Note: Descriptions are shown in the official language in which they were submitted.
CA 02920500 2016-04-14
REPLACEMENT PAGE
FLUID HEATER
CROSS-REFERENCE TO RELATED APPLICATIONS
This application claims the benefit of the August 1,
2014 priority date of U.S. Application No. 61/999,582.
FIELD OF DISCLOSURE
This disclosure relates to heat transfer systems, and
in particular to devices for transferring heat to a fluid.
BACKGROUND
Many heat transfer systems use hot fluids as a heat
transfer medium. Such systems include a heat generator for
generating heat, a heat transfer medium in thermal
communication with the energy source, and a pump to move
the heated medium to wherever the heat is needed. Because
of its high heat capacity and its abundance, a common heat
transfer fluid is water, both in its liquid and gas phase.
A variety of heat generators are in common use. For
instance, in nuclear power plants, nuclear fission provides
energy for heating water. There also exist solar water
heaters that use solar energy. However, most heat transfer
sources rely on an exothermal chemical reaction, and in
particular, on combustion of some fuel.
SUMMARY
In one aspect, the invention features an apparatus for
heating fluid, the apparatus including a tank for holding
fluid to be heated, and a fuel wafer in fluid communication
with the fluid, the fuel wafer including a fuel mixture
including reagents and a catalyst, and a heat source or
ignition source in thermal communication with the fuel
mixture and the catalyst. The heat source or ignition
1
CA 029200 2016--134
WO 2016/018851 PCT/US2015/042353
source can be an electrical resistor, or a heat source that
relies on either heat from combustion, such as combustion
of natural gas, or a heat source that relies on inductive
heating.
Among the embodiments are those in which the fuel
mixture includes lithium and lithium aluminum hydride,
those in which the catalyst includes a group 10 element,
such as nickel in powdered form, or in any combination
thereof.
In other embodiments, the catalyst in powdered form,
has been treated to enhance its porosity. For example, the
catalyst can be nickel powder that has been treated to
enhance porosity thereof. The apparatus can also include an
electrical energy source, such as a voltage source and/or
current source in electrical communication with the heat
source.
Among the other embodiments are those in which the
fuel wafer includes a multi-layer structure having a layer
of the fuel mixture in thermal communication with a layer
containing the heat source.
In yet other embodiments, the fuel wafer includes a
central heating insert and a pair of fuel inserts disposed
on either side of the heating insert.
A variety of tanks can be used. For example, in some
embodiments, the tank includes a recess for receiving the
fuel wafer therein. Among these are embodiments in which
the tank further includes a door for sealing the recess. In
yet other embodiments the tank includes a radiation shield.
2
CA 029200 2016--134
WO 2016/018851 PCT/US2015/042353
Also included among the embodiments are those that
further include a controller in communication with the
voltage source. Among these are controllers that are
configured to vary the voltage in response to temperature
of the fluid to be heated.
In another aspect, the invention features an apparatus
for heating a fluid, the apparatus including means for
containing the fluid, and means for holding a fuel mixture
containing a catalyst and a reagent, and means for
initiating a reaction sequence mediated by the catalyst to
cause an exothermic reaction.
Another aspect of the invention is a composition of
matter for generating heat, the composition including a
mixture of porosity-enhanced nickel powder, lithium powder,
and lithium aluminum powder. A heat source in thermal
communication with the mixture can be used for initiating a
nickel catalyzed exothermic reaction.
Yet another aspect features a for generating heat. The
composition includes a fuel mixture and a catalyst. The
catalyst comprises a group 10 element.
Embodiments include those in which the catalyst
comprises nickel. Among these are embodiments in which the
nickel is in the form of nickel powder and those in which
the nickel powder has been treated to enhance porosity
thereof.
Another aspect of the invention is a method of heating
a fluid, the method including placing a mixture of nickel
powder, lithium powder, and lithium aluminum hydride in
thermal communication with the fluid; and heating the
3
CA 02920500 2016-02-04
WO 2016/018851
PCT/US2015/042353
mixture, thereby initiating an exothermic reaction in the
mixture.
