Note: Descriptions are shown in the official language in which they were submitted.
CA 02916447 2015-12-21
1
Method for extracting highly viscous oils and/or bitumen
The present invention relates to a method for extracting highly viscous oils
and/or
bitumen.
In Alberta, there is at present a rapid expansion in the process of exploiting
the oil sands
located there. The reason for this is the comparatively low cost of extraction
which is
attributable, inter alia, to the fact that the location and yield of the
individual extraction
sites in the oil sand region of Alberta are known in detail so that
exploration projects
costing billions are not required. Traditionally, open mining activities were
effected out
in the open. However, this is no longer possible in the case of newer projects
because, in
the meanwhile, the deposits are located at greater depths so that different
extraction
techniques have had to be adopted.
These extraction techniques are mostly very energy-intensive and are based on
the use of
natural gas and steam as well as heat for heating and cracking the extracted
bitumen. The
water used to obtain the steam must be cleaned and recycled after use.
In one extraction technique, the oil sand is extracted and crushed in an open
mining
operation. Subsequently, the bitumen contained in the oil sand is extracted by
washing it
with hot water at a temperature of 50 to 80 C for example. This results in a
bitumen
foam containing approximately 60 % bitumen, 30 % water and 10 % sand which is
then
cleaned. The bitumen is then melted (in order to enable it to be pumped) and
decompose
into smaller hydrocarbons in a hydrocracker. In the case of the above
technique, the
processes of heating the water and melting the bitumen and the hydrocracking
process (>
380 C) are very energy-intensive.
In another technique relating to underground mining, steam is forced into the
deeper
lying bitumen seams in order to thermally soften bitumen and make it more
viscous. The
more viscous bitumen is taken up in a deeper lying pipe and pumped to the
surface.
CA 02916447 2015-12-21
2
From there, it is pumped in its hot, still viscous state to the hydrocracker
and it is
decomposed into lighter hydrocarbons.
In the case of this ¨ so-called SDAG - technique, about 34 m3 of natural gas
are currently
consumed per barrel of cracked bitumen. These 34 m3 of natural gas result in
approx. 80
kg of CO2 emissions. That corresponds to about 50 % of the weight of the
extracted
crude oil (the cracked bitumen then still has to be pumped to the refinery) or
20 % of the
energy content of the cracked bitumen. Compared with conventional crude oil,
both of
these values are very high and have a corresponding environmental impact,
particularly
due to the high CO2 emissions.
In an alternative technique, which is known as VAPEX (vapour extraction
process),
gaseous organic solvents are used instead of steam. The bitumen is dissolved
thereby and
is afterwards pumped away in the form of a viscous liquid. Thus, it functions
in a similar
manner to SDAG, only with a liquid with a lower boiling point and thus with
significantly lower expenditure of energy.
The lower the viscosity of the bitumen, the easier the extraction and the
further
transportation thereof by pipeline. However, as low boiling and thus thin-
bodied
hydrocarbons are not generally present in most oil sand regions, a
disadvantage of the
VAPEX process lies in the expenditure involved in providing the necessary
solvents on
site. In this case, the relevant fractions from refinery processes are not a
solution since an
energy-intensive refining process must then be implemented twice. Thus, in
practice,
there is merely a displacement of, but not a reduction in energy consumption.
Furthermore, a method is known from US 2013 045 902 Al in which a solvent
consisting
of at least one alkane having at least nine carbon atoms, an ether, and an
aromatic
hydrocarbon is utilised for improving the oil recovery process. The solvent
can be
employed in liquid form, however preferably it is in the form of a vapour.
CA 02916447 2015-12-21
3
Consequently, the object of the invention is to provide an alternative
extraction process
which overcomes or at least reduces one or more of the problems encountered in
the
methods specified above. In particular, a reduction of CO2 emissions is
desired.
In accordance with the invention, this object is achieved by a method
according to Claim
1 or Claim 3. Further embodiments of the invention are apparent, inter alia,
from the
appendant Claims.
In particular, there is provided a method for extracting highly viscous oils
and/or bitumen
wherein a hydrocarbon in liquid form is brought into contact with a material
containing
highly viscous oil and/or bitumen, in order to at least partly dissolve or
absorb the highly
viscous oil and/or bitumen in the hydrocarbon, and wherein the highly viscous
oil and/or
bitumen that has been dissolved in this manner is subsequently conveyed away
with the
hydrocarbon in liquid form. The hydrocarbon is one which is liquid at ambient
temperature and normal pressure and which is produced from another hydrocarbon
that is
gaseous, highly viscous or solid at ambient temperature and normal pressure,
by:
decomposing the other hydrocarbon in absence of oxygen into carbon and
hydrogen;
mixing at least part of the resulting mixture of carbon and hydrogen with CO2
at a
higher temperature in the region of at least 800 C in order to convert the
mixture into
a synthesis gas; and feeding the synthesis gas into a CO converter for forming
the
liquid hydrocarbon; or
decomposing the other hydrocarbon in absence of oxygen into carbon and
hydrogen,
mixing at least part of the carbon obtained from the decomposing process with
CO2
at a higher temperature in the region of at least 800 C in order to convert
the mixture
into CO, mixing at least part of the hydrogen obtained from the decomposing
process
with the CO before and/or within a CO converter for forming the liquid
hydrocarbon.
In the first variant, additional hydrogen that is obtained from a process of
decomposing a
hydrocarbon that is gaseous, highly viscous or solid at ambient temperature
and normal
pressure can be added to the synthesis gas before and/or within the CO
converter.
CA 02916447 2015-12-21
4
Thus, there is provided a method that works with a medium which absorbs highly
viscous
oil and/or bitumen and which needs no special treatment under normal
environmental
conditions such as an ambient temperature of approximately 25 C and normal
pressure
for example and is thus easy to handle. Furthermore, the absorbent medium can
also be
utilised directly for conveying the highly viscous oil and/or bitumen over a
pipeline.
