Note: Descriptions are shown in the official language in which they were submitted.
CA 02920724 2016-02-08
WO 2015/023579
PCT/US2014/050525
APPARATUS AND METHODS FOR IMPLANTING A REPLACEMENT
HEART VALVE
Cross-Reference to Related Applications
[0001] This application claims the priority of U.S. Provisional
Application
Serial No. 61/864,860, filed August 12, 2013 (pending); U.S. Provisional
Application Serial No. 61/867,287, filed August 19, 2013 (pending); and U.S.
Provisional Application Serial No. 61/878,280, filed September 16, 2013
(pending), the disclosures of which are hereby incorporated by reference
herein.
Technical Field
[0002] The present invention generally relates to medical procedures
and
devices pertaining to heart valves such as replacement techniques and
apparatus. More specifically, the invention relates to the replacement of
heart
valves having various malformations and dysfunctions.
Background
[0003] Complications of the mitral valve, which controls the flow of
blood
from the left atrium into the left ventricle of the human heart, have been
known
to cause fatal heart failure. In the developed world, one of the most common
forms of valvular heart disease is mitral valve leak, also known as mitral
regurgitation, which is characterized by the abnormal leaking of blood from
the
left ventricle through the mitral valve and back into the left atrium. This
occurs
most commonly due to ischemic heart disease when the leaflets of the mitral
valve no longer meet or close properly after multiple infarctions, idiopathic
and
hypertensive cardiomyopathies where the left ventricle enlarges, and with
leaflet and chordal abnormalities, such as those caused by a degenerative
disease.
[0004] In addition to mitral regurgitation, mitral narrowing or
stenosis is
most frequently the result of rheumatic disease. While this has been virtually
eliminated in developed countries, it is still common where living standards
are
not as high.
[0005] Similar to complications of the mitral valve are complications
of
the aortic valve, which controls the flow of blood from the left ventricle
into the
-1-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
aorta. For example, many older patients develop aortic valve stenosis.
Historically, the traditional treatment had been valve replacement by a large
open heart procedure. The procedure takes a considerable amount of time for
recovery since it is so highly invasive. Fortunately, in the last decade great
advances have been made in replacing this open heart surgery procedure with
a catheter procedure that can be performed quickly without surgical incisions
or
the need for a heart-lung machine to support the circulation while the heart
is
stopped. Using catheters, valves are mounted on stents or stent-like
structures,
which are compressed and delivered through blood vessels to the heart. The
stents are then expanded and the valves begin to function. The diseased valve
is not removed, but instead it is crushed or deformed by the stent which
contains the new valve. The deformed tissue serves to help anchor the new
prosthetic valve.
[0006] Delivery of the valves can be accomplished from arteries which
can be easily accessed in a patient. Most commonly this is done from the groin
where the femoral and iliac arteries can be cannulated. The shoulder region is
also used, where the subclavian and axillary arteries can also be accessed.
Recovery from this procedure is remarkably quick.
[0007] Not all patients can be served with a pure catheter procedure.
In
some cases the arteries are too small to allow passage of catheters to the
heart, or the arteries are too diseased or tortuous. In these cases, surgeons
can make a small chest incision (thoractomy) and then place these catheter-
based devices directly into the heart. Typically, a purse string suture is
made in
the apex of the left ventricle and the delivery system is place through the
apex
of the heart. The valve is then delivered into its final position. These
delivery
systems can also be used to access the aortic valve from the aorta itself.
Some
surgeons introduce the aortic valve delivery system directly in the aorta at
the
time of open surgery. The valves vary considerably. There is a mounting
structure that is often a form of stent. Prosthetic leaflets are carried
inside the
stent on mounting and retention structure. Typically, these leaflets are made
from biologic material that is used in traditional surgical valves. The valve
can
be actual heart valve tissue from an animal or more often the leaflets are
made
from pericardial tissue from cows, pigs or horses. These leaflets are treated
to
reduce their immunogenicity and improve their durability. Many tissue
processing techniques have been developed for this purpose. In the future
-2-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
biologically engineered tissue may be used or polymers or other non-biologic
materials may be used for valve leaflets. All of these can be incorporated
into
the inventions described in this disclosure.
[0008] There are in fact more patients with mitral valve disease
than
aortic valve disease. In the course of the last decade many companies have
been successful in creating catheter or minimally invasive implantable aortic
valves, but implantation of a mitral valve is more difficult and to date there
has
been no good solution. Patients would be benefited by implanting a device by a
surgical procedure employing a small incision or by a catheter implantation
such as from the groin. From the patient's point of view, the catheter
procedure
is very attractive. At this time there is no commercially available way to
replace
the mitral valve with a catheter procedure. Many patients who require mitral
valve replacement are elderly and an open heart procedure is painful, risky
and
takes time for recovery. Some patients are not even candidates for surgery due
to advanced age and frailty. Therefore, there exists a particular need for a
remotely placed mitral valve replacement device.
[0009] While previously it was thought that mitral valve replacement
rather than valve repair was associated with a more negative long term
prognosis for patients with mitral valve disease, this belief has come into
question. It is now believed that the outcome for patients with mitral valve
leak
or regurgitation is almost equal whether the valve is repaired or replaced.
Furthermore, the durability of a mitral valve surgical repair is now under
question. Many patients, who have undergone repair, redevelop a leak over
several years. As many of these are elderly, a repeat intervention in an older
patient is not welcomed by the patient or the physicians.
[0010] The most prominent obstacle for catheter mitral valve
replacement
is retaining the valve in position. The mitral valve is subject to a large
cyclic
load. The pressure in the left ventricle is close to zero before contraction
and
then rises to the systolic pressure (or higher if there is aortic stenosis)
and this
can be very high if the patient has systolic hypertension. Often the load on
the
valve is 150mmHg or more. Since the heart is moving as it beats, the
movement and the load can combine to dislodge a valve. Also the movement
and rhythmic load can fatigue materials leading to fractures of the materials.
Thus, there is a major problem associated with anchoring a valve.
-3-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
[0011] Another problem with creating a catheter delivered mitral
valve
replacement is size. The implant must have strong retention and leak
avoidance features and it must contain a valve. Separate prostheses may
contribute to solving this problem, by placing an anchor or dock first and
then
implanting the valve second. However, in this situation the patient must
remain
stable between implantation of the anchor or dock and implantation of the
valve.
If the patient's native mitral valve is rendered non-functional by the anchor
or
dock, then the patient may quickly become unstable and the operator may be
forced to hastily implant the new valve or possibly stabilize the patient by
removing the anchor or dock and abandoning the procedure.
[0012] Another problem with mitral replacement is leak around the
valve,
or paravalvular leak. If a good seal is not established around the valve,
blood
can leak back into the left atrium. This places extra load on the heart and
can
damage the blood as it travels in jets through sites of leaks. Hemolysis or
breakdown of red blood cells is a frequent complication if this occurs.
Paravalvular leak was one of the common problems encountered when the
aortic valve was first implanted on a catheter. During surgical replacement, a
surgeon has a major advantage when replacing the valve as he or she can see
a gap outside the valve suture line and prevent or repair it. With catheter
insertion, this is not possible. Furthermore, large leaks may reduce a
patient's
survival and may cause symptoms that restrict mobility and make the patient
'
uncomfortable (e.g. short of breathe, edematous, fatigued). Therefore,
devices,
systems, and methods which relate to mitral valve replacement should also
incorporate means to prevent and repair leaks around the replacement valve.
[0013] A patient's mitral valve annulus can also be quite large. When
companies develop surgical replacement valves, this problem is solved by
restricting the number of sizes of the actual valve produced and then adding
more fabric cuff around the margin of the valve to increase the valve size.
For
example, a patient may have a 45mm valve annulus. In this case, the actual
prosthetic valve diameter may be 30mm and the difference is made up by
adding a larger band of fabric cuff material around the prosthetic valve.
However, in catheter procedures, adding more material to a prosthetic valve is
problematic since the material must be condensed and retained by small
delivery systems. Often this method is very difficult and impractical, so
alternative solutions are necessary.
-4-
CA 02920724 2016-02-08
WO 2015/023579
PCT/US2014/050525
[0014] Since numerous valves have been developed for the aortic
position, it is desirable to avoid repeating valve development and to take
advantage of existing valves. These valves have been very expensive to
develop and bring to market, so extending their application can save
considerable amounts of time and money. It would be useful then to create a
mitral anchor or docking station for such a valve. An existing valve developed
for the aortic position, perhaps with some modification, could then be
implanted
in the docking station. Some previously developed valves may fit well with no
modification, such as the Edwards Sapien TM valve. Others, such as the
Corevalve TM may be implantable but require some modification for an optimal
engagement with the anchor and fit inside the heart.
[0015] A number of further complications may arise from a poorly
retained or poorly positioned mitral valve replacement prosthesis. Namely, a
valve can be dislodged into the atrium or ventricle, which could be fatal for
a
patient. Prior prosthetic anchors have reduced the risk of dislodgement by
puncturing tissue to retain the prosthesis. However, this is a risky maneuver
since the penetration must be accomplished by a sharp object at a long
distance, leading to a risk of perforation of the heart and patient injury.
[0016] Orientation of the mitral prosthesis is also important. The
valve
must allow blood to flow easily from the atrium to the ventricle. A prosthesis
that enters at an angle may lead to poor flow, obstruction of the flow by the
wall
of the heart or a leaflet and a poor hemodynamic result. Repeated contraction
against the ventricular wall can also lead to rupture of the back wall of the
heart
and sudden death of the patient.
[0017] With surgical mitral valve repair or replacement, sometimes
the
anterior leaflet of the mitral valve leaflet is pushed into the area of the
left
ventricular outflow and this leads to poor left ventricular emptying. This
syndrome is known as left ventricular tract outflow obstruction. The
replacement valve itself can cause left ventricular outflow tract obstruction
if it is
situated close to the aortic valve.
[0018] Yet another obstacle faced when implanting a replacement
mitral
valve is the need for the patient's native mitral valve to continue to
function
regularly during placement of the prosthesis so that the patient can remain
stable without the need for a heart-lung machine to support circulation.
