Note: Descriptions are shown in the official language in which they were submitted.
SYSTEM AND METHOD FOR INCREASING PATIENT ADHERENCE
TO MEDICATION TREATMENT REGIMENS
Cross-Reference to Related Applications
[0001] The present application is a continuation-in-part of United States
Patent Application Serial No.
13/565,164 filed August 2, 2012, which in turn is a continuation-in-part of
United States Patent
Application Serial No. 13/544,531 filed July 9,2012, which in turn is a
continuation-in-part of United
States Patent Application Serial No. 13/462,486, filed May 2, 2012, which in
turn claims the benefit
of United Stated Provisional Application Serial No. 61/635,613, filed April
19, 2012, and United
Stated Provisional Application Serial No. 61/611,942, filed March 16, 2012.
Field of the Invention
[0002] The present invention generally relates to systems and methods for
improving patient adherence
to medical treatment regimens, and more particularly to computer processor
based systems and methods
for electronically enhancing patient adherence to prescription medicine
treatment regimens.
Background of the Invention
[0003] Poor patient adherence to taking and complying with treatment regimens
is a major concern
within the health care industry. After visiting their health care provider and
receiving at least one
prescription for medication such as a pharmaceutical substance, many patients
thereafter fail to maintain
a level of compliance with their prescribed treatment regimen and adherence to
diligently taking their
mediation as prescribed and/or continuing their medication regimen by timely
seeking refills on their
medication. This practice results in increased costs to all parties involved
in the health care industry,
including, but not limited to the patient, the health care provider, the
pharmacies, the pharmaceutical
companies, and the health insurance companies. There are currently some
employed with the goal of
increasing patient adherence, such as by the distribution of patient
educational health materials,
discount coupons, and patient reminder services. However, such approaches have
generally lacked
sufficient coordination and/or timely provision of patient compliance
information to the health care
provider or other entities to make a significant improvement in
1
Date Recue/Date Received 2021-04-15
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
the overall care of the patient.
[0004] One important element to increasing patient adherence is good health
care
provider-patient interaction. Because this interaction is most effective when
it takes place at
the point-of-care while the patient is thinking about their current physical
state, this
interaction is crucial for facilitating patient health, awareness, and
adherence. However,
there is currently not a system that allows for a health care provider to be
fully informed of
whether their patients are adhering to diligently taking their prescriptions
contemporaneously
while the patients are at the point-of-care. Further, the current methods of
increasing patient
adherence through educational and financial incentives require the health care
provider to not
only know whether or not their patient is adhering to taking their prescribed
medications, but
also requires the health care provider to have the specific education material
and discount
coupons available for each patient's specific diagnoses and prescribed
medications at the
point-of-care. This is overly burdensome and practically impossible for a
health care
provider who has patients with a wide variety of health care needs. Therefore,
there is also
a need for a system that can more easily and efficiently coordinate and
distribute patient
educational materials, coupons, and other supplemental programs at the point-
of-care.
[0005] Additionally, pharmaceutical companies are restricted in the number of
coupons and
other incentives they may distribute. Currently, the coupons and other
incentives are
distributed to patients without taking into consideration whether the patient
receiving the
coupon or other incentive needs will be incentivized by them. Therefore, there
also remains
a need for a system that can aid pharmaceutical companies in distributing
coupons and other
incentives to their customers in a more efficient manner.
[0006] Improved systems and methods are desired for providing contemporaneous
information about patient adherence to following prescription medication
regimens and
enhancing the effectiveness of the foregoing patient incentives.
Summary of the Invention
[0007] The systems and methods described herein help to fill the needs and
solve the issues
described above. These systems and methods may be embodied in the form of
computer-
implemented processes and apparatuses configured for implementing and
practicing those
processes, as further described herein.
[0008] The present disclosure provides a patient adherence system and
compliance dashboard
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
for determining patient behavior and adherence to prescription medication
regimens based on
patient medication histories, and further providing that adherence infoimation
to a user such
as a health care provider contemporaneous with the time and at the point of
service such as in
the doctor's office during a patient visit. Advantageously, this allows the
health care
provider to make an intervention as needed to get the patient to regularly
fill and take their
prescribed medications which will benefit the patient's health. Embodiments of
the
compliance dashboard include an interactive toolbar that allows the health
care provider to
navigate to and display important medication adherence information made
available by the
patient adherence system. In one embodiment, the compliance dashboard may
conveniently
be integrated into the electronic prescription system user interface to
provide ready access to
the patient medication adherence infoimation, as further described herein.
[0009] According to one aspect of the present invention, a medication data
processing and
communication system includes: a health care provider system comprising a
first processor
and a display device; an electronic prescription system comprising a second
processor and a
non-transitory computer readable medium; and a patient adherence system
comprising a third
processor, a non-transitory computer readable medium, and an adherence
database residing
on the computer readable medium. The adherence database includes medication
adherence
data for prescription drugs prescribed for the patient. The health care
provider system,
electronic prescription system, and patient adherence system are in operable
communication,
which in one embodiment may be via the Internet. The third processor of the
patient
adherence system is configured by program instructions to retrieve the
medication adherence
data from the adherence database and transmit the medication adherence data
for display on
the display device of the health care provider system. The medication
adherence data
displayed comprises an adherence chart showing the medication adherence data
in a first
graphical form comprising a plurality of individual drug timelines, and an
adherence graphic
displaying the medication adherence data in a second graphical form different
than the first
form. The adherence graphic provides a readily discernible visual object
presented on a
graphical user interface of a user's display device which represents the
patient medication
adherence data by a compliance metric having a single numeric value which
summarizes the
patient's overall compliance with taking a prescribed drug represented by one
of the drug
timelines. In one embodiment, the adherence graphic comprises a bar chart
having an
adherence bar which changes length directly proportionate to the numeric value
of the patient
medication adherence data or metric associated with at least one of the drug
timelines
3
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
displayed. In one embodiment, the metric may be medication adherence rate
which is
represented by a percentage between 1 and 100 percent.
[0010] By contrast, each drug timeline may visually depict plural and more
detailed
chronological information regarding the prescribed drug regimen such as
without limitation
prescription start date, fill date, refill date, end date, and other
information which the system
uses to calculate a medication adherence data or metric having a single
numerical value
cumulatively reflecting patient medication regimen compliance. Advantageously,
the
adherence graphic provides a health care provider with a quick snapshot of
patient
compliance without the need to extract that information by undertaking a more
in-depth
review of the individual drug timelines. This saves time for the physician or
other health
care provider. In one embodiment, each drug timeline may have its own
associated
adherence graphic which are all displayed simultaneously with the drug
timelines. In other
embodiments, a single visually enlarged adherence graphic may be displayed at
a given time
showing the medication adherence metric for one of the single drug timeline,
which may be
alternatingly selected by a user to another drug timeline, as further
described herein.
[0011] According to another embodiment of the present invention, a computer
processor
based system for generating and displaying patient medication adherence data
is provided.
The system includes: a health care provider system comprising a processor and
a display
device having a graphical user interface; a patient adherence system
comprising one or more
processors in operable communication with the health care provider system; a
first software
module stored on computer readable medium and executed by the one or more
processors of
the patient adherence system, the first software module including instructions
which
configure the one or more processors to (1) retrieve patient medication
history from
electronic records of a pharmacy system, and (2) to store the patient
medication history in a
first database; and a second software module stored on computer readable
medium and
executed by the one or more processors of the patient adherence system, the
second software
module including instructions which configure the one or more processors to
(1) retrieve
patient medication history from the first database, (2) generate patient
medication adherence
metrics based on the patient medication history which are indicative of a
patient's compliance
with a prescribed medication treatment regimen, and (3) store the patient
medication
adherence in a second database. The second software module further includes
instructions
which configure the one or more processors of the patient adherence system to
(1) receive a
request for patient medication adherence metrics from the health care provider
system, (2)
4
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
retrieve the patient medication adherence metrics from the second database,
and (3) transmit
the patient medication adherence metrics directly to the health care provider
system for
display. The medication adherence metrics displayed comprises a report card
including a
plurality of individual drug timelines and an adherence graphic displaying a
medication
adherence metric for at least one of the drug timelines in an additional
graphical form
different than the timeline. In one embodiment, the adherence graphic
comprises a bar chart
having an adherence bar which changes length directly proportionate to the
numeric value of
the patient medication adherence data associated with at least one of the drug
timelines
displayed.
[0012] According to another embodiment of the present invention, a medication
treatment
regimen adherence system is provided. The system includes: a health care
provider system
comprising a first processor and a display device having a graphical user
interface comprising
a toolbar having a plurality of user selectable dynamic content tabs; an
electronic prescription
system comprising a second processor and a non-transitory computer readable
medium; and a
patient adherence system comprising a third processor and a non-transitory
computer
readable medium including software program instructions. The third processor
executes the
software program instructions which causes the third processor to: retrieve
patient medication
history from a first database; based on the patient medication history,
generate a first patient
medication adherence metric for a first drug prescribed for the patient having
a single
numeric value indicative of a patient's overall compliance with following a
prescribed
medication treatment regimen; store the patient medication adherence in an
adherence
database; retrieve the patient medication adherence metric from the adherence
database in
response to a user selecting a first content tab in the toolbar of the health
care provider system;
and transmit the first patient medication adherence metric to the health care
provider system
for display in the graphical user interface. The graphical user interface of
the display device
comprises an adherence chart having a plurality of individual drug timelines
and an
adherence graphic showing the first patient medication adherence metric in a
graphical form
different than the timelines. The first patient medication adherence metric
has a single
numeric value which summarizes the patient's overall compliance with taking a
drug
represented by one of the drug timelines. In one embodiment, the metric may be
medication
adherence rate.
[0013] According to one embodiment, the present invention is directed to a
method of
provisioning a combined educational coupon, the method comprising: a)
receiving, on a
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
computer apparatus, electronic prescription data for a prescribed substance
for a patient; b)
the computer apparatus determining educational data relating to the prescribed
substance and
coupon data relating to the prescribed substance; and c) the computer
apparatus generating a
single data file comprising the educational data relating to the prescribed
substance and the
coupon data relating to the prescribed substance.
[0014] According to another embodiment, the present invention is directed to a
non-transitory computer-readable storage medium encoded with instructions
which, when
executed on a processor, perfoim a method comprising: a) receiving data
relating to an
electronic prescription of a prescribed substance for a patient; b) searching
one or more
databases for educational data relating to the prescribed substance and coupon
data relating to
the prescribed substance; c) determining educational data relating to the
prescribed substance
and coupon data relating to the prescribed substance; d) retrieving from the
one or more
databases the educational data relating to the prescribed substance and the
coupon data
relating to the prescribed substance; and e) generating a single data file
comprising the
educational data relating to the prescribed substance and the coupon data
relating to the
prescribed substance.
[0015] According to yet another embodiment, the present invention is directed
to a computer
system for electronically generating educational coupons for a prescribed
substance, the
computer system comprising: a processor; a storage device; a network
interface; and
instructions residing on the storage unit, which when executed by the
processor, causes the
processor to: a) receive electronic prescription data for a prescribed
substance for a patient; b)
determine educational data relating to the prescribed substance and coupon
data relating to
the prescribed substance; and c) generate a single data file comprising the
educational data
relating to the prescribed substance and the coupon data relating to the
prescribed substance.
[0016] According to another embodiment, the present invention is directed to a
method of
supplementing an electronic prescription issued by a health care provider, the
method
comprising: a) receiving, on a computer apparatus, electronic prescription
data generated by a
health care provider for a patient for a prescribed substance; b) the computer
apparatus
determining, from a plurality of available supplemental programs stored on one
or more
databases, supplemental programs for which the patient is eligible based on
the electronic
prescription data; c) presenting to the health care provider, in a display
device, a list of the
eligible supplemental programs, each of the eligible supplemental programs
being selectable
and de-selectable by the health care provider in the display device; and d)
the computer
6
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
apparatus activating each supplemental program from the plurality of available
supplemental
programs that have been selected and confirmed by the health care provider in
the display
device; and wherein one of the activated supplemental programs is a coupon
service, and
wherein step d) further comprises retrieving coupon data relating to the
prescribed substance
from the one or more databases, and provisioning a coupon based on the coupon
data to the
patient; and wherein one of the activated supplemental programs is a
prescribed substance
education service, and wherein step d) further comprises retrieving education
content relating
to the prescribed substance from the one or more databases, and transmitting
said education
content to the patient, said coupon data being integrated into the education
content to create a
combined educational coupon.
[0017] According to another embodiment, the present invention is directed to a
method of
providing educational materials to a patient, the method comprising: a)
receiving, on a
computer apparatus, electronic prescription data for a prescribed substance
for a patient, said
electronic prescription data including a diagnostic code; 1)) searching one or
more databases,
using the computer apparatus, to deteimine: (1) general educational data
relating to the
prescribed substance and independent of the diagnostic code; and (2) specific
educational
data relating to the prescribed substance and based on the diagnostic code;
and c) presenting
to a health care provider, in a display device, a list of the general
educational data and the
specific educational data determined in step b) for provisioning to the
patient.
[0018] According to another embodiment, the present invention is directed to a
non-transitory computer-readable storage medium encoded with instructions
which, when
executed on a processor, perform a method comprising: a) receiving electronic
prescription
data for a prescribed substance for a patient, said electronic prescription
data including a
diagnostic code; b) searching one or more databases to determine: (1) general
educational
data relating to the prescribed substance independent of the diagnostic code;
and (2) specific
educational data relating to the prescribed substance based on the diagnostic
code; and c)
presenting to a health care provider, in a display device, a list of the
general educational data
and the specific educational data determined in step b) for provisioning to
the patient.
[0019] According to yet another embodiment, the present invention is directed
to a computer
system for electronically generating coupons for a prescribed substance, the
computer system
comprising: a processor; a storage device: a network interface; and
instructions residing on
the storage unit, which when executed by the processor, causes the processor
to: a) receive
electronic prescription data for a prescribed substance for a patient, said
electronic
7
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
prescription data including a diagnostic code; b) search one or more databases
to determine:
(1) general educational data relating to the prescribed substance independent
of the diagnostic
code; and (2) specific educational data relating to the prescribed substance
based on the
diagnostic code; and c) present to a health care provider, in a display
device, a list of the
general educational data and the specific educational data detemiined in step
b) for
provisioning to the patient.
Brief Description of the Drawings
[0020] The features of the exemplary embodiments of the present invention will
be described
with reference to the following drawings where like elements are labeled
similarly, and in
which:
[0021] FIG. 1 is a schematic diagram of a system for supplementing patient and
provider
interactions to increase patient adherence according to one embodiment of the
present
invention;
[0022] FIG. 2 is a schematic diagram of a health care provider system for
supplementing
patient and provider interactions to increase patient adherence according to
one embodiment
of the present invention;
[0023] FIG. 3 is a schematic diagram of an electronic prescription system for
supplementing
patient and provider interactions to increase patient adherence according to
one embodiment
of the present invention;
[0024] FIG. 4 is a schematic diagram of a supplemental program system for
supplementing
patient and provider interactions to increase patient adherence according to
one embodiment
of the present invention;
[0025] FIG. 5 is a schematic diagram of a plurality of third party program
vendors for
supplementing patient and provider interactions to increase patient adherence
according to
one embodiment of the present invention;
[0026] FIG. 6 is a schematic diagram of a pharmacy system for supplementing
patient and
provider interactions to increase patient adherence according to one
embodiment of the
present invention;
[0027] FIG. 7 is a schematic diagram of payor system for supplementing patient
and provider
interactions to increase patient adherence according to one embodiment of the
present
8
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
invention;
[0028] FIGS. 8a-8c is a flow chart of a system for supplementing patient and
provider
interactions to increase patient adherence according to one embodiment of the
present
invention;
[0029] FIG. 9 is an illustration of a combined educational material and coupon
document
according to one embodiment of the present invention;
[0030] FIGS. 10-15 are screen shots of graphical user interfaces used for
supplementing
patient and provider interactions to increase patient adherence according to
one embodiment
of the present invention;
[0031] FIG. 16 is a flow diagram of a method of acquiring patient medication
history data
according to an embodiment of the present invention;
[0032] FIGS. 17-28 are event diagrams of methods for supplementing patient and
provider
interactions to increase patient adherence according to embodiments of the
present invention;
[0033] FIG. 29 is a schematic diagram of a system for increasing patient
adherence through
the activation of supplemental programs according to one embodiment of the
present
invention;
[0034] FIG. 30 is a flow chart of a method for defining a plurality of cohorts
of a program
cohort group according to one embodiment of the present invention;
[0035] FIG. 31 is a schematic diagram depicting how the SP module defines a
plurality of
cohorts according to one embodiment of the present invention;
[0036] FIG. 32 is a schematic diagram of a method of defining a plurality of
different
permutations of eligible supplemental programs for a target drug according to
one
embodiment of the present invention;
[0037] FIG. 33 is a schematic diagram of a method of assigning a different
permutation of
eligible supplemental programs to each cohort out of a plurality of cohorts of
a program
cohort group according to one embodiment of the present invention;
[0038] FIG. 34 is a schematic diagram of a cohort relation table according to
one
embodiment of the present invention;
[0039] FIGS. 35a-35b are a flow chart of a method for receiving data relating
to an electronic
9
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
prescription for the target drug and activating the permutation of
supplemental programs
associated with the health care provider's cohort for the target drug
according to one
embodiment of the present invention;
[0040] FIG. 36a is a flow chart of a method of retrieving supplemental program
data in
accordance with an alternate embodiment of the present invention;
[0041] FIG. 36b is a flow chart of a method of generating a document list of
all eligible
supplemental programs that are selected and accepted by a provider for an
electronic
prescription according to an alternate embodiment of the present invention;
[0042] FIG. 36c is a flow chart of a method of retrieving the document
associated with
supplemental programs that are selected and accepted by a provider for an
electronic
prescription according to an alternate embodiment of the present invention;
[0043] FIGS. 37a-37b are a flow chart of a method of parsing patient adherence
data into
data grouping based on a plurality of different cohorts according to an
embodiment of the
present invention;
[0044] FIG. 38a is a graphical representation of the effectiveness of
different permutations of
supplemental programs on patient's first fill compliance according to one
embodiment of the
present invention;
[0045] FIG. 38b is a graphical representation of the effectiveness of
different permutations of
supplemental programs on patient's medication persistency rate according to
one
embodiment of the present invention;
[0046] FIG. 39 is a schematic diagram of a patient adherence system for
tracking and
reporting compliance with drug treatment regimens;
[0047] FIG. 40 is a flow chart of an exemplary process showing how the patient
adherence
system of FIG. 39 may process prescription data;
[0048] FIG. 41 is a flow chart of an exemplary process showing how the patient
adherence
system of FIG. 39 may obtain medication history data;
[0049] FIGS. 42-45 are exemplary screen shots of a graphical user interface
(GUI) including
a patient advisor system compliance dashboard with user interactive patient
advisor toolbar
for navigating the patient adherence system and retrieving patient medication
history and
adherence data in addition to other useful medication related information;
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
[0050] FIGS. 46-53 are exemplary GUI screen shots showing a patient advisor
report card
containing patient medication adherence and related data for a plurality of
drugs accessed via
a Report Card tab in the toolbar;
[0051] FIGS. 54-55 are exemplary GUI screen shots showing medication related
studies
accessed via an Outcome Studies tab in the toolbar;
[0052] FIGS. 56-57 are exemplary GUI screen shots showing medication
educational
materials accessed via an Education tab in the toolbar;
[0053] FIGS. 58-59 are exemplary GUI screen shots showing medication discount
information and coupons accessed via a Coupon tab in the toolbar;
[0054] FIG. 60 is a flow chart of a process for generating and transmitting
patient medication
adherence data to the patient advisor system compliance dashboard for display
to a user such
as the health care provider or others;
[0055] FIG. 61 is a flow chart of a process for retrieving and displaying the
Report Card tab
in the patient advisor system toolbar in two different display modes; and
[0056] FIG. 62 is an exemplary GUI screen shot showing an alternative display
of the patient
advisor report card.
[0057] All drawings are schematic.
Detailed Description of Embodiments
[0058] The features and benefits of the present disclosure are illustrated and
described herein
by reference to exemplary embodiments and is in no way intended to limit the
invention, its
application, or uses. This description of exemplary embodiments is intended to
be read in
connection with the accompanying drawings, which are to be considered part of
the entire
written description. Accordingly, the present disclosure expressly should not
be limited to
such embodiments illustrating some possible non-limiting combination of
features that may
exist alone or in other combinations of features; the scope of the claimed
invention being
defined by the claims appended hereto.
[0059] In the description of embodiments disclosed herein, any reference to
direction or
orientation is merely intended for convenience of description and is not
intended in any way
to limit the scope of the present invention. Relative terms such as "lower,"
"upper,"
"horizontal," "vertical,", "above," "below," "up," "down," "top" and "bottom"
as well as
11
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
derivative thereof (e.g., "horizontally." "downwardly," "upwardly," etc.)
should be construed
to refer to the orientation as then described or as shown in the drawing under
discussion.
These relative terms are for convenience of description only and do not
require that the
apparatus be constructed or operated in a particular orientation. Terms such
as "attached,"
"coupled," "affixed," "connected," "interconnected," and the like refer to a
relationship
wherein structures are secured or attached to one another either directly or
indirectly through
intervening structures, as well as both movable or rigid attachments or
relationships, unless
expressly described otherwise. The tenits "medication," "drug" and "substance"
may be
used interchangeably herein unless specifically noted to the contrary to
define a medication
prescribed by a health care provider having a potential health effect.
[0060] Computer-executable instructions/programs (e.g. code) and data
described herein may
be programmed into and tangibly embodied in non-transitory computer readable
medium that
is accessible to and retrievable by a respective processor as described herein
which
configures and directs the processor to perform the desired functions and
processes by
executing the instructions encoded in the medium. It should be noted that non-
transitory
"computer readable medium" as described herein may include, without
limitation, any
suitable volatile or non-volatile memory including random access memory (RAM)
and
various types thereof, read-only memory (ROM) and various types thereof, USB
flash
memory, and magnetic or optical data storage devices (e.g. internal/external
hard disks,
floppy discs, magnetic tape CD-ROM, DVD-ROM, optical disk, 7JTM drive, Blu-ray
disk,
and others), which may be written to and/or read by a processor operably
connected to the
medium.
[0061] Aspects of the present invention may be implemented in software,
hardware,
firmware, or combinations thereof. The computer programs described herein are
not limited
to any particular embodiment, and may be implemented in an operating system,
application
program, foreground or background process, driver, or any combination thereof,
executing on
a single computer or server processor or multiple computer or server
processors
[0062] Processors described herein may be any computer/server central
processing unit
(CPU), microprocessor, micro-controller, or computational device or circuit
configured for
executing computer program instructions (e.g. code).
[0063] The present invention may be embodied in the form of computer-
implemented
processes and apparatus for practicing those processes. The present invention
may also be
12
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
embodied in the form of computer program code embodied in tangible media, such
as floppy
diskettes, read only memories (ROMs), CD-ROMs, DVDs, hard drives, ZIPTM disks,
memory
sticks, or any other computer-readable storage medium, wherein, when the
computer program
code is loaded into and executed by a computer, the computer becomes an
apparatus for
practicing the invention. The present invention may also be embodied in the
form of
computer program code, for example, whether stored in a storage medium, loaded
into and/or
executed by a computer, or transmitted over some transmission medium, such as
over the
electrical wiring or cabling, through fiber optics, or via electromagnetic
radiation, wherein,
when the computer program code is loaded into and executed by a computer, the
computer
becomes an apparatus for practicing the invention. When implemented on a
general-purpose
processor, the computer program code segments configure the processor to
create specific
logic circuits
System Overview
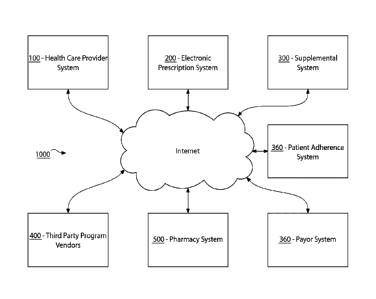
[0064] Referring to FIG. 1, a schematic diagram of a system 1000 for
supplementing patient
and provider interactions to increase patient adherence according to one
embodiment of the
present invention is illustrated. System 1000
is a networked data processing and
communication system. Generally, the system 1000 comprises a health care
provider (HCP)
system 100, an electronic prescription (EP) system 200, a supplemental program
(SP) system
300, at least one third party program vendor 400, a pharmacy system 500, and a
payor system
600 all in operable communication with one another to form a wide area network
(WAN).
In some embodiments, as further described herein, system 1000 may further
include a patient
adherence system 360.
[0065] The individual systems 100-500 and 360 shown in FIG. 1 which comprises
system
1000 and other systems which may operably communicate and exchange data with
any of
these systems through system 1000 shall be broadly construed to be health care
systems. As
the term is used herein, a health care system shall broadly mean any type of
data processing
and/or communication system which creates, processes, accesses, uploads,
downloads, stores,
collects, displays, manipulates, or otherwise utilizes in any manner or degree
whatsoever any
type of information/data directly or tangentially related to a patient,
medication, and health
and dental care for a patient (human or animal) and any related activities and
services.
[0066] As exemplified by FIG. 1, the components of the system 1000 may be in
operable
communication via the internet. However, the invention is not so limited and
other
13
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
electronic communication means or links may be utilized, such as a satellite
network, a
cellular network, a common carrier network(s), Wi-Fi, Bluetooth, WiMAX, wired
connections, or any combination thereof as some possible but non-limiting
examples.
Further, it should be noted that operable communication includes any means of
electronic
communication, such as but not limited to wired and wireless electronic
communication, in
which data can be transmitted and received between the systems and modules of
the system
1000. Moreover, it should also be noted that operable communication includes
both direct
and indirect communication, as well as bi-directional communication between
the systems
and modules of the system 1000.
[0067] As discussed in more detail below, the system 1000 of the present
invention may be
configured in other ways. Therefore, it should be noted that the invention is
not limited only
to those configuration explicitly described herein and, in alternate
embodiments the system
1000 may take on other configurations and/or layouts. For instance, any of the
systems
and/or modules or components of the system 1000 may be connected via a local
area network
(LAN). For example, according to one embodiment of the present invention, the
EP system
200 and the SP system 300 reside on the same LAN, and therefore, may
communicate via
Ethernet and/or Wi-Fi over a LAN.
[0068] Referring to FIG. 2, a schematic diagram of an IICP system 100
according to one
embodiment of the present invention is illustrated. The HCP system 100
comprises a server
110, a terminal 120, and a printer 130 in operable communication. Further, as
discussed in
more detail below, the HCP system 100 may also be said to comprise at least
one health care
provider 101. Although exemplified as comprising the above components, the
IICP system
200 may comprise any number, more or less, of the components listed above. For
example,
a particular HCP system 100 may comprises a plurality of providers 101, a
plurality of
servers 110, a plurality of terminals 120, and/or a plurality of printers 130.
[0069] Generally, the HCP system 100 may be associated with an individual,
institution or
organization that provides general and/or specific health care for those
patients in need. For
example, an HCP system 100 may be associated with an entire hospital or health
care system,
a specialized practice group within a larger hospital or health care system, a
private general
practice, or a private specialized practice. The health care provider 101 may
be a medical
doctor, a nurse practitioner, or a staff administrator who is authorized to
issue prescriptions.
As noted above, the IICP system 100 may be associated with any number of
providers 101,
14
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
and a particular provider 101 may be associated with more than one HCP system
100 at any
given time.
[0070] The server 110 of the HCP system 100 comprises a properly programmed
processor
(e.g. central processing unit (CPU) or microprocessor) 111 executing computer
instructions, a
network interface 112, and a memory device or unit 113 (also referred to
herein as simply
"memory") all in operable communication via a system bus. In one embodiment,
memory
113 may preferably be a non-volatile form of non-transitory computer readable
medium
which retains data even after power is removed or lost from the system. It
should be noted
the processor 111 may be considered the processor of the HCP system 100.
Further,
although exemplified as a single server 110, the invention is not so limited
and in alternate
embodiments the HCP system 100 may comprise any number of servers 110.
Additionally,
although not exemplified, it should be understood that the processor 111 can
have integrated
memory. The network interface 112 connects the server 110 to the over systems
and
modules of the system 1000 via the internet. The properly programmed processor
111 of
the HCP system 100 effectuates the performing of the processes and functions
described
below, including but not limited to, the storage of data to the memory 113 of
the HCP system
100, the performance of the processes and functions of a thin-client version
or portion or of
an electronic prescription (EP) module 203 and a supplemental program (SP)
module widget
302, and the transfer (transmission and receipt) of data from HCP system 100
to the other
systems and modules of the system 1000.
[0071] Although not specifically enumerated herein, it should be noted that
server 110 (and
other servers as variously described herein) include all of the usual
appurtenances necessary
to form a fully functional and interconnected data processing and storage
system.
Accordingly, server 110 will generally include a bus which operably
interconnects the
processor (e.g. processor 111), read-only memory (ROM), random access memory
(RAM),
computer readable medium such as non-volatile memory (e.g. memory 113), and
others.
[0072] In the exemplified embodiment, the memory 113 comprises the thin-client
portion of
the EP module 203 and the SP module widget 302, both of which are described in
more detail
below. Although exemplified schematically as a single memory unit, it should
be noted that
the memory 113 may comprise any number or types of computer readable medium
which
may include one or more databases used to store data, modules, or other
information. For
example, the memory may be used to store provider information, patient
information,
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
prescribed substance or medication information, and appropriate software (e.g.
computer
instructions, program or application) operable to direct operation of
processor 111 and to
allow the provider 101 to interact with the thin-client portion of the EP
module 203 and the
SP module widget 302.
[0073] Although exemplified as part of the memory 113, in other embodiments
the
thin-client portion of the EP module 203 may reside elsewhere on the HCP
system 100 or on
another system altogether accessible to HCP system 100. Further, in the
exemplified
embodiment, the SP module widget 302 is integrated into the thin-client
portion of the EP
module 203. However, it should be noted that the invention is not so limited
and in
alternate embodiments, any portion of the SP module may be integrated into any
portion of
the HCP system 100. Further, in one embodiment of the present invention, the
SP module is
not integrated with the EP module, but is rather a completely separate module
altogether.
[0074] The terminal 120 of the HCP system 100 may be a personal computer (PC)
or any
mobile processor-based electronic unit, such as without limitation for example
a notebook or
laptop computer, tablet or pad computer, personal digital assistant,
mobile/smart phone, or
equivalent which is in operable communication with server HCP 110 by wired or
wireless
communication protocols such as Wi-Fi, Bluetooth, etc. Each terminal 120 of
the HCP
system 100 comprises a properly programmed processor (not shown), a memory
device (not
shown), a power supply (not shown), a video card (not shown), a display device
121,
firmware (not shown), software (not shown), a network interface (not shown)
and a user input
device 122 (e.g., a keyboard, mouse and/or touch screen). Although not
exemplified, it
should be understood that the processor of the terminal 120 can have
integrated memory.
The properly programmed processor of the terminal 120 is configured to
effectuate the
processes and functions described below, including, but not limited to the
effectuation of the
graphical user interfaces (GUI) for display on the display device 121 of the
terminal 120 for
the provider 101 and the transmission of user inputs from the provider 101 via
the input
device 122 to the other systems and modules of the system 1000.
[0075] As discussed in more detail below, after the provider 101 generates a
prescription for
a substance using the thin-client portion of the EP module 203, the electronic
prescription is
transmitted by the HCP system 100 to the phaimacy system 500 for processing.
Further, as
also discussed in more detail below, at any point during the prescription
writing processes
using the thin-client portion of EP module 203, the SP module widget 302 may
receive
16
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
electronic prescription data relating to the electronic prescription and
transmits the electronic
prescription data to the to the SP system 300 for further processing.
[0076] Referring to FIG. 3, a schematic diagram of an EP system 200 according
to one
embodiment of the present invention is illustrated. Generally, the EP system
200 comprises
a server 210 which comprises a properly programmed processor (CPU) 211
executing
computer instructions or control logic, a network interface 212, and a non-
transitory
computer readable medium such as memory unit 213 in operable communication. In
one
embodiment, memory 213 is non-volatile. It should be noted that the processor
211 may be
considered the processor of the EP system 200. Further, although exemplified
as a single
server 210, the invention is not so limited and in alternate embodiments the
EP system 200
may comprise any number of servers 210. Additionally, although not
exemplified, it should
be understood that the processor 211 can have integrated memory. The network
interface
212 connects the server 210 to the over systems and modules of the system 1000
via the
internet.
[0077] As discussed in more detail below, the processor 211 of the EP system
200 effectuates
the performance of the processes and functions described herein, including but
not limited to
the performance of the processes carried out by the central portion of the EP
module 202, the
storage of data to the EP database 201, and the transfer of data between the
EP system 200
and the other systems and modules of the system 1000.
[0078] 'Me memory 213 of the EP system 200 comprises an electronic
prescription (EP)
database 201 and a central portion of the electronic prescription (EP) module
202. The EP
database 201 stores infoimation relating to electronic prescriptions that are
generated using
and effectuated by the EP module, such as, but not limited to, provider data,
patient data,
prescribed substance data, payor data, and patient medication history data.
Further,
although exemplified as a single memory unit, it should be noted that the
memory 213 may
comprise any number of databases used to store data, modules, or other
information.
[0079] Generally, the EP module is one or more computer programs configured to
allow a
provider 101 to generate and transmit electronic prescriptions to the pharmacy
system 500.
In embodiments where the EP module comprises a central portion 202 and a
client portion
203, the central portion 202 is configured to do most of the heavy processing
of the EP
module. Further, in such embodiments, the client portion 203 is a thin-client
portion that
does light processing and generates/displays user interfaces for the provider
101 on the
17
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
display device 121 of their terminal 120.
[0080] As used herein, the central portion 202 and the thin-client portion 203
of the EP
module may he collectively defined as the "EP module." Although exemplified as
comprising a thin-client portion 203 that resides within the memory 113 of the
IICP system
100 and a central portion 202 that resides within the memory 213 of the EP
system 200, the
EP module is not so limited. In alternate embodiments, the central portion 202
of the EP
module may reside elsewhere on the system 1000 or be combined with the thin-
client portion
203 of the EP module and reside on any of the systems of the system 1000. In
embodiments
where the central portion 202 and the thin-client portion 203 are combined,
the provider 101
may access the EP module via a web interface (portal) or an applicant user
interface using
their terminal 120. One non-limiting example of an EP module is Rcopia by
DrFirst . In
other possible embodiments, the central portion 202 and the thin-client
portion 203 may be
combined and located in the HCP system 100 such as in HCP memory 113
accessible locally
to server 110 such that the provider 101 may access the combined EP system
without resort
to an external communication link such as via the web interface and Internet.
[0081] Referring to FIG. 4, a schematic diagram of a SP system 300 according
to one
embodiment of the present invention is illustrated. Generally, the SP system
300 comprises
a server 310 which comprises a properly programmed processor (CPU) 311, a
network
interface 312, and a non-transitory computer readable medium such as memory
unit 313 in
operable communication. In one embodiment, memory 313 is non-volatile. It
should be
noted that the server 310 may be considered the supplemental program server
and the
processor 311 may be considered the processor of the SP system 300. Further,
although
exemplified as a single server 310, the invention is not so limited and
alternate embodiments
the SP system 300 may comprise any number of servers 310. Additionally,
although not
exemplified, it should be understood that the processor 311 can have
integrated memory.
The network interface 312 connects the server 310 to the over systems and
modules of the
system 1000 via the internet.
[0082] As discussed in more detail below, the processor 311 of the SP system
300 effectuates
the performance of the processes and functions described herein, including but
not limited to
the perfoimance of the processes carried out by the central portion 301 of a
supplemental
program (SP) module (e.g., the determination of eligibility performed by the
SP module, the
transfer of content to a patient or the IICP system 100, the enrollment of a
patient into a
18
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
service, etc.), the storage of data to a supplemental program database 303 and
a record
database 304, and the transfer of data between the SP system 300 and the other
systems and
modules of the system 1000.
[0083] The memory 313 of the SP system 300 comprises a central portion of the
SP module
301, a supplemental program database 303, and a records database 304. Although
exemplified as a single memory unit, it should be noted that the memory 113
may comprise
any number of databases used to store data, modules, or other information. As
used herein,
the central portion 301 and the widget 302 of the SP module may be
collectively defined as
the "SP module."
[0084] Generally, the SP module is one or more computer programs configured to
determine,
from a plurality of available supplemental programs, specific supplemental
programs for
which a patient is eligible. Further, as also discussed in more detail below,
the SP module is
configured to, among other things: (1) receive electronic prescription data
generated by a
provider 101 for a patient for a prescribed substance from either the IICP
system 100, the EP
module, or the EP system 200; (2) retrieve patient data, prescribed substance
data, provider
data, and/or payor data from one or more databases of the system 1000; (3)
deteimine, from a
plurality of available supplemental programs, specific supplemental programs
for which a
patient is eligible; (4) determine delivery modes that are available for each
supplemental
program in which the patient is eligible; (5) generate graphical user
interfaces (GUIs) that are
displayed to the provider 101 on the display device 121 of the HCP system 100;
(6) receive
inputs front the provider 101 via the input device 122 of the HCP system 100;
(7) generate an
activation signal for each supplemental program that is selected by the
provider 101; (8)
receive the activation signal from the HCP system 100; (9) activate
supplemental programs
that are selected and confirmed by the provider 101; (10) tailor content
associated with an
activated supplemental program for a specific delivery mode; and (11) deliver
the content
associated with the activated supplemental program to the patient.
[0085] As also discussed in more detail below, the central portion 301 of the
SP module
determines eligible supplemental programs, out of a plurality of available
supplemental
programs, for a patient based on at least electronic prescription data and the
rules of each
available supplemental programs. Although not exemplified, the central portion
301 of the
SP module comprises a rules engine that determines the eligibility of each of
the available
supplemental programs for a patient being prescribed a particular substance.
Further, the
19
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
central portion 301 of the SP module also comprises agents that reach out to
the third party
content providers 400 to retrieve content relating to the plurality of
supplemental programs.
However, the invention is not so limited and in one embodiment, the central
portion 301 of
the SP module does not comprise the rules engine, but rather just transmits at
least the
electronic prescription data to the third party content providers 400, which
in turn determines
the eligibility of each of the available supplemental programs.
[0086] In the exemplified embodiments, the central portion 301 does most of
the heavy
processing of the SP module, while the SP widget 302 routes data to the
central portion 301
and provides an interface for the provider 101 to access the SP module.
Although
exemplified as comprising a SP module widget 302 that resides within the
memory 113 of the
HCP system 100 (and more specifically, a SP module widget 302 that is
integrated into the
EP module) and a central portion 301 that resides within the memory 313 of the
SP system
300, the SP module is not so limited. In alternate embodiments of the present
invention, the
central portion 301 and/or the SP widget 302 may reside elsewhere on the
system 1000, or
the central portion 301 may be combined with the SP module widget 302 and the
combined
SP module may reside on any system or module of the system 1000. Further, as
described
herein, it should be understood that any of the processes or functions
performed by either the
central portion 301 or the SP widget 302 may be performed partially or wholly
by the other
portion of the SP module in an alternate embodiment of the present invention.
[0087] The supplemental program database 303 stores general supplemental
program data,
including, but not limited to the names of a plurality of available
supplemental programs,
general infoimation relating to each of the plurality of available
supplemental programs, and
the rules of each of the available supplemental program. As discussed in more
detail below,
according to one embodiment of the present invention, a supplemental program
is a document
or service that is activated for a patient based on the defined rules of the
supplemental
program. Further,
according to one embodiment of the present invention, each
supplemental program is designed to increase the patient's adherence to a
prescribed
sub stance.