Hereby, the absorbent medium can partially dissolve highly viscous oils and/or
bitumen
or else hold them in suspension. Hereby, when being fed-in, the liquid
hydrocarbon
should preferably have a viscosity of less than 1.0 mPa s (Ns/m2). CO2 coming
from
another process such as that of energy production for example can preferably
be fixed
during the production process for the liquid hydrocarbon so that emissions of
CO2 on site
can be reduced or prevented substantially in their entirety.
In order to assist the process of dissolving the highly viscous oils and/or
the bitumen, the
hydrocarbon can be heated up to a temperature of over 50 C and in particular
over 100 C
before being brought into contact with the material containing highly viscous
oil and/or
bitumen. At these temperatures, liquid hydrocarbons having a viscosity of 0.5-
1.0 Ns/m2
under standard conditions are preferred.
In particular, the other hydrocarbon can be a gaseous hydrocarbon which is
possibly
available locally or which can be supplied over a pipeline in a simple manner.
In one
embodiment, the decomposing of the hydrocarbon is effected with the help of a
plasma.
Preferably, the carbon obtained from the decomposing process has a temperature
of over
800 C after the decomposing process, and the carbon is mixed with the CO2,
before it has
cooled down to a temperature of below 700 C. The heat used for the decomposing
process can then be utilised for further processes.
Preferably, the CO2 originates from the burning of fossil fuels and/or biomass
for
producing the energy that is utilised at least partly for the process of
decomposing the
other hydrocarbon. One can thereby obtain a substantially closed system which
provides
the energy required for the extraction process but manages to do without CO2
emissions,
CA 02916447 2015-12-21
or at least manages to reduce CO2 emissions due to the fact that the CO2
produced for the
extraction process is fixed in the hydrocarbon.
In one embodiment, the hydrocarbon in liquid form is supplied over a first
line and fed
into an underground deposit of highly viscous oil and/or bitumen, and it is
subsequently
fed out of the underground deposit through the first line or through a second
line after the
absorption of the highly viscous oil and/or bitumen in the hydrocarbon. The
underground
deposit can be exploited in a simple manner by means of this process. In
particular, oil
fields that have already been abandoned can be further exploited by using this
method.
Preferably, the hydrocarbon can be forwarded over a pipeline to a distantly
located
further processing plant immediately after it has been fed out i.e. without
being subjected
to any further treatment on site.
In addition to the hydrocarbon in liquid form, a gas can be fed into the
underground
deposit in order to promote thorough mixing of the hydrocarbon with the highly
viscous
oil and/or the bitumen that is to be extracted.
In accordance with the invention, a liquid hydrocarbon serving as a solvent is
preferably
provided at the extraction site thereby enabling the process of extracting the
highly
viscous oils and/or bitumen to be effected with substantial savings of primary
energy. In
particular, the provision of the solvent can be effected in CO2-neutral manner
in
accordance with one aspect of the invention so that, for example, the
exploitation of the
oil sand can take place in an environmentally compatible manner in the future.
The invention is described in more detail hereinafter with the aid of the
drawings; in the
drawings:
Fig. 1 shows a schematic view of a plant for extracting highly viscous oils
and/or
bitumen in accordance with one embodiment;
Fig. 2 a schematic view of a plant for extracting highly viscous oils and/or
bitumen in
accordance with a further embodiment;
CA 02916447 2015-12-21
6
Fig. 3 a schematic illustration of a further system for the production of
synthetic
hydrocarbon in accordance with one embodiment;
Fig. 4 a schematic illustration of a further system for the production of
synthetic
hydrocarbon in accordance with a further embodiment; and
Fig. 5 a schematic illustration of a further system for the production of
synthetic
hydrocarbon in accordance with yet another embodiment;
It should be noted that, in the following description, the expressions above,
below, right
and left and also similar indications refer to the orientations or
arrangements illustrated in
the Figures and serve only for describing the exemplary embodiments. These
expressions are not however to be understood in a restrictive sense.
Furthermore, the
same reference symbols are used to some extent in the different Figures
insofar as the
same or similar parts are designated.
Fig. 1 shows a simplified schematic view of a plant 1 for extracting highly
viscous oils
and/or bitumen from an underground deposit 3 of highly viscous oils and/or
bitumen.
The plant consists, in essence, of a supply unit 5 for feeding liquid
hydrocarbon into the
deposit 3 and an extraction unit 7 for extracting a mixture of the liquid
hydrocarbon and
highly viscous oils and/or bitumen from the deposit 3.
The supply unit 5 can be supplied via a pipeline 9 with a hydrocarbon which is
liquid at
ambient temperature and normal pressure which is introduced through a first
bore 10 into
the deposit 3. This can take place at a higher temperature and/or at a greater
pressure as
long as the hydrocarbon continues to be present in liquid form. The bore 10
extends with
arbitrary orientation from an above-ground point to the deposit 3 and ends
preferably in
an upper region of the deposit 3. Thus, in the simplest case, the supply unit
5 can be
limited to a pumping station provided with an appropriate supply (pipeline 9)
of a liquid
hydrocarbon and the first bore 10. Although this is not illustrated, a gas
supply may
additionally be provided so that the liquid hydrocarbon may be introduced
together with a
gas through the first bore 10 into the deposit 3 in order to promote thorough
mixing with
the highly viscous oils and/or bitumen in the deposit 3.
CA 02916447 2015-12-21
7
The extraction unit 7 is connected, on the one hand, to the deposit 3 via a
second bore 12
and on the other hand, it is connected to a pipeline 13 for removing a mixture
of the
liquid hydrocarbon and the oils and/or bitumen that are dissolved and/or
absorbed therein
from the deposit 3. The second bore 12 extends with arbitrary orientation from
an above-
ground point to the deposit 3 and ends preferably in a lower region of the
deposit 3. It is
advantageous in particular if, in terms of height, the bore 12 ends below the
bore 10. It is
to be generally understood here that a bore is a line which has been inserted
into the
deposit 3 by means of a drilling process. The mixture of the liquid
hydrocarbon and the
oils and/or bitumen dissolved and/or absorbed therein can be extracted from
the deposit 3
by means of a pump (that is not illustrated in detail) through the bore 12 and
supplied to
the pipeline 13. The pipeline 13 can be connected to a plant for further
processing of the
mixture.