-5-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
[0019] In addition, it is desirable to provide devices and methods
that can
be utilized in a variety of implantation approaches. Depending on a particular
patient's anatomy and clinical situation, a medical professional may wish to
make a determination regarding the optimal method of implantation, such as
inserting a replacement valve directly into the heart in an open procedure
(open
heart surgery or a minimally invasive surgery) or inserting a replacement
valve
from veins and via arteries in a closed procedure (such as a catheter-based
implantation). It is preferable to allow a medical professional a plurality of
implantation options to choose from. For example, a medical professional may
wish to insert a replacement valve either from the ventricle or from the
atrial
side of the mitral valve.
[0020] Therefore, the present invention provides devices and methods
that address these and other challenges in the art.
Summary
[0021] In one illustrative embodiment, a system for docking a heart
valve
prosthesis is provided and includes a helical anchor formed as multiple coils
adapted to support a heart valve prosthesis with coil portions positioned
above
and below the heart valve annulus and a seal coupled with the helical anchor.
The seal includes portions extending between adjacent coils for preventing
blood leakage through the helical anchor and past the heart valve prosthesis.
[0022] The system can further include a heart valve prosthesis
capable
of being delivered to a native heart valve position of a patient and expanded
inside the multiple coils and into engagement with leaflets of the heart
valve.
The seal is engaged with both the helical anchor and the heart valve
prosthesis.
The coils of the helical anchor may be formed of a superelastic or a shape
memory material, or other suitable material. The seal may be a membrane or
panel extending over at least two coils of the helical anchor. The membrane or
panel is moved between an undeployed state and a deployed state, the
undeployed state being adapted for delivery to a site of implantation and the
deployed state being adapted for implanting the system and anchoring the heart
valve prosthesis. The undeployed state may be a rolled up state on one of the
coils of the helical anchor or any other collapsed state. The membrane or
panel
may include a support element affixed therewith, such as an internal, spring-
biased wire. The seal may further include one or more seal elements carried by
-6-
CA 02920724 2016-02-08
WO 2015/023579
PCT/US2014/050525
the helical anchor with overlapping portions configured to seal a space
between
adjacent coils of the helical anchor. The one or more seal elements may each
include a support element such as an internal wire, which may be a spring-
biased coil or other configuration, affixed therewith. The one or more seal
elements may be cross sectional shape, with examples being generally circular
or oblong. The one or more seal elements may each have a connecting portion
affixed to one of the coils and an extension portion extending toward an
adjacent coil for providing the seal function between coils.
[0023] In another illustrative embodiment a system for replacing a
native
heart valve includes an expansible helical anchor formed as multiple coils
adapted to support a heart valve prosthesis. At least one of the coils is
normally defined by a first diameter, and is expandable to a second, larger
diameter upon application of radial outward force from within the helical
anchor.
The system further includes an expansible heart valve prosthesis capable of
being delivered into the helical anchor and expanded inside the multiple coils
into engagement with the at least one coil to move the at least one coil from
the
first diameter to the second diameter while securing the helical anchor and
the
heart valve prosthesis together.
[0024] As a further aspect the helical anchor may include another
coil
that moves from a larger diameter to a smaller diameter as the heart valve
prosthesis is expanded inside the multiple coils. At least two adjacent coils
of
the helical anchor may be spaced apart, and the adjacent coils move toward
each other as the heart valve prosthesis is expanded inside the multiple
coils.
The helical anchor may further includes a plurality of fasteners, and the
fasteners are moved from an undeployed state to a deployed state as the at
least one coil moves from the first diameter to the second, larger diameter. A
seal may be coupled with the helical anchor and include portions extending
between adjacent coils for preventing blood leakage through the helical anchor
and past the heart valve prosthesis. The system can further include at least
one compressible element on the helical anchor, the compressible element
being engaged by the heart valve prosthesis as the heart valve prosthesis is
expanded inside the multiple coils to assist with affixing the heart valve
prosthesis to the helical anchor. The compressible element may take any of
several forms, such as fabric or other soft material, or resilient, springy
material
such as polymer or foam. The at least one compressible element further may
-7..
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
include multiple compressible elements spaced along the multiple coils or a
continuous compressible element extending along the multiple coils. The heart
valve prosthesis may further include an expansible structure including
openings. The openings are engaged by the at least one compressible element
as the heart valve prosthesis is expanded inside the multiple coils for
purposes
of strengthening the connection between the anchor and the prosthesis. The
multiple coils of the helical anchor may include at least two coils that cross
over
each other. This system may include any feature or features of the system that
uses the seal, and vice versa, depending on the functions and effects desired.
[0025] Methods of implanting a heart valve prosthesis in the heart of
a
patient are also provided. In one illustrative embodiment, the method includes
delivering a helical anchor in the form of multiple coils such that a portion
of the
helical anchor is above the native heart valve and a portion is below the
native
heart valve. The heart valve prosthesis is implanted within the multiple coils
of
the helical anchor such that the heart valve prosthesis is supported by the
helical anchor. A seal is positioned between at least two adjacent coils of
the
helical anchor and the heart valve prosthesis for preventing leakage of blood
flow during operation of the heart valve prosthesis.
[0026] Positioning the seal can further comprise positioning a
membrane
or panel extending over at least two coils of the helical anchor. The method
further includes delivering the membrane or panel in an undeployed state to
the
site of the native heart valve and then deploying the membrane or panel within
the helical anchor, and expanding the heart valve prosthesis against the
membrane or panel. The undeployed state includes a rolled up state or other
collapsed state. Positioning the seal may further include positioning one or
more seal elements carried by the helical anchor such that overlapping
portions
seal a space between adjacent coils of the helical anchor. The one or more
seal elements may each include a support element affixed therewith.
[0027] In another embodiment, a method of implanting an expansible
heart valve prosthesis in the heart of a patient is provided. This method
includes delivering an expansible helical anchor in the form of multiple coils
such that a portion of the expansible helical anchor is above the native heart
valve and a portion is below the native heart valve. The expansible heart
valve
prosthesis is positioned within the multiple coils of the expansible helical
anchor
with the expansible heart valve prosthesis and the expansible helical anchor
in
-8-
CA 02920724 2016-02-08
WO 2015/023579
PCT/US2014/050525
unexpanded states. The expansible heart valve prosthesis in then expanded
against the expansible helical anchor thereby securing the expansible heart
valve prosthesis to the expansible helical anchor. By "expansible" it is meant
that at least one coil of the anchor enlarges in diameter.
[0028] The method may further include moving a coil from a larger
diameter to a smaller diameter as the heart valve prosthesis is expanded
inside
the multiple coils. At least two adjacent coils of the helical anchor may be
spaced apart, and the method further comprises moving the at least two
adjacent coils toward each other as the heart valve prosthesis is expanded
inside the multiple coils. The helical anchor further may comprise a plurality
of
fasteners, and the method further comprises moving the fasteners from an
undeployed state to a deployed state as the expansible heart valve prosthesis
is expanded against the expansible helical anchor. A seal may be positioned
between adjacent coils for preventing blood leakage through the helical anchor
and past the heart valve prosthesis and the fasteners engage the seal in the
deployed state. The fasteners may instead engage a portion of the anchor
which is not a seal. Any other aspects of the methods or systems disclosed
herein may also or alternatively be used in this method depending on the
desired outcome.
[0029] Various additional advantages, methods, devices, systems and
features will become more readily apparent to those of ordinary skill in the
art
upon review of the following detailed description of the illustrative
embodiments
taken in conjunction with the accompanying drawings.
Brief Description of the Drawings
[0030] FIG. 1A is a schematic cross-sectional view illustrating a
replacement heart valve implanted in a native valve position using a helical
anchor.
[0031] FIG. 1B is a schematic cross-sectional view similar to the
FIG. 1A,
but illustrating the use of seals in conjunction with the helical anchor.
[0032] FIG. 2A is a perspective view illustrating one method of
applying
the seal structure to the helical anchor.
[0033] FIGS. 2B is a perspective view illustrating a further step in
the
method illustrated in figure 2A.
-9-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
[0034] FIG. 20 is a cross-sectional view showing the helical anchor after
application of the seal.
[0035] FIG. 2D is an enlarged cross-sectional view of the helical anchor
having one form of seal applied.
[0036] FIG. 2E is a cross-sectional view similar to FIG. 2D, but
illustrating
an alternative embodiment of the seal.
[0037] FIG. 2F is another enlarged cross-sectional view similar to FIG.
2E but illustrating another alternative embodiment for the seal.
[0038] FIG. 3A is a schematic perspective view illustrating another
alternative embodiment of the helical anchor and seal.
[0039] FIG. 3B is a cross-sectional view of the embodiment shown in
FIG. 3A, with the helical adjacent coils compressed together for delivery.
[0040] FIG. 30 is a cross-sectional view showing the helical anchor and
seal expanded after delivery.
[0041] FIG. 3D is a partial perspective view illustrating another
illustrative
embodiment of the helical anchor.
[0042] FIG. 3E is a schematic elevational view, partially fragmented, to
show the application of a seal to the helical anchor structure of FIG. 3D.
[0043] FIG. 3F is an enlarged cross-sectional view illustrating another
embodiment of a helical coil structure with a seal.
[0044] FIG. 3G is a cross-sectional view similar to FIG. 3F, but
illustrating
the structure after delivery and unfolding of the seal.
[0045] FIG. 3H is a cross-sectional view similar to FIG. 3G but
illustrating
multiple parts of the helical anchor structure and associated seal expanded
after delivery.
[0046] FIG. 4A is a perspective view illustrating a helical anchor in
combination with another alternative embodiment of a seal.
[0047] FIG. 4B is a perspective view of the seal illustrating an
alternative
embodiment which adds support structure to the seal.
[0048] FIG. 4C is a schematic cross-sectional view illustrating the
embodiment of FIG. 4A implanted in a native heart valve position.
[0049] FIG. 4D is a schematic cross-sectional view illustrating a
replacement heart valve implanted within the helical anchor and seal structure
of FIG. 40.
-10-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
[0050] FIG. 5A is a perspective view of a helical anchor with a membrane
or panel seal being applied.
[0051] FIG. 5B is a perspective view of the helical anchor with the
membrane or panel seal of FIG. 5A deployed or unfolded.
[0052] FIG. 5C illustrates a perspective view of the membrane or panel
seal with an internal support structure.
[0053] FIG. 5D is an enlarged cross-sectional view of the helical coil and
undeployed membrane seal.
[0054] FIG. 5E is a cross-sectional view similar to FIG. 5D but
illustrating
a membrane seal which has been collapsed or folded rather than wound around
a coil of the helix.
[0055] FIG. 5F is a perspective view of a portion of the coil and
membrane seal illustrating further details including the internal support
structure
and a suture line.
