Note: Descriptions are shown in the official language in which they were submitted.
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
LIQUID ADDITIVE FOR CEMENT RESILIENCY
CROSS-REFERENCE TO RELATED APPLICATIONS
Not applicable.
TECHNICAL FIELD
[0001] The disclosure is in the field of hydraulic cement compositions. Such
compositions can be useful in producing crude oil or natural gas from
subterranean formations,
such as cementing of wells.
BACKGROUND
[0002] Prior materials used to modify the mechanical properties of set cement
include
elastomeric-based particles. Such elastomeric particulates primarily affect
the Young's modulus
and Poisson's ratio of a set cement.
[0003] Examples of such solid particulates include: LATEX 2000Tm and LATEX
30O0, which are very small elastomer particles (specific gravity about 1)
suspended in an
aqueous solution (about 50% by weight), and LAP-11m, which is a solid
particulate of a
polyvinyl alcohol that is slightly crosslinked (specific gravity about 1.33),
all of which are
commercially available from Halliburton Energy Services, Inc. in Duncan,
Oklahoma. Other
examples include styrene-butadiene copolymer ("SBC") particulates (specific
gravity about 1).
SBC particulates are often provided as dry, solid materials that can be dry-
blended.
[0004] Cement slurries usually have specific gravities in the range of about
1.44 (12
lb/gal) to about 2.28 (19 lb/gal). Because solid elastomeric materials such as
SBC have a
specific gravity lower than the specific gravities for common cement slurries,
they tend to float
to the surface rather than stay suspended in the cement slurry, resulting in
cement slurry stability
issues.
[0005] An elastomeric material can have a weighting agents, such as barite
powder,
incorporated into the matrix of the elastomeric material to increase the
apparent specific gravity
1
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
of a particulate formed with such a mixed material; however, twice as much of
such a product
(by weight) is required to meet the same active elastomer content.
GENERAL DESCRIPTION OF EMBODIMENTS
[0006] The disclosure provides a polymer can be included in a cement slurry to
provide
a set cement that has lower Young's Modulus values and, hence, is a more
resilient set cement.
[0007] The polymer can be dissolved in an aqueous phase that can be included
in a
cement composition; however, upon heating, which can be, for example, due to
downhole
temperature conditions in a well, the dissolved polymer will precipitate to
form solid particles.
Due to this change from a liquid state to a solid state, there are no cement
slurry stability issues
relating to surface mixing of the dissolved polymer.
[0008] According to an embodiment of the disclosure, a hydraulic cement
composition
including: (A) a hydraulic cement; (B) at least a sufficient concentration of
water to form a
pumpable slurry with the hydraulic cement; and (C) a polymer selected from the
group
consisting of: (i) a homopolymer of one monomer selected from the group
consisting of:
N-isopropylacrylamide, N-propyl acrylamide, and /V,N-diethyl acrylamide; (ii)
a copolymer
consisting of two or more monomers selected from the group consisting of:
N-isopropylacrylamide, N-propyl acrylamide, and N,N-diethyl acrylamide; and
(iii) a copolymer
comprising: (a) one or more first monomers selected from the group consisting
of:
N-isopropylacrylamide, N-propyl acrylamide, N,N-diethyl acrylamide, and any
combination
thereof; and (b) one or more second monomers selected from the group
consisting of:
acrylamide, an acrylamide derivative other than one of the first monomers,
methacrylamide, an
N-alkyl methacrylamide, N-methyl-N-vinylacetamide, N-vinylformamide, a vinyl
pyrrolidone, a
vinyl pyridine, N-vinylcaprolactam, N-methyl-N-vinylacetamide, a styrene
sulfonic acid, a
styrene sulfonate, a vinyl sulfonic acid, a vinyl sulfonate, and any
combination of the foregoing.
[0009] In cases where the setting temperature is greater than a lower critical
solution
temperature ("LCST") of the polymer, at least some of any such dissolved
polymer will
precipitate to increase the resiliency of the set cement.
2
CA 2918017 2017-05-03
[0001] According to an embodiment of the disclosure, a method of cementing in
a well includes: (A) forming such a hydraulic cement composition according to
this
disclosure; and (B) introducing the hydraulic cement composition into a
treatment zone of
the well. In cases where the design temperature of the treatment zone in the
well is
greater than a lower critical solution temperature ("LCST") of the polymer, at
least some
of any such dissolved polymer will precipitate to increase the resiliency of
the set cement.
[0002] These and other embodiments of the disclosure will be apparent to one
skilled in the art upon reading the following detailed description. While the
disclosure is
susceptible to various modifications and alternative forms, specific
embodiments thereof
will be described in detail and shown by way of example. It should be
understood,
however, that it is not intended to limit the disclosure to the particular
forms disclosed.
BRIEF DESCRIPTION OF THE DRAWING
[0003] The accompanying drawing is incorporated into the specification to help
illustrate examples according to a presently preferred embodiment of the
disclosure.
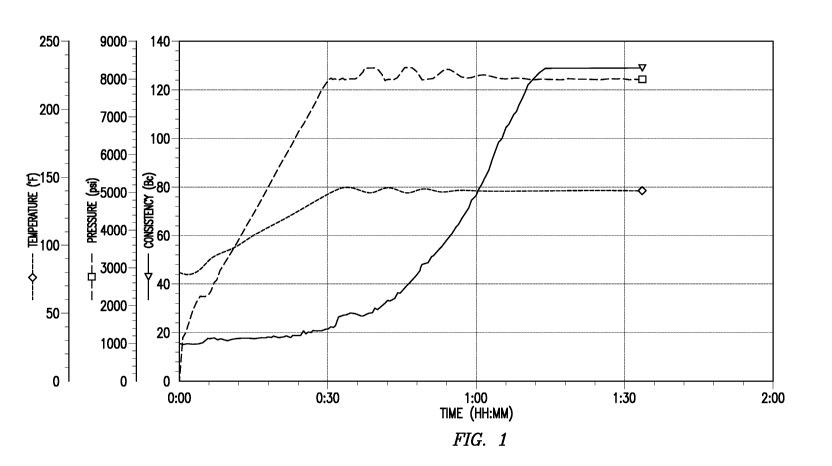
[0004] Figure 1 is a graph showing the setting of a control cement without any
NIPAM/DMAC polymer. (PRIOR ART)
[0005] Figure 2 is a graph showing the setting of a cement having 1.82
gal/sack
(7.5 eq. % bwoc) low molecular weight NIPAM/DMAC copolymer.
DETAILED DESCRIPTION OF PRESENTLY PREFERRED EMBODIMENTS
AND BEST MODE
Definitions and Usages
General Interpretation
[0006] The words or terms used herein have their plain, ordinary meaning in
the
field of this disclosure, except to the extent explicitly and clearly defined
in this
disclosure or unless the specific context otherwise requires a different
meaning.
[0007] The words "comprising," "containing," "including," "having," and all
grammatical variations thereof are intended to have an open, non-limiting
meaning. For
3
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
example, a composition comprising a component does not exclude it from having
additional
components, an apparatus comprising a part does not exclude it from having
additional parts, and
a method having a step does not exclude it having additional steps. When such
terms are used,
the compositions, apparatuses, and methods that "consist essentially of" or
"consist of' the
specified components, parts, and steps are specifically included and
disclosed. As used herein,
the words "consisting essentially of," and all grammatical variations thereof
are intended to limit
the scope of a claim to the specified materials or steps and those that do not
materially affect the
basic and novel characteristic(s) of the claimed invention.
[0017] The indefinite articles "a" or "an" mean one or more than one of the
component,
part, or step that the article introduces.
[0018] Whenever a numerical range of degree or measurement with a lower limit
and
an upper limit is disclosed, any number and any range falling within the range
is also intended to
be specifically disclosed. For example, every range of values (in the form
"from a to b," or
"from about a to about b," or "from about a to b," "from approximately a to
b," and any similar
expressions, where "a" and "b" represent numerical values of degree or
measurement) is to be
understood to set forth every number and range encompassed within the broader
range of values.