The simple operation of the plant 1 is now described wherein initially a
hydrocarbon that
is liquid at ambient temperature and normal pressure is brought in through the
pipeline 9
and the supply unit 5 and is introduced via the first bore 10 into the deposit
3. Within the
deposit 3, the liquid hydrocarbon comes into contact with the highly viscous
oils and/or
bitumen in the deposit 3 and partially dissolves them. Furthermore, highly
viscous oils
and/or bitumen could also be absorbed as a suspension in the liquid
hydrocarbon. The
liquid hydrocarbon together with the highly viscous oils and/or bitumen
absorbed therein
flows within the deposit 3 to a deeper point such as the region around the end
of the bore
12 for example. From there, the liquid hydrocarbon together with the highly
viscous oils
and/or bitumen absorbed therein are pumped out through the bore 12 and
supplied to the
pipeline 13. Hereby, the liquid hydrocarbon together with the highly viscous
oils and/or
bitumen absorbed therein is preferably of sufficiently low viscosity as to
enable it to be
conveyed over some distance to a distantly located further processing plant.
Costly
cracking processes or other processes for preparing the extracted highly
viscous oils
and/or bitumen for onward transportation can thereby be dispensed with in the
vicinity of
the deposit 3.
CA 02916447 2015-12-21
8
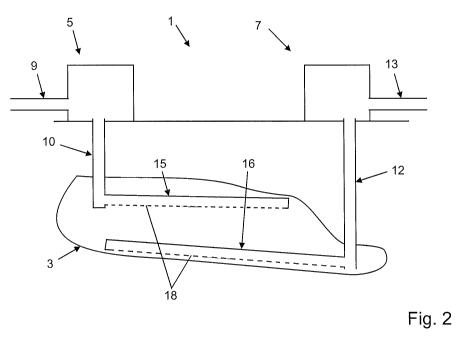
Fig. 2 shows a simplified schematic view of an alternative plant 1 for
extracting highly
viscous oils and/or bitumen from an underground deposit 3. The plant 1
basically
resembles the previously described one and comprises a supply unit 5, an
extraction unit
7, a pipeline 9, a first bore 10, a second bore 12 and a further pipeline 13.
These
elements can be disposed in the same way as for the first embodiment.
However, additional transverse bores 15, 16 incorporating a respective
plurality of
passage openings 18, 19 are provided in the deposit 3 at the respective ends
of the bores
10, 12. Each of the transverse bores 15, 16 extends transversely to the bores
10, 12
within the deposit 3, wherein they may run horizontally or be inclined so as
to match the
contours of the deposit 3. In the illustrated embodiment, the transverse bore
15
connected to the bore 10 runs, in terms of height, above the transverse bore
16 that is
connected to the bore 12. Furthermore, the transverse bores 15, 16 overlap in
the
longitudinal extent thereof. When in operation, the transverse bore 15 serves
to provide
better distribution of the liquid hydrocarbon in the deposit 3 and the
transverse bore 16
provides better uptake of the mixture of liquid hydrocarbon and highly viscous
oils
and/or bitumen from the deposit 3.
Although at least two bores 10, 12 to the deposit 3 are provided in each of
the above
embodiments, it would also be possible to provide just a single bore, such as
the bore 12
for example, through which in a first step a liquid hydrocarbon is brought
into the deposit
3 and then in a second step a mixture of liquid hydrocarbon together with the
highly
viscous oils and/or bitumen absorbed therein is subsequently extracted from
the deposit
3. It should also be noted that by using the basic principle of supplying a
liquid
hydrocarbon, highly viscous oils and/or bitumen could also be removed from
above-
ground deposits. Recycling of bituminous and/or asphalt-containing materials
in a
reprocessing plant is also possible by using the basic principle.
Nothing has been said in the above embodiments about the origin of the
hydrocarbon
which is liquid at ambient temperature and normal pressure. This can originate
from
CA 02916447 2015-12-21
9
many varied sources. In a preferred embodiment however, a synthetically
produced
hydrocarbon are used.
A plant 100 for the production of such a synthetic hydrocarbon is described
hereinafter
with reference to Fig. 3, this preferably being used in combination with a
plant and/or a
process for extracting highly viscous oils and/or bitumen of the type
described above.
Fig. 3 shows a plant 100 for the production of synthetic functionalised and/or
non-
functionalised hydrocarbons which comprises a plant-part 101 for the
conversion of
carbon dioxide into carbon monoxide and a CO converter 131. An alternative
plant-part
101' for the conversion of H20 into synthesis gas which takes the place of the
plant-part
101 is provided in another implementation (Fig. 5).
The plant-part 101 comprises a hydrocarbon converter 103 which comprises a
hydrocarbon inlet 104 and also a first carbon outlet 105, an optional hydrogen
outlet 106
and also an optional second carbon outlet 107. The plant-part 101 for the
production of
carbon monoxide further comprises a CO2 converter 109 having a CO2 inlet 110,
a
carbon inlet 111 (also called a C-inlet) and an outlet 112. The hydrocarbon
converter 103
and the CO2 converter 109 are arranged in such a manner that the carbon outlet
105 of the
hydrocarbon converter 103 is connected by a direct connection 108 to the
carbon inlet
111 of the CO2 converter 109, wherein the outlet 105 could also directly form
the carbon
inlet 111 of the CO2 converter 109. Carbon can thus be transported from the
hydrocarbon
converter of 103 directly into the CO2 converter 109.
The alternative plant-part 101' likewise comprises the hydrocarbon converter
103 which
comprises a hydrocarbon inlet 104 and also a first carbon outlet 105, an
optional
hydrogen outlet 106 and also an optional second carbon outlet 107. The plant-
part 101'
for the production of synthesis gas further comprises a C-converter 109'
having an H20-
inlet 110', a carbon inlet 111 (also called a C-inlet) and an outlet 112. The
hydrocarbon
converter 103 and the C-converter 109' are arranged in such a manner that the
carbon
outlet 105 of the hydrocarbon converter 103 is connected by a direct
connection 108 to
CA 02916447 2015-12-21
the carbon inlet 111 of the C-converter 109', wherein the outlet 105 could
also directly
form the carbon inlet 111 of the C-converter 109. Carbon can thus be
transported from
the hydrocarbon converter 103 directly into the C-converter 109'.
