Note: Descriptions are shown in the official language in which they were submitted.
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
IMPLANTABLE MEDICAL DEVICES
CROSS REFERENCE
[0001] This application claims the benefit of and priority to, continuation-
in-part U.S.
Application serial number 14/458,561, filed August 13, 2014, and continuation-
in-part
U.S. Patent Application serial number 14/458,549, filed August 13, 2014, which
both
are continuation-in-part patent applications which claims the benefit of U.S.
patent
application serial number 13/973,818, filed August 22, 2013, which the entire
disclosures of all are incorporated herein by reference.
BACKGROUND
[0002] The present invention relates to implantable medical devices made
entirely
or partially of silk, including silk medical devices with at least one surface
(i.e. a top
surface or a bottom surface of the silk medical device) made or prepared so
that, after
in vivo implantation of the silk medical device (such as implantation in
conjunction with
a medical or surgical procedure, such as an abdominal procedure, such as a
hernia
repair procedure) adhesion or attachment of a tissue (such as a human
abdominal,
bowel or intestinal tissue) to that surface or surfaces of the silk medical
device is
prevented, substantially prevented, discouraged and/or not facilitated (hence
an "anti-
adhesive" surface). In particular the present invention relates to single
layer and multi-
laminate, anti-adhesive surface silk based devices comprising one or more of a
silk
film, a silk sponge, and a knitted silk fiber or fabric as well as methods for
making and
using, for example in abdominal surgery. The devices can be combined with or
coated
with a hyaluronic acid or other macromolecule (such as for example dextran,
heparin
and sulphates thereof)
[0003] Silk is a natural (non-synthetic) protein that can be processed into
high
strength fibroin fibers with mechanical properties similar to or better than
many of
i
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
synthetic high performance fibers. Silk is stable at physiological
temperatures in a wide
range of pH, and is insoluble in most aqueous and organic solvents. As a
protein,
unlike the case with most if not all synthetic polymers, the degradation
products (e.g.
peptides, amino acids) of silk are biocompatible. Silk is non-mammalian
derived and
carries far less bioburden than other comparable natural biomaterials (e.g.
bovine or
porcine derived collagen). Silk, as the term is generally known in the art,
means a
filamentous fiber product secreted by an organism such as a silkworm or
spider. Silks
can be made by certain insects such as for example Bombyx mori silkworms, and
Nephilia clavipes spiders. There are many variants of natural silk. Fibroin is
produced
and secreted by a silkworm's two silk glands. As fibroin leaves the glands it
is coated
with sericin a glue-like substance. Spider silk is produced as a single
filament lacking
the immunogenic protein sericin.
[0004] Silk has been used in biomedical applications. The Bombyx mori
species of
silkworm produces a silk fiber (a "bave") and uses the fiber to build its
cocoon. The
bave as produced include two fibroin filaments or broins which are surrounded
with a
coating of the gummy, antigenic protein sericin. Silk fibers harvested for
making
textiles, sutures and clothing are not sericin extracted or are sericin
depleted or only to
a minor extent and typically the silk remains at least 10% to 26% by weight
sericin.
Retaining the sericin coating protects the frail fibroin filaments from
fraying during
textile manufacture. Hence textile grade silk is generally made of sericin
coated silk
fibroin fibers. Medical grade silkworm silk is used as either as virgin silk
suture, where
the sericin has not been removed, or as a silk suture from which the sericin
has been
removed and replaced with a wax or silicone coating to provide a barrier
between the
silk fibroin and the body tissue and cells.
[0005] Hyaluronic acid (HA) (synonymously hyaluron or hyaluronate) is a
naturally
occurring glucosaminoglycan that has been used as a constituent of a dermal
filler for
wrinkle reduction and tissue volumizing. Hyaluronan is an anionic, nonsulfated
glycosaminoglycan distributed widely throughout connective, epithelial, and
neural
tissues. Polymeric hyaluronic acid can have a molecular weight of several
million
Da!tons. An individual can typically have about 15 grams of hyaluronan in his
body
about a third of which every day is degraded by endogenous enzymes and free
radicals within a few hours or days and replaced by hyaluronic acid newly
synthesized
by the body.
2
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
[0006] Bioconjugate Chemistry, 2010, 21, 240-247: Joem Y., et al., Effect
of cross-
linking reagents for hyaluronic acid hydrogel dermal fillers on tissue
augmentation and
regeneration, discusses use of a particular cross-linker HMDA to prepare a
cross-
linked hyaluronic acid dermal filler, and also discloses use of a variety of
hyaluronic
acid cross linkers and hyaluronic activators including BDDE and EDC.
[0007] Carbohydrate Polymers, 2007, 70, 251-257: Jeon, O., et al.,
Mechanical
properties and degradation behaviors of hyaluronic acid hydrogels cross-linked
at
various cross-linking densities, discusses properties of hyaluronic acid cross
linked with
a polyethylene glycol diamine (a PEG-diamine).
[0008] J. Am. Chem. Soc., 1955, 77 (14), 3908-3913: Schroeder W., et al.,
The
amino acid composition of Bombyx mori silk fibroin and of Tussah silk fibroin,
compares the amino acid compositions of the silk from two silkworm species.
[0009] US Patent Application Publication. Pub. No. US 2008/0004421 A1:
Chenault,
H., et al., Tissue adhesives with modified elasticity discloses an adhesive
hydrogel
useful as a medical tissue adhesive for example to assist wound closure can be
made
by preparing a chain extended, multi-arm polyether amine (such as an 8 arm PEG
amine) cross linked (using for example PEG 4000 dimesylate) to an oxidized
polysaccharide (such as dextran), by mixing the cross linked molecule in a
syringe at
the point of injection or administration with a hydrogel such as a solution of
dextran
dialdehyde.
[00010] US Patent Application Publication. Pub. No. US 2010/0016886 A1: Lu,
H.,
High swell, long lived hydrogel sealant; discusses reacting a multi-arm amine
(i.e. an 9
arm polyethelene glycol (PEG) with an oxidized (i.e. to introduce aldehyde
groups)
polysaccharide (such as hyaluronic acid), useful for tissue augmentation or a
tissue
adhesive/sealant.
[00011] US patent 6,903,199 to Moon. T., et al., Crosslinked amide derivatives
of
hyaluronic acid and manufacturing method thereof discusses cross linking
hyaluronic
acid with a chitosan or with a deacetylated hyaluronic acid with reactive
amide groups,
using (for example) EDC or NHS.
3
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
[00012] International Patent Application WO/2010/123945, Altman, G., et al.,
Silk
fibroin hydrogels and uses thereof discusses silk hydrogels made by, for
example,
digesting degummed silk hydrogels made by, for example, digesting degummed
Bombyx mori silk at 60 C for 4 hours in 9.3M lithium bromide to thereby
obtain a 20%
silk solution, an 8% silk solution of which was induced to gel using 23RGD
and/or
ethanol, which can be present in a hyaluronic acid carrier. Altman also
discusses
possible use as a dermal filler and to promote wound closure, and (in
paragraph
[0210]) a silk hydrogel coating on a silk mesh. Altman also discusses silk
cross linked
to hyaluronic acid (see paragraphs [213] to [220], using various cross
linkers.
[00013] International Patent Application. Pub. No. WO/2008/008857: Prestwich,
G.,
et al., Tholated macromolecules and methods for making and using thereof
discloses a
thioethyl ether substituted hyaluronic acid made by oxidating coupling useful,
for
example, in arthritis treatment.
[00014] International Patent Application. Pub. No. WO/2008/008859: Prestwich,
G.,
et al., Macromolecules modified with electrophilic groups and methods of
making and
using thereof discloses a haloacetate derivative hyaluronic acid reacted with
thiol
modified hyaluronic acid to make a hydrogel, with various medical uses.
[00015] Biomacromolecules, 2010, 11 (9), 2230-2237: Serban, M., et. Al.,
Modular
elastic patches: mechanical and biological effects discusses how to make an
elastic
patch by cross linking elastin, hyaluronic acid and silk, by adding an
aminated
hyaluronic acid (made using EDC) with a 20% silk solution and elastin, in PBS
with
BS3 (bissulfosuccinimidyl suberate, as cross linker) at 37 C. for 12 hours.
[00016] Biomaterials, 2008, 29(10), 1388-1399: Serban, M., et al., Synthesis,
characterization and chondroprotective properties of a hyaluronan thioethyl
ether
derivative discusses a viscous 2-thioethyl ether hyaluronic acid derivative
solution
useful for viscosupplementation in arthritis treatment. The abstract mentions
use of
hyaluronic acid with multiple thio groups for adhesion prevention.
[00017] Methods, 2008, 45, 93-98: Serban, M., et al., Modular extracellular
matrices:
solutions to the puzzle discusses cross linked thio modified hyaluronic acid
hydrogel
useful as a semi synthetic extracellular matrix for cell culture.
4
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
[00018] Biomacromolecules, 2007, 8(9), 2821-2828: Serban, M., et al.,
Synthesis of
hyaluronan haloacetates and biology of novel cross linker free synthetic
extracellular
matrix hydrogels discusses cross linking haloacetate substituted hyaluronic
acids
reacted with a thiol substituted hyaluronic acid to make a hydrogel useful for
cell culture
or adhesion prevention or medical device coating.
[00019] Journal of Materials Chemistry, 2009, 19, 6443-6450: Murphy A., et
al.,
Biomedical applications of chemically modified silk fibroin is a review of
methods to
make silk conjugates, including silk conjugated to oligosaccharides, modified
silk and
medical uses.
[00020] Biomacromolecules, 2004, 5, 751-757: Sohn, S., et al., Phase behavior
and
hydration of silk fibroin discusses a study of Bombyx mori silk in vitro using
osmotic
stress, determining that silk l (a-silk) but not silk 11(13-sheet, spun silk
fiber) is hydrated.
[00021] US patent 8,071,722 to Kaplan, D., et al., Silk Biomaterials and
methods of
use thereof discloses silk films, use of 9-12m LiBr to dissolve extracted
silk, adding
hyaluronic acid to a silk solution to make fibers from the composition. See
also eg the
Kaplan patents and application 7,674,882; 8,178,656; 2010 055438, and; 2011
223153.
[00022] US patent application 2011 071239 by Kaplan, D., et al., PH induced
silk
gels and uses thereof discloses methods for making silk fibroin gel from silk
fibroin
solution, useful to coat a medical device (see paragraph [0012]), as an
injectable gel to
fill a tissue void, making an adhesive silk gel (with or without a hyaluronic
acid),
adhering the adhesive silk gel to a subject for example for use as a wound
bioadhesive, a multi-layered silk gel.
[00023] US patent application 2009 0202614 by Kaplan, D., et al., Methods for
stepwise deposition of silk fibroin coatings discusses layered silk coatings,
silk films
made using silk fibroin solutions which can include a hyaluronic acid, useful,
for
example, as wound healing patches, to coat an implantable medical device.
[00024] US patent 4,818,291 to lwatsuki M., et al., Silk-fibroin and human-
fibrinogen
adhesive composition discusses surgical adhesive useful in tissue repair made
as a
mixture of LiBr dissolved silk and fibrinogen.
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
[00025] Implantable, knitted silk fabrics for surgical use are known. See eg
US patent
applications 2004/0224406 and 2012/0029537. Post operative adhesions are a
common occurrence after surgery and are undesirable. For example postoperative
intra-abdominal and pelvic adhesions are the leading cause of infertility,
chronic pelvic
pain, and intestinal obstruction. Adhesions form as a result of the body's
natural
healing response and imply migration of fibroblasts to the trauma/wound site,
cell
proliferation, de novo extracellular matrix secretion and wound closing
through
adhesion formations. Post-operative adhesions can occur at the tissue-tissue
interface
(i.e. peritendinous tissue adhesion involves adhesion between the repaired
tendon and
the surrounding tissue) or at a tissue-biomaterial interface, in cases where a
biomaterial (i.e. a supporting scaffold) is used to reinforce the mechanical
properties of
the repaired tissue. For example in hernia repair where a biomaterial mesh is
used to
reinforce the reconstructed abdominal wall, adhesions commonly form between
the
mesh and underlying bowel tissue.
[00026] Thus there is a need for an implantable biomaterial mesh that can
decrease
or eliminate formation of post-operative adhesions.
6
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
SUMMARY
[00027] The present invention meets these needs and provides silk based
medical
devices that can reduce or prevent post-operative tissue to tissue or tissue
to scaffold
adhesion formation. Important to the invention was discovery of a
biocompatible
material that does not promote cell attachment, provides a smooth surface that
hinders
cell attachment, eliminates the introduction of foreign chemical agents,
exploit silk's
intrinsic physical cross linking capacity via hydrogen-bond mediated beta-
sheet
formation; and provides a robust, pliable, and user friendly implantable
medical device.
[00028] The present invention also includes an entirely silk based self
adherent
medical devices. This device is: biocompatible and can stick (adhere) to a
physiological
surface (such as skin or other tissue surface); provides a smooth surface that
can
prevent cell adherence and/or tissue abrasions; circumvent the introduction of
any
external agents or chemicals; makes use of silk's intrinsic physical
crosslinking
capacity via hydrogen-bond mediated beta-sheet formation; and (e) robust,
pliable,
cost-efficient and a user friendly medical device.
[00029] An embodiment of the present invention is a laminate, implantable silk
medical device having a first layer comprising a knitted silk fabric, the
first later having
a top side and a bottom side, and a second layer comprising a silk film or
sponge
fused to at least a portion of the bottom side of the first layer, thereby
obtaining a
laminate, implantable silk medical device. The silk film or sponge can
comprise silk
and a compound selected from the group consisting of polyethylene glycol,
ethylene
oxide, propylene oxide block copolymer, hyaluronic acid, dextran, and alginate
and
salts and combinations thereof. Additionally, the silk film or sponge can be
water
resistant and the silk film can be fused to the silk fabric by drying the silk
film or sponge
after placing the silk film or sponge onto the silk fabric.
[00030] Additional embodiments of the present invention can include an
implantable
silk medical device with or without pores, knitted using one to 36 filament
silk yarn
prepared at a various twist rates; an implantable silk medical device which is
about 0.5
mm to about 4 mm thick; an implantable silk medical device knitted as a flat
sheet with
a top side and a bottom side wherein the bottom is has a low profile, anti-
adhesive
surface, and; a laminate, implantable silk medical device comprising: (a) a
first layer
7
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
comprising a knitted silk fabric, the first later having a top side and a
bottom side; (b) a
second joining layer comprising a knitted, non-silk fabric having a top side
and a
bottom side, the second joining layer joining the bottom side of the first
layer to the top
side of the second joining layer and the bottom side of the second joining
layer a top
side of a third sacrificial layer, and; (c) the third sacrificial layer
comprising a knitted,
non-silk fabric having a top side and a bottom side, the top side of the third
sacrificial
layer attached to at least a portion of the bottom side of the second layer,
thereby
obtaining a laminate, implantable silk medical device, wherein the first layer
biodegrades over about 1 years to about 3 years after implantation of the
device, and
the second joining layer biodegrades over about 10 to 30 days after
implantation of the
device biodegradation of the second joining layer thereby releasing the third
sacrificial
layer from indirect attachment to the first layer through the second joining
layer.
[00031] Another embodiment of the present invention is a process for making a
laminate, implantable silk medical device by (a) knitting a fabric from
sericin depleted
silk thereby making a first layer having a top side and a bottom side, (b)
preparing a silk
solution by dissolving silk into a solvent; (c) casting a silk film or sponge
from the silk
solution; (d) treating the silk film or sponge so that at least one side of
the silk film is
water resistant, thereby forming a second layer; and (e) fusing the second
layer to at
least a portion of the bottom side of the first layer, thereby obtaining a
laminate,
implantable silk medical device.
[00032] The present invention also includes a method for providing tissue
support
and reducing adhesion formation by implanting the device, including an
abdominal
surgical method comprising the step of implanting the device.
[00033] A detailed embodiment of the present invention can be a laminate,
implantable silk medical device comprising: (a) a first layer comprising a
water
resistant, non-adherent silk film, the first layer having a top side and a
bottom side,
and;(b) a second layer comprising a water soluble, adherent silk film or
sponge formed
on or placed on the top side of the first layer, thereby obtaining a laminate,
implantable
silk medical device.
[00034] Additional embodiments of the present invention can be:an implantable
silk
medical device with an average pore size of about 4 mm by about 4 mm, knitted
using
8
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
six or nine filament silk yard prepared at a twist rate of 2(6S) 3(3(Z); an
implantable silk
medical device which is about 3 mm to about 4 mm thick made with a pick
density of
about 26 picks per centimeter; an implantable silk medical device knitted as a
flat sheet
with a top side and a bottom side wherein the bottom is has a smooth, anti-
adhesive
surface made with a pick density of about 18 picks per centimeter, and; a
laminate,
implantable silk medical device comprising: (a) a first layer comprising a
knitted silk
fabric, the first later having a top side and a bottom side; (b) a second
joining layer
comprising a knitted, non-silk fabric having a top side and a bottom side, the
second
joining layer joining the bottom side of the first layer to the top side of
the second
joining layer and the bottom side of the second joining layer a top side of a
third
sacrificial layer, and; (c) the third sacrificial layer comprising a knitted,
non-silk fabric
having a top side and a bottom side, the top side of the third sacrificial
layer attached to
at least a portion of the bottom side of the second layer, thereby obtaining a
laminate,
implantable silk medical device, wherein the first layer biodegrades over
about 1 years
to about 3 years after implantation of the device, and the second joining
layer
biodegrades over about 30 days after implantation of the device biodegradation
of the
second joining layer thereby releasing the third sacrificial layer from
indirect attachment
to the first layer through the second joining layer.
