Note: Descriptions are shown in the official language in which they were submitted.
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
RECOMBINANT VECTOR WITH OPTIMIZED A-BULGE
CROSS REFERENCE TO RELATED APPLICATIONS
[0001] This application claims priority to U.S. Provisional
Application Serial No. 61/862,433, filed August 5, 2013, the
disclosure of which is incorporated herein by reference.
TECHNICAL FIELD
[0002] This disclosure relates to optimized internal ribosome
entry sites (IRES), compositions containing such optimized IRESs
including vectors. More particularly, the disclosure relates to
replication competent retroviral vectors for treating cell
proliferative disorders. The disclosure further relates to the use
of such replication competent retroviral vectors for delivery and
expression of heterologous nucleic acids.
BACKGROUND
[0003] Effective methods of delivering genes and heterologous
nucleic acids to cells and subjects has been a goal researchers for
scientific development and for possible treatments of diseases and
disorders.
INCORPORATION OF SEQUENCE LISTING
[0004] The present application is filed with a Sequence Listing
in electronic format. The Sequence Listing is provided as a file
entitled 00014-019W01Sequence 5T25.txt created on August 5, 2014,
which is 202 Kb in size. The information in the electronic format
of the sequence listing is incorporated herein by reference in its
entirety.
SUMMARY
[0005] The disclosure provides recombinant replication competent
retrovirus comprising: a retroviral GAG protein; a retroviral POL
protein; a retroviral envelope; a retroviral polynucleotide
comprising Long-Terminal Repeat (LTR) sequences at the 3' end of
the retroviral polynucleotide sequence, a promoter sequence at the
5' end of the retroviral polynucleotide, said promoter being
suitable for expression in a mammalian cell, a gag nucleic acid
domain, a pol nucleic acid domain and an env nucleic acid domain; a
cassette comprising an internal ribosome entry site (IRES)
consisting of 6A's in the A-bulge in the bifurcation region of the
IRES, wherein the IRES is operably linked to a heterologous
1
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
polynucleotide, wherein the cassette is positioned 5' to the 3' LTR
and 3' to the env nucleic acid domain encoding the retroviral
envelope; and cis-acting sequences necessary for reverse
transcription, packaging and integration in a target cell, wherein
the RCR maintains higher replication competency after 6 passages
compared to a vector comprising SEQ ID NO:21 (pACE). In one
embodiment, the virus infects a target cell multiple times
resulting in an average number of copies/diploid genome of 5 or
greater. In another embodiment of any of the foregoing, the
retroviral polynucleotide sequence is derived from a virus selected
from the group consisting of murine leukemia virus (MLV), Moloney
murine leukemia virus (MoMLV), Feline leukemia virus (FeLV), Baboon
endogenous retrovirus (BEV), porcine endogenous virus (PERV), the
cat derived retrovirus RD114, squirrel monkey retrovirus,
Xenotropic murine leukemia virus-related virus(XMRV), avian
reticuloendotheliosis virus (REV), or Gibbon ape leukemia virus
(GALV). In another embodiment of any of the foregoing, the
retroviral envelope is an amphotropic MLV envelope. In another
embodiment of any of the foregoing, the retrovirus is a
gammaretrovirus. In another embodiment of any of the foregoing, the
target cell is a cell having a cell proliferative disorder. In
another embodiment of any of the foregoing, target cell is a
neoplastic cell. In another embodiment of any of the foregoing, the
cell proliferative disorder is selected from the group consisting
of lung cancer, colon-rectum cancer, breast cancer, prostate
cancer, urinary tract cancer, uterine cancer, brain cancer, head
and neck cancer, pancreatic cancer, melanoma, stomach cancer and
ovarian cancer, rheumatoid arthritis or other autoimmune disease.
In another embodiment of any of the foregoing, the promoter
sequence is associated with a growth regulatory gene. In another
embodiment of any of the foregoing, the promoter sequence comprises
a tissue-specific promoter sequence. In another embodiment of any
of the foregoing, the tissue-specific promoter sequence comprises
at least one androgen response element (ARE). In another embodiment
of any of the foregoing, the promoter comprises a CMV promoter
having a sequence as set forth in SEQ ID NO:19, 20, 22 or 42 from
nucleotide 1 to about nucleotide 582 and may include modification
2
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
to one or more nucleic acid bases and which is capable of directing
and initiating transcription In another embodiment of any of the
foregoing, the promoter comprises a CMV-R-U5 domain polynucleotide.
In another embodiment of any of the foregoing, the CMV-R-U5 domain
comprises the immediately early promoter from human cytomegalovirus
linked to an MLV R-U5 region. In another embodiment of any of the
foregoing, the CMV-R-U5 domain polynucleotide comprises a sequence
as set forth in SEQ ID NO: 19, 20, 22 or 42 from about nucleotide 1
to about nucleotide 1202 or sequences that are at least 95%
identical to a sequence as set forth in SEQ ID NO:19, 20, 22 or 42,
wherein the polynucleotide promotes transcription of a nucleic acid
molecule operably linked thereto. In another embodiment of any of
the foregoing, the gag polynucleotide is derived from a
gammaretrovirus. In another embodiment of any of the foregoing, the
gag nucleic acid domain comprises a sequence from about nucleotide
number 1203 to about nucleotide 2819 of SEQ ID NO: 19, 20, 22 or 42
or a sequence having at least 95%, 98%, 99% or 99.8% identity
thereto. In another embodiment of any of the foregoing, the pol
domain of the polynucleotide is derived from a gammaretrovirus. In
another embodiment of any of the foregoing, the pol domain
comprises a sequence from about nucleotide number 2820 to about
nucleotide 6358 of SEQ ID NO: 19, 20, 22 or 42 or a sequence having
at least 95%, 98%, 99% or 99.9% identity thereto. In another
embodiment of any of the foregoing, the env domain comprises a
sequence from about nucleotide number 6359 to about nucleotide 8323
of SEQ ID NO: 19, 20, 22 or 42 or a sequence having at least 95%,
98%, 99% or 99.8% identity thereto. In another embodiment of any
of the foregoing,the IRES consists of the sequence set forth in SEQ
ID NO:41. In another embodiment of any of the foregoing, the
retroviral polynucleotide sequence comprises (i) the sequence set
forth in SEQ ID NO:42 or (ii) the sequence as set forth in SEQ ID
NO:42, wherein T is U. In another embodiment of any of the
foregoing, the heterologous nucleic acid comprises a polynucleotide
having a sequence as set forth in SEQ ID NO:3, 5, 11, 13, 15 or 17.
In another embodiment of any of the foregoing, the heterologous
nucleic acid encodes a polypeptide comprising a sequence as set
forth in SEQ ID NO:4. In another embodiment of any of the
3
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
foregoing, the heterologous nucleic acid is human codon optimized
and encodes a polypeptide as set forth in SEQ ID NO:4. In another
embodiment of any of the foregoing, the heterologous nucleic acid
comprises a sequence as set forth in SEQ ID NO: 19 or 22 from about
nucleotide number 8877 to about 9353. In another embodiment of any
of the foregoing, the 3' LTR is derived from a gammaretrovirus. In
another embodiment of any of the foregoing, the 3' LTR comprises a
U3-R-U5 domain. In another embodiment of any of the foregoing,the
3' LTR comprises a sequence as set forth in SEQ ID NO: 19 or 22
from about nucleotide 9405 to about 9998 or a sequence that is at
least 95%, 98% or 99.5% identical thereto. In another embodiment of
any of the foregoing, the heterologous nucleic acid sequence
encodes a biological response modifier or an immunopotentiating
cytokine. In another embodiment of any of the foregoing, the
immunopotentiating cytokine is selected from the group consisting
of interleukins 1 through 15, interferon, tumor necrosis factor
(TNF), and granulocyte-macrophage-colony stimulating factor (GM-
CSF). In another embodiment of any of the foregoing, the
immunopotentiating cytokine is interferon gamma. In another
embodiment of any of the foregoing,the heterologous nucleic acid
encodes a polypeptide that converts a nontoxic prodrug in to a
toxic drug. In another embodiment of any of the foregoing,the
polypeptide that converts a nontoxic prodrug in to a toxic drug is
thymidine kinase, purine nucleoside phosphorylase (PNP), or
cytosine deaminase. In another embodiment of any of the foregoing,
the heterologous nucleic acid sequence encodes a receptor domain,
an antibody, or antibody fragment. In another embodiment of any of
the foregoing, the heterologous nucleic acid sequence comprises an
inhibitory polynucleotide. In another embodiment of any of the
foregoing, the inhibitory polynucleotide comprises an miRNA, RNAi
or siRNA sequence.
[0006] The disclosure also provides a recombinant retroviral
polynucleotide genome for producing a replication competent
retrovirus as described above.
[0007] The disclosure also provides a method of treating a cell
proliferative disorder comprising contacting the subject with a
recombinant replication competent retrovirus of the disclosure
4
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
under conditions such that the cytosine deaminase polynucleotide is
expressed and contacting the subject with 5-fluorocytosine. In
another embodiment, the cell proliferative disorder is glioblastoma
multiforme. In another embodiment of any of the foregoing,the cell
proliferative disorder is selected from the group consisting of
lung cancer, colon-rectum cancer, breast cancer, prostate cancer,
urinary tract cancer, uterine cancer, brain cancer, head and neck
cancer, pancreatic cancer, melanoma, stomach cancer and ovarian
cancer.
[0008] The disclosure also provides a vector that expresses a
heterologous gene in a mammalian cell from an ECMV IRES with 6As in
the A bulge in the J-K bifurcation region. In another embodiment,
the vector is a viral vector. In another embodiment of any of the
foregoing, the vector is a retroviral replicating vector. In
another embodiment of any of the foregoing, the vector is a
retroviral replicating vector derived from a gamma-retrovirus. In
another embodiment of any of the foregoing, the gamma-retrovirus is
derived from one of Murine Leukemia Virus, Baboon Endogenous Virus,
Gibbon Ape Leukemia virus, Feline leukemia virus. In another
embodiment of any of the foregoing, the heterologous gene is a gene
with a therapeutic activity in mammals In another embodiment of any
of the foregoing, the therapeutic activity is an anticancer
activity. In another embodiment of any of the foregoing, the
heterologous gene is a prodrug activating gene. In another
embodiment of any of the foregoing, the vector can express a
heterologous gene in a mammalian cell from an ECMV IRES in the
absence of the protein PTB-1.
[0009] The disclosure also provides a method of treating cancer,
by administering a vector as described above.
[0010] The disclosure also provides a recombinant replication
competent retrovirus comprising: a retroviral GAG protein; a
retroviral POL protein; a retroviral envelope; a retroviral
polynucleotide comprising Long-Terminal Repeat (LTR) sequences at
the 3' end of the retroviral polynucleotide sequence, a promoter
sequence at the 5' end of the retroviral polynucleotide, said
promoter being suitable for expression in a mammalian cell, a gag
nucleic acid domain, a pol nucleic acid domain and an env nucleic
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
acid domain; a cassette comprising (i) a minimal internal ribosome
entry site (IRES), wherein the minimal IRES is operably linked to a
heterologous polynucleotide, (ii) a polIII promoter linked to an
miRNA or (iii) a mini-promoter operably linked to a heterologous
polynucleotide that is proceeds or follows (ii), wherein the
cassette is positioned 5' to the 3' LTR and 3' to the env nucleic
acid domain encoding the retroviral envelope; and cis-acting
sequences necessary for reverse transcription, packaging and
integration in a target cell. In one embodiment, the minimal IRES
consists of a sequence from about base 123 to 544 of SEQ ID NO:41.
In another embodiment of any of the foregoing, the minimum IRES
consists of a sequence from about base 183 to 544 of SEQ ID NO:41.
In another embodiment of any of the foregoing, the IRES has 6As in
the A bulge. In another embodiment of any of the foregoing, the
virus infects a target cell multiple times resulting in an average
number of copies/diploid genome of 5 or greater. In another
embodiment of any of the foregoing, the retroviral polynucleotide
sequence is derived from a virus selected from the group consisting
of murine leukemia virus (MLV), Moloney murine leukemia virus
(MoMLV), Feline leukemia virus (FeLV), Baboon endogenous retrovirus
(BEV), porcine endogenous virus (PERV), the cat derived retrovirus
RD114, squirrel monkey retrovirus, Xenotropic murine leukemia
virus-related virus(XMRV), avian reticuloendotheliosis virus (REV),
or Gibbon ape leukemia virus (GALV). In another embodiment of any
of the foregoing, the retroviral envelope is an amphotropic MLV
envelope. In another embodiment of any of the foregoing, the
retrovirus is a gammaretrovirus. In another embodiment of any of
the foregoing, the target cell is a cell having a cell
proliferative disorder. In another embodiment of any of the
foregoing, the target cell is a neoplastic cell. In another
embodiment of any of the foregoing, the cell proliferative disorder
is selected from the group consisting of lung cancer, colon-rectum
cancer, breast cancer, prostate cancer, urinary tract cancer,
uterine cancer, brain cancer, head and neck cancer, pancreatic
cancer, melanoma, stomach cancer and ovarian cancer, rheumatoid
arthritis or other autoimmune disease. In another embodiment of any
of the foregoing, the promoter sequence is associated with a growth
6
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
regulatory gene. In another embodiment of any of the foregoing, the
promoter sequence comprises a tissue-specific promoter sequence. In
another embodiment of any of the foregoing, the tissue-specific
promoter sequence comprises at least one androgen response element
(ARE). In another embodiment of any of the foregoing, the promoter
comprises a CMV promoter having a sequence as set forth in SEQ ID
NO:19, 20, 22 or 42 from nucleotide 1 to about nucleotide 582 and
may include modification to one or more nucleic acid bases and
which is capable of directing and initiating transcription. In
another embodiment of any of the foregoing, the promoter comprises
a CMV-R-U5 domain polynucleotide. In another embodiment of any of
the foregoing, the CMV-R-U5 domain comprises the immediately early
promoter from human cytomegalovirus linked to an MLV R-U5 region.
In another embodiment of any of the foregoing, the CMV-R-U5 domain
polynucleotide comprises a sequence as set forth in SEQ ID NO: 19,
20, 22 or 42 from about nucleotide 1 to about nucleotide 1202 or
sequences that are at least 95% identical to a sequence as set
forth in SEQ ID NO:19, 20, 22 or 42, wherein the polynucleotide
promotes transcription of a nucleic acid molecule operably linked
thereto. In another embodiment of any of the foregoing, the gag
polynucleotide is derived from a gammaretrovirus. In another
embodiment of any of the foregoing, the gag nucleic acid domain
comprises a sequence from about nucleotide number 1203 to about
nucleotide 2819 of SEQ ID NO: 19, 20, 22 or 42 or a sequence having
at least 95%, 98%, 99% or 99.8% identity thereto. In another
embodiment of any of the foregoing, the pol domain of the
polynucleotide is derived from a gammaretrovirus. In another
embodiment of any of the foregoing, the pol domain comprises a
sequence from about nucleotide number 2820 to about nucleotide 6358
of SEQ ID NO: 19, 20, 22 or 42 or a sequence having at least 95%,
98%, 99% or 99.9% identity thereto. In another embodiment of any
of the foregoing, the env domain comprises a sequence from about
nucleotide number 6359 to about nucleotide 8323 of SEQ ID NO: 19,
20, 22 or 42 or a sequence having at least 95%, 98%, 99% or 99.8%
identity thereto. In another embodiment of any of the foregoing,
the heterologous nucleic acid comprises a polynucleotide having a
sequence as set forth in SEQ ID NO:3, 5, 11, 13, 15 or 17. In
7
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
another embodiment of any of the foregoing, the heterologous
nucleic acid encodes a polypeptide comprising a sequence as set
forth in SEQ ID NO:4. In another embodiment of any of the
foregoing, the heterologous nucleic acid is human codon optimized
and encodes a polypeptide as set forth in SEQ ID NO:4. In another
embodiment of any of the foregoing, the heterologous nucleic acid
comprises a sequence as set forth in SEQ ID NO: 19 or 22 from about
nucleotide number 8877 to about 9353. In another embodiment of any
of the foregoing, the 3' LTR is derived from a gammaretrovirus. In
another embodiment of any of the foregoing, the 3' LTR comprises a
U3-R-U5 domain. In another embodiment of any of the foregoing, the
3' LTR comprises a sequence as set forth in SEQ ID NO: 19 or 22
from about nucleotide 9405 to about 9998 or a sequence that is at
least 95%, 98% or 99.5% identical thereto. In another embodiment of
any of the foregoing, the heterologous nucleic acid sequence
encodes a biological response modifier or an immunopotentiating
cytokine. In another embodiment of any of the foregoing, the
immunopotentiating cytokine is selected from the group consisting
of interleukins 1 through 15, interferon, tumor necrosis factor
(TNF), and granulocyte-macrophage-colony stimulating factor (GM-
CSF). In another embodiment of any of the foregoing, the
immunopotentiating cytokine is interferon gamma. In another
embodiment of any of the foregoing, the heterologous nucleic acid
encodes a polypeptide that converts a nontoxic prodrug in to a
toxic drug. In another embodiment of any of the foregoing,the
polypeptide that converts a nontoxic prodrug in to a toxic drug is
thymidine kinase, purine nucleoside phosphorylase (PNP), or
cytosine deaminase. In another embodiment of any of the foregoing,
the heterologous nucleic acid sequence encodes a receptor domain,
an antibody, or antibody fragment. In another embodiment of any of
the foregoing, the heterologous nucleic acid sequence comprises an
inhibitory polynucleotide. In another embodiment of any of the
foregoing, the inhibitory polynucleotide comprises an miRNA, RNAi
or siRNA sequence.