In both of the plant-parts 101 or 101', the carbon can also be fed together
with a portion
of the hydrogen from the hydrocarbon converter 103 into the CO2 converter 109
or into
the C-converter 109'. In this case, only a small amount of carbon or hydrogen
is fed out
from the optional hydrogen outlet 106 and the optional second carbon outlet
107. In the
event that all the carbon and all the hydrogen are fed out together from the
hydrocarbon
converter 103, the optional hydrogen outlet 106 and also the optional second
carbon
outlet 107 can naturally be omitted.
The hydrocarbon converter 103 is any type of hydrocarbon converter which can
convert
or decompose the hydrocarbons that are being fed-in into carbon and hydrogen.
The
hydrocarbon converter 103 comprises a processing area having an inlet for a
fluid
containing hydrocarbon, at least one unit for supplying decomposing energy to
the fluid
and at least one outlet. The decomposing energy is provided, at least
partially, by heat
which is produced by a plasma for example. However, it can be made available
in any
other way and, if the decomposing process is effected primarily by heat, then
the fluid
should be heated to over 1000 C, in particular, to a temperature of over 1500
C.
A Kvaerner reactor is used in the illustrated embodiment, such a reactor
providing the
necessary heat by means of a plasma arc in a plasma burner. However, other
types of
reactor are known which work, in particular, at lower temperatures under 1000
C and
which, apart from heat, inject additional energy into the hydrocarbon, such as
by way of a
microwave plasma for example. As will be described in more detail hereinafter,
the
invention takes both types of reactor (and also those that work without a
plasma) into
consideration, especially too, in combination with one another. Hydrocarbon
converters
which work at a temperature of more than 1000 C are referred to as high-
temperature
reactors hereinafter, whilst those which work at temperatures below 1000 C and
in
CA 02916447 2015-12-21
11
particular at a temperature of between 200 C and 1000 C are referred to as low-
temperature reactors.
Hydrogen and carbon are generated from hydrocarbons (CõH.) in the reactor by
means of
heat and/or a plasma. Hereby, the hydrocarbons are preferably introduced into
the
reactor in gaseous form. In the case of hydrocarbons that are fluid under
standard
conditions, these can be turned into gaseous form before being introduced into
the
reactor, or they could also be fed-in in a finely atomised form. Both forms
are referred to
as fluids hereinafter.
The process of separating the hydrocarbons should take place if possible in
absence of
oxygen in order to prevent the unwanted formation of carbon oxides or water.
Here again
however, small quantities of oxygen that are introduced with the hydrocarbons
for
example are not detrimental to the process.
In the Kvaerner reactor described above, hydrocarbon-containing fluids are
decomposed
in a plasma burner at high temperature into pure carbon (in the form of
activated
charcoal, carbon black, graphite or industrial soot for example) and hydrogen
together
with possible impurities. The hydrocarbon-containing fluids serving as input
materials
for the hydrocarbon converter 103 are, for example, methane, natural gas, bio
gases,
liquid gases or heavy oil, but synthetic, functionalised and/or non-
functionalised
hydrocarbons could also be used as input materials for the hydrocarbon
converter 103.
After the original decomposition process, the elements are usually present as
a mixture
especially in the form of an aerosol. As will be described hereinafter, this
mixture can be
supplied in this form to a further process, or it could also be separated into
its individual
elements in an appropriate, not illustrated separating unit. Naturally, in the
context of
this application, such a separating unit is regarded as being part of the
hydrocarbon
converter 103 even though it could be implemented as a separate unit. If a
separating
unit is not provided, then the carbon outlet 105 is the only outlet of the
hydrocarbon
converter 103 which feeds a mixture (an aerosol) of carbon and hydrogen
directly into the
CO2 converter 109. If there is a separating unit, the carbon that has been
separated at
CA 02916447 2015-12-21
12
least partly from the hydrogen can be fed via the carbon outlet 105 into the
CO2 converter
109. The separated hydrogen and possibly further carbon can then be fed out
via the
optional outlets 106 and 107.
The CO2 converter 109 can be any suitable type of CO2 converter which can
produce
carbon monoxide (CO) from carbon (C) and carbon dioxide (CO2). In the
embodiment of
Fig. 3, the CO2 converter 109 works according to a part of the reaction known
in the art
as a blast furnace reaction which runs at temperatures of between approx. 750
C and
1200 C without the necessity for a catalyst. Preferably, the CO2 converter 109
works at a
temperature of between 800 C and 1000 C, wherein the heat required for
reaching this
temperature is made available primarily by the product stream of the
hydrocarbon
converter 103 as will be described in more detail hereinafter. In the CO2
converter 109,
CO2 is fed over hot carbon or mixed therewith (and possibly with hydrogen) in
order to
be converted in accordance with the chemical equation CO2 + C ¨> 2 CO. The CO2
converter 109 works best at the Boudouard equilibrium and at a temperature of
1000 C.
At temperatures of 800 C, about 94% carbon monoxide is produced, and at
temperatures
of around 1000 C, about 99% carbon monoxide is produced. A further rise in
temperature does not result in substantial changes.