[0088] As also discussed in more detail below, the rules of each supplemental
program
dictate which patients are eligible for the supplemental program. Generally,
each rule may
be based on, among other things, a substance currently being prescribed to the
patient, the
patient's medical history, information relating to the provider, and/or
information relating to
CA 02918798 2016-01-19
WO 2015/013695
PCT/1JS2014/048330
the patient's payor or health insurance company. According to one embodiment
of the
present invention, the rules of the supplemental programs are defined by a
combination of an
administrator of the SP system 300 and one or more pharmaceutical companies.
However,
the invention is not so limited, and in alternate embodiments the rules may be
defined by any
combination of the administrator of the SP system 300, the pharmaceutical
companies, and/or
the third party program vendors 400.
[0089] It should be noted that although exemplified as residing entirely in
the memory 313 of
the SP system 300, in alternate embodiments, the supplemental program database
303 may
reside entirely on another system of the system 1000 or be broken up and
reside partially on
two or more of the systems of the system 1000. Specifically, in one alternate
embodiment
the supplemental program database 303 resides entirely on the HCP system 100,
while in
another alternate embodiment the supplemental program database 303 resides
entirely on the
EP system 200.
[0090] Further, as also discussed in more detail below, in one embodiment of
the present
invention the supplemental program database 303 may comprise the underlying
supplemental
programs themselves. In such embodiments, the SP module does not have to reach
out to
the third party content providers 400 to retrieve content relating to a
supplemental program or
to enroll a patient in a supplemental program.
[0091] The record database 304 stores information relating to the parties and
the processes
involved in supplementing an electronic prescription, such as, but not limited
to, patient data,
prescribed substance data, provider data, payor data, and patient medication
history data.
Further, the record database 304 may further store provider preference data
and patient
preference data. It should be noted that although exemplified as residing
entirely on the SP
system 300, in alternate embodiments, the record database 304 may reside
entirely on another
system of the system 1000 or be broken up and reside partially on two or more
of the systems
of the system 1000. Specifically, in one alternate embodiment the record
database 304
resides entirely on the HCP system 100, while in another alternate embodiment
the record
database 304 resides entirely on the EP system 200.
[0092] Finally, according to one embodiment of the present invention, the SP
system 300
further comprises an administrator. The administrator is an individual (or
group of
individuals) who has access to the databases, modules, and engines of the SP
system 300, and
may configured to databases, modules, and engines as they see fit. For
example, in some
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
embodiments of the present invention, the administrator may configure the
settings of the SP
module (both central portion 301 and SP widget 302), may configure the data
stored in the
one or more databases of the SP system 300, may configure the rules of the
available
supplemental programs, and/or may configure the rules engine of the SP module.
Therefore,
the administrator of the SP system 300 has the ability to access and control
the processes and
functions of all of the components of the SP system 300.
[0093] Referring to FIG. 5, a schematic diagram of a plurality of third party
content providers
400 according to one embodiment of the present invention is illustrated.
Generally, the
present invention is not limited to any specific number of third party content
providers 400.
Therefore, although four third party content providers (410, 420, 430, 440)
are illustrated in
FIG. 5, the present invention may comprise more or less than four third party
content
providers 400. For example, in an alternate embodiment of the present
invention, one or
more of the third party content providers 400 may be combined.
[0094] Each third party content provider 400 comprises a server 410, 420, 430,
440 which
comprises a properly programmed processor (CPU) 411, 421, 431, 441, a network
interface
412, 422, 432, 442, and a non-transitory computer readable medium such as
memory unit 413,
423, 433, 443 in operable communication. In one embodiment, memory 413, 423,
433, 443
are non-volatile. Although each third party content provider 400 is
exemplified as
comprising a single server 410 (or 420, 430, 440), the invention is not so
limited and in
alternate embodiments any of the third party content provider 400 may comprise
any number
of servers. Additionally, although not exemplified, it should be understood
that the
processors 411, 421, 431, 441 can have integrated memory. Finally, the network
interfaces
412, 422, 432, 442 connects their respective server 410, 420, 430, 440 to the
over systems
and modules of the system 1000 via the internet.
[0095] As discussed in more detail below, the processors 411, 421, 431, 441 of
each third
party content providers 400 effectuates the performance of the processes and
functions
described herein, including but not limited to the storage of data to the
databases 401, 402,
403, and 404, the transfer of content to a patient, the enrollment of a
patient into a service,
and the transfer of data between each third party content providers 400 and
the other systems
(specifically the SP system 300) of the system 1000.
[0096] The memory unit 413 comprises a coupon database 401, the memory unit
423
comprises an educational information database 402, the memory unit 433
comprises a
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
patient/medication reminder service database 403, and the memory unit 443
comprises a
patient adherence service database 404. Although exemplified as a single
memory unit, it
should be noted that any of the memory units 413, 423, 433, 443 may comprise
any number
of databases used to store data, modules, or other information.
[0097] The coupon database 401 stores supplemental program coupon data
relating to a
plurality of different coupon documents and coupon services for a plurality of
substances that
may be prescribed to a patient. The supplemental program coupon data may
include the
amount of a coupon, the rules relating to the eligibility of a coupon or a
coupon service, the
delivery modes of the coupon or coupon service, and other information relating
to a particular
coupon or coupon service. Further, it should be noted that a coupon may be,
but is not
limited to, a discount for prescribed substances, a rebate for prescribed
substances, or a
voucher for a free trial of prescribed substances.
[0098] The educational information database 402 stores supplemental program
educational
data relating to a plurality of different educational documents for a
plurality of different
substances and diseases states for which a patient may be prescribed or
diagnosed. The
supplemental program educational data may include general educational
documents relating
to a plurality of different substances or disease states, specific educational
documents relating
to a plurality of different substances or disease states, general educational
services that relate
to a plurality of different substances or disease states, specific educational
services that relate
to a plurality of different substances or disease states, and the rules
relating to the eligibility
of the documents and services listed above.
[0099] The patient/medication reminder service database 403 stores
supplemental program
reminder data relating to a plurality of different patient reminder services
and substance (or
medication) reminder services. The supplemental program reminder data may
include
information relating to appointment reminder services for a patient,
prescription filling
reminder services for a patient, refill reminder services for a patient, and
the rules relating to
the eligibility of the services listed above.
[00100] The patient
adherence service database 404 stores supplemental program
adherence data relating to a plurality of adherence services for patients. The
supplemental
program adherence data may include information relating to a variety of
different adherence
programs and services for patients, including the rules relating to the
eligibility of the
services.
23
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
[00101] Referring to
FIG. 6, a schematic diagram of a pharmacy system 500 according
to one embodiment of the present invention is illustrated. In the exemplified
embodiment,
the pharmacy system 500 comprises a prescription routing sub-system 501 and at
least one
prescription filling sub-system 502, all in operable communication with one
another.
Generally, the prescription routing sub-system 501 is configured to
electronically receive a
prescription for a substance from the HCP system 100 or the EP system 200 and
route the
prescription to a prescription filling sub-system 502.
[00102] The
prescription routing sub-system 501 comprises a server 510 that
comprises a properly programmed processor 511, a network interface 512, and a
non-transitory computer readable medium such as memory device or unit 513 in
operable
communication. In one embodiment, memory 513 is non-volatile. Although not
exemplified, each of the prescription filling sub-systems 502 comprises a
properly
programmed processor, a network interface, and a memory unit. Although
exemplified as a
single server 510, the invention is not so limited and in alternate
embodiments the
prescription routing sub-system 501 may comprise any number of servers 510.
Additionally,
although not exemplified, it should he understood that the processor 511 can
have integrated
memory. The network interface 512 connects the server 510 to the over systems
and
modules of the system 1000 via the internet. The processor 511 of the pharmacy
system
500 effectuates the processes and functions described herein, including but
not limited to, the
reception of prescription data from the EP module, the transfer of
prescription history
information to the SP module, and the transfer of data between the pharmacy
system 500 and
the other systems and modules of the system 1000.
[00103] In the
exemplified embodiment, the memory 513 of the prescription routing
sub-system 501 comprises a prescription filling system database 504 and a
patient
prescription history database 505. The prescription filling system database
504 stores the
names, addresses and other information relating to each of the prescription
filling
sub-system(s) 502. The patient prescription history database 505 stores
information relating
to previous prescriptions routed by the pharmacy system 500 for patients.
[00104] The
prescription filling sub-system 502 is a system that fills the prescribed
substance for an end user. For example, prescription filling sub-system 502
may be a local
pharmacy used by a patient.
[00105] Referring to
FIG. 7, a schematic diagram of a payor system 600 according to
24
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
one embodiment of the present invention is illustrated. Generally, the payor
system 600
comprises a server 610 which comprises a properly programmed processor (CPU)
611, a
network interface 612, and a non-transitory computer readable medium such as
memory unit
613 in operable communication. In one embodiment, memory 613 is non-volatile.
It
should be noted that the processor 611 may be considered the processor of the
payor system
600. Further, although exemplified as a single server 610, the invention is
not so limited
and in alternate embodiments the payor system 600 may comprise any number of
servers 610.
Additionally, although not exemplified, it should be understood that the
processor 611 can
have integrated memory. The network interface 612 connects the server 610 to
the over
systems and modules of the system 1000 via the internet.
[00106] As discussed
in more detail below, the processor 611 of the payor system 600
effectuates the performance of the processes and functions described herein,
including but not
limited to the storage of data to the database 601 of the memory 613 and the
transfer of data
(e.g., patient insurance information) between the payor system 600 and the
other systems and
modules of the system 1000.
[00107] The memory
613 of the payor system 600 comprises a patient insurance
database 601. The patient insurance database 601 stores information relating
to the payor of
prescriptions for substances of patients, such as, but not limited to, the
patient's insurance
company, the patient's co-pay amount, and the patient's other deductibles.
Further,
although exemplified as a single memory unit, it should be noted that the
memory 613 may
comprise any number of databases used to store data, modules, or other
information.
[00108] Therefore,
it may be said that the system 1000 comprises a plurality of
databases or one or more databases. Specifically, as noted above, the system
1000
comprises the EP database 201 on the EP system 200, the supplemental program
database
303 and the records database 304 on the SP system 300, the coupon database
401, the
educational information database 402, the patient medication/reminder service
database 403,
and the patient adherence service database 404 of the third party content
providers 400, the
prescription filling sub-system database 504 and the patient prescription
history database 505
of the pharmacy system 500, and the patient insurance database 601 of the
payor system 600.
Supplemental Programs
[00109] A
supplemental program, as used herein, may be any document that is
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
provided to a patient or any service in which a patient is enrolled that is
designed for
increasing patient adherence to a prescribed substance. Stated another way, a
supplemental
program may be a document or service designed to help patients understand
their medication
regimen and comply with it. For example, a supplemental program may be a
coupon (or a
coupon service) that is provided to a patient for a particular prescribed
substance, educational
material (either general or specific) that is provided to a patient for a
particular prescribed
substance or disease state, a combined coupon/educational document (referred
to herein as an
"EduSAVETM" document, one example of which is exemplified in FIG. 9), a
loyalty card, a
prescription reminder service, an appointment reminder service, a health care
coaching
service, or any other patient adherence service or document. In one
embodiment, the
available supplemental programs are all patient adherence programs. However,
the
invention is not so limited and in alternate embodiments, some or all of the
available
supplemental programs may not relate to patient adherence.
[00110] As discussed
in more detail below, eligibility of a supplemental program is
determined by comparing one or more of a plurality of different data elements
(such as, but
not limited to, a patient's general information, a patient's medical history,
a brand name or
formula of a substance prescribed to a patient, other information relating to
a substance
prescribed to a patient, a patient's payor's information (e.g., a patient's
health insurance
company and/or health insurance plan), a provider's general or specific
information) with the
rules of each of the available supplemental programs. If the data element(s)
meets the rules
for a specific supplemental program, then the patient is determined to be
"eligible" for that
program. For example, a specific program may only be eligible to patients who
are being
prescribed a particular substance, patients who reside within a particular
geographic region,
patients who have a specific history with a particular substance, patients who
have at least
specific co-pay for a particular substance, or patients whose providers meet
certain
qualifications.
[00111] As discussed
in detail below, the determination of eligibility is determined by
the SP module, and more specifically, by the rules engine of the SP module.
Generally, the
SP module receives and/or retrieves a plurality of data relating to the
patient, the prescribed
substance, the provider, and/or the payor, and applies that data to the rules
of each of the
available supplemental programs to determine supplemental programs in which
the patient is
eligible.
26
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
Method for Supplementing Patient and Provider Interactions
[00112] Generally
and in accordance with one embodiment of the present invention, a
method for supplementing patient and provider interactions to increase patient
adherence
generally comprises three steps: (1) determining, from a plurality of
available supplemental
programs, supplemental programs for which a particular patient receiving a
prescription for a
particular substance is eligible; (2) receiving confirmation/approval from the
patient's health
care provider that the eligible supplemental program should be activated; and
(3) activating
the eligible supplemental programs that the provider has confirmed/approved in
order to
increase patient adherence to the prescribed substance.
1. Determining Eligible Supplemental Programs for a Patient
[00113] Referring to
FIGS. 8a-8c, a flow chart of a system for supplementing patient
and provider interactions to increase patient adherence according to one
embodiment of the
present invention is illustrated.
[00114] According to
one embodiment of the present invention, the process begins
when a patient visits their health care provider 101 seeking health care
advice, and the
provider 101, after diagnosing the patient, decides to write an electronic
prescription for a
particular substance for the patient. The electronic prescription is typically
generated by the
provider 201 using the EP module. Specifically, in one embodiment of the
present
invention, the provider 101 drafts an electronic prescription using the thin-
client portion of
the EP module 203, which resides on the HCP system 100.
[00115] Referring to
FIG. 10, a screen shot of a graphical user interface (GUI)
generated by the EP module (and specifically the thin-client portion 203 of
the EP module) to
allow the provider 101 to generate an electronic prescription for a patient
according to one
embodiment of the present invention is illustrated. As shown in FIG. 10, the
GUI comprises
information relating to the provider (at least their name) 1001, information
relating to the
patient 1002, infoimation relating to the pharmacy 1003 where the electronic
prescription
will be transmitted, information relating to the formulary of the patient
1004, information
relating to the patient's medical history 1005, and information relating to a
substance 1006 to
be prescribed to the patient. Moreover, the GUI is not so limited and may
further comprise
27
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
information relating to the other medications previously prescribed to the
patient, current
allergies or adverse reactions of the patient, or other previously recorded
problems of the
patient.
[00116] Referring to
FIGS. 11-14, multiple, sequential graphical user interfaces (GUIs)
1011, 1012, 1013, 1014 generated by the EP module to allow the provider 101 to
generate an
electronic prescription for a patient according to one embodiment of the
present invention are
illustrated. In the GUI 1011 exemplified in FIG. 11, the provider 101 may
select a
medication for prescription. As shown, the provider 101 may search for a new
substance to
prescribe by name or may choose a substance from a pre-established list of
favorites. As
shown in the GUI 1012 of FIG. 12, after a provider 101 chooses a substance to
prescribe to
the patient, the EP module generates and displays the GUI 1012, which
comprises drug
interaction warnings, formulary alerts based on the patient's formulary
status, and other
medication alerts and warnings.
[00117] After the
provider 101 confirms the substance in the GUI 1012, the EP module
generates and displays GUI 1013 (shown in FIG. 13), which allows the provider
to enter the
details of the substance to be prescribed 1006. For example, substance details
such as the
names, dosage, strength, form, duration, quantity, and refills are displayed
for provider 101
input, along with directions to the patient and/or pharmacist and other
details relating to the
filling pharmacy and provider 101. After the provider 101, has entered all the
required
information using the input device 122 of their terminal 120 of the HCP system
100, the EP
module generates a displays GUI 1014. As exemplified in FIG. 14, the GUI 1014
provides
a summary of the electronic prescription for the substance for the provider's
review. After
the provider 101 reviews and confirms that the prescription is accurate, the
electronic
prescription for the substance is created.
[00118] Still also
referring to FIGS. 8a-8c, in the exemplified embodiment, after an
electronic prescription for the substance is created by the provider 101, the
SP widget 302
retrieves data relating to the electronic prescription from the thin-client
portion of the EP
module 203. The SP widget 302 then transmits the electronic prescription data
to the central
portion 301 of the SP module residing on the SP system 300, such that the
central portion 301
of the SP module receives the electronic prescription data, thereby completing
step 801 in
FIG. 8a. The electronic prescription data comprises first patient data that is
specific to the
patient, first prescribed substance data that is specific to the prescribed
substance, first
28
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
provider data that is specific to the provider 101, and first payor data that
is specific to the
payor.
[00119] The first
patient data comprises the information that is part of the prescription
and relates to the patient, such as but not limited to, the patient's name,
gender, date of birth
(DOB), contact information (telephone and address), and the patient's
formulary status.
[00120] The first
prescribed substance data comprises information that is part of the
prescription and relates to the prescribed substance, such as but not limited
to, the name of
the prescribed substance, the dosage, strength, fon'', duration, and quantity
of the prescribed
substance, and the number of refills listed on the prescription.
[00121] The first
provider data comprises information that is part of the prescription
and relates to the provider 101, such as but not limited to, the provider's
name, the address
and phone number of the provider's practice, and national provider identifier
(NPI) number.
[00122] The first
payor data comprises information that is part of the prescription and
relates to the payor of the patient, such as but not limited to, the payor's
name and the
formulary status (or health care plan) of the patient.
[00123] However, the
invention is not so limited, and in an alternate embodiment of
the present invention, the electronic prescription data may not relate to an
electronic
prescription currently being prescribed by the provider 101 for the patient,
but rather relate to
a refill, a renewal, or a previously prescribed substance. In such
embodiments, the
electronic prescription data may be received by the SP module from one of the
other
databases of the system 1000 (e.g., the EP database 201, the records database
304, the patient
prescription history database 505, etc.).
[00124] Once the
central portion 301 of the SP module receives the electronic
prescription data, the central portion 301 of the SP module retrieves
additional data prior to
determining supplemental programs for which the patient is eligible. However,
it should be
noted that the invention is not so limited and in alternate embodiments, the
central portion
301 of the SP module may only retrieve a portion of the data listed herein or
may not retrieve
any additional data prior to determining supplemental programs for which the
patient is
eligible.
[0100] In the exemplified embodiment, the central portion 301 of the SP module
retrieves
patient data that is specific to the patient from the record database 304,
thereby completing
29
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
step 802. The retrieved patient data may be referred to as "additional" or
"second" patient
data, or simply patient data. The patient data comprises one or more of the
patient's current
medication, the patient's recent drug fills, the patient's drug fill history,
the patient's
demographics, the patient's health care plan or payor information, the
patient's adherence
information, and the patient's clinical trial cohort (if the patient is part
of a clinical trial
cohort). The patient adherence information may relate to the patient's past
adherence to
prescriptions for the same prescribed substance, for prescriptions to
prescribed substances for
the same disease state, and/or the patient's general adherence to any
combination of the
substances they have previously been prescribed to the patient. It should be
noted that this
information is in addition to the first patient data that was retrieved by the
centralized portion
of the SP module 301 from the created electronic prescription.
[0101] Further, the central portion 301 of the SP module may also retrieve
prescribed
substance data relating to the substance prescribed by the electronic
prescription from the
record database 304, thereby completing step 803. The retrieved prescribed
substance data
may be referred to as "additional" or "second" prescribed substance data, or
simply
prescribed substance data. The prescribed substance data comprises one or more
of the
prescribed substance's drug properties, the prescribed substance's therapeutic
class(es), a
prescribed substance substitution code, the prescribed substance's formulary
data, and a
prescription indicator. It should be noted that this information is in
addition to the first
prescribed substance data that was retrieved by the central portion 301 of the
SP module from
the created electronic prescription.
[0102] The central portion 301 of the SP module may also retrieve provider
data relating to
the health care provider 101 from the record database 304, thereby completing
step 804.
The retrieved provider data may be referred to as "additional" or "second"
provider data, or
simply provider data. The provider data comprises one or more of the
provider's
geographic location, the provider's state of residency, and the specialty of
the provider 101.
It should be noted that this information is in addition to the first provider
data that was
retrieved by the central portion 301 of the SP module from the created
electronic prescription.
[0103] "[he central portion 301 of the SP module may also retrieve payor data
relating to the
payor (e.g., a health care insurance company) of the patient from the record
database 304,
thereby completing step 805. The retrieved payor data may be referred to as
"additional" or
"second" payor data, or simply payor data. Further, according to one
embodiment, if the
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
record database 304 does not have any of the patient's payor information
stored therein (or
even if it does), then the central portion 301 of the SP module may transmit a
request to the
payor system 600 and receive back the patient's payor information from the
patient insurance
database 601. The payor data comprises one or more of the formulary status (or
health care
plan) of the patient, the co-pay of the patient, and any other information
relating to the payor
of the patient. It should be noted that this information is in addition to the
first payor data
that was retrieved by the central portion 301 of the SP module from the
created electronic
prescription.
[0104] According to one embodiment of the present invention, the central
portion 301 of the
SP module first attempts to retrieve the relevant data from the records
database 304.
Thereafter, if the records database 304 does not comprise the relevant data
required by the
central portion 301 to determine the eligible supplemental programs, then the
central portion
301 reaches out to at least one other database on the system 1000, such as,
but not limited to
the EP database 201 and the patient insurance database 601. In one embodiment,
upon
retrieving the relevant data from one of the other databases of the system
1000, the central
portion 301 stores the relevant data in the records database 304 for future
processing.
[0105] After the central portion 301 of the SP module retrieves the additional
data required,
the central portion 301, using the rules engine, determines, from a plurality
of available
supplemental programs, supplemental programs for which the patient is eligible
based on the
electronic prescription for the substance. As discussed above, a plurality of
available
supplemental programs are stored within one or more databases, which includes
but is not
limited to the supplemental program database 303 and the databases 401. 402,
403, 404 of the
third party content providers 400. As noted above, according to one embodiment
of the
present invention each available supplemental program is a document that is
provided to a
patient or a service in which a patient is enrolled. Moreover, according to
one embodiment,
each available supplemental program is designed to increase patient adherence
to the
prescribed substance.
[0106] As also noted above, each supplemental program out of the plurality of
available
supplemental programs comprises one or more rules. Generally, the rules of a
supplemental
program must be met in order for the patient to be "eligible" for the
supplemental program.
The rules may relate to information relating to the patient, the prescribed
substance, the
provider, and/or the payor of the patient. Therefore, the rules of each of the
available
31
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
supplemental programs may act as constraints and/or restrictions dictating the
eligibility of a
patient for a particular available supplemental program.
[0107] Examples of rules include, but are not limited to, restricting a
supplemental program
to a specific prescribed substance or disease state, restricting a
supplemental program to a
specific prescribed substance of a specific dosage strength, restricting a
supplemental
program to patients or providers of a specific geographic region, restricting
a supplemental
program to patients who have a certain adherence history (whether with the
prescribed
substance or in general), restricting a supplemental program to patients who
have a specific
persistency rate for the prescribed substance (e.g., a persistency rate under
60%, a persistency
rate between 30%-60%, or a persistency rate between 10%-85%), restricting a
supplemental
program to patients of a certain age or age range, restricting a supplemental
program to
patients who have been prescribed the substance for at least a predetermined
time period,
restricting a supplemental program to patient's who have a certain co-pay for
a specific
prescribed substance, restricting a supplemental program to patient's having a
certain health
insurance carrier, etc.
[0108] Therefore, for example, a specific supplemental program is only
eligible to patients
who meet the rules described above. Restricting the dissemination of
supplemental
programs on the basis of the rules listed above may be beneficial since
supplemental
programs will only go to those patients with which they will have the greatest
effect.
Therefore, for example, a pharmaceutical company is not blindly handing out
coupons to
patients whose habits may not be affected by the receipt of a coupon. Rather,
the coupons
are distributed on the basis of predetermined rules to increase the likelihood
that the coupons
will result in increased adherence by the patient, and in turn, sales of the
prescribed substance
and reduced costs to the other parties involved. Further, since the
determination of
eligibility is performed by the rules engine of the SP module, the health care
provider 101,
may, but is not required to calculate or analyze whether a patient would he
incentivized by a
supplemental program. This helps to alleviate some of the burden typically
placed on health
care providers 101 with regards to disseminating documentation to their
patients.
[0109] Further, according to another embodiment of the present invention,
rules may further
include a patient's specific usage stage for a substance. Therefore, in one
embodiment, a
patient may only be eligible for supplemental program that provides a specific
coupon if they
are at a specific usage stage for a particular substance. For example, a
patient may only be
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
eligible for a coupon if they are after their second refill for a particular
substance, or if they
are between their third and fourth refill of a particular substance. In such
embodiments,
providing coupons to a patient based on their specific usage stage for a
substance may
encourage continued patient adherence for that substance.
[0110] The rules engine of the central portion 301 of the SP module determines
the eligibility
of each of the plurality of available supplemental programs for the patient by
comparing the
data received/retrieved by the SP module with the rules of each available
supplemental
program. As noted above, the data used in the comparison includes, but is not
limited to,
the first patient data received from the electronic prescription, the first
prescribed substance
data from the electronic prescription, the first provider data from the
electronic prescription,
the first payor data from the electronic prescription, the patient data
retrieved by the central
portion 301 of the SP module, the prescribed substance data retrieved by the
central portion
301 of the SP module, the provider data retrieved by the central portion 301
of the SP module,
and the payor data retrieved by the central portion 301 of the SP module.
Therefore, the
eligibility of each of the available supplemental programs is determined by
the rules engine
of the central portion 301 of the SP module using any combination of the data
(or data
elements) listed above.
[0111] Still referring to FIG. 8a, in decision step 806, the central portion
301 of the SP
module, using the rules engine, determines the eligibility of the plurality of
available
supplemental programs by comparing the data received and retrieved (e.g., the
patient data,
the prescribed substance data, the provider data, and the payor data discussed
above) with the
rules of each of the available supplemental programs. If the
received/retrieved data does not
meet the rules of any of the plurality of available supplemental programs,
then the process
ends at step 807. However, if the received/retrieved data meets the rules of
at least one
available supplemental program, then the process continues to step 808. It
should be noted
that those supplemental programs whose rules are determined by the rules
engine to meet the
received and retrieved data are considered eligible supplemental programs.
[0112] Further, it should be noted that although exemplified as a single
determination step,
the invention is not so limited. In one embodiment of the present invention,
the
determination of eligible supplemental programs by the rules engine of the SP
module is a
multi-step comparison process. For example, in one embodiment of the present
invention,
during a first comparison step the central portion 301 of the SP module
compares the
33
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
prescribed substance data (including either the first prescribed substance
data retrieved from
the electronic prescription and/or the prescribed substance data retrieved
from the record
database 304) with the rules of each of the available supplemental programs.
More
specifically, the rules engine of the SP module may compare the brand name or
formula of
the prescribed substance with each of the plurality of available supplemental
programs. If
the brand name or formula of the prescribed substance matches the brand name
or formula of
a rule an available supplemental program, then that supplemental program
passes the first
comparison step of the rules engine.
[0113] If the prescribed substance data does meet the rules of at least one
available
supplemental program, then the central portion 301 of the SP module performs a
second
comparison step, whereby the SP module compares the patient data (including
either the first
patient data retrieved from the electronic prescription and/or the patient
data retrieved from
the record database 304) with the rules of each of the supplemental programs
that passed the
first comparison step. Thereafter, the SP module may perform subsequent
comparison steps
using the provider data and/or the payor data. In such embodiments, a patient
is "eligible"
for a supplemental program, if and only if, the supplemental program passes
each step of the
multi-step comparison process.
[0114] It should be noted that in such multi-step comparison embodiments, the
invention is
not limited to any specific number of comparison steps, the order of the
comparison steps, or
the types of comparison steps (e.g., steps using prescribed substance data,
using patient data,
using provider data, or using payor data).
[0115] Further, it should be noted that in an alternate embodiment of the
present invention,
the central portion 301 of the SP module transmits the data received and
retrieved from the
one or more databases (e.g., the patient, prescribed substance, provider, and
payor data
discussed above) to a third party system (e.g., one of the third party content
providers 400).
Thereafter, the third party system compares the data against the rules of each
of the available
supplemental programs to determine eligibility. After testing the data against
the rules, the
third party system transmits a signal back to the central portion 301 of the
SP module
indicating which of the available supplemental programs are eligible.
Therefore, in such
embodiments, the SP module determines the eligibility of the available
supplemental
programs by transmitting the appropriate data to a third party system and
receiving back a
signal indicating for which of the available supplemental programs the patient
is eligible.
34
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
[0116] Although not exemplified, in one embodiment of the present invention,
prior to
performing step 806, the central portion 301 of the SP module retrieves
provider preference
data (and/or patient preference data) from the records database 304 and/or the
supplemental
program database 303. Provider preference data is information that relates to
the
preferences of the specific provider 101 who drafted the prescribed substance.
Similarly,
patient preference data is information that relates to the preferences of the
patient which
whom the substance is being prescribed. The preference data includes, but is
not limited to,
specific modes of delivery (e.g., print, email, SMS, etc.) and supplemental
program types
(e.g., educational material, coupons, reminder services, etc.) that the
provider 101 and/or
patient prefers.
[0117] If the SP module locates and retrieves preferences for the provider 101
and/or patient,
then the following steps are limited to those supplemental programs and
delivery modes that
are preferred by the provider and/or patient. For example, if the provider 101
sets their
preferences to select only a specific type of supplemental program (e.g.,
educational material),
then the SP module will only determine eligible supplemental programs that are
of that
specific type of supplemental program. For purposes of this discussion, we
will assume that
the SP module does not retrieve any provider or patient preference data.
[0118] According to one embodiment of the present invention, the SP module
receives
provider 101 and/or patient preference data directly from the provider 101 via
the input
device 122 of the HCP system 100. However, it should be noted that in other
embodiments
of the present invention, the SP module may learn the preferences of a
provider 101 and/or a
patient based on one or more previous instances where the provider 101 and/or
patient used
the SP module. Upon receiving or learning provider 101 and/or patient
preference data, the
central portion 301 of the SP module stores the preference data in the record
database 304.
[0119] After the SP module determines which of the available supplemental
programs the
patient is eligible, the central portion 301 of the SP module retrieves
supplemental program
data relating to each of the eligible supplemental programs from the
supplemental program
database 303, thereby completing step 808. It should be noted that, according
to one
embodiment of the present invention, the supplemental program data is not the
actual
supplemental program itself, but rather information relating to each of the
supplemental
programs.
[0120] In the exemplified embodiment, the supplemental program data comprises
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
information about the eligible supplemental program, such as, but not limited
to, the name of
the eligible supplemental program, the specific type of document or service
the eligible
supplemental program comprises, and delivery mode data relating to the
available delivery
modes of each of the eligible supplemental programs. Further, it should be
noted that if the
received/retrieved data meets the rules of more than one available
supplemental program,
then the central portion 301 of the SP module retrieves supplemental program
data relating to
each of the plurality of eligible supplemental programs from the supplemental
program
database 303.
[0121] However, the invention is not so limited, and in another embodiment of
the present
invention, the central portion 301 of the SP module retrieves the supplemental
program data
from the one or more databases 401, 402, 403, 404 of the appropriate third
party content
provider 400. Further, in another alternate embodiment, the central portion
301 of the SP
module may actual receive the supplemental programs itself at step 808.
[0122] After retrieving the supplemental program data in step 808, the central
portion 310 of
the SP module retrieves patient delivery mode data relating to the delivery
modes that are
available for the patient from the record database 304 of the SP system 300,
thereby
completing step 809. The patient delivery mode data comprises information
relating to the
patient, such as but not limited to, an email address of the patient, a phone
number of the
patient, and a mailing address of the patient. It should be noted that the
central portion 301
of the SP module can retrieve information about the patient that is currently
stored in the
record database 304, along with patient data that is stored in the other, one
or more databases
of the system 1000.
[0123] After retrieving patient delivery mode data, the central portion 301 of
the SP module
compares the patient delivery mode data with the delivery mode data for each
of the eligible
supplemental programs, thereby completing step 810. As noted above, the
delivery mode
data for the eligible supplemental programs is retrieved by the central
portion 301 in step 808.
The comparison is done in order to determine qualified delivery modes for each
of the
eligible supplemental programs. A qualified delivery mode is a delivery mode
that is
available for a supplemental program and a delivery mode in which the patient
delivery mode
data (e.g., the patient's email address, phone number, etc.) relating to that
delivery mode is
stored in the SP system 300 and has been retrieved by the SP module.
[0124] As discussed in more detail below and according to one embodiment of
the present
36
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
invention, eligible supplemental programs that do have qualified delivery
modes may be
preselected by the SP module for those specific delivery modes. Further, in
another
embodiment of the present invention, eligible supplemental programs that do
not have at least
one qualified delivery modes associated therewith may be locked so as to be
incapable of
selection by the provider 101. In such embodiments, if the provider 101 may be
required to
enter patient delivery mode information into the GUI of FIG. 15 described
below in order to
unlock the selection mechanism for that particular supplemental program.
Further, it should
be noted that the invention is not so limited, and in other alternate
embodiments the qualified
delivery modes may just be preselected by the SP module, while the non-
qualified delivery
modes are grayed-out or only selectable upon the provider 101 entering the
appropriate
patient delivery mode information.
[0125] Further, in yet another embodiment of the present invention, the SP
module does not
retrieve patient delivery mode data and, therefore a comparison between
patient delivery
mode data and delivery mode data for each of the eligible supplemental
programs is not
performed by the SP module. In such instances, all of the available delivery
modes for each
of the eligible supplemental programs may be displayed in the GUI to the
provider 101 using
the display device 121.
2. Receiving Confirmation from the Health Care Provider to Activate the
Supplemental Programs
[0126] After the SP module has determined supplemental programs for which the
patient is
eligible, the SP module generates and displays a GUI to the provider 101 in
order to receive
confirmation from the provider 101 regarding which of the eligible
supplemental programs
should be activated.
[0127] Referring to FIG. 8b and after step 810, the SP module generates a GUI
that
comprises a list of the eligible supplemental programs for the provider's
selection and
confirmation by the health care provider 101, thereby completing step 811. In
one
embodiment of the present invention, the central portion 301 of the SP module
generates the
GUI that comprises the list of eligible supplemental programs for the patient,
and then
transmits the GUI to the SP widget 302 for display to the provider 101 in the
display device
121. However, in alternate embodiments of the present invention, the GUI is
generated and
37
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
displayed by the SP widget 302.
[0128] After the GUI is generated, the SP widget 302 displays the GUI in the
display device
121 of the terminal 120 to the provider 101, thereby completing step 812. One
example of a
GUI is shown in FIG. 15. As exemplified by the GUI of FIG. 15, the GUI
comprises a
pop-up window 1010, which comprises information relating to the substance 1011
for which
eligible supplemental programs are being presented, information relating to
the eligible
supplemental programs 1012, selection mechanisms 1013 for each of the eligible
supplemental programs, information relating to delivery modes 1014 available
for the eligible
supplemental programs, delivery mode selection mechanisms 1015 for each of the
delivery
modes available for each of the eligible supplemental programs, delivery mode
input fields
1016, a confirmation mechanism 1017, and a cancellation mechanism 1018.
[0129] Still referring to FIG. 15, although only one substance is listed in
the window 1010, it
should be noted that section 1011 may comprise information relating to a
plurality of
prescribed substances. For instance, if the provider 101 drafts more than one
prescription
relating to more than one substance for the patient and the SP module
determines that there
are eligible supplemental programs relating to more than one of the prescribed
substances,
then the window 1010 will comprises a list of each of the substances 1011
along with a list of
each of their associated eligible supplemental programs 1012.
[0130] As further exemplified by the window 1010, section 1012 which comprises
a list of
the eligible supplemental programs for each of the prescribed substances, also
comprises a
selection mechanism 1013 to allow the provider 101 to determine which of the
eligible
supplemental programs they would like to activate for their patient. The
selection
mechanism 1013 allows each of the eligible supplemental programs to be
selected and/or
deselected by the provider 101 using the input means 122 of the IICP system
100. As
discussed in more detail below, the eligible supplemental programs that are
selected (e.g., via
a check box) by the provider 101 using the selection mechanism 1013 when the
confirmation
mechanism 1017 is actuated by the provider 101 will be activated by the SP
module for the
patient upon the provider 101 actuating the confirmation mechanism 1017.
Although
exemplified as a check box, the invention is not so limited and in alternate
embodiments the
selection mechanism 1013 may be changed to include any selection mechanism
known in the
art.
[0131] Moreover, as discussed above, it should be noted that if the
supplemental program
38
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
database 303 and/or the records database 304 comprises provider preference
data and/or
patient preference data, then the SP module will upload that information and
generate the
window 1010 based on that information. For instance, if the preference data
relates to
specific types of supplemental programs or delivery modes preferred by the
provider 101 or
patient, then the selection mechanisms 1013, 1015 for those supplemental
program and/or
delivery modes will be pre-selected when the window 1010 is generated by the
central
portion 301 of the SP module and displayed by the SP widget 302 on the display
device 121
for the provider 101.
[0132] Still referring to the window 1010 shown in FIG. 15, a list of
available delivery
modes 1014 for the eligible supplemental programs is also displayed for the
provider 101.
As shown, each delivery mode comprises a delivery mode selection mechanism
1015 that
may be selected and/or deselected by the provider 101 using the input means
122. The
delivery mode selection mechanisms 1015 allow the provider 101 to determine
how the
supplemental programs will be delivered to the patient. Further, it should be
noted that
more than one delivery mode may be selected by the provider 101. In such
instances, the
supplemental programs will be delivered to the patient via all of the selected
delivery modes.
Although the delivery modes are shown to comprise print, email, and text/SMS,
the invention
is not so limited and in alternate embodiments, the delivery modes may also
include mailing
to the patient's address, along with other methods of delivering documents to
the patient.
[0133] Further, the window 1010 also comprises the delivery mode input fields
1016, which
allow a provider 101 to manually enter in the patient's mobile phone number,
email address,
or other patient delivery mode information required for delivery of a
supplemental program.
If the provider 101 enters patient delivery mode information into a delivery
mode input field
1016, then upon the provider 101 actuating the confirmation mechanism 1017,
the SP module
stores the patient's delivery mode infoimation in one or more databases of the
system 1000
(e.g., the records database 304) for future instances. Additionally, it should
be noted that if
the patient's delivery mode information (e.g., email, phone number, address,
etc.) is
previously stored in one or more of the databases of the system 1000 (e.g.,
the record
database 304, the EP database 201, etc.), then the SP module will retrieve the
patient's
delivery mode information from the one or more databases and auto-populate the
delivery
mode input fields 1016 in window 1010.
[0134] Further, one of the delivery mode input field 1016 shown in the window
1010 is a
39
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
preview field. The preview field allows the provider 101 to preview the
eligible
supplemental program(s) before activating the program(s) for the patient.
If the
supplemental program is a coupon, education material, or other document, then
another
window displaying the document or information relating to the document will be
generated
and displayed by the SP module in the display device 121. According to one
embodiment
of the present invention, if the supplemental program is a service, then
another window
displaying general information relating to the service will be generated and
displayed by the
SP module in the display device 121.
[0135] As noted above, according to one embodiment of the present invention,
prior to
generating and displaying the window 1010, the SP module retrieves delivery
mode data
relating to the eligible supplemental program and the patient. In the list of
delivery modes
1014 exemplified in FIG. 15, "print" is a qualified delivery mode and the
delivery mode input
field 1016 for print has been pre-selected by the SP widget 302. Since the
other delivery
modes, such as email and mobile, do not comprise patient delivery mode data,
they are not
qualified delivery modes and are not pre-selected by the SP module.