[0056] FIG. 5G is a cross-sectional view illustrating the helical coil and
membrane seal implanted at a native heart valve site.
[0057] FIG. 5I-f is a cross-sectional view similar to FIG. 5G, but further
illustrating a replacement or prosthetic heart valve implanted within the
helical
coil and membrane seal.
[0058] FIG. 6A is a cross-sectional view illustrating a helical coil
implanted and at a native heart valve site being expanded by a balloon.
[0059] FIG. 6B is a cross-sectional view illustrating a stented,
replacement or prosthetic heart valve implanted within a helical coil and
membrane seal structure.
[0060] FIG. 7A is a cross-sectional view schematically illustrating a
helical anchor having approximately two turns or coils having a first diameter
and another coil having a second, larger diameter.
[0061] FIG. 7B illustrates an initial step during implantation of
the helical
anchor shown in FIG. 7A at a native heart valve site with a stent mounted
replacement heart valve ready for implantation within the helical anchor.
[0062] FIG. 7C illustrates a further portion of the procedure in
which the
stented replacement heart valve is expanded using a balloon catheter.
[0063] FIG. 7D is a further portion of the procedure and illustrates
a
cross-sectional view of the implanted replacement heart valve within the
helical
anchor.
-11-
CA 02920724 2016-02-08
WO 2015/023579
PCT/US2014/050525
[0064] FIG. 7D-1 is a cross-sectional view of an implanted
replacement
heart valve within a helical anchor, similar to FIG. 7D but illustrating
alternative
configurations for the replacement heart valve and the anchor.
[0065] FIG. 8A is an elevational view of another embodiment of a
helical
anchor being expanded by a balloon catheter.
[0066] FIG. 8B is a view similar to FIG. 8A, but illustrating further
expansion of the balloon catheter.
[0067] FIG. 8C is a view similar to FIG. 88 but illustrating even
further
expansion of the balloon catheter.
[0068] FIG. 8D is an enlarged cross-sectional view showing
compression
of the helical coils from FIG. 8C.
[0069] FIG. 9A is an elevational view of another embodiment of a
helical
anchor being expanded by a balloon catheter.
[0070] FIG. 9B is a view similar to FIG. 9A, but illustrating further
expansion of the balloon catheter.
[0071] FIG. 9C is a view similar to FIG. 9B but illustrating even
further
expansion of the balloon catheter.
[0072] FIG. 9D is an enlarged cross-sectional view showing
compression
of the helical coils from FIG. 9C.
[0073] FIG. 10A is a partial cross-sectional view illustrating
another
embodiment of a helical anchor inserted or implanted at a native heart valve
site and insertion of a stent mounted replacement heart valve within the
helical
anchor and native heart valve site.
[0074] FIG. 10B is a cross-sectional view similar to FIG. 10A, but
illustrating expansion and implantation of the stent mounted replacement heart
valve within the helical anchor.
[0075] FIG. 10C is a cross-sectional view, partially fragmented, of
the
implanted replacement heart valve and helical anchor shown in FIG. 10B.
[0076] FIG. 10C-1 is an enlarged cross-sectional view showing
engagement between the stent of the replacement heart valve and the helical
anchor.
[0077] FIG. 10D is a top view illustrating the process of expanding
the
stent mounted replacement heart valve within the helical anchor of FIG. 100.
[0078] FIG. 10E is a top view similar to FIG 10D, but illustrating
full
expansion and implantation of the stent mounted replacement heart valve.
-12-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
[0079] FIG. 11A is a partial cross-sectional view illustrating
another
embodiment of a helical anchor inserted or implanted at a native heart valve
site and insertion of a stent mounted replacement heart valve within the
helical
anchor and native heart valve site.
[0080] FIG. 11B is a cross-sectional view similar to FIG. 11A, but
illustrating expansion and implantation of the stent mounted replacement heart
valve within the helical anchor.
[0081] FIG. 11C is a top view illustrating the process of expanding
the
stent mounted replacement heart valve within the helical anchor of FIG. 11B.
[0082] FIG. 11D is a top view illustrating full expansion of the
stent
mounted replacement heart valve within the helical anchor of FIG. 11C.
[0083] FIG. 12A is an elevational view of another embodiment of a
helical
anchor.
[0084] FIG. 12B is a cross-sectional view of another embodiment of a
helical anchor.
[0085] FIG. 12C is an enlarged cross-sectional view of the helical
anchor
taken along line 12C-12C of FIG. 12B.
[0086] FIG. 12D is a top view of a helical anchor schematically
illustrating
expansion by a balloon catheter.
[0087] FIG. 12E is a cross-sectional view of the helical anchor shown
in
FIG. 12D, but expanded to show deployment of the parts into the fabric seal.
[0088] FIG. 13A is an elevational view of another embodiment of a
helical
anchor.
[0089] FIG. 13B is a cross-sectional view of another embodiment of a
helical anchor.
[0090] FIG. 13C is an enlarged cross-sectional view of the helical
anchor
taken along line 13C-13C of FIG. 13B with deployment of the barbs into the
outer seal layer.
[0091] FIG. 14A is a perspective view of an alternative helical
anchor.
[0092] FIG. 14B is a top perspective view of the helical anchor shown
in
FIG. 14A.
[0093] FIG. 14C is a front view of the helical anchor shown in FIGS.
14A
and 14B.
-13-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
Detailed Description of the Illustrative Embodiments
[0094] It will be appreciated that like reference numerals are used
to refer
to generally like structure or features in each of the drawings. Differences
between such elements will generally be described, as needed, but the same
structure need not be described repeatedly for each figure as prior
description
may be referred to instead for purposes of clarity and conciseness. FIG. 1
schematically illustrates a typical replacement heart valve or prosthesis 10
that
may be implanted in the position of a native heart valve, such as the mitral
valve 12, using a catheter (not shown). A sealed condition is desired around
the valve 10, i.e., between the periphery of the replacement valve 10 and the
native biologic tissue, in order to prevent leakage of blood around the
periphery
of the replacement valve 10 as the leaflets 14, 16 of the replacement valve 10
open and close during systolic and biastolyic phases of the heart. The portion
of the replacement heart valve 10 intended to be positioned in contact with
native tissue includes a fabric or polymeric covering 18 to prevent
regurgitation
of blood flow. In FIG. 1A, the fabric cover 18 is shown adjacent to the
replacement valve leaflets 14, 16 within the stent mounted replacement valve
10. These replacement valve leaflets 14, 16 are typically formed from biologic
material, such as from a cow or a pig, but may synthetic or other bioforms.
Approximately half of this replacement valve 10 has no seal, i.e., it is more
or
less exposed stent 24 with openings 24a. This is because when the
replacement valve 10 is placed in the aortic native position, the coronary
arteries arise just above the aortic valve. If the seal 18 extended the entire
length of the stent portion 24 of the replacement valve 10, the coronary
artery
could be blocked. In FIG. 1A, an unmodified aortic replacement valve 10 is
shown implanted in a helical anchor 30 comprised of coils 32. Leakage of
blood flow may occur as depicted schematically by the arrows 36, because
there is a gap between the seal 18 on the stented valve 10 and the attachment
to the patient's mitral valve 12. The leakage of blood flow may occur in any
direction. Here, the arrows 36 depict the leak occurring from the ventricle 40
to
the atrium 42 since the ventricular pressure is higher than the atrial
pressure.
An unmodified aortic valve 10 placed in the native mitral valve position will
be
prone to develop a leak. To avoid this problem, two major approaches may be
taken. First, a seal may be added to the system, for example, the helical
anchor 30 may have sealing features added. Second, the location where the
-14-
CA 02920724 2016-02-08
WO 2015/023579
PCT/US2014/050525
stent mounted replacement heart valve 10 sits may be changed. In this regard,
if the replacement heart valve 10 is positioned lower inside the ventricle 40,
the
seal 18 on the replacement heart valve 10 will be situated such that there is
no
leak. One drawback to seating the valve 10 lower inside the left ventricle 40
is
that the replacement heart 10 valve may cause damage inside the left ventricle
40 or the valve 10 may obstruct ventricular contraction. The replacement heart
valve 10 may damage the ventricular wall or block the outflow of blood from
the
ventricle 40 into the aorta. Rather than simply seating the replacement heart
valve 10 more deeply or lower into the left ventricle 40, it may be useful to
keep
the position of the stent mounted replacement valve 10 more atrially
positioned
as it is depicted in FIG. 1A (i.e., positioned higher and extending into the
atrium
42).
[0095] FIG. 1B illustrates one embodiment of providing seal structure
50
at the upper portion of a replacement heart valve 10 to prevent blood flow
leakage as discussed above and shown in FIG. 1A. In this regard, one or more
seals 52 have been added to the helical anchor 30. Specifically, a fabric
covered oval seal structure 52 is added to the helical anchor 30 to provide a
seal. The seal 52 may be formed from fabric, or any other material that
provides a sufficient seal and does not allow blood to flow through. The seal
52
extends down to the level of the attachment between the stent mounted
replacement valve 10 and the native mitral leaflets 12a, 12b. The seal 52, in
this illustrative embodiment is a continuous tube and comprises one or more
seal elements or portions 52a, 52b, 52c in the form of overlapping segments of
fabric or other sealing material. These segments 52a, 52b, 52c of sealing
structure act as siding structure or shingles to seal the space between the
coils
32 or turns of the helical anchor 30.
[0096] FIG. 2A illustrates one manner of applying the overlapping
seal
structure 50 such as shown in FIG. 1B or otherwise integrating the seal
structure 50 on the helical anchor 30. In this regard, the seal structure 50
may
be integrated with the helical anchor 30 for delivery purposes. The shingles
or
overlapping seal portions 52a-c (FIG. 1B) may be collapsed and extruded from
a catheter 60. Alternatively, once the helical anchor 30 has been delivered to
the native heart valve site, the fabric or other seal structure 50 may be
delivered
over the coils 32 of the anchor 30 from the same delivery catheter 60.