The C-converter 109' in the alternative plant-part 101' can be any suitable
type of C-
converter which can produce synthesis gas (Syngas, CO + H2) from carbon (C)
and water
(H20). In the embodiment of Fig. 5, H2O is fed over hot carbon in the C-
converter 109
or else is introduced in the form of steam in a stream of carbon and hydrogen
(H2/C-
Aerosol) and is mixed therewith in order to be converted in accordance with
the chemical
equation C + H2O ¨> CO + 112. The following reactions occur in the C-converter
109':
C + H2O ¨> CO + 142 + 131.38 kJ/mol endothermic
CO + 1120 ¨> CO2 + H2 - 41.19 kJ/mol exothermic
This reaction takes place at the Boudouard equilibrium:
2 C + 02 ¨> 2 CO + 172.58 kJ/mol endothermic
CA 02916447 2015-12-21
13
Since all three reactions are in equilibrium with one another, the process in
the C-
converter 109' preferably takes place at high temperatures of from 800 to 1700
C,
preferably 1000 to 1200 C, since the second reaction would be preferred at
lower
temperatures, wherein the heat required for reaching this temperature is
primarily made
available by the raw material for the hydrocarbon converter 103. The water
(H20) in the
C-converter 109' is vaporous under these conditions and can be immediately
introduced
in the form of steam. In operation, the addition of water is controlled in
such a way that a
surplus of water is avoided in order to prevent rapid cooling. In the event of
excessive
cooling in the C-converter 109', the second reaction would likewise preferably
occur.
The C-converter 109' works best at high temperatures of from 1000 to 1200 C in
order to
repress the exothermic Watergas-Shift reaction CO + H20 ¨> CO2 + H2 and thus
optimize the fraction of CO in the synthesis gas. The reactions in the C-
converter 109'
are known to the skilled person and so will not be described in more detail
here.
The operation of the plant 101 for the conversion of carbon dioxide into
carbon monoxide
is explained in more detail hereinafter with reference to Fig. 1. It is
assumed hereinafter
that the hydrocarbon converter 103 is a high temperature reactor of the
Kvaerner type.
Hydrocarbon-containing fluids (especially in gaseous form) are introduced into
the
hydrocarbon converter 103 through the hydrocarbon inlet 104. If the
hydrocarbon is
methane (CH4) for example, then 1 mol carbon and 2 mol hydrogen are produced
from 1
mol methane. The hydrocarbons are converted in the hydrocarbon converter 103
at about
1600 C in accordance with the following reaction equation, wherein the
supplied energy
is heat which is produced in the plasma by means of electrical energy:
CnH + energy n C + m/2 H2
By appropriate execution of the process, the Kvaerner reactor can achieve
almost 100%
conversion of the hydrocarbon into its constituents when in continuous
operation.
CA 02916447 2015-12-21
14
It is assumed hereinafter that the carbon and the hydrogen are separated in
the
hydrocarbon converter 103 and are fed out separately insofar as possible.
However, it is
also possible that separation does not take place and that the carbon and the
hydrogen are
fed out as a mixture and then are supplied to the CO2 converter 109. The
hydrogen does
not impair the conversion process in the CO2 converter 109 but it can serve as
an
additional heat carrier. The carbon is fed, at least partially, via the carbon
outlet 105
directly into the carbon inlet 111 of the CO2 converter 109. Herein, the
"direct" feed
from the outlet 105 of the hydrocarbon converter 103 to the carbon inlet 111
of the CO2
converter 109 shall cover all such variants wherein the cooling effect amounts
to no more
than 50% (preferably to no more than 20%) with respect to the temperature of
the
materials being fed-in. Since the carbon exiting from the hydrocarbon
converter 103 has
a high temperature of preferably over 1000 C, the heat energy contained
therein can be
used for maintaining the temperature required for the conversion process in
the CO2
converter 109 which preferably works at a temperature of approx. 1000 C.
The connection 108 between the hydrocarbon converter 103 and the CO2 converter
109 is
designed in such a way that the carbon does not cool down excessively (by less
than
50%, preferably by less than 20% with respect to temperature) on its way from
the
hydrocarbon converter 103 to the CO2 converter 109. In particular, the
connection 108
can be insulated or even actively heated for example, wherein preferably, no
further heat
- apart from the heat that is produced in the hydrocarbon converter 103 - is
supplied to
the system. Optionally however, it is also possible to provide a heat
exchanger 125 in the
vicinity of the connection 108 in order to derive heat for preheating a flow
of CO2 into the
CO2 converter 109. The hydrogen produced in the hydrocarbon converter 103
likewise
contains heat energy due to the operating temperature in the hydrocarbon
converter 103.
Consequently, there is an optional possibility of using the heat energy of the
hydrogen
being fed out from the hydrogen outlet 106 directly or indirectly by means of
a heat
exchanger arrangement 127 for heating the flow of CO2 into the CO2 converter
109, the
connection 108 and/or the CO2 converter 109.
CA 02916447 2015-12-21
CO2 which is being fed-in through the CO2 inlet 110 of the CO2 converter 109
is directed
over the hot carbon and/or is mixed therewith in the CO2 converter 109. The
CO2
converter 109 works best at the Boudouard equilibrium which occurs during the
conversion of carbon dioxide with hot carbon. This reaction which is known to
the
skilled person is dependent on pressure and temperature and will not be
described in
detail here. Either the quantity of the CO2 being fed into the CO2 converter
109 or the
quantity of carbon can be controlled and/or regulated by suitable means.
CO2 + C ¨> 2C0; AH = +172.45 kJ/mol
The CO2 can, for example, originate from any combustion-type power station
(coal-, gas-
and/or oil-fired power station), wherein however, it preferably originates
from a gas-fired
power station which on the one hand generates the energy required for the
hydrocarbon
converter 103 and/or for the plant 1 (pumps, heating units etc..). It is
preferable that
substantially all of the CO2 output of such a power station is supplied to the
CO2
converter 109 and is converted there. The effect can thereby be achieved that
the entire
plant and thus the process of extracting highly viscous oils and/or bitumen
can be carried
out without any substantial CO2 emissions. In dependence on the location
however, the
CO2 can originate entirely or else additionally from another process (such as
a steel or a
cement production process for example) which produces suitable quantities of
CO2,
insofar as there is an appropriate industry in the locality. This will most
likely be the case
where there is recycling of bitumen and/or asphalt-containing materials in an
appropriate
reprocessing plant in industrialized areas.
In dependence on the temperature of the CO2 from the CO2 source, it is
advantageous for
the CO2 that is being fed into the CO2 inlet 110 of the CO2 converter 109 to
be preheated
since the CO2 converter 109 works at a temperature of between 800 and 1200 C.