[0136] Referring to both FIG. 8b and FIG. 15, after the provider 101 has
selected the eligible
supplemental programs and the delivery mode(s) for the eligible supplemental
programs that
they would like to activate for the patient, the provider 101 actuates the
confirmation
mechanism 1017. Upon actuating the confirmation mechanism 1017, the SP widget
302
generates and transmits an activation signal for each of the supplemental
programs that have
been selected by the provider 101 to the central portion 301 of the SP module,
thereby
completing step 813. Each of the activation signals comprises information
relating to the
eligible supplemental program itself, along with the deliver mode selected by
the provider
101. However, the invention is not so limited and in alternate embodiments,
the SP widget
302 generates and transmits a single activation signal that comprises
information relating to
all of the eligible supplemental programs that were selected by the provider
101.
[0137] Although exemplified as an icon in the window 1010, the confirmation
mechanism
1017 is not so limited. In alternate embodiments, the confirmation mechanism
1017 may be
a button, switch, lever, etc. that can be actuated by the provider to confirm
the selected
eligible supplemental programs and delivery modes.
[0138] However, if the provider 101 decides that they do not want to have any
of the eligible
supplemental programs activated for the patient, then the provider 101 may
actuate the
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
cancellation mechanism 1018. Upon actuating the cancellation mechanism 1018,
the SP
widget 302 generates and transmits a cancellation signal to the central
portion 301 of the SP
module. In such instances, none of the eligible supplemental programs are
activated for the
patient.
[0139] As shown in FIG. 15, the window 1010 is displayed concurrently with the
electronic
prescription interface that was used to generate the electronic prescription
data previously
received by the SP module. More specifically, the window 1010 overlays the
electronic
prescription interface and is automatically generated and displayed by the SP
module in the
display device 121 during the electronic prescriptions session undertaken by
the provider 101.
By using such a system, the provider 101 does not have to leave their
electronic prescription
writing interface in order to be presented with eligible supplemental programs
for their
patients. Stated simply, the SP module, due in part to the SP widget 302 being
integrated
into the EP module, provides one continuous interface for the provider 101
during their
prescription writing / supplemental program activating process.
[0140] This is beneficial because it allows the provider 101 to know what
sorts of
supplemental programs are available for their patient and, specifically for
the substance the
provider 101 is currently prescribing for their patient, without having to
leave their electronic
prescription writing interface. Such a system encourages providers 101 to
disseminate
documents and enroll their patients in services to increase their patient
adherence in their
prescribed substances.
[0141] Further, additional benefits arise from granting the provider 101 the
ability not only to
select what specific supplemental programs will be activated for each and
every one of their
patients, but also the ability to preview the supplemental programs before
they are activated
for the patient. Additionally, the provider 101 may select the specific
delivery mode for
each patient. Therefore, the provider 101 may tailor the supplemental programs
depending
on the particular preferences of the patient, as well as what the provider 101
believes will
result in the most beneficial results. Finally, allowing the provider 101 to
be the gatekeeper
between the supplemental programs and the patient encourages communication
between the
provider 101 and patient, which ultimately results in better care for the
patient.
[0142] Although exemplified as pop-up window 1010, it should be noted that the
invention is
not so limited. In alternate embodiments, the provider interface created by
the SP module
may be any interface designed to allow the provider 101 to select and confirm
the specific
41
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
supplemental programs they would like to be delivered to the patient. For
example, the
interface may be a screen that takes up the entirety of the display device 121
or a window that
is separate from the EP module (as opposed to pop-up window 1010, which is
displayed on
top of the electronic prescription writing interface). Stated simply, the
current invention is
not limited to the type of interface generated and displayed to the provider
101.
3. Activating the Eligible Supplemental Programs that have been Confirmed
by the Health Care Provider
[0143] In general, activation of an eligible supplemental program begins when
the provider
101 actuates the confirmation mechanism 1017 after selecting the programs they
would like
to be activated for their patient. In the embodiments discussed with reference
to FIGS.
8a-8c, activation begins with step 813 and continues to step 818. However, it
should be
noted that in alternate embodiments of the present invention, activation
further includes step
819 and sometimes even step 820. Moreover, in another embodiment of the
present
invention, activation only includes step 813, which comprises the SP widget
302 generating
and transmitting an activation signal to the central portion of the SP module
301 upon the
provider 101 actuating the confirmation mechanism 1017.
[0144] Referring to FIG. 8b, after the SP widget 302 generates and transmits
the activation
signal for each of the supplemental programs, the central portion 301 of the
SP module
receives the activation signals, thereby completing step 814. Using the
received activation
signals, the central portion 301 of the SP module detennines which of the
eligible
supplemental programs the provider 101 has confirmed.
[0145] Thereafter, the central portion 301 of the SP module transmits the
relevant data for
one of the confirmed supplemental program to the appropriate third party
content provider
400, thereby completing step 815. For instance, if the confirmed supplemental
program is a
document (e.g., a coupon, educational material, EduSAVETM, etc.), then the
central portion
301 of the SP module transmits at least a request for the document(s) to the
appropriate third
party content provider 400. Similarly, if the supplemental program is a
service (e.g., a
prescription reminder service, a medication reminder service, an appointment
reminder
service, a patient adherence service, etc.), then the central portion 301 of
the SP module
transmits a request for patient enrollment in the service. Therefore,
depending on the
42
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
specific document or service requested, the central portion 301 of the SP
module transmits
the relevant request to the appropriate server 410, 420, 430, 440 of the third
party content
provider 400.
[0146] It should be noted that in some embodiments of the present invention,
the central
portion 301 of the SP module may also transmit patient delivery mode data to
the third party
content provider 400. This may be required if the third party content provider
400 is to
deliver the content directly to the patient or enroll the patient directly
into the service.
[0147] Upon receiving the request from the central portion 301 of the SP
module, the
appropriate third party content provider 400 determines whether the request is
for a document
or a service. If the request is for a document, then the third party content
provider 400
generates the document and returns the document to the central portion 301 of
the SP module.
If the request is for a service, then the third party content provider 400
configures the service
and transmits a configuration signal back to the central portion 301 of the SP
module.
Specifically, the corresponding document or service is retrieved from the
appropriate one of
the databases 401, 402, 403, 404.
[0148] Thereafter, the central portion 301 of the SP module receives the
document(s) and/or
enrollment confirmation signal from the third party content system 400,
thereby completing
step 816. Next, the central portion 301 of the SP module deteimines if there
are any other
eligible supplemental programs for which a request has yet to be delivered to
the third party
vendor system 400 at decision step 817. If there are additional confirmed
supplemental
programs for which relevant data has not yet been transmitted to the third
party content
system 400, then the process returns to step 815 and the central portion 301
of the SP module
transmits the relevant data for another of the confirmed supplemental program
to the
appropriate third party content provider 400. However, if the relevant data
has been
transmitted to the third party content system 400 for each of the confirmed
supplemental
programs, then the process continues to step 819.
[0149] It should be noted that in one embodiment of the present invention, the
central portion
301 of the SP module transmits the relevant data for all of the confirmed
supplemental
programs to the appropriate third party content provider 400 at step 815. In
such instances,
decision step 817 may be omitted.
[0150] Therefore, after a request has been delivered by the central portion
301 of the SP
43
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
module to the appropriate third party content provide 400 for all of the
eligible supplemental
programs that were confirmed by the provider 101, the SP module has activated
each of the
supplemental programs. Generally, by activating a supplemental program the SP
module
either receives content, such as a document to the patient, that is to be
delivered to the patient
or enrolls the patient in one of the aforementioned services via the
appropriate third party
content provider 400.
[0151] A non-limiting list of examples whereby the SP module activates a
supplemental
program is discussed below. It should be noted that the invention is not
limited to the
explicit examples presented herein. In one embodiment, in which the
supplemental program
is a coupon service, the SP module activates the supplemental program by
retrieving coupon
data relating to the prescribed substance from the coupon database 401 of the
appropriate
third party content provider 400 and integrating the coupon data into the
electronic
prescription. The integration of the coupon data into the electronic
prescription is done by
the SP widget 302, which is integrated into the thin-client portion 320 of the
EP module
residing on the HCP system 100. Thereafter, the HCP system 100 may transmit
the
electronic prescription with integration coupon to the pharmacy system 500 for
further
processing.
[0152] In another embodiment, in which the supplemental program is a coupon
service, the
SP module activates the supplemental program by retrieving the coupon data and
provisioning a coupon based on the coupon data. Further, in one embodiment,
activation
further includes the SP module delivering the coupon to the patient via the
selected delivery
mode. For instance, the coupon may be delivered to the IICP system 100 so the
provider
101 may print the coupon out for the patient using the printer 130, or the
coupon may be
delivered directly to the patient via one of the delivery modes discussed
above.
[0153] For further example, in one embodiment in which the supplemental
program is a
prescribed substance education service, the SP module activates the
supplemental program by
retrieving educational content relating to the prescribed substance from the
educational
information database 402 of the appropriate third party content provider 400.
Thereafter,
according to one embodiment, activation may further include the SP module
delivering the
education content to the patient via the selected delivery mode. Therefore,
the education
content may be delivered to the patient by transmitting the educational
content to the HCP
system 100 so the content may be printed by the provider 101 for the patient
using the printer
44
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
130, or the educational content may be delivered directly to the patient via
one of the delivery
modes discussed above.
[0154] Additionally, in another embodiment in which the supplemental programs
is a
prescribed substance education service and/or a coupon service, the SP module
activates the
supplemental program by retrieving education content relating to the
prescribed substance
from the educational information database 402 of the appropriate third party
content provider
400 and integrating the educational content into a coupon (such as the
EduSAVETm
document shown in FIG. 9). Further, according to one embodiment of the present
invention,
activation may further include delivering the combined educational coupon to
the patient via
the selected delivery mode. Similarly, the combined educational coupon may be
delivered
to the patient by transmitting the educational content to the HCP system 100
so the
educational coupon may be printed by the provider 101 for the patient using
the printer 130,
or the educational coupon may be delivered directly to the patient via one of
the delivery
modes discussed above.
[0155] In one embodiment, in which the supplemental program is a patient
adherence service,
the SP module activates the supplemental program by enrolling the patient in
the patient
adherence service. According to another embodiment of the present invention,
in which the
activated supplemental program is a prescription reminder service, the SP
module activates
the supplemental program by enrolling the patient in the prescription reminder
service. In
yet another embodiment of the present invention, in which the activated
supplemental
programs is an appointment reminder service, the SP module activates the
supplemental
program by enrolling the patient in the reminder service.
[0156] In the exemplified embodiments, the SP module enrolls the patient in
the service by
transmitting the relevant data to the appropriate third party content provider
400, and the
appropriate third party content provider 400 signs the patient up for the
service. For
example, the relevant data may include data relating to the patient, data
relating to the
patient's past adherence, data relating to the electronic prescription, and
data relating to the
patient's appointment schedule. However, in alternate embodiments of the
present
invention the SP module may enroll the patient into the service without the
use of the
appropriate third party content provider 400. In such embodiments, the SP
module may
further comprise an enrollment engine in order to effectuate the enrollment of
the patient in
the appropriate service directly.
CA 02918798 2016-01-19
WO 2015/013695
PCT/1JS2014/048330
[0157] For example, in embodiments where the SP module further comprises an
enrollment
engine, the central portion 301 of the SP module would effectuate the
enrollment of patients
into the services that were activated for them, without the need of the SP
module transmitting
patient enrollment data to a third party content provider 400.
[0158] Referring back to FIG. 8c, after the SP module is done activating the
eligible
supplemental programs that have been confirmed by the health care provider
101, the SP
module tailors the content relating to each of the activated supplemental
programs for the
specific delivery mode that was selected and confirmed by the provider 101,
thereby
completing step 819. Generally, the central portion 301 of the SP module
alters the specific
document depending on the specific delivery mode selected and confirmed by the
provider
101. For instance, if the delivery mode is selected to be via email, then the
content is
configured to be most easily viewable by a web browser. If the delivery mode
is selected to
be via text/SMS to the patient's mobile phone, then the content is configured
to be most
easily viewable on the smaller screen of a mobile device. Further, if the
delivery mode is
selected so that the content is printed at the printer 130, then the content
is configured to be
most easily printed.
[0159] It should be noted that if the supplemental program is a service, the
step of tailoring
the content is typically not be performed. However, in some embodiments, the
SP module
may tailor a confirmation message of the patient's enrollment in the service
for delivery to
the patient via the specific delivery mode selected and confirmed above.
[0160] After the SP module tailors the content for the selected and confirmed
delivery mode,
the SP module delivers the content of each of the activated supplemental
programs to the
patient via the selected and confirmed delivery modes, thereby completing step
820.
Generally, the central portion 301 of the SP module will delivery the content
if the selected
delivery mode is to the patient's mobile phone, email, or mailing address.
However, if the
selected delivery mode is for the content to be printed at the printer 130,
then the central
portion 301 of the SP module will transmit the content to the SP widget 302
residing on the
HCP system 100, and the SP widget 302 thereby effectuates the printing of the
content by a
printer 130 of the HCP system 100.
[0161] As noted above, according to one embodiment of the present invention,
one or both of
steps 819 and 820 may be considered part of the activation step performed by
the SP module.
However, as also noted above, the invention is not so limited and the
processing performed
46
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
by steps 819 and 820 may also be considered separate, subsequent steps that
are performed
after the activation step of the SP module.
[0162] In one alternate embodiment, the supplemental program data relating to
all of the
available supplemental programs resides on the SP system 300 in its one or
more databases.
In such embodiments, the third party vendor system 400 is omitted, and the
central portion
301 of the SP module does not have to reach out to the third party vendor
system 400 to
provide the patient with the documents or enroll the patient in the services.
[0163] In another embodiment of the present invention, the third party vendor
system 400
may transmit the document directly to the patient or enroll the patient in the
service upon
receiving the request and the patient delivery module data. Therefore, in such
embodiments,
the central portion 301 of the SP module does not receive a document or
confirmation signal
from the third party content provider 400.
[0164] Moreover, it should be noted that some of the services require the
patient to confirm
their enrollment in the service. Therefore, enrolhnent cannot be fully
effectuated by the SP
module or the third party vendor system 400. In such instance, the SP module
or the third
party vendor system 400 would transmit the appropriate confirmation to the
patient via the
delivery mode chosen by the provider. Thereafter, if the confirmation is
received by the SP
module, then the SP module would transmit another enrollment signal back to
the third party
content provider 400. However, if the confirmation is received by the third
party content
provider 400, then the third party content provider 400 would enroll the
patient in the service
upon receiving the confirmation from the patient.
[0165] Further, in one embodiment of the present invention, a clinical staff
personnel may
perform the steps initiated by the provider 101. A clinical staff personnel
may be a nurse,
an office or hospital administrator, or any other personnel involved in the
health care industry.
In such embodiments, the clinical staff personnel would choose a previously
prescribed
substance to begin the process. Thereafter, the process would continue as
described above,
ultimately resulting in the patient receiving a document (e.g., coupon,
educational material,
etc.) or being enrolled in a service.
[0166] Finally, it should be noted that the SP system 300, and specifically
the SP module, of
the present invention further comprises control and management options for the
provider 101
or an administrator of the IICP system 100. Therefore, using the control and
management
47
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
options, the provider 101 or administrator may adjust the look, functionality,
and processes of
the SP module, including but not limited to, adding, removing, or editing
provider and patient
preferences, altering the GUIs generated and displayed by the SP module, etc.
[0167] Referring now to FIG. 16, a flow diagram of one method of acquiring
patient
medication history data according to an embodiment of the present invention is
illustrated.
As shown, the process begins when the SP module residing on the SP system 300
retrieves
data relating to an electronic prescription from the EP module, thereby
completing step 1601.
This may be accomplished in a manner similar to as discussed above.
[0168] Upon receiving the electronic prescription data, the SP module parses
the data to
determine information relating to the patient, such as, but not limited to the
name of the
patient, the age of the patient, and other identifying information. Further,
the SP module
may also parse the electronic prescription data to determine information
relating to the
prescribed substance, the provider, and/or the payor. Next, the SP module
transmits the
retrieved patient data (potentially along with other relevant data) to a
Medication History
Poller System 350 (also referred to herein as Medication Hx Poller for
brevity), thereby
completing step 1602.
[0169] The Medication History Poller System 350 receives and stores the
patient data. Next,
the Medication History Poller System transmits some of the patient data along
with a request
for patient medication history information to the pharmacy system 500 and/or
the payor
system 600, thereby completing step 1603. Thereafter, the Medication History
Poller
System 350 receives medication history data relating to the patient from the
pharmacy system
500 and/or the payor system 600. It should be noted that in other embodiments
of the
present invention, the Medication History Poller System may be part of the SP
module.
Further, it should be noted that, as discussed above, the pharmacy system 500
may comprise
a prescription routing sub-system 501 (e.g., Surescripts ) and the
prescription filling
sub-systems 502.
[0170] Upon receiving the medication history data relating to the patient, the
Medication
History Poller System transmits the medication history data relating to the
patient back to the
SP module residing on the SP system 300. It should be noted that in some
embodiments of
the present invention, the Medication History Poller System parses and
analyzes the
medication history data relating to the patient to determine adherence data
relating to the
patient, including but not limited to, the patient's adherence history in
general, the patient's
48
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
adherence history over a specific time frame, and/or the patient's adherence
history in
relation to a specific prescribed substance or plurality of prescribed
substances for the same
disease state.
[0171] Upon receiving the medication history data relating to the patient from
the Medication
History Poller System, the central portion 301 of the SP module uses the
patient's medication
history data when determining eligibility of each of the plurality of
available supplemental
programs. Further, the central portion 301 of the SP module may further store
the patient's
medication history information in either or both of the records database 304
or a patient
adherence database residing within the memory 313 of the SP system 300.
[0172] Referring now to FIGS. 17-28, event diagrams for supplementing patient
and provider
interactions to increase patient adherence according to other embodiments of
the present
invention are illustrated. It should be noted that the diagrams and methods
described in
reference to FIGS. 17-28 are in no way limiting of the present invention.
[0173] Referring to FIG. 17, an event diagram of one method for acquiring
provider 101
preference data according to an embodiment of the present invention is
illustrated. The
method of FIG. 17 begins when the health care provider 101 logs into the EP
module using
their terminal 120. Thereafter, the EP module displays the startup screen to
the provider
101 on the display device 121. Next, the EP module prompts the SP widget 302
to acquire
provider preference information from the SP module. Upon being prompted by the
EP
module, the SP widget 302 calls the central portion 301 of the SP module.
Specifically, as
exemplified in FIG. 17, the SP widget 302 calls a layout portion of the
central portion 301 of
the SP module to set the provider's preferences.
[0174] According to one embodiment of the present invention, the central
portion 301 of the
SP module comprises two sub-portions, a layout and an adapter. The layout of
the SP
module generates the GUIs that are displayed to the provider 101 via the
display device 121.
The adapter of the SP module performs the transmission and receipt of data
between the
central portion 301 of the SP module and the other modules and systems of the
system 1000.
[0175] After being called by the SP widget 302, the layout calls the adapter
to set the
provider's preferences. Thereafter, the adapter obtains a plurality of
different provider
preferences offered by the SP module. Next, the layout determines whether any
provider
preferences had been previously set by the provider 101 by searching the
records database
49
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
304 of the SP system 300. If all of the preferences have been set by the
provider 101, then
the process ends. However, if there is at least one unset provider preference,
then the layout
generates a GUI comprising the unset preferences and transmits the GUI to the
SP widget
302. Upon receiving the GUI, the SP widget 302 displays the GUI comprising the
unset
provider preferences to the provider 101 via the display device 121.
[0176] Next, the provider 101 sets their preferences using the input means 122
and via the
GUI displayed on the display device 121. Thereafter, the SP widget 302
transmits a signal
to the layout to set the provider's preferences. Upon receiving the provider's
preferences,
the layout calls the adapter to set the provider's preferences, and the
adapter stores the
provider preference information in the records database 304 of the SP system
300.
[0177] Referring to FIG. 18, an event diagram of one method for storing
provider 101
preference data according to an embodiment of the present invention is
illustrated. The
method begins after the provider 101 selects their preferences in an
appropriate GUI
generated by the SP module and displayed via the display device 121. After
selecting their
preferences, the provider 101 actuates a save button on a GUI displayed by the
SP widget 302.
Actuating the save button causes the SP widget 302 to call the layout of the
SP module,
which in turn calls the adapter of the SP module, which causes the SP module
to store the
prescribed preference data in the records database 304.
[0178] Referring to FIG. 19, an event diagram of one method for cancelling
provider 101
preferences according to an embodiment of the present invention is
illustrated. The method
begins when the provider 101 actuates a cancel button on a GUI displayed by
the SP widget
302. This causes the SP widget to close or cancel out of the preference GUI.
[0179] Referring to FIG. 20, an event diagram of one method for selecting
supplemental
programs according to an embodiment of the present invention is illustrated.
The method
begins when the provider 101 uses the EP module to create a new electronic
prescription for a
substance. After creating the electronic prescription, the provider 101 has
the ability to
modify the prescription using the EP module. Thereafter, the EP module prompts
the SP
widget 302 with data relating to the electronic prescription. After being
prompted by the EP
module, the SP widget 302 retrieves electronic prescription data relating to
the electronic
prescription from the EP module. Upon retrieving the electronic prescription
data, the SP
widget 302 transmits the data to the layout of the SP module, which in turn
transmits the
electronic prescription data to the adapter of the SP module.
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
[0180] Upon receiving the electronic prescription data, the adapter determines
if there are
any programs, out of the plurality of available supplemental programs, for
which the patient
and the electronic prescription are eligible. If there are not any eligible
programs, then the
process ends. However, if there are eligible supplemental programs, then the
layout of the
SP module formats the supplemental program option in a created GUI. Formatting
may
include a selection of the available delivery modes of each supplemental
program and the
generation of the GUIs that are to present the eligible supplemental programs
to the provider
101. At least one GUI is then displayed by the SP widget 302 to the provider
101, so that
the provider 101 may select and confirm which of the eligible supplemental
programs they
would like activated for the patient.
[0181] After the provider 101 makes a selection of eligible supplemental
programs, the SP
widget 302 transmits the provider's selections to layout of the SP module. The
layout then
transmits the provider's selection to the adapter. Thereafter, the adapter
retrieves the
selected supplemental programs from the supplemental program database 303 and
transmits
the selected eligible supplemental programs to the SP widget 302 for display
to the provider
101 via the display device 121. Thereafter, the provider 101 may print the
eligible
supplemental programs for delivery to the patient.
[0182] Referring to FIG. 21, an event diagram of another method for selecting
supplemental
programs according to an embodiment of the present invention is illustrated.
The method of
FIG. 21 is vastly similar to the method of FIG. 20. It should be noted that
processes
performed by the layout of the SP module in FIG. 20 are instead performed by
the SP widget
302 in the method of FIG. 21. Such a system and method may be preferred to
reduce the
processing that occurs outside of the HCP system 100.
[0183] Referring to FIG. 22, an event diagram of one method for presenting
unexercised
options according to an embodiment of the present invention is illustrated. An
unexercised
option is an eligible supplemental program that the provider 101 did not
select and confirm
for activation. In one embodiment, the provider 101 may go back at a later
time, and select
unexercised options for activation by the SP module. This allows the provider
101 to
activate supplemental programs for a patient outside of the prescription
writing workflow.
[0184] According to one embodiment of the present invention, the method begins
when the
provider 101 selects and views a prescription report generated and display by
the EP module.
The EP module displays a prescription report that comprises icon placeholders,
the icon
51
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
placeholders representing prescriptions that the provider 101 previously
selected for a
subsequent selection of eligible supplemental programs. Next, the EP module
prompts the
SP widget 302 with report prescription identification data. Although
exemplified as being
displayed by the EP module, it should be noted that in alternate embodiments,
the
prescription report may be generated and displayed by the SP module.
[0185] Upon receiving the report prescription identification data, the SP
widget 302 calls the
layout of the SP module for the unexercised options that relate to each of the
prescriptions
identified by the report prescription identification data. Thereafter, the
adapter obtains the
unexercised options off the identified prescriptions, and the layout generates
HyperText
Markup Language (HTML) for placeholders for the unexercised options. Finally,
the SP
widget 302 instantiates icon Uniform Resource Locators (URLs) for the
prescription report.
[0186] Referring to FIG. 23, an event diagram of one method for displaying
supplemental
program options according to an embodiment of the present invention is
illustrated. This
process begins when the provider 101 actuates a program icon from the
prescription report,
discussed above with reference to FIG. 22. The EP module receives the
provider's input
and prompts the SP widget 302 with the prescription identification. The SP
widget 302 then
obtains the prescription content from the prescription identification,
retrieves the
supplemental program options for the prescription and displays the
supplemental program
options to the provider 101 in an overlay, similar to that exemplified in FIG.
15. It should
be noted that the SP widget 302 does not have to reach back out to the central
portion of the
SP module, because in such embodiments, the SP widget 302 comprises local
memory in
which the program options are stored.
[0187] Referring to FIG. 24, an event diagram of one method of the adapter
acquiring
unexercised options according to an embodiment of the present invention is
illustrated. The
method exemplified in FIG. 24 is used when the SP widget 302 does not have
stored the
unexercised options of the prescriptions identified in the prescription report
cached locally on
the HCP system 100. As shown, the method of FIG. 24 begins with the adapter
retrieving
the unexercised options. Next, the adapter of the SP module checks to see if
the prescription
statuses are cached, and determines whether all the prescriptions have
statuses that are cached.
If not, then the adapter calls the SP module to get prescription statuses for
all uncached
prescriptions, and the SP module retrieves the prescription statuses from the
records database
304. Thereafter, the adapter combines the statuses of the prescriptions and
returns ITRLs for
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
the unexercised options of each of the electronic prescriptions to the SP
widget 302 for
provider input.
[0188] Referring to FIG. 25, an event diagram of one method for displaying
supplemental
program options according to an embodiment of the present invention is
illustrated. The
method begins with the SP widget 302 displaying supplemental program options
to the
provider 101 via the display device 121. The SP widget 302 then reads the
electronic
prescription context XML and calls the adapter of the central portion 301 of
the SP module to
get the options for the supplemental program. The SP module then evaluates the
prescription context using the rules engine to obtain a list of eligible
supplemental programs
for the electronic prescriptions. After a list is obtained, the SP module
obtains preference
data relating to the provider 101 and the patient and composes a display for
the supplemental
program options. Finally, the SP widget receives a GUI comprising the
supplemental
program options and displays the GUI to the provider 101 via the display
device 121.
[0189] Referring to FIG. 26, an event diagram of one method for acquiring
patient preference
data according to an embodiment of the present invention is illustrated. The
method begins
with the adapter of the SP module receiving a request for patient preference
data from the SP
widget 302. The adapter determines whether the patient preferences are cached,
and if so
the adapter retrieves the patient preferences from the cached memory. If not,
then the
adapter retrieves the patient preferences from the record database 304.
[0190] Referring to FIG. 27, an event diagram of another method for acquiring
provider 101
preference data according to an embodiment of the present invention is
illustrated. The
method of FIG. 27 is very similar to that of FIG. 26. The method begins with
the adapter of
the SP module receiving a request for provider preference data from the SP
widget 302.
The adapter determines whether the provider preferences are cached, and if so
the adapter
retrieves the provider preferences from the cached memory. If not, then the
adapter
retrieves the provider preferences from the record database 304.
[0191] Referring to FIG. 28, an event diagram of one method for acquiring
supplemental
programs from a third party content provider 400 according to an embodiment of
the present
invention is illustrated. The method begins with the adapter calling the SP
module for
supplemental program documents that have been selected and confirmed by the
provider 101.
The SP module obtains one supplemental program at a time. The SP module first
determines which supplemental program to fetch and then retrieves the third
party content
53
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
provider 400 identifiers for that particular supplemental program. The
identifiers comprise
information relating to the third party content provider 400 that stores the
supplemental
program. After retrieving the identifiers, the SP module calls the third party
content
provider system 400.
[0192] Upon receiving the signal from the SP module, the third party content
provider 400
determines whether the request is for a document or a service based on the
identifier of the
supplemental program. If the request is for a document, then the third party
content
provider 400 generates the document. If the request is for a service, then the
third party
content provider 400 configures the service for the patient. Thereafter, the
third party
content provider 400 transmits a response to the SP module. The SP module then
adds the
document to a response and repeats the process for each of the supplemental
programs that
were selected and confirmed by the provider 101. After documents for all of
the
supplemental programs has been received by the SP module, the SP module
returns the
documents to the adapter, which in turn returns the documents to the SP widget
302 for
presentation to the provider 101 via display device 121 and provider input.
[0193] Referring to FIG. 29, a schematic diagram of a system for increasing
patient
adherence through the activation of supplemental programs according to another
embodiment
of the present invention is illustrated. As shown, the system comprises an
IICP system 100,
an EP system 200, an SP system 300, and third party content providers 400.
EduSave m
[0194] Referring to FIG. 9, an EduSAVETM 900 document according to one
embodiment of
the present invention is illustrated. The EduSAVETM 900 document is one type
of
combined educational coupon document and comprises a general information
section 901, an
educational information section 902, and a coupon section 903. The general
information
section 901 may comprise information relating to the health care provider 101
who issued the
prescription for the patient, information relating to the patient, information
relating to the
pharmacy filling sub-system 502, and/or information relating to the patient's
payor (e.g.,
health insurance company). The educational information section 902 comprises
either
general and/or specific information relating to the substance being prescribed
and/or the
disease state of the patient for which the substance is being prescribed.
Finally, the coupon
section 903 comprises coupon infoimation relating to a discount, a rebate, or
a voucher for
the patient for the substance being prescribed. Although described as
combining general
54
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
information, educational information, and coupon information, it should be
noted that in
alternate embodiments of the present invention, the EduSAVETM 900 document may
comprises just educational infoimation and coupon infoimation, or any
combination of health
care provider 101 information, patient information, payor infoimation, and
pharmacy
information along with educational information and coupon information.
[0195] One reason the EduSAVETM 900 document is beneficial is because it
provides for a
single document that comprises both educational information and coupon
information.
Therefore, when the patient brings the EduSAVETM 900 document to their
pharmacy for
redemption of the coupon, they are encouraged to read the educational
information provided
therewith. Further, if the patient has any questions or concerns regarding the
substance after
they have left the provider's office, the patient may easily access the
provider's contact
information on the EduSAVETM 900 document.
[0196] According to one embodiment of the present invention, after an
electronic
prescription for the substance is created by a health care provider 101, the
SP widget 302
retrieves data relating to the electronic prescription from the thin-client
portion of the EP
module 203. The SP widget 302 then transmits the electronic prescription data
to the central
portion 301 of the SP module residing on the SP system 300, such that the
central portion 301
of the SP module receives the electronic prescription data. The electronic
prescription data
comprises first patient data that is specific to the patient, first prescribed
substance data that is
specific to the prescribed substance, first provider data that is specific to
the provider 101,
and first payor data that is specific to the payor. It should be noted that
the specifics of the
electronic prescription data are discussed in detail above.
[0197] However, the invention is not so limited, and in an alternate
embodiment of the
present invention, the electronic prescription data may not relate to an
electronic prescription
currently being prescribed by a health care provider 101 for a patient, but
rather relate to a
refill, a renewal, or a previously prescribed substance. In such embodiments,
the electronic
prescription data may be received by the SP module from one of the other
databases of the
system 1000 (e.g., the EP database 201, the records database 304, the patient
prescription
history database 505, etc.). Stated another way, the electronic prescription
data may, but
does not necessarily have to be generated by a health care provider 101.
[0198] Once the central portion 301 of the SP module receives the electronic
prescription
data, the central portion 301 of the SP module retrieves additional data prior
to determining
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
educational data and coupon data for the prescribed substance. The additional
(or second)
data may comprise one or more of patient data, prescribed substance data,
provider data, and
payor data. Further, the additional (or second) patient data may comprise
patient adherence
data. According to one embodiment of the present invention, the additional (or
second) data
is retrieved by the SP module from the records database 304 of the SP system
300.
However, it should be noted that the invention is not so limited and in
alternate embodiments,
the central portion 301 of the SP module may only retrieve a portion of the
data listed herein
or may not retrieve any additional data prior to determining educational data
and coupon data
for the prescribed substance.
[0199] After receiving electronic prescription data, and possibly after
retrieving additional
data from the records database 304, the SP module determines educational data
relating to the
prescribed substance and coupon data relating to the prescribed substance. In
one
embodiment of the present invention, the deteimination is accomplished by the
central
portion 301 of the SP module in response to receiving the electronic
prescription data. Thus,
it may be said that the SP module determines the educational data and coupon
data
automatically upon receiving electronic prescription data according to one
embodiment of the
present invention. In one embodiment of the present invention, the
determination of both
the educational data and coupon data comprises the central portion 301 of the
SP module
searching one or more databases, such as the supplemental program database 303
or one of
the databases 401, 402, 403, 404 of the appropriate third party content
provider 400, for
educational data and coupon data both relating to the prescribed substance. It
should be
noted that the determination of both the educational data and coupon data may
be
accomplished by the central portion 301 of the SP module using any combination
of the first
patient data, first prescribed substance data, first provider data, and first
payor received from
the electronic prescription data, potentially along with any of the additional
(or second) data
(patient data, prescribed substance data, provider data, and payor data)
retrieved by the SP
module.
[0200] In one embodiment of the present invention, the determination of
educational data and
coupon data for the prescribed substance requires the central portion 301 of
the SP module to
determine both educational data and coupon data for which the patient is
eligible based on the
electronic prescription. This is similar to as discussed above with reference
to the
determination of eligible supplemental programs. Therefore, in such
embodiments, the
56
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
educational data and coupon data may comprise rules that must be met in order
for the
educational data and coupon data to be eligible for the electronic
prescription of the patient.
Thus, in accordance with one embodiment of the present invention, the
determination of both
the educational data and coupon data may be accomplished by the SP module in a
manner
similar to as discussed above with reference to supplemental programs (FIG. 8a
and steps
806-808).
[0201] However, the invention is not so limited, and in another embodiment of
the present
invention, the SP module simply transmits any combination of data (electronic
prescription
data and additional, retrieved data) to a third party system. This, in turn,
causes the third
party system to determine the educational data and/or the coupon data upon
receiving the
appropriate data from the SP module. Thus, in this embodiment of the present
invention,
the third party system determines the educational data and/or the coupon data
that relates to
the prescribed substance. Finally, upon determining the educational data
and/or the coupon
data, the third party system transmits the educational data and/or the coupon
data to the SP
module. Therefore, it may be said that the central portion 301 of the SP
module receives the
educational data and/or the coupon data from one or more of the databases 401,
402, 403, 404
of the appropriate third party content provider 400.
[0202] Upon determining the educational data and coupon data that relates to
the prescribed
substance, the central portion 301 of the SP module retrieves from one or more
databases,
such as the supplemental program database 303 or one of the databases 401,
402, 403, 404 of
the appropriate third party content provider 400, the educational data and the
coupon data.
Thereafter, the central portion 301 of the SP module combines the educational
data and the
coupon data into a single data file, which may be considered a combined
educational coupon
since the file comprises both educational data and coupon data. It should be
noted that
when the central portion 301 of the SP module combines the educational data
and the coupon
data into a single data file, the central portion 301 of the SP module may
combine a portion
of or the entirety of the educational data and/or coupon data. Thus, the
single data file may
comprise just a portion of either or both of the educational data and the
coupon data.
Further, in one embodiment of the present invention, the single data file may
be a text-based
file, an image-based file, or a combined text and image based file. One
example of a
combined educational coupon is the EduSAVErm 900 document exemplified in FIG.
9.
[0203] Further, in one embodiment of the present invention, prior to
generating a single data
57
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
file comprising the educational data and the coupon data, the central portion
301 of the SP
module presents to a health care provider 101, in the display device 121, a
list of the
education data and the coupon data for selection and acceptance by the health
care provider
101. Thereafter, the central portion 301 of the SP module generates the single
data file, or
the combined educational coupon, upon both the educational data and the coupon
data being
selected and accepted by the health care provider 101. It should be noted that
this may be
accomplished in any manner discussed above with reference to steps 811-814 of
FIG. 8b.
Finally, in one embodiment of the present invention, the central portion 301
of the SP module
determines more than one separate portion of educational data and/or coupon
data and
presents the one or more educational data and/or coupon data to the health
care provider 101
in a list via the display device 121. '[hereafter, the health care provider
101 may select one
or more of the educational data and/or coupon data, and the central portion
301 of the health
care provider 101 combines the one or more of the educational data and/or
coupon data into a
single data file, the single data file being a combined educational coupon.
Thus, the
combined educational coupon is not limited to just one educational data file
and one coupon
data file, but may comprise more than one educational data file and/or coupon
data file.
Finally, after generating the combined educational coupon, the central portion
301 of the SP
module may then store the combined education coupon in one or more databases
(e.g., the
records database 304 or the supplemental program database 303) of the SP
system 300 for
subsequent applications.
[0204] Finally, after the central portion 301 of the SP module has generated
the single data
file comprising the educational data and coupon data relating to the
prescribed substance, the
central portion 301 of the SP module may transmit the single data file to one
or more systems
or devices. These include, but are note limited to, a pharmacy filling sub-
system 502, an
IICP system 100, and a patient computer device. It may be beneficial to
transmit the single
data file to a pharmacy filling sub-system 502 so that the patient does not
have to remember
to bring the combined educational coupon with them when picking up the
prescription.
Rather, the combined educational coupon is waiting for them at the pharmacy
filling
sub-system 502, such that the pharmacist may automatically deduct the coupon
from the
purchase price of the prescription and the educational material may be
provided to the patient
upon receiving their prescription. Further, it may be beneficial to transmit
the single data
file to the HCP system 100 so that the HCP system 100 may make the combined
educational
coupon available to patient while they are still in the presence of their
health care provider
58
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
101. Therefore, if the patient has any questions or concerns, they may
inunediately speak
with their health care provider 101 upon receiving the combined educational
coupon.
Finally, it may be beneficial to transmit the single data file to a patient
computer device, such
as a personal computer, smart phone, printer, fax machine, or other electronic
device owned
or controlled by the patient. This allows the patient to receive and view the
combined
educational coupon at their convenience.
[0205] Nonetheless, it should be noted that in accordance with one embodiment
of the
present invention, the central portion 301 of the SP module generates a
combined educational
coupon using the process described above with respect to supplemental
programs. In such
embodiments, the central portion 301 of the SP module parses out educational
information
data from an eligible supplemental program that relates to educational
material, and coupon
data from an eligible supplemental program that relates to a coupon.
Thereafter, the central
portion 301 of the SP module combines the educational information data and the
coupon data,
potentially along with other general data (e.g., provider data, patient data,
etc.) into a single
data file to create the combined educational coupon. Thereafter, the central
portion 301 of
the SP module may store the combined education coupon in the supplemental
program
database 303 of the SP system 300 for subsequent applications. Further, after
creating the
combined educational coupon, the central portion 301 of the SP module may
transmit the
combined educational coupon to the HCP system 100 as a single data file.
[0206] Further, it should be noted that in one embodiment of the present
invention, the
central portion 301 of the SP module may be considered a non-transitory
computer-readable
storage medium that is encoded with instructions which, when executed by the
processor 311,
perform a method of receiving data relating to an electronic prescription of a
prescribed
substance for a patient; searching one or more databases for educational data
relating to the
prescribed substance and coupon data relating to the prescribed substance;
determining
educational data relating to the prescribed substance and coupon data relating
to the
prescribed substance; retrieving from the one or more databases the
educational data relating
to the prescribed substance and the coupon data relating to the prescribed
substance; and
generating a single data file comprising the educational data relating to the
prescribed
substance and the coupon data relating to the prescribed substance.
