and topical
(including dermal/epicutaneous, transdermal, mucosa!, transmucosal, intranasal
[e.g., by nasal spray or
drop], intraocular [e.g., by eye drop], pulmonary [e.g., by inhalation],
buccal, sublingual, rectal and
vaginal). In certain embodiments, serlopitant is administered orally.
[0099] In other embodiments, serlopitant is administered topically via a
buccal or sublingual tablet or
pill. The buccal or sublingual tablet or pill can be designed to provide
faster release of serlopitant for
more rapid uptake of it into systemic circulation. In addition to a
therapeutically effective amount of
serlopitant, the buccal or sublingual tablet or pill can contain suitable
excipients, including without
limitation any combination of fillers and diluents (e.g., mannitol and
sorbitol), binding agents (e.g.,
sodium carbonate), wetting agents (e.g., sodium carbonate), disintegrants
(e.g., crospovidone and
croscarmellose sodium), lubricants (e.g., silicon dioxide [including colloidal
silicon dioxide] and sodium
stearyl fumarate), stabilizers (e.g., sodium bicarbonate), flavoring agents
(e.g., spearmint flavor),
sweetening agents (e.g., sucralose), and coloring agents (e.g., yellow iron
oxide). A non-limiting
example of a patient population that can benefit from a buccal or sublingual
tablet or pill of a sleep aid
is patients who wake up prematurely and have difficulty falling asleep again.
32
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
[00100] In some embodiments, an (one or more) additional sleep-aiding agent is
administered in
combination with serlopitant to aid sleep. The additional sleep-aiding agent
can be administered
concurrently with or sequentially to (before or after) administration of
serlopitant. If administered
concurrently with serlopitant, the additional sleep-aiding agent can be
contained in the same
composition as serlopitant or in separate compositions. Use of serlopitant may
reduce the dosage of
and/or the length of treatment with the additional sleep-aiding agent which
would otherwise be required
and thereby minimize or avoid any adverse effects (e.g., dependence or
addiction) of the additional
sleep-aiding agent.
[00101] The additional sleep-aiding agent can be selected for its soporific
property or for its ability to
treat the sleep disorder or the underlying cause of the sleep disorder (e.g.,
stress, anxiety, depression
or a neurological condition). In some embodiments, the additional sleep-aiding
agent is selected from
the group consisting of hypnotics, sedatives, anxiolytics, antipsychotics and
antidepressants. A
particular sleep-aiding agent can have pharmacological effects that fall in
multiple categories (e.g.,
benzodiazepines can have a sedative or anxiolytic effect at a lower dose and a
hypnotic effect at a
higher dose). In further embodiments, the additional sleep-aiding agent is
selected from the group
consisting of:
antidepressants, including tricyclic antidepressants (e.g., amitriptyline,
amitriptylinoxide,
amoxapine, clomipramine, desipramine, dosulepin [dothiepin], doxepin,
imipramine, lofepramine,
melitracen, nortriptyline, protriptyline and trimipramine), tetracyclic
antidepressants (e.g., amoxapine,
maprotiline, mazindol, mianserin, mirtazapine and setiptiline), selective
serotonin reuptake inhibitors
(SSR1s, e.g., citaloprann, dapoxetine, escitalopram, fluoxetine, fluvoxamine,
paroxetine and sertraline),
serotonin antagonist and reuptake inhibitors (SARls, e.g., etoperidone,
lorpiprazole, lubazodone,
mepiprazole, nefazodone and trazodone), serotonin-norepinephrine reuptake
inhibitors (SNRIs, e.g.,
bicifadine, duloxetine, milnacipran, levomilnacipran, sibutramine,
venlafaxine, desvenlafaxine and SEP-
227162), and monoamine oxidase (MAO) inhibitors (including selective MAO-A
inhibitors, such as
moclobemide, pirlindole [pirazidolj and toloxatone [humory1]), and analogs and
derivatives thereof;
antipsychotics, including first-generation (or typical) antipsychotics
(including phenothiazines
[e.g., chlorpromazine, fluphenazine, levomepromazine, perazine, pericyazine,
perphenazine,
pipotiazine, prochlorperazine, promazine, promethazine, thioproperazine,
thioridazine and
trffluoperazine] and thioxanthenes [e.g., clopenthixol, zuclopenthixol,
flupentixol and thiotixeneD and
second-generation (or atypical) antipsychotics (e.g., amisulpride,
aripiprazole, asenapine, dozapine,
iloperidone, loxapine, amoxapine, lurasidone, olanzapine, quetiapine,
norquetiapine, risperidone,
paliperidone, sertindole, trimiprannine, ziprasidone and zotepine), and
analogs and derivatives thereof;
33
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
antihistamines that inhibit action at the histamine H1 receptor, including
first-generation
antihistamines such as alimemazine (trimeprazine), antazoline, azatadine,
bromazine, carbinoxamine,
chlorpromazine, clemastine, clocinizine, cyclizine, chlorcyclizine,
cyproheptadine, dimenhydrinate,
dimetindene, diphenhydramine, bromodiphenhydramine, chlorodiphenhydramine,
doxylamine,
hydroxyzine, meclizine, mepyramine [pyrilamine], methdilazine, oxatomide,
phenindamine,
pheniramine, brompheniramine, chlorpheniramine, fluorpheniramine,
orphenadrine, phenyltoloxamine,
promethazine, tripelennamine and triprolidine, and analogs and derivatives
thereof;
benzodiazepines that enhance the effect of gamma-aminobutyric acid (GABA) at
the GABAA