Preliminary heating of the CO2 can be achieved for example by using the heat
energy that
is contained in the hot hydrogen directly or indirectly with the aid of a heat
exchanger
arrangement for preheating the CO2. However preferably, the heat contained in
the
carbon will suffice for bringing the CO2 up to the desired temperature. It is
only in the
CA 02916447 2015-12-21
16
=
case where the heat generated in the hydrocarbon converter 103 is not
sufficient for
achieving the desired conversion temperature of approximately 1000 C that an
optional
additional heating unit need be provided for heating the CO2 converter 109 or
the
elements contained within it. Such a thing can also be used as a preheating
unit in the
vicinity of a feed line for the CO2 or the carbon. It could also be used just
for the start-up
period of the plant in order to initially bring the CO2 converter 109 or the
media-carrying
parts of the plant up to an initial temperature so that the system will
achieve a desired
temperature state more quickly.
Hot carbon monoxide (CO) at a temperature of approximately 800 to 1000 C
emerges
from the CO2 converter 109 (in dependence on the operating temperature of the
CO2
converter 109). The carbon monoxide emerging from the CO2 converter 109
likewise
contains heat energy which can optionally be used directly or indirectly by a
heat
exchanger indicated in Fig. 3 by reference 126 for e.g. preheating the CO2
being fed into
the CO2 inlet 110.
As was mentioned above, the hydrocarbon converter 103 may comprise a second
carbon
outlet 107 for feeding out carbon. The carbon produced in the hydrocarbon
converter
103 can ¨ after an appropriate separation process (or else as a C-H2 mixture) -
be fed out
in different proportions from the first carbon outlet 105 and the second
carbon outlet 107.
The second carbon outlet 107 is used in order to remove, if necessary, a
portion of the
carbon that has been produced which is not used in the CO2 converter 109 for
the
production of carbon monoxide. The carbon removed via the second carbon outlet
107
can be removed in the form of activated charcoal, graphite, carbon black or
some other
modification such as carbon cones or carbon discs. In dependence on the form
and
quality of the removed carbon, the removed carbon can be used industrially as
a raw
material. If suitable onward transportation should not be possible due to the
location of
the deposit 3, utilization thereof for producing energy would also be
conceivable.
With the help of the method for the conversion of carbon dioxide into CO that
is
illustrated above, it is possible to convert the hot carbon from the
hydrocarbon converter
CA 02916447 2015-12-21
17
103 into carbon monoxide with the aid of warm to hot carbon dioxide from the
exhaust
air of industrial processes in the CO2 converter 109 without a supply of
external energy
or at least without the need for a substantial amount of external energy.
Preferably, at
least 80% and in particular at least 90% of the heat required for reaching the
conversion
temperature should originate from the hydrocarbon converter 103.
Alternatively, water is
converted into synthesis gas using the hot carbon or an H2/C aerosol from the
hydrocarbon converter 103 in the C-converter 109' without or at least without
considerable supply of external energy.
The CO converter 131 is arranged downstream of the CO2 converter 109 and
comprises a
CO inlet 132 for feeding-in CO, an H2 inlet 133 for feeding-in hydrogen and a
hydrocarbon outlet 134 for feeding-out synthetic functionalised and/or non-
functionalised
hydrocarbons. The CO inlet 132 of the CO converter 131 is connected to the CO
outlet
112 of the CO2 converter 109 by a CO connection 135. The H2 inlet 133 of the
CO
converter 131 is connected by an H2 connection 136 to the H2 outlet 106 of the
hydrocarbon converter 103. As an alternative however, it is also possible for
the CO
converter to comprise only one synthesis gas inlet for synthesis gas which is
produced in
a mixer. Such a mixer can, for example, be connected to the CO outlet 112 of
the CO2
converter 109 and the H2 outlet 106 of the hydrocarbon converter 103 in order
to enable
mixing of the gases for forming a synthesis gas outside the CO converter 131.
The outlet
of the mixer would then be connected to the synthesis gas inlet of the CO
converter. The
CO converter 131 can cooperate in the same way with the alternative plant-part
101'
because the C-converter 109' already produces a synthesis gas. The synthesis
gas from
the C-converter 109' has an H2/C ratio which depends on whether just carbon is
being fed
into the C-converter 109' or whether a portion of the hydrogen from the
decomposing
process in the hydrocarbon converter 103 is also being fed-in. In this case
too, additional
hydrogen can be added to the synthesis gas from the C-converter 109' in order
to achieve
a desired H2/C ratio.
The CO converter 131 may be any type of CO converter for producing synthetic
functionalised and/or non-functionalised hydrocarbons. In the embodiment shown
in Fig.
CA 02916447 2015-12-21
18
3, the CO converter is preferably either a Fischer Tropsch converter, a
Bergius Pier
converter or a Pier converter having an appropriate catalyst and a temperature
and/or
pressure control unit.
In one embodiment, the CO converter 131 comprises a Fischer Tropsch converter.
A
Fischer Tropsch converter catalytically converts a synthesis gas into
hydrocarbons and
water. Various versions of Fischer Tropsch reactors and Fischer Tropsch
processes
which are not illustrated in detail here are known to the skilled person. The
main reaction
equations read as follows:
n CO + (2n+1) H2 ¨* CnH2r1+2 n H20 for alkanes
n CO + (2n) H2 ¨> C11H2/1 n H2O for alkenes
n CO + (2n) H2 ¨ CnH2n4-1 OH + (n-1) H2O for alcohols
The Fischer Tropsch processes can be carried out as high-temperature processes
or as low
temperature processes, wherein the process temperatures generally lie at
between 200 and
400 C. Known variants of the Fischer Tropsch process are, inter alia, the high
load
synthesis process, the Synthol synthesis process and the SMDS process of the
company
Shell (SMDS = Shell Middle Distillate Synthesis). Typically a hydrocarbon
mixture
consisting of liquid gases (propane, butane), benzene, kerosene (diesel oil),
soft paraffin,
hard paraffin, methanol, methane-diesel fuel or a mixture of several of these
is produced
by a Fischer Tropsch converter. Herein, the process should be set-up and/or
controlled in
such a way that it results in as large as possible a proportion of liquid
hydrocarbons that
are suitable for the extraction of highly viscous oils and/or bitumen in the
deposit 3. The
Fischer-Tropsch synthesis process is exothermic as is known to the skilled
person. The
heat of reaction from the Fischer Tropsch process can be used by means of a
heat
exchanger (not shown in the Figures), for example, for preheating CO2. For
example, a
two-stage preliminary heating of the CO2 being fed into the CO2 converter 109
is
considered, wherein preliminary heating by means of the waste heat from the CO
converter 131 (in the implementation as a Fischer Tropsch converter) takes
place first,
CA 02916447 2015-12-21
19
=
and wherein thereafter further heating of the CO2 is effected by means of the
heat from
one or more of the heat exchangers 125, 126, 127.
