Note: Descriptions are shown in the official language in which they were submitted.
CA 02915571 2015-12-16
GRAVITY DRAINAGE PROCESS FOR RECOVERING VISCOUS OIL USING
NEAR-AZEOTROPIC INJECTION
FIELD OF THE INVENTION
[0001] The disclosure relates generally to hydrocarbon recovery from
underground
reservoirs. More specifically, the disclosure relates to gravity drainage
processes for
recovering viscous oil.
BACKGROUND OF THE INVENTION
[0002] This section is intended to introduce various aspects of the art,
which may be
associated with the present disclosure. This discussion is believed to assist
in providing a
framework to facilitate a better understanding of particular aspects of the
present disclosure.
Accordingly, it should be understood that this section should be read in this
light, and not
necessarily as admissions of prior art.
[0003] Modern society is greatly dependent on the use of hydrocarbon
resources for
fuels and chemical feedstocks. Hydrocarbons are generally found in subsurface
formations
that can be termed "reservoirs". Removing hydrocarbons from the reservoirs
depends on
numerous physical properties of the subsurface formations, such as the
permeability of the
rock containing the hydrocarbons, the ability of the hydrocarbons to flow
through the
subsurface formations, and the proportion of hydrocarbons present, among other
things.
Easily harvested sources of hydrocarbons are dwindling, leaving less
accessible sources to
satisfy future energy needs. As the prices of hydrocarbons increase, the less
accessible
sources become more economically attractive.
[0004] Recently, the harvesting of oil sands to remove heavy oil has
become more
economical. Hydrocarbon removal from oil sands may be performed by several
techniques.
For example, a well can be drilled to an oil sand reservoir and steam, hot
gas, solvents, or a
combination thereof, can be injected to release the hydrocarbons. The released
hydrocarbons may be collected by wells and brought to the surface.
[0005] Bitumen and heavy oil (collectively referred to herein as "viscous
oil" as further
defined below) reserves exist at varying depths beneath the earth's surface.
More shallow
reserves are often mined followed by surface extraction. Deeper reserves are
often exploited
by in situ processes.
1
CA 02915571 2015-12-16
[0006] Solvents have been used for both in situ and surface extraction
processes to
dilute viscous oil. Solvents reduce the viscosity of viscous oil by dilution,
while steam reduces
the viscosity of viscous oil by raising the viscous oil temperature. Reducing
the viscosity of in
situ viscous oil is done to permit or facilitate its production.
[0007] Where deposits lie well below the surface, viscous oil may be
extracted using
in situ ("in place") processes. Thermal recovery processes are one category of
in situ
processes, where steam is used to reduce the viscosity of the viscous oil.
These processes
are referred to as steam-based processes. One example of an in situ thermal
process is the
steam-assisted gravity drainage method (SAGD). In SAGD, directional drilling
is employed to
place two horizontal wells in the oil sands ¨ a lower well and an upper well
positioned above
it. Steam is injected into the upper well to heat the bitumen and lower its
viscosity. The
bitumen and condensed steam will then drain downward through the reservoir
under the
action of gravity and flow into the lower production well, whereby these
liquids can be
pumped to the surface. At the surface of the well, the condensed steam and
bitumen are
separated, and the bitumen is diluted with appropriate light hydrocarbons for
transport to a
refinery or an upgrader. An example of SAGD is described in U.S. Patent No.
4,344,485
(Butler).
[0008] Other steam-based thermal processes include Solvent-Assisted
Steam-Assisted Gravity Drainage (SA-SAGD), an example of which is described in
Canadian
Patent No. 2,323,029 (Nasr); Liquid Addition to Steam for Enhanced Recovery
(LASER), an
example of which is described in U.S. Patent No. 6,708,759 (Leaute et al.);
Combined Steam
and Vapor Extraction Process (SAVEX), an example of which is described in U.S.
Patent No.
6,662,872 (Gutek et al.), and derivatives thereof. These processes employ a
solvent with
steam.
[0009] Solvent-dominated recovery processes (SDRPs) are another category
of in
situ processes, where solvent is used to reduce the viscosity of the viscous
oil. At the present
time, solvent-dominated recovery processes (SDRPs) are rarely used to produce
highly
viscous oil. Vapor Extraction (VAPEX) is an example of a SDRP, which is
described in U.S.
Patent No. 5,899,274 (Frauenfeld). In certain described SDRPs, the solvent is
heated as in,
for example, heated-VAPEX (H-VAPEX), which is a VAPEX process using a heated
solvent
(Butler 1991 and 1993).
2
CA 02915571 2015-12-16
[0010] It is desirable to provide an improved or alternative gravity
drainage process
for recovering viscous oil from an underground reservoir.
SUMMARY OF THE INVENTION
[0011] Generally, described herein is a gravity drainage process for
recovering
viscous oil from an underground reservoir, the process comprising: (a)
injecting steam and a
solvent into the reservoir to mobilize the viscous oil, wherein the solvent is
in a vapor state,
and the steam and solvent are injected wherein the solvent molar fraction of
the combined
steam and solvent is 70-100% of the azeotropic solvent molar fraction of the
steam and the
solvent as measured at the reservoir operating pressure; and (b) producing at
least a
fraction of the mobilized oil, the solvent, and water.
[0012] Other aspects and features of the present invention will become
apparent to
those ordinarily skilled in the art upon review of the following description
of specific
embodiments of the invention in conjunction with the accompanying figures.
BRIEF DESCRIPTION OF THE DRAWINGS
[0013] These and other features, aspects and advantages of the disclosure
will
become apparent from the following description, appending claims and the
accompanying
drawings, which are briefly described below.