[0207] Finally, it should be noted that in one embodiment of the present
invention, the SP
system 300 may be considered a computer system for electronically generating
educational
59
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
coupons for a prescribed substance, which comprises a processor 311; a storage
device 313; a
network interface 312; and instructions residing on the storage unit 313,
which when
executed by the processor 311, causes the processor 311 to: a) receive
electronic prescription
data for a prescribed substance for a patient; b) determine educational data
relating to the
prescribed substance and coupon data relating to the prescribed substance; and
c) generate a
single data file comprising the educational data relating to the prescribed
substance and the
coupon data relating to the prescribed substance.
Tailoring General and Specific Educational Content
[0208] As noted above, the supplemental programs comprise both general
educational
content and specific educational content. In one embodiment of the present
invention, the
general educational content comprises information relating to the prescribed
substance, while
the specific educational content comprises information relating, not only to
the prescribed
substance, but also the specific diagnostic reason(s) that the substance was
prescribed to the
patient, such as but not limited to, the specific disease(s) for which the
substance was
prescribed, along with a wide variety of signs, symptoms, abnormal findings,
complaints,
social circumstances, and external causes of injury or disease for which the
substance was
prescribed. Thus, the educational content (or material), both general and
specific, is tailored
to the specific needs and diagnoses of the patient.
[0209] Similar to as discussed above and according to one embodiment of the
present
invention, after an electronic prescription for the substance is created by a
health care
provider 101, the SP widget 302 retrieves data relating to the electronic
prescription from the
thin-client portion of the EP module 203. Further, the SP widget 302 may also
retrieve a
diagnostic code that is entered by the health care provider 101. Specifically,
the provider
101 enters a diagnostic code into the SP widget 302 using the input device 122
of the
terminal 120. The diagnostic code may be entered prior to, during, or after
the creation of
the electronic prescription by the health care provider 101.
[0210] A diagnostic code is a string of characters and/or numbers that are
used to represent
medical diseases and/or a wide variety of signs, symptoms, abnormal findings,
complaints,
social circumstances, and external causes of injury or disease. According to
one
embodiment, the diagnostic code is entered or provided by the health care
provider 101 and
provides a more specific reasoning as to why the patient is being prescribed a
particular
substance. For example, in one embodiment, the diagnostic code is an
International
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
Classification of Diseases, Ninth Revision (ICD-9) code entered by the
provider 101 during
the patient's visit.
[0211] After receiving data relating to an electronic prescription and a
diagnostic code, the
SP widget 302 then transmits the electronic prescription data and the
diagnostic code to the
central portion 301 of the SP module residing on the SP system 300, such that
the central
portion 301 of the SP module receives the electronic prescription data and the
diagnostic code.
It should be noted that the diagnostic code may, but does not necessarily have
to be received
in conjunction with the electronic prescription data. As discussed in detail
above, the
electronic prescription data comprises first patient data that is specific to
the patient, first
prescribed substance data that is specific to the prescribed substance, first
provider data that
is specific to the provider 101, and first payor data that is specific to the
payor. Further, in
one embodiment of the present invention, the electronic prescription data
further comprises a
National Drug Code identifier of the prescribed substance. The National Drug
Code
identifier is a unique product identifier for prescribed substances that are
intended for human
use. Finally, it should be noted that in one embodiment of the present
invention, the
electronic prescription data comprises the diagnostic code.
[0212] Further, in an alternate embodiment of the present invention, the
electronic
prescription data does not relate to an electronic prescription currently
being prescribed by a
health care provider 101 for a patient, but rather relates to a refill, a
renewal, or a previously
prescribed substance. In such embodiments, the electronic prescription data
may be
received by the SP module from one of the other databases of the system 1000
(e.g., the EP
database 201, the records database 304, the patient prescription history
database 505, etc.).
Stated another way, the electronic prescription data may, but does not
necessarily have to be
generated by a health care provider 101.
[0213] Once the central portion 301 of the SP module receives the electronic
prescription
data and the diagnostic code, the central portion 301 of the SP module
retrieves additional
data prior to determining general and specific educational data. As discussed
in more detail
above, the additional (or second) data may comprise one or more of patient
data, prescribed
substance data, provider data, and payor data. Further, the additional (or
second) patient
data may comprise patient adherence data. According to one embodiment of the
present
invention, the additional (or second) data is retrieved by the SP module from
the records
database 304 of the SP system 300. However, it should be noted that the
invention is not so
61
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
limited and in alternate embodiments, the central portion 301 of the SP module
may only
retrieve a portion of the data listed herein or may not retrieve any
additional data prior to
determining general and specific educational data.
[0214] After receiving electronic prescription data and the diagnostic code,
and possibly after
retrieving additional data from the records database 304, the central portion
301 of the SP
module searches one or more databases, such as the supplemental program
database 303 or
one of the databases 401, 402, 403, 404 of the appropriate third party content
provider 400,
for general educational data and specific educational data both relating to
the prescribed
substance. The general educational data relates to the prescribed substance,
but is
independent of the diagnostic code. Thus, the general educational data
comprises broad and
wide-ranging information that relates generally to the prescribed substance or
the disease
state for which the substance was prescribed to the patient. For example,
general
educational data may comprise information relating to high blood pressure in
general.
[0215] By contrast, the specific educational data relates to the prescribed
substance and is
based on the received diagnostic code. Thus, the specific educational data
comprises
specific information about the particular prescribed substance or the disease
state of the
prescribed substance, and is particular to the exact reasons for which the
provider 101 has
written the prescription for the patient. For example, specific educational
data may
comprise information relating to malignant hypertension or renal hypertension,
whereby the
general educational data simply relates to high blood pressure in a broader or
more general
sense. Stated another way, the specific educational content may relate to one
or more of the
specific disease(s) for which the substance was prescribed, and/or the signs,
symptoms,
abnormal findings, complaints, social circumstances, and/or external causes of
injury or
disease for which the substance was prescribed to the patient. Further, as
discussed in detail
above, the educational data (both general and specific) may relate to an
educational document
or an educational service, as discussed above in more detail.
[0216] It should be noted that the determination of both the general and
specific educational
data may be accomplished by the central portion 301 of the SP module using any
combination of the diagnostic code, the first patient data, first prescribed
substance data, first
provider data, and first payor received from the electronic prescription data,
potentially along
with any of the additional (or second) data (patient data, prescribed
substance data, provider
data, and payor data) retrieved by the SP module. Further, in one embodiment
of the
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
present invention, the general educational data is searched using the National
Drug Code
identifier, along with any combination of the electronic prescription data and
retrieved data
discussed above. Similarly, it should be noted that the specific educational
data is searched
using the diagnostic code received by the central portion 301 of the SP
module, along with
any combination of the electronic prescription data and retrieved data
discussed above.
[0217] In one embodiment of the present invention, in order for the central
portion 301 of the
SP module to determine both the general educational data and the specific
educational data,
the central portion 301 of the SP module must also determine which general and
specific
educational data the patient is eligible for based on the electronic
prescription. This is
similar to as discussed above with reference to the determination of eligible
supplemental
programs. Therefore, in such embodiments, the general educational data and the
specific
educational data may comprise rules that must be met in order for the general
educational
data and the specific educational data to be eligible for the electronic
prescription of the
patient. Moreover, in accordance with one embodiment of the present invention,
the
determination of both the general educational data and the specific
educational data may be
accomplished by the central portion 301 of the SP module in a manner similar
to as discussed
above with reference to supplemental programs (FIG. 8a and steps 806-808).
[0218] However, the invention is not so limited, and in another embodiment of
the present
invention, the SP module simply transmits any combination of data (electronic
prescription
data, diagnostic code, National Drug Code identifier, and additional,
retrieved data) to a third
party system. This, in turn, causes the third party system to determine the
general
educational data and/or the specific educational data upon receiving the
appropriate data from
the SP module. Thus, in this embodiment of the present invention, the third
party system
determines the general educational data and/or the specific educational data
that relates to the
prescribed substance. Finally, upon determining the general educational data
and/or the
specific educational data, the third party system transmits the general
educational data and/or
the specific educational data to the SP module. Therefore, it may be said that
the central
portion 301 of the SP module receives the general educational data and/or the
specific
educational data from one or more of the databases 401, 402, 403, 404 of the
appropriate
third party content provider 400.
[0219] Upon determining the general educational data and the specific
educational data that
relates to the prescribed substance and the diagnostic code, the central
portion 301 of the SP
63
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
module retrieves from one or more databases, such as the supplemental program
database 303
or one of the databases 401, 402, 403, 404 of the appropriate third party
content provider 400,
the general educational data and the specific educational data. According to
one
embodiment of the present invention, the central portion 301 of the SP module
retrieves two
data files, a first data file that is the general educational data and a
second data file that is the
specific education data. Thereafter, the central portion 301 of the SP module
may combine
the first data file (general educational data) and the second data file
(specific educational data)
into a single data file, thus creating a single data file that comprises both
general educational
data and specific educational data. It should be noted that when the central
portion 301 of
the SP module combines the general and specific educational data into a single
data file, the
central portion 301 of the SP module may combine a portion of or the entirety
of the general
educational data and the specific educational data. Thus, the single data file
may comprise
just a portion of either or both of the general and specific educational data.
Moreover,
according to one embodiment of the present invention, the central portion 301
of the SP
module may combine one or more general education data file with one or more
specific
educational data file. However, it should be noted that the invention is not
so limited, and in
some embodiments of the present invention, the general educational data and
the specific
educational data is not combined into a single data file.
[0220] Further, in one embodiment of the present invention, the central
portion 301 of the SP
module presents to a health care provider 101, in the display device 121, a
list that comprises
the general education data and the specific educational data for selection and
acceptance by
the health care provider 101. In such embodiments, each of the general
education data and
the specific education data presented in the display device 121 is selectable
and de-selectable
by the health care provider 101 using the input device 122. It should be noted
that the
display of the list and the selection and acceptance of the general and
specific educational
data may be accomplished in any manner similar to as discussed above with
reference to
supplemental programs in steps 811-814 of FIG. 8b. Further, it should be noted
that the list
is not limited to only one general educational data file and one specific
educational file, but
rather may include any number of general and/or specific educational files for
selection and
acceptance by the health care provider 101.
[0221] According to one embodiment of the present invention, after a list of
the general
education data and the specific educational data is presented in the display
device 121 to the
64
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
health care provider 101 for provisioning to the patient, the central portion
301 of the SP
module makes the general educational data and the specific educational data
that is selected
and confirmed by the health care provider 101 available to the patient. In one
embodiment,
this is accomplished by the SP module transmitting the general education data
and the
specific educational data that is selected and confirmed by the health care
provider 101 to a
patient computer device. This may be accomplished using at least one of email,
SMS, WAP,
and a mobile application.
[0222] Therefore, the diagnostic code may be used, in addition to the
electronic prescription
data and any additional retrieved data, by the central portion 301 of the SP
module to
determine if there is both general educational data and specific educational
data for which the
patient is eligible. Therefore, by using a diagnostic code (e.g., the ICD-9
code) to determine
if there is any eligible specific educational data relating to why the
prescribed substance was
prescribed to the patient, the present invention may provide both general and
specific
educational material to the patient. For instance, the patient may receive
general
information relating to the substance they are being prescribed or the disease
state in which
they are diagnosed, while also receiving specific educational material
directed to the specific
reason(s) the patient has been prescribed the particular substance. This is
beneficial because
the educational documents and/or services help the patient in understanding
not only their
general health concerns and prescribed substances, but also their specific
health concerns
along with their specific diagnoses. Thus, the educational material (both
general and
specific) that is made available to the patient may be tailored to the
specific needs and
diagnoses of the patient.
[0223] Further, it should be noted that in one embodiment of the present
invention, the
central portion 301 of the SP module may be considered a non-transitory
computer-readable
storage medium that is encoded with instructions which, when executed by the
processor 311,
perform a method of receiving electronic prescription data for a prescribed
substance for a
patient, said electronic prescription data including a diagnostic code;
searching one or more
databases to determine: (1) general educational data relating to the
prescribed substance
independent of the diagnostic code; and (2) specific educational data relating
to the
prescribed substance based on the diagnostic code; and presenting to a health
care provider,
in a display device, a list of the general educational data and the specific
educational data
determined in step b) for provisioning to the patient.
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
[0224] Finally, it should be noted that in one embodiment of the present
invention, the SP
system 300 may be considered a computer system for electronically generating
educational
coupons for a prescribed substance, which comprises a processor 311; a storage
device 313; a
network interface 312; and instructions residing on the storage unit 313,
which when
executed by the processor 311, causes the processor 311 to: a) receive
electronic prescription
data for a prescribed substance for a patient, said electronic prescription
data including a
diagnostic code; b) search one or more databases to determine: (1) general
educational data
relating to the prescribed substance independent of the diagnostic code; and
(2) specific
educational data relating to the prescribed substance based on the diagnostic
code; and c)
present to a health care provider, in a display device, a list of the general
educational data and
the specific educational data determined in step b) for provisioning to the
patient.
Method of Using Cohorts to Determine Effectiveness of Supplemental Programs
[0225] According to another embodiment of the present invention, the system
1000 described
above may be used to determine the effectiveness of supplemental programs on
patient
adherence through the use of a plurality of cohorts. Generally, as used
herein, a cohort is a
group of health care providers 101 who activate the same supplemental programs
for a
particular prescribed substance. As discussed in more detail below, in
accordance with one
embodiment of the present invention a plurality of cohorts, each comprising
different
permutations (or groupings) of supplemental programs, are used to determine
the respective
effectiveness of the different permutations of supplemental programs on
patient adherence to
one or more prescribed substances.
[0226] According to one embodiment of the present invention, a method of
determining the
effectiveness of a plurality of available supplemental programs on patient
adherence
comprises four steps: (1) defining a plurality of cohorts, each cohort
comprising at least one
health care provider; (2) receiving data relating to an electronic
prescription of the one or
more prescribed substances generated by a health care provider and activating
the
supplemental programs associated with the cohort to which the health care
provider belongs;
(3) receiving patient adherence data relating to electronic prescriptions for
the one or more
prescribed substances issued by the plurality of health care providers; and
(4) analyzing the
patient adherence data to determine the effectiveness of the different
permutations of the
supplemental programs on patient adherence.
[0227] Although the processes and functions described below are described and
exemplified
66
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
as being performed by the SP module in general, it should be understood that
the invention is
not so limited, and in alternate embodiments the processes and function
described herein with
reference to cohorts may be preformed by any single portion, the central
portion 301 or the
SP widget 302, or a combination of the portions of the SP module.
[0228] Referring to FIG. 4, it should be noted that the memory 313 of the
server 310 of the
SP system 300 further comprises a cohort database 305, a Patient Adherence
Generation
Module 306, and a patient adherence database 307. As explained in more detail
below, the
cohort database 305 stores, among other things, information relating to a
plurality of health
care providers 101 who are part of at least one cohort, along with information
relating to each
of the cohorts defined by the SP module. For instance, after defining the
cohorts, the SP
module stores information relating to each cohort (e.g., the target drug data,
the provider
cohort data, the assigned health care providers 101, the assigned permutation
of supplemental
programs, the cohort rules, the maximum counter, etc.) in the cohort database
305 of the SP
system 300.
[0229] As also discussed in more detail below, the Patient Adherence
Generation Module
306 receives data relating to the patient's prescription history for the
target drug from the
pharmacy system 500 and/or the payor system 600. This information is referred
to as
patient medication history data. After receiving the patient medication
history data, the
Patient Adherence Generation Module 306 stores the patient medication history
data in one
or more databases, such as, but not limited to the patient adherence database
307.
Thereafter, the Patient Adherence Generation Module 306 analyzes the patient
medication
history data to generate patient adherence data from the received patient
medication history
data. Finally, the Patient Adherence Generation Module 306 stores the patient
adherence
data in the patient adherence database 307 for further processing. In certain
embodiments,
the Patient Adherence Generation Module 306 obtains all of the drug fill data
that it can for a
patient for all drugs, not just the drugs for which the patient has
prescriptions. Although in
the exemplified embodiment the Patient Adherence Generation Module 306 does
not use the
data for drugs without a known prescription, it is contemplated that in
certain other
embodiments the Patient Adherence Generation Module 306 could use the data
from both
drugs with a known prescription and drugs without a known prescription.
[0230] Generally, and as discussed in more detail below, patient medication
history data
comprises information relating to the medication history of the patient and
the patient's fill
67
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
history for various prescriptions. As also discussed in more detail below,
patient adherence
data comprises information relating to the patient's adherence to previous
prescriptions.
Although sometimes described with reference to the target drug of a cohort, it
should be
noted that the patient medication history data and patient adherence data is
not so limited, and
in alternate embodiments of the present invention the patient medication
history data and/or
the patient adherence data may refer to prescriptions for any substances of
the patient.
Moreover, it should be noted that the patient adherence database 307 may store
patient
adherence data relating to various different cohorts for the same target drug,
along with
patient adherence data relating to various different cohorts for different
target thugs. One
non-limiting example of a Patient Adherence Generation Module 306 is the
HMACSTm
system by DrFirst .
[0231] As discussed in more detail below, the Patient Adherence Generation
Module 306
receives the patient medication history data either directly or indirectly
from another system,
such as but not limited to the pharmacy system 500 and the payor system 600.
After
receiving the patient medication history data, the Patient Adherence
Generation Module 306
generates the patient adherence data using one or more algorithms that are
stored in the
patient adherence database 307. 'thereafter, the SP module receives the
patient adherence
data from the patient adherence database 307 so that the SP module may parse
and analyze
the patient adherence data by cohort to determine the effectiveness of a
plurality of different
permutations of supplemental programs on patient adherence.
[0100] As noted above and as discussed in more detail below, the patient
adherence database
307 stores information relating to one or more previously prescribed
substances of one or
more patients, such as, but not limited to, patient medication history data
and patient
adherence data. It should be noted that in other embodiments of the present
invention,
prescription data, patient medication history data, and patient adherence data
may be stored
on separate databases.
[0232] Since, in the exemplified embodiment of FIG. 4, the Patient Adherence
Generation
Module 306 resides within the memory 313 of the SP system 300, it may be said
that the SP
system 300 receives patient medication history data, parses the patient
medication history
data, and analyzes the data to generate adherence data for the patient for the
target drug.
Nonetheless, it should be noted that in other embodiments of the present
invention, the
Patient Adherence Generation Module 306 may reside on another system or within
its own
68
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
system as part of system 1000. Similarly, in other embodiments of the present
invention,
the adherence database 307 may also reside within the memory of another system
of system
1000. For example, according to one embodiment of the present invention and as
discussed
in more detail below, the Patient Adherence Generation Module 306 resides on a
separate
system as part of the system 1000, and the adherence database 307 resides
within the memory
of the separate system, such that the Patient Adherence Generation Module 306
and
adherence database 307 reside on their own separate system (e.g., a patient
adherence system).
In one embodiment, as shown in FIG. 39 and described below, the separate
system may be
patient adherence system 360.
1. Defining a Plurality of Cohorts
[0233] As noted above, the first step of determining the effectiveness of a
plurality of
supplemental programs on patient adherence comprises the defining (or
creating) of a
plurality of cohorts, whereby each cohort comprises a plurality of health care
providers 101.
Generally, the process of defining the plurality of cohorts comprises two
steps: (1) assigning
a sub-set of a plurality of health care providers 101 to each of the plurality
of cohorts; and (2)
assigning a different permutation of supplemental programs to each of the
plurality of
cohorts.
[0234] As used herein, the plurality of cohorts is used to test the
effectiveness of different
permutations of supplemental programs on patient adherence to a particular
substance or a
plurality of prescribed substances for a particular disease state. Therefore,
each cohort out
of the plurality of cohorts (of the same program cohort group) all relate to
the same
prescribed substance(s). For example, a particular prescribed substance, such
as Lipitor ,
may be assigned to a plurality of cohorts (of the same program cohort group),
so that
different permutations of supplemental programs may be tested to determine
their
effectiveness on the patients' adherence to Lipitor . For further example, a
plurality of
prescribed substances, such as Lipitor , Lescol , Mevacor , Pravachol , and
Zocor , for the
same disease state, high cholesterol, may be assigned to a plurality of
cohorts so that different
permutations of supplemental programs may be tested to dete, ____ mine their
effectiveness on the
patients' adherence to the prescribed substance for that particular disease
state (high
cholesterol).
[0235] It should be noted that when a plurality of cohorts are all related to
one another (e.g.,
are used together as a single test group for the same prescribed substance(s))
they are
69
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
considered to be part of the same program cohort group. Therefore, according
to one
embodiment of the present invention, a program cohort group is a group of a
plurality of
cohorts for the same prescribed substance or plurality of prescribed
substances for a
particular disease state. However, it should be noted that there may be
different program
cohort groups that relate to the same prescribed substance. Further, as
discussed in more
detail below, the related program cohort group for each of the plurality of
cohorts is stored
within the cohort database 305 of the SP system 300.
[0236] As noted above, each cohort of a plurality of cohorts is assigned a sub-
set of health
care providers 101. Further, as discussed in more detail below, each sub-set
of health care
providers 101 has a commonality of prescribing factors with relation to the
prescribed
substance(s) of the program cohort group. It should be noted that although a
provider 101
may be associated with only one out of a plurality of cohorts for a particular
prescribed
substance (or plurality of prescribed substances for a particular disease
state), a provider 101
may be a part of other, unrelated program cohort groups. Stated more simply, a
provider
101 may only be associated with one cohort of a particular program cohort
group, but the
provider 101 may be associated with different cohorts of different program
cohort groups.
[0237] Referring to FIG. 30, a flow chart of a method 3000 for defining a
plurality of cohorts
of a program cohort group according to one embodiment of the present invention
is illustrated.
To begin, the SP module first receives target drug data, thereby completing
step 3001. The
target drug data comprises information relating to a particular prescribed
substance or a
plurality of prescribed substances for a particular disease state to which the
plurality of
cohorts will relate. After receiving the target drug data, the SP module
stores the target drug
data in the cohort database 305 of the SP system 300 in correlation with a
program cohort
group.
[0238] Therefore, the target drug data defines for which prescribed
substance(s) the
effectiveness of the available supplemental programs on patient adherence will
be analyzed
by the SP module. Stated another way, the SP module defines a plurality of
cohorts to
analyze the effectiveness of the available supplemental programs on patient
adherence for
only the prescribed substance(s) identified by the target drug data. For
example, using the
examples set forth above, the target drug data may be just Lipitoe) or it may
be the
combination of Lipitor , Lescol , Mevacor , Pravachol , and Zocor . For
purposes of
simplicity, the teini "target drug" will be used to denote the particular
prescribed substance or
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
the plurality of prescribed substances for a particular disease state that is
defined by the
received target drug data. Thus, the term target drug may comprise one or more
than one
prescribed substance.
[0239] According to one embodiment of the present invention, the target drug
data is
received by the SP module via inputs from the administrator of the SP system
300. It should
be noted that an administrator of the SP system 300 may be one or more
individuals who
have access to and may control the SP system 300 (including the modules
residing thereon).
In such embodiments, by defining the target drug data, the administrator of
the SP system
300 selects the particular prescribed substance(s) that will be used for the
cohorts of a
program cohort group. It should be noted that the invention is not so limited,
and in
alternate embodiments the SP module may receive the target drug data from a
pharmaceutical
company or the target drug data may be derived jointly by both the
administrator of the SP
system 300 and a third party (e.g., a pharmaceutical company).
[0240] Still referring to FIG. 30, after the SP module receives the target
drug data, the SP
module retrieves provider cohort data from the cohort database 305 of the SP
system 300,
thereby completing step 3002. As discussed in more detail below, the provider
cohort data
comprises information relating to each of the plurality of health care
providers 101, and more
specifically, comprises information relating to the provider's history of
prescribing the target
drug.
[0241] According to one embodiment of the present invention, the provider
cohort data
comprises a decile level for one or more prescribed substances and/or a
medical specialty of
the health care provider 101. As understood in the art, a decile level is a
rating or level, on a
scale from 1 to 10, for which the provider 101 has prescribed a particular
prescribed
substance or prescribed substances for a particular disease state. For
example, a provider
101 who has prescribed Lipitor more than another provider 101 will have a
higher decile
level. Therefore, the SP module assigns a sub-set of the plurality of health
care providers
101 to each of the plurality of cohorts such that the average decile level of
the health care
providers 101 assigned to each cohort of a program cohort group is similar. By
assigning
health care providers 101 to the cohorts based on their decile level, each of
the plurality of
cohorts of a program cohort group has a commonality of prescribing factors
relating to the
target drug. However, as discussed below, the invention is not so limited and
in alternate
embodiments, the SP module assigns health care providers 101 to a cohort based
on other
71
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
factors so long as each cohort of a program cohort group has a commonality of
prescribing
factors.
[0242] As noted above, the use of a decile level is one way to define the
cohorts such that
there is a commonality of prescribing factors between the health care
providers 101 of each
cohort of a program cohort group. The present invention may utilize any number
of other
methods to assign health care providers 101 such that each cohort has a
commonality of
prescribing factors. Therefore, the commonality of prescribing factors relates
to the target
drug, ensures that each cohort has a similar cross-section of providers 101 as
they relate to
the target drug, and may be defined by the SP module in any manner. For
example, the SP
module may define the commonality of prescribing factors between the plurality
of health
care provider 101 as a decile level, as a rate of prescription of the
prescribed substance(s)
defined by the target drug data, or a total number of prescriptions written by
each provider
101 for the prescribed substance(s) defined by the target drug data. A
commonality of
prescribing factors could also be determined by the age groups of the
prescriber's patients, or
age and demographics of the town in which the prescriber is located.
[0243] Still Referring to FIG. 30, after retrieving the provider cohort data
relating to each of
the plurality of health care providers 101, the SP module assigns a sub-set of
a plurality of
health care providers 101 to each of a plurality of cohorts based on the
retrieved provider
cohort data, thereby completing step 3003. Stated another way, the SP module
assigns at
least one health care provider 101, and preferably more than one health care
provider 101 to
each of the plurality of cohorts of a program cohort group. In one embodiment
of the
present invention, the SP module assigns the health care providers 101 to the
plurality of
cohorts using the provider's NPI numbers. After assigning a sub-set of the
plurality of
providers 101 to each of the cohorts of a program cohort group, the SP module
stores the
provider's NPI number in the cohort database 305 in association with the a
cohort identifier,
as discussed below in more detail. However, the invention is not so limited
and in alternate
embodiments the assignment of health care providers 101 to cohorts may be done
using name
or any other identifying factor of the health care providers 101.
[0244] Further, it should be noted that the plurality of health care providers
101 that is broken
down in sub-sets and assigned to the cohorts of a program control group may be
chosen by
the SP module via one or more methods. For example, the plurality of health
care providers
101 may be selected using geographic location, commonality of prescribing
factors, and/or
72
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
input by an administrator of the SP module.
[0245] Referring to FIG. 31, a schematic diagram depicting how the SP module
defines a
plurality of cohorts according to one embodiment of the present invention is
illustrated. The
SP module retrieving provider 101 information (e.g., provider NPI numbers)
from the cohort
database 305 or the records database 303. After retrieving the provider 101
information, the
SP module defines a plurality of cohorts such that each of the plurality of
cohorts comprises a
sub-set of the plurality of health care providers 101. It should be noted that
in the preferred
embodiment, each of the plurality of health care providers 101 is assigned to
only one cohort
of a program cohort group. For example, as shown in FIG. 31, cohort 1 is
defined to
comprise health care providers (HCP) 101 number 1, 3 and 8, cohort 2 is
defined to comprise
HCP 101 number 2, 4, and 10, cohort 3 is defined to comprise HCP 101 number 5,
9, and 11,
and cohort 4 is defined to comprise HCP 101 number 6, 7, and 12. Although not
exemplified, the health care providers 101 are assigned to each of the cohorts
such that each
cohort has a commonality of prescribing factors between their assigned
providers 101.
[0246] Although four cohorts are defined by the SP module in the example
exemplified by
FIG. 31, the invention is not so limited and in alternate embodiments the SP
module may
define any number of cohorts for a particular program cohort group. Stated
another way,
the plurality of cohorts comprises at least two cohorts and may comprise any
number of
cohorts. Further, although each cohort of FIG. 31 is assigned only three
health care
providers 101, the invention is not so limited and in alternate embodiments,
each cohort may
be defined to comprise any number of health care providers 101 so long as each
cohort has a
commonality of prescribing factors between its assigned health care providers
101.
Therefore, according to one embodiment of the present invention, different
cohorts of a
program cohort group may comprise a different number of health care providers
101.
[0247] Referring back to FIG. 30, and as mentioned above, after assigning a
sub-set of health
care providers 101 to each of the plurality of cohorts, the SP module
generates a cohort
identifier for each of the plurality of cohorts. In one embodiment, the cohort
identifier is a
unique string of numbers used by the SP module to identify that particular
cohort After
generating a cohort identifier for each of the plurality of cohorts, the SP
module associates
the cohort identifier with each of the plurality of health care providers 101
of that particular
cohort, thereby completing step 3004. Therefore, each health care provider 101
of a sub-set
of health care providers 101 of a particular cohort is associated with the
same unique cohort
73
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
identifier of that cohort. Thereafter, the SP module stores the association of
health care
provider 101 (e.g. NPI number) and cohort identifier in the cohort database
305 of the SP
system 300. As a result, and as discussed in more detail below, the SP module
may more
easily identify the associated cohort of a particular health care provider 101
using the
provider's associated cohort identifier stored in the cohort database 305.
[0248] As noted above and in accordance with one embodiment of the present
invention, the
assignment of providers 101 between cohorts is accomplished by the SP module
via inputs
from the administrator of the SP system 300. In one embodiment, the
instructions from the
administrator of the SP system 300 specify which provider 101 is assigned to
which cohort.
In another embodiment, the instructions from the administrator of the SP
system 300 specify
rules by which the providers 101 will be assigned to each cohort (e.g.,
geographic location,
specialty, prescribing history of the target drug, etc.). In yet another
embodiment of the
present invention, the SP module does not receive instructions, but rather
automatically
assigns providers 101 to each cohort using an algorithm stored within the
cohort database 305,
such that each cohort has a commonality of prescribing factors.
[0249] After assigning a sub-set of a plurality of health care providers 101
to each of the
cohorts, the SP module assigns a different permutation of eligible
supplemental programs to
each cohort. As discussed above, the supplemental program database 303 of the
SP system
300 stores general supplemental program data, including, but not limited to
the name of the
supplemental program, general information relating to the supplemental
program, and the
rules of each supplemental program. As noted above, each supplemental program
comprises rules (non-cohort rules), such as a particular prescribed
substance(s) for which the
program is eligible and information relating to which patients are eligible
for the
supplemental program.
[0250] Still referring to FIG. 30, prior to assigning a different permutation
of eligible
supplemental programs to each cohort, the SP module determines which
supplemental
programs out of the plurality of available supplemental programs are eligible
for target drug
of the plurality of cohorts, thereby completing step 3005. According to one
embodiment of
the present invention, eligibility is determined by the SP module based on the
target drug
defined by the received target drug data. Therefore, the SP module determines
which
supplemental programs out of the available supplemental programs are eligible
for the
cohorts based on the received target drug data.
74
CA 02918798 2016-01-19
WO 2015/013695
PCT/1JS2014/048330
[0251] After determining which of the available supplemental programs are
eligible for the
plurality of cohorts based on the received target drug data, the SP module
retrieves
information relating to the plurality of eligible supplemental programs from
the supplemental
program database 303 of the SP system 300. After retrieving information
relating to the
plurality of eligible supplemental programs, the SP module defines a plurality
of different
permutations of the eligible supplemental programs, whereby the number of
permutations of
eligible supplemental programs equals the number of difference cohorts of the
program
cohort group, thereby completing step 3006. Therefore, the SP module creates
an equal
number of permutations of eligible supplemental programs and cohorts. Finally,
after
defining a plurality of different permutations of the eligible supplemental
programs for the
target drug, the SP module assigns a different permutation of supplemental
programs with
each cohort of the program cohort group, thereby completing step 3007.
[0252] According to one embodiment of the present invention, the SP module
receives
instructions regarding how to define the different peimutations of eligible
supplemental
programs from the administrator of the SP system 300. For example, the
administrator of
the SP system 300 may transmit instructions to the SP module which dictate the
exact
configurations of each of the different permutations of eligible supplemental
programs.
This may be beneficial if the administrator would like to test specifically
the effectiveness of
particular combinations of supplemental programs on patient adherence. In
such
embodiments, if the instructions comprise the specific permutations of
eligible supplemental
programs, then the SP module may not have to deteimine which supplemental
programs are
eligible for the target drug, define a plurality of different permutations,
and/or assign the
different permutations to each of the cohorts.
[0253] In the preferred embodiment of the present invention, one of the
permutations of
supplemental programs is created such that the cohort of which it is
associated is a control
group. A control group is a cohort which is assigned a permutation of
supplemental
programs that either does not comprise any supplemental programs or comprises
one or more
supplemental programs that are also common to all of the plurality of
permutations of
supplemental programs assigned to the other cohorts of the program cohort
group. Stated
another way, a permutation of supplemental programs may be used as a control
group if: (1)
it does not comprise any supplemental programs; or (2) only comprises
supplemental
programs that are also included in each of the other permutations of
supplemental programs
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
of a program cohort group. The use of a control group is beneficial because it
provides a
baseline for the SP module to analyze the effectiveness of the other
supplemental programs
on patient adherence. Nonetheless, although it is preferred that one of the
plurality of
cohorts is a control group, it should be noted that in alternate embodiments
of the present
invention a control group may be omitted.
[0254] Further, according to one embodiment of the present invention, each
cohort further
comprises a current counter and a maximum counter. The maximum counter
defines/stores
the maximum number of times the permutation of supplemental programs for a
cohort may
be activated by the SP module, while the current counter defines/stores the
number of times
the permutation of supplemental programs for a particular cohort has been
activated to date.
Therefore, by using both a current count and a maximum counter, the SP module
may ensure
that the permutation of supplemental programs for each cohort of a program
cohort group get
activated an equal number of times. As discussed in more detail below, by
limiting the total
number of times the SP module may activate the permutation of supplemental
programs for
each cohort, each permutation of supplemental programs will have been
activated the same
number of times when the SP module determines the effectiveness of each
permutation.
This helps to provide a more accurate representation of the effectiveness of
the supplemental
programs on patient adherence. Further, in one embodiment of the present
invention, when
the current counter of all the cohorts of a program cohort group reach the
maximum counter,
the SP module ceases to activate the permutations of supplemental programs for
the cohorts
of the program cohort group, and analyzes patient adherence data to determine
the
effectiveness of the different permutations of the supplemental programs on
patient
adherence.
[0255] Referring to FIG. 32, a schematic diagram of a method of defining a
plurality of
different permutations of eligible supplemental programs for a target thug
according to one
embodiment of the present invention is illustrated. As exemplified, the SP
module retrieves
supplemental program data from the supplemental program database 303, the
supplemental
program data relating to supplemental programs which are eligible for the
target drug. After
retrieving the supplemental program data, the SP module defines a plurality of
different
permutations of eligible supplemental programs. This may be accomplished by
the rules
engine of the SP module or via instructions received by the SP module from the
administrator
of the SP system 300, as discussed in detail above.
76
CA 02918798 2016-01-19
WO 2015/013695
PCT/1JS2014/048330
[0256] Referring to FIG. 32, the permutations of supplemental programs are
defined by the
SP module such that permutation number 1 does not comprise any eligible
supplemental
programs, permutation number 2 comprises supplemental program number 1,
permutation
number 3 comprises supplemental program number 2 and 3, and permutation number
4
comprises supplemental program number 1 and 3. It should be noted that FIG. 32
is but just
one example of the SP module defining a plurality of different permutations of
supplemental
programs. It should be noted that the present invention is not limited to the
number of
permutations of supplemental programs or the number of supplemental programs
per
permutation.
[0257] Referring to FIG. 33, a schematic diagram of a method of assigning a
different
pemiutation of eligible supplemental programs to each cohort out of a
plurality of cohorts of
a program cohort group according to one embodiment of the present invention is
illustrated.
After creating the plurality of permutations of the eligible supplemental
programs, the SP
module assigns a different permutation of eligible supplemental programs to
each cohort.
According to one embodiment of the present invention, the SP module may
receive
instructions from the administrator of the SP system 300 that defines how the
SP module is to
assign the different permutations of eligible supplemental programs to each
cohort.
However, the invention is not so limited, and in alternate embodiments of the
present
invention, the SP module may assign the permutation of supplemental programs
to each
cohort using an algorithm stored within the cohort database 305 that dictates
how the
permutations are to be assigned to each cohort.
[0258] For example and as exemplified in FIG. 33, the central portion 301 the
SP module
assigns program permutation number 2 to cohort number 1, program permutation
number 1 to
cohort number 2, program permutation number 3 to cohort number 3, and program
permutation number 4 to cohort number 4. It should be noted that this is just
one
non-limiting example of the assignment of permutations of supplemental
programs to cohorts
in accordance with the present invention. Specifically, it should be noted
that the
permutations of the present invention are not limited to any specific number
of supplemental
programs. Therefore, although only a maximum of two supplemental programs are
exemplified in any one permutation, in alternate embodiments of the present
invention, a
permutation may comprise any number of eligible supplemental programs.
[0259] As discussed in more detail below, since permutation number 1 does not
comprise any
77
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
supplemental programs, cohort number 2, which was assigned permutation number
1, is a
control group. Stated another way, cohort 2 (and supplemental program
permutation
number 1) is a control group in which no supplemental programs are available
for the target
drug. For further example, it should be noted that a control group may
comprise at least one
supplemental program, as long as the supplemental program(s) of the control
group are also
assigned to the other cohorts of the program cohort group.
[0260] Referring back to FIG. 30, after assigning a different permutation of
eligible
supplemental programs to each of the cohorts, the SP module stores the
association of each
permutation of eligible supplemental programs with its associated cohort in
the cohort
database 305 of the SP system 300, thereby completing step 3008. Therefore,
the cohort
database 305 comprises a correlated list of the cohorts, the assigned health
care providers 101
of each cohort, and the assigned permutation of supplemental programs of each
cohort.
[0261] According to one embodiment of the present invention, the SP module
generates a
program identifier for each of the different permutations of supplemental
programs. Similar
to the cohort identifier discussed above, in one embodiment of the present
invention the
program identifier is a unique string of numbers used by the SP module to
identify that
particular permutation of supplemental programs. After generating a program
identifier for
each of the different permutations of supplemental programs, the SP module
associates a
program identifier with each of the plurality of health care providers 101
based on their
associated cohort, thereby completing step 3009. Thereafter, the SP module
stores the
association in the cohort database 305 of the SP system 300.
[0262] Therefore, according to one embodiment of the present invention, all of
the health
care providers 101 of a particular cohort are associated with the unique
cohort identifier of
their associated cohort and the unique program identifier of the associated
peimutation of
supplemental programs of their cohort in the cohort database 305. As a result,
and as
discussed in more detail below, the SP module may more easily identify the
associated cohort
and permutation of supplemental programs of a particular health care provider
101 using the
cohort identifier and the program identifier stored in the cohort database
305.
[0263] Next, the SP module generates at least one cohort rule, and assigns at
least one cohort
rule to each cohort of the plurality of cohorts, thereby completing step 3010.
A cohort rule
is similar to a rule, as discussed in detail above, but a cohort rule is a
restriction assigned to
each of the plurality of cohorts, whereas the rules discussed above are
restrictions assigned to
78
CA 02918798 2016-01-19
WO 2015/013695
PCT/1JS2014/048330
at least one supplemental program. Therefore, the cohort rules further
restrict a cohort to
additional constraints in addition to the target drug and provider 101
restrictions discussed
above. Thus, in order for a prescription to qualify for the permutation of
supplemental
programs of a cohort, the prescription must meet the target drug, provider
101, and cohort
rules of a particular cohort. After generating and assigning cohort rules to
each of the
cohorts of a program cohort group, the SP module stores the cohort rules in
association with
each cohort in the cohort database 305.