[0011] The details of one or more embodiments of the disclosure
are set forth in the accompanying drawings and the description
8
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
below. Other features, objects, and advantages will be apparent
from the description and drawings, and from the claims.
BRIEF DESCRIPTION OF DRAWINGS
[0012] Figure 1A-C shows replicating retroviral vectors containing
IRES with various numbers of A's in the A bulge and their titers.
(A) Predicted secondary structure of the EMCV internal ribosomal
entry site. The sequences start from position 680. Circled capital
letter J, K, L and M indicate defined region in the IRES. Arrow
indicates the bifurcation loop in the J-K region. AUG8, AUG9, AUG10
and AUG11 are underlined. (B) Diagram of the A bulge in the J-K
bifurcation region in EMCV IRES incorporated into RRV expressing
yCD2 or GFP. The native ATG8 (AUG in RNA) and ATG9 are underlined;
enlarged and underlined sequence represents the A bulge in the J-K
bifurcation region; lower case letters indicate the 5' sequences in
the polypyrimidine tract in the 3' IRES; (C) Viral titer of RRV
containing various numbers of As in the A bulge produced by
infected HT1080 cells.
[0013] Figure 2A-D shows cellular viral derived RNA and protein
expression by RRV with various numbers of A's in the A bulge. (A)
Schematic diagram of cellular viral RNA isoforms. Env2 primers and
probe, and yCD2 primers and probe recognize both unspliced and
spliced viral RNA in the env and the yCD2 region, respectively,
were used to measure the level of cellular viral RNA by qRT-PCR.
Filled triangles: env2 primer and probe set; open triangles: yCD2
primer and probe set. (B) Immunoblot of yCD2 and GAPDH protein.
Twenty micrograms of cell lysate were loaded to each lane and
equivalent loading and blotting efficiency controlled for by
detection of the ubiquitous marker GAPDH. PC, positive control; NC,
negative control. Graph represents the RNA and protein expression
levels relative to the yCD2-6A vector. (C) RNA and GFP expression
levels relative to the GFP-6A vector. The percentage GFP positive
cells were determined by flow cytometry using proper gating to
exclude GFP-negative cells. GFP protein expression levels were
quantified by using mean fluorescent intensity (D) Proviral vector
copy number of infected U87-MG cells (MOI of 0.01) by qPCR.
Genomic DNA is isolated day 14 post infection at which the vector
with 7A is expected to be maximally infected. The data show that
9
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
there is no significant difference in vector copy of number of
maximally infected U87-MG cells. This is consistent with viral
production data in which no significant effect on viral titer is
observed among the variants.
[0014] Figure 3 shows a vector sequence (SEQ ID NO:22) with an
A-bulge containing 7A's underlined and bolded.
[0015] Figure 4A-B shows vector stability data. (A) Vectors
stability in infected U87-MG cells (MOI of 0.01) by end-point PCR.
Genomic DNA is isolated day 14 post infection and the IRES-yCD2
region is amplified using the primer set spanning the 3' of the env
and 3'UTR region (Perez et al., 2012). (B) Assessment of vector
stability by serial infection. Approximately 105 naive U87-MG cells
were initially infected with the viral vectors at a MOI of 0.1 and
grown for 1 week to complete a single cycle of infection. 100 pL of
the 2 ml of viral supernatant from fully infected cells is used to
infect naive cells and repeated up to 12 cycles. Vector stability
of the IRES-yCD2 region is assessed by PCR amplification of the
integrated provirus from the infected cells. The expected PCR
product size is approximately 1.2 kb. The appearance of any bands
smaller than 1.2 kb indicate deletion in the IRES-yCD2 region.
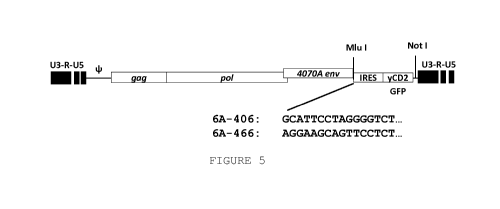
[0016] Figure 5 shows a diagram of a construct of the disclosure
designed with minimal IRESs (SEQ ID NO:41 from base 123-139; and
183 to 198).
DETAILED DESCRIPTION
[0017] As used herein and in the appended claims, the singular
forms "a," "and," and "the" include plural referents unless the
context clearly dictates otherwise. Thus, for example, reference
to "a cell" includes a plurality of such cells and reference to
"the agent" includes reference to one or more agents known to those
skilled in the art, and so forth.
[0018] Also, the use of "or" means "and/or" unless stated
otherwise. Similarly, "comprise," "comprises," "comprising"
"include," "includes," and "including" are interchangeable and not
intended to be limiting.
[0019] It is to be further understood that where descriptions
of various embodiments use the term "comprising," those skilled in
the art would understand that in some specific instances, an
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
embodiment can be alternatively described using language
"consisting essentially of" or "consisting of."
[0020] Unless defined otherwise, all technical and scientific
terms used herein have the same meaning as commonly understood to
one of ordinary skill in the art to which this disclosure belongs.
Although methods and materials similar or equivalent to those
described herein can be used in the practice of the disclosed
methods and compositions, the exemplary methods, devices and
materials are described herein.
[0021] General texts which describe molecular biological
techniques useful herein, including the use of vectors, promoters
and many other relevant topics, include Berger and Kimmel, Guide to
Molecular Cloning Techniques, Methods in Enzymology Volume 152,
(Academic Press, Inc., San Diego, Calif.) ("Berger"); Sambrook et
al., Molecular Cloning--A Laboratory Manual, 2d ed., Vol. 1-3, Cold
Spring Harbor Laboratory, Cold Spring Harbor, N.Y., 1989
("Sambrook") and Current Protocols in Molecular Biology, F. M.
Ausubel et al., eds., Current Protocols, a joint venture between
Greene Publishing Associates, Inc. and John Wiley & Sons, Inc.,
(supplemented through 1999) ("Ausubel"). Examples of protocols
sufficient to direct persons of skill through in vitro
amplification methods, including the polymerase chain reaction
(PCR), the ligase chain reaction (LCR), QP-replicase amplification
and other RNA polymerase mediated techniques (e.g., NASBA), e.g.,
for the production of the homologous nucleic acids of the
disclosure are found in Berger, Sambrook, and Ausubel, as well as
in Mullis et al. (1987) U.S. Pat. No. 4,683,202; Innis et al., eds.
(1990) PCR Protocols: A Guide to Methods and Applications (Academic
Press Inc. San Diego, Calif.) ("Innis"); Arnheim & Levinson (Oct.
1, 1990) C&EN 36-47; The Journal Of NIH Research (1991) 3: 81-94;
Kwoh et al. (1989) Proc. Natl. Acad. Sci. USA 86: 1173; Guatelli et
al. (1990) Proc. Nat'l. Acad. Sci. USA 87: 1874; Lomell et al.
(1989) J. Clin. Chem 35: 1826; Landegren et al. (1988) Science 241:
1077-1080; Van Brunt (1990) Biotechnology 8: 291-294; Wu and
Wallace (1989) Gene 4:560; Barringer et al. (1990) Gene 89:117; and
Sooknanan and Malek (1995) Biotechnology 13: 563-564. Improved
methods for cloning in vitro amplified nucleic acids are described
11
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
in Wallace et al., U.S. Pat. No. 5,426,039. Improved methods for
amplifying large nucleic acids by PCR are summarized in Cheng et
al. (1994) Nature 369: 684-685 and the references cited therein, in
which PCR amplicons of up to 40 kb are generated. One of skill will
appreciate that essentially any RNA can be converted into a double
stranded DNA suitable for restriction digestion, PCR expansion and
sequencing using reverse transcriptase and a polymerase. See, e.g.,
Ausubel, Sambrook and Berger, all supra.
[0022] The publications discussed throughout the text are
provided solely for their disclosure prior to the filing date of
the present application. Nothing herein is to be construed as an
admission that the inventors are not entitled to antedate such
disclosure by virtue of prior disclosure.
[0023] The disclosure provides methods and compositions useful
for gene or protein delivery to a cell or subject. Such methods
and compositions can be used to treat various diseases and
disorders in a subject including cancer and other cell
proliferative diseases and disorders. In one aspect, the
disclosure provides optimized IRESs. Such optimized IRESs can be
used in various vectors to facilitate protein expression. In
another aspect, the disclosure provides replication competent
retroviral vectors for gene delivery. The disclosure demonstrates
that commonly used IRESs containing 7A's in the A-bulge in the J-K
bifurcation region are not optimal and thus the disclosure provides
an IRES with an optimal A bulge sequence having improved
polypeptide expression compared to IRESs with fewer (3-5) or more
(7-8) As.
[0024] An internal ribosome entry sites ("IRES") refers to a
segment of nucleic acid that promotes the entry or retention of a
ribosome during translation of a coding sequence usually 3' to the
IRES. In some embodiments the IRES may comprise a splice
acceptor/donor site, however, preferred IRESs lack a splice
acceptor/donor site. Normally, the entry of ribosomes into
messenger RNA takes place via the cap located at the 5' end of all
eukaryotic mRNAs. However, there are exceptions to this universal
rule. The absence of a cap in some viral mRNAs suggests the
existence of alternative structures permitting the entry of
12
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
ribosomes at an internal site of these RNAs. To date, a number of
these structures, designated IRES on account of their function,
have been identified in the 5' noncoding region of uncapped viral
mRNAs, including, for example, that of picornaviruses such as
poliomyelitis virus (Pelletier et al., 1988, Mol. Cell. Biol., 8,
1103-1112) and the EMCV virus (encephalo-myocarditis virus) (Jang
et al., J. Virol., 1988, 62, 2636-2643). The disclosure provides
the use of an optimized IRES in the context of a vector and more
particularly a replication-competent retroviral (RCR) vector.
[0025] The internal ribosomal entry site (IRES) allows
translation of viral RNAs in a cap-independent manner. The IRES
from encephalomyocarditis virus (EMCV) has been studied extensively
and is widely used in retroviral and other mammalian expression
vectors. The proper folding and secondary structure of the IRES
dictate its functionality, and sequence changes may or may not
affect this. Palmenberg and coworkers showed that, independent of
the 5'-IRES region, the J-K elements in the 3' end of the IRES play
a critical role in translation initiation, (Figure 1A). The
sequence of the IRES in various vectors can be found to contain
various numbers of polyAs in the A-bulge. For example, Logg et al.
(J. Virol. 75:6989-6998, 2001) describes an IRES that carries seven
adenosine residues (As) instead of the six As in the A bulge in the
bifurcation region (see, e.g., Duke et al., J. Virol. 66:1924-1932,
1992). As described more fully elsewhere herein, the number of A's
in the A-bulge affects the expression of an operably associated
heterologous sequence. For example, the disclosure identifies an
optimal number of A's in the A-bulge as peaking at 6 A's and
expression decreasing slightly the further from the optimal number
of A's on either sides. For example, 4 A's is less effective than
6 A's and 8 A's is less effective than 6 A's.
[0026] As used herein an "optimized IRES" refers to an IRES
derived from an encephalomyocarditis virus having 6As in the A-
bulge of the J-K bifurcation region. The optimized IRES can be
part of a cassette that comprises a gene or sequence to be
expressed ("heterologous polynucleotide" or "gene"). In such
instances the optimized IRES is operably linked and upstream of the
heterologous polynucleotide sequence and is operably to cause
13
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
translation of the linked heterologous polynucleotide. The
optimized IRES cassette demonstrates increased protein expression
from a linked heterologous polynucleotide compared to a non-
optimized IRES (e.g., and IRES having 3-5 or 7-8 A's in the A-
bulge). An optimized IRES or IRES-cassette can be cloned into any
number of vectors for expression of a linked heterologous
polynucleotide. For example, vectors that can contain and be used
with an optimized IRES or IRES-cassette of the disclosure include
plasmids, expression vectors, viral vectors (replication defective
and replication competent) and the like.
[0027] In one embodiment, the disclosure provides an optimized
IRES comprising a sequence selected from the group consisting of:
(i) a sequence having 95% identity to SEQ ID NO:41 and having 6A's
in the J-K bifurcation region; (ii) a truncated IRES comprising a
sequence as set forth in SEQ ID NO:41 containing 6A's in the
bifurcation region and begins anywhere following base pair 1 to
about base 183 and continues to 544 of SEQ ID NO:41 (e.g., about
123 to 544 or about 183 to 544 of SEQ ID NO:41) and has improved
polypeptide expression compared to a similar IRES with 7As in the
bifurcation region; or ; (iii) a sequence as set forth in SEQ ID
NO:41 and (iv) any of the foregoing wherein T can be U (e.g., an
RNA version).
[0028] A heterologous nucleic acid sequence is operably linked
to an optimized IRES consisting of, in one embodiment, 6 "As" in
the A-bulge region. As used herein, the term "heterologous" nucleic
acid sequence or transgene refers to (i) a sequence that does not
normally exist in a wild-type retrovirus, (ii) a sequence that
originates from a foreign species, (iii) a sequence that is not
normally found downstream of an IRES, or (iv) if from the same
species, it may be substantially modified from its original form.
Alternatively, an unchanged nucleic acid sequence that is not
normally expressed in a cell is a heterologous nucleic acid
sequence.
[0029] In one embodiment, the disclosure provides a vector
comprising an optimized IRES in a cassette comprising an A-bulge in
the J-K bifurcation region consisting of 6As operably linked to a
polynucleotide sequence to be expressed. As described in more
14
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
detail below, an A-bulge consisting of 6A's unexpectedly provides
superior protein expression compared to similar IRES cassettes
containing 3-5 or 7-8 A's. As will be recognized, particularly in
gene delivery, protein expression from a recombinant vector is
important not only for in vitro protein production but also for
therapeutic protein production in vivo. For example, Logg et al.
(J. Virol. 75:6989-6998, 2001) describes an IRES that carries seven
adenosine residues (As) instead of the six A's in the A bulge in
the bifurcation region.
[0030] The optimized IRES cassette can be cloned into any number
of art recognized vectors. Such vectors are described below, but
include plasmids and viral vectors. For example, the disclosure
contemplates an optimized IRES of the disclosure cloned into an
expression vector wherein the optimized IRES is located just
upstream (e.g., 0 to about 50 bp upstream) of a heterologous
polynucleotide to be expressed. Of particular interest is the use
of replication competent gamma retroviral vectors that are capable
of infecting and spreading in mammalian tissue without the need for
recombinant receptors or helper cells. Such RCR vectors include
gamma retroviruses such as mo-MLV, MLV, GALV, FELV and the like. A
typical gamma retrovirus comprises LTRs, gag, poi and env gene, and
factors necessary for reverse transcription and integration into a
host genome (e.g., psi factors). Modifications of the typical
gamma retroviral vector have been performed for nearly 20 years
including generating replication incompetent vectors, vectors
carrying heterologous genes in various locations and vectors
containing IRES cassettes. For example, Kasahara et al. describes
the generation of a replication competent retroviral vector derived
from MLV in U.S. Patent No. 6,410,313 that carries an IRES cassette
downstream of the env gene and upstream of the 3' LTR. Gruber et
al. (U.S. Patent No. 8,722,867) describe a further optimized vector
comprising an IRES cassette just downstream of the env gene and
upstream of the 3'LTR. In Gruber et al. the IRES cassette shows an
A-bulge of 7As in the JK bifurcation region.
[0031] The disclosure provides, in one embodiment, a replication
competent gammaretroviral vector (RCR) comprising an optimized IRES
cassette just downstream of the env gene and upstream of the 3'
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
LTR, wherein the optimized IRES of the optimized IRES cassette
consists of an A-bulge in the bifurcation region of 6As. In a
further embodiment, the RCR has increased protein expression
compared to a vector containing an A-bulge having 3-5 or 7-8A's.
[0032] The disclosure provides vectors having an A-bulge in the J-
K bifurcation region consisting of 6A's compared to that found in
prior replication competent retroviral vectors (e.g., see U.S.
Patent Publ. Nos: 2011/0287020-A1; and 2011/0217267-A1, which show
7A's in the A-bulge, the disclosures of which are incorporated
herein by reference). Unexpectedly the change in a single A (i.e.,
7A's to 6A's) provides increased protein production compared to
that of 7A's. Thus, a vector comprising 6A's would have improved
protein expression of a heterologous gene linked to an IRES
cassette having a "6A" A-bulge.