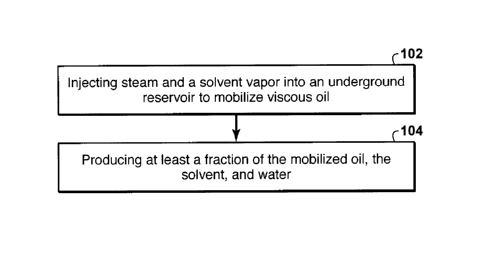
[0014] Fig. 1 is a flow chart of a gravity drainage process for
recovering viscous oil
from an underground reservoir.
[0015] Fig. 2 is a plot of the phase behavior for a steam-hexane system.
[0016] Fig. 3 is a series of contour plots comparing reservoir properties
for heated
pentane (C5 H-VAPEX) and heated pentane with steam at the azeotrope
concentration (C5
Azeotropic H-VAPEX), at the same operating pressure.
[0017] Fig. 4 is a graph of cumulative solvent to oil ratio (CS010R)
versus time for
H-VAPEX and Azeotropic H-VAPEX utilizing C5 and C7 injection.
[0018] Fig. 5 is a graph of cumulative produced oil over retained solvent
versus time
for Heated Vapex and Azeotropic H-VAPEX utilizing C5 and C7 injection.
[0019] Fig. 6 is a graph of oil recovery versus time for H-VAPEX and
Azeotropic
Heated Vapex utilizing C5 and 07 injection.
3
CA 02915571 2015-12-16
[0020] Fig. 7 is a graph of semi-azeotropic behaviour of a steam-
multicomponent
solvent (diluent) system.
[0021] Fig. 8 is a graph of cumulative solvent to oil ratio (CSõIOR)
versus time in high
and low initial water saturation reservoir conditions.
[0022] Fig. 9 graph of produced oil to retained solvent versus time in
high and low
initial water saturation reservoir conditions.
[0023] Fig. 10 is a collective dew point temperature plots for vapor
mixtures of
individual hydrocarbon solvents of C4-C9 with water as a function of solvent
mole fraction.
[0024] It should be noted that the figures are merely an example and no
limitations
on the scope of the present disclosure are intended thereby. Further, the
figures are
generally not drawn to scale, but are drafted for purposes of convenience and
clarity in
illustrating various aspects of the disclosure.
DETAILED DESCRIPTION
[0025] For the purpose of promoting an understanding of the principles of
the
disclosure, reference will now be made to the features illustrated in the
drawings and specific
language will be used to describe the same. It will nevertheless be understood
that no
limitation of the scope of the disclosure is thereby intended. Any alterations
and further
modifications, and any further applications of the principles of the
disclosure as described
herein are contemplated as would normally occur to one skilled in the art to
which the
disclosure relates. It will be apparent to those skilled in the relevant art
that some features
that are not relevant to the present disclosure may not be shown in the
drawings for the sake
of clarity.
[0026] At the outset, for ease of reference, certain terms used in this
application and
their meaning, as used in this context, are set forth below. To the extent a
term used herein
is not defined below, it should be given the broadest definition persons in
the pertinent art
have given that term as reflected in at least one printed publication or
issued patent. Further,
the present processes are not limited by the usage of the terms shown below,
as all
equivalents, synonyms, new developments and terms or processes that serve the
same or a
similar purpose are considered to be within the scope of the present
disclosure.
[0027] A "hydrocarbon" is an organic compound that primarily includes the
elements
of hydrogen and carbon, although nitrogen, sulfur, oxygen, metals, or any
number of other
4
CA 02915571 2015-12-16
elements may be present in small amounts. Hydrocarbons generally refer to
components
found in heavy oil or in oil sands. Hydrocarbon compounds may be aliphatic or
aromatic, and
may be straight chained, branched, or partially or fully cyclic.
[0028] "Bitumen" is a naturally occurring heavy oil material. Generally,
it is the
hydrocarbon component found in oil sands. Bitumen can vary in composition
depending
upon the degree of loss of more volatile components. It can vary from a very
viscous, tar-like,
semi-solid material to solid forms. The hydrocarbon types found in bitumen can
include
aliphatics, aromatics, resins, and asphaltenes. A typical bitumen might be
composed of:
19 weight (wt.) percent (%) aliphatics (which can range from 5 wt. % - 30 wt.
%, or
higher);
19 wt. % asphaltenes (which can range from 5 wt. % - 30 wt. %, or higher);
30 wt. % aromatics (which can range from 15 wt. % -50 wt. %, or higher);
32 wt. % resins (which can range from 15 wt. % - 50 wt. %, or higher); and
some amount of sulfur (which can range from 2 to 7 wt. %, or higher%).
In addition, bitumen can contain some water and nitrogen compounds ranging
from less than
0.4 wt. A to in excess of 0.7 wt. %. The percentage of the hydrocarbon found
in bitumen can
vary. The term "heavy oil" includes bitumen as well as lighter materials that
may be found in
a sand or carbonate reservoir.
[0029] "Heavy oil" includes oils which are classified by the American
Petroleum
Institute ("API"), as heavy oils, extra heavy oils, or bitumens. The term
"heavy oil" includes
bitumen. Heavy oil may have a viscosity of about 1,000 centipoise (cP) or
more, 10,000 cP
or more, 100,000 cP or more, or 1,000,000 cP or more. In general, a heavy oil
has an API
gravity between 22.3 API (density of 920 kilograms per meter cubed (kg/m3) or
0.920 grams
per centimeter cubed (g/cm3)) and 10.0 API (density of 1,000 kg/m3 or 1
g/cm3). An extra
heavy oil, in general, has an API gravity of less than 10.0 API (density
greater than 1,000
kg/m3 or 1 g/cm3). For example, a source of heavy oil includes oil sand or
bituminous sand,
which is a combination of clay, sand, water and bitumen.