receptor by positive allosteric modulation of the receptor, such as
adinazolam, alprazolam,
chlordiazepoxide, climazolam, clonazepam, clorazepate, diazepam, estazolam,
etizolam (a
benzodiazepine analog), flunitrazepam, flurazepam, halazepam, loprazolam,
lorazepam,
lormetazepam, midazolam, nimetazepam, nitrazepam, oxazepam, prazepam,
quazepam, temazepam
and triazolam, and analogs and derivatives thereof;
non-benzodiazepines (also called Z-drugs) that are positive allosteric
modulators of the GABAA
receptor, such as beta-carbolines (e.g., abecarnil, gedocarnil and ZK-93423),
cyclopyrrolones (e.g.,
pagoclone, pazinaclone, suproclone, suriclone, zopiclone and eszopiclone,),
imidazopyridines (e.g.,
alpidem, necopidem, saripidem and zolpidem), pyrazolopyrimidines (e.g.,
divaplon, fasiplon, indiplon,
lorediplon, ocinaplon, panadiplon, taniplon and zaieplon), and
triazolopyridazines (e.g., CL-218,872),
and analogs and derivatives thereof;
barbiturates that are positive allosteric modulators of the GABAA receptor,
such as allobarbital,
amobarbital, aprobarbital, alphenal, barbital, brallobarbital, butabarbital,
mephobarbital, pentobarbital,
phenobarbital, secobarbital and sodium thiopental, and analogs and derivatives
thereof;
GABA analogs, such as gabapentin and pregabalin, and analogs and derivatives
thereof;
melatonin receptor (e.g., MT., and/or MT2) agonists, such as melatonin,
agomelatine, LY-
156,735, piromelatine, ramelteon and tasimelteon, and analogs and derivatives
thereof;
orexin receptor (e.g., OX, and/or OX2) antagonists, such as almorexant,
suvorexant, SB-
334,867, SB-408,124, SB-649,868, TCS-0X2-29, and N-Ethyl-2-[(6-methoxy-pyridin-
3-y1)-(toluene-2-
sulfony1)-amino]-N-pyridin-3-ylmethyl-acetamide (EMPA), and analogs and
derivatives thereof;
4-quinazolinones, such as afloqualone, cloroqualone, diproqualone, etaqualone,
mebroqualone,
mecloqualone, methaqualone, methylmethaqualone and nitromethaqualone, and
analogs and
derivatives thereof;
opioids (e.g., for pain-associated sleep disorders), such as buprenorphine,
codeine, fentanyl,
hydrocodone, hydromorphone, levorphanol, methadone, morphine, ethylmorphine,
oxycodone,
34
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
oxymorphone, pethidine, propoxyphene, dextropropoxyphene, thebaine and
tramadol, and analogs and
derivatives thereof;
herbs, such as Cannabis (including cannabinoids such as cannabidiol [CM] and
tetrahydrocannabinol [THCD, Duboisia hopwoodii (pituri), Humulus lupulus
(hops), Hypericum
perforatum (St. John's wort), Lactuca virosa (opium lettuce), Lavandula
(lavender), Matricaria
chamomilla (chamomile), Nepeta cataria (catnip), Passiflora (passion flowers)
(e.g., P. incamata), Piper
methysticum (kava), Prostanthera striatitiora (striped mintbush), Sceletium
tortuosum (kanna),
Scutellaria (skullcaps) (e.g., S. canescens, S. cordifolia, S. galericulata
and S. lateriflora), Valeriana
officinalis (valerian), and Withania somnifera (ashwagandha); and
other kinds of substances, such as S-adenosyl-L-homocysteine, L-tryptophan, L-
arginine-L-
aspartate, delta sleep-inducing peptide (DS1P), chloral hydrate, ethanol, 2-
methyl-2-butanol, gamma-
hydroxybutyric acid (GHB), glutethimide, medetomidine, dexmedetomidine,
menthyl isovalerate
(validol), S32212, a2 adrenergic agonists (e.g., clonidine), and carbonic
anhydrase inhibitors (e.g.,
acetazolamide and topiramate), and analogs and derivatives thereof.
[00102] The additional sleep-aiding agent can also be selected for its ability
to treat a condition that
contributes to sleep difficulty (e.g., abnormal bodily movement or behavior).
For example, an
anticonvulsant can be used in combination with serlopitant to treat a
parasomnia, such as restless legs
syndrome, periodic limb movement disorder or nocturnal eating syndrome.
Examples of
anticonvulsants include without limitation carbamazepine, gabapentin,
pregabalin, valproic acid and
salts thereof (e.g., sodium valproate), and analogs and derivatives thereof.
[00103] The additional sleep-aiding agent can be administered via any suitable
mode. In certain
embodiments, the additional sleep-aiding agent is administered orally, bucally
or sublingually.
Therapeutic Administration and Doses
[00104] The terms "administration of' or "administering a" compound should be
understood to mean
providing a compound of the invention to the individual in need of treatment
in a form that can be
introduced into that individuals body in a therapeutically useful form and
therapeutically effective
amount, including, but not limited to, oral dosage forms, such as tablets,
capsules, syrups,
suspensions, and the like.
[00105] The terms "treat", "treating" and "treatment" of chronic pruritus all
refer to reducing the
frequency of symptoms of acute or chronic pruritus (including eliminating them
entirely), avoiding the
occurrence of acute or chronic pruritus and/or reducing the severity of
symptoms of acute or chronic
pruritus.
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
[00106] The term "therapeutically effective amount" refers to a sufficient
quantity of the compounds of
the present invention, in a suitable composition, and in a suitable dosage
form to treat the noted
disease conditions. The "therapeutically effective amount" will vary depending
on the compound, the
severity of the condition causing the pruritus, and the age, weight, etc., of
the patient to be treated.