Alternatively, the overlapping seal structure 50 may be added to the helical
-15-
CA 02920724 2016-02-08
WO 2015/023579
PCT/US2014/050525
anchor 30 as the helical anchor 30 is being extruded or extended from the
delivery catheter 60. FIG. 2A specifically illustrates a helical anchor 30
with a
fabric or other seal structure 50 being fed over the helical coils 32 from a
sheath
or delivery catheter 60. The seal structure 50 may be generally circular in
cross
section or any other shape, such as a shape that is better configured for
overlapping as generally shown in FIG. 1B above. FIG. 2B illustrates fabric 62
and an internal support coil 64 being added to the helical anchor 30 in a
further
portion or step of the procedure illustrated in FIG. 2A. FIG. 2C illustrates
one
embodiment of a completed assembly, shown in cross section, comprising the
helical anchor 30 covered by the coil 64 and fabric 62 and delivered by a
sheath
or delivery catheter 60. The delivery sheath or catheter 60 may remain over
the
coil and fabric combination or it may be used to merely deliver these sealing
elements 62, 64 over the helical anchor 30.
[0097] FIG. 2D illustrates a cross sectional view of the sealing
elements
62, 64 which, in this case, are circular in cross section. These sealing
elements
62, 64, including, for example, a coil support and fabric combination, may be
virtually any shape as long as they provide a seal when placed together.
Sealing elements 62, 64 may not overlap in use but instead contact each other
as shown to create a seal therebetween.
[0098] FIG. 2E shows an oblong or oval cross sectionally shaped seal
structure 70 similar to the seal 50 shown in FIG. 1 B in which segments 70a,
70b
overlap each other to produce a secure and fluidtight seal. It is possible to
have
the oblong seal structure 70 compressed for delivery and then spring or bias
open once the seal structure 70 is extruded from a delivery catheter or
sheath.
A coil 74 internally supporting fabric 72 may be made of Nitinol
(superelastic)
wire or spring steel wire so that it may be collapsed and then bias or spring
into
a predetermined shape as needed.
[0099] FIG. 2F shows another alternative seal structure 80. In this
case,
a sealing fabric 82 or other material is wrapped around the helical anchor 30.
The fabric is stitched together with suitable thread to form stiff, structural
panels
84 extending from the connecting portion 86 that is affixed to a coil 32 of
the
helical anchor 30. The panels 84 again overlap, similar to a shingle effect,
to
provide a fluidtight seal. This configuration may be delivered in a similar
manner to the previously described fabric covered coil designs above by
passing the panel structure over the helical anchor 30 as shown.
-16-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
[00100] FIG. 3A illustrates another embodiment for providing a sealing
structure. In order to provide further shape and support to a seal structure
90,
there may be two or more "framing" segments 92, 94 inside a fabric covering 96
or other material seal. This will give a shape to the seal structure 90 and
provide for more reliable overlap of the seal segments (only one shown in FIG.
3A). This may be achieved by using a double helix in which two wires 92, 94
run parallel to each other to form a helical shape. The two wires 92, 94 may
be
connected at their ends with a curved section 98 as shown in FIG. 3A. The
fabric or other material sleeve or coating 96 may be passed over the double
helix during or after delivery of this helical seal structure 90.
[00101] FIG. 3B illustrates a cross sectional view of the seal structure 90
compressed with wires 92, 94 inside the outer fabric or other material 96.
This
can provide for easier delivery to the site of implantation.
[00102] FIG. 3C illustrates the double helix seal 90 spread apart and
overlapping after delivery. Two segments 90a, 90b of the helical seal 90 can
expand as they are being delivered to form overlapping seal segments 90a, 90b
similar to the "shingle" configuration discussed above. Here, two overlapping
seal segments 90a, 90b are supported by two double helix frames 92, 94
positioned adjacent and overlapping to each other to produce an effective,
fluidtight seal.
[00103] FIG. 3D illustrates another alternative method for coupling frame
segments 92, 94 of a seal and, specifically, biasing the frame segments 92, 94
apart. Interconnecting segments 100 between the two frame parts or wires 92,
94 can push the frame segments 92, 94 into a desired final shape. This double
helix design may be made from multiple wire pieces or may be made from a
single solid Nitinol or steel tube or wire, similar to stent manufacture
techniques.
The seal frame 92, 94 may also have a sinusoidal or generally back and forth
configuration (not shown) to hold a shingle-type shape rather than two rails
or
wires inside of the outer seal material or fabric 96 (FIG. 3C).
[00104] FIG. 3E details how the outer seal material or fabric 96 may be
placed over the expanded frame 92, 94. The seal material 96 may be
preattached to the double helix frame 92, 94 and the two may be delivered
together. Alternatively, the seal material 96 may be delivered onto the double
helix frame 92, 94 after the double helix frame 92, 94 is already in place at
the
implantation site, such as the site of a native mitral valve. In the
unexpanded
-17-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
state, the double helix 92, 94 may be extruded through a catheter as
previously
described.
[00105] FIGS. 3F, 3G and 3H generally show the progression of delivery
and implantation of the seal 90. In these figures, the seal material or fabric
96
extends beyond the frame 92, 94 to form flaps or panels 102 of seal material.
These flaps or panels 102 may be stiffened and reinforced with heavy suture,
or
the material may be soaked or coated in a stiffening agent. This may be useful
to ensure a fluidtight seal. In FIG. 3F, the internal wire frame 92, 94 is
collapsed and the fabric cover 96, 102 is folded within a delivery sheath 60
for
delivery. In FIG. 3G the frame 92, 94 has been delivered and the segments or
flaps 102 of seal material 96 that extend beyond the frame 92, 94 have
unfolded. FIG. 3H illustrates the frame parts 92, 94 expanded, in a manner
similar to a stent. This provides a solid and secure seal. The cross members
or biasing members 100 that were collapsed inside the double helix frame 92,
94 are now biased outward and lengthened or straightened. These cross
members 100 may be made of Nitinol or other spring material and expand the
frame 92, 94 with a spring force as the frame 92, 94 is delivered from a
catheter
or sheath 60. Alternatively, there may be another mechanism or manner for
activating and expanding the frame 92, 94 as needed during the implantation
procedure.
[00106] FIG. 4A illustrates another embodiment for adding sealing
features to a helical anchor 30. Here, a fabric windsock-type shape or
panel/membrane structure 110 has been mounted to an upper turn or coil 32 of
the helical anchor 30. This panel 110 unfolds or extends within the helical
anchor 30 to provide a sealing membrane. The fabric or other seal material
may be sewed or permanently fastened to the helical anchor 30. Alternatively,
this seal panel 110 may be delivered onto the helical anchor 30 after the
helical
anchor 30 is placed at the site of implantation within a native heart valve.
The
seal material 110 may be attached on any portion of the helical anchor 30 at
any level of the anchor 30. In FIG. 4A, the seal panel 110 is attached to the
uppermost coil 32 of the helical anchor 30 such that the panel 110 can then
expand to the full length of the helical anchor 30 and provide a full length,
fluidtight seal.
[00107] FIG. 4B illustrates the seal panel 110 opened and an internal
support structure 112, in the form of a wire or sinusoidal-type support
element
-18-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
inside or within layers of the seal material. This support structure 112 for
the
seal 110 may be made of, for example, Nitinol or steel. The support 112 may
be sewn into the fabric or otherwise secured to the seal material. The fabric
may, for example, contain a channel for the support 112 and the support 112
could be pushed into the channel, expanding the seal material 110 as needed.
If the support 112 is made from Nitinol or superelastic material, and imbedded
inside the fabric or seal material 110, it may straighten and fold up the
fabric or
other seal material inside a delivery catheter or sheath. While being
delivered,
the Nitinol or superelastic support would return to its initial zigzag or
sinusoidal
shape, expanding the fabric as it is released and extruded from the delivery
sheath or catheter.
[00108] FIG. 40 is a cross sectional view illustrating a helical anchor 30
and fabric seal panel 110, such as shown in FIG. 4A delivered and implanted at
a native valve site, such as within the mitral valve 12 of a patient. The seal
panel 110 is annular in shape and generally follows the interior of the
helical
anchor 30. As shown here, the fabric panel 110 is stitched to the upper turn
or
coil 32 of the helical anchor 30 and the fabric is folded on itself and
stitched
together as shown. Stitching 114 can also provide structural support to help
the
fabric shape itself correctly. The stitching may be made of steel wire or
Nitinol
wire that may assist in providing shape stability to the membrane or panel
structure 110. The stitching 114 may also be suture or thread. The heavier the
stitching material, the more support it will provide for the fabric. Here, the
stitching is in horizontal lines, however, it may instead be other
configurations
such as vertical, zigzag, or any other suitable configuration.
[00109] FIG. 4D illustrates a stent mounted heart valve 10 expanded
within the helical anchor 30 and seal structure 110 of FIG. 40. The seal 110
prevents any leakage of blood around the valve 10 and covers any areas of the
stent portion 24 of the valve 10 that are not already covered and sealed. The
seal 110 allows the replacement heart valve 10 to be seated higher toward the
atrium 42, thereby reducing the risk of left ventricle injury or left
ventricle blood
outflow obstruction.
[00110] FIG. 5A illustrates a helical anchor 30 with an attached membrane
or panel seal 110 being delivered onto the coils 32 of the helical anchor 30.
It
should also be noted that the membrane or panel seal 110 can also improve the
attachment of the replacement heart valve 10. In this regard, a bare helical
-19-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
anchor 30, particularly one made of metal that attaches to a metal stent will
result in metal surfaces contacting each other. As the heart beats and
pressure
rises with each contraction, e.g., about 100,000 times per day, there is a
risk of
slippage between the metal surfaces and potential valve dislodgement.
Therefore, the addition of a membrane, panel 110 or other seal structure can
reduce the tendency for the valves to slip and even fail. The membrane or seal
panel 110 may be smooth or have various degrees of texture or roughness to
help maintain fixation of the replacement heart valve 10. Textured or
roughened surfaces will increase friction and therefore reduce slippage. Also,
the fabric or other seal material 110 may be forced inside the openings or
cells
of the stent portion 24 of the replacement heart valve 10 thereby improving or
creating a locking effect and anchoring the stent mounted replacement valve 10
to the helical anchor 30, including the seal material 110. In FIG. 5A, the
membrane or panel seal 110 is attached to the helical anchor 30 and as
previously described, the membrane or panel seal 110 may be attached prior to
implantation within the patient or added at any point during the implantation
procedure. It may be advantageous to add the membrane or panel seal 110
after the helical anchor 30 is placed at the implantation site in order to
reduce
complication during delivery of the helical anchor 30. FIG. 5B illustrates the
membrane seal or panel seal 110 unfolded or expanded within the helical
anchor 30. As previously described, the membrane or panel seal 110 is
attached to the uppermost turn 32 of the helical anchor 30, however, it may be
attached anywhere along the helical anchor 30. The membrane or panel seal
110 may be continuous or intermittent, and may be comprised of overlapping
panel portions similar to a shingle effect. Although the membrane or panel
seal
110 makes a complete annulus as shown in FIG. 5B within the helical anchor
30, it may instead be formed as less than a complete annulus.