[0030] The term "viscous oil" as used herein means a hydrocarbon, or
mixture of
hydrocarbons, that occurs naturally and that has a viscosity of at least 10 cP
(centipoise) at
initial reservoir conditions. Viscous oil includes oils generally defined as
"heavy oil" or
"bitumen". Bitumen is classified as an extra heavy oil, with an API gravity of
about 10 or
less, referring to its gravity as measured in degrees on the American
Petroleum Institute
CA 02915571 2015-12-16
(API) Scale. Heavy oil has an API gravity in the range of about 22.3 to about
100. The terms
viscous oil, heavy oil, and bitumen are used interchangeably herein since they
may be
extracted using similar processes.
[0031] In situ is a Latin phrase for "in the place" and, in the context
of hydrocarbon
recovery, refers generally to a subsurface hydrocarbon-bearing reservoir. For
example, in
situ temperature means the temperature within the reservoir. In another usage,
an in situ oil
recovery technique is one that recovers oil from a reservoir below the earth's
surface.
[0032] The term "subterranean formation" refers to the material existing
below the
earth's surface. The subterranean formation may comprise a range of
components, e.g.
minerals such as quartz, siliceous materials such as sand and clays, as well
as the oil and/or
gas that is extracted. The subterranean formation may be a subterranean body
of rock that is
distinct and continuous. The terms "reservoir" and "formation" may be used
interchangeably.
[0033] "Pressure" is the force exerted per unit area by the gas on the
walls of the
volume. Pressure may be shown in this disclosure as pounds per square inch
(psi),
kilopascals (kPa) or megapascals (MPa). "Atmospheric pressure" refers to the
local
pressure of the air. "Absolute pressure" (psia) refers to the sum of the
atmospheric pressure
(14.7 psia at standard conditions) plus the gauge pressure. "Gauge pressure"
(psig) refers to
the pressure measured by a gauge, which indicates only the pressure exceeding
the local
atmospheric pressure (i.e., a gauge pressure of 0 psig corresponds to an
absolute pressure
of 14.7 psia). The term "vapor pressure" has the usual thermodynamic meaning.
For a pure
component in an enclosed system at a given pressure, the component vapor
pressure is
essentially equal to the total pressure in the system. Unless otherwise
specified, the
pressures in the present disclosure are absolute pressures.
[0034] The terms "approximately," "about," "substantially," and similar
terms are
intended to have a broad meaning in harmony with the common and accepted usage
by
those of ordinary skill in the art to which the subject matter of this
disclosure pertains. It
should be understood by those of skill in the art that these terms are
intended to allow a
description of certain features described and claimed without restricting the
scope of these
features to the precise numeral ranges provided. Accordingly, these terms
should be
interpreted as indicating that insubstantial or inconsequential modifications
or alterations of
the subject matter described and are considered to be within the scope of the
disclosure.
6
CA 02915571 2015-12-16
[0035] The articles "the", "a" and "an" are not necessarily limited to
mean only one,
but rather are inclusive and open ended so as to include, optionally, multiple
such elements.
[0036] "At least one," in reference to a list of one or more entities
should be
understood to mean at least one entity selected from any one or more of the
entity in the list
of entities, but not necessarily including at least one of each and every
entity specifically
listed within the list of entities and not excluding any combinations of
entities in the list of
entities. This definition also allows that entities may optionally be present
other than the
entities specifically identified within the list of entities to which the
phrase "at least one"
refers, whether related or unrelated to those entities specifically
identified. Thus, as a
non-limiting example, "at least one of A and B" (or, equivalently, "at least
one of A or B," or,
equivalently "at least one of A and/or B") may refer, to at least one,
optionally including more
than one, A, with no B present (and optionally including entities other than
B); to at least one,
optionally including more than one, B, with no A present (and optionally
including entities
other than A); to at least one, optionally including more than one, A, and at
least one,
optionally including more than one, B (and optionally including other
entities). In other words,
the phrases "at least one," "one or more," and "and/or" are open-ended
expressions that are
both conjunctive and disjunctive in operation. For example, each of the
expressions "at least
one of A, B and C," "at least one of A, B, or C," "one or more of A, B, and
C," "one or more of
A, B, or C" and "A, B, and/or C" may mean A alone, B alone, C alone, A and B
together, A
and C together, B and C together, A, B and C together, and optionally any of
the above in
combination with at least one other entity.
[0037] Where two or more ranges are used, such as but not limited to 1 to
5 or 2 to 4,
any number between or inclusive of these ranges is implied.
[0038] As used herein, the phrase, "for example," the phrase, "as an
example,"
and/or simply the term "example," when used with reference to one or more
components,
features, details, structures, and/or methods according to the present
disclosure, are
intended to convey that the described component, feature, detail, structure,
and/or method is
an illustrative, non-exclusive example of components, features, details,
structures, and/or
methods according to the present disclosure. Thus, the described component,
feature, detail,
structure, and/or method is not intended to be limiting, required, or
exclusive/exhaustive; and
other components, features, details, structures, and/or methods, including
structurally and/or
7
CA 02915571 2015-12-16
functionally similar and/or equivalent components, features, details,
structures, and/or
methods, are also within the scope of the present disclosure.
[0039] Steam to Oil Ratio ("SOR") is the ratio of a volume of steam (in
cold water
equivalents) required to produce a volume of oil. Cumulative SOR ("CSOR") is
the average
volume of steam (in cold water equivalents) over the life of the operation
required to produce
a volume of oil. Instantaneous ("ISOR") is the instantaneous rate of steam (in
cold water
equivalents) required to produce a volume of oil. SOR, CSOR, and ISOR are
calculated at
standard temperature and pressure ("STP", 15 C and 100kPa or 60 F and 14.696
psi).
[0040] Likewise, Solvent to Oil Ratio ("SoIOR") is the ratio of a volume
of solvent (in
cold liquid equivalents) required to produce a volume of oil. Cumulative SoIOR
("CSolOR") is
the average volume of solvent (in cold liquid equivalents) over the life of
the operation
required to produce a volume of oil. Instantaneous ("ISolOR") is the
instantaneous rate of
solvent required to produce a volume of oil. SolOR, CS010R, and ISolOR are
calculated at
STP.