[00107] The term "loading dose" refers to the amount of the compounds or
compositions of the present
invention that is often larger than subsequent doses, administered for the
purpose of establishing a
therapeutic level of the drug. More generally, a loading dose is the amount of
Compound I, or a
pharmaceutically acceptable salt, solvate or polymorph thereof, administered
to a patient with pruritus
given sometime after presentation but before initiation of one or more
maintenance doses.
Alternatively, a loading dose refers to one or a series of doses that may be
given at the onset of therapy
to achieve a target concentration of an active ingredient quickly.
[00108] The present methods for treatment of pruritus require administration
of serlopitant, or a
pharmaceutical composition containing serlopitant, to a patient in need of
such treatment. The
compound and/or pharmaceutical compositions are preferably administered
orally. Various delivery
systems are known, (e.g., encapsulation in liposomes, microparticles,
microcapsules, capsules, etc.)
can be used to administer a serlopitant compound and/or composition. The
compound and/or
pharmaceutical compositions may be delivered via sustained release dosage
forms.
[00109] The amount of serlopitant, a pharmaceutically acceptable salt, solvate
or polymorph thereof,
that will be effective in the treatment pruritus in a patient will depend on
the specific nature of the
condition, and can be determined by standard clinical techniques known in the
art. In addition, in vitro
or in vivo assays may optionally be employed to help identify optimal dosage
ranges. The specific dose
level for any particular individual will depend upon a variety of factors
including the activity of the
composition, the age, body weight, general physical and mental health, genetic
factors, environmental
influences, sex, diet, time of administration, route of administration, rate
of excretion, and the severity of
the pruritus being treated.
[00110] Preferably, the dosage forms are adapted to be administered to a
patient three, two or one time
a day. More preferably, a therapeutically effective amount is taken once per
day. Alternatively, a dose
may be taken every other day, every third day, every fourth day or once a
week. In some
embodiments, serlopitant is administered under a chronic dosing regimen. In
certain embodiments, a
therapeutically effective amount of serlopitant is administered over a period
of at least 2 weeks, 3
weeks, 1 month, 1.5 months, 2 months, 3 months, 4 months, 5 months, 6 months
or longer.
36
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
[00111 Doses may be taken at any time convenient to the patient. However, to
minimize side effects
such as dizziness or drowsiness, a daily dose may be taken at bedtime. NK-1
receptor antagonists
have been shown to cause drowsiness in human clinical trials for uses other
than treating pruritus. For
example, Ratti et aL reported as much as a doubling in the incidence of
somnolence vs. placebo in
patients treated with casopitant for major depressive disorder (J. Clin.
PsychopharmacoL, 2011,
31:727-733). Somnolence was also seen in a similar clinical trial testing NK-1
receptor antagonist L-
759274 as an anti-depressant (M. S. Kramer et al., Neuropsychopharm., 2004,
29:385-392). Thus, in
one embodiment of the present invention, serlopitant is administered before
the patient goes to bed.
[00112] Dosing may be provided alone or in combination with other drugs and
may continue as long as
required for effective treatment pruritus. For example, the compounds of the
present invention may be
administered in combination with another substance that has a complimentary
effect to the tachykinin
and substance P inhibitory effect of the present invention. Appropriate
compounds include other NK-1
receptor antagonists such as, but not limited to, casopitant (GW679769), L-
759274, L-733060,
CPI 22,721, BIIF 1149CL, DNK333, M516102, ezlopitant, rolapitant, orvepitant,
LY-686017, lanepitant
(LY-303870), maropitant, vestipitant, vofopitant, aprepitant, fosaprepitant,
AV-818, and TA-5538.
[00113] Dosage ranges of compounds of the present invention for oral
administration may be stated in
terms of amount of drug administered per time period. A certain amount of
active ingredient may be
given one or more times a day as appropriate according to the factors
described above. For example,
doses may be taken once a day, twice a day, three times a day, four times a
day, or more. Suitable
dosages range from about 0.1 mg to about 30 mg, and preferably, from about 1
mg to about 7.5 mg.
Suitable dosages are typically 0.10 mg, 0.15 mg, 0.20 mg, 0.25 mg, 0.5 mg,
0.75 mg, 1 mg, 2 mg, 2.5
mg, 3 mg, 4 mg, 5 mg, 6 mg, 7 mg, 8 mg, 9 mg, 10 mg, 15 mg, 20 mg, 25 mg, 30
mg, 35 mg, 40 mg,
50 mg, 100 mg or 200 mg one or more times a day. Preferably, a dose of 0.25
mg, 1 mg or 5 mg is
administered once a day.
[001141 Alternatively, suitable dosage ranges of compounds of the present
invention for oral
administration are generally about 0.001 mg to about 500 mg of drug per
kilogram body weight,
preferably from about 0.1 mg to about 200 mg of drug per kilogram body weight,
and more preferably
about 1 to about 100 mg/kg-body wt. per day. Dosage ranges may be readily
determined by methods
known to the skilled artisan. The amount of active ingredient that may be, for
instance, combined with
carrier materials to produce a single dosage form will vary depending upon the
patient treated and the
particular mode of administration. Dosage unit forms will generally contain
between about 0.25 mg to
about 500 mg of active ingredient.
37
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
[00115] In cases in which longer-term persistence of active drug is desirable,
for example but not
limited to, in the treatment of chronic pruritus, a dosing schedule is used
where a loading dose is
administered, followed by either (1) a second loading dose, or doses, and a
maintenance dose (or
doses), or (ii) a maintenance dose or doses, without a second loading dose, as
determined to be
appropriate by one skilled in the art. The schedule for administration of the
loading and maintenance
doses may be determined based upon the individual requirements of a particular
patient. In one
embodiment of the present invention, one loading dose is administered,
followed by administration of a
therapeutically effective maintenance dose after an appropriate interval, such
as after one day. In
another embodiment, a loading dose is administered on day 1, a second loading
dose on day 2, and
the maintenance dose is administered on day 3 and thereafter for the duration
of therapy. The loading
dose may be five, four, three or two times the maintenance dose. Preferably,
the loading dose is three
times the maintenance dose. The active drug can be administered via any
suitable mode (e.g., orally).