[0033] The terms "vector", "vector construct" and "expression
vector" mean the vehicle by which a DNA or RNA sequence (e.g., a
foreign gene) can be introduced into a host cell, so as to
transform the host and promote expression (e.g., transcription and
translation) of the introduced sequence. Vectors typically comprise
the DNA or RNA of a transmissible agent, into which foreign DNA or
RNA encoding a protein is inserted by restriction enzyme
technology. A common type of vector is a "plasmid", which generally
is a self-contained molecule of double-stranded DNA that can
readily accept additional (foreign) DNA and which can readily
introduced into a suitable host cell. A large number of vectors,
including plasmid and fungal vectors, have been described for
replication and/or expression in a variety of eukaryotic and
prokaryotic hosts. Non-limiting examples include pKK plasmids
(Clonetech), pUC plasmids, pET plasmids (Novagen, Inc., Madison,
Wis.), pRSET or pREP plasmids (Invitrogen, San Diego, Calif.), or
pMAL plasmids (New England Biolabs, Beverly, Mass.), and many
appropriate host cells, using methods disclosed or cited herein or
otherwise known to those skilled in the relevant art. Recombinant
cloning vectors will often include one or more replication systems
for cloning or expression, one or more markers for selection in the
host, e.g., antibiotic resistance, and one or more expression
cassettes.
16
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
[0034] The terms "express" and "expression" mean allowing or
causing the information in a gene or DNA sequence to become
manifest, for example producing a protein by activating the
cellular functions involved in transcription and translation of a
corresponding gene, RNA or DNA sequence. A DNA or RNA sequence is
expressed in or by a cell to form an "expression product" such as a
protein. The expression product itself, e.g. the resulting protein,
may also be said to be "expressed" by the cell. A polynucleotide or
polypeptide is expressed recombinantly, for example, when it is
expressed or produced in a foreign host cell under the control of a
foreign or native promoter, or wherein a native gene in a native
host cell is expressed under the control of a foreign promoter.
[0035] The disclosure provides modified retroviral vectors.
The modified retroviral vectors can be derived from members of the
retroviridae family. The Retroviridae family consists of three
groups: the spumaviruses-(or foamy viruses) such as the human foamy
virus (HFV); the lentiviruses, as well as visna virus of sheep; and
the oncoviruses (although not all viruses within this group are
oncogenic). The term "lentivirus" is used in its conventional sense
to describe a genus of viruses containing reverse transcriptase.
The lentiviruses include the "immunodeficiency viruses" which
include human immunodeficiency virus (HIV) type 1 and type 2 (HIV-1
and HIV-2) and simian immunodeficiency virus (SIV). The oncoviruses
have historically been further subdivided into groups A, B, C and D
on the basis of particle morphology, as seen under the electron
microscope during viral maturation. A-type particles represent the
immature particles of the B- and D-type viruses seen in the
cytoplasm of infected cells. These particles are not infectious. B-
type particles bud as mature virion from the plasma membrane by the
enveloping of intracytoplasmic A-type particles. At the membrane
they possess a toroidal core of 75 nm, from which long glycoprotein
spikes project. After budding, B-type particles contain an
eccentrically located, electron-dense core. The prototype B-type
virus is mouse mammary tumor virus (MMTV). No intracytoplasmic
particles can be observed in cells infected by C-type viruses.
Instead, mature particles bud directly from the cell surface via a
crescent 'C'-shaped condensation which then closes on itself and is
17
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
enclosed by the plasma membrane. Envelope glycoprotein spikes may
be visible, along with a uniformly electron-dense core. Budding may
occur from the surface plasma membrane or directly into
intracellular vacuoles. The C-type viruses are the most commonly
studied and include many of the avian and murine leukemia viruses
(MLV). Bovine leukemia virus (BLV), and the human T-cell leukemia
virus types I and II (HTLV-I/II) are similarly classified as C-type
particles because of the morphology of their budding from the cell
surface. However, they also have a regular hexagonal morphology and
more complex genome structures than the prototypic C-type viruses
such as the murine leukemia viruses (MLV). D-type particles
resemble B-type particles in that they show as ring-like structures
in the infected cell cytoplasm, which bud from the cell surface,
but the virion incorporate short surface glycoprotein spikes. The
electron-dense cores are also eccentrically located within the
particles. Mason Pfizer monkey virus (MPMV) is the prototype D-type
virus.
[0036] Retroviruses have been classified in various ways but
the nomenclature has been standardized in the last decade (see
ICTVdB - The Universal Virus Database, v 4 on the World Wide Web
(www) at ncbi.nlm.nih.gov/ICTVdb/ICTVdB/ and the text book
"Retroviruses" Eds Coffin, Hughs and Varmus, Cold Spring Harbor
Press 1997; the disclosures of which are incorporated herein by
reference). In one embodiment, the replication competent
retroviral vector can comprise an Orthoretrovirus or more typically
a gamma retrovirus vector.
[0037] Retroviruses are defined by the way in which they
replicate their genetic material. During replication the RNA is
converted into DNA. Following infection of the cell a double-
stranded molecule of DNA is generated from the two molecules of RNA
which are carried in the viral particle by the molecular process
known as reverse transcription. The DNA form becomes covalently
integrated in the host cell genome as a provirus, from which viral
RNAs are expressed with the aid of cellular and/or viral factors.
The expressed viral RNAs are packaged into particles and released
as infectious virion.
18
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
[0038] The retrovirus particle is composed of two identical RNA
molecules. Each wild-type genome has a positive sense, single-
stranded RNA molecule, which is capped at the 5' end and
polyadenylated at the 3' tail. The diploid virus particle contains
the two RNA strands complexed with gag proteins, viral enzymes (pol
gene products) and host tRNA molecules within a 'core' structure of
gag proteins. Surrounding and protecting this capsid is a lipid
bilayer, derived from host cell membranes and containing viral
envelope (env) proteins. The env proteins bind to a cellular
receptor for the virus and the particle typically enters the host
cell via receptor-mediated endocytosis and/or membrane fusion.
[0039] After the outer envelope is shed, the viral RNA is
copied into DNA by reverse transcription. This is catalyzed by the
reverse transcriptase enzyme encoded by the pol region and uses the
host cell tRNA packaged into the virion as a primer for DNA
synthesis. In this way the RNA genome is converted into the more
complex DNA genome.
[0040] The double-stranded linear DNA produced by reverse
transcription may, or may not, have to be circularized in the
nucleus. The provirus now has two identical repeats at either end,
known as the long terminal repeats (LTR). The termini of the two
LTR sequences produces the site recognized by a pol product--the
integrase protein--which catalyzes integration, such that the
provirus is always joined to host DNA two base pairs (bp) from the
ends of the LTRs. A duplication of cellular sequences is seen at
the ends of both LTRs, reminiscent of the integration pattern of
transposable genetic elements. Retroviruses can integrate their
DNAs at many sites in host DNA, but different retroviruses have
different integration site preferences. HIV-1 and simian
immunodeficiency virus DNAs preferentially integrate into expressed
genes, murine leukemia virus (MLV) DNA preferentially integrates
near transcriptional start sites (TSSs), and avian sarcoma leukosis
virus (ASLV) and human T cell leukemia virus (HTLV) DNAs integrate
nearly randomly, showing a slight preference for genes (Derse D, et
al. (2007) Human T-cell leukemia virus type 1 integration target
sites in the human genome: comparison with those of other
retroviruses. J Virol 81:6731-6741; Lewinski MK, et al. (2006)
19
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
Retroviral DNA integration: viral and cellular determinants of
target-site selection. PLoS Pathog 2:e601).
[0041] Transcription, RNA splicing and translation of the
integrated viral DNA is mediated by host cell proteins. Variously
spliced transcripts are generated. In the case of the human
retroviruses HIV-1/2 and HTLV-I/II viral proteins are also used to
regulate gene expression. The interplay between cellular and viral
factors is a factor in the control of virus latency and the
temporal sequence in which viral genes are expressed.
[0042] Retroviruses can be transmitted horizontally and
vertically. Efficient infectious transmission of retroviruses
requires the expression on the target cell of receptors which
specifically recognize the viral envelope proteins, although
viruses may use receptor-independent, nonspecific routes of entry
at low efficiency. Normally a viral infection leads to a single or
few copies of viral genome per cell because of receptor masking or
down-regulation that in turn leads to resistance to superinfection
(Ch3 p104 in "Retroviruses", JM Coffin, SH Hughes, & HE Varmus 1997
Cold Spring Harbor Laboratory Press, Cold Spring Harbor NY; Fan et
al. J.Virol 28:802, 1978). In addition, the target cell type must
be able to support all stages of the replication cycle after virus
has bound and penetrated. Vertical transmission occurs when the
viral genome becomes integrated in the germ line of the host. The
provirus will then be passed from generation to generation as
though it were a cellular gene. Hence endogenous proviruses become
established which frequently lie latent, but which can become
activated when the host is exposed to appropriate agents.
[0043] In many situations for using a recombinant replication
competent retrovirus therapeutically, it is advantageous to have
high levels of expression of the transgene that is encoded by the
recombinant replication competent retrovirus. For example, with a
prodrug activating gene such as the cytosine deaminase gene it is
advantageous to have higher levels of expression of the CD protein
in a cell so that the conversion of the prodrug 5-FC to 5-FU is
more efficient. Similarly high levels of expression of siRNA or
shRNA lead to more efficient suppression of target gene expression.
Also for cytokines or single chain antibodies (scAbs) it is usually
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
advantageous to express high levels of the cytokine or scAb. In
addition, in the case that there are mutations in some copies of
the vector that inactivate or impair the activity of the vector or
transgene, it is advantageous to have multiple copies of the vector
in the target cell as this provides a high probability of efficient
expression of the intact transgene. The disclosure provides
recombinant replication competent retroviruses capable of infecting
a target cell or target cell population multiple times resulting in
an average number of copies/diploid genome of 5 or greater. The
disclosure also provides methods of testing for this property.
Also provided are methods of treating a cell proliferative
disorder, using a recombinant replication competent retrovirus
capable of infecting a target cell or target cell population
multiple times resulting in an average number of copies/diploid
genome of 5 or greater.
[0044] As mentioned above, the integrated DNA intermediate is
referred to as a provirus. Prior gene therapy or gene delivery
systems use methods and retroviruses that require transcription of
the provirus and assembly into infectious virus while in the
presence of an appropriate helper virus or in a cell line
containing appropriate sequences enabling encapsidation without
coincident production of a contaminating helper virus. As
described below, a helper virus is not required for the production
of the recombinant retrovirus of the disclosure, since the
sequences for encapsidation are provided in the genome thus
providing a replication competent retroviral vector for gene
delivery or therapy.
[0045] Other existing replication competent retroviral vectors
also tend to be unstable and lose sequences during horizontal or
vertical transmission to an infected cell or host cell and during
replication. This may be due in-part from the presence of extra
nucleotide sequences that include repeats or which reduce the
efficiency of a polymerase.
[0046] The retroviral genome and the proviral DNA of the
disclosure have at least three genes: the gag, the poi, and the
env, these genes may be flanked by one or two long terminal (LTR)
repeat, or in the provirus are flanked by two long terminal repeat
21
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
(LTR) and sequences containing cis-acting sequences such as psi.
The gag gene encodes the internal structural (matrix, capsid, and
nucleocapsid) proteins; the poi gene encodes the RNA-directed DNA
polymerase (reverse transcriptase), protease and integrase; and the
env gene encodes viral envelope glycoproteins. The 5' and/or 3'
LTRs serve to promote transcription and polyadenylation of the
virion RNAs. The LTR contains all other cis-acting sequences
necessary for viral replication. Lentiviruses have additional genes
including vif, vpr, tat, rev, vpu, nef, and vpx (in HIV-1, HIV-2
and/or SIV).
[0047] Adjacent to the 5' LTR are sequences necessary for
reverse transcription of the genome (the tRNA primer binding site)
and for efficient encapsidation of viral RNA into particles (the
Psi site). If the sequences necessary for encapsidation (or
packaging of retroviral RNA into infectious virion) are missing
from the viral genome, the result is a cis defect which prevents
encapsidation of genomic viral RNA. This type of modified vector is
what has typically been used in prior gene delivery systems (i.e.,
systems lacking elements which are required for encapsidation of
the virion) as 'helper' elements providing viral proteins in trans
that package a non-replicating, but packageable, RNA genome.
[0048] The disclosure provides vectors that contain an
optimized IRES. The optimized IRES is typically linked to a
heterologous polynucleotide encoding, for example, a cytosine
deaminase or mutant thereof, a thymidine kinase or mutant thereof,
an miRNA or siRNA, a cytokine, an antibody binding domain etc.,
that can be delivered to a cell or subject. In one embodiment, the
vector is a viral vector. The viral vector can be an adenoviral
vector, a measles vector, a herpes vector, a retroviral vector
(including a lentiviral vector), a rhabdoviral vector such as a
Vesicular Stomatitis viral vector, a reovirus vector, a Seneca
Valley Virus vector, a poxvirus vector (including animal pox or
vaccinia derived vectors), a parvovirus vector (including an AAV
vector), an alphavirus vector or other viral vector known to one
skilled in the art (see also, e.g., Concepts in Genetic Medicine,
ed. Boro Dropulic and Barrie Carter, Wiley, 2008, Hoboken, NJ.; The
Development of Human Gene Therapy, ed. Theodore Friedmann, Cold
22
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
Springs Harbor Laboratory Press, Cold springs Harbor, New York,
1999; Gene and Cell Therapy, ed. Nancy Smyth Templeton, Marcel
Dekker Inc., New York, New York, 2000 and Gene Therapy: Therapeutic
Mechanism and Strategies, ed. Nancy Smyth Templetone and Danilo D
Lasic, Marcel Dekker, Inc., New York, New York, 2000; the
disclosures of which are incorporated herein by reference).
[0049] In one embodiment, the retroviral genome of the
disclosure contains an optimized IRES comprising a cloning site
downstream of the optimized IRES for insertion of a
desired/heterologous polynucleotide. In one embodiment, the
optimized IRES is located 3' to the env gene in a retroviral
vector, but 5' to the desired heterologous polynucleotide and 5' to
the 3' LTR. In all of the foregoing embodiments, the optimized IRES
comprises an A-bulge with 6A's. A heterologous polynucleotide
encoding a desired polypeptide may be operably linked to the
optimized IRES.
[0050] In one embodiment, the viral vector can be a replication
competent retroviral vector obtained or derived from a
gammaretrovirus capable of infecting replicating mammalian cells.
The replication competent retroviral vector comprises an optimized
internal ribosomal entry site (IRES) comprising an A-bulge
consisting of 6 A's located 5' to a heterologous polynucleotide
encoding, e.g., a cytosine deaminase (SEQ ID NO:3), thymidine
kinase (SEQ ID NO:37), miRNA, siRNA, cytokine, receptor, antibody
or the like. When the heterologous polynucleotide encodes a non¨
translated RNA such as siRNA, miRNA or RNAi then an IRES is not
necessary, but may be included for another translated
polynucleotide. In one embodiment, an optimized IRES cassette
containing the heterologous polynucleotide is 3' to a ENV
polynucleotide of a retroviral vector, but 5' to the 3' LTR. In
one embodiment the viral vector is a retroviral vector capable of
infecting target cells multiple times (e.g., 5 or more per diploid
cell).
[0051] The disclosure provides replication competent retroviral
vectors having increased stability relative to prior retroviral
vectors and containing an optimized IRES having 6A's in the A-
bulge. Such increased stability during infection and replication
23
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
is important for the treatment of cell proliferative disorders. In
addition, the increased protein expression from the optimized A-
bulge provides additional delivery of therapeutic proteins to a
target cell/tissue. The combination of transduction efficiency,
transgene stability, transgene expression and target selectivity is
provided by the replication competent retrovirus. The compositions
and methods provide insert stability and maintain transcription
activity of the transgene and the translational viability of the
encoded polypeptide.
[0052] Depending upon the intended use of a vector or the
retroviral vector of the disclosure any number of heterologous
polynucleotide or nucleic acid sequences may be inserted into the
vector or retroviral vector. For example, for in vitro studies
commonly used marker genes or reporter genes may be used,
including, antibiotic resistance and fluorescent molecules (e.g.,
GFP). Additional polynucleotide sequences encoding any desired
polypeptide sequence may also be inserted into the vector of the
disclosure. Where in vivo delivery of a heterologous nucleic acid
sequence is sought both therapeutic and non-therapeutic sequences
may be used. For example, the heterologous sequence can encode a
therapeutic molecule including antisense molecules (miRNA, siRNA)
or ribozymes directed to a particular gene associated with a cell
proliferative disorder or other gene-associated disease or
disorder, the heterologous sequence can be a suicide gene (e.g.,
HSV-tk or PNP or cytosine deaminase; either modified or unmodified,
humanized or non-humanized), a growth factor or a therapeutic
protein (e.g., Factor IX, IL2, and the like). Other therapeutic
proteins applicable to the disclosure are easily identified in the
art.
[0053] In one embodiment, the heterologous polynucleotide within
the vector comprises a cytosine deaminase that has been optimized
for expression in a human cell. In a further embodiment, the
cytosine deaminase comprises a sequence that has been human codon
optimized and comprises mutations that increase the cytosine
deaminase's stability (e.g., reduced degradation or increased
thermo-stability) compared to a wild-type cytosine deaminase (see,
e.g., SEQ ID NO:4). In yet another embodiment, the heterologous
24
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
polynucleotide encodes a fusion construct comprising a cytosine
deaminase (either human codon optimized or non-optimized, either
mutated or non-mutated) operably linked to a polynucleotide
encoding a polypeptide having UPRT or OPRT activity (see, e.g., SEQ
ID NO:11, 13, 15 and 17). Examples of such polypeptides having
cytosine deaminase and polynucleotides encoding such polypeptides
can be found in International Publication No. WO 2010/045002, which
is incorporated herein by reference.
