Note: Descriptions are shown in the official language in which they were submitted.
81786497
SYSTEM AND METHOD FOR DEFORMING AND ANALYZING PARTICLES
CROSS-REFERENCE TO RELATED APPLICATIONS
[0001] This Application claims priority to U.S. Provisional Patent
Application No.
61/718,077 filed on October, 24, 2012, U.S. Provisional Patent Application No.
61/718,092
filed on October 24, 2012, and U.S. Provisional Patent Application No.
61/719,171 filed on
October 26, 2012. Priority is claimed pursuant to 35 U.S.C. 119.
STATEMENT REGARDING FEDERALLY SPONSORED
RESEARCH AND DEVELOPMENT
[0002] This invention was made with Government support under Grant No. N66001-
11-1-
4125 awarded by the Defense Advanced Research Projects Agency and Grant No.
1150588
awarded by the National Science Foundation. The Government has certain rights
in this
invention.
TECHNICAL FIELD
[0003] This invention relates generally to the cytometer field, and more
specifically to an
improved system and method for deforming and analyzing particles such as cells
in the
cytometer field.
BACKGROUND
[0004] There is growing evidence that cell deformability is a useful
indicator of abnormal
cytoskeletal changes, and may provide a label-free biomarker for determining
cell states or
properties, such as metastatic potential, cell cycle stage, degree of
differentiation, and
leukocyte activation. Clinically, a measure of metastatic potential could
guide treatment
decisions, or a measure of degree of differentiation could prevent
transplantation of
undifferentiated tumorigenic stem cells in regenerative therapies. For drug
discovery and
personalized medicine, a measure of cytoskeletal integrity could allow
screening for
cytoskeletal-acting drugs or evaluation of cytoskeletal drug resistance in
biopsied samples.
Cell deformability can further provide insight into mechanotransduction
pathways for
1
CA 2920132 2020-01-22
CA 02920132 2016-02-01
WO 2014/113110
PCMJS2013/065747
different cell lines, opening new avenues of discovery in cellular
biomechanics. Currently,
implementation of these techniques and analyses is cost-prohibitive and labor-
intensive,
which is a substantial limiting factor in clinical and research applications.
Current platforms
for cell deformation techniques and analyses suffer from a large number of
limitations,
including one or more of the following: limited throughput, inconsistency,
limited
characterization of sample heterogeneity, speed, and labor intensity. In
particular, platforms
optimized for biophysics research operate at rates of approximately 1
cell/minute, which
significantly hampers one's ability to process and analyze a large number of
heterogeneous
particles.
[0005] Thus, there is a need in the cytometer field to create a new and
improved system
and method for deforming and analyzing particles. This invention provides such
a new and
improved system and method.
SUMMARY
[0006] In one embodiment, a system for deforming and analyzing a plurality
of particles
carried in a sample volume includes a substrate defining an inlet, configured
to receive the
sample volume, and an outlet; a fluidic pathway fluidly coupled to the inlet
and the outlet and
defining a delivery region located upstream of a deformation region configured
to deform
one or more particles in the plurality of particles, wherein the fluidic
pathway includes a first
branch configured to deliver a first portion of the sample volume in a first
flow, and a second
branch configured to deliver a second portion of the sample volume in a second
flow that
opposes the first flow, wherein an intersection of the first flow and the
second flow defines
the deformation region, and wherein the delivery region is fluidically coupled
with at least
one of the first and the second branches; a detection module comprising a
sensor configured
to generate a morphology dataset characterizing deformation of one or more
particles in the
plurality of particles, and comprising a photodetector configured to generate
a fluorescence
dataset characterizing fluorescence of one or more particles in the plurality
of particles; and a
processor configured to output an analysis of the plurality of particles based
at least in part on
the morphology dataset and the fluorescent dataset for the plurality of
particles.
[0007] In another embodiment, a system for deforming and analyzing a
plurality of
particles carried in a sample volume, the system includes a substrate defining
an inlet and an
outlet and a fluidic pathway interposed there between; a focusing region
disposed in the
2
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
fluidic pathway and coupled at a downstream end thereof to a trifurcation
comprising a
central branch, a first side branch, and a second side branch; a deformation
region disposed
downstream of the trifurcation comprising an intersection formed between the
central branch,
the first side branch, and the second side branch, wherein first side branch
and the second
side branch intersect with the central branch is a substantially orthogonal
orientation; a
detection module comprising a sensor configured to generate a morphology
dataset
characterizing deformation of one or more particles in the plurality of
particles, and
comprising a photodetector configured to generate a fluorescence dataset
characterizing
fluorescence of one or more particles in the plurality of particles; and
a processor configured to output an analysis of the plurality of particles
based at least in part
on the deformation dataset and the fluorescent dataset for the plurality of
particles.
[0008] In another embodiment, a method for deforming and analyzing a
plurality of
particles carried in a sample volume, the method includes: receiving the
sample volume
comprising the plurality of particles; diverting a first portion of the sample
volume in a first
flow and a second portion of the sample volume in a second flow, substantially
opposed to
the first flow, such that an intersection of the first and the second flows
defines a deformation
region; delivering the plurality of particles into the deformation region;
generating a
morphology dataset characterizing deformation of one or more particles of the
plurality of
particles within the deformation region;
generating a fluorescence dataset characterizing fluorescence of one or more
particles of the
plurality of particles within the deformation region; and outputting an
analysis of the plurality
of particles based at least in part on the deformation dataset and the
fluorescent dataset for the
plurality of particles.
[0009] In another embodiment, a system for deforming and analyzing a
plurality of
particles carried in a sample volume, the system includes: a substrate
defining an inlet,
configured to receive the sample volume, and an outlet; and a fluidic pathway
fluidly coupled
to the inlet and the outlet. The fluidic pathway includes: a delivery region
configured to
receive the plurality of particles from the inlet and focus the plurality of
particles from a
random distribution to a focused state, a deformation region defining an
intersection located
downstream of the delivery region and coupled to the outlet, and wherein the
deformation
region is configured to receive the plurality of particles from the delivery
region and to
transmit each particle in the plurality of particles into the intersection
from a single direction,
3
' 81786497
a first branch fluidly coupled to the deformation region and configured to
transmit a first flow
into the intersection, and a second branch fluidly coupled to the deformation
region and
configured to transmit a second flow, substantially opposing the first flow,
into the
intersection, wherein the first flow and the second flow are configured to
induce extension of
one or more particles in the plurality of particles.
[0010] In another embodiment, a system for deforming and analyzing a plurality
of
particles carried in a sample volume, the system includes: a substrate
defining an inlet,
configured to receive the sample volume, and an outlet; and a fluidic pathway
fluidly coupled
to the inlet and the outlet. The fluidic pathway includes: a delivery region
configured to
receive the plurality of particles from the inlet and focus the plurality of
particles from a
random distribution to a focused state, a deformation region coupled to the
outlet and defining
an intersection configured to receive and deform one or more particles of the
plurality of
particles, and a trifurcation fluidly coupled to the delivery region and the
intersection of the
deformation region by a first branch and a second branch, wherein the delivery
region is
configured to direct substantially all particles of the plurality of particles
into the first branch
in a first flow toward the intersection, and wherein the second branch is
configured to transmit
a second flow, substantially devoid of any particles of the plurality of
particles, wherein first
flow and second flow at the intersection induces extension of one or more
particles of the
plurality of particles.
[0010a] In another embodiment, there is provided a system for deforming and
analyzing a
plurality of particles carried in a sample volume, the system comprising: a
substrate defining
an inlet, configured to receive the sample volume, and an outlet; a fluidic
pathway fluidly
coupled to the inlet and the outlet and defining a delivery region comprising
a particle
focusing region located upstream of a deformation region configured to deform
one or more
particles in the plurality of particles, wherein the fluidic pathway includes
a first branch
configured to deliver a first portion of the sample volume in a first flow,
and a second branch
configured to deliver a second portion of the sample volume in a second flow
that opposes the
first flow and wherein the delivery region is configured to direct
substantially all of the
plurality of particles into the first branch and wherein the second branch
containing the second
portion of the sample volume is substantially free of particles, wherein an
intersection of the
4
CA 2920132 2020-01-22
81786497
first flow and the second flow defines the deformation region, and wherein the
delivery region
is fluidically coupled with at least one of the first and the second branches;
a detection module
comprising a sensor configured to generate a morphology dataset characterizing
deformation
of one or more particles in the plurality of particles, and comprising a
photodetector
configured to generate a fluorescence dataset characterizing fluorescence of
one or more
particles in the plurality of particles; and a processor configured to output
an analysis of the
plurality of particles based at least in part on the morphology dataset and
the fluorescent
dataset for the plurality of particles.
[0010b1 In another embodiment, there is provided a system for deforming and
analyzing a
plurality of particles carried in a sample volume, the system comprising: a
substrate defining
an inlet and an outlet and a fluidic pathway interposed therebetween; a
focusing region
disposed in the fluidic pathway and coupled at a downstream end thereof to a
trifurcation
comprising a central branch, a first side branch, and a second side branch; a
deformation
region disposed downstream of the trifurcation comprising an intersection
formed between the
central branch, the first side branch, and the second side branch, wherein
first side branch and
the second side branch intersect with the central branch is a substantially
orthogonal
orientation and wherein the central branch contains substantially all of the
particles from the
focusing region and the first side branch and the second side branch are
substantially free of
particles; a detection module comprising a sensor configured to generate a
morphology
dataset characterizing deformation of one or more particles in the plurality
of particles, and
comprising a photodetector configured to generate a fluorescence dataset
characterizing
fluorescence of one or more particles in the plurality of particles; and a
processor configured
to output an analysis of the plurality of particles based at least in part on
the deformation
dataset and the fluorescent dataset for the plurality of particles.
[0010c] In another embodiment, there is provided a method for deforming and
analyzing a
plurality of particles carried in a sample volume, the method comprising:
receiving the sample
volume comprising the plurality of particles; focusing the plurality of
particles from a random
distribution to a focused state in a microfluidic channel; diverting a first
portion of the sample
volume from the microfluidic channel in a first flow and a second portion of
the sample
volume from the microfluidic channel in a second flow, substantially opposed
to the first
4a
CA 2920132 2020-01-22
81786497
flow, such that an intersection of the first and the second flows defines a
deformation region,
and wherein the first flow contains substantially all of the plurality of
particles and the second
flow is substantially free of particles; delivering the plurality of particles
into the deformation
region from the first flow; generating a morphology dataset characterizing
deformation of one
or more particles of the plurality of particles within the deformation region;
generating a
fluorescence dataset characterizing fluorescence of one or more particles of
the plurality of
particles within the deformation region; and outputting an analysis of the
plurality of particles
based at least in part on the deformation dataset and the fluorescent dataset
for the plurality of
particles.
[0010d] In another embodiment, there is provided a system for deforming and
analyzing a
plurality of particles carried in a sample volume, the system comprising: a
substrate defining
an inlet, configured to receive the sample volume, and an outlet; and a
fluidic pathway fluidly
coupled to the inlet and the outlet and comprising: a delivery region
configured to receive the
plurality of particles from the inlet and focus the plurality of particles
from a random
distribution to a focused state, a deformation region defining an intersection
located
downstream of the delivery region and coupled to the outlet, and wherein the
deformation
region is configured to receive the plurality of particles from the delivery
region and to
transmit each particle in the plurality of particles into the intersection
from a single direction,
a first branch fluidly coupled to the deformation region and configured to
transmit a first flow
into the intersection, and a second branch fluidly coupled to the deformation
region and
configured to transmit a second flow, substantially opposing the first flow,
into the
intersection, wherein substantially all particles of the plurality of
particles are disposed in the
first branch and the second branch is substantially free of particles, and
wherein the first flow
and the second flow are configured to induce extension of one or more
particles in the
plurality of particles.
[0010e] In another embodiment, there is provided a system for deforming and
analyzing a
plurality of particles carried in a sample volume, the system comprising: a
substrate defining
an inlet, configured to receive the sample volume, and an outlet; and a
fluidic pathway fluidly
coupled to the inlet and the outlet and comprising: a delivery region
configured to receive the
plurality of particles from the inlet and focus the plurality of particles
from a random
4b
CA 2920132 2020-01-22
81786497
distribution to a focused state, a deformation region coupled to the outlet
and defining an
intersection configured to receive and deform one or more particles of the
plurality of
particles, and a trifurcation fluidly coupled to the delivery region and the
intersection of the
deformation region by a first branch, a second branch, and a third branch
wherein the delivery
region is configured to direct substantially all particles of the plurality of
particles into the first
branch in a first flow toward the intersection, and wherein the second branch
and the third
branch are configured to transmit second, and third flows, respectively,
substantially devoid of
any particles of the plurality of particles, wherein the second and third
flows at the
intersection induces extension of one or more particles of the plurality of
particles.