[0041] "Azeotrope" means the thermodynamic azeotrope" as described
further
herein.
[0042] Described herein, with reference to Fig. 1, is a gravity drainage
process for
recovering viscous oil from an underground reservoir, the process comprising:
(a) injecting
(102) steam and a solvent into the reservoir to mobilize the viscous oil,
wherein the solvent is
in a vapor state, and the steam and solvent are injected wherein the solvent
molar fraction of
the combined steam and solvent is 70-100% of the azeotropic solvent molar
fraction of the
steam and the solvent as measured at the reservoir operating pressure; and
(b) producing (104) at least a fraction of the mobilized oil, the solvent, and
water.
[0043] For ease of reference, the above described injection is referred
to herein as
"near-azeotropic injection".
[0044] Prior to step (a), a target reservoir operating pressure may be
selected and a
solvent may be selected which is a vapor at this pressure. Then, an azeotropic
solvent molar
fraction at the target reservoir operating pressure and the azeotropic
temperature at the
target reservoir operating pressure may be determined. By way of example, Fig.
2
graphically illustrates the azeotrope for a hexane-water system at a pressure
of 2.5 MPA
where the azeotropic solvent molar ratio is 0.64 and the azeotropic
temperature is 182 C.
Continuing with this example, 70-100% of an azeotropic solvent molar fraction
of 0.64 is
8
CA 02915571 2015-12-16
0.45-0.64. In this example, near-azeotropic injection means using a solvent
molar fraction of
0.45-0.64 in vapor mixture of hexane and steam.
[0045] The solvent molar fraction may alternatively be 80-100% or 90-100%
of the
azeotropic solvent molar fraction at the reservoir operating pressure.
[0046] For practical purposes, the selection of the solvent molar
fraction and the
operating pressure constrains the temperature of the vapor phase assuming
saturated
conditions.
[0047] In practice, as will become more apparent by the description
below, one may
select a solvent that has a favorable operating temperature and solvent molar
fraction at the
azeotrope condition when combined with steam. A favorable operating
temperature is a
temperature that results in economic production rates while delivering
adequate or good
thermal efficiency. A favorable solvent molar fraction is one that reduces the
Solvent to Oil
Ratio (SolOR) as compared, for instance, with a heated VAPEX process.
[0048] A physical phenomenon that increases the 5010R for heated VAPEX
and
therefore reduces the efficiency of the process is that when a heated solvent
is injected, it
vaporizes all the in-situ water, including a large fraction of the bound or
irreducible water, in
the vicinity of the injector.
[0049] As a result of this vaporization, at the boundary of the VAPEX
chamber, both
water and solvent condense together under conditions that are at or close to
the azeotrope.
This is a lower temperature than that of the injected heated solvent.
Furthermore, because
the boundary is relatively narrow, the idealistic benefits of a solvent-only
process with no
flowing water are not practically achieved.
[0050] An important feature of the azeotrope pressure-temperature
conditions is that
the two fluids largely behave as a single fluid. That is, both fluids condense
together in the
same molar ratios of concentrations as they exist in the gas. Additionally,
there is no
tendency for either fluid to preferentially flash from the liquid state into
the vapor state (i.e.
vaporize additional in-situ water in the vicinity of the injector well). As
such, the combined
fluids can behave effectively as a single fluid with modified properties
compared to either
single fluid.
[0051] Water is a very effective working fluid for transferring heat
whereas
hydrocarbon solvents tend to be relatively inefficient working fluids for that
purpose.
Conversely, hydrocarbon solvents are very effective viscosity reducing agents
for heavy oils
9
CA 02915571 2015-12-16
whereas water is practically immiscible. However, mixtures of hydrocarbons and
water at the
azeotrope behave largely as a single fluid with beneficial qualitative
features of both the
water and the hydrocarbon solvent.
[0052]
Without intending to be bound by theory, a near optimal injection ratio of
solvent and steam vapor, where the fluid enters the reservoir, may be a ratio
that is at or
close to the azeotrope for the solvent-water mixture. Consider the case of
injecting a mixture
of water and hexane at 2.5 MPa. As shown in Figure 2, the molar fraction of
solvent at the
azeotrope is approximately 0.64 or 64% so that the water molar fraction is
0.36 or 36%. On
mass basis, this converts to a 10.5% water fraction and on a volumetric basis
this converts to
a 7% water fraction. The heat of vaporization of hexane at the azeotrope
temperature is
approximately 220 kJ/kg and that for water is about 2000 kJ/kg. The combined
fluids have
an effective heat capacity of about 410 kJ/kg. As a result, the mass of fluid
required to
deliver the same heat to the reservoir is approximately half and accordingly
the operating
solvent-to-oil ratio would be expected to be about half.
[0053] If
hexane vapor at 2.5 MPa is injected into the reservoir it will be at a
temperature of 220 C (see Figure 2). As the hot hydrocarbon vapor enters the
reservoir and
enters pore spaces with liquid water, the water will vaporize and a fraction
of the solvent will
condense. The temperature will also decline until the water-hydrocarbon system
in vapor
phase finds an equilibrium point near the azeotrope. At
that point, any additional
condensation will result in the water and solvent condensing with the mole
fraction ratio of
the azeotrope. The solvent-steam mixture that progresses to the boundary of
the vapor
chamber will be a mixture at or close to the azeotrope. This phenomenon has
several of
important implications:
[0054] 1.
A significant volume of reservoir rock will be increased in temperature to as
much as 220 C which is an additional heat sink compared to injection at the
azeotrope
temperature of 182 C.
[0055] 2.
Due to the hotter injection fluids and conductive heating in the vicinity of
the
producer, produced fluids will be at a higher temperature. Hence more heat
will be produced
back from the reservoir which is less thermally efficient.