[0054] In another embodiment, a vector or replication competent
retroviral vector can comprise a heterologous polynucleotide
encoding a polypeptide comprising a cytosine deaminase (as
described herein) and may further comprise a polynucleotide
comprising a miRNA or siRNA molecule either as part of the primary
transcript from the viral promoter or linked to a promoter, which
can be cell-type or tissue specific.
[0055] In yet further embodiments, the heterologous
polynucleotide may comprise a cytokine such as an interleukin,
interferon gamma or the like. Cytokines that may expressed from a
retroviral vector of the disclosure include, but are not limited
to, IL-1alpha, IL-1beta, IL-2 (SEQ ID NO:40), IL-3, IL-4, IL-5, IL-
6, IL-7, IL-8, IL-9, IL-10, IL-11, IL-12, IL-13, IL-14, IL-15, IL-
16, IL-17, IL-18, IL-19, IL-20, and IL-21, anti-CD40, CD4OL, IFN-
gamma (human - SEQ ID NO:38; mouse - SEQ ID NO:39) and TNF-alpha,
soluble forms of TNF-alpha, lymphotoxin-alpha (LT-alpha, also known
as TNF-beta), LT-beta (found in complex heterotrimer LT-alpha2-
beta), OPGL, FasL, CD27L, CD3OL, CD4OL, 4-1BBL, DcR3, OX4OL, TNF-
gamma (International Publication No. WO 96/14328), AIM-I
(International Publication No. WO 97/33899), endokine-alpha
(International Publication No. WO 98/07880), OPG, and neutrokine-
alpha (International Publication No. WO 98/18921, 0X40, and nerve
growth factor (NGF), and soluble forms of Fas, CD30, CD27, CD40 and
4-IBB, TR2 (International Publication No. WO 96/34095), DR3
(International Publication No. WO 97/33904), DR4 (International
Publication No. WO 98/32856), TR5 (International Publication No. WO
98/30693), TRANK, TR9 (International Publication No. WO 98/56892),
TRIO (International Publication No. WO 98/54202), 312C2
(International Publication No. WO 98/06842), and TR12, and soluble
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
forms CD154, CD70, and CD153. Angiogenic proteins may be useful in
some embodiments, particularly for protein production from cell
lines. Such angiogenic factors include, but are not limited to,
Glioma Derived Growth Factor (GDGF), Platelet Derived Growth
Factor-A (PDGF-A), Platelet Derived Growth Factor-B (PDGF-B),
Placental Growth Factor (PIGF), Placental Growth Factor-2 (PIGF-2),
Vascular Endothelial Growth Factor (VEGF), Vascular Endothelial
Growth Factor-A (VEGF-A), Vascular Endothelial Growth Factor-2
(VEGF-2), Vascular Endothelial Growth Factor B (VEGF-3), Vascular
Endothelial Growth Factor B-1 86 (VEGF-B186), Vascular Endothelial
Growth Factor-D (VEGF-D), Vascular Endothelial Growth Factor-D
(VEGF-D), and Vascular Endothelial Growth Factor-E (VEGF-E).
Fibroblast Growth Factors may be delivered by a vector of the
disclosure and include, but are not limited to, FGF-1, FGF-2, FGF-
3, FGF-4, FGF-5, FGF-6, FGF-7, FGF-8, FGF-9, FGF-10, FGF-11, FGF-
12, FGF-13, FGF-14, and FGF-15. Hematopoietic growth factors may
be delivered using vectors of the disclosure, such growth factors
include, but are not limited to, granulocyte macrophage colony
stimulating factor (GM-CSF) (sargramostim), granulocyte colony
stimulating factor (G-CSF) (filgrastim), macrophage colony
stimulating factor (M-CSF, CSF-1) erythropoietin (epoetin alfa),
stem cell factor (SCF, c-kit ligand, steel factor), megakaryocyte
colony stimulating factor, PIXY321 (a GMCSF/IL-3) fusion protein
and the like.
[0056] MicroRNAs (miRNA) are small, non-coding RNAs. They are
located within introns of coding or non-coding gene, exons of non-
coding genes or in inter-genic regions. miRNA genes are
transcribed by RNA polymerase II that generate precursor
polynucleotides called primary precursor miRNA (pri-miRNA). The
pri-miRNA in the nucleus is processed by the ribonuclease Drosha to
produce the miRNA precursor (pre-miRNA) that forms a short hairpin
structure. Subsequently, pre-miRNA is transported to the cytoplasm
via Exportin 5 and further processed by another ribonuclease called
Dicer to generate an active, mature miRNA.
[0057] A mature miRNA is approximately 21 nucleotides in
length. It exerts in function by binding to the 3' untranslated
region of mRNA of targeted genes and suppressing protein expression
26
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
either by repression of protein translation or degradation of mRNA.
miRNA are involved in biological processes including development,
cell proliferation, differentiation and cancer progression.
Studies of miRNA profiling indicate that some miRNA expressions are
tissue specific or enriched in certain tissues. For example, miR-
142-3p, miR-181 and miR-223 expressions have demonstrated to be
enriched in hematopoietic tissues in human and mouse (Baskerville
et al., 2005 RNA 11, 241-247; Chen et al., 2004 Science 303, 83-
86). The target sequence of miR-142-3p is shown in SEQ ID NO:35.
The target of miR-142-3p4X is shown in SEQ ID NO:36.
[0058] Some miRNAs have been observed to be up-regulated
(oncogenic miRNA) or down-regulated (repressor)in several tumors
(Spizzo et al., 2009 Cell 137, 586e1). For example, miR-21 is
overexpressed in glioblastoma, breast, lung, prostate, colon,
stomach, esophageal, and cervical cancer, uterine leiomyosarcoma,
DLBCL, head and neck cancer. In contrast, members of let-7 have
reported to be down-regulated in glioblastoma, lung, breast,
gastric, ovary, prostate and colon cancers. Re-establishment of
homeostasis of miRNA expression in cancer is an imperative
mechanism to inhibit or reverse cancer progression.
[0059] As a consequence of the vital functions modulated by
miRNAs in cancers, focus in developing potential therapeutic
approaches has been directed toward antisense-mediated inhibition
(antigomers) of oncogenic miRNAs. However, miRNA replacement might
represent an equally efficacious strategy. In this approach, the
most therapeutically useful miRNAs are the ones expressed at low
levels in tumors but at high level, and therefore tolerated, in
normal tissues.
[0060] miRNAs that are down-regulated in cancers could be
useful as anticancer agents. Examples include mir-128-1/2 (SEQ ID
NO:31 and 32 respectively), let-7, miR-26, miR-124, and miR-137
(Esquela-Kerscher et al., 2008 Cell Cycle 7, 759-764; Kumar et al.,
2008 Proc Natl Acad Sci USA 105, 3903-3908; Kota et al., 2009
Cell 137, 1005-1017; Silber et al., 2008 BMC Medicine 6:14 1-17).
miR-128 expression has reported to be enriched in the central
nervous system and has been observed to be down-regulated in
glioblastomas (Sempere et al., 2004 Genome Biology 5:R13.5-11;
27
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
Godlewski et al., 2008 Cancer Res 68: (22) 9125-9130). miR-128 is
encoded by two distinct genes, miR-128-1 and miR-128-2. Both are
processed into identical mature sequence. Bmi-i and E2F3a have
been reported to be the direct targets of miR-128 (Godlewski et
al., 2008 Cancer Res 68:(22) 9125-9130; Zhang et al., 2009 J. Mol
Med 87:43-51). In addition, Bmi-1 expression has been observed to
be up-regulated in a variety of human cancers, including gliomas,
mantle cell lymphomas, non-small cell lung cancer B-cell non-
Hodgkin's lymphoma, breast, colorectal and prostate cancer.
Furthermore, Bmi-1 has been demonstrated to be required for the
self-renewal of stem cells from diverse tissues, including neuronal
stem cells as well as "stem-like" cell population in gliomas.
[0061] Although there have been a number of in vitro
demonstrations of the possibilities of miRNA mediated inhibition of
cellular function, it has been difficult to deliver these as
oligonucleotides or in viral vectors as efficiently as necessary to
have in vivo effects (e.g., Li et al., Cell Cycle 5:2103-2109
2006), as has been true for other molecules.
[0062] Replication-defective retroviral and lentiviral vectors
have been used to stably express pri-miRNA by a polymerase II
promoter such as CMV or LTR and demonstrated production of mature
miRNA. The, incorporation of type III RNA polymerase III promoters
such as the U6 and the H1 promoter in non-replicative retroviral
and lentiviral vectors has been used widely to express functional
small interference RNA (siRNA) producing a short hairpin structured
RNA (Bromberg-White et al., 2004 J Virol 78:9, 4914-4916; Silva et
al., 2006 Virology 351, 218-225; Haga et al., 2006, Transplant Proc
38(10):3184-8). The loop sequence is cleaved by Dicer producing
the mature siRNAs that are 21-22 nucleotides in length. shRNA can
be stably expressed in cells to down-regulate target gene
expression. SEQ ID NO:33 and 34 comprise a pre-miR-128 linked to
an H1 promoter.
[0063] In another embodiment, an optimized IRES comprising 6A's in
the A-bulge can be used in combination with a core promoter,
wherein an optimized IRES is operably linked to a first
heterologous coding sequence and the core promoter or minipromoter
is linked to a second heterologous coding sequence or an siRNA,
28
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
miRNA, or shRNA sequence (see, e.g., WO 2014/066700, incorporated
herein by reference).
[0064] As used herein, a "core promoter" refers to a minimal
promoter comprising about 50-100 bp and lacks enhancer elements.
Such core promoters include, but are not limited to, SCP1, AdML and
CMV core promoters. More particularly, where a core-promoter
cassette is present a second cassette (e.g., a second mini-promoter
cassette, a polIII promoter cassette or IRES cassette) will be
present. In some embodiments, a vector comprising a cassette with
a core promoter specifically excludes the use of SCP1, AdML and CMV
core promoters, but rather utilize designer core promoters as
described further herein and below.
[0065] Core promoters include certain viral promoters. Viral
promoters, as used herein, are promoters that have a core sequence
but also usually some further accessory elements. For example, the
early promoter for SV40 contains three types of elements: a TATA
box, an initiation site and a GC repeat (Barrera-Saldana et al.,
EMBO J, 4:3839-3849, 1985; Yaniv, Virology, 384:369-374, 2009). The
TATA box is located approximately 20 base-pairs upstream from the
transcriptional start site. The GC repeat regions is a 21 base-pair
repeat containing six GC boxes and is the site that determines the
direction of transcription. This core promoter sequence is around
100 bp. Adding an additional 72 base-pair repeats, thus making it
a "mini-promoter," is useful as a transcriptional enhancer that
increase the functionality of the promoter by a factor of about 10.
When the SP1 protein interacts with the 21 bp repeats it binds
either the first or the last three GC boxes. Binding of the first
three initiates early expression, and binding of the last three
initiates late expression. The function of the 72 bp repeats is to
enhance the amount of stable RNA and increase the rate of
synthesis. This is done by binding (dimerization) with the AP1
(activator protein 1) to give a primary transcript that is 3'
polyadenylated and 5' capped. Other viral promoters, such as the
Rous Sarcoma Virus (RSV), the HBV X gene promoter, and the Herpes
Thymidine kinase core promoter can also be used as the basis for
selection desired function.
29
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
[0 0 6 6] A core promoter typically encompasses -40 to +40
relative to the +1 transcription start site (Juven-Gershon and
Kadonaga, Dev. Biol. 339:225-229, 2010), which defines the location
at which the RNA polymerase II machinery initiates transcription.
Typically, RNA polymerase II interacts with a number of
transcription factors that bind to DNA motifs in the promoter.
These factors are commonly known as "general" or "basal"
transcriptions factors and include, but are not limited to, TFIIA
(transcription factor for RNA polymerase IA), TFIIB, TFIID, TFIIE,
TFIIF, and TFIIH. These factors act in a "general" manner with all
core promoters; hence they are often referred to as the "basal"
transcription factors.
[0067] Juven-Gershon et al., (Nat. Methods, 3(11):917-922,
2006), describe elements of core promoters. For example, the
pRC/CMV core promoter consists of a TATA box and is 81 bp in
length; the CMV core promoter consists of a TATA box and a
initiator site; while the SCP synthetic core promoters (SCP1 and
SCP2) consist of a TATA box, an Inr (initiator), an MTE site (Motif
Ten Element), and a DPE site (Downstream promoter element) and is
about 81 bp in length. The SCP synthetic promoter has improved
expression compared to the simple pRC/CMV core promoter.
[0068] As used herein a "mini-promoter" or "small promoter"
refers to a regulatory domain that promotes transcription of an
operably linked gene or coding nucleic acid sequence. The mini-
promoter, as the name implies, includes the minimal amount of
elements necessary for effective transcription and/or translation
of an operably linked coding sequence. A mini-promoter can
comprise a "core promoter" in combination with additional
regulatory elements or a "modified core promoter". Typically, the
mini-promoter or modified core promoter will be about 100-600 bp in
length while a core promoter is typically less than about 100bp
(e.g., about 70-80 bp). In other embodiments, where a core
promoter is present, the cassette will typically comprise an
enhancer element or another element either upstream or downstream
of the core promoter sequence that facilitates expression of an
operably linked coding sequence above the expression levels of the
core promoter alone.
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
[0 0 6 9] Accordingly, the disclosure provides mini-promoters
(e.g., modified core promoters) derived from cellular elements as
determined for "core promoter" elements (<100, <200, <400 or <600
bp) that allow ubiquitous expression at significant levels in
target cells and are useful for stable incorporation into vectors,
in general, and replicating retroviral vectors, in particular, to
allow efficient expression of transgenes. Also provided are mini-
promoters comprising core promoters plus minimal enhancer sequences
and/or Kozak sequences to allow better gene expression compared to
a core-promoter lacking such sequences that are still under 200,
400 or 600bp. Such mini-promoters include modified core promoters
and naturally occurring tissue specific promoters such as the
elastin promoter (specific for pancreatic acinar cells, (204 bp;
Hammer et al., Mol Cell Biol., 7:2956-2967, 1987) and the promoter
from the cell cycle dependent ASK gene from mouse and man (63-380
bp; Yamada et al., J. Biol. Chem., 277: 27668-27681, 2002).
Ubiquitously expressed small promoters also include viral promoters
such as the 5V40 early and late promoters (about 340 bp), the RSV
LTR promoter (about 270 bp) and the HBV X gene promoter (about 180
bp) (e.g., R Anish et al., PLoS One, 4: 5103, 2009) that has no
canonical "TATTAA box" and has a 13 bp core sequence of 5'-
CCCCGTTGCCCGG-3' (SEQ ID NO:43). In yet other embodiments, the
therapeutic cassette comprising at least one mini-promoter cassette
will have expression levels that exceed, are about equal to, or
about about 1 fold to 2.5 fold less than the expression levels of
an IRES cassette present in an RRV.
[0070] Transcription from a core- or mini-promoter occurs
through the interaction of various elements. In focused
transcription, for example, there is either a single major
transcription start site or several start sites within a narrow
region of several nucleotides. Focused transcription is the
predominant mode of transcription in simpler organisms. In
dispersed transcription, there are several weak transcription start
sites over a broad region of about 50 to 100 nucleotides. Dispersed
transcription is the most common mode of transcription in
vertebrates. For instance, dispersed transcription is observed in
about two-thirds of human genes. In vertebrates, focused
31
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
transcription tends to be associated with regulated promoters,
whereas dispersed transcription is typically observed in
constitutive promoters in CpG islands.
[0071] Table 1: Binding sites that can contribute to a focused
core promoter (almost always with a "TATA box and a single
transcription start site (TSS)), or a dispersed promoter without a
TATA box, usually with a DPE element (see R. Dickstein,
Trasncription, 2(5):201-206, 2011; Juven-Gershon et al., Nat.
Methods, 2006, supra). Symbols for nucleotides follow the
international convention (world wide web:
chem.qmul.ac.uk/iubmb/misc/naseq.html).
Transcription Full name Binding site wrt to transcription start site
(TSS +1)
factor
BREu TFIIB recognition element, Upstream of TATA Box, SSRCGCC
upstream
TATA box TATA box T at -31/-30 TATAWAAR, key focused promoter
element
BREd TFIIB recognition element, -23 to -17 RTDKKKK
downstream
XCPE1 HBV X core promoter -8 to +2 DSGYGGRASM from HBV Xgene
element 1
XCPE2 HBV X core promoter VCYCRTTRCMY from HBV Xgene
element 2
Inr initiator -2 to +4 YYANWYY
DOE SI Downstream core element +6 to +11 CTTC
site 1
DOE SII Downstream core element +16 to +21 CTGT
site II
DOE SIII Downstream core element +30 to +34 AGO
site III
MTE Motif ten element +18 to +27 CSARCSSAAC mostly in Drosophila
DPE Downstream promoter +28 to +33 RGWYVT common in Drosophila,
key
element dispersed promoter element
[0072] Table 2 sets forth oligonucleotides that can be used to
construct and clone enhancer elements into core promoter regions.