[0264] As discussed in more detail below, the rules engine of the SP module
applies the
cohort rule to the prescription for the target drug to determine whether the
prescription
qualifies for the cohort, and thus the permutation of supplemental programs of
that cohort.
Therefore, although a prescription may be for a target drug of a cohort in
which the provider
101 belongs, the cohort may still not be eligible for the permutation of
supplemental
programs of the cohort unless the prescription also meets the cohort rule(s).
Thus, in such
embodiments, a prescription will only be deemed eligible for a cohort if the
rules engine of
the SP module determines that the prescribed substance is the target drug of a
cohort, the
provider 101 is assigned to the cohort, and the prescription meets the cohort
rules of the
cohort. Nonetheless, it should be noted that the invention is not so limited,
and in alternate
embodiments of the present invention, each of the plurality of cohorts may not
be assigned
any cohort rules.
[0265] It should further be noted that the cohort rules are exclusionary
rules, meaning that if
there is an applicable cohort rule for a prescription, then other non-cohort
rules (or "rules" as
discussed above) are not applied by the rules engine of the SP module when
determining
whether the prescription qualifies for the cohort, and in turn the permutation
of supplemental
programs of that cohort. Additionally, in one embodiment of the present
invention, the
cohort rules comprise the name of the target drug so that the SP module may
more easily
apply the cohort rules to received prescription data. However, the invention
is not so
limited, an in alternate embodiments of the present invention, the SP module
may apply both
the cohort rules and non-conflicting non-cohort rules.
[0266] A cohort rule may be similar to any of the non-cohort rules discussed
above. For
further example, a cohort rule may relate to the dosage strength of the
substance (e.g., the
prescription of the target drug must be for 60 mg pills or the prescription of
the target drug
must be equal to or greater than 30 mg pills), the duration in which the
patient has been
79
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
receiving prescriptions for the target drug (e.g., the patient must have been
prescribed the
drug for at least 90 days prior to the current prescription), the age of the
patient (e.g., the
prescription must be for a patient who is greater than 18 years old or a
patient who is less
than or equal to 60 years old), the Medication Persistency Rate (MPR) of the
patient, or any
other patient adherence data.
[0267] Similar to the set-up of the permutations of supplemental programs,
according to one
embodiment of the present invention, the SP module receives instruction from
an
administrator of the SP system relating to the generation of the cohort rules
of a plurality of
cohorts. For example, the administrator of the SP system 300 may transmit
instructions to
the SP module which dictate the exact cohort rules for the plurality of
cohorts. This may be
beneficial if the administrator would like to specifically test the
effectiveness of supplemental
programs on a particular patient type, patients at a particular usage stage of
the target drug, or
prescriptions for a particular strength of the target drug. However, in an
alternate
embodiment of the present invention, the rules engine of the SP module
generates the cohort
rules such that each of the plurality of cohorts are configured to be
activated for the most
common types of patients receiving prescriptions for the target drug or for
patients with a
specific MPR range (e.g. patients who have an MPR between 30%-80%).
[0268] In accordance with one embodiment of the present invention, the cohort
rules may be
reconfigured or altered by the SP module at any time. For example, the
administrator of the
SP system 300 may determine that the results being received from a particular
cohort are not
ideal. Therefore, the administrator may remove, alter, or reconfigure any
cohort rules at any
stage after the cohorts are created. By allowing the administrator to alter
the cohort rules
after the cohorts are in use, the SP module enables the administrator to
correct or alter the
qualifications required for each cohort. For instance, in one embodiment, the
SP module
may allow an administrator to use a sliding scale to adjust the range of a
particular cohort rule
(e.g., a cohort rules restriction qualification of the cohort to prescriptions
whose patients have
an MPR in a certain range).
[0269] Further, in another embodiment of the present invention, the SP module
may
comprise an algorithm that is configured to automatically adjust a cohort rule
based on a
percentage of prescription that pass or fail the cohort rule. For example, the
SP module may
automatically adjust a cohort rules restriction qualification of the cohort to
prescriptions
whose patients have an MPR between 40-80% to an MPR between 30-90% in order to
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
increase the number of prescriptions qualifying for the cohort.
[0270] Further, according to one embodiment of the present invention, one or
more cohort
rules may be used by the SP module to define the target drug of each cohort,
select and
allocate providers 101 for each cohort, define the maximum counter of each
cohort, and/or
select and allocate the permutation of supplemental programs for each cohort.
In such
instances, the SP module may not receive instructions from the administrator
of the SP
system 300 that define how to assign providers 101 between cohorts and/or
select and
allocate the permutation of supplemental programs for each cohort. For
example, instead of
receiving instructions relating to the specific assignment of providers 101
between cohorts,
the SP module may receive instructions defining a cohort rule that
automatically allocates
specific permutations of supplemental programs to specific providers (e.g.,
providers living in
a certain geographic location are allocated a particular permutation of
supplemental programs
for a particular prescribed substance(s)). Therefore, in such embodiments, the
cohorts will
be automatically defined and created by the SP module using cohort rules.
[0271] Still referring to FIG. 30 and as discussed above, the SP module stores
a correlated
list of the information relating to cohorts in the cohort database 305 to aid
the SP module in
performing additional processing steps. In step 3011, the SP module stores the
cohort
information in a cohort relation table created by the SP module, the cohort
relation table
being stored by the SP module in the cohort database 305 of the SP system 300.
The cohort
relation table associates or links a plurality of data relating to each
cohort. For example, the
cohort relation table may associate or link any combination of the data
relating to each cohort,
such as but not limited to, the cohort identifier of a cohort, the provider
NPI numbers of a
cohort, the target drug of a cohort, the permutation of supplemental programs
of a cohort, the
current and maximum counter of a cohort and/or the cohort rules of a cohort.
Therefore,
when the SP module receives prescription data, the rules engine may use the
cohort relation
table to determine whether a cohort is applicable to the received prescription
data, the
associated supplemental programs of a cohort, and the current and maximum
counter of a
cohort.
[0272] Referring to FIG. 34, a cohort relation table 3400 according to one
embodiment of the
present invention is illustrated. As discussed above, according to one
embodiment of the
present invention, the cohort database 305 stores a cross-referenced listing
of cohort
identifiers, health care provider NPI numbers, target drug(s), permutation of
supplemental
81
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
programs, current/maximum counter, and cohort rules. Nonetheless, it should be
noted that
the cohort relation table may comprise a cross-referencing of any number of
cohort related
data items. Further, it should be noted that the information listed in the
cohort relation table
3400 is generically illustrated for purposes of simplicity.
[0273] The cohort relation table 3400 may be utilized by the SP module when
cross-referencing the cohort database 305, such that the cross-referencing is
accomplished
using the cohort relation table 3400. This enables the SP module to determine
whether a
health care provider 101 is associated with a cohort, and if so, what specific
cohort, what
permutation of supplemental programs associated with the cohort, etc. the SP
module should
apply for an electronic prescription.
[0274] Although exemplified as a single table, it should be noted that in
alternate
embodiments of the present invention, the cohort relation table may consist of
multiple
separate tables that are linked to one another (e.g., mapping tables).
Therefore, the SP
module may determine associated data of one element (e.g., a cohort, a health
care provider
101, a target drug, etc.) by searching through the appropriate mapping tables
stored within the
cohort database 305 of the SP system 300.
[0275] Further, in accordance with one embodiment of the present invention,
the SP module
may change a provider 101 between cohorts using the cohort relation table.
Therefore, the
SP module may move providers 101 between cohorts, and such changes would
update their
cohort association stored within the cohort relation table in the cohort
database 305.
[0276] After storing the cohort relation table in the cohort database 305, the
SP module has
defined the plurality of cohorts of a program cohort group.
2. Receiving Data Relating to an Electronic Prescription and Activating the
Supplemental Programs Associated with the Health Care Provider's Cohort
[0277] After a plurality of cohorts is defined by the SP module, the SP module
is ready to
analyze the effectiveness of the different peimutations of supplemental
programs on patient
adherence for the target drug. However, the first step is for the SP module to
activate the
permutation of supplemental programs of each of the cohorts for electronic
prescriptions.
As discussed in more detail below, the SP module receives information relating
to an
electronic prescription for the target drug written by one of the health care
providers 101,
82
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
determines whether the health care provider 101 is part of an existing cohort,
and activates
that provider's permutation of supplemental programs for the patient upon the
provider's
request. Thereafter, the SP module receives patient adherence data and
analyzes the
effectiveness of the activated supplemental programs on the patient's
adherence to the target
drug. It should be noted, as discussed above, that the target drug may be just
one or a
plurality of prescribed substances as indicated by the target drug data.
[0278] Referring to FIGS. 35a-35b, a flow chart of a method 3500 for receiving
data relating
to an electronic prescription for the target drug and activating the
permutation of
supplemental programs associated with the health care provider's cohort for
the target drug
according to one embodiment of the present invention is illustrated. It should
be noted that
the method 3500 of FIGS. 35a-35b may be considered an intervening method that
works in
conjunction with the method 800 exemplified in FIGS. 8a-8c. For example,
according to
one embodiment of the present invention, the method 3500 picks up after the
health care
provider 101 writes an electronic prescription for a prescribed substance
using the thin client
portion 203 of the EP module and the SP widget 302 retrieves data relating to
the electronic
prescription from the thin-client portion of the EP module 203. Therefore, the
method 3500
begins with the central portion 301 of the SP module receiving data relating
to the electronic
prescription of the prescribed substance for the patient, similar to as
discussed above with
respect to step 801 of FIG. 8a, thereby completing step 3501.
[0279] Further, it should be noted that the method 3500 occurs after the
performance of
method 3000 by the SP module. Finally, as discussed above with respect to the
method 800,
the method 3500 is not a one-time transmission of information, but rather a
recurrent process
that occurs each time a health care provider 101 writes and/or transmits an
electronic
prescription.
[0280] After receiving the electronic prescription data, the SP module
extracts the NPI
number of the health care provider 101 who wrote the prescription and the
prescribed
substance of the electronic prescription from the received electronic
prescription data, thereby
completing step 3502. As noted above, the electronic prescription data
comprises first
patient data that is specific to the patient, first prescribed substance data
that is specific to the
prescribed substance, first provider data that is specific to the health care
provider 101, and
first payor data that is specific to the payor.
[0281] Next, the central portion 301 of the SP module determines whether the
prescribed
83
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
substance of the electronic prescription is part of a particular cohort in
decision step 3503.
According to one embodiment of the present invention, the central portion 301
of the SP
module cross-references the prescribed substance data from the electronic
prescription with
cohort relation table stored in the cohort database 305 of the SP system 300
to deteimine if
the prescribed substance of the electronic prescription is the target drug of
one or more
cohorts. For example, according to one embodiment of the present invention,
the SP
module determines whether the target drug name is associated with any of the
defined
cohorts.
[0282] If the prescribed substance is not associated with any cohorts, then
the method moves
to step 3504, and as a result, returns to step 802 in FIG. 8a. In such
instances, the prescribed
substance is not associated with any cohorts and, therefore the method returns
to step 802 of
FIG. 8. Nonetheless, the SP module may still determine eligible supplemental
programs for
the patient based on the received electronic prescription data, as discussed
above in detail
with respect to FIGS. 8a-8b. However, if the prescribed substance is the same
as the (or a)
target drug of one or more cohorts, then the method continues to step 3505.
[0283] Thereafter, the SP module determines whether the health care provider
101 who wrote
the prescription is part of one of the cohorts identified in decision step
3505. According to
one embodiment of the present invention, the SP module cross-references the
provider data
from the electronic prescription with the cohort database 305 of the SP system
300 to
determine if the provider 101 has an associated cohort, and further if the
target drug of the
associated cohort is the same as the prescribed substance. For example, in one
embodiment
of the present invention, the SP module deteimines whether the provider's NPI
number is
associated with any of the cohorts for the target drug.
[0284] If the health care provider 101 is not associated with any cohorts,
then the method
moves to step 3506, and as a result, returns to step 802 in FIG. 8a. In such
instances, even
though the prescribed substance is the target drug of one or more cohorts, the
provider 101 is
not associated with any of the cohorts of that target drug, and therefore the
cohort(s) of the
target drug are not relevant for detei __________________________ mining
supplemental programs. Nonetheless, the SP
module may still determine eligible supplemental programs for the patient
based on the
received electronic prescription data, as discussed above in detail with
respect to FIGS. 8a-8b.
However, if the health care provider 101 is associated with a cohort of the
prescribed
substance, then the process continues on to step 3507.
84
CA 02918798 2016-01-19
WO 2015/013695
PCT/1JS2014/048330
[0285] If the method continues to step 3507, then the health care provider 101
is associated
with at least one cohort and the prescribed substance is the (or a) target
drug of the provider's
associated cohort. At that point, the rules engine of the SP module retrieves
the cohort rules
for that cohort from the cohort relation table stored within the cohort
database 305. As
noted above, the cohort rules define rules or restrictions that are used by
the rules engine to
determine whether or not the electronic prescription is eligible for the
cohort, and in turn the
permutation of supplemental programs of the cohort. It should be noted that in
accordance
with one embodiment of the present invention, the rules engine of the SP
module further
retrieves patient data, prescribed substance data, provider data, and/or payor
data from the
records database 304 of the SP module. This is similar to as discussed with
reference to
steps 802-805 of FIG. 8a.
[0286] Referring to FIG. 35b, at step 3508 the rules engine of the SP module
determines
whether the received (and potentially retrieved) data meets the cohort rules
of the provider's
cohort. If the received/retrieved data does not meet the cohort rules, then
the method moves
to step 3509 and returns to step 802 of FIG. 8a. In such instances, even
though the provider
101 is a part of a cohort and the prescribed substance is the target drug of
that cohort, the
electronic prescription does not meet the other requirements, or cohort rules,
of that cohort.
Therefore, the electronic prescription is not eligible for the permutation of
supplemental
programs for that cohort.
[0287] However, if the received/retrieved data does meet the cohort rules,
then electronic
prescription is eligible for the cohort and the method continues to step 3510.
At step 3510,
the SP module deteimines whether the current counter of the permutation of
supplemental
programs meets or exceeds the maximum counter of the permutation of
supplemental
programs. The SP module makes the determination by checking the cohort
relation table
stored within the cohort database 305. If the current counter does meet or
exceed the
maximum counter of the permutation of supplemental programs, then the method
continues
to step 3511 and returns to step 802 in FIG. 8a. In such instances, even
though the provider
101 is a part of a cohort, the prescribed substance is the target drug of that
cohort, and the
electronic prescription meets the cohort rules of that cohort, the permutation
of supplemental
programs for that cohort has been met or exceeded, so the SP module will not
continue to
activate that permutation of supplemental programs. However, if the current
counter does
not meet or exceed the maximum counter of the permutation of supplemental
programs, then
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
the method continues to step 3512. Further, it should be noted that in
embodiments when
the cohort does not comprise a current and maximum counter for the permutation
of
supplemental programs, steps 3510 and 3511 may be omitted.
[0288] At step 3512, the SP module retrieves the permutation of supplemental
programs
associated with the provider's cohort from the cohort database 305. According
to one
embodiment of the present invention, the SP module retrieves the cohort
identifier of the
provider's cohort from the cohort database 305, and in turn, using the cohort
identifier,
retrieves the program identifier of the cohort from the cohort database 305.
The program
identifier identifying each of the supplemental programs of the permutation of
supplemental
programs associated with the provider's cohort.
[0289] After retrieving the permutation of supplemental programs from the
cohort database
305, the SP module retrieves data relating to each of the supplemental
programs associated
with the permutation of supplemental programs, thereby completing step 3513.
Next, the
method continues to step 3514, and as a result, returns to step 809 in FIG.
8a. Thereafter,
the method may continue as discussed above with reference to FIGS. 8a-8c. The
method
may include, but not limited to, any of the embodiments described above.
[0290] Therefore, according to one embodiment of the present invention and as
discussed in
greater detail above, the SP module generates and displays a GUI comprising a
list of the
supplemental programs associated with the provider's cohort on the display
device 121 for
the provider's input. Therefore, each of the supplemental programs of the
permutation of
supplemental programs associated with the cohort to which the health care
provider 101
belongs is displayed to the health care provider 101 for selection and
activation. Also
similar to as discussed above, the SP module will activate each of the
supplemental programs
associated with the cohort to which the health care provider 101 belongs that
are selected and
activated by the health care provider 101 using the input device 122 of the
HCP system 100.
As noted above, activation of a supplemental program may comprise many
different steps,
including, but not limited to, the delivery or transmission of content to the
patient, the
transmitting of patient information to sign a patient up for a service, or any
other form as
discussed above in detail.
[0291] However, the invention is not so limited and according to one
embodiment of the
present invention, and unlike as described above, the provider 101 does not
have the
opportunity to select or de-select the supplemental programs. Rather, since
the provider 101
86
CA 02918798 2016-01-19
WO 2015/013695
PCT/1JS2014/048330
is associated with the particular cohort, all of the supplemental programs
associated with that
cohort are automatically activated by the SP module. In such embodiments, a
GUI may be,
but is not necessarily, displayed to the provider 101 via the display device
121. The
supplemental programs are simply activated by the SP module for the patient.
[0292] It should be noted that among the processes discussed above that may be
incorporated
into the embodiments where the prescribing health care provider 101 is a part
of a cohort, the
use of delivery modes may be incorporated into herein. For example, in one
embodiment of
the present invention the SP module may retrieve delivery mode data relating
to the patient
and the supplemental programs of the cohort from the record database 304 and
supplemental
program database 303 respectively, as discussed in detail above. Thereafter,
the SP module
may compare delivery mode data relating to the patient with the delivery mode
data relating
to the supplemental programs to determine common delivery modes, and
displaying the
common delivery modes of the supplemental programs in the GUI via the display
device 121
for selection and acceptance by the health care provider 101, as also
discussed above in
greater detail. Finally, in such embodiments, if activation of a supplemental
program of the
cohort causes content to be delivered to the patient, the delivery of the
content is via the
delivery mode that is selected and accepted by the health care provider 101.
[0293] Furthermore, it should be noted that in alternate embodiments of the
present invention
the SP module widget 302 and not the central portion 301 of the SP module
performs the
processes relating to the use of the plurality of cohorts. For example, in one
embodiment of
the present invention, the SP module widget 302 and not the central portion
301 of the SP
module determines whether the provider 101 is a part of a particular cohort
and/or whether
the prescribed substance of the electronic prescription is the (or a) target
drug of the
provider's associated cohort. In such embodiments, the SP module widget 302
may track
electronic prescriptions being prescribed by each one of the plurality of
health care providers
101 that are part of one of the plurality of cohorts. Similar to above, the SP
module widget
302 may cross-reference electronic prescription data with the cohort database
305. In such
embodiments, the cohort database 305 may reside within the memory 113 of the
HCP system
100. Thereafter, when a health care provider 101 out of the plurality of
health care
providers 101 that is a part of one of the plurality of cohorts writes an
electronic prescription
for the (or a) target drug of that cohort, the SP module widget 302 retrieves
electronic
prescription data for the target drug from the thin-client portion of the EP
module 203 and
87
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
transmits the electronic prescription data for the target drug to the central
portion 301 of the
SP module.
[0294] Generally, it should be noted that since each cohort is assigned a
different permutation
of supplemental programs, each cohort may be used to analyze the effectiveness
of a specific
permutation of supplemental programs on patient adherence. Stated another way,
and as
discussed in more detail below, each of the plurality of cohorts analyzes the
effectiveness of
its assigned supplemental programs on patient adherence for the prescribed
substance(s)
identified by the target drug data.
2a. Alternate Embodiment of Processing a Prescription to Determine
Eligible Supplemental Programs via Cohorts
[0295] Referring to FIG. 36a, a flow chart of a method 3600 of retrieving
supplemental
program data in accordance with an alternate embodiment of the present
invention is
illustrated. Referring to FIG. 36b, a method 3600 of generating a document
list of all
eligible supplemental programs that are selected and accepted by a provider
101 for an
electronic prescription according to one embodiment of the present invention
is illustrated.
Referring to FIG. 36c, a method 3600 of retrieving the document associated
with
supplemental programs that are selected and accepted by a provider 101 for an
electronic
prescription according to one embodiment of the present invention is
illustrated.
[0296] It should be noted that the method 3600 is an alternate method to
method 3500
discussed in reference to FIGS. 35a-35b above. Specifically, as opposed to the
method
3500, in which the rules engine first determines whether the electronic
prescription is eligible
for a supplemental program prior to determining if there are any eligible
supplemental
programs, in the method 3600 the rules engine first determines if there are
any supplemental
programs for which the electronic prescription is eligible, and then
subsequently determines
whether any of the eligible supplemental programs are part of a predefined
cohort of the
provider 101. It should be further noted that in alternate embodiments of the
present
invention, the SP module may perform a combination of any of the steps of
methods 3500
and 3600 when determining eligible supplemental programs for cohort related
electronic
prescriptions.
[0297] Further, it should be noted that steps 3601-3631 are performed by the
rules engine of
the SP module, while steps 3632-3634 are performed by the central portion 301
of the SP
88
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
module. Therefore, although the process of steps 3601-3631 is discussed with
reference to
the "SP module," it should be noted that the steps 3601-3631 are performed by
the rules
engine of the SP module. Nonetheless, the invention is not so limited and
according to other
embodiments of the present invention, the central portion 301 of the SP module
and/or the
rules engine may perform any of the steps 3601-3634 discussed below.
[0298] The method begins at step 3601 with the SP module storing received
electronic
prescription data in the memory 313 of the SP system 300. According to one
embodiment
of the present invention, the electronic prescription data may be stored in
the record database
304. However, it should be noted that in other embodiments of the present
invention, the
electronic prescription data may be stored in its own database or in temporary
memory within
the SP system 300.
[0299] After storing the electronic prescription data, the SP module transmits
the electronic
prescription data to the rules engine for evaluation, thereby completing step
3602. Next, the
rules engine evaluates the electronic prescription data to deteimine if there
are any eligible
supplemental programs out of the plurality available supplemental programs for
the
electronic prescription, thereby completing step 3603. If there are no
eligible supplemental
programs for the prescription, then an empty response is returned in step
3604, and the
method ends. However, if there is at least one eligible supplemental program
returned, then
the method continues to step 3605.
[0300] At step 3605, the SP module stores, in a failed programs log in the
record database
304, a listing of all of the supplemental programs of the target drug for
which the electronic
prescription was not eligible. For example, if the prescribed substance met
the rules for the
supplemental program, but the provider 101 or dosage of the substance did not
meet the rules
of the supplemental program, then that information is stored within the failed
programs log.
By storing all of the ineligible supplemental programs for each received
prescription, the
failed programs log provides a means by which the administrator of the SP
system 300 may
analyze why certain supplemental programs are being activated more or less
than others.
This is beneficial in determining how to increase or decrease the activation
of certain
supplemental programs.
[0301] 'Thereafter, the SP module processes each eligible supplemental
program, preferably
one at a time, in step 3606. First, at decision step 3607, the rule engine of
the SP module
determines whether the supplemental program is cohort related. According to
one
89
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
embodiment, the SP module cross references the program identifier of the
supplemental
program with the cohort relation table (or mapping tables) stored within the
cohort database
305. This is beneficial if some of the supplemental programs returned by the
rule engine in
step 3603 are cohort related while others are not.
[0302] If the returned supplemental program is not cohort related (i.e., the
provider 101
and/or the substance is not part of the same cohort, or the cohort rules are
not met), then the
method 3600 continues to step 3610. However, if the supplemental program is
cohort
related, then the method continues to step 3608. At step 3608, the rules
engine of the SP
module searches for the provider's cohort using the supplemental program
identifier and the
NPI number of the provider 101. Specifically, the rules engine cross-
references the cohort
relation table (or mapping tables) stored within the cohort database 305 to
determine the
specific cohort in which the supplemental program and electronic prescription
belong.
[0303] Upon determining the provider's cohort for the substance of the
electronic
prescription, the rules engine of the SP module determines whether the
provider's cohort is a
control cohort (or control group), thereby completing decision step 3609. As
discussed
above, a control group is a cohort which is assigned a permutation of
supplemental programs
that either does not comprise any supplemental programs or comprises one or
more
supplemental programs that are also common to all of the plurality of
permutations of
supplemental programs of the other related plurality of cohorts. If the
provider's cohort is a
control group, then the method continues to step 3614, and a program option
for the eligible
supplemental program is not generated for display to the provider 101.
However, if the
provider's cohort is not the control group, then the method continues to step
3610.
[0304] Nonetheless, it should be noted that in some embodiments of the present
invention,
the method may continue to step 3610 although the provider's cohort is the
control group.
Specifically, this may be the case if the provider's control group is assigned
a plurality of
supplemental programs that are common to all of the plurality of permutations
of
supplemental programs assigned to each of the other cohorts of the program
cohort group.
In such instances, it is important that the supplemental programs assigned to
the control
group are also activated. Therefore, in such instance, the method may continue
to step
3610.
[0305] At step 3610, the rules engine of the SP module retrieves the program
cohort group of
the provider's cohort, which may comprise a current counter and a max counter
of the cohort,
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
from the cohort database 305 of the SP system 300. After retrieving the
program cohort
group (and the current and max counter of the supplemental program for the
provider's
cohort), the SP module determines whether activation of the supplemental
program is
controlled by the provider's cohort in decision step 3611. It should be noted
that one
method of controlling the activation of a supplemental program is through the
use of a current
and max counter. If the activation of the supplemental program is not
controlled by a
current and max counter (e.g., the supplemental program is not related to a
cohort, or the
provider's cohort does not comprise a corresponding max counter for the
supplemental
program), then the method continues to step 3613. However, if the activation
of the
supplemental program is controlled by the current and max counter, then the
method
continues to step 3612.
[0306] At decision step 3612, the SP module determines whether the current
counter of the
supplemental program for the provider's cohort is greater than or equal to the
maximum
counter of the supplemental program for the provider's cohort. If the current
counter is
greater than or equal to the maximum counter, then the supplemental program
has been
activated its allotted amount of times for the particular cohort, and as such,
the method
continues to step 3614. At step 3614, the SP module skips the supplemental
program such
that the eligible supplemental program is not activated. However, if the
current counter is
less than the maximum counter, then the supplemental program has not been
activated its
allotted amount of times for the particular cohort, and as such, the method
continues to step
3613.
[0307] At step 3613, the SP module generates a supplemental program option for
the
supplemental program in a response. As discussed below with reference to FIG.
36b, the
response will be transmitted by the central portion 301 of the SP module to
the SP widget 302
residing on the HCP system 100 so that the SP module may receive the
provider's selection
and activation of the eligible supplemental programs. According to one
embodiment of the
present invention, a response is a pop-up window 1010, such as that
exemplified in FIG. 15,
and the supplemental program option is the information relating to the
eligible supplemental
program option 1012 display in the pop-up window 1010 on the display device
121 to the
provider 101 for the provider's selection and acceptance of each eligible
supplemental
program. It should be noted that the pop-up window 1010 and the infounation
relating to
the eligible supplemental program 1012 is but just one non-limiting example of
a response for
91
CA 02918798 2016-01-19
WO 2015/013695
PCT/1JS2014/048330
the supplemental program in accordance with the present invention.
[0308] It should be noted that the response may comprise information relating
to
supplemental programs that are part of the provider's cohort, along with
information relating
to supplemental programs that are not part of the provider's cohort However,
in one
embodiment of the present invention, the SP module removes from the response
the
information relating to all non-cohort relating supplemental programs so that
only those
supplemental programs that are part of the provider's cohort are displayed to
the provider
101.
[0309] After generating the supplemental program option in the response, the
SP module
continues to decision step 3615. At decision step 3615, the SP module
determines whether
the any more eligible supplemental programs remaining to be processed at step
3606. In
one embodiment, this determination is made by the SP module through a cross-
referencing of
the supplemental programs determined to be eligible by the rules engine in
step 3603. If
there remains additional eligible supplemental programs that need to be
processed through
steps 3606 ¨ 3615, then the method returns to step 3606 and another eligible
supplemental
program is processed. After all of the supplemental programs determined by the
rules
engine in step 3603 to be eligible have been processed through step 3606 ¨
3615, the method
of FIG. 36a is complete.
[0310] Referring to FIG. 36b, a method 3600 of generating a document list of
all eligible
supplemental programs that are selected and accepted by a provider 101 for an
electronic
prescription according to one embodiment of the present invention is
illustrated. It should
be noted that the method of FIG. 36b is a continuation of the method of FIG.
36a. Therefore,
as illustrated, the method 3600 of FIG. 36b begins with step 3616. It should
be noted that
between step 3615 and step 3616, the central portion 301 of the SP module has
transmitted
the response comprising the information relating to the eligible supplemental
programs to the
SP widget 302, the SP widget 302 has displayed a list of the eligible
supplemental programs
on the display device 121 to the provider 101, the provider 101 has selected
and accepted the
eligible supplemental programs they would like to activate for the patient,
and the SP widget
302 has generated a signal indicating the supplemental programs that were both
selected and
accepted by the provider 101.
[0311] At step 3616, the SP module receives a signal from the SP widget 320
indicating
which of the supplemental programs are both selected and accepted by the
provider 101. In
92
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
one embodiment, the signal may be the activation signal as discussed above
with reference to
FIGS. 8a-8c. Next, at step 3617, the SP module retrieves the list of eligible
supplemental
programs that were displayed for the provider's selection and acceptance. The
submitted
program list comprising the information relating to each of the eligible
supplemental
programs, regardless of whether the supplemental programs were selected and
accepted by
the provider 101 for activation.
[0312] Thereafter, at decision step 3618, the SP module determines whether
there are any
supplemental programs that remain to be processed by the SP module. If so,
then the SP
module selects one supplemental program from the supplemental program list and
determines
whether the signal received from the SP widget 302 indicates that the
supplemental program
was selected and accepted by the provider 101. If the supplemental program was
not
selected and accepted by the provider 101, then the method continues to step
3620 and the
supplemental program does not get activated. However, if the supplemental
program was
selected and accepted by the provider 101, then the method continues to step
3621.
[0313] It should be noted that the process of steps 3621-3623 is very similar
to the process of
steps 3607-3609 discussed above with respect to FIG. 36a. At decision step
3621, the SP
module determines whether the supplemental program is cohort related. As noted
above, in
one embodiment, the SP module cross references the program identifier of the
supplemental
program with the cohort relation table (or mapping tables) stored within the
cohort database
305. Further, it should be noted that in one embodiment of the present
invention, steps
3621-3623 are omitted. In such embodiments, the method goes from step 3620
directly to
step 3624.
[0314] If the supplemental program is not cohort related, then the method
continues to step
3624. However, if the supplemental program is cohort related, then the method
continues to
step 3622. At step 3622, the rules engine of the SP module searches for the
provider's
cohort using the supplemental program identifier and the NPI number of the
provider 101.
Specifically, the rules engine cross-references the cohort relation table (or
mapping tables)
stored within the cohort database 305 to determine the specific cohort in
which the
supplemental program and electronic prescription belong.
[0315] Upon determining the provider's cohort, the rules engine of the SP
module determines
whether the provider's cohort is a control cohort (or control group), thereby
completing
decision step 3623. If the provider's cohort is a control group, then the
method continues to
93
CA 02918798 2016-01-19
WO 2015/013695
PCT/1JS2014/048330
step 3620, and the eligible supplemental program is not activated. However, if
the
provider's cohort is not the control group, then the method continues to step
3624.
[0316] Nonetheless, similar to as discussed above, in some embodiments of the
present
invention, the method may continue to step 3624 although the provider's cohort
is the control
group. Specifically, this may be the case if the provider's control group is
assigned a
plurality of supplemental programs that are common to all of the plurality of
permutations of
supplemental programs assigned to each of the other cohorts of the program
cohort group.
In such instances, it is important that the supplemental programs assigned to
the control
group are also activated. Therefore, in such instance, the method may continue
to step
3624.
[0317] At step 3624, the rules engine of the SP module retrieves the program
cohort group of
the provider's cohort, which may comprises a current counter and a max counter
of the cohort,
from the cohort database 305 of the SP system 300. After retrieving the
program cohort
group (and the current and max counter of the supplemental program for the
provider's
cohort), the SP module determines whether activation of the supplemental
program is
controlled by the provider's cohort in decision step 3625. If the activation
of the
supplemental program is not controlled by a current and max counter, then the
method
continues to step 3627. However, if the activation of the supplemental program
is
controlled by the current and max counter, then the method continues to step
3626.
[0318] At decision step 3626, the SP module determines whether the current
counter of the
supplemental program for the provider's cohort is greater than or equal to the
maximum
counter of the supplemental program for the provider's cohort. If the current
counter is
greater than or equal to the maximum counter, then the supplemental program
has been
activated its allotted amount of times for the particular cohort, and as such,
the method
continues to step 3620. At step 3620, the SP module skips the supplemental
program such
that the eligible supplemental program is not activated. However, if the
current counter is
less than the maximum counter, then the supplemental program has not been
activated its
allotted amount of times for the particular cohort, and as such, the method
continues to step
3627.
[0319] At step 3627, the SP module retrieves the service configuration from
the supplemental
program database 303 for calling a third party program vendor 400. The service
configuration comprises information relating to the specific third party
program vendor 400
94
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
that comprises the supplemental program. As noted above, in accordance with
one
embodiment of the present invention, the SP module does not store the physical
documents or
run the services that are the supplemental programs. Rather, the SP module
simply stores
information relating to the supplemental programs so that upon activation, the
SP module
may retrieve the actual document from the appropriate third party program
vendor 400 or
enroll the patient in the service by transmitting patient data to the
appropriate third party
program vendor 400. Nonetheless, in one embodiment of the present invention,
the SP
module does store the actual documents and run the actual services that are
the supplemental
programs. In such embodiments, steps 3627-3629 may be omitted.
[0320] Next, at step 3628, the SP module transmits a signal to the appropriate
third party
program vendor 400 to invoke the vendor 400 to confirm that the third part
program vendor
400 does in fact have stored the actual document or service relating to the
supplemental
program. Thereafter, the SP module receives the confirmation signal from the
appropriate
third party program vendor 400, and logs the confirmation from the third party
program
vendor 400 in a log table at step 3629, the log table being stored within the
cohort database
305.
[0321] Next, at step 3630. the SP module generates the supplemental program
title and puts
the supplemental program title in a response list, the response list stored in
the cohort
database 305. As discussed in more detail below, the response list comprises
the titles of the
supplemental programs that were selected and accepted by the provider 101.
After putting
the document title in the response list, the method returns to steps 3617 and
3618 and the SP
module determines if there are any remaining supplemental programs that need
to be
processed. Upon the SP module processing each of the selected and accepted
supplemental
programs, the method continues to step 3631 and the SP module generates a
response, the
response comprising the response list that comprises the titles of the
supplemental programs
that were selected and accepted by the provider 101.
[0322] Referring to FIG. 36c, a method 3600 of retrieving the document
associated with
supplemental programs that are selected and accepted by a provider 101 for an
electronic
prescription according to one embodiment of the present invention is
illustrated. It should
be noted that the method of FIG. 36c is a continuation of the method of FIG.
36b.
[0323] At step 3632, the central portion 301 of the SP module receives a
request from the
rules engine which comprises the response listing a log identifier for the
supplemental
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
programs that were selected and accepted by the provider 101 for activation.
The log
identifier has the program identifiers for the supplemental programs that were
selected and
accepted by the health care provider 101.
[0324] Next, in step 3633, the central portion 301 of the SP module retrieves
the
supplemental program service log from one or more of the databases of the SP
system 300.
The supplemental program service log indicates the supplemental programs that
were
selected and accepted by the provider 101 for activation.
[0325] Next, at decision step 3634, the central portion 301 of the SP module
determines
whether the supplemental program is related to the provider's cohort. If the
supplemental
program is cohort related, then the method continues to step 3635 and the
central portion 301
of the SP module retrieves the program group of the supplemental program,
which comprises
the current and maximum counter. However, if the supplemental program is not
cohort
related, then the method continues to step 3639, as discussed in more detail
below.
[0326] Assuming the supplemental program is cohort related and after
retrieving the current
and maximum counter of the supplemental program for the provider's cohort, the
central
portion 301 of the SP module determines whether the current counter is less
than the
maximum counter at decision step 3636. If the current counter is not less than
the
maximum counter, then the central portion 301 of the SP module generates a
message
indicating that the supplemental program is not available for activation
(e.g., "No Coupon
Available"), and includes that message in a response that is transmitted to
the SP widget 302
for display to the provider 101 on the display device 121. However, if the
current count is
less than the maximum counter, then the central portion 301 of the SP module
increases the
current counter by one, such increase being stored in the cohort database 305,
thereby
completing step 3637.
[0327] Next, the central portion 301 of the SP module calls the appropriate
third party
program vendor 400 and retrieves the actual document or information relating
to the service
of the supplemental program. In one embodiment, the central portion 301 of the
SP module
transmits the program identifier to the third party program vendor 400 and
receives the actual
document or information relating to the service of the supplemental program
from the third
party program vendor 400. However, the invention is not so limited, and in
alternate
embodiments the central portion 301 of the SP module may transmits any signal
indicating
the specific supplemental program to the appropriate third party program
vendor 400.
96
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
[0328] Referring back to step 3634, if the supplemental program is not cohort
related, then
the central portion 301 of the SP module calls the appropriate third party
program vendor 400
and retrieves the actual document or infoimation relating to the service of
the supplemental
program, thereby completing step 3639. This is similar to the process
described with
respect to step 3638.
[0329] Thereafter, the SP module receives the actual document or information
relating to the
service of the supplemental program from the appropriate third party program
vendor 400,
and stores the actual document or information relating to the service of the
supplemental
program in the supplemental program database 303 (or other temporary memory).
Thus, the
SP module has, within one or more of its databases, the actual document or
information
relating to the service of the supplemental program. Thereafter, the SP module
logs the
response from the third party program vendor 400 in a log table at step 3640,
the log table
being stored within the cohort database 305.
[0330] Next, the central portion 301 of the SP module generates a response
that comprises
the actual document or information relating to the service of the supplemental
program. It
should be noted that the response may comprise more than one document or other
supplemental program related information. Thereafter, the central portion 301
of the SP
module transmits the response, along with the associated documents and
information, to the
SP widget 302, thereby completing step 3643.
[0331] Upon receiving the response, the SP widget 302 may display a GUI to the
provider
101 that allows the provider 101 to distribute the document or other
supplemental program
information to the patient. In an alternate embodiment of the present
invention, the SP
widget 302 may transmit the documents directly to a printer without requiring
provider
interaction. Nonetheless, it should be noted that, upon receiving the
response, the SP widget
302 may cause the documents and other information to be disseminated in any
manner
consistent with the present invention.
3. Receiving Patient Adherence Data
[0332] After the SP module activates the supplemental programs of the cohort
that were
selected and accepted by the provider 101, the patient receives the activated
supplemental
programs. Thereafter, as discussed in detail below, the SP module receives
patient
adherence data relating to the electronic prescription. It should be noted
that since there are
97
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
a plurality of different cohorts and since each cohort comprises a sub-set of
health care
providers 101, the SP module will receive patient adherence data relating to a
plurality of
different electronic prescriptions generated by different health care
providers 101, some of
these prescriptions for a target drug of a cohort and other not. Further, the
SP module will
be reaching patient adherence data while the program cohort groups are still
active.
Thereafter, upon receiving patient adherence data, the SP module must parse
the patient
adherence data by the cohort in which the patient adherence data relates.
[0333] As noted above, according to one embodiment of the present invention,
the memory
313 of the SP system 300 further comprises a Patient Adherence Generation
Module 306.