[00035] The present invention also includes a laminate, implantable silk
medical
device comprising: (a) a first base layer comprising a knitted silk fabric,
the first layer
having a top side and a bottom side; (b) a second anti-adhesive layer
comprising a
knitted silk fabric having a top side and a bottom side, the second anti-
adhesive layer
being attached at least in part on the bottom side of the first layer, wherein
the first and
second layer biodegrades over about 1 years to about 3 years after
implantation of the
device. The present invention also includes a laminate, implantable silk
medical
device comprising: (a) a first base layer comprising a knitted silk fabric,
the first layer
having a top side and a bottom side, and; (b) a second sacrificial layer
comprising a
knitted, non-silk fabric having a top side and a bottom side, the second
sacrificial layer
being attached at least in part on the bottom side of the first layer, wherein
the first
layer biodegrades over about 1 years to about 3 years after implantation of
the device,
and the second sacrificial layer biodegrades over about 10 to 30 days after
implantation of the device. The present invention also includes a laminate,
implantable
silk medical device comprising: (a) a first base layer comprising a knitted
silk fabric,
9
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
the first layer having a top side and a bottom side; (b) a second (middle)
layer
comprising a knitted, non-silk fabric having a top side and a bottom side, the
second
joining layer joining the bottom side of the first layer to the top side of
the second
joining layer and the bottom side of the second joining layer a top side of a
third
sacrificial layer, and; (c) the third detaching layer comprising a knitted,
non-silk or silk
fabric having a top side and a bottom side, the top side of the third
sacrificial layer
attached to at least a portion of the bottom side of the second layer, thereby
obtaining a
laminate, implantable silk medical device, wherein the first layer biodegrades
over
about 1 years to about 3 years after implantation of the device, the second
joining layer
biodegrades over about 10 to 30 days after implantation, releasing the third
sacrificial
layer from tissue attachment, the thirds sacrificial layer biodegrading over
about 10
days to about 3 years after implantation of the device.
[00036] The present invention also includes a laminate, implantable silk
medical
device comprising: (a) a first base layer comprising a knitted silk fabric,
the first later
having a top side and a bottom side, and; (b) a second layer comprising a silk
film or
sponge fused to at least a portion of the bottom side of the first layer,
thereby obtaining
a laminate, implantable silk medical device, wherein the silk film is water
resistant,
wherein the silk film or sponge is fused to the silk fabric by drying the silk
film or
sponge after placing the silk film onto the silk fabric.
[00037] The present invention also includes a process for making a laminate,
implantable silk medical device, the process comprising (a) knitting a fabric
from sericin
depleted silk thereby making a first layer having a top side and a bottom
side, and; (b)
preparing a silk solution by dissolving silk into a solvent; (c) casting a
silk film or
sponge from the silk solution; (d) treating the silk film or sponge so that at
least one
side of the silk film is water resistant, thereby forming a second layer; and
(e) fusing the
second layer to at least a portion of the bottom side of the first layer,
thereby obtaining
a laminate, implantable silk medical device.
[00038] The present invention also includes a laminate, implantable silk
medical
device comprising: (a) a first layer comprising a water resistant, non-
adherent silk film
or sponge , the first layer having a top side and a bottom side, and; (b) a
second layer
comprising a water soluble, adherent silk film or sponge formed on or placed
on the top
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
side of the first layer, thereby obtaining a laminate, implantable silk
medical device,
wherein the silk film or sponge comprises silk and a compound selected from
the group
consisting of polyethylene glycol, ethylene oxide, propylene oxide block
copolymer,
hyaluronic acid, dextran, and alginate and salts and combinations thereof.
DRAWINGS
[00039] Aspects of the present invention are illustrated by the following
drawings.
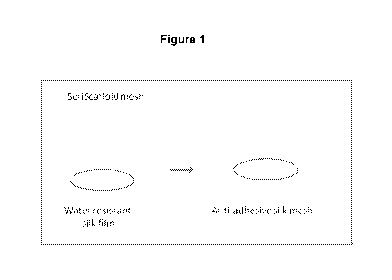
[00040] Figure 1 illustrates the procedure for casting a silk form from a silk
solution to
thereby make a water resistant silk film. The middle drawing in Figure 1 shows
the silk
solution being dispensed from a pipette. "Et0H" in Figure 1 means application
of
ethanol to the silk film.
[00041] Figure 2 illustrates the procedure for making a multi laminate medical
device
using the water resistant silk film made by the Figure 1 process. In Figure 2
the water
resistant silk film is shown fused onto a knitted silk mesh (the particular
knitted silk
mesh used was SERI Surgical Scaffold, available from Allergan, Irvine,
California).
[00042] Figure 3 is a graph obtained by use of FTIR showing on the x axis the
absorbance wavelength (nm) and on the y axis the absorbance (arbitrary units
or AU)
confirming beta sheet induction through silk film treatment with the ethanol
solution.
[00043] Figure 4 shows on the left hand side of Figure 4 a side view
photograph and
on the right hand side of Figure 4 a top view photograph of the water
resistant silk film
made by the process of Figure 1.
[00044] Figure 5 is a pictorial representation of how the silk film made by
the process
of Figure 1 can be used to wrapped around a portion of a tendon so as to
isolate the
tendon from adjacent tissues.
[00045] Figure 6 top - shows on the left hand side of Figure 6 a bottom view
photograph (the "smooth side") of a multi laminate medical device comprising a
water
resistant silk film fused to the knitted silk fabric. The right hand side of
Figure 6 is a top
view photograph (the "rough side") of the multi laminate silk device. Figure 6
bottom ¨
contrasts the device comprising of a water resistant silk film fused to the
knitted silk
11
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
fabric with the device comprising of a water resistant silk sponge fused to
the knitted
silk fabric.
[00046] Figure 7 is a pictorial representation showing in the top portion of
Figure 7
knit characteristics of the knitted silk fabric used (SERI Surgical
Scaffold), and in the
bottom portion of Figure 7.
[00047] Figure 8 is a pictorial representation of the use of the fused silk-
film mesh
medical device for post-operative adhesion prevention in an abdominal wall
repair
model.
[00048] Figure 9 is an illustration of the casting process of a double layered
self-
adherent silk film.
[00049] Figure 10 is a graph obtained by use of FTIR showing on the x axis the
absorbance wavelength (nm) and on the y axis the absorbance (AU) confirming
beta
sheet induction through silk film treatment with the ethanol solution.
[00050] Figure 11 presents two photographs of a multi laminate (two layers of
silk
film) medical device, showing in the left hand side photograph adherence to
the top of
a Petri dish and in the right hand side photograph adherence to a moistened
nitrile
surgical glove.
[00051] Figure 12 is a pictorial illustration of the silk film adherence
mechanism to
wet or moist surfaces. The hydrophilicity of the contact surface probably
triggers silk
fibroin structural rearrangements that lead to the reorientation of the
hydrophilic and
hydrophobic regions of the protein to promote the most energetically favorable
interactions.
[00052] Figure 13 presents two bar graphs: evaluation of cell numbers after 48
hours
(the upper graph in Figure 13) and after 6 days (the lower graph in Figure 13)
incubation on different biomaterial formulations, by colorimetric MTS (344,5-
dimethylthiazol-2-y1)-5-(3-carboxymethoxypheny1)-2-(4-su Ifopheny1)-2H-
tetrazoliu m)
(tetrazolium dye) assay.
[00053] Figure 14 comprises five bar graphs showing the additive dose
dependent
cell responses on different biomaterial (second layer) formulations (24 hour
incubation
12
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
with 2x105 cells/well). The dotted box in each of the five Figure 14 graphs
shows the
preferred second layer for the formulation set forth by that bar graph.
[00054] Figure 15 is a diagram showing the knit pattern used to make the
single bed
102 (base layer) device.
[00055] Figure 16 is showing the appearance of the technical back and
technical
front of the single bad 102 (base layer) device that was knitted with the
pattern shown
in Figure 15.
[00056] Figure 17 is a diagram showing the knit pattern used to make satin
devices -
both anti-adhesive and sacrificial (prototypes SS-P02-02-01, SS-P02-02-02, SS-
P02-
02-04 and SS-P02-02-10).
[00057] Figure 18 is showing the appearance of the technical back and
technical
front of a representative anti-adhesive satin device (SS-P02-02-01) that was
knitted
with the pattern shown in Figure 17.
[00058] Figure 19 is a diagram showing the knit pattern used to make "shag
carpet"
devices.
[00059] Figure 20 is showing the appearance of the technical back, technical
front
and cross-section of a representative "shag carpet" device (SS-P02-03-09) that
was
knitted with the pattern shown in Figure 19.
[00060] Figure 21 is showing the appearance of the technical back and
technical
front of a representative sacrificial layer satin device (SS-P02-02-10) that
was knitted
with the pattern shown in Figure 17.
[00061] Figure 22 is a depiction of the detachable layer device concept.
[00062] Figure 23 is a diagram showing the knit pattern used to make
representative
detachable layer devices (SS-PO4-01 and SS-PO4-02-0X).
[00063] Figure 24 is showing the appearance of the technical back, technical
front
and cross-section of a representative detachable layer device (SS-PO4-03).
13
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
[00064] Figure 25 is a bar graph showing the measured thickness in millimeters
of
the SERI Surgical Scaffold ("SERI Standard") and six of the devices made.
[00065] Figure 26 is a bar graph showing the measured burst strength (in MPa)
of the
SERI Surgical Scaffold ("SERI Standard") and the same six devices measured
in
Figure 25.
[00066] Figure 27 is a bar graph showing the stiffness (in Newtons per
millimeter) of
the SERI Surgical Scaffold ("SERI Standard") and the same six devices
measured
in Figure 25.
[00067] Figure 28 is a bar graph showing the measured suture pull out strength
(in
Newtons per suture) of the SERI Surgical Scaffold ("SERI Standard") and the
same
six devices measured in Figure 25.
[00068] Figure 29 is a bar graph showing the maximum load in the machine
(fabric
length) direction measured in Newtons for the SERI Surgical Scaffold ("SERI
Standard") and for the same six devices measured in Figure 25.
[00069] Figure 30 is a bar graph showing the measured percent elongation at
break
in the machine (fabric length) direction for the SERI Surgical Scaffold
("SERI
Standard") and for the same six devices measured in Figure 25.
[00070] Figure 31 is a bar graph showing shows the maximum load in the course
(fabric width) direction in Newtons for the SERI Surgical Scaffold ("SERI
Standard")
and for the same six devices measured in Figure 25.
[00071] Figure 32 is a bar graph shows the percent elongation at break in the
course
(fabric width) direction for the SERI Surgical Scaffold ("SERI Standard")
and for the
same six devices measured in Figure 25.
14
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
DESCRIPTION
[00072] The present invention is based on the discovery of laminate silk
medical
devices that can be implanted to separate adjoining tissues, provide soft
tissue support
and/or reduce formation of adhesions.
[00073] The silk films and the silk fabrics set forth herein can be made from
silkworm
cocoons substantially depleted of sericin. A preferred source of raw silk is
from the
silkworm B. mori. Other sources of silk include other strains of Bombycidae
including
Antheraea pemyi, Antheraea yamamai, Antheraea mylitta, Antheraea assama, and
Philosamia cynthia ricini, as well as silk producing members of the families
Satumidae,
Thaumetopoeidae, and silk-producing members of the order Araneae. Suitable
silk can
also be obtained from other spider, caterpillar, or recombinant sources.
Methods for
performing sericin extraction have been described in pending U.S. Patent
Application
Ser. No. 10/008,924, U.S. Publication No. 2003/0100108, Matrix for the
production of
tissue engineered ligaments, tendons and other tissue.
[00074] Extractants such as urea solution, hot water, enzyme solutions
including
papain among others which are known in the art to remove sericin from fibroin
would
also be acceptable for generation of the silk. Mechanical methods may also be
used for
the removal of sericin from silk fibroin. This includes but is not limited to
ultrasound,
abrasive scrubbing and fluid flow. The rinse post-extraction is conducted
preferably
with vigorous agitation to remove substantially any ionic contaminants,
soluble, and
insoluble debris present on the silk as monitored through microscopy and
solution
electrochemical measurements. A criterion is that the extractant predictably
and
repeatably remove the sericin coat of the source silk without significantly
compromising
the molecular structure of the fibroin. For example, an extraction may be
evaluated for
sericin removal via mass loss, amino acid content analysis, and scanning
electron
microscopy. Fibroin degradation may in turn be monitored by FTIR analysis,
standard
protein gel electrophoresis and scanning electron microscopy.
[00075] In certain cases, the silk utilized for making the composition has
been
substantially depleted of its native sericin content (i.e., 4%
(w/w) residual sericin in
the final extracted silk). Alternatively, higher concentrations of residual
sericin may be
left on the silk following extraction or the extraction step may be omitted.
In preferred
aspects of this embodiment, the sericin-depleted silk fibroin has, e.g. about
0% to
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
about 4% (w/w) residual sericin. In the most preferred aspects of this
embodiment, the
sericin-depleted silk fibroin has, e.g. about 1% to 3% (w/w) residual sericin.
[00076] In
certain cases, the silk utilized for generation of a medical device within the
scope of the present invention is entirely free of its native sericin content.
As used
herein, the term "entirely free (i.e. "consisting of" terminology) means that
within the
detection range of the instrument or process being used, the substance cannot
be
detected or its presence cannot be confirmed.
[00077] The water soluble or dissolved silk can be prepared by a 4 hour
solubilization
(process of silk into solution) at 60 C of pure silk fibroin at a
concentration of 200 g/L in
a 9.3 M aqueous solution of lithium bromide to a silk concentration of 20%
(w/v). This
process may be conducted by other means provided that they deliver a similar
degree
of dissociation to that provided by a 4 hour solubilization at 60 C of pure
silk fibroin at
a concentration of 200 g/L in a 9.3 M aqueous solution of lithium bromide. The
primary
goal of this is to create uniformly and repeatably dissociated silk fibroin
molecules to
ensure similar fibroin solution properties and, subsequently, device
properties. Less
substantially dissociated silk solution may have altered gelation kinetics
resulting in
differing final gel properties. The degree of dissociation may be indicated by
Fourier-
transform Infrared Spectroscopy (FTIR) or x-ray diffraction (XRD) and other
modalities
that quantitatively and qualitatively measure protein structure. Additionally,
one may
confirm that heavy and light chain domains of the silk fibroin dimer have
remained
intact following silk processing and dissolution. This may be achieved by
methods such
as standard protein sodium-dodecyl-sulfate polyacrylamide gel electrophoresis
(SDS-
PAGE) which assess molecular weight of the independent silk fibroin domains.
[00078] System parameters which may be modified in the initial dissolution of
silk
include but are not limited to solvent type, silk concentration, temperature,
pressure,
and addition of mechanical disruptive forces. Solvent types other than aqueous
lithium
bromide may include but are not limited to aqueous solutions, alcohol
solutions,
1,1,1,3,3,3-hexafluoro-2-propanol, and hexafluoroacetone, 1-
butyl-3-
methylimidazolium. These solvents may be further enhanced by addition of urea
or
ionic species including lithium bromide, calcium chloride, lithium
thiocyanate, zinc
chloride, magnesium salts, sodium thiocyanate, and other lithium and calcium
halides
16
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
would be useful for such an application. These solvents may also be modified
through
adjustment of pH either by addition of acidic of basic compounds.
[00079] The medical devices disclosed herein are preferably biodegradable,
bioerodible, and/or bioresorbable. In a particular embodiment the medical
device (for
example as a silk film) can entirely or substantially biodegrade between about
10 days
to about 120 days after implantation. In another embodiment the medical device
(for
example formed as a laminate silk device comprising both a silk film and a
knitted silk
fabric) can entirely or substantially biodegrade over a period of time between
about 3
years or about 4 years after implantation.
[00080] Aspects of the present specification provide, in part, a silk film
having a
transparency and/or translucency. Transparency (also called pellucidity or
diaphaneity)
is the physical property of allowing light to pass through a material, whereas
translucency (also called translucence or translucidity) only allows light to
pass through
diffusely. The opposite property is opacity. Transparent materials are clear,
while
translucent ones cannot be seen through clearly. The silk films disclosed
herein may,
or may not, exhibit optical properties such as transparency and translucency.
In certain
cases, e.g., superficial line filling, it would be an advantage to have an
opaque silk film.
In other cases such as development of a lens or a "humor" for filling the eye,
it would
be an advantage to have a translucent silk film. These properties could be
modified by
affecting the structural distribution of the silk film. Factors used to
control a hydrogel's
optical properties include, without limitation, silk fibroin concentration,
gel crystallinity,
and silk homogeneity.
[00081] When light encounters a material, it can interact with it in several
different
ways. These interactions depend on the nature of the light (its wavelength,
frequency,
energy, etc.) and the nature of the material. Light waves interact with an
object by
some combination of reflection, and transmittance with refraction. As such, an
optically
transparent material allows much of the light that falls on it to be
transmitted, with little
light being reflected. Materials which do not allow the transmission of light
are called
optically opaque or simply opaque.
[00082] In an embodiment, a silk film is optically transparent. In aspects of
this
embodiment, a silk film transmits, e.g., between about 75% to about 100% of
the light.
In some preferred aspects of this embodiment, a silk film transmits, e.g.,
between
17
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
about 80% to about 90% of the light. In the most preferred aspects of this
embodiment,
a silk film transmits, e.g., between about 85% to about 90% of the light.
[00083] In an embodiment, a silk sponge is optically transparent. In aspects
of this
embodiment, a silk sponge transmits, e.g., between about 75% to about 100% of
the
light. In some preferred aspects of this embodiment, a silk film transmits,
e.g., between
about 80% to about 90% of the light. In the most preferred aspects of this
embodiment,
a silk sponge transmits, e.g., between about 85% to about 90% of the light.
[00084] Aspects of the present specification provide, in part, a medical
device
comprising a hyaluronan. As used herein, the term "hyaluronic acid" is
synonymous
with "HA", "hyaluronic acid", and "hyaluronate" refers to an anionic, non-
sulfated
glycosaminoglycan polymer comprising disaccharide units, which themselves
include
D-glucuronic acid and D-N-acetylglucosamine monomers, linked together via
alternating 13 -1 ,4 and 13 -1 ,3 glycosidic bonds and pharmaceutically
acceptable salts
thereof. Hyaluronan can be purified from animal and non-animal sources.