[0056] 3.
Vaporized solvent is condensing in the reservoir in order to vaporize water
which is then carrying the heat to the boundary of the steam chamber, which is
not effectively
using the benefits of the solvent. That is, as described above, hydrocarbon
solvents tend to
CA 02915571 2015-12-16
be relatively inefficient working fluids for transferring heat but are very
effective viscosity
reducing agents.
[0057] 4. A region will develop around the injector which is nearly
completely water
free (sometimes called a desiccation zone). It is possible that this could be
advantageous in
some circumstances. However it could also be a disadvantage due to factors
such as salt or
scale deposition and pore plugging, fines movement causing pore plugging and a
shift from a
water wet system to an oil wet system resulting in less favorable residual oil
saturations and
relative permeabilities.
[0058] Practical implications of using near-azeotropic injection are
partly illustrated by
the results of some analyses that are provided in Tables 1 and 2. Table 1
shows the
azeotropic temperatures and molar concentrations for pentane, hexane and
heptane at 1
MPa and 2.5 MPa pressures. Table 1 also provides mass fractions, standard
volumetric
fractions and enthalpies for steam and solvent at their respective, ideal
partial pressures for
the azeotropic temperature. It can be seen from Table 1 that the azeotrope
molar
concentration of steam increases significantly from lighter to heavier
solvents. However, for
a given solvent there is limited variation with pressure. It can also be seen
from Table 1 that
the heat of vaporization for the combined fluids increases much more
substantially for
heavier solvents at the azeotrope than it does for the lighter solvents. Table
2 lists an
assumed injected solvent-to-oil ratio (SolOR) for each of the cases shown in
Table 1. For
illustrative purposes, the assumed SolOR increases with solvent type and
pressure
proportionally to the difference between the azeotrope operating temperature
and an
assumed initial reservoir temperature of 7 C. The fifth and sixth columns of
the Table 2
show the equivalent combined steam to oil ratio (SOR) and SoIOR when operating
with
azeotropic injection. In all cases, the required solvent recirculation is
significantly reduced.
For pentane, it is estimated to be about a 23% reduction at 1 MPa. The benefit
is predicted
to increase with pressure and with the use of heavier solvents. The required
solvent
recycling is predicted to be reduced by 50% or more for heavier solvents.
11
CA 02915571 2015-12-16
[0059] Table 1. Properties for steam-solvent
Design Parameters Azeotrope Properties (Approximate) Steam -Solvent
Ratios Thermal Properties
Solvent Pressure Temperature Steam Solvent Steam Solvent Steam Solvent
Steam Heat Solvent Heat Combined
Molar Molar Mass Mass Volume Volume of of Fluid
Heat of
Fraction Fraction Fraction Fraction Fraction Fraction Vaporization
Vaporization Vaporization
Mpa deg. C. kJ/kg kJ/kg kJ/kg
Pentane 1 116 0.16 0.84 0.045 0.955 0.029 0.971
2213 319 405
Hexane , 1 140.5 0.34 0.66 0.097 0.903 0.066 0.934
2142 270 452
Heptane 1 155.7 0.54 0.46 0.174 0.826 0.125 0.875
2095 226 551
Pentane 2.5 157.7 0.2 0.8 0.059 0.941 0.038 0.962
2089 208 318
Hexane 2.5 182 0.36 0.64 0.105 0.895 0.071 0.929
2006 220 408
Heptane 2.5 196.9 0.54 0.46 0.174 0.826 0.125
0.875 1951 210 513
[0060] Table 2. Representative Solvent-only and Isotropic Steam-Solvent
Ratios
Solvent Pressure Temperature Solvent-only Azeotrope Combined
SolOR Reduction
Mpa deg. C. SdOR Steam SOR Solvent SoiOR %
Pentane 1 116 8.8 0.20 6.8 23
Hexane 1 140.5 12.1 0.49 7.0 42
Heptane 1 155.7 15.6 0.86 6.0 61
Pentane 2.5 157.7 18.6 0.46 11.9 36
Hexane 2.5 182 19.4 0.78 10.1 48
Heptane 2.5 196.9 21.4 1.18 8.3 61
[0061] Simulation Results
[0062] The concept described herein is examined by a numerical simulation
in a
typical Athabasca reservoir. Figure 3 shows the reservoir properties map for
single
component solvent H-VAPEX (n-05 or normal-pentane) and Azeotropic H-VAPEX
("AH-VAPEX") at the same operating pressure condition. As seen in the
temperature map,
the average temperature in the depleted zone for AH-VAPEX with no near
wellbore heating
is lower than the H-VAPEX case. It is also noted from the water saturation map
that in
AH-Vapex, the co-injected steam at the azeotropic concentrations inhibited the
vaporization
of the initial in-situ water and minimized solvent condensation to provide the
required energy
for water vaporization. The improvement in SolOR for this case is shown in
Figure 4. Figure 4
also shows the improvement in the SoIOR for AH-VAPEX in azeotropic steam-nC7
(steam-normal-heptane) system. As described above, the azeotropic systems for
heavier
solvents results in a higher energy content in the injected azeotropic fluid
compared to lighter
solvents and therefore results in a higher reduction in SolOR, as is shown
Table 1 and Table
2 and in Figure 4.
12
CA 02915571 2015-12-16
[0063] The vaporized in-situ water in H-VAPEX in the depleted zone is
replaced with
hydrocarbon liquid phase which is mainly condensed liquid solvent. Prevention
(or limitation)
of in-situ water vaporization in the AH-VAPEX results in reduction of liquid
hydrocarbon
phase in the depleted chamber and therefore reduction in solvent retention in
the depleted
reservoir. This is seen in the liquid phase saturation map in Figure 3 as a
reduced residual
liquid phase saturation region within depleted chamber in AH-VAPEX compared to
H-VAPEX. The reduction in solvent retention in reservoir is reflected in
Figure 5 in terms of
an increase in produced oil-to-retained solvent ratio (PBRSR). It is noted
that nC7
AH-VAPEX has a higher increase in PBRSR compared to the nC5 AH-VAPEX. The oil
recovery rates in the azeotropic H-VAPEX and H-VAPEX is generally similar as
shown in
Figure 6.