The SP system 300, and more particularly the Patient Adherence Generation
Module 306,
receives patient medication history data relating to a plurality of electronic
prescriptions for
which supplemental programs were previously activated. It should be noted that
the
received patient medication history may, but does not have to relate to
prescriptions that met
all of the cohort rules and caused the activation of the permutation of
supplemental programs
for a cohort. Stated another way, the Patient Adherence Generation Module 306
receives
patient medication history data relating to a plurality of electronic
prescriptions, regardless of
whether supplemental programs of a cohort (as opposed to supplemental programs
in general)
were activated for the prescription. As discussed in more detail below, the
Patient
Adherence Generation Module 306 receives the patient medication history data
from another
system, such as but not limited to the pharmacy system 500 and the payor
system 600.
[0334] Generally, the patient medication history data comprises information
relating to the
medication history of the patient for one or more prescribed substances. This
may, but does
not necessary include prescriptions for the target drug of a cohort. For
example, patient
medication history may comprise any of the substance data described above
(e.g., substance
details such as the dosage, strength, form, duration, quantity, date, and
refills) with reference
to a prescription, the prescription start date and stop date, information
relating to whether the
patient picked up the prescription, the duration in which the prescription sat
at the pharmacy
prior to patient pickup, the number of refills of the prescription that the
patient filled, and the
time frame between refills in comparison to the duration of each prescription.
Further, it
should he noted that the patient medication history data may comprise
information relating to
any number of prescriptions for any number of patients, regardless of whether
supplemental
programs were activated by the SP module for those prescriptions.
98
CA 02918798 2016-01-19
WO 2015/013695
PCT/1JS2014/048330
[0335] However, in one embodiment of the present invention, the Patient
Adherence
Generation Module 306 only receives patient medication history data for
prescriptions in
which supplemental programs were activated. Further, in other embodiments, the
Patient
Adherence Generation Module 306 only receives patient medication history data
for
prescriptions of a target drug of a cohort in which the permutation of
supplemental program
of that cohort were activated.
[0336] After receiving the patient medication history data, the Patient
Adherence Generation
Module 306 generates patient adherence data from the received patient
medication history
data. After generating the patient adherence data, the Patient Adherence
Generation Module
306 stores the patient adherence data in the patient adherence database 307.
According to
one embodiment of the present invention, the Patient Adherence Generation
Module 306
generates the patient adherence data by applying the received patient
medication history data
to one or more algorithms configured to deteimine patient adherence metrics
from the
received patient medication history data. Through use of the algorithms, the
Patient
Adherence Generation Module 306 generates one or more adherence analytics for
a particular
prescription and/or medication history data of a patient. In such embodiments,
the
algorithms are stored in the patient adherence database 307 of the SP system
300.
[0337] It should be noted that, in accordance with one embodiment of the
present invention, a
prescription and drug fill (which is a type of patient medication history data
¨ e.g., the date on
which a prescription was filled by a patient) must qualify for calculating
patient adherence for
a given patient and substance in order to be used by the SP module when
determining patient
adherence data. A prescription qualifies if the prescription matches the
target drug by name,
has a stop date that is after the compliance interval start date, and is
either the most recent
matching prescription or has a stop date that is within N days (e.g. 30) of
the start date of the
next most recent prescription. A drug fill qualifies if it matches the target
drug by name, has
a fill date that is after the compliance interval start date, and has a fill
date that is between the
start and stop date of a qualifying prescription. The compliance interval
start date is the
start of a compliance interval of interest.
[0338] Generally, patient adherence data comprises information relating to a
patient's
adherence, compliance, and/or persistency to prescriptions issued to the
patient. As noted
above, the prescriptions may, but do not necessarily have to be for the target
drug of a cohort.
For example, the patient adherence data may comprise information relating to
the patient's
99
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
first fill compliance of a prescription, the patient's first fill interval of
a prescription, the
patient's compliance interval of a prescription, and any other information
relating to the
patient's adhere, compliance, or persistency to a prescription.
[0339] For further example, in one embodiment of the present invention,
patient adherence
data comprises first fill compliance (FFC) data and patient Medication
Persistency Rate
(MPR) data. Generally, FFC data comprises information relating to whether or
not the
patient has been prescribed a particular prescribed substance (e.g., the
target drug) in the past
and/or whether the patient picked-up or took the particular prescribed
substance (e.g., the
target drug) in the past.
[0340] Further, in one embodiment of the present invention, FFC data of a
prescription has
three possible values: (1) present; (2) absent; or (3) unknown. The FFC data
has a value of
present if there exists a qualifying drug fill where: (1) the fill date of the
prescription is after
the start date of the prescription: and (2) the fill date of the prescription
is before the end of
the end of the first fill interval of the prescription. In such instances, the
prescription was
filled by the patient after the prescription was written by the health care
provider 101, but
before the end of the first fill interval of the prescription. Therefore, the
prescription is first
fill compliant.
[0341] The FFC data has a value of unknown if the start date of the
prescription is after the
end of the first fill interval of the prescription. In such instances, the
prescription occurred
after the first fill interval and therefore may be a refill of the
prescription. Thus, information
relating to a refill of a prescription does not indicate whether the patient
was compliant with
filling their prescription on the first fill. In all other instances, the FFC
data has a value of
absent.
[0342] Generally, MPR data comprises information relating to the patient's
adherence to a
particular prescribed substance (e.g., the target drug). For example, MPR data
may
comprise information relating to the degree or extent of conformity of the
patient to the
recommendations about day-to-day treatment by the health care provider 101
with respect to
the timing, dosage, and/or frequency of a particular prescribed substance
(e.g., the target
drug), information relating to the extent to which a patient acts in
accordance with the
prescribed interval and dose of a dosing regimen of the particular prescribed
substance (e.g.,
the target drug), and information relating to the continuation the treatment
by the patient for
the prescribed duration of the particular prescribed substance (e.g., the
target drug).
100
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
Therefore, in one embodiment of the present invention, the MPR data comprises,
as a
percentage, information relating to how often the patient filled a
prescription compared with
how often s/he could have filled it optimally. Finally, it should be noted
that in some
embodiments of the present invention, MPR data may be referred to as
medication possession
ratio data.
[0343] According to one embodiment of the present invention if there are fewer
than three
qualifying fills of a particular prescription, then the MPR data for that
prescription has a
value of unknown. Otherwise, if there are three or more qualifying fills of a
particular
prescription, then the MPR data is calculated as follows. First, the Patient
Adherence
Generation Module 306 of the SP system 300 calculates compliance interval days
for the
prescription. Compliance interval days are the number of days in a compliance
interval, or
the number of days that a supply of prescription is available to the patient
during the
compliance interval. For each qualifying prescription, the Patient Adherence
Generation
Module 306 determines the adjusted prescription start date (the later of the
start date of the
prescription and the compliance interval start date), the adjusted
prescription stop date (the
earlier of the prescription stop date or the current date), and the compliance
interval days of
the prescription (the adjusted stop date minus the adjusted start date).
[0344] Next, the Patient Adherence Generation Module 306 calculates the total
fill days of
the prescription. Total fill days is a measure of the number of days that a
supply of a
prescription is actually filled by the patient during the compliance interval.
For each
qualifying prescription, the Patient Adherence Generation Module 306
calculates the adjusted
fill start date (the later of the fill date of the prescription and the
compliance interval start date
of the prescription) and the adjusted fill stop date (the earlier of the fill
date plus the fill days
and the current date). Next, the Patient Adherence Generation Module 306
calculates the
total fill days of the prescription (the adjusted fill stop date minus the
adjust fill start date).
Finally, the Patient Adherence Generation Module 306 calculates the MPR data
for the
prescription (the total fill days divided by the compliance interval days ¨
multiplied by 100 to
show as percentage).
[0345] After the patient adherence data is generated by the Patient Adherence
Generation
Module 306, the Patient Adherence Generation Module 306 transmits the patient
adherence
data to the central portion 301 of the SP module so that the SP module may
parse and analyze
the patient adherence data by cohort to determine the effectiveness of a
plurality of different
101
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
permutations of supplemental programs on patient adherence.
[0346] Referring to FIGS. 37a-37b, a flow chart of a method of parsing patient
adherence
data into data grouping based on a plurality of different cohorts according to
an embodiment
of the present invention is illustrated. The method 3700 begins when the SP
module
receives patient adherence data relating to a plurality of electronic
prescriptions, thereby
completing step 3701. It should be noted that the SP module may receive
patient adherence
data on a periodic basis (e.g., once a day), or the SP module may receive
patient adherence
data on a continuous basis. Further, the patient adherence data may relate to
prescriptions
that were associated with a particular cohort or not. As discussed in more
detail below, the
SP module parses the patient adherence data on the basis of cohort to
determine the
effectiveness of the permutation of supplemental programs of each cohort.
[0347] After receiving patient adherence data, the SP module processes the
data and extracts
the provider's NPI number and prescribed substance name from the electronic
prescription
associated with the patient adherence data, thereby completing step 3702 and
3703. It
should be noted that the patient adherence data comprises information that
relates to a
corresponding electronic prescription processed by the EP module, and is
therefore stored
within the record database 304 of the SP system 300. As a result, the SP
module can extract
the provider's NPI number and substance name from the underlying electronic
prescription
associated with the patient adherence data.
[0348] After extracting the provider's NPI number and substance name, the SP
module
determines whether the health care provider 101 identified by the NPI number
is associated
or part of a cohort, thereby completing decision step 3704. According to one
embodiment
of the present invention, the determination is made by the SP module by a
cross-referencing
of the cohort relation table discussed above. In such instances, the SP module
may
cross-reference the provider's NPI number with the cohort relation table to
determine
whether the provider is part of a cohort. If the provider 101 identified by
the NPI number is
not associated with a cohort, then the method returns to step 3702 and the SP
module
processes patient adherence data relating to another prescription. However, if
the provider
101 identified by the NPI number is associated with a cohort, then the method
continues to
decision step 3705.
[0349] At decision step 3705, the SP module deteimines whether the prescribed
substance
identified by the electronic prescription is associated with one of the
provider's cohort(s)
102
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
identified in step 3704. Similar to above and according to one embodiment of
the present
invention, the determination is made by the SP module by a cross-referencing
of the cohort
relation table discussed above. In such instances, the SP module may cross-
reference the
prescribed substance name with the cohort relation table to determine whether
the prescribed
substance is associated with one of the provider's cohort(s) identified in
step 3704. If the
prescribed substance name does not correspond with the prescribed substance of
one of the
provider's cohort(s), then the method returns to step 3702 and the SP module
processes
patient adherence data relating to another prescription. However, if the
prescribed substance
name does correspond with the provider's cohort, then the method continues to
step 3706.
[0350] It should be noted that in such instances, the patient adherence data
relates to an
electronic prescription that meets all the cohort rules of the provider's
cohort. However, at
decision step 3706, the SP module determines whether the electronic
prescription caused the
permutation of supplemental programs of the provider's cohort were activated
by the SP
module. Stated another way, the SP module determines whether the cohort rules
were met
by the electronic prescription and whether the current counter was at or below
the maximum
counter when the SP module processed the electronic prescription when
determining if the
permutations of supplemental programs for the cohort would be activated.
[0351] In one embodiment of the present invention, when a permutation of
supplemental
programs of a cohort is activated for a prescription, the SP module tags the
corresponding
prescription. The corresponding prescription, the permutation of supplemental
programs,
and the tag are all stored in correlation with each other in the records
database 304 or another
databases of the SP system 300. Thereafter, the SP module uses the tag to
determine
whether the permutation of supplemental programs of a cohort was activated for
an electronic
prescription. For example,
when receiving patient adherence data, SP module
cross-references the records database 304 to see if the appropriate tag is
associated with the
corresponding prescription, thereby indicating that the permutation of
supplemental programs
was activated. Nonetheless, the invention is not so limited, and in alternate
embodiments of
the present invention, the Patient Adherence Generation Module 306 may
determine whether
the permutation of supplemental programs was activated for a particular
prescription using
other methods.
[0352] Next, after the SP module determines that the electronic prescription
caused the
permutation of supplemental programs of the provider's cohort to be activated
by the SP
103
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
module, the SP module associates the patient adherence data with the
corresponding cohort of
the health care provider 101, thereby completing step 3707. Thereafter, the SP
module
stores the patient adherence data in association with the health care
provider's cohort in the
cohort database 305, thereby completing step 3708.
[0353] Next, at decision step 3709, the SP module determines whether all of
the received
patient adherence data for all the prescriptions has been parsed by cohort. If
so, then the
process ends at step 3711. However, if there remains patient adherence data
that has not bee
parsed by the SP module, then the method 3700 continues to step 3710, and as
such, returns
to step 3702 discussed above.
[0354] According to one embodiment of the present invention, more than one
prescription of
a patient may be combined in order to determine compliance data. Thus, if a
patient has
more than one prescription for a given substance, it may be the case that the
prescriptions
should be combined for the purposes of calculating compliance data. For
example,
prescriptions may be combined if they are for the same patient and the same
substance, and
the prescriptions have overlapping prescription dates. For example, if
prescription 1 has a
start date of 3/1/11 and stop date of 6/1/11 and prescription 2 has a start
date of 5/1/11 and a
stop date of 10/1/11, then the prescriptions may be combined and considered
one prescription
for the purposes of determining patient adherence data.
4. Analyzing the Patient Adherence Data to Determine the Effectiveness of the
Different Permutations of Supplemental Programs on Patient Adherence
[0355] After parsing the patient adherence data by cohort, the SP module
analyzes the patient
adherence data to determine the effectiveness of each of the different
permutations of
supplemental programs on patient adherence. In one embodiment of the present
invention,
when the current counter of all the cohorts of a program cohort group reach
the maximum
counter, the SP module ceases to activate the permutations of supplemental
programs for the
cohorts of the program cohort group, and begins to analyze patient adherence
data to
determine the effectiveness of the different permutations of the supplemental
programs of a
program cohort group on patient adherence. However, in other embodiments of
the present
invention, the SP module may analyze patient adherence data continuously in
real-time, even
as the SP module is also still activating permutations of supplemental
programs for a program
104
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
cohort group.
[0356] According to one embodiment of the present invention, one of the
cohorts out of the
plurality of cohorts of the program cohort group is a control group. Since the
control group
either does not comprise any associated supplemental programs (i.e., the
permutation of
supplemental programs of the control group is empty) or the control group
comprises one or
more supplemental programs that are also associated with every one of the
other cohorts of
the program cohort group, then the control group can be used as a basis of
comparison to
determine the effectiveness of the supplemental programs of the other cohorts
of the program
cohort group. Stated more simply, the SP module may determine the
effectiveness of the
permutations of supplemental programs by using the control group as a baseline
indicator of
standard patient adherence to the target drug.
[0357] In embodiments that do not comprise a control group, the basis of
comparison may be
the average patient adherence for the target drug in general. This may be
determined by the
SP module in many ways, including but not limited to, the patient adherence
data for the
target drug that is stored within the patient adherence database 307 and was
generated by the
Patient Adherence Generation Module 306. Further, in other embodiments that do
not
comprise a control group, the different cohorts may be simply compared to one
another, with
the assumption that the permutations of supplemental programs have a
beneficial effect on
patient adherence. After determining a basis of comparison (e.g., the control
group), the SP
module will analyze the patient adherence data of each cohort of a program
cohort group to
determine the effectiveness of each of the different permutations of
supplemental programs
on patient adherence.
[0358] In one embodiment of the present invention, the SP module determines
the
effectiveness using one or more algorithms that are configured to compare
multiple sets of
data to one another. According to one embodiment of the present invention, the
algorithms
are stored within the cohort database 305. The data that is compared to
determine the
effectiveness of the permutations of supplemental programs on patient
adherence includes,
but is not limited to, patient adherence data, coupon redemption data, and
patient feedback
data.
[0359] After the data is compared using the one or more algorithms, the SP
module may
display effectiveness data on a display device to an administrator of the SP
system 300.
This allows the administrator of the SP system 300 to visually interpret which
permutation of
105
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
supplemental programs was most effective for encouraging patient adherence to
the target
drug. As discussed in more detail below, the effectiveness data may be
displayed in
graphical representations or in numerical values.
[0360] Referring to FIG. 38a, a graphical representation of the effectiveness
of different
permutations of supplemental programs on patient's first fill compliance
according to one
embodiment of the present invention is illustrated. For example, in one
embodiment of the
present invention. the SP module determines the effectiveness of the
permutations of
supplemental programs by comparing the first fill compliance data of the
prescriptions of a
cohort to the other cohorts of the program cohort group. It should be noted
that first fill
compliance (FFC) data indicates whether the patient filled the medication
promptly after
receiving the prescription. Further, in embodiments where one of the cohorts
out of the
plurality of cohorts is a control cohort, the SP module will compare the FFC
data of the
prescriptions of each cohort with the control cohort in order to determine the
effectiveness of
the supplemental programs of each cohort. The permutation of supplemental
programs of
the cohort whose prescriptions have the highest FFC data will be considered
the most
effective on patient adherence. This is because the patients receiving that
permutation of
supplemental programs of that cohort were more likely to comply with the first
fill of the
prescription, and this increase in adherence is determined to be due from the
supplemental
programs those patients received.
[0361] Referring to FIG. 38b, a graphical representation of the effectiveness
of different
permutations of supplemental programs on patient's medication persistency rate
displayed on
a display device according to one embodiment of the present invention is
illustrated. For
further example, in one embodiment of the present invention, the SP module
determines the
effectiveness of the permutations of supplemental programs by comparing the
patient
Medication Persistency Rate (MPR) data of the prescriptions of a cohort to the
other cohorts
of the program cohort group. It should be noted that MPR data is a percentage
(from
1-100%) that indicates how often a patient filled a medication compared with
how often s/he
could have done so. If there is no available MPR data, a result is returned
indicating N/A in
place of an MPR percentage. Specifically, in embodiments where one of the
cohorts out of
the plurality of cohorts is a control cohort, the SP module will compare the
MPR data of the
prescriptions of each cohort with the control cohort in order to determine the
effectiveness of
the supplemental programs of each cohort. The permutation of supplemental
programs of
106
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
the cohort whose prescriptions have the highest MPR data will be considered
the most
effective on patient adherence. This is because the patients receiving that
peimutation of
supplemental programs of that cohort were more likely to fill their
prescription when having
the opportunity.
[0362] Specifically, referring to FIGS. 38a and 38b concurrently, cohort
number 1 is the
control cohort, while cohort numbers 2-6 are other cohorts of the program
cohort group that
were each assigned a different permutation of supplemental programs to measure
the
effectiveness of those programs on patient adherence. It should be noted that
cohort number
1 was not assigned any supplemental programs (i.e., its permutation of
supplemental
programs is empty). Further, the program control group is program control
group 8, and the
target drug is Lipitor . As illustrated in FIG. 38a, cohort number 1, which
was the control
group, has a FFC of 60%. This means that 60% of the prescriptions that were
written by the
health care providers 101 of cohort 1 for the target drug were filled within
the first fill
interval of the prescription. Further, as illustrated in FIG. 38b, cohort
number 1, which was
the control group, has a MPR of 45%. This means that, on average, the patients
who were
prescribed the target drug by the health care providers 101 of cohort 1 had an
MPR of 45%.
Cohort number l's PVC of 60% and MPR of 45% may be used as a baseline to
determine the
effectiveness of the other permutations of supplemental programs were assigned
to the other
cohorts.
[0363] As noted above, FIGS. 38a and 38b are two examples of graphical
representations of
the effectiveness of different permutations of supplemental programs on
patient adherence.
As exemplified in FIG. 38a, cohort number 5 has the highest FFC rate of 90%,
and therefore,
the permutation of supplemental programs assigned to cohort number 5 is the
most effective
at increasing patient's first fill compliance to the target drug. By contrast,
cohort number 2
has the lowest FFC rate of the non-control cohorts of 63%, and therefore, the
peimutation so
supplemental programs assigned to cohort number 2 is the least effective in
increasing
patient's first fill compliance to the target drug. Moreover, as exemplified
in FIG. 38b,
cohort number 4 exemplifies the highest MPR of 81%, and therefore, the
peimutation of
supplemental programs assigned to cohort number 4 is the most effective at
increasing a
patient's persistence to the target drug regimen. By contrast, cohort number 3
exemplifies
the lowest MPR rate of the non-control cohorts of 58%, and therefore, the
peimutation of
supplemental programs assigned to cohort number 3 is the least effective at
increasing a
107
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
patient's persistence to the target drug regimen. Thus, as illustrated,
depending on the
means of measurement of patient adherence (FFC, MPR, etc.), different
permutations of
supplemental programs may be most effective. As a result, by using cohorts to
test the
effectiveness of supplemental programs on a variety of different measurements
of patient
adherence, the SP module may be used to determine the most effective
combination of
supplemental programs to increase patient adherence in the most desired
manner.
[0364] However, it should be noted that the invention is not so limited, and
in other
embodiments of the present invention, the SP module determines the
effectiveness of the
permutations of supplemental programs by comparing one or more forms of
patient
adherence data of the prescriptions of the plurality of cohorts of the program
cohort group.
Stated another way, the invention is not limited to comparing FFC data and MPR
data when
determining the effectiveness of the plurality of cohorts of a program cohort
group. Any
patient adherence data may be used by the SP module. Finally, it should be
noted that the
graphic representation exemplified in FIGS. 38a and 38b may be displayed on a
display
device of the administrator of the SP system 300, to a pharmaceutical company,
or to any
other person or system of the system 1000.
[0365] For even further example, in another embodiment of the present
invention, every
cohort of a program cohort group, including a control cohort comprises a
supplemental
program that distributes a coupon for the target drug to the patient upon
activation. In such
instances, the non-control cohorts of the program cohort group all comprise
different
supplemental programs in addition to the supplemental program that distributes
a coupon.
As a result, the SP module measures the effectiveness of each permutation of
supplemental
programs by comparing the rates of coupon redemption of the prescriptions of
each cohort of
the program cohort group. Therefore, the patients who are receiving the
permutation of
supplemental programs of the cohort with the highest effectiveness on patient
adherence will
have redeemed their coupon more often than the patients receiving the
permutations of
supplemental programs of the other cohorts.
[0366] According to one embodiment of the present invention, after determining
the
effectiveness of the supplemental programs on patient adherence, the SP module
generates
patient adherence reports based on the determined effectiveness. After
generation of the
reports, the SP module mail deliver the reports to any one of the
administrators of the SP
system 300, any other system or module of the system 1000, or a particular
pharmaceutical
108
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
company. Examples of graphical reports are exemplified in FIGS. 38a and 38b.
Nonetheless, it should be noted that the invention is not limited to any
specific type or foimat
or graphical report. Further, in alternate embodiments of the present
invention, the patient
adherence data of each cohort may be displayed purely as numerical value, as
numerical
values and graphical representations, or just graphical representations.
[0367] After the graphical report is generated by the SP module, the graphical
report is saved
to the cohort database 305 of the SP system 300. Further, according to one
embodiment of
the present invention, the cohort relation table is updated with the graphical
report stored in
association with the associated health care provider 101 and cohort of the
graphical report.
Patient Advisor System
[0368] According to another aspect of the invention, a patient advisor system
is provided for
tracking patient adherence to following a prescription drug or medication
treatment regimen,
and timely reporting details on the same to the health care provider
contemporaneous with the
time and at the point of service. In one embodiment, as shown in FIG. 1, the
patient advisor
system may comprise a patient adherence system 360 which is in operable
communication
with other component systems of the overall system such as via the internet or
other suitable
communication links. The patient adherence system 360 is configured and
operable to
retrieve, analyze, calculate, and transmit display patient adherence
infoimation via interacting
with the other sub-systems. The patient adherence system 360 includes a
Patient Adherence
Generation Module 306 previously described herein.
[0369] In one embodiment, the patient advisor system further is configured to
generate a user
interface which may be in the form of an interactive toolbar 2004 (see, e.g.
FIG. 42) that is
presented on a display of the HCP system or another display, as further
described below.
The toolbar provides a GUI interface that provides a health care provider or
other user with
access to various content related to patient medication adherence information
and metrics,
educational materials, clinical studies, and other information relevant to
prescription
medications for improving patient compliance with their medication regimens.
[0370] Embodiments of the toolbar 2004 may include a visual indicia displayed
on the
toolbar which alerts the health care provider of an "at risk" patient that is
not following his or
her prescribed medication regimen so that the health care provider can
intervene with the
patient at the time and point of service. In further embodiments of the
patient adherence
system, the degree to which the "at risk- patient has not been following the
prescribed
109
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
medication regimen is also graphically displayed in a manner which is visually
and/or
audibly readily recognizable by the health care provider using various indicia
and/or sounds
as described herein. The toolbar 2004 is further operable to access a patient
advisor report
card 2200 (see, e.g. FIG. 46) which gives the user a graphical overview of the
patient's active
medication list and associated medication adherence information, as further
described below.
[0371] The patient advisor system advantageously enables health care providers
to support
patients between office visits with evidence-based patient education, co-pay
savings, tools
and resources. The system aims to help reduce first fill prescription
abandonment, improve
medication adherence rates with sustainable results, and allow providers to
gain insight into
patient behaviors. In one embodiment, the patient advisor system includes a
set of services
presented which may be accessed via the toolbar 2004 displayed across the EP
system GUI
such that when a patient has been identified, relevant information and
services will be made
accessible. In addition, a series of web service APIs are provided between the
EP system
200 and the patient advisor system such that during the course of the drug
prescribing event,
the patient advisor system can provide context based co-pay savings and
adherence materials
for diseases and illnesses related to the drug being prescribed. Moreover, the
patient advisor
system is helpful to the health care provider in assessing whether symptoms
observed during
the present patient visit may possibly be attributable to the patient's lack
of compliance with
their prescribed medication regimen so that an appropriate intervention can be
implemented.
[0372] FIG. 39 illustrates an exemplary embodiment of a system 1000 which
includes a
patient adherence system 360 having patient adherence reporting and display
capabilities.
[0373] Referring initially to FIG. 1, the system 1000 includes the HCP system
100, EP
system 200, SP system 300, pharmacy system 500, payor system 600, and a
patient adherence
system 360. Although all of the elements of the IICP system 100, EP system
200, SP
system 300, pharmacy system 500, and payor system 600 are not explicitly
depicted in FIG.
39, it should be recognized these systems are nonetheless included as shown in
FIG. 1 and
may be similar as discussed above in detail with reference to FIGS. 1-7. It
should be noted
that the communication links between the foregoing systems and components
forming each
part of the system (as illustrated via lines with arrows showing the flow of
data/information
exchange and signal transmission) may be accomplished by any suitable data
communication
link such as without limitation via the Internet (see, e.g. FIG. 1) and/or
another form of
wireless or wired communication links depending on the physical location and
proximity of
110
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
each system and components which operably interface with patient adherence
system 360.
[0374] With reference to FIG. 39, the patient adherence system 360 in one
embodiment
generally includes a Medication History ("Medication Hx" for short) Service
382, a Patient
Adherence Core 388, and a Payor Engine 318. Authentication server 380 is also
shown
which interacts with the patient adherence system 360 and EP system 200, as
further
described herein.
[0375] Patient adherence system 360 may further include a patient adherence
database 307, a
patient medication history database 308, and a candidate prescription database
309 as shown
in FIG. 39. In general, patient adherence database 307 stores patient
medication adherence
data and calculated adherence metrics (e.g. MAR/MPR, FFC, etc.) which may be
categorized
by each patient. The patient medication history database 308 stores patient
medication
history data which may be categorized by each patient. The candidate
prescription database
309 stores prescription data which may be categorized and associated with each
patient. In
one embodiment, databases 307, 308, and 309 may be embodied in computer
readable
medium 384 accessible to one or more of the servers which are configured for
storing,
retrieving, and organizing data stored in each database, as described below.
In various
embodiments, two of the foregoing databases may be combined and/or additional
databases
may be provided.
[0376] Medication Hx Service 382 is a functional sub-system of patient
adherence system
360 which includes Candidate Prescription Server 315 and Medication Hx Poller
350.
Medication Hx Poller 350 is a software program or routine running on and which
configures
Candidate Prescription Server 315 to perform the functions further described
herein. In one
embodiment, Candidate Prescription Server 315 is operably connected to patient
medication
history database 308 and a candidate prescription database 309 for data
exchange shown in
FIG. 39.
[0377] Patient Adherence Core 388 is a functional sub-system of the patient
adherence
system 360 which includes Adherence Server 316 and Patient Adherence
Generation Module
306. Patient Adherence Generation Module 306 is a software program or routine
running
on and which configures Adherence Server 316 to perform the functions further
described
herein. In one embodiment, Adherence Server 316 is operably connected to
patient
adherence database 307 for data exchange as shown in FIG. 39.
111
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
[0378] In patient adherence system 360 shown in FIG. 39, it should be noted
that the Patient
Adherence Generation Module 306 (see, e.g. FIG. 4), the Medication History
Poller 350 (see,
e.g. FIG. 16), and the patient adherence database 307 (see, e.g. FIG. 4) of
patient adherence
system 360 may generally be similar in configuration and function to those as
described
above and previously shown in the parenthetical figures. In other embodiments,
these
components may the different.
[0379] The patient medication history database 308 stores information relating
to the
medication history of patients. Generally, the patient medication history data
comprises
information relating to the medication history of the patient for one or more
prescriptions of
one or more prescribed substances or drugs. Patient medication history data
includes, but is
not limited to, information relating to prescriptions written for a patient
and information
relating to prescription fills and refills (i.e., drug fill and refill data).
More specifically,
patient medication history may comprise any of the drug-related data described
above (e.g.,
drug details such as the dosage, strength, form, duration, quantity, date, and
refills) with
reference to a prescription, the prescription start date and stop date,
information relating to
whether the patient picked up the prescription, the duration in which the
prescription sat at
the pharmacy prior to patient pickup, the number of refills of the
prescription that the patient
filled, the time frame between refills in comparison to the duration of each
prescription,
information relating to the prescription filling sub-system 502, information
relating to the
payor, and information relating to the patient. In one embodiment of the
present invention,
the medication history relates to prescriptions that were processed by the EP
module or the
SP module. However, the invention is not so limited, and in other embodiments,
the patient
medication history relates to all prescriptions that were previously processed
for any patients.
Further, it should be noted that the patient medication history data may be
similar to the
patient medication history data stored in the EP database 201 and the record
database 304, as
discussed in more detail above.
[0380] Although exemplified as two separate databases, it should be noted that
the invention
is not so limited, and in other embodiments of the present invention, the
candidate
prescription database 309 and the patient medication history database 308 may
be combined.
Further, in one embodiment of the present invention, the candidate
prescription database 309,
the patient medication history database 308, and the patient adherence
database 307 may be
combined into a single database. For example, in one such embodiment, the
candidate
112
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
prescription database 309 and the patient medication history database 308 may
be combined
into the patient adherence database 307. Thus, in such embodiments, the
patient adherence
database 307 stores prescription data, patient medication history data, and
patient adherence
data.
[0381] According to the one embodiment, with reference to FIG. 39, the
Candidate
Prescription Server 315 of the Medication Hx Service 382 may receive data from
the SP
system 300 relating to patient prescriptions in which supplemental programs
were activated.
by the SP Candidate Prescription Module 317. Specifically, the Candidate
Prescription
Server 315 in this case receives supplemental program (SP) events relating to
electronic
prescriptions from a SP Candidate Prescription Module 317 which is a software
program or
routine that runs on SP Server 310 (see also FIG. 4) and resides on the SP
system 300 in a
non-transitory computer readable medium such as without limitation memory 313.
[0382] In general, the SP Candidate Prescription Module 317 polls the record
database 304 as
shown in FIG. 39 for information relating to electronic prescriptions in which
supplemental
programs were activated. Thus, in one embodiment of the present invention,
when the SP
module activates one or more supplemental programs for an electronic
prescription, the SP
Candidate Prescription Module 317 generates a record of such and stores the
record in the SP
record database 304. These records are called "SP events" herein and comprise
information
relating to the patient, the prescribed substance, and the activated
supplemental programs for
the electronic prescription. However, in other embodiments of the present
invention, the SP
module generates SP events after activating supplemental programs.
[0383] Therefore, according to one embodiment of the present invention, the SP
candidate
prescription server 317 periodically polls the record database 304 for SP
events. Upon
identifying SP events, the SP candidate prescription server 317 creates a log
of SP events and
stores the log in the record database 304. Thereafter, the SP candidate
prescription server
317 transmits the log comprising the SP events to the candidate prescription
server 315.
According to one embodiment of the present invention, the SP candidate
prescription server
317 transmits the log comprising the SP events to the Candidate Prescription
Server 315 on a
daily basis. However, the invention is not so limited, and in other
embodiments, the SP
candidate prescription server 317 transmits the log comprising the SP events
to the candidate
prescription server 315 on a periodic basis other than daily including "on the
fly" (i.e. on
demand) upon receiving a request.
113
CA 02918798 2016-01-19
WO 2015/013695
PCT/1JS2014/048330
[0384] According to one embodiment of the present invention, after receiving
the log of SP
events, the Candidate Prescription Server 315 transmits the log of SP events
to the Candidate
Prescription Module 220. After receiving the log of SP events, the Candidate
Prescription
Module 220 periodically polls the EP database 201 for prescriptions that match
the
prescriptions identified in the log of SP events. After identifying the
relevant prescriptions,
the Candidate Prescription Module 220 retrieves information relating to those
prescriptions
and transmits the prescription data to the Candidate Prescription Server 315.
Thus, the
Candidate Prescription Server 315 operably receives prescription data from the
EP database
201; the prescription data relating specifically to those prescriptions
identified in the log of
SP events and in which supplemental programs were activated by the SP module.
According to one embodiment of the present invention, the candidate
prescription module
220 matches prescriptions to SP events using the patient information, the drug
name, the
insurance companies, the drug classifications, etc. and only transmits
prescription data
relating to those prescriptions to the candidate prescription server 315.
[0385] Therefore, after receiving the log of SP events from the SP Candidate
Prescription
Module 317, the Candidate Prescription Server 315 receives prescription data
relating to the
prescriptions identified in the log of SP events from a Candidate Prescription
Module 220.
According to one embodiment of the present invention, the Candidate
Prescription Module
220 is a software program or routine that resides on the EP system 200 and
polls the EP
database 201 for new prescription data. Thus, by providing the Candidate
Prescription
Server 315 with the log of SP events, the candidate prescription server, and
ultimately the
Medication Hx Poller 350, may receive more detailed information relating to
the
prescriptions in which supplemental programs were activated from the EP
database 201. In
turn, the Patient Adherence Generation Module 306 may then generate patient
adherence data
for those prescriptions in order to perform any subsequent processing (e.g.,
determining the
effectiveness of different permutations of supplemental programs for cohort
analysis).
However, the invention is not so limited, and in alternate embodiments, the
Candidate
Prescription Module 220 may reside on the patient adherence system 360 or the
SP system
300.
[0386] According to another embodiment of the present invention, the Candidate
Prescription
Server 315 simply receives information relating to all prescription events
from both the SP
Candidate Prescription Module 317 and/or the Candidate Prescription Module
220. In such
114
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
embodiments, whenever a prescription is processed by either the EP module or
the SP
module, an event is recorded by the respective module of the EP system or the
SP system.
Thereafter, the Candidate Prescription Module 220 or SP Candidate Prescription
Module 317
transmits information relating to that prescription (e.g., prescription data)
to the Candidate
Prescription Server 315. Thus, in such embodiments, the Candidate Prescription
Server 315
receives prescription data for all prescriptions that were processed by either
the EP module or
the SP module. Nonetheless, it should be noted that in other embodiments of
the present
invention, any number of constraints may be placed on the transmission of
prescription data
to the Candidate Prescription Server 315.
[0387] In yet other embodiments, the Candidate Prescription Server 315 may
receive
information relating to all prescription events from only the Candidate
Prescription Module
220 of the EP system for which prescriptions are processed. Accordingly, it
will be
appreciated that any of the foregoing process and data flow paths for
transmitting prescription
data to Candidate Prescription Server 315 may be accomplished by configuring
the Candidate
Prescription Server with an appropriately constructed computer program
instructions.
[0388] The prescription data is information relating to prescriptions that
were written by a
health care provider 101 for a patient. Prescription data includes, but is not
limited to,
patient information, prescribed substance information (name, date, dosage,
refills, strength,
etc.), health care provider information, and payor information. In one
embodiment of the
present invention, the received prescription data is only for electronic
prescriptions in which
supplemental programs were activated for the patient by the SP module.
However, the
invention is not so limited, and in alternate embodiments, the candidate
prescription server
315 may receive prescription data for prescriptions in which supplemental
programs were not
activated. After receiving the prescription data, the candidate prescription
server 315 stores
the prescription data in the candidate prescription database 309. Thus, the
candidate
prescription database 309 stores information relating to prescriptions that
were processed by
the EP module and/or the SP module.
[0389] The foregoing components of the patient adherence system 360 and
related processes
are described in further detail below.
[0390] Candidate Prescription Server
[0391] Referring to FIG. 40, a schematic flow chart of the processing of the
Candidate
115
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
Prescription Server 315 according to one embodiment of the present invention
is illustrated.
FIG. 40 exemplifies one non-limiting example of a method 4000 depicting steps
showing
how the Candidate Prescription Server 315 may receive prescription data
according to one
embodiment of the present invention. In step 4001, the Candidate Prescription
Server 315
receives a list of prescriptions. As noted above, this may be from either the
SP Candidate
Prescription Module 317 or the Candidate Prescription Module 220. After
receiving the list
of prescriptions, the Candidate Prescription Server 315 logs the request in
step 4002, and
picks a prescription off of the list in step 4003. Moreover, it should be
noted that the
Candidate Prescription Server 315 may receive information relating to all
prescriptions
processed by the SP module and the EP module, such information received via
the SP
Candidate Prescription Module 317and the Candidate Prescription Module 220,
respectively.
[0392] Next, in decision step 4004, the Candidate Prescription Server 315
determines
whether the candidate prescription database 309 has stored prescription data
that relates to the
prescription indicated on the received prescription list. This determination
may be made
using the prescription data, the patient data, and the prescribed substance
data. For example,
in one embodiment of the present invention, the Candidate Prescription Server
315
determines that prescription data exists if there is existing prescription
data that matches in
both patient information and prescribed substance information.
[0393] If no matching information exists in the candidate prescription
database 309, the
Candidate Prescription Server 315 creates a record of prescription data
relating to the
prescription, and stores such in the candidate prescription database 309,
thereby completing
step 4006. However, if prescription data already exists for the prescription,
then the method
continues to step 4005, and the Candidate Prescription Server 315 determines
if there is any
additional information received from either the SP Candidate Prescription
Module 317 or the
Candidate Prescription Module 220 relating to the prescription. There may be
new or
additional prescription information if information relating to the
prescription was stored in
the candidate prescription database 309 prior to the prescription being
processing by either
the EP module or the SP module. If there is any new or additional information
relating to
the prescription, then the Candidate Prescription Server 315 adds the
additional prescription
information to the associated prescription data and stores such in the
candidate prescription
database 309, thereby completing step 4006. However, if no new information
exists, then
the method returns to step 4003, and the Candidate Prescription Server 315
retrieves another
116
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
prescription from the list. Further, it should be noted that after completing
step 4006, the
Candidate Prescription Server 315 also returns to step 4003 and retrieves
another prescription
from the list.
[0394] This method continues until the Candidate Prescription Server 315 has
analyzed each
prescription of the received list of prescriptions. Thereafter, the Candidate
Prescription
Server 315 formulates and logs a response, thereby completing steps 4007 and
4008, and
transmits the response back to either the SP Candidate Prescription Module 317
or the
Candidate Prescription Module 220. Thus, by performing the method 400,
Candidate
Prescription Server 315 may populate the candidate prescription database 309
with updated
prescription records for all new prescriptions processed by either the EP
module or the SP
module.
[0395] Medication History Poller
[0396] In general, the Medication History Poller (Medication Hx Poller) 350
includes a
software program or routine in some embodiments that polls the candidate
prescription
database 309 for eligible prescriptions, obtains medication history data for
patients of eligible
prescriptions, and stores the medication history data in the patient
medication history
database 308. According to one embodiments of the present invention, with
reference to
FIG. 39, the Medication Hx Poller 350 begins by first polling the candidate
prescription
database 309 to obtain all of the patient prescriptions that are still active.