[0019] Terms such as "first," "second," "third," etc. may be assigned
arbitrarily and
are merely intended to differentiate between two or more components, parts, or
steps that are
otherwise similar or corresponding in nature, structure, function, or action.
For example, the
words "first" and "second" serve no other purpose and are not part of the name
or description of
the following name or descriptive terms. The mere use of the term "first" does
not require that
there be any "second" similar or corresponding component, part, or step.
Similarly, the mere use
of the word "second" does not require that there be any "first" or "third"
similar or
corresponding component, part, or step. Further, it is to be understood that
the mere use of the
term "first" does not require that the element or step be the very first in
any sequence, but merely
that it is at least one of the elements or steps. Similarly, the mere use of
the terms "first" and
"second" does not necessarily require any sequence. Accordingly, the mere use
of such terms
does not exclude intervening elements or steps between the "first" and
"second" elements or
steps, etc.
4
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
Oil and Gas Reservoirs
[0020] In the context of production from a well, "oil" and "gas" are
understood to refer
to crude oil and natural gas, respectively. Oil and gas are naturally
occurring hydrocarbons in
certain subterranean formations.
[0021] A "subterranean formation" is a body of rock that has sufficiently
distinctive
characteristics and is sufficiently continuous for geologists to describe,
map, and name it.
[0022] A subterranean formation having a sufficient porosity and permeability
to store
and transmit fluids is sometimes referred to as a "reservoir."
[0023] A subterranean formation containing oil or gas may be located under
land or
under the seabed off shore. Oil and gas reservoirs are typically located in
the range of a few
hundred feet (shallow reservoirs) to a few tens of thousands of feet (ultra-
deep reservoirs) below
the surface of the land or seabed.
Well Servicing and Fluids
[0024] To produce oil or gas from a reservoir, a wellbore is drilled into a
subterranean
formation, which may be the reservoir or adjacent to the reservoir. Typically,
a wellbore of a
well must be drilled hundreds or thousands of feet into the earth to reach a
hydrocarbon-bearing
formation.
[0025] Generally, well services include a wide variety of operations that may
be
performed in oil, gas, geothermal, or water wells, such as drilling,
cementing, completion, and
intervention. Well services are designed to facilitate or enhance the
production of desirable
fluids such as oil or gas from or through a subterranean formation. A well
service usually
involves introducing a fluid into a well.
[0026] Drilling is the process of drilling the wellbore. After a portion of
the wellbore is
drilled, sections of steel pipe, referred to as casing, which are slightly
smaller in diameter than
the borehole, are placed in at least the uppermost portions of the wellbore.
The casing provides
structural integrity to the newly drilled borehole.
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
[0027] Cementing is a common well operation. For example, hydraulic cement
compositions can be used in cementing operations in which a string of pipe,
such as casing or
liner, is cemented in a wellbore. The cement stabilizes the pipe in the
wellbore and prevents
undesirable migration of fluids along the annulus between the wellbore and the
outside of the
casing or liner from one zone along the wellbore to the next. Where the
wellbore penetrates into
a hydrocarbon-bearing zone of a subterranean formation, the casing can later
be perforated to
allow fluid communication between the zone and the wellbore. The cemented
casing also
enables subsequent or remedial separation or isolation of one or more
production zones of the
wellbore by using downhole tools, such as packers or plugs, or by using other
techniques, such
as forming sand plugs or placing cement in the perforations. Hydraulic cement
compositions can
also be utilized in intervention operations, such as in plugging highly
permeable zones, or
fractures in zones, that may be producing too much water, plugging cracks or
holes in pipe
strings, and the like.
[0028] Completion is the process of making a well ready for production or
injection.
This principally involves preparing a zone of the wellbore to the required
specifications, running
in the production tubing and associated downhole equipment, as well as
perforating and
stimulating as required.
[0029] Intervention is any operation carried out on a well during or at the
end of its
productive life that alters the state of the well or well geometry, provides
well diagnostics, or
manages the production of the well.
[0030] Drilling, cementing, completion, and intervention operations can
include various
types of treatments that are commonly performed on a well or subterranean
formation. Among
other types of treatments, cementing or remedial cementing may be useful
treatments as part of
such operations.
Wells
[0031] A "well" includes a wellhead and at least one wellbore from the
wellhead
penetrating the earth. The "wellhead" is the surface termination of a
wellbore, which surface
may be on land or on a seabed.
6
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
[0032] A "well site" is the geographical location of a wellhead of a well. It
may
include related facilities, such as a tank battery, separators, compressor
stations, heating or other
equipment, and fluid pits. If offshore, a well site can include a platform.
[0033] The "wellbore refers to the drilled hole, including any cased or
uncased
portions of the well or any other tubulars in the well. The "borehole" usually
refers to the inside
wellbore wall, that is, the rock surface or wall that bounds the drilled hole.
A wellbore can have
portions that are vertical, horizontal, or anything in between, and it can
have portions that are
straight, curved, or branched. As used herein, "uphole," "downhole," and
similar terms are
relative to the direction of the wellhead, regardless of whether a wellbore
portion is vertical or
horizontal.
[0034] As used herein, introducing "into a well" means introducing at least
into and
through the wellhead. According to various techniques known in the art,
tubulars, equipment,
tools, or fluids can be directed from the wellhead into any desired portion of
the wellbore.
[0035] As used herein, the word "tubular" means any kind of structural body in
the
general form of a tube. Examples of tubulars in oil wells include, but are not
limited to, a drill
pipe, a casing, a tubing string, a line pipe, and a transportation pipe.
[0036] As used herein, the term "annulus" means the space between two
generally
cylindrical objects, one inside the other. The objects can be concentric or
eccentric. Without
limitation, one of the objects can be a tubular and the other object can be an
enclosed conduit.
The enclosed conduit can be a wellbore or borehole or it can be another
tubular. The following
are some non-limiting examples illustrating some situations in which an
annulus can exist.
Referring to an oil, gas, or water well, in an open hole well, the space
between the outside of a
tubing string and the borehole of the wellbore is an annulus. In a cased hole,
the space between
the outside of the casing and the borehole is an annulus. In addition, in a
cased hole there may
be an annulus between the outside cylindrical portion of a tubular, such as a
production tubing
string, and the inside cylindrical portion of the casing. An annulus can be a
space through which
a fluid can flow or it can be filled with a material or object that blocks
fluid flow, such as a
packing element. Unless otherwise clear from the context, as used herein an
"annulus" is a space
through which a fluid can flow.
7
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
[0037] A fluid can be, for example, a drilling fluid, a setting composition, a
treatment
fluid, or a spacer fluid.
[0038] As used herein, the word "treatment" refers to any treatment for
changing a
condition of a portion of a wellbore or a subterranean formation adjacent a
wellbore; however,
the word "treatment" does not necessarily imply any particular treatment
purpose. A treatment
usually involves introducing a fluid for the treatment, in which case it may
be referred to as a
treatment fluid, into a well. As used herein. a "treatment fluid" is a fluid
used in a treatment.
The word "treatment" in the term "treatment fluid" does not necessarily imply
any particular
treatment or action by the fluid.
[0039] As used herein, the terms spacer fluid, wash fluid, and inverter fluid
can be used
interchangeably. A spacer fluid is a fluid used to physically separate one
special-purpose fluid
from another. It may be undesirable for one special-purpose fluid to mix with
another used in
the well, so a spacer fluid compatible with each is used between the two. A
spacer fluid is
usually used when changing between fluids used in a well.
[0040] In the context of a well or wellbore, a "portion" or "interval" refers
to any
downhole portion or interval along the length of a wellbore.
[0041] A "zone" refers to an interval of rock along a wellbore that is
differentiated from
uphole and downhole zones based on hydrocarbon content or other features, such
as
permeability, composition, perforations or other fluid communication with the
wellbore, faults,
or fractures. A zone of a wellbore that penetrates a hydrocarbon-bearing zone
that is capable of
producing hydrocarbon is referred to as a "production zone." A "treatment
zone" refers to an
interval of rock along a wellbore into which a fluid is directed to flow from
the wellbore. As
used herein, "into a treatment zone" means into and through the wellhead and,
additionally,
through the wellbore and into the treatment zone.