[0064] For field applications, the commercially available solvents are
generally a
mixture of hydrocarbon compounds rather than a pure single compound.
Commercial gas
condensate, diluents, and naphtha are among the used solvents. The phase
behavior of
these multicomponent solvents with steam is more complicated than the single
compound
solvents. However, their phase behavior when mixed with steam can be
considered as
superposition of individual pure compounds behavior. These systems exhibit a
semi-azeotropic behavior with a minimum boiling characteristic similar to
single compound
solvents. Figure 7 shows the semi-azeotropic behavior of steam-diluent system.
The
minimum dew point temperature in this system is the co-condensation point of
steam-solvent
components at a semi-azeotriopic water concentration similar to azeotropic
point in a single
component solvent-steam system. Figures 8 and 9 show the enhancement effects
in SoIOR
and PBRSR of semi-azeotropic steam-diluent AH-VAPEX compared to diluent H-
VAPEX.
Figures 8 and 9 also compare the SoIOR and PBRSR improvement in a high initial
water
saturation reservoir (lean reservoir, So=0.61), compared to an Athabasca
reservoir with
typical initial water saturation (So=0.87). Oil saturation of "So" is a
fraction of oil volume
based on pore volume.
[0065] Potential advantages in terms of efficiency of near-azeotropic
injection in
AH-VAPEX relative to heated VAPEX include:
[0066] 1. The average temperatures in the vapor chamber are reduced while
the
temperature at the chamber boundary remains near the azeotrope temperature.
13
CA 02915571 2015-12-16
[0067] 2. The temperatures at the top of the steam chamber will also be
reduced
resulting in less heat loss to the overburden.
[0068] 3. There is virtually no thermodynamic tendency to vaporize water
(mobile,
immobile or bound) within the vapor chamber. This eliminates (or reduces) the
complexities
and potential problems associated with a dry (or desiccation) zone.
[0069] 4. Preventing in-situ water (mobile, immobile or bound)
vaporization in the
near-azeotropic injection results in a reduction of the liquid hydrocarbon
phase in the
depleted chamber, reduction in solvent concentration in the vapor phase in the
depleted
chamber, and therefore a reduction in solvent retention in reservoir.
[0070] 5. Since water is a much more effective thermal working fluid than
hydrocarbon solvents, the combined fluids have a greater average working
enthalpy
associated with the condensation of the vapor.
[0071] 6. Oil rates from the process will remain largely unchanged since
in either
process water is condensing at the boundary with the solvent at similar water
to solvent
ratios.
[0072] 7. Heat loss from the wellbore can result in significant
condensation of fluids.
An additional volume or molar concentration of water can be added to the
injected stream at
surface such that water preferentially condenses in the wellbore and injection
at the sand
face is then near the azeotrope.
[0073] 8. It may also be advantageous to inject vapor at the sand face
with a water
concentration marginally above the azeotrope concentration so that, for
example, in later life,
primarily water condenses at the top of the reservoir. In particular, solvent
that condenses
on the top of the steam chamber and drains down is not as effective as solvent
that
condenses on the oil interface. If one injects above the steam azeotrope
concentration, it will
be water that condenses first at the top of the steam chamber. As a result,
the optimal molar
fraction of steam may start at or near the azeotrope and increase with time.
There will likely
be a reduction in the volume of vapor being injected into the reservoir which
may allow for
smaller wellbore sizes and tubulars.
[0074] Since thermal separation will be required in order to recycle
solvent, process
facilities may be designed to flash water at a desired concentration.
[0075] Overall, advantages of near-azeotropic injection may include
reductions in the
solvent-to-oil ratio (SolOR) relative to solvent-only heated VAPEX, a
potentially broader
14
CA 02915571 2015-12-16
applicability to higher initial water saturation resources, a reduction in
solvent storage, and an
improvement in the produced oil-to-retained solvent ratio.
[0076] The solvent may be a fluid of a lower viscosity and lower density
than those of
the viscous oil being recovered. Its viscosity may, for example, be 0.2 to 5
cP (centipoise) at
room temperature and at a pressure high enough to make it liquid. Its density
may be, for
example, 450 to 950 kg/m3 at 15 C and at a pressure high enough to make it
liquid. The
mixture or the blend of solvent and viscous oil may have a viscosity and a
density that is in
between those of the solvent and the viscous oil. The solvent may or may not
precipitate
asphaltenes if its concentration exceeds a critical concentration.
[0077] The solvent may be a single hydrocarbon compound or a mixture of
hydrocarbon compounds having a number of carbon atoms in the range of Cl to
C30+. The
solvent may have at least one hydrocarbon in the range of C3 to C12 and this
at least one
hydrocarbon may comprise at least 50 wt. % of the solvent. The mixture may
have aliphatic,
naphthenic, aromatic, and/or olefinic fractions.
[0078] The solvent may comprise at least at least 50 wt. A) of one or
more C3-C12
hydrocarbons, at least 50 wt. % of one or more C4-C10 hydrocarbons, at least
50 wt. % of
one or more C5-C7 hydrocarbons, or a natural gas condensate or a crude oil
refinery
naphtha.
[0079] The solvent may comprise alkanes, iso-alkanes, naphthenic
hydrocarbons,
aromatic hydrocarbons, and/or olefin hydrocarbons. In general, normal alkanes
may have a
highest tendency of causing phase separation of asphaltenes, with a decreasing
tendency
for phase separation being observed when moving from iso-alkanes to naphthenic
hydrocarbons to aromatic hydrocarbons.