BRIEF DESCRIPTION OF THE FIGURES
[0011] FIGURE 1 is a schematic representation of an embodiment of a system for
deforming and analyzing particles;
[0012] FIGURES 2A and 2B are schematic representations of an embodiment of a
portion
of a system for deforming and analyzing particles;
[0013] FIGURES 3A and 3B depict a variation of a delivery region in an
embodiment of a
system for deforming and analyzing particles;
[0014] FIGURES 4A and 4B depict a variation of a delivery region in an
embodiment of a
system for deforming and analyzing particles;
[0015] FIGURE 5 depicts a variation of a deformation region in an embodiment
of a
system for deforming and analyzing particles;
4c
CA 2920132 2020-01-22
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
[0016] FIGURE 6A-6C depict variations of a deformation region in an
embodiment of a
system for deforming and analyzing particles;
[0017] FIGURE 7 depicts an example of a fluidic pathway in an embodiment of
a system
for deforming and analyzing particles;
[0018] FIGURE 8A depicts an example of a fluidic pathway in an embodiment of a
system for deforming and analyzing particles;
[0019] FIGURE 8B depicts another example of a fluidic pathway in an
embodiment of a
system for deforming and analyzing particles;
[0020] FIGURE 9A depicts an alternative example of a deformation region in
an
embodiment of a system for deforming and analyzing particles;
[0021] FIGURE 9B depicts an example of a fluidic pathway in an embodiment of a
system for deforming and analyzing particles using the deformation region
illustrated in
FIGURE 9A;
[0022] FIGURE 9C illustrates the fluidic pathway of FIGURE 9B with the
resistances
labeled for various branch and inlet channels;
[0023] FIGURE 9D illustrates an embodiment of a fluidic pathway that
combines off-axis
squeezing at a first deformation region followed by a secondary deformation
region in which
particles are subject to deformation at an intersection of opposing flows;
[0024] FIGURE 9E illustrates a simplified resistor diagram of the combined
HA-DC
device of FIGURE 9D.
[0025] FIGURE 9F illustrates another embodiment of a fluidic pathway in
which
hydropipette aspiration is combined with rapid inertial solution exchange for
integrated
sample preparation and analysis.
[0026] FIGURE 9G illustrates series of magnified images of selected regions
of the device
of FIGURE 9F.
[0027] FIGURES 10A-10C depict variations of a detection module in an
embodiment of a
system for deforming and analyzing particles;
[0028] FIGURE 11A-11C depict alternative variations of a detection module
in an
embodiment of a system for deforming and analyzing particles;
[0029] FIGURES 12A-12C depict alternative variations of a detection module
in an
embodiment of a system for deforming and analyzing particles;
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
[0030] FIGURE 13A-13C depict example particle characteristics extracted
using an
embodiment of a system for deforming and analyzing particles;
[0031] FIGURE 14 depicts an example synchronization method for an
embodiment of a
system for deforming and analyzing particles;
[0032] FIGURE 15 is a flowchart of an embodiment of a method for deforming and
analyzing particles; and
[0033] FIGURE 16 is a flowchart of an embodiment of a method for deforming and
analyzing particles.
DESCRIPTION OF THE ILLUSTRATED EMBODIMENTS
[0034] The following descriptions of the illustrated embodiments of the
invention are not
intended to limit the invention to this preferred embodiment, but rather to
enable any person
skilled in the art of flow cytometers to make and use this invention.
[0035] 1. System
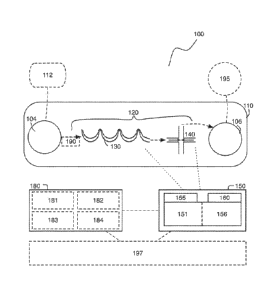
[0036] As shown in FIGURE 1, a system 100 according to one embodiment is
disclosed
for deforming and analyzing a plurality of particles carried in a sample
fluid. As used herein,
the terms "particle" or "particles" are meant to encompass small objects that
can be contained
within fluid flow. A particle may include a biological object such as a cell
or even an
organelle. According to this embodiment, the system 100 includes a substrate
110 defining
an inlet 104 and an outlet 106; a fluidic pathway 120 fluidly coupled to the
inlet 104 and the
outlet 106 and defining a delivery region 130 located upstream of a
deformation region 140
configured to deform one or more particles that enter the deformation region
140; a detection
module 150 including a sensor 155 configured to generate data characterizing
the
deformation of one or more particles contained within a plurality of particles
flowing through
the system 100 and a photodetector 160 configured to generate data
characterizing
fluorescence of each particle in the plurality of particles; and a processor
180 configured to
generate an analysis based upon deformation and fluorescence of the one or
more particles.
[0037] The system 100 functions to enable the deformation of single
particles in a high-
throughput and consistent manner, with the ability to simultaneously generate
and analyze
multiple data types characterizing the single particles. Preferably, the
system 100 further
functions to enable the generation of data that directly correlates surface
biomarkers of
phenotype with mechanical properties at the single-particle level. This can
allow the
6
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
generation of a direct quantitative comparison between biomolecular properties
and
mechanical properties. Preferably, the system 100 is used to process and
analyze biological
particles, such as cells, and in specific applications, the system 100 can be
used to analyze
leukocyte activation, stem cell differentiation, cellular response to drugs,
and cancer cell
malignancy by way of correlating cellular deformation with biomolecular
phenotypes using
fluorescence assays. Besides correlating to biomolecular phenotypes, combining
biomolecular and deformability-based data can provide additional
classification accuracy.
However, the system 100 can alternatively be used to process, deform, and
analyze any other
suitable biological particle or non-biological particles.
[0038] 1.1 System - Substrate
[0039] The substrate 110 functions to provide a platform by which particles
of interest can
be deformed and analyzed. The substrate preferably comprises microfluidic
elements that
enable deformation of the particles of interest, and facilitates data
generation from the
deformed particles of interest by defining a suitable configuration of the
microfluidic
elements relative to other elements of the system 100 (e.g., pump, detection
module, waste
chamber). In one variation, the microfluidic elements of the substrate include
an inlet 104 and
an outlet 106 for receiving a sample volume and transmitting a processed
sample volume,
respectively, from the substrate 110. In a first specific example, as shown in
FIGURES 2A
and 2B, the substrate 110 includes a single inlet 104 defmed at a first
surface of one end of
the substrate 110 and two outlets 106 defined at an opposite end of the
substrate 110.
However, other variations of the substrate 110 can comprise any other suitable
element(s) in
any suitable configuration that facilitates coupling with elements external to
the substrate 110
for deforming, processing, and analyzing a sample volume containing particles
of interest.
For example, the substrate 100 may include multiple inlets 104 and multiple
outlets 106. The
inlet(s) 104 and outlet(s) 106 of the substrate 110 can be defined at any
suitable end, at any
suitable surface, and/or within any suitable region of the substrate 110.
Furthermore, an inlet
104 can be configured to receive any suitable processing fluid (e.g., sheath
fluid, reagent,
buffer, wash, etc.) to facilitate sample processing.
[0040] In some variations, the substrate 110 can be configured to be a
reusable element
and in other variations, the substrate 110 can be configured to be a
disposable element. In
variations wherein the substrate 110 is reusable, the substrate 110 can be
configured to couple
to a module for washing or flushing the substrate 110 (e.g., through the inlet
or outlet) after
7
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
uses of the substrate. Alternatively, in these variations of a reusable
substrate 110, the
substrate 110 can be configured to be self-cleaning or self-washing (e.g.,
using surface
coatings, by geometric configuration of fluidic pathways, etc.). In other
variations, the
substrate can be configured to be reusable for a certain number of uses or
until failure (e.g.,
failure by clogging), and then disposed to be replaced. In any of these
variations, the substrate
110 can comprise aligners (e.g., slots, pins, guides, etc.) configured to
facilitate alignment of
the substrate 110 within the system 100 and relatively to other elements of
the system 100.
The substrate 110 may be a monolithic substrate or the substrate 110 may be
formed from
multiple layers that are bonded or otherwise secured to one another to form
the appropriate
micro fluidic elements within the substrate 110.
[0041] The inlet 104 functions to receive a sample volume, including a
plurality of
particles of interest, to initiate processing and analysis of the particles
within the substrate
110. Preferably, the inlet 104 is configured to receive the sample volume and
the plurality of
particles from a fluid delivery module including a pump 112, as shown in
FIGURE 1;
however, the inlet can be configured to receive the sample volume in any other
suitable
manner. In other variations, the pump 112 can be a syringe pump containing the
sample
volume and the plurality of particles, or any other fluid pump configured to
provide at least
one of a positive pressure and a negative pressure, in order to deliver the
sample volume and
the plurality of particles into the inlet 104. Additionally, the pump 112 can
be manually or
automatically operated, but is preferably configured to transmit the sample
volume into the
inlet 104 at a uniform flow rate that can be adjusted. Furthermore, the pump
112 can be
coupled to any suitable conduit (e.g., tubing, conduit, manifold) configured
to transmit the
sample volume (e.g., from a sample well coupled to the substrate) into the
inlet 104, and can
comprise a valve and/or a pressure sensor in order to control and detect flow
parameters. In
one specific example, the pump 112 is automatically controlled and configured
to provide an
adjustable flow rate that enables particle focusing and achieves a desired
particle
deformation. Alternatively, the pump 112 may be controlled to achieve a
particular particle
throughput. Furthermore, in still other alternative examples, the pump 112 may
be
configured to deliver a sample volume including cells (i.e., particles of
interest) with a
density between 200,000 cells/mL and 8 million cells/mL.
[0042] In specific applications with biological particles, the plurality of
particles (e.g.,
cells) can be prepared for fluorescence¨based assays prior to delivery into
the inlet 104 of the
8
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
substrate 110. Preferably, the plurality of particles is prepared using an
approach that omits
fixation, which can affect deformation of the particles in unknown and/or
unpredictable ways.
The cells are preferably labeled with at least one fluorescently-labeled
biochemical probe
(e.g., SSEA4 probe, 0ct4 probe, TRA-1-60 probe, CD34 probe, CD38 probe, HLA-DR
probe, CD64 probe, etc.) bound to cell surface proteins or other biomarkers,
which facilitates
identification of biomolecular markers that can be extracted as fluorescence
data. The cells
can additionally be processed with cell-permeable stains to facilitate
identification. However,
the plurality of particles can be processed in any other suitable manner prior
to delivery into
an inlet 104, and/or during transmission through any element of the system 100
(e.g., fluidic
pathway, etc.).
[0043] Preferably, the inlet 104 is configured to form a hermetic seal
about the fluid
delivery module and/or the pump 112, such that the sample volume does not leak
from the
inlet 104; furthermore, the inlet 104 is preferably configured to be
reversibly coupled to the
fluid delivery module and/or the pump 112. However, the inlet 104 can be
configured to
couple to the fluid delivery module and/or the pump 112 in any other suitable
manner. In one
variation, the inlet 104 is configured to couple to the pump 112 by a threaded
male-female
coupling configured to produce a hermetic seal. In another variation, the
inlet 104 can
additionally or alternatively comprise an o-ring configured to facilitate
generation of the
hermetic seal. In still other variations, the inlet 104 can additionally or
alternatively comprise
any other suitable sealant (e.g., resealable septum, silicone sealant, sealing
putty) for
generation of the hermetic seal.
[0044] The outlet 106 functions to transmit the sample volume including the
plurality of
particles of interest from the substrate 110, after the sample volume has been
processed.
Preferably, the outlet 106 is configured to transmit the processed sample
volume as waste
from the substrate 110; however, the outlet 106 can alternatively be
configured to transmit
the processed sample volume from the substrate 110 for further processing and
analysis. In
one variation, the outlet 106 can be configured to couple to a waste chamber
as seen in
FIGURE 2A that is configured to receive waste fluids from the outlet 106. In
this variation,
the waste chamber can be integrated (e.g., of unitary construction, physically
coextensive)
with the substrate 110, such that the outlet 106 is configured to deliver
waste fluids into the
waste chamber of the substrate 110. In another variation, the outlet 106 can
be configured to
couple to a fluid conduit that delivers the processed sample volume to another
module for
9
81786497
further processing. Similar to the inlet 104, the outlet 106 is preferably
configured to form a
hermetic seal about a point of coupling (e.g., to a waste chamber, to a module
for further
processing), and can comprise any one or more of: a male-female threaded
coupling, an o-
ring, septum, and a sealant that facilitates generation of the hermetic seal.
In other variations,
however, the outlet 106 can be configured to couple to any other suitable
element in any
other suitable manner, for example, using one or more microfluidic conduits or
channels.
[0045] The substrate 110 is preferably composed of an optically
transparent material with
no autofluorescence, in order to facilitate detection of sample particle
characteristics (e.g.,
deformation characteristics, mechanical properties, fluorescence
characteristics) without
optical interference from the substrate 110. However, the substrate 110 can be
sufficiently
transparent and/or composed of a material with sufficiently low
autofluorescence in order to
enable detection of particle characteristics. Additionally, the substrate 110
can comprise any
structures or elements configured to reflect light toward particles passing
through the
substrate 110, in order to enhance detection of particle characteristics and
parameters by a
detection module 150. Furthermore, the substrate 110 can include any suitable
structure(s) for
microfluidic applications, including glass structures, polymeric structures,
or composite
structures. In one variation, the substrate 110 can be composed of a polymeric
material that is
processable to form the inlet(s) 104, the outlet(s) 106, and/or any other
suitable element(s) of
the substrate 110. In a specific example of this variation, the substrate 110
is composed of
polydimethylsiloxane (PDMS) contained on a optically transparent solid surface
such as
glass, with inlet(s) 104, outlet(s) 106, and microfluidic elements defined by
a lithographic
process (e.g., photolithography), such as a process described in U.S. Pub. No.
2013/0177935,
entitled "Method and Device for High Throughput Cell Deformability
Measurements".
In other variations of this example, substrate features can be additionally or
alternatively
defined by any other suitable process (e.g., micromachining, molding, etching,
3D printing, etc.). Alternatively, the substrate 110 can comprise or be
composed of any
other suitable material, processable by any other suitable method to form
features of the
substrate 110 (e.g., inlets, outlets, fluidic pathways, etc.).