[0042] Generally, the greater the depth of the formation, the higher the
static
temperature and pressure of the formation. Initially, the static pressure
equals the initial pressure
in the formation before production. After production begins, the static
pressure approaches the
average reservoir pressure.
8
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
[0043] A "design" refers to the estimate or measure of one or more parameters
planned
or expected for a particular fluid or stage of a well service or treatment.
For example, a fluid can
be designed to have components that provide a minimum density or viscosity for
at least a
specified time under expected downhole conditions. A well service may include
design
parameters such as fluid volume to be pumped, required pumping time for a
treatment, or the
shear conditions of the pumping.
[0044] The term "design temperature" refers to an estimate or measurement of
the
actual temperature at the downhole environment during the time of a treatment.
For example,
the design temperature for a well treatment takes into account not only the
bottom hole static
temperature ("BHST"), but also the effect of the temperature of the fluid on
the BHST during
treatment. The design temperature for a fluid is sometimes referred to as the
bottom hole
circulation temperature ("BHCT"). Because fluids may be considerably cooler
than BHST, the
difference between the two temperatures can be quite large. Ultimately, if
left undisturbed a
subterranean formation will return to the BHST.
Phases, Physical States, and Materials
[0045] As used herein, "phase" is used to refer to a substance having a
chemical
composition and physical state that is distinguishable from an adjacent phase
of a substance
having a different chemical composition or a different physical state.
[0046] As used herein, if not other otherwise specifically stated, the
physical state or
phase of a substance (or mixture of substances) and other physical properties
are determined at a
temperature of 77 F (25 C) and a pressure of l atmosphere (Standard
Laboratory
Conditions) without applied shear.
[0047] The word "material" refers to the substance, constituted of one or more
phases,
of a physical entity or object. Rock, water, air, metal, cement slurry, sand,
and wood are all
examples of materials. The word "material" can refer to a single phase of a
substance on a bulk
scale (larger than a particle) or a bulk scale of a mixture of phases,
depending on the context.
9
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
Particles and Particulates
[0048] As used herein, a "particle" refers to a body having a finite mass and
sufficient
cohesion such that it can be considered as an entity but having relatively
small dimensions. A
particle can be of any size ranging from molecular scale to macroscopic,
depending on context.
[0049] A particle can be in any physical state. For example, a particle of a
substance in
a solid state can be as small as a few molecules on the scale of nanometers up
to a large particle
on the scale of a few millimeters, such as large grains of sand. Similarly, a
particle of a
substance in a liquid state can be as small as a few molecules on the scale of
nanometers up to a
large drop on the scale of a few millimeters.
[0050] As used herein, particulate or particulate material refers to matter in
the physical
form of distinct particles in a solid or liquid state (which means such an
association of a few
atoms or molecules). As used herein, a particulate is a grouping of particles
having similar
chemical composition and particle size ranges anywhere in the range of about
0.5 micrometer
(500 nm), for example, microscopic clay particles, to about 3 millimeters, for
example, large
grains of sand.
[0051] A particulate can be of solid or liquid particles. As used herein,
however, unless
the context otherwise requires, particulate refers to a solid particulate.
[0052] As used herein, -particle density" or -true density" means the density
of a
particulate is the density of the individual particles that make up the
particulate, in contrast to the
bulk density, which measures the average density of a large volume of the
powder in a specific
medium (usually air). The particle density is a relatively well-defined
quantity, as it is not
dependent on the degree of compaction of the solid, whereas the bulk density
has different values
depending on whether it is measured in the freely settled or compacted state
(tap density).
However, a variety of definitions of particle density are available, which
differ in terms of
whether pores are included in the particle volume, and whether voids are
included. As used
herein, particle density includes the apparent density of a particle having
any pores or voids into
which water does not penetrate.
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
Dispersions
[0053] A dispersion is a system in which particles of a substance of one
chemical
composition and physical state are dispersed in another substance of a
different chemical
composition or physical state. In addition, phases can be nested. If a
substance has more than
one phase, the most external phase is referred to as the continuous phase of
the substance as a
whole, regardless of the number of different internal phases or nested phases.
[0054] A dispersion can be classified in different ways, including, for
example, based
on the size of the dispersed particles, the uniformity or lack of uniformity
of the dispersion, and,
if a fluid, by whether or not precipitation occurs.
[0055] A dispersion is considered to be heterogeneous if the dispersed
particles are not
dissolved and are greater than about 1 nanometer in size. (For reference, the
diameter of a
molecule of toluene is about 1 nm and a molecule of water is about 0.3 nm).
[0056] Heterogeneous dispersions can have gas, liquid, or solid as an external
phase.
For example, in a case where the dispersed-phase particles are liquid in an
external phase that is
another liquid, this kind of heterogeneous dispersion is more particularly
referred to as an
emulsion. A solid dispersed phase in a continuous liquid phase is referred to
as a sol,
suspension, or slurry, partly depending on the size of the dispersed solid
particulate.
[0057] A dispersion is considered to be homogeneous if the dispersed particles
are
dissolved in solution or the particles are less than about 1 nanometer in
size. Even if not
dissolved, a dispersion is considered to be homogeneous if the dispersed
particles are less than
about 1 nanometer in size.
[0058] Heterogeneous dispersions can be further classified based on the
dispersed
particle size.
[0059] A heterogeneous dispersion is a "suspension" where the dispersed
particles are
larger than about 50 micrometers. Such particles can be seen with a
microscope, or if larger than
about 50 micrometers (0.05 mm), with the unaided human eye. The dispersed
particles of a
suspension in a liquid external phase may eventually separate on standing, for
example, settle in
cases where the particles have a higher density than the liquid phase.
Suspensions having a
11
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
liquid external phase are essentially unstable from a thermodynamic point of
view; however,
they can be kinetically stable over a long period depending on temperature and
other conditions.
[0060] A heterogeneous dispersion is a "slun-y" where the dispersed particles
are larger
than about 50 micrometers, which can be seen with the unaided human eye. A
hydraulic cement
dispersed in water is usually considered to be a slurry.
Solutions and Hydratability or Solubility
[0061] A solution is a special type of homogeneous mixture. A solution is
considered
homogeneous: (a) because the ratio of solute to solvent is the same throughout
the solution; and
(b) because solute will never settle out of solution, even under powerful
centrifugation, which is
due to intermolecular attraction between the solvent and the solute. An
aqueous solution, for
example, saltwater, is a homogenous solution in which water is the solvent and
salt is the solute.
[0062] One may also refer to the solvated state, in which a solute ion or
molecule is
complexed by solvent molecules. A chemical that is dissolved in solution is in
a solvated state.
The solvated state is distinct from dissolution and solubility. Dissolution is
a kinetic process,
and is quantified by its rate. Solubility quantifies the concentration of the
solute at which there is
dynamic equilibrium between the rate of dissolution and the rate of
precipitation of the solute.
Dissolution and solubility can be dependent on temperature and pressure, and
may be dependent
on other factors, such as salinity or pH of an aqueous phase.
[0063] As referred to herein, "hydratable" means capable of being hydrated by
contacting the hydratable material with water. Regarding a hydratable material
that includes a
polymer, this means, among other things, to associate sites on the polymer
with water molecules
and to unravel and extend the polymer chain in the water.
[0064] The term "solution" is intended to include not only true molecular
solutions but
also dispersions of a polymer wherein the polymer is so highly hydrated as to
cause the
dispersion to be visually clear and having essentially no particulate matter
visible to the unaided
eye. The term "soluble" is intended to have a meaning consistent with these
meanings of
solution.
12
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
[0065] As used herein, a substance is considered to be "soluble" in a liquid
if at least 10
grams of the substance can be hydrated or dissolved in one liter of the liquid
when tested at 60 F
(15 C) and 1 atmosphere pressure for 2 hours, considered to be "insoluble" if
less than 1 gram
per liter, and considered to be "sparingly soluble" for intermediate
solubility values.