[0080] Upon selecting an operating temperature range (for instance 60-140
C), a
solvent may be selected that has a vapor pressure that does not exceed a
selected
maximum pressure.
[0081] The solvent may be chosen to be compatible with the desired
reservoir
operating pressure such that economics of the process will be optimized
through a
combination maximizing the producing oil rate, minimizing the injected solvent
to oil ratio,
minimizing the injected steam to oil ratio, maximizing the produced oil-to-
retained solvent
ratio, and selecting lower cost-of-supply solvents.
CA 02915571 2015-12-16
[0082] The steam may have a quality (defined as the wt. % of total steam
present as
steam vapour, and the remainder as liquid) of at least 5%, or 10-100%. The
steam may be
present in a near-azeotropic injection stream in an amount of 2-85 vol. % and
solvent may be
present in an amount of 15-98 vol.%, both calculated at standard temperature
and pressure
(STP) and in cold liquid equivalents. The volume percentage range must be
determined for
each solvent at given pressure. By way of example, the cold liquid equivalent
volume
percentage range for C4 is 2-7 vol% and for C12 is 80-85 vol%.
[0083] The solvent molar fraction may be decreased over time.
[0084] The steam and solvent may be injected with other components, such
as:
diesel, aromatic light catalytic gas oil, or another solvent, to provide flow
assurance, or CO2,
natural gas, C3+ hydrocarbons, ketones, or alcohols.
[0085] The process may further comprise separating and reusing the
solvent and
water in a separation, purification, revaporization and reinjection facility.
[0086] The gravity drainage process may involve directional drilling to
place two
horizontal wells in the viscous oil reservoir ¨ a lower well and an upper well
positioned above
it. The solvent and steam may be injected into the upper well to dilute and
reduce the
viscosity of the viscous oil. The viscous oil, solvent, and condensed steam
will then drain
downward through the reservoir under the action of gravity and flow into the
lower production
well, whereby these fluids can be pumped to the surface. At the surface of the
well, all or a
fraction of the solvent or a mixture of reduced-viscosity hydrocarbons may be
separated from
the produced fluids and reused as the solvent for injection with the steam.
All or a fraction of
the solvent or reduced-viscosity hydrocarbons may also remain mixed with the
oil to aid in
transport to a refinery or an upgrader.
[0087] Light hydrocarbon gases may also be separated from the produced
fluids and
may include hydrocarbons and/or carbon compounds with four or fewer carbon
atoms, such
as methane, ethane, propane, and/or butane. Light hydrocarbon gases may be
used
upstream in the process, for instance, as fuel to heat the solvent and steam
prior to injection.
[0088] The operating pressure for the process may be informed by many
external
factors such as needing to be close to the pressure of nearby water zones, gas
zones or
other operations such that the injected fluids do not migrate away from the
production well
and unwanted fluids do not migrate to the production well. Additionally, the
potential for
16
CA 02915571 2015-12-16
formation fracturing may limit the maximum pressure. As such, the choice of
solvent may be
driven by the acceptable range of operating pressures
[0089] A
threshold maximum pressure also may be related to and/or based upon the
characteristic pressure of the subterranean formation. The reservoir operating
pressure may
be 5-95% of a fracture pressure of the reservoir, or 0.2 to 4 MPa, or 1 to 2.5
MPa.
[0090] The
injection temperature of the solvent and steam, when it is injected into the
injection well, may be affected by the selection of the molar concentration of
the steam and
the solvent once the optimal solvent has been selected. The thermodynamic
phase behavior
will dictate that injection temperature is the saturation temperature
corresponding to the
molar concentration of the steam and the solvent in absence of any degrees of
superheat.
The molar concentration of the steam will most often be higher than the
azeotropic
concentration in order to most efficiently manage heat losses.
Correspondingly, the
temperature will be higher than the azeotropic temperature as well. The
injection
temperature may be 30-250 C or 80-150 C.
[0091] The
heat of vaporization of the hydrocarbon solvents is much smaller than
steam. Therefore, one may add excess steam to an azeotropic mixture of
hydrocarbon vapor
and steam. The thermodynamic phase behavior dictates that the excess steam
will condense
first to provide the required energy for heat losses. By way of example, a
mixture of steam
and hydrocarbon vapor may be prepared at a central processing facility with a
solvent molar
fraction (X1) less than the azeotropic vapor solvent molar fraction (Xaz) and
a temperature
(Ti) greater than the azeotropic temperature (Taz). As the mixture flows
through the
pipelines toward the wellhead, some of excess steam will condense due to heat
losses. At
the wellhead, the vapor mixture may have a higher solvent molar fraction (X2,
i.e.
X2>X1>Xaz) and a lower temperature (T2, i.e. T2<T1). At the wellhead,
preferably X2>Xaz
and T2>Taz. As the mixture flows down the well, some of the excess steam will
again
condense due to heat losses. At the sand face, the vapor mixture may have a
solvent molar
fraction (X3) where X3>X2 and a temperature (T3) where T3<T2. Preferably, at
the sand
face X3>Xaz and T3>Taz. In this way, one can inject the mixture at the sand
face with some
extra steam as compared to an azeotropic mixture to provide the energy
required to account
for heat losses to the overburden. Heaters can also be utilized on the surface
or downhole to
add some degree of superheat to the solvent and vapor mixture in order to
ensure single
phase flow. Examples are surface heaters and downhole electrical heaters.
Therefore, the
17
CA 02915571 2015-12-16
solvent and steam vapor mixture may be injected at 1-50 C or 1-20 C of
superheat,
measured at the sand face, with respect to the saturation temperature of the
solvent molar
fraction at the reservoir operating pressure.
[0092] As described above, near-azeotropic injection of solvent and steam
means
using a solvent molar fraction of 70-100% of the azeotropic solvent molar
fraction.