[0046] 1.2 System - Fluidic Pathway
[0047] The fluidic pathway 120, as shown in FIGURES 1 and 2A-2B, is preferably
fluidically coupled to the inlet(s) 104 and the outlet(s) 106 of the substrate
110, and functions
CA 2920132 2020-01-22
81786497
to facilitate focusing and deformation of the plurality of particles of the
sample volume. The
fluidic pathway 120 is also preferably configured between the inlet(s) 104 and
the outlet(s)
106, such that any pressure differential (e.g., generated by the pump 112)
along the fluidic
pathway 120 facilitates fluid flow through at least a portion of the fluidic
pathway 120.
Preferably, the fluidic pathway 120 is at least partially defined within the
interior of the
substrate 110 (e.g., by a lithographic process, by etching, by micromachining,
by 3D printing,
etc.); however, the fluidic pathway 120 can be partially or completely defined
external to the
substrate 110. Preferably, the fluidic pathway 120 comprises a delivery region
130 that is
located upstream of a deformation region 140, such that the plurality of
particles of the
sample volume can be transmitted from an inlet 104, focused within the
delivery region 130,
and transmitted to the deformation region 140 for deformation and analysis. In
this
configuration, the delivery region 130 is interposed between the inlet(s) 104
and the
deformation region 140.
[0048] The delivery region 130 functions to focus at least a subset of
the plurality of
particles into the deformation region 140 along a common equilibrium point or
streamline,
such that each particle in the plurality of particles experiences sufficiently
uniform flow and
deformation conditions in a manner that limits experimental variability.
Additionally, the
delivery region 130 is preferably configured to cooperate with conditions
provided by the
pump 112, such that the plurality of particles flows in single file at a
substantially uniform
velocity (e.g., with particle size-dependent fluctuations in velocity in 5-10%
range) into the
deformation region 140. Alternatively, the delivery region 130 and the pump
112 can be
configured to transmit the plurality of particles in non-single file, and/or
with any suitable
velocity profile (e.g., variable velocity profile) into the deformation region
140. Preferably,
the delivery region 130 provides inertial focusing and can comprise at least
one curved
confined channel 132 configured to provide inertial focusing of the plurality
of particles into
the deformation region 140. In a first variation of the delivery region 130',
an example of
which is shown in FIGURE 3A, the curved channel 132 can be characterized by a
profile
described, for example, in D.R. Gossett et al., "Particle focusing mechanisms
in curving confined flows," Analytical Chemistry, 81, 8459 (2009).
The curved channels 132 may be symmetric or asymmetric although
asymmetric curved channels 132 are generally preferred. Furthermore, in this
variation, the delivery region 130 can comprise multiple curved confined
channels 132
11
CA 2920132 2020-01-22
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
coupled in series, as shown in FIGURE 3A, that enable focusing of particles
into the
deformation region 140. In the first variation of the delivery region 130, the
curved channel
132 configuration focuses the plurality of particles along a single projected
line, with each
particle positioned within one of two focal planes, as shown in FIGURE 3B.
While this
embodiment focuses particles at two focal planes as seen in FIGURE 3B,
particles at both
locations can be imaged using a single detection module 150 that operates at a
relatively low
magnification. At higher magnifications, image processing may be needed to
extract images
at the two focal planes for deformation analysis.
[0049] In a second variation of the delivery region 130 as shown in FIGURES
4A and
4B, the delivery region comprises a straight channel 133 that is interspersed
with a plurality
of serially arrayed constrictions in height 134, orthogonally arranged
relative to the flow
direction that provides focusing based upon inertial focusing and geometry-
induced
secondary flows. The straight channel 133 in the second variation is
preferably defined by a
low aspect ratio (defined as height divided by width), and the combination of
inertial
focusing upstream and a pair of local helical secondary flows induced by the
height
constrictions 134 provides focusing of each particle in the plurality of
particles, in sequence,
to a single position. In this variation of the delivery region 130", at a
finite Reynolds number
(Re), particle migration in the straight channel 133 occurs due to a balance
of two inertial lift
forces: shear-gradient (FSL) and wall-effect (FWL) lift forces. An interaction
between a
particle wake and a wall of a channel of the delivery region 130 produces a
FWL directed
toward the channel centerline, while a parabolic velocity profile causes a
shear-gradient
induced FSL directed toward a channel wall throughout the channel, except
where it is zero at
the channel centerline; the balance of the FSL and FWL forces thus leads to
well-defined
equilibrium particle positions (e.g., along centerlines of channel walls for a
channel with a
rectangular cross section, as in FIGURE 4B. Then, the plurality of height
constrictions 134
induce a pair of helical secondary flows configured to induce lateral motions
that compete
with the inertial lift forces to direct the plurality of particles into a
single particle position on
a channel wall opposite to the plurality of height constrictions 134, as shown
in FIGURES 4A
and 4B.
[0050] In a specific example of the second variation of the delivery region
130, the
straight channel 133 is a rectangular channel with an aspect ratio of
approximately 0.5 with a
width of 84 micrometers, a height of 41.5 micrometers, and a length of 6 cm.
In the specific
12
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
example, the delivery region comprises thirty (30) constrictions in height
that are 21
micrometers in height, 40 micrometers in length, and spaced apart by 1 mm. It
should be
understood that the particular dimensions discussed above should be regarded
as exemplary
as other dimensions for the channel and the constrictions may be used.
Further, as disclosed
herein, a different number of constrictions (e.g., fewer than thirty (30)) may
be used to focus
the plurality of particles. Prior to entering a height constriction 134, the
plurality of particles
are focused along centerlines proximal to each of two to four walls of the
straight channel
133, depending on aspect ratio. Then, after successively entering each height
constriction 134
in the plurality of height constrictions 134, the particles of the plurality
of particles deviate
toward a single equilibrium position based upon a balance between strong FSL
forces and
weaker FWL forces. In the specific example, focusing to a single stream
defining a single
equilibrium position achieved a focusing efficiency (i.e., percentage of
particles reaching the
equilibrium position) of 99.77% after the plurality of particles entered
approximately twenty-
five (25) height constrictions of the plurality of height constrictions 134.
The height
constrictions 134 may project upward from a lower base or, alternatively,
project downward
from an upper surface. Furthermore, the full width at half maximum (FWHM)
defining
focusing tightness was 10.995 micrometers in the delivery region for 10
micrometer diameter
particles, indicating sufficiently narrow particle focusing. Additionally,
focusing in the
specific example of the second variation improved with Re, such that at Re =
83.33, all
particles in the plurality of particles were focused at a single equilibrium
position, facilitating
measurements by a detection module 150 (e.g., a module defining a single focal
depth). In
alternatives to the second variation, the straight channel 133 can be replaced
by a curved
channel 132, such as a curved channel described in the first variation of the
delivery region
130 described above. Variations using a curved channel 132 can decrease a
total channel
length used for the delivery region 130.
[0051] In alternative variations, the delivery region 130 can be configured
for any one or
more of the following types of focusing: hydrodynamic focusing, focusing using
a sheath
fluid, dielectrophoretic focusing, ultrasonic focusing, magnetic focusing, and
any other
suitable focusing method. In one example, the delivery region 130 can be
configured to direct
the plurality of particles into a branch of the fluidic pathway 120 along a
common streamline,
and simultaneously, to direct portions of the sample volume not including the
plurality of
particles into other branches of the fluidic pathway 120. As such, the
delivery region 130 can
13
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
be used to separate the plurality of particles from the sample volume, and to
utilize a portion
of the sample volume for a subsequent use. For example, one subsequent use of
a sample
volume that does not contain particles includes using the diverted sample
volume to squeeze
particles. This can be seen, for example, in the trifurcation structure of
FIGURE 7 whereby
two branches 121', 123' divert fluid that is free of particles that is later
used in a deformation
region 140. Furthermore, the delivery region 130 is preferably configured to
direct the
plurality of particles along a centerline of a channel of the fluidic pathway
120 to facilitate
measurements by a detection module 150; however, the delivery region 130 can
be
additionally or alternatively be configured to direct the plurality of
particles along any
suitable portion (e.g., centerline, periphery) of a channel of the fluidic
pathway 120 or a
branch of the fluidic pathway 120, in order to divert the plurality of
particles into specific
regions for processing.
[0052] The deformation region 140 functions to deform one or more of the
plurality of
particles by using opposing flows, according to one embodiment, as shown in
FIGURE 5. In
this embodiment, the deformation region 140 is formed at an intersection of
opposing flows,
whereby a particle entering the intersection of the opposing flows undergoes
deceleration and
is compressed by the opposing flows, leading to compression of a particle
along one axis and
extension of each particle along another axis. However, alternative variations
of the
deformation region can mechanically deform the plurality of particles using
any other
suitable mechanism. In the embodiment of FIGURE 5, the opposing flows are
substantially
coaxially aligned and flow anti-parallel to each other; however, the opposing
flows can be
unaligned and/or not flow in anti-parallel directions. In the embodiment of
FIGURE 5, the
particles enter from only one side of the extension region 140. The opposing
flow enters the
extension region 140 but is free of particles. Preferably, a first flow and a
second flow in the
opposing flows are generated from the sample volume (i.e., in a self-sheathing
manner), such
that a first portion of the sample volume is used to generate the first flow
and a second
portion of the sample volume is used to generated the second flow that opposes
the first flow.
This can be achieved at branches of the fluidic pathway 120 that are
configured to diverge
and/or converge (e.g., by way of bifurcations, trifurcations, etc.). This is
seen, for example,
in the embodiments of FIGURES 1. 2A, 7, 8A, 8B, 9B, 9C, and 9D.
[0053] Preferably, the deformation region 140, in cooperation with flow
conditions
provided by the pump and the delivery region 140, generates a suitable amount
of
14
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
deformation that is substantially uniform across the plurality of particles
that have the same
mechanical characteristics and does not result in saturation of measurements.
For instance, a
low flow rate generated by the pump can result in non-uniform deformation at
the
deformation region 140, and a high flow rate generated by the pump can result
in particles
being deformed beyond an imaging window and/or particle lysis, leading to
measurement
saturation. The flow rate(s) used to deform the plurality of particles at the
deformation region
140 is preferably associated with a cross-sectional dimension (e.g., diameter,
width) of at
least a portion of the fluidic pathway 120 (e.g., branch, delivery region,
deformation region),
with higher flow rates required for larger cross-sections. In one variation,
the flow conditions
provided by the pump can be governed based upon an analysis of channel
resistances (e.g., a
ratio of resistances between flow branches), which at least partially depend
upon a cross-
sectional dimension. In examples of this variation, as shown in FIGURES 6A-6C,
a first flow
and a second flow in the opposing flows arc designed to have a ratio of
resistances that
generates a suitable opposing flow profile, while maintaining a sufficient
number of particles
(e.g., 95% of the plurality of particles) within one of the first flow and the
second flow. For
example, with respect to FIGURE 6A, the first flow may include substantially
all of the
plurality of particles while the second, opposing flow is substantially free
of particles. In
other examples, a first flow and a second flow in the opposing flows can have
matched or
unmatched resistances, in order to generate a desired deformation of each
particle in the
plurality of particles. For example, with reference to FIGURE 6B, the
resistance of Routlet 2
may be larger than the resistance of R-
- 1 in which case a larger percentage of particles
will
exit the deformation region 140 via R-
- 1 =
[0054] In one embodiment, the deformation region 140 receives the plurality
of particles
from only one flow in the opposing flows that enter the deformation region
140, such that a
first flow provides the focused plurality of particles (i.e., from the
delivery region 130) and at
least one other flow opposes the first flow at an intersection to generate the
deformation
region 140. The plurality of particles is thus configured to enter the
deformation region 140
from a single direction. The single-direction design aspect is important when
used in
conjunction with fluorescent detection because fluorescent measurements can be
made in a
single location upstream of the deformation region 140 where the velocity of
entering
particles is substantially uniform. However, the plurality of particles can
alternatively be
divided into multiple flows of the opposing flows, and configured to enter an
intersection of
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
the opposing flows (i.e., a deformation region 140) from at least two
directions for
deformation. In variations wherein the plurality of particles is divided into
multiple flows, the
multiple flows each preferably comprise a delivery region 130 to focus
particles along
common streamlines prior to deformation. However, any portion of the multiple
flows can
omit a delivery region 130 in other variations.
[0055] In one variation, the fluidic pathway 120 comprises a first branch
configured to
deliver a first portion of the sample volume in a first flow, and a second
portion of the sample
volume in a second flow, such that sample volume is divided into at least two
flows that
cooperate to focus and deform the plurality of particles. In a first example
of this variation, as
shown in FIGURE 7, the fluidic pathway 120' includes a trifurcation 125' that
divides the
sample volume into a first branch 121' in a first flow, a second branch 122'
in a second flow,
and a third branch 123' in a third flow. In the first example, the delivery
region 130' is
coupled to the first branch 121', the second branch 122', and the third branch
123' of the
trifurcation 125', in a manner that focuses substantially all of the plurality
of particles into the
second branch 122' of the trifurcation. Additionally, in the first example,
the first and the
third flows are substantially devoid of particles of the plurality of
particles, and the first and
the third branches 121', 123' are configured to direct the first and the third
flows,
respectively, in a direction that opposes the second flow of the second branch
122'
(illustrated by arrow A in FIGURE 7). In the first example, the intersection
of the first, the
second, and the third flows at a point of opposition, forms the deformation
region 140 for
deformation of the plurality of particles. Furthermore, in the first example,
the deformation
region 140 is configured to couple to a first outlet 106 and a second outlet
106', for
transmission of processed sample fluid out of the substrate 110. In variations
of the first
example, the delivery region 130' can be configured to divert the plurality of
particles into
any one or more of the first, the second, and the third branches 121', 122,
123', and the
fluidic pathway 120' can be configured to couple to any suitable number of
inlets 104 and
outlets 106 for reception of the sample volume (or other fluids) and
transmission of fluids
from the substrate 110.