As mentioned above, the modified/optimized core promoters of the
disclosure can include a core sequence with the addition of
elements from Table 1 and may further include enhancers cloned as
set forth in Table 2. In doing so, the size of the mini-promoter
may be increased. However, the final mini-promoter should not
exceed 600 bp and will typically be about 100 bp, 200 bp, 300 bp,
400 bp, 500 bp and any integer there between.
[0073] Table 2. Oligonucleotides Used for Constructing
Enhancer segments.
32
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
No. Oligonucleotide Motif Sequence Reference
1 AP-1 5'-TGTCTCAG-3' Hallahanet al. Int. J. Radiat.
Oncol.
Biol. Phys. 36:355-3601996.
2 CArG 5'-CCATATAAGG-3' Datta et al. Proc. Natl. Acad. Sci.
USA 89:10149-10153. 1992
3 NF-KB1 5'-GGAAAT0000-3' Ueda et al. FEBS Lett. 491:40-44
2001
4 NF-KB2 5'-GGAAAGTCCCC-3' Kanno etal. EMBO J. 8:4205-4214
1989
NF-KB3 5'-GGAGTTCCC-3' Hong etal. J. Biol.Chem.
275:18022-18028 2000.
6 NF-Y 5'-CATTGGG-3' Hu etal. J. Biol. Chem. 275:2979-
2985 2000.
AP-1, activating protein-1; NF-K13, nuclear factor KB.
[0074] In one embodiment, the disclosure provides a recombinant
replication competent retrovirus capable of infecting a non-
dividing host cell, a host dividing cell, or a host cell having a
cell proliferative disorder. The recombinant replication competent
retrovirus of the disclosure comprises a polynucleotide sequence
encoding a viral GAG, a viral POL, a viral ENV, a heterologous
polynucleotide preceded by an optimized internal ribosome entry
site (TRES) having 6 A's in the A-bulge of the TRES encapsulated
within a virion.
[0075] Generally, the recombinant vector of the disclosure is
capable of transferring a nucleic acid sequence into a target cell.
The phrase "non-dividing" cell refers to a cell that does not go
through mitosis. Non-dividing cells may be blocked at any point in
the cell cycle, (e.g., Go/G1, Gi/s, G2/M), as long as the cell is not
actively dividing. For ex vivo infection, a dividing cell can be
treated to block cell division by standard techniques used by those
of skill in the art, including, irradiation, aphidocolin treatment,
serum starvation, and contact inhibition. However, it should be
understood that ex vivo infection is often performed without
blocking the cells since many cells are already arrested (e.g.,
stem cells). For example, a recombinant lentivirus vector is
capable of infecting non-dividing cells. Examples of pre-existing
non-dividing cells in the body include neuronal, muscle, liver,
skin, heart, lung, and bone marrow cells, and their derivatives.
For dividing cells onco-retroviral vectors can be used.
[0076] By "dividing" cell is meant a cell that undergoes active
mitosis, or meiosis. Such dividing cells include stem cells, skin
cells (e.g., fibroblasts and keratinocytes), gametes, and other
33
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
dividing cells known in the art. Of particular interest and
encompassed by the term dividing cell are cells having cell
proliferative disorders, such as neoplastic cells. The term "cell
proliferative disorder" refers to a condition characterized by an
abnormal number of cells. The condition can include both
hypertrophic (the continual multiplication of cells resulting in an
overgrowth of a cell population within a tissue) and hypotrophic (a
lack or deficiency of cells within a tissue) cell growth or an
excessive influx or migration of cells into an area of a body. The
cell populations are not necessarily transformed, tumorigenic or
malignant cells, but can include normal cells as well. Cell
proliferative disorders include disorders associated with an
overgrowth of connective tissues, such as various fibrotic
conditions, including scleroderma, arthritis and liver cirrhosis.
Cell proliferative disorders include neoplastic disorders such as
head and neck carcinomas. Head and neck carcinomas would include,
for example, carcinoma of the mouth, esophagus, throat, larynx,
thyroid gland, tongue, lips, salivary glands, nose, paranasal
sinuses, nasopharynx, superior nasal vault and sinus tumors,
esthesioneuroblastoma, squamous cell cancer, malignant melanoma,
sinonasal undifferentiated carcinoma (SNUC), brain (including
glioblastomas) or blood neoplasia. Also included are carcinoma's of
the regional lymph nodes including cervical lymph nodes,
prelaryngeal lymph nodes, pulmonary juxtaesophageal lymph nodes and
submandibular lymph nodes (Harrison's Principles of Internal
Medicine (eds., Isselbacher, et al., McGraw-Hill, Inc., 13th
Edition, pp1850-1853, 1994). Other cancer types, include, but are
not limited to, lung cancer, colon-rectum cancer, breast cancer,
prostate cancer, urinary tract cancer, uterine cancer lymphoma,
oral cancer, pancreatic cancer, leukemia, melanoma, stomach cancer,
skin cancer and ovarian cancer. The cell proliferative disease also
includes rheumatoid arthritis (O'Dell NEJM 350:2591 2004)and other
auto-immune disorders (Mackay et al NEJM 345:340 2001) that are
often characterized by inappropriate proliferation of cells of the
immune system.
[0077] In other embodiments, host cells transfected with a
replication competent retroviral vector of the disclosure are
34
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
provided. Host cells include eukaryotic cells such as yeast cells,
insect cells, or animal cells. Host cells also include prokaryotic
cells such as bacterial cells. In other embodiments, the host cells
have been modified or selected to be continuously grown in serum
free suspension (see, e.g., U.S. Patent Publ. No. 2012/0087894-A1,
which is incorporated herein by reference).
[0078] Also provided are engineered host cells that are
transduced (transformed or transfected) with a vector provided
herein (e.g., a replication competent retroviral vector). The
engineered host cells can be cultured in conventional nutrient
media modified as appropriate for activating promoters, selecting
transformants, or amplifying a coding polynucleotide. Culture
conditions, such as temperature, pH and the like, are those
previously used with the host cell selected for expression, and
will be apparent to those skilled in the art and in the references
cited herein, including, e.g., Sambrook, Ausubel and Berger, as
well as e.g., Freshney (1994) Culture of Animal Cells: A Manual of
Basic Technique, 3rd ed. (Wiley-Liss, New York) and the references
cited therein.
[0079] Examples of appropriate expression hosts include:
mammalian cells such as CHO, COS, BHK, HEK 293 br Bowes melanoma
etc. Typically human cells or cell lines will be used; however, it
may be desirable to clone vectors and polynucleotides of the
disclosure into non-human host cells for purposes of sequencing,
amplification and cloning.
[0080] In another embodiment, a targeting polynucleotide
sequence is included as part of a recombinant retroviral vector of
the disclosure. The targeting polynucleotide sequence is a
targeting ligand (e.g., peptide hormones such as heregulin, a
single-chain antibody, a receptor or a ligand for a receptor), a
tissue-specific or cell-type specific regulatory element (e.g., a
tissue-specific or cell-type specific promoter or enhancer), or a
combination of a targeting ligand and a tissue-specific/cell-type
specific regulatory element. The targeting ligand is operably
linked to the env protein of the retrovirus, creating a chimeric
retroviral env protein. The viral GAG, viral POL and viral ENV
proteins can be derived from any suitable retrovirus (e.g., MLV or
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
lentivirus-derived). In another embodiment, the viral ENV protein
is non-retrovirus-derived (e.g., CMV or VSV).
[0081] In one embodiment, the retroviral vector is targeted to
the cell by binding to cells having a molecule on the external
surface of the cell. This method of targeting the retrovirus
utilizes expression of a targeting ligand on the coat of the
retrovirus to assist in targeting the virus to cells or tissues
that have a receptor or binding molecule which interacts with the
targeting ligand on the surface of the retrovirus. After infection
of a cell by the virus, the virus injects its nucleic acid into the
cell and the retrovirus genetic material can integrate into the
host cell genome.
[0082] Thus, the disclosure includes in one embodiment, a
chimeric env protein comprising a retroviral ENV protein operably
linked to a targeting polypeptide. The targeting polypeptide can be
a cell specific receptor molecule, a ligand for a cell specific
receptor, an antibody or antibody fragment to a cell specific
antigenic epitope or any other ligand easily identified in the art
which is capable of binding or interacting with a target cell.
Examples of targeting polypeptides or molecules include bivalent
antibodies using biotin-streptavidin as linkers (Etienne-Julan et
al., J. Of General Virol., 73, 3251-3255 (1992); Roux et al., Proc.
Natl. Acad. Sci USA 86, 9079-9083 (1989)), recombinant virus
containing in its envelope a sequence encoding a single-chain
antibody variable region against a hapten (Russell et al., Nucleic
Acids Research, 21, 1081-1085 (1993)), cloning of peptide hormone
ligands into the retrovirus envelope (Kasahara et al., Science,
266, 1373-1376 (1994)), chimeric EPO/env constructs (Kasahara et
al., 1994), single-chain antibody against the low density
lipoprotein (LDL) receptor in the ecotropic MLV envelope, resulting
in specific infection of HeLa cells expressing LDL receptor (Somia
et al., Proc. Natl. Acad. Sci USA, 92, 7570-7574 (1995)), similarly
the host range of ALV can be altered by incorporation of an
integrin ligand, enabling the virus to now cross species to
specifically infect rat glioblastoma cells (Valsesia-Wittmann et
al., J. Virol. 68, 4609-4619 (1994)), and Dornberg and co-workers
(Chu and Dornburg, J. Virol 69, 2659-2663 (1995);M. Engelstadter
36
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
et a/.Gene Therapy 8,1202-1206 (2001)) have reported tissue-
specific targeting of spleen necrosis virus (SNV), an avian
retrovirus, using envelopes containing single-chain antibodies
directed against tumor markers.
[0083] In one embodiment, the recombinant retrovirus of the
disclosure is genetically modified in such a way that the virus is
targeted to a particular cell type (e.g., smooth muscle cells,
hepatic cells, renal cells, fibroblasts, keratinocytes, mesenchymal
stem cells, bone marrow cells, chondrocyte, epithelial cells,
intestinal cells, mammary cells, neoplastic cells, glioma cells,
neuronal cells and others known in the art) such that the
recombinant genome of the retroviral vector is delivered to a
target non-dividing, a target dividing cell, or a target cell
having a cell proliferative disorder.
[0084] In another embodiment, targeting uses cell- or tissue-
specific regulatory elements to promote expression and
transcription of the viral genome in a targeted cell which actively
utilizes the regulatory elements, as described more fully below.
The transferred retrovirus genetic material is then transcribed and
translated into proteins within the host cell. The targeting
regulatory element is typically linked to the 5' and/or 3' LTR,
creating a chimeric LTR.
[0085] The disclosure provides in one embodiment a replication
competent retrovirus that does not require helper virus or
additional nucleic acid sequence or proteins in order to propagate
and produce virion. For example, the nucleic acid sequences of the
retrovirus of the disclosure encode a group specific antigen and
reverse transcriptase, (and integrase and protease-enzymes
necessary for maturation and reverse transcription), respectively,
as discussed above. The viral gag and pol can be derived from a
lentivirus, such as HIV or an oncovirus or gammaretrovirus such as
MoMLV. In addition, the nucleic acid genome of the retrovirus of
the disclosure includes a sequence encoding a viral envelope (ENV)
protein. The env gene can be derived from any retroviruses. The env
may be an amphotropic envelope protein which allows transduction of
cells of human and other species, or may be an ecotropic envelope
protein, which is able to transduce only mouse and rat cells.
37
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
Further, it may be desirable to target the recombinant virus by
linkage of the envelope protein with an antibody or a particular
ligand for targeting to a receptor of a particular cell-type. As
mentioned above, retroviral vectors can be made target specific by
inserting, for example, a glycolipid, or a protein. Targeting is
often accomplished by using an antibody to target the retroviral
vector to an antigen on a particular cell-type (e.g., a cell type
found in a certain tissue, or a cancer cell type). Those of skill
in the art will know of, or can readily ascertain without undue
experimentation, specific methods to achieve delivery of a
retroviral vector to a specific target. In one embodiment, the env
gene is derived from a non-retrovirus (e.g., CMV or VSV). Examples
of retroviral-derived env genes include, but are not limited to:
Moloney murine leukemia virus (MoMuLV), Harvey murine sarcoma virus
(HaMuSV), murine mammary tumor virus (MuMTV), gibbon ape leukemia
virus (GaLV), human immunodeficiency virus (HIV) and Rous Sarcoma
Virus (RSV). Other env genes such as Vesicular stomatitis virus
(VSV) (Protein G), cytomegalovirus envelope (CMV), or influenza
virus hemagglutinin (HA) can also be used.
[0086] In one embodiment, the retroviral genome is derived from
an onco-retrovirus, and more particularly a mammalian onco-
retrovirus. In a further embodiment, the retroviral genome is
derived from a gamma retrovirus, and more particularly a mammalian
gamma retrovirus. By "derived" is meant that the parent
polynucleotide sequence is an wild-type oncovirus which has been
modified by insertion or removal of naturally occurring sequences
(e.g., insertion of an IRES, insertion of a heterologous
polynucleotide encoding a polypeptide or inhibitory nucleic acid of
interest, swapping of a more effective promoter from a different
retrovirus or virus in place of the wild-type promoter and the
like).
[0087] Unlike recombinant retroviruses produced by standard
methods in the art that are defective and require assistance in
order to produce infectious vector particles, the disclosure
provides a retrovirus that is replication-competent.
[0088] In another embodiment, the disclosure provides
retroviral vectors that are targeted using regulatory sequences.
38
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
Cell- or tissue-specific regulatory sequences (e.g., promoters) can
be utilized to target expression of gene sequences in specific cell
populations. Suitable mammalian and viral promoters for the
disclosure are described elsewhere herein. Accordingly, in one
embodiment, the disclosure provides a retrovirus having tissue-
specific promoter elements at the 5' end of the retroviral genome.
Typically, the tissue-specific regulatory elements/sequences are in
the U3 region of the LTR of the retroviral genome, including for
example cell- or tissue-specific promoters and enhancers to
neoplastic cells (e.g., tumor cell-specific enhancers and
promoters), and inducible promoters (e.g., tetracycline).
[0089] In some circumstances, it may be desirable to regulate
expression. For example, different viral promoters with varying
strengths of activity may be utilized depending on the level of
expression desired. In mammalian cells, the CMV immediate early
promoter if often used to provide strong transcriptional
activation. Modified versions of the CMV promoter that are less
potent have also been used when reduced levels of expression of the
transgene are desired. When expression of a transgene in
hematopoietic cells is desired, retroviral promoters such as the
LTRs from MLV or MMTV can be used. Other viral promoters that can
be used include SV40, RSV LTR, HIV-1 and HIV-2 LTR, adenovirus
promoters such as from the E1A, E2A, or MLP region, AAV LTR,
cauliflower mosaic virus, HSV-TK, and avian sarcoma virus.
[0090] Similarly tissue specific or selective promoters may be
used to effect transcription in specific tissues or cells so as to
reduce potential toxicity or undesirable effects to non-targeted
tissues. For example, promoters such as the PSA, probasin,
prostatic acid phosphatase or prostate-specific glandular
kallikrein (hK2) may be used to target gene expression in the
prostate. The Whey accessory protein (WAP) may be used for breast
tissue expression (Andres et al., PNAS 84:1299-1303, 1987). Other
promoters/regulatory domains that can be used are set forth in
Table 3.
[0091] "Tissue-specific regulatory elements" are regulatory
elements (e.g., promoters) that are capable of driving
transcription of a gene in one tissue while remaining largely
39
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
"silent" in other tissue types. It will be understood, however,
that tissue-specific promoters may have a detectable amount of
"background" or "base" activity in those tissues where they are
silent. The degree to which a promoter is selectively activated in
a target tissue can be expressed as a selectivity ratio (activity
in a target tissue/activity in a control tissue). In this regard, a
tissue specific promoter useful in the practice of the disclosure
typically has a selectivity ratio of greater than about 5.
Preferably, the selectivity ratio is greater than about 15.