In an alternative embodiment, the CO converter 131 comprises a Bergius Pier
converter
or a combination of a Pier converter with an MtL converter (MtL = methanol-to-
liquid).
The Bergius Pier process that is of course well known to the skilled person
takes place in
a Bergius Pier converter, wherein hydrocarbons are produced by the
hydrogenation of
carbon with hydrogen in an exothermic chemical reaction. The spectrum of
output
products from the Bergius Pier process depends on the reaction conditions and
the way in
which the reaction is carried out. Mainly fluidic end products are produced
which can
then be used as fuels such as heavy and medium oils for example. Known
developments
of the Bergius Pier process are the console process and the H-coal-process for
example.
In the above mentioned combination of a Pier converter with an MtL converter,
synthesis
gas is first converted into methanol in accord with the known Pier process.
The MtL
converter is a converter in which methanol is converted into benzene. One wide-
spread
process is the MtL process from the companies ExxonMobil or Esso. The input
product
of the MtL converter is typically the methanol that is produced by the Pier
converter for
example. The output product which is produced by the MtL converter is
typically
benzene which is suitable as a liquid hydrocarbon for the extraction of highly
viscous oils
and/or bitumen in the deposit 3.
In summary, it may be said that end products comprising inter alia liquid
hydrocarbons
that are suitable for the extraction of highly viscous oils and/or bitumen in
the deposit 3
can be produced from CO and H2 in the CO converter 131 irrelevant of the
principles
illustrated above by which it works. The process heat which arises during the
exothermic
conversion process in the CO converter 131 can in turn be used by a heat
exchanger for
heating different areas of the plant or for producing electric current in
order to improve
the efficiency of the plants described here.
CA 02916447 2015-12-21
As the product of the CO converter 131, there may be a mixture of hydrocarbons
that
contains a proportion of hydrocarbons which, after a separation process, are
not suitable
as liquid hydrocarbons for the extraction of the highly viscous oils and/or
bitumen in the
deposit 3. This portion, insofar as it cannot be further processed directly or
profitably
sold as an end product, such as methane or paraffin for example, can be fed
back into the
process described here. For this purpose, the plant 100 comprises a return
pipe
connection 139 with the assistance of which part of the synthetically produced
hydrocarbons can be fed back to the hydrocarbon inlet 104 of the hydrocarbon
converter
103. Further processing or separation of non-suitable hydrocarbons is effected
before the
process of returning them to the hydrocarbon inlet 104 in dependence on the
composition
of the synthetically produced hydrocarbons that are being fed back.
Fig. 4 shows an example of a plant having a plurality of adjacently operating
hydrocarbon converters as a further embodiment of the plant 100 for the
production of
synthetic functionalised and/or non-functionalised hydrocarbons. In Fig. 4,
the same
reference symbols as were used in the previous embodiment are utilised insofar
as
equivalent or similar elements are described. In the case of the embodiment
shown in
Fig. 4, a combination of a high temperature hydrocarbon converter 103a and a
low-
temperature hydrocarbon converter 103b is provided instead of a single
hydrocarbon
converter 103.
The high temperature hydrocarbon converter 103a comprises a hydrocarbon inlet
104a, a
first outlet 105a for feeding out carbon and a second outlet 106a for feeding
out
hydrogen. Once again however, a single outlet 105a for a mixture (especially
an aerosol)
of carbon and hydrogen can be provided. The outlet 105a is connected by a
connection
108 to the inlet 111 of the CO2 converter 109. The optional outlet 106a of the
high
temperature hydrocarbon converter 103a is connected to the H2 inlet 133 of the
CO
converter 131. The high temperature hydrocarbon converter 103a can optionally
comprise a further outlet for carbon that is not shown in Fig. 105.
CA 02916447 2015-12-21
21
The low-temperature hydrocarbon converter 103b comprises a processing area
having a
hydrocarbon inlet 104b, a first outlet 105b for feeding out carbon, a second
outlet 106b
for feeding out hydrogen, and an optional third outlet 107b for feeding out
carbon.
Preferably, the low-temperature hydrocarbon converter 103b comprises a
separating unit
for separating the hydrogen and carbon resulting from the decomposing process
and
feeding them to the respective outlets. Optionally, the first outlet 105b is
connected by
the connection 108 to the inlet 111 of the CO2 converter 109, but it could
also be
connected to a carbon collecting unit. The outlet 106b of the low-temperature
hydrocarbon converter 103b is connected to the H2 inlet 133 of the CO
converter 131.
The optional third outlet 107b is connected to a carbon collecting unit from
which the
collected carbon can be removed for example in the form of carbon black,
activated
charcoal or some other form.
The hydrocarbon that is being fed into the hydrocarbon inlet 104a and into the
hydrocarbon inlet 104b can be one and the same hydrocarbon, or they could be
different
hydrocarbons. Hydrocarbon, such as natural gas from a natural gas reservoir
for
example, can be fed into the hydrocarbon inlet 104a from a first source of
hydrocarbon.
In contrast thereto, a functionalised and/or non-functionalised synthetically
produced
hydrocarbon for example can be fed into the hydrocarbon inlet 104b of the low-
temperature hydrocarbon converter 103b via the previously mentioned optional
return
pipe connection 139 for example. By the use of several hydrocarbon converters
103a,
103b operating in parallel, the plant 100 is more easily scalable, more easily
controllable
and different types of carbon can be produced.