[0056] FIGURE 6C schematically illustrates the fluidic resistances of the
trifurcation
embodiment of FIGURE 7. R1 represents the fluidic resistance in the second
branch 122'.
R2 represents the fluidic resistance in the return sheath flow entering the
deformation region
16
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
140. R3 represents the fluidic resistance in the first and third branches
121', 123'. In this
embodiment, as part of the design criteria, R1=R3/2+R2.
[0057] In another embodiment, as shown in FIGURE 8A, the fluidic pathway
120"
includes a bifurcation 124" that divides the sample volume into a first branch
121" in a first
flow and a second branch 122" in a second flow, wherein the first flow and the
second flow
each contain a subset of the plurality of particles of the sample volume. In
the second
example, the delivery region 130" is coupled to the first branch 121"
downstream of the
bifurcation 124", and a second delivery region 131" is coupled to the second
branch 122'
downstream of the bifurcation 124", such that the subsets of the plurality of
particles are
focused within the delivery region 130" and the second delivery region 131".
Furthermore,
in the second example the first branch 121" and the second branch 122" are
configured to
direct the first flow and the second flow, respectively, in opposing
directions downstream of
the delivery regions 130", 131", such that an intersection of the first flow
and the second
flow defines the deformation region 140". The deformation region 140" in the
second
example is configured to couple to a first outlet 106 and a second outlet
106', for
transmission of processed sample fluid out of the substrate 110. In one
alternative
embodiment, the fluidic pathway 120" can be configured to divert a first
portion of the
sample volume (e.g., by inertial focusing, by using multiple inlets), with
substantially all
particles of the plurality of particles, into the first branch 121 such that
the second branch
122" does not receive any particle of the plurality of particles in the second
flow (or vice
versa). In this variation of the second example, the second delivery region
131" can be
omitted, such that the first branch is configured to focus the plurality of
particles into the
deformation region 140" formed at the intersection of the first and the second
branches
121", 122". In this variation of the second example, the plurality of
particles is thus
configured to enter the deformation region 140 from a single direction.
[0058] FIGURE 8B illustrates the alternative embodiment discussed above
wherein
substantially all particles of the plurality of particles are diverted into
the first branch 121"
while the second branch 122" is substantially free of particles. In this
example, the
bifurcation 124" ' initiates from a curved portion of an upstream focusing
region 127
whereby the particles are preferentially aligned along fluid streamlines that
are shunted to the
first branch 121'". The particles then pass through a delivery region 130"
prior to entering
an imaging region 129 located immediately upstream of a deformation region
140'".
17
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
Particles leave the deformation region 140" via one or both outlets 106",
106". A filter
190 is illustrated coupled to the upstream of the focusing region 127.
[0059] Furthermore, in alternative variations, each particle in the
plurality of particles can
be deformed by an opposing flow that has a direction component that is
transverse to a
prevailing direction of the flow containing the particles. In these
alternative variations, at
least one opposing flow can be generated with or without using any portion of
the sample
volume (e.g., by an outside flow that is injected or pumped to generate an
opposing flow). In
one alternative variation, an opposing flow that is coaxially aligned with,
but anti-parallel to a
flow containing at least a portion of the plurality of particles, can be
generated by an outside
flow that is transmitted through an inlet. In another alternative variation,
at least one opposing
flow can be generated in a direction not coaxially aligned with a flow
containing at least a
portion of the plurality of particles, such that the opposing flow has a
direction component
that is transverse to a prevailing direction of the flow containing the
particles. In this
alternative variation, the opposing flow is preferably substantially
orthogonal to a prevailing
direction of the flow containing the particles; however, the opposing flow can
alternatively be
non-orthogonal to and non-parallel to the flow containing the particles.
[0060] In one example of an alternative variation, as shown in FIGURE 9A, a
first flow
containing the plurality of particles is configured to enter the deformation
region 140 along a
first direction via central branch channel 122, after being focused in an
embodiment of the
delivery region 130 described above. A first inlet 135 and a second inlet 135'
at the
deformation region 140 are configured to provide a first opposing flow and a
second
opposing flow that are anti-parallel (i.e., off-axis) to the first opposing
flow. In one
embodiment, the first opposing flow and the second opposing flow are both
substantially
orthogonal to the first flow containing the plurality of particles. The first
opposing flow and
the second opposing flow in this example are equal and opposite; however, the
first opposing
flow and the second opposing flow can alternatively be non-equal and/or non-
opposite in
variations of this example. In addition, while the first inlet 135 and the
second inlet 135' are
illustrated as being substantially orthogonal to the axis of the first flow
containing the
plurality of particles in other alternative embodiments, the first inlet 135
and the second inlet
135' may intersect in the deformation region 140 in an off-axis manner yet not
be
substantially orthogonal to the axis of first flow.
18
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
[0061] In one embodiment of FIGURE 9A, the fluid that enters the first
inlet 135 and the
second inlet 135' are siphoned off from an upstream channel that contains the
focused
particles as seen in FIGURE 9B. Because the particles are aligned in the
center of the
channel due to focusing, side streams can be siphoned off the main flow while
letting the
focused particles remain in the central branch channel 122". This particular
embodiment is
referred to as hydropipette aspiration (HA). The branch channels 135, 135' are
subsequently
returned to apply a pinching flow at the deformation region 140. Particles are
then deformed
by the rejoining cell-free (in some embodiments) "sheath" fluid. In contrast
with
deformability cytometry (DC) whereby cells are subject to a head-on flow and
quickly
slowed and then accelerated in a transverse direction, the HA device and
method is able to
achieve a much higher particle throughput. For example, a throughput of 65,000
cells/sec.
has been achieved using this design compared to a throughput of around 2,000
cells/sec.
achieved using the DC design.
[0062] FIGURE 9B illustrates an example of a fluidic pathway that utilizes
the off-axis
configuration illustrated in FIGURE 9A. As seen in FIGURE 9B, fluid containing
the
plurality of particles passes first through a filter 190. The outlet of the
filter 190 is coupled to
a delivery region 130 as described herein that is used to substantially focus
the plurality of
particles along a common axis as seen inset image at point c in the fluidic
pathway. The
particles then enter a trifurcation 125 The particles continue along via
central branch
channel 122" while a portion of the substantially particle-free fluid is
shunted to inlets 135,
135' where they recombine with the central branch channel 122' in the
deformation region
140 to squeeze and deform the particles as illustrated. The particles continue
on in the same
direction to outlet 106.
[0063] Furthermore, the first opposing flow and the second opposing flow
can be
generated from the sample volume by siphoning portions of the sample volume
(e.g., into a
trifurcation or bifurcation that rejoins at the deformation region), or by
flows (e.g., injected
sheath flows) not generated from the sample volume. In this example, the
particles are thus
compressed in a direction substantially orthogonal to a direction in which the
particle flows,
and extends along the direction in which the particle flows. In the
configuration provided in
this example, particles do not undergo substantial deceleration (e.g., slow
down or stop) upon
entering the deformation region 140, and the throughput of the system 100 can
be increased
because multiple particles of the plurality of particles can enter the
deformation region 140
19
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
simultaneously. Furthermore, a variable range of forces used to deform
particles of the
plurality of particles can be generated by the first opposing flow and the
second opposing
flow, by modulating flow parameters of any one or more of the first flow, the
first opposing
flow, and the second opposing flow. Small forces used to deform the particles
can, in
particular, be interesting for probing intrinsic particle properties and/or
properties of smaller
particles (e.g., < 10 micrometers in diameter), and can provide insight into
membrane
elasticity, particle relaxation behavior and other properties of particles
that are difficult to
assess with large deformation forces.
[0064] In still other variations, the delivery region 130 and the
deformation region 140 can
be configured using any suitable number of branches and in any other suitable
manner that
enables focusing of the plurality of particles and deformation of the
plurality of particles. For
example, a variation of the fluidic pathway 120 can comprise multiple delivery
regions 130
configured upstream and downstream of a deformation region 140, such that the
plurality of
particles is focused before and after deformation. In other examples, multiple
branches (e.g.,
more than two branches) can be configured to convene upon the deformation
region 140, in
order to provide alternative modes of deformation. In still other examples,
the plurality of
particles can be configured to enter a first deformation region 140 configured
to provide
deformation from flows that are orthogonal to a direction of the flow carrying
the plurality of
particles, and can be configured to subsequently enter a second deformation
region 140'
configured to provide a deformation force from a flow that is anti-parallel to
a flow carrying
the plurality of particles. Additionally or alternatively, the plurality of
particles can be
configured to be actively sorted or directed (e.g., by focusing, by flow
diversion, based upon
channel resistance), into a specific outlet 106. This example could facilitate
additional
processing of the plurality of particles, as enabled by uniform flow
conditions within the
additional delivery region 130 and/or active sorting downstream of the
deformation region
140.
[0065] FIGURE 9C illustrates the fluidic pathway of FIGURE 9B with the
resistances
labeled for the central branch channel 122" and inlets 135, 135' for the
deformation region
140 according to one design. As seen in FIGURE 9C, R2=1.7*R1. This leads to a
decreased
fraction of flow down the central branch channel 122" but allows for
sufficient Reynolds
number for efficient inertial focusing. The outer branches have a lower
resistance to allow
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
for a higher flow rate and velocity. This enables a larger squeezing flow on
the cells as they
pass through the deformation region 140.
[0066] FIGURE 9D illustrates an embodiment of a fluidic pathway that
combines off-axis
squeezing at a first deformation region 140 followed by a secondary
deformation region 140'
in which particles are subject to deformation at an intersection of opposing
flows. In this
embodiment, fluid containing the plurality of particles passes first through a
filter 190. The
outlet of the filter 190 is coupled to a delivery region 130 as described
herein that is used to
substantially focus the plurality of particles along a common axis. The
particles then enter a
junction 125 of five (5) branch channels 141, 141', 141", 141", 141". Central
branch
channel 141 contains substantially all the particles. Outer branch channels
141', 141",
141", and 141" are substantially free of particles and contain portions of
fluid shunted
from junction 125" Inner branches 141', 141" recombine with the central branch
channel
141 in an off-axis manner to squeeze the particles at the first deformation
region 140. The
particles continue to a second deformation region 140' whereby fluid from
branch channels
141 141'" recombine and intersect with the central branch channel 141 in an
opposing
flow. Particles passing through this second deformation region 140' can then
exit the fluidic
pathway via one or both outlets 106', 106".
[0067] FIGURE 9E illustrates a simplified resistor diagram of the combined
design of
FIGURE 9D that uses off-axis squeezing of particles (hydropipette aspiration
or "HA") in
conjunction with deformability cytometry ("DC"). Tuning of resistance is used
to ensure
equal flow through the two branches of channels creating the extensional flow
(RDC and
RHA). In this embodiment, RDC RHA.
[0068] FIGURE 9F illustrates another embodiment of a fluidic pathway in
which
hydropipette aspiration is combined with rapid inertial solution exchange for
integrated
sample preparation and analysis. In the embodiment of FIGURE 9F, a solution
containing a
plurality of particles is delivered to inlet 104 which then passes through a
filter 190. The
outlet of the filter 190 terminates in a bifurcation 142 that then recombine
in an anti-parallel,
off-axis junction J with a central channel 143 fluidically coupled to a wash
inlet 104'. As
seen in FIGURE 9F, a filter 190' is interposed between the outlet of the wash
inlet 104' and
the central channel 143. The central channel 143 continues until another
trifurcation 144 that
results in a first branch channel 145, a second branch channel 146 and a
continuation of the
central channel 143 which may include a focusing or delivery region as
described herein.
21
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
The first and second branch channels 145, 146 are configured to siphon off a
portion of fluid
flow within the central channel 143. In the embodiment of FIGURE 9F, the first
and second
branch channels 145, 146 act as waste channels which are fluidically coupled
to outlet 106.
A deformation region 140 is formed downstream of the trifurcation 144 by an
intersection of
the central channel 143 as well as first and second side channels 147, 148.
The first and
second side channels 147, 148 are oriented substantially orthogonal to the
central channel 143
and are coupled to an inlet 159 that is configured to be fluidically coupled
to a pressurized
source of fluid. In this regard, sheathing fluid enters inlet 159 and passes
into channels 147,
148 which then recombine with the central channel 143 at the deformation
region 140. This
fluid flow effectuates side squeezing or sheathing of the particles as
described herein. After
passing through the deformation region 140, the particles can then exit the
device via outlet
106'.
[0069] FIGURE 9G illustrates series of magnified images of selected regions
of the device
of FIGURE 9F. As seen in FIGURES 9F and 9G, in this particular example, a
solution
containing a mixture of cells (e.g., a blood sample containing a mixture of
cells) is delivered
to the inlet 104. A wash solution is delivered to the wash inlet 104'. The
wash solution may
include, for example, phosphate buffered saline (PBS). At the junction J, the
outer channels
that combine with the central channel 143. After the junction J, in the
central channel 143
size-dependent lift forces act upon the larger cells (e.g., cancer cells) to
transfer them to the
central wash solution contained in the central channel 143. Still referring to
FIGURE 9F,
when the cells reach the trifurcation 144, the smaller blood cells (e.g.,
white blood cells) are
siphoned off to the first and second branch channels 145, 146. The cancer
cells continue on
in the central channel 143 past the trifurcation 144. Meanwhile, during
operation of the
device, a solution such as PBS is delivered to the inlet 159 using a pump or
the like to create
the squeezing sheathing flow at the deformation region 140. At or adjacent to
the
deformation region 140, the cells can be imaged using a detection module 150
(described in
more detail below) that can generate a morphology dataset and/or fluorescent
dataset for the
cells.