Polymers of
hyaluronan can range in size from about 5,000 Da to about 20,000,000 Da. Any
hyaluronan is useful in the compositions disclosed herein with the proviso
that the
hyaluronan improves a condition of the skin, such as, e.g., hydration or
elasticity. Non-
limiting examples of pharmaceutically acceptable salts of hyaluronan include
sodium
hyaluronan, potassium hyaluronan, magnesium hyaluronan, calcium hyaluronan,
and
combinations thereof.
[00085] Aspects of the present specification provide, in part, a composition
comprising a crosslinked matrix polymer. As used herein, the term
"crosslinked" refers
to the intermolecular physical or chemical bonds joining the individual
polymer
molecules, or monomer chains, into a more stable structure like a gel. As
such, a
crosslinked matrix polymer has at least one intermolecular physical or
chemical bond
joining at least one individual polymer molecule to another one. Matrix
polymers
disclosed herein may be chemically crosslinked using dialdehydes and disufides
crosslinking agents including, without limitation, multifunctional PEG-based
cross
linking agents, divinyl sulfones, diglycidyl ethers, and bis-epoxides. Non-
limiting
examples of hyaluronan crosslinking agents include divinyl sulfone (DVS), 1,4-
butanediol diglycidyl ether (BDDE), 1,2-bis(2,3-epoxypropoxy)ethylene (EGDGE),
1,2,7,8-diepoxyoctane (DEO), biscarbodiimide (BCD!), pentaerythritol
tetraglycidyl
18
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
ether (PETGE), adipic dihydrazide (ADH), bis(sulfosuccinimidyl)suberate (BS),
hexamethylenediamine (H M DA), 1-(2,3-epoxypropyI)-2,3-epoxycyclohexane, or
combinations thereof.
[00086] Aspects of the present specification provide, in part, a composition
comprising a crosslinked matrix polymer having a degree of crosslinking. As
used
herein, the term "degree of crosslinking" refers to the percentage of matrix
polymer
monomeric units that are bound to a cross-linking agent, such as, e.g., the
disaccharide monomer units of hyaluronan. Thus, a composition that that has a
crosslinked matrix polymer with a 4% degree of crosslinking means that on
average
there are four crosslinking molecules for every 100 monomeric units. Every
other
parameter being equal, the greater the degree of crosslinking, the harder the
gel
becomes. Non-limiting examples of a degree of crosslinking include about 1% to
about
15%.
[00087] In an embodiment, a composition comprises an uncrosslinked hyaluronan
where the uncrosslinked hyaluronan comprises a combination of both high
molecular
weight hyaluronan and low molecular weight hyaluronan in a ratio of about
20:1, about
15:1, about 10:1, about 5:1, about 1:1, about 1:5 about 1:10, about 1:15, or
about 1:20.
[00088] In another embodiment, a composition comprises an uncrosslinked
hyaluronan where the uncrosslinked hyaluronan comprises a combination of both
high
molecular weight hyaluronan and low molecular weight hyaluronan, in various
ratios.
As used herein, the term "high molecular weight hyaluronan" refers to a
hyaluronan
polymer that has a molecular weight of 1,000,000 Da or greater. Non-limiting
examples
of a high molecular weight hyaluronan include a hyaluronan of about 1,500,000
Da, a
hyaluronan of about 2,000,000 Da, a hyaluronan of about 2,500,000 Da, a
hyaluronan
of about 3,000,000 Da, a hyaluronan of about 3,500,000 Da, a hyaluronan of
about
4,000,000 Da, a hyaluronan of about 4,500,000 Da, and a hyaluronan of about
5,000,000 Da. As used herein, the term "low molecular weight hyaluronan"
refers to a
hyaluronan polymer that has a molecular weight of less than 1,000,000 Da. Non-
limiting examples of a low molecular weight hyaluronan include a hyaluronan of
about
200,000 Da, a hyaluronan of about 300,000 Da, a hyaluronan of about 400,000
Da, a
hyaluronan of about 500,000 Da, a hyaluronan of about 600,000 Da, a hyaluronan
of
19
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
about 700,000 Da, a hyaluronan of about 800,000 Da, and a hyaluronan of about
900,000 Da.
[00089] In other aspects of this embodiment, a composition comprises a
crosslinked
hyaluronan where the crosslinked hyaluronan has a mean molecular weight of,
e.g.,
about 1,000,000 Da, about 1,500,000 Da, about 2,000,000 Da, about 2,500,000
Da,
about 3,000,000 Da, about 3,500,000 Da, about 4,000,000 Da, about 4,500,000
Da, or
about 5,000,000 Da. In yet other aspects of this embodiment, a composition
comprises
a crosslinked hyaluronan where the crosslinked hyaluronan has a mean molecular
weight of, e.g., at least 1,000,000 Da, at least 1,500,000 Da, at least
2,000,000 Da, at
least 2,500,000 Da, at least 3,000,000 Da, at least 3,500,000 Da, at least
4,000,000
Da, at least 4,500,000 Da, or at least 5,000,000 Da. In still other aspects of
this
embodiment, a composition comprises a crosslinked hyaluronan where the
crosslinked
hyaluronan has a mean molecular weight of, e.g., about 1,000,000 Da to about
5,000,000 Da, about 1,500,000 Da to about 5,000,000 Da, about 2,000,000 Da to
about 5,000,000 Da, about 2,500,000 Da to about 5,000,000 Da, about 2,000,000
Da
to about 3,000,000 Da, about 2,500,000 Da to about 3,500,000 Da, or about
2,000,000
Da to about 4,000,000 Da.
[00090] In other aspects of this embodiment, a composition comprises an
uncrosslinked hyaluronan where the uncrosslinked hyaluronan has a mean
molecular
weight of, e.g., about 1,000,000 Da, about 1,500,000 Da, about 2,000,000 Da,
about
2,500,000 Da, about 3,000,000 Da, about 3,500,000 Da, about 4,000,000 Da,
about
4,500,000 Da, or about 5,000,000 Da. In yet other aspects of this embodiment,
a
composition comprises an uncrosslinked hyaluronan where the uncrosslinked
hyaluronan has a mean molecular weight of, e.g., at least 1,000,000 Da, at
least
1,500,000 Da, at least 2,000,000 Da, at least 2,500,000 Da, at least 3,000,000
Da, at
least 3,500,000 Da, at least 4,000,000 Da, at least 4,500,000 Da, or at least
5,000,000
Da. In still other aspects of this embodiment, a composition comprises an
uncrosslinked hyaluronan where the uncrosslinked hyaluronan has a mean
molecular
weight of, e.g., about 1,000,000 Da to about 5,000,000 Da, about 1,500,000 Da
to
about 5,000,000 Da, about 2,000,000 Da to about 5,000,000 Da, about 2,500,000
Da
to about 5,000,000 Da, about 2,000,000 Da to about 3,000,000 Da, about
2,500,000
Da to about 3,500,000 Da, or about 2,000,000 Da to about 4,000,000 Da. In
further
aspects, a composition comprises an uncrosslinked hyaluronan where the
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
uncrosslinked hyaluronan has a mean molecular weight of, e.g., greater than
2,000,000
Da and less than about 3,000,000 Da, greater than 2,000,000 Da and less than
about
3,500,000 Da, greater than 2,000,000 Da and less than about 4,000,000 Da,
greater
than 2,000,000 Da and less than about 4,500,000 Da, greater than 2,000,000 Da
and
less than about 5,000,000 Da.
EXAMPLES
[00091] The following examples illustrate embodiments of the present
invention.
Example 1
Preparation of a Silk Based Biomaterial useful as an Adhesion Barrier
The materials used in this Example 1 to make a silk based biomaterial useful
as an
adhesion barrier included: an aqueous silk fibroin solution (7-12% w/v
concentration of
silk); sterile 60-mm Petri dishes (used as casting molds); ethanol solution
90% v/v, and;
a knitted silk fabric (the particular knitted silk fabric used was SERI
Surgical Scaffold.
SERI Surgical Scaffold is available from Allergan, Inc., Irvine, California).
SERI
Surgical Scaffold is an embodiment of the knitted silk medical devices set
forth in US
patent applications serial numbers 13/715,872; 13/587,040; 13/843,519;
13/088,706,
and; 12/680,404.
[00092] As a first step to obtain a solution of water-soluble silk fibroin,
either Bombyx
Mori silk cocoons or silk fibroin yarn made by processing Bombyx Mori silk
cocoons
were soaked in a warm basic solution to thereby remove the immunogenic protein
sericin naturally present on the silkworm silk. The sericin depleted silk was
then
digested (solubilized) by dissolving the sericin depleted silk in 9.3M LiBr
followed by
dialysis into an aqueous solution. The amino acid composition of Bombyx Mori
silk
fibroin shows a low amount of aspartic acid/glutamic acid (carboxylic groups),
even
lower amount of lysine (amine groups) and a high amount of serine (hydroxyl
groups).
Silk beta-sheet formation can be induced with accelerants (pH, temperature,
vortexing,
sonication, ethanol treatment, etc.).
[00093] A first device was made as follows. Silk fibroin solution (1 ml) was
cast on
the bottom of an inverted 60 mm Petri dish and allowed to dry between 2-12
hours (
21
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
see Figure 1). The dried films were then immersed for two 2 hours in the
ethanol
solution to induce beta-sheet formation in the silk.
[00094] A second device was made as follows. Silk fibroin films cast as
described
above were allowed to dry for 50 minutes in a laminar flow hood then, prior to
complete
drying of the surface, were overlayed with precut SERI Surgical Scaffold
meshes (4x5
cm) (see Figure 2). The film was allowed to fuse with the mesh for 2-12 hours,
then the
construct was immersed in ethanol solution for 2 hours to induce physical
crosslinking
via beta sheets.
[00095] For both devices made in this Example 1, the ability of silk to become
water
resistant by physical crosslinking of the silk molecules was made use of.
Through this
cross linking process, the silk fibroin protein underwent structural
rearrangements to a
beta-sheet rich conformation. Temperature, pH, ionic strength and treatment
with polar
agents such as alcohols are all factors known to induce such structural
transitions. For
the two devices made in this Example 1, beta sheet formation was induced via
ethanol
treatment (see Figure 3).
[00096] The first device was a monolayer of transparent water-resistant silk
film, as
shown by Figure 4. The thickness of the film was controllable and depended on
the
silk fibroin solution concentration and the casting area. We found that an 8%
w/v silk
fibroin solution cast on a 4.6 cm diameter mold would yield a 50 [im tick
film. The film
was pliable, moldable, stretchable, with good mechanical integrity (average
maximum
load of 8.8 1.9 N for a 50 [im thick film versus an average maximum load of
71.7 1.0
N of SERI Surgical Scaffold) and can be used to wrap the target tissue (i.e.
tendon) to
isolate it from the surrounding tissues to with it may non-specifically adhere
(Figure 5).
Additionally, the first device can be used in conjunction with other devices
(meshes,
sheets). Moreover, the transparency of device 1 is a convenient feature as it
allows the
user to correctly evaluate the positioning of the device 1 film on the tissue.
[00097] The second device made in this Example 1 consisted of a single layer
silk
film fused with the SERI Surgical Scaffold (see Figure 6). The fusion of the
silk with
the mesh was driven by the partial encasing of the mesh filaments by the silk
solution
prior its complete drying (Figure 7). After complete drying of the film the
construct was
treated with ethanol solution to render it water insoluble via beta-sheet
formation. The
key features of this second device were: (a) ¨ its smooth surface on one side
and (b) ¨
22
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
the rugged surface provided by the mesh pores on the other side. In the case
of the
abdominal wall repair for example, the smooth side is intended to contact the
bowel
and prevent adhesion formations, while the rugged surface will face the
abdominal wall
and will integrate well with the surrounding tissue by promoting cells to
adhere to its
groove (Figure 8).
[00098] Both device 1 and device 2 have the advantage of being both entirely
silk
fibroin based. The sterility of both these devices can be ensured either by
using
autoclaved silk fibroin solution for film casting (and fusing them with
sterile meshed for
device 2) or via ethylene oxide sterilization. Moreover, both devices are
compatible to
be used with a variety of other mesh medical such as Vicryl and Mersilene.
These
devices: (a) ¨ are biocompatible and do not intrinsically sustain cell
attachment as
previously established by large bodies of scientific literature; (b) ¨ provide
a smooth
surface that further hinders cell attachment; (c) ¨ do not contain any
"foreign" chemical
agents; (d) ¨ are physically crosslinked through intra- and inter-molecular
beta-sheets;
and (e) - are robust, drapable and easy to handle.
Example 2
Self Adherent Silk based Biomaterials
[00099] The materials used in this Example 2 included: an aqueous silk fibroin
solution (7-12% w/v) made by the same methods set forth in Example 2; sterile
60-mm
Petri dishes (used as casting molds), and; an ethanol solution 90% v/v.
[000100] Silk fibroin solution (8% w/v, 1 ml) was cast on the bottom of two
inverted 60
mm Petri dish and allowed to dry between 2-12 hours. Half of the films were
then
immersed for 2 hours in ethanol solution to induce beta-sheet formation.
Subsequently,
the ethanol treated films were rinsed with deionized water and repositioned on
the
molds. The remaining films ( non-treated, water soluble silk films) were then
deposited
on top of the wet ethanol treated films and the double layered films was
allowed to air
dry for 2-12 hours (Figure 9). Alternatively, a second layer of silk fibroin
solution was
deposited on top of the ethanol treated films, then allowed to dry, to yield
the double
layered self-adherent films.
23
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
[000101] This Example 2 also made use of silk's natural ability to become
water
resistant via physical crosslinking. Through this process, the silk fibroin
protein
undergoes structural rearrangements to a beta-sheet rich conformation.
Temperature,
pH, ionic strength and treatment with polar agents such as alcohols are all
factors
known to induce such structural transitions. For the device made in this
Example 2,
beta sheet formation was induced via ethanol treatment (Figure 10).
[000102] The devices made were smooth, double layered, self-adherent silk film
consisting of a waterproof, physically crosslinked side and a water soluble,
adherent
side. The adhesiveness of the water soluble silk film is responsible for the
cohesiveness of the double layered constructs as it intimately blended with
the surface
of the ethanol treated film. The dried device can be easily handled with dried
gloves or
hands. When applied to a wet or moist surface, the water soluble side of
construct
rehydrates and tightly adheres to the contact surface (Figure 11). The ethanol
treated
side then provides a beta sheet rich, waterproof barrier.
[000103] The film adherence mechanism probably implies structural
rearrangements
of the silk fibroin in which the hydrophilic regions of the protein get
oriented toward and
interact with the hydrophilic regions of the contact surface and analogously,
the
hydrophobic regions of the protein re-orient toward and interact with the
hydrophobic,
beta sheet rich interface of the ethanol treated silk film (Figure 12).
The device can be used for example in: (a) ¨ hemostasis (by attaching it or by
juxtaposing it to bleeding blood vessels) ; (b) ¨ wound dressing (by attaching
it or by
juxtaposing it to superficial wounds); (c) ¨ burn dressings (by substituting
skin grafts) ;
(d) ¨ small defect repair patch (by patching small defects such a tympanic
membrane
holes); (e) ¨ tissue enforcing/supporting patch (by wrapping it against
weakened
tissues, i.e. cervix to prevent pre-term deliveries); or (f) ¨ post-operative
adhesion
barrier (by attaching it to the affected tissue with the "sticky' side, then
the waterproof
side would serve as a barrier to attachment to surrounding tissues). The
versatility of
this device is further highlighted by its transparency ¨ which would enhance
the ability
to control the exact placement of the device; ease of sterilization ¨ since it
can be
sterilely manufactured from autoclaved silk fibroin solution; control over the
thickness
and mechanical strength ¨ since these parameters are dictated by the
concentration of
24
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
the silk solution used and the cast mold area; prolonged stability and cost
effective
manufacturing process.
Example 3
Use of Silk Medical Device in Abdominal Surgery
[000104] Briefly, a hernia is a bulge of intestine, another organ, or fat
through the
muscles of the abdomen, where tissue structure and function is lost at the
load-bearing
muscle, tendon and fascia! layer. Thus, a hernia can occur when there is
weakness in
the muscle wall that allows part of an internal organ to push through. The
silk medical
device within the scope of the present invention can be used to assist in the
repair of
an inguinal (inner groin), incisional (resulting from an incision), femoral
(outer groin),
umbilical (belly button), or hiatal (upper stomach) hernia, using either an
open or
laproscopic technique. A ventral hernia is a type of abdominal hernia ¨ it can
develop
as a defect at birth, resulting from incomplete closure of part of the
abdominal wall, or
develop where an incision was made during an abdominal surgery, occurring when
the
incision doesn't heal properly.
[000105] A silk medical device within the scope of the present invention can
be used
in both open and laparoscopic procedures to assist in the repair of a ventral
hernia as
follows: the patient lies on the operating table, either flat on the back or
on the side,
depending on the location of the hernia. General anesthesia is usually given,
though
some patients can have local or regional anesthesia, depending on the location
of the
hernia and complexity of the repair. A catheter is inserted into the bladder
to remove
urine and decompress the bladder. If the hernia is near the stomach, a gastric
(nose or
mouth to stomach) tube can be inserted to decompress the stomach. In an open
procedure, an incision is made just large enough to remove fat and scar tissue
from the
abdominal wall near the hernia. The outside edges of the weakened hernial area
are
defined and excess tissue removed from within the area. The silk medical
device is
then applied so that it overlaps the weakened area by several inches
(centimeters) in
all directions. Non-absorbable sutures are placed into the full thickness
of the
abdominal wall. The sutures are tied down and knotted.
[000106] In the less-invasive laparoscopic procedure, two or three small
incisions are
made to access the hernia site - the laparoscope is inserted in one incision
and
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
surgical instruments in the others to remove tissue and place the silk medical
device in
the same fashion as in an open procedure. Significantly less abdominal wall
tissue is
removed in laparoscopic repair. The surgeon views the entire procedure on a
video
monitor to guide the placement and suturing of the silk medical device.