[0066] As will be appreciated by a person of skill in the art, the
hydratability,
dispersibility, or solubility of a substance in water can be dependent on the
salinity, pH, or other
substances in the water. Accordingly, the salinity, pH, and additive selection
of the water can be
modified to facilitate the hydratability, dispersibility, or solubility of a
substance in aqueous
solution. To the extent not specified, the hydratability, dispersibility, or
solubility of a substance
in water is determined in deionized water, at neutral pH, and without any
other additives.
[0067] As used herein, the term "polar" means having a dielectric constant
greater than
30. The term "relatively polar" means having a dielectric constant greater
than about 2 and less
than about 30. "Non-polar" means having a dielectric constant less than 2.
Fluids
[0068] A fluid can be a homogeneous or heterogeneous. In general, a fluid is
an
amorphous substance that is or has a continuous phase of particles that are
smaller than about 1
micrometer that tends to flow and to conform to the outline of its container.
[0069] Examples of fluids are gases and liquids. A gas (in the sense of a
physical
state) refers to an amorphous substance that has a high tendency to disperse
(at the molecular
level) and a relatively high compressibility. A liquid refers to an amorphous
substance that has
little tendency to disperse (at the molecular level) and relatively high
incompressibility. The
tendency to disperse is related to Intermolecular Forces (also known as van
der Waal's Forces).
(A continuous mass of a particulate, for example, a powder or sand, can tend
to flow as a fluid
depending on many factors such as particle size distribution, particle shape
distribution, the
proportion and nature of any wetting liquid or other surface coating on the
particles, and many
other variables. Nevertheless, as used herein, a fluid does not refer to a
continuous mass of
particulate as the sizes of the solid particles of a mass of a particulate are
too large to be
appreciably affected by the range of Intermolecular Forces.)
13
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
[0070] Every fluid inherently has at least a continuous phase. A fluid can
have more
than one phase. The continuous phase of a treatment fluid is a liquid under
Standard Laboratory
Conditions. For example, a fluid can be in the form of a suspension (larger
solid particles
dispersed in a liquid phase), such as a slurry, an emulsion (liquid particles
dispersed in another
liquid phase), or a foam (a gas phase dispersed in a liquid phase).
Apparent Viscosity of a Fluid
[0071] Viscosity is a measure of the resistance of a fluid to flow. In
everyday terms,
viscosity is "thickness" or "internal friction." Therefore, pure water is
"thin," having a relatively
low viscosity whereas honey is "thick," having a relatively higher viscosity.
Put simply, the less
viscous the fluid is, the greater its ease of movement (fluidity). More
precisely, viscosity is
defined as the ratio of shear stress to shear rate.
[0072] A fluid moving along solid boundary will incur a shear stress on that
boundary.
The no-slip condition dictates that the speed of the fluid at the boundary
(relative to the
boundary) is zero, but at some distance from the boundary, the flow speed must
equal that of the
fluid. The region between these two points is named the boundary layer.
[0073] A Newtonian fluid (named after Isaac Newton) is a fluid for which
stress versus
strain rate curve is linear and passes through the origin. The constant of
proportionality is known
as the viscosity. Examples of Newtonian fluids include water and most gases.
Newton's law of
viscosity is an approximation that holds for some substances but not others.
[0074] Non-Newtonian fluids exhibit a more complicated relationship between
shear
stress and velocity gradient (i.e., shear rate) than simple linearity.
Therefore, there exist a
number of forms of non-Newtonian fluids. Shear thickening fluids have an
apparent viscosity
that increases with increasing the rate of shear. Shear thinning fluids have a
viscosity that
decreases with increasing rate of shear. Thixotropic fluids become less
viscous over time at a
constant shear rate. Rheopectic fluids become more viscous over time at a
constant shear rate.
A Bingham plastic is a material that behaves as a solid at low stresses but
flows as a viscous
fluid at high yield stresses.
14
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
[0075] Most fluids are non-Newtonian fluids. Accordingly, the apparent
viscosity of a
fluid applies only under a particular set of conditions including shear stress
versus shear rate,
which must be specified or understood from the context. As used herein, a
reference to viscosity
is actually a reference to an apparent viscosity. Apparent viscosity is
commonly expressed in
units of mPa.s or centipoise (cP), which are equivalent.
Cement and Cement Compositions
[0076] As used herein, the term "set" means the process of becoming solid or
hard by
curing.
[0077] In the most general sense of the word, a "cement" is a binder, that is,
a
substance that sets. As used herein, "cement" refers to an inorganic cement
that, when mixed
with water, will begin to set and harden into a concrete material.
[0078] As used herein. a "cement composition" is a material including at least
one
inorganic cement. A cement composition can also include additives. Some cement
compositions can include water or be mixed with water. Depending on the type
of cement, the
chemical proportions, when a cement composition is mixed with water it can
begin setting to
form a solid material.
[0079] A cement can be characterized as non-hydraulic or hydraulic.
[0080] Hydraulic cements (for example, Portland cement) harden because of
hydration,
chemical reactions that occur independently of the mixture's water content;
they can harden even
underwater or when constantly exposed to wet weather. The chemical reaction
that results when
the dry cement powder is mixed with water produces hydrates that have
extremely low solubility
in water. The cement composition sets by a hydration process, and it passes
through a gel phase
to solid phase.
[0081] More particularly, Portland cement is formed from a clinker such as a
clinker
according to the European Standard EN197-1: "Portland cement clinker is a
hydraulic material
which shall consist of at least two-thirds by mass of calcium silicates (3
CaO. 5i02 and 2
CaO=Si02), the remainder consisting of aluminium- and iron-containing clinker
phases and other
compounds. The ratio of CaO to 5i02 shall not be less than 2Ø The magnesium
oxide content
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
(MgO) shall not exceed 5.0% by mass." The American Society of Testing
Materials
("ASTM") standard "C 150" defines Portland cement as "hydraulic cement (cement
that not only
hardens by reacting with water but also forms a water-resistant product)
produced by pulverizing
clinkers consisting essentially of hydraulic calcium silicates, usually
containing one or more of
the forms of calcium sulfate as an inter ground addition." In addition,
Portland cements typically
have a ratio of CaO to SiG) of less than 4Ø
[0082] Clinkers are nodules (diameters about 0.2 inch to about 1.0 inch [about
5 mm
to about 25 mm]) of a sintered material that is produced when a raw mixture of
predetermined
composition is heated to high temperature.
[0083] Portland cement clinker is made by heating to sintering temperature a
mixture of
raw materials, which is about 1450 C for modern cements. The alumina and iron
oxide are
present as a flux and contribute little to the strength.
[0084] The American Society for Testing and Materials (ASTM) has established a
set
of standards for a Portland cement to meet to be considered an ASTM cement.
These standards
include Types I, II, III, IV, and V.
[0085] The American Petroleum Institute (API) has established a set of
standards that a
Portland cement must meet to be considered an API cement. The standards
include Classes A,
B, C, D, E, F, G, H, I, and J.
[0086] A blended cement is a hydraulic cement produced by intergrinding
Portland
cement clinker with other materials, by blending Portland cement with other
materials, or by a
combination of intergrinding and blending.
Cementing and Other Uses for Cement Compositions
[0087] During well completion, it is common to introduce a cement composition
into
an annulus in the wellbore. For example, in a cased hole, the cement
composition is placed into
and allowed to set in the annulus between the wellbore and the casing in order
to stabilize and
secure the casing in the wellbore. After setting, the set cement composition
should have a low
permeability. Consequently, oil or gas can be produced in a controlled manner
by directing the
flow of oil or gas through the casing and into the wellhead.
16
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
[0088] Cement compositions can also be used, for example, in well-plugging
operations
or gravel-packing operations. Cement compositions can also be used to control
fluid loss or
migration in zones.
[0089] During placement of a cement composition, it is necessary for the
cement
composition to remain pumpable during introduction into the subterranean
formation or the well
and until the cement composition is situated in the portion of the
subterranean formation or the
well to be cemented. After the cement composition has reached the portion of
the well to be
cemented, the cement composition ultimately sets. A cement composition that
thickens too
quickly while being pumped can damage pumping equipment or block tubing or
pipes, and a
cement composition that sets too slowly can cost time and money while waiting
for the cement
composition to set.