[00111] FIG. 5C is similar to FIG. 4B described above and simply
illustrates that in this embodiment, the delivered and deployed membrane seal
110 may also include a similar internal support 116. It is also possible that
the
membrane or panel seal 110 is intrinsically stiff and springs open without
internal support structure of any sort. Many other ways to open or deploy the
membrane or panel seal 110 may used instead. For example, the panel seal
110 may contain pillars or other supports (not shown) that are collapsed for
delivery but that allow the membrane or panel 110 to be biased open once the
-20-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
membrane or panel 110 is delivered from a suitable catheter or sheath. These
pillars or other supports may, for example, be formed from shape memory or
superelastic material, or other suitable spring biased material.
[00112] FIG. 5D illustrates the panel seal 110 unwinding or being
deployed. The panel seal 110, in this illustrative embodiment, is formed of
two
layers with a support 116 between these two layers. The support 116, as
described above, is suitably secured between the layers of the panel seal 110.
Although shown as a sinusoidal configuration, the support 116 may be of any
desired and suitable configuration, or may be comprised of separate support
structures such as generally circular or oval support structures (not shown).
Other useful structures in this regard may include any of those shown and
described in U.S. Provisional Patent Application Serial No. 61/864,860, filed
on
August 12, 2013, the disclosure of which is hereby fully incorporated by
reference herein. Finally, drawstrings (not shown) may be added to the end of
the membrane seal 110 or to any part or parts of the membrane seal '110 that
may be used to pull the membrane seal 110 open and unfold it or otherwise
deploy it.
[00113] FIG. 5E illustrates a membrane or panel seal 100 which has been
collapsed or folded onto itself rather than wound around the coil 32 of the
helical anchor 30. A collapsed membrane seal 110 such as this may be more
practical. The membrane or panel seal 110 can be opened with the support
structure 116 normally biased to a deployed state as shown previously, or it
may be deployed by containing structural support elements 116, such as shape
memory support elements. As also previously discussed, drawstrings (not
shown) might be added for deployment purposes.
[00114] FIG. 5F illustrates a cross sectional, enlarged view of the helical
anchor 30 with the seal membrane 110 or panel extending adjacent to coils 32
of the helical anchor 30. The panel seal 110 includes a suture line 118 that
keeps the seal 110 in place within the helical anchor 30, shown as a dotted
line.
This need not be a suture, instead, the securement may be provided by any
suitable fasteners, glue, or other elements that maintain the membrane or
panel
seal 110 in position. In addition, the panel seal 110 may be glued or attached
to the helical anchor 30 and this would eliminate the need for sutures or
separate fasteners. As described previously, the panel seal 110 may be fabric
or any other suitable biocompatible material. For example, the seal material
in
-21-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
this and any other embodiment may be Dacron or Goretex, or may be biologic
material from an animal or human. Other examples of seal material include
engineered biomaterials or any combination of biologic and/or synthetic
materials. The panel seal 110, in this embodiment, is opened with a spring
biased support wire 116 as generally described above, but may be opened in
any suitable manner during or after deployment and implantation of the helical
anchor 30.
[00115] FIG. 5G illustrates the helical anchor 30 and panel seal 1'10
combination implanted at the site of a native mitral valve 12 of a patient.
FIG.
51-1 illustrates a replacement heart valve 10, and specifically a stent
mounted
replacement heart valve 10 secured within the helical anchor 30 and panel seal
110 combination. These figures are described above with regard to FIGS. 40
and 4D. Thus, it will be appreciated that the panel seal structure 110 and
helical anchor 30, regardless of deployment and delivery techniques, provide
fluidtight sealing as previously described. It will be appreciated that
additional
features may be used to help deploy the panel seal or membrane 110 open as
shown in FIGS. 5G and 5H. A foam layer (not shown) may also be positioned
at any desired location, for example, to aid in sealing and/or valve
retention.
The membrane or panel seal 110 may extend the full length of the helical
anchor 30 or only a portion of the length. In these figures, FIG. 5G
illustrates
the membrane or panel 110 extending only part of the length while FIG. 5H
illustrates the panel or membrane 110 extending almost the entire length of
the
valve 10. As shown in FIG. 5H, the replacement heart valve 10 is positioned
within the native mitral valve 12 such that much of the replacement heart
valve
sits within the atrium. It will be appreciated that the replacement heart
valve
10 may be positioned anywhere along the helical anchor 30. The helical anchor
30 may contain the entire prosthetic or replacement heart valve 10 or the
replacement heart valve 10 may project at either end of the helical anchor 30
or
from both ends of the helical anchor 30. The number of coils or turns 32 of
the
helical anchor 30 may also be varied. The key arrangement is to prevent as
much leakage as possible, and maintain the replacement heart valve 10
securely in position after implantation.
[00116] In FIG. 5H one coil 32 of the anchor 30 extends beyond the
stented prosthetic valve 10 inside the left ventricle 40. This may serve a
number of functions. The end of the stent valve 10 is sharp and may damage
-22-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
structures inside the left ventricle 40. By leaving a turn 32 of the anchor 30
beyond the end of the valve 10, it may be possible to protect the structures
inside the heart from contacting the sharp end of the valve 10. The lowest
turn
32 of the anchor 30 may act as a "bumper" that is smooth and prevents injury
to
structures inside the ventricle 40. A smooth metallic (such as Nitinol) anchor
coil 32 may be very well tolerated and prevent wear and abrasion inside the
left
ventricle 40.
[00117] The lowest turn or coil 32 of the anchor 30 may also wrap native
mitral valve leaflet tissue around the end of the valve 10. This may also
shield
the sharp end of the prosthetic valve 10 from structures inside the heart.
[00118] The lowest turn or coil 32 of the helical anchor 30 may also
provide tension on chordal structures. The function of the left ventricle 40
is
improved and the shape of the left ventricle 40 can be optimized by placing
tension on chordal structures. In FIG. 5H, the lowest coil 32 pulls the
chordae
toward the center of the ventricle 40 and shapes the left ventricle 40
optimally
for contraction. It may be useful to have multiple coils 32 of the anchor 30
extending inside the left ventricle 40 beyond the anchor 30. These coils 32
could pull the chordae inward over a longer distance inside the heart. For
example, if a patient had a very large left ventricle 40, it may be desirable
to
improve his left ventricular function by having a helical extension well
beyond
the valve 10. This would tighten the chordae and reshape the left ventricle
40.
The coils 32 of the anchor 30 could also be heavier/thicker diameter to assist
in
reshaping the heart. The diameter of the coils 32 could also be varied to
optimize the left ventricle shape change.
[00119] The concept of reshaping the left ventricle 40 with the anchor 30
does not need to apply to just mitral valve replacement. The helical anchors
30
shown in these descriptions can also be used for mitral valve repair.
Extensions of the helix coils 32 inside the left ventricle 40 can also re-
shape the
left ventricle 40 even when a replacement prosthetic valve 10 is not used. As
described previously, various numbers of coils 32, diameter of coils 32,
thickness of materials, etc. could be used to achieve an optimal result.
[00120] It is also useful to use the helical anchor 30 to repair a native
heart valve 12 and reshape the left ventricle 40 and leave open the
possibility to
add a prosthetic replacement valve 10 later if the repair fails over time.
After
surgical valve repair, this is not uncommon. An anchor 30 that serves as a
-23-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
repair device with or without left ventricular reshaping with coils 32 that
extend
into the left ventricle 40 may be useful as an anchor 30 if a prosthetic valve
replacement is needed later.
[00121] FIG. 6A illustrates a helical anchor 30 implanted at the native
mitral valve position. In general, it will be important to seat the helical
anchor
30 close to the under surface of the native mitral valve 12. If the diameter
of the
coils 32 or turns under the mitral valve 12 is relatively small, the helical
anchor
30 is forced to slip down into the left ventricle 40. The helical anchor 30
attachment to the native valve 12 will be away from the annulus 12c and once
the heart starts beating, the helical anchor 30 will be sitting inside the
left
ventricle 40 and, when there is mitral valve tissue between the helical anchor
30
and the mitral valve annulus 12c, the helical anchor 30 is not firmly attached
in
the annular region of the mitral valve 12, but rather to the leaflets 12a, 12b
lower in the left ventricle 40, and this is not desirable. In FIG. 6A, a
relatively
large diameter turn or coil 32 of the helical anchor 30 is positioned just
under
the mitral valve leaflets 12a, 12b. This position is directly adjacent to the
native
mitral valve annulus 12c. Relatively smaller diameter coils 32 are positioned
lower in the left ventricle 40. It may be useful to have a gap 120 between the
relative larger coil 32 that is positioned under the valve leaflets 12a, 12b
at the
valve annulus 12c and the relatively smaller coil 32 positioned farther into
the
left ventricle 40. This will prevent the entire helical anchor 30 from being
pulled
down farther into the left ventricle 40 after implantation. Relatively smaller
diameter coils 32 of the helical anchor 30 are positioned above the mitral
valve
12, i.e., above the mitral valve native leaflets 12a, 12b. For illustrative
purposes, a balloon 122 is shown for purposes of expanding the smaller
diameter coils 32. This causes the larger diameter coil portions 32 to move
relatively inward in a radial direction thereby tightening all of the coils 32
along a
more similar diameter and tightening the connection between the helical anchor
30 and the native mitral valve tissue. Most importantly, the coil or turn 32
under
the native mitral valve leaflets 12a 12b tends to grip against the underside
of
the mitral annulus 12c and pull the annulus radially inward, reducing the
diameter of the native mitral annulus 12c. Annular reduction in this manner is
important to improve left ventricular function when the heart is enlarged.
Annular diameter reduction of a native mitral valve 12 is also important
during
mitral valve repair. The smaller diameter annulus adds to the improvement in
-24-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
left ventricular function. The concept of annular reduction using a sliding
helical
anchor 30 to control the leaflets 12a, 12b and pull the native mitral leaflets
12a,
12b and annulus 12c radially inward is specifically useful in mitral valve
repair.