Determination of Therapeutic Effectiveness
[00116] The effectiveness of compositions of the present invention can be
tested in experimental
animal models of pruritus known to those skilled in the art. For example,
various mouse models have
been utilized to evaluate treatments for itching. Tsukumo et al. describe a
model in which 4-
ethoxymethylene-2-pheny1-2-oxazolin-5-one (oxazolone) induces chronic
dermatitis with an associated
itch response in BALB/c mice that can be used to determine whether an anti-
pruritic treatment is
effective (J. Pharmacol. Sci., 2010, 113:255-262). Costa etal. report a
similar model in which
Phoneutria nigriventer spider venom is used as the itch inducer (VascuL
Pharmacol., 2006, 46(4)209-
14). Analogously, Ohmura et at. use picrylchloride in NC/Nga mice to stimulate
scratching behavior
(Eur. J. Phannacol., 2004; 491:191-194). Essentially, itching is induced in
the subject animal with an
irritating agent, the test compound or a placebo is administered, and the
animal observed under
controlled conditions. Scratching behavior is quantified and analyzed using
standard statistical
techniques. A test compound is considered effective if either continuous or
severe scratching is
suppressed.
[00117] The efficacy of the methods and compositions of the present invention
in the treatment of acute
and chronic pruritus can also optionally be evaluated in human clinical trials
conducted under
appropriate standards and ethical guidelines as set forth by the U.S. Food and
Drug Administration
(FDA). After the general safety of a drug is determined in Phase I clinical
trials conducted in healthy
volunteers, Phase ll trials assessing the safety and efficacy of the drug in
patients with the condition
being treated are conducted. Typically, such trials are double-blinded and
placebo-controlled, and may
38
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
be dose-ranging. Phase Ill studies gather more information about safety and
effectiveness by studying
different populations and different dosages and by using the drug in
combination with other drugs.
[00118] Because amelioration of pruritus is subject to a patient's own
perceptions, it can be difficult to
evaluate with typical clinical endpoints. However, two standardized assessment
tools have been
created and may be used in clinical trials demonstrating the utility of the
present invention. The Visual
Analog Scale (VAS) is the most commonly used tool to evaluate the intensity of
pruritus (N. 0. Phan et
al., Acta Derm. Venereol., 2012; 92:502-507). The VAS is a graphic tool with a
100-mm horizontal line
with the left end labeled "no symptom" and the right end labeled "worst
imaginable symptom". The
patient is asked to draw a vertical line to indicate the horizontal scale at a
point that corresponded to
the intensity of the symptom. The length from the left end to the vertical
mark made by the patient is
measured in millimeters. Separation in one-hundredths is regarded as
sufficiently sensitive (R. C.
Aitken, Proc. R. Soc. Med., 1969, 62:989-993). The results may be analyzed
using standard statistical
techniques known to those skilled in the art.
[00119] In addition to the VAS, the Dermatology Life Quality Index (DLQI) may
be used to evaluate the
efficacy of a chronic pruritus treatment. The DLQI, a self-administered
general dermatology quality of
life questionnaire, was originally developed and published in a dermatology
clinic at University Hospital
of Wales (A. Y. Finlay and G. K. Khan, Clin. Exper. Derm., 1994,19:210-216).
Independent studies
have verified that the DLQI is an easy and efficient method for assessing
quality of life in dermatology
patients (H. B. Hahn et al., J. Am. Acad. DermatoL, 2001, 45(1):44-8). A
current version of the simple,
ten-question validated questionnaire, with instructions for use and scoring is
available from the School
of Medicine, Cardiff University, Wales, UK (world wide web URL
dermatology.org.uk/quality/).
[00120] The following examples are offered by way of illustration and not by
way of limitation.
EXAMPLES
[00121] All of the inactive pharmaceutical ingredients in the examples below
comply with United States
Pharmacopeia and The National Formulary requirements and are tested and
released according to the
monograph for each ingredient specified in the USP/NF compendium.
Example 1. Preparation of Serlopitant Tablets
[00122] Serlopitant, 3-[(3aR,4R,5S,7aS)-5-[(1R)-143,5-
bis(trifluoromethyl)phenyllethoxy]-4-(4-
fluoropheny1)-1,3,3a,4,5,6,7,7a-octahydroisoindol-2-yljcyclopent-2-en-1-one,
Compound 1, may be
formulated as a tablet for oral use. Table 1 shows the
qualitative/quantitative composition of exemplary
39
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
dosages. Minor variations in the excipient quantities (+/-10 %) may occur
during the drug development
process.
Table '1
Components Function % of
composition
Compound 1 Active agent 1-6 %
Microcrystalline cellulose Diluent 50-60%
Mannitol Diluent 20-30%
Croscarmellose Sodium Disintegrant 1-3%
Colloidal silica Disintegrant 0.25-0.5%
Sodium Lauryl Sulfate Surfactant 5-6%
Magnesium Stearate Lubricant 0.25-2%
Total Tablet Composition 100%
[00123] Tablet potencies of 0.25, 1 and 5 mg are prepared as a compressed
tablet formulation.