[0092] In certain indications, it may be desirable to activate
transcription at specific times after administration of the
recombinant replication competent retrovirus of the disclosure
(RRCR). This may be done with promoters that are hormone or
cytokine regulatable. For example in therapeutic applications where
the indication is a gonadal tissue where specific steroids are
produced or routed to, use of androgen or estrogen regulated
promoters may be advantageous. Such promoters that are hormone
regulatable include MMTV, MT-1, ecdysone and RuBisco. Other hormone
regulated promoters such as those responsive to thyroid, pituitary
and adrenal hormones may be used. Cytokine and inflammatory protein
responsive promoters that could be used include K and T Kininogen
(Kageyama et al., 1987), c-fos, TNF-alpha, C-reactive protein
(Arcone et al., 1988), haptoglobin (Oliviero et al., 1987), serum
amyloid A2, C/EBP alpha, IL-1, IL-6 (Poli and Cortese, 1989),
Complement C3 (Wilson et al., 1990), IL-8, alpha-1 acid
glycoprotein (Prowse and Baumann, 1988), alpha-1 antitypsin,
lipoprotein lipase (Zechner et al., 1988), angiotensinogen (Ron et
al., 1990), fibrinogen, c-jun (inducible by phorbol esters, TNF-
alpha, UV radiation, retinoic acid, and hydrogen peroxide),
collagenase (induced by phorbol esters and retinoic acid),
metallothionein (heavy metal and glucocorticoid inducible),
Stromelysin (inducible by phorbol ester, interleukin-1 and EGF),
alpha-2 macroglobulin and alpha-1 antichymotrypsin. Tumor specific
promoters such as osteocalcin, hypoxia-responsive element (HRE),
MAGE-4, CEA, alpha-fetoprotein, GRP78/BiP and tyrosinase may also
be used to regulate gene expression in tumor cells.
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
[0 0 9 3] In addition, this list of promoters should not be
construed to be exhaustive or limiting, those of skill in the art
will know of other promoters that may be used in conjunction with
the promoters and methods disclosed herein.
TABLE 3 TISSUE SPECIFIC PROMOTERS
Tissue Promoter
Pancreas Insulin Elastin Amylase
pdr-1 pdx-1 glucokinase
Liver Albumin PEPCK HBV enhancer
a fetoprotein apolipoprotein C a-1
antitrypsin vitellogenin, NF-AB
Transthyretin
Skeletal muscle Myosin H chain Muscle creatine kinase
Dystrophin Calpain p94 Skeletal
alpha-actin fast troponin 1
Skin Keratin K6 Keratin K1
Lung CFTR Human cytokeratin 18 (K18)
Pulmonary surfactant proteins A, B
and C CC-10 P1
Smooth muscle sm22 a SM-alpha-actin
Endothelium Endothelin-1 E-selectin von
Willebrand factor TIE (Korhonen et
al., 1995) KDR/flk-1 Melanocytes
Tyrosinase
Adipose tissue Lipoprotein lipase (Zechner et al.,
1988) Adipsin (Spiegelman et al., 1989)
acetyl-CoA carboxylase (Pape and Kim,
1989) glycerophosphate dehydrogenase
(Dani et al., 1989) adipocyte P2 (Hunt
et al., 1986)
Breast Whey Acidic Protien (WAP)(Andres et al.
PNAS 84:1299-1303 1987
Blood Z-globin
[0094] It will be further understood that certain promoters,
while not restricted in activity to a single tissue type, may
nevertheless show selectivity in that they may be active in one
group of tissues, and less active or silent in another group. Such
promoters are also termed "tissue specific", and are contemplated
for use with the disclosure. For example, promoters that are active
in a variety of central nervous system (CNS) neurons may be
therapeutically useful in protecting against damage due to stroke,
which may affect any of a number of different regions of the brain.
Accordingly, the tissue-specific regulatory elements used in the
disclosure, have applicability to regulation of the heterologous
proteins as well as an applicability as a targeting polynucleotide
sequence in the present retroviral vectors.
41
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
[0 0 9 5] In yet another embodiment, the disclosure provides
plasmids comprising a recombinant retroviral derived construct.
The plasmid can be directly introduced into a target cell or a cell
culture such as NIH 3T3 or other tissue culture cells. The
resulting cells release the retroviral vector into the culture
medium.
[0096] In view of the foregoing, and the following example, the
disclosure provides in one embodiment, a recombinant replication
competent retrovirus (RCR) comprising an optimized IRES cassette.
In one embodiment, the retroviral polynucleotide sequence is
derived from a virus selected from the group consisting of murine
leukemia virus (MLV), Moloney murine leukemia virus (MoMLV), Feline
leukemia virus (FeLV), Baboon endogenous retrovirus (BEV), porcine
endogenous virus (PERV), the cat derived retrovirus RD114, squirrel
monkey retrovirus, Xenotropic murine leukemia virus-related virus
(XMRV), avian reticuloendotheliosis virus (REV), or Gibbon ape
leukemia virus (GALV). In another embodiment the RCR comprises a
retroviral GAG protein; retroviral POL protein; a retroviral
envelope (which can be chimeric, ecotropic and amphotropic); a
retroviral polynucleotide comprising Long-Terminal Repeat (LTR)
sequences at the 3' end of the retroviral polynucleotide sequence,
gag, pol and env genes and an optimized IRES cassette (and/or
optional additional elements including core promoter, inhibitory
nucleic acid such as miRNA and the like) and a promoter within the
LTR at the 5' end of the retroviral polynucleotide. In one
embodiment, the 3' LTR comprises a sequence that is at least 98%
identical to the sequence from about nucleotide 9405 to about 9998
of SEQ ID NO:19, 22 or 42. In another embodiment, the promoter
sequence at the 5' end of the retroviral polynucleotide is suitable
for expression in a mammalian cell. In another embodiment of any
of the foregoing, the promoter, gag, poi and env domains comprise a
sequence that is at least 98% identical to the sequence from about
1 to about 8323 of SEQ ID NO: 19, 22 or 42 and wherein the
retroviral polynucleotide lacks 70 base pairs of MLV sequence
downstream form the 3'LTR compared to a vector of SEQ ID NO:21
(pACE). In yet another embodiment of any of the foregoing, a
cassette comprising an optimized internal ribosome entry site
42
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
(IRES) comprising a sequence that is at least 98% identical to the
sequence from about 8327 to 8875 of SEQ ID NO: 19, 22 or 42 and
consisting of 6As in the A-bulge in the J-K bifurcation region. In
a further embodiment, the optimized IRES is operably linked to a
heterologous polynucleotide, wherein the cassette is positioned 5'
to the 3' LTR and 3' to the env nucleic acid domain encoding the
retroviral envelope and lacking small repeats on either side of the
cassette compared to the pACE vector of SEQ ID NO:21 (pACE-CD). In
yet another embodiment of any of the foregoing, the vector includes
cis-acting sequences necessary for reverse transcription, packaging
and integration in a target cell. In still another embodiment, the
RCR maintains higher replication competency after 6 passages
compared to a vector comprising SEQ ID NO:21 (pACE) and wherein
when the heterologous polynucleotide is expressed it produces at
least 20%, 30%, 40%, 50% or more expressed heterologous polypeptide
compared to a pAC3-yCD2 (SEQ ID NO:22) vector. In another
embodiment, the RCR infects a target cell multiple times resulting
in an average number of copies/diploid genome of 5 or greater.
In another embodiment, the retroviral envelope is an amphotropic
MLV envelope. In one embodiment, the promoter comprises a CMV
promoter having a sequence as set forth in SEQ ID NO:19, 20, 22 or
42 from nucleotide 1 to about nucleotide 582 and may include
modification to one or more nucleic acid bases and which is capable
of directing and initiating transcription. In another embodiment,
the promoter comprises a CMV-R-U5 domain polynucleotide. In still
a further embodiment, the CMV-R-U5 domain comprises the immediately
early promoter from human cytomegalovirus linked to an MLV R-U5
region. In yet a further embodiment, the CMV-R-U5 domain
polynucleotide comprises a sequence as set forth in SEQ ID NO:19,
20, 22 or 42 from about nucleotide 1 to about nucleotide 1202 or
sequences that are at least 99% identical to a sequence as set
forth in SEQ ID NO:19, 20, 22 or 42, wherein the polynucleotide
promotes transcription of a nucleic acid molecule operably linked
thereto. In another embodiment, the gag nucleic acid domain
comprises a sequence from about nucleotide number 1203 to about
nucleotide 2819 of SEQ ID NO: 19, 22 or 42 or a sequence having at
least 99% or 99.8% identity thereto. In another embodiment,
43
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
embodiment, the poi domain of the polynucleotide is derived from a
gammaretrovirus. In a further embodiment, the poi domain comprises
a sequence from about nucleotide number 2820 to about nucleotide
6358 of SEQ ID NO: 19, 22 or 42 or a sequence having at least 99%
or 99.9% identity thereto. In yet another embodiment, the env
domain comprises a sequence from about nucleotide number 6359 to
about nucleotide 8323 of SEQ ID NO: 19, 22 or 42 or a sequence
having at least 99% or 99.8% identity thereto. In yet another
embodiment, the IRES comprises a sequence as set forth in SEQ ID
NO:41. In yet another embodiment, the heterologous nucleic acid
comprises a polynucleotide having a sequence as set forth in SEQ ID
NO:3, 5, 11, 13, 15 or 17. In another embodiment, the
heterologous nucleic acid encodes a polypeptide comprising a
sequence as set forth in SEQ ID NO:4. In a further embodiment, the
heterologous nucleic acid is human codon optimized and encodes a
polypeptide as set forth in SEQ ID NO:4. In yet another
embodiment, the heterologous nucleic acid comprises a sequence as
set forth in SEQ ID NO: 19, 22 or 42 from about nucleotide number
8877 to about 9353. In another embodiment, the 3' LTR comprises a
U3-R-U5 domain. In yet a further embodiment, the 3' LTR comprises
a sequence as set forth in SEQ ID NO: 19, 22 or 42 from about
nucleotide 9405 to about 9998 or a sequence that is at least 95%,
98% or 99.5% identical thereto. In one embodiment, the disclosure
provides a retroviral polynucleotide comprising SEQ ID NO:42. In
another embodiment the retroviral polynucleotide of SEQ ID NO:42 is
an RNA sequence wherein T is replaced with U. In yet another
embodiment, a retroviral RNA polynucleotide according to SEQ ID
NO:42, wherein T is U is encapsulated in a viral capsid. In yet
another embodiment, of any of the foregoing, the retroviral
polynucleotide can further comprise and miRNA, siRNA or shRNA
sequence to be delivered to a target cell. The miRNA, siRNA or
shRNA can be operably linked to a polIII promoter. The miRNA may
be located upstream or downstream of the optimized IRES cassette.
In another embodiment, the heterologous polynucleotide can be any
number of coding sequences including cytokines, immunopotentiating
agents, thymidine kinase, cytosine deaminase, purine nucleoside
phophorylase, receptors, antibody and fragments etc.
44
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
[0 0 9 7] The disclosure also provides a method of treating a cell
proliferative disorder comprising contacting the subject with a
retrovirus as described herein. In one embodiment, the retrovirus
containing an optimized IRES under conditions such that a
heterologous polynucleotide linked to the optimized IRES comprises
cytosine deaminase activity and contacting the subject with 5-
fluorocytosine. In one embodiment, the retrovirus infects a cell
resulting in integration of a polynucleotide comprising SEQ ID
NO:42. In another embodiment, the cell proliferative disorder is
glioblastoma multiforme. In another embodiment, the cell
proliferative disorder is selected from the group consisting of
lung cancer, colon-rectum cancer, breast cancer, prostate cancer,
urinary tract cancer, uterine cancer, brain cancer, head and neck
cancer, pancreatic cancer, melanoma, stomach cancer and ovarian
cancer. The method can include a combination therapy, wherein a
subject to be treated is contacted with a retrovirus and further
contacted with an anticancer agent or chemotherapeutic agent. For
example, the anticancer or chemotherapeutic agent can be selected
from the group consisting of bevacizumab, pegaptanib, ranibizumab,
sorafenib, sunitinib, AE-941, VEGF Trap, pazopanib, vandetanib,
vatalanib, cediranib, fenretinide, squalamine, INGN-241, oral
tetrathiomolybdate, tetrathiomolybdate, Panzem NCD, 2-
methoxyestradiol, AEE-788, AG-013958, bevasiranib sodium, AMG-706,
axitinib, BIBF-1120, CDP-791, CP-547632, PI-88, SU-14813, SU-6668,
XL-647, XL-999, IMC-1121B, ABT-869, BAY-57-9352, BAY-73-4506, BMS-
582664, CEP-7055, CHIR-265, CT-322, CX-3542, E-7080, ENMD-1198,
OSI-930, PTC-299, Sirna-027, TKI-258, Veglin, XL-184, or ZK-304709.
[0098] In another embodiment of any of the foregoing, a
retrovirus is administered from about 10 to 107 TU/g brain weight.
In another embodiment, the retrovirus is administered from about
10' to 10' TU/g brain weight.
[0099] The disclosure provides a polynucleotide construct
comprising from 5' to 3': a promoter or regulatory region useful
for initiating transcription; a psi packaging signal; a gag
encoding nucleic acid sequence, a poi encoding nucleic acid
sequence; an env encoding nucleic acid sequence; an internal
ribosome entry site nucleic acid sequence comprising 6 A's in the
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
A-bulge; a heterologous polynucleotide encoding a marker,
therapeutic or diagnostic polypeptide; and a LTR nucleic acid
sequence. As described elsewhere herein and as follows the various
segment of the polynucleotide construct of the disclosure (e.g., a
recombinant replication competent retroviral polynucleotide) are
engineered depending in part upon the desired host cell, expression
timing or amount, and the heterologous polynucleotide. A
replication competent retroviral construct of the disclosure can be
divided up into a number of domains that may be individually
modified by those of skill in the art.
[00100] For example, the promoter can comprise a CMV promoter
having a sequence as set forth in SEQ ID NO:19, 20, 22 or 42 from
nucleotide 1 to about nucleotide 582 and may include modification
to one or more (e.g., 2-5, 5-10, 10-20, 20-30, 30-50 or more
nucleic acid bases) so long as the modified promoter is capable of
directing and initiating transcription. In one embodiment, the
promoter or regulatory region comprises a CMV-R-U5 domain
polynucleotide. The CMV-R-U5 domain comprises the immediately
early promoter from human cytomegalovirus to the MLV R-U5 region.
In one embodiment, the CMV-R-U5 domain polynucleotide comprises a
sequence as set forth in SEQ ID NO: 19, 20, 22 or 42 from about
nucleotide 1 to about nucleotide 1202 or sequences that are at
least 95% identical to a sequence as set forth in SEQ ID NO: 19,
20, 22 or 42 from about nucleotide 1 to about nucleotide 1202,
wherein the polynucleotide promotes transcription of a nucleic acid
molecule operably linked thereto. The gag domain of the
polynucleotide may be derived from any number of retroviruses, but
will typically be derived from an oncoretrovirus and more
particularly from a mammalian oncoretrovirus. In one embodiment
the gag domain comprises a sequence from about nucleotide number
1203 to about nucleotide 2819 of a sequence as set forth in SEQ ID
NO: 19, 20, 22 or 42 or a sequence having at least 95%, 98%, 99% or
99.8% (rounded to the nearest 10th) identity thereto. The poi domain
of the polynucleotide may be derived from any number of
retroviruses, but will typically be derived from an oncoretrovirus
and more particularly from a mammalian oncoretrovirus. In one
embodiment the poi domain comprises a sequence from about
46
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
nucleotide number 2820 to about nucleotide 6358 of a sequence as
set forth in SEQ ID NO: 19, 20, 22 or 42 or a sequence having at
least 95%, 98%, 99% or 99.9% (roundest to the nearest 10th) identity
thereto. The env domain of the polynucleotide may be derived from
any number of retroviruses, but will typically be derived from an
oncoretrovirus or gamma-retrovirus and more particularly from a
mammalian oncoretrovirus or gamma-retrovirus. In some embodiments
the env coding domain comprises an amphotropic env domain. In one
embodiment the env domain comprises a sequence from about
nucleotide number 6359 to about nucleotide 8323 of a sequence as
set forth in SEQ ID NO: 19, 20, 22 or 42 or a sequence having at
least 95%, 98%, 99% or 99.8% (roundest to the nearest 10th) identity
thereto. The optimized IRES domain of the polynucleotide may be
obtained from any number of internal ribosome entry sites. In one
embodiment, optimized IRES is derived from an encephalomyocarditis
virus. In one embodiment the optimized IRES domain comprises as set
forth in SEQ ID NO:41 or a sequence having at least 95%, 98%, or
99% (roundest to the nearest 10th) identity thereto so long as the
domain allows for entry of a ribosome and comprises 6 A's in the A-
bulge. The heterologous domain can comprise a cytosine deaminase
(CD) of the disclosure. In one embodiment, the CD polynucleotide
comprises a human codon optimized sequence. In yet another
embodiment, the CD polynucleotide encodes a mutant polypeptide
having cytosine deaminase, wherein the mutations confer increased
thermal stabilization that increase the melting temperature (Tm) by
C allowing sustained kinetic activity over a broader
temperature range and increased accumulated levels of protein. In
another embodiment, the disclosure comprises a human codon
optimized thymidine kinase. The heterologous domain may be
followed by a polypurine rich domain. The 3' LTR can be derived
from any number of retroviruses, typically an oncoretrovirus and
preferably a mammalian oncoretrovirus. In one embodiment, the 3'
LTR comprises a U3-R-U5 domain. In yet another embodiment the LTR
comprises a sequence as set forth in SEQ ID NO:19, 20, 22 or 42
from about nucleotide 9405 to about 9998 or a sequence that is at
least 95%, 98% or 99.5% (rounded to the nearest 10th) identical
thereto.