These and other features of the invention will be
apparent from the following detailed description and the
accompanying figures, in which:
BRIEF DESCRIPTION OF THE FIGURES
FIG. 1 shows a heat transfer system having a heat
source;
FIG. 2 is a cut-away view of the heat source in FIG.
1;
FIG. 3 is a cross-section of the wafer for use in the
heat source of FIG. 2;
FIG. 4 shows an exemplary resistor in the central
layer of the wafer shown in FIG. 3.
FIG. 5 shows the heat source of FIG. 1 operating with
a conventional furnace.
FIG. 6 shows plural heat sources like that in FIG. 2
connected in series.
FIG. 7 shows plural heat sources like that in FIG. 2
connected in parallel.
DETAILED DESCRIPTION
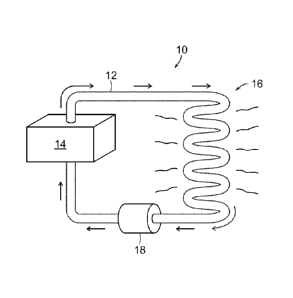
Referring to FIG. 1, a heat transfer system 10
includes a pipe 12 for transporting a heated fluid in a
closed loop between a heat source 14 and a thermal load 16.
In most cases, for example where there is hydraulic
resistance to be overcome, a pump 18 propels the heated
fluid. However, in some cases, such as where the heated
4
CA 02920500 2016-02-04
WO 2016/018851 PCT/US2015/042353
fluid is steam, the fluid's own pressure is sufficient to
propel the fluid. A typical thermal load 16 includes
radiators such as those commonly used for heating interior
spaces.
As shown in FIG. 2, the heat source 14 is a tank 20
having a lead composite shield, an inlet 22 and an outlet
24, both of which are connected to the pipe 12. The
interior of the tank 20 contains fluid to be heated. In
many cases, the fluid is water. However, other fluids can
be used. In addition, the fluid need not be a liquid fluid
but can also be a gas, such as air.
The tank 20 further includes a door 26 that leads to a
receptacle 28 protruding into the tank 20. Radiating fins
30 protrude from walls of the receptacle 28 into the tank
20. To maximize heat transfer, the receptacle 28 and the
fins 30 are typically made of a material having high
thermal conductivity, such as metal. A suitable metal is
one not subject to corrosion, such as stainless steel.
The receptacle 28 holds a multi-layer wafer 32 for
generating heat. A voltage source 33 is connected to the
wafer 32, and a controller 35 for controlling the voltage
source 33 in response to temperature of fluid in the tank
12 as sensed by a sensor 37.
As shown in FIG. 3, the multilayer fuel wafer 32
includes a heating section 34 sandwiched between two fuel
sections 36, 38. The heating section 34 features a central
layer 40 made of an insulating material, such as mica, that
supports a resistor 42.It should be noted that other
heating sources can be used, including heat sources that
5
CA 02920500 2016-02-04
WO 2016/018851 PCT/US2015/042353
rely on combustion of, for example, natural gas, as well as
heat sources that rely on electrical induction. The use of
gas thus avoids the need to have a source of electrical
energy for initiating the reaction.
FIG. 4 shows an exemplary central layer 40 having
holes 44 through which a resistive wire 42 has been wound.
This resistive wire 42 is connected to the voltage source
33. First and second insulating layers 46, 48, such as mica
layers, encase the central layer 40 to provide electrical
insulation from the adjacent fuel sections 36, 38.
Each fuel section 36, 38 features a pair of thermally
conductive layers 50, 52, such as steel layers. Sandwiched
between each pair of conductive layers 50, 52 is a fuel
layer 54 that contains a fuel mixture having nickel,
lithium, and lithium aluminum hydride LiA1H4 ("LAH"), all in
powdered form. Preferably, the nickel has been treated to
increase its porosity, for example by heating the nickel
powder to for times and temperatures selected to superheat
any water present in micro-cavities that are inherently in
each particle of nickel powder. The resulting steam
pressure causes explosions that create larger cavities, as
well as additional smaller nickel particles.