The concepts, methods and devices for improving left ventricular function in
mitral valve prosthetic replacement, i.e., replacements that reduce the
annulus
diameter and tension chordae and reshape the left ventricle 40, will be
invoked
herein demonstrating mitral repair devices, concepts and methods. A smooth
turn or coil 32 of the helical anchor 30 under the native mitral annulus 12
will
have less tendency to grip against the mitral valve tissue and reduce the
mitral
valve annulus diameter. It may be useful to increase the "grip" of the turn or
coil
32 under the annulus 12c for this reason. This may be accomplished in many
ways including roughening the surface of the coil 32 such as by texturing the
metal or by adding a high friction coating or fabric. The coating, fabric or
other
high friction material may be fixed to the helical anchor 30 or it may slide
along
the helical anchor 30. The high friction portion of the helical anchor 30 may
be
continuous or discontinuous.
[00122] FIG. 6B illustrates
the final position of the prosthetic replacement
heart valve 10 inside the helical anchor 30 and its relation to the native
mitral
valve 12 and left ventricle structures. The left ventricle chordate 130 have
been
tensioned and, therefore, the left ventricle 40 has been appropriately
reshaped.
The sharp end 132 of the prosthetic replacement heart valve 10 has been
covered by seal material 134, native valve tissue 136 and a "bumper" 138 of a
lowest turn or coil 32 of the helical anchor 30. This provides multiple types
of
protection from injury inside the left ventricle 40 due to the sharp end of
the
stented prosthetic valve 10. Also note that the stented prosthetic heart valve
10
is positioned higher toward the atrium 42, and away from the structure in the
left
ventricle 40. This provides further protection from injury to the left
ventricle 40
by the replacement heart valve 10. The fabric membrane seal, or other type of
panel seal 110, may extend for any length. In this illustration it extends
beyond
the replacement heart valve 10. The fabric or other seal material may also
extend beyond the end of the helical anchor 30 within the left ventricle 40.
The
fabric or other seal material 110 should cover the end of the replacement
heart
valve 10 until there is a seal at the level of the mitral valve 12. There is
no need
for a seal if the prosthetic replacement valve 10 has an attached seal or a
seal
is otherwise attached to the prosthetic replacement valve 10. In this case,
-25-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
useful features disclosed relate mainly to the attachment of the replacement
valve 10 to the helical anchor 30 and the ability of the helical anchor 30 to
reshape the left ventricle 40.
[00123] FIGS. 7A-7D illustrates devices, methods and procedures relating
to the interaction of the helical anchor 30, helical anchor design features
and
the stent mounted replacement heart valve 10 delivered or mounted on a
balloon 140. Various catheters may be manipulated to take advantage of a
design of the helical anchor 30 to improve valve implantation. For example,
the
stent mounted replacement valve 10 may be partially deployed and the helical
anchor 30 manipulated with the stent mounted replacement valve 10 in a
partially deployed state before the final deployment position is reached. FIG.
6A illustrates the helical anchor 30 with three coils or turns 32. The top two
coils 32 have a relatively smaller dimension d2 while the lowest turn or coil
32
has a relatively larger dimension or diameter di. FIG. 7B illustrates a stent
mounted replacement valve 10 with a balloon 140 inside to deploy the valve 10
once the valve 10 has been positioned inside the helical anchor 30. The
helical
anchor 30 is placed with two of the coils or turns 32 positioned above the
native
mitral valve 12 and one coil or turn 32 positioned below the native mitral
valve
leaflets 12a, 12b and adjacent to the mitral valve native annulus 12c. The
arrows 142 indicate the radially outward direction of balloon inflation and
the
resulting expansion of the stent mounted replacement heart valve 10.
[00124] FIG. 70 illustrates expansion of the balloon 140 and stent
mounted replacement heart valve 10. Since the diameter of the upper two coils
or turns 32 of the helical anchor 30 are smaller, as the balloon 140 is
expanded,
the stent mounted replacement heart valve 10 first contacts the smaller turns
32
of the helical anchor 30. The stent mounted heart valve 10 becomes engaged
against these two smaller diameter turns or coils 32. While in this position,
the
catheter deploying the balloon 140 may be used to manipulate or reposition the
helical anchor 30. The movement of the balloon catheter 140, such as in the
direction of the large arrow 146, will result in the large turn 32 of the
helical
anchor 30 being moved upwardly toward the native mitral annulus 12c in this
illustrative example. That is, the turn or coil portion 32 adjacent to the
native
mitral annulus 12c will move in the direction of the small arrows 148 adjacent
thereto. This also results in an upper movement of the turns or coil portions
32
above the native mitral valve annulus 12c. In fact, with enough force, once
the
-26-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
turn or coil portion 32 below the annulus 12c comes in contact with the
leaflet
12a or 12b or annulus tissue 12c below the mitral valve 12, the helical anchor
30 can actually be sprung open such that a segment of the helical anchor 30
that connects the turn or coil portion 32 above the leaflet 12a or 12b and
below
the leaflet 12a or 12b, becomes extended. This can increase the gap between
segments of the helical anchor 30.
[00125] FIG. 7D illustrates a stent mounted replacement heart valve 10
fully expanded after deployment and expansion by a balloon catheter 140,
which has been removed. The largest turn or coil 32 of the helical anchor 30
is
positioned relatively high just under the native mitral annulus 12c. After
full
inflation of the balloon catheter 140, the system cannot move because the
native mitral valve of leaflets 12a, 12b are now trapped between the helical
anchor 30 and the stent mounted replacement heart valve 10. The balloon
catheter 140 that holds the replacement heart valve 10 may be moved in any
direction. In this figure, up and down motions are clearly possible as these
would be made by moving the balloon catheter 140 in and out of the patient.
There are many deflectable catheters which would allow the balloon catheter
140 to move laterally also.
[00126] This series of figures is intended to show how procedures can be
conducted with a helical anchor 30. The anchor 30 can be engaged and
manipulated by the stent mounted valve 10 prior to the final positioning and
full
expansion of the stent valve 10.
[00127] It is also possible to manipulate the anchor 30 prior to its
release.
The anchor 30 can have a catheter or other element attached to it during this
procedure. So both the anchor 30 and the stent mounted valve 10 could be
remotely manipulated to achieve a desired result.
[00128] FIGS. 7A-7D also show how inflating the balloon 140 inside
smaller turns 32 of the anchor 30 can serve to "tighten" a larger turn 32.
Part of
the larger turn or coil 32 under the annulus 12c is drawn up above the annulus
12c when the smaller turn or coil 32 is expanded, thus shortening the coil 32
under the annulus 12c. This allows the large coil 32 to tighten around the
stent
valve 10. This effect is more pronounced when a larger coil 32 is located
between two smaller coils 32 of the anchor 30. The two small coils 32 on each
side of the larger coil 32 expand and thus decrease the diameter of the larger
coil 32 so the larger coil 32 can trap and assist in anchoring the valve 10.
-27-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
[00129] It is very important to position the anchor 30 as close to the
annulus 12c as possible. This is the natural anatomic location for the valve
10.
If the anchor 30 is attached to leaflet tissue 12a, 12b remote from the
annulus
12c, the leaflet tissue 12a, 12b moves with each beat of the heart. This can
cause rocking of the anchor 30 and the valve 10. Repeated motion can lead to
valve dislodgement. So strategies to allow placement of large coils 32 of the
anchor 30 near the annulus 12c are important. It is also useful to convert a
larger coil 32 to a smaller coil 32 so that the coil 32 can actually function
to trap
the stent valve 10.
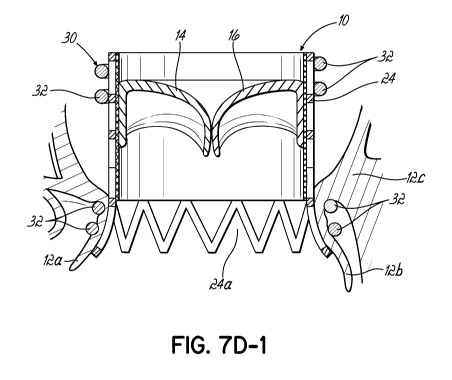
[00130] FIG. 7D-1 illustrates another embodiment of a replacement valve
and helical anchor 30 combination in which the upper end of the
replacement valve 10 does not flare outward but rather is retained in a
generally
cylindrical shape, for example, by upper coils 32 of the anchor 30. The lower
end or outflow end is flared radially outward as shown. It will be appreciated
that structure, such as a seal (not shown) may be included between the stent
24 and the lower coils 32 for both sealing purposes as previously described as
well as or alternatively to provide a softer, more compliant surface against
the
native mitral leaflets 12a, 12b. In addition, it will be appreciated that the
upper
coils 32 create a gap and do not engage or trap the tissue adjacent the native
mitral valve in the atrium. On the other hand, the lower coils 32 engage
tissue
just underneath the native mitral annulus 12c. The embodiment of replacement
valve 10 shown in FIG. 7D-1 stands in contrast to valves 10 configured as
previously shown, such as in FIGS. 1A and 1B, in which the valve retains a
cylindrical shape after implantation and application of a helical anchor 30,
and,
for example, that shown in FIG. 7D in which the valve 10 includes a very
slight
outwardly directed configuration at the lower or outflow end but does not
result
in any significant flare.
[00131] FIGS. 8A-8D illustrate the use of a balloon catheter 140 to expand
a helical anchor 30 without the presence of a stent mounted replacement heart
valve 10. Specifically, FIG. 8A illustrates a helical anchor 30 with
approximately
four coils or turns 32. There are two coils 32 on each side of a joining
segment
32a which separates them to create a gap. Mitral valve native leaflets (not
shown) could easily be positioned between the coils 32 at the position of the
gap created by the joining segment 32a. In this figure, the balloon 140 is
beginning to be expanded as shown by the radially outward directed arrows
-28-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
150. FIG. 8B illustrates further expansion of the balloon 140 thereby causing
the helical anchor 30 to create an indentation in the balloon 140 around the
helical anchor 30. The balloon 140 on both sides of the helical anchor 30
expands further. This results in a force on the turns or coils 32 of the
helical
anchor 30 that moves them together generally shown by the arrows 152. As
the balloon 140 is expanded further, as shown in FIG. 80, the gap between the
turns or coils 32, 32a diminishes and eventually may be completely closed such
that the two main portions of the helical anchor 30 are compressed against
each other in the direction of the blood flow or central axis of the helical
anchor
30 (i.e., along the length of the balloon 140). FIG. 8D illustrates a cross
sectional view showing the turns or coils 32, 32a of the helical anchor 30
compressed together. As shown in these figures, the coils 32, 32a of the
helical
anchor 30 may be compressed against each other by inflating a balloon 140
inside the helical anchor 30. There does not need to be a joining segment 32a
or gap for this to occur. The helical coils 32 would be compressed tightly
against each other with or without the gap illustrated in this embodiment.