Simulation results have shown than the total injected energy per volume of
bitumen
produced and the bitumen production rate are not be considerably affected by
varying the
composition of the injected fluid in this range. As an example, for C5
(pentane) one may
inject with a solvent molar fraction of 0.62-0.88, and for C9 (nonane) one may
inject with a
solvent molar fraction of 0.13-0.18, both at a pressure of 500 kPa. These
compositions
translate to different dew point temperature ranges for each solvent, and as
illustrated in
Figure 10, namely, 87-119 C for C5, and 145-147 C for C9. In general, as
pressure
increases the temperature range corresponding to 70% to 100% of the azeotropic
solvent
molar fraction becomes narrower.
[0093] Separation of the produced fluid may be effected in any suitable
separation
system or structure, such as a single stage separation vessel, a multistage
distillation
assembly, a liquid-liquid separation or extraction assembly and/or any
suitable gas-liquid
separation, or extraction assembly.
[0094] Purification of the solvent may be effected in any suitable system
or structure,
such as any suitable liquid-liquid separation or extraction assembly, any
suitable gas-liquid
separation or extraction assembly, any suitable gas-gas separation or
extraction assembly, a
single stage separation vessel, and/or any suitable multistage distillation
assembly.
[0095] Vaporization of the solvent may be effected by any suitable system
or
structure above ground or downhole.
[0096] The injection well may be spaced apart from the production well.
The
production well may extend at least partially below the injection well, may
extend at least
partially vertically below the injection well, and/or may define a greater
distance (or average
distance) from the surface when compared to the injection well. At least a
portion of the
production well may be parallel to, or at least substantially parallel to, a
corresponding
portion of the injection well. At least a portion of the injection well,
and/or of the production
well, may include a horizontal, or at least substantially horizontal, portion.
18
CA 02915571 2015-12-16
[0097] The process may include preheating or providing thermal energy to
at least a
portion of the subterranean formation in any suitable manner. The preheating
may include
electrically preheating the subterranean formation, chemically preheating the
subterranean
formation, and/or injecting a preheating steam stream into the subterranean
formation. The
preheating may include preheating any suitable portion of the subterranean
formation, such
as a portion of the subterranean formation that is proximal to the injection
well, a portion of
the subterranean formation that is proximal to the production well, and/or a
portion of the
subterranean formation that defines a vapor chamber that receives the solvent
and steam.
[0098] Heating the solvent may include directly heating the solvent in a
surface
region or using the co-injection with the steam.
[0099] Condensing the solvent and steam within the subterranean formation
may
include condensing any suitable portion of the solvent and steam to release a
latent heat of
condensation of the solvent and steam, heat the subterranean formation, heat
the viscous
oil, and/or generate the reduced-viscosity hydrocarbons within the
subterranean formation.
The condensing may include condensing a majority, at least 50 wt. %, at least
60 wt. %, at
least 70 wt. %, at least 80 wt. %, at least 90 wt. %, at least 95 wt. %, at
least 99 wt. %, or
substantially all of the solvent and steam within the subterranean formation.
The condensing
may include regulating a temperature within the subterranean formation to
facilitate, or
permit, the condensing.
[00100] Producing the reduced-viscosity hydrocarbons may include producing
the
reduced-viscosity hydrocarbons via any suitable production well, which may
extend within
the subterranean formation and/or may be spaced apart from the injection well.
This may
include flowing the reduced-viscosity hydrocarbons from the subterranean
formation, through
the production well, and to, proximal to, and/or toward the surface region.
[00101] The producing may include producing asphaltenes. The asphaltenes
may be
present within the subterranean formation and/or within the viscous oil. The
asphaltenes may
be produced as a portion of the reduced-viscosity hydrocarbons (and/or the
reduced-viscosity hydrocarbons may include, or comprise, asphaltenes). The
injecting may
include injecting into a stimulated region of the subterranean formation that
includes
asphaltenes, and the producing may include producing at least a threshold
fraction of the
asphaltenes from the stimulated region. This may include producing at least 10
wt. %, at
least 20 wt. %, at least 30 wt. %, at least 40 wt. %, at least 50 wt. %, at
least 60 wt. %, at
19
CA 02915571 2015-12-16
least 70 wt. %, at least 80 wt. %, or at least 90 wt. % of the asphaltenes
that are, or were,
present within the stimulated region prior to the injecting. The fractions of
the asphaltenes
that are produced and left in the reservoir is a function of the operating
temperature,
pressure and the choice of solvents. The determination of these parameters may
be
influenced by the fraction of asphaltenes that is produced and associated
value of the
produced hydrocarbons.
[00102] Recycling the solvent may include recycling the solvent in any
suitable
manner. The recycling may include separating at least a separated portion of
the solvent
from the reduced-viscosity hydrocarbon mixture and/or from the reduced-
viscosity
hydrocarbons. The recycling also may include utilizing at least a recycled
portion of the
solvent as, or as a portion of, the hydrocarbon solvent mixture and/or
returning the recycled
portion of the condensate to the subterranean formation via the injection
well. The recycling
may include purifying the recycled portion of the solvent prior to utilizing
the recycled portion
of the solvent and/or prior to returning the recycled portion of the solvent
to the subterranean
formation.
[00103] The properties of the azeotropic mixture which condenses at the
boundary of
the vapour chamber are strongly influenced by the lightest hydrocarbons
present in the
injected solvent so the recycling process may have facilities designed to
specifically remove
the lightest components.
[00104] It should be understood that numerous changes, modifications, and
alternatives to the preceding disclosure can be made without departing from
the scope of the
disclosure. The preceding description, therefore, is not meant to limit the
scope of the
disclosure. Rather, the scope of the disclosure is to be determined only by
the appended
claims and their equivalents. It is also contemplated that structures and
features in the
present examples can be altered, rearranged, substituted, deleted, duplicated,
combined, or
added to each other.