The entire set of layers is welded together on all
sides to form a sealed unit. The size of the wafer 32 is
not important to its function. However, the wafer 32 is
easier to handle if it is on the order of 1/3 inch thick
and 12 inches on each side. The steel layers 50, 52 are
typically 1 mm thick, and the mica layers 40, 48, which are
covered by a protective polymer coating, are on the order
of 0.1 mm thick. However, other thicknesses can also be
6
CA 02920500 2016-02-04
WO 2016/018851
PCT/US2015/042353
used.
In operation, a voltage is applied by the voltage
source 33 to heat the resistor 42. Heat from the resistor
42 is then transferred by conduction to the fuel layers 54,
where it initiates a sequence of reactions, the last of
which is reversible. These reactions, which are catalyzed
by the presence of the nickel powder, are:
3LiA1H4-4 Li3A1H6 + 2A1 + 3H2
2Li3A1146-4 6LiH + 2A1 + 3H2
2LiH + 2A1 2LiA1 + H2
Once the reaction sequence is initiated, the voltage
source 33 can be turned off, as the reaction sequence is
self-sustaining. However, the reaction rate may not be
constant. Hence, it may be desirable to turn on the voltage
source 33 at certain times to reinvigorate the reaction. To
determine whether or not the voltage source 33 should be
turned on, the temperature sensor 37 provides a signal to
the controller 35, which then determines whether or not to
apply a voltage in response to the temperature signal. It
has been found that after the reaction has generated
approximately 6 kilowatt hours of energy, it is desirable
to apply approximately 1 kilowatt hour of electrical energy
to reinvigorate the reaction sequence.
Eventually, the efficiency of the wafer 32 will
decrease to the point where it is uneconomical to
continually reinvigorate the reaction sequence. At this
point, the wafer 32 can simply be replaced. Typically, the
wafer 32 will sustain approximately 180 days of continuous
7
CA 029200 2016--134
WO 2016/018851 PCT/US2015/042353
operation before replacement becomes desirable.
The powder in the fuel mixture consists largely of
spherical particles having diameters in the nanometer to
micrometer range, for example between 1 nanometer and 100
micrometers. Variations in the ratio of reactants and
catalyst tend to govern reaction rate and are not critical.
However, it has been found that a suitable mixture would
include a starting mixture of 50% nickel, 20% lithium, and
30% LAH. Within this mixture, nickel acts as a catalyst for
the reaction, and is not itself a reagent. While nickel is
particularly useful because of its relative abundance, its
function can also be carried out by other elements in
column 10 of the periodic table, such as platinum or
palladium.
FIGS. 5-7 show a variety of ways to connect the heat
source 14 in FIG. 1.
In FIG. 5, the heat source 14 is placed downstream
from a conventional furnace 56. In this case, the
controller 35 is optionally connected to control the
conventional furnace. As a result, the conventional furnace
56 will remain off unless the output temperature of the
heat source 14 falls below some threshold, at which point
the furnace 56 will start. In this configuration, the
conventional furnace 56 functions as a back-up unit.
In FIG. 6, first and second heat sources 58, 60 like
that described in FIGS. 1-4 are connected in series. This
configuration provides a hotter output temperature than can
be provided with only a single heat source 58 by itself.
Additional heat sources can be added in series to further
8
CA 02920500 2016-02-04
WO 2016/018851
PCT/US2015/042353
increase the temperature.
In FIG. 7, first and second heat sources 62, 64 like
that described in FIGS. 1-4 are connected in parallel. In
this configuration, the output volume can be made greater
than what could be provided by a single heat transfer unit
by itself. Additional heat transfer units can be added in
parallel to further increase volume.
In one embodiment, the reagents are placed in the
reaction chamber at a pressure of 3-6 bar and a temperature
of from 400 C to 600 C. An anode is placed at one side of
the reactor and a cathode is placed at the other side of
the reactor. This accelerates electrons between them to an
extent sufficient to have very high energy, in excess of
100 Key. Regulation of the electron energy can be carried
out by regulating the electric field between the cathode
and the anode.
Having described the invention, and a preferred
embodiment thereof, what I claim as new and secured by
letters patent is:
9