Moreover, the high temperature hydrocarbon converter 103a can be used to
advantage to
produce "hot" carbon preferably having a temperature of over 1000 C for the
CO2
conversion process in the CO2 converter 109 for example. Hereby in particular,
the high
temperature hydrocarbon converter 103a can manage to work without a separating
unit
because the C-H2 mixture obtained from the decomposing process can be
introduced
directly into the CO2 converter 109. In this case, the CO2 converter 109 then
gives out a
CA 02916447 2015-12-21
22
synthesis gas having a C-H2 mix-proportion of approximately 1:1 at the outlet
for
example.
By contrast, the low-temperature hydrocarbon converter 103b is used primarily
for the
production of additional hydrogen in order to enable the production of a
synthesis gas or
a C-H2 mixture having a C-H2 mix-proportion of more than 1:1, and in
particular, of
more than 1:2 for the CO converter 131. Since a transfer of heat from the low-
temperature hydrocarbon converter 103b to a following process is not required
here, it
can be operated to advantage at temperatures of under 1000 C and preferably at
the
lowest possible temperature.
Thus, when the plant 100 is in operation, a portion of the carbon produced in
the
hydrocarbon converters 103a, 103b (preferably that from the high temperature
hydrocarbon converter 103a) can be fed into the CO2 converter 109, whilst
another
portion (preferably that from the low-temperature hydrocarbon converter 103b)
can be
fed out from the process as a raw material for the production of further
products. If
necessary, surplus hydrogen can be otherwise used, for example, for the
production of
electric current (by burning), wherein the low-temperature hydrocarbon
converter 103b is
preferably operated in such a way that it only produces the required
additional hydrogen.
From the above description, there thus results a method which makes it
possible to
provide good and pollution-free extraction of highly viscous oils and/or
bitumen by the
employment of a preferably synthetically produced liquid hydrocarbon.
If one uses a modern gas-fired power station having power-heat coupling for
producing
the necessary energy by means of gas, then this power station can provide heat
energy
and electric current for the operation of the plant 1 and also the plant 100.
The
production of the liquid hydrocarbon is effected with part of the electric
current, whereby
the CO2 emissions of the gas-fired power station are completely or at least to
the greater
part absorbed into the liquid hydrocarbons (particularly benzene, kerosene,
diesel) which
is used for the extraction of the highly viscous oils and/or bitumen in the
deposit 3. At
CA 02916447 2015-12-21
23
the same time, sufficient electricity can be provided for pumping the
resulting liquids and
if necessary too, for the operation of a cracker insofar as such a device is
to be operated
on site.
The decomposing process can be effected by dissolving out the bitumen by means
of the
locally produced liquid hydrocarbon. In practice, there is produced an
artificial crude oil
which can be pumped out of the deposit and then sent by pipeline to a
refinery.
Reprocessing of the artificial crude oil produced in this manner may then take
place not
before the refinery so that an on-site distillation step can be saved. In
contrast to the
steam process, one can thereby dispense with the process of producing steam at
the site of
the deposit itself and the environmental problems that are associated
therewith.
Yet another advantage can result for the extraction companies when there is a
local
shortage of labour as is already the case in Alberta for example since, in
comparison with
existing methods, some working steps are completely dispensed with and other
working
steps may be shifted to a distantly located refinery. Furthermore, there are
environmental
benefits because water consumption is substantially reduced and, in
particular, CO2
emissions may be reduced or even avoided entirely.
The invention has been described in detail on the basis of special embodiments
and on
the basis of some examples, but is not restricted thereto. In particular, the
elements of the
individual embodiments are combinable with one another and/or are exchangeable
insofar as they are compatible. Numerous modifications and deviations which
fall within
the scope of the following claims will occur to the skilled person. In
particular, above-
ground extraction of highly viscous oils and/or bitumen, or, recycling of
bitumen and/or
asphalt-containing materials in a reprocessing plant is also conceivable. In
particular, oil
sands can also be brought into contact with the liquid hydrocarbon in order to
dissolve
the oil/bitumen and to subsequently filter out solid constituents of the oil
sands and
especially the sand. Consideration could additionally be given to "flushing-
out" almost
fully exhausted oil fields since these are deposits of highly viscous
oil/bitumen.
Although the plant 100 for the production of synthetic functional and/or non-
functional
CA 02916447 2015-12-21
24
hydrocarbons has been described as being located at a deposit 3, a
corresponding plant
100 could also be located at a certain distance from the deposit 3. In this
case, the liquid
hydrocarbon that has been produced could be sent to the deposit 3 over a
pipeline for
example. In such a constellation for example, several deposits 3 could also be
supplied
with liquid hydrocarbon by just a single plant 100. Then, for example, such a
plant 100
could also bind CO2 emissions from other plants and processes in the liquid
hydrocarbon
and thus prevent them from initially being released into the environment.
In a particularly simple embodiment of a plant for the production of synthetic
functional
and/or non-functional hydrocarbons, the CO2 converter can, for example, be in
the form
of a simple pipe (e.g. an output pipe of a high temperature hydrocarbon
converter that
does not comprise a separating unit) into which a CO2 line opens out. Thereby,
the CO2
line should open out into the pipe in such a way as to achieve thorough mixing
of the
respective media streams. The pipe should be surrounded by insulation and a
section of
the inlet end thereof could be attached to a heating unit for example in order
to preheat
the pipe up to the operating temperature especially at the beginning of the
operation.
Further downstream, the pipe could again be attached to a heat exchanger which
extracts
the surplus heat which can possibly be utilised for heating other areas of the
plant and/or
for the production of electric current. In addition, an inlet line for
hydrogen may open
out into the pipe (e.g. downstream of the heat exchanger) so that the same
pipe can carry
out not only the function of a CO2 converter but also that of a mixer for
producing a
synthesis gas. The hydrogen supply line could extend from a hydrogen outlet of
a low-
temperature hydrocarbon converter (having a separating unit) for example. The
output
end of the pipe, at which a synthesis gas having a pre-determined mix-
proportion can be
expelled, could then end in a CO converter.