[0070] 1.3 System - Detection Module
[0071] As shown in FIGURE 1, the detection module 150 includes an imaging
subsystem
151 and a fluorescence subsystem 156, and functions to generate a morphology
dataset
characterizing deformation of each particle, and a fluorescence dataset
characterizing
22
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
fluorescence of each particle in the plurality of particles. Preferably, the
deformation region
140 substantially coincides with a field of view of the at least one of the
imaging subsystem
151 and the fluorescence subsystem 156, and additionally, the detection module
150 is
preferably configured to capture a field of view extending beyond the
deformation region
140. As such, the imaging module 151 and the fluorescence module 155 can be
configured to
focus upon any suitable region including and extending before or beyond the
deformation
region 140. For example, in some embodiments, fluorescent images are obtained
prior to the
particles entering the deformation region 140. Preferably, the detection
module 150
generates the morphology dataset and the fluorescence dataset simultaneously;
however, the
detection module 150 can alternatively be configured to generate the
morphology dataset and
the fluorescence dataset non-simultaneously (e.g., sequentially). In
variations wherein the
detection module 150 generates the morphology dataset and the fluorescence
dataset
simultaneously, the detection module 150 is preferably configured such that
light (e.g., white
light) used to generate the morphology dataset does not interfere with
generation of the
fluorescence dataset. Interference can take the form of unwanted excitation of
fluorescent
labels and/or saturation of fluorescence detectors (e.g., photodetectors)
during generation of
the fluorescence dataset.
[0072] The imaging subsystem 151 functions to generate a morphology dataset
characterizing deformation of the particles. Referring now to FIGURE 10A, the
imaging
subsystem 151 preferably comprises a first light source 152 and a first filter
153 configured
to transmit light from the first light source 152, through the deformation
region 140 and onto
an objective lens 154, the objective lens configured to magnify light from the
deformation
region onto an image sensor 155 for generating the morphology dataset. The
imaging
subsystem 151 can additionally comprise any suitable number of lenses, for
example, for
focusing light from the first light source 152 through the first filter 153,
for focusing light
from the first filter 153 onto the deformation region 140, and for focusing
light from the
objective lens 154 onto the image sensor 154. The lenses thus function as
collection and
condensing optics elements, and preferably comprise aspheric lenses; however,
the lenses can
alternatively comprise plano-convex lenses and/or any other suitable lenses
configured to
collect and condense light.
[0073] The first light source 152 functions to provide enough illumination
for generating a
morphology dataset at the image sensor 155, without producing unwanted
excitation of
23
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
fluorescent labels at the plurality of particles and/or saturation of a
fluorescence detector
(e.g., photodetector). As such, the first light source 152 preferably provides
a specified range
of wavelengths that minimally overlaps with range of wavelengths of
fluorescent emission
generated in response to fluorescence subsystem 156. The first light source
152 thus
preferably provides a sufficient intensity of light that enables proper
illumination during short
exposure times used in high-speed image data capture. As such, the first light
source 152 can
be filtered by the first filter 153, in order to reduce interference at a
photodetector of the
fluorescence subsystem 156 while still providing sufficient illumination at
the image sensor
155. In a first variation, the first light source 152 is a xenon light source,
which can be used in
high-speed imaging applications and fluorescence imaging. Alternatively, the
first light
source 152 can comprise a halogen light source and/or any other suitable light
source in other
variations. Furthermore, variations of the detection module 150 can include
interchangeable/adjustable light sources, in order to provide varying ranges
of light
wavelengths, varying intensities of light, and/or any other suitable varying
light parameter.
[0074] The first filter 153 functions to filter light from the first light
source 152 and to
transmit filtered light toward the deformation region 140, in order to avoid
spectral overlap
between the imaging subsystem 151 and the fluorescence subsystem 156. As such,
the first
filter 153 is preferably coaxially aligned with the first light source 152, in
order to properly
filter light from the first light source 152. Preferably, the first filter 153
is a bandpass filter
configured to only pass light that does not excite fluorophores at the
plurality of particles, and
additionally, to only pass light that is not detected by a photodetector of
the fluorescence
subsystem 156. In a specific example, the first filter is configured to filter
out wavelengths
around 532nm and around 580 nm, in order to not excite fluorescent labels
bound to particles
and to avoid light interference at a photodetector of the fluorescence
subsystem 156
respectively. In alternative variations the first filter 153 can comprise a
lowpass filter, a
highpass filter, and/or any other suitable filter for filtering interfering
light wavelengths.
Furthermore, variations of the detection module 150 can comprise
interchangeable filters for
filtering light from the first light source 152.
[0075] The objective lens 154 functions to receive light from the first
filter 153 and
passing through the deformation region 140 and to magnify light onto an image
sensor 155,
in order to facilitate generation of a morphology dataset characterizing
deformation of each
particle in the plurality of particles. The objective lens 154 is preferably
substantially aligned
24
81786497
between the first filter 153 and the image sensor; however, the objective lens
154 can
alternatively have any other suitable configuration relative to other elements
of the detection
module 150. The objective lens is preferably characterized by a magnification
that enables an
entire deformed particle of the plurality of particles to be captured within a
window defined
by the image sensor 155, wherein the desired magnification depends upon the
focal length of
the objective lens and/or focal length(s) of any additional optics element(s)
(e.g., tube lens),
and the position of the image sensor 155 relative to the objective lens and/or
optics
element(s). In a specific example, the objective lens provides a 10X
magnification; however,
in other variations, the objective lens can provide any other suitable
alternative magnification.
In variations, the detection module 150 can include interchangeable/adjustable
objective
lenses 154, in order to provide an adjustable magnification. Different levels
of magnification
can enhance the morphology dataset generated at the image sensor 155, by
providing, for
example, magnification of features not seen at all magnification levels.
[0076] The image sensor 155 functions to receive light from the
deformation region 140
and passing through the objective lens 154, in order to generate a morphology
dataset
characterizing deformation of each particle in the plurality of particles.
Preferably, the image
sensor 155 is substantially aligned with the objective lens 154; however, the
image sensor
155 can have any other suitable configuration relative to other elements of
the detection
module 150. The image sensor 155 can be integrated into a high-speed/high
frame-rate
imaging module (e.g., camera), configured to generate image data that captures
multiple
stages of deformation for each particle in the plurality of particles. As
such, specifications of
the image sensor 155 and the light source are preferably codependent in order
to provide
sufficient light parameters (e.g., intensity) for image data generation. The
image sensor 155
can comprise a variation of the image sensor described in U.S. Pub. No.
2013/0177935,
entitled "Method and Device for High Throughput Cell Deformability
Measurements";
however, the image sensor 155 can comprise any other suitable image sensor for
generating the morphology dataset.
[0077] The fluorescence subsystem 156 functions to generate a
fluorescence dataset
characterizing the fluorescence (or absence of fluorescence) of each particle
in the plurality of
particles. The fluorescence subsystem 156 can thus comprise a second light
source 157 and a
second filter 158 configured to transmit light from the second light source
157, through a
fiber optic unit 159, through a portion of the fluidic pathway 120, and onto
an objective lens
CA 2920132 2020-01-22
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
154, the objective lens 154 configured to magnify light from the fluidic
pathway 120 onto a
photodetector 160 for generating the fluorescence dataset. Light from the
fluidic pathway 120
can further be passed through a third filter 161 prior to reception at the
photodetector 160, in
order to reduce or eliminate effects of interfering wavelengths of light.
[0078] The second light source 157 functions to provide excitation
wavelengths of light,
and to transmit light at excitation wavelengths toward each particle in the
plurality of
particles in a portion of the fluidic pathway 120. The second light source
preferably directs
light toward the second filter 158 and the fiber optic unit 159, onto a
portion of the fluidic
pathway 120, such that fluorescent labels bound to particles passing through
the portion of
the fluidic pathway 120 are excited by excitation wavelengths of light. In
response, the
excited fluorescent labels emit emission wavelengths of light, indicative of
biomolecular
characteristics of the particles, which can be detected at a photodetector
160. The second light
source 157 is preferably a light source that provides a specific excitation
wavelength of light,
and can be a laser (e.g., a 532nm laser). However, the second light source 157
can
alternatively be configured to provide a range of excitation wavelengths of
light. In one
variation, the second light source 157 can be a broad-spectrum light source
(e.g., white light
LEDs) that transmits light through at least one excitation filter to generate
a specific
wavelength or range of wavelengths of light for fluorescent labels(s)
excitation. In variations
including the excitation filter(s) and a broad-spectrum light source, the
excitation filter(s) can
be interchangeable in order to provide an adjustable excitation wavelength or
an adjustable
range of excitation wavelengths.
[0079] The second filter 158 functions to modify a parameter of light
transmitted from the
second light source 157, in order to condition light provided by the second
light source 158.
The second filter 158 is preferably aligned between the second light source
157 and the fiber
optic unit 159; however, in variations omitting the fiber optic unit 159, the
second filter 158
can be aligned with the second light source 157 or can have any other suitable
configuration.
The second filter 158 is preferably a neutral density filter, which is
configured to modify or
reduce an intensity of light transmitted from the second light source 157. As
such, the neutral
density filter can function to prevent signal saturation due to high-intensity
light, and can
additionally function to protect sensitive elements of the detection module
150 from high-
intensity light. The second filter 158 can, however, comprise any other
suitable filter for
conditioning light from the second light source 157.
26
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
[00801 The fiber optic unit 159 functions to redirect light transmitted
through the second
filter 158 from the second light source 157, in order to satisfy space
requirements of the
system 100. Furthermore, the fiber optic unit 159 can function to alter a beam
shape (e.g., by
a fiber collimator to produce a more spatially uniform beam), and can
facilitate translation by
coupling to a mount for fine resolution translation in one or more directions
(e.g., two
dimensions by an x-y mount). As such, the fiber optic unit 159 can include a
fiber coupler
coupled to a fiber optic-fiber probe assembly that allows light to be
transmitted through the
fiber-optic-fiber probe assembly. The fiber probe is preferably configured to
direct light into
a portion of the fluidic pathway through which the plurality of particles
pass, such that
fluorescent labels bound to the plurality of particles can be properly
excited. The portion of
the fluidic pathway can comprise the deformation region 140, such that the
detection module
150 is configured to simultaneously or nearly simultaneously capture
deformation and
fluorescence characteristics at the same location along the fluidic pathway;
however, the
portion of the fluidic pathway can alternatively comprise any other suitable
region of the
fluidic pathway, for example, a region upstream of the deformation region 140
and
downstream of a delivery region 130, or any other suitable region of the
fluidic pathway. In
one variation, light from the second light source 157 can be directed toward a
region
immediately upstream of the deformation region (e.g., 100 micrometers to lmm
upstream),
wherein flow conditions are sufficiently uniform. In some variations, wherein
space is less of
a constraint, the fluorescence module 156 can omit the fiber optic unit 159,
light from the
second light source 157 through the second filter 158 can be transmitted
directly in a straight
line from the second light source 157 to the portion of the fluidic pathway
120. Some
variations of the fluorescence subsystem 156 can, however, omit the fiber
optic unit 159 and
instead comprise beam steering mirrors to translate a beam provided by the
second light
source 157 in multiple dimensions and/or a movable stage (e.g., x-y stage)
configured to
facilitate translation of a beam in one or more directions.
[00811 Similar to the objective lens of the imaging subsystem 151, the
objective lens 154
functions to receive light from the second filter 158 passing through the
portion of the fluidic
pathway 120, and to magnify light onto a photodetector 160, in order to
facilitate generation
of a fluorescence dataset characterizing fluorescence of each particle in the
plurality of
particles. The objective lens 154 can be positioned between the fiber probe of
the fiber optic
unit 159 and the photodetector 161 in any suitable configuration relative to
other elements of
27
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
the detection module 150. The objective lens is preferably characterized by a
magnification
that enables an entire fluorescing particle of the plurality of particles to
be captured within a
window defined by the photodetector 160, wherein the desired magnification
depends upon
the focal length of the objective lens and the position of the photodetector
160 relative to the
objective lens 154. In a specific example, the objective lens provides a 10X
magnification;
however, in other variations, the objective lens can provide any other
suitable alternative
magnification. In variations, the detection module 150 can include
interchangeable/adjustable
objective lenses 154, in order to provide an adjustable magnification.
[0082] The photodetector 160 functions to receive light emitted upon
excitation of
fluorescent labels bound to particles of the plurality of particles. The
photodetector 160
additionally functions to facilitate generation of a fluorescence dataset
characterizing
fluorescence characteristics for each particle in the plurality of particles.
As such, the
photodetector 160 is preferably configured to detect ultraviolet, visible, and
infrared light,
emitted from excited fluorescent labels. In one variation, the photodetector
160 can comprise
a photomultiplier configured to operate by a photoelectric effect upon
reception of incident
light; however, in other variations, the photodetector 160 can include any
other suitable
photodetector configured to detect any suitable wavelength of light, by any
other suitable
mechanism.