47
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
[00101] The disclosure also provides a recombinant retroviral
vector comprising from 5' to 3' a CMV-R-U5, fusion of the immediate
early promoter from human cytomegalovirus to the MLV R-U5 region; a
PBS, primer binding site for reverse transcriptase; a 5' splice
site; a * packaging signal; a gag, ORE for MLV group specific
antigen; a pol, ORE for MLV polymerase polyprotein; a 3' splice
site; a 4070A env, ORE for envelope protein of MLV strain 4070A; an
optimized IRES, consisting of 6A's in the A-bulge; a modified
cytosine deaminase (thermostabilized and codon optimized) or human
codon optimized thymidine kinase; a PPT, polypurine tract; and a
U3-R-U5, MLV long terminal repeat.
[00102] The disclosure also provides a retroviral vector
comprising a sequence as set forth in SEQ ID NO:42 (or SEQ ID NO:42
wherein T can be U) comprising an optimized A-bulge for expression.
In one embodiment, the optimized A-bulge of the IRES consists of
6A' s.
[00103] The retroviral vectors can be used to treat a wide range
of disease and disorders including a number of cell proliferative
diseases and disorders (see, e.g., U.S. Pat. Nos. 4,405,712 and
4,650,764; Friedmann, 1989, Science, 244:1275-1281; Mulligan, 1993,
Science, 260:926-932, R. Crystal, 1995, Science 270:404-410, each
of which are incorporated herein by reference in their entirety,
see also, The Development of Human Gene Therapy, Theodore
Friedmann, Ed., Cold Spring Harbor Laboratory Press, Cold Spring
Harbor, N.Y., 1999. ISBN 0-87969-528-5, which is incorporated
herein by reference in its entirety).
[00104] The disclosure also provides gene therapy for the
treatment of cell proliferative disorders. Such therapy would
achieve its therapeutic effect by introduction of an appropriate
therapeutic polynucleotide (e.g., antisense, ribozymes, suicide
genes, siRNA), into cells of subject having the proliferative
disorder. Delivery of polynucleotide constructs can be achieved
using the recombinant retroviral vector of the disclosure,
particularly if it is based on MLV, which is capable of infecting
dividing cells.
[00105] In addition, the therapeutic methods (e.g., the gene
therapy or gene delivery methods) as described herein can be
48
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
performed in vivo or ex vivo. It may be preferable to remove the
majority of a tumor prior to gene therapy, for example surgically
or by radiation. In some aspects, the retroviral therapy may be
preceded or followed by surgery, chemotherapy or radiation therapy.
[00106] Thus, the disclosure provides a recombinant retrovirus
capable of infecting a non-dividing cell, a dividing cell or a
neoplastic cell, therein the recombinant retrovirus comprises a
viral GAG; a viral POL; a viral ENV; a heterologous nucleic acid
operably linked to an IRES consisting of 6A's in the A-bulge; and
cis-acting nucleic acid sequences necessary for packaging, reverse
transcription and integration. The recombinant retrovirus can be a
lentivirus, such as HIV, or can be an oncovirus. As described above
for the method of producing a recombinant retrovirus, the
recombinant retrovirus of the disclosure may further include at
least one of VPR, VIE, NEF, VPX, TAT, REV, and VPU protein. While
not wanting to be bound by a particular theory, it is believed that
one or more of these genes/protein products are important for
increasing the viral titer of the recombinant retrovirus produced
(e.g., NEF) or may be necessary for infection and packaging of
virion.
[00107] The disclosure also provides a method of nucleic acid
transfer to a target cell to provide expression of a particular
nucleic acid (e.g., a heterologous sequence). Therefore, in another
embodiment, the disclosure provides a method for introduction and
expression of a heterologous nucleic acid in a target cell
comprising infecting the target cell with the recombinant virus of
the disclosure and expressing the heterologous nucleic acid in the
target cell. As mentioned above, the target cell can be any cell
type including dividing, non-dividing, neoplastic, immortalized,
modified and other cell types recognized by those of skill in the
art, so long as they are capable of infection by a retrovirus.
[00108] It may be desirable to modulate the expression of a gene
in a cell by the introduction of a nucleic acid sequence (e.g., the
heterologous nucleic acid sequence) by the method of the
disclosure, wherein the nucleic acid sequence give rise, for
example, to an antisense or ribozyme molecule. The term "modulate"
envisions the suppression of expression of a gene when it is over-
49
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
expressed, or augmentation of expression when it is under-
expressed. Where a cell proliferative disorder is associated with
the expression of a gene, nucleic acid sequences that interfere
with the gene's expression at the translational level can be used.
This approach utilizes, for example, antisense nucleic acid,
ribozymes, or triplex agents to block transcription or translation
of a specific mRNA, either by masking that mRNA with an antisense
nucleic acid or triplex agent, or by cleaving it with a ribozyme.
[00109] It may be
desirable to transfer a nucleic acid encoding
a biological response modifier (e.g., a cytokine) into a cell or
subject. Included in this category are immunopotentiating agents
including nucleic acids encoding a number of the cytokines
classified as "interleukins". These include, for example,
interleukins 1 through 15, as well as other response modifiers and
factors described elsewhere herein. Also included in this category,
although not necessarily working according to the same mechanisms,
are interferons, and in particular gamma interferon, tumor necrosis
factor (TNF) and granulocyte-macrophage-colony stimulating factor
(GM-CSF). Other polypeptides include, for example, angiogenic
factors and anti-angiogenic factors. It may be desirable to deliver
such nucleic acids to bone marrow cells or macrophages to treat
enzymatic deficiencies or immune defects. Nucleic acids encoding
growth factors, toxic peptides, ligands, receptors, or other
physiologically important proteins can also be introduced into
specific target cells.
[00110] The
disclosure can be used for delivery of heterologous
polynucleotides that promote drug specific targeting and effects.
For example, HER2, a member of the EGF receptor family, is the
target for binding of the drug trastuzumab (Herceptin'TM, Genentech).
Trastuzumab is a mediator of antibody-dependent cellular
cytotoxicity (ADCC). Activity is preferentially targeted to HER2-
expressing cells with 2+ and 3+ levels of overexpression by
immunohistochemistry rather than 1+ and non-expressing cells
(Herceptin prescribing information, Crommelin 2002).
Enhancement
of expression of HER2 by introduction of vector expressing HER2 or
truncated HER2 (expressing only the extracellular and transmembrane
domains) in HER2 low tumors may facilitate optimal triggering of
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
ADCC and overcome the rapidly developing resistance to Herceptin
that is observed in clinical use.
[00111] The substitution of yCD2 (comprising SEQ ID NO:19 from
about 8877 to 9353) for the intracellular domain of HER2 allows for
cell surface expression of HER2 and cytosolic localization of yCD2.
The HER2 extracellular domain (ECD) and transmembrane domain (TM)
(approximately 2026 bp from about position 175 to 2200 of SEQ ID
NO:23) can be amplified by PCR (Yamamoto et al., Nature 319:230-
234, 1986; Chen et al., Canc. Res., 58:1965-1971, 1998) or
chemically synthesized (BioBasic Inc., Markham, Ontario, Canada)
and inserted between the IRES and yCD2 gene in the vector pAC3-yCD2
SEQ ID NO: 19 (e.g., between about nucleotide 8876 and 8877 of SEQ
ID NO:19). Alternatively, the yCD gene can be excised and replaced
with a polynucleotide encoding a HER2 polypeptide or fragment
thereof. A further truncated HER2 with only the Herceptin binding
domain IV of the ECD and TM domains (approximately 290 bp from
position 1910 to 2200) can be amplified or chemically synthesized
and used as above (Landgraf 2007; Garrett et al., J. of Immunol.,
178:7120-7131, 2007). A further modification of this truncated
form with the native signal peptide (approximately 69 bp from
position 175-237) fused to domain IV and the TM can be chemically
synthesized and used as above. The resulting viruses can be used
to treat a cell proliferative disorder in a subject in combination
with trastuzumab or trastuzumab and 5-EC.
[00112] Alternatively, HER2 and the modifications described
above can be expressed in a separate vector containing a different
ENV gene or other appropriate surface protein. This vector can be
replication competent (Logg et al. J.Mol Biol. 369:1214 2007) or
non-replicative "first generation" retroviral vector that encodes
the envelope and the gene of interest (Emi et al. J.Virol 65:1202
1991). In the latter case the pre-existing viral infection will
provide complementary gag and pol to allow infective spread of the
"non-replicative" vector from any previously infected cell.
Alternate ENV and glycoproteins include xenotropic and polytropic
ENV and glycoproteins capable of infecting human cells, for example
ENV sequences from the NZB strain of MLV and glycoproteins from
MCF, VSV, GALV and other viruses (Palu 2000, Baum et al., Mol.
51
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
Therapy, 13(6):1050-1063, 2006). For example, a polynucleotide can
comprise a sequence wherein the GAG and POL and yCD2 genes of SEQ
ID NO: 19 are deleted, the ENV corresponds to a xenotropic ENV
domain of NZB MLV or VSV-g, and the IRES or a promoter such as RSV
is operatively linked directly to HER2, HER2 ECDTM, HER2 ECDIVTM,
or HER2 SECDIVTM.
[00113] Mixed infection of cells by VSVG pseudotyped virus and
amphotropic retrovirus results in the production of progeny virions
bearing the genome of one virus encapsidated by the envelope
proteins of the other. The same is true for other envelopes that
pseudotype retroviral particles. For example, infection by
retroviruses derived as above results in production of progeny
virions capable of encoding yCD2 and HER2 (or variant) in infected
cells. The resulting viruses can be used to treat a cell
proliferative disorder in a subject in combination with trastuzumab
or trastuzumab and 5-FC.
[00114] Another aspect of the development of resistance to
trastuzumab relates to the interference with intracellular
signaling required for the activity of trastuzumab. Resistant
cells show loss of PTEN and lower expression of p27kip1 [Fujita,
Brit J. Cancer, 94:247, 2006; Lu et al., Journal of the National
Cancer Institute, 93(24): 1852-1857, 2001; Kute et al., Cytometry
Part A 57A:86-93, 2004). For example, a polynucleotide encoding
PTEN can be recombinantly generated or chemically synthesized
(BioBasic Inc., Markham, Canada) and operably inserted directly
after the yCD2 polynucleotide in the vector pAC3-yCD2 SEQ ID NO: 19
or 22, or with a linker sequence as previously described, or as a
replacement for yCD2. In a further example, the PTEN encoding
polynucleotide (SEQ ID NO:25) can be synthesized as above and
inserted between the IRES and yCD2 sequences or with a linker as
previously described.
[00115] Alternatively, PTEN can be expressed in a separate
vector containing a different ENV gene or other appropriate surface
protein. This vector can be replication competent (Logg et al.
J.Mol Biol. 369:1214 2007) or non-replicative "first generation"
retroviral vector that encodes the envelope and the gene of
interest (Emi et al., J.Virol 65:1202 1991). In the latter case the
52
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
pre-existing viral infection will provide complementary gag and pol
to allow infective spread of the "non-replicative" vector from any
previously infected cell. Alternate ENV and glycoproteins include
xenotropic and polytropic ENV and glycoproteins capable of
infecting human cells, for example ENV sequences from the NZB
strain of MLV and glycoproteins from MCF, VSV, GALV and other
viruses (Palu, Rev Med Virol. 2000, Baum, Mol. Ther. 13(6):1050-
1063, 2006). For example, a polynucleotide can comprise a
sequence wherein the GAG and POL and yCD2 genes of SEQ ID NO: 19
are deleted, the ENV corresponds to a xenotropic ENV domain of NZB
MLV or VSV-g, and the IRES or a promoter such as RSV is operatively
linked directly to PTEN.
[00116] Mixed infection of cells by VSVG pseudotyped virus and
amphotropic retrovirus results in the production of progeny virions
bearing the genome of one virus encapsidated by the envelope
proteins of the other [Emi 1991]. The same is true for other
envelopes that pseudotype retroviral particles. For example,
infection by retroviruses derived as above results in production of
progeny virions capable of encoding yCD2 and PTEN (or variant) or
PTEN alone in infected cells. The resulting viruses can be used to
treat a cell proliferative disorder in a subject in combination
with trastuzumab or trastuzumab and 5-FC.
[00117] Similarly, a polynucleotide encoding p27kip1 (SEQ ID
NO:27 and 28) can be chemically synthesized (BioBasic Inc.,
Markham, Canada) and operably inserted directly after the yCD2 gene
in the vector pAC3-yCD2 SEQ ID NO:19 or SEQ ID NO:42 or with a
linker sequence. In a further example, the p27kip1 encoding
polynucleotide can be synthesized as above and inserted between the
IRES consisting of 6A's in the A-bulge and yCD2 sequences or with a
linker as previously described or in place of the yCD2 gene.
[00118] Alternatively, p27kip1 can be expressed in a separate
vector containing a different ENV gene or other appropriate surface
protein. This vector can be replication competent (Logg et al.
J.Mol Biol. 369:1214 2007) or non-replicative "first generation"
retroviral vector that encodes the envelope and the gene of
interest (Emi et al. J.Virol 65:1202 1991). In the latter case the
pre-existing viral infection will provide complementary gag and pol
53
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
to allow infective spread of the "non-replicative" vector from any
previously infected cell. Alternate ENV and glycoproteins include
xenotropic and polytropic ENV and glycoproteins capable of
infecting human cells, for example ENV sequences from the NZB
strain of MLV and glycoproteins from MCF, VSV, GALV and other
viruses (Palu 2000, Baum 2006, supra). For example, a
polynucleotide can comprise a sequence wherein the GAG and POL and
yCD2 genes of SEQ ID NO: 19 are deleted, the ENV corresponds to a
xenotropic ENV domain of NZB MLV or VSV-g, and the IRES consisting
of 6A's in the A-bulge or a promoter such as RSV is operatively
linked directly to p27kip1.
[00119] Mixed infection of cells by VSVG pseudotyped virus and
amphotropic retrovirus results in the production of progeny virions
bearing the genome of one virus encapsidated by the envelope
proteins of the other [Emi 1991]. The same is true for other
envelopes that pseudotype retroviral particles. For example,
infection by retroviruses derived as above from both SEQ ID NO:19,
22 and 42 results in production of progeny virions capable of
encoding yCD2 and p27kip1 (or variant) in infected cells. The
resulting viruses can be used to treat a cell proliferative
disorder in a subject in combination with trastuzumab or
trastuzumab and 5-FC.
[00120] In another example, CD20 is the target for binding of
the drug rituximab (Rituxan'TM, Genentech). Rituximab is a mediator
of complement-dependent cytotoxicity (CDC) and ADCC. Cells with
higher mean fluorescence intensity by flow cytometry show enhanced
sensitivity to rituximab (van Meerten et al., Clin Cancer Res 2006;
12(13):4027-4035, 2006). Enhancement of expression of CD20 by
introduction of vector expressing CD20 in CD20 low B cells may
facilitate optimal triggering of ADCC.
[00121] For example, a polynucleotide encoding CD20 (SEQ ID
NO:29 and 30) can be chemically synthesized (BioBasic Inc.,
Markham, Canada) and operably inserted directly after the yCD2 gene
in the vector pAC3-yCD2 (-2) SEQ ID NO: 19, 22 or 42 with a linker
sequence as previously described, or as a replacement for the yCD2
gene. In a further example, the CD20 encoding polynucleotide can be
synthesized as above and inserted between the IRES consisting of
54
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
6A's in the A-bulge and yCD2 sequences or with a linker as
previously described. As a further alternative the CD20 sequence
can be inserted into the pAC3-yCD2 vector after excision of the CD
gene by Psi1 and Not1 digestion.
[00122] In still a further example, a polynucleotide encoding
CD20 (SEQ ID NO:29 and 30) can be chemically synthesized (BioBasic
Inc., Markham, Canada)and inserted into a vector containing a non
amphotropic ENV gene or other appropriate surface protein (Tedder
et al., PNAS, 85:208-212, 1988). Alternate ENV and glycoproteins
include xenotropic and polytropic ENV and glycoproteins capable of
infecting human cells, for example ENV sequences from the NZB
strain of MLV and glycoproteins from MCF, VSV, GALV and other
viruses [Palu 2000, Baum 2006]. For
example, a polynucleotide can
comprise a sequence wherein the GAG and POL and yCD2 genes of SEQ
ID NO: 19 are deleted, the ENV corresponds to a xenotropic ENV
domain of NZB MLV or VSV-g, and the IRES consisting of 6A's in the
A-bulge or a promoter such as RSV is operatively linked directly to
CD20.
[00123] Mixed infection of cells by VSVG pseudotyped virus and
amphotropic retrovirus results in the production of progeny virions
bearing the genome of one virus encapsidated by the envelope
proteins of the other (Emi 1991). The same is true for other
envelopes that pseudotype retroviral particles. For example,
infection by retroviruses derived as above from SEQ ID NO:19, 22 or
42 results in production of progeny virions capable of encoding
yCD2 and CD20 in infected cells. The resulting viruses can be used
to treat a cell proliferative disorder in a subject in combination
with Rituxan and/or 5-FC. Similarly, infection of a tumor with a
vector encoding only the CD20 marker can make the tumor treatable
by the use of Rituxan.