Example 4
Anti-Adhesive Silk Medical Devices
This Example 4 details the experiments we carried out to make and characterize
various multi-component, multilayer or fused layers silk (or silk based)
medical devices
("the device" or "the devices"). The devices we made are intended for
implantation in
humans or other mammals in a surgical or medical procedure, such as in a
hernia
repair surgical procedure, to assist in the repair and/or support of various
soft tissues
and prevent, or at least substantial reduce, adhesion formation onto the
implanted
devices or to adjacent tissues. Soft tissue can be tissues that connect,
support, or
surround other structures and organs of a mammalian (and in particular a
human)
body, such as tendons, ligaments, fascia, skin, fibrous tissues, fat, synovial
membranes, connective tissue, muscles, nerves, blood vessels, as well as
various soft
tissue organs such as the breast.
The device is preferably made as a flat sheet. The device can comprise one
layer or
several layers of material. One layer or one side (i.e. the front) of the
device is made of
silk or is silk based, for example it is made of sericin extracted, knitted,
silk fibroin yarn.
When the device comprises only one layer of material the back or bottom side
of the
device has an adhesive property. When the device comprises two layers, the
second
layer on the opposite (i.e. the back side of the second layer) side of the
device
(attached to or fused the bottom side of the first layer) has the anti-
adhesive property.
The first layer can be and is preferably a silk fabric, such as SERI Surgical
Scaffold
(available from Allergan, Inc., Irvine, California). The anti-adhesive
property of the
second layer of a two layer device prevents the second layer once the device
is
abdominally implanted (or subsequent to the implantation of the device where
the
second layer is a sacrificial layer) facing the bowel, from attaching (or
adhering) to the
bowel.
26
CA 02921966 2016-02-19
WO 2015/027144
PCT/US2014/052264
The two layer devices are made by a multiple-step fabrication process, and can
comprise a silk film or a silk fabric or mesh (a suitable and preferred silk
fabric is SERI
Surgical Scaffold), as a first layer of the device, attached to a second layer
which
second layer forms an anti-adhesive barrier layer (when this version of the
second
layer faces the bowel the second layer is made of a biomaterial that does not
promote
cell attachment and proliferation).
Thus, as explained above the silk medical devices we developed have an anti-
adhesive property either because the second layer does not promote cell
attachment
and proliferation or because the second layer is a sacrificial layer.
In this Example 4:
= we carried out two in vitro cell screening assays to determine
characteristics of
various biomaterial substrates to use as the second layer of the device;
= three devices comprising oxidized regenerated cellulose ("ORC") as the
second
were made and characterized in vivo;
= we made fourteen devices;
= we tested in vitro devices which comprised a silk film ("SF") and a
hyaluronic acid
("HA"), an alginate ("ALG"), dextran sulfate ("DS"), a polyethylene glycol
("PEG") or
Pluronico F127 ("F127"), and;
= we made use of film casting and sponge casting technologies, as well an e-
beam
sterilization technique.
Table 1 shows the second layer materials (for a two or multi-layer device) we
examined. Further details of each of these materials are provided in this
Example 4.
G
Anti-adhesive Biomaterial Structure Source properties
==
Low cell attachment
Anionic, linear, non- Bacterial, avian or ¨ due to
Hyaluronic acid (HA)
sulfated polysaccharide mammalian hydrophilicity and
negative charge
27
CA 02921966 2016-02-19
WO 2015/027144
PCT/US2014/052264
Anti-adhesive
Biomaterial Structure Source
properties
=
=
Very low cell
Bacterial(dextran)/ attachment - due to
Anionic, linear, highly
Dextran sulfate (DS) negative charge
sulfated polysaccharide synthetic (sulfated
dextran) inherent to high
sulfate content
Low cell attachment
Anionic, linear, non- due to
Alginate (ALG) Brown algae
sulfated polysaccharide hydrophilicity and
negative charge
Low cell
Polyethylene glycol Hydrophilic, non-ionic
Synthetic attachment¨ due to
(PEG) polymer
hydrophilicity
Cytotoxic at higher
concentration 5%
Hydrophilic, non-ionic
Pluronic F127 (F127) Synthetic v/w), low cell
polymer
attachment due to
hydrophilicity
Fibers supports cell
attachment because
of local 3D
Oxidized regenerated Anionic, linear, oxidized Plants topography, but
cellulose (ORC) polysaccharide ORC rapidly
degrades and can
be used as
"sacrificial layer"
Table 1. Anti-Adhesive Device Second Layer Materials Examined.
Selection of Anti-Adhesive Layer
An in vitro biomaterial screening experiment was carried out to:
- rapidly evaluate the anti-adherence capacities of a large number of
materials;
- identify the most effective anti-adhesive material, and;
- limit the number of devices tested in vivo.
This in vitro screening process involved the use of primary human fibroblasts
("the
cells", which are similar to the cells present at injury/surgery sites), and
assessment of
cell attachment, phenotype, proliferation and overall cell health, when
cultured on
different biomaterials. Thus our screening for suitable anti-adhesive material
involved
28
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
two main components: (a) the cells and (b) substrate biomaterials (the second
layer).
Additionally, the screening process was designed to allow the microscopical
evaluation
of the cells. For this purpose, we chose to prepare and evaluate the selected
biomaterials (the second layer) as thin films, cast in wells of multi-well
tissue culture
plates. Although generally substrates can be presented to cells in a variety
of physical
forms such as gels, films, sponges, spheroids, etc., for our purpose it was
considered
that evaluation of cells on thin films was:
= reflective of the cellular responses to formulation components
= conveniently allow for microscopic cell phenotype evaluation because a
light
beam can easily pass through films
= served the purpose of the pre-screening process by differentiating
between
substrate induced cellular changes
Materials (equivalent materials can also be used)
= 70% (v/v) ethanol solution (Fisher Scientific, cat # 25467025)
= Clorox bleach (Fisher Scientifics, cat # 509387879)
= Human dermal fibroblasts, adult ( PCS-201-012, American Type Culture
Collection (ATCC))
= Fibroblast Basal Medium (ATCC, cat # PCS-201-030)
= Fibroblast Growth Kit-Serum-free (ATCC, cat # PCS-201-040)
= Fetal bovine serum (FBS) (ATCC, cat # 30-2021)
= Penicillin-Streptomycin-Amphotericin B solution (ATCC, cat # PCS-999-002)
= Dulbecco's Phosphate Buffered Saline 1X (DPBS) (ATCC, cat # 30-2200)
= Eppendorf micropipetter set (Fisher Scientific, cat # 13-684-251)
= Filter top bottles (VWR, cat # 154-0020)
29
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
= Kimwipes (Fisher Scientific, cat # 06-666-1A)
= Cell culture flasks (T75 flasks, Fisher Scientific, cat # 10-126-37)
= Cell culture multi-well plates (24 well plates, Fisher Scientific, cat #
08-772-4G)
= Sterile serological pipettes, 1-50 ml (VWR, cat # 89130)
= Sterile aspirating pipettes, 2 ml (VWR, cat # 414004-265)
= Hemacytometer (Fisher Scientific, cat # 02-672-5)
= Cell dissociation reagent (Accutase) (lnvitrogen, cat # A1110501)
= Sterile conical tubes (50 ml) (Fisher Scientific, cat # 07201332)
= LIVE/DEADO Viability/Cytotoxicity Kit, for mammalian cells (Invitrogen,
cat #
L3224)
= Cell proliferation assay (Promega CellTiter 96 Aqueous One Cell
Proliferation
MTS Assay) (Fisher Scientific, cat # PR-G3580)
= Sterile Petri dishes (60 mm diameter) (VWR)
= Deionized water (Siemens (US Filter) RO/DI Water Purification System)
= Parafilm0 Wrap (VWR)
Equipment
= Humidified incubator (New Brunswick Excella E24R, VWR, Bridgeport, NJ)
= Laminar flow hood (SterilGard III Biohood; Allergan # 0116)
= Sterile surgical scissors (VWR)
= Sterile forceps (VWR)
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
Cell culture
Primary human adult fibroblasts (HDFs) were obtained from the American Type
Culture
Collection (the ATCC, Manassas, Virginia USA 20110) and cell cultures were
initiated
as per the ATCC instructions provided. Briefly, fibroblast specific cell
culture media
was prepared in the laminar flow hood, then the cell vial was thawed in a
water bath at
37 C for 1 minute. The cell suspension was then transferred to a T75 culture
flask that
contained 25 ml of culture medium. Cells were then incubated at 37 C and 5%
CO2
and the culture medium was changed every 72 h until cells were needed for
assays or
became ¨80% confluent. When confluent, cells were subcultured in new flasks.
Cells
were propagated for a maximum of 6 passages throughout the duration of the
study
(HDFs have a maximum cycle of 10 propagations).
Biomaterial (second layer) casting methods
Biomaterial films were prepared in the laminar flow hood from sterile filtered
solutions, as described in Table 3. The solution concentrations used were
chosen
based on practical reasons:
= The SF preparation process typically yields solutions with a silk
concentration of
6-8% v/w. Higher of silk in the solution concentrations can be obtained by
further
processing, however, silk fibroin solution gels rapidly at concentrations over
8% v/v
which make its handling difficult.
= HA is a polymeric material with good aqueous solubility, however at
concentrations over 2% w/v the solutions are highly viscous which makes their
handling difficult
= ALG is similar to HA, therefore we chose to use both these
polysaccharides at
2% w/v
= DS and PEG yielded low viscosity solutions at 10% w/v ¨ higher
concentrations
would produce more viscous solutions that could not be sterile filtered
31
CA 02921966 2016-02-19
WO 2015/027144
PCT/US2014/052264
= F127 has a critical gel transition temperature at 25 C when used at 20%
w/v,
therefore this was chosen as stock concentration
Overall, the intent of this experiment was to have the working solution as
concentrated as possible while keeping the viscosities at level that permitted
pipetting,
sterile filtration and transfer.
The volume ratios chosen for formulation screening were based on the need to
obtain silk based solutions that would be physically crosslinkable via beta
sheet
interactions. This requirement ensures that the final scaffolds would not
readily
dissolved when placed in an aqueous environment and that no chemical
crosslinkers
are used in the process.
The film volumes (200 pi/well) was chosen based on the well area ¨ this volume
ensures uniform surface coverage while eliminating capillary tension effect
(thicker film
edges, thin film centers). It also provided minimal interference with the
microscope
light beam.
The crosslinking of the films prepared was performed with ethanol. For
alginate
based films, CaCl2 was added to ethanol, as alginate gels in the presence of
Ca2+ but
is soluble in ethanol. HA, DS, PEG and F127 are also soluble in ethanol,
however the
SF crosslinking process entraps these macromolecules in the silk network even
though
some nano- and micro- scale heterogeneity arises in films because of the
differential
solubility of the components. The crosslinking solution volume (0.5 ml was
chosen
based on the volume of the tissue culture plate wells.
The biomaterial films were prepared as shown in Table 2.
Biomaterial SF/DS SF/DS
SF SF/HA ALG SF/ALG SF/PEG
SF/F127 F127
formulation (HMW) (LMW)
SF, SF, SF, SF, SF, 7.8- SF, 7.8- SF,
Starting SF, 7.8- 7.8- SF, 7.8-
7.8- 7.8- 8.1% 8.10/0 7.8-
concentrations 7.8- 8.1% 8.1% 8.1%
8.1% 8.1% 8.1%
ALG, F127,
(w/v) 8.1% HA, ALG,
2% DS, DS, PEG, F127,
20%
2% 2% 10% 10% 10% 10%
32
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
1:1 1:1
Volume ratios NA 2:1 NA 2:1 8:1 8:1 8:1 8:1 NA
3:1 3:1
Casting
200 ill/well of 24-well tissue culture plate
volume
Drying 18 h in laminar flow
hood
0.5 ml/well of 1:1
0.5 ml/well of v/v of 90% Et0H
Crosslinking 0.5 ml/well of 90% Et0H for 30 min
90% Et0H for 30 and 0.5M CaCl2 NA
procedure
min in DPBS for 30
min
Wash steps 3X with sterile 1X DPBS, 0.5 ml/well
Table 2. Summary of the biomaterial formulations screened for the development
of
anti-adhesive devices and the casting method used.
Film surface investigation
For the screening of biomaterials biological effects, the following
biomaterials
formulations were evaluated: SF; SF/HA (1:1; 2:1 and 3:1 v/v), ALG, SF/ALG
(1:1; 2:1
and 3:1 v/v), SF/DS (8:1 v/v), SF/PEG (8:1 v/v), F127, SF/F127 (8:1 v/v). The
1:1 ratios
were be well stabilized via SF crosslinking. However, higher SF content in the
formulation conferred higher aqueous stability to the final formulation.
For this we
tested formulations with gradually increasing silk amounts. The surfaces of
films cast
as above were investigated microscopically at 100X magnification.
SF films had a smooth surface with cracks that originated most likely during
the
physical crosslinking process. SF/HA formulations showed a heterogeneous
surface,
most likely due to the fact that HA is insoluble in ethanol and tends to fall
out of solution
during the physical crosslinking process of silk. ALG (alginate) undergoes
crosslinking
in the presence of Ca2+ . This caused the film to wrinkle and detach form the
edges of
the well. Due to the presence of the ethanol, needed to ensure similar
treatment of all
wells and also as an added measure of sterility, some amounts of alginate
appeared to
fall out of solution, similar to HA. SF/ALG formulations showed a
heterogeneous
surface, most likely due to the fact that ALG is insoluble in ethanol and
tends to fall out
33
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
of solution during the physical crosslinking process of silk. SF/PEG films
were smooth
due to the presence of PEG, which acts as a plasticizer and reduces the inter-
and
intra-molecular tension between silk molecules during the physical
crosslinking
process. The
SF/DS films were smooth with some crater-like irregularities, most likely
generated by
the differences in solubility between SF and DS. F127 poloxamer, at
concentrations of
15% w/v and above, gels at room temperature and showed a smooth surface. F127
is
however soluble in ethanol and some material most likely washed off during the
ethanol treatment. The SF/F127 films surfaces were heterogeneous. F127 was
expected to act as plasticizer, however the differences in solubility between
SF and
F127 are probably the cause for the observed surface irregularities.
Cell Attachment Evaluation
In the context of the development of a device with or with a layer of the
device that has
an anti-adhesive property, cell attachment was evaluated as a primary
indicator of the
device or the layer's anti-adhesive efficiency (the lower the cell attachment
the better
the anti-adhesive properties of the biomaterial). Primary human dermal
fibroblasts
(adult, HDF) passage 5 were cultured on the biomaterial films made at a
density of
2x105 cells/ml corresponding to 5000 cells/well in 2500 culture medium. The
cell
seeding concentration was chosen based on the culture surface area, the HDF
proliferation pattern observed during cell culturing and assay duration (slow
proliferating cells would be seeded at high numbers, while fast proliferating
cells would
be seeded at low numbers to avoid contact inhibition issues at longer than 24
h (hour)
assay time points). Cell morphology and attachment were visually assessed
after 24
hours and 6 days incubation.
On the tissue culture plate ("TCP") control , HDFs show the fibroblast
specific, spindle-
shaped morphology, both at 24 h and at 6 days. The 6 day data revealed a
healthy cell
phenotype with good proliferation. This data set represented our positive
control:
because the TCP surface is designed to support and promote cell attachment and
viability (ATCC animal cell culture guide). For
all our anti-adhesive device
formulations, we targeted lower cell attachment than that observed on the TCP.
34
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
The 24 h data images were representative for the observed phenotypes on the
entire
film surface and revealed atypical fibroblast phenotypes on all formulations,
with SF,
SF/PEG and SF/DS films still induced elongated, somewhat spindle-like
phenotypes,
but the overall cell morphology was different than on TCP showing that cell
attachment
was impaired, as desired. SF/HA and SF/ALG prevented cell attachment to the
point
where cells were rounded and clustered together.
The 6 day data revealed further cellular changes. On SF, cells were covering
the
surface unevenly and were anchored to few attachment points most probably
corresponding to cracks in the film surface. This showed that SF enhanced anti-
adhesive properties compared to the TCP. On SF/HA some cell spreading was
noticed, however the surface coverage appeared to be less than the TCP
control, as
estimated via microscopic evaluation. SF/ALG and SF/DS prevented cell
spreading
and a few rounded cell clusters were observed on the surface of these
biomaterials.
Significantly, all the biomaterial formulations we made and evaluated showed
decreased cell attachment and surface coverage as compared to the TCP control.
This showed that each of the chosen second layer materials evaluated can be
used as
the anti-adhesive layer of the device. It is important to note that although
referred to
above as a second layer, the biomaterials used were in fact fused to the silk
film layer
(SF) used. The anti-adhesive second layer can alternately be attached or fused
to a
first layer which is in the form of a silk fabric or a silk mesh.
Cell Viability Assay
The cell attachment assay offered a visual assessment of the desired anti-
adhesive/cell-repellant properties of different biomaterial formulations. In
addition to
this feature, it was important to evaluate the actual effects of SF and the
second layer
materials ("additives" or "biomaterials") on cell viability.
The cytocompatibility of biomaterials was evaluated after 48 h and 6 day
incubation
period. For this, a LIVE/DEAD cytotoxicity kit was used. This kit has two
components:
fluorescein (green fluorescence) - a dye that binds to the membrane of intact,
live cells;
and ethidium homodimer (red fluorescence) ¨ a nucleic acid specific dye that
binds to
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
the nucleus of damaged/dead cells, but cannot permeate the membrane of healthy
cells.
Post-plating, the duration of cell attachment is dependent on the cell type
and
substrate, and might take up to 24 h to complete (ATCC animal cell culture
guide).