[0083] As described earlier, the fluorescence module 150 can include a
third filter 161
configured to filter light prior to reception at the photodetector 160. The
third filter 161 thus
functions to reduce or eliminate any effect of interfering light generated
from any source
(e.g., the first light source 152). Preferably, the third filter 161 is
substantially aligned with
the photodetector 160, such that incident light on the photodetector 160 is
configured to pass
through the third filter 161. Additionally or alternatively, the third filter
161 can be
configured along any suitable portion of a light path from the objective lens
154 to the
photodetector 160. The third filter 161 preferably comprises a bandpass
filter; however, the
third filter 161 can alternatively or additionally comprise a lowpass filter
or a highpass filter.
[0084] Preferably, the imaging subsystem 151 and the fluorescence subsystem
155 are
integrated, in order to reduce space and cost demands of the detection module
150. As such,
in some variations, the imaging subsystem 151 and the fluorescence subsystem
155 can share
elements. In one such variation, the imaging subsystem 151 and the
fluorescence subsystem
155 can share a single light source, with flow parameters correspondingly
adjusted to ensure
28
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
that there is only a single particle at a time in an illumination spot
provided by the light
source. In other variations, other elements can be additionally or
alternatively be shared
between the subsystems 151, 155.
[0085] In an example, as shown in FIGURE 10A, the imaging subsystem 151 and
the
fluorescence subsystem 156 share an objective lens 154 that simultaneously
receives and
transmits light originating from the first light source 152 and the second
light source 157
toward an image sensor 155 and a photodetector 160, respectively. In the
example, the
imaging subsystem 151 and the fluorescence subsystem 156 further share a
dichroic mirror
162 configured to transmit specific wavelengths of light from the objective
lens 154, and to
reflect other wavelengths of light from the objective lens 154 (e.g., to
another light sensing
module). The dichroic mirror 162 can be configured to reflect light emitted by
fluorescent
labels, in response to excitation, toward the photodetector 160 to generate
the fluorescence
dataset, and to transmit light from the first light source 152 directly toward
the image sensor
155 to generate the morphology dataset. Alternatively, the dichroic mirror 162
can be
configured to transmit light emitted by fluorescent labels toward the
photodetector 160 and to
reflect light from the first light source toward the image sensor 155. The
dichroic mirror 162
is preferably a short-pass dichroic mirror, but can alternatively be a long-
pass dichroic mirror
or any other suitable dichroic mirror.
[0086] In another example, as shown in FIGURE 10B, the imaging subsystem
151 and the
fluorescence subsystem 155 share an objective lens 154 that simultaneously
receives and
transmits light originating from the first light source 152 and the second
light source 157
toward an image sensor 155 and a photodetector 160, respectively. In this
example, the
detection module 150 includes a first xenon light source 152 configured to
transmit light
through a lowpass filter 153 and a plurality of lenses separated by an
aperture and a lowpass
filter 153, toward the deformation region 140 of the fluidic pathway 120, and
through a 10X
objective lens to be reflected off of a first and a second dichroic mirror
162, 162' toward an
image sensor 155. In this example, the detection module 150 further includes a
second 532nm
laser light source 157 configured to transmit light through the first dichroic
mirror 162, to be
reflected off of the second dichroic mirror 162' toward the 10X objective
lens. Excitation
light from the second light source 157 is configured to focus upon the
deformation region
140, and light emitted from fluorescent labels at the deformation region is
configured to be
transmitted back through the second dichroic mirror 162', through a bandpass
filter and a lens
29
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
and a bandpass filter, toward a photodetector 160. In variations of this
example, light emitted
from fluorescent labels at the deformation region 140 can be transmitted
through the second
dichroic mirror 162' and toward additional photodetectors 160', 160" by
additional dichroic
mirrors 162" 162", bandpass filters, and lenses, wherein the photodetectors
160, 160',
and 160¨ are configured to receive different wavelengths (or ranges of
wavelengths) of light.
[0087] In still another example, as shown in FIGURE 10C, the imaging
subsystem 151
and the fluorescence subsystem 155 share an objective lens 154 that
simultaneously receives
and transmits light originating from the first light source 152 and the second
light source 157
toward an image sensor 155 and a photodetector 160, respectively. In this
example, the
detection module 150 includes a first light source 152 configured to transmit
light through a
lowpass filter 153 and a plurality of lenses separated by an aperture and a
lowpass filter 153,
toward the deformation region 140 of the fluidic pathway 120, and through a
10X objective
lens 154 and a first short pass dichroic mirror 162 toward an image sensor
155. In this
example, the detection module 150 further includes a second 532nm laser light
source 157
coupled to a fiber probe and configured to transmit light through collimating
optics, through a
beam steering element (e.g., a set of mirrors, as in FIGURE 10C, or an x-y
translating fiber
mount), through a second short pass dichroic mirror 162, to be reflected off
of the first short
pass dichroic mirror and through the objective lens 154 to the deformation
region 140. Light
emitted from fluorescent labels at the deformation region is then configured
to pass into the
objective lens 154, to be reflected off the first short pass dichroic mirror
162 and the second
dichroic mirror 162 to a set of long pass dichroic mirrors 162'. The set of
long pass dichroic
mirrors is configured to reflect and transmit specific wavelengths of light,
through band-pass
filters 161, toward specific photodetectors 160 for fluorescence detection, as
shown in
FIGURE 10C.
[0088] In other variations, the detection module 150 can include any other
suitable
element(s) and/or configuration of elements that allows simultaneous or near
simultaneous
generation of the morphology dataset and the fluorescence dataset. In
examples, the detection
module can comprise any one or more of a beam splitter, an aperture, an
additional dichroic
mirror, a collimator, any number of lenses, and any other suitable element
configured to
manipulate light from a light source. Furthermore, any element can be coupled
to an actuator
(e.g., manual, automatic actuator) that enables alignment of optics and/or
adjustment of focal
lengths. In one example, the objective lens 154 can be coupled to a linear
actuator (e.g., a z-
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
axis control) that enables adjustment along one or more axes. Additionally or
alternatively,
the substrate itself 110 can be coupled to an actuator (e.g., by a stage) that
provides linear
actuation along one or more axes (e.g., by an x-y control).
[0089] 1.3.1 System - Detection Module Alternatives
[0090] In some embodiments, the detection module 150 can additionally or
alternatively
include a one-dimensional detection module 163 configured to facilitate an
increase in data
acquisition rates and a decrease in analysis times. The one-dimensional
detection module 163
functions to enable extraction of particle deformation characteristics,
without generation of
two-dimensional or three-dimensional data, in order to generate a morphology
dataset.
[0091] In a first variation, as shown in FIGURE 11A, the one-dimensional
detection
module 163 comprises an optical mask including a set of slits 164 configured
to facilitate
generation of particle transit time measurements with an increased signal-to-
noise ratio. In
the first variation, the optical mask preferably includes at least one slit
situated upstream of
the deformation region 140 and at least one slit situated downstream of the
deformation
region, which enables detection of a difference in a particle dimension (e.g.,
cell length)
before and after particle deformation. A photodetector 169 configured to
receive light
through the optical mask, and to generate an electrical signal (e.g., a
voltage drop) upon a
change in incident light produced by a particle passing a slit of the optical
mask, can be used
to provide a correlation between an electrical signal (e.g., voltage drop,
duration of a voltage
drop) and a particle dimension (e.g., cell length). In the first variation,
the slit width is
governed by an anticipated particle dimension, and in specific examples, is
preferably smaller
than the smallest expected cell size in order to directly infer a cell
dimension from the one-
dimensional detection module 163. However, the optical mask can alternatively
include a slit
with a width greater than an anticipated particle dimension (e.g., to
facilitate optical mask
fabrication), and signals generated by a photodetector cooperating with the
optical mask can
be configured to produce deformation measurements based upon deconvolution
with mean
transit signal characteristics. In an alternative to the first variation, the
one-dimensional
detection module 163 comprises an optical mask including a set of patterns and
a
photodetector 169 configured to receive light through the optical mask, and to
generate an
electrical signal (e.g., a voltage drop, duration of a voltage drop) upon a
change in incident
light produced by a particle passing a pattern of the optical mask. In this
alternative, a signal
produced by the photodetector can be matched to a library of generated signals
in order to
31
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
extract particle dimensional parameters (e.g., cell length). However, the
optical mask can
alternatively include any other features configured to enable detection of a
particle dimension
without generation of two-dimensional or three-dimensional data.
[0092] In a second variation, the one-dimensional detection module 163 can
comprise a
set of lenses 166 with a liquid waveguide 167 coupled to a light source 168
(e.g., fiber optic
coupled to a light source) and a detector 169, wherein a light ribbon
generated by light
passing from the light source 168, through the liquid waveguide 167, and
through the set of
lenses 166, can be used to generate transit time measurements resulting from a
voltage drop
induced by a particle passing the light ribbon. The liquid waveguide 167 and
the set of lenses
166 are preferably integrated (e.g., physically coextensive) with the
substrate 110, as shown
in FIGURE 11B; however, the liquid waveguide 167 and/or the set of lenses 166
can
alternatively be configured in any other suitable alternative manner.
Furthermore, the light
ribbon can be directed directly to the detector 169, such that the light
source 168 is directly
opposed to the detector 169 as in FIGURE 11B, or can be directed between the
light source
168 and the detector 169 in any other suitable manner (e.g., using
positionally offset
waveguides). In a specific example of the second variation, the liquid
waveguide comprises
high-refractive index oil (e.g., index = 1.6), and the lenses have a
refractive index of 1 to
facilitate generation of the light ribbon.
[0093] In a third variation, the one-dimensional detection module 163 can
comprise a
detector 169 configured to enable generation of a particle dimension
measurement during
deformation using forward and/or side-scatter measurements, as shown in FIGURE
11C.
Scattered-light features (e.g., profiles, parameters) detected as a particle
enters and leaves the
deformation region 140 can be used to infer particle deformation
characteristics. For
example, light scattering, as detected by the detector 169, can produce a
voltage drop that
increases in magnitude with increasing deformation, as shown in FIGURE 11C. At
least a
portion of the third variation of the one-dimensional detection module 163 can
be integrated
into the substrate 160; however, the third variation of the one-dimensional
detection module
163 can alternatively be physically distinct from the substrate 110.
[0094] In still other embodiments, the detection module 150 can
additionally or
alternatively include a two-dimensional detection module 170 configured to
facilitate an
increase in data acquisition rates and a decrease in analysis times. The two-
dimensional
detection module 170 functions to enable rapid extraction of particle
deformation
32
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
characteristics based upon alternative element compositions and/or
configurations, in order to
generate a morphology dataset. An exemplary two-dimensional detection module
170 is
illustrated in FIGURE 12A.
[0095] In one embodiment, the two-dimensional detection module 170 includes
a first
position-sensitive detector 171 (PSD) configured to detect a particle
deformation along a first
axis (e.g., x-axis deformation of a particle) as the particle is deformed
within the deformation
region 140, and a second PSD 172, oriented orthogonally to the first PSD 171
and configured
to detect a particle deformation along a second axis (e.g., y-axis deformation
of a particle) as
the particle is deformed within the deformation region 140. The first and the
second PSDs
171, 172 are each preferably configured to generate an electrical signal
(e.g., voltage drop,
duration of a voltage drop) indicative of a particle dimension (e.g., length)
during particle
deformation within the deformation region 140, as shown in FIGURE 12A. Signals
provided
by the first PSD can be passed in a first channel and signals provided by the
second PSD can
be passed in a second channel, and in an alternative variation, signals
provided by the first
PSD and the second PSD 171, 172 can be multiplexed in a single channel to
reduce resource
requirements during signal processing. In a specific example of the first
variation, the first
and the second PSDs are defined by a 100 kHz bandwidth and a 2 micrometer
spatial
resolution, configured to enable deformation measurements within a 70
micrometer x 70
micrometer region of the deformation region 140.
[0096] in a second embodiment, as shown in FIGURE 12C, the two-dimensional
detection
module 170 comprises an image sensor 173 and a field programmable gate array
(FPGA) 174
configured to cooperate with the image sensor 173 to identify a particle
event, and to
selectively trigger signal capture of a particle undergoing deformation upon
identification of
the particle event. The image sensor 173 is preferably configured to capture
image data at the
deformation region 140, but can be configured in any other suitable manner. In
the second
variation of the two-dimensional detection module 170, the detection module
150 can thus
avoid collecting a substantial number of blank frames (i.e., frames not
providing any particle-
related data), which significantly reduces computational workload. In a
specific application,
the FPGA can be configured to screen a trigger region 175 upstream of the
deformation
region 140, and upon identification of a particle within the trigger region
175 (i.e., the
particle event) by the FPGA, the image sensor 173 can be configured to capture
image data of
the particle undergoing deformation within a limited time window (e.g., 30
frames).
33
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
[0097] In a third embodiment, the two-dimensional detection module 170
comprises an
image sensor 176 configured to capture deformation of a particle within the
deformation
region 140, a light source 177 configured to emit light toward particles
entering the
deformation region 140 at a location upstream of the deformation region, a
photodetector 178
configured to receive light from the light source 177, thus facilitating
identification of a
particle about to enter the deformation region 140, and a strobe 179
configured to flash
multiple times in synchronization with motion of the particle within the
deformation region
140. Flashing of the strobe 179 thus enables capturing of multiple positions
and/or
deformations of a particle within a single image frame, which allows a single
image frame to
provide more useful data related to particle deformation characteristics. The
strobe can be
configured to flash multiple times, with a fixed time interval between strobe
flashes, and can
alternatively be configured to flash without a fixed time interval between
strobe flashes, as
guided by the photodetector 178. In a specific example of the third variation,
the image
sensor 176 is characterized by a frame rate of 2,000-10,000 frames per second
and a field of
view of 150 micrometer x 150 micrometer. The light source 177 in the example
is a laser
focused upstream of the deformation region 140, and the photodetector 178
comprises at least
one of a photomultiplier tube (PMT) and an amplified photodiodc configured to
detect
scattered laser light produced when a particles passes through the laser beam.
