Note: Descriptions are shown in the official language in which they were submitted.
UNITARY BODY SYSTEMS AND DEVICES AND METHODS TO USE
THE SAME FOR RETROPERFUSION
BACKGROUND
Peripheral arterial disease involves inadequate blood supply to the peripheral
limbs due to arterial
damage, defect, or blockage. In view of the same, devices, systems, and
methods of using the same to
facilitate adequate blood supply to the peripheral limbs would be well
appreciated in the marketplace.
BRIEF SUMMARY
The present disclosure includes disclosure of various perfusion and/or
retroperfusion devices and
systems and methods of using the same, configured for use in connection with
various coronary, peripheral,
and other retroperfusion methods/procedures and/or to treat various conditions
of ischemia and/or to
facilitate/promote local venous arterialization.
In at least one embodiment of a perfusion device of the present disclosure,
the perfusion device
comprises a unitary body having a wall and a lumen defined therethrough, a
first portion terminating at a
first end and configured for at least partial placement within a mammalian
artery, and a second portion
terminating at a second end and configured for at least partial placement
within a mammalian vein. In
another embodiment, the first portion is relatively shorter than the second
portion. In yet another
embodiment, the first portion has a first length, wherein the second portion
has a second length, and wherein
the first length is less than the second length. In an additional embodiment,
the device further comprises a
first one-way valve positioned at or near an end of the first portion opposite
the first end. In yet an additional
embodiment, the device further comprises a second one-way valve positioned at
or near an end of the second
portion opposite the second end.
1
Date Recue/Date Received 2020-12-23
CA 02919981 2016-01-29
WO 2015/017714 PCT/US2014/049270
In at least one embodiment of a perfusion device of the present disclosure,
the device
further comprises an optional segment between the first one-way valve and the
second one-way
valve. In an additional embodiment, the first one-way valve and the second one-
way valve are
each sized and shaped to be immediately adjacent to one another. In yet an
additional
embodiment, the one-way valve is sized and shaped to receive at least part of
a first guidewire
therethrough. In another embodiment, the second one-way valve is sized and
shaped to receive at
least part of a second guidewire therethrough.
In at least one embodiment of a perfusion device of the present disclosure,
and when in
use, at least part of the first portion could be positioned within a
subclavian artery or axiallary
artery, and wherein at least part of the second portion could be positioned
within a subclavi an vein
or an axillary vein for use and/or treatment at or near the heart. In another
embodiment, in use at
least part of the first portion could be positioned within an iliac artery,
and wherein at least part of
the second portion could be positioned within a saphenous vein or a femoral
vein. In yet another
embodiment, the entire body is flexible or one or more portions of the body
is/are flexible. In an
.. additional embodiment, the body is able to deform easily without collapsing
so that the lumen
remains open to allow blood to flow from the first end, through the body, and
out of the second
end when in use.
In at least one embodiment of a perfusion device of the present disclosure,
the body
comprises a coil-reinforced wall having one or more coils. In an additional
embodiment, the one
or more coils are used in connection with an impeimeable coating. In yet an
additional
embodiment, the device further comprises a balloon positioned within or
coupled to the second
portion. In another embodiment, the device further comprises a balloon tube
having a balloon
port, the balloon tube coupled to the balloon. In at least one embodiment of a
perfusion device of
the present disclosure, introduction of a gas and/or a liquid into the balloon
port can be used to
inflate the balloon, and removal of the gas and/or the liquid via the balloon
port can be used to
deflate the balloon. In an additional embodiment, the balloon is used to
ensure retrograde flow of
blood. In yet an additional embodiment, at least a portion of the device is
sized and shaped to fit
within a splittable introducer sheath.
In at least one embodiment of a perfusion device of the present disclosure,
the device
further comprises a flarable tip defined at or coupled to the second end of
the device. In another
embodiment, the flarable tip is configured to shift from a first configuration
to a second
configuration and back to the first configuration. In yet another embodiment,
the first
configuration is generally tapered or unflared, and wherein the second
configuration is generally
flared. In an additional embodiment, the first configuration is not expanded,
and wherein the
.. second configuration is expanded. In at least one embodiment of a perfusion
device of the present
2
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
disclosure, the first configuration exists under typical venous blood
pressure, and wherein the
second configuration exists due to a relatively higher arterial blood
pressure. In an additional
embodiment, the flarable Lip is generally configured so that the second end
distends to a luminal
perimeter of a portion of a vein having the second end positioned therein so
that blood flow
therethrough is retrograde. In yet an additional embodiment, the flarable tip
comprises a
membrane reinforced by a plurality of struts. In another embodiment, the
membrane comprises a
material selected from the group consisting of polytetrafluoroethylene,
mammalian tissue, and/or
one or more other biologically-compatible thin or relatively thin materials.
In yet another
embodiment, the struts comprise a material selected from the group consisting
of nitinol, stainless
steel, and/or one or more other biologically-compatible rigid compositions.
In at least one embodiment of a perfusion device of the present disclosure,
the second
portion comprises a first tapered portion. In another embodiment, the first
tapered portion
comprises part of the second portion. In yet another embodiment, the first
tapered portion
comprises all or substantially all of second portion. In an additional
embodiment, the second
portion is sized and shaped to conform to dimensions of the mammalian vein. In
yet an additional
embodiment, the device is configured so that the second portion is sized and
shaped to facilitate
implantation within the mammalian vein. In at least one embodiment of a
perfusion device of the
present disclosure, the device is configured so that the second portion is
sized and shaped to
reduce a risk of rupture of the mammalian vein. In an additional embodiment,
the tapered portion
tapers distally from a first diameter to a second diameter, wherein the first
diameter is greater than
the second diameter. In yet an additional embodiment, the second portion
comprises a second
tapered portion. In another embodiment, the second portion comprises one or
more additional
tapered portions. In yet another embodiment, the first tapered portion is the
only tapered portion.
In at least one embodiment of a perfusion device of the present disclosure, a
degree,
number, and length of tapered portions can be selected to regulate the degree
of pressure drop
along the device in order to reduce the transmission of arterial pressure to
the venous system and
to generally avoid over-pressurization of the venous system. In another
embodiment, a blood
pressure is decreased during blood flow through the device. In yet another
embodiment, the
decrease in blood pressure is facilitated at the first tapered portion. In at
least one embodiment of
a perfusion device of the present disclosure, when the first portion is
immediately adjacent to the
second portion, the first portion meets the second portion at a central
junction. In another
embodiment, part of the first portion adjacent to the central junction is
flexible. In yet another
embodiment, part of the second portion adjacent to the central junction is
flexible. In an
additional embodiment, the first portion is configured to bend at a first
amount, the first amount
having a range of above 00 to 1800. In yet an additional embodiment, the
second portion is
3
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
configured to bend at a second amount, the second amount having a range of
above 00 to 180 . In
at least one embodiment of a perfusion device of the present disclosure, the
device is configured
so that a first angle ranging from above 0' to 180' can be formed within the
first portion and/or a
second angle ranging from above 0 to 180 can be formed within the second
portion. In an
additional embodiment, when the device comprises a segment between the first
one-way valve
and the second one way-valve, the device is configured so that a first angle
ranging from above 0
to 180' can be formed relative to the first portion and the segment and/or a
second angle ranging
from above 0 to 180 can be formed relative to the second portion and the
segment. In yet an
additional embodiment, the bend at the first amount corresponds to the first
angle in various
embodiments and wherein the bend at the second amount corresponds to the
second angle in
various embodiments.
In at least one embodiment of a perfusion device of the present disclosure, a
pressure drop
along the device can vary depending upon the bend at the first amount, the
first angle, the bend at
the second amount, and/or the second angle. In another embodiment, the
pressure drop can be
regulated up to at least 32% due to an extent of the first amount, the first
angle, the bend at the
second amount, and/or the second angle. In yet another embodiment, the
pressure drop is a
function of a device length, a device diameter, a flow friction factor, and a
relative flow condition
between two vessels which are connected by the device. In at least one
embodiment of a
retroperfusion system of the present disclosure, the system comprises an
exemplary retroperfusion
device of the present disclosure, and at least one other item, such as, for
example, one or more of
a first guide wire, a second guide wire, a splittable introducer sheath,
and/or a data wire.
The various device and systems referenced herein may be configured for use in
connection
with various coronary, peripheral, and other retroperfusion methods/procedures
and/or to treat
various conditions of ischemia and/or to facilitate/promote local venous
arterialization, depending
on device and/or system configuration. In at least one embodiment of a method
of the present
disclosure, the method comprises, one or more of the steps of, in any order,
(a) implanting an
exemplary retroperfusion device of the present disclosure into a patient so
that a first part of the
device is in communication with an artery and that a second part of the device
is in
communication with a vein and so that blood can flow from the artery, through
the device, and
into the vein; (b) bending the device so that one or more desired angles
and/or bends are present
along at least part of the device, so to obtain a desired pressure drop
through the device; (c)
configuring one or more lengths and/or one or more diameters of the device
and/or of parts of the
device based upon use within the patient, a height of the patient, a blood
pressure of the patient,
and/or a flow of blood through the artery and/or the vein of the patient;
and/or (d) selecting a
suitable device from a plurality of available devices, the suitable device
selected based upon the
4
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
one or more lengths and/or the one or more diameters of the device and/or of
parts of the device
based upon use within the patient, the height of the patient, the blood
pressure of the patient,
and/or the flow of blood through the artery and/or the vein of the patient. In
at least one method,
the method comprises the steps of positioning a first portion of a perfusion
device within an
artery, wherein a first guidewire is positioned through part of the first
portion of the device into
the artery, and positioning a second portion of a perfusion device within an
vein, wherein a
second guidewire is positioned through part of the second portion of the
device into the vein, and
removing the first guidewire from the first part of the device and removing
the second guidewire
from the second part of the device. In another embodiment, the part of the
first portion is the first
one-way valve, and the part of the second portion is the second one-way valve.
In various
embodiments, blood can flow from the artery, through the perfusion device, and
into the vein. In
another method, the method further comprises the step of bending the perfusion
device to form a
desired angle within the device. In an exemplary method embodiment, the method
comprises the
step of positioning a device of the present disclosure within a mammalian
patient so that the first
portion is positioned within an artery and so that the second portion is
positioned within a vein. In
another embodiment, the positioning step is performed by positioning a first
portion of a perfusion
device within the artery, wherein a first guidewire is positioned through part
of the first portion of
the device into the artery, and positioning a second portion of a perfusion
device within the vein,
wherein a second guidewire is positioned through part of the second portion of
the device into the
vein. In another embodiment, the positioning step is further performed by
advancing a first
dilator over the first guidewire before positioning the first portion of the
perfusion device into the
artery and by advancing a second dilator over the second guidewire before
positioning the second
portion of the perfusion device into the vein.
BRIEF DESCRIPTION OF THE DRAWINGS
The disclosed embodiments and other features, advantages, and disclosures
contained
herein, and the matter of attaining them, will become apparent and the present
disclosure will be
better understood by reference to the following description of various
exemplary embodiments of
the present disclosure taken in conjunction with the accompanying drawings,
wherein:
FIG. 1 shows a side view of a catheter for placement within an arterial vessel
and that may
be used to deliver retroperfusion therapy, according to at least one
embodiment of the present
disclosure;
FIG. 2A shows a side view of the catheter of FIG. 1 in a collapsed position,
according to at
least one embodiment of the present disclosure;
FIG. 2B shows a side view of the catheter of FIG. 1 in an extended position,
according to
at least one embodiment of the present disclosure;
5
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
FIG. 3 shows a side view of an autoretroperfusion system positioned to deliver
retroperfusion therapy to a heart, according to at least one embodiment of the
present disclosure;
FIGS. 4A and 4B show perspective views of the distal end of a venous catheter
used in the
autoretroperfusion system of FIG. 3, according to at least one embodiment of
the present
disclosure;
FIG. 5 shows the components of an autoretroperfusion system that can be used
to deliver
retroperfusion therapy to ischemic tissue, according to at least one
embodiment of the present
disclosure;
FIG. 6 shows a view of the base and diaphragmatic surface of a heart with the
distal ends
of two components of the autoretroperfusion system of FIG. 5 positioned
therein such that the
autoretroperfusion system can deliver simultaneous selective
autoretroperfusion therapy thereto,
according to at least one embodiment of the present disclosure;
FIG. 7 shows a flow chart of a method for delivering autoretroperfusion
therapy,
according to at least one embodiment of the present disclosure;
FIG. 8A shows a side view of the catheter of FIG. 1 in a collapsed position
within an
introducer, according to at least one embodiment of the present disclosure;
FIG. 8B, shows a side view of the catheter of FIG. 1 being introduced via an
introducer
into an arterial vessel, according to at least one embodiment of the present
disclosure;
FIGS. 8C and 8D show side views of the introducer of FIG. 8A being removed
from an
.. arterial vessel, thereby deploying the projection cannula of the catheter
of FIG. 1, according to at
least one embodiment of the present disclosure;
FIG. 8E shows a side view of the catheter of FIG. 1 anchored within an
arterial vessel
through the use of an expandable balloon, according to at least one embodiment
of the present
disclosure;
FIG. 9 shows a schematic view of the autoretroperfusion system of FIG. 5 as
applied to a
heart, according to at least one embodiment of the present disclosure;
FIG. 10 shows a schematic view of the autoretroperfusion system of FIG. 5 as
applied to a
heart, according to at least one embodiment of the present disclosure;
FIG. 11 shows a schematic view of a step of the method of FIG. 7 as the method
is applied
to a heart, according to at least one embodiment of the present disclosure;
FIG. 12 shows a flow chart of a method for delivering simultaneously selective
autoretroperfusion therapy, according to at least one embodiment of the
present disclosure;
FIG. 13 shows a schematic view of a step of the method of FIG. 12 as the
method is
applied to a heart, according to at least one embodiment of the present
disclosure;
6
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
FIG. 14 shows a schematic view of a step of the method of FIG. 12 as the
method is
applied to a heart, according to at least one embodiment of the present
disclosure;
FIG. 15 shows an exemplary retroperfusion system, according to at least one
embodiment
of the present disclosure;
FIG. 16 shows a portion of an exemplary retroperfusion system, according to at
least one
embodiment of the present disclosure; and
FIG. 17 shows a block diagram of components of an exemplary retroperfusion
system
coupled to a blood supply, according to at least one embodiment of the present
disclosure;
FIG. 18 shows a schematic of the retroperfusion system showing the arterial
and
retroperfusion catheters, according to a study in connection with the present
disclosure;
FIG. 19 shows a diagram of steps of an exemplary method of organ perfusion,
according
to at least one embodiment of the present disclosure;
FIG. 20 shows a block diagram of a regional hypothermia system and kit used in
connection with an exemplary device or system of the present disclosure;
FIG. 21 shows an intravenous arterialization catheter, according to an
exemplary
embodiment of the present disclosure;
FIG. 22 shows a biodegradable intravenous arterialization catheter, according
to an
exemplary embodiment of the present disclosure;
FIG. 23A shows steps of a method of using an intravenous arterialization
catheter,
according to an exemplary embodiment of the present disclosure;
FIG. 23B shows an embodiment of a catheter positioned within a vein and
connected to a
graft in communication with an artery, according to an exemplary embodiment of
the present
disclosure;
FIG. 23C shows steps of another method of using an intravenous arterialization
catheter,
according to an exemplary embodiment of the present disclosure;
FIG. 24A shows an intravenous arterialization catheter, according to an
exemplary
embodiment of the present disclosure;
FIG. 24B shows an embodiment of a catheter positioned subcutaneously and into
a vein
and connected to a graft in communication with an artery, according to an
exemplary embodiment
of the present disclosure;
FIG. 25 shows an intravenous arterialization catheter, according to an
exemplary
embodiment of the present disclosure; and
FIGS. 26A and 26B show embodiments of catheters positioned into a human and
animal
vein, respectively, according to exemplary embodiments of the present
disclosure.
7
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
FIG. 27A shows a retroperfusion device, according to an exemplary embodiment
of the
present disclosure;
FIG. 27B shows a portion of a retroperfusion device according to an exemplary
embodiment of the present disclosure;
FIG. 27C shows part of a retroperfusion device, according to an exemplary
embodiment of
the present disclosure;
FIG. 28 shows a retroperfusion device positioned at least partially within a
mammalian
vasculature, according to an exemplary embodiment of the present disclosure;
FIG. 29 shows a retroperfusion device, according to an exemplary embodiment of
the
present disclosure;
FIG. 30 shows a retroperfusion device positioned at least partially within a
mammalian
vasculature, according to an exemplary embodiment of the present disclosure;
FIGS. 31A and 31B show a portion of a retroperfusion device having a flarable
tip,
according to an exemplary embodiment of the present disclosure;
FIG. 32 shows a retroperfusion device with a tapered portion, according to an
exemplary
embodiment of the present disclosure;
FIG. 33 shows several curvature profiles tied to mathematical functions,
according to
exemplary embodiments of the present disclosure;
FIG. 34 shows various device configurations based upon sigmoi dal functions,
according to
exemplary embodiments of the present disclosure;
FIG. 35 shows in vivo wave fonns obtained from a femoral artery;
FIG. 36 shows a pulsatile inlet velocity profile, according to an exemplary
embodiment of
the present disclosure;
FIG. 37 shows a device having an extreme curvature (180' bend), according to
an
exemplary embodiment of the present disclosure;
FIG. 38 shows a depiction of a relative pressure drop as compared to a
relative pressure
drop in view of device curvature angles, according to an exemplary embodiment
of the present
disclosure; and
FIG. 39 shows a device positioned within a mammalian artery and vein,
according to an
exemplary embodiment of the present disclosure.
DETAILED DESCRIPTION
The embodiments discussed herein include devices, systems, and methods useful
for
providing selective autoretroperfusion to the venous system. In addition, and
with various
embodiments of devices and systems of the present disclosure, said devices
and/or systems can
also be used to achieve a controlled arterialization of the venous system. For
the purposes of
8
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
promoting an understanding of the principles of the present disclosure,
reference will now be
made to the embodiments illustrated in the drawings, and specific language
will be used to
describe the same. It will nevertheless be understood that no limitation of
the scope of this
disclosure is thereby intended.
The devices, systems and methods disclosed herein can be used to safely and
selectively
arterialize venous vessels in order to decrease the stress thereon and prevent
rupture of the same.
Accordingly, through the use of the devices, systems and methods disclosed
herein, long-term
autoretroperfusion of oxygenated blood through the coronary venous system can
be achieved,
thereby providing a continuous supply of oxygen-rich blood to an ischemic area
of a tissue or
organ. While the devices, systems and methods disclosed herein are described
in connection with
a heart, it will be understood that such devices, systems and methods are not
limited in their
application solely to the heart and the same may be used in connection with
any ischemic tissue
and/or organ in need of an oxygen-rich blood supply.
Selective auto-retroperfusion (SARP) can be indicated for both chronic and
acute
applications, and exemplary catheters 10 and/or systems 100 of the present
disclosure (and as
referenced in further detail herein) can be used in connection therewith.
References to "acute" for
SARP applications are used generally to indicate the amount of time that an
exemplary catheter
10 and/or system 100 of the present disclosure may be in use on a given
patient. In at least one
embodiment, catheter 10 and/or system 100, or portions thereof, will be
sterile and intended for
disposal after a single use. In at least one embodiment of a system 100 useful
in connection with
an acute indication, use of system 100 could be limited to less than 24 hrs.