Therefore, the 48 h time point was chosen as it is the earliest point that
would allow
evaluation of substrate related cytotoxic effects after cell attachment has
occurred. The
6 day time point was chosen to evaluate the longer term cytocompatibility of
the
substrates as the potential effects of additive leaching was expected to be
detectable at
this time point (after 6 days, cells on some substrates reached confluence,
therefore
we chose not to investigate later time points). Cell viability on TCP was used
as the
positive control.
At both time points all the second layer films we had prepared showed minimal
cytotoxic effects, with cell viability exceeding 95% as determined by
microscopic
evaluation. The only second layer material the appeared to induce cell death
was
pluronic F127 as stand-alone formulation. We also noted that based on the
substrate
(second layer) formulations, the cells had different phenotypes ¨ a more
rounded
appearance indicative of lower attachment while a spindle-like phenotype was
indicative of better attachment, comparable to the control.
In summary, the cytocompatibility assay showed that all the tested second
layers were
cytocompatible and did not induce cell death. This showed that SF and the
tested
additives (the second layer materials) can be used in the device.
Cell Proliferation Assay
Another method we used to evaluate the affinity of cells to a surface and the
cell-
substrate interaction was to perform a cell proliferation assay (MTS assay).
This assay
relied on the cell mediated enzymatic reduction of a soluble methyl
tetrazolium salt
(MTS) to its reduced, colored format, therefore eliminating the possibility of
any
artifacts or false positives. This enzymatic reduction process gave a direct
correlation
between the number of living cells on a surface and the color intensity of the
reduced
MTS.
36
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
The cell numbers present on the screened biomaterial surfaces were evaluated
at 48 h
and 6 days post incubation. The 48 h time point was chosen as an early
indicator of
cellular affinity to the films, however the 6 day readings were more
representative since
the assay is sensitive to the overall cell number and yields better results
for higher cell
densities, such as those observed at later incubation time points (Figure 13).
At 48 h,
the A450 values were lower than those observed at 6 days. This was consistent
with a
lower initial cell number/well correlating with attachment differences. As
attached cells
proliferate and their number increases in the test well, when assayed, the
intensity of
the colorimetric reagent increases and this translates into higher A450
values, as seen
at day 6. Nevertheless, the 48 h and 6 day data trends correlated well and
supported
earlier observations indicating that all screened biomaterial formulations
(the second
layer) showed anti-adhesive potential as cell attachment was 50%
lower on all
formulations compared to the TCP control.
Device Formulation Screening
The results of the aforementioned cell screening data showed that the chosen
formulations (second layer materials) had the desirable anti-adhesive feature.
We
wished to minimize the amount of additives (second layer material) in order to
maintain
the silk (first layer) characteristic physical crosslinking and to avoid any
potential or
unknown negative interactions these might cause so we therefore additionally
screened the formulations (second layer materials) with increased silk
content. For SF
as the first layer with five different second layer materials, Table 3
summarizes the five
tested SF/additive formulations and the results are illustrated in the five
Figure 14 bar
graphs.
Biomaterial
SF/HA SF/ALG SFIDS SF/PEG SF/F127
formulation
1
3:1 3:1 8:1 8:1 8:1
5:1 5:1 10:1 10:1 10:1
Volume ratios
8:1 8:1 15:1 15:1 15:1
(v/v)
15:1 15:1 20:1 20:1 20:1
20:1 20:1 25:1 25:1 25:1
37
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
Table 3. Summary of SF/additive formulations tested in order to minimize the
amount
of additives while maintaining the biological properties.
The biomaterial (second layer) films were prepared as described above. HDF
cells
were plated at a density of 2x105 cells/well and incubated for 24 h before
assayed for
cell number (MTS assay). A higher cell seeding density was chosen for this
short
duration assay in order to maximize assay sensitivity. For SF/HA formulations,
the data
showed that the 3:1 volume ratio yielded the best biological outcome
(equivalent to the
lowest cell concentration) and that decreasing the HA amount in the
formulation can
increase cell adhesion. However, during the device casting, the sponge surface
appeared to shed upon rubbing. Therefore, a 10:1 SF to HA ratio was chosen for
device evaluation since it was the highest HA containing formulation that
yielded a
robust sponge surface. For SF/ALG the 20:1 ratio yielded similar biological
effects to
higher ALG ratios, therefore devices were prepared with 20:1 SF to ALG. For
SF/PEG
the 8:1 ratio produced the best biological outcomes, therefore devices were
prepared
with 8:1 SF to PEG. For SF/F127, when F127 was used at 10% w/v concentration,
the
cell adherence was similar on all formulations. When 20% w/v F127 was used, at
an
8:1 volume ratio, the cell repellent effects were more pronounced than for its
10% w/v
counterpart. However, because of cytotoxicity concerns as evident from cells
seeded
pure F127, devices were made with 8:1 SF to F127 (10% w/v). For SF/DS, the
tested
formulations elicited a clear dose response, with cell attachment being the
lowest in the
presence of the highest amount of DS (corresponding to the 8:1 SF/DS
formulation). In
our assays DS showed good biocompatibility. Since
all ratios were more anti-
adhesive than the SF control, we made devices with a 15:1 SF to DS ratio, to
minimize
the amount of additive but still maintain the significantly increased anti-
adherent
properties.
Device Preparation
Materials
= SERI Surgical Scaffold (Allergan), a knitted silk mesh.
38
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
= Silk fibroin solution (prepared as set forth in International Patent
Application
WO/2010/123945; see paragraph [0011], ibid.)
= Silk yarn (9-filament)
= HA, high molecular weight (HMW) (intrinsic viscosity 2.84 m3/kg)
= HA, low molecular weight (HMW) (intrinsic viscosity 0.41 m3/kg)
= DS (HMW) (Sigma, cat # 67578, lot #, MW 200 kDa)
= DS (LMW) (Sigma, cat # 42867, lot # BCBK1677V, MW 40 kDa)
= ORC as Surgicel SNoW absorbable hemostat (ref # 2083, lot ELB5821,
Ethicon)
= ORC as Surgicel NuKnit absorbable hemostat(ref # 1946, lot # 3650291,
Ethicon)
= ORC as Surgicel Fibrillar absorbable hemostat (ref #1963, lot # 3653407,
Ethicon)
= ORC as Surgicel Original absorbable hemostat(ref # 1952, lot # 3649196,
Ethicon)
= ALG (NovaMatrix ProNova, SLG100, lot # 271108/3)
= PEG (Alfa Aesar, cat # 43443, lot # J04Y009, MW 8 kDa)
= F127 (Sigma, cat # P2443, lot # 5LBC8439V). F127 and Pluronic F127 is an
ethylene oxide, propylene oxide block copolymer.
= Conical tubes (50 ml, Fisher Scientific, cat # 339653)
= Petri dishes (100 mm diameter, VWR, cat # 25384-342)
= Square dishes (110 mm x 15 mm, VWR, 100501-176)
= OmniTray (Nunc 86x128mm, Fisher Scientific, cat # 242811)
= Cleanroom Wipes (Berkshire, DR670.1212.20)
= Ethanol (100%, Fisher Scientific, cat # 50-980-460)
39
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
Equipment
= Freezer (-80 C) (VWR Model 5708)
= Lyophilizer (VirTis Model Benchtop K)
= Water bath (Thermo Fisher Precision, Fisher Scientific)
= Laminar flow hood (SterilGARDI1Advance, The Baker Company)
= Incubator (Forma Scientific Model 3326, Fisher Scientific)
= Centrifuge (Eppendorf Model 5804, Fisher Scientific)
= Refrigerator (NorLake Scientific, Fisher Scientific)
= Bright field microscope (Leica Model DMI3000B)
= Vacuum oven (VVVR Model 1410)
= Stainless steel scissors (VWR)
= Tweezers (VVVR)
= Lead rings (Fisher Scientific, cat # 22-260-103)
= Sewing machine (JUKI Corporation Model DDL-5530N)
= Sterilizations pouches metallic (MPPE) (PeelMaster Medical Packaging,
item #
1854-024)
= Sterilization pouches foil (PPFP) (PeelMaster Medical packaging, item #
1854-024)
= Pouch sealer (Accu-Seal Model 630)
= Self-seal sterilization pouches (VWR, cat # 89140-800)
= Flatiron (Black & Decker, cat # A5870 Type 1)
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
General Considerations
The devices were with the second layers selected based on the results set
forth above.
Depending on the properties, some formulations were prepared as films and some
as
sponges. Biomaterial (second layer) mixes that yielded homogeneous
formulations,
with good pliability were cast as films (SF/PEG and SF/F127), while based on
the same
considerations, sponges appeared to be a more suitable option for
heterogeneous
materials such as SF/HA, SF/ ALG and SF/DS. Films fused with silk mesh were
easily
sutured as films and were transparent, however the sponges fused with silk
mesh
appeared to have more robustness during handling (film devices can delaminate
when
crumpled in hand, while crumpling was not an issue with the sponges made). For
certain devices made additional composition adjustment were made to improve
their
processability.
For sterilization, some devices were processed dry, with ethylene oxide, while
other
were processed moist, with e-beam sterilization. The intent was to sterilize
all samples
dry, however, with film and certain sponges, drying caused curling and
cracking of the
scaffold. Based on this, we chose to process films and sponges moist, sealed
in
pouches with moisture barrier. Since ethylene oxide cannot be used with such
pouches, samples were processed via e-beam treatment. The sterilization of all
devices was performed with standard sterilization cycles and parameters and no
additional sterility control was performed on any of the devices.
Process
Device 1
Description: SERI Surgical Scaffold fused with Surgicel SNoW (ORC) (6x6 cm)
Execution: Sterile SERI Surgical Scaffolds were cut in the laminar flow hood
with
sterile stainless steel scissors into 6 x 6 cm squares. Similarly, Surgicel
SNoW were
cut in the laminar flow hood with sterile stainless steel scissors into 6 x 6
cm squares.
Autoclaved silk fibroin solution (c = 7.5% w/v) was used to mount Surgicel
SNoW onto
the mesh. Specifically, 2 ml of silk solution were added to the lid of a
sterile 100 cm
Petri dish and was evenly spread with a sterile pipette tip. The mesh was then
placed
in the dish until its surface was uniformly wet. The mesh was transferred onto
a
41
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
Surgicel SNoW square and pressed down with sterile tweezers for 1 minute. All
assembled devices were then allowed to dry for 1 h in the laminar flow hood
then
ethanol treated (100% v/v) for 30 minutes. The ethanol was then allowed to
evaporate
and devices were individually washed with 150 ml of sterile PBS. The washing
step
was done by using a vacuum filtration flask - the device was laid flat on the
top filter,
the filter was connected to vacuum and 15 ml of PBS were poured onto the
device. The
vacuum helped remove most of the PBS from the devices. The prototypes were
then
further dried on the laminar flow hood for 12 h in partially covered sterile
rectangular
plates (OmniTrays).
Sterilization: these devices were assembled in the laminar flow hood from
sterile
starting materials. No additional sterilization was performed.
Packaging/Storage: Devices prepared as above were places in autoclaved
containers
and covered with sterile PBS. They were kept under ambient conditions for 24 h
before
use.
Testing: Device 1 was used as "wet lab" material to consolidate the surgical
procedure.
No additional testing was performed.
Observations: partial delamination of the two layers was observed for some of
the
device 1 samples made.
Device 1A
Description: SERI Surgical Scaffold sewn with Surgicel SNoW (ORC) (6x6 cm)
Execution: Non-sterile SERI Surgical Scaffolds were cut with sterile
stainless steel
scissors into 6 x 6 cm squares. Similarly, Surgicel SNoW were cut under non-
sterile
conditions with stainless steel scissors into 6 x 6 cm squares. For each
device one
mesh square was sewn with a sewing machine to one SNoW square by using
extracted 9-filament silk yarn.
Sterilization: devices were placed in self-sealing pouches and ethylene oxide
(EO)
sterilized.
42
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
Packaging/Storage: Devices 1A prepared were placed in self-sealing
sterilization
pouches, were EO sterilized then aerated for at least 3 day prior use. During
the
aeration period, devices were kept under environmental conditions.
Testing: Prototype 1A was tested in vivo
Device 2
Description: SERI Surgical Scaffold fused with Surgicel NuKnit (ORC) (6x6
cm)
Execution: Sterile SERI Surgical Scaffolds were cut in the laminar flow hood
with
sterile stainless steel scissors into 6 x 6 cm squares. Similarly, Surgicel
NuKnit were
cut in the laminar flow hood with sterile stainless steel scissors into 6 x 6
cm squares.
Autoclaved silk fibroin solution (c = 7.5% w/v) was used to mount Surgicel
NuKnit
(patterned side up) onto the mesh. Specifically, 1 ml of silk solution was
added to the
lid of a sterile 100 cm Petri dish and was evenly spread with a sterile
pipette tip (the
amount of silk used for fusing the two layers was reduced to 1 ml in this case
as
dipping of the mesh into 2 ml of silk caused wetting of NuKnit and impaired
the fusion
of the layers). The mesh was then placed in the dish until its surface was
uniformly wet.
The mesh was transferred onto a NuKnit square and pressed down by rolling the
bottom of a Petri dish on its side. All assembled devices were then allowed to
dry for 1
h in the laminar flow hood then ethanol treated (100% v/v) for 30 minutes. The
ethanol
was then allowed to evaporated and devices were individually washed with 150
ml of
sterile PBS. The washing step was done by using a vacuum filtration flask -
the device
was laid flat on the top filter, the filter was connected to vacuum and 15 ml
were poured
onto the device. The vacuum helped remove most of the PBS from the devices.
The
prototypes were then further dried on the laminar flow hood for 12 h in
partially covered
sterile rectangular plates (OmniTrays).
Sterilization: these devices were assembled in the laminar flow hood from
sterile
starting materials. No additional sterilization was performed.
Packaging/Storage: Devices prepared as above were places in autoclaved
containers
(see image above) and covered with sterile PBS. They were kept under ambient
conditions for 24 h before use.
43
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
Testing: Prototype 2 was used as "wet lab" material to consolidate the
surgical
procedure. No additional testing was performed.
Observations: partial delamination of the two layers was observed for some of
the
Device 2 samples.
Device 2A
Description: SERI Surgical Scaffold sewn with Surgicel NuKnit (ORC) (6x6 cm)
Execution: Non-sterile SERI Surgical Scaffolds were cut with sterile
stainless steel
scissors into 6 x 6 cm squares. Similarly, Surgicel NuKnit were cut under non-
sterile
conditions with stainless steel scissors into 6 x 6 cm squares. Based on the
tightly knit
pattern of the ORC, the sacrificial layer of Prototype 2A is expected to
degrade slower
than that of Prototype 1A. For each device one mesh square was sewn with a
sewing
machine to one NuKnit square (patterned side up) by using extracted 9-filament
silk
yarn.
Sterilization: devices were placed in self-sealing pouches and ethylene oxide
(EO)
sterilized.
Packaging/Storage: Devices prepared as above were placed in self-sealing
sterilization
pouches, were EO sterilized then aerated for at least 3 day prior use.
Testing: Device 2A was tested in vivo.
Device 3
Description: SERI Surgical Scaffold sewn with Surgicel Fibrillar (2 sheets)
and
Surgicel Original (ORC) (6x6 cm)
Execution: Non-sterile SERI Surgical Scaffolds were cut with sterile
stainless steel
scissors into 6 x 6 cm squares. Similarly, Surgicel Fibrillar and Surgicel
Original
44
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
were cut under non-sterile conditions with stainless steel scissors into 7 x 7
cm
squares. For each device one mesh square was sewn with a sewing machine to two
sheets of Surgicel Fibrillar and topped with one layer of Surgicel Original
by using
extracted 9-filament silk yarn. The combination of the two ORC materials
ensured a
thicker sacrificial layer that could potentially degrade at a slower rate than
that of
Device 1A or Device 2A. The assembled device was then trimmed to a size of 6x6
cm.
Sterilization: devices were placed in self-sealing pouches and ethylene oxide
(EO)
sterilized.
Packaging/Storage: Devices prepared as above were placed in self-sealing
sterilization
pouches, were EO sterilized then aerated for at least 3 day prior use.
Testing: Device 2A was tested in vivo.
Device 4 and Device 5 were silk based control devices (SBR-202 and SERI 3D).
Device 6
Description: SERI Surgical Scaffold fused with SF/PEG film (6x6 cm)
Execution: Non-sterile SERI Surgical Scaffolds were cut with stainless steel
scissors
into 6 x 6 cm squares. Separately, silk fibroin solution (c = 8.1 % w/v) was
mixed with
PEG (c = 10% w/v) in a 8:1 volume ratio then the mix was homogenized by
pipetting up
and down. The solution (8 ml) was cast in 10 cm square Petri dish bottoms and
dried in
the vacuum oven for 18 h. Dried films in dishes were then treated with 6 ml of
ethanol
(100% v/v) for 5 min. Films were then removed from dishes and briefly hydrated
by a 5
second dip in deionized water, followed by 5 second dip in 90% v/v ethanol.
Subsequently, films were placed face down (the side that was exposed to air
during
drying) and stretched on the lid of a 100 mm Petri dish then allowed to dry
flat with a
Petri dish bottom and a lead ring sitting on top.
Silk fibroin solution (c = 8.1% w/v) was used to mount the mesh onto the films
("c"
means "concentration"). Specifically, 2 ml of silk solution were added to the
lid of a
sterile 100 cm Petri dish and were evenly spread with a sterile pipette tip.
The mesh
was placed in the dish until its surface was uniformly wet. The mesh was then
added
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
onto the dried film and smoothed down with gloved fingers to ensure uniform
surface
attachment. The constructs were dried flat with the bottom of a 100mm Petri
dish and
a lead ring resting on top for 15 minutes. The devices were then placed in 90%
ethanol
for 10 minutes, blotted, then placed in deionized water for 5 minutes for
first wash. After
the first wash, films were trimmed down to the size of the 6x6cm mesh and then
placed
into second wash for 5 minutes. The devices were washed one more time then
pouched.