Pumping Time and Thickening Time
[0090] As used herein, the "pumping time" is the total time required for
pumping a
hydraulic cementing composition into a desired portion or zone of the well in
a cementing
operation plus a safety factor.
[0091] As used herein, the "thickening time" is how long it takes for a cement
composition to become unpumpable at a specified temperature and specified
pressure. The
pumpability of a cement composition is related to the consistency of the
composition. The
consistency of a cement composition is measured in Bearden units of
consistency (Bc), a
dimensionless unit with no direct conversion factor to the more common units
of viscosity. As
used herein, a setting fluid is considered to be "pumpable" so long as the
fluid has an apparent
viscosity less than 30,000 mPa=s (cP) (independent of any gel characteristic)
or a consistency of
less than 70 Bc. A setting fluid becomes "unpumpable" when the consistency of
the composition
reaches at least 70 Bc.
[0092] As used herein, the consistency of a cement composition is measured
according
to ANSI/API Recommended Practice 10B-2 as follows. The cement composition is
mixed and
then placed in the test cell of a High-Temperature, High-Pressure (HTHP)
consistometer, such as
a FANNTm Model 290 or a CHANDLERTm Model 8340. The cement composition is
tested in
17
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
the HTHP consistometer at the specified temperature and pressure. Consistency
measurements
are taken continuously until the consistency of the cement composition exceeds
70 Bc.
[0093] Of course, the thickening time should be greater than the pumping time
for a
cementing operation.
Setting and Compressive Strength
[0094] Depending on the composition and the conditions, it can take just a few
minutes
up to 72 hours or longer for some cement compositions to initially set. A
cement composition
sample that is at least initially set is suitable for destructive compressive
strength testing.
[0095] Compressive strength is defined as the capacity of a material to
withstand
axially directed pushing forces. The compressive strength a setting
composition attains is a
function of both curing time and temperature, among other things.
[0096] The compressive strength of a cement composition can be used to
indicate
whether the cement composition has set. As used herein, a cement composition
is considered
"initially set" when the cement composition has developed a compressive
strength of 50 psi
using the non-destructive compressive strength method. As used herein, the
"initial setting time"
is the difference in time between when the cement is mixed with water and when
the cement
composition is initially set. Some cement compositions can continue to develop
a compressive
strength greater than 50 psi over the course of several days. The compressive
strength of certain
kinds of cement compositions can reach over 10,000 psi.
[0097] Compressive strength is typically measured at a specified time after
the cement
composition has been mixed and at a specified temperature and pressure
conditions. If not
otherwise stated, the setting and the initial setting time is determined at a
temperature of 212 F
and an atmospheric pressure of 3,000 psi. Compressive strength can also be
measured at a
specific time and temperature after the cement composition has been mixed, for
example, in the
range of about 24 to about 72 hours at a design temperature and pressure, for
example, a
temperature of 212 F and 3,000 psi. According to ANSI/API Recommended
Practice 10B-2,
compressive strength can be measured by either a destructive method or non-
destructive method.
18
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
[0098] The destructive method mechanically tests the strength of cement
composition
samples at various points in time by crushing the samples in a compression-
testing machine. The
destructive method is peifonned as follows. The cement composition is mixed
and then cured.
The cured cement composition sample is placed in a compressive strength
testing device, such as
a Super L Universal testing machine model 602, available from Tinius Olsen,
Horsham in
Pennsylvania, USA. According to the destructive method, the compressive
strength is calculated
as the force required to break the sample divided by the smallest cross-
sectional area in contact
with the load-bearing plates of the compression device. The actual compressive
strength is
reported in units of pressure, such as pound-force per square inch (psi) or
megapascals (MPa).
[0099] The non-destructive method continually measures a correlated
compressive
strength of a cement composition sample throughout the test period by
utilizing a non-destructive
sonic device such as an Ultrasonic Cement Analyzer (UCA) available from Fann
Instruments in
Houston, TX. As used herein, the "compressive strength" of a cement
composition is measured
utilizing an Ultrasonic Cement Analyzer as follows. The cement composition is
mixed. The
cement composition is placed in an Ultrasonic Cement Analyzer, in which the
cement
composition is heated to the specified temperature and pressurized to the
specified pressure. The
UCA continually measures the transit time of the acoustic signal through the
sample. The UCA
device contains preset algorithms that correlate transit time through the
sample to compressive
strength. The UCA reports the compressive strength of the cement composition
in units of
pressure, such as psi or megapascals (MPa).
Young's Modulus (Elastic Modulus)
[0100] Young's modulus, named after Thomas Young, is also known as the elastic
modulus. It is a measure of the stiffness of an elastic material. It is
defined as the ratio of the
stress along an axis over the strain along that axis in the range of stress in
which Hooke's law
holds. The slope of the stress-strain curve at any point is called the tangent
modulus. The
tangent modulus of the initial, linear portion of a stress-strain curve is
called Young's modulus.
It can be experimentally determined from the slope of a stress-strain curve
created during tensile
tests conducted on a sample of the material. Young's modulus is the ratio of
stress (which has
19
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
units of pressure) to strain (which is dimensionless); therefore, Young's
modulus has units of
pressure.
Poisson's Ratio
[0101] Poisson's ratio, named after Simeon Poisson, is the negative ratio of
transverse
to axial strain. When a material is compressed in one direction, it usually
tends to expand in the
other two directions perpendicular to the direction of compression. This
phenomenon is called
the Poisson effect. Poisson's ratio is a measure of this effect. The Poisson
ratio is the ratio of the
fraction (or percent) of expansion divided by the fraction (or percent) of
compression, for small
values of these changes. Conversely, if the material is stretched rather than
compressed, it
usually tends to contract in the directions transverse to the direction of
stretching. In this case,
the Poisson ratio will be the ratio of relative contraction to relative
stretching, and will have the
same value as above.
Cement Testing Conditions
[0102] As used herein, if any test (for example, thickening time or
compressive
strength, requires the step of mixing the setting composition, cement
composition, or the like,
then the mixing step is performed according to ANSI/API Recommended Practice
10B-2 as
follows. Any of the ingredients that are a dry particulate substance are pre-
blended. The liquid
is added to a mixing container and the container is then placed on a mixer
base. For example,
the mixer can be a Lightning Mixer. The motor of the base is then turned on
and maintained at
about 4,000 revolutions per minute (rpm). The pre-blended dry ingredients are
added to the
container at a uniform rate in not more than 15 seconds (s). After all the dry
ingredients have
been added to the liquid ingredients in the container, a cover is then placed
on the container, and
the composition is mixed at 12,000 rpm (+/- 500 rpm) for 35 s (+/- 1 s). It is
to be understood
that the composition is mixed under Standard Laboratory Conditions (about 77
F and about 1
atmosphere pre s sure).
[0103] It is also to be understood that if any test (for example, thickening
time or
compressive strength or permeability) specifies the test be performed at a
specified temperature
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
and possibly a specified pressure, then the temperature and pressure of the
cement composition is
ramped up to the specified temperature and pressure after being mixed at
ambient temperature
and pressure. For example, the cement composition can be mixed at 77 F and
then placed into
the testing apparatus and the temperature of the cement composition can be
ramped up to the
specified temperature. As used herein, the rate of ramping up the temperature
is in the range of
about 3 F/min to about 5 F/min. After the cement composition is ramped up to
the specified
temperature and possibly pressure, the cement composition is maintained at
that temperature and
pressure for the duration of the testing.
[0104] As used herein, if any test (for example, compressive strength or
permeability) requires the step of "curing the cement composition" or the
like, then the curing
step is performed according to ANSI/API Recommended Practice 10B-2 as follows.
After the
cement composition has been mixed, it is poured into a curing mold. The curing
mold is placed
into a pressurized curing chamber and the curing chamber is maintained at a
temperature of 212
F and a pressure of 3000 psi. The cement composition is allowed to cure for
the length of time
necessary for the composition to set. After the composition has set, the
curing mold is placed
into a water cooling bath until the cement composition sample is tested.