[00132] This compression can serve as a "motor" to allow various
functions to occur. For example, it can be possible to mount pins or fasteners
(not shown) to the turns 32, 32a of the anchor 30 that can be driven and
activated by the inflation of the balloon 140. The pins or fasteners could be
positioned so they pass through the native valve leaflet. The fasteners could
also traverse the native leaflets and move into the anchor 30 on the opposite
side of the leaflet. A fabric coating, spongy coating or another receptive
material on the anchor 30 would improve the retention of fasteners.
[00133] Generally, these methods and devices would allow for areas of
the mitral valve 12 near the annulus 12c or on the annulus 12c to be fastened
to
a helical anchor 30. The fasteners could traverse the valve tissue and engage
coils 32 on the one or on both sides of the leaflets. Leaflet trapping by
balloon
inflation can allow the mitral valve 12 and its annulus 12c to be manipulated
and
to perform therapeutic procedures. For example, the anchor coils 32 once
fastened to a valve leaflet 12a, 12b could be reduced in size to create a
purse
string effect on the valve annulus 12c ¨ resulting in an annular reduction or
annuloplasty procedure. A drawstring (not shown) could be added to the
anchor 30 to reduce the diameter.
-29-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
[00134] The fasteners could be used to join segments of the helical
anchor 30 together. For example, turns or coils 32 of the anchor 30 above the
leaflet 12a, 12b could be joined together. Fabric or other material could be
wrapped around or otherwise placed on the anchor coils 32 and pins or
fasteners from one coil 32 could engage and trap themselves in the fabric of
an
adjacent coil 32. Adjacent coils 32 could engage each other. This can create a
greater mass on each side of the leaflet 12a, 12b to control the mitral
annulus
12c. In summary, balloon inflation inside a helical anchor 30 can drive coils
32
of the anchor 30 together. This maneuver can be used as a motor or drive
mechanism to activate mechanical systems. It can also move anchor coils 32
tightly together.
[00135] FIGS. 9A-9D illustrate another ability of the helical anchor 30 as
the helical anchor 30 is expanded by a balloon 140. In this regard, the actual
total length of the helical coils 32 forming the anchor 30 remains the same.
Therefore, to increase the diameter of the helical anchor 30, the ends 30a,
30b
of the helical anchor 30 must move to accommodate the expansion. This
movement may also be used as a motor or drive mechanism to activate
additional functions. More specifically, FIG. 9A illustrates a balloon 140
being
expanded inside the helical anchor 30. As the balloon 140 expands, the
diameter of the helical anchor 30 increases and the opposite ends 30a, 30b of
the helical anchor move to accommodate the expansion. As shown by the
arrows 160, the ends 30a, 30b of the coils 32 move or rotate in opposite
directions. FIG. 9B illustrates continuation of the balloon expansion and the
previous figures of FIGS. 8A-8D show how the balloon 140 also compresses
the coils 32 of the helical anchor 30 together. FIG. 9B highlights how the
coils
32 of the helical anchor 30 rotate generally as the balloon 140 expands. This
rotation is helpful in retaining a stent mounted replacement heart valve as
the
tension around the stent portion of the heart valve (not shown) increases.
FIG.
90 illustrates that the helical anchor 30 has unwound as it expands under the
force of the balloon 140. There are fewer turns or coils 32 and the remaining
turns or coils 32 are now larger in diameter. FIG. 9D shows a cross sectional
view of the expanded helical anchor 30. The motion of the ends 30a, 30b of the
helical anchor 30 can be used to perform functions. As further described
below,
for example, the movement of the coils 32 of the helical anchor 30 may be used
to drive anchors, or perform other functions.
-30-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
[00136] FIGS. 10A-10E illustrate the effect of a cover or coating 170 on
the helical anchor 30. Also, the replacement valve 10 as shown, for example,
in
FIGS. 10B and 100, takes on an outward flare at both the upper and lower
ends. This may not be desirable for various reasons, but rather, at least one
end of the valve 10 may be desired to have and retain a generally cylindrical
cross sectional shape (as viewed from above or below). The coating or
covering 170 may be in the form of any type of sheath or material applied to
the
helical anchor 30 and may be comprised of any biocompatible material. For
example the coating 170 may be made of fabric material, such as Dacron,
Teflon or other material. It may be formed from PTFE or EPTFE in fabric form
that has a fabric texture or as a plastic sleeve, or cover or coating that is
smooth. There may be a foam material under the coating 170 as is commonly
used in, for example, surgical valves. The foam material may consist of rolls
of
fabric or folds of fabric. Other possible materials include resilient
materials or,
more specifically, material such as medical grade silicone. Biological
materials
may also be used, and may include animal, human, or bioengineered materials.
Some materials commonly used in cardiac repair procedures are pericardium
and intestinal wall materials. FIG. 10A illustrates a helical anchor 30 which
is
covered by a coating 170 comprised of a fabric backed by a foam material. The
helical anchor 30 is positioned inside the native mitral heart valve 12 with
two
turns or coils 32 above and two turns or coils 32 below the native mitral
valve
annulus 12c. A stent mounted replacement heart valve 10 is placed inside of
the helical anchor 30 and inflation of the balloon delivery catheter 140
inside the
replacement heart valve 10 has begun as indicated by the arrows 172. In FIG.
10B, the replacement stent mounted valve 10 is shown fully expanded against
the helical anchor 30. Typically, the stent portion 24 of the valve 10 is
comprised of thin metal material that includes openings or cells. These
openings or cells become embedded against the coating or covering 170. The
stent 24 therefore firmly engages with the helical anchor 30 creating a very
strong attachment for the replacement valve 10 inside the helical anchor 30.
FIG. 10C more specifically illustrates an enlarged view demonstrating how the
stent portion 24 has deformed the fabric and foam coating 170 of the helical
anchor 30. This engagement is very strong and prevents the replacement heart
valve 10 from becoming dislodged. FIG. 100-1 is an even further enlarged
view showing a cell or opening 24a of the stent 24 that is engaged against the
-31-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
foam and fabric covering 170, creating a very strong physical connection
between these two components. FIG. 10D illustrates a balloon catheter 140
expanding a replacement valve 10 inside of the coated helical anchor 30 from a
view above the helical anchor 30. FIG. 10E illustrates the same view from
above the helical anchor 30, but illustrating full expansion of the valve 10
after
inflation of the balloon catheter 140 (FIG. 10A). The stent portion 24 of the
replacement heart valve 10 is then fully engaged into the resilient,
frictional
coating 170 on the helical anchor 30.
[00137] FIGS. 11A-11D illustrate an embodiment that includes a covering
or coating 180 on the helical anchor 30 which is intermittent, as opposed to
the
continuous coating 170 shown in the previous figures. In this regard, there
are
segments of coating 180 along the helical anchor 30 and these segments 180
may be rigidly fixed to the helical anchor 30. However, there may also be an
advantage to allowing these segments 180 to slide along the helical anchor 30
as the helical anchor 30 is expanded using, for example, balloon inflation as
previously described. The segments 180 may slide along the coils 32 of the
helical anchor 30 to allow the helical anchor 30 to tighten and at the same
time
the segments 180 can firmly engage with the cells or openings 24a of the
replacement heart valve stent 24.
[00138] FIG. 11A illustrates a helical anchor 30 with a covering that is
intermittent and formed with segments 180. The covering segments 180 are
shown with a taper at each end to allow the anchor 30 to be turned into
position
without a flat leading edge to impair placement. The taper is not necessary,
but
assists if desired in this regard. This taper may be of any suitable design
and
may be angular, or curved in any shape that promotes easy motion of the
helical anchor 30. A balloon catheter 140 is positioned inside of a stent
mounted replacement valve 10 as previously described and is initiating its
inflation as indicated by the arrows 182. FIG. 11B illustrates the stent
mounted
replacement heart valve 10 fully expanded. The coating segments 180 have
become fully engaged within the cells or openings of the heart valve stent 24.
Once these segments 180 engage with the stent 24 and enter one or more cells
or openings, they become fixed to the stent 24 and they will begin to slide
along
the helical anchor 30. The helical anchor 30 can expand and tighten against
the stent portion 24 of the replacement valve 10 and at the same time there
will
still be the beneficial effect of intermittent and strong attachment to the
helical
-32-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
anchor 30 afforded by the segments 180 of high friction and resilient and/or
compressible material. FIGS. 11C and 11D illustrate the process from above
the helical anchor 30 showing initial expansion of the stent mounted
replacement heart valve 10 in FIG. 110 and full expansion and engagement
between the segments 180 and the stent 24 in FIG. 11D firmly attaching these
two structures together during the implantation procedure within a patient.
[00139] FIGS. 12A-12E illustrate a helical anchor 30 and the motor or
drive function provided when the helical anchor 30 expands and the ends 30a,
30b of the coils 32 move. FIG. 12A illustrates a helical anchor 30 with about
four turns or coils 32, while FIG. 12B illustrates a helical anchor 30 with
about
three turns or coils 32. As further shown in FIG. 12B the helical anchor 30 is
attached to barbed fasteners 190 for delivery into a replacement heart valve
10.
A fabric or other material coating or exterior 192 is applied around the barbs
190 and around the helical anchor 30. When a balloon 140 is inflated inside of
the helical anchor 30, the two ends 30a, 30b of the helical anchor 30 move in
opposite directions as the helical anchor 30 is expanded. In this manner, the
barbs 190 are oriented in opposite directions to the movement of the helical
anchor 30 so that these barbs 190 will be activated or move when the helical
anchor 30 is expanded. FIG. 120 illustrates a cross section of the helical
anchor 30 with the fabric or other covering or coating 192 and a fastener
system 190 coupled with the helical coil 30. It was previously described as to
how the turns or coils 32 of the helical anchor 30 may be driven together by
inflation of a balloon 140. Balloon inflation also drives or moves the turns
32 of
the helical anchor 30 together, increasing the penetration of the barbs 190.