Sterilization: the devices were placed in metallized peelable polyester
polyethylene
film (MMPE) and paper polyethylene foil polyethylene barrier (PPFP) pouches,
sealed
using Accu-Seal Sealer Model 630, and e-beam sterilized.
Packaging/Storage: The devices prepared as above were placed in PPFP pouches,
heat sealed and e-beam sterilized. The devices were then kept in pouches and
stored.
Testing: The devices in both MMPE and PPFP pouches were examined two weeks
after sterilization for overall integrity, delamination, pliability and
suturability.
Observations: Films remained moist during and after sterilization. Devices
appeared to
have good pliability but when crumpled in hand, the films separated from the
mesh.
Devices were easy to suture through as the films are transparent and mesh
pores are
clearly visible.
Device 7
Description: SERI Surgical Scaffold fused with SF/F127 film (6x6 cm)
Execution: Non-sterile SERI Surgical Scaffolds were cut with stainless steel
scissors
into 6 x 6 cm squares. Separately, silk fibroin solution (c = 8.1 % w/v) was
mixed with
F127 (c = 10% w/v) in a 8:1 volume ratio then the mix was homogenized by
pipetting
up and down. The solution (8 ml) was then cast in 10 cm square Petri dish
bottoms
and dried on the bench top for 26 h.
Silk fibroin solution (c = 8.1% w/v) was used to mount the mesh onto the
films.
Specifically, 2 ml of silk solution were added to the lid of a sterile 100 cm
Petri dish
and were evenly spread with a sterile pipette tip. The mesh was placed in the
dish until
46
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
its surface was uniformly wet. The mesh was then added onto the dried film and
smoothed down with gloved fingers to ensure uniform surface attachment. The
construct was dried flat on bench top for 45 minutes, then placed in 90%
ethanol for 45
minutes. Subsequently, the prototype was placed in 1L deionized water for 1h
then
dried on bench top. The drying process caused films to shrink, curl and detach
from the
mesh. Out of seven constructs prepared, two appeared well fused and smooth and
were sent for sterilization.
Sterilization: devices were placed in self-sealing pouches and ethylene oxide
(EO)
sterilized.
Packaging/Storage: Sterile devices were kept in pouches for under
environmental
conditions
Testing: Pouches were opened in the laminar flow hood and prototypes were
assessed for integrity and biological properties in vitro.
Observations: Devices remained intact during and after sterilization and when
tested
for cell adherence, the results were comparable to the pre-sterilization data
indicating
that EO sterilization did not alter the device's biological properties.
Device 7A
Description: SERI Surgical Scaffold fused with SF/F127 film (6x6 cm)
Execution: Non-sterile SERI Surgical Scaffolds were cut with stainless steel
scissors
into 6 x 6 cm squares. Separately, silk fibroin solution (c = 8.1 % w/v) was
mixed with
F127 (c = 10% w/v) in a 8:1 volume ratio then the mix was homogenized by
pipetting
up and down. The solution (6 ml) was then cast in 10 cm square Petri dish
bottoms and
dried in the vacuum oven for 18 h. Dried films in dishes were then treated
with 6 ml of
ethanol (100% v/v) for 5 min. Films were then removed from dishes and briefly
hydrated by a 5 second dip in deionized water, followed by a 5 second dip in
90% v/v
ethanol. Subsequently, films were placed face down (the side that was exposed
to air
during drying) and stretched on the lid of a 100 mm Petri dish then allowed to
dry flat
with a Petri dish bottom and a lead ring sitting on top.
47
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
Silk fibroin solution (c = 8.1% w/v) was used to mount the mesh onto the
films.
Specifically, 2 ml of silk solution were added to the lid of a sterile 100 cm
Petri dish
and were evenly spread with a sterile pipette tip. The mesh was placed in the
dish until
its surface was uniformly wet. The mesh was then added onto the dried film and
smoothed down with gloved fingers to ensure uniform surface attachment.
Constructs
were dried flat with the bottom of a 100mm Petri dish and a lead ring resting
on top for
15 minutes. Prototypes were then placed in 90% ethanol for 10 minutes,
blotted, then
placed in deionized water for 5 minutes for first wash. After the first wash,
films were
trimmed down to the size of the 6x6cm mesh and then places into second wash
for 5
minutes. Prototypes were washed one more time then pouched.
Sterilization: Devices were placed in metallized peelable polyester
polyethylene film
(MMPE) and paper polyethylene foil polyethylene barrier (PPFP) pouches, sealed
using Accu-Seal Sealer Model 630, and e-beam sterilized
Packaging/Storage: Devices prepared as above were placed in PPFP pouches, heat
sealed and e-beam sterilized. Devices were then kept in pouches and stored.
Testing: Devices in both MMPE and PPFP pouches were examined two weeks after
sterilization for overall integrity, delamination, pliability and
suturability.
Observations: Films remained moist during and after sterilization. Devices had
good
pliability but when crumpled in hand, the films separated for from the mesh.
Devices
were easy to suture through as the films are transparent and mesh pores are
clearly
visible.
Device 8
Description: SERI Surgical Scaffold fused with SF
Execution: Non-sterile SERI Surgical Scaffolds were cut with stainless steel
scissors
into 6 x 6 cm squares. Separately, silk fibroin solution (c = 8.1 % w/v) was
mixed with
HA (LMW, c = 2% w/v) in a 3:1 volume ratio then the mix was homogenized by
pipetting up and down. To obtain a sponge-like biomaterial, the solution (15
ml) was
then cast in OmniTray lids and put into the -80 C freezer for two hours.
Frozen
48
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
samples were lyophilized for 24 hours to dry, then treated with 15 ml of
ethanol (100%
v/v) for 45 min. Sponges were then removed from the tray, the edges were cut
off ,
then were returned to the tray for an additional 30 minutes ethanol
incubation.
Subsequently, sponges were dried flat covered with OmniTray lids and lead
rings.
Silk fibroin solution (c = 8.1% w/v) was used to mount the mesh onto the
films.
Specifically, 2 ml of silk solution were added to the lid of a sterile 100 cm
Petri dish
and were evenly spread with a sterile pipette tip. The mesh was placed in the
dish until
its surface was uniformly wet. The mesh was added onto the dried sponge (to
the side
that contacted the tray while freezing) then smoothed down with gloved fingers
to
ensure uniform surface attachment. Prototypes were dried for 1 hour, then
placed in
90% ethanol for 30 minutes and blotted. Subsequently, constructs were placed
in
deionized water for 5 minutes for first wash. After the first wash, sponges
were trimmed
down to the size of the 6x6cm mesh and then placed into second wash for 5
minutes.
Prototypes were washed one more time then pouched.
Sterilization: Devices were placed in MMPE and PPFP pouches, sealed and e-beam
sterilized.
Testing: Devices in both MMPE and PPFP pouches were examined two weeks after
sterilization for overall integrity, delamination, pliability and
suturability.
Observations: Devices remained moist during and after sterilization. The
devices had
good pliability and did not delaminate when crumpled in hand. However, the
sponge
side appeared to shed when rubbed with gloved hands. The devices were easy to
suture through.
Device 8A
Description: SERI Surgical Scaffold fused with SF/HA sponge (6x6 cm)
Execution: Non-sterile SERI Surgical Scaffolds were cut with stainless steel
scissors
into 6 x 6 cm squares. Separately, silk fibroin solution (c = 8.1 % w/v) was
mixed with
HA (LMW, c = 2% w/v) in a 10:1 volume ratio then the mix was homogenized by
pipetting up and down. To obtain a sponge-like biomaterial, the solution (15
ml) was
49
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
then cast in OmniTray lids and put into the -80 C freezer for two hours.
Frozen
samples were lyophilized for 24 hours to dry, then treated with 15 ml of
ethanol (100%
v/v) for 45 min. Sponges were then removed from the tray, the edges were cut
off ,
then were returned to the tray for an additional 30 minutes ethanol
incubation.
Subsequently, sponges were dried flat covered with OmniTray lids and lead
rings. The
sponge can be viewed as a particular type of film (a sponge like film).
Silk fibroin solution (c = 8.1% w/v) was used to mount the mesh onto the
films.
Specifically, 2 ml of silk solution were added to the lid of a sterile 100 cm
Petri dish
and were evenly spread with a sterile pipette tip. The mesh was placed in the
dish until
its surface was uniformly wet. The mesh was added onto the dried sponge (to
the side
that contacted the tray while freezing) then smoothed down with gloved fingers
to
ensure uniform surface attachment. Prototypes were dried for 1 hour, then
placed in
90% ethanol for 30 minutes and blotted. Subsequently, constructs were placed
in
deionized water for 5 minutes for first wash. After the first wash, sponges
were trimmed
down to the size of the 6x6cm mesh and then placed into second wash for 5
minutes.
The devices were washed one more time then pouched.
Sterilization: Devices were placed in MMPE and PPFP pouches, sealed and e-beam
sterilized.
Packaging/Storage: Devices were then kept in pouches and stored in a plastic
bin
under environmental conditions.
Testing: Devices in both MMPE and PPFP pouches were examined two weeks after
sterilization for overall integrity, delamination, pliability and
suturability.
Observations: The devices remained moist during and after sterilization, had
good
pliability and did not delaminate when crumpled in hand. This specific SF/HA
formulation yielded sponges that did not shed when rubbed with gloved hands
and the
devices were easy to suture through.
Device 9
Description: SERI Surgical Scaffold fused with SF/DS sponge (6x6 cm)
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
Execution: Non-sterile SERI Surgical Scaffolds were cut with stainless steel
scissors
into 6 x 6 cm squares. Separately, silk fibroin solution (c = 8.1 % w/v) was
mixed with
DS (LMW, c = 10% w/v) in a 15:1 volume ratio then the mix was homogenized by
pipetting up and down. To obtain a sponge-like biomaterial the solution (15
ml) was
then cast in OmniTray lids and put into the -80 C freezer for two hours.
Frozen
samples were lyophilized for 24 hours to dry, then treated with 15 ml of
ethanol (100%
v/v) for 45 min. Sponges were then removed from the tray, the edges were cut
off ,
then were returned to the tray for an additional 30 minutes ethanol
incubation.
Subsequently, sponges were dried flat covered with OmniTray lids and lead
rings. The
sponge can be viewed as a particular type of film (a sponge like film).
Silk fibroin solution (c = 8.1% w/v) was used to mount the mesh onto the
films.
Specifically, 2 ml of silk solution were added to the lid of a sterile 100 cm
Petri dish
and were evenly spread with a sterile pipette tip. The mesh was placed in the
dish until
its surface was uniformly wet. The mesh was added onto the dried sponge (to
the side
that contacted the tray while freezing) then smoothed down with gloved fingers
to
ensure uniform surface attachment. Prototypes were dried for 1 hour, then
placed in
90% ethanol for 30 minutes and blotted. Subsequently, the devices were placed
in
deionized water for 5 minutes for first wash. After the first wash, sponges
were trimmed
down to the size of the 6x6cm mesh and then placed into second wash for 5
minutes.
The devices were washed one more time then pouched.
Sterilization: Devices were placed in metallized peelable polyester
polyethylene film
(MMPE) and paper polyethylene foil polyethylene barrier (PPFP) pouches, sealed
using Accu-Seal Sealer Model 630, and e-beam sterilized.
Packaging/Storage: Devices prepared as above were placed in PPFP pouches,
sealed
using Accu-Seal Sealer Model 630, and e-beam sterilized. Devices were then
kept in
pouches and stored under ambient conditions.
Testing: Devices in both MMPE and PPFP pouches were examined two weeks after
sterilization for overall integrity, delamination, pliability and
suturability.
51
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
Observations: The devices remained moist during and after sterilization, had
good
pliability and did not delaminate when crumpled in hand. The sponges did not
shed
when rubbed with gloved hands and the devices were easy to suture through.
Device 10
Description: SERI Surgical Scaffold fused with SF/ALG sponge (6x6 cm)
Execution: Non-sterile SERI Surgical Scaffolds were cut with stainless steel
scissors
into 6 x 6 cm squares. Separately, silk fibroin solution (c = 8.1 % w/v) was
mixed with
ALG (c = 2% w/v) in a 20:1 volume ratio then the mix was homogenized by
pipetting up
and down. To obtain a sponge-like biomaterial the solution (15 ml) was then
cast in
OmniTray lids and put into the -80 C freezer for two hours. Frozen samples
were
lyophilized for 24 hours to dry, then treated with 15 ml of ethanol (100% v/v)
for 45 min.
Sponges were then removed from the tray, the edges were cut off, then were
returned
to the tray for an additional 30 minutes ethanol incubation. Subsequently,
sponges
were dried flat covered with OmniTray lids and lead rings. The sponge can be
viewed
as a particular type of film (a sponge like film).
Silk fibroin solution (c = 8.1% w/v) was used to mount the mesh onto the
films.
Specifically, 2 ml of silk solution were added to the lid of a sterile 100 cm
Petri dish
and were evenly spread with a sterile pipette tip. The mesh was placed in the
dish until
its surface was uniformly wet. The mesh was added onto the dried sponge (to
the side
that contacted the tray while freezing) then smoothed down with gloved fingers
to
ensure uniform surface attachment. Prototypes were dried for 1 hour, then
placed in
90% ethanol for 30 minutes and blotted. Subsequently, constructs were placed
in
deionized water for 5 minutes for first wash. After the first wash, sponges
were trimmed
down to the size of the 6x6cm mesh and then placed into second wash for 5
minutes.
The devices were washed one more time then pouched.
Sterilization: Devices were placed in metallized peelable polyester
polyethylene film
(MMPE) and paper polyethylene foil polyethylene barrier (PPFP) pouches, sealed
using Accu-Seal Sealer Model 630, and e-beam sterilized.
52
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
Packaging/Storage: Devices prepared as above were placed in PPFP pouches,
sealed
using Accu-Seal Sealer Model 630, and e-beam sterilized. The devices were then
kept
in pouches and stored under ambient conditions.
Testing: in both MM PE and PPFP pouches were examined two weeks after
sterilization
for overall integrity, delamination, pliability and suturability.
Observations: Devices remained moist during and after sterilization. Devices
appeared
to have good pliability and did not delaminate when crumpled in hand. The
sponges did
not shed when rubbed with gloved hands. Devices were easy to suture through.
Device 11
Description: SERI Surgical Scaffold fused with SF sponge (6x6 cm)
Execution: Non-sterile SERI Surgical Scaffolds were cut with sterile
stainless steel
scissors into 6 x 6 cm squares. Separately, to obtain a sponge-like
biomaterial, silk
fibroin solution (c = 8.1 % w/v) (7.5 ml) was cast in OmniTray lids and put
into the -
80 C freezer for two hours. Frozen samples were lyophilized for 24 hours to
dry, then
treated with 15 ml of ethanol (100% v/v) for 45 min. Sponges were then removed
from
the tray, the edges were cut off, flipped over and returned to the tray for an
additional
30 minutes of ethanol incubation. Subsequently, sponges were dried flat
between 3
lint-free wipes, underneath a plastic tray with a lead ring on top. The sponge
can be
viewed as a particular type of film (a sponge like film).
Silk fibroin solution (c = 8.1% w/v) was used to mount the mesh onto the
films.
Specifically, 2 ml of silk solution were added to the lid of a sterile 100 cm
Petri dish
and were evenly spread with a sterile pipette tip. The mesh was placed in the
dish until
its surface was uniformly wet Then the mesh was added onto the dried sponge
(to the
side that was in contact with the plate while freezing) and smoothed down with
gloved
fingers to ensure uniform surface attachment. Prototypes were allowed to dry
flat under
OmniTray lids for 1 hour. When dry, sponges were roughly trimmed, placed in
90%
ethanol for 30 minutes, blotted, then put in deionized water for 5 minutes for
first wash.
After first wash, sponges were trimmed down to the size of the 6x6 cm mesh and
then
53
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
put into second wash for 5 minutes. The devices were then dried covered with
OmniTrays and lead rings.
Sterilization: devices were placed in self-sealing pouches and ethylene oxide
(EO)
sterilized.
Packaging/Storage: Devices prepared as above were placed in self-sealing
sterilization
pouches, were EO sterilized then aerated for at least 3 day prior use. During
the
aeration period, devices were kept under environmental conditions.
Testing: Samples were visually assessed for integrity.
Observations: Devices maintained their integrity during and after
sterilization. No
delamination, change in color or sponge cracking was notices upon visual
inspection of
the pouched devices.
Device 11A
Description: SERI Surgical Scaffold fused with SF sponge (6x6 cm)
Execution: Non-sterile SERI Surgical Scaffolds were cut with stainless steel
scissors
into 6 x 6 cm squares. Separately, to obtain a sponge-like biomaterial, silk
fibroin
solution (c = 8.1 % w/v) (15 ml) was then cast in OmniTray lids and put into
the -80 C
freezer for two hours. Frozen samples were lyophilized for 24 hours to dry,
then treated
with 15 ml of ethanol (100% v/v) for 45 min. Sponges were then removed from
the tray,
the edges were cut off , then were returned to the tray for an additional 30
minutes
ethanol incubation. Subsequently, sponges were dried flat covered with
OmniTray lids
and lead rings. The sponge can be viewed as a particular type of film (a
sponge like
film).
Silk fibroin solution (c = 8.1% w/v) was used to mount the mesh onto the
films.
Specifically, 2 ml of silk solution were added to the lid of a sterile 100 cm
Petri dish
and were evenly spread with a sterile pipette tip. The mesh was placed in the
dish until
its surface was uniformly wet. The mesh was added onto the dried sponge (to
the side
54
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
that contacted the tray while freezing) then smoothed down with gloved fingers
to
ensure uniform surface attachment. Prototypes were dried for 1 hour, then
placed in
90% ethanol for 30 minutes and blotted. Subsequently, constructs were placed
in
deionized water for 5 minutes for first wash. After the first wash, sponges
were trimmed
down to the size of the 6x6cm mesh and then placed into second wash for 5
minutes.
The devices were washed one more time then pouched.