Cement Retarders
[0105] As used herein, a -retarder" is a chemical agent used to increase the
thickening
time of a cement composition. The need for retarding the thickening time of a
cement
composition tends to increase with depth of the zone to be cemented due to the
greater time
required to complete the cementing operation and the effect of increased
temperature on the
setting of the cement. A longer thickening time at the design temperature
allows for a longer
pumping time that may be required.
Other Cement Additives
[0106] Cement compositions can contain other additives, including but not
limited to
resins, latex, stabilizers, silica, microspheres, aqueous superabsorbers,
viscosifying agents,
suspending agents, dispersing agents, salts, accelerants, surfactants,
retardants, defoamers, high-
21
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
density materials, low-density materials, fluid-loss control agents,
elastomers, vitrified shale, gas
migration control additives, formation conditioning agents, or other additives
or modifying
agents, or combinations thereof.
[0107] An example of an additive is a high-density additive. As used herein, a
"high-
density" additive is an additive that has a density greater than 3 g/cm3. Some
metal oxides can
be used as a high-density additive. As used herein, a "metal oxide" is a metal
cation or transition
metal cation with an oxide anion. Examples of metal oxides include, but are
not limited to, iron
oxide (Fe203) and manganese oxide (Mn304). A commercially available example of
an iron
oxide high-density additive is HI-DENSETm and an example of a commercially
available
manganese oxide is MICROMAXTm, both available from Halliburton Energy
Services, Inc. in
Duncan, Oklahoma.
[0108] For example, MICROMAXTm weight additive increases slurry density with
hausmannite ore ground to an average particle size of 5 microns. Unlike most
weighting
materials, MICROMAXTm weight additive remains in suspension when added
directly to mixing
water. MICROMAXIm weight additive can be used at bottomhole circulating
temperatures
between 80 F and 500 F (27 C to 260 C). In deep wells with high
temperatures and
pressures, MICROMAXTm weight additive can help restrain formation pressures
and improve
mud displacement. Additive concentrations depend on the slurry weight designed
for individual
wells. Because of the fine-ground ore in MICROMAXThi weight additive, higher
concentrations
of retarders might be required to achieve the thickening times provided by
other types of weight
additives. Slurries of cement compositions containing MICROMAXTm weight
additive might
also require the addition of dispersants. MICROMAXTm weight additive is
commercially
available from Halliburton Energy Services, Inc. in Duncan, Oklahoma.
[0109] Some oil and gas wells can have a corrosive environment. As used
herein, a
"corrosive environment" is an environment containing corrosive materials.
Examples of
corrosive materials include, but are not limited to, liquids with a pH below
5, acid gas, or fluids
containing dissolved acid gas. As used herein, the term "acid gas" means any
gas that can mix
with water to form an acidic solution having a pH below 5. The most common
acid gases are
22
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
hydrogen sulfide (H2S) and carbon dioxide (CO2). For example, CO, reacts with
water to form
carbonic acid in an aqueous solution.
[0110] A cement composition that contains a metal oxide, high-density additive
is
prone to corrosion if introduced into a well having a corrosive environment.
For example, after
the cement composition has set in the portion of the well, the corrosive
materials in the well can
corrode a portion of the cement composition. Consequently, for example, oil or
gas can flow
more easily through the annulus and it can be more difficult to produce oil or
gas in a controlled
manner through the casing. Moreover, as the permeability of the set
composition increases, the
corrosive materials can flow through the set composition and come in contact
with the casing.
The corrosive materials can then corrode portions of the casing. Moreover, if
the set cement
composition comes into contact with corrosive materials, some of the metal
oxide of the cement
composition can dissolve out of the composition and then precipitate elsewhere
to plug up other
areas of the well. As a result, it can become more difficult to produce oil or
gas.
General Measurement Terms
[0111] Unless otherwise specified or unless the context otherwise clearly
requires, any
ratio or percentage means by weight.
[0112] Unless otherwise specified or unless the context otherwise clearly
requires, the
phrase "by weight of the water" means the weight of the water of an aqueous
phase of the fluid
without the weight of any viscosity-increasing agent, dissolved salt,
suspended particulate, or
other materials or additives that may be present in the water.
[0113] If there is any difference between U.S. or Imperial units, U.S. units
are intended.
[0114] As used herein, a "sack" ("sk") is an amount that weighs 94 pounds (94
lb/sk).
[0115] As used herein, the conversion between gallon per sack (gal/sk) and
percent by
weight of cement (% bwoc) is 1 gal/sk = 3.96% bwoc.
[0116] The conversion between pound per gallon (lb/gal or ppg) and kilogram
per cubic
meter (kg/m3) is: 1 lb/gal = (0.4536 kg/lb) x (ga1/0.003785 m3) = 120 kg/m3.
[0117] The conversion between pound per square foot (1b/ft2) and kilogram per
square
meter (kg/m2) is: 1 lb/ft2= 4.9 kg/m2.
23
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
General Approach
[0118] As stated above, this disclosure provides a polymer that can be
included in a
cement slurry to provide a set cement that has a lower Young's Modulus value
and, hence, is a
more resilient set cement.
[0119] The polymer can be dissolved in an aqueous phase that can be included
in a
cement composition; however, upon heating, which can be, for example, due to
downhole
temperature conditions in a well, the dissolved polymer will precipitate to
form solid particles.
Due to this change from a liquid state to a solid state, there are no cement
slurry stability issues
relating to surface mixing of the dissolved polymer.
[0120] As used herein, unless the context otherwise requires, a "polymer" or
"polymeric material" includes homopolymers, copolymers, terpolymers, etc. In
addition, the
term "copolymer" as used herein is not limited to the combination of polymers
having two
monomeric units, but includes any combination of monomeric units, for example,
terpolymers,
tetrapolymers. etc.
[0121] According to an embodiment of the disclosure, a hydraulic cement
composition
is provided, the composition including: (A) a hydraulic cement; (B) at least a
sufficient
concentration of water to form a pumpable slurry with the hydraulic cement;
and (C) a polymer
selected from the group consisting of: (i) a homopolymer of one monomer
selected from the
group consisting of: N-isopropylacrylamide, N-propyl acrylamide, and N,N-
diethyl acrylamide;
(ii) a copolymer consisting of two or more monomers selected from the group
consisting of:
N-isopropylacrylamide, N-propyl acrylamide, and N,N-diethyl acrylamide; and
(iii) a copolymer
comprising: (a) one or more first monomers selected from the group consisting
of:
N-i sopropyl acryl amide, N-propyl acryl amide, N,N-diethyl acryl amide, and
any combination
thereof; and (b) one or more second monomers selected from the group
consisting of:
acrylamide, an acrylamide derivative other than one of the first monomers,
methacrylamide, an
N-alkyl methacrylamide, N-methyl-N-vinylacetamide, N-vinylformamide, a vinyl
pyrrolidone, a
vinyl pyridine, N-vinylcaprolactam, N-methyl-N-vinylacetamide, a styrene
sulfonic acid, a
styrene sulfonate, a vinyl sulfonic acid, a vinyl sulfonate, and any
combination of the foregoing.
24
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
[0122] In an embodiment, the acrylamide derivative is an N-alkyl acrylamide.
In a
further embodiment, the N-alkyl acrylamide is selected from the group
consisting of: N,N-
dimethylacrylamide, sodium 2-acrylamido-2-methylpropanesulfonate, 2-acrylamido-
2-
methylpropanesulfonic acid, N-(hydroxymethyl)acrylamide, N-
(hydroxyethyl)acrylamide,
acrylamide, and N-acryloyl morpholine.
[0123] In an embodiment, the vinyl pyrrolidone is 1-viny1-2-pyrrolidone.
[0124] In an embodiment. the styrene sulfonate is an alkali metal 4-
styrenesulfonate.
[0125] In an embodiment, the polymer comprises at least 25 mole% of
N-isopropylacrylamide, N-propyl acrylamide, N,N-diethyl acrylamide; and any
combination
thereof.