The tablet manufacturing process is the same for all proposed potencies. The
process consists of the
following steps: 1) Compound 1, mannitol and sodium lauryl sulfate are
blended; 2) the remaining
mannitol is added to the blender and mixed; 3) microcrystalline cellulose,
croscarmellose sodium, and
colloidal silica are added to the blender containing the mixture above to
complete the mixing and
the blend is de-agglomerated if necessary; 4) the blend is lubricated with
magnesium stearate
which has been previously screened, if necessary; 5) the lubricated blend is
roller compacted
and milled, and then lubricated with magnesium stearate, which has been
previously screened, if
necessary; and 6) the mixture is then compressed into tablets of the
appropriate weight.
Example 2. Preparation of Serlopitant Capsules
[00124] Serlopitant (Compound 1) may also be supplied to the clinic as liquid-
filled capsules. Table
2 shows the qualitative/quantitative composition of exemplary dosages. Minor
variations in the
excipient quantities (+1-10 %) may occur during the drug development process.
Table 2
Unit Strength
Components Function
0.25 mg 1 mg
4 mg
Capsule Fill
Compound 1 Active agent 0.25 mg 1 mg
4 mg
Mono- & Di-glycerides Solubilizer 399 mg 398.6 mg 395.6
mg
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
Butylated Hydroxyanisole Antioxidant 0.40 mg 0.40 mg 0.40
mg
Capsule Shell
#0 White Opaque Hard Gelatin Capsule shell 96 mg** 96 mg** 96
mg**
Capsule*
Gelatin*** Banding ¨ ¨ ¨
component
Polysorbate 80*** Banding
component
*Capsules are provided by Capsugel (Morristown, NJ) and contain gelatin and
titanium dioxide
**Approximate weight of empty capsule shell
***As needed to seal the capsule shells
[00125] The formulation is prepared by dissolving the drug substance in mono-
and di-glycerides.
Furthermore, 0.1 wt% butylated hydroxyanisole is added as an antioxidant.
Initial capsule strengths
are dispensed into hard gelatin capsules and sealed by spraying with a 1:1
(wt/wt) water:ethanol
solution. Subsequent potencies including 0.25, 1, and 4 mg are dispensed into
hard gelatin
capsules and sealed with a band of gelatin/polysorbate 80. Corresponding
placebo formulations are
prepared in a similar manner, but without the addition of the drug substance
and the antioxidant.
[00126] The capsule manufacturing process is the same for all potencies. The
process consists of
the following steps: 1) the mono- and di-glycerides excipient is melted at 40
C, if necessary; 2) the
mono- and diglycerides are added to an appropriately sized, jacketed vessel
and mixing is initiated; 3)
the butylated hydroxyanisole is added to the mono- and di-glycerides and mixed
until dissolved
(minimum of 10 min); 4) Compound 1 is slowly added to the mixture and mixed
until dissolved (visual
confirmation); 5) the solution is filled into hard gelatin capsules; 6) the
filled capsules are sealed with a
mixture of gelatin and polysorbate 80; 7) the sealed capsules are allowed to
dry overnight and then the
capsules are visually inspected for leaking; 8) the acceptable capsules may be
weighed sorted, if
necessary; and 9) the finished product is then packaged in appropriate
containers.
Example 3. Clinical Study of Serlopitant in Chronic Pruritus
[00127] A well-controlled human clinical trial testing the efficacy of three
dosages of serlopitant in the
treatment of chronic pruritus is conducted in accordance with the ICH
Guidelines for Good Clinical
Practices, the U.S. Code of Federal Regulations, the Health Insurance
Portability and Accountability
Act (H(PAA), and any local regulatory requirements. The study is a Phase II
randomized, double-blind,
parallel group, placebo-controlled, multicenter trial designed to test the
efficacy and safety of several
doses of serlopitant versus placebo in patients with chronic pruritus. The
study patient population
41
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
includes adult, males or females, 18 to 72 years of age. The patients must be
previously diagnosed
with chronic pruritus caused by any etiology, except uremia, hepatic failure,
cancer or cancer therapy,
with chronic pruritus defined as greater than 6 weeks of itching and a VAS
score of greater than 7.
[00128] Patients are randomized to receive either placebo or one of three
doses of active agent.
Patients take active drug or placebo once daily by mouth for a total of 2 to 8
weeks. The maximum
study duration for each subject is approximately 14 weeks and includes a
screening period of up to 2
weeks, a treatment period of 2-8 weeks, and a follow-up period of up to 4
weeks. The study
parameters are summarized in Table 3.
Table 3
Study Title: Phase II Study of Serlopitant In Patients with
Chronic Pruritus
Development Phase: Phase II
Study Objectives: Dose finding, efficacy and safety
Study Design: Multicenter, double blind, parallel group, dose
finding
Sample Size: 80-240 subjects evaluable for analysis
Study Population: Patients with chronic pruritus (over 6 weeks
duration)
unresponsive to standard treatment
Investigational Product: Oral daily tablet
Dosage and frequency:
Day 1: loading dose of 3 times of drug dose (0.25 mg, 1 mg, or
mg), followed by Drug A, Drug B, or Drug C
Drug A: 0.25 mg serlopitant daily for 2 to 8 weeks
Drug B: 1 mg serlopitant daily for 2 to 8 weeks
Drug C: 5 mg serlopitant daily for 2 to 8 weeks
Reference Product(s): None
Control Product(s): Matching placebo daily for 2 to 8 weeks
Efficacy Evaluation Criteria: Efficacy is measured daily by patient diary.
Patients record
pruritus level on a 10 point VAS scale. Clinical response is
measured by a change in VAS score between the active agent
and the placebo. Secondary endpoints will include measures
of the Dermatology Life Quality Index (DLQI),Iesion healing,
and patient and physician global assessments.