Now referring to FIG. 1, a side view of a catheter 10 is shown. The catheter
10 is
configured to be placed within an arterial vessel and comprises a flexible,
elongated tube having a
proximal end 12, a distal end 14 and at least one lumen 15 extending between
the proximal end 12
and the distal end 14. The dimensions of the catheter 10 may vary depending on
the particulars of
a specific patient or with respect to the artery to be cannulated. For example
and without
limitation, where the catheter 10 is used to in a system for
autoretroperfusion of the coronary
sinus, the catheter 10 may comprise a diameter of about 2.7 millimeters to
about 4 millimeters
(about 8 Fr to about 12 Fr). Furthermore, the at least one lumen 15 of the
catheter 10 comprises a
sufficient diameter such that blood can flow therethrough. In addition, the
catheter 10 may be
comprised of any appropriate material, including without limitation,
polyurethane or silicone
rubber. Furthermore, the catheter 10 may be coated with heparin or any other
suitable anti-
coagulant such that the catheter 10 may be placed within a vessel for an
extended period of time
without inhibiting blood flow due to coagulation. The distal end 14 of the
catheter 10 is
configured to allow arterial blood to flow therethrough and into the at least
one lumen 15 of the
9
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
catheter 10. Similarly, the proximal end 12 of the catheter 10 is configured
to allow blood within
the at least one lumen 15 to flow out of the catheter 10. Accordingly, when
the catheter 10 is
positioned within an arterial vessel, the oxygenated blood is allowed to flow
into the catheter 10
through the distal end 14 of the catheter 10, through the at least one lumen
15, and out of the
catheter 10 through the proximal end 12 of the catheter 10. In this manner,
placement of the
catheter 10 within a vessel does not inhibit the flow of blood through the
vessel or significantly
affect the pressure of the blood flow within the vessel.
As shown in FIG. 1, the catheter 10 further comprises a projection cannula 16
that extends
from the proximal end 12 of the catheter 10 and forms a Y-shaped configuration
therewith. The
projection cannula 16 comprises a flexible tube of material that is
appropriate for insertion within
a vessel and placement within an opening in a vessel wall. Furthermore, the
projection cannula
16 comprises at least one lumen 18, a proximal end 20, and a distal end 22.
The distal end 22 of
the projection cannula 16 is coupled with the body of the catheter 10 and
configured to allow the
lumen 18 of the projection cannula 16 to communicate with at least one of the
at least one lumens
15 of the catheter 10. Accordingly, when blood flows through the at least one
lumen of the
catheter 10, a portion of the blood flow enters the lumen 18 of the projection
cannula 16 through
the distal end 22 thereof and flows out through the proximal end 20 of the
projection cannula 16.
In this manner, the catheter 10 is capable of bifurcating the flow of blood
through the vessel in
which it is inserted and routing some of that blood flow out of the vessel and
to another location.
This bifurcation can be exploited to modify the pressure of the blood flowing
through the
projection cannula 16 and/or through the proximal end 12 of the catheter 10 by
manipulating the
dimensions of the projection cannula 16 and the body of the catheter 10. For
example, and
without limitation, if the diameter of the projection cannula 16 is less than
the diameter of the at
least one lumen 15 of the catheter 10, the majority of the blood will flow
through the proximal
end 12 of the catheter 10 and the pressure of the remaining blood that flows
through the smaller
projection cannula 16 will necessarily be reduced. Predictably, the smaller
the diameter of the
lumen 18 of the projection cannula 16, the greater the pressure drop that can
be achieved in the
blood flowing through the lumen 18 of the projection cannula 16. Accordingly,
with respect to
the catheter's 10 application to autoretroperfusion therapies, the projection
cannula 16 can be used
to re-route blood flow from an artery to a vein while simultaneously achieving
the necessary
pressure drop in the re-routed blood between the arterial system and
unarterialized venous system.
Moreover, the catheter 10 is capable of maintaining substantially normal blood
flow through the
artery in which it is housed as the arterial blood not re-routed through the
projection cannula 16 is
allowed to flow through the open proximal end 12 of the catheter 10 and back
into the artery in
the normal antegrade fashion.
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
Due to the configuration of the projection cannula 16 and the material of
which it is
comprised, the projection cannula 16 is capable of hingedly moving relative to
the body of the
catheter 10 between a collapsed position and an extended position. Now
referring to FIGS. 2A
and 2B, the projection cannula 16 is shown in the collapsed position (FIG. 2A)
and in the
extended position (FIG. 2B). When the projection cannula 16 is in the
collapsed position, the
projection cannula 16 is positioned substantially parallel with the body of
the catheter 10.
Alternatively, when the projection cannula 16 is in the extended position, the
projection cannula
16 is positioned such that the projection cannula 16 fomis an angle 0 with the
proximal end 12 of
the catheter 10. The value of angle 0 may be selected depending on the desired
application of the
catheter 10. For example, in at least one embodiment, the angle 0 may comprise
any value
ranging between about 15' and about 90 . In another example, the angle 0 may
comprise about
45 when the projection cannula 16 is in the extended position. The projection
cannula 16 is
biased such that, when it is not subject to a downward force, the projection
cannula 16 rests in the
expanded position. Conversely, when a downward force is applied to the
projection cannula 16
by way of an introducer or otherwise, the projection cannula 16 moves into and
remains in the
collapsed position until the downward force is removed. In this manner, the
projection cannula 16
may be introduced into a vessel in the collapsed position through the use of
an introducer or shaft
and thereafter move into the expanded position when the catheter 10 is
properly positioned within
the vessel and the introducer or shaft is removed.
Optionally, as shown in FIG. 1, the catheter 10 may further comprise an
expandable
balloon 58 coupled with an intermediary portion of the external surface of the
catheter 10 such
that the expandable balloon 58 encases the catheter 10 and the distal end 22
of the projection
cannula 18. The expandable balloon 58 may be any expandable balloon 58 that is
appropriate for
insertion within a vessel and may comprise any material suitable for this
function, including
without limitation, polyethylene, latex, polyestherurethane, polyurethane,
sylastic, silicone rubber,
or combinations thereof. In operation, the expandable balloon 58 can be used
to anchor the
catheter 10 in a desired position within a vessel wall and prevent leakage
from the opening in the
vessel wall through which the projection cannula 16 traverses. The expandable
balloon 58 is
capable of being controlled by a clinician such that it can inflate and/or
deflate to the proper size.
The sizing of the expandable balloon 58 will differ between patients and
applications. The
expandable balloon 58 may be in fluid communication with a balloon inflation
port 62 through a
secondary lumen 60 within the lumen 18 of the projection cannula 16.
Alternatively, the
expandable balloon 58 may be in fluid communication with the balloon inflation
port 62 through a
tube or other means that is positioned within the lumen 18 of the projection
cannula 16 as shown
in FIG. 1. The balloon port 62 may be positioned subcutaneously or otherwise
such that a
11
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
clinician can easily access the balloon port 62 when the catheter 10 is
positioned within a vessel.
In this manner the balloon port 62 can be accessed by a clinician,
subcutaneously, percutaneously
or otherwise, and used to inflate or deflate the expandable balloon 58 with no
or minimal invasion
to the patient.
Now referring to FIG. 3, an autoretroperfusion system 100 is shown positioned
to allow
arterial blood to irrigate the coronary sinus of a heart 101. With respect to
the heart 101, the
autoretroperfusion system 100 may be used for treatment of myocardial
infarctions by injecting
arterial blood into the coronary sinus in synchronism with the patient's
heartbeat. Furthermore,
the autoretroperfusion system 100 is capable of controlling the pressure of
the arterial blood flow
as it enters the venous vessel such that when the arterial blood flow is first
introduced into the
venous system, the pressure of the re-routed arterial blood flow is reduced to
protect the thinner
venous vessels. In this manner, the venous system is allowed to gradually
arterialize. Further,
after the selected venous vessel has sufficiently arterialized, the
autoretroperfusion system 100 is
capable of reducing or ceasing its influence on the pressure of the re-routed
arterial blood flow
such that the standard arterial blood flow pressure is thereafter allowed to
flow into the
arterialized venous vessel.
Autoretroperfusion system 100 comprises the catheter 10, a second catheter
150, and a
connector 170. The catheter 10 is for placement within an arterial vessel and
is configured as
previously described in connection with FIGS. 1-2B. The second catheter 150 is
configured for
placement within the venous system. The connector 170 is configured to form an
anastomosis
between the catheter 10 and the second catheter 150 and further functions to
monitor various data
points on the blood flow flowing therethrough. In addition, in at least one
embodiment, the
connector 170 is capable of controlling the pressure of arterial blood flowing
therethrough. The
second catheter 150 is configured for placement within a venous vessel wall
114 and comprises a
flexible tube having a proximal end 152, a distal end 154 and at least one
lumen 156 extending
between the proximal end 152 and the distal end 154. Both the proximal end 152
and the distal
end 154 of the second catheter 150 are open and in communication with the at
least one lumen
156 of the second catheter 150, thereby allowing blood to flow into the at
least one lumen 156
through the proximal end 152 and out of the distal end 154 back into the
venous vessel 114. The
second catheter 150 may be any catheter known in the art that is capable of
intravascular insertion
and advancement through the venous system and may comprise any appropriate
material,
including without limitation, polyurethane or silicone rubber. In at least one
embodiment, the
second catheter 150 is configured to receive a guidewire 510 (see FIGS. 4A and
4B) through the
at least one lumen 156 to facilitate the intravascular delivery of the distal
end 154 of the second
catheter 150 into the desired location of the venous vessel 114. Furthermore,
similar to the
12
catheter 10, the second catheter 150 may be coated with heparin or any other
suitable anti-coagulant
prior to insertion in order to facilitate the extended placement of the second
catheter 150 within the
venous vessel 114. Accordingly, the autoretroperfusion system 100 may be used
to deliver chronic
retroperfusion treatment to an ischemic area of a body.
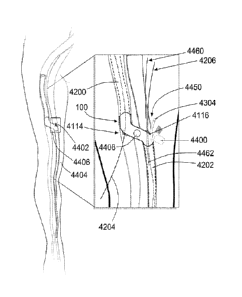
FIGS. 4A and 4B show side views of the distal end 154 of the second catheter
150 positioned
within the venous vessel wall 114. As shown in FIG. 4A, the distal end 154 of
the second catheter
150 may further comprise an expandable balloon 158 coupled with the external
surface of the second
catheter 150. In operation, the expandable balloon 158 can be used to anchor
the distal end 154 of
the second catheter 150 in the desired location within the venous vessel wall
114. The expandable
balloon 158 may be any expandable balloon that is appropriate for insertion
within a vessel and can
be formed of any material suitable for this function, including without
limitation, polyethylene, latex,
polyestherurethane, polyurethane, sylastic, silicone rubber, or combinations
thereof.
The expandable balloon 158 is capable of being controlled by a clinician such
that it can
inflate and/or deflate to the proper size. The sizing of the expandable
balloon 158 will differ between
patients and applications and it is often important to determine the proper
sizing of the expandable
balloon 158 to ensure the distal end 154 of the second catheter 150 is
securely anchored within the
desired location of the vessel wall 114. The accurate size of the expandable
balloon 158 can be
determined through any technique known in the art, including without
limitation, by measuring the
compliance of the expandable balloon 158 ex vivo or in vivo. In addition, the
distal end 154 of the
second catheter 150 may further comprise a plurality of electrodes that are
capable of accurately
measuring the cross-sectional area of the vessel of interest as is known in
the art. For example, the
plurality of electrodes may comprise a combination of excitation and detection
electrodes as
described in detail in the currently pending U.S. Patent Application No.
11/891,981 entitled System
and Method for Measuring Cross-Sectional Areas and Pressure Gradients in
Luminal Organs, and
filed on August 14, 2007. In at least one embodiment, such electrodes may
comprise impedence and
conductance electrodes and may be used in connection with ports for the
suction of fluid from the
vessel and/or the infusion of fluid therein. The expandable balloon 158 may be
in fluid
communication with a secondary lumen 160 disposed within the at least one
lumen 156 of the second
catheter 150. In this example, the secondary lumen 160 is coupled with a
balloon port 162 that
extends from the proximal end 152 of the second catheter 150 (see FIG. 3).
Accordingly, when the
autoretroperfusion system 100 is positioned within a patient, the balloon port
162 can be easily
accessed by a clinician, subcutaneously, percutaneously
13
Date Recue/Date Received 2020-12-23
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
or otherwise, and used to inflate or deflate the expandable balloon 158 with
no or minimal
invasion to the patient.
As shown in FIGS. 4A and 4B, the distal end 154 of the second catheter 150 may
further
comprise at least one sensor 166 coupled therewith. In at least one
embodiment, the at least one
sensor 166 is disposed on the distal end 154 of the second catheter 150
distally of the expandable
balloon 158; however, it will be understood that the at least one sensor 166
may be disposed in
any location on the distal end 154 of the second catheter 150. The at least
one sensor 166 may be
used for monitoring purposes and, for example, may be capable of periodically
or continuously
monitoring the pressure of the blood flow flowing through the at least one
lumen 156 of the first
catheter 150 or the venous vessel 14 in which the second catheter 150 is
inserted. Additionally,
one of the at least one sensors 166 may be used to monitor the pH or the
concentrations of carbon
dioxide, lactate, or cardiac enzymes within the blood. Furthermore, the at
least one sensor 166 is
capable of wirelessly communicating the information it has gathered to a
remote module through
the use of telemetry technology, the internet, or other wireless means, such
that the information
can be easily accessed by a clinician on a real-time basis or otherwise.
Now referring back to FIG. 3, the autoretroperfusion system 100 further
comprises a
connector 170. The connector 170 comprises any connector or quick connector
known in the
medical arts that is capable of forming an anastomosis between an artery and a
vein such that
oxygenated blood from the arterial system can flow into the venous system. For
example, the
connector 170 may comprise an annular connector that is capable of coupling
with the proximal
end 20 of the projection cannula 16 of the catheter 10 and with the proximal
end 152 of the
second catheter 150 such that arterial blood can flow continuously from the at
least one lumen 15
of the catheter 10 to the at least one lumen 156 of the second catheter 150.
The connector 170
may be formed of any suitable material known in the art including, but not
limited to, silicon
rubber, poly(tetrafluoroethene), and/or polyurethane. The connector 170 of the
autoretroperfusion
system 100 may comprise a pressure/flow regulator unit that is capable of
measuring the flow rate
of the blood moving therethrough, the pressure of the blood moving
therethrough, and/or other
data regarding the blood flowing through the anastomosis. The connector 170
may also be
capable of transmitting such gathered data to a remote module 180 through a
lead placed
intravascularly or, in the alternative, through telemetry or another wireless
means. The remote
module 180 may comprise any device capable of receiving the data collected by
the connector
170 and displaying the same. For example, and without limitation, the remote
module 180 may
comprise any display device known in the art or a computer, a microprocessor,
hand-held
computing device or other processing means.
14
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
Additionally, the connector 170 may further comprise a means for regulating
the blood
flow through the anastomosis. One of the main challenges of successfully
delivering
retroperfusion therapies is that the arterial blood pressure must be reduced
prior to being
introduced into a vein due to the thinner and more fragile anatomy of venous
walls. Indeed,
.. subjecting a non-arterialized venous vessel to the high pressures of
arterial blood flow typically
results in rupture of the venous vessel. Accordingly, with retroperfusion
therapies, it is critical to
ensure that the pressure of the arterial blood flow is at least initially
controlled such that the
venous vessel can arterialize prior to being subjected to the unregulated
pressure of the arterial
blood flow. In at least one embodiment the connector 170 may comprise an
external compression
device to facilitate the control of the flow rate of the blood moving through
the anastomosis.
Alternatively, other means that are known in the art may be employed to
regulate the blood flow
and pressure of the blood flowing through the anastomosis formed by the
connector 170. In at
least one embodiment, the means for regulating the blood flow through the
anastomosis formed
by the connector 170 is capable of regulating the pressure and/or flow
velocity of the blood
flowing through the anastomosis. For example, the means for regulating blood
flow can be
adjusted to ensure that about a 50 mg hg pressure drop occurs in the blood
flow between the
arterial vessel and the venous vessel.
The connector 170 is capable of not only transmitting data to the remote
module 180, but
also receiving commands from the remote module 180 and adjusting the means for
regulating
blood flow pursuant to such commands. Accordingly, when the autoretroperfusion
system 100 is
positioned within a patient for retroperfusion therapy, a clinician can use
the remote module 180
to view the blood flow data collected by the connector 170 and non-invasively
adjust the
connector 170 to achieve the desired pressure and/or flow through the
anastomosis. Such remote
control of the connector 170 is particularly useful as a clinician may
incrementally decrease the
.. connector's 170 regulation of the blood flow without surgical intervention
during the venous
arterialization process and/or after the venous vessel arterializes. Further,
where the remote
module 180 comprises a computer or other processing means, the remote module
180 is also
capable of being programmed to automatically analyze the data received from
the connector 170
and, based on the results thereof, suggest how to adjust the means of
regulating the blood flow of
the connector 170 and/or automatically adjust the means of regulating the
blood flow of the
connector 170 to achieve the optimal result. For example, and without
limitation, when the
autoretroperfusion system 100 is implanted into a patient and the anastomosis
is first perfornled,
the remote module 180 can automatically adjust the means for regulating the
blood flow of the
connector 170 based on the initial blood flow data received by the remote
module 180. In this
manner, the desired pressure drop between the arterial system and the venous
system is immediately
achieved and the risk of venous rupture is significantly reduced.
Alternatively, where the connector 170 of the autoretroperfusion system 100
does not comprise
a means for regulating blood flow, the gradual arterialization of the venous
vessel can be achieved
through other techniques known in the art. For example, in at least one
embodiment, the
autoretroperfusion system 100 further comprises a coil designed to at least
partially occlude the vein
of interest. In this manner, the pressure is allowed to build in front of the
portion of the vein at least
partially occluded by the coil and the vein gradually arterializes. In this at
least one embodiment, the
coil may comprise a metallic memory coil (made of nitinol, stainless steel or
other acceptable materials
that are radioopaque) and is covered with polytetrafluorethylene, polyethylene
terephthalate,
polyurethane or any other protective covering available in the medical arts.
Additionally, gradual
arterialization can be performed by the second catheter 150. In this
embodiment of autoretroperfusion
system 100, the at least one lumen 156 of the second catheter 150 is designed
to provide an optimal
stenosis geometry to facilitate the desired pressure drop as the arterial
blood flows therethrough and
into the venous system. For example, and without limitation, the at least one
lumen 156 may further
comprise an internal balloon or resorbable stenosis as disclosed in
International Patent Application No.
PCT/US2006/029223, entitled "Devices and Methods for Controlling Blood
Perfusion Pressure Using
a Retrograde Cannula," filed July 28, 2006.
In at least one embodiment, the stenosis comprises an internal expandable
balloon (not shown)
positioned within the lumen 156 of the second catheter 150. In this at least
one embodiment, the
internal expandable balloon can be used to provide a pressure drop between the
arterial and venous
systems as is required to achieve the gradual arterialization of the target
vein. The internal expandable
balloon and the external expandable balloon 158 of the second catheter 150 may
positioned
concentrically or, alternatively, the internal expandable balloon and the
expandable balloon 158 may
be coupled with distinct portions of the second catheter 150. The internal
expandable balloon may
comprise any material suitable in the medical arts, including, without
limitation, polyethylene, latex,
polyestherurethane, polyurethane, sylastic, silicone rubber, or combinations
thereof. Further, the
internal expandable balloon may be in fluid communication with a tertiary
lumen (not shown) disposed
within the at least one lumen 156 of the second catheter 150. In this
embodiment, the tertiary lumen is also
in fluid communication with an internal balloon port that extends from the
proximal end 152 of the second
catheter 150. Accordingly, the internal balloon port can be easily accessed by
a clinician, subcutaneously,
percutaneously or otherwise, and the internal balloon port can be used to
inflate or deflate the internal
expandable balloon with minimal or no discomfort to the patient when the
system 100 is
16
Date Recue/Date Received 2020-12-23
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
in operation. Alternatively, the internal expandable balloon may be in fluid
communication with
the at least one lumen 156 of the second catheter 150. In this example, the
arterial blood flow
through the at least one lumen 156 functions to inflate and deflate the
internal expandable balloon
in conjunction with the systolic and diastolic components of a heartbeat.
The internal expandable balloon may be sized to a specific configuration in
order to
achieve the desired stenosis. In one embodiment, the size of the desired
stenosis may be obtained
by measuring the pressure at the tip of the distal end 156 of the second
catheter 150 with the at
least one sensor 166 while the internal expandable balloon is being inflated.
Once the desired
intermediate pressure is obtained, the internal expandable balloon volume may
then be finalized
and the vein is thereafter allowed to arterialize at the modified pressure for
a defined period of
time. At the end of the defined period (typically about 2-3 weeks), the
internal expandable
balloon may be removed from the at least one lumen 156 of the second catheter
150. Insertion
and/or removal of the internal expandable balloon from the system 100 may be
achieved through
the internal balloon port and the related tertiary lumen of the second
catheter 150. For example, if
the internal expandable balloon is no longer necessary to control the pressure
on the venous
system because the arterialization of the vein is substantially complete, the
internal expandable
balloon can be deflated through use of internal balloon port and withdrawn
from the system 100
through the tertiary lumen and the internal balloon port.
Other embodiments of the system 100 may comprise other suitable means for
providing a
stenosis within the at least one lumen 156 of the second catheter 150 such
that a pressure drop is
achieved in blood flowing therethrough. For example, while a stenosis can be
imposed by
inflation of the internal expandable balloon, it may also be imposed through
positioning a
resorbable material within the at least one lumen 156 of the second catheter
150. The resorbable
stenosis may be comprised of a variety of materials including, for example and
without limitation,
magnesium alloy and polyols such as mannitol, sorbitol and maltitol. The
degradation rate of the
resulting resorbable stenosis will be dependent, at least in part, upon on
what type of material(s) is
selected to make-up the resorbable stenosis and the same may be manipulated to
achieve the
desired effect.
In addition to the aforementioned components of the autoretroperfusion system
100, the
autoretroperfusion system 100 may further include a first graft 185 and a
second graft 190 as
shown in FIG. 3. In this embodiment, the first graft 185 is coupled with the
proximal end 20 of
the projection cannula 16 (that extends through the exterior arterial wall
116) and the connector
170. Further, the second graft 190 is coupled with the proximal end 152 of the
second catheter
150 (positioned within the venous vessel wall 114) and the connector 170.
Accordingly, in this at
least one embodiment, the second graft 190 is capable of traversing the venous
vessel wall 114 in
17
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
such a manner that the anastomosis is sealed and no blood flow is allowed to
leak from the
anastomosed vein 114. In this manner, the first and second grafts 185, 190
facilitate the formation
of an elongated anastomosis between the venous and arterial vessels 114, 116
and thereby relieve
any pressure that may be applied to the two vessels 114, 116 due to the
anastomosis formed
therebetween. For example and without limitation, in at least one embodiment
the combined
length of the grafts 185, 190 and the connector 170 is about 6 centimeters.
However, it will be
understood that the grafts 185, 190 may comprise any length(s) so long as the
dimensions allow
for an anastomosis to form between the applicable vessels and a fully
developed blood flow is
achieved from the artery to the venous vessel of interest.
Alternatively, the autoretroperfusion system 100 may only comprise the second
graft 190
in addition to the catheter 10, the second catheter 150 and the connector 170.
In this embodiment,
the connector 170 is coupled with the proximal end 20 of the projection
cannula 16 and the
second graft 190. Furthermore, the second graft 190 is further coupled with
the proximal end 152
of the second catheter 150 such that the second graft 190 traverses an opening
within the venous
vessel wall 114 (see FIG. 5). The grafts 185, 190 may comprise any
biocompatible, non-
resorbable material having the necessary strength to support the surrounding
tissue and withstand
the pressure asserted by the blood flow therethrough. Furthemiore, the grafts
185, 190 must
exhibit the necessary flexibility to form an anastomosis between the vein and
the artery within
which the catheter 10 and the second catheter 150 are respectively housed. For
example, and
without limitation, the grafts 185, 190 may comprise any conventional implant
including synthetic
and natural prosthesis, grafts, and the like. The grafts 185, 190 may also
comprise a variety of
suitable materials, including those conventionally used in anastomosis
procedures, including,
without limitation, natural and synthetic materials such as heterologous
tissue, homologous tissue,
polymeric materials, Dacron, fluoropolymers, and polyurethanes. For example,
and without
limitation, the first and second grafts 185, 190 may comprise a material such
as GORE-TEX
(polytetraflouroethylene). The grafts 185, 190 may be coated with heparin or
any other suitable
anti-coagulant. Accordingly, the first graft 185 and the second graft 190 may
he placed within a
vessel or have blood flow therethrough for an extended period of time without
inhibiting blood
flow due to coagulation.
In at least one embodiment of the autoretroperfusion system 100, the
components of the
system 100 are available in a package. here, the package may also contain at
least one sterile
syringe containing the fluid to be injected into the balloon port 62 to
inflate the expandable
balloon 58 of the catheter 10 and/or the balloon port 162 to inflate the
expandable balloon 158 of
the second catheter 150. Furthermore, the package may also contain devices to
facilitate delivery
of the autoretroperfusion system 100 such as venous and arterial access
devices, a delivery
18
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
catheter, a guidewire and/or mandrel, an introducer to maintain the catheter
10 in the collapsed
position during delivery and, in those embodiments where a coil is used to
arterialize the vein of
interest, a pusher bar as is known in the art. The guidewire used to
facilitate the delivery of the
autoretroperfusion system 100 into a vessel by providing support to the
components thereof. The
guidewire may comprise any guidewire known in the art. Furthermore, the distal
end of the
guidewire may comprise a plurality of impedance electrodes that are capable of
taking
measurements of the size the vessel in which the guidewire is inserted through
the use of
impedance technology. Additionally, in at least one embodiment, the impedance
electrodes may
be further capable of communicating such measurements to the remote module 180
through
telemetry or other wireless means in a manner similar to the at least one
sensor 166 of the distal
end 154 of the second catheter 150. In at least one embodiment, the distal end
of the guidewire
may comprise two tetrapolar sets of impedance electrodes disposed on its
distal-most tip.
Based on the information gathered by the impedance electrodes, a clinician can
obtain
accurate measurements of a selective region of a vessel. In this manner, the
expandable balloon
158 coupled with the distal end 154 of the second catheter 150 may be properly
sized and the
amount of fluid or gas needed to inflate the expandable balloon 158 can be
determined prior to
introducing the second catheter 150 into the vein of interest. For example, a
clinician can use the
plurality of impedance electrodes on the guidewire to obtain measurements of
the size and shape
of the sub-branches of the coronary sinus. Now referring to FIG. 5, components
of a simultaneous
selective autoretroperfusion system 300 are shown. The
simultaneous selective
autoretroperfusion system 300 (the "SSA system 300) are configured identically
to the
autoretroperfusion system 100 except that the SSA system 300 further comprises
a third catheter
350 and a Y connector 320, both configured for placement within the venous
vessel wall 114.
Specifically, the SSA system 300 comprises the catheter 10, the second
catheter 150, the third
catheter 350, the connector 170, and the Y connector 320. It will be
understood that the SSA
system 300 can also further comprise the first graft 185 and/or the second
graft 190, and the
remote module 180 as described in connection with autoretroperfusion system
100. The third
catheter 350 is configured for placement within the venous vessel wall 114
adjacent to the second
catheter 150. The third catheter 350 is configured identically to the second
catheter 150 and
comprises a flexible tube having a proximal end 352, a distal end 354 and at
least one lumen 356
extending between the proximal end 352 and the distal end 354. Both the
proximal end 352 and
the distal end 354 of the third catheter 350 are open and in communication
with the at least one
lumen 356 of the third catheter 350, thereby allowing blood to flow into the
at least one lumen
356 through the proximal end 352 and out of the distal end 354 back into the
venous vessel 114.
The third catheter 350 may be any catheter known in the art that is capable of
intravascular
19
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
insertion and advancement through the venous system. The third catheter 350
may comprise any
appropriate material, including without limitation, polyurethane or silicone
rubber. In at least one
embodiment, the third catheter 350 is configured to receive a guidewire 310
(see FIGS. 5 and 6)
through the at least one lumen 356 in order to facilitate the intravascular
delivery of the distal end
354 of the third catheter 350 into the desired location of the venous vessel
114. Furthermore, the
third catheter 350 is coated with heparin or any other suitable anti-coagulant
prior to insertion in
order to facilitate the extended placement of the third catheter 350 within
the venous vessel 114.
As shown in FIG. 5, the distal end 354 of the third catheter 350 further
comprises an
expandable balloon 358 coupled with the external surface of the third catheter
350. In operation,
the expandable balloon 358 can be used to anchor the distal end 354 of the
third catheter 350 in
the desired location within the venous vessel wall 114. The expandable balloon
358 may be any
expandable balloon that is appropriate for insertion within a vessel and can
be foitned of any
material suitable for this function, including without limitation,
polyethylene, latex,
polyestherurethane, polyurethane, sylastic, silicone rubber, or combinations
thereof. Similar to the
expandable balloon 158 of the second catheter 150, the expandable balloon 358
is capable of
being controlled by a clinician such that it can inflate and/or deflate to the
proper size. The
appropriate size of the expandable balloon 358 can be deteimined through any
technique known
in the art, including without limitation, by measuring the compliance of the
expandable balloon
358 ex vivo or in vivo. Furthermore, when the guidewire 310 is used to
facilitate the delivery of
the distal end 354 of the third catheter 350 into the desired location within
the venous vessel wall
114, the electrodes on the distal end of the guidewire 310 may be used to
accurately measure the
cross-sectional area of the venous vessel 114 such that the expandable balloon
358 can be
precisely sized prior to insertion into the vein 114. In this at least one
embodiment, the
expandable balloon 358 is in fluid communication with a secondary lumen 360
disposed within
the at least one lumen 356 of the third catheter 350. In this example, the
secondary lumen 360 is
coupled with a balloon port 362 that extends from the proximal end 352 of the
third catheter 350.
Accordingly, when the SSA system 300 is positioned within a patient, the
balloon port 362 can be
easily accessed by a clinician, subcutaneously, percutaneously or otherwise,
and used to inflate or
deflate the expandable balloon 358 with no or minimal invasion to the patient.
Similar to the second catheter 150, the distal end 354 of the third catheter
350 may further
comprise at least one sensor 366 coupled therewith. The at least one sensor
366 may be
configured identically to the at least one sensor 166 of the second catheter
150 and, accordingly,
the at least one sensor 366 may be used to monitor the pressure of blood flow
through the at least
one lumen 356 of the third catheter 350 or the venous vessel 114 or to monitor
the pH or the
concentrations of carbon dioxide, lactate, or cardiac enzymes within the
blood. Furthennore, the
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
at least one sensor 366 is capable of communicating the data it gathers to the
remote module 180
through the use of a wireless technology such that a clinician can easily
access the gathered
information on a real-time basis or otherwise. In at least one embodiment, the
at least one sensor
366 is disposed on the distal end 354 of the third catheter 350 distally of
the expandable balloon
358; however, it will be understood that the at least one sensor 366 may be
disposed in any
location on the distal end 354 of the third catheter 350. The Y connector 320
of the SSA system
300 comprises flexible material and has a proximal end 322, a distal end 324
and at least one
lumen 326 extending between the proximal and distal ends 322, 324. The
proximal end 322 of
the Y connector 322 is open and configured to be securely coupled with the
graft 190. The distal
end 324 of the Y connector 322 comprises two open ends which extend from the
body of the Y
connector 322 in a substantially Y-shaped configuration. The two open ends of
the distal end 324
of the Y connector 322 thereby divide the at least one lumen 326 into two
separate channels and
thus the blood flowing through the at least one lumen 326 is yet again
bifurcated. The proximal
end 152 of the second catheter 150 is coupled with one of the two open ends of
the distal end 324
of the Y connector 322, thereby receiving a portion of the blood flow that
flows through the at
least one lumen 326 of the Y-connector. Similarly, the proximal end 352 of the
third catheter 350
is coupled with the other open end of the distal end 324 of the Y connector
322 and, thus, the third
catheter receives a portion of the blood flow that flows through the at least
one lumen 326 of the
Y-connector. In this manner, the SSA system 300 can be used to simultaneously
retroperfuse
more than one ischemic area of the body.
In application, the second catheter 150 and the third catheter 350 are
positioned adjacent to
each other within the venous vessel wall 114 as shown in FIG. 5. Furthermore,
the distal ends
154, 354 of the second and third catheters 150, 350, respectively, may be
placed within different
veins such that the arterial blood is delivered to selective portions of
ischemic tissue. For
example, as shown in FIG. 6, in at least one embodiment the SSA system 300 can
be applied to a
heart 314 to provide an arterial blood supply to two separate coronary veins,
or sub-branches,
simultaneously. In this at least one embodiment, the distal ends 154, 354 of
the second and third
catheters 150, 350 are both advanced through the coronary sinus 370. As the
diameter of the
coronary sinus 370 ranges from about 10 to about 20 millimeters, cannulating
the coronary sinus
370 with both the second and third catheters 150, 350 does not occlude the
normal antegrade flow
of the blood therethrough. Upon reaching the veins or sub-branches of
interest, the distal ends
154, 354 of the second and third catheters 150, 350 are each independently
positioned within the
veins of interest. In the example shown in FIG. 6, the second catheter 150 is
positioned within the
interventricular vein 374 and the distal end 354 of the third catheter 350 is
positioned within the
middle cardiac vein 376. As with autoretroperfusion system 100, the expandable
balloons 158,
21
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
358 are inflated through balloon ports 162, 362, respectively (shown in FIG.
5), such that the
distal ends 154, 354 of the second and third catheters 150, 350 are securely
anchored in the
desired location within the veins of interest. In this manner, the SSA system
300 can deliver
controlled arterial blood flow to, and thus arterialize, two areas of the
heart 314 simultaneously.
In at least one embodiment of the SSA system 300, the components of the system
300 are
available in a package. Here, the package may also contain sterile syringes
with the fluids to be
injected into the balloon ports 162, 362 to inflate the expandable balloons
158, 358, respectively.
Furtheimore, the package may also contain devices to facilitate delivery of
the SSA system 300
such as arterial and venous access devices, a delivery catheter, at least two
guidewires (configured
as described in connection with the delivery of autoretroperfusion system
100), an introducer to
maintain the catheter 10 in the collapsed position during delivery and, in
those embodiments
where a coil is used to arterialize the vein of interest, a pusher bar as is
known in the art. Now
referring to FIG. 7, a flow chart of a method 400 for performing automatic
retroperfusion using
the system 100 is shown. While the method 400 is described herein in
connection with treating a
heart through catheterization of the coronary sinus, it will be understood
that the method 400 may
be used to perfohn autoretroperfusion on any organ or tissue in need of
retroperfusion treatment
and/or other areas near the coronary sinus, such as the great cardiac vein,
for example. Method
400, and the embodiments thereof, can be performed under local anesthesia and
do not require
any arterial sutures. Further, once implanted, the system 100 can deliver
chronic treatment to the
patient as the system 100 is capable of remaining within a patient's vascular
system for an
extended period of time. In this manner, the system 100 and method 400 can be
used to treat no-
option patients and greatly enhance their quality of life. As shown in FIG. 7,
in one approach to
the method 400, at step 402 an artery 502 of interest is percutaneously
punctured under local
anesthesia with a conventional artery access device or as otherwise known in
the art. For example
and without limitation, in at least one embodiment, an 18 gauge needle is
inserted into the femoral
or subclavian artery. At step 404, the catheter 10 housed in a collapsed
position within an
introducer 504 (see FIG. 8A) is inserted into the artery 502 of interest.
After the distal end 14 of
the catheter 10 is positioned in the desired location within the artery 502,
the introducer 504 is
proximally withdrawn from the artery 502 as shown in FIG. 8B, leaving the
catheter 10 positioned
therein.
In at least one embodiment, the projection cannula 16 is configured such that
when the
introducer 504 is withdrawn in a proximal direction, the proximal end 12 of
the catheter 10 is
released from the introducer 504 before the proximal end 20 of the projection
cannula 16 is
released from the introducer 504. In this manner, the proximal end 12 of the
catheter 10 is
delivered within the interior of the arterial wall 502, while the projection
cannula 16 remains
22
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
housed within the interior of the introducer 504 as shown in FIG. 8C.
Furthermore, because the
introducer 504 no longer applies downward pressure to the projection cannula
16 relative to the
proximal end 12 of the catheter 10, the projection cannula 16 is allowed to
shift from the
collapsed position to the expanded position and therefore extends in a
direction that is not parallel
with the artery 502 or the body of the catheter 10. In this manner, as shown
in FIGS. 8C and 8D,
the proximal end 20 of the projection cannula 16 is directed through the
opening formed in the
arterial wall 502 by the introducer 504. Accordingly, when the catheter 10 is
positioned within
the artery 502, the antegrade blood arterial blood flow is allowed to continue
through the artery
502 through the proximal end 12 of the catheter 10, while only a portion of
the arterial blood is
rerouted through the projection cannula 16 and into the veins 506 of interest.
In this manner, the
normal blood flow through the artery 502 is not inhibited by operation of the
autoretroperfusion
system 100. Furthermore, in addition to bifurcating the blood flowing through
the artery 502, the
projection cannula 16 traversing the arterial wall 502 further functions to
anchor the catheter 10 in
the desired position within the artery 502.
In the embodiment where the catheter 10 further comprises the expandable
balloon 58 (see
FIG. 1), step 404 may further comprise inflating the expandable balloon 58 to
the desired size by
injecting fluid into the balloon port 62. In this manner, the expandable
balloon 58 functions to
further anchor the catheter 10 in the desired location within the artery 502
and seal the opening in
the artery 502 through which the projection cannula 16 projects (see FIG. 8E).
At step 406, a vein
506 of interest is percutaneously punctured under local anesthesia with a
conventional venous
access device or as otherwise known in the art. For example and without
limitation, in at least
one embodiment, an 18 gauge needle is inserted into the femoral or subclavian
vein. At step 408,
a delivery catheter 508 is inserted into and advanced through the vein 506 to
catheterize the
coronary sinus ostium. A guidewire 510 is then inserted at step 410 into the
delivery catheter 510
and advanced into the lumen of the vein 506 through the distal end of the
delivery catheter 510.
Furthermore, the guidewire 510 is advanced into the region of interest by use
of x-ray (i.e.
fluoroscopy), direct vision, transesophageal echocardiogram, or other suitable
means or
visualization techniques.
FIGS. 9 and 10 show schematic views of the method 400 as applied to a heart
500.
Specifically, in this at least one embodiment, at steps 402 and 404 the artery
502, which in FIG. 9
comprises the subclavian artery, is punctured and the catheter 10 is inserted
and positioned
therein. Further, at step 406 the vein 506, which in FIG. 9 comprises the
subclavian vein, is
punctured and at step 408 the delivery catheter 508 is advanced through the
superior vena cava
518 and into the coronary ostium of the coronary sinus 520. As shown in FIG.
10, at step 410, the
23
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
guidewire 510 is advanced through the coronary sinus 520 and into the vein of
interest, which, in
this at least one embodiment, comprises the posterior vein 522 of the heart
500.
Now referring back to FIG. 7, the guidewire 510 inserted into the vein 506 at
step 410 may
further comprise a plurality of impedance electrodes as previously described
herein. In this
approach, the guidewire 510 may be used at optional step 411 to determine the
size of the vessel
of interest through use of the plurality of impedance electrodes disposed
thereon. In this manner,
a clinician can use the measurements generated by the impedance electrodes to
select a properly
sized expandable balloon 158 for use in connection with the second catheter
150. By using a
precisely sized expandable balloon 158 and inflation volume, the clinician can
ensure that the
distal end 154 of the second catheter 150 is securely anchored within the
vessel of interest without
imposing an undue force on the venous vessel walls. After the guidewire 510
has been advanced
into the vessel of interest at step 410 and, optionally, the dimensions of the
vessel of interest have
been measured at step 411, the method 400 advances to step 412. At step 412,
the distal end 154
of the second catheter 150 is inserted into the delivery catheter 508 over the
guidewire 510.
Accordingly, the guidewire 510 is slidably received by the at least one lumen
156 of the second
catheter 150. The distal end 154 of the second catheter 150 is then advanced
over the guidewire
510 to the region of interest and the expandable balloon 158 of the second
catheter 150 is inflated
to anchor the distal end 154 within the targeted vessel. FIG. 11 shows a
schematic view of the
method 400, as applied to the heart 500, after step 412 has been completed. It
will be understood
.. that at any point after the distal end 154 of the second catheter 150 is
positioned and anchored
within the desired location in the targeted vessel, the delivery catheter 508
and the guidewire 510
may be withdrawn from the vein of interest. After the distal end 154 of the
second catheter 150 is
secured within the targeted vessel, at step 414 the anastomosis between the
vein 506 and the
artery 502 is formed. Specifically, in at least one approach, the proximal end
20 of the projection
cannula 16 of the catheter 10 is coupled with the proximal end 152 of the
second catheter 150 by
way of the connector 170. In the at least one embodiment of the system 100
comprising the first
graft 185 and the second graft 190, the connector 170 may be coupled with the
catheter 10 and the
second catheter 150 via the first graft 185 and the second graft 190 to foim
an elongated
anastomosis. Alternatively, in yet another approach, the connector 185 may be
coupled with the
catheter 10 via the proximal end 20 of the projection cannula 16 and the
second catheter 150 via
only the second graft 190. It will be understood that any combination of the
catheter 10, the
second catheter 150 and the first and second grafts 185, 190 may be used in
connection with the
connector 170 to form the desired anastomosis between the vein 506 and the
artery 502.
After the anastomosis is formed and the arterial blood is allowed to flow
through the
anastomosis and thereby through the connector 170, at step 416 the connector
170 measures the
24
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
flow rate, pressure and any other desired data of the arterial blood flow. The
connector 170
transmits the collected data to the remote module 180 either through
intravascularly placed leads
or wirelessly, through telemetry or other means. In this manner, a clinician
may easily view the
blood flow data on the remote module 180 and assess the degree of pressure
drop that will be
required to preserve and gradually arterialize the vein 506. At step 418, the
pressure of the
arterial blood flow through the system 100 is modified to transmit the desired
pressure to the
venous system. In this step 418 the pressure modification can be achieved
through a clinician
modifying the means of regulating the blood flow of the connector 170 through
remote means or,
in at least one embodiment of the system 100, inflating the internal
expandable balloon of the
second catheter 150 using the internal balloon port in order to partially
occlude the flow of arterial
blood through the at least one lumen 156 of the second catheter 150.
Furthermore, in at least one
alternative embodiment of the system 100, a clinician may deliver a resorbable
stenosis
configured to achieve the necessary pressure drop into the at least one lumen
156 of the second
catheter 150 through means known in the art. Alternatively, as previously
described in
connection with autoretroperfusion system 100, the remote module 180 may
further comprise a
computer or other processing means capable of being programmed to
automatically analyze the
data received from the connector 170 and, based on such data, determine the
proper degree of
adjustment required in the blood pressure flowing through the anastomosis. In
this embodiment,
at step 418, the remote module 180 automatically adjusts the means of
regulating the blood flow
of the connector 170 to achieve the optimal pressure drop. In this manner, the
desired pressure
drop between the arterial system and the venous system is immediately achieved
and the risk of
venous rupture is significantly reduced. In step 420 the method 400 allows the
arterial blood
having a modified pressure to irrigate the vein 506 for a period of time such
that the vein 506
properly arterializes. For example, and without limitation, the patient's
venous system may be
subjected to the reduced arterial pressure for about fourteen days to allow
the vein 506 to adapt to
the elevated blood pressure flowing therethrough.
After arterialization of the vein 506 is achieved, at step 422 the patient may
optionally
undergo a coronary venous bypass graft surgery and the components of the
autoretroperfusion
system 100 may be removed. However, as previously discussed, even with a
properly arterialized
vein 506, many patients that require retroperfusion therapy may still not be
candidates for a
coronary vein bypass graft surgery. In the event that the patient is unable to
tolerate such a
procedure, after the vein 506 has arterialized at step 420, the method 400 can
progress directly to
step 424. At step 424, the pressure modification of the arterial blood flowing
through the second
catheter 150 is ceased. Accordingly, pre-arterialized veins 506 are subjected
to the full arterial
pressure of the blood flowing through the anastomosis and second catheter 150.
In at least one
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
embodiment, a clinician can cease the pressure modification by adjusting the
controller 170.
Alternatively, in the at least one embodiment where the controller 170 can be
automatically
adjusted by the remote module 180, the remote module 180 can automatically
adjust the controller
170 after the veins 506 have pre-arterialized. Further, where the pressure
drop is achieved
.. through the use of an internal expandable balloon positioned within the at
least one lumen 156 of
the second catheter, the clinician may deflate the internal expandable balloon
through the internal
balloon port and thereafter withdraw the deflated internal expandable balloon
through the tertiary
lumen of the second catheter and the internal balloon port. In yet another
embodiment where a
resorbable stenosis is used to achieve the pressure drop in the arterial blood
as it flows through the
second catheter 150, the resorbable stenosis can be configured to dissolve
after the desired period
of time, thereby gradually decreasing the influence the resorbable stenosis
has on the pressure of
the blood flowing through the at least one lumen 156 of the second catheter
over a period of time.
Accordingly, the autoretroperfusion system 100 can remain chronically
implanted within the
patient to deliver oxygen-rich blood to a targeted area of tissue over an
extended period of time.
Now referring to FIG. 12, a flow chart of a method 600 for performing
simultaneous
selective retroperfusion using the SSA system 300 is shown. While the method
600 is described
herein in connection with treating a heart 500 through catheterization of the
coronary sinus 520, it
will be understood that the method 600 may be used to perform
autoretroperfusion on any organ
or tissue in need of retroperfusion treatment. The reference numerals used to
identify the steps of
method 600 that are included in the description of method 400 designate like
steps between the
two methods 400, 600. As such, like steps between the two methods 400, 600
will not be
discussed in detail with respect to the method 600 and it will be understood
that such description
can be obtained through the description of the method 400. Method 600, and the
embodiments
thereof, can be performed under local anesthesia and does not require arterial
sutures. Further,
once implanted, the SSA system 300 can deliver simultaneous chronic treatment
to multiple
ischemic locations as the system 300 is capable of remaining within a
patient's vascular system
for an extended period of time and selectively retroperfusion more than one
sub-branch of a vein
506. The method 600 progresses through steps 402 through 410 as previously
described in
connection with the method 400. After the guidewire 510 is advanced through
the coronary sinus
520 and into the first vein of interest, a second guidewire 610 is inserted at
step 602 into the
delivery catheter 508 adjacent to the guidewire 510, and advanced into the
lumen of the vein 506
through the distal end of the delivery catheter 510. The second guidewire 610
is then advanced
into a second region of interest by use of x-ray (i.e. fluoroscopy), direct
vision, transesophageal
echocardiogram, or other suitable means or visualization techniques. The
second guidewire 610
is configured similar to the guidewire 510 and is capable of functioning the
in the same manner.
26
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
FIG. 13 shows a schematic view of the method 600 as applied to a heart 500.
Specifically,
in this at least one embodiment, FIG. 13 shows the method 600 at step 602
wherein the guidewire
510 is inserted a first vein of interest, which comprises the posterior vein
522 of the heart 500, and
the second guidewire 610 is inserted into a second vein of interest, which
comprises the
interventricular vein 622 of the heart 500. Now referring back to FIG. 12, the
guidewire 610
inserted into the second vein of interest in step 602 may further comprise a
plurality of impedance
electrodes as previously described with respect to the guidewire 510. In this
embodiment, the
guidewire 610 may be used at optional step 603 to determine the size of the
second vessel of
interest through use of the plurality of impedance electrodes disposed
thereon. In this manner, a
clinician can use the measurements generated by the impedance electrodes to
select a properly
sized expandable balloon 358 for use in connection with the third catheter
350. By using a
precisely sized expandable balloon 358 and inflation volume, a clinician can
ensure that the distal
end 354 of the third catheter 350 is securely anchored within the second
vessel of interest without
imposing an undue force on the venous vessel walls. After the guidewire 610
has been advanced
into the second vessel of interest at step 602 and, optionally, the dimensions
of the second vessel
of interest have been measured at step 603, the method 600 advances to step
412 wherein the
second catheter 150 is inserted over the guidewire 510 as described in
connection with method
400. At step 604, the distal end 354 of the third catheter 350 is inserted
into the delivery catheter
508 over the second guidewire 610. Accordingly, the second guidewire 610 is
slidably received
by the at least one lumen 356 of the third catheter 350. The distal end 354 of
the third catheter
350 is then advanced over the second guidewire 610 to the second region of
interest and the
expandable balloon 358 of the third catheter 350 is inflated to anchor the
distal end 354 within the
targeted vessel. FIG. 14 shows a schematic view of the method 600 at step 604
as applied to the
heart 500. It will be understood that at any point after the distal ends 154,
354 of the second and
third catheters 150, 350 are positioned and anchored in the desired locations
within the targeted
vessels, the delivery catheter 508 and the guidewires 510, 610 may be
withdrawn from the vein
506.
After both the distal end 154 of the second catheter 150 and the distal end
354 of the third
catheter 350 are secured within the targeted vessels, the method 600 proceeds
to step 414 where
the anastomosis is formed between the vein 506 and the artery 502 as described
in connection
with method 400. Thereafter, the method 600 advances through steps 416 through
424 as
described in connection with the method 400. Furthermore, at step 418, it will
be recognized that
a clinician can independently adjust the pressure drop through the second and
third catheters 150,
350 in the event that an internal expandable balloon is used in either or both
catheters 150, 350 or
resorbable stenosis are employed within the at least one lumens 156, 356 of
the second and third
27
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
catheters 150, 350. Alternatively, in the at least one embodiment where the
controller 170
comprises a means for regulating the blood flow through the anastomosis, the
pressure of the
arterial blood flowing through both the second and third catheters 150, 350
may be substantially
the same. As described herein, the method 600 may be used to simultaneously
and immediately
treat two different ischemic areas of a tissue through the use of one
minimally to non-invasive
procedure. Furthermore, the method 600 can provide no-option patients with a
viable treatment
option that is not associated with contraindications for congestive heart
failure, diabetes, or drug
treatment.
An additional embodiment of a perfusion system 100 of the present disclosure
is shown in
.. FIG. 15. As shown in FIG. 15, system 100 comprises a first catheter 1000
having a distal end
1002, a proximal end 1004, and defining a lumen 1006 therethrough, wherein at
least a portion of
first catheter 1000 is configured for insertion into a body of a patient, such
as into a patient's heart
or a patient's vein, for example. First catheter 1000, after insertion into a
patient's vein or heart,
for example, is capable of providing arterial blood (which is relatively rich
in oxygen and other
.. nutrients) thereto by way of transfer of arterial blood from, for example,
a patient's artery, as
described below, into a proximal catheter opening 1008, through lumen 1006,
and out of distal
catheter opening 1010. In such a fashion, for example, a system 100 can be
referred to as an
autoretroperfusion system 100, noting that no outside pumps are necessary (as
the patient's own
heart serves as the pump), and due to the retrograde nature of the perfusion
with respect to such a
.. use. Exemplary uses, as provided in detail herein, are to provide arterial
blood, using system 100,
to a patient's femoral vein, internal jugular vein, subclavian vein, and/or
brachial cephalic vein. In
an exemplary embodiment, first catheter 1000 may be tapered toward distal end
1002 to facilitate
insertion into a patient. In at least one embodiment of system 100, and as
shown in FIGS. 15 and
16, system 100 comprises a coupler 1012 having an outlet port 1013 and one or
more additional
.. ports to facilitate connection outside of the patient's body. For example,
and as shown in FIGS.
15 and 16, coupler 1012 comprises an inflation port 1014, whereby fluid and/or
gas introduced
into inflation port 1014 can he used to inflate an expandable balloon 1016
positioned along first
catheter 1000 at or near the distal end 1004 of first catheter 1000. As shown
in the figures, and in
at least one embodiment, an inflation tube 1018 may be coupled to inflation
port 1014 at a distal
.. end 1020 of inflation tube 1018, whereby inflation tube 1018 may also have
an optional flow
regulator 1022 positioned relative thereto to regulate the flow and/or
pressure of fluid and/or gas
in and out of a lumen 1024 of inflation tube 1018 to inflate and deflate
expandable balloon 1016.
Inflation tube 1018 may further comprise a proximal connector 1026 configured
to receive fluid
and/or gas from a fluid/gas source (not shown), whereby proximal connector
1026 can be
positioned at or near a proximal end 1028 of inflation tube 1018, for example.
Inflation of
28
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
expandable balloon 1016, for example, can be used to anchor first catheter
1000 to a desired
position within a luminal organ of a patient. An exemplary coupler 1012 of the
present disclosure
further comprises an arterial blood port 1030 configured to receive
arterial/oxygenated blood
from, for example, an arterial blood tube 1032 coupled thereto at or near a
distal end 1034 of
arterial blood tube 1032. As shown in FIGS. 15 and 16, a blood flow regulator
1036 may be
positioned relative to arterial blood tube 1032 and operate to regulate the
flow and/or pressure of
arterial/oxygenated blood flow therethrough. In at least one embodiment, blood
flow regulator
1036 comprises a rotatable dial capable of rotation to apply and/or remove
pressure to/from
arterial blood tube 1032 to regulate the flow and/or pressure of blood through
a lumen 1038 of
arterial blood tube 1032 and/or to adjust pressure therein based upon
identified blood pressure
measurements. Such a blood flow regulator 1036, for example, can be used to
control blood
pressure to limit injury to the patient's luminal organs (such as the
patient's venous system and/or
myocardium) and/or to minimize potential edema with respect to the same
luminal organs.
Arterial blood tube 1032 may further comprise a proximal connector 1040
configured to receive
arterial/oxygenated blood from a blood supply, whereby proximal connector can
be positioned at
or near a proximal end 1040 of arterial blood tube 1032, for example. A
coupler catheter 1042, as
shown in the component block diagram of system 100 shown in FIG. 17, may be
used to couple
arterial blood tube 1032 to a blood supply 1044, which, as described herein,
could be a patient's
own artery using the patient's heart as a pump, or could be an external supply
that provides blood
to arterial blood tube 1032, which may then be used in connection with an
apparatus to remove
blood from the patient as well.
Furthermore, and in at least one embodiment, an exemplary coupler 1012 of the
present
disclosure further comprises a medicament port 1046 configured to receive a
medicament, saline,
and/or the like, so that the same can enter the patient by way of first
catheter 1000. Medicament
port 1046, as shown in FIGS. 15 and 16, may receive a medicament tube 1048
defining a lumen
1050 therethrough, whereby a distal end 1052 of medicament tube 1048 can
couple to
medicament port 1046 so that a medicament, saline, and/or the like can be
introduced from a
medicament source (not shown) coupled to medicament tube 1052 at or near a
proximal end 1054
of medicament tube 1048. Exemplary medicaments may include, but are not
limited to,
fibrinolitic drugs, cardiotonic drugs, antirrhytmic drugs, scavengers, cells
or angiogenic growth
factors, for example, through the coronary vein or another luminal organ. In
at least one
embodiment, and as shown in FIGS. 15 and 16, medicament tube 1048 can be
branched, whereby
a second proximal end 1056 of medicament tube 1048 can receive a medicament
and control the
flow of medicament therethrough, for example, by way of a medicament regulator
1058
positioned relative to medicament tube 1048, for example. Furtheimore, one or
more of proximal
29
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
end 1054 and second proximal end 1056 may be configured to receive a wire
therein, such as, for
example, a .035" guidewire and/or a .014" pressure wire. As generally
referenced herein, any
blood, air, fluid, medicament, wire, etc. that enters coupler 1012 by way of
inflation port 1014,
arterial blood port 1030, and/or medicament port 1046 and eventually enters a
lumen of first
catheter 1000 will enter one or more of said ports of coupler 1012 and exit
outlet port 1013 at the
time of entry into first catheter 1000. FIG. 17, as referenced above, is a
block diagram of various
components of an exemplary system 100 of the present disclosure. As shown
therein, an
exemplary embodiment of a system 100 of the present disclosure comprises a
first catheter 1000,
a coupler 1012, an arterial blood tube 1032 with a blood flow regulator 1036,
and a coupler
catheter 1042 configured to for connection to a blood supply 1044, wherein the
blood supply may
or may not be considered as part of a formal system 100. In addition, an
exemplary system 100
may comprise an inflation tube 1018 with a flow regulator 1022, whereby an end
of inflation tube
1018 is configured for connection to a gas/liquid source 1060. Various
embodiments of systems
100 of the present disclosure may have more or less components than shown in
FIG. 17, and
exemplary embodiments of systems 100 of the present disclosure may be
configured to engage
various embodiments of catheters 10 as referenced herein.
In use, for example, first catheter 1000 of system 100 may be positioned
within a luminal
organ of a patient within the patient's venous system. Inflation of expandable
balloon 1016 to
secure first catheter 1000 can not only provide oxygenated arterial blood to
the patient's venous
system, but can also continue to allow coronary venous return to continue due
to the selective
autoretroperfusion nature of an exemplary embodiment of system 100 and use
thereof and due to
the redundancy of the patient's venous system. In the event that an increased
pressure, edema, or
other undesired condition may occur at or near the site of inflated expandable
balloon 1016, a user
of system 100 could, if desired, temporarily deflate expandable balloon 1016
to allow the
increased pressure and or edema to alleviate itself. For example, system 100
could be used for a
relatively long period of time (an hour, by way of example), and expandable
balloon 1016 could
be deflated for a relatively short period of time (seconds, for example), to
alleviate a high pressure
or edema occurrence, and then expandable balloon 1016 could be re-inflated to
again secure first
catheter 1000 at a desired location within the patient. The type of patients
for whom the device
will be utilized in the acute application may fall into various categories,
including, but not limited
to, S-T segment Elevated Myocardial Infarction (STEMI) patients, cardiogenic
shock patients,
and high risk Percutaneous Coronary Intervention (PCI) patients (such as those
undergoing PCI of
the left main coronary artery). STEMI is the traditional "emergent" patient
who presents with
classic heart attack symptoms, and when diagnosed in a hospital emergency room
for example,
the patient would traditionally be immediately moved to a Cath Lab to receive
PCI to open an
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
occluded coronary artery and restore blood flow to the myocardium. These
patients are
hemodynamically unstable and need support for the left ventricle.
In such a use, for example, an exemplary system 100 of the present disclosure
could be
used to, for example: (i) provide cardiac support to a patient who does not
have immediate access
to the Cath Lab and PCI. These patients may present in rural or community
hospitals that do not
have Cath Labs. They will need some type of temporary support while being
transferred to an
appropriate facility. These patients might also present at a hospital with a
Cath Lab, but the Cath
Lab is either understaffed to treat the patient, or does not have an available
room to treat. In these
cases, the system 100 of the present disclosure operates as a bridge to
provide support until
definitive treatment (primary PCI) is available; and/or (ii) provide cardiac
support before,
during, and after primary PCI. Many patients enter the Cath Lab in an unstable
condition, and the
insertion of balloons and stents adds to hemodynamic instability. An exemplary
system 100 can
provide cardiac support and improve hemodynamics such that the physician can
operate in a more
stable/controlled environment. It is also believed that by reperfusing
ischemic myocardium
before/during/and after primary PCI, one may reduce the amount of myocardium
that is damaged
by the ischemic event. This is clinically referred to as a "reduction in
infarct size." Initial animal
studies (as referenced in further detail herein) have suggested that the use
of SARP in support of
STEMI patients could cause a reduction in infarct size, which would have a
significant impact on
the outcomes for the patient in both the near and long term. Reduction in
infarct size would slow
the progression of any subsequent heart failure and reduce long term
hospitalization and costs for
this group of patients.
Cardiogenic shock is marked by a significant lowering of blood pressure and
cardiac
output that if not reversed, will ultimately lead to multisystem organ failure
and death.
Cardiogenic shock patients have a mortality exceeding 60%. In many cases,
cardiogenic shock
patients are too unstable to undergo surgery or PCI.
Pharmacologics are used to increase
pressure and cardiac output. Intra Aortic Balloon Pumps (IABP) and other LVAD
type products
are also employed to improve hemodynamics in an attempt to reverse the
downward cycle of
cardiogenic shock patients Exemplary embodiments of systems 100 of the present
disclosure
could be used in much the same fashion. High Risk PCI is typically defined as
patients who have
disease of the left main coronary artery, are diabetic, have multivessel
disease, are above 75 years
of age, have a prior history of MI, have renal insufficiency, etc.. These are
very sick patients, who
are considered at high risk of adverse events before, during, and after
undergoing PCI. Mortality
rates and Major Adverse Cardiac Event (MACE) rates are much higher in this
patient population.
IABP's are commonly used in this patient population. In this population,
systems 100 of the
present disclosure may be used to provide cardiac support for a high risk PCI
patient who is, at the
31
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
time of the procedure, found to be hemodynamically unstable. It is evident to
the operator that
cardiac support is and will be needed during the procedure, and an exemplary
system 100 of the
present disclosure would be deployed from the outset. The patient's
hemodynamics improve and
the operator feels more comfortable working in the coronary system. IABP use
is common in
these patients.
Systems 100 of the present disclosure may also be used in this high risk
population when
it is anticipated that cardiac support may be needed during the procedure. In
this case, an
exemplary system 100 is deployed prior to the case, in order to provide
support when and if it is
needed. The patient is hemodynamically stable at the outset, and remains so
throughout. IABP's
are currently used in this fashion. This is commonly referred to as
prophylactic use of cardiac
support.
Acute Applications: In this setting, exemplary systems 100 of the present
disclosure will
be used for cardiac support and to protect myocardium for a period of time
that will generally be
less than 24 hours. The clinical condition that precipitated the need for SARP
will have typically
been resolved in that 24 hour period, and the system 100 would be removed.
However, use of
systems 100 of the present disclosure are not limited to a 24 hour period, as
in some cases, IABPs
and other short term cardiac support devices are left in for periods exceeding
24 hours. Typically,
the longest period of time that a short term device might be left in place is
4-6 days, at which
point the clinician would begin to consider longer term implanted left
Ventricular Assist Devices
(LVADs), which can support a patient for an extended period of time (weeks),
and are often used
as a bridge to heart transplant.
Clinical conditions that would require the acute application of an exemplary
system 100 of
the present disclosure include, but are not limited to: (i) Emergent treatment
of STEMI and/or
other Acute Myocardial Infarction (AM!) patients; (ii) Cardiogenic shock;
(iii) High Risk PCI;
(iv) Failed or aborted PCI where severe hemodynamic instability presents after
initiation of the
procedure. These patients are often transferred to immediate cardiac surgery,
and require cardiac
support while waiting for the surgical intervention; and/or (v) Weaning from a
cardiopulmonary
bypass machine in cardiac surgery. Some cardiac surgery patients have
difficulty returning to
normal cardiac condition when the cardiopulmonary bypass machine is turned off
and the heart is
restarted after successful revascularization in cardiac surgery. Exemplary
systems 100 of the
present disclosure could be used to support the heart until notmal cardiac
parameters return.
Insertion could occur in the surgical suite, and the device would be left in
place while the patient
was transferred to a Cardiac Critical Care Unit (CCU). These exemplary
clinical conditions cover
the majority of potential applications for an acute embodiment of a system 100
of the present
disclosure. Currently, more than 95% of all IABP and other short term support
devices are used
32
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
for these applications. In such applications, the goal of using an exemplary
system 100 of the
present disclosure is to deliver arterial (oxygenated) blood to the
myocardium, in a retrograde
manner using the venous system, in order to create hemodynamic stability for
the patient and to
protect and preserve myocardial tissue until the clinical event resolves or
primary intervention
(PCI or CABG) and revascularization can occur.
Chronic Applications: In this setting it is intended that an exemplary
embodiment of a
system 100 of the present disclosure be implanted for 2 weeks or longer, for
example, noting that
ultimate implantation may be somewhat shorter in duration. Initial animal
studies suggest that
within 2 weeks, arterialization of the venous system is achieved, such that
the venous system can
become the conduit for a constant flow of arterial blood at arterial pressure.
A clinical condition
where the chronic application of a system 100 would be utilized is often
referred to as "no option"
patients, that is, patients for which there are no options available through
which their clinical
condition can be resolved. More specifically, these are patients with diffuse
coronary artery
disease (CAD) or refractory angina, where PCI and/or Coronary Artery Bypass
Graft Surgery
(CABG) is not an option. Patients that are diabetic, or have other co-
morbidities, and are not
candidates for interventions, would be candidates for a chronic application of
a system 100 of the
present disclosure. As previously referenced herein, the chronic application
will generally require
10-14 days of retroperfusion in order to allow arterialization of the venous
system. In certain
instances, retroperfusion could be required for a longer period (such as 2-3
weeks, for example),
or a lesser period, such as less than 10 days, for example. 'These patients,
dependent upon their
complete clinical situation, may be hospitalized for that period, or they may
reside outside of the
hospital. When residing outside of the hospital, the device utilized may be a
catheter 10
embodiment with a branched implantable portion, such as shown in FIG. I, for
example. The
catheter 10, including method of pressure regulation, would be implanted in
the patient.
For those chronic patients, who must remain in the hospital for one of the
aforementioned
time periods, an acute embodiment of a system 100, for example, may be
applicable. In such an
embodiment, for example, system 100 may be percutaneously inserted and
utilized during that
time frame. Once arterialization occurs, a more peimanent conduit may be
constructed
percutaneously or surgically to provide the permanent arterial blood source.
When using an
exemplary system 100 of the present disclosure, standard guide catheters can
be used by the
clinician to locate the coronary sinus and/or the great cardiac vein, for
example.. An .035"
guidewire can be inserted to further establish access to the coronary sinus or
the great cardiac
vein. An exemplary system 100 can then be inserted over the .035" guidewire
and advanced to
the coronary sinus or the great cardiac vein, for example, via one of the
ports as referenced herein.
The distal end 1004 of the first catheter 1000 is intended to be located at
the left main vein. The
33
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
operator may advance the tip (distal end 1014) of first catheter 1000 to other
vein sites dependent
on clinical need. A balloon 1016, which in at least one embodiment may be
located
approximately 2cm back from the distal end 1004, would then be inflated to
secure the position of
first catheter 1000 within the coronary sinus or the great cardiac vein, for
example, allowing for
the distal end 1004 of first catheter 1000 to locate at the left main vein.
The inflated balloon 1016
will also work to ensure that arterial blood will flow in the retrograde
fashion. Once the distal
balloon 1016 is inflated, the .035" guidewire can be exchanged for an .014"
pressure measurement
wire, which will be used to measure the pressure at the distal end 1004 of
first catheter 1000, to
ensure that the portions of system 100 are not over pressurizing the vein, and
to tell the operator
how much pressure change will be required from the external pressure
regulator. The proximal
end of the pressure wire will be connected to its appropriate monitor.
When the catheter is located in the coronary sinus or the great cardiac vein,
for example,
the operator can now make the external (outside the body) connection to the
arterial blood supply
1044. This is typically, but not limited to, the femoral or radial arteries.
The physician will have
previously inserted a standard procedural sheath into the arterial source in
order to gain access to
the source. This arterial sheath can also be used to provide access for
catheters, guidewires,
balloons, stents, or other devices that might be utilized while treating the
patient. That arterial
sheath will have a connector which can connect to the arterial supply cannula
(with regulator) on
the acute device (an embodiment of system 100). Once the connection is
established and flow
commences, the pressure wire will indicate the distal pressure measurement and
the regulator can
be adjusted to the proper setting (not to exceed 60mmhg, for example).
Monitoring of the distal
pressure will be on-going throughout the period of time that the device is in-
vivo. The regulator
allows the operator to provide the correct distal pressures and to adjust
those pressures, dependent
on changes in the patient's pressure. With the pressure set and monitored, the
patient is now
receiving oxygenated blood to the myocardium in a retrograde fashion thru the
coronary venous
system. Such an operation (namely to retrogradly provide oxygenated blood) can
be used to save
a significant amount of ischemic tissue at the level of the border zone. In at
least one
embodiment, such a system 100 is used to perfuse the left anterior descending
vein to supply
oxygenated blood to the LAD artery occluded territory. Depending upon patient
need and
circumstance, the acute device (an embodiment of system 100) will be removed
typically within
the first 24 hours of insertion. The physician will make that determination.
The insertion site will
be closed per hospital protocol.
Validation of Methodology
As referenced in detail herein, coronary artery disease (CAD) is the number
one cause of
morbidity and mortality in the U.S. and worldwide. Even today, with
percutaneous transluminal
34
CA 02919981 2016-01-29
WO 2015/017714 PCT/1JS2014/049270
coronary angioplasty (VIVA) and coronary artery bypass grafting (CABG),
optimal and timely
treatment is still not available for all patients. Bridge therapies to
complement existing gold
standards of reperfusion therapy would be of significant value to a large
number of patients.
Because the coronary venous system rarely develops atherosclerosis, the use of
the venous system
for delivery of oxygenated blood has been well explored. Synchronized
retrograde perfusion
(SRP) and pressure-controlled intermittent coronary sinus occlusion (PICSO)
are two
retroperfusion methods for acute treatment of myocardial ischemia through the
coronary venous
system. PICSO and SRP have been used in conjunction with a balloon-tipped
catheter positioned
just beyond the orifice of the coronary sinus connected to a pneumatic pump,
and either passively
redirect coronary sinus blood (PICSO) or actively pump arterial blood during
diastole (SRP) to
the ischemic myocardium. These techniques have been shown to decrease ischemic
changes,
infarct size, myocardial hemorrhage, and no-reflow phenomenon, and improve
left ventricular
(LV) function when coronary blood flow is reinstituted after an acute
occlusion. Wide application
of these techniques, however, has been limited by concerns over their safety
and complexity, and
in particular, the need for repeated occlusion of the coronary sinus with a
balloon. High pressure
(SRP and PICSO) and flow (SRP) can cause damage to the coronary sinus with
thrombosis and
chronic myocardial edema.
We have validated in animal studies both the acute and chronic application of
the
methodologies referenced herein. In a recent acute study, we showed that
preservation of the
contractile function of the ischemic myocardium can be accomplished with
selective
autoretroperfusion (SARP) without the use of an external pump during acute LAD
artery ligation.
The hypothesis that SARP can preserve myocardial function at regulated
pressures without
hemorrhage of vessels or damage of myocytes was verified. In connection with
this animal work,
a bolus of Heparin was given before instrumentation and was then supplemented
as needed to
keep an activated clotting time (ACT) over 200 seconds. The right femoral
artery was cannulated
with a 7Fr catheter and connected to a pressure transducer (TSD104A ¨ Biopac
Systems, Inc) for
monitoring of arterial pressure. Before the sternotomy, the right carotid
artery was cannulated
with a 10Fr polyethylene catheter through a ventrolateral incision on the neck
to reach the
brachiocephalic artery to supply the LAD vein during retroperfusion. The
catheter had a roller
clamp that was used to control the arterial pressure transmitted to the LAD
vein. The right jugular
vein was cannulated with an 8Fr catheter for administration of drugs and
fluids. Lidocaine
hydrochloride was infused at a rate of 60 ug/kg/min before opening the chest
and during the rest
of the procedure. Magnesium sulfate (10 mg/min IV) along with lidocain was
also used to treat
extrasystole in the case of the control group. A vasopressor (LevophedC),
Norepinephrine
Bitartrate Injection, Minneapolis, MN, 2-6 vg/min IV) was used during the
procedure, and was
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
adjusted accordingly to maintain a constant arterial blood pressure (70.0 8.9
mmHg, mean) in
both the experimental and the control groups. Finally, heparin and
nitroglycerine were diluted in
60 mL of 0.9% sodium chloride and infused using a syringe pump at a rate of 1
ml/min. The
chest was opened through a midsternal thoracotomy, and an incision was made in
the pericardium
with the creation of a sling to support the heart with pericardial stay
sutures.
A pair of piezoelectric ultrasonic crystals (2 mm in diameter on 34 gauge
copper wire -
Sonometrics Corporation) were implanted through small stab incisions in the
anterior wall of the
LV (area at risk) distal to the planned site (below first diagonal branch in
the SARP group, and
second diagonal branch in the control group) of LAD artery ligation, for
assessment of regional
myocardial function through measurement of midwall segment length changes. An
additional
pair of crystals was also implanted in the anterior wall of the LV within the
nounal perfusion bed
(control area) of the proximal portion of the LAD artery. FIG. 18 shows a
schematic of the
retroperfusion system showing the arterial and retroperfusion catheters. Each
pair of crystals
were positioned in the midmyocardium (about 7 mm from the epicardium)
approximately 10-15
mm apart and oriented parallel to the minor axis of the heart. The acoustical
signal of the crystals
was verified by an oscilloscope. In the SARP group (ligation + retroperfusion)
the LAD artery
was dissected free from the surrounding tissue distal to the first diagonal
branch for subsequent
ligation. A 2.5 mm flow probe was placed around the LAD artery and connected
to a flow meter
(T403 ¨ Transonic Systems, Inc). The LAD vein was also dissected close to the
junction with the
great cardiac vein, and the proximal portion ligated with 2-0 silk suture in
order to prevent runoff
to the coronary sinus. The LAD vein was then cannulated below the ligation
with a 10Fr cannula
that was attached to the brachiocephalic catheter through one of two four-way
stopcocks. A flow
probe was placed between the stopcocks for measurement of coronary venous
flow. Venous
pressure was recorded through the pressure monitoring line from the
retroperfusion cannula (as
.. shown in FIG. 18). Retroperfusion was initiated immediately after ligation
of the LAD artery and
was maintained for a period of 3 hours. Arterial blood samples were taken at
baseline and at the
end of the first, second and third hours of ligation + retroperfusion for
monitoring of pH,
hematocrit, electrolytes, activated clotting time, and cardiac troponin I.
Coronary venous SARP
may be an effective method of protecting the myocardium during acute ischemia
before definitive
treatment is established as referenced herein regarding various catheter 10
and system 100
embodiments of the present disclosure. SARP may not only offer protection to
the ischemic
myocardium through retrograde perfusion of oxygenated blood but may also serve
as a route for
administration of thrombolytics, antiarrhythmics, and cell and gene therapy to
the jeopardized
myocardium before PTCA or CABG can be implemented in patients eligible for
these procedures.
36
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
In addition to the foregoing, various devices and systems of the present
disclosure can be
used to perform methods for retroperfusion of various bodily organs to treat
many different types
of conditions. As referenced above, providing blood from one bodily vessel to
another bodily
vessel can be performed using devices and systems of the present disclosure,
but in accordance
with the following, said devices and systems can also be used to perfoun the
following novel
methods and procedures.
As generally referenced above, the concept of using veins to deliver
oxygenated nutrient-
tilled blood (arterial blood) is predicated on the fact that despite any
extent of the coronary arterial
disease, the corresponding venous counterpart is atherosclerosis-free. An
additional fact is that
the upper body arterial system has much less predilection for atherosclerosis
than the lower body.
As such, the present disclosure identifies that the upper body can generally
serve as the source of
arterial blood to the venous systems of organs with arterial disease, and that
devices and systems
of the present disclosure can also be used in that regard. An additional
characteristic of the venous
system necessary to facilitate SARP (as referenced herein) is the existence of
a redundancy of the
venous system (namely multiple veins per artery as well as interconnections
between venous
vessels) to ensure proper venous drainage when portion of the system is used
for SARP.
In view of the foregoing, a number of embodiments for retroperfusion of
various organs or
bodily regions that identify arterial blood donor and organ (venous system)
are identified with the
present disclosure, including, hut not limited to, the following:
(i). Peripheral
vessels. Embodiments of devices and systems of the present disclosure
can be used to provide oxygenated blood from the femoral artery, the internal
femoral artery, or
the iliac artery, for example, to the distal saphenous vein or to deep muscle
veins for
arterialization in diabetic patients (a diffuse disease) to treat, for example
a leg pre-amputation or
a necrotic or gangrenous foot ulcer. This venous system has valves (typically
larger than 1-1.5
mm in diameter) which can be overcome (inverted) through catheterization
(namely the insertion
of guidewire and SARP catheter, with guidewire dimensions down to 0.35 mm for
0.014"
standard guidewire) to facilitate said peripheral vessel treatment.
(ii).
Kidney-Renal Vein. Embodiments of devices and systems of the present
disclosure can also be used to facilitate arterialization of the renal vein,
which can be partial (polar
vein) or total (left or right main veins) by way of the femoral or iliac
arteries (if disease free), or
from the axillary, brachial, or subclavian arteries of the upper body, if
desired. Said procedure
could be performed to, for example, treat acute or chronic renal ischemia due
to diffuse
atherosclerosis, severe intima hyperplasia, and to treat the kidney in
connection with various
collagen- vascular diseases.
37
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
(iii). Intestine (Bowel). A number of arterial sources, such as the femoral,
iliac,
axiallary, brachial, subclavian, or epigastric arteries, can be used with
devices and systems of the
present disclosure to facilitate regional arterialization following vein
anastomosis (at the vein
arch) to treat mesenteric arterial ischemia. In at least one embodiment, said
arterialization is
performed to treat an acute embolic or thrombotic mesenteric artery occlusion
in patients with a
severe bowel ischemia.
(iv). Spine. The first of the two main divisions of the spinal system, namely
the
intracranial veins, includes the cortical veins, the dural sinuses, the
cavernous sinuses, and the
ophthalmic veins. The second main division, namely the vertebral venous system
(VVS), includes
the vertebral venous plexuses which course along the entire length of the
spine. The intracranial
veins richly anastomose with the VVS in the suboccipital region, and caudally,
the cerebrospinal
venous system (CSVS) freely communicates with the sacral and pelvic veins and
the prostatic
venous plexus. The CSVS constitutes a unique, large-capacity, valve-less
venous network in
which flow is bidirectional. The CSVS plays important roles in the regulation
of intracranial
pressure with changes in posture, and in venous outflow from the brain. In
addition, the CSVS
provides a direct vascular route for the spread of a tumor, an infection, or
an emboli among its
different components in either direction. Various embodiments of devices and
systems of the
present disclosure can be used to provide oxygenated blood from the external
carotid artery, the
brachial artery, or the axiallary artery, directly to the jugular vein to
treat any number of potential
spinal injuries or conditions, including spinal cord ischemia.
(v). Penis. Various embodiments of devices and systems of the present
disclosure can
also be used to provide arterial blood from the epigastrie artery to the
penile dorsal vein to the
cavernous system of the penis to treat erectile dysfunction.
The foregoing examples of organ-specific perfusion protocols are not intended
to be
exhaustive, but merely exemplary of various novel uses of perfusion devices
and systems of the
present disclosure. Accordingly, the present disclosure includes various
methods for treating
organ-related diseases, various methods of providing arterial (oxygenated)
blood to veins at or
near various organs, and various methods of potentially arterializing veins at
or near various
bodily organs using devices and systems of the present disclosure. For
example, and as shown in
FIG. 19, an exemplary method of organ perfusion of the present disclosure is
provided. Method
1900, in at least one embodiment, comprises the steps of positioning at least
a portion of a device
into a patient's artery (an exemplary artery positioning step 1902),
positioning at least a portion of
the same or a different device into a patient's vein at or near a target organ
(an exemplary vein
positioning step 1904), and facilitating operation of the positioned portions
to allow blood to flow
from the artery to the vein to treat a condition or disease of the target
organ (an exemplary
38
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
operation step 1906). By way of example, an exemplary artery positioning step
1902 could be
performed by positioning at least part of a first catheter 10 having a cannula
16 within an artery of
a patient, the first catheter 10 configured to permit arterial blood to flow
therethrough and further
configured to pefinit a portion of the arterial blood to flow through the
cannula 16, and an
exemplary vein positioning step 1904 could be perfonned by positioning at
least part of a second
catheter 150 within a vein of the patient at or near a target organ, the
second catheter 150
configured to receive sonic or all of the portion of the arterial blood. In
such an embodiment,
which may be referred to as a chronic treatment using catheter 10 and catheter
150, an exemplary
operation step 1906 involves connecting the cannula 16 of the first catheter
10 to a portion of the
second catheter 150 so that some or all of the portion of the arterial blood
flowing through the
cannula 16 is provided into the vein to treat a condition or disease of the
target organ. Further, and
by way of another example, an exemplary artery positioning step 1902 could be
perfonned by
positioning at least a portion of an arterial tube 1032 of a perfusion system
100 within an artery of
a patient, the arterial tube 1032 configured to pennit arterial blood to flow
therethrough, and an
exemplary vein positioning step 1904 could be performed by positioning at
least a portion of a
first catheter 1000 of the perfusion system 100 into a vein of the patient at
or near a target organ,
the first catheter 1000 configured to receive some or all of the arterial
blood from the arterial tube
1032. In such an embodiment, which may be referred to as an acute treatment
using system 100
of the present disclosure, an exemplary operation step 1906 involves operating
a first flow
regulator 1036 of the perfusion system 100 so that some or all of the arterial
blood flowing
through the arterial tube 1032 is provided into the vein to treat a condition
or disease of the target
organ.
In addition to the foregoing, and in various embodiments of devices (such as
catheters 10
and/or cannulas 16), systems 100, and/or SSA systems 300, for example, of the
present disclosure,
such catheters 10, cannulas 16, and/or systems 100 may optionally comprise a
regional
hypothermia system 4000 configured in accordance with the following. Various
regional
hypothermia systems 4000 of the present disclosure, as shown in component
block diagram of
FIG. 20 and as referenced in further detail herein, are configured for use to
cool (reduce the
temperature of) blood and/or other fluids within the body for targeted
delivery to a location within
the body. Such cooling can be from, for example, at or about 0.5 C to as much
as 10 C cooler,
for example, than the native temperature of blood within the mammalian body.
In some
embodiments, localized blood cooling of greater than 10 C may be desired and
accomplished
using one or more regional hypothermia systems 4000 of the present disclosure.
In various
embodiments, regional hypothermia systems 4000 are configured for use within a
mammalian
body even at tissues that are relatively difficult to reach due to, for
example, potential occlusion of
39
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
one or more coronary and/or cerebral arteries. Such regional hypothermia
systems 4000 of the
present disclosure may be useful in connection with the reduction of perfusion
injuries by cooling
the region of risk, whether it be at, near, or in the heart and/or brain, may
be critical to reduce
reperfusion injury and to decrease infarct size, for example, prior to opening
an artery in the heart
or brain. Retroperfusion, as referenced generally herein, provides an ideal
mechanism to deliver
blood at a target location, and the use of a regional hypothermia system 4000
of the present
disclosure in connection with one or more catheters 10, cannulas 16, systems
100, and/or SSA
systems 300 of the present disclosure can effectively deliver blood at a
desired/targeted
temperature by way of delivery through open veins, for example, to the region
at risk, such as a
heart or brain. In general, such catheters 10, cannulas 16, systems 100,
and/or SSA systems 300,
in connection with the use of one or more regional hypothermia systems 4000 of
the present
disclosure, can allow perfusion/retroperfusion of oxygenated blood, control
blood perfusion
pressure within a vessel, condition a blood vessel to operate under higher
blood pressure (such as
arterialization of a vein), increase flow of oxygenated blood to ischemic
myocardium, and/or
decrease the acute ischeinic area during a myocardial infarct event, all at a
relatively colder
temperature than would otherwise be allowed without the use of a regional
hypothermia system.
In at least one embodiment of a regional hypothermia system 4000 of the
present
disclosure, and as shown in FIG. 20, regional hypothermia system 4000
comprises a heat
exchanger 4002 coupled to one or more components of catheters 10, cannulas 16,
systems 100,
.. and/or SSA systems 300 of the present disclosure, such as, for example,
catheter 10, cannula 16,
second catheter 150, connector 170, first graft 185, second graft 190, Y
connector 320, third
catheter 350, first catheter 1000, arterial blood tube 1032, coupler catheter
1042, and/or other
components referenced herein. Heat exchanger 4002, in various embodiments, is
configured to
reduce the temperature of blood passing through one or more components of
catheters 10,
cannulas 16, systems 100, and/or SSA systems 300, so that the blood that is
ultimately delivered
to the targeted area of interest, such as being at, near, or in the heart
and/or brain, is at a lower
temperature than normal (or without the use of a regional hypothermia system
4000). For
example, and in at least one embodiment, regional hypothermia system 4000 is
used to reduce the
temperature of blood delivered at, near, or in the heart and/or brain by or
about 3 C to 4 C via the
general blood circuit created using various catheters 10, cannulas 16, systems
100, and/or SSA
systems 300. IIeat exchanger 4002, as referenced herein, can utilize one or
more cooling products
4004, such as perfluorocarbon, liquid carbon dioxide, helium, another cooled
gas, and/or another
refrigerant or refrigeration mechanism known in the art, that facilitates the
cooling of blood, and
ultimately tissues at or near the cooled blood, through components of
catheters 10, cannulas 16,
systems 100, and/or SSA systems 300 of the present disclosure. Furthermore,
one or more
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
temperature sensors 4006 can be coupled to various components of catheters 10,
cannulas 16,
systems 100, and/or SSA systems 300 of the present disclosure, catheter 10,
cannula 16, second
catheter 150, connector 170, first graft 185, second graft 190, Y connector
320, third catheter 350,
first catheter 1000, arterial blood tube 1032, coupler catheter 1042, and/or
other components
referenced herein, so that blood and/or tissue temperature(s) (including
temperatures at, near, or in
the heart and/or brain, depending on the type of catheters 10, cannulas 16,
systems 100, and/or
SSA systems 300 used) can be detected by temperature sensors 4006 and
transmitted (via wire or
wirelessly) to a remote module 270 and/or another data acquisition and
processing
system/mechanism so that a user of regional hypothermia system 4000 can
regulate localized
temperature (at, near, or in the heart or brain, for example), as desired. A
generic device 4008 is
shown in FIG. 20 as being operably coupled to an exemplary regional
hypothermia system 4000
of the present disclosure, whereby generic device 4008 may comprise one or
more catheters 10,
cannulas 16, systems 100, SSA systems 300, other devices and/or systems of the
present
disclosure, and/or individual components thereof. An exemplary kit 4010 of the
present
disclosure, as shown in the figures, comprises an exemplary regional
hypothermia system 4000
operably coupled to an exemplary generic device 4008 of the present
disclosure.
Further, and in various embodiments, heat exchanger 4004 can be at the level
of an
arterial-venous connector, a double-lumen catheter, and/or another component
of one or more
catheters 10, cannulas 16, systems 100, and/or SSA systems 300 of the present
disclosure. For the
heart, this can be particularly important for patients with a door-to-balloon
time of greater than
two hours, for patients with ST segment elevation myocardial infarction
(STEMI) that are at high
risk for reperfusion injury, and/or patients with hemodynamics instability.
There are several
advantages to using a regional hypothermia system 400 of the present
disclosure, including hut
not limited to rapid percutaneous insertion and rapid cooling of the
myocardial area before
opening the culprit artery to avoid the cascade of inflammatory reactions
responsible for
reperfusion injury. As referenced generally above, various regional
hypothermia systems 4000 of
the present disclosure are configured and operable to introduce mild
hypothermia to reduce
cardiac infarct size and general severity of the same. Such systems 4000, in
connection with
various catheters 10, cannulas 16, systems 100, and/or SSA systems 300 of the
present disclosure,
can treat chronic and acute heart failure, as needed, and generally reduce the
severity of an injury
and/or reduce inflammation as referenced herein, by way of regionally reducing
blood
temperature.
The disclosure of the present application also relates to a potential goal of
translating the
efficacy of a currently invasive open surgery that requires destruction of
vein valves and induces
edema due to the transmission of arterial blood pressure to the veins to a
mini-
41
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
surgical/percutaneous procedure that is much less invasive, takes less time
and does not require
removal of valves and damps the pressure to the veins to reduce the edema. In
lower extremities
with total or near complete obstruction of arterial blood flow, the perfusion
of the limb in a
retrograde manner through the venous system with arterial blood using various
devices of the
present disclosure will provide adequate oxygen and nutrient supply/demand
matching to salvage
limb function. Accordingly, the present disclosure includes methods of using
venous circulation
as an alternative method of limb salvage to deliver arterial blood in a
retrograde manner to the
ischemic extremity through a novel retroperfusion devices that will transform
a lengthy surgical
procedure into a simpler surgical/percutaneous hybrid procedure. In the
absence of substantial
forward native arterial pressure in the capillaries, arterial blood fed into
the venous system at
higher pressure than the native venous pressure will stimulate the development
of significant
collateral network between the native arteries and newly arterialized veins to
supply nutritive flow
and adequate oxygenation to the ischemic tissue and thus salvage the limb (to
avoid amputation).
An exemplary catheter for facilitating intravenous arterialization of the
present disclosure
is shown in FIG. 21. Catheter 3100, as shown in FIG. 21, is configured as a
hybrid endovascular
catheter and comprises an elongated body 3102 having a proximal end 3104 and a
distal end
3106. A balloon 3108 (which may be any number of inflatable members used in
the catheter
arts), in at least one embodiment, is positioned along elongated body 3102 and
may be located
closer to distal end 3106 than proximal end 3104. Balloon 3108, in various
embodiments, may
either be expandable (inflatable) as desired, using a gas and/or a liquid for
example, or may be
inflated automatically using a gas and/or a liquid, the latter referred to
herein as being
"autoexpandable." As shown in FIG. 21, exemplary catheters 3100 of the present
disclosure have
a plurality of apertures 3110 defined through elongated body 3102 at or near
distal end 3106.
Apertures 3110 are configured to allow fluid, such as oxygenated arterial
blood, to flow from
within a catheter lumen 3112 defined along a longitudinal length of elongated
body 3102 out of
apertures 3110 and into a luminal organ of interest, such as to an ischemic
venous blood vessel.
Apertures 3110, in certain other embodiments, may extend either an entire,
substantial, or partial
length of catheter 3110, and the number, concentration, and/or size of
apertures 3110 can vary, as
can the dimensions (such as internal diameter or cross-sectional area of
catheter 3100) so to
control the pressure by way of a pressure drop so that oxygenated arterial
blood flowing through
catheter 3100 and out of apertures 3110 is at a pressure or pressure range
that the venous system
can handle. Accordingly, various catheter 3100 features (such as length and
diameter) can be
tested to ensure proper pressure/flow relationships for the types of
resistances that will be
experienced in-vivo.
42
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
To facilitate proper guidance and positioning within a luminal organ of
interest, various
catheter 3100 embodiments of the present disclosure are configured to receive
a guidewire 3114
therein (such as within lumen 3112 of catheter 3100), whereby guidewire 3114
could be
positioned within catheter 3100 between a proximal opening 3116 (also referred
to as a "lateral
entrance") and a distal opening 3118 of catheter 3100 as shown in FIG. 21, for
example. In
addition, and in at least one embodiment of a catheter 3100 of the present
disclosure, the proximal
end 3104 of catheter 3100 is configured to attach to a graft 3120 (which may
also be referred to
herein as a "prosthesis"), with said connection by way of an optional
connector 3122 (also
referred to herein as a "quick connector") in some embodiments. In embodiments
using one or
more connectors 3122, proximal end 3104 of catheter 3100 may be configured
with a "female"
end or using a connector 3122 with a male or female end, and graft 3120 may be
configured with
a "male" end or using a connector 3122 with a male or female end. In other
embodiments,
opposing gender connections may appear on said components. As referenced
herein, a general
system 3150, as identified in FIG. 21, may comprise an exemplary catheter 3100
of the present
disclosure and one or more additional elements, such as, for example, an
exemplary graft 312, and
exemplary guidewire 3114, and/or an exemplary dilator 3402, as shown in FIG.
24B and
referenced in further detail herein.
Graft 3120, as shown in FIG. 21, can be used to effectively anastomose an
artery of
interest to a vein of interest. For example, and as shown in FIG. 23B, a
proximal end 3124 of
graft 3120 can be positioned within an artery 3220 (such as a femoral artery,
as shown in FIG.
23B), and a distal end 3126 of graft 3120 can be positioned within a vein 3222
(such as a
saphenous vein, also shown in FIG. 23B) so that oxygenated blood from artery
3220 can flow
through a lumen 3128 of graft 3120 and into catheter 3100 coupled thereto,
either directly or via
the use of a connector 3122. Desired dimensions of graft 3120 would be such
that the risk of
lumen 3128 closing off (via thrombosis) would be reduced or eliminated. As
shown in FIG. 3B,
graft 3120 would be positioned within artery 3220 at a location proximal to an
area of artery 3220
having diffuse disease (such as atherosclerotic plaques 3224 as shown in the
figure), so that the
user placing graft 3120 has a level of confidence that sufficient oxygenated
arterial blood flow
will exist at that location of artery 3220. If properly placed and connected,
blood can flow from
artery 3220, through graft 3120, into lumen 3112 of catheter 3100, and out of
apertures 3110 so to
introduce oxygenated blood to the peripheral/collateral veins 3206 at or near
the distal end 3106
of catheter 3100 within vein 3222. In various embodiments of catheters 3100 of
the present
disclosure, shown in FIG. 21 or otherwise and/or as referenced herein,
catheters 3100 may
comprise one or more biologically compatible materials, such as polyurethane
and/or other
synthetic polymers. Grafts 3120 and/or catheters 3100 of the present
disclosure may comprise the
43
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
same or different materials, such as polytetrafluoroethylene ("PTEE"),
polyethylene terephthalate
(such as Dacron ), and/or other synthetic polymers. In addition, at least one
embodiment of a
catheter 3100 of the present disclosure is at least partially coated with an
anticoagulant and/or an
antithrombotic material, such as heparin, for example. An exemplary catheter
3100 and an
.. exemplary graft 3120 of the present disclosure may couple to one another by
way of their inherent
coupling characteristics and/or using one or more connectors 3122 for
anastomosis of graft 3120.
The use of graft 3120 with catheter 3100, in at least one embodiment, allows
for a controlled flow
of oxygenated blood from an artery into a venous area of interest.
Arterialization of a vein, as
generally referenced herein, should preferably occur in a controlled or
gradual fashion, as a rapid
.. increase in blood flow and pressure to a vein can cause significant
swelling, localized blood
accumulation, and potential venous rupture. Graft 3120, in various
embodiments, can be sutured
to the artery and/or vein so to prevent unintended or undesired migration so
to stabilize the same.
Furtheimore, the dimensions of graft 3120 (length, inner diameter or cross-
sectional area, etc.)
can be varied so to provide an initial controlled measure (flow or pressure)
of blood therethrough
upon implantation. By controlling the dimensions of graft 3120 and/or catheter
3100, as
referenced above, side effects such as edema can be controlled/minimized by
reducing the
pressure of blood flowing into the vein or veins of interest. Implantation of
graft 3120, as
referenced in further detail herein, can be performed percutaneously.
An additional catheter 3100 embodiment of the present disclosure is shown in
FIG. 2. As
shown in FIG. 22, catheter 3100 is configured to fit within an external shaft
3200, with external
shaft 3200 being split at its proximal end 3202. Catheter 3100, in such an
embodiment, also
defines a plurality of apertures 3110 within elongated body 3102 so that fluid
can flow through a
lumen 3112 of elongated body 3102 and out of apertures 3110. Exemplary
catheters 3100 of the
present disclosure, such as shown in FIG. 22, are partially or completely
biodegradable and/or
bioabsorbable. Various polymers, such as poly(lactic-co-glycolic acid)
(`PLGA"), may be used
within various catheter 3100 components, such as nodes 3204 shown in FIG. 22.
Nodes 3204, as
shown therein, would be located on the external wall of catheter 3100 (such as
on elongated body
3102) for segmental occlusion at different levels of a luminal organ, such as
the saphein vein.
Exemplary nodes 3204 can resorb at different times, such as in one or more
days, weeks, or
months, and differing resorption rates can allow oxygenated blood to be
introduced into other
areas of the vein proximal to the initial introduction over time to facilitate
gradual arterialization
of the vein proximal to the initial introduction location. External shaft
3200, in various
embodiments, is used/configured to cover apertures 3110, so that if it is
desired to arterialize
different locations within the vein, external shaft 3200 can be retracted so
that additional apertures
3110 proximal to the originally exposed aperture(s) 3100 are exposed to
irrigate oxygenated blood
44
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
to the additional targeted vein area(s). External shaft 3200, in various
embodiments, is
used/configured to cover nodes 3204, whereby retraction of external shaft 3200
to expose nodes
3204 to blood flow would start/facilitate the process of resorption of nodes
3204. An exemplary
biodegradable and/or bioabsorbable catheter 3100, such as shown in FIG. 22,
may have additional
.. features such as those shown in FIG. 21 or as otherwise shown or described
herein, such as, for
example, a connector 3122.
Exemplary catheters 3100 of the present disclosure may be used in accordance
with the
following methods, as depicted in step foimat in FIG. 23A with mammalian body
placement
shown in FIG. 23B. In an exemplary method 3300 of the present disclosure, a
small incision is
made at the level of the peripheral artery source, such as the iliac, femoral,
or popliteal artery (an
exemplary arterial incision step 3302), and the proximal end 3124 of graft
3120 is positioned into
the artery and the distal end 3126 of graft 3120 is positioned into the vein
of interest, such as the
saphenous vein, to anastomose the same (an exemplary graft anastomosis step
3304). Method
3300, in at least one embodiment, further comprises one or more of the steps
of puncturing the
vein of interest (such as the saphenous vein, for example) (an exemplary
venous puncture step
3306), introducing at least part of a guidewire 3114 into the vein through the
puncture aperture
(an exemplary guidewire insertion step 3308), and the distal advancement
(progression) of
guidewire 3114 to a location at or near the portion of the vein of interest
(such as, for example, the
malleolus saphenous vein segment), while avoiding any venous valves along the
way if possible
(an exemplary guidewire advancement step 3310). Various methods 3300 of the
present
disclosure further comprise the steps of advancing (progressing) catheter 3100
over guidewire
3114 so that the distal end 3106 of guidewire 3100 is located within the vein
at the region of
interest (an exemplary catheter advancement step 3312), and connecting
catheter 3100 (at, for
example, the proximal end 3104 of catheter 3100) to the graft 3120 (at, for
example, the distal end
.. 3126 of graft 3120), either directly or using connector 3122, releasing the
oxygenated arterial
blood and allowing it to flow from the artery into lumen 3112 of catheter 3100
and out of
apertures 3110 (an exemplary catheter-graft connection step 3314). Such a mini-
surgical
procedure, namely the perfoimance of catheter-graft step 3314, will create a
graft anastomosis
with an artery, such as the femoral artery. This would complete the procedure
to allow arterial
oxygenated blood to flow from the artery to the vein via graft 3120 and
catheter 3100 to various
extremities, including the lower extremities. Steps 3310 and/or 3312, or other
method 3300 steps
of the present disclosure, may be performed using fluoroscopy, intravascular
ultrasound
("IVUS"), a surface ultrasound, or other scanning methods so that the user of
guidewire 3114
and/or catheter 3100 is aware of the locations of portions of said devices
within the patient's
vasculature. To avoid or reduce retrograde flow and/or to secure a portion of
catheter 3100 within
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
the vein of interest, an exemplary method 3300 of the present disclosure may
further comprise the
step of inflating balloon 3108 (by way of manually or automatically operating
an inflation source
operably coupled to balloon 3108) (an exemplary balloon inflation step 3316).
Balloon 3108,
which in at least one embodiment may be positioned approximately 1-2 cm from
the distal end
3106 of catheter 3100, will be inflated to ensure selective retroperfusion of
the region of interest
(minimize edema) and to prevent antegmde flow of the blood once retroperfusion
is established.
Steps of methods 3300, as referenced above, may be performed in a different
order than described
above. For example, step 3304 may be performed after steps 3310 and 3312.
Over time, such as after two to four weeks for example after use of catheter
3100 within
the patient, the venous vessels in the area at or distal to the distal end
3106 of catheter 3100 will
arterialize, and over a period of approximately four to six weeks, the native
arterial system will
form collaterals with the newly arterialized venous vessels to revascularize
the limb, such as the
leg or portions thereof, such as the foot. After arterialization has been
achieved, catheter 3100 can
be removed from the patient (an exemplary catheter removal step 3318).
However, and prior to
catheter 3100 removal, catheter removal step 3318 may further comprise the
additional step of
connecting the vein to the artery so to provide oxygenated blood to the distal
arterialized venous
area. Such a step may also include the step of occluding the vein by way of a
tying and/or
clipping the proximal portion of the vein. In general, removal of catheter
3100 would discontinue
the supply of oxygenated blood to the venous region of interest, and
connecting the artery to the
vein would allow oxygenated blood to continue to flow through the vein. 'The
tying and/or
clipping of the vein proximal to the region of interest, using a tie and/or a
cutting tool, for
example, would eliminate undesired retrograde blood flow through the vein. The
above-
referenced exemplary methods 3300, or other methods whereby some of all of an
exemplary
catheter 3100 of the present disclosure is positioned within a patient's
vasculature, would allow
the patient to resume or pursue certain mobility, such as walking and sitting
if catheter 3100 is
positioned within the patient's leg. In such embodiments, catheter 3100 may
comprise malleable
and non-collapsible biologically-compatible material(s) so to improve overall
comfort. However,
certain patient's either may not wish to have the majority or all of catheter
3100 positioned within
their vasculature, or the treating physician/interventionalist may determine
that using catheter
3100 in a different fashion, or a different catheter 3100 embodiment, may be
preferred.
Accordingly, at least one additional method 3300 of the present disclosure is
depicted in
step format in FIG. 23C and described as follows. In at least one additional
method 3300 of the
present disclosure, method 3300 comprises the steps of implanting catheter
3300 within the
patient through a subcutaneous tunnel 3400 parallel or substantially parallel
to the length of the
vein of interest (such as the saphenous vein), reaching the desired area of
interest (such as the
46
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
malleolus saphein vein segment) (an exemplary catheter implantation step
3350), and making an
incision in the skin and isolating the distal end 3106 of catheter 3100 at the
level of the malleolus
saphein vein segment, for example (an exemplary skin incision step 3352). Step
3350 may be
performed via skin puncture as well, using an optional guidewire 3114 and/or
an optional dilator
3402, as shown in FIG. 24B if desired. Dilator 3402, in at least one
embodiment, comprises an
elongated body having a cross-section larger than a cross-section of catheter
3100, so that when
dilator 3402 is advanced subcutaneously, catheter 3100 can be positioned
within the subcutaneous
tunnel created using dilator 3402. In at least another embodiment, and as
shown in FIG. 24B,
dilator comprises a dilator lumen 3404 defined therethrough along a
longitudinal length of dilator
3402, terminating at or near one end with a distal dilator aperture 3406,
whereby a guidewire
3114 can be positioned within dilator lumen 3404, and/or whereby device 3100
can be positioned
within dilator lumen 3404. In view of the same, catheter implantation step
3350 may be
performed in various manners. For example, catheter implantation step 3350 can
be performed by
creating a subcutaneous tunnel using dilator 3402, and advancing at least a
portion of catheter
3100 within the subcutaneous tunnel. In another embodiment, catheter
implantation step 3350
may be performed by introducing and subcutaneously advancing guidewire 3114
into the
mammalian patient and advancing at least a portion of catheter 3100 over
guidewire 3114. In yet
an additional embodiment, catheter implantation step 3350 can be performed by
introducing and
subcutaneously advancing a guidewire into the mammalian patient, advancing a
dilator over the
guidewire to create a subcutaneous tunnel, and advancing at least a portion of
the catheter within
the dilator. In another embodiment, catheter implantation step 3350 can be
performed by
introducing and subcutaneously advancing a dilator having a dilator lumen
defined therein and a
guidewire positioned within the guidewire lumen into the mammalian patient to
create a
subcutaneous tunnel, removing the dilator, and advancing at least a portion of
the catheter within
over the guidewire. In yet another embodiment, catheter implantation step 3350
can be performed
by introducing and subcutaneously advancing a dilator having a dilator lumen
defined therein and
a guidewire positioned within the guidewire lumen into the mammalian patient
to create a
subcutaneous tunnel, removing the dilator, and advancing at least a portion of
the catheter within
over the guidewire.
Exemplary methods 3300 may further comprise the steps of puncturing the vein
of interest
(such as the saphenous vein) via traditional venous puncture or incision so to
form a venous
entrance 3408 (an exemplary venous puncture step 3306), and introducing the
distal end 3106 of
catheter 3100 into the vein of interest (such as the distal malleolus
saphenous vein segment) (an
exemplary catheter introduction step 3354). Various methods 3300 further
comprise the steps of
implanting an exemplary graft 3120 (such as by performing arterial incision
step 3302) so that the
47
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
proximal end 3124 of graft 3120 is positioned into the artery and the distal
end 3126 of graft 3120
is available to be connected to catheter 3100 at, for example, the proximal
end 3104 of catheter
3100, and connecting catheter 3100 (at, for example, the proximal end 3104 of
catheter 3100) to
the graft 3120 (at, for example, the distal end 3126 of graft 3120), either
directly or using
.. connector 3122, releasing the oxygenated arterial blood and allowing it to
flow from the artery
into lumen 3112 of catheter 3100 and out of apertures 3110 (an exemplary
catheter-graft
connection step 3314). Over time, such as after two to four weeks for example,
the venous
vessels in the area at or distal to the distal end 3106 of catheter 3100 will
become fully
arterialized, and over a period of approximately four to six weeks, the native
arterial system will
.. form collaterals with the newly arterialized venous vessels to
revascularize the limb, such as the
leg. After arterialization has been achieved, catheter 3100, or remaining non-
biodegradable
portions thereof, can be removed from the patient (an exemplary catheter
removal step 3318). If
the entire catheter 3100 is biodegradable or bioresorbable, catheter removal
step 3318 may not be
required. The term "collaterals", as referenced herein, refers generally to
the phenomenon that
.. occurs during and after initial arterialization. Arteries and veins tend to
run generally parallel to
one another, with the veins forming a general drainage system that allows
blood to flow back to
the heart. By performing one or more methods 3300 as referenced herein,
oxygenated blood
flows to a vein, for which the increased blood pressure and increased overall
blood nutrients
facilitates arterializations. Arteries generally do not collateralize with
veins, as veins generally
have nothing to offer with respect to oxygenated blood or other blood
nutrients. Arties having
oxygen-deficient or nutrient-deficient blood flowing therethrough will want to
connect with
arteries having oxygen and/or nutrient rich blood flowing therethrough, but
that process is
generally limited naturally as arteries would need to be adjacent to one
another to facilitate the
collateralization process. As arteries and veins overlap one another, various
methods 3300 of the
present disclosure effectively turn portions of veins into arteries, and the
newly-formed arteries
can then collateralize with other adjacent arteries and potentially adjacent
veins. Various
additional methods 3300 of the present disclosure may further comprise the
step of moving
catheter 3100 to another location within the vein of interest, or moving
catheter 3100 to another
vein of interest, so to facilitate arterialization of a second region within
the patient's venous
vasculature (an exemplary second region arterialization step 3375, such as
shown in FIGS. 23A
and 23C). For example, catheter-graft connection step 3314, as referenced
above, may be
performed at a first location, and, after a desired amount of time has
elapsed, catheter 3100 can be
moved to a second location within the patient's body, allowing for additional
localized
arterialization to take place via step 3375.
48
CA 02919981 2016-01-29
WO 2015/017714 PCMTS2014/049270
FIG. 24A shows selected components of an exemplary catheter 3100 of the
present
disclosure useful in connection with method 3300 as depicted in FIG. 23C and
referenced above.
As shown in FIG. 24A, exemplary catheter 3100 comprises an elongated body
3102, an
autoexpandable balloon 3108 and a plurality of apertures 3110 at or near
distal end 3106 of
elongated body 3102, and a quick connector 3122 at proximal end 3104 of
elongated body to
connect graft 3120 to elongated body 3102 of catheter 3100. FIG. 24B shows
placement of an
exemplary catheter 3100 of the present disclosure in connection with one or
more above-
referenced methods 3300 whereby catheter 3100 is positioned subcutaneously
through a
subcutaneous tunnel 3400. As shown therein, the distal end 3106 of catheter
3100 is positioned
through a venous entrance 3408 so that arterial (oxygenated) blood can flow
through graft 3120,
through lumen 3112 of catheter 3100, and out of apertures 3110 into vein 3222
so to arterialize
peripheral/collateral veins 3206. FIG. 25 shows an exemplary catheter 3100 of
the present
disclosure with certain identified components. As shown therein, catheter 3100
comprises an
elongated body 3102 having a proximal end 3104 and a distal end 3106, a
balloon 3108
positioned at or near distal end 3106, and a graft 3120 coupled to catheter
3100 at or near
proximal end 3104 of catheter 3100. FIGS. 26A and 26B show embodiments of
catheters 3100 of
the present disclosure positioned within a veins 3222 of a mammalian
circulatory system. As
shown therein (human leg in FIG. 26A, animal leg in FIG. 26B), catheter 3100
is positioned
within the great saphenous vein (vein 3222), distal to the femoral vein 3600,
while an anastomosis
3602 is present between graft 3120 and the femoral artery 3220. Balloon 3108
is shown in its
inflated stated, potentially to anchor catheter 3100 within vein 3222 and to
prevent retrograde
flow of arterial blood through the great saphenous vein 3222 proximal to
balloon 3108.
In addition, the use of a graft 3120 and a catheter 3100 of the present
disclosure can not
only control pressure and flow of blood therethrough to a vein of interest,
catheter 3100 can be
used in a way to preserve (not destroy) any valves present in the vein where
catheter 3100 is
implanted. For example, advancement of a guidewire 3114 through lumen 3112 of
catheter 3100
and out of distal opening 3118, as shown in FIG. 21, can facilitate
advancement of catheter 3100
within the vein of interest, allowing any valves passed by catheter 3100 to
resume operation upon
withdrawal or bioabsorption of catheter 3100. In addition to the foregoing,
catheter 3100 and/or
graft 3120 can be implanted percutaneously, which may be a preferred
implantation method for
high risk or otherwise compromised patient conditions. For example, graft 3120
can be inserted
percutaneously by puncture of the targeted arterial site (identified using
echodoppler,
angiography, or another scanning method), and catheter 3100 can be inserted
percutaneously into
the vein (such as the saphein vein, identified using echodoppler, angiography,
or another scanning
method). Furthermore, connecting catheter 3100 and graft 3120 using a quick
connector 3122
49
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
percutaneously can also facilitate the movement of catheter 3100 to a second
location within the
patient or removal out of the patient altogether. As generally referenced
above, exemplary
methods 3300 of the present disclosure, and potentially other uses of
exemplary catheters 3100 of
the present disclosure, have a number of advantages over current invasive
surgical procedures.
For example, certain traditional surgical procedures not only take several
hours to perform, but
also are invasive open surgeries where most, if not all, branches off of the
vein of interest are
ligatal, and certain other surgeries actually remove the vein of interest
itself, reverse it, and
reconnect it, creating additional potential complications. Uses of catheters
3100 of the present
disclosure are far less invasive, do not require complicated open surgical
procedures, and can be
used to treat inoperable lower limbs via gradual and selective
retroperfusion/revascularization.
Furthermore, and as referenced above, destruction of venous valves is avoided
using catheters
3100 of the present disclosure, while certain surgical procedures either
intentionally or
intentionally destroy or reduce the functionality of said valves. In addition
to the foregoing,
various methods 3300 of the present disclosure may be used to direct blood to
and arterialize
other areas of the mammalian body, not just the peripheral venous system of a
patient's leg or
foot. For example, other areas of a patient, such as the patient's hands,
arms, torso, and other
areas, may be targeted as locations to receive arterialized blood using one or
more catheters 3100
of the present disclosure.
The present disclosure also includes disclosure of various perfusion and/or
retroperfusion
devices and systems and methods of using the same, configured for use in
connection with
various coronary, peripheral, and other retroperfusion methods/procedures
and/or to treat various
ischemic conditions. Various devices and systems of the present disclosure are
configured to
facilitate blood flow in a retrograde direction, from a patient's artery to
the patient's vein, so that a
supply of oxygenated blood can reach parts of the patient's body that can
benefit from that blood.
Various devices and systems of the present disclosure can also be used to
generally facilitate
arterialization of a venous vessel over time at or by way of a gradual
increase in pressure, so that
the arterialized vessel can ultimately withstand full or substantially full
blood pressures that
otherwise exist within a patient's arterial system. Furthermore, various
devices and systems of
the present disclosure can be used to generally regulate blood pressures
therethrough.
An exemplary perfusion/retroperfusion device of the present disclosure is
shown in FIG.
27A. As shown therein, device 100 comprises a unitary body 4100 having a wall
4102, a first
portion 4104 terminating at a first end 4106 and configured for at least
partial placement within a
mammalian artery 4200 (as shown in FIG. 28), and a second portion 4108
terminating at a second
end 4110 and configured for at least partial placement within a mammalian vein
4202 (also as
shown in FIG. 28). As shown in FIG. 27A, device 100 has a lumen 4112 defined
therethrough
inside of wall 4102. Devices 100, as referenced herein, may also generally be
referred to as "catheters"
or "catheter devices" given that they have some sort of lumen defined
therethrough. At least one
advantage of unitary body 4100 over other retroperfusion devices 100 known in
the art is that unitary
body 4100 is a single continuous component, and does not comprises at least
two components that
must be coupled to one another. Such a unitary body 4100 therefore eliminates
all risk of the two or
more components becoming disconnected while in use. In at least one
embodiment, and as shown in
FIG. 27A, first portion 4104 of device 100 is relatively shorter than second
portion 4108 of device 100.
Phrased differently, and as shown in the figure, first portion 4104 has a
first length (Li), and second
portion 4108 has a second length (L2), whereby Li is less than L2. For
example, and in certain device
100 embodiments, Li can range from or about 5-10cm, while L2 can range from or
about 50-70cm.
Other embodiments, such as devices 100 where Li is under 5cm or over 10cm,
and/or where L2 is
under 50cm or over 70cm, are also within the scope of the present disclosure.
Such a configuration is
indicative of the potential need to deliver the source of oxygenated blood to
a peripheral area of a
patient, such as to the patient's feet, and access to a suitable artery 4200
may be significantly away
from that ultimate delivery location. The ability to deliver oxygenated blood
to a peripheral area of a
patient, such as to a patient's foot, may ultimately facilitate being able to
prevent potential foot
amputation. As such, a relatively longer second portion 4108 would allow blood
to travel within device
100 a relatively longer distance in the second portion 4108 as compared to the
first portion 4104. As
such a longer portion would not be required within the patient's artery 4200,
first portion 4104 can be
somewhat shorter than second portion 4108, and in at least certain device 100
embodiments, it may be
desirable to have a shorter first portion 4104.
An exemplary device 100 of the present disclosure, as shown in FIG. 27A, has a
first one-way
valve 4114 and a second one-way valve 4116. A segment 4118 may exist between
first one-way valve
4114 and second one-way valve 4116, or first one-way valve 4114 and second one-
way valve 4116
may be sized and shaped to be immediately adjacent to one another. First one-
way valve 4114 is sized
and shaped to receive at least part of a first guidewire 4204 (as shown in
FIG. 28) therethrough, and
second one-way valve 4116 is sized and shaped to receive at least part of a
second guidewire 4206
(also shown in FIG. 28) therethrough. As shown in FIG. 27A, first one-way
valve 4114 may be
positioned at or near an end of first portion 4104 opposite first end 4106,
and second one-way valve
4116 may be positioned at or near an end of second portion 4108 opposite
second end 4110. Various
retroperfusion devices 100 of the present disclosure can be used in connection
with various coronary,
peripheral, and other retroperfusion methods and/or to treat various
conditions as referenced in the
various patent applications. For example, at least part of first portion 4104
of device 100 could be
51
Date Recue/Date Received 2020-12-23
positioned within a subclavian or axillary artery (exemplary arteries 4200),
and at least part of second
portion 4108 of device 100 could be positioned within a subclavian or an
axillary vein (exemplary
veins 4202) for use and/or treatment at or near the heart (such as, for
example, coronary retroperfusion).
Peripheral uses, for example, may include positioning at least part of first
portion 4104 of device within
an iliac artery (an exemplary artery 4200) and positioning at least part of a
second portion 4108 of
device 100 within a saphenous vein (an exemplary vein 4202), as shown in FIG.
28, for example.
Various arteries 4200, such as the femoral artery, the iliac artery, and in
potentially more extreme
instances/circumstances, the aorta itself, can be used as potential sources of
oxygenated blood for
retrograde delivery to a patient's venous system.
Furthermore, various devices 100 of the present disclosure have a flexible
body 4100/wall
4102, which is able to deform easily without collapsing (so that lumen 4112
remains open to allow
blood to flow from first end 4106, through body 4100, and out of second end
4110). The entire body
4100, or portions thereof, can be flexible. Such an embodiment of body 4102
may comprise a coil-
reinforced wall, whereby one or more coils 4150, as shown in FIG. 27B, are
used in connection with
an impermeable coating 4152. Use of an exemplary device 100 of the present
disclosure may include
the following steps/tasks. An exemplary device 100 is shown in FIG. 28
positioned in part within an
artery 4200 and in part within a vein 4202 in a patient's arm. A second
guidewire 4206 may be inserted
into device 100 through one-way valve 4116 so to position at least part of the
second portion 4108
within vein 4202. A first guidewire 4204 may be inserted into device 100
through one-way valve 4114
so to position at least part of the first portion 4104 within artery 4200.
Upon positioning device 100
or portions thereof as desired, first guidewire 4204 and second guidewire 4206
can be removed, and
one-way valves 4114, 4116 prevent blood from leaking out of device 100. An
optional dilator 3402,
as referenced in detail herein, can be used to facilitate a transition between
the first guidewire 4204
and/or the second guidewire 4206 and the artery 4200 and/or vein 4202 of
interest, so that the artery
4200 and/or vein 4202 is gradually expanded to receive portions of device 100
after use of dilator 3402.
For example, one or more dilators 3402 may be advanced over first guidewire
4204 and/or second
guidewire 4206 into artery 4200 and/or vein 4202, noting that dilators 3402
would have a larger outer
perimeter than the guidewires 4204, 4206, and then portions of device 100
could be positioned into
artery 4200 and/or vein 4202 over dilator(s) 3402. When such a device 100 is
properly positioned,
blood can flow from artery 4200 into device 100 and into vein 4202. As shown
in FIG. 28, device 100
does not need to enter an artery 4200 and an adjacent vein 4202 within the
same plane, and that such
an angle (at or near 90 relative to artery 4200 and vein 4202) may not be
preferred due to potential
fluid shear with said angles. FIGS. 27A and 27C
52
Date Recue/Date Received 2020-12-23
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
show exemplary angle A1 and an exemplary angle A2, whereby A1 and A2, as shown
therein, are
each greater than 90 , and said angles are also greater than 900 as shown in
FIG. 28. Should
angles A1 and/or A2 approach 90 (implying a device bend approaching 90 ), be
90 (implying a
device bend of 90"), or be less than 90' (implying a device bend of greater
than 90"), blood flow
would become disturbed given the sharp turn which would result in energy
losses, which is
generally not preferred. A larger angle Al also allows first portion 4104 of
device 100 to be
positioned in artery 4200 relatively higher up, for example, than second
portion 4108 positioned
within vein 4202, in at least one exemplary use, such as shown in FIG. 28.
General
defommbility/flexibility of at least part of device 100 would allow a user not
only to more easily
position the various areas of device 100 within a patient's vasculature, but
also would allow for
desired angles (such as angle A1 and/or A2) to be achieved upon device 100
implantation. For
example, device 100 could have a general s-shape when being positioned or
ultimately positioned
within a patient, such as shown in FIGS. 27A and 28.
FIG. 29 shows an additional embodiment of a device 100 of the present
disclosure. As
shown therein, device 100 comprises a balloon 4300 positioned within or
coupled to second
portion 4108 of device 100, with balloon 4300 in communication with a balloon
inflation tube
4302 having a balloon port 4304. Introduction of a gas and/or a liquid into
balloon port 4304 can
be used to inflate balloon 4300, and removal of gas and/or a liquid via
balloon port 4304 can be
used to deflate balloon 4300. Balloon 4300, in at least one embodiment, is
useful to hold/anchor a
portion of device 100 in place within a patient's vasculature. Balloon 4300
may also, or
alternatively, be used to ensure that blood flowing through device 100 flows
in a retrograde
direction, and that blood flowing therethrough flows at a desirable rate,
which may be less than a
full rate which would occur should no balloon 4300 be present. For example,
inflation and/or
deflation of balloon 4300 could be used to control blood flow through that
portion of device 100,
so that a desired flow rate, volume, and/or pressure of blood flows through
device 100 to vein
4202, which can be determined using a pressure/flow guidewire, for example. As
such, devices
100 can effectively regulate blood pressures through device 100 and the amount
(rate, volume,
and/or pressure) of blood into vein 4202. In at least one embodiment, general
dimensions
(diameter(s) and/or length(s) of various regions of device 100) may be
specified so that blood
flow therethrough is at a desired rate, consistent with the general teachings
of Pouiselle' s law.
Balloon 4300 can also be used to prevent the backflow of blood from vein 4202
into device 100.
The entirety of device 100 does not need to be deformable, but in at least
some embodiments,
portions of first portion 4104 and/or second portion 4108 are deformable as
referenced herein.
FIG. 30 shows an embodiment of a device 100 of the present disclosure
partially positioned
53
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
within vein 4202, with such placement facilitated using a splittable
introducer sheath 4400.
Splittable introducer sheath 4400 can be positioned within an opening of vein
4202, and a portion
of device 100 (such as second portion 4108) can be positioned inside of
splittable introducer
sheath, with such placement being facilitated using a second guide wire 4206,
such as shown in
.. FIG. 28. In at least one method (or step(s) of a method), splitttable
introducer sheath 4400 can be
positioned within an opening of an artery 4200 and/or a vein 4202 (noting that
two splittable
introducer sheaths can be used, one in artery 4200 and one in vein 4202, or
only one splittable
introducer sheath can be used in either artery 4200 or vein 4202), and using
guidewire 4204 or
4206, a portion of device 100 can be advanced into artery 4200 and/or vein
4202. Guidevvire
4206, for example, can be advance through various vein 4202 valves (such as in
the middle/center
of each valve), so that said valves remain functional after ultimate removal
of guidewire 4206 (so
not to destroy said valves). After placement of the portion of device 100
therein, splittable
introducer sheath 4400 can be split so that it can be removed from vein 4202
while the portion of
device 100 remains within vein. One or more optional sutures 4402, as shown in
FIG. 30, can be
used to secure device 100 to the patient's skin 4404, and/or one or more
bandages 4406 can be
used to cover the skin 4404 to potentially facilitate healing and/or maintain
a relatively clean
location. After a period of time, such as 4-6 weeks to allow vein 4202 to
generally and locally
arterialize due to the higher pressure of blood flowing therethrough from
device 100, device 100
can be removed from the patient. Should it be desired to have device 100
positioned
subcutaneously, instead of being exposed from the patient, an incision and/or
other type of
puncture can be made through skin 4404 to position the device subcutaneously.
Procedurally, and
in at least one method, second portion 4108 would be first positioned within
vein 4202 prior to
positioning first portion 4104 into artery 4200. In such a method, blood from
artery 4200 would
be routed through device 100 to vein 4202. However, should first portion 4104
be positioned into
artery 4200 before second portion 4108 is positioned within vein 4202, blood
from artery 4200
may exit device 100 outside of vein 4202, which would not be preferable.
In addition to the foregoing, at least one exemplary device 100 of the present
disclosure
may comprise a pressure controlling element 4408, such as shown in FIG. 30.
Pressure
controlling element 4408 would allow a user to control/regulate the general
blood pressure
through at least part of device 100, and may be positioned at various regions
along device.
Pressure controlling element 4408, in at least one embodiment, may be
positioned at or near
segment 4118, which, in various embodiments and/or uses, may be exposed
outside of a patient
when device 100 is implanted and in use. Pressure controlling element 4408, in
at least one
embodiment, is adjustable by a user of device 100 and/or a person positioning
or otherwise
maintaining device 100. For example, pressure controlling element 4408 may
comprise an
54
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
occluder (to partially occlude lumen 4112 at a location within device 100), a
pressure/flow wire
(operable to detect blood pressure within lumen 4112), or another device
useful to obtain blood
pressure/flow data and/or adjust a local dimension of device 100 as desired.
There are several
ways pressure can be controlled/regulated to avoid over-pressurization of
veins, including the
foregoing as well as, for example, use of an external constrictor (another
exemplary pressure
controlling element 4408), an internal stenosis that resorbs over time
(poly(lactic-co-glycolic
acid) (PLGA), another exemplary pressure controlling element 4408), and/or by
design, to reduce
the dimension of the arterial portion (first portion 4104) of device 100. As
noted above, and
based on Pouseulle's law, diameter and length can be selected of the arterial
portion (first portion
4104) to obtain the desired pressure drop, which will, in at least one
embodiment, involve a
reduction of the diameter of (first portion 4104) of the catheter, which is
desirable as it would
imply a lower profile device 100 in the patient's arterial system. As shown in
FIG. 30, an
exemplary system 4450 of the present disclosure includes an exemplary device
100 of the present
disclosure and at least one other item, such as, for example, one or more of a
first guide wire
4204, a second guide wire 4206, a splittable introducer sheath 4400, and/or a
data wire 4460.
An additional embodiment of a portion of an exemplary device 100 of the
present
disclosure is shown in FIGS. 31A and 31B. As shown in FIG. 31A, part of a
second portion 4108
(of unitary body 4100) of an exemplary device 100 is shown therein, wherein
distal end 4110
comprises, defines, and/or is coupled to a flarable tip 4500. Flarable tip
4500, as shown in FIG.
31A, can be coupled to second end 4110 of second portion 4108, or can be part
of or defined at
second end 4110. Flarable tip 4500, as shown in FIG. 31B and in various
embodiments, is
configured to shift from a first configuration 4502, which is generally
tapered or unflared, to a
second configuration 4504, which is generally flared, and back again. Phrased
differently,
flarable tip 4500, in various embodiments, is configured to shift from a first
configuration 4502,
which is not expanded, to a second configuration 4504, which is generally
expanded, and back
again. Said configurations, as referenced above, occur based upon pressures of
fluid flowing
therethrough when device 100 is positioned within a lumina] organ having a
fluid therein, as
described in further detail below. For example, and as shown on the left side
of FIG. 31B, if
device 100 was positioned wholly within a vein 4202 and not susceptible to
arterial blood
pressures, flarable tip 4500 would be in a first configuration 4502 or
relatively close to a first
configuration 4502, as flarable tip 4500, configured consistent with the
present disclosure, would
not shift to a second configuration 4504 because venous blood pressure (P1 in
FIG. 31B) would
not be high enough to cause flarable tip 4500 to shift to a second
configuration 4504. However,
for example and as shown on the right side of FIG. 31B, when device 100 is
positioned within a
vasculature as generally referenced herein (wherein a first portion 4104 of
device 100 is
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
positioned within an artery 4200 and a second portion 4108 of device 100 is
positioned within a
vein 4202), flarable tip 4500 would be in or shift to a second configuration
4504 or relatively
close to a second configuration 4504, as flarable tip 4500, configured
consistent with the present
disclosure, would flare or generally expand due to higher arterial blood
pressure (P2 in FIG. 31B)
as compared to the relatively lower venous blood pressure (P1 in FIG. 31B).
Harable tip 4500, as
referenced herein, is generally configured so that the second end 4110 of the
second portion 4108
of device 100 distends to the size (luminal perimeter) of the portion of vein
4202 having second
end 4110 positioned therein so that blood flow therethrough is retrograde. In
such a
configuration, flarable tip 4500 serves as a flow direction regulator. In at
least one embodiment,
and as shown in FIG. 31B, an exemplary flarable tip 4500 may comprise a
membrane 4510 of one
or more thin or relatively thin materials, such as polytetrafluoroethylene
(PTFE), mammalian
tissue, and/or one or more other biologically-compatible thin or relatively
thin materials,
reinforced by a number of struts 4512 (also referred to as structural fibers),
which may comprise
nitinol, stainless steel, and/or one or more other biologically-compatible
rigid compositions, so
that a general axial integrity of flarable tip 4500 exists whereby flarable
tip 4500 does not crumple
or fold over during insertion into vein 4202 when it is in its first
configuration 4502. As noted
above, second configuration 4504 of flarable tip 4500 can be induced by
pressure, such as when
device 100 is connected to arterial pressure as generally referenced herein,
since membrane 4510
can be distended open. The converse occurs when the pressure is disconnected
(the flarable tip
4500 collapses) for removal. Flarable tip 4500 deployment, as referenced
herein, can also flare
open (shift to second configuration 4504) based upon a memory aspect of one or
more component
materials, such as nitinol, whereby struts 4512 have a general memory that
permits struts 4512 to
flare open due to some sort of engagement or general touching of struts 4512
in one or more
particular fashions.
An additional embodiment of a device 100 of the present disclosure is shown in
FIG. 32.
As shown in FIG. 32, device 100 comprises a number of components as shown in
FIG. 27A as
well as flarable tip 4500 as shown in FIGS. 31A and 31B. In addition, and in
at least this
embodiment, device 100 further comprises a tapered portion 4600, which
comprises a portion of
second portion 4108 (as shown in FIG. 32), or even all or substantially all of
second portion 4108,
extending from second one-way valve 4116 to flarable tip 4500, for example. As
venous cross-
sectional areas generally decrease toward the more peripheral areas of the
body, such a device 100
embodiment may not only be easier to position within a patient, but also may
provide more
comfort to the patient upon implantation. In addition, a narrower or tapered
second portion 4108
would reduce the risk of venous rupture during and after implantation, as such
a device 100 would
56
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
better confoun to the patient's actual vein, such as a peripheral vein in a
patient's leg. As shown
in FIG. 32, tapered portion 4600 tapers distally from a first diameter
(identified as D1 in the
figure) to a second diameter (identified as D2 in the figure), whereby the
first diameter is greater
than the second diameter. Furthermore, one or more device embodiments of the
present
disclosure may have more than one tapered portion 4600 within second portion
4108 of device
100. The degree, number, and length of tapered portions 4600 can be selected
to regulate the
degree of pressure drop along the device 100 in order to reduce the
transmission of arterial
pressure to the venous system and to generally avoid over-pressurization of
the venous system.
For example, if the arterial pressure into the first portion 4104 (the
aiterial portion of device 100)
may be between 80 and 100 mmHg, it may be desirable to reduce this pressure,
such as to
between 40 and 60 mmHg, in the patient's venous system through a 40 mmHg (or
other
comparable) pressure drop along device 100. Such a determination of pressure
drop can be
implemented through a computational fluid dynamic simulation.
As referenced above, the present disclosure includes a novel approach for the
reperfusion
of a diseased peripheral artery, where an exemplary device 100 of the present
disclosure can be
used to connect a vein 4202 and an artery 4200 that run alongside one another.
In this approach,
the diseased vein 4202 is bypassed and `retro-perfused' through the adjacent
artery 4200 with
arterial blood flow through a simple, flexible tube-type device 100. The
diseased vein 4202 is not
only provided with oxygen-rich blood immediately hut, as referenced herein,
may also be
arterialized chronically (such as over a period of several weeks, for
example). The pressure level
and flow rate in the peripheral vein are usually much lower than in the artery
4200 at the
corresponding level (e.g., femoral vein vs. femoral artery). As the vein 4202
is perfused by the
blood flow from the artery 4200 through the device 100, the venous pressure
may change
depending on the relative flow conditions between the vein 4202 and the artery
4200. One factor,
which may be a relatively important one, for the arterialization is how much
the vein 4202 is
pressurized by the arterial blood flow. Too much pressure would cause damage,
while too little
pressure would not have the desired effect. Hence, the right amount of
pressure increased (such
as approximately the average of arterial and venous pressure; i.e., -40-60
mmHg as previously
referenced herein) would be necessary. Device 100 embodiments of the present
disclosure can
regulate the pressure through the degree of articulation (bending) of said
device 100. The
pressure drop or energy loss in a circular pipe occurs to various degrees
depending on the
geometry of the device and the inlet and outlet boundary conditions. The
geometric change is the
primary determinant of pressure drop (e.g., sudden expansion or contraction of
the lumen area).
For an isodiametric circular tube, such as device 100 embodiments of the
present disclosure, a
simple but effective way of manipulating the pressure drop along the unitary
body 4100 is to
57
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
make a local curvature in the unitary body 4100 as referenced above and as
shown in FIGS. 27A
and 27C for example (with respect to the localized device 100 bending
corresponding to angles
A1 and A2).
The present disclosure also includes a determination of the relation between
the degree of
local bend in the device 100 and the pressure drop along the device 100 in
connection with the
local bend. To demonstrate the same, a sigmoidal function was adopted to model
the local
curvature of a circular tube since a wide range of profiles can be
systematically generated by
varying two arguments of the function according to the situations where the
device is deployed.
The adopted sigmoidal function has two primary arguments (A and B in Equation
1) that can be
changed in the profile as follows:
f = _____
(1.+e-Bs )
[1]
where A and B are varied depending on the vein and artery size, the distance
between two
vessels alongside one another, and degree of local bending of the device. For
the present testing,
the diameter (D) and the distance between the inlet and outlet of the device
(L) was assumed to be
4 and 60 mm, respectively, such as shown in the exemplary devices shown in
FIG. 34. FIG. 33
shows that given the vein and artery dimension (i.e., A is deteimined by the
vein and artery
diameter and distance between two vessels alongside), a variety of local
curvature profiles of the
device are generated by changing the argument B. As the B value increases, the
profile becomes
steeper which may lead to the larger pressure drop across the device. FIG. 34
depicts the actual
device configurations constructed from the sigmoidal function with various
arguments (i.e., B
varies from 0.25 to 0.75).
The ability of the device to adjust the pressure change along the device with
various local
geometric changes subject to in vivo flow conditions (such as shown in FIG.
35) was then
determined. The temporal velocity profile at the device inlet (as shown in
FIG. 36) was specified
by digitizing in vivo flow wave folui shown in FIG. 35 for flow modeling
purposes. The flow
field was then obtained by solving the Navier-Stoke Equation as follows:
Continuity:
V V= 0 [2]
Momentum:
[31
58
CA 02919981 2016-01-29
WO 2015/017714 PCMJS2014/049270
where V , p, ,u, and r are pressure, dynamic viscosity of blood, and blood
density,
respectively. Blood was assumed as an incompressible and Newtonian viscous
fluid with a
constant density of 1060 kg/m3 and a constant dynamic viscosity of 0.0035 kg/m-
s.
Regarding pressure regulation, the pressure drop across the device is a
function of the
device configuration modeled by the local curvature profile. In the laminar
flow regime such as
the present flow condition adopted (14<Re<400, Remean = 147), the pressure
drop along a straight
circular tube with a specific length was established, for example:
Ap = L f. f 64= 2 ___________ Re
D
where L, D, and f denote tube length, diameter, and flow friction factor,
respectively. A
pressure drop is also a general function of a relative flow condition between
two vessels (such as
artery 4200 and vein 4202), which are connected to one another using a device
100 as generally
referenced herein. Such a flow condition could vary from patient to patient,
and could also vary
depending on the various positions device 100 would be positioned within
artery 4200 and vein
4202. With respect to relative flow conditions, the medical personnel involved
with selection
and/or implantation of device 100 into a patient could, for example, refer to
a table or other
database of information that relate to various patient arterial blood
pressures, a length of device
100 needed (such as L2, referenced herein) which would depend on the patient's
height, and
blood flow, which can be measured using one or more other devices, such as a
data wire 4460
(which may include, but is not limited to, a pressure wire or catheter, a flow
wire or catheter, or a
combination pressure and flow wire or catheter, for example), having a sensor
4462 thereon or
therein (such as a pressure sensor or flow sensor) so to identify flow and so
that one or more
devices angles and/or bends referenced herein can be adjusted to provide the
desired pressure
drop. Since the pressure drop varies with the curvature applied to a straight
circular tube, the
pressure drop of a curved circular tube was calculated relative to a straight
pipe subject to an
identical flow condition. This is an indicator of how much additional pressure
drop is induced by
the local geometric change of the device. Five different configurations of the
curved tube were
investigated (with results shown in Table 1), and the cycle-average relative
pressure drop was
calculated for each configuration.
59
Case I Case II Case III Case IV
Case V
Config. Straight 61(1 e -0.25x) 61(1 e -0.5x) 610 e -0.75x) 61(1 e -
X)
180 Bend
APcu
1.09 1.19 1.28 1.37 2.34
APst
(Alicu/ Lcu)
1 1.08 1.17 1.24 1.32
1.86
(Apt /1st)
Device
bend (0 = 0 35 42.5 53.5 69.5
180
straight)
Table 1: Cycle-average Relative Pressure Drop
A circular tube with 180 bending (such as shown in FIG. 37) was examined as
an extreme
case. The results demonstrated that the relative pressure drop increases as
the curvature becomes
steeper (i.e., from Case I up to Case IV, as shown in Table 1 and depicted in
FIG. 38). The increase in
pressure drop shown in FICi. 38 relates the conditions shown in FICIS. 33-37,
for example, and not
under the circumstance depicted in FIG. 39. The angles referenced in FIG. 38
correspond to 180
minus Ai or 180 minus A2, as appropriate. Two different definitions of
relative pressure drop were
considered (i.e., Apcu / Apst and ( Apcu /Lcu ) / ( Apst / Lst)),
demonstrating that they result in only
slight difference for the sigmoidal function-based curved tubes (Case II ¨
Case IV) while they can lead
to a significant discrepancy for the extremely bent configuration (Case V).
Although the distance
between inlet and outlet of the device is identical for all the tube
configurations considered (i.e., L
remains same as 60 mm, as shown in FIG. 34), the length of flow path (i.e.,
L.) increases when the
curvature is applied to a straight tube. Since the actual flow path length is
a primary regulator of the
pressure drop along the tube, the length needs to be taken into account to
calculate the relative pressure
drop. Thus, the normalized relative pressure drop or ( Ap. /L) I ( Apt /Lt)
seems to be a relevant
indicator of the pressure drop in a circular tube with local curvature. As
such, the results indicate that
the approaches referenced herein are a useful and inventive tool to generate a
wide range of variations
in pressure drop across the device to pressurize the vein to different
extents.
FIG. 39 shows a flexible tube-type device 3902 positioned within a mammalian
artery 3904
and a vein 3906 according to an exemplary embodiment of the present
disclosure. Oxygen-poor blood
is represented by arrow 3908 and oxygen-rich blood is represented by arrow
3910.
Date Recue/Date Received 2020-12-23
In summary, the devices and methods to regulate the degree of pressure drop
through the
change in catheter configuration (at the bend) at the junction between the
arterial and venous portion
of the device are disclosed herein. The pressure drop across the device can be
regulated up to any
number of percentages, such as 10%, 20%, 30%, 40%, or higher or lower, for
practical changes in
.. curvature or shape (as indicated below). These changes in curvature can be
ensured, for example,
through a suture anchor in the device and in the patient's tissue at the time
of implant. In addition, and
in various device 100 embodiments, the degree of pressure drop can be
regulated based on one or more
device diameters (such as Di and D2 referenced herein and shown in FIG. 32),
and on one or more
device 100 lengths (such as Li and L2 referenced herein and shown in FIG.
27A). For example, and in
.. at least one embodiment, the degree of pressure drop through device 100 is
based, in at least part, upon
a device diameter, length, and device 100 bend/curvature. As noted above, and
in at least the
aforementioned embodiment or other embodiments, the degree of pressure drop
through device 100 is
based, in at least part, upon a device diameter, device length, and device
100, a flow friction factor,
and a relative flow condition between two vessels (such as artery 4200 and
vein 4202), which are
connected to one another using a device 100 as generally referenced herein.
While various embodiments of retroperfusion devices and systems and methods of
using the
same have been described in considerable detail herein, the embodiments are
merely offered as non-
limiting examples of the disclosure described herein. It will therefore be
understood that various
changes and modifications may be made, and equivalents may be substituted for
elements thereof,
without departing from the scope of the present disclosure. The present
disclosure is not intended to
be exhaustive or limiting with respect to the content thereof.
Further, in describing representative embodiments, the present disclosure may
have presented
a method and/or a process as a particular sequence of steps. However, to the
extent that the method or
process does not rely on the particular order of steps set forth therein, the
method or process should
not be limited to the particular sequence of steps described, as other
sequences of steps may be possible.
Therefore, the particular order of the steps disclosed herein should not be
construed as limitations of
the present disclosure. In addition, disclosure directed to a method and/or
process should not be limited
to the performance of their steps in the order written. Such sequences may be
varied and still remain
within the scope of the present disclosure.
61
Date Recue/Date Received 2020-12-23