[0126] In cases where the setting temperature is greater than a lower critical
solution
temperature ("LCST") of the polymer, at least some of any such dissolved
polymer will
precipitate to increase the resiliency of the set cement. At least in such
cases, the polymer is not
expected to interact with the chemistry of the setting of the hydraulic cement
or the rate, and,
therefore, the precipitating polymer is not expected to retard the thickening
time or setting of the
hydraulic cement.
[0127] In various embodiments, the polymer can be selected for having a lower
critical
solution temperature (-LCST") to be in the range of about 60 F (15 C) to
about 212 F
(100 C). In some of these embodiments, the polymer can be selected such that
the LCST is at
least 120 F (49 C) to avoid undesired precipitation of polymer during
storage or shipping, for
example, on a hot day at a well site.
Hydraulic Cement
[0128] While various hydraulic cements can be utilized in the cement
compositions,
Portland cement is generally preferred, and can be, for example, one or more
of the various types
identified as API Classes A-H and J cements. These cements are classified and
defined in API
Specification for Materials and Testing for Well Cements, API Specification
10A, 21st Edition
dated Sep. 1. 1991, of the American Petroleum Institute, Washington, D.C. API
Portland
cements generally have a maxi mum particle size of about 90 microns and a
specific surface
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
(sometimes referred to as Blaine Fineness) of about 3900 square centimeters
per gram. A highly
useful and effective cement slurry base for use in accordance with this
invention comprises API
Class H Portland cement mixed with water to provide a density of from about
11.3 lb/gal to
about 18.0 lb/gal of water.
Water
[0129] The term "water" is used generally herein to include fresh water or
brine, unless
the context otherwise requires. As used herein, the term "brine" is intended
to include, unless
the context otherwise requires, any aqueous solution having greater than 1,000
ppm total
dissolved mineral salts.
Polymer
[0130] Polymers or certain copolymers of N-isopropylacrylamide, N-propyl
acrylamide, and N,N-diethyl acrylamide can have the characteristic of
precipitating from solution
with increasing temperature.
[0131] For example. poly(N-isopropylacrylamide) (VNIPAM") and copolymers
containing N-isopropylacrylamide ("NIPAM") exhibit a thermoresponsive behavior
in aqueous
solution. NIPAM can be copolymerized, for example, with one or more other
monomers to alter
or adjust the thermoresponsive and solubility characteristics in aqueous
solution.
[0132] PNIPAM chains have hydrophilic domains below the lower critical
solution
temperature ("LCST") and hydrophobic domains above the LCST. R Pelton, Poly(N-
isopropylacrylamide) (PNIPAM) is never hydrophobic, J Colloid Interface Sci.,
2010 Aug
l5;348(2):673-4.
[0133] The NIPAM is believed to be responsible for the thermoresponsive
precipitation
of such a polymer. A lower critical solution temperature (LCST) describes a
temperature above
which hydrophobic domains are formed along the polymer chain leading to the
formation of
colloidal suspensions. Depending on the monomer ratio of the NIPAM/DMAC
copolymer, the
temperature at which the hydrophobic domains are formed can vary.
26
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
[0134] A description of the mechanism of precipitation is included in: I. C.
Barker, J.
M. G. Cowie, T. N. Huckerby, D. A. Shaw, I. Soutar, and L. Swanson, Studies of
the "Smart"
The rmoresponsive Behavior of
Copolymers of N-Isopropylacrylamide and
N,N-Dimethylacrylamide in Dilute Aqueous Solution, Macromolecules 2003, 36,
7765-7770).
As described in the Abstract of this reference: "Various fluorescence
techniques and cloud point
measurements have been used to study the effects of altering the
hydrophilic/hydrophobic
balance in a series of N-isopropylacrylamide (NIPAM)/N,N-dimethylacrylamide
(DMAC) statistical copolymers upon the smart thermal responses of these
systems in dilute
aqueous solution. As expected, incorporation of DMAC into the polymer
structure raises its
lower critical solution temperature to an extent dependent upon DMAC content.
However, use
of such a hydrophilic modifier reduces the magnitude of the collapse
transition that characterizes
the macromolecule's thermal response. In PNIPAM, the LCST is associated with a
conformational transition between a coil and a globule. However, introduction
of DMAC
derivatives into the polymer expands its 'globular' form into a much more open
structure that
progressively loses its capacity for solubilization of organic guests.
Consequently, although
copolymerization with more polar monomers can be used to raise the LCST of
NIPAM-based
thermoresponsive polymers, the value of this approach will be limited in
applications requiring
switchable carrier/release properties." As the DMAC content of the polymer is
increased, the
LCST is raised, the transition becomes more diffuse, and its -intensity"
reduces. In addition, the
article discusses that cross-linking of the polymer increases the critical
temperature.
[0135] This property of NIPAM/DMAC copolymers is adapted to provide a
polymeric
material that can be dissolved in water at a lower temperature, and then as
the temperature
increases the polymeric material will precipitate.
[0136] This additive can be dissolved in a liquid at the surface and,
therefore, has no
stability issues when included in a cement slurry and pumped downhole.
Although it may be
useful in some application, it is not necessary for such a NIPAM copolymer to
be included in a
cement slurry as an emulsion or suspension. Upon heating, the dissolved
additive will
precipitate to form particles that lower the Young's modulus of the set
cement. An additive
according to this disclosure can be tailored to precipitate solid material at
varying temperatures.
27
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
_______________________________ 0 0
HN
Poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide)
[0137] For the poly(N-isopropylacrylamide-co-N,N-dimethylacrylamide shown
above,
x and y represent the numbers of the different monomeric units of the polymer,
which can be the
same or different. In addition, it should be understood that the polymer can
be a random co-
polymer or a block co-polymer.
Examples
[0138] To facilitate a better understanding of the present disclosure, the
following
examples of certain aspects of some embodiments are given. In no way should
the following
examples be read to limit, or define, the entire scope of the disclosure.
The chart below shows a comparison of set cement properties for various SBC
elastomeric
particulates and the liquid-based additive of this disclosure.
[0139] A 16.4 lb/gal cement slurry was used in the examples consisting of 100%
by
weight of cement Texas Lehigh Class H Cement, sodium silicate (finely ground
powder form) at
2% by weight of cement), the respective additive listed, and fresh water.
[0140] The elastomeric-based particulates used in some of the example cement
compositions were as follows. SBC #1 is a particulate of a styrene butadiene
copolymer
("SBC") (monomer ratio about 30:70), which has a specific gravity around 1.
SBC #2 is a
particulate of a styrene butadiene copolymer ("SBC") (monomer ratio about
20:80), which has a
28
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
specific gravity around 1. SBC #1 with barite is a particulate of SBC #1 that
includes a
sufficient concentration of incorporated barite in the matrix of the solid SBC
#1 to obtain a
specific gravity of about 2. Similarly, SBC #2 with barite is a particulate of
SBC #1 that
includes a sufficient concentration of incorporated barite in the matrix of
the solid SBC #2 to
obtain a specific gravity of about 2.
[0141] A high molecular weight NIPAM/DMAC copolymer is a copolymer of N-
isopropylacrylamide (NIPAM) and N,N-dimethylacrylamide ("DMAC") (about 50/50
mole
percent). Without a chain transfer agent, the molecular weight of the formed
polymer is very
high. At standard laboratory conditions, only up to about 5% by weight of the
high molecular
weight NIPAM/DMAC copolymer can be dissolved in water without the solution
being
excessively viscous such that it is difficult to mix with a dry blend of the
cementitious material.
In an embodiment, the viscosity of an aqueous solution of the polymer is less
than about 5,000
cP, and preferably less than about 4,000 cP, which viscosities are measured at
a low shear rate of
about 1 sec-land standard laboratory conditions.
[0142] A low molecular weight NIPAM/DMAC copolymer is a copolymer of N-
isopropylacrylamide (NIPAM) and N,N-dimethylacrylamide (DMAC) (about 50/50
mole
percent). This polymer is synthesized with a chain transfer agent to keep the
molecular weight
of the polymer sufficiently low to thereby allow a high concentration (that
is, solubility) of the
polymer in water to be achieved. At standard laboratory conditions, up to
about 40% by weight
of the low molecular weight NIPAM/DMAC copolymer can be dissolved in water
without the
solution being excessively viscous such that it is difficult to mix with a dry
blend of the
cementitious material. In an embodiment, the viscosity of an aqueous solution
of the polymer is
less than about 5,000 cP, and preferably less than about 4,000 cP, which
viscosities are measured
at a low shear rate of about 1 sec-land standard laboratory conditions.