Safety Evaluation Criteria: All local and systemic adverse events observed
by or reported
to the investigators are evaluated. The intensity, duration, and
causal relationship to the study product are rated for all
adverse events.
Statistical Methods: The primary study endpoint is the difference in
VAS score at
baseline and on treatment between placebo and active agent.
Study Sites: Multicenter
42
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
[00129] Additional clinical trials according to a similar design may be
conducted to test different dosage
levels of the active ingredient or to differentiate between optimal doses or
dosing schedules. Further,
the efficacy of the drug in specific populations, such as the elderly,
children, or patients with uremia,
hepatic failure, cancer or patients undergoing cancer therapy, may be
determined in additional clinical
trials conducted in a similar fashion.
Example 4. Topical Formulations Containing Serlopitant
[00130] Table 5 shows various topical formulations containing serlopitant. The
formulations contain
VanicreamTm Moisturizing Skin Cream ("VM"), VanicreamTm Lite Lotion ("VLL") or
Aquaphor Healing
Ointment ("AP", from Eucerin) as the base or carrier. VM and VLL are oil-in-
water emulsion and AP
has an oil base. A stock solution of free base serlopitant (Compound 1, or
"Cpd 1") in ethanol (Et0H)
was prepared by dissolving free base serlopitant in ethanol to the maximum
extent and then filtering the
resulting solution through an Anotopt 25 inorganic filter having a 0.02 micron
pore size. Free base
serlopitant has a maximum solubility in ethanol of 64.5 mg/g Et0H, or 6.45%
w/w. To prepare a topical
formulation, the stock solution of serlopitant/ethanol was added to a tared
tube containing a particular
amount of the base until the resulting mixture weighed 25.0 g. The mixture was
mixed vigorously for
2 minutes using a vibration stand and then was rotated slowly for 4 days. For
the "C" formulations,
ethanol containing no serlopitant was added so that the "B" and "C"
formulations would contain the
same amount of base and ethanol.
Table 5
Mixture Lot Size (g) Base (g) Cpd 1/Et0H Blank Et0H % Cpd 1 % Et0H
Stock SoIn (g) (9) (w/w) (w/w)
VM-A 25.0 23.06 1.94 0.0 0.5 7.8
VM-B 25.0 21.12 3.88 0.0 1.0 15.5
VM-C 25.0 21.12 1.94 1.94 0.5 15.5
VLL-A 25.0 23.06 1.94 0.0 0.5 7.8
VLL-B 25.0 21.12 3.88 0.0 1.0 15.5
VLL-C 25.0 21.12 1.94 1.94 0.5 15.5
AP-A 25.0 23.06 1.94 0.0 0.5 7.8
AP-B 25.0 21.12 3.88 0.0 1.0 15.5
AP-C 25.0 21.12 1.94 1.94 0.5 15.5
[00131] AP was determined to be an unsuitable base for an ethanol solution
containing serlopitant
because of ethanol insolubility in that base. The VM base appeared
stable/unchanged under 15x
43
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
microscopic magnification after 4 days of mixing with 15.5% ethanol. The VLL
base showed some
aggregation of lamellar structures under 15x microscopic magnification after 4
days of mixing with
15.5% ethanol, but the overall change to the base appeared minor. The UM and
VLL formulations can
be tested, e.g., for the skin permeation of serlopitant.
Example 5. In Vitro Skin Permeation of Serlopitant in Topical Formulations
[00132] Topical formulations A-D used in the in vitro skin permeation studies
are shown in Table 6.
The bases "VM" and "VLL" of formulations A-D are described in Example 4.
Formulations A-D were
prepared according to the procedures described in Example 4.
Table 6
Formul'n Final Mass Base (g) Cpd 1/Et0H Blank Et0H % Cpd 1 % Et0H
(Base) (9) Stock SoIn (g) (9) (w/w) (w/w)
A (VM) 25.28 21.27 0.0 4.01 0.0 15.9
B (VLL) 25.12 21.19 3.93 0.0 1.0 15.6
C (VM) 13.80 11.63 2.17 0.0 1.0 15.7
D (VLL) 25.02 21.15 0.0 3.87 0.0 15.5
[00133]/n vitro skin permeation of serlopitant in topical formulations A-D was
evaluated using a Franz
diffusion cell. FIG. 2 illustrates a Franz diffusion cell. A Franz diffusion
cell having a circular
permeation area of 4.15 cm2 and a receptor chamber volume of 19 mL was set up
with a thermo-
regulated outer water jacket to maintain the temperature at 37 C. The
receptor chamber was filled
with 19 mL 1xPBS (pH 7.5) containing 10% ethanol and 1% TweergED 80.
Solubility test indicated that
serlopitant remained soluble at concentrations of 0.5, 5 and 50 ug/mL in this
solution after 1 hour of
incubation at 37 C. The solubility of serlopitant decreased significantly if
Tween 80 was not used
and decreased slightly if ethanol was not used.
00134] Human skin was pretreated to remove all subcutaneous fat and was
cleaned with 70% ethanol
before use. The skin was visually inspected to ensure that it was free of any
surface irregularity or
small holes and was equally divided into four pieces. The skin was then
mounted onto the receptor
chamber with the stratum comeum side facing up. About 100 mg of topical
formulation A, B, C or D
was applied to the skin (actual weight: A, 103.8 mg; B, 101.3 mg; C, 103.2 mg;
and D, 103.8 mg),
which was then covered with parafilm to avoid evaporation.
[00135] About 0.5 mL of solution was withdrawn through the sampling port of
the Franz diffusion cell at
0.5, 1, 2, 4, 6, 18 and 22 hours. The receptor chamber was replenished with
equal volume of fresh
44
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
diffusion buffer after each sampling. At the end of the experiment (after 22
hours of incubation), the
skin was wiped clean with methanol, and the formulation-treated area was
weighed and frozen for
cryosectioning.