The
barbs 190 in FIGS. 12B-12E are oriented obliquely relative to the central axis
of
the helical anchor 30, however, the barbs 190 may instead deploy in a straight
or parallel direction relative to the axis of the helical anchor 30, straight
toward
an adjacent turn or coil 32 of the helical anchor 30, driven by the
compression
of the helical coils 32 together by the inflating balloon 140. With expansion,
the
ends 30a, 30b of the helical anchor 30 move considerably, but the central part
of the anchor 30 does not turn or rotate considerably. Barbs 190 without an
oblique orientation may be preferred at the center coils 32. The angle of the
barbs 190 may increase and their length can be increased in areas toward the
ends 30a, 30b of the helical anchor 30 where the movement during inflation of
a
balloon 140 is more pronounced. FIG. 12D illustrates a top view of the helical
-33-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
anchor 30. As the balloon catheter 140 is inflated, the helical anchor 30
increases in diameter and the ends 30a, 30b of the helical anchor 30 rotate to
allow this diameter expansion. As shown in FIG. 12E, the expansion of the
helical anchor 30 has mobilized or deployed the barbs 190 and the barbs 190
engage into the fabric or other material coating 192 within the middle or
central
turn or coil 32. This locks the turns or coils 32 of the helical anchor 30
together.
No native valve leaflet tissue is shown in FIG. 12E, however, it will be
appreciated that leaflet tissue could be located between the turns or coils 32
and the barbs 190 could entail and engage the leaflet tissue for further
securing
the helical anchor 30 to the native mitral valve tissue.
[00140] FIGS. 13A-13C
illustrate another embodiment in which a helical
anchor 30 is used having relatively larger diameter turns or coils 32 at the
ends
of the anchor 30 and a relatively smaller turn or turns in a middle or central
portion of the helical anchor 30. The helical anchor 30 is attached to barbs
190
and covered by a suitable coating material 192, such as fabric or other
material.
When the balloon 140 is inflated the ends of the helical anchor 30 begin to
move and the barbs 190 are activated as the central, smaller helical turn 30
is
expanded outwardly. This particular arrangement is ideal to attach to the
native
mitral valve of a patient. One barbed turn or coil 32 of the helical anchor 30
may be placed above the native mitral valve leaflets and one barbed turn or
coil
32 may be placed below the native mitral valve leaflets. The smaller diameter
turn or coil 32 may sit above or below the native mitral valve leaflets. When
the
balloon (not shown) is inflated, the large helical turns or coils 32 above and
below the native mitral valve leaflets will be driven towards each other as
generally shown and described above in FIGS. 8A-8D. Also, the anchor ends
will rotate and barbs 190 will deploy through the mitrel valve leaflet tissue
positioned between the larger turns or coils 32 close to the native annulus.
The
two large helical turns or coils 32 can also be bound together as the barbs
190
cross the mitrel tissue and penetrate the covering 192 on the helical coil 32
at
the opposite side of the native mitre! valve. These actions will trap the
mitrel
valve between the turns or coils 32 of the helical anchor 30, although it is
not
necessary for this to occur. It is also apparent that the large diameter turns
or
coils 32 at the opposite ends of the helical anchor 30 will become smaller in
diameter as the balloon is expanded. In this regard, the upper and lower turns
or coils 32 "donate" to the middle coil or turn 32. This will result in a
diameter
-34-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
reduction for the upper and lower coils 32. After the coils 32 have been
fastened to the native mitral valve perimeter or annulus, this will result in
a
downsizing of the diameter of the mitral valve, i.e., an annuloplasty
procedure
will result. When the barbs 190 are retained in the native mitral valve tissue
firmly, they should not dislodge or withdraw after penetration. FIG. 130
illustrates a cross sectional view of a helical anchor 30 from FIG. 13B, as
well
as a barb system 190 and coating 192, such as fabric or other material. As
described previously, barbs 190 can deploy directly from the helical anchor 30
at a roughly 90 angle relative to the coil 32. This may be driven simply by
compressing coils 32 relative to one another as described above in connection
with FIGS. 8A-8D. The movement of the helical coil or anchor turns 32
longitudinally or rotationally also allows barbs 190 or other types of
fasteners to
be applied in a direction which is more parallel or oblique relative to the
turns or
coils 32 of the helical anchor 30.
[00141] FIGS. 14A-140 illustrate a different configuration for a helical
anchor 30. This anchor 30 has generally four coils 32. There are two upper
coils 32 followed by a joining segment 32a (gap segment). The joining segment
32a is typically used to separate the coils 32 of the anchor 30 that sit above
the
valve leaflets from those that are below (in the atrium and in the ventricle,
respectively). There is a coil 32b of similar size as the two upper coils 32
at the
end of the joining segment 32a. This is the lowest coil 32b on the anchor 30.
The final coil 32c changes direction ¨ instead of continuing on downward, it
coils back up and overlaps or crosses over an adjacent coil 32 of the anchor
30.
This coil 32c is shown as the "larger convolution" in Figure 14B. The figure
shows a directional change (like the joining segment) in the anchor 30 that
allows the final coil 32c to be directed upward. The final coil 32c is also
larger
to allow it to sit on the outside of the other coils. This larger coil 32c is
the
middle coil of the anchor 30 but is actually turned into the native valve
first when
being delivered. The important feature of this anchor 30 is that as it is
turned
into position, the upward bend in the joining segment 32a forces the anchor 30
up toward the annulus. This anchor 30, when positioned with two coils above
and two coils below the leaflets, sits with the larger coil 32c of the anchor
30
sitting right under the mitral valve annulus. The anchor 30 does not tend to
fall
into the ventricle. The lowest coils do not necessarily have to cross on the
-35-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
same point when viewed from the side (producing an X). They could cross for
example on opposite sides.
[00142] The key element in
the embodiment of FIGS. 14A-14C is for the
turning of the anchor 30 into position to result in an upward motion of the
end of
the anchor 30 which drives the anchor 30 into position right under the mitral
valve. As this anchor is "screwed in" the lowest coil 32b forces the anchor 30
upward against the mitral annulus. The larger diameter coil 32c in the middle
of
the anchor 30 also helps the anchor 30 positioning right under the leaflets
and
close to the annulus. The mitral annulus has a certain diameter and by
matching this diameter with the diameter of the largest anchor coil 32c, the
anchor 30 is able to sit right under the annulus. If this coil 32c is too
small, the
anchor 30 can drag against the leaflet tissue and inhibit the anchor 30 from
riding upward toward the annulus as it is placed. It will be appreciated that
crossing coils 32a, 32b in an anchor 30 may also be useful for valve anchoring
when using an anchor 30. The crossing coil 32a occurs in the lowest coil of
this
anchor 30. But a crossing segment 32a could occur in any location. It could
occur at the top, in the middle or at the bottom of the anchor 30. The amount
of
crossover could also vary. Here the cross over includes the lowest two coils
32.
There could be more coils that overlap. FIG. 14C shows the overlapping coil
32a with the lowest coil being outside the prior coils. The overlapping coil
32a
or crossing segment could occur inside the prior coils. FIG. 14C also shows an
abrupt change in pitch to cause an overlap. The overlap can also occur with a
gentle change of pitch. In FIGS. 14A through 14C, the spacing between coils in
both the top to bottom and side to side dimensions are exaggerated for
clarity.
The coils will apply compression from the top and bottom toward the center.
[00143] A major advantage of
the configuration shown in FIGS. 14A-14C
is that the number of coils 32 available to attach the valve is increased, but
the
length of the anchor 30 does not increase. This allows a shorter anchor. For
example, it may be useful to have less anchor length positioned in the left
ventricle 40 so the valve 10 can sit more towards the atrium 42. The
overlapping or crossing coils 32a may crossover in a desired manner and allow
the valve 10 to be retained with strong force and a shorter overall length
inside
the left ventricle 40. The overlap 32a in the anchor could also be positioned
at
the level where the native leaflets 12a, 12b are sitting. This would increase
the
trapping of the leaflets 12a, 12b ¨the anchor 30 could be positioned such that
-36-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
overlapping coils had leaflet between them. If the gap between the coils 32 of
the anchor 30 were sufficiently small, the leaflets 12a, 12b could be trapped
between the coils 32 without the need for additional fasteners. This
arrangement may also position the leaflets 12a, 12b to be fastened to the
anchor 30 or an anchoring system attached or guided by the anchor 30. This
particular anchor arrangement is also useful because the lowest coil of the
anchor coils 32 extends in the opposite direction to the remainder of the
anchor
30 ¨ while the other coils 32 are biased downward, this is biased upward. As
this anchor 30 is turned into position, the lowest coil 32b will tend to move
back
upward. This is actually creating a virtual reverse thread. A typical helical
anchor is screwed into the valve leaflets 12a, 12b like a corkscrew and as it
is
turned, it moves downward. With this configuration, once the first coil of the
anchor 30 is turned into the valve 12 and the joining segment 32a is reached,
the anchor 30 actually begins to turn upward instead of downward as the lowest
coil 32b is being turned in. This means this particular anchor arrangement
will
tend to sit right under the annulus 12. This is useful in optimally
positioning the
anchor 30 close to the underside of the annulus 12. An anchor 30 attached to
the leaflets 12a, 12b away from the annulus 12 will tend to move and rock as
the heart contracts. This is because of leaflet motion away from the annulus
12
as the heart beats. In contrast the annulus 12 itself moves very little as the
heart beats. By placing the anchor 30 closer to the annulus 12 (away from the
leaflets), the amount of movement of the anchor 30 is reduced. Each day the
heart beats about 100,000 times. This repetitive motion will produce a risk of
anchor and valve dislodgement. Thus minimizing the motion by placing the
anchor 30 close to the annulus 12 will reduce the risk of valve implant
failure.
In FIGS. 14A-14C, the crossing points for the anchor coils 32 are both on the
same side of the anchor 30. This creates an X. It is not necessary for the
crossing points to occur at the same side. For example, they could be on
opposite sides of the anchor 30.
[00144] While the present
invention has been illustrated by a description
of preferred embodiments and while these embodiments have been described
in some detail, it is not the intention of the Applicants to restrict or in
any way
limit the scope of the appended claims to such detail. Additional advantages
and modifications will readily appear to those skilled in the art. The various
features and concepts of the invention may be used alone or in any
-37-
CA 02920724 2016-02-08
WO 2015/023579 PCT/US2014/050525
combination depending on the needs and preferences of the operator. This has
been a description of the present invention, along with the preferred methods
of
practicing the present invention as currently known. However, the invention
itself should only be defined by the appended claims.
-38-