[0143] Table 1 shows a comparison of compressive strength, Young's modulus,
and
Poisson's ratio between the above additives in the 16.4 lb/gal Class LI Cement
base cement slurry
after setting.
[0144] Regarding the high molecular weight NIPAM/DMAC copolymer, the % by
weight of cement shown in Table 1 is after solidification of the copolymer.
Regarding the low
29
CA 02913017 2016-01-11
WO 2015/034475
PCT/US2013/057911
molecular weight NIPAM/DMAC copolymer, the % by weight of cement ("% bwoc")
shown in
Table 1 is after solidification of the copolymer.
Table 1
Total % Solids by Compressive Young's
Poisson's
Additive Weight of Cement Strength Modulus
Ratio
(% bwoc) (psi) (psi)
SBC #1 7.5 5060 1.76E6 0.20
SBC #2 7.5 5340 1.76E6 0.19
SBC #1 with barite 15 3810 1.45E6 0.19
SBC #2 with barite 15 4340 1.46E6 0.19
high molecular weight
NIPAM/DMAC
copolymer (5% by 7.5 1170 1.19E6 0.16
weight aqueous
solution)
low molecular weight
NIPAM/DMAC
copolymer (40% by 7.5 2710 1.26E6 0.18
weight aqueous
solution)
[0145] The pump time charts are shown in Figure 1 and Figure 2, the control
has no
additive and the low molecular weight NIPAM/DMAC copolymer test has an
equivalent of 7.5
% by weight of cement of the active polymer.
[0146] In addition, thickening time tests were performed on a control cement
compositions without any NIPAM polymer and on another cement composition
including a
NIPAM polymer according to the disclosure.
[0147] Figure 1 shows the consistency of a cement slurry as a control, without
low
molecular weight NIPAM/DMAC copolymer (NIPAM/DMAC copolymer 40% active aqueous
solution). This control composition reached 40 Bc at about 46 minutes, 50 Bc
at about 51
minutes, 70 Bc at about 58 minutes, and 100 Bc at about 1 hour and 5 minutes.
[0148] Figure 2 shows the consistency of a cement slurry comprising 1.82
gal/sack (7.5
eq. %bwoc) low molecular weight NIPAM/DMAC copolymer (NIPAM/DMAC copolymer 40%
active aqueous solution). This composition reached 40 Bc at 42 minutes, 50 Bc
at about 50
minutes, 70 Bc at about 57 minutes, and 100 Bc at about 1 hour and 7 minutes.
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
[0149] These thickening time tests of Figure 1 and Figure 2 show that there
were no
significant retarding effects by the polymer on the set time of the cement.
Essentially, it is inert
to the thickening time and setting of the cement slurry.
Method of Cementing in a Well
[0150] According to an embodiment of the disclosure, a method of cementing in
a well
is provided, the method including the steps of: forming a hydraulic cement
composition
according to the disclosure; and introducing the composition into the well.
[0151] According to a further embodiment, the design temperature of the
treatment
zone in the well is greater than a LCST of the polymer.
Forming Fluid
[0152] A fluid such as a hydraulic cement composition according to this
disclosure can
be prepared at the job site, prepared at a plant or facility prior to use, or
certain components of
the fluid can be pre-mixed prior to use and then transported to the job site.
Certain components
of the fluid may be provided as a "dry mix" to be combined with fluid or other
components prior
to or during introducing the fluid into the well.
[0153] In certain embodiments, the preparation of a fluid can be done at the
job site in a
method characterized as being performed -on the fly." The term -on-the-fly" is
used herein to
include methods of combining two or more components wherein a flowing stream
of one
element is continuously introduced into flowing stream of another component so
that the streams
are combined and mixed while continuing to flow as a single stream as part of
the on-going
treatment. Such mixing can also be described as "real-time" mixing.
Introducing Into Well or Treatment Zone
[0154] Often the step of delivering a fluid into a well or treatment zone of a
well is
within a relatively short period after forming the fluid, for example, less
within 30 minutes to one
hour. More preferably, the step of delivering the fluid is immediately after
the step of forming
the fluid, which is "on the fly."
31
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
[0155] It should be understood that the step of delivering a fluid into a well
can
advantageously include the use of one or more fluid pumps.
Allowing Time for Setting in the Well
[0156] After the step of introducing a cementing composition according to the
disclosure, time should allowed for the setting of the composition in place in
the well, unless it
was not properly placed and needs to be cleaned out before setting. This
preferably occurs with
time under the conditions in the zone of the subterranean fluid.
Flow Back Conditions
[0157] In an embodiment, a step of flowing back from the well is after at
least
sufficient time for setting of the cement composition in the well.
Producing Hydrocarbon from Subterranean Formation
[0158] After such use of a cement composition according to the disclosure, a
step of
producing hydrocarbon from the well or a particular zone is often a desirable
objective. In some
applications, however, an objective may be plugging a wellbore or sealing a
zone.
Conclusion
[0159] Therefore, the present disclosure is well adapted to attain the ends
and
advantages mentioned as well as those that are inherent therein.
[0160] The exemplary hydraulic cement compositions disclosed herein may
directly or
indirectly affect one or more components or pieces of equipment associated
with the preparation,
delivery, recapture, recycling, reuse, or disposal of the disclosed hydraulic
cement compositions.
For example, the disclosed hydraulic cement compositions may directly or
indirectly affect one
or more mixers, related mixing equipment, mud pits, storage facilities or
units, fluid separators,
heat exchangers, sensors, gauges, pumps, compressors, and the like used
generate, store,
monitor, regulate, or recondition the exemplary hydraulic cement compositions.
The disclosed
hydraulic cement compositions may also directly or indirectly affect any
transport or delivery
32
CA 02913017 2016-01-11
WO 2015/034475 PCT/US2013/057911
equipment used to convey the hydraulic cement compositions to a well site or
downhole such as,
for example, any transport vessels, conduits, pipelines, trucks, tubulars, or
pipes used to
fluidically move the hydraulic cement compositions from one location to
another, any pumps,
compressors, or motors (for example, topside or downhole) used to drive the
hydraulic cement
compositions into motion, any valves or related joints used to regulate the
pressure or flow rate
of the hydraulic cement compositions, and any sensors (i.e., pressure and
temperature), gauges,
or combinations thereof, and the like. The disclosed hydraulic cement
compositions may also
directly or indirectly affect the various downhole equipment and tools that
may come into
contact with the chemicals/fluids such as, but not limited to, drill string,
coiled tubing, drill pipe,
drill collars, mud motors, downhole motors or pumps, floats, MWD/LWD tools and
related
telemetry equipment, drill bits (including roller cone, PDC, natural diamond,
hole openers,
reamers, and coring bits), sensors or distributed sensors, downhole heat
exchangers, valves and
corresponding actuation devices, tool seals, packers and other wellbore
isolation devices or
components, and the like.
[0161] The particular embodiments disclosed above are illustrative only, as
the present
disclosure may be modified and practiced in different but equivalent manners
apparent to those
skilled in the art having the benefit of the teachings herein. It is,
therefore, evident that the
particular illustrative embodiments disclosed above may be altered or modified
and all such
variations are considered within the scope of the present disclosure.
[0162] The various elements or steps according to the disclosed elements or
steps can
be combined advantageously or practiced together in various combinations or
sub-combinations
of elements or sequences of steps to increase the efficiency and benefits that
can be obtained
from the disclosure.
[0163] It will be appreciated that one or more of the above embodiments may be
combined with one or more of the other embodiments, unless explicitly stated
otherwise.
[0164] The illustrative disclosure can be practiced in the absence of any
element or step
that is not specifically disclosed or claimed.
[0165] Furthermore, no limitations are intended to the details of
construction,
composition, design, or steps herein shown, other than as described in the
claims.
33