[00136] All samples were processed by solid-phase extraction (SPE) before LC-
MS/MS analysis.
Briefly, a Strata-X 33 urn Polymeric Reverse-Phase column with 30 mg sorbent
mass /1 mL volume
(Phenomenex) was conditioned with 1 mL of methanol and equilibrated with 1 mL
of water. 300 uL of
sample was loaded to the column followed by a wash with 1 mL of 30% methanol.
Serlopitant was
eluted with 2% formic acid in acetonitrile. The sample then was concentrated
by blow drying with
nitrogen and re-suspended in 50 uL of 50% methanol. A working standard was
first generated by
spiking the diffusion buffer with known concentrations of serlopitant, which
was then processed using
the same SPE method. A sensitivity of 0.1 ng/mL was achieved. Serlopitant
concentrations in samples
resulting from formulations A-D were determined by comparison to the standard.
Serlopitant was not
detected in samples resulting from topical formulations A and D, as expected.
FIG. 3 shows the
cumulative release of serlopitant from topical formulations B and C into the
receptor chamber at 0.5, 1,
2, 4, 6, 18 and 22 hours. After an initial lag, serlopitant was detected by LC-
MS/MS in the receptor
chamber at 6 hours. FIG. 3 indicates that topical formulation B resulted in
greater penetration of
serlopitant through the skin than topical formulation C in this in vitro
study.
[00137] The amount of serlopitant retained in the skin was determined at the
end of the experiment.
The skin was wiped and washed with methanol. The formulation-treated area was
cut into horizontal
sections of 25 urn using a cryostat. Every 10 sections were pooled, placed in
Eppendorf tubes,
weighed and digested with twice the volume of 1 mg/mL liberase at 37 C for 1
hour. Digested skin
sections were further homogenized with a probe sonicator. To 25 uL of the skin
homogenate were
added 25 uL of 50% methanol and 100 uL of acetonitrilelmethanol to extract
serlopitant. For spiked
standards, 25 uL of a solution of serlopitant in 50% methanol (from 5 ng/mL to
5000 ng/mL) was added
to 25 uL of blank skin homogenate followed by 100 uL of acetonitrile/methanol.
Extracted serlopitant
was quantified by LC-MS/MS. FIG. 4 shows the amount of serlopitant (called
"VPD737" in FIG. 4)
retained in the skin at the end of the experiment. Each bar represents ug of
serlopitant/g of skin in
250 urn skin layers. For each of topical formulations B and C, the bars from
left to right represent the
amount of serlopitant retained in skin layers from the stratum corneum to the
dermis.
Example 6. Representative Topical Formulations Containing Serlopitant
[00138] Table 7 provides non-limiting examples of topical formulations that
can be prepared with
serlopitant or a salt, solvate or polymorph thereof, and optionally an
additional therapeutic agent.
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
Table 7
Dosage Ingredients in Addition to Serlopitant
Form
cream sorbitol, cetyl alcohol, isopropyl myristate, glyceryl stearate, PEG-
100 stearate,
petrolatum, benzyl alcohol, titanium dioxide and water
cream propylene glycol, cetostearyl alcohol, Cremophor A6, Cremophor A25,
liquid
paraffin, parabens and water
cream glycerol, sorbitol, isopropyl palmitate, emulsifying wax, benzyl
alcohol, a pH
adjuster (e.g., NaOH or lactic acid), and water
cream glycerol, stearic acid, glyceryl monostearate, triethanolamine,
parabens and water
cream propylene glycol, cetostearyl alcohol, mineral oil, white petrolatum,
ceteareth-30,
chlorocresol, sodium phosphate monobasic, phosphoric acid, water, and
optionally NaOH
cream glycerol, cetostearyl alcohol, mineral oil, petrolatum, ceteth-20,
diazolidinyl urea,
dichlorobenzyl alcohol, edetic acid (EDTA) or disodium edetate, dibasic sodium
phosphate and water
cream propylene glycol, stearyl alcohol, white petrolatum, polysorbate 60,
parabens, and
optionally water
cream propylene glycol, stearyl alcohol, cetyl alcohol, leyl alcohol, mono-
, di- and/or tri-
glycerides, sodium cetostearyl sulphate, benzyl alcohol, citric acid, a pH
adjuster
(e.g., NaOH or lactic acid), and water
cream hexylene glycol, stearyl alcohol, propylene glycol stearate, white
wax, white
petrolatum, aluminum starch octenylsuccinate, ceteareth-20, titanium dioxide,
phosphoric acid and water
cream propylene glycol, sorbitol, glyceryl monoisostearate, polyglycery1-3
oleate, mineral
oil, microcrystalline wax, colloidal silicon dioxide, parabens, EDTA or
disodium
edetate, and water
cream propylene glycol, stearic acid, isopropyl palmitate, emulsifying wax,
beeswax,
polysorbate 60, an antioxidant (e.g., propyl gallate), a preservative (e.g.,
sorbic
acid and/or potassium sorbate), a pH adjuster (e.g., NaOH and/or citric acid),
and
water
cream cetostearyl alcohol, lanolin alcohols, isopropyl myristate, aluminum
stearate,
magnesium stearate, mineral oil, white petrolatum, water, and optionally
disodium
edetate and/or lactic acid
cream propylene glycol, cetostearyl alcohol, white soft paraffin, liquid
paraffin, lanolin,
simethicone M30, Tween 60, parabens and water
cream cetostearyl alcohol, mineral oil, white petrolatum, ceteth-20,