The scattered
light, as detected by the photodetector 178 in the specific example, is used
to trigger the
strobe 179 to flash twice (e.g., with a 500ns exposure time) with a fixed time
interval
corresponding to a time required for the particle (i.e., the particle
scattering light from the
laser) to transit between two positions about the deformation region 140. In
the specific
example, each image frame thus comprises information related to two positions
and two
morphological characterizations of a particle undergoing deformation in the
deformation
region 140.
[0098] Other alternative variations of the detection module 150 can include
any other
suitable element(s) or combination of elements that enable measurement and
detection of
particle morphological data that yield deformation based upon single-dimension
acquisition
and/or multi-dimension acquisition.
[0099] 1.4 System - Other Elements
[00100] Referring back to FIGURE 1, the processor 180 functions to transform
the
morphology dataset into a set of deformation characteristics characterizing
deformation of
34
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
each particle in the plurality of particles, to transform the fluorescence
dataset into a set of
fluorescence parameters characterizing biomolecular properties of each
particle in the
plurality of particles, and to generate an analysis based upon the set of
deformation
characteristics and the set of fluorescence parameters. Preferably, the
morphology dataset and
the fluorescence dataset are temporally synchronized, to facilitate matching
of image and
fluorescence data with specific particles in the plurality of particles;
however, the image and
the fluorescence datasets can be synchronized by any other metric. As such,
the processor
180 preferably includes a first module 181 configured to extract a set of
deformation
characteristics from the morphology dataset, a second module 182 configured to
extract a set
of fluorescence parameters from the fluorescence dataset, a third module 183
configured to
synchronize the morphology dataset and the fluorescence dataset, and a fourth
module 184
configured to generate an analysis based upon the set of deformation
characteristics and the
set of fluorescence parameters. It should be understood that, in some
alternative
embodiments, any of the modules 181, 182, 183, 184 may be combined with one
another.
The modules 181, 182, 183, 184 can include instructions or algorithms executed
by the
processor 180. These modules 181, 182, 183, 184 may be stored in memory or
other data
storage device operatively coupled to the processor 180. Further, while
reference is made to
a single processor 180 it should be understood that one or more additional
processors 180
may function together as a single processing unit.
[00101] The first module 181 functions to extract a set of deformation
characteristics from
the morphology dataset that can be used to synchronize the morphology dataset
with the
fluorescence dataset, and can be used to generate an analysis by the fourth
module 184. The
first module 181 can extract the set of deformation characteristics
continuously or near-
continuously and in real time (e.g., such that deformation of a particle is
tracked in real time);
however, the first module 181 can alternatively be configured to extract
characteristics non-
continuously and/or in non-real time. The set of deformation characteristics
preferably
provide morphological and/or structural characteristics indicative of
phenotype, such as
nuclear size, chromatin decondensation, cytoskeletal disassembly/fluidization,
and membrane
compromise/lysis. The set of deformation characteristics can thus provide
information related
to the cell membrane and/or the cell nucleus. In some variations, the set of
deformation
characteristics can include any one or more of: particle deformability (e.g.,
a ratio of particle
length to width), particle elastic modulus (e.g., a ratio of strain measured
in an initial high
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
frequency deformation regime, to stress provided by a library of simulated
fluid-induced
stresses and particle dimensions), particle viscosity (e.g., a measurement of
strain rate in a
low frequency deformation regime), particle hydrodynamic viscosity (e.g.,
based upon an
inertial equilibrium position of a particle), particle circularity (e.g.,
based upon a ratio of
particle projected area to particle projected perimeter), particle roughness
(e.g., a standard
deviation of particle radius measurements), particle size (e.g., volume, area,
diameter, etc.),
particle topological characteristics, particle asymmetry, and any other
suitable morphological
or structural characteristic, as shown in FIGURES 13A-13C. The first module
181 can also
be configured to extract baseline morphological particle characteristics,
including one or
more of: initial particle volume, initial particle diameter, initial particle
asymmetry, and any
other suitable baseline characteristic. Extracting particle characteristics
can be performed as
in U.S. Pub. No. 2013/0177935, entitled "Method and Device for High Throughput
Cell
Deformability Measurements", or in any other suitable manner. In variations
wherein the first
module 181 is configured to extract baseline morphological particle
characteristics, the
baseline characteristics can be used to normalize the set of deformation
characteristics for
each particle in the plurality of particles, and/or can be used by the fourth
module 184 to
generate the analysis in any other suitable manner. In one embodiment, the
first module 181
outputs a sequence indicator (e.g., frame number(s) of an image used to
extract a deformation
characteristic, time stamp, etc.) along with at least one extracted
deformation characteristic
for each particle in the plurality of particles; however, the first module can
provide any other
suitable output. In a specific example, the first module 181 is configured to
output particle
deformability along with the frame number(s) of an image used to extract
deformability.
[00102] The second module 182 functions to extract a set of fluorescence
parameters from
the fluorescence dataset that can be used to synchronize the fluorescence
dataset with the
morphology dataset, and can be used to generate an analysis by the fourth
module 184. The
second module 182 can extract the set of fluorescence parameters continuously
or near-
continuously and in real time (e.g., such that fluorescence of a particle is
tracked in real
time); however, the first module 181 can alternatively be configured to
extract characteristics
non-continuously and/or in non-real time. The set of fluorescence parameters
preferably
provide characteristics indicative of biomolecular phenotype (e.g., surface
markers, nucleic
acid composition, membrane integrity, receptor characteristics) and can
include any one or
more of: an intensity of emitted light (e.g., average intensity, peak
intensity), a wavelength of
36
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
emitted light, kinetic parameters of fluorescence, and any other suitable
fluorescence
parameter. The second module 182 can also be configured to extract baseline
fluorescence
parameters (i.e., prior to particle deformation), including one or more of:
initial intensity
(e.g., initial average or peak intensity), initial emitted wavelength prior to
deformation, initial
kinetic parameter(s) prior to deformation, and any other suitable baseline
parameter. In
variations wherein the second module 182 is configured to extract baseline
fluorescence
parameters, the baseline parameters can be used to normalize the set of
fluorescence
parameters for each particle in the plurality of particles, and/or can be used
by the fourth
module 184 to generate the analysis in any other suitable manner. Preferably,
the second
module 182 outputs a sequence indicator (e.g., time stamp, frame number(s) of
an image used
to extract a fluorescence parameter, etc.) along with at least one extracted
fluorescence
parameter for each particle in the plurality of particles; however, the first
module can provide
any other suitable output. In a specific example, the second module is
configured to output a
continuous signal of intensity and time. For example, the signal may comprise
a continuous
voltage signal from a PMT as described herein. Peaks corresponding to the
detected
fluorescent particles may be extracted from the generated dataset using the
second module
182.
[00103] The third module 183 functions to synchronize the morphology dataset
and the
fluorescence dataset. Preferably, the morphology dataset and the fluorescence
dataset are
output from the image sensor 155 and the photodetector 160 using the same
clock, such that
time points across the image data and the fluorescence data are substantially
synchronized.
Synchronization may be accomplished by subtracting an elapsed time that
corresponds to the
time delay when the particle passes from the fluorescence interrogation region
to the
morphology detection region. In some variations, however, the morphology
dataset and the
fluorescence dataset may not be associated with the same clock, motivating
synchronization
of the morphology dataset and the fluorescence dataset. The third module 183
can be
configured to perform any suitable signal conditioning step (e.g., noise
removal by filtering
and peak-finding). In one specific example, wherein the first module 181 is
configured to
output particle deformability along with a frame number of an image used to
extract
deformability and the second module 182 is configured to output a continuous
signal of
intensity and time, the third module 183 is configured to apply signal filters
to remove signal
noise and apply a peak-finding algorithm to identify a time-dependent sequence
of particles.
37
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
A sequence matching or cross-correlation algorithm, an example of which is
shown in
FIGURE 14, is then used to align the event vs. time signals of the morphology
dataset with
the event vs. time signals of the fluorescence dataset. In variations of the
example, calibration
particles (e.g., rigid or deformable fluorescent calibration microspheres)
characterized by
identifiable deformability and fluorescence signatures can be used to
synchronize the
morphology dataset with the fluorescence dataset, irrespective of time stamps.
In still other
variations, relationships between deformability (or any other suitable
deformation
characteristic) and emitted fluorescence intensity can be used to synchronize
the sets of data.
However, the morphology dataset and the fluorescence dataset can be
synchronized in any
other suitable manner.
[00104] The fourth module 184 functions to generate an analysis based upon the
set of
deformation characteristics and the set of fluorescence parameters. The
analysis can comprise
a correlation between mechanical and biochemical/biomolecular markers for the
particles of
interest, which can be used to identify mechanical (e.g., deformation)
characteristics,
fluorescence parameters, and/or combinations of mechanical and fluorescence
parameters
useful for characterizing particles of the plurality of particles. In specific
applications, the
analysis generated by the fourth module 184 can be used to identify activation
states of
specific cell types (e.g., blood mononuclear cell activation by mitogens or
inflammatory
processes, granulocyte activation with cytokines or blood stream infections,
as identified by
deformability and surface expression of activation markers), with important
implications in
label-free monitoring of diseases, diagnosis of diseases, treatment of
diseases, and prediction
of transplant rejection. In additional applications, the analysis generated by
the fourth module
184 can be used to identify phenotypic connections between stem cells and
cancers (e.g.,
Jurkat and HL60), used to identify differentiation indicators for stem cells,
and used for
identification of subpopulations of cells within diverse populations of cells
in body fluid
samples from healthy or diseased patients (e.g., resting or activated
leuokocytes, PBMCs, and
granulocytes as in blood, or pleural fluid). As such, the fourth module 184
can be used to
aggregate a library of data of multiple types of phenotypic markers (e.g.,
mechanical,
deformation, fluorescence, etc.) for a variety of biological particles, using
a high-throughput
approach.
[00105] The fourth module 184 can be configured to conduct a statistical
analysis (e.g.,
correlation, t-test, ANOVA, etc.), which functions to investigate
relationships between
38
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
deformation and fluorescence parameters. Additionally or alternatively,
classification and
regression trees (CARTs) generated by the fourth module 184 can be used, with
deformation
and fluorescence parameters used to enhance identification. Receiver operating
characteristic
(ROC) curves can be used to assess an ability to correctly identify particles
for purposes of
generating predictive models. Furthermore, linear discriminate analyses (or
other machine
learning approaches) can be used to identify similarities and/or differences
between different
sample volumes, which can be used, for example, to stratify samples from
different patients.
In some variations, the processor 180 can further be configured to render the
analysis at a
user interface (e.g., as a flow cytometry 2D or 3D density plot of single
cells, etc.) such as a
display or monitor.
[00106] As shown in FIGURE 1, the system 100 can further comprise a filter 190
located
upstream of the delivery region 130. The filter 190 functions to separate
particles of interest
from other particles or debris in the sample volume and to allow the particles
of interest to
pass into the fluidic pathway 120. Preferably, the filter 190 is configured
between an inlet
104 and the fluidic pathway 102, such that the sample volume is substantially
filtered prior to
delivery into the fluidic pathway 120, delivery region 130, and/or deformation
region 140.
Additionally or alternatively, the system 100 can include a filter 190
positioned at any other
suitable location of the system 100, and/or any suitable number of filters in
any other suitable
configuration. The filter 190 preferably separates the particles based upon
size (e.g., using
suitably sized pores in a porous structure or mesh); however, the filter 190
can alternatively
separate particles of interest from other particles in the sample volume based
upon any other
suitable separation mechanism (e.g., chemical, affinity moiety, electric,
magnetic, etc.).
[00107] Also shown in FIGURE 1, the system 100 can further comprise a
processed sample
volume receiver 195, which functions to receive a processed sample fluid from
an outlet 106
of the substrate 110. As briefly described earlier, the processed sample
volume receiver 195
can be a waste chamber configured to fluidly couple to the outlet 106 to
collect the processed
sample fluid as waste. Furthermore, the waste chamber can be integrated (e.g.,
physically
coextensive, of unitary construction) with the substrate 110 in any suitable
manner.
Alternatively, the processed sample volume receiver 195 can be configured to
collect and
transmit the processed sample volume, including the plurality of particles, to
another module
for additional assays and analyses. As such, the processed sample volume
receiver can
comprise one or more conduits and/or valves that facilitate sample
transmission. In still other
39
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
variations, the processed sample volume receiver 195 can be a composite
receiver that
receives a portion of the sample volume as waste, and facilitates collection
of another portion
of the sample volume for further analyses.
[00108] In some variations, the system 100 can further comprise a storage
module 197 with
accessible memory, which functions to receive and/or store at least one of the
morphology
dataset, the fluorescence dataset, an analysis, system 100 parameters (e.g.,
flow parameters,
detection module parameters, etc.), sample volume identifiers (e.g., name,
contents, date),
and module algorithms. The accessible memory permits a user to access stored
information
about sample runs using the system 100 and the system parameters that were
utilized during
those runs. Any stored information is preferably accessible by a user and/or
any other suitable
entity. The storage module can be implemented using any suitable computing
device (e.g.,
desktop computer, hardware storage device, server, cloud).
[00109] The system 100 can, however, include any other suitable element(s) or
combination of elements that facilitate the deformation, assaying, and/or
analysis of particles
of a sample volume. As a person skilled in the art will recognize from the
previous detailed
description and from the figures and claims, modifications and changes can be
made to the
embodiments of the system 100 without departing from the scope of the system
100.