It should be noted
that, in accordance with one embodiment of the present invention, the
candidate prescription
database 309 stores prescription data for the prescriptions in which
supplemental programs
were activated (i.e. SP events). Thus, in such embodiments. the Medication Hx
Poller 350
gathers prescription data from the candidate prescription database 309 for
patients for whom
supplemental programs were activated such that patient adherence data may be
generated for
those patients.
[0397] As noted above, however, the Candidate Prescription Server 315 receives
prescription
data from both the EP system 200 and/or the SP system 300, and stores the
prescription data
in the candidate prescription database 309. Consequently, the Medication Hx
Poller 350
periodically polls the candidate prescription database 309 to obtain all
active prescription (Rx)
data stored in the database regardless of the source of the prescriptions.
[0398] As noted above, according to one embodiment of the present invention,
the
117
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
Medication Hx Poller 350 polls the candidate prescription database 309 to
obtain prescription
data for all of the prescriptions that are still active. Such prescriptions
are considered to be
eligible prescriptions. The Medication Hx Poller 350 determines that a
particular
prescription is "active" if the stop date of the prescription is greater than
or equal to the
current date. It should be noted that the stop data of the prescription is the
total duration of
the prescription, which includes all of the possible refills, from the start
date of the
prescription. For example, if a prescription is written on January 1, 2012
(the prescription
start date), comprises 30 pills, directs the patient to take 1 pill per day,
and has 2 refills, then
the total duration of the prescription is 90 days. Thus, the stop date of the
prescription is
March 30, 2012 (90 days from January 1, 2012). Further, in an alternate
embodiment, a
prescription will only be considered to be "active" if the current date is not
within a
predetermined number of days (e.g. 7 days) from the stop date of the
prescription.
[0399] Further, according to one embodiment of the present invention, the
Medication Hx
Poller 350 may be configured to only obtain prescription data for those
patients who have not
had their medication history queried for a predetermined period of time. This
may be
referred to as the drug history fetch interval. Thus, the Medication Hx Poller
350 will only
obtain prescription data if the patient of the prescription has not had their
medication history
obtained in the past predetermined number of days (e.g., 14 days). This helps
to improve
the overall efficiency of the Medication Hx Poller 350. Nonetheless, it should
be noted that
the invention is not so limited, and in other embodiments of the present
invention, the
Medication Hx Poller 350 polls the candidate prescription database 309 for
prescription data
of prescriptions regardless of how recent the Medication Hx Poller 350 had
last obtained
patient medication history data for the patient of those prescriptions.
Further, it should be
noted that the invention is not limited to the specific order of processing
performed by the
Medication IIx Poller 350.
[0400] According to one embodiment of the present invention, after obtaining
the
prescription data for all active prescriptions (whose patients have not had
their medication
history queried for a predetermined period of time in some embodiments), the
Medication Hx
Poller 350 parses the prescription data to determine information relating to
the patient, such
as, but not limited to the name of the patient, the age of the patient, and
other identifying
information. Further, the Medication Hx Poller 350 may also parse the
prescription data to
determine information relating to the prescribed substance, the provider,
and/or the payor.
118
CA 02918798 2016-01-19
WO 2015/013695
PCT/1JS2014/048330
[0401] Next, with continuing reference to FIG. 39, in addition to obtaining
active
prescription data from candidate prescription database 309 as described above,
Medication
Hx Poller 350 then also requests and obtains the medication history data for
the eligible
active prescriptions. Specifically, the Medication Hx Poller 350 may transmit
at least some
of the patient data, the prescribed substance or drug data, the provider data,
and/or the payor
data obtained from the prescription data stored in the candidate prescription
database 309,
along with a request for patient medication history information to the Payor
Engine 318. In
one embodiment, the Payor Engine 318 is a computer program or set of
instructions (e.g.
software) residing in patient adherence system 360 on any one of the computer
readable
medium or memory devices described herein which may be accessible to and
running on
Candidate Prescription Server 315 or Adherence Server 316.
[0402] Upon receiving the information, the Payor Engine 318 transmits the
information along
with a request to the pharmacy system 500 (e.g. Surescripts ) for patient
medication history
data. Thereafter, the Payor Engine 318 receives the requested medication
history data
relating to active prescriptions for each patient requested from the pharmacy
system 500.
Upon receiving the medication history data relating to the prescription for
the patient, the
Payor Engine 318 transmits the medication history data to the Medication Hx
Poller 350,
which in turn stores the patient medication data in the patient medication
history database 308.
This patient medication history data will be used by the Patient Adherence
Core 388 to
calculate patent medication adherence metrics, as further described herein.
[0403] Referring to FIG. 41, a schematic flow chart is shown illustrating an
exemplary
method for the Medication IIx Poller 350 to obtain medication history data via
the Payor
Engine 318 according to one embodiment of the present invention. The method
4100
begins at step 4101 with the Medication Hx Poller 350 scheduling a job to
obtain patient
medication history data. This may be scheduled through either (1) Poller 350
selecting an
eligible prescription from candidate prescription database 309 for which to
obtain medication
history data, which in some embodiments may be initiated via a predetermined
polling
schedule programmed into the Poller 350, or (2) upon receipt of a specific
request for
medication history data for a particular patient or another triggering event
received by the
Medication Hx Service 382 from an external source (e.g. EP system 200 or HP
system 100).
[0404] After selecting an eligible prescription from either the candidate
prescription database
309 or receiving a patient specific request, the Medication 'Ix Poller 350
transmits
119
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
information relating to the eligible prescription with a request to obtain
medication history
data to the Payor Engine 318 for a specific patient, thereby completing step
4102. Next, at
step 4103, the Payor Engine 318 receives the information and processes the
request, and
thereafter transmits the request to one or more systems, such as without
limitation the
pharmacy system 500. Thereafter, the Payor Engine 318 receives the requested
medication
history data from pharmacy system 500 relating and transmits the medication
history data to
the Medication Hx Poller 350. Next, at steps 4104 and 4105, the Medication Hx
Poller 350
aggregates the data received from the Payor Engine 318 and saves the
medication history data
("response") from the Payor Engine 318 in patient medication history database
308.
[0405] After saving the response, in step 4106, the Medication Hx Poller 350
next checks the
patient medication history database 308 for existing medication history data
that corresponds
to the received medication history data, and combines or associates the
aggregated
medication history data received from the Payor Engine 318 with corresponding
medication
history data previously stored in the patient medication history database 308.
Thereafter,
the Medication Hx Poller 350 stores the combined medication history data in
the patient
medication history database 308 in correlation with the associated
prescription. Thus, when
completed, the patient medication history database 308 has an up-to-date and
aggregated
collection of medication history data stored by prescription on a per patient
basis.
Medication history data comprised of fill data for all active medications
prescribed for each
patient over an interval of time is therefore stored in patient medication
history database 308.
[0406] Patient Adherence Generation Module
[0407] As noted above, the patient adherence system 360 further comprises
Patient
Adherence Generation Module 306. In general, the Patient Adherence Generation
Module
306 is a software program or routine running on Adherence Server 316 that
retrieves/ loads
medication history data and prescription data as required from patient
medication history
database 308 which is populated by Medication Hx Poller 350, and then
calculates and
generates patient adherence analytics or data (e.g. "adherence metrics") using
appropriately
configured algorithms. The adherence metrics may be MAR (aka MPR), FIT, and
others
which typically have a calculated numeric value as further described herein.
[0408] According to one non-limiting embodiment of the present invention, the
Patient
Adherence Generation Module 306 may calculate and generate patient adherence
data/metrics through essentially six primary steps described below to provide
one
120
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
non-limiting example for illustrating the basic process: (1) loading
prescription data and
prescription fill data (patient medication history data) for a given patient
and all active patient
prescriptions; (2) merge prescription and prescription fill data; (3)
calculate patient adherence
data/metrics (e.g. MAR, etc.); (4) calculate risk level for controlling
patient advisor
compliance dashboard display; (5) build drug list for medication menu; and (6)
generate fill
event timelines for each drug on list. These steps are further described
below. As noted
above, one example of a Patient Adherence Generation Module 306 is the HMACS
module
by DrFirst .
[0409] (1) Load Qualified Prescriptions
[0410] Referring to FIG. 39, the Patient Adherence Generation Module 306
begins by
loading data relating to a selected given patient's active prescriptions from
the candidate
prescription database 309 which has been populated by Candidate Prescription
Server 315
and historical prescription fill data (patient medication history data) for
the given patient from
patient medication history database 308 which has been populated by the
Medication IIx
Poller 350.
[0411] To complete this step in the process, the Patient Adherence Generation
Module 306
polls the patient medication history database 308 for all drug fills for a
given prescription and
patient. According to one embodiment, the Patient Adherence Generation Module
306 may
identify the drug fill data for a prescription using the patient's
identification information (e.g.,
the patient's name, social security number, etc.). Upon identifying all active
drug fill data
that relates to prescriptions for the given patient, the Patient Adherence
Generation Module
306 then retrieves and loads the drug fill data from the patient medication
history database
308.
[0412] Similarly, the Patient Adherence Generation Module 306 polls the
candidate
prescription database 309 for all drug prescriptions for the same given
patient based on the
patient's identification information (e.g., the patient's name, social
security number, etc.).
Upon identifying all active drug prescriptions for the given patient, the
Patient Adherence
Generation Module 306 then retrieves and loads a list of active prescriptions
from the
candidate prescription database 309.
[0413] (2) Merge Prescription and Fill Data
[0414] Next, Patient Adherence Generation Module 306 compares and merges the
121
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
prescription data (i.e. active prescriptions written and submitted via the EP
system 200 for a
given patient, as described herein) for the given patient and their active
prescriptions with the
medication history data such as drug fill data. As noted above, the drug fill
data comprises
information relating to the fill days, the fill quantity, the first fill
interval, and the fill date of
the prescription. Specifically, according to one embodiment, the Patient
Adherence
Generation Module 306 searches for and matches the prescription data for each
given patient
prescription with its corresponding historical drug fill data, which in one
embodiment is
sourced from the pharmacy system 500 as further described herein.
[0415] The patient adherence generation module 306 may match the drug fill
data with the
prescription data by using at least one of the drug name (including the
matching of brand
names to generics and vice versa), the drug fill date with the start date of
the prescription (e.g.,
if the drug fill date is greater than or equal to the start date of the
prescription), and the drug
fill date with the stop date of the prescription (e.g., if the drug fill date
is less than or equal to
the stop date of the prescription). Further, according to one embodiment of
the present
invention, the substance of a drug fill data matches a prescribed substance by
strength if the
substance of the drug fill and the prescribed substance is equivalent in name
and strength. It
should be noted that in one embodiment of the present invention, if there is
more than one
prescription that matches with drug fill data, then the patient adherence
generation module
306 will only match the most recent prescription with the drug fill data.
[0416] Further, it should be noted that, in accordance with some embodiments
of the present
invention, if a patient has more than one prescription for a given prescribed
substance, the
patient adherence generation module 306 may combine the prescription data for
those
prescriptions for purposes of calculating adherence data. Thus, in such
embodiments, the
patient adherence generation module 306 combines all the related prescription
data for the
given patient. Specifically, the patient adherence generation module 306 may
combine the
prescription data for two or more prescriptions for purposes of determining
patient adherence
data if the two or more prescriptions are for the same substance and the
prescription total
days of the two or more prescriptions overlap. In such instances, the patient
adherence
generation module 306 sets the prescription start date of the combined
prescriptions are the
earliest of the start dates of the prescriptions individually. Similarly, the
patient adherence
generation module 306 sets the prescription stop date of the combined
prescriptions are the
latest of the stop dates of the prescriptions individually. After combining
the two or more
122
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
prescriptions, the patient adherence generation module 306 treats the combined
prescription
as one for all other purposes.
[0417] (3) Calculate and Generate Patient Adherence Data/Metrics
[0418] In the next step, the Patient Adherence Generation Module 306
calculates and
generates patient adherence data and metrics. In general, patient adherence
data comprises
information relating to a patient's adherence, compliance, and/or persistency
to taking the
prescription medications prescribed for the patient by the health care
provider. The patient
adherence data may comprise information of value to the health care provider
which are a
numerical measure of medication regimen adherence metrics such as without
limitation the
patient's Medication Adherence Rate (MAR) data (also referred to as Medication
Persistency
Rate-MPR), the patient's first fill compliance (FFC) data, and other relevant
adherence data.
In one embodiment, MAR is calculated. The Patient Adherence Generation Module
306 is
configured with the appropriate algorithms necessary to calculate the MAR or
other
adherence data/metrics of interest using the patient medication history data
previously
downloaded from the patient medication history database 308.
[0419] After generating the patient adherence metric data for one of the given
patient's
prescription medications, the patient adherence generation module 306 stores
the adherence
data in the patient adherence database 307. After generating patient adherence
data for the
foregoing single prescription drug associated with the given patent, the
patient adherence
generation module 306 then repeats the process to calculate and generate
patient adherence
data for each additional drug prescribed for the patient until adherence data
has been
generated for all of the active prescriptions associated with the patient.
Further, it should be
noted that if the patient adherence generation module 306 loaded existing
adherence data for
the patient prior to generating the additional or updated patient adherence
data, the patient
adherence generation module 306 updates the existing patient adherence data in
the patient
adherence database 307 to reflect the newly generated patient adherence data.
Thus, the
patient adherence database 307 comprises up-to-date adherence data for all
active
prescriptions associated with the given patient.
[0420] As noted above, in one embodiment of the present invention, patient
adherence data
may include first fill compliance (FTC) data and patient Medication Adherence
Rate (MAR)
data. These two parameters provide are valuable to the health care provider
101 as relevant
indicators which yield quick snapshot of how diligent a given patient has been
in following
123
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
their prescription medication treatment regimen for one or a plurality of
prescribed
medications or substances. Generally. FFC data comprises information relating
to whether
or not the patient has been prescribed a particular prescribed substance
(e.g., the target drug)
in the past and/or whether the patient picked-up or took the particular
prescribed substance
(e.g., the target drug) in the past.
[0421] In one embodiment of the present invention, FTC data of a prescription
has three
possible values: (1) present; (2) absent; or (3) unknown. The FFC data has a
value of
present if there exists a qualifying drug fill where: (1) the fill date of the
prescription may be
on the same or after the start date of the prescription; and (2) the fill date
of the prescription is
before the end of the end of the first fill interval of the prescription.
Thus, the prescription
has a FFC value of present if the prescription was filled by the patient after
the prescription
was written by the health care provider 101, but before the end of the first
fill interval of the
prescription. If the prescription has a FFC value of present, then the
prescription is first fill
compliant.
[0422] The FFC data has a value of unknown if the start date of the
prescription is after the
end of the first fill interval of the prescription. In such instances, the
prescription occurred
after the first fill interval and therefore may be a refill of the
prescription. Thus, since
information relating to a refill of a prescription does not indicate whether
the patient was
compliant with filling their prescription on the first fill, the prescription
has a FFC value of
unknown. In all other instances, the FFC data has a value of absent.
[0423] Generally, MAR data comprises information relating to the patient's
adherence to a
particular prescribed substance. For example, MAR data may comprise
information relating
to the degree or extent of conformity of the patient to the recommendations
about day-to-day
treatment by the health care provider 101 with respect to the timing, dosage,
and/or frequency
of a particular prescribed substance, information relating to the extent to
which a patient acts
in accordance with the prescribed interval and dose of a dosing regimen of the
particular
prescribed substance, and information relating to the continuation the
treatment by the patient
for the prescribed duration of the particular prescribed substance. Therefore,
in one
embodiment of the present invention, the MAR data comprises, as a numerical
percentage,
information relating to how often the patient filled a prescription compared
with how often
they could have filled it optimally.
[0424] According to one embodiment of the present invention, if there are
fewer than two
124
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
drug fills or refills of a particular prescription, then the MAR data for that
prescription may
be assigned a value of "unknown" and displayed in the toolbar as "N/A" in some
embodiments. In one embodiment, four events are needed to calculate MAR
including (1)
start date of prescription, (2) first fill, (3) a refill, and (4) end date of
prescription. The
MAR data is calculated as follows:
[0425] First, the Patient Adherence Generation Module 306 calculates adherence
interval
days for the prescription. As noted above, the adherence interval is the
number of days in
an adherence interval, or the number of days that a supply of prescription is
available to the
patient during the adherence interval. For each qualifying prescription, the
Patient
Adherence Generation Module 306 determines the adjusted prescription start
date (the later
of the start date of the prescription or the adherence interval start date),
the adjusted
prescription stop date (the earlier of the prescription stop date or the
current date), and the
adherence interval days of the prescription (the adjusted stop date minus the
adjusted start
date).
[0426] Next, the Patient Adherence Generation Module 306 calculates the total
fill days of
the prescription. As noted above, the total fill days are a measure of the
number of days that
a supply of a prescription is actually filled by the patient during the
adherence interval. For
each qualifying prescription, the patient adherence generation module 306
calculates the
adjusted fill start date (the later of the fill date of the prescription or
the adherence interval
start date of the prescription) and the adjusted fill stop date (the earlier
of the fill date plus the
fill days or the current date). Next, the Patient Adherence Generation Module
306
calculates the total fill days of the prescription (the adjusted fill stop
date minus the adjust fill
start date). Finally, the patient adherence generation module 306 calculates
the MAR data
for the prescription (the total fill days divided by the adherence interval
days ¨ multiplied by
100 to show as numerical percentage).
[0427] As already noted herein, it should be recognized that the patient
medication adherence
metric Medication Adherence Rate (MAR) may alternatively be expressed as
Medication
Persistency Rate (MPR) which has the same meaning and is calculated in the
same foregoing
manner as MAR using historical phaimacy fill information. Accordingly, the
teims MPR
and MAR may be used interchangeably herein. The MPR or MAR value, which will
numerically be a fraction, is multiplied by 100 herein to calculate a numeric
MAR % for each
medication where possible. In general, the theoretical range of possible
MPR/MAR values
125
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
expressed as a percentage may be 1% to 100%.
[0428] (4) Calculate Risk Level
[0429] In some embodiments, the Patient Adherence Generation Module 306 may
optionally
calculate a "risk level" associated with the patient's adherence data such as
MAR. In general,
the lower the MAR %, the greater the patient risk for experiencing health
related problems
associate with not following their prescribed medication regimen. In one
embodiment, the
calculated risk level may be used to control the display and/or appearance of
information
and/or icons appearing on the GUI of display device 121 of the HCP system 100
for
interacting with the EP (electronic prescription) system 200, as more fully
described below.
Briefly, in one non-limiting example, the Report Card tab 2010 appearing in
the patient
advisor toolbar 2004 may change color as follows: Green ¨ MAR is 71% or
greater; Yellow ¨
MAR is between 60% to 70%; Red ¨ MAR is less than 60%; which visually presents
the
level of patient risk (see, e.g. FIGS. 42-45). This same approach may
analogously be used
to change of the appearance and/or color of the patient advisor report card
summary field
2240 which displays the MAR % numeric value (see, e.g. FIG. 46). It will be
appreciated
that other uses of the risk level may be employed for the benefit of the
health care provider
and patient.
[0430] (5) Build Drug List
[0431] The Patient Adherence Generation Module 306 may construct or build a
list of all
active drugs which have been retrieved and analyzed in the foregoing steps
associated with
the given patient presently being analyzed for medication adherence data. In
one
embodiment, as further described below, the list of active drugs may be used
to compile and
generate a medication menu 2210 displayable in the patient advisor report card
2200 (see, e.g.
FIG. 46). The list of drugs is saved to patient adherence database 307 by
Patient Adherence
Generation Module 306.
[0432] (6) Generate Prescription Fill Events
[0433] Using the list of active drugs for the given patient, the Patient
Adherence Generation
Module 306 identifies and generates prescription fill events for each
medication. The fill
events are based on the historical patient medication history downloaded to
Module 360 from
the patient medication history database 308, as described above. In one
embodiment,
Patient Adherence Generation Module 306 is operable to generate individual
drug timelines
126
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
for each medication which includes information such as without limitation
prescription date),
fill date, expected fill date, prescription end date, gap in fill date, and
prescription duration.
A drug timeline is construction and associate with each drug by brand or
generic name where
sufficient data exists to generate a timeline (e.g. a sufficient number of
fill/refill events).
The drug timelines and other fills event information is saved to patient
adherence database
307 by Patient Adherence Generation Module 306.
[0434] It will be appreciated that the foregoing steps may be performed in any
suitable
sequence which may be different than presented herein, and some of the steps
may be
performed simultaneously in some embodiments. Additional steps and sub-steps
may be
provided in other embodiments. Accordingly, the present invention is not
limited to the
foregoing steps and sequence.
[0435] Adherence Server - Generation and Display of Adherence Data
[0436] According to one aspect of the patient advisor system, a graphical user
interface (GUI)
comprising a compliance dashboard with interactive toolbar is provided which
is displayable
on a video display device such as without limitation display 121 of the HCP
system 100.
The compliance dashboard may include lists of all active medications/drugs for
each patient
and charts which graphically depict patient medication adherence data in
visual and graphical
founats which can be readily viewed and easily interpreted by a health care
provider 101.
The compliance dashboard may further include a medication report card for each
patient
which displays patient medication adherence data and metrics.
[0437] In general, with reference to FIG. 39, the Adherence Server 316 is a
front end device
of the patient adherence system 360 that receives and processes requests for
patient
medication adherence data originating from the EP module 202 of the electronic
prescription
system 200 (see also FIGS. 1 and 4) or other systems that may be operably
connected to
patient adherence system 360. In some embodiments, the adherence data
requested may
include without adherence metrics such as without limitation MAR (MPR), FFC,
or others
based on programming Patient Adherence Generation Module 306 with the
appropriate
calculation algorithms for the metric to be determined.
[0438] In one embodiment, as further described herein, the request for patient
adherence data
may be automatically generated by EP module 202 in conjunction with a health
care provider
101 electronically prescribing a medication or pharmaceutical substance for a
patient via their
127
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
terminal 120 using the EP system 200 such as by the health care provider
selecting/inputting
a patient name in the EP system, as further described herein.
[0439] Referring to FIG. 39, after the Adherence Server 316 receives the
patient adherence
data request from EP module 202, the Adherence Server 316 routes the request
to the Patient
Adherence Generation Module 306. Thereafter, the Patient Adherence Generation
Module
306, based on the received request, retrieves the respective adherence data
from patient
adherence database 307 for the patient.
[0440] In some embodiments, the Patient Adherence Generation Module 306 is
further
operable to generate interactive adherence GUIs comprising visual data images
and icons
such as charts, graphs, symbols, etc. in one or more colors which are
representative of patient
medication adherence data and presented to health care providers 101 in easy
to view formats.
The Patient Adherence Generation Module 306 is operable to generate the
patient advisor
GUIs comprising the requested patient adherence data in a visual format for
display on the
display device 121 of the IICP system 100. Thus, the Patient Adherence
Generation
Module 306 not only generates the patient adherence data, but also generates
the GUIs
comprising user selectable images or icons for display on the display device
121 of the health
care provider 101.
[0441] As discussed in more detail below, one example of a GUI generated by
the patient
advisor system that provides access to and comprises patient adherence data
generated by the
patient adherence system 360 is a graphical compliance dashboard which
includes an
interactive toolbar, as further described in detail below.
[0442] Referring back again now to FIG. 39, the process which causes the
Adherence Server
316 to route the request for patient adherence data to the Patient Adherence
Generation
Module 306 begins with the Adherence Server 316 receiving the request from EP
module 202
via EP interface 212. EP module 202 may reside on EP system 200 or
alternatively on an
FICP system 100, as discussed in above. In yet other embodiments, a request
for adherence
data may be received by Adherence Server 316 which originates from a third
party electronic
prescription system other than EP system 200. Accordingly, the request for
adherence data
may originate from a variety of sources and the present invention is not
limited to any
particular source.
[0443] According to one embodiment of the present invention, the incoming
request for
128
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
patient medication adherence data may include specific patient data (e.g.
name, birthdate,
social security number, or other relevant identifiers) and prescribed
medication or substance
data (e.g. name of drug). The Adherence Server 316 then routes the request to
the Patient
Adherence Generation Module 306 for processing. In yet other possible
embodiments, the
adherence data request may include only patient identifier data and a request
for adherence
data related to all prescription medications taken by the patient within a
predetermined
timeframe (e.g. last year, 2 years, 3 years, etc.).
[0444] The Patient Adherence Generation Module 306 searches the patient
adherence
database 307 for the most recent adherence data for the specific patient and
prescribed
substance or drug identified in the request if a single drug is identified, or
for all prescribed
substances. After identifying the relevant adherence data, the Patient
Adherence Generation
Module 306 loads the adherence data from the patient adherence database 307
and generates
a GUI that includes the requested patient adherence data in a combination of
both numerical
and/or graphic formats, as further described below. Thereafter, the Patient
Adherence
Generation Module 306 transmits the GUI to the Adherence Server 316 which
transmits the
GUI back through the EP interface 212 to PE module 202, which in turn relays
the GUI to the
HCP system 100 for display on the display device 121 for viewing by the health
care provider
101 (see FIG. 39).
[0445] In some embodiments, the adherence GUI may be previously generated in
advance by
the Patient Adherence Generation Module 306 and stored in patient adherence
database 307
prior to receiving the request for adherence data. However, in other
embodiments of the
present invention, the Patient Adherence Generation Module 306 generates the
adherence
GUI upon receiving the corresponding request for patient medication adherence
data from the
EP module 200.
[0446] According to one embodiment of the present invention, the Patient
Adherence
Generation Module 306 generates an adherence graph when there is new drug fill
data for
corresponding to an existing graph or a new adherence event occurs. According
to one
embodiment, the Patient Adherence Generation Module 306 generates an image of
an
adherence graph. In such embodiments, after corresponding adherence data is
calculated by
the Patient Adherence Generation Module 306, the Patient Adherence Generation
Module
306 generates a new graph or amends an existing graph with the updated
adherence data.
Next, the Patient Adherence Generation Module 306 stores the image in the
patient adherence
129
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
database 307. Thereafter, the Patient Adherence Generation Module 306 loads
the image
when a request is received, and the image is embedded into a GUI that is
ultimately displayed
on the display device 121 to the provider 101. However, the invention is not
so limited, and
according to another embodiment of the present invention, the Patient
Adherence Generation
Module 306 generates and stores the graph at the time the request is received
from the EP
module.
[0447] Further, it should be noted that in some embodiments of the present
invention, the
Adherence Server 316 may receive requests comprising more than one
corresponding pairs of
patient data and prescribed substance data, such that the Patient Adherence
Generation
Module 306 may process multiple different requests at one time.
[0448] FIG. 60 shows a flow chart summarizing the foregoing exemplary computer
processor-implemented method executed by patient adherence system 360 which
utilizes the
Patient Adherence Generation Module 306 in the manner described above to
generate and
transmit patient medication adherence data to patient advisor system
compliance dashboard
for display to a user such as the health care provider 101. Additional
reference should be
made to FIGS. 1-7 and 39 as appropriate, in description of the process which
follows.
[0449] Process 2300 begins in step 2305 with a health care provider 101
logging into the EP
system 200 and manually selecting a patient via name in the main electronic
prescription (EP)
system GITI 2002 on display device 121 of the HCP system 100. This interface
may be
enabled on the HCP system 100 by the thin client 203 portion of the EP Module
which
resides in the non-transitory computer readable medium (memory 113 in this
case) accessible
to server 110 (see FIG. 2). Selecting the patient's name automatically
generates and sends a
request from the EP system to the Payor Engine 318 specific to the selected
patient. In other
possible embodiments, the patient name may be selected in any type of
healthcare related
system operably linked to communicate with the patient adherence system 360
other than an
EP; the invention not being limited to an EP system interface alone.
[0450] Next, in step 2310, the Payor Engine 318 receives the eligibility
request and
automatically sends a request for patient medication history and pharmacy fill
data to the
pharmacy system 500. The request is patient specific for the given patient
selected in the
preceding step. In response, the pharmacy system 500 compiles and sends the
requested
medication history and raw fill data to Payor Engine 318 in step 2315. The
Payor Engine
318 in turn transmits/sends the medication history and raw fill data to
Medication Hx Poller
130
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
350 of the Medication Hx Service 382. The Medication Hx Poller 350 may check
for and
resolve duplication in the raw fill data. In step 2320. the Medication IIx
Poller 350 may
then store the raw fill data and medication history data in the patient
medication history
database 308. In some embodiments, the Medication Hx Poller 350 may be
configured to
also poll and retrieve prescription data (Rx data) from candidate prescription
database 309,
and may also save the prescription data to the patient medication history
database 308 in
addition to the raw fill data. In other embodiments, the prescription data may
remain only in
candidate prescription database 309 and will be accessed later in the process
by Patient
Adherence Generation Module 306 as described herein.
[0451] With continuing reference to FIG. 60, upon selecting the patient's name
in the in the
main electronic prescription (EP) system in step 2305, or alternatively after
step 2320, the
patient adherence system 360 system triggers display of the compliance
dashboard 2000 with
interactive toolbar 2004 on display 121 of the HCP system in step 2325, as
further described
in detail below. The compliance dashboard 2000 then posts/sends the relevant
patient
demographics (e.g. name, date of birth, etc.) and a request for patient
medication adherence
data to the Patient Adherence Core 388, and more particularly in one
embodiment to
Adherence Server 316 and the Patient Adherence Generation Module 306 (step
2340)
running on the Adherence Server. In one embodiment, the Patient Adherence Core
388
searches for current (e.g. on date corresponding to the adherence data request
date) calculated
patient medication adherence data in patient adherence database 307 for the
selected patient.
If a result (calculated adherence data) is found and not expired (i.e. current
date), control
moves to the next step 2345. If outdated or no result is found, Patient
Adherence
Generation Module 306 may send a request for prescription data and medication
history/fill
data to the Medication Hx Service 382 for retrieval in the manner already
described herein
to enable patient medication adherence data to be calculated based on current
information.
[0452] In step 2345, the Patient Adherence Generation Module 306 retrieves
medication
history/fill data and prescription data (Rx data) for the selected patient. In
step 2350, the
Patient Adherence Generation Module 306 calculates and generates patient
medication
adherence data metrics such as medication adherence rate (MAR)% in the manner
already
described herein. The Patient Adherence Generation Module 306 stores the
patient
medication adherence data in patient adherence database 307 in step 2355.
[0453] In step 2360, the patient adherence system 360, specifically Adherence
Server 316 in
131
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
one embodiment, sends the patient medication adherence data to the patient
advisor
compliance dashboard 2000. The patient medication adherence data is then
displayed in
step 2365 by EP server 210 on the GUI of the health care provider display
121via the
compliance dashboard 2000, as further described in additional detail below. In
other
embodiments, as shown in FIG. 39, the HCP system 100 may communicate directly
with the
adherence server 316 (following the token authentication process described
herein) via
communication links as shown in FIG. 1 wherein the HCP server 110 may display
the patient
medication adherence data.
[0454] Patient Advisor System Toolbar and Compliance Dashboard
[0455] As noted herein, the Patient Adherence Generation Module 306 calculates
and stores
patient medication adherence data in patient adherence database 307 (see FIG.
39). The
Adherence Server 316, upon receiving a request initiated by the health care
provider through
the GUI 2002 on display 121 of the HCP system 100, retrieves and transmits
adherence data
back the IICP system 100 for viewing on display 121.
[0456] In one embodiment, the adherence data is incorporated into a GUI in the
form of a
patient advisor compliance dashboard 2000 as initially shown in FIG. 42. In
general, the
compliance dashboard is an application specific interactive GUI, or a
plurality of GUIs, that
offers a health care provider 101 with graphical and alphanumerical
representations of the
applicable patient medication adherence data. The adherence data may be
displayed in the
form of any number and type of graphical images or icons and alphanumeric
characters. In
one embodiment, the compliance dashboard 2000 includes an interactive toolbar
2004.
[0457] According to one embodiment of the present invention, the compliance
dashboard
2000 with toolbar 2004 may be provided as a separate software module 386 which
is hosted
on the EP system server 210, or alternatively a third party EP system server.
As such, the
health care provider 101 may easily switch between prescription writing for a
patient and the
compliance dashboard that provides access to the patient's past adherence data
at the point
and time of service at the health care provider's facilities. Thus, the health
care provider
101 may engage the patient regarding their adherence history relating to
previous
prescriptions prior to drafting a new prescription for that patient in hopes
of increasing the
patient adherence to filling/refilling, picking up, and taking their
prescribed medications per
their treatment regimen. Advantageously, the compliance dashboard 2000
provides a
quantitative means for the health care provider 101 to engage their patients
in prescription
132
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
adherence discussions.
[0458] It should be noted that the present invention allows for both
synchronous and
asynchronous methods of displaying patient compliance data via the compliance
dashboard
2000 to the provider 101 through the health care provider's display device 121
(se e FIG. 2).
In the synchronous model, a single continuous user interface (which can
include multiple
sequential GUIs) is displayed and interfaced with by the health care provider
101 for both
generating an electronic prescription using the EP module 202 and viewing
patient
compliance data generated by the Patient Adherence Generation Module 306 in
the
compliance dashboard 2000 (reference FIGS. 3 and 39). Therefore, the
synchronous model
may be used when the EP module 202 and the Patient Adherence Generation Module
306
utilize the same GUI (e.g., when the EP module is Rcopia ). When using the
synchronous
model, the provider 101 may view patient compliance data generated by the
Patient
Adherence Generation Module 306 via compliance dashboard 2000 and fill out
prescriptions
for the patient using the EP module, using the same user interface and display
screen. FIGS.
42-59 show an example of various synchronous models of compliance dashboard
2000 in
which the compliance dashboard GUI is overlaid on the patient summary screen
of the main
EP system GUI 2002 which are both simultaneously displayed on the health care
provider's
display 121. It should be noted that in some embodiments, the EP system GUI
2002 may be
generated by the thin client EP module 203 residing on the HCP system 100 (see
FIG. 2).
[0459] In the asynchronous model, the EP module 202 and the compliance
dashboard 2000
of the Patient Adherence Generation Module 306 will have different GUIs
displayed
separately in different windows of the health care provider display 121. In
this manner, the
Patient Adherence Generation Module 306 may be configured for use with a
plurality of
different third party EP systems and EP modules, regardless of their GUIs.
According to
one embodiment of the present invention using the asynchronous model, upon
receiving a
request from a provider 101 to view patient compliance data, the Patient
Adherence
Generation Module 306 generates and displays a GUI that is distinct from the
GUIs generated
and displayed by the third party EP module.
[0460] The compliance dashboard 2000 and associated system and user processes
will now
be described in greater detail. Referring to FIG. 42, the patient summary
screen of the main
EP system GUI 2002 viewed by the health care provider 101 on display 121 may
include
several fields including a top header navigation field 2002a having a
plurality of
133
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
user-selectable buttons or links as shown which provides a user interface to
access and
operate the EP system 200 for electronically writing scripts and ordering
medications for a
patient as described herein. A patient information field 2002b is provided
which may
include patient name and personal demographic details, preferred pharmacy,
health care
provider's name, and other information. GUI 2002 may further include a
prescription entry
field 2002c for entry and/or selection of a prescription drug or medication
and a medication
history field 2002d which displays a list of active medications taken by the
patient. It will
be appreciated that these fields 2002a-2002d and other fields that may be
provided in the
context of the EP system interface for the health care provider may be
arranged in any
suitable manner or order and have many different appearances other than those
explicitly
depicted in FIG. 42. Accordingly, the invention is not limited to the layout
and appearance
of GUI 2002 shown.
[0461] Referring to FIG. 42, in the main EP system GUI 2002, access to the
compliance
dashboard 2000 may start with a health care provider 101 first selecting a
patient name by
selecting the "Select Patient" active link or button 2006 in field 2002a. It
should be noted
that button 2006 and other "active" buttons or graphical icons in GUI 2002 may
be selected
by any suitable input device 122 (reference FIG. 2), such as for example
without limitation a
cursor or pointer displayed in the HCP display 121 which is controlled via a
mouse, touch
pad, trackball, keyboard, etc. or directly via a finger or stylus contact with
the on-screen
active button/icon in the case where display 121 is a touch screen. It should
further be noted
that "active" buttons or alphanumerical indicia are defined herein as those
icons which may
be considered dynamic links (e.g. dynamic icons) in nature wherein when
selected by a user
trigger a further action to be implemented and executed by the system (e.g.
data retrieval,
display, etc.), in contrast to "inactive" screen icons or alphanumerical
indicia which display
static information in graphical and/or alphanumerical forms.
[0462] Selection of a patient name in the EP system 200 by the health care
provider 101 via
the main EP system GUI 2002 (see, e.g. FIG. 39) is a triggering event in one
embodiment
which causes the patient advisor module 386 to then display the patient
advisor compliance
dashboard 2000 GUI with interactive toolbar 2004 in the main EP system GUI
2002. The
dashboard with toolbar is displayed and viewable by the health care provider
on display 121
of the HCP system 100, as shown. In other suitable embodiments, the compliance
dashboard 2000 may remain visible at all times whenever the main EP system GUI
2000 is
134
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
displayed on the health care provider's display 121. The invention is not
limited to either
display scenario or configuration of the patient advisor system.
[0463] It should be noted that the patient advisor module 386 which generates
the
compliance dashboard 2000 and interactive toolbar 2004 GUI can be hosted on
either EP
server 210 of the EP system 200 (see FIG. 39) or a third party electronic
prescription server
(not shown). In the present embodiment being described, the patient advisor
module 386 is
hosted on EP server 210.
[0464] In one embodiment, the triggering event of selecting a patient name in
the EP system
200 as noted above may cause the patient advisor compliance toolbar 2000 to be
display
through operation of Authentication Server 380 shown in FIG. 39. The user such
as a health
care provider initially logs into the EP system 200 through the EP interface
212 which
communicates with central portion 202 of the EP module (see also FIG. 3). A
token is first
obtained at the login and transmitted to the Authentication Server 380. The
Authentication
Server 380 then returns the token to the EP interface of the EP system. Next,
the token is
passed (transmitted) to the HCP system 100 via the network interface 112 (see
FIG. 1), and in
turn then passed onto Adherence Server 316 of the patient adherence system
360. The
Adherence Server 316 passes the token to the Authentication Server 380 for
verification, and
receives notice back that the token is valid and authenticated. The Adherence
Server 316
may then display the patient advisor compliance dashboard 2000 with
interactive toolbar
2004 in the user's display 121 and is operable to transmit patient medication
adherence
data/metrics to the user for display via the dashboard.
[0465] In one embodiment, the interactive toolbar 2004 may be displayed
horizontally across
the display screen of the HCP display device 121. In other embodiments, the
toolbar may
be vertically oriented. Toolbar 2004 may be interspersed between existing
fields
2002a-2002d of GUI 2002 in a manner that does not obscure and allows access to
the
information and active links embedded in the fields simultaneously with use of
the toolbar.
[0466] The toolbar 2004 may comprise a plurality of user-selectable active
icons or buttons
(see, e.g. FIGS. 42-45), which in one non-limiting embodiment may be in the
form of content
type dynamic tabs 2010, 2020, 2030, 2040. 2050, and 2060 in some embodiments.
In one
embodiment, content tabs 2010-2060 are axially aligned in a horizontal line
across GUI 2002
as shown and may have the appearance of folder tabs which serve as portals for
a user to
access and reveal different type information in each. Any suitable number and
types of
135
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
user-selectable tabs may be used. Tabs 2010-2060 may be active/dynamic links
which
display content, data, and information related to the subject matter shown on
the tab when
selected and activated by the user. In one embodiment, for example without
limitation, the
tabs may include the following indicia: "Report Card" (2010), "Outcome
Studies" (2020),
"Education" (2030), "Coupons" (2040), "Adherence Plan" (2050), and "Clinical
Trials"
(2060). Additional or less tabs may be used as well as other type information
content.