parabens, citric acid,
sodium citrate, and water
cream propylene glycol, cetostearyl alcohol, polyoxyl 20 cetostearyl ether,
mineral oil
(liquid paraffin), petrolatum (white soft paraffin), chlorocresol, parabens,
sodium
phosphate monobasic, and water
46
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
cream propylene glycol, cetostearyl alcohol, stearic acid, cetyl palmitate,
sorbitan
monostearate, mineral oil, polysorbate 60, benzyl alcohol and water
ointment hexylene glycol, propylene glycol stearate, white wax, white
petrolatum,
phosphoric acid and water
ointment propylene glycol, mineral oil, petrolatum, steareth-2, tocopherol,
EDTA or
disodium edetate, dibasic sodium phosphate and water
ointment propylene glycol, fatty alcohol citrate, fatty acid pentaerythritol
ester, sorbitan
sesquioleate, white petrolatum, beeswax, aluminum stearate, butylated
hydroxyanisole (BHA), citric acid, and optionally water
ointment an alcohol (e.g., ethanol and/or propylene glycol), polyethylene or
white
petrolatum, mineral oil, and optionally water
gel ethanol, carbomer 934P, triethanolamine and water
gel glycerol, carbomer 940, poloxamer, dimethicone, disodium lauryl
sulfosuccinate,
silicon dioxide, a preservative (e.g., benzoyl peroxide and/or methyl
paraben),
EDTA or disodium edetate, a pH adjuster (e.g., NaOH or lactic acid), and water
gel glycerol, hydroxy-beta-cyclodextrin, hydroxyethyl cellulose,
parabens, EDTA or
disodium edetate, and water
gel propylene glycol, polyacrylic acid, medium-chain triglycerides,
lecithin,
polysorbate 80, a preservative (e.g., benzoic acid), EDTA or disodium edetate,
a
pH adjuster (e.g., NaOH or lactic acid), and water
gel ethanol, isopropyl myristate, carbomer 940, triethanolamine, docusate
sodium,
EDTA or disodium edetate, and water
gel propylene glycol, Carbopole 941, PEG 400, methyl paraben, a pH
adjuster (e.g.,
NaOH or lactic acid), and water
gel propylene glycol, PEG 400, carbomer 934P, allantoin, methyl paraben,
a pH
adjuster (e.g., NaOH or lactic acid), and water
gel an alcohol (e.g., ethanol and/or propylene glycol), carbomer, dioctyl
sodium
sulfosuccinate, a preservative (e.g., benzoyl peroxide), a pH adjuster (e.g.,
NaOH
or lactic acid), and water
gel glycerol, propylene glycol, aloe vera gel, diazolidinyl urea,
capryl/capramidopropyl
betaine, parabens, citric acid, sodium citrate, and water
gel ethanol, hydroxypropyl cellulose and water
lotion glycerol, stearyl alcohol, glyceryl stearate, PEG-100 stearate, PEG
400, carbomer
941, cyclomethicone, light mineral oil, steareth-21, benzyl alcohol, sorbic
acid or
potassium sorbate, a pH adjuster (e.g., NaOH or lactic acid), and water
lotion isopropanol, propylene glycol, hydroxypropyl cellulose, sodium
phosphate
nrionobasic, phosphoric acid and water
lotion propylene glycol, cetyl alcohol, stearyl alcohol, glyceryl stearate,
sorbitan
monostearate, light mineral oil, sodium lauryl sulfate, parabens, EDTA or
disodium edetate, water, and optionally a pH adjuster (e.g., NaOH or citric
acid)
47
CA 02915474 2015-12-14
WO 2014/209962
PCT/US2014/043811
lotion glycerol, cetostearyl alcohol, isostearyl alcohol, stearic acid,
glyceryl stearate,
sodium lauroyl sarcosinate, methyl paraben and water
suppo- an alcohol (e.g., ethanol and/or propylene glycol) and glycerides of
saturated fatty
sitory acids
suppo- 95% ethanol and Suppocire AM (glyceride base containing saturated Ca-
C18
sitory triglyceride fatty acids)
pledget isopropanol, propylene glycol and water
foam ethanol, propylene glycol, cetyl alcohol, stearyl alcohol, polysorbate
60, KOH and
water, and pressurized with a propane/butane propellant
spray ethanol, undecylenic acid, isopropyl myristate, sodium lauryl
sulfate, and water
(dermal)
spray glycerol, lactose, cetostearyl alcohol, mineral oil, ceteth-20
phosphate, dicetyl
(dermal) phosphate, urea, potassium phosphate monobasic, parabens, a pH
adjuster (e.g.,
NaOH or lactic acid), and water
spray microcrystalline cellulose, carboxymethyl cellulose sodium, dextrose,
polysorbate
(nasal) 80, disodium edetate, potassium sorbate, a pH adjuster (e.g., HCI),
water, and
optionally an alcohol (e.g., ethanol)
spray microcrystalline cellulose, carboxymethyl cellulose sodium, dextrose,
polysorbate
(nasal) 80, benzalkonium chloride, phenylethyl alcohol, water, and
optionally an alcohol
(e.g., ethanol)
spray hypromellose, benzalkonium chloride, NaCI, EDTA, citric acid, sodium
phosphate
(nasal) dibasic, water, and optionally an alcohol (e.g., ethanol)
[001391 All publications and patent applications mentioned in this
specification are herein incorporated
by reference to the same extent as if each individual publication or patent
application were specifically
and individually indicated to be incorporated by reference.
[001401 From the foregoing it will be appreciated that, although specific
embodiments of the invention
have been described herein for purposes of illustration, various modifications
may be made without
deviating from the spirit and scope of the invention. Accordingly, the
invention is not limited except as
by the appended claims.
48