Sterilization: Devices were placed in paper polyethylene foil polyethylene
barrier
(PPFP) pouches, sealed using Accu-Seal Sealer Model 630, and e-beam
sterilized.
Packaging/Storage: Devices prepared as above were placed in PPFP pouches,
sealed
using Accu-Seal Sealer Model 630, and e-beam sterilized. The devices were then
kept in pouches and stored under ambient conditions.
Testing: in both MM PE and PPFP pouches were examined two weeks after
sterilization
for overall integrity, delamination, pliability and suturability.
Observations: Devices remained moist during and after sterilization. Devices
appeared
to have good pliability and did not delaminate when crumpled in hand. The
sponges did
not shed when rubbed with gloved hands. The devices were easy to suture
through.
Device Characterization
Materials
= The devices made (as set forth above)
= SERI Surgical Scaffold (Allergan)
= Dulbecco's Phosphate Buffered Saline 1X (DPBS) (ATCC, cat # 30-2200)
Equipment
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
= Thickness dial gauge (SNAP-004, Kafer J100 type C)
= Mechanical testing equipment (lnstron Model E3000)
= Mechanical testing equipment (lnstron Model 8871)
Device swelling
Swelling characterization is crucial for implantable devices since after
surgical
implantation of the device it is important that the device not cause tissue or
nerve
compression due to device volume increases. To determine the extent of any
swelling
of the anti-adhesive devices made, we compared dry device sample thicknesses
to that
of device samples incubated under physiological conditions. Thickness
measurements
were performed with a thickness dial gauge and 15 measurements were taken per
6x6
cm device. To mimic physiological environments, the devices were incubated in
DPBS
at 37 C, 50 rpm for 24 h. The results showed that devices 6, 7A, 8A, 9, 10 has
no or
an insignificant or a clearly unsubstantial amount of swelling ( none or
essentially no
swelling at all, that is + or ¨ 5% of the reference value) (T TEST, p > 0.05)
when
incubated under physiological conditions. The ORC prototypes (PIA, P2A and P3)
were not included in this evaluation since ORC gels in the presence of water
and would
yield erroneous measurements.
Mechanical Testing
The mechanical properties of the devices (devices 1A, 2A, 3, 6, 7A, 8A, 9, 10
and 11A)
assessed their suitability for use in surgical soft tissue repair procedures.
SERI
Surgical Scaffold (SERI mesh) was used as the reference material. Both tear
testing
and burst testing was carried out. Briefly, samples were cut into 40x40 mm for
burst
testing and 10x60 mm for tear testing and were immersed in PBS for 2 h at room
temperature. For burst testing, samples were mounted on the specimen clamp and
the
ball burst fixture was pushed against the sample at a constant rate of 60mm/in
until
sample failure. For tear testing, samples were affixed with clamps and pulled
at a
constant rate of 2400 mm/min until sample failure.
56
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
The burst strength results showed that the fusion of films or sponges to SERI
Surgical
Scaffold did not improve and did or deteriorate the reference's intrinsic
mechanical
properties. All devices showed comparable to or very similar (that is + or ¨
10% of the
reference value) of to the burst strength values of the SERI Surgical
Scaffold control
(t-test, p > 0.05).
The tensile testing of devices 1A, 2A, 3, 6, 7A, 8A, 9, 10 and 11A) also
showed close
similarity to the control with comparable (i.e. + or ¨ about 10% of the
reference value)
"Elongation at break: values obtained for all devices (t-test, p > 0.05).
However, device
was able to withstand about a 10% higher tensile loads at break as compared to
the
SERI reference control (t-test p = 0.02).
Overall, this Example 4 showed that the swelling of the tested devices was
negligible
and they would not pose any risk of compression to the surrounding tissue post-
implantation. The ORC materials could not be tested because the sacrificial
layer
gelled and disintegrated when hydrated, making sample manipulation impossible.
Additionally, the mechanical properties of the devices tested were very
similar to SERI
Surgical Scaffold showing that the devices can provide sufficient mechanical
support
when implanted to assist soft tissue repair.
Example 5
Single Layer and Two Layered Anti-Adhesive Surface Silk Medical Devices
This Example 5 discloses two types of silk medical devices we made and
characterized. Both types of devices we made included a particular new,
knitted silk
mesh (or scaffold). The first type of device we made was a particular knitted
silk mesh
(or scaffold) prepared with at least one surface or side of the device having
an anti-
adhesive (i.e. having a smooth or low profile with full coverage of open space
or pores)
surface. This silk mesh of this first type of device bioresorbs after
implantation over
about 1-3 years. The second type of device we made also comprised a first
layer of
knitted silk mesh (scaffold), as with the first device, and with the anti-
adhesive property
provided by a sacrificial (second) layer attached to or fused to one side of
the first
knitted mesh layer of the second device. The sacrificial layer is comprised
entirely or
mainly of a faster (preferably over at least about 10 days and no more than
about 30
days) bioresorbable yarn. Thus this two layer, second type of device has a
front or top
57
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
side made of the knitted silk mesh (which does not have an anti-adhesive
property) and
a back or bottom side formed by an anti-adhesive, sacrificial layer, which
sacrificial
layer can be made of quickly bioresorbing fibers, such as PGA, PLGA and/or ORC
fibers. The anti-adhesive property of either the first type or device or of
the second
type of device prevents or reduces tissue (for example bowel tissue and/or
abdominal
viscera) adhesion to the (bottom or back) side of the device placed in contact
with the
bowel tissue or the abdominal viscera. It is important to note that the (top
or front) side
of the device is the knitted silk mesh layer of the device which top or front
side of the
device does not have an anti-adhesive property, and in fact the pores on the
top or
front side of the device (for both the first and the second type of device)
facilitates
tissue ingrowth onto and into the front or top side of the device. In this
manner the
device, as with the SERI Surgical Scaffold, provides soft tissue mechanical
support
and a soft tissue load bearing function as new connective tissue forms onto
and into
the slowly biodegrading top or font side of the implanted device.
Thus both types of devices made in this Example 5 had an anti-adhesive
property and
additionally were made using a single step fabrication (textile knitting)
process.
Thus we developed new silk based medical devices (with a knitted silk mesh
layer) for
use in various medical and surgical procedures, including in hernia repair
procedures
which silk based devices due to their anti-adhesive property can resist or
prevent post-
operative adhesion formations on the anti-adhesive side of the device.
Materials
The four types of yarns we used for the embodiments of our invention made
were:
= 6 filament, low twist, sericin extracted silk yarn.
= 9 filament, high twist, sericin extracted silk yarn.
= 45D PGA yarn (for example made by Teleflex Medical as Deknate1 ). This is
an18
filament, 45 denier, violet, polyglycolic acid (PGA) fiber processed with
water-
washable spin finish (up to 4% w/w).
58
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
= 128D PGA yarn (for example also made Teleflex Medical under the Deknatel
brand name). This is a 48 filament, 128 denier, undyed, polyglycolic acid
(PGA) fiber
processed with water-washable spin finish (up to 4% w/w).
The silk yarn was used to make the first type of device or to make the top of
front side
layer of the second type of device. The PGA (non-silk) yarns were used to make
the
sacrificial layer of the second type of device. A mineral oil (such as a
heavy, white,
mineral oil available from Avantor) was used to coat the yarns to facilitate
their knitting.
Oil residue can be later removed from the yarns and/or from the knitted device
by a
variety of methods including soaking (washing) and/or carbon dioxide
treatment.
Equipment
The backwinder (single head) used was made by SIMET as model number SE-01.
The knitting machine used was made by COM EZ as model number EL-800-8B. This
is
a double needle bed warp knitter with 8 bar capability including two long
throw bars.
The thickness gauge made by Kafer as model number J-100 C
The testing equipment we used was made by lnstron as model number E3000
(tensile
tester).
As set forth above we made two types of silk based medical devices in this
Example 5.
All the devices made included at least a knitted silk mesh (scaffold), for
example as the
base layer. The first type of device made comprised only the knitted silk mesh
with one
side having a low profile, low sheer, full coverage, anti-adhesive property
(i.e. satin
knit). This first type of silk device was made to bioresorb over about 1-3
years after
implantation. The second type of silk device we made comprised the knitted
silk mesh
layer of the first device attached or fused to a second, anti-adhesive,
sacrificial knitted
non-silk fiber layer . The sacrificial layer comprised entirely or mainly a
faster (over at
least about 10 days but over less than about 30 days) bioresorbable non-silk
fibers.
59
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
Devices without a Sacrificial Layer (Base Layer Meshes)
For the base layer mesh of the devices we made with a sacrificial layer or
with an anti-
adhesive we developed a single layer using low denier, low twist yarn using a
knit
pattern that provides a low profile (smooth) surface to the material to
thereby eliminate
or minimize irritation to the bowel and hence remove or substantially reduce
adhesion
formation onto the side of the device (i.e. the smooth side facing the bowel
tissue.
Embodiment we made of such a suitable device we refer to as "the Single Bed
102" or
as the "SBR 202". The SBR 202 devices are six filament mesh devices.
Specifically, the Single Bed 102 devices were made as a series of "SS-P01-0X"
devices, where X is an integer 1 and higher (i.e. the devices referenced as
the series
of devices P01-01-0X). Thus several versions of this device were made (i.e.
the SS-
P01-01-OX device versions). This device has a low profile (smooth) surface on
the
bowel facing side made on a single bed (front bed) knitting machine with about
half the
stitch density used for SERI Surgical Scaffold, resulting in a thinner, low
sheer, full
coverage knitted silk fabric and a low (smooth) loop profile.
Number of bars used: 03 (bars # 4, 5, and 7)
Knitting beds: Front only (10 gauge).
Bed Spacing:0.8 mm
Pick density: 18 picks/cm
Type of needle used: Latch needle (Comez part #61326, Groz-Beckert part # SN-S
51.60 G01)
Number of needles used: 25
Pattern length: 12
Creel setup:
Front creel: 28 ends on left side for bar # 7 (lay-in)
25 ends on right side for bar # 5 (pillar stitch).
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
Back creel: 25 ends on left side for bar # 4 (pillar stitch)
Feed rollers setup:
Feeder # 18: Feeding 25 ends to bar # 4
Feeder # 20: Feeding 02 ends to bar # 7 (ends for outer most edges)
Feeder # 21: Feeding 02 ends to bar # 7 (second-in from outer edges)
Feeder # 22: Feeding 25 ends to bar # 5
Feeder # 23: Feeding 24 ends to bar # 7 (bulk of the lay-in)
Bar swing setup: 15.5 mm with centered swing
Chain links and bar threading
Bar # 7 (lay-in) full set as 9-9, 9-9, 7-7, 7-7, 9-9, 9-9, 1-1, 1-1, 3-3, 3-3,
1-1, 1-1
Bar # 5 (pillar) full set as (3-1, 1-1, 1-3, 3-3) x 3
Bar # 4 (pillar) full set as (1-3, 3-3, 3-1, 1-1) x 3
Figure 15 shows the knit pattern diagram for Single Bed 102 mesh device and
Figure 16 shows the appearance of the Single Bed 102 device.
Anti-adhesive knitted (satin) devices
With this device, the anti-adhesive layer (side facing bowel) was made using
silk yarn
and a satin knit pattern combined to the SS-P01-01 (single bed 102 design).
The satin
knitting consists of long strides of yarn crossing back and forth along more
than one
needle. This back and forth motion of the yarn creates a "wood stack" type of
design
that runs along the fabric course direction leading to a lustrous appearance
and the
"smooth" hand characteristic of satin fabrics. The percent coverage of the
surface can
be controlled by the crossing angle and the amount of yarn crossing at a given
time.
The crossing angle is controlled by the number of needles across which the
yarn
61
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
crosses and the amount of yarn is controlled by the number of threads per
guide and
the yarn denier (reference number satin series: SS-P02-02-0X).
Number of bars used: 04 (bars # 2, 4, 5, and 7)
Knitting beds: Front only (10 gauge).
Bed Spacing: 1.0 mm
Pick density: 18 picks/cm
Type of needle used: Latch needle (Gomez part #61326, Groz-Beckert part # SN-S
51.60
G01)
Number of needles used: 25 for S-P02-02-01 through 03
30 for S-P02-02-08 through 13
Pattern length: 12 for SS-P02-02-01, 02, 03, and 10
08 for SS-P02-02-13
04 for SS-P02-02-08, and 09
Creel setup: the settings below are for patterns made with 30 needles
Front creel: 33 ends on left side for bar # 7 (lay-in)
30 ends on right side for bar # 5 (pillar).
Back creel: 30 ends on left side for bar # 4 (pillar)
Nx(30-C) ends on left side for bar # 2 (satin)
Where N is the number of ends/threads per guide. C is the number of needles
that the
satin yarn is crosses in each stride.
Feed rollers setup:
Feeder # 17: Feeding Nx(30-C) ends to bar # 2
Feeder # 18: Feeding 30 ends to bar # 4
62
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
Feeder # 20: Feeding 02 ends to bar # 7 (ends for outer most edges)
Feeder # 21: Feeding 02 ends to bar # 7 (second-in from outer edges)
Feeder # 22: Feeding 30 ends to bar # 5
Feeder # 23: Feeding 29 ends to bar # 7 (bulk of the lay-in)
Bar swing setup: 15.5 mm with centered swing.
Bar # 7 (lay-in) full threading: 9-9, 9-9, 7-7, 7-7, 9-9, 9-9, 1-1, 1-1, 3-3,
3-3, 1-1, 1-1
Bar # 5 (pillar) full threading: (3-1, 1-1, 1-3, 3-3) x 3
Bar # 4 (pillar) full threading: (1-3, 3-3, 3-1, 1-1) x 3
Bar # 2 (satin) full threading: (3-1, 5-5, 9-11, 5-5) x 3
Figure 17 shows the knit pattern diagram for certain satin devices and
Figure 18 shows the appearance of a silk based satin device.
Devices with a Sacrificial Layer
The second type of devices we made in this Example 5 had a sacrificial layer.
The (top
or front) side facing the abdominal wall or muscle area was made primarily or
entirely
of knitted silk (that is porous and mechanically strong) and promotes tissue
integration.
The (bottom or back) side facing the bowel comprises the sacrificial layer.
This bottom
or back side layer is made of a material or a composite that allows temporary
tissue
adhesion to the sacrificial layer to occur. Shortly after implantation (within
about 10
days to about 30 days after implantation), the sacrificial layer is
mechanically
compromised by being biodegraded and bioresorbed leading to the separation of
the
adhering tissue (i.e. bowel tissue) from the device. The sacrificial layer
comprising
devices are biocompatible, made by knitting (textile machinery) of yarns by a
twisting,
backwinding, and warp knitting process, and as noted can for example lose at
least
50% of their mechanical integrity or strength within 10-30 days after
implantation of the
63
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
device with such a sacrificial layer. We determined that suitable materials to
comprise
the sacrificial layer can be knittable non-silk fibers of polyglycolic acid
(PGA), poly
lactic-co-glycolic acid (PLGA), oxidized regenerated cellulose (ORC),
carboxymethylcellulose (CMC) and combinations thereof. The
sacrificial layers of
embodiment made were made using the 45D PGA yarn but can also be made using
various deniers of PGA, PLGA, ORC, CMC or a combination thereof.
Embodiments of Sacrificial Layer comprising devices made :
1. Shag carpet device (several versions of this shag carpet device were made,
as the
S-P02-02-0X and SS-P02-03-0X device versions). These devices had a shag carpet
like structure with the protruding loops act as the sacrificial layer (side
facing bowel).
The shag carpet devices consisted of two components or layers. The first was
the
base layer of (knitted silk) fabric that provided the overall fabric integrity
and the load
distribution when subjected to external mechanical forces. The second layer
was a
loose (non-silk) knitted yarn that forms extended loops protruding vertically
away from
the base fabric plane, hence giving the fabric its characteristic loopy or
shag like
texture. The percent loop coverage of the surface can be controlled by the
loop length
(controlled by feed rate), the amount of loose yarn per loop (controlled both
by feed
rate, yarn count, and number of threads per count), and the number of loops
per
surface area (controlled by machine gauge used and pattern).
In the case of the devices described, loops can be formed on the base silk
fabric
(device SS-P01-01) by using a simple closed (or open) tricot stitch swinging
back and
forth between adjacent needles with high feed rate.
Number of bars used: 04 (bars # 2, 4, 5, and 7)
Knitting beds: Front only (10 gauge)
Bed Spacing: 1.0 mm
Pick density: 18 picks/cm
Type of needle used: Latch needle (Comez part #61326, Groz-Beckert part # SN-S
51.60 G01)
64
CA 02921966 2016-02-19
WO 2015/027144
PCT/US2014/052264
Number of needles used: 25 for SS-P02-02-05 and 06 and SS-P02-03-02 through 09
30 for SS-P02-02-06, 07, and 12
Pattern length:04 for SS-P02-02-12 and 12 for other than SS-P02-02-12
Creel setup: the settings below are for the patterns made with 30 needles
Front creel: 33 ends on left side for bar # 7 (lay-in)
30 ends on right side for bar # 5 (pillar).