Accordingly, the invention is not limited to the tabs or content shown and
described herein.
[0467] The Report Card tab 2010 is an active button which operably retrieves
and displays
patient medication adherence data in alphanumerical and/or graphical form on
the EP system
GUI 2002. In one embodiment, Report Card tab 2010 may be displayed in two
different
modes having a different appearance and indicia in each display mode. The
display modes
may include a "normal" display mode and an "alert" display mode. In one
embodiment, the
display mode which is displayed is determined and triggered by correlation to
a quantitative
value of patient adherence data generated by Patient Adherence Generation
Module 306 (see
FIG. 39) which is stored in patient adherence database 307 of the patient
adherence system
360. In one embodiment, the triggering adherence data may he MAR (medication
adherence rate) as already described herein which provides a snapshot of
patient medication
adherence habits.
[0468] In one exemplary embodiment, simultaneously with triggering the
generation and
display of the patient advisor toolbar 2004 by selecting the patient name,
this user action also
causes the EP module 202 (see, e.g. FIG. 39) to send a request for retrieval
of patient
medication adherence data to the Adherence Server 316 as described herein. The
Adherence Server 316 retrieves the requested adherence data from patient
adherence database
307.
[0469] In one embodiment, the adherence data requested for retrieval by EP
module 202 may
be MAR. The MAR or other adherence data retrieved by the patient advisor
module may be
for all active medications prescribed for the patient, which may or may not
include the
specific drug presently being prescribed. Accordingly, the compliance
dashboard 2000 may
provide patient medication adherence data to the health care provider for the
patient's entire
prescription medication regimen in a snapshot. In one embodiment, the patient
advisor
module 386 uses the MAR data to determine which of the two foregoing display
modes of
Report Card tab 2010 (i.e. "normal" or "alert") to implement, as described in
further below.
136
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
[0470] It will be appreciated that any one or all of the tabs 2010-2060 may be
displayable in
at least two different display modes comprised of a "normal" display mode and
an "alert"
display mode.
[0471] FIG. 42 shows toolbar 2004 with Report Card tab 2010 in a first
"normal" display
mode being of a first color, a first size, and with first alphanumerical
indicia which may
simply read "Report Card." The health care provider uses this tab to access
the patient's
medication history adherence report card, as further described below. In one
embodiment,
the color of Report Card tab 2010 in the noimal display mode may be the same
size and color
as the remaining tabs 2020-2060 so as to be visually indistinguishable by size
or color alone
in appearance. Any suitable color may be used for the tabs.
[0472] In one embodiment, the first "normal" display mode for the Report Card
tab 2010
may be displayed by the patient advisor module 386 to the health care provider
on display
121 when the MAR value calculated by the Patient Adherence Generation Module
306
exceeds a predetermined MAR threshold value, thereby indicating that the
patient is at least
fairly diligent in filling, refilling, and taking their prescribed
medications. In one
embodiment, for example, an MAR of greater 70% may be used as the threshold
value for
displaying the Report Card tab 2010 in the "normal" display mode. Other
suitable MAR
threshold values may be used.
[0473] Hovering or rolling the cursor of an input device over a tab 2010-2060
may also
change the appearance (e.g. size, color, and/or indicia) of a tab. In FIG. 43,
the Report Card
tab 2010 for example is shown enlarged in size in response to hovering or
rolling the cursor
over this tab. The Report Card tab 2010 has a second size which is larger than
the first size
when a cursor is not placed over the tab in the display. Hovering the curser
over the tab
may also change the indicia on the tab as shown. here, a triangle with
exclamation mark is
added to the tab above the words "Report Card."
[0474] In one embodiment, a second "alert" display mode for the Report Card
tab 2010 may
be displayed by the patient advisor module 386 to the health care provider on
display 121
when the MAR value calculated by the Patient Adherence Generation Module 306
is less
than a predetermined MAR threshold value. In one embodiment, an MAR of less
than or
equal to 70% may be used, thereby indicating that the patient has not been
diligent in filling,
refilling, and taking their prescribed medications. It will be appreciated
that other MAR
threshold values may instead be used.
137
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
[0475] FIG. 44 shows toolbar 2004 with Report Card tab 2010 in the second
"alert" display
mode being of a second color and with second alphanumerical indicia, which in
some
embodiments may read "At Risk Patient" or something equivalent. In one
embodiment, the
size of the Report Card tab 2010 may be the same as the first noimal size, or
in other
embodiments an enlarged tab in contrast to the normal size of tabs 2010-2060
may be
displayed in the "alert" display mode further urging the health care provider
to select the tab
for adherence information. The appearance of the tab (size, color, and/or
indicia) in the
"alert" display mode visually communicates a greater sense of urgency for the
health care
provider to view the patient's medication adherence information. In one
embodiment,
hovering or rolling the cursor of an input device over the "At Risk Patient"
tab may enlarge
the tab as shown in FIG. 45 and change the alphanumeric indicia on the tab as
shown (see
exclamation point triangle). It should be noted that the health care provider
still uses this
same tab 2010 to access the patient's medication history adherence report
card, as noted
above.
[0476] In one embodiment, the color of Report Card tab 2010 in the second
"alert" display
mode is a different and preferably more vibrant color than in the first color
in the normal
display mode to visually communicate that the patient being seen is "at risk"
in terms of
potential medical complications stemming from a lack of adherence to their
prescription
medication regimen. Any suitable color may be used to signify an "alert" to
the health care
provider. In some embodiments, the second color of the Report Card tab 2010 in
the "alert"
display mode may be for example without limitation yellow, orange, or red. In
yet other
embodiments, the patient advisor module 386 may be programmed to change the
display
color of Report Card tab 2010 in the "alert'. display mode to a color directly
correlated to the
degree of a patient's MAR compliance with taking their medications. In one non-
limiting
example, the Report Card tab 2010 may change color as follows: Green ¨ MAR is
71% or
greater; Yellow ¨ MAR is between 60% to 70%; Red ¨ MAR is less than 60%. Other
suitable colors and/or ranges may be used. It will be appreciated that these
colors and
numerical ranges are for example only and are in no way intended to be
limiting, but rather
are used to broadly demonstrate the concept.
[0477] FIG. 61 shows a flow chart summarizing the foregoing computer
processor-implemented process of retrieving and displaying either the "normal"
display mode
of Report Card tab 2010 or the "alert" display mode ("At Risk Patient") of the
tab.
138
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
Additional reference is made to FIG. 39. Process 2100 begins in step 2105 with
a health
care provider 101 manually selecting a patient in the main EP system GUI 2002
on display
device 121 of the HCP system 100. In one embodiment, the selection
automatically
generates and sends a request to Adherence Server 316 of the patient adherence
system 360
to retrieve MAR adherence data for all active prescription medications
currently prescribed
for the selected patient (step 2110), as already described herein. The MAR
data for all
active prescriptions related to the selected patient is stored in patient
adherence database 307,
having previously been generated by Patient Adherence Generation Module 306
(step 2106)
and stored in the database 307 (step 2108) in the form of graphs, charts,
numerical data, etc
(see also FIG. 60 and discussion herein). In one embodiment, the MAR data may
b
generated "on the fly" (i.e. on demand) for a single specific patient at a
time when a specific
request is made such as by selecting a patient name in the EP system 200, as
already
described herein with reference to FIG. 60. This approach minimizes the demand
on Patient
Adherence Generation Module 306 to analyze, generate, and store patient
adherence database
307 at a given time. In alternative embodiments, patient medication adherence
data may be
generated and stored in advance for a plurality of patients by configuring the
patient
adherence system 360 to cause the Patient Adherence Generation Module 306 to
routinely
poll patient medication history database 308 and retrieve patient medication
history data (step
2102) from patient medication history database 308, which may be routinely
populated with
medication history data by Med Hx Poller 350 as described herein. Patient
Adherence
Generation Module 306 then routinely analyzes the medication history data
(step 2104)
including calculation of MAR% for all medications, generates the adherence
data associated
with all active prescription medications taken by a plurality of patients
polled via the Payor
Engine 318, and stores the adherence data in patient adherence database 307.
[0478] With continuing reference to FIGS. 39 and 61, in step 2115 the patient
advisor
module 386 analyzes the MAR data for the selected patient, compares the
predetermined
threshold MAR criteria (value) to the MAR data for all active prescriptions,
and determines if
any one of the plurality of active prescription medication values falls below
the threshold
value as failure to take a single prescribed drug may be responsible for
symptoms exhibited
by the patient during the visit to the health care provider 101 or may
exacerbate the patient's
health in the future. In addition, the health care provider may be able to
determine whether
the failure to fill/refill and take the prescribed medications may be related
to financial reasons,
lack of education or belief about the effectiveness of the medication, or
other reasons which
139
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
can then be addressed to get the patent to take their medications regularly.
[0479] If in step 2115 the patient advisor module 386 determines that the MAR%
is below
the predetermined threshold value (e.g. less than or equal to 70% MAR in this
non-limiting
example) indicating failure of the patient to adhere to their medication
regimen. a "Yes"
condition is returned which causes the process flow to proceed to step 2120.
The "At Risk
Patient" alert display mode of the Report Card tab 2010 is displayed to the
health care
provider. In some embodiments, this visual tab display event may be coupled
with an
audible indication of non-adherence that is produced by the IICP terminal 120
when the alert
display mode is initiated such as any suitable preprogrammed notification
sound to further
get the health care provider's attention. The health care provider may then
select (e.g. click)
this alert condition tab in step 2125 in which case the patient advisor MAR
report card 2200
is displayed (see, e.g. FIG. 46) in step 2140, the contents of which are
further described
herein below. The health care provider may now explore the extent and degree
of the
patient's non-compliance with their medication treatment regimen in greater
detail to take
corrective action.
[0480] If in step 2115 the patient advisor module 386 determines that the MAR%
is instead
above the predetermined threshold value (e.g. greater than 70% MAR in this non-
limiting
example) indicating that the patient generally adheres to their medication
regimen, a "No"
condition is returned which causes the process flow to proceed to step 2130.
The "Report
Card" nominal display mode of the Report Card tab 2010 is displayed to the
health care
provider. The health care provider may then select (e.g. click) this normal
condition tab in
step 2135 in which case the patient advisor MAR report card 2200 is displayed
(see, e.g. FIG.
46) in step 2140, the contents of which are further described herein below.
The health care
provider may now explore patient's adherence to their medication treatment
regimen in
greater detail if desired, albeit with a lesser degree of urgency for a
compliant patient.
[0481] The patient advisor report card 2200 accessed via Report Card tab 2010
in step 2140
will now be described in greater detail. In one enthodiment, the report card
is based on
MAR (medication adherence rate); however, other relevant patient medication
adherence data
may be used in other embodiments.
[0482] Patient Advisor Report Card Tab
[0483] FIG. 46 shows an embodiment of a patient advisor report card 2200 which
is
140
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
displayed when the health care provider selects the Report Card tab 2010. In
one
embodiment, report card 2200 may be an enlarged pop-up screen or window which
is an
interactive GUI that may be displayed in overlay fashion onto the patient
summary screen of
the main EP system GUI 2002. Report card 2200 may include a prescription
medication list
or menu 2210 having one or more items comprised of active medications
currently prescribed
for the patient, patient medication adherence chart 2220 comprising a
graphical display of
statistics pertaining to the patient's medication regimen adherence habits,
dynamic chart
navigation control tags 2230, and a quick-view report card summary field 2240.
[0484] In some embodiments, the drugs listed in active medications menu 2210
may match
the list of active drugs shown in the medication history field 2002d of the
patient summary
screen in the main EP system GUI 2002. Each of the drugs listed by name in the
medication
menu 2210 are dynamic links which are user selectable to control and change
the MAR
displayed in the report card summary field 2240. In the example shown in FIG.
46, the drug
name hydrocodone-acetaminophen has been selected by the health care provider
which may
be emboldened or otherwise highlighted for visual contrast relative to the
other unselected
drugs listed whose names are displayed normally in a different and preferably
more subdued
manner. The drug name may be selected via a cursor click associated with an
input device
or by finger or stylus touch if a touch screen input device is used as
terminal 120 (see FIG. 2).
Selecting hydrocodone-acetaminophen causes its MAR to be displayed in summary
field
2240, further described below. Selecting a different drug causes its
corresponding MAR to
be displayed instead in summary field 2240 (see, e.g. FIG. 47 showing
Humulin).
Accordingly, the health care provider can advantageously quickly navigate
through the drugs
listed in the menu to obtain an immediate snapshot of the patient's MAR with
respect to each
medication.
[0485] In one embodiment, a scroll bar 2229a may be provided on the right or
alternatively
left side that allows a user to scroll up or down the list of drugs in
medication menu 2210
which also vertically changes the corresponding display in the adherence chart
2220.
Alternatively or in addition, a user may place the cursor of an input device
such as a mouse
within the GUI display of the medication menu 2210 and/or adherence chart 2220
and roll the
center wheel of the mouse up or down as an alternative way of scrolling
vertically through
the list of drugs and chart.
[0486] In one embodiment, the list of active medications in the patient
medication menu
141
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
2210 may be sorted by ascending MAR order with the lowest MAR percentage at
the top of
the list. The medications without an MAR may be located at the bottom of the
list.
Generic drug names may be displayed in lower case letters. Brand drug names
may be
displayed with the first letter capitalized with the generic name placed under
it in parentheses
(see, e.g. FIGS. 46 and 47).
[0487] Referring to FIG. 46, report card summary field 2240 may include an
adherence
graphic 2241 representing the MAR percentage rate in a graphical and/or
numeric value form,
the name 2242 of the pertinent drug, and an adherence data identifier 2243
confirming the
type of adherence data being displayed in the summary field and Report Card
tab 2010 at
present (e.g. MAR as shown here). To get the MAR for each active medication in
the
patient medication menu 2210, the user clicks on the name of the drug of
interest listed in the
menu. The MAR will change for each drug when selected and display the
corresponding
MAR percentage. The name 2242 of the drug chosen will also change in the
report card
summary field 2240 to correspond to the drug that was selected from the active
medication
menu 2210.
[0488] In one embodiment, without limitation, the adherence graphic 2241
includes a bar
graph which may be a circular bar graph (also referred to as a wheel or donut
chart) including
an arcuately-shaped bar, as shown in FIG. 46. Advantageously, the circular
format of a
circular bar graph permits a visually large display of adherence data or
metric in a compact
area which does consume an excessive amount of space in the patient advisor
report card
2200. The circular bar graph may include a display of the numeric value of the
adherence
data or metric such as the MAR percentage value displayed and positioned in
the center of
the circular graph which further contributes to the compactness of the
adherence graphic and
patient advisor report card 2200 in general. The numeric MAR value, however,
may be
located elsewhere in the report card summary field 2240 in other embodiments.
The
circular bar graph may be in the form of a partial circle with a gap as shown
or alternatively a
complete circle without a gap (not shown). Preferably, in one embodiment, the
adherence
bar representing the MAR percentage value graphically displayed in the
circular bar graph
has a circumferential or arcuate length which is directly proportionate to the
actual MAR
numeric value (compare, e.g. FIG. 46 showing MAR of 30% and FIG. 47 showing
MAR of
27% with bar having a shorter extent or length). Accordingly, the bar has an
extent or
length (based on MAR %) which is relative to the total available graph space.
For example,
142
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
a bar representing an MAR of 100% would completely fill the available circular
bar graph
space whereas a MAR of 50% will only fill half of the available graph space
(e.g. to
approximately the 12 o'clock position). The adherence bar for the MAR of 30%
in FIG. 46
occupies only about 30% of the available graph space.
[0489] Preferably, the adherence wheel or bar in the circular bar graph of the
adherence
graphic 2241 may have a contrasting color and/or pattern in comparison to the
unused
available circumferential portion of the circular bar graph to be readily
visibility noticeable to
the health care provider for ease of reference. In some embodiments, the
adherence bar may
change color based on the MAR percentage corresponding to the selected drug
from the
patient medication menu 2210. In one non-limiting example, the adherence bar
may change
color as follows: Green ¨ MAR is 71% or greater; Yellow ¨ MAR is between 60%
to 70%;
Red ¨ MAR is less than 60%. Other suitable colors and/or ranges may be used.
[0490] In instances there is insufficient data to calculate an MAR for a drug
selected from
patient medication menu 2210, "N/A" or something similar may be displayed for
the
adherence graphic 2241. A minimum of two "fills" for a single prescription
drug are
required in order to calculate the MAR.
[0491] It will be appreciated that in other embodiments, the adherence graphic
2241 may
have other configuration and shapes other than a circular bar graph which are
typically used
to visually depict relative numeric data values, such as for example without
limitation a linear
bar graph, a line graph, a pie chart, and others. Accordingly, the type and
configuration of
the adherence graphic 2241 is not limited to circular bar graphs or donut
charts alone. A
preferred type of adherence graphic 2241 displayed, however, should not be
complex so that
the graphic representation of the MAR value is easily and quickly recognizable
by the health
care provider without undue examination to save time and expedite the patient
visit.
[0492] With continuing reference to FIG. 46, the chart navigation control
buttons 2230 are
dynamic links which provide one-click access to one or more different types of
patient
medication adherence data to be displayed in patient advisor report card 2200.
The
non-limiting example shown includes four dynamic control buttons; however,
more or less
buttons may be provided. The button currently selected is preferably
highlighted in some
manner (e.g. change in appearance such as size, color, etc.) so as to be
distinguishable from
non-selected buttons. In this example shown in FIG. 46, the MAR button 2230 is
highlighted indicating that it has been selected (whereas the remaining
buttons may be grayed
143
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
out) and the information displayed in the report card summary field 2240 and
adherence chart
2220 is MAR information for the selected drug (hydrocodone-acetaminophen in
this
example). Preferably, when the user such as a health care provider initially
selects (e.g.
click on) and activates the Report Card tab 2010, a predetermined one of the
default chart
navigation control buttons 2230 is automatically activated/selected for
display of the patient
advisor report card 2200 to the user. The user may then switch to display
another type of
patient medication adherence data by selecting a different one of the
available chart
navigation control buttons 2230. Non-limiting examples of some other type
adherence data
that may be used for buttons 2230 include first fill compliance (FFC) data and
patient
Medication Adherence Rate (MAR)/Medication Persistency Rate (MPR) data as
already
described which also offer information in summary form with respect to patient
compliance
in following their medication regimen.
[0493] The patient medication adherence chart 2220 is a graphical display of
the patient's
medication fill history for all their active medications. Adherence chart 2220
is a graphical
display of medication fill history. In one embodiment, the graphical display
of every
medication may he placed on a time graph as further described below. At a
glance, the
health care provider will be able to see if a patient has "filled" their
medications or if there
has been a gap in care because they have not filled it.
[0494] Referring to FIG. 46, patient medication adherence chart 2220 (also
referred to as
simply "adherence chart" for brevity herein) in the present embodiment may
generally
include a master timeline 2224 and a plurality of individual historical drug
timelines 2222
each corresponding to one of the drugs listed in the patient medication menu
2210 located
adjacent to the chart for ease of reference. In one non-limiting embodiment as
shown, the
master timeline may be displayed at the bottom of the chart 2220; however, in
other
embodiments the timeline 2224 may be displayed at the top of the chart or
another suitable
location. The master timeline 2224 includes past, present, and future dates.
[0495] When the patient advisor report card 2200 and patient medication
adherence chart
2220 are initially rendered via selecting the Report Card tab 2010, the
present date 2221 (i.e.
today's date of patient visit and/or health care provider's access to the
patient advisor system)
is displayed as shown in FIG. 46. The present date 2221 may be displayed in a
highlighted
or otherwise visually emphasized manner that is readily discernible to the
user. In one
embodiment, a vertical dateline 2228 may be displayed in the adherence chart
2220
144
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
corresponding to the present date. Advantageously, the adherence chart 2220
projects and
displays future information about each drug including expected refill dates
and prescription
end dates so that the health care provider gets a future look at the
medications which may
continue to be taken to consider when prescribing a new medication or
additional refills for
an existing medication.
[0496] In one embodiment, a slidable date marker 2223 (see, e.g. FIGS. 46 and
particularly
FIG. 47) may be provided that allows the user to slide the dateline 2228 to
the right or left
over any the information in an individual drug timeline 2222 on the adherence
chart 2220
(corresponding to the drug currently selected for display in medication menu
2210) to quickly
get the adherence infounation for the date located at the present position of
the dateline
without having to click on the chart for details. The slidable date marker
2223 is moveable
left or right (i.e. backwards or forward in time) along the master timeline
2224. The
adherence information may be displayed in a pop-up window or box in a manner
similar to
that shown in FIGS. 47-52, as further described below. However, an input
device cursor
need not be placed directly over the individual drug timeline 2222 as in FIGS.
47-52 to
access the pop-up box and information displayed therein. Instead, the cursor
is used to lock
on the slidable date marker 2223 via a click and hold action by the user
followed by sliding
the marker to the right or left in the adherence chart 2220.
[0497] In addition to the slidable date marker 2223, a stationary present date
marker 2227
may also be provided (see, e.g. FIG. 47) which remains fixed in position on
master timeline
2224 at the present date position. Present date marker 2227 displays the
present date 2221
and allows the user to see the chart lined up against the current day. In one
embodiment,
when the patient advisor report card 2220 is first rendered in the user
interface as shown in
FIG. 46, the present date marker 2227 is positioned underneath the slidable
date marker 2223
which is superimposed over top of the present date marker. When the user moves
the
slidahle date marker 2223 right or left, the present date marker 2227 becomes
visible and
remains in the position fixed on master timeline 2224. In one embodiment, the
present date
marker 2227 may further have an associated vertical dateline 2225 displayed in
the adherence
chart 2220.
[0498] In one embodiment, a chart scroll bar 2229b may be provided such as at
the bottom of
the adherence chart 2220 or alternatively at the top of the chart. The scroll
bar 2229b
provides another or an alternate means to slidable date marker 2223 for a user
to navigate
145
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
forward or backward in time in the chart by moving the bar from left to right
and vice-versa.
Similarly to sliding date marker 2223 left or right, the date range displayed
at a given time in
the adherence chart timeline changes forward or backward in time.
[0499] Referring to FIGS. 46-52, the following icons or symbols and acronyms
may be used
in the adherence chart 2220 to represent various possible elements displayed
in each
historical drug timeline 2222: Rx = prescription date; F = Fill Date; R =
Expected Refill Date
(calculated from total quantity of prescription and prescribed dosage); X =
prescription end
date; solid colored bar = gap in fill date; and striped colored bar =
prescription duration. It
will be appreciated that more or less and/or different icons and/or may be
used to represent
the elements of the timelines. In addition, additional or other information
may be displayed
in the timelines. Furthermore, the solid and striped colored bars may also be
displayed in a
variety of alternative different colors and/or patterns or plain other than
those foregoing
non-limiting examples given herein. Accordingly, the display and information
content of
the drug timelines 2222 are described herein by way of the foregoing exemplary
embodiments for convenient and are not intended to limit the invention.
[0500] In one embodiment, a chart legend 2260 defining these foregoing
timeline elements
may be accessed via a chart legend icon 2226 which causes a pop-up window to
be displayed
with the legend information when selected or an input device cursor is
hovered/rolled over
the icon, as shown in FIG. 53.
[0501] In certain embodiments, each of the drug timelines 2222 may include one
or more
dynamic links to medication and adherence summary data and information for
each drug.
Each or some of the foregoing elements of a drug timeline 2222 displayed in
the adherence
chart 2220 (e.g. F, R, X, plain bar, striped bar, etc.) may themselves be an
active dynamic
link that causes a pop-up box with complementary information to be displayed
as shown in
FIGS. 47-52 by rolling or hovering the cursor of an input device over the
element.
[0502] FIG. 47 shows an exemplary pop-up box 2250 that is displayed in
adherence chart
2220 by placing an input device cursor over the Rx (prescription date)
element. The pop-up
box shows the name of the drug (e.g. "Humulin 70/30"), prescription start date
(e.g.
"10/20/12"), and MAR (e.g. "27%"). Dosage information may also be displayed.
[0503] FIG. 48 shows an exemplary pop-up box 2251that is displayed in
adherence chart
2220 by placing an input device cursor over the F (fill date) element. The pop-
up box
146
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
shows the name of the drug (e.g. "Humulin 70/30"), prescription fill date
(e.g. "10/26/12"),
and dosage (e.g. "100 units/mL (70-30)"). The Fill data indicates the date in
which the
medication was filled at a pharmacy.
[0504] FIG. 49 shows an exemplary pop-up box 2252 that is displayed in
adherence chart
2220 by placing an input device cursor over the striped colored bar
(prescription duration)
element. The pop-up box shows the identity of the selected element (e.g.
"Duration"), the
duration in days (e.g. "10 days"), and the calendar dates corresponding to the
days duration
(e.g. "10/26/12-11/4/12"). The duration bar or element indicates the duration
of the fill for
that medication before a refill is required or when it ends. The number of
days plus the date
range it covers may be displayed. For a drug fill that did not fall within a
prescription's start
and end date, there will be no duration bar that follows. The reason for this
is because there
is no clear indication for which prescription that prescription fill was
associated with.
[0505] FIG. 50 shows an exemplary pop-up box 2253 that is displayed in
adherence chart
2220 by placing an input device cursor over the R (expected refill date)
element. The
pop-up box shows the name of the drug (e.g. "Humulin 70/30") and expected
refill date (e.g.
"11/5/12"). The Refill (R) element indicates to the health care provider that
there was a
refill event that should have occurred but was missed. Accordingly, the pop-up
box shows
the name of the drug and date that it was expected to have been refilled based
on the original
prescription. The refill date R takes into account when the prior fill
actually occurred. For
example, if a prescription with 10 pills taken once a day was written on
January 1 but was not
filled until January 15, then the first refill is expected to occur on January
25. The "R" will
thus appear in chart 2220 indicating to the provider that a refill date was
missed according to
the original prescription.
[0506] FIG. 51 shows an exemplary pop-up box 2254 that is displayed in
adherence chart
2220 by placing an input device cursor over the solid colored bar (gap in fill
date) element.
The pop-up box shows the identity of the selected element (e.g. "Refill Gap"),
the duration in
days (e.g. "25 days"), and the calendar dates corresponding to the days
duration (e.g.
"11/5/12-11/29/12"). The refill gap bar shows the number of days and the dates
between the
time the patient should have had their medication filled and the next event.
The refill gap
bar can show up between an "Rx" and an "F" and between a "R" and an "F" or the
end of a
prescription.
[0507] FIG. 52 shows an exemplary pop-up box 2255 that is displayed in
adherence chart
147
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
2220 by placing an input device cursor over the X (prescription end data)
element. The
pop-up box shows the name of the drug (e.g. "llumulin 70/30") and prescription
end date (e.g.
"11/29/12"). The end of a prescription is calculated and readjusted based on
the dates that
the prescription was filled.
[0508] In one embodiment, as shown in FIG. 46, the adherence graphic 2241
representing the
MAR percentage in graphical and/or numeric value form is displayed adjacent to
the patient
medication adherence chart 2220 in the graphical user interface in a manner
that does not
visually obscure the individual historical drug timelines 2222. The adherence
graphic 2241
is visually enlarged having a vertical height which is higher than the
vertical height of an
individual drug timeline 2222, and preferably at least as vertically high as
two individual
historical drug timelines 2222 spaced vertically apart in the patient
medication adherence
chart 2220 so that the graphic readily stands out from the chart 2220 and
timelines 2222 to
the user. Furthermore, it should be noted that the patient medication
adherence data (e.g.
MAR in this embodiment) shown in adherence graphic 2241 which has a numeric
value
calculated from the patient medication history data graphically represented in
the individual
historical drug timelines 2222 cannot be obtained with a single glance by the
health care
provider from the timelines without more in-depth time consuming manual
calculations by
the health care provider from the adherence chart 2220. Accordingly, the
report card
summary field 2240 and particularly adherence graphic 2241 provides a
different form of
adherence data to the health care provider than adherence chart 2220 (e.g.
numeric MAR
value) albeit that numeric data is calculated from the same patient medication
history data
used by Patient Adherence Generation Module 306 to generate the individual
historical drug
timelines 2222.
[0509] Advantageously, the report card summary field 2240 including the
adherence graphic
2241 provides a quantitative summary of patient adherence to their medication
regimen for
each drug identified in the medication menu 2210 and adherence chart 2220
based on an
actual numeric value or measure of adherence (e.g. MAR/MPR, FFC, etc.) instead
of on a
statistically less meaningful qualitative basis to the health care provider
which may be less
informative as to the degree of the patient's non-compliance (e.g. star
ratings,
poor/average/good, etc.). In addition, by providing the ability for the health
care provider to
select an individual drug and display its associated patient adherence
information in the
visually enlarged report card summary field 2240 (in height relative to the
height of each
148
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
smaller individual historical drug timelines displayed in the adherence chart
2220), the degree
of non-compliance is more easily discernible without requiring the health care
provider to
scan through the more detailed adherence chart 2220 timelines and data to
obtain information
about the patient's adherence data. Accordingly, the patient advisor report
card 2200
advantageously offers an easier to navigate, use, and meaningful approach for
graphically
displaying medication adherence data on a GUI which saves time for both the
health care
provider and patient. Since the patient advisor report card 2200 is accessible
directly from
the summary screen of the main EP system GUI 2002 via the patient advisor
toolbar 2004, no
additional searching or accessing other program modules or system are required
for the health
care provider making the patient advisor system user friendly and improving
efficiency for
the user.
[0510] The patient advisor report card 2200 may be closed in one embodiment by
a user
selecting and clicking a "close" tab 2229c (see, e.g. FIG. 46) which returns
the display to the
summary screen of the main EP system GUI 2002 as shown in FIG. 42.
Alternatively, a
user may select one of the other tabs 2020-2060 in the toolbar 2004 which
switches the
display to the selected new tab. The contents of the additional tabs will now
he described.
[0511] FIG. 62 shows an alternative embodiment of a report card summary field
2240 which
displays the MAR % numeric value in a similar manner to that shown in FIG. 46
described
herein, but with MAR% being simultaneously displayed for each drug listed in
the
medication menu 2210. Individual drug adherence graphics 2270 representing the
MAR
percentage rate are displayed for each drug with a numeric value MAR (e.g.
25%, 53%, etc.).
In some illustrative embodiments, without limitation, an adherence graphic
2270 may be
configured as a square as shown, recognizing that alternative shapes may be
used such as
rectangles, triangles, circles, etc. The adherence graphics 2270 may include a
color indicia
which changes color in dependent upon the MAR% for each drug in a similar
manner to the
color changing operation of adherence graphic 2241 as already described herein
(e.g. Green ¨
MAR is 71% or greater; Yellow ¨ MAR is between 60% to 70%; Red ¨ MAR is less
than
60%). Other suitable colors and/or ranges may be used.
[0512] Outcome Studies Tab
[0513] Referring to FIG. 54, the Outcome Studies tab 2020 may optionally be
selected by a
user (e.g. health care provider) from the toolbar 2004 in a manner similar to
Report Card tab
2010 already described herein in detail. Upon selecting this tab, a pop-up
window 2021
149
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
appears in the summary screen of the EP system GUI 2002, such as below the
patient advisor
compliance dashboard 2000 and toolbar 2004 as shown. In one embodiment, window
2021
may be enlarged and overlaid on top of GUI 2002. Outcome Studies is a list of
studies (e.g.
clinical studies) that have evidence-based data with proven metrics showing
that medication
adherence has a positive outcome on a patient's overall well-being. The user
will be able to
review the article within Patient Advisor, send it to the printer, or save it
to their local storage
drive. Window 2021 displays a list of one or more relevant study articles 2022
with
bibliographic summary information such as title of article, source, author,
date, etc. for each
article. The article name 2024 is a user selectable dynamic link which allows
the user to
access and display a copy of the article selected, as shown in FIG. 55. In one
embodiment,
the Outcome Studies tab 2020 may include a studies counter icon 2023 which is
displayed
with tab 2020 and includes a numeric identifier showing the user at a glance
the total number
of articles available on topic. The studies counter icon 2023 may be displayed
as being at
least partially connected or part of the Outcome Studies tab 2020 and may have
a different
color than the tab to be visibly discernible by the user.
[0514] In the non-limiting example shown in FIGS. 54 and 55, the study
articles 2022 pertain
to the benefits realized by patient's adhering to their prescription
medication regimen. It
should be noted that the Outcome Studies listed in this section are not
patient specific and
general or generic in nature.
[0515] In other embodiments contemplated, the system 1000 may be programmed
and
configured to compile and provide user access (e.g. health care provider) to
drug-specific
study articles via the Outcome Studies tab 2020. Accordingly, the content of
articles
accessed by the Outcome Studies tab 2020 corresponds to studies which are drug-
specific in
nature based on the medications shown in the medication history field 2002d of
the patient
summary screen of the EP system GUI 2002 which displays a list of all active
medications
prescribed for the patient (see, e.g. FIG. 42). The total number of available
articles which
may be accessed via Outcome Studies tab 2020 (and shown in studies counter
icon 2023)
may correspond to all drugs listed in the medication history field 2002d, or
alternatively only
articles corresponding to one or a group of some drugs that has been selected
(e.g.
highlighted or marked) by the user from the list within the medication history
field 2002d by
selecting or de-selecting the drug in the list. The foregoing method of
selecting study
articles 2022 for retrieval and display by the system involve user actions
which are taken in
150
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
the medication history field 2002d of the patient summary screen of the EP
system GUI 2002.
[0516] In other embodiments, selecting study articles 2022 for retrieval and
display by the
system may involve user actions which are taken in the medication menu 2210
displayed in
the patient advisor report card 2200. For example, if a user (e.g. health care
provider)
selects Humulin in the medication menu 2210 of the patient advisor report card
2200 for
viewing the MAR percentage (shown highlighted in FIG. 53), the content of the
clinical study
articles accessible by selecting the Outcome Studies tab 2020 directly
correspond to the
beneficial effects experienced by patients taking Humulin. The article content
will change
when the user selects a different drug in the medication menu 2210 to
correspond to the new
drug selected. The studies counter icon 2023 will change each time a different
drug is
selected by the user. If no articles 2022 are available related to the drug
selected, the
counter icon 2023 may not be displayed by the system or alternatively "0" may
be indicated
in the icon.
[0517] FIG. 55 shows an exemplary image of a clinical study article 2022 which
is displayed
by clicking on (i.e. selecting) the article name 2024 in the pop-up window
2021 shown in FIG.
56. A return tab 2023b allows the user to close the displayed article
window and return to
the previous screen shown in FIG. 546 with a list of all available articles.
[0518] To close the Outcome Studies tab 2020 and return to the patient summary
screen of
the EP system GUI 2002 (see, e.g. FIG. 42), a close tab 2023a may be provided
as shown in
FIG. 54.
[0519] Education Tab
[0520] Referring to FIG. 56, the Education tab 2030 may optionally be selected
by a user (e.g.
health care provider) from the toolbar 2004 in a manner similar to Report Card
tab 2010
already described herein in detail. Upon selecting this tab, a pop-up window
2031 appears
in the summary screen of the EP system GUI 2002 as shown displaying a list of
educational
sheets or articles 2032 which are drug-specific in nature. In one embodiment,
window 2031
may be enlarged and overlaid on top of Gill 2002.
[0521] The content of the Education tab 2030 is mapped by the system 1000 to a
patient's
active medication list in one embodiment (see, e.g. medication menu 2210 of
patient advisor
report card 2200 in FIG. 46). If the patient has an active medication for
which an
educational type health sheet or article 2032 is available for a drug on the
medication list, it
151
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
will be accessible and listed in the Education tab. The education counter icon
2034, which
functions similarly to studies counter icon 2023 discussed above, will be
displayed with a
numeric indication of the number of articles 2032 that are available for
viewing by clicking
on the Education tab 2030.
[0522] In other or additional embodiments, the health care provider may take
one of the
following actions in the EP system 200 with a prescription to be written and
filled which will
also display if educational articles 2032 are available for the prescribed
medications in the
Education tab 2030: (1) electronically sending/submitting the prescription to
the pharmacy
system (see, e.g. FIGS. 1 and 6); (2) electronically sending/submitting and
printing
prescription; (3) printing prescription without electronically
sending/submitting; and (4)
electronically signing the prescription without electronically
sending/submitting.
[0523] To select an educational health sheet or article 2032 for immediate
viewing on display
121 of the health care provider system within the main EP system GUI 2002, the
user can
click on the article icon 2035 shown in FIG. 56. In some embodiments, an
action options
field 2033 may be provided which includes a plurality of user selectable
action buttons 2036
which gives the health care provider several options for providing a displayed
article 2032 to
the patient based on the format preferences of the patient for obtaining the
health information.
[0524] To select an educational health sheet or article 2032 to print, the
user can click on the
"Print" button on the same line as the article. To select multiple documents
to print at a
time, the user can click on the check box 2037 next to each educational health
sheet or article
2032 and then click on the "Print Selected" icon 2038. Other available user
action buttons
2036 may include "E-mail" which triggers the system to send a copy of the
article 2032 to the
patient's email address of record in the EP system 200, and "Send Text" which
sends a text
message to the patient's cell phone number of record in the EP system of the
article. Other
action buttons may also be provided.
[0525] FIG. 57 shows an exemplary image of an educational health sheet or
article 2032
which is displayed by clicking on (i.e. selecting) the article icon 2035 shown
in FIG. 56.
The article may be displayed in a pop-up window below patient advisor
compliance
dashboard 2000 and interactive toolbar 2004 in one embodiment as shown. A
return tab
2039b allows the user to close the displayed article window and return to the
previous screen
shown in FIG. 56 with a list of all available articles on the topic.
152
CA 02918798 2016-01-19
WO 2015/013695
PCT/US2014/048330
[0526] To close the Education tab 2030 and return to the patient summary
screen of the EP
system GUI 2002 (see, e.g. FIG. 42), a close tab 2039a may be provided as
shown in FIG. 56.
[0527] Coupons and Co-Pay
[0528] Selecting Coupon tab 2040 of the toolbar 2004 shown in FIG. 42 provides
a health
care provider with access to and displays available coupons and co-pay savings
for the patient
based on active medications currently prescribed for the patient or those new
medications to
be presently prescribed. In one embodiment, the patient adherence system 360
may be
configured to automatically display a relevant coupon for a drug presently
being
electronically prescribed via the EP system 200 (e.g. new prescription)
without the health
care provider or other user manually selecting the Coupon tab 2040 simply by
the action of
the health care provider or other user electronically writing the prescription
in the EP system.
Coupons and co-pay savings for branded drugs which have been prescribed by the
provider
may be available for printing after the prescription is written in the EP
system 200. If a
coupon is available for a particular medication, the provider will see a
preview window with
the savings offer, as shown in FIG. 58. The relevant adjudication information
of this offer
will automatically be amended to the electronic prescription as part of the
notes to pharmacist
as shown in FIG. 59. In some enthodiments, if the provider uses the notes to
pharmacist
field to write a message, the coupon information may not be amended in that
field.
[0529] While the embodiment of the present invention has been described with
reference to
the accompanying drawings, it will be understood by those skilled in the art
that the present
invention can be embodied in other specific forms without departing from its
spirit or
essential characteristics. Therefore, the foregoing embodiments and advantages
are merely
exemplary and are not to be construed as limiting the present invention. The
present teaching
can be readily applied to other types of apparatuses. The description of the
foregoing
embodiments is intended to be illustrative, and not to limit the scope of the
claims. Many
alternatives, modifications, and variations will be apparent to those skilled
in the art. In the
claims, means-plus-function clauses if provided are intended to cover the
structures described
herein as perfoi ming the recited function and also equivalent structures.
153