[00110] 2. Method
[00111] As shown in FIGURE 15, a method 200 for deforming and analyzing a
plurality of
particles carried in a sample volume includes: receiving the sample volume
including the
plurality of particles S210; diverting a first portion of the sample volume in
a first flow and a
second portion of the sample volume in a second flow, opposed to the first
flow, wherein an
intersection of the first and the second flows defines a deformation region
S220; focusing the
plurality of particles into the deformation region S230; generating a
morphology dataset
characterizing deformation of each particle in the plurality of particles
within the deformation
region S240; generating a fluorescence dataset characterizing fluorescence of
each particle of
the plurality of particles within the deformation region S250; and outputting
an analysis of
the plurality of particles based at least in part on the morphology dataset
and the fluorescent
dataset for the plurality of particles S260.
[00112] The method 200 functions to enable the deformation of single particles
in a high-
throughput and consistent manner, with the ability to simultaneously generate
and analyze
multiple data types characterizing the single particles. Preferably, the
method 200 further
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
functions to enable the generation of data that directly correlates surface
biomarkers of
phenotype with mechanical properties at the single-particle level. This can
allow the
generation of a direct quantitative comparison between biomolecular properties
and
mechanical properties. Preferably, the method 200 is used to process and
analyze biological
particles, such as cells, and in specific applications, the method 200 can be
used to analyze
leukocyte activation, stem cell differentiation, and cancer cell malignancy by
way of
correlating cellular deformation with biomolecular phenotypes using
fluorescence assays.
However, the system 100 can alternatively be used to process, deform, and
analyze any other
suitable biological particle or non-biological particle using any other
suitable analysis.
[00113] Block S210 recites: receiving the sample volume including the
plurality of
particles, and functions to receive a sample volume, including the plurality
of particles, to
initiate processing and analysis of the plurality of particles. The sample
volume is preferably
received at an inlet of a substrate, using a pump, as in an embodiment of the
system 100
described above; however, the sample volume can be received and/or delivered
in any other
suitable manner. In some variations, Block S210 can further include filtering
the sample
volume S212, as shown in FIGURE 15, which functions to separate particles of
interest from
other particles in the sample volume and to allow the particles of interest to
pass into a fluidic
pathway for further processing and analysis. Block S212 can be implemented
using any
suitable variation of the filter described above, or using any other suitable
method of
separating particles of interest from other particles in a sample volume.
[00114] Block S220 recites: diverting a first portion of the sample volume in
a first flow
and a second portion of the sample volume in a second flow, opposed to the
first flow,
wherein an intersection of the first and the second flows defines a
deformation region. Block
S220 functions to generate opposing flows configured to deform each particle
in the plurality
of particles. Block S220 is preferably implemented at an embodiment of the
fluidic pathway
of the system 100 described above, wherein the fluidic pathway includes at
least two
branches configured to generate the first and the second flows from the sample
volume.
Additionally or alternatively, an injected flow, not derived sample volume,
can be used to
generate at least one flow in the opposing flows. However, Block S220 can be
implemented
using any other suitable method of generating opposing flows, at least
partially from a sample
volume. In some variations, Block S220 can include diverting a first portion
of the sample
volume in the first flow, wherein the first flow comprises substantially all
of the particles of
41
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
interest and diverting a second portion of the sample volume in the second
flow, wherein the
second flow is substantially free of particles of interest; however, in other
variations, the first
flow and the second flow can both comprise a subset of the plurality of
particles.
[00115] Block S230 recites: focusing the plurality of particles into the
deformation region,
and functions to transmit the plurality of particles, along at least one
streamline into the
deformation region, such that each particle in the plurality of particles
experiences uniform
flow conditions prior to deformation within the deformation region. Block S230
is preferably
implemented at a delivery region in an embodiment of the system 100 described
above, but
can be implemented at any other suitable portion of a fluid pathway configured
to focus
particles. Preferably, any flow including particles of the plurality of
particles is focused into
the deformation region in Block S230; however, in alternative variations,
Block S230 can
omit focusing of any subset of the plurality of particles, and/or focusing of
flows not
including particles of the plurality of particles. In one embodiment, focusing
in Block S230
includes focusing using inertial focusing in at least one of a confined curved
channel and a
channel including a set of height restrictions, as described above; however,
focusing in Block
S230 can comprise any one or more of: hydrodynamic focusing, focusing using a
sheath
fluid, dielectrophoretic focusing, ultrasonic focusing, magnetic focusing, and
any other
suitable focusing method.
[00116] Block S240 recites: generating a morphology dataset characterizing
deformation of
each particle in the plurality of particles within the deformation region, and
functions to
generate a dataset that can be used to extract a set of deformation
characteristics for
generation of an analysis based upon deformation characteristics. The
morphology dataset is
preferably generated in Block S240 using an embodiment of the detection module
and
imaging subsystem described above; however, the morphology dataset can
additionally or
alternatively be generated using any suitable module including an image sensor
configured to
capture image data for particles undergoing deformation. Preferably, the
morphology dataset
generated is characterized by a high frame rate, such that the morphology
dataset
characterizes multiple stages of deformation for each particle in the
plurality of particles.
Furthermore, the morphology dataset is preferably generated in a continuous
manner and in
real time; however, the morphology dataset can alternatively be generated in
any other
suitable manner.
42
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
[00117] Block S250 recites: generating a fluorescence dataset characterizing
fluorescence
of each particle of the plurality of particles within the deformation region,
and functions to
generate a dataset that can be used to extract a set of fluorescence
parameters for generation
of an analysis based upon fluorescence parameters. The fluorescence dataset is
preferably
generated in Block S250 using an embodiment of the detection module and
fluorescence
subsystem described above; however, the fluorescence dataset can additionally
or
alternatively be generated using any suitable module including a photodetector
configured to
detect light emitted by fluorescent labels being excited by excitation
wavelengths of light.
Preferably, the fluorescence dataset is generated in a continuous manner and
in real time;
however, the fluorescence dataset can alternatively be generated in any other
suitable manner.
Furthermore, Block S250 is performed concurrently with Block S240, such that
the
morphology dataset and the fluorescence dataset are simultaneously or nearly
simultaneously
generated, and deformation characteristics and fluorescence parameters can be
temporally
matched or otherwise synchronized to each particle in the plurality of
particles.
[00118] Block S260 recites: outputting an analysis of the plurality of
particles based at least
in part on the morphology dataset and the fluorescent dataset for the
plurality of particles
S260, and functions to produce an analysis characterizing the particles of
interest based upon
multiple types of parameters (e.g., mechanical, deformation, fluorescence,
biochemical, etc.).
Block S260 is preferably implemented at an embodiment of the processor
described above;
however, Block S260 can additionally or alternatively be performed using any
suitable
processing element configured to generate an analysis based upon the
morphology dataset
and the fluorescence dataset. In variations, Block S260 can thus be
implemented at a
processor including a first module that extracts the set of deformation
characteristics from the
morphology dataset; a second module that extracts the set of fluorescence
parameters from
the fluorescence dataset; a fourth module configured to synchronize the
morphology dataset
and the fluorescence dataset based upon a deformation characteristic and a
fluorescence
parameter; and a fourth module configured to generate the analysis. As such,
Block S260 can
further include, as illustrated in FIGURE 16: extracting a set of deformation
characteristics
from the morphology dataset S261; extracting a set of fluorescence parameters
from the
fluorescence dataset S262; and temporally synchronizing the morphology dataset
and the
fluorescence dataset based upon a deformation characteristic and a
fluorescence parameter
S263, as shown in FIGURE 16.
43
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
[00119] In Blocks S260 and S261, the set of deformation characteristics
preferably provide
morphological characteristics indicative of phenotype, such as nuclear size,
chromatin
decondensation, cytoskeletal disassembly/fluidization, and membrane
compromise/lysis. The
set of deformation characteristics can thus include any one or more of:
particle deformability,
particle circularity, particle size (e.g., volume, area, etc.), particle
asymmetry, and any other
suitable morphological characteristic. In relation to Blocks S260 and S261 in
FIGURE 16,
the method can additionally comprise extracting baseline morphological
particle
characteristics S264, including one or more of: initial particle volume,
initial particle
diameter, initial particle asymmetry, and any other suitable baseline
characteristic. Extracting
particle characteristics in Block S264 can be performed as in U.S. Pub. No.
2013/0177935,
entitled "Method and Device for High Throughput Cell Deformability
Measurements", or in
any other suitable manner. In variations of the method 200 including Blocks
S260, S261, and
S264, the baseline characteristics can be used to normalize the set of
deformation
characteristics for each particle in the plurality of particles, and/or can be
used to generate the
analysis in any other suitable manner.
[00120] In Blocks S260 and S262, the set of fluorescence parameters preferably
provide
characteristics indicative of biomolecular phenotype and can include any one
or more of: an
intensity of emitted light (e.g., average intensity, peak intensity), a
wavelength of emitted
light, kinetic parameters of fluorescence, and any other suitable fluorescence
parameter.
Similar to variations of the method 200 including Block S264, the method 200
can also
include extracting baseline fluorescence parameters S265 (i.e., prior to
particle deformation),
including one or more of: initial intensity (e.g., initial average or peak
intensity), initial
emitted wavelength prior to deformation, initial kinetic parameter(s) prior to
deformation,
and any other suitable baseline parameter. The baseline parameters can be used
to normalize
the set of fluorescence parameters for each particle in the plurality of
particles, and/or can be
used to generate the analysis in any other suitable manner.
[00121] In Blocks S260 and S263, synchronizing the morphology dataset and the
fluorescence dataset can include conditioning at least one of the morphology
dataset and the
fluorescence dataset S266, wherein conditioning comprises at least one of
noise removal by
filtering and peak-finding. Block S266 can include applying signal filters to
remove signal
noise and applying a peak-finding algorithm to identify a time-dependent
sequence of
particles. Block S263 can further include implementing a sequence matching
algorithm S267,
44
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
an example of which is shown in FIGURE 14, to align event vs. time signals of
the
morphology dataset with event vs. time signals of the fluorescence dataset. In
variations,
Block S263 can further include synchronizing the morphology dataset and the
fluorescence
dataset based upon data generated from calibration particles of the sample
volume S268. In
examples, the calibration particles can comprise rigid fluorescent calibration
microspheres
characterized by identifiable deformability and fluorescence signatures can be
used to
synchronize the morphology dataset with the fluorescence dataset, irrespective
of time
stamps. In still other variations, relationships between deformability (or any
other suitable
deformation characteristic) and emitted fluorescence intensity can be used to
synchronize the
sets of data in Block S263. However, the morphology dataset and the
fluorescence dataset
can be synchronized in any other suitable manner.
[00122] The analysis generated in Block S260 can comprise a correlation
between
mechanical and biochemical/biomolecular markers for the particles of interest,
which can be
used to identify mechanical (e.g., deformation) characteristics, fluorescence
parameters,
and/or combinations of mechanical and fluorescence parameters useful for
characterizing
particles of the plurality of particles. In specific applications, the
analysis generated in Block
S260 can be used identify activation states of specific cell lines (e.g.,
blood mononuclear cell
activation by mitogens or inflammatory processes, granulocyte activation with
cytokines or
blood streams infections, as identified by deformability and surface
expression of activation
markers), with important implications in label-free monitoring of diseases,
diagnosis of
diseases, treatment of diseases, and prediction of transplant rejection. In
additional
applications, the analysis generated can be used to identify phenotypic
connections between
stem cells and cancers (e.g., Jurkat and HL60), used to identify
differentiation indicators for
stem cells, and used for identification of cells within a diverse populations
of cells within
diverse populations of cells in body fluid samples from healthy or diseased
patients (e.g.,
resting or activated leuokocytes, PBMCs, and granulocytes as in blood, or
pleural fluid). As
such, Block S260 can be further include aggregating a library of data of
multiple types of
phenotypic markers based upon the analysis S269, wherein the library
characterizes
phenotypic markers (e.g., mechanical, deformation, fluorescence, etc.) for a
variety of
biological particles, using a high-throughput approach.
[00123] Generating an analysis in Block S260 can thus comprise conducting a
statistical
analysis (e.g., correlation, t-test, ANOVA, etc.) to investigate relationships
between
CA 02920132 2016-02-01
WO 2014/113110
PCT/US2013/065747
deformation and fluorescence parameters. Additionally or alternatively, Block
S260 can
include generating a classification and regression tree (CART) to enhance
identification, and
can further include using a receiver operating characteristic (ROC) curves to
assess correct
identification of particles Furthermore, linear discriminate analyses can be
used in Block
S260 to identify similarities and/or differences between different sample
volumes, which can
be used, for example, to stratify samples from different patients.
[00124] As shown in FIGURE 15, the method can further comprise Block S270,
which
recites: storing at least one of the morphology dataset, the fluorescence
dataset, and the
analysis. Block S270 functions to receive data related to deformation
characteristics of the
plurality of particles, fluorescence parameters of the plurality of particles,
and correlations
between deformation and fluorescence parameters for each particle in the
plurality of
particles. Block S270 can additionally function to store system parameters
used to generate
the datasets and/or the analyses, and can further function to enable data
transmission to a user
or another entity involved with the analysis. Block S270 is preferably
implemented using an
embodiment of the storage module described above; however, Block S270 can be
implementing using any other suitable storage module.
[00125] As a person skilled in the art will recognize from the previous
detailed description
and from the figures and claims, modifications and changes can be made to the
preferred
embodiments of the invention without departing from the scope of this
invention defined in
the following claims.
46