Back creel: 30 ends on left side for bar # 4 (pillar)
29 ends on left side for bar # 2 (loops)
Feed rollers setup:
Feeder # 17: Feeding 29 ends to bar # 2 (loops)
Feeder # 18: Feeding 30 ends to bar # 4 (pillar)
Feeder # 20: Feeding 02 ends to bar # 7 (ends for outer most edges)
Feeder # 21: Feeding 02 ends to bar # 7 (second-in from outer edges)
Feeder # 22: Feeding 30 ends to bar # 5 (pillar)
Feeder # 23: Feeding 29 ends to bar # 7 (bulk of the lay-in)
Bar swing setup: 15.5 mm with centered swing
The pattern for all 'shag carpet' devices except for SS-P02-02-12 was;
Bar # 7 (lay-in) full threading as 9-9, 9-9, 7-7, 7-7, 9-9, 9-9, 1-1, 1-1, 3-
3, 3-3, 1-1, 1-1
Bar # 5 (pillar) full threading as (3-1, 1-1, 1-3, 3-3) x 3
Bar # 4 (pillar) full threading as (1-3, 3-3, 3-1, 1-1) x 3
Bar # 2 (satin) full threading as (3-1, 3-3, 3-5, 3-3) x 3
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
The Pattern for device SS-P02-02-12 was:
Bar # 7 (lay-in) full threading as 9-9, 9-9, 1-1, 1-1
Bar # 5 (pillar) full threading as 3-1, 1-1, 1-3, 3-3
Bar # 4 (pillar) full threading as 1-3, 3-3, 3-1, 1-1
Bar # 2 (satin) full threading as 3-1, 3-3, 3-5, 3-3
Guide (heddle) threading: For all prototypes, Bars # 4, 5, and 7 were single
threaded
(one end per heddle) . As for bar # 2, the following threading was used:
Single for SS-P02-03-02 through 09 and SS-P02-02-05
Double for SS-P02-02-06
Triple 45D PGA violet for SS-P02-02-07
Triple 45D PGA violet for SS-P02-02-12.
Figure 19 shows a pattern diagram and chain links for an exemplary Shag Carpet
device.
Figures 20 shows the appearance of an exemplary knitted fabric (shag carpet)
device.
2. Another embodiment of a sacrificial layer comprising device made was the
satin
series reference number SS-P02-02-0X. With these devices, the sacrificial, non-
silk
layer (the side facing the bowel, and made of PGA, PLGA, ORC, CMC or
combinations
thereof) was made using a 'satin' knit pattern combined to the SS-P01-01
(single bed
102 design). 'Satin' consists of long strides of non-silk yarn crossing back
and forth
along more than one needle. This back and forth motion of the yarn creates
'wood
stack' type of design that run along the fabric course direction leading to a
lustrous
66
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
appearance and smooth hand characteristic of 'satin' fabrics. The percent
coverage of
the surface can be controlled by the crossing angle and the amount of yarn
crossing at
a given time. The crossing angle is controlled by the number of needles across
which
the yarn crosses and the amount of yarn is controlled by the number of threads
per
guide and the yarn denier.
Number of bars used: 04 (bars # 2, 4, 5, and 7)
Knitting beds: Front only (10 gauge).
Bed Spacing: 1.0 mm
Pick density: 18 picks/cm
Type of needle used: Latch needle (Gomez part #61326, Groz-Beckert part # SN-S
51.60
G01)
Number of needles used: 25 for S-P02-02-01 through 03
30 for S-P02-02-08 through 13
Pattern length: 12 for SS-P02-02-01, 02, 03, and 10
08 for SS-P02-02-13
04 for SS-P02-02-08, and 09
Creel setup: the settings below are for patterns made with 30 needles
Front creel: 33 ends on left side for bar # 7 (lay-in)
30 ends on right side for bar # 5 (pillar).
Back creel: 30 ends on left side for bar # 4 (pillar)
Nx(30-C) ends on left side for bar # 2 (satin)
Where N is the number of ends/threads per guide. C is the number of needles
that the
'satin' yarn is crosses in each stride.
67
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
Feed rollers setup:
Feeder # 17: Feeding Nx(30-C) ends to bar # 2
Feeder # 18: Feeding 30 ends to bar # 4
Feeder # 20: Feeding 02 ends to bar # 7 (ends for outer most edges)
Feeder # 21: Feeding 02 ends to bar # 7 (second-in from outer edges)
Feeder # 22: Feeding 30 ends to bar # 5
Feeder # 23: Feeding 29 ends to bar # 7 (bulk of the lay-in)
Bar swing setup: 15.5 mm with centered swing.
Bar # 7 (lay-in) full threading: 9-9, 9-9, 7-7, 7-7, 9-9, 9-9, 1-1, 1-1, 3-3,
3-3, 1-1, 1-1
Bar # 5 (pillar) full threading: (3-1, 1-1, 1-3, 3-3) x 3
Bar # 4 (pillar) full threading: (1-3, 3-3, 3-1, 1-1) x 3
Bar # 2 (satin) full threading: (3-1, 5-5, 9-11, 5-5) x 3
Figure 21 shows the appearance of a representative sacrificial layer
comprising satin
device.
Devices with Detachable Layers (several versions of this device were made, as
the SS-
PO4-0X detachable layer version series)
Unlike the satin and the shag carpet devices, the non-silk sacrificial layer
in this device
was not integrated with the base knitted silk fabric. It
instead constituted an
independent layer that peeled away from the base fabric within 30 days of
implantation.
It was typically a tightly knit non-silk fabric with small pore size (70 ¨ 200
micron
diameter). As depicted in Figure 22. The non-silk sacrificial layer and the
knitted silk
base fabric were linked together using a fast resorbing/degrading yarn that
was
68
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
designed to be the first component of the assemble to fail mechanically
leading
therefore to the separation of the two layers.
To do so, a double needle bed was needed. The front bed was used to knit the
base
fabric as in SS-P01-01 (bars # 4, 5, and 7) made out of silk and integrates
with the
abdominal wall tissue and act as the main load carrier. Meanwhile, the back
bed was
used to knit the sacrificial layer (bar # 1). This layer can be made using
either slow
bioresorbing material like silk or fast resorbing material like PGA or ORC.
Finally, the
yarn used to link both fabrics (layers) was threaded through a dedicated bar
(bar # 2)
and knits on both beds. This yarn consisted of low denier PGA or any other
fast
resorbing/degrading yarn, e.g. PLGA (90-10) and ORC yarns.
The detachable (triple) layered devices (reference number of detachable
layered
devices: SS-PO4-0X), unlike for satin and "shag carpet" devices, these
embodiments
knit independently two textile layers (the base layer and the detaching layer)
and then
knit them together with a fast resorbing/degrading yarn (resulting in a three
layered
device). The middle layer disintegrated and so separated of the two outer
layers within
10-30 days of implantation (see Figure 21), thus preventing the adhesion of
tissue to
the base layer while keeping the tissue covered with the detaching layer. The
detaching layer consisted of a tightly knit non-silk fabric with small pore
size (70 ¨ 200
micron diameter). A double needle bed knitter was used. The front bed was used
to
knit the base fabric as in SS-P01-01 (bars # 4, 5, and 7). Meanwhile, the back
bed was
used to knit the detaching layer (bar # 1). This layer can be made using
either slow
bioresorbing material like silk or fast resorbing material like PGA, PLGA or
ORC.
Finally, the yarn used to link both fabrics (layers) was threaded through a
dedicated bar
(bar # 2) and knits on both beds (constituting the middle layer). This yarn
consisted of
low denier PGA or any other fast resorbing/degrading yarn, e.g. PLGA (90-10)
and
ORC yarns.
The percent coverage of the surface was controlled by the gauge used on the
back
bed, crossing angle, and the amount of yarn crossing at a given time. The
crossing
angle was controlled by the number of needles across which the yarn crosses
and the
amount of yarn is controlled by the number of threads per guide and the yarn
denier.
Weft insertion between both fabric layers was an additional option to increase
surface
coverage and shield direct exposure between silk (in the base fabric) and
bowel
69
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
surface. Figure 22 illustrates this three layer device and Figure 23 shows the
pattern
diagram and chain links used for an embodiment of such a device.
Number of bars used: 05 (bars # 1, 2, 4, 5, and 7)
Weft insertion: none for SS-PO4-01
Single 45D PGA for SS-PO4-02-01 and 03
Triple 45D PGA for SS-PO4-02-02
Note: the weft insertion bar was place at the bar position # 3
Knitting beds: Front (10 gauge) and back (20 gauge) beds.
Bed spacing: 1.0 mm
Pick density: 22 picks/cm
Type of needle used: Latch needle (Gomez part #61326, Groz-Beckert part # SN-S
51.60
G01)
Number of needles used:30
Pattern length: 12
Creel setup: the settings was for patterns made with 30 needles
Front creel: 33 ends on left side for bar # 7 (lay-in ¨ base fabric)
30 ends on right side for bar # 5 (pillar ¨ base fabric)
Back creel: 30 ends on left side for bar # 4 (pillar ¨ base fabric)
30 ends on right side for bar # 2 (pillar ¨ linker)
60 ends on right side for bar # 1 (tricot ¨ sacrificial fabric)
1-4 ends in the middle for weft insertion
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
Feed rollers setup: Feeder # 16: Feeding 60 ends to bar # 1
Feeder # 17: Feeding 30 ends to bar # 2
Feeder # 18: Feeding 30 ends to bar # 4
Feeder # 20: Feeding 02 ends to bar # 7 (ends for outer most edges)
Feeder # 21: Feeding 02 ends to bar # 7 (second-in from outer edges)
Feeder # 22: Feeding 30 ends to bar # 5
Feeder # 23: Feeding 29 ends to bar # 7 (bulk of the lay-in)
Bar swing setup: 3.5 mm with centered swing
Bar # 7 (lay-in) full 10gg threading: 9-9, 9-9, 7-7, 7-7, 9-9, 9-9, 1-1, 1-1,
3-3, 3-3, 1-1, 1-
1
Bar # 5 (pillar) full 10gg threading: (3-1, 1-1, 1-3, 3-3) x 3
Bar # 4 (pillar) full 10gg threading: (1-3, 3-3, 3-1, 1-1) x 3
Bar # 2 (linker) full 10gg threading: (1-3, 3-1) x 6
Bar # 2 (tricot) full 20gg threading: (3-3, 1-3, 3-3, 5-3) x 3
Note: Variants of the 'detachable layer concept can be made by changing the
chain
links on bar # 1 from closed to open loops and by varying the number of
needled (C)
crossed per swing.
Guide (heddle) threading: For all prototypes, Bars # 4, 5, and 7 were single
threaded
(one end per heddle) using SUB-YNO9E-001.
As for bar # 2 the following threading was used:
Single for SS-PO4-01
Single 45D PGA violet for SS-PO4-02-0X
71
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
As for bar # 1 the following threading was used:
Single for SS-PO4-03
Double 45D PGA violetfor SS-PO4-02-01 and 02.
Figures 24 shows the appearance of the knitted fabric
Device Testing and Characterization
All device testing was performed with a sample size of n = 15.
Thickness
Device (n =15) thickness was measured using the J-100 Kafer thickness gauge.
The
average thickness values one standard deviation are shown in the Figure 25
graph
below. All SS-PDX devices were 22 to 37% percent thinner than SERI Surgical
Scaffold
(also known as Standard 102 or as SERI Standard). This confirms the thinner
profile
produced from knitting on a single bed with low pick density. The thinner
profile along
with the non-looping course design resulted in a smoother fabric (hand feel).
SS-P01-
01-01 had the lowest thickness value that correlates with the absence of any
added
material (e.g. PGA, ORC, etc. to the back of the fabric.
Burst Strength
Device burst testing was carried out. Average burst strength and stiffness
values one
standard deviation are shown in Figure 26 and 27.
Except for SS-P01-01-01, all other tested SS-PDX series had 10% to 150%
increase in
the burst strength and stiffness at time 0 when compared to the SERI
Standard. The
lower values recorded for SS-P01-01-01 can be explained by the lower densities
of silk
used to make the prototype and lack of any other yarns attached to the back,
i.e. PGA.
72
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
For abdominal wall reconstruction, the maximal anatomical (intra-abdominal)
pressure
is about 20 kPa. To withstand this, the device has a minimal burst strength of
about
0.11 MPa Based on these considerations, the burst strength values determined
for the
silk/PGA prototypes indicated that they were suitable for abdominal
reconstruction
procedures. In terms burst stiffness, any device with a stiffness value equal
or higher
than the SERI Standard can be suitable for abdominal wall reconstruction.
Suture pull out
Device suture pull out testing was carried out. Average suture pull-out
strength values
one standard deviation are reported in Figure 28.
The suture pull-out strength for all SS-PDX prototypes was equal or higher (up
to 70 %
higher in the case of SS-P02-02-10) to that of the SERI Standard at time
zero. Given
that the SERI Standard suture pull-out strength is compatible with abdominal
wall
repair procedures, all SS-PDX prototypes can perform adequately in the
abdominal
setting.
Tensile testing
Device tensile testing was performed both in the wale and course directions.
Tensile
testing (single pull to failure) was performed in the direction of fabric
formation and in
the fabric width (course) direction. Average tensile strength, % elongation at
break, and
values one standard deviation are reported in Figures 29 to 32. Figure 29
shows the
maximum load in the machine (fabric length) direction. Figure 30 shows the
percent
elongation at break in the machine (fabric length) direction. Figure 31 shows
the
maximum load in the course (fabric width) direction. Figure 32 shows the
percent
elongation at break in the course (fabric width) direction.
Based on these results, all SS-PDX were 77 -150% stronger in the machine
direction
then the SERI Standard. Along the fabric width however, the strength was
ranging
from half as strong (SS-P01-01-01) to two and half times stronger (SS-P02-02-
08) than
SERI Standard. Additionally, the pattern change described for the satin
devices, that
73
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
decrease the device pore size 3x, lead to a threefold increase in the strength
along the
fabric width. This is a result of a threefold increase of courses per unit
length (SS-P02-
02-02 and SS-P02-02-08). These results illustrate how the mechanical
properties of the
knitted devices can be modulated by controlling the knit design.
[000107] In closing, it is to be understood that although aspects of the
present
specification have been described with reference to the various embodiments,
one
skilled in the art will readily appreciate that the specific examples
disclosed are only
illustrative of the principles of the subject matter disclosed herein.
Therefore, it should
be understood that the disclosed subject matter is in no way limited to a
particular
methodology, protocol, and/or reagent, etc., described herein. As such,
various
modifications or changes to or alternative configurations of the disclosed
subject matter
can be made in accordance with the teachings herein without departing from the
spirit
of the present specification. Lastly, the terminology used herein is for the
purpose of
describing particular embodiments only, and is not intended to limit the scope
of the
present invention, which is defined solely by the claims. Accordingly, the
present
invention is not limited to that precisely as shown and described.
[000108] Certain embodiments of this invention are described herein, including
the
best mode known to the inventors for carrying out the invention. Of course,
variations
on these described embodiments will become apparent to those of ordinary skill
in the
art upon reading the foregoing description. The inventor expects skilled
artisans to
employ such variations as appropriate, and the inventors intend for the
invention to be
practiced otherwise than specifically described herein. Accordingly, this
invention
includes all modifications and equivalents of the subject matter recited in
the claims
appended hereto as permitted by applicable law. Moreover, any combination of
the
above-described elements in all possible variations thereof is encompassed by
the
invention unless otherwise indicated herein or otherwise clearly contradicted
by
context.
[000109] Groupings of alternative elements or embodiments of the invention
disclosed
herein are not to be construed as limitations. Each group member may be
referred to
74
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
and claimed individually or in any combination with other members of the group
or
other elements found herein. It is anticipated that one or more members of a
group
may be included in, or deleted from, a group for reasons of convenience and/or
patentability. When any such inclusion or deletion occurs, the specification
is deemed
to contain the group as modified thus fulfilling the written description of
all Markush
groups used in the appended claims.
[000110] Unless otherwise indicated, all numbers expressing quantities of
ingredients,
properties such as molecular weight, reaction conditions, and so forth used in
the
specification and claims are to be understood as being modified in all
instances by the
term "about." As used herein, the term "about" means that the item, parameter
or term
so qualified encompasses a range of plus or minus ten percent above and below
the
value of the stated item, parameter or term. Accordingly, unless indicated to
the
contrary, the numerical parameters set forth in the specification and attached
claims
are approximations that may vary depending upon the desired properties sought
to be
obtained by the present invention. At the very least, and not as an attempt to
limit the
application of the doctrine of equivalents to the scope of the claims, each
numerical
parameter should at least be construed in light of the number of reported
significant
digits and by applying ordinary rounding techniques. Notwithstanding that the
numerical ranges and parameters setting forth the broad scope of the invention
are
approximations, the numerical values set forth in the specific examples are
reported as
precisely as possible. Any numerical value, however, inherently contains
certain errors
necessarily resulting from the standard deviation found in their respective
testing
measurements
[000111] The terms "a," "an," "the" and similar referents used in the context
of
describing the invention (especially in the context of the following claims)
are to be
construed to cover both the singular and the plural, unless otherwise
indicated herein
or clearly contradicted by context. Recitation of ranges of values herein is
merely
intended to serve as a shorthand method of referring individually to each
separate
value falling within the range. Unless otherwise indicated herein, each
individual value
is incorporated into the specification as if it were individually recited
herein. All methods
described herein can be performed in any suitable order unless otherwise
indicated
herein or otherwise clearly contradicted by context. The use of any and all
examples, or
exemplary language (e.g., "such as") provided herein is intended merely to
better
CA 02921966 2016-02-19
WO 2015/027144 PCT/US2014/052264
illuminate the invention and does not pose a limitation on the scope of the
invention
otherwise claimed. No language in the specification should be construed as
indicating
any non-claimed element essential to the practice of the invention.
[000112] Specific embodiments disclosed herein may be further limited in the
claims
using consisting of or consisting essentially of language. When used in the
claims,
whether as filed or added per amendment, the transition term "consisting of"
excludes
any element, step, or ingredient not specified in the claims. The transition
term
"consisting essentially of" limits the scope of a claim to the specified
materials or steps
and those that do not materially affect the basic and novel characteristic(s).
Embodiments of the invention so claimed are inherently or expressly described
and
enabled herein.
[000113] All patents, patent publications, and other publications referenced
and
identified in the present specification are individually and expressly
incorporated herein
by reference in their entirety for the purpose of describing and disclosing,
for example,
the compositions and methodologies described in such publications that might
be used
in connection with the present invention. These publications are provided
solely for
their disclosure prior to the filing date of the present application. Nothing
in this regard
should be construed as an admission that the inventors are not entitled to
antedate
such disclosure by virtue of prior invention or for any other reason. All
statements as to
the date or representation as to the contents of these documents is based on
the
information available to the applicants and does not constitute any admission
as to the
correctness of the dates or contents of these documents.
76