[00124] Levels of the enzymes and cofactors involved in
pyrimidine anabolism can be limiting. OPRT, thymidine kinase (TK),
Uridine monophosphate kinase, and pyrimidine nucleoside
phosphorylase expression is low in 5-FU resistant cancer cells
compared to sensitive lines (Wang et al., Cancer Res., 64:8167-
8176, 2004). Large population analyses show correlation of enzyme
levels with disease outcome (Fukui et al., Int'l. J. OF Mol. Med.,
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
22:709-716, 2008). Coexpression of CD and other pyrimidine
anabolism enzymes (PAE) can be exploited to increase the activity
and therefore therapeutic index of fluoropyrimidine drugs.
[00125] The disclosure provides methods for treating cell
proliferative disorders such as cancer and neoplasms comprising
administering an RCR vector of the disclosure followed by treatment
with a chemotherapeutic agent or anti-cancer agent. In one aspect,
the RCR vector is administered to a subject for a period of time
prior to administration of the chemotherapeutic or anti-cancer
agent that allows the RCR to infect and replicate. The subject is
then treated with a chemotherapeutic agent or anti-cancer agent for
a period of time and dosage to reduce proliferation or kill the
cancer cells. In one aspect, if the treatment with the
chemotherapeutic or anti-cancer agent reduces, but does not kill
the cancer/tumor (e.g., partial remission or temporary remission),
the subject may then be treated with a non-toxic therapeutic agent
(e.g., 5-EC) that is converted to a toxic therapeutic agent in
cells expression a cytotoxic gene (e.g., cytosine deaminase) from
the RCR.
[00126] Using such methods the RCR vectors of the disclosure are
spread during a replication process of the tumor cells, such cells
can then be killed by treatment with an anti-cancer or
chemotherapeutic agent and further killing can occur using the RCR
treatment process described herein.
[00127] In yet another embodiment of the disclosure, the
heterologous gene can comprise a coding sequence for a target
antigen (e.g., a cancer antigen). In this embodiment, cells
comprising a cell proliferative disorder are infected with an RCR
comprising a heterologous polynucleotide encoding the target
antigen to provide expression of the target antigen (e.g.,
overexpression of a cancer antigen). An anticancer agent
comprising a targeting cognate moiety that specifically interacts
with the target antigen is then administered to the subject. The
targeting cognate moiety can be operably linked to a cytotoxic
agent or can itself be an anticancer agent. Thus, a cancer cell
infected by the RCR comprising the targeting antigen coding
56
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
sequences increases the expression of target on the cancer cell
resulting in increased efficiency/efficacy of cytotoxic targeting.
[00128] In yet another embodiment, an RCR of the disclosure can
comprise a coding sequence comprising a binding domain (e.g., an
antibody, antibody fragment, antibody domain or receptor ligand)
that specifically interacts with a cognate antigen or ligand. The
RCR comprising the coding sequence for the binding domain can then
be used to infect cells in a subject comprising a cell
proliferative disorder such as a cancer cell or neoplastic cell.
The infected cell will then express the binding domain or antibody.
An antigen or cognate operably linked to a cytotoxic agent or which
is cytotoxic itself can then be administered to a subject. The
cytotoxic cognate will then selectively kill infected cells
expressing the binding domain. Alternatively the binding domain
itself can be an anti-cancer agent.
[00129] As used herein, the term "antibody" refers to a protein
that includes at least one immunoglobulin variable domain or
immunoglobulin variable domain sequence. For example, an antibody
can include a heavy (H) chain variable region (abbreviated herein
as VH), and a light (L) chain variable region (abbreviated herein
as VL). In another example, an antibody includes two heavy (H)
chain variable regions and two light (L) chain variable regions.
The term "antibody" encompasses antigen-binding fragments of
antibodies (e.g., single chain antibodies, Fab fragments, F(ab')2,
a Fd fragment, a Fv fragments, and dAb fragments) as well as
complete antibodies.
[00130] The disclosure provides a method of treating a subject
having a cell proliferative disorder. The subject can be any
mammal, and is preferably a human. The subject is contacted with a
recombinant replication competent retroviral vector of the
disclosure. The contacting can be in vivo or ex vivo. Methods of
administering the retroviral vector of the disclosure are known in
the art and include, for example, systemic administration, topical
administration, intraperitoneal administration, intra-muscular
administration, intracranial, cerebrospinal, as well as
administration directly at the site of a tumor or cell-
57
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
proliferative disorder. Other routes of administration known in
the art.
[00131] Thus, the disclosure includes various pharmaceutical
compositions useful for treating a cell proliferative disorder. The
pharmaceutical compositions according to the disclosure are
prepared by bringing a retroviral vector containing a heterologous
polynucleotide sequence useful in treating or modulating a cell
proliferative disorder according to the disclosure into a form
suitable for administration to a subject using carriers, excipients
and additives or auxiliaries. Frequently used carriers or
auxiliaries include magnesium carbonate, titanium dioxide, lactose,
mannitol and other sugars, talc, milk protein, gelatin, starch,
vitamins, cellulose and its derivatives, animal and vegetable oils,
polyethylene glycols and solvents, such as sterile water, alcohols,
glycerol and polyhydric alcohols. Intravenous vehicles include
fluid and nutrient replenishers. Preservatives include
antimicrobial, anti-oxidants, chelating agents and inert gases.
Other pharmaceutically acceptable carriers include aqueous
solutions, non-toxic excipients, including salts, preservatives,
buffers and the like, as described, for instance, in Remington's
Pharmaceutical Sciences, 15th ed. Easton: Mack Publishing Co.,
1405-1412, 1461-1487 (1975) and The National Formulary XIV., 14th
ed. Washington: American Pharmaceutical Association (1975), the
contents of which are hereby incorporated by reference. The pH and
exact concentration of the various components of the pharmaceutical
composition are adjusted according to routine skills in the art.
See Goodman and Gilman's The Pharmacological Basis for Therapeutics
(7th ed.).
[00132] For example, and not by way of limitation, a retroviral
vector useful in treating a cell proliferative disorder will
include an amphotropic ENV protein, GAG, and POL proteins, a
promoter sequence in the U3 region retroviral genome, and all cis-
acting sequence necessary for replication, packaging and
integration of the retroviral genome into the target cell.
[00133] The following Examples are intended to illustrate, but
not to limit the disclosure. While such Examples are typical of
58
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
those that might be used, other procedures known to those skilled
in the art may alternatively be utilized.
EXAMPLES
[00134] The expression level of yCD2 and the conversion of 5-FC to
5-FU by yCD2 have been demonstrated to be efficient and stable both
in vitro and in vivo when cells are maximally infected with Toca
511 (pAC3-yCD2; SEQ ID NO:22). However, in an in vivo pilot study
in long-term (180 days approximately) infected Balb/c mice
integrated proviruses from some tissues were shown to carry
expanded or contracted oligo A sequences in the J-K bifurcation
loop. In tissues from four mice of a biolocalization study
analyzed by molecular PCR cloning, a heterogeneous expansion of 7A
to 8A, 9A, 10A, 11A and 12A and a contraction of 7A to 6A was
observed. This observation and the 7As in pEMCF as opposed to the
6As in ECMV IRES originally described, led to the investigation of
the impact of the yCD2 expression mediated by IRES with various
numbers of As in the A bulge, and, in particular, the impact on
protein translation in the context of RRV. Accordingly, a series
of deletion and insertion mutants specifically in the A bulge in
the bifurcation region were generated. The data show that neither
deletion nor insertion of the oligo A sequence in the A bulge
affects RRV production, that 6 As provide maximal CD and green
fluorescent protein (GFP) expression and that small changes in the
number of As from the 6As have moderate effect, but that larger
changes have drastic effects on efficiency of the IRES-mediated
translation of mRNA from the transgene.
[00135] Construction of RRVs containing various numbers of A's in
the A bulge of the J-K bifurcation region. RRVs containing an EMCV
IRES and encoding CD or GFP were generated to have 4, 5, 6, 7, 8,
or 12As in the A-bulge in the J-K bifurcation region. Each
construct was generated by DNA synthesis (BioBasics Inc.) of the
entire IRES cassette with a Mlu I at the 5' end and a Psi I at the
3'end, respectively, for direct replacement of the equivalent
cassette in the RRV backbone (Figure 1B). All DNA fragments were
confirmed by sequencing analysis prior and post cloning into the
RRV backbone. The RRV constructs containing the yCD2 transgene
were designated using the name of the transgene followed by the
59
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
number of A's in the A bulge (e.g., yCD2-4A contains yCD2 transgene
and 4As in the A bulge in the IRES).
[00136] RRVs containing various numbers of A's in the A bulge
produce similar titers. Virus stock was produced by transient
transfection of 293T cells using calcium phosphate precipitation
method. Viral supernatant was collected approximately 42 hours post
transfection. Viral infection to determine titers was performed.
Viral supernatant of each vector was subsequently used to infect
HT1080 cells to generate RRV-producer cells. The viral titers
obtained were measured before infecting naive U87-MG cells.
Figure 1C shows that HT1080 cells infected with RRVs containing
various numbers of As produced similar levels of virus, suggesting
that the number of the As in the bifurcation loop does not affect
viral replication.
[00137] RRVs containing various numbers of A's in the J-K
bifurcation region express similar levels of transcripts but
different levels of protein expression. The viral supernatant from
HT1080 cells was then used to infect naive U87-MG cells at
multiplicity of infection (MOI) of 0.1. At day 10 post infection,
when the cells were fully infected, cellular viral RNA levels were
measured by quantitative real-time polymerase chain reaction (qRT-
PCR), and protein expression level of yCD2 was examined by
immunoblotting (Perez et al., 2012). The cellular viral RNA
expression levels were measured using two different primer sets,
located in the env (5fEnv2: 5'-ACCCTCAACCTCCCCTACAAGT-3', 3fEnv2:
5'-GTTAAGCGCCTGATAGGCTC-3', probe: 5'FAM-AGCCACCCCCAGGAACTGGAGA
TAGA-3fBHQ) and in yCD2 region (5fyCD2: 5f-ATCATCATGTACGGCATCCCTAG-
3', 3fyCD2: 5f-TGAA CTGCTTCATCA GCTTCTTAC-3f, probe: 5fFAM-
TCATCGTCAACAACCACCACCTCGT-3fBHQ), respectively, (Figure 2A). The
relative level of RNA from each vector was calculated using 2-AL(Ct)
method with respect to the vector containing the 6As. The cellular
viral RNA level ratios range from 0.8 to 1.1 (Figure 2B),
suggesting that there is no significant difference in viral RNA
transcript due to modifications in the IRES. In examining the yCD2
protein expression level of these vectors by Western blot, yCD2
protein expression levels of the vectors containing the 5 and 7As
were identified as being 69% and 77% that of the yCD2-6A vector.
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
In contrast, a substantial reduction of yCD2 protein expression was
observed in the vectors containing the 4, 8, 10 and 12As. The CD
protein expression levels of these vectors range from 4 to 25% that
of the yCD2-6A vector (Figure 2B). The drastic reduction of the
yCD2 protein expression with similar expression levels of the
cellular viral RNA suggested that the length of oligo A in the
bifurcation region in the IRES can have a large effect on gene
expression at the post-transcriptional level. Relative
intracellular CD enzymatic activity was also measure by adding 5 FC
to the cultures and measuring 5-FU after an hour. The differences
in activity were ranked similarly to the Western blot data, but
were not as marked. This can be attributed to limitations in a
cell-based assay and to the low availability of intracellular 5-FC
which was below the Km for the enzyme in the assay utilized.
Therefore, the effect of the number of A's in the loop were
analyzed with another transgene for which the protein expression
assay was well defined. Also, using a different transgene would
allow a determination of whether or not the alteration in yCD2
protein expression with change in number of A's in the A bulge is
transgene-specific.
[00138] An equivalent set of RRVs encoding GFP were generated. The
viral titers of these vectors were also comparable to one another
and this data looked very similar to that with the yCD2 transgene
(Fig 1C). The GFP expression levels were measured using flow
cytometry by gating the GFP-positive cells. The mean fluorescent
intensity (MFI) of each vector was normalized to the cellular viral
RNA level and calculated relative to the GFP-6A vector. The
results (Figure 2C), from this set of vector were consistent with
those observed with yCD2 vectors (Figure 2B) and the vectors
containing the 6As expresses the highest level of protein from the
transgene in both sets of vectors. Furthermore, due to the
sensitivity of the detection method, a remarkable difference in GFP
expression level was revealed, showing approximately 96% and 99%
decrease in GFP expressed by the vectors containing the 10As and
12As, respectively. In both sets of the vectors, RRV with 7As
showed an approximately 30% decrease in protein expression.
Consistent with findings reported by Hoffman et al., RRV with 4As
61
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
and 5As, respectively, showed similar phenotype as 868A4 described
by Hoffman et al. with markedly reduced protein translation
efficiency compared to RRV with 6As.
[00139] The disclosure demonstrates that the length of the A-bulge
in the J-K bifurcation region affects expression of the transgene
downstream of the IRES presumably through effects on the
translation efficiency. Previous findings implying that the
context around AUG11, the spacing between the polypyrimidine tract
located in the 3' IRES and the first AUG in the cistron as well as
the arrangement of cistron on the mRNA all play a role in
modulating protein translation. The data show that the presence of
6 As provides the highest level of transgene protein expression and
alteration of the numbers of As in the A bulge by contraction or
expansion of 2-4 nucleotides could significantly affect the
expression level of the transgene downstream of the IRES. The
protein expression results suggest that the optimum IRES
configuration in general is with 6As in the bifurcation loop, while
7As is acceptable probably due to the rescue by polypyrimidine
tract binding protein (PTB) previously described by Kaminiski et
al., showing that lengthening the bulge A from 6 As to 7As rendered
IRES function dependent on polypyrimidine tract binding protein
(PTB). It is possible the vector variants with 4, 5, 8, 10 and 12As
also require binding of PBT to the polypyrimidine tract for
efficient protein translation and that these vector variants
significantly distort the secondary and tertiary structure of the
IRES and thus compromise the binding of PBT and/or other trans-
acting factors to the polypyrimidine tract, and hence diminish the
PBT-mediated rescue of translational activity. Other than the EMCV
IRES synthetic constructs made for bicistronic expression vectors,
the mutations in the number of adenosine residues in the A-bulge
has not been described in EMCV. It seems unlikely that the
alterations in number of adenosine residue are driven by any kind
of selective pressure, but rather happen during extensive RRV
replication over 180 days in the mice, due to its mutation-prone
reverse transcriptase activity. In conclusion, in RRVs including
the ECMV IRES, it is preferable to use the 6A version of the IRES,
not only because of the enhanced transgene expression, but also
62
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
because of the more frequent direction of oligo A number drift
seems to be preferentially towards longer oligo A in the bulge.
Thus, if the bulge starts with 6 A's there is more tolerance in
terms of transgene expression to the acquisition of a single extra
adenosine nucleotide.
[00140] Construction of RRVs containing a minimum IRES with 6A
produce similar level of titer, viral transcript and transgene
protein expression as the RRV containing the 6A alone. It has been
shown that mutants generated by progressive deletion from the 5'
EMCV IRES have differential translational efficiencies in vitro
(Duke et al., J Virol. 66:1602-9 1992). Here, RRVs containing
various lengths of minimum IRES are generated, designated 6A-406
(e.g., base 123 to 544 of SEQ ID NO:41) and 6A-466 (base 183 to 544
of SEQ ID NO:41) (see, Figure 5). Other similar constructs with
other numbers of A's and either the 406 or 466 IRES sequence can be
constructed (designated 7A-406 and 7A-466 (referring to a 7A
containing minimal IRES, etc.) and perform approximately in
proportion to constructs with the equivalent number of A's and the
full length IRES. Each construct is generated by DNA synthesis
(BioBasics Inc.) of the entire IRES cassette with a Mlu I at the 5'
end and a Psi I at the 3'end, respectively, for direct replacement
of the equivalent cassette in the RRV backbone. All DNA fragments
are confirmed by sequencing prior and post cloning into the RRV
backbone. The RRV constructs containing the yCD2 transgene were
designated using the name of the transgene followed by the number
of A's in the A bulge (/.e. yCD2-4A contains yCD2 transgene and 4As
in the A bulge in the IRES). The data show that titer from
transiently transfected 293T and maximally infected HT1080 cells
are similar to that of the bulge A variants. Protein expression of
yCD2 is measured from fully infected U87-MG cells. The 6A-406
variant expresses similar level (within 2, 5 or 10 fold) of yCD2
protein in a comparison to the 6A variant with full-length IRES.
The 6A-466 variant which carries a further deletion of the 5' IRES
shows expression of yCD2. In addition, data from replication
kinetics and vector stability by serial infection also show that
both 6A-406 and 6A-466 vectors are stable up to at least 10 cycles
of infection.
63
CA 02920049 2016-01-28
WO 2015/021077
PCT/US2014/049831
[0 0 1 4 1 ] A number of embodiments of the disclosure have been
described. Nevertheless, it will be understood that various
modifications may be made without departing from the spirit and
scope of the disclosure. Accordingly, other embodiments are within
the